Recommendations for missed doses
Each LAIA has specific recommendations for missed dosing. Carpenter and Wong12 reviewed the recommendations for managing missed LAIA doses in Current Psychiatry July 2018. This article is available at mdedge.com/psychiatry/article/168776/schizophrenia-other-psychotic-disorders/long-acting-injectable.12
Consider patient preference
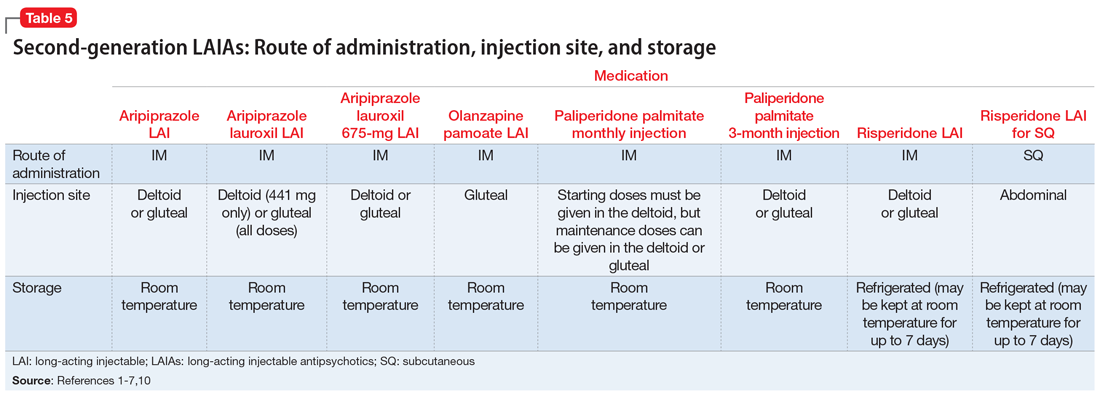
Patient preference for the type and location of the injection may factor into a clinician’s choice of LAIA (Table 51-7,10). Risperidone LAI for SQ is the only LAIA that is administered as an SQ abdominal injection.7 All other LAIAs are IM injections in the deltoid or gluteal muscle.1-6 All doses of risperidone LAI, paliperidone palmitate 3-month LAI, aripiprazole LAI, and aripiprazole lauroxil 675-mg LAI can be administered in the deltoid or gluteal muscle.1,3,4,10 Deltoid administration is required for the 2 starting doses of paliperidone palmitate monthly injection, but maintenance doses can be administered in the deltoid or gluteal muscle. Because administration into the deltoid results in a higher concentration of the drug compared with gluteal administration, administering the 2 starting doses of paliperidone palmitate monthly injection into the deltoid helps to rapidly attain therapeutic concentrations.2 Olanzapine pamoate LAI should be administered only in the gluteal muscle.5 The 441-mg dose of aripiprazole lauroxil LAI may be administered in the deltoid or gluteal muscle, but all other doses of aripiprazole lauroxil LAI should be administered only in the gluteal muscle.6
Storage
Most LAIAs can be stored at room temperature2-6; however, risperidone LAI and risperidone LAI for SQ need to be stored in the refrigerator. Both risperidone LAI and risperidone LAI for SQ may be kept at room temperature for up to 7 days. If they are not used within 7 days at room temperature, they should be discarded.1,7
Clinical pearls for specific LAIAs
Aripiprazole LAI. The recommended starting and maintenance dose for aripiprazole LAI is 400 mg monthly, unless the patient has drug interactions or other factors that require dose adjustment. If patients experience adverse reactions to the 400-mg dose, a reduction to 300 mg monthly could be considered.4
Olanzapine pamoate LAI has a Risk Evaluation and Mitigation Strategy (REMS) due to the potential for post-injection delirium/sedation syndrome (PDSS). Prescribing clinicians, dispensing pharmacies, and administering health care facilities must all be certified to prescribe, dispense, or administer olanzapine pamoate LAI. The patient must also be enrolled in the REMS program.13 Patients must be observed by health care staff for 3 hours after receiving a dose of olanzapine pamoate LAI to monitor for signs and symptoms of PDSS.5
Continue to: Risperidone LAI