User login
FDA clears nonstimulant for ADHD in children aged 6 years and up
The Food and Drug Administration has approved the nonstimulant medication viloxazine extended-release capsules (Qelbree, Supernus Pharmaceuticals) for the treatment of attention deficit hyperactivity disorder (ADHD) in children aged 6-17 years, the company has announced.
Viloxazine (formerly SPN-812) is a selective norepinephrine reuptake inhibitor. Capsules may be swallowed whole or opened and the entire contents sprinkled onto applesauce, as needed.
The approval of viloxazine is supported by data from four phase 3 clinical trials involving more than 1,000 pediatric patients aged 6-17 years, the company said.
In one randomized, placebo-controlled phase 3 study that included more than 400 children, viloxazine reduced symptoms of ADHD as soon as 1 week after dosing and was well tolerated.
As reported by this news organization, the study was published last July in Clinical Therapeutics.
In addition to its fast onset of action, the fact that it was effective for both inattentive and hyperactive/impulsive clusters of symptoms is “impressive,” study investigator Andrew Cutler, MD, clinical associate professor of psychiatry, SUNY Upstate Medical University, Syracuse, N.Y., said in an interview.
Also noteworthy was the improvement in measures of quality of life and function, “especially function in the areas of school, home life, family relations, and peer relationships, which can be really disrupted with ADHD,” Dr. Cutler said.
The prescribing label for viloxazine includes a boxed warning regarding the potential for suicidal thoughts and behaviors in some children with ADHD treated with the drug, especially within the first few months of treatment or when the dose is changed.
In clinical trials, higher rates of suicidal thoughts and behavior were reported in pediatric patients treated with viloxazine than in patients treated with placebo. Patients taking viloxazine should be closely monitored for any new or sudden changes in mood, behavior, thoughts, and feelings.
Viloxazine has shown promise in a phase 3 trial involving adults with ADHD.
The company plans to submit a supplemental new drug application to the FDA for viloxazine in adults later this year.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the nonstimulant medication viloxazine extended-release capsules (Qelbree, Supernus Pharmaceuticals) for the treatment of attention deficit hyperactivity disorder (ADHD) in children aged 6-17 years, the company has announced.
Viloxazine (formerly SPN-812) is a selective norepinephrine reuptake inhibitor. Capsules may be swallowed whole or opened and the entire contents sprinkled onto applesauce, as needed.
The approval of viloxazine is supported by data from four phase 3 clinical trials involving more than 1,000 pediatric patients aged 6-17 years, the company said.
In one randomized, placebo-controlled phase 3 study that included more than 400 children, viloxazine reduced symptoms of ADHD as soon as 1 week after dosing and was well tolerated.
As reported by this news organization, the study was published last July in Clinical Therapeutics.
In addition to its fast onset of action, the fact that it was effective for both inattentive and hyperactive/impulsive clusters of symptoms is “impressive,” study investigator Andrew Cutler, MD, clinical associate professor of psychiatry, SUNY Upstate Medical University, Syracuse, N.Y., said in an interview.
Also noteworthy was the improvement in measures of quality of life and function, “especially function in the areas of school, home life, family relations, and peer relationships, which can be really disrupted with ADHD,” Dr. Cutler said.
The prescribing label for viloxazine includes a boxed warning regarding the potential for suicidal thoughts and behaviors in some children with ADHD treated with the drug, especially within the first few months of treatment or when the dose is changed.
In clinical trials, higher rates of suicidal thoughts and behavior were reported in pediatric patients treated with viloxazine than in patients treated with placebo. Patients taking viloxazine should be closely monitored for any new or sudden changes in mood, behavior, thoughts, and feelings.
Viloxazine has shown promise in a phase 3 trial involving adults with ADHD.
The company plans to submit a supplemental new drug application to the FDA for viloxazine in adults later this year.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the nonstimulant medication viloxazine extended-release capsules (Qelbree, Supernus Pharmaceuticals) for the treatment of attention deficit hyperactivity disorder (ADHD) in children aged 6-17 years, the company has announced.
Viloxazine (formerly SPN-812) is a selective norepinephrine reuptake inhibitor. Capsules may be swallowed whole or opened and the entire contents sprinkled onto applesauce, as needed.
The approval of viloxazine is supported by data from four phase 3 clinical trials involving more than 1,000 pediatric patients aged 6-17 years, the company said.
In one randomized, placebo-controlled phase 3 study that included more than 400 children, viloxazine reduced symptoms of ADHD as soon as 1 week after dosing and was well tolerated.
As reported by this news organization, the study was published last July in Clinical Therapeutics.
In addition to its fast onset of action, the fact that it was effective for both inattentive and hyperactive/impulsive clusters of symptoms is “impressive,” study investigator Andrew Cutler, MD, clinical associate professor of psychiatry, SUNY Upstate Medical University, Syracuse, N.Y., said in an interview.
Also noteworthy was the improvement in measures of quality of life and function, “especially function in the areas of school, home life, family relations, and peer relationships, which can be really disrupted with ADHD,” Dr. Cutler said.
The prescribing label for viloxazine includes a boxed warning regarding the potential for suicidal thoughts and behaviors in some children with ADHD treated with the drug, especially within the first few months of treatment or when the dose is changed.
In clinical trials, higher rates of suicidal thoughts and behavior were reported in pediatric patients treated with viloxazine than in patients treated with placebo. Patients taking viloxazine should be closely monitored for any new or sudden changes in mood, behavior, thoughts, and feelings.
Viloxazine has shown promise in a phase 3 trial involving adults with ADHD.
The company plans to submit a supplemental new drug application to the FDA for viloxazine in adults later this year.
A version of this article first appeared on Medscape.com.
FDA okays novel dual-action stimulant med for ADHD
The Food and Drug Administration has approved a new, once-daily oral stimulant medication for treatment of ADHD in people aged 6 years and older.
Azstarys (KemPharm) combines extended-release serdexmethylphenidate (SDX), KemPharm’s prodrug of dexmethylphenidate (d-MPH), coformulated with immediate-release d-MPH.
Following absorption in the gastrointestinal tract, SDX is converted to d-MPH, which is gradually released throughout the day, providing symptom control both rapidly with the d-MPH and for an extended duration with SDX.
The dual action of Azstarys addresses an unmet need for a medication that has early onset of action and long duration of therapy, with steady ADHD symptom control in one capsule, Corium, the company that will lead U.S. commercialization of the drug, stated in a news release.
“The data documenting the efficacy and safety of this new dual-action medicine, the first ever to use the novel prodrug serdexmethylphenidate together with dexmethylphenidate, is welcome news for clinicians and families to consider when choosing an appropriate ADHD therapy for children,” Ann Childress, MD, president of the Center for Psychiatry and Behavioral Medicine in Las Vegas, who led the phase 3 trial of the drug, said in the release.
The study included 150 children aged 6-12 years with ADHD. Compared with placebo, treatment with Azstarys led to significant improvement in ADHD symptoms, as measured by the primary endpoint, the change from baseline in Swanson, Kotkin, Agler, M-Flynn, and Pelham Rating Scale–Combined scores averaged over 13 hours.
Adverse events seen more often with Azstarys than placebo were headache (5.4% vs. 1.3%), upper abdominal pain (4.1% vs. 1.3%), insomnia (2.7% vs. 1.3%) and pharyngitis (2.7% vs. 0%). No serious adverse events were reported.
The FDA has recommended a schedule II controlled substance classification for Azstarys and the Drug Enforcement Administration will decide on scheduling within 90 days.
Pending the DEA’s action, the launch of Azstarys is anticipated this summer. Azstarys will be available in three once-daily dosage strengths of SDX/d-MPH: 26.1/5.2 mg, 39.2/7.8 mg, and 52.3/10.4 mg.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a new, once-daily oral stimulant medication for treatment of ADHD in people aged 6 years and older.
Azstarys (KemPharm) combines extended-release serdexmethylphenidate (SDX), KemPharm’s prodrug of dexmethylphenidate (d-MPH), coformulated with immediate-release d-MPH.
Following absorption in the gastrointestinal tract, SDX is converted to d-MPH, which is gradually released throughout the day, providing symptom control both rapidly with the d-MPH and for an extended duration with SDX.
The dual action of Azstarys addresses an unmet need for a medication that has early onset of action and long duration of therapy, with steady ADHD symptom control in one capsule, Corium, the company that will lead U.S. commercialization of the drug, stated in a news release.
“The data documenting the efficacy and safety of this new dual-action medicine, the first ever to use the novel prodrug serdexmethylphenidate together with dexmethylphenidate, is welcome news for clinicians and families to consider when choosing an appropriate ADHD therapy for children,” Ann Childress, MD, president of the Center for Psychiatry and Behavioral Medicine in Las Vegas, who led the phase 3 trial of the drug, said in the release.
The study included 150 children aged 6-12 years with ADHD. Compared with placebo, treatment with Azstarys led to significant improvement in ADHD symptoms, as measured by the primary endpoint, the change from baseline in Swanson, Kotkin, Agler, M-Flynn, and Pelham Rating Scale–Combined scores averaged over 13 hours.
Adverse events seen more often with Azstarys than placebo were headache (5.4% vs. 1.3%), upper abdominal pain (4.1% vs. 1.3%), insomnia (2.7% vs. 1.3%) and pharyngitis (2.7% vs. 0%). No serious adverse events were reported.
The FDA has recommended a schedule II controlled substance classification for Azstarys and the Drug Enforcement Administration will decide on scheduling within 90 days.
Pending the DEA’s action, the launch of Azstarys is anticipated this summer. Azstarys will be available in three once-daily dosage strengths of SDX/d-MPH: 26.1/5.2 mg, 39.2/7.8 mg, and 52.3/10.4 mg.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved a new, once-daily oral stimulant medication for treatment of ADHD in people aged 6 years and older.
Azstarys (KemPharm) combines extended-release serdexmethylphenidate (SDX), KemPharm’s prodrug of dexmethylphenidate (d-MPH), coformulated with immediate-release d-MPH.
Following absorption in the gastrointestinal tract, SDX is converted to d-MPH, which is gradually released throughout the day, providing symptom control both rapidly with the d-MPH and for an extended duration with SDX.
The dual action of Azstarys addresses an unmet need for a medication that has early onset of action and long duration of therapy, with steady ADHD symptom control in one capsule, Corium, the company that will lead U.S. commercialization of the drug, stated in a news release.
“The data documenting the efficacy and safety of this new dual-action medicine, the first ever to use the novel prodrug serdexmethylphenidate together with dexmethylphenidate, is welcome news for clinicians and families to consider when choosing an appropriate ADHD therapy for children,” Ann Childress, MD, president of the Center for Psychiatry and Behavioral Medicine in Las Vegas, who led the phase 3 trial of the drug, said in the release.
The study included 150 children aged 6-12 years with ADHD. Compared with placebo, treatment with Azstarys led to significant improvement in ADHD symptoms, as measured by the primary endpoint, the change from baseline in Swanson, Kotkin, Agler, M-Flynn, and Pelham Rating Scale–Combined scores averaged over 13 hours.
Adverse events seen more often with Azstarys than placebo were headache (5.4% vs. 1.3%), upper abdominal pain (4.1% vs. 1.3%), insomnia (2.7% vs. 1.3%) and pharyngitis (2.7% vs. 0%). No serious adverse events were reported.
The FDA has recommended a schedule II controlled substance classification for Azstarys and the Drug Enforcement Administration will decide on scheduling within 90 days.
Pending the DEA’s action, the launch of Azstarys is anticipated this summer. Azstarys will be available in three once-daily dosage strengths of SDX/d-MPH: 26.1/5.2 mg, 39.2/7.8 mg, and 52.3/10.4 mg.
A version of this article first appeared on Medscape.com.
Does L-methylfolate have a role in ADHD management?
Editor’s note: Readers’ Forum is a new department for correspondence from readers that is not in response to articles published in
Since the completion of the human genome project, the role of pharmacogenomics in treating mental health disorders has become more prevalent. Recently discovered genetic polymorphisms and mutations in the methylenetetrahydrofolate reductase (MTHFR) gene have led clinicians to seek out new therapeutic approaches to personalize mental health care. MTHFR is a key enzyme of folate metabolism, and changes in its gene can result in reduced enzyme activity, which has been associated with psychiatric illnesses such as schizophrenia, major depressive disorder (MDD), attention-deficit/hyperactivity disorder (ADHD), and autism.1 Supplementation with L-methylfolate, the active form of folate, has been found to improve clinical and social recovery in patients with psychiatric illnesses such as schizophrenia and MDD.2 While L-methylfolate is classified as an FDA-approved medicinal food for patients with depression and schizophrenia, its role in ADHD remains controversial.3 L-methylfolate modulates the synthesis of monoamines such as dopamine and norepinephrine, which are pivotal in reducing inattentiveness and hyperactivity in patients with ADHD.4,5 As a result, it could play an important role in the management of ADHD in patients with MTHFR deficiency.
Despite its high prevalence in many children, ADHD can persist into adulthood with impairing symptoms that have long-term social and economic impacts. Conventional methods of treating ADHD include stimulant medications such as methylphenidate, which can increase the levels of dopamine and norepinephrine in the brain. Unfortunately, stimulants’ cost, adverse effect profile, and high potential for abuse can hinder their use and contribute to treatment resistance.6 Because L-methylfolate can cross the blood-brain barrier and lacks the adverse effect profile of stimulants, it represents an alternative that could improve the quality of life for ADHD patients, particularly those with MTHFR polymorphisms or mutations.
Conflicting evidence
Several researchers have investigated the role of L-methylfolate as a supplement or alternative to stimulant therapy for patients with ADHD. While some preliminary studies have found some benefit, others have not. Here we describe 2 studies with differing results.
Quilliin7 (2013). In an open-label study at a children’s hospital in Texas, Quillin7 investigated L-methylfolate for alleviating attention-deficit disorder/ADHD symptoms in 59 patients age 5 to 18. Twenty-seven patients received stimulant therapy. All patients were treated with L-methylfolate, 0.2 mg/kg/d in a chewable tablet form, for 6 weeks. The primary endpoint was change on the average Vanderbilt Assessment Scale Total Symptom Score (TSS), which was 30 at baseline. At the study’s conclusion, the average TSS score was 22, a 27% reduction. Patients who were taking only L-methylfolate had an average score of 21 at the end of the study, which was a 34% improvement, compared with an average TSS score of 23 in those who were taking stimulants.
Surman et al3 (2019). In this 12-week, double-blind, placebo-controlled clinical trial, researchers assessed the efficacy and tolerability of L-methylfolate when added to osmotic-release oral system methylphenidate (OROS-MPH).3 Surman et al3 randomized 44 adult patients (age 18 to 55) who met the DSM-5 criteria for ADHD to a placebo group or an active group. The placebo group was treated with placebo plus OROS-MPH, while the active group received L-methylfolate, 15 mg/d, plus OROS-MPH. OROS-MPH was started at 36 mg/d and titrated to optimal response. The primary endpoint was change in score from baseline on the Adult ADHD Investigator Symptom Report scale. Although it was well tolerated, L-methylfolate was not associated with a significant change in measures of ADHD or mental health function.3 However, researchers noticed that patients who received L-methylfolate needed to receive higher doses of methylphenidate over time. This suggests that supplementation with L-methylfolate could reduce the effectiveness of methylphenidate in adult patients with ADHD.3
While more research is needed, the contradictory results of these studies suggests that the relationship between L-methylfolate and ADHD could be impacted by dosing, as well as by differences in adult and childhood ADHD that are not yet fully understood.
Continue to: An area warranting future research
An area warranting future research
The growth of pharmacogenomics represents an important opportunity to bridge the gap between our understanding of psychiatric illnesses and new ways to treat them. Using L-methylfolate to treat ADHD might help bridge this gap. For this to occur, psychiatrists need to use evidence-based pharmacogenetic research to inform their decision-making. The differing results in studies evaluating the use of L-methylfolate in adult and pediatric patients pose interesting questions that will require more robust research to answer. Clinicians should be cautious in the use of L-methylfolate and recognize the importance of evaluating every patient with ADHD for MTHFR deficiency. This could help personalize care in ways that may improve the quality of life for patients and their families.
1. Wan L, Li Y, Zhang Z, et al. Methylenetetrahydrofolate reductase and psychiatric diseases. Transl Psychiatry. 2018;8. doi: 10.1038/s41398-018-0276-6
2. Godfrey PSA, Toone BK, Bottiglien T, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
3. Surman C, Ceranoglu A, Vaudreuil C, et al. Does L-methylfolate supplement methylphenidate pharmacotherapy in attention-deficit/hyperactivity disorder?: evidence of lack of benefit from a double-blind, placebo-controlled, randomized clinical trial. J Clin Psychopharmacol. 2019;39(1):28-38.
4. Stahl SM. L-methylfolate: a vitamin for your monoamines. J Clin Psychiatry. 2008;69(9):1352-1353.
5. Arnsten AFT. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31(11):2376-2383.
6. Childress A, Tran C. Current investigational drugs for the treatment of attention-deficit/hyperactivity disorder. Expert Opin Investig Drugs. 2016;25(4):463-474.
7. Quillin R. High dose L-methylfolate as novel therapy in ADHD. Abstract presented at: 2013 American Academy of Pediatrics National Conference and Exhibition; October 28, 2013; Orlando, FL.
Editor’s note: Readers’ Forum is a new department for correspondence from readers that is not in response to articles published in
Since the completion of the human genome project, the role of pharmacogenomics in treating mental health disorders has become more prevalent. Recently discovered genetic polymorphisms and mutations in the methylenetetrahydrofolate reductase (MTHFR) gene have led clinicians to seek out new therapeutic approaches to personalize mental health care. MTHFR is a key enzyme of folate metabolism, and changes in its gene can result in reduced enzyme activity, which has been associated with psychiatric illnesses such as schizophrenia, major depressive disorder (MDD), attention-deficit/hyperactivity disorder (ADHD), and autism.1 Supplementation with L-methylfolate, the active form of folate, has been found to improve clinical and social recovery in patients with psychiatric illnesses such as schizophrenia and MDD.2 While L-methylfolate is classified as an FDA-approved medicinal food for patients with depression and schizophrenia, its role in ADHD remains controversial.3 L-methylfolate modulates the synthesis of monoamines such as dopamine and norepinephrine, which are pivotal in reducing inattentiveness and hyperactivity in patients with ADHD.4,5 As a result, it could play an important role in the management of ADHD in patients with MTHFR deficiency.
Despite its high prevalence in many children, ADHD can persist into adulthood with impairing symptoms that have long-term social and economic impacts. Conventional methods of treating ADHD include stimulant medications such as methylphenidate, which can increase the levels of dopamine and norepinephrine in the brain. Unfortunately, stimulants’ cost, adverse effect profile, and high potential for abuse can hinder their use and contribute to treatment resistance.6 Because L-methylfolate can cross the blood-brain barrier and lacks the adverse effect profile of stimulants, it represents an alternative that could improve the quality of life for ADHD patients, particularly those with MTHFR polymorphisms or mutations.
Conflicting evidence
Several researchers have investigated the role of L-methylfolate as a supplement or alternative to stimulant therapy for patients with ADHD. While some preliminary studies have found some benefit, others have not. Here we describe 2 studies with differing results.
Quilliin7 (2013). In an open-label study at a children’s hospital in Texas, Quillin7 investigated L-methylfolate for alleviating attention-deficit disorder/ADHD symptoms in 59 patients age 5 to 18. Twenty-seven patients received stimulant therapy. All patients were treated with L-methylfolate, 0.2 mg/kg/d in a chewable tablet form, for 6 weeks. The primary endpoint was change on the average Vanderbilt Assessment Scale Total Symptom Score (TSS), which was 30 at baseline. At the study’s conclusion, the average TSS score was 22, a 27% reduction. Patients who were taking only L-methylfolate had an average score of 21 at the end of the study, which was a 34% improvement, compared with an average TSS score of 23 in those who were taking stimulants.
Surman et al3 (2019). In this 12-week, double-blind, placebo-controlled clinical trial, researchers assessed the efficacy and tolerability of L-methylfolate when added to osmotic-release oral system methylphenidate (OROS-MPH).3 Surman et al3 randomized 44 adult patients (age 18 to 55) who met the DSM-5 criteria for ADHD to a placebo group or an active group. The placebo group was treated with placebo plus OROS-MPH, while the active group received L-methylfolate, 15 mg/d, plus OROS-MPH. OROS-MPH was started at 36 mg/d and titrated to optimal response. The primary endpoint was change in score from baseline on the Adult ADHD Investigator Symptom Report scale. Although it was well tolerated, L-methylfolate was not associated with a significant change in measures of ADHD or mental health function.3 However, researchers noticed that patients who received L-methylfolate needed to receive higher doses of methylphenidate over time. This suggests that supplementation with L-methylfolate could reduce the effectiveness of methylphenidate in adult patients with ADHD.3
While more research is needed, the contradictory results of these studies suggests that the relationship between L-methylfolate and ADHD could be impacted by dosing, as well as by differences in adult and childhood ADHD that are not yet fully understood.
Continue to: An area warranting future research
An area warranting future research
The growth of pharmacogenomics represents an important opportunity to bridge the gap between our understanding of psychiatric illnesses and new ways to treat them. Using L-methylfolate to treat ADHD might help bridge this gap. For this to occur, psychiatrists need to use evidence-based pharmacogenetic research to inform their decision-making. The differing results in studies evaluating the use of L-methylfolate in adult and pediatric patients pose interesting questions that will require more robust research to answer. Clinicians should be cautious in the use of L-methylfolate and recognize the importance of evaluating every patient with ADHD for MTHFR deficiency. This could help personalize care in ways that may improve the quality of life for patients and their families.
Editor’s note: Readers’ Forum is a new department for correspondence from readers that is not in response to articles published in
Since the completion of the human genome project, the role of pharmacogenomics in treating mental health disorders has become more prevalent. Recently discovered genetic polymorphisms and mutations in the methylenetetrahydrofolate reductase (MTHFR) gene have led clinicians to seek out new therapeutic approaches to personalize mental health care. MTHFR is a key enzyme of folate metabolism, and changes in its gene can result in reduced enzyme activity, which has been associated with psychiatric illnesses such as schizophrenia, major depressive disorder (MDD), attention-deficit/hyperactivity disorder (ADHD), and autism.1 Supplementation with L-methylfolate, the active form of folate, has been found to improve clinical and social recovery in patients with psychiatric illnesses such as schizophrenia and MDD.2 While L-methylfolate is classified as an FDA-approved medicinal food for patients with depression and schizophrenia, its role in ADHD remains controversial.3 L-methylfolate modulates the synthesis of monoamines such as dopamine and norepinephrine, which are pivotal in reducing inattentiveness and hyperactivity in patients with ADHD.4,5 As a result, it could play an important role in the management of ADHD in patients with MTHFR deficiency.
Despite its high prevalence in many children, ADHD can persist into adulthood with impairing symptoms that have long-term social and economic impacts. Conventional methods of treating ADHD include stimulant medications such as methylphenidate, which can increase the levels of dopamine and norepinephrine in the brain. Unfortunately, stimulants’ cost, adverse effect profile, and high potential for abuse can hinder their use and contribute to treatment resistance.6 Because L-methylfolate can cross the blood-brain barrier and lacks the adverse effect profile of stimulants, it represents an alternative that could improve the quality of life for ADHD patients, particularly those with MTHFR polymorphisms or mutations.
Conflicting evidence
Several researchers have investigated the role of L-methylfolate as a supplement or alternative to stimulant therapy for patients with ADHD. While some preliminary studies have found some benefit, others have not. Here we describe 2 studies with differing results.
Quilliin7 (2013). In an open-label study at a children’s hospital in Texas, Quillin7 investigated L-methylfolate for alleviating attention-deficit disorder/ADHD symptoms in 59 patients age 5 to 18. Twenty-seven patients received stimulant therapy. All patients were treated with L-methylfolate, 0.2 mg/kg/d in a chewable tablet form, for 6 weeks. The primary endpoint was change on the average Vanderbilt Assessment Scale Total Symptom Score (TSS), which was 30 at baseline. At the study’s conclusion, the average TSS score was 22, a 27% reduction. Patients who were taking only L-methylfolate had an average score of 21 at the end of the study, which was a 34% improvement, compared with an average TSS score of 23 in those who were taking stimulants.
Surman et al3 (2019). In this 12-week, double-blind, placebo-controlled clinical trial, researchers assessed the efficacy and tolerability of L-methylfolate when added to osmotic-release oral system methylphenidate (OROS-MPH).3 Surman et al3 randomized 44 adult patients (age 18 to 55) who met the DSM-5 criteria for ADHD to a placebo group or an active group. The placebo group was treated with placebo plus OROS-MPH, while the active group received L-methylfolate, 15 mg/d, plus OROS-MPH. OROS-MPH was started at 36 mg/d and titrated to optimal response. The primary endpoint was change in score from baseline on the Adult ADHD Investigator Symptom Report scale. Although it was well tolerated, L-methylfolate was not associated with a significant change in measures of ADHD or mental health function.3 However, researchers noticed that patients who received L-methylfolate needed to receive higher doses of methylphenidate over time. This suggests that supplementation with L-methylfolate could reduce the effectiveness of methylphenidate in adult patients with ADHD.3
While more research is needed, the contradictory results of these studies suggests that the relationship between L-methylfolate and ADHD could be impacted by dosing, as well as by differences in adult and childhood ADHD that are not yet fully understood.
Continue to: An area warranting future research
An area warranting future research
The growth of pharmacogenomics represents an important opportunity to bridge the gap between our understanding of psychiatric illnesses and new ways to treat them. Using L-methylfolate to treat ADHD might help bridge this gap. For this to occur, psychiatrists need to use evidence-based pharmacogenetic research to inform their decision-making. The differing results in studies evaluating the use of L-methylfolate in adult and pediatric patients pose interesting questions that will require more robust research to answer. Clinicians should be cautious in the use of L-methylfolate and recognize the importance of evaluating every patient with ADHD for MTHFR deficiency. This could help personalize care in ways that may improve the quality of life for patients and their families.
1. Wan L, Li Y, Zhang Z, et al. Methylenetetrahydrofolate reductase and psychiatric diseases. Transl Psychiatry. 2018;8. doi: 10.1038/s41398-018-0276-6
2. Godfrey PSA, Toone BK, Bottiglien T, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
3. Surman C, Ceranoglu A, Vaudreuil C, et al. Does L-methylfolate supplement methylphenidate pharmacotherapy in attention-deficit/hyperactivity disorder?: evidence of lack of benefit from a double-blind, placebo-controlled, randomized clinical trial. J Clin Psychopharmacol. 2019;39(1):28-38.
4. Stahl SM. L-methylfolate: a vitamin for your monoamines. J Clin Psychiatry. 2008;69(9):1352-1353.
5. Arnsten AFT. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31(11):2376-2383.
6. Childress A, Tran C. Current investigational drugs for the treatment of attention-deficit/hyperactivity disorder. Expert Opin Investig Drugs. 2016;25(4):463-474.
7. Quillin R. High dose L-methylfolate as novel therapy in ADHD. Abstract presented at: 2013 American Academy of Pediatrics National Conference and Exhibition; October 28, 2013; Orlando, FL.
1. Wan L, Li Y, Zhang Z, et al. Methylenetetrahydrofolate reductase and psychiatric diseases. Transl Psychiatry. 2018;8. doi: 10.1038/s41398-018-0276-6
2. Godfrey PSA, Toone BK, Bottiglien T, et al. Enhancement of recovery from psychiatric illness by methylfolate. Lancet. 1990;336(8712):392-395.
3. Surman C, Ceranoglu A, Vaudreuil C, et al. Does L-methylfolate supplement methylphenidate pharmacotherapy in attention-deficit/hyperactivity disorder?: evidence of lack of benefit from a double-blind, placebo-controlled, randomized clinical trial. J Clin Psychopharmacol. 2019;39(1):28-38.
4. Stahl SM. L-methylfolate: a vitamin for your monoamines. J Clin Psychiatry. 2008;69(9):1352-1353.
5. Arnsten AFT. Stimulants: therapeutic actions in ADHD. Neuropsychopharmacology. 2006;31(11):2376-2383.
6. Childress A, Tran C. Current investigational drugs for the treatment of attention-deficit/hyperactivity disorder. Expert Opin Investig Drugs. 2016;25(4):463-474.
7. Quillin R. High dose L-methylfolate as novel therapy in ADHD. Abstract presented at: 2013 American Academy of Pediatrics National Conference and Exhibition; October 28, 2013; Orlando, FL.
Tips offered for treating co-occurring ADHD and SUDs
When Frances R. Levin, MD, began her clinical psychiatry career in the mid-1990s, she spent a lot of time educating colleagues about the validity of an ADHD diagnosis in adults.
“That’s no longer an issue,” Dr. Levin, the Kennedy-Leavy Professor of Psychiatry at Columbia University, New York, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “But at the time, we often thought, ‘ADHD is something that’s specific to people who are stimulant users.’ In fact, what we found over the years was that these rates are elevated in a range of substance use populations.”
According to National Comorbidity Survey, a nontreatment sample of more than 3,000 adults, individuals who have SUD have two to three times the risk of having ADHD, while individuals who have ADHD have about three times the rate of having an SUD, compared with those who don’t (Am J Psychiatry. 2006;163[4]:716-23). “When you move to treatment samples, the rates also remain quite high,” said Dr. Levin, who is also chief of the division of substance use disorders at the medical center.
“In the general population, the rates of ADHD are 2%-4%. When we look at people who are coming in specifically for treatment of their SUD, the rates are substantially higher, ranging from 10% to 24%.”
According to a 2014 review of medical literature, potential reasons for the association between ADHD and SUD vary and include underlying biologic deficits, such as parental SUDs and genetics; conduct disorder symptoms, such as defiance, rule breaking, and delinquency; poor performance in school, such as low grades, grade retention, or drop-out; and social difficulties, such as rejection from conventional groups or few quality friendships (Annu Rev Clin Psychol. 2014;10:607-39). Other potential pathways include neurocognitive deficits, stress-negative affect models, impulsive anger, and other underlying traits.
One key reason to treat ADHD in patients with SUDs is that they tend to develop the SUD earlier when the ADHD is present, Dr. Levin said. They’re also less likely to be retained in treatment and have a reduced likelihood of going into remission if dependence develops. “Even when they do achieve remission, it seems to take longer for people to reach remission,” she said. “They have more treatment exposure yet do less well in treatment. This can make it more challenging to treat this population.”
One common assumption from clinicians regarding patients with ADHD and a concomitant SUD is that standard treatments for ADHD do not work in active substance users. Another is that, even if treatments work for ADHD, they do not affect the substance use disorder. “Understandably, there is also concern that active substance abusers will misuse and divert their medications,” she said. “Finally, there are often additional psychiatric comorbidities that may make it harder to effectively treat individuals with ADHD and SUD.”
Since 2002, 15 double-blind outpatient studies using stimulants/atomoxetine to treat substance abusers with ADHD have appeared in the medical literature, Dr. Levin said. Only three have included adolescents. “That’s surprising, because up to 40% of kids who come in for treatment, often for cannabis use disorder, will have ADHD, yet there is very little guidance from empirical studies as to how to best treat them,” she said. “There have been several studies looking at atomoxetine to treat substance abusers with ADHD, but results have been mixed. In the cannabis use populations, atomoxetine has not been shown to be effective in treating the substance use disorder, and results are mixed regarding superiority in reducing ADHD symptoms. There is one study showing that ADHD is more likely to be improved in adults with alcohol use disorders with mixed results regarding the alcohol use.”
Overall, most of the outpatient and inpatient studies conducted in this population have demonstrated some signal in terms of reducing ADHD, she said, while a minority of the outpatient studies suggest some benefit in terms of substance use. “What’s interesting is that when you see a response in terms of the ADHD, you often see an improvement in the substance use as well,” Dr. Levin said. “This potentially suggests that patients may be self-medicating their ADHD symptoms or that if the ADHD responds to treatment, then the patient may benefit from the psychosocial interventions that targets the SUD.”
A separate meta-analysis involving more than 1,000 patients found mixed results from pharmacologic interventions and concluded that, while they modestly improved ADHD symptoms, no beneficial effect was seen on drug abstinence or on treatment discontinuation (J Psychopharmacol. 2015 Jan;29[1]:15-23). “I would argue that you don’t need to be as nihilistic about this as the meta-analysis might suggest, because the devil’s in the details,” said Dr. Levin, whose own research was included in the work.
“First of all, many of the studies had high drop-out rates. The outcome measures were variable, and some of the studies used formulations with poor bioavailability. Also, trials that evaluated atomoxetine or stimulants were combined, which may be problematic given the different mechanisms of action. Further, the meta-analysis did not include two recent placebo-controlled trials in adults with stimulant-use disorders that both found that higher dosing of a long-acting stimulant resulted in greater improvements in ADHD symptoms and stimulant use” (Addict. 2014;109[3]:440-9 and JAMA Psychiatry. 2015;72[6]:593-602).
Dr. Levin went on to note that there are few empirical data to guide treatment for those who have multiple psychiatric disorders, let alone treatment for ADHD and SUDs without additional psychiatric disorders. The challenge is what to treat first and/or how to treat the concomitant conditions safely.
“Generally, if possible, treat what is most clinically impairing first,” she said. “Overall, both stimulants and atomoxetine may work for ADHD even in the presence of additional depression, anxiety disorders, and substance use disorders.”
She cautioned against treating a patient with ADHD medication if there is a preexisting psychosis or bipolar illness. “If you start a stimulant or atomoxetine and psychosis or mania occurs, you clearly want to stop the medication and reassess,” she said. Researchers found that the risk of precipitating mania with a stimulant is uncommon if you alleviate symptoms first with a mood stabilizer. “This is a situation where you probably want to treat the bipolar illness first, but it does not preclude the treatment of ADHD once the mood stabilization has occurred,” she said.
In patients with ADHD and anxiety, she often treats the ADHD first, “because oftentimes the anxiety is driven by the procrastination and the inability to get things done,” she explained. “It’s important to determine whether the anxiety is an independent disorder rather than symptoms of ADHD. Inner restlessness can be described as anxiety.”
When there are concerns that preclude the use of a controlled medication, there are medications, in addition to atomoxetine, that might be considered. While bupropion is not Food and Drug Administration approved for ADHD, it might be useful in comorbid mood disorders for nicotine dependence. Other off-label medications that may help include guanfacine, modafinil, and tricyclic antidepressants.
“To date, robust dosing of long-acting amphetamine or methylphenidate formulations have been shown to be effective for patients with stimulant-use disorder, but as mentioned earlier, the data only come from two studies,” she said.
In order to determine whether stimulant treatment is yielding a benefit in a patient with co-occurring ADHD and SUD, she recommends carrying out a structured assessment of ADHD symptoms. Monitoring for functional improvement is also key.
“If there is no improvement in social, occupational, or academic settings and the patient is still actively using drugs, then there is no reason to keep prescribing,” she said. Close monitoring for cardiovascular or other psychiatric symptoms are key as well. Further, for those individuals with both ADHD and a substance-use disorder, it is critical that both are targeted for treatment.
Dr. Levin reported that she has received research, training, or salary support from the National Institute on Drug Abuse, New York state, and the Substance Abuse and Mental Health Services Administration. She has also received or currently receives industry support from Indivior and U.S. World Meds and for medication and from Major League Baseball. In addition, Dr. Levin has been an unpaid scientific advisory board member for Alkermes, Indivior, and Novartis.
When Frances R. Levin, MD, began her clinical psychiatry career in the mid-1990s, she spent a lot of time educating colleagues about the validity of an ADHD diagnosis in adults.
“That’s no longer an issue,” Dr. Levin, the Kennedy-Leavy Professor of Psychiatry at Columbia University, New York, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “But at the time, we often thought, ‘ADHD is something that’s specific to people who are stimulant users.’ In fact, what we found over the years was that these rates are elevated in a range of substance use populations.”
According to National Comorbidity Survey, a nontreatment sample of more than 3,000 adults, individuals who have SUD have two to three times the risk of having ADHD, while individuals who have ADHD have about three times the rate of having an SUD, compared with those who don’t (Am J Psychiatry. 2006;163[4]:716-23). “When you move to treatment samples, the rates also remain quite high,” said Dr. Levin, who is also chief of the division of substance use disorders at the medical center.
“In the general population, the rates of ADHD are 2%-4%. When we look at people who are coming in specifically for treatment of their SUD, the rates are substantially higher, ranging from 10% to 24%.”
According to a 2014 review of medical literature, potential reasons for the association between ADHD and SUD vary and include underlying biologic deficits, such as parental SUDs and genetics; conduct disorder symptoms, such as defiance, rule breaking, and delinquency; poor performance in school, such as low grades, grade retention, or drop-out; and social difficulties, such as rejection from conventional groups or few quality friendships (Annu Rev Clin Psychol. 2014;10:607-39). Other potential pathways include neurocognitive deficits, stress-negative affect models, impulsive anger, and other underlying traits.
One key reason to treat ADHD in patients with SUDs is that they tend to develop the SUD earlier when the ADHD is present, Dr. Levin said. They’re also less likely to be retained in treatment and have a reduced likelihood of going into remission if dependence develops. “Even when they do achieve remission, it seems to take longer for people to reach remission,” she said. “They have more treatment exposure yet do less well in treatment. This can make it more challenging to treat this population.”
One common assumption from clinicians regarding patients with ADHD and a concomitant SUD is that standard treatments for ADHD do not work in active substance users. Another is that, even if treatments work for ADHD, they do not affect the substance use disorder. “Understandably, there is also concern that active substance abusers will misuse and divert their medications,” she said. “Finally, there are often additional psychiatric comorbidities that may make it harder to effectively treat individuals with ADHD and SUD.”
Since 2002, 15 double-blind outpatient studies using stimulants/atomoxetine to treat substance abusers with ADHD have appeared in the medical literature, Dr. Levin said. Only three have included adolescents. “That’s surprising, because up to 40% of kids who come in for treatment, often for cannabis use disorder, will have ADHD, yet there is very little guidance from empirical studies as to how to best treat them,” she said. “There have been several studies looking at atomoxetine to treat substance abusers with ADHD, but results have been mixed. In the cannabis use populations, atomoxetine has not been shown to be effective in treating the substance use disorder, and results are mixed regarding superiority in reducing ADHD symptoms. There is one study showing that ADHD is more likely to be improved in adults with alcohol use disorders with mixed results regarding the alcohol use.”
Overall, most of the outpatient and inpatient studies conducted in this population have demonstrated some signal in terms of reducing ADHD, she said, while a minority of the outpatient studies suggest some benefit in terms of substance use. “What’s interesting is that when you see a response in terms of the ADHD, you often see an improvement in the substance use as well,” Dr. Levin said. “This potentially suggests that patients may be self-medicating their ADHD symptoms or that if the ADHD responds to treatment, then the patient may benefit from the psychosocial interventions that targets the SUD.”
A separate meta-analysis involving more than 1,000 patients found mixed results from pharmacologic interventions and concluded that, while they modestly improved ADHD symptoms, no beneficial effect was seen on drug abstinence or on treatment discontinuation (J Psychopharmacol. 2015 Jan;29[1]:15-23). “I would argue that you don’t need to be as nihilistic about this as the meta-analysis might suggest, because the devil’s in the details,” said Dr. Levin, whose own research was included in the work.
“First of all, many of the studies had high drop-out rates. The outcome measures were variable, and some of the studies used formulations with poor bioavailability. Also, trials that evaluated atomoxetine or stimulants were combined, which may be problematic given the different mechanisms of action. Further, the meta-analysis did not include two recent placebo-controlled trials in adults with stimulant-use disorders that both found that higher dosing of a long-acting stimulant resulted in greater improvements in ADHD symptoms and stimulant use” (Addict. 2014;109[3]:440-9 and JAMA Psychiatry. 2015;72[6]:593-602).
Dr. Levin went on to note that there are few empirical data to guide treatment for those who have multiple psychiatric disorders, let alone treatment for ADHD and SUDs without additional psychiatric disorders. The challenge is what to treat first and/or how to treat the concomitant conditions safely.
“Generally, if possible, treat what is most clinically impairing first,” she said. “Overall, both stimulants and atomoxetine may work for ADHD even in the presence of additional depression, anxiety disorders, and substance use disorders.”
She cautioned against treating a patient with ADHD medication if there is a preexisting psychosis or bipolar illness. “If you start a stimulant or atomoxetine and psychosis or mania occurs, you clearly want to stop the medication and reassess,” she said. Researchers found that the risk of precipitating mania with a stimulant is uncommon if you alleviate symptoms first with a mood stabilizer. “This is a situation where you probably want to treat the bipolar illness first, but it does not preclude the treatment of ADHD once the mood stabilization has occurred,” she said.
In patients with ADHD and anxiety, she often treats the ADHD first, “because oftentimes the anxiety is driven by the procrastination and the inability to get things done,” she explained. “It’s important to determine whether the anxiety is an independent disorder rather than symptoms of ADHD. Inner restlessness can be described as anxiety.”
When there are concerns that preclude the use of a controlled medication, there are medications, in addition to atomoxetine, that might be considered. While bupropion is not Food and Drug Administration approved for ADHD, it might be useful in comorbid mood disorders for nicotine dependence. Other off-label medications that may help include guanfacine, modafinil, and tricyclic antidepressants.
“To date, robust dosing of long-acting amphetamine or methylphenidate formulations have been shown to be effective for patients with stimulant-use disorder, but as mentioned earlier, the data only come from two studies,” she said.
In order to determine whether stimulant treatment is yielding a benefit in a patient with co-occurring ADHD and SUD, she recommends carrying out a structured assessment of ADHD symptoms. Monitoring for functional improvement is also key.
“If there is no improvement in social, occupational, or academic settings and the patient is still actively using drugs, then there is no reason to keep prescribing,” she said. Close monitoring for cardiovascular or other psychiatric symptoms are key as well. Further, for those individuals with both ADHD and a substance-use disorder, it is critical that both are targeted for treatment.
Dr. Levin reported that she has received research, training, or salary support from the National Institute on Drug Abuse, New York state, and the Substance Abuse and Mental Health Services Administration. She has also received or currently receives industry support from Indivior and U.S. World Meds and for medication and from Major League Baseball. In addition, Dr. Levin has been an unpaid scientific advisory board member for Alkermes, Indivior, and Novartis.
When Frances R. Levin, MD, began her clinical psychiatry career in the mid-1990s, she spent a lot of time educating colleagues about the validity of an ADHD diagnosis in adults.
“That’s no longer an issue,” Dr. Levin, the Kennedy-Leavy Professor of Psychiatry at Columbia University, New York, said during an annual psychopharmacology update held by the Nevada Psychiatric Association. “But at the time, we often thought, ‘ADHD is something that’s specific to people who are stimulant users.’ In fact, what we found over the years was that these rates are elevated in a range of substance use populations.”
According to National Comorbidity Survey, a nontreatment sample of more than 3,000 adults, individuals who have SUD have two to three times the risk of having ADHD, while individuals who have ADHD have about three times the rate of having an SUD, compared with those who don’t (Am J Psychiatry. 2006;163[4]:716-23). “When you move to treatment samples, the rates also remain quite high,” said Dr. Levin, who is also chief of the division of substance use disorders at the medical center.
“In the general population, the rates of ADHD are 2%-4%. When we look at people who are coming in specifically for treatment of their SUD, the rates are substantially higher, ranging from 10% to 24%.”
According to a 2014 review of medical literature, potential reasons for the association between ADHD and SUD vary and include underlying biologic deficits, such as parental SUDs and genetics; conduct disorder symptoms, such as defiance, rule breaking, and delinquency; poor performance in school, such as low grades, grade retention, or drop-out; and social difficulties, such as rejection from conventional groups or few quality friendships (Annu Rev Clin Psychol. 2014;10:607-39). Other potential pathways include neurocognitive deficits, stress-negative affect models, impulsive anger, and other underlying traits.
One key reason to treat ADHD in patients with SUDs is that they tend to develop the SUD earlier when the ADHD is present, Dr. Levin said. They’re also less likely to be retained in treatment and have a reduced likelihood of going into remission if dependence develops. “Even when they do achieve remission, it seems to take longer for people to reach remission,” she said. “They have more treatment exposure yet do less well in treatment. This can make it more challenging to treat this population.”
One common assumption from clinicians regarding patients with ADHD and a concomitant SUD is that standard treatments for ADHD do not work in active substance users. Another is that, even if treatments work for ADHD, they do not affect the substance use disorder. “Understandably, there is also concern that active substance abusers will misuse and divert their medications,” she said. “Finally, there are often additional psychiatric comorbidities that may make it harder to effectively treat individuals with ADHD and SUD.”
Since 2002, 15 double-blind outpatient studies using stimulants/atomoxetine to treat substance abusers with ADHD have appeared in the medical literature, Dr. Levin said. Only three have included adolescents. “That’s surprising, because up to 40% of kids who come in for treatment, often for cannabis use disorder, will have ADHD, yet there is very little guidance from empirical studies as to how to best treat them,” she said. “There have been several studies looking at atomoxetine to treat substance abusers with ADHD, but results have been mixed. In the cannabis use populations, atomoxetine has not been shown to be effective in treating the substance use disorder, and results are mixed regarding superiority in reducing ADHD symptoms. There is one study showing that ADHD is more likely to be improved in adults with alcohol use disorders with mixed results regarding the alcohol use.”
Overall, most of the outpatient and inpatient studies conducted in this population have demonstrated some signal in terms of reducing ADHD, she said, while a minority of the outpatient studies suggest some benefit in terms of substance use. “What’s interesting is that when you see a response in terms of the ADHD, you often see an improvement in the substance use as well,” Dr. Levin said. “This potentially suggests that patients may be self-medicating their ADHD symptoms or that if the ADHD responds to treatment, then the patient may benefit from the psychosocial interventions that targets the SUD.”
A separate meta-analysis involving more than 1,000 patients found mixed results from pharmacologic interventions and concluded that, while they modestly improved ADHD symptoms, no beneficial effect was seen on drug abstinence or on treatment discontinuation (J Psychopharmacol. 2015 Jan;29[1]:15-23). “I would argue that you don’t need to be as nihilistic about this as the meta-analysis might suggest, because the devil’s in the details,” said Dr. Levin, whose own research was included in the work.
“First of all, many of the studies had high drop-out rates. The outcome measures were variable, and some of the studies used formulations with poor bioavailability. Also, trials that evaluated atomoxetine or stimulants were combined, which may be problematic given the different mechanisms of action. Further, the meta-analysis did not include two recent placebo-controlled trials in adults with stimulant-use disorders that both found that higher dosing of a long-acting stimulant resulted in greater improvements in ADHD symptoms and stimulant use” (Addict. 2014;109[3]:440-9 and JAMA Psychiatry. 2015;72[6]:593-602).
Dr. Levin went on to note that there are few empirical data to guide treatment for those who have multiple psychiatric disorders, let alone treatment for ADHD and SUDs without additional psychiatric disorders. The challenge is what to treat first and/or how to treat the concomitant conditions safely.
“Generally, if possible, treat what is most clinically impairing first,” she said. “Overall, both stimulants and atomoxetine may work for ADHD even in the presence of additional depression, anxiety disorders, and substance use disorders.”
She cautioned against treating a patient with ADHD medication if there is a preexisting psychosis or bipolar illness. “If you start a stimulant or atomoxetine and psychosis or mania occurs, you clearly want to stop the medication and reassess,” she said. Researchers found that the risk of precipitating mania with a stimulant is uncommon if you alleviate symptoms first with a mood stabilizer. “This is a situation where you probably want to treat the bipolar illness first, but it does not preclude the treatment of ADHD once the mood stabilization has occurred,” she said.
In patients with ADHD and anxiety, she often treats the ADHD first, “because oftentimes the anxiety is driven by the procrastination and the inability to get things done,” she explained. “It’s important to determine whether the anxiety is an independent disorder rather than symptoms of ADHD. Inner restlessness can be described as anxiety.”
When there are concerns that preclude the use of a controlled medication, there are medications, in addition to atomoxetine, that might be considered. While bupropion is not Food and Drug Administration approved for ADHD, it might be useful in comorbid mood disorders for nicotine dependence. Other off-label medications that may help include guanfacine, modafinil, and tricyclic antidepressants.
“To date, robust dosing of long-acting amphetamine or methylphenidate formulations have been shown to be effective for patients with stimulant-use disorder, but as mentioned earlier, the data only come from two studies,” she said.
In order to determine whether stimulant treatment is yielding a benefit in a patient with co-occurring ADHD and SUD, she recommends carrying out a structured assessment of ADHD symptoms. Monitoring for functional improvement is also key.
“If there is no improvement in social, occupational, or academic settings and the patient is still actively using drugs, then there is no reason to keep prescribing,” she said. Close monitoring for cardiovascular or other psychiatric symptoms are key as well. Further, for those individuals with both ADHD and a substance-use disorder, it is critical that both are targeted for treatment.
Dr. Levin reported that she has received research, training, or salary support from the National Institute on Drug Abuse, New York state, and the Substance Abuse and Mental Health Services Administration. She has also received or currently receives industry support from Indivior and U.S. World Meds and for medication and from Major League Baseball. In addition, Dr. Levin has been an unpaid scientific advisory board member for Alkermes, Indivior, and Novartis.
FROM NPA 2021
Brain connectivity patterns reliably identify ADHD
Functional brain connectivity patterns are a stable biomarker of attention-deficit/hyperactivity disorder, new research suggests.
By applying a machine-learning approach to brain-imaging data, investigators were able to identify with 99% accuracy the adult study participants who had been diagnosed with ADHD in childhood.
“Even though the symptoms of ADHD may be less apparent in adulthood, the brain-wiring signature seems to be persistent,” study investigator Christopher McNorgan, PhD, of the department of psychology, State University of New York at Buffalo told this news organization.
The findings were published online Dec. 17, 2020, in Frontiers of Psychology.
Deep-learning neural networks
The researchers analyzed archived functional magnetic resonance imaging (fMRI) and behavioral data for 80 adults (mean age, 24 years; 64 male). Of these participants, 55 were diagnosed with ADHD in childhood and 25 were not.
The fMRI data were obtained during a response inhibition task that tested the individual’s ability to not respond automatically; for example, not saying “Simon Says” after someone else makes the comment.
The behavioral data included scores on the Iowa Gambling Task (IGT), which is used to measure impulsivity and risk taking.
“Usually, but not always, people with ADHD make riskier choices on this task,” Dr. McNorgan noted.
The investigators measured the amount of interconnectedness among different brain regions during the response inhibition task, which was repeated four times.
Patterns of interconnectivity were then fed into a deep-learning neural network that learned which patterns belonged to the ADHD group vs. those without ADHD (control group) and which patterns belonged to the high vs. low scorers on the IGT.
Caveats, cautionary notes
“The trained models are then tested on brain patterns they had never seen before, and we found the models would make the correct ADHD diagnosis and could tell apart the high and low scorers on the IGT 99% of the time,” Dr. McNorgan reported.
“The trained classifiers make predictions by calculating probabilities, and the neural networks learned how each of the brain connections contributes towards the final classification probability. We identified the set of brain connections that had the greatest influence on these probability calculations,” he noted.
Because the network classified both ADHD diagnosis and gambling task performance, the researchers were able to distinguish between connections that predicted ADHD when gambling performance was poor, as is typical for patients with ADHD, and those predicting ADHD when gambling performance was uncharacteristically good.
While more work is needed, the findings have potential clinical relevance, Dr. McNorgan said.
“ADHD can be difficult to diagnose reliably. If expense wasn’t an issue, fMRI may be able to help make diagnosis more reliable and objective,” he added.
However, because individuals with ADHD have different behavioral profiles, such as scoring atypically well on the IGT, additional studies using this approach may help identify brain networks “that are more or less active in those with ADHD that show a particular diagnostic trait,” he said.
“This could help inform what treatments might be more effective for those individuals,” Dr. McNorgan said.
Of course, he added, “clinicians’ diagnostic expertise is still required, as I would not base an ADHD diagnosis solely on the results of a single brain scan.”
No cross-validation
Commenting on the findings for this news organization, Vince Calhoun, PhD, neuroscientist and founding director of the Center for Translational Research in Neuroimaging and Data Science, Atlanta, a joint effort between Georgia State, Georgia Tech, and Emory University, noted some study limitations.
One cautionary note is that the investigators “appear to select relevant regions to include in the model based on activation to the task, then computed the predictions using the subset of regions that showed strong activation. The issue is this was done on the same data, so there was no cross-validation of this ‘feature selection’ step,” said Dr. Calhoun, who was not involved with the research. “This is a type of circularity which can lead to inflated accuracies,” he added.
Dr. Calhoun also noted that “multiple ADHD classification studies” have reported accuracies above 90%. In addition, there were only 80 participants in the current dataset.
“That’s relatively small for making strong claims about high accuracies as has been reported elsewhere,” he said.
Dr. McNorgan and Dr. Calhoun have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Functional brain connectivity patterns are a stable biomarker of attention-deficit/hyperactivity disorder, new research suggests.
By applying a machine-learning approach to brain-imaging data, investigators were able to identify with 99% accuracy the adult study participants who had been diagnosed with ADHD in childhood.
“Even though the symptoms of ADHD may be less apparent in adulthood, the brain-wiring signature seems to be persistent,” study investigator Christopher McNorgan, PhD, of the department of psychology, State University of New York at Buffalo told this news organization.
The findings were published online Dec. 17, 2020, in Frontiers of Psychology.
Deep-learning neural networks
The researchers analyzed archived functional magnetic resonance imaging (fMRI) and behavioral data for 80 adults (mean age, 24 years; 64 male). Of these participants, 55 were diagnosed with ADHD in childhood and 25 were not.
The fMRI data were obtained during a response inhibition task that tested the individual’s ability to not respond automatically; for example, not saying “Simon Says” after someone else makes the comment.
The behavioral data included scores on the Iowa Gambling Task (IGT), which is used to measure impulsivity and risk taking.
“Usually, but not always, people with ADHD make riskier choices on this task,” Dr. McNorgan noted.
The investigators measured the amount of interconnectedness among different brain regions during the response inhibition task, which was repeated four times.
Patterns of interconnectivity were then fed into a deep-learning neural network that learned which patterns belonged to the ADHD group vs. those without ADHD (control group) and which patterns belonged to the high vs. low scorers on the IGT.
Caveats, cautionary notes
“The trained models are then tested on brain patterns they had never seen before, and we found the models would make the correct ADHD diagnosis and could tell apart the high and low scorers on the IGT 99% of the time,” Dr. McNorgan reported.
“The trained classifiers make predictions by calculating probabilities, and the neural networks learned how each of the brain connections contributes towards the final classification probability. We identified the set of brain connections that had the greatest influence on these probability calculations,” he noted.
Because the network classified both ADHD diagnosis and gambling task performance, the researchers were able to distinguish between connections that predicted ADHD when gambling performance was poor, as is typical for patients with ADHD, and those predicting ADHD when gambling performance was uncharacteristically good.
While more work is needed, the findings have potential clinical relevance, Dr. McNorgan said.
“ADHD can be difficult to diagnose reliably. If expense wasn’t an issue, fMRI may be able to help make diagnosis more reliable and objective,” he added.
However, because individuals with ADHD have different behavioral profiles, such as scoring atypically well on the IGT, additional studies using this approach may help identify brain networks “that are more or less active in those with ADHD that show a particular diagnostic trait,” he said.
“This could help inform what treatments might be more effective for those individuals,” Dr. McNorgan said.
Of course, he added, “clinicians’ diagnostic expertise is still required, as I would not base an ADHD diagnosis solely on the results of a single brain scan.”
No cross-validation
Commenting on the findings for this news organization, Vince Calhoun, PhD, neuroscientist and founding director of the Center for Translational Research in Neuroimaging and Data Science, Atlanta, a joint effort between Georgia State, Georgia Tech, and Emory University, noted some study limitations.
One cautionary note is that the investigators “appear to select relevant regions to include in the model based on activation to the task, then computed the predictions using the subset of regions that showed strong activation. The issue is this was done on the same data, so there was no cross-validation of this ‘feature selection’ step,” said Dr. Calhoun, who was not involved with the research. “This is a type of circularity which can lead to inflated accuracies,” he added.
Dr. Calhoun also noted that “multiple ADHD classification studies” have reported accuracies above 90%. In addition, there were only 80 participants in the current dataset.
“That’s relatively small for making strong claims about high accuracies as has been reported elsewhere,” he said.
Dr. McNorgan and Dr. Calhoun have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Functional brain connectivity patterns are a stable biomarker of attention-deficit/hyperactivity disorder, new research suggests.
By applying a machine-learning approach to brain-imaging data, investigators were able to identify with 99% accuracy the adult study participants who had been diagnosed with ADHD in childhood.
“Even though the symptoms of ADHD may be less apparent in adulthood, the brain-wiring signature seems to be persistent,” study investigator Christopher McNorgan, PhD, of the department of psychology, State University of New York at Buffalo told this news organization.
The findings were published online Dec. 17, 2020, in Frontiers of Psychology.
Deep-learning neural networks
The researchers analyzed archived functional magnetic resonance imaging (fMRI) and behavioral data for 80 adults (mean age, 24 years; 64 male). Of these participants, 55 were diagnosed with ADHD in childhood and 25 were not.
The fMRI data were obtained during a response inhibition task that tested the individual’s ability to not respond automatically; for example, not saying “Simon Says” after someone else makes the comment.
The behavioral data included scores on the Iowa Gambling Task (IGT), which is used to measure impulsivity and risk taking.
“Usually, but not always, people with ADHD make riskier choices on this task,” Dr. McNorgan noted.
The investigators measured the amount of interconnectedness among different brain regions during the response inhibition task, which was repeated four times.
Patterns of interconnectivity were then fed into a deep-learning neural network that learned which patterns belonged to the ADHD group vs. those without ADHD (control group) and which patterns belonged to the high vs. low scorers on the IGT.
Caveats, cautionary notes
“The trained models are then tested on brain patterns they had never seen before, and we found the models would make the correct ADHD diagnosis and could tell apart the high and low scorers on the IGT 99% of the time,” Dr. McNorgan reported.
“The trained classifiers make predictions by calculating probabilities, and the neural networks learned how each of the brain connections contributes towards the final classification probability. We identified the set of brain connections that had the greatest influence on these probability calculations,” he noted.
Because the network classified both ADHD diagnosis and gambling task performance, the researchers were able to distinguish between connections that predicted ADHD when gambling performance was poor, as is typical for patients with ADHD, and those predicting ADHD when gambling performance was uncharacteristically good.
While more work is needed, the findings have potential clinical relevance, Dr. McNorgan said.
“ADHD can be difficult to diagnose reliably. If expense wasn’t an issue, fMRI may be able to help make diagnosis more reliable and objective,” he added.
However, because individuals with ADHD have different behavioral profiles, such as scoring atypically well on the IGT, additional studies using this approach may help identify brain networks “that are more or less active in those with ADHD that show a particular diagnostic trait,” he said.
“This could help inform what treatments might be more effective for those individuals,” Dr. McNorgan said.
Of course, he added, “clinicians’ diagnostic expertise is still required, as I would not base an ADHD diagnosis solely on the results of a single brain scan.”
No cross-validation
Commenting on the findings for this news organization, Vince Calhoun, PhD, neuroscientist and founding director of the Center for Translational Research in Neuroimaging and Data Science, Atlanta, a joint effort between Georgia State, Georgia Tech, and Emory University, noted some study limitations.
One cautionary note is that the investigators “appear to select relevant regions to include in the model based on activation to the task, then computed the predictions using the subset of regions that showed strong activation. The issue is this was done on the same data, so there was no cross-validation of this ‘feature selection’ step,” said Dr. Calhoun, who was not involved with the research. “This is a type of circularity which can lead to inflated accuracies,” he added.
Dr. Calhoun also noted that “multiple ADHD classification studies” have reported accuracies above 90%. In addition, there were only 80 participants in the current dataset.
“That’s relatively small for making strong claims about high accuracies as has been reported elsewhere,” he said.
Dr. McNorgan and Dr. Calhoun have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cannabis tied to self-harm, death in youth with mood disorders
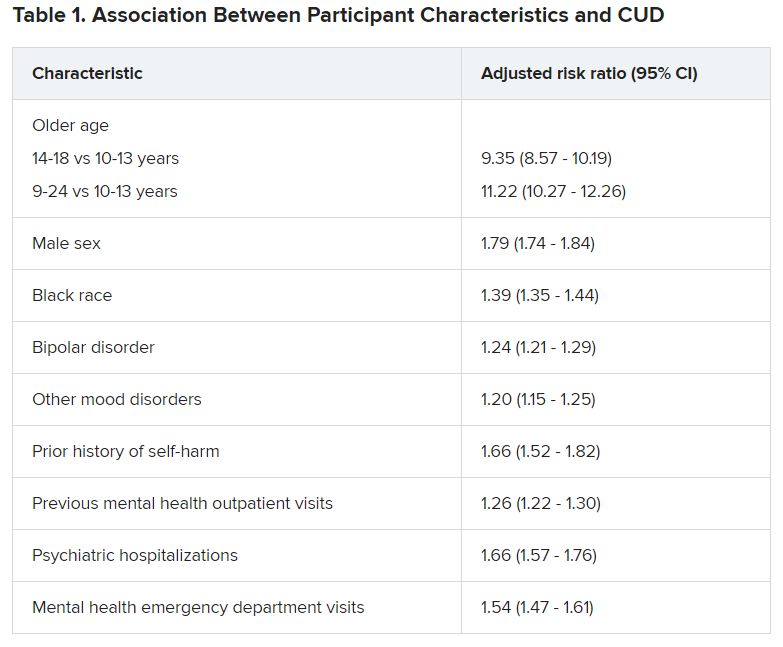
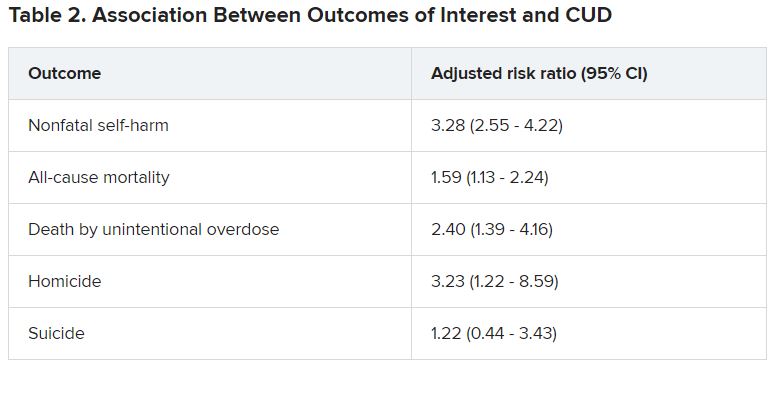
Adolescents and young adults with mood disorders and cannabis use disorder (CUD) are at significantly increased risk for self-harm, all-cause mortality, homicide, and death by unintentional overdose, new research suggests.
Investigators found the risk for self-harm was three times higher, all-cause mortality was 59% higher, unintentional overdose was 2.5 times higher, and homicide was more than three times higher in those with versus without CUD.
“The take-home message of these findings is that we need to be aware of the perception that cannabis use is harmless, when it’s actually not,” lead author Cynthia Fontanella, PhD, associate professor of psychiatry, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“We need to educate parents and clinicians that there are risks associated with cannabis, including increased risk for self-harm and death, and we need to effectively treat both cannabis use disorder and mood disorders,” she said.
The study was published online Jan. 19, 2021, in JAMA Pediatrics.
Little research in youth
“There has been very little research conducted on CUD in the adolescent population, and most studies have been conducted with adults,” Dr. Fontanella said.
Research on adults has shown that, even in people without mood disorders, cannabis use is associated with the early onset of mood disorders, psychosis, and anxiety disorders and has also been linked with suicidal behavior and increased risk for motor vehicle accidents, Dr. Fontanella said.
“We were motivated to conduct this study because we treat kids with depression and bipolar disorder and we noticed a high prevalence of CUD in this population, so we were curious about what its negative effects might be,” Dr. Fontanella recounted.
The researchers analyzed 7-year data drawn from Ohio Medicaid claims and linked to data from death certificates in 204,780 youths between the ages of 10 and 24 years (mean age was 17.2 years at the time of mood disorder diagnosis). Most were female, non-Hispanic White, enrolled in Medicaid because of poverty, and living in a metropolitan area (65.0%, 66.9%, 87.6%, and 77.1%, respectively).
Participants were followed up to 1 year from diagnosis until the end of enrollment, a self-harm event, or death.
Researchers included demographic, clinical, and treatment factors as covariates.
Close to three-quarters (72.7%) of the cohort had a depressive disorder, followed by unspecified/persistent mood disorder and bipolar disorder (14.9% and 12.4%, respectively). Comorbidities included ADHD (12.4%), anxiety disorder (12.3%), and other mental disorders (13.1%).
One -tenth of the cohort (10.3%) were diagnosed with CUD.
CUD treatment referrals
“Although CUD was associated with suicide in the unadjusted model, it was not significantly associated in adjusted models,” the authors reported.
Dr. Fontanella noted that the risk for these adverse outcomes is greater among those who engage in heavy, frequent use or who use cannabis that has higher-potency tetrahydrocannabinol (THC) content.
Reasons why CUD might be associated with these adverse outcomes are that it can increase impulsivity, poor judgment, and clouded thinking, which may in turn increase the risk for self-harm behaviors, she said.
She recommended that clinicians refer youth with CUD for “effective treatments,” including family-based models and individual approaches, such as cognitive behavioral therapy and motivational enhancement therapy.
Open dialogue
In a comment, Wilfrid Noel Raby, MD, PhD, adjunct clinical professor, Albert Einstein College of Medicine, New York, noted that psychosis can occur in patients with CUD and mood disorders – especially bipolar disorder – but was not included as a study outcome. “I would have liked to see more data about that,” he said.
However, “The trend is that cannabis use is starting at younger and younger ages, which has all kinds of ramifications in terms of cerebral development.”
Christopher Hammond, MD, PhD, assistant professor of psychiatry, Johns Hopkins University, Baltimore, said: “Three major strengths of the study are the size of the sample, its longitudinal analysis, and that the authors controlled for a number of potential confounding variables.”
In light of the findings, Dr. Hammond recommended clinicians and other health professionals who work with young people “should screen for cannabis-related problems in youth with mood disorders.”
Dr. Hammond, who is the director of the Co-occurring Disorders in Adolescents and Young Adults Clinical and Research Program, Johns Hopkins Bayview Medical Center, Baltimore, and was not involved with the study, recommended counseling youth with mood disorders and their parents and families “regarding the potential adverse health effects related to cannabis use.”
He also recommended “open dialogue with youth with and without mental health conditions about misleading reports in the national media and advertising about cannabis’ health benefits.”
The study was funded by the National Institute of Mental Health. Dr. Fontanella reported receiving grants from the National Institute of Mental Health during the conduct of the study. Dr. Raby reported no relevant financial relationships. Dr. Hammond reported receiving research grant funding from the National Institutes of Health, the American Academy of Child & Adolescent Psychiatry, Substance Abuse Mental Health Services Administration, the National Network of Depression Centers, and the Armstrong Institute at Johns Hopkins Bayview and serves as a scientific adviser for the National Courts and Science Institute and as a subject matter expert for SAMHSA related to co-occurring substance use disorders and severe emotional disturbance in youth.
A version of this article first appeared on Medscape.com.
Adolescents and young adults with mood disorders and cannabis use disorder (CUD) are at significantly increased risk for self-harm, all-cause mortality, homicide, and death by unintentional overdose, new research suggests.
Investigators found the risk for self-harm was three times higher, all-cause mortality was 59% higher, unintentional overdose was 2.5 times higher, and homicide was more than three times higher in those with versus without CUD.
“The take-home message of these findings is that we need to be aware of the perception that cannabis use is harmless, when it’s actually not,” lead author Cynthia Fontanella, PhD, associate professor of psychiatry, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“We need to educate parents and clinicians that there are risks associated with cannabis, including increased risk for self-harm and death, and we need to effectively treat both cannabis use disorder and mood disorders,” she said.
The study was published online Jan. 19, 2021, in JAMA Pediatrics.
Little research in youth
“There has been very little research conducted on CUD in the adolescent population, and most studies have been conducted with adults,” Dr. Fontanella said.
Research on adults has shown that, even in people without mood disorders, cannabis use is associated with the early onset of mood disorders, psychosis, and anxiety disorders and has also been linked with suicidal behavior and increased risk for motor vehicle accidents, Dr. Fontanella said.
“We were motivated to conduct this study because we treat kids with depression and bipolar disorder and we noticed a high prevalence of CUD in this population, so we were curious about what its negative effects might be,” Dr. Fontanella recounted.
The researchers analyzed 7-year data drawn from Ohio Medicaid claims and linked to data from death certificates in 204,780 youths between the ages of 10 and 24 years (mean age was 17.2 years at the time of mood disorder diagnosis). Most were female, non-Hispanic White, enrolled in Medicaid because of poverty, and living in a metropolitan area (65.0%, 66.9%, 87.6%, and 77.1%, respectively).
Participants were followed up to 1 year from diagnosis until the end of enrollment, a self-harm event, or death.
Researchers included demographic, clinical, and treatment factors as covariates.
Close to three-quarters (72.7%) of the cohort had a depressive disorder, followed by unspecified/persistent mood disorder and bipolar disorder (14.9% and 12.4%, respectively). Comorbidities included ADHD (12.4%), anxiety disorder (12.3%), and other mental disorders (13.1%).
One -tenth of the cohort (10.3%) were diagnosed with CUD.
CUD treatment referrals
“Although CUD was associated with suicide in the unadjusted model, it was not significantly associated in adjusted models,” the authors reported.
Dr. Fontanella noted that the risk for these adverse outcomes is greater among those who engage in heavy, frequent use or who use cannabis that has higher-potency tetrahydrocannabinol (THC) content.
Reasons why CUD might be associated with these adverse outcomes are that it can increase impulsivity, poor judgment, and clouded thinking, which may in turn increase the risk for self-harm behaviors, she said.
She recommended that clinicians refer youth with CUD for “effective treatments,” including family-based models and individual approaches, such as cognitive behavioral therapy and motivational enhancement therapy.
Open dialogue
In a comment, Wilfrid Noel Raby, MD, PhD, adjunct clinical professor, Albert Einstein College of Medicine, New York, noted that psychosis can occur in patients with CUD and mood disorders – especially bipolar disorder – but was not included as a study outcome. “I would have liked to see more data about that,” he said.
However, “The trend is that cannabis use is starting at younger and younger ages, which has all kinds of ramifications in terms of cerebral development.”
Christopher Hammond, MD, PhD, assistant professor of psychiatry, Johns Hopkins University, Baltimore, said: “Three major strengths of the study are the size of the sample, its longitudinal analysis, and that the authors controlled for a number of potential confounding variables.”
In light of the findings, Dr. Hammond recommended clinicians and other health professionals who work with young people “should screen for cannabis-related problems in youth with mood disorders.”
Dr. Hammond, who is the director of the Co-occurring Disorders in Adolescents and Young Adults Clinical and Research Program, Johns Hopkins Bayview Medical Center, Baltimore, and was not involved with the study, recommended counseling youth with mood disorders and their parents and families “regarding the potential adverse health effects related to cannabis use.”
He also recommended “open dialogue with youth with and without mental health conditions about misleading reports in the national media and advertising about cannabis’ health benefits.”
The study was funded by the National Institute of Mental Health. Dr. Fontanella reported receiving grants from the National Institute of Mental Health during the conduct of the study. Dr. Raby reported no relevant financial relationships. Dr. Hammond reported receiving research grant funding from the National Institutes of Health, the American Academy of Child & Adolescent Psychiatry, Substance Abuse Mental Health Services Administration, the National Network of Depression Centers, and the Armstrong Institute at Johns Hopkins Bayview and serves as a scientific adviser for the National Courts and Science Institute and as a subject matter expert for SAMHSA related to co-occurring substance use disorders and severe emotional disturbance in youth.
A version of this article first appeared on Medscape.com.
Adolescents and young adults with mood disorders and cannabis use disorder (CUD) are at significantly increased risk for self-harm, all-cause mortality, homicide, and death by unintentional overdose, new research suggests.
Investigators found the risk for self-harm was three times higher, all-cause mortality was 59% higher, unintentional overdose was 2.5 times higher, and homicide was more than three times higher in those with versus without CUD.
“The take-home message of these findings is that we need to be aware of the perception that cannabis use is harmless, when it’s actually not,” lead author Cynthia Fontanella, PhD, associate professor of psychiatry, Ohio State University Wexner Medical Center, Columbus, said in an interview.
“We need to educate parents and clinicians that there are risks associated with cannabis, including increased risk for self-harm and death, and we need to effectively treat both cannabis use disorder and mood disorders,” she said.
The study was published online Jan. 19, 2021, in JAMA Pediatrics.
Little research in youth
“There has been very little research conducted on CUD in the adolescent population, and most studies have been conducted with adults,” Dr. Fontanella said.
Research on adults has shown that, even in people without mood disorders, cannabis use is associated with the early onset of mood disorders, psychosis, and anxiety disorders and has also been linked with suicidal behavior and increased risk for motor vehicle accidents, Dr. Fontanella said.
“We were motivated to conduct this study because we treat kids with depression and bipolar disorder and we noticed a high prevalence of CUD in this population, so we were curious about what its negative effects might be,” Dr. Fontanella recounted.
The researchers analyzed 7-year data drawn from Ohio Medicaid claims and linked to data from death certificates in 204,780 youths between the ages of 10 and 24 years (mean age was 17.2 years at the time of mood disorder diagnosis). Most were female, non-Hispanic White, enrolled in Medicaid because of poverty, and living in a metropolitan area (65.0%, 66.9%, 87.6%, and 77.1%, respectively).
Participants were followed up to 1 year from diagnosis until the end of enrollment, a self-harm event, or death.
Researchers included demographic, clinical, and treatment factors as covariates.
Close to three-quarters (72.7%) of the cohort had a depressive disorder, followed by unspecified/persistent mood disorder and bipolar disorder (14.9% and 12.4%, respectively). Comorbidities included ADHD (12.4%), anxiety disorder (12.3%), and other mental disorders (13.1%).
One -tenth of the cohort (10.3%) were diagnosed with CUD.
CUD treatment referrals
“Although CUD was associated with suicide in the unadjusted model, it was not significantly associated in adjusted models,” the authors reported.
Dr. Fontanella noted that the risk for these adverse outcomes is greater among those who engage in heavy, frequent use or who use cannabis that has higher-potency tetrahydrocannabinol (THC) content.
Reasons why CUD might be associated with these adverse outcomes are that it can increase impulsivity, poor judgment, and clouded thinking, which may in turn increase the risk for self-harm behaviors, she said.
She recommended that clinicians refer youth with CUD for “effective treatments,” including family-based models and individual approaches, such as cognitive behavioral therapy and motivational enhancement therapy.
Open dialogue
In a comment, Wilfrid Noel Raby, MD, PhD, adjunct clinical professor, Albert Einstein College of Medicine, New York, noted that psychosis can occur in patients with CUD and mood disorders – especially bipolar disorder – but was not included as a study outcome. “I would have liked to see more data about that,” he said.
However, “The trend is that cannabis use is starting at younger and younger ages, which has all kinds of ramifications in terms of cerebral development.”
Christopher Hammond, MD, PhD, assistant professor of psychiatry, Johns Hopkins University, Baltimore, said: “Three major strengths of the study are the size of the sample, its longitudinal analysis, and that the authors controlled for a number of potential confounding variables.”
In light of the findings, Dr. Hammond recommended clinicians and other health professionals who work with young people “should screen for cannabis-related problems in youth with mood disorders.”
Dr. Hammond, who is the director of the Co-occurring Disorders in Adolescents and Young Adults Clinical and Research Program, Johns Hopkins Bayview Medical Center, Baltimore, and was not involved with the study, recommended counseling youth with mood disorders and their parents and families “regarding the potential adverse health effects related to cannabis use.”
He also recommended “open dialogue with youth with and without mental health conditions about misleading reports in the national media and advertising about cannabis’ health benefits.”
The study was funded by the National Institute of Mental Health. Dr. Fontanella reported receiving grants from the National Institute of Mental Health during the conduct of the study. Dr. Raby reported no relevant financial relationships. Dr. Hammond reported receiving research grant funding from the National Institutes of Health, the American Academy of Child & Adolescent Psychiatry, Substance Abuse Mental Health Services Administration, the National Network of Depression Centers, and the Armstrong Institute at Johns Hopkins Bayview and serves as a scientific adviser for the National Courts and Science Institute and as a subject matter expert for SAMHSA related to co-occurring substance use disorders and severe emotional disturbance in youth.
A version of this article first appeared on Medscape.com.
Kids already coping with mental disorders spiral as pandemic topples vital support systems
A bag of Doritos, that’s all Princess wanted.
Her mom calls her Princess, but her real name is Lindsey. She’s 17 and lives with her mom, Sandra, a nurse, outside Atlanta. On May 17, 2020, a Sunday, Lindsey decided she didn’t want breakfast; she wanted Doritos. So she left home and walked to Family Dollar, taking her pants off on the way, while her mom followed on foot, talking to the police on her phone as they went.
Lindsey has autism. It can be hard for her to communicate and navigate social situations. She thrives on routine and gets special help at school. Or got help, before the coronavirus pandemic closed schools and forced tens of millions of children to stay home. Sandra said that’s when their living hell started.
“It’s like her brain was wired,” she said. “She’d just put on her jacket, and she’s out the door. And I’m chasing her.”
On May 17, Sandra chased her all the way to Family Dollar. Hours later, Lindsey was in jail, charged with assaulting her mom. (KHN and NPR are not using the family’s last name.)
Lindsey is 1 of almost 3 million children in the United States who have a serious emotional or behavioral health condition. When the pandemic forced schools and doctors’ offices to close last spring, it also cut children off from the trained teachers and therapists who understand their needs.
As a result, many, like Lindsey, spiraled into EDs and even police custody. Federal data shows a nationwide surge of children in mental health crisis during the pandemic – a surge that’s further taxing an already overstretched safety net.
‘Take her’
Even after schools closed, Lindsey continued to wake up early, get dressed and wait for the bus. When she realized it had stopped coming, Sandra said, her daughter just started walking out of the house, wandering, a few times a week.
In those situations, Sandra did what many families in crisis report they’ve had to do since the pandemic began: Race through the short list of places she could call for help.
First, her state’s mental health crisis hotline. But they often put Sandra on hold.
“This is ridiculous,” she said of the wait. “It’s supposed to be a crisis team. But I’m on hold for 40, 50 minutes. And by the time you get on the phone, [the crisis] is done!”
Then there’s the local hospital’s ED, but Sandra said she had taken Lindsey there for previous crises and been told there isn’t much they can do.
That’s why, on May 17, when Lindsey walked to Family Dollar in just a red T-shirt and underwear to get that bag of Doritos, Sandra called the last option on her list: the police.
Sandra arrived at the store before the police and paid for the chips. According to Sandra and police records, when an officer approached, Lindsey grew agitated and hit her mom on the back, hard.
Sandra said she explained to the officer: “‘She’s autistic. You know, I’m okay. I’m a nurse. I just need to take her home and give her her medication.’ ”
Lindsey takes a mood stabilizer, but because she left home before breakfast, she hadn’t taken it that morning. The officer asked if Sandra wanted to take her to the nearest hospital.
The hospital wouldn’t be able to help Lindsey, Sandra said. It hadn’t before. “They already told me: ‘Ma’am, there’s nothing we can do.’ They just check her labs, it’s fine, and they ship her back home. There’s nothing [the hospital] can do,” she recalled telling the officer.
Sandra asked if the police could drive her daughter home so the teen could take her medication, but the officer said no, they couldn’t. The only other thing they could do, the officer said, was take Lindsey to jail for hitting her mom.
“I’ve tried everything,” Sandra said, exasperated. She paced the parking lot, feeling hopeless, sad and out of options. Finally, in tears, she told the officers: “Take her.”
Lindsey does not like to be touched and fought back when authorities tried to handcuff her. Several officers wrestled her to the ground. At that point, Sandra protested and said an officer threatened to arrest her, too, if she didn’t back away. Lindsey was taken to jail, where she spent much of the night until Sandra was able to post bail.
Clayton County Solicitor-General Charles Brooks denied that Sandra was threatened with arrest and said that, while Lindsey’s case is still pending, his office “is working to ensure that the resolution in this matter involves a plan for medication compliance and not punitive action.”
Sandra isn’t alone in her experience. Multiple families interviewed for this story reported similar experiences of calling in the police when a child was in crisis because caretakers didn’t feel they had any other option.
‘The whole system is really grinding to a halt’
Roughly 6% of U.S. children ages 6-17 years are living with serious emotional or behavioral difficulties, including children with autism, severe anxiety, depression and trauma-related mental health conditions.
Many of these children depend on schools for access to vital therapies. When schools and doctors’ offices stopped providing in-person services last spring, kids were untethered from the people and supports they rely on.
“The lack of in-person services is really detrimental,” said Susan Duffy, MD,a pediatrician and professor of emergency medicine at Brown University, Providence, R.I.
Marjorie, a mother in Florida, said her 15-year-old son has suffered during these disruptions. He has ADHD and oppositional defiant disorder, a condition marked by frequent and persistent hostility. Little things – like being asked to do schoolwork – can send him into a rage, leading to holes punched in walls, broken doors and violent threats. (The family’s last name or her son’s first name are not used to protect her son’s privacy and future prospects.)
The pandemic has shifted both school and her son’s therapy sessions online. But Marjorie said virtual therapy isn’t working because her son doesn’t focus well during sessions and tries to watch television instead. Lately, she has simply been canceling them.
“I was paying for appointments and there was no therapeutic value,” Marjorie said.
The issues cut across socioeconomic lines – affecting families with private insurance, like Marjorie, as well as those who receive coverage through Medicaid, a federal-state program that provides health insurance to low-income people and those with disabilities.
In the first few months of the pandemic, between March and May, children on Medicaid received 44% fewer outpatient mental health services – including therapy and in-home support – compared with the same time period in 2019, according to the Centers for Medicare & Medicaid Services. That’s even after accounting for increased telehealth appointments.
And while the nation’s EDs have seen a decline in overall visits, there was a relative increase in mental health visits for kids in 2020, compared with 2019.
The Centers for Disease Control and Prevention found that, from April to October 2020, hospitals across the United States saw a 24% increase in the proportion of mental health emergency visits for children aged 5-11 years, and a 31% increase for children aged 12-17.
“Not only are we seeing more children, more children are being admitted” to inpatient care.
That’s because there are fewer outpatient services now available to children, she said, and because the conditions of the children showing up at EDs “are more serious.”
This crisis is not only making life harder for these kids and their families, but it’s also stressing the entire health care system.
Child and adolescent psychiatrists working in hospitals around the country said children are increasingly “boarding” in EDs for days, waiting for inpatient admission to a regular hospital or psychiatric hospital.
Before the pandemic, there was already a shortage of inpatient psychiatric beds for children, said Christopher Bellonci, MD, a child psychiatrist at Judge Baker Children’s Center in Boston. That shortage has only gotten worse as hospitals cut capacity to allow for more physical distancing within psychiatric units.
“The whole system is really grinding to a halt at a time when we have unprecedented need,” Dr. Bellonci said.
‘A signal that the rest of your system doesn’t work’
Psychiatrists on the front lines share the frustrations of parents struggling to find help for their children.
Part of the problem is there have never been enough psychiatrists and therapists trained to work with children, intervening in the early stages of their illness, said Jennifer Havens, MD, a child psychiatrist at New York University.
“Tons of people showing up in emergency rooms in bad shape is a signal that the rest of your system doesn’t work,” she said.
Too often, Dr. Havens said, services aren’t available until children are older – and in crisis. “Often for people who don’t have access to services, we wait until they’re too big to be managed.”
While the pandemic has made life harder for Marjorie and her son in Florida, she said it has always been difficult to find the support and care he needs. Last fall, he needed a psychiatric evaluation, but the nearest specialist who would accept her commercial insurance was 100 miles away, in Alabama.
“Even when you have the money or you have the insurance, it is still a travesty,” Marjorie said. “You cannot get help for these kids.”
Parents are frustrated, and so are psychiatrists on the front lines. C.J. Glawe, MD, who leads the psychiatric crisis department at Nationwide Children’s Hospital in Columbus, Ohio, said that once a child is stabilized after a crisis it can be hard to explain to parents that they may not be able to find follow-up care anywhere near their home.
“Especially when I can clearly tell you I know exactly what you need, I just can’t give it to you,” Dr. Glawe said. “It’s demoralizing.”
When states and communities fail to provide children the services they need to live at home, kids can deteriorate and even wind up in jail, like Lindsey. At that point, Dr. Glawe said, the cost and level of care required will be even higher, whether that’s hospitalization or long stays in residential treatment facilities.
That’s exactly the scenario Sandra, Lindsey’s mom, is hoping to avoid for her Princess.
“For me, as a nurse and as a provider, that will be the last thing for my daughter,” she said. “It’s like [state and local leaders] leave it to the school and the parent to deal with, and they don’t care. And that’s the problem. It’s sad because, if I’m not here...”
Her voice trailed off as tears welled.
“She didn’t ask to have autism.”
To help families like Sandra’s and Marjorie’s, advocates said, all levels of government need to invest in creating a mental health system that’s accessible to anyone who needs it.
But given that many states have seen their revenues drop because of the pandemic, there’s a concern services will instead be cut – at a time when the need has never been greater.
This story is part of a reporting partnership that includes NPR, Illinois Public Media and Kaiser Health News. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
A bag of Doritos, that’s all Princess wanted.
Her mom calls her Princess, but her real name is Lindsey. She’s 17 and lives with her mom, Sandra, a nurse, outside Atlanta. On May 17, 2020, a Sunday, Lindsey decided she didn’t want breakfast; she wanted Doritos. So she left home and walked to Family Dollar, taking her pants off on the way, while her mom followed on foot, talking to the police on her phone as they went.
Lindsey has autism. It can be hard for her to communicate and navigate social situations. She thrives on routine and gets special help at school. Or got help, before the coronavirus pandemic closed schools and forced tens of millions of children to stay home. Sandra said that’s when their living hell started.
“It’s like her brain was wired,” she said. “She’d just put on her jacket, and she’s out the door. And I’m chasing her.”
On May 17, Sandra chased her all the way to Family Dollar. Hours later, Lindsey was in jail, charged with assaulting her mom. (KHN and NPR are not using the family’s last name.)
Lindsey is 1 of almost 3 million children in the United States who have a serious emotional or behavioral health condition. When the pandemic forced schools and doctors’ offices to close last spring, it also cut children off from the trained teachers and therapists who understand their needs.
As a result, many, like Lindsey, spiraled into EDs and even police custody. Federal data shows a nationwide surge of children in mental health crisis during the pandemic – a surge that’s further taxing an already overstretched safety net.
‘Take her’
Even after schools closed, Lindsey continued to wake up early, get dressed and wait for the bus. When she realized it had stopped coming, Sandra said, her daughter just started walking out of the house, wandering, a few times a week.
In those situations, Sandra did what many families in crisis report they’ve had to do since the pandemic began: Race through the short list of places she could call for help.
First, her state’s mental health crisis hotline. But they often put Sandra on hold.
“This is ridiculous,” she said of the wait. “It’s supposed to be a crisis team. But I’m on hold for 40, 50 minutes. And by the time you get on the phone, [the crisis] is done!”
Then there’s the local hospital’s ED, but Sandra said she had taken Lindsey there for previous crises and been told there isn’t much they can do.
That’s why, on May 17, when Lindsey walked to Family Dollar in just a red T-shirt and underwear to get that bag of Doritos, Sandra called the last option on her list: the police.
Sandra arrived at the store before the police and paid for the chips. According to Sandra and police records, when an officer approached, Lindsey grew agitated and hit her mom on the back, hard.
Sandra said she explained to the officer: “‘She’s autistic. You know, I’m okay. I’m a nurse. I just need to take her home and give her her medication.’ ”
Lindsey takes a mood stabilizer, but because she left home before breakfast, she hadn’t taken it that morning. The officer asked if Sandra wanted to take her to the nearest hospital.
The hospital wouldn’t be able to help Lindsey, Sandra said. It hadn’t before. “They already told me: ‘Ma’am, there’s nothing we can do.’ They just check her labs, it’s fine, and they ship her back home. There’s nothing [the hospital] can do,” she recalled telling the officer.
Sandra asked if the police could drive her daughter home so the teen could take her medication, but the officer said no, they couldn’t. The only other thing they could do, the officer said, was take Lindsey to jail for hitting her mom.
“I’ve tried everything,” Sandra said, exasperated. She paced the parking lot, feeling hopeless, sad and out of options. Finally, in tears, she told the officers: “Take her.”
Lindsey does not like to be touched and fought back when authorities tried to handcuff her. Several officers wrestled her to the ground. At that point, Sandra protested and said an officer threatened to arrest her, too, if she didn’t back away. Lindsey was taken to jail, where she spent much of the night until Sandra was able to post bail.
Clayton County Solicitor-General Charles Brooks denied that Sandra was threatened with arrest and said that, while Lindsey’s case is still pending, his office “is working to ensure that the resolution in this matter involves a plan for medication compliance and not punitive action.”
Sandra isn’t alone in her experience. Multiple families interviewed for this story reported similar experiences of calling in the police when a child was in crisis because caretakers didn’t feel they had any other option.
‘The whole system is really grinding to a halt’
Roughly 6% of U.S. children ages 6-17 years are living with serious emotional or behavioral difficulties, including children with autism, severe anxiety, depression and trauma-related mental health conditions.
Many of these children depend on schools for access to vital therapies. When schools and doctors’ offices stopped providing in-person services last spring, kids were untethered from the people and supports they rely on.
“The lack of in-person services is really detrimental,” said Susan Duffy, MD,a pediatrician and professor of emergency medicine at Brown University, Providence, R.I.
Marjorie, a mother in Florida, said her 15-year-old son has suffered during these disruptions. He has ADHD and oppositional defiant disorder, a condition marked by frequent and persistent hostility. Little things – like being asked to do schoolwork – can send him into a rage, leading to holes punched in walls, broken doors and violent threats. (The family’s last name or her son’s first name are not used to protect her son’s privacy and future prospects.)
The pandemic has shifted both school and her son’s therapy sessions online. But Marjorie said virtual therapy isn’t working because her son doesn’t focus well during sessions and tries to watch television instead. Lately, she has simply been canceling them.
“I was paying for appointments and there was no therapeutic value,” Marjorie said.
The issues cut across socioeconomic lines – affecting families with private insurance, like Marjorie, as well as those who receive coverage through Medicaid, a federal-state program that provides health insurance to low-income people and those with disabilities.
In the first few months of the pandemic, between March and May, children on Medicaid received 44% fewer outpatient mental health services – including therapy and in-home support – compared with the same time period in 2019, according to the Centers for Medicare & Medicaid Services. That’s even after accounting for increased telehealth appointments.
And while the nation’s EDs have seen a decline in overall visits, there was a relative increase in mental health visits for kids in 2020, compared with 2019.
The Centers for Disease Control and Prevention found that, from April to October 2020, hospitals across the United States saw a 24% increase in the proportion of mental health emergency visits for children aged 5-11 years, and a 31% increase for children aged 12-17.
“Not only are we seeing more children, more children are being admitted” to inpatient care.
That’s because there are fewer outpatient services now available to children, she said, and because the conditions of the children showing up at EDs “are more serious.”
This crisis is not only making life harder for these kids and their families, but it’s also stressing the entire health care system.
Child and adolescent psychiatrists working in hospitals around the country said children are increasingly “boarding” in EDs for days, waiting for inpatient admission to a regular hospital or psychiatric hospital.
Before the pandemic, there was already a shortage of inpatient psychiatric beds for children, said Christopher Bellonci, MD, a child psychiatrist at Judge Baker Children’s Center in Boston. That shortage has only gotten worse as hospitals cut capacity to allow for more physical distancing within psychiatric units.
“The whole system is really grinding to a halt at a time when we have unprecedented need,” Dr. Bellonci said.
‘A signal that the rest of your system doesn’t work’
Psychiatrists on the front lines share the frustrations of parents struggling to find help for their children.
Part of the problem is there have never been enough psychiatrists and therapists trained to work with children, intervening in the early stages of their illness, said Jennifer Havens, MD, a child psychiatrist at New York University.
“Tons of people showing up in emergency rooms in bad shape is a signal that the rest of your system doesn’t work,” she said.
Too often, Dr. Havens said, services aren’t available until children are older – and in crisis. “Often for people who don’t have access to services, we wait until they’re too big to be managed.”
While the pandemic has made life harder for Marjorie and her son in Florida, she said it has always been difficult to find the support and care he needs. Last fall, he needed a psychiatric evaluation, but the nearest specialist who would accept her commercial insurance was 100 miles away, in Alabama.
“Even when you have the money or you have the insurance, it is still a travesty,” Marjorie said. “You cannot get help for these kids.”
Parents are frustrated, and so are psychiatrists on the front lines. C.J. Glawe, MD, who leads the psychiatric crisis department at Nationwide Children’s Hospital in Columbus, Ohio, said that once a child is stabilized after a crisis it can be hard to explain to parents that they may not be able to find follow-up care anywhere near their home.
“Especially when I can clearly tell you I know exactly what you need, I just can’t give it to you,” Dr. Glawe said. “It’s demoralizing.”
When states and communities fail to provide children the services they need to live at home, kids can deteriorate and even wind up in jail, like Lindsey. At that point, Dr. Glawe said, the cost and level of care required will be even higher, whether that’s hospitalization or long stays in residential treatment facilities.
That’s exactly the scenario Sandra, Lindsey’s mom, is hoping to avoid for her Princess.
“For me, as a nurse and as a provider, that will be the last thing for my daughter,” she said. “It’s like [state and local leaders] leave it to the school and the parent to deal with, and they don’t care. And that’s the problem. It’s sad because, if I’m not here...”
Her voice trailed off as tears welled.
“She didn’t ask to have autism.”
To help families like Sandra’s and Marjorie’s, advocates said, all levels of government need to invest in creating a mental health system that’s accessible to anyone who needs it.
But given that many states have seen their revenues drop because of the pandemic, there’s a concern services will instead be cut – at a time when the need has never been greater.
This story is part of a reporting partnership that includes NPR, Illinois Public Media and Kaiser Health News. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
A bag of Doritos, that’s all Princess wanted.
Her mom calls her Princess, but her real name is Lindsey. She’s 17 and lives with her mom, Sandra, a nurse, outside Atlanta. On May 17, 2020, a Sunday, Lindsey decided she didn’t want breakfast; she wanted Doritos. So she left home and walked to Family Dollar, taking her pants off on the way, while her mom followed on foot, talking to the police on her phone as they went.
Lindsey has autism. It can be hard for her to communicate and navigate social situations. She thrives on routine and gets special help at school. Or got help, before the coronavirus pandemic closed schools and forced tens of millions of children to stay home. Sandra said that’s when their living hell started.
“It’s like her brain was wired,” she said. “She’d just put on her jacket, and she’s out the door. And I’m chasing her.”
On May 17, Sandra chased her all the way to Family Dollar. Hours later, Lindsey was in jail, charged with assaulting her mom. (KHN and NPR are not using the family’s last name.)
Lindsey is 1 of almost 3 million children in the United States who have a serious emotional or behavioral health condition. When the pandemic forced schools and doctors’ offices to close last spring, it also cut children off from the trained teachers and therapists who understand their needs.
As a result, many, like Lindsey, spiraled into EDs and even police custody. Federal data shows a nationwide surge of children in mental health crisis during the pandemic – a surge that’s further taxing an already overstretched safety net.
‘Take her’
Even after schools closed, Lindsey continued to wake up early, get dressed and wait for the bus. When she realized it had stopped coming, Sandra said, her daughter just started walking out of the house, wandering, a few times a week.
In those situations, Sandra did what many families in crisis report they’ve had to do since the pandemic began: Race through the short list of places she could call for help.
First, her state’s mental health crisis hotline. But they often put Sandra on hold.
“This is ridiculous,” she said of the wait. “It’s supposed to be a crisis team. But I’m on hold for 40, 50 minutes. And by the time you get on the phone, [the crisis] is done!”
Then there’s the local hospital’s ED, but Sandra said she had taken Lindsey there for previous crises and been told there isn’t much they can do.
That’s why, on May 17, when Lindsey walked to Family Dollar in just a red T-shirt and underwear to get that bag of Doritos, Sandra called the last option on her list: the police.
Sandra arrived at the store before the police and paid for the chips. According to Sandra and police records, when an officer approached, Lindsey grew agitated and hit her mom on the back, hard.
Sandra said she explained to the officer: “‘She’s autistic. You know, I’m okay. I’m a nurse. I just need to take her home and give her her medication.’ ”
Lindsey takes a mood stabilizer, but because she left home before breakfast, she hadn’t taken it that morning. The officer asked if Sandra wanted to take her to the nearest hospital.
The hospital wouldn’t be able to help Lindsey, Sandra said. It hadn’t before. “They already told me: ‘Ma’am, there’s nothing we can do.’ They just check her labs, it’s fine, and they ship her back home. There’s nothing [the hospital] can do,” she recalled telling the officer.
Sandra asked if the police could drive her daughter home so the teen could take her medication, but the officer said no, they couldn’t. The only other thing they could do, the officer said, was take Lindsey to jail for hitting her mom.
“I’ve tried everything,” Sandra said, exasperated. She paced the parking lot, feeling hopeless, sad and out of options. Finally, in tears, she told the officers: “Take her.”
Lindsey does not like to be touched and fought back when authorities tried to handcuff her. Several officers wrestled her to the ground. At that point, Sandra protested and said an officer threatened to arrest her, too, if she didn’t back away. Lindsey was taken to jail, where she spent much of the night until Sandra was able to post bail.
Clayton County Solicitor-General Charles Brooks denied that Sandra was threatened with arrest and said that, while Lindsey’s case is still pending, his office “is working to ensure that the resolution in this matter involves a plan for medication compliance and not punitive action.”
Sandra isn’t alone in her experience. Multiple families interviewed for this story reported similar experiences of calling in the police when a child was in crisis because caretakers didn’t feel they had any other option.
‘The whole system is really grinding to a halt’
Roughly 6% of U.S. children ages 6-17 years are living with serious emotional or behavioral difficulties, including children with autism, severe anxiety, depression and trauma-related mental health conditions.
Many of these children depend on schools for access to vital therapies. When schools and doctors’ offices stopped providing in-person services last spring, kids were untethered from the people and supports they rely on.
“The lack of in-person services is really detrimental,” said Susan Duffy, MD,a pediatrician and professor of emergency medicine at Brown University, Providence, R.I.
Marjorie, a mother in Florida, said her 15-year-old son has suffered during these disruptions. He has ADHD and oppositional defiant disorder, a condition marked by frequent and persistent hostility. Little things – like being asked to do schoolwork – can send him into a rage, leading to holes punched in walls, broken doors and violent threats. (The family’s last name or her son’s first name are not used to protect her son’s privacy and future prospects.)
The pandemic has shifted both school and her son’s therapy sessions online. But Marjorie said virtual therapy isn’t working because her son doesn’t focus well during sessions and tries to watch television instead. Lately, she has simply been canceling them.
“I was paying for appointments and there was no therapeutic value,” Marjorie said.
The issues cut across socioeconomic lines – affecting families with private insurance, like Marjorie, as well as those who receive coverage through Medicaid, a federal-state program that provides health insurance to low-income people and those with disabilities.
In the first few months of the pandemic, between March and May, children on Medicaid received 44% fewer outpatient mental health services – including therapy and in-home support – compared with the same time period in 2019, according to the Centers for Medicare & Medicaid Services. That’s even after accounting for increased telehealth appointments.
And while the nation’s EDs have seen a decline in overall visits, there was a relative increase in mental health visits for kids in 2020, compared with 2019.
The Centers for Disease Control and Prevention found that, from April to October 2020, hospitals across the United States saw a 24% increase in the proportion of mental health emergency visits for children aged 5-11 years, and a 31% increase for children aged 12-17.
“Not only are we seeing more children, more children are being admitted” to inpatient care.
That’s because there are fewer outpatient services now available to children, she said, and because the conditions of the children showing up at EDs “are more serious.”
This crisis is not only making life harder for these kids and their families, but it’s also stressing the entire health care system.
Child and adolescent psychiatrists working in hospitals around the country said children are increasingly “boarding” in EDs for days, waiting for inpatient admission to a regular hospital or psychiatric hospital.
Before the pandemic, there was already a shortage of inpatient psychiatric beds for children, said Christopher Bellonci, MD, a child psychiatrist at Judge Baker Children’s Center in Boston. That shortage has only gotten worse as hospitals cut capacity to allow for more physical distancing within psychiatric units.
“The whole system is really grinding to a halt at a time when we have unprecedented need,” Dr. Bellonci said.
‘A signal that the rest of your system doesn’t work’
Psychiatrists on the front lines share the frustrations of parents struggling to find help for their children.
Part of the problem is there have never been enough psychiatrists and therapists trained to work with children, intervening in the early stages of their illness, said Jennifer Havens, MD, a child psychiatrist at New York University.
“Tons of people showing up in emergency rooms in bad shape is a signal that the rest of your system doesn’t work,” she said.
Too often, Dr. Havens said, services aren’t available until children are older – and in crisis. “Often for people who don’t have access to services, we wait until they’re too big to be managed.”
While the pandemic has made life harder for Marjorie and her son in Florida, she said it has always been difficult to find the support and care he needs. Last fall, he needed a psychiatric evaluation, but the nearest specialist who would accept her commercial insurance was 100 miles away, in Alabama.
“Even when you have the money or you have the insurance, it is still a travesty,” Marjorie said. “You cannot get help for these kids.”
Parents are frustrated, and so are psychiatrists on the front lines. C.J. Glawe, MD, who leads the psychiatric crisis department at Nationwide Children’s Hospital in Columbus, Ohio, said that once a child is stabilized after a crisis it can be hard to explain to parents that they may not be able to find follow-up care anywhere near their home.
“Especially when I can clearly tell you I know exactly what you need, I just can’t give it to you,” Dr. Glawe said. “It’s demoralizing.”
When states and communities fail to provide children the services they need to live at home, kids can deteriorate and even wind up in jail, like Lindsey. At that point, Dr. Glawe said, the cost and level of care required will be even higher, whether that’s hospitalization or long stays in residential treatment facilities.
That’s exactly the scenario Sandra, Lindsey’s mom, is hoping to avoid for her Princess.
“For me, as a nurse and as a provider, that will be the last thing for my daughter,” she said. “It’s like [state and local leaders] leave it to the school and the parent to deal with, and they don’t care. And that’s the problem. It’s sad because, if I’m not here...”
Her voice trailed off as tears welled.
“She didn’t ask to have autism.”
To help families like Sandra’s and Marjorie’s, advocates said, all levels of government need to invest in creating a mental health system that’s accessible to anyone who needs it.
But given that many states have seen their revenues drop because of the pandemic, there’s a concern services will instead be cut – at a time when the need has never been greater.
This story is part of a reporting partnership that includes NPR, Illinois Public Media and Kaiser Health News. Kaiser Health News is a nonprofit news service covering health issues. It is an editorially independent program of KFF (Kaiser Family Foundation), which is not affiliated with Kaiser Permanente.
Maternal autoimmune disease raises children’s risk of ADHD
Maternal autoimmune diseases significantly increased the risk of ADHD in children, based on data from a large cohort study of more than 800,000 mothers and children and a subsequent meta-analysis.
“There is growing evidence that immune-related cells and proteins play a role in brain development and function and that maternal immune activation, including infection, autoimmune disease, and chronic inflammation during pregnancy, increases the risk of neurodevelopmental disorders among children,” wrote Timothy C. Nielsen, MPH, of the University of Sydney, and colleagues.
Previous research has examined a link between maternal autoimmune disorders and autism spectrum disorders in children, but associations with ADHD have not been well studied, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers identified 831,718 mothers and their 831,718 singleton infants in Australia. A total of 12,787 infants were born to mothers with an autoimmune diagnosis; 12,610 of them were matched to 50,440 control infants. ADHD was determined based on prescription for a stimulant treatment or a hospital diagnosis; children with a first ADHD event younger than 3 years were excluded.
In the total cohort of 63,050 infants, the presence of any maternal autoimmune disease was associated with a significantly increased risk of ADHD (hazard ratio, 1.30) as was the presence of several specific conditions: type 1 diabetes (HR, 2.23), psoriasis (HR, 1.66), and rheumatic fever or rheumatic carditis (HR, 1.75).
In addition, the researchers conducted a meta-analysis of the current study and four additional studies that yielded similar results. In the meta-analysis, the risk of ADHD was significantly associated with any maternal autoimmune disease in two studies (HR, 1.20); with maternal type 1 diabetes in four studies (HR, 1.53); with maternal hyperthyroidism in three studies (HR 1.15); and with maternal psoriasis in two studies (HR, 1.31).
Type 1 diabetes (T1D) had the highest HR and was the most often studied condition. However, “the observed association may also be related to nonimmune aspects of T1D, such as glycemic control, as nonautoimmune diabetes has been associated with ADHD among children,” the researchers wrote.
The study findings were limited by several factors, including the lack of outpatient and primary care records to identify maternal autoimmune disease, and lack of data on any medication used to managed diseases during pregnancy, as well as a lack of data on children with ADHD who might not have been treated with medication, the researchers noted. In addition, “given differences in study design and definitions, the pooled HRs presented in the meta-analysis need to be treated cautiously.”
However, the results were strengthened by the hybrid study design and large study population, and were generally consistent with previous research supporting an effect of maternal immune function on fetal neurodevelopment, they noted.
“Our study provides justification for future studies that examine the effect of maternal autoimmune diseases, including biomarkers, condition severity, and management in pregnancy and in the periconception period, on neurodevelopmental disorders in children,” they concluded.
Studies need to explore mechanism of action
The current study, with its hybrid design, adds support to the evidence of an association between any maternal autoimmune disease and ADHD in children, as well as an association between the specific conditions of type 1 diabetes, hyperthyroidism, and psoriasis in mothers and ADHD in children, Søren Dalsgaard, MD, of Aarhus (Denmark) University, wrote in an accompanying editorial.
“Importantly, Nielsen et al. emphasized in their article that, for the many different autoimmune diseases, different underlying mechanisms for the associations with disorders of the central nervous system were likely. They mentioned that, for T1D, low glycemic control may play a role, as type 2 diabetes has been associated with ADHD,” said Dr. Dalsgaard.
“Overall, these mechanisms are thought to include shared genetic and environmental risk factors or direct effects of maternal autoantibodies or cytokines crossing the placenta and altering the fetal immune response, which in turns leads to changes in the central nervous system,” Dr. Dalsgaard explained. However, the current study and previous studies have not identified the mechanisms to explain the association between ADHD in children and maternal autoimmune disease.
“To understand more about these associations, future studies should include researchers and data from different scientific disciplines, such as epidemiology, animal modeling, genetics, and neuroimmunology,” he concluded.
Association is not causality
Overall, the study findings add to the evidence of a correlation between autoimmune diseases and neurologic disease, said Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., in an interview. “Anything that might contribute to behavioral problems is worth investigating.” However, it is important to remember that association is not causation.
“There is some literature and evidence that autoimmune disease is associated with mental health issues, but the mechanisms of action are unknown,” said Dr. Lessin. ADHD is highly heritable, so the association may be caused by a similar genetic predisposition, or it may be something related to autoimmunity that is impacting the fetus by passing through the placenta.
The current study’s strengths include the large size and hybrid design, but limitations such as the identification of ADHD based on medication prescriptions may have led to underreporting, and identifying maternal autoimmune disease via inpatient hospital diagnosis could have selected for more severe disease, he said.
From a clinical standpoint, the study suggests a correlation that should be noted in a family history and potentially used to inform a diagnosis, especially in cases of type 1 diabetes where the association was strongest, Dr. Lessin said. The findings also support the value of further research to look for mechanisms that might explain whether the association between autoimmune disease and ADHD is autoimmune system causality or shared genetic susceptibility.
The study received no outside funding. One coauthor disclosed receiving grants from the National Blood Authority Australia and the Australian National Health and Medical Research Council during the conduct of the study. Dr. Dalsgaard had no financial conflicts to disclose. Dr. Lessin disclosed serving as editor of the ADHD toolkit for the American Academy of Pediatrics and coauthor of the current ADHD clinical guidelines. He also serves in advisory capacity to Cognoa, a company involved in diagnosis of autism, and Corium/KemPharm, companies involved in the development of ADHD treatments.
Maternal autoimmune diseases significantly increased the risk of ADHD in children, based on data from a large cohort study of more than 800,000 mothers and children and a subsequent meta-analysis.
“There is growing evidence that immune-related cells and proteins play a role in brain development and function and that maternal immune activation, including infection, autoimmune disease, and chronic inflammation during pregnancy, increases the risk of neurodevelopmental disorders among children,” wrote Timothy C. Nielsen, MPH, of the University of Sydney, and colleagues.
Previous research has examined a link between maternal autoimmune disorders and autism spectrum disorders in children, but associations with ADHD have not been well studied, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers identified 831,718 mothers and their 831,718 singleton infants in Australia. A total of 12,787 infants were born to mothers with an autoimmune diagnosis; 12,610 of them were matched to 50,440 control infants. ADHD was determined based on prescription for a stimulant treatment or a hospital diagnosis; children with a first ADHD event younger than 3 years were excluded.
In the total cohort of 63,050 infants, the presence of any maternal autoimmune disease was associated with a significantly increased risk of ADHD (hazard ratio, 1.30) as was the presence of several specific conditions: type 1 diabetes (HR, 2.23), psoriasis (HR, 1.66), and rheumatic fever or rheumatic carditis (HR, 1.75).
In addition, the researchers conducted a meta-analysis of the current study and four additional studies that yielded similar results. In the meta-analysis, the risk of ADHD was significantly associated with any maternal autoimmune disease in two studies (HR, 1.20); with maternal type 1 diabetes in four studies (HR, 1.53); with maternal hyperthyroidism in three studies (HR 1.15); and with maternal psoriasis in two studies (HR, 1.31).
Type 1 diabetes (T1D) had the highest HR and was the most often studied condition. However, “the observed association may also be related to nonimmune aspects of T1D, such as glycemic control, as nonautoimmune diabetes has been associated with ADHD among children,” the researchers wrote.
The study findings were limited by several factors, including the lack of outpatient and primary care records to identify maternal autoimmune disease, and lack of data on any medication used to managed diseases during pregnancy, as well as a lack of data on children with ADHD who might not have been treated with medication, the researchers noted. In addition, “given differences in study design and definitions, the pooled HRs presented in the meta-analysis need to be treated cautiously.”
However, the results were strengthened by the hybrid study design and large study population, and were generally consistent with previous research supporting an effect of maternal immune function on fetal neurodevelopment, they noted.
“Our study provides justification for future studies that examine the effect of maternal autoimmune diseases, including biomarkers, condition severity, and management in pregnancy and in the periconception period, on neurodevelopmental disorders in children,” they concluded.
Studies need to explore mechanism of action
The current study, with its hybrid design, adds support to the evidence of an association between any maternal autoimmune disease and ADHD in children, as well as an association between the specific conditions of type 1 diabetes, hyperthyroidism, and psoriasis in mothers and ADHD in children, Søren Dalsgaard, MD, of Aarhus (Denmark) University, wrote in an accompanying editorial.
“Importantly, Nielsen et al. emphasized in their article that, for the many different autoimmune diseases, different underlying mechanisms for the associations with disorders of the central nervous system were likely. They mentioned that, for T1D, low glycemic control may play a role, as type 2 diabetes has been associated with ADHD,” said Dr. Dalsgaard.
“Overall, these mechanisms are thought to include shared genetic and environmental risk factors or direct effects of maternal autoantibodies or cytokines crossing the placenta and altering the fetal immune response, which in turns leads to changes in the central nervous system,” Dr. Dalsgaard explained. However, the current study and previous studies have not identified the mechanisms to explain the association between ADHD in children and maternal autoimmune disease.
“To understand more about these associations, future studies should include researchers and data from different scientific disciplines, such as epidemiology, animal modeling, genetics, and neuroimmunology,” he concluded.
Association is not causality
Overall, the study findings add to the evidence of a correlation between autoimmune diseases and neurologic disease, said Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., in an interview. “Anything that might contribute to behavioral problems is worth investigating.” However, it is important to remember that association is not causation.
“There is some literature and evidence that autoimmune disease is associated with mental health issues, but the mechanisms of action are unknown,” said Dr. Lessin. ADHD is highly heritable, so the association may be caused by a similar genetic predisposition, or it may be something related to autoimmunity that is impacting the fetus by passing through the placenta.
The current study’s strengths include the large size and hybrid design, but limitations such as the identification of ADHD based on medication prescriptions may have led to underreporting, and identifying maternal autoimmune disease via inpatient hospital diagnosis could have selected for more severe disease, he said.
From a clinical standpoint, the study suggests a correlation that should be noted in a family history and potentially used to inform a diagnosis, especially in cases of type 1 diabetes where the association was strongest, Dr. Lessin said. The findings also support the value of further research to look for mechanisms that might explain whether the association between autoimmune disease and ADHD is autoimmune system causality or shared genetic susceptibility.
The study received no outside funding. One coauthor disclosed receiving grants from the National Blood Authority Australia and the Australian National Health and Medical Research Council during the conduct of the study. Dr. Dalsgaard had no financial conflicts to disclose. Dr. Lessin disclosed serving as editor of the ADHD toolkit for the American Academy of Pediatrics and coauthor of the current ADHD clinical guidelines. He also serves in advisory capacity to Cognoa, a company involved in diagnosis of autism, and Corium/KemPharm, companies involved in the development of ADHD treatments.
Maternal autoimmune diseases significantly increased the risk of ADHD in children, based on data from a large cohort study of more than 800,000 mothers and children and a subsequent meta-analysis.
“There is growing evidence that immune-related cells and proteins play a role in brain development and function and that maternal immune activation, including infection, autoimmune disease, and chronic inflammation during pregnancy, increases the risk of neurodevelopmental disorders among children,” wrote Timothy C. Nielsen, MPH, of the University of Sydney, and colleagues.
Previous research has examined a link between maternal autoimmune disorders and autism spectrum disorders in children, but associations with ADHD have not been well studied, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers identified 831,718 mothers and their 831,718 singleton infants in Australia. A total of 12,787 infants were born to mothers with an autoimmune diagnosis; 12,610 of them were matched to 50,440 control infants. ADHD was determined based on prescription for a stimulant treatment or a hospital diagnosis; children with a first ADHD event younger than 3 years were excluded.
In the total cohort of 63,050 infants, the presence of any maternal autoimmune disease was associated with a significantly increased risk of ADHD (hazard ratio, 1.30) as was the presence of several specific conditions: type 1 diabetes (HR, 2.23), psoriasis (HR, 1.66), and rheumatic fever or rheumatic carditis (HR, 1.75).
In addition, the researchers conducted a meta-analysis of the current study and four additional studies that yielded similar results. In the meta-analysis, the risk of ADHD was significantly associated with any maternal autoimmune disease in two studies (HR, 1.20); with maternal type 1 diabetes in four studies (HR, 1.53); with maternal hyperthyroidism in three studies (HR 1.15); and with maternal psoriasis in two studies (HR, 1.31).
Type 1 diabetes (T1D) had the highest HR and was the most often studied condition. However, “the observed association may also be related to nonimmune aspects of T1D, such as glycemic control, as nonautoimmune diabetes has been associated with ADHD among children,” the researchers wrote.
The study findings were limited by several factors, including the lack of outpatient and primary care records to identify maternal autoimmune disease, and lack of data on any medication used to managed diseases during pregnancy, as well as a lack of data on children with ADHD who might not have been treated with medication, the researchers noted. In addition, “given differences in study design and definitions, the pooled HRs presented in the meta-analysis need to be treated cautiously.”
However, the results were strengthened by the hybrid study design and large study population, and were generally consistent with previous research supporting an effect of maternal immune function on fetal neurodevelopment, they noted.
“Our study provides justification for future studies that examine the effect of maternal autoimmune diseases, including biomarkers, condition severity, and management in pregnancy and in the periconception period, on neurodevelopmental disorders in children,” they concluded.
Studies need to explore mechanism of action
The current study, with its hybrid design, adds support to the evidence of an association between any maternal autoimmune disease and ADHD in children, as well as an association between the specific conditions of type 1 diabetes, hyperthyroidism, and psoriasis in mothers and ADHD in children, Søren Dalsgaard, MD, of Aarhus (Denmark) University, wrote in an accompanying editorial.
“Importantly, Nielsen et al. emphasized in their article that, for the many different autoimmune diseases, different underlying mechanisms for the associations with disorders of the central nervous system were likely. They mentioned that, for T1D, low glycemic control may play a role, as type 2 diabetes has been associated with ADHD,” said Dr. Dalsgaard.
“Overall, these mechanisms are thought to include shared genetic and environmental risk factors or direct effects of maternal autoantibodies or cytokines crossing the placenta and altering the fetal immune response, which in turns leads to changes in the central nervous system,” Dr. Dalsgaard explained. However, the current study and previous studies have not identified the mechanisms to explain the association between ADHD in children and maternal autoimmune disease.
“To understand more about these associations, future studies should include researchers and data from different scientific disciplines, such as epidemiology, animal modeling, genetics, and neuroimmunology,” he concluded.
Association is not causality
Overall, the study findings add to the evidence of a correlation between autoimmune diseases and neurologic disease, said Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., in an interview. “Anything that might contribute to behavioral problems is worth investigating.” However, it is important to remember that association is not causation.
“There is some literature and evidence that autoimmune disease is associated with mental health issues, but the mechanisms of action are unknown,” said Dr. Lessin. ADHD is highly heritable, so the association may be caused by a similar genetic predisposition, or it may be something related to autoimmunity that is impacting the fetus by passing through the placenta.
The current study’s strengths include the large size and hybrid design, but limitations such as the identification of ADHD based on medication prescriptions may have led to underreporting, and identifying maternal autoimmune disease via inpatient hospital diagnosis could have selected for more severe disease, he said.
From a clinical standpoint, the study suggests a correlation that should be noted in a family history and potentially used to inform a diagnosis, especially in cases of type 1 diabetes where the association was strongest, Dr. Lessin said. The findings also support the value of further research to look for mechanisms that might explain whether the association between autoimmune disease and ADHD is autoimmune system causality or shared genetic susceptibility.
The study received no outside funding. One coauthor disclosed receiving grants from the National Blood Authority Australia and the Australian National Health and Medical Research Council during the conduct of the study. Dr. Dalsgaard had no financial conflicts to disclose. Dr. Lessin disclosed serving as editor of the ADHD toolkit for the American Academy of Pediatrics and coauthor of the current ADHD clinical guidelines. He also serves in advisory capacity to Cognoa, a company involved in diagnosis of autism, and Corium/KemPharm, companies involved in the development of ADHD treatments.
FROM JAMA PEDIATRICS
ADHD meds may boost treatment retention in comorbid addiction
Judicious use of stimulants may help patients with attention-deficit hyperactivity disorder (ADHD) and comorbid substance use disorder (SUD) stay in addiction treatment programs, research shows.
Results of a 5-year retrospective cohort study showed adult patients with ADHD attending an addiction recovery program were five times less likely to drop out of care if they were receiving stimulant medication within the first 90 days, compared with their peers who received no medication.
“When considering the risks and benefits of ADHD pharmacotherapy and particularly stimulant therapy in the addiction clinic, we should really be thinking about the risk of treatment dropout and poor retention if we do not treat the ADHD syndrome,” study investigator Kristopher A. Kast, MD, Vanderbilt University, Nashville, Tenn., told this news organization.
The findings were presented at the American Academy of Addiction Psychiatry annual meeting, which was held online this year.
Comorbidity common
“This study matters because this clinical situation comes up a lot, where you have patients who are presenting in the substance use disorder clinic who are experiencing symptoms of ADHD and who have been on stimulant therapy either as a child or young adult in the past,” said Dr. Kast, who conducted this study while he was at Massachusetts General Hospital in Boston.
About 25% of patients presenting to outpatient substance use care meet criteria for an ADHD diagnosis, and having both conditions worsens ADHD and SUD outcomes, he noted.
“ADHD treatment would be helpful to these people, but often clinicians are reluctant to prescribe stimulant medication because it’s a controlled substance. Especially early on in treatment, we’re often worried that such a medication could destabilize the patient,” said Dr. Kast.
To examine the relationship between ADHD pharmacotherapy and retention in SUD treatment participants, the investigators assessed electronic medical record data from Mass General over a period of 5.5 years, from July 2014 to January 2020.
The data included information on 2,163 patients (63% men; mean age, 44 years) admitted to the addiction clinic. A total of 203 had a clinical diagnosis of ADHD (9.4%). Of these 203 participants, 171 were receiving ADHD pharmacotherapy and 32 were untreated.
Among all participants, the group with ADHD was significantly younger than the non-ADHD group (mean age, 38 vs. 45 years, respectively) and more likely to use cocaine (31% vs. 12%) and have private insurance (64% vs. 44%) (P < .001 for all comparisons).
Results showed ADHD stimulant therapy within the first 90 days of SUD treatment was a robust indication of retention. After adjusting for several variables, only ADHD pharmacotherapy was significantly associated with retention (hazard ratio, 0.59; 95% confidence interval, 0.4-0.9; P = .008).
“It was the only variable in a multivariate regression analysis that predicted longer-term retention. It was an even stronger predictor than Suboxone [buprenorphine and naloxone] therapy, with is traditionally strongly associated with retention,” Dr. Kast noted.
He added that, because this was a retrospective, nonrandomized study, it limited the ability to address confounding and unmeasured covariates.
“Our findings may not generalize to the undiagnosed group of patients who would be identified by standardized diagnostic instruments,” Kast said. “Future studies should address risk and number-needed-to-harm associated with ADHD pharmacotherapy.”
High dropout rate
Commenting on the findings for this news organization, Frances Levin, MD, professor of psychiatry at Columbia University Irving Medical Center, New York, noted that previous research has shown that patients with ADHD tend to do less well in addiction treatment and drop out of programs more frequently.
What has not been shown as effectively, at least in substance use treatment settings, is that treating ADHD makes a difference in terms of retention, she said.
Although Dr. Levin wasn’t involved in this study, she is currently part of a European study that is assessing SUD treatment-retention outcomes in patients with ADHD who have been randomly assigned to receive either stimulant or nonstimulant medication.
Clinicians are too often focused on risks for overtreatment, diversion, and misuse but what is underappreciated is the risk for undertreatment, Dr. Levin noted.
“ Not using the right drugs may make people less likely to stay in treatment and continue their drug use,” she said.
“Misuse and diversion are much higher with immediate-release preparations, and for this reason it’s important to use the long-acting stimulants in this population. Often people do not make that distinction,” Dr. Levin added.
As an expert in the field for more than 2 decades, Dr. Levin said she has learned a lot about treating this type of patient. “You have to monitor them very closely, and never prescribe in a cavalier way,” she said.
“I have the same discussion with these patients that I have when I talk about buprenorphine for opioid use disorder. It is a tremendously powerful medication, saves many lives and prevents overdose, but there is a risk of misuse and diversion, albeit pretty low. It’s there, and you have to use it carefully, but I think being careful vs. never prescribing are two different things,” Dr. Levin said.
‘Guidance and reassurance’
The traditional belief among the general medical community that controlled substances should always be avoided in patients with SUD has hindered treatment for many with comorbid ADHD, said Cornel Stanciu, MD, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., when asked for comment.
“I have encountered many non–addiction-trained physicians who provide buprenorphine treatment for OUD, and they hesitate not only to assess for ADHD but also to implement standard of care treatment when such a diagnosis is made,” Dr. Stanciu told said in an interview.
He added that this practice often stems from fear of “being under the radar” of the U.S. Drug Enforcement Administration for what it might consider an aberrant prescribing pattern involving two controlled substances.
“Hopefully, studies such as Dr. Kast’s will continue to shine light on this issue and offer guidance and reassurance to those treating addictive disorders,” Dr. Stanciu said.
Dr. Kast, Dr. Levin, and Dr. Stanciu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Judicious use of stimulants may help patients with attention-deficit hyperactivity disorder (ADHD) and comorbid substance use disorder (SUD) stay in addiction treatment programs, research shows.
Results of a 5-year retrospective cohort study showed adult patients with ADHD attending an addiction recovery program were five times less likely to drop out of care if they were receiving stimulant medication within the first 90 days, compared with their peers who received no medication.
“When considering the risks and benefits of ADHD pharmacotherapy and particularly stimulant therapy in the addiction clinic, we should really be thinking about the risk of treatment dropout and poor retention if we do not treat the ADHD syndrome,” study investigator Kristopher A. Kast, MD, Vanderbilt University, Nashville, Tenn., told this news organization.
The findings were presented at the American Academy of Addiction Psychiatry annual meeting, which was held online this year.
Comorbidity common
“This study matters because this clinical situation comes up a lot, where you have patients who are presenting in the substance use disorder clinic who are experiencing symptoms of ADHD and who have been on stimulant therapy either as a child or young adult in the past,” said Dr. Kast, who conducted this study while he was at Massachusetts General Hospital in Boston.
About 25% of patients presenting to outpatient substance use care meet criteria for an ADHD diagnosis, and having both conditions worsens ADHD and SUD outcomes, he noted.
“ADHD treatment would be helpful to these people, but often clinicians are reluctant to prescribe stimulant medication because it’s a controlled substance. Especially early on in treatment, we’re often worried that such a medication could destabilize the patient,” said Dr. Kast.
To examine the relationship between ADHD pharmacotherapy and retention in SUD treatment participants, the investigators assessed electronic medical record data from Mass General over a period of 5.5 years, from July 2014 to January 2020.
The data included information on 2,163 patients (63% men; mean age, 44 years) admitted to the addiction clinic. A total of 203 had a clinical diagnosis of ADHD (9.4%). Of these 203 participants, 171 were receiving ADHD pharmacotherapy and 32 were untreated.
Among all participants, the group with ADHD was significantly younger than the non-ADHD group (mean age, 38 vs. 45 years, respectively) and more likely to use cocaine (31% vs. 12%) and have private insurance (64% vs. 44%) (P < .001 for all comparisons).
Results showed ADHD stimulant therapy within the first 90 days of SUD treatment was a robust indication of retention. After adjusting for several variables, only ADHD pharmacotherapy was significantly associated with retention (hazard ratio, 0.59; 95% confidence interval, 0.4-0.9; P = .008).
“It was the only variable in a multivariate regression analysis that predicted longer-term retention. It was an even stronger predictor than Suboxone [buprenorphine and naloxone] therapy, with is traditionally strongly associated with retention,” Dr. Kast noted.
He added that, because this was a retrospective, nonrandomized study, it limited the ability to address confounding and unmeasured covariates.
“Our findings may not generalize to the undiagnosed group of patients who would be identified by standardized diagnostic instruments,” Kast said. “Future studies should address risk and number-needed-to-harm associated with ADHD pharmacotherapy.”
High dropout rate
Commenting on the findings for this news organization, Frances Levin, MD, professor of psychiatry at Columbia University Irving Medical Center, New York, noted that previous research has shown that patients with ADHD tend to do less well in addiction treatment and drop out of programs more frequently.
What has not been shown as effectively, at least in substance use treatment settings, is that treating ADHD makes a difference in terms of retention, she said.
Although Dr. Levin wasn’t involved in this study, she is currently part of a European study that is assessing SUD treatment-retention outcomes in patients with ADHD who have been randomly assigned to receive either stimulant or nonstimulant medication.
Clinicians are too often focused on risks for overtreatment, diversion, and misuse but what is underappreciated is the risk for undertreatment, Dr. Levin noted.
“ Not using the right drugs may make people less likely to stay in treatment and continue their drug use,” she said.
“Misuse and diversion are much higher with immediate-release preparations, and for this reason it’s important to use the long-acting stimulants in this population. Often people do not make that distinction,” Dr. Levin added.
As an expert in the field for more than 2 decades, Dr. Levin said she has learned a lot about treating this type of patient. “You have to monitor them very closely, and never prescribe in a cavalier way,” she said.
“I have the same discussion with these patients that I have when I talk about buprenorphine for opioid use disorder. It is a tremendously powerful medication, saves many lives and prevents overdose, but there is a risk of misuse and diversion, albeit pretty low. It’s there, and you have to use it carefully, but I think being careful vs. never prescribing are two different things,” Dr. Levin said.
‘Guidance and reassurance’
The traditional belief among the general medical community that controlled substances should always be avoided in patients with SUD has hindered treatment for many with comorbid ADHD, said Cornel Stanciu, MD, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., when asked for comment.
“I have encountered many non–addiction-trained physicians who provide buprenorphine treatment for OUD, and they hesitate not only to assess for ADHD but also to implement standard of care treatment when such a diagnosis is made,” Dr. Stanciu told said in an interview.
He added that this practice often stems from fear of “being under the radar” of the U.S. Drug Enforcement Administration for what it might consider an aberrant prescribing pattern involving two controlled substances.
“Hopefully, studies such as Dr. Kast’s will continue to shine light on this issue and offer guidance and reassurance to those treating addictive disorders,” Dr. Stanciu said.
Dr. Kast, Dr. Levin, and Dr. Stanciu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Judicious use of stimulants may help patients with attention-deficit hyperactivity disorder (ADHD) and comorbid substance use disorder (SUD) stay in addiction treatment programs, research shows.
Results of a 5-year retrospective cohort study showed adult patients with ADHD attending an addiction recovery program were five times less likely to drop out of care if they were receiving stimulant medication within the first 90 days, compared with their peers who received no medication.
“When considering the risks and benefits of ADHD pharmacotherapy and particularly stimulant therapy in the addiction clinic, we should really be thinking about the risk of treatment dropout and poor retention if we do not treat the ADHD syndrome,” study investigator Kristopher A. Kast, MD, Vanderbilt University, Nashville, Tenn., told this news organization.
The findings were presented at the American Academy of Addiction Psychiatry annual meeting, which was held online this year.
Comorbidity common
“This study matters because this clinical situation comes up a lot, where you have patients who are presenting in the substance use disorder clinic who are experiencing symptoms of ADHD and who have been on stimulant therapy either as a child or young adult in the past,” said Dr. Kast, who conducted this study while he was at Massachusetts General Hospital in Boston.
About 25% of patients presenting to outpatient substance use care meet criteria for an ADHD diagnosis, and having both conditions worsens ADHD and SUD outcomes, he noted.
“ADHD treatment would be helpful to these people, but often clinicians are reluctant to prescribe stimulant medication because it’s a controlled substance. Especially early on in treatment, we’re often worried that such a medication could destabilize the patient,” said Dr. Kast.
To examine the relationship between ADHD pharmacotherapy and retention in SUD treatment participants, the investigators assessed electronic medical record data from Mass General over a period of 5.5 years, from July 2014 to January 2020.
The data included information on 2,163 patients (63% men; mean age, 44 years) admitted to the addiction clinic. A total of 203 had a clinical diagnosis of ADHD (9.4%). Of these 203 participants, 171 were receiving ADHD pharmacotherapy and 32 were untreated.
Among all participants, the group with ADHD was significantly younger than the non-ADHD group (mean age, 38 vs. 45 years, respectively) and more likely to use cocaine (31% vs. 12%) and have private insurance (64% vs. 44%) (P < .001 for all comparisons).
Results showed ADHD stimulant therapy within the first 90 days of SUD treatment was a robust indication of retention. After adjusting for several variables, only ADHD pharmacotherapy was significantly associated with retention (hazard ratio, 0.59; 95% confidence interval, 0.4-0.9; P = .008).
“It was the only variable in a multivariate regression analysis that predicted longer-term retention. It was an even stronger predictor than Suboxone [buprenorphine and naloxone] therapy, with is traditionally strongly associated with retention,” Dr. Kast noted.
He added that, because this was a retrospective, nonrandomized study, it limited the ability to address confounding and unmeasured covariates.
“Our findings may not generalize to the undiagnosed group of patients who would be identified by standardized diagnostic instruments,” Kast said. “Future studies should address risk and number-needed-to-harm associated with ADHD pharmacotherapy.”
High dropout rate
Commenting on the findings for this news organization, Frances Levin, MD, professor of psychiatry at Columbia University Irving Medical Center, New York, noted that previous research has shown that patients with ADHD tend to do less well in addiction treatment and drop out of programs more frequently.
What has not been shown as effectively, at least in substance use treatment settings, is that treating ADHD makes a difference in terms of retention, she said.
Although Dr. Levin wasn’t involved in this study, she is currently part of a European study that is assessing SUD treatment-retention outcomes in patients with ADHD who have been randomly assigned to receive either stimulant or nonstimulant medication.
Clinicians are too often focused on risks for overtreatment, diversion, and misuse but what is underappreciated is the risk for undertreatment, Dr. Levin noted.
“ Not using the right drugs may make people less likely to stay in treatment and continue their drug use,” she said.
“Misuse and diversion are much higher with immediate-release preparations, and for this reason it’s important to use the long-acting stimulants in this population. Often people do not make that distinction,” Dr. Levin added.
As an expert in the field for more than 2 decades, Dr. Levin said she has learned a lot about treating this type of patient. “You have to monitor them very closely, and never prescribe in a cavalier way,” she said.
“I have the same discussion with these patients that I have when I talk about buprenorphine for opioid use disorder. It is a tremendously powerful medication, saves many lives and prevents overdose, but there is a risk of misuse and diversion, albeit pretty low. It’s there, and you have to use it carefully, but I think being careful vs. never prescribing are two different things,” Dr. Levin said.
‘Guidance and reassurance’
The traditional belief among the general medical community that controlled substances should always be avoided in patients with SUD has hindered treatment for many with comorbid ADHD, said Cornel Stanciu, MD, Dartmouth-Hitchcock Medical Center, Lebanon, N.H., when asked for comment.
“I have encountered many non–addiction-trained physicians who provide buprenorphine treatment for OUD, and they hesitate not only to assess for ADHD but also to implement standard of care treatment when such a diagnosis is made,” Dr. Stanciu told said in an interview.
He added that this practice often stems from fear of “being under the radar” of the U.S. Drug Enforcement Administration for what it might consider an aberrant prescribing pattern involving two controlled substances.
“Hopefully, studies such as Dr. Kast’s will continue to shine light on this issue and offer guidance and reassurance to those treating addictive disorders,” Dr. Stanciu said.
Dr. Kast, Dr. Levin, and Dr. Stanciu have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Concussion linked to risk for dementia, Parkinson’s disease, and ADHD
new research suggests. Results from a retrospective, population-based cohort study showed that controlling for socioeconomic status and overall health did not significantly affect this association.
The link between concussion and risk for ADHD and for mood and anxiety disorder was stronger in the women than in the men. In addition, having a history of multiple concussions strengthened the association between concussion and subsequent mood and anxiety disorder, dementia, and Parkinson’s disease compared with experiencing just one concussion.
The findings are similar to those of previous studies, noted lead author Marc P. Morissette, PhD, research assistant at the Pan Am Clinic Foundation in Winnipeg, Manitoba, Canada. “The main methodological differences separating our study from previous studies in this area is a focus on concussion-specific injuries identified from medical records and the potential for study participants to have up to 25 years of follow-up data,” said Dr. Morissette.
The findings were published online July 27 in Family Medicine and Community Health, a BMJ journal.
Almost 190,000 participants
Several studies have shown associations between head injury and increased risk for ADHD, depression, anxiety, Alzheimer’s disease, and Parkinson’s disease. However, many of these studies relied on self-reported medical history, included all forms of traumatic brain injury, and failed to adjust for preexisting health conditions.
An improved understanding of concussion and the risks associated with it could help physicians manage their patients’ long-term needs, the investigators noted.
In the current study, the researchers examined anonymized administrative health data collected between the periods of 1990–1991 and 2014–2015 in the Manitoba Population Research Data Repository at the Manitoba Center for Health Policy.
Eligible patients had been diagnosed with concussion in accordance with standard criteria. Participants were excluded if they had been diagnosed with dementia or Parkinson’s disease before the incident concussion during the study period. The investigators matched three control participants to each included patient on the basis of age, sex, and location.
Study outcome was time from index date (date of first concussion) to diagnosis of ADHD, mood and anxiety disorder, dementia, or Parkinson’s disease. The researchers controlled for socioeconomic status using the Socioeconomic Factor Index, version 2 (SEFI2), and for preexisting medical conditions using the Charlson Comorbidity Index (CCI).
The study included 28,021 men (mean age, 25 years) and 19,462 women (mean age, 30 years) in the concussion group and 81,871 men (mean age, 25 years) and 57,159 women (mean age, 30 years) in the control group. Mean SEFI2 score was approximately −0.05, and mean CCI score was approximately 0.2.
Dose effect?
Results showed that concussion was associated with an increased risk for ADHD (hazard ratio [HR], 1.39), mood and anxiety disorder (HR, 1.72), dementia (HR, 1.72), and Parkinson’s disease (HR, 1.57).
After a concussion, the risk of developing ADHD was 28% higher and the risk of developing mood and anxiety disorder was 7% higher among women than among men. Gender was not associated with risk for dementia or Parkinson’s disease after concussion.
Sustaining a second concussion increased the strength of the association with risk for dementia compared with sustaining a single concussion (HR, 1.62). Similarly, sustaining more than three concussions increased the strength of the association with the risk for mood and anxiety disorders (HR for more than three vs one concussion, 1.22) and Parkinson›s disease (HR, 3.27).
A sensitivity analysis found similar associations between concussion and risk for mood and anxiety disorder among all age groups. Younger participants were at greater risk for ADHD, however, and older participants were at greater risk for dementia and Parkinson’s disease.
Increased awareness of concussion and the outcomes of interest, along with improved diagnostic tools, may have influenced the study’s findings, Dr. Morissette noted. “The sex-based differences may be due to either pathophysiological differences in response to concussive injuries or potentially a difference in willingness to seek medical care or share symptoms, concussion-related or otherwise, with a medical professional,” he said.
“We are hopeful that our findings will encourage practitioners to be cognizant of various conditions that may present in individuals who have previously experienced a concussion,” Dr. Morissette added. “If physicians are aware of the various associations identified following a concussion, it may lead to more thorough clinical examination at initial presentation, along with more dedicated care throughout the patient’s life.”
Association versus causation
Commenting on the research, Steven Erickson, MD, sports medicine specialist at Banner–University Medicine Neuroscience Institute, Phoenix, Ariz., noted that although the study showed an association between concussion and subsequent diagnosis of ADHD, anxiety, and Parkinson’s disease, “this association should not be misconstrued as causation.” He added that the study’s conclusions “are just as likely to be due to labeling theory” or a self-fulfilling prophecy.
“Patients diagnosed with ADHD, anxiety, or Parkinson’s disease may recall concussion and associate the two diagnoses; but patients who have not previously been diagnosed with a concussion cannot draw that conclusion,” said Dr. Erickson, who was not involved with the research.
Citing the apparent gender difference in the strength of the association between concussion and the outcomes of interest, Dr. Erickson noted that women are more likely to report symptoms in general “and therefore are more likely to be diagnosed with ADHD and anxiety disorders” because of differences in reporting rather than incidence of disease.
“Further research needs to be done to definitively determine a causal relationship between concussion and any psychiatric or neurologic diagnosis,” Dr. Erickson concluded.
The study was funded by the Pan Am Clinic Foundation. Dr. Morissette and Dr. Erickson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Results from a retrospective, population-based cohort study showed that controlling for socioeconomic status and overall health did not significantly affect this association.
The link between concussion and risk for ADHD and for mood and anxiety disorder was stronger in the women than in the men. In addition, having a history of multiple concussions strengthened the association between concussion and subsequent mood and anxiety disorder, dementia, and Parkinson’s disease compared with experiencing just one concussion.
The findings are similar to those of previous studies, noted lead author Marc P. Morissette, PhD, research assistant at the Pan Am Clinic Foundation in Winnipeg, Manitoba, Canada. “The main methodological differences separating our study from previous studies in this area is a focus on concussion-specific injuries identified from medical records and the potential for study participants to have up to 25 years of follow-up data,” said Dr. Morissette.
The findings were published online July 27 in Family Medicine and Community Health, a BMJ journal.
Almost 190,000 participants
Several studies have shown associations between head injury and increased risk for ADHD, depression, anxiety, Alzheimer’s disease, and Parkinson’s disease. However, many of these studies relied on self-reported medical history, included all forms of traumatic brain injury, and failed to adjust for preexisting health conditions.
An improved understanding of concussion and the risks associated with it could help physicians manage their patients’ long-term needs, the investigators noted.
In the current study, the researchers examined anonymized administrative health data collected between the periods of 1990–1991 and 2014–2015 in the Manitoba Population Research Data Repository at the Manitoba Center for Health Policy.
Eligible patients had been diagnosed with concussion in accordance with standard criteria. Participants were excluded if they had been diagnosed with dementia or Parkinson’s disease before the incident concussion during the study period. The investigators matched three control participants to each included patient on the basis of age, sex, and location.
Study outcome was time from index date (date of first concussion) to diagnosis of ADHD, mood and anxiety disorder, dementia, or Parkinson’s disease. The researchers controlled for socioeconomic status using the Socioeconomic Factor Index, version 2 (SEFI2), and for preexisting medical conditions using the Charlson Comorbidity Index (CCI).
The study included 28,021 men (mean age, 25 years) and 19,462 women (mean age, 30 years) in the concussion group and 81,871 men (mean age, 25 years) and 57,159 women (mean age, 30 years) in the control group. Mean SEFI2 score was approximately −0.05, and mean CCI score was approximately 0.2.
Dose effect?
Results showed that concussion was associated with an increased risk for ADHD (hazard ratio [HR], 1.39), mood and anxiety disorder (HR, 1.72), dementia (HR, 1.72), and Parkinson’s disease (HR, 1.57).
After a concussion, the risk of developing ADHD was 28% higher and the risk of developing mood and anxiety disorder was 7% higher among women than among men. Gender was not associated with risk for dementia or Parkinson’s disease after concussion.
Sustaining a second concussion increased the strength of the association with risk for dementia compared with sustaining a single concussion (HR, 1.62). Similarly, sustaining more than three concussions increased the strength of the association with the risk for mood and anxiety disorders (HR for more than three vs one concussion, 1.22) and Parkinson›s disease (HR, 3.27).
A sensitivity analysis found similar associations between concussion and risk for mood and anxiety disorder among all age groups. Younger participants were at greater risk for ADHD, however, and older participants were at greater risk for dementia and Parkinson’s disease.
Increased awareness of concussion and the outcomes of interest, along with improved diagnostic tools, may have influenced the study’s findings, Dr. Morissette noted. “The sex-based differences may be due to either pathophysiological differences in response to concussive injuries or potentially a difference in willingness to seek medical care or share symptoms, concussion-related or otherwise, with a medical professional,” he said.
“We are hopeful that our findings will encourage practitioners to be cognizant of various conditions that may present in individuals who have previously experienced a concussion,” Dr. Morissette added. “If physicians are aware of the various associations identified following a concussion, it may lead to more thorough clinical examination at initial presentation, along with more dedicated care throughout the patient’s life.”
Association versus causation
Commenting on the research, Steven Erickson, MD, sports medicine specialist at Banner–University Medicine Neuroscience Institute, Phoenix, Ariz., noted that although the study showed an association between concussion and subsequent diagnosis of ADHD, anxiety, and Parkinson’s disease, “this association should not be misconstrued as causation.” He added that the study’s conclusions “are just as likely to be due to labeling theory” or a self-fulfilling prophecy.
“Patients diagnosed with ADHD, anxiety, or Parkinson’s disease may recall concussion and associate the two diagnoses; but patients who have not previously been diagnosed with a concussion cannot draw that conclusion,” said Dr. Erickson, who was not involved with the research.
Citing the apparent gender difference in the strength of the association between concussion and the outcomes of interest, Dr. Erickson noted that women are more likely to report symptoms in general “and therefore are more likely to be diagnosed with ADHD and anxiety disorders” because of differences in reporting rather than incidence of disease.
“Further research needs to be done to definitively determine a causal relationship between concussion and any psychiatric or neurologic diagnosis,” Dr. Erickson concluded.
The study was funded by the Pan Am Clinic Foundation. Dr. Morissette and Dr. Erickson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests. Results from a retrospective, population-based cohort study showed that controlling for socioeconomic status and overall health did not significantly affect this association.
The link between concussion and risk for ADHD and for mood and anxiety disorder was stronger in the women than in the men. In addition, having a history of multiple concussions strengthened the association between concussion and subsequent mood and anxiety disorder, dementia, and Parkinson’s disease compared with experiencing just one concussion.
The findings are similar to those of previous studies, noted lead author Marc P. Morissette, PhD, research assistant at the Pan Am Clinic Foundation in Winnipeg, Manitoba, Canada. “The main methodological differences separating our study from previous studies in this area is a focus on concussion-specific injuries identified from medical records and the potential for study participants to have up to 25 years of follow-up data,” said Dr. Morissette.
The findings were published online July 27 in Family Medicine and Community Health, a BMJ journal.
Almost 190,000 participants
Several studies have shown associations between head injury and increased risk for ADHD, depression, anxiety, Alzheimer’s disease, and Parkinson’s disease. However, many of these studies relied on self-reported medical history, included all forms of traumatic brain injury, and failed to adjust for preexisting health conditions.
An improved understanding of concussion and the risks associated with it could help physicians manage their patients’ long-term needs, the investigators noted.
In the current study, the researchers examined anonymized administrative health data collected between the periods of 1990–1991 and 2014–2015 in the Manitoba Population Research Data Repository at the Manitoba Center for Health Policy.
Eligible patients had been diagnosed with concussion in accordance with standard criteria. Participants were excluded if they had been diagnosed with dementia or Parkinson’s disease before the incident concussion during the study period. The investigators matched three control participants to each included patient on the basis of age, sex, and location.
Study outcome was time from index date (date of first concussion) to diagnosis of ADHD, mood and anxiety disorder, dementia, or Parkinson’s disease. The researchers controlled for socioeconomic status using the Socioeconomic Factor Index, version 2 (SEFI2), and for preexisting medical conditions using the Charlson Comorbidity Index (CCI).
The study included 28,021 men (mean age, 25 years) and 19,462 women (mean age, 30 years) in the concussion group and 81,871 men (mean age, 25 years) and 57,159 women (mean age, 30 years) in the control group. Mean SEFI2 score was approximately −0.05, and mean CCI score was approximately 0.2.
Dose effect?
Results showed that concussion was associated with an increased risk for ADHD (hazard ratio [HR], 1.39), mood and anxiety disorder (HR, 1.72), dementia (HR, 1.72), and Parkinson’s disease (HR, 1.57).
After a concussion, the risk of developing ADHD was 28% higher and the risk of developing mood and anxiety disorder was 7% higher among women than among men. Gender was not associated with risk for dementia or Parkinson’s disease after concussion.
Sustaining a second concussion increased the strength of the association with risk for dementia compared with sustaining a single concussion (HR, 1.62). Similarly, sustaining more than three concussions increased the strength of the association with the risk for mood and anxiety disorders (HR for more than three vs one concussion, 1.22) and Parkinson›s disease (HR, 3.27).
A sensitivity analysis found similar associations between concussion and risk for mood and anxiety disorder among all age groups. Younger participants were at greater risk for ADHD, however, and older participants were at greater risk for dementia and Parkinson’s disease.
Increased awareness of concussion and the outcomes of interest, along with improved diagnostic tools, may have influenced the study’s findings, Dr. Morissette noted. “The sex-based differences may be due to either pathophysiological differences in response to concussive injuries or potentially a difference in willingness to seek medical care or share symptoms, concussion-related or otherwise, with a medical professional,” he said.
“We are hopeful that our findings will encourage practitioners to be cognizant of various conditions that may present in individuals who have previously experienced a concussion,” Dr. Morissette added. “If physicians are aware of the various associations identified following a concussion, it may lead to more thorough clinical examination at initial presentation, along with more dedicated care throughout the patient’s life.”
Association versus causation
Commenting on the research, Steven Erickson, MD, sports medicine specialist at Banner–University Medicine Neuroscience Institute, Phoenix, Ariz., noted that although the study showed an association between concussion and subsequent diagnosis of ADHD, anxiety, and Parkinson’s disease, “this association should not be misconstrued as causation.” He added that the study’s conclusions “are just as likely to be due to labeling theory” or a self-fulfilling prophecy.
“Patients diagnosed with ADHD, anxiety, or Parkinson’s disease may recall concussion and associate the two diagnoses; but patients who have not previously been diagnosed with a concussion cannot draw that conclusion,” said Dr. Erickson, who was not involved with the research.
Citing the apparent gender difference in the strength of the association between concussion and the outcomes of interest, Dr. Erickson noted that women are more likely to report symptoms in general “and therefore are more likely to be diagnosed with ADHD and anxiety disorders” because of differences in reporting rather than incidence of disease.
“Further research needs to be done to definitively determine a causal relationship between concussion and any psychiatric or neurologic diagnosis,” Dr. Erickson concluded.
The study was funded by the Pan Am Clinic Foundation. Dr. Morissette and Dr. Erickson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
From Family Medicine and Community Health