User login
Things We Do for No Reason™: Routine Correction of Elevated INR and Thrombocytopenia Prior to Paracentesis in Patients with Cirrhosis

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 52-year-old man with alcoholic cirrhosis for tense ascites and altered mentation. Home medications include furosemide, spironolactone, lactulose, and rifaximin, but his family notes he ran out last week. Although afebrile and hemodynamically stable, the patient’s coagulopathy, with an international normalized ratio (INR) of 2.3, and thrombocytopenia, with a platelet count of 37,000/μL, worries the hospitalist. The hospitalist wonders whether to transfuse fresh frozen plasma (FFP) and platelets prior to diagnostic paracentesis to reduce the risk of procedural bleeding.
WHY ROUTINELY DOING THIS MIGHT SEEM HELPFUL
Many patients undergoing paracentesis have severe liver disease and present with both thrombocytopenia and elevated INRs. While platelet count and INR serve as surrogate markers for bleeding risk in many settings, clinicians often extrapolate this concept to patients with cirrhosis. Many hospitalists routinely check INR and platelet count and administer FFP and platelets prior to diagnostic or therapeutic paracentesis to mitigate procedure-related bleeding risk. Some medical resources recommend this practice,1 while case reports and personal experiences with bleeding in these patients create availability bias that influences perception of bleeding risk.2 One recent study of patients with decompensated cirrhosis presenting to a US tertiary care center found that, of those receiving large-volume paracentesis, 22.2% received prophylactic FFP and 17.3% received prophylactic platelets before paracentesis.3
WHY ROUTINELY DOING THIS IS NOT HELPFUL
Advances in our understanding of coagulation in cirrhosis demonstrate neither INR nor platelet count accurately predict bleeding risk in this population. Additionally, evidence demonstrates the overall safety of paracentesis in cirrhosis—even in the presence of high INR and thrombocytopenia—and the lack of benefit from prophylactic transfusions with FFP or platelets.
Substantial evidence in patients with cirrhosis demonstrates that changes in coagulation and platelet function confer a “balanced coagulopathy” in which patients oscillate between hyper- and hypocoagulable states. In a cirrhotic liver, hepatic synthetic dysfunction results in a complex milieu through reduced production and plasma concentrations of both pro- and anticoagulant factors that can lead to either bleeding or clotting.4 This “rebalancing” makes prothrombin time (PT) and INR unreliable indicators of bleeding or clotting risk. Similarly, in patients with cirrhosis, thrombocytopenia does not necessarily reflect impaired clotting ability. These patients experience an increase in production of von Willebrand Factor, which may compensate for low platelet counts by producing stronger platelet adhesion to collagen.4 Unfortunately, we currently lack a reliable test or risk score to assess true bleeding risk in patients with cirrhosis.
Observational studies support these laboratory findings. Large case series consistently demonstrate no association between INR or platelet counts and bleeding risk in either diagnostic or therapeutic paracentesis, including large-volume paracentesis (See Appendix for a list of recent representative studies).5-10 Moreover, prophylactic transfusion of FFP or platelets does not significantly reduce bleeding risk.
In a 1991 study by McVay et al, the researchers examined bleeding outcomes of 441 paracenteses performed on hospitalized patients.11 Among patients who did not receive FFP prior to paracentesis, only one required a transfusion for procedure-related bleeding, an event rate of 0.25%. This single patient had a normal platelet count and an elevated PT to the same extent as 261 others who underwent paracentesis without complication. In a pooled analysis that included 391 paracenteses and 207 thoracenteses, the authors concluded neither PT nor platelet level predicted bleeding risk. Similarly, the largest published case series on this topic examined 4,729 paracenteses over a decade on a liver unit and found low rates of major bleeding (0.19%).9 Furthermore, preprocedure INR or platelet count did not correlate with bleeding risk. The authors did not report preprocedure transfusion rates, but they noted transfusions occurred only “occasionally.”
Subsequent observational studies have consistently revealed low bleeding risks even in settings of high coagulopathy prevalence. Grabau et al reviewed all large-volume paracenteses performed in a gastroenterology clinic over 7 years.10 In over 1,100 procedures, no major bleeding events occurred despite 27% of patients having INR greater than 2.0 and 54% having platelet counts less than 50,000/μL. Kurup et al examined bleeding risk among 304 procedures performed on patients with platelet counts less than 50,000/μL referred to radiology for ultrasound-guided paracentesis.7 Three bleeding events occurred, an overall event rate of 0.99%. They also found no association between preprocedure platelet count and bleeding risk.
In addition to observational data, one randomized, controlled trial evaluated the effects of FFP and platelet administration on bleeding risk among 60 patients with cirrhosis undergoing invasive procedures, including 19 paracenteses.6 Enrollment criteria included INR greater than 1.8 and/or platelet count less than 50,000/μL. One hundred percent of patients randomized to the usual care control arm received platelets or FFP as compared to 17% in the thromboelastography (TEG)–guided transfusion strategy arm. TEG assesses the viscoelastic properties of evolving clot formation in whole blood. Only one patient, a patient in the control arm who received FFP, developed procedure-related bleeding. Although receiving many fewer transfusions, the TEG-guided group experienced no bleeding.
In the presence of multiple studies demonstrating lack of benefit from FFP and platelet transfusion, guidelines published by the American Association for the Study of Liver Disease (AASLD), the American Gastroenterological Association (AGA), and the Society of Interventional Radiology (SIR) acknowledge the inaccuracy of platelet count and INR in predicting bleeding risk.12-14 Both AASLD and AGA recommend against routine transfusion of platelets and FFP prior to paracentesis.12,13 SIR guidelines from 2019 recommend against using an INR threshold for low-risk procedures like paracentesis and lowered their recommended platelet transfusion threshold from less than 50,000/μL to less than 20,000/μL.14 While we have limited safety data for paracentesis in patients with very low platelet counts, Kurup et al observed no bleeding events in the 19 patients in their cohort with platelets less than 20,000/μL undergoing ultrasound-guided paracentesis.7
In addition to lack of proven benefit, preprocedure transfusion exposes patients to objective risk. Transfusion-related acute lung injury and transfusion-associated circulatory overload develop at a rate of 0.48 and 3.8 per 100,000 components transfused, respectively.15 FFP transfusions also risk anaphylactic reactions with incidence ranging from 1:18,000 to 1:172,000.16 Platelets carry additional risk of bacterial contamination and resultant sepsis estimated at 1:5,000 to 1:8,000 per unit.17 Volume expansion from transfusions may contribute to portal hypertension and increase risk of variceal bleeding in decompensated liver disease.
Finally, FFP and platelet transfusions carry a significant cost. Rowley et al estimated eliminating preprocedure transfusions over 2 years and 3,116 paracenteses saved their institution $816,000.5 Furthermore, checking and correcting INR and thrombocytopenia can lead to procedural delay. Studies have demonstrated increased mortality from delaying paracentesis.18
WHEN IT IS HELPFUL
While most patients undergoing paracentesis have cirrhosis, patients without cirrhosis also undergo this procedure. Although several cited studies examined paracentesis among all-comers with ascites, our recommendations specifically apply to patients with ascites from cirrhosis.
Furthermore, although no paracentesis data in patients with severe coagulopathy (INR >2.5 or platelet count <20,000/μL) suggest periprocedural transfusion helps, we also lack data to prove it does not help.
Current recommendations from the AASLD suggest correcting coagulopathy in patients with clinically evident disseminated intravascular coagulation or hyperfibrinolysis prior to procedures.12 While no clear guidance related to paracentesis exists on when to assess for these entities, we recommend evaluating for them only when the clinical situation otherwise merits doing so and not solely for the purpose of screening prior to paracentesis. Measuring fibrinogen before paracentesis to predict bleeding risk is an emerging concept, but it cannot be routinely recommended at this time.13 Other factors that may play an important role in bleeding risk—ultrasound guidance, operator experience, and ability to avoid epigastric vessels and collateral veins—are beyond the scope of this article.
WHAT SHOULD BE DONE INSTEAD
Given that laboratory evaluations like INR and platelet count cannot predict which patients with cirrhosis will experience major bleeding complications after paracentesis and given that routinely transfusing FFP or platelets does not confer benefit and may cause serious harm, providers should avoid measuring INR or platelet count to prepare for paracentesis. Likewise, providers should avoid routinely transfusing FFP and platelets prior to paracentesis even in the presence of abnormal laboratory values because such values do not accurately reflect bleeding risk in patients with cirrhosis. Perform clinically indicated paracentesis without the delays that accompany unnecessary laboratory evaluations or transfusions.
RECOMMENDATIONS
Keep the following in mind with patients presenting with ascites from cirrhosis:
- Do not routinely use platelet count or INR when preparing for paracentesis, whether diagnostic or therapeutic, because no evidence-based “cutoff” for safe performance of paracentesis exists.
- Do not routinely transfuse FFP or platelets for prophylaxis prior to paracentesis in patients with cirrhosis.
- Reserve preprocedure transfusion of FFP or platelets for patients with disseminated intravascular coagulation, hyperfibrinolysis, or other indications for transfusion unrelated to procedural prophylaxis.
CONCLUSION
Case series representing diverse institutional experiences with thousands of patients consistently demonstrate that bleeding after paracentesis is rare (<1%), mortality from bleeding occurs very infrequently, and neither INR nor platelet counts predict bleeding risk during paracentesis in cirrhosis. These studies demonstrate that abandoning routine correction of coagulopathy does not lead to worse outcomes, can avoid potentially significant transfusion-related adverse events, and can save scarce resources.
Returning to our clinical scenario, the hospitalist should not transfuse FFP or platelets and should not delay the diagnostic paracentesis.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
Acknowledgments
The authors wish to acknowledge James Burton, MD, H Raymond Tahhan, MD, John Hess, MD, MPH, and Terry Gernsheimer, MD, for directing the authors to useful references cited in the manuscript.
1. Shlamovitz G. Paracentesis. Medscape. 2018. Accessed April 16, 2019. https://emedicine.medscape.com/article/80944-overview
2. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124-1131. https://doi.org/10.1126/science.185.4157.1124
3. Barnhill M, Lee A, Montero A. Adherence rates to recommended guidelines for paracentesis in cirrhotic patients at a tertiary care center and associated complications. Am J Gastroenterol. 2017;112:S504.
4. Tripodi A, Primignani M, Mannucci PM, Caldwell SH. Changing concepts of cirrhotic coagulopathy. Am J Gastroenterol. 2017;112(2):274-281. https://doi.org/10.1038/ajg.2016.498
5. Rowley MW, Agarwal S, Seetharam AB, Hirsch KS. Real-time ultrasound-guided paracentesis by radiologists: near zero risk of hemorrhage without correction of coagulopathy. J Vasc Interv Radiol. 2019;30(2):259-264. https://doi.org/10.1016/j.jvir.2018.11.001
6. De Pietri L, Bianchini M, Montalti R, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology. 2016;63(2):566-573. https://doi.org/10.1002/hep.28148
7. Kurup AN, Lekah A, Reardon ST, et al. Bleeding rate for ultrasound-guided paracentesis in thrombocytopenic patients. J Ultrasound Med. 2015;34(10):1833-1838. https://doi.org/10.7863/ultra.14.10034
8. De Gottardi A, Thévenot T, Spahr L, et al. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin Gastroenterol Hepatol. 2009;7(8):906-909. https://doi.org/10.1016/j.cgh.2009.05.004
9. Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther. 2005;21(5):525-529. https://doi.org/10.1111/j.1365-2036.2005.02387.x
10. Grabau CM, Crago SF, Hoff LK, et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40(2):484-488. https://doi.org/10.1002/hep.20317
11. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. https://doi.org/10.1046/j.1537-2995.1991.31291142949.x
12. Runyon BA. AASLD Practice Guideline: Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012. The American Association for the Study of Liver Diseases; 2012. Accessed April 16, 2019. https://www.aasld.org/sites/default/files/2019-06/141020_Guideline_Ascites_4UFb_2015.pdf
13. O’Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA clinical practice update: coagulation in cirrhosis. Gastroenterology. 2019;157(1):34-43.e1. https://doi.org/10.1053/j.gastro.2019.03.070
14. Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions—part ii: recommendations. J Vasc Interv Radiol. 2019;30(8):1168-1184.e1. https://doi.org/10.1016/j.jvir.2019.04.017
15. Blumberg N, Heal JM, Gettins K, et al. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion. 2010;50(12):2738-2744. https://doi.org/10.1111/j.1537-2995.2010.02748.x
16. Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012; 52(Suppl 1):65S-79S. https://doi.org/10.1111/j.1537-2995.2012.03663.x
17. Kleinman S, Reed W, Stassinopoulos A. A patient-oriented risk-benefit analysis of pathogen-inactivated blood components: application to apheresis platelets in the United States. Transfusion. 2013;53(7):1603-1618. https://doi.org/10.1111/j.1537-2995.2012.03928.x
18. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. https://doi.org/10.1038/ajg.2014.212

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 52-year-old man with alcoholic cirrhosis for tense ascites and altered mentation. Home medications include furosemide, spironolactone, lactulose, and rifaximin, but his family notes he ran out last week. Although afebrile and hemodynamically stable, the patient’s coagulopathy, with an international normalized ratio (INR) of 2.3, and thrombocytopenia, with a platelet count of 37,000/μL, worries the hospitalist. The hospitalist wonders whether to transfuse fresh frozen plasma (FFP) and platelets prior to diagnostic paracentesis to reduce the risk of procedural bleeding.
WHY ROUTINELY DOING THIS MIGHT SEEM HELPFUL
Many patients undergoing paracentesis have severe liver disease and present with both thrombocytopenia and elevated INRs. While platelet count and INR serve as surrogate markers for bleeding risk in many settings, clinicians often extrapolate this concept to patients with cirrhosis. Many hospitalists routinely check INR and platelet count and administer FFP and platelets prior to diagnostic or therapeutic paracentesis to mitigate procedure-related bleeding risk. Some medical resources recommend this practice,1 while case reports and personal experiences with bleeding in these patients create availability bias that influences perception of bleeding risk.2 One recent study of patients with decompensated cirrhosis presenting to a US tertiary care center found that, of those receiving large-volume paracentesis, 22.2% received prophylactic FFP and 17.3% received prophylactic platelets before paracentesis.3
WHY ROUTINELY DOING THIS IS NOT HELPFUL
Advances in our understanding of coagulation in cirrhosis demonstrate neither INR nor platelet count accurately predict bleeding risk in this population. Additionally, evidence demonstrates the overall safety of paracentesis in cirrhosis—even in the presence of high INR and thrombocytopenia—and the lack of benefit from prophylactic transfusions with FFP or platelets.
Substantial evidence in patients with cirrhosis demonstrates that changes in coagulation and platelet function confer a “balanced coagulopathy” in which patients oscillate between hyper- and hypocoagulable states. In a cirrhotic liver, hepatic synthetic dysfunction results in a complex milieu through reduced production and plasma concentrations of both pro- and anticoagulant factors that can lead to either bleeding or clotting.4 This “rebalancing” makes prothrombin time (PT) and INR unreliable indicators of bleeding or clotting risk. Similarly, in patients with cirrhosis, thrombocytopenia does not necessarily reflect impaired clotting ability. These patients experience an increase in production of von Willebrand Factor, which may compensate for low platelet counts by producing stronger platelet adhesion to collagen.4 Unfortunately, we currently lack a reliable test or risk score to assess true bleeding risk in patients with cirrhosis.
Observational studies support these laboratory findings. Large case series consistently demonstrate no association between INR or platelet counts and bleeding risk in either diagnostic or therapeutic paracentesis, including large-volume paracentesis (See Appendix for a list of recent representative studies).5-10 Moreover, prophylactic transfusion of FFP or platelets does not significantly reduce bleeding risk.
In a 1991 study by McVay et al, the researchers examined bleeding outcomes of 441 paracenteses performed on hospitalized patients.11 Among patients who did not receive FFP prior to paracentesis, only one required a transfusion for procedure-related bleeding, an event rate of 0.25%. This single patient had a normal platelet count and an elevated PT to the same extent as 261 others who underwent paracentesis without complication. In a pooled analysis that included 391 paracenteses and 207 thoracenteses, the authors concluded neither PT nor platelet level predicted bleeding risk. Similarly, the largest published case series on this topic examined 4,729 paracenteses over a decade on a liver unit and found low rates of major bleeding (0.19%).9 Furthermore, preprocedure INR or platelet count did not correlate with bleeding risk. The authors did not report preprocedure transfusion rates, but they noted transfusions occurred only “occasionally.”
Subsequent observational studies have consistently revealed low bleeding risks even in settings of high coagulopathy prevalence. Grabau et al reviewed all large-volume paracenteses performed in a gastroenterology clinic over 7 years.10 In over 1,100 procedures, no major bleeding events occurred despite 27% of patients having INR greater than 2.0 and 54% having platelet counts less than 50,000/μL. Kurup et al examined bleeding risk among 304 procedures performed on patients with platelet counts less than 50,000/μL referred to radiology for ultrasound-guided paracentesis.7 Three bleeding events occurred, an overall event rate of 0.99%. They also found no association between preprocedure platelet count and bleeding risk.
In addition to observational data, one randomized, controlled trial evaluated the effects of FFP and platelet administration on bleeding risk among 60 patients with cirrhosis undergoing invasive procedures, including 19 paracenteses.6 Enrollment criteria included INR greater than 1.8 and/or platelet count less than 50,000/μL. One hundred percent of patients randomized to the usual care control arm received platelets or FFP as compared to 17% in the thromboelastography (TEG)–guided transfusion strategy arm. TEG assesses the viscoelastic properties of evolving clot formation in whole blood. Only one patient, a patient in the control arm who received FFP, developed procedure-related bleeding. Although receiving many fewer transfusions, the TEG-guided group experienced no bleeding.
In the presence of multiple studies demonstrating lack of benefit from FFP and platelet transfusion, guidelines published by the American Association for the Study of Liver Disease (AASLD), the American Gastroenterological Association (AGA), and the Society of Interventional Radiology (SIR) acknowledge the inaccuracy of platelet count and INR in predicting bleeding risk.12-14 Both AASLD and AGA recommend against routine transfusion of platelets and FFP prior to paracentesis.12,13 SIR guidelines from 2019 recommend against using an INR threshold for low-risk procedures like paracentesis and lowered their recommended platelet transfusion threshold from less than 50,000/μL to less than 20,000/μL.14 While we have limited safety data for paracentesis in patients with very low platelet counts, Kurup et al observed no bleeding events in the 19 patients in their cohort with platelets less than 20,000/μL undergoing ultrasound-guided paracentesis.7
In addition to lack of proven benefit, preprocedure transfusion exposes patients to objective risk. Transfusion-related acute lung injury and transfusion-associated circulatory overload develop at a rate of 0.48 and 3.8 per 100,000 components transfused, respectively.15 FFP transfusions also risk anaphylactic reactions with incidence ranging from 1:18,000 to 1:172,000.16 Platelets carry additional risk of bacterial contamination and resultant sepsis estimated at 1:5,000 to 1:8,000 per unit.17 Volume expansion from transfusions may contribute to portal hypertension and increase risk of variceal bleeding in decompensated liver disease.
Finally, FFP and platelet transfusions carry a significant cost. Rowley et al estimated eliminating preprocedure transfusions over 2 years and 3,116 paracenteses saved their institution $816,000.5 Furthermore, checking and correcting INR and thrombocytopenia can lead to procedural delay. Studies have demonstrated increased mortality from delaying paracentesis.18
WHEN IT IS HELPFUL
While most patients undergoing paracentesis have cirrhosis, patients without cirrhosis also undergo this procedure. Although several cited studies examined paracentesis among all-comers with ascites, our recommendations specifically apply to patients with ascites from cirrhosis.
Furthermore, although no paracentesis data in patients with severe coagulopathy (INR >2.5 or platelet count <20,000/μL) suggest periprocedural transfusion helps, we also lack data to prove it does not help.
Current recommendations from the AASLD suggest correcting coagulopathy in patients with clinically evident disseminated intravascular coagulation or hyperfibrinolysis prior to procedures.12 While no clear guidance related to paracentesis exists on when to assess for these entities, we recommend evaluating for them only when the clinical situation otherwise merits doing so and not solely for the purpose of screening prior to paracentesis. Measuring fibrinogen before paracentesis to predict bleeding risk is an emerging concept, but it cannot be routinely recommended at this time.13 Other factors that may play an important role in bleeding risk—ultrasound guidance, operator experience, and ability to avoid epigastric vessels and collateral veins—are beyond the scope of this article.
WHAT SHOULD BE DONE INSTEAD
Given that laboratory evaluations like INR and platelet count cannot predict which patients with cirrhosis will experience major bleeding complications after paracentesis and given that routinely transfusing FFP or platelets does not confer benefit and may cause serious harm, providers should avoid measuring INR or platelet count to prepare for paracentesis. Likewise, providers should avoid routinely transfusing FFP and platelets prior to paracentesis even in the presence of abnormal laboratory values because such values do not accurately reflect bleeding risk in patients with cirrhosis. Perform clinically indicated paracentesis without the delays that accompany unnecessary laboratory evaluations or transfusions.
RECOMMENDATIONS
Keep the following in mind with patients presenting with ascites from cirrhosis:
- Do not routinely use platelet count or INR when preparing for paracentesis, whether diagnostic or therapeutic, because no evidence-based “cutoff” for safe performance of paracentesis exists.
- Do not routinely transfuse FFP or platelets for prophylaxis prior to paracentesis in patients with cirrhosis.
- Reserve preprocedure transfusion of FFP or platelets for patients with disseminated intravascular coagulation, hyperfibrinolysis, or other indications for transfusion unrelated to procedural prophylaxis.
CONCLUSION
Case series representing diverse institutional experiences with thousands of patients consistently demonstrate that bleeding after paracentesis is rare (<1%), mortality from bleeding occurs very infrequently, and neither INR nor platelet counts predict bleeding risk during paracentesis in cirrhosis. These studies demonstrate that abandoning routine correction of coagulopathy does not lead to worse outcomes, can avoid potentially significant transfusion-related adverse events, and can save scarce resources.
Returning to our clinical scenario, the hospitalist should not transfuse FFP or platelets and should not delay the diagnostic paracentesis.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
Acknowledgments
The authors wish to acknowledge James Burton, MD, H Raymond Tahhan, MD, John Hess, MD, MPH, and Terry Gernsheimer, MD, for directing the authors to useful references cited in the manuscript.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 52-year-old man with alcoholic cirrhosis for tense ascites and altered mentation. Home medications include furosemide, spironolactone, lactulose, and rifaximin, but his family notes he ran out last week. Although afebrile and hemodynamically stable, the patient’s coagulopathy, with an international normalized ratio (INR) of 2.3, and thrombocytopenia, with a platelet count of 37,000/μL, worries the hospitalist. The hospitalist wonders whether to transfuse fresh frozen plasma (FFP) and platelets prior to diagnostic paracentesis to reduce the risk of procedural bleeding.
WHY ROUTINELY DOING THIS MIGHT SEEM HELPFUL
Many patients undergoing paracentesis have severe liver disease and present with both thrombocytopenia and elevated INRs. While platelet count and INR serve as surrogate markers for bleeding risk in many settings, clinicians often extrapolate this concept to patients with cirrhosis. Many hospitalists routinely check INR and platelet count and administer FFP and platelets prior to diagnostic or therapeutic paracentesis to mitigate procedure-related bleeding risk. Some medical resources recommend this practice,1 while case reports and personal experiences with bleeding in these patients create availability bias that influences perception of bleeding risk.2 One recent study of patients with decompensated cirrhosis presenting to a US tertiary care center found that, of those receiving large-volume paracentesis, 22.2% received prophylactic FFP and 17.3% received prophylactic platelets before paracentesis.3
WHY ROUTINELY DOING THIS IS NOT HELPFUL
Advances in our understanding of coagulation in cirrhosis demonstrate neither INR nor platelet count accurately predict bleeding risk in this population. Additionally, evidence demonstrates the overall safety of paracentesis in cirrhosis—even in the presence of high INR and thrombocytopenia—and the lack of benefit from prophylactic transfusions with FFP or platelets.
Substantial evidence in patients with cirrhosis demonstrates that changes in coagulation and platelet function confer a “balanced coagulopathy” in which patients oscillate between hyper- and hypocoagulable states. In a cirrhotic liver, hepatic synthetic dysfunction results in a complex milieu through reduced production and plasma concentrations of both pro- and anticoagulant factors that can lead to either bleeding or clotting.4 This “rebalancing” makes prothrombin time (PT) and INR unreliable indicators of bleeding or clotting risk. Similarly, in patients with cirrhosis, thrombocytopenia does not necessarily reflect impaired clotting ability. These patients experience an increase in production of von Willebrand Factor, which may compensate for low platelet counts by producing stronger platelet adhesion to collagen.4 Unfortunately, we currently lack a reliable test or risk score to assess true bleeding risk in patients with cirrhosis.
Observational studies support these laboratory findings. Large case series consistently demonstrate no association between INR or platelet counts and bleeding risk in either diagnostic or therapeutic paracentesis, including large-volume paracentesis (See Appendix for a list of recent representative studies).5-10 Moreover, prophylactic transfusion of FFP or platelets does not significantly reduce bleeding risk.
In a 1991 study by McVay et al, the researchers examined bleeding outcomes of 441 paracenteses performed on hospitalized patients.11 Among patients who did not receive FFP prior to paracentesis, only one required a transfusion for procedure-related bleeding, an event rate of 0.25%. This single patient had a normal platelet count and an elevated PT to the same extent as 261 others who underwent paracentesis without complication. In a pooled analysis that included 391 paracenteses and 207 thoracenteses, the authors concluded neither PT nor platelet level predicted bleeding risk. Similarly, the largest published case series on this topic examined 4,729 paracenteses over a decade on a liver unit and found low rates of major bleeding (0.19%).9 Furthermore, preprocedure INR or platelet count did not correlate with bleeding risk. The authors did not report preprocedure transfusion rates, but they noted transfusions occurred only “occasionally.”
Subsequent observational studies have consistently revealed low bleeding risks even in settings of high coagulopathy prevalence. Grabau et al reviewed all large-volume paracenteses performed in a gastroenterology clinic over 7 years.10 In over 1,100 procedures, no major bleeding events occurred despite 27% of patients having INR greater than 2.0 and 54% having platelet counts less than 50,000/μL. Kurup et al examined bleeding risk among 304 procedures performed on patients with platelet counts less than 50,000/μL referred to radiology for ultrasound-guided paracentesis.7 Three bleeding events occurred, an overall event rate of 0.99%. They also found no association between preprocedure platelet count and bleeding risk.
In addition to observational data, one randomized, controlled trial evaluated the effects of FFP and platelet administration on bleeding risk among 60 patients with cirrhosis undergoing invasive procedures, including 19 paracenteses.6 Enrollment criteria included INR greater than 1.8 and/or platelet count less than 50,000/μL. One hundred percent of patients randomized to the usual care control arm received platelets or FFP as compared to 17% in the thromboelastography (TEG)–guided transfusion strategy arm. TEG assesses the viscoelastic properties of evolving clot formation in whole blood. Only one patient, a patient in the control arm who received FFP, developed procedure-related bleeding. Although receiving many fewer transfusions, the TEG-guided group experienced no bleeding.
In the presence of multiple studies demonstrating lack of benefit from FFP and platelet transfusion, guidelines published by the American Association for the Study of Liver Disease (AASLD), the American Gastroenterological Association (AGA), and the Society of Interventional Radiology (SIR) acknowledge the inaccuracy of platelet count and INR in predicting bleeding risk.12-14 Both AASLD and AGA recommend against routine transfusion of platelets and FFP prior to paracentesis.12,13 SIR guidelines from 2019 recommend against using an INR threshold for low-risk procedures like paracentesis and lowered their recommended platelet transfusion threshold from less than 50,000/μL to less than 20,000/μL.14 While we have limited safety data for paracentesis in patients with very low platelet counts, Kurup et al observed no bleeding events in the 19 patients in their cohort with platelets less than 20,000/μL undergoing ultrasound-guided paracentesis.7
In addition to lack of proven benefit, preprocedure transfusion exposes patients to objective risk. Transfusion-related acute lung injury and transfusion-associated circulatory overload develop at a rate of 0.48 and 3.8 per 100,000 components transfused, respectively.15 FFP transfusions also risk anaphylactic reactions with incidence ranging from 1:18,000 to 1:172,000.16 Platelets carry additional risk of bacterial contamination and resultant sepsis estimated at 1:5,000 to 1:8,000 per unit.17 Volume expansion from transfusions may contribute to portal hypertension and increase risk of variceal bleeding in decompensated liver disease.
Finally, FFP and platelet transfusions carry a significant cost. Rowley et al estimated eliminating preprocedure transfusions over 2 years and 3,116 paracenteses saved their institution $816,000.5 Furthermore, checking and correcting INR and thrombocytopenia can lead to procedural delay. Studies have demonstrated increased mortality from delaying paracentesis.18
WHEN IT IS HELPFUL
While most patients undergoing paracentesis have cirrhosis, patients without cirrhosis also undergo this procedure. Although several cited studies examined paracentesis among all-comers with ascites, our recommendations specifically apply to patients with ascites from cirrhosis.
Furthermore, although no paracentesis data in patients with severe coagulopathy (INR >2.5 or platelet count <20,000/μL) suggest periprocedural transfusion helps, we also lack data to prove it does not help.
Current recommendations from the AASLD suggest correcting coagulopathy in patients with clinically evident disseminated intravascular coagulation or hyperfibrinolysis prior to procedures.12 While no clear guidance related to paracentesis exists on when to assess for these entities, we recommend evaluating for them only when the clinical situation otherwise merits doing so and not solely for the purpose of screening prior to paracentesis. Measuring fibrinogen before paracentesis to predict bleeding risk is an emerging concept, but it cannot be routinely recommended at this time.13 Other factors that may play an important role in bleeding risk—ultrasound guidance, operator experience, and ability to avoid epigastric vessels and collateral veins—are beyond the scope of this article.
WHAT SHOULD BE DONE INSTEAD
Given that laboratory evaluations like INR and platelet count cannot predict which patients with cirrhosis will experience major bleeding complications after paracentesis and given that routinely transfusing FFP or platelets does not confer benefit and may cause serious harm, providers should avoid measuring INR or platelet count to prepare for paracentesis. Likewise, providers should avoid routinely transfusing FFP and platelets prior to paracentesis even in the presence of abnormal laboratory values because such values do not accurately reflect bleeding risk in patients with cirrhosis. Perform clinically indicated paracentesis without the delays that accompany unnecessary laboratory evaluations or transfusions.
RECOMMENDATIONS
Keep the following in mind with patients presenting with ascites from cirrhosis:
- Do not routinely use platelet count or INR when preparing for paracentesis, whether diagnostic or therapeutic, because no evidence-based “cutoff” for safe performance of paracentesis exists.
- Do not routinely transfuse FFP or platelets for prophylaxis prior to paracentesis in patients with cirrhosis.
- Reserve preprocedure transfusion of FFP or platelets for patients with disseminated intravascular coagulation, hyperfibrinolysis, or other indications for transfusion unrelated to procedural prophylaxis.
CONCLUSION
Case series representing diverse institutional experiences with thousands of patients consistently demonstrate that bleeding after paracentesis is rare (<1%), mortality from bleeding occurs very infrequently, and neither INR nor platelet counts predict bleeding risk during paracentesis in cirrhosis. These studies demonstrate that abandoning routine correction of coagulopathy does not lead to worse outcomes, can avoid potentially significant transfusion-related adverse events, and can save scarce resources.
Returning to our clinical scenario, the hospitalist should not transfuse FFP or platelets and should not delay the diagnostic paracentesis.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
Acknowledgments
The authors wish to acknowledge James Burton, MD, H Raymond Tahhan, MD, John Hess, MD, MPH, and Terry Gernsheimer, MD, for directing the authors to useful references cited in the manuscript.
1. Shlamovitz G. Paracentesis. Medscape. 2018. Accessed April 16, 2019. https://emedicine.medscape.com/article/80944-overview
2. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124-1131. https://doi.org/10.1126/science.185.4157.1124
3. Barnhill M, Lee A, Montero A. Adherence rates to recommended guidelines for paracentesis in cirrhotic patients at a tertiary care center and associated complications. Am J Gastroenterol. 2017;112:S504.
4. Tripodi A, Primignani M, Mannucci PM, Caldwell SH. Changing concepts of cirrhotic coagulopathy. Am J Gastroenterol. 2017;112(2):274-281. https://doi.org/10.1038/ajg.2016.498
5. Rowley MW, Agarwal S, Seetharam AB, Hirsch KS. Real-time ultrasound-guided paracentesis by radiologists: near zero risk of hemorrhage without correction of coagulopathy. J Vasc Interv Radiol. 2019;30(2):259-264. https://doi.org/10.1016/j.jvir.2018.11.001
6. De Pietri L, Bianchini M, Montalti R, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology. 2016;63(2):566-573. https://doi.org/10.1002/hep.28148
7. Kurup AN, Lekah A, Reardon ST, et al. Bleeding rate for ultrasound-guided paracentesis in thrombocytopenic patients. J Ultrasound Med. 2015;34(10):1833-1838. https://doi.org/10.7863/ultra.14.10034
8. De Gottardi A, Thévenot T, Spahr L, et al. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin Gastroenterol Hepatol. 2009;7(8):906-909. https://doi.org/10.1016/j.cgh.2009.05.004
9. Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther. 2005;21(5):525-529. https://doi.org/10.1111/j.1365-2036.2005.02387.x
10. Grabau CM, Crago SF, Hoff LK, et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40(2):484-488. https://doi.org/10.1002/hep.20317
11. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. https://doi.org/10.1046/j.1537-2995.1991.31291142949.x
12. Runyon BA. AASLD Practice Guideline: Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012. The American Association for the Study of Liver Diseases; 2012. Accessed April 16, 2019. https://www.aasld.org/sites/default/files/2019-06/141020_Guideline_Ascites_4UFb_2015.pdf
13. O’Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA clinical practice update: coagulation in cirrhosis. Gastroenterology. 2019;157(1):34-43.e1. https://doi.org/10.1053/j.gastro.2019.03.070
14. Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions—part ii: recommendations. J Vasc Interv Radiol. 2019;30(8):1168-1184.e1. https://doi.org/10.1016/j.jvir.2019.04.017
15. Blumberg N, Heal JM, Gettins K, et al. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion. 2010;50(12):2738-2744. https://doi.org/10.1111/j.1537-2995.2010.02748.x
16. Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012; 52(Suppl 1):65S-79S. https://doi.org/10.1111/j.1537-2995.2012.03663.x
17. Kleinman S, Reed W, Stassinopoulos A. A patient-oriented risk-benefit analysis of pathogen-inactivated blood components: application to apheresis platelets in the United States. Transfusion. 2013;53(7):1603-1618. https://doi.org/10.1111/j.1537-2995.2012.03928.x
18. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. https://doi.org/10.1038/ajg.2014.212
1. Shlamovitz G. Paracentesis. Medscape. 2018. Accessed April 16, 2019. https://emedicine.medscape.com/article/80944-overview
2. Tversky A, Kahneman D. Judgment under uncertainty: heuristics and biases. Science. 1974;185(4157):1124-1131. https://doi.org/10.1126/science.185.4157.1124
3. Barnhill M, Lee A, Montero A. Adherence rates to recommended guidelines for paracentesis in cirrhotic patients at a tertiary care center and associated complications. Am J Gastroenterol. 2017;112:S504.
4. Tripodi A, Primignani M, Mannucci PM, Caldwell SH. Changing concepts of cirrhotic coagulopathy. Am J Gastroenterol. 2017;112(2):274-281. https://doi.org/10.1038/ajg.2016.498
5. Rowley MW, Agarwal S, Seetharam AB, Hirsch KS. Real-time ultrasound-guided paracentesis by radiologists: near zero risk of hemorrhage without correction of coagulopathy. J Vasc Interv Radiol. 2019;30(2):259-264. https://doi.org/10.1016/j.jvir.2018.11.001
6. De Pietri L, Bianchini M, Montalti R, et al. Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology. 2016;63(2):566-573. https://doi.org/10.1002/hep.28148
7. Kurup AN, Lekah A, Reardon ST, et al. Bleeding rate for ultrasound-guided paracentesis in thrombocytopenic patients. J Ultrasound Med. 2015;34(10):1833-1838. https://doi.org/10.7863/ultra.14.10034
8. De Gottardi A, Thévenot T, Spahr L, et al. Risk of complications after abdominal paracentesis in cirrhotic patients: a prospective study. Clin Gastroenterol Hepatol. 2009;7(8):906-909. https://doi.org/10.1016/j.cgh.2009.05.004
9. Pache I, Bilodeau M. Severe haemorrhage following abdominal paracentesis for ascites in patients with liver disease. Aliment Pharmacol Ther. 2005;21(5):525-529. https://doi.org/10.1111/j.1365-2036.2005.02387.x
10. Grabau CM, Crago SF, Hoff LK, et al. Performance standards for therapeutic abdominal paracentesis. Hepatology. 2004;40(2):484-488. https://doi.org/10.1002/hep.20317
11. McVay PA, Toy PT. Lack of increased bleeding after paracentesis and thoracentesis in patients with mild coagulation abnormalities. Transfusion. 1991;31(2):164-171. https://doi.org/10.1046/j.1537-2995.1991.31291142949.x
12. Runyon BA. AASLD Practice Guideline: Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012. The American Association for the Study of Liver Diseases; 2012. Accessed April 16, 2019. https://www.aasld.org/sites/default/files/2019-06/141020_Guideline_Ascites_4UFb_2015.pdf
13. O’Leary JG, Greenberg CS, Patton HM, Caldwell SH. AGA clinical practice update: coagulation in cirrhosis. Gastroenterology. 2019;157(1):34-43.e1. https://doi.org/10.1053/j.gastro.2019.03.070
14. Patel IJ, Rahim S, Davidson JC, et al. Society of Interventional Radiology consensus guidelines for the periprocedural management of thrombotic and bleeding risk in patients undergoing percutaneous image-guided interventions—part ii: recommendations. J Vasc Interv Radiol. 2019;30(8):1168-1184.e1. https://doi.org/10.1016/j.jvir.2019.04.017
15. Blumberg N, Heal JM, Gettins K, et al. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion. 2010;50(12):2738-2744. https://doi.org/10.1111/j.1537-2995.2010.02748.x
16. Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012; 52(Suppl 1):65S-79S. https://doi.org/10.1111/j.1537-2995.2012.03663.x
17. Kleinman S, Reed W, Stassinopoulos A. A patient-oriented risk-benefit analysis of pathogen-inactivated blood components: application to apheresis platelets in the United States. Transfusion. 2013;53(7):1603-1618. https://doi.org/10.1111/j.1537-2995.2012.03928.x
18. Kim JJ, Tsukamoto MM, Mathur AK, et al. Delayed paracentesis is associated with increased in-hospital mortality in patients with spontaneous bacterial peritonitis. Am J Gastroenterol. 2014;109(9):1436-1442. https://doi.org/10.1038/ajg.2014.212
© 2020 Society of Hospital Medicine
Things We Do for No Reason™: Routine Coverage of Anaerobes in Aspiration Pneumonia

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
An 88-year-old woman with a history of dementia presents to the emergency room with new-onset dyspnea following 2 days of a self-limited gastrointestinal illness associated with nausea, vomiting, and diarrhea. After noting a new supplemental oxygen requirement of 4 L and a temperature of 38.6 °C, the hospitalist’s exam finds an edentulous patient with bibasilar lung crackles and a nontender abdomen. Taking into account her elevated white blood cell count and chest radiograph with right greater than left bibasilar opacities, the admitting hospitalist diagnoses aspiration pneumonia (AP) and specifically selects an antibiotic regimen with anaerobic coverage.
BACKGROUND
Aspiration, the inhalation of oropharyngeal or gastric materials into the lung, takes one of the following three forms: (1) “microaspiration,” wherein a small number of virulent organisms from oropharynx gains entry into the alveoli, (2) “macroaspiration,” wherein a large volume of typically less virulent organisms gains entry into the airways, or (3) a combination of the two. Hospitalists may struggle to distinguish unwitnessed macroaspiration causing AP from other typical causes of pneumonia, such as community-acquired pneumonia (CAP) or hospital-acquired pneumonia (HAP).1 A hospitalist should suspect macroaspiration—the most common cause of AP—in patients with risk factors such as dysphagia, diminished cough reflex or impaired swallowing, and infiltrates in the dependent bronchopulmonary segments, or of course, in cases of witnessed aspiration.2
Moreover, hospitalists must differentiate AP, an infectious entity, from aspiration pneumonitis, a noninfectious entity caused by macroaspiration of mostly sterile gastric content. Aspiration pneumonitis presents with acute lung injury within hours of an aspiration event, whereas AP entails a gradual onset of symptoms and signs of pneumonia.2 Although aspiration pneumonitis can present dramatically with hypoxemia and pulmonary edema and may evolve into AP, patients do not initially benefit from empiric antibiotics.1
WHY YOU MIGHT THINK SPECIFIC ANAEROBIC COVERAGE IS ESSENTIAL
In the 1970s, several studies of patients who were presumed to have AP because of risk factors for macroaspiration, such as alcohol use disorder, illicit drug use, and seizure disorder, identified anaerobes as major etiologic pathogens. These studies reported the presence of putrid sputum and obtained samples through invasive methods (eg, transtracheal aspirates, thoracentesis, and blood cultures).3,4 Many of the patients studied had radiographic findings of pleuropulmonary disease. For example, in the study by Bartlett et al, 70% of patients had radiographic evidence of abscess or pulmonary necrosis. These findings led to the assumption that anaerobes play a significant role in all cases of aspiration-related pulmonary syndromes. Because anaerobic bacteria live in the gingival sulcus, with an especially high burden in dental plaques, their role as a potential pathogen in AP may seem logical.5 Given the backdrop of those concerns, Kioka et al found that providers treated 90% of presumed AP patients in the intensive care unit with antibiotics that have anaerobic activity despite only 30% meeting the criteria for anaerobic coverage.6
WHY ANAEROBIC COVERAGE IS NOT ROUTINELY NECESSARY
In contrast to the population of patients with AP described from the 1970s, we now diagnose AP more frequently in nursing home residents, the elderly with cognitive impairment, and those with tube feed dependence, dysphagia, or gastrointestinal motility disorders.1 Concurrent with this change in the epidemiology of AP, we have witnessed a shift in recovered bacteria from anaerobes to aerobes in recent studies.7,8 In an intensive care unit study from 1999, respiratory tract organisms of patients with suspected aspiration mirrored those of patients with CAP or HAP.9 In a systematic review of eight observational studies that included studies from 1993 to 2014 and involved elderly patients with uncomplicated AP, only two out of eight studies demonstrated the presence of anaerobes in respiratory cultures. Even in those two studies, anaerobic bacteria frequently coexisted with aerobes. The majority of organisms in all eight studies consisted of aerobic gram-positives, gram-negatives, or both.10
A study by El-Solh et al most frequently isolated pathogenic aerobic gram-negative bacteria (49% of cases), followed by anaerobic bacteria (16%), among institutionalized elderly patients with severe AP diagnosed by clinical features. In that same study, most anaerobes coexisted with aerobic gram-negative bacteria, and the clinical illness promptly resolved in the absence of specific anaerobic coverage.11 AP can be successfully treated without anaerobic coverage due to a variety of factors: the insignificant role of anaerobes in the pathogenesis of uncomplicated AP, lower severity of illness in the absence of abscesses or pulmonary necrosis (uncomplicated), and altered local redox-potential from the elimination of aerobic pathogens, which effectively also treats anaerobes.1 Moreover, anaerobes possess generally less virulence in comparison with aerobes. AP from these organisms typically requires risk for excessive oral growth (eg, periodontal disease) and macroaspiration of a large number of organisms.5
There are also potential harms associated with the unnecessary treatment of anaerobic bacteria. Since anaerobes account for the majority of the bacteria present in the bowel, targeting anaerobes can result in gut dysbiosis.1 Moreover, a prospective study showed an increase in the incidence of vancomycin-resistant enterococci and antibiotic-resistant gram-negative bacteria associated with the empiric use of antibiotics with anaerobic activity.12 Finally, a systematic review detailed the high incidence of Clostridioides difficile infections among patients receiving clindamycin and carbapenems.13
WHEN ANAEROBIC COVERAGE IS INDICATED
Despite the predominance of aerobic organisms in the respiratory tract specimens of patients diagnosed with AP in the current era, situations still exist that require treatment of anaerobes. These include necrotizing pneumonia, empyema, or lung abscess.2 Additionally, patients with severe periodontal disease may harbor anaerobic bacteria such as Bacteroides species, Peptostreptococcus species, and Actinomyces israelii.5 When we suspect macroaspiration leading to AP, patients with severe periodontal disease may benefit from anaerobic coverage. Putrid sputum generation may indicate the presence of anaerobic organisms that produce the characteristic foul odor of short-chain volatile fatty acids observed in patients with lung abscess or empyema.2 It often takes about 8 to 14 days after an aspiration event for lung cavitation or empyema to develop.14 Therefore, a longer duration of illness or putrid sputum production may signal a significant concurrent burden of anaerobes. The 2019 official guidelines of the American Thoracic Society and Infectious Disease Society of America recommend adding anaerobic coverage to CAP only when empyema or lung abscess is suspected (conditional recommendation, very low quality of evidence).15
WHAT YOU SHOULD DO INSTEAD
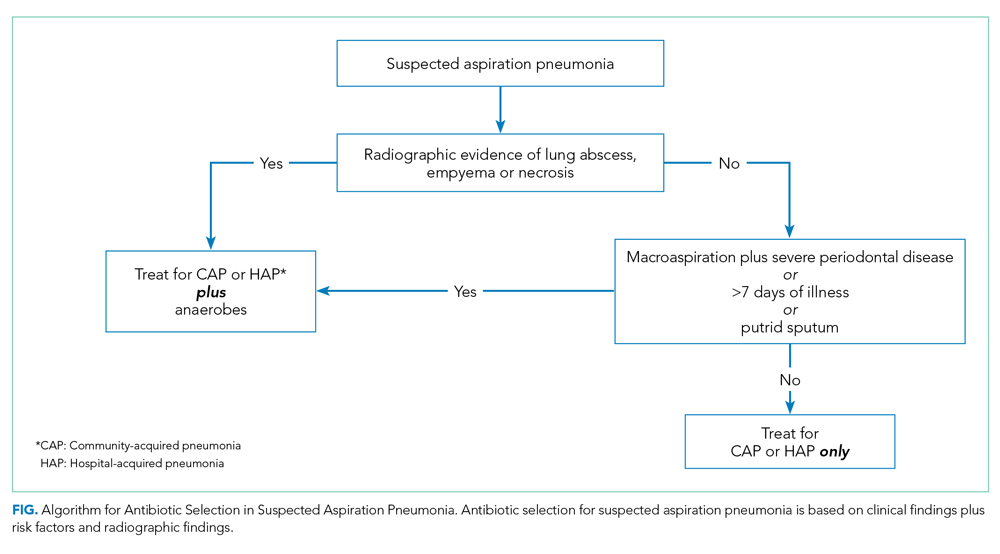
When you suspect AP in a patient, categorize it as either community or hospital acquired based on risk factors similar to CAP or HAP. For patients with witnessed macroaspiration or in patients with substantial macroaspiration risk factors, perform a radiologic evaluation and a thorough oral examination to evaluate for poor dentition, gingival disease (marked redness, tendency to bleed, ulceration), and tongue coating. For patients presenting from the community with suspected AP without complications, treat with the standard therapy (without additional anaerobic coverage) for CAP. Provide empiric anaerobic coverage for complicated AP (eg, lung abscess, necrosis, or empyema) or for macroaspiration in the setting of severe periodontal disease, putrid sputum, or longer duration of illness. Similarly, treat hospital-acquired AP as HAP (Figure).
When prescribing anaerobic coverage of AP, use combination drugs that include a ß-lactamase inhibitor (eg, ampicillin-sulbactam), clindamycin (either alone or in combination with ß-lactams), or moxifloxacin.1 Most anaerobes have ß-lactamase or cephalosporinase activity, which renders penicillin and cephalosporins ineffective. Despite its potential side effects, such as C difficile infection, treating with clindamycin has the benefit of a relatively low cost and its association with lower rates of methicillin-resistant Staphylococcus aureus emergence after treatment.16 Piperacillin-tazobactam and carbapenems also have excellent anaerobic coverage, but we should reserve them for more severe and complicated cases of AP given their extensive antibacterial activity and concern for the emergence of resistance.8 Although well known and used for decades for its activity against clinically important anaerobes, avoid metronidazole due to its reduced cure rate in lung abscess caused by microaerophilic streptococci of the oral cavity.17 Due to a lack of evidence, we do not recommend the use of metronidazole in lung infections.
RECOMMENDATIONS
- Empirically treat most suspected cases of AP with regimens similar to the standard antibiotics for CAP and HAP. In the absence of specific risk factors for anaerobic infections, do not routinely provide anaerobic coverage.
- Provide anaerobic coverage empirically for AP associated with macroaspiration in the setting of severe periodontal disease, putrid sputum, or longer duration of illness.
- Provide anaerobic coverage in AP with evidence of necrotizing pneumonia, empyema, or lung abscess.
CONCLUSION
Current evidence does not support routine anaerobic coverage of AP in the absence of identifiable risk factors for an anaerobic lung infection.
In consideration of the clinical case, importantly, she has no periodontal disease and no evidence for necrotizing pneumonia, empyema, or lung abscess radiographically. For these reasons, select an empiric antibiotic regime that targets CAP organisms predominantly and forgo additional anaerobic coverage.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason ™ ”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason ™ ” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
The authors have no conflicts of interest relevant to this article.
1. Mandell LA, Niederman MS. Aspiration pneumonia. N Engl J Med. 2019;380(7):651-663. https://doi.org/10.1056/nejmra1714562
2. Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671. https://doi.org/10.1056/nejm200103013440908
3. Bartlett JG, Gorbach SL, Finegold SM. The bacteriology of aspiration pneumonia. Am J Med. 1974;56(2):202-207. https://doi.org/10.1016/0002-9343(74)90598-1
4. Bartlett JG, Gorbach SL. The triple threat of aspiration pneumonia. Chest. 1975;68(4):560-566. https://doi.org/10.1378/chest.68.4.560
5. Sutter VL. Anaerobes as normal oral flora. Rev Infect Dis. 1984;6(suppl 1):S62-S66. https://doi.org/10.1093/clinids/6.supplement_1.s62
6. Kioka MJ, DiGiovine B, Rezik M, Jennings JH. Anaerobic antibiotic usage for pneumonia in the medical intensive care unit. Respirology. 2017;22(8):1656-1661. https://doi.org/10.1111/resp.13111
7. Ott SR, Allewelt M, Lorenz J, Reimnitz P, Lode H; German Lung Abscess Study Group. Moxifloxacin vs ampicillin/sulbactam in aspiration pneumonia and primary lung abscess. Infection. 2008;36(1):23-30. https://doi.org/10.1007/s15010-007-7043-6
8. Tokuyasu H, Harada T, Watanabe E, et al. Effectiveness of meropenem for the treatment of aspiration pneumonia in elderly patients. Intern Med. 2009;48(3):129-135. https://doi.org/10.2169/internalmedicine.48.1308
9. Marik PE, Careau P. The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. Chest. 1999;115(1):178-183. https://doi.org/10.1378/chest.115.1.178
10. Bowerman TJ, Zhang J, Waite LM. Antibacterial treatment of aspiration pneumonia in older people: a systematic review. Clin Interv Aging. 2018;13:2201-2213. https://doi.org/10.2147/cia.s183344
11. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167(12):1650-1654. https://doi.org/10.1164/rccm.200212-1543oc
12. Bhalla A, Pultz NJ, Ray AJ, Hoyen CK, Eckstein EC, Donskey CJ. Antianaerobic antibiotic therapy promotes overgrowth of antibiotic-resistant, gram-negative bacilli and vancomycin-resistant enterococci in the stool of colonized patients. Infect Control Hosp Epidemiol. 2003;24(9):644-649. https://doi.org/10.1086/502267
13. Vardakas KZ, Trigkidis KK, Boukouvala E, Falagas ME. Clostridium difficile infection following systemic antibiotic administration in randomised controlled trials: a systematic review and meta-analysis. Int J Antimicrob Agents. 2016;48(1):1-10. https://doi.org/10.1016/j.ijantimicag.2016.03.008
14. Leatherman JW, Iber C, F Davies SF. Cavitation in bacteremic pneumococcal pneumonia. Causal role of mixed infection with anaerobic bacteria. Am Rev Respir Dis. 1984;129(2):317-321.
15. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581st
16. Kadowaki M, Demura Y, Mizuno S, et al. Reappraisal of clindamycin IV monotherapy for treatment of mild-to-moderate aspiration pneumonia in elderly patients. Chest. 2005;127(4):1276-1282. https://doi.org/10.1378/chest.127.4.1276
17. Perlino CA. Metronidazole vs clindamycin treatment of anaerobic pulmonary infection. Failure of metronidazole therapy. Arch Intern Med. 1981;141(11):1424-1427.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
An 88-year-old woman with a history of dementia presents to the emergency room with new-onset dyspnea following 2 days of a self-limited gastrointestinal illness associated with nausea, vomiting, and diarrhea. After noting a new supplemental oxygen requirement of 4 L and a temperature of 38.6 °C, the hospitalist’s exam finds an edentulous patient with bibasilar lung crackles and a nontender abdomen. Taking into account her elevated white blood cell count and chest radiograph with right greater than left bibasilar opacities, the admitting hospitalist diagnoses aspiration pneumonia (AP) and specifically selects an antibiotic regimen with anaerobic coverage.
BACKGROUND
Aspiration, the inhalation of oropharyngeal or gastric materials into the lung, takes one of the following three forms: (1) “microaspiration,” wherein a small number of virulent organisms from oropharynx gains entry into the alveoli, (2) “macroaspiration,” wherein a large volume of typically less virulent organisms gains entry into the airways, or (3) a combination of the two. Hospitalists may struggle to distinguish unwitnessed macroaspiration causing AP from other typical causes of pneumonia, such as community-acquired pneumonia (CAP) or hospital-acquired pneumonia (HAP).1 A hospitalist should suspect macroaspiration—the most common cause of AP—in patients with risk factors such as dysphagia, diminished cough reflex or impaired swallowing, and infiltrates in the dependent bronchopulmonary segments, or of course, in cases of witnessed aspiration.2
Moreover, hospitalists must differentiate AP, an infectious entity, from aspiration pneumonitis, a noninfectious entity caused by macroaspiration of mostly sterile gastric content. Aspiration pneumonitis presents with acute lung injury within hours of an aspiration event, whereas AP entails a gradual onset of symptoms and signs of pneumonia.2 Although aspiration pneumonitis can present dramatically with hypoxemia and pulmonary edema and may evolve into AP, patients do not initially benefit from empiric antibiotics.1
WHY YOU MIGHT THINK SPECIFIC ANAEROBIC COVERAGE IS ESSENTIAL
In the 1970s, several studies of patients who were presumed to have AP because of risk factors for macroaspiration, such as alcohol use disorder, illicit drug use, and seizure disorder, identified anaerobes as major etiologic pathogens. These studies reported the presence of putrid sputum and obtained samples through invasive methods (eg, transtracheal aspirates, thoracentesis, and blood cultures).3,4 Many of the patients studied had radiographic findings of pleuropulmonary disease. For example, in the study by Bartlett et al, 70% of patients had radiographic evidence of abscess or pulmonary necrosis. These findings led to the assumption that anaerobes play a significant role in all cases of aspiration-related pulmonary syndromes. Because anaerobic bacteria live in the gingival sulcus, with an especially high burden in dental plaques, their role as a potential pathogen in AP may seem logical.5 Given the backdrop of those concerns, Kioka et al found that providers treated 90% of presumed AP patients in the intensive care unit with antibiotics that have anaerobic activity despite only 30% meeting the criteria for anaerobic coverage.6
WHY ANAEROBIC COVERAGE IS NOT ROUTINELY NECESSARY
In contrast to the population of patients with AP described from the 1970s, we now diagnose AP more frequently in nursing home residents, the elderly with cognitive impairment, and those with tube feed dependence, dysphagia, or gastrointestinal motility disorders.1 Concurrent with this change in the epidemiology of AP, we have witnessed a shift in recovered bacteria from anaerobes to aerobes in recent studies.7,8 In an intensive care unit study from 1999, respiratory tract organisms of patients with suspected aspiration mirrored those of patients with CAP or HAP.9 In a systematic review of eight observational studies that included studies from 1993 to 2014 and involved elderly patients with uncomplicated AP, only two out of eight studies demonstrated the presence of anaerobes in respiratory cultures. Even in those two studies, anaerobic bacteria frequently coexisted with aerobes. The majority of organisms in all eight studies consisted of aerobic gram-positives, gram-negatives, or both.10
A study by El-Solh et al most frequently isolated pathogenic aerobic gram-negative bacteria (49% of cases), followed by anaerobic bacteria (16%), among institutionalized elderly patients with severe AP diagnosed by clinical features. In that same study, most anaerobes coexisted with aerobic gram-negative bacteria, and the clinical illness promptly resolved in the absence of specific anaerobic coverage.11 AP can be successfully treated without anaerobic coverage due to a variety of factors: the insignificant role of anaerobes in the pathogenesis of uncomplicated AP, lower severity of illness in the absence of abscesses or pulmonary necrosis (uncomplicated), and altered local redox-potential from the elimination of aerobic pathogens, which effectively also treats anaerobes.1 Moreover, anaerobes possess generally less virulence in comparison with aerobes. AP from these organisms typically requires risk for excessive oral growth (eg, periodontal disease) and macroaspiration of a large number of organisms.5
There are also potential harms associated with the unnecessary treatment of anaerobic bacteria. Since anaerobes account for the majority of the bacteria present in the bowel, targeting anaerobes can result in gut dysbiosis.1 Moreover, a prospective study showed an increase in the incidence of vancomycin-resistant enterococci and antibiotic-resistant gram-negative bacteria associated with the empiric use of antibiotics with anaerobic activity.12 Finally, a systematic review detailed the high incidence of Clostridioides difficile infections among patients receiving clindamycin and carbapenems.13
WHEN ANAEROBIC COVERAGE IS INDICATED
Despite the predominance of aerobic organisms in the respiratory tract specimens of patients diagnosed with AP in the current era, situations still exist that require treatment of anaerobes. These include necrotizing pneumonia, empyema, or lung abscess.2 Additionally, patients with severe periodontal disease may harbor anaerobic bacteria such as Bacteroides species, Peptostreptococcus species, and Actinomyces israelii.5 When we suspect macroaspiration leading to AP, patients with severe periodontal disease may benefit from anaerobic coverage. Putrid sputum generation may indicate the presence of anaerobic organisms that produce the characteristic foul odor of short-chain volatile fatty acids observed in patients with lung abscess or empyema.2 It often takes about 8 to 14 days after an aspiration event for lung cavitation or empyema to develop.14 Therefore, a longer duration of illness or putrid sputum production may signal a significant concurrent burden of anaerobes. The 2019 official guidelines of the American Thoracic Society and Infectious Disease Society of America recommend adding anaerobic coverage to CAP only when empyema or lung abscess is suspected (conditional recommendation, very low quality of evidence).15
WHAT YOU SHOULD DO INSTEAD
When you suspect AP in a patient, categorize it as either community or hospital acquired based on risk factors similar to CAP or HAP. For patients with witnessed macroaspiration or in patients with substantial macroaspiration risk factors, perform a radiologic evaluation and a thorough oral examination to evaluate for poor dentition, gingival disease (marked redness, tendency to bleed, ulceration), and tongue coating. For patients presenting from the community with suspected AP without complications, treat with the standard therapy (without additional anaerobic coverage) for CAP. Provide empiric anaerobic coverage for complicated AP (eg, lung abscess, necrosis, or empyema) or for macroaspiration in the setting of severe periodontal disease, putrid sputum, or longer duration of illness. Similarly, treat hospital-acquired AP as HAP (Figure).

When prescribing anaerobic coverage of AP, use combination drugs that include a ß-lactamase inhibitor (eg, ampicillin-sulbactam), clindamycin (either alone or in combination with ß-lactams), or moxifloxacin.1 Most anaerobes have ß-lactamase or cephalosporinase activity, which renders penicillin and cephalosporins ineffective. Despite its potential side effects, such as C difficile infection, treating with clindamycin has the benefit of a relatively low cost and its association with lower rates of methicillin-resistant Staphylococcus aureus emergence after treatment.16 Piperacillin-tazobactam and carbapenems also have excellent anaerobic coverage, but we should reserve them for more severe and complicated cases of AP given their extensive antibacterial activity and concern for the emergence of resistance.8 Although well known and used for decades for its activity against clinically important anaerobes, avoid metronidazole due to its reduced cure rate in lung abscess caused by microaerophilic streptococci of the oral cavity.17 Due to a lack of evidence, we do not recommend the use of metronidazole in lung infections.
RECOMMENDATIONS
- Empirically treat most suspected cases of AP with regimens similar to the standard antibiotics for CAP and HAP. In the absence of specific risk factors for anaerobic infections, do not routinely provide anaerobic coverage.
- Provide anaerobic coverage empirically for AP associated with macroaspiration in the setting of severe periodontal disease, putrid sputum, or longer duration of illness.
- Provide anaerobic coverage in AP with evidence of necrotizing pneumonia, empyema, or lung abscess.
CONCLUSION
Current evidence does not support routine anaerobic coverage of AP in the absence of identifiable risk factors for an anaerobic lung infection.
In consideration of the clinical case, importantly, she has no periodontal disease and no evidence for necrotizing pneumonia, empyema, or lung abscess radiographically. For these reasons, select an empiric antibiotic regime that targets CAP organisms predominantly and forgo additional anaerobic coverage.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason ™ ”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason ™ ” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
The authors have no conflicts of interest relevant to this article.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
An 88-year-old woman with a history of dementia presents to the emergency room with new-onset dyspnea following 2 days of a self-limited gastrointestinal illness associated with nausea, vomiting, and diarrhea. After noting a new supplemental oxygen requirement of 4 L and a temperature of 38.6 °C, the hospitalist’s exam finds an edentulous patient with bibasilar lung crackles and a nontender abdomen. Taking into account her elevated white blood cell count and chest radiograph with right greater than left bibasilar opacities, the admitting hospitalist diagnoses aspiration pneumonia (AP) and specifically selects an antibiotic regimen with anaerobic coverage.
BACKGROUND
Aspiration, the inhalation of oropharyngeal or gastric materials into the lung, takes one of the following three forms: (1) “microaspiration,” wherein a small number of virulent organisms from oropharynx gains entry into the alveoli, (2) “macroaspiration,” wherein a large volume of typically less virulent organisms gains entry into the airways, or (3) a combination of the two. Hospitalists may struggle to distinguish unwitnessed macroaspiration causing AP from other typical causes of pneumonia, such as community-acquired pneumonia (CAP) or hospital-acquired pneumonia (HAP).1 A hospitalist should suspect macroaspiration—the most common cause of AP—in patients with risk factors such as dysphagia, diminished cough reflex or impaired swallowing, and infiltrates in the dependent bronchopulmonary segments, or of course, in cases of witnessed aspiration.2
Moreover, hospitalists must differentiate AP, an infectious entity, from aspiration pneumonitis, a noninfectious entity caused by macroaspiration of mostly sterile gastric content. Aspiration pneumonitis presents with acute lung injury within hours of an aspiration event, whereas AP entails a gradual onset of symptoms and signs of pneumonia.2 Although aspiration pneumonitis can present dramatically with hypoxemia and pulmonary edema and may evolve into AP, patients do not initially benefit from empiric antibiotics.1
WHY YOU MIGHT THINK SPECIFIC ANAEROBIC COVERAGE IS ESSENTIAL
In the 1970s, several studies of patients who were presumed to have AP because of risk factors for macroaspiration, such as alcohol use disorder, illicit drug use, and seizure disorder, identified anaerobes as major etiologic pathogens. These studies reported the presence of putrid sputum and obtained samples through invasive methods (eg, transtracheal aspirates, thoracentesis, and blood cultures).3,4 Many of the patients studied had radiographic findings of pleuropulmonary disease. For example, in the study by Bartlett et al, 70% of patients had radiographic evidence of abscess or pulmonary necrosis. These findings led to the assumption that anaerobes play a significant role in all cases of aspiration-related pulmonary syndromes. Because anaerobic bacteria live in the gingival sulcus, with an especially high burden in dental plaques, their role as a potential pathogen in AP may seem logical.5 Given the backdrop of those concerns, Kioka et al found that providers treated 90% of presumed AP patients in the intensive care unit with antibiotics that have anaerobic activity despite only 30% meeting the criteria for anaerobic coverage.6
WHY ANAEROBIC COVERAGE IS NOT ROUTINELY NECESSARY
In contrast to the population of patients with AP described from the 1970s, we now diagnose AP more frequently in nursing home residents, the elderly with cognitive impairment, and those with tube feed dependence, dysphagia, or gastrointestinal motility disorders.1 Concurrent with this change in the epidemiology of AP, we have witnessed a shift in recovered bacteria from anaerobes to aerobes in recent studies.7,8 In an intensive care unit study from 1999, respiratory tract organisms of patients with suspected aspiration mirrored those of patients with CAP or HAP.9 In a systematic review of eight observational studies that included studies from 1993 to 2014 and involved elderly patients with uncomplicated AP, only two out of eight studies demonstrated the presence of anaerobes in respiratory cultures. Even in those two studies, anaerobic bacteria frequently coexisted with aerobes. The majority of organisms in all eight studies consisted of aerobic gram-positives, gram-negatives, or both.10
A study by El-Solh et al most frequently isolated pathogenic aerobic gram-negative bacteria (49% of cases), followed by anaerobic bacteria (16%), among institutionalized elderly patients with severe AP diagnosed by clinical features. In that same study, most anaerobes coexisted with aerobic gram-negative bacteria, and the clinical illness promptly resolved in the absence of specific anaerobic coverage.11 AP can be successfully treated without anaerobic coverage due to a variety of factors: the insignificant role of anaerobes in the pathogenesis of uncomplicated AP, lower severity of illness in the absence of abscesses or pulmonary necrosis (uncomplicated), and altered local redox-potential from the elimination of aerobic pathogens, which effectively also treats anaerobes.1 Moreover, anaerobes possess generally less virulence in comparison with aerobes. AP from these organisms typically requires risk for excessive oral growth (eg, periodontal disease) and macroaspiration of a large number of organisms.5
There are also potential harms associated with the unnecessary treatment of anaerobic bacteria. Since anaerobes account for the majority of the bacteria present in the bowel, targeting anaerobes can result in gut dysbiosis.1 Moreover, a prospective study showed an increase in the incidence of vancomycin-resistant enterococci and antibiotic-resistant gram-negative bacteria associated with the empiric use of antibiotics with anaerobic activity.12 Finally, a systematic review detailed the high incidence of Clostridioides difficile infections among patients receiving clindamycin and carbapenems.13
WHEN ANAEROBIC COVERAGE IS INDICATED
Despite the predominance of aerobic organisms in the respiratory tract specimens of patients diagnosed with AP in the current era, situations still exist that require treatment of anaerobes. These include necrotizing pneumonia, empyema, or lung abscess.2 Additionally, patients with severe periodontal disease may harbor anaerobic bacteria such as Bacteroides species, Peptostreptococcus species, and Actinomyces israelii.5 When we suspect macroaspiration leading to AP, patients with severe periodontal disease may benefit from anaerobic coverage. Putrid sputum generation may indicate the presence of anaerobic organisms that produce the characteristic foul odor of short-chain volatile fatty acids observed in patients with lung abscess or empyema.2 It often takes about 8 to 14 days after an aspiration event for lung cavitation or empyema to develop.14 Therefore, a longer duration of illness or putrid sputum production may signal a significant concurrent burden of anaerobes. The 2019 official guidelines of the American Thoracic Society and Infectious Disease Society of America recommend adding anaerobic coverage to CAP only when empyema or lung abscess is suspected (conditional recommendation, very low quality of evidence).15
WHAT YOU SHOULD DO INSTEAD
When you suspect AP in a patient, categorize it as either community or hospital acquired based on risk factors similar to CAP or HAP. For patients with witnessed macroaspiration or in patients with substantial macroaspiration risk factors, perform a radiologic evaluation and a thorough oral examination to evaluate for poor dentition, gingival disease (marked redness, tendency to bleed, ulceration), and tongue coating. For patients presenting from the community with suspected AP without complications, treat with the standard therapy (without additional anaerobic coverage) for CAP. Provide empiric anaerobic coverage for complicated AP (eg, lung abscess, necrosis, or empyema) or for macroaspiration in the setting of severe periodontal disease, putrid sputum, or longer duration of illness. Similarly, treat hospital-acquired AP as HAP (Figure).

When prescribing anaerobic coverage of AP, use combination drugs that include a ß-lactamase inhibitor (eg, ampicillin-sulbactam), clindamycin (either alone or in combination with ß-lactams), or moxifloxacin.1 Most anaerobes have ß-lactamase or cephalosporinase activity, which renders penicillin and cephalosporins ineffective. Despite its potential side effects, such as C difficile infection, treating with clindamycin has the benefit of a relatively low cost and its association with lower rates of methicillin-resistant Staphylococcus aureus emergence after treatment.16 Piperacillin-tazobactam and carbapenems also have excellent anaerobic coverage, but we should reserve them for more severe and complicated cases of AP given their extensive antibacterial activity and concern for the emergence of resistance.8 Although well known and used for decades for its activity against clinically important anaerobes, avoid metronidazole due to its reduced cure rate in lung abscess caused by microaerophilic streptococci of the oral cavity.17 Due to a lack of evidence, we do not recommend the use of metronidazole in lung infections.
RECOMMENDATIONS
- Empirically treat most suspected cases of AP with regimens similar to the standard antibiotics for CAP and HAP. In the absence of specific risk factors for anaerobic infections, do not routinely provide anaerobic coverage.
- Provide anaerobic coverage empirically for AP associated with macroaspiration in the setting of severe periodontal disease, putrid sputum, or longer duration of illness.
- Provide anaerobic coverage in AP with evidence of necrotizing pneumonia, empyema, or lung abscess.
CONCLUSION
Current evidence does not support routine anaerobic coverage of AP in the absence of identifiable risk factors for an anaerobic lung infection.
In consideration of the clinical case, importantly, she has no periodontal disease and no evidence for necrotizing pneumonia, empyema, or lung abscess radiographically. For these reasons, select an empiric antibiotic regime that targets CAP organisms predominantly and forgo additional anaerobic coverage.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason ™ ”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason ™ ” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
The authors have no conflicts of interest relevant to this article.
1. Mandell LA, Niederman MS. Aspiration pneumonia. N Engl J Med. 2019;380(7):651-663. https://doi.org/10.1056/nejmra1714562
2. Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671. https://doi.org/10.1056/nejm200103013440908
3. Bartlett JG, Gorbach SL, Finegold SM. The bacteriology of aspiration pneumonia. Am J Med. 1974;56(2):202-207. https://doi.org/10.1016/0002-9343(74)90598-1
4. Bartlett JG, Gorbach SL. The triple threat of aspiration pneumonia. Chest. 1975;68(4):560-566. https://doi.org/10.1378/chest.68.4.560
5. Sutter VL. Anaerobes as normal oral flora. Rev Infect Dis. 1984;6(suppl 1):S62-S66. https://doi.org/10.1093/clinids/6.supplement_1.s62
6. Kioka MJ, DiGiovine B, Rezik M, Jennings JH. Anaerobic antibiotic usage for pneumonia in the medical intensive care unit. Respirology. 2017;22(8):1656-1661. https://doi.org/10.1111/resp.13111
7. Ott SR, Allewelt M, Lorenz J, Reimnitz P, Lode H; German Lung Abscess Study Group. Moxifloxacin vs ampicillin/sulbactam in aspiration pneumonia and primary lung abscess. Infection. 2008;36(1):23-30. https://doi.org/10.1007/s15010-007-7043-6
8. Tokuyasu H, Harada T, Watanabe E, et al. Effectiveness of meropenem for the treatment of aspiration pneumonia in elderly patients. Intern Med. 2009;48(3):129-135. https://doi.org/10.2169/internalmedicine.48.1308
9. Marik PE, Careau P. The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. Chest. 1999;115(1):178-183. https://doi.org/10.1378/chest.115.1.178
10. Bowerman TJ, Zhang J, Waite LM. Antibacterial treatment of aspiration pneumonia in older people: a systematic review. Clin Interv Aging. 2018;13:2201-2213. https://doi.org/10.2147/cia.s183344
11. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167(12):1650-1654. https://doi.org/10.1164/rccm.200212-1543oc
12. Bhalla A, Pultz NJ, Ray AJ, Hoyen CK, Eckstein EC, Donskey CJ. Antianaerobic antibiotic therapy promotes overgrowth of antibiotic-resistant, gram-negative bacilli and vancomycin-resistant enterococci in the stool of colonized patients. Infect Control Hosp Epidemiol. 2003;24(9):644-649. https://doi.org/10.1086/502267
13. Vardakas KZ, Trigkidis KK, Boukouvala E, Falagas ME. Clostridium difficile infection following systemic antibiotic administration in randomised controlled trials: a systematic review and meta-analysis. Int J Antimicrob Agents. 2016;48(1):1-10. https://doi.org/10.1016/j.ijantimicag.2016.03.008
14. Leatherman JW, Iber C, F Davies SF. Cavitation in bacteremic pneumococcal pneumonia. Causal role of mixed infection with anaerobic bacteria. Am Rev Respir Dis. 1984;129(2):317-321.
15. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581st
16. Kadowaki M, Demura Y, Mizuno S, et al. Reappraisal of clindamycin IV monotherapy for treatment of mild-to-moderate aspiration pneumonia in elderly patients. Chest. 2005;127(4):1276-1282. https://doi.org/10.1378/chest.127.4.1276
17. Perlino CA. Metronidazole vs clindamycin treatment of anaerobic pulmonary infection. Failure of metronidazole therapy. Arch Intern Med. 1981;141(11):1424-1427.
1. Mandell LA, Niederman MS. Aspiration pneumonia. N Engl J Med. 2019;380(7):651-663. https://doi.org/10.1056/nejmra1714562
2. Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671. https://doi.org/10.1056/nejm200103013440908
3. Bartlett JG, Gorbach SL, Finegold SM. The bacteriology of aspiration pneumonia. Am J Med. 1974;56(2):202-207. https://doi.org/10.1016/0002-9343(74)90598-1
4. Bartlett JG, Gorbach SL. The triple threat of aspiration pneumonia. Chest. 1975;68(4):560-566. https://doi.org/10.1378/chest.68.4.560
5. Sutter VL. Anaerobes as normal oral flora. Rev Infect Dis. 1984;6(suppl 1):S62-S66. https://doi.org/10.1093/clinids/6.supplement_1.s62
6. Kioka MJ, DiGiovine B, Rezik M, Jennings JH. Anaerobic antibiotic usage for pneumonia in the medical intensive care unit. Respirology. 2017;22(8):1656-1661. https://doi.org/10.1111/resp.13111
7. Ott SR, Allewelt M, Lorenz J, Reimnitz P, Lode H; German Lung Abscess Study Group. Moxifloxacin vs ampicillin/sulbactam in aspiration pneumonia and primary lung abscess. Infection. 2008;36(1):23-30. https://doi.org/10.1007/s15010-007-7043-6
8. Tokuyasu H, Harada T, Watanabe E, et al. Effectiveness of meropenem for the treatment of aspiration pneumonia in elderly patients. Intern Med. 2009;48(3):129-135. https://doi.org/10.2169/internalmedicine.48.1308
9. Marik PE, Careau P. The role of anaerobes in patients with ventilator-associated pneumonia and aspiration pneumonia: a prospective study. Chest. 1999;115(1):178-183. https://doi.org/10.1378/chest.115.1.178
10. Bowerman TJ, Zhang J, Waite LM. Antibacterial treatment of aspiration pneumonia in older people: a systematic review. Clin Interv Aging. 2018;13:2201-2213. https://doi.org/10.2147/cia.s183344
11. El-Solh AA, Pietrantoni C, Bhat A, et al. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am J Respir Crit Care Med. 2003;167(12):1650-1654. https://doi.org/10.1164/rccm.200212-1543oc
12. Bhalla A, Pultz NJ, Ray AJ, Hoyen CK, Eckstein EC, Donskey CJ. Antianaerobic antibiotic therapy promotes overgrowth of antibiotic-resistant, gram-negative bacilli and vancomycin-resistant enterococci in the stool of colonized patients. Infect Control Hosp Epidemiol. 2003;24(9):644-649. https://doi.org/10.1086/502267
13. Vardakas KZ, Trigkidis KK, Boukouvala E, Falagas ME. Clostridium difficile infection following systemic antibiotic administration in randomised controlled trials: a systematic review and meta-analysis. Int J Antimicrob Agents. 2016;48(1):1-10. https://doi.org/10.1016/j.ijantimicag.2016.03.008
14. Leatherman JW, Iber C, F Davies SF. Cavitation in bacteremic pneumococcal pneumonia. Causal role of mixed infection with anaerobic bacteria. Am Rev Respir Dis. 1984;129(2):317-321.
15. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45-e67. https://doi.org/10.1164/rccm.201908-1581st
16. Kadowaki M, Demura Y, Mizuno S, et al. Reappraisal of clindamycin IV monotherapy for treatment of mild-to-moderate aspiration pneumonia in elderly patients. Chest. 2005;127(4):1276-1282. https://doi.org/10.1378/chest.127.4.1276
17. Perlino CA. Metronidazole vs clindamycin treatment of anaerobic pulmonary infection. Failure of metronidazole therapy. Arch Intern Med. 1981;141(11):1424-1427.
© 2020 Society of Hospital Medicine
Things We Do For No Reason™: Treatment of Infection-Related Fever in Hospitalized Patients

CLINICAL SCENARIO
The hospitalist admitted a 56-year-old man with hypertension and hyperlipidemia to the general medical unit for community-acquired pneumonia and started him on appropriate antimicrobial therapy. On the evening of admission, the nurse woke the patient to take his vital signs and noted a fever of 39.1°C (102.4°F). The patient had a pulse of 90 beats per minute, normal blood pressure, and a stable supplemental oxygen requirement via nasal cannula. The nurse noted an oral acetaminophen “as needed” order for fever. She woke the patient again to administer acetaminophen and notified the hospitalist.
BACKGROUND
Hospitalists frequently encounter febrile patients. According to one large hospital survey, fever occurs in 25% of pediatric and 31% of adult medical patients.1 Fever in hospitalized patients most commonly results from infection, but autoimmune disease, malignancy, and an array of other inflammatory conditions cause fevers as well.1
Defined as an elevated body temperature resulting from a raised hypothalamic set point2, hospitalists often treat fever with acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs). These routinely administered medications act centrally to temporarily lower the hypothalamic set point and relieve fever.2,3 Standard hospital admission order sets commonly include an as-needed antipyretic every 4 to 6 hours for treatment of fever, regardless of the presence of fever-related symptoms.
Fever is differentiated from hyperthermia, where temperature increases because of dysregulated peripheral processes despite a normal hypothalamic set point.2 Examples of hyperthermia include heat stroke, malignant hyperthermia, and neuroleptic malignant syndrome. Notably, antipyretic medications have no effect on hyperthermia, but physical means, such as cooling blankets, can lead to temperature reduction.2
WHY YOU MIGHT THINK TREATMENT OF INFECTION-RELATED FEVER IS HELPFUL IN HOSPITALIZED PATIENTS
Hospitalists prescribe antipyretic medication to alleviate fever-related symptoms, including headache, chills and sweats, and joint and muscle aches.3 While researchers have sparingly studied this practice, available evidence and experience suggest that fever-related symptoms decline in parallel with defervescence after administration of acetaminophen or NSAIDs in both adult and pediatric populations.4,5 One randomized, controlled, double-blind study of nearly 400 adult outpatients in Germany with febrile upper respiratory tract infections showed that both aspirin and acetaminophen bested the placebo in reducing fever and associated headache, achiness, and discomfort over a span of 6 hours.4 In another study, this time with pediatric patients hospitalized with fever and uncomplicated respiratory tract infections, patients who received acetaminophen had statistically significant improvements in activity, alertness, mood, comfort, appetite, and fluid intake 6 hours after receiving that therapy.5
Physicians, nurses, and caregivers also commonly believe that fever is inherently noxious and that treatment of infection-related fever contributes to fighting the infection itself.2,3,6 The pediatric literature describes parents, caretakers, and clinicians who suffer from “fever phobia,” the worry that fevers contribute to long-term neurologic complications, recurrent febrile seizures, and death.6,7
Finally, healthcare providers administer antipyretic medication to mitigate the demand fever places on the cardiovascular and pulmonary systems.3 An elevated temperature increases the body’s metabolic rate, oxygen consumption, and cardiac output that critically ill patients who have acute and/or chronic compromise to those systems may not tolerate. For example, patients requiring pressor support for hemodynamic shock or mechanical ventilation for respiratory failure may not tolerate an elevated temperature.8
WHY THERE IS NO REASON TO TREAT INFECTION-RELATED FEVER IN ASYMPTOMATIC HOSPITALIZED PATIENTS
Fever serves as an adaptive host response to infection, boosting innate and adaptive immunity in a multitude of ways.8 In animal models, fever slows the replication of pathogenic bacteria and enhances the activity of antibiotic agents.8 In vitro studies demonstrate that fever increases mobility of leukocytes, phagocytic activity, and proliferation of T cells.8 Retrospective case-control studies of patients hospitalized with severe bacterial illnesses, including gram-negative bacteremia, spontaneous bacterial peritonitis, and community-acquired pneumonia, found that patients with a documented febrile response had increased survival compared with those who remained afebrile during the infection.9 In addition, a large retrospective cohort study of septic ICU patients found a progressive decline in mortality in association with increasing peak temperature on the day of ICU admission.10
In addition to the above studies supporting the important role of fever in fighting infection, recent evidence definitively demonstrates no mortality or morbidity benefit of using antipyretic medications in infected patients. A 2017 meta-analysis that included eight observational and eight randomized studies, totaling 18,939 adult septic ICU patients, demonstrated no difference in hospital and 28-day mortality in patients treated with antipyretics vs those who were not.11 The authors again found no mortality benefit with antipyretic use when separately analyzing data from only the randomized controlled trials (1,507 patients) or when stratifying patients based on the type of antipyretic received (acetaminophen, NSAIDs, or physical cooling).11 They reported no differences in predefined secondary outcomes of shock reversal or nosocomial infections. The authors commented that these robust results likely would not change even with more data from additional trials. In children, a recent meta-analysis of three randomized controlled trials (540 patients) did not find the use of acetaminophen, ibuprofen, or diclofenac effective in preventing febrile seizures.12Pediatric practice guidelines consistently recommend using antipyretic medication to alleviate discomfort caused by fever and not solely to reduce temperature.13,14
Antipyretic agents interfere with the effectiveness of the body’s immune response, as demonstrated in a number of infectious diseases.2,15-18 Two randomized controlled studies conducted in healthy adult volunteers challenged with rhinovirus reported increased viral shedding and decreased antibody response in those subjects who received aspirin or acetaminophen, compared with those given placebo.15,16 In another randomized controlled trial conducted in African children with malaria, paracetamol use delayed parasite clearance by 16 hours.17 A large case-control study correlated the use of NSAIDs with an increased risk of severe skin and soft-tissue complications in children with varicella and in adults with varicella zoster. 18 The international scientific community has raised concerns about worse outcomes with NSAID use in patients with COVID-19, the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); NSAIDs should be avoided in stable patients with COVID-19 until more data are available. 19
Additional risks and potential harms accompany antipyretic fever therapy. First, NSAIDs or acetaminophen may adversely affect patients with renal or hepatic insufficiency.2,3 Second, masking fevers may impair the clinician’s ability to diagnose or evaluate response to treatment. Third, unnecessarily waking a sleeping patient to check temperature or administer unneeded antipyretics can contribute to hospital-associated problems, including delirium, insomnia, and falls. Treating these iatrogenic problems in turn may require additional medications or interventions. These unintended consequences may potentially prolong hospital stays, increase medication errors and polypharmacy, and detract from a patient’s overall healing and recovery.
While the use of antipyretic medications improves fever-related symptoms, it comes at the cost of blunting a protective host response and exposes patients to medication risks without providing a clinical benefit. In sleeping, asymptomatic, or minimally symptomatic hospitalized patients, the risks of administering antipyretic medications clearly outweigh the benefits.
WHEN TREATING FEVER IS INDICATED
Treatment with antipyretic medication can alleviate fever-related symptoms in those patients who have significant headache, body aches, chills, or sweats and in pediatric patients with notable malaise, irritability, or poor oral intake. Debate continues on the use of antipyretics in the ICU setting when managing critically ill patients with severe cardiopulmonary compromise who may not tolerate the additional hemodynamic strain a fever produces (eg, patients with shock requiring vasopressor support or respiratory failure requiring mechanical ventilation). Remember, decrease body temperature in hyperthermia syndromes by physical means.
WHAT WE SHOULD DO INSTEAD
Withhold antipyretic medication (ie, allow permissive fever) in well-appearing general medical patients with asymptomatic infection-related fevers. In patients who tolerate fever with minimal or no symptoms, potential benefits of permissive fever include decreased time to infection resolution and/or decreased risk of hospital-acquired infections. This may result in shorter hospital stays and significant cost savings. If we do not treat patients with asymptomatic fevers, then it follows that we should not check overnight temperatures in hospitalized patients sleeping comfortably.
RECOMMENDATIONS
- Do not order as-needed antipyretic medication for stable patients on general medical units with infection solely to reduce temperature or achieve normothermia.
- Only treat infected febrile patients with antipyretic medications for fever-related symptoms (headache, chills, or body aches or, in pediatric patients, irritability, malaise, or poor oral intake).
- Treat pathologically elevated temperatures (ie, hyperthermia syndromes) with physical measures because antipyretic medications will be ineffective.
CONCLUSIONS
In the clinical scenario, the hospitalist admitted the patient in stable condition for treatment of a community-acquired pneumonia. He mounted a febrile response to infection, which suggests that his active immune system may aid in recovery. The nurse noted the fever while the patient slept comfortably without fever-related symptoms.
After discussing these facts with the patient’s concerned nurse, the clinician should discontinue the order for as-needed acetaminophen for fever and instead recommend permissive fever without administration of antipyretic medication. This may facilitate recovery, avoid unnecessary polypharmacy, and allow the medical care team to follow his fever curve to ensure that the infection is adequately treated. If the patient develops bothersome fever-related symptoms, the hospitalist can reasonably treat with a single-dose of acetaminophen or NSAID.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. McGowan JE Jr, Rose RC, Jacobs NF, Schaberg DR, Haley RW. Fever in hospitalized patients, with special reference to the medical service. Am J Med. 1987;82(3):580-586. https://doi.org/10.1016/0002-9343(87)90103-3.
2. Plaisance K, Mackowiak P. Antipyretic therapy. Arch Intern Med. 2000;160:449-456. https://doi.org/10.1001/archinte.160.4.449.
3. Greisman LA, Mackowiak PA. Fever: beneficial and detrimental effects of antipyretics. Curr Opin Infect Dis. 2002;15:241-245. https://doi.org/10.1097/00001432-200206000-00005.
4. Bachert C, Chuchalin AG, Eisebitt R, Netayzhenko VZ, Voelker M. Aspirin compared with acetaminophen in the treatment of fever and other symptoms of upper respiratory tract infection in adults: a multicenter, randomized, double-blind, double-dummy, placebo-controlled, parallel-group, single-dose, 6-hour dose-ranging study. Clin Ther. 2005;27(7):993-1003. https://doi.org/10.1016/j.clinthera.2005.06.002.
5. Gupta H, Shah D, Gupta P, Sharma KK. Role of paracetamol in treatment of childhood fever: a double-blind randomized placebo controlled trial. Indian Pediatr. 2007;44:903-911.
6. Schmitt BD. Fever phobia: misconceptions of parents about fevers. Am J Dis Child. 1980;134(2):176-181.
7. Karwowska A, Nijssen-Jordan C, Johnson D, Davies HD. Parental and health care provider understanding of childhood fever: a Canadian perspective. CJEM. 2002;4(6):394-400. https://doi.org/10.1017/s1481803500007892.
8. Kiekkas P, Aretha D, Bakalis N, Karpouhtsi I, Marneras C, Baltopoulos GI. Fever effects and treatment in critical care: literature review. Aust Crit Care. 2013;26:130-135. https://doi.org/10.1016/j.aucc.2012.10.004.
9. Hasday JD, Fairchild KD, Shanholtz C. The role of fever in the infected host. Microbes Infect. 2000;2(15):1891-1894. https://doi.org/10.1016/s1286-4579(00)01337-x.
10. Young PJ, Saxena M, Beasley R, et al. Early peak temperature and mortality in critically ill patients with or without infection. Intensive Care Med. 2012;38:437-444. https://doi.org/10.1007/s00134-012-2478-3.
11. Drewry A, Ablordeppey E, Murray E, et al. Antipyretic therapy in critically ill septic patients: a systematic review and meta-analysis. Crit Care Med. 2017;45(5):806-813. https://doi.org/10.1097/CCM.0000000000002285.
12. Rosenbloom E, Finkelstein Y, Adams-Webber T, Kozer E. Do antipyretics prevent the recurrence of febrile seizures in children? a systematic review of randomized controlled trials and meta-analysis. Eur J Paediatr Neuro. 2013;17:585-588. https://doi.org/10.1016/j.ejpn.2013.04.008.
13. Chiappini J, Venturini E, Remaschi G. 2016 Update of the Italian Pediatric Society Guidelines for management of fever in children. J Pediatr. 2017;180:177-183. https://doi.org/10.1016/j.jpeds.2016.09.043.
14. Fields E, Chard J, Murphy MS, Richardson M, Guideline Development Group and Technical Team. Assessment and initial management of feverish illness in children younger than five years: summary of updated NICE guidance. BMJ. 2013;346:f2866. https://doi.org/10.1136/bmj.f2866.
15. Stanley ED, Jackson GG, Panusarn C, Rubenis M, Dirda V. Increased viral shedding with aspirin treatment of rhinovirus infection. JAMA. 1975;231:1248-1251. https://doi.org/10.1001/jama.1975.03240240018017.
16. Graham NM, Burrell CJ, Douglas RM, Debelle P, Davies L. Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus-infected volunteers. J Infect Dis. 1990;162:1277-1282. https://doi.org/10.1093/infdis/162.6.1277.
17. Brandts CH, Ndjave M, Graninger W, Kremsner PG. Effect of paracetamol on parasite clearance time in Plasmodium falciparum malaria. Lancet. 1997;350:704-709. https://doi.org/10.1016/S0140-6736(97)02255-1.
18. Mikaeloff Y, Kezouh A, Suissa S. Nonsteroidal anti-inflammatory drug use and the risk of severe skin and soft tissue complications in patients with varicella or zoster disease. Br J Clin Pharmacol. 2007;65:2:203-209. https://doi.org/10.1016/S0140-6736(97)02255-1.
19. Day M. COVID-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368:m1086. https://doi.org/10.1136/bmj.m1086.

CLINICAL SCENARIO
The hospitalist admitted a 56-year-old man with hypertension and hyperlipidemia to the general medical unit for community-acquired pneumonia and started him on appropriate antimicrobial therapy. On the evening of admission, the nurse woke the patient to take his vital signs and noted a fever of 39.1°C (102.4°F). The patient had a pulse of 90 beats per minute, normal blood pressure, and a stable supplemental oxygen requirement via nasal cannula. The nurse noted an oral acetaminophen “as needed” order for fever. She woke the patient again to administer acetaminophen and notified the hospitalist.
BACKGROUND
Hospitalists frequently encounter febrile patients. According to one large hospital survey, fever occurs in 25% of pediatric and 31% of adult medical patients.1 Fever in hospitalized patients most commonly results from infection, but autoimmune disease, malignancy, and an array of other inflammatory conditions cause fevers as well.1
Defined as an elevated body temperature resulting from a raised hypothalamic set point2, hospitalists often treat fever with acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs). These routinely administered medications act centrally to temporarily lower the hypothalamic set point and relieve fever.2,3 Standard hospital admission order sets commonly include an as-needed antipyretic every 4 to 6 hours for treatment of fever, regardless of the presence of fever-related symptoms.
Fever is differentiated from hyperthermia, where temperature increases because of dysregulated peripheral processes despite a normal hypothalamic set point.2 Examples of hyperthermia include heat stroke, malignant hyperthermia, and neuroleptic malignant syndrome. Notably, antipyretic medications have no effect on hyperthermia, but physical means, such as cooling blankets, can lead to temperature reduction.2
WHY YOU MIGHT THINK TREATMENT OF INFECTION-RELATED FEVER IS HELPFUL IN HOSPITALIZED PATIENTS
Hospitalists prescribe antipyretic medication to alleviate fever-related symptoms, including headache, chills and sweats, and joint and muscle aches.3 While researchers have sparingly studied this practice, available evidence and experience suggest that fever-related symptoms decline in parallel with defervescence after administration of acetaminophen or NSAIDs in both adult and pediatric populations.4,5 One randomized, controlled, double-blind study of nearly 400 adult outpatients in Germany with febrile upper respiratory tract infections showed that both aspirin and acetaminophen bested the placebo in reducing fever and associated headache, achiness, and discomfort over a span of 6 hours.4 In another study, this time with pediatric patients hospitalized with fever and uncomplicated respiratory tract infections, patients who received acetaminophen had statistically significant improvements in activity, alertness, mood, comfort, appetite, and fluid intake 6 hours after receiving that therapy.5
Physicians, nurses, and caregivers also commonly believe that fever is inherently noxious and that treatment of infection-related fever contributes to fighting the infection itself.2,3,6 The pediatric literature describes parents, caretakers, and clinicians who suffer from “fever phobia,” the worry that fevers contribute to long-term neurologic complications, recurrent febrile seizures, and death.6,7
Finally, healthcare providers administer antipyretic medication to mitigate the demand fever places on the cardiovascular and pulmonary systems.3 An elevated temperature increases the body’s metabolic rate, oxygen consumption, and cardiac output that critically ill patients who have acute and/or chronic compromise to those systems may not tolerate. For example, patients requiring pressor support for hemodynamic shock or mechanical ventilation for respiratory failure may not tolerate an elevated temperature.8
WHY THERE IS NO REASON TO TREAT INFECTION-RELATED FEVER IN ASYMPTOMATIC HOSPITALIZED PATIENTS
Fever serves as an adaptive host response to infection, boosting innate and adaptive immunity in a multitude of ways.8 In animal models, fever slows the replication of pathogenic bacteria and enhances the activity of antibiotic agents.8 In vitro studies demonstrate that fever increases mobility of leukocytes, phagocytic activity, and proliferation of T cells.8 Retrospective case-control studies of patients hospitalized with severe bacterial illnesses, including gram-negative bacteremia, spontaneous bacterial peritonitis, and community-acquired pneumonia, found that patients with a documented febrile response had increased survival compared with those who remained afebrile during the infection.9 In addition, a large retrospective cohort study of septic ICU patients found a progressive decline in mortality in association with increasing peak temperature on the day of ICU admission.10
In addition to the above studies supporting the important role of fever in fighting infection, recent evidence definitively demonstrates no mortality or morbidity benefit of using antipyretic medications in infected patients. A 2017 meta-analysis that included eight observational and eight randomized studies, totaling 18,939 adult septic ICU patients, demonstrated no difference in hospital and 28-day mortality in patients treated with antipyretics vs those who were not.11 The authors again found no mortality benefit with antipyretic use when separately analyzing data from only the randomized controlled trials (1,507 patients) or when stratifying patients based on the type of antipyretic received (acetaminophen, NSAIDs, or physical cooling).11 They reported no differences in predefined secondary outcomes of shock reversal or nosocomial infections. The authors commented that these robust results likely would not change even with more data from additional trials. In children, a recent meta-analysis of three randomized controlled trials (540 patients) did not find the use of acetaminophen, ibuprofen, or diclofenac effective in preventing febrile seizures.12Pediatric practice guidelines consistently recommend using antipyretic medication to alleviate discomfort caused by fever and not solely to reduce temperature.13,14
Antipyretic agents interfere with the effectiveness of the body’s immune response, as demonstrated in a number of infectious diseases.2,15-18 Two randomized controlled studies conducted in healthy adult volunteers challenged with rhinovirus reported increased viral shedding and decreased antibody response in those subjects who received aspirin or acetaminophen, compared with those given placebo.15,16 In another randomized controlled trial conducted in African children with malaria, paracetamol use delayed parasite clearance by 16 hours.17 A large case-control study correlated the use of NSAIDs with an increased risk of severe skin and soft-tissue complications in children with varicella and in adults with varicella zoster. 18 The international scientific community has raised concerns about worse outcomes with NSAID use in patients with COVID-19, the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); NSAIDs should be avoided in stable patients with COVID-19 until more data are available. 19
Additional risks and potential harms accompany antipyretic fever therapy. First, NSAIDs or acetaminophen may adversely affect patients with renal or hepatic insufficiency.2,3 Second, masking fevers may impair the clinician’s ability to diagnose or evaluate response to treatment. Third, unnecessarily waking a sleeping patient to check temperature or administer unneeded antipyretics can contribute to hospital-associated problems, including delirium, insomnia, and falls. Treating these iatrogenic problems in turn may require additional medications or interventions. These unintended consequences may potentially prolong hospital stays, increase medication errors and polypharmacy, and detract from a patient’s overall healing and recovery.
While the use of antipyretic medications improves fever-related symptoms, it comes at the cost of blunting a protective host response and exposes patients to medication risks without providing a clinical benefit. In sleeping, asymptomatic, or minimally symptomatic hospitalized patients, the risks of administering antipyretic medications clearly outweigh the benefits.
WHEN TREATING FEVER IS INDICATED
Treatment with antipyretic medication can alleviate fever-related symptoms in those patients who have significant headache, body aches, chills, or sweats and in pediatric patients with notable malaise, irritability, or poor oral intake. Debate continues on the use of antipyretics in the ICU setting when managing critically ill patients with severe cardiopulmonary compromise who may not tolerate the additional hemodynamic strain a fever produces (eg, patients with shock requiring vasopressor support or respiratory failure requiring mechanical ventilation). Remember, decrease body temperature in hyperthermia syndromes by physical means.
WHAT WE SHOULD DO INSTEAD
Withhold antipyretic medication (ie, allow permissive fever) in well-appearing general medical patients with asymptomatic infection-related fevers. In patients who tolerate fever with minimal or no symptoms, potential benefits of permissive fever include decreased time to infection resolution and/or decreased risk of hospital-acquired infections. This may result in shorter hospital stays and significant cost savings. If we do not treat patients with asymptomatic fevers, then it follows that we should not check overnight temperatures in hospitalized patients sleeping comfortably.
RECOMMENDATIONS
- Do not order as-needed antipyretic medication for stable patients on general medical units with infection solely to reduce temperature or achieve normothermia.
- Only treat infected febrile patients with antipyretic medications for fever-related symptoms (headache, chills, or body aches or, in pediatric patients, irritability, malaise, or poor oral intake).
- Treat pathologically elevated temperatures (ie, hyperthermia syndromes) with physical measures because antipyretic medications will be ineffective.
CONCLUSIONS
In the clinical scenario, the hospitalist admitted the patient in stable condition for treatment of a community-acquired pneumonia. He mounted a febrile response to infection, which suggests that his active immune system may aid in recovery. The nurse noted the fever while the patient slept comfortably without fever-related symptoms.
After discussing these facts with the patient’s concerned nurse, the clinician should discontinue the order for as-needed acetaminophen for fever and instead recommend permissive fever without administration of antipyretic medication. This may facilitate recovery, avoid unnecessary polypharmacy, and allow the medical care team to follow his fever curve to ensure that the infection is adequately treated. If the patient develops bothersome fever-related symptoms, the hospitalist can reasonably treat with a single-dose of acetaminophen or NSAID.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.

CLINICAL SCENARIO
The hospitalist admitted a 56-year-old man with hypertension and hyperlipidemia to the general medical unit for community-acquired pneumonia and started him on appropriate antimicrobial therapy. On the evening of admission, the nurse woke the patient to take his vital signs and noted a fever of 39.1°C (102.4°F). The patient had a pulse of 90 beats per minute, normal blood pressure, and a stable supplemental oxygen requirement via nasal cannula. The nurse noted an oral acetaminophen “as needed” order for fever. She woke the patient again to administer acetaminophen and notified the hospitalist.
BACKGROUND
Hospitalists frequently encounter febrile patients. According to one large hospital survey, fever occurs in 25% of pediatric and 31% of adult medical patients.1 Fever in hospitalized patients most commonly results from infection, but autoimmune disease, malignancy, and an array of other inflammatory conditions cause fevers as well.1
Defined as an elevated body temperature resulting from a raised hypothalamic set point2, hospitalists often treat fever with acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs). These routinely administered medications act centrally to temporarily lower the hypothalamic set point and relieve fever.2,3 Standard hospital admission order sets commonly include an as-needed antipyretic every 4 to 6 hours for treatment of fever, regardless of the presence of fever-related symptoms.
Fever is differentiated from hyperthermia, where temperature increases because of dysregulated peripheral processes despite a normal hypothalamic set point.2 Examples of hyperthermia include heat stroke, malignant hyperthermia, and neuroleptic malignant syndrome. Notably, antipyretic medications have no effect on hyperthermia, but physical means, such as cooling blankets, can lead to temperature reduction.2
WHY YOU MIGHT THINK TREATMENT OF INFECTION-RELATED FEVER IS HELPFUL IN HOSPITALIZED PATIENTS
Hospitalists prescribe antipyretic medication to alleviate fever-related symptoms, including headache, chills and sweats, and joint and muscle aches.3 While researchers have sparingly studied this practice, available evidence and experience suggest that fever-related symptoms decline in parallel with defervescence after administration of acetaminophen or NSAIDs in both adult and pediatric populations.4,5 One randomized, controlled, double-blind study of nearly 400 adult outpatients in Germany with febrile upper respiratory tract infections showed that both aspirin and acetaminophen bested the placebo in reducing fever and associated headache, achiness, and discomfort over a span of 6 hours.4 In another study, this time with pediatric patients hospitalized with fever and uncomplicated respiratory tract infections, patients who received acetaminophen had statistically significant improvements in activity, alertness, mood, comfort, appetite, and fluid intake 6 hours after receiving that therapy.5
Physicians, nurses, and caregivers also commonly believe that fever is inherently noxious and that treatment of infection-related fever contributes to fighting the infection itself.2,3,6 The pediatric literature describes parents, caretakers, and clinicians who suffer from “fever phobia,” the worry that fevers contribute to long-term neurologic complications, recurrent febrile seizures, and death.6,7
Finally, healthcare providers administer antipyretic medication to mitigate the demand fever places on the cardiovascular and pulmonary systems.3 An elevated temperature increases the body’s metabolic rate, oxygen consumption, and cardiac output that critically ill patients who have acute and/or chronic compromise to those systems may not tolerate. For example, patients requiring pressor support for hemodynamic shock or mechanical ventilation for respiratory failure may not tolerate an elevated temperature.8
WHY THERE IS NO REASON TO TREAT INFECTION-RELATED FEVER IN ASYMPTOMATIC HOSPITALIZED PATIENTS
Fever serves as an adaptive host response to infection, boosting innate and adaptive immunity in a multitude of ways.8 In animal models, fever slows the replication of pathogenic bacteria and enhances the activity of antibiotic agents.8 In vitro studies demonstrate that fever increases mobility of leukocytes, phagocytic activity, and proliferation of T cells.8 Retrospective case-control studies of patients hospitalized with severe bacterial illnesses, including gram-negative bacteremia, spontaneous bacterial peritonitis, and community-acquired pneumonia, found that patients with a documented febrile response had increased survival compared with those who remained afebrile during the infection.9 In addition, a large retrospective cohort study of septic ICU patients found a progressive decline in mortality in association with increasing peak temperature on the day of ICU admission.10
In addition to the above studies supporting the important role of fever in fighting infection, recent evidence definitively demonstrates no mortality or morbidity benefit of using antipyretic medications in infected patients. A 2017 meta-analysis that included eight observational and eight randomized studies, totaling 18,939 adult septic ICU patients, demonstrated no difference in hospital and 28-day mortality in patients treated with antipyretics vs those who were not.11 The authors again found no mortality benefit with antipyretic use when separately analyzing data from only the randomized controlled trials (1,507 patients) or when stratifying patients based on the type of antipyretic received (acetaminophen, NSAIDs, or physical cooling).11 They reported no differences in predefined secondary outcomes of shock reversal or nosocomial infections. The authors commented that these robust results likely would not change even with more data from additional trials. In children, a recent meta-analysis of three randomized controlled trials (540 patients) did not find the use of acetaminophen, ibuprofen, or diclofenac effective in preventing febrile seizures.12Pediatric practice guidelines consistently recommend using antipyretic medication to alleviate discomfort caused by fever and not solely to reduce temperature.13,14
Antipyretic agents interfere with the effectiveness of the body’s immune response, as demonstrated in a number of infectious diseases.2,15-18 Two randomized controlled studies conducted in healthy adult volunteers challenged with rhinovirus reported increased viral shedding and decreased antibody response in those subjects who received aspirin or acetaminophen, compared with those given placebo.15,16 In another randomized controlled trial conducted in African children with malaria, paracetamol use delayed parasite clearance by 16 hours.17 A large case-control study correlated the use of NSAIDs with an increased risk of severe skin and soft-tissue complications in children with varicella and in adults with varicella zoster. 18 The international scientific community has raised concerns about worse outcomes with NSAID use in patients with COVID-19, the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); NSAIDs should be avoided in stable patients with COVID-19 until more data are available. 19
Additional risks and potential harms accompany antipyretic fever therapy. First, NSAIDs or acetaminophen may adversely affect patients with renal or hepatic insufficiency.2,3 Second, masking fevers may impair the clinician’s ability to diagnose or evaluate response to treatment. Third, unnecessarily waking a sleeping patient to check temperature or administer unneeded antipyretics can contribute to hospital-associated problems, including delirium, insomnia, and falls. Treating these iatrogenic problems in turn may require additional medications or interventions. These unintended consequences may potentially prolong hospital stays, increase medication errors and polypharmacy, and detract from a patient’s overall healing and recovery.
While the use of antipyretic medications improves fever-related symptoms, it comes at the cost of blunting a protective host response and exposes patients to medication risks without providing a clinical benefit. In sleeping, asymptomatic, or minimally symptomatic hospitalized patients, the risks of administering antipyretic medications clearly outweigh the benefits.
WHEN TREATING FEVER IS INDICATED
Treatment with antipyretic medication can alleviate fever-related symptoms in those patients who have significant headache, body aches, chills, or sweats and in pediatric patients with notable malaise, irritability, or poor oral intake. Debate continues on the use of antipyretics in the ICU setting when managing critically ill patients with severe cardiopulmonary compromise who may not tolerate the additional hemodynamic strain a fever produces (eg, patients with shock requiring vasopressor support or respiratory failure requiring mechanical ventilation). Remember, decrease body temperature in hyperthermia syndromes by physical means.
WHAT WE SHOULD DO INSTEAD
Withhold antipyretic medication (ie, allow permissive fever) in well-appearing general medical patients with asymptomatic infection-related fevers. In patients who tolerate fever with minimal or no symptoms, potential benefits of permissive fever include decreased time to infection resolution and/or decreased risk of hospital-acquired infections. This may result in shorter hospital stays and significant cost savings. If we do not treat patients with asymptomatic fevers, then it follows that we should not check overnight temperatures in hospitalized patients sleeping comfortably.
RECOMMENDATIONS
- Do not order as-needed antipyretic medication for stable patients on general medical units with infection solely to reduce temperature or achieve normothermia.
- Only treat infected febrile patients with antipyretic medications for fever-related symptoms (headache, chills, or body aches or, in pediatric patients, irritability, malaise, or poor oral intake).
- Treat pathologically elevated temperatures (ie, hyperthermia syndromes) with physical measures because antipyretic medications will be ineffective.
CONCLUSIONS
In the clinical scenario, the hospitalist admitted the patient in stable condition for treatment of a community-acquired pneumonia. He mounted a febrile response to infection, which suggests that his active immune system may aid in recovery. The nurse noted the fever while the patient slept comfortably without fever-related symptoms.
After discussing these facts with the patient’s concerned nurse, the clinician should discontinue the order for as-needed acetaminophen for fever and instead recommend permissive fever without administration of antipyretic medication. This may facilitate recovery, avoid unnecessary polypharmacy, and allow the medical care team to follow his fever curve to ensure that the infection is adequately treated. If the patient develops bothersome fever-related symptoms, the hospitalist can reasonably treat with a single-dose of acetaminophen or NSAID.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. McGowan JE Jr, Rose RC, Jacobs NF, Schaberg DR, Haley RW. Fever in hospitalized patients, with special reference to the medical service. Am J Med. 1987;82(3):580-586. https://doi.org/10.1016/0002-9343(87)90103-3.
2. Plaisance K, Mackowiak P. Antipyretic therapy. Arch Intern Med. 2000;160:449-456. https://doi.org/10.1001/archinte.160.4.449.
3. Greisman LA, Mackowiak PA. Fever: beneficial and detrimental effects of antipyretics. Curr Opin Infect Dis. 2002;15:241-245. https://doi.org/10.1097/00001432-200206000-00005.
4. Bachert C, Chuchalin AG, Eisebitt R, Netayzhenko VZ, Voelker M. Aspirin compared with acetaminophen in the treatment of fever and other symptoms of upper respiratory tract infection in adults: a multicenter, randomized, double-blind, double-dummy, placebo-controlled, parallel-group, single-dose, 6-hour dose-ranging study. Clin Ther. 2005;27(7):993-1003. https://doi.org/10.1016/j.clinthera.2005.06.002.
5. Gupta H, Shah D, Gupta P, Sharma KK. Role of paracetamol in treatment of childhood fever: a double-blind randomized placebo controlled trial. Indian Pediatr. 2007;44:903-911.
6. Schmitt BD. Fever phobia: misconceptions of parents about fevers. Am J Dis Child. 1980;134(2):176-181.
7. Karwowska A, Nijssen-Jordan C, Johnson D, Davies HD. Parental and health care provider understanding of childhood fever: a Canadian perspective. CJEM. 2002;4(6):394-400. https://doi.org/10.1017/s1481803500007892.
8. Kiekkas P, Aretha D, Bakalis N, Karpouhtsi I, Marneras C, Baltopoulos GI. Fever effects and treatment in critical care: literature review. Aust Crit Care. 2013;26:130-135. https://doi.org/10.1016/j.aucc.2012.10.004.
9. Hasday JD, Fairchild KD, Shanholtz C. The role of fever in the infected host. Microbes Infect. 2000;2(15):1891-1894. https://doi.org/10.1016/s1286-4579(00)01337-x.
10. Young PJ, Saxena M, Beasley R, et al. Early peak temperature and mortality in critically ill patients with or without infection. Intensive Care Med. 2012;38:437-444. https://doi.org/10.1007/s00134-012-2478-3.
11. Drewry A, Ablordeppey E, Murray E, et al. Antipyretic therapy in critically ill septic patients: a systematic review and meta-analysis. Crit Care Med. 2017;45(5):806-813. https://doi.org/10.1097/CCM.0000000000002285.
12. Rosenbloom E, Finkelstein Y, Adams-Webber T, Kozer E. Do antipyretics prevent the recurrence of febrile seizures in children? a systematic review of randomized controlled trials and meta-analysis. Eur J Paediatr Neuro. 2013;17:585-588. https://doi.org/10.1016/j.ejpn.2013.04.008.
13. Chiappini J, Venturini E, Remaschi G. 2016 Update of the Italian Pediatric Society Guidelines for management of fever in children. J Pediatr. 2017;180:177-183. https://doi.org/10.1016/j.jpeds.2016.09.043.
14. Fields E, Chard J, Murphy MS, Richardson M, Guideline Development Group and Technical Team. Assessment and initial management of feverish illness in children younger than five years: summary of updated NICE guidance. BMJ. 2013;346:f2866. https://doi.org/10.1136/bmj.f2866.
15. Stanley ED, Jackson GG, Panusarn C, Rubenis M, Dirda V. Increased viral shedding with aspirin treatment of rhinovirus infection. JAMA. 1975;231:1248-1251. https://doi.org/10.1001/jama.1975.03240240018017.
16. Graham NM, Burrell CJ, Douglas RM, Debelle P, Davies L. Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus-infected volunteers. J Infect Dis. 1990;162:1277-1282. https://doi.org/10.1093/infdis/162.6.1277.
17. Brandts CH, Ndjave M, Graninger W, Kremsner PG. Effect of paracetamol on parasite clearance time in Plasmodium falciparum malaria. Lancet. 1997;350:704-709. https://doi.org/10.1016/S0140-6736(97)02255-1.
18. Mikaeloff Y, Kezouh A, Suissa S. Nonsteroidal anti-inflammatory drug use and the risk of severe skin and soft tissue complications in patients with varicella or zoster disease. Br J Clin Pharmacol. 2007;65:2:203-209. https://doi.org/10.1016/S0140-6736(97)02255-1.
19. Day M. COVID-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368:m1086. https://doi.org/10.1136/bmj.m1086.
1. McGowan JE Jr, Rose RC, Jacobs NF, Schaberg DR, Haley RW. Fever in hospitalized patients, with special reference to the medical service. Am J Med. 1987;82(3):580-586. https://doi.org/10.1016/0002-9343(87)90103-3.
2. Plaisance K, Mackowiak P. Antipyretic therapy. Arch Intern Med. 2000;160:449-456. https://doi.org/10.1001/archinte.160.4.449.
3. Greisman LA, Mackowiak PA. Fever: beneficial and detrimental effects of antipyretics. Curr Opin Infect Dis. 2002;15:241-245. https://doi.org/10.1097/00001432-200206000-00005.
4. Bachert C, Chuchalin AG, Eisebitt R, Netayzhenko VZ, Voelker M. Aspirin compared with acetaminophen in the treatment of fever and other symptoms of upper respiratory tract infection in adults: a multicenter, randomized, double-blind, double-dummy, placebo-controlled, parallel-group, single-dose, 6-hour dose-ranging study. Clin Ther. 2005;27(7):993-1003. https://doi.org/10.1016/j.clinthera.2005.06.002.
5. Gupta H, Shah D, Gupta P, Sharma KK. Role of paracetamol in treatment of childhood fever: a double-blind randomized placebo controlled trial. Indian Pediatr. 2007;44:903-911.
6. Schmitt BD. Fever phobia: misconceptions of parents about fevers. Am J Dis Child. 1980;134(2):176-181.
7. Karwowska A, Nijssen-Jordan C, Johnson D, Davies HD. Parental and health care provider understanding of childhood fever: a Canadian perspective. CJEM. 2002;4(6):394-400. https://doi.org/10.1017/s1481803500007892.
8. Kiekkas P, Aretha D, Bakalis N, Karpouhtsi I, Marneras C, Baltopoulos GI. Fever effects and treatment in critical care: literature review. Aust Crit Care. 2013;26:130-135. https://doi.org/10.1016/j.aucc.2012.10.004.
9. Hasday JD, Fairchild KD, Shanholtz C. The role of fever in the infected host. Microbes Infect. 2000;2(15):1891-1894. https://doi.org/10.1016/s1286-4579(00)01337-x.
10. Young PJ, Saxena M, Beasley R, et al. Early peak temperature and mortality in critically ill patients with or without infection. Intensive Care Med. 2012;38:437-444. https://doi.org/10.1007/s00134-012-2478-3.
11. Drewry A, Ablordeppey E, Murray E, et al. Antipyretic therapy in critically ill septic patients: a systematic review and meta-analysis. Crit Care Med. 2017;45(5):806-813. https://doi.org/10.1097/CCM.0000000000002285.
12. Rosenbloom E, Finkelstein Y, Adams-Webber T, Kozer E. Do antipyretics prevent the recurrence of febrile seizures in children? a systematic review of randomized controlled trials and meta-analysis. Eur J Paediatr Neuro. 2013;17:585-588. https://doi.org/10.1016/j.ejpn.2013.04.008.
13. Chiappini J, Venturini E, Remaschi G. 2016 Update of the Italian Pediatric Society Guidelines for management of fever in children. J Pediatr. 2017;180:177-183. https://doi.org/10.1016/j.jpeds.2016.09.043.
14. Fields E, Chard J, Murphy MS, Richardson M, Guideline Development Group and Technical Team. Assessment and initial management of feverish illness in children younger than five years: summary of updated NICE guidance. BMJ. 2013;346:f2866. https://doi.org/10.1136/bmj.f2866.
15. Stanley ED, Jackson GG, Panusarn C, Rubenis M, Dirda V. Increased viral shedding with aspirin treatment of rhinovirus infection. JAMA. 1975;231:1248-1251. https://doi.org/10.1001/jama.1975.03240240018017.
16. Graham NM, Burrell CJ, Douglas RM, Debelle P, Davies L. Adverse effects of aspirin, acetaminophen, and ibuprofen on immune function, viral shedding, and clinical status in rhinovirus-infected volunteers. J Infect Dis. 1990;162:1277-1282. https://doi.org/10.1093/infdis/162.6.1277.
17. Brandts CH, Ndjave M, Graninger W, Kremsner PG. Effect of paracetamol on parasite clearance time in Plasmodium falciparum malaria. Lancet. 1997;350:704-709. https://doi.org/10.1016/S0140-6736(97)02255-1.
18. Mikaeloff Y, Kezouh A, Suissa S. Nonsteroidal anti-inflammatory drug use and the risk of severe skin and soft tissue complications in patients with varicella or zoster disease. Br J Clin Pharmacol. 2007;65:2:203-209. https://doi.org/10.1016/S0140-6736(97)02255-1.
19. Day M. COVID-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368:m1086. https://doi.org/10.1136/bmj.m1086.
© 2020 Society of Hospital Medicine
Things We Do For No Reason™: Routine Overnight Vital Sign Checks

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™”(TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 73-year-old man with non–insulin dependent diabetes and essential hypertension to the general medicine ward for lower extremity cellulitis. The hospitalist uses standard admission orders, encourages him to elevate his leg above his heart, starts intravenous antibiotics, and monitors him throughout the day and night with regular vital signs. On his second day of admission, the patient’s cellulitis clinically improves, and the team prepares for discharge. However, the nurse notes that the patient did not sleep well and has not slept since his 4
WHY YOU MIGHT THINK Q4 VITAL SIGNS OVERNIGHT ARE HELPFUL
General medicine floors commonly default frequency for measuring vital signs to every 4 hours (Q4), a practice that dates back more than a century to the time of Florence Nightingale.This custom remains in place to ensure the ability to identify and intervene for those at risk for clinical deterioration and preventable death. Research supports the notion that frequent and consistent vital sign checks can minimize mortality and morbidity in the hospital. In fact, validated scoring systems incorporate vital signs with other clinical findings as a way of quickly identifying a patient with worsening clinical status.1 Further, trends and trajectories in vital signs may enable us to identify those with impending decompensation.2 A 2008 consensus statement made by experts in patient safety encouraged hospitals to use frequent vital sign monitoring of patients when available and affordable.3 These interventions aim to help identify and treat patients with early clinical deterioration to prevent poor outcomes.
WHY Q4 VITAL SIGNS OVERNIGHT MIGHT NOT BE NECESSARY
The practice of checking vital signs every 4 hours throughout the night dates to long before the modern era of evidence-based medicine. Research thus far has not focused on the necessity of vital sign checks every 4 hours throughout the night, despite affecting almost every hospitalized patient. Further, patient acuity or need for monitoring does not drive the frequency of overnight vital signs; instead habit and defaults do. We often monitor patients at high risk for clinical deterioration just as frequently as patients at low risk.4
While evidence-based medicine influences much of clinical care, “real-world” needs encountered at the bedside often drive early adapters to innovate. Nurses, who spend the most time at the bedside and conduct the most regular patient assessments, have recognized that not all patients need vital signs checked every 4 hours throughout the night. In 2013, Hands et al conducted a chart review of hospital patterns and found that nurses obtained complete vital sign checks on patients less frequently throughout the night than during the day.5 Their work further showed that nurses used their clinical judgment to make decisions about risk: Those patients deemed low risk by the nurses received fewer vital sign checks while the sicker patients received monitoring every 4 hours throughout the night.
Few researchers have quantitatively identified reasons why nurses may choose to not conduct frequent observations for some patients, beyond the providers’ own experience and judgment. In one study, Hope et al conducted a qualitative analysis of nurses to better understand their reasoning behind who should and should not receive overnight monitoring.6 The results of the analysis revealed that nurses recognize the importance of sleep in support of health and healing and use their clinical judgement when deciding which patients and conditions can forgo frequent observations.Stiver et al conducted trailblazing work that examines the outcomes of decreasing overnight vital sign checks for low-risk hospitalized patients through a randomized pilot study.7 In order to ensure patient safety, their group employed regular nurse observations throughout the night without waking the patient. Those patients assigned to less monitoring overnight reported a trend toward better sleep during hospitalization without the occurrence of any adverse events or escalation in care.
Most important, evidence indicates that sleep disruptions in the hospital worsen health and impede healing; further supporting nurses’ instincts and practices. Hospitalized adults without comorbidities who experience inadequate sleep during hospitalization have a higher perception of pain.8 Similarly, research has associated hospital-induced sleep deprivation and a higher odds of elevated blood glucose in those without diabetes, or “hyperglycemia of hospitalization.” 9 Furthermore, national organizations have recognized the importance of sleep. The American Academy of Nursing, as part of its Choosing Wisely™ campaign, states that, in the hospital, nurses should not disturb a patient’s sleep “unless the patient’s condition or care specifically requires it.”10
Finally, in the era of COVID-19, any opportunity to support physical distancing and to limit face-to-face interaction could protect our patients and staff from acquiring SARS-CoV-2.
WHAT WE SHOULD DO INSTEAD
While consistent vital sign checks allow for early identification of those trending toward clinical deterioration, risk stratification of ward patients can identify those who may benefit from overnight Q4 vital sign checks. While clinicians often use their judgment to identify a subset of low-risk patients for de-escalation of overnight care, artificial intelligence such as Modified Early Warning Score (MEWS) and Pediatric Early Warning Signs (PEWS) may have a role to play. These validated systems use physiologic symptoms that present prior to significant vital sign alterations to identify patients at risk for clinical deterioration.11 As an example, one randomized, controlled trial used a risk stratification tool to eliminate overnight monitoring for low-risk patients. Patients slept more soundly and reported fewer noise disruptions and higher satisfaction with the nursing staff. No adverse events were reported for those who were electronically stratified as low risk.12Further, forcing clinicians to decide on the need for overnight vitals by removing the Q4 vital sign default in the electronic health records (EHR) may minimize overnight disruptions. The University of Chicago in Illinois has implemented “sleep-friendly” options for vital sign ordering in the EHR for both children and adults. Enhanced order sets force providers to consider whether patients qualify for fewer overnight interventions. This change, alongside staff education and empowerment, reduced interruptions overnight for both populations and improved patient experience.13 This patient-centered practice mirrors a recent recommendation from the American Academy of Nursing to minimize sleep disruptions for hospitalized patients by letting low-risk patients sleep.10
RECOMMENDATIONS
- Use clinical judgment or an existing risk stratification system, such as MEWS or PEWS, to identify patients who may benefit from more or less monitoring.
- Forgo overnight vital sign checks for low-risk patients.
- Check overnight vitals for low-risk patients at 10
pm and 6am. - Use pulse oximetry or regular nurse checks as a balancing measure, especially in the pediatric population.
CONCLUSION
Minimizing unnecessary sleep disruptors for hospitalized patients is essential for healing and health. The patient in the clinical scenario had iatrogenic comorbidities added during his hospitalization and an increase in length of stay that resulted from sleep-associated delirium. Hospitalists should take the lead in developing sleep protocols that can leverage current technology to “nudge” clinicians to improve patient sleep. We can modify the frequency of checking vital signs for low-acuity patients and alter environmental factors that may impair sleep, such as noise, light, and temperature, for high-risk patients who cannot forgo overnight vital sign checks. In addition to clinical judgment, artificial intelligence can enable hospitalists and nurses to determine which patients may benefit least from overnight vital sign checks. Finally, if we stop disrupting low-risk patients’ sleep, we can better target resources to patients at high risk for clinical deterioration. Let’s start improving inpatient sleep by eliminating the disruptive things we do for no reason.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. Moon A, Cosgrove JF, Lea D, Fairs A, Cressey DM. An eight-year audit before and after the introduction of Modified Early Warning Score (MEWS) charts, of patients admitted to a tertiary referral intensive care unit after CPR. Resuscitation. 2011;82(2):150-154. https://doi.org/10.1016/j.resuscitation.2010.09.480.
2. Churpek MM, Adhikari R, Edelson DP. The value of vital sign trends for detecting clinical deterioration on the wards. Resuscitation 2016;102(5):1-5. https://doi.org/10.1016/j.resuscitation.2016.02.005.
3. DeVita MA, Smith GB, Adam SK, et al. ‘‘Identifying the hospitalized patient in crisis’’—a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375-382. https://doi.org/10.1016/j.resuscitation.2009.12.008.
4. Yoder JC, Yuen TC, Churpek MM, Arora VM, Edelson DP. A prospective study of nighttime vital sign monitoring frequency and risk of clinical deterioration. JAMA Intern Med. 2013;173(16):1554-1555. https://doi.org/10.1001/jamainternmed.2013.7791
5. Hands C, Reid E, Meredith P, et al. Patterns in the recording of vital sign and early warning scores: compliance with a clinical escalation protocol. BMJ Qual Saf. 2013;22(9):719-726. https://doi.org/10.1136/bmjqs-2013-001954
6. Hope J, Recio-Saucedo A, Fogg C, et al. A fundamental conflict of care: nurses’ accounts of balancing patients’ sleep with taking vital sign observations at night. J Clin Nurs. 2018;27:1860-1871. https://doi.org/10.1111/jocn.14234.
7. Stiver K, Sharma N, Geller K, Smith L, Stephens J. “Quiet at night”: reduced overnight vital sign monitoring linked to both safety and improvements in patients’ perception of hospital sleep quality. Patient Exp J. 2017;4(1):Article 10. https://doi.org/10.35680/2372-0247.1185.
8. Raymond I, Nielsen TA, Lavigne G, Manzini C, Choiniere M. Quality of sleep and its daily relationship to pain intensity in hospitalized adult burn patients. Pain. 2001;93(3):381-388. https://doi.org/10.1016/s0304-3959(01)00282-2.
9. DePietro RH, Knutson KL, Spampinato L, et al. Association between inpatient sleep loss and hyperglycemia of hospitalization. Diabetes Care. 2017;40(2):188-193. https://doi.org/10.2337/dc16-1683.
10. American Academy of Nursing. Choosing Wisely. Twenty-Five Things Nurses and Patients Should Question. July 2018. https://www.choosingwisely.org/wp-content/uploads/2015/02/AANursing-Choosing-Wisely-List.pdf.
11. van Galen LS, Dijkstra CC, Ludikhuize J, Kramer MHH, Nanayakkara PWB. A protocolised once a day Modified Early Warning Score (MEWS) measurement is an appropriate screening tool for major adverse events in a general hospital population. PLoS One. 2016;11(8):e0160811. https://doi.org/10.1371/journal.pone.0160811.
12. Edelson DP, Carey K, Twu NM, et al. Acuity-based nighttime vital sign assessments: a randomized controlled trial. Abstract presented at: Hospital Medicine 2019; March 24-27, 2019; National Harbor, Maryland. https://www.shmabstracts.com/abstract/acuity-based-nighttime-vital-sign-assessments-a-randomized-controlled-trial/. Accessed March 20, 2020
13. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™”(TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 73-year-old man with non–insulin dependent diabetes and essential hypertension to the general medicine ward for lower extremity cellulitis. The hospitalist uses standard admission orders, encourages him to elevate his leg above his heart, starts intravenous antibiotics, and monitors him throughout the day and night with regular vital signs. On his second day of admission, the patient’s cellulitis clinically improves, and the team prepares for discharge. However, the nurse notes that the patient did not sleep well and has not slept since his 4
WHY YOU MIGHT THINK Q4 VITAL SIGNS OVERNIGHT ARE HELPFUL
General medicine floors commonly default frequency for measuring vital signs to every 4 hours (Q4), a practice that dates back more than a century to the time of Florence Nightingale.This custom remains in place to ensure the ability to identify and intervene for those at risk for clinical deterioration and preventable death. Research supports the notion that frequent and consistent vital sign checks can minimize mortality and morbidity in the hospital. In fact, validated scoring systems incorporate vital signs with other clinical findings as a way of quickly identifying a patient with worsening clinical status.1 Further, trends and trajectories in vital signs may enable us to identify those with impending decompensation.2 A 2008 consensus statement made by experts in patient safety encouraged hospitals to use frequent vital sign monitoring of patients when available and affordable.3 These interventions aim to help identify and treat patients with early clinical deterioration to prevent poor outcomes.
WHY Q4 VITAL SIGNS OVERNIGHT MIGHT NOT BE NECESSARY
The practice of checking vital signs every 4 hours throughout the night dates to long before the modern era of evidence-based medicine. Research thus far has not focused on the necessity of vital sign checks every 4 hours throughout the night, despite affecting almost every hospitalized patient. Further, patient acuity or need for monitoring does not drive the frequency of overnight vital signs; instead habit and defaults do. We often monitor patients at high risk for clinical deterioration just as frequently as patients at low risk.4
While evidence-based medicine influences much of clinical care, “real-world” needs encountered at the bedside often drive early adapters to innovate. Nurses, who spend the most time at the bedside and conduct the most regular patient assessments, have recognized that not all patients need vital signs checked every 4 hours throughout the night. In 2013, Hands et al conducted a chart review of hospital patterns and found that nurses obtained complete vital sign checks on patients less frequently throughout the night than during the day.5 Their work further showed that nurses used their clinical judgment to make decisions about risk: Those patients deemed low risk by the nurses received fewer vital sign checks while the sicker patients received monitoring every 4 hours throughout the night.
Few researchers have quantitatively identified reasons why nurses may choose to not conduct frequent observations for some patients, beyond the providers’ own experience and judgment. In one study, Hope et al conducted a qualitative analysis of nurses to better understand their reasoning behind who should and should not receive overnight monitoring.6 The results of the analysis revealed that nurses recognize the importance of sleep in support of health and healing and use their clinical judgement when deciding which patients and conditions can forgo frequent observations.Stiver et al conducted trailblazing work that examines the outcomes of decreasing overnight vital sign checks for low-risk hospitalized patients through a randomized pilot study.7 In order to ensure patient safety, their group employed regular nurse observations throughout the night without waking the patient. Those patients assigned to less monitoring overnight reported a trend toward better sleep during hospitalization without the occurrence of any adverse events or escalation in care.
Most important, evidence indicates that sleep disruptions in the hospital worsen health and impede healing; further supporting nurses’ instincts and practices. Hospitalized adults without comorbidities who experience inadequate sleep during hospitalization have a higher perception of pain.8 Similarly, research has associated hospital-induced sleep deprivation and a higher odds of elevated blood glucose in those without diabetes, or “hyperglycemia of hospitalization.” 9 Furthermore, national organizations have recognized the importance of sleep. The American Academy of Nursing, as part of its Choosing Wisely™ campaign, states that, in the hospital, nurses should not disturb a patient’s sleep “unless the patient’s condition or care specifically requires it.”10
Finally, in the era of COVID-19, any opportunity to support physical distancing and to limit face-to-face interaction could protect our patients and staff from acquiring SARS-CoV-2.
WHAT WE SHOULD DO INSTEAD
While consistent vital sign checks allow for early identification of those trending toward clinical deterioration, risk stratification of ward patients can identify those who may benefit from overnight Q4 vital sign checks. While clinicians often use their judgment to identify a subset of low-risk patients for de-escalation of overnight care, artificial intelligence such as Modified Early Warning Score (MEWS) and Pediatric Early Warning Signs (PEWS) may have a role to play. These validated systems use physiologic symptoms that present prior to significant vital sign alterations to identify patients at risk for clinical deterioration.11 As an example, one randomized, controlled trial used a risk stratification tool to eliminate overnight monitoring for low-risk patients. Patients slept more soundly and reported fewer noise disruptions and higher satisfaction with the nursing staff. No adverse events were reported for those who were electronically stratified as low risk.12Further, forcing clinicians to decide on the need for overnight vitals by removing the Q4 vital sign default in the electronic health records (EHR) may minimize overnight disruptions. The University of Chicago in Illinois has implemented “sleep-friendly” options for vital sign ordering in the EHR for both children and adults. Enhanced order sets force providers to consider whether patients qualify for fewer overnight interventions. This change, alongside staff education and empowerment, reduced interruptions overnight for both populations and improved patient experience.13 This patient-centered practice mirrors a recent recommendation from the American Academy of Nursing to minimize sleep disruptions for hospitalized patients by letting low-risk patients sleep.10
RECOMMENDATIONS
- Use clinical judgment or an existing risk stratification system, such as MEWS or PEWS, to identify patients who may benefit from more or less monitoring.
- Forgo overnight vital sign checks for low-risk patients.
- Check overnight vitals for low-risk patients at 10
pm and 6am. - Use pulse oximetry or regular nurse checks as a balancing measure, especially in the pediatric population.
CONCLUSION
Minimizing unnecessary sleep disruptors for hospitalized patients is essential for healing and health. The patient in the clinical scenario had iatrogenic comorbidities added during his hospitalization and an increase in length of stay that resulted from sleep-associated delirium. Hospitalists should take the lead in developing sleep protocols that can leverage current technology to “nudge” clinicians to improve patient sleep. We can modify the frequency of checking vital signs for low-acuity patients and alter environmental factors that may impair sleep, such as noise, light, and temperature, for high-risk patients who cannot forgo overnight vital sign checks. In addition to clinical judgment, artificial intelligence can enable hospitalists and nurses to determine which patients may benefit least from overnight vital sign checks. Finally, if we stop disrupting low-risk patients’ sleep, we can better target resources to patients at high risk for clinical deterioration. Let’s start improving inpatient sleep by eliminating the disruptive things we do for no reason.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™”(TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
The hospitalist admits a 73-year-old man with non–insulin dependent diabetes and essential hypertension to the general medicine ward for lower extremity cellulitis. The hospitalist uses standard admission orders, encourages him to elevate his leg above his heart, starts intravenous antibiotics, and monitors him throughout the day and night with regular vital signs. On his second day of admission, the patient’s cellulitis clinically improves, and the team prepares for discharge. However, the nurse notes that the patient did not sleep well and has not slept since his 4
WHY YOU MIGHT THINK Q4 VITAL SIGNS OVERNIGHT ARE HELPFUL
General medicine floors commonly default frequency for measuring vital signs to every 4 hours (Q4), a practice that dates back more than a century to the time of Florence Nightingale.This custom remains in place to ensure the ability to identify and intervene for those at risk for clinical deterioration and preventable death. Research supports the notion that frequent and consistent vital sign checks can minimize mortality and morbidity in the hospital. In fact, validated scoring systems incorporate vital signs with other clinical findings as a way of quickly identifying a patient with worsening clinical status.1 Further, trends and trajectories in vital signs may enable us to identify those with impending decompensation.2 A 2008 consensus statement made by experts in patient safety encouraged hospitals to use frequent vital sign monitoring of patients when available and affordable.3 These interventions aim to help identify and treat patients with early clinical deterioration to prevent poor outcomes.
WHY Q4 VITAL SIGNS OVERNIGHT MIGHT NOT BE NECESSARY
The practice of checking vital signs every 4 hours throughout the night dates to long before the modern era of evidence-based medicine. Research thus far has not focused on the necessity of vital sign checks every 4 hours throughout the night, despite affecting almost every hospitalized patient. Further, patient acuity or need for monitoring does not drive the frequency of overnight vital signs; instead habit and defaults do. We often monitor patients at high risk for clinical deterioration just as frequently as patients at low risk.4
While evidence-based medicine influences much of clinical care, “real-world” needs encountered at the bedside often drive early adapters to innovate. Nurses, who spend the most time at the bedside and conduct the most regular patient assessments, have recognized that not all patients need vital signs checked every 4 hours throughout the night. In 2013, Hands et al conducted a chart review of hospital patterns and found that nurses obtained complete vital sign checks on patients less frequently throughout the night than during the day.5 Their work further showed that nurses used their clinical judgment to make decisions about risk: Those patients deemed low risk by the nurses received fewer vital sign checks while the sicker patients received monitoring every 4 hours throughout the night.
Few researchers have quantitatively identified reasons why nurses may choose to not conduct frequent observations for some patients, beyond the providers’ own experience and judgment. In one study, Hope et al conducted a qualitative analysis of nurses to better understand their reasoning behind who should and should not receive overnight monitoring.6 The results of the analysis revealed that nurses recognize the importance of sleep in support of health and healing and use their clinical judgement when deciding which patients and conditions can forgo frequent observations.Stiver et al conducted trailblazing work that examines the outcomes of decreasing overnight vital sign checks for low-risk hospitalized patients through a randomized pilot study.7 In order to ensure patient safety, their group employed regular nurse observations throughout the night without waking the patient. Those patients assigned to less monitoring overnight reported a trend toward better sleep during hospitalization without the occurrence of any adverse events or escalation in care.
Most important, evidence indicates that sleep disruptions in the hospital worsen health and impede healing; further supporting nurses’ instincts and practices. Hospitalized adults without comorbidities who experience inadequate sleep during hospitalization have a higher perception of pain.8 Similarly, research has associated hospital-induced sleep deprivation and a higher odds of elevated blood glucose in those without diabetes, or “hyperglycemia of hospitalization.” 9 Furthermore, national organizations have recognized the importance of sleep. The American Academy of Nursing, as part of its Choosing Wisely™ campaign, states that, in the hospital, nurses should not disturb a patient’s sleep “unless the patient’s condition or care specifically requires it.”10
Finally, in the era of COVID-19, any opportunity to support physical distancing and to limit face-to-face interaction could protect our patients and staff from acquiring SARS-CoV-2.
WHAT WE SHOULD DO INSTEAD
While consistent vital sign checks allow for early identification of those trending toward clinical deterioration, risk stratification of ward patients can identify those who may benefit from overnight Q4 vital sign checks. While clinicians often use their judgment to identify a subset of low-risk patients for de-escalation of overnight care, artificial intelligence such as Modified Early Warning Score (MEWS) and Pediatric Early Warning Signs (PEWS) may have a role to play. These validated systems use physiologic symptoms that present prior to significant vital sign alterations to identify patients at risk for clinical deterioration.11 As an example, one randomized, controlled trial used a risk stratification tool to eliminate overnight monitoring for low-risk patients. Patients slept more soundly and reported fewer noise disruptions and higher satisfaction with the nursing staff. No adverse events were reported for those who were electronically stratified as low risk.12Further, forcing clinicians to decide on the need for overnight vitals by removing the Q4 vital sign default in the electronic health records (EHR) may minimize overnight disruptions. The University of Chicago in Illinois has implemented “sleep-friendly” options for vital sign ordering in the EHR for both children and adults. Enhanced order sets force providers to consider whether patients qualify for fewer overnight interventions. This change, alongside staff education and empowerment, reduced interruptions overnight for both populations and improved patient experience.13 This patient-centered practice mirrors a recent recommendation from the American Academy of Nursing to minimize sleep disruptions for hospitalized patients by letting low-risk patients sleep.10
RECOMMENDATIONS
- Use clinical judgment or an existing risk stratification system, such as MEWS or PEWS, to identify patients who may benefit from more or less monitoring.
- Forgo overnight vital sign checks for low-risk patients.
- Check overnight vitals for low-risk patients at 10
pm and 6am. - Use pulse oximetry or regular nurse checks as a balancing measure, especially in the pediatric population.
CONCLUSION
Minimizing unnecessary sleep disruptors for hospitalized patients is essential for healing and health. The patient in the clinical scenario had iatrogenic comorbidities added during his hospitalization and an increase in length of stay that resulted from sleep-associated delirium. Hospitalists should take the lead in developing sleep protocols that can leverage current technology to “nudge” clinicians to improve patient sleep. We can modify the frequency of checking vital signs for low-acuity patients and alter environmental factors that may impair sleep, such as noise, light, and temperature, for high-risk patients who cannot forgo overnight vital sign checks. In addition to clinical judgment, artificial intelligence can enable hospitalists and nurses to determine which patients may benefit least from overnight vital sign checks. Finally, if we stop disrupting low-risk patients’ sleep, we can better target resources to patients at high risk for clinical deterioration. Let’s start improving inpatient sleep by eliminating the disruptive things we do for no reason.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
1. Moon A, Cosgrove JF, Lea D, Fairs A, Cressey DM. An eight-year audit before and after the introduction of Modified Early Warning Score (MEWS) charts, of patients admitted to a tertiary referral intensive care unit after CPR. Resuscitation. 2011;82(2):150-154. https://doi.org/10.1016/j.resuscitation.2010.09.480.
2. Churpek MM, Adhikari R, Edelson DP. The value of vital sign trends for detecting clinical deterioration on the wards. Resuscitation 2016;102(5):1-5. https://doi.org/10.1016/j.resuscitation.2016.02.005.
3. DeVita MA, Smith GB, Adam SK, et al. ‘‘Identifying the hospitalized patient in crisis’’—a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375-382. https://doi.org/10.1016/j.resuscitation.2009.12.008.
4. Yoder JC, Yuen TC, Churpek MM, Arora VM, Edelson DP. A prospective study of nighttime vital sign monitoring frequency and risk of clinical deterioration. JAMA Intern Med. 2013;173(16):1554-1555. https://doi.org/10.1001/jamainternmed.2013.7791
5. Hands C, Reid E, Meredith P, et al. Patterns in the recording of vital sign and early warning scores: compliance with a clinical escalation protocol. BMJ Qual Saf. 2013;22(9):719-726. https://doi.org/10.1136/bmjqs-2013-001954
6. Hope J, Recio-Saucedo A, Fogg C, et al. A fundamental conflict of care: nurses’ accounts of balancing patients’ sleep with taking vital sign observations at night. J Clin Nurs. 2018;27:1860-1871. https://doi.org/10.1111/jocn.14234.
7. Stiver K, Sharma N, Geller K, Smith L, Stephens J. “Quiet at night”: reduced overnight vital sign monitoring linked to both safety and improvements in patients’ perception of hospital sleep quality. Patient Exp J. 2017;4(1):Article 10. https://doi.org/10.35680/2372-0247.1185.
8. Raymond I, Nielsen TA, Lavigne G, Manzini C, Choiniere M. Quality of sleep and its daily relationship to pain intensity in hospitalized adult burn patients. Pain. 2001;93(3):381-388. https://doi.org/10.1016/s0304-3959(01)00282-2.
9. DePietro RH, Knutson KL, Spampinato L, et al. Association between inpatient sleep loss and hyperglycemia of hospitalization. Diabetes Care. 2017;40(2):188-193. https://doi.org/10.2337/dc16-1683.
10. American Academy of Nursing. Choosing Wisely. Twenty-Five Things Nurses and Patients Should Question. July 2018. https://www.choosingwisely.org/wp-content/uploads/2015/02/AANursing-Choosing-Wisely-List.pdf.
11. van Galen LS, Dijkstra CC, Ludikhuize J, Kramer MHH, Nanayakkara PWB. A protocolised once a day Modified Early Warning Score (MEWS) measurement is an appropriate screening tool for major adverse events in a general hospital population. PLoS One. 2016;11(8):e0160811. https://doi.org/10.1371/journal.pone.0160811.
12. Edelson DP, Carey K, Twu NM, et al. Acuity-based nighttime vital sign assessments: a randomized controlled trial. Abstract presented at: Hospital Medicine 2019; March 24-27, 2019; National Harbor, Maryland. https://www.shmabstracts.com/abstract/acuity-based-nighttime-vital-sign-assessments-a-randomized-controlled-trial/. Accessed March 20, 2020
13. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091.
1. Moon A, Cosgrove JF, Lea D, Fairs A, Cressey DM. An eight-year audit before and after the introduction of Modified Early Warning Score (MEWS) charts, of patients admitted to a tertiary referral intensive care unit after CPR. Resuscitation. 2011;82(2):150-154. https://doi.org/10.1016/j.resuscitation.2010.09.480.
2. Churpek MM, Adhikari R, Edelson DP. The value of vital sign trends for detecting clinical deterioration on the wards. Resuscitation 2016;102(5):1-5. https://doi.org/10.1016/j.resuscitation.2016.02.005.
3. DeVita MA, Smith GB, Adam SK, et al. ‘‘Identifying the hospitalized patient in crisis’’—a consensus conference on the afferent limb of rapid response systems. Resuscitation. 2010;81(4):375-382. https://doi.org/10.1016/j.resuscitation.2009.12.008.
4. Yoder JC, Yuen TC, Churpek MM, Arora VM, Edelson DP. A prospective study of nighttime vital sign monitoring frequency and risk of clinical deterioration. JAMA Intern Med. 2013;173(16):1554-1555. https://doi.org/10.1001/jamainternmed.2013.7791
5. Hands C, Reid E, Meredith P, et al. Patterns in the recording of vital sign and early warning scores: compliance with a clinical escalation protocol. BMJ Qual Saf. 2013;22(9):719-726. https://doi.org/10.1136/bmjqs-2013-001954
6. Hope J, Recio-Saucedo A, Fogg C, et al. A fundamental conflict of care: nurses’ accounts of balancing patients’ sleep with taking vital sign observations at night. J Clin Nurs. 2018;27:1860-1871. https://doi.org/10.1111/jocn.14234.
7. Stiver K, Sharma N, Geller K, Smith L, Stephens J. “Quiet at night”: reduced overnight vital sign monitoring linked to both safety and improvements in patients’ perception of hospital sleep quality. Patient Exp J. 2017;4(1):Article 10. https://doi.org/10.35680/2372-0247.1185.
8. Raymond I, Nielsen TA, Lavigne G, Manzini C, Choiniere M. Quality of sleep and its daily relationship to pain intensity in hospitalized adult burn patients. Pain. 2001;93(3):381-388. https://doi.org/10.1016/s0304-3959(01)00282-2.
9. DePietro RH, Knutson KL, Spampinato L, et al. Association between inpatient sleep loss and hyperglycemia of hospitalization. Diabetes Care. 2017;40(2):188-193. https://doi.org/10.2337/dc16-1683.
10. American Academy of Nursing. Choosing Wisely. Twenty-Five Things Nurses and Patients Should Question. July 2018. https://www.choosingwisely.org/wp-content/uploads/2015/02/AANursing-Choosing-Wisely-List.pdf.
11. van Galen LS, Dijkstra CC, Ludikhuize J, Kramer MHH, Nanayakkara PWB. A protocolised once a day Modified Early Warning Score (MEWS) measurement is an appropriate screening tool for major adverse events in a general hospital population. PLoS One. 2016;11(8):e0160811. https://doi.org/10.1371/journal.pone.0160811.
12. Edelson DP, Carey K, Twu NM, et al. Acuity-based nighttime vital sign assessments: a randomized controlled trial. Abstract presented at: Hospital Medicine 2019; March 24-27, 2019; National Harbor, Maryland. https://www.shmabstracts.com/abstract/acuity-based-nighttime-vital-sign-assessments-a-randomized-controlled-trial/. Accessed March 20, 2020
13. Arora VM, Machado N, Anderson SL, et al. Effectiveness of SIESTA on objective and subjective metrics of nighttime hospital sleep disruptors. J Hosp Med. 2019;14(1):38-41. https://doi.org/10.12788/jhm.3091.
© 2020 Society of Hospital Medicine
Things We Do for No Reason™: Obtaining an Abdominal X-ray to Assess for Constipation in Children

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 5-year old boy is admitted to the hospital for a bowel clean-out after presenting with abdominal pain and having an abdominal x-ray that demonstrated a “moderate stool burden.” After ingestion of the bowel prep, he develops worsening abdominal cramping and diarrhea. Upon reviewing the bowel history with his mother afterward, the team learns that he has had a bowel movement every 1-2 days as usual and has been having soft stools without any straining, pain, or blood present.
BACKGROUND
Functional constipation is a common clinical problem in pediatrics and constitutes a large number of admissions into hospitals and visits to clinics and emergency departments. In the United States, up to 36% of children are affected.Associated healthcare costs for children with constipation are estimated at $5.9 billion per year, which is $3.9 billion more than the general pediatric population without constipation.1 In 2011, American children aged 17 years and younger had more than 270,000 visits to the emergency department for constipation.2 As many as 70% of children who are given a diagnosis of constipation in the emergency department have an abdominal x-ray completed.3 The carcinogenic effects of radiation from radiography are well known. Unnecessary imaging places the child at risk for these effects while adding to the overall cost of medical care.4
WHY AN ABDOMINAL X-RAY MAY SEEM HELPFUL
The overall utilization of diagnostic imaging is increasing in pediatric emergency departments.4 When questioning why this is the case, one should consider the method of problem solving used by most physicians. After formulating initial hypotheses based on available information, prior knowledge, and experience, physicians aim to obtain additional data to confirm or reject each hypothesis.5
A 2017 study surveyed 24 pediatric gastroenterologists after 72 patient encounters and found that the most common cause for obtaining an abdominal x-ray was for evaluation of stool burden (70%).5 Other reasons included assessing the need for a bowel clean-out (35%), diagnosing fecal impaction (27%), finding the cause for abdominal pain (24%), and demonstrating stool burden to a family (14%). This same study found that most of the polled providers used an abdominal x-ray to assess for constipation, and nearly half changed their management based on the findings.
WHY ABDOMINAL X-RAYS ARE NOT HELPFUL
Many systematic reviews and retrospective studies have investigated the efficacy of abdominal x-rays for diagnosing constipation. One retrospective review involving 160 children with defecation complaints assessed the accuracy of different radiologic scoring methods in identifying children with constipation.6 Three pediatric gastroenterologists and 1 pediatric radiologist blindly applied 4 scoring methods: colonic transit time, Leech score, Barr score, and fecal loading. The results showed that all x-ray scoring methods had low sensitivity for diagnosing constipation, variable specificity, and low interobserver reproducibility of scores.6 There was also poor ability to differentiate between patients with constipation and nonretentive fecal incontinence. Fecal loading had the worst performance in differentiating between these 2. Greater than 20% of children with clinically diagnosed constipation had normal Barr and Leech scores.6 Another systematic review also found no evidence for a diagnostic association between clinical symptoms of constipation and fecal loading on abdominal x-rays.7 In this study, the sensitivity and specificity of the x-ray were as low as 61% and 55%, respectively, which indicate poor overall diagnostic accuracy. Abdominal x-rays are subjective, not standardized, and represent a single observation in time. The amount of fecal loading seen on imaging is subject to daily variation depending on the timing of last food intake and timing of last defecation. There is a large variance in interpretation of fecal loading, and any stool seen on an x-ray does not rule out another potential diagnosis causing abdominal pain.
In 2014, the North American Society for Pediatric Gastroenterology, Hepatology, & Nutrition (NASPGHAN) and the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) released joint clinical guidelines that the evidence supports not obtaining an abdominal x-ray to diagnose functional constipation.8 Similarly, the National Institute for Health and Care Excellence (NICE) stated that abdominal x-rays should not be recommended as an additional test for constipation in children.9 These groups advocate for diagnosing functional constipation clinically by using a careful history and physical exam.
WHY ABDOMINAL X-RAYS ARE POTENTIALLY HARMFUL
The primary patient harm associated with abdominal x-rays is radiation exposure. While the amount of radiation in a single x-ray is low, children with constipation tend to have frequent revisits, significantly more than children with other common chronic conditions (eg, asthma and migraine headaches).10
One multicenter retrospective cohort study that included approximately 282,000 children diagnosed with constipation found that children who received an abdominal x-ray were twice as likely to return to the emergency department with a clinically significant alternate diagnosis (0.33% vs 0.17%). The 2 most common missed diagnoses were acute appendicitis and intussusception.3 Another retrospective study that included about 3,700 children also found that x-rays were performed more frequently in children who were misdiagnosed than in those who did not have a significant alternate diagnosis (75% vs 46%).11 In this case, both of these groups had a similar amount of stool on the x-rays as determined by the mean Leech scores. While this study identified an association between abdominal x-ray use and misdiagnoses, a causative effect was not necessarily discovered between the 2. The authors felt that even relatively large amounts of stool on an x-ray should not discount serious causes of abdominal pain or tenderness.11 A third retrospective study determined that children who received an abdominal x-ray and were diagnosed with constipation were significantly more likely to be admitted to the hospital, further raising healthcare costs.12 In this study, having an x-ray reduced the odds of being discharged home by about half. They also found that abdominal x-rays could be avoided if digital rectal exams were performed.12
HOW CONSTIPATION SHOULD BE DIAGNOSED
Functional constipation is a clinical diagnosis based on a thorough collection of history and a complete physical exam in children of all ages, including digital examination of the rectum to assess for fecal impaction, if necessary.
The Rome IV criteria for chronic constipation can be helpful and includes at least 2 of the following features for at least 1 month in infants up to 4 years of age: 2 or fewer stools per week, history of excessive stool retention, history of painful or hard bowel movements, history of large-diameter stools, and presence of a large fecal mass in the rectum.13 In children who are toilet trained, 2 additional criteria may be used: at least 1 episode of fecal incontinence per week after being toilet-trained and history of large-diameter stools that may obstruct the toilet.13
The NASPGHAN and ESPGHAN joint guidelines from 2014 state that, while constipation is based on history and physical exam, a major role of the history and physical exam is to exclude other disorders that also present with difficulty in defecation.8 This can help identify red-flag features or complications and guide further investigation. While evidence did not support routine use of a digital rectal exam in diagnosing constipation, the guidelines stated that a rectal exam (visual and digital) helps to evaluate for anorectal malformations, anal stenosis, rectal tone, distension, erythema, skin tags, anal fissures, or a fecal mass.8
In regard to history, approximately 0.4%-20% of healthy children without constipation have at least 1 clinical feature listed above. Therefore, the use of a single clinical finding to diagnose constipation, such as decreased bowel frequency, can result in an inappropriate diagnosis. Children experience large variations in stool output depending on diet, genetics, and environmental factors.10 The usual pattern of bowel habits in humans range from 3 times daily to every 3 days.14 Importantly, there are times to order an abdominal x-ray for patients with abdominal pain. The NASPGHAN and ESPGHAN joint guidelines recommend obtaining abdominal x-rays to evaluate children who have concerning features, such as previous abdominal surgeries, known genetic conditions or malformations, bilious emesis, or severe abdominal distension.8
RECOMMENDATIONS
- Functional constipation should be diagnosed based purely on a thorough history and physical examination, including a rectal exam
- Abdominal x-rays (ordered for any reason) should not be used to diagnose or assess for functional constipation
CONCLUSIONS
Performing abdominal x-rays to assess for pediatric functional constipation is not beneficial and potentially harmful to patients. Multiple retrospective studies revealed no diagnostic association between clinical symptoms or severity of constipation and findings on abdominal radiography. X-rays have very low sensitivity and specificity for diagnosing constipation. In the pediatric emergency department, abdominal x-rays completed for patients diagnosed with constipation have been associated with missed diagnoses, false reassurance of constipation, more frequent admissions into the hospital, longer hospital stays, higher healthcare costs, and unnecessary radiation exposure. The NICE as well as 2014 NASPGHAN and ESPGHAN clinical guidelines recommend against obtaining x-rays to diagnose constipation. The most effective way to diagnose functional constipation in children is with a thorough collection of history and physical exam. In the introductory case, the boy received an osmotic laxative based on abdominal x-ray findings, which resulted in the adverse effect of diarrhea. This case demonstrates how using abdominal x-rays to assess for constipation can be misleading and emphasizes the importance of collecting a thorough history and physical exam.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Afzal NA, Tighe MP, Thomson MA. (2011, June 13). Constipation in children. Ital J Pediatr. 2011;37:28. https://doi.org/10.1186/1824-7288-37-28.
2. Sommers T, Corban C, Sengupta N, et al. Emergency department burden of constipation in the United States from 2006 to 2011. Am J Gastroenterol. 2015;110(4):572-579. https://doi.org/10.1038/ajg.2015.64.
3. Freedman SB, Rodean J, Hall M, et al. (2017). Delayed diagnoses in children with constipation: multicenter retrospective cohort study. J Pediatr. 186, 87-94.e16. https://doi.org/10.1016/j.jpeds.2017.03.061.
4. Reed MH. Imaging utilization commentary: A radiology perspective. Pediatr Radiol. 2008;38 (Suppl 4):S660-S663. https://doi.org/10.1007/s00247-008-0982-y.
5. Beinvogl B, Sabharwal S, McSweeney M, Nurko S. Are we using abdominal radiographs appropriately in the management of pediatric constipation? J Pediatr. 2017;191:179-183. https://doi.org/10.1016/j.jpeds.2017.08.075.
6. Pensabene L, Buonomo C, Fishman L, Chitkara D, Nurko S. Lack of utility of abdominal x-rays in the evaluation of children with constipation: Comparison of different scoring methods. J Pediatr Gastroenterol Nutr. 2010;51(2):155-159. https://doi.org/10.1097/MPG.0b013e3181cb4309.
7. Berger MY, Tabbers MM, Kurver MJ, Boluyt N, Benninga MA. Value of abdominal radiography, colonic transit time, and rectal ultrasound scanning in the diagnosis of idiopathic constipation in children: A systematic review. J Pediatr. 2012;161(1):44–50.e502. https://doi.org/10.1016/j.jpeds.2011.12.045.
8. Tabbers MM, DiLorenzo C, Berger MY, et al. Evaluation and treatment of functional constipation in infants and children: Evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. 2014;58(2):258-274. https://doi.org/10.1097/mpg.0000000000000266.
9. Bardisa-Ezcurra L, Ullman R, Gordon J; Guideline Development Group. Diagnosis and management of idiopathic childhood constipation: summary of NICE guidance. BMJ. 2010;340:c2585. https://doi.org/10.1136/bmj.c2585.
10. Rajindrajith S, Manjuri Devanarayana N, Benninga MA. Defecation Disorders in Children: Constipation and Functional Fecal Incontinence. In: Guandalini S, Dhawan A, Branski D. eds. Textbook of Pediatric Gastroenterology, Hepatology and Nutrition: A Comprehensive Guide to Practice (1st ed.). Basingstoke, England: Springer; 2016:247-260.
11. Freedman SB, Thull-Freedman J, Manson D, et al. Pediatric abdominal radiograph use, constipation, and significant misdiagnoses. J Pediatr. 2014;164(1):83-88.e2. https://doi.org/10.1016/j.jpeds.2013.08.074.
12. Chumpitazi CE, Rees CA, Camp EA, Henkel EB, Valdez KL, Chumpitazi BP. Diagnostic approach to constipation impacts pediatric emergency department disposition. Am J Emerg Med. 2017;35(10):1490-1493. https://doi.org/10.1016/j.ajem.2017.04.060.
13. Benninga MA, Nurko S, Faure C, Hyman PE, St. James Roberts I, Schechter NL. Childhood functional GI disorders: Neonate/toddler. Gastroenterology. 2016;150(6):1443-1455. https://doi.org/10.1053/j.gastro.2016.02.016.
14. Walter SA, Kjellström L, Nyhlin H, Talley NJ, Agréus L. Assessment of normal bowel habits in the general adult population: the Popcol study. Scand J Gastroenterol. 2010;45(5):556-566. https://doi.org/10.3109/00365520903551332.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 5-year old boy is admitted to the hospital for a bowel clean-out after presenting with abdominal pain and having an abdominal x-ray that demonstrated a “moderate stool burden.” After ingestion of the bowel prep, he develops worsening abdominal cramping and diarrhea. Upon reviewing the bowel history with his mother afterward, the team learns that he has had a bowel movement every 1-2 days as usual and has been having soft stools without any straining, pain, or blood present.
BACKGROUND
Functional constipation is a common clinical problem in pediatrics and constitutes a large number of admissions into hospitals and visits to clinics and emergency departments. In the United States, up to 36% of children are affected.Associated healthcare costs for children with constipation are estimated at $5.9 billion per year, which is $3.9 billion more than the general pediatric population without constipation.1 In 2011, American children aged 17 years and younger had more than 270,000 visits to the emergency department for constipation.2 As many as 70% of children who are given a diagnosis of constipation in the emergency department have an abdominal x-ray completed.3 The carcinogenic effects of radiation from radiography are well known. Unnecessary imaging places the child at risk for these effects while adding to the overall cost of medical care.4
WHY AN ABDOMINAL X-RAY MAY SEEM HELPFUL
The overall utilization of diagnostic imaging is increasing in pediatric emergency departments.4 When questioning why this is the case, one should consider the method of problem solving used by most physicians. After formulating initial hypotheses based on available information, prior knowledge, and experience, physicians aim to obtain additional data to confirm or reject each hypothesis.5
A 2017 study surveyed 24 pediatric gastroenterologists after 72 patient encounters and found that the most common cause for obtaining an abdominal x-ray was for evaluation of stool burden (70%).5 Other reasons included assessing the need for a bowel clean-out (35%), diagnosing fecal impaction (27%), finding the cause for abdominal pain (24%), and demonstrating stool burden to a family (14%). This same study found that most of the polled providers used an abdominal x-ray to assess for constipation, and nearly half changed their management based on the findings.
WHY ABDOMINAL X-RAYS ARE NOT HELPFUL
Many systematic reviews and retrospective studies have investigated the efficacy of abdominal x-rays for diagnosing constipation. One retrospective review involving 160 children with defecation complaints assessed the accuracy of different radiologic scoring methods in identifying children with constipation.6 Three pediatric gastroenterologists and 1 pediatric radiologist blindly applied 4 scoring methods: colonic transit time, Leech score, Barr score, and fecal loading. The results showed that all x-ray scoring methods had low sensitivity for diagnosing constipation, variable specificity, and low interobserver reproducibility of scores.6 There was also poor ability to differentiate between patients with constipation and nonretentive fecal incontinence. Fecal loading had the worst performance in differentiating between these 2. Greater than 20% of children with clinically diagnosed constipation had normal Barr and Leech scores.6 Another systematic review also found no evidence for a diagnostic association between clinical symptoms of constipation and fecal loading on abdominal x-rays.7 In this study, the sensitivity and specificity of the x-ray were as low as 61% and 55%, respectively, which indicate poor overall diagnostic accuracy. Abdominal x-rays are subjective, not standardized, and represent a single observation in time. The amount of fecal loading seen on imaging is subject to daily variation depending on the timing of last food intake and timing of last defecation. There is a large variance in interpretation of fecal loading, and any stool seen on an x-ray does not rule out another potential diagnosis causing abdominal pain.
In 2014, the North American Society for Pediatric Gastroenterology, Hepatology, & Nutrition (NASPGHAN) and the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) released joint clinical guidelines that the evidence supports not obtaining an abdominal x-ray to diagnose functional constipation.8 Similarly, the National Institute for Health and Care Excellence (NICE) stated that abdominal x-rays should not be recommended as an additional test for constipation in children.9 These groups advocate for diagnosing functional constipation clinically by using a careful history and physical exam.
WHY ABDOMINAL X-RAYS ARE POTENTIALLY HARMFUL
The primary patient harm associated with abdominal x-rays is radiation exposure. While the amount of radiation in a single x-ray is low, children with constipation tend to have frequent revisits, significantly more than children with other common chronic conditions (eg, asthma and migraine headaches).10
One multicenter retrospective cohort study that included approximately 282,000 children diagnosed with constipation found that children who received an abdominal x-ray were twice as likely to return to the emergency department with a clinically significant alternate diagnosis (0.33% vs 0.17%). The 2 most common missed diagnoses were acute appendicitis and intussusception.3 Another retrospective study that included about 3,700 children also found that x-rays were performed more frequently in children who were misdiagnosed than in those who did not have a significant alternate diagnosis (75% vs 46%).11 In this case, both of these groups had a similar amount of stool on the x-rays as determined by the mean Leech scores. While this study identified an association between abdominal x-ray use and misdiagnoses, a causative effect was not necessarily discovered between the 2. The authors felt that even relatively large amounts of stool on an x-ray should not discount serious causes of abdominal pain or tenderness.11 A third retrospective study determined that children who received an abdominal x-ray and were diagnosed with constipation were significantly more likely to be admitted to the hospital, further raising healthcare costs.12 In this study, having an x-ray reduced the odds of being discharged home by about half. They also found that abdominal x-rays could be avoided if digital rectal exams were performed.12
HOW CONSTIPATION SHOULD BE DIAGNOSED
Functional constipation is a clinical diagnosis based on a thorough collection of history and a complete physical exam in children of all ages, including digital examination of the rectum to assess for fecal impaction, if necessary.
The Rome IV criteria for chronic constipation can be helpful and includes at least 2 of the following features for at least 1 month in infants up to 4 years of age: 2 or fewer stools per week, history of excessive stool retention, history of painful or hard bowel movements, history of large-diameter stools, and presence of a large fecal mass in the rectum.13 In children who are toilet trained, 2 additional criteria may be used: at least 1 episode of fecal incontinence per week after being toilet-trained and history of large-diameter stools that may obstruct the toilet.13
The NASPGHAN and ESPGHAN joint guidelines from 2014 state that, while constipation is based on history and physical exam, a major role of the history and physical exam is to exclude other disorders that also present with difficulty in defecation.8 This can help identify red-flag features or complications and guide further investigation. While evidence did not support routine use of a digital rectal exam in diagnosing constipation, the guidelines stated that a rectal exam (visual and digital) helps to evaluate for anorectal malformations, anal stenosis, rectal tone, distension, erythema, skin tags, anal fissures, or a fecal mass.8
In regard to history, approximately 0.4%-20% of healthy children without constipation have at least 1 clinical feature listed above. Therefore, the use of a single clinical finding to diagnose constipation, such as decreased bowel frequency, can result in an inappropriate diagnosis. Children experience large variations in stool output depending on diet, genetics, and environmental factors.10 The usual pattern of bowel habits in humans range from 3 times daily to every 3 days.14 Importantly, there are times to order an abdominal x-ray for patients with abdominal pain. The NASPGHAN and ESPGHAN joint guidelines recommend obtaining abdominal x-rays to evaluate children who have concerning features, such as previous abdominal surgeries, known genetic conditions or malformations, bilious emesis, or severe abdominal distension.8
RECOMMENDATIONS
- Functional constipation should be diagnosed based purely on a thorough history and physical examination, including a rectal exam
- Abdominal x-rays (ordered for any reason) should not be used to diagnose or assess for functional constipation
CONCLUSIONS
Performing abdominal x-rays to assess for pediatric functional constipation is not beneficial and potentially harmful to patients. Multiple retrospective studies revealed no diagnostic association between clinical symptoms or severity of constipation and findings on abdominal radiography. X-rays have very low sensitivity and specificity for diagnosing constipation. In the pediatric emergency department, abdominal x-rays completed for patients diagnosed with constipation have been associated with missed diagnoses, false reassurance of constipation, more frequent admissions into the hospital, longer hospital stays, higher healthcare costs, and unnecessary radiation exposure. The NICE as well as 2014 NASPGHAN and ESPGHAN clinical guidelines recommend against obtaining x-rays to diagnose constipation. The most effective way to diagnose functional constipation in children is with a thorough collection of history and physical exam. In the introductory case, the boy received an osmotic laxative based on abdominal x-ray findings, which resulted in the adverse effect of diarrhea. This case demonstrates how using abdominal x-rays to assess for constipation can be misleading and emphasizes the importance of collecting a thorough history and physical exam.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 5-year old boy is admitted to the hospital for a bowel clean-out after presenting with abdominal pain and having an abdominal x-ray that demonstrated a “moderate stool burden.” After ingestion of the bowel prep, he develops worsening abdominal cramping and diarrhea. Upon reviewing the bowel history with his mother afterward, the team learns that he has had a bowel movement every 1-2 days as usual and has been having soft stools without any straining, pain, or blood present.
BACKGROUND
Functional constipation is a common clinical problem in pediatrics and constitutes a large number of admissions into hospitals and visits to clinics and emergency departments. In the United States, up to 36% of children are affected.Associated healthcare costs for children with constipation are estimated at $5.9 billion per year, which is $3.9 billion more than the general pediatric population without constipation.1 In 2011, American children aged 17 years and younger had more than 270,000 visits to the emergency department for constipation.2 As many as 70% of children who are given a diagnosis of constipation in the emergency department have an abdominal x-ray completed.3 The carcinogenic effects of radiation from radiography are well known. Unnecessary imaging places the child at risk for these effects while adding to the overall cost of medical care.4
WHY AN ABDOMINAL X-RAY MAY SEEM HELPFUL
The overall utilization of diagnostic imaging is increasing in pediatric emergency departments.4 When questioning why this is the case, one should consider the method of problem solving used by most physicians. After formulating initial hypotheses based on available information, prior knowledge, and experience, physicians aim to obtain additional data to confirm or reject each hypothesis.5
A 2017 study surveyed 24 pediatric gastroenterologists after 72 patient encounters and found that the most common cause for obtaining an abdominal x-ray was for evaluation of stool burden (70%).5 Other reasons included assessing the need for a bowel clean-out (35%), diagnosing fecal impaction (27%), finding the cause for abdominal pain (24%), and demonstrating stool burden to a family (14%). This same study found that most of the polled providers used an abdominal x-ray to assess for constipation, and nearly half changed their management based on the findings.
WHY ABDOMINAL X-RAYS ARE NOT HELPFUL
Many systematic reviews and retrospective studies have investigated the efficacy of abdominal x-rays for diagnosing constipation. One retrospective review involving 160 children with defecation complaints assessed the accuracy of different radiologic scoring methods in identifying children with constipation.6 Three pediatric gastroenterologists and 1 pediatric radiologist blindly applied 4 scoring methods: colonic transit time, Leech score, Barr score, and fecal loading. The results showed that all x-ray scoring methods had low sensitivity for diagnosing constipation, variable specificity, and low interobserver reproducibility of scores.6 There was also poor ability to differentiate between patients with constipation and nonretentive fecal incontinence. Fecal loading had the worst performance in differentiating between these 2. Greater than 20% of children with clinically diagnosed constipation had normal Barr and Leech scores.6 Another systematic review also found no evidence for a diagnostic association between clinical symptoms of constipation and fecal loading on abdominal x-rays.7 In this study, the sensitivity and specificity of the x-ray were as low as 61% and 55%, respectively, which indicate poor overall diagnostic accuracy. Abdominal x-rays are subjective, not standardized, and represent a single observation in time. The amount of fecal loading seen on imaging is subject to daily variation depending on the timing of last food intake and timing of last defecation. There is a large variance in interpretation of fecal loading, and any stool seen on an x-ray does not rule out another potential diagnosis causing abdominal pain.
In 2014, the North American Society for Pediatric Gastroenterology, Hepatology, & Nutrition (NASPGHAN) and the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) released joint clinical guidelines that the evidence supports not obtaining an abdominal x-ray to diagnose functional constipation.8 Similarly, the National Institute for Health and Care Excellence (NICE) stated that abdominal x-rays should not be recommended as an additional test for constipation in children.9 These groups advocate for diagnosing functional constipation clinically by using a careful history and physical exam.
WHY ABDOMINAL X-RAYS ARE POTENTIALLY HARMFUL
The primary patient harm associated with abdominal x-rays is radiation exposure. While the amount of radiation in a single x-ray is low, children with constipation tend to have frequent revisits, significantly more than children with other common chronic conditions (eg, asthma and migraine headaches).10
One multicenter retrospective cohort study that included approximately 282,000 children diagnosed with constipation found that children who received an abdominal x-ray were twice as likely to return to the emergency department with a clinically significant alternate diagnosis (0.33% vs 0.17%). The 2 most common missed diagnoses were acute appendicitis and intussusception.3 Another retrospective study that included about 3,700 children also found that x-rays were performed more frequently in children who were misdiagnosed than in those who did not have a significant alternate diagnosis (75% vs 46%).11 In this case, both of these groups had a similar amount of stool on the x-rays as determined by the mean Leech scores. While this study identified an association between abdominal x-ray use and misdiagnoses, a causative effect was not necessarily discovered between the 2. The authors felt that even relatively large amounts of stool on an x-ray should not discount serious causes of abdominal pain or tenderness.11 A third retrospective study determined that children who received an abdominal x-ray and were diagnosed with constipation were significantly more likely to be admitted to the hospital, further raising healthcare costs.12 In this study, having an x-ray reduced the odds of being discharged home by about half. They also found that abdominal x-rays could be avoided if digital rectal exams were performed.12
HOW CONSTIPATION SHOULD BE DIAGNOSED
Functional constipation is a clinical diagnosis based on a thorough collection of history and a complete physical exam in children of all ages, including digital examination of the rectum to assess for fecal impaction, if necessary.
The Rome IV criteria for chronic constipation can be helpful and includes at least 2 of the following features for at least 1 month in infants up to 4 years of age: 2 or fewer stools per week, history of excessive stool retention, history of painful or hard bowel movements, history of large-diameter stools, and presence of a large fecal mass in the rectum.13 In children who are toilet trained, 2 additional criteria may be used: at least 1 episode of fecal incontinence per week after being toilet-trained and history of large-diameter stools that may obstruct the toilet.13
The NASPGHAN and ESPGHAN joint guidelines from 2014 state that, while constipation is based on history and physical exam, a major role of the history and physical exam is to exclude other disorders that also present with difficulty in defecation.8 This can help identify red-flag features or complications and guide further investigation. While evidence did not support routine use of a digital rectal exam in diagnosing constipation, the guidelines stated that a rectal exam (visual and digital) helps to evaluate for anorectal malformations, anal stenosis, rectal tone, distension, erythema, skin tags, anal fissures, or a fecal mass.8
In regard to history, approximately 0.4%-20% of healthy children without constipation have at least 1 clinical feature listed above. Therefore, the use of a single clinical finding to diagnose constipation, such as decreased bowel frequency, can result in an inappropriate diagnosis. Children experience large variations in stool output depending on diet, genetics, and environmental factors.10 The usual pattern of bowel habits in humans range from 3 times daily to every 3 days.14 Importantly, there are times to order an abdominal x-ray for patients with abdominal pain. The NASPGHAN and ESPGHAN joint guidelines recommend obtaining abdominal x-rays to evaluate children who have concerning features, such as previous abdominal surgeries, known genetic conditions or malformations, bilious emesis, or severe abdominal distension.8
RECOMMENDATIONS
- Functional constipation should be diagnosed based purely on a thorough history and physical examination, including a rectal exam
- Abdominal x-rays (ordered for any reason) should not be used to diagnose or assess for functional constipation
CONCLUSIONS
Performing abdominal x-rays to assess for pediatric functional constipation is not beneficial and potentially harmful to patients. Multiple retrospective studies revealed no diagnostic association between clinical symptoms or severity of constipation and findings on abdominal radiography. X-rays have very low sensitivity and specificity for diagnosing constipation. In the pediatric emergency department, abdominal x-rays completed for patients diagnosed with constipation have been associated with missed diagnoses, false reassurance of constipation, more frequent admissions into the hospital, longer hospital stays, higher healthcare costs, and unnecessary radiation exposure. The NICE as well as 2014 NASPGHAN and ESPGHAN clinical guidelines recommend against obtaining x-rays to diagnose constipation. The most effective way to diagnose functional constipation in children is with a thorough collection of history and physical exam. In the introductory case, the boy received an osmotic laxative based on abdominal x-ray findings, which resulted in the adverse effect of diarrhea. This case demonstrates how using abdominal x-rays to assess for constipation can be misleading and emphasizes the importance of collecting a thorough history and physical exam.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Afzal NA, Tighe MP, Thomson MA. (2011, June 13). Constipation in children. Ital J Pediatr. 2011;37:28. https://doi.org/10.1186/1824-7288-37-28.
2. Sommers T, Corban C, Sengupta N, et al. Emergency department burden of constipation in the United States from 2006 to 2011. Am J Gastroenterol. 2015;110(4):572-579. https://doi.org/10.1038/ajg.2015.64.
3. Freedman SB, Rodean J, Hall M, et al. (2017). Delayed diagnoses in children with constipation: multicenter retrospective cohort study. J Pediatr. 186, 87-94.e16. https://doi.org/10.1016/j.jpeds.2017.03.061.
4. Reed MH. Imaging utilization commentary: A radiology perspective. Pediatr Radiol. 2008;38 (Suppl 4):S660-S663. https://doi.org/10.1007/s00247-008-0982-y.
5. Beinvogl B, Sabharwal S, McSweeney M, Nurko S. Are we using abdominal radiographs appropriately in the management of pediatric constipation? J Pediatr. 2017;191:179-183. https://doi.org/10.1016/j.jpeds.2017.08.075.
6. Pensabene L, Buonomo C, Fishman L, Chitkara D, Nurko S. Lack of utility of abdominal x-rays in the evaluation of children with constipation: Comparison of different scoring methods. J Pediatr Gastroenterol Nutr. 2010;51(2):155-159. https://doi.org/10.1097/MPG.0b013e3181cb4309.
7. Berger MY, Tabbers MM, Kurver MJ, Boluyt N, Benninga MA. Value of abdominal radiography, colonic transit time, and rectal ultrasound scanning in the diagnosis of idiopathic constipation in children: A systematic review. J Pediatr. 2012;161(1):44–50.e502. https://doi.org/10.1016/j.jpeds.2011.12.045.
8. Tabbers MM, DiLorenzo C, Berger MY, et al. Evaluation and treatment of functional constipation in infants and children: Evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. 2014;58(2):258-274. https://doi.org/10.1097/mpg.0000000000000266.
9. Bardisa-Ezcurra L, Ullman R, Gordon J; Guideline Development Group. Diagnosis and management of idiopathic childhood constipation: summary of NICE guidance. BMJ. 2010;340:c2585. https://doi.org/10.1136/bmj.c2585.
10. Rajindrajith S, Manjuri Devanarayana N, Benninga MA. Defecation Disorders in Children: Constipation and Functional Fecal Incontinence. In: Guandalini S, Dhawan A, Branski D. eds. Textbook of Pediatric Gastroenterology, Hepatology and Nutrition: A Comprehensive Guide to Practice (1st ed.). Basingstoke, England: Springer; 2016:247-260.
11. Freedman SB, Thull-Freedman J, Manson D, et al. Pediatric abdominal radiograph use, constipation, and significant misdiagnoses. J Pediatr. 2014;164(1):83-88.e2. https://doi.org/10.1016/j.jpeds.2013.08.074.
12. Chumpitazi CE, Rees CA, Camp EA, Henkel EB, Valdez KL, Chumpitazi BP. Diagnostic approach to constipation impacts pediatric emergency department disposition. Am J Emerg Med. 2017;35(10):1490-1493. https://doi.org/10.1016/j.ajem.2017.04.060.
13. Benninga MA, Nurko S, Faure C, Hyman PE, St. James Roberts I, Schechter NL. Childhood functional GI disorders: Neonate/toddler. Gastroenterology. 2016;150(6):1443-1455. https://doi.org/10.1053/j.gastro.2016.02.016.
14. Walter SA, Kjellström L, Nyhlin H, Talley NJ, Agréus L. Assessment of normal bowel habits in the general adult population: the Popcol study. Scand J Gastroenterol. 2010;45(5):556-566. https://doi.org/10.3109/00365520903551332.
1. Afzal NA, Tighe MP, Thomson MA. (2011, June 13). Constipation in children. Ital J Pediatr. 2011;37:28. https://doi.org/10.1186/1824-7288-37-28.
2. Sommers T, Corban C, Sengupta N, et al. Emergency department burden of constipation in the United States from 2006 to 2011. Am J Gastroenterol. 2015;110(4):572-579. https://doi.org/10.1038/ajg.2015.64.
3. Freedman SB, Rodean J, Hall M, et al. (2017). Delayed diagnoses in children with constipation: multicenter retrospective cohort study. J Pediatr. 186, 87-94.e16. https://doi.org/10.1016/j.jpeds.2017.03.061.
4. Reed MH. Imaging utilization commentary: A radiology perspective. Pediatr Radiol. 2008;38 (Suppl 4):S660-S663. https://doi.org/10.1007/s00247-008-0982-y.
5. Beinvogl B, Sabharwal S, McSweeney M, Nurko S. Are we using abdominal radiographs appropriately in the management of pediatric constipation? J Pediatr. 2017;191:179-183. https://doi.org/10.1016/j.jpeds.2017.08.075.
6. Pensabene L, Buonomo C, Fishman L, Chitkara D, Nurko S. Lack of utility of abdominal x-rays in the evaluation of children with constipation: Comparison of different scoring methods. J Pediatr Gastroenterol Nutr. 2010;51(2):155-159. https://doi.org/10.1097/MPG.0b013e3181cb4309.
7. Berger MY, Tabbers MM, Kurver MJ, Boluyt N, Benninga MA. Value of abdominal radiography, colonic transit time, and rectal ultrasound scanning in the diagnosis of idiopathic constipation in children: A systematic review. J Pediatr. 2012;161(1):44–50.e502. https://doi.org/10.1016/j.jpeds.2011.12.045.
8. Tabbers MM, DiLorenzo C, Berger MY, et al. Evaluation and treatment of functional constipation in infants and children: Evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. 2014;58(2):258-274. https://doi.org/10.1097/mpg.0000000000000266.
9. Bardisa-Ezcurra L, Ullman R, Gordon J; Guideline Development Group. Diagnosis and management of idiopathic childhood constipation: summary of NICE guidance. BMJ. 2010;340:c2585. https://doi.org/10.1136/bmj.c2585.
10. Rajindrajith S, Manjuri Devanarayana N, Benninga MA. Defecation Disorders in Children: Constipation and Functional Fecal Incontinence. In: Guandalini S, Dhawan A, Branski D. eds. Textbook of Pediatric Gastroenterology, Hepatology and Nutrition: A Comprehensive Guide to Practice (1st ed.). Basingstoke, England: Springer; 2016:247-260.
11. Freedman SB, Thull-Freedman J, Manson D, et al. Pediatric abdominal radiograph use, constipation, and significant misdiagnoses. J Pediatr. 2014;164(1):83-88.e2. https://doi.org/10.1016/j.jpeds.2013.08.074.
12. Chumpitazi CE, Rees CA, Camp EA, Henkel EB, Valdez KL, Chumpitazi BP. Diagnostic approach to constipation impacts pediatric emergency department disposition. Am J Emerg Med. 2017;35(10):1490-1493. https://doi.org/10.1016/j.ajem.2017.04.060.
13. Benninga MA, Nurko S, Faure C, Hyman PE, St. James Roberts I, Schechter NL. Childhood functional GI disorders: Neonate/toddler. Gastroenterology. 2016;150(6):1443-1455. https://doi.org/10.1053/j.gastro.2016.02.016.
14. Walter SA, Kjellström L, Nyhlin H, Talley NJ, Agréus L. Assessment of normal bowel habits in the general adult population: the Popcol study. Scand J Gastroenterol. 2010;45(5):556-566. https://doi.org/10.3109/00365520903551332.
© 2020 Society of Hospital Medicine
Things We Do for No Reason™: Card Flipping Rounds

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 32-year-old man with a history of polysubstance use disorder is hospitalized with endocarditis. The senior resident on the inpatient medical team suggests that the team “card flip” on this patient, citing a large number of patients on the team census, time constraints, and concerns that his substance use history will make bedside rounds uncomfortable.
BACKGROUND
“Rounds” is an inpatient care model in which teams of practitioners assess patients, determine care plans, and communicate with patients, families, and other healthcare professionals.1 One form of rounds is bedside rounding (BSR) through which an entire patient presentation occurs at the bedside, analogous to family-centered rounds common in pediatric inpatient care.2 This style of rounding is distinct from “walk rounding” that involves presentations occurring separately from a patient followed by a brief team bedside encounter. BSR is also different from “card flipping” or “table rounding” that involves presentations of a case separately without a team-patient encounter. The frequency of BSR at academic institutions has markedly decreased across the United States, and the time spent at the bedside is only a small fraction of rounding time.3
WHY YOU MIGHT THINK CARD FLIPPING IS HELPFUL
There are several reasons to employ strategies such as card-flipping or walk-rounding for discussing patient care away from the bedside. These BSR risks can be organized into patient harm, inefficiency, and risks to healthcare professional training.
First, BSR may result in patient harm. For example, discussing private health information in a semiprivate room may not only be uncomfortable for patients but may also violate patient privacy.4 Care teams are often large in number and rounding at the bedside can simultaneously trigger anxiety among patients, cause confusion about plans, or result in lack of clarity on the role of each provider.4 Furthermore, delivering bad news during BSR, or discussing sensitive topics such as substance use, psychiatric illness, or concerns of malingering behavior, may be difficult and uncomfortable.4,5 Additionally, some potential diagnoses, such as cancer or human immunodeficiency virus, even if unlikely, could induce panic among patients when they hear them being discussed.5 Trainees may also lose situational awareness because they focus on the agenda of bedside rounds and fail to respond to patients’ emotional needs.6
Efficiency is another reason to avoid BSR. The systemic factors of changing hospital demographics, such as short length of stay and increasing patient volumes, generate a substantial administrative burden on trainees.7 Modern trainees are also constrained by work hour restrictions, engagement with mandatory curricula, and other professional development opportunities. Furthermore, changes in a medical work environment cause trainees to rely heavily on electronic health records, which forces them to be at a computer instead of in a patient’s room.8 This confluence of factors results in substantial time pressure, and BSR is perceived as an inefficient use of time.9
The impact on education and trainee development is another concern of BSR. Rounding away from a patient ensures a safe environment for learners to interpret data and articulate clinical reasoning without the risk of embarrassment in front of a patient. This time outside a patient room also allows the team to have a shared mental model so that communication is aligned when a patient encounter does occur. Card flipping may result in improved trainee autonomy because the constant presence of attending supervision, particularly in front of patients, can risk undermining resident leadership and patient trust.9
WHY WE SHOULD RETURN TO THE BEDSIDE
The cited reasons for provider hesitancy to BSR, including possible patient harm and inefficiency, may be mostly related to individual perceptions and have recently been questioned.10,11
Several studies have suggested that bedside rounds may be better for patients’ experience over traditional walk-rounding or card-flipping models. In these studies, patients signal a preference for bedside rounds and suggest that discussing sensitive issues or concerning differential diagnoses during BSR may not be as concerning as physicians worry.11 For example, one randomized trial found that 87% of patients are untroubled by bedside discussions,12 and another trial revealed no difference between rounding models in emotional distress to patients or families.11 Patients and families also report higher levels of clarity from physicians, and they cited significantly improved levels of understanding their illness10 and test results.9 Furthermore, patients describe that physicians spend about twice as much time on their care when BSR is used.12 In many related studies, patients report a preference for BSR as a rounding strategy.2,11-13 For example, one study found that 99% of patients prefer BSR.13 Another study showed that 85% of families request to be part of bedside family-centered rounds over traditional walk rounding.2
Rounding away from a patient via card flipping or walk rounding seems more efficient, but this idea may be illusory. Although these strategies may seem faster, the lack of communication and coordination between team members and the patient may cause inefficiencies and delays in care throughout the day.14 For example, one study has demonstrated that family-centered bedside rounds are about 20% longer than walk rounding, but everyone involved, including housestaff, felt it was more efficient and saved time later in the day.2 Additionally, a study comparing BSR with walk rounding13 found no difference in time spent per patient, and another study has shown similar results in terms of family-centered rounds.15 Both studies have reported a similar amount of time spent per patient.
Physicians should return to BSR not only to improve patient experience but also to develop the clinical skills of trainees. The direct observation of trainees with patients allows high-level impactful clinical feedback and provides a basis for calibrating how much autonomy to allow.16 Trainees also indicate that teaching is more impactful during BSR than during walk rounding or card flipping, and clinical skill training during BSR is superior to a discussion in a conference room or a hallway context.2,3,15,17,18 One study has even suggested that the education of bedside rounds may help improve clinical skills in comparison with traditional models.18
The lack of BSR during medical school and residency training results in a deleterious cycle. Trainees become less proficient and less comfortable with BSR skills and therefore graduate as faculty members who are unskilled or uncomfortable insisting on BSR. As such, the cycle continues. As a result and as the traditional cornerstone of clinical training and inpatient care, BSR is recommended as standard practice by some professional organizations.19
WHAT WE SHOULD DO INSTEAD
Developing buy-in is an important first step for engaging in BSR. We recommend starting by demonstrating the value of BSR to overcome initial team or trainee hesitancy. Regardless of systems established to improve the efficiency of BSR, it is our experience that learners hesitantly engage if they do not understand the value of a given activity. We also urge attendings to demonstrate value by articulating how BSR fits in a patient-centered approach to emphasize the evidence-based positive impacts of BSR on patients.9 Beyond reviewing the benefits, faculty should set an expectation that the team will carry out BSR.9 Doing so sets an informal curriculum showing that BSR is important and sets the standard of care, which allows an inpatient team to adapt early in a rotation.
Next, faculty should ensure that BSR remains efficient.9 We believe that efficiency starts by setting expectations with patients. Patient expectations can be set by an attending or a supervising resident and should include a preview about how each encounter will progress, who will be in the room, how large the team will be, and what their role is during the encounter. Patients should be invited to be part of the discussion, offered an opportunity to opt out, and informed that questions arising from or clarifications needed following encounters can be addressed later within the day or after BSR. Nurses should be invited to actively participate during patient presentations. Each bedside encounter should be kept brief and standardized.20,21 To maximize efficiency, we also believe that roles should be delegated ahead of time and positioning in the room should be deliberate.22 Team members should know who is speaking when and in what order, who is accessing the electronic health record, and who will be examining the patient. Ideally, goals should be set ahead of time and tailored to each individual encounter. Finally, ensure everyone is on the same page by huddling briefly before each encounter to establish goals and roles and huddle afterward to debrief for learning and teamwork calibration.
In order to mitigate the learner’s anxiety about presenting in front of patients, build a partnership with the trainee, and time should be allotted to establish a safe learning environment.9 Sustain a supportive learning environment by providing positive feedback to learners in front of patients and teams. Faculty members should demonstrate how to bedside round effectively by leading initial encounters and generate momentum by selecting initial patient encounters that are most likely to succeed.23 Checklists can also be useful cognitive aids to facilitate an encounter and manage the cognitive load of learners.24 Ultimately, hesitancies can be overcome with experience.
Faculty members should ensure that bedside encounters are educationally valuable for an entire team.9 This initiative starts by preparing ahead of time, which allows the mental energy during encounters to be directly observed by learners in action.16 Preparation also allows the presentation to focus more on clinical reasoning rather than data gathering.20 Faculty members should also consider ways to foster resident autonomy and establish the role of a supervising resident as the team leader. Positioning in the room is critical22; we suggest that faculty members should position themselves near the head of the bed, out of a patient’s direct eyesight. In this way, they can observe how individual team members and the team as a whole interact with patients. The supervising resident should be at the foot of the bed, central to the team and the focal point of a patient’s view. The presenting intern or student should be seated near the head of the bed and opposite the supervising attending. Clinical teaching should also be kept short and pertinent to the patient, and questions should be phrased as “how” or “why” rather than “what” to reduce the risk of “wrong” answers in front of patients and the team.
WHEN IS CARD FLIPPING APPROPRIATE?
We believe that bedside rounds are most consistent with patient-centered inpatient care and should be considered the first-line approach. We also acknowledge that it is not always possible to bedside round on every patient on an inpatient census. For example, at an average of 13-15 minutes per patient,2,13 a census of 16 patients can take up to 4 hours to round. This timeline is not always feasible given the timing of training program didactics, interprofessional or case management rounds, and pressure for early discharges. Similar to all aspects of medicine, many approaches have been established to provide patient care, and context is important. Therefore, card flipping and walk rounding are beneficial to patients in some instances. For example, consider BSR for new, sick, or undifferentiated patients or when the history or exam findings need clarification; walk rounding or card flipping is suitable for patients with clear plans in place or when an encounter will be too disruptive to the rounding flow.21 Census size and individual patient or family concerns should dictate the style of rounding; in most situations, BSR may be equally efficient because it offers significant benefits to patients and families.
RECOMMENDATIONS
- Expectations should be set early with both trainees and patients. Patients should be informed that the team can come back later for more in-depth discussions.
- Trainees should be taught evidence-based approaches supporting the value of bedside rounds for patients.
- Faculty should consider leading initial encounters to demonstrate how to bedside round and to model behaviors.
- Positive feedback should be provided in front of patients and the team to build confidence.
- Encounters should be kept brief and efficient.
- A sufficient space for resident autonomy should be ensured through deliberate positioning, delegation of responsibilities, and huddling before and after encounters.
- Bedside rounds should be educationally worthwhile.
CONCLUSION
BSR is a traditional cornerstone of clinical training and inpatient care. Teaching at the bedside has many established benefits, such as connecting with patients and families, affording educators a valuable opportunity to assess learners and role model, and solidifying medical content by integrating teaching with clinical care. Concerns about bedside rounding may be based more on conjecture than on available evidence and can be overcome with deliberate education and proper planning. We propose several recommendations to successfully implement efficient, patient-centered, and educationally valuable bedside rounds.
For this (and most) patient(s), we recommend BSR. If this BSR is the first encounter, we suggest that the team should start with a more straightforward patient and come back to the new admission after the team has a chance to practice with other patients.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Gonzalo JD, Wolpaw DR, Lehman E, Chuang CH. Patient-centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040-1047. https://doi.org/10.1007/s11606-014-2817-x.
2. Muething SE, Kotagal UR, Schoettker PJ, del Rey JG, DeWitt TG. Family-centered bedside rounds: a new approach to patient care and teaching. Pediatrics. 2007;119(4):829-832. https://doi.org/10.1542/peds.2006-2528.
3. Ngo TL, Blankenburg R, Yu CE. Teaching at the bedside: strategies for optimizing education on patient and family centered rounds. Pediatr Clin North Am. 2019;66(4):881-889. https://doi.org/10.1016/j.pcl.2019.03.012.
4. Berkwitt A, Grossman M. A Qualitative analysis of pediatric patient attitudes regarding family-centered rounds. Hosp Pediatr. 2015;5(7):357. https://doi.org/10.1542/hpeds.2014-0198.
5. Rabinowitz R, Farnan J, Hulland O, et al. Rounds today: a qualitative study of internal medicine and pediatrics resident perceptions. J Grad Med Educ. 2016;8(4):523-531. https://doi.org/10.4300/JGME-D-15-00106.1.
6. Pingree EW, Freed JA, Riviello ED, et al. A tale of two rounds: managing conflict during the worst of times in family-centered rounds. Hosp Pediatr. 2019;9(7):563-565. https://doi.org/10.1542/hpeds.2019-0047.
7. Mamykina L, Vawdrey DK, Hripcsak G. How do residents spend their shift time? A time and motion study with a particular focus on the use of computers. Acad Med. 2016;91(6):827-832. https://doi.org/10.1097/ACM.0000000000001148.
8. Verghese A. Culture shock--patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. https://doi.org/10.1056/NEJMp0807461.
9. Gonzalo JD, Heist BS, Duffy BL, et al. Identifying and overcoming the barriers to bedside rounds: a multicenter qualitative study. Acad Med. 2014;89(2):326-334. https://doi.org/10.1097/ACM.0000000000000100.
10. Rogers HD, Carline JD, Paauw DS. Examination room presentations in general internal medicine clinic: patients’ and students’ perceptions. Acad Med. 2003;78(9):945-949. https://doi.org/10.1097/00001888-200309000-00023.
11. Landry M-A, Lafrenaye S, Roy M-C, Cyr C. A randomized, controlled trial of bedside versus conference-room case presentation in a pediatric intensive care unit. Pediatrics. 2007;120(2):275-280. https://doi.org/10.1542/peds.2007-0107.
12. Lehmann LS, Brancati FL, Chen M-C, Roter D, Dobs AS. The effect of bedside case presentations on patients’ perceptions of their medical care. N Engl J Med. 1997;336(16):1150-1156. https://doi.org/10.1056/NEJM199704173361606.
13. Gonzalo JD, Chuang CH, Huang G, Smith C. The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792-798. https://doi.org/10.1007/s11606-010-1344-7.
14. Okoniewska B, Santana MJ, Groshaus H, et al. Barriers to discharge in an acute care medical teaching unit: a qualitative analysis of health providers’ perceptions. J Multidiscip Healthc. 2015;8:83-89. https://doi.org/10.2147/JMDH.S72633.
15. Kelly MM, Xie A, Li Y, et al. System factors influencing the use of a family-centered rounds checklist. Pediatr Qual Saf. 2019;4(4):e196. https://doi.org/10.1097/pq9.0000000000000196.
16. Kogan JR, Hatala R, Hauer KE, Holmboe E. Guidelines: The do’s, don’ts and don’t knows of direct observation of clinical skills in medical education. Perspect Med Educ. 2017;6(5):286-305. https://doi.org/10.1007/s40037-017-0376-7.
17. Williams KN, Ramani S, Fraser B, Orlander JD. Improving bedside teaching: findings from a focus group study of learners. Acad Med. 2008;83(3):257-264. https://doi.org/10.1097/ACM.0b013e3181637f3e.
18. Heckmann JG, Bleh C, Dütsch M, Lang CJG, Neundörfer B. Does improved problem-based teaching influence students’ knowledge at the end of their neurology elective? An observational study of 40 students. J Neurol. 2003;250(12):1464-1468. https://doi.org/10.1007/s00415-003-0255-5.
19. Committee on hospital care and institute for patient and family centered care. Patient- and family-centered care and the pediatrician’s role. Pediatrics. 2012;129(2):394-404. https://doi.org/10.1542/peds.2011-3084.
20. Dhaliwal G, Hauer KE. The oral patient presentation in the era of night float admissions. JAMA. 2013;310(21):2247. https://doi.org/10.1001/jama.2013.282322.
21. Wiese JG. Teaching in the Hospital. Philadelphia, PA: ACP PRess; 2010. https://books.google.co.uk/books?hl=en&lr=&id=qquGWP4d2Q4C&oi=fnd&pg=PR13&dq=Wiese+J.+2010.+ACP+Teaching+Medicine+Series:+Teaching+in+the+Hospital.+Philadelphia,+PA:+ACP+Press&ots=JSRFojkBSn&sig=c33tapsL9DzV9nuFhENA6eObISA#v=onepage&q=bedside round&f=fals. Accessed November 29, 2019.
22. Lopez M, Vaks Y, Wilson M, et al. Impacting satisfaction, learning, and efficiency through structured interdisciplinary rounding in a pediatric intensive care unit. Pediatr Qual Saf. 2019;4(3):e176. https://doi.org/10.1097/pq9.0000000000000176.
23. Benbassat J. Role modeling in medical education: the importance of a reflective imitation. Acad Med. 2014;89(4):550-554. https://doi.org/10.1097/ACM.0000000000000189.
24. Cox ED, Jacobsohn GC, Rajamanickam VP, et al. A family-centered rounds checklist, family engagement, and patient safety: a randomized trial. Pediatrics. 2017;139(5):e20161688. https://doi.org/10.1542/peds.2016-1688.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 32-year-old man with a history of polysubstance use disorder is hospitalized with endocarditis. The senior resident on the inpatient medical team suggests that the team “card flip” on this patient, citing a large number of patients on the team census, time constraints, and concerns that his substance use history will make bedside rounds uncomfortable.
BACKGROUND
“Rounds” is an inpatient care model in which teams of practitioners assess patients, determine care plans, and communicate with patients, families, and other healthcare professionals.1 One form of rounds is bedside rounding (BSR) through which an entire patient presentation occurs at the bedside, analogous to family-centered rounds common in pediatric inpatient care.2 This style of rounding is distinct from “walk rounding” that involves presentations occurring separately from a patient followed by a brief team bedside encounter. BSR is also different from “card flipping” or “table rounding” that involves presentations of a case separately without a team-patient encounter. The frequency of BSR at academic institutions has markedly decreased across the United States, and the time spent at the bedside is only a small fraction of rounding time.3
WHY YOU MIGHT THINK CARD FLIPPING IS HELPFUL
There are several reasons to employ strategies such as card-flipping or walk-rounding for discussing patient care away from the bedside. These BSR risks can be organized into patient harm, inefficiency, and risks to healthcare professional training.
First, BSR may result in patient harm. For example, discussing private health information in a semiprivate room may not only be uncomfortable for patients but may also violate patient privacy.4 Care teams are often large in number and rounding at the bedside can simultaneously trigger anxiety among patients, cause confusion about plans, or result in lack of clarity on the role of each provider.4 Furthermore, delivering bad news during BSR, or discussing sensitive topics such as substance use, psychiatric illness, or concerns of malingering behavior, may be difficult and uncomfortable.4,5 Additionally, some potential diagnoses, such as cancer or human immunodeficiency virus, even if unlikely, could induce panic among patients when they hear them being discussed.5 Trainees may also lose situational awareness because they focus on the agenda of bedside rounds and fail to respond to patients’ emotional needs.6
Efficiency is another reason to avoid BSR. The systemic factors of changing hospital demographics, such as short length of stay and increasing patient volumes, generate a substantial administrative burden on trainees.7 Modern trainees are also constrained by work hour restrictions, engagement with mandatory curricula, and other professional development opportunities. Furthermore, changes in a medical work environment cause trainees to rely heavily on electronic health records, which forces them to be at a computer instead of in a patient’s room.8 This confluence of factors results in substantial time pressure, and BSR is perceived as an inefficient use of time.9
The impact on education and trainee development is another concern of BSR. Rounding away from a patient ensures a safe environment for learners to interpret data and articulate clinical reasoning without the risk of embarrassment in front of a patient. This time outside a patient room also allows the team to have a shared mental model so that communication is aligned when a patient encounter does occur. Card flipping may result in improved trainee autonomy because the constant presence of attending supervision, particularly in front of patients, can risk undermining resident leadership and patient trust.9
WHY WE SHOULD RETURN TO THE BEDSIDE
The cited reasons for provider hesitancy to BSR, including possible patient harm and inefficiency, may be mostly related to individual perceptions and have recently been questioned.10,11
Several studies have suggested that bedside rounds may be better for patients’ experience over traditional walk-rounding or card-flipping models. In these studies, patients signal a preference for bedside rounds and suggest that discussing sensitive issues or concerning differential diagnoses during BSR may not be as concerning as physicians worry.11 For example, one randomized trial found that 87% of patients are untroubled by bedside discussions,12 and another trial revealed no difference between rounding models in emotional distress to patients or families.11 Patients and families also report higher levels of clarity from physicians, and they cited significantly improved levels of understanding their illness10 and test results.9 Furthermore, patients describe that physicians spend about twice as much time on their care when BSR is used.12 In many related studies, patients report a preference for BSR as a rounding strategy.2,11-13 For example, one study found that 99% of patients prefer BSR.13 Another study showed that 85% of families request to be part of bedside family-centered rounds over traditional walk rounding.2
Rounding away from a patient via card flipping or walk rounding seems more efficient, but this idea may be illusory. Although these strategies may seem faster, the lack of communication and coordination between team members and the patient may cause inefficiencies and delays in care throughout the day.14 For example, one study has demonstrated that family-centered bedside rounds are about 20% longer than walk rounding, but everyone involved, including housestaff, felt it was more efficient and saved time later in the day.2 Additionally, a study comparing BSR with walk rounding13 found no difference in time spent per patient, and another study has shown similar results in terms of family-centered rounds.15 Both studies have reported a similar amount of time spent per patient.
Physicians should return to BSR not only to improve patient experience but also to develop the clinical skills of trainees. The direct observation of trainees with patients allows high-level impactful clinical feedback and provides a basis for calibrating how much autonomy to allow.16 Trainees also indicate that teaching is more impactful during BSR than during walk rounding or card flipping, and clinical skill training during BSR is superior to a discussion in a conference room or a hallway context.2,3,15,17,18 One study has even suggested that the education of bedside rounds may help improve clinical skills in comparison with traditional models.18
The lack of BSR during medical school and residency training results in a deleterious cycle. Trainees become less proficient and less comfortable with BSR skills and therefore graduate as faculty members who are unskilled or uncomfortable insisting on BSR. As such, the cycle continues. As a result and as the traditional cornerstone of clinical training and inpatient care, BSR is recommended as standard practice by some professional organizations.19
WHAT WE SHOULD DO INSTEAD
Developing buy-in is an important first step for engaging in BSR. We recommend starting by demonstrating the value of BSR to overcome initial team or trainee hesitancy. Regardless of systems established to improve the efficiency of BSR, it is our experience that learners hesitantly engage if they do not understand the value of a given activity. We also urge attendings to demonstrate value by articulating how BSR fits in a patient-centered approach to emphasize the evidence-based positive impacts of BSR on patients.9 Beyond reviewing the benefits, faculty should set an expectation that the team will carry out BSR.9 Doing so sets an informal curriculum showing that BSR is important and sets the standard of care, which allows an inpatient team to adapt early in a rotation.
Next, faculty should ensure that BSR remains efficient.9 We believe that efficiency starts by setting expectations with patients. Patient expectations can be set by an attending or a supervising resident and should include a preview about how each encounter will progress, who will be in the room, how large the team will be, and what their role is during the encounter. Patients should be invited to be part of the discussion, offered an opportunity to opt out, and informed that questions arising from or clarifications needed following encounters can be addressed later within the day or after BSR. Nurses should be invited to actively participate during patient presentations. Each bedside encounter should be kept brief and standardized.20,21 To maximize efficiency, we also believe that roles should be delegated ahead of time and positioning in the room should be deliberate.22 Team members should know who is speaking when and in what order, who is accessing the electronic health record, and who will be examining the patient. Ideally, goals should be set ahead of time and tailored to each individual encounter. Finally, ensure everyone is on the same page by huddling briefly before each encounter to establish goals and roles and huddle afterward to debrief for learning and teamwork calibration.
In order to mitigate the learner’s anxiety about presenting in front of patients, build a partnership with the trainee, and time should be allotted to establish a safe learning environment.9 Sustain a supportive learning environment by providing positive feedback to learners in front of patients and teams. Faculty members should demonstrate how to bedside round effectively by leading initial encounters and generate momentum by selecting initial patient encounters that are most likely to succeed.23 Checklists can also be useful cognitive aids to facilitate an encounter and manage the cognitive load of learners.24 Ultimately, hesitancies can be overcome with experience.
Faculty members should ensure that bedside encounters are educationally valuable for an entire team.9 This initiative starts by preparing ahead of time, which allows the mental energy during encounters to be directly observed by learners in action.16 Preparation also allows the presentation to focus more on clinical reasoning rather than data gathering.20 Faculty members should also consider ways to foster resident autonomy and establish the role of a supervising resident as the team leader. Positioning in the room is critical22; we suggest that faculty members should position themselves near the head of the bed, out of a patient’s direct eyesight. In this way, they can observe how individual team members and the team as a whole interact with patients. The supervising resident should be at the foot of the bed, central to the team and the focal point of a patient’s view. The presenting intern or student should be seated near the head of the bed and opposite the supervising attending. Clinical teaching should also be kept short and pertinent to the patient, and questions should be phrased as “how” or “why” rather than “what” to reduce the risk of “wrong” answers in front of patients and the team.
WHEN IS CARD FLIPPING APPROPRIATE?
We believe that bedside rounds are most consistent with patient-centered inpatient care and should be considered the first-line approach. We also acknowledge that it is not always possible to bedside round on every patient on an inpatient census. For example, at an average of 13-15 minutes per patient,2,13 a census of 16 patients can take up to 4 hours to round. This timeline is not always feasible given the timing of training program didactics, interprofessional or case management rounds, and pressure for early discharges. Similar to all aspects of medicine, many approaches have been established to provide patient care, and context is important. Therefore, card flipping and walk rounding are beneficial to patients in some instances. For example, consider BSR for new, sick, or undifferentiated patients or when the history or exam findings need clarification; walk rounding or card flipping is suitable for patients with clear plans in place or when an encounter will be too disruptive to the rounding flow.21 Census size and individual patient or family concerns should dictate the style of rounding; in most situations, BSR may be equally efficient because it offers significant benefits to patients and families.
RECOMMENDATIONS
- Expectations should be set early with both trainees and patients. Patients should be informed that the team can come back later for more in-depth discussions.
- Trainees should be taught evidence-based approaches supporting the value of bedside rounds for patients.
- Faculty should consider leading initial encounters to demonstrate how to bedside round and to model behaviors.
- Positive feedback should be provided in front of patients and the team to build confidence.
- Encounters should be kept brief and efficient.
- A sufficient space for resident autonomy should be ensured through deliberate positioning, delegation of responsibilities, and huddling before and after encounters.
- Bedside rounds should be educationally worthwhile.
CONCLUSION
BSR is a traditional cornerstone of clinical training and inpatient care. Teaching at the bedside has many established benefits, such as connecting with patients and families, affording educators a valuable opportunity to assess learners and role model, and solidifying medical content by integrating teaching with clinical care. Concerns about bedside rounding may be based more on conjecture than on available evidence and can be overcome with deliberate education and proper planning. We propose several recommendations to successfully implement efficient, patient-centered, and educationally valuable bedside rounds.
For this (and most) patient(s), we recommend BSR. If this BSR is the first encounter, we suggest that the team should start with a more straightforward patient and come back to the new admission after the team has a chance to practice with other patients.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 32-year-old man with a history of polysubstance use disorder is hospitalized with endocarditis. The senior resident on the inpatient medical team suggests that the team “card flip” on this patient, citing a large number of patients on the team census, time constraints, and concerns that his substance use history will make bedside rounds uncomfortable.
BACKGROUND
“Rounds” is an inpatient care model in which teams of practitioners assess patients, determine care plans, and communicate with patients, families, and other healthcare professionals.1 One form of rounds is bedside rounding (BSR) through which an entire patient presentation occurs at the bedside, analogous to family-centered rounds common in pediatric inpatient care.2 This style of rounding is distinct from “walk rounding” that involves presentations occurring separately from a patient followed by a brief team bedside encounter. BSR is also different from “card flipping” or “table rounding” that involves presentations of a case separately without a team-patient encounter. The frequency of BSR at academic institutions has markedly decreased across the United States, and the time spent at the bedside is only a small fraction of rounding time.3
WHY YOU MIGHT THINK CARD FLIPPING IS HELPFUL
There are several reasons to employ strategies such as card-flipping or walk-rounding for discussing patient care away from the bedside. These BSR risks can be organized into patient harm, inefficiency, and risks to healthcare professional training.
First, BSR may result in patient harm. For example, discussing private health information in a semiprivate room may not only be uncomfortable for patients but may also violate patient privacy.4 Care teams are often large in number and rounding at the bedside can simultaneously trigger anxiety among patients, cause confusion about plans, or result in lack of clarity on the role of each provider.4 Furthermore, delivering bad news during BSR, or discussing sensitive topics such as substance use, psychiatric illness, or concerns of malingering behavior, may be difficult and uncomfortable.4,5 Additionally, some potential diagnoses, such as cancer or human immunodeficiency virus, even if unlikely, could induce panic among patients when they hear them being discussed.5 Trainees may also lose situational awareness because they focus on the agenda of bedside rounds and fail to respond to patients’ emotional needs.6
Efficiency is another reason to avoid BSR. The systemic factors of changing hospital demographics, such as short length of stay and increasing patient volumes, generate a substantial administrative burden on trainees.7 Modern trainees are also constrained by work hour restrictions, engagement with mandatory curricula, and other professional development opportunities. Furthermore, changes in a medical work environment cause trainees to rely heavily on electronic health records, which forces them to be at a computer instead of in a patient’s room.8 This confluence of factors results in substantial time pressure, and BSR is perceived as an inefficient use of time.9
The impact on education and trainee development is another concern of BSR. Rounding away from a patient ensures a safe environment for learners to interpret data and articulate clinical reasoning without the risk of embarrassment in front of a patient. This time outside a patient room also allows the team to have a shared mental model so that communication is aligned when a patient encounter does occur. Card flipping may result in improved trainee autonomy because the constant presence of attending supervision, particularly in front of patients, can risk undermining resident leadership and patient trust.9
WHY WE SHOULD RETURN TO THE BEDSIDE
The cited reasons for provider hesitancy to BSR, including possible patient harm and inefficiency, may be mostly related to individual perceptions and have recently been questioned.10,11
Several studies have suggested that bedside rounds may be better for patients’ experience over traditional walk-rounding or card-flipping models. In these studies, patients signal a preference for bedside rounds and suggest that discussing sensitive issues or concerning differential diagnoses during BSR may not be as concerning as physicians worry.11 For example, one randomized trial found that 87% of patients are untroubled by bedside discussions,12 and another trial revealed no difference between rounding models in emotional distress to patients or families.11 Patients and families also report higher levels of clarity from physicians, and they cited significantly improved levels of understanding their illness10 and test results.9 Furthermore, patients describe that physicians spend about twice as much time on their care when BSR is used.12 In many related studies, patients report a preference for BSR as a rounding strategy.2,11-13 For example, one study found that 99% of patients prefer BSR.13 Another study showed that 85% of families request to be part of bedside family-centered rounds over traditional walk rounding.2
Rounding away from a patient via card flipping or walk rounding seems more efficient, but this idea may be illusory. Although these strategies may seem faster, the lack of communication and coordination between team members and the patient may cause inefficiencies and delays in care throughout the day.14 For example, one study has demonstrated that family-centered bedside rounds are about 20% longer than walk rounding, but everyone involved, including housestaff, felt it was more efficient and saved time later in the day.2 Additionally, a study comparing BSR with walk rounding13 found no difference in time spent per patient, and another study has shown similar results in terms of family-centered rounds.15 Both studies have reported a similar amount of time spent per patient.
Physicians should return to BSR not only to improve patient experience but also to develop the clinical skills of trainees. The direct observation of trainees with patients allows high-level impactful clinical feedback and provides a basis for calibrating how much autonomy to allow.16 Trainees also indicate that teaching is more impactful during BSR than during walk rounding or card flipping, and clinical skill training during BSR is superior to a discussion in a conference room or a hallway context.2,3,15,17,18 One study has even suggested that the education of bedside rounds may help improve clinical skills in comparison with traditional models.18
The lack of BSR during medical school and residency training results in a deleterious cycle. Trainees become less proficient and less comfortable with BSR skills and therefore graduate as faculty members who are unskilled or uncomfortable insisting on BSR. As such, the cycle continues. As a result and as the traditional cornerstone of clinical training and inpatient care, BSR is recommended as standard practice by some professional organizations.19
WHAT WE SHOULD DO INSTEAD
Developing buy-in is an important first step for engaging in BSR. We recommend starting by demonstrating the value of BSR to overcome initial team or trainee hesitancy. Regardless of systems established to improve the efficiency of BSR, it is our experience that learners hesitantly engage if they do not understand the value of a given activity. We also urge attendings to demonstrate value by articulating how BSR fits in a patient-centered approach to emphasize the evidence-based positive impacts of BSR on patients.9 Beyond reviewing the benefits, faculty should set an expectation that the team will carry out BSR.9 Doing so sets an informal curriculum showing that BSR is important and sets the standard of care, which allows an inpatient team to adapt early in a rotation.
Next, faculty should ensure that BSR remains efficient.9 We believe that efficiency starts by setting expectations with patients. Patient expectations can be set by an attending or a supervising resident and should include a preview about how each encounter will progress, who will be in the room, how large the team will be, and what their role is during the encounter. Patients should be invited to be part of the discussion, offered an opportunity to opt out, and informed that questions arising from or clarifications needed following encounters can be addressed later within the day or after BSR. Nurses should be invited to actively participate during patient presentations. Each bedside encounter should be kept brief and standardized.20,21 To maximize efficiency, we also believe that roles should be delegated ahead of time and positioning in the room should be deliberate.22 Team members should know who is speaking when and in what order, who is accessing the electronic health record, and who will be examining the patient. Ideally, goals should be set ahead of time and tailored to each individual encounter. Finally, ensure everyone is on the same page by huddling briefly before each encounter to establish goals and roles and huddle afterward to debrief for learning and teamwork calibration.
In order to mitigate the learner’s anxiety about presenting in front of patients, build a partnership with the trainee, and time should be allotted to establish a safe learning environment.9 Sustain a supportive learning environment by providing positive feedback to learners in front of patients and teams. Faculty members should demonstrate how to bedside round effectively by leading initial encounters and generate momentum by selecting initial patient encounters that are most likely to succeed.23 Checklists can also be useful cognitive aids to facilitate an encounter and manage the cognitive load of learners.24 Ultimately, hesitancies can be overcome with experience.
Faculty members should ensure that bedside encounters are educationally valuable for an entire team.9 This initiative starts by preparing ahead of time, which allows the mental energy during encounters to be directly observed by learners in action.16 Preparation also allows the presentation to focus more on clinical reasoning rather than data gathering.20 Faculty members should also consider ways to foster resident autonomy and establish the role of a supervising resident as the team leader. Positioning in the room is critical22; we suggest that faculty members should position themselves near the head of the bed, out of a patient’s direct eyesight. In this way, they can observe how individual team members and the team as a whole interact with patients. The supervising resident should be at the foot of the bed, central to the team and the focal point of a patient’s view. The presenting intern or student should be seated near the head of the bed and opposite the supervising attending. Clinical teaching should also be kept short and pertinent to the patient, and questions should be phrased as “how” or “why” rather than “what” to reduce the risk of “wrong” answers in front of patients and the team.
WHEN IS CARD FLIPPING APPROPRIATE?
We believe that bedside rounds are most consistent with patient-centered inpatient care and should be considered the first-line approach. We also acknowledge that it is not always possible to bedside round on every patient on an inpatient census. For example, at an average of 13-15 minutes per patient,2,13 a census of 16 patients can take up to 4 hours to round. This timeline is not always feasible given the timing of training program didactics, interprofessional or case management rounds, and pressure for early discharges. Similar to all aspects of medicine, many approaches have been established to provide patient care, and context is important. Therefore, card flipping and walk rounding are beneficial to patients in some instances. For example, consider BSR for new, sick, or undifferentiated patients or when the history or exam findings need clarification; walk rounding or card flipping is suitable for patients with clear plans in place or when an encounter will be too disruptive to the rounding flow.21 Census size and individual patient or family concerns should dictate the style of rounding; in most situations, BSR may be equally efficient because it offers significant benefits to patients and families.
RECOMMENDATIONS
- Expectations should be set early with both trainees and patients. Patients should be informed that the team can come back later for more in-depth discussions.
- Trainees should be taught evidence-based approaches supporting the value of bedside rounds for patients.
- Faculty should consider leading initial encounters to demonstrate how to bedside round and to model behaviors.
- Positive feedback should be provided in front of patients and the team to build confidence.
- Encounters should be kept brief and efficient.
- A sufficient space for resident autonomy should be ensured through deliberate positioning, delegation of responsibilities, and huddling before and after encounters.
- Bedside rounds should be educationally worthwhile.
CONCLUSION
BSR is a traditional cornerstone of clinical training and inpatient care. Teaching at the bedside has many established benefits, such as connecting with patients and families, affording educators a valuable opportunity to assess learners and role model, and solidifying medical content by integrating teaching with clinical care. Concerns about bedside rounding may be based more on conjecture than on available evidence and can be overcome with deliberate education and proper planning. We propose several recommendations to successfully implement efficient, patient-centered, and educationally valuable bedside rounds.
For this (and most) patient(s), we recommend BSR. If this BSR is the first encounter, we suggest that the team should start with a more straightforward patient and come back to the new admission after the team has a chance to practice with other patients.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Gonzalo JD, Wolpaw DR, Lehman E, Chuang CH. Patient-centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040-1047. https://doi.org/10.1007/s11606-014-2817-x.
2. Muething SE, Kotagal UR, Schoettker PJ, del Rey JG, DeWitt TG. Family-centered bedside rounds: a new approach to patient care and teaching. Pediatrics. 2007;119(4):829-832. https://doi.org/10.1542/peds.2006-2528.
3. Ngo TL, Blankenburg R, Yu CE. Teaching at the bedside: strategies for optimizing education on patient and family centered rounds. Pediatr Clin North Am. 2019;66(4):881-889. https://doi.org/10.1016/j.pcl.2019.03.012.
4. Berkwitt A, Grossman M. A Qualitative analysis of pediatric patient attitudes regarding family-centered rounds. Hosp Pediatr. 2015;5(7):357. https://doi.org/10.1542/hpeds.2014-0198.
5. Rabinowitz R, Farnan J, Hulland O, et al. Rounds today: a qualitative study of internal medicine and pediatrics resident perceptions. J Grad Med Educ. 2016;8(4):523-531. https://doi.org/10.4300/JGME-D-15-00106.1.
6. Pingree EW, Freed JA, Riviello ED, et al. A tale of two rounds: managing conflict during the worst of times in family-centered rounds. Hosp Pediatr. 2019;9(7):563-565. https://doi.org/10.1542/hpeds.2019-0047.
7. Mamykina L, Vawdrey DK, Hripcsak G. How do residents spend their shift time? A time and motion study with a particular focus on the use of computers. Acad Med. 2016;91(6):827-832. https://doi.org/10.1097/ACM.0000000000001148.
8. Verghese A. Culture shock--patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. https://doi.org/10.1056/NEJMp0807461.
9. Gonzalo JD, Heist BS, Duffy BL, et al. Identifying and overcoming the barriers to bedside rounds: a multicenter qualitative study. Acad Med. 2014;89(2):326-334. https://doi.org/10.1097/ACM.0000000000000100.
10. Rogers HD, Carline JD, Paauw DS. Examination room presentations in general internal medicine clinic: patients’ and students’ perceptions. Acad Med. 2003;78(9):945-949. https://doi.org/10.1097/00001888-200309000-00023.
11. Landry M-A, Lafrenaye S, Roy M-C, Cyr C. A randomized, controlled trial of bedside versus conference-room case presentation in a pediatric intensive care unit. Pediatrics. 2007;120(2):275-280. https://doi.org/10.1542/peds.2007-0107.
12. Lehmann LS, Brancati FL, Chen M-C, Roter D, Dobs AS. The effect of bedside case presentations on patients’ perceptions of their medical care. N Engl J Med. 1997;336(16):1150-1156. https://doi.org/10.1056/NEJM199704173361606.
13. Gonzalo JD, Chuang CH, Huang G, Smith C. The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792-798. https://doi.org/10.1007/s11606-010-1344-7.
14. Okoniewska B, Santana MJ, Groshaus H, et al. Barriers to discharge in an acute care medical teaching unit: a qualitative analysis of health providers’ perceptions. J Multidiscip Healthc. 2015;8:83-89. https://doi.org/10.2147/JMDH.S72633.
15. Kelly MM, Xie A, Li Y, et al. System factors influencing the use of a family-centered rounds checklist. Pediatr Qual Saf. 2019;4(4):e196. https://doi.org/10.1097/pq9.0000000000000196.
16. Kogan JR, Hatala R, Hauer KE, Holmboe E. Guidelines: The do’s, don’ts and don’t knows of direct observation of clinical skills in medical education. Perspect Med Educ. 2017;6(5):286-305. https://doi.org/10.1007/s40037-017-0376-7.
17. Williams KN, Ramani S, Fraser B, Orlander JD. Improving bedside teaching: findings from a focus group study of learners. Acad Med. 2008;83(3):257-264. https://doi.org/10.1097/ACM.0b013e3181637f3e.
18. Heckmann JG, Bleh C, Dütsch M, Lang CJG, Neundörfer B. Does improved problem-based teaching influence students’ knowledge at the end of their neurology elective? An observational study of 40 students. J Neurol. 2003;250(12):1464-1468. https://doi.org/10.1007/s00415-003-0255-5.
19. Committee on hospital care and institute for patient and family centered care. Patient- and family-centered care and the pediatrician’s role. Pediatrics. 2012;129(2):394-404. https://doi.org/10.1542/peds.2011-3084.
20. Dhaliwal G, Hauer KE. The oral patient presentation in the era of night float admissions. JAMA. 2013;310(21):2247. https://doi.org/10.1001/jama.2013.282322.
21. Wiese JG. Teaching in the Hospital. Philadelphia, PA: ACP PRess; 2010. https://books.google.co.uk/books?hl=en&lr=&id=qquGWP4d2Q4C&oi=fnd&pg=PR13&dq=Wiese+J.+2010.+ACP+Teaching+Medicine+Series:+Teaching+in+the+Hospital.+Philadelphia,+PA:+ACP+Press&ots=JSRFojkBSn&sig=c33tapsL9DzV9nuFhENA6eObISA#v=onepage&q=bedside round&f=fals. Accessed November 29, 2019.
22. Lopez M, Vaks Y, Wilson M, et al. Impacting satisfaction, learning, and efficiency through structured interdisciplinary rounding in a pediatric intensive care unit. Pediatr Qual Saf. 2019;4(3):e176. https://doi.org/10.1097/pq9.0000000000000176.
23. Benbassat J. Role modeling in medical education: the importance of a reflective imitation. Acad Med. 2014;89(4):550-554. https://doi.org/10.1097/ACM.0000000000000189.
24. Cox ED, Jacobsohn GC, Rajamanickam VP, et al. A family-centered rounds checklist, family engagement, and patient safety: a randomized trial. Pediatrics. 2017;139(5):e20161688. https://doi.org/10.1542/peds.2016-1688.
1. Gonzalo JD, Wolpaw DR, Lehman E, Chuang CH. Patient-centered interprofessional collaborative care: factors associated with bedside interprofessional rounds. J Gen Intern Med. 2014;29(7):1040-1047. https://doi.org/10.1007/s11606-014-2817-x.
2. Muething SE, Kotagal UR, Schoettker PJ, del Rey JG, DeWitt TG. Family-centered bedside rounds: a new approach to patient care and teaching. Pediatrics. 2007;119(4):829-832. https://doi.org/10.1542/peds.2006-2528.
3. Ngo TL, Blankenburg R, Yu CE. Teaching at the bedside: strategies for optimizing education on patient and family centered rounds. Pediatr Clin North Am. 2019;66(4):881-889. https://doi.org/10.1016/j.pcl.2019.03.012.
4. Berkwitt A, Grossman M. A Qualitative analysis of pediatric patient attitudes regarding family-centered rounds. Hosp Pediatr. 2015;5(7):357. https://doi.org/10.1542/hpeds.2014-0198.
5. Rabinowitz R, Farnan J, Hulland O, et al. Rounds today: a qualitative study of internal medicine and pediatrics resident perceptions. J Grad Med Educ. 2016;8(4):523-531. https://doi.org/10.4300/JGME-D-15-00106.1.
6. Pingree EW, Freed JA, Riviello ED, et al. A tale of two rounds: managing conflict during the worst of times in family-centered rounds. Hosp Pediatr. 2019;9(7):563-565. https://doi.org/10.1542/hpeds.2019-0047.
7. Mamykina L, Vawdrey DK, Hripcsak G. How do residents spend their shift time? A time and motion study with a particular focus on the use of computers. Acad Med. 2016;91(6):827-832. https://doi.org/10.1097/ACM.0000000000001148.
8. Verghese A. Culture shock--patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. https://doi.org/10.1056/NEJMp0807461.
9. Gonzalo JD, Heist BS, Duffy BL, et al. Identifying and overcoming the barriers to bedside rounds: a multicenter qualitative study. Acad Med. 2014;89(2):326-334. https://doi.org/10.1097/ACM.0000000000000100.
10. Rogers HD, Carline JD, Paauw DS. Examination room presentations in general internal medicine clinic: patients’ and students’ perceptions. Acad Med. 2003;78(9):945-949. https://doi.org/10.1097/00001888-200309000-00023.
11. Landry M-A, Lafrenaye S, Roy M-C, Cyr C. A randomized, controlled trial of bedside versus conference-room case presentation in a pediatric intensive care unit. Pediatrics. 2007;120(2):275-280. https://doi.org/10.1542/peds.2007-0107.
12. Lehmann LS, Brancati FL, Chen M-C, Roter D, Dobs AS. The effect of bedside case presentations on patients’ perceptions of their medical care. N Engl J Med. 1997;336(16):1150-1156. https://doi.org/10.1056/NEJM199704173361606.
13. Gonzalo JD, Chuang CH, Huang G, Smith C. The return of bedside rounds: an educational intervention. J Gen Intern Med. 2010;25(8):792-798. https://doi.org/10.1007/s11606-010-1344-7.
14. Okoniewska B, Santana MJ, Groshaus H, et al. Barriers to discharge in an acute care medical teaching unit: a qualitative analysis of health providers’ perceptions. J Multidiscip Healthc. 2015;8:83-89. https://doi.org/10.2147/JMDH.S72633.
15. Kelly MM, Xie A, Li Y, et al. System factors influencing the use of a family-centered rounds checklist. Pediatr Qual Saf. 2019;4(4):e196. https://doi.org/10.1097/pq9.0000000000000196.
16. Kogan JR, Hatala R, Hauer KE, Holmboe E. Guidelines: The do’s, don’ts and don’t knows of direct observation of clinical skills in medical education. Perspect Med Educ. 2017;6(5):286-305. https://doi.org/10.1007/s40037-017-0376-7.
17. Williams KN, Ramani S, Fraser B, Orlander JD. Improving bedside teaching: findings from a focus group study of learners. Acad Med. 2008;83(3):257-264. https://doi.org/10.1097/ACM.0b013e3181637f3e.
18. Heckmann JG, Bleh C, Dütsch M, Lang CJG, Neundörfer B. Does improved problem-based teaching influence students’ knowledge at the end of their neurology elective? An observational study of 40 students. J Neurol. 2003;250(12):1464-1468. https://doi.org/10.1007/s00415-003-0255-5.
19. Committee on hospital care and institute for patient and family centered care. Patient- and family-centered care and the pediatrician’s role. Pediatrics. 2012;129(2):394-404. https://doi.org/10.1542/peds.2011-3084.
20. Dhaliwal G, Hauer KE. The oral patient presentation in the era of night float admissions. JAMA. 2013;310(21):2247. https://doi.org/10.1001/jama.2013.282322.
21. Wiese JG. Teaching in the Hospital. Philadelphia, PA: ACP PRess; 2010. https://books.google.co.uk/books?hl=en&lr=&id=qquGWP4d2Q4C&oi=fnd&pg=PR13&dq=Wiese+J.+2010.+ACP+Teaching+Medicine+Series:+Teaching+in+the+Hospital.+Philadelphia,+PA:+ACP+Press&ots=JSRFojkBSn&sig=c33tapsL9DzV9nuFhENA6eObISA#v=onepage&q=bedside round&f=fals. Accessed November 29, 2019.
22. Lopez M, Vaks Y, Wilson M, et al. Impacting satisfaction, learning, and efficiency through structured interdisciplinary rounding in a pediatric intensive care unit. Pediatr Qual Saf. 2019;4(3):e176. https://doi.org/10.1097/pq9.0000000000000176.
23. Benbassat J. Role modeling in medical education: the importance of a reflective imitation. Acad Med. 2014;89(4):550-554. https://doi.org/10.1097/ACM.0000000000000189.
24. Cox ED, Jacobsohn GC, Rajamanickam VP, et al. A family-centered rounds checklist, family engagement, and patient safety: a randomized trial. Pediatrics. 2017;139(5):e20161688. https://doi.org/10.1542/peds.2016-1688.
© 2020 Society of Hospital Medicine
Things We Do for No Reason™: Routinely Prescribing Transfusion Premedication To Prevent Acute Transfusion Reactions

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 68-year-old woman with a known history of myelodysplastic syndrome is admitted for fatigue and shortness of breath on exertion. Her hemoglobin concentration decreased from 9.1 g/dL to 6.5 g/dL. Her physical examination is unremarkable except for mild tachycardia with a heart rate of 105. She is scheduled to receive her first red blood cell (RBC) transfusion. The hospitalist orders premedication with acetaminophen and/or diphenhydramine to prevent an acute transfusion reaction.
BACKGROUND
The most frequent complications of blood transfusion are allergic transfusion reactions (ATRs) and febrile nonhemolytic transfusion reactions (FNHTRs), with a combined incidence of approximately 1%-4% per transfusion.1 ATRs may range in severity from mild urticaria to life-threatening anaphylaxis. FNHTRs manifest as a fever (oral temperature greater than or equal to 38°C/100.4°F and an increase of at least 1°C/1.8°F from pretransfusion values) or chills/rigors. With approximately 17 million blood transfusions, including RBCs, plasma, platelet, and cryoprecipitate components, administered annually in the United States, often to those with severe illnesses, ATRs and FNHTRs confer a substantial public health burden. Currently, the prevalence of premedication to prevent acute transfusion reactions in the United States and Canada is variable, ranging from 1.6% in one Canadian institution to as high as 80% in one large US hospital.2,3
WHY YOU MIGHT THINK PREMEDICATION IS HELPFUL TO PREVENT TRANSFUSION REACTIONS
FNHTRs are thought to be caused by cytokines elaborated by donor leukocytes that remain in blood products and/or by recipient antibodies reacting with donor leukocytes.1 While the clinical course is self-limited, these reactions can cause patients significant distress. The rationale behind acetaminophen premedication is to blunt the febrile response.
ATRs are usually mild, but anaphylaxis (which may include respiratory compromise, hypotension, and even death) can occur. They are caused by recipient histamine release in response to exposure to donor plasma proteins.1 This provides the theoretical rationale for antihistamine (eg, diphenhydramine) premedication as a prevention strategy.
Data on pretransfusion medication originate from the mid-20th century. In 1952, Ferris et al. published results showing a significant decrease in both febrile and ATRs when blood bottles were injected with an antihistamine.4 This was followed, in 1956, by Winter and Taplin’s further demonstration that both febrile and allergic reactions were significantly reduced when patients received units of blood injected with both oral acetylsalicylic acid and an antihistamine (chlorprophenpyridamine).5 These trials notably lacked appropriate controls and blinding, and numerous transfusion practice changes have taken place during the subsequent decades.
WHY PREMEDICATION TO PREVENT TRANSFUSION REACTION IS NOT HELPFUL
In the past 20 years, three double-blind randomized controlled trials published show that premedication with a combination of acetaminophen and an antihistamine (either diphenhydramine or chlorpheniramine) does not reduce the risk of ATR and FNHTR. The first study, published in 2002, randomized 51 patients with hematological malignancies receiving prestorage-irradiated, leukocyte-reduced, single-donor apheresis platelets to premedication with either acetaminophen and diphenhydramine or placebo.6 Patients with a history of either ATR or FNHTR were included, but patients with a history of hemolytic transfusion reaction were excluded.6 The study found that premedication did not significantly lower the incidence of these transfusion reactions (15.4%) as compared with placebo (15.2%; P = .94).6
In a larger study published in 2008, Kennedy et al. randomized 315 patients with hematological malignancies receiving RBC or platelet transfusion to either pretransfusion acetaminophen and diphenhydramine or placebo.7 Patients with a documented history of an ATR or FNHTR were excluded, which may have contributed to the lower incidence compared with the aforementioned earlier clinical trial. There was no significant difference in the overall rate of transfusion reactions between the two groups (1.44 per 100 transfusions vs 1.51 per 100 transfusions, P = .433). When the rates of ATRs and FNHTRs were analyzed separately, there was no significant difference between the treatment and control groups for either reaction type (P = .899 and P = .084, respectively). There was a trend toward a reduction in FNHTRs, but the authors calculated that we would need to premedicate approximately 344 transfusions to prevent one febrile reaction.7
A more recent study published in 2018 evaluated 147 Thai children and adolescents with thalassemia receiving leukoreduced blood products.8 Researchers randomized them to either premedication with acetaminophen and chlorpheniramine or placebo.8 The incidences of FNHTR were not statistically significantly different: 6.9% in the intervention group, compared with 9.5% in the placebo group (P = .565).8 These three studies constitute the best currently available evidence and suggest that pretransfusion antihistamines and/or antipyretics are not effective.
Beyond a lack of proven benefit, the use of premedication is not without risk. Diphenhydramine, the most commonly used antihistamine for premedication, can cause cognitive impairment, sedation, and delirium.9 Such adverse effects are potentially heightened in the elderly and seriously ill populations where transfusion commonly occurs. Acetaminophen, although generally safe, can result in hepatotoxicity in patients who are fasting, regularly consume alcohol, or have underlying liver disease. Since there is both a lack of clinical benefit and potential for harm, avoid premedication.
WHAT YOU SHOULD DO INSTEAD
Rather than pretreating the patient, consider modifying the blood product selected for transfusion. Administering platelet and/or RBC components with certain modifications (a product-centered approach) is effective at reducing mild transfusion reactions.10 A well-known product-centered modification method includes prestorage leukoreduction of RBC and platelet components to remove donor leukocytes to a level <5 × 106 per unit. This intervention reduces the incidence of FNHTRs by approximately 50%.11 A recent large, national survey demonstrated 90% of institutions (2,712/3,032) use universal leukoreduction.12 This widely employed and effective prevention strategy has likely helped reduce FNHTRs nationwide, so there are now fewer to prevent.12
Irradiation is another common modification of blood components used to prevent transfusion-associated graft-vs-host-disease (TA-GVHD) for recipients with significantly compromised cellular immunity. TA-GVHD is a rare but nearly universally fatal delayed complication of transfusion. Note that irradiation does not prevent FNHTRs or ATRs.
Under the premise that platelet-related allergic reactions are the result of recipient reaction to donor plasma proteins, reducing the plasma volume administered should decrease the coadministration of allergy-inducing plasma proteins.1 Reducing plasma volume can be achieved by two means: using a platelet additive solution that replaces two-thirds of the plasma content in a platelet unit or plasma removal by centrifugation. These two strategies decrease the plasma volume from 300 mL to ~100 mL per unit transfused, which effectively reduces the incidence of platelet-associated ATRs by 50%.10 For patients with recurrent severe ATRs, blood banks can wash RBC and platelet components, virtually removing all plasma proteins from the units.13 Epinephrine should be available at the bedside for patients with a history of severe ATRs.
Volume reduction and washing do negatively affect the quality of the unit: Platelets activate during the process, and transfusions result in a 20%-30% reduction in posttransfusion platelet counts.14 In addition, product manipulation takes significant blood bank processing time and results in an open system with greater risk of bacterial contamination, leading to a significantly shortened product expiration (24 hours for washed RBCs and 4 hours for washed or volume-reduced platelets).1 Reserve volume reduction and washing for patients with a history of multiple recurrent or severe ATRs, respectively. Platelet additive solution results in a reduction in posttransfusion count but does not require additional manipulation. Platelet additive solution products may not be available at many centers but could be used selectively (similar to volume reduction) depending on availability and cost.
Avoiding unnecessary transfusions is an essential strategy to prevent ATRs and FNHTRs. Evidence-based patient blood management (PBM), now considered the standard of care, is defined as optimizing anemia and hemostasis in patients with the goal of restricting blood transfusions. Evidence supporting restrictive transfusion strategies continues to accumulate, and numerous hospital systems have implemented PBM programs resulting in a significant nationwide reduction in transfusions since 2008. An effective PBM program reduces unnecessary transfusions and subsequent transfusion reactions.
Finally, appropriate close monitoring of patients undergoing blood transfusion and after completion of a transfusion is highly important. Paying close attention to signs and symptoms can alert the transfusing team to a developing adverse reaction and should prompt immediate cessation of an ongoing transfusion, the critical first step when a transfusion reaction is suspected. Hospitalists may need to take additional actions to treat the patient (eg, antihistamines after an ATR manifests or a diuretic in the setting of transfusion-associated circulatory overload). Report suspected transfusion reactions to the transfusion service. Failing to report a suspected transfusion reaction can lead to catastrophic consequences that can even be fatal.15
RECOMMENDATIONS
- Do not prescribe an antihistamine or acetaminophen prior to transfusion.
- Reduce the risk of FNHTRs in all transfusion recipients with universal prestorage leukoreduction.
- For individuals with multiple recurrent ATRs to platelets, employ platelet additive solution or platelet volume reduction.
- Reserve washing RBC and platelet components for patients with a history of severe ATRs. Make sure epinephrine is at the patient’s bedside.
- Curb unnecessary blood transfusions to reduce avoidable transfusion reactions.
- Monitor patients undergoing transfusion closely.
CONCLUSION
In our clinical scenario, there is no indication for premedication with acetaminophen and/or an antihistamine. Routine premedication is a low-value practice. Our RBC and platelet components are leukoreduced to prevent FNHTRs (and lower the risk of human leukocyte antigen alloimmunization and cytomegalovirus transmission). For individuals with multiple recurrent ATRs to platelets, we recommend platelet additive solution–stored or volume-reduced platelet components to lower the risk of future reactions. For patients with a history of severe ATRs, some blood banks may be able to provide washed components. Make sure epinephrine is at the patient’s bedside. Avoiding unnecessary transfusion is also essential to prevent adverse events related to blood transfusion—if a transfusion does not occur, then neither will a transfusion reaction. Finally, monitor patients undergoing transfusion closely.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
The authors have nothing to disclose.
1. Fung MK, Eder A, Spitalnik SL, Westhoff CM. American Association of Blood Banks Technical Manual. 19th Ed: Bethesda, Md: AABB; 2017.
2. Ezidiegwu CN, Lauenstein KJ, Rosales LG, Kelly KC, Henry JB. Febrile nonhemolytic transfusion reactions: management by premedication and cost implications in adult patients. Arch Pathol Lab Med. 2004;128(9):991-995. doi: 10.1043/1543-2165(2004)128<991:FNTR>2.0.CO;2.
3. Fry JL, Arnold DM, Clase CM, et al. Transfusion premedication to prevent acute transfusion reactions: a retrospective observational study to assess current practices. Transfusion. 2010;50(8):1722-1730. doi: 10.1111/j.1537-2995.2010.02636.x.
4. Ferris HE, Alpert S, Coakley CS. Prevention of allergic transfusion reactions; the prophylactic use of antihistamine in blood to prevent allergic transfusion reactions. Am Pract Dig Treat. 1952;3(3):177-183.
5. Winter CC, Taplin GV. Prevention of acute allergic and febrile reactions to blood transfusions by prophylactic use of an antihistamine plus an antipyretic. Ann Allergy. 1956;14(1):76-81.
6. Wang SE, Lara PN, Jr., Lee-Ow A, et al. Acetaminophen and diphenhydramine as premedication for platelet transfusions: a prospective randomized double-blind placebo-controlled trial. Am J Hematol. 2002;70(3):191-194. doi: 10.1002/ajh.10119.
7. Kennedy LD, Case LD, Hurd DD, Cruz JM, Pomper GJ. A prospective, randomized, double-blind controlled trial of acetaminophen and diphenhydramine pretransfusion medication versus placebo for the prevention of transfusion reactions. Transfusion. 2008;48(11):2285-2291. doi: 10.1111/j.1537-2995.2008.01858.x.
8. Rujkijyanont P, Monsereenusorn C, Manoonphol P, Traivaree C. Efficacy of oral acetaminophen and intravenous chlorpheniramine maleate versus placebo to prevent red cell transfusion reactions in children and adolescent with thalassemia: a prospective, randomized, double-blind controlled trial. Anemia. 2018;2018:9492303. doi: 10.1155/2018/9492303.
9. By the American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246. doi: 10.1111/jgs.13702.
10. Pagano MB, Katchatag BL, Khoobyari S, et al. Evaluating safety and cost-effectiveness of platelets stored in additive solution (PAS-F) as a hemolysis risk mitigation strategy. Transfusion. 2019;59(4):1246-1251. doi: 10.1111/trf.15138.
11. King KE, Shirey RS, Thoman SK, Bensen-Kennedy D, Tanz WS, Ness PM. Universal leukoreduction decreases the incidence of febrile nonhemolytic transfusion reactions to RBCs. Transfusion. 2004;44(1):25-29. doi: 10.1046/j.0041-1132.2004.00609.x.
12. Weisberg SP, Staley EM, Williams LA 3rd, et al. Survey on transfusion-transmitted cytomegalovirus and cytomegalovirus disease mitigation. Arch Pathol Lab Med. 2017;141(12):1705-1711. doi: 10.5858/arpa.2016-0461-OA.
13. Tobian AA, Savage WJ, Tisch DJ, Thoman S, King KE, Ness PM. Prevention of allergic transfusion reactions to platelets and red blood cells through plasma reduction. Transfusion. 2011;51(8):1676-1683. doi: 10.1111/j.1537-2995.2010.03008.x.
14. Veeraputhiran M, Ware J, Dent J, et al. A comparison of washed and volume-reduced platelets with respect to platelet activation, aggregation, and plasma protein removal. Transfusion. 2011;51(5):1030-1036. doi: 10.1111/j.1537-2995.2010.02897.x.
15. Corean J, Al-Tigar R, Pysher T, Blaylock R, Metcalf RA. Quality improvement after multiple fatal transfusion-transmitted bacterial infections. Am J Clin Pathol. 2018;149(4):293-299. doi: 10.1111/j.1537-2995.2010.02897.x.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 68-year-old woman with a known history of myelodysplastic syndrome is admitted for fatigue and shortness of breath on exertion. Her hemoglobin concentration decreased from 9.1 g/dL to 6.5 g/dL. Her physical examination is unremarkable except for mild tachycardia with a heart rate of 105. She is scheduled to receive her first red blood cell (RBC) transfusion. The hospitalist orders premedication with acetaminophen and/or diphenhydramine to prevent an acute transfusion reaction.
BACKGROUND
The most frequent complications of blood transfusion are allergic transfusion reactions (ATRs) and febrile nonhemolytic transfusion reactions (FNHTRs), with a combined incidence of approximately 1%-4% per transfusion.1 ATRs may range in severity from mild urticaria to life-threatening anaphylaxis. FNHTRs manifest as a fever (oral temperature greater than or equal to 38°C/100.4°F and an increase of at least 1°C/1.8°F from pretransfusion values) or chills/rigors. With approximately 17 million blood transfusions, including RBCs, plasma, platelet, and cryoprecipitate components, administered annually in the United States, often to those with severe illnesses, ATRs and FNHTRs confer a substantial public health burden. Currently, the prevalence of premedication to prevent acute transfusion reactions in the United States and Canada is variable, ranging from 1.6% in one Canadian institution to as high as 80% in one large US hospital.2,3
WHY YOU MIGHT THINK PREMEDICATION IS HELPFUL TO PREVENT TRANSFUSION REACTIONS
FNHTRs are thought to be caused by cytokines elaborated by donor leukocytes that remain in blood products and/or by recipient antibodies reacting with donor leukocytes.1 While the clinical course is self-limited, these reactions can cause patients significant distress. The rationale behind acetaminophen premedication is to blunt the febrile response.
ATRs are usually mild, but anaphylaxis (which may include respiratory compromise, hypotension, and even death) can occur. They are caused by recipient histamine release in response to exposure to donor plasma proteins.1 This provides the theoretical rationale for antihistamine (eg, diphenhydramine) premedication as a prevention strategy.
Data on pretransfusion medication originate from the mid-20th century. In 1952, Ferris et al. published results showing a significant decrease in both febrile and ATRs when blood bottles were injected with an antihistamine.4 This was followed, in 1956, by Winter and Taplin’s further demonstration that both febrile and allergic reactions were significantly reduced when patients received units of blood injected with both oral acetylsalicylic acid and an antihistamine (chlorprophenpyridamine).5 These trials notably lacked appropriate controls and blinding, and numerous transfusion practice changes have taken place during the subsequent decades.
WHY PREMEDICATION TO PREVENT TRANSFUSION REACTION IS NOT HELPFUL
In the past 20 years, three double-blind randomized controlled trials published show that premedication with a combination of acetaminophen and an antihistamine (either diphenhydramine or chlorpheniramine) does not reduce the risk of ATR and FNHTR. The first study, published in 2002, randomized 51 patients with hematological malignancies receiving prestorage-irradiated, leukocyte-reduced, single-donor apheresis platelets to premedication with either acetaminophen and diphenhydramine or placebo.6 Patients with a history of either ATR or FNHTR were included, but patients with a history of hemolytic transfusion reaction were excluded.6 The study found that premedication did not significantly lower the incidence of these transfusion reactions (15.4%) as compared with placebo (15.2%; P = .94).6
In a larger study published in 2008, Kennedy et al. randomized 315 patients with hematological malignancies receiving RBC or platelet transfusion to either pretransfusion acetaminophen and diphenhydramine or placebo.7 Patients with a documented history of an ATR or FNHTR were excluded, which may have contributed to the lower incidence compared with the aforementioned earlier clinical trial. There was no significant difference in the overall rate of transfusion reactions between the two groups (1.44 per 100 transfusions vs 1.51 per 100 transfusions, P = .433). When the rates of ATRs and FNHTRs were analyzed separately, there was no significant difference between the treatment and control groups for either reaction type (P = .899 and P = .084, respectively). There was a trend toward a reduction in FNHTRs, but the authors calculated that we would need to premedicate approximately 344 transfusions to prevent one febrile reaction.7
A more recent study published in 2018 evaluated 147 Thai children and adolescents with thalassemia receiving leukoreduced blood products.8 Researchers randomized them to either premedication with acetaminophen and chlorpheniramine or placebo.8 The incidences of FNHTR were not statistically significantly different: 6.9% in the intervention group, compared with 9.5% in the placebo group (P = .565).8 These three studies constitute the best currently available evidence and suggest that pretransfusion antihistamines and/or antipyretics are not effective.
Beyond a lack of proven benefit, the use of premedication is not without risk. Diphenhydramine, the most commonly used antihistamine for premedication, can cause cognitive impairment, sedation, and delirium.9 Such adverse effects are potentially heightened in the elderly and seriously ill populations where transfusion commonly occurs. Acetaminophen, although generally safe, can result in hepatotoxicity in patients who are fasting, regularly consume alcohol, or have underlying liver disease. Since there is both a lack of clinical benefit and potential for harm, avoid premedication.
WHAT YOU SHOULD DO INSTEAD
Rather than pretreating the patient, consider modifying the blood product selected for transfusion. Administering platelet and/or RBC components with certain modifications (a product-centered approach) is effective at reducing mild transfusion reactions.10 A well-known product-centered modification method includes prestorage leukoreduction of RBC and platelet components to remove donor leukocytes to a level <5 × 106 per unit. This intervention reduces the incidence of FNHTRs by approximately 50%.11 A recent large, national survey demonstrated 90% of institutions (2,712/3,032) use universal leukoreduction.12 This widely employed and effective prevention strategy has likely helped reduce FNHTRs nationwide, so there are now fewer to prevent.12
Irradiation is another common modification of blood components used to prevent transfusion-associated graft-vs-host-disease (TA-GVHD) for recipients with significantly compromised cellular immunity. TA-GVHD is a rare but nearly universally fatal delayed complication of transfusion. Note that irradiation does not prevent FNHTRs or ATRs.
Under the premise that platelet-related allergic reactions are the result of recipient reaction to donor plasma proteins, reducing the plasma volume administered should decrease the coadministration of allergy-inducing plasma proteins.1 Reducing plasma volume can be achieved by two means: using a platelet additive solution that replaces two-thirds of the plasma content in a platelet unit or plasma removal by centrifugation. These two strategies decrease the plasma volume from 300 mL to ~100 mL per unit transfused, which effectively reduces the incidence of platelet-associated ATRs by 50%.10 For patients with recurrent severe ATRs, blood banks can wash RBC and platelet components, virtually removing all plasma proteins from the units.13 Epinephrine should be available at the bedside for patients with a history of severe ATRs.
Volume reduction and washing do negatively affect the quality of the unit: Platelets activate during the process, and transfusions result in a 20%-30% reduction in posttransfusion platelet counts.14 In addition, product manipulation takes significant blood bank processing time and results in an open system with greater risk of bacterial contamination, leading to a significantly shortened product expiration (24 hours for washed RBCs and 4 hours for washed or volume-reduced platelets).1 Reserve volume reduction and washing for patients with a history of multiple recurrent or severe ATRs, respectively. Platelet additive solution results in a reduction in posttransfusion count but does not require additional manipulation. Platelet additive solution products may not be available at many centers but could be used selectively (similar to volume reduction) depending on availability and cost.
Avoiding unnecessary transfusions is an essential strategy to prevent ATRs and FNHTRs. Evidence-based patient blood management (PBM), now considered the standard of care, is defined as optimizing anemia and hemostasis in patients with the goal of restricting blood transfusions. Evidence supporting restrictive transfusion strategies continues to accumulate, and numerous hospital systems have implemented PBM programs resulting in a significant nationwide reduction in transfusions since 2008. An effective PBM program reduces unnecessary transfusions and subsequent transfusion reactions.
Finally, appropriate close monitoring of patients undergoing blood transfusion and after completion of a transfusion is highly important. Paying close attention to signs and symptoms can alert the transfusing team to a developing adverse reaction and should prompt immediate cessation of an ongoing transfusion, the critical first step when a transfusion reaction is suspected. Hospitalists may need to take additional actions to treat the patient (eg, antihistamines after an ATR manifests or a diuretic in the setting of transfusion-associated circulatory overload). Report suspected transfusion reactions to the transfusion service. Failing to report a suspected transfusion reaction can lead to catastrophic consequences that can even be fatal.15
RECOMMENDATIONS
- Do not prescribe an antihistamine or acetaminophen prior to transfusion.
- Reduce the risk of FNHTRs in all transfusion recipients with universal prestorage leukoreduction.
- For individuals with multiple recurrent ATRs to platelets, employ platelet additive solution or platelet volume reduction.
- Reserve washing RBC and platelet components for patients with a history of severe ATRs. Make sure epinephrine is at the patient’s bedside.
- Curb unnecessary blood transfusions to reduce avoidable transfusion reactions.
- Monitor patients undergoing transfusion closely.
CONCLUSION
In our clinical scenario, there is no indication for premedication with acetaminophen and/or an antihistamine. Routine premedication is a low-value practice. Our RBC and platelet components are leukoreduced to prevent FNHTRs (and lower the risk of human leukocyte antigen alloimmunization and cytomegalovirus transmission). For individuals with multiple recurrent ATRs to platelets, we recommend platelet additive solution–stored or volume-reduced platelet components to lower the risk of future reactions. For patients with a history of severe ATRs, some blood banks may be able to provide washed components. Make sure epinephrine is at the patient’s bedside. Avoiding unnecessary transfusion is also essential to prevent adverse events related to blood transfusion—if a transfusion does not occur, then neither will a transfusion reaction. Finally, monitor patients undergoing transfusion closely.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
The authors have nothing to disclose.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 68-year-old woman with a known history of myelodysplastic syndrome is admitted for fatigue and shortness of breath on exertion. Her hemoglobin concentration decreased from 9.1 g/dL to 6.5 g/dL. Her physical examination is unremarkable except for mild tachycardia with a heart rate of 105. She is scheduled to receive her first red blood cell (RBC) transfusion. The hospitalist orders premedication with acetaminophen and/or diphenhydramine to prevent an acute transfusion reaction.
BACKGROUND
The most frequent complications of blood transfusion are allergic transfusion reactions (ATRs) and febrile nonhemolytic transfusion reactions (FNHTRs), with a combined incidence of approximately 1%-4% per transfusion.1 ATRs may range in severity from mild urticaria to life-threatening anaphylaxis. FNHTRs manifest as a fever (oral temperature greater than or equal to 38°C/100.4°F and an increase of at least 1°C/1.8°F from pretransfusion values) or chills/rigors. With approximately 17 million blood transfusions, including RBCs, plasma, platelet, and cryoprecipitate components, administered annually in the United States, often to those with severe illnesses, ATRs and FNHTRs confer a substantial public health burden. Currently, the prevalence of premedication to prevent acute transfusion reactions in the United States and Canada is variable, ranging from 1.6% in one Canadian institution to as high as 80% in one large US hospital.2,3
WHY YOU MIGHT THINK PREMEDICATION IS HELPFUL TO PREVENT TRANSFUSION REACTIONS
FNHTRs are thought to be caused by cytokines elaborated by donor leukocytes that remain in blood products and/or by recipient antibodies reacting with donor leukocytes.1 While the clinical course is self-limited, these reactions can cause patients significant distress. The rationale behind acetaminophen premedication is to blunt the febrile response.
ATRs are usually mild, but anaphylaxis (which may include respiratory compromise, hypotension, and even death) can occur. They are caused by recipient histamine release in response to exposure to donor plasma proteins.1 This provides the theoretical rationale for antihistamine (eg, diphenhydramine) premedication as a prevention strategy.
Data on pretransfusion medication originate from the mid-20th century. In 1952, Ferris et al. published results showing a significant decrease in both febrile and ATRs when blood bottles were injected with an antihistamine.4 This was followed, in 1956, by Winter and Taplin’s further demonstration that both febrile and allergic reactions were significantly reduced when patients received units of blood injected with both oral acetylsalicylic acid and an antihistamine (chlorprophenpyridamine).5 These trials notably lacked appropriate controls and blinding, and numerous transfusion practice changes have taken place during the subsequent decades.
WHY PREMEDICATION TO PREVENT TRANSFUSION REACTION IS NOT HELPFUL
In the past 20 years, three double-blind randomized controlled trials published show that premedication with a combination of acetaminophen and an antihistamine (either diphenhydramine or chlorpheniramine) does not reduce the risk of ATR and FNHTR. The first study, published in 2002, randomized 51 patients with hematological malignancies receiving prestorage-irradiated, leukocyte-reduced, single-donor apheresis platelets to premedication with either acetaminophen and diphenhydramine or placebo.6 Patients with a history of either ATR or FNHTR were included, but patients with a history of hemolytic transfusion reaction were excluded.6 The study found that premedication did not significantly lower the incidence of these transfusion reactions (15.4%) as compared with placebo (15.2%; P = .94).6
In a larger study published in 2008, Kennedy et al. randomized 315 patients with hematological malignancies receiving RBC or platelet transfusion to either pretransfusion acetaminophen and diphenhydramine or placebo.7 Patients with a documented history of an ATR or FNHTR were excluded, which may have contributed to the lower incidence compared with the aforementioned earlier clinical trial. There was no significant difference in the overall rate of transfusion reactions between the two groups (1.44 per 100 transfusions vs 1.51 per 100 transfusions, P = .433). When the rates of ATRs and FNHTRs were analyzed separately, there was no significant difference between the treatment and control groups for either reaction type (P = .899 and P = .084, respectively). There was a trend toward a reduction in FNHTRs, but the authors calculated that we would need to premedicate approximately 344 transfusions to prevent one febrile reaction.7
A more recent study published in 2018 evaluated 147 Thai children and adolescents with thalassemia receiving leukoreduced blood products.8 Researchers randomized them to either premedication with acetaminophen and chlorpheniramine or placebo.8 The incidences of FNHTR were not statistically significantly different: 6.9% in the intervention group, compared with 9.5% in the placebo group (P = .565).8 These three studies constitute the best currently available evidence and suggest that pretransfusion antihistamines and/or antipyretics are not effective.
Beyond a lack of proven benefit, the use of premedication is not without risk. Diphenhydramine, the most commonly used antihistamine for premedication, can cause cognitive impairment, sedation, and delirium.9 Such adverse effects are potentially heightened in the elderly and seriously ill populations where transfusion commonly occurs. Acetaminophen, although generally safe, can result in hepatotoxicity in patients who are fasting, regularly consume alcohol, or have underlying liver disease. Since there is both a lack of clinical benefit and potential for harm, avoid premedication.
WHAT YOU SHOULD DO INSTEAD
Rather than pretreating the patient, consider modifying the blood product selected for transfusion. Administering platelet and/or RBC components with certain modifications (a product-centered approach) is effective at reducing mild transfusion reactions.10 A well-known product-centered modification method includes prestorage leukoreduction of RBC and platelet components to remove donor leukocytes to a level <5 × 106 per unit. This intervention reduces the incidence of FNHTRs by approximately 50%.11 A recent large, national survey demonstrated 90% of institutions (2,712/3,032) use universal leukoreduction.12 This widely employed and effective prevention strategy has likely helped reduce FNHTRs nationwide, so there are now fewer to prevent.12
Irradiation is another common modification of blood components used to prevent transfusion-associated graft-vs-host-disease (TA-GVHD) for recipients with significantly compromised cellular immunity. TA-GVHD is a rare but nearly universally fatal delayed complication of transfusion. Note that irradiation does not prevent FNHTRs or ATRs.
Under the premise that platelet-related allergic reactions are the result of recipient reaction to donor plasma proteins, reducing the plasma volume administered should decrease the coadministration of allergy-inducing plasma proteins.1 Reducing plasma volume can be achieved by two means: using a platelet additive solution that replaces two-thirds of the plasma content in a platelet unit or plasma removal by centrifugation. These two strategies decrease the plasma volume from 300 mL to ~100 mL per unit transfused, which effectively reduces the incidence of platelet-associated ATRs by 50%.10 For patients with recurrent severe ATRs, blood banks can wash RBC and platelet components, virtually removing all plasma proteins from the units.13 Epinephrine should be available at the bedside for patients with a history of severe ATRs.
Volume reduction and washing do negatively affect the quality of the unit: Platelets activate during the process, and transfusions result in a 20%-30% reduction in posttransfusion platelet counts.14 In addition, product manipulation takes significant blood bank processing time and results in an open system with greater risk of bacterial contamination, leading to a significantly shortened product expiration (24 hours for washed RBCs and 4 hours for washed or volume-reduced platelets).1 Reserve volume reduction and washing for patients with a history of multiple recurrent or severe ATRs, respectively. Platelet additive solution results in a reduction in posttransfusion count but does not require additional manipulation. Platelet additive solution products may not be available at many centers but could be used selectively (similar to volume reduction) depending on availability and cost.
Avoiding unnecessary transfusions is an essential strategy to prevent ATRs and FNHTRs. Evidence-based patient blood management (PBM), now considered the standard of care, is defined as optimizing anemia and hemostasis in patients with the goal of restricting blood transfusions. Evidence supporting restrictive transfusion strategies continues to accumulate, and numerous hospital systems have implemented PBM programs resulting in a significant nationwide reduction in transfusions since 2008. An effective PBM program reduces unnecessary transfusions and subsequent transfusion reactions.
Finally, appropriate close monitoring of patients undergoing blood transfusion and after completion of a transfusion is highly important. Paying close attention to signs and symptoms can alert the transfusing team to a developing adverse reaction and should prompt immediate cessation of an ongoing transfusion, the critical first step when a transfusion reaction is suspected. Hospitalists may need to take additional actions to treat the patient (eg, antihistamines after an ATR manifests or a diuretic in the setting of transfusion-associated circulatory overload). Report suspected transfusion reactions to the transfusion service. Failing to report a suspected transfusion reaction can lead to catastrophic consequences that can even be fatal.15
RECOMMENDATIONS
- Do not prescribe an antihistamine or acetaminophen prior to transfusion.
- Reduce the risk of FNHTRs in all transfusion recipients with universal prestorage leukoreduction.
- For individuals with multiple recurrent ATRs to platelets, employ platelet additive solution or platelet volume reduction.
- Reserve washing RBC and platelet components for patients with a history of severe ATRs. Make sure epinephrine is at the patient’s bedside.
- Curb unnecessary blood transfusions to reduce avoidable transfusion reactions.
- Monitor patients undergoing transfusion closely.
CONCLUSION
In our clinical scenario, there is no indication for premedication with acetaminophen and/or an antihistamine. Routine premedication is a low-value practice. Our RBC and platelet components are leukoreduced to prevent FNHTRs (and lower the risk of human leukocyte antigen alloimmunization and cytomegalovirus transmission). For individuals with multiple recurrent ATRs to platelets, we recommend platelet additive solution–stored or volume-reduced platelet components to lower the risk of future reactions. For patients with a history of severe ATRs, some blood banks may be able to provide washed components. Make sure epinephrine is at the patient’s bedside. Avoiding unnecessary transfusion is also essential to prevent adverse events related to blood transfusion—if a transfusion does not occur, then neither will a transfusion reaction. Finally, monitor patients undergoing transfusion closely.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing TWDFNR@hospitalmedicine.org.
Disclosures
The authors have nothing to disclose.
1. Fung MK, Eder A, Spitalnik SL, Westhoff CM. American Association of Blood Banks Technical Manual. 19th Ed: Bethesda, Md: AABB; 2017.
2. Ezidiegwu CN, Lauenstein KJ, Rosales LG, Kelly KC, Henry JB. Febrile nonhemolytic transfusion reactions: management by premedication and cost implications in adult patients. Arch Pathol Lab Med. 2004;128(9):991-995. doi: 10.1043/1543-2165(2004)128<991:FNTR>2.0.CO;2.
3. Fry JL, Arnold DM, Clase CM, et al. Transfusion premedication to prevent acute transfusion reactions: a retrospective observational study to assess current practices. Transfusion. 2010;50(8):1722-1730. doi: 10.1111/j.1537-2995.2010.02636.x.
4. Ferris HE, Alpert S, Coakley CS. Prevention of allergic transfusion reactions; the prophylactic use of antihistamine in blood to prevent allergic transfusion reactions. Am Pract Dig Treat. 1952;3(3):177-183.
5. Winter CC, Taplin GV. Prevention of acute allergic and febrile reactions to blood transfusions by prophylactic use of an antihistamine plus an antipyretic. Ann Allergy. 1956;14(1):76-81.
6. Wang SE, Lara PN, Jr., Lee-Ow A, et al. Acetaminophen and diphenhydramine as premedication for platelet transfusions: a prospective randomized double-blind placebo-controlled trial. Am J Hematol. 2002;70(3):191-194. doi: 10.1002/ajh.10119.
7. Kennedy LD, Case LD, Hurd DD, Cruz JM, Pomper GJ. A prospective, randomized, double-blind controlled trial of acetaminophen and diphenhydramine pretransfusion medication versus placebo for the prevention of transfusion reactions. Transfusion. 2008;48(11):2285-2291. doi: 10.1111/j.1537-2995.2008.01858.x.
8. Rujkijyanont P, Monsereenusorn C, Manoonphol P, Traivaree C. Efficacy of oral acetaminophen and intravenous chlorpheniramine maleate versus placebo to prevent red cell transfusion reactions in children and adolescent with thalassemia: a prospective, randomized, double-blind controlled trial. Anemia. 2018;2018:9492303. doi: 10.1155/2018/9492303.
9. By the American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246. doi: 10.1111/jgs.13702.
10. Pagano MB, Katchatag BL, Khoobyari S, et al. Evaluating safety and cost-effectiveness of platelets stored in additive solution (PAS-F) as a hemolysis risk mitigation strategy. Transfusion. 2019;59(4):1246-1251. doi: 10.1111/trf.15138.
11. King KE, Shirey RS, Thoman SK, Bensen-Kennedy D, Tanz WS, Ness PM. Universal leukoreduction decreases the incidence of febrile nonhemolytic transfusion reactions to RBCs. Transfusion. 2004;44(1):25-29. doi: 10.1046/j.0041-1132.2004.00609.x.
12. Weisberg SP, Staley EM, Williams LA 3rd, et al. Survey on transfusion-transmitted cytomegalovirus and cytomegalovirus disease mitigation. Arch Pathol Lab Med. 2017;141(12):1705-1711. doi: 10.5858/arpa.2016-0461-OA.
13. Tobian AA, Savage WJ, Tisch DJ, Thoman S, King KE, Ness PM. Prevention of allergic transfusion reactions to platelets and red blood cells through plasma reduction. Transfusion. 2011;51(8):1676-1683. doi: 10.1111/j.1537-2995.2010.03008.x.
14. Veeraputhiran M, Ware J, Dent J, et al. A comparison of washed and volume-reduced platelets with respect to platelet activation, aggregation, and plasma protein removal. Transfusion. 2011;51(5):1030-1036. doi: 10.1111/j.1537-2995.2010.02897.x.
15. Corean J, Al-Tigar R, Pysher T, Blaylock R, Metcalf RA. Quality improvement after multiple fatal transfusion-transmitted bacterial infections. Am J Clin Pathol. 2018;149(4):293-299. doi: 10.1111/j.1537-2995.2010.02897.x.
1. Fung MK, Eder A, Spitalnik SL, Westhoff CM. American Association of Blood Banks Technical Manual. 19th Ed: Bethesda, Md: AABB; 2017.
2. Ezidiegwu CN, Lauenstein KJ, Rosales LG, Kelly KC, Henry JB. Febrile nonhemolytic transfusion reactions: management by premedication and cost implications in adult patients. Arch Pathol Lab Med. 2004;128(9):991-995. doi: 10.1043/1543-2165(2004)128<991:FNTR>2.0.CO;2.
3. Fry JL, Arnold DM, Clase CM, et al. Transfusion premedication to prevent acute transfusion reactions: a retrospective observational study to assess current practices. Transfusion. 2010;50(8):1722-1730. doi: 10.1111/j.1537-2995.2010.02636.x.
4. Ferris HE, Alpert S, Coakley CS. Prevention of allergic transfusion reactions; the prophylactic use of antihistamine in blood to prevent allergic transfusion reactions. Am Pract Dig Treat. 1952;3(3):177-183.
5. Winter CC, Taplin GV. Prevention of acute allergic and febrile reactions to blood transfusions by prophylactic use of an antihistamine plus an antipyretic. Ann Allergy. 1956;14(1):76-81.
6. Wang SE, Lara PN, Jr., Lee-Ow A, et al. Acetaminophen and diphenhydramine as premedication for platelet transfusions: a prospective randomized double-blind placebo-controlled trial. Am J Hematol. 2002;70(3):191-194. doi: 10.1002/ajh.10119.
7. Kennedy LD, Case LD, Hurd DD, Cruz JM, Pomper GJ. A prospective, randomized, double-blind controlled trial of acetaminophen and diphenhydramine pretransfusion medication versus placebo for the prevention of transfusion reactions. Transfusion. 2008;48(11):2285-2291. doi: 10.1111/j.1537-2995.2008.01858.x.
8. Rujkijyanont P, Monsereenusorn C, Manoonphol P, Traivaree C. Efficacy of oral acetaminophen and intravenous chlorpheniramine maleate versus placebo to prevent red cell transfusion reactions in children and adolescent with thalassemia: a prospective, randomized, double-blind controlled trial. Anemia. 2018;2018:9492303. doi: 10.1155/2018/9492303.
9. By the American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246. doi: 10.1111/jgs.13702.
10. Pagano MB, Katchatag BL, Khoobyari S, et al. Evaluating safety and cost-effectiveness of platelets stored in additive solution (PAS-F) as a hemolysis risk mitigation strategy. Transfusion. 2019;59(4):1246-1251. doi: 10.1111/trf.15138.
11. King KE, Shirey RS, Thoman SK, Bensen-Kennedy D, Tanz WS, Ness PM. Universal leukoreduction decreases the incidence of febrile nonhemolytic transfusion reactions to RBCs. Transfusion. 2004;44(1):25-29. doi: 10.1046/j.0041-1132.2004.00609.x.
12. Weisberg SP, Staley EM, Williams LA 3rd, et al. Survey on transfusion-transmitted cytomegalovirus and cytomegalovirus disease mitigation. Arch Pathol Lab Med. 2017;141(12):1705-1711. doi: 10.5858/arpa.2016-0461-OA.
13. Tobian AA, Savage WJ, Tisch DJ, Thoman S, King KE, Ness PM. Prevention of allergic transfusion reactions to platelets and red blood cells through plasma reduction. Transfusion. 2011;51(8):1676-1683. doi: 10.1111/j.1537-2995.2010.03008.x.
14. Veeraputhiran M, Ware J, Dent J, et al. A comparison of washed and volume-reduced platelets with respect to platelet activation, aggregation, and plasma protein removal. Transfusion. 2011;51(5):1030-1036. doi: 10.1111/j.1537-2995.2010.02897.x.
15. Corean J, Al-Tigar R, Pysher T, Blaylock R, Metcalf RA. Quality improvement after multiple fatal transfusion-transmitted bacterial infections. Am J Clin Pathol. 2018;149(4):293-299. doi: 10.1111/j.1537-2995.2010.02897.x.
© 2020 Society of Hospital Medicine
Things We Do for No Reason™: Routine Thyroid-Stimulating Hormone Testing in the Hospital

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 62-year-old woman with chronic obstructive pulmonary disease (COPD) presents to the emergency department with shortness of breath, wheezing, and altered mental status (AMS). She is diagnosed with an acute COPD exacerbation with hypercarbic respiratory failure and is treated with nebulized albuterol/ipratropium and intravenous methylprednisolone. The hospitalist orders basic admission laboratory tests, including a thyroid-stimulating hormone (TSH) test for completeness, although she suspects that the patient’s AMS is secondary to hypercapnia. Upon review, the TSH level is low (0.12 mIU/L). A free T4 (FT4) level is ordered and returns mildly low (0.6 ng/dL). Somewhat puzzled, the hospitalist wonders if the patient might have central hypothyroidism and if further testing is needed.
BACKGROUND
Thyroid disease has a prevalence in adults of 4.6% and 1.3% for hypo- and hyperthyroidism, respectively.1 Severe manifestations of thyroid disease are rare, with an annual incidence of 0.2 per 100,0002 for thyroid storm and 1.08 per 1,000,0003 for myxedema coma in adults. Although most thyroid disease is mild and managed in the outpatient setting, inpatient thyroid testing is common, with evidence suggesting that 21%-100% of internal medicine admissions receive thyroid testing.4-7
WHY YOU MIGHT THINK ORDERING TSH ROUTINELY IS HELPFUL
Despite the rarity of severe thyroid disease, symptomatic hypo- or hyperthyroidism is often included in the differential diagnosis for a multitude of presenting problems to the hospital. Providers may view TSH as a simple means to rule out thyroid illness and narrow the diagnostic differential, particularly given the speed and availability of testing. In addition, cultural norms may encourage the routine assessment of thyroid function as a part of a thorough inpatient evaluation, even when alternative diagnoses could explain the patient’s symptoms.8 In many hospitals, TSH is included in emergency department laboratory panels and hospital admission order sets (sometimes as a preselected default), which can significantly influence prescriber ordering.4,6,7,9
Hardwick et al. conducted structured interviews with primary care providers to explore the factors contributing to high thyroid testing variability. Among the potential contributing factors identified were fear of a missed diagnosis, as well as the complexity and poor integration of electronic health records, which makes repeat testing easier than requesting outside records.10 Most importantly, providers may assume that all abnormal results indicate clinically relevant thyroid dysfunction despite differences between TSH test characteristics in inpatient vs outpatient settings.11
WHY ORDERING TSH ROUTINELY IS NOT HELPFUL AND IS UNNECCESSARY
The most important confounder of thyroid function testing in the hospital is nonthyroidal illness syndrome (NTIS), also known as sick euthyroid syndrome. Although the prevalence of unrecognized thyroid disease in hospitalized patients is 1%-2.5%,11 NTIS is observed in up to 62% of hospitalized patients and not exclusively in critically ill patients as previously thought.8 Risk factors include infection, stroke, myocardial infarction, kidney or liver injury, burns, malnutrition, malignancy, and recent surgery, as well as multiple medications.12 Contributing factors may include the effect of cytokines on thyroid-releasing hormone and TSH secretion, decreased deiodinase activity, and changes in thyroid hormone receptor activity.8 No one pattern of thyroid function testing is pathognomonic of NTIS.8,12
The high prevalence of NTIS reduces the specificity of TSH testing in hospitalized patients. In this population, Attia et al. determined that mild abnormalities (TSH 0.1-0.6 mIU/L or 6.7-20 mIU/L) have a positive likelihood ratio (LR+) of true thyroid disease of 0.0 and 0.74, respectively, counterintuitively reducing rather than increasing the posttest probability of thyroid disease. Although TSH levels <0.01 and >20 mIU/L carry a higher LR+ (7.7 and 11.1, respectively), the vast majority of abnormal TSH results in the hospital are mild, self-resolving, and do not change clinical management.5,11,13 Adlan et al. reported that only 1.2% of tested patients have very abnormal TSH results (4/751 with TSH <0.01 and 5/751 with TSH >10 mIU/L).5
Spencer et al. measured TSH and other thyroid function tests in 1,580 adult patients admitted to a large county hospital in the United States, without regard to symptoms or prior diagnosis of thyroid disease. They found that 519/1,580 (33%) had TSH values outside the laboratory reference range. Of the 1,580 patients, 329 were randomly selected for further analysis, and 29/329 (8.8%) were found to have true thyroid disease. The vast majority of these patients (22/29, 75.8%) had TSH levels <0.1 mIU/L or >20 mIU/L. Importantly, the authors did not indicate how many of the 29 patients had known preexisiting thyroid disease or clinical symptoms.13
Similarly, an Israeli study examined the utility of routine TSH testing upon admission to an internal medicine service. More than 1 in 10 patients had abnormal TSH results (11.8%, 232/1,966). After chart review, the majority of the abnormal results (52.2%, 121/232) were felt to be secondary to NTIS. Subclinical thyrotoxicosis and subclinical hypothyroidism were noted in a further 20.7% (48/232) and 18.5% (43/232) of the patients, respectively. Overall, in only nine patients (0.5%, 9/1,966) did TSH testing lead to a change in clinical management. In all these cases, patients were either already on a medication known to affect thyroid function (eg, levothyroxine, amiodarone) or the pretest probability of thyroid-related illness was elevated because of clinical presentation.4
Several institutions have implemented quality improvement (QI) initiatives to reduce inappropriate thyroid function testing without apparent compromise to clinical care.14 Although none included balancing measures within their QI design, the implementation of simple appropriateness guidelines, for example, has been shown to reduce the frequency of TSH ordering by as much as 50%, which suggests significant overtesting.5,15,16 Similarly, in a clustered randomized control trial, Thomas et al. demonstrated a significant reduction (odds ratio [OR] 0.82) in outpatient TSH ordering after the addition of a simple educational message to the order.17
HARMS ASSOCIATED WITH ROUTINE TSH TESTING
NTIS may cause TSH, T4, and even FT4 to increase or decrease, even in discordant patterns, such as in the case above. This makes interpretation difficult for the hospitalist, who may wonder
WHEN TO CONSIDER TSH TESTING
Given the limitations of TSH testing in hospitalized patients due to NTIS, the AACE/ATA recommend TSH measurement in hospitalized patients only in cases of high clinical suspicion for thyroid dysfunction (Grade A, Best Level Evidence 2).19 The specificity of TSH testing in the hospital setting is too low to justify screening for mild or subclinical disease.8 Instead, directed thyroid function testing should be performed for hospitalized patients with sufficient signs and symptoms to raise the pretest probability of a clinically relevant result (Table). According to Attia et al., the total number of signs and symptoms (rather than one particular sign or symptom) may be the most reliable indicator. In two outpatient studies (no inpatient data available), the presence of one to two signs or symptoms of thyroid disease yielded an LR+ of 0.11-0.2, three to four signs or symptoms yielded an LR+ of 0.74-1.14, and five or more signs or symptoms yielded an LR+ of 6.75-18.6.11 For example, if a general medical patient (prevalence of undiagnosed hypothyroidism estimated to be 0.6%) has constipation and fatigue (LR+ 0.2), then the pretest probability would be approximately 0.1%. If the TSH level results between 6.7 and 20 mIU/L (LR+ 0.74), the posttest probability of thyroid disease would remain only 0.1%. Alternatively, a general medical patient with five symptoms consistent with hypothyroidism (LR+ 18.6) would have a pretest probability of 10%. If the TSH level results >20 mIU/L (LR+ 11.1), then the posttest probability of hypothyroidism would be 55%.11
For patients on stable doses of thyroid hormone replacement, although it may seem logical to check a TSH level upon admission to the hospital, guidelines recommend monitoring levels routinely in the outpatient setting, at most once every 12 months. More frequent monitoring should be undertaken only if clinical symptoms suggest that a dose change may be needed,19 and routine hospital testing should be avoided because of the potential for misleading results.
However, in some specific clinical scenarios, it may be reasonable to test for thyroid disease. Guidelines suggest TSH testing in the evaluation of certain conditions such as atrial fibrillation20 and syndrome of inappropriate antidiuretic hormone (SIADH).21 In addition, in the evaluation of unexplained sinus tachycardia, it is reasonable to test for hyperthyroidism after more common causes (pain, anxiety, infection, anemia, drug ingestion, and beta-blocker withdrawal) have been excluded.22 In the evaluation of delirium, TSH may be an appropriate “second tier” test after more likely contributors have been excluded.23
RECOMMENDATIONS
- Do not routinely order TSH on admission given the low pretest probability of clinically significant thyroid disease.
- Do not routinely check TSH for inpatients on stable outpatient doses of thyroid hormone replacement.
- Reserve TSH testing for clinical scenarios in which there is either a high pretest probability of thyroid disease (five or more symptoms) or for the evaluation of specific clinical syndromes for which thyroid dysfunction is a known reversible contributor (such as atrial fibrillation, SIADH, unexplained sinus tachycardia, and delirium).
- Do not attempt to diagnose subclinical thyroid disease in the hospital.
- If NTIS is suspected, avoid further testing in the hospital. Repeating TFTs as an outpatient may be appropriate after resolution of the acute illness.
CONCLUSION
Routine TSH testing in hospitalized patients is unhelpful and often yields confusing results because of the low prevalence of unrecognized thyroid disease, the high prevalence of NTIS, and the resulting difficulty with interpretation of results. Mild TSH abnormalities in hospitalized patients do not predict clinically significant thyroid disease.4,11 The patient in the previously described clinical scenario has NTIS caused by acute on chronic illness and the effect of glucocorticoids. As the hospitalist suspected, the patient’s AMS was caused by hypercapnia. Reserving TSH testing for patients with clinical signs and symptoms of thyroid disease or for those with specific conditions has the potential to save healthcare dollars, prevent harm to patients associated with overtesting or overtreatment, and decrease time spent interpreting abnormal results of unclear significance.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Hollowell J, Staehling N, Flanders W, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499. https://doi.org/10.1210/jcem.87.2.8182.
2. Akamizu T, Satoh T, Isozaki O, et al. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid. 2012;22(7):661-679. https://doi.org/10.1089/thy.2011.0334.
3. Ono Y, Ono S, Yasunaga H, Matsui H, Fushimi K, Tanaka Y. Clinical characteristics and outcomes of myxedema coma: Analysis of a national inpatient database in Japan. J Epidemiol. 2017;27(3):117-122. https://doi.org/10.1016/j.je.2016.04.002.
4. Bashkin A, Yaakobi E, Nodelman M, Ronen O. Is routine measurement of TSH in hospitalized patients necessary? Endocr Connect. 2018;7(4):567-572. https://doi.org/10.1530/EC-18-0004.
5. Adlan M, Neel V, Lakra S, Bondugulapati LN, Premawardhana LD. Targeted thyroid testing in acute illness: Achieving success through audit. J Endocrinol Invest. 2011;34(8):e210-e213. https://doi.org/10.3275/7480.
6. Roti E, Gardini E, Magotti M, et al. Are thyroid function tests too frequently and inappropriately requested?. J Endocrinol Invest. 1999;22(3):184-190. https://doi.org/10.1007/bf03343539.
7. Dalal S, Bhesania S, Silber S, Mehta P. Use of electronic clinical decision support and hard stops to decrease unnecessary thyroid function testing. BMJ Qual Improv Rep. 2017;6(1):u223041.w8346. https://doi.org/10.1136/bmjquality.u223041.w8346.
8. Premawardhana L. Thyroid testing in acutely ill patients may be an expensive distraction. Biochem Med (Zagreb). 2017;27(300):300-307. https://doi.org/10.11613/bm.2017.033.
9. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. https://doi.org/10.1056/nejmsb071595.
10. Hardwick R, Heaton, J, Vaidya B, et al. Exploring reasons for variation in ordering thyroid function tests in primary care: A qualitative study. Qual Prim Care. 2014;22(6):256-261.
11. Attia J, Margetts P, Guyatt G. Diagnosis of thyroid disease in hospitalized patients: a systematic review. Arch Intern Med. 1999;159(7):658-665. https://doi.org/10.1001/archinte.159.7.658.
12. Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab. 2013;27(6):745-762. https://doi.org/10.1016/j.beem.2013.10.003.
13. Spencer C, Elgen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem. 1987;33(8):1391-1396.
14. Zhelev Z, Abbott R, Rogers M, et al. Effectiveness of interventions to reduce ordering of thyroid function tests: a systematic review. BMJ Open. 2016;6:e010065. https://doi.org/10.1136/bmjopen-2015-010065.
15. Daucort V, Saillour-Glenisson F, Michel P, Jutand MA, Abouelfath A. A multicenter cluster randomized controlled trial of strategies to improve thyroid function testing. Med Care. 2003;41(3):432-441. https://doi.org/10.1097/01.mlr.0000053216.33277.a4.
16. Toubert M, Chavret S, Cassinat B, Schlageter MH, Beressi JP, Rain JD. From guidelines to hospital practice: reducing inappropriate ordering of thyroid hormone and antibody tests. Eur J Endocrinol. 2000:605-610. https://doi.org/10.1530/eje.0.1420605.
17. Thomas RE, Croal BL, Ramsay C, Eccles M, Grimshaw J. Effect of enhanced feedback and brief educational reminder messages on laboratory test requesting in primary care: A cluster randomised trial. Lancet. 2006;367(9527):1990-1996. https://doi.org/10.1016/s0140-6736(06)68888-0.
18. Taylor P, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels—balancing benefits and risks. JAMA Intern Med. 2014;174(1):32. https://doi.org/10.1001/jamainternmed.2013.11312.
19. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American association of clinical endocrinologists and the American thyroid association. Thyroid. 2012;22(12):1200-1235. https://doi.org/ 10.1089/thy.2012.0205.
20. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1-e76. https://doi.org/10.1016/j.jacc.2014.03.022.
21. Verbalis J, Goldsmith S, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: Expert panel recommendations. Am J Med. 2013;126(10):S1-S42. https://doi.org/10.1016/j.amjmed.2013.07.006.
22. Olshansky B, Sullivan R. Inappropriate sinus tachycardia. J Am Coll Cardiol. 2013;61(8):793-801. https://doi.org/10.1016/j.jacc.2012.07.074.
23. Josephson SA, Miller BL. Confusion and delirium. In: Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine, 20e. New York, NY: McGraw-Hill; http://accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192011608. Accessed January 29, 2019.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 62-year-old woman with chronic obstructive pulmonary disease (COPD) presents to the emergency department with shortness of breath, wheezing, and altered mental status (AMS). She is diagnosed with an acute COPD exacerbation with hypercarbic respiratory failure and is treated with nebulized albuterol/ipratropium and intravenous methylprednisolone. The hospitalist orders basic admission laboratory tests, including a thyroid-stimulating hormone (TSH) test for completeness, although she suspects that the patient’s AMS is secondary to hypercapnia. Upon review, the TSH level is low (0.12 mIU/L). A free T4 (FT4) level is ordered and returns mildly low (0.6 ng/dL). Somewhat puzzled, the hospitalist wonders if the patient might have central hypothyroidism and if further testing is needed.
BACKGROUND
Thyroid disease has a prevalence in adults of 4.6% and 1.3% for hypo- and hyperthyroidism, respectively.1 Severe manifestations of thyroid disease are rare, with an annual incidence of 0.2 per 100,0002 for thyroid storm and 1.08 per 1,000,0003 for myxedema coma in adults. Although most thyroid disease is mild and managed in the outpatient setting, inpatient thyroid testing is common, with evidence suggesting that 21%-100% of internal medicine admissions receive thyroid testing.4-7
WHY YOU MIGHT THINK ORDERING TSH ROUTINELY IS HELPFUL
Despite the rarity of severe thyroid disease, symptomatic hypo- or hyperthyroidism is often included in the differential diagnosis for a multitude of presenting problems to the hospital. Providers may view TSH as a simple means to rule out thyroid illness and narrow the diagnostic differential, particularly given the speed and availability of testing. In addition, cultural norms may encourage the routine assessment of thyroid function as a part of a thorough inpatient evaluation, even when alternative diagnoses could explain the patient’s symptoms.8 In many hospitals, TSH is included in emergency department laboratory panels and hospital admission order sets (sometimes as a preselected default), which can significantly influence prescriber ordering.4,6,7,9
Hardwick et al. conducted structured interviews with primary care providers to explore the factors contributing to high thyroid testing variability. Among the potential contributing factors identified were fear of a missed diagnosis, as well as the complexity and poor integration of electronic health records, which makes repeat testing easier than requesting outside records.10 Most importantly, providers may assume that all abnormal results indicate clinically relevant thyroid dysfunction despite differences between TSH test characteristics in inpatient vs outpatient settings.11
WHY ORDERING TSH ROUTINELY IS NOT HELPFUL AND IS UNNECCESSARY
The most important confounder of thyroid function testing in the hospital is nonthyroidal illness syndrome (NTIS), also known as sick euthyroid syndrome. Although the prevalence of unrecognized thyroid disease in hospitalized patients is 1%-2.5%,11 NTIS is observed in up to 62% of hospitalized patients and not exclusively in critically ill patients as previously thought.8 Risk factors include infection, stroke, myocardial infarction, kidney or liver injury, burns, malnutrition, malignancy, and recent surgery, as well as multiple medications.12 Contributing factors may include the effect of cytokines on thyroid-releasing hormone and TSH secretion, decreased deiodinase activity, and changes in thyroid hormone receptor activity.8 No one pattern of thyroid function testing is pathognomonic of NTIS.8,12
The high prevalence of NTIS reduces the specificity of TSH testing in hospitalized patients. In this population, Attia et al. determined that mild abnormalities (TSH 0.1-0.6 mIU/L or 6.7-20 mIU/L) have a positive likelihood ratio (LR+) of true thyroid disease of 0.0 and 0.74, respectively, counterintuitively reducing rather than increasing the posttest probability of thyroid disease. Although TSH levels <0.01 and >20 mIU/L carry a higher LR+ (7.7 and 11.1, respectively), the vast majority of abnormal TSH results in the hospital are mild, self-resolving, and do not change clinical management.5,11,13 Adlan et al. reported that only 1.2% of tested patients have very abnormal TSH results (4/751 with TSH <0.01 and 5/751 with TSH >10 mIU/L).5
Spencer et al. measured TSH and other thyroid function tests in 1,580 adult patients admitted to a large county hospital in the United States, without regard to symptoms or prior diagnosis of thyroid disease. They found that 519/1,580 (33%) had TSH values outside the laboratory reference range. Of the 1,580 patients, 329 were randomly selected for further analysis, and 29/329 (8.8%) were found to have true thyroid disease. The vast majority of these patients (22/29, 75.8%) had TSH levels <0.1 mIU/L or >20 mIU/L. Importantly, the authors did not indicate how many of the 29 patients had known preexisiting thyroid disease or clinical symptoms.13
Similarly, an Israeli study examined the utility of routine TSH testing upon admission to an internal medicine service. More than 1 in 10 patients had abnormal TSH results (11.8%, 232/1,966). After chart review, the majority of the abnormal results (52.2%, 121/232) were felt to be secondary to NTIS. Subclinical thyrotoxicosis and subclinical hypothyroidism were noted in a further 20.7% (48/232) and 18.5% (43/232) of the patients, respectively. Overall, in only nine patients (0.5%, 9/1,966) did TSH testing lead to a change in clinical management. In all these cases, patients were either already on a medication known to affect thyroid function (eg, levothyroxine, amiodarone) or the pretest probability of thyroid-related illness was elevated because of clinical presentation.4
Several institutions have implemented quality improvement (QI) initiatives to reduce inappropriate thyroid function testing without apparent compromise to clinical care.14 Although none included balancing measures within their QI design, the implementation of simple appropriateness guidelines, for example, has been shown to reduce the frequency of TSH ordering by as much as 50%, which suggests significant overtesting.5,15,16 Similarly, in a clustered randomized control trial, Thomas et al. demonstrated a significant reduction (odds ratio [OR] 0.82) in outpatient TSH ordering after the addition of a simple educational message to the order.17
HARMS ASSOCIATED WITH ROUTINE TSH TESTING
NTIS may cause TSH, T4, and even FT4 to increase or decrease, even in discordant patterns, such as in the case above. This makes interpretation difficult for the hospitalist, who may wonder
WHEN TO CONSIDER TSH TESTING
Given the limitations of TSH testing in hospitalized patients due to NTIS, the AACE/ATA recommend TSH measurement in hospitalized patients only in cases of high clinical suspicion for thyroid dysfunction (Grade A, Best Level Evidence 2).19 The specificity of TSH testing in the hospital setting is too low to justify screening for mild or subclinical disease.8 Instead, directed thyroid function testing should be performed for hospitalized patients with sufficient signs and symptoms to raise the pretest probability of a clinically relevant result (Table). According to Attia et al., the total number of signs and symptoms (rather than one particular sign or symptom) may be the most reliable indicator. In two outpatient studies (no inpatient data available), the presence of one to two signs or symptoms of thyroid disease yielded an LR+ of 0.11-0.2, three to four signs or symptoms yielded an LR+ of 0.74-1.14, and five or more signs or symptoms yielded an LR+ of 6.75-18.6.11 For example, if a general medical patient (prevalence of undiagnosed hypothyroidism estimated to be 0.6%) has constipation and fatigue (LR+ 0.2), then the pretest probability would be approximately 0.1%. If the TSH level results between 6.7 and 20 mIU/L (LR+ 0.74), the posttest probability of thyroid disease would remain only 0.1%. Alternatively, a general medical patient with five symptoms consistent with hypothyroidism (LR+ 18.6) would have a pretest probability of 10%. If the TSH level results >20 mIU/L (LR+ 11.1), then the posttest probability of hypothyroidism would be 55%.11
For patients on stable doses of thyroid hormone replacement, although it may seem logical to check a TSH level upon admission to the hospital, guidelines recommend monitoring levels routinely in the outpatient setting, at most once every 12 months. More frequent monitoring should be undertaken only if clinical symptoms suggest that a dose change may be needed,19 and routine hospital testing should be avoided because of the potential for misleading results.
However, in some specific clinical scenarios, it may be reasonable to test for thyroid disease. Guidelines suggest TSH testing in the evaluation of certain conditions such as atrial fibrillation20 and syndrome of inappropriate antidiuretic hormone (SIADH).21 In addition, in the evaluation of unexplained sinus tachycardia, it is reasonable to test for hyperthyroidism after more common causes (pain, anxiety, infection, anemia, drug ingestion, and beta-blocker withdrawal) have been excluded.22 In the evaluation of delirium, TSH may be an appropriate “second tier” test after more likely contributors have been excluded.23
RECOMMENDATIONS
- Do not routinely order TSH on admission given the low pretest probability of clinically significant thyroid disease.
- Do not routinely check TSH for inpatients on stable outpatient doses of thyroid hormone replacement.
- Reserve TSH testing for clinical scenarios in which there is either a high pretest probability of thyroid disease (five or more symptoms) or for the evaluation of specific clinical syndromes for which thyroid dysfunction is a known reversible contributor (such as atrial fibrillation, SIADH, unexplained sinus tachycardia, and delirium).
- Do not attempt to diagnose subclinical thyroid disease in the hospital.
- If NTIS is suspected, avoid further testing in the hospital. Repeating TFTs as an outpatient may be appropriate after resolution of the acute illness.
CONCLUSION
Routine TSH testing in hospitalized patients is unhelpful and often yields confusing results because of the low prevalence of unrecognized thyroid disease, the high prevalence of NTIS, and the resulting difficulty with interpretation of results. Mild TSH abnormalities in hospitalized patients do not predict clinically significant thyroid disease.4,11 The patient in the previously described clinical scenario has NTIS caused by acute on chronic illness and the effect of glucocorticoids. As the hospitalist suspected, the patient’s AMS was caused by hypercapnia. Reserving TSH testing for patients with clinical signs and symptoms of thyroid disease or for those with specific conditions has the potential to save healthcare dollars, prevent harm to patients associated with overtesting or overtreatment, and decrease time spent interpreting abnormal results of unclear significance.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 62-year-old woman with chronic obstructive pulmonary disease (COPD) presents to the emergency department with shortness of breath, wheezing, and altered mental status (AMS). She is diagnosed with an acute COPD exacerbation with hypercarbic respiratory failure and is treated with nebulized albuterol/ipratropium and intravenous methylprednisolone. The hospitalist orders basic admission laboratory tests, including a thyroid-stimulating hormone (TSH) test for completeness, although she suspects that the patient’s AMS is secondary to hypercapnia. Upon review, the TSH level is low (0.12 mIU/L). A free T4 (FT4) level is ordered and returns mildly low (0.6 ng/dL). Somewhat puzzled, the hospitalist wonders if the patient might have central hypothyroidism and if further testing is needed.
BACKGROUND
Thyroid disease has a prevalence in adults of 4.6% and 1.3% for hypo- and hyperthyroidism, respectively.1 Severe manifestations of thyroid disease are rare, with an annual incidence of 0.2 per 100,0002 for thyroid storm and 1.08 per 1,000,0003 for myxedema coma in adults. Although most thyroid disease is mild and managed in the outpatient setting, inpatient thyroid testing is common, with evidence suggesting that 21%-100% of internal medicine admissions receive thyroid testing.4-7
WHY YOU MIGHT THINK ORDERING TSH ROUTINELY IS HELPFUL
Despite the rarity of severe thyroid disease, symptomatic hypo- or hyperthyroidism is often included in the differential diagnosis for a multitude of presenting problems to the hospital. Providers may view TSH as a simple means to rule out thyroid illness and narrow the diagnostic differential, particularly given the speed and availability of testing. In addition, cultural norms may encourage the routine assessment of thyroid function as a part of a thorough inpatient evaluation, even when alternative diagnoses could explain the patient’s symptoms.8 In many hospitals, TSH is included in emergency department laboratory panels and hospital admission order sets (sometimes as a preselected default), which can significantly influence prescriber ordering.4,6,7,9
Hardwick et al. conducted structured interviews with primary care providers to explore the factors contributing to high thyroid testing variability. Among the potential contributing factors identified were fear of a missed diagnosis, as well as the complexity and poor integration of electronic health records, which makes repeat testing easier than requesting outside records.10 Most importantly, providers may assume that all abnormal results indicate clinically relevant thyroid dysfunction despite differences between TSH test characteristics in inpatient vs outpatient settings.11
WHY ORDERING TSH ROUTINELY IS NOT HELPFUL AND IS UNNECCESSARY
The most important confounder of thyroid function testing in the hospital is nonthyroidal illness syndrome (NTIS), also known as sick euthyroid syndrome. Although the prevalence of unrecognized thyroid disease in hospitalized patients is 1%-2.5%,11 NTIS is observed in up to 62% of hospitalized patients and not exclusively in critically ill patients as previously thought.8 Risk factors include infection, stroke, myocardial infarction, kidney or liver injury, burns, malnutrition, malignancy, and recent surgery, as well as multiple medications.12 Contributing factors may include the effect of cytokines on thyroid-releasing hormone and TSH secretion, decreased deiodinase activity, and changes in thyroid hormone receptor activity.8 No one pattern of thyroid function testing is pathognomonic of NTIS.8,12
The high prevalence of NTIS reduces the specificity of TSH testing in hospitalized patients. In this population, Attia et al. determined that mild abnormalities (TSH 0.1-0.6 mIU/L or 6.7-20 mIU/L) have a positive likelihood ratio (LR+) of true thyroid disease of 0.0 and 0.74, respectively, counterintuitively reducing rather than increasing the posttest probability of thyroid disease. Although TSH levels <0.01 and >20 mIU/L carry a higher LR+ (7.7 and 11.1, respectively), the vast majority of abnormal TSH results in the hospital are mild, self-resolving, and do not change clinical management.5,11,13 Adlan et al. reported that only 1.2% of tested patients have very abnormal TSH results (4/751 with TSH <0.01 and 5/751 with TSH >10 mIU/L).5
Spencer et al. measured TSH and other thyroid function tests in 1,580 adult patients admitted to a large county hospital in the United States, without regard to symptoms or prior diagnosis of thyroid disease. They found that 519/1,580 (33%) had TSH values outside the laboratory reference range. Of the 1,580 patients, 329 were randomly selected for further analysis, and 29/329 (8.8%) were found to have true thyroid disease. The vast majority of these patients (22/29, 75.8%) had TSH levels <0.1 mIU/L or >20 mIU/L. Importantly, the authors did not indicate how many of the 29 patients had known preexisiting thyroid disease or clinical symptoms.13
Similarly, an Israeli study examined the utility of routine TSH testing upon admission to an internal medicine service. More than 1 in 10 patients had abnormal TSH results (11.8%, 232/1,966). After chart review, the majority of the abnormal results (52.2%, 121/232) were felt to be secondary to NTIS. Subclinical thyrotoxicosis and subclinical hypothyroidism were noted in a further 20.7% (48/232) and 18.5% (43/232) of the patients, respectively. Overall, in only nine patients (0.5%, 9/1,966) did TSH testing lead to a change in clinical management. In all these cases, patients were either already on a medication known to affect thyroid function (eg, levothyroxine, amiodarone) or the pretest probability of thyroid-related illness was elevated because of clinical presentation.4
Several institutions have implemented quality improvement (QI) initiatives to reduce inappropriate thyroid function testing without apparent compromise to clinical care.14 Although none included balancing measures within their QI design, the implementation of simple appropriateness guidelines, for example, has been shown to reduce the frequency of TSH ordering by as much as 50%, which suggests significant overtesting.5,15,16 Similarly, in a clustered randomized control trial, Thomas et al. demonstrated a significant reduction (odds ratio [OR] 0.82) in outpatient TSH ordering after the addition of a simple educational message to the order.17
HARMS ASSOCIATED WITH ROUTINE TSH TESTING
NTIS may cause TSH, T4, and even FT4 to increase or decrease, even in discordant patterns, such as in the case above. This makes interpretation difficult for the hospitalist, who may wonder
WHEN TO CONSIDER TSH TESTING
Given the limitations of TSH testing in hospitalized patients due to NTIS, the AACE/ATA recommend TSH measurement in hospitalized patients only in cases of high clinical suspicion for thyroid dysfunction (Grade A, Best Level Evidence 2).19 The specificity of TSH testing in the hospital setting is too low to justify screening for mild or subclinical disease.8 Instead, directed thyroid function testing should be performed for hospitalized patients with sufficient signs and symptoms to raise the pretest probability of a clinically relevant result (Table). According to Attia et al., the total number of signs and symptoms (rather than one particular sign or symptom) may be the most reliable indicator. In two outpatient studies (no inpatient data available), the presence of one to two signs or symptoms of thyroid disease yielded an LR+ of 0.11-0.2, three to four signs or symptoms yielded an LR+ of 0.74-1.14, and five or more signs or symptoms yielded an LR+ of 6.75-18.6.11 For example, if a general medical patient (prevalence of undiagnosed hypothyroidism estimated to be 0.6%) has constipation and fatigue (LR+ 0.2), then the pretest probability would be approximately 0.1%. If the TSH level results between 6.7 and 20 mIU/L (LR+ 0.74), the posttest probability of thyroid disease would remain only 0.1%. Alternatively, a general medical patient with five symptoms consistent with hypothyroidism (LR+ 18.6) would have a pretest probability of 10%. If the TSH level results >20 mIU/L (LR+ 11.1), then the posttest probability of hypothyroidism would be 55%.11
For patients on stable doses of thyroid hormone replacement, although it may seem logical to check a TSH level upon admission to the hospital, guidelines recommend monitoring levels routinely in the outpatient setting, at most once every 12 months. More frequent monitoring should be undertaken only if clinical symptoms suggest that a dose change may be needed,19 and routine hospital testing should be avoided because of the potential for misleading results.
However, in some specific clinical scenarios, it may be reasonable to test for thyroid disease. Guidelines suggest TSH testing in the evaluation of certain conditions such as atrial fibrillation20 and syndrome of inappropriate antidiuretic hormone (SIADH).21 In addition, in the evaluation of unexplained sinus tachycardia, it is reasonable to test for hyperthyroidism after more common causes (pain, anxiety, infection, anemia, drug ingestion, and beta-blocker withdrawal) have been excluded.22 In the evaluation of delirium, TSH may be an appropriate “second tier” test after more likely contributors have been excluded.23
RECOMMENDATIONS
- Do not routinely order TSH on admission given the low pretest probability of clinically significant thyroid disease.
- Do not routinely check TSH for inpatients on stable outpatient doses of thyroid hormone replacement.
- Reserve TSH testing for clinical scenarios in which there is either a high pretest probability of thyroid disease (five or more symptoms) or for the evaluation of specific clinical syndromes for which thyroid dysfunction is a known reversible contributor (such as atrial fibrillation, SIADH, unexplained sinus tachycardia, and delirium).
- Do not attempt to diagnose subclinical thyroid disease in the hospital.
- If NTIS is suspected, avoid further testing in the hospital. Repeating TFTs as an outpatient may be appropriate after resolution of the acute illness.
CONCLUSION
Routine TSH testing in hospitalized patients is unhelpful and often yields confusing results because of the low prevalence of unrecognized thyroid disease, the high prevalence of NTIS, and the resulting difficulty with interpretation of results. Mild TSH abnormalities in hospitalized patients do not predict clinically significant thyroid disease.4,11 The patient in the previously described clinical scenario has NTIS caused by acute on chronic illness and the effect of glucocorticoids. As the hospitalist suspected, the patient’s AMS was caused by hypercapnia. Reserving TSH testing for patients with clinical signs and symptoms of thyroid disease or for those with specific conditions has the potential to save healthcare dollars, prevent harm to patients associated with overtesting or overtreatment, and decrease time spent interpreting abnormal results of unclear significance.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Hollowell J, Staehling N, Flanders W, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499. https://doi.org/10.1210/jcem.87.2.8182.
2. Akamizu T, Satoh T, Isozaki O, et al. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid. 2012;22(7):661-679. https://doi.org/10.1089/thy.2011.0334.
3. Ono Y, Ono S, Yasunaga H, Matsui H, Fushimi K, Tanaka Y. Clinical characteristics and outcomes of myxedema coma: Analysis of a national inpatient database in Japan. J Epidemiol. 2017;27(3):117-122. https://doi.org/10.1016/j.je.2016.04.002.
4. Bashkin A, Yaakobi E, Nodelman M, Ronen O. Is routine measurement of TSH in hospitalized patients necessary? Endocr Connect. 2018;7(4):567-572. https://doi.org/10.1530/EC-18-0004.
5. Adlan M, Neel V, Lakra S, Bondugulapati LN, Premawardhana LD. Targeted thyroid testing in acute illness: Achieving success through audit. J Endocrinol Invest. 2011;34(8):e210-e213. https://doi.org/10.3275/7480.
6. Roti E, Gardini E, Magotti M, et al. Are thyroid function tests too frequently and inappropriately requested?. J Endocrinol Invest. 1999;22(3):184-190. https://doi.org/10.1007/bf03343539.
7. Dalal S, Bhesania S, Silber S, Mehta P. Use of electronic clinical decision support and hard stops to decrease unnecessary thyroid function testing. BMJ Qual Improv Rep. 2017;6(1):u223041.w8346. https://doi.org/10.1136/bmjquality.u223041.w8346.
8. Premawardhana L. Thyroid testing in acutely ill patients may be an expensive distraction. Biochem Med (Zagreb). 2017;27(300):300-307. https://doi.org/10.11613/bm.2017.033.
9. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. https://doi.org/10.1056/nejmsb071595.
10. Hardwick R, Heaton, J, Vaidya B, et al. Exploring reasons for variation in ordering thyroid function tests in primary care: A qualitative study. Qual Prim Care. 2014;22(6):256-261.
11. Attia J, Margetts P, Guyatt G. Diagnosis of thyroid disease in hospitalized patients: a systematic review. Arch Intern Med. 1999;159(7):658-665. https://doi.org/10.1001/archinte.159.7.658.
12. Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab. 2013;27(6):745-762. https://doi.org/10.1016/j.beem.2013.10.003.
13. Spencer C, Elgen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem. 1987;33(8):1391-1396.
14. Zhelev Z, Abbott R, Rogers M, et al. Effectiveness of interventions to reduce ordering of thyroid function tests: a systematic review. BMJ Open. 2016;6:e010065. https://doi.org/10.1136/bmjopen-2015-010065.
15. Daucort V, Saillour-Glenisson F, Michel P, Jutand MA, Abouelfath A. A multicenter cluster randomized controlled trial of strategies to improve thyroid function testing. Med Care. 2003;41(3):432-441. https://doi.org/10.1097/01.mlr.0000053216.33277.a4.
16. Toubert M, Chavret S, Cassinat B, Schlageter MH, Beressi JP, Rain JD. From guidelines to hospital practice: reducing inappropriate ordering of thyroid hormone and antibody tests. Eur J Endocrinol. 2000:605-610. https://doi.org/10.1530/eje.0.1420605.
17. Thomas RE, Croal BL, Ramsay C, Eccles M, Grimshaw J. Effect of enhanced feedback and brief educational reminder messages on laboratory test requesting in primary care: A cluster randomised trial. Lancet. 2006;367(9527):1990-1996. https://doi.org/10.1016/s0140-6736(06)68888-0.
18. Taylor P, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels—balancing benefits and risks. JAMA Intern Med. 2014;174(1):32. https://doi.org/10.1001/jamainternmed.2013.11312.
19. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American association of clinical endocrinologists and the American thyroid association. Thyroid. 2012;22(12):1200-1235. https://doi.org/ 10.1089/thy.2012.0205.
20. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1-e76. https://doi.org/10.1016/j.jacc.2014.03.022.
21. Verbalis J, Goldsmith S, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: Expert panel recommendations. Am J Med. 2013;126(10):S1-S42. https://doi.org/10.1016/j.amjmed.2013.07.006.
22. Olshansky B, Sullivan R. Inappropriate sinus tachycardia. J Am Coll Cardiol. 2013;61(8):793-801. https://doi.org/10.1016/j.jacc.2012.07.074.
23. Josephson SA, Miller BL. Confusion and delirium. In: Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine, 20e. New York, NY: McGraw-Hill; http://accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192011608. Accessed January 29, 2019.
1. Hollowell J, Staehling N, Flanders W, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489-499. https://doi.org/10.1210/jcem.87.2.8182.
2. Akamizu T, Satoh T, Isozaki O, et al. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid. 2012;22(7):661-679. https://doi.org/10.1089/thy.2011.0334.
3. Ono Y, Ono S, Yasunaga H, Matsui H, Fushimi K, Tanaka Y. Clinical characteristics and outcomes of myxedema coma: Analysis of a national inpatient database in Japan. J Epidemiol. 2017;27(3):117-122. https://doi.org/10.1016/j.je.2016.04.002.
4. Bashkin A, Yaakobi E, Nodelman M, Ronen O. Is routine measurement of TSH in hospitalized patients necessary? Endocr Connect. 2018;7(4):567-572. https://doi.org/10.1530/EC-18-0004.
5. Adlan M, Neel V, Lakra S, Bondugulapati LN, Premawardhana LD. Targeted thyroid testing in acute illness: Achieving success through audit. J Endocrinol Invest. 2011;34(8):e210-e213. https://doi.org/10.3275/7480.
6. Roti E, Gardini E, Magotti M, et al. Are thyroid function tests too frequently and inappropriately requested?. J Endocrinol Invest. 1999;22(3):184-190. https://doi.org/10.1007/bf03343539.
7. Dalal S, Bhesania S, Silber S, Mehta P. Use of electronic clinical decision support and hard stops to decrease unnecessary thyroid function testing. BMJ Qual Improv Rep. 2017;6(1):u223041.w8346. https://doi.org/10.1136/bmjquality.u223041.w8346.
8. Premawardhana L. Thyroid testing in acutely ill patients may be an expensive distraction. Biochem Med (Zagreb). 2017;27(300):300-307. https://doi.org/10.11613/bm.2017.033.
9. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. https://doi.org/10.1056/nejmsb071595.
10. Hardwick R, Heaton, J, Vaidya B, et al. Exploring reasons for variation in ordering thyroid function tests in primary care: A qualitative study. Qual Prim Care. 2014;22(6):256-261.
11. Attia J, Margetts P, Guyatt G. Diagnosis of thyroid disease in hospitalized patients: a systematic review. Arch Intern Med. 1999;159(7):658-665. https://doi.org/10.1001/archinte.159.7.658.
12. Koulouri O, Moran C, Halsall D, Chatterjee K, Gurnell M. Pitfalls in the measurement and interpretation of thyroid function tests. Best Pract Res Clin Endocrinol Metab. 2013;27(6):745-762. https://doi.org/10.1016/j.beem.2013.10.003.
13. Spencer C, Elgen A, Shen D, et al. Specificity of sensitive assays of thyrotropin (TSH) used to screen for thyroid disease in hospitalized patients. Clin Chem. 1987;33(8):1391-1396.
14. Zhelev Z, Abbott R, Rogers M, et al. Effectiveness of interventions to reduce ordering of thyroid function tests: a systematic review. BMJ Open. 2016;6:e010065. https://doi.org/10.1136/bmjopen-2015-010065.
15. Daucort V, Saillour-Glenisson F, Michel P, Jutand MA, Abouelfath A. A multicenter cluster randomized controlled trial of strategies to improve thyroid function testing. Med Care. 2003;41(3):432-441. https://doi.org/10.1097/01.mlr.0000053216.33277.a4.
16. Toubert M, Chavret S, Cassinat B, Schlageter MH, Beressi JP, Rain JD. From guidelines to hospital practice: reducing inappropriate ordering of thyroid hormone and antibody tests. Eur J Endocrinol. 2000:605-610. https://doi.org/10.1530/eje.0.1420605.
17. Thomas RE, Croal BL, Ramsay C, Eccles M, Grimshaw J. Effect of enhanced feedback and brief educational reminder messages on laboratory test requesting in primary care: A cluster randomised trial. Lancet. 2006;367(9527):1990-1996. https://doi.org/10.1016/s0140-6736(06)68888-0.
18. Taylor P, Iqbal A, Minassian C, et al. Falling threshold for treatment of borderline elevated thyrotropin levels—balancing benefits and risks. JAMA Intern Med. 2014;174(1):32. https://doi.org/10.1001/jamainternmed.2013.11312.
19. Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American association of clinical endocrinologists and the American thyroid association. Thyroid. 2012;22(12):1200-1235. https://doi.org/ 10.1089/thy.2012.0205.
20. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1-e76. https://doi.org/10.1016/j.jacc.2014.03.022.
21. Verbalis J, Goldsmith S, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: Expert panel recommendations. Am J Med. 2013;126(10):S1-S42. https://doi.org/10.1016/j.amjmed.2013.07.006.
22. Olshansky B, Sullivan R. Inappropriate sinus tachycardia. J Am Coll Cardiol. 2013;61(8):793-801. https://doi.org/10.1016/j.jacc.2012.07.074.
23. Josephson SA, Miller BL. Confusion and delirium. In: Jameson J, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J, eds. Harrison’s Principles of Internal Medicine, 20e. New York, NY: McGraw-Hill; http://accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192011608. Accessed January 29, 2019.
© 2020 Society of Hospital Medicine
Things We Do for No Reason™: Lumbar Punctures in Low-Risk Febrile Infants with Bronchiolitis

CLINICAL SCENARIO
A 22-day-old full-term previously healthy male infant was evaluated in the emergency department (ED). The patient’s mother reported a three-day history of nasal congestion, cough and labored breathing, decreased oral intake, and subjective fever.
In the ED, the patient was found to have a rectal temperature of 101.3 °F (38.3 °C), heart rate of 112 beats per minute, and a respiratory rate of 54 breaths per minute, with subcostal retractions and diffuse expiratory wheezing. His appearance was otherwise unremarkable. His evaluation in the ED included a normal complete blood count (CBC) with differential, a normal urinalysis, and a chest radiograph with diffuse peribronchial thickening. Blood and catheterized urine cultures were also collected. The patient’s provider informs the parents that a lumbar puncture (LP) would be performed to rule out bacterial meningitis. Is it necessary for this patient to receive an LP?
INTRODUCTION
Fever in an infant <90 days old is a common clinical presentation.1 Because a newborn’s immune system is still developing, there is a heightened concern for bacterial infections in this age group. These include bloodstream infections, meningitis, pneumonia, urinary tract infections (UTIs), skin/soft tissue infections, and osteoarticular infections. Bacterial infections collectively account for approximately 10% of illness in young febrile infants <90 days.2 Of these, UTIs are the most common. The most recent literature has narrowed the focus on infants <60 days old as the risk of serious infection is inversely correlated with age. Meningitis accounts for 1% of infections or less in children <60 days of age who present with a fever.3
Frequently, the evaluation of fever in young infants leads to cerebrospinal fluid (CSF) collection and hospitalization.4 Among febrile infants, current practice patterns regarding LPs vary across institutions.5 Some clinical practice guidelines recommend universal CSF testing for all febrile infants ≤56 days old.6
Bronchiolitis is also a common presentation. Up to 90% of children are infected with respiratory syncytial virus, the most common viral cause of bronchiolitis, within the first two years of life.7 Fever may be a presenting symptom in infants with bronchiolitis and one study found approximately 11% of febrile infants less than 90 days old met clinical criteria for bronchiolitis.8
WHY YOU MIGHT THINK LUMBAR PUNCTURE IN FEBRILE INFANTS WITH BRONCHIOLITIS IS HELPFUL
While clinical guidelines for bronchiolitis are well established,7 the evaluation and management of fever in an infant <90 days old remains a challenge because of concern for missing a bloodstream infection or meningitis. Meningitis can devastate an infant neurologically.9 Signs and symptoms of bacterial meningitis in infants are not specific, including the physical exam.10 Blood cultures are only concomitantly positive in 62% of cases of culture-confirmed bacterial meningitis.11
Several risk stratification algorithms exist to evaluate the likelihood of bacterial infections in febrile infants (Table). Two of the most common criteria—the Boston and Philadelphia—were validated using CSF cell count data. Other algorithms do not require an LP.12-15 All of the fever criteria algorithms have several limitations including lack of robust validation studies, under-powered methodologies (particularly for meningitis), and different inclusion criteria.2 Even with these risk stratification algorithms, some providers may continue to feel more comfortable obtaining CSF due to fear of missing meningitis in well-appearing, low-risk infants.
WHY LUMBAR PUNCTURE IN LOW-RISK FEBRILE INFANTS WITH BRONCHIOLITIS IS NOT NECESSARY
Bacterial meningitis, even in young infants, is rare. A recent meta-analysis estimated the general prevalence of meningitis in febrile neonates (regardless of risk stratification or bronchiolitis symptoms) in their first and second months of life were 1.2% (95% CI, 0.8%-1.9%) and 0.4% (95% CI, 0.2%-1.0%), respectively.3
Febrile infant risk stratification algorithms have high negative predictive values (NPVs) in ruling out meningitis. The Rochester criteria, which does not utilize CSF, has an NPV of greater than 98%.12 A recent Pediatric Emergency Care Applied Research Network Clinical Prediction Rule has an NPV of 99.9% among febrile infants <60 days, using only absolute neutrophil count, urinalysis, and procalcitonin.15
Among the patients that are already a low risk, concomitant viral infections further decrease the pretest probability. Febrile infants with lab-confirmed respiratory viral infections are at lower risk for serious bacterial infections.16,17 Multiple retrospective and prospective observational studies have demonstrated that low-risk patients with bronchiolitis symptoms are extremely unlikely to have bacterial meningitis.8,18-22 A systematic review of 1749 febrile patients under 90 days of age with clinical bronchiolitis demonstrated no cases of meningitis.23 Many of these studies included infants aged <28 days. Though the total number of neonates (<28 days) in all studies is somewhat unclear, it suggests that the cut-off to avoid an LP may be even lower.
Recent literature has advocated outpatient observation without an LP for low-risk infants as a cost-effective management tool,24 and this is particularly true in patients with concomitant viral bronchiolitis.
Based on the latest data confirming the low prevalence of meningitis among all infants,3 the ability to identify low-risk infants based on risk stratification algorithms (Table), and the decreased prevalence of meningitis in patients with clinical bronchiolitis,23 low-risk infants with bronchiolitis seem to have minimal, if any, risk of meningitis. Therefore, low-risk infants with bronchiolitis do not warrant an LP.
Importantly, LPs are not risk neutral. Their benefit versus harm should be weighed every time they are considered. Approximately 19% of LP attempts in infants under 90 days old are either traumatic or unsuccessful.25 Infants aged 28 to 60 days with traumatic or unsuccessful LPs are more frequently hospitalized.25 Increased hospitalizations are associated with higher costs.4 The majority of positive CSF cultures are deemed to be “contaminants” (87% in one study26), but the positive result still leads to unnecessary further evaluation, hospitalization, repeated invasive procedures, and family distress.27 These data further support refraining from pursuing an LP in low-risk infants with bronchiolitis.
WHY LUMBAR PUNCTURE MIGHT BE HELPFUL IN CERTAIN CIRCUMSTANCES
If the patient is not low risk based on criteria or does not have clinical bronchiolitis, consider performing an LP. A recent study demonstrated a 0.4% incidence of bacterial meningitis in febrile infants with viral co-infection,29 though it is not determined if the patients presented with symptoms of bronchiolitis or were risk-stratified using the algorithms discussed.
In the studies looking at viral infections in febrile infants, each has important exclusion criteria including prematurity, comorbidities, and recent antibiotic administration.23 For these patients, an LP may be warranted (though the evidence is lacking). In addition, in very young infants (less than seven-14 days old), viral infections may be less common than in older infants, resulting in a desire to rule out bacterial infections more thoroughly in this population.
WHAT YOU SHOULD DO INSTEAD: AVOID AN LP IN LOW-RISK FEBRILE INFANTS WITH BRONCHIOLITIS
For low-risk febrile infants with signs of bronchiolitis, evaluation for bacterial meningitis is not necessary. The low prevalence of meningitis in this age range along with the even lower likelihood of meningitis when bronchiolitis is identified suggests that the procedure is unnecessary. Moreover, the risks associated with LP—including trauma, hospitalization, costs, and family stress—likely outweigh the benefits of CSF analysis.
RECOMMENDATIONS
- In febrile infants, determine the risk of serious bacterial infections using published algorithms (Table) before considering lumbar puncture.
- In low-risk febrile infants with typical bronchiolitis, evaluation for bacterial meningitis with an LP is not necessary.
CONCLUSION
Infants under 90 days of age often present to care with fever. While there is a concern for missing bacterial meningitis, the prevalence of such an infection in infants is very low. Moreover, in low-risk patients that present with typical bronchiolitis symptoms, the prevalence is effectively zero. LP practices vary by institution and can be associated with risks. In low-risk infants with typical bronchiolitis symptoms, an LP is one of the Things We Do for No Reason™.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Cioffredi L-A, Jhaveri R. Evaluation and management of febrile children. JAMA Pediatr. 2016;170(8):794. https://doi.org/10.1001/jamapediatrics.2016.0596.
2. Huppler AR, Eickhoff JC, Wald ER. Performance of low-risk criteria in the evaluation of young infants with fever: review of the literature. Pediatrics. 2010;125(2):228-233. https://doi.org/10.1542/peds.2009-1070.
3. Biondi EA, Lee B, Ralston SL, et al. Prevalence of bacteremia and bacterial meningitis in febrile neonates and infants in the second month of life a systematic review and meta-analysis + supplemental content. JAMA Netw Open. 2019;2(3):190874. https://doi.org/10.1001/jamanetworkopen.2019.0874.
4. Aronson PL, Thurm C, Williams DJ, et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10(6):358-365. https://doi.org/10.1002/jhm.2329.
5. Aronson PL, Thurm C, Alpern ER, et al. Variation in care of the febrile young infant <90 days in us pediatric emergency departments. Pediatrics. 2014;134(4):667-677. https://doi.org/10.1542/peds.2014-1382.
6. Aronson PL, Thurm C, Williams DJ, et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10(6):358-365. https://doi.org/10.1002/jhm.2329.
7. Mendonca EA, Meissner HC, Gadomski AM, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742.
8. Melendez E, Harper MB. Utility of sepsis evaluation in infants 90 days of age or younger with fever and clinical bronchiolitis. Pediatr Infect Dis J. 2003;22(12):1053-1056. https://doi.org/10.1097/01.inf.0000101296.68993.4d.
9. Pruitt CM, Neuman MI, Shah SS, et al. Factors associated with adverse outcomes among febrile young infants with invasive bacterial infections. J. Pediatr. 2018;204:177-182. https://doi.org/10.1016/j.jpeds.2018.08.066.
10. Casper TC, Mahajan PV., Tzimenatos L, et al. The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics. 2017;140(1):e20170695. https://doi.org/10.1542/peds.2017-0695.
11. Garges HP. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics. 2006;117(4):1094-1100. https://doi.org/10.1542/peds.2005-1132.
12. Jaskiewicz JA, McCarthy CA, Richardson AC, et al. Febrile infants at low risk for serious bacterial infection-an appraisal of the Rochester criteria and implications for management. Pediatrics. 1994;94(3):390-396. http://www.ncbi.nlm.nih.gov/pubmed/8065869. Accessed March 23, 2019.
13. Aronson P, Wang M, Shapiro E, et al. Risk stratification of febrile infants ≤60 days old without routine lumbar puncture. Pediatrics. 2018;142(6):e20181879. https://doi.org/10.1542/peds.2018-1879.
14. Galetto-Lacour A, Zamora SA, Andreola B, et al. Validation of a laboratory risk index score for the identification of severe bacterial infection in children with fever without source. Arch Dis Child. 2010;95(12):968-973. https://doi.org/10.1136/adc.2009.176800.
15. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342. https://doi.org/10.1001/jamapediatrics.2018.5501.
16. Byington CL, Enriquez FR, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004;113(6):1662-1666. https://doi.org/10.1542/peds.113.6.1662.
17. Cioffredi LA, Jhaveri R. Evaluation and management of febrile children: a review. JAMA Pediatr. 2016;170(8):794-800. https://doi.org/10.1001/jamapediatrics.2016.0596.
18. Dayan PS, Roskind CG, Levine DA, Kuppermann N. Controversies in the management of children with bronchiolitis. Clin Pediatr Emerg Med. 2004;5(1):41-53. https://doi.org/10.1016/j.cpem.2003.11.001.
19. Oray-Schrom P, Phoenix C, St. Martin D, Amoateng-Adjepong Y. Sepsis workup in febrile infants 0-90 days of age with respiratory syncytial virus infection. Pediatr Emerg Care. 2003;19(5):314-319. https://doi.org/10.1097/01.pec.0000092576.40174.28.
20. Purcell K, Fergie J. Concurrent serious bacterial infections in 2396 infants and children hospitalized with respiratory syncytial virus lower respiratory tract infections. Arch Pediatr Adolesc Med. 2002;156(4):322-324. https://doi.org/10.1001/archpedi.156.4.322.
21. Purcell K, Fergie J. Concurrent serious bacterial infections in 912 infants and children hospitalized for treatment of respiratory syncytial virus lower respiratory tract infection. Pediatr Infect Dis J. 2004;23(3):267-269. https://doi.org/10.1097/01.inf.0000116759.21252.29.
22. Yarden-Bilavsky H, Ashkenazi-Hoffnung L, Livni G, Amir J, Bilavsky E. Month-by-month age analysis of the risk for serious bacterial infections in febrile infants with bronchiolitis. Clin Pediatr (Phila). 2011;50(11):1052-1056. https://doi.org/10.1177/0009922811412949.
23. Ralston S, Hill V, Waters A. Occult serious bacterial infection in infants younger than 60 to 90 days with bronchiolitis: a systematic review. Arch Pediatr Adolesc Med. 2011;165(10):951-956. https://doi.org/10.1001/archpediatrics.2011.155.
24. Lee TJ, Aronson PL. To spinal tap or not to spinal tap, that is the question. Hosp Pediatr. 2018;8(4):236-238. https://doi.org/10.1542/hpeds.2017-0207.
25. Pingree EW, Kimia AA, Nigrovic LE. The effect of traumatic lumbar puncture on hospitalization rate for febrile infants 28 to 60 days of age. Acad Emerg Med. 2015;22(2):240-243. https://doi.org/10.1111/acem.12582.
26. Leazer R, Erickson N, Paulson J, et al. epidemiology of cerebrospinal fluid cultures and time to detection in term infants. Pediatrics. 2017;139(5):e20163268. https://doi.org/10.1542/peds.2016-3268.
27. Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71-77. https://doi.org/10.1177/000992280104000202.
28. Mahajan P, Br owne LR, Levine DA, et al. Risk of bacterial coinfections in febrile infants 60 days old and younger with documented viral infections. J Pediatr. 2018;203:86-91.e2. https://doi.org/10.1016/j.jpeds.2018.07.073.

CLINICAL SCENARIO
A 22-day-old full-term previously healthy male infant was evaluated in the emergency department (ED). The patient’s mother reported a three-day history of nasal congestion, cough and labored breathing, decreased oral intake, and subjective fever.
In the ED, the patient was found to have a rectal temperature of 101.3 °F (38.3 °C), heart rate of 112 beats per minute, and a respiratory rate of 54 breaths per minute, with subcostal retractions and diffuse expiratory wheezing. His appearance was otherwise unremarkable. His evaluation in the ED included a normal complete blood count (CBC) with differential, a normal urinalysis, and a chest radiograph with diffuse peribronchial thickening. Blood and catheterized urine cultures were also collected. The patient’s provider informs the parents that a lumbar puncture (LP) would be performed to rule out bacterial meningitis. Is it necessary for this patient to receive an LP?
INTRODUCTION
Fever in an infant <90 days old is a common clinical presentation.1 Because a newborn’s immune system is still developing, there is a heightened concern for bacterial infections in this age group. These include bloodstream infections, meningitis, pneumonia, urinary tract infections (UTIs), skin/soft tissue infections, and osteoarticular infections. Bacterial infections collectively account for approximately 10% of illness in young febrile infants <90 days.2 Of these, UTIs are the most common. The most recent literature has narrowed the focus on infants <60 days old as the risk of serious infection is inversely correlated with age. Meningitis accounts for 1% of infections or less in children <60 days of age who present with a fever.3
Frequently, the evaluation of fever in young infants leads to cerebrospinal fluid (CSF) collection and hospitalization.4 Among febrile infants, current practice patterns regarding LPs vary across institutions.5 Some clinical practice guidelines recommend universal CSF testing for all febrile infants ≤56 days old.6
Bronchiolitis is also a common presentation. Up to 90% of children are infected with respiratory syncytial virus, the most common viral cause of bronchiolitis, within the first two years of life.7 Fever may be a presenting symptom in infants with bronchiolitis and one study found approximately 11% of febrile infants less than 90 days old met clinical criteria for bronchiolitis.8
WHY YOU MIGHT THINK LUMBAR PUNCTURE IN FEBRILE INFANTS WITH BRONCHIOLITIS IS HELPFUL
While clinical guidelines for bronchiolitis are well established,7 the evaluation and management of fever in an infant <90 days old remains a challenge because of concern for missing a bloodstream infection or meningitis. Meningitis can devastate an infant neurologically.9 Signs and symptoms of bacterial meningitis in infants are not specific, including the physical exam.10 Blood cultures are only concomitantly positive in 62% of cases of culture-confirmed bacterial meningitis.11
Several risk stratification algorithms exist to evaluate the likelihood of bacterial infections in febrile infants (Table). Two of the most common criteria—the Boston and Philadelphia—were validated using CSF cell count data. Other algorithms do not require an LP.12-15 All of the fever criteria algorithms have several limitations including lack of robust validation studies, under-powered methodologies (particularly for meningitis), and different inclusion criteria.2 Even with these risk stratification algorithms, some providers may continue to feel more comfortable obtaining CSF due to fear of missing meningitis in well-appearing, low-risk infants.
WHY LUMBAR PUNCTURE IN LOW-RISK FEBRILE INFANTS WITH BRONCHIOLITIS IS NOT NECESSARY
Bacterial meningitis, even in young infants, is rare. A recent meta-analysis estimated the general prevalence of meningitis in febrile neonates (regardless of risk stratification or bronchiolitis symptoms) in their first and second months of life were 1.2% (95% CI, 0.8%-1.9%) and 0.4% (95% CI, 0.2%-1.0%), respectively.3
Febrile infant risk stratification algorithms have high negative predictive values (NPVs) in ruling out meningitis. The Rochester criteria, which does not utilize CSF, has an NPV of greater than 98%.12 A recent Pediatric Emergency Care Applied Research Network Clinical Prediction Rule has an NPV of 99.9% among febrile infants <60 days, using only absolute neutrophil count, urinalysis, and procalcitonin.15
Among the patients that are already a low risk, concomitant viral infections further decrease the pretest probability. Febrile infants with lab-confirmed respiratory viral infections are at lower risk for serious bacterial infections.16,17 Multiple retrospective and prospective observational studies have demonstrated that low-risk patients with bronchiolitis symptoms are extremely unlikely to have bacterial meningitis.8,18-22 A systematic review of 1749 febrile patients under 90 days of age with clinical bronchiolitis demonstrated no cases of meningitis.23 Many of these studies included infants aged <28 days. Though the total number of neonates (<28 days) in all studies is somewhat unclear, it suggests that the cut-off to avoid an LP may be even lower.
Recent literature has advocated outpatient observation without an LP for low-risk infants as a cost-effective management tool,24 and this is particularly true in patients with concomitant viral bronchiolitis.
Based on the latest data confirming the low prevalence of meningitis among all infants,3 the ability to identify low-risk infants based on risk stratification algorithms (Table), and the decreased prevalence of meningitis in patients with clinical bronchiolitis,23 low-risk infants with bronchiolitis seem to have minimal, if any, risk of meningitis. Therefore, low-risk infants with bronchiolitis do not warrant an LP.
Importantly, LPs are not risk neutral. Their benefit versus harm should be weighed every time they are considered. Approximately 19% of LP attempts in infants under 90 days old are either traumatic or unsuccessful.25 Infants aged 28 to 60 days with traumatic or unsuccessful LPs are more frequently hospitalized.25 Increased hospitalizations are associated with higher costs.4 The majority of positive CSF cultures are deemed to be “contaminants” (87% in one study26), but the positive result still leads to unnecessary further evaluation, hospitalization, repeated invasive procedures, and family distress.27 These data further support refraining from pursuing an LP in low-risk infants with bronchiolitis.
WHY LUMBAR PUNCTURE MIGHT BE HELPFUL IN CERTAIN CIRCUMSTANCES
If the patient is not low risk based on criteria or does not have clinical bronchiolitis, consider performing an LP. A recent study demonstrated a 0.4% incidence of bacterial meningitis in febrile infants with viral co-infection,29 though it is not determined if the patients presented with symptoms of bronchiolitis or were risk-stratified using the algorithms discussed.
In the studies looking at viral infections in febrile infants, each has important exclusion criteria including prematurity, comorbidities, and recent antibiotic administration.23 For these patients, an LP may be warranted (though the evidence is lacking). In addition, in very young infants (less than seven-14 days old), viral infections may be less common than in older infants, resulting in a desire to rule out bacterial infections more thoroughly in this population.
WHAT YOU SHOULD DO INSTEAD: AVOID AN LP IN LOW-RISK FEBRILE INFANTS WITH BRONCHIOLITIS
For low-risk febrile infants with signs of bronchiolitis, evaluation for bacterial meningitis is not necessary. The low prevalence of meningitis in this age range along with the even lower likelihood of meningitis when bronchiolitis is identified suggests that the procedure is unnecessary. Moreover, the risks associated with LP—including trauma, hospitalization, costs, and family stress—likely outweigh the benefits of CSF analysis.
RECOMMENDATIONS
- In febrile infants, determine the risk of serious bacterial infections using published algorithms (Table) before considering lumbar puncture.
- In low-risk febrile infants with typical bronchiolitis, evaluation for bacterial meningitis with an LP is not necessary.
CONCLUSION
Infants under 90 days of age often present to care with fever. While there is a concern for missing bacterial meningitis, the prevalence of such an infection in infants is very low. Moreover, in low-risk patients that present with typical bronchiolitis symptoms, the prevalence is effectively zero. LP practices vary by institution and can be associated with risks. In low-risk infants with typical bronchiolitis symptoms, an LP is one of the Things We Do for No Reason™.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.

CLINICAL SCENARIO
A 22-day-old full-term previously healthy male infant was evaluated in the emergency department (ED). The patient’s mother reported a three-day history of nasal congestion, cough and labored breathing, decreased oral intake, and subjective fever.
In the ED, the patient was found to have a rectal temperature of 101.3 °F (38.3 °C), heart rate of 112 beats per minute, and a respiratory rate of 54 breaths per minute, with subcostal retractions and diffuse expiratory wheezing. His appearance was otherwise unremarkable. His evaluation in the ED included a normal complete blood count (CBC) with differential, a normal urinalysis, and a chest radiograph with diffuse peribronchial thickening. Blood and catheterized urine cultures were also collected. The patient’s provider informs the parents that a lumbar puncture (LP) would be performed to rule out bacterial meningitis. Is it necessary for this patient to receive an LP?
INTRODUCTION
Fever in an infant <90 days old is a common clinical presentation.1 Because a newborn’s immune system is still developing, there is a heightened concern for bacterial infections in this age group. These include bloodstream infections, meningitis, pneumonia, urinary tract infections (UTIs), skin/soft tissue infections, and osteoarticular infections. Bacterial infections collectively account for approximately 10% of illness in young febrile infants <90 days.2 Of these, UTIs are the most common. The most recent literature has narrowed the focus on infants <60 days old as the risk of serious infection is inversely correlated with age. Meningitis accounts for 1% of infections or less in children <60 days of age who present with a fever.3
Frequently, the evaluation of fever in young infants leads to cerebrospinal fluid (CSF) collection and hospitalization.4 Among febrile infants, current practice patterns regarding LPs vary across institutions.5 Some clinical practice guidelines recommend universal CSF testing for all febrile infants ≤56 days old.6
Bronchiolitis is also a common presentation. Up to 90% of children are infected with respiratory syncytial virus, the most common viral cause of bronchiolitis, within the first two years of life.7 Fever may be a presenting symptom in infants with bronchiolitis and one study found approximately 11% of febrile infants less than 90 days old met clinical criteria for bronchiolitis.8
WHY YOU MIGHT THINK LUMBAR PUNCTURE IN FEBRILE INFANTS WITH BRONCHIOLITIS IS HELPFUL
While clinical guidelines for bronchiolitis are well established,7 the evaluation and management of fever in an infant <90 days old remains a challenge because of concern for missing a bloodstream infection or meningitis. Meningitis can devastate an infant neurologically.9 Signs and symptoms of bacterial meningitis in infants are not specific, including the physical exam.10 Blood cultures are only concomitantly positive in 62% of cases of culture-confirmed bacterial meningitis.11
Several risk stratification algorithms exist to evaluate the likelihood of bacterial infections in febrile infants (Table). Two of the most common criteria—the Boston and Philadelphia—were validated using CSF cell count data. Other algorithms do not require an LP.12-15 All of the fever criteria algorithms have several limitations including lack of robust validation studies, under-powered methodologies (particularly for meningitis), and different inclusion criteria.2 Even with these risk stratification algorithms, some providers may continue to feel more comfortable obtaining CSF due to fear of missing meningitis in well-appearing, low-risk infants.
WHY LUMBAR PUNCTURE IN LOW-RISK FEBRILE INFANTS WITH BRONCHIOLITIS IS NOT NECESSARY
Bacterial meningitis, even in young infants, is rare. A recent meta-analysis estimated the general prevalence of meningitis in febrile neonates (regardless of risk stratification or bronchiolitis symptoms) in their first and second months of life were 1.2% (95% CI, 0.8%-1.9%) and 0.4% (95% CI, 0.2%-1.0%), respectively.3
Febrile infant risk stratification algorithms have high negative predictive values (NPVs) in ruling out meningitis. The Rochester criteria, which does not utilize CSF, has an NPV of greater than 98%.12 A recent Pediatric Emergency Care Applied Research Network Clinical Prediction Rule has an NPV of 99.9% among febrile infants <60 days, using only absolute neutrophil count, urinalysis, and procalcitonin.15
Among the patients that are already a low risk, concomitant viral infections further decrease the pretest probability. Febrile infants with lab-confirmed respiratory viral infections are at lower risk for serious bacterial infections.16,17 Multiple retrospective and prospective observational studies have demonstrated that low-risk patients with bronchiolitis symptoms are extremely unlikely to have bacterial meningitis.8,18-22 A systematic review of 1749 febrile patients under 90 days of age with clinical bronchiolitis demonstrated no cases of meningitis.23 Many of these studies included infants aged <28 days. Though the total number of neonates (<28 days) in all studies is somewhat unclear, it suggests that the cut-off to avoid an LP may be even lower.
Recent literature has advocated outpatient observation without an LP for low-risk infants as a cost-effective management tool,24 and this is particularly true in patients with concomitant viral bronchiolitis.
Based on the latest data confirming the low prevalence of meningitis among all infants,3 the ability to identify low-risk infants based on risk stratification algorithms (Table), and the decreased prevalence of meningitis in patients with clinical bronchiolitis,23 low-risk infants with bronchiolitis seem to have minimal, if any, risk of meningitis. Therefore, low-risk infants with bronchiolitis do not warrant an LP.
Importantly, LPs are not risk neutral. Their benefit versus harm should be weighed every time they are considered. Approximately 19% of LP attempts in infants under 90 days old are either traumatic or unsuccessful.25 Infants aged 28 to 60 days with traumatic or unsuccessful LPs are more frequently hospitalized.25 Increased hospitalizations are associated with higher costs.4 The majority of positive CSF cultures are deemed to be “contaminants” (87% in one study26), but the positive result still leads to unnecessary further evaluation, hospitalization, repeated invasive procedures, and family distress.27 These data further support refraining from pursuing an LP in low-risk infants with bronchiolitis.
WHY LUMBAR PUNCTURE MIGHT BE HELPFUL IN CERTAIN CIRCUMSTANCES
If the patient is not low risk based on criteria or does not have clinical bronchiolitis, consider performing an LP. A recent study demonstrated a 0.4% incidence of bacterial meningitis in febrile infants with viral co-infection,29 though it is not determined if the patients presented with symptoms of bronchiolitis or were risk-stratified using the algorithms discussed.
In the studies looking at viral infections in febrile infants, each has important exclusion criteria including prematurity, comorbidities, and recent antibiotic administration.23 For these patients, an LP may be warranted (though the evidence is lacking). In addition, in very young infants (less than seven-14 days old), viral infections may be less common than in older infants, resulting in a desire to rule out bacterial infections more thoroughly in this population.
WHAT YOU SHOULD DO INSTEAD: AVOID AN LP IN LOW-RISK FEBRILE INFANTS WITH BRONCHIOLITIS
For low-risk febrile infants with signs of bronchiolitis, evaluation for bacterial meningitis is not necessary. The low prevalence of meningitis in this age range along with the even lower likelihood of meningitis when bronchiolitis is identified suggests that the procedure is unnecessary. Moreover, the risks associated with LP—including trauma, hospitalization, costs, and family stress—likely outweigh the benefits of CSF analysis.
RECOMMENDATIONS
- In febrile infants, determine the risk of serious bacterial infections using published algorithms (Table) before considering lumbar puncture.
- In low-risk febrile infants with typical bronchiolitis, evaluation for bacterial meningitis with an LP is not necessary.
CONCLUSION
Infants under 90 days of age often present to care with fever. While there is a concern for missing bacterial meningitis, the prevalence of such an infection in infants is very low. Moreover, in low-risk patients that present with typical bronchiolitis symptoms, the prevalence is effectively zero. LP practices vary by institution and can be associated with risks. In low-risk infants with typical bronchiolitis symptoms, an LP is one of the Things We Do for No Reason™.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Cioffredi L-A, Jhaveri R. Evaluation and management of febrile children. JAMA Pediatr. 2016;170(8):794. https://doi.org/10.1001/jamapediatrics.2016.0596.
2. Huppler AR, Eickhoff JC, Wald ER. Performance of low-risk criteria in the evaluation of young infants with fever: review of the literature. Pediatrics. 2010;125(2):228-233. https://doi.org/10.1542/peds.2009-1070.
3. Biondi EA, Lee B, Ralston SL, et al. Prevalence of bacteremia and bacterial meningitis in febrile neonates and infants in the second month of life a systematic review and meta-analysis + supplemental content. JAMA Netw Open. 2019;2(3):190874. https://doi.org/10.1001/jamanetworkopen.2019.0874.
4. Aronson PL, Thurm C, Williams DJ, et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10(6):358-365. https://doi.org/10.1002/jhm.2329.
5. Aronson PL, Thurm C, Alpern ER, et al. Variation in care of the febrile young infant <90 days in us pediatric emergency departments. Pediatrics. 2014;134(4):667-677. https://doi.org/10.1542/peds.2014-1382.
6. Aronson PL, Thurm C, Williams DJ, et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10(6):358-365. https://doi.org/10.1002/jhm.2329.
7. Mendonca EA, Meissner HC, Gadomski AM, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742.
8. Melendez E, Harper MB. Utility of sepsis evaluation in infants 90 days of age or younger with fever and clinical bronchiolitis. Pediatr Infect Dis J. 2003;22(12):1053-1056. https://doi.org/10.1097/01.inf.0000101296.68993.4d.
9. Pruitt CM, Neuman MI, Shah SS, et al. Factors associated with adverse outcomes among febrile young infants with invasive bacterial infections. J. Pediatr. 2018;204:177-182. https://doi.org/10.1016/j.jpeds.2018.08.066.
10. Casper TC, Mahajan PV., Tzimenatos L, et al. The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics. 2017;140(1):e20170695. https://doi.org/10.1542/peds.2017-0695.
11. Garges HP. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics. 2006;117(4):1094-1100. https://doi.org/10.1542/peds.2005-1132.
12. Jaskiewicz JA, McCarthy CA, Richardson AC, et al. Febrile infants at low risk for serious bacterial infection-an appraisal of the Rochester criteria and implications for management. Pediatrics. 1994;94(3):390-396. http://www.ncbi.nlm.nih.gov/pubmed/8065869. Accessed March 23, 2019.
13. Aronson P, Wang M, Shapiro E, et al. Risk stratification of febrile infants ≤60 days old without routine lumbar puncture. Pediatrics. 2018;142(6):e20181879. https://doi.org/10.1542/peds.2018-1879.
14. Galetto-Lacour A, Zamora SA, Andreola B, et al. Validation of a laboratory risk index score for the identification of severe bacterial infection in children with fever without source. Arch Dis Child. 2010;95(12):968-973. https://doi.org/10.1136/adc.2009.176800.
15. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342. https://doi.org/10.1001/jamapediatrics.2018.5501.
16. Byington CL, Enriquez FR, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004;113(6):1662-1666. https://doi.org/10.1542/peds.113.6.1662.
17. Cioffredi LA, Jhaveri R. Evaluation and management of febrile children: a review. JAMA Pediatr. 2016;170(8):794-800. https://doi.org/10.1001/jamapediatrics.2016.0596.
18. Dayan PS, Roskind CG, Levine DA, Kuppermann N. Controversies in the management of children with bronchiolitis. Clin Pediatr Emerg Med. 2004;5(1):41-53. https://doi.org/10.1016/j.cpem.2003.11.001.
19. Oray-Schrom P, Phoenix C, St. Martin D, Amoateng-Adjepong Y. Sepsis workup in febrile infants 0-90 days of age with respiratory syncytial virus infection. Pediatr Emerg Care. 2003;19(5):314-319. https://doi.org/10.1097/01.pec.0000092576.40174.28.
20. Purcell K, Fergie J. Concurrent serious bacterial infections in 2396 infants and children hospitalized with respiratory syncytial virus lower respiratory tract infections. Arch Pediatr Adolesc Med. 2002;156(4):322-324. https://doi.org/10.1001/archpedi.156.4.322.
21. Purcell K, Fergie J. Concurrent serious bacterial infections in 912 infants and children hospitalized for treatment of respiratory syncytial virus lower respiratory tract infection. Pediatr Infect Dis J. 2004;23(3):267-269. https://doi.org/10.1097/01.inf.0000116759.21252.29.
22. Yarden-Bilavsky H, Ashkenazi-Hoffnung L, Livni G, Amir J, Bilavsky E. Month-by-month age analysis of the risk for serious bacterial infections in febrile infants with bronchiolitis. Clin Pediatr (Phila). 2011;50(11):1052-1056. https://doi.org/10.1177/0009922811412949.
23. Ralston S, Hill V, Waters A. Occult serious bacterial infection in infants younger than 60 to 90 days with bronchiolitis: a systematic review. Arch Pediatr Adolesc Med. 2011;165(10):951-956. https://doi.org/10.1001/archpediatrics.2011.155.
24. Lee TJ, Aronson PL. To spinal tap or not to spinal tap, that is the question. Hosp Pediatr. 2018;8(4):236-238. https://doi.org/10.1542/hpeds.2017-0207.
25. Pingree EW, Kimia AA, Nigrovic LE. The effect of traumatic lumbar puncture on hospitalization rate for febrile infants 28 to 60 days of age. Acad Emerg Med. 2015;22(2):240-243. https://doi.org/10.1111/acem.12582.
26. Leazer R, Erickson N, Paulson J, et al. epidemiology of cerebrospinal fluid cultures and time to detection in term infants. Pediatrics. 2017;139(5):e20163268. https://doi.org/10.1542/peds.2016-3268.
27. Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71-77. https://doi.org/10.1177/000992280104000202.
28. Mahajan P, Br owne LR, Levine DA, et al. Risk of bacterial coinfections in febrile infants 60 days old and younger with documented viral infections. J Pediatr. 2018;203:86-91.e2. https://doi.org/10.1016/j.jpeds.2018.07.073.
1. Cioffredi L-A, Jhaveri R. Evaluation and management of febrile children. JAMA Pediatr. 2016;170(8):794. https://doi.org/10.1001/jamapediatrics.2016.0596.
2. Huppler AR, Eickhoff JC, Wald ER. Performance of low-risk criteria in the evaluation of young infants with fever: review of the literature. Pediatrics. 2010;125(2):228-233. https://doi.org/10.1542/peds.2009-1070.
3. Biondi EA, Lee B, Ralston SL, et al. Prevalence of bacteremia and bacterial meningitis in febrile neonates and infants in the second month of life a systematic review and meta-analysis + supplemental content. JAMA Netw Open. 2019;2(3):190874. https://doi.org/10.1001/jamanetworkopen.2019.0874.
4. Aronson PL, Thurm C, Williams DJ, et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10(6):358-365. https://doi.org/10.1002/jhm.2329.
5. Aronson PL, Thurm C, Alpern ER, et al. Variation in care of the febrile young infant <90 days in us pediatric emergency departments. Pediatrics. 2014;134(4):667-677. https://doi.org/10.1542/peds.2014-1382.
6. Aronson PL, Thurm C, Williams DJ, et al. Association of clinical practice guidelines with emergency department management of febrile infants ≤56 days of age. J Hosp Med. 2015;10(6):358-365. https://doi.org/10.1002/jhm.2329.
7. Mendonca EA, Meissner HC, Gadomski AM, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. 2014;134(5):e1474-e1502. https://doi.org/10.1542/peds.2014-2742.
8. Melendez E, Harper MB. Utility of sepsis evaluation in infants 90 days of age or younger with fever and clinical bronchiolitis. Pediatr Infect Dis J. 2003;22(12):1053-1056. https://doi.org/10.1097/01.inf.0000101296.68993.4d.
9. Pruitt CM, Neuman MI, Shah SS, et al. Factors associated with adverse outcomes among febrile young infants with invasive bacterial infections. J. Pediatr. 2018;204:177-182. https://doi.org/10.1016/j.jpeds.2018.08.066.
10. Casper TC, Mahajan PV., Tzimenatos L, et al. The Yale Observation Scale Score and the risk of serious bacterial infections in febrile infants. Pediatrics. 2017;140(1):e20170695. https://doi.org/10.1542/peds.2017-0695.
11. Garges HP. Neonatal meningitis: what is the correlation among cerebrospinal fluid cultures, blood cultures, and cerebrospinal fluid parameters? Pediatrics. 2006;117(4):1094-1100. https://doi.org/10.1542/peds.2005-1132.
12. Jaskiewicz JA, McCarthy CA, Richardson AC, et al. Febrile infants at low risk for serious bacterial infection-an appraisal of the Rochester criteria and implications for management. Pediatrics. 1994;94(3):390-396. http://www.ncbi.nlm.nih.gov/pubmed/8065869. Accessed March 23, 2019.
13. Aronson P, Wang M, Shapiro E, et al. Risk stratification of febrile infants ≤60 days old without routine lumbar puncture. Pediatrics. 2018;142(6):e20181879. https://doi.org/10.1542/peds.2018-1879.
14. Galetto-Lacour A, Zamora SA, Andreola B, et al. Validation of a laboratory risk index score for the identification of severe bacterial infection in children with fever without source. Arch Dis Child. 2010;95(12):968-973. https://doi.org/10.1136/adc.2009.176800.
15. Kuppermann N, Dayan PS, Levine DA, et al. A clinical prediction rule to identify febrile infants 60 days and younger at low risk for serious bacterial infections. JAMA Pediatr. 2019;173(4):342. https://doi.org/10.1001/jamapediatrics.2018.5501.
16. Byington CL, Enriquez FR, Hoff C, et al. Serious bacterial infections in febrile infants 1 to 90 days old with and without viral infections. Pediatrics. 2004;113(6):1662-1666. https://doi.org/10.1542/peds.113.6.1662.
17. Cioffredi LA, Jhaveri R. Evaluation and management of febrile children: a review. JAMA Pediatr. 2016;170(8):794-800. https://doi.org/10.1001/jamapediatrics.2016.0596.
18. Dayan PS, Roskind CG, Levine DA, Kuppermann N. Controversies in the management of children with bronchiolitis. Clin Pediatr Emerg Med. 2004;5(1):41-53. https://doi.org/10.1016/j.cpem.2003.11.001.
19. Oray-Schrom P, Phoenix C, St. Martin D, Amoateng-Adjepong Y. Sepsis workup in febrile infants 0-90 days of age with respiratory syncytial virus infection. Pediatr Emerg Care. 2003;19(5):314-319. https://doi.org/10.1097/01.pec.0000092576.40174.28.
20. Purcell K, Fergie J. Concurrent serious bacterial infections in 2396 infants and children hospitalized with respiratory syncytial virus lower respiratory tract infections. Arch Pediatr Adolesc Med. 2002;156(4):322-324. https://doi.org/10.1001/archpedi.156.4.322.
21. Purcell K, Fergie J. Concurrent serious bacterial infections in 912 infants and children hospitalized for treatment of respiratory syncytial virus lower respiratory tract infection. Pediatr Infect Dis J. 2004;23(3):267-269. https://doi.org/10.1097/01.inf.0000116759.21252.29.
22. Yarden-Bilavsky H, Ashkenazi-Hoffnung L, Livni G, Amir J, Bilavsky E. Month-by-month age analysis of the risk for serious bacterial infections in febrile infants with bronchiolitis. Clin Pediatr (Phila). 2011;50(11):1052-1056. https://doi.org/10.1177/0009922811412949.
23. Ralston S, Hill V, Waters A. Occult serious bacterial infection in infants younger than 60 to 90 days with bronchiolitis: a systematic review. Arch Pediatr Adolesc Med. 2011;165(10):951-956. https://doi.org/10.1001/archpediatrics.2011.155.
24. Lee TJ, Aronson PL. To spinal tap or not to spinal tap, that is the question. Hosp Pediatr. 2018;8(4):236-238. https://doi.org/10.1542/hpeds.2017-0207.
25. Pingree EW, Kimia AA, Nigrovic LE. The effect of traumatic lumbar puncture on hospitalization rate for febrile infants 28 to 60 days of age. Acad Emerg Med. 2015;22(2):240-243. https://doi.org/10.1111/acem.12582.
26. Leazer R, Erickson N, Paulson J, et al. epidemiology of cerebrospinal fluid cultures and time to detection in term infants. Pediatrics. 2017;139(5):e20163268. https://doi.org/10.1542/peds.2016-3268.
27. Paxton RD, Byington CL. An examination of the unintended consequences of the rule-out sepsis evaluation: a parental perspective. Clin Pediatr (Phila). 2001;40(2):71-77. https://doi.org/10.1177/000992280104000202.
28. Mahajan P, Br owne LR, Levine DA, et al. Risk of bacterial coinfections in febrile infants 60 days old and younger with documented viral infections. J Pediatr. 2018;203:86-91.e2. https://doi.org/10.1016/j.jpeds.2018.07.073.
© 2019 Society of Hospital Medicine
Things We Do for No Reason™: Supplemental Oxygen for Patients without Hypoxemia

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 65-year-old woman with hypertension presents to the emergency department with three days of dyspnea, malaise, and pleuritic chest pain. Her temperature is 100.1°F, heart rate 110 beats per minute, and blood pressure 110/60 mm Hg. She is breathing 24 times per minute and has an oxygen saturation (SpO2) of 94% on room air. Her exam is remarkable for dry mucous membranes and right lower lung crackles. Her nurse places her on 3 L of oxygen per minute via nasal cannula, and her SpO2 rises to 99%.
WHY YOU MIGHT THINK SUPPLEMENTAL OXYGEN FOR NORMOXEMIC PATIENTS IS HELPFUL
Shortly after the discovery of oxygen in the late 18th century, physicians began using it to treat a variety of conditions including tuberculosis, pneumonia, respiratory failure, and angina. By the 1970s, most medical texts recommended oxygen use in suspected myocardial infarction (MI) because of the theoretical appeal of increasing delivery of oxygen to the heart and other vital organs.1 Additionally, there is a tendency to believe that supplemental oxygen alleviates dyspnea regardless of etiology or oxygen saturation. Recent studies have shown widespread use of oxygen in scenarios without clear indications and without oxygen saturation goals. A 2010 survey of clinicians managing acute MI found that 98% “always or usually” used oxygen and 55% believed that oxygen “definitely or probably reduces the risk of death.”2 In a Danish prehospital study, supplemental oxygen was used in 34% of ambulance patients even though only 17% of these patients had an SpO2 less than 94%.3 A study of critically ill patients found that most of the time, SpO2 exceeded 98%. Even when the fraction of inspired oxygen (FiO2) was between 0.3 and 0.4, no one adjusted the oxygen dose.4
WHY IT IS NOT HELPFUL TO PROVIDE SUPPLEMENTAL OXYGEN TO NORMOXEMIC PATIENTS
The reflexive use of oxygen in patients with acute respiratory or cardiovascular illness is problematic for several reasons. First, when oxygen saturation is near-normal, the potential benefit from supplemental oxygen lacks physiologic plausibility. More compellingly, evidence exists that hyperoxemia may cause significant harm. Finally, the unnecessary use of supplemental oxygen incurs practical inconveniences and expenses.
To understand why the physiologic basis for reflexive oxygen use is weak, it is important to distinguish hypoxemia (low arterial oxygen tension and hemoglobin oxygen saturation), tissue hypoxia (which can occur from hypoxemia or focal abnormalities in perfusion), and dyspnea (a subjective experience of breathing discomfort). A variety of mechanisms cause dyspnea, most of which do not involve hypoxemia. A patient with acute heart failure may experience severe dyspnea caused by activation of pressure-sensitive J-receptors in the lung, even if oxygen saturation and tissue perfusion are intact. This process will be relieved by reducing pulmonary capillary pressures, but it is unaffected by supplemental oxygen. Coronary occlusion causes hypoxia of the heart muscle, but restoring perfusion is the most effective treatment. The instinct to maximize the oxygen-carrying capacity of the remaining blood flow is understandable. However, in a normoxemic patient, increasing the inspired fraction of oxygen has a marginal effect on oxygen-carrying capacity, since hemoglobin saturation and concentration rather than arterial oxygen tension (PaO2) predominantly determine oxygen-carrying capacity. On the other hand, supraphysiologic levels of dissolved oxygen may lead to toxicity.5
For over a century, we have known the potential harms of hyperoxia. Original studies in animal models showed that hyperoxia led to lung injury, altered hemodynamics, endothelial cell dysfunction, and inflammatory activation.5 Many of these detrimental effects involve the generation of reactive oxygen species and oxidative stress.5 High levels of inspired oxygen can also cause increased pulmonary shunting through inhibition of physiologic hypoxic vasoconstriction and due to absorption atelectasis.6 Oxygen negatively affects cardiovascular function by reducing coronary blood flow, increasing systemic vascular resistance, and reducing cardiac output.1
Chronic obstructive pulmonary disease (COPD) is the clinical setting in which risks of supplemental oxygen are most well-recognized historically. In patients with COPD at risk for hypercarbia, oxygen titrated to a goal SpO2 outside 88%-92% is associated with a two-fold risk of mortality.7 Worsening ventilation-perfusion matching and the Haldane effect (decreased affinity of hemoglobin for carbon dioxide as the PaO2 rises), rather than the previously theorized decrease in hypoxic drive, are now believed to contribute most to hyperoxia-induced hypercarbia. These unintended consequences may also occur in patients with other forms of acute and chronic lung disease.
The British Medical Journal published the first randomized controlled trial of oxygen use in suspected MI in 1976.1 Patients who received oxygen at 6 L per minute for 24 hours had more episodes of sinus tachycardia without any improvement in mortality, analgesic use, or infarct size.1 More recent and robust trials comparing outcomes in normoxemic patients randomized to supplemental oxygen versus room air have had similar findings: no difference in mortality, infarct size, or pain ratings.8,9 One found a significantly increased rate of MI recurrence with the use of oxygen.8 These data have led the latest guidelines for the management of ST-elevation MI from the European Society of Cardiology to discourage the use of supplemental oxygen unless SpO2 is <90%.10
Two recent trials investigated the effects of hyperoxia in critically ill patients.11,12 Girardis and colleagues randomized 480 critically ill patients in an Italian medical-surgical intensive care unit to conservative (SpO2 between 94% and 98% or PaO2 between 70 and 100 mm Hg) versus conventional oxygenation targets (SpO2 between 97% and 100% and PaO2 up to 150 mm Hg). Compared with conventional oxygen targets, conservative oxygen use was associated with an absolute risk reduction in mortality of 8.6% (11.6% vs 20.2%; P =.01).11 Another trial from 22 centers in France compared outcomes in mechanically ventilated patients with septic shock who received FiO2 at 1.0 compared with those with oxygen titration to SpO2 between 88% and 95%. The trial was stopped early for safety concerns. Those in the hyperoxemia group had a higher incidence of serious adverse events (85% vs 76%; P =.02), including pneumothorax, clinically relevant bleeding, myocardial infarction, and arrhythmias, as well as a trend toward increased mortality.12
Trials of liberal oxygen use in other settings of acute illness,13 including ischemic stroke,14 traumatic brain injury,15 and postcardiac arrest,16 have also linked liberal oxygen use with increased risk of mortality and other adverse events. “Liberal” use in these trials ranged from an FiO2 of 0.28 (equivalent to 2 L of nasal cannula) to 1.0. Significant secondary outcomes included fewer hospital-free and ventilator-free days in patients with liberal oxygen use. Furthermore, a meta-analysis of 25 trials including over 16,000 patients found dose-dependent toxicity: for every 1% increase in SpO2 above 94%-96% (the median SpO2 in the liberal oxygen groups), there was a 25% relative increase in in-hospital mortality.13
In addition to the data above, there are practical reasons to avoid unnecessary use of supplemental oxygen. Providing supplemental oxygen to a patient who is not hypoxemic may delay the recognition of cardiopulmonary decompensation by delaying detection of hypoxemia.6 Beyond the effects of oxygen itself, oxygen delivery methods carry their own potential adverse effects. These include epistaxis (with nasal cannula), claustrophobia (with face masks), decreased mobility, falls, and delirium.17 Finally, oxygen administration has direct and indirect financial costs, including those of supplies, care coordination, and monitoring.
WHEN SUPPLEMENTAL OXYGEN MIGHT BE HELPFUL
Importantly, the above discussion pertains to normoxemic patients receiving supplemental oxygen. There is no dispute that significantly hypoxemic patients should receive supplemental oxygen. There are also instances where the use of supplemental oxygen in normoxemic patients may be beneficial, such as in carbon monoxide poisoning, decompression injury, gas embolism, cluster headaches, sickle cell crisis, and pneumothorax.17
WHAT YOU SHOULD DO INSTEAD
Like any other drug, oxygen should be administered after assessment of its indications, intended benefits, and possible harms. Both significant hypoxemia and hyperoxemia should be avoided. In patients with neither hypoxemia nor the indications above, clinicians should not administer supplemental oxygen. Recent society guidelines can be applied in various clinical contexts. In patients with suspected MI, oxygen should be administered if SpO2 is <90%.10 For most other acutely ill patients, clinicians should administer supplemental oxygen if SpO2 <90%-92% and target an SpO2 of no higher than 94%-96%,18-19 as meta-analyses found evidence of harm above this level.13 Results of randomized trials currently underway should add supporting evidence for more specific oxygenation targets in different patient populations. With respect to implementation, it must be noted that factors beyond physician decision influence the use of supplemental oxygen. Appropriate institutional policies, standards of care, and educational efforts to all hospital providers must be enacted in order to reduce the unnecessary use of supplemental oxygen.
RECOMMENDATIONS
- For most acutely ill patients, do not administer supplemental oxygen when SpO2 >92%. If supplemental oxygen is used, the SpO2 should not exceed 94%-96%.
- For patients with suspected MI, only start supplemental oxygen for SpO2 <90%.
- For patients at risk for hypercapnic respiratory failure (eg, COPD patients), target SpO2 of 88%-92%.
- Provide supplemental oxygen to normoxemic patients with carbon monoxide poisoning, decompression injury, gas embolism, cluster headache, sickle cell crisis, and pneumothorax.
- Review and revise institutional practices and policies that contribute to unnecessary use of supplemental oxygen.
CONCLUSIONS
In the opening case, the patient is acutely ill and requires further workup. Her current SpO2 of 99% puts her at risk for adverse events and death, and supplemental oxygen should be titrated down or stopped to avoid SpO2 greater than 94%-96%. For years, clinicians have erred on the side of using supplemental oxygen, without recognizing its dangers. However, over a century of evidence from pathophysiologic experiments and randomized trials across multiple clinical settings have associated hyperoxemia with adverse outcomes and increased mortality. Professional societies are adopting this evidence into their guideline recommendations, and clinicians should use supplemental oxygen judiciously in their daily practice.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Rawles JM, Kenmure AC. Controlled trial of oxygen in uncomplicated myocardial infarction. Br Med J. 1976;1(6018):1121-1123. https://doi.org/10.1136/bmj.1.6018.1121.
2. Burls A, Emparanza JI, Quinn T, Cabello J. Oxygen use in acute myocardial infarction: an online survey of health professionals’ practice and beliefs. Emerg Med J. 2010;27(4):283-286. https://doi.org/10.1136/emj.2009.077370.
3. Hale KE, Gavin C, O’Driscoll BR. Audit of oxygen use in emergency ambulances and in a hospital emergency department. Emerg Med J. 2008;25(11):773-776. https://doi.org/10.1136/emj.2008.059287.
4. Suzuki S, Eastwood G, Peck L, Glassford N, Bellomo R. Oxygen management in mechanically ventilated patients: a prospective observational cohort study. Aust Crit Care. 2014;27(1):50-51. https://doi.org/10.1016/j.aucc.2013.10.025.
5. Helmerhorst HJ, Schultz MJ, van der Voort PH, de Jonge E, van Wasterloo DJ. Bench-to-bedside review: the effects of hyperoxia during critical illness. Crit Care. 2015;19(1):284. https://doi.org/10.1186/s13054-015-0996-4.
6. Downs JB. Has oxygen administration delayed appropriate respiratory care? Fallacies regarding oxygen therapy. Respir Care. 2003;48(6):611-620.
7. Austin MA, Willis KE, Blizzard L, Walters EH, Wood-Baker R. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341:c5462. https://doi.org/10.2307/20800296.
8. Stub D, Smith K, Bernard S, et al. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation. 2015;131(24):2143-2150. https://doi.org/10.1161/CIRCULATIONAHA.114.014494.
9. Hofman R. Witt N, Lagergvist B, et al. Oxygen therapy in ST-elevation myocardial infarction. Eur Heart J. 2018;39(29):2730-2739. https://doi.org/10.1093/eurheartj/ehy326.
10. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018:39(2):119-177. https://doi.org/10.1093/eurheartj/ehx393.
11. Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit. JAMA. 2016;316(15):1583-1589. https://doi.org/10.1001/jama.2016.11993.
12. Asfar P, Schortgen F, Boisramé-Helms J, et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med. 2017:5(3):180-190. https://doi.org/10.1016/S2213-2600(17)30046-2.
13. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. https://doi.org/10.1016/S0140-6736(18)30479-3.
14. Rincon F, Kang J, Maltenfort M, et al. Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med. 2014;42(2):387-396. https://doi.org/10.1097/CCM.0b013e3182a27732.
15. Brenner M, Stein D, Hu P, Kufera J, Woodford M, Scalea T. Association between early hyperoxia and worse outcomes after traumatic brain injury. Arch Surg. 2012;147(11):1042-1046. https://doi.org/10.1001/archsurg.2012.1560.
16. Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165-2171. https://doi.org/10.1
17. Siemieniuk RA, Chu DK, Kim L, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. https://doi.org/10.1136/bmj.k4169.
18. O’Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(1):ii1-ii90. https://doi.org/10.1136/thoraxjnl-2016-209729.
19. Beasley R, Chien J, Douglas J, et al. Thoracic Society of Australia and New Zealand oxygen guidelines for acute oxygen use in adults: ‘Swimming between the flags’. Respirology. 2015;20(8):1182-1191. https://doi.org/10.1111/resp.12620.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 65-year-old woman with hypertension presents to the emergency department with three days of dyspnea, malaise, and pleuritic chest pain. Her temperature is 100.1°F, heart rate 110 beats per minute, and blood pressure 110/60 mm Hg. She is breathing 24 times per minute and has an oxygen saturation (SpO2) of 94% on room air. Her exam is remarkable for dry mucous membranes and right lower lung crackles. Her nurse places her on 3 L of oxygen per minute via nasal cannula, and her SpO2 rises to 99%.
WHY YOU MIGHT THINK SUPPLEMENTAL OXYGEN FOR NORMOXEMIC PATIENTS IS HELPFUL
Shortly after the discovery of oxygen in the late 18th century, physicians began using it to treat a variety of conditions including tuberculosis, pneumonia, respiratory failure, and angina. By the 1970s, most medical texts recommended oxygen use in suspected myocardial infarction (MI) because of the theoretical appeal of increasing delivery of oxygen to the heart and other vital organs.1 Additionally, there is a tendency to believe that supplemental oxygen alleviates dyspnea regardless of etiology or oxygen saturation. Recent studies have shown widespread use of oxygen in scenarios without clear indications and without oxygen saturation goals. A 2010 survey of clinicians managing acute MI found that 98% “always or usually” used oxygen and 55% believed that oxygen “definitely or probably reduces the risk of death.”2 In a Danish prehospital study, supplemental oxygen was used in 34% of ambulance patients even though only 17% of these patients had an SpO2 less than 94%.3 A study of critically ill patients found that most of the time, SpO2 exceeded 98%. Even when the fraction of inspired oxygen (FiO2) was between 0.3 and 0.4, no one adjusted the oxygen dose.4
WHY IT IS NOT HELPFUL TO PROVIDE SUPPLEMENTAL OXYGEN TO NORMOXEMIC PATIENTS
The reflexive use of oxygen in patients with acute respiratory or cardiovascular illness is problematic for several reasons. First, when oxygen saturation is near-normal, the potential benefit from supplemental oxygen lacks physiologic plausibility. More compellingly, evidence exists that hyperoxemia may cause significant harm. Finally, the unnecessary use of supplemental oxygen incurs practical inconveniences and expenses.
To understand why the physiologic basis for reflexive oxygen use is weak, it is important to distinguish hypoxemia (low arterial oxygen tension and hemoglobin oxygen saturation), tissue hypoxia (which can occur from hypoxemia or focal abnormalities in perfusion), and dyspnea (a subjective experience of breathing discomfort). A variety of mechanisms cause dyspnea, most of which do not involve hypoxemia. A patient with acute heart failure may experience severe dyspnea caused by activation of pressure-sensitive J-receptors in the lung, even if oxygen saturation and tissue perfusion are intact. This process will be relieved by reducing pulmonary capillary pressures, but it is unaffected by supplemental oxygen. Coronary occlusion causes hypoxia of the heart muscle, but restoring perfusion is the most effective treatment. The instinct to maximize the oxygen-carrying capacity of the remaining blood flow is understandable. However, in a normoxemic patient, increasing the inspired fraction of oxygen has a marginal effect on oxygen-carrying capacity, since hemoglobin saturation and concentration rather than arterial oxygen tension (PaO2) predominantly determine oxygen-carrying capacity. On the other hand, supraphysiologic levels of dissolved oxygen may lead to toxicity.5
For over a century, we have known the potential harms of hyperoxia. Original studies in animal models showed that hyperoxia led to lung injury, altered hemodynamics, endothelial cell dysfunction, and inflammatory activation.5 Many of these detrimental effects involve the generation of reactive oxygen species and oxidative stress.5 High levels of inspired oxygen can also cause increased pulmonary shunting through inhibition of physiologic hypoxic vasoconstriction and due to absorption atelectasis.6 Oxygen negatively affects cardiovascular function by reducing coronary blood flow, increasing systemic vascular resistance, and reducing cardiac output.1
Chronic obstructive pulmonary disease (COPD) is the clinical setting in which risks of supplemental oxygen are most well-recognized historically. In patients with COPD at risk for hypercarbia, oxygen titrated to a goal SpO2 outside 88%-92% is associated with a two-fold risk of mortality.7 Worsening ventilation-perfusion matching and the Haldane effect (decreased affinity of hemoglobin for carbon dioxide as the PaO2 rises), rather than the previously theorized decrease in hypoxic drive, are now believed to contribute most to hyperoxia-induced hypercarbia. These unintended consequences may also occur in patients with other forms of acute and chronic lung disease.
The British Medical Journal published the first randomized controlled trial of oxygen use in suspected MI in 1976.1 Patients who received oxygen at 6 L per minute for 24 hours had more episodes of sinus tachycardia without any improvement in mortality, analgesic use, or infarct size.1 More recent and robust trials comparing outcomes in normoxemic patients randomized to supplemental oxygen versus room air have had similar findings: no difference in mortality, infarct size, or pain ratings.8,9 One found a significantly increased rate of MI recurrence with the use of oxygen.8 These data have led the latest guidelines for the management of ST-elevation MI from the European Society of Cardiology to discourage the use of supplemental oxygen unless SpO2 is <90%.10
Two recent trials investigated the effects of hyperoxia in critically ill patients.11,12 Girardis and colleagues randomized 480 critically ill patients in an Italian medical-surgical intensive care unit to conservative (SpO2 between 94% and 98% or PaO2 between 70 and 100 mm Hg) versus conventional oxygenation targets (SpO2 between 97% and 100% and PaO2 up to 150 mm Hg). Compared with conventional oxygen targets, conservative oxygen use was associated with an absolute risk reduction in mortality of 8.6% (11.6% vs 20.2%; P =.01).11 Another trial from 22 centers in France compared outcomes in mechanically ventilated patients with septic shock who received FiO2 at 1.0 compared with those with oxygen titration to SpO2 between 88% and 95%. The trial was stopped early for safety concerns. Those in the hyperoxemia group had a higher incidence of serious adverse events (85% vs 76%; P =.02), including pneumothorax, clinically relevant bleeding, myocardial infarction, and arrhythmias, as well as a trend toward increased mortality.12
Trials of liberal oxygen use in other settings of acute illness,13 including ischemic stroke,14 traumatic brain injury,15 and postcardiac arrest,16 have also linked liberal oxygen use with increased risk of mortality and other adverse events. “Liberal” use in these trials ranged from an FiO2 of 0.28 (equivalent to 2 L of nasal cannula) to 1.0. Significant secondary outcomes included fewer hospital-free and ventilator-free days in patients with liberal oxygen use. Furthermore, a meta-analysis of 25 trials including over 16,000 patients found dose-dependent toxicity: for every 1% increase in SpO2 above 94%-96% (the median SpO2 in the liberal oxygen groups), there was a 25% relative increase in in-hospital mortality.13
In addition to the data above, there are practical reasons to avoid unnecessary use of supplemental oxygen. Providing supplemental oxygen to a patient who is not hypoxemic may delay the recognition of cardiopulmonary decompensation by delaying detection of hypoxemia.6 Beyond the effects of oxygen itself, oxygen delivery methods carry their own potential adverse effects. These include epistaxis (with nasal cannula), claustrophobia (with face masks), decreased mobility, falls, and delirium.17 Finally, oxygen administration has direct and indirect financial costs, including those of supplies, care coordination, and monitoring.
WHEN SUPPLEMENTAL OXYGEN MIGHT BE HELPFUL
Importantly, the above discussion pertains to normoxemic patients receiving supplemental oxygen. There is no dispute that significantly hypoxemic patients should receive supplemental oxygen. There are also instances where the use of supplemental oxygen in normoxemic patients may be beneficial, such as in carbon monoxide poisoning, decompression injury, gas embolism, cluster headaches, sickle cell crisis, and pneumothorax.17
WHAT YOU SHOULD DO INSTEAD
Like any other drug, oxygen should be administered after assessment of its indications, intended benefits, and possible harms. Both significant hypoxemia and hyperoxemia should be avoided. In patients with neither hypoxemia nor the indications above, clinicians should not administer supplemental oxygen. Recent society guidelines can be applied in various clinical contexts. In patients with suspected MI, oxygen should be administered if SpO2 is <90%.10 For most other acutely ill patients, clinicians should administer supplemental oxygen if SpO2 <90%-92% and target an SpO2 of no higher than 94%-96%,18-19 as meta-analyses found evidence of harm above this level.13 Results of randomized trials currently underway should add supporting evidence for more specific oxygenation targets in different patient populations. With respect to implementation, it must be noted that factors beyond physician decision influence the use of supplemental oxygen. Appropriate institutional policies, standards of care, and educational efforts to all hospital providers must be enacted in order to reduce the unnecessary use of supplemental oxygen.
RECOMMENDATIONS
- For most acutely ill patients, do not administer supplemental oxygen when SpO2 >92%. If supplemental oxygen is used, the SpO2 should not exceed 94%-96%.
- For patients with suspected MI, only start supplemental oxygen for SpO2 <90%.
- For patients at risk for hypercapnic respiratory failure (eg, COPD patients), target SpO2 of 88%-92%.
- Provide supplemental oxygen to normoxemic patients with carbon monoxide poisoning, decompression injury, gas embolism, cluster headache, sickle cell crisis, and pneumothorax.
- Review and revise institutional practices and policies that contribute to unnecessary use of supplemental oxygen.
CONCLUSIONS
In the opening case, the patient is acutely ill and requires further workup. Her current SpO2 of 99% puts her at risk for adverse events and death, and supplemental oxygen should be titrated down or stopped to avoid SpO2 greater than 94%-96%. For years, clinicians have erred on the side of using supplemental oxygen, without recognizing its dangers. However, over a century of evidence from pathophysiologic experiments and randomized trials across multiple clinical settings have associated hyperoxemia with adverse outcomes and increased mortality. Professional societies are adopting this evidence into their guideline recommendations, and clinicians should use supplemental oxygen judiciously in their daily practice.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent clear-cut conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 65-year-old woman with hypertension presents to the emergency department with three days of dyspnea, malaise, and pleuritic chest pain. Her temperature is 100.1°F, heart rate 110 beats per minute, and blood pressure 110/60 mm Hg. She is breathing 24 times per minute and has an oxygen saturation (SpO2) of 94% on room air. Her exam is remarkable for dry mucous membranes and right lower lung crackles. Her nurse places her on 3 L of oxygen per minute via nasal cannula, and her SpO2 rises to 99%.
WHY YOU MIGHT THINK SUPPLEMENTAL OXYGEN FOR NORMOXEMIC PATIENTS IS HELPFUL
Shortly after the discovery of oxygen in the late 18th century, physicians began using it to treat a variety of conditions including tuberculosis, pneumonia, respiratory failure, and angina. By the 1970s, most medical texts recommended oxygen use in suspected myocardial infarction (MI) because of the theoretical appeal of increasing delivery of oxygen to the heart and other vital organs.1 Additionally, there is a tendency to believe that supplemental oxygen alleviates dyspnea regardless of etiology or oxygen saturation. Recent studies have shown widespread use of oxygen in scenarios without clear indications and without oxygen saturation goals. A 2010 survey of clinicians managing acute MI found that 98% “always or usually” used oxygen and 55% believed that oxygen “definitely or probably reduces the risk of death.”2 In a Danish prehospital study, supplemental oxygen was used in 34% of ambulance patients even though only 17% of these patients had an SpO2 less than 94%.3 A study of critically ill patients found that most of the time, SpO2 exceeded 98%. Even when the fraction of inspired oxygen (FiO2) was between 0.3 and 0.4, no one adjusted the oxygen dose.4
WHY IT IS NOT HELPFUL TO PROVIDE SUPPLEMENTAL OXYGEN TO NORMOXEMIC PATIENTS
The reflexive use of oxygen in patients with acute respiratory or cardiovascular illness is problematic for several reasons. First, when oxygen saturation is near-normal, the potential benefit from supplemental oxygen lacks physiologic plausibility. More compellingly, evidence exists that hyperoxemia may cause significant harm. Finally, the unnecessary use of supplemental oxygen incurs practical inconveniences and expenses.
To understand why the physiologic basis for reflexive oxygen use is weak, it is important to distinguish hypoxemia (low arterial oxygen tension and hemoglobin oxygen saturation), tissue hypoxia (which can occur from hypoxemia or focal abnormalities in perfusion), and dyspnea (a subjective experience of breathing discomfort). A variety of mechanisms cause dyspnea, most of which do not involve hypoxemia. A patient with acute heart failure may experience severe dyspnea caused by activation of pressure-sensitive J-receptors in the lung, even if oxygen saturation and tissue perfusion are intact. This process will be relieved by reducing pulmonary capillary pressures, but it is unaffected by supplemental oxygen. Coronary occlusion causes hypoxia of the heart muscle, but restoring perfusion is the most effective treatment. The instinct to maximize the oxygen-carrying capacity of the remaining blood flow is understandable. However, in a normoxemic patient, increasing the inspired fraction of oxygen has a marginal effect on oxygen-carrying capacity, since hemoglobin saturation and concentration rather than arterial oxygen tension (PaO2) predominantly determine oxygen-carrying capacity. On the other hand, supraphysiologic levels of dissolved oxygen may lead to toxicity.5
For over a century, we have known the potential harms of hyperoxia. Original studies in animal models showed that hyperoxia led to lung injury, altered hemodynamics, endothelial cell dysfunction, and inflammatory activation.5 Many of these detrimental effects involve the generation of reactive oxygen species and oxidative stress.5 High levels of inspired oxygen can also cause increased pulmonary shunting through inhibition of physiologic hypoxic vasoconstriction and due to absorption atelectasis.6 Oxygen negatively affects cardiovascular function by reducing coronary blood flow, increasing systemic vascular resistance, and reducing cardiac output.1
Chronic obstructive pulmonary disease (COPD) is the clinical setting in which risks of supplemental oxygen are most well-recognized historically. In patients with COPD at risk for hypercarbia, oxygen titrated to a goal SpO2 outside 88%-92% is associated with a two-fold risk of mortality.7 Worsening ventilation-perfusion matching and the Haldane effect (decreased affinity of hemoglobin for carbon dioxide as the PaO2 rises), rather than the previously theorized decrease in hypoxic drive, are now believed to contribute most to hyperoxia-induced hypercarbia. These unintended consequences may also occur in patients with other forms of acute and chronic lung disease.
The British Medical Journal published the first randomized controlled trial of oxygen use in suspected MI in 1976.1 Patients who received oxygen at 6 L per minute for 24 hours had more episodes of sinus tachycardia without any improvement in mortality, analgesic use, or infarct size.1 More recent and robust trials comparing outcomes in normoxemic patients randomized to supplemental oxygen versus room air have had similar findings: no difference in mortality, infarct size, or pain ratings.8,9 One found a significantly increased rate of MI recurrence with the use of oxygen.8 These data have led the latest guidelines for the management of ST-elevation MI from the European Society of Cardiology to discourage the use of supplemental oxygen unless SpO2 is <90%.10
Two recent trials investigated the effects of hyperoxia in critically ill patients.11,12 Girardis and colleagues randomized 480 critically ill patients in an Italian medical-surgical intensive care unit to conservative (SpO2 between 94% and 98% or PaO2 between 70 and 100 mm Hg) versus conventional oxygenation targets (SpO2 between 97% and 100% and PaO2 up to 150 mm Hg). Compared with conventional oxygen targets, conservative oxygen use was associated with an absolute risk reduction in mortality of 8.6% (11.6% vs 20.2%; P =.01).11 Another trial from 22 centers in France compared outcomes in mechanically ventilated patients with septic shock who received FiO2 at 1.0 compared with those with oxygen titration to SpO2 between 88% and 95%. The trial was stopped early for safety concerns. Those in the hyperoxemia group had a higher incidence of serious adverse events (85% vs 76%; P =.02), including pneumothorax, clinically relevant bleeding, myocardial infarction, and arrhythmias, as well as a trend toward increased mortality.12
Trials of liberal oxygen use in other settings of acute illness,13 including ischemic stroke,14 traumatic brain injury,15 and postcardiac arrest,16 have also linked liberal oxygen use with increased risk of mortality and other adverse events. “Liberal” use in these trials ranged from an FiO2 of 0.28 (equivalent to 2 L of nasal cannula) to 1.0. Significant secondary outcomes included fewer hospital-free and ventilator-free days in patients with liberal oxygen use. Furthermore, a meta-analysis of 25 trials including over 16,000 patients found dose-dependent toxicity: for every 1% increase in SpO2 above 94%-96% (the median SpO2 in the liberal oxygen groups), there was a 25% relative increase in in-hospital mortality.13
In addition to the data above, there are practical reasons to avoid unnecessary use of supplemental oxygen. Providing supplemental oxygen to a patient who is not hypoxemic may delay the recognition of cardiopulmonary decompensation by delaying detection of hypoxemia.6 Beyond the effects of oxygen itself, oxygen delivery methods carry their own potential adverse effects. These include epistaxis (with nasal cannula), claustrophobia (with face masks), decreased mobility, falls, and delirium.17 Finally, oxygen administration has direct and indirect financial costs, including those of supplies, care coordination, and monitoring.
WHEN SUPPLEMENTAL OXYGEN MIGHT BE HELPFUL
Importantly, the above discussion pertains to normoxemic patients receiving supplemental oxygen. There is no dispute that significantly hypoxemic patients should receive supplemental oxygen. There are also instances where the use of supplemental oxygen in normoxemic patients may be beneficial, such as in carbon monoxide poisoning, decompression injury, gas embolism, cluster headaches, sickle cell crisis, and pneumothorax.17
WHAT YOU SHOULD DO INSTEAD
Like any other drug, oxygen should be administered after assessment of its indications, intended benefits, and possible harms. Both significant hypoxemia and hyperoxemia should be avoided. In patients with neither hypoxemia nor the indications above, clinicians should not administer supplemental oxygen. Recent society guidelines can be applied in various clinical contexts. In patients with suspected MI, oxygen should be administered if SpO2 is <90%.10 For most other acutely ill patients, clinicians should administer supplemental oxygen if SpO2 <90%-92% and target an SpO2 of no higher than 94%-96%,18-19 as meta-analyses found evidence of harm above this level.13 Results of randomized trials currently underway should add supporting evidence for more specific oxygenation targets in different patient populations. With respect to implementation, it must be noted that factors beyond physician decision influence the use of supplemental oxygen. Appropriate institutional policies, standards of care, and educational efforts to all hospital providers must be enacted in order to reduce the unnecessary use of supplemental oxygen.
RECOMMENDATIONS
- For most acutely ill patients, do not administer supplemental oxygen when SpO2 >92%. If supplemental oxygen is used, the SpO2 should not exceed 94%-96%.
- For patients with suspected MI, only start supplemental oxygen for SpO2 <90%.
- For patients at risk for hypercapnic respiratory failure (eg, COPD patients), target SpO2 of 88%-92%.
- Provide supplemental oxygen to normoxemic patients with carbon monoxide poisoning, decompression injury, gas embolism, cluster headache, sickle cell crisis, and pneumothorax.
- Review and revise institutional practices and policies that contribute to unnecessary use of supplemental oxygen.
CONCLUSIONS
In the opening case, the patient is acutely ill and requires further workup. Her current SpO2 of 99% puts her at risk for adverse events and death, and supplemental oxygen should be titrated down or stopped to avoid SpO2 greater than 94%-96%. For years, clinicians have erred on the side of using supplemental oxygen, without recognizing its dangers. However, over a century of evidence from pathophysiologic experiments and randomized trials across multiple clinical settings have associated hyperoxemia with adverse outcomes and increased mortality. Professional societies are adopting this evidence into their guideline recommendations, and clinicians should use supplemental oxygen judiciously in their daily practice.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™”? Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing TWDFNR@hospitalmedicine.org.
1. Rawles JM, Kenmure AC. Controlled trial of oxygen in uncomplicated myocardial infarction. Br Med J. 1976;1(6018):1121-1123. https://doi.org/10.1136/bmj.1.6018.1121.
2. Burls A, Emparanza JI, Quinn T, Cabello J. Oxygen use in acute myocardial infarction: an online survey of health professionals’ practice and beliefs. Emerg Med J. 2010;27(4):283-286. https://doi.org/10.1136/emj.2009.077370.
3. Hale KE, Gavin C, O’Driscoll BR. Audit of oxygen use in emergency ambulances and in a hospital emergency department. Emerg Med J. 2008;25(11):773-776. https://doi.org/10.1136/emj.2008.059287.
4. Suzuki S, Eastwood G, Peck L, Glassford N, Bellomo R. Oxygen management in mechanically ventilated patients: a prospective observational cohort study. Aust Crit Care. 2014;27(1):50-51. https://doi.org/10.1016/j.aucc.2013.10.025.
5. Helmerhorst HJ, Schultz MJ, van der Voort PH, de Jonge E, van Wasterloo DJ. Bench-to-bedside review: the effects of hyperoxia during critical illness. Crit Care. 2015;19(1):284. https://doi.org/10.1186/s13054-015-0996-4.
6. Downs JB. Has oxygen administration delayed appropriate respiratory care? Fallacies regarding oxygen therapy. Respir Care. 2003;48(6):611-620.
7. Austin MA, Willis KE, Blizzard L, Walters EH, Wood-Baker R. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341:c5462. https://doi.org/10.2307/20800296.
8. Stub D, Smith K, Bernard S, et al. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation. 2015;131(24):2143-2150. https://doi.org/10.1161/CIRCULATIONAHA.114.014494.
9. Hofman R. Witt N, Lagergvist B, et al. Oxygen therapy in ST-elevation myocardial infarction. Eur Heart J. 2018;39(29):2730-2739. https://doi.org/10.1093/eurheartj/ehy326.
10. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018:39(2):119-177. https://doi.org/10.1093/eurheartj/ehx393.
11. Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit. JAMA. 2016;316(15):1583-1589. https://doi.org/10.1001/jama.2016.11993.
12. Asfar P, Schortgen F, Boisramé-Helms J, et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med. 2017:5(3):180-190. https://doi.org/10.1016/S2213-2600(17)30046-2.
13. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. https://doi.org/10.1016/S0140-6736(18)30479-3.
14. Rincon F, Kang J, Maltenfort M, et al. Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med. 2014;42(2):387-396. https://doi.org/10.1097/CCM.0b013e3182a27732.
15. Brenner M, Stein D, Hu P, Kufera J, Woodford M, Scalea T. Association between early hyperoxia and worse outcomes after traumatic brain injury. Arch Surg. 2012;147(11):1042-1046. https://doi.org/10.1001/archsurg.2012.1560.
16. Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165-2171. https://doi.org/10.1
17. Siemieniuk RA, Chu DK, Kim L, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. https://doi.org/10.1136/bmj.k4169.
18. O’Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(1):ii1-ii90. https://doi.org/10.1136/thoraxjnl-2016-209729.
19. Beasley R, Chien J, Douglas J, et al. Thoracic Society of Australia and New Zealand oxygen guidelines for acute oxygen use in adults: ‘Swimming between the flags’. Respirology. 2015;20(8):1182-1191. https://doi.org/10.1111/resp.12620.
1. Rawles JM, Kenmure AC. Controlled trial of oxygen in uncomplicated myocardial infarction. Br Med J. 1976;1(6018):1121-1123. https://doi.org/10.1136/bmj.1.6018.1121.
2. Burls A, Emparanza JI, Quinn T, Cabello J. Oxygen use in acute myocardial infarction: an online survey of health professionals’ practice and beliefs. Emerg Med J. 2010;27(4):283-286. https://doi.org/10.1136/emj.2009.077370.
3. Hale KE, Gavin C, O’Driscoll BR. Audit of oxygen use in emergency ambulances and in a hospital emergency department. Emerg Med J. 2008;25(11):773-776. https://doi.org/10.1136/emj.2008.059287.
4. Suzuki S, Eastwood G, Peck L, Glassford N, Bellomo R. Oxygen management in mechanically ventilated patients: a prospective observational cohort study. Aust Crit Care. 2014;27(1):50-51. https://doi.org/10.1016/j.aucc.2013.10.025.
5. Helmerhorst HJ, Schultz MJ, van der Voort PH, de Jonge E, van Wasterloo DJ. Bench-to-bedside review: the effects of hyperoxia during critical illness. Crit Care. 2015;19(1):284. https://doi.org/10.1186/s13054-015-0996-4.
6. Downs JB. Has oxygen administration delayed appropriate respiratory care? Fallacies regarding oxygen therapy. Respir Care. 2003;48(6):611-620.
7. Austin MA, Willis KE, Blizzard L, Walters EH, Wood-Baker R. Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010;341:c5462. https://doi.org/10.2307/20800296.
8. Stub D, Smith K, Bernard S, et al. Air versus oxygen in ST-segment-elevation myocardial infarction. Circulation. 2015;131(24):2143-2150. https://doi.org/10.1161/CIRCULATIONAHA.114.014494.
9. Hofman R. Witt N, Lagergvist B, et al. Oxygen therapy in ST-elevation myocardial infarction. Eur Heart J. 2018;39(29):2730-2739. https://doi.org/10.1093/eurheartj/ehy326.
10. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018:39(2):119-177. https://doi.org/10.1093/eurheartj/ehx393.
11. Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit. JAMA. 2016;316(15):1583-1589. https://doi.org/10.1001/jama.2016.11993.
12. Asfar P, Schortgen F, Boisramé-Helms J, et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med. 2017:5(3):180-190. https://doi.org/10.1016/S2213-2600(17)30046-2.
13. Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693-1705. https://doi.org/10.1016/S0140-6736(18)30479-3.
14. Rincon F, Kang J, Maltenfort M, et al. Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med. 2014;42(2):387-396. https://doi.org/10.1097/CCM.0b013e3182a27732.
15. Brenner M, Stein D, Hu P, Kufera J, Woodford M, Scalea T. Association between early hyperoxia and worse outcomes after traumatic brain injury. Arch Surg. 2012;147(11):1042-1046. https://doi.org/10.1001/archsurg.2012.1560.
16. Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303(21):2165-2171. https://doi.org/10.1
17. Siemieniuk RA, Chu DK, Kim L, et al. Oxygen therapy for acutely ill medical patients: a clinical practice guideline. BMJ. 2018;363:k4169. https://doi.org/10.1136/bmj.k4169.
18. O’Driscoll BR, Howard LS, Earis J, et al. BTS guideline for oxygen use in adults in healthcare and emergency settings. Thorax. 2017;72(1):ii1-ii90. https://doi.org/10.1136/thoraxjnl-2016-209729.
19. Beasley R, Chien J, Douglas J, et al. Thoracic Society of Australia and New Zealand oxygen guidelines for acute oxygen use in adults: ‘Swimming between the flags’. Respirology. 2015;20(8):1182-1191. https://doi.org/10.1111/resp.12620.
© 2019 Society of Hospital Medicine