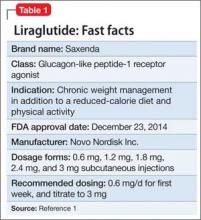
Liraglutide (rDNA origin) injection, approved by the FDA in 2010 for managing type 2 diabetes mellitus (T2DM), has a new formulation and indication for treating obesity in adults as an adjunct to a reduced-calorie diet and increased physical activity (Table 1).1
Liraglutide, recommended dosage 3 mg/d (under the brand name Saxenda), is approved for adults with a body mass index (BMI) ≥30, or those with a BMI of ≥27 and a weight-related condition such as hypertension, T2DM, or high cholesterol.1 (A 1.8-mg formulation, under the brand name Victoza, is FDA-approved for managing T2DM, but is not indicated for weight management.)
How it works
Liraglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist. GLP-1, which regulates appetite and calorie intake, is found in several regions of the brain that are involved in regulating appetite. Patients taking liraglutide lose weight because of decreased calorie intake, not increased energy expenditure.
Liraglutide is endogenously metabolized without a specific organ as a major route of elimination.1
Dosage and administration
Liraglutide is administered using a prefilled, multi-dose pen that can be injected in the abdomen, thigh, or upper arm. Recommended dosage is 3 mg/d, administered any time of day. Initiate dosage at 0.6 mg/d the first week, then titrate by 0.6 mg a week—to reduce the likelihood of adverse gastrointestinal symptoms—until 3 mg/d is reached.
Discontinue liraglutide if a patient has not lost at least 4% of body weight after 16 weeks of treatment, because it is unlikely the patient will achieve and sustain weight loss.
Efficacy
Liraglutide was studied in 3 clinical trials of obese and overweight participants who had a weight-related condition. Patients who had a history of major depressive disorder or suicide attempt were excluded from the studies. All participants in Studies 1 and 2 received instruction about following a reduced-calorie diet and increasing physical activity. In Study 3, patients were randomized to treatment after losing >5% of their body weight through reduced calorie intake and exercise; those who did not meet the required weight loss were excluded from the study. In these 56-week clinical studies:
• of 3,731 participants without diabetes or a weight-related comorbidity, such as high blood pressure or high cholesterol, 62% of patients (n = 2,313) who took liraglutide lost ≥5% of their body weight from baseline, compared with 34% of participants who received placebo
• of 635 participants with T2DM, 49% of patients (n = 311) treated with liraglutide lost ≥5% of their body weight compared with 16% placebo patients
• of 422 participants with a weight-related comorbidity, 42% of patients (n = 177) lost ≥5% of their body weight compared with 21.7% of placebo patients.
Improvements in some cardiovascular disease risk factors were observed. Long-term follow up was not studied.
Contraindictations
Liraglutide is contraindicated in patients who have a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2. In a 104-week study, malignant thyroid C-cell carcinomas were detected in rats and mice given liraglutide, 1 and 3 mg/kg/d; however, it was not detected in groups given 0.03 and 0.2 mg/kg/d. It isn’t known whether liraglutide can cause thyroid C-cell tumors in humans.
Patients should not take liraglutide if they have hypersensitivity to liraglutide or any product components, are using insulin, are taking any other GLP-1 receptor agonist, or are pregnant.
Adverse effects
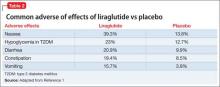
The most common reported adverse effects are nausea (39.3%), hypoglycemia in patients with T2DM (23%), diarrhea (20.9%), constipation (19.4%), and vomiting (15.7%) (Table 2). In clinical trials, 9.8% of patients discontinued treatment because of adverse effects, compared with 4.3% of those receiving placebo.
Liraglutide has low potential for pharmacokinetic drug-drug interactions related to cytochrome P450 and plasma protein binding. For a full list of drug-drug interactions, see the full prescribing information.1