User login
Do Probiotics Reduce C diff Risk in Hospitalized Patients?
A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
Several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI, although not all of them followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines or focused specifically on hospitalized patients, who are at increased risk.4-6 The largest high-quality randomized controlled trial (RCT) on the use of probiotics to prevent CDI, the PLACIDE trial, found no difference in CDI incidence between inpatients (ages 65 and older) who did and those who did not receive probiotics in addition to their oral or parenteral antibiotics; however, this trial had a lower incidence of CDI than was assumed in the power calculations.7 Guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America do not include a recommendation for the use of probiotics in CDI prevention.8,9
Given the conflicting and poor-quality evidence and lack of recommendations, an additional systematic review and meta-analysis was performed, following PRISMA guidelines and focusing on studies conducted only in hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in this population
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 hospitalized adults taking antibiotics. All patients were 18 or older (mean age, 68-69) and received antibiotics orally, intravenously, or via both routes, for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotic strains used were Lactobacillus, Saccharomyces, Bifidobacterium, or Streptococcus (alone or in combination). Probiotic doses ranged from 4 billion to 900 billion colony-forming U/d and were started from 1 to 7 days after the first antibiotic dose. Duration of probiotic use was either fixed at 14 to 21 days or varied based on the duration of antibiotics (extending 3-14 d after the last antibiotic dose).
Control groups received matching placebo in all but 2 trials; those 2 used usual care of no probiotics as the control. Exclusion criteria included pregnancy, immunocompromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
[polldaddy:10452484]
Continue to: The risk for CDI...
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%), with no heterogeneity when the data from all 19 studies were pooled (relative risk [RR], 0.42). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43.
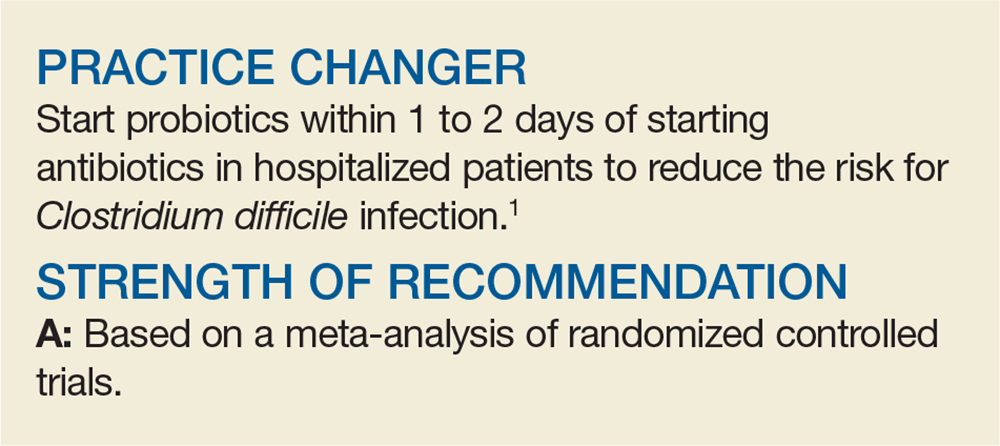
The researchers examined the NNT at varying incidence rates. If the CDI incidence was 1.2%, the NNT to prevent 1 case of CDI was 144; if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR, 0.32), but not if they were started at 3 to 7 days (RR, 0.70). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%).
WHAT’S NEW
Added benefit if probiotics taken sooner
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Limited applicability, lack of recommendations
Findings from this meta-analysis do not apply to patients who are pregnant; who have an immunocompromising condition, a prosthetic heart valve, or a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis); or who require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
CHALLENGES TO IMPLEMENTATION
Limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adults is their availability on local hospital formularies. Probiotics are not technically a medication; t
Continue to: ACKNOWLEDGMENT
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[6]:351-352,354).
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152(8):1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(24):2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158(12):706-707.
7. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249-1257.
8. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478-498.
9. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.

A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
Several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI, although not all of them followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines or focused specifically on hospitalized patients, who are at increased risk.4-6 The largest high-quality randomized controlled trial (RCT) on the use of probiotics to prevent CDI, the PLACIDE trial, found no difference in CDI incidence between inpatients (ages 65 and older) who did and those who did not receive probiotics in addition to their oral or parenteral antibiotics; however, this trial had a lower incidence of CDI than was assumed in the power calculations.7 Guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America do not include a recommendation for the use of probiotics in CDI prevention.8,9
Given the conflicting and poor-quality evidence and lack of recommendations, an additional systematic review and meta-analysis was performed, following PRISMA guidelines and focusing on studies conducted only in hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in this population
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 hospitalized adults taking antibiotics. All patients were 18 or older (mean age, 68-69) and received antibiotics orally, intravenously, or via both routes, for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotic strains used were Lactobacillus, Saccharomyces, Bifidobacterium, or Streptococcus (alone or in combination). Probiotic doses ranged from 4 billion to 900 billion colony-forming U/d and were started from 1 to 7 days after the first antibiotic dose. Duration of probiotic use was either fixed at 14 to 21 days or varied based on the duration of antibiotics (extending 3-14 d after the last antibiotic dose).
Control groups received matching placebo in all but 2 trials; those 2 used usual care of no probiotics as the control. Exclusion criteria included pregnancy, immunocompromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
[polldaddy:10452484]
Continue to: The risk for CDI...
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%), with no heterogeneity when the data from all 19 studies were pooled (relative risk [RR], 0.42). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43.
The researchers examined the NNT at varying incidence rates. If the CDI incidence was 1.2%, the NNT to prevent 1 case of CDI was 144; if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR, 0.32), but not if they were started at 3 to 7 days (RR, 0.70). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%).
WHAT’S NEW
Added benefit if probiotics taken sooner
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Limited applicability, lack of recommendations
Findings from this meta-analysis do not apply to patients who are pregnant; who have an immunocompromising condition, a prosthetic heart valve, or a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis); or who require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
CHALLENGES TO IMPLEMENTATION
Limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adults is their availability on local hospital formularies. Probiotics are not technically a medication; t
Continue to: ACKNOWLEDGMENT
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[6]:351-352,354).

A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
Several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI, although not all of them followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines or focused specifically on hospitalized patients, who are at increased risk.4-6 The largest high-quality randomized controlled trial (RCT) on the use of probiotics to prevent CDI, the PLACIDE trial, found no difference in CDI incidence between inpatients (ages 65 and older) who did and those who did not receive probiotics in addition to their oral or parenteral antibiotics; however, this trial had a lower incidence of CDI than was assumed in the power calculations.7 Guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America do not include a recommendation for the use of probiotics in CDI prevention.8,9
Given the conflicting and poor-quality evidence and lack of recommendations, an additional systematic review and meta-analysis was performed, following PRISMA guidelines and focusing on studies conducted only in hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in this population
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 hospitalized adults taking antibiotics. All patients were 18 or older (mean age, 68-69) and received antibiotics orally, intravenously, or via both routes, for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotic strains used were Lactobacillus, Saccharomyces, Bifidobacterium, or Streptococcus (alone or in combination). Probiotic doses ranged from 4 billion to 900 billion colony-forming U/d and were started from 1 to 7 days after the first antibiotic dose. Duration of probiotic use was either fixed at 14 to 21 days or varied based on the duration of antibiotics (extending 3-14 d after the last antibiotic dose).
Control groups received matching placebo in all but 2 trials; those 2 used usual care of no probiotics as the control. Exclusion criteria included pregnancy, immunocompromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
[polldaddy:10452484]
Continue to: The risk for CDI...
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%), with no heterogeneity when the data from all 19 studies were pooled (relative risk [RR], 0.42). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43.
The researchers examined the NNT at varying incidence rates. If the CDI incidence was 1.2%, the NNT to prevent 1 case of CDI was 144; if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR, 0.32), but not if they were started at 3 to 7 days (RR, 0.70). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%).
WHAT’S NEW
Added benefit if probiotics taken sooner
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Limited applicability, lack of recommendations
Findings from this meta-analysis do not apply to patients who are pregnant; who have an immunocompromising condition, a prosthetic heart valve, or a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis); or who require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
CHALLENGES TO IMPLEMENTATION
Limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adults is their availability on local hospital formularies. Probiotics are not technically a medication; t
Continue to: ACKNOWLEDGMENT
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[6]:351-352,354).
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152(8):1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(24):2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158(12):706-707.
7. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249-1257.
8. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478-498.
9. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152(8):1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(24):2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L, et al. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158(12):706-707.
7. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382(9900):1249-1257.
8. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108(4):478-498.
9. Cohen SH, Gerding DN, Johnson S, et al; Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31(5):431-455.
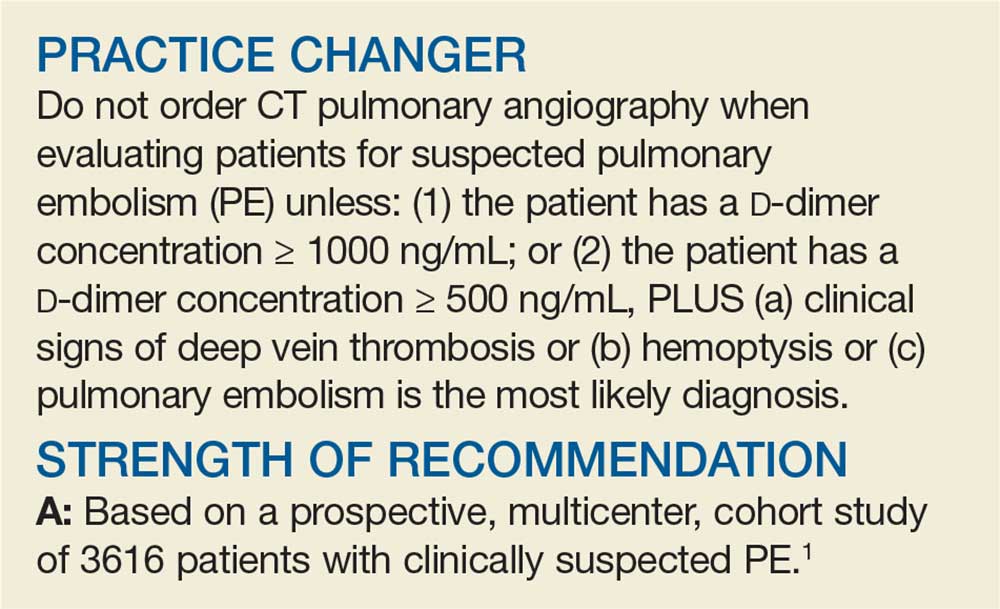
A Better Approach to the Diagnosis of PE
Penny E, a 48-year-old woman with a history of asthma, presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. PE is not your most likely diagnosis, but it is included in the differential, so you order a D
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2/1000 individuals and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
A diagnostic algorithm that includes the Wells criteria and a
Further, it is common for a
Three items of the original Wells criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 A total of 151 patients met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA). Investigators managed the remaining 3465 study patients according to the YEARS algorithm, which calls for obtaining a
PE was considered excluded if a patient had a
[polldaddy:10428150]
Continue to: Of the 1743 patients...
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a D
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%), with 6 patients (0.20%) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43%, which is similar to the 0.34% reported in a previous meta-analysis of the Wells rule algorithm.13 Overall, fatal PE occurred in 0.3% of patients in the YEARS cohort vs 0.6% in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells algorithm, for an absolute difference of 13% and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference) when compared with using the Wells rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria do not consider an age-adjusted
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[5]:286-287,295).
1. van der Hulle T, Cheung WY, Kooij S, et al; YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(suppl 4):S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al; Prometheus Study Group. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and d -dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al; EMDEPU Study Group. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted d -dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including d -dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating d -dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal d -dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
Penny E, a 48-year-old woman with a history of asthma, presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. PE is not your most likely diagnosis, but it is included in the differential, so you order a D
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2/1000 individuals and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
A diagnostic algorithm that includes the Wells criteria and a
Further, it is common for a
Three items of the original Wells criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 A total of 151 patients met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA). Investigators managed the remaining 3465 study patients according to the YEARS algorithm, which calls for obtaining a
PE was considered excluded if a patient had a
[polldaddy:10428150]
Continue to: Of the 1743 patients...
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a D
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%), with 6 patients (0.20%) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43%, which is similar to the 0.34% reported in a previous meta-analysis of the Wells rule algorithm.13 Overall, fatal PE occurred in 0.3% of patients in the YEARS cohort vs 0.6% in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells algorithm, for an absolute difference of 13% and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference) when compared with using the Wells rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria do not consider an age-adjusted
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[5]:286-287,295).
Penny E, a 48-year-old woman with a history of asthma, presents with wheezing and respiratory distress. There are no clinical signs of deep vein thrombosis or hemoptysis. PE is not your most likely diagnosis, but it is included in the differential, so you order a D
PE is the third most common type of cardiovascular disease after coronary artery disease and stroke, with an estimated incidence in the United States of 1-2/1000 individuals and a 30-day mortality rate between 10% and 30%.2 Improved adherence to a clinical decision support system has been shown to significantly decrease the number of diagnostic tests performed and the number of diagnostic failures.3
A diagnostic algorithm that includes the Wells criteria and a
Further, it is common for a
Three items of the original Wells criteria—clinical signs of deep vein thrombosis, hemoptysis, and whether PE is the most likely diagnosis—are the most predictive for PE.8 The development of a more efficient algorithm based on these 3 items that uses differential D
STUDY SUMMARY
Simplified algorithm diagnoses PE with fewer CTPAs
The YEARS study was a prospective cohort study conducted in 12 hospitals in the Netherlands that included 3616 patients with clinically suspected PE.1 A total of 151 patients met exclusion criteria (life expectancy < 3 months, ongoing anticoagulation treatment, pregnancy, and contraindication to CTPA). Investigators managed the remaining 3465 study patients according to the YEARS algorithm, which calls for obtaining a
PE was considered excluded if a patient had a
[polldaddy:10428150]
Continue to: Of the 1743 patients...
Of the 1743 patients who had none of the 3 YEARS items, 1320 had a D
Eighteen of the 2964 patients who had PE ruled out by the YEARS algorithm at baseline were found to have symptomatic VTE during the follow-up period (0.61%), with 6 patients (0.20%) sustaining a fatal PE. The 3-month incidence of VTE in patients who did not have CTPA was 0.43%, which is similar to the 0.34% reported in a previous meta-analysis of the Wells rule algorithm.13 Overall, fatal PE occurred in 0.3% of patients in the YEARS cohort vs 0.6% in a meta-analysis of studies using standard algorithms.14
Using an intention-to-diagnose analysis, 1611 (46%) patients did not have a CTPA indicated by the YEARS algorithm compared with 1174 (34%) using the Wells algorithm, for an absolute difference of 13% and estimated cost savings of $283,176 in this sample. The per-protocol analysis also had a decrease of CTPA examinations in favor of the YEARS algorithm, ruling out 1651 (48%) patients—a decrease of 14% and an estimated savings of $309,096.
WHAT’S NEW
High-level evidence says 14% fewer CTPAs
The YEARS study provides a high level of evidence that a new, simple diagnostic algorithm can reliably and efficiently exclude PE and decrease the need for CTPA by 14% (absolute difference) when compared with using the Wells rule and fixed
CAVEATS
No adjusting D -dimer for age
The YEARS criteria do not consider an age-adjusted
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
None to speak of
We see no challenges to the implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[5]:286-287,295).
1. van der Hulle T, Cheung WY, Kooij S, et al; YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(suppl 4):S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al; Prometheus Study Group. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and d -dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al; EMDEPU Study Group. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted d -dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including d -dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating d -dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal d -dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
1. van der Hulle T, Cheung WY, Kooij S, et al; YEARS study group. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017;390:289-297.
2. Beckman MG, Hooper WC, Critchley SE, et al. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(suppl 4):S495-S501.
3. Douma RA, Mos ICM, Erkens PMG, et al; Prometheus Study Group. Performance of 4 clinical decision rules in the diagnostic management of acute pulmonary embolism. Ann Intern Med. 2011;154:709-718.
4. van Es N, van der Hulle T, van Es J, et al. Wells Rule and d -dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016;165:253-261.
5. Roy P-M, Meyer G, Vielle B, et al; EMDEPU Study Group. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Ann Intern Med. 2006;144:157-164.
6. Newnham M, Stone H, Summerfield R, et al. Performance of algorithms and pre-test probability scores is often overlooked in the diagnosis of pulmonary embolism. BMJ. 2013;346:f1557.
7. Righini M, Van Es J, Den Exter PL, et al. Age-adjusted d -dimer cutoff levels to rule out pulmonary embolism. JAMA. 2014;311:1117-1124.
8. van Es J, Beenen LFM, Douma RA, et al. A simple decision rule including d -dimer to reduce the need for computed tomography scanning in patients with suspected pulmonary embolism. J Thromb Haemost. 2015;13:1428-1435.
9. Kooiman J, Klok FA, Mos ICM, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010;8:409-411.
10. Sarma A, Heilbrun ME, Conner KE, et al. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142:750-760.
11. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071-2077.
12. Verma K, Legnani C, Palareti G. Cost-minimization analysis of venous thromboembolism diagnosis: comparison of standalone imaging with a strategy incorporating d -dimer for exclusion of venous thromboembolism. Res Pract Thromb Haemost. 2017;1:57-61.
13. Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the Wells rule and normal d -dimer concentration: a meta-analysis. Thromb Res. 2010;125:e123-e127.
14. Mos ICM, Klok FA, Kroft LJM, et al. Safety of ruling out acute pulmonary embolism by normal computed tomography pulmonary angiography in patients with an indication for computed tomography: systematic review and meta-analysis. J Thromb Haemost. 2009;7:1491-1498.
Can sleep apnea be accurately diagnosed at home?
ILLUSTRATIVE CASE
A 50-year-old overweight male with a history of hypertension presents to your office for a yearly physical. On review of symptoms, he notes feeling constantly tired, despite reported good sleep hygiene practices. He scores 11 on the Epworth Sleepiness Scale, and his wife complains about his snoring. You have a high suspicion of obstructive sleep apnea. What is your next step?
Obstructive sleep apnea (OSA) is quite common, affecting at least 2% to 4% of the general adult population.2 The gold standard for OSA diagnosis has been laboratory polysomnography (PSG) to measure the apnea-hypopnea index (AHI), which is the average number of apneas and hypopneas per hour of sleep, and the respiratory event index (REI), which is the average number of apneas, hypopneas, and respiratory effort-related arousals per hour of sleep. A minimum of 5 on the AHI or REI, along with clinical symptoms, is required for diagnosis.
Many adults go undiagnosed and untreated, however, due to barriers to diagnosis including the inconvenience of laboratory PSG.3 Sleep laboratories often have a significant wait time for evaluation, and sleeping in an unfamiliar place can be inconvenient or intolerable for some patients, making diagnosis difficult despite high clinical suspicion. Untreated sleep apnea is associated with an increased risk of hypertension, coronary artery disease, congestive heart failure, stroke, atrial fibrillation, and type 2 diabetes.4
Home sleep studies are an alternative for patients with a high risk of OSA without comorbid sleep conditions, heart failure, or chronic obstructive pulmonary disease (COPD). This study investigated the long-term effectiveness of diagnosis by home respiratory polygraphy (HRP) vs laboratory PSG in patients with an intermediate to high clinical suspicion for OSA.
STUDY SUMMARY
Home Dx is noninferior to lab Dx in all aspects studied
This multicenter, noninferiority randomized controlled trial and cost analysis study conducted in Spain randomized 430 adults referred to pulmonology for suspected OSA to receive either in-lab PSG or HRP. Patients received treatment with continuous positive airway pressure (CPAP) if their REI was ≥ 5 for HRP or their AHI was ≥ 5 for PSG with significant clinical symptoms, which is consistent with the Spanish Sleep Network guidelines.5 All patients in both arms received sleep hygiene instruction, nutrition education, and single-session auto-CPAP titration, and were evaluated at 1 and 3 months to assess for compliance. At 6 months, all patients were evaluated with PSG.
HRP was found to be non-inferior to PSG based on Epworth Sleepiness Scale (ESS) scores evaluated at baseline and at 6-month follow-up (HRP mean = -4.2 points; 95% confidence interval [CI], -4.8 to -3.6 and PSG mean -4.9; 95% CI, -5.4 to -4.3; P = .14). Both groups had similar secondary outcomes. Quality-of-life as measured by the 30-point Functional Outcomes of Sleep Questionnaire improved by an average of 6.7 (standard deviation [SD] = 16.7) in the HRP group vs 6.5 (SD = 18.1) in the PSG group (P = .92). Systolic and diastolic blood pressure improved significantly in both groups without any statistically significant difference between the groups. HRP was also found to be more cost-effective than PSG with a savings equivalent to more than half the cost of PSG, or about $450 per study (depending on the exchange rate).
WHAT’S NEW
HRP offers advantages for low-risk patients
In the majority of patients, OSA can be diagnosed at home with outcomes similar to those for lab diagnosis, decreased cost, and decreased time from suspected diagnosis to treatment. HRP is acceptable for patients with a high probability of OSA without significant comorbidities if monitoring includes at least airflow, respiratory effort, and blood oxygenation.6
Continue to: CAVEATS
CAVEATS
Recommendations are somewhat ambiguous
This study, as well as current guidelines, recommend home sleep studies for patients with a high clinical suspicion or high pre-test probability of OSA and who lack comorbid conditions that could affect sleep. The comorbid conditions are well identified: COPD, heart failure hypoventilation syndromes, insomnia, hypersomnia, parasomnia, periodic limb movement disorder, narcolepsy, and chronic opioid use.6 However, what constitutes “a high clinical suspicion” or “high pre-test probability” was not well defined in this study.
Several clinical screening tools are available and include the ESS, Berlin Questionnaire, and STOP-BANG Scoring System (Snoring, Tiredness, Observed apnea, Pressure [systemic hypertension], Body mass index > 35, Age > 50 years, Neck circumference > 16 inches, male Gender). An ESS score ≥ 10 warrants further evaluation, but is not very sensitive. Two or more positive categories on the Berlin Questionnaire indicates a high risk of OSA with a sensitivity of 76%, 77%, and 77% for mild, moderate, and severe OSA, respectively.7 A score of ≥ 3 on the STOP-BANG Scoring System has been validated and has a sensitivity of 83.6%, 92.9%, and 100% for an AHI > 5, > 15, and > 30, respectively.8
Home sleep studies should not be used to screen the general population.
CHALLENGES TO IMPLEMENTATION
Recommendations may present a challenge but insurance should not
The American Academy of Sleep Medicine recommends that portable monitoring must record airflow, respiratory effort, and blood oxygenation, and the device must be able to display the raw data to be interpreted by a board-certified sleep medicine physician according to current published standards.6 Implementation would require appropriate selection of a home monitoring device, consultation with a sleep medicine specialist, and significant patient education to ensure interpretable results.
Insurance should not be a barrier to implementation as the Centers for Medicare and Medicaid Services accept home sleep apnea testing results for CPAP prescriptions.9 However, variability currently exists regarding the extent to which private insurers provide coverage for home sleep apnea testing.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Corral J, Sánchez-Quiroga MÁ, Carmona-Bernal C, et al. Conventional polysomnography is not necessary for the management of most patients with suspected obstructive sleep apnea. Noninferiority, randomized controlled trial. Am J Respir Crit Care Med. 2017;196:1181-1190.
2. Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
3. Colten H, Abboud F, Block G, et al. Sleep disorders and sleep deprivation: an unmet public health problem. 2006. Washington, DC: National Academy of Sciences.
4. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136-143.
5. Lloberes P, Durán-Cantolla J, Martinez-Garcia MA, et al. Diagnosis and treatment of sleep apnea-hypopnea syndrome. Spanish Society of Pulmonology and Thoracic Surgery. Arch Bronconeumol. 2011;47:143-156.
6. Rosen IM, Kirsch DB, Chervin RD; American Academy of Sleep Medicine Board of Directors. Clinical use of a home sleep apnea test: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2017;13:1205-1207.
7. Chiu HY, Chen PY, Chuang, LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP and Epworth Sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57-70.
8. Chung, F, Yegneswaran B, Lio P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812-821.
9. Centers for Medicare and Medicaid Services. Decision Memo for Continuous Positive Airway Pressure (CPAP) Therapy for Obstructive Sleep Apnea (OSA) (CAG-00093R2). March 13, 2008. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=204. Accessed September 6, 2019.
ILLUSTRATIVE CASE
A 50-year-old overweight male with a history of hypertension presents to your office for a yearly physical. On review of symptoms, he notes feeling constantly tired, despite reported good sleep hygiene practices. He scores 11 on the Epworth Sleepiness Scale, and his wife complains about his snoring. You have a high suspicion of obstructive sleep apnea. What is your next step?
Obstructive sleep apnea (OSA) is quite common, affecting at least 2% to 4% of the general adult population.2 The gold standard for OSA diagnosis has been laboratory polysomnography (PSG) to measure the apnea-hypopnea index (AHI), which is the average number of apneas and hypopneas per hour of sleep, and the respiratory event index (REI), which is the average number of apneas, hypopneas, and respiratory effort-related arousals per hour of sleep. A minimum of 5 on the AHI or REI, along with clinical symptoms, is required for diagnosis.
Many adults go undiagnosed and untreated, however, due to barriers to diagnosis including the inconvenience of laboratory PSG.3 Sleep laboratories often have a significant wait time for evaluation, and sleeping in an unfamiliar place can be inconvenient or intolerable for some patients, making diagnosis difficult despite high clinical suspicion. Untreated sleep apnea is associated with an increased risk of hypertension, coronary artery disease, congestive heart failure, stroke, atrial fibrillation, and type 2 diabetes.4
Home sleep studies are an alternative for patients with a high risk of OSA without comorbid sleep conditions, heart failure, or chronic obstructive pulmonary disease (COPD). This study investigated the long-term effectiveness of diagnosis by home respiratory polygraphy (HRP) vs laboratory PSG in patients with an intermediate to high clinical suspicion for OSA.
STUDY SUMMARY
Home Dx is noninferior to lab Dx in all aspects studied
This multicenter, noninferiority randomized controlled trial and cost analysis study conducted in Spain randomized 430 adults referred to pulmonology for suspected OSA to receive either in-lab PSG or HRP. Patients received treatment with continuous positive airway pressure (CPAP) if their REI was ≥ 5 for HRP or their AHI was ≥ 5 for PSG with significant clinical symptoms, which is consistent with the Spanish Sleep Network guidelines.5 All patients in both arms received sleep hygiene instruction, nutrition education, and single-session auto-CPAP titration, and were evaluated at 1 and 3 months to assess for compliance. At 6 months, all patients were evaluated with PSG.
HRP was found to be non-inferior to PSG based on Epworth Sleepiness Scale (ESS) scores evaluated at baseline and at 6-month follow-up (HRP mean = -4.2 points; 95% confidence interval [CI], -4.8 to -3.6 and PSG mean -4.9; 95% CI, -5.4 to -4.3; P = .14). Both groups had similar secondary outcomes. Quality-of-life as measured by the 30-point Functional Outcomes of Sleep Questionnaire improved by an average of 6.7 (standard deviation [SD] = 16.7) in the HRP group vs 6.5 (SD = 18.1) in the PSG group (P = .92). Systolic and diastolic blood pressure improved significantly in both groups without any statistically significant difference between the groups. HRP was also found to be more cost-effective than PSG with a savings equivalent to more than half the cost of PSG, or about $450 per study (depending on the exchange rate).
WHAT’S NEW
HRP offers advantages for low-risk patients
In the majority of patients, OSA can be diagnosed at home with outcomes similar to those for lab diagnosis, decreased cost, and decreased time from suspected diagnosis to treatment. HRP is acceptable for patients with a high probability of OSA without significant comorbidities if monitoring includes at least airflow, respiratory effort, and blood oxygenation.6
Continue to: CAVEATS
CAVEATS
Recommendations are somewhat ambiguous
This study, as well as current guidelines, recommend home sleep studies for patients with a high clinical suspicion or high pre-test probability of OSA and who lack comorbid conditions that could affect sleep. The comorbid conditions are well identified: COPD, heart failure hypoventilation syndromes, insomnia, hypersomnia, parasomnia, periodic limb movement disorder, narcolepsy, and chronic opioid use.6 However, what constitutes “a high clinical suspicion” or “high pre-test probability” was not well defined in this study.
Several clinical screening tools are available and include the ESS, Berlin Questionnaire, and STOP-BANG Scoring System (Snoring, Tiredness, Observed apnea, Pressure [systemic hypertension], Body mass index > 35, Age > 50 years, Neck circumference > 16 inches, male Gender). An ESS score ≥ 10 warrants further evaluation, but is not very sensitive. Two or more positive categories on the Berlin Questionnaire indicates a high risk of OSA with a sensitivity of 76%, 77%, and 77% for mild, moderate, and severe OSA, respectively.7 A score of ≥ 3 on the STOP-BANG Scoring System has been validated and has a sensitivity of 83.6%, 92.9%, and 100% for an AHI > 5, > 15, and > 30, respectively.8
Home sleep studies should not be used to screen the general population.
CHALLENGES TO IMPLEMENTATION
Recommendations may present a challenge but insurance should not
The American Academy of Sleep Medicine recommends that portable monitoring must record airflow, respiratory effort, and blood oxygenation, and the device must be able to display the raw data to be interpreted by a board-certified sleep medicine physician according to current published standards.6 Implementation would require appropriate selection of a home monitoring device, consultation with a sleep medicine specialist, and significant patient education to ensure interpretable results.
Insurance should not be a barrier to implementation as the Centers for Medicare and Medicaid Services accept home sleep apnea testing results for CPAP prescriptions.9 However, variability currently exists regarding the extent to which private insurers provide coverage for home sleep apnea testing.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 50-year-old overweight male with a history of hypertension presents to your office for a yearly physical. On review of symptoms, he notes feeling constantly tired, despite reported good sleep hygiene practices. He scores 11 on the Epworth Sleepiness Scale, and his wife complains about his snoring. You have a high suspicion of obstructive sleep apnea. What is your next step?
Obstructive sleep apnea (OSA) is quite common, affecting at least 2% to 4% of the general adult population.2 The gold standard for OSA diagnosis has been laboratory polysomnography (PSG) to measure the apnea-hypopnea index (AHI), which is the average number of apneas and hypopneas per hour of sleep, and the respiratory event index (REI), which is the average number of apneas, hypopneas, and respiratory effort-related arousals per hour of sleep. A minimum of 5 on the AHI or REI, along with clinical symptoms, is required for diagnosis.
Many adults go undiagnosed and untreated, however, due to barriers to diagnosis including the inconvenience of laboratory PSG.3 Sleep laboratories often have a significant wait time for evaluation, and sleeping in an unfamiliar place can be inconvenient or intolerable for some patients, making diagnosis difficult despite high clinical suspicion. Untreated sleep apnea is associated with an increased risk of hypertension, coronary artery disease, congestive heart failure, stroke, atrial fibrillation, and type 2 diabetes.4
Home sleep studies are an alternative for patients with a high risk of OSA without comorbid sleep conditions, heart failure, or chronic obstructive pulmonary disease (COPD). This study investigated the long-term effectiveness of diagnosis by home respiratory polygraphy (HRP) vs laboratory PSG in patients with an intermediate to high clinical suspicion for OSA.
STUDY SUMMARY
Home Dx is noninferior to lab Dx in all aspects studied
This multicenter, noninferiority randomized controlled trial and cost analysis study conducted in Spain randomized 430 adults referred to pulmonology for suspected OSA to receive either in-lab PSG or HRP. Patients received treatment with continuous positive airway pressure (CPAP) if their REI was ≥ 5 for HRP or their AHI was ≥ 5 for PSG with significant clinical symptoms, which is consistent with the Spanish Sleep Network guidelines.5 All patients in both arms received sleep hygiene instruction, nutrition education, and single-session auto-CPAP titration, and were evaluated at 1 and 3 months to assess for compliance. At 6 months, all patients were evaluated with PSG.
HRP was found to be non-inferior to PSG based on Epworth Sleepiness Scale (ESS) scores evaluated at baseline and at 6-month follow-up (HRP mean = -4.2 points; 95% confidence interval [CI], -4.8 to -3.6 and PSG mean -4.9; 95% CI, -5.4 to -4.3; P = .14). Both groups had similar secondary outcomes. Quality-of-life as measured by the 30-point Functional Outcomes of Sleep Questionnaire improved by an average of 6.7 (standard deviation [SD] = 16.7) in the HRP group vs 6.5 (SD = 18.1) in the PSG group (P = .92). Systolic and diastolic blood pressure improved significantly in both groups without any statistically significant difference between the groups. HRP was also found to be more cost-effective than PSG with a savings equivalent to more than half the cost of PSG, or about $450 per study (depending on the exchange rate).
WHAT’S NEW
HRP offers advantages for low-risk patients
In the majority of patients, OSA can be diagnosed at home with outcomes similar to those for lab diagnosis, decreased cost, and decreased time from suspected diagnosis to treatment. HRP is acceptable for patients with a high probability of OSA without significant comorbidities if monitoring includes at least airflow, respiratory effort, and blood oxygenation.6
Continue to: CAVEATS
CAVEATS
Recommendations are somewhat ambiguous
This study, as well as current guidelines, recommend home sleep studies for patients with a high clinical suspicion or high pre-test probability of OSA and who lack comorbid conditions that could affect sleep. The comorbid conditions are well identified: COPD, heart failure hypoventilation syndromes, insomnia, hypersomnia, parasomnia, periodic limb movement disorder, narcolepsy, and chronic opioid use.6 However, what constitutes “a high clinical suspicion” or “high pre-test probability” was not well defined in this study.
Several clinical screening tools are available and include the ESS, Berlin Questionnaire, and STOP-BANG Scoring System (Snoring, Tiredness, Observed apnea, Pressure [systemic hypertension], Body mass index > 35, Age > 50 years, Neck circumference > 16 inches, male Gender). An ESS score ≥ 10 warrants further evaluation, but is not very sensitive. Two or more positive categories on the Berlin Questionnaire indicates a high risk of OSA with a sensitivity of 76%, 77%, and 77% for mild, moderate, and severe OSA, respectively.7 A score of ≥ 3 on the STOP-BANG Scoring System has been validated and has a sensitivity of 83.6%, 92.9%, and 100% for an AHI > 5, > 15, and > 30, respectively.8
Home sleep studies should not be used to screen the general population.
CHALLENGES TO IMPLEMENTATION
Recommendations may present a challenge but insurance should not
The American Academy of Sleep Medicine recommends that portable monitoring must record airflow, respiratory effort, and blood oxygenation, and the device must be able to display the raw data to be interpreted by a board-certified sleep medicine physician according to current published standards.6 Implementation would require appropriate selection of a home monitoring device, consultation with a sleep medicine specialist, and significant patient education to ensure interpretable results.
Insurance should not be a barrier to implementation as the Centers for Medicare and Medicaid Services accept home sleep apnea testing results for CPAP prescriptions.9 However, variability currently exists regarding the extent to which private insurers provide coverage for home sleep apnea testing.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Corral J, Sánchez-Quiroga MÁ, Carmona-Bernal C, et al. Conventional polysomnography is not necessary for the management of most patients with suspected obstructive sleep apnea. Noninferiority, randomized controlled trial. Am J Respir Crit Care Med. 2017;196:1181-1190.
2. Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
3. Colten H, Abboud F, Block G, et al. Sleep disorders and sleep deprivation: an unmet public health problem. 2006. Washington, DC: National Academy of Sciences.
4. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136-143.
5. Lloberes P, Durán-Cantolla J, Martinez-Garcia MA, et al. Diagnosis and treatment of sleep apnea-hypopnea syndrome. Spanish Society of Pulmonology and Thoracic Surgery. Arch Bronconeumol. 2011;47:143-156.
6. Rosen IM, Kirsch DB, Chervin RD; American Academy of Sleep Medicine Board of Directors. Clinical use of a home sleep apnea test: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2017;13:1205-1207.
7. Chiu HY, Chen PY, Chuang, LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP and Epworth Sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57-70.
8. Chung, F, Yegneswaran B, Lio P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812-821.
9. Centers for Medicare and Medicaid Services. Decision Memo for Continuous Positive Airway Pressure (CPAP) Therapy for Obstructive Sleep Apnea (OSA) (CAG-00093R2). March 13, 2008. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=204. Accessed September 6, 2019.
1. Corral J, Sánchez-Quiroga MÁ, Carmona-Bernal C, et al. Conventional polysomnography is not necessary for the management of most patients with suspected obstructive sleep apnea. Noninferiority, randomized controlled trial. Am J Respir Crit Care Med. 2017;196:1181-1190.
2. Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
3. Colten H, Abboud F, Block G, et al. Sleep disorders and sleep deprivation: an unmet public health problem. 2006. Washington, DC: National Academy of Sciences.
4. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136-143.
5. Lloberes P, Durán-Cantolla J, Martinez-Garcia MA, et al. Diagnosis and treatment of sleep apnea-hypopnea syndrome. Spanish Society of Pulmonology and Thoracic Surgery. Arch Bronconeumol. 2011;47:143-156.
6. Rosen IM, Kirsch DB, Chervin RD; American Academy of Sleep Medicine Board of Directors. Clinical use of a home sleep apnea test: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2017;13:1205-1207.
7. Chiu HY, Chen PY, Chuang, LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP and Epworth Sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57-70.
8. Chung, F, Yegneswaran B, Lio P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812-821.
9. Centers for Medicare and Medicaid Services. Decision Memo for Continuous Positive Airway Pressure (CPAP) Therapy for Obstructive Sleep Apnea (OSA) (CAG-00093R2). March 13, 2008. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=204. Accessed September 6, 2019.
PRACTICE CHANGER
Consider ordering home respiratory polygraphy vs laboratory sleep studies for patients suspected of having obstructive sleep apnea.1
Corral J, Sánchez-Quiroga MÁ, Carmona-Bernal C, et al. Conventional polysomnography is not necessary for the management of most patients with suspected obstructive sleep apnea. Noninferiority, randomized controlled trial. Am J Respir Crit Care Med. 2017;196:1181-1190.
STRENGTH OF RECOMMENDATION
B: Based on a multicenter, noninferiority randomized controlled trial and cost analysis study.
Can Vitamin D Prevent Acute Respiratory Infections?
Ms. M, a generally healthy 55-year-old woman, was diagnosed recently with severe vitamin D deficiency (serum 25-hydroxyvitamin D [25(OH)D] level of 8 ng/mL). She presents with her second episode of acute viral bronchitis in the past 6 months. She has no history of significant smoking or exposure or history of asthma and does not take respiratory medications. Standard treatment for her level of vitamin D deficiency is 50,000 IU/wk in bolus dosing—but is that your best option for the patient?
ARTIs include nonspecific upper respiratory illnesses, otitis media, sinusitis (~70% viral), pharyngitis, acute bronchitis (also ~70% viral), influenza, respiratory syncytial virus, and pneumonia.1,2 In the United States, ARTIs strain the health care system and are the most common reason for ambulatory care visits, accounting for almost 120 million (about 10% of all) visits per year.3 In addition, ARTIs account for almost 50% of antibiotic prescriptions for adults and almost 75% of antibiotic prescriptions for children—many of which are unnecessary.2,4
While patient and parent education, antibiotic stewardship programs, and demand management may reduce inappropriate antibiotic use and the overall burden of ARTIs on the health care system, prevention of infections is a powerful tool within the overall approach to managing ARTIs.
STUDY SUMMARY
Vitamin D is protective in smaller doses
This 2017 systematic review and meta-analysis of 25 trials (N = 10,933) evaluated vitamin D supplementation for the prevention of ARTIs in the primary care setting. Individual participant data were reevaluated to reduce risk for bias. The Cochrane risk-for-bias tool was used to address threats to validity.
The study included institutional review board–approved, randomized, double-blind, placebo-controlled trials of vitamin D3 or D2 supplementation of any duration and in any language. The incidence of ARTI was a prespecified efficacy outcome. Duration of the included randomized controlled trials (RCTs) ranged from 7 weeks to 1.5 years.
Outcomes. The primary outcome was an incidence of at least 1 ARTI. Secondary outcomes included incidence of upper and lower ARTIs; incidence of adverse reactions to vitamin D; incidence of emergency department visits or hospital admission or both for ARTI; use of antimicrobials for ARTI; absence from work or school due to ARTI; and mortality (ARTI-related and all-cause).
Findings. Daily or weekly vitamin D supplementation (in doses ranging from < 20 to ≥ 50 µg/d) reduced the risk for ARTI (adjusted odds ratio [AOR], 0.88; number needed to treat [NNT], 33). In subgroup analysis, daily or weekly vitamin D was protective (AOR, 0.81), but bolus dosing (≥ 30,000 IU) was not (AOR, 0.97).
In 2-step analysis, patients benefited if they had baseline circulating 25(OH)D concentrations < 10 ng/mL (AOR, 0.30; NNT, 4); had baseline circulating 25(OH)D levels of 10 to 28 ng/mL (AOR, 0.75; NNT, 15); were ages 1.1 to 15.9 (AOR, 0.59); were ages 16 to 65 (AOR, 0.79); or had a BMI < 25 (AOR, 0.82).
Higher D levels are a different story. Vitamin D supplementation in people with circulating levels of 25(OH)D ≥ 30 ng/mL did not appear to provide benefit (AOR, 0.96). Supplementation in this population did not influence any of the secondary outcomes, including risk for all-cause serious adverse events (AOR, 0.98).
WHAT’S NEW
A more accurate snapshot
Previous studies of vitamin D and respiratory tract infections were mostly observational in nature. Those that were RCTs used variable doses of vitamin D, had variable baseline 25(OH)D levels, and employed various methods to monitor ARTI symptoms/incidence.5-8 This is the first systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials with supplementation using vitamin D3 or D2 that used individual participant-level data, which gives a more accurate estimate of outcomes when compared with traditional meta-analyses.
CAVEATS
Only the most deficient benefit?
Vitamin D supplementation was safe and protected against ARTIs overall, but the greatest effect was noted in those who were most severely vitamin D deficient (those with circulating 25(OH)D levels < 10 ng/mL [NNT, 4] and those with circulating 25(OH)D levels 10-28 ng/mL [NNT, 15]). There was no demonstrable effect once circulating 25(OH)D levels reached 30 ng/mL.
CHALLENGES TO IMPLEMENTATION
Breaking tradition
The study found that both daily and weekly doses of vitamin D were effective in reducing the incidence of ARTIs. However, the doses studied were much lower than those commonly used (10,000 to 50,000 IU bolus), which were ineffective in reducing ARTIs in this meta-analysis. Changing from bolus dosing may prove challenging, a
In addition, the authors of the study suggest that one way to provide this level of vitamin D is through food fortification. But this method is often complicated by emotional and/or political issues that could thwart implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[4]:230-231).
1. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
2. Renati S, Linder JA. Necessity of office visits for acute respiratory infections in primary care. Fam Pract. 2016,33:312-317.
3. CDC National Center for Health Statistics. National Health Care Surveys. www.cdc.gov/nchs/dhcs.htm. Accessed September 5, 2019.
4. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758-766.
5. Rees JR, Hendricks K, Barry EL, et al. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin Infect Dis. 2013;57:1384-1392.
6. Murdoch DR, Slow S, Chambers ST, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308:1333-1339.
7. Laaksi I, Ruohola J-P, Mattila V, et al. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blinded trial among young Finnish men. Infect Dis. 2010;202:809-814.
8. Bergman P, Norlin A-C, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2:e001663.
Ms. M, a generally healthy 55-year-old woman, was diagnosed recently with severe vitamin D deficiency (serum 25-hydroxyvitamin D [25(OH)D] level of 8 ng/mL). She presents with her second episode of acute viral bronchitis in the past 6 months. She has no history of significant smoking or exposure or history of asthma and does not take respiratory medications. Standard treatment for her level of vitamin D deficiency is 50,000 IU/wk in bolus dosing—but is that your best option for the patient?
ARTIs include nonspecific upper respiratory illnesses, otitis media, sinusitis (~70% viral), pharyngitis, acute bronchitis (also ~70% viral), influenza, respiratory syncytial virus, and pneumonia.1,2 In the United States, ARTIs strain the health care system and are the most common reason for ambulatory care visits, accounting for almost 120 million (about 10% of all) visits per year.3 In addition, ARTIs account for almost 50% of antibiotic prescriptions for adults and almost 75% of antibiotic prescriptions for children—many of which are unnecessary.2,4
While patient and parent education, antibiotic stewardship programs, and demand management may reduce inappropriate antibiotic use and the overall burden of ARTIs on the health care system, prevention of infections is a powerful tool within the overall approach to managing ARTIs.
STUDY SUMMARY
Vitamin D is protective in smaller doses
This 2017 systematic review and meta-analysis of 25 trials (N = 10,933) evaluated vitamin D supplementation for the prevention of ARTIs in the primary care setting. Individual participant data were reevaluated to reduce risk for bias. The Cochrane risk-for-bias tool was used to address threats to validity.
The study included institutional review board–approved, randomized, double-blind, placebo-controlled trials of vitamin D3 or D2 supplementation of any duration and in any language. The incidence of ARTI was a prespecified efficacy outcome. Duration of the included randomized controlled trials (RCTs) ranged from 7 weeks to 1.5 years.
Outcomes. The primary outcome was an incidence of at least 1 ARTI. Secondary outcomes included incidence of upper and lower ARTIs; incidence of adverse reactions to vitamin D; incidence of emergency department visits or hospital admission or both for ARTI; use of antimicrobials for ARTI; absence from work or school due to ARTI; and mortality (ARTI-related and all-cause).
Findings. Daily or weekly vitamin D supplementation (in doses ranging from < 20 to ≥ 50 µg/d) reduced the risk for ARTI (adjusted odds ratio [AOR], 0.88; number needed to treat [NNT], 33). In subgroup analysis, daily or weekly vitamin D was protective (AOR, 0.81), but bolus dosing (≥ 30,000 IU) was not (AOR, 0.97).
In 2-step analysis, patients benefited if they had baseline circulating 25(OH)D concentrations < 10 ng/mL (AOR, 0.30; NNT, 4); had baseline circulating 25(OH)D levels of 10 to 28 ng/mL (AOR, 0.75; NNT, 15); were ages 1.1 to 15.9 (AOR, 0.59); were ages 16 to 65 (AOR, 0.79); or had a BMI < 25 (AOR, 0.82).
Higher D levels are a different story. Vitamin D supplementation in people with circulating levels of 25(OH)D ≥ 30 ng/mL did not appear to provide benefit (AOR, 0.96). Supplementation in this population did not influence any of the secondary outcomes, including risk for all-cause serious adverse events (AOR, 0.98).
WHAT’S NEW
A more accurate snapshot
Previous studies of vitamin D and respiratory tract infections were mostly observational in nature. Those that were RCTs used variable doses of vitamin D, had variable baseline 25(OH)D levels, and employed various methods to monitor ARTI symptoms/incidence.5-8 This is the first systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials with supplementation using vitamin D3 or D2 that used individual participant-level data, which gives a more accurate estimate of outcomes when compared with traditional meta-analyses.
CAVEATS
Only the most deficient benefit?
Vitamin D supplementation was safe and protected against ARTIs overall, but the greatest effect was noted in those who were most severely vitamin D deficient (those with circulating 25(OH)D levels < 10 ng/mL [NNT, 4] and those with circulating 25(OH)D levels 10-28 ng/mL [NNT, 15]). There was no demonstrable effect once circulating 25(OH)D levels reached 30 ng/mL.
CHALLENGES TO IMPLEMENTATION
Breaking tradition
The study found that both daily and weekly doses of vitamin D were effective in reducing the incidence of ARTIs. However, the doses studied were much lower than those commonly used (10,000 to 50,000 IU bolus), which were ineffective in reducing ARTIs in this meta-analysis. Changing from bolus dosing may prove challenging, a
In addition, the authors of the study suggest that one way to provide this level of vitamin D is through food fortification. But this method is often complicated by emotional and/or political issues that could thwart implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[4]:230-231).
Ms. M, a generally healthy 55-year-old woman, was diagnosed recently with severe vitamin D deficiency (serum 25-hydroxyvitamin D [25(OH)D] level of 8 ng/mL). She presents with her second episode of acute viral bronchitis in the past 6 months. She has no history of significant smoking or exposure or history of asthma and does not take respiratory medications. Standard treatment for her level of vitamin D deficiency is 50,000 IU/wk in bolus dosing—but is that your best option for the patient?
ARTIs include nonspecific upper respiratory illnesses, otitis media, sinusitis (~70% viral), pharyngitis, acute bronchitis (also ~70% viral), influenza, respiratory syncytial virus, and pneumonia.1,2 In the United States, ARTIs strain the health care system and are the most common reason for ambulatory care visits, accounting for almost 120 million (about 10% of all) visits per year.3 In addition, ARTIs account for almost 50% of antibiotic prescriptions for adults and almost 75% of antibiotic prescriptions for children—many of which are unnecessary.2,4
While patient and parent education, antibiotic stewardship programs, and demand management may reduce inappropriate antibiotic use and the overall burden of ARTIs on the health care system, prevention of infections is a powerful tool within the overall approach to managing ARTIs.
STUDY SUMMARY
Vitamin D is protective in smaller doses
This 2017 systematic review and meta-analysis of 25 trials (N = 10,933) evaluated vitamin D supplementation for the prevention of ARTIs in the primary care setting. Individual participant data were reevaluated to reduce risk for bias. The Cochrane risk-for-bias tool was used to address threats to validity.
The study included institutional review board–approved, randomized, double-blind, placebo-controlled trials of vitamin D3 or D2 supplementation of any duration and in any language. The incidence of ARTI was a prespecified efficacy outcome. Duration of the included randomized controlled trials (RCTs) ranged from 7 weeks to 1.5 years.
Outcomes. The primary outcome was an incidence of at least 1 ARTI. Secondary outcomes included incidence of upper and lower ARTIs; incidence of adverse reactions to vitamin D; incidence of emergency department visits or hospital admission or both for ARTI; use of antimicrobials for ARTI; absence from work or school due to ARTI; and mortality (ARTI-related and all-cause).
Findings. Daily or weekly vitamin D supplementation (in doses ranging from < 20 to ≥ 50 µg/d) reduced the risk for ARTI (adjusted odds ratio [AOR], 0.88; number needed to treat [NNT], 33). In subgroup analysis, daily or weekly vitamin D was protective (AOR, 0.81), but bolus dosing (≥ 30,000 IU) was not (AOR, 0.97).
In 2-step analysis, patients benefited if they had baseline circulating 25(OH)D concentrations < 10 ng/mL (AOR, 0.30; NNT, 4); had baseline circulating 25(OH)D levels of 10 to 28 ng/mL (AOR, 0.75; NNT, 15); were ages 1.1 to 15.9 (AOR, 0.59); were ages 16 to 65 (AOR, 0.79); or had a BMI < 25 (AOR, 0.82).
Higher D levels are a different story. Vitamin D supplementation in people with circulating levels of 25(OH)D ≥ 30 ng/mL did not appear to provide benefit (AOR, 0.96). Supplementation in this population did not influence any of the secondary outcomes, including risk for all-cause serious adverse events (AOR, 0.98).
WHAT’S NEW
A more accurate snapshot
Previous studies of vitamin D and respiratory tract infections were mostly observational in nature. Those that were RCTs used variable doses of vitamin D, had variable baseline 25(OH)D levels, and employed various methods to monitor ARTI symptoms/incidence.5-8 This is the first systematic review and meta-analysis of randomized, double-blind, placebo-controlled trials with supplementation using vitamin D3 or D2 that used individual participant-level data, which gives a more accurate estimate of outcomes when compared with traditional meta-analyses.
CAVEATS
Only the most deficient benefit?
Vitamin D supplementation was safe and protected against ARTIs overall, but the greatest effect was noted in those who were most severely vitamin D deficient (those with circulating 25(OH)D levels < 10 ng/mL [NNT, 4] and those with circulating 25(OH)D levels 10-28 ng/mL [NNT, 15]). There was no demonstrable effect once circulating 25(OH)D levels reached 30 ng/mL.
CHALLENGES TO IMPLEMENTATION
Breaking tradition
The study found that both daily and weekly doses of vitamin D were effective in reducing the incidence of ARTIs. However, the doses studied were much lower than those commonly used (10,000 to 50,000 IU bolus), which were ineffective in reducing ARTIs in this meta-analysis. Changing from bolus dosing may prove challenging, a
In addition, the authors of the study suggest that one way to provide this level of vitamin D is through food fortification. But this method is often complicated by emotional and/or political issues that could thwart implementation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[4]:230-231).
1. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
2. Renati S, Linder JA. Necessity of office visits for acute respiratory infections in primary care. Fam Pract. 2016,33:312-317.
3. CDC National Center for Health Statistics. National Health Care Surveys. www.cdc.gov/nchs/dhcs.htm. Accessed September 5, 2019.
4. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758-766.
5. Rees JR, Hendricks K, Barry EL, et al. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin Infect Dis. 2013;57:1384-1392.
6. Murdoch DR, Slow S, Chambers ST, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308:1333-1339.
7. Laaksi I, Ruohola J-P, Mattila V, et al. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blinded trial among young Finnish men. Infect Dis. 2010;202:809-814.
8. Bergman P, Norlin A-C, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2:e001663.
1. Martineau AR, Jolliffe DA, Hooper RL, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
2. Renati S, Linder JA. Necessity of office visits for acute respiratory infections in primary care. Fam Pract. 2016,33:312-317.
3. CDC National Center for Health Statistics. National Health Care Surveys. www.cdc.gov/nchs/dhcs.htm. Accessed September 5, 2019.
4. Grijalva CG, Nuorti JP, Griffin MR. Antibiotic prescription rates for acute respiratory tract infections in US ambulatory settings. JAMA. 2009;302:758-766.
5. Rees JR, Hendricks K, Barry EL, et al. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin Infect Dis. 2013;57:1384-1392.
6. Murdoch DR, Slow S, Chambers ST, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308:1333-1339.
7. Laaksi I, Ruohola J-P, Mattila V, et al. Vitamin D supplementation for the prevention of acute respiratory tract infection: a randomized, double-blinded trial among young Finnish men. Infect Dis. 2010;202:809-814.
8. Bergman P, Norlin A-C, Hansen S, et al. Vitamin D3 supplementation in patients with frequent respiratory tract infections: a randomised and double-blind intervention study. BMJ Open. 2012;2:e001663.
Antidepressant Tx for anxiety disorders: How long?
ILLUSTRATIVE CASE
A 42-year-old woman with generalized anxiety disorder and panic attacks has been treated with sertraline 100 mg/d for the past 8 months. She has also engaged in cognitive behavioral therapy (CBT) for 6 months. Her Generalized Anxiety Disorder-7 score has decreased from 19 prior to treatment to 5 at present. Now she would like to stop her antidepressant medication because she feels better. Would you recommend that she discontinue her medication at this point?
Anxiety disorders are common, often chronic, and can cause significant morbidity and impairment.2,3 First-line treatments for anxiety disorders include CBT and antidepressants, particularly selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors.4-6
There is limited evidence regarding duration of antidepressant therapy for anxiety disorders. Previous studies have shown a high risk of relapse after discontinuation of antidepressants.6 A review of current practice patterns regarding pharmacologic treatment of depression and anxiety indicates an uptick in longer term antidepressant use for up to 2 years.7 However, long-term studies to guide treatment decisions are lacking.
STUDY SUMMARY
Clear benefit of continuing treatment up to 1 year
This systematic review and meta-analysis evaluated studies that looked at relapse rates and time to relapse in patients treated for anxiety disorders.1 The authors used PubMed, Cochrane, and Embase to identify studies involving patients treated for a variety of disorders, including generalized anxiety disorder (GAD), posttraumatic stress disorder (PTSD), panic disorder (PD), obsessive-compulsive disorder (OCD), and social phobia. Eligible studies enrolled patients with anxiety disorders who had a positive response to an antidepressant and then randomized them in a double-blind fashion to either discontinuation of antidepressants and starting placebo (stopping group) or continuation of antidepressants (continuation group) for a duration of 8 to 52 weeks. The primary outcomes were relapse rate and time to relapse.
Twenty-eight studies met the inclusion criteria for the meta-analysis, with a total of 5233 patients (2625 patients in the antidepressant group and 2608 patients in the placebo group). A breakdown of the trials by indiication included OCD (7), PD (6), GAD (6), social phobia (5), and PTSD (4). The authors graded the overall risk of bias to be low but noted that attrition bias was present in most studies.
Results. Relapse was more likely in the stopping group (odds ratio [OR] = 3.11; 95% confidence interval [CI], 2.48-3.89; n = 28 studies). Heterogeneity for relapse rate was low (I2 = 8.07%). Subgroup analyses by type of antidepressant, mode of discontinuation, and exclusion of patient comorbidities yielded similar results. Relapse prevalence was 16.4% in the antidepressant group and 36.4% in the stopping group. Additionally, time to relapse was shorter when antidepressants were discontinued (hazard ratio [HR] = 3.63; 95% CI, 2.58-5.10; n = 11 studies). Again, the heterogeneity for relapse rate was low (I2 = 0%). The original publications did not consistently report medication tolerability or withdrawal symptoms, preventing analysis of these. Dropout rates were higher in the stopping group (OR = 1.31; 95% CI, 1.06-1.63; n = 27 studies).
WHAT’S NEW
No more guessing about how long to treat
Previously, there was limited evidence to guide decisions about the duration of antidepressant treatment for anxiety disorders. This study provides evidence that stopping antidepressant treatment before 1 year increases the risk of relapse.
Continue to: CAVEATS
CAVEATS
Potential bias … bias … and more bias
While the authors used standard and appropriate methodologies for this type of study, some significant threats to validity remained. All but 2 studies in the analysis were industry funded. Publication bias is another potential issue, even though the authors identified and included 6 unpublished studies, 4 of which had negative results.
Additionally, the authors graded 11 of 28 trials as having a high likelihood of selective reporting bias, meaning that important portions of the original studies’ results may not have been published. Most studies were at high risk for attrition bias, resulting in loss of information when patients dropped out of the study. While this happened more often in the stopping groups, it is still possible that there are unidentified harms or unexpected outcomes in the medication groups.
While PTSD and OCD are no longer considered anxiety disorders, subgroup analyses found no difference in relapse rates between these diagnoses and the others included in the studies. Finally, treatment duration longer than 52 weeks has not been studied, so the optimal treatment duration is unknown.
CHALLENGES TO IMPLEMENTATION
Patients may resist continuing treatment once symptoms abate
Some patients may want to discontinue antidepressant treatment if their anxiety symptoms improve prior to 1 year. It may be difficult to convince them that continuing treatment will prevent relapse of their condition. Providing patients with information about the increased relapse rate with stopping medication early (with an estimated number needed to treat of 5) may help patients make a more informed decision.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Batelaan NM, Bosman RC, Muntingh A, et al. Risk of relapse after antidepressant discontinuation in anxiety disorders, obsessive-compulsive disorder, and post-traumatic stress disorder: systematic review and meta-analysis of relapse prevention trials. BMJ. 2017;358:j3927. Erratum in: BMJ. 2017;358:j4461.
2. National Institute of Mental Health. Prevalence of any anxiety disorder among adults. https://www.nimh.nih.gov/health/statistics/any-anxiety-disorder.shtml#part_155094. Updated November 2017. Accessed July 11, 2019.
3. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184.
4. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77-84.
5. Kaczkurkin AN, Foa EB. Cognitive-behavioral therapy for anxiety disorders: an update on the empirical evidence. Dialogues Clin Neurosci. 2015;17:337-346.
6. Donovan MR, Glue P, Kolluri S, et al. Comparative efficacy of antidepressants in preventing relapse in anxiety disorders—a meta-analysis. J Affect Disord. 2010;123:9-16.
7. Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the U.S. National Health and Nutrition Examination Survey. J Clin Psychiatry. 2014;75:169-177.
ILLUSTRATIVE CASE
A 42-year-old woman with generalized anxiety disorder and panic attacks has been treated with sertraline 100 mg/d for the past 8 months. She has also engaged in cognitive behavioral therapy (CBT) for 6 months. Her Generalized Anxiety Disorder-7 score has decreased from 19 prior to treatment to 5 at present. Now she would like to stop her antidepressant medication because she feels better. Would you recommend that she discontinue her medication at this point?
Anxiety disorders are common, often chronic, and can cause significant morbidity and impairment.2,3 First-line treatments for anxiety disorders include CBT and antidepressants, particularly selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors.4-6
There is limited evidence regarding duration of antidepressant therapy for anxiety disorders. Previous studies have shown a high risk of relapse after discontinuation of antidepressants.6 A review of current practice patterns regarding pharmacologic treatment of depression and anxiety indicates an uptick in longer term antidepressant use for up to 2 years.7 However, long-term studies to guide treatment decisions are lacking.
STUDY SUMMARY
Clear benefit of continuing treatment up to 1 year
This systematic review and meta-analysis evaluated studies that looked at relapse rates and time to relapse in patients treated for anxiety disorders.1 The authors used PubMed, Cochrane, and Embase to identify studies involving patients treated for a variety of disorders, including generalized anxiety disorder (GAD), posttraumatic stress disorder (PTSD), panic disorder (PD), obsessive-compulsive disorder (OCD), and social phobia. Eligible studies enrolled patients with anxiety disorders who had a positive response to an antidepressant and then randomized them in a double-blind fashion to either discontinuation of antidepressants and starting placebo (stopping group) or continuation of antidepressants (continuation group) for a duration of 8 to 52 weeks. The primary outcomes were relapse rate and time to relapse.
Twenty-eight studies met the inclusion criteria for the meta-analysis, with a total of 5233 patients (2625 patients in the antidepressant group and 2608 patients in the placebo group). A breakdown of the trials by indiication included OCD (7), PD (6), GAD (6), social phobia (5), and PTSD (4). The authors graded the overall risk of bias to be low but noted that attrition bias was present in most studies.
Results. Relapse was more likely in the stopping group (odds ratio [OR] = 3.11; 95% confidence interval [CI], 2.48-3.89; n = 28 studies). Heterogeneity for relapse rate was low (I2 = 8.07%). Subgroup analyses by type of antidepressant, mode of discontinuation, and exclusion of patient comorbidities yielded similar results. Relapse prevalence was 16.4% in the antidepressant group and 36.4% in the stopping group. Additionally, time to relapse was shorter when antidepressants were discontinued (hazard ratio [HR] = 3.63; 95% CI, 2.58-5.10; n = 11 studies). Again, the heterogeneity for relapse rate was low (I2 = 0%). The original publications did not consistently report medication tolerability or withdrawal symptoms, preventing analysis of these. Dropout rates were higher in the stopping group (OR = 1.31; 95% CI, 1.06-1.63; n = 27 studies).
WHAT’S NEW
No more guessing about how long to treat
Previously, there was limited evidence to guide decisions about the duration of antidepressant treatment for anxiety disorders. This study provides evidence that stopping antidepressant treatment before 1 year increases the risk of relapse.
Continue to: CAVEATS
CAVEATS
Potential bias … bias … and more bias
While the authors used standard and appropriate methodologies for this type of study, some significant threats to validity remained. All but 2 studies in the analysis were industry funded. Publication bias is another potential issue, even though the authors identified and included 6 unpublished studies, 4 of which had negative results.
Additionally, the authors graded 11 of 28 trials as having a high likelihood of selective reporting bias, meaning that important portions of the original studies’ results may not have been published. Most studies were at high risk for attrition bias, resulting in loss of information when patients dropped out of the study. While this happened more often in the stopping groups, it is still possible that there are unidentified harms or unexpected outcomes in the medication groups.
While PTSD and OCD are no longer considered anxiety disorders, subgroup analyses found no difference in relapse rates between these diagnoses and the others included in the studies. Finally, treatment duration longer than 52 weeks has not been studied, so the optimal treatment duration is unknown.
CHALLENGES TO IMPLEMENTATION
Patients may resist continuing treatment once symptoms abate
Some patients may want to discontinue antidepressant treatment if their anxiety symptoms improve prior to 1 year. It may be difficult to convince them that continuing treatment will prevent relapse of their condition. Providing patients with information about the increased relapse rate with stopping medication early (with an estimated number needed to treat of 5) may help patients make a more informed decision.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 42-year-old woman with generalized anxiety disorder and panic attacks has been treated with sertraline 100 mg/d for the past 8 months. She has also engaged in cognitive behavioral therapy (CBT) for 6 months. Her Generalized Anxiety Disorder-7 score has decreased from 19 prior to treatment to 5 at present. Now she would like to stop her antidepressant medication because she feels better. Would you recommend that she discontinue her medication at this point?
Anxiety disorders are common, often chronic, and can cause significant morbidity and impairment.2,3 First-line treatments for anxiety disorders include CBT and antidepressants, particularly selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors.4-6
There is limited evidence regarding duration of antidepressant therapy for anxiety disorders. Previous studies have shown a high risk of relapse after discontinuation of antidepressants.6 A review of current practice patterns regarding pharmacologic treatment of depression and anxiety indicates an uptick in longer term antidepressant use for up to 2 years.7 However, long-term studies to guide treatment decisions are lacking.
STUDY SUMMARY
Clear benefit of continuing treatment up to 1 year
This systematic review and meta-analysis evaluated studies that looked at relapse rates and time to relapse in patients treated for anxiety disorders.1 The authors used PubMed, Cochrane, and Embase to identify studies involving patients treated for a variety of disorders, including generalized anxiety disorder (GAD), posttraumatic stress disorder (PTSD), panic disorder (PD), obsessive-compulsive disorder (OCD), and social phobia. Eligible studies enrolled patients with anxiety disorders who had a positive response to an antidepressant and then randomized them in a double-blind fashion to either discontinuation of antidepressants and starting placebo (stopping group) or continuation of antidepressants (continuation group) for a duration of 8 to 52 weeks. The primary outcomes were relapse rate and time to relapse.
Twenty-eight studies met the inclusion criteria for the meta-analysis, with a total of 5233 patients (2625 patients in the antidepressant group and 2608 patients in the placebo group). A breakdown of the trials by indiication included OCD (7), PD (6), GAD (6), social phobia (5), and PTSD (4). The authors graded the overall risk of bias to be low but noted that attrition bias was present in most studies.
Results. Relapse was more likely in the stopping group (odds ratio [OR] = 3.11; 95% confidence interval [CI], 2.48-3.89; n = 28 studies). Heterogeneity for relapse rate was low (I2 = 8.07%). Subgroup analyses by type of antidepressant, mode of discontinuation, and exclusion of patient comorbidities yielded similar results. Relapse prevalence was 16.4% in the antidepressant group and 36.4% in the stopping group. Additionally, time to relapse was shorter when antidepressants were discontinued (hazard ratio [HR] = 3.63; 95% CI, 2.58-5.10; n = 11 studies). Again, the heterogeneity for relapse rate was low (I2 = 0%). The original publications did not consistently report medication tolerability or withdrawal symptoms, preventing analysis of these. Dropout rates were higher in the stopping group (OR = 1.31; 95% CI, 1.06-1.63; n = 27 studies).
WHAT’S NEW
No more guessing about how long to treat
Previously, there was limited evidence to guide decisions about the duration of antidepressant treatment for anxiety disorders. This study provides evidence that stopping antidepressant treatment before 1 year increases the risk of relapse.
Continue to: CAVEATS
CAVEATS
Potential bias … bias … and more bias
While the authors used standard and appropriate methodologies for this type of study, some significant threats to validity remained. All but 2 studies in the analysis were industry funded. Publication bias is another potential issue, even though the authors identified and included 6 unpublished studies, 4 of which had negative results.
Additionally, the authors graded 11 of 28 trials as having a high likelihood of selective reporting bias, meaning that important portions of the original studies’ results may not have been published. Most studies were at high risk for attrition bias, resulting in loss of information when patients dropped out of the study. While this happened more often in the stopping groups, it is still possible that there are unidentified harms or unexpected outcomes in the medication groups.
While PTSD and OCD are no longer considered anxiety disorders, subgroup analyses found no difference in relapse rates between these diagnoses and the others included in the studies. Finally, treatment duration longer than 52 weeks has not been studied, so the optimal treatment duration is unknown.
CHALLENGES TO IMPLEMENTATION
Patients may resist continuing treatment once symptoms abate
Some patients may want to discontinue antidepressant treatment if their anxiety symptoms improve prior to 1 year. It may be difficult to convince them that continuing treatment will prevent relapse of their condition. Providing patients with information about the increased relapse rate with stopping medication early (with an estimated number needed to treat of 5) may help patients make a more informed decision.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Batelaan NM, Bosman RC, Muntingh A, et al. Risk of relapse after antidepressant discontinuation in anxiety disorders, obsessive-compulsive disorder, and post-traumatic stress disorder: systematic review and meta-analysis of relapse prevention trials. BMJ. 2017;358:j3927. Erratum in: BMJ. 2017;358:j4461.
2. National Institute of Mental Health. Prevalence of any anxiety disorder among adults. https://www.nimh.nih.gov/health/statistics/any-anxiety-disorder.shtml#part_155094. Updated November 2017. Accessed July 11, 2019.
3. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184.
4. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77-84.
5. Kaczkurkin AN, Foa EB. Cognitive-behavioral therapy for anxiety disorders: an update on the empirical evidence. Dialogues Clin Neurosci. 2015;17:337-346.
6. Donovan MR, Glue P, Kolluri S, et al. Comparative efficacy of antidepressants in preventing relapse in anxiety disorders—a meta-analysis. J Affect Disord. 2010;123:9-16.
7. Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the U.S. National Health and Nutrition Examination Survey. J Clin Psychiatry. 2014;75:169-177.
1. Batelaan NM, Bosman RC, Muntingh A, et al. Risk of relapse after antidepressant discontinuation in anxiety disorders, obsessive-compulsive disorder, and post-traumatic stress disorder: systematic review and meta-analysis of relapse prevention trials. BMJ. 2017;358:j3927. Erratum in: BMJ. 2017;358:j4461.
2. National Institute of Mental Health. Prevalence of any anxiety disorder among adults. https://www.nimh.nih.gov/health/statistics/any-anxiety-disorder.shtml#part_155094. Updated November 2017. Accessed July 11, 2019.
3. Kessler RC, Petukhova M, Sampson NA, et al. Twelve-month and lifetime prevalence and lifetime morbid risk of anxiety and mood disorders in the United States. Int J Methods Psychiatr Res. 2012;21:169-184.
4. Bandelow B, Sher L, Bunevicius R, et al. Guidelines for the pharmacological treatment of anxiety disorders, obsessive-compulsive disorder and posttraumatic stress disorder in primary care. Int J Psychiatry Clin Pract. 2012;16:77-84.
5. Kaczkurkin AN, Foa EB. Cognitive-behavioral therapy for anxiety disorders: an update on the empirical evidence. Dialogues Clin Neurosci. 2015;17:337-346.
6. Donovan MR, Glue P, Kolluri S, et al. Comparative efficacy of antidepressants in preventing relapse in anxiety disorders—a meta-analysis. J Affect Disord. 2010;123:9-16.
7. Mojtabai R, Olfson M. National trends in long-term use of antidepressant medications: results from the U.S. National Health and Nutrition Examination Survey. J Clin Psychiatry. 2014;75:169-177.
PRACTICE CHANGER
Keep patients on antidepressant therapy for anxiety disorders for a year or longer before considering a taper.
STRENGTH OF RECOMMENDATION
A: Based on a systematic review/meta-analysis of several good quality randomized controlled trials.1
Batelaan NM, Bosman RC, Muntingh A, et al. Risk of relapse after antidepressant discontinuation in anxiety disorders, obsessive-compulsive disorder, and post-traumatic stress disorder: systematic review and meta-analysis of relapse prevention trials. BMJ. 2017;358:j3927. Erratum in: BMJ. 2017;358:j4461.
Should You Switch the DAPT Agent a Month After ACS?
A 60-year-old man visits your clinic 30 days after he was hospitalized for acute coronary syndrome (ACS) due to ST-elevation myocardial infarction (STEMI). The patient underwent percutaneous coronary intervention (PCI) with placement of a stent and received aspirin and a loading dose of ticagrelor for antiplatelet therapy. He was discharged on dual antiplatelet therapy (DAPT) consisting of daily aspirin and ticagrelor. He asks about the risk for bleeding associated with these medications. Should you recommend any changes?
Platelet inhibition during and after ACS to prevent recurrent ischemic events is a cornerstone of treatment for patients after a myocardial infarction (MI).2 Current American College of Cardiology/American Heart Association and European Society of Cardiology guidelines recommend that patients with coronary artery disease who recently had an MI continue DAPT with aspirin and a P2Y12 blocker (clopidogrel, ticlopidine, ticagrelor, prasugrel, or cangrelor) for 12 months following ACS to reduce recurrent ischemia.2-4
Studies have shown that using the newer P2Y12 inhibitors (prasugrel and ticagrelor) after PCI leads to a significant reduction in recurrent ischemic events, compared with clopidogrel.5-7 These data prompted a guideline change recommending the use of the newer agents over clopidogrel for 12 months following PCI.2 Follow-up studies show strong evidence for the use of the newer P2Y12 agents in the first month following PCI, but they also demonstrate an increased bleeding risk in the maintenance phase (from 30 days to 12 months post-PCI).6,7 This increased risk is the basis for the study by Cuisset et al, which examined switching from a newer P2Y12 agent to clopidogrel after the initial 30-day period following PCI.
STUDY SUMMARY
Switched DAPT is superior
This open-label RCT (N = 646) evaluated changing DAPT from aspirin plus a newer P2Y12 blocker (prasugrel or ticagrelor) to a combination of aspirin and clopidogrel after the first month of DAPT post-ACS.1 Prior to PCI, patients received a loading dose of ticagrelor (180 mg) or prasugrel (60 mg). Subsequently, all patients took aspirin (75 mg/d) and either prasugrel (10 mg/d) or ticagrelor (90 mg bid) for 1 month. After 30 days, participants who had no adverse events were randomly assigned in a 1:1 ratio to continue the aspirin and newer P2Y12 blocker regimen or switch to aspirin and clopidogrel (75 mg/d). In the following year, researchers examined the composite outcome of cardiovascular death, urgent revascularization, stroke, and major bleeding (defined by a Bleeding Academic Research Consortium [BARC] classification ≥ Type 2 at 1-year post-ACS).
Of the participants (average age, 60), 40% had a STEMI and 60% had a non-STEMI. Overall, 43% of patients were prescribed ticagrelor and 57% prasugrel. At 1 year, 86% of the switched-DAPT group and 75% of the unchanged-DAPT group were still taking their medication. The composite outcome at 1-year follow-up was lower in the switched group compared with the unchanged group (13.4% vs 26.3%; hazard ratio [HR], 0.48; 95% confidence interval [CI], 0.34-0.68; number needed to treat [NNT], 8).
Bleeding events (ranging from minimal to fatal) were lower in the switched group (9.3% vs 23.5%; HR, 0.39; 95% CI, 0.27-0.57; NNT, 7) and events identified as BARC ≥ Type 2 (defined as needing medical treatment) were also lower in this group (4% vs 14.9%; HR, 0.30, 95% CI, 0.18-0.50; NNT, 9). There were no significant differences in reported recurrent cardiovascular ischemic events (9.3% vs 11.5%; HR, 0.80, 95% CI, 0.50-1.29).
WHAT’S NEW
Less bleeding, no increase in ischemic events
Cardiology guidelines recommend the newer P2Y12 blockers as part of DAPT after ACS, but this trial showed switching to clopidogrel for DAPT after 30 days of treatment lowers bleeding events with no difference in recurrent ischemic events.2-4
Continue to: CAVEATS
CAVEATS
Less-than-ideal study methods
In this open-label and unblinded study, the investigators adjudicating critical events were blinded to the treatment allocation. However, patients could self-report minor bleeding and medication discontinuation for which no consultation was sought. In addition, the investigators used opaque envelopes—a less-than-ideal method—to conceal allocation at enrollment.
CHALLENGES TO IMPLEMENTATION
PCP may not change cardiologist’s prescription
Implementing this practice is facilitated by the comparatively lower cost of clopidogrel versus the newer P2Y12 blockers. However, after ACS and PCI treatment, cardiologists usually initiate antiplatelet therapy and may continue to manage patients after discharge. The primary care provider (PCP) may not be responsible for the DAPT switch initially; furthermore, ordering a switch may require coordination if the PCP is hesitant to change the cardiologist’s prescription. Lastly, guidelines currently recommend using the newer P2Y12 blockers for 12 months.2 CR
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[3]:162,164).
1. Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38(41):3070-3078.
2. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68(10):1082-1115.
3. Steg PG, James SK, Atar D, et al; Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33(20):2569-2619.
4. Roffi M, Patrono C, Collet J-P, et al; ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2015;37(3):267-315.
5. Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Cardiol. 2008;51(21): 2028-2033.
6. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045-1057.
7. Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-2015.
A 60-year-old man visits your clinic 30 days after he was hospitalized for acute coronary syndrome (ACS) due to ST-elevation myocardial infarction (STEMI). The patient underwent percutaneous coronary intervention (PCI) with placement of a stent and received aspirin and a loading dose of ticagrelor for antiplatelet therapy. He was discharged on dual antiplatelet therapy (DAPT) consisting of daily aspirin and ticagrelor. He asks about the risk for bleeding associated with these medications. Should you recommend any changes?
Platelet inhibition during and after ACS to prevent recurrent ischemic events is a cornerstone of treatment for patients after a myocardial infarction (MI).2 Current American College of Cardiology/American Heart Association and European Society of Cardiology guidelines recommend that patients with coronary artery disease who recently had an MI continue DAPT with aspirin and a P2Y12 blocker (clopidogrel, ticlopidine, ticagrelor, prasugrel, or cangrelor) for 12 months following ACS to reduce recurrent ischemia.2-4
Studies have shown that using the newer P2Y12 inhibitors (prasugrel and ticagrelor) after PCI leads to a significant reduction in recurrent ischemic events, compared with clopidogrel.5-7 These data prompted a guideline change recommending the use of the newer agents over clopidogrel for 12 months following PCI.2 Follow-up studies show strong evidence for the use of the newer P2Y12 agents in the first month following PCI, but they also demonstrate an increased bleeding risk in the maintenance phase (from 30 days to 12 months post-PCI).6,7 This increased risk is the basis for the study by Cuisset et al, which examined switching from a newer P2Y12 agent to clopidogrel after the initial 30-day period following PCI.
STUDY SUMMARY
Switched DAPT is superior
This open-label RCT (N = 646) evaluated changing DAPT from aspirin plus a newer P2Y12 blocker (prasugrel or ticagrelor) to a combination of aspirin and clopidogrel after the first month of DAPT post-ACS.1 Prior to PCI, patients received a loading dose of ticagrelor (180 mg) or prasugrel (60 mg). Subsequently, all patients took aspirin (75 mg/d) and either prasugrel (10 mg/d) or ticagrelor (90 mg bid) for 1 month. After 30 days, participants who had no adverse events were randomly assigned in a 1:1 ratio to continue the aspirin and newer P2Y12 blocker regimen or switch to aspirin and clopidogrel (75 mg/d). In the following year, researchers examined the composite outcome of cardiovascular death, urgent revascularization, stroke, and major bleeding (defined by a Bleeding Academic Research Consortium [BARC] classification ≥ Type 2 at 1-year post-ACS).
Of the participants (average age, 60), 40% had a STEMI and 60% had a non-STEMI. Overall, 43% of patients were prescribed ticagrelor and 57% prasugrel. At 1 year, 86% of the switched-DAPT group and 75% of the unchanged-DAPT group were still taking their medication. The composite outcome at 1-year follow-up was lower in the switched group compared with the unchanged group (13.4% vs 26.3%; hazard ratio [HR], 0.48; 95% confidence interval [CI], 0.34-0.68; number needed to treat [NNT], 8).
Bleeding events (ranging from minimal to fatal) were lower in the switched group (9.3% vs 23.5%; HR, 0.39; 95% CI, 0.27-0.57; NNT, 7) and events identified as BARC ≥ Type 2 (defined as needing medical treatment) were also lower in this group (4% vs 14.9%; HR, 0.30, 95% CI, 0.18-0.50; NNT, 9). There were no significant differences in reported recurrent cardiovascular ischemic events (9.3% vs 11.5%; HR, 0.80, 95% CI, 0.50-1.29).
WHAT’S NEW
Less bleeding, no increase in ischemic events
Cardiology guidelines recommend the newer P2Y12 blockers as part of DAPT after ACS, but this trial showed switching to clopidogrel for DAPT after 30 days of treatment lowers bleeding events with no difference in recurrent ischemic events.2-4
Continue to: CAVEATS
CAVEATS
Less-than-ideal study methods
In this open-label and unblinded study, the investigators adjudicating critical events were blinded to the treatment allocation. However, patients could self-report minor bleeding and medication discontinuation for which no consultation was sought. In addition, the investigators used opaque envelopes—a less-than-ideal method—to conceal allocation at enrollment.
CHALLENGES TO IMPLEMENTATION
PCP may not change cardiologist’s prescription
Implementing this practice is facilitated by the comparatively lower cost of clopidogrel versus the newer P2Y12 blockers. However, after ACS and PCI treatment, cardiologists usually initiate antiplatelet therapy and may continue to manage patients after discharge. The primary care provider (PCP) may not be responsible for the DAPT switch initially; furthermore, ordering a switch may require coordination if the PCP is hesitant to change the cardiologist’s prescription. Lastly, guidelines currently recommend using the newer P2Y12 blockers for 12 months.2 CR
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[3]:162,164).
A 60-year-old man visits your clinic 30 days after he was hospitalized for acute coronary syndrome (ACS) due to ST-elevation myocardial infarction (STEMI). The patient underwent percutaneous coronary intervention (PCI) with placement of a stent and received aspirin and a loading dose of ticagrelor for antiplatelet therapy. He was discharged on dual antiplatelet therapy (DAPT) consisting of daily aspirin and ticagrelor. He asks about the risk for bleeding associated with these medications. Should you recommend any changes?
Platelet inhibition during and after ACS to prevent recurrent ischemic events is a cornerstone of treatment for patients after a myocardial infarction (MI).2 Current American College of Cardiology/American Heart Association and European Society of Cardiology guidelines recommend that patients with coronary artery disease who recently had an MI continue DAPT with aspirin and a P2Y12 blocker (clopidogrel, ticlopidine, ticagrelor, prasugrel, or cangrelor) for 12 months following ACS to reduce recurrent ischemia.2-4
Studies have shown that using the newer P2Y12 inhibitors (prasugrel and ticagrelor) after PCI leads to a significant reduction in recurrent ischemic events, compared with clopidogrel.5-7 These data prompted a guideline change recommending the use of the newer agents over clopidogrel for 12 months following PCI.2 Follow-up studies show strong evidence for the use of the newer P2Y12 agents in the first month following PCI, but they also demonstrate an increased bleeding risk in the maintenance phase (from 30 days to 12 months post-PCI).6,7 This increased risk is the basis for the study by Cuisset et al, which examined switching from a newer P2Y12 agent to clopidogrel after the initial 30-day period following PCI.
STUDY SUMMARY
Switched DAPT is superior
This open-label RCT (N = 646) evaluated changing DAPT from aspirin plus a newer P2Y12 blocker (prasugrel or ticagrelor) to a combination of aspirin and clopidogrel after the first month of DAPT post-ACS.1 Prior to PCI, patients received a loading dose of ticagrelor (180 mg) or prasugrel (60 mg). Subsequently, all patients took aspirin (75 mg/d) and either prasugrel (10 mg/d) or ticagrelor (90 mg bid) for 1 month. After 30 days, participants who had no adverse events were randomly assigned in a 1:1 ratio to continue the aspirin and newer P2Y12 blocker regimen or switch to aspirin and clopidogrel (75 mg/d). In the following year, researchers examined the composite outcome of cardiovascular death, urgent revascularization, stroke, and major bleeding (defined by a Bleeding Academic Research Consortium [BARC] classification ≥ Type 2 at 1-year post-ACS).
Of the participants (average age, 60), 40% had a STEMI and 60% had a non-STEMI. Overall, 43% of patients were prescribed ticagrelor and 57% prasugrel. At 1 year, 86% of the switched-DAPT group and 75% of the unchanged-DAPT group were still taking their medication. The composite outcome at 1-year follow-up was lower in the switched group compared with the unchanged group (13.4% vs 26.3%; hazard ratio [HR], 0.48; 95% confidence interval [CI], 0.34-0.68; number needed to treat [NNT], 8).
Bleeding events (ranging from minimal to fatal) were lower in the switched group (9.3% vs 23.5%; HR, 0.39; 95% CI, 0.27-0.57; NNT, 7) and events identified as BARC ≥ Type 2 (defined as needing medical treatment) were also lower in this group (4% vs 14.9%; HR, 0.30, 95% CI, 0.18-0.50; NNT, 9). There were no significant differences in reported recurrent cardiovascular ischemic events (9.3% vs 11.5%; HR, 0.80, 95% CI, 0.50-1.29).
WHAT’S NEW
Less bleeding, no increase in ischemic events
Cardiology guidelines recommend the newer P2Y12 blockers as part of DAPT after ACS, but this trial showed switching to clopidogrel for DAPT after 30 days of treatment lowers bleeding events with no difference in recurrent ischemic events.2-4
Continue to: CAVEATS
CAVEATS
Less-than-ideal study methods
In this open-label and unblinded study, the investigators adjudicating critical events were blinded to the treatment allocation. However, patients could self-report minor bleeding and medication discontinuation for which no consultation was sought. In addition, the investigators used opaque envelopes—a less-than-ideal method—to conceal allocation at enrollment.
CHALLENGES TO IMPLEMENTATION
PCP may not change cardiologist’s prescription
Implementing this practice is facilitated by the comparatively lower cost of clopidogrel versus the newer P2Y12 blockers. However, after ACS and PCI treatment, cardiologists usually initiate antiplatelet therapy and may continue to manage patients after discharge. The primary care provider (PCP) may not be responsible for the DAPT switch initially; furthermore, ordering a switch may require coordination if the PCP is hesitant to change the cardiologist’s prescription. Lastly, guidelines currently recommend using the newer P2Y12 blockers for 12 months.2 CR
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2019. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2019;68[3]:162,164).
1. Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38(41):3070-3078.
2. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68(10):1082-1115.
3. Steg PG, James SK, Atar D, et al; Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33(20):2569-2619.
4. Roffi M, Patrono C, Collet J-P, et al; ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2015;37(3):267-315.
5. Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Cardiol. 2008;51(21): 2028-2033.
6. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045-1057.
7. Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-2015.
1. Cuisset T, Deharo P, Quilici J, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38(41):3070-3078.
2. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2016;68(10):1082-1115.
3. Steg PG, James SK, Atar D, et al; Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33(20):2569-2619.
4. Roffi M, Patrono C, Collet J-P, et al; ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2015;37(3):267-315.
5. Antman EM, Wiviott SD, Murphy SA, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Cardiol. 2008;51(21): 2028-2033.
6. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045-1057.
7. Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-2015.
Do probiotics reduce C diff risk in hospitalized patients?
ILLUSTRATIVE CASE
A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
While several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI,4-6 guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America did not incorporate a recommendation for the use of probiotics in their CDI prevention strategy.7,8
The PLACIDE trial studied the use of probiotics in inpatients ages ≥ 65 years receiving either oral or parenteral antibiotics and found no difference in the incidence of CDI in those who received probiotics vs those who did not.9 Even though the PLACIDE trial was the largest, high-quality, randomized controlled trial (RCT) on the use of probiotics to prevent CDI, it had a lower incidence of CDI than was assumed in the power calculations. Additionally, previous systematic reviews did not always follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and did not focus specifically on hospitalized patients, who are at higher risk for CDI.
Given the conflicting and poor evidence and recommendations, an additional systematic review and meta-analysis was performed following PRISMA guidelines and focusing on studies conducted only on hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in hospitalized patients receiving antibiotics
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 adult hospitalized patients taking antibiotics. All patients were ≥ 18 years (mean age 68-69 years) and received antibiotics orally, intravenously, or via both routes for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotics used were 1 or a combination of 4 strains (Lactobacillus, Saccharomyces, Bifidobacterium, Streptococcus). Probiotic doses ranged from 4 billion to 900 billion colony-forming u/day and were started from 1 to 7 days after first antibiotic dose. Duration of probiotic use was either fixed at between 14 and 21 days or varied based on the duration of antibiotics (extending 3-14 days after the last antibiotic dose).
Continue to: Control groups received...
Control groups received matching placebo in all trials but 2; those 2 used usual care of no probiotics as the control. Common patient exclusions were pregnancy, immune system compromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%) with no heterogeneity (I2 = 0.0%; P = .56) when the data were pooled from all 19 studies (relative risk [RR] = 0.42; 95% confidence interval [CI], 0.30-0.57). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43 (95% CI, 36-58).
The researchers examined the NNT at varying incidence rates. If the incidence of CDI was 1.2%, the NNT to prevent 1 case of CDI was 144, and if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR = 0.32; 95% CI, 0.22-0.48), but not if they were started at 3 to 7 days (RR = 0.70; 95% CI, 0.40-1.2). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%; P = .35).
WHAT’S NEW
Probiotics provide added benefit if taken sooner rather than later
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Findings do not apply to all patients; specific recommendations are lacking
Findings from this meta-analysis do not apply to patients who have an immunocompromising condition, are pregnant, have a prosthetic heart valve, have a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis), or require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Lack of “medication” status leads to limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adult patients is the availability of probiotics on local hospital formularies. Probiotics are not technically a medication; they are not regulated or approved by the US Food and Drug Administration and thus, insurance coverage and availability for inpatient use are limited. Lastly, US cost-effectiveness data are lacking, although such data would likely be favorable given the high costs associated with treatment of CDI.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152:1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(Suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158:706-707.
7. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
8. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
9. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
ILLUSTRATIVE CASE
A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
While several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI,4-6 guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America did not incorporate a recommendation for the use of probiotics in their CDI prevention strategy.7,8
The PLACIDE trial studied the use of probiotics in inpatients ages ≥ 65 years receiving either oral or parenteral antibiotics and found no difference in the incidence of CDI in those who received probiotics vs those who did not.9 Even though the PLACIDE trial was the largest, high-quality, randomized controlled trial (RCT) on the use of probiotics to prevent CDI, it had a lower incidence of CDI than was assumed in the power calculations. Additionally, previous systematic reviews did not always follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and did not focus specifically on hospitalized patients, who are at higher risk for CDI.
Given the conflicting and poor evidence and recommendations, an additional systematic review and meta-analysis was performed following PRISMA guidelines and focusing on studies conducted only on hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in hospitalized patients receiving antibiotics
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 adult hospitalized patients taking antibiotics. All patients were ≥ 18 years (mean age 68-69 years) and received antibiotics orally, intravenously, or via both routes for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotics used were 1 or a combination of 4 strains (Lactobacillus, Saccharomyces, Bifidobacterium, Streptococcus). Probiotic doses ranged from 4 billion to 900 billion colony-forming u/day and were started from 1 to 7 days after first antibiotic dose. Duration of probiotic use was either fixed at between 14 and 21 days or varied based on the duration of antibiotics (extending 3-14 days after the last antibiotic dose).
Continue to: Control groups received...
Control groups received matching placebo in all trials but 2; those 2 used usual care of no probiotics as the control. Common patient exclusions were pregnancy, immune system compromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%) with no heterogeneity (I2 = 0.0%; P = .56) when the data were pooled from all 19 studies (relative risk [RR] = 0.42; 95% confidence interval [CI], 0.30-0.57). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43 (95% CI, 36-58).
The researchers examined the NNT at varying incidence rates. If the incidence of CDI was 1.2%, the NNT to prevent 1 case of CDI was 144, and if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR = 0.32; 95% CI, 0.22-0.48), but not if they were started at 3 to 7 days (RR = 0.70; 95% CI, 0.40-1.2). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%; P = .35).
WHAT’S NEW
Probiotics provide added benefit if taken sooner rather than later
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Findings do not apply to all patients; specific recommendations are lacking
Findings from this meta-analysis do not apply to patients who have an immunocompromising condition, are pregnant, have a prosthetic heart valve, have a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis), or require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Lack of “medication” status leads to limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adult patients is the availability of probiotics on local hospital formularies. Probiotics are not technically a medication; they are not regulated or approved by the US Food and Drug Administration and thus, insurance coverage and availability for inpatient use are limited. Lastly, US cost-effectiveness data are lacking, although such data would likely be favorable given the high costs associated with treatment of CDI.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 68-year-old woman is admitted to the hospital with a diagnosis of community-acquired pneumonia. Should you add probiotics to her antibiotic regimen to prevent infection with Clostridium difficile?
Clostridium difficile infection (CDI) leads to significant morbidity, mortality, and treatment failures. In 2011, it culminated in a cost of $4.8 billion and 29,000 deaths.2,3 Risk factors for infection include antibiotic use, hospitalization, older age, and medical comorbidities.2 Probiotics have been proposed as one way to prevent CDI.
While several systematic reviews have demonstrated efficacy for probiotics in the prevention of CDI,4-6 guidelines from the American College of Gastroenterology and the Society for Healthcare Epidemiology of America did not incorporate a recommendation for the use of probiotics in their CDI prevention strategy.7,8
The PLACIDE trial studied the use of probiotics in inpatients ages ≥ 65 years receiving either oral or parenteral antibiotics and found no difference in the incidence of CDI in those who received probiotics vs those who did not.9 Even though the PLACIDE trial was the largest, high-quality, randomized controlled trial (RCT) on the use of probiotics to prevent CDI, it had a lower incidence of CDI than was assumed in the power calculations. Additionally, previous systematic reviews did not always follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, and did not focus specifically on hospitalized patients, who are at higher risk for CDI.
Given the conflicting and poor evidence and recommendations, an additional systematic review and meta-analysis was performed following PRISMA guidelines and focusing on studies conducted only on hospitalized adults.
STUDY SUMMARY
Probiotics prevent CDI in hospitalized patients receiving antibiotics
This meta-analysis of 19 RCTs evaluated the efficacy of probiotics for the prevention of CDI in 6261 adult hospitalized patients taking antibiotics. All patients were ≥ 18 years (mean age 68-69 years) and received antibiotics orally, intravenously, or via both routes for any medical indication.
Trials were included if the intervention was for CDI prevention and if the probiotics used were 1 or a combination of 4 strains (Lactobacillus, Saccharomyces, Bifidobacterium, Streptococcus). Probiotic doses ranged from 4 billion to 900 billion colony-forming u/day and were started from 1 to 7 days after first antibiotic dose. Duration of probiotic use was either fixed at between 14 and 21 days or varied based on the duration of antibiotics (extending 3-14 days after the last antibiotic dose).
Continue to: Control groups received...
Control groups received matching placebo in all trials but 2; those 2 used usual care of no probiotics as the control. Common patient exclusions were pregnancy, immune system compromise, intensive care, a prosthetic heart valve, and pre-existing gastrointestinal disorders.
The risk for CDI was lower in the probiotic group (range 0%-11%) than in the control group (0%-40%) with no heterogeneity (I2 = 0.0%; P = .56) when the data were pooled from all 19 studies (relative risk [RR] = 0.42; 95% confidence interval [CI], 0.30-0.57). The median incidence of CDI in the control groups from all studies was 4%, which yielded a number needed to treat (NNT) of 43 (95% CI, 36-58).
The researchers examined the NNT at varying incidence rates. If the incidence of CDI was 1.2%, the NNT to prevent 1 case of CDI was 144, and if the incidence was 7.4%, the NNT was 23. Compared with control groups, there was a significant reduction in CDI if probiotics were started within 1 to 2 days of antibiotic initiation (RR = 0.32; 95% CI, 0.22-0.48), but not if they were started at 3 to 7 days (RR = 0.70; 95% CI, 0.40-1.2). There was no significant difference in adverse events (ie, cramping, nausea, fever, soft stools, flatulence, taste disturbance) between probiotic and control groups (14% vs 16%; P = .35).
WHAT’S NEW
Probiotics provide added benefit if taken sooner rather than later
This high-quality meta-analysis shows that administration of probiotics to hospitalized patients—particularly when started within 1 to 2 days of initiating antibiotic therapy—can prevent CDI.
CAVEATS
Findings do not apply to all patients; specific recommendations are lacking
Findings from this meta-analysis do not apply to patients who have an immunocompromising condition, are pregnant, have a prosthetic heart valve, have a pre-existing gastrointestinal disorder (eg, irritable bowel disease, pancreatitis), or require intensive care. In addition, specific recommendations as to the optimal probiotic species, dose, formulation, and duration of use cannot be made based on this meta-analysis. Lastly, findings from this study do not apply to patients treated with antibiotics in the ambulatory care setting.
Continue to: CHALLENGES TO IMPLEMENTATION
CHALLENGES TO IMPLEMENTATION
Lack of “medication” status leads to limited availability in hospitals
The largest barrier to giving probiotics to hospitalized adult patients is the availability of probiotics on local hospital formularies. Probiotics are not technically a medication; they are not regulated or approved by the US Food and Drug Administration and thus, insurance coverage and availability for inpatient use are limited. Lastly, US cost-effectiveness data are lacking, although such data would likely be favorable given the high costs associated with treatment of CDI.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152:1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(Suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158:706-707.
7. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
8. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
9. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
1. Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152:1889-1900.e9.
2. Evans CT, Safdar N. Current trends in the epidemiology and outcomes of Clostridium difficile infection. Clin Infect Dis. 2015;60(Suppl 2):S66-S71.
3. Lessa FC, Winston LG, McDonald LC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:2369-2370.
4. Goldenberg JZ, Yap C, Lytvyn L. Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
5. Lau CS, Chamberlain RS. Probiotics are effective at preventing Clostridium difficile–associated diarrhea: a systematic review and meta-analysis. Int J Gen Med. 2016:22:27-37.
6. Johnston BC, Goldenberg JZ, Guyatt GH. Probiotics for the prevention of Clostridium difficile–associated diarrhea. In response. Ann Intern Med. 2013;158:706-707.
7. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;108:478-498.
8. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455.
9. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2013;382:1249-1257.
PRACTICE CHANGER
Start probiotics within 1 to 2 days of starting antibiotics in hospitalized patients to reduce the risk of Clostridium difficile infection.1
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of randomized controlled trials.
Shen NT, Maw A, Tmanova LL, et al. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: a systematic review with meta-regression analysis. Gastroenterology. 2017;152:1889-1900. e9.