How should I be approaching cervical cancer screening: with primary human papillomavirus (HPV) testing, or cotesting? We get this question all the time from clinicians. Although they have heard of the latest cervical cancer screening guidelines for stand-alone “primary” HPV testing, they are still ordering cervical cytology (Papanicolaou, or Pap, test) for women aged 21 to 29 years and cotesting (cervical cytology with HPV testing) for women with a cervix aged 30 and older.
Changes in cervical cancer testing guidance
Cervical cancer occurs in more than 13,000 women in the United States annually.1 High-risk types of HPV—the known cause of cervical cancer—also cause a large majority of cancers of the anus, vagina, vulva, and oropharynx.2
Cervical cancer screening programs in the United States have markedly decreased the incidence of and mortality from cervical cancer since introduction of the Pap smear in the 1950s. In 2000, HPV testing was approved by the US Food and Drug Administration (FDA) as a reflex test to a Pap smear result of atypical squamous cells of undetermined significance (ASC-US). HPV testing was then approved for use with cytology as a cotest in 2003 and subsequently as a primary stand-alone test in 2014.
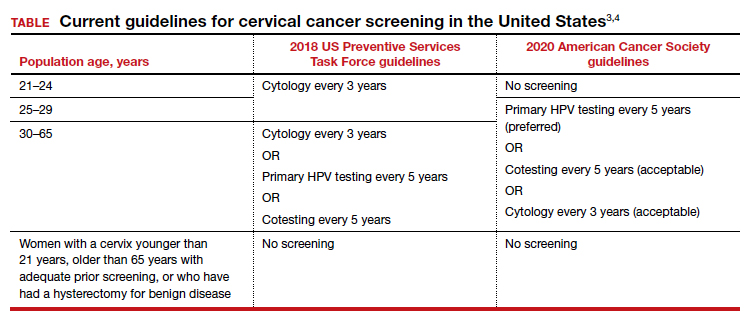
Recently, the American Cancer Society (ACS) released new cervical screening guidelines that depart from prior guidelines.3 They recommend not to screen 21- to 24-year-olds and to start screening at age 25 until age 65 with the preferred strategy of primary HPV testing every 5 years, using an FDA-approved HPV test. Alternative screening strategies are cytology (Pap) every 3 years or cotesting every 5 years.
The 2018 US Preventive Services Task Force (USPSTF) guidelines differ from the ACS guidelines. The USPSTF recommends cytology every 3 years as the preferred method for women with a cervix who are aged 21 to 29 years and, for women with a cervix who are aged 30 to 65 years, the option for cytology every 3 years, primary HPV testing every 5 years, or cotesting every 5 years (TABLE).4
Why the reluctance to switch to HPV testing?
Despite FDA approval in 2014 for primary HPV testing and concurrent professional society guidance to use this testing strategy in women with a cervix who are aged 25 years and older, few practices in the United States have switched over to primary HPV testing for cervical cancer screening.5,6 Several reasons underlie this inertia:
- Many practices currently use HPV tests that are not FDA approved for primary HPV testing.
- Until recently, national screening guidelines did not recommend primary HPV testing as the preferred testing strategy.
- Long-established guidance on the importance of regular cervical cytology screening promoted by the ACS and others (which especially impacts women with a cervix older than age 50 who guide their younger daughters) will rely on significant re-education to move away from the established “Pap smear” cultural icon to a new approach.
- Last but not least, companies that manufacture HPV tests and laboratories integrated to offer such tests not yet approved for primary screening are promoting reliance on the prior proven cotest strategy. They have lobbied to preserve cotesting as a primary test, with some laboratory database studies showing gaps in detection with HPV test screening alone.7-9
Currently, the FDA-approved HPV tests for primary HPV screening include the Cobas HPV test (Roche) and the BD Onclarity HPV assay (Becton, Dickinson and Company). Both are DNA tests for 14 high-risk types of HPV that include genotyping for HPV 16 and 18.
Continue to: Follow the evidence...