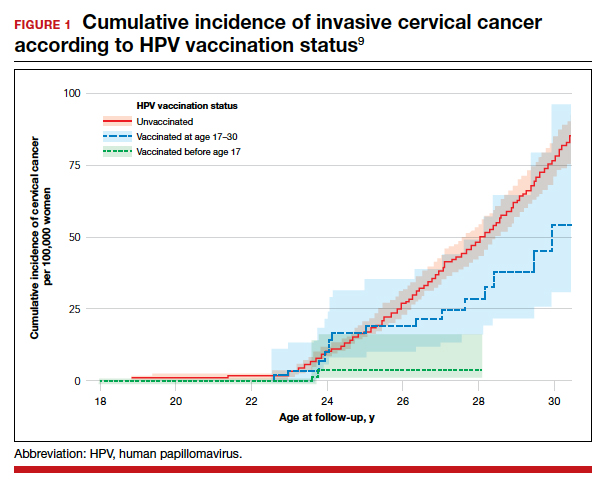
Cervical cancer rates were lowest in women vaccinated before age 17
A total of 1,672,983 women were included in the study; 527,871 received at least one dose of the HPV vaccine. During the study period, cervical cancer was diagnosed in 19 women who had received the quadrivalent HPV vaccine and in 538 women who had not received the vaccine. Women who initiated vaccination before age 17 had the lowest rates of cervical cancer (4 cases per 100,000 persons), followed by women vaccinated after age 17 (54 cases per 100,000 persons) and then those who were not vaccinated (94 cases per 100,000 persons).
After adjusting for confounders, the incidence rate ratio (RR) of cervical cancer was significantly lower among vaccinated women compared with unvaccinated women (RR, 0.37; 95% confidence interval [CI], 0.21– 0.57) (FIGURE 1).9 In addition, women who were vaccinated before age 17 demonstrated the greatest benefit. For those vaccinated before age 17 versus those who were unvaccinated, the RR was 0.12 (95% CI, 0.00–0.34). For women vaccinated between age 17 and 30 versus unvaccinated women, the RR was 0.47 (95% CI, 0.27–0.75).
The study by Lei and colleagues showed that HPV vaccination was associated with a substantially lower risk of invasive cervical cancer. While all women who received the vaccine had reduced rates of invasive cervical cancer, those who received the vaccine earlier (before age 17) showed the greatest reduction in invasive cervical cancer. On a population level, this study demonstrates that a program of HPV vaccination can reduce the burden of cervical cancer.
Promising option for patients with advanced endometrial cancer: Lenvatinib plus pembrolizumab
Makker V, Taylor MH, Aghajanian C, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer. J Clin Oncol. 2020;38:2981-2992.
Advanced stage endometrial cancer is associated with a 17% 5-year survival rate.10 Paclitaxel with carboplatin is the standard first-line treatment for advanced, recurrent, and metastatic endometrial cancer; for women who do not respond to this regimen, effective treatment options are limited.11,12
The immunotherapy approach
Immunotherapy is a more recently developed treatment, an approach in which the immune system is activated to target cancer cells. Pembrolizumab is a commonly used agent for many solid tumors.13 This drug binds to the programmed cell death receptor 1 (PD-1) or PD-ligand 1 (PD-L1), a component of the immune checkpoint, which then allows the immune system to target and destroy cancer cells.14
Prembrolizumab is FDA approved for use in the treatment of microsatellite instability-high (MSI-H)/mismatch repair deficient (dMMR) solid tumors that have progressed after prior therapy and for which there are no satisfactory alternative treatment options.15 Endometrial cancers frequently display microsatellite instability and mismatch repair defects.16
Lenvatinib is an oral multikinase inhibitor that targets vascular endothelial growth factor receptors 1, 2, and 3; fibroblast growth factor receptors 1, 2, 3, and 4; and platelet derived growth factor receptor alpha, RET, and KIT.17-19 In a phase 2 study of lenvatinib monotherapy for advanced previously treated endometrial cancer, the response rate was 14.3%.20
While some preclinical studies have examined the combination of immune checkpoint inhibitors with lenvatinib,21-23 a recent study is the first to evaluate this combination in patients with advanced tumors.24
Continue to: Prembrolizumab-lenvatinib combination therapy...