User login
Device positioning may be culprit behind post-LVAD pump thrombosis
TORONTO – Device positioning may help explain significant increases in pump thrombosis after left ventricular assist device implantation, according to a single-center study presented at the 2014 annual meeting of the American Association for Thoracic Surgery.
Dr. Jay Bhama, associate director of lung and heart transplantation at the University of Pittsburgh, found that more device-positioning issues coincided with more occurrences of pump thrombosis (PT). The adequacy of anticoagulation, major adverse events, and medical noncompliance were not found to be contributing factors, Dr. Bhama said.
His investigation joins recently published data indicating that left ventricular assist device (LVAD) thrombosis nearly quadrupled in less than 2 years in a multicenter study.
The purported mechanisms of PT in patients supported with the HeartMate II LVAD are thought to be multifactorial, but possibly related to design modifications, expansion of use to the destination therapy indication, nonuniform surgical implant technique, and nonuniform anticoagulation strategies across centers and over time.
"Starting in 2010, we started to notice a rapid and sudden increase in the rate of pump thrombosis, which has increased steadily over the last 3 years," said Dr. Bhama, who reported that PT occurred in 10 of 62 patients (16%) treated at the University of Pittsburgh Medical Center, with an overall event rate of 0.281 per patient-year.
In response to the increase, the group at the medical center investigated how potential contributing factors may have changed over time. They retrospectively assessed all primary LVAD implants in patients who survived hospitalization (62 of 74 total implants) between 2004 and 2012, grouping patients according to the era of implant: from June 2004 to December 2009 (era 1; n = 24) and from January 2010 (when FDA approval was given to expand use to destination therapy) to December 2012 (era 2; n = 38).
None of those who died during the index hospitalization experienced PT, Dr. Bhama noted.
PT was defined as either visualized thrombus within the pump at device exchange or significant hemolysis in the setting of heart failure symptoms or pump malfunction.
The actuarial freedom from PT at 24 months was significantly lower in era 2 than in era 1 (57% vs. 100%; P = .016).
Effective anticoagulation (percent of all international normalized ratio [INR] measurements greater than 1.8) was more reliably achieved in era 2 than in era 1 (50% vs. 34%; P less than .001).
To assess device positioning, the researchers looked at the angle of the inflow cannula, defining malposition as either less than the 5th or greater than the 95th percentile of the median of all the inflow cannula angles. Regarding the outflow cannula, they looked at patients who had bend-relief disconnects, either partial or complete, and those who had radiographic evidence of outflow graft malposition or kink.
Device positioning issues were significantly more prevalent during era 2 than during era 1 (29% vs. 4%). Most of this difference was driven by inflow cannula positioning problems, Dr. Bhama noted.
When the patients with concerns related to device positioning were excluded, the freedom from PT at 24 months no longer differed significantly between groups (P = .094).
The groups were demographically similar except for age, which was higher in the era 2 group (57 years, vs. 50 years for era 1; P = .037). More patients in the era 2 group received an LVAD for destination therapy, although this difference actually wasn’t significant (61% vs. 38%; P = .066).
The groups were also similar with regard to early major adverse events (right ventricular failure, bleeding, infection, and stroke) and medical noncompliance.
In the earlier multicenter study, Dr. Randall C. Starling, of the Cleveland Clinic, and his colleagues reported an abrupt increase in LVAD thrombosis: Between March 2011 and Jan. 1, 2013, the occurrence of PT at 3 months after implantation increased from 2.2% to 8.4% (N. Engl. J. Med. 2014;370:33-40).
"Dissecting the root cause of this problem is an extremely difficult task," said Dr. Nader Moazami, the invited discussant for Dr. Bhama’s presentation and the second author on the Starling paper. Dr. Moazami is surgical director of the Kaufman Center for Heart Failure at the Cleveland Clinic.
"While recent advances in LVAD technology with continuous-flow pumps have saved the lives of thousands of dying patients, issues related to adverse events and the associated morbidity and mortality are of immense importance, specifically as we consider the relevance of this technology to the more ambulatory heart failure patients," said Dr. Moazami, commenting on the study.
However, he suggested that the real cause of the recent increase in PT has not yet been discovered, and questioned the validity of assessing inflow cannula angulation based on a chest x-ray. "This to my knowledge has never been validated and was a concern in about half of the patients in the pump thrombosis group," Dr. Moazami said. Patients with "a demonstrable mechanical reason for pump thrombosis" were excluded from the Starling team’s analysis, he added.
In response, Dr. Bhama cited a study by Dr. Abeel Mangi, a cardiac surgeon at Yale University, New Haven, Conn., which found that greater angulation of the HeartMate II inflow cannula, along with the depth of the pump pocket, correlated with the development of PT (Ann. Thorac. Surg. 2013;96:1259-65).
"These aren’t just angles that are slightly off here and there," noted Dr. Bhama. "These are splayed very widely, situations I think where we all would say this is something we should be concerned about."
Dr. Bhama reported no relevant disclosures.
TORONTO – Device positioning may help explain significant increases in pump thrombosis after left ventricular assist device implantation, according to a single-center study presented at the 2014 annual meeting of the American Association for Thoracic Surgery.
Dr. Jay Bhama, associate director of lung and heart transplantation at the University of Pittsburgh, found that more device-positioning issues coincided with more occurrences of pump thrombosis (PT). The adequacy of anticoagulation, major adverse events, and medical noncompliance were not found to be contributing factors, Dr. Bhama said.
His investigation joins recently published data indicating that left ventricular assist device (LVAD) thrombosis nearly quadrupled in less than 2 years in a multicenter study.
The purported mechanisms of PT in patients supported with the HeartMate II LVAD are thought to be multifactorial, but possibly related to design modifications, expansion of use to the destination therapy indication, nonuniform surgical implant technique, and nonuniform anticoagulation strategies across centers and over time.
"Starting in 2010, we started to notice a rapid and sudden increase in the rate of pump thrombosis, which has increased steadily over the last 3 years," said Dr. Bhama, who reported that PT occurred in 10 of 62 patients (16%) treated at the University of Pittsburgh Medical Center, with an overall event rate of 0.281 per patient-year.
In response to the increase, the group at the medical center investigated how potential contributing factors may have changed over time. They retrospectively assessed all primary LVAD implants in patients who survived hospitalization (62 of 74 total implants) between 2004 and 2012, grouping patients according to the era of implant: from June 2004 to December 2009 (era 1; n = 24) and from January 2010 (when FDA approval was given to expand use to destination therapy) to December 2012 (era 2; n = 38).
None of those who died during the index hospitalization experienced PT, Dr. Bhama noted.
PT was defined as either visualized thrombus within the pump at device exchange or significant hemolysis in the setting of heart failure symptoms or pump malfunction.
The actuarial freedom from PT at 24 months was significantly lower in era 2 than in era 1 (57% vs. 100%; P = .016).
Effective anticoagulation (percent of all international normalized ratio [INR] measurements greater than 1.8) was more reliably achieved in era 2 than in era 1 (50% vs. 34%; P less than .001).
To assess device positioning, the researchers looked at the angle of the inflow cannula, defining malposition as either less than the 5th or greater than the 95th percentile of the median of all the inflow cannula angles. Regarding the outflow cannula, they looked at patients who had bend-relief disconnects, either partial or complete, and those who had radiographic evidence of outflow graft malposition or kink.
Device positioning issues were significantly more prevalent during era 2 than during era 1 (29% vs. 4%). Most of this difference was driven by inflow cannula positioning problems, Dr. Bhama noted.
When the patients with concerns related to device positioning were excluded, the freedom from PT at 24 months no longer differed significantly between groups (P = .094).
The groups were demographically similar except for age, which was higher in the era 2 group (57 years, vs. 50 years for era 1; P = .037). More patients in the era 2 group received an LVAD for destination therapy, although this difference actually wasn’t significant (61% vs. 38%; P = .066).
The groups were also similar with regard to early major adverse events (right ventricular failure, bleeding, infection, and stroke) and medical noncompliance.
In the earlier multicenter study, Dr. Randall C. Starling, of the Cleveland Clinic, and his colleagues reported an abrupt increase in LVAD thrombosis: Between March 2011 and Jan. 1, 2013, the occurrence of PT at 3 months after implantation increased from 2.2% to 8.4% (N. Engl. J. Med. 2014;370:33-40).
"Dissecting the root cause of this problem is an extremely difficult task," said Dr. Nader Moazami, the invited discussant for Dr. Bhama’s presentation and the second author on the Starling paper. Dr. Moazami is surgical director of the Kaufman Center for Heart Failure at the Cleveland Clinic.
"While recent advances in LVAD technology with continuous-flow pumps have saved the lives of thousands of dying patients, issues related to adverse events and the associated morbidity and mortality are of immense importance, specifically as we consider the relevance of this technology to the more ambulatory heart failure patients," said Dr. Moazami, commenting on the study.
However, he suggested that the real cause of the recent increase in PT has not yet been discovered, and questioned the validity of assessing inflow cannula angulation based on a chest x-ray. "This to my knowledge has never been validated and was a concern in about half of the patients in the pump thrombosis group," Dr. Moazami said. Patients with "a demonstrable mechanical reason for pump thrombosis" were excluded from the Starling team’s analysis, he added.
In response, Dr. Bhama cited a study by Dr. Abeel Mangi, a cardiac surgeon at Yale University, New Haven, Conn., which found that greater angulation of the HeartMate II inflow cannula, along with the depth of the pump pocket, correlated with the development of PT (Ann. Thorac. Surg. 2013;96:1259-65).
"These aren’t just angles that are slightly off here and there," noted Dr. Bhama. "These are splayed very widely, situations I think where we all would say this is something we should be concerned about."
Dr. Bhama reported no relevant disclosures.
TORONTO – Device positioning may help explain significant increases in pump thrombosis after left ventricular assist device implantation, according to a single-center study presented at the 2014 annual meeting of the American Association for Thoracic Surgery.
Dr. Jay Bhama, associate director of lung and heart transplantation at the University of Pittsburgh, found that more device-positioning issues coincided with more occurrences of pump thrombosis (PT). The adequacy of anticoagulation, major adverse events, and medical noncompliance were not found to be contributing factors, Dr. Bhama said.
His investigation joins recently published data indicating that left ventricular assist device (LVAD) thrombosis nearly quadrupled in less than 2 years in a multicenter study.
The purported mechanisms of PT in patients supported with the HeartMate II LVAD are thought to be multifactorial, but possibly related to design modifications, expansion of use to the destination therapy indication, nonuniform surgical implant technique, and nonuniform anticoagulation strategies across centers and over time.
"Starting in 2010, we started to notice a rapid and sudden increase in the rate of pump thrombosis, which has increased steadily over the last 3 years," said Dr. Bhama, who reported that PT occurred in 10 of 62 patients (16%) treated at the University of Pittsburgh Medical Center, with an overall event rate of 0.281 per patient-year.
In response to the increase, the group at the medical center investigated how potential contributing factors may have changed over time. They retrospectively assessed all primary LVAD implants in patients who survived hospitalization (62 of 74 total implants) between 2004 and 2012, grouping patients according to the era of implant: from June 2004 to December 2009 (era 1; n = 24) and from January 2010 (when FDA approval was given to expand use to destination therapy) to December 2012 (era 2; n = 38).
None of those who died during the index hospitalization experienced PT, Dr. Bhama noted.
PT was defined as either visualized thrombus within the pump at device exchange or significant hemolysis in the setting of heart failure symptoms or pump malfunction.
The actuarial freedom from PT at 24 months was significantly lower in era 2 than in era 1 (57% vs. 100%; P = .016).
Effective anticoagulation (percent of all international normalized ratio [INR] measurements greater than 1.8) was more reliably achieved in era 2 than in era 1 (50% vs. 34%; P less than .001).
To assess device positioning, the researchers looked at the angle of the inflow cannula, defining malposition as either less than the 5th or greater than the 95th percentile of the median of all the inflow cannula angles. Regarding the outflow cannula, they looked at patients who had bend-relief disconnects, either partial or complete, and those who had radiographic evidence of outflow graft malposition or kink.
Device positioning issues were significantly more prevalent during era 2 than during era 1 (29% vs. 4%). Most of this difference was driven by inflow cannula positioning problems, Dr. Bhama noted.
When the patients with concerns related to device positioning were excluded, the freedom from PT at 24 months no longer differed significantly between groups (P = .094).
The groups were demographically similar except for age, which was higher in the era 2 group (57 years, vs. 50 years for era 1; P = .037). More patients in the era 2 group received an LVAD for destination therapy, although this difference actually wasn’t significant (61% vs. 38%; P = .066).
The groups were also similar with regard to early major adverse events (right ventricular failure, bleeding, infection, and stroke) and medical noncompliance.
In the earlier multicenter study, Dr. Randall C. Starling, of the Cleveland Clinic, and his colleagues reported an abrupt increase in LVAD thrombosis: Between March 2011 and Jan. 1, 2013, the occurrence of PT at 3 months after implantation increased from 2.2% to 8.4% (N. Engl. J. Med. 2014;370:33-40).
"Dissecting the root cause of this problem is an extremely difficult task," said Dr. Nader Moazami, the invited discussant for Dr. Bhama’s presentation and the second author on the Starling paper. Dr. Moazami is surgical director of the Kaufman Center for Heart Failure at the Cleveland Clinic.
"While recent advances in LVAD technology with continuous-flow pumps have saved the lives of thousands of dying patients, issues related to adverse events and the associated morbidity and mortality are of immense importance, specifically as we consider the relevance of this technology to the more ambulatory heart failure patients," said Dr. Moazami, commenting on the study.
However, he suggested that the real cause of the recent increase in PT has not yet been discovered, and questioned the validity of assessing inflow cannula angulation based on a chest x-ray. "This to my knowledge has never been validated and was a concern in about half of the patients in the pump thrombosis group," Dr. Moazami said. Patients with "a demonstrable mechanical reason for pump thrombosis" were excluded from the Starling team’s analysis, he added.
In response, Dr. Bhama cited a study by Dr. Abeel Mangi, a cardiac surgeon at Yale University, New Haven, Conn., which found that greater angulation of the HeartMate II inflow cannula, along with the depth of the pump pocket, correlated with the development of PT (Ann. Thorac. Surg. 2013;96:1259-65).
"These aren’t just angles that are slightly off here and there," noted Dr. Bhama. "These are splayed very widely, situations I think where we all would say this is something we should be concerned about."
Dr. Bhama reported no relevant disclosures.
AT THE AATS ANNUAL MEETING
Key clinical point: Cannula malpositioning may be related to pump thrombosis after LVAD placement.
Major finding: The rate of pump thrombosis after LVAD implantation has rapidly increased since 2010, increasing steadily over the past 3 years. Excluding patients with device positioning concerns eliminated the significant difference seen in pump thrombosis across time.
Data source: Single-center, retrospective study of 63 LVAD implant patient records.
Disclosures: Dr. Bhama reported no relevant disclosures.
Systemwide disparities seen in diagnosis, care of women with heart disease
MELBOURNE – Women with heart disease are frequently underdiagnosed, undertreated, and underrepresented in clinical trials, and experience poorer outcomes both from inpatient and outpatient care.
Furthermore, while women have a tremendous amount of cardiovascular risk, they themselves are failing to recognize that heart disease is their No. 1 killer, Dr. Joanne M. Foody of Harvard Medical School, Boston, said at the World Congress of Cardiology 2014.
"The challenge is to ensure that women understand their risk, that the health care provider taking care of them understand their risk, and only by doing that can we really then impact their risk factors and treat them appropriately," said Dr. Foody, also director of the Pollin Cardiovascular Wellness Center at Brigham and Women’s Hospital, Boston.
Heart disease often presents differently in women than in men, with symptoms such as fatigue and breathlessness, which many women themselves would likely dismiss as just being part of a busy life.
While men tend to develop more focal plaques and narrowing of the arteries, women have smaller coronary arteries, even after body size is adjusted for, and therefore often have a more diffuse distribution of atherosclerosis.
"Women tend not to get that acute heart attack; they tend to have symptoms more related to small vessel disease which can lead to heart failure–like symptoms," she said.
Dr. Foody said that while the same cardiovascular risk factors apply to women and men, hormonal changes with menopause have a significant impact that is frequently underestimated by women and health care providers.
"Women undergo significant changes in their cholesterol levels, their blood pressure, and even their insulin resistance as they go through perimenopause and menopause, so it puts women at a unique transition point," Dr. Foody said. "Unfortunately, in women who were completely healthy and had no risk factors, that can change dramatically within the course of a couple of years."
Dr. Foody said that given the differences in presentation and treatment of heart disease in women, it is hardly surprising that women are more likely to die in the hospital, are more likely to experience reinfarction, and have a higher risk of heart failure, stroke, bleeding, and transfusion.
Women are also less likely to have an ECG performed within 10 minutes of hospital presentation, less likely to receive care from a cardiologist, and less likely to undergo diagnostic catheterization and revascularization procedures.
"These differences in care and in treatment can easily explain at least part of the disparities we see in outcomes for women," Dr. Foody said at the conference, which was sponsored by the World Heart Federation.
However, women themselves are also often more wary or skeptical of medication, Dr. Foody said, with evidence suggesting that married men are the most adherent to medication while married women are the least adherent.
Recent initiatives such as the global Go Red for Women and U.S.-based Screen Us campaigns were both aimed at raising awareness of heart disease among women and health care providers, but Dr. Foody said a system-level approach is required.
"That has to be coupled though with comparable programs that help inform health care providers, that help really put funding into appropriate screenings as well as appropriate research."
Dr. Foody said she had no relevant financial disclosures.
MELBOURNE – Women with heart disease are frequently underdiagnosed, undertreated, and underrepresented in clinical trials, and experience poorer outcomes both from inpatient and outpatient care.
Furthermore, while women have a tremendous amount of cardiovascular risk, they themselves are failing to recognize that heart disease is their No. 1 killer, Dr. Joanne M. Foody of Harvard Medical School, Boston, said at the World Congress of Cardiology 2014.
"The challenge is to ensure that women understand their risk, that the health care provider taking care of them understand their risk, and only by doing that can we really then impact their risk factors and treat them appropriately," said Dr. Foody, also director of the Pollin Cardiovascular Wellness Center at Brigham and Women’s Hospital, Boston.
Heart disease often presents differently in women than in men, with symptoms such as fatigue and breathlessness, which many women themselves would likely dismiss as just being part of a busy life.
While men tend to develop more focal plaques and narrowing of the arteries, women have smaller coronary arteries, even after body size is adjusted for, and therefore often have a more diffuse distribution of atherosclerosis.
"Women tend not to get that acute heart attack; they tend to have symptoms more related to small vessel disease which can lead to heart failure–like symptoms," she said.
Dr. Foody said that while the same cardiovascular risk factors apply to women and men, hormonal changes with menopause have a significant impact that is frequently underestimated by women and health care providers.
"Women undergo significant changes in their cholesterol levels, their blood pressure, and even their insulin resistance as they go through perimenopause and menopause, so it puts women at a unique transition point," Dr. Foody said. "Unfortunately, in women who were completely healthy and had no risk factors, that can change dramatically within the course of a couple of years."
Dr. Foody said that given the differences in presentation and treatment of heart disease in women, it is hardly surprising that women are more likely to die in the hospital, are more likely to experience reinfarction, and have a higher risk of heart failure, stroke, bleeding, and transfusion.
Women are also less likely to have an ECG performed within 10 minutes of hospital presentation, less likely to receive care from a cardiologist, and less likely to undergo diagnostic catheterization and revascularization procedures.
"These differences in care and in treatment can easily explain at least part of the disparities we see in outcomes for women," Dr. Foody said at the conference, which was sponsored by the World Heart Federation.
However, women themselves are also often more wary or skeptical of medication, Dr. Foody said, with evidence suggesting that married men are the most adherent to medication while married women are the least adherent.
Recent initiatives such as the global Go Red for Women and U.S.-based Screen Us campaigns were both aimed at raising awareness of heart disease among women and health care providers, but Dr. Foody said a system-level approach is required.
"That has to be coupled though with comparable programs that help inform health care providers, that help really put funding into appropriate screenings as well as appropriate research."
Dr. Foody said she had no relevant financial disclosures.
MELBOURNE – Women with heart disease are frequently underdiagnosed, undertreated, and underrepresented in clinical trials, and experience poorer outcomes both from inpatient and outpatient care.
Furthermore, while women have a tremendous amount of cardiovascular risk, they themselves are failing to recognize that heart disease is their No. 1 killer, Dr. Joanne M. Foody of Harvard Medical School, Boston, said at the World Congress of Cardiology 2014.
"The challenge is to ensure that women understand their risk, that the health care provider taking care of them understand their risk, and only by doing that can we really then impact their risk factors and treat them appropriately," said Dr. Foody, also director of the Pollin Cardiovascular Wellness Center at Brigham and Women’s Hospital, Boston.
Heart disease often presents differently in women than in men, with symptoms such as fatigue and breathlessness, which many women themselves would likely dismiss as just being part of a busy life.
While men tend to develop more focal plaques and narrowing of the arteries, women have smaller coronary arteries, even after body size is adjusted for, and therefore often have a more diffuse distribution of atherosclerosis.
"Women tend not to get that acute heart attack; they tend to have symptoms more related to small vessel disease which can lead to heart failure–like symptoms," she said.
Dr. Foody said that while the same cardiovascular risk factors apply to women and men, hormonal changes with menopause have a significant impact that is frequently underestimated by women and health care providers.
"Women undergo significant changes in their cholesterol levels, their blood pressure, and even their insulin resistance as they go through perimenopause and menopause, so it puts women at a unique transition point," Dr. Foody said. "Unfortunately, in women who were completely healthy and had no risk factors, that can change dramatically within the course of a couple of years."
Dr. Foody said that given the differences in presentation and treatment of heart disease in women, it is hardly surprising that women are more likely to die in the hospital, are more likely to experience reinfarction, and have a higher risk of heart failure, stroke, bleeding, and transfusion.
Women are also less likely to have an ECG performed within 10 minutes of hospital presentation, less likely to receive care from a cardiologist, and less likely to undergo diagnostic catheterization and revascularization procedures.
"These differences in care and in treatment can easily explain at least part of the disparities we see in outcomes for women," Dr. Foody said at the conference, which was sponsored by the World Heart Federation.
However, women themselves are also often more wary or skeptical of medication, Dr. Foody said, with evidence suggesting that married men are the most adherent to medication while married women are the least adherent.
Recent initiatives such as the global Go Red for Women and U.S.-based Screen Us campaigns were both aimed at raising awareness of heart disease among women and health care providers, but Dr. Foody said a system-level approach is required.
"That has to be coupled though with comparable programs that help inform health care providers, that help really put funding into appropriate screenings as well as appropriate research."
Dr. Foody said she had no relevant financial disclosures.
EXPERT ANALYSIS FROM WCC 2014
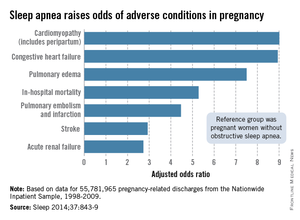
Sleep apnea raises cardiomyopathy risk ninefold in pregnancy
Pregnant women with obstructive sleep apnea are more likely to experience adverse clinical conditions than are pregnant women who do not have sleep apnea, according to an analysis of over 55 million pregnancy-related hospital discharges from 1998 to 2009.
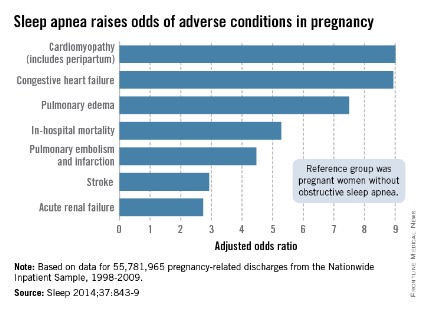
Women with obstructive sleep apnea (OSA) were nine times more likely to have cardiomyopathy and 8.9 times more likely to have congestive heart failure during pregnancy than were women without OSA, after adjustment for numerous factors, including maternal age and obesity, race/ethnicity, household income, heart disease, hyperlipidemia, and prepregnancy diabetes, reported Dr. Judette M. Louis of the University of South Florida, Tampa, and her associates.
Women with OSA also were more likely to have pulmonary edema (adjusted odds ratio, 7.5), in-hospital mortality (AOR, 5.3), pulmonary embolism and infarction (AOR, 4.5), stroke (AOR, 2.9), and acute renal failure (AOR, 2.7), Dr. Louis and her associates said (Sleep 2014;37:843-9).
The investigators analyzed data on 55,781,965 maternal hospital discharges from 1998 to 2009 in the Agency for Healthcare Research and Quality’s Nationwide Inpatient Sample. The institution of one investigator received a grant from ResMed Inc. and equipment from ResMed and Philips Respironics for use in clinical trials.
Pregnant women with obstructive sleep apnea are more likely to experience adverse clinical conditions than are pregnant women who do not have sleep apnea, according to an analysis of over 55 million pregnancy-related hospital discharges from 1998 to 2009.
Women with obstructive sleep apnea (OSA) were nine times more likely to have cardiomyopathy and 8.9 times more likely to have congestive heart failure during pregnancy than were women without OSA, after adjustment for numerous factors, including maternal age and obesity, race/ethnicity, household income, heart disease, hyperlipidemia, and prepregnancy diabetes, reported Dr. Judette M. Louis of the University of South Florida, Tampa, and her associates.
Women with OSA also were more likely to have pulmonary edema (adjusted odds ratio, 7.5), in-hospital mortality (AOR, 5.3), pulmonary embolism and infarction (AOR, 4.5), stroke (AOR, 2.9), and acute renal failure (AOR, 2.7), Dr. Louis and her associates said (Sleep 2014;37:843-9).
The investigators analyzed data on 55,781,965 maternal hospital discharges from 1998 to 2009 in the Agency for Healthcare Research and Quality’s Nationwide Inpatient Sample. The institution of one investigator received a grant from ResMed Inc. and equipment from ResMed and Philips Respironics for use in clinical trials.

Pregnant women with obstructive sleep apnea are more likely to experience adverse clinical conditions than are pregnant women who do not have sleep apnea, according to an analysis of over 55 million pregnancy-related hospital discharges from 1998 to 2009.
Women with obstructive sleep apnea (OSA) were nine times more likely to have cardiomyopathy and 8.9 times more likely to have congestive heart failure during pregnancy than were women without OSA, after adjustment for numerous factors, including maternal age and obesity, race/ethnicity, household income, heart disease, hyperlipidemia, and prepregnancy diabetes, reported Dr. Judette M. Louis of the University of South Florida, Tampa, and her associates.
Women with OSA also were more likely to have pulmonary edema (adjusted odds ratio, 7.5), in-hospital mortality (AOR, 5.3), pulmonary embolism and infarction (AOR, 4.5), stroke (AOR, 2.9), and acute renal failure (AOR, 2.7), Dr. Louis and her associates said (Sleep 2014;37:843-9).
The investigators analyzed data on 55,781,965 maternal hospital discharges from 1998 to 2009 in the Agency for Healthcare Research and Quality’s Nationwide Inpatient Sample. The institution of one investigator received a grant from ResMed Inc. and equipment from ResMed and Philips Respironics for use in clinical trials.

FROM SLEEP
FDA’s olmesartan probe showed no increased CV risk in diabetes
The Food and Drug Administration’s review of the safety of olmesartan has found "no clear evidence" of increased cardiovascular risks associated with the use of the drug in people with diabetes, but some concern remains about the possible risk with high doses, the agency reported on June 24.
The review, prompted by the ROADMAP (Randomized Olmesartan and Diabetes Microalbuminuria Prevention) study of patients with type 2 diabetes – which unexpectedly found an increased risk of cardiovascular death among patients on olmesartan, compared with those on placebo – has been completed, and recommendations for the use of the angiotensin receptor blocker (ARB) in people with diabetes will remain unchanged, according to the FDA.
Information about some of the studies reviewed by the FDA, however, including a large observational study of Medicare patients, will be included in the labels of olmesartan products, which include Benicar, Benicar HCT, AZO, Tribenzor, and generic formulations, the statement said.
"While data from the ROADMAP trial and the Medicare study have suggested that high-dose olmesartan may increase CV risk in diabetic patients, when considering the data from all trials and studies, they are not conclusive," the FDA statement said, adding, "Overall, we determined these studies do not clearly show an increased cardiovascular risk. Thus, the collective evidence available at this time does not support changing our recommendations for olmesartan use and does not support recommending that its use be avoided in patients with diabetes."
The risk of nonfatal MI, however, was lower among those on olmesartan in the ROADMAP study, which was designed to determine whether olmesartan could delay kidney damage in patients with type 2 diabetes (N. Engl. J. Med. 2011;364:907-17).
In the Medicare study of patients aged 65 years and older, the risk of death was increased among patients with diabetes, who received the highest (40 mg/day) dose of olmesartan for more than 6 months (hazard ratio, 2.0). Among patients who did not have diabetes, however, the highest dose was associated with a reduced risk of death (HR, 0.46). The conflicting results in these two patient groups in the study "are difficult to reconcile and raise uncertainty about the credibility of the findings in either group," the FDA said.
Other studies reviewed include a U.K. study of primary care medical records that compared outcomes of patients receiving high-dose olmesartan with those treated with high doses of other ARBs in over 58,000 patients. The study found an increased risk of overall death and of acute myocardial infarction associated with the use of high-dose olmesartan, which was not statistically significant (Pharmacoepidemiol. Drug Saf. 2014;23:340-7).
"Overall, these data raise concern of possible increased cardiovascular risk associated with the use of high-dose olmesartan in diabetic patients," the FDA concluded.
The FDA’s first safety communication on this issue was posted in June 2010, followed by an update in April 2011.
In 2013, about 1.8 million people received a dispensed prescription for olmesartan products from U.S. outpatient retail pharmacies, according to the FDA.
Serious adverse events in patients treated with olmesartan products should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/medwatch.
The Food and Drug Administration’s review of the safety of olmesartan has found "no clear evidence" of increased cardiovascular risks associated with the use of the drug in people with diabetes, but some concern remains about the possible risk with high doses, the agency reported on June 24.
The review, prompted by the ROADMAP (Randomized Olmesartan and Diabetes Microalbuminuria Prevention) study of patients with type 2 diabetes – which unexpectedly found an increased risk of cardiovascular death among patients on olmesartan, compared with those on placebo – has been completed, and recommendations for the use of the angiotensin receptor blocker (ARB) in people with diabetes will remain unchanged, according to the FDA.
Information about some of the studies reviewed by the FDA, however, including a large observational study of Medicare patients, will be included in the labels of olmesartan products, which include Benicar, Benicar HCT, AZO, Tribenzor, and generic formulations, the statement said.
"While data from the ROADMAP trial and the Medicare study have suggested that high-dose olmesartan may increase CV risk in diabetic patients, when considering the data from all trials and studies, they are not conclusive," the FDA statement said, adding, "Overall, we determined these studies do not clearly show an increased cardiovascular risk. Thus, the collective evidence available at this time does not support changing our recommendations for olmesartan use and does not support recommending that its use be avoided in patients with diabetes."
The risk of nonfatal MI, however, was lower among those on olmesartan in the ROADMAP study, which was designed to determine whether olmesartan could delay kidney damage in patients with type 2 diabetes (N. Engl. J. Med. 2011;364:907-17).
In the Medicare study of patients aged 65 years and older, the risk of death was increased among patients with diabetes, who received the highest (40 mg/day) dose of olmesartan for more than 6 months (hazard ratio, 2.0). Among patients who did not have diabetes, however, the highest dose was associated with a reduced risk of death (HR, 0.46). The conflicting results in these two patient groups in the study "are difficult to reconcile and raise uncertainty about the credibility of the findings in either group," the FDA said.
Other studies reviewed include a U.K. study of primary care medical records that compared outcomes of patients receiving high-dose olmesartan with those treated with high doses of other ARBs in over 58,000 patients. The study found an increased risk of overall death and of acute myocardial infarction associated with the use of high-dose olmesartan, which was not statistically significant (Pharmacoepidemiol. Drug Saf. 2014;23:340-7).
"Overall, these data raise concern of possible increased cardiovascular risk associated with the use of high-dose olmesartan in diabetic patients," the FDA concluded.
The FDA’s first safety communication on this issue was posted in June 2010, followed by an update in April 2011.
In 2013, about 1.8 million people received a dispensed prescription for olmesartan products from U.S. outpatient retail pharmacies, according to the FDA.
Serious adverse events in patients treated with olmesartan products should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/medwatch.
The Food and Drug Administration’s review of the safety of olmesartan has found "no clear evidence" of increased cardiovascular risks associated with the use of the drug in people with diabetes, but some concern remains about the possible risk with high doses, the agency reported on June 24.
The review, prompted by the ROADMAP (Randomized Olmesartan and Diabetes Microalbuminuria Prevention) study of patients with type 2 diabetes – which unexpectedly found an increased risk of cardiovascular death among patients on olmesartan, compared with those on placebo – has been completed, and recommendations for the use of the angiotensin receptor blocker (ARB) in people with diabetes will remain unchanged, according to the FDA.
Information about some of the studies reviewed by the FDA, however, including a large observational study of Medicare patients, will be included in the labels of olmesartan products, which include Benicar, Benicar HCT, AZO, Tribenzor, and generic formulations, the statement said.
"While data from the ROADMAP trial and the Medicare study have suggested that high-dose olmesartan may increase CV risk in diabetic patients, when considering the data from all trials and studies, they are not conclusive," the FDA statement said, adding, "Overall, we determined these studies do not clearly show an increased cardiovascular risk. Thus, the collective evidence available at this time does not support changing our recommendations for olmesartan use and does not support recommending that its use be avoided in patients with diabetes."
The risk of nonfatal MI, however, was lower among those on olmesartan in the ROADMAP study, which was designed to determine whether olmesartan could delay kidney damage in patients with type 2 diabetes (N. Engl. J. Med. 2011;364:907-17).
In the Medicare study of patients aged 65 years and older, the risk of death was increased among patients with diabetes, who received the highest (40 mg/day) dose of olmesartan for more than 6 months (hazard ratio, 2.0). Among patients who did not have diabetes, however, the highest dose was associated with a reduced risk of death (HR, 0.46). The conflicting results in these two patient groups in the study "are difficult to reconcile and raise uncertainty about the credibility of the findings in either group," the FDA said.
Other studies reviewed include a U.K. study of primary care medical records that compared outcomes of patients receiving high-dose olmesartan with those treated with high doses of other ARBs in over 58,000 patients. The study found an increased risk of overall death and of acute myocardial infarction associated with the use of high-dose olmesartan, which was not statistically significant (Pharmacoepidemiol. Drug Saf. 2014;23:340-7).
"Overall, these data raise concern of possible increased cardiovascular risk associated with the use of high-dose olmesartan in diabetic patients," the FDA concluded.
The FDA’s first safety communication on this issue was posted in June 2010, followed by an update in April 2011.
In 2013, about 1.8 million people received a dispensed prescription for olmesartan products from U.S. outpatient retail pharmacies, according to the FDA.
Serious adverse events in patients treated with olmesartan products should be reported to the FDA’s MedWatch program at 800-332-1088 or www.fda.gov/medwatch.
Women benefit from CRT at shorter QRS durations
Women with mild heart failure and left bundle branch block benefit from cardiac resynchronization therapy at a shorter QRS duration – less than 150 milliseconds – than do men, according to a report published online June 23 in JAMA Internal Medicine.
Current guidelines limit the class I indication for CRT to patients with mild heart failure, LBBB, and a QRS interval of 150 msec or longer. They are based on clinical trials in which women were underrepresented, comprising only about 20% of participants. Noting that QRS duration is generally shorter in women than in men, Food and Drug Administration researchers examined whether women might benefit from CRT at a shorter QRS duration than men do, said Dr. Robbert Zusterzeel and his associates at the FDA’s Center for Devices and Radiological Health.
They performed a pooled analysis of patient-level data from three large, randomized clinical trials submitted to the FDA as part of an application for premarketing approval of the CRT devices: 1,820 participants in MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy), 1,663 from the RAFT (Resynchronization-Defibrillation for Ambulatory Heart Failure Trial) study, and 593 from the REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial.
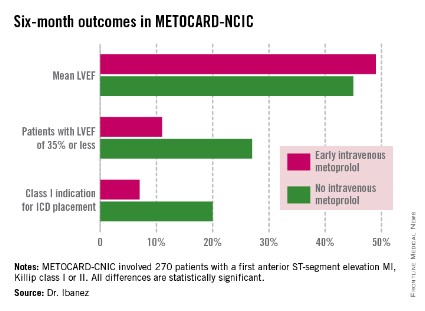
In women overall, CRT produced a 60% relative reduction in the combined end point of heart failure event or death and a 55% relative reduction in death alone, compared with relative reductions of only 26% and 15% in men. In the subgroup of women who had QRS durations of 130-139 msec, CRT resulted in an 85% relative reduction in heart failure event or death; and in women with QRS durations of 140-149 msec, CRT resulted in a 69% relative reduction. In contrast, men with QRS durations in this low range did not benefit from CRT, Dr. Zusterzeel and his associates said (JAMA Intern. Med. 2014 June 23 [doi:10.1001/jamainternmed.2014.2717]).
"Considering that women receive CRT less often than men, we believe that the current findings are important to communicate" to clinicians and patients, they said.
Their study also "highlights the importance of sex-specific analysis in medical device clinical studies and the public health value of combining individual-patient data from clinical trials submitted to the FDA," the investigators added.
This study was supported in part by the FDA Office of Women’s Health, the Oak Ridge Institute for Science and Education, and the U.S. Department of Energy. No financial conflicts of interest were reported.
These new findings indicate that CRT is likely underused in women, who show greater benefit from the treatment than men do.
These results also shed light on a major contributor to the misdiagnosis and suboptimal treatment of CVD in women: Guidelines are typically based on a male standard and do not address important differences in women.
Women remain the minority of research subjects even though they are the majority of persons dying of CVD.
Dr. C. Noel Bairey Merz is at the Barbra Streisand Women’s Heart Center, Cedars Sinai Heart Institute, Los Angeles. Dr. Vera Regitz-Zagrosek is at the Institute for Gender in Medicine at Charité University and at the German Cardiovascular Research Center, both in Berlin. They reported no financial conflicts of interest. These remarks were taken from their commentary accompanying Dr. Zusterzeel’s report (JAMA Inter. Med. 2014 June 23 [doi:10.1001/jamainternmed.2014.320]). This work was supported by the National Institutes of Health, the National Center for Research Resources, the Gustavus and Louise Pfeiffer Research Foundation, the Women’s Guild of Cedars-Sinai Medical Center, and several others.
These new findings indicate that CRT is likely underused in women, who show greater benefit from the treatment than men do.
These results also shed light on a major contributor to the misdiagnosis and suboptimal treatment of CVD in women: Guidelines are typically based on a male standard and do not address important differences in women.
Women remain the minority of research subjects even though they are the majority of persons dying of CVD.
Dr. C. Noel Bairey Merz is at the Barbra Streisand Women’s Heart Center, Cedars Sinai Heart Institute, Los Angeles. Dr. Vera Regitz-Zagrosek is at the Institute for Gender in Medicine at Charité University and at the German Cardiovascular Research Center, both in Berlin. They reported no financial conflicts of interest. These remarks were taken from their commentary accompanying Dr. Zusterzeel’s report (JAMA Inter. Med. 2014 June 23 [doi:10.1001/jamainternmed.2014.320]). This work was supported by the National Institutes of Health, the National Center for Research Resources, the Gustavus and Louise Pfeiffer Research Foundation, the Women’s Guild of Cedars-Sinai Medical Center, and several others.
These new findings indicate that CRT is likely underused in women, who show greater benefit from the treatment than men do.
These results also shed light on a major contributor to the misdiagnosis and suboptimal treatment of CVD in women: Guidelines are typically based on a male standard and do not address important differences in women.
Women remain the minority of research subjects even though they are the majority of persons dying of CVD.
Dr. C. Noel Bairey Merz is at the Barbra Streisand Women’s Heart Center, Cedars Sinai Heart Institute, Los Angeles. Dr. Vera Regitz-Zagrosek is at the Institute for Gender in Medicine at Charité University and at the German Cardiovascular Research Center, both in Berlin. They reported no financial conflicts of interest. These remarks were taken from their commentary accompanying Dr. Zusterzeel’s report (JAMA Inter. Med. 2014 June 23 [doi:10.1001/jamainternmed.2014.320]). This work was supported by the National Institutes of Health, the National Center for Research Resources, the Gustavus and Louise Pfeiffer Research Foundation, the Women’s Guild of Cedars-Sinai Medical Center, and several others.
Women with mild heart failure and left bundle branch block benefit from cardiac resynchronization therapy at a shorter QRS duration – less than 150 milliseconds – than do men, according to a report published online June 23 in JAMA Internal Medicine.
Current guidelines limit the class I indication for CRT to patients with mild heart failure, LBBB, and a QRS interval of 150 msec or longer. They are based on clinical trials in which women were underrepresented, comprising only about 20% of participants. Noting that QRS duration is generally shorter in women than in men, Food and Drug Administration researchers examined whether women might benefit from CRT at a shorter QRS duration than men do, said Dr. Robbert Zusterzeel and his associates at the FDA’s Center for Devices and Radiological Health.
They performed a pooled analysis of patient-level data from three large, randomized clinical trials submitted to the FDA as part of an application for premarketing approval of the CRT devices: 1,820 participants in MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy), 1,663 from the RAFT (Resynchronization-Defibrillation for Ambulatory Heart Failure Trial) study, and 593 from the REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial.
In women overall, CRT produced a 60% relative reduction in the combined end point of heart failure event or death and a 55% relative reduction in death alone, compared with relative reductions of only 26% and 15% in men. In the subgroup of women who had QRS durations of 130-139 msec, CRT resulted in an 85% relative reduction in heart failure event or death; and in women with QRS durations of 140-149 msec, CRT resulted in a 69% relative reduction. In contrast, men with QRS durations in this low range did not benefit from CRT, Dr. Zusterzeel and his associates said (JAMA Intern. Med. 2014 June 23 [doi:10.1001/jamainternmed.2014.2717]).
"Considering that women receive CRT less often than men, we believe that the current findings are important to communicate" to clinicians and patients, they said.
Their study also "highlights the importance of sex-specific analysis in medical device clinical studies and the public health value of combining individual-patient data from clinical trials submitted to the FDA," the investigators added.
This study was supported in part by the FDA Office of Women’s Health, the Oak Ridge Institute for Science and Education, and the U.S. Department of Energy. No financial conflicts of interest were reported.
Women with mild heart failure and left bundle branch block benefit from cardiac resynchronization therapy at a shorter QRS duration – less than 150 milliseconds – than do men, according to a report published online June 23 in JAMA Internal Medicine.
Current guidelines limit the class I indication for CRT to patients with mild heart failure, LBBB, and a QRS interval of 150 msec or longer. They are based on clinical trials in which women were underrepresented, comprising only about 20% of participants. Noting that QRS duration is generally shorter in women than in men, Food and Drug Administration researchers examined whether women might benefit from CRT at a shorter QRS duration than men do, said Dr. Robbert Zusterzeel and his associates at the FDA’s Center for Devices and Radiological Health.
They performed a pooled analysis of patient-level data from three large, randomized clinical trials submitted to the FDA as part of an application for premarketing approval of the CRT devices: 1,820 participants in MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy), 1,663 from the RAFT (Resynchronization-Defibrillation for Ambulatory Heart Failure Trial) study, and 593 from the REVERSE (Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction) trial.
In women overall, CRT produced a 60% relative reduction in the combined end point of heart failure event or death and a 55% relative reduction in death alone, compared with relative reductions of only 26% and 15% in men. In the subgroup of women who had QRS durations of 130-139 msec, CRT resulted in an 85% relative reduction in heart failure event or death; and in women with QRS durations of 140-149 msec, CRT resulted in a 69% relative reduction. In contrast, men with QRS durations in this low range did not benefit from CRT, Dr. Zusterzeel and his associates said (JAMA Intern. Med. 2014 June 23 [doi:10.1001/jamainternmed.2014.2717]).
"Considering that women receive CRT less often than men, we believe that the current findings are important to communicate" to clinicians and patients, they said.
Their study also "highlights the importance of sex-specific analysis in medical device clinical studies and the public health value of combining individual-patient data from clinical trials submitted to the FDA," the investigators added.
This study was supported in part by the FDA Office of Women’s Health, the Oak Ridge Institute for Science and Education, and the U.S. Department of Energy. No financial conflicts of interest were reported.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Women, especially those with QRS durations of 130-139 msec, benefit more from CRT therapy than men do.
Major finding: In women who had QRS durations of 130-139 msec, CRT produced an 85% relative reduction in heart failure event or death, and in women with QRS durations of 140-149 msec, CRT produced a 69% relative reduction; in contrast, men with QRS durations in this low range did not benefit from CRT.
Data source: A patient-level pooled analysis of data from three large randomized clinical trials involving 4,076 patients with mild heart failure and LBBB who received either CRT or an ICD were followed for up to 3 years.
Disclosures: This study was supported in part by the FDA Office of Women’s Health, the Oak Ridge Institute for Science and Education, and the U.S. Department of Energy. No financial conflicts of interest were reported.
ZS-9 may be a safer hyperkalemia therapy
LAS VEGAS – A novel, highly selective, oral potassium ion–binding agent called ZS-9 effectively reversed hyperkalemia in patients with underlying diabetes, heart failure, or chronic kidney disease.
ZS-9’s safety and tolerability matched those of placebo in the double-blind trial, which was the largest phase III treatment trial ever conducted in patients with hyperkalemia, Dr. Bhupinder Singh said at a meeting sponsored by the National Kidney Foundation.
ZS-9 is an inorganic, microporous crystal composed of zirconium silicate. It is insoluble, highly stable, and not systemically absorbed. In vitro it is more than 125 times more selective for potassium ions than is sodium polystyrene sulfate (SPS, brand name Kayexalate), the resin-based therapy traditionally used in hyperkalemia. ZS-9 also has nine times greater potassium ion binding capacity than SPS, according to Dr. Singh, of Apex Research in Riverside, Calif.
He reported on 753 patients with serum potassium levels of 5.0-6.5 mEq/L; 60% had chronic kidney disease, 40% had heart failure, and 60% had diabetes. Two-thirds of the patients were on an ACE inhibitor or an angiotensin receptor blocker (ARB). Importantly, 28% of subjects had an estimated glomerular filtration rate below 29 mL/min per 1.73 m2.
Subjects were randomized to one of four dosing regimens of ZS-9 or to placebo. For the first 48 hours of the study, patients received oral ZS-9 at doses of 1.25, 2.5, 5, or 10 g t.i.d. or placebo. Patients whose serum potassium levels normalized after 48 hours were then switched to once-daily doses of the same strengths or to placebo for 12 days in order to evaluate ZS-9’s safety and efficacy as a maintenance therapy.
The average reduction from baseline at 48 hours in serum potassium (as measured 14 hours after the last dose) was 0.46 mEq/L in the 2.5-g t.i.d. group, 0.54 mEq/L in 5-mg t.i.d. group, and 0.73 mEq/L in the 10-mg t.i.d. group. Normokalemia was attained at a similar rate in all patient subgroups, regardless of their underlying hyperkalemia-promoting disease. ZS-9 at 1.25 g t.i.d. wasn’t significantly more effective than placebo.
The higher a patient’s baseline serum potassium, the larger the absolute drop in response to a given dose. At 10 mg t.i.d., for example, patients with a baseline serum potassium level of 5.3 mEq/L or less averaged a 0.58-mEq/L reduction. Those who started at 5.4-5.5 mEq/L averaged a 0.98-mEq/L decrease, and patients with a baseline serum potassium level in excess of 5.5 mEq/L had a 1.10-mEq/L reduction.
Of patients who were on ZS-9 at 10 mg t.i.d. for the acute phase of the study, 99% were normokalemic at 48 hours.
All told, 542 patients entered the 12-day maintenance therapy phase. The two higher dosing regimens – 5 and 10 mg once daily – provided clinically and statistically significant benefit, with serum potassium levels remaining flat throughout the study period. Those who were switched double-blind to placebo after 12 days on ZS-9 immediately experienced a rise in their serum potassium. For example, patients with a serum potassium at 4.6 mEq/L during 12 days on ZS-9 at 10 mg climbed on average to a serum potassium of 5.0 mEq/L after 1 week on placebo.
Serum sodium, magnesium, and calcium did not change, nor did blood pressure or body weight in ZS-9-treated patients. There were no cases of significant hypokalemia (values below 3.0 mEq/L) in the study. Two of 11,000 serum potassium measures were less than 3.5 mEq/L. The side-effect profile of ZS-9 was basically the same as that of placebo. The incidence of nausea, vomiting, and other gastrointestinal adverse effects was actually more than twice as high with placebo than it was with active therapy, according to Dr. Singh.
Ongoing and planned studies of ZS-9 include an open-label, 12-month study and a 1-month, randomized treatment withdrawal with long-term extension.
Dr. Singh cited two major candidates for ZS-9 in clinical practice. One is in the large subgroup of patients with heart failure, diabetic kidney disease, or proteinuric nephropathy who become hyperkalemic on optimal doses of guideline-recommended therapy with an ACE inhibitor or ARB. The other potential beneficiaries are patients who would like to follow a healthy diet emphasizing vegetables, fruits, and low-fat dairy products, many of which are quite high in potassium content.
Dr. Singh reported serving as a consultant to ZS Pharma, which is developing ZS-9, as well as to Keryx Biopharmaceuticals and Concert.
 |
|
The phase III ZS-9 results are extremely encouraging. There is a major unmet need for a safe and effective agent for acute and chronic management of hyperkalemia. Concern is mounting regarding chronic use of sodium polystyrene sulfate. A systematic review of published reports highlighted the risk of potentially fatal colonic necrosis and other gastrointestinal injuries with this traditional therapy (Am. J. Med. 2013;126:264.e9-e.24).
This information calls into question the use of SPS as a chronic therapy. So we need other options, and we perhaps have some on the horizon with ZS-9. Patiromer is another novel therapy well along in the developmental pipeline. This high-capacity, nonabsorbed, oral ion-exchange polymer also performed well in a phase III randomized trial presented by Dr. Matthew Weir of the University of Maryland, Baltimore, last fall at the annual meeting of the American Society of Nephrology.
Dr. John P. Middleton, a nephrologist at Duke University in Durham, N.C., made these remarks in a separate presentation at the meeting. Dr. Middleton reported having no financial conflicts.
 |
|
The phase III ZS-9 results are extremely encouraging. There is a major unmet need for a safe and effective agent for acute and chronic management of hyperkalemia. Concern is mounting regarding chronic use of sodium polystyrene sulfate. A systematic review of published reports highlighted the risk of potentially fatal colonic necrosis and other gastrointestinal injuries with this traditional therapy (Am. J. Med. 2013;126:264.e9-e.24).
This information calls into question the use of SPS as a chronic therapy. So we need other options, and we perhaps have some on the horizon with ZS-9. Patiromer is another novel therapy well along in the developmental pipeline. This high-capacity, nonabsorbed, oral ion-exchange polymer also performed well in a phase III randomized trial presented by Dr. Matthew Weir of the University of Maryland, Baltimore, last fall at the annual meeting of the American Society of Nephrology.
Dr. John P. Middleton, a nephrologist at Duke University in Durham, N.C., made these remarks in a separate presentation at the meeting. Dr. Middleton reported having no financial conflicts.
 |
|
The phase III ZS-9 results are extremely encouraging. There is a major unmet need for a safe and effective agent for acute and chronic management of hyperkalemia. Concern is mounting regarding chronic use of sodium polystyrene sulfate. A systematic review of published reports highlighted the risk of potentially fatal colonic necrosis and other gastrointestinal injuries with this traditional therapy (Am. J. Med. 2013;126:264.e9-e.24).
This information calls into question the use of SPS as a chronic therapy. So we need other options, and we perhaps have some on the horizon with ZS-9. Patiromer is another novel therapy well along in the developmental pipeline. This high-capacity, nonabsorbed, oral ion-exchange polymer also performed well in a phase III randomized trial presented by Dr. Matthew Weir of the University of Maryland, Baltimore, last fall at the annual meeting of the American Society of Nephrology.
Dr. John P. Middleton, a nephrologist at Duke University in Durham, N.C., made these remarks in a separate presentation at the meeting. Dr. Middleton reported having no financial conflicts.
LAS VEGAS – A novel, highly selective, oral potassium ion–binding agent called ZS-9 effectively reversed hyperkalemia in patients with underlying diabetes, heart failure, or chronic kidney disease.
ZS-9’s safety and tolerability matched those of placebo in the double-blind trial, which was the largest phase III treatment trial ever conducted in patients with hyperkalemia, Dr. Bhupinder Singh said at a meeting sponsored by the National Kidney Foundation.
ZS-9 is an inorganic, microporous crystal composed of zirconium silicate. It is insoluble, highly stable, and not systemically absorbed. In vitro it is more than 125 times more selective for potassium ions than is sodium polystyrene sulfate (SPS, brand name Kayexalate), the resin-based therapy traditionally used in hyperkalemia. ZS-9 also has nine times greater potassium ion binding capacity than SPS, according to Dr. Singh, of Apex Research in Riverside, Calif.
He reported on 753 patients with serum potassium levels of 5.0-6.5 mEq/L; 60% had chronic kidney disease, 40% had heart failure, and 60% had diabetes. Two-thirds of the patients were on an ACE inhibitor or an angiotensin receptor blocker (ARB). Importantly, 28% of subjects had an estimated glomerular filtration rate below 29 mL/min per 1.73 m2.
Subjects were randomized to one of four dosing regimens of ZS-9 or to placebo. For the first 48 hours of the study, patients received oral ZS-9 at doses of 1.25, 2.5, 5, or 10 g t.i.d. or placebo. Patients whose serum potassium levels normalized after 48 hours were then switched to once-daily doses of the same strengths or to placebo for 12 days in order to evaluate ZS-9’s safety and efficacy as a maintenance therapy.
The average reduction from baseline at 48 hours in serum potassium (as measured 14 hours after the last dose) was 0.46 mEq/L in the 2.5-g t.i.d. group, 0.54 mEq/L in 5-mg t.i.d. group, and 0.73 mEq/L in the 10-mg t.i.d. group. Normokalemia was attained at a similar rate in all patient subgroups, regardless of their underlying hyperkalemia-promoting disease. ZS-9 at 1.25 g t.i.d. wasn’t significantly more effective than placebo.
The higher a patient’s baseline serum potassium, the larger the absolute drop in response to a given dose. At 10 mg t.i.d., for example, patients with a baseline serum potassium level of 5.3 mEq/L or less averaged a 0.58-mEq/L reduction. Those who started at 5.4-5.5 mEq/L averaged a 0.98-mEq/L decrease, and patients with a baseline serum potassium level in excess of 5.5 mEq/L had a 1.10-mEq/L reduction.
Of patients who were on ZS-9 at 10 mg t.i.d. for the acute phase of the study, 99% were normokalemic at 48 hours.
All told, 542 patients entered the 12-day maintenance therapy phase. The two higher dosing regimens – 5 and 10 mg once daily – provided clinically and statistically significant benefit, with serum potassium levels remaining flat throughout the study period. Those who were switched double-blind to placebo after 12 days on ZS-9 immediately experienced a rise in their serum potassium. For example, patients with a serum potassium at 4.6 mEq/L during 12 days on ZS-9 at 10 mg climbed on average to a serum potassium of 5.0 mEq/L after 1 week on placebo.
Serum sodium, magnesium, and calcium did not change, nor did blood pressure or body weight in ZS-9-treated patients. There were no cases of significant hypokalemia (values below 3.0 mEq/L) in the study. Two of 11,000 serum potassium measures were less than 3.5 mEq/L. The side-effect profile of ZS-9 was basically the same as that of placebo. The incidence of nausea, vomiting, and other gastrointestinal adverse effects was actually more than twice as high with placebo than it was with active therapy, according to Dr. Singh.
Ongoing and planned studies of ZS-9 include an open-label, 12-month study and a 1-month, randomized treatment withdrawal with long-term extension.
Dr. Singh cited two major candidates for ZS-9 in clinical practice. One is in the large subgroup of patients with heart failure, diabetic kidney disease, or proteinuric nephropathy who become hyperkalemic on optimal doses of guideline-recommended therapy with an ACE inhibitor or ARB. The other potential beneficiaries are patients who would like to follow a healthy diet emphasizing vegetables, fruits, and low-fat dairy products, many of which are quite high in potassium content.
Dr. Singh reported serving as a consultant to ZS Pharma, which is developing ZS-9, as well as to Keryx Biopharmaceuticals and Concert.
LAS VEGAS – A novel, highly selective, oral potassium ion–binding agent called ZS-9 effectively reversed hyperkalemia in patients with underlying diabetes, heart failure, or chronic kidney disease.
ZS-9’s safety and tolerability matched those of placebo in the double-blind trial, which was the largest phase III treatment trial ever conducted in patients with hyperkalemia, Dr. Bhupinder Singh said at a meeting sponsored by the National Kidney Foundation.
ZS-9 is an inorganic, microporous crystal composed of zirconium silicate. It is insoluble, highly stable, and not systemically absorbed. In vitro it is more than 125 times more selective for potassium ions than is sodium polystyrene sulfate (SPS, brand name Kayexalate), the resin-based therapy traditionally used in hyperkalemia. ZS-9 also has nine times greater potassium ion binding capacity than SPS, according to Dr. Singh, of Apex Research in Riverside, Calif.
He reported on 753 patients with serum potassium levels of 5.0-6.5 mEq/L; 60% had chronic kidney disease, 40% had heart failure, and 60% had diabetes. Two-thirds of the patients were on an ACE inhibitor or an angiotensin receptor blocker (ARB). Importantly, 28% of subjects had an estimated glomerular filtration rate below 29 mL/min per 1.73 m2.
Subjects were randomized to one of four dosing regimens of ZS-9 or to placebo. For the first 48 hours of the study, patients received oral ZS-9 at doses of 1.25, 2.5, 5, or 10 g t.i.d. or placebo. Patients whose serum potassium levels normalized after 48 hours were then switched to once-daily doses of the same strengths or to placebo for 12 days in order to evaluate ZS-9’s safety and efficacy as a maintenance therapy.
The average reduction from baseline at 48 hours in serum potassium (as measured 14 hours after the last dose) was 0.46 mEq/L in the 2.5-g t.i.d. group, 0.54 mEq/L in 5-mg t.i.d. group, and 0.73 mEq/L in the 10-mg t.i.d. group. Normokalemia was attained at a similar rate in all patient subgroups, regardless of their underlying hyperkalemia-promoting disease. ZS-9 at 1.25 g t.i.d. wasn’t significantly more effective than placebo.
The higher a patient’s baseline serum potassium, the larger the absolute drop in response to a given dose. At 10 mg t.i.d., for example, patients with a baseline serum potassium level of 5.3 mEq/L or less averaged a 0.58-mEq/L reduction. Those who started at 5.4-5.5 mEq/L averaged a 0.98-mEq/L decrease, and patients with a baseline serum potassium level in excess of 5.5 mEq/L had a 1.10-mEq/L reduction.
Of patients who were on ZS-9 at 10 mg t.i.d. for the acute phase of the study, 99% were normokalemic at 48 hours.
All told, 542 patients entered the 12-day maintenance therapy phase. The two higher dosing regimens – 5 and 10 mg once daily – provided clinically and statistically significant benefit, with serum potassium levels remaining flat throughout the study period. Those who were switched double-blind to placebo after 12 days on ZS-9 immediately experienced a rise in their serum potassium. For example, patients with a serum potassium at 4.6 mEq/L during 12 days on ZS-9 at 10 mg climbed on average to a serum potassium of 5.0 mEq/L after 1 week on placebo.
Serum sodium, magnesium, and calcium did not change, nor did blood pressure or body weight in ZS-9-treated patients. There were no cases of significant hypokalemia (values below 3.0 mEq/L) in the study. Two of 11,000 serum potassium measures were less than 3.5 mEq/L. The side-effect profile of ZS-9 was basically the same as that of placebo. The incidence of nausea, vomiting, and other gastrointestinal adverse effects was actually more than twice as high with placebo than it was with active therapy, according to Dr. Singh.
Ongoing and planned studies of ZS-9 include an open-label, 12-month study and a 1-month, randomized treatment withdrawal with long-term extension.
Dr. Singh cited two major candidates for ZS-9 in clinical practice. One is in the large subgroup of patients with heart failure, diabetic kidney disease, or proteinuric nephropathy who become hyperkalemic on optimal doses of guideline-recommended therapy with an ACE inhibitor or ARB. The other potential beneficiaries are patients who would like to follow a healthy diet emphasizing vegetables, fruits, and low-fat dairy products, many of which are quite high in potassium content.
Dr. Singh reported serving as a consultant to ZS Pharma, which is developing ZS-9, as well as to Keryx Biopharmaceuticals and Concert.
AT SCM 14
Key clinical point: ZS-9 may become a safer treatment alternative to sodium polystyrene sulfate.
Major finding: After 48 hours on the investigational selective potassium ion trapper known as ZS-9 at 10 mg t.i.d., 99% of initially hyperkalemic patients with chronic kidney disease, diabetes, and/or heart failure were normokalemic.
Data source: This phase III, double-blind trial included 753 hyperkalemic patients randomized to one of four doses of ZS-9 or to placebo.
Disclosures: The study was sponsored by ZS Pharma. Dr. Singh reported serving as a consultant to ZS Pharma, which is developing ZS-9.
Cardio-oncology clinics needed to deal with cardiotoxic chemotherapy sequelae
MELBOURNE – Specialist cardio-oncology clinics may be needed to address the long-term cardiac care of cancer patients treated with cardiotoxic chemotherapy regimens such as anthracyclines and trastuzumab, presenters told the World Congress of Cardiology 2014.
Dr. Puja K. Mehta, codirector of the cardio-oncology program at the Barbra Streisand Women’s Heart Center, presented poster data showing that 12% of women who attended the clinic over a 7-month period had a previous diagnosis of cancer, nearly half of which were cases of breast cancer.
The study of 892 patients seen at the center found that 92% of all breast cancer survivors had at least one cardiac risk factor – 55% had hypertension, 64% had hyperlipidemia, 14% had diabetes, 18% coronary artery disease, and 23% ischemic heart disease.
There is growing awareness of the long-term sequelae of cardiotoxic chemotherapy regimens but the challenge was how to take a preventive approach to the problem, Dr. Mehta said at the meeting sponsored by the World Heart Federation.
"People who have had a history of breast cancer ... often they’re only seeing oncologists to follow up on breast cancer recurrence so they are getting yearly mammograms and those things, but what they’ve missed is a little bit of hypertension that’s been there for years and then you get the accelerated atherosclerosis," Dr. Mehta said in an interview with Cardiology News.
The study’s authors suggest that cardio-oncology clinics could play an important role in implementing lifestyle modification and preventive interventions to reduce the risk of cardiac sequelae in cancer survivors.
In another presentation, American Heart Association President Mariell Jessup, Medical Director of the Penn Heart and Vascular Center, said early-onset cardiotoxicity occurs in a relatively small percent of patients – around 1%-2% – within the first year after chemotherapy treatment.
However late-onset cardiotoxicity was far more insidious as it affected a wide range of patients and the signs may not manifest until long after chemotherapy.
"We used to be taught that it came within the first year or so, and so when someone showed up 10 years or 15 years from treatment we discounted the fact that it was cardiotoxicity from anthracycline," Dr. Jessup told Cardiology News.
Dr. Jessup said an estimated 20%-30% of patients who had been treated with anthracyclines would likely show some kind of cardiac problems, but the addition of trastuzumab to breast cancer chemotherapy further increased the risk.
"They can present with heart failure ... but if they have radiotherapy on top of chemotherapy they are more susceptible to proximal coronary disease," she said.
"Then we’re seeing a group of patients that end up at cardiac transplant because of their heart failure, and they have very fragile bone marrows so they don’t tolerate immunosuppression as well, and they tend to be very anemic."
Dr. Jessup told the conference that more research was critical to understand the mechanisms of anthracycline cardiotoxicity, and to identify predictive markers of cardiac damage, but she stressed clinician awareness was also fundamental.
"We need to educate clinicians about not only the importance of these chemotherapeutic agents to the survival of patients following their cancer but how to be particularly attuned to the possibility that exposure many years before can lead to cardiac toxicity."
A member of the audience raised the question about treating patients with angiotensin-converting enzyme inhibitors at the same time as their chemotherapy to reduce the cardiac impact of the chemotherapy.
Dr. Jessup said while there were some intriguing data emerging about the concurrent use of ACE inhibitors and beta-blockers in patients treated with anthracyclines, there still remained questions about when the drugs should be used as they may not be well tolerated during chemotherapy.
Dr. Jessup had no disclosures. Dr. Mehta declared grant/research support from Gilead, and a coauthor declared grant/research support from Gilead and consultancies and honoraria from a range of organizations.
MELBOURNE – Specialist cardio-oncology clinics may be needed to address the long-term cardiac care of cancer patients treated with cardiotoxic chemotherapy regimens such as anthracyclines and trastuzumab, presenters told the World Congress of Cardiology 2014.
Dr. Puja K. Mehta, codirector of the cardio-oncology program at the Barbra Streisand Women’s Heart Center, presented poster data showing that 12% of women who attended the clinic over a 7-month period had a previous diagnosis of cancer, nearly half of which were cases of breast cancer.
The study of 892 patients seen at the center found that 92% of all breast cancer survivors had at least one cardiac risk factor – 55% had hypertension, 64% had hyperlipidemia, 14% had diabetes, 18% coronary artery disease, and 23% ischemic heart disease.
There is growing awareness of the long-term sequelae of cardiotoxic chemotherapy regimens but the challenge was how to take a preventive approach to the problem, Dr. Mehta said at the meeting sponsored by the World Heart Federation.
"People who have had a history of breast cancer ... often they’re only seeing oncologists to follow up on breast cancer recurrence so they are getting yearly mammograms and those things, but what they’ve missed is a little bit of hypertension that’s been there for years and then you get the accelerated atherosclerosis," Dr. Mehta said in an interview with Cardiology News.
The study’s authors suggest that cardio-oncology clinics could play an important role in implementing lifestyle modification and preventive interventions to reduce the risk of cardiac sequelae in cancer survivors.
In another presentation, American Heart Association President Mariell Jessup, Medical Director of the Penn Heart and Vascular Center, said early-onset cardiotoxicity occurs in a relatively small percent of patients – around 1%-2% – within the first year after chemotherapy treatment.
However late-onset cardiotoxicity was far more insidious as it affected a wide range of patients and the signs may not manifest until long after chemotherapy.
"We used to be taught that it came within the first year or so, and so when someone showed up 10 years or 15 years from treatment we discounted the fact that it was cardiotoxicity from anthracycline," Dr. Jessup told Cardiology News.
Dr. Jessup said an estimated 20%-30% of patients who had been treated with anthracyclines would likely show some kind of cardiac problems, but the addition of trastuzumab to breast cancer chemotherapy further increased the risk.
"They can present with heart failure ... but if they have radiotherapy on top of chemotherapy they are more susceptible to proximal coronary disease," she said.
"Then we’re seeing a group of patients that end up at cardiac transplant because of their heart failure, and they have very fragile bone marrows so they don’t tolerate immunosuppression as well, and they tend to be very anemic."
Dr. Jessup told the conference that more research was critical to understand the mechanisms of anthracycline cardiotoxicity, and to identify predictive markers of cardiac damage, but she stressed clinician awareness was also fundamental.
"We need to educate clinicians about not only the importance of these chemotherapeutic agents to the survival of patients following their cancer but how to be particularly attuned to the possibility that exposure many years before can lead to cardiac toxicity."
A member of the audience raised the question about treating patients with angiotensin-converting enzyme inhibitors at the same time as their chemotherapy to reduce the cardiac impact of the chemotherapy.
Dr. Jessup said while there were some intriguing data emerging about the concurrent use of ACE inhibitors and beta-blockers in patients treated with anthracyclines, there still remained questions about when the drugs should be used as they may not be well tolerated during chemotherapy.
Dr. Jessup had no disclosures. Dr. Mehta declared grant/research support from Gilead, and a coauthor declared grant/research support from Gilead and consultancies and honoraria from a range of organizations.
MELBOURNE – Specialist cardio-oncology clinics may be needed to address the long-term cardiac care of cancer patients treated with cardiotoxic chemotherapy regimens such as anthracyclines and trastuzumab, presenters told the World Congress of Cardiology 2014.
Dr. Puja K. Mehta, codirector of the cardio-oncology program at the Barbra Streisand Women’s Heart Center, presented poster data showing that 12% of women who attended the clinic over a 7-month period had a previous diagnosis of cancer, nearly half of which were cases of breast cancer.
The study of 892 patients seen at the center found that 92% of all breast cancer survivors had at least one cardiac risk factor – 55% had hypertension, 64% had hyperlipidemia, 14% had diabetes, 18% coronary artery disease, and 23% ischemic heart disease.
There is growing awareness of the long-term sequelae of cardiotoxic chemotherapy regimens but the challenge was how to take a preventive approach to the problem, Dr. Mehta said at the meeting sponsored by the World Heart Federation.
"People who have had a history of breast cancer ... often they’re only seeing oncologists to follow up on breast cancer recurrence so they are getting yearly mammograms and those things, but what they’ve missed is a little bit of hypertension that’s been there for years and then you get the accelerated atherosclerosis," Dr. Mehta said in an interview with Cardiology News.
The study’s authors suggest that cardio-oncology clinics could play an important role in implementing lifestyle modification and preventive interventions to reduce the risk of cardiac sequelae in cancer survivors.
In another presentation, American Heart Association President Mariell Jessup, Medical Director of the Penn Heart and Vascular Center, said early-onset cardiotoxicity occurs in a relatively small percent of patients – around 1%-2% – within the first year after chemotherapy treatment.
However late-onset cardiotoxicity was far more insidious as it affected a wide range of patients and the signs may not manifest until long after chemotherapy.
"We used to be taught that it came within the first year or so, and so when someone showed up 10 years or 15 years from treatment we discounted the fact that it was cardiotoxicity from anthracycline," Dr. Jessup told Cardiology News.
Dr. Jessup said an estimated 20%-30% of patients who had been treated with anthracyclines would likely show some kind of cardiac problems, but the addition of trastuzumab to breast cancer chemotherapy further increased the risk.
"They can present with heart failure ... but if they have radiotherapy on top of chemotherapy they are more susceptible to proximal coronary disease," she said.
"Then we’re seeing a group of patients that end up at cardiac transplant because of their heart failure, and they have very fragile bone marrows so they don’t tolerate immunosuppression as well, and they tend to be very anemic."
Dr. Jessup told the conference that more research was critical to understand the mechanisms of anthracycline cardiotoxicity, and to identify predictive markers of cardiac damage, but she stressed clinician awareness was also fundamental.
"We need to educate clinicians about not only the importance of these chemotherapeutic agents to the survival of patients following their cancer but how to be particularly attuned to the possibility that exposure many years before can lead to cardiac toxicity."
A member of the audience raised the question about treating patients with angiotensin-converting enzyme inhibitors at the same time as their chemotherapy to reduce the cardiac impact of the chemotherapy.
Dr. Jessup said while there were some intriguing data emerging about the concurrent use of ACE inhibitors and beta-blockers in patients treated with anthracyclines, there still remained questions about when the drugs should be used as they may not be well tolerated during chemotherapy.
Dr. Jessup had no disclosures. Dr. Mehta declared grant/research support from Gilead, and a coauthor declared grant/research support from Gilead and consultancies and honoraria from a range of organizations.
AT WCC 2014
Major finding: Specialist cardio-oncology clinics may be needed to address the long-term cardiac care of cancer patients treated with cardiotoxic chemotherapy regimens such as anthracyclines, say experts, with a study finding 12% of patients attending a specialist heart clinic had a previous diagnosis of cancer, particularly breast cancer.
Data source: Poster presentation of data from a retrospective chart review of 892 women attending a specialist heart clinic, and a review presentation.
Disclosures: Dr. Jessup had no disclosures while Dr. Mehta declared grant/research support from Gilead, and a coauthor declared grant/research support from Gilead and consultancies and honorarium from a range of organizations.
AHRQ report examines interventions for lowering readmissions in heart failure patients
Home-visiting programs, multidisciplinary heart failure clinics, and structured telephone support interventions "should receive the greatest consideration by systems or providers seeking to implement transitional care interventions" for people hospitalized for heart failure, according to a report issued May 27 by the Agency for Healthcare Research and Quality Effective Health Care Program.
But for these interventions to be effective, "you need a very mature infrastructure – communication infrastructure, information technology infrastructure, and systems-level infrastructure," Dr. Hiren Shah, medical director of the cardiac telemetry unit at Northwestern Memorial Hospital, Chicago, said in an interview about the implications of the report. "A lot of people point to cost and the fact that these interventions are expensive. But even if you have the ability to invest in these interventions, do you have the infrastructure that’s necessary to implement them?"
Further, the report lacks details on the cost-effectiveness of these interventions, a vital bit of information for hospitals looking to potentially implement them, Dr. Shah said. "It’s very important that not only do we know what may potentially work as an intervention, but we need to know whether that intervention or those interventions are cost effective. Within the scientific literature, we have very little information on the cost effectiveness of interventions."
The report’s conclusions are based on a systematic review and meta-analysis of the efficacy, comparative effectiveness, and harms of transitional care interventions aimed at reducing hospital readmissions and mortality for adults hospitalized with heart failure. The researchers, led by Dr. Cynthia Feltner of Duke University Medical Center in Durham, N.C., drew from 53 published articles on 47 randomized, controlled trials. Most studies compared a transitional care intervention with usual care, though the report notes that usual care was not consistently or well described.
In general, the trials included patients around age 70 years with moderate to severe heart failure. Most patients were prescribed an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker; the percentage of patients prescribed a beta-blocker varied widely across the study populations. Trial settings included academic medical centers, Veteran Affairs hospitals, and community hospitals.
Few trials reported 30-day readmission rates, as most measured outcomes over 3-6 months. Home-visiting programs and multidisciplinary heart failure clinic interventions reduced all-cause readmissions and mortality; structured telephone support reduced heart failure–specific readmissions and mortality.
Successful programs and interventions included heart failure education that emphasized self-care and recognition of symptoms and weight monitoring; patient education about medications and adherence to regimens as well as evidence-based therapies before and after discharge; face-to-face contact with visiting or clinic personnel within a week following discharge; streamlined mechanisms for contacting care delivery personnel outside of scheduled visits; and mechanisms for postdischarge medication adjustment.
"Separating out individual components from the overall categories [or "bundles"] of interventions that showed efficacy was not possible," the report notes.
None of the investigators have affiliations or financial involvements that constitute a conflict of interest with the material presented in the report.
Home-visiting programs, multidisciplinary heart failure clinics, and structured telephone support interventions "should receive the greatest consideration by systems or providers seeking to implement transitional care interventions" for people hospitalized for heart failure, according to a report issued May 27 by the Agency for Healthcare Research and Quality Effective Health Care Program.
But for these interventions to be effective, "you need a very mature infrastructure – communication infrastructure, information technology infrastructure, and systems-level infrastructure," Dr. Hiren Shah, medical director of the cardiac telemetry unit at Northwestern Memorial Hospital, Chicago, said in an interview about the implications of the report. "A lot of people point to cost and the fact that these interventions are expensive. But even if you have the ability to invest in these interventions, do you have the infrastructure that’s necessary to implement them?"
Further, the report lacks details on the cost-effectiveness of these interventions, a vital bit of information for hospitals looking to potentially implement them, Dr. Shah said. "It’s very important that not only do we know what may potentially work as an intervention, but we need to know whether that intervention or those interventions are cost effective. Within the scientific literature, we have very little information on the cost effectiveness of interventions."
The report’s conclusions are based on a systematic review and meta-analysis of the efficacy, comparative effectiveness, and harms of transitional care interventions aimed at reducing hospital readmissions and mortality for adults hospitalized with heart failure. The researchers, led by Dr. Cynthia Feltner of Duke University Medical Center in Durham, N.C., drew from 53 published articles on 47 randomized, controlled trials. Most studies compared a transitional care intervention with usual care, though the report notes that usual care was not consistently or well described.
In general, the trials included patients around age 70 years with moderate to severe heart failure. Most patients were prescribed an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker; the percentage of patients prescribed a beta-blocker varied widely across the study populations. Trial settings included academic medical centers, Veteran Affairs hospitals, and community hospitals.
Few trials reported 30-day readmission rates, as most measured outcomes over 3-6 months. Home-visiting programs and multidisciplinary heart failure clinic interventions reduced all-cause readmissions and mortality; structured telephone support reduced heart failure–specific readmissions and mortality.
Successful programs and interventions included heart failure education that emphasized self-care and recognition of symptoms and weight monitoring; patient education about medications and adherence to regimens as well as evidence-based therapies before and after discharge; face-to-face contact with visiting or clinic personnel within a week following discharge; streamlined mechanisms for contacting care delivery personnel outside of scheduled visits; and mechanisms for postdischarge medication adjustment.
"Separating out individual components from the overall categories [or "bundles"] of interventions that showed efficacy was not possible," the report notes.
None of the investigators have affiliations or financial involvements that constitute a conflict of interest with the material presented in the report.
Home-visiting programs, multidisciplinary heart failure clinics, and structured telephone support interventions "should receive the greatest consideration by systems or providers seeking to implement transitional care interventions" for people hospitalized for heart failure, according to a report issued May 27 by the Agency for Healthcare Research and Quality Effective Health Care Program.
But for these interventions to be effective, "you need a very mature infrastructure – communication infrastructure, information technology infrastructure, and systems-level infrastructure," Dr. Hiren Shah, medical director of the cardiac telemetry unit at Northwestern Memorial Hospital, Chicago, said in an interview about the implications of the report. "A lot of people point to cost and the fact that these interventions are expensive. But even if you have the ability to invest in these interventions, do you have the infrastructure that’s necessary to implement them?"
Further, the report lacks details on the cost-effectiveness of these interventions, a vital bit of information for hospitals looking to potentially implement them, Dr. Shah said. "It’s very important that not only do we know what may potentially work as an intervention, but we need to know whether that intervention or those interventions are cost effective. Within the scientific literature, we have very little information on the cost effectiveness of interventions."
The report’s conclusions are based on a systematic review and meta-analysis of the efficacy, comparative effectiveness, and harms of transitional care interventions aimed at reducing hospital readmissions and mortality for adults hospitalized with heart failure. The researchers, led by Dr. Cynthia Feltner of Duke University Medical Center in Durham, N.C., drew from 53 published articles on 47 randomized, controlled trials. Most studies compared a transitional care intervention with usual care, though the report notes that usual care was not consistently or well described.
In general, the trials included patients around age 70 years with moderate to severe heart failure. Most patients were prescribed an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker; the percentage of patients prescribed a beta-blocker varied widely across the study populations. Trial settings included academic medical centers, Veteran Affairs hospitals, and community hospitals.
Few trials reported 30-day readmission rates, as most measured outcomes over 3-6 months. Home-visiting programs and multidisciplinary heart failure clinic interventions reduced all-cause readmissions and mortality; structured telephone support reduced heart failure–specific readmissions and mortality.
Successful programs and interventions included heart failure education that emphasized self-care and recognition of symptoms and weight monitoring; patient education about medications and adherence to regimens as well as evidence-based therapies before and after discharge; face-to-face contact with visiting or clinic personnel within a week following discharge; streamlined mechanisms for contacting care delivery personnel outside of scheduled visits; and mechanisms for postdischarge medication adjustment.
"Separating out individual components from the overall categories [or "bundles"] of interventions that showed efficacy was not possible," the report notes.
None of the investigators have affiliations or financial involvements that constitute a conflict of interest with the material presented in the report.
Fatty heart may bring on diabetes
SAN FRANCISCO – A high volume of pericardial adipose tissue was associated with the prevalence of diabetes, independent of overall obesity, results from a long-term, diverse cohort study showed.
"Obesity is associated with an increased risk for cardiovascular disease and type 2 diabetes, and specific fat deposits may increase the risk more than others," Amy C. Alman, Ph.D., said at the annual scientific sessions of the American Diabetes Association.
The findings come from an analysis of year-25 exam data among 3,079 participants in CARDIA (Coronary Artery Risk Development in Young Adults), a longitudinal cohort study of the development of cardiovascular risk and disease that began in 1985 and was conducted at centers in Alabama, Minnesota, Illinois, and California.
Visceral adiposity "is metabolically active and more strongly associated with cardiovascular risk than subcutaneous adiposity. Ectopic adipose depots are metabolically active fat found in and around tissues and organs throughout the body, including the liver, muscles, and around the heart," explained Dr. Alman of the department of epidemiology and biostatistics at the University of South Florida, Tampa.
Pericardial adipose tissue (PAT) is an ectopic fat depot composed of epicardial adipose tissue deep to the pericardium and surrounding the coronary arteries and paracardial tissue, which is located along the surface of the parietal pericardium. She and her associates tested whether PAT was positively associated with prevalent diabetes at the year-25 exam of the CARDIA study.
Examinations including volume of PAT measures from chest CT scans were performed during 2010-2011, and the researchers used multivariable logistic regression to examine the relation between quartiles of PAT and diabetes. There were four PAT quartiles: less than 33.5 cm3 (quartile 1/referent volume), between 33.5 and less than 48.7 cm3 (quartile 2), between 48.7 and less than 71.7 cm3 (quartile 3), and greater than 71.7 cm3 (quartile 4).
The mean age of the 3,079 study participants was 51 years, 44% were male, and their average body mass index was 31 kg/m2. Of these, 419 had prevalent diabetes. The prevalence of diabetes was highest in the fourth quartile of PAT volume (46% vs. 12% in the first quartile, 18% in the second, and 24% in the third), Dr. Alman reported.
In a logistic regression model of PAT volume on diabetes status by obesity, only PAT volume in the fourth quartile was significantly associated with diabetes status (odds ratio, 2.47), adjusted for field center, gender, age, race, systolic blood pressure, total cholesterol, triglycerides, and treatment with blood pressure– and cholesterol-lowering medications. A similar association was observed among nonobese patients (OR, 3.78).
"Deposition of a higher proportion of metabolically active ectopic fat is associated with diabetes in both obese and nonobese individuals," Dr. Alman concluded.
The analysis was "a very well characterized multicenter cohort study with a diverse racial cohort of middle-aged men and women," she added. Limitations of the study, she said, included its cross-sectional design, the fact that PAT was measured at a single point in time, and that it did not account for other ectopic fat depots such as liver fat. A longitudinal analysis of the participants is planned.
Dr. Alman had no relevant financial conflicts to disclose.
SAN FRANCISCO – A high volume of pericardial adipose tissue was associated with the prevalence of diabetes, independent of overall obesity, results from a long-term, diverse cohort study showed.
"Obesity is associated with an increased risk for cardiovascular disease and type 2 diabetes, and specific fat deposits may increase the risk more than others," Amy C. Alman, Ph.D., said at the annual scientific sessions of the American Diabetes Association.
The findings come from an analysis of year-25 exam data among 3,079 participants in CARDIA (Coronary Artery Risk Development in Young Adults), a longitudinal cohort study of the development of cardiovascular risk and disease that began in 1985 and was conducted at centers in Alabama, Minnesota, Illinois, and California.
Visceral adiposity "is metabolically active and more strongly associated with cardiovascular risk than subcutaneous adiposity. Ectopic adipose depots are metabolically active fat found in and around tissues and organs throughout the body, including the liver, muscles, and around the heart," explained Dr. Alman of the department of epidemiology and biostatistics at the University of South Florida, Tampa.
Pericardial adipose tissue (PAT) is an ectopic fat depot composed of epicardial adipose tissue deep to the pericardium and surrounding the coronary arteries and paracardial tissue, which is located along the surface of the parietal pericardium. She and her associates tested whether PAT was positively associated with prevalent diabetes at the year-25 exam of the CARDIA study.
Examinations including volume of PAT measures from chest CT scans were performed during 2010-2011, and the researchers used multivariable logistic regression to examine the relation between quartiles of PAT and diabetes. There were four PAT quartiles: less than 33.5 cm3 (quartile 1/referent volume), between 33.5 and less than 48.7 cm3 (quartile 2), between 48.7 and less than 71.7 cm3 (quartile 3), and greater than 71.7 cm3 (quartile 4).
The mean age of the 3,079 study participants was 51 years, 44% were male, and their average body mass index was 31 kg/m2. Of these, 419 had prevalent diabetes. The prevalence of diabetes was highest in the fourth quartile of PAT volume (46% vs. 12% in the first quartile, 18% in the second, and 24% in the third), Dr. Alman reported.
In a logistic regression model of PAT volume on diabetes status by obesity, only PAT volume in the fourth quartile was significantly associated with diabetes status (odds ratio, 2.47), adjusted for field center, gender, age, race, systolic blood pressure, total cholesterol, triglycerides, and treatment with blood pressure– and cholesterol-lowering medications. A similar association was observed among nonobese patients (OR, 3.78).
"Deposition of a higher proportion of metabolically active ectopic fat is associated with diabetes in both obese and nonobese individuals," Dr. Alman concluded.
The analysis was "a very well characterized multicenter cohort study with a diverse racial cohort of middle-aged men and women," she added. Limitations of the study, she said, included its cross-sectional design, the fact that PAT was measured at a single point in time, and that it did not account for other ectopic fat depots such as liver fat. A longitudinal analysis of the participants is planned.
Dr. Alman had no relevant financial conflicts to disclose.
SAN FRANCISCO – A high volume of pericardial adipose tissue was associated with the prevalence of diabetes, independent of overall obesity, results from a long-term, diverse cohort study showed.
"Obesity is associated with an increased risk for cardiovascular disease and type 2 diabetes, and specific fat deposits may increase the risk more than others," Amy C. Alman, Ph.D., said at the annual scientific sessions of the American Diabetes Association.
The findings come from an analysis of year-25 exam data among 3,079 participants in CARDIA (Coronary Artery Risk Development in Young Adults), a longitudinal cohort study of the development of cardiovascular risk and disease that began in 1985 and was conducted at centers in Alabama, Minnesota, Illinois, and California.
Visceral adiposity "is metabolically active and more strongly associated with cardiovascular risk than subcutaneous adiposity. Ectopic adipose depots are metabolically active fat found in and around tissues and organs throughout the body, including the liver, muscles, and around the heart," explained Dr. Alman of the department of epidemiology and biostatistics at the University of South Florida, Tampa.
Pericardial adipose tissue (PAT) is an ectopic fat depot composed of epicardial adipose tissue deep to the pericardium and surrounding the coronary arteries and paracardial tissue, which is located along the surface of the parietal pericardium. She and her associates tested whether PAT was positively associated with prevalent diabetes at the year-25 exam of the CARDIA study.
Examinations including volume of PAT measures from chest CT scans were performed during 2010-2011, and the researchers used multivariable logistic regression to examine the relation between quartiles of PAT and diabetes. There were four PAT quartiles: less than 33.5 cm3 (quartile 1/referent volume), between 33.5 and less than 48.7 cm3 (quartile 2), between 48.7 and less than 71.7 cm3 (quartile 3), and greater than 71.7 cm3 (quartile 4).
The mean age of the 3,079 study participants was 51 years, 44% were male, and their average body mass index was 31 kg/m2. Of these, 419 had prevalent diabetes. The prevalence of diabetes was highest in the fourth quartile of PAT volume (46% vs. 12% in the first quartile, 18% in the second, and 24% in the third), Dr. Alman reported.
In a logistic regression model of PAT volume on diabetes status by obesity, only PAT volume in the fourth quartile was significantly associated with diabetes status (odds ratio, 2.47), adjusted for field center, gender, age, race, systolic blood pressure, total cholesterol, triglycerides, and treatment with blood pressure– and cholesterol-lowering medications. A similar association was observed among nonobese patients (OR, 3.78).
"Deposition of a higher proportion of metabolically active ectopic fat is associated with diabetes in both obese and nonobese individuals," Dr. Alman concluded.
The analysis was "a very well characterized multicenter cohort study with a diverse racial cohort of middle-aged men and women," she added. Limitations of the study, she said, included its cross-sectional design, the fact that PAT was measured at a single point in time, and that it did not account for other ectopic fat depots such as liver fat. A longitudinal analysis of the participants is planned.
Dr. Alman had no relevant financial conflicts to disclose.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Key clinical point: The greater the volume of pericardial adipose tissue, the greater the risk of diabetes.
Major finding: In a logistic regression model of PAT volume on diabetes status by obesity, only PAT volume in the fourth quartile was significantly associated with diabetes status (OR, 2.47).
Data source: An analysis of data from 3,079 participants at the year-25 exam of the CARDIA study.
Disclosures: Dr. Alman said that she had no relevant financial conflicts.
STEMI: Prereperfusion IV metoprolol shows long-term benefits
WASHINGTON – Early administration of intravenous metoprolol prior to primary percutaneous coronary intervention in patients with Killip class I or II anterior ST-elevation myocardial infarction reaped impressive long-term benefits in updated results from the Spanish METOCARD-CNIC trial.
At 6 months post infarct, the mean left ventricular ejection fraction (LVEF) was significantly higher in the early IV beta-blocker group than in controls. Also, the prevalence of a severely depressed LVEF of 35% or less was significantly lower, as was the proportion of patients having a class I indication for an implantable cardioverter-defibrillator, Dr. Borja Ibanez reported at the annual meeting of the American College of Cardiology.
METOCARD-CNIC
The primary study endpoint was infarct size as measured by magnetic resonance imaging 7 days post STEMI. As previously reported, infarct size was 20% smaller in the IV metoprolol recipients (Circulation 2013;128:1495-1503). This finding was encouraging, Dr. Ibanez observed, because infarct size is a major determinant of long-term morbidity and mortality. And while primary PCI for STEMI results in very low acute mortality, there is a high residual risk of subsequent heart failure and death. So the search is on for treatments that reduce infarct size. And prior to METOCARD-CNIC there had been no randomized controlled trials of early IV beta-blocker therapy during the primary PCI era.
At ACC 14, Dr. Ibanez presented prespecified secondary endpoints based upon outcomes at 6 months (see chart) and 2 years post STEMI.
At a median follow-up of 2 years, the composite endpoint comprised of death, reinfarction, hospital admission for heart failure, or malignant arrhythmia had occurred in 10.8% of the IV metoprolol group, compared with 18.3% of controls. This translated to an adjusted 45% relative risk reduction which didn’t quite reach statistical significance, but then again the trial wasn’t of sufficient size to be powered to evaluate clinical endpoints.
Of note, the heart failure hospital admission component of the composite endpoint occurred in 2.2% of the IV beta-blocker group, compared with 6.9% of controls, for a 68% relative risk reduction that was statistically significant. The curves began to split at 12 months and continued to diverge through 24 months, according to Dr. Ibanez.
Based upon the encouraging findings of METOCARD-CNIC, planning is underway for a large randomized trial powered to evaluate hard clinical endpoints. It will be called the MOVE ON! trial, the cardiologist added.
METOCARD-CNIC was funded by the Carlos III National Center for Cardiovascular Investigations and the Spanish Ministry of Health and Social Policy. Dr. Ibanez reported having no financial conflicts of interest.
WASHINGTON – Early administration of intravenous metoprolol prior to primary percutaneous coronary intervention in patients with Killip class I or II anterior ST-elevation myocardial infarction reaped impressive long-term benefits in updated results from the Spanish METOCARD-CNIC trial.
At 6 months post infarct, the mean left ventricular ejection fraction (LVEF) was significantly higher in the early IV beta-blocker group than in controls. Also, the prevalence of a severely depressed LVEF of 35% or less was significantly lower, as was the proportion of patients having a class I indication for an implantable cardioverter-defibrillator, Dr. Borja Ibanez reported at the annual meeting of the American College of Cardiology.

METOCARD-CNIC
The primary study endpoint was infarct size as measured by magnetic resonance imaging 7 days post STEMI. As previously reported, infarct size was 20% smaller in the IV metoprolol recipients (Circulation 2013;128:1495-1503). This finding was encouraging, Dr. Ibanez observed, because infarct size is a major determinant of long-term morbidity and mortality. And while primary PCI for STEMI results in very low acute mortality, there is a high residual risk of subsequent heart failure and death. So the search is on for treatments that reduce infarct size. And prior to METOCARD-CNIC there had been no randomized controlled trials of early IV beta-blocker therapy during the primary PCI era.
At ACC 14, Dr. Ibanez presented prespecified secondary endpoints based upon outcomes at 6 months (see chart) and 2 years post STEMI.
At a median follow-up of 2 years, the composite endpoint comprised of death, reinfarction, hospital admission for heart failure, or malignant arrhythmia had occurred in 10.8% of the IV metoprolol group, compared with 18.3% of controls. This translated to an adjusted 45% relative risk reduction which didn’t quite reach statistical significance, but then again the trial wasn’t of sufficient size to be powered to evaluate clinical endpoints.
Of note, the heart failure hospital admission component of the composite endpoint occurred in 2.2% of the IV beta-blocker group, compared with 6.9% of controls, for a 68% relative risk reduction that was statistically significant. The curves began to split at 12 months and continued to diverge through 24 months, according to Dr. Ibanez.
Based upon the encouraging findings of METOCARD-CNIC, planning is underway for a large randomized trial powered to evaluate hard clinical endpoints. It will be called the MOVE ON! trial, the cardiologist added.
METOCARD-CNIC was funded by the Carlos III National Center for Cardiovascular Investigations and the Spanish Ministry of Health and Social Policy. Dr. Ibanez reported having no financial conflicts of interest.
WASHINGTON – Early administration of intravenous metoprolol prior to primary percutaneous coronary intervention in patients with Killip class I or II anterior ST-elevation myocardial infarction reaped impressive long-term benefits in updated results from the Spanish METOCARD-CNIC trial.
At 6 months post infarct, the mean left ventricular ejection fraction (LVEF) was significantly higher in the early IV beta-blocker group than in controls. Also, the prevalence of a severely depressed LVEF of 35% or less was significantly lower, as was the proportion of patients having a class I indication for an implantable cardioverter-defibrillator, Dr. Borja Ibanez reported at the annual meeting of the American College of Cardiology.

METOCARD-CNIC
The primary study endpoint was infarct size as measured by magnetic resonance imaging 7 days post STEMI. As previously reported, infarct size was 20% smaller in the IV metoprolol recipients (Circulation 2013;128:1495-1503). This finding was encouraging, Dr. Ibanez observed, because infarct size is a major determinant of long-term morbidity and mortality. And while primary PCI for STEMI results in very low acute mortality, there is a high residual risk of subsequent heart failure and death. So the search is on for treatments that reduce infarct size. And prior to METOCARD-CNIC there had been no randomized controlled trials of early IV beta-blocker therapy during the primary PCI era.
At ACC 14, Dr. Ibanez presented prespecified secondary endpoints based upon outcomes at 6 months (see chart) and 2 years post STEMI.
At a median follow-up of 2 years, the composite endpoint comprised of death, reinfarction, hospital admission for heart failure, or malignant arrhythmia had occurred in 10.8% of the IV metoprolol group, compared with 18.3% of controls. This translated to an adjusted 45% relative risk reduction which didn’t quite reach statistical significance, but then again the trial wasn’t of sufficient size to be powered to evaluate clinical endpoints.
Of note, the heart failure hospital admission component of the composite endpoint occurred in 2.2% of the IV beta-blocker group, compared with 6.9% of controls, for a 68% relative risk reduction that was statistically significant. The curves began to split at 12 months and continued to diverge through 24 months, according to Dr. Ibanez.
Based upon the encouraging findings of METOCARD-CNIC, planning is underway for a large randomized trial powered to evaluate hard clinical endpoints. It will be called the MOVE ON! trial, the cardiologist added.
METOCARD-CNIC was funded by the Carlos III National Center for Cardiovascular Investigations and the Spanish Ministry of Health and Social Policy. Dr. Ibanez reported having no financial conflicts of interest.
AT ACC 14
Key clinical point: Prereperfusion IV metoprolol may reduce heart failure readmissions.
Major finding: Patients with anterior ST-elevation MI who received intravenous metoprolol prior to primary PCI had a significantly greater left ventricular ejection fraction at 6 months follow-up than those who didn’t. They also were 68% less likely to be hospitalized for heart failure during 2 years of follow-up.
Data source: A six-center prospective trial in which 270 Spanish patients with a first anterior STEMI were randomized to have administration of IV metoprolol or not while being transported for primary PCI.
Disclosures: The METOCARD-CNIC trial was sponsored by the Spanish Ministry of Health and Social Policy and the Carlos III National Center for Cardiovascular Investigations. The presenter reported having no financial conflicts.