VIENNA – Topical crisaborole 2% ointment administered twice a day was consistently associated with clinically meaningful quality of life improvement scores on multiple measures in the two pivotal phase III, randomized, controlled trials of atopic dermatitis (AD) patients aged 2 years old through adulthood, Amy S. Paller, MD, reported at the annual congress of the European Academy of Dermatology and Venereology.
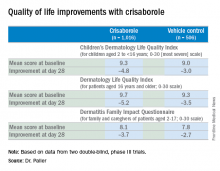
AD in children and adolescents is infamous for the adverse quality of life impact it imposes upon the patients’ parents, family, and caregivers. So the significant improvement seen with crisaborole, compared with its vehicle on the Dermatitis Family Impact (DFI) Questionnaire was particularly gratifying. The DFI questionnaire assesses quality of life in key domains, including family, parent, and caregiver sleep, emotional distress, relationships, family leisure, and ability to do housework or go shopping.
“If approved, crisaborole ... could improve the quality of life for patients with mild to moderate atopic dermatitis and, very importantly, for their families as well,” declared Dr. Paller, professor of dermatology and chair of the department of dermatology and professor of pediatrics at Northwestern University, Chicago.
Crisaborole’s developer, Anacor Pharmaceuticals, has filed an application for approval for treating mild to moderate AD in patients aged 2 years and older, now under review at the Food and Drug Administration.
In a separate presentation at the EADV congress, Lawrence F. Eichenfield, MD, presented the results of a long-term, open-label crisaborole safety study of 48-52 weeks duration. The long-term study involved 517 participants in the two pivotal phase III trials. There were no serious adverse events and no long-term cutaneous adverse events such as the skin atrophy or telangiectasias that can occur with topical steroids. The safety profile was favorable for long-term treatment of patients 2 years of age or older with mild to moderate AD.
“What’s nice about this study is that the number of grams of drug used was enough to provide a good picture of safety. Patients used a total of 760 g on average over the course of 11 or 12 months, or about 45 to 70 g per month of b.i.d. utilization, so they had reasonable exposure to the medication,” observed Dr. Eichenfield, professor of dermatology and pediatrics at the University of California, San Diego, and chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego.Crisaborole 2% topical ointment is a novel, boron-based, nonsteroidal inhibitor of phosphodiesterase 4 (PDE-4). AD is marked by overactivity of PDE-4, which results in decreased levels of cyclic AMP and resultant increased release of inflammatory cytokines.
Dr. Paller noted that, in the previously reported efficacy results of the two pivotal, double-blind, 28-day, phase III trials, crisaborole treatment reduced global disease severity and provided early and sustained improvement in itch severity. She presented the prespecified quality of life results for the two identically designed, parallel pivotal trials, which totaled 1,016 patients on crisaborole and 506 on its vehicle. At baseline, 39% of subjects had mild AD, and 61% had moderate AD. The mean body surface area affected was 18%. Participants’ mean age was 12.3 years, and 14% were aged 8 years old or older.
All three quality of life instruments featured 10 questions addressing key quality of life domains. The response to each question could be scored from 0 (not at all) to 3 (very much).The structure of the long-term safety study suggests how crisaborole might be used in clinical practice. During the year-long, open-label study, patients were evaluated every 28 days. If their skin was deemed clear or almost clear on the basis of an Investigator’s Static Global Assessment (ISGA) score of 0 or 1, they were taken off crisaborole and could use only emollients for the next 28 days, at which time they would be reevaluated. At that point, if they had an ISGA of 2 or more, they went back on crisaborole twice a day for 28 days until their next evaluation.
Dr. Eichenfield reported that 10.2% of participants in the long-term safety study reported treatment-related adverse events, which were mild to moderate. The most frequently reported of these were mild to moderate flares of AD during a 28-day off-treatment period in 1.3% of patients, application site pain in 2.3%, and application site infection in 1.2%.
Dr. Paller and Dr. Eichenfield reported serving as consultants to Anacor Pharmaceuticals.
The FDA review of crisaborole is expected to be completed by early January 2017, according to Anacor.