Clinical Review

5 IUD myths dispelled
Although it is gaining popularity in the United States, the IUD is often overlooked as a contraceptive option because of several common...
Deborah Reale, Managing Editor

A spherical device is undergoing multinational testing to determine its efficacy versus traditional IUDs
Most intrauterine devices (IUDs) worldwide, and all IUDs currently available in the United States have a 2-dimensional T-shaped design. A new device, called the intrauterine ball (IUB), consists of 17 copper spheres threaded over a plastic-coated wire constructed of nitinol, an alloy with elastic characteristics.1,2 The wire and copper spheres are placed in the uterus using a 3.2-mm outer-diameter tube. When released from the tube, the wire and spheres assume a circular configuration within the endometrial cavity that is larger than the cervical canal.1,2
Details of a small study
Dr. Ilan Baram and colleagues recently published a case series in the journal Contraception in which 15 women (mean age 33.9 years; one woman was nulliparous) were followed for one year. The study was supported in part by the National Institutes of Health (NIH) and also by Ocon Medical, the device manufacturer (two of the authors reported being officers of Ocon Medical).1
The report authors noted that the IUB was easily inserted in each woman. No perforations, expulsions, malpositioned devices, or pregnancies were observed. Due to heavy uterine bleeding, the IUB was removed from one woman at 17 weeks; curettage revealed simple endometrial hyperplasia. Three women reported transient abdominal discomfort. At one year, 12 women indicated they were “very satisfied” with this contraceptive; one indicated she was “satisfied”; and one indicated she was “moderately satisfied”.1 (One woman discontinued the study at 119 days after insertion for a reason not related to the device.)
What is a practitioner’s point of view regarding this new IUD design? Andrew M. Kaunitz, MD, Professor and Associate Chairman of the Department of Obstetrics and Gynecology at the University of Florida College of Medicine in Jacksonville, and member of the OBG Management Board of Editors, says, “Although the design of the IUB as well as the findings from this small study are intriguing, it will be important to learn how the IUB’s performance (including rates of failure, infection, perforation, expulsion, device malposition, and continuation as well as insertion pain and impact on bleeding) compare with existing IUDs.”
Additional trials planned
The study authors indicate that a larger randomized trial comparing the IUB with the Copper T380 is now underway in Europe.1
A North American clinical trial3 has begun to evaluate early expulsion rates of Ocon’s second product, the IUB SCu380A, with a larger copper surface area (380 mm2 copper surface area) than the original SCu300A (300 mm2 copper surface area).2 The trial site, located in Vancouver, Canada, will recruit 50 women. The trial will examine expulsion rates after 2 months and will continue to follow these women for 12 months.
According to a press release, Ocon hopes to gather clinical evidence to understand the IUB’s advantages over current IUDs, especially with respect to its safety profile and quality of life attributes. To accomplish this, Ocon will initiate additional multinational clinical trials in the next several months.3,4
To watch a video that highlights the design of the Ocon Medical IUB, click here.

Although it is gaining popularity in the United States, the IUD is often overlooked as a contraceptive option because of several common...

In this in-depth interview, hear headache expert Dr. Anne Calhoun discuss how oral contraceptives can help your patients with migraine—despite...

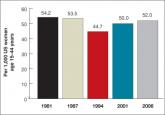
Why we have not yet reduced the unintended pregnancy rate
