User login
Intimate partner violence: Opening the door to a safer future
THE CASE
Louise T* is a 42-year-old woman who presented to her family medicine office for a routine annual visit. During the exam, her physician noticed bruises on Ms. T’s arms and back. Upon further inquiry, Ms. T reported that she and her husband had argued the night before the appointment. With some hesitancy, she went on to say that this was not the first time this had happened. She said that she and her husband had been arguing frequently for several years and that 6 months earlier, when he lost his job, he began hitting and pushing her.
●
*The patient’s name has been changed to protect her identity.
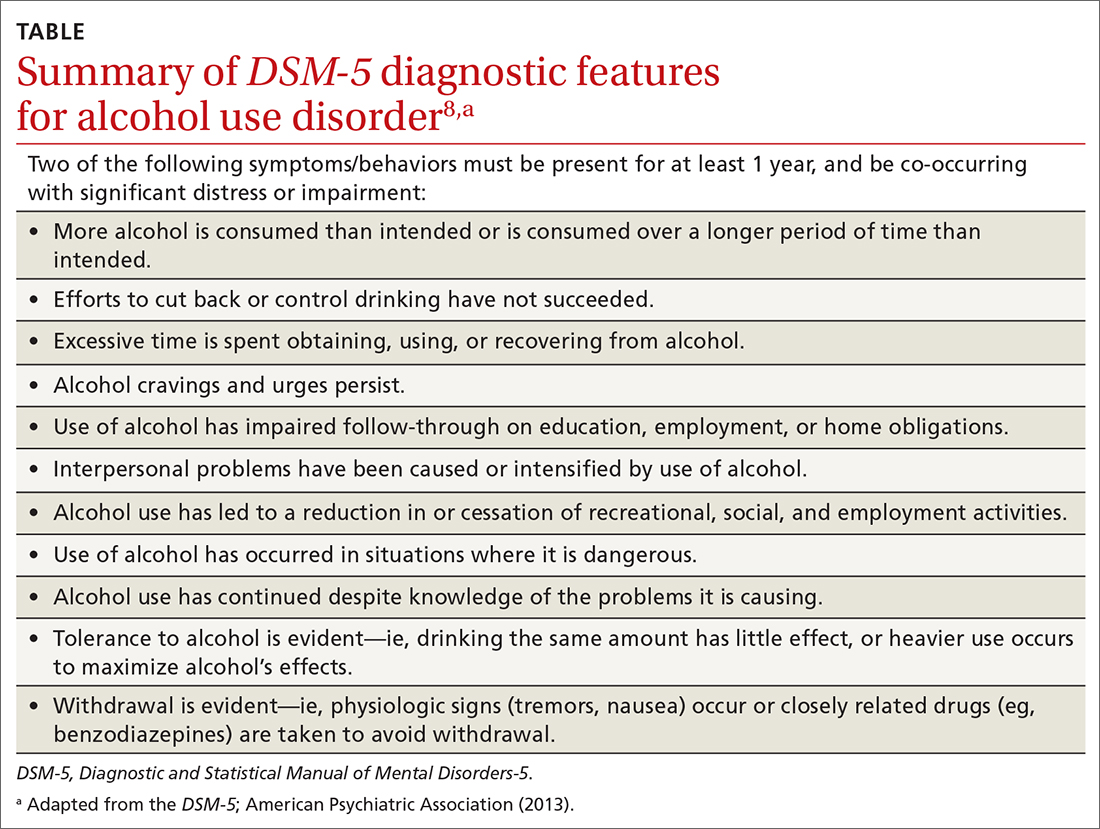
Intimate partner violence (IPV) includes physical, sexual, or psychological aggression or stalking perpetrated by a current or former relationship partner.1 IPV affects more than 12 million men and women living in the United States each year.2 According to a national survey of IPV, approximately one-third (35.6%) of women and one-quarter (28.5%) of men living in the United States experience rape, physical violence, or stalking by an intimate partner during their lifetime.2 Lifetime exposure to psychological IPV is even more prevalent, affecting nearly half of women and men (48.4% and 48.8%, respectively).2
Lifetime prevalence of any form of IPV is higher among women who identify as bisexual (59.8%) and lesbian (46.3%) compared with those who identify as heterosexual (37.2%); rates are comparable among men who identify as heterosexual (31.9%), bisexual (35.3%), and gay (35.1%).3 Preliminary data suggest that IPV may have increased in frequency and severity during the COVID-19 pandemic, particularly in the context of mandated shelter-in-place and stay-at-home orders.4-6
IPV is associated with numerous negative health consequences. They include fear and concern for safety, mental health disorders such as posttraumatic stress disorder (PTSD), and physical health problems including physical injury, chronic pain, sleep disturbance, and frequent headaches.2 IPV is also associated with a greater number of missed days from school and work and increased utilization of legal, health care, and housing services.2,7 The overall annual cost of IPV against women is estimated at $5.8 billion, with health care costs accounting for approximately $4.1 billion.7 Family physicians can play an important role in curbing the devastating effects of IPV by screening patients and providing resources when needed.
Facilitate disclosure using screening tools and protocol
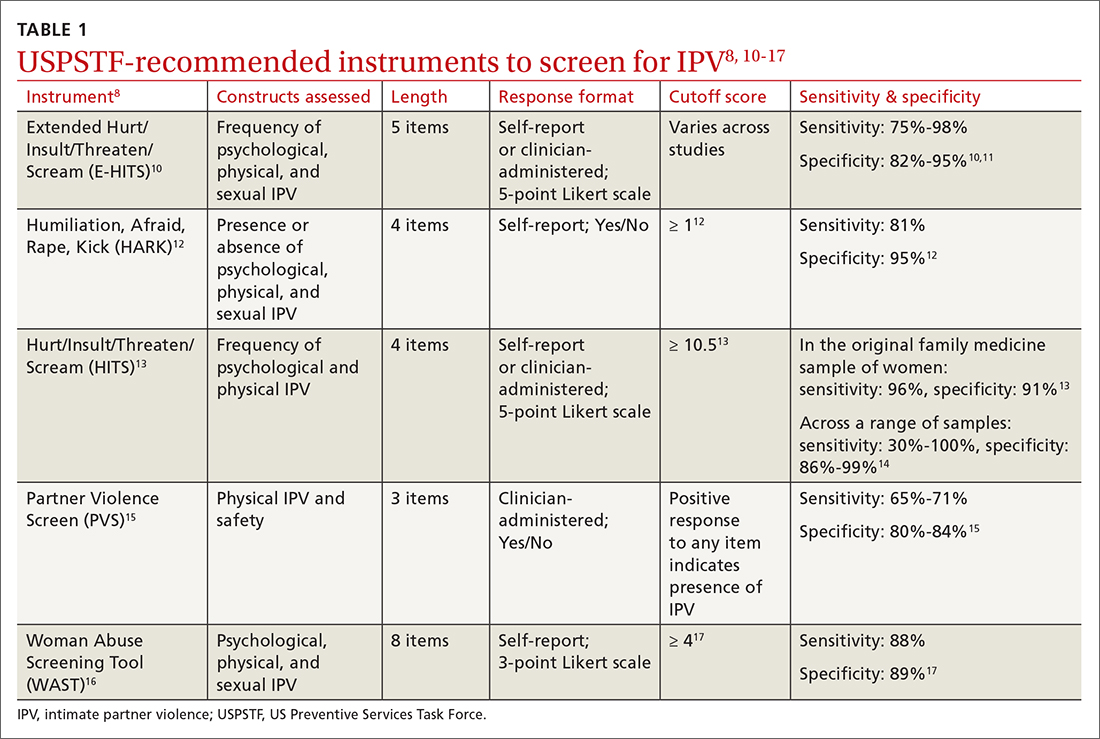
In Ms. T’s case, evidence of violence was clearly visible. However, not all instances of IPV leave physical marks. The US Preventive Services Task Force (USPSTF) recommends that all women of childbearing age be screened for IPV, whether or not they exhibit signs of violence.8 While the USPSTF has only published recommendations regarding screening women for IPV, there has been a recent push to screen all patients given that men also experience high rates of IPV.9
Utilize a brief screening tool. Directly ask patients about IPV; this can help reduce stigma, facilitate disclosure, and initiate the process of connecting patients to potentially lifesaving resources. The USPSTF lists several brief screening measures that can be used in primary care settings to assess exposure to IPV (TABLE 18,10-17). The brevity of these screening tools makes them well suited for busy physicians; cutoff scores facilitate the rapid identification of positive screens. While the USPSTF has not made specific recommendations regarding a screening interval, many studies examining the utility of these measures have reported on annual screenings.8 While there is limited evidence that brief screening alone leads to reductions in IPV,8 discussing IPV in a supportive and empathic manner and connecting patients to resources, such as supportive counseling, does have an important benefit: It can reduce symptoms of depression.18
Continue to: Screen patients in private; this protocol can help
Screen patients in private; this protocol can help. Given the sensitive nature of IPV and the potential danger some patients may be facing, it is important to screen patients in a safe and supportive environment.19,20 Screening should be conducted by the primary care clinician, ideally when a trusting relationship already has been formed. Screen patients only when they are alone in a private room; avoid screening in public spaces such as clinic waiting rooms or in the vicinity of the patient’s partner or children older than age 2 years.19,20
To provide all patients with an opportunity for private and safe IPV screening, clinics are encouraged to develop a clinic-wide policy whereby patients are routinely escorted to the exam room alone for the first portion of their visit, after which any accompanying individuals may be invited to join.21 Clinic staff can inform patients and accompanying individuals of this policy when they first arrive. Once in the exam room, and before the screening process begins, clearly state reporting requirements to ensure that patients can make an informed decision about whether to disclose IPV.19
Set a receptive tone. The manner in which clinicians discuss IPV with their patients is just as important as the setting. Demonstrating sensitivity and genuine concern for the patient’s safety and well-being may increase the patient’s comfort level throughout the screening process and may facilitate disclosures of IPV.19,22 When screening patients for IPV, sit face to face rather than standing over them, maintain warm and open body language, and speak in a soft tone of voice.22
Patients may feel more comfortable if you ask screening questions in a straightforward, nonjudgmental manner, as this helps to normalize the screening experience. We also recommend using behaviorally specific language (eg, “Do arguments [with your partner] ever result in hitting, kicking, or pushing?”16 or “How often does your partner scream or curse at you?”),13 as some patients who have experienced IPV will not label their experiences as “abuse” or “violence.” Not every patient who experiences IPV will be ready to disclose these events; however, maintaining a positive and supportive relationship during routine IPV screening and throughout the remainder of the medical visit may help facilitate future disclosures if, and when, a patient is ready to seek support.19
CRITICAL INTERVENTION ELEMENTS: EMPATHY AND SAFETY
A physician’s response to an IPV disclosure can have a lasting impact on the patient. We encourage family physicians to respond to IPV disclosures with empathy. Maintain eye contact and warm body language, validate the patient’s experiences (“I am sorry this happened to you,” “that must have been terrifying”), tell the patient that the violence was not their fault, and thank the patient for disclosing.23
Continue to: Assess patient safety
Assess patient safety. Another critical component of intervention is to assess the patient’s safety and engage in safety planning. If the patient agrees to this next step, you may wish to provide a warm handoff to a trained social worker, nurse, or psychologist in the clinic who can spend more time covering this information with the patient. Some key components of a safety assessment include determining whether the violence or threat of violence is ongoing and identifying who lives in the home (eg, the partner, children, and any pets). You and the patient can also discuss red flags that would indicate elevated risk. You should discuss red flags that are unique to the patient’s relationship as well as common factors that have been found to heighten risk for IPV (eg, partner engaging in heavy alcohol use).1
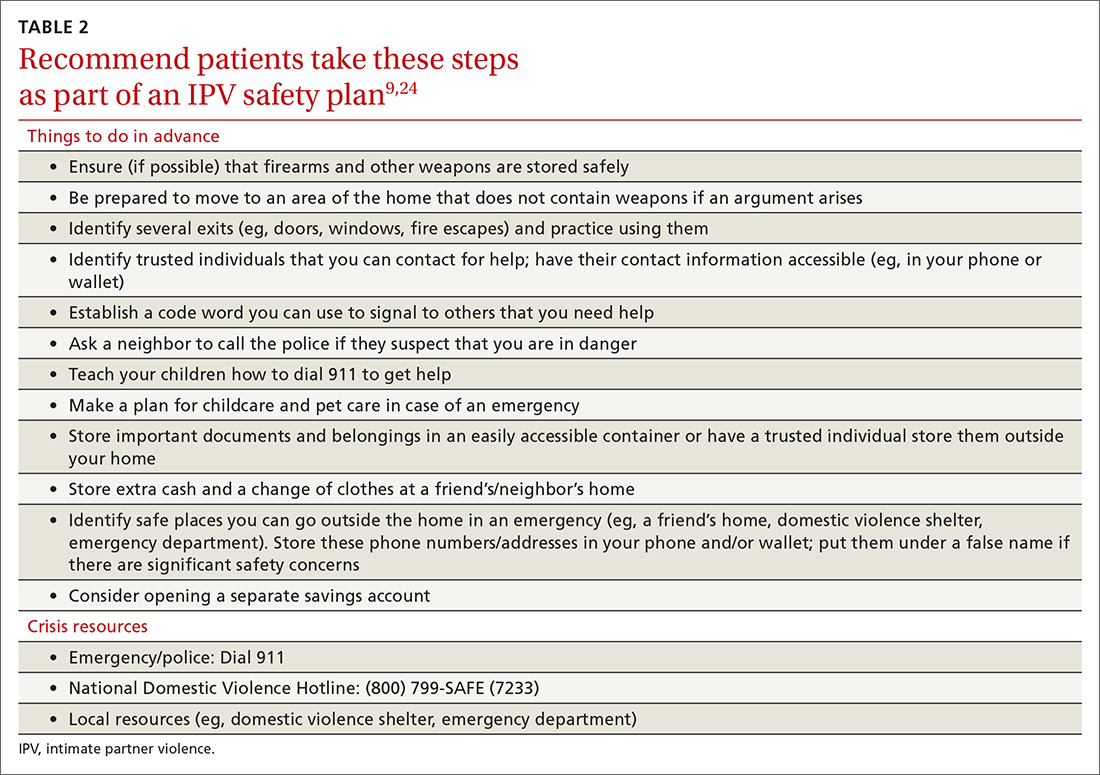
With the patient’s permission, collaboratively construct a safety plan that details how the patient can stay safe on a daily basis and how to safely leave should a dangerous situation arise (TABLE 29,24). The interactive safety planning tool available on the National Domestic Violence Hotline’s website can be a valuable resource (www.thehotline.org/plan-for-safety/).24 Finally, if a patient is experiencing mental health concerns associated with IPV (eg, PTSD, depression, substance misuse, suicidal ideation), consider a referral to a domestic violence counseling center or mental health provider.
Move at the patient’s pace. Even if patients are willing to disclose IPV, they will differ in their readiness to discuss psychoeducation, safety planning, and referrals. Similarly, even if a patient is experiencing severe violence, they may not be ready to leave the relationship. Thus, it’s important to ask the patient for permission before initiating each successive step of the follow-up intervention. You and the patient may wish to schedule additional appointments to discuss this information at a pace the patient finds appropriate.
You may need to spend some time helping the patient recognize the severity of their situation and to feel empowered to take action. In addition, offer information and resources to all patients, even those who do not disclose IPV. Some patients may want to receive this information even if they do not feel comfortable sharing their experiences during the appointment.20 You can also inform patients that they are welcome to bring up issues related to IPV at any future appointments in order to leave the door open to future disclosures.
THE CASE
The physician determined that Ms. T had been experiencing physical and psychological IPV in her current relationship. After responding empathically and obtaining the patient’s consent, the physician provided a warm handoff to the psychologist in the clinic. With Ms. T’s permission, the psychologist provided psychoeducation about IPV, and they discussed Ms. T’s current situation and risk level. They determined that Ms. T was at risk for subsequent episodes of IPV and they collaborated on a safety plan, making sure to discuss contact information for local and national crisis resources.
Continue to: Ms. T saved the phone number...
Ms. T saved the phone number for her local domestic violence shelter in her phone under a false name in case her husband looked through her phone. She said she planned to work on several safety plan items when her husband was away from the house and it was safe to do so. For example, she planned to identify additional ways to exit the house in an emergency and she was going to put together a bag with a change of clothes and some money and drop it off at a trusted friend’s house.
Ms. T and the psychologist agreed to follow up with an office visit in 1 week to discuss any additional safety concerns and to determine whether Ms. T could benefit from a referral to domestic violence counseling services or mental health treatment. The psychologist provided a summary of the topics she and Ms. T had discussed to the physician. The physician scheduled a follow-up appointment with Ms. T in 3 weeks to assess her current safety, troubleshoot any difficulties in implementing her safety plan, and offer additional resources, as needed.
CORRESPONDENCE
Andrea Massa, PhD, 125 Doughty Street, Suite 300, Charleston, SC 29403; massa@musc.edu
1. CDC. National Center for Injury Prevention and Control. Preventing intimate partner violence. 2021. Accessed June 27, 2022. www.cdc.gov/violenceprevention/intimatepartnerviolence/fastfact.html
2. CDC. Black MC, Basile KC, Breiding MJ, et al. The National Intimate Partner and Sexual Violence Survey: 2010 Summary Report. Accessed June 27, 2022. www.cdc.gov/violenceprevention/pdf/nisvs_executive_summary-a.pdf
3. Chen J, Walters ML, Gilbert LK, et al. Sexual violence, stalking, and intimate partner violence by sexual orientation, United States. Psychol Violence. 2020;10:110-119. doi:10.1037/vio0000252
4. Kofman YB, Garfin DR. Home is not always a haven: the domestic violence crisis amid the COVID-19 pandemic. Psychol Trauma. 2020;12:S199-S201. doi:10.1037/tra0000866
5. Lyons M, Brewer G. Experiences of intimate partner violence during lockdown and the COVID-19 pandemic. J Fam Violence. 2021:1-9. doi:10.1007/s10896-021-00260-x
6. Parrott DJ, Halmos MB, Stappenbeck CA, et al. Intimate partner aggression during the COVID-19 pandemic: associations with stress and heavy drinking. Psychol Violence. 2021;12:95-103. doi:10.1037/vio0000395
7. CDC. National Center for Injury Prevention and
8. US Preventive Services Task Force. Screening for intimate partner violence, elder abuse, and abuse of vulnerable adults: US Preventive Services Task Force final recommendation statement. JAMA. 2018;320:1678-1687. doi:10.1001/jama.2018.14741
9. Sprunger JG, Schumacher JA, Coffey SF, et al. It’s time to start asking all patients about intimate partner violence. J Fam Pract. 2019;68:152-161.
10. Chan CC, Chan YC, Au A, et al. Reliability and validity of the “Extended - Hurt, Insult, Threaten, Scream” (E-HITS) screening tool in detecting intimate partner violence in hospital emergency departments in Hong Kong. Hong Kong J Emerg Med. 2010;17:109-117. doi:10.1177/102490791001700202
11. Iverson KM, King MW, Gerber MR, et al. Accuracy of an intimate partner violence screening tool for female VHA patients: a replication and extension. J Trauma Stress. 2015;28:79-82. doi:10.1002/jts.21985
12. Sohal H, Eldridge S, Feder G. The sensitivity and specificity of four questions (HARK) to identify intimate partner violence: a diagnostic accuracy study in general practice. BMC Fam Pract. 2007;8:49. doi:10.1186/1471-2296-8-49
13. Sherin KM, Sinacore JM, Li X, et al. HITS: a short domestic violence screening tool for use in a family practice setting. Fam Med. 1998;30:508-512.
14. Rabin RF, Jennings JM, Campbell JC, et al. Intimate partner violence screening tools: a systematic review. Am J Prev Med. 2009;36:439-445.e4. doi:10.1016/j.amepre.2009.01.024
15. Feldhaus KM, Koziol-McLain J, Amsbury HL, et al. Accuracy of 3 brief screening questions for detecting partner violence in the emergency department. JAMA. 1997;277:1357-1361. doi:10.1001/jama.1997.03540410035027
16. Brown JB, Lent B, Schmidt G, et al. Application of the Woman Abuse Screening Tool (WAST) and WAST-short in the family practice setting. J Fam Pract. 2000;49:896-903.
17. Wathen CN, Jamieson E, MacMillan HL, MVAWRG. Who is identified by screening for intimate partner violence? Womens Health Issues. 2008;18:423-432. doi:10.1016/j.whi.2008.08.003
18. Hegarty K, O’Doherty L, Taft A, et al. Screening and counselling in the primary care setting for women who have experienced intimate partner violence (WEAVE): a cluster randomised controlled trial. Lancet. 2013;382:249-258. doi: 10.1016/S0140-6736(13)60052-5
19. Correa NP, Cain CM, Bertenthal M, et al. Women’s experiences of being screened for intimate partner violence in the health care setting. Nurs Womens Health. 2020;24:185-196. doi:10.1016/j.nwh.2020.04.002
20. Chang JC, Decker MR, Moracco KE, et al. Asking about intimate partner violence: advice from female survivors to health care providers. Patient Educ Couns. 2005;59:141-147. doi:10.1016/j.pec.2004.10.008
21. Paterno MT, Draughon JE. Screening for intimate partner violence. J Midwifery Womens Health. 2016;61:370-375. doi:10.1111/jmwh.12443
22. Iverson KM, Huang K, Wells SY, et al. Women veterans’ preferences for intimate partner violence screening and response procedures within the Veterans Health Administration. Res Nurs Health. 2014;37:302-311. doi:10.1002/nur.21602
23. National Sexual Violence Research Center. Assessing patients for sexual violence: A guide for health care providers. 2011. Accessed June 28, 2022. www.nsvrc.org/publications/assessing-patients-sexual-violence-guide-health-care-providers
24. National Domestic Violence Hotline. Interactive guide to safety planning. Accessed August 22, 2022. https://www.thehotline.org/plan-for-safety/create-a-safety-plan/
THE CASE
Louise T* is a 42-year-old woman who presented to her family medicine office for a routine annual visit. During the exam, her physician noticed bruises on Ms. T’s arms and back. Upon further inquiry, Ms. T reported that she and her husband had argued the night before the appointment. With some hesitancy, she went on to say that this was not the first time this had happened. She said that she and her husband had been arguing frequently for several years and that 6 months earlier, when he lost his job, he began hitting and pushing her.
●
*The patient’s name has been changed to protect her identity.
Intimate partner violence (IPV) includes physical, sexual, or psychological aggression or stalking perpetrated by a current or former relationship partner.1 IPV affects more than 12 million men and women living in the United States each year.2 According to a national survey of IPV, approximately one-third (35.6%) of women and one-quarter (28.5%) of men living in the United States experience rape, physical violence, or stalking by an intimate partner during their lifetime.2 Lifetime exposure to psychological IPV is even more prevalent, affecting nearly half of women and men (48.4% and 48.8%, respectively).2
Lifetime prevalence of any form of IPV is higher among women who identify as bisexual (59.8%) and lesbian (46.3%) compared with those who identify as heterosexual (37.2%); rates are comparable among men who identify as heterosexual (31.9%), bisexual (35.3%), and gay (35.1%).3 Preliminary data suggest that IPV may have increased in frequency and severity during the COVID-19 pandemic, particularly in the context of mandated shelter-in-place and stay-at-home orders.4-6
IPV is associated with numerous negative health consequences. They include fear and concern for safety, mental health disorders such as posttraumatic stress disorder (PTSD), and physical health problems including physical injury, chronic pain, sleep disturbance, and frequent headaches.2 IPV is also associated with a greater number of missed days from school and work and increased utilization of legal, health care, and housing services.2,7 The overall annual cost of IPV against women is estimated at $5.8 billion, with health care costs accounting for approximately $4.1 billion.7 Family physicians can play an important role in curbing the devastating effects of IPV by screening patients and providing resources when needed.
Facilitate disclosure using screening tools and protocol
In Ms. T’s case, evidence of violence was clearly visible. However, not all instances of IPV leave physical marks. The US Preventive Services Task Force (USPSTF) recommends that all women of childbearing age be screened for IPV, whether or not they exhibit signs of violence.8 While the USPSTF has only published recommendations regarding screening women for IPV, there has been a recent push to screen all patients given that men also experience high rates of IPV.9
Utilize a brief screening tool. Directly ask patients about IPV; this can help reduce stigma, facilitate disclosure, and initiate the process of connecting patients to potentially lifesaving resources. The USPSTF lists several brief screening measures that can be used in primary care settings to assess exposure to IPV (TABLE 18,10-17). The brevity of these screening tools makes them well suited for busy physicians; cutoff scores facilitate the rapid identification of positive screens. While the USPSTF has not made specific recommendations regarding a screening interval, many studies examining the utility of these measures have reported on annual screenings.8 While there is limited evidence that brief screening alone leads to reductions in IPV,8 discussing IPV in a supportive and empathic manner and connecting patients to resources, such as supportive counseling, does have an important benefit: It can reduce symptoms of depression.18

Continue to: Screen patients in private; this protocol can help
Screen patients in private; this protocol can help. Given the sensitive nature of IPV and the potential danger some patients may be facing, it is important to screen patients in a safe and supportive environment.19,20 Screening should be conducted by the primary care clinician, ideally when a trusting relationship already has been formed. Screen patients only when they are alone in a private room; avoid screening in public spaces such as clinic waiting rooms or in the vicinity of the patient’s partner or children older than age 2 years.19,20
To provide all patients with an opportunity for private and safe IPV screening, clinics are encouraged to develop a clinic-wide policy whereby patients are routinely escorted to the exam room alone for the first portion of their visit, after which any accompanying individuals may be invited to join.21 Clinic staff can inform patients and accompanying individuals of this policy when they first arrive. Once in the exam room, and before the screening process begins, clearly state reporting requirements to ensure that patients can make an informed decision about whether to disclose IPV.19
Set a receptive tone. The manner in which clinicians discuss IPV with their patients is just as important as the setting. Demonstrating sensitivity and genuine concern for the patient’s safety and well-being may increase the patient’s comfort level throughout the screening process and may facilitate disclosures of IPV.19,22 When screening patients for IPV, sit face to face rather than standing over them, maintain warm and open body language, and speak in a soft tone of voice.22
Patients may feel more comfortable if you ask screening questions in a straightforward, nonjudgmental manner, as this helps to normalize the screening experience. We also recommend using behaviorally specific language (eg, “Do arguments [with your partner] ever result in hitting, kicking, or pushing?”16 or “How often does your partner scream or curse at you?”),13 as some patients who have experienced IPV will not label their experiences as “abuse” or “violence.” Not every patient who experiences IPV will be ready to disclose these events; however, maintaining a positive and supportive relationship during routine IPV screening and throughout the remainder of the medical visit may help facilitate future disclosures if, and when, a patient is ready to seek support.19
CRITICAL INTERVENTION ELEMENTS: EMPATHY AND SAFETY
A physician’s response to an IPV disclosure can have a lasting impact on the patient. We encourage family physicians to respond to IPV disclosures with empathy. Maintain eye contact and warm body language, validate the patient’s experiences (“I am sorry this happened to you,” “that must have been terrifying”), tell the patient that the violence was not their fault, and thank the patient for disclosing.23
Continue to: Assess patient safety
Assess patient safety. Another critical component of intervention is to assess the patient’s safety and engage in safety planning. If the patient agrees to this next step, you may wish to provide a warm handoff to a trained social worker, nurse, or psychologist in the clinic who can spend more time covering this information with the patient. Some key components of a safety assessment include determining whether the violence or threat of violence is ongoing and identifying who lives in the home (eg, the partner, children, and any pets). You and the patient can also discuss red flags that would indicate elevated risk. You should discuss red flags that are unique to the patient’s relationship as well as common factors that have been found to heighten risk for IPV (eg, partner engaging in heavy alcohol use).1
With the patient’s permission, collaboratively construct a safety plan that details how the patient can stay safe on a daily basis and how to safely leave should a dangerous situation arise (TABLE 29,24). The interactive safety planning tool available on the National Domestic Violence Hotline’s website can be a valuable resource (www.thehotline.org/plan-for-safety/).24 Finally, if a patient is experiencing mental health concerns associated with IPV (eg, PTSD, depression, substance misuse, suicidal ideation), consider a referral to a domestic violence counseling center or mental health provider.

Move at the patient’s pace. Even if patients are willing to disclose IPV, they will differ in their readiness to discuss psychoeducation, safety planning, and referrals. Similarly, even if a patient is experiencing severe violence, they may not be ready to leave the relationship. Thus, it’s important to ask the patient for permission before initiating each successive step of the follow-up intervention. You and the patient may wish to schedule additional appointments to discuss this information at a pace the patient finds appropriate.
You may need to spend some time helping the patient recognize the severity of their situation and to feel empowered to take action. In addition, offer information and resources to all patients, even those who do not disclose IPV. Some patients may want to receive this information even if they do not feel comfortable sharing their experiences during the appointment.20 You can also inform patients that they are welcome to bring up issues related to IPV at any future appointments in order to leave the door open to future disclosures.
THE CASE
The physician determined that Ms. T had been experiencing physical and psychological IPV in her current relationship. After responding empathically and obtaining the patient’s consent, the physician provided a warm handoff to the psychologist in the clinic. With Ms. T’s permission, the psychologist provided psychoeducation about IPV, and they discussed Ms. T’s current situation and risk level. They determined that Ms. T was at risk for subsequent episodes of IPV and they collaborated on a safety plan, making sure to discuss contact information for local and national crisis resources.
Continue to: Ms. T saved the phone number...
Ms. T saved the phone number for her local domestic violence shelter in her phone under a false name in case her husband looked through her phone. She said she planned to work on several safety plan items when her husband was away from the house and it was safe to do so. For example, she planned to identify additional ways to exit the house in an emergency and she was going to put together a bag with a change of clothes and some money and drop it off at a trusted friend’s house.
Ms. T and the psychologist agreed to follow up with an office visit in 1 week to discuss any additional safety concerns and to determine whether Ms. T could benefit from a referral to domestic violence counseling services or mental health treatment. The psychologist provided a summary of the topics she and Ms. T had discussed to the physician. The physician scheduled a follow-up appointment with Ms. T in 3 weeks to assess her current safety, troubleshoot any difficulties in implementing her safety plan, and offer additional resources, as needed.
CORRESPONDENCE
Andrea Massa, PhD, 125 Doughty Street, Suite 300, Charleston, SC 29403; massa@musc.edu
THE CASE
Louise T* is a 42-year-old woman who presented to her family medicine office for a routine annual visit. During the exam, her physician noticed bruises on Ms. T’s arms and back. Upon further inquiry, Ms. T reported that she and her husband had argued the night before the appointment. With some hesitancy, she went on to say that this was not the first time this had happened. She said that she and her husband had been arguing frequently for several years and that 6 months earlier, when he lost his job, he began hitting and pushing her.
●
*The patient’s name has been changed to protect her identity.
Intimate partner violence (IPV) includes physical, sexual, or psychological aggression or stalking perpetrated by a current or former relationship partner.1 IPV affects more than 12 million men and women living in the United States each year.2 According to a national survey of IPV, approximately one-third (35.6%) of women and one-quarter (28.5%) of men living in the United States experience rape, physical violence, or stalking by an intimate partner during their lifetime.2 Lifetime exposure to psychological IPV is even more prevalent, affecting nearly half of women and men (48.4% and 48.8%, respectively).2
Lifetime prevalence of any form of IPV is higher among women who identify as bisexual (59.8%) and lesbian (46.3%) compared with those who identify as heterosexual (37.2%); rates are comparable among men who identify as heterosexual (31.9%), bisexual (35.3%), and gay (35.1%).3 Preliminary data suggest that IPV may have increased in frequency and severity during the COVID-19 pandemic, particularly in the context of mandated shelter-in-place and stay-at-home orders.4-6
IPV is associated with numerous negative health consequences. They include fear and concern for safety, mental health disorders such as posttraumatic stress disorder (PTSD), and physical health problems including physical injury, chronic pain, sleep disturbance, and frequent headaches.2 IPV is also associated with a greater number of missed days from school and work and increased utilization of legal, health care, and housing services.2,7 The overall annual cost of IPV against women is estimated at $5.8 billion, with health care costs accounting for approximately $4.1 billion.7 Family physicians can play an important role in curbing the devastating effects of IPV by screening patients and providing resources when needed.
Facilitate disclosure using screening tools and protocol
In Ms. T’s case, evidence of violence was clearly visible. However, not all instances of IPV leave physical marks. The US Preventive Services Task Force (USPSTF) recommends that all women of childbearing age be screened for IPV, whether or not they exhibit signs of violence.8 While the USPSTF has only published recommendations regarding screening women for IPV, there has been a recent push to screen all patients given that men also experience high rates of IPV.9
Utilize a brief screening tool. Directly ask patients about IPV; this can help reduce stigma, facilitate disclosure, and initiate the process of connecting patients to potentially lifesaving resources. The USPSTF lists several brief screening measures that can be used in primary care settings to assess exposure to IPV (TABLE 18,10-17). The brevity of these screening tools makes them well suited for busy physicians; cutoff scores facilitate the rapid identification of positive screens. While the USPSTF has not made specific recommendations regarding a screening interval, many studies examining the utility of these measures have reported on annual screenings.8 While there is limited evidence that brief screening alone leads to reductions in IPV,8 discussing IPV in a supportive and empathic manner and connecting patients to resources, such as supportive counseling, does have an important benefit: It can reduce symptoms of depression.18

Continue to: Screen patients in private; this protocol can help
Screen patients in private; this protocol can help. Given the sensitive nature of IPV and the potential danger some patients may be facing, it is important to screen patients in a safe and supportive environment.19,20 Screening should be conducted by the primary care clinician, ideally when a trusting relationship already has been formed. Screen patients only when they are alone in a private room; avoid screening in public spaces such as clinic waiting rooms or in the vicinity of the patient’s partner or children older than age 2 years.19,20
To provide all patients with an opportunity for private and safe IPV screening, clinics are encouraged to develop a clinic-wide policy whereby patients are routinely escorted to the exam room alone for the first portion of their visit, after which any accompanying individuals may be invited to join.21 Clinic staff can inform patients and accompanying individuals of this policy when they first arrive. Once in the exam room, and before the screening process begins, clearly state reporting requirements to ensure that patients can make an informed decision about whether to disclose IPV.19
Set a receptive tone. The manner in which clinicians discuss IPV with their patients is just as important as the setting. Demonstrating sensitivity and genuine concern for the patient’s safety and well-being may increase the patient’s comfort level throughout the screening process and may facilitate disclosures of IPV.19,22 When screening patients for IPV, sit face to face rather than standing over them, maintain warm and open body language, and speak in a soft tone of voice.22
Patients may feel more comfortable if you ask screening questions in a straightforward, nonjudgmental manner, as this helps to normalize the screening experience. We also recommend using behaviorally specific language (eg, “Do arguments [with your partner] ever result in hitting, kicking, or pushing?”16 or “How often does your partner scream or curse at you?”),13 as some patients who have experienced IPV will not label their experiences as “abuse” or “violence.” Not every patient who experiences IPV will be ready to disclose these events; however, maintaining a positive and supportive relationship during routine IPV screening and throughout the remainder of the medical visit may help facilitate future disclosures if, and when, a patient is ready to seek support.19
CRITICAL INTERVENTION ELEMENTS: EMPATHY AND SAFETY
A physician’s response to an IPV disclosure can have a lasting impact on the patient. We encourage family physicians to respond to IPV disclosures with empathy. Maintain eye contact and warm body language, validate the patient’s experiences (“I am sorry this happened to you,” “that must have been terrifying”), tell the patient that the violence was not their fault, and thank the patient for disclosing.23
Continue to: Assess patient safety
Assess patient safety. Another critical component of intervention is to assess the patient’s safety and engage in safety planning. If the patient agrees to this next step, you may wish to provide a warm handoff to a trained social worker, nurse, or psychologist in the clinic who can spend more time covering this information with the patient. Some key components of a safety assessment include determining whether the violence or threat of violence is ongoing and identifying who lives in the home (eg, the partner, children, and any pets). You and the patient can also discuss red flags that would indicate elevated risk. You should discuss red flags that are unique to the patient’s relationship as well as common factors that have been found to heighten risk for IPV (eg, partner engaging in heavy alcohol use).1
With the patient’s permission, collaboratively construct a safety plan that details how the patient can stay safe on a daily basis and how to safely leave should a dangerous situation arise (TABLE 29,24). The interactive safety planning tool available on the National Domestic Violence Hotline’s website can be a valuable resource (www.thehotline.org/plan-for-safety/).24 Finally, if a patient is experiencing mental health concerns associated with IPV (eg, PTSD, depression, substance misuse, suicidal ideation), consider a referral to a domestic violence counseling center or mental health provider.

Move at the patient’s pace. Even if patients are willing to disclose IPV, they will differ in their readiness to discuss psychoeducation, safety planning, and referrals. Similarly, even if a patient is experiencing severe violence, they may not be ready to leave the relationship. Thus, it’s important to ask the patient for permission before initiating each successive step of the follow-up intervention. You and the patient may wish to schedule additional appointments to discuss this information at a pace the patient finds appropriate.
You may need to spend some time helping the patient recognize the severity of their situation and to feel empowered to take action. In addition, offer information and resources to all patients, even those who do not disclose IPV. Some patients may want to receive this information even if they do not feel comfortable sharing their experiences during the appointment.20 You can also inform patients that they are welcome to bring up issues related to IPV at any future appointments in order to leave the door open to future disclosures.
THE CASE
The physician determined that Ms. T had been experiencing physical and psychological IPV in her current relationship. After responding empathically and obtaining the patient’s consent, the physician provided a warm handoff to the psychologist in the clinic. With Ms. T’s permission, the psychologist provided psychoeducation about IPV, and they discussed Ms. T’s current situation and risk level. They determined that Ms. T was at risk for subsequent episodes of IPV and they collaborated on a safety plan, making sure to discuss contact information for local and national crisis resources.
Continue to: Ms. T saved the phone number...
Ms. T saved the phone number for her local domestic violence shelter in her phone under a false name in case her husband looked through her phone. She said she planned to work on several safety plan items when her husband was away from the house and it was safe to do so. For example, she planned to identify additional ways to exit the house in an emergency and she was going to put together a bag with a change of clothes and some money and drop it off at a trusted friend’s house.
Ms. T and the psychologist agreed to follow up with an office visit in 1 week to discuss any additional safety concerns and to determine whether Ms. T could benefit from a referral to domestic violence counseling services or mental health treatment. The psychologist provided a summary of the topics she and Ms. T had discussed to the physician. The physician scheduled a follow-up appointment with Ms. T in 3 weeks to assess her current safety, troubleshoot any difficulties in implementing her safety plan, and offer additional resources, as needed.
CORRESPONDENCE
Andrea Massa, PhD, 125 Doughty Street, Suite 300, Charleston, SC 29403; massa@musc.edu
1. CDC. National Center for Injury Prevention and Control. Preventing intimate partner violence. 2021. Accessed June 27, 2022. www.cdc.gov/violenceprevention/intimatepartnerviolence/fastfact.html
2. CDC. Black MC, Basile KC, Breiding MJ, et al. The National Intimate Partner and Sexual Violence Survey: 2010 Summary Report. Accessed June 27, 2022. www.cdc.gov/violenceprevention/pdf/nisvs_executive_summary-a.pdf
3. Chen J, Walters ML, Gilbert LK, et al. Sexual violence, stalking, and intimate partner violence by sexual orientation, United States. Psychol Violence. 2020;10:110-119. doi:10.1037/vio0000252
4. Kofman YB, Garfin DR. Home is not always a haven: the domestic violence crisis amid the COVID-19 pandemic. Psychol Trauma. 2020;12:S199-S201. doi:10.1037/tra0000866
5. Lyons M, Brewer G. Experiences of intimate partner violence during lockdown and the COVID-19 pandemic. J Fam Violence. 2021:1-9. doi:10.1007/s10896-021-00260-x
6. Parrott DJ, Halmos MB, Stappenbeck CA, et al. Intimate partner aggression during the COVID-19 pandemic: associations with stress and heavy drinking. Psychol Violence. 2021;12:95-103. doi:10.1037/vio0000395
7. CDC. National Center for Injury Prevention and
8. US Preventive Services Task Force. Screening for intimate partner violence, elder abuse, and abuse of vulnerable adults: US Preventive Services Task Force final recommendation statement. JAMA. 2018;320:1678-1687. doi:10.1001/jama.2018.14741
9. Sprunger JG, Schumacher JA, Coffey SF, et al. It’s time to start asking all patients about intimate partner violence. J Fam Pract. 2019;68:152-161.
10. Chan CC, Chan YC, Au A, et al. Reliability and validity of the “Extended - Hurt, Insult, Threaten, Scream” (E-HITS) screening tool in detecting intimate partner violence in hospital emergency departments in Hong Kong. Hong Kong J Emerg Med. 2010;17:109-117. doi:10.1177/102490791001700202
11. Iverson KM, King MW, Gerber MR, et al. Accuracy of an intimate partner violence screening tool for female VHA patients: a replication and extension. J Trauma Stress. 2015;28:79-82. doi:10.1002/jts.21985
12. Sohal H, Eldridge S, Feder G. The sensitivity and specificity of four questions (HARK) to identify intimate partner violence: a diagnostic accuracy study in general practice. BMC Fam Pract. 2007;8:49. doi:10.1186/1471-2296-8-49
13. Sherin KM, Sinacore JM, Li X, et al. HITS: a short domestic violence screening tool for use in a family practice setting. Fam Med. 1998;30:508-512.
14. Rabin RF, Jennings JM, Campbell JC, et al. Intimate partner violence screening tools: a systematic review. Am J Prev Med. 2009;36:439-445.e4. doi:10.1016/j.amepre.2009.01.024
15. Feldhaus KM, Koziol-McLain J, Amsbury HL, et al. Accuracy of 3 brief screening questions for detecting partner violence in the emergency department. JAMA. 1997;277:1357-1361. doi:10.1001/jama.1997.03540410035027
16. Brown JB, Lent B, Schmidt G, et al. Application of the Woman Abuse Screening Tool (WAST) and WAST-short in the family practice setting. J Fam Pract. 2000;49:896-903.
17. Wathen CN, Jamieson E, MacMillan HL, MVAWRG. Who is identified by screening for intimate partner violence? Womens Health Issues. 2008;18:423-432. doi:10.1016/j.whi.2008.08.003
18. Hegarty K, O’Doherty L, Taft A, et al. Screening and counselling in the primary care setting for women who have experienced intimate partner violence (WEAVE): a cluster randomised controlled trial. Lancet. 2013;382:249-258. doi: 10.1016/S0140-6736(13)60052-5
19. Correa NP, Cain CM, Bertenthal M, et al. Women’s experiences of being screened for intimate partner violence in the health care setting. Nurs Womens Health. 2020;24:185-196. doi:10.1016/j.nwh.2020.04.002
20. Chang JC, Decker MR, Moracco KE, et al. Asking about intimate partner violence: advice from female survivors to health care providers. Patient Educ Couns. 2005;59:141-147. doi:10.1016/j.pec.2004.10.008
21. Paterno MT, Draughon JE. Screening for intimate partner violence. J Midwifery Womens Health. 2016;61:370-375. doi:10.1111/jmwh.12443
22. Iverson KM, Huang K, Wells SY, et al. Women veterans’ preferences for intimate partner violence screening and response procedures within the Veterans Health Administration. Res Nurs Health. 2014;37:302-311. doi:10.1002/nur.21602
23. National Sexual Violence Research Center. Assessing patients for sexual violence: A guide for health care providers. 2011. Accessed June 28, 2022. www.nsvrc.org/publications/assessing-patients-sexual-violence-guide-health-care-providers
24. National Domestic Violence Hotline. Interactive guide to safety planning. Accessed August 22, 2022. https://www.thehotline.org/plan-for-safety/create-a-safety-plan/
1. CDC. National Center for Injury Prevention and Control. Preventing intimate partner violence. 2021. Accessed June 27, 2022. www.cdc.gov/violenceprevention/intimatepartnerviolence/fastfact.html
2. CDC. Black MC, Basile KC, Breiding MJ, et al. The National Intimate Partner and Sexual Violence Survey: 2010 Summary Report. Accessed June 27, 2022. www.cdc.gov/violenceprevention/pdf/nisvs_executive_summary-a.pdf
3. Chen J, Walters ML, Gilbert LK, et al. Sexual violence, stalking, and intimate partner violence by sexual orientation, United States. Psychol Violence. 2020;10:110-119. doi:10.1037/vio0000252
4. Kofman YB, Garfin DR. Home is not always a haven: the domestic violence crisis amid the COVID-19 pandemic. Psychol Trauma. 2020;12:S199-S201. doi:10.1037/tra0000866
5. Lyons M, Brewer G. Experiences of intimate partner violence during lockdown and the COVID-19 pandemic. J Fam Violence. 2021:1-9. doi:10.1007/s10896-021-00260-x
6. Parrott DJ, Halmos MB, Stappenbeck CA, et al. Intimate partner aggression during the COVID-19 pandemic: associations with stress and heavy drinking. Psychol Violence. 2021;12:95-103. doi:10.1037/vio0000395
7. CDC. National Center for Injury Prevention and
8. US Preventive Services Task Force. Screening for intimate partner violence, elder abuse, and abuse of vulnerable adults: US Preventive Services Task Force final recommendation statement. JAMA. 2018;320:1678-1687. doi:10.1001/jama.2018.14741
9. Sprunger JG, Schumacher JA, Coffey SF, et al. It’s time to start asking all patients about intimate partner violence. J Fam Pract. 2019;68:152-161.
10. Chan CC, Chan YC, Au A, et al. Reliability and validity of the “Extended - Hurt, Insult, Threaten, Scream” (E-HITS) screening tool in detecting intimate partner violence in hospital emergency departments in Hong Kong. Hong Kong J Emerg Med. 2010;17:109-117. doi:10.1177/102490791001700202
11. Iverson KM, King MW, Gerber MR, et al. Accuracy of an intimate partner violence screening tool for female VHA patients: a replication and extension. J Trauma Stress. 2015;28:79-82. doi:10.1002/jts.21985
12. Sohal H, Eldridge S, Feder G. The sensitivity and specificity of four questions (HARK) to identify intimate partner violence: a diagnostic accuracy study in general practice. BMC Fam Pract. 2007;8:49. doi:10.1186/1471-2296-8-49
13. Sherin KM, Sinacore JM, Li X, et al. HITS: a short domestic violence screening tool for use in a family practice setting. Fam Med. 1998;30:508-512.
14. Rabin RF, Jennings JM, Campbell JC, et al. Intimate partner violence screening tools: a systematic review. Am J Prev Med. 2009;36:439-445.e4. doi:10.1016/j.amepre.2009.01.024
15. Feldhaus KM, Koziol-McLain J, Amsbury HL, et al. Accuracy of 3 brief screening questions for detecting partner violence in the emergency department. JAMA. 1997;277:1357-1361. doi:10.1001/jama.1997.03540410035027
16. Brown JB, Lent B, Schmidt G, et al. Application of the Woman Abuse Screening Tool (WAST) and WAST-short in the family practice setting. J Fam Pract. 2000;49:896-903.
17. Wathen CN, Jamieson E, MacMillan HL, MVAWRG. Who is identified by screening for intimate partner violence? Womens Health Issues. 2008;18:423-432. doi:10.1016/j.whi.2008.08.003
18. Hegarty K, O’Doherty L, Taft A, et al. Screening and counselling in the primary care setting for women who have experienced intimate partner violence (WEAVE): a cluster randomised controlled trial. Lancet. 2013;382:249-258. doi: 10.1016/S0140-6736(13)60052-5
19. Correa NP, Cain CM, Bertenthal M, et al. Women’s experiences of being screened for intimate partner violence in the health care setting. Nurs Womens Health. 2020;24:185-196. doi:10.1016/j.nwh.2020.04.002
20. Chang JC, Decker MR, Moracco KE, et al. Asking about intimate partner violence: advice from female survivors to health care providers. Patient Educ Couns. 2005;59:141-147. doi:10.1016/j.pec.2004.10.008
21. Paterno MT, Draughon JE. Screening for intimate partner violence. J Midwifery Womens Health. 2016;61:370-375. doi:10.1111/jmwh.12443
22. Iverson KM, Huang K, Wells SY, et al. Women veterans’ preferences for intimate partner violence screening and response procedures within the Veterans Health Administration. Res Nurs Health. 2014;37:302-311. doi:10.1002/nur.21602
23. National Sexual Violence Research Center. Assessing patients for sexual violence: A guide for health care providers. 2011. Accessed June 28, 2022. www.nsvrc.org/publications/assessing-patients-sexual-violence-guide-health-care-providers
24. National Domestic Violence Hotline. Interactive guide to safety planning. Accessed August 22, 2022. https://www.thehotline.org/plan-for-safety/create-a-safety-plan/
Helping patients move forward following traumatic brain injury
THE CASE
Declan M*, a 42-year-old man, presents as a new patient for general medical care. One year ago, he sustained a severe frontal traumatic brain injury (TBI) when he was hit by a car while crossing a street. He developed a subdural hematoma and was in a coma for 6 days. He also had fractured ribs and a fractured left foot. When he regained consciousness, he had posttraumatic amnesia. He also had executive function deficits and memory difficulties, so a guardian was appointed.
Mr. M no longer works as an auto mechanic, a career he once greatly enjoyed. Mr. M’s guardian reports that recently, Mr. M has lost interest in activities he’d previously enjoyed, is frequently irritable, has poor sleep, is socially isolated, and is spending increasing amounts of time at home. When his new primary care physician (PCP) enters the examining room, Mr. M is seated in a chair with his arms folded across his chest. He states that he is “fine” and just needs to “get a doctor.”
●
*This patient is an amalgam of patients for whom the author has provided care.
TBI ranges from mild to severe and can produce a number of profound effects that are a direct—or indirect—result of the physical injury.1 The location and the severity of the injury affect symptoms.2 Even mild TBI can cause impairment, and severe TBI can lead to broad cognitive, behavioral, and physical difficulties. As numbers of TBI cases increase globally, primary care providers need to recognize the symptoms and assess accordingly.1 The Acute Concussion Evaluation (ACE; Physician/Clinician Office Version) facilitates a structured evaluation for patients presenting with possible TBI symptoms. It can easily be accessed on the Centers for Disease Control and Prevention website.3
Direct effects of TBI include impulsivity, depression, reduced frustration tolerance, reduced motivation, low awareness, and insomnia and other sleep difficulties.4,5 Depression may also result indirectly from, or be exacerbated by, new posttraumatic limitations and lifestyle changes as well as loss of career and community.4 Both direct and indirect depression often manifest as feelings of hopelessness and worthlessness and a lack of interest in once enjoyable activities. Depression can worsen other TBI sequelae such as difficulty concentrating, lack of initiation, flat affect, irritability, reduced independence, reduced functional performance, loss of inhibition, and physical pain.6
Nationwide, most mental health concerns continue to be addressed in the primary care setting.7 Individuals with TBI experience major depression at a rate 5 to 6 times higher than those in the general population, with a prevalence rate of 45%.8
Suicide. The subject of suicide must be explored with survivors of TBI; evidence suggests a correlation between TBI, depression, and increased risk for suicide.9 Among those who have TBI, as many as 22% experience suicidal ideation; the risk of suicide in survivors of severe TBI is 3 to 4 times the risk in the general population.10 Additionally, suicidality in this context appears to be a chronic concern; therefore, carefully assess for its presence no matter how long ago the TBI occurred.10
Additional TBI-associated health concerns
Grief and loss. We so often focus on death as the only cause for grief, but grief can occur for other types of loss, as well. Individuals with TBI often experience a radical negative change in self-concept after their injury, which is associated with feelings of grief.11 Helping patients recognize that they are grieving the loss of the person they once were can help set a framework for their experience.
Continue to: Relationship loss
Relationship loss. Many people with TBI lose close relationships.12 This can be due to life changes such as job loss, loss of function or ability to do previously enjoyed activities, or personality changes. These relationship losses can affect a person profoundly.12 Going forward, they may have difficulty trusting others, for example.
Existential issues. Many people with TBI also find that cognitive deficits prevent them from engaging in formerly meaningful work. For example, Mr. M lost his longstanding career as an auto mechanic and therefore part of his identity. Not being able to find purpose and meaning can be a strong contributor to coping difficulties in those with TBI.13
Chronic pain. More than half of people with TBI experience chronic pain. Headaches are the most common pain condition among all TBI survivors.14
Substance use disorders. The directionality of substance use disorders and TBI is not always clear; however, most evidence suggests that substance abuse is highly prevalent, premorbid, and often a contributing factor in TBI (eg, car accidents).15 Alcohol abuse is the most common risk factor, followed by drug abuse.16 Substance abuse may be exacerbated after TBI when it becomes a coping mechanism under worsening stressors; additionally, executive function deficits or other neurologic problems may result in poor decision-making with regard to substance use.15 While substance abuse may decline in the immediate post-TBI period, it can return to pre-injury levels within a year.17
Selective serotonin reuptake inhibitors may help
Few studies have explored the efficacy of antidepressant medication in TBI survivors. In a controlled study of patients with TBI, Fann and colleagues18 found no significant improvement in depression symptoms between sertraline and a placebo. However, they did note some possibilities for this lack of significance: socially isolated TBI survivors in the placebo group may have demonstrated improvement in depression symptoms because of increased social interaction;
Continue to: Other research has found...
Other research has found that sertraline improved both depression and quality of life for men with post-TBI depression.19 In a meta-analysis of 4 studies, Paraschakis and Katsanos20 found that sertraline demonstrated a “trend toward significance” in the treatment of depression among patients with TBI. Silverberg and Panenka21 argue that selective serotonin reuptake inhibitors should be used as first-line treatment for depression in survivors of TBI. They note that in non-randomized studies, treatment effects with antidepressants are significant. Additionally, patients who do not respond to the first antidepressant prescribed will often respond to adjunctive or different medications. Finally, they argue that depression measures can capture symptoms related to the physical brain injury, in addition to symptoms of depression, thus confounding results.
THE CASE
Mr. M’s chart showed that he was not taking any medication and that he had no history of substance abuse or tobacco use. He refused to fill out the Patient Health Questionnaire (PHQ)-2. His guardian said that Mr. M was spending much of his time at home, and that he used to be an avid painter and guitar player but had not engaged in either activity for months. Furthermore, Mr. M used to enjoy working out but did so rarely now.
During the interview, the PCP was careful to make eye contact with Mr. M as well as his guardian, thereby making sure Mr. M was part of the conversation about his care. Pacing of questions was deliberate and unhurried; a return visit would be scheduled to further explore any concerns not covered in this visit. This collaborative, inclusive, patient-centered approach to the clinical interview seemed to place Mr. M at ease. When his guardian said he thought Mr. M was depressed, Mr. M agreed. Although Mr. M still refused to fill out the PHQ-2, he was now willing to answer questions about depression. He acknowledged that he was feeling hopeless and took little pleasure in activities he used to enjoy, thereby indicating a positive screen for depression.
The PCP opted to read the PHQ-9 questions aloud, and Mr. M agreed with most of the items but strongly denied suicidal ideation, citing his religious faith.
The PCP determined that Mr. M’s depression was likely a combination of the direct and indirect effects of his TBI. A quantitative estimate based on Mr. M’s report yielded a PHQ-9 score of 17, indicating moderately severe depression.
Continue to: In addition to building rapport...
In addition to building rapport, careful listening garnered important information about Mr. M. For example, until his accident and subsequent depression, Mr. M had long prioritized his physical health through diet and exercise. He followed a vegetarian diet but recently had little appetite and was eating one microwaveable meal a day. He had an irregular sleep schedule and struggled with insomnia. He lost his closest long-term relationship after his accident due to difficulties with affect regulation. He also lost his job as he could no longer cognitively handle the tasks required.
Hearing Mr. M’s story provided the opportunity to customize education about self-management skills including regular diet, exercise, and sleep hygiene. Due to limited visit time, the PCP elected to use this first visit to focus on sleep and depression. As cognitive behavioral therapy (CBT) for insomnia is first-line treatment for both primary insomnia and insomnia due to a medical condition such as TBI,5 a sleep aid was not prescribed. Fortunately, the clinic psychologist who offered CBT was able to join the interview to meet Mr. M and explain the treatment.
Mr. M expressed some initial reluctance to try an antidepressant. However, acknowledging he “just hasn’t been the same” since his TBI, he agreed to a prescription for sertraline and said he hoped it could make him “more like [he] was.”
RETURN VISIT
Four weeks after Mr. M began taking sertraline and participating in weekly CBT sessions, he returned for a follow-up visit with his PCP. He had a noticeably brighter affect, and his guardian reported that he had been playing the guitar again. Mr. M said that he had more energy as a result of improved sleep and mood, and that he felt like his “thinking was clearer.” Mr. M noted that he never thought he would meet with a psychologist but was finding CBT for insomnia helpful.
The psychologist’s notes proposed a treatment plan that would also include targeted grief and existential therapies to address Mr. M’s sudden life changes. At this visit, Mr. M admitted that his reading comprehension and speed were negatively affected by the accident and said this is why he did not wish to fill out the PHQ-2. But he was again willing to have the PHQ-9 questions read to him with his guardian’s support. Results showed a score of 6, indicating mild depression.
A follow-up appointment with Mr. M was scheduled for 6 weeks later, and the team was confident he was getting the behavioral and mental health support he needed through medication and therapy.
CORRESPONDENCE
Elizabeth Imbesi, PhD, VA Ann Arbor Healthcare System, 2215 Fuller Road, Ann Arbor, MI 48105; elizabeth.imbesi@va.gov
1. CDC. Traumatic brain injury & concussion. 2020. Accessed May 19, 2022. www.cdc.gov/traumaticbraininjury/index.html
2. Finset A, Anderson S. Coping strategies in patients with acquired brain injury: relationships between coping, apathy, depression and lesion location. Brain Inj. 2009;14:887-905. doi: 10.1080/026990500445718
3. CDC. Gioia G, Collins M. Acute concussion evaluation. 2006. Accessed May 19, 2022. www.cdc.gov/headsup/pdfs/providers/ace_v2-a.pdf
4. Prigatano GP. Psychotherapy and the process of coping with a brain disorder. Oral presentation at: American Psychological Association annual convention. August 2015; Toronto, Canada.
5. Ouellet M, Beaulieu-Bonneau S, Savard J, Morin C. Insomnia and Fatigue After Traumatic Brain Injury: A CBT Approach to Assessment and Treatment. Elsevier Academic Press: 2019.
6. Lewis FD, Horn GH. Depression following traumatic brain injury: impact on post-hospital residential rehabilitation outcomes. NeuroRehabilitation. 2017;40:401-410. doi: 10.3233/NRE-161427
7. Barkil-Oteo A. Collaborative care for depression in primary care: how psychiatry could “troubleshoot” current treatments and practices. Yale J Bio Med. 2013;86:139-146.
8. Whelan-Goodinson R, Ponsford J, Johnston L, et al. Psychiatric disorders following traumatic brain injury: their nature and frequency. J Head Trauma Rehabil. 2009;24:324-332. doi: 10.1097/HTR.0b013e3181a712aa
9. Reeves RR, Laizer JT. Traumatic brain injury and suicide. J Psychosoc Nurs Ment Health Serv. 2012;50:32-38. doi: 10.3928/02793695-20120207-02
10. Simpson G, Tate R. Suicidality in people surviving a traumatic brain injury: Prevalence, risk factors and implications for clinical management. Brain Inj. 2007;21:1335-1351. doi: 10.1080/02699050701785542
11. Carroll E, Coetzer R. Identity, grief and self-awareness after traumatic brain injury. Neuropsychol Rehabil. 2011;21:289-305. doi: 10.1080/09602011.2011.555972
12. Salas CE, Casassus M, Rowlands L, et al. “Relating through sameness”: a qualitative study of friendship and social isolation in chronic traumatic brain injury. Neuropsychol Rehabil. 2018;28:1161-1178. doi: 10.1080/09602011.2016.1247730
13. Hinkebein JA, Stucky R. Coping with traumatic brain injury: existential challenges and managing hope. In: Martz E, Livneh H, eds. Coping with Chronic Illness and Disability: Theoretical, Empirical, and Clinical Aspects. Springer Science & Business Media; 2007:389-409.
14. Khoury S, Benavides R. Pain with traumatic brain injury and psychological disorders. Prog Neuropsychopharmacol and Biol Psychiatry. 2018;87:224-233. doi: 10.1016/j.pnpbp.2017.06.007
15. Bjork JM, Grant SJ. Does traumatic brain injury increase risk for substance abuse? J Neurotrauma. 2009;26:1077-1082. doi: 10.1089/neu.2008.0849
16. Unsworth DJ, Mathias JL. Traumatic brain injury and alcohol/substance abuse: a Bayesian meta-analysis comparing the outcomes of people with and without a history of abuse. J Clin Exp Neuropsychol. 2017,39:547-562. doi: 10.1080/13803395.2016.1248812
17. Beaulieu-Bonneau S, St-Onge F, Blackburn M, et al. Alcohol and drug use before and during the first year after traumatic brain injury. J Head Trauma Rehabil. 2018;33:E51-E60. doi: 10.1097/HTR.0000000000000341
18. Fann JR, Bombardier CH, Temkin N, et al. Sertraline for major depression during the year following traumatic brain injury: a randomized control trial. J Head Trauma Rehabil. 2017;32:332-342. doi: 10.1097/HTR.0000000000000322
19. Ansari A, Jain A, Sharma A, et al. Role of sertraline in posttraumatic brain injury depression and quality of life in TBI. Asian J Neurosurg. 2014;9:182-188. doi: 10.4103/1793-5482.146597
20. Paraschakis A, Katsanos AH. Antidepressants for depression associated with traumatic brain injury: a meta-analytical study of randomized control trials. East Asian Arch Psychiatry. 2017;27:142-149.
21. Silverberg ND, Panenka WJ. Antidepressants for depression after concussion and traumatic brain injury are still best practice. BMC Psychiatry. 2019;19:100. doi: 10.1186/s12888-019-2076-9
THE CASE
Declan M*, a 42-year-old man, presents as a new patient for general medical care. One year ago, he sustained a severe frontal traumatic brain injury (TBI) when he was hit by a car while crossing a street. He developed a subdural hematoma and was in a coma for 6 days. He also had fractured ribs and a fractured left foot. When he regained consciousness, he had posttraumatic amnesia. He also had executive function deficits and memory difficulties, so a guardian was appointed.
Mr. M no longer works as an auto mechanic, a career he once greatly enjoyed. Mr. M’s guardian reports that recently, Mr. M has lost interest in activities he’d previously enjoyed, is frequently irritable, has poor sleep, is socially isolated, and is spending increasing amounts of time at home. When his new primary care physician (PCP) enters the examining room, Mr. M is seated in a chair with his arms folded across his chest. He states that he is “fine” and just needs to “get a doctor.”
●
*This patient is an amalgam of patients for whom the author has provided care.
TBI ranges from mild to severe and can produce a number of profound effects that are a direct—or indirect—result of the physical injury.1 The location and the severity of the injury affect symptoms.2 Even mild TBI can cause impairment, and severe TBI can lead to broad cognitive, behavioral, and physical difficulties. As numbers of TBI cases increase globally, primary care providers need to recognize the symptoms and assess accordingly.1 The Acute Concussion Evaluation (ACE; Physician/Clinician Office Version) facilitates a structured evaluation for patients presenting with possible TBI symptoms. It can easily be accessed on the Centers for Disease Control and Prevention website.3
Direct effects of TBI include impulsivity, depression, reduced frustration tolerance, reduced motivation, low awareness, and insomnia and other sleep difficulties.4,5 Depression may also result indirectly from, or be exacerbated by, new posttraumatic limitations and lifestyle changes as well as loss of career and community.4 Both direct and indirect depression often manifest as feelings of hopelessness and worthlessness and a lack of interest in once enjoyable activities. Depression can worsen other TBI sequelae such as difficulty concentrating, lack of initiation, flat affect, irritability, reduced independence, reduced functional performance, loss of inhibition, and physical pain.6
Nationwide, most mental health concerns continue to be addressed in the primary care setting.7 Individuals with TBI experience major depression at a rate 5 to 6 times higher than those in the general population, with a prevalence rate of 45%.8
Suicide. The subject of suicide must be explored with survivors of TBI; evidence suggests a correlation between TBI, depression, and increased risk for suicide.9 Among those who have TBI, as many as 22% experience suicidal ideation; the risk of suicide in survivors of severe TBI is 3 to 4 times the risk in the general population.10 Additionally, suicidality in this context appears to be a chronic concern; therefore, carefully assess for its presence no matter how long ago the TBI occurred.10
Additional TBI-associated health concerns
Grief and loss. We so often focus on death as the only cause for grief, but grief can occur for other types of loss, as well. Individuals with TBI often experience a radical negative change in self-concept after their injury, which is associated with feelings of grief.11 Helping patients recognize that they are grieving the loss of the person they once were can help set a framework for their experience.
Continue to: Relationship loss
Relationship loss. Many people with TBI lose close relationships.12 This can be due to life changes such as job loss, loss of function or ability to do previously enjoyed activities, or personality changes. These relationship losses can affect a person profoundly.12 Going forward, they may have difficulty trusting others, for example.
Existential issues. Many people with TBI also find that cognitive deficits prevent them from engaging in formerly meaningful work. For example, Mr. M lost his longstanding career as an auto mechanic and therefore part of his identity. Not being able to find purpose and meaning can be a strong contributor to coping difficulties in those with TBI.13
Chronic pain. More than half of people with TBI experience chronic pain. Headaches are the most common pain condition among all TBI survivors.14
Substance use disorders. The directionality of substance use disorders and TBI is not always clear; however, most evidence suggests that substance abuse is highly prevalent, premorbid, and often a contributing factor in TBI (eg, car accidents).15 Alcohol abuse is the most common risk factor, followed by drug abuse.16 Substance abuse may be exacerbated after TBI when it becomes a coping mechanism under worsening stressors; additionally, executive function deficits or other neurologic problems may result in poor decision-making with regard to substance use.15 While substance abuse may decline in the immediate post-TBI period, it can return to pre-injury levels within a year.17
Selective serotonin reuptake inhibitors may help
Few studies have explored the efficacy of antidepressant medication in TBI survivors. In a controlled study of patients with TBI, Fann and colleagues18 found no significant improvement in depression symptoms between sertraline and a placebo. However, they did note some possibilities for this lack of significance: socially isolated TBI survivors in the placebo group may have demonstrated improvement in depression symptoms because of increased social interaction;
Continue to: Other research has found...
Other research has found that sertraline improved both depression and quality of life for men with post-TBI depression.19 In a meta-analysis of 4 studies, Paraschakis and Katsanos20 found that sertraline demonstrated a “trend toward significance” in the treatment of depression among patients with TBI. Silverberg and Panenka21 argue that selective serotonin reuptake inhibitors should be used as first-line treatment for depression in survivors of TBI. They note that in non-randomized studies, treatment effects with antidepressants are significant. Additionally, patients who do not respond to the first antidepressant prescribed will often respond to adjunctive or different medications. Finally, they argue that depression measures can capture symptoms related to the physical brain injury, in addition to symptoms of depression, thus confounding results.
THE CASE
Mr. M’s chart showed that he was not taking any medication and that he had no history of substance abuse or tobacco use. He refused to fill out the Patient Health Questionnaire (PHQ)-2. His guardian said that Mr. M was spending much of his time at home, and that he used to be an avid painter and guitar player but had not engaged in either activity for months. Furthermore, Mr. M used to enjoy working out but did so rarely now.
During the interview, the PCP was careful to make eye contact with Mr. M as well as his guardian, thereby making sure Mr. M was part of the conversation about his care. Pacing of questions was deliberate and unhurried; a return visit would be scheduled to further explore any concerns not covered in this visit. This collaborative, inclusive, patient-centered approach to the clinical interview seemed to place Mr. M at ease. When his guardian said he thought Mr. M was depressed, Mr. M agreed. Although Mr. M still refused to fill out the PHQ-2, he was now willing to answer questions about depression. He acknowledged that he was feeling hopeless and took little pleasure in activities he used to enjoy, thereby indicating a positive screen for depression.
The PCP opted to read the PHQ-9 questions aloud, and Mr. M agreed with most of the items but strongly denied suicidal ideation, citing his religious faith.
The PCP determined that Mr. M’s depression was likely a combination of the direct and indirect effects of his TBI. A quantitative estimate based on Mr. M’s report yielded a PHQ-9 score of 17, indicating moderately severe depression.
Continue to: In addition to building rapport...
In addition to building rapport, careful listening garnered important information about Mr. M. For example, until his accident and subsequent depression, Mr. M had long prioritized his physical health through diet and exercise. He followed a vegetarian diet but recently had little appetite and was eating one microwaveable meal a day. He had an irregular sleep schedule and struggled with insomnia. He lost his closest long-term relationship after his accident due to difficulties with affect regulation. He also lost his job as he could no longer cognitively handle the tasks required.
Hearing Mr. M’s story provided the opportunity to customize education about self-management skills including regular diet, exercise, and sleep hygiene. Due to limited visit time, the PCP elected to use this first visit to focus on sleep and depression. As cognitive behavioral therapy (CBT) for insomnia is first-line treatment for both primary insomnia and insomnia due to a medical condition such as TBI,5 a sleep aid was not prescribed. Fortunately, the clinic psychologist who offered CBT was able to join the interview to meet Mr. M and explain the treatment.
Mr. M expressed some initial reluctance to try an antidepressant. However, acknowledging he “just hasn’t been the same” since his TBI, he agreed to a prescription for sertraline and said he hoped it could make him “more like [he] was.”
RETURN VISIT
Four weeks after Mr. M began taking sertraline and participating in weekly CBT sessions, he returned for a follow-up visit with his PCP. He had a noticeably brighter affect, and his guardian reported that he had been playing the guitar again. Mr. M said that he had more energy as a result of improved sleep and mood, and that he felt like his “thinking was clearer.” Mr. M noted that he never thought he would meet with a psychologist but was finding CBT for insomnia helpful.
The psychologist’s notes proposed a treatment plan that would also include targeted grief and existential therapies to address Mr. M’s sudden life changes. At this visit, Mr. M admitted that his reading comprehension and speed were negatively affected by the accident and said this is why he did not wish to fill out the PHQ-2. But he was again willing to have the PHQ-9 questions read to him with his guardian’s support. Results showed a score of 6, indicating mild depression.
A follow-up appointment with Mr. M was scheduled for 6 weeks later, and the team was confident he was getting the behavioral and mental health support he needed through medication and therapy.
CORRESPONDENCE
Elizabeth Imbesi, PhD, VA Ann Arbor Healthcare System, 2215 Fuller Road, Ann Arbor, MI 48105; elizabeth.imbesi@va.gov
THE CASE
Declan M*, a 42-year-old man, presents as a new patient for general medical care. One year ago, he sustained a severe frontal traumatic brain injury (TBI) when he was hit by a car while crossing a street. He developed a subdural hematoma and was in a coma for 6 days. He also had fractured ribs and a fractured left foot. When he regained consciousness, he had posttraumatic amnesia. He also had executive function deficits and memory difficulties, so a guardian was appointed.
Mr. M no longer works as an auto mechanic, a career he once greatly enjoyed. Mr. M’s guardian reports that recently, Mr. M has lost interest in activities he’d previously enjoyed, is frequently irritable, has poor sleep, is socially isolated, and is spending increasing amounts of time at home. When his new primary care physician (PCP) enters the examining room, Mr. M is seated in a chair with his arms folded across his chest. He states that he is “fine” and just needs to “get a doctor.”
●
*This patient is an amalgam of patients for whom the author has provided care.
TBI ranges from mild to severe and can produce a number of profound effects that are a direct—or indirect—result of the physical injury.1 The location and the severity of the injury affect symptoms.2 Even mild TBI can cause impairment, and severe TBI can lead to broad cognitive, behavioral, and physical difficulties. As numbers of TBI cases increase globally, primary care providers need to recognize the symptoms and assess accordingly.1 The Acute Concussion Evaluation (ACE; Physician/Clinician Office Version) facilitates a structured evaluation for patients presenting with possible TBI symptoms. It can easily be accessed on the Centers for Disease Control and Prevention website.3
Direct effects of TBI include impulsivity, depression, reduced frustration tolerance, reduced motivation, low awareness, and insomnia and other sleep difficulties.4,5 Depression may also result indirectly from, or be exacerbated by, new posttraumatic limitations and lifestyle changes as well as loss of career and community.4 Both direct and indirect depression often manifest as feelings of hopelessness and worthlessness and a lack of interest in once enjoyable activities. Depression can worsen other TBI sequelae such as difficulty concentrating, lack of initiation, flat affect, irritability, reduced independence, reduced functional performance, loss of inhibition, and physical pain.6
Nationwide, most mental health concerns continue to be addressed in the primary care setting.7 Individuals with TBI experience major depression at a rate 5 to 6 times higher than those in the general population, with a prevalence rate of 45%.8
Suicide. The subject of suicide must be explored with survivors of TBI; evidence suggests a correlation between TBI, depression, and increased risk for suicide.9 Among those who have TBI, as many as 22% experience suicidal ideation; the risk of suicide in survivors of severe TBI is 3 to 4 times the risk in the general population.10 Additionally, suicidality in this context appears to be a chronic concern; therefore, carefully assess for its presence no matter how long ago the TBI occurred.10
Additional TBI-associated health concerns
Grief and loss. We so often focus on death as the only cause for grief, but grief can occur for other types of loss, as well. Individuals with TBI often experience a radical negative change in self-concept after their injury, which is associated with feelings of grief.11 Helping patients recognize that they are grieving the loss of the person they once were can help set a framework for their experience.
Continue to: Relationship loss
Relationship loss. Many people with TBI lose close relationships.12 This can be due to life changes such as job loss, loss of function or ability to do previously enjoyed activities, or personality changes. These relationship losses can affect a person profoundly.12 Going forward, they may have difficulty trusting others, for example.
Existential issues. Many people with TBI also find that cognitive deficits prevent them from engaging in formerly meaningful work. For example, Mr. M lost his longstanding career as an auto mechanic and therefore part of his identity. Not being able to find purpose and meaning can be a strong contributor to coping difficulties in those with TBI.13
Chronic pain. More than half of people with TBI experience chronic pain. Headaches are the most common pain condition among all TBI survivors.14
Substance use disorders. The directionality of substance use disorders and TBI is not always clear; however, most evidence suggests that substance abuse is highly prevalent, premorbid, and often a contributing factor in TBI (eg, car accidents).15 Alcohol abuse is the most common risk factor, followed by drug abuse.16 Substance abuse may be exacerbated after TBI when it becomes a coping mechanism under worsening stressors; additionally, executive function deficits or other neurologic problems may result in poor decision-making with regard to substance use.15 While substance abuse may decline in the immediate post-TBI period, it can return to pre-injury levels within a year.17
Selective serotonin reuptake inhibitors may help
Few studies have explored the efficacy of antidepressant medication in TBI survivors. In a controlled study of patients with TBI, Fann and colleagues18 found no significant improvement in depression symptoms between sertraline and a placebo. However, they did note some possibilities for this lack of significance: socially isolated TBI survivors in the placebo group may have demonstrated improvement in depression symptoms because of increased social interaction;
Continue to: Other research has found...
Other research has found that sertraline improved both depression and quality of life for men with post-TBI depression.19 In a meta-analysis of 4 studies, Paraschakis and Katsanos20 found that sertraline demonstrated a “trend toward significance” in the treatment of depression among patients with TBI. Silverberg and Panenka21 argue that selective serotonin reuptake inhibitors should be used as first-line treatment for depression in survivors of TBI. They note that in non-randomized studies, treatment effects with antidepressants are significant. Additionally, patients who do not respond to the first antidepressant prescribed will often respond to adjunctive or different medications. Finally, they argue that depression measures can capture symptoms related to the physical brain injury, in addition to symptoms of depression, thus confounding results.
THE CASE
Mr. M’s chart showed that he was not taking any medication and that he had no history of substance abuse or tobacco use. He refused to fill out the Patient Health Questionnaire (PHQ)-2. His guardian said that Mr. M was spending much of his time at home, and that he used to be an avid painter and guitar player but had not engaged in either activity for months. Furthermore, Mr. M used to enjoy working out but did so rarely now.
During the interview, the PCP was careful to make eye contact with Mr. M as well as his guardian, thereby making sure Mr. M was part of the conversation about his care. Pacing of questions was deliberate and unhurried; a return visit would be scheduled to further explore any concerns not covered in this visit. This collaborative, inclusive, patient-centered approach to the clinical interview seemed to place Mr. M at ease. When his guardian said he thought Mr. M was depressed, Mr. M agreed. Although Mr. M still refused to fill out the PHQ-2, he was now willing to answer questions about depression. He acknowledged that he was feeling hopeless and took little pleasure in activities he used to enjoy, thereby indicating a positive screen for depression.
The PCP opted to read the PHQ-9 questions aloud, and Mr. M agreed with most of the items but strongly denied suicidal ideation, citing his religious faith.
The PCP determined that Mr. M’s depression was likely a combination of the direct and indirect effects of his TBI. A quantitative estimate based on Mr. M’s report yielded a PHQ-9 score of 17, indicating moderately severe depression.
Continue to: In addition to building rapport...
In addition to building rapport, careful listening garnered important information about Mr. M. For example, until his accident and subsequent depression, Mr. M had long prioritized his physical health through diet and exercise. He followed a vegetarian diet but recently had little appetite and was eating one microwaveable meal a day. He had an irregular sleep schedule and struggled with insomnia. He lost his closest long-term relationship after his accident due to difficulties with affect regulation. He also lost his job as he could no longer cognitively handle the tasks required.
Hearing Mr. M’s story provided the opportunity to customize education about self-management skills including regular diet, exercise, and sleep hygiene. Due to limited visit time, the PCP elected to use this first visit to focus on sleep and depression. As cognitive behavioral therapy (CBT) for insomnia is first-line treatment for both primary insomnia and insomnia due to a medical condition such as TBI,5 a sleep aid was not prescribed. Fortunately, the clinic psychologist who offered CBT was able to join the interview to meet Mr. M and explain the treatment.
Mr. M expressed some initial reluctance to try an antidepressant. However, acknowledging he “just hasn’t been the same” since his TBI, he agreed to a prescription for sertraline and said he hoped it could make him “more like [he] was.”
RETURN VISIT
Four weeks after Mr. M began taking sertraline and participating in weekly CBT sessions, he returned for a follow-up visit with his PCP. He had a noticeably brighter affect, and his guardian reported that he had been playing the guitar again. Mr. M said that he had more energy as a result of improved sleep and mood, and that he felt like his “thinking was clearer.” Mr. M noted that he never thought he would meet with a psychologist but was finding CBT for insomnia helpful.
The psychologist’s notes proposed a treatment plan that would also include targeted grief and existential therapies to address Mr. M’s sudden life changes. At this visit, Mr. M admitted that his reading comprehension and speed were negatively affected by the accident and said this is why he did not wish to fill out the PHQ-2. But he was again willing to have the PHQ-9 questions read to him with his guardian’s support. Results showed a score of 6, indicating mild depression.
A follow-up appointment with Mr. M was scheduled for 6 weeks later, and the team was confident he was getting the behavioral and mental health support he needed through medication and therapy.
CORRESPONDENCE
Elizabeth Imbesi, PhD, VA Ann Arbor Healthcare System, 2215 Fuller Road, Ann Arbor, MI 48105; elizabeth.imbesi@va.gov
1. CDC. Traumatic brain injury & concussion. 2020. Accessed May 19, 2022. www.cdc.gov/traumaticbraininjury/index.html
2. Finset A, Anderson S. Coping strategies in patients with acquired brain injury: relationships between coping, apathy, depression and lesion location. Brain Inj. 2009;14:887-905. doi: 10.1080/026990500445718
3. CDC. Gioia G, Collins M. Acute concussion evaluation. 2006. Accessed May 19, 2022. www.cdc.gov/headsup/pdfs/providers/ace_v2-a.pdf
4. Prigatano GP. Psychotherapy and the process of coping with a brain disorder. Oral presentation at: American Psychological Association annual convention. August 2015; Toronto, Canada.
5. Ouellet M, Beaulieu-Bonneau S, Savard J, Morin C. Insomnia and Fatigue After Traumatic Brain Injury: A CBT Approach to Assessment and Treatment. Elsevier Academic Press: 2019.
6. Lewis FD, Horn GH. Depression following traumatic brain injury: impact on post-hospital residential rehabilitation outcomes. NeuroRehabilitation. 2017;40:401-410. doi: 10.3233/NRE-161427
7. Barkil-Oteo A. Collaborative care for depression in primary care: how psychiatry could “troubleshoot” current treatments and practices. Yale J Bio Med. 2013;86:139-146.
8. Whelan-Goodinson R, Ponsford J, Johnston L, et al. Psychiatric disorders following traumatic brain injury: their nature and frequency. J Head Trauma Rehabil. 2009;24:324-332. doi: 10.1097/HTR.0b013e3181a712aa
9. Reeves RR, Laizer JT. Traumatic brain injury and suicide. J Psychosoc Nurs Ment Health Serv. 2012;50:32-38. doi: 10.3928/02793695-20120207-02
10. Simpson G, Tate R. Suicidality in people surviving a traumatic brain injury: Prevalence, risk factors and implications for clinical management. Brain Inj. 2007;21:1335-1351. doi: 10.1080/02699050701785542
11. Carroll E, Coetzer R. Identity, grief and self-awareness after traumatic brain injury. Neuropsychol Rehabil. 2011;21:289-305. doi: 10.1080/09602011.2011.555972
12. Salas CE, Casassus M, Rowlands L, et al. “Relating through sameness”: a qualitative study of friendship and social isolation in chronic traumatic brain injury. Neuropsychol Rehabil. 2018;28:1161-1178. doi: 10.1080/09602011.2016.1247730
13. Hinkebein JA, Stucky R. Coping with traumatic brain injury: existential challenges and managing hope. In: Martz E, Livneh H, eds. Coping with Chronic Illness and Disability: Theoretical, Empirical, and Clinical Aspects. Springer Science & Business Media; 2007:389-409.
14. Khoury S, Benavides R. Pain with traumatic brain injury and psychological disorders. Prog Neuropsychopharmacol and Biol Psychiatry. 2018;87:224-233. doi: 10.1016/j.pnpbp.2017.06.007
15. Bjork JM, Grant SJ. Does traumatic brain injury increase risk for substance abuse? J Neurotrauma. 2009;26:1077-1082. doi: 10.1089/neu.2008.0849
16. Unsworth DJ, Mathias JL. Traumatic brain injury and alcohol/substance abuse: a Bayesian meta-analysis comparing the outcomes of people with and without a history of abuse. J Clin Exp Neuropsychol. 2017,39:547-562. doi: 10.1080/13803395.2016.1248812
17. Beaulieu-Bonneau S, St-Onge F, Blackburn M, et al. Alcohol and drug use before and during the first year after traumatic brain injury. J Head Trauma Rehabil. 2018;33:E51-E60. doi: 10.1097/HTR.0000000000000341
18. Fann JR, Bombardier CH, Temkin N, et al. Sertraline for major depression during the year following traumatic brain injury: a randomized control trial. J Head Trauma Rehabil. 2017;32:332-342. doi: 10.1097/HTR.0000000000000322
19. Ansari A, Jain A, Sharma A, et al. Role of sertraline in posttraumatic brain injury depression and quality of life in TBI. Asian J Neurosurg. 2014;9:182-188. doi: 10.4103/1793-5482.146597
20. Paraschakis A, Katsanos AH. Antidepressants for depression associated with traumatic brain injury: a meta-analytical study of randomized control trials. East Asian Arch Psychiatry. 2017;27:142-149.
21. Silverberg ND, Panenka WJ. Antidepressants for depression after concussion and traumatic brain injury are still best practice. BMC Psychiatry. 2019;19:100. doi: 10.1186/s12888-019-2076-9
1. CDC. Traumatic brain injury & concussion. 2020. Accessed May 19, 2022. www.cdc.gov/traumaticbraininjury/index.html
2. Finset A, Anderson S. Coping strategies in patients with acquired brain injury: relationships between coping, apathy, depression and lesion location. Brain Inj. 2009;14:887-905. doi: 10.1080/026990500445718
3. CDC. Gioia G, Collins M. Acute concussion evaluation. 2006. Accessed May 19, 2022. www.cdc.gov/headsup/pdfs/providers/ace_v2-a.pdf
4. Prigatano GP. Psychotherapy and the process of coping with a brain disorder. Oral presentation at: American Psychological Association annual convention. August 2015; Toronto, Canada.
5. Ouellet M, Beaulieu-Bonneau S, Savard J, Morin C. Insomnia and Fatigue After Traumatic Brain Injury: A CBT Approach to Assessment and Treatment. Elsevier Academic Press: 2019.
6. Lewis FD, Horn GH. Depression following traumatic brain injury: impact on post-hospital residential rehabilitation outcomes. NeuroRehabilitation. 2017;40:401-410. doi: 10.3233/NRE-161427
7. Barkil-Oteo A. Collaborative care for depression in primary care: how psychiatry could “troubleshoot” current treatments and practices. Yale J Bio Med. 2013;86:139-146.
8. Whelan-Goodinson R, Ponsford J, Johnston L, et al. Psychiatric disorders following traumatic brain injury: their nature and frequency. J Head Trauma Rehabil. 2009;24:324-332. doi: 10.1097/HTR.0b013e3181a712aa
9. Reeves RR, Laizer JT. Traumatic brain injury and suicide. J Psychosoc Nurs Ment Health Serv. 2012;50:32-38. doi: 10.3928/02793695-20120207-02
10. Simpson G, Tate R. Suicidality in people surviving a traumatic brain injury: Prevalence, risk factors and implications for clinical management. Brain Inj. 2007;21:1335-1351. doi: 10.1080/02699050701785542
11. Carroll E, Coetzer R. Identity, grief and self-awareness after traumatic brain injury. Neuropsychol Rehabil. 2011;21:289-305. doi: 10.1080/09602011.2011.555972
12. Salas CE, Casassus M, Rowlands L, et al. “Relating through sameness”: a qualitative study of friendship and social isolation in chronic traumatic brain injury. Neuropsychol Rehabil. 2018;28:1161-1178. doi: 10.1080/09602011.2016.1247730
13. Hinkebein JA, Stucky R. Coping with traumatic brain injury: existential challenges and managing hope. In: Martz E, Livneh H, eds. Coping with Chronic Illness and Disability: Theoretical, Empirical, and Clinical Aspects. Springer Science & Business Media; 2007:389-409.
14. Khoury S, Benavides R. Pain with traumatic brain injury and psychological disorders. Prog Neuropsychopharmacol and Biol Psychiatry. 2018;87:224-233. doi: 10.1016/j.pnpbp.2017.06.007
15. Bjork JM, Grant SJ. Does traumatic brain injury increase risk for substance abuse? J Neurotrauma. 2009;26:1077-1082. doi: 10.1089/neu.2008.0849
16. Unsworth DJ, Mathias JL. Traumatic brain injury and alcohol/substance abuse: a Bayesian meta-analysis comparing the outcomes of people with and without a history of abuse. J Clin Exp Neuropsychol. 2017,39:547-562. doi: 10.1080/13803395.2016.1248812
17. Beaulieu-Bonneau S, St-Onge F, Blackburn M, et al. Alcohol and drug use before and during the first year after traumatic brain injury. J Head Trauma Rehabil. 2018;33:E51-E60. doi: 10.1097/HTR.0000000000000341
18. Fann JR, Bombardier CH, Temkin N, et al. Sertraline for major depression during the year following traumatic brain injury: a randomized control trial. J Head Trauma Rehabil. 2017;32:332-342. doi: 10.1097/HTR.0000000000000322
19. Ansari A, Jain A, Sharma A, et al. Role of sertraline in posttraumatic brain injury depression and quality of life in TBI. Asian J Neurosurg. 2014;9:182-188. doi: 10.4103/1793-5482.146597
20. Paraschakis A, Katsanos AH. Antidepressants for depression associated with traumatic brain injury: a meta-analytical study of randomized control trials. East Asian Arch Psychiatry. 2017;27:142-149.
21. Silverberg ND, Panenka WJ. Antidepressants for depression after concussion and traumatic brain injury are still best practice. BMC Psychiatry. 2019;19:100. doi: 10.1186/s12888-019-2076-9
Somatic symptom disorder in primary care: A collaborative approach
THE CASE
James R* is a 30-year-old man who presented for a primary care walk-in visit due to dizziness, 2 days after he visited an emergency department (ED) for the same concern. He reported episodic symptoms lasting seconds to minutes, specifically when lying down. He said he had not fallen or experienced other physical trauma, did not have blurred vision or hearing loss, and was taking no medications. He also reported panic attacks, during which he experienced palpitations, trembling, paresthesia, and fear of dying. He stated that dizziness did not occur exclusively during panic episodes. His medical history was significant for hypertension; however, he reported significant anxiety related to medical visits. All home blood pressure readings he reported were within normal limits.
Upon examination, the patient had a blood pressure reading of 142/90 mm Hg and no evidence of nystagmus at rest. A neurologic exam was normal and a Dix-Hallpike maneuver reproduced subjective vertigo without nystagmus. Laboratory findings from the patient’s ED visit were negative for troponin and drug use, and blood oxygenation levels were within normal limits. At the time of this current visit, an electrocardiogram was unremarkable, with the exception of some tachycardia.
The presumptive diagnosis was benign paroxysmal positional vertigo (BPPV). An Epley maneuver was performed in the clinic and resulted in minimal symptom improvement. The physician taught Mr. R how to perform the Epley maneuver himself, prescribed a short course of meclizine, and referred him to the integrated mental health care service to address his panic attacks and anxiety.
Over the next few months, Mr. R continued to report significant distress about the dizzy spells, which persisted even after performing the Epley maneuver, and he reported that the meclizine was causing worsening vertigo. He received an ear-nose-and-throat consultation and cognitive behavioral therapy (CBT)–based consultation/interventions. He also reported avoiding multiple activities due to concerns about his dizziness.
●
*The patient’s name and other personally identifying information have been changed to protect his identity.
Somatic symptom disorder (SSD) is characterized by one or more physical symptoms associated with “excessive thoughts, feelings, or behaviors that result in distress and/or functional impairment.”1 Individuals with SSD are preoccupied with symptom-related severity, experience high symptom-related anxiety, or devote significant time and energy to the symptoms or heath concerns. With a diagnosis of SSD, physical symptoms experienced by the patient may or may not be medically explained. The same symptom need not be continuously present as long as the overall symptomatic presentation lasts 6 months or longer.
The specifier “with predominant pain” is used when pain dominates the presentation.1 Estimated prevalence of SSD in primary care ranges from 5% to 35%.2 The true scope of SSD is difficult to assess accurately since research tends to focus on medically unexplained symptoms, rather than excessive symptom-related concerns. Furthermore, terms such as “medically unexplained symptoms” and “functional syndromes” (including fibromyalgia and irritable bowel syndrome) are frequently used when describing SSD.3
One or more factors may contribute to unexplained symptoms: limitations of medical procedures and techniques, partial clinical information, patients’ inability to follow management recommendations, challenges in differential diagnostics, and access-to-care limitations preventing regular care and appropriate diagnostic work up.
What’s important to remember is that it’s the patient’s reaction to physical symptoms, rather than the presence of symptoms per se, that defines SSD.
Considerations in the differential diagnosis
When making a diagnosis of SSD, symptoms cannot:4
- be feigned or deliberately produced as in malingering or factitious disorder.
- result from physiologic effects of a substance (eg, intoxication, withdrawal, or adverse medication effects).
- constitute somatic delusions, as occur in psychotic disorders.
- constitute symptoms or deficits affecting voluntary motor or sensory function that are better explained by neurologic, medical, or psychiatric conditions (consider conversion disorder).
- be preoccupations with physical appearance flaws, as in body dysmorphic disorder.
- be accounted for by an anxiety disorder (eg, palpitations associated with panic attacks).
Continue to: Illness anxiety disorder...
Illness anxiety disorder is also characterized by significant health-related concerns; however, physical symptoms are either mild or absent.
Possible causes of SSD are varied and complex, including genetic and biological factors, family dynamics, behavioral modeling/learning, personality traits, difficulties with emotional regulation, and awareness.5 Patients may present with ongoing requests for symptom explanations, feelings of helplessness, fear of having concerns dismissed, and low motivation for change.3
Aids in supporting a diagnosis of SSD
It’s not appropriate to rely solely on questionnaires to make the diagnosis of SSD. However, brief screening tools are a time-efficient way to capture patients’ experiences and perceptions.6 Along with other components of clinical evaluation, brief symptom screens can both support the diagnosis and help in longitudinal symptom assessment.
Patient Health Questionnaire-15 (PHQ-15), developed for self-report screening in primary care, has desirable psychometric properties including appropriate internal reliability; convergent validity with measures of functional status, disability days, and symptom-related burden; and discriminant validity from measures of depressive symptoms.7 The PHQ-15 is an open access tool that is available in several languages. The respondent is asked to rate the extent of being bothered by a range of medical symptoms in the proceeding 4 weeks. Total scores range from 0 to 30, with higher scores indicating greater symptom aggravation. Cutoffs of 5, 10, and 15 correspond to mild, moderate, and severe symptom levels.8
Somatic Symptom Disorder - B Criteria Scale (SSD-12) aims to capture SSD symptoms in line with Diagnostic and Statistical Manual of Mental Disorders (DSM-5) diagnostic criteria. It assesses cognitive, affective, and behavioral aspects of SSD.9 The SSD-12 is copyrighted and its use requires registration and purchase. Cutoffs by age and gender are available. SSD-12 has demonstrated appropriate reliability and validity.9
Continue to: Structured Clinical Interview for DSM Disorders
Structured Clinical Interview for DSM Disorders (SCID)10,11 is perhaps the most rigorous differential diagnostic tool. However, SCID administration requires training and skill; time for administration and cost of the materials may be prohibitive in primary care.
Finally, SSD symptoms are highly associated with depression and anxiety. Ongoing elevated screening scores for anxiety and depression refractory to interventions may indicate the possibility of overlooked SSD. Furthermore, use of SSD screening tools with anxiety and depression screening tools can provide a more comprehensive picture of impairment, as well as symptom progress.
Treatment: Avoid a split approach
Diagnosing and treating SSD can be challenging for physicians who focus on biomedically based approaches in patient care. Additional tests, studies, and prescriptions are likely to fuel (rather than pacify) patients’ concerns, as such steps divert attention from the underlying psychological needs and mechanisms which maintain SSD. Avoid using a split biopsychosocial approach—ie, beginning the inquiry and treatment planning from a biomedical perspective, and then falling back on psychosocial formulation when treatment efforts have been ineffective. Such an approach leads to understandable patient dissatisfaction and can be interpreted by them as the caregiver suggesting that physical symptoms are “all in [their] head.”12
These 4 tips can help
1. Use a biopsychosocial formulation when initiating treatment. Be familiar with biopsychosocial factors in SSD and develop a narrative for discussing this formulation with patients. For example: “Mr. R, we are going to use the following [medical tests/studies/medications] to understand the cause of your symptoms and better manage them. We also need to think about the role of stress and distress in your symptoms because these can also be at play with dizziness.” This may be particularly beneficial for a functional disorder, such as chronic pain. Incorporating patient education resources is an important step toward shared understanding (see Hunter Integrated Pain Service for chronic pain educational videos; www.tga.gov.au/chronic-pain-management-video-resource-brainman13).
2. Combine education about pathophysiology with patient-centered interviewing. Significant SSD symptom improvements were noted following a single 30-minute educational session, while motivational interviewing techniques were used to probe patients’ concerns.2
Continue to: Maintain professionalism and good clinical practice
3. Maintain professionalism and good clinical practice. Consider SSD a medical matter and address it accordingly: explore concerns fully, provide evidence-based responses, communicate empathy, and employ objective management strategies.14
4. Do not overlook the value of the relationship. A recent systematic review concluded that the relationship between the patient and care provider was central to the success of the interventions for symptom reduction.15
A controversial approach. Pharmacotherapy for SSD is controversial. While several trials of antidepressants and St. John’s wort have been positive and some authors have stated that all classes of antidepressants are effective for SSD, others maintain that questions regarding dosing, treatment duration, and sustainability of improvement have not been sufficiently addressed in research.16,17
Coordination of care issues
Primary care continues to be the de facto mental health system, and specialty services may be unavailable or declined by patients.18 CBT delivered in person or online is empirically supported as a treatment approach to medically unexplained symptoms and SSD.17,19-22
A recent meta-analysis of randomized controlled trials published by Jing and colleagues23 reported that CBT was effective for SSD symptom reduction, and that treatment gains were maintained 3 to 12 months post treatment. However, concerns about the practical implementation of CBT in primary care were raised because CBT was not shown to be effective in improving social functioning or reducing the number of medical visits. Symptom improvement was maximized with longer durations of treatment (> 10 sessions) and greater session lengths (> 50 minutes). Additionally, Abbass and colleagues24 brought up several methodologic (sampling and analysis) concerns related to Jing et al’s work.
Continue to: Overally, CBT's effect sizes...
Overall, CBT’s effect sizes are small, and patients who are open to biopsychosocial explanations for their symptoms and to receiving psychological services may differ from most patients seen in primary care practices.21 Furthermore, mental health providers may hesitate to diagnose SSD because they are concerned about missing a somatic illness.3 Therefore, when coordinating care with mental health providers, it may be beneficial to discuss the treatment approach, assess familiarity with the SSD diagnosis, and closely coordinate and collaborate on the treatment plan.
While physicians cannot be expected to function as psychotherapists, an understanding of CBT and techniques for SSD treatment can be beneficial. Integrated mental health services may hold promise in addressing SSD in primary care. Onsite availability of a behavioral health provider competent in providing evidence-based care can target SSD symptoms and support both patients and physicians.
THE CASE
Mr. R’s treatment course included multiple primary care appointments (scheduled and walk in), ED visits, and specialist visits (ENT/vestibular rehabilitation). He sought care as symptoms intensified, lasted longer, or occurred in new circumstances. He reported persistent fear of the symptoms and anxiety that serious medical causes had been overlooked. He also described distress associated with vertigo and his anxiety sensitivity (anxiety about being anxious).
The behavioral health consultant (BHC; psychologist) and physician talked to the patient about the biopsychosocial antecedents of his condition and the factors that perpetuate the anxiety and stress response. The BHC described the fight/flight/freeze response to the patient and explained its role in the physiologic stress response associated with somatic symptoms and panic. Educational materials (videos and handouts) were also provided to the patient to further illustrate these concepts. The BHC also discussed the role of interoceptive and situational avoidance and active coping (eg, engaging in safe activities); taught the patient relaxation and grounding techniques; and used cognitive disputation aimed at challenging catastrophic symptom interpretations.
The BHC and the patient’s physician established joint treatment goals that included improving functioning, promoting active coping, and decreasing distress associated with symptoms. After the initial medical and BHC visits, both vertigo and anxiety symptoms appeared to abate somewhat, but symptoms have been ongoing and distress and impairment have been variable. The patient’s family physician and BHC continue to work with him to optimize the care plan and treatment goals.
CORRESPONDENCE
Nataliya Pilipenko, PhD, ABPP, Center for Family and Community Medicine, Columbia University Vagelos College of Physicians and Surgeons, 630 West 168th Street, New York, NY 10032; np2615@cumc.columbia.edu
ACKNOWLEDGEMENT
The author thanks Dr. Molly Warren for her collaboration and guidance.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
2. Johnson KK, Bennett C, Rochani H. Significant improvement of somatic symptom disorder with brief psychoeducational intervention by PMHNP in primary care. J Am Psychiatr Nurses Assoc. 2020;28:171-180. doi: 10.1177/1078390320960524
3. Weigel A, Maehder K, Witt M, et al. Psychotherapists’ perspective on the treatment of patients with somatic symptom disorders. J Psychosom Res. 2020;138:110228. doi: 10.1016/j.jpsychores.2020.110228
4. American Psychiatric Association. Handbook of Differential Diagnosis. American Psychiatric Publishing; 2014;234-235.
5. Mayo Clinic. Somatic symptom disorder. Accessed February 21, 2022. www.mayoclinic.org/diseases-conditions/somatic-symptom-disorder/symptoms-causes/syc-20377776?p=1
6. Toussaint A, Riedl B, Kehrer S, et al. Validity of the Somatic Symptom Disorder-B Criteria Scale (SSD-12) in primary care. Fam Pract. 2018;35:342-347. doi: 10.1093/fampra/cmx116
7. Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258-66. doi: 10.1097/00006842-200203000-00008
8. Kroenke K, Spitzer RL, Williams JB, et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345-359. doi: 10.1016/j.genhosppsych.2010.03.006
9. Toussaint A, Löwe B, Brähler E, et al. The Somatic Symptom Disorder - B Criteria Scale (SSD-12): factorial structure, validity and population-based norms. J Psychosom Res. 2017;97:9-17. doi: 10.1016/j.jpsychores.2017.03.017
10. First MB, Williams JBW, Karg RS, Spitzer RL, eds. Structured Clinical Interview for DSM-5 Disorders, Research Version. American Psychiatric Association, 2015.
11. First MB, Williams JBW, Karg RS, Spitzer RL, eds. Structured Clinical Interview for DSM-5 Disorders, Clinician Version. American Psychiatric Publishing; 2016.
12. McDaniel SH, Hepworth J, Campbell TL, et al, eds. Family Oriented Primary Care. Springer Publishing Co; 2005:1-15.
13. Hunter Integrated Pain Service (2016). Brainman videos. Hunter New England Local Health District. New South Wales, Australia. Accessed February 21, 2022. www.tga.gov.au/chronic-pain-management-video-resource-brainman
14. Murray AM, Toussaint A, Althaus A, et al. The challenge of diagnosing non-specific, functional, and somatoform disorders: a systematic review of barriers to diagnosis in primary care. J Psychosom Res. 2016;80:1-10. doi: 10.1016/j.jpsychores.2015.11.002
15. Leaviss J, Davis S, Ren S, et al. Behavioral modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation. Health Technol Assess. 2020;24:1-490. doi: 10.3310/hta24460
16. Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med. 2007;69:881-888. doi: 10.1097/PSY.0b013e31815b00c4
17. Steinbrecher N, Koerber S, Frieser D, et al. The prevalence of medically unexplained symptoms in primary care. Psychosomatics. 2011;52:263-271. doi: 10.1016/j.psym.2011.01.007
18. Kessler R, Stafford D. Primary care is the de facto mental health system. In Kessler R, Stafford D, eds. Collaborative Medicine Case Studies: Evidence in Practice. Springer Publishing Co, 2008; 9-21.
19. Barsky AJ, Ahern DK, Bauer MR, et al. A randomized trial of treatments for high-utilizing somatizing patients. J Gen Intern Med. 2013;28:1396-1404. doi: 10.1007/s11606-013-2392-6
20. Newby JM, Smith J, Uppal S, et al. Internet-based cognitive behavioral therapy versus psychoeducation control for illness anxiety disorder and somatic symptom disorder: A randomized controlled trial. J Consult Clin Psychol. 2018;86:89-98. doi: 10.1037/ccp0000248
21. van Dessel N, den Boeft M, van der Wouden JC, et al. Non-pharmacological interventions for somatoform disorders and medically unexplained physical symptoms (MUPS) in adults. Cochrane Database Syst Rev. 2014(11):CD011142. doi: 10.1002/14651858.CD011142.pub2
22. Verdurmen MJ, Videler AC, Kamperman AM, et al. Cognitive behavioral therapy for somatic symptom disorders in later life: a prospective comparative explorative pilot study in two clinical populations. Neuropsychiatr Dis Treat. 2017;13:2331-2339. doi: 10.2147/NDT.S141208
23. Liu J, Gill NS, Teodorczuk A, et al. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;245:98-112. doi: 10.1016/j.jad.2018.10.114
24. Abbass A, Leichsenring F, Steinert C. Re: Jing et al., the efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;255:S0165-0327(18)33197-5. doi: 10.1016/j.jad.2019.02.055
THE CASE
James R* is a 30-year-old man who presented for a primary care walk-in visit due to dizziness, 2 days after he visited an emergency department (ED) for the same concern. He reported episodic symptoms lasting seconds to minutes, specifically when lying down. He said he had not fallen or experienced other physical trauma, did not have blurred vision or hearing loss, and was taking no medications. He also reported panic attacks, during which he experienced palpitations, trembling, paresthesia, and fear of dying. He stated that dizziness did not occur exclusively during panic episodes. His medical history was significant for hypertension; however, he reported significant anxiety related to medical visits. All home blood pressure readings he reported were within normal limits.
Upon examination, the patient had a blood pressure reading of 142/90 mm Hg and no evidence of nystagmus at rest. A neurologic exam was normal and a Dix-Hallpike maneuver reproduced subjective vertigo without nystagmus. Laboratory findings from the patient’s ED visit were negative for troponin and drug use, and blood oxygenation levels were within normal limits. At the time of this current visit, an electrocardiogram was unremarkable, with the exception of some tachycardia.
The presumptive diagnosis was benign paroxysmal positional vertigo (BPPV). An Epley maneuver was performed in the clinic and resulted in minimal symptom improvement. The physician taught Mr. R how to perform the Epley maneuver himself, prescribed a short course of meclizine, and referred him to the integrated mental health care service to address his panic attacks and anxiety.
Over the next few months, Mr. R continued to report significant distress about the dizzy spells, which persisted even after performing the Epley maneuver, and he reported that the meclizine was causing worsening vertigo. He received an ear-nose-and-throat consultation and cognitive behavioral therapy (CBT)–based consultation/interventions. He also reported avoiding multiple activities due to concerns about his dizziness.
●
*The patient’s name and other personally identifying information have been changed to protect his identity.
Somatic symptom disorder (SSD) is characterized by one or more physical symptoms associated with “excessive thoughts, feelings, or behaviors that result in distress and/or functional impairment.”1 Individuals with SSD are preoccupied with symptom-related severity, experience high symptom-related anxiety, or devote significant time and energy to the symptoms or heath concerns. With a diagnosis of SSD, physical symptoms experienced by the patient may or may not be medically explained. The same symptom need not be continuously present as long as the overall symptomatic presentation lasts 6 months or longer.
The specifier “with predominant pain” is used when pain dominates the presentation.1 Estimated prevalence of SSD in primary care ranges from 5% to 35%.2 The true scope of SSD is difficult to assess accurately since research tends to focus on medically unexplained symptoms, rather than excessive symptom-related concerns. Furthermore, terms such as “medically unexplained symptoms” and “functional syndromes” (including fibromyalgia and irritable bowel syndrome) are frequently used when describing SSD.3
One or more factors may contribute to unexplained symptoms: limitations of medical procedures and techniques, partial clinical information, patients’ inability to follow management recommendations, challenges in differential diagnostics, and access-to-care limitations preventing regular care and appropriate diagnostic work up.
What’s important to remember is that it’s the patient’s reaction to physical symptoms, rather than the presence of symptoms per se, that defines SSD.
Considerations in the differential diagnosis
When making a diagnosis of SSD, symptoms cannot:4
- be feigned or deliberately produced as in malingering or factitious disorder.
- result from physiologic effects of a substance (eg, intoxication, withdrawal, or adverse medication effects).
- constitute somatic delusions, as occur in psychotic disorders.
- constitute symptoms or deficits affecting voluntary motor or sensory function that are better explained by neurologic, medical, or psychiatric conditions (consider conversion disorder).
- be preoccupations with physical appearance flaws, as in body dysmorphic disorder.
- be accounted for by an anxiety disorder (eg, palpitations associated with panic attacks).
Continue to: Illness anxiety disorder...
Illness anxiety disorder is also characterized by significant health-related concerns; however, physical symptoms are either mild or absent.
Possible causes of SSD are varied and complex, including genetic and biological factors, family dynamics, behavioral modeling/learning, personality traits, difficulties with emotional regulation, and awareness.5 Patients may present with ongoing requests for symptom explanations, feelings of helplessness, fear of having concerns dismissed, and low motivation for change.3
Aids in supporting a diagnosis of SSD
It’s not appropriate to rely solely on questionnaires to make the diagnosis of SSD. However, brief screening tools are a time-efficient way to capture patients’ experiences and perceptions.6 Along with other components of clinical evaluation, brief symptom screens can both support the diagnosis and help in longitudinal symptom assessment.
Patient Health Questionnaire-15 (PHQ-15), developed for self-report screening in primary care, has desirable psychometric properties including appropriate internal reliability; convergent validity with measures of functional status, disability days, and symptom-related burden; and discriminant validity from measures of depressive symptoms.7 The PHQ-15 is an open access tool that is available in several languages. The respondent is asked to rate the extent of being bothered by a range of medical symptoms in the proceeding 4 weeks. Total scores range from 0 to 30, with higher scores indicating greater symptom aggravation. Cutoffs of 5, 10, and 15 correspond to mild, moderate, and severe symptom levels.8
Somatic Symptom Disorder - B Criteria Scale (SSD-12) aims to capture SSD symptoms in line with Diagnostic and Statistical Manual of Mental Disorders (DSM-5) diagnostic criteria. It assesses cognitive, affective, and behavioral aspects of SSD.9 The SSD-12 is copyrighted and its use requires registration and purchase. Cutoffs by age and gender are available. SSD-12 has demonstrated appropriate reliability and validity.9
Continue to: Structured Clinical Interview for DSM Disorders
Structured Clinical Interview for DSM Disorders (SCID)10,11 is perhaps the most rigorous differential diagnostic tool. However, SCID administration requires training and skill; time for administration and cost of the materials may be prohibitive in primary care.
Finally, SSD symptoms are highly associated with depression and anxiety. Ongoing elevated screening scores for anxiety and depression refractory to interventions may indicate the possibility of overlooked SSD. Furthermore, use of SSD screening tools with anxiety and depression screening tools can provide a more comprehensive picture of impairment, as well as symptom progress.
Treatment: Avoid a split approach
Diagnosing and treating SSD can be challenging for physicians who focus on biomedically based approaches in patient care. Additional tests, studies, and prescriptions are likely to fuel (rather than pacify) patients’ concerns, as such steps divert attention from the underlying psychological needs and mechanisms which maintain SSD. Avoid using a split biopsychosocial approach—ie, beginning the inquiry and treatment planning from a biomedical perspective, and then falling back on psychosocial formulation when treatment efforts have been ineffective. Such an approach leads to understandable patient dissatisfaction and can be interpreted by them as the caregiver suggesting that physical symptoms are “all in [their] head.”12
These 4 tips can help
1. Use a biopsychosocial formulation when initiating treatment. Be familiar with biopsychosocial factors in SSD and develop a narrative for discussing this formulation with patients. For example: “Mr. R, we are going to use the following [medical tests/studies/medications] to understand the cause of your symptoms and better manage them. We also need to think about the role of stress and distress in your symptoms because these can also be at play with dizziness.” This may be particularly beneficial for a functional disorder, such as chronic pain. Incorporating patient education resources is an important step toward shared understanding (see Hunter Integrated Pain Service for chronic pain educational videos; www.tga.gov.au/chronic-pain-management-video-resource-brainman13).
2. Combine education about pathophysiology with patient-centered interviewing. Significant SSD symptom improvements were noted following a single 30-minute educational session, while motivational interviewing techniques were used to probe patients’ concerns.2
Continue to: Maintain professionalism and good clinical practice
3. Maintain professionalism and good clinical practice. Consider SSD a medical matter and address it accordingly: explore concerns fully, provide evidence-based responses, communicate empathy, and employ objective management strategies.14
4. Do not overlook the value of the relationship. A recent systematic review concluded that the relationship between the patient and care provider was central to the success of the interventions for symptom reduction.15
A controversial approach. Pharmacotherapy for SSD is controversial. While several trials of antidepressants and St. John’s wort have been positive and some authors have stated that all classes of antidepressants are effective for SSD, others maintain that questions regarding dosing, treatment duration, and sustainability of improvement have not been sufficiently addressed in research.16,17
Coordination of care issues
Primary care continues to be the de facto mental health system, and specialty services may be unavailable or declined by patients.18 CBT delivered in person or online is empirically supported as a treatment approach to medically unexplained symptoms and SSD.17,19-22
A recent meta-analysis of randomized controlled trials published by Jing and colleagues23 reported that CBT was effective for SSD symptom reduction, and that treatment gains were maintained 3 to 12 months post treatment. However, concerns about the practical implementation of CBT in primary care were raised because CBT was not shown to be effective in improving social functioning or reducing the number of medical visits. Symptom improvement was maximized with longer durations of treatment (> 10 sessions) and greater session lengths (> 50 minutes). Additionally, Abbass and colleagues24 brought up several methodologic (sampling and analysis) concerns related to Jing et al’s work.
Continue to: Overally, CBT's effect sizes...
Overall, CBT’s effect sizes are small, and patients who are open to biopsychosocial explanations for their symptoms and to receiving psychological services may differ from most patients seen in primary care practices.21 Furthermore, mental health providers may hesitate to diagnose SSD because they are concerned about missing a somatic illness.3 Therefore, when coordinating care with mental health providers, it may be beneficial to discuss the treatment approach, assess familiarity with the SSD diagnosis, and closely coordinate and collaborate on the treatment plan.
While physicians cannot be expected to function as psychotherapists, an understanding of CBT and techniques for SSD treatment can be beneficial. Integrated mental health services may hold promise in addressing SSD in primary care. Onsite availability of a behavioral health provider competent in providing evidence-based care can target SSD symptoms and support both patients and physicians.
THE CASE
Mr. R’s treatment course included multiple primary care appointments (scheduled and walk in), ED visits, and specialist visits (ENT/vestibular rehabilitation). He sought care as symptoms intensified, lasted longer, or occurred in new circumstances. He reported persistent fear of the symptoms and anxiety that serious medical causes had been overlooked. He also described distress associated with vertigo and his anxiety sensitivity (anxiety about being anxious).
The behavioral health consultant (BHC; psychologist) and physician talked to the patient about the biopsychosocial antecedents of his condition and the factors that perpetuate the anxiety and stress response. The BHC described the fight/flight/freeze response to the patient and explained its role in the physiologic stress response associated with somatic symptoms and panic. Educational materials (videos and handouts) were also provided to the patient to further illustrate these concepts. The BHC also discussed the role of interoceptive and situational avoidance and active coping (eg, engaging in safe activities); taught the patient relaxation and grounding techniques; and used cognitive disputation aimed at challenging catastrophic symptom interpretations.
The BHC and the patient’s physician established joint treatment goals that included improving functioning, promoting active coping, and decreasing distress associated with symptoms. After the initial medical and BHC visits, both vertigo and anxiety symptoms appeared to abate somewhat, but symptoms have been ongoing and distress and impairment have been variable. The patient’s family physician and BHC continue to work with him to optimize the care plan and treatment goals.
CORRESPONDENCE
Nataliya Pilipenko, PhD, ABPP, Center for Family and Community Medicine, Columbia University Vagelos College of Physicians and Surgeons, 630 West 168th Street, New York, NY 10032; np2615@cumc.columbia.edu
ACKNOWLEDGEMENT
The author thanks Dr. Molly Warren for her collaboration and guidance.
THE CASE
James R* is a 30-year-old man who presented for a primary care walk-in visit due to dizziness, 2 days after he visited an emergency department (ED) for the same concern. He reported episodic symptoms lasting seconds to minutes, specifically when lying down. He said he had not fallen or experienced other physical trauma, did not have blurred vision or hearing loss, and was taking no medications. He also reported panic attacks, during which he experienced palpitations, trembling, paresthesia, and fear of dying. He stated that dizziness did not occur exclusively during panic episodes. His medical history was significant for hypertension; however, he reported significant anxiety related to medical visits. All home blood pressure readings he reported were within normal limits.
Upon examination, the patient had a blood pressure reading of 142/90 mm Hg and no evidence of nystagmus at rest. A neurologic exam was normal and a Dix-Hallpike maneuver reproduced subjective vertigo without nystagmus. Laboratory findings from the patient’s ED visit were negative for troponin and drug use, and blood oxygenation levels were within normal limits. At the time of this current visit, an electrocardiogram was unremarkable, with the exception of some tachycardia.
The presumptive diagnosis was benign paroxysmal positional vertigo (BPPV). An Epley maneuver was performed in the clinic and resulted in minimal symptom improvement. The physician taught Mr. R how to perform the Epley maneuver himself, prescribed a short course of meclizine, and referred him to the integrated mental health care service to address his panic attacks and anxiety.
Over the next few months, Mr. R continued to report significant distress about the dizzy spells, which persisted even after performing the Epley maneuver, and he reported that the meclizine was causing worsening vertigo. He received an ear-nose-and-throat consultation and cognitive behavioral therapy (CBT)–based consultation/interventions. He also reported avoiding multiple activities due to concerns about his dizziness.
●
*The patient’s name and other personally identifying information have been changed to protect his identity.
Somatic symptom disorder (SSD) is characterized by one or more physical symptoms associated with “excessive thoughts, feelings, or behaviors that result in distress and/or functional impairment.”1 Individuals with SSD are preoccupied with symptom-related severity, experience high symptom-related anxiety, or devote significant time and energy to the symptoms or heath concerns. With a diagnosis of SSD, physical symptoms experienced by the patient may or may not be medically explained. The same symptom need not be continuously present as long as the overall symptomatic presentation lasts 6 months or longer.
The specifier “with predominant pain” is used when pain dominates the presentation.1 Estimated prevalence of SSD in primary care ranges from 5% to 35%.2 The true scope of SSD is difficult to assess accurately since research tends to focus on medically unexplained symptoms, rather than excessive symptom-related concerns. Furthermore, terms such as “medically unexplained symptoms” and “functional syndromes” (including fibromyalgia and irritable bowel syndrome) are frequently used when describing SSD.3
One or more factors may contribute to unexplained symptoms: limitations of medical procedures and techniques, partial clinical information, patients’ inability to follow management recommendations, challenges in differential diagnostics, and access-to-care limitations preventing regular care and appropriate diagnostic work up.
What’s important to remember is that it’s the patient’s reaction to physical symptoms, rather than the presence of symptoms per se, that defines SSD.
Considerations in the differential diagnosis
When making a diagnosis of SSD, symptoms cannot:4
- be feigned or deliberately produced as in malingering or factitious disorder.
- result from physiologic effects of a substance (eg, intoxication, withdrawal, or adverse medication effects).
- constitute somatic delusions, as occur in psychotic disorders.
- constitute symptoms or deficits affecting voluntary motor or sensory function that are better explained by neurologic, medical, or psychiatric conditions (consider conversion disorder).
- be preoccupations with physical appearance flaws, as in body dysmorphic disorder.
- be accounted for by an anxiety disorder (eg, palpitations associated with panic attacks).
Continue to: Illness anxiety disorder...
Illness anxiety disorder is also characterized by significant health-related concerns; however, physical symptoms are either mild or absent.
Possible causes of SSD are varied and complex, including genetic and biological factors, family dynamics, behavioral modeling/learning, personality traits, difficulties with emotional regulation, and awareness.5 Patients may present with ongoing requests for symptom explanations, feelings of helplessness, fear of having concerns dismissed, and low motivation for change.3
Aids in supporting a diagnosis of SSD
It’s not appropriate to rely solely on questionnaires to make the diagnosis of SSD. However, brief screening tools are a time-efficient way to capture patients’ experiences and perceptions.6 Along with other components of clinical evaluation, brief symptom screens can both support the diagnosis and help in longitudinal symptom assessment.
Patient Health Questionnaire-15 (PHQ-15), developed for self-report screening in primary care, has desirable psychometric properties including appropriate internal reliability; convergent validity with measures of functional status, disability days, and symptom-related burden; and discriminant validity from measures of depressive symptoms.7 The PHQ-15 is an open access tool that is available in several languages. The respondent is asked to rate the extent of being bothered by a range of medical symptoms in the proceeding 4 weeks. Total scores range from 0 to 30, with higher scores indicating greater symptom aggravation. Cutoffs of 5, 10, and 15 correspond to mild, moderate, and severe symptom levels.8
Somatic Symptom Disorder - B Criteria Scale (SSD-12) aims to capture SSD symptoms in line with Diagnostic and Statistical Manual of Mental Disorders (DSM-5) diagnostic criteria. It assesses cognitive, affective, and behavioral aspects of SSD.9 The SSD-12 is copyrighted and its use requires registration and purchase. Cutoffs by age and gender are available. SSD-12 has demonstrated appropriate reliability and validity.9
Continue to: Structured Clinical Interview for DSM Disorders
Structured Clinical Interview for DSM Disorders (SCID)10,11 is perhaps the most rigorous differential diagnostic tool. However, SCID administration requires training and skill; time for administration and cost of the materials may be prohibitive in primary care.
Finally, SSD symptoms are highly associated with depression and anxiety. Ongoing elevated screening scores for anxiety and depression refractory to interventions may indicate the possibility of overlooked SSD. Furthermore, use of SSD screening tools with anxiety and depression screening tools can provide a more comprehensive picture of impairment, as well as symptom progress.
Treatment: Avoid a split approach
Diagnosing and treating SSD can be challenging for physicians who focus on biomedically based approaches in patient care. Additional tests, studies, and prescriptions are likely to fuel (rather than pacify) patients’ concerns, as such steps divert attention from the underlying psychological needs and mechanisms which maintain SSD. Avoid using a split biopsychosocial approach—ie, beginning the inquiry and treatment planning from a biomedical perspective, and then falling back on psychosocial formulation when treatment efforts have been ineffective. Such an approach leads to understandable patient dissatisfaction and can be interpreted by them as the caregiver suggesting that physical symptoms are “all in [their] head.”12
These 4 tips can help
1. Use a biopsychosocial formulation when initiating treatment. Be familiar with biopsychosocial factors in SSD and develop a narrative for discussing this formulation with patients. For example: “Mr. R, we are going to use the following [medical tests/studies/medications] to understand the cause of your symptoms and better manage them. We also need to think about the role of stress and distress in your symptoms because these can also be at play with dizziness.” This may be particularly beneficial for a functional disorder, such as chronic pain. Incorporating patient education resources is an important step toward shared understanding (see Hunter Integrated Pain Service for chronic pain educational videos; www.tga.gov.au/chronic-pain-management-video-resource-brainman13).
2. Combine education about pathophysiology with patient-centered interviewing. Significant SSD symptom improvements were noted following a single 30-minute educational session, while motivational interviewing techniques were used to probe patients’ concerns.2
Continue to: Maintain professionalism and good clinical practice
3. Maintain professionalism and good clinical practice. Consider SSD a medical matter and address it accordingly: explore concerns fully, provide evidence-based responses, communicate empathy, and employ objective management strategies.14
4. Do not overlook the value of the relationship. A recent systematic review concluded that the relationship between the patient and care provider was central to the success of the interventions for symptom reduction.15
A controversial approach. Pharmacotherapy for SSD is controversial. While several trials of antidepressants and St. John’s wort have been positive and some authors have stated that all classes of antidepressants are effective for SSD, others maintain that questions regarding dosing, treatment duration, and sustainability of improvement have not been sufficiently addressed in research.16,17
Coordination of care issues
Primary care continues to be the de facto mental health system, and specialty services may be unavailable or declined by patients.18 CBT delivered in person or online is empirically supported as a treatment approach to medically unexplained symptoms and SSD.17,19-22
A recent meta-analysis of randomized controlled trials published by Jing and colleagues23 reported that CBT was effective for SSD symptom reduction, and that treatment gains were maintained 3 to 12 months post treatment. However, concerns about the practical implementation of CBT in primary care were raised because CBT was not shown to be effective in improving social functioning or reducing the number of medical visits. Symptom improvement was maximized with longer durations of treatment (> 10 sessions) and greater session lengths (> 50 minutes). Additionally, Abbass and colleagues24 brought up several methodologic (sampling and analysis) concerns related to Jing et al’s work.
Continue to: Overally, CBT's effect sizes...
Overall, CBT’s effect sizes are small, and patients who are open to biopsychosocial explanations for their symptoms and to receiving psychological services may differ from most patients seen in primary care practices.21 Furthermore, mental health providers may hesitate to diagnose SSD because they are concerned about missing a somatic illness.3 Therefore, when coordinating care with mental health providers, it may be beneficial to discuss the treatment approach, assess familiarity with the SSD diagnosis, and closely coordinate and collaborate on the treatment plan.
While physicians cannot be expected to function as psychotherapists, an understanding of CBT and techniques for SSD treatment can be beneficial. Integrated mental health services may hold promise in addressing SSD in primary care. Onsite availability of a behavioral health provider competent in providing evidence-based care can target SSD symptoms and support both patients and physicians.
THE CASE
Mr. R’s treatment course included multiple primary care appointments (scheduled and walk in), ED visits, and specialist visits (ENT/vestibular rehabilitation). He sought care as symptoms intensified, lasted longer, or occurred in new circumstances. He reported persistent fear of the symptoms and anxiety that serious medical causes had been overlooked. He also described distress associated with vertigo and his anxiety sensitivity (anxiety about being anxious).
The behavioral health consultant (BHC; psychologist) and physician talked to the patient about the biopsychosocial antecedents of his condition and the factors that perpetuate the anxiety and stress response. The BHC described the fight/flight/freeze response to the patient and explained its role in the physiologic stress response associated with somatic symptoms and panic. Educational materials (videos and handouts) were also provided to the patient to further illustrate these concepts. The BHC also discussed the role of interoceptive and situational avoidance and active coping (eg, engaging in safe activities); taught the patient relaxation and grounding techniques; and used cognitive disputation aimed at challenging catastrophic symptom interpretations.
The BHC and the patient’s physician established joint treatment goals that included improving functioning, promoting active coping, and decreasing distress associated with symptoms. After the initial medical and BHC visits, both vertigo and anxiety symptoms appeared to abate somewhat, but symptoms have been ongoing and distress and impairment have been variable. The patient’s family physician and BHC continue to work with him to optimize the care plan and treatment goals.
CORRESPONDENCE
Nataliya Pilipenko, PhD, ABPP, Center for Family and Community Medicine, Columbia University Vagelos College of Physicians and Surgeons, 630 West 168th Street, New York, NY 10032; np2615@cumc.columbia.edu
ACKNOWLEDGEMENT
The author thanks Dr. Molly Warren for her collaboration and guidance.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
2. Johnson KK, Bennett C, Rochani H. Significant improvement of somatic symptom disorder with brief psychoeducational intervention by PMHNP in primary care. J Am Psychiatr Nurses Assoc. 2020;28:171-180. doi: 10.1177/1078390320960524
3. Weigel A, Maehder K, Witt M, et al. Psychotherapists’ perspective on the treatment of patients with somatic symptom disorders. J Psychosom Res. 2020;138:110228. doi: 10.1016/j.jpsychores.2020.110228
4. American Psychiatric Association. Handbook of Differential Diagnosis. American Psychiatric Publishing; 2014;234-235.
5. Mayo Clinic. Somatic symptom disorder. Accessed February 21, 2022. www.mayoclinic.org/diseases-conditions/somatic-symptom-disorder/symptoms-causes/syc-20377776?p=1
6. Toussaint A, Riedl B, Kehrer S, et al. Validity of the Somatic Symptom Disorder-B Criteria Scale (SSD-12) in primary care. Fam Pract. 2018;35:342-347. doi: 10.1093/fampra/cmx116
7. Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258-66. doi: 10.1097/00006842-200203000-00008
8. Kroenke K, Spitzer RL, Williams JB, et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345-359. doi: 10.1016/j.genhosppsych.2010.03.006
9. Toussaint A, Löwe B, Brähler E, et al. The Somatic Symptom Disorder - B Criteria Scale (SSD-12): factorial structure, validity and population-based norms. J Psychosom Res. 2017;97:9-17. doi: 10.1016/j.jpsychores.2017.03.017
10. First MB, Williams JBW, Karg RS, Spitzer RL, eds. Structured Clinical Interview for DSM-5 Disorders, Research Version. American Psychiatric Association, 2015.
11. First MB, Williams JBW, Karg RS, Spitzer RL, eds. Structured Clinical Interview for DSM-5 Disorders, Clinician Version. American Psychiatric Publishing; 2016.
12. McDaniel SH, Hepworth J, Campbell TL, et al, eds. Family Oriented Primary Care. Springer Publishing Co; 2005:1-15.
13. Hunter Integrated Pain Service (2016). Brainman videos. Hunter New England Local Health District. New South Wales, Australia. Accessed February 21, 2022. www.tga.gov.au/chronic-pain-management-video-resource-brainman
14. Murray AM, Toussaint A, Althaus A, et al. The challenge of diagnosing non-specific, functional, and somatoform disorders: a systematic review of barriers to diagnosis in primary care. J Psychosom Res. 2016;80:1-10. doi: 10.1016/j.jpsychores.2015.11.002
15. Leaviss J, Davis S, Ren S, et al. Behavioral modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation. Health Technol Assess. 2020;24:1-490. doi: 10.3310/hta24460
16. Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med. 2007;69:881-888. doi: 10.1097/PSY.0b013e31815b00c4
17. Steinbrecher N, Koerber S, Frieser D, et al. The prevalence of medically unexplained symptoms in primary care. Psychosomatics. 2011;52:263-271. doi: 10.1016/j.psym.2011.01.007
18. Kessler R, Stafford D. Primary care is the de facto mental health system. In Kessler R, Stafford D, eds. Collaborative Medicine Case Studies: Evidence in Practice. Springer Publishing Co, 2008; 9-21.
19. Barsky AJ, Ahern DK, Bauer MR, et al. A randomized trial of treatments for high-utilizing somatizing patients. J Gen Intern Med. 2013;28:1396-1404. doi: 10.1007/s11606-013-2392-6
20. Newby JM, Smith J, Uppal S, et al. Internet-based cognitive behavioral therapy versus psychoeducation control for illness anxiety disorder and somatic symptom disorder: A randomized controlled trial. J Consult Clin Psychol. 2018;86:89-98. doi: 10.1037/ccp0000248
21. van Dessel N, den Boeft M, van der Wouden JC, et al. Non-pharmacological interventions for somatoform disorders and medically unexplained physical symptoms (MUPS) in adults. Cochrane Database Syst Rev. 2014(11):CD011142. doi: 10.1002/14651858.CD011142.pub2
22. Verdurmen MJ, Videler AC, Kamperman AM, et al. Cognitive behavioral therapy for somatic symptom disorders in later life: a prospective comparative explorative pilot study in two clinical populations. Neuropsychiatr Dis Treat. 2017;13:2331-2339. doi: 10.2147/NDT.S141208
23. Liu J, Gill NS, Teodorczuk A, et al. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;245:98-112. doi: 10.1016/j.jad.2018.10.114
24. Abbass A, Leichsenring F, Steinert C. Re: Jing et al., the efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;255:S0165-0327(18)33197-5. doi: 10.1016/j.jad.2019.02.055
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition (DSM-5). American Psychiatric Publishing; 2013.
2. Johnson KK, Bennett C, Rochani H. Significant improvement of somatic symptom disorder with brief psychoeducational intervention by PMHNP in primary care. J Am Psychiatr Nurses Assoc. 2020;28:171-180. doi: 10.1177/1078390320960524
3. Weigel A, Maehder K, Witt M, et al. Psychotherapists’ perspective on the treatment of patients with somatic symptom disorders. J Psychosom Res. 2020;138:110228. doi: 10.1016/j.jpsychores.2020.110228
4. American Psychiatric Association. Handbook of Differential Diagnosis. American Psychiatric Publishing; 2014;234-235.
5. Mayo Clinic. Somatic symptom disorder. Accessed February 21, 2022. www.mayoclinic.org/diseases-conditions/somatic-symptom-disorder/symptoms-causes/syc-20377776?p=1
6. Toussaint A, Riedl B, Kehrer S, et al. Validity of the Somatic Symptom Disorder-B Criteria Scale (SSD-12) in primary care. Fam Pract. 2018;35:342-347. doi: 10.1093/fampra/cmx116
7. Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64:258-66. doi: 10.1097/00006842-200203000-00008
8. Kroenke K, Spitzer RL, Williams JB, et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32:345-359. doi: 10.1016/j.genhosppsych.2010.03.006
9. Toussaint A, Löwe B, Brähler E, et al. The Somatic Symptom Disorder - B Criteria Scale (SSD-12): factorial structure, validity and population-based norms. J Psychosom Res. 2017;97:9-17. doi: 10.1016/j.jpsychores.2017.03.017
10. First MB, Williams JBW, Karg RS, Spitzer RL, eds. Structured Clinical Interview for DSM-5 Disorders, Research Version. American Psychiatric Association, 2015.
11. First MB, Williams JBW, Karg RS, Spitzer RL, eds. Structured Clinical Interview for DSM-5 Disorders, Clinician Version. American Psychiatric Publishing; 2016.
12. McDaniel SH, Hepworth J, Campbell TL, et al, eds. Family Oriented Primary Care. Springer Publishing Co; 2005:1-15.
13. Hunter Integrated Pain Service (2016). Brainman videos. Hunter New England Local Health District. New South Wales, Australia. Accessed February 21, 2022. www.tga.gov.au/chronic-pain-management-video-resource-brainman
14. Murray AM, Toussaint A, Althaus A, et al. The challenge of diagnosing non-specific, functional, and somatoform disorders: a systematic review of barriers to diagnosis in primary care. J Psychosom Res. 2016;80:1-10. doi: 10.1016/j.jpsychores.2015.11.002
15. Leaviss J, Davis S, Ren S, et al. Behavioral modification interventions for medically unexplained symptoms in primary care: systematic reviews and economic evaluation. Health Technol Assess. 2020;24:1-490. doi: 10.3310/hta24460
16. Kroenke K. Efficacy of treatment for somatoform disorders: a review of randomized controlled trials. Psychosom Med. 2007;69:881-888. doi: 10.1097/PSY.0b013e31815b00c4
17. Steinbrecher N, Koerber S, Frieser D, et al. The prevalence of medically unexplained symptoms in primary care. Psychosomatics. 2011;52:263-271. doi: 10.1016/j.psym.2011.01.007
18. Kessler R, Stafford D. Primary care is the de facto mental health system. In Kessler R, Stafford D, eds. Collaborative Medicine Case Studies: Evidence in Practice. Springer Publishing Co, 2008; 9-21.
19. Barsky AJ, Ahern DK, Bauer MR, et al. A randomized trial of treatments for high-utilizing somatizing patients. J Gen Intern Med. 2013;28:1396-1404. doi: 10.1007/s11606-013-2392-6
20. Newby JM, Smith J, Uppal S, et al. Internet-based cognitive behavioral therapy versus psychoeducation control for illness anxiety disorder and somatic symptom disorder: A randomized controlled trial. J Consult Clin Psychol. 2018;86:89-98. doi: 10.1037/ccp0000248
21. van Dessel N, den Boeft M, van der Wouden JC, et al. Non-pharmacological interventions for somatoform disorders and medically unexplained physical symptoms (MUPS) in adults. Cochrane Database Syst Rev. 2014(11):CD011142. doi: 10.1002/14651858.CD011142.pub2
22. Verdurmen MJ, Videler AC, Kamperman AM, et al. Cognitive behavioral therapy for somatic symptom disorders in later life: a prospective comparative explorative pilot study in two clinical populations. Neuropsychiatr Dis Treat. 2017;13:2331-2339. doi: 10.2147/NDT.S141208
23. Liu J, Gill NS, Teodorczuk A, et al. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;245:98-112. doi: 10.1016/j.jad.2018.10.114
24. Abbass A, Leichsenring F, Steinert C. Re: Jing et al., the efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: a meta-analysis of randomized controlled trials. J Affect Disord. 2019;255:S0165-0327(18)33197-5. doi: 10.1016/j.jad.2019.02.055
How to screen for and treat teen alcohol use
THE CASE
Paul F* is a 16-year-old White boy who lives with his mother and spends some weekends with his father who has shared custody. He recently presented to the clinic for treatment due to an arrest for disorderly conduct at school. He and a friend were found drinking liquor outside the school building when they were scheduled to be in class. Paul reported that he and his friends often drink at school and at extracurricular functions. He has been using alcohol for the past 2 years, with escalating consumption (5 or more drinks per episode) in the past year. Paul has been drinking most days of the week and has even driven under the influence at times. He said, “I just feel happier when I am drinking.” An accomplished soccer player recruited by colleges, Paul recently was suspended from the team due to his poor grades. His response was, “It’s stupid anyway. What’s the point of playing?”
●
* The patient’s name and some personal details have been changed to protect his identity.
Alcohol is the number 1 substance of abuse for adolescents, used more than tobacco or drugs.1-3 In 2007 and again in 2016, the Surgeon General of the United States issued reports to highlight this important topic,1,2 noting that early and repeated exposure to alcohol during this crucial time of brain development increases the risk for future problems, including addiction.2
Adolescent alcohol use is often underestimated by parents and physicians, including misjudging how much, how often, and how young children are when they begin to drink.1 Boys and girls tend to start drinking at similar ages (13.9 and 14.4 years, respectively),3 but as girls age, they tend to drink more and binge more.4 In 2019, 1 in 4 adolescents reported drinking and more than 4 million reported at least 1 episode of binge drinking in the prior month.4 These numbers have further ramifications: early drinking is associated with alcohol dependence, relapse, use of other substances, risky sexual behaviors, injurious behaviors, suicide, motor vehicle accidents, and dating violence.4-6
Diagnosing alcohol use disorder
The range of alcohol use includes consumption, bingeing, abuse, and dependence.7,8 Consumption is defined as the drinking of alcoholic beverages. Bingeing is the consumption of more than 5 drinks for men or 4 drinks for women in 2 hours, according to the National Institute on Alcohol Abuse and Alcoholism.7 However, the criterion is slightly different for the Substance Abuse and Mental Health Services Administration, which broadens the timeframe to “on the same occasion.”9 While previously known as separate disorders, alcohol abuse (or misuse) and alcohol dependence are now diagnostically classified together as alcohol use disorders (AUDs), per the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5).8 AUD is further stratified as mild, moderate, or severe, depending on the number of criteria that are met by the patient (TABLE).8,10
Alcohol screening
Currently, the US Preventive Services Task Force (USPSTF) does not recommend screening adolescents ages 12 to 17 for AUD, and has instead issued an “I” statement (insufficient evidence).11 While the USPSTF recognizes the potential burdens of adolescent alcohol use, the potential harms of screening include “stigma, anxiety, labeling, discrimination, privacy concerns, and interference with the patient–clinician relationship.”11 The USPSTF also notes that it “did not find any evidence that specifically examined the harms of screening for alcohol use in adolescents.”11
This is at odds with recommendations from the American Academy of Pediatrics (AAP), which in 2011 released a policy statement advocating screening, brief intervention, and referral to treatment for adolescent substance use.12 In the United States, even though 83% of adolescents see a physician at least once each year,12,13 alcohol misuse screening still varies, occurring in the range of 50% to 86% of office visits.12 When screening does occur, it is often based on clinical impression only.12 Studies have shown that when a screening tool is not used, up to two-thirds of substance use disorders may be missed.12-15
Continue to: A full and complete biopsychosocial interview
A full and complete biopsychosocial interview with adolescents is a necessity, and should include queries about alcohol, drugs, and other substances. Acknowledgment of use should trigger further investigation into the substance use areas. Interviews may start with open-ended questions about alcohol use at home or at school before moving to more personalized and detailed questioning and use of screening tools.16
While various screening instruments exist, for the sake of brevity we provide as an example the Screening to Brief Intervention (S2BI) tool. It is an efficient, single-page tool that can help clinicians in their routine care of adolescents to quickly stratify the patient risk of substance use disorder as none/low, moderate, or severe.12 It can be found here: www.mcpap.com/pdf/S2Bi%20Toolkit.pdf (see page 10).
For all patients, but particularly for adolescents, confidentiality is important, and many specialty societies have created language to address this issue.12 Discuss confidentiality with both the adolescent patient and the patient’s caregiver simultaneously, with dialogue that includes: (a) the need to speak with adolescents alone during the office visit, (b) the benefits of confidentiality in the physician–patient relationship, and (c) the need to disclose selected information to keep patients safe.12 Describing the process for required disclosures is essential. Benefits of disclosure include further support for the adolescent patient as well as appropriate parental participation and support for possible referrals.12
Treating AUD
Treatment for AUD should be multifaceted. Screen for comorbid mood disorders, such as generalized anxiety,17,18 social anxiety,18 and depression,19 as well as for insomnia.18 Studies have demonstrated a strong link between insomnia and anxiety, and again between anxiety and AUD.17-19 Finally, screen for adverse childhood events such as trauma, victimization, and abuse.20 Addressing issues discovered in screening allows for more targeted and personalized treatment of AUD.
The National Institute on Drug Abuse categorizes evidence-based treatment into 3 areas: behavioral therapies, family therapies, and medications.21
Continue to: Behavioral therapies
Behavioral therapies can include group therapy, cognitive behavioral therapy (CBT), motivational enhancement therapy, 12-Step facilitation, and contingency management, in which small rewards or incentives are given for participation in treatment to reinforce positive behaviors.21
Family-based therapies, such as brief strategic family therapy, functional family therapy, and multisystem therapy recognize that adolescents exist in systems of families in communities, and that the patient’s success in treatment may be supported by these relationships.21
Some medications may achieve modest benefit for treatment of adolescents with AUD. Naltrexone, acamprosate, and disulfiram have all been used successfully to treat AUD in adults21; some physicians may choose to use these medications “off label” in adolescents. Bupropion has been used successfully in the treatment of nicotine use disorder,21 and a small study in 2005 showed some success with bupropion in treating adolescents with attention-deficit/hyperactivity disorder, comorbid depression, and substance use disorder.22 Naltrexone has also been studied in adolescents with opioid use disorder, although these were not large studies.23
Adolescents with serious, sustained issues with AUD may require more in-depth treatments such as an intensive outpatient program, a partial hospitalization program, or a residential treatment program.15 The least-restrictive environment is preferable.15 Families are generally included as part of the treatment and recovery process in those settings.21 Some patients may require detoxification prior to referral to residential treatment settings; the American Society of Addiction Medicine has published a comprehensive guideline on alcohol withdrawal.24
Paul’s family physician diagnosed his condition as AUD and referred him for CBT with a psychologist, who treated him for both the AUD and an underlying depressive disorder that was later identified. CBT focused on cognitive restructuring of depressive thoughts as well as support for continued abstinence from alcohol. The patient, with family support, declined antidepressant medication.
After 6 months of treatment, Paul and his parents were pleased with his progress. His grades improved to the point that he was permitted to play soccer again, and he was seriously looking at his future college options.
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; sfields@hsc.wvu.edu
1. US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2007.
2. US Department of Health and Human Services. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2016.
3. Hingson R, White A. New research findings since the 2007 Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking: A review. J Stud Alcohol Drugs Suppl. 2014; 75:158-169.
4. National Institute on Alcohol Abuse and Alcoholism. Underage drinking. National Institute of Health. Accessed December 22, 2021. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/underage-drinking.
5. Hingson R, Zha W, Iannotti R, et al. Physician advice to adolescents about drinking and other health behaviors. Pediatrics. 2013;131:249-257.
6. Schaus JF, Sole ML, McCoy TP, et al. Screening for high-risk drinking in a college student health center: characterizing students based on quantity, frequency, and harms. J Stud Alcohol Drugs Suppl. 2009;16:34-44.
7. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed December 27, 2021. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Arlington, VA; American Psychiatric Association. 2013.
9. Substance Abuse and Mental Health Services Administration. Bringing down binge drinking. Accessed December 27, 2021. www.samhsa.gov/sites/default/files/programs_campaigns/nation_prevention_week/data-binge-drinking.pdf
10. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 Alcohol Use Disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
11. USPSTF. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:1899-1909.
12. Levy SJ, Williams JF, Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161211.
13. MacKay AP, Duran CP. Adolescent Health in the United States. National Center for Health Statistics, Centers for Disease Control and Prevention. 2007.
14. Haller DM, Meynard A, Lefebvre D, et al. Effectiveness of training family physicians to deliver a brief intervention to address excessive substance use among young patients: a cluster randomized controlled trial. CMAJ. 2014;186:E263-E272.
15. Borus J, Parhami I, Levy S. Screening, brief intervention, and referral to treatment. Child Adolesc Psychiatric Clin N Am. 2016;25:579-601.
16. Knight J, Roberts T, Gabrielli J, et al. Adolescent alcohol and substance use and abuse. Performing preventive services: A bright futures handbook. Accessed December 22, 2021. American Academy of Pediatrics. https://ocfcpacourts.us/wp-content/uploads/2020/06/Adolescent_Alcohol_and_Substance_Abuse_001005.pdf
17. Dyer ML, Heron J, Hickman M, et al. Alcohol use in late adolescence and early adulthood: the role of generalized anxiety disorder and drinking to cope motives. Drug Alcohol Depend. 2019;204:107480.
18. Blumenthal H, Taylor DJ, Cloutier RM, et al. The links between social anxiety disorder, insomnia symptoms, and alcohol use disorders: findings from a large sample of adolescents in the United States. Behav Ther. 2019;50:50-59.
19. Pedrelli P, Shapero B, Archibald A, et al. Alcohol use and depression during adolescence and young adulthood: a summary and interpretation of mixed findings. Curr Addict Rep. 2016;3:91-97.
20. Davis JP, Dworkin ER, Helton J, et al. Extending poly-victimization theory: differential effects of adolescents’ experiences of victimization on substance use disorder diagnoses upon treatment entry. Child Abuse Negl. 2019; 89:165-177.
21. NIDA. Principles of adolescent substance use disorder treatment: a research-based guide. Accessed December 22, 2021. www.drugabuse.gov/publications/principles-adolescent-substance-use-disorder-treatment-research-based-guide
22. Solhkhah R, Wilens TE, Daly J, et al. Bupropion SR for the treatment of substance-abusing outpatient adolescents with attention-deficit/hyperactivity disorder and mood disorders. J Child Adolesc Psychopharmacol. 2005:15:777-786.
23. Camenga DR, Colon-Rivera HA, Muvvala SB. Medications for maintenance treatment of opioid use disorder in adolescents. J Stud Alcohol Drugs. 2019;80:393-402.
24. American Society of Addiction Medicine. The ASAM clinical practice guideline on alcohol withdrawal management. Accessed December 22, 2021. www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
THE CASE
Paul F* is a 16-year-old White boy who lives with his mother and spends some weekends with his father who has shared custody. He recently presented to the clinic for treatment due to an arrest for disorderly conduct at school. He and a friend were found drinking liquor outside the school building when they were scheduled to be in class. Paul reported that he and his friends often drink at school and at extracurricular functions. He has been using alcohol for the past 2 years, with escalating consumption (5 or more drinks per episode) in the past year. Paul has been drinking most days of the week and has even driven under the influence at times. He said, “I just feel happier when I am drinking.” An accomplished soccer player recruited by colleges, Paul recently was suspended from the team due to his poor grades. His response was, “It’s stupid anyway. What’s the point of playing?”
●
* The patient’s name and some personal details have been changed to protect his identity.
Alcohol is the number 1 substance of abuse for adolescents, used more than tobacco or drugs.1-3 In 2007 and again in 2016, the Surgeon General of the United States issued reports to highlight this important topic,1,2 noting that early and repeated exposure to alcohol during this crucial time of brain development increases the risk for future problems, including addiction.2
Adolescent alcohol use is often underestimated by parents and physicians, including misjudging how much, how often, and how young children are when they begin to drink.1 Boys and girls tend to start drinking at similar ages (13.9 and 14.4 years, respectively),3 but as girls age, they tend to drink more and binge more.4 In 2019, 1 in 4 adolescents reported drinking and more than 4 million reported at least 1 episode of binge drinking in the prior month.4 These numbers have further ramifications: early drinking is associated with alcohol dependence, relapse, use of other substances, risky sexual behaviors, injurious behaviors, suicide, motor vehicle accidents, and dating violence.4-6
Diagnosing alcohol use disorder
The range of alcohol use includes consumption, bingeing, abuse, and dependence.7,8 Consumption is defined as the drinking of alcoholic beverages. Bingeing is the consumption of more than 5 drinks for men or 4 drinks for women in 2 hours, according to the National Institute on Alcohol Abuse and Alcoholism.7 However, the criterion is slightly different for the Substance Abuse and Mental Health Services Administration, which broadens the timeframe to “on the same occasion.”9 While previously known as separate disorders, alcohol abuse (or misuse) and alcohol dependence are now diagnostically classified together as alcohol use disorders (AUDs), per the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5).8 AUD is further stratified as mild, moderate, or severe, depending on the number of criteria that are met by the patient (TABLE).8,10
Alcohol screening
Currently, the US Preventive Services Task Force (USPSTF) does not recommend screening adolescents ages 12 to 17 for AUD, and has instead issued an “I” statement (insufficient evidence).11 While the USPSTF recognizes the potential burdens of adolescent alcohol use, the potential harms of screening include “stigma, anxiety, labeling, discrimination, privacy concerns, and interference with the patient–clinician relationship.”11 The USPSTF also notes that it “did not find any evidence that specifically examined the harms of screening for alcohol use in adolescents.”11
This is at odds with recommendations from the American Academy of Pediatrics (AAP), which in 2011 released a policy statement advocating screening, brief intervention, and referral to treatment for adolescent substance use.12 In the United States, even though 83% of adolescents see a physician at least once each year,12,13 alcohol misuse screening still varies, occurring in the range of 50% to 86% of office visits.12 When screening does occur, it is often based on clinical impression only.12 Studies have shown that when a screening tool is not used, up to two-thirds of substance use disorders may be missed.12-15
Continue to: A full and complete biopsychosocial interview
A full and complete biopsychosocial interview with adolescents is a necessity, and should include queries about alcohol, drugs, and other substances. Acknowledgment of use should trigger further investigation into the substance use areas. Interviews may start with open-ended questions about alcohol use at home or at school before moving to more personalized and detailed questioning and use of screening tools.16
While various screening instruments exist, for the sake of brevity we provide as an example the Screening to Brief Intervention (S2BI) tool. It is an efficient, single-page tool that can help clinicians in their routine care of adolescents to quickly stratify the patient risk of substance use disorder as none/low, moderate, or severe.12 It can be found here: www.mcpap.com/pdf/S2Bi%20Toolkit.pdf (see page 10).
For all patients, but particularly for adolescents, confidentiality is important, and many specialty societies have created language to address this issue.12 Discuss confidentiality with both the adolescent patient and the patient’s caregiver simultaneously, with dialogue that includes: (a) the need to speak with adolescents alone during the office visit, (b) the benefits of confidentiality in the physician–patient relationship, and (c) the need to disclose selected information to keep patients safe.12 Describing the process for required disclosures is essential. Benefits of disclosure include further support for the adolescent patient as well as appropriate parental participation and support for possible referrals.12
Treating AUD
Treatment for AUD should be multifaceted. Screen for comorbid mood disorders, such as generalized anxiety,17,18 social anxiety,18 and depression,19 as well as for insomnia.18 Studies have demonstrated a strong link between insomnia and anxiety, and again between anxiety and AUD.17-19 Finally, screen for adverse childhood events such as trauma, victimization, and abuse.20 Addressing issues discovered in screening allows for more targeted and personalized treatment of AUD.
The National Institute on Drug Abuse categorizes evidence-based treatment into 3 areas: behavioral therapies, family therapies, and medications.21
Continue to: Behavioral therapies
Behavioral therapies can include group therapy, cognitive behavioral therapy (CBT), motivational enhancement therapy, 12-Step facilitation, and contingency management, in which small rewards or incentives are given for participation in treatment to reinforce positive behaviors.21
Family-based therapies, such as brief strategic family therapy, functional family therapy, and multisystem therapy recognize that adolescents exist in systems of families in communities, and that the patient’s success in treatment may be supported by these relationships.21
Some medications may achieve modest benefit for treatment of adolescents with AUD. Naltrexone, acamprosate, and disulfiram have all been used successfully to treat AUD in adults21; some physicians may choose to use these medications “off label” in adolescents. Bupropion has been used successfully in the treatment of nicotine use disorder,21 and a small study in 2005 showed some success with bupropion in treating adolescents with attention-deficit/hyperactivity disorder, comorbid depression, and substance use disorder.22 Naltrexone has also been studied in adolescents with opioid use disorder, although these were not large studies.23
Adolescents with serious, sustained issues with AUD may require more in-depth treatments such as an intensive outpatient program, a partial hospitalization program, or a residential treatment program.15 The least-restrictive environment is preferable.15 Families are generally included as part of the treatment and recovery process in those settings.21 Some patients may require detoxification prior to referral to residential treatment settings; the American Society of Addiction Medicine has published a comprehensive guideline on alcohol withdrawal.24
Paul’s family physician diagnosed his condition as AUD and referred him for CBT with a psychologist, who treated him for both the AUD and an underlying depressive disorder that was later identified. CBT focused on cognitive restructuring of depressive thoughts as well as support for continued abstinence from alcohol. The patient, with family support, declined antidepressant medication.
After 6 months of treatment, Paul and his parents were pleased with his progress. His grades improved to the point that he was permitted to play soccer again, and he was seriously looking at his future college options.
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; sfields@hsc.wvu.edu
THE CASE
Paul F* is a 16-year-old White boy who lives with his mother and spends some weekends with his father who has shared custody. He recently presented to the clinic for treatment due to an arrest for disorderly conduct at school. He and a friend were found drinking liquor outside the school building when they were scheduled to be in class. Paul reported that he and his friends often drink at school and at extracurricular functions. He has been using alcohol for the past 2 years, with escalating consumption (5 or more drinks per episode) in the past year. Paul has been drinking most days of the week and has even driven under the influence at times. He said, “I just feel happier when I am drinking.” An accomplished soccer player recruited by colleges, Paul recently was suspended from the team due to his poor grades. His response was, “It’s stupid anyway. What’s the point of playing?”
●
* The patient’s name and some personal details have been changed to protect his identity.
Alcohol is the number 1 substance of abuse for adolescents, used more than tobacco or drugs.1-3 In 2007 and again in 2016, the Surgeon General of the United States issued reports to highlight this important topic,1,2 noting that early and repeated exposure to alcohol during this crucial time of brain development increases the risk for future problems, including addiction.2
Adolescent alcohol use is often underestimated by parents and physicians, including misjudging how much, how often, and how young children are when they begin to drink.1 Boys and girls tend to start drinking at similar ages (13.9 and 14.4 years, respectively),3 but as girls age, they tend to drink more and binge more.4 In 2019, 1 in 4 adolescents reported drinking and more than 4 million reported at least 1 episode of binge drinking in the prior month.4 These numbers have further ramifications: early drinking is associated with alcohol dependence, relapse, use of other substances, risky sexual behaviors, injurious behaviors, suicide, motor vehicle accidents, and dating violence.4-6
Diagnosing alcohol use disorder
The range of alcohol use includes consumption, bingeing, abuse, and dependence.7,8 Consumption is defined as the drinking of alcoholic beverages. Bingeing is the consumption of more than 5 drinks for men or 4 drinks for women in 2 hours, according to the National Institute on Alcohol Abuse and Alcoholism.7 However, the criterion is slightly different for the Substance Abuse and Mental Health Services Administration, which broadens the timeframe to “on the same occasion.”9 While previously known as separate disorders, alcohol abuse (or misuse) and alcohol dependence are now diagnostically classified together as alcohol use disorders (AUDs), per the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5).8 AUD is further stratified as mild, moderate, or severe, depending on the number of criteria that are met by the patient (TABLE).8,10
Alcohol screening
Currently, the US Preventive Services Task Force (USPSTF) does not recommend screening adolescents ages 12 to 17 for AUD, and has instead issued an “I” statement (insufficient evidence).11 While the USPSTF recognizes the potential burdens of adolescent alcohol use, the potential harms of screening include “stigma, anxiety, labeling, discrimination, privacy concerns, and interference with the patient–clinician relationship.”11 The USPSTF also notes that it “did not find any evidence that specifically examined the harms of screening for alcohol use in adolescents.”11
This is at odds with recommendations from the American Academy of Pediatrics (AAP), which in 2011 released a policy statement advocating screening, brief intervention, and referral to treatment for adolescent substance use.12 In the United States, even though 83% of adolescents see a physician at least once each year,12,13 alcohol misuse screening still varies, occurring in the range of 50% to 86% of office visits.12 When screening does occur, it is often based on clinical impression only.12 Studies have shown that when a screening tool is not used, up to two-thirds of substance use disorders may be missed.12-15
Continue to: A full and complete biopsychosocial interview
A full and complete biopsychosocial interview with adolescents is a necessity, and should include queries about alcohol, drugs, and other substances. Acknowledgment of use should trigger further investigation into the substance use areas. Interviews may start with open-ended questions about alcohol use at home or at school before moving to more personalized and detailed questioning and use of screening tools.16
While various screening instruments exist, for the sake of brevity we provide as an example the Screening to Brief Intervention (S2BI) tool. It is an efficient, single-page tool that can help clinicians in their routine care of adolescents to quickly stratify the patient risk of substance use disorder as none/low, moderate, or severe.12 It can be found here: www.mcpap.com/pdf/S2Bi%20Toolkit.pdf (see page 10).
For all patients, but particularly for adolescents, confidentiality is important, and many specialty societies have created language to address this issue.12 Discuss confidentiality with both the adolescent patient and the patient’s caregiver simultaneously, with dialogue that includes: (a) the need to speak with adolescents alone during the office visit, (b) the benefits of confidentiality in the physician–patient relationship, and (c) the need to disclose selected information to keep patients safe.12 Describing the process for required disclosures is essential. Benefits of disclosure include further support for the adolescent patient as well as appropriate parental participation and support for possible referrals.12
Treating AUD
Treatment for AUD should be multifaceted. Screen for comorbid mood disorders, such as generalized anxiety,17,18 social anxiety,18 and depression,19 as well as for insomnia.18 Studies have demonstrated a strong link between insomnia and anxiety, and again between anxiety and AUD.17-19 Finally, screen for adverse childhood events such as trauma, victimization, and abuse.20 Addressing issues discovered in screening allows for more targeted and personalized treatment of AUD.
The National Institute on Drug Abuse categorizes evidence-based treatment into 3 areas: behavioral therapies, family therapies, and medications.21
Continue to: Behavioral therapies
Behavioral therapies can include group therapy, cognitive behavioral therapy (CBT), motivational enhancement therapy, 12-Step facilitation, and contingency management, in which small rewards or incentives are given for participation in treatment to reinforce positive behaviors.21
Family-based therapies, such as brief strategic family therapy, functional family therapy, and multisystem therapy recognize that adolescents exist in systems of families in communities, and that the patient’s success in treatment may be supported by these relationships.21
Some medications may achieve modest benefit for treatment of adolescents with AUD. Naltrexone, acamprosate, and disulfiram have all been used successfully to treat AUD in adults21; some physicians may choose to use these medications “off label” in adolescents. Bupropion has been used successfully in the treatment of nicotine use disorder,21 and a small study in 2005 showed some success with bupropion in treating adolescents with attention-deficit/hyperactivity disorder, comorbid depression, and substance use disorder.22 Naltrexone has also been studied in adolescents with opioid use disorder, although these were not large studies.23
Adolescents with serious, sustained issues with AUD may require more in-depth treatments such as an intensive outpatient program, a partial hospitalization program, or a residential treatment program.15 The least-restrictive environment is preferable.15 Families are generally included as part of the treatment and recovery process in those settings.21 Some patients may require detoxification prior to referral to residential treatment settings; the American Society of Addiction Medicine has published a comprehensive guideline on alcohol withdrawal.24
Paul’s family physician diagnosed his condition as AUD and referred him for CBT with a psychologist, who treated him for both the AUD and an underlying depressive disorder that was later identified. CBT focused on cognitive restructuring of depressive thoughts as well as support for continued abstinence from alcohol. The patient, with family support, declined antidepressant medication.
After 6 months of treatment, Paul and his parents were pleased with his progress. His grades improved to the point that he was permitted to play soccer again, and he was seriously looking at his future college options.
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; sfields@hsc.wvu.edu
1. US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2007.
2. US Department of Health and Human Services. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2016.
3. Hingson R, White A. New research findings since the 2007 Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking: A review. J Stud Alcohol Drugs Suppl. 2014; 75:158-169.
4. National Institute on Alcohol Abuse and Alcoholism. Underage drinking. National Institute of Health. Accessed December 22, 2021. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/underage-drinking.
5. Hingson R, Zha W, Iannotti R, et al. Physician advice to adolescents about drinking and other health behaviors. Pediatrics. 2013;131:249-257.
6. Schaus JF, Sole ML, McCoy TP, et al. Screening for high-risk drinking in a college student health center: characterizing students based on quantity, frequency, and harms. J Stud Alcohol Drugs Suppl. 2009;16:34-44.
7. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed December 27, 2021. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Arlington, VA; American Psychiatric Association. 2013.
9. Substance Abuse and Mental Health Services Administration. Bringing down binge drinking. Accessed December 27, 2021. www.samhsa.gov/sites/default/files/programs_campaigns/nation_prevention_week/data-binge-drinking.pdf
10. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 Alcohol Use Disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
11. USPSTF. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:1899-1909.
12. Levy SJ, Williams JF, Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161211.
13. MacKay AP, Duran CP. Adolescent Health in the United States. National Center for Health Statistics, Centers for Disease Control and Prevention. 2007.
14. Haller DM, Meynard A, Lefebvre D, et al. Effectiveness of training family physicians to deliver a brief intervention to address excessive substance use among young patients: a cluster randomized controlled trial. CMAJ. 2014;186:E263-E272.
15. Borus J, Parhami I, Levy S. Screening, brief intervention, and referral to treatment. Child Adolesc Psychiatric Clin N Am. 2016;25:579-601.
16. Knight J, Roberts T, Gabrielli J, et al. Adolescent alcohol and substance use and abuse. Performing preventive services: A bright futures handbook. Accessed December 22, 2021. American Academy of Pediatrics. https://ocfcpacourts.us/wp-content/uploads/2020/06/Adolescent_Alcohol_and_Substance_Abuse_001005.pdf
17. Dyer ML, Heron J, Hickman M, et al. Alcohol use in late adolescence and early adulthood: the role of generalized anxiety disorder and drinking to cope motives. Drug Alcohol Depend. 2019;204:107480.
18. Blumenthal H, Taylor DJ, Cloutier RM, et al. The links between social anxiety disorder, insomnia symptoms, and alcohol use disorders: findings from a large sample of adolescents in the United States. Behav Ther. 2019;50:50-59.
19. Pedrelli P, Shapero B, Archibald A, et al. Alcohol use and depression during adolescence and young adulthood: a summary and interpretation of mixed findings. Curr Addict Rep. 2016;3:91-97.
20. Davis JP, Dworkin ER, Helton J, et al. Extending poly-victimization theory: differential effects of adolescents’ experiences of victimization on substance use disorder diagnoses upon treatment entry. Child Abuse Negl. 2019; 89:165-177.
21. NIDA. Principles of adolescent substance use disorder treatment: a research-based guide. Accessed December 22, 2021. www.drugabuse.gov/publications/principles-adolescent-substance-use-disorder-treatment-research-based-guide
22. Solhkhah R, Wilens TE, Daly J, et al. Bupropion SR for the treatment of substance-abusing outpatient adolescents with attention-deficit/hyperactivity disorder and mood disorders. J Child Adolesc Psychopharmacol. 2005:15:777-786.
23. Camenga DR, Colon-Rivera HA, Muvvala SB. Medications for maintenance treatment of opioid use disorder in adolescents. J Stud Alcohol Drugs. 2019;80:393-402.
24. American Society of Addiction Medicine. The ASAM clinical practice guideline on alcohol withdrawal management. Accessed December 22, 2021. www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
1. US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2007.
2. US Department of Health and Human Services. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2016.
3. Hingson R, White A. New research findings since the 2007 Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking: A review. J Stud Alcohol Drugs Suppl. 2014; 75:158-169.
4. National Institute on Alcohol Abuse and Alcoholism. Underage drinking. National Institute of Health. Accessed December 22, 2021. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/underage-drinking.
5. Hingson R, Zha W, Iannotti R, et al. Physician advice to adolescents about drinking and other health behaviors. Pediatrics. 2013;131:249-257.
6. Schaus JF, Sole ML, McCoy TP, et al. Screening for high-risk drinking in a college student health center: characterizing students based on quantity, frequency, and harms. J Stud Alcohol Drugs Suppl. 2009;16:34-44.
7. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed December 27, 2021. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Arlington, VA; American Psychiatric Association. 2013.
9. Substance Abuse and Mental Health Services Administration. Bringing down binge drinking. Accessed December 27, 2021. www.samhsa.gov/sites/default/files/programs_campaigns/nation_prevention_week/data-binge-drinking.pdf
10. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 Alcohol Use Disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
11. USPSTF. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:1899-1909.
12. Levy SJ, Williams JF, Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161211.
13. MacKay AP, Duran CP. Adolescent Health in the United States. National Center for Health Statistics, Centers for Disease Control and Prevention. 2007.
14. Haller DM, Meynard A, Lefebvre D, et al. Effectiveness of training family physicians to deliver a brief intervention to address excessive substance use among young patients: a cluster randomized controlled trial. CMAJ. 2014;186:E263-E272.
15. Borus J, Parhami I, Levy S. Screening, brief intervention, and referral to treatment. Child Adolesc Psychiatric Clin N Am. 2016;25:579-601.
16. Knight J, Roberts T, Gabrielli J, et al. Adolescent alcohol and substance use and abuse. Performing preventive services: A bright futures handbook. Accessed December 22, 2021. American Academy of Pediatrics. https://ocfcpacourts.us/wp-content/uploads/2020/06/Adolescent_Alcohol_and_Substance_Abuse_001005.pdf
17. Dyer ML, Heron J, Hickman M, et al. Alcohol use in late adolescence and early adulthood: the role of generalized anxiety disorder and drinking to cope motives. Drug Alcohol Depend. 2019;204:107480.
18. Blumenthal H, Taylor DJ, Cloutier RM, et al. The links between social anxiety disorder, insomnia symptoms, and alcohol use disorders: findings from a large sample of adolescents in the United States. Behav Ther. 2019;50:50-59.
19. Pedrelli P, Shapero B, Archibald A, et al. Alcohol use and depression during adolescence and young adulthood: a summary and interpretation of mixed findings. Curr Addict Rep. 2016;3:91-97.
20. Davis JP, Dworkin ER, Helton J, et al. Extending poly-victimization theory: differential effects of adolescents’ experiences of victimization on substance use disorder diagnoses upon treatment entry. Child Abuse Negl. 2019; 89:165-177.
21. NIDA. Principles of adolescent substance use disorder treatment: a research-based guide. Accessed December 22, 2021. www.drugabuse.gov/publications/principles-adolescent-substance-use-disorder-treatment-research-based-guide
22. Solhkhah R, Wilens TE, Daly J, et al. Bupropion SR for the treatment of substance-abusing outpatient adolescents with attention-deficit/hyperactivity disorder and mood disorders. J Child Adolesc Psychopharmacol. 2005:15:777-786.
23. Camenga DR, Colon-Rivera HA, Muvvala SB. Medications for maintenance treatment of opioid use disorder in adolescents. J Stud Alcohol Drugs. 2019;80:393-402.
24. American Society of Addiction Medicine. The ASAM clinical practice guideline on alcohol withdrawal management. Accessed December 22, 2021. www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
Twice exceptionality: A hidden diagnosis in primary care
THE CASE
Michael T,* a 20-year-old cisgender male, visited one of our clinic’s primary care physicians (PCPs). He was reserved and quiet and spoke of his concerns about depression and social anxiety that had been present for several years. He also spoke of his inability to succeed at work and school. Following a thorough PCP review leading to diagnoses of major depressive disorder and social anxiety, the patient agreed to try medication. Over a period of 15 months, trials of medications including fluoxetine, sertraline, aripiprazole, and duloxetine did little to improve the patient’s mood. The PCP decided to consult with our clinic’s integrated health team.
The team reviewed several diagnostic possibilities (TABLE 1) and agreed with the PCP’s diagnoses of major depression and social anxiety. But these disorders alone did not explain the full picture. Team members noted the patient’s unusual communication style, characterized by remarkably long response times and slow processing speed. In particular, when discussing mood, he took several seconds to respond but would respond thoughtfully and with few words.
We administered the Wechsler Adult Intelligence Scale (WAIS-IV). Due to differences between the 4 indices within the WAIS-IV, the Full Scale Intelligence Quotient may under- or overestimate abilities across domains; this was the case for this patient. His General Ability Index (GAI) score was 130, in the very superior range and at the 98th percentile, placing him in the category of gifted intelligence. The patient’s processing speed, however, was at the 18th percentile, which explained his delayed response style and presence of developmental asynchrony, a concept occasionally reported when interpreting socio-emotional and educational maladjustment in gifted individuals.
We determined that Mr. T was twice exceptional—intellectually gifted and also having one or more areas of disability.
●
* The patient’s name has been changed to protect his identity .
In individuals with gifted intelligence, a discrepancy between cognitive and emotional development can make them vulnerable to behavioral and emotional challenges. It is not uncommon for gifted individuals to experience co-occurring distress, anxiety, depression, social withdrawal, difficulty coping with challenging tasks and experiences, low self-esteem, and excessive perfectionism.1-6 Giftedness accompanied by a delay in general abilities and processing speed (significant verbal-performance discrepancy) places an individual in the category of twice-exceptionality, or “2E”—having the potential for high achievement while displaying evidence of 1 or more disabilities including emotional or behavioral difficulties.7
2E Individuals: Prevalence, characteristics, and outcomes
Reported prevalence of twice-exceptionality varies, from approximately 180,000 to 360,000 students in the United States.7 In 2009, the National Commission on Twice Exceptional Students provided the following definition of twice exceptionality:7,8
“Twice-exceptional learners are students who demonstrate the potential for high achievement or creative productivity in one or more domains such as math, science, technology, the social arts, the visual, spatial, or performing arts or other areas of human productivity AND who manifest one or more disabilities as defined by federal or state eligibility criteria. These disabilities include specific learning disabilities; speech and language disorders; emotional/behavioral disorders; physical disabilities; Autism Spectrum Disorders (ASD); or other health impairments, such as Attention Deficit/Hyperactivity Disorder (ADHD).”
How twice-exceptionality might manifest. The literature describes 3 unique groupings of 2E children: those who excel early due to strong language abilities, but later show signs of disability, often when curricular demands rise in junior high, high school, or even college; students diagnosed with disability, but who show exceptional gifts in some areas that may be masked by their learning difficulties; and highly intelligent students who seem to be average, because their disabilities mask their giftedness or their talents mask their difficulties.9,10
Unique behavioral and emotional challenges of 2E individuals may include lower motivation and academic self-efficacy, low self-worth and feelings of failure, or disruptive behaviors.7,11,12 Anxiety and depression often result from the functional impact of twice-exceptionality as well as resultant withdrawal, social isolation, and delay or hindrance of social skills (such as difficulty interpreting social cues).13,14 The individual in our case displayed many of these challenges, including lower motivation, self-worth, and self-esteem, and comorbid anxiety and depression (TABLE 1), further clouding diagnostic clarity.
Continue to: The need for improved recognition
The need for improved recognition. Twice-exceptionality commonly manifests as children reach grade-school age, but they are underrepresented in programs for the gifted due to misunderstanding and misdiagnosis by professionals.15,16 Best practices in identifying 2E children incorporate multidimensional assessments including pre-referral and screening, preliminary intervention, evaluation procedures, and educational planning.16 Despite research asserting that 2E individuals need more support services, knowing how to best identify and support individuals across various settings can prove difficult.7,17-19
Primary care, as we will discuss in a bit, is an interdisciplinary setting in which identification and comprehensive and collaborative support can occur. Historically, though, mental and physical health care have been “siloed” and mental health professionals’ functions in medical settings have often been circumscribed.20,21
A lesson from how our case unfolded
Our integrated health team, known as Integrated Behavioral Health Plus (IBH+), was developed at the University of Colorado School of Medicine, and is a system-level integration of behavioral health professionals working with medical providers to improve outcomes and satisfaction.22 Psychology supervisors and trainees, telepsychiatrists and psychiatry residents, social workers, and pharmacists work together with PCPs and residents to deliver comprehensive patient care. Our model includes a range of behavioral health access points for patients (TABLE 2) and the use of complex patient databases and care team meetings.
In the case we have described here, the nature of the patient’s presentation did not trigger any of the clinical procedures described in TABLE 2, and he fell under the radar of complex patient cases in the clinic. Instead, informal, asynchronous clinical conversations between providers were what eventually lead to diagnostic clarification. Team consultation and psychometric testing provided by IBH+ helped uncover the “hidden diagnosis” of this patient in primary care and identified him as twice-exceptional, experiencing both giftedness and significant emotional suffering (major depression and social anxiety, low self-esteem and self-worth).
Takeaways for primary care
Not all PCPs, of course, have immediate onsite access to a program such as ours. However, innovative ways to tap into available resources might include establishing a partnership with 1 or more behavioral health professionals or bridging less formal relationships with such providers in the community and schools to more easily share patient records.
Continue to: Other presentations within 2E populations
Other presentations within 2E populations. 2E individuals may have other presentations coupled with high cognitive ability7: symptoms of hyperactivity disorders; specific learning disabilities; a diagnosis of autism spectrum disorder (previously termed Asperger type); attention, organizational, social, and behavioral issues; and impulsivity or emotional volatility.
Of note, the perspective of our care team shifted from a “bugs and drugs” perspective of diagnosis and treatment—biological explanations and pharmaceutical solutions—to an approach that explored the underlying interplay between cognitive and emotional functioning for this individual. Our treatment focused on a strengths-based and patient-centered approach. Even without the resources of a full IBH+ model, primary care practices may be able to adapt our experience to their ever-growing complex populations.
Our team shifted treatment planning to the needs of the patient. The 2E identification changed the patient’s perspective about himself. After learning of his giftedness, the patient was able to reframe himself as a highly intelligent, capable individual in need of treatment for depression and social anxiety, as opposed to questioning his intelligence and experiencing confusion and hopelessness within the medical system. His PCP collaborated with the team via telecommunication to maintain an efficacious antidepressant plan and to use a strengths-based approach focused on increasing the patient’s self-view and changing the illness narrative. This narrative was changed by practicing skills, such as challenging unhelpful thought patterns, setting beneficial boundaries, and supporting assertive communication to oppose thoughts and relationships that perpetuated old, negative beliefs and assumptions.
CORRESPONDENCE
Kathryn S. Saldaña, PhD, University of Colorado, 12631 East 17th Avenue, AO1 L15, 3rd Floor, Aurora, CO 80045; kathryn. saldana@ucdenver.edu.
ACKNOWLEDGEMENTS
Our thanks to A.F. Williams Family Medicine Clinic and the University of Colorado Anschutz Medical Campus School of Medicine for their unparalleled models of resident training and multidisciplinary care.
1. Guénolé F, Louis J, Creveuil C, et al. Behavioral profiles of clinically referred children with intellectual giftedness. BioMed Res Int. 2013;2013:540153.
2. Alesi M, Rappo G, Pepi A. Emotional profile and intellectual functioning: A comparison among children with borderline intellectual functioning, average intellectual functioning, and gifted intellectual functioning. SAGE Open. 2015;5:2158244015589995.
3. Alsop G. Asynchrony: intuitively valid and theoretically reliable. Roeper Rev. 2003;25:118-127.
4. Guignard J-H, Jacquet A-Y, Lubart TI. Perfectionism and anxiety: a paradox in intellectual giftedness? PloS One. 2012;7:e41043.
5. Reis SM, McCoach DB. The underachievement of gifted students: What do we know and where do we go? Gifted Child Quarterly. 2000;44:152-170.
6. Barchmann H, Kinze W. Behaviour and achievement disorders in children with high intelligence. Acta Paedopsychiatr. 1990;53:168-172.
7. Reis SM, Baum SM, Burke E. An operational definition of twice-exceptional learners: implications and applications. Gifted Child Quarterly. 2014;58:217-230.
8. NAGC Position Statements & White Papers. Accessed September 18, 2021. http://www.nagc.org/index.aspx?id=5094
9. Neihart M. Identifying and providing services to twice exceptional children. In: Handbook of Giftedness in Children. Pfeiffer SI, ed. Springer; 2008:115-137.
10. Baum SM, Owen SV. To Be Gifted & Learning Disabled: Strategies for Helping Bright Students with Learning & Attention Difficulties. Prufrock Press Inc; 2004.
11. Reis SM. Talents in two places: case studies of high ability students with learning disabilities who have achieved. [Research Monograph 95114]. 1995.
12. Schiff MM, Kaufman AS, Kaufman NL. Scatter analysis of WISC-R profiles for learning disabled children with superior intelligence. J Learn Disabil. 1981;14:400-404.
13. King EW. Addressing the social and emotional needs of twice-exceptional students. Teaching Exceptional Child. 2005;38:16-21.
14. Stormont M, Stebbins MS, Holliday G. Characteristics and educational support needs of underrepresented gifted adolescents. Psychol Schools. 2001;38:413-423.
15. Morrison WF, Rizza MG. Creating a toolkit for identifying twice-exceptional students. J Educ Gifted. 2007;31:57-76.
16. Rizza MG, Morrison WF. Identifying twice exceptional children: a toolkit for success. Accessed September 17, 2021. https://files.eric.ed.gov/fulltext/EJ967126.pdf
17. Cohen SS, Vaughn S. Gifted students with learning disabilities: what does the research say? Learn Disabil. 1994;5:87-94.
18. National Center for Education Statistics. Students with disabilities. Accessed September 18, 2021. https://nces.ed.gov/programs/coe/indicator_cgg.asp
19. The Hechinger Report. Twice exceptional, doubly disadvantaged? How schools struggle to serve gifted students with disabilities. Accessed September 18, 2021. https://hechingerreport.org/twice-exceptional-doubly-disadvantaged-how-schools-struggle-to-serve-gifted-students-with-disabilities
20. Mendaglio S. Heightened multifaceted sensitivity of gifted students: implications for counseling. J Secondary Gifted Educ. 2002;14:72-82.
21. Pereles DA, Omdal S, Baldwin L. Response to intervention and twice-exceptional learners: a promising fit. Gifted Child Today. 2009;32:40-51.
22. Gerrity M. Evolving models of behavioral health integration: evidence update 2010-2015. Milbank Memorial Fund. 2016. Accessed September 18, 2021. www.milbank.org/wp-content/uploads/2016/05/Evolving-Models-of-BHI.pdf
THE CASE
Michael T,* a 20-year-old cisgender male, visited one of our clinic’s primary care physicians (PCPs). He was reserved and quiet and spoke of his concerns about depression and social anxiety that had been present for several years. He also spoke of his inability to succeed at work and school. Following a thorough PCP review leading to diagnoses of major depressive disorder and social anxiety, the patient agreed to try medication. Over a period of 15 months, trials of medications including fluoxetine, sertraline, aripiprazole, and duloxetine did little to improve the patient’s mood. The PCP decided to consult with our clinic’s integrated health team.
The team reviewed several diagnostic possibilities (TABLE 1) and agreed with the PCP’s diagnoses of major depression and social anxiety. But these disorders alone did not explain the full picture. Team members noted the patient’s unusual communication style, characterized by remarkably long response times and slow processing speed. In particular, when discussing mood, he took several seconds to respond but would respond thoughtfully and with few words.
We administered the Wechsler Adult Intelligence Scale (WAIS-IV). Due to differences between the 4 indices within the WAIS-IV, the Full Scale Intelligence Quotient may under- or overestimate abilities across domains; this was the case for this patient. His General Ability Index (GAI) score was 130, in the very superior range and at the 98th percentile, placing him in the category of gifted intelligence. The patient’s processing speed, however, was at the 18th percentile, which explained his delayed response style and presence of developmental asynchrony, a concept occasionally reported when interpreting socio-emotional and educational maladjustment in gifted individuals.
We determined that Mr. T was twice exceptional—intellectually gifted and also having one or more areas of disability.
●
* The patient’s name has been changed to protect his identity .
In individuals with gifted intelligence, a discrepancy between cognitive and emotional development can make them vulnerable to behavioral and emotional challenges. It is not uncommon for gifted individuals to experience co-occurring distress, anxiety, depression, social withdrawal, difficulty coping with challenging tasks and experiences, low self-esteem, and excessive perfectionism.1-6 Giftedness accompanied by a delay in general abilities and processing speed (significant verbal-performance discrepancy) places an individual in the category of twice-exceptionality, or “2E”—having the potential for high achievement while displaying evidence of 1 or more disabilities including emotional or behavioral difficulties.7
2E Individuals: Prevalence, characteristics, and outcomes
Reported prevalence of twice-exceptionality varies, from approximately 180,000 to 360,000 students in the United States.7 In 2009, the National Commission on Twice Exceptional Students provided the following definition of twice exceptionality:7,8
“Twice-exceptional learners are students who demonstrate the potential for high achievement or creative productivity in one or more domains such as math, science, technology, the social arts, the visual, spatial, or performing arts or other areas of human productivity AND who manifest one or more disabilities as defined by federal or state eligibility criteria. These disabilities include specific learning disabilities; speech and language disorders; emotional/behavioral disorders; physical disabilities; Autism Spectrum Disorders (ASD); or other health impairments, such as Attention Deficit/Hyperactivity Disorder (ADHD).”
How twice-exceptionality might manifest. The literature describes 3 unique groupings of 2E children: those who excel early due to strong language abilities, but later show signs of disability, often when curricular demands rise in junior high, high school, or even college; students diagnosed with disability, but who show exceptional gifts in some areas that may be masked by their learning difficulties; and highly intelligent students who seem to be average, because their disabilities mask their giftedness or their talents mask their difficulties.9,10
Unique behavioral and emotional challenges of 2E individuals may include lower motivation and academic self-efficacy, low self-worth and feelings of failure, or disruptive behaviors.7,11,12 Anxiety and depression often result from the functional impact of twice-exceptionality as well as resultant withdrawal, social isolation, and delay or hindrance of social skills (such as difficulty interpreting social cues).13,14 The individual in our case displayed many of these challenges, including lower motivation, self-worth, and self-esteem, and comorbid anxiety and depression (TABLE 1), further clouding diagnostic clarity.
Continue to: The need for improved recognition
The need for improved recognition. Twice-exceptionality commonly manifests as children reach grade-school age, but they are underrepresented in programs for the gifted due to misunderstanding and misdiagnosis by professionals.15,16 Best practices in identifying 2E children incorporate multidimensional assessments including pre-referral and screening, preliminary intervention, evaluation procedures, and educational planning.16 Despite research asserting that 2E individuals need more support services, knowing how to best identify and support individuals across various settings can prove difficult.7,17-19
Primary care, as we will discuss in a bit, is an interdisciplinary setting in which identification and comprehensive and collaborative support can occur. Historically, though, mental and physical health care have been “siloed” and mental health professionals’ functions in medical settings have often been circumscribed.20,21
A lesson from how our case unfolded
Our integrated health team, known as Integrated Behavioral Health Plus (IBH+), was developed at the University of Colorado School of Medicine, and is a system-level integration of behavioral health professionals working with medical providers to improve outcomes and satisfaction.22 Psychology supervisors and trainees, telepsychiatrists and psychiatry residents, social workers, and pharmacists work together with PCPs and residents to deliver comprehensive patient care. Our model includes a range of behavioral health access points for patients (TABLE 2) and the use of complex patient databases and care team meetings.
In the case we have described here, the nature of the patient’s presentation did not trigger any of the clinical procedures described in TABLE 2, and he fell under the radar of complex patient cases in the clinic. Instead, informal, asynchronous clinical conversations between providers were what eventually lead to diagnostic clarification. Team consultation and psychometric testing provided by IBH+ helped uncover the “hidden diagnosis” of this patient in primary care and identified him as twice-exceptional, experiencing both giftedness and significant emotional suffering (major depression and social anxiety, low self-esteem and self-worth).
Takeaways for primary care
Not all PCPs, of course, have immediate onsite access to a program such as ours. However, innovative ways to tap into available resources might include establishing a partnership with 1 or more behavioral health professionals or bridging less formal relationships with such providers in the community and schools to more easily share patient records.
Continue to: Other presentations within 2E populations
Other presentations within 2E populations. 2E individuals may have other presentations coupled with high cognitive ability7: symptoms of hyperactivity disorders; specific learning disabilities; a diagnosis of autism spectrum disorder (previously termed Asperger type); attention, organizational, social, and behavioral issues; and impulsivity or emotional volatility.
Of note, the perspective of our care team shifted from a “bugs and drugs” perspective of diagnosis and treatment—biological explanations and pharmaceutical solutions—to an approach that explored the underlying interplay between cognitive and emotional functioning for this individual. Our treatment focused on a strengths-based and patient-centered approach. Even without the resources of a full IBH+ model, primary care practices may be able to adapt our experience to their ever-growing complex populations.
Our team shifted treatment planning to the needs of the patient. The 2E identification changed the patient’s perspective about himself. After learning of his giftedness, the patient was able to reframe himself as a highly intelligent, capable individual in need of treatment for depression and social anxiety, as opposed to questioning his intelligence and experiencing confusion and hopelessness within the medical system. His PCP collaborated with the team via telecommunication to maintain an efficacious antidepressant plan and to use a strengths-based approach focused on increasing the patient’s self-view and changing the illness narrative. This narrative was changed by practicing skills, such as challenging unhelpful thought patterns, setting beneficial boundaries, and supporting assertive communication to oppose thoughts and relationships that perpetuated old, negative beliefs and assumptions.
CORRESPONDENCE
Kathryn S. Saldaña, PhD, University of Colorado, 12631 East 17th Avenue, AO1 L15, 3rd Floor, Aurora, CO 80045; kathryn. saldana@ucdenver.edu.
ACKNOWLEDGEMENTS
Our thanks to A.F. Williams Family Medicine Clinic and the University of Colorado Anschutz Medical Campus School of Medicine for their unparalleled models of resident training and multidisciplinary care.
THE CASE
Michael T,* a 20-year-old cisgender male, visited one of our clinic’s primary care physicians (PCPs). He was reserved and quiet and spoke of his concerns about depression and social anxiety that had been present for several years. He also spoke of his inability to succeed at work and school. Following a thorough PCP review leading to diagnoses of major depressive disorder and social anxiety, the patient agreed to try medication. Over a period of 15 months, trials of medications including fluoxetine, sertraline, aripiprazole, and duloxetine did little to improve the patient’s mood. The PCP decided to consult with our clinic’s integrated health team.
The team reviewed several diagnostic possibilities (TABLE 1) and agreed with the PCP’s diagnoses of major depression and social anxiety. But these disorders alone did not explain the full picture. Team members noted the patient’s unusual communication style, characterized by remarkably long response times and slow processing speed. In particular, when discussing mood, he took several seconds to respond but would respond thoughtfully and with few words.
We administered the Wechsler Adult Intelligence Scale (WAIS-IV). Due to differences between the 4 indices within the WAIS-IV, the Full Scale Intelligence Quotient may under- or overestimate abilities across domains; this was the case for this patient. His General Ability Index (GAI) score was 130, in the very superior range and at the 98th percentile, placing him in the category of gifted intelligence. The patient’s processing speed, however, was at the 18th percentile, which explained his delayed response style and presence of developmental asynchrony, a concept occasionally reported when interpreting socio-emotional and educational maladjustment in gifted individuals.
We determined that Mr. T was twice exceptional—intellectually gifted and also having one or more areas of disability.
●
* The patient’s name has been changed to protect his identity .
In individuals with gifted intelligence, a discrepancy between cognitive and emotional development can make them vulnerable to behavioral and emotional challenges. It is not uncommon for gifted individuals to experience co-occurring distress, anxiety, depression, social withdrawal, difficulty coping with challenging tasks and experiences, low self-esteem, and excessive perfectionism.1-6 Giftedness accompanied by a delay in general abilities and processing speed (significant verbal-performance discrepancy) places an individual in the category of twice-exceptionality, or “2E”—having the potential for high achievement while displaying evidence of 1 or more disabilities including emotional or behavioral difficulties.7
2E Individuals: Prevalence, characteristics, and outcomes
Reported prevalence of twice-exceptionality varies, from approximately 180,000 to 360,000 students in the United States.7 In 2009, the National Commission on Twice Exceptional Students provided the following definition of twice exceptionality:7,8
“Twice-exceptional learners are students who demonstrate the potential for high achievement or creative productivity in one or more domains such as math, science, technology, the social arts, the visual, spatial, or performing arts or other areas of human productivity AND who manifest one or more disabilities as defined by federal or state eligibility criteria. These disabilities include specific learning disabilities; speech and language disorders; emotional/behavioral disorders; physical disabilities; Autism Spectrum Disorders (ASD); or other health impairments, such as Attention Deficit/Hyperactivity Disorder (ADHD).”
How twice-exceptionality might manifest. The literature describes 3 unique groupings of 2E children: those who excel early due to strong language abilities, but later show signs of disability, often when curricular demands rise in junior high, high school, or even college; students diagnosed with disability, but who show exceptional gifts in some areas that may be masked by their learning difficulties; and highly intelligent students who seem to be average, because their disabilities mask their giftedness or their talents mask their difficulties.9,10
Unique behavioral and emotional challenges of 2E individuals may include lower motivation and academic self-efficacy, low self-worth and feelings of failure, or disruptive behaviors.7,11,12 Anxiety and depression often result from the functional impact of twice-exceptionality as well as resultant withdrawal, social isolation, and delay or hindrance of social skills (such as difficulty interpreting social cues).13,14 The individual in our case displayed many of these challenges, including lower motivation, self-worth, and self-esteem, and comorbid anxiety and depression (TABLE 1), further clouding diagnostic clarity.
Continue to: The need for improved recognition
The need for improved recognition. Twice-exceptionality commonly manifests as children reach grade-school age, but they are underrepresented in programs for the gifted due to misunderstanding and misdiagnosis by professionals.15,16 Best practices in identifying 2E children incorporate multidimensional assessments including pre-referral and screening, preliminary intervention, evaluation procedures, and educational planning.16 Despite research asserting that 2E individuals need more support services, knowing how to best identify and support individuals across various settings can prove difficult.7,17-19
Primary care, as we will discuss in a bit, is an interdisciplinary setting in which identification and comprehensive and collaborative support can occur. Historically, though, mental and physical health care have been “siloed” and mental health professionals’ functions in medical settings have often been circumscribed.20,21
A lesson from how our case unfolded
Our integrated health team, known as Integrated Behavioral Health Plus (IBH+), was developed at the University of Colorado School of Medicine, and is a system-level integration of behavioral health professionals working with medical providers to improve outcomes and satisfaction.22 Psychology supervisors and trainees, telepsychiatrists and psychiatry residents, social workers, and pharmacists work together with PCPs and residents to deliver comprehensive patient care. Our model includes a range of behavioral health access points for patients (TABLE 2) and the use of complex patient databases and care team meetings.
In the case we have described here, the nature of the patient’s presentation did not trigger any of the clinical procedures described in TABLE 2, and he fell under the radar of complex patient cases in the clinic. Instead, informal, asynchronous clinical conversations between providers were what eventually lead to diagnostic clarification. Team consultation and psychometric testing provided by IBH+ helped uncover the “hidden diagnosis” of this patient in primary care and identified him as twice-exceptional, experiencing both giftedness and significant emotional suffering (major depression and social anxiety, low self-esteem and self-worth).
Takeaways for primary care
Not all PCPs, of course, have immediate onsite access to a program such as ours. However, innovative ways to tap into available resources might include establishing a partnership with 1 or more behavioral health professionals or bridging less formal relationships with such providers in the community and schools to more easily share patient records.
Continue to: Other presentations within 2E populations
Other presentations within 2E populations. 2E individuals may have other presentations coupled with high cognitive ability7: symptoms of hyperactivity disorders; specific learning disabilities; a diagnosis of autism spectrum disorder (previously termed Asperger type); attention, organizational, social, and behavioral issues; and impulsivity or emotional volatility.
Of note, the perspective of our care team shifted from a “bugs and drugs” perspective of diagnosis and treatment—biological explanations and pharmaceutical solutions—to an approach that explored the underlying interplay between cognitive and emotional functioning for this individual. Our treatment focused on a strengths-based and patient-centered approach. Even without the resources of a full IBH+ model, primary care practices may be able to adapt our experience to their ever-growing complex populations.
Our team shifted treatment planning to the needs of the patient. The 2E identification changed the patient’s perspective about himself. After learning of his giftedness, the patient was able to reframe himself as a highly intelligent, capable individual in need of treatment for depression and social anxiety, as opposed to questioning his intelligence and experiencing confusion and hopelessness within the medical system. His PCP collaborated with the team via telecommunication to maintain an efficacious antidepressant plan and to use a strengths-based approach focused on increasing the patient’s self-view and changing the illness narrative. This narrative was changed by practicing skills, such as challenging unhelpful thought patterns, setting beneficial boundaries, and supporting assertive communication to oppose thoughts and relationships that perpetuated old, negative beliefs and assumptions.
CORRESPONDENCE
Kathryn S. Saldaña, PhD, University of Colorado, 12631 East 17th Avenue, AO1 L15, 3rd Floor, Aurora, CO 80045; kathryn. saldana@ucdenver.edu.
ACKNOWLEDGEMENTS
Our thanks to A.F. Williams Family Medicine Clinic and the University of Colorado Anschutz Medical Campus School of Medicine for their unparalleled models of resident training and multidisciplinary care.
1. Guénolé F, Louis J, Creveuil C, et al. Behavioral profiles of clinically referred children with intellectual giftedness. BioMed Res Int. 2013;2013:540153.
2. Alesi M, Rappo G, Pepi A. Emotional profile and intellectual functioning: A comparison among children with borderline intellectual functioning, average intellectual functioning, and gifted intellectual functioning. SAGE Open. 2015;5:2158244015589995.
3. Alsop G. Asynchrony: intuitively valid and theoretically reliable. Roeper Rev. 2003;25:118-127.
4. Guignard J-H, Jacquet A-Y, Lubart TI. Perfectionism and anxiety: a paradox in intellectual giftedness? PloS One. 2012;7:e41043.
5. Reis SM, McCoach DB. The underachievement of gifted students: What do we know and where do we go? Gifted Child Quarterly. 2000;44:152-170.
6. Barchmann H, Kinze W. Behaviour and achievement disorders in children with high intelligence. Acta Paedopsychiatr. 1990;53:168-172.
7. Reis SM, Baum SM, Burke E. An operational definition of twice-exceptional learners: implications and applications. Gifted Child Quarterly. 2014;58:217-230.
8. NAGC Position Statements & White Papers. Accessed September 18, 2021. http://www.nagc.org/index.aspx?id=5094
9. Neihart M. Identifying and providing services to twice exceptional children. In: Handbook of Giftedness in Children. Pfeiffer SI, ed. Springer; 2008:115-137.
10. Baum SM, Owen SV. To Be Gifted & Learning Disabled: Strategies for Helping Bright Students with Learning & Attention Difficulties. Prufrock Press Inc; 2004.
11. Reis SM. Talents in two places: case studies of high ability students with learning disabilities who have achieved. [Research Monograph 95114]. 1995.
12. Schiff MM, Kaufman AS, Kaufman NL. Scatter analysis of WISC-R profiles for learning disabled children with superior intelligence. J Learn Disabil. 1981;14:400-404.
13. King EW. Addressing the social and emotional needs of twice-exceptional students. Teaching Exceptional Child. 2005;38:16-21.
14. Stormont M, Stebbins MS, Holliday G. Characteristics and educational support needs of underrepresented gifted adolescents. Psychol Schools. 2001;38:413-423.
15. Morrison WF, Rizza MG. Creating a toolkit for identifying twice-exceptional students. J Educ Gifted. 2007;31:57-76.
16. Rizza MG, Morrison WF. Identifying twice exceptional children: a toolkit for success. Accessed September 17, 2021. https://files.eric.ed.gov/fulltext/EJ967126.pdf
17. Cohen SS, Vaughn S. Gifted students with learning disabilities: what does the research say? Learn Disabil. 1994;5:87-94.
18. National Center for Education Statistics. Students with disabilities. Accessed September 18, 2021. https://nces.ed.gov/programs/coe/indicator_cgg.asp
19. The Hechinger Report. Twice exceptional, doubly disadvantaged? How schools struggle to serve gifted students with disabilities. Accessed September 18, 2021. https://hechingerreport.org/twice-exceptional-doubly-disadvantaged-how-schools-struggle-to-serve-gifted-students-with-disabilities
20. Mendaglio S. Heightened multifaceted sensitivity of gifted students: implications for counseling. J Secondary Gifted Educ. 2002;14:72-82.
21. Pereles DA, Omdal S, Baldwin L. Response to intervention and twice-exceptional learners: a promising fit. Gifted Child Today. 2009;32:40-51.
22. Gerrity M. Evolving models of behavioral health integration: evidence update 2010-2015. Milbank Memorial Fund. 2016. Accessed September 18, 2021. www.milbank.org/wp-content/uploads/2016/05/Evolving-Models-of-BHI.pdf
1. Guénolé F, Louis J, Creveuil C, et al. Behavioral profiles of clinically referred children with intellectual giftedness. BioMed Res Int. 2013;2013:540153.
2. Alesi M, Rappo G, Pepi A. Emotional profile and intellectual functioning: A comparison among children with borderline intellectual functioning, average intellectual functioning, and gifted intellectual functioning. SAGE Open. 2015;5:2158244015589995.
3. Alsop G. Asynchrony: intuitively valid and theoretically reliable. Roeper Rev. 2003;25:118-127.
4. Guignard J-H, Jacquet A-Y, Lubart TI. Perfectionism and anxiety: a paradox in intellectual giftedness? PloS One. 2012;7:e41043.
5. Reis SM, McCoach DB. The underachievement of gifted students: What do we know and where do we go? Gifted Child Quarterly. 2000;44:152-170.
6. Barchmann H, Kinze W. Behaviour and achievement disorders in children with high intelligence. Acta Paedopsychiatr. 1990;53:168-172.
7. Reis SM, Baum SM, Burke E. An operational definition of twice-exceptional learners: implications and applications. Gifted Child Quarterly. 2014;58:217-230.
8. NAGC Position Statements & White Papers. Accessed September 18, 2021. http://www.nagc.org/index.aspx?id=5094
9. Neihart M. Identifying and providing services to twice exceptional children. In: Handbook of Giftedness in Children. Pfeiffer SI, ed. Springer; 2008:115-137.
10. Baum SM, Owen SV. To Be Gifted & Learning Disabled: Strategies for Helping Bright Students with Learning & Attention Difficulties. Prufrock Press Inc; 2004.
11. Reis SM. Talents in two places: case studies of high ability students with learning disabilities who have achieved. [Research Monograph 95114]. 1995.
12. Schiff MM, Kaufman AS, Kaufman NL. Scatter analysis of WISC-R profiles for learning disabled children with superior intelligence. J Learn Disabil. 1981;14:400-404.
13. King EW. Addressing the social and emotional needs of twice-exceptional students. Teaching Exceptional Child. 2005;38:16-21.
14. Stormont M, Stebbins MS, Holliday G. Characteristics and educational support needs of underrepresented gifted adolescents. Psychol Schools. 2001;38:413-423.
15. Morrison WF, Rizza MG. Creating a toolkit for identifying twice-exceptional students. J Educ Gifted. 2007;31:57-76.
16. Rizza MG, Morrison WF. Identifying twice exceptional children: a toolkit for success. Accessed September 17, 2021. https://files.eric.ed.gov/fulltext/EJ967126.pdf
17. Cohen SS, Vaughn S. Gifted students with learning disabilities: what does the research say? Learn Disabil. 1994;5:87-94.
18. National Center for Education Statistics. Students with disabilities. Accessed September 18, 2021. https://nces.ed.gov/programs/coe/indicator_cgg.asp
19. The Hechinger Report. Twice exceptional, doubly disadvantaged? How schools struggle to serve gifted students with disabilities. Accessed September 18, 2021. https://hechingerreport.org/twice-exceptional-doubly-disadvantaged-how-schools-struggle-to-serve-gifted-students-with-disabilities
20. Mendaglio S. Heightened multifaceted sensitivity of gifted students: implications for counseling. J Secondary Gifted Educ. 2002;14:72-82.
21. Pereles DA, Omdal S, Baldwin L. Response to intervention and twice-exceptional learners: a promising fit. Gifted Child Today. 2009;32:40-51.
22. Gerrity M. Evolving models of behavioral health integration: evidence update 2010-2015. Milbank Memorial Fund. 2016. Accessed September 18, 2021. www.milbank.org/wp-content/uploads/2016/05/Evolving-Models-of-BHI.pdf