BERLIN – A switch to long-acting second-generation injectable antipsychotics brought improved attitudes and beliefs regarding antipsychotic therapy among patients with schizoaffective disorder who already were stabilized on a single oral antipsychotic agent, Dr. Francesco Pietrini reported at the annual congress of the European College of Neuropsychopharmacology.
The conventional view has been that long-acting injectable antipsychotics are most appropriate as an alternative option for patients with poor adherence to oral antipsychotics. But this small observational study suggests that the switch is beneficial in a broader population, according to Dr. Pietrini of the University of Florence (Italy).
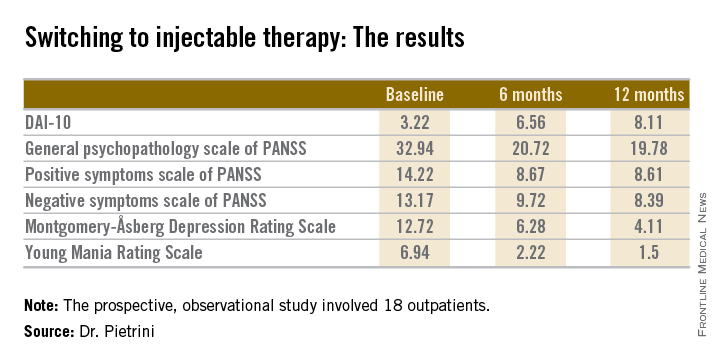
The 18 outpatients who participated in this 12-month, prospective, observational study showed significant improvements in a variety of measures of psychopathology after the switch to long-acting injectable therapy. These benefits were accompanied by a steadily improving attitude toward treatment as reflected in rising scores on the Drug Attitude Inventory-10 (DAI-10).
All patients were stable at baseline on either oral olanzapine or oral paliperidone. They were then switched to the corresponding dose of the long-acting injectable formulation of the same drug and followed prospectively for 12 months.
Mean scores on the 0-10 DAI-10 rose from 3.22 at baseline to 6.56 after 6 months on a long-acting injectable antipsychotic and 8.11 at 12 months. These improvements in scores reflect the patients’ growing endorsement of inventory items such as “taking medication will prevent me from having a breakdown,” “my thoughts are clearer on medication,” and “for me, the good things about medication outweigh the bad.”
Scores on most measures of psychopathology improved significantly from baseline during the first 6 months on long-acting therapy. The improvement was maintained but not expanded upon during the second 6 months. The exception was negative psychotic symptoms as assessed by the negative symptom scale of the Positive and Negative Syndrome Scale (PANSS), which didn’t show significant improvement during the first 6 months but did in the second half of the year-long study (see chart).
Dr. Pietrini reported having no financial conflicts regarding this study, which was conducted free of commercial support.