Presently, FDA-approved first-line treatments and standard adjunctive strategies (eg, lithium, thyroid supplementation, stimulants, second-generation antipsychotics) for major depressive disorder (MDD) often produce less-than-desired outcomes while carrying a potentially substantial safety and tolerability burden. The lack of clinically useful and individual-based biomarkers (eg, genetic, neurophysiological, imaging) is a major obstacle to enhancing treatment efficacy and/or decreasing associated adverse effects (AEs). While the discovery of such tools is being aggressively pursued and ultimately will facilitate a more precision-based choice of therapy, empirical strategies remain our primary approach.
In controlled trials, several nontraditional treatments used primarily as adjuncts to standard antidepressants have shown promise. These include “repurposed” (off-label) medications, herbal/nutraceuticals, anti-inflammatory/immune system therapies, device-based treatments, and other alternative approaches.
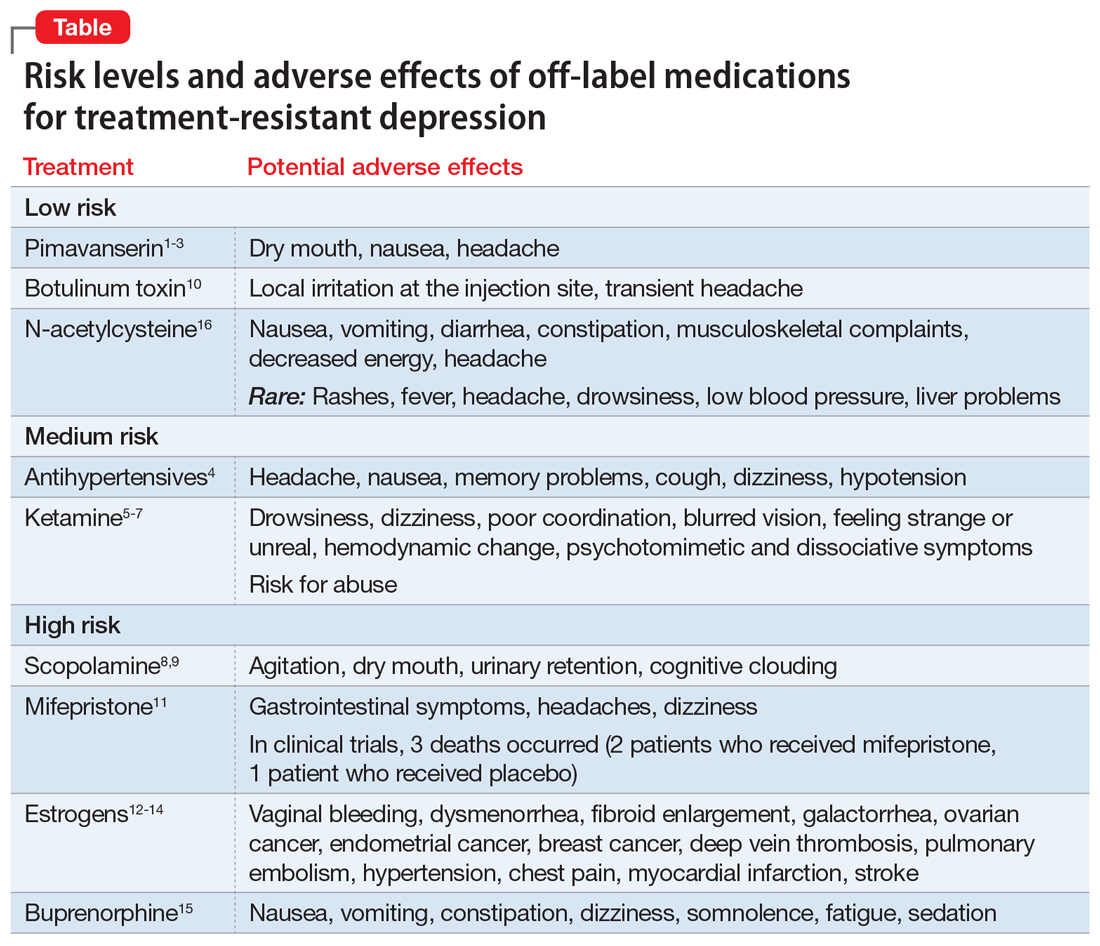
Importantly, some nontraditional treatments also demonstrate AEs (Table1-16). With a careful consideration of the risk/benefit balance, this article reviews some of the better-studied treatment options for patients with treatment-resistant depression (TRD). In Part 1, we will examine off-label medications. In Part 2, we will review other nontraditional approaches to TRD, including herbal/nutraceuticals, anti-inflammatory/immune system therapies, device-based treatments, and other alternative approaches.
We believe this review will help clinicians who need to formulate a different approach after their patient with depression is not helped by traditional first-, second-, and third-line treatments. The potential options discussed in Part 1 of this article are categorized based on their putative mechanism of action (MOA) for depression.
Serotonergic and noradrenergic strategies
Pimavanserin is FDA-approved for treatment of Parkinson’s psychosis. Its potential MOA as an adjunctive strategy for MDD may involve 5-HT2A antagonist and inverse agonist receptor activity, as well as lesser effects at the 5-HT2Creceptor.
A 2-stage, 5-week randomized controlled trial (RCT) (CLARITY; N = 207) found adjunctive pimavanserin (34 mg/d) produced a robust antidepressant effect vs placebo in patients whose depression did not respond to selective serotonin reuptake inhibitors (SSRIs) or serotonin-norepinephrine reuptake inhibitors (SNRIs).1 Furthermore, a secondary analysis of the data suggested that pimavanserin also improved sleepiness (P < .0003) and daily functioning (P < .014) at Week 5.2
Unfortunately, two 6-week, Phase III RCTs (CLARITY-2 and -3; N = 298) did not find a statistically significant difference between active treatment and placebo. This was based on change in the primary outcome measure (Hamilton Depression Rating Scale-17 score) when adjunctive pimavanserin (34 mg/d) was added to an SSRI or SNRI in patients with TRD.3 There was, however, a significant difference favoring active treatment over placebo based on the Clinical Global Impression–Severity score.
Continue to: In these trials...