User login
Things We Do for No Reason™: Universal Venous Thromboembolism Chemoprophylaxis in Low-Risk Hospitalized Medical Patients

Inspired by the ABIM Foundation’s Choosing Wisel y ® campaign, the “Things We Do for No Reason ™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A hospitalist admits a 68-year-old woman for community-acquired pneumonia with a past medical history of hypertension, gastroesophageal reflux disease, and osteoarthritis. Her hospitalist consults physical therapy to maximize mobility; continues her home medications including pantoprazole, hydrochlorothiazide, and acetaminophen; and initiates antimicrobial therapy with ceftriaxone and azithromycin. The hospital admission order set requires administration of subcutaneous unfractionated heparin for venous thromboembolism chemoprophylaxis.
WHY YOU MIGHT THINK UNIVERSAL CHEMOPROPHYLAXIS IS NECESSARY
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), ranks among the leading preventable causes of morbidity and mortality in hospitalized patients.1 DVTs can rapidly progress to a PE, which account for 5% to 10% of in-hospital deaths.1 The negative sequelae of in-hospital VTE, including prolonged hospital stay, increased healthcare costs, and greater risks associated with pharmacologic treatment, add $9 to $18.2 billion in US healthcare expenditures each year.2 Various risk-assessment models (RAMs) identify medical patients at high risk for developing VTE based on the presence of risk factors including acute heart failure, prior history of VTE, and reduced mobility.3 Since hospitalization may itself increase the risk for VTE, medical patients often receive universal chemoprophylaxis with anticoagulants such as unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), or fondaparinux.3 A meta-analysis of randomized controlled trials (RCTs) published by Wein et al supports the use of VTE chemoprophylaxis in high-risk patients.4 It showed statistically significant reductions in rates of PE in high-risk hospitalized medical patients with UFH (risk ratio [RR], 0.64; 95% CI, 0.50-0.82) or LMWH chemoprophylaxis (RR, 0.37; 95% CI, 0.21-0.64), compared with controls.
In recognition of the magnitude of the problem, national organizations have emphasized routine chemoprophylaxis for prevention of in-hospital VTE as a top-priority measure for patient safety.5,6 The Joint Commission includes chemoprophylaxis as a quality core metric and failure to adhere to such standards compromises hospital accreditation.5 Since 2008, the Centers for Medicare & Medicaid Services no longer reimburses hospitals for preventable VTE and requires institutions to document the rationale for omitting chemoprophylaxis if not commenced on hospital admission.6
WHY CHEMOPROPHYLAXIS FOR LOW-RISK MEDICAL PATIENTS IS UNNECESSARY
In order to understand why chemoprophylaxis fails to benefit low-risk medical patients, it is necessary to critically examine the benefits identified in trials of high-risk patients. Although RCTs and meta-analysis of chemoprophylaxis have consistently demonstrated a reduction in VTE, prevention of asymptomatic VTE identified on screening with ultrasound or venography accounts for more than 90% of the composite outcome in the three key trials.7-9 Hospitalists do not routinely screen for asymptomatic VTE, and incorporation of these events into composite VTE outcomes inflates the magnitude of benefit gained by chemoprophylaxis. Importantly, the standard of care does not include screening for asymptomatic DVTs, and studies have estimated that only 10% to 15% of asymptomatic DVTs progress to a symptomatic VTE.10
A meta-analysis of trials evaluating unselected general medical patients (ie, not those with specific high-risk conditions such as acute myocardial infarction) did not show a reduction in symptomatic VTE with chemoprophylaxis (odds ratio [OR], 0.59; 95% CI, 0.29-1.23).11 In the meta-analysis by Wein et al, which did include patients with specific high-risk conditions, chemoprophylaxis produced a small absolute risk reduction, resulting in a number needed to treat (NNT) of 345 to prevent one PE.4 This demonstrates that, even in high-risk patients, the magnitude of benefit is small. Population-level data also question the benefit of chemoprophylaxis. Flanders et al stratified 35 Michigan hospitals into high-, moderate-, and low-performance tertiles, with performance based on the rate of chemoprophylaxis use on admission for general medical patients at high-risk for VTE. The authors found no significant difference in the rate of VTE at 90 days among tertiles.12 These findings question the usefulness of universal chemoprophylaxis when applied in a real-world setting.
The high rates of VTE in the absence of chemoprophylaxis reported in historic trials may overestimate the contemporary risk. A 2019 multicenter, observational study examined the rate of hospital-acquired DVT for 1,170 low- and high-risk patients with acute medical illness admitted to the internal medicine ward.13 Of them, 250 (21%) underwent prophylaxis with parenteral anticoagulants (mean Padua Prediction Score, 4.5). The remaining 920 (79%) were not treated with prophylaxis (mean Padua Prediction Score, 2.5). All patients underwent ultrasound at admission and discharge. The average length of stay was 13 days, and just three patients (0.3%) experienced in-hospital DVT, two of whom were receiving chemoprophylaxis. Only one (0.09%) DVT was symptomatic.
It should be emphasized that any evidence favoring chemoprophylaxis comes from studies of patients at high-risk of VTE. No data show benefit for low-risk patients. Therefore, any risk of chemoprophylaxis likely outweighs the benefits in low-risk patients. Importantly, the risks are underappreciated. A 2014 meta-analysis reported an increased risk of major hemorrhage (OR, 1.81; 95% CI, 1.10-2.98; P = .02) in high-risk medically ill patients on chemoprophylaxis.14 This results in a number needed to harm for major bleeding of 336, a value similar to the NNT for benefit reported by Wein et al.4 Heparin-induced thrombocytopenia, a potentially limb- and life-threatening complication of UFH or LMWH exposure, has an overall incidence of 0.3% to 0.7% in hospitalized patients on chemoprophylaxis.3 Finally, the most commonly used chemoprophylaxis medications are administered subcutaneously, resulting in injection site pain. Unsurprisingly, hospitalized patients refuse chemoprophylaxis more frequently than any other medication.15
The negative implications of inappropriate chemoprophylaxis extend beyond direct harms to patients. Poor stratification and overuse results in unnecessary healthcare costs. One single-center retrospective review demonstrated that, after integration of chemoprophylaxis into hospital order sets, 76% of patients received unnecessary administration of chemoprophylaxis, resulting in an annualized expenditure of $77,652.16 This does not take into account costs associated with major bleeds.
Unfortunately, the pendulum has shifted from an era of underprescribing chemoprophylaxis to hospitalized medical patients to one of overprescribing. Data published in 2018 suggest that providers overuse chemoprophylaxis in low-risk medical patients at more than double the rate of underusing it in high-risk patients (57% vs 21%).17
Several national societies, including the often cited American College of Chest Physicians (ACCP) and American Society of Hematology (ASH), provide guidance on the use of VTE chemoprophylaxis in acutely ill medical inpatients.3,18 The ASH guidelines conditionally recommend VTE chemoprophylaxis rather than no chemoprophylaxis.18 However, the guidelines do not provide guidance on a risk-stratified approach and disclose that this recommendation is supported by a low certainty in the evidence of the net health benefit gained.18 Guidelines from ACCP lean towards individualized care and recommend against the use of VTE chemoprophylaxis for hospitalized acutely ill, low-risk medical patients.3
WHAT YOU SHOULD DO INSTEAD
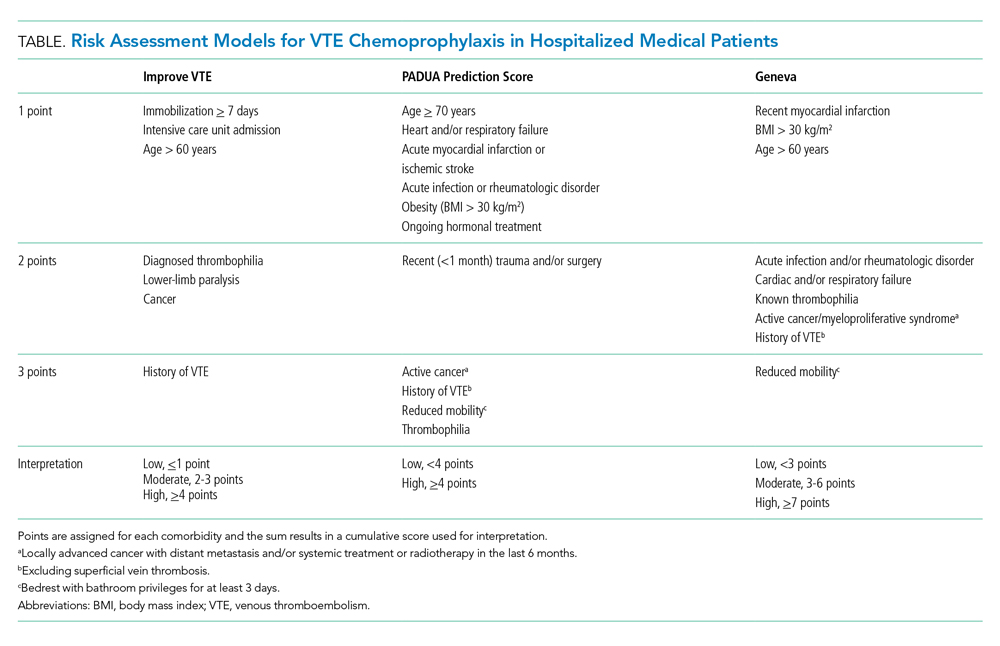
Clinicians should risk stratify using validated RAMs when making a patient-centered treatment plan on admission. The table outlines the most common RAMs with evidence for use in acute medically ill hospitalized patients. Although RAMs have limitations (eg, lack of prospective validation and complexity), the ACCP guidelines advocate for their use.3
Given that immobility independently increases risk for VTE, early mobilization is a simple and cost-effective way to potentially prevent VTE in low-risk patients. In addition to this potential benefit, early mobilization shortens the length of hospital stay, improves functional status and rates of delirium in hospitalized elderly patients, and hastens postoperative recovery after major surgeries.19
RECOMMENDATIONS
- Incorporate a patient-centered, risk-stratified approach to identify low-risk patients. This can be done manually or with use of RAMS embedded in the electronic health record.
- Do not prescribe chemoprophylaxis to low-risk hospitalized medical patients.
- Emphasize the importance of early mobilization in hospitalized patients.
CONCLUSION
In regard to the case, the hospitalist should use a RAM developed for the nonsurgical, non–critically ill patient to determine her need for chemoprophylaxis. Based on the clinical data presented, the three RAMs available would classify the patient as low risk for developing an in-hospital VTE. She should not receive chemoprophylaxis given the lack of data demonstrating benefit in this population. To mitigate the potential risk of bleeding, heparin-induced thrombocytopenia, and painful injections, the hospitalist should discontinue heparin. The hospitalist should advocate for early mobilization and minimize the duration of hospital stay as appropriate.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
- Francis CW. Clinical practice. prophylaxis for thromboembolism in hospitalized medical patients. N Engl J Med. 2007;356(14):1438-1444. https://doi.org/10.1056/nejmcp067264
- Mahan CE, Borrego ME, Woersching AL, et al. Venous thromboembolism: annualised United States models for total, hospital-acquired and preventable costs utilising long-term attack rates. Thromb Haemost. 2012;108(2):291-302. https://doi.org/10.1160/th12-03-0162
- Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e195S-e226S. https://doi.org/10.1378/chest.11-2296
- Wein L, Wein S, Haas SJ, Shaw J, Krum H. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167(14):1476-1486. https://doi.org/10.1001/archinte.167.14.1476
- Performance Measurement. The Joint Commission. Updated October 26, 2020. Accessed November 8, 2019. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/VTE.htm
- Venous Thromboembolism Prophylaxis. Centers for Medicare & Medicaid Services. Updated May 6, 2020. Accessed November 8, 2019. https://ecqi.healthit.gov/ecqm/eh/2019/cms108v7
- Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325-329. https://doi.org/10.1136/bmj.38733.466748.7c
- Leizorovicz A, Cohen AT, Turpie AG, et al. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874-879. https://doi.org/10.1161/01.cir.0000138928.83266.24
- Samama MM, Cohen AT, Darmon JY, et. al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. prophylaxis in medical patients with enoxaparin study group. N Engl J Med. 1999;341(11):793-800. https://doi.org/10.1056/nejm199909093411103
- Segers AE, Prins MH, Lensing AW, Buller HR. Is contrast venography a valid surrogate outcome measure in venous thromboembolism prevention studies? J Thromb Haemost. 2005;3(5):1099-1102. https://doi.org/10.1111/j.1538-7836.2005.01317.x
- Vardi M, Steinberg M, Haran M, Cohen S. Benefits versus risks of pharmacological prophylaxis to prevent symptomatic venous thromboembolism in unselected medical patients revisited. Meta-analysis of the medical literature. J Thromb Thrombolysis. 2012;34(1):11-19. https://doi.org/10.1007/s11239-012-0730-x
- Flanders SA, Greene MT, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577-1584. https://doi.org/10.1001/jamainternmed.2014.3384
- Loffredo L, Arienti V, Vidili G, et al. Low rate of intrahospital deep venous thrombosis in acutely ill medical patients: results from the AURELIO study. Mayo Clin Proc. 2019;94(1):37-43. https://doi.org/10.1016/j.mayocp.2018.07.020
- Alikhan R, Bedenis R, Cohen AT. Heparin for the prevention of venous thromboembolism in acutely ill medical patients (excluding stroke and myocardial infarction). Cochrane Database Syst Rev. 2014;2014(5):CD003747. https://doi.org/10.1002/14651858.cd003747.pub4
- Popoola VO, Lau BD, Tan E, et al. Nonadministration of medication doses for venous thromboembolism prophylaxis in a cohort of hospitalized patients. Am J Health Syst Pharm. 2018;75(6):392-397. https://doi.org/10.2146/ajhp161057
- Chaudhary R, Damluji A, Batukbhai B, et al. Venous Thromboembolism prophylaxis: inadequate and overprophylaxis when comparing perceived versus calculated risk. Mayo Clin Proc Innov Qual Outcomes. 2017;1(3):242-247. https://doi.org/10.1016/j.mayocpiqo.2017.10.003
- Grant PJ, Conlon A, Chopra V, Flanders SA. Use of venous thromboembolism prophylaxis in hospitalized patients. JAMA Intern Med. 2018;178(8):1122-1124. https://doi.org/10.1001/jamainternmed.2018.2022
- Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198-3225. https://doi.org/10.1182/bloodadvances.2018022954
- Pashikanti L, Von Ah D. Impact of early mobilization protocol on the medical-surgical inpatient population: an integrated review of literature. Clin Nurse Spec. 2012;26(2):87-94. https://doi.org/10.1097/nur.0b013e31824590e6

Inspired by the ABIM Foundation’s Choosing Wisel y ® campaign, the “Things We Do for No Reason ™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A hospitalist admits a 68-year-old woman for community-acquired pneumonia with a past medical history of hypertension, gastroesophageal reflux disease, and osteoarthritis. Her hospitalist consults physical therapy to maximize mobility; continues her home medications including pantoprazole, hydrochlorothiazide, and acetaminophen; and initiates antimicrobial therapy with ceftriaxone and azithromycin. The hospital admission order set requires administration of subcutaneous unfractionated heparin for venous thromboembolism chemoprophylaxis.
WHY YOU MIGHT THINK UNIVERSAL CHEMOPROPHYLAXIS IS NECESSARY
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), ranks among the leading preventable causes of morbidity and mortality in hospitalized patients.1 DVTs can rapidly progress to a PE, which account for 5% to 10% of in-hospital deaths.1 The negative sequelae of in-hospital VTE, including prolonged hospital stay, increased healthcare costs, and greater risks associated with pharmacologic treatment, add $9 to $18.2 billion in US healthcare expenditures each year.2 Various risk-assessment models (RAMs) identify medical patients at high risk for developing VTE based on the presence of risk factors including acute heart failure, prior history of VTE, and reduced mobility.3 Since hospitalization may itself increase the risk for VTE, medical patients often receive universal chemoprophylaxis with anticoagulants such as unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), or fondaparinux.3 A meta-analysis of randomized controlled trials (RCTs) published by Wein et al supports the use of VTE chemoprophylaxis in high-risk patients.4 It showed statistically significant reductions in rates of PE in high-risk hospitalized medical patients with UFH (risk ratio [RR], 0.64; 95% CI, 0.50-0.82) or LMWH chemoprophylaxis (RR, 0.37; 95% CI, 0.21-0.64), compared with controls.
In recognition of the magnitude of the problem, national organizations have emphasized routine chemoprophylaxis for prevention of in-hospital VTE as a top-priority measure for patient safety.5,6 The Joint Commission includes chemoprophylaxis as a quality core metric and failure to adhere to such standards compromises hospital accreditation.5 Since 2008, the Centers for Medicare & Medicaid Services no longer reimburses hospitals for preventable VTE and requires institutions to document the rationale for omitting chemoprophylaxis if not commenced on hospital admission.6
WHY CHEMOPROPHYLAXIS FOR LOW-RISK MEDICAL PATIENTS IS UNNECESSARY
In order to understand why chemoprophylaxis fails to benefit low-risk medical patients, it is necessary to critically examine the benefits identified in trials of high-risk patients. Although RCTs and meta-analysis of chemoprophylaxis have consistently demonstrated a reduction in VTE, prevention of asymptomatic VTE identified on screening with ultrasound or venography accounts for more than 90% of the composite outcome in the three key trials.7-9 Hospitalists do not routinely screen for asymptomatic VTE, and incorporation of these events into composite VTE outcomes inflates the magnitude of benefit gained by chemoprophylaxis. Importantly, the standard of care does not include screening for asymptomatic DVTs, and studies have estimated that only 10% to 15% of asymptomatic DVTs progress to a symptomatic VTE.10
A meta-analysis of trials evaluating unselected general medical patients (ie, not those with specific high-risk conditions such as acute myocardial infarction) did not show a reduction in symptomatic VTE with chemoprophylaxis (odds ratio [OR], 0.59; 95% CI, 0.29-1.23).11 In the meta-analysis by Wein et al, which did include patients with specific high-risk conditions, chemoprophylaxis produced a small absolute risk reduction, resulting in a number needed to treat (NNT) of 345 to prevent one PE.4 This demonstrates that, even in high-risk patients, the magnitude of benefit is small. Population-level data also question the benefit of chemoprophylaxis. Flanders et al stratified 35 Michigan hospitals into high-, moderate-, and low-performance tertiles, with performance based on the rate of chemoprophylaxis use on admission for general medical patients at high-risk for VTE. The authors found no significant difference in the rate of VTE at 90 days among tertiles.12 These findings question the usefulness of universal chemoprophylaxis when applied in a real-world setting.
The high rates of VTE in the absence of chemoprophylaxis reported in historic trials may overestimate the contemporary risk. A 2019 multicenter, observational study examined the rate of hospital-acquired DVT for 1,170 low- and high-risk patients with acute medical illness admitted to the internal medicine ward.13 Of them, 250 (21%) underwent prophylaxis with parenteral anticoagulants (mean Padua Prediction Score, 4.5). The remaining 920 (79%) were not treated with prophylaxis (mean Padua Prediction Score, 2.5). All patients underwent ultrasound at admission and discharge. The average length of stay was 13 days, and just three patients (0.3%) experienced in-hospital DVT, two of whom were receiving chemoprophylaxis. Only one (0.09%) DVT was symptomatic.
It should be emphasized that any evidence favoring chemoprophylaxis comes from studies of patients at high-risk of VTE. No data show benefit for low-risk patients. Therefore, any risk of chemoprophylaxis likely outweighs the benefits in low-risk patients. Importantly, the risks are underappreciated. A 2014 meta-analysis reported an increased risk of major hemorrhage (OR, 1.81; 95% CI, 1.10-2.98; P = .02) in high-risk medically ill patients on chemoprophylaxis.14 This results in a number needed to harm for major bleeding of 336, a value similar to the NNT for benefit reported by Wein et al.4 Heparin-induced thrombocytopenia, a potentially limb- and life-threatening complication of UFH or LMWH exposure, has an overall incidence of 0.3% to 0.7% in hospitalized patients on chemoprophylaxis.3 Finally, the most commonly used chemoprophylaxis medications are administered subcutaneously, resulting in injection site pain. Unsurprisingly, hospitalized patients refuse chemoprophylaxis more frequently than any other medication.15
The negative implications of inappropriate chemoprophylaxis extend beyond direct harms to patients. Poor stratification and overuse results in unnecessary healthcare costs. One single-center retrospective review demonstrated that, after integration of chemoprophylaxis into hospital order sets, 76% of patients received unnecessary administration of chemoprophylaxis, resulting in an annualized expenditure of $77,652.16 This does not take into account costs associated with major bleeds.
Unfortunately, the pendulum has shifted from an era of underprescribing chemoprophylaxis to hospitalized medical patients to one of overprescribing. Data published in 2018 suggest that providers overuse chemoprophylaxis in low-risk medical patients at more than double the rate of underusing it in high-risk patients (57% vs 21%).17
Several national societies, including the often cited American College of Chest Physicians (ACCP) and American Society of Hematology (ASH), provide guidance on the use of VTE chemoprophylaxis in acutely ill medical inpatients.3,18 The ASH guidelines conditionally recommend VTE chemoprophylaxis rather than no chemoprophylaxis.18 However, the guidelines do not provide guidance on a risk-stratified approach and disclose that this recommendation is supported by a low certainty in the evidence of the net health benefit gained.18 Guidelines from ACCP lean towards individualized care and recommend against the use of VTE chemoprophylaxis for hospitalized acutely ill, low-risk medical patients.3
WHAT YOU SHOULD DO INSTEAD
Clinicians should risk stratify using validated RAMs when making a patient-centered treatment plan on admission. The table outlines the most common RAMs with evidence for use in acute medically ill hospitalized patients. Although RAMs have limitations (eg, lack of prospective validation and complexity), the ACCP guidelines advocate for their use.3

Given that immobility independently increases risk for VTE, early mobilization is a simple and cost-effective way to potentially prevent VTE in low-risk patients. In addition to this potential benefit, early mobilization shortens the length of hospital stay, improves functional status and rates of delirium in hospitalized elderly patients, and hastens postoperative recovery after major surgeries.19
RECOMMENDATIONS
- Incorporate a patient-centered, risk-stratified approach to identify low-risk patients. This can be done manually or with use of RAMS embedded in the electronic health record.
- Do not prescribe chemoprophylaxis to low-risk hospitalized medical patients.
- Emphasize the importance of early mobilization in hospitalized patients.
CONCLUSION
In regard to the case, the hospitalist should use a RAM developed for the nonsurgical, non–critically ill patient to determine her need for chemoprophylaxis. Based on the clinical data presented, the three RAMs available would classify the patient as low risk for developing an in-hospital VTE. She should not receive chemoprophylaxis given the lack of data demonstrating benefit in this population. To mitigate the potential risk of bleeding, heparin-induced thrombocytopenia, and painful injections, the hospitalist should discontinue heparin. The hospitalist should advocate for early mobilization and minimize the duration of hospital stay as appropriate.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.

Inspired by the ABIM Foundation’s Choosing Wisel y ® campaign, the “Things We Do for No Reason ™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A hospitalist admits a 68-year-old woman for community-acquired pneumonia with a past medical history of hypertension, gastroesophageal reflux disease, and osteoarthritis. Her hospitalist consults physical therapy to maximize mobility; continues her home medications including pantoprazole, hydrochlorothiazide, and acetaminophen; and initiates antimicrobial therapy with ceftriaxone and azithromycin. The hospital admission order set requires administration of subcutaneous unfractionated heparin for venous thromboembolism chemoprophylaxis.
WHY YOU MIGHT THINK UNIVERSAL CHEMOPROPHYLAXIS IS NECESSARY
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), ranks among the leading preventable causes of morbidity and mortality in hospitalized patients.1 DVTs can rapidly progress to a PE, which account for 5% to 10% of in-hospital deaths.1 The negative sequelae of in-hospital VTE, including prolonged hospital stay, increased healthcare costs, and greater risks associated with pharmacologic treatment, add $9 to $18.2 billion in US healthcare expenditures each year.2 Various risk-assessment models (RAMs) identify medical patients at high risk for developing VTE based on the presence of risk factors including acute heart failure, prior history of VTE, and reduced mobility.3 Since hospitalization may itself increase the risk for VTE, medical patients often receive universal chemoprophylaxis with anticoagulants such as unfractionated heparin (UFH), low-molecular-weight heparin (LMWH), or fondaparinux.3 A meta-analysis of randomized controlled trials (RCTs) published by Wein et al supports the use of VTE chemoprophylaxis in high-risk patients.4 It showed statistically significant reductions in rates of PE in high-risk hospitalized medical patients with UFH (risk ratio [RR], 0.64; 95% CI, 0.50-0.82) or LMWH chemoprophylaxis (RR, 0.37; 95% CI, 0.21-0.64), compared with controls.
In recognition of the magnitude of the problem, national organizations have emphasized routine chemoprophylaxis for prevention of in-hospital VTE as a top-priority measure for patient safety.5,6 The Joint Commission includes chemoprophylaxis as a quality core metric and failure to adhere to such standards compromises hospital accreditation.5 Since 2008, the Centers for Medicare & Medicaid Services no longer reimburses hospitals for preventable VTE and requires institutions to document the rationale for omitting chemoprophylaxis if not commenced on hospital admission.6
WHY CHEMOPROPHYLAXIS FOR LOW-RISK MEDICAL PATIENTS IS UNNECESSARY
In order to understand why chemoprophylaxis fails to benefit low-risk medical patients, it is necessary to critically examine the benefits identified in trials of high-risk patients. Although RCTs and meta-analysis of chemoprophylaxis have consistently demonstrated a reduction in VTE, prevention of asymptomatic VTE identified on screening with ultrasound or venography accounts for more than 90% of the composite outcome in the three key trials.7-9 Hospitalists do not routinely screen for asymptomatic VTE, and incorporation of these events into composite VTE outcomes inflates the magnitude of benefit gained by chemoprophylaxis. Importantly, the standard of care does not include screening for asymptomatic DVTs, and studies have estimated that only 10% to 15% of asymptomatic DVTs progress to a symptomatic VTE.10
A meta-analysis of trials evaluating unselected general medical patients (ie, not those with specific high-risk conditions such as acute myocardial infarction) did not show a reduction in symptomatic VTE with chemoprophylaxis (odds ratio [OR], 0.59; 95% CI, 0.29-1.23).11 In the meta-analysis by Wein et al, which did include patients with specific high-risk conditions, chemoprophylaxis produced a small absolute risk reduction, resulting in a number needed to treat (NNT) of 345 to prevent one PE.4 This demonstrates that, even in high-risk patients, the magnitude of benefit is small. Population-level data also question the benefit of chemoprophylaxis. Flanders et al stratified 35 Michigan hospitals into high-, moderate-, and low-performance tertiles, with performance based on the rate of chemoprophylaxis use on admission for general medical patients at high-risk for VTE. The authors found no significant difference in the rate of VTE at 90 days among tertiles.12 These findings question the usefulness of universal chemoprophylaxis when applied in a real-world setting.
The high rates of VTE in the absence of chemoprophylaxis reported in historic trials may overestimate the contemporary risk. A 2019 multicenter, observational study examined the rate of hospital-acquired DVT for 1,170 low- and high-risk patients with acute medical illness admitted to the internal medicine ward.13 Of them, 250 (21%) underwent prophylaxis with parenteral anticoagulants (mean Padua Prediction Score, 4.5). The remaining 920 (79%) were not treated with prophylaxis (mean Padua Prediction Score, 2.5). All patients underwent ultrasound at admission and discharge. The average length of stay was 13 days, and just three patients (0.3%) experienced in-hospital DVT, two of whom were receiving chemoprophylaxis. Only one (0.09%) DVT was symptomatic.
It should be emphasized that any evidence favoring chemoprophylaxis comes from studies of patients at high-risk of VTE. No data show benefit for low-risk patients. Therefore, any risk of chemoprophylaxis likely outweighs the benefits in low-risk patients. Importantly, the risks are underappreciated. A 2014 meta-analysis reported an increased risk of major hemorrhage (OR, 1.81; 95% CI, 1.10-2.98; P = .02) in high-risk medically ill patients on chemoprophylaxis.14 This results in a number needed to harm for major bleeding of 336, a value similar to the NNT for benefit reported by Wein et al.4 Heparin-induced thrombocytopenia, a potentially limb- and life-threatening complication of UFH or LMWH exposure, has an overall incidence of 0.3% to 0.7% in hospitalized patients on chemoprophylaxis.3 Finally, the most commonly used chemoprophylaxis medications are administered subcutaneously, resulting in injection site pain. Unsurprisingly, hospitalized patients refuse chemoprophylaxis more frequently than any other medication.15
The negative implications of inappropriate chemoprophylaxis extend beyond direct harms to patients. Poor stratification and overuse results in unnecessary healthcare costs. One single-center retrospective review demonstrated that, after integration of chemoprophylaxis into hospital order sets, 76% of patients received unnecessary administration of chemoprophylaxis, resulting in an annualized expenditure of $77,652.16 This does not take into account costs associated with major bleeds.
Unfortunately, the pendulum has shifted from an era of underprescribing chemoprophylaxis to hospitalized medical patients to one of overprescribing. Data published in 2018 suggest that providers overuse chemoprophylaxis in low-risk medical patients at more than double the rate of underusing it in high-risk patients (57% vs 21%).17
Several national societies, including the often cited American College of Chest Physicians (ACCP) and American Society of Hematology (ASH), provide guidance on the use of VTE chemoprophylaxis in acutely ill medical inpatients.3,18 The ASH guidelines conditionally recommend VTE chemoprophylaxis rather than no chemoprophylaxis.18 However, the guidelines do not provide guidance on a risk-stratified approach and disclose that this recommendation is supported by a low certainty in the evidence of the net health benefit gained.18 Guidelines from ACCP lean towards individualized care and recommend against the use of VTE chemoprophylaxis for hospitalized acutely ill, low-risk medical patients.3
WHAT YOU SHOULD DO INSTEAD
Clinicians should risk stratify using validated RAMs when making a patient-centered treatment plan on admission. The table outlines the most common RAMs with evidence for use in acute medically ill hospitalized patients. Although RAMs have limitations (eg, lack of prospective validation and complexity), the ACCP guidelines advocate for their use.3

Given that immobility independently increases risk for VTE, early mobilization is a simple and cost-effective way to potentially prevent VTE in low-risk patients. In addition to this potential benefit, early mobilization shortens the length of hospital stay, improves functional status and rates of delirium in hospitalized elderly patients, and hastens postoperative recovery after major surgeries.19
RECOMMENDATIONS
- Incorporate a patient-centered, risk-stratified approach to identify low-risk patients. This can be done manually or with use of RAMS embedded in the electronic health record.
- Do not prescribe chemoprophylaxis to low-risk hospitalized medical patients.
- Emphasize the importance of early mobilization in hospitalized patients.
CONCLUSION
In regard to the case, the hospitalist should use a RAM developed for the nonsurgical, non–critically ill patient to determine her need for chemoprophylaxis. Based on the clinical data presented, the three RAMs available would classify the patient as low risk for developing an in-hospital VTE. She should not receive chemoprophylaxis given the lack of data demonstrating benefit in this population. To mitigate the potential risk of bleeding, heparin-induced thrombocytopenia, and painful injections, the hospitalist should discontinue heparin. The hospitalist should advocate for early mobilization and minimize the duration of hospital stay as appropriate.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
- Francis CW. Clinical practice. prophylaxis for thromboembolism in hospitalized medical patients. N Engl J Med. 2007;356(14):1438-1444. https://doi.org/10.1056/nejmcp067264
- Mahan CE, Borrego ME, Woersching AL, et al. Venous thromboembolism: annualised United States models for total, hospital-acquired and preventable costs utilising long-term attack rates. Thromb Haemost. 2012;108(2):291-302. https://doi.org/10.1160/th12-03-0162
- Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e195S-e226S. https://doi.org/10.1378/chest.11-2296
- Wein L, Wein S, Haas SJ, Shaw J, Krum H. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167(14):1476-1486. https://doi.org/10.1001/archinte.167.14.1476
- Performance Measurement. The Joint Commission. Updated October 26, 2020. Accessed November 8, 2019. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/VTE.htm
- Venous Thromboembolism Prophylaxis. Centers for Medicare & Medicaid Services. Updated May 6, 2020. Accessed November 8, 2019. https://ecqi.healthit.gov/ecqm/eh/2019/cms108v7
- Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325-329. https://doi.org/10.1136/bmj.38733.466748.7c
- Leizorovicz A, Cohen AT, Turpie AG, et al. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874-879. https://doi.org/10.1161/01.cir.0000138928.83266.24
- Samama MM, Cohen AT, Darmon JY, et. al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. prophylaxis in medical patients with enoxaparin study group. N Engl J Med. 1999;341(11):793-800. https://doi.org/10.1056/nejm199909093411103
- Segers AE, Prins MH, Lensing AW, Buller HR. Is contrast venography a valid surrogate outcome measure in venous thromboembolism prevention studies? J Thromb Haemost. 2005;3(5):1099-1102. https://doi.org/10.1111/j.1538-7836.2005.01317.x
- Vardi M, Steinberg M, Haran M, Cohen S. Benefits versus risks of pharmacological prophylaxis to prevent symptomatic venous thromboembolism in unselected medical patients revisited. Meta-analysis of the medical literature. J Thromb Thrombolysis. 2012;34(1):11-19. https://doi.org/10.1007/s11239-012-0730-x
- Flanders SA, Greene MT, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577-1584. https://doi.org/10.1001/jamainternmed.2014.3384
- Loffredo L, Arienti V, Vidili G, et al. Low rate of intrahospital deep venous thrombosis in acutely ill medical patients: results from the AURELIO study. Mayo Clin Proc. 2019;94(1):37-43. https://doi.org/10.1016/j.mayocp.2018.07.020
- Alikhan R, Bedenis R, Cohen AT. Heparin for the prevention of venous thromboembolism in acutely ill medical patients (excluding stroke and myocardial infarction). Cochrane Database Syst Rev. 2014;2014(5):CD003747. https://doi.org/10.1002/14651858.cd003747.pub4
- Popoola VO, Lau BD, Tan E, et al. Nonadministration of medication doses for venous thromboembolism prophylaxis in a cohort of hospitalized patients. Am J Health Syst Pharm. 2018;75(6):392-397. https://doi.org/10.2146/ajhp161057
- Chaudhary R, Damluji A, Batukbhai B, et al. Venous Thromboembolism prophylaxis: inadequate and overprophylaxis when comparing perceived versus calculated risk. Mayo Clin Proc Innov Qual Outcomes. 2017;1(3):242-247. https://doi.org/10.1016/j.mayocpiqo.2017.10.003
- Grant PJ, Conlon A, Chopra V, Flanders SA. Use of venous thromboembolism prophylaxis in hospitalized patients. JAMA Intern Med. 2018;178(8):1122-1124. https://doi.org/10.1001/jamainternmed.2018.2022
- Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198-3225. https://doi.org/10.1182/bloodadvances.2018022954
- Pashikanti L, Von Ah D. Impact of early mobilization protocol on the medical-surgical inpatient population: an integrated review of literature. Clin Nurse Spec. 2012;26(2):87-94. https://doi.org/10.1097/nur.0b013e31824590e6
- Francis CW. Clinical practice. prophylaxis for thromboembolism in hospitalized medical patients. N Engl J Med. 2007;356(14):1438-1444. https://doi.org/10.1056/nejmcp067264
- Mahan CE, Borrego ME, Woersching AL, et al. Venous thromboembolism: annualised United States models for total, hospital-acquired and preventable costs utilising long-term attack rates. Thromb Haemost. 2012;108(2):291-302. https://doi.org/10.1160/th12-03-0162
- Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e195S-e226S. https://doi.org/10.1378/chest.11-2296
- Wein L, Wein S, Haas SJ, Shaw J, Krum H. Pharmacological venous thromboembolism prophylaxis in hospitalized medical patients: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167(14):1476-1486. https://doi.org/10.1001/archinte.167.14.1476
- Performance Measurement. The Joint Commission. Updated October 26, 2020. Accessed November 8, 2019. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/VTE.htm
- Venous Thromboembolism Prophylaxis. Centers for Medicare & Medicaid Services. Updated May 6, 2020. Accessed November 8, 2019. https://ecqi.healthit.gov/ecqm/eh/2019/cms108v7
- Cohen AT, Davidson BL, Gallus AS, et al. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006;332(7537):325-329. https://doi.org/10.1136/bmj.38733.466748.7c
- Leizorovicz A, Cohen AT, Turpie AG, et al. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation. 2004;110(7):874-879. https://doi.org/10.1161/01.cir.0000138928.83266.24
- Samama MM, Cohen AT, Darmon JY, et. al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. prophylaxis in medical patients with enoxaparin study group. N Engl J Med. 1999;341(11):793-800. https://doi.org/10.1056/nejm199909093411103
- Segers AE, Prins MH, Lensing AW, Buller HR. Is contrast venography a valid surrogate outcome measure in venous thromboembolism prevention studies? J Thromb Haemost. 2005;3(5):1099-1102. https://doi.org/10.1111/j.1538-7836.2005.01317.x
- Vardi M, Steinberg M, Haran M, Cohen S. Benefits versus risks of pharmacological prophylaxis to prevent symptomatic venous thromboembolism in unselected medical patients revisited. Meta-analysis of the medical literature. J Thromb Thrombolysis. 2012;34(1):11-19. https://doi.org/10.1007/s11239-012-0730-x
- Flanders SA, Greene MT, Grant P, et al. Hospital performance for pharmacologic venous thromboembolism prophylaxis and rate of venous thromboembolism: a cohort study. JAMA Intern Med. 2014;174(10):1577-1584. https://doi.org/10.1001/jamainternmed.2014.3384
- Loffredo L, Arienti V, Vidili G, et al. Low rate of intrahospital deep venous thrombosis in acutely ill medical patients: results from the AURELIO study. Mayo Clin Proc. 2019;94(1):37-43. https://doi.org/10.1016/j.mayocp.2018.07.020
- Alikhan R, Bedenis R, Cohen AT. Heparin for the prevention of venous thromboembolism in acutely ill medical patients (excluding stroke and myocardial infarction). Cochrane Database Syst Rev. 2014;2014(5):CD003747. https://doi.org/10.1002/14651858.cd003747.pub4
- Popoola VO, Lau BD, Tan E, et al. Nonadministration of medication doses for venous thromboembolism prophylaxis in a cohort of hospitalized patients. Am J Health Syst Pharm. 2018;75(6):392-397. https://doi.org/10.2146/ajhp161057
- Chaudhary R, Damluji A, Batukbhai B, et al. Venous Thromboembolism prophylaxis: inadequate and overprophylaxis when comparing perceived versus calculated risk. Mayo Clin Proc Innov Qual Outcomes. 2017;1(3):242-247. https://doi.org/10.1016/j.mayocpiqo.2017.10.003
- Grant PJ, Conlon A, Chopra V, Flanders SA. Use of venous thromboembolism prophylaxis in hospitalized patients. JAMA Intern Med. 2018;178(8):1122-1124. https://doi.org/10.1001/jamainternmed.2018.2022
- Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv. 2018;2(22):3198-3225. https://doi.org/10.1182/bloodadvances.2018022954
- Pashikanti L, Von Ah D. Impact of early mobilization protocol on the medical-surgical inpatient population: an integrated review of literature. Clin Nurse Spec. 2012;26(2):87-94. https://doi.org/10.1097/nur.0b013e31824590e6
© 2020 Society of Hospital Medicine
Email: blba249@uky.edu; Telephone: 267-627-4207; Twitter @theABofPharmaC.
Things We Do for No Reason™: Routine Correction of Elevated INR and Thrombocytopenia Prior to Paracentesis in Patients with Cirrhosis

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 52-year-old man with alcoholic cirrhosis for tense ascites and altered mentation. Home medications include furosemide, spironolactone, lactulose, and rifaximin, but his family notes he ran out last week. Although afebrile and hemodynamically stable, the patient’s coagulopathy, with an international normalized ratio (INR) of 2.3, and thrombocytopenia, with a platelet count of 37,000/μL, worries the hospitalist. The hospitalist wonders whether to transfuse fresh frozen plasma (FFP) and platelets prior to diagnostic paracentesis to reduce the risk of procedural bleeding.
WHY ROUTINELY DOING THIS MIGHT SEEM HELPFUL
Many patients undergoing paracentesis have severe liver disease and present with both thrombocytopenia and elevated INRs. While platelet count and INR serve as surrogate markers for bleeding risk in many settings, clinicians often extrapolate this concept to patients with cirrhosis. Many hospitalists routinely check INR and platelet count and administer FFP and platelets prior to diagnostic or therapeutic paracentesis to mitigate procedure-related bleeding risk. Some medical resources recommend this practice,1 while case reports and personal experiences with bleeding in these patients create availability bias that influences perception of bleeding risk.2 One recent study of patients with decompensated cirrhosis presenting to a US tertiary care center found that, of those receiving large-volume paracentesis, 22.2% received prophylactic FFP and 17.3% received prophylactic platelets before paracentesis.3
WHY ROUTINELY DOING THIS IS NOT HELPFUL
Advances in our understanding of coagulation in cirrhosis demonstrate neither INR nor platelet count accurately predict bleeding risk in this population. Additionally, evidence demonstrates the overall safety of paracentesis in cirrhosis—even in the presence of high INR and thrombocytopenia—and the lack of benefit from prophylactic transfusions with FFP or platelets.
Substantial evidence in patients with cirrhosis demonstrates that changes in coagulation and platelet function confer a “balanced coagulopathy” in which patients oscillate between hyper- and hypocoagulable states. In a cirrhotic liver, hepatic synthetic dysfunction results in a complex milieu through reduced production and plasma concentrations of both pro- and anticoagulant factors that can lead to either bleeding or clotting.4 This “rebalancing” makes prothrombin time (PT) and INR unreliable indicators of bleeding or clotting risk. Similarly, in patients with cirrhosis, thrombocytopenia does not necessarily reflect impaired clotting ability. These patients experience an increase in production of von Willebrand Factor, which may compensate for low platelet counts by producing stronger platelet adhesion to collagen.4 Unfortunately, we currently lack a reliable test or risk score to assess true bleeding risk in patients with cirrhosis.
Observational studies support these laboratory findings. Large case series consistently demonstrate no association between INR or platelet counts and bleeding risk in either diagnostic or therapeutic paracentesis, including large-volume paracentesis (See Appendix for a list of recent representative studies).5-10 Moreover, prophylactic transfusion of FFP or platelets does not significantly reduce bleeding risk.
In a 1991 study by McVay et al, the researchers examined bleeding outcomes of 441 paracenteses performed on hospitalized patients.11 Among patients who did not receive FFP prior to paracentesis, only one required a transfusion for procedure-related bleeding, an event rate of 0.25%. This single patient had a normal platelet count and an elevated PT to the same extent as 261 others who underwent paracentesis without complication. In a pooled analysis that included 391 paracenteses and 207 thoracenteses, the authors concluded neither PT nor platelet level predicted bleeding risk. Similarly, the largest published case series on this topic examined 4,729 paracenteses over a decade on a liver unit and found low rates of major bleeding (0.19%).9 Furthermore, preprocedure INR or platelet count did not correlate with bleeding risk. The authors did not report preprocedure transfusion rates, but they noted transfusions occurred only “occasionally.”
Subsequent observational studies have consistently revealed low bleeding risks even in settings of high coagulopathy prevalence. Grabau et al reviewed all large-volume paracenteses performed in a gastroenterology clinic over 7 years.10 In over 1,100 procedures, no major bleeding events occurred despite 27% of patients having INR greater than 2.0 and 54% having platelet counts less than 50,000/μL. Kurup et al examined bleeding risk among 304 procedures performed on patients with platelet counts less than 50,000/μL referred to radiology for ultrasound-guided paracentesis.7 Three bleeding events occurred, an overall event rate of 0.99%. They also found no association between preprocedure platelet count and bleeding risk.
In addition to observational data, one randomized, controlled trial evaluated the effects of FFP and platelet administration on bleeding risk among 60 patients with cirrhosis undergoing invasive procedures, including 19 paracenteses.6 Enrollment criteria included INR greater than 1.8 and/or platelet count less than 50,000/μL. One hundred percent of patients randomized to the usual care control arm received platelets or FFP as compared to 17% in the thromboelastography (TEG)–guided transfusion strategy arm. TEG assesses the viscoelastic properties of evolving clot formation in whole blood. Only one patient, a patient in the control arm who received FFP, developed procedure-related bleeding. Although receiving many fewer transfusions, the TEG-guided group experienced no bleeding.
In the presence of multiple studies demonstrating lack of benefit from FFP and platelet transfusion, guidelines published by the American Association for the Study of Liver Disease (AASLD), the American Gastroenterological Association (AGA), and the Society of Interventional Radiology (SIR) acknowledge the inaccuracy of platelet count and INR in predicting bleeding risk.12-14 Both AASLD and AGA recommend against routine transfusion of platelets and FFP prior to paracentesis.12,13 SIR guidelines from 2019 recommend against using an INR threshold for low-risk procedures like paracentesis and lowered their recommended platelet transfusion threshold from less than 50,000/μL to less than 20,000/μL.14 While we have limited safety data for paracentesis in patients with very low platelet counts, Kurup et al observed no bleeding events in the 19 patients in their cohort with platelets less than 20,000/μL undergoing ultrasound-guided paracentesis.7
In addition to lack of proven benefit, preprocedure transfusion exposes patients to objective risk. Transfusion-related acute lung injury and transfusion-associated circulatory overload develop at a rate of 0.48 and 3.8 per 100,000 components transfused, respectively.15 FFP transfusions also risk anaphylactic reactions with incidence ranging from 1:18,000 to 1:172,000.16 Platelets carry additional risk of bacterial contamination and resultant sepsis estimated at 1:5,000 to 1:8,000 per unit.17 Volume expansion from transfusions may contribute to portal hypertension and increase risk of variceal bleeding in decompensated liver disease.
Finally, FFP and platelet transfusions carry a significant cost. Rowley et al estimated eliminating preprocedure transfusions over 2 years and 3,116 paracenteses saved their institution $816,000.5 Furthermore, checking and correcting INR and thrombocytopenia can lead to procedural delay. Studies have demonstrated increased mortality from delaying paracentesis.18
WHEN IT IS HELPFUL
While most patients undergoing paracentesis have cirrhosis, patients without cirrhosis also undergo this procedure. Although several cited studies examined paracentesis among all-comers with ascites, our recommendations specifically apply to patients with ascites from cirrhosis.
Furthermore, although no paracentesis data in patients with severe coagulopathy (INR >2.5 or platelet count <20,000/μL) suggest periprocedural transfusion helps, we also lack data to prove it does not help.
Current recommendations from the AASLD suggest correcting coagulopathy in patients with clinically evident disseminated intravascular coagulation or hyperfibrinolysis prior to procedures.12 While no clear guidance related to paracentesis exists on when to assess for these entities, we recommend evaluating for them only when the clinical situation otherwise merits doing so and not solely for the purpose of screening prior to paracentesis. Measuring fibrinogen before paracentesis to predict bleeding risk is an emerging concept, but it cannot be routinely recommended at this time.13 Other factors that may play an important role in bleeding risk—ultrasound guidance, operator experience, and ability to avoid epigastric vessels and collateral veins—are beyond the scope of this article.
WHAT SHOULD BE DONE INSTEAD
Given that laboratory evaluations like INR and platelet count cannot predict which patients with cirrhosis will experience major bleeding complications after paracentesis and given that routinely transfusing FFP or platelets does not confer benefit and may cause serious harm, providers should avoid measuring INR or platelet count to prepare for paracentesis. Likewise, providers should avoid routinely transfusing FFP and platelets prior to paracentesis even in the presence of abnormal laboratory values because such values do not accurately reflect bleeding risk in patients with cirrhosis. Perform clinically indicated paracentesis without the delays that accompany unnecessary laboratory evaluations or transfusions.
RECOMMENDATIONS
Keep the following in mind with patients presenting with ascites from cirrhosis:
- Do not routinely use platelet count or INR when preparing for paracentesis, whether diagnostic or therapeutic, because no evidence-based “cutoff” for safe performance of paracentesis exists.
- Do not routinely transfuse FFP or platelets for prophylaxis prior to paracentesis in patients with cirrhosis.
- Reserve preprocedure transfusion of FFP or platelets for patients with disseminated intravascular coagulation, hyperfibrinolysis, or other indications for transfusion unrelated to procedural prophylaxis.
CONCLUSION
Case series representing diverse institutional experiences with thousands of patients consistently demonstrate that bleeding after paracentesis is rare (<1%), mortality from bleeding occurs very infrequently, and neither INR nor platelet counts predict bleeding risk during paracentesis in cirrhosis. These studies demonstrate that abandoning routine correction of coagulopathy does not lead to worse outcomes, can avoid potentially significant transfusion-related adverse events, and can save scarce resources.
Returning to our clinical scenario, the hospitalist should not transfuse FFP or platelets and should not delay the diagnostic paracentesis.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
Acknowledgments
The authors wish to acknowledge James Burton, MD, H Raymond Tahhan, MD, John Hess, MD, MPH, and Terry Gernsheimer, MD, for directing the authors to useful references cited in the manuscript.
1. Shlamovitz G. Paracentesis. Medscape. 2018. Accessed April 16, 2019. https://emedicine.medscape.com/article/80944-overview
2. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124-1131. https://doi.org/10.1126/science.185.4157.1124
3. Barnhill M, Lee A, Montero A. Adherence rates to recommended guidelines for paracentesis in cirrhotic patients at a tertiary care center and associated complications. Am J Gastroenterol. 2017;112:S504.
4. Tripodi A, Primignani M, Mannucci PM, Caldwell SH. Changing concepts of cirrhotic coagulopathy. Am J Gastroenterol. 2017;112(2):274-281. https://doi.org/10.1038/ajg.2016.498
5. Rowley MW, Agarwal S, Seetharam AB, Hirsch KS. Real-time ultrasound-guided paracentesis by radiologists: near zero risk of hemorrhage without correction of coagulopathy. J Vasc Interv Radiol. 2019;30(2):259-264. https://doi.org/10.1016/j.jvir.2018.11.001
6. De Pietri L, Bianchini M, Montalti R, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology. 2016;63(2):566-573. https://doi.org/10.1002/hep.28148
7. Kurup AN, Lekah A, Reardon ST, et al. Bleeding rate for ultrasound-guided paracentesis in thrombocytopenic patients. J Ultrasound Med. 2015;34(10):1833-1838. https://doi.org/10.7863/ultra.14.10034
8. De Gottardi A, Thévenot T, Spahr L, et al. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin Gastroenterol Hepatol. 2009;7(8):906-909. https://doi.org/10.1016/j.cgh.2009.05.004
9. Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther. 2005;21(5):525-529. https://doi.org/10.1111/j.1365-2036.2005.02387.x
10. Grabau CM, Crago SF, Hoff LK, et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40(2):484-488. https://doi.org/10.1002/hep.20317
11. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. https://doi.org/10.1046/j.1537-2995.1991.31291142949.x
12. Runyon BA. AASLD Practice Guideline: Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012. The American Association for the Study of Liver Diseases; 2012. Accessed April 16, 2019. https://www.aasld.org/sites/default/files/2019-06/141020_Guideline_Ascites_4UFb_2015.pdf
13. O’Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA clinical practice update: coagulation in cirrhosis. Gastroenterology. 2019;157(1):34-43.e1. https://doi.org/10.1053/j.gastro.2019.03.070
14. Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions—part ii: recommendations. J Vasc Interv Radiol. 2019;30(8):1168-1184.e1. https://doi.org/10.1016/j.jvir.2019.04.017
15. Blumberg N, Heal JM, Gettins K, et al. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion. 2010;50(12):2738-2744. https://doi.org/10.1111/j.1537-2995.2010.02748.x
16. Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012; 52(Suppl 1):65S-79S. https://doi.org/10.1111/j.1537-2995.2012.03663.x
17. Kleinman S, Reed W, Stassinopoulos A. A patient-oriented risk-benefit analysis of pathogen-inactivated blood components: application to apheresis platelets in the United States. Transfusion. 2013;53(7):1603-1618. https://doi.org/10.1111/j.1537-2995.2012.03928.x
18. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. https://doi.org/10.1038/ajg.2014.212

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 52-year-old man with alcoholic cirrhosis for tense ascites and altered mentation. Home medications include furosemide, spironolactone, lactulose, and rifaximin, but his family notes he ran out last week. Although afebrile and hemodynamically stable, the patient’s coagulopathy, with an international normalized ratio (INR) of 2.3, and thrombocytopenia, with a platelet count of 37,000/μL, worries the hospitalist. The hospitalist wonders whether to transfuse fresh frozen plasma (FFP) and platelets prior to diagnostic paracentesis to reduce the risk of procedural bleeding.
WHY ROUTINELY DOING THIS MIGHT SEEM HELPFUL
Many patients undergoing paracentesis have severe liver disease and present with both thrombocytopenia and elevated INRs. While platelet count and INR serve as surrogate markers for bleeding risk in many settings, clinicians often extrapolate this concept to patients with cirrhosis. Many hospitalists routinely check INR and platelet count and administer FFP and platelets prior to diagnostic or therapeutic paracentesis to mitigate procedure-related bleeding risk. Some medical resources recommend this practice,1 while case reports and personal experiences with bleeding in these patients create availability bias that influences perception of bleeding risk.2 One recent study of patients with decompensated cirrhosis presenting to a US tertiary care center found that, of those receiving large-volume paracentesis, 22.2% received prophylactic FFP and 17.3% received prophylactic platelets before paracentesis.3
WHY ROUTINELY DOING THIS IS NOT HELPFUL
Advances in our understanding of coagulation in cirrhosis demonstrate neither INR nor platelet count accurately predict bleeding risk in this population. Additionally, evidence demonstrates the overall safety of paracentesis in cirrhosis—even in the presence of high INR and thrombocytopenia—and the lack of benefit from prophylactic transfusions with FFP or platelets.
Substantial evidence in patients with cirrhosis demonstrates that changes in coagulation and platelet function confer a “balanced coagulopathy” in which patients oscillate between hyper- and hypocoagulable states. In a cirrhotic liver, hepatic synthetic dysfunction results in a complex milieu through reduced production and plasma concentrations of both pro- and anticoagulant factors that can lead to either bleeding or clotting.4 This “rebalancing” makes prothrombin time (PT) and INR unreliable indicators of bleeding or clotting risk. Similarly, in patients with cirrhosis, thrombocytopenia does not necessarily reflect impaired clotting ability. These patients experience an increase in production of von Willebrand Factor, which may compensate for low platelet counts by producing stronger platelet adhesion to collagen.4 Unfortunately, we currently lack a reliable test or risk score to assess true bleeding risk in patients with cirrhosis.
Observational studies support these laboratory findings. Large case series consistently demonstrate no association between INR or platelet counts and bleeding risk in either diagnostic or therapeutic paracentesis, including large-volume paracentesis (See Appendix for a list of recent representative studies).5-10 Moreover, prophylactic transfusion of FFP or platelets does not significantly reduce bleeding risk.
In a 1991 study by McVay et al, the researchers examined bleeding outcomes of 441 paracenteses performed on hospitalized patients.11 Among patients who did not receive FFP prior to paracentesis, only one required a transfusion for procedure-related bleeding, an event rate of 0.25%. This single patient had a normal platelet count and an elevated PT to the same extent as 261 others who underwent paracentesis without complication. In a pooled analysis that included 391 paracenteses and 207 thoracenteses, the authors concluded neither PT nor platelet level predicted bleeding risk. Similarly, the largest published case series on this topic examined 4,729 paracenteses over a decade on a liver unit and found low rates of major bleeding (0.19%).9 Furthermore, preprocedure INR or platelet count did not correlate with bleeding risk. The authors did not report preprocedure transfusion rates, but they noted transfusions occurred only “occasionally.”
Subsequent observational studies have consistently revealed low bleeding risks even in settings of high coagulopathy prevalence. Grabau et al reviewed all large-volume paracenteses performed in a gastroenterology clinic over 7 years.10 In over 1,100 procedures, no major bleeding events occurred despite 27% of patients having INR greater than 2.0 and 54% having platelet counts less than 50,000/μL. Kurup et al examined bleeding risk among 304 procedures performed on patients with platelet counts less than 50,000/μL referred to radiology for ultrasound-guided paracentesis.7 Three bleeding events occurred, an overall event rate of 0.99%. They also found no association between preprocedure platelet count and bleeding risk.
In addition to observational data, one randomized, controlled trial evaluated the effects of FFP and platelet administration on bleeding risk among 60 patients with cirrhosis undergoing invasive procedures, including 19 paracenteses.6 Enrollment criteria included INR greater than 1.8 and/or platelet count less than 50,000/μL. One hundred percent of patients randomized to the usual care control arm received platelets or FFP as compared to 17% in the thromboelastography (TEG)–guided transfusion strategy arm. TEG assesses the viscoelastic properties of evolving clot formation in whole blood. Only one patient, a patient in the control arm who received FFP, developed procedure-related bleeding. Although receiving many fewer transfusions, the TEG-guided group experienced no bleeding.
In the presence of multiple studies demonstrating lack of benefit from FFP and platelet transfusion, guidelines published by the American Association for the Study of Liver Disease (AASLD), the American Gastroenterological Association (AGA), and the Society of Interventional Radiology (SIR) acknowledge the inaccuracy of platelet count and INR in predicting bleeding risk.12-14 Both AASLD and AGA recommend against routine transfusion of platelets and FFP prior to paracentesis.12,13 SIR guidelines from 2019 recommend against using an INR threshold for low-risk procedures like paracentesis and lowered their recommended platelet transfusion threshold from less than 50,000/μL to less than 20,000/μL.14 While we have limited safety data for paracentesis in patients with very low platelet counts, Kurup et al observed no bleeding events in the 19 patients in their cohort with platelets less than 20,000/μL undergoing ultrasound-guided paracentesis.7
In addition to lack of proven benefit, preprocedure transfusion exposes patients to objective risk. Transfusion-related acute lung injury and transfusion-associated circulatory overload develop at a rate of 0.48 and 3.8 per 100,000 components transfused, respectively.15 FFP transfusions also risk anaphylactic reactions with incidence ranging from 1:18,000 to 1:172,000.16 Platelets carry additional risk of bacterial contamination and resultant sepsis estimated at 1:5,000 to 1:8,000 per unit.17 Volume expansion from transfusions may contribute to portal hypertension and increase risk of variceal bleeding in decompensated liver disease.
Finally, FFP and platelet transfusions carry a significant cost. Rowley et al estimated eliminating preprocedure transfusions over 2 years and 3,116 paracenteses saved their institution $816,000.5 Furthermore, checking and correcting INR and thrombocytopenia can lead to procedural delay. Studies have demonstrated increased mortality from delaying paracentesis.18
WHEN IT IS HELPFUL
While most patients undergoing paracentesis have cirrhosis, patients without cirrhosis also undergo this procedure. Although several cited studies examined paracentesis among all-comers with ascites, our recommendations specifically apply to patients with ascites from cirrhosis.
Furthermore, although no paracentesis data in patients with severe coagulopathy (INR >2.5 or platelet count <20,000/μL) suggest periprocedural transfusion helps, we also lack data to prove it does not help.
Current recommendations from the AASLD suggest correcting coagulopathy in patients with clinically evident disseminated intravascular coagulation or hyperfibrinolysis prior to procedures.12 While no clear guidance related to paracentesis exists on when to assess for these entities, we recommend evaluating for them only when the clinical situation otherwise merits doing so and not solely for the purpose of screening prior to paracentesis. Measuring fibrinogen before paracentesis to predict bleeding risk is an emerging concept, but it cannot be routinely recommended at this time.13 Other factors that may play an important role in bleeding risk—ultrasound guidance, operator experience, and ability to avoid epigastric vessels and collateral veins—are beyond the scope of this article.
WHAT SHOULD BE DONE INSTEAD
Given that laboratory evaluations like INR and platelet count cannot predict which patients with cirrhosis will experience major bleeding complications after paracentesis and given that routinely transfusing FFP or platelets does not confer benefit and may cause serious harm, providers should avoid measuring INR or platelet count to prepare for paracentesis. Likewise, providers should avoid routinely transfusing FFP and platelets prior to paracentesis even in the presence of abnormal laboratory values because such values do not accurately reflect bleeding risk in patients with cirrhosis. Perform clinically indicated paracentesis without the delays that accompany unnecessary laboratory evaluations or transfusions.
RECOMMENDATIONS
Keep the following in mind with patients presenting with ascites from cirrhosis:
- Do not routinely use platelet count or INR when preparing for paracentesis, whether diagnostic or therapeutic, because no evidence-based “cutoff” for safe performance of paracentesis exists.
- Do not routinely transfuse FFP or platelets for prophylaxis prior to paracentesis in patients with cirrhosis.
- Reserve preprocedure transfusion of FFP or platelets for patients with disseminated intravascular coagulation, hyperfibrinolysis, or other indications for transfusion unrelated to procedural prophylaxis.
CONCLUSION
Case series representing diverse institutional experiences with thousands of patients consistently demonstrate that bleeding after paracentesis is rare (<1%), mortality from bleeding occurs very infrequently, and neither INR nor platelet counts predict bleeding risk during paracentesis in cirrhosis. These studies demonstrate that abandoning routine correction of coagulopathy does not lead to worse outcomes, can avoid potentially significant transfusion-related adverse events, and can save scarce resources.
Returning to our clinical scenario, the hospitalist should not transfuse FFP or platelets and should not delay the diagnostic paracentesis.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
Acknowledgments
The authors wish to acknowledge James Burton, MD, H Raymond Tahhan, MD, John Hess, MD, MPH, and Terry Gernsheimer, MD, for directing the authors to useful references cited in the manuscript.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 52-year-old man with alcoholic cirrhosis for tense ascites and altered mentation. Home medications include furosemide, spironolactone, lactulose, and rifaximin, but his family notes he ran out last week. Although afebrile and hemodynamically stable, the patient’s coagulopathy, with an international normalized ratio (INR) of 2.3, and thrombocytopenia, with a platelet count of 37,000/μL, worries the hospitalist. The hospitalist wonders whether to transfuse fresh frozen plasma (FFP) and platelets prior to diagnostic paracentesis to reduce the risk of procedural bleeding.
WHY ROUTINELY DOING THIS MIGHT SEEM HELPFUL
Many patients undergoing paracentesis have severe liver disease and present with both thrombocytopenia and elevated INRs. While platelet count and INR serve as surrogate markers for bleeding risk in many settings, clinicians often extrapolate this concept to patients with cirrhosis. Many hospitalists routinely check INR and platelet count and administer FFP and platelets prior to diagnostic or therapeutic paracentesis to mitigate procedure-related bleeding risk. Some medical resources recommend this practice,1 while case reports and personal experiences with bleeding in these patients create availability bias that influences perception of bleeding risk.2 One recent study of patients with decompensated cirrhosis presenting to a US tertiary care center found that, of those receiving large-volume paracentesis, 22.2% received prophylactic FFP and 17.3% received prophylactic platelets before paracentesis.3
WHY ROUTINELY DOING THIS IS NOT HELPFUL
Advances in our understanding of coagulation in cirrhosis demonstrate neither INR nor platelet count accurately predict bleeding risk in this population. Additionally, evidence demonstrates the overall safety of paracentesis in cirrhosis—even in the presence of high INR and thrombocytopenia—and the lack of benefit from prophylactic transfusions with FFP or platelets.
Substantial evidence in patients with cirrhosis demonstrates that changes in coagulation and platelet function confer a “balanced coagulopathy” in which patients oscillate between hyper- and hypocoagulable states. In a cirrhotic liver, hepatic synthetic dysfunction results in a complex milieu through reduced production and plasma concentrations of both pro- and anticoagulant factors that can lead to either bleeding or clotting.4 This “rebalancing” makes prothrombin time (PT) and INR unreliable indicators of bleeding or clotting risk. Similarly, in patients with cirrhosis, thrombocytopenia does not necessarily reflect impaired clotting ability. These patients experience an increase in production of von Willebrand Factor, which may compensate for low platelet counts by producing stronger platelet adhesion to collagen.4 Unfortunately, we currently lack a reliable test or risk score to assess true bleeding risk in patients with cirrhosis.
Observational studies support these laboratory findings. Large case series consistently demonstrate no association between INR or platelet counts and bleeding risk in either diagnostic or therapeutic paracentesis, including large-volume paracentesis (See Appendix for a list of recent representative studies).5-10 Moreover, prophylactic transfusion of FFP or platelets does not significantly reduce bleeding risk.
In a 1991 study by McVay et al, the researchers examined bleeding outcomes of 441 paracenteses performed on hospitalized patients.11 Among patients who did not receive FFP prior to paracentesis, only one required a transfusion for procedure-related bleeding, an event rate of 0.25%. This single patient had a normal platelet count and an elevated PT to the same extent as 261 others who underwent paracentesis without complication. In a pooled analysis that included 391 paracenteses and 207 thoracenteses, the authors concluded neither PT nor platelet level predicted bleeding risk. Similarly, the largest published case series on this topic examined 4,729 paracenteses over a decade on a liver unit and found low rates of major bleeding (0.19%).9 Furthermore, preprocedure INR or platelet count did not correlate with bleeding risk. The authors did not report preprocedure transfusion rates, but they noted transfusions occurred only “occasionally.”
Subsequent observational studies have consistently revealed low bleeding risks even in settings of high coagulopathy prevalence. Grabau et al reviewed all large-volume paracenteses performed in a gastroenterology clinic over 7 years.10 In over 1,100 procedures, no major bleeding events occurred despite 27% of patients having INR greater than 2.0 and 54% having platelet counts less than 50,000/μL. Kurup et al examined bleeding risk among 304 procedures performed on patients with platelet counts less than 50,000/μL referred to radiology for ultrasound-guided paracentesis.7 Three bleeding events occurred, an overall event rate of 0.99%. They also found no association between preprocedure platelet count and bleeding risk.
In addition to observational data, one randomized, controlled trial evaluated the effects of FFP and platelet administration on bleeding risk among 60 patients with cirrhosis undergoing invasive procedures, including 19 paracenteses.6 Enrollment criteria included INR greater than 1.8 and/or platelet count less than 50,000/μL. One hundred percent of patients randomized to the usual care control arm received platelets or FFP as compared to 17% in the thromboelastography (TEG)–guided transfusion strategy arm. TEG assesses the viscoelastic properties of evolving clot formation in whole blood. Only one patient, a patient in the control arm who received FFP, developed procedure-related bleeding. Although receiving many fewer transfusions, the TEG-guided group experienced no bleeding.
In the presence of multiple studies demonstrating lack of benefit from FFP and platelet transfusion, guidelines published by the American Association for the Study of Liver Disease (AASLD), the American Gastroenterological Association (AGA), and the Society of Interventional Radiology (SIR) acknowledge the inaccuracy of platelet count and INR in predicting bleeding risk.12-14 Both AASLD and AGA recommend against routine transfusion of platelets and FFP prior to paracentesis.12,13 SIR guidelines from 2019 recommend against using an INR threshold for low-risk procedures like paracentesis and lowered their recommended platelet transfusion threshold from less than 50,000/μL to less than 20,000/μL.14 While we have limited safety data for paracentesis in patients with very low platelet counts, Kurup et al observed no bleeding events in the 19 patients in their cohort with platelets less than 20,000/μL undergoing ultrasound-guided paracentesis.7
In addition to lack of proven benefit, preprocedure transfusion exposes patients to objective risk. Transfusion-related acute lung injury and transfusion-associated circulatory overload develop at a rate of 0.48 and 3.8 per 100,000 components transfused, respectively.15 FFP transfusions also risk anaphylactic reactions with incidence ranging from 1:18,000 to 1:172,000.16 Platelets carry additional risk of bacterial contamination and resultant sepsis estimated at 1:5,000 to 1:8,000 per unit.17 Volume expansion from transfusions may contribute to portal hypertension and increase risk of variceal bleeding in decompensated liver disease.
Finally, FFP and platelet transfusions carry a significant cost. Rowley et al estimated eliminating preprocedure transfusions over 2 years and 3,116 paracenteses saved their institution $816,000.5 Furthermore, checking and correcting INR and thrombocytopenia can lead to procedural delay. Studies have demonstrated increased mortality from delaying paracentesis.18
WHEN IT IS HELPFUL
While most patients undergoing paracentesis have cirrhosis, patients without cirrhosis also undergo this procedure. Although several cited studies examined paracentesis among all-comers with ascites, our recommendations specifically apply to patients with ascites from cirrhosis.
Furthermore, although no paracentesis data in patients with severe coagulopathy (INR >2.5 or platelet count <20,000/μL) suggest periprocedural transfusion helps, we also lack data to prove it does not help.
Current recommendations from the AASLD suggest correcting coagulopathy in patients with clinically evident disseminated intravascular coagulation or hyperfibrinolysis prior to procedures.12 While no clear guidance related to paracentesis exists on when to assess for these entities, we recommend evaluating for them only when the clinical situation otherwise merits doing so and not solely for the purpose of screening prior to paracentesis. Measuring fibrinogen before paracentesis to predict bleeding risk is an emerging concept, but it cannot be routinely recommended at this time.13 Other factors that may play an important role in bleeding risk—ultrasound guidance, operator experience, and ability to avoid epigastric vessels and collateral veins—are beyond the scope of this article.
WHAT SHOULD BE DONE INSTEAD
Given that laboratory evaluations like INR and platelet count cannot predict which patients with cirrhosis will experience major bleeding complications after paracentesis and given that routinely transfusing FFP or platelets does not confer benefit and may cause serious harm, providers should avoid measuring INR or platelet count to prepare for paracentesis. Likewise, providers should avoid routinely transfusing FFP and platelets prior to paracentesis even in the presence of abnormal laboratory values because such values do not accurately reflect bleeding risk in patients with cirrhosis. Perform clinically indicated paracentesis without the delays that accompany unnecessary laboratory evaluations or transfusions.
RECOMMENDATIONS
Keep the following in mind with patients presenting with ascites from cirrhosis:
- Do not routinely use platelet count or INR when preparing for paracentesis, whether diagnostic or therapeutic, because no evidence-based “cutoff” for safe performance of paracentesis exists.
- Do not routinely transfuse FFP or platelets for prophylaxis prior to paracentesis in patients with cirrhosis.
- Reserve preprocedure transfusion of FFP or platelets for patients with disseminated intravascular coagulation, hyperfibrinolysis, or other indications for transfusion unrelated to procedural prophylaxis.
CONCLUSION
Case series representing diverse institutional experiences with thousands of patients consistently demonstrate that bleeding after paracentesis is rare (<1%), mortality from bleeding occurs very infrequently, and neither INR nor platelet counts predict bleeding risk during paracentesis in cirrhosis. These studies demonstrate that abandoning routine correction of coagulopathy does not lead to worse outcomes, can avoid potentially significant transfusion-related adverse events, and can save scarce resources.
Returning to our clinical scenario, the hospitalist should not transfuse FFP or platelets and should not delay the diagnostic paracentesis.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
Acknowledgments
The authors wish to acknowledge James Burton, MD, H Raymond Tahhan, MD, John Hess, MD, MPH, and Terry Gernsheimer, MD, for directing the authors to useful references cited in the manuscript.
1. Shlamovitz G. Paracentesis. Medscape. 2018. Accessed April 16, 2019. https://emedicine.medscape.com/article/80944-overview
2. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124-1131. https://doi.org/10.1126/science.185.4157.1124
3. Barnhill M, Lee A, Montero A. Adherence rates to recommended guidelines for paracentesis in cirrhotic patients at a tertiary care center and associated complications. Am J Gastroenterol. 2017;112:S504.
4. Tripodi A, Primignani M, Mannucci PM, Caldwell SH. Changing concepts of cirrhotic coagulopathy. Am J Gastroenterol. 2017;112(2):274-281. https://doi.org/10.1038/ajg.2016.498
5. Rowley MW, Agarwal S, Seetharam AB, Hirsch KS. Real-time ultrasound-guided paracentesis by radiologists: near zero risk of hemorrhage without correction of coagulopathy. J Vasc Interv Radiol. 2019;30(2):259-264. https://doi.org/10.1016/j.jvir.2018.11.001
6. De Pietri L, Bianchini M, Montalti R, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology. 2016;63(2):566-573. https://doi.org/10.1002/hep.28148
7. Kurup AN, Lekah A, Reardon ST, et al. Bleeding rate for ultrasound-guided paracentesis in thrombocytopenic patients. J Ultrasound Med. 2015;34(10):1833-1838. https://doi.org/10.7863/ultra.14.10034
8. De Gottardi A, Thévenot T, Spahr L, et al. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin Gastroenterol Hepatol. 2009;7(8):906-909. https://doi.org/10.1016/j.cgh.2009.05.004
9. Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther. 2005;21(5):525-529. https://doi.org/10.1111/j.1365-2036.2005.02387.x
10. Grabau CM, Crago SF, Hoff LK, et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40(2):484-488. https://doi.org/10.1002/hep.20317
11. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. https://doi.org/10.1046/j.1537-2995.1991.31291142949.x
12. Runyon BA. AASLD Practice Guideline: Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012. The American Association for the Study of Liver Diseases; 2012. Accessed April 16, 2019. https://www.aasld.org/sites/default/files/2019-06/141020_Guideline_Ascites_4UFb_2015.pdf
13. O’Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA clinical practice update: coagulation in cirrhosis. Gastroenterology. 2019;157(1):34-43.e1. https://doi.org/10.1053/j.gastro.2019.03.070
14. Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions—part ii: recommendations. J Vasc Interv Radiol. 2019;30(8):1168-1184.e1. https://doi.org/10.1016/j.jvir.2019.04.017
15. Blumberg N, Heal JM, Gettins K, et al. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion. 2010;50(12):2738-2744. https://doi.org/10.1111/j.1537-2995.2010.02748.x
16. Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012; 52(Suppl 1):65S-79S. https://doi.org/10.1111/j.1537-2995.2012.03663.x
17. Kleinman S, Reed W, Stassinopoulos A. A patient-oriented risk-benefit analysis of pathogen-inactivated blood components: application to apheresis platelets in the United States. Transfusion. 2013;53(7):1603-1618. https://doi.org/10.1111/j.1537-2995.2012.03928.x
18. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. https://doi.org/10.1038/ajg.2014.212
1. Shlamovitz G. Paracentesis. Medscape. 2018. Accessed April 16, 2019. https://emedicine.medscape.com/article/80944-overview
2. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124-1131. https://doi.org/10.1126/science.185.4157.1124
3. Barnhill M, Lee A, Montero A. Adherence rates to recommended guidelines for paracentesis in cirrhotic patients at a tertiary care center and associated complications. Am J Gastroenterol. 2017;112:S504.
4. Tripodi A, Primignani M, Mannucci PM, Caldwell SH. Changing concepts of cirrhotic coagulopathy. Am J Gastroenterol. 2017;112(2):274-281. https://doi.org/10.1038/ajg.2016.498
5. Rowley MW, Agarwal S, Seetharam AB, Hirsch KS. Real-time ultrasound-guided paracentesis by radiologists: near zero risk of hemorrhage without correction of coagulopathy. J Vasc Interv Radiol. 2019;30(2):259-264. https://doi.org/10.1016/j.jvir.2018.11.001
6. De Pietri L, Bianchini M, Montalti R, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology. 2016;63(2):566-573. https://doi.org/10.1002/hep.28148
7. Kurup AN, Lekah A, Reardon ST, et al. Bleeding rate for ultrasound-guided paracentesis in thrombocytopenic patients. J Ultrasound Med. 2015;34(10):1833-1838. https://doi.org/10.7863/ultra.14.10034
8. De Gottardi A, Thévenot T, Spahr L, et al. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin Gastroenterol Hepatol. 2009;7(8):906-909. https://doi.org/10.1016/j.cgh.2009.05.004
9. Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther. 2005;21(5):525-529. https://doi.org/10.1111/j.1365-2036.2005.02387.x
10. Grabau CM, Crago SF, Hoff LK, et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40(2):484-488. https://doi.org/10.1002/hep.20317
11. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. https://doi.org/10.1046/j.1537-2995.1991.31291142949.x
12. Runyon BA. AASLD Practice Guideline: Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012. The American Association for the Study of Liver Diseases; 2012. Accessed April 16, 2019. https://www.aasld.org/sites/default/files/2019-06/141020_Guideline_Ascites_4UFb_2015.pdf
13. O’Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA clinical practice update: coagulation in cirrhosis. Gastroenterology. 2019;157(1):34-43.e1. https://doi.org/10.1053/j.gastro.2019.03.070
14. Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions—part ii: recommendations. J Vasc Interv Radiol. 2019;30(8):1168-1184.e1. https://doi.org/10.1016/j.jvir.2019.04.017
15. Blumberg N, Heal JM, Gettins K, et al. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion. 2010;50(12):2738-2744. https://doi.org/10.1111/j.1537-2995.2010.02748.x
16. Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012; 52(Suppl 1):65S-79S. https://doi.org/10.1111/j.1537-2995.2012.03663.x
17. Kleinman S, Reed W, Stassinopoulos A. A patient-oriented risk-benefit analysis of pathogen-inactivated blood components: application to apheresis platelets in the United States. Transfusion. 2013;53(7):1603-1618. https://doi.org/10.1111/j.1537-2995.2012.03928.x
18. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. https://doi.org/10.1038/ajg.2014.212
© 2020 Society of Hospital Medicine