Training medical students and residents
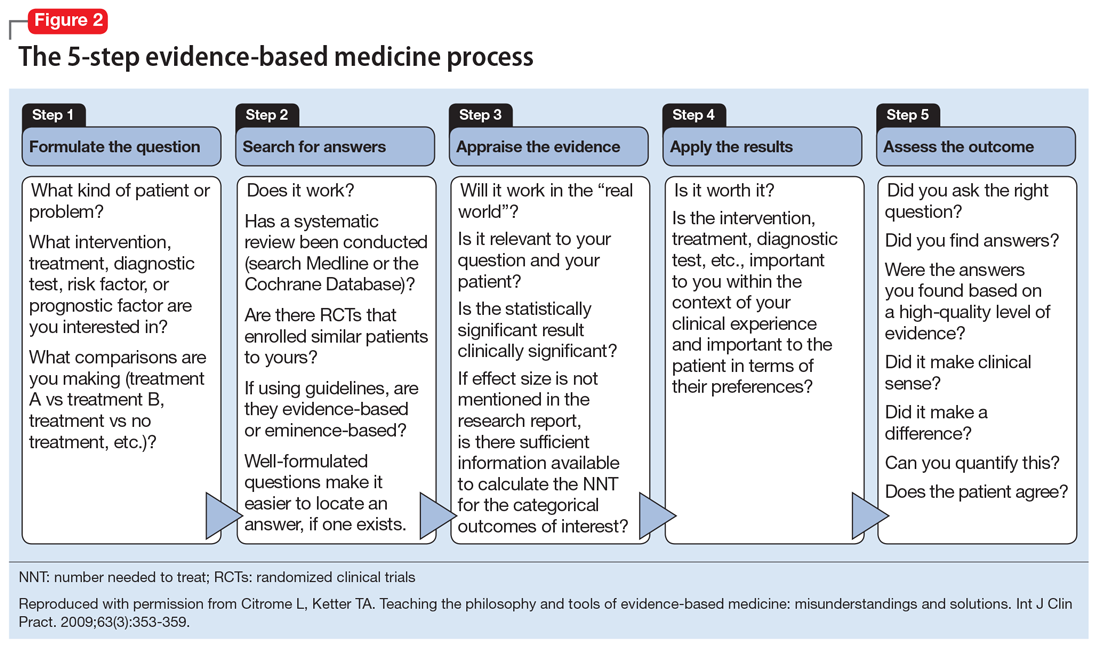
Although there is some variation in how EBM is taught to medical students and residents,7,8 the expectation is that such education occurs. The Accreditation Council for Graduate Medical Education requirements for a residency program state that “the program must advance residents’ knowledge and practice of the scholarly approach to evidence-based patient care.”9 The topic has been part of the American Society of Clinical Psychopharmacology Model Psychopharmacology Curriculum, but only in an optional lecture.10 The formal teaching of EBM includes how to find relevant biomedical publications for the clinical issues at hand, understand the different hierarchies of evidence, interpret results in terms of effect size, and apply this knowledge in the care of patients. This 5-step process is illustrated in Figure 28. See Related Resources for 3 books that provide a scholarly yet clinically relevant approach to EBM.
Continuing medical education
Most Current Psychiatry readers have been out of residency for some time and have not necessarily been exposed to the philosophy and tools of EBM. It may be easier to simply jump in and learn about effect sizes and then use information already curated and apply this knowledge. A good starting point is a recap11,12 of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) for schizophrenia that answers the questions: “How large is the effect being measured?” “Is it clinically important?” and “How are we dealing with a result that may be statistically significant but irrelevant for day-to-day patient care?” Number needed to treat (NNT) and number needed to harm (NNH) can help explain this by allowing one to judge the clinical significance of a statistically significant result.13
Practical applications
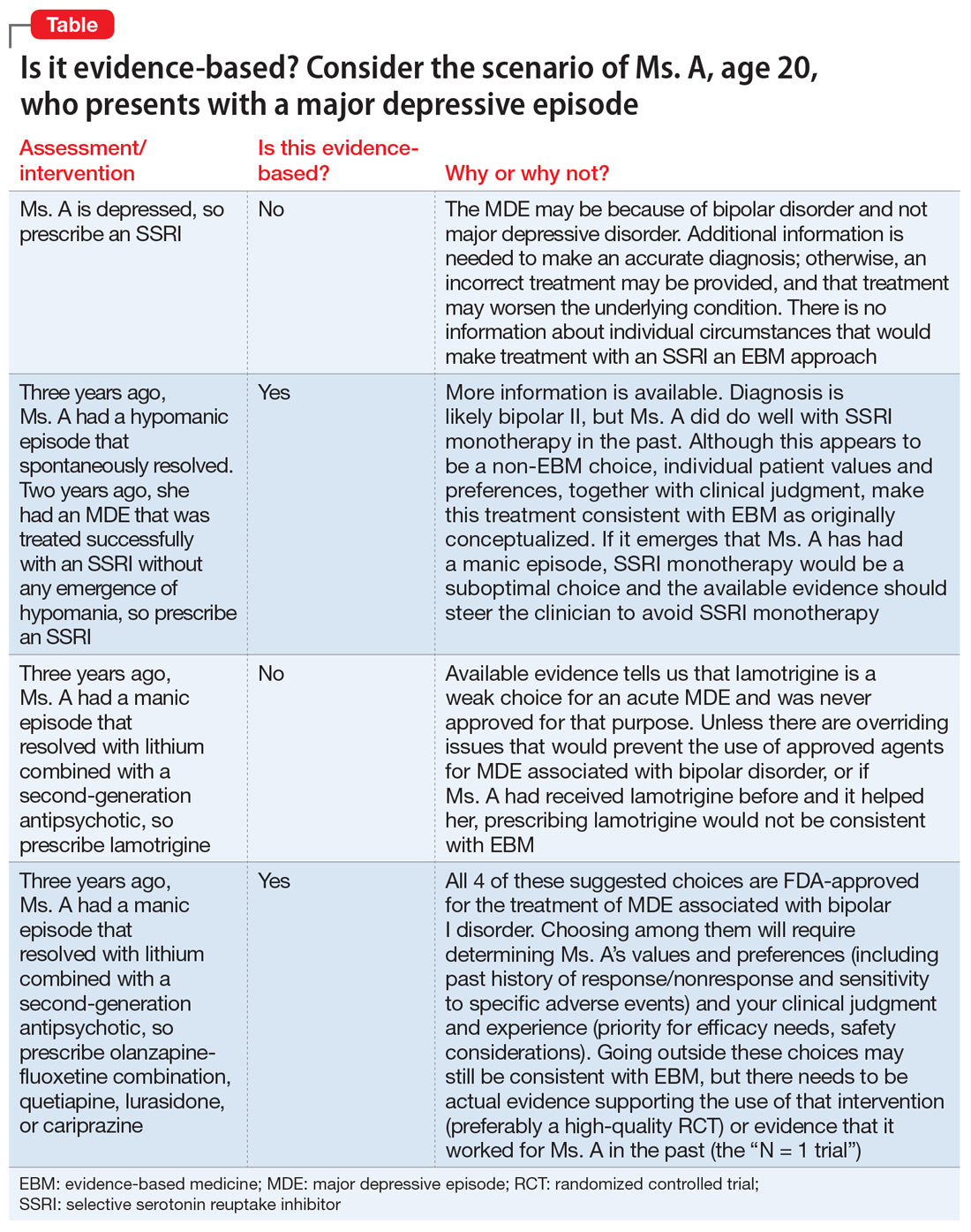
There are common clinical scenarios where evidence is ignored, or where it is overvalued. For example, the treatment of bipolar depression can be made worse with the use of antidepressants.14 Does this mean that antidepressants should never be used? What about patient history and preference? What if the approved agents fail to relieve symptoms or are not well tolerated? Available FDA-approved choices may not always be suitable.15 The Table illustrates some of these scenarios.