Heavy menstrual bleeding (HMB) is a major gynecologic problem, affecting more than 30% of women at some point in their life. Evaluation of HMB can be extensive, including a history and physical exam; transvaginal ultrasonography; hysteroscopy or sonohysterography; assessment for anemia; and, when indicated, endometrial sampling. Some women who have HMB may need to be evaluated for a bleeding diathesis, such as von Willebrand disease or a platelet disorder.
Many women who have HMB have a structural abnormality of the uterus, such as a submucous fibroid or endometrial polyp. For them, surgical treatment (if the condition is amenable) typically resolves their heavy bleeding.
Others have ovulatory bleeding, but no detectable structural uterine abnormality or identifiable bleeding diathesis. In community practice, these women are typically treated with a two-step protocol:
- medication, such as a nonsteroidal anti-inflammatory agent or a hormonal preparation (estrogen-progestin or progestin only)
- procedure-based treatment if medical therapy is unsuccessful: insertion of a levonorgestrel-releasing intrauterine system (LNG-IUS), endometrial ablation, or hysterectomy.
Tranexamic acid (Lysteda) (FIGURE 1) is a recently approved nonhormonal treatment for HMB. It provides an additional non-hormonal option that would be used in Step 1 of this protocol.
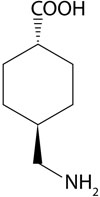
FIGURE 1 Chemical structure of tranexamic acid
How tranexamic acid produces its effect
Endometrial concentrations of tissue plasminogen activator and plasmin are elevated in women who have HMB. Tranexamic acid blocks the action of plasmin, thereby reducing fibrin degradation, stabilizing clots, and reducing menstrual bleeding (FIGURE 2). It has no effect on standard blood coagulation parameters, such as the platelet count, activated partial thromboplastin time, and prothrombin time. Tranexamic acid is eliminated, largely intact, in urine. Its terminal half-life is approximately 2 hours.
Randomized clinical trials have demonstrated that tranexamic acid is effective for HMB.
Study #1. 294 women who had HMB were randomized to one of three arms: placebo; tranexamic acid, 1,950 mg/d for 5 days each menstrual cycle; or tranexamic acid, 3,900 mg/d for the same duration. Menstrual blood loss was reduced in the three groups by 5%, 25%, and 39%, respectively. Self-reported quality of life was most improved among women who received active drug at 3,900 mg/d.1
Study #2. 187 women who had HMB were randomized to placebo or tranexamic acid, 3,900 mg/d, for 5 days. Menstrual blood loss was reduced by 12% in the placebo group and 38% in the group taking tranexamic acid.2
Notably, women who had uterine myomata were allowed to enter this trial. In a preliminary analysis (reported as an abstract), the reduction in menstrual blood loss was similar in women who did not (38%) and who did (34%) have myomata.
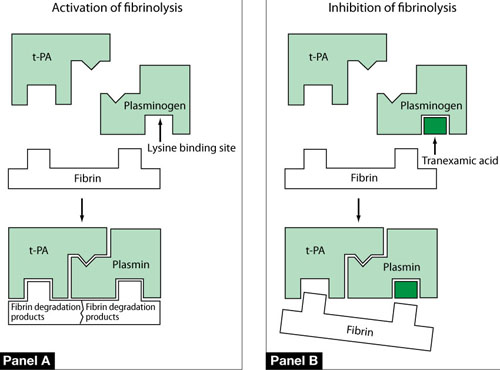
FIGURE 2 One mechanism by which tranexamic acid acts on fibrinolysis
Panel A In a healthy, untreated person, plasminogen binds to fibrin at a lysine binding site and is converted to plasmin in the presence of tissue plasminogen activator (t-PA). Plasmin is able to degrade fibrin filaments into fibrin degradation products.
Panel B Tranexamic acid binds to plasminogen and its activated plasmin form, thereby blocking binding sites that would bind lysine residues on fibrin. Consequently, cleavage of fibrin is inhibited.
Source: Modified from Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;57(6):1005–1032.
Dosing, adverse effects, contraindications
The FDA has approved tranexamic acid at a dosage of 1,300 mg (two 650-mg tablets) three times daily for 5 days each menstrual cycle. The medication cycle is typically initiated on the first day of menstrual bleeding. Cost of a single 5-day treatment cycle (i.e., six tablets daily for 5 days—30 650-mg tablets, total) in the United States is approximately $175.
The most common side effects reported in clinical trials were:
- headache
- sinus and nasal symptoms
- back pain
- musculoskeletal pain.
Women who have a history of venous thromboembolism (VTE) or strong risk factors for VTE should not use tranexamic acid because of concern that, by reducing fibrin degradation, the drug may increase the tendency to clot. A strong scientific link between tranexamic acid and VTE is controversial, however. In two observational studies from Sweden (where tranexamic acid is available over the counter), investigators reported no increase in VTE events associated with the drug.3,4
For HMB associated with a clotting disorder
Women who have von Willebrand disease or a platelet aggregation and release disorder are at increased risk of HMB. They are often prescribed intranasal desmopressin (DDAVP), to be used during menses to improve coagulation and reduce HMB.
In one head-to-head trial of DDAVP and tranexamic acid for HMB in women who had abnormal clotting, tranexamic acid (1 g, four times daily for the first 5 days of the menstrual cycle) resulted in a greater reduction in menstrual blood loss than DDAVP.5


