User login
HM16 Session Analysis: ICD-10 Coding Tips
Presenter: Aziz Ansari, DO, FHM
Summary: With the implementation of ICD-10, correct and specific documentation to ensure proper patient diagnosis categorization has become increasingly important. Hospitalists are urged to understand the impact CDI has on quality and reimbursement.
Quality Impact: Documentation has a direct impact on quality reporting for mortality and complication rates, risk of mortality, as well as severity of illness. Documenting present on admission (POA) also directly impacts the hospital-acquired condition (HAC) classifications.
Reimbursement Impact: Documentation has a direct impact on expected length of stay, case mix index (CMI), cost reporting, and appropriate hospital reimbursement.
HM Takeaways:
- Be clear and specific.
- Document principle diagnosis and secondary diagnoses, and their associated interactions, are critically important.
- Ensure all diagnoses are a part of the discharge summary.
- Avoid saying “History of.”
- It’s OK to document “possible,” “probably,” “likely,” or “suspected.”
- Document “why” the patient has the diagnosis.
- List all differentials, and identify if ruled in or ruled out.
- Indicate acuity, even if obvious.
This presenter also reviewed common CDI opportunities in hospital medicine.
Note: This discussion was specific to the needs of the hospital patient diagnosis and billing, and not related to physician billing and CPT codes.
Presenter: Aziz Ansari, DO, FHM
Summary: With the implementation of ICD-10, correct and specific documentation to ensure proper patient diagnosis categorization has become increasingly important. Hospitalists are urged to understand the impact CDI has on quality and reimbursement.
Quality Impact: Documentation has a direct impact on quality reporting for mortality and complication rates, risk of mortality, as well as severity of illness. Documenting present on admission (POA) also directly impacts the hospital-acquired condition (HAC) classifications.
Reimbursement Impact: Documentation has a direct impact on expected length of stay, case mix index (CMI), cost reporting, and appropriate hospital reimbursement.
HM Takeaways:
- Be clear and specific.
- Document principle diagnosis and secondary diagnoses, and their associated interactions, are critically important.
- Ensure all diagnoses are a part of the discharge summary.
- Avoid saying “History of.”
- It’s OK to document “possible,” “probably,” “likely,” or “suspected.”
- Document “why” the patient has the diagnosis.
- List all differentials, and identify if ruled in or ruled out.
- Indicate acuity, even if obvious.
This presenter also reviewed common CDI opportunities in hospital medicine.
Note: This discussion was specific to the needs of the hospital patient diagnosis and billing, and not related to physician billing and CPT codes.
Presenter: Aziz Ansari, DO, FHM
Summary: With the implementation of ICD-10, correct and specific documentation to ensure proper patient diagnosis categorization has become increasingly important. Hospitalists are urged to understand the impact CDI has on quality and reimbursement.
Quality Impact: Documentation has a direct impact on quality reporting for mortality and complication rates, risk of mortality, as well as severity of illness. Documenting present on admission (POA) also directly impacts the hospital-acquired condition (HAC) classifications.
Reimbursement Impact: Documentation has a direct impact on expected length of stay, case mix index (CMI), cost reporting, and appropriate hospital reimbursement.
HM Takeaways:
- Be clear and specific.
- Document principle diagnosis and secondary diagnoses, and their associated interactions, are critically important.
- Ensure all diagnoses are a part of the discharge summary.
- Avoid saying “History of.”
- It’s OK to document “possible,” “probably,” “likely,” or “suspected.”
- Document “why” the patient has the diagnosis.
- List all differentials, and identify if ruled in or ruled out.
- Indicate acuity, even if obvious.
This presenter also reviewed common CDI opportunities in hospital medicine.
Note: This discussion was specific to the needs of the hospital patient diagnosis and billing, and not related to physician billing and CPT codes.
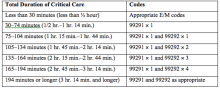
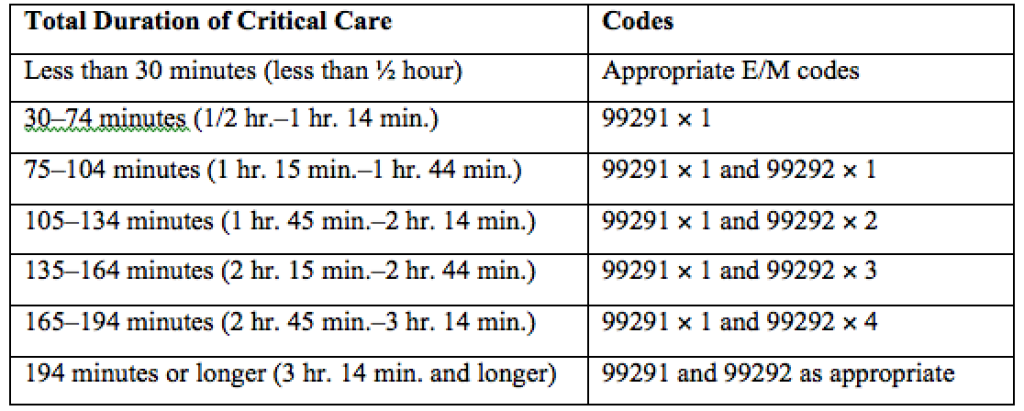
Key Elements of Critical Care
Code 99291 is used for critical care, evaluation, and management of the critically ill or critically injured patient, first 30–74 minutes.1 It is to be reported only once per day per physician or group member of the same specialty.
Code 99292 is for critical care, evaluation, and management of the critically ill or critically injured patient, each additional 30 minutes. It is to be listed separately in addition to the code for primary service.1 Code 99292 is categorized as an add-on code. It must be reported on the same invoice as its primary code, 99291. Multiple units of code 99292 can be reported per day per physician/group.
Despite the increased resources and references for critical care billing, critical care reporting issues persist. Medicare data analysis continues to identify 99291 as high risk for claim payment errors, perpetuating prepayment claim edits for outlier utilization and location discrepancies (i.e., settings other than inpatient hospital, outpatient hospital, or the emergency department). 2,3,4
Bolster your documentation with these three key elements.
Critical Illness, Injury Management
Current Procedural Terminology (CPT) and the Centers for Medicare & Medicaid Services (CMS) define “critical illness or injury” as a condition that acutely impairs one or more vital organ systems such that there is a high probability of imminent or life-threatening deterioration in the patient’s condition (e.g., central nervous system failure; circulatory failure; shock; renal, hepatic, metabolic, and/or respiratory failure).5
Hospitalists providing care to the critically ill patient must perform highly complex decision making and interventions of high intensity that are required to prevent the patient’s inevitable decline. CMS further elaborates that “the patient shall be critically ill or injured at the time of the physician’s visit.”6 This is to ensure that hospitalists and other specialists support the medical necessity of the service and do not continue to report critical care codes on days after the patient has become stable and improved.
Consider the following scenarios:
CMS examples of patients whose medical condition may warrant critical care services (99291, 99292):6
- An 81-year-old male patient is admitted to the ICU following abdominal aortic aneurysm resection. Two days after surgery, he requires fluids and pressors to maintain adequate perfusion and arterial pressures. He remains ventilator dependent.
- A 67-year-old female patient is three days post mitral valve repair. She develops petechiae, hypotension, and hypoxia requiring respiratory and circulatory support.
- A 70-year-old admitted for right lower lobe pneumococcal pneumonia with a history of COPD becomes hypoxic and hypotensive two days after admission.
- A 68-year-old admitted for an acute anterior wall myocardial infarction continues to have symptomatic ventricular tachycardia that is marginally responsive to antiarrhythmic therapy.
CMS examples of patients who may not satisfy Medicare medical necessity criteria, or do not meet critical care criteria, or who do not have a critical care illness or injury and, therefore, are not eligible for critical care payment but may be reported using another appropriate hospital care code, such as subsequent hospital care codes (99231–99233), initial hospital care codes (99221–99223), or hospital consultation codes (99251–99255) when applicable:1,6
- Patients admitted to a critical care unit because no other hospital beds were available;
- Patients admitted to a critical care unit for close nursing observation and/or frequent monitoring of vital signs (e.g., drug toxicity or overdose);
- Patients admitted to a critical care unit because hospital rules require certain treatments (e.g., insulin infusions) to be administered in the critical care unit; and
- Patients receiving only care of a chronic illness in absence of care for a critical illness (e.g., daily management of a chronic ventilator patient, management of or care related to dialysis for end-stage renal disease). Services considered palliative in nature as this type of care do not meet the definition of critical care services.7
Concurrent Care
Critically ill patients often require the care of hospitalists and other specialists throughout the course of treatment. Payors are sensitive to the multiple hours billed by multiple providers for a single patient on a given day. Claim logic provides an automated response to only allow reimbursement for 99291 once per day when reported by physicians of the same group and specialty.8 Physicians of different specialties can separately report critical care hours as long as they are caring for a condition that meets the definition of critical care.
The CMS example of this: A dermatologist evaluates and treats a rash on an ICU patient who is maintained on a ventilator and nitroglycerine infusion that are being managed by an intensivist. The dermatologist should not report a service for critical care.6
Similarly for hospitalists, if an intensivist is taking care of the critical condition and there is nothing more for the hospitalist to add to the plan of care for the critical condition, critical care services may not be justified.
When different specialists are reporting critical care on the same day, it is imperative for the documentation to demonstrate that care is not duplicative of any other provider’s care (i.e., identify management of different conditions or revising elements of the plan). The care cannot overlap the same time period of any other physician reporting critical care services.
Calculating Time
Critical care time constitutes bedside time and time spent on the patient’s unit/floor where the physician is immediately available to the patient (see Table 1). Certain labs, diagnostic studies, and procedures are considered inherent to critical care services and are not reported separately on the claim form: cardiac output measurements (93561, 93562); chest X-rays (71010, 71015, 71020); pulse oximetry (94760, 94761, 94762); blood gases and interpretation of data stored in computers, such as ECGs, blood pressures, and hematologic data (99090); gastric intubation (43752, 43753); temporary transcutaneous pacing (92953); ventilation management (94002–94004, 94660, 94662); and vascular access procedures (36000, 36410, 36415, 36591, 36600).1
Instead, physician time associated with the performance and/or interpretation of these services is toward the cumulative critical care time of the day. Services or procedures that are considered separately billable (e.g., central line placement, intubation, CPR) cannot contribute to critical care time.
When separately billable procedures are performed by the same provider/specialty group on the same day as critical care, physicians should make a notation in the medical record indicating the non-overlapping service times (e.g., “central line insertion is not included as critical care time”). This may assist with securing reimbursement when the payor requests the documentation for each reported claim item.
Activities on the floor/unit that do not directly contribute to patient care or management (e.g., review of literature, teaching rounds) cannot be counted toward critical care time. Do not count time associated with indirect care provided outside of the patient’s unit/floor (e.g., reviewing data or calling the family from the office) toward critical care time.
Family discussions can be counted toward critical care time but must take place at bedside or on the patient’s unit/floor. The patient must participate in the discussion unless medically unable or clinically incompetent to participate. If unable to participate, a notation in the chart must delineate the patient’s inability to participate and the reason.
Credited time can only involve obtaining a medical history and/or discussing treatment options or limitation(s) of treatment. The conversation must bear directly on patient management.1,7 Do not count time associated with providing periodic condition updates to the family, answering questions about the patient’s condition that are unrelated to decision making, or counseling the family during their grief process. If the conversation must take place via phone, it may be counted toward critical care time if the physician is calling from the patient’s unit/floor and the conversation involves the same criterion identified for face-to-face family meetings.10
Physicians should keep track of their critical care time throughout the day. Since critical care time is a cumulative service, each entry should include the total time that critical care services were provided (e.g., 45 minutes).10 Some payors may still impose the notation of “start-and-stop time” per encounter (e.g., 2–2:50 a.m.).
Same-specialty physicians (i.e., two hospitalists from the same group practice) may require separate claims. The initial critical care hour (99291) must be met by a single physician. Medically necessary critical care time beyond the first hour (99292) may be met individually by the same physician or collectively with another physician from the same group. The physician performing the additional time, beyond the first hour, reports the appropriate units of 99292 (see Table 1) under the corresponding NPI.11
CMS has issued instructions for contractors to recognize this atypical reporting method. However, non-Medicare payors may not recognize this newer reporting method and maintain that the cumulative service (by the same-specialty physician in the same provider group) should be reported under one physician name. Be sure to query the payors for appropriate reporting methods. TH
References
- Abraham M, Ahlman J, Boudreau A, Connelly J, Crosslin, R. Current Procedural Terminology 2015 Professional Edition. Chicago: American Medical Association Press; 2014. 23-25.
- Widespread prepayment targeted review notification—CPT 99291. Cahaba website. Available at: www.cahabagba.com/news/widespread-prepayment-targeted-review-notification-part-b/. Accessed December 17, 2015.
- Critical care CPT 99291 widespread prepayment targeted review results. Cahaba website. Available at: https://www.cahabagba.com/news/critical-care-cpt-99291-widespread-prepayment-targeted-review-results-2/. Accessed December 17, 2015.
- Prepayment edit of evaluation and management (E/M) code 99291. First Coast Service Options, Inc. website. Available at: medicare.fcso.com/Publications_B/2013/251608.pdf. Accessed December 17, 2015.
- Medicare claims processing manual: chapter 12, section 30.6.12A. Centers for Medicare & Medicaid Services website. Available at: www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c12.pdf. Accessed December 17, 2015.
- Medicare claims processing manual: chapter 12, section 30.6.12B. Centers for Medicare & Medicaid Services website. Available at: www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c12.pdf. Accessed December 17, 2015.
- Critical care fact sheet. CGS Administrators, LLC website. Available at: www.cgsmedicare.com/partb/mr/pdf/critical_care_fact_sheet.pdf. Accessed December 17, 2015.
- Same day same service policy. United Healthcare website. Available at: www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Main%20Menu/Tools%20&%20Resources/Policies%20and%20Protocols/Medicare%20Advantage%20Reimbursement%20Policies/S/SameDaySameService.pdf. Accessed December 17, 2015.
- Medicare claims processing manual: chapter 12, section 30.6.12G. Centers for Medicare & Medicaid Services website. Available at: www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c12.pdf. Accessed December 17, 2015.
- Medicare claims processing manual: chapter 12, section 30.6.12E. Centers for Medicare & Medicaid Services website. Available at: www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c12.pdf. Accessed December 17, 2015.
- Medicare claims processing manual: chapter 12, section 30.6.12I. Centers for Medicare & Medicaid Services website. Available at: www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c12.pdf. Accessed December 17, 2015.
Code 99291 is used for critical care, evaluation, and management of the critically ill or critically injured patient, first 30–74 minutes.1 It is to be reported only once per day per physician or group member of the same specialty.
Code 99292 is for critical care, evaluation, and management of the critically ill or critically injured patient, each additional 30 minutes. It is to be listed separately in addition to the code for primary service.1 Code 99292 is categorized as an add-on code. It must be reported on the same invoice as its primary code, 99291. Multiple units of code 99292 can be reported per day per physician/group.
Despite the increased resources and references for critical care billing, critical care reporting issues persist. Medicare data analysis continues to identify 99291 as high risk for claim payment errors, perpetuating prepayment claim edits for outlier utilization and location discrepancies (i.e., settings other than inpatient hospital, outpatient hospital, or the emergency department). 2,3,4
Bolster your documentation with these three key elements.
Critical Illness, Injury Management
Current Procedural Terminology (CPT) and the Centers for Medicare & Medicaid Services (CMS) define “critical illness or injury” as a condition that acutely impairs one or more vital organ systems such that there is a high probability of imminent or life-threatening deterioration in the patient’s condition (e.g., central nervous system failure; circulatory failure; shock; renal, hepatic, metabolic, and/or respiratory failure).5
Hospitalists providing care to the critically ill patient must perform highly complex decision making and interventions of high intensity that are required to prevent the patient’s inevitable decline. CMS further elaborates that “the patient shall be critically ill or injured at the time of the physician’s visit.”6 This is to ensure that hospitalists and other specialists support the medical necessity of the service and do not continue to report critical care codes on days after the patient has become stable and improved.
Consider the following scenarios:
CMS examples of patients whose medical condition may warrant critical care services (99291, 99292):6
- An 81-year-old male patient is admitted to the ICU following abdominal aortic aneurysm resection. Two days after surgery, he requires fluids and pressors to maintain adequate perfusion and arterial pressures. He remains ventilator dependent.
- A 67-year-old female patient is three days post mitral valve repair. She develops petechiae, hypotension, and hypoxia requiring respiratory and circulatory support.
- A 70-year-old admitted for right lower lobe pneumococcal pneumonia with a history of COPD becomes hypoxic and hypotensive two days after admission.
- A 68-year-old admitted for an acute anterior wall myocardial infarction continues to have symptomatic ventricular tachycardia that is marginally responsive to antiarrhythmic therapy.
CMS examples of patients who may not satisfy Medicare medical necessity criteria, or do not meet critical care criteria, or who do not have a critical care illness or injury and, therefore, are not eligible for critical care payment but may be reported using another appropriate hospital care code, such as subsequent hospital care codes (99231–99233), initial hospital care codes (99221–99223), or hospital consultation codes (99251–99255) when applicable:1,6
- Patients admitted to a critical care unit because no other hospital beds were available;
- Patients admitted to a critical care unit for close nursing observation and/or frequent monitoring of vital signs (e.g., drug toxicity or overdose);
- Patients admitted to a critical care unit because hospital rules require certain treatments (e.g., insulin infusions) to be administered in the critical care unit; and
- Patients receiving only care of a chronic illness in absence of care for a critical illness (e.g., daily management of a chronic ventilator patient, management of or care related to dialysis for end-stage renal disease). Services considered palliative in nature as this type of care do not meet the definition of critical care services.7
Concurrent Care
Critically ill patients often require the care of hospitalists and other specialists throughout the course of treatment. Payors are sensitive to the multiple hours billed by multiple providers for a single patient on a given day. Claim logic provides an automated response to only allow reimbursement for 99291 once per day when reported by physicians of the same group and specialty.8 Physicians of different specialties can separately report critical care hours as long as they are caring for a condition that meets the definition of critical care.
The CMS example of this: A dermatologist evaluates and treats a rash on an ICU patient who is maintained on a ventilator and nitroglycerine infusion that are being managed by an intensivist. The dermatologist should not report a service for critical care.6
Similarly for hospitalists, if an intensivist is taking care of the critical condition and there is nothing more for the hospitalist to add to the plan of care for the critical condition, critical care services may not be justified.
When different specialists are reporting critical care on the same day, it is imperative for the documentation to demonstrate that care is not duplicative of any other provider’s care (i.e., identify management of different conditions or revising elements of the plan). The care cannot overlap the same time period of any other physician reporting critical care services.
Calculating Time
Critical care time constitutes bedside time and time spent on the patient’s unit/floor where the physician is immediately available to the patient (see Table 1). Certain labs, diagnostic studies, and procedures are considered inherent to critical care services and are not reported separately on the claim form: cardiac output measurements (93561, 93562); chest X-rays (71010, 71015, 71020); pulse oximetry (94760, 94761, 94762); blood gases and interpretation of data stored in computers, such as ECGs, blood pressures, and hematologic data (99090); gastric intubation (43752, 43753); temporary transcutaneous pacing (92953); ventilation management (94002–94004, 94660, 94662); and vascular access procedures (36000, 36410, 36415, 36591, 36600).1
Instead, physician time associated with the performance and/or interpretation of these services is toward the cumulative critical care time of the day. Services or procedures that are considered separately billable (e.g., central line placement, intubation, CPR) cannot contribute to critical care time.
When separately billable procedures are performed by the same provider/specialty group on the same day as critical care, physicians should make a notation in the medical record indicating the non-overlapping service times (e.g., “central line insertion is not included as critical care time”). This may assist with securing reimbursement when the payor requests the documentation for each reported claim item.
Activities on the floor/unit that do not directly contribute to patient care or management (e.g., review of literature, teaching rounds) cannot be counted toward critical care time. Do not count time associated with indirect care provided outside of the patient’s unit/floor (e.g., reviewing data or calling the family from the office) toward critical care time.
Family discussions can be counted toward critical care time but must take place at bedside or on the patient’s unit/floor. The patient must participate in the discussion unless medically unable or clinically incompetent to participate. If unable to participate, a notation in the chart must delineate the patient’s inability to participate and the reason.
Credited time can only involve obtaining a medical history and/or discussing treatment options or limitation(s) of treatment. The conversation must bear directly on patient management.1,7 Do not count time associated with providing periodic condition updates to the family, answering questions about the patient’s condition that are unrelated to decision making, or counseling the family during their grief process. If the conversation must take place via phone, it may be counted toward critical care time if the physician is calling from the patient’s unit/floor and the conversation involves the same criterion identified for face-to-face family meetings.10
Physicians should keep track of their critical care time throughout the day. Since critical care time is a cumulative service, each entry should include the total time that critical care services were provided (e.g., 45 minutes).10 Some payors may still impose the notation of “start-and-stop time” per encounter (e.g., 2–2:50 a.m.).
Same-specialty physicians (i.e., two hospitalists from the same group practice) may require separate claims. The initial critical care hour (99291) must be met by a single physician. Medically necessary critical care time beyond the first hour (99292) may be met individually by the same physician or collectively with another physician from the same group. The physician performing the additional time, beyond the first hour, reports the appropriate units of 99292 (see Table 1) under the corresponding NPI.11
CMS has issued instructions for contractors to recognize this atypical reporting method. However, non-Medicare payors may not recognize this newer reporting method and maintain that the cumulative service (by the same-specialty physician in the same provider group) should be reported under one physician name. Be sure to query the payors for appropriate reporting methods. TH
References
- Abraham M, Ahlman J, Boudreau A, Connelly J, Crosslin, R. Current Procedural Terminology 2015 Professional Edition. Chicago: American Medical Association Press; 2014. 23-25.
- Widespread prepayment targeted review notification—CPT 99291. Cahaba website. Available at: www.cahabagba.com/news/widespread-prepayment-targeted-review-notification-part-b/. Accessed December 17, 2015.
- Critical care CPT 99291 widespread prepayment targeted review results. Cahaba website. Available at: https://www.cahabagba.com/news/critical-care-cpt-99291-widespread-prepayment-targeted-review-results-2/. Accessed December 17, 2015.
- Prepayment edit of evaluation and management (E/M) code 99291. First Coast Service Options, Inc. website. Available at: medicare.fcso.com/Publications_B/2013/251608.pdf. Accessed December 17, 2015.
- Medicare claims processing manual: chapter 12, section 30.6.12A. Centers for Medicare & Medicaid Services website. Available at: www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c12.pdf. Accessed December 17, 2015.
- Medicare claims processing manual: chapter 12, section 30.6.12B. Centers for Medicare & Medicaid Services website. Available at: www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c12.pdf. Accessed December 17, 2015.
- Critical care fact sheet. CGS Administrators, LLC website. Available at: www.cgsmedicare.com/partb/mr/pdf/critical_care_fact_sheet.pdf. Accessed December 17, 2015.
- Same day same service policy. United Healthcare website. Available at: www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Main%20Menu/Tools%20&%20Resources/Policies%20and%20Protocols/Medicare%20Advantage%20Reimbursement%20Policies/S/SameDaySameService.pdf. Accessed December 17, 2015.
- Medicare claims processing manual: chapter 12, section 30.6.12G. Centers for Medicare & Medicaid Services website. Available at: www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c12.pdf. Accessed December 17, 2015.
- Medicare claims processing manual: chapter 12, section 30.6.12E. Centers for Medicare & Medicaid Services website. Available at: www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c12.pdf. Accessed December 17, 2015.
- Medicare claims processing manual: chapter 12, section 30.6.12I. Centers for Medicare & Medicaid Services website. Available at: www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c12.pdf. Accessed December 17, 2015.
Code 99291 is used for critical care, evaluation, and management of the critically ill or critically injured patient, first 30–74 minutes.1 It is to be reported only once per day per physician or group member of the same specialty.
Code 99292 is for critical care, evaluation, and management of the critically ill or critically injured patient, each additional 30 minutes. It is to be listed separately in addition to the code for primary service.1 Code 99292 is categorized as an add-on code. It must be reported on the same invoice as its primary code, 99291. Multiple units of code 99292 can be reported per day per physician/group.
Despite the increased resources and references for critical care billing, critical care reporting issues persist. Medicare data analysis continues to identify 99291 as high risk for claim payment errors, perpetuating prepayment claim edits for outlier utilization and location discrepancies (i.e., settings other than inpatient hospital, outpatient hospital, or the emergency department). 2,3,4
Bolster your documentation with these three key elements.
Critical Illness, Injury Management
Current Procedural Terminology (CPT) and the Centers for Medicare & Medicaid Services (CMS) define “critical illness or injury” as a condition that acutely impairs one or more vital organ systems such that there is a high probability of imminent or life-threatening deterioration in the patient’s condition (e.g., central nervous system failure; circulatory failure; shock; renal, hepatic, metabolic, and/or respiratory failure).5
Hospitalists providing care to the critically ill patient must perform highly complex decision making and interventions of high intensity that are required to prevent the patient’s inevitable decline. CMS further elaborates that “the patient shall be critically ill or injured at the time of the physician’s visit.”6 This is to ensure that hospitalists and other specialists support the medical necessity of the service and do not continue to report critical care codes on days after the patient has become stable and improved.
Consider the following scenarios:
CMS examples of patients whose medical condition may warrant critical care services (99291, 99292):6
- An 81-year-old male patient is admitted to the ICU following abdominal aortic aneurysm resection. Two days after surgery, he requires fluids and pressors to maintain adequate perfusion and arterial pressures. He remains ventilator dependent.
- A 67-year-old female patient is three days post mitral valve repair. She develops petechiae, hypotension, and hypoxia requiring respiratory and circulatory support.
- A 70-year-old admitted for right lower lobe pneumococcal pneumonia with a history of COPD becomes hypoxic and hypotensive two days after admission.
- A 68-year-old admitted for an acute anterior wall myocardial infarction continues to have symptomatic ventricular tachycardia that is marginally responsive to antiarrhythmic therapy.
CMS examples of patients who may not satisfy Medicare medical necessity criteria, or do not meet critical care criteria, or who do not have a critical care illness or injury and, therefore, are not eligible for critical care payment but may be reported using another appropriate hospital care code, such as subsequent hospital care codes (99231–99233), initial hospital care codes (99221–99223), or hospital consultation codes (99251–99255) when applicable:1,6
- Patients admitted to a critical care unit because no other hospital beds were available;
- Patients admitted to a critical care unit for close nursing observation and/or frequent monitoring of vital signs (e.g., drug toxicity or overdose);
- Patients admitted to a critical care unit because hospital rules require certain treatments (e.g., insulin infusions) to be administered in the critical care unit; and
- Patients receiving only care of a chronic illness in absence of care for a critical illness (e.g., daily management of a chronic ventilator patient, management of or care related to dialysis for end-stage renal disease). Services considered palliative in nature as this type of care do not meet the definition of critical care services.7
Concurrent Care
Critically ill patients often require the care of hospitalists and other specialists throughout the course of treatment. Payors are sensitive to the multiple hours billed by multiple providers for a single patient on a given day. Claim logic provides an automated response to only allow reimbursement for 99291 once per day when reported by physicians of the same group and specialty.8 Physicians of different specialties can separately report critical care hours as long as they are caring for a condition that meets the definition of critical care.
The CMS example of this: A dermatologist evaluates and treats a rash on an ICU patient who is maintained on a ventilator and nitroglycerine infusion that are being managed by an intensivist. The dermatologist should not report a service for critical care.6
Similarly for hospitalists, if an intensivist is taking care of the critical condition and there is nothing more for the hospitalist to add to the plan of care for the critical condition, critical care services may not be justified.
When different specialists are reporting critical care on the same day, it is imperative for the documentation to demonstrate that care is not duplicative of any other provider’s care (i.e., identify management of different conditions or revising elements of the plan). The care cannot overlap the same time period of any other physician reporting critical care services.
Calculating Time
Critical care time constitutes bedside time and time spent on the patient’s unit/floor where the physician is immediately available to the patient (see Table 1). Certain labs, diagnostic studies, and procedures are considered inherent to critical care services and are not reported separately on the claim form: cardiac output measurements (93561, 93562); chest X-rays (71010, 71015, 71020); pulse oximetry (94760, 94761, 94762); blood gases and interpretation of data stored in computers, such as ECGs, blood pressures, and hematologic data (99090); gastric intubation (43752, 43753); temporary transcutaneous pacing (92953); ventilation management (94002–94004, 94660, 94662); and vascular access procedures (36000, 36410, 36415, 36591, 36600).1
Instead, physician time associated with the performance and/or interpretation of these services is toward the cumulative critical care time of the day. Services or procedures that are considered separately billable (e.g., central line placement, intubation, CPR) cannot contribute to critical care time.
When separately billable procedures are performed by the same provider/specialty group on the same day as critical care, physicians should make a notation in the medical record indicating the non-overlapping service times (e.g., “central line insertion is not included as critical care time”). This may assist with securing reimbursement when the payor requests the documentation for each reported claim item.
Activities on the floor/unit that do not directly contribute to patient care or management (e.g., review of literature, teaching rounds) cannot be counted toward critical care time. Do not count time associated with indirect care provided outside of the patient’s unit/floor (e.g., reviewing data or calling the family from the office) toward critical care time.
Family discussions can be counted toward critical care time but must take place at bedside or on the patient’s unit/floor. The patient must participate in the discussion unless medically unable or clinically incompetent to participate. If unable to participate, a notation in the chart must delineate the patient’s inability to participate and the reason.
Credited time can only involve obtaining a medical history and/or discussing treatment options or limitation(s) of treatment. The conversation must bear directly on patient management.1,7 Do not count time associated with providing periodic condition updates to the family, answering questions about the patient’s condition that are unrelated to decision making, or counseling the family during their grief process. If the conversation must take place via phone, it may be counted toward critical care time if the physician is calling from the patient’s unit/floor and the conversation involves the same criterion identified for face-to-face family meetings.10
Physicians should keep track of their critical care time throughout the day. Since critical care time is a cumulative service, each entry should include the total time that critical care services were provided (e.g., 45 minutes).10 Some payors may still impose the notation of “start-and-stop time” per encounter (e.g., 2–2:50 a.m.).
Same-specialty physicians (i.e., two hospitalists from the same group practice) may require separate claims. The initial critical care hour (99291) must be met by a single physician. Medically necessary critical care time beyond the first hour (99292) may be met individually by the same physician or collectively with another physician from the same group. The physician performing the additional time, beyond the first hour, reports the appropriate units of 99292 (see Table 1) under the corresponding NPI.11
CMS has issued instructions for contractors to recognize this atypical reporting method. However, non-Medicare payors may not recognize this newer reporting method and maintain that the cumulative service (by the same-specialty physician in the same provider group) should be reported under one physician name. Be sure to query the payors for appropriate reporting methods. TH
References
- Abraham M, Ahlman J, Boudreau A, Connelly J, Crosslin, R. Current Procedural Terminology 2015 Professional Edition. Chicago: American Medical Association Press; 2014. 23-25.
- Widespread prepayment targeted review notification—CPT 99291. Cahaba website. Available at: www.cahabagba.com/news/widespread-prepayment-targeted-review-notification-part-b/. Accessed December 17, 2015.
- Critical care CPT 99291 widespread prepayment targeted review results. Cahaba website. Available at: https://www.cahabagba.com/news/critical-care-cpt-99291-widespread-prepayment-targeted-review-results-2/. Accessed December 17, 2015.
- Prepayment edit of evaluation and management (E/M) code 99291. First Coast Service Options, Inc. website. Available at: medicare.fcso.com/Publications_B/2013/251608.pdf. Accessed December 17, 2015.
- Medicare claims processing manual: chapter 12, section 30.6.12A. Centers for Medicare & Medicaid Services website. Available at: www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c12.pdf. Accessed December 17, 2015.
- Medicare claims processing manual: chapter 12, section 30.6.12B. Centers for Medicare & Medicaid Services website. Available at: www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c12.pdf. Accessed December 17, 2015.
- Critical care fact sheet. CGS Administrators, LLC website. Available at: www.cgsmedicare.com/partb/mr/pdf/critical_care_fact_sheet.pdf. Accessed December 17, 2015.
- Same day same service policy. United Healthcare website. Available at: www.unitedhealthcareonline.com/ccmcontent/ProviderII/UHC/en-US/Main%20Menu/Tools%20&%20Resources/Policies%20and%20Protocols/Medicare%20Advantage%20Reimbursement%20Policies/S/SameDaySameService.pdf. Accessed December 17, 2015.
- Medicare claims processing manual: chapter 12, section 30.6.12G. Centers for Medicare & Medicaid Services website. Available at: www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c12.pdf. Accessed December 17, 2015.
- Medicare claims processing manual: chapter 12, section 30.6.12E. Centers for Medicare & Medicaid Services website. Available at: www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c12.pdf. Accessed December 17, 2015.
- Medicare claims processing manual: chapter 12, section 30.6.12I. Centers for Medicare & Medicaid Services website. Available at: www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c12.pdf. Accessed December 17, 2015.
Medicare Grants Billing Code for Hospitalists
PHILADELPHIA—The Society of Hospital Medicine (SHM) is pleased to announce the introduction of a dedicated billing code for hospitalists by the Centers for Medicare & Medicaid Services (CMS). This decision comes in response to concerted advocacy efforts from SHM for CMS to recognize the specialty. This is a monumental step for hospital medicine, which continues to be the fastest growing medical specialty in the United States with over 48,000 practitioners identifying as hospitalists, growing from approximately 1,000 in the mid-1990s.
“We see each day that hospitalists are driving positive change in healthcare, and this recognition by CMS affirms that hospital medicine is growing both in scope and impact,” notes Laurence Wellikson, MD, MHM, CEO of SHM. “The ability for hospital medicine practitioners to differentiate themselves from providers in other specialties will have a huge impact, particularly for upcoming value-based or pay-for-performance programs.”
Until now, hospitalists could only compare performance to that of practitioners in internal medicine or another related specialty. This new billing code will allow hospitalists to appropriately benchmark and focus improvement efforts with others in the hospital medicine specialty, facilitating more accurate comparisons and fairer assessments of hospitalist performance.
Despite varied training backgrounds, hospitalists have become focused within their own unique specialty, dedicated to providing care to hospitalized patients and working toward high-quality, patient-centered care in the hospital. They have developed institutional-based skills that differentiate them from practitioners in other specialties, such as internal and family medicine. Their specialized expertise includes improving both the efficiency and safety of care for hospitalized patients and the ability to manage and innovate in a hospital’s team-based environment.
This momentous decision coincides with the twenty-year anniversary of the coining of the term ‘hospitalist’ by Robert Wachter, MD, MHM, and Lee Goldman, MD in the New England Journal of Medicine. In recognition of this anniversary, SHM introduced a year-long celebration, the “Year of the Hospitalist,” to commemorate the specialty’s continued success and bright future.
“We have known who we are for years, and the special role that hospitalists play in the well-being of our patients, communities and health systems,” explains Brian Harte, MD, SFHM, president-elect of SHM. “The hospitalist provider code will provide Medicare and other players in the healthcare system an important new tool to better understand and acknowledge the critical role we play in the care of hospitalized patients nationwide.”
Lisa Zoks is SHM's Vice-President of Communications.
ABOUT SHM
Representing the fastest growing specialty in modern healthcare, SHM is the leading medical society for more than 48,000 hospitalists and their patients. SHM is dedicated to promoting the highest quality care for all hospitalized patients and overall excellence in the practice of hospital medicine through quality improvement, education, advocacy and research. Over the past decade, studies have shown that hospitalists can contribute to decreased patient lengths of stay, reductions in hospital costs and readmission rates, and increased patient satisfaction.
PHILADELPHIA—The Society of Hospital Medicine (SHM) is pleased to announce the introduction of a dedicated billing code for hospitalists by the Centers for Medicare & Medicaid Services (CMS). This decision comes in response to concerted advocacy efforts from SHM for CMS to recognize the specialty. This is a monumental step for hospital medicine, which continues to be the fastest growing medical specialty in the United States with over 48,000 practitioners identifying as hospitalists, growing from approximately 1,000 in the mid-1990s.
“We see each day that hospitalists are driving positive change in healthcare, and this recognition by CMS affirms that hospital medicine is growing both in scope and impact,” notes Laurence Wellikson, MD, MHM, CEO of SHM. “The ability for hospital medicine practitioners to differentiate themselves from providers in other specialties will have a huge impact, particularly for upcoming value-based or pay-for-performance programs.”
Until now, hospitalists could only compare performance to that of practitioners in internal medicine or another related specialty. This new billing code will allow hospitalists to appropriately benchmark and focus improvement efforts with others in the hospital medicine specialty, facilitating more accurate comparisons and fairer assessments of hospitalist performance.
Despite varied training backgrounds, hospitalists have become focused within their own unique specialty, dedicated to providing care to hospitalized patients and working toward high-quality, patient-centered care in the hospital. They have developed institutional-based skills that differentiate them from practitioners in other specialties, such as internal and family medicine. Their specialized expertise includes improving both the efficiency and safety of care for hospitalized patients and the ability to manage and innovate in a hospital’s team-based environment.
This momentous decision coincides with the twenty-year anniversary of the coining of the term ‘hospitalist’ by Robert Wachter, MD, MHM, and Lee Goldman, MD in the New England Journal of Medicine. In recognition of this anniversary, SHM introduced a year-long celebration, the “Year of the Hospitalist,” to commemorate the specialty’s continued success and bright future.
“We have known who we are for years, and the special role that hospitalists play in the well-being of our patients, communities and health systems,” explains Brian Harte, MD, SFHM, president-elect of SHM. “The hospitalist provider code will provide Medicare and other players in the healthcare system an important new tool to better understand and acknowledge the critical role we play in the care of hospitalized patients nationwide.”
Lisa Zoks is SHM's Vice-President of Communications.
ABOUT SHM
Representing the fastest growing specialty in modern healthcare, SHM is the leading medical society for more than 48,000 hospitalists and their patients. SHM is dedicated to promoting the highest quality care for all hospitalized patients and overall excellence in the practice of hospital medicine through quality improvement, education, advocacy and research. Over the past decade, studies have shown that hospitalists can contribute to decreased patient lengths of stay, reductions in hospital costs and readmission rates, and increased patient satisfaction.
PHILADELPHIA—The Society of Hospital Medicine (SHM) is pleased to announce the introduction of a dedicated billing code for hospitalists by the Centers for Medicare & Medicaid Services (CMS). This decision comes in response to concerted advocacy efforts from SHM for CMS to recognize the specialty. This is a monumental step for hospital medicine, which continues to be the fastest growing medical specialty in the United States with over 48,000 practitioners identifying as hospitalists, growing from approximately 1,000 in the mid-1990s.
“We see each day that hospitalists are driving positive change in healthcare, and this recognition by CMS affirms that hospital medicine is growing both in scope and impact,” notes Laurence Wellikson, MD, MHM, CEO of SHM. “The ability for hospital medicine practitioners to differentiate themselves from providers in other specialties will have a huge impact, particularly for upcoming value-based or pay-for-performance programs.”
Until now, hospitalists could only compare performance to that of practitioners in internal medicine or another related specialty. This new billing code will allow hospitalists to appropriately benchmark and focus improvement efforts with others in the hospital medicine specialty, facilitating more accurate comparisons and fairer assessments of hospitalist performance.
Despite varied training backgrounds, hospitalists have become focused within their own unique specialty, dedicated to providing care to hospitalized patients and working toward high-quality, patient-centered care in the hospital. They have developed institutional-based skills that differentiate them from practitioners in other specialties, such as internal and family medicine. Their specialized expertise includes improving both the efficiency and safety of care for hospitalized patients and the ability to manage and innovate in a hospital’s team-based environment.
This momentous decision coincides with the twenty-year anniversary of the coining of the term ‘hospitalist’ by Robert Wachter, MD, MHM, and Lee Goldman, MD in the New England Journal of Medicine. In recognition of this anniversary, SHM introduced a year-long celebration, the “Year of the Hospitalist,” to commemorate the specialty’s continued success and bright future.
“We have known who we are for years, and the special role that hospitalists play in the well-being of our patients, communities and health systems,” explains Brian Harte, MD, SFHM, president-elect of SHM. “The hospitalist provider code will provide Medicare and other players in the healthcare system an important new tool to better understand and acknowledge the critical role we play in the care of hospitalized patients nationwide.”
Lisa Zoks is SHM's Vice-President of Communications.
ABOUT SHM
Representing the fastest growing specialty in modern healthcare, SHM is the leading medical society for more than 48,000 hospitalists and their patients. SHM is dedicated to promoting the highest quality care for all hospitalized patients and overall excellence in the practice of hospital medicine through quality improvement, education, advocacy and research. Over the past decade, studies have shown that hospitalists can contribute to decreased patient lengths of stay, reductions in hospital costs and readmission rates, and increased patient satisfaction.
A Guide to Ultrasound of the Shoulder, Part 1: Coding and Reimbursement
Although ultrasound has been around for many years, the technology is underutilized. It has been used primarily by the radiologists and obstetricians-gynecologists. However, orthopedic surgeons and sports medicine doctors are beginning to realize the utility of this imaging modality for their specialties. Ultrasound has classically been used as a diagnostic tool. This usage is beneficial to sports medicine specialists for on-field coverage at sports competitions to efficiently evaluate injuries without the need for taking the athletes back to the locker room for an x-ray or magnetic resonance imaging (MRI). Ultrasound can quickly assess for damage to soft tissue, joints, and superficial bones. Another of ultrasound’s benefits is its use as an adjunct to treatment. Ultrasound has been shown to vastly increase the accuracy of injections and can be used in surgery to accurately guide percutaneous techniques or to identify structures that previously required radiation-exposing fluoroscopy or large incisions to find by feel or eye.
Ultrasound is a technician-dependent modality. The surgeon and physician must become facile with the use of the probe and how ultrasound works. The use of the probe is similar to an arthroscope, requiring small movements of the hand to reveal the best imaging of the tissues. Rather than relying on just the patient’s position with an immobile machine, the user must use the probe position and the placement of the patient’s limb or body to optimize the use of ultrasound. Doing so saves time, money, and exposure to dangerous radiation. In a retrospective study of 1012 patients treated over a 10-month period, Sivan and colleagues1 concluded that the use of clinic-based musculoskeletal (MSK) ultrasound enables a one-stop approach, reduces repeat hospital appointments, and improves quality of care.With the increased use of ultrasound comes the need to accurately code and bill for the use of ultrasound. According to the College of Radiology, Medicare reimbursements for MSK ultrasound studies has increased by 316% from 2000-2009.2 Paradoxically, ultrasound has still been relatively underutilized when compared to the use of MSK MRI.
Diagnostic Ultrasound
Ultrasound is based off sound waves, emitted from a transducer, which are then bounced back off the underlying structures based on the density of that structure. The computer interprets the returning sound waves and produces an image reflecting the quality and strength of those returning waves. When the sound waves are bounced back strongly and quickly, like when hitting bone, we see an image that is intensely white (“hyperechoic”). When the sound waves encounter a substance that transmits those waves easily and do not return, like air or fluid, the image is dark (“hypoechoic”).
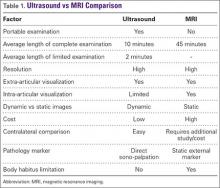
Ultrasound’s fundamental advantages start with every patient being able to have an ultrasound: no interference from metal, pacemakers, claustrophobia, or obesity. Contralateral comparisons, sono-palpation at the site of pathology, and real-time dynamic studies allow for a more comprehensive diagnostic evaluation. Doppler capabilities can further expand the usefulness of the evaluation and guide safer interventions. With the advent of high-resolution portable ultrasound machines, these studies can essentially be performed anywhere, and are typically done in a timely and cost-effective manner.
Ultrasound has many diagnostic uses for soft tissue, joint, and bone disorders. For soft tissues, ultrasound can image tears of muscles, tendons, and ligaments; show inflammation like tenosynovitis; demonstrate masses like hematomas, cysts, solid tumors, or calcific tendonitis; display nerve disorders like Morton’s neuroma; or confirm foreign bodies or infections.3-5 For joint disorders, ultrasound can show erosions on bones, loose bodies, pannus, inflammation, or effusions. For bone disorders, ultrasound can diagnose fractures and, sometimes, even stress fractures. Tomer and colleagues6 compared 51 patients with bone contusions and fractures; they determined that ultrasound was most reliable in the diagnosis of long bone diaphyseal fractures. The one disadvantage, especially when compared to MRI, is ultrasound’s inability to fully evaluate intra-articular or deep structures such as articular cartilage, the glenohumeral labrum, the biceps’ anchor, etc.
Magnetic Resonance Imaging
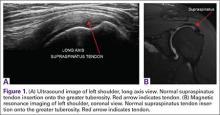
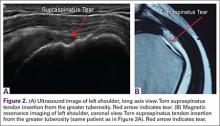
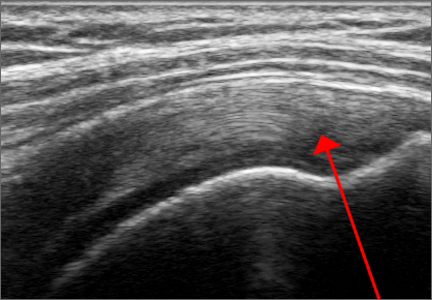
Ultrasound is similar to MRI as it images tissues and gives us ideas whether that tissue is normal, damaged, or diseased (Figures 1A, 1B). MRI is based on magnetics and large machines that cannot be moved. MRI yields planar images that can only be changed by changing the position of the limb or body in the MRI tube. This can create an issue with obese patients or with postoperative patients who cannot maintain the operated body part in one position for the length of the MRI scan. Ultrasound is better tolerated by patients without the need for claustrophobic large machines (Table 1). In 2004, Middleton and colleagues7 surveyed 118 patients who obtained an ultrasound and MRI of the shoulder for suspected rotator cuff pathology; ultrasound had higher satisfaction levels, and 93% of patients preferred ultrasound to MRI.
For rotator cuff tears, ultrasound is also comparable diagnostically with MRI (Figures 2A, 2B). In a prospective study of 124 patients, MRI and ultrasound had comparable accuracy for identifying and measuring the size of full-thickness and partial-thickness rotator cuff tears, with arthroscopic findings used as the standard.8 A 2015 meta-analysis published in the British Journal of Sports Medicine showed that the diagnostic accuracy of ultrasound, MRI, and MR arthrography in the characterization of full thickness rotator cuff tears had >90% sensitivity and specificity. As for partial rotator cuff tears and tendinopathy, overall estimates of specificity were also high (>90%), while sensitivity was as high as 83%. Diagnostic accuracy of ultrasound was similar whether it was performed by a trained radiologist, sonographer, or orthopedist.9
Medicare reimbursements for MSK ultrasound studies has increased by 316% in the past decade.2 Private practice MSK ultrasound procedures increased from 19,372 in 2000 to 158,351 in 2009.2 In 2010, non-radiologists accounted for more ultrasound-guided procedures than radiologists for the first time.10 MSK ultrasound is still underutilized compared to MRI. This underutilization is also unfortunate economically. The cost of MRIs is significantly higher. According to Parker and colleagues10, the projected Medicare cost for MSK imaging in 2020 is $3.6 billion, with MRI accounting for $2 billion. They also concluded that replacing MSK MRI with MSK ultrasound when clinically indicated could save over $6.9 billion between 2006 and 2020.11
Ultrasound-Guided Procedures
MSK ultrasound has gained significant ground on other imaging modalities when it comes to procedures, both in office and in the operating room. The ability to have a small, mobile, inexpensive machine that can be used in real time has dramatically changed how interventions are done. Most imaging modalities used to perform injections or percutaneous surgery use fluoroscopy machines. This exposes the patients to significant radiation, costs significantly more, and usually requires a secondary consultation with radiologists in a different facility. This wastes time and money, and results in potentially unnecessary exposure to radiation.
Accuracy is the most common reason for referral for guided injections. The guidance can help avoid nerves, vessels, and other sensitive tissues. However, accuracy is also important to make sure the injection is placed in the correct location. When injections are placed into a muscle, tendon, or ligament, it causes significant pain; however, injections placed into a bursal space or joint do not cause pain. Numerous studies have shown that even in the hands of experts, “simple” injections can still miss their mark over 30% of the time.12-19 Therefore, if a patient experiences pain during a bursal space or joint injection, the injection was not placed properly.
The American Medical Society for Sports Medicine Position Paper on MSK ultrasound is based on a systematic review of the literature, including 124 studies. It states that ultrasound-guided joint injections (USGI) are more accurate and efficacious than landmark guided injections (LMGI), with a strength of recommendation taxonomy (SORT) evidence rating of A and B, respectively.19 In terms of patient satisfaction, in a randomized controlled trial of 148 patients undergoing knee injections, Sibbitt and colleagues20 showed that USGI had a 48% reduction (P < .001) in procedural pain, a 58.5% reduction (P < .001) in absolute pain scores at the 2-week outcome mark, and a 75% reduction (P < .001) in significant pain and 62% reduction in nonresponder rate.20 From a financial point of view, Sibbitt and colleagues20 also demonstrated a 13% reduction in cost per patient per year, and a 58% reduction in cost per responder per year for a hospital outpatient center (P < .001).
Coding
Coding for diagnostic MSK ultrasound requires an understanding of a few current procedural terminology (CPT) codes (Table 2). Ultrasound usage should follow the usual requirements of medical necessity and the CPT code selected should be based on the elements of the study performed. A complete examination, described by CPT code 76881, includes the examination and documentation of the muscles, tendons, joint, and other soft tissue structures and any identifiable abnormality of the joint being evaluated. If anything less is done, then the CPT code 76882 should be used.
New CPT codes for joint injections became effective January 2015 (Table 3). The new changes affect only the joint injection series (20600-20610). Previously, injections could be billed with CPT code 76942, which was “Ultrasonic guidance for needle placement (eg, biopsy, aspiration, injection, localization device), imaging supervision and interpretation.” This code can still be used, but with only specific injections, when the verbiage “with ultrasound/image guidance” is not included in the injection CPT code descriptor (Table 4).
Under the National Correct Coding Initiative (NCCI), which sets Centers for Medicare & Medicaid Services (CMS) payment policy as well as that of many private payers, one unit of service is allowed for CPT code 76942 in a single patient encounter regardless of the number of needle placements performed. Per NCCI, “The unit of service for these codes is the patient encounter, not number of lesions, number of aspirations, number of biopsies, number of injections, or number of localizations.”
Per the Radiology section of the NCCI, “Ultrasound guidance and diagnostic ultrasound (echography) procedures may be reported separately only if each service is distinct and separate. If a diagnostic ultrasound study identifies a previously unknown abnormality that requires a therapeutic procedure with ultrasound guidance at the same patient encounter, both the diagnostic ultrasound and ultrasound guidance procedure codes may be reported separately. However, a previously unknown abnormality identified during ultrasound guidance for a procedure should not be reported separately as a diagnostic ultrasound procedure.”
Under the Medicare program, the International Classification of Diseases 10th Revision (ICD-10) code selected should be based on the test results, with 2 exceptions. If the test does not yield a diagnosis or was normal, the physician should use the pre-service signs, symptoms, and conditions that prompted the study. If the test is a screening examination ordered in the absence of any signs or symptoms of illness or injury, the physician should select “screening” as the primary reason for the service and record the test results, if any, as additional diagnoses.
Modifiers must be used in specific settings. In the office, physicians who own the equipment and perform the service themselves (or the service is performed by an employed or contracted sonographer) may bill the global fee without any modifiers. However, if billing for a procedure on the same day as an office visit, the -25 modifier must be used. This indicates “[a] significant, separately identifiable evaluation and management service.” This modifier should not be used routinely. If the service is performed in a hospital, the -26 modifier must be used to indicate that the professional service only was provided when the physician does not own the machine (Tables 2, 3, 4). The payers will not reimburse physicians for the technical component in the hospital setting.
Reimbursement
In general, medical insurance plans will cover ultrasound studies when they are medically indicated. However, we recommend checking with each individual private payer directly, including Medicare. Medicare Part B will generally reimburse physicians for medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors require that the physician who performs and/or interprets some types of ultrasound examinations be capable of demonstrating relevant, documented training through recent residency training or post-graduate continuing medical education (CME) and experience. Medicare does not differentiate by medical specialty with respect to billing medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors have coverage policies regarding either the diagnostic study or ultrasound guidance of certain injections, or both.
Payment policies for beneficiaries enrolled in Medicare Part C, known as the Medicare Advantage plans, will reflect those of the private insurance administrator. The Medicare Advantage plan may be either a health maintenance organization (HMO) or a preferred provider organization (PPO). Private insurance payment rules vary by payer and plan with respect to which specialties may perform and receive reimbursement for ultrasound services. Some payers will reimburse providers of any specialty for ultrasound services, while others may restrict imaging procedures to specific specialties or providers possessing specific certifications or accreditations. Some insurers require physicians to submit applications requesting ultrasound be added to their list of services performed in their practice. Physicians should contact private payers before submitting claims to determine their requirements and request that they add ultrasound to the list of services.
When contacting the private payers, ask the following questions:
- What do I need to do to have ultrasound added to my practice’s contract or list of services?
- Are there any specific training requirements that I must meet or credentials that I must obtain in order to be privileged to perform ultrasound in my office?
- Do I need to send a letter or can I submit the request verbally?
- Is there an application that must be completed?
- If there is a privileging program, how long will it take after submission of the application before we are accepted?
- What is the fee schedule associated with these codes?
- Are there any bundling edits in place covering any of the services I am considering performing? (Be prepared to provide the codes for any non-ultrasound services you will be performing in conjunction with the ultrasound services.)
- Are there any preauthorization requirements for specific ultrasound studies?
- Are there any preauthorization requirements for specific ultrasound studies?
Documentation Requirements
All diagnostic ultrasound examinations, including those when ultrasound is used to guide a procedure, require that permanently recorded images be maintained in the patient record. The images can be kept in the patient record or some other archive—they do not need to be submitted with the claim. Images can be stored as printed images, on a tape or electronic medium. Documentation of the study must be available to the insurer upon request.
A written report of all ultrasound studies should be maintained in the patient’s record. In the case of ultrasound guidance, the written report may be filed as a separate item in the patient’s record or it may be included within the report of the procedure for which the guidance is utilized.
As examples of our documentation in the office, copies of 3 of our standard forms are available: “Ultrasound report of the shoulder” (Appendix 1), “Procedure note for an ultrasound-guided injection of cortisone” (Appendix 2), and “Procedure note for an ultrasound-guided injection of platelet-rich plasma” (Appendix 3).
Appropriate Use Criteria (AUC)
The Protecting Access to Medicare Act of 2014 was an effort to help reduce unnecessary imaging services and reduce costs; the Secretary of Health and Human Services was to establish a program to promote the use of “appropriate use criteria” (AUC) for advanced imaging services such as MRI, computed tomography, positron emission tomography, and nuclear cardiology. AUC are criteria that are developed or endorsed by national professional medical specialty societies or other provider-led entities to assist ordering professionals and furnishing professionals in making the most appropriate treatment decision for a specific clinical condition for an individual. The law also noted that the criteria should be evidence-based, meaning they should have stakeholder consensus, be scientifically valid, and be based on studies that are published and reviewable by stakeholders.
By April 2016, the Secretary will identify and publish the list of qualified clinical decision support mechanisms, which are tools that could be used by ordering professionals to ensure that AUC is met for applicable imaging services. These may include certified health electronic record technology, private sector clinical decision support mechanisms, and others. Actual use of the AUC will begin in January 2017. This legislation applies only to Medicare services, but other payers have cited concerns and may follow in the future.
Conclusion
Ultrasound is being increasingly used in varying specialties, especially orthopedic surgery. It provides a time- and cost-efficient modality with diagnostic power comparable to MRI. Portability and a high safety profile allows for ease of implementation as an in-office or sideline tool. Needle guidance and other intraoperative applications highlight its versatility as an adjunct to orthopedic treatments. This article provides a comprehensive guide to billing and coding for both diagnostic and therapeutic MSK ultrasound of the shoulder. Providers should stay up to date with upcoming appropriate use criteria and adjustments to current billing procedures.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Sharpe R, Nazarian L, Parker L, Rao V, Levin D. Dramatically increased musculoskeletal ultrasound utilization from 2000 to 2009, especially by podiatrists in private offices. Department of Radiology Faculty Papers. Paper 16. http://jdc.jefferson.edu/radiologyfp/16. Accessed January 7, 2016.
3. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: The orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
4. Sinha TP, Bhoi S, Kumar S, et al. Diagnostic accuracy of bedside emergency ultrasound screening for fractures in pediatric trauma patients. J Emerg Trauma Shock. 2011;4(4);443-445.
5. Bica D, Armen J, Kulas AS, Young K, Womack Z. Reliability and precision of stress sonography of the ulnar collateral ligament. J Ultrasound Med. 2015;34(3):371-376.
6. Tomer K, Kleinbaum Y, Heyman Z, Dudkiewicz I, Blankstein A. Ultrasound diagnosis of fractures in adults. Akt Traumatol. 2006;36(4):171-174.
7. Middleton W, Payne WT, Teefey SA, Hidebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol. 2004;183(5):1449-1452.
8. Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance and arthroscopic finding in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A(4):708-716.
9. Roy-JS, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis. Br J Sports Med. 2015;49(20):1316-1328.
10. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal Imaging: Medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5(3):182-188.
11. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
12. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
13. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
14. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
15. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
16. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
17. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
18. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
19. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
20. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intra-articular injections? J Rheumatol. 2009;36(9):1892-1902.
Although ultrasound has been around for many years, the technology is underutilized. It has been used primarily by the radiologists and obstetricians-gynecologists. However, orthopedic surgeons and sports medicine doctors are beginning to realize the utility of this imaging modality for their specialties. Ultrasound has classically been used as a diagnostic tool. This usage is beneficial to sports medicine specialists for on-field coverage at sports competitions to efficiently evaluate injuries without the need for taking the athletes back to the locker room for an x-ray or magnetic resonance imaging (MRI). Ultrasound can quickly assess for damage to soft tissue, joints, and superficial bones. Another of ultrasound’s benefits is its use as an adjunct to treatment. Ultrasound has been shown to vastly increase the accuracy of injections and can be used in surgery to accurately guide percutaneous techniques or to identify structures that previously required radiation-exposing fluoroscopy or large incisions to find by feel or eye.
Ultrasound is a technician-dependent modality. The surgeon and physician must become facile with the use of the probe and how ultrasound works. The use of the probe is similar to an arthroscope, requiring small movements of the hand to reveal the best imaging of the tissues. Rather than relying on just the patient’s position with an immobile machine, the user must use the probe position and the placement of the patient’s limb or body to optimize the use of ultrasound. Doing so saves time, money, and exposure to dangerous radiation. In a retrospective study of 1012 patients treated over a 10-month period, Sivan and colleagues1 concluded that the use of clinic-based musculoskeletal (MSK) ultrasound enables a one-stop approach, reduces repeat hospital appointments, and improves quality of care.With the increased use of ultrasound comes the need to accurately code and bill for the use of ultrasound. According to the College of Radiology, Medicare reimbursements for MSK ultrasound studies has increased by 316% from 2000-2009.2 Paradoxically, ultrasound has still been relatively underutilized when compared to the use of MSK MRI.
Diagnostic Ultrasound
Ultrasound is based off sound waves, emitted from a transducer, which are then bounced back off the underlying structures based on the density of that structure. The computer interprets the returning sound waves and produces an image reflecting the quality and strength of those returning waves. When the sound waves are bounced back strongly and quickly, like when hitting bone, we see an image that is intensely white (“hyperechoic”). When the sound waves encounter a substance that transmits those waves easily and do not return, like air or fluid, the image is dark (“hypoechoic”).
Ultrasound’s fundamental advantages start with every patient being able to have an ultrasound: no interference from metal, pacemakers, claustrophobia, or obesity. Contralateral comparisons, sono-palpation at the site of pathology, and real-time dynamic studies allow for a more comprehensive diagnostic evaluation. Doppler capabilities can further expand the usefulness of the evaluation and guide safer interventions. With the advent of high-resolution portable ultrasound machines, these studies can essentially be performed anywhere, and are typically done in a timely and cost-effective manner.
Ultrasound has many diagnostic uses for soft tissue, joint, and bone disorders. For soft tissues, ultrasound can image tears of muscles, tendons, and ligaments; show inflammation like tenosynovitis; demonstrate masses like hematomas, cysts, solid tumors, or calcific tendonitis; display nerve disorders like Morton’s neuroma; or confirm foreign bodies or infections.3-5 For joint disorders, ultrasound can show erosions on bones, loose bodies, pannus, inflammation, or effusions. For bone disorders, ultrasound can diagnose fractures and, sometimes, even stress fractures. Tomer and colleagues6 compared 51 patients with bone contusions and fractures; they determined that ultrasound was most reliable in the diagnosis of long bone diaphyseal fractures. The one disadvantage, especially when compared to MRI, is ultrasound’s inability to fully evaluate intra-articular or deep structures such as articular cartilage, the glenohumeral labrum, the biceps’ anchor, etc.
Magnetic Resonance Imaging
Ultrasound is similar to MRI as it images tissues and gives us ideas whether that tissue is normal, damaged, or diseased (Figures 1A, 1B). MRI is based on magnetics and large machines that cannot be moved. MRI yields planar images that can only be changed by changing the position of the limb or body in the MRI tube. This can create an issue with obese patients or with postoperative patients who cannot maintain the operated body part in one position for the length of the MRI scan. Ultrasound is better tolerated by patients without the need for claustrophobic large machines (Table 1). In 2004, Middleton and colleagues7 surveyed 118 patients who obtained an ultrasound and MRI of the shoulder for suspected rotator cuff pathology; ultrasound had higher satisfaction levels, and 93% of patients preferred ultrasound to MRI.
For rotator cuff tears, ultrasound is also comparable diagnostically with MRI (Figures 2A, 2B). In a prospective study of 124 patients, MRI and ultrasound had comparable accuracy for identifying and measuring the size of full-thickness and partial-thickness rotator cuff tears, with arthroscopic findings used as the standard.8 A 2015 meta-analysis published in the British Journal of Sports Medicine showed that the diagnostic accuracy of ultrasound, MRI, and MR arthrography in the characterization of full thickness rotator cuff tears had >90% sensitivity and specificity. As for partial rotator cuff tears and tendinopathy, overall estimates of specificity were also high (>90%), while sensitivity was as high as 83%. Diagnostic accuracy of ultrasound was similar whether it was performed by a trained radiologist, sonographer, or orthopedist.9
Medicare reimbursements for MSK ultrasound studies has increased by 316% in the past decade.2 Private practice MSK ultrasound procedures increased from 19,372 in 2000 to 158,351 in 2009.2 In 2010, non-radiologists accounted for more ultrasound-guided procedures than radiologists for the first time.10 MSK ultrasound is still underutilized compared to MRI. This underutilization is also unfortunate economically. The cost of MRIs is significantly higher. According to Parker and colleagues10, the projected Medicare cost for MSK imaging in 2020 is $3.6 billion, with MRI accounting for $2 billion. They also concluded that replacing MSK MRI with MSK ultrasound when clinically indicated could save over $6.9 billion between 2006 and 2020.11
Ultrasound-Guided Procedures
MSK ultrasound has gained significant ground on other imaging modalities when it comes to procedures, both in office and in the operating room. The ability to have a small, mobile, inexpensive machine that can be used in real time has dramatically changed how interventions are done. Most imaging modalities used to perform injections or percutaneous surgery use fluoroscopy machines. This exposes the patients to significant radiation, costs significantly more, and usually requires a secondary consultation with radiologists in a different facility. This wastes time and money, and results in potentially unnecessary exposure to radiation.
Accuracy is the most common reason for referral for guided injections. The guidance can help avoid nerves, vessels, and other sensitive tissues. However, accuracy is also important to make sure the injection is placed in the correct location. When injections are placed into a muscle, tendon, or ligament, it causes significant pain; however, injections placed into a bursal space or joint do not cause pain. Numerous studies have shown that even in the hands of experts, “simple” injections can still miss their mark over 30% of the time.12-19 Therefore, if a patient experiences pain during a bursal space or joint injection, the injection was not placed properly.
The American Medical Society for Sports Medicine Position Paper on MSK ultrasound is based on a systematic review of the literature, including 124 studies. It states that ultrasound-guided joint injections (USGI) are more accurate and efficacious than landmark guided injections (LMGI), with a strength of recommendation taxonomy (SORT) evidence rating of A and B, respectively.19 In terms of patient satisfaction, in a randomized controlled trial of 148 patients undergoing knee injections, Sibbitt and colleagues20 showed that USGI had a 48% reduction (P < .001) in procedural pain, a 58.5% reduction (P < .001) in absolute pain scores at the 2-week outcome mark, and a 75% reduction (P < .001) in significant pain and 62% reduction in nonresponder rate.20 From a financial point of view, Sibbitt and colleagues20 also demonstrated a 13% reduction in cost per patient per year, and a 58% reduction in cost per responder per year for a hospital outpatient center (P < .001).
Coding
Coding for diagnostic MSK ultrasound requires an understanding of a few current procedural terminology (CPT) codes (Table 2). Ultrasound usage should follow the usual requirements of medical necessity and the CPT code selected should be based on the elements of the study performed. A complete examination, described by CPT code 76881, includes the examination and documentation of the muscles, tendons, joint, and other soft tissue structures and any identifiable abnormality of the joint being evaluated. If anything less is done, then the CPT code 76882 should be used.
New CPT codes for joint injections became effective January 2015 (Table 3). The new changes affect only the joint injection series (20600-20610). Previously, injections could be billed with CPT code 76942, which was “Ultrasonic guidance for needle placement (eg, biopsy, aspiration, injection, localization device), imaging supervision and interpretation.” This code can still be used, but with only specific injections, when the verbiage “with ultrasound/image guidance” is not included in the injection CPT code descriptor (Table 4).
Under the National Correct Coding Initiative (NCCI), which sets Centers for Medicare & Medicaid Services (CMS) payment policy as well as that of many private payers, one unit of service is allowed for CPT code 76942 in a single patient encounter regardless of the number of needle placements performed. Per NCCI, “The unit of service for these codes is the patient encounter, not number of lesions, number of aspirations, number of biopsies, number of injections, or number of localizations.”
Per the Radiology section of the NCCI, “Ultrasound guidance and diagnostic ultrasound (echography) procedures may be reported separately only if each service is distinct and separate. If a diagnostic ultrasound study identifies a previously unknown abnormality that requires a therapeutic procedure with ultrasound guidance at the same patient encounter, both the diagnostic ultrasound and ultrasound guidance procedure codes may be reported separately. However, a previously unknown abnormality identified during ultrasound guidance for a procedure should not be reported separately as a diagnostic ultrasound procedure.”
Under the Medicare program, the International Classification of Diseases 10th Revision (ICD-10) code selected should be based on the test results, with 2 exceptions. If the test does not yield a diagnosis or was normal, the physician should use the pre-service signs, symptoms, and conditions that prompted the study. If the test is a screening examination ordered in the absence of any signs or symptoms of illness or injury, the physician should select “screening” as the primary reason for the service and record the test results, if any, as additional diagnoses.
Modifiers must be used in specific settings. In the office, physicians who own the equipment and perform the service themselves (or the service is performed by an employed or contracted sonographer) may bill the global fee without any modifiers. However, if billing for a procedure on the same day as an office visit, the -25 modifier must be used. This indicates “[a] significant, separately identifiable evaluation and management service.” This modifier should not be used routinely. If the service is performed in a hospital, the -26 modifier must be used to indicate that the professional service only was provided when the physician does not own the machine (Tables 2, 3, 4). The payers will not reimburse physicians for the technical component in the hospital setting.
Reimbursement
In general, medical insurance plans will cover ultrasound studies when they are medically indicated. However, we recommend checking with each individual private payer directly, including Medicare. Medicare Part B will generally reimburse physicians for medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors require that the physician who performs and/or interprets some types of ultrasound examinations be capable of demonstrating relevant, documented training through recent residency training or post-graduate continuing medical education (CME) and experience. Medicare does not differentiate by medical specialty with respect to billing medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors have coverage policies regarding either the diagnostic study or ultrasound guidance of certain injections, or both.
Payment policies for beneficiaries enrolled in Medicare Part C, known as the Medicare Advantage plans, will reflect those of the private insurance administrator. The Medicare Advantage plan may be either a health maintenance organization (HMO) or a preferred provider organization (PPO). Private insurance payment rules vary by payer and plan with respect to which specialties may perform and receive reimbursement for ultrasound services. Some payers will reimburse providers of any specialty for ultrasound services, while others may restrict imaging procedures to specific specialties or providers possessing specific certifications or accreditations. Some insurers require physicians to submit applications requesting ultrasound be added to their list of services performed in their practice. Physicians should contact private payers before submitting claims to determine their requirements and request that they add ultrasound to the list of services.
When contacting the private payers, ask the following questions:
- What do I need to do to have ultrasound added to my practice’s contract or list of services?
- Are there any specific training requirements that I must meet or credentials that I must obtain in order to be privileged to perform ultrasound in my office?
- Do I need to send a letter or can I submit the request verbally?
- Is there an application that must be completed?
- If there is a privileging program, how long will it take after submission of the application before we are accepted?
- What is the fee schedule associated with these codes?
- Are there any bundling edits in place covering any of the services I am considering performing? (Be prepared to provide the codes for any non-ultrasound services you will be performing in conjunction with the ultrasound services.)
- Are there any preauthorization requirements for specific ultrasound studies?
- Are there any preauthorization requirements for specific ultrasound studies?
Documentation Requirements
All diagnostic ultrasound examinations, including those when ultrasound is used to guide a procedure, require that permanently recorded images be maintained in the patient record. The images can be kept in the patient record or some other archive—they do not need to be submitted with the claim. Images can be stored as printed images, on a tape or electronic medium. Documentation of the study must be available to the insurer upon request.
A written report of all ultrasound studies should be maintained in the patient’s record. In the case of ultrasound guidance, the written report may be filed as a separate item in the patient’s record or it may be included within the report of the procedure for which the guidance is utilized.
As examples of our documentation in the office, copies of 3 of our standard forms are available: “Ultrasound report of the shoulder” (Appendix 1), “Procedure note for an ultrasound-guided injection of cortisone” (Appendix 2), and “Procedure note for an ultrasound-guided injection of platelet-rich plasma” (Appendix 3).
Appropriate Use Criteria (AUC)
The Protecting Access to Medicare Act of 2014 was an effort to help reduce unnecessary imaging services and reduce costs; the Secretary of Health and Human Services was to establish a program to promote the use of “appropriate use criteria” (AUC) for advanced imaging services such as MRI, computed tomography, positron emission tomography, and nuclear cardiology. AUC are criteria that are developed or endorsed by national professional medical specialty societies or other provider-led entities to assist ordering professionals and furnishing professionals in making the most appropriate treatment decision for a specific clinical condition for an individual. The law also noted that the criteria should be evidence-based, meaning they should have stakeholder consensus, be scientifically valid, and be based on studies that are published and reviewable by stakeholders.
By April 2016, the Secretary will identify and publish the list of qualified clinical decision support mechanisms, which are tools that could be used by ordering professionals to ensure that AUC is met for applicable imaging services. These may include certified health electronic record technology, private sector clinical decision support mechanisms, and others. Actual use of the AUC will begin in January 2017. This legislation applies only to Medicare services, but other payers have cited concerns and may follow in the future.
Conclusion
Ultrasound is being increasingly used in varying specialties, especially orthopedic surgery. It provides a time- and cost-efficient modality with diagnostic power comparable to MRI. Portability and a high safety profile allows for ease of implementation as an in-office or sideline tool. Needle guidance and other intraoperative applications highlight its versatility as an adjunct to orthopedic treatments. This article provides a comprehensive guide to billing and coding for both diagnostic and therapeutic MSK ultrasound of the shoulder. Providers should stay up to date with upcoming appropriate use criteria and adjustments to current billing procedures.
Although ultrasound has been around for many years, the technology is underutilized. It has been used primarily by the radiologists and obstetricians-gynecologists. However, orthopedic surgeons and sports medicine doctors are beginning to realize the utility of this imaging modality for their specialties. Ultrasound has classically been used as a diagnostic tool. This usage is beneficial to sports medicine specialists for on-field coverage at sports competitions to efficiently evaluate injuries without the need for taking the athletes back to the locker room for an x-ray or magnetic resonance imaging (MRI). Ultrasound can quickly assess for damage to soft tissue, joints, and superficial bones. Another of ultrasound’s benefits is its use as an adjunct to treatment. Ultrasound has been shown to vastly increase the accuracy of injections and can be used in surgery to accurately guide percutaneous techniques or to identify structures that previously required radiation-exposing fluoroscopy or large incisions to find by feel or eye.
Ultrasound is a technician-dependent modality. The surgeon and physician must become facile with the use of the probe and how ultrasound works. The use of the probe is similar to an arthroscope, requiring small movements of the hand to reveal the best imaging of the tissues. Rather than relying on just the patient’s position with an immobile machine, the user must use the probe position and the placement of the patient’s limb or body to optimize the use of ultrasound. Doing so saves time, money, and exposure to dangerous radiation. In a retrospective study of 1012 patients treated over a 10-month period, Sivan and colleagues1 concluded that the use of clinic-based musculoskeletal (MSK) ultrasound enables a one-stop approach, reduces repeat hospital appointments, and improves quality of care.With the increased use of ultrasound comes the need to accurately code and bill for the use of ultrasound. According to the College of Radiology, Medicare reimbursements for MSK ultrasound studies has increased by 316% from 2000-2009.2 Paradoxically, ultrasound has still been relatively underutilized when compared to the use of MSK MRI.
Diagnostic Ultrasound
Ultrasound is based off sound waves, emitted from a transducer, which are then bounced back off the underlying structures based on the density of that structure. The computer interprets the returning sound waves and produces an image reflecting the quality and strength of those returning waves. When the sound waves are bounced back strongly and quickly, like when hitting bone, we see an image that is intensely white (“hyperechoic”). When the sound waves encounter a substance that transmits those waves easily and do not return, like air or fluid, the image is dark (“hypoechoic”).
Ultrasound’s fundamental advantages start with every patient being able to have an ultrasound: no interference from metal, pacemakers, claustrophobia, or obesity. Contralateral comparisons, sono-palpation at the site of pathology, and real-time dynamic studies allow for a more comprehensive diagnostic evaluation. Doppler capabilities can further expand the usefulness of the evaluation and guide safer interventions. With the advent of high-resolution portable ultrasound machines, these studies can essentially be performed anywhere, and are typically done in a timely and cost-effective manner.
Ultrasound has many diagnostic uses for soft tissue, joint, and bone disorders. For soft tissues, ultrasound can image tears of muscles, tendons, and ligaments; show inflammation like tenosynovitis; demonstrate masses like hematomas, cysts, solid tumors, or calcific tendonitis; display nerve disorders like Morton’s neuroma; or confirm foreign bodies or infections.3-5 For joint disorders, ultrasound can show erosions on bones, loose bodies, pannus, inflammation, or effusions. For bone disorders, ultrasound can diagnose fractures and, sometimes, even stress fractures. Tomer and colleagues6 compared 51 patients with bone contusions and fractures; they determined that ultrasound was most reliable in the diagnosis of long bone diaphyseal fractures. The one disadvantage, especially when compared to MRI, is ultrasound’s inability to fully evaluate intra-articular or deep structures such as articular cartilage, the glenohumeral labrum, the biceps’ anchor, etc.
Magnetic Resonance Imaging
Ultrasound is similar to MRI as it images tissues and gives us ideas whether that tissue is normal, damaged, or diseased (Figures 1A, 1B). MRI is based on magnetics and large machines that cannot be moved. MRI yields planar images that can only be changed by changing the position of the limb or body in the MRI tube. This can create an issue with obese patients or with postoperative patients who cannot maintain the operated body part in one position for the length of the MRI scan. Ultrasound is better tolerated by patients without the need for claustrophobic large machines (Table 1). In 2004, Middleton and colleagues7 surveyed 118 patients who obtained an ultrasound and MRI of the shoulder for suspected rotator cuff pathology; ultrasound had higher satisfaction levels, and 93% of patients preferred ultrasound to MRI.
For rotator cuff tears, ultrasound is also comparable diagnostically with MRI (Figures 2A, 2B). In a prospective study of 124 patients, MRI and ultrasound had comparable accuracy for identifying and measuring the size of full-thickness and partial-thickness rotator cuff tears, with arthroscopic findings used as the standard.8 A 2015 meta-analysis published in the British Journal of Sports Medicine showed that the diagnostic accuracy of ultrasound, MRI, and MR arthrography in the characterization of full thickness rotator cuff tears had >90% sensitivity and specificity. As for partial rotator cuff tears and tendinopathy, overall estimates of specificity were also high (>90%), while sensitivity was as high as 83%. Diagnostic accuracy of ultrasound was similar whether it was performed by a trained radiologist, sonographer, or orthopedist.9
Medicare reimbursements for MSK ultrasound studies has increased by 316% in the past decade.2 Private practice MSK ultrasound procedures increased from 19,372 in 2000 to 158,351 in 2009.2 In 2010, non-radiologists accounted for more ultrasound-guided procedures than radiologists for the first time.10 MSK ultrasound is still underutilized compared to MRI. This underutilization is also unfortunate economically. The cost of MRIs is significantly higher. According to Parker and colleagues10, the projected Medicare cost for MSK imaging in 2020 is $3.6 billion, with MRI accounting for $2 billion. They also concluded that replacing MSK MRI with MSK ultrasound when clinically indicated could save over $6.9 billion between 2006 and 2020.11
Ultrasound-Guided Procedures
MSK ultrasound has gained significant ground on other imaging modalities when it comes to procedures, both in office and in the operating room. The ability to have a small, mobile, inexpensive machine that can be used in real time has dramatically changed how interventions are done. Most imaging modalities used to perform injections or percutaneous surgery use fluoroscopy machines. This exposes the patients to significant radiation, costs significantly more, and usually requires a secondary consultation with radiologists in a different facility. This wastes time and money, and results in potentially unnecessary exposure to radiation.
Accuracy is the most common reason for referral for guided injections. The guidance can help avoid nerves, vessels, and other sensitive tissues. However, accuracy is also important to make sure the injection is placed in the correct location. When injections are placed into a muscle, tendon, or ligament, it causes significant pain; however, injections placed into a bursal space or joint do not cause pain. Numerous studies have shown that even in the hands of experts, “simple” injections can still miss their mark over 30% of the time.12-19 Therefore, if a patient experiences pain during a bursal space or joint injection, the injection was not placed properly.
The American Medical Society for Sports Medicine Position Paper on MSK ultrasound is based on a systematic review of the literature, including 124 studies. It states that ultrasound-guided joint injections (USGI) are more accurate and efficacious than landmark guided injections (LMGI), with a strength of recommendation taxonomy (SORT) evidence rating of A and B, respectively.19 In terms of patient satisfaction, in a randomized controlled trial of 148 patients undergoing knee injections, Sibbitt and colleagues20 showed that USGI had a 48% reduction (P < .001) in procedural pain, a 58.5% reduction (P < .001) in absolute pain scores at the 2-week outcome mark, and a 75% reduction (P < .001) in significant pain and 62% reduction in nonresponder rate.20 From a financial point of view, Sibbitt and colleagues20 also demonstrated a 13% reduction in cost per patient per year, and a 58% reduction in cost per responder per year for a hospital outpatient center (P < .001).
Coding
Coding for diagnostic MSK ultrasound requires an understanding of a few current procedural terminology (CPT) codes (Table 2). Ultrasound usage should follow the usual requirements of medical necessity and the CPT code selected should be based on the elements of the study performed. A complete examination, described by CPT code 76881, includes the examination and documentation of the muscles, tendons, joint, and other soft tissue structures and any identifiable abnormality of the joint being evaluated. If anything less is done, then the CPT code 76882 should be used.
New CPT codes for joint injections became effective January 2015 (Table 3). The new changes affect only the joint injection series (20600-20610). Previously, injections could be billed with CPT code 76942, which was “Ultrasonic guidance for needle placement (eg, biopsy, aspiration, injection, localization device), imaging supervision and interpretation.” This code can still be used, but with only specific injections, when the verbiage “with ultrasound/image guidance” is not included in the injection CPT code descriptor (Table 4).
Under the National Correct Coding Initiative (NCCI), which sets Centers for Medicare & Medicaid Services (CMS) payment policy as well as that of many private payers, one unit of service is allowed for CPT code 76942 in a single patient encounter regardless of the number of needle placements performed. Per NCCI, “The unit of service for these codes is the patient encounter, not number of lesions, number of aspirations, number of biopsies, number of injections, or number of localizations.”
Per the Radiology section of the NCCI, “Ultrasound guidance and diagnostic ultrasound (echography) procedures may be reported separately only if each service is distinct and separate. If a diagnostic ultrasound study identifies a previously unknown abnormality that requires a therapeutic procedure with ultrasound guidance at the same patient encounter, both the diagnostic ultrasound and ultrasound guidance procedure codes may be reported separately. However, a previously unknown abnormality identified during ultrasound guidance for a procedure should not be reported separately as a diagnostic ultrasound procedure.”
Under the Medicare program, the International Classification of Diseases 10th Revision (ICD-10) code selected should be based on the test results, with 2 exceptions. If the test does not yield a diagnosis or was normal, the physician should use the pre-service signs, symptoms, and conditions that prompted the study. If the test is a screening examination ordered in the absence of any signs or symptoms of illness or injury, the physician should select “screening” as the primary reason for the service and record the test results, if any, as additional diagnoses.
Modifiers must be used in specific settings. In the office, physicians who own the equipment and perform the service themselves (or the service is performed by an employed or contracted sonographer) may bill the global fee without any modifiers. However, if billing for a procedure on the same day as an office visit, the -25 modifier must be used. This indicates “[a] significant, separately identifiable evaluation and management service.” This modifier should not be used routinely. If the service is performed in a hospital, the -26 modifier must be used to indicate that the professional service only was provided when the physician does not own the machine (Tables 2, 3, 4). The payers will not reimburse physicians for the technical component in the hospital setting.
Reimbursement
In general, medical insurance plans will cover ultrasound studies when they are medically indicated. However, we recommend checking with each individual private payer directly, including Medicare. Medicare Part B will generally reimburse physicians for medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors require that the physician who performs and/or interprets some types of ultrasound examinations be capable of demonstrating relevant, documented training through recent residency training or post-graduate continuing medical education (CME) and experience. Medicare does not differentiate by medical specialty with respect to billing medically necessary diagnostic ultrasound services, provided the services are within the scope of the physician’s license. Some Medicare contractors have coverage policies regarding either the diagnostic study or ultrasound guidance of certain injections, or both.
Payment policies for beneficiaries enrolled in Medicare Part C, known as the Medicare Advantage plans, will reflect those of the private insurance administrator. The Medicare Advantage plan may be either a health maintenance organization (HMO) or a preferred provider organization (PPO). Private insurance payment rules vary by payer and plan with respect to which specialties may perform and receive reimbursement for ultrasound services. Some payers will reimburse providers of any specialty for ultrasound services, while others may restrict imaging procedures to specific specialties or providers possessing specific certifications or accreditations. Some insurers require physicians to submit applications requesting ultrasound be added to their list of services performed in their practice. Physicians should contact private payers before submitting claims to determine their requirements and request that they add ultrasound to the list of services.
When contacting the private payers, ask the following questions:
- What do I need to do to have ultrasound added to my practice’s contract or list of services?
- Are there any specific training requirements that I must meet or credentials that I must obtain in order to be privileged to perform ultrasound in my office?
- Do I need to send a letter or can I submit the request verbally?
- Is there an application that must be completed?
- If there is a privileging program, how long will it take after submission of the application before we are accepted?
- What is the fee schedule associated with these codes?
- Are there any bundling edits in place covering any of the services I am considering performing? (Be prepared to provide the codes for any non-ultrasound services you will be performing in conjunction with the ultrasound services.)
- Are there any preauthorization requirements for specific ultrasound studies?
- Are there any preauthorization requirements for specific ultrasound studies?
Documentation Requirements
All diagnostic ultrasound examinations, including those when ultrasound is used to guide a procedure, require that permanently recorded images be maintained in the patient record. The images can be kept in the patient record or some other archive—they do not need to be submitted with the claim. Images can be stored as printed images, on a tape or electronic medium. Documentation of the study must be available to the insurer upon request.
A written report of all ultrasound studies should be maintained in the patient’s record. In the case of ultrasound guidance, the written report may be filed as a separate item in the patient’s record or it may be included within the report of the procedure for which the guidance is utilized.
As examples of our documentation in the office, copies of 3 of our standard forms are available: “Ultrasound report of the shoulder” (Appendix 1), “Procedure note for an ultrasound-guided injection of cortisone” (Appendix 2), and “Procedure note for an ultrasound-guided injection of platelet-rich plasma” (Appendix 3).
Appropriate Use Criteria (AUC)
The Protecting Access to Medicare Act of 2014 was an effort to help reduce unnecessary imaging services and reduce costs; the Secretary of Health and Human Services was to establish a program to promote the use of “appropriate use criteria” (AUC) for advanced imaging services such as MRI, computed tomography, positron emission tomography, and nuclear cardiology. AUC are criteria that are developed or endorsed by national professional medical specialty societies or other provider-led entities to assist ordering professionals and furnishing professionals in making the most appropriate treatment decision for a specific clinical condition for an individual. The law also noted that the criteria should be evidence-based, meaning they should have stakeholder consensus, be scientifically valid, and be based on studies that are published and reviewable by stakeholders.
By April 2016, the Secretary will identify and publish the list of qualified clinical decision support mechanisms, which are tools that could be used by ordering professionals to ensure that AUC is met for applicable imaging services. These may include certified health electronic record technology, private sector clinical decision support mechanisms, and others. Actual use of the AUC will begin in January 2017. This legislation applies only to Medicare services, but other payers have cited concerns and may follow in the future.
Conclusion
Ultrasound is being increasingly used in varying specialties, especially orthopedic surgery. It provides a time- and cost-efficient modality with diagnostic power comparable to MRI. Portability and a high safety profile allows for ease of implementation as an in-office or sideline tool. Needle guidance and other intraoperative applications highlight its versatility as an adjunct to orthopedic treatments. This article provides a comprehensive guide to billing and coding for both diagnostic and therapeutic MSK ultrasound of the shoulder. Providers should stay up to date with upcoming appropriate use criteria and adjustments to current billing procedures.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Sharpe R, Nazarian L, Parker L, Rao V, Levin D. Dramatically increased musculoskeletal ultrasound utilization from 2000 to 2009, especially by podiatrists in private offices. Department of Radiology Faculty Papers. Paper 16. http://jdc.jefferson.edu/radiologyfp/16. Accessed January 7, 2016.
3. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: The orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
4. Sinha TP, Bhoi S, Kumar S, et al. Diagnostic accuracy of bedside emergency ultrasound screening for fractures in pediatric trauma patients. J Emerg Trauma Shock. 2011;4(4);443-445.
5. Bica D, Armen J, Kulas AS, Young K, Womack Z. Reliability and precision of stress sonography of the ulnar collateral ligament. J Ultrasound Med. 2015;34(3):371-376.
6. Tomer K, Kleinbaum Y, Heyman Z, Dudkiewicz I, Blankstein A. Ultrasound diagnosis of fractures in adults. Akt Traumatol. 2006;36(4):171-174.
7. Middleton W, Payne WT, Teefey SA, Hidebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol. 2004;183(5):1449-1452.
8. Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance and arthroscopic finding in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A(4):708-716.
9. Roy-JS, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis. Br J Sports Med. 2015;49(20):1316-1328.
10. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal Imaging: Medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5(3):182-188.
11. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
12. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
13. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
14. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
15. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
16. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
17. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
18. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
19. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
20. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intra-articular injections? J Rheumatol. 2009;36(9):1892-1902.
1. Sivan M, Brown J, Brennan S, Bhakta B. A one-stop approach to the management of soft tissue and degenerative musculoskeletal conditions using clinic-based ultrasonography. Musculoskeletal Care. 2011;9(2):63-68.
2. Sharpe R, Nazarian L, Parker L, Rao V, Levin D. Dramatically increased musculoskeletal ultrasound utilization from 2000 to 2009, especially by podiatrists in private offices. Department of Radiology Faculty Papers. Paper 16. http://jdc.jefferson.edu/radiologyfp/16. Accessed January 7, 2016.
3. Blankstein A. Ultrasound in the diagnosis of clinical orthopedics: The orthopedic stethoscope. World J Orthop. 2011;2(2):13-24.
4. Sinha TP, Bhoi S, Kumar S, et al. Diagnostic accuracy of bedside emergency ultrasound screening for fractures in pediatric trauma patients. J Emerg Trauma Shock. 2011;4(4);443-445.
5. Bica D, Armen J, Kulas AS, Young K, Womack Z. Reliability and precision of stress sonography of the ulnar collateral ligament. J Ultrasound Med. 2015;34(3):371-376.
6. Tomer K, Kleinbaum Y, Heyman Z, Dudkiewicz I, Blankstein A. Ultrasound diagnosis of fractures in adults. Akt Traumatol. 2006;36(4):171-174.
7. Middleton W, Payne WT, Teefey SA, Hidebolt CF, Rubin DA, Yamaguchi K. Sonography and MRI of the shoulder: comparison of patient satisfaction. AJR Am J Roentgenol. 2004;183(5):1449-1452.
8. Teefey SA, Rubin DA, Middleton WD, Hildebolt CF, Leibold RA, Yamaguchi K. Detection and quantification of rotator cuff tears. Comparison of ultrasonographic, magnetic resonance and arthroscopic finding in seventy-one consecutive cases. J Bone Joint Surg Am. 2004;86-A(4):708-716.
9. Roy-JS, Braën C, Leblond J, et al. Diagnostic accuracy of ultrasonography, MRI and MR arthrography in the characterization of rotator cuff disorders: a meta-analysis. Br J Sports Med. 2015;49(20):1316-1328.
10. Parker L, Nazarian LN, Carrino JA, et al. Musculoskeletal Imaging: Medicare use, costs, and potential for cost substitution. J Am Coll Radiol. 2008;5(3):182-188.
11. Eustace J, Brophy D, Gibney R, Bresnihan B, FitzGerald O. Comparison of the accuracy of steroid placement with clinical outcome in patients with shoulder symptoms. Ann Rheum Dis. 1997;56(1):59-63.
12. Partington P, Broome G. Diagnostic injection around the shoulder: Hit and miss? A cadaveric study of injection accuracy. J Shoulder Elbow Surg. 1998;7(2):147-150.
13. Rutten M, Maresch B, Jager G, de Waal Malefijt M. Injection of the subacromial-subdeltoid bursa: Blind or ultrasound-guided? Acta Orthop. 2007;78(2):254-257.
14. Kang M, Rizio L, Prybicien M, Middlemas D, Blacksin M. The accuracy of subacromial corticosteroid injections: A comparison of multiple methods. J Shoulder Elbow Surg. 2008;17(1 Suppl):61S-66S.
15. Yamakado K. The targeting accuracy of subacromial injection to the shoulder: An arthrographic evaluation. Arthroscopy. 2002;19(8):887-891.
16. Henkus HE, Cobben M, Coerkamp E, Nelissen R, van Arkel E. The accuracy of subacromial injections: A prospective randomized magnetic resonance imaging study. Arthroscopy. 2006;22(3):277-282.
17. Sethi PM, El Attrache N. Accuracy of intra-articular injection of the glenohumeral joint: a cadaveric study. Orthopedics. 2006;29(2):149-152.
18. Naredo E, Cabero F, Beneyto P, et al. A randomized comparative study of short term response to blind injection versus sonographic-guided injection of local corticosteroids in patients with painful shoulder. J Rheumatol. 2004;31(2):308-314.
19. Finnoff JT, Hall MM, Adams E, et al. American Medical Society for Sports Medicine (AMSSM) position statement: interventional musculoskeletal ultrasound in sports medicine. Br J Sports Med. 2015;49(3):145-150.
20. Sibbitt WL Jr, Peisajovich A, Michael AA, et al. Does sonographic needle guidance affect the clinical outcome of intra-articular injections? J Rheumatol. 2009;36(9):1892-1902.
Documentation for Mohs Surgery
In 2013, the Centers for Medicare and Medicaid Services (CMS) issued a guidance to reduce reimbursement issues for Mohs micrographic surgery (MMS).1 One crucial question that remains is when and if these documentation guidelines will be formally implemented. The guidelines outlined by the CMS currently are regarded as suggestions until Medicare contractors adopt them into the local coverage determinations (LCDs).
Key Documentation Guidelines
To reduce MMS reimbursement issues, documentation in the patient’s medical record should support the medical necessity of the procedure and reflect the number and anatomic locations of specimens taken and the reason for the procedure should be clearly communicated. The specific tumor type also should be approved for treatment with MMS in the respective LCD.
Nonphysician providers are not authorized by Medicare to perform MMS. To ensure proper coding, both surgery and pathology must be performed by a single physician and should be supported by documentation in the patient’s medical record (eg, relevant chart notes should be made under the provider’s signature). These documentation guidelines are not new but are included in the CMS guidance to reiterate their importance in reducing MMS reimbursement issues.
Per customary clinical practice, the CMS guidance specifies that MMS documentation should include gross description of the tissue removed, including the location, number, and size of the lesions, as well as how many specimens were removed for each stage. However, the guidance diverges from routine MMS documentation requirements in its emphasis on providing a histologic description of the tissue removed. The guidance suggests that the depth of tumor invasion, pathologic pattern, cell morphology (which is not typically specified for skin cancers), and, if present, the existence of perineural invasion or scar tissue should be documented. If these features are constant across stages, they only need to be noted for the first stage.
Adapting Guidelines for Clinical Practice
The CMS guidance may create some conundrums for physicians regarding MMS documentation; for instance, if a tumor is cleared in one stage, as is often the case, no tumor will be seen on glass slides prepared to assess tissue margins during the procedure and therefore documentation of characteristics like depth and pattern will be impossible. Similarly, cell morphology is not a feature that usually is relevant for most squamous and basal cell carcinomas, although it may be useful in certain unusual instances, such as in cases of rare tumors with particular histologic features that may influence management and/or prognosis. When in doubt regarding the appropriate documentation method for MMS, the surgeon should use his or her best judgment based on clinical experience rather than simply following guidelines that may not be applicable.
Final Thoughts
The CMS guidance serves as a reminder of the documentation requirements for MMS and extends current practice by suggesting a detailed microscopic description of the removed tissue. The American Academy of Dermatology has developed a guide to help Mohs surgeons provide the necessary documentation without creating cumbersome chart notes.2 Mohs surgeons should consult the most recent version of the LCD that applies to their geographic area to determine if the new documentation guidelines have been adopted.
- Centers for Medicare and Medicaid Services. Guidance to Reduce Mohs Surgery Reimbursement Issues. Bethesda, MD: Centers for Medicare and Medicaid Services, US Department of Health and Human Services; 2013. MLN Matters SE1318.
- Position statement on documentation of frozen section specimens during Mohs micrographic surgery. American Academy of Dermatology Web site. https://www.aad.org/forms/policies/Uploads/PS/PS%20-%20Documentation%20of%20Frozen%20Section%20Specimens%20during%20Mohs%20Micrographic%20Surgery.pdf. Accessed November 30, 2015.
In 2013, the Centers for Medicare and Medicaid Services (CMS) issued a guidance to reduce reimbursement issues for Mohs micrographic surgery (MMS).1 One crucial question that remains is when and if these documentation guidelines will be formally implemented. The guidelines outlined by the CMS currently are regarded as suggestions until Medicare contractors adopt them into the local coverage determinations (LCDs).
Key Documentation Guidelines
To reduce MMS reimbursement issues, documentation in the patient’s medical record should support the medical necessity of the procedure and reflect the number and anatomic locations of specimens taken and the reason for the procedure should be clearly communicated. The specific tumor type also should be approved for treatment with MMS in the respective LCD.
Nonphysician providers are not authorized by Medicare to perform MMS. To ensure proper coding, both surgery and pathology must be performed by a single physician and should be supported by documentation in the patient’s medical record (eg, relevant chart notes should be made under the provider’s signature). These documentation guidelines are not new but are included in the CMS guidance to reiterate their importance in reducing MMS reimbursement issues.
Per customary clinical practice, the CMS guidance specifies that MMS documentation should include gross description of the tissue removed, including the location, number, and size of the lesions, as well as how many specimens were removed for each stage. However, the guidance diverges from routine MMS documentation requirements in its emphasis on providing a histologic description of the tissue removed. The guidance suggests that the depth of tumor invasion, pathologic pattern, cell morphology (which is not typically specified for skin cancers), and, if present, the existence of perineural invasion or scar tissue should be documented. If these features are constant across stages, they only need to be noted for the first stage.
Adapting Guidelines for Clinical Practice
The CMS guidance may create some conundrums for physicians regarding MMS documentation; for instance, if a tumor is cleared in one stage, as is often the case, no tumor will be seen on glass slides prepared to assess tissue margins during the procedure and therefore documentation of characteristics like depth and pattern will be impossible. Similarly, cell morphology is not a feature that usually is relevant for most squamous and basal cell carcinomas, although it may be useful in certain unusual instances, such as in cases of rare tumors with particular histologic features that may influence management and/or prognosis. When in doubt regarding the appropriate documentation method for MMS, the surgeon should use his or her best judgment based on clinical experience rather than simply following guidelines that may not be applicable.
Final Thoughts
The CMS guidance serves as a reminder of the documentation requirements for MMS and extends current practice by suggesting a detailed microscopic description of the removed tissue. The American Academy of Dermatology has developed a guide to help Mohs surgeons provide the necessary documentation without creating cumbersome chart notes.2 Mohs surgeons should consult the most recent version of the LCD that applies to their geographic area to determine if the new documentation guidelines have been adopted.
In 2013, the Centers for Medicare and Medicaid Services (CMS) issued a guidance to reduce reimbursement issues for Mohs micrographic surgery (MMS).1 One crucial question that remains is when and if these documentation guidelines will be formally implemented. The guidelines outlined by the CMS currently are regarded as suggestions until Medicare contractors adopt them into the local coverage determinations (LCDs).
Key Documentation Guidelines
To reduce MMS reimbursement issues, documentation in the patient’s medical record should support the medical necessity of the procedure and reflect the number and anatomic locations of specimens taken and the reason for the procedure should be clearly communicated. The specific tumor type also should be approved for treatment with MMS in the respective LCD.
Nonphysician providers are not authorized by Medicare to perform MMS. To ensure proper coding, both surgery and pathology must be performed by a single physician and should be supported by documentation in the patient’s medical record (eg, relevant chart notes should be made under the provider’s signature). These documentation guidelines are not new but are included in the CMS guidance to reiterate their importance in reducing MMS reimbursement issues.
Per customary clinical practice, the CMS guidance specifies that MMS documentation should include gross description of the tissue removed, including the location, number, and size of the lesions, as well as how many specimens were removed for each stage. However, the guidance diverges from routine MMS documentation requirements in its emphasis on providing a histologic description of the tissue removed. The guidance suggests that the depth of tumor invasion, pathologic pattern, cell morphology (which is not typically specified for skin cancers), and, if present, the existence of perineural invasion or scar tissue should be documented. If these features are constant across stages, they only need to be noted for the first stage.
Adapting Guidelines for Clinical Practice
The CMS guidance may create some conundrums for physicians regarding MMS documentation; for instance, if a tumor is cleared in one stage, as is often the case, no tumor will be seen on glass slides prepared to assess tissue margins during the procedure and therefore documentation of characteristics like depth and pattern will be impossible. Similarly, cell morphology is not a feature that usually is relevant for most squamous and basal cell carcinomas, although it may be useful in certain unusual instances, such as in cases of rare tumors with particular histologic features that may influence management and/or prognosis. When in doubt regarding the appropriate documentation method for MMS, the surgeon should use his or her best judgment based on clinical experience rather than simply following guidelines that may not be applicable.
Final Thoughts
The CMS guidance serves as a reminder of the documentation requirements for MMS and extends current practice by suggesting a detailed microscopic description of the removed tissue. The American Academy of Dermatology has developed a guide to help Mohs surgeons provide the necessary documentation without creating cumbersome chart notes.2 Mohs surgeons should consult the most recent version of the LCD that applies to their geographic area to determine if the new documentation guidelines have been adopted.
- Centers for Medicare and Medicaid Services. Guidance to Reduce Mohs Surgery Reimbursement Issues. Bethesda, MD: Centers for Medicare and Medicaid Services, US Department of Health and Human Services; 2013. MLN Matters SE1318.
- Position statement on documentation of frozen section specimens during Mohs micrographic surgery. American Academy of Dermatology Web site. https://www.aad.org/forms/policies/Uploads/PS/PS%20-%20Documentation%20of%20Frozen%20Section%20Specimens%20during%20Mohs%20Micrographic%20Surgery.pdf. Accessed November 30, 2015.
- Centers for Medicare and Medicaid Services. Guidance to Reduce Mohs Surgery Reimbursement Issues. Bethesda, MD: Centers for Medicare and Medicaid Services, US Department of Health and Human Services; 2013. MLN Matters SE1318.
- Position statement on documentation of frozen section specimens during Mohs micrographic surgery. American Academy of Dermatology Web site. https://www.aad.org/forms/policies/Uploads/PS/PS%20-%20Documentation%20of%20Frozen%20Section%20Specimens%20during%20Mohs%20Micrographic%20Surgery.pdf. Accessed November 30, 2015.
ICD-10 Flexibility Helps Transition to New Coding Systems, Principles, Payer Policy Requirements
Effective October 1, providers submit claims with ICD-10-CM codes. As they adapt to this new system, physicians, clinical staff, and billers should be relying on feedback from each other to achieve a successful transition. On July 6, the Centers for Medicare and Medicaid Services (CMS), in conjunction with the AMA, issued a letter to the provider community offering ICD-10-CM guidance. The joint announcement and guidance regarding ICD-10 flexibilities minimizes the anxiety that often accompanies change and clarifies a few key points about claim scrutiny.1
According to the correspondence, “CMS is releasing additional guidance that will allow for flexibility in the claims auditing and quality reporting process as the medical community gains experience using the new ICD-10 code set.”1 The guidance specifies the flexibility that will be used during the first 12 months of ICD-10-CM use.
This “flexibility” is an opportunity and should not be disregarded. Physician practices can effectively use this time to become accustomed to the ICD-10-CM system, correct coding principles, and payer policy requirements. Internal audit and review processes should increase in order to correct or confirm appropriate coding and claim submission.
Valid Codes
Medicare review contractors are instructed “not to deny physician or other practitioner claims billed under the Part B physician fee schedule through either automated medical review or complex medical review based solely on the specificity of the ICD-10 diagnosis code as long as the physician/practitioner used a valid code from the right family.”2 This “flexibility” will only occur for the first 12 months of ICD-10-CM implementation; the ultimate goal is for providers to assign the correct diagnosis code and the appropriate level of specificity after one year.
The “family code” allowance should not be confused with provision of an incomplete or truncated diagnosis code; these types of codes will always result in claim denial. The ICD-10-CM code presented on the claim form must be carried out to the highest character available for that particular code.
For example, an initial encounter involving an infected peripherally inserted central catheter (PICC) is reported with ICD-10-CM T80.212A (local infection due to central venous catheter). An individual unfamiliar with ICD-10-CM nomenclature may not realize that the seventh extension character of the code is required to carry the code out to its highest level of specificity. If T880.212 is mistakenly reported because the encounter detail (i.e., initial encounter [A], subsequent encounter [D], or sequela [S]) was not documented or provided to the biller, the payers’ claim edit system will identify this as a truncated or invalid diagnosis and reject the claim. Therefore, the code is required to be complete. The “flexibility” refers to reporting the code that best reflects the documented condition. As long as the reported code comes from the same family of codes and is valid, the claim cannot be denied.
Code Families
Code families are “codes within a category [that] are clinically related and provide differences in capturing specific information on the type of condition.”3 Upon review, Medicare will allow ICD-10-CM codes from the same code family to be reported on the claim without penalty if the most accurate code is not selected.
For example, a patient with COPD with acute exacerbation is admitted to the hospital. During the 12-month “flexibility” period, the claim could include J44.9 (COPD, unspecified) without being considered erroneous. The most appropriate code, however, is J44.1 (COPD with acute exacerbation). During the course of the hospitalization, if the physician determines that the COPD exacerbation was caused by an acute lower respiratory infection, J44.0 (COPD with acute lower respiratory infection) is the best option.
The provider goal for this flexibility period is to identify all of the “unspecified codes” used on their claims, review the documentation, and determine the most appropriate code. The practice staff assigned to this task would then provide feedback to the physicians to enhance their future reporting strategies. Although “unspecified” codes are often reported by default, physicians and staff should attempt to reduce usage of this code type unless the patient’s condition is unable to be further specified or categorized at a given point in time.
For example, it would not be acceptable to report R10.8 (unspecified abdominal pain) when a more specific diagnosis code can be easily determined by patient history or exam findings (e.g. right upper quadrant abdominal pain, R10.11).
Affected Claims
As previously stated, “Medicare review contractors will not deny physician or other practitioner claims billed under the Part B physician fee schedule through either automated medical review or complex medical record review.”3 The review contractors included are as follows:
- Medicare Administrative Contractors (MACs) process claims submitted by physicians, hospitals, and other healthcare professionals and submit payment to those providers according to Medicare rules and regulations (including identifying and correcting underpayments and overpayments);
- Recovery Auditors (RACs) review claims to identify potential underpayments and overpayments in Medicare fee-for-service, as part of the Recovery Audit Program;
- Zone Program Integrity Contractors (ZPICs) perform investigations that are unique and tailored to the specific circumstances and occur only in situations where there is potential fraud and take appropriate corrective actions; and
- Supplemental Medical Review Contractor (SMRCs) conduct nationwide medical review as directed by CMS (including identifying underpayments and overpayments).4
This instruction applies to claims that are typically selected for review due to the ICD-10-CM code used on the claim but does not affect claims that are selected for review for other reasons (e.g. modifier 25 [separately identifiable visit performed on the same day as another procedure or service], unbundling, service-specific current procedural terminology code). If a claim is selected for one of these other reasons and does not meet the corresponding criterion, the service will be denied. This instruction also excludes claims for services that correspond to an existing local coverage determination (LCD) or national coverage determination (NCD).
For example, an esophagogastroduodenoscopy (EGD) is not considered “medically necessary” when reported with R10.8 (unspecified abdominal pain) and would be denied. EGD requires a more specific diagnosis (e.g. right upper quadrant abdominal pain, R10.11) per Medicare LCD.
Non-Medicare Payer Considerations
Most payers that are required to convert to ICD-10-CM have also provided some guidance about claim submission. Although most do not address the audit and review process, payers will follow some basic principles:
- Claims submitted with service dates on or after October 1 must use ICD-10-CM codes.
- Claims submitted with service dates prior to October 1 must use ICD-9-CM codes; this includes claims that are initially submitted after October 1 or require correction and resubmission after October 1.
- Physician claims will be held to medical necessity guidelines identified by specific ICD-10-CM codes represented in existing payer policies.
- General equivalence mappings (GEMs) should only be used as a starting point to convert large databases and large code lists from ICD-9 to ICD-10. Many ICD-9-CM codes do not crosswalk directly to an ICD-10-CM code. Physician and staff should continue to use the ICD-10-CM coding books and resources to determine the most accurate code selection.
- “Unspecified” codes are only for use when the information in the medical record is insufficient to assign a more specific code.5,6,7
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is on the faculty of SHM’s inpatient coding course.
References
- Centers for Medicare and Medicaid Services. CMS and AMA announce efforts to help providers get ready for ICD-10. July 6, 2015. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. CMS and AMA announce efforts to help providers get ready for ICD-10: frequently asked questions. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. Clarifying questions and answers related to the July 6, 2015 CMS/AMA joint announcement and guidance regarding ICD-10 flexibilities. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. Medicare Learning Network: Medicare claim review programs. May 2015. Accessed October 3, 2015.
- Aetna. Preparation for ICD-10-CM: frequently asked questions. Accessed October 3, 2015.
- Independence Blue Cross. Transition to ICD-10: frequently asked questions. Accessed October 3, 2015.
- Cigna. Ready, Set, Switch: Know Your ICD-10 Codes. Accessed November 16, 2015.
Effective October 1, providers submit claims with ICD-10-CM codes. As they adapt to this new system, physicians, clinical staff, and billers should be relying on feedback from each other to achieve a successful transition. On July 6, the Centers for Medicare and Medicaid Services (CMS), in conjunction with the AMA, issued a letter to the provider community offering ICD-10-CM guidance. The joint announcement and guidance regarding ICD-10 flexibilities minimizes the anxiety that often accompanies change and clarifies a few key points about claim scrutiny.1
According to the correspondence, “CMS is releasing additional guidance that will allow for flexibility in the claims auditing and quality reporting process as the medical community gains experience using the new ICD-10 code set.”1 The guidance specifies the flexibility that will be used during the first 12 months of ICD-10-CM use.
This “flexibility” is an opportunity and should not be disregarded. Physician practices can effectively use this time to become accustomed to the ICD-10-CM system, correct coding principles, and payer policy requirements. Internal audit and review processes should increase in order to correct or confirm appropriate coding and claim submission.
Valid Codes
Medicare review contractors are instructed “not to deny physician or other practitioner claims billed under the Part B physician fee schedule through either automated medical review or complex medical review based solely on the specificity of the ICD-10 diagnosis code as long as the physician/practitioner used a valid code from the right family.”2 This “flexibility” will only occur for the first 12 months of ICD-10-CM implementation; the ultimate goal is for providers to assign the correct diagnosis code and the appropriate level of specificity after one year.
The “family code” allowance should not be confused with provision of an incomplete or truncated diagnosis code; these types of codes will always result in claim denial. The ICD-10-CM code presented on the claim form must be carried out to the highest character available for that particular code.
For example, an initial encounter involving an infected peripherally inserted central catheter (PICC) is reported with ICD-10-CM T80.212A (local infection due to central venous catheter). An individual unfamiliar with ICD-10-CM nomenclature may not realize that the seventh extension character of the code is required to carry the code out to its highest level of specificity. If T880.212 is mistakenly reported because the encounter detail (i.e., initial encounter [A], subsequent encounter [D], or sequela [S]) was not documented or provided to the biller, the payers’ claim edit system will identify this as a truncated or invalid diagnosis and reject the claim. Therefore, the code is required to be complete. The “flexibility” refers to reporting the code that best reflects the documented condition. As long as the reported code comes from the same family of codes and is valid, the claim cannot be denied.
Code Families
Code families are “codes within a category [that] are clinically related and provide differences in capturing specific information on the type of condition.”3 Upon review, Medicare will allow ICD-10-CM codes from the same code family to be reported on the claim without penalty if the most accurate code is not selected.
For example, a patient with COPD with acute exacerbation is admitted to the hospital. During the 12-month “flexibility” period, the claim could include J44.9 (COPD, unspecified) without being considered erroneous. The most appropriate code, however, is J44.1 (COPD with acute exacerbation). During the course of the hospitalization, if the physician determines that the COPD exacerbation was caused by an acute lower respiratory infection, J44.0 (COPD with acute lower respiratory infection) is the best option.
The provider goal for this flexibility period is to identify all of the “unspecified codes” used on their claims, review the documentation, and determine the most appropriate code. The practice staff assigned to this task would then provide feedback to the physicians to enhance their future reporting strategies. Although “unspecified” codes are often reported by default, physicians and staff should attempt to reduce usage of this code type unless the patient’s condition is unable to be further specified or categorized at a given point in time.
For example, it would not be acceptable to report R10.8 (unspecified abdominal pain) when a more specific diagnosis code can be easily determined by patient history or exam findings (e.g. right upper quadrant abdominal pain, R10.11).
Affected Claims
As previously stated, “Medicare review contractors will not deny physician or other practitioner claims billed under the Part B physician fee schedule through either automated medical review or complex medical record review.”3 The review contractors included are as follows:
- Medicare Administrative Contractors (MACs) process claims submitted by physicians, hospitals, and other healthcare professionals and submit payment to those providers according to Medicare rules and regulations (including identifying and correcting underpayments and overpayments);
- Recovery Auditors (RACs) review claims to identify potential underpayments and overpayments in Medicare fee-for-service, as part of the Recovery Audit Program;
- Zone Program Integrity Contractors (ZPICs) perform investigations that are unique and tailored to the specific circumstances and occur only in situations where there is potential fraud and take appropriate corrective actions; and
- Supplemental Medical Review Contractor (SMRCs) conduct nationwide medical review as directed by CMS (including identifying underpayments and overpayments).4
This instruction applies to claims that are typically selected for review due to the ICD-10-CM code used on the claim but does not affect claims that are selected for review for other reasons (e.g. modifier 25 [separately identifiable visit performed on the same day as another procedure or service], unbundling, service-specific current procedural terminology code). If a claim is selected for one of these other reasons and does not meet the corresponding criterion, the service will be denied. This instruction also excludes claims for services that correspond to an existing local coverage determination (LCD) or national coverage determination (NCD).
For example, an esophagogastroduodenoscopy (EGD) is not considered “medically necessary” when reported with R10.8 (unspecified abdominal pain) and would be denied. EGD requires a more specific diagnosis (e.g. right upper quadrant abdominal pain, R10.11) per Medicare LCD.
Non-Medicare Payer Considerations
Most payers that are required to convert to ICD-10-CM have also provided some guidance about claim submission. Although most do not address the audit and review process, payers will follow some basic principles:
- Claims submitted with service dates on or after October 1 must use ICD-10-CM codes.
- Claims submitted with service dates prior to October 1 must use ICD-9-CM codes; this includes claims that are initially submitted after October 1 or require correction and resubmission after October 1.
- Physician claims will be held to medical necessity guidelines identified by specific ICD-10-CM codes represented in existing payer policies.
- General equivalence mappings (GEMs) should only be used as a starting point to convert large databases and large code lists from ICD-9 to ICD-10. Many ICD-9-CM codes do not crosswalk directly to an ICD-10-CM code. Physician and staff should continue to use the ICD-10-CM coding books and resources to determine the most accurate code selection.
- “Unspecified” codes are only for use when the information in the medical record is insufficient to assign a more specific code.5,6,7
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is on the faculty of SHM’s inpatient coding course.
References
- Centers for Medicare and Medicaid Services. CMS and AMA announce efforts to help providers get ready for ICD-10. July 6, 2015. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. CMS and AMA announce efforts to help providers get ready for ICD-10: frequently asked questions. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. Clarifying questions and answers related to the July 6, 2015 CMS/AMA joint announcement and guidance regarding ICD-10 flexibilities. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. Medicare Learning Network: Medicare claim review programs. May 2015. Accessed October 3, 2015.
- Aetna. Preparation for ICD-10-CM: frequently asked questions. Accessed October 3, 2015.
- Independence Blue Cross. Transition to ICD-10: frequently asked questions. Accessed October 3, 2015.
- Cigna. Ready, Set, Switch: Know Your ICD-10 Codes. Accessed November 16, 2015.
Effective October 1, providers submit claims with ICD-10-CM codes. As they adapt to this new system, physicians, clinical staff, and billers should be relying on feedback from each other to achieve a successful transition. On July 6, the Centers for Medicare and Medicaid Services (CMS), in conjunction with the AMA, issued a letter to the provider community offering ICD-10-CM guidance. The joint announcement and guidance regarding ICD-10 flexibilities minimizes the anxiety that often accompanies change and clarifies a few key points about claim scrutiny.1
According to the correspondence, “CMS is releasing additional guidance that will allow for flexibility in the claims auditing and quality reporting process as the medical community gains experience using the new ICD-10 code set.”1 The guidance specifies the flexibility that will be used during the first 12 months of ICD-10-CM use.
This “flexibility” is an opportunity and should not be disregarded. Physician practices can effectively use this time to become accustomed to the ICD-10-CM system, correct coding principles, and payer policy requirements. Internal audit and review processes should increase in order to correct or confirm appropriate coding and claim submission.
Valid Codes
Medicare review contractors are instructed “not to deny physician or other practitioner claims billed under the Part B physician fee schedule through either automated medical review or complex medical review based solely on the specificity of the ICD-10 diagnosis code as long as the physician/practitioner used a valid code from the right family.”2 This “flexibility” will only occur for the first 12 months of ICD-10-CM implementation; the ultimate goal is for providers to assign the correct diagnosis code and the appropriate level of specificity after one year.
The “family code” allowance should not be confused with provision of an incomplete or truncated diagnosis code; these types of codes will always result in claim denial. The ICD-10-CM code presented on the claim form must be carried out to the highest character available for that particular code.
For example, an initial encounter involving an infected peripherally inserted central catheter (PICC) is reported with ICD-10-CM T80.212A (local infection due to central venous catheter). An individual unfamiliar with ICD-10-CM nomenclature may not realize that the seventh extension character of the code is required to carry the code out to its highest level of specificity. If T880.212 is mistakenly reported because the encounter detail (i.e., initial encounter [A], subsequent encounter [D], or sequela [S]) was not documented or provided to the biller, the payers’ claim edit system will identify this as a truncated or invalid diagnosis and reject the claim. Therefore, the code is required to be complete. The “flexibility” refers to reporting the code that best reflects the documented condition. As long as the reported code comes from the same family of codes and is valid, the claim cannot be denied.
Code Families
Code families are “codes within a category [that] are clinically related and provide differences in capturing specific information on the type of condition.”3 Upon review, Medicare will allow ICD-10-CM codes from the same code family to be reported on the claim without penalty if the most accurate code is not selected.
For example, a patient with COPD with acute exacerbation is admitted to the hospital. During the 12-month “flexibility” period, the claim could include J44.9 (COPD, unspecified) without being considered erroneous. The most appropriate code, however, is J44.1 (COPD with acute exacerbation). During the course of the hospitalization, if the physician determines that the COPD exacerbation was caused by an acute lower respiratory infection, J44.0 (COPD with acute lower respiratory infection) is the best option.
The provider goal for this flexibility period is to identify all of the “unspecified codes” used on their claims, review the documentation, and determine the most appropriate code. The practice staff assigned to this task would then provide feedback to the physicians to enhance their future reporting strategies. Although “unspecified” codes are often reported by default, physicians and staff should attempt to reduce usage of this code type unless the patient’s condition is unable to be further specified or categorized at a given point in time.
For example, it would not be acceptable to report R10.8 (unspecified abdominal pain) when a more specific diagnosis code can be easily determined by patient history or exam findings (e.g. right upper quadrant abdominal pain, R10.11).
Affected Claims
As previously stated, “Medicare review contractors will not deny physician or other practitioner claims billed under the Part B physician fee schedule through either automated medical review or complex medical record review.”3 The review contractors included are as follows:
- Medicare Administrative Contractors (MACs) process claims submitted by physicians, hospitals, and other healthcare professionals and submit payment to those providers according to Medicare rules and regulations (including identifying and correcting underpayments and overpayments);
- Recovery Auditors (RACs) review claims to identify potential underpayments and overpayments in Medicare fee-for-service, as part of the Recovery Audit Program;
- Zone Program Integrity Contractors (ZPICs) perform investigations that are unique and tailored to the specific circumstances and occur only in situations where there is potential fraud and take appropriate corrective actions; and
- Supplemental Medical Review Contractor (SMRCs) conduct nationwide medical review as directed by CMS (including identifying underpayments and overpayments).4
This instruction applies to claims that are typically selected for review due to the ICD-10-CM code used on the claim but does not affect claims that are selected for review for other reasons (e.g. modifier 25 [separately identifiable visit performed on the same day as another procedure or service], unbundling, service-specific current procedural terminology code). If a claim is selected for one of these other reasons and does not meet the corresponding criterion, the service will be denied. This instruction also excludes claims for services that correspond to an existing local coverage determination (LCD) or national coverage determination (NCD).
For example, an esophagogastroduodenoscopy (EGD) is not considered “medically necessary” when reported with R10.8 (unspecified abdominal pain) and would be denied. EGD requires a more specific diagnosis (e.g. right upper quadrant abdominal pain, R10.11) per Medicare LCD.
Non-Medicare Payer Considerations
Most payers that are required to convert to ICD-10-CM have also provided some guidance about claim submission. Although most do not address the audit and review process, payers will follow some basic principles:
- Claims submitted with service dates on or after October 1 must use ICD-10-CM codes.
- Claims submitted with service dates prior to October 1 must use ICD-9-CM codes; this includes claims that are initially submitted after October 1 or require correction and resubmission after October 1.
- Physician claims will be held to medical necessity guidelines identified by specific ICD-10-CM codes represented in existing payer policies.
- General equivalence mappings (GEMs) should only be used as a starting point to convert large databases and large code lists from ICD-9 to ICD-10. Many ICD-9-CM codes do not crosswalk directly to an ICD-10-CM code. Physician and staff should continue to use the ICD-10-CM coding books and resources to determine the most accurate code selection.
- “Unspecified” codes are only for use when the information in the medical record is insufficient to assign a more specific code.5,6,7
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is on the faculty of SHM’s inpatient coding course.
References
- Centers for Medicare and Medicaid Services. CMS and AMA announce efforts to help providers get ready for ICD-10. July 6, 2015. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. CMS and AMA announce efforts to help providers get ready for ICD-10: frequently asked questions. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. Clarifying questions and answers related to the July 6, 2015 CMS/AMA joint announcement and guidance regarding ICD-10 flexibilities. Accessed October 3, 2015.
- Centers for Medicare and Medicaid Services. Medicare Learning Network: Medicare claim review programs. May 2015. Accessed October 3, 2015.
- Aetna. Preparation for ICD-10-CM: frequently asked questions. Accessed October 3, 2015.
- Independence Blue Cross. Transition to ICD-10: frequently asked questions. Accessed October 3, 2015.
- Cigna. Ready, Set, Switch: Know Your ICD-10 Codes. Accessed November 16, 2015.
ICD-10 Under ACP Scrutiny
NEW YORK - While the new International Classification of Diseases, Tenth Revision, Clinical
Modification (ICD-10-CM) codes offer greater diagnostic precision, their implementation will require training of clinicians, coders, and other staff to minimize payment denials or delays from both public and private payers.
Brian Outland and colleagues from the American College of Physicians in Washington, D.C., outline some of the promises and challenges of ICD-10-CM implementation in a report online Sept. 22 in Annals of Internal Medicine.
Although completed and endorsed by the World Health Assembly in 1990, ICD-10-CM's implementation date has repeatedly been delayed, and was scheduled to take effect on Oct. 1.
The authors suggest that "the newer coding system will produce data that will indicate the clinical trajectory and other factors that will enable the data to be used in meaningful ways to better understand complications, design robust algorithms for clinical decision support, and track outcomes. Having these details built into the codes will decrease the need for health care providers to include supporting documentation with claims."
The new ICD-10-CM alphanumeric codes will contain as many as seven characters that specify categories, subcategories, laterality, severity and other features.
The use of codes that are not specific enough can result in payment denials or delays, so practices will need to keep current on payer reimbursement policies to ensure the reporting of ICD-10-CM codes that support reimbursement, the authors note.
The cost for the training of clinicians and staffs will depend on practice size, specialty, the method of training, current documentation quality, and technology readiness and availability.
Dr. Susan H. Fenton from UTHealth School of Biomedical Informatics in Houston, Texas, said by email, "One of the thoughts I cannot get away from is that the U.S. is trying to manage a 21st-century, rapidly evolving healthcare system with a 1970s technology. I can think of little else in healthcare that has remained as static since the 1970s."
"The diagnostic system added lots of codes, but the basic structure is the same," she said.
"Certainly, with more detail such as laterality, as well as first encounter, subsequent encounter, and sequelae, it will be much easier to track care for specific conditions across providers," Dr. Fenton said. "I think the issue of claims denials will have to play out over time."
Resources and tools from the Centers for Medicare & Medicaid Services (CMS) can be found online at www.roadto10.org.
The American College of Physicians also has helpful information available at www.acponline.org/ICD10.
Outland did not respond to a request for comment.
NEW YORK - While the new International Classification of Diseases, Tenth Revision, Clinical
Modification (ICD-10-CM) codes offer greater diagnostic precision, their implementation will require training of clinicians, coders, and other staff to minimize payment denials or delays from both public and private payers.
Brian Outland and colleagues from the American College of Physicians in Washington, D.C., outline some of the promises and challenges of ICD-10-CM implementation in a report online Sept. 22 in Annals of Internal Medicine.
Although completed and endorsed by the World Health Assembly in 1990, ICD-10-CM's implementation date has repeatedly been delayed, and was scheduled to take effect on Oct. 1.
The authors suggest that "the newer coding system will produce data that will indicate the clinical trajectory and other factors that will enable the data to be used in meaningful ways to better understand complications, design robust algorithms for clinical decision support, and track outcomes. Having these details built into the codes will decrease the need for health care providers to include supporting documentation with claims."
The new ICD-10-CM alphanumeric codes will contain as many as seven characters that specify categories, subcategories, laterality, severity and other features.
The use of codes that are not specific enough can result in payment denials or delays, so practices will need to keep current on payer reimbursement policies to ensure the reporting of ICD-10-CM codes that support reimbursement, the authors note.
The cost for the training of clinicians and staffs will depend on practice size, specialty, the method of training, current documentation quality, and technology readiness and availability.
Dr. Susan H. Fenton from UTHealth School of Biomedical Informatics in Houston, Texas, said by email, "One of the thoughts I cannot get away from is that the U.S. is trying to manage a 21st-century, rapidly evolving healthcare system with a 1970s technology. I can think of little else in healthcare that has remained as static since the 1970s."
"The diagnostic system added lots of codes, but the basic structure is the same," she said.
"Certainly, with more detail such as laterality, as well as first encounter, subsequent encounter, and sequelae, it will be much easier to track care for specific conditions across providers," Dr. Fenton said. "I think the issue of claims denials will have to play out over time."
Resources and tools from the Centers for Medicare & Medicaid Services (CMS) can be found online at www.roadto10.org.
The American College of Physicians also has helpful information available at www.acponline.org/ICD10.
Outland did not respond to a request for comment.
NEW YORK - While the new International Classification of Diseases, Tenth Revision, Clinical
Modification (ICD-10-CM) codes offer greater diagnostic precision, their implementation will require training of clinicians, coders, and other staff to minimize payment denials or delays from both public and private payers.
Brian Outland and colleagues from the American College of Physicians in Washington, D.C., outline some of the promises and challenges of ICD-10-CM implementation in a report online Sept. 22 in Annals of Internal Medicine.
Although completed and endorsed by the World Health Assembly in 1990, ICD-10-CM's implementation date has repeatedly been delayed, and was scheduled to take effect on Oct. 1.
The authors suggest that "the newer coding system will produce data that will indicate the clinical trajectory and other factors that will enable the data to be used in meaningful ways to better understand complications, design robust algorithms for clinical decision support, and track outcomes. Having these details built into the codes will decrease the need for health care providers to include supporting documentation with claims."
The new ICD-10-CM alphanumeric codes will contain as many as seven characters that specify categories, subcategories, laterality, severity and other features.
The use of codes that are not specific enough can result in payment denials or delays, so practices will need to keep current on payer reimbursement policies to ensure the reporting of ICD-10-CM codes that support reimbursement, the authors note.
The cost for the training of clinicians and staffs will depend on practice size, specialty, the method of training, current documentation quality, and technology readiness and availability.
Dr. Susan H. Fenton from UTHealth School of Biomedical Informatics in Houston, Texas, said by email, "One of the thoughts I cannot get away from is that the U.S. is trying to manage a 21st-century, rapidly evolving healthcare system with a 1970s technology. I can think of little else in healthcare that has remained as static since the 1970s."
"The diagnostic system added lots of codes, but the basic structure is the same," she said.
"Certainly, with more detail such as laterality, as well as first encounter, subsequent encounter, and sequelae, it will be much easier to track care for specific conditions across providers," Dr. Fenton said. "I think the issue of claims denials will have to play out over time."
Resources and tools from the Centers for Medicare & Medicaid Services (CMS) can be found online at www.roadto10.org.
The American College of Physicians also has helpful information available at www.acponline.org/ICD10.
Outland did not respond to a request for comment.
Coding for Biopsies, Shave Removals, and Excisions
In dermatology, samples of skin and subcutaneous tissue are routinely removed to establish a diagnosis, treat symptomatic lesions, or remove potential tumors. The Current Procedural Terminology (CPT) codes used in billing for these procedures typically are generic, but it is important to differentiate between 3 degrees of tissue removal—biopsy, shave removal, and excision—when billing for these services since different codes may be appropriate in each of these circumstances.
Biopsy
Specifically, biopsy (CPT codes 11100/11101) is described as an “independent…procedure to obtain tissue for pathologic examination.”1 The method of biopsy is not specified by CPT and can include any of the following, as long as the primary purpose of the procedure is to remove tissue for analysis: removal by scissors, shaving with a blade or specialized instrument to any level including the subcutaneous fat, extraction using a punch, and excision down to the subcutaneous fat with a scalpel. The feature that differentiates biopsy from shave removal or excision is not depth or extent of tissue mobilization but the intent “to remove a portion of skin, suspect lesion, or entire lesion so that it can be examined histologically.”2 The underlying assumption is that neither definitive clinical nor histologic diagnosis exists prior to biopsy, the purpose of which is to help establish the identity of the lesion.
If the tissue within a large, single lesion is sampled at several separate locations at the same visit, then only a single unit of a single biopsy code (eg, either 11100, 11101, or some site-specific code) should be reported.In contrast, if a number of discrete lesions in the same approximate anatomic area were sampled for diagnoses, each sample taken from separate lesions would constitute a distinct biopsy and would be billed as a separate unit of service.
Shave Removals and Excisions
Shave removal of skin lesions (CPT codes 11300–11313) includes the removal of tangential or saucerized skin lesions to a level no deeper than the base of the dermis. The CPT provides no detailed guidance regarding differentiation of codes for shave removal versus biopsy when a specimen is submitted for histopathologic examination other than the definition of biopsy that was discussed previously. If the tissue is removed specifically for establishing diagnosis, then by definition the procedure should be coded as a biopsy. On the other hand, shave removal implies the intent to completely remove a lesion that already has a presumptive clinical or histologic diagnosis or is being removed for some purpose other than diagnosis (eg, symptomatic relief).
Shave removals are, however, clearly different than excisions (CPT codes 11400–11646), which must proceed through the entire dermis to the subcutis. Additionally, skin lesion excisions include margins, as the intent of an excision procedure is to remove the entire lesion along with a margin of normal skin around it.2
Specialized Biopsy and Excision Codes
While most biopsies, shave removals, and excisions are performed using generic codes, there are specialized circumstances when more specific codes may be preferable. For instance, there are site-specific skin biopsy codes for the nail unit (11755), vermilion and mucosal lip (40490), penis (54100), vulva (56605), and external ear (69100) that take into account the additional complexity of biopsy at these anatomic locations. There also is a site-specific code for eyelid biopsy (67810), which was redefined in 2013 as an “incisional biopsy of eyelid skin including lid margin.”1 Therefore, biopsies of eyelid skin that do not remove the eyelid margin must be coded as 11100/11101, or if the entire cutaneous lesion was removed, can be reclassified as shave removals, which would be coded in the 11310 to 11313 range.
Specialized excision codes include those of the soft tissue. Soft tissue excision codes typically used by dermatologists are not numbered consecutively, are site-specific, and are typically used for resection of benign tumors confined to the subcutaneous tissue below the skin but above the deep fascia. Cysts of all types, including epidermoid and pilar cysts, are specifically excluded from this code set regardless of how large or complex they may be, as they protrude into the dermis or above and are not exclusively in the subcutis. However, lipomas meet the definition for soft-tissue excision, and therefore site-specific soft tissue excision codes can be used in lieu of traditional skin excision codes. The soft-tissue excision codes are distributed throughout the CPT manual, with distinct codes for the abdominal wall (22902, 22903); leg or ankle (27618, 27632); back or flank (21930, 21931); external auditory canal (69145); upper arm or elbow (24075, 24071); face or scalp (21011, 21012); hand or finger (26115, 26111); foot or toe (28043, 28039); forearm or wrist (25075, 25071); hip or pelvis (27047, 27043); thigh or knee (27327, 27337); neck or anterior thorax (21555, 21552); and shoulder (23075, 23071). In general, there are 2 codes for each area—one for smaller and one for larger excisions—but they frequently are out of order (ie, the code associated with a higher numerical value may correspond with the smaller excision). Care should be taken in selecting the correct code. The specific size cutoffs for the various soft tissue excision code sets are different, so it is important to be familiar with the particular CPT descriptions for each.
Final Thoughts
In summary, biopsies, shave removals, and excisions are different procedures and therefore should be coded differently. Although the distinction between biopsies and shave removals is ill defined, remember that biopsies are intended to establish a diagnosis and shave removals are intended to remove the entire lesion. By definition, excisions must include margins and proceed through the dermis to the subcutis. In particular circumstances, site-specific biopsy codes may be appropriate and can be used to code for lipoma excisions.
1. Current Procedural Terminology 2015, Professional Edition. Chicago, Illinois: American Medical Association; 2014.
2. American Medical Association. Biopsy. CPT Assistant. Chicago, IL: American Medical Association: October 2004:4.
In dermatology, samples of skin and subcutaneous tissue are routinely removed to establish a diagnosis, treat symptomatic lesions, or remove potential tumors. The Current Procedural Terminology (CPT) codes used in billing for these procedures typically are generic, but it is important to differentiate between 3 degrees of tissue removal—biopsy, shave removal, and excision—when billing for these services since different codes may be appropriate in each of these circumstances.
Biopsy
Specifically, biopsy (CPT codes 11100/11101) is described as an “independent…procedure to obtain tissue for pathologic examination.”1 The method of biopsy is not specified by CPT and can include any of the following, as long as the primary purpose of the procedure is to remove tissue for analysis: removal by scissors, shaving with a blade or specialized instrument to any level including the subcutaneous fat, extraction using a punch, and excision down to the subcutaneous fat with a scalpel. The feature that differentiates biopsy from shave removal or excision is not depth or extent of tissue mobilization but the intent “to remove a portion of skin, suspect lesion, or entire lesion so that it can be examined histologically.”2 The underlying assumption is that neither definitive clinical nor histologic diagnosis exists prior to biopsy, the purpose of which is to help establish the identity of the lesion.
If the tissue within a large, single lesion is sampled at several separate locations at the same visit, then only a single unit of a single biopsy code (eg, either 11100, 11101, or some site-specific code) should be reported.In contrast, if a number of discrete lesions in the same approximate anatomic area were sampled for diagnoses, each sample taken from separate lesions would constitute a distinct biopsy and would be billed as a separate unit of service.
Shave Removals and Excisions
Shave removal of skin lesions (CPT codes 11300–11313) includes the removal of tangential or saucerized skin lesions to a level no deeper than the base of the dermis. The CPT provides no detailed guidance regarding differentiation of codes for shave removal versus biopsy when a specimen is submitted for histopathologic examination other than the definition of biopsy that was discussed previously. If the tissue is removed specifically for establishing diagnosis, then by definition the procedure should be coded as a biopsy. On the other hand, shave removal implies the intent to completely remove a lesion that already has a presumptive clinical or histologic diagnosis or is being removed for some purpose other than diagnosis (eg, symptomatic relief).
Shave removals are, however, clearly different than excisions (CPT codes 11400–11646), which must proceed through the entire dermis to the subcutis. Additionally, skin lesion excisions include margins, as the intent of an excision procedure is to remove the entire lesion along with a margin of normal skin around it.2
Specialized Biopsy and Excision Codes
While most biopsies, shave removals, and excisions are performed using generic codes, there are specialized circumstances when more specific codes may be preferable. For instance, there are site-specific skin biopsy codes for the nail unit (11755), vermilion and mucosal lip (40490), penis (54100), vulva (56605), and external ear (69100) that take into account the additional complexity of biopsy at these anatomic locations. There also is a site-specific code for eyelid biopsy (67810), which was redefined in 2013 as an “incisional biopsy of eyelid skin including lid margin.”1 Therefore, biopsies of eyelid skin that do not remove the eyelid margin must be coded as 11100/11101, or if the entire cutaneous lesion was removed, can be reclassified as shave removals, which would be coded in the 11310 to 11313 range.
Specialized excision codes include those of the soft tissue. Soft tissue excision codes typically used by dermatologists are not numbered consecutively, are site-specific, and are typically used for resection of benign tumors confined to the subcutaneous tissue below the skin but above the deep fascia. Cysts of all types, including epidermoid and pilar cysts, are specifically excluded from this code set regardless of how large or complex they may be, as they protrude into the dermis or above and are not exclusively in the subcutis. However, lipomas meet the definition for soft-tissue excision, and therefore site-specific soft tissue excision codes can be used in lieu of traditional skin excision codes. The soft-tissue excision codes are distributed throughout the CPT manual, with distinct codes for the abdominal wall (22902, 22903); leg or ankle (27618, 27632); back or flank (21930, 21931); external auditory canal (69145); upper arm or elbow (24075, 24071); face or scalp (21011, 21012); hand or finger (26115, 26111); foot or toe (28043, 28039); forearm or wrist (25075, 25071); hip or pelvis (27047, 27043); thigh or knee (27327, 27337); neck or anterior thorax (21555, 21552); and shoulder (23075, 23071). In general, there are 2 codes for each area—one for smaller and one for larger excisions—but they frequently are out of order (ie, the code associated with a higher numerical value may correspond with the smaller excision). Care should be taken in selecting the correct code. The specific size cutoffs for the various soft tissue excision code sets are different, so it is important to be familiar with the particular CPT descriptions for each.
Final Thoughts
In summary, biopsies, shave removals, and excisions are different procedures and therefore should be coded differently. Although the distinction between biopsies and shave removals is ill defined, remember that biopsies are intended to establish a diagnosis and shave removals are intended to remove the entire lesion. By definition, excisions must include margins and proceed through the dermis to the subcutis. In particular circumstances, site-specific biopsy codes may be appropriate and can be used to code for lipoma excisions.
In dermatology, samples of skin and subcutaneous tissue are routinely removed to establish a diagnosis, treat symptomatic lesions, or remove potential tumors. The Current Procedural Terminology (CPT) codes used in billing for these procedures typically are generic, but it is important to differentiate between 3 degrees of tissue removal—biopsy, shave removal, and excision—when billing for these services since different codes may be appropriate in each of these circumstances.
Biopsy
Specifically, biopsy (CPT codes 11100/11101) is described as an “independent…procedure to obtain tissue for pathologic examination.”1 The method of biopsy is not specified by CPT and can include any of the following, as long as the primary purpose of the procedure is to remove tissue for analysis: removal by scissors, shaving with a blade or specialized instrument to any level including the subcutaneous fat, extraction using a punch, and excision down to the subcutaneous fat with a scalpel. The feature that differentiates biopsy from shave removal or excision is not depth or extent of tissue mobilization but the intent “to remove a portion of skin, suspect lesion, or entire lesion so that it can be examined histologically.”2 The underlying assumption is that neither definitive clinical nor histologic diagnosis exists prior to biopsy, the purpose of which is to help establish the identity of the lesion.
If the tissue within a large, single lesion is sampled at several separate locations at the same visit, then only a single unit of a single biopsy code (eg, either 11100, 11101, or some site-specific code) should be reported.In contrast, if a number of discrete lesions in the same approximate anatomic area were sampled for diagnoses, each sample taken from separate lesions would constitute a distinct biopsy and would be billed as a separate unit of service.
Shave Removals and Excisions
Shave removal of skin lesions (CPT codes 11300–11313) includes the removal of tangential or saucerized skin lesions to a level no deeper than the base of the dermis. The CPT provides no detailed guidance regarding differentiation of codes for shave removal versus biopsy when a specimen is submitted for histopathologic examination other than the definition of biopsy that was discussed previously. If the tissue is removed specifically for establishing diagnosis, then by definition the procedure should be coded as a biopsy. On the other hand, shave removal implies the intent to completely remove a lesion that already has a presumptive clinical or histologic diagnosis or is being removed for some purpose other than diagnosis (eg, symptomatic relief).
Shave removals are, however, clearly different than excisions (CPT codes 11400–11646), which must proceed through the entire dermis to the subcutis. Additionally, skin lesion excisions include margins, as the intent of an excision procedure is to remove the entire lesion along with a margin of normal skin around it.2
Specialized Biopsy and Excision Codes
While most biopsies, shave removals, and excisions are performed using generic codes, there are specialized circumstances when more specific codes may be preferable. For instance, there are site-specific skin biopsy codes for the nail unit (11755), vermilion and mucosal lip (40490), penis (54100), vulva (56605), and external ear (69100) that take into account the additional complexity of biopsy at these anatomic locations. There also is a site-specific code for eyelid biopsy (67810), which was redefined in 2013 as an “incisional biopsy of eyelid skin including lid margin.”1 Therefore, biopsies of eyelid skin that do not remove the eyelid margin must be coded as 11100/11101, or if the entire cutaneous lesion was removed, can be reclassified as shave removals, which would be coded in the 11310 to 11313 range.
Specialized excision codes include those of the soft tissue. Soft tissue excision codes typically used by dermatologists are not numbered consecutively, are site-specific, and are typically used for resection of benign tumors confined to the subcutaneous tissue below the skin but above the deep fascia. Cysts of all types, including epidermoid and pilar cysts, are specifically excluded from this code set regardless of how large or complex they may be, as they protrude into the dermis or above and are not exclusively in the subcutis. However, lipomas meet the definition for soft-tissue excision, and therefore site-specific soft tissue excision codes can be used in lieu of traditional skin excision codes. The soft-tissue excision codes are distributed throughout the CPT manual, with distinct codes for the abdominal wall (22902, 22903); leg or ankle (27618, 27632); back or flank (21930, 21931); external auditory canal (69145); upper arm or elbow (24075, 24071); face or scalp (21011, 21012); hand or finger (26115, 26111); foot or toe (28043, 28039); forearm or wrist (25075, 25071); hip or pelvis (27047, 27043); thigh or knee (27327, 27337); neck or anterior thorax (21555, 21552); and shoulder (23075, 23071). In general, there are 2 codes for each area—one for smaller and one for larger excisions—but they frequently are out of order (ie, the code associated with a higher numerical value may correspond with the smaller excision). Care should be taken in selecting the correct code. The specific size cutoffs for the various soft tissue excision code sets are different, so it is important to be familiar with the particular CPT descriptions for each.
Final Thoughts
In summary, biopsies, shave removals, and excisions are different procedures and therefore should be coded differently. Although the distinction between biopsies and shave removals is ill defined, remember that biopsies are intended to establish a diagnosis and shave removals are intended to remove the entire lesion. By definition, excisions must include margins and proceed through the dermis to the subcutis. In particular circumstances, site-specific biopsy codes may be appropriate and can be used to code for lipoma excisions.
1. Current Procedural Terminology 2015, Professional Edition. Chicago, Illinois: American Medical Association; 2014.
2. American Medical Association. Biopsy. CPT Assistant. Chicago, IL: American Medical Association: October 2004:4.
1. Current Procedural Terminology 2015, Professional Edition. Chicago, Illinois: American Medical Association; 2014.
2. American Medical Association. Biopsy. CPT Assistant. Chicago, IL: American Medical Association: October 2004:4.
Practice Points
- Biopsies are coded when there is an independent procedure to remove skin for histologic analysis to help establish a definitive histologic diagnosis.
- Coding for shave removals and excisions requires the intent to remove the entire lesion.
- Unlike shave removals, excisions can be coded only if the lesion is removed to the level of the subcutaneous fat.
- When available, site-specific biopsy or soft tissue excision codes may better describe a procedure than standard biopsy or excision codes.
Which Hospitalist Should Bill for Inpatient Stays with Multiple Providers?
During a facility stay, a patient could be attended to by more than one hospitalist. For example, perhaps one hospitalist is the admitting physician, but the patient has a three-day stay and may be seen by three different hospitalists. Are there any guidelines as to which physician should be billed on the facility claim? Thank you for any remarks, suggestions, or references.
—Anonymous
Dr. Hospitalist responds:
Most of us can definitely relate to the concerns you have about properly billing during the patient’s hospital stay. By facility claim, I’m assuming you mean the physician’s bill for services rendered to a hospitalized patient. After querying the Centers for Medicare and Medicaid (CMS) website and discussing the question with several of our coding and billing gurus, as far as I can tell, there are no specific guidelines pertaining to which physician in a multiphysician group should bill. CMS guidelines are clear that you should only bill for the services you provide. CMS is very specific about allowing only one physician of the same specialty billing per day (reference the CMS Manual, Chapter 12, 30.6.9-Payment for Inpatient Hospital Visits).
In our very large group, we bill daily for the individual inpatient services we provide. That way, when the bill goes out, the clinician author is responsible for its validity and can support the level of care as documented.
Billing and coding is such an arduous process, I can’t imagine attempting it without an electronic interface. Most hospitalist groups have some form of electronic billing software that has integrated checks and balances to catch the common mistakes. Improper billing done by anyone in the group can expose the entire group to an audit. With ICD-10 now upon us, this becomes ever more important.
Good luck!
Do you have a problem or concern that you’d like Dr. Hospitalist to address? Email your questions to drhospit@wiley.com.
During a facility stay, a patient could be attended to by more than one hospitalist. For example, perhaps one hospitalist is the admitting physician, but the patient has a three-day stay and may be seen by three different hospitalists. Are there any guidelines as to which physician should be billed on the facility claim? Thank you for any remarks, suggestions, or references.
—Anonymous
Dr. Hospitalist responds:
Most of us can definitely relate to the concerns you have about properly billing during the patient’s hospital stay. By facility claim, I’m assuming you mean the physician’s bill for services rendered to a hospitalized patient. After querying the Centers for Medicare and Medicaid (CMS) website and discussing the question with several of our coding and billing gurus, as far as I can tell, there are no specific guidelines pertaining to which physician in a multiphysician group should bill. CMS guidelines are clear that you should only bill for the services you provide. CMS is very specific about allowing only one physician of the same specialty billing per day (reference the CMS Manual, Chapter 12, 30.6.9-Payment for Inpatient Hospital Visits).
In our very large group, we bill daily for the individual inpatient services we provide. That way, when the bill goes out, the clinician author is responsible for its validity and can support the level of care as documented.
Billing and coding is such an arduous process, I can’t imagine attempting it without an electronic interface. Most hospitalist groups have some form of electronic billing software that has integrated checks and balances to catch the common mistakes. Improper billing done by anyone in the group can expose the entire group to an audit. With ICD-10 now upon us, this becomes ever more important.
Good luck!
Do you have a problem or concern that you’d like Dr. Hospitalist to address? Email your questions to drhospit@wiley.com.
During a facility stay, a patient could be attended to by more than one hospitalist. For example, perhaps one hospitalist is the admitting physician, but the patient has a three-day stay and may be seen by three different hospitalists. Are there any guidelines as to which physician should be billed on the facility claim? Thank you for any remarks, suggestions, or references.
—Anonymous
Dr. Hospitalist responds:
Most of us can definitely relate to the concerns you have about properly billing during the patient’s hospital stay. By facility claim, I’m assuming you mean the physician’s bill for services rendered to a hospitalized patient. After querying the Centers for Medicare and Medicaid (CMS) website and discussing the question with several of our coding and billing gurus, as far as I can tell, there are no specific guidelines pertaining to which physician in a multiphysician group should bill. CMS guidelines are clear that you should only bill for the services you provide. CMS is very specific about allowing only one physician of the same specialty billing per day (reference the CMS Manual, Chapter 12, 30.6.9-Payment for Inpatient Hospital Visits).
In our very large group, we bill daily for the individual inpatient services we provide. That way, when the bill goes out, the clinician author is responsible for its validity and can support the level of care as documented.
Billing and coding is such an arduous process, I can’t imagine attempting it without an electronic interface. Most hospitalist groups have some form of electronic billing software that has integrated checks and balances to catch the common mistakes. Improper billing done by anyone in the group can expose the entire group to an audit. With ICD-10 now upon us, this becomes ever more important.
Good luck!
Do you have a problem or concern that you’d like Dr. Hospitalist to address? Email your questions to drhospit@wiley.com.
Billing, Coding Documentation to Support Services, Minimize Risks
The electronic health record (EHR) has many benefits:
- Improved patient care;
- Improved care coordination;
- Improved diagnostics and patient outcomes;
- Increased patient participation; and
- Increased practice efficiencies and cost savings.1
EHRs also introduce risks, however. Heightened concern about EHR misuse and vulnerability elevates the level of scrutiny placed on provider documentation as it relates to billing and coding. Without clear guidelines from the Centers for Medicare and Medicaid Services (CMS) or other payers, the potential for unintentional misapplication exists. Auditor misinterpretation is also possible. Providers should utilize simple defensive documentation principles to support their services and minimize their risks.
Reason for Encounter
Under section 1862 (a)(1)(A) of the Social Security Act, the Medicare Program may only pay for items and services that are “reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member,” unless there is another statutory authorization for payment (e.g. colorectal cancer screening).2
A payer can determine if a service is “reasonable and necessary” based on the service indication. The reason for the patient encounter, otherwise known as the chief complaint, must be evident. This can be a symptom, problem, condition, diagnosis, physician-recommended return, or another factor that necessitates the encounter.1 It cannot be inferred and must be clearly stated in the documentation. Without it, a payer may question the medical necessity of the service, especially if it involves hospital-based services in the course of which multiple specialists will see the patient on any given date. Payers are likely to deny services that cannot be easily differentiated (e.g. “no c/o”). Furthermore, payers can deny concurrent care services for the following reasons:3
- Services exceed normal frequency or duration for a given condition without documented circumstances requiring additional care; or
- Services by one physician duplicate/overlap those of the other provider without any recognizable distinction.
Providers should be specific in identifying the encounter reason, as in the following examples: “Patient seen for shortness of breath” or “Patient with COPD, feeling improved with 3L O2 NC.”
Assessment and Plan
Accurately representing patient complexity for every visit throughout the hospitalization presents its challenges. Although the problem list may not dramatically change day to day, providers must formulate an assessment of the patient’s condition with a corresponding plan of care for each encounter. Documenting problems without a corresponding plan of care does not substantiate physician participation in the management of that problem. Providing a brief, generalized comment (e.g. “DM, CKD, CHF: Continue current treatment plan”) minimizes the complexity and effort put forth in the encounter and could result in auditor downgrading upon documentation review.
Developing shortcuts might falsely minimize the provider’s documentation burden. An electronic documentation system might make it possible to copy previous progress notes into the current encounter to save time; however, the previously entered information could include elements that do not require reassessment during a subsequent encounter or contain information about conditions that are being managed concurrently by another specialist (e.g. CKD being managed by the nephrologist). Leaving the copied information unmodified may not accurately reflect the patient’s current condition or the care provided by the hospitalist during the current encounter. Information that is pulled forward or copied and pasted from a previous entry should be modified to demonstrate updated content and nonoverlapping care relevant to that date.
According to the Office of Inspector General (OIG), “inappropriate copy-pasting could facilitate attempts to inflate claims and duplicate or create fraudulent claims.”4
An equally problematic EHR function involves “overdocumentation,” the practice of inserting false or irrelevant documentation to create the appearance of support for billing higher level services.4 EHR technology has the ability to auto-populate fields using templates built into the system or generate extensive documentation on the basis of a single click. The OIG cautions providers to use these features carefully, because they can produce information suggesting the practitioner performed more comprehensive services than were actually rendered.4
An example is the inclusion of the same lab results more than once. Although clinicians include this information as a reference to avoid having to “find it somewhere in the chart” when it is needed—as a basis for comparison, for example—auditors mistake this as an attempt to gain credit for the daily review of the same “old” information. Including only relevant data will mitigate this concern.
Authorship
Dates and signatures are essential to each encounter. Medicare requires services provided/ordered to be authenticated by the author.5 A reviewer must be able to identify each individual who performs, documents, and bills for a service on a given date. Progress notes that fail to identify the service date or service provider will likely result in denial.
Additionally, a service is questioned when two different sets of handwriting appear on a note, yet only one signature is provided. Since the reviewer cannot confirm the credentials of the unidentified individual and cannot be sure which portion belongs to the identified individual, the entire note is disregarded.
Notes that contain an illegible signature are equally problematic. If the legibility of the signature prevents the reviewer from correctly identifying the rendering provider, the service may be denied.
CMS has instructed Medicare contractors to request a signed provider attestation before issuing a denial.5 The provider should print his/her name beside the signature or include a separate signature sheet with the requested documentation to assist the reviewer in provider identification. Stamped signatures are not acceptable under any circumstance. Medicare accepts only handwritten or electronic signatures.5
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
References
- HealthIT.gov. Benefits of electronic health records (EHRs). Accessed August 1, 2015.
- Social Security Administration. Exclusions from coverage and Medicare as secondary payer. Accessed August 1, 2015.
- Centers for Medicare and Medicaid Services. Medicare Benefit Policy Manual: Chapter 15—Covered medical and other health services. Chapter 15, Section 30.E. Concurrent care. Accessed August 1, 2015.
- Department of Health and Human Services. Office of Inspector General. CMS and its contractors have adopted few program integrity practices to address vulnerabilities in EHRs. Accessed August 1, 2015.
- Centers for Medicare and Medicaid Services. Signature guidelines for medical review purposes. Accessed August 1, 2015.
- Centers for Medicare and Medicaid Services. 1995 documentation guidelines for evaluation and management services. Accessed August 1, 2015.
The electronic health record (EHR) has many benefits:
- Improved patient care;
- Improved care coordination;
- Improved diagnostics and patient outcomes;
- Increased patient participation; and
- Increased practice efficiencies and cost savings.1
EHRs also introduce risks, however. Heightened concern about EHR misuse and vulnerability elevates the level of scrutiny placed on provider documentation as it relates to billing and coding. Without clear guidelines from the Centers for Medicare and Medicaid Services (CMS) or other payers, the potential for unintentional misapplication exists. Auditor misinterpretation is also possible. Providers should utilize simple defensive documentation principles to support their services and minimize their risks.
Reason for Encounter
Under section 1862 (a)(1)(A) of the Social Security Act, the Medicare Program may only pay for items and services that are “reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member,” unless there is another statutory authorization for payment (e.g. colorectal cancer screening).2
A payer can determine if a service is “reasonable and necessary” based on the service indication. The reason for the patient encounter, otherwise known as the chief complaint, must be evident. This can be a symptom, problem, condition, diagnosis, physician-recommended return, or another factor that necessitates the encounter.1 It cannot be inferred and must be clearly stated in the documentation. Without it, a payer may question the medical necessity of the service, especially if it involves hospital-based services in the course of which multiple specialists will see the patient on any given date. Payers are likely to deny services that cannot be easily differentiated (e.g. “no c/o”). Furthermore, payers can deny concurrent care services for the following reasons:3
- Services exceed normal frequency or duration for a given condition without documented circumstances requiring additional care; or
- Services by one physician duplicate/overlap those of the other provider without any recognizable distinction.
Providers should be specific in identifying the encounter reason, as in the following examples: “Patient seen for shortness of breath” or “Patient with COPD, feeling improved with 3L O2 NC.”
Assessment and Plan
Accurately representing patient complexity for every visit throughout the hospitalization presents its challenges. Although the problem list may not dramatically change day to day, providers must formulate an assessment of the patient’s condition with a corresponding plan of care for each encounter. Documenting problems without a corresponding plan of care does not substantiate physician participation in the management of that problem. Providing a brief, generalized comment (e.g. “DM, CKD, CHF: Continue current treatment plan”) minimizes the complexity and effort put forth in the encounter and could result in auditor downgrading upon documentation review.
Developing shortcuts might falsely minimize the provider’s documentation burden. An electronic documentation system might make it possible to copy previous progress notes into the current encounter to save time; however, the previously entered information could include elements that do not require reassessment during a subsequent encounter or contain information about conditions that are being managed concurrently by another specialist (e.g. CKD being managed by the nephrologist). Leaving the copied information unmodified may not accurately reflect the patient’s current condition or the care provided by the hospitalist during the current encounter. Information that is pulled forward or copied and pasted from a previous entry should be modified to demonstrate updated content and nonoverlapping care relevant to that date.
According to the Office of Inspector General (OIG), “inappropriate copy-pasting could facilitate attempts to inflate claims and duplicate or create fraudulent claims.”4
An equally problematic EHR function involves “overdocumentation,” the practice of inserting false or irrelevant documentation to create the appearance of support for billing higher level services.4 EHR technology has the ability to auto-populate fields using templates built into the system or generate extensive documentation on the basis of a single click. The OIG cautions providers to use these features carefully, because they can produce information suggesting the practitioner performed more comprehensive services than were actually rendered.4
An example is the inclusion of the same lab results more than once. Although clinicians include this information as a reference to avoid having to “find it somewhere in the chart” when it is needed—as a basis for comparison, for example—auditors mistake this as an attempt to gain credit for the daily review of the same “old” information. Including only relevant data will mitigate this concern.
Authorship
Dates and signatures are essential to each encounter. Medicare requires services provided/ordered to be authenticated by the author.5 A reviewer must be able to identify each individual who performs, documents, and bills for a service on a given date. Progress notes that fail to identify the service date or service provider will likely result in denial.
Additionally, a service is questioned when two different sets of handwriting appear on a note, yet only one signature is provided. Since the reviewer cannot confirm the credentials of the unidentified individual and cannot be sure which portion belongs to the identified individual, the entire note is disregarded.
Notes that contain an illegible signature are equally problematic. If the legibility of the signature prevents the reviewer from correctly identifying the rendering provider, the service may be denied.
CMS has instructed Medicare contractors to request a signed provider attestation before issuing a denial.5 The provider should print his/her name beside the signature or include a separate signature sheet with the requested documentation to assist the reviewer in provider identification. Stamped signatures are not acceptable under any circumstance. Medicare accepts only handwritten or electronic signatures.5
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
References
- HealthIT.gov. Benefits of electronic health records (EHRs). Accessed August 1, 2015.
- Social Security Administration. Exclusions from coverage and Medicare as secondary payer. Accessed August 1, 2015.
- Centers for Medicare and Medicaid Services. Medicare Benefit Policy Manual: Chapter 15—Covered medical and other health services. Chapter 15, Section 30.E. Concurrent care. Accessed August 1, 2015.
- Department of Health and Human Services. Office of Inspector General. CMS and its contractors have adopted few program integrity practices to address vulnerabilities in EHRs. Accessed August 1, 2015.
- Centers for Medicare and Medicaid Services. Signature guidelines for medical review purposes. Accessed August 1, 2015.
- Centers for Medicare and Medicaid Services. 1995 documentation guidelines for evaluation and management services. Accessed August 1, 2015.
The electronic health record (EHR) has many benefits:
- Improved patient care;
- Improved care coordination;
- Improved diagnostics and patient outcomes;
- Increased patient participation; and
- Increased practice efficiencies and cost savings.1
EHRs also introduce risks, however. Heightened concern about EHR misuse and vulnerability elevates the level of scrutiny placed on provider documentation as it relates to billing and coding. Without clear guidelines from the Centers for Medicare and Medicaid Services (CMS) or other payers, the potential for unintentional misapplication exists. Auditor misinterpretation is also possible. Providers should utilize simple defensive documentation principles to support their services and minimize their risks.
Reason for Encounter
Under section 1862 (a)(1)(A) of the Social Security Act, the Medicare Program may only pay for items and services that are “reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member,” unless there is another statutory authorization for payment (e.g. colorectal cancer screening).2
A payer can determine if a service is “reasonable and necessary” based on the service indication. The reason for the patient encounter, otherwise known as the chief complaint, must be evident. This can be a symptom, problem, condition, diagnosis, physician-recommended return, or another factor that necessitates the encounter.1 It cannot be inferred and must be clearly stated in the documentation. Without it, a payer may question the medical necessity of the service, especially if it involves hospital-based services in the course of which multiple specialists will see the patient on any given date. Payers are likely to deny services that cannot be easily differentiated (e.g. “no c/o”). Furthermore, payers can deny concurrent care services for the following reasons:3
- Services exceed normal frequency or duration for a given condition without documented circumstances requiring additional care; or
- Services by one physician duplicate/overlap those of the other provider without any recognizable distinction.
Providers should be specific in identifying the encounter reason, as in the following examples: “Patient seen for shortness of breath” or “Patient with COPD, feeling improved with 3L O2 NC.”
Assessment and Plan
Accurately representing patient complexity for every visit throughout the hospitalization presents its challenges. Although the problem list may not dramatically change day to day, providers must formulate an assessment of the patient’s condition with a corresponding plan of care for each encounter. Documenting problems without a corresponding plan of care does not substantiate physician participation in the management of that problem. Providing a brief, generalized comment (e.g. “DM, CKD, CHF: Continue current treatment plan”) minimizes the complexity and effort put forth in the encounter and could result in auditor downgrading upon documentation review.
Developing shortcuts might falsely minimize the provider’s documentation burden. An electronic documentation system might make it possible to copy previous progress notes into the current encounter to save time; however, the previously entered information could include elements that do not require reassessment during a subsequent encounter or contain information about conditions that are being managed concurrently by another specialist (e.g. CKD being managed by the nephrologist). Leaving the copied information unmodified may not accurately reflect the patient’s current condition or the care provided by the hospitalist during the current encounter. Information that is pulled forward or copied and pasted from a previous entry should be modified to demonstrate updated content and nonoverlapping care relevant to that date.
According to the Office of Inspector General (OIG), “inappropriate copy-pasting could facilitate attempts to inflate claims and duplicate or create fraudulent claims.”4
An equally problematic EHR function involves “overdocumentation,” the practice of inserting false or irrelevant documentation to create the appearance of support for billing higher level services.4 EHR technology has the ability to auto-populate fields using templates built into the system or generate extensive documentation on the basis of a single click. The OIG cautions providers to use these features carefully, because they can produce information suggesting the practitioner performed more comprehensive services than were actually rendered.4
An example is the inclusion of the same lab results more than once. Although clinicians include this information as a reference to avoid having to “find it somewhere in the chart” when it is needed—as a basis for comparison, for example—auditors mistake this as an attempt to gain credit for the daily review of the same “old” information. Including only relevant data will mitigate this concern.
Authorship
Dates and signatures are essential to each encounter. Medicare requires services provided/ordered to be authenticated by the author.5 A reviewer must be able to identify each individual who performs, documents, and bills for a service on a given date. Progress notes that fail to identify the service date or service provider will likely result in denial.
Additionally, a service is questioned when two different sets of handwriting appear on a note, yet only one signature is provided. Since the reviewer cannot confirm the credentials of the unidentified individual and cannot be sure which portion belongs to the identified individual, the entire note is disregarded.
Notes that contain an illegible signature are equally problematic. If the legibility of the signature prevents the reviewer from correctly identifying the rendering provider, the service may be denied.
CMS has instructed Medicare contractors to request a signed provider attestation before issuing a denial.5 The provider should print his/her name beside the signature or include a separate signature sheet with the requested documentation to assist the reviewer in provider identification. Stamped signatures are not acceptable under any circumstance. Medicare accepts only handwritten or electronic signatures.5
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She is also on the faculty of SHM’s inpatient coding course.
References
- HealthIT.gov. Benefits of electronic health records (EHRs). Accessed August 1, 2015.
- Social Security Administration. Exclusions from coverage and Medicare as secondary payer. Accessed August 1, 2015.
- Centers for Medicare and Medicaid Services. Medicare Benefit Policy Manual: Chapter 15—Covered medical and other health services. Chapter 15, Section 30.E. Concurrent care. Accessed August 1, 2015.
- Department of Health and Human Services. Office of Inspector General. CMS and its contractors have adopted few program integrity practices to address vulnerabilities in EHRs. Accessed August 1, 2015.
- Centers for Medicare and Medicaid Services. Signature guidelines for medical review purposes. Accessed August 1, 2015.
- Centers for Medicare and Medicaid Services. 1995 documentation guidelines for evaluation and management services. Accessed August 1, 2015.