CASE Patient concerned about hormonal contraception’s impact on lactation
A 19-year-old woman (G2P1102) is postpartum day 1 after delivering a baby at 26 weeks’ gestation. When you see her on postpartum rounds, she states that she does not want any hormonal contraception because she heard that it will decrease her milk supply. What are your next steps?
The American Academy of Pediatrics recently updated its policy statement on breastfeeding and the use of human milk to recommend exclusive breastfeeding for 6 months and continued breastfeeding, with complementary foods, as mutually desired for 2 years or beyond given evidence of maternal health benefits with breastfeeding longer than 1 year.1
Breastfeeding prevalence—and challenges
Despite maternal and infant benefits associated with lactation, current breastfeeding prevalence in the United States remains suboptimal. In 2019, 24.9% of infants were exclusively breastfed through 6 months and 35.9% were breastfeeding at 12 months.2 Furthermore, disparities in breastfeeding exist, which contribute to health inequities. For example, non-Hispanic Black infants had lower rates of exclusive breastfeeding at 6 months (19.1%) and any breastfeeding at 12 months (24.1%) compared with non-Hispanic White infants (26.9% and 39.4%, respectively).3
While many new mothers intend to breastfeed and initiate breastfeeding in the hospital after delivery, overall and exclusive breastfeeding continuation rates are low, indicating that patients face challenges with breastfeeding after hospital discharge. Many structural and societal barriers to breastfeeding exist, including inadequate social support and parental leave policies.4 Suboptimal maternity care practices during the birth hospitalization may lead to challenges with breastfeeding initiation. Health care practitioners may present additional barriers to breastfeeding due to a lack of knowledge of available resources for patients or incomplete training in breastfeeding counseling and support.
To address our case patient’s concerns, clinicians should be aware of how exogenous progestins may affect breastfeeding physiology, risk factors for breastfeeding difficulty, and the available evidence for safety of hormonal contraception use while breastfeeding.
Physiology of breastfeeding
During the second half of pregnancy, secretory differentiation (lactogenesis I) of mammary alveolar epithelial cells into secretory cells occurs to allow the mammary gland to eventually produce milk.5 After delivery of the placenta, progesterone withdrawal triggers secretory activation (lactogenesis II), which refers to the onset of copious milk production within 2 to 3 days postpartum.5 Most patients experience secretory activation within 72 hours; however, a delay in secretory activation past 72 hours is associated with cessation of any and exclusive breastfeeding at 4 weeks postpartum.6
Impaired lactation can be related to a delay in secretory activation or to insufficient lactation related to low milk supply. Maternal medical comorbidities (for example, diabetes mellitus, thyroid dysfunction, obesity), breast anatomy (such as insufficient glandular tissue, prior breast reduction surgery), pregnancy-related events (preeclampsia, retained placenta, postpartum hemorrhage), and infant conditions (such as multiple gestation, premature birth, congenital anomalies) all contribute to a risk of impaired lactation.7
Guidance on breastfeeding and hormonal contraception initiation
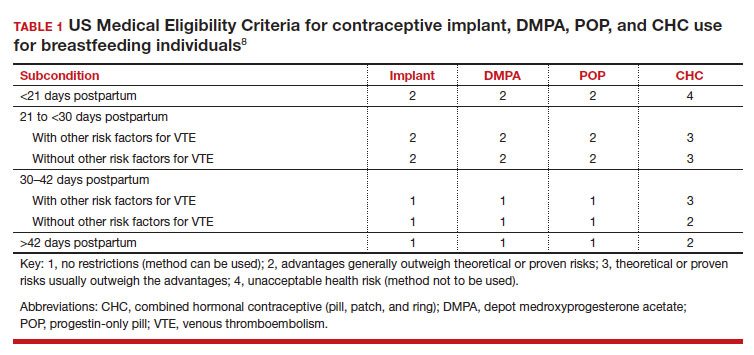
Early initiation of hormonal contraception poses theoretical concerns about breastfeeding difficulty if exogenous progestin interferes with endogenous signals for onset of milk production. The Centers for Disease Control and Prevention US Medical Eligibility Criteria (MEC) for Contraceptive Use provide recommendations on the safety of contraceptive use in the setting of various medical conditions or patient characteristics based on available data. The MEC uses 4 categories in assessing the safety of contraceptive method use for individuals with specific medical conditions or characteristics: 1, no restrictions exist for use of the contraceptive method; 2, advantages generally outweigh theoretical or proven risks; 3, theoretical or proven risks usually outweigh the advantages; and 4, conditions that represent an unacceptable health risk if the method is used.8
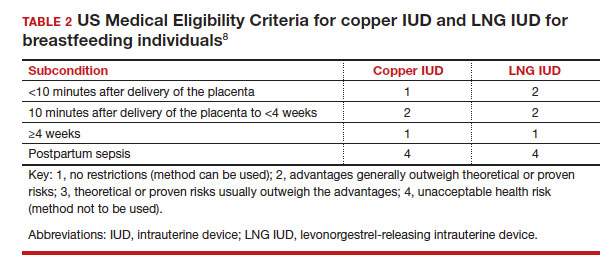
In the 2016 guidelines, combined hormonal contraceptives are considered category 4 at less than 21 days postpartum, regardless of breastfeeding status, due to the increased risk of venous thromboembolism in the immediate postpartum period (TABLE 1).8 Progestin-only contraception is considered category 1 in nonbreastfeeding individuals and category 2 in breastfeeding individuals based on overall evidence that found no adverse outcome with breastfeeding or infant outcomes with early initiation of progestin-only contraception (TABLE 1, TABLE 2).8
Since the publication of the 2016 MEC guidelines, several studies have continued to examine breastfeeding and infant outcomes with early initiation of hormonal contraception.
- In a noninferiority randomized controlled trial of immediate versus delayed initiation of a levonorgestrel intrauterine device (LNG IUD), any breastfeeding at 8 weeks in the immediate group was 78% (95% confidence interval [CI], 70%–85%), which was lower than but within the specified noninferiority margin of the delayed breastfeeding group (83%; 95% CI, 75%–90%), indicating that breastfeeding outcomes with immediate initiation of an LNG IUD were not worse compared with delayed initiation.9
- A secondary analysis of a randomized trial that compared intracesarean versus LNG IUD placement at 6 or more weeks postpartum showed no difference in breastfeeding at 6, 12, and 24 weeks after LNG IUD placement.10
- A randomized trial of early (up to 48 hours postpartum) versus placement of an etonogestrel (ENG) implant at 6 or more weeks postpartum showed no difference between groups in infant weight at 12 months.11
- A randomized trial of immediate (within 5 days of delivery) or interval placement of the 2-rod LNG implant (not approved in the United States) showed no difference in change in infant weight from birth to 6 months after delivery, onset of secretory activation, or breastfeeding continuation at 3 and 6 months postpartum.12
- In a prospective cohort study that compared immediate postpartum initiation of ENG versus a 2-rod LNG implant (approved by the FDA but not marketed in the United States), there were no differences in breastfeeding continuation at 24 months and exclusive breastfeeding at 6 months postpartum.13
- In a noninferiority randomized controlled trial that compared ENG implant initiation in the delivery room (0–2 hours postdelivery) versus delayed initiation (24–48 hours postdelivery), the time to secretory activation in those who initiated an ENG implant in the delivery room (66.8 [SD, 25.2] hours) was noninferior to delayed initiation (66.0 [SD, 35.3] hours). There also was no difference in ongoing breastfeeding over the first year after delivery and implant use at 12 months.14
- A secondary analysis of a randomized controlled trial examined breastfeeding outcomes with receipt of depot medroxyprogesterone acetate (DMPA) prior to discharge in women who delivered infants who weighed 1,500 g or less at 32 weeks’ or less gestation. Time to secretory activation was longer in 29 women who received DMPA (103.7 hours) compared with 141 women who did not (88.6 hours; P = .028); however, there was no difference in daily milk production, lactation duration, or infant consumption of mother’s own milk.15
While the overall evidence suggests that early initiation of hormonal contraception does not affect breastfeeding or infant outcomes, it is important for clinicians to recognize the limitations of available data with regard to the populations included in these studies. Specifically, most studies did not include individuals with premature, low birth weight, or multiple gestation infants, who are at higher risk of impaired lactation, and individuals with a higher prevalence of breastfeeding were not included to determine whether early initiation of hormonal contraception would impact breastfeeding. Furthermore, while these studies enrolled participants who planned to breastfeed, data indicate that intentions to initiate and continue exclusive breastfeeding can vary.16 As the reported rates of any and exclusive breastfeeding are consistent with or lower than current US breastfeeding rates, any decrease in breastfeeding exclusivity or duration that may be attributable to hormonal contraception may be unacceptable to those who are strongly motivated to breastfeed.
Continue to: How can clinicians integrate evidence into contraception counseling?...