User login
A New Protocol for RhD-negative Pregnant Women?
A 30-year-old G1P0 woman presents to your office for routine obstetric care at 18 weeks’ gestation. Her pregnancy has been uncomplicated, but her prenatal lab evaluation is notable for blood type A-negative. She wants to know if she really needs the anti-D immune globulin injection.
Rhesus (Rh)D-negative women carrying an RhD-positive fetus are at risk for anti-D antibodies, placing the fetus at risk for hemolytic disease of the fetus and newborn (HDFN). If undiagnosed and/or untreated, HDFN carries significant risk for perinatal morbidity and mortality.2
With routine postnatal anti-D immunoglobulin prophylaxis of RhD-negative women who delivered an RhD-positive child (which began around 1970), the risk for maternal alloimmunization was reduced from 16% to 1.12%-1.3%.3-5 The risk was further reduced to approximately 0.28% with the addition of consistent prophylaxis at 28 weeks’ gestation.4 As a result, the current standard of care is to administer anti-D immunoglobulin at 28 weeks’ gestation, within 72 hours of delivery of an RhD-positive fetus, and after events with risk for fetal-to-maternal transfusion (eg, spontaneous, threatened, or induced abortion; invasive prenatal diagnostic procedures such as amniocentesis; blunt abdominal trauma; external cephalic version; second or third trimester antepartum bleeding).6
The problem of unnecessary Tx. However, under this current practice, many RhD-negative women are receiving anti-D immunoglobulin unnecessarily. This is because the fetus’s RhD status is not routinely known during the prenatal period.
Enter cell-free DNA testing. Cell-free DNA testing analyzes fragments of fetal DNA found in maternal blood. The use of cell-free DNA testing at 10 to 13 weeks’ gestation to screen for fetal chromosomal abnormalities is reliable (91%-99% sensitivity for trisomies 21, 18, and 137) and becoming increasingly more common.
A notable meta-analysis. A 2017 meta-analysis of 30 studies of cell-free DNA testing of RhD status in the first and second trimesters calculated a sensitivity of 99.3% and a specificity of 98.4%.7 Denmark, the Netherlands, Sweden, France, and Finland are using this method routinely. As of this writing, the American College of Obstetricians and Gynecologists (ACOG) has not recommended the use of cell-free DNA RhD testing in the United States, but they do note that as the cost of the assay declines, this method may become preferred.8 The National Institute for Health and Care Excellence in England recommends its use as long as its cost remains below a set threshold.9
This study evaluated the accuracy of using cell-free DNA testing at 27 weeks’ gestation to determine fetal RhD status compared with serologic typing of cord blood at delivery.
Continue to: STUDY SUMMARY
STUDY SUMMARY
Test gets high marks in Netherlands trial
This large observational cohort trial from the Netherlands examined the accuracy of identifying RhD-positive fetuses using cell-free DNA isolates in maternal plasma. Over the 15-month study period, fetal RhD testing was conducted during Week 27 of gestation, and results were compared with those obtained using neonatal cord blood at birth. If the fetal RhD test was positive, providers administered 200 µg anti-D immunoglobulin during the 30th week of gestation and within 48 hours of birth. If fetal RhD was negative, providers were told immunoglobulin was unnecessary.
More than 32,000 RhD-negative women were screened. The cell-free DNA test showed fetal RhD-positive results 62% of the time and RhD-negative results in the remainder. Cord blood samples were available for 25,789 pregnancies (80%).
Sensitivity, specificity. The sensitivity for identifying fetal RhD was 99% and the specificity was 98%. Both negative and positive predictive values were 99%. Overall, there were 225 false-positive results and nine false-negative results. In the nine false negatives, six were due to a lack of fetal DNA in the sample and three were due to technical error (defined as an operator ignoring a failure of the robot pipetting the plasma or other technical failures).
The false-negative rate (0.03%) was lower than the predetermined estimated false-negative rate of cord blood serology (0.25%). In 22 of the supposed false positives, follow-up serology or molecular testing found an RhD gene was actually present, meaning the results of the neonatal cord blood serology in these cases were falsely negative. If you recalculate with these data in mind, the false-negative rate for fetal DNA testing was actually less than half that of typical serologic determination.
Continue to: WHAT'S NEW
WHAT’S NEW
Accurate test, potential to reduce unnecessary Tx
Fetal RhD testing at 27 weeks’ gestation appears to be highly accurate and could reduce the unnecessary use of anti-D immunoglobulin when the fetal RhD is negative.
CAVEATS
Different results by ethnicity?
Dutch participants are not necessarily reflective of the US population. Known variation in the rate of fetal RhD positivity among RhD-negative pregnant women by race and ethnicity could mean that the number of women able to forego anti-D immunoglobulin prophylaxis would be different in the United States than in other countries.
Also, in this study, polymerase chain reaction for two RhD sequences was run in triplicate, and a computer-based algorithm was used to automatically score samples to provide results. For safe implementation, the cell-free fetal RhD DNA testing process would need to follow similar methods.
CHALLENGES TO IMPLEMENTATION
Cost and availability are big unknowns
Cost and availability of the test may be barriers, but there is currently too little information on either subject in the United States to make a determination. A 2013 study indicated that the use of cell-free DNA testing to determine fetal RhD status was then approximately $682.10
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[5]: 306, 308, 319).
1. de Haas M, Thurik FF, van der Ploeg CP, et al. Sensitivity of fetal RHD screening for safe guidance of targeted anti-D immunoglobulin prophylaxis: prospective cohort study of a nationwide programme in the Netherlands. BMJ. 2016;355:i5789.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 75: Management of alloimmunization during pregnancy. Obstet Gynecol. 2006; 108:457-464.
3. Urbaniak SJ, Greiss MA. RhD haemolytic disease of the fetus and the newborn. Blood Rev. 2000;14(1):44-61.
4. Mayne S, Parker JH, Harden TA, et al. Rate of RhD sensitisation before and after implementation of a community based antenatal prophylaxis programme. BMJ. 1997;315(7122):1588.
5. MacKenzie IZ, Bowell P, Gregory H, et al. Routine antenatal Rhesus D immunoglobulin prophylaxis: the results of a prospective 10 year study. Br J Obstet Gynecol. 1999;106:492-497.
6. Zolotor AJ, Carlough MC. Update on prenatal care. Am Fam Physician. 2014;89(3):199-208.
7. Mackie FL, Hemming K, Allen S, et al. The accuracy of cell-free fetal DNA-based non-invasive prenatal testing in singleton pregnancies: a systematic review and bivariate meta-analysis. BJOG. 2017;124(1):32-46.
8. American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 181: Prevention of Rh D Alloimmunization. Obstet Gynecol. 2017;130:e57-e70.
9. National Institute for Health and Care Excellence. High-throughput non-invasive prenatal testing for fetal RHD genotype 1: Recommendations. www.nice.org.uk/guidance/dg25/chapter/1-Recommendations. Accessed May 7, 2018.
10. Hawk AF, Chang EY, Shields SM, Simpson KN. Costs and clinical outcomes of noninvasive fetal RhD typing for targeted prophylaxis. Obstet Gynecol. 2013;122(3):579-585.
A 30-year-old G1P0 woman presents to your office for routine obstetric care at 18 weeks’ gestation. Her pregnancy has been uncomplicated, but her prenatal lab evaluation is notable for blood type A-negative. She wants to know if she really needs the anti-D immune globulin injection.
Rhesus (Rh)D-negative women carrying an RhD-positive fetus are at risk for anti-D antibodies, placing the fetus at risk for hemolytic disease of the fetus and newborn (HDFN). If undiagnosed and/or untreated, HDFN carries significant risk for perinatal morbidity and mortality.2
With routine postnatal anti-D immunoglobulin prophylaxis of RhD-negative women who delivered an RhD-positive child (which began around 1970), the risk for maternal alloimmunization was reduced from 16% to 1.12%-1.3%.3-5 The risk was further reduced to approximately 0.28% with the addition of consistent prophylaxis at 28 weeks’ gestation.4 As a result, the current standard of care is to administer anti-D immunoglobulin at 28 weeks’ gestation, within 72 hours of delivery of an RhD-positive fetus, and after events with risk for fetal-to-maternal transfusion (eg, spontaneous, threatened, or induced abortion; invasive prenatal diagnostic procedures such as amniocentesis; blunt abdominal trauma; external cephalic version; second or third trimester antepartum bleeding).6
The problem of unnecessary Tx. However, under this current practice, many RhD-negative women are receiving anti-D immunoglobulin unnecessarily. This is because the fetus’s RhD status is not routinely known during the prenatal period.
Enter cell-free DNA testing. Cell-free DNA testing analyzes fragments of fetal DNA found in maternal blood. The use of cell-free DNA testing at 10 to 13 weeks’ gestation to screen for fetal chromosomal abnormalities is reliable (91%-99% sensitivity for trisomies 21, 18, and 137) and becoming increasingly more common.
A notable meta-analysis. A 2017 meta-analysis of 30 studies of cell-free DNA testing of RhD status in the first and second trimesters calculated a sensitivity of 99.3% and a specificity of 98.4%.7 Denmark, the Netherlands, Sweden, France, and Finland are using this method routinely. As of this writing, the American College of Obstetricians and Gynecologists (ACOG) has not recommended the use of cell-free DNA RhD testing in the United States, but they do note that as the cost of the assay declines, this method may become preferred.8 The National Institute for Health and Care Excellence in England recommends its use as long as its cost remains below a set threshold.9
This study evaluated the accuracy of using cell-free DNA testing at 27 weeks’ gestation to determine fetal RhD status compared with serologic typing of cord blood at delivery.
Continue to: STUDY SUMMARY
STUDY SUMMARY
Test gets high marks in Netherlands trial
This large observational cohort trial from the Netherlands examined the accuracy of identifying RhD-positive fetuses using cell-free DNA isolates in maternal plasma. Over the 15-month study period, fetal RhD testing was conducted during Week 27 of gestation, and results were compared with those obtained using neonatal cord blood at birth. If the fetal RhD test was positive, providers administered 200 µg anti-D immunoglobulin during the 30th week of gestation and within 48 hours of birth. If fetal RhD was negative, providers were told immunoglobulin was unnecessary.
More than 32,000 RhD-negative women were screened. The cell-free DNA test showed fetal RhD-positive results 62% of the time and RhD-negative results in the remainder. Cord blood samples were available for 25,789 pregnancies (80%).
Sensitivity, specificity. The sensitivity for identifying fetal RhD was 99% and the specificity was 98%. Both negative and positive predictive values were 99%. Overall, there were 225 false-positive results and nine false-negative results. In the nine false negatives, six were due to a lack of fetal DNA in the sample and three were due to technical error (defined as an operator ignoring a failure of the robot pipetting the plasma or other technical failures).
The false-negative rate (0.03%) was lower than the predetermined estimated false-negative rate of cord blood serology (0.25%). In 22 of the supposed false positives, follow-up serology or molecular testing found an RhD gene was actually present, meaning the results of the neonatal cord blood serology in these cases were falsely negative. If you recalculate with these data in mind, the false-negative rate for fetal DNA testing was actually less than half that of typical serologic determination.
Continue to: WHAT'S NEW
WHAT’S NEW
Accurate test, potential to reduce unnecessary Tx
Fetal RhD testing at 27 weeks’ gestation appears to be highly accurate and could reduce the unnecessary use of anti-D immunoglobulin when the fetal RhD is negative.
CAVEATS
Different results by ethnicity?
Dutch participants are not necessarily reflective of the US population. Known variation in the rate of fetal RhD positivity among RhD-negative pregnant women by race and ethnicity could mean that the number of women able to forego anti-D immunoglobulin prophylaxis would be different in the United States than in other countries.
Also, in this study, polymerase chain reaction for two RhD sequences was run in triplicate, and a computer-based algorithm was used to automatically score samples to provide results. For safe implementation, the cell-free fetal RhD DNA testing process would need to follow similar methods.
CHALLENGES TO IMPLEMENTATION
Cost and availability are big unknowns
Cost and availability of the test may be barriers, but there is currently too little information on either subject in the United States to make a determination. A 2013 study indicated that the use of cell-free DNA testing to determine fetal RhD status was then approximately $682.10
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[5]: 306, 308, 319).
A 30-year-old G1P0 woman presents to your office for routine obstetric care at 18 weeks’ gestation. Her pregnancy has been uncomplicated, but her prenatal lab evaluation is notable for blood type A-negative. She wants to know if she really needs the anti-D immune globulin injection.
Rhesus (Rh)D-negative women carrying an RhD-positive fetus are at risk for anti-D antibodies, placing the fetus at risk for hemolytic disease of the fetus and newborn (HDFN). If undiagnosed and/or untreated, HDFN carries significant risk for perinatal morbidity and mortality.2
With routine postnatal anti-D immunoglobulin prophylaxis of RhD-negative women who delivered an RhD-positive child (which began around 1970), the risk for maternal alloimmunization was reduced from 16% to 1.12%-1.3%.3-5 The risk was further reduced to approximately 0.28% with the addition of consistent prophylaxis at 28 weeks’ gestation.4 As a result, the current standard of care is to administer anti-D immunoglobulin at 28 weeks’ gestation, within 72 hours of delivery of an RhD-positive fetus, and after events with risk for fetal-to-maternal transfusion (eg, spontaneous, threatened, or induced abortion; invasive prenatal diagnostic procedures such as amniocentesis; blunt abdominal trauma; external cephalic version; second or third trimester antepartum bleeding).6
The problem of unnecessary Tx. However, under this current practice, many RhD-negative women are receiving anti-D immunoglobulin unnecessarily. This is because the fetus’s RhD status is not routinely known during the prenatal period.
Enter cell-free DNA testing. Cell-free DNA testing analyzes fragments of fetal DNA found in maternal blood. The use of cell-free DNA testing at 10 to 13 weeks’ gestation to screen for fetal chromosomal abnormalities is reliable (91%-99% sensitivity for trisomies 21, 18, and 137) and becoming increasingly more common.
A notable meta-analysis. A 2017 meta-analysis of 30 studies of cell-free DNA testing of RhD status in the first and second trimesters calculated a sensitivity of 99.3% and a specificity of 98.4%.7 Denmark, the Netherlands, Sweden, France, and Finland are using this method routinely. As of this writing, the American College of Obstetricians and Gynecologists (ACOG) has not recommended the use of cell-free DNA RhD testing in the United States, but they do note that as the cost of the assay declines, this method may become preferred.8 The National Institute for Health and Care Excellence in England recommends its use as long as its cost remains below a set threshold.9
This study evaluated the accuracy of using cell-free DNA testing at 27 weeks’ gestation to determine fetal RhD status compared with serologic typing of cord blood at delivery.
Continue to: STUDY SUMMARY
STUDY SUMMARY
Test gets high marks in Netherlands trial
This large observational cohort trial from the Netherlands examined the accuracy of identifying RhD-positive fetuses using cell-free DNA isolates in maternal plasma. Over the 15-month study period, fetal RhD testing was conducted during Week 27 of gestation, and results were compared with those obtained using neonatal cord blood at birth. If the fetal RhD test was positive, providers administered 200 µg anti-D immunoglobulin during the 30th week of gestation and within 48 hours of birth. If fetal RhD was negative, providers were told immunoglobulin was unnecessary.
More than 32,000 RhD-negative women were screened. The cell-free DNA test showed fetal RhD-positive results 62% of the time and RhD-negative results in the remainder. Cord blood samples were available for 25,789 pregnancies (80%).
Sensitivity, specificity. The sensitivity for identifying fetal RhD was 99% and the specificity was 98%. Both negative and positive predictive values were 99%. Overall, there were 225 false-positive results and nine false-negative results. In the nine false negatives, six were due to a lack of fetal DNA in the sample and three were due to technical error (defined as an operator ignoring a failure of the robot pipetting the plasma or other technical failures).
The false-negative rate (0.03%) was lower than the predetermined estimated false-negative rate of cord blood serology (0.25%). In 22 of the supposed false positives, follow-up serology or molecular testing found an RhD gene was actually present, meaning the results of the neonatal cord blood serology in these cases were falsely negative. If you recalculate with these data in mind, the false-negative rate for fetal DNA testing was actually less than half that of typical serologic determination.
Continue to: WHAT'S NEW
WHAT’S NEW
Accurate test, potential to reduce unnecessary Tx
Fetal RhD testing at 27 weeks’ gestation appears to be highly accurate and could reduce the unnecessary use of anti-D immunoglobulin when the fetal RhD is negative.
CAVEATS
Different results by ethnicity?
Dutch participants are not necessarily reflective of the US population. Known variation in the rate of fetal RhD positivity among RhD-negative pregnant women by race and ethnicity could mean that the number of women able to forego anti-D immunoglobulin prophylaxis would be different in the United States than in other countries.
Also, in this study, polymerase chain reaction for two RhD sequences was run in triplicate, and a computer-based algorithm was used to automatically score samples to provide results. For safe implementation, the cell-free fetal RhD DNA testing process would need to follow similar methods.
CHALLENGES TO IMPLEMENTATION
Cost and availability are big unknowns
Cost and availability of the test may be barriers, but there is currently too little information on either subject in the United States to make a determination. A 2013 study indicated that the use of cell-free DNA testing to determine fetal RhD status was then approximately $682.10
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[5]: 306, 308, 319).
1. de Haas M, Thurik FF, van der Ploeg CP, et al. Sensitivity of fetal RHD screening for safe guidance of targeted anti-D immunoglobulin prophylaxis: prospective cohort study of a nationwide programme in the Netherlands. BMJ. 2016;355:i5789.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 75: Management of alloimmunization during pregnancy. Obstet Gynecol. 2006; 108:457-464.
3. Urbaniak SJ, Greiss MA. RhD haemolytic disease of the fetus and the newborn. Blood Rev. 2000;14(1):44-61.
4. Mayne S, Parker JH, Harden TA, et al. Rate of RhD sensitisation before and after implementation of a community based antenatal prophylaxis programme. BMJ. 1997;315(7122):1588.
5. MacKenzie IZ, Bowell P, Gregory H, et al. Routine antenatal Rhesus D immunoglobulin prophylaxis: the results of a prospective 10 year study. Br J Obstet Gynecol. 1999;106:492-497.
6. Zolotor AJ, Carlough MC. Update on prenatal care. Am Fam Physician. 2014;89(3):199-208.
7. Mackie FL, Hemming K, Allen S, et al. The accuracy of cell-free fetal DNA-based non-invasive prenatal testing in singleton pregnancies: a systematic review and bivariate meta-analysis. BJOG. 2017;124(1):32-46.
8. American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 181: Prevention of Rh D Alloimmunization. Obstet Gynecol. 2017;130:e57-e70.
9. National Institute for Health and Care Excellence. High-throughput non-invasive prenatal testing for fetal RHD genotype 1: Recommendations. www.nice.org.uk/guidance/dg25/chapter/1-Recommendations. Accessed May 7, 2018.
10. Hawk AF, Chang EY, Shields SM, Simpson KN. Costs and clinical outcomes of noninvasive fetal RhD typing for targeted prophylaxis. Obstet Gynecol. 2013;122(3):579-585.
1. de Haas M, Thurik FF, van der Ploeg CP, et al. Sensitivity of fetal RHD screening for safe guidance of targeted anti-D immunoglobulin prophylaxis: prospective cohort study of a nationwide programme in the Netherlands. BMJ. 2016;355:i5789.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 75: Management of alloimmunization during pregnancy. Obstet Gynecol. 2006; 108:457-464.
3. Urbaniak SJ, Greiss MA. RhD haemolytic disease of the fetus and the newborn. Blood Rev. 2000;14(1):44-61.
4. Mayne S, Parker JH, Harden TA, et al. Rate of RhD sensitisation before and after implementation of a community based antenatal prophylaxis programme. BMJ. 1997;315(7122):1588.
5. MacKenzie IZ, Bowell P, Gregory H, et al. Routine antenatal Rhesus D immunoglobulin prophylaxis: the results of a prospective 10 year study. Br J Obstet Gynecol. 1999;106:492-497.
6. Zolotor AJ, Carlough MC. Update on prenatal care. Am Fam Physician. 2014;89(3):199-208.
7. Mackie FL, Hemming K, Allen S, et al. The accuracy of cell-free fetal DNA-based non-invasive prenatal testing in singleton pregnancies: a systematic review and bivariate meta-analysis. BJOG. 2017;124(1):32-46.
8. American College of Obstetricians and Gynecologists Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 181: Prevention of Rh D Alloimmunization. Obstet Gynecol. 2017;130:e57-e70.
9. National Institute for Health and Care Excellence. High-throughput non-invasive prenatal testing for fetal RHD genotype 1: Recommendations. www.nice.org.uk/guidance/dg25/chapter/1-Recommendations. Accessed May 7, 2018.
10. Hawk AF, Chang EY, Shields SM, Simpson KN. Costs and clinical outcomes of noninvasive fetal RhD typing for targeted prophylaxis. Obstet Gynecol. 2013;122(3):579-585.
Let low-risk moms eat during labor?
Illustrative Case
A 23-year-old nulliparous female at term with an uncomplicated pregnancy presents to labor and delivery. She reports regular contractions for the last several hours and is admitted in labor for an anticipated vaginal delivery. She has not had anything to eat or drink for the last 3 hours and says she’s hungry.
What type of diet should you order for this patient? Should you place any restrictions in the diet order?
Since the first reports of Mendelson Syndrome (aspiration during general anesthesia) in the early 1940s,2 many health care providers managing laboring women restrict their diets to clear liquids or less with little evidence to support the decision. In a recent survey of Canadian hospitals, for example, 51% of laboring women who did not receive an epidural during the active phase of labor were placed on restricted diets of only clear fluids and/or ice chips; this number rose to 83% for women who did receive an epidural.3
Dietary restrictions continue to be enforced despite the fact that only about 5% of obstetric patients require general anesthesia.1 In a study of 172,334 patients ≥18 years of age in the general population undergoing a total of 215,488 emergency or elective surgeries with general anesthesia, the risk of aspiration was 1:895 and 1:3886, respectively.4 Of the 66 patients who aspirated, 42 had no respiratory sequelae.
Similarly, Robinson et al noted that anesthesia-associated aspiration fatalities have been much lower in more recent studies than in historical ones—approximately 1 in 350,000 anesthesia events compared with 1 in 45,000 to 240,000—and are more commonly observed during intubation for emergency surgery.5
The current American College of Obstetricians and Gynecologists guidance is to restrict oral intake to clear liquids during labor for low-risk patients, with further restriction for those at increased risk for aspiration.6 The meta-analysis described here looked at the risks and benefits of a less restrictive diet during labor.
Continue to: STUDY SUMMARY
STUDY SUMMARY
Meta-analysis finds not one case of aspiration
This meta-analysis of 10 RCTs, including 3982 laboring women, analyzed the effect of food intake on labor and the risks and benefits associated with less restrictive diets for low-risk women in labor.1 Women were included in the trials if they had singleton pregnancies with cephalic presentation at the time of delivery. The women had varying cervical dilation at the time of presentation. Seven of 10 studies involved women with a gestational age ≥37 weeks, 2 studies set the gestational age threshold at 36 weeks, and one study included women with a gestational age ≥30 weeks.
In the intervention groups, the authors studied varying degrees of diets and/or intakes, ranging from oral carbohydrate solutions to low-fat food to a completely unrestricted diet. One study accounted for 61% of the patients in this review and compared intake of low-fat foods to ice chips, water, or sips of water until delivery. The primary outcome of the meta-analysis was duration of labor.
Results. The authors of the meta-analysis found that the patients in the intervention groups, compared with the control groups, had a shorter mean duration of labor by 16 minutes (95% confidence interval [CI], -25 to -7). Apgar scores and the rates of Cesarean delivery, operative vaginal delivery, epidural analgesia, and admission to the neonatal intensive care unit were similar in the intervention and control groups. Maternal vomiting was also similar: 37.6% in the intervention group and 36.5% in the control group (relative risk=1.00; 95% CI, 0.81-1.23). None of the 3982 patients experienced aspiration pneumonia or pneumonitis.1
WHAT’S NEW
Restricting diets during labor is outdated
For years, women’s diets have been restricted during labor without sufficient evidence to support the practice. In this systematic review and meta-analysis, Ciardulli and colleagues did not find a single case of aspiration pneumonitis—the outcome on which the rationale for restricting diets during labor is based. A 2013 Cochrane review by Singata et al also found no harm in less restrictive diets for low-risk women in labor.7 Ciardulli et al concluded that dietary restrictions for women at low risk of complications/surgery during labor are not justified based on current data.
Continue to: CAVEATS
CAVEATS
Underpowered and missing information
This meta-analysis found no occurrences of aspiration pneumonia or pneumonitis; however, it was underpowered to identify these rare complications. This is partially due to the unusual need for general anesthesia in low-risk patients, as noted earlier. Data on the total number of women who underwent general anesthesia in the current review were limited, as not every study within the meta-analysis included this information.
CHALLENGES TO IMPLEMENTATION
Stemming the cultural tide
One challenge to implementation is changing the culture of practice regarding low-risk pregnant women in labor, as well as the opinions of other health care providers and hospital policies that oppose less restrictive oral intake during labor.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ciardulli A, Saccone G, Anastasio H, et al. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129:473-480.
2. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205.
3. Chackowicz A, Spence AR, Abenhaim HA. Restrictions on oral and parenteral intake for low-risk labouring women in hospitals across Canada: a cross-sectional study. J Obstet Gynaecol Can. 2016;38:1009-1014.
4. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during perioperative period. Anesthesiology. 1993;78:56-62.
5. Robinson M, Davidson A. Aspiration under anaesthesia: risk assessment and decision-making. Cont Educ Anaesth Crit Care Pain. 2014;14:171-175.
6. Committee on Obstetric Practice. ACOG Committee Opinion No. 441. Oral intake during labor. Obstet Gynecol. 2009;114:714. Reaffirmed 2017.
7. Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2013;(8):CD003930.
Illustrative Case
A 23-year-old nulliparous female at term with an uncomplicated pregnancy presents to labor and delivery. She reports regular contractions for the last several hours and is admitted in labor for an anticipated vaginal delivery. She has not had anything to eat or drink for the last 3 hours and says she’s hungry.
What type of diet should you order for this patient? Should you place any restrictions in the diet order?
Since the first reports of Mendelson Syndrome (aspiration during general anesthesia) in the early 1940s,2 many health care providers managing laboring women restrict their diets to clear liquids or less with little evidence to support the decision. In a recent survey of Canadian hospitals, for example, 51% of laboring women who did not receive an epidural during the active phase of labor were placed on restricted diets of only clear fluids and/or ice chips; this number rose to 83% for women who did receive an epidural.3
Dietary restrictions continue to be enforced despite the fact that only about 5% of obstetric patients require general anesthesia.1 In a study of 172,334 patients ≥18 years of age in the general population undergoing a total of 215,488 emergency or elective surgeries with general anesthesia, the risk of aspiration was 1:895 and 1:3886, respectively.4 Of the 66 patients who aspirated, 42 had no respiratory sequelae.
Similarly, Robinson et al noted that anesthesia-associated aspiration fatalities have been much lower in more recent studies than in historical ones—approximately 1 in 350,000 anesthesia events compared with 1 in 45,000 to 240,000—and are more commonly observed during intubation for emergency surgery.5
The current American College of Obstetricians and Gynecologists guidance is to restrict oral intake to clear liquids during labor for low-risk patients, with further restriction for those at increased risk for aspiration.6 The meta-analysis described here looked at the risks and benefits of a less restrictive diet during labor.
Continue to: STUDY SUMMARY
STUDY SUMMARY
Meta-analysis finds not one case of aspiration
This meta-analysis of 10 RCTs, including 3982 laboring women, analyzed the effect of food intake on labor and the risks and benefits associated with less restrictive diets for low-risk women in labor.1 Women were included in the trials if they had singleton pregnancies with cephalic presentation at the time of delivery. The women had varying cervical dilation at the time of presentation. Seven of 10 studies involved women with a gestational age ≥37 weeks, 2 studies set the gestational age threshold at 36 weeks, and one study included women with a gestational age ≥30 weeks.
In the intervention groups, the authors studied varying degrees of diets and/or intakes, ranging from oral carbohydrate solutions to low-fat food to a completely unrestricted diet. One study accounted for 61% of the patients in this review and compared intake of low-fat foods to ice chips, water, or sips of water until delivery. The primary outcome of the meta-analysis was duration of labor.
Results. The authors of the meta-analysis found that the patients in the intervention groups, compared with the control groups, had a shorter mean duration of labor by 16 minutes (95% confidence interval [CI], -25 to -7). Apgar scores and the rates of Cesarean delivery, operative vaginal delivery, epidural analgesia, and admission to the neonatal intensive care unit were similar in the intervention and control groups. Maternal vomiting was also similar: 37.6% in the intervention group and 36.5% in the control group (relative risk=1.00; 95% CI, 0.81-1.23). None of the 3982 patients experienced aspiration pneumonia or pneumonitis.1
WHAT’S NEW
Restricting diets during labor is outdated
For years, women’s diets have been restricted during labor without sufficient evidence to support the practice. In this systematic review and meta-analysis, Ciardulli and colleagues did not find a single case of aspiration pneumonitis—the outcome on which the rationale for restricting diets during labor is based. A 2013 Cochrane review by Singata et al also found no harm in less restrictive diets for low-risk women in labor.7 Ciardulli et al concluded that dietary restrictions for women at low risk of complications/surgery during labor are not justified based on current data.
Continue to: CAVEATS
CAVEATS
Underpowered and missing information
This meta-analysis found no occurrences of aspiration pneumonia or pneumonitis; however, it was underpowered to identify these rare complications. This is partially due to the unusual need for general anesthesia in low-risk patients, as noted earlier. Data on the total number of women who underwent general anesthesia in the current review were limited, as not every study within the meta-analysis included this information.
CHALLENGES TO IMPLEMENTATION
Stemming the cultural tide
One challenge to implementation is changing the culture of practice regarding low-risk pregnant women in labor, as well as the opinions of other health care providers and hospital policies that oppose less restrictive oral intake during labor.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Illustrative Case
A 23-year-old nulliparous female at term with an uncomplicated pregnancy presents to labor and delivery. She reports regular contractions for the last several hours and is admitted in labor for an anticipated vaginal delivery. She has not had anything to eat or drink for the last 3 hours and says she’s hungry.
What type of diet should you order for this patient? Should you place any restrictions in the diet order?
Since the first reports of Mendelson Syndrome (aspiration during general anesthesia) in the early 1940s,2 many health care providers managing laboring women restrict their diets to clear liquids or less with little evidence to support the decision. In a recent survey of Canadian hospitals, for example, 51% of laboring women who did not receive an epidural during the active phase of labor were placed on restricted diets of only clear fluids and/or ice chips; this number rose to 83% for women who did receive an epidural.3
Dietary restrictions continue to be enforced despite the fact that only about 5% of obstetric patients require general anesthesia.1 In a study of 172,334 patients ≥18 years of age in the general population undergoing a total of 215,488 emergency or elective surgeries with general anesthesia, the risk of aspiration was 1:895 and 1:3886, respectively.4 Of the 66 patients who aspirated, 42 had no respiratory sequelae.
Similarly, Robinson et al noted that anesthesia-associated aspiration fatalities have been much lower in more recent studies than in historical ones—approximately 1 in 350,000 anesthesia events compared with 1 in 45,000 to 240,000—and are more commonly observed during intubation for emergency surgery.5
The current American College of Obstetricians and Gynecologists guidance is to restrict oral intake to clear liquids during labor for low-risk patients, with further restriction for those at increased risk for aspiration.6 The meta-analysis described here looked at the risks and benefits of a less restrictive diet during labor.
Continue to: STUDY SUMMARY
STUDY SUMMARY
Meta-analysis finds not one case of aspiration
This meta-analysis of 10 RCTs, including 3982 laboring women, analyzed the effect of food intake on labor and the risks and benefits associated with less restrictive diets for low-risk women in labor.1 Women were included in the trials if they had singleton pregnancies with cephalic presentation at the time of delivery. The women had varying cervical dilation at the time of presentation. Seven of 10 studies involved women with a gestational age ≥37 weeks, 2 studies set the gestational age threshold at 36 weeks, and one study included women with a gestational age ≥30 weeks.
In the intervention groups, the authors studied varying degrees of diets and/or intakes, ranging from oral carbohydrate solutions to low-fat food to a completely unrestricted diet. One study accounted for 61% of the patients in this review and compared intake of low-fat foods to ice chips, water, or sips of water until delivery. The primary outcome of the meta-analysis was duration of labor.
Results. The authors of the meta-analysis found that the patients in the intervention groups, compared with the control groups, had a shorter mean duration of labor by 16 minutes (95% confidence interval [CI], -25 to -7). Apgar scores and the rates of Cesarean delivery, operative vaginal delivery, epidural analgesia, and admission to the neonatal intensive care unit were similar in the intervention and control groups. Maternal vomiting was also similar: 37.6% in the intervention group and 36.5% in the control group (relative risk=1.00; 95% CI, 0.81-1.23). None of the 3982 patients experienced aspiration pneumonia or pneumonitis.1
WHAT’S NEW
Restricting diets during labor is outdated
For years, women’s diets have been restricted during labor without sufficient evidence to support the practice. In this systematic review and meta-analysis, Ciardulli and colleagues did not find a single case of aspiration pneumonitis—the outcome on which the rationale for restricting diets during labor is based. A 2013 Cochrane review by Singata et al also found no harm in less restrictive diets for low-risk women in labor.7 Ciardulli et al concluded that dietary restrictions for women at low risk of complications/surgery during labor are not justified based on current data.
Continue to: CAVEATS
CAVEATS
Underpowered and missing information
This meta-analysis found no occurrences of aspiration pneumonia or pneumonitis; however, it was underpowered to identify these rare complications. This is partially due to the unusual need for general anesthesia in low-risk patients, as noted earlier. Data on the total number of women who underwent general anesthesia in the current review were limited, as not every study within the meta-analysis included this information.
CHALLENGES TO IMPLEMENTATION
Stemming the cultural tide
One challenge to implementation is changing the culture of practice regarding low-risk pregnant women in labor, as well as the opinions of other health care providers and hospital policies that oppose less restrictive oral intake during labor.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Ciardulli A, Saccone G, Anastasio H, et al. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129:473-480.
2. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205.
3. Chackowicz A, Spence AR, Abenhaim HA. Restrictions on oral and parenteral intake for low-risk labouring women in hospitals across Canada: a cross-sectional study. J Obstet Gynaecol Can. 2016;38:1009-1014.
4. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during perioperative period. Anesthesiology. 1993;78:56-62.
5. Robinson M, Davidson A. Aspiration under anaesthesia: risk assessment and decision-making. Cont Educ Anaesth Crit Care Pain. 2014;14:171-175.
6. Committee on Obstetric Practice. ACOG Committee Opinion No. 441. Oral intake during labor. Obstet Gynecol. 2009;114:714. Reaffirmed 2017.
7. Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2013;(8):CD003930.
1. Ciardulli A, Saccone G, Anastasio H, et al. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129:473-480.
2. Mendelson CL. The aspiration of stomach contents into the lungs during obstetric anesthesia. Am J Obstet Gynecol. 1946;52:191-205.
3. Chackowicz A, Spence AR, Abenhaim HA. Restrictions on oral and parenteral intake for low-risk labouring women in hospitals across Canada: a cross-sectional study. J Obstet Gynaecol Can. 2016;38:1009-1014.
4. Warner MA, Warner ME, Weber JG. Clinical significance of pulmonary aspiration during perioperative period. Anesthesiology. 1993;78:56-62.
5. Robinson M, Davidson A. Aspiration under anaesthesia: risk assessment and decision-making. Cont Educ Anaesth Crit Care Pain. 2014;14:171-175.
6. Committee on Obstetric Practice. ACOG Committee Opinion No. 441. Oral intake during labor. Obstet Gynecol. 2009;114:714. Reaffirmed 2017.
7. Singata M, Tranmer J, Gyte GM. Restricting oral fluid and food intake during labour. Cochrane Database Syst Rev. 2013;(8):CD003930.
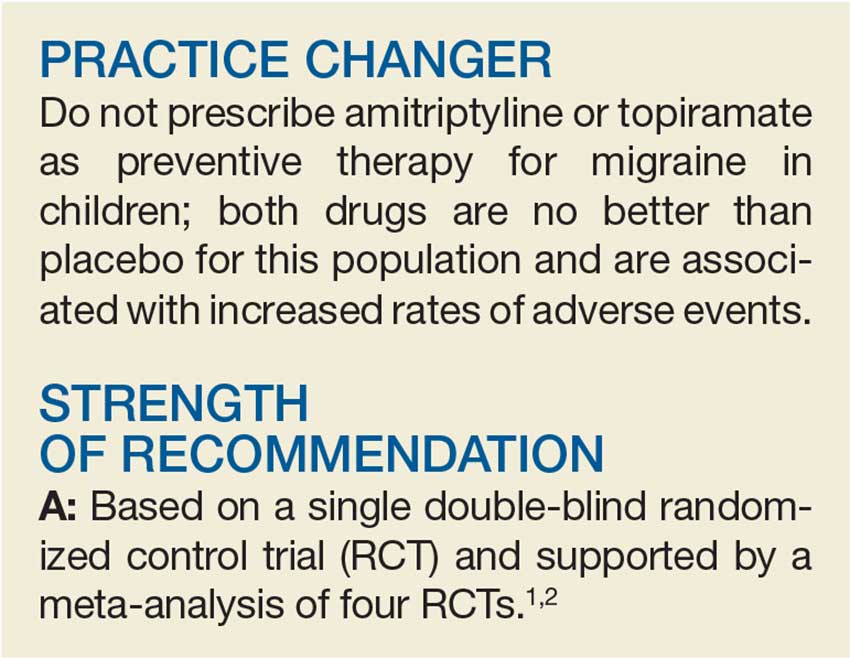
PRACTICE CHANGER
Allowing low-risk patients planning for a vaginal delivery less restrictive diets during labor does not seem to increase the risk of aspiration or other harms and may shorten labor.1
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of 10 randomized controlled trials (RCTs) in tertiary hospitals.
Ciardulli A, Saccone G, Anastasio H, et al. Less-restrictive food intake during labor in low-risk singleton pregnancies: a systematic review and meta-analysis. Obstet Gynecol. 2017;129:473-480.
Treating Migraines: It’s Different for Kids
A 15-year-old girl presents to your clinic with poorly controlled chronic migraines that prevent her from attending school three to four days per month. As part of her treatment regimen, you are considering migraine prevention strategies. Should you prescribe amitriptyline or topiramate?
Migraine headaches are the most common reason for headache presentation in pediatric neurology outpatient clinics, affecting 5% to 10% of the pediatric population worldwide.2 Current recommendations regarding prophylactic migraine therapy in childhood are based on consensus opinions.3,4 While the FDA has not approved any medications for migraine prevention in children younger than 12, surveys of pediatric headache specialists suggest that amitriptyline and topiramate are among the most commonly prescribed medications for childhood migraine prophylaxis.3,4
There is low-quality evidence from individual RCTs about the effectiveness of topiramate. A meta-analysis by El-Chammas and colleagues included three RCTs comparing topiramate to placebo for the prevention of episodic migraines (migraine headaches that occur < 15 x/mo) in a combined total of 283 children younger than 18.5 Topiramate demonstrated a nonclinically significant, but statistically significant, reduction of less than one headache per month (–0.71). This is based on moderate-quality evidence due to a high placebo response rate and study durations of only 12 weeks.5 The FDA has approved topiramate for migraine prevention in children ages 12 to 17.6
Adult guidelines. The findings described above are consistent with the most recent adult guidelines from the American Academy of Neurology and the American Headache Society.7 In a joint publication from 2012, these societies recommended both topiramate and amitriptyline for the prevention of migraines in adults based on high-quality (Level A) and medium-quality (Level B) evidence, respectively.7
STUDY SUMMARY
No better than placebo in children
A multicenter, double-blind RCT by Powers and colleagues compared the effectiveness of amitriptyline, topiramate, and placebo in the prevention of pediatric migraines.1 Target dosing for amitriptyline and topiramate was set at 1 mg/kg/d and 2 mg/kg/d, respectively. Titration toward these doses occurred over an eight-week period, based on reported adverse effects. Patients then continued their maximum tolerated dose for an additional 16 weeks.
Patients were predominantly white (70%), female (68%), and 8 to 17 years of age. They had at least four headache days over a prospective 28-day pretreatment period and a Pediatric Migraine Disability Assessment Scale (PedMIDAS) score of 11 to 139 (scores of 11-50 indicate mild-to-moderate disability; of > 50, severe disability).1,8 The primary endpoint consisted of at least a 50% relative reduction (RR) in the number of headache days in the final 28 days of the trial, compared to the 28-day pretherapy (baseline) period.1
The authors of the study included 328 patients in the primary efficacy analysis and randomly assigned them in a 2:2:1 ratio to receive either amitriptyline (132 patients), topiramate (130 patients), or placebo (66 patients). After 24 weeks of therapy, there was no significant difference between the amitriptyline, topiramate, and placebo groups in the primary endpoint (52% amitriptyline, 55% topiramate, 61% placebo; adjusted odds ratio [OR], 0.71 between amitriptyline and placebo; OR, 0.81 between topiramate and placebo; OR, 0.88 between amitriptyline and topiramate).
Continue to: There was also no difference...
There was also no difference in the secondary outcomes of absolute reduction in headache days and headache-related disability as determined by PedMIDAS. The study was stopped early for futility. Compared with placebo, amitriptyline significantly increased fatigue (number needed to harm [NNH], 8) and dry mouth (NNH, 9) and was associated with three serious adverse events of altered mood. Compared with placebo, topiramate significantly increased paresthesia (NNH, 4) and weight loss (NNH, 13) and was associated with one serious adverse event—a suicide attempt.1
WHAT’S NEW?
Higher-level evidence, lack of efficacy
This RCT provides new, higher-level evidence that demonstrates the lack of efficacy of amitriptyline and topiramate in the prevention of pediatric migraines. It also highlights the risk for increased adverse events with topiramate and amitriptyline.
Two of the three topiramate trials used in the older meta-analysis by El-Chammas and colleagues and this new RCT were included in an updated meta-analysis by Le and colleagues (total participants, 465) published in 2017.1,2,5 This newer meta-analysis found no statistical benefit associated with the use of topiramate over placebo. It demonstrated a nonsignificant decrease in the number of patients with at least a 50% relative reduction in headache frequency (risk ratio, 1.26) and in the overall number of headache days (mean difference, –0.77) in patients younger than 18.2 Both meta-analyses, however, showed an increase in the rate of adverse events in patients using topiramate versus placebo.2,5
CAVEATS
Is there a gender predominance?
El-Chammas and colleagues describe male pediatric patients as being the predominant pediatric gender with migraines.5 However, they do not quote an incidence rate or cite the reference for this statement. No other reference to gender predominance was noted in the literature. The current study, in addition to the total population of the meta-analysis by Le and colleagues, included women as the predominant patient population.1,2 Hopefully, future studies will help to delineate whether there is a gender predominance and, if so, whether the current treatment data apply to both genders.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
There are no barriers to implementing this recommendation immediately.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67 [4]:238-239, 241).
1. Powers SW, Coffey CS, Chamberlin LA, et al; for the CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017; 376:115-124.
2. Le K, Yu D, Wang J, et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.
3. Lewis D, Ashwal S, Hershey A, et al. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology. 2004;63:2215-2224.
4. Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurology. 2010;9:190-204.
5. El-Chammas K, Keyes J, Thompson N, et al. Pharmacologic treatment of pediatric headaches: a meta-analysis. JAMA Pediatr. 2013;167:250-258.
6. Qudexy XR. Highlights of prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2017/205122s003s005lbl.pdf. Accessed April 6, 2018.
7. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
8. Hershey AD, Powers SW, Vockell AL, et al. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034-2039.
A 15-year-old girl presents to your clinic with poorly controlled chronic migraines that prevent her from attending school three to four days per month. As part of her treatment regimen, you are considering migraine prevention strategies. Should you prescribe amitriptyline or topiramate?
Migraine headaches are the most common reason for headache presentation in pediatric neurology outpatient clinics, affecting 5% to 10% of the pediatric population worldwide.2 Current recommendations regarding prophylactic migraine therapy in childhood are based on consensus opinions.3,4 While the FDA has not approved any medications for migraine prevention in children younger than 12, surveys of pediatric headache specialists suggest that amitriptyline and topiramate are among the most commonly prescribed medications for childhood migraine prophylaxis.3,4
There is low-quality evidence from individual RCTs about the effectiveness of topiramate. A meta-analysis by El-Chammas and colleagues included three RCTs comparing topiramate to placebo for the prevention of episodic migraines (migraine headaches that occur < 15 x/mo) in a combined total of 283 children younger than 18.5 Topiramate demonstrated a nonclinically significant, but statistically significant, reduction of less than one headache per month (–0.71). This is based on moderate-quality evidence due to a high placebo response rate and study durations of only 12 weeks.5 The FDA has approved topiramate for migraine prevention in children ages 12 to 17.6
Adult guidelines. The findings described above are consistent with the most recent adult guidelines from the American Academy of Neurology and the American Headache Society.7 In a joint publication from 2012, these societies recommended both topiramate and amitriptyline for the prevention of migraines in adults based on high-quality (Level A) and medium-quality (Level B) evidence, respectively.7
STUDY SUMMARY
No better than placebo in children
A multicenter, double-blind RCT by Powers and colleagues compared the effectiveness of amitriptyline, topiramate, and placebo in the prevention of pediatric migraines.1 Target dosing for amitriptyline and topiramate was set at 1 mg/kg/d and 2 mg/kg/d, respectively. Titration toward these doses occurred over an eight-week period, based on reported adverse effects. Patients then continued their maximum tolerated dose for an additional 16 weeks.
Patients were predominantly white (70%), female (68%), and 8 to 17 years of age. They had at least four headache days over a prospective 28-day pretreatment period and a Pediatric Migraine Disability Assessment Scale (PedMIDAS) score of 11 to 139 (scores of 11-50 indicate mild-to-moderate disability; of > 50, severe disability).1,8 The primary endpoint consisted of at least a 50% relative reduction (RR) in the number of headache days in the final 28 days of the trial, compared to the 28-day pretherapy (baseline) period.1
The authors of the study included 328 patients in the primary efficacy analysis and randomly assigned them in a 2:2:1 ratio to receive either amitriptyline (132 patients), topiramate (130 patients), or placebo (66 patients). After 24 weeks of therapy, there was no significant difference between the amitriptyline, topiramate, and placebo groups in the primary endpoint (52% amitriptyline, 55% topiramate, 61% placebo; adjusted odds ratio [OR], 0.71 between amitriptyline and placebo; OR, 0.81 between topiramate and placebo; OR, 0.88 between amitriptyline and topiramate).
Continue to: There was also no difference...
There was also no difference in the secondary outcomes of absolute reduction in headache days and headache-related disability as determined by PedMIDAS. The study was stopped early for futility. Compared with placebo, amitriptyline significantly increased fatigue (number needed to harm [NNH], 8) and dry mouth (NNH, 9) and was associated with three serious adverse events of altered mood. Compared with placebo, topiramate significantly increased paresthesia (NNH, 4) and weight loss (NNH, 13) and was associated with one serious adverse event—a suicide attempt.1
WHAT’S NEW?
Higher-level evidence, lack of efficacy
This RCT provides new, higher-level evidence that demonstrates the lack of efficacy of amitriptyline and topiramate in the prevention of pediatric migraines. It also highlights the risk for increased adverse events with topiramate and amitriptyline.
Two of the three topiramate trials used in the older meta-analysis by El-Chammas and colleagues and this new RCT were included in an updated meta-analysis by Le and colleagues (total participants, 465) published in 2017.1,2,5 This newer meta-analysis found no statistical benefit associated with the use of topiramate over placebo. It demonstrated a nonsignificant decrease in the number of patients with at least a 50% relative reduction in headache frequency (risk ratio, 1.26) and in the overall number of headache days (mean difference, –0.77) in patients younger than 18.2 Both meta-analyses, however, showed an increase in the rate of adverse events in patients using topiramate versus placebo.2,5
CAVEATS
Is there a gender predominance?
El-Chammas and colleagues describe male pediatric patients as being the predominant pediatric gender with migraines.5 However, they do not quote an incidence rate or cite the reference for this statement. No other reference to gender predominance was noted in the literature. The current study, in addition to the total population of the meta-analysis by Le and colleagues, included women as the predominant patient population.1,2 Hopefully, future studies will help to delineate whether there is a gender predominance and, if so, whether the current treatment data apply to both genders.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
There are no barriers to implementing this recommendation immediately.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67 [4]:238-239, 241).
A 15-year-old girl presents to your clinic with poorly controlled chronic migraines that prevent her from attending school three to four days per month. As part of her treatment regimen, you are considering migraine prevention strategies. Should you prescribe amitriptyline or topiramate?
Migraine headaches are the most common reason for headache presentation in pediatric neurology outpatient clinics, affecting 5% to 10% of the pediatric population worldwide.2 Current recommendations regarding prophylactic migraine therapy in childhood are based on consensus opinions.3,4 While the FDA has not approved any medications for migraine prevention in children younger than 12, surveys of pediatric headache specialists suggest that amitriptyline and topiramate are among the most commonly prescribed medications for childhood migraine prophylaxis.3,4
There is low-quality evidence from individual RCTs about the effectiveness of topiramate. A meta-analysis by El-Chammas and colleagues included three RCTs comparing topiramate to placebo for the prevention of episodic migraines (migraine headaches that occur < 15 x/mo) in a combined total of 283 children younger than 18.5 Topiramate demonstrated a nonclinically significant, but statistically significant, reduction of less than one headache per month (–0.71). This is based on moderate-quality evidence due to a high placebo response rate and study durations of only 12 weeks.5 The FDA has approved topiramate for migraine prevention in children ages 12 to 17.6
Adult guidelines. The findings described above are consistent with the most recent adult guidelines from the American Academy of Neurology and the American Headache Society.7 In a joint publication from 2012, these societies recommended both topiramate and amitriptyline for the prevention of migraines in adults based on high-quality (Level A) and medium-quality (Level B) evidence, respectively.7
STUDY SUMMARY
No better than placebo in children
A multicenter, double-blind RCT by Powers and colleagues compared the effectiveness of amitriptyline, topiramate, and placebo in the prevention of pediatric migraines.1 Target dosing for amitriptyline and topiramate was set at 1 mg/kg/d and 2 mg/kg/d, respectively. Titration toward these doses occurred over an eight-week period, based on reported adverse effects. Patients then continued their maximum tolerated dose for an additional 16 weeks.
Patients were predominantly white (70%), female (68%), and 8 to 17 years of age. They had at least four headache days over a prospective 28-day pretreatment period and a Pediatric Migraine Disability Assessment Scale (PedMIDAS) score of 11 to 139 (scores of 11-50 indicate mild-to-moderate disability; of > 50, severe disability).1,8 The primary endpoint consisted of at least a 50% relative reduction (RR) in the number of headache days in the final 28 days of the trial, compared to the 28-day pretherapy (baseline) period.1
The authors of the study included 328 patients in the primary efficacy analysis and randomly assigned them in a 2:2:1 ratio to receive either amitriptyline (132 patients), topiramate (130 patients), or placebo (66 patients). After 24 weeks of therapy, there was no significant difference between the amitriptyline, topiramate, and placebo groups in the primary endpoint (52% amitriptyline, 55% topiramate, 61% placebo; adjusted odds ratio [OR], 0.71 between amitriptyline and placebo; OR, 0.81 between topiramate and placebo; OR, 0.88 between amitriptyline and topiramate).
Continue to: There was also no difference...
There was also no difference in the secondary outcomes of absolute reduction in headache days and headache-related disability as determined by PedMIDAS. The study was stopped early for futility. Compared with placebo, amitriptyline significantly increased fatigue (number needed to harm [NNH], 8) and dry mouth (NNH, 9) and was associated with three serious adverse events of altered mood. Compared with placebo, topiramate significantly increased paresthesia (NNH, 4) and weight loss (NNH, 13) and was associated with one serious adverse event—a suicide attempt.1
WHAT’S NEW?
Higher-level evidence, lack of efficacy
This RCT provides new, higher-level evidence that demonstrates the lack of efficacy of amitriptyline and topiramate in the prevention of pediatric migraines. It also highlights the risk for increased adverse events with topiramate and amitriptyline.
Two of the three topiramate trials used in the older meta-analysis by El-Chammas and colleagues and this new RCT were included in an updated meta-analysis by Le and colleagues (total participants, 465) published in 2017.1,2,5 This newer meta-analysis found no statistical benefit associated with the use of topiramate over placebo. It demonstrated a nonsignificant decrease in the number of patients with at least a 50% relative reduction in headache frequency (risk ratio, 1.26) and in the overall number of headache days (mean difference, –0.77) in patients younger than 18.2 Both meta-analyses, however, showed an increase in the rate of adverse events in patients using topiramate versus placebo.2,5
CAVEATS
Is there a gender predominance?
El-Chammas and colleagues describe male pediatric patients as being the predominant pediatric gender with migraines.5 However, they do not quote an incidence rate or cite the reference for this statement. No other reference to gender predominance was noted in the literature. The current study, in addition to the total population of the meta-analysis by Le and colleagues, included women as the predominant patient population.1,2 Hopefully, future studies will help to delineate whether there is a gender predominance and, if so, whether the current treatment data apply to both genders.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
There are no barriers to implementing this recommendation immediately.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67 [4]:238-239, 241).
1. Powers SW, Coffey CS, Chamberlin LA, et al; for the CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017; 376:115-124.
2. Le K, Yu D, Wang J, et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.
3. Lewis D, Ashwal S, Hershey A, et al. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology. 2004;63:2215-2224.
4. Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurology. 2010;9:190-204.
5. El-Chammas K, Keyes J, Thompson N, et al. Pharmacologic treatment of pediatric headaches: a meta-analysis. JAMA Pediatr. 2013;167:250-258.
6. Qudexy XR. Highlights of prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2017/205122s003s005lbl.pdf. Accessed April 6, 2018.
7. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
8. Hershey AD, Powers SW, Vockell AL, et al. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034-2039.
1. Powers SW, Coffey CS, Chamberlin LA, et al; for the CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017; 376:115-124.
2. Le K, Yu D, Wang J, et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.
3. Lewis D, Ashwal S, Hershey A, et al. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology. 2004;63:2215-2224.
4. Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurology. 2010;9:190-204.
5. El-Chammas K, Keyes J, Thompson N, et al. Pharmacologic treatment of pediatric headaches: a meta-analysis. JAMA Pediatr. 2013;167:250-258.
6. Qudexy XR. Highlights of prescribing information. www.accessdata.fda.gov/drugsatfda_docs/label/2017/205122s003s005lbl.pdf. Accessed April 6, 2018.
7. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
8. Hershey AD, Powers SW, Vockell AL, et al. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034-2039.
A new protocol for RhD-negative pregnant women?
ILLUSTRATIVE CASE
A 30-year-old G1P0 woman presents to your office for routine obstetric care at 18 weeks’ gestation. Her pregnancy has been uncomplicated, but her prenatal lab evaluation is notable for blood type A-negative. She wants to know if she really needs the anti-D immune globulin injection.
Rhesus (Rh)D-negative women carrying an RhD-positive fetus are at risk of developing anti-D antibodies, placing the fetus at risk for HDFN (hemolytic disease of the fetus and newborn). If undiagnosed and/or untreated, HDFN carries significant risk of perinatal morbidity and mortality.2
With routine postnatal anti-D immunoglobulin prophylaxis of RhD-negative women who delivered an RhD-positive child (which began around 1970), the risk of maternal alloimmunization was reduced from 16% to 1.12%-1.3%.3-5 The risk was further reduced to approximately 0.28% with the addition of consistent prophylaxis at 28 weeks’ gestation.4 As a result, the current standard of care is to administer anti-D immunoglobulin at 28 weeks’ gestation, within 72 hours of delivery of an RhD-positive fetus, and after events with risk of fetal-to-maternal transfusion (eg, spontaneous, threatened, or induced abortion; invasive prenatal diagnostic procedures such as amniocentesis; blunt abdominal trauma; external cephalic version; second or third trimester antepartum bleeding).6
The problem of unnecessary Tx. However, under this current practice, many RhD-negative women are receiving anti-D immunoglobulin unnecessarily. This is because the fetus’s RhD status is not routinely known during the prenatal period.
Enter cell-free DNA testing. Cell-free DNA testing analyzes fragments of fetal DNA found in maternal blood. The use of cell-free DNA testing at 10 to 13 weeks’ gestation to screen for fetal chromosomal abnormalities is reliable (91%-99% sensitivity for trisomies 21, 18, and 137) and becoming increasingly more common.
A notable meta-analysis. A 2017 meta-analysis of 30 studies of cell-free DNA testing of RhD status in the first and second trimester calculated a sensitivity of 99.3% (95% confidence interval [CI], 98.2-99.7) and a specificity of 98.4% (95% CI, 96.4-99.3).7
This study evaluated the accuracy of using cell-free DNA testing at 27 weeks’ gestation to determine fetal RhD status compared with serologic typing of cord blood at delivery.
STUDY SUMMARY
Cell-free DNA test gets high marks in Netherlands trial
This large observational cohort trial from the Netherlands examined the accuracy of identifying RhD-positive fetuses using cell-free DNA isolates in maternal plasma. Over the 15-month study period, fetal RhD testing was conducted during Week 27 of gestation, and results were compared with those obtained using neonatal cord blood at birth. If the fetal RhD test was positive, providers administered 200 mcg anti-D immunoglobulin during the 30th week of gestation and within 48 hours of birth. If fetal RhD was negative, providers were told immunoglobulin was unnecessary.
More than 32,000 RhD-negative women were screened. The cell-free DNA test showed fetal RhD-positive results 62% of the time and RhD-negative results in the remainder. Cord blood samples were available for 25,789 pregnancies (80%).
Sensitivity, specificity. The sensitivity for identifying fetal RhD was 99% and the specificity was 98%. Both negative and positive predictive values were 99%. Overall, there were 225 false-positive results and 9 false-negative results. In the 9 false negatives, 6 were due to a lack of fetal DNA in the sample and 3 were due to technical error (defined as an operator ignoring a failure of the robot pipetting the plasma or other technical failures).
The false-negative rate (0.03%) was lower than the predetermined estimated false-negative rate of cord blood serology (0.25%). In 22 of the supposed false positives, follow-up serology or molecular testing found an RhD gene was actually present, meaning the results of the neonatal cord blood serology in these cases were falsely negative. If you recalculate with these data in mind, the false-negative rate for fetal DNA testing was actually less than half that of typical serologic determination.
WHAT’S NEW
An accurate test with the potential to reduce unnecessary Tx
Fetal RhD testing at 27 weeks’ gestation appears to be highly accurate and could reduce the unnecessary use of anti-D immunoglobulin when the fetal RhD is negative.
CAVEATS
Different results with different ethnicities?
Dutch participants are not necessarily reflective of the US population. Known variation in the rate of fetal RhD positivity among RhD-negative pregnant women by race and ethnicity could mean that the number of women able to forego anti-D-immunoglobulin prophylaxis would be different in the United States from that in other countries.
Also, in this study, polymerase chain reaction (PCR) for 2 RhD sequences was run in triplicate, and a computer-based algorithm was used to automatically score samples to provide results. For safe implementation, the cell-free fetal RhD DNA testing process would need to follow similar methods.
CHALLENGES TO IMPLEMENTATION
Test cost and availability are big unknowns
Cost and availability of the test may be barriers, but there is currently too little information on either subject in the United States to make a determination. A 2013 study indicated that the use of cell-free DNA testing to determine fetal RhD status was then approximately $682.10
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. de Haas M, Thurik FF, van der Ploeg CP, et al. Sensitivity of fetal RHD screening for safe guidance of targeted anti-D immunoglobulin prophylaxis: prospective cohort study of a nationwide programme in the Netherlands. BMJ. 2016;355:i5789.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 75: Management of alloimmunization during pregnancy. Obstet Gynecol. 2006;108:457-464.
3. Urbaniak S, Greiss MA. RhD haemolytic disease of the fetus and the newborn. Blood Rev. 2000;14:44-61.
4. Mayne S, Parker JH, Harden TA, et al. Rate of RhD sensitisation before and after implementation of a community based antenatal prophylaxis programme. BMJ. 1997;315:1588-1588.
5. MacKenzie IZ, Bowell P, Gregory H, et al. Routine antenatal Rhesus D immunoglobulin prophylaxis: the results of a prospective 10 year study. Br J Obstet Gynecol: 1999;106:492-497.
6. Zolotor AJ, Carlough MC. Update on prenatal care. Am Fam Physician. 2014;89:199-208.
7. Mackie FL, Hemming K, Allen S, et al. The accuracy of cell-free fetal DNA-based non-invasive prenatal testing in singleton pregnancies: a systematic review and bivariate meta-analysis. BJOG. 2017;124:32-46.
8. Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 181: Prevention of Rh D Alloimmunization. Obstet Gynecol. 2017;130:e57-e70.
9. National Institute for Health and Care Excellence. High-throughput non-invasive prenatal testing for fetal RHD genotype 1: Recommendations. Available at: https://www.nice.org.uk/guidance/dg25/chapter/1-Recommendations. Accessed August 9, 2017.
10. Hawk AF, Chang EY, Shields SM, et al. Costs and clinical outcomes of noninvasive fetal RhD typing for targeted prophylaxis. Obstet Gynecol. 2013;122:579-585.
ILLUSTRATIVE CASE
A 30-year-old G1P0 woman presents to your office for routine obstetric care at 18 weeks’ gestation. Her pregnancy has been uncomplicated, but her prenatal lab evaluation is notable for blood type A-negative. She wants to know if she really needs the anti-D immune globulin injection.
Rhesus (Rh)D-negative women carrying an RhD-positive fetus are at risk of developing anti-D antibodies, placing the fetus at risk for HDFN (hemolytic disease of the fetus and newborn). If undiagnosed and/or untreated, HDFN carries significant risk of perinatal morbidity and mortality.2
With routine postnatal anti-D immunoglobulin prophylaxis of RhD-negative women who delivered an RhD-positive child (which began around 1970), the risk of maternal alloimmunization was reduced from 16% to 1.12%-1.3%.3-5 The risk was further reduced to approximately 0.28% with the addition of consistent prophylaxis at 28 weeks’ gestation.4 As a result, the current standard of care is to administer anti-D immunoglobulin at 28 weeks’ gestation, within 72 hours of delivery of an RhD-positive fetus, and after events with risk of fetal-to-maternal transfusion (eg, spontaneous, threatened, or induced abortion; invasive prenatal diagnostic procedures such as amniocentesis; blunt abdominal trauma; external cephalic version; second or third trimester antepartum bleeding).6
The problem of unnecessary Tx. However, under this current practice, many RhD-negative women are receiving anti-D immunoglobulin unnecessarily. This is because the fetus’s RhD status is not routinely known during the prenatal period.
Enter cell-free DNA testing. Cell-free DNA testing analyzes fragments of fetal DNA found in maternal blood. The use of cell-free DNA testing at 10 to 13 weeks’ gestation to screen for fetal chromosomal abnormalities is reliable (91%-99% sensitivity for trisomies 21, 18, and 137) and becoming increasingly more common.
A notable meta-analysis. A 2017 meta-analysis of 30 studies of cell-free DNA testing of RhD status in the first and second trimester calculated a sensitivity of 99.3% (95% confidence interval [CI], 98.2-99.7) and a specificity of 98.4% (95% CI, 96.4-99.3).7
This study evaluated the accuracy of using cell-free DNA testing at 27 weeks’ gestation to determine fetal RhD status compared with serologic typing of cord blood at delivery.
STUDY SUMMARY
Cell-free DNA test gets high marks in Netherlands trial
This large observational cohort trial from the Netherlands examined the accuracy of identifying RhD-positive fetuses using cell-free DNA isolates in maternal plasma. Over the 15-month study period, fetal RhD testing was conducted during Week 27 of gestation, and results were compared with those obtained using neonatal cord blood at birth. If the fetal RhD test was positive, providers administered 200 mcg anti-D immunoglobulin during the 30th week of gestation and within 48 hours of birth. If fetal RhD was negative, providers were told immunoglobulin was unnecessary.
More than 32,000 RhD-negative women were screened. The cell-free DNA test showed fetal RhD-positive results 62% of the time and RhD-negative results in the remainder. Cord blood samples were available for 25,789 pregnancies (80%).
Sensitivity, specificity. The sensitivity for identifying fetal RhD was 99% and the specificity was 98%. Both negative and positive predictive values were 99%. Overall, there were 225 false-positive results and 9 false-negative results. In the 9 false negatives, 6 were due to a lack of fetal DNA in the sample and 3 were due to technical error (defined as an operator ignoring a failure of the robot pipetting the plasma or other technical failures).
The false-negative rate (0.03%) was lower than the predetermined estimated false-negative rate of cord blood serology (0.25%). In 22 of the supposed false positives, follow-up serology or molecular testing found an RhD gene was actually present, meaning the results of the neonatal cord blood serology in these cases were falsely negative. If you recalculate with these data in mind, the false-negative rate for fetal DNA testing was actually less than half that of typical serologic determination.
WHAT’S NEW
An accurate test with the potential to reduce unnecessary Tx
Fetal RhD testing at 27 weeks’ gestation appears to be highly accurate and could reduce the unnecessary use of anti-D immunoglobulin when the fetal RhD is negative.
CAVEATS
Different results with different ethnicities?
Dutch participants are not necessarily reflective of the US population. Known variation in the rate of fetal RhD positivity among RhD-negative pregnant women by race and ethnicity could mean that the number of women able to forego anti-D-immunoglobulin prophylaxis would be different in the United States from that in other countries.
Also, in this study, polymerase chain reaction (PCR) for 2 RhD sequences was run in triplicate, and a computer-based algorithm was used to automatically score samples to provide results. For safe implementation, the cell-free fetal RhD DNA testing process would need to follow similar methods.
CHALLENGES TO IMPLEMENTATION
Test cost and availability are big unknowns
Cost and availability of the test may be barriers, but there is currently too little information on either subject in the United States to make a determination. A 2013 study indicated that the use of cell-free DNA testing to determine fetal RhD status was then approximately $682.10
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 30-year-old G1P0 woman presents to your office for routine obstetric care at 18 weeks’ gestation. Her pregnancy has been uncomplicated, but her prenatal lab evaluation is notable for blood type A-negative. She wants to know if she really needs the anti-D immune globulin injection.
Rhesus (Rh)D-negative women carrying an RhD-positive fetus are at risk of developing anti-D antibodies, placing the fetus at risk for HDFN (hemolytic disease of the fetus and newborn). If undiagnosed and/or untreated, HDFN carries significant risk of perinatal morbidity and mortality.2
With routine postnatal anti-D immunoglobulin prophylaxis of RhD-negative women who delivered an RhD-positive child (which began around 1970), the risk of maternal alloimmunization was reduced from 16% to 1.12%-1.3%.3-5 The risk was further reduced to approximately 0.28% with the addition of consistent prophylaxis at 28 weeks’ gestation.4 As a result, the current standard of care is to administer anti-D immunoglobulin at 28 weeks’ gestation, within 72 hours of delivery of an RhD-positive fetus, and after events with risk of fetal-to-maternal transfusion (eg, spontaneous, threatened, or induced abortion; invasive prenatal diagnostic procedures such as amniocentesis; blunt abdominal trauma; external cephalic version; second or third trimester antepartum bleeding).6
The problem of unnecessary Tx. However, under this current practice, many RhD-negative women are receiving anti-D immunoglobulin unnecessarily. This is because the fetus’s RhD status is not routinely known during the prenatal period.
Enter cell-free DNA testing. Cell-free DNA testing analyzes fragments of fetal DNA found in maternal blood. The use of cell-free DNA testing at 10 to 13 weeks’ gestation to screen for fetal chromosomal abnormalities is reliable (91%-99% sensitivity for trisomies 21, 18, and 137) and becoming increasingly more common.
A notable meta-analysis. A 2017 meta-analysis of 30 studies of cell-free DNA testing of RhD status in the first and second trimester calculated a sensitivity of 99.3% (95% confidence interval [CI], 98.2-99.7) and a specificity of 98.4% (95% CI, 96.4-99.3).7
This study evaluated the accuracy of using cell-free DNA testing at 27 weeks’ gestation to determine fetal RhD status compared with serologic typing of cord blood at delivery.
STUDY SUMMARY
Cell-free DNA test gets high marks in Netherlands trial
This large observational cohort trial from the Netherlands examined the accuracy of identifying RhD-positive fetuses using cell-free DNA isolates in maternal plasma. Over the 15-month study period, fetal RhD testing was conducted during Week 27 of gestation, and results were compared with those obtained using neonatal cord blood at birth. If the fetal RhD test was positive, providers administered 200 mcg anti-D immunoglobulin during the 30th week of gestation and within 48 hours of birth. If fetal RhD was negative, providers were told immunoglobulin was unnecessary.
More than 32,000 RhD-negative women were screened. The cell-free DNA test showed fetal RhD-positive results 62% of the time and RhD-negative results in the remainder. Cord blood samples were available for 25,789 pregnancies (80%).
Sensitivity, specificity. The sensitivity for identifying fetal RhD was 99% and the specificity was 98%. Both negative and positive predictive values were 99%. Overall, there were 225 false-positive results and 9 false-negative results. In the 9 false negatives, 6 were due to a lack of fetal DNA in the sample and 3 were due to technical error (defined as an operator ignoring a failure of the robot pipetting the plasma or other technical failures).
The false-negative rate (0.03%) was lower than the predetermined estimated false-negative rate of cord blood serology (0.25%). In 22 of the supposed false positives, follow-up serology or molecular testing found an RhD gene was actually present, meaning the results of the neonatal cord blood serology in these cases were falsely negative. If you recalculate with these data in mind, the false-negative rate for fetal DNA testing was actually less than half that of typical serologic determination.
WHAT’S NEW
An accurate test with the potential to reduce unnecessary Tx
Fetal RhD testing at 27 weeks’ gestation appears to be highly accurate and could reduce the unnecessary use of anti-D immunoglobulin when the fetal RhD is negative.
CAVEATS
Different results with different ethnicities?
Dutch participants are not necessarily reflective of the US population. Known variation in the rate of fetal RhD positivity among RhD-negative pregnant women by race and ethnicity could mean that the number of women able to forego anti-D-immunoglobulin prophylaxis would be different in the United States from that in other countries.
Also, in this study, polymerase chain reaction (PCR) for 2 RhD sequences was run in triplicate, and a computer-based algorithm was used to automatically score samples to provide results. For safe implementation, the cell-free fetal RhD DNA testing process would need to follow similar methods.
CHALLENGES TO IMPLEMENTATION
Test cost and availability are big unknowns
Cost and availability of the test may be barriers, but there is currently too little information on either subject in the United States to make a determination. A 2013 study indicated that the use of cell-free DNA testing to determine fetal RhD status was then approximately $682.10
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. de Haas M, Thurik FF, van der Ploeg CP, et al. Sensitivity of fetal RHD screening for safe guidance of targeted anti-D immunoglobulin prophylaxis: prospective cohort study of a nationwide programme in the Netherlands. BMJ. 2016;355:i5789.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 75: Management of alloimmunization during pregnancy. Obstet Gynecol. 2006;108:457-464.
3. Urbaniak S, Greiss MA. RhD haemolytic disease of the fetus and the newborn. Blood Rev. 2000;14:44-61.
4. Mayne S, Parker JH, Harden TA, et al. Rate of RhD sensitisation before and after implementation of a community based antenatal prophylaxis programme. BMJ. 1997;315:1588-1588.
5. MacKenzie IZ, Bowell P, Gregory H, et al. Routine antenatal Rhesus D immunoglobulin prophylaxis: the results of a prospective 10 year study. Br J Obstet Gynecol: 1999;106:492-497.
6. Zolotor AJ, Carlough MC. Update on prenatal care. Am Fam Physician. 2014;89:199-208.
7. Mackie FL, Hemming K, Allen S, et al. The accuracy of cell-free fetal DNA-based non-invasive prenatal testing in singleton pregnancies: a systematic review and bivariate meta-analysis. BJOG. 2017;124:32-46.
8. Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 181: Prevention of Rh D Alloimmunization. Obstet Gynecol. 2017;130:e57-e70.
9. National Institute for Health and Care Excellence. High-throughput non-invasive prenatal testing for fetal RHD genotype 1: Recommendations. Available at: https://www.nice.org.uk/guidance/dg25/chapter/1-Recommendations. Accessed August 9, 2017.
10. Hawk AF, Chang EY, Shields SM, et al. Costs and clinical outcomes of noninvasive fetal RhD typing for targeted prophylaxis. Obstet Gynecol. 2013;122:579-585.
1. de Haas M, Thurik FF, van der Ploeg CP, et al. Sensitivity of fetal RHD screening for safe guidance of targeted anti-D immunoglobulin prophylaxis: prospective cohort study of a nationwide programme in the Netherlands. BMJ. 2016;355:i5789.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 75: Management of alloimmunization during pregnancy. Obstet Gynecol. 2006;108:457-464.
3. Urbaniak S, Greiss MA. RhD haemolytic disease of the fetus and the newborn. Blood Rev. 2000;14:44-61.
4. Mayne S, Parker JH, Harden TA, et al. Rate of RhD sensitisation before and after implementation of a community based antenatal prophylaxis programme. BMJ. 1997;315:1588-1588.
5. MacKenzie IZ, Bowell P, Gregory H, et al. Routine antenatal Rhesus D immunoglobulin prophylaxis: the results of a prospective 10 year study. Br J Obstet Gynecol: 1999;106:492-497.
6. Zolotor AJ, Carlough MC. Update on prenatal care. Am Fam Physician. 2014;89:199-208.
7. Mackie FL, Hemming K, Allen S, et al. The accuracy of cell-free fetal DNA-based non-invasive prenatal testing in singleton pregnancies: a systematic review and bivariate meta-analysis. BJOG. 2017;124:32-46.
8. Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 181: Prevention of Rh D Alloimmunization. Obstet Gynecol. 2017;130:e57-e70.
9. National Institute for Health and Care Excellence. High-throughput non-invasive prenatal testing for fetal RHD genotype 1: Recommendations. Available at: https://www.nice.org.uk/guidance/dg25/chapter/1-Recommendations. Accessed August 9, 2017.
10. Hawk AF, Chang EY, Shields SM, et al. Costs and clinical outcomes of noninvasive fetal RhD typing for targeted prophylaxis. Obstet Gynecol. 2013;122:579-585.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Employ cell-free DNA testing at 27 weeks’ gestation in your RhD-negative obstetric patients to reduce unnecessary use of anti-D immunoglobulin.1
STRENGTH OF RECOMMENDATION
B: Based on a single, prospective, cohort study.
de Haas M, Thurik FF, van der Ploeg CP, et al. Sensitivity of fetal RHD screening for safe guidance of targeted anti-D immunoglobulin prophylaxis: prospective cohort study of a nationwide programme in the Netherlands. BMJ. 2016;355:i5789.
An Easy Approach to Obtaining Clean-catch Urine From Infants
A fussy 6-month-old infant is brought to the emergency department (ED) with a rectal temperature of 101.5°F. She is consolable,
A febrile infant in a family practice office or ED is a familiar clinical situation that may require an invasive diagnostic workup. Up to 7% of infants ages 2 to 24 months with fever of unknown origin may have a UTI.2 Collecting a urine sample from pre–toilet-trained children can be time consuming. In fact, in one RCT, obtaining a clean-catch urine sample in this age group took more than an hour, on average.3 But more convenient methods of urine collection, such as placing a cotton ball in the diaper or using a perineal collection bag, have contamination rates of up to 63%.4
In its guidelines for evaluating possible UTI in a febrile child younger than age 2, the American Academy of Pediatrics (AAP) recommends obtaining a sample for urinalysis “through the most convenient means.”5 If urinalysis is positive, only urine obtained by catheterization or suprapubic aspiration should be cultured. Guidelines from the National Institute for Health and Care Excellence in the United Kingdom are similar, but allow for culture of clean-catch urine samples.6
A recent prospective cohort study examined a noninvasive alternating lumbar-bladder tapping method to stimulate voiding in infants ages 6 months or younger.7 Within five minutes, 49% of the infants provided a clean-catch sample, with contamination rates similar to those of samples obtained using invasive methods.7 Younger infants were more likely to void within the time allotted. Another trial of bladder tapping conducted in hospitalized infants younger than 30 days old showed similar results.8 There are, however, no previously reported randomized trials demonstrating the efficacy of a noninvasive urine collection technique in the outpatient setting.
Use of invasive collection methods requires skilled personnel and may cause significant discomfort for patients (and parents). Noninvasive methods, such as bag urine collection, have unacceptable contamination rates. In addition, waiting to catch a potentially cleaner urine sample is time consuming, so better strategies to collect urine from infants are needed. This RCT is the first to examine the efficacy of a unique stimulation technique to obtain a clean-catch urine sample from infants ages 1 to 12 months.
STUDY SUMMARY
Noninvasive stimulation triggers faster samples
A nonblinded, single-center RCT conducted in Australia compared two methods for obtaining a clean-catch urine sample within five minutes: the Quick-Wee method (suprapubic stimulation with gauze soaked in cold fluid) or usual care (waiting for spontaneous voiding with no stimulation).1 A total of 354 infants (ages 1-12 mo) who required urine sample collection were randomized in a 1:1 ratio; allocation was concealed. Infants with anatomic or neurologic abnormalities and those needing immediate antibiotic therapy were excluded.
The most common reasons for obtaining the urine sample were fever of unknown origin and “unsettled baby,” followed by poor feeding and suspected UTI. The primary outcome was voiding within five minutes; secondary outcomes included time to void, whether urine was successfully caught, contamination rate, and parent/clinician satisfaction.
Study personnel removed the diaper, then cleaned the genitals of all patients with room temperature sterile water. A caregiver or clinician was ready and waiting to catch urine when the patient voided. In the Quick-Wee group, a clinician rubbed the patient’s suprapubic area in a circular fashion with gauze soaked in refrigerated saline (2.8°C). At five minutes, clinicians recorded the voiding status and decided how to proceed.
Using intention-to-treat analysis, 31% of the patients in the Quick-Wee group voided within five minutes, compared with 12% of the usual-care patients. Similarly, 30% of patients in the Quick-Wee group provided a successful clean-catch sample within five minutes, compared with 9% in the usual-care group (number needed to treat, 4.7).
Contamination rates were no different between the Quick-Wee and usual-care samples. Both parents and clinicians were more satisfied with the Quick-Wee method than with usual care (median score of 2 vs 3 on a 5-point Likert scale, in which 1 is “most satisfied”). There was no difference when results were adjusted for age or sex. No adverse events occurred.
Continue to: WHAT'S NEW
WHAT’S NEW
Method could reduce need for invasive sampling
A simple suprapubic stimulation technique increased the number of infants who provided a clean-catch voided urine sample within five minutes—a clinically relevant and satisfying outcome. In appropriate patients, use of the Quick-Wee method to obtain a clean-catch voided sample for initial urinalysis, rather than attempting methods with known high contamination rates, may potentially reduce the need for invasive sampling using catheterization or suprapubic aspiration.
CAVEATS
Complete age range & ideal storage temperature are unknown
Neonates and precontinent children older than 12 months were not included in this trial, so these conclusions do not apply to those groups. The intervention period lasted only five minutes, but other published studies suggest that this amount of time is adequate for voiding to occur.6,7 Although this study used soaking fluid stored at 2.8°C, the ideal storage temperature is unknown.
CHALLENGES TO IMPLEMENTATION
AAP doesn’t endorse clean-catch urine samples
The Quick-Wee method is simple and easy to implement, and requires no specialized training or equipment. AAP guidelines do not endorse the use of clean-catch voided urine for culture, which may be a barrier to changing urine collection practices in some settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[3]: 166, 168-169).
1. Kaufman J, Fitzpatrick P, Tosif S, et al. Faster clean catch urine collection (Quick-Wee method) from infants: randomised controlled trial. BMJ. 2017;357:j1341.
2. Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008;27(4):302-308.
3. Davies P, Greenwood R, Benger J. Randomised trial of a vibrating bladder stimulator—the time to pee study. Arch Dis Child. 2008;93(5):423-424.
4. Al-Orifi F, McGillivray D, Tange S, Kramer MS. Urine culture from bag specimens in young children: are the risks too high? J Pediatr. 2000;137(2):221-226.
5. Roberts KB, Downs SM, Finnell SM, et al; Subcommittee on Urinary Tract Infection. Reaffirmation of AAP clinical practice guideline: the diagnosis and management of the initial urinary tract infection in febrile infants and young children 2-24 months of age. Pediatrics. 2016;138(6): e20163026.
6. National Institute for Health and Care Excellence. Urinary tract infection in under 16s: diagnosis and management [clinical guideline CG54]. www.nice.org.uk/guidance/cg54/chapter/1-guidance. Accessed March 1, 2018.
7. Labrosse M, Levy A, Autmizguine J, Gravel J. Evaluation of a new strategy for clean-catch urine in infants. Pediatrics. 2016;138(3):e20160573.
8. Herreros Fernández ML, González Merino N, Tagarro García A, et al. A new technique for fast and safe collection of urine in newborns. Arch Dis Child. 2013;98(1):27-29.
A fussy 6-month-old infant is brought to the emergency department (ED) with a rectal temperature of 101.5°F. She is consolable,
A febrile infant in a family practice office or ED is a familiar clinical situation that may require an invasive diagnostic workup. Up to 7% of infants ages 2 to 24 months with fever of unknown origin may have a UTI.2 Collecting a urine sample from pre–toilet-trained children can be time consuming. In fact, in one RCT, obtaining a clean-catch urine sample in this age group took more than an hour, on average.3 But more convenient methods of urine collection, such as placing a cotton ball in the diaper or using a perineal collection bag, have contamination rates of up to 63%.4
In its guidelines for evaluating possible UTI in a febrile child younger than age 2, the American Academy of Pediatrics (AAP) recommends obtaining a sample for urinalysis “through the most convenient means.”5 If urinalysis is positive, only urine obtained by catheterization or suprapubic aspiration should be cultured. Guidelines from the National Institute for Health and Care Excellence in the United Kingdom are similar, but allow for culture of clean-catch urine samples.6
A recent prospective cohort study examined a noninvasive alternating lumbar-bladder tapping method to stimulate voiding in infants ages 6 months or younger.7 Within five minutes, 49% of the infants provided a clean-catch sample, with contamination rates similar to those of samples obtained using invasive methods.7 Younger infants were more likely to void within the time allotted. Another trial of bladder tapping conducted in hospitalized infants younger than 30 days old showed similar results.8 There are, however, no previously reported randomized trials demonstrating the efficacy of a noninvasive urine collection technique in the outpatient setting.
Use of invasive collection methods requires skilled personnel and may cause significant discomfort for patients (and parents). Noninvasive methods, such as bag urine collection, have unacceptable contamination rates. In addition, waiting to catch a potentially cleaner urine sample is time consuming, so better strategies to collect urine from infants are needed. This RCT is the first to examine the efficacy of a unique stimulation technique to obtain a clean-catch urine sample from infants ages 1 to 12 months.
STUDY SUMMARY
Noninvasive stimulation triggers faster samples
A nonblinded, single-center RCT conducted in Australia compared two methods for obtaining a clean-catch urine sample within five minutes: the Quick-Wee method (suprapubic stimulation with gauze soaked in cold fluid) or usual care (waiting for spontaneous voiding with no stimulation).1 A total of 354 infants (ages 1-12 mo) who required urine sample collection were randomized in a 1:1 ratio; allocation was concealed. Infants with anatomic or neurologic abnormalities and those needing immediate antibiotic therapy were excluded.
The most common reasons for obtaining the urine sample were fever of unknown origin and “unsettled baby,” followed by poor feeding and suspected UTI. The primary outcome was voiding within five minutes; secondary outcomes included time to void, whether urine was successfully caught, contamination rate, and parent/clinician satisfaction.
Study personnel removed the diaper, then cleaned the genitals of all patients with room temperature sterile water. A caregiver or clinician was ready and waiting to catch urine when the patient voided. In the Quick-Wee group, a clinician rubbed the patient’s suprapubic area in a circular fashion with gauze soaked in refrigerated saline (2.8°C). At five minutes, clinicians recorded the voiding status and decided how to proceed.
Using intention-to-treat analysis, 31% of the patients in the Quick-Wee group voided within five minutes, compared with 12% of the usual-care patients. Similarly, 30% of patients in the Quick-Wee group provided a successful clean-catch sample within five minutes, compared with 9% in the usual-care group (number needed to treat, 4.7).
Contamination rates were no different between the Quick-Wee and usual-care samples. Both parents and clinicians were more satisfied with the Quick-Wee method than with usual care (median score of 2 vs 3 on a 5-point Likert scale, in which 1 is “most satisfied”). There was no difference when results were adjusted for age or sex. No adverse events occurred.
Continue to: WHAT'S NEW
WHAT’S NEW
Method could reduce need for invasive sampling
A simple suprapubic stimulation technique increased the number of infants who provided a clean-catch voided urine sample within five minutes—a clinically relevant and satisfying outcome. In appropriate patients, use of the Quick-Wee method to obtain a clean-catch voided sample for initial urinalysis, rather than attempting methods with known high contamination rates, may potentially reduce the need for invasive sampling using catheterization or suprapubic aspiration.
CAVEATS
Complete age range & ideal storage temperature are unknown
Neonates and precontinent children older than 12 months were not included in this trial, so these conclusions do not apply to those groups. The intervention period lasted only five minutes, but other published studies suggest that this amount of time is adequate for voiding to occur.6,7 Although this study used soaking fluid stored at 2.8°C, the ideal storage temperature is unknown.
CHALLENGES TO IMPLEMENTATION
AAP doesn’t endorse clean-catch urine samples
The Quick-Wee method is simple and easy to implement, and requires no specialized training or equipment. AAP guidelines do not endorse the use of clean-catch voided urine for culture, which may be a barrier to changing urine collection practices in some settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[3]: 166, 168-169).
A fussy 6-month-old infant is brought to the emergency department (ED) with a rectal temperature of 101.5°F. She is consolable,
A febrile infant in a family practice office or ED is a familiar clinical situation that may require an invasive diagnostic workup. Up to 7% of infants ages 2 to 24 months with fever of unknown origin may have a UTI.2 Collecting a urine sample from pre–toilet-trained children can be time consuming. In fact, in one RCT, obtaining a clean-catch urine sample in this age group took more than an hour, on average.3 But more convenient methods of urine collection, such as placing a cotton ball in the diaper or using a perineal collection bag, have contamination rates of up to 63%.4
In its guidelines for evaluating possible UTI in a febrile child younger than age 2, the American Academy of Pediatrics (AAP) recommends obtaining a sample for urinalysis “through the most convenient means.”5 If urinalysis is positive, only urine obtained by catheterization or suprapubic aspiration should be cultured. Guidelines from the National Institute for Health and Care Excellence in the United Kingdom are similar, but allow for culture of clean-catch urine samples.6
A recent prospective cohort study examined a noninvasive alternating lumbar-bladder tapping method to stimulate voiding in infants ages 6 months or younger.7 Within five minutes, 49% of the infants provided a clean-catch sample, with contamination rates similar to those of samples obtained using invasive methods.7 Younger infants were more likely to void within the time allotted. Another trial of bladder tapping conducted in hospitalized infants younger than 30 days old showed similar results.8 There are, however, no previously reported randomized trials demonstrating the efficacy of a noninvasive urine collection technique in the outpatient setting.
Use of invasive collection methods requires skilled personnel and may cause significant discomfort for patients (and parents). Noninvasive methods, such as bag urine collection, have unacceptable contamination rates. In addition, waiting to catch a potentially cleaner urine sample is time consuming, so better strategies to collect urine from infants are needed. This RCT is the first to examine the efficacy of a unique stimulation technique to obtain a clean-catch urine sample from infants ages 1 to 12 months.
STUDY SUMMARY
Noninvasive stimulation triggers faster samples
A nonblinded, single-center RCT conducted in Australia compared two methods for obtaining a clean-catch urine sample within five minutes: the Quick-Wee method (suprapubic stimulation with gauze soaked in cold fluid) or usual care (waiting for spontaneous voiding with no stimulation).1 A total of 354 infants (ages 1-12 mo) who required urine sample collection were randomized in a 1:1 ratio; allocation was concealed. Infants with anatomic or neurologic abnormalities and those needing immediate antibiotic therapy were excluded.
The most common reasons for obtaining the urine sample were fever of unknown origin and “unsettled baby,” followed by poor feeding and suspected UTI. The primary outcome was voiding within five minutes; secondary outcomes included time to void, whether urine was successfully caught, contamination rate, and parent/clinician satisfaction.
Study personnel removed the diaper, then cleaned the genitals of all patients with room temperature sterile water. A caregiver or clinician was ready and waiting to catch urine when the patient voided. In the Quick-Wee group, a clinician rubbed the patient’s suprapubic area in a circular fashion with gauze soaked in refrigerated saline (2.8°C). At five minutes, clinicians recorded the voiding status and decided how to proceed.
Using intention-to-treat analysis, 31% of the patients in the Quick-Wee group voided within five minutes, compared with 12% of the usual-care patients. Similarly, 30% of patients in the Quick-Wee group provided a successful clean-catch sample within five minutes, compared with 9% in the usual-care group (number needed to treat, 4.7).
Contamination rates were no different between the Quick-Wee and usual-care samples. Both parents and clinicians were more satisfied with the Quick-Wee method than with usual care (median score of 2 vs 3 on a 5-point Likert scale, in which 1 is “most satisfied”). There was no difference when results were adjusted for age or sex. No adverse events occurred.
Continue to: WHAT'S NEW
WHAT’S NEW
Method could reduce need for invasive sampling
A simple suprapubic stimulation technique increased the number of infants who provided a clean-catch voided urine sample within five minutes—a clinically relevant and satisfying outcome. In appropriate patients, use of the Quick-Wee method to obtain a clean-catch voided sample for initial urinalysis, rather than attempting methods with known high contamination rates, may potentially reduce the need for invasive sampling using catheterization or suprapubic aspiration.
CAVEATS
Complete age range & ideal storage temperature are unknown
Neonates and precontinent children older than 12 months were not included in this trial, so these conclusions do not apply to those groups. The intervention period lasted only five minutes, but other published studies suggest that this amount of time is adequate for voiding to occur.6,7 Although this study used soaking fluid stored at 2.8°C, the ideal storage temperature is unknown.
CHALLENGES TO IMPLEMENTATION
AAP doesn’t endorse clean-catch urine samples
The Quick-Wee method is simple and easy to implement, and requires no specialized training or equipment. AAP guidelines do not endorse the use of clean-catch voided urine for culture, which may be a barrier to changing urine collection practices in some settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2018;67[3]: 166, 168-169).
1. Kaufman J, Fitzpatrick P, Tosif S, et al. Faster clean catch urine collection (Quick-Wee method) from infants: randomised controlled trial. BMJ. 2017;357:j1341.
2. Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008;27(4):302-308.
3. Davies P, Greenwood R, Benger J. Randomised trial of a vibrating bladder stimulator—the time to pee study. Arch Dis Child. 2008;93(5):423-424.
4. Al-Orifi F, McGillivray D, Tange S, Kramer MS. Urine culture from bag specimens in young children: are the risks too high? J Pediatr. 2000;137(2):221-226.
5. Roberts KB, Downs SM, Finnell SM, et al; Subcommittee on Urinary Tract Infection. Reaffirmation of AAP clinical practice guideline: the diagnosis and management of the initial urinary tract infection in febrile infants and young children 2-24 months of age. Pediatrics. 2016;138(6): e20163026.
6. National Institute for Health and Care Excellence. Urinary tract infection in under 16s: diagnosis and management [clinical guideline CG54]. www.nice.org.uk/guidance/cg54/chapter/1-guidance. Accessed March 1, 2018.
7. Labrosse M, Levy A, Autmizguine J, Gravel J. Evaluation of a new strategy for clean-catch urine in infants. Pediatrics. 2016;138(3):e20160573.
8. Herreros Fernández ML, González Merino N, Tagarro García A, et al. A new technique for fast and safe collection of urine in newborns. Arch Dis Child. 2013;98(1):27-29.
1. Kaufman J, Fitzpatrick P, Tosif S, et al. Faster clean catch urine collection (Quick-Wee method) from infants: randomised controlled trial. BMJ. 2017;357:j1341.
2. Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: a meta-analysis. Pediatr Infect Dis J. 2008;27(4):302-308.
3. Davies P, Greenwood R, Benger J. Randomised trial of a vibrating bladder stimulator—the time to pee study. Arch Dis Child. 2008;93(5):423-424.
4. Al-Orifi F, McGillivray D, Tange S, Kramer MS. Urine culture from bag specimens in young children: are the risks too high? J Pediatr. 2000;137(2):221-226.
5. Roberts KB, Downs SM, Finnell SM, et al; Subcommittee on Urinary Tract Infection. Reaffirmation of AAP clinical practice guideline: the diagnosis and management of the initial urinary tract infection in febrile infants and young children 2-24 months of age. Pediatrics. 2016;138(6): e20163026.
6. National Institute for Health and Care Excellence. Urinary tract infection in under 16s: diagnosis and management [clinical guideline CG54]. www.nice.org.uk/guidance/cg54/chapter/1-guidance. Accessed March 1, 2018.
7. Labrosse M, Levy A, Autmizguine J, Gravel J. Evaluation of a new strategy for clean-catch urine in infants. Pediatrics. 2016;138(3):e20160573.
8. Herreros Fernández ML, González Merino N, Tagarro García A, et al. A new technique for fast and safe collection of urine in newborns. Arch Dis Child. 2013;98(1):27-29.
Treating migraines: It’s different for kids
ILLUSTRATIVE CASE
A 15-year-old girl presents to your clinic with poorly controlled chronic migraines that are preventing her from attending school 3 to 4 days per month. As part of her treatment regimen, you are considering migraine prevention strategies.
Should you prescribe amitriptyline or topiramate for preventive migraine therapy?
Migraine headaches are the most common reason for headache presentation in pediatric neurology outpatient clinics, affecting 5% to 10% of the pediatric population worldwide.2 Current recommendations regarding prophylactic migraine therapy in childhood are based on consensus opinions.3,4 And the US Food and Drug Administration (FDA) has not approved any medications for preventing migraines in children younger than 12 years of age. However, surveys of pediatric headache specialists suggest that amitriptyline and topiramate are among the most commonly prescribed medications for childhood migraine prophylaxis.3,4
There is low-quality evidence from individual randomized controlled trials (RCTs) about the effectiveness of topiramate. A meta-analysis by El-Chammas and colleagues included 3 RCTs comparing topiramate to placebo for the prevention of episodic migraines (migraine headaches that occur <15 times/month) in a combined total of 283 children younger than 18 years of age.5 Topiramate demonstrated a nonclinically significant, but statistically significant, reduction of less than one headache per month (-0.71; 95% confidence interval [CI], -1.19 to -0.24). This is based on moderate quality evidence due to a high placebo response rate and study durations of only 12 weeks.5 The FDA has approved topiramate for migraine prevention in children ages 12 to 17 years.6
Adult guidelines. The findings described above are consistent with the most recent adult guidelines from the American Academy of Neurology and the American Headache Society.7 In a joint publication from 2012, these societies recommended both topiramate and amitriptyline for the prevention of migraines in adults based on high-quality (Level A evidence) and medium-quality evidence (Level B), respectively.7
[polldaddy:9973304]
STUDY SUMMARY
Both drugs are no better than placebo in children
A multicenter, double-blind RCT by Powers and colleagues compared the effectiveness of amitriptyline, topiramate, and placebo in the prevention of pediatric migraines.1 Target dosing for amitriptyline and topiramate was set at 1 mg/kg/d and 2 mg/kg/d, respectively. Titration toward these doses occurred over an 8-week period based on reported adverse effects. Patients then continued their maximum tolerated dose for an additional 16 weeks.
Patients were predominantly white (70%), female (68%), and 8 to 17 years of age. They had at least 4 headache days over a prospective 28-day pre-treatment period and a Pediatric Migraine Disability Assessment Scale (PedMIDAS) score of 11 to 139 (mild to moderate disability=11-50; severe disability >50).1,8 The primary endpoint consisted of at least a 50% relative reduction (RR) in the number of headache days over the 28-day pre-therapy (baseline) period compared with the final 28 days of the trial.1
The authors of the study included 328 patients in the primary efficacy analysis and randomly assigned them in a 2:2:1 ratio to receive either amitriptyline (132 patients), topiramate (130 patients), or placebo (66 patients), respectively. After 24 weeks of therapy, there was no significant difference between the amitriptyline, topiramate, and placebo groups in the primary endpoint (52% amitriptyline, 55% topiramate, 61% placebo; adjusted odds ratio [OR]=0.71; 98% CI, 0.34-1.48; P=.26 between amitriptyline and placebo; OR=0.81; 98% CI, 0.39-1.68; P=.48 between topiramate and placebo; OR=0.88; 98% CI, 0.49-1.59; P=.49 between amitriptyline and topiramate).
There was also no difference in the secondary outcomes of absolute reduction in headache days and headache-related disability as determined by PedMIDAS. The study was stopped early for futility. Compared with placebo, amitriptyline significantly increased fatigue (number needed to harm [NNH]=8) and dry mouth (NNH=9) and was associated with 3 serious adverse events of altered mood. Compared with placebo, topiramate significantly increased paresthesia (NNH=4) and weight loss (NNH=13) and was associated with one serious adverse event—a suicide attempt.1
WHAT’S NEW?
Higher-level evidence demonstrates lack of efficacy
This RCT provides new, higher-level evidence that demonstrates the lack of efficacy of amitriptyline and topiramate in the prevention of pediatric migraines. It also highlights the risk of increased adverse events with topiramate and amitriptyline.
Two of the 3 topiramate trials used in the older meta-analysis by El-Chammas and colleagues5 and this new RCT1 were included in an updated meta-analysis by Le and colleagues (total participants 465) published in 2017.2 This newer meta-analysis found no statistical benefit associated with the use of topiramate over placebo. It demonstrated a nonsignificant decrease in the number of patients with at least a 50% relative reduction in headache frequency (risk ratio = 1.26; 95% CI, 0.94-1.67) and in the overall number of headache days (mean difference = -0.77; 95% CI, -2.31 to 0.76) in patients younger than 18 years of age.2 Both meta-analyses, however, showed an increase in the rate of adverse events in patients using topiramate vs placebo.2,5
CAVEATS
Is there a gender predominance?
El-Chammas and colleagues5 describe male pediatric patients as being the predominant pediatric gender with migraines. However, they do not quote an incidence rate or cite the reference for this statement. No other reference to gender predominance was noted in the literature. The current study,1 in addition to the total population of the meta-analysis by Le and colleagues,2 included women as the predominant patient population. Hopefully, future studies will help to delineate if there is a gender predominance and, if so, whether the current treatment data apply to both genders.
CHALLENGES TO IMPLEMENTATION
None to speak of
There are no barriers to implementing this recommendation immediately in all primary care settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Powers SW, Coffey CS, Chamberlin LA, et al; for the CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
2. Le K, Yu D, Wang J, et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.
3. Lewis D, Ashwal S, Hershey A, et al. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology. 2004;63:2215-2224.
4. Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurology. 2010;9:190-204.
5. El-Chammas K, Keyes J, Thompson N, et al. Pharmacologic treatment of pediatric headaches: a meta-analysis. JAMA Pediatr. 2013;167:250-258.
6. Qudexy XR. Highlights of prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205122s003s005lbl.pdf. Accessed March 15, 2018.
7. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
8. Hershey AD, Powers SW, Vockell AL, et al. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034-2039.
ILLUSTRATIVE CASE
A 15-year-old girl presents to your clinic with poorly controlled chronic migraines that are preventing her from attending school 3 to 4 days per month. As part of her treatment regimen, you are considering migraine prevention strategies.
Should you prescribe amitriptyline or topiramate for preventive migraine therapy?
Migraine headaches are the most common reason for headache presentation in pediatric neurology outpatient clinics, affecting 5% to 10% of the pediatric population worldwide.2 Current recommendations regarding prophylactic migraine therapy in childhood are based on consensus opinions.3,4 And the US Food and Drug Administration (FDA) has not approved any medications for preventing migraines in children younger than 12 years of age. However, surveys of pediatric headache specialists suggest that amitriptyline and topiramate are among the most commonly prescribed medications for childhood migraine prophylaxis.3,4
There is low-quality evidence from individual randomized controlled trials (RCTs) about the effectiveness of topiramate. A meta-analysis by El-Chammas and colleagues included 3 RCTs comparing topiramate to placebo for the prevention of episodic migraines (migraine headaches that occur <15 times/month) in a combined total of 283 children younger than 18 years of age.5 Topiramate demonstrated a nonclinically significant, but statistically significant, reduction of less than one headache per month (-0.71; 95% confidence interval [CI], -1.19 to -0.24). This is based on moderate quality evidence due to a high placebo response rate and study durations of only 12 weeks.5 The FDA has approved topiramate for migraine prevention in children ages 12 to 17 years.6
Adult guidelines. The findings described above are consistent with the most recent adult guidelines from the American Academy of Neurology and the American Headache Society.7 In a joint publication from 2012, these societies recommended both topiramate and amitriptyline for the prevention of migraines in adults based on high-quality (Level A evidence) and medium-quality evidence (Level B), respectively.7
[polldaddy:9973304]
STUDY SUMMARY
Both drugs are no better than placebo in children
A multicenter, double-blind RCT by Powers and colleagues compared the effectiveness of amitriptyline, topiramate, and placebo in the prevention of pediatric migraines.1 Target dosing for amitriptyline and topiramate was set at 1 mg/kg/d and 2 mg/kg/d, respectively. Titration toward these doses occurred over an 8-week period based on reported adverse effects. Patients then continued their maximum tolerated dose for an additional 16 weeks.
Patients were predominantly white (70%), female (68%), and 8 to 17 years of age. They had at least 4 headache days over a prospective 28-day pre-treatment period and a Pediatric Migraine Disability Assessment Scale (PedMIDAS) score of 11 to 139 (mild to moderate disability=11-50; severe disability >50).1,8 The primary endpoint consisted of at least a 50% relative reduction (RR) in the number of headache days over the 28-day pre-therapy (baseline) period compared with the final 28 days of the trial.1
The authors of the study included 328 patients in the primary efficacy analysis and randomly assigned them in a 2:2:1 ratio to receive either amitriptyline (132 patients), topiramate (130 patients), or placebo (66 patients), respectively. After 24 weeks of therapy, there was no significant difference between the amitriptyline, topiramate, and placebo groups in the primary endpoint (52% amitriptyline, 55% topiramate, 61% placebo; adjusted odds ratio [OR]=0.71; 98% CI, 0.34-1.48; P=.26 between amitriptyline and placebo; OR=0.81; 98% CI, 0.39-1.68; P=.48 between topiramate and placebo; OR=0.88; 98% CI, 0.49-1.59; P=.49 between amitriptyline and topiramate).
There was also no difference in the secondary outcomes of absolute reduction in headache days and headache-related disability as determined by PedMIDAS. The study was stopped early for futility. Compared with placebo, amitriptyline significantly increased fatigue (number needed to harm [NNH]=8) and dry mouth (NNH=9) and was associated with 3 serious adverse events of altered mood. Compared with placebo, topiramate significantly increased paresthesia (NNH=4) and weight loss (NNH=13) and was associated with one serious adverse event—a suicide attempt.1
WHAT’S NEW?
Higher-level evidence demonstrates lack of efficacy
This RCT provides new, higher-level evidence that demonstrates the lack of efficacy of amitriptyline and topiramate in the prevention of pediatric migraines. It also highlights the risk of increased adverse events with topiramate and amitriptyline.
Two of the 3 topiramate trials used in the older meta-analysis by El-Chammas and colleagues5 and this new RCT1 were included in an updated meta-analysis by Le and colleagues (total participants 465) published in 2017.2 This newer meta-analysis found no statistical benefit associated with the use of topiramate over placebo. It demonstrated a nonsignificant decrease in the number of patients with at least a 50% relative reduction in headache frequency (risk ratio = 1.26; 95% CI, 0.94-1.67) and in the overall number of headache days (mean difference = -0.77; 95% CI, -2.31 to 0.76) in patients younger than 18 years of age.2 Both meta-analyses, however, showed an increase in the rate of adverse events in patients using topiramate vs placebo.2,5
CAVEATS
Is there a gender predominance?
El-Chammas and colleagues5 describe male pediatric patients as being the predominant pediatric gender with migraines. However, they do not quote an incidence rate or cite the reference for this statement. No other reference to gender predominance was noted in the literature. The current study,1 in addition to the total population of the meta-analysis by Le and colleagues,2 included women as the predominant patient population. Hopefully, future studies will help to delineate if there is a gender predominance and, if so, whether the current treatment data apply to both genders.
CHALLENGES TO IMPLEMENTATION
None to speak of
There are no barriers to implementing this recommendation immediately in all primary care settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 15-year-old girl presents to your clinic with poorly controlled chronic migraines that are preventing her from attending school 3 to 4 days per month. As part of her treatment regimen, you are considering migraine prevention strategies.
Should you prescribe amitriptyline or topiramate for preventive migraine therapy?
Migraine headaches are the most common reason for headache presentation in pediatric neurology outpatient clinics, affecting 5% to 10% of the pediatric population worldwide.2 Current recommendations regarding prophylactic migraine therapy in childhood are based on consensus opinions.3,4 And the US Food and Drug Administration (FDA) has not approved any medications for preventing migraines in children younger than 12 years of age. However, surveys of pediatric headache specialists suggest that amitriptyline and topiramate are among the most commonly prescribed medications for childhood migraine prophylaxis.3,4
There is low-quality evidence from individual randomized controlled trials (RCTs) about the effectiveness of topiramate. A meta-analysis by El-Chammas and colleagues included 3 RCTs comparing topiramate to placebo for the prevention of episodic migraines (migraine headaches that occur <15 times/month) in a combined total of 283 children younger than 18 years of age.5 Topiramate demonstrated a nonclinically significant, but statistically significant, reduction of less than one headache per month (-0.71; 95% confidence interval [CI], -1.19 to -0.24). This is based on moderate quality evidence due to a high placebo response rate and study durations of only 12 weeks.5 The FDA has approved topiramate for migraine prevention in children ages 12 to 17 years.6
Adult guidelines. The findings described above are consistent with the most recent adult guidelines from the American Academy of Neurology and the American Headache Society.7 In a joint publication from 2012, these societies recommended both topiramate and amitriptyline for the prevention of migraines in adults based on high-quality (Level A evidence) and medium-quality evidence (Level B), respectively.7
[polldaddy:9973304]
STUDY SUMMARY
Both drugs are no better than placebo in children
A multicenter, double-blind RCT by Powers and colleagues compared the effectiveness of amitriptyline, topiramate, and placebo in the prevention of pediatric migraines.1 Target dosing for amitriptyline and topiramate was set at 1 mg/kg/d and 2 mg/kg/d, respectively. Titration toward these doses occurred over an 8-week period based on reported adverse effects. Patients then continued their maximum tolerated dose for an additional 16 weeks.
Patients were predominantly white (70%), female (68%), and 8 to 17 years of age. They had at least 4 headache days over a prospective 28-day pre-treatment period and a Pediatric Migraine Disability Assessment Scale (PedMIDAS) score of 11 to 139 (mild to moderate disability=11-50; severe disability >50).1,8 The primary endpoint consisted of at least a 50% relative reduction (RR) in the number of headache days over the 28-day pre-therapy (baseline) period compared with the final 28 days of the trial.1
The authors of the study included 328 patients in the primary efficacy analysis and randomly assigned them in a 2:2:1 ratio to receive either amitriptyline (132 patients), topiramate (130 patients), or placebo (66 patients), respectively. After 24 weeks of therapy, there was no significant difference between the amitriptyline, topiramate, and placebo groups in the primary endpoint (52% amitriptyline, 55% topiramate, 61% placebo; adjusted odds ratio [OR]=0.71; 98% CI, 0.34-1.48; P=.26 between amitriptyline and placebo; OR=0.81; 98% CI, 0.39-1.68; P=.48 between topiramate and placebo; OR=0.88; 98% CI, 0.49-1.59; P=.49 between amitriptyline and topiramate).
There was also no difference in the secondary outcomes of absolute reduction in headache days and headache-related disability as determined by PedMIDAS. The study was stopped early for futility. Compared with placebo, amitriptyline significantly increased fatigue (number needed to harm [NNH]=8) and dry mouth (NNH=9) and was associated with 3 serious adverse events of altered mood. Compared with placebo, topiramate significantly increased paresthesia (NNH=4) and weight loss (NNH=13) and was associated with one serious adverse event—a suicide attempt.1
WHAT’S NEW?
Higher-level evidence demonstrates lack of efficacy
This RCT provides new, higher-level evidence that demonstrates the lack of efficacy of amitriptyline and topiramate in the prevention of pediatric migraines. It also highlights the risk of increased adverse events with topiramate and amitriptyline.
Two of the 3 topiramate trials used in the older meta-analysis by El-Chammas and colleagues5 and this new RCT1 were included in an updated meta-analysis by Le and colleagues (total participants 465) published in 2017.2 This newer meta-analysis found no statistical benefit associated with the use of topiramate over placebo. It demonstrated a nonsignificant decrease in the number of patients with at least a 50% relative reduction in headache frequency (risk ratio = 1.26; 95% CI, 0.94-1.67) and in the overall number of headache days (mean difference = -0.77; 95% CI, -2.31 to 0.76) in patients younger than 18 years of age.2 Both meta-analyses, however, showed an increase in the rate of adverse events in patients using topiramate vs placebo.2,5
CAVEATS
Is there a gender predominance?
El-Chammas and colleagues5 describe male pediatric patients as being the predominant pediatric gender with migraines. However, they do not quote an incidence rate or cite the reference for this statement. No other reference to gender predominance was noted in the literature. The current study,1 in addition to the total population of the meta-analysis by Le and colleagues,2 included women as the predominant patient population. Hopefully, future studies will help to delineate if there is a gender predominance and, if so, whether the current treatment data apply to both genders.
CHALLENGES TO IMPLEMENTATION
None to speak of
There are no barriers to implementing this recommendation immediately in all primary care settings.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Powers SW, Coffey CS, Chamberlin LA, et al; for the CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
2. Le K, Yu D, Wang J, et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.
3. Lewis D, Ashwal S, Hershey A, et al. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology. 2004;63:2215-2224.
4. Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurology. 2010;9:190-204.
5. El-Chammas K, Keyes J, Thompson N, et al. Pharmacologic treatment of pediatric headaches: a meta-analysis. JAMA Pediatr. 2013;167:250-258.
6. Qudexy XR. Highlights of prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205122s003s005lbl.pdf. Accessed March 15, 2018.
7. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
8. Hershey AD, Powers SW, Vockell AL, et al. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034-2039.
1. Powers SW, Coffey CS, Chamberlin LA, et al; for the CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
2. Le K, Yu D, Wang J, et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.
3. Lewis D, Ashwal S, Hershey A, et al. Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology Quality Standards Subcommittee and the Practice Committee of the Child Neurology Society. Neurology. 2004;63:2215-2224.
4. Hershey AD. Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurology. 2010;9:190-204.
5. El-Chammas K, Keyes J, Thompson N, et al. Pharmacologic treatment of pediatric headaches: a meta-analysis. JAMA Pediatr. 2013;167:250-258.
6. Qudexy XR. Highlights of prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205122s003s005lbl.pdf. Accessed March 15, 2018.
7. Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1337-1345.
8. Hershey AD, Powers SW, Vockell AL, et al. PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034-2039.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Do not prescribe amitriptyline or topiramate as preventive therapy for migraine in children; both drugs are no better than placebo for this population and are associated with increased rates of adverse events.1,2
STRENGTH OF RECOMMENDATION
A: Based on a single double-blind randomized control trial (RCT) and supported by a meta-analysis of 4 RCTs.
1. Powers SW, Coffey CS, Chamberlin LA, et al; for the CHAMP Investigators. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115-124.
2. Le K, Yu D, Wang J, et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain. 2017;18:69.