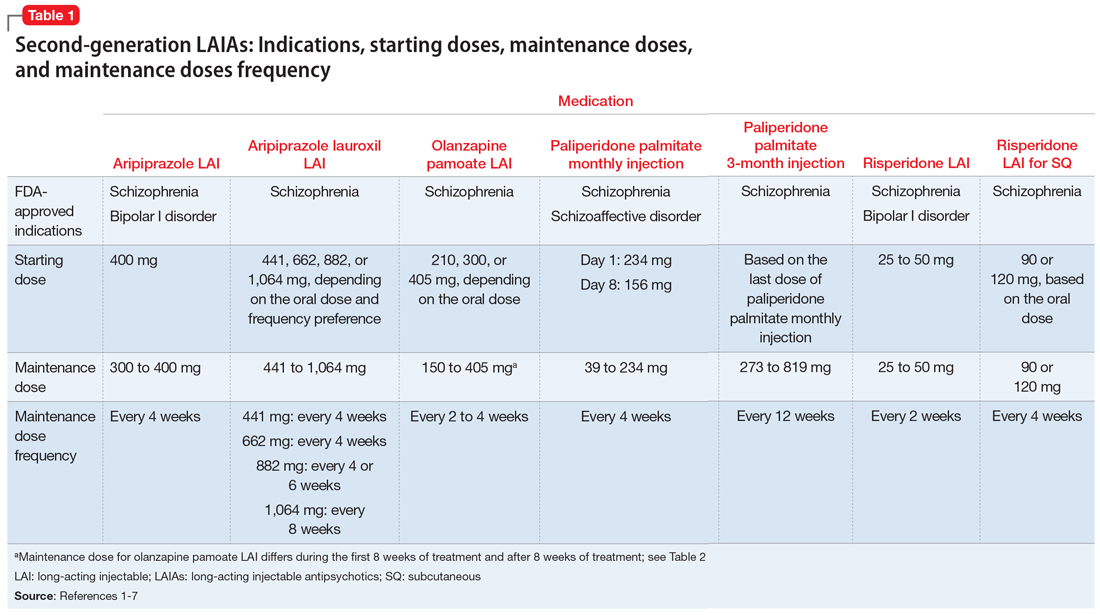
There are currently 7 FDA-approved second-generation long-acting injectable antipsychotics (LAIAs).1-7 These LAIAs provide a unique dosage form that allows patients to receive an antipsychotic without taking oral medications every day, or multiple times per day. This may be an appealing option for patients and clinicians, but because there are several types of LAIAs available, it may be difficult to determine which LAIA characteristics are best for a given patient.
Since the FDA approved the first second-generation LAIA, risperidone long-acting injectable (LAI),1 in 2003, 6 additional second-generation LAIAs have been approved:
- aripiprazole LAI
- aripiprazole lauroxil LAI
- olanzapine pamoate LAI
- paliperidone palmitate monthly injection
- paliperidone palmitate 3-month LAI
- risperidone LAI for subcutaneous (SQ) injection.
When discussing medication options with patients, clinicians need to consider factors that are unique to each LAIA. In this article, I describe the similarities and differences among the second-generation LAIAs, and address common questions about these medications.
A major potential benefit: Increased adherence
One potential benefit of all LAIAs is increased medication adherence compared with oral antipsychotics. One meta-analysis of 21 randomized controlled trials (RCTs) that compared LAIAs with oral antipsychotics and included 5,176 patients found that LAIAs had a similar efficacy to oral antipsychotics in preventing relapse.8 However, a meta-analysis of 25 mirror-image studies comparing LAIAs with oral antipsychotics that included 5,940 patients found that LAIAs were superior in preventing hospitalization.9 In these mirror-image studies, participants received oral antipsychotics first and then switched to LAIAs, and the 2 study periods were compared. Because mirror-image studies are observational, participants do not engage with research teams to the extent that they do in RCTs.9 Although mirror-image studies have limitations, participants in these studies may be a better representation of patients encountered in clinical practice due to the extensive monitoring and follow-up RCT participants typically receive.9
Differences in FDA-approved indications
The 7 currently available LAIAs vary in terms of FDA-approved indications, dose options, frequency, need for oral antipsychotic overlap, route of administration, and other factors. Table 11-7 summarizes some of these differences. Although all second-generation LAIAs are approved for schizophrenia,1-7 risperidone LAI and aripiprazole LAI are also approved for bipolar I disorder.1,4 Paliperidone palmitate monthly injection is the only LAIA approved for treating patients with schizoaffective disorder.2
Starting doses
For most LAIAs, the starting dose is the same as the maintenance dose (Table 11-7). One exception is paliperidone palmitate monthly injection, which requires a 234-mg dose on Day 1 followed by a 156-mg dose on Day 8 for all patients, regardless of the maintenance dose required.2 The 156-mg dose may be given 4 days before or after Day 8.2 The first maintenance dose of paliperidone palmitate monthly injection should be administered 5 weeks after the 234-mg dose on Day 1.2 Before starting paliperidone palmitate 3-month injection, patients should be stable on paliperidone palmitate monthly injection for 4 months, and the 2 most recent doses of paliperidone palmitate monthly injection should be the same.3
Maintenance doses
Dosing frequency may be an important factor for some patients when deciding to receive a LAIA. The frequency of the maintenance doses for all second-generation LAIAs varies from every 2 weeks to 12 weeks (Table 11-7). Paliperidone palmitate 3-month LAI is the only LAIA that is administered every 12 weeks.3 Some dosages of aripiprazole lauroxil LAI are administered every 6 or 8 weeks.6 All other second-generation LAIAs are given every 2 to 4 weeks.
Continue to: Start with an oral antipsychotic