User login
Post–COVID-19 cardiac involvement in college athletes much rarer than thought
In a multicenter study conducted during September-December 2020, only 0.7% of 3,018 collegiate athletes who tested positive for SARS-CoV-2 infection were found to have definite, probable, or possible infection-related cardiac involvement.
None experienced an adverse cardiac event and only five (0.2%) required hospitalization for noncardiac complications of COVID-19.
“The take-home message is that cardiac involvement does not happen as much as we had initially feared. It’s in the range of 0.5% to 3%, depending on how you define cardiac involvement, which is not nothing, but it’s not the 30% or 50% that some early studies hinted at,” said Kimberly G. Harmon, MD, of the University of Washington, Seattle.
Dr. Harmon, along with Jeffrey A. Drezner, MD, also from UW, and Aaron L. Baggish, MD, of Massachusetts General Hospital, Boston, were co–primary investigators of the Outcomes Registry for Cardiac Conditions in Athletes (ORCCA) study. The group’s findings were published April 17 in Circulation.
Nearly 20,000 athletes tested
The researchers prospectively tested 19,378 athletes for SARS-CoV-2 infection from 42 U.S. colleges and universities during the study period. A total of 3,018 (16%; mean age, 20 years; 32% female) tested positive and underwent cardiac evaluation.
“We didn’t prescribe what the schools had to do in terms of cardiac evaluation, but most of these colleges are well resourced, and about 74% of athletes were evaluated using the triad testing strategy of 12-lead electrocardiography, cardiac troponin, and transthoracic echocardiography [TEE], with cardiac magnetic resonance [CMR ]when indicated,” explained Dr. Harmon. Only 198 athletes underwent primary screening with CMR.
Athletes were often tested multiple times for SARS-CoV-2 infection by participating institutions and were included in this study if they had any positive test and underwent postinfection cardiac screening.
The cohort includes athletes representing 26 distinct sporting disciplines, including American-style football (36%), basketball (9%), and cross country/track and field (8%). Most were asymptomatic or had only mild COVID-19 symptoms (33% and 29%, respectively).
‘Exercise appears to be protective’
Abnormal findings suggestive of SARS-CoV-2 cardiac involvement were detected by ECG in 0.7% of athletes (21 of 2,999), cardiac troponin elevation in 0.9% (24/2,719), and abnormal TTE findings in 0.9% (24/2,556).
The odds of having cardiac involvement was 3.1 times higher in athletes with cardiopulmonary symptoms.
“One thing we’ve seen in the literature and in this cohort, is that exercise appears to be protective to some extent from COVID-19. We had a lot of cases, but in the whole cohort, only five athletes were hospitalized with COVID and those were for noncardiac reasons,” said Dr. Harmon.
During a median clinical surveillance of 113 days, there was one (0.03%) adverse cardiac event likely unrelated to SARS-CoV-2 infection.
The diagnostic yield for probable or definite cardiac involvement was 6.7 times higher for a CMR obtained for clinical reasons (10.1%) versus a primary screening CMR (1.5%).
“This is data we desperately needed. Small, single-center studies early in the pandemic had indicated a higher prevalence of cardiac involvement, which led us to be very conservative about return-to-play in the early days,” said Jeffrey Lander, MD, who was not involved in the study.
The study is complementary, he noted, to one published in March that looked at professional athletes post–COVID-19 and also found cardiac pathology in fewer than 1%. The mean age in that study was 25 years.
“They saw a similarly low rate of cardiac involvement in professional athletes, and together with this study, it gives us new information that is also reassuring,” added Dr. Lander, codirector of sports cardiology at Saint Barnabas Medical Center in Livingston, N.J., an RWJBarnabas Health facility, and team cardiologist for Seton Hall University in South Orange, N.J.
Limit CMR to symptomatic athletes
“I think this data can be extended beyond the college athlete. And it’s fair to say to high school athletes and young recreational athletes who have had asymptomatic or mild infection, you probably don’t need further workup if you’re feeling fine,” suggested Dr. Harmon.
“For those with moderate or severe illness, then the triple screen protocol is a good idea, particularly if they are having any symptoms,” she added.
Dr. Lander agrees that athletes should be screened by appropriate providers before returning to sports, but that CMR should not be used routinely for return-to-play screening.
“We’ve never taken a group of, say, 1,000 college athletes who just recovered from the flu and done cardiac MRIs on them, so it’s a bit like opening Pandora’s box when it’s used too liberally. It’s difficult to assess if the findings are secondary to COVID infection or from something entirely unrelated,” he noted.
ORCCA is a collaboration of the American Heart Association and the American Medical Society for Sports Medicine to track COVID-19 cases among National Collegiate Athletic Association (NCAA) athletes. The current study was supported by a grant from the American Medical Society for Sports Medicine.
In a multicenter study conducted during September-December 2020, only 0.7% of 3,018 collegiate athletes who tested positive for SARS-CoV-2 infection were found to have definite, probable, or possible infection-related cardiac involvement.
None experienced an adverse cardiac event and only five (0.2%) required hospitalization for noncardiac complications of COVID-19.
“The take-home message is that cardiac involvement does not happen as much as we had initially feared. It’s in the range of 0.5% to 3%, depending on how you define cardiac involvement, which is not nothing, but it’s not the 30% or 50% that some early studies hinted at,” said Kimberly G. Harmon, MD, of the University of Washington, Seattle.
Dr. Harmon, along with Jeffrey A. Drezner, MD, also from UW, and Aaron L. Baggish, MD, of Massachusetts General Hospital, Boston, were co–primary investigators of the Outcomes Registry for Cardiac Conditions in Athletes (ORCCA) study. The group’s findings were published April 17 in Circulation.
Nearly 20,000 athletes tested
The researchers prospectively tested 19,378 athletes for SARS-CoV-2 infection from 42 U.S. colleges and universities during the study period. A total of 3,018 (16%; mean age, 20 years; 32% female) tested positive and underwent cardiac evaluation.
“We didn’t prescribe what the schools had to do in terms of cardiac evaluation, but most of these colleges are well resourced, and about 74% of athletes were evaluated using the triad testing strategy of 12-lead electrocardiography, cardiac troponin, and transthoracic echocardiography [TEE], with cardiac magnetic resonance [CMR ]when indicated,” explained Dr. Harmon. Only 198 athletes underwent primary screening with CMR.
Athletes were often tested multiple times for SARS-CoV-2 infection by participating institutions and were included in this study if they had any positive test and underwent postinfection cardiac screening.
The cohort includes athletes representing 26 distinct sporting disciplines, including American-style football (36%), basketball (9%), and cross country/track and field (8%). Most were asymptomatic or had only mild COVID-19 symptoms (33% and 29%, respectively).
‘Exercise appears to be protective’
Abnormal findings suggestive of SARS-CoV-2 cardiac involvement were detected by ECG in 0.7% of athletes (21 of 2,999), cardiac troponin elevation in 0.9% (24/2,719), and abnormal TTE findings in 0.9% (24/2,556).
The odds of having cardiac involvement was 3.1 times higher in athletes with cardiopulmonary symptoms.
“One thing we’ve seen in the literature and in this cohort, is that exercise appears to be protective to some extent from COVID-19. We had a lot of cases, but in the whole cohort, only five athletes were hospitalized with COVID and those were for noncardiac reasons,” said Dr. Harmon.
During a median clinical surveillance of 113 days, there was one (0.03%) adverse cardiac event likely unrelated to SARS-CoV-2 infection.
The diagnostic yield for probable or definite cardiac involvement was 6.7 times higher for a CMR obtained for clinical reasons (10.1%) versus a primary screening CMR (1.5%).
“This is data we desperately needed. Small, single-center studies early in the pandemic had indicated a higher prevalence of cardiac involvement, which led us to be very conservative about return-to-play in the early days,” said Jeffrey Lander, MD, who was not involved in the study.
The study is complementary, he noted, to one published in March that looked at professional athletes post–COVID-19 and also found cardiac pathology in fewer than 1%. The mean age in that study was 25 years.
“They saw a similarly low rate of cardiac involvement in professional athletes, and together with this study, it gives us new information that is also reassuring,” added Dr. Lander, codirector of sports cardiology at Saint Barnabas Medical Center in Livingston, N.J., an RWJBarnabas Health facility, and team cardiologist for Seton Hall University in South Orange, N.J.
Limit CMR to symptomatic athletes
“I think this data can be extended beyond the college athlete. And it’s fair to say to high school athletes and young recreational athletes who have had asymptomatic or mild infection, you probably don’t need further workup if you’re feeling fine,” suggested Dr. Harmon.
“For those with moderate or severe illness, then the triple screen protocol is a good idea, particularly if they are having any symptoms,” she added.
Dr. Lander agrees that athletes should be screened by appropriate providers before returning to sports, but that CMR should not be used routinely for return-to-play screening.
“We’ve never taken a group of, say, 1,000 college athletes who just recovered from the flu and done cardiac MRIs on them, so it’s a bit like opening Pandora’s box when it’s used too liberally. It’s difficult to assess if the findings are secondary to COVID infection or from something entirely unrelated,” he noted.
ORCCA is a collaboration of the American Heart Association and the American Medical Society for Sports Medicine to track COVID-19 cases among National Collegiate Athletic Association (NCAA) athletes. The current study was supported by a grant from the American Medical Society for Sports Medicine.
In a multicenter study conducted during September-December 2020, only 0.7% of 3,018 collegiate athletes who tested positive for SARS-CoV-2 infection were found to have definite, probable, or possible infection-related cardiac involvement.
None experienced an adverse cardiac event and only five (0.2%) required hospitalization for noncardiac complications of COVID-19.
“The take-home message is that cardiac involvement does not happen as much as we had initially feared. It’s in the range of 0.5% to 3%, depending on how you define cardiac involvement, which is not nothing, but it’s not the 30% or 50% that some early studies hinted at,” said Kimberly G. Harmon, MD, of the University of Washington, Seattle.
Dr. Harmon, along with Jeffrey A. Drezner, MD, also from UW, and Aaron L. Baggish, MD, of Massachusetts General Hospital, Boston, were co–primary investigators of the Outcomes Registry for Cardiac Conditions in Athletes (ORCCA) study. The group’s findings were published April 17 in Circulation.
Nearly 20,000 athletes tested
The researchers prospectively tested 19,378 athletes for SARS-CoV-2 infection from 42 U.S. colleges and universities during the study period. A total of 3,018 (16%; mean age, 20 years; 32% female) tested positive and underwent cardiac evaluation.
“We didn’t prescribe what the schools had to do in terms of cardiac evaluation, but most of these colleges are well resourced, and about 74% of athletes were evaluated using the triad testing strategy of 12-lead electrocardiography, cardiac troponin, and transthoracic echocardiography [TEE], with cardiac magnetic resonance [CMR ]when indicated,” explained Dr. Harmon. Only 198 athletes underwent primary screening with CMR.
Athletes were often tested multiple times for SARS-CoV-2 infection by participating institutions and were included in this study if they had any positive test and underwent postinfection cardiac screening.
The cohort includes athletes representing 26 distinct sporting disciplines, including American-style football (36%), basketball (9%), and cross country/track and field (8%). Most were asymptomatic or had only mild COVID-19 symptoms (33% and 29%, respectively).
‘Exercise appears to be protective’
Abnormal findings suggestive of SARS-CoV-2 cardiac involvement were detected by ECG in 0.7% of athletes (21 of 2,999), cardiac troponin elevation in 0.9% (24/2,719), and abnormal TTE findings in 0.9% (24/2,556).
The odds of having cardiac involvement was 3.1 times higher in athletes with cardiopulmonary symptoms.
“One thing we’ve seen in the literature and in this cohort, is that exercise appears to be protective to some extent from COVID-19. We had a lot of cases, but in the whole cohort, only five athletes were hospitalized with COVID and those were for noncardiac reasons,” said Dr. Harmon.
During a median clinical surveillance of 113 days, there was one (0.03%) adverse cardiac event likely unrelated to SARS-CoV-2 infection.
The diagnostic yield for probable or definite cardiac involvement was 6.7 times higher for a CMR obtained for clinical reasons (10.1%) versus a primary screening CMR (1.5%).
“This is data we desperately needed. Small, single-center studies early in the pandemic had indicated a higher prevalence of cardiac involvement, which led us to be very conservative about return-to-play in the early days,” said Jeffrey Lander, MD, who was not involved in the study.
The study is complementary, he noted, to one published in March that looked at professional athletes post–COVID-19 and also found cardiac pathology in fewer than 1%. The mean age in that study was 25 years.
“They saw a similarly low rate of cardiac involvement in professional athletes, and together with this study, it gives us new information that is also reassuring,” added Dr. Lander, codirector of sports cardiology at Saint Barnabas Medical Center in Livingston, N.J., an RWJBarnabas Health facility, and team cardiologist for Seton Hall University in South Orange, N.J.
Limit CMR to symptomatic athletes
“I think this data can be extended beyond the college athlete. And it’s fair to say to high school athletes and young recreational athletes who have had asymptomatic or mild infection, you probably don’t need further workup if you’re feeling fine,” suggested Dr. Harmon.
“For those with moderate or severe illness, then the triple screen protocol is a good idea, particularly if they are having any symptoms,” she added.
Dr. Lander agrees that athletes should be screened by appropriate providers before returning to sports, but that CMR should not be used routinely for return-to-play screening.
“We’ve never taken a group of, say, 1,000 college athletes who just recovered from the flu and done cardiac MRIs on them, so it’s a bit like opening Pandora’s box when it’s used too liberally. It’s difficult to assess if the findings are secondary to COVID infection or from something entirely unrelated,” he noted.
ORCCA is a collaboration of the American Heart Association and the American Medical Society for Sports Medicine to track COVID-19 cases among National Collegiate Athletic Association (NCAA) athletes. The current study was supported by a grant from the American Medical Society for Sports Medicine.
FROM CIRCULATION
Low concordance between troponin assays for ACS
Clinicians should be aware that the discordance between high-sensitivity cardiac troponin (hs-cTn) assays is significant enough that management recommendations may change, for example, for a patient assessed for suspected acute coronary syndrome (ACS) in one hospital and transferred to another that uses a different assay, according to a team of international researchers.
When hs-cTn concentrations were measured using the three Food and Drug Administration–approved assays, only 37.4% (384 of 1,027 samples) of blood samples were classified into the same analytical benchmark category.
“We didn’t expect such low concordance, to be honest, but I have to stress that this first assessment used just one-time blood testing and serial testing is what is more commonly recommended now,” said Júlia Karády, MD, from Massachusetts General Hospital and Harvard Medical School, both in Boston.
To see if concordance improved with serial testing, the researchers looked at the 242 patients for whom serial samples were available and saw concordance of management recommendations across assays rise to 74.8%.
“We tested the 0/2-hour algorithm and found that the overall agreement almost doubled, so I think that a very important message from our study is that serial testing improves the agreement between the assays in terms of clinical management and patient stratification,” said Dr. Karády.
Dr. Karády and colleagues published their findings in the Journal of the American College of Cardiology.
The researchers tested three assays referred to clinically as high-sensitivity assays: Elecsys 2010 platform (Roche Diagnostics); ARCHITECT i2000SR (Abbott Diagnostics); and hsVista (Siemens Diagnostics). All three have received FDA approval, starting with Elecsys in 2017.
The proportion of patients with similar management recommendations differed between the assays for both “rule-out” (87.2%, 73.1%, and 78.5% for Roche, Abbott, and Siemens, respectively) and “observe” (9.5%, 24%, and 17.8%; both P < .001). For the purposes of “rule-in,” no difference was noted (3.3%, 2.9%, and 3.7%).
“It’s important to note that this was a highly selected population of patients with an intermediate likelihood for ACS, not an all-comer population. This group comprises about 20% of the [emergency department] population and actually is the group we struggle with the most, which is hardest to diagnose because it excludes the very low– and very high–risk patients,” said Dr. Karády.
The patients included in this study all had suspected ACS and were enrolled in the ROMICAT-I and II trials.
Among 1,027 samples from 624 patients (mean age, 52.8 years; 39.4% women), samples were classified as below the limit of detection (LOD) in 56.3%, 10.4%, and 41.2% (P < .001) by Roche, Abbott, and Siemens, respectively.
The proportion of sample with a troponin measurement between LOD to the 99th percentile also differed significantly between the assays at 36.5%, 83.5%, and 52.6%, respectively (P < .001).
Only the proportion classified greater than 99th percentile did not differ (7.2%, 6.0%, and 6.2%; P = .114).
When the researchers looked at sex-specific difference, no differences were seen in rule-in numbers for men, but significant differences were seen for women.
“One possible explanation for this could be differences in the representation of men and women in the various reference populations used to develop the 99th percentile values for these assays,” suggested Dr. Karády.
They estimate around 30%-40% of U.S. centers are currently using high-sensitivity troponin assays and this number is “rapidly rising.”
The diagnostic algorithms developed for use with high-sensitivity assays, such as the 0/2-h algorithm, acknowledge differences in performance characteristics and recommend that assay-specific cut points be used for clinical decision-making rather than relying on generally applicable thresholds.
Joseph S. Alpert, MD, University of Arizona, Tucson, and coauthors of an accompanying editorial said the take-home message here is caveat emptor.
“First, ‘let the buyer (i.e., the clinician) beware’ when patients are transferred from one hospital to another, where different hs-cTn assays may be used,” they wrote. This is particularly true in women and in those with troponin levels in the “observe (gray zone)” clinical management recommendation.
Dr. Karády has received grant support from the Fulbright Visiting Researcher Grant and the Rosztoczy Foundation. One of the coauthors of the editorial comment consults or has consulted for most of the major diagnostic companies, including the manufacturers of the three assays tested in this study. Dr. Alpert disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians should be aware that the discordance between high-sensitivity cardiac troponin (hs-cTn) assays is significant enough that management recommendations may change, for example, for a patient assessed for suspected acute coronary syndrome (ACS) in one hospital and transferred to another that uses a different assay, according to a team of international researchers.
When hs-cTn concentrations were measured using the three Food and Drug Administration–approved assays, only 37.4% (384 of 1,027 samples) of blood samples were classified into the same analytical benchmark category.
“We didn’t expect such low concordance, to be honest, but I have to stress that this first assessment used just one-time blood testing and serial testing is what is more commonly recommended now,” said Júlia Karády, MD, from Massachusetts General Hospital and Harvard Medical School, both in Boston.
To see if concordance improved with serial testing, the researchers looked at the 242 patients for whom serial samples were available and saw concordance of management recommendations across assays rise to 74.8%.
“We tested the 0/2-hour algorithm and found that the overall agreement almost doubled, so I think that a very important message from our study is that serial testing improves the agreement between the assays in terms of clinical management and patient stratification,” said Dr. Karády.
Dr. Karády and colleagues published their findings in the Journal of the American College of Cardiology.
The researchers tested three assays referred to clinically as high-sensitivity assays: Elecsys 2010 platform (Roche Diagnostics); ARCHITECT i2000SR (Abbott Diagnostics); and hsVista (Siemens Diagnostics). All three have received FDA approval, starting with Elecsys in 2017.
The proportion of patients with similar management recommendations differed between the assays for both “rule-out” (87.2%, 73.1%, and 78.5% for Roche, Abbott, and Siemens, respectively) and “observe” (9.5%, 24%, and 17.8%; both P < .001). For the purposes of “rule-in,” no difference was noted (3.3%, 2.9%, and 3.7%).
“It’s important to note that this was a highly selected population of patients with an intermediate likelihood for ACS, not an all-comer population. This group comprises about 20% of the [emergency department] population and actually is the group we struggle with the most, which is hardest to diagnose because it excludes the very low– and very high–risk patients,” said Dr. Karády.
The patients included in this study all had suspected ACS and were enrolled in the ROMICAT-I and II trials.
Among 1,027 samples from 624 patients (mean age, 52.8 years; 39.4% women), samples were classified as below the limit of detection (LOD) in 56.3%, 10.4%, and 41.2% (P < .001) by Roche, Abbott, and Siemens, respectively.
The proportion of sample with a troponin measurement between LOD to the 99th percentile also differed significantly between the assays at 36.5%, 83.5%, and 52.6%, respectively (P < .001).
Only the proportion classified greater than 99th percentile did not differ (7.2%, 6.0%, and 6.2%; P = .114).
When the researchers looked at sex-specific difference, no differences were seen in rule-in numbers for men, but significant differences were seen for women.
“One possible explanation for this could be differences in the representation of men and women in the various reference populations used to develop the 99th percentile values for these assays,” suggested Dr. Karády.
They estimate around 30%-40% of U.S. centers are currently using high-sensitivity troponin assays and this number is “rapidly rising.”
The diagnostic algorithms developed for use with high-sensitivity assays, such as the 0/2-h algorithm, acknowledge differences in performance characteristics and recommend that assay-specific cut points be used for clinical decision-making rather than relying on generally applicable thresholds.
Joseph S. Alpert, MD, University of Arizona, Tucson, and coauthors of an accompanying editorial said the take-home message here is caveat emptor.
“First, ‘let the buyer (i.e., the clinician) beware’ when patients are transferred from one hospital to another, where different hs-cTn assays may be used,” they wrote. This is particularly true in women and in those with troponin levels in the “observe (gray zone)” clinical management recommendation.
Dr. Karády has received grant support from the Fulbright Visiting Researcher Grant and the Rosztoczy Foundation. One of the coauthors of the editorial comment consults or has consulted for most of the major diagnostic companies, including the manufacturers of the three assays tested in this study. Dr. Alpert disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians should be aware that the discordance between high-sensitivity cardiac troponin (hs-cTn) assays is significant enough that management recommendations may change, for example, for a patient assessed for suspected acute coronary syndrome (ACS) in one hospital and transferred to another that uses a different assay, according to a team of international researchers.
When hs-cTn concentrations were measured using the three Food and Drug Administration–approved assays, only 37.4% (384 of 1,027 samples) of blood samples were classified into the same analytical benchmark category.
“We didn’t expect such low concordance, to be honest, but I have to stress that this first assessment used just one-time blood testing and serial testing is what is more commonly recommended now,” said Júlia Karády, MD, from Massachusetts General Hospital and Harvard Medical School, both in Boston.
To see if concordance improved with serial testing, the researchers looked at the 242 patients for whom serial samples were available and saw concordance of management recommendations across assays rise to 74.8%.
“We tested the 0/2-hour algorithm and found that the overall agreement almost doubled, so I think that a very important message from our study is that serial testing improves the agreement between the assays in terms of clinical management and patient stratification,” said Dr. Karády.
Dr. Karády and colleagues published their findings in the Journal of the American College of Cardiology.
The researchers tested three assays referred to clinically as high-sensitivity assays: Elecsys 2010 platform (Roche Diagnostics); ARCHITECT i2000SR (Abbott Diagnostics); and hsVista (Siemens Diagnostics). All three have received FDA approval, starting with Elecsys in 2017.
The proportion of patients with similar management recommendations differed between the assays for both “rule-out” (87.2%, 73.1%, and 78.5% for Roche, Abbott, and Siemens, respectively) and “observe” (9.5%, 24%, and 17.8%; both P < .001). For the purposes of “rule-in,” no difference was noted (3.3%, 2.9%, and 3.7%).
“It’s important to note that this was a highly selected population of patients with an intermediate likelihood for ACS, not an all-comer population. This group comprises about 20% of the [emergency department] population and actually is the group we struggle with the most, which is hardest to diagnose because it excludes the very low– and very high–risk patients,” said Dr. Karády.
The patients included in this study all had suspected ACS and were enrolled in the ROMICAT-I and II trials.
Among 1,027 samples from 624 patients (mean age, 52.8 years; 39.4% women), samples were classified as below the limit of detection (LOD) in 56.3%, 10.4%, and 41.2% (P < .001) by Roche, Abbott, and Siemens, respectively.
The proportion of sample with a troponin measurement between LOD to the 99th percentile also differed significantly between the assays at 36.5%, 83.5%, and 52.6%, respectively (P < .001).
Only the proportion classified greater than 99th percentile did not differ (7.2%, 6.0%, and 6.2%; P = .114).
When the researchers looked at sex-specific difference, no differences were seen in rule-in numbers for men, but significant differences were seen for women.
“One possible explanation for this could be differences in the representation of men and women in the various reference populations used to develop the 99th percentile values for these assays,” suggested Dr. Karády.
They estimate around 30%-40% of U.S. centers are currently using high-sensitivity troponin assays and this number is “rapidly rising.”
The diagnostic algorithms developed for use with high-sensitivity assays, such as the 0/2-h algorithm, acknowledge differences in performance characteristics and recommend that assay-specific cut points be used for clinical decision-making rather than relying on generally applicable thresholds.
Joseph S. Alpert, MD, University of Arizona, Tucson, and coauthors of an accompanying editorial said the take-home message here is caveat emptor.
“First, ‘let the buyer (i.e., the clinician) beware’ when patients are transferred from one hospital to another, where different hs-cTn assays may be used,” they wrote. This is particularly true in women and in those with troponin levels in the “observe (gray zone)” clinical management recommendation.
Dr. Karády has received grant support from the Fulbright Visiting Researcher Grant and the Rosztoczy Foundation. One of the coauthors of the editorial comment consults or has consulted for most of the major diagnostic companies, including the manufacturers of the three assays tested in this study. Dr. Alpert disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Heart failure redefined with new classifications, staging
The terminology and classification scheme for heart failure (HF) is changing in ways that experts hope will directly impact patient outcomes.
In a new consensus statement, a multisociety group of experts proposed a new universal definition of heart failure and made substantial revisions to the way in which the disease is staged and classified.
The authors of the statement, led by writing committee chair and immediate past president of the Heart Failure Society of America Biykem Bozkurt, MD, PhD, hope their efforts will go far to improve standardization of terminology, but more importantly will facilitate better management of the disease in ways that keep pace with current knowledge and advances in the field.
“There is a great need for reframing and standardizing the terminology across societies and different stakeholders, and importantly for patients because a lot of the terminology we were using was understood by academicians, but were not being translated in important ways to ensure patients are being appropriately treated,” said Dr. Bozkurt, of Baylor College of Medicine, Houston.
The consensus statement was a group effort led by the HFSA, the Heart Failure Association of the European Society of Cardiology, and the Japanese Heart Failure Society, with endorsements from the Canadian Heart Failure Society, the Heart Failure Association of India, the Cardiac Society of Australia and New Zealand, and the Chinese Heart Failure Association.
The article was published March 1 in the Journal of Cardiac Failure and the European Journal of Heart Failure, authored by a writing committee of 38 individuals with domain expertise in HF, cardiomyopathy, and cardiovascular disease.
“This is a very thorough and very carefully written document that I think will be helpful for clinicians because they’ve tapped into important changes in the field that have occurred over the past 10 years and that now allow us to do more for patients than we could before,” Eugene Braunwald, MD, said in an interview.
Dr. Braunwald and Elliott M. Antman, MD, both from TIMI Study Group at Brigham and Women’s Hospital and Harvard Medical School in Boston, wrote an editorial that accompanied the European Journal of Heart Failure article.
A new universal definition
“[Heart failure] is a clinical syndrome with symptoms and or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion.”
This proposed definition, said the authors, is designed to be contemporary and simple “but conceptually comprehensive, with near universal applicability, prognostic and therapeutic viability, and acceptable sensitivity and specificity.”
Both left and right HF qualifies under this definition, said the authors, but conditions that result in marked volume overload, such as chronic kidney disease, which may present with signs and symptoms of HF, do not.
“Although some of these patients may have concomitant HF, these patients have a primary abnormality that may require a specific treatment beyond that for HF,” said the consensus statement authors.
For his part, Douglas L. Mann, MD, is happy to see what he considers a more accurate and practical definition for heart failure.
“We’ve had some wacky definitions in heart failure that haven’t made sense for 30 years, the principal of which is the definition of heart failure that says it’s the inability of the heart to meet the metabolic demands of the body,” Dr. Mann, of Washington University, St. Louis, said in an interview.
“I think this description was developed thinking about people with end-stage heart failure, but it makes no sense in clinical practice. Does it make sense to say about someone with New York Heart Association class I heart failure that their heart can’t meet the metabolic demands of the body?” said Dr. Mann, who was not involved with the writing of the consensus statement.
Proposed revised stages of the HF continuum
Overall, minimal changes have been made to the HF stages, with tweaks intended to enhance understanding and address the evolving role of biomarkers.
The authors proposed an approach to staging of HF:
- At-risk for HF (stage A), for patients at risk for HF but without current or prior symptoms or signs of HF and without structural or biomarkers evidence of heart disease.
- Pre-HF (stage B), for patients without current or prior symptoms or signs of HF, but evidence of structural heart disease or abnormal cardiac function, or elevated natriuretic peptide levels.
- HF (stage C), for patients with current or prior symptoms and/or signs of HF caused by a structural and/or functional cardiac abnormality.
- Advanced HF (stage D), for patients with severe symptoms and/or signs of HF at rest, recurrent hospitalizations despite guideline-directed management and therapy (GDMT), refractory or intolerant to GDMT, requiring advanced therapies such as consideration for transplant, mechanical circulatory support, or palliative care.
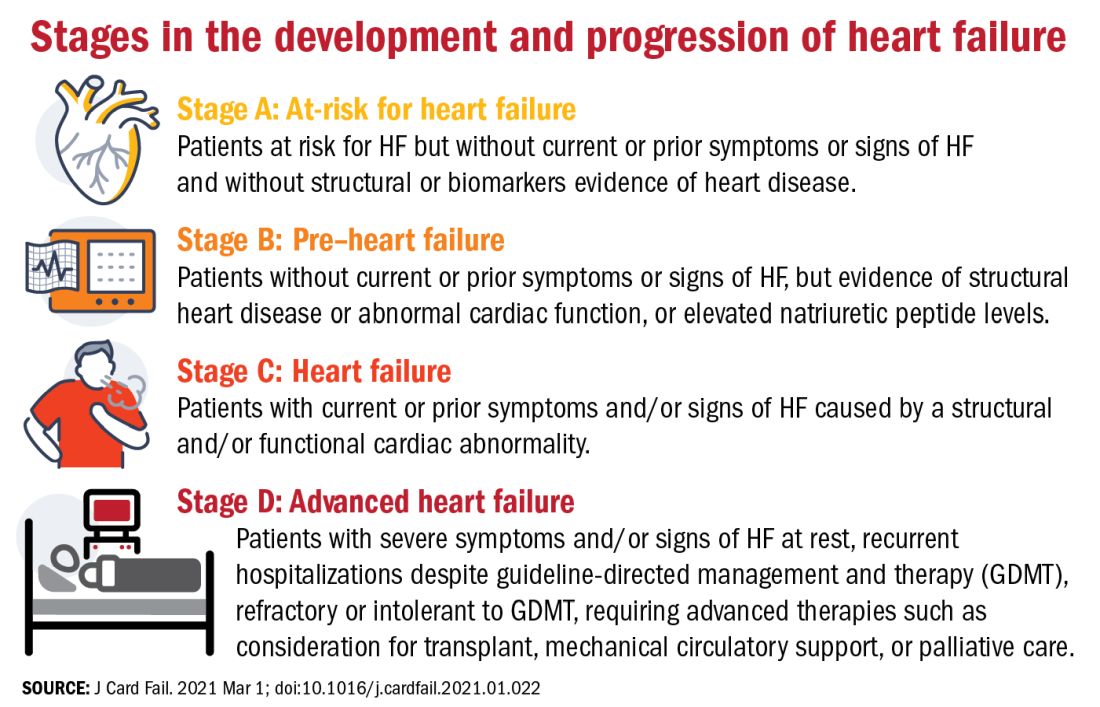
One notable change to the staging scheme is stage B, which the authors have reframed as “pre–heart failure.”
“Pre-cancer is a term widely understood and considered actionable and we wanted to tap into this successful messaging and embrace the pre–heart failure concept as something that is treatable and preventable,” said Dr. Bozkurt.
“We want patients and clinicians to understand that there are things we can do to prevent heart failure, strategies we didn’t have before, like SGLT2 inhibitors in patients with diabetes at risk for HF,” she added.
The revision also avoids the stigma of HF before the symptoms are manifest.
“Not calling it stage A and stage B heart failure you might say is semantics, but it’s important semantics,” said Dr. Braunwald. “When you’re talking to a patient or a relative and tell them they have stage A heart failure, it’s scares them unnecessarily. They don’t hear the stage A or B part, just the heart failure part.”
New classifications according to LVEF
And finally, in what some might consider the most obviously needed modification, the document proposes a new and revised classification of HF according to left ventricular ejection fraction (LVEF). Most agree on how to classify heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF), but although the middle range has long been understood to be a clinically relevant, it has no proper name or clear delineation.
“For standardization across practice guidelines, to recognize clinical trajectories in HF, and to facilitate the recognition of different heart failure entities in a sensitive and specific manner that can guide therapy, we want to formalize the heart failure categories according to ejection fraction,” said Dr. Bozkurt.
To this end, the authors propose the following four classifications of EF:
- HF with reduced EF (HFrEF): LVEF of up to 40%.
- HF with mildly reduced EF (HFmrEF): LVEF of 41-49%.
- HF with preserved EF (HFpEF)HF with an LVEF of at least 50%.
- HF with improved EF (HFimpEF): HF with a baseline LVEF of 40% or less, an increase of at least 10 points from baseline LVEF, and a second measurement of LVEF of greater than 40%.
HFmrEF is usually a transition period, noted Dr. Bozkurt. “Patients with HF in this range may represent a population whose EF is likely to change, either increase or decrease over time and it’s important to be cognizant of that trajectory. Understanding where your patient is headed is crucial for prognosis and optimization of guideline-directed treatment,” she said.
Improved, not recovered, HF
The last classification of heart failure with improved ejection fraction (HFimpEF) represents an important change to the current classification scheme.
“We want to clarify what terms to use but also which not to use. For example, we don’t want people to use recovered heart failure or heart failure in remission, partly because we don’t want the medication to be stopped. We don’t want to give the false message that there has been full recovery,” said Dr. Bozkurt.
As seen in the TRED-HF trial, guideline-directed medical therapy should be continued in patients with HF with improved EF regardless of whether it has improved to a normal range of above 50% in subsequent measurements.
“This is a distinct group of people, and for a while the guidelines were lumping them in with HFpEF, which I think is totally wrong,” said Dr. Mann.
“I think it’s very important that we emphasize heart failure as a continuum, rather than a one-way street of [inevitable] progression. Because we do see improvements in ejection fraction and we do see that we can prevent heart failure if we do the right things, and this should be reflected in the terminology we use,” he added.
Dr. Bozkurt stressed that HFimpEF only applies if the EF improves to above 40%. A move from an EF of 10%-20% would still see the patient classified as having HFrEF, but a patient whose EF improved from, say, 30% to 45% would be classified as HFimpEF.
“The reason for this, again, is because a transition from, say an EF of 10%-20% does not change therapy, but a move upward over 40% might, especially regarding decisions for device therapies, so the trajectory as well as the absolute EF is important,” she added.
“Particularly in the early stages, people are responsive to therapy and it’s possible in some cases to reverse heart failure, so I think this change helps us understand when that’s happened,” said Dr. Braunwald.
One step toward universality
“The implementation of this terminology and nomenclature into practice will require a variety of tactics,” said Dr. Bozkurt. “For example, the current ICD 10 codes need to incorporate the at-risk and pre–heart failure categories, as well as the mid-range EF, preserved, and improved EF classifications, because the treatment differs between those three domains.”
In terms of how these proposed changes will be worked into practice guidelines, Dr. Bozkurt declined to comment on this to avoid any perception of conflict of interest as she is the cochair of the American College of Cardiology/American Heart Association HF guideline writing committee.
Dr. Braunwald and Dr. Antman suggest it may be premature to call the new terminology and classifications “universal.” In an interview, Dr. Braunwald lamented the absence of the World Heart Federation, the ACC, and the AHA as active participants in this effort and suggested this paper is only the first step of a multistep process that requires input from many stakeholders.
“It’s important that these organizations be involved, not just to bless it, but to contribute their expertise to the process,” he said.
For his part, Dr. Mann hopes these changes will gain widespread acceptance and clinical traction. “The problem sometimes with guidelines is that they’re so data driven that you just can’t come out and say the obvious, so making a position statement is a good first step. And they got good international representation on this, so I think these changes will be accepted in the next heart failure guidelines.”
To encourage further discussion and acceptance, Robert J. Mentz, MD, and Anuradha Lala, MD, editor-in-chief and deputy editor of the Journal of Cardiac Failure, respectively, announced a series of multidisciplinary perspective pieces to be published in the journal monthly, starting in May with editorials from Dr. Clyde W Yancy, MD, MSc, and Carolyn S.P. Lam, MBBS, PhD, both of whom were authors of the consensus statement.
Dr. Bozkurt reports being a consultant for Abbott, Amgen, Baxter, Bristol Myers Squibb, Liva Nova Relypsa/Vifor Pharma, Respicardia, and being on the registry steering committee for Sanofi-Aventis. Dr. Braunwald reports research grant support through Brigham and Women’s Hospital from AstraZeneca, Daiichi Sankyo, Merck, and Novartis; and consulting for Amgen, Boehringer-Ingelheim/Lilly, Cardurion, MyoKardia, Novo Nordisk, and Verve. Dr. Mann has been a consultant to Novartis, is on the steering committee for the PARADISE trial, and is on the scientific advisory board for MyoKardia/Bristol Myers Squibb.
The terminology and classification scheme for heart failure (HF) is changing in ways that experts hope will directly impact patient outcomes.
In a new consensus statement, a multisociety group of experts proposed a new universal definition of heart failure and made substantial revisions to the way in which the disease is staged and classified.
The authors of the statement, led by writing committee chair and immediate past president of the Heart Failure Society of America Biykem Bozkurt, MD, PhD, hope their efforts will go far to improve standardization of terminology, but more importantly will facilitate better management of the disease in ways that keep pace with current knowledge and advances in the field.
“There is a great need for reframing and standardizing the terminology across societies and different stakeholders, and importantly for patients because a lot of the terminology we were using was understood by academicians, but were not being translated in important ways to ensure patients are being appropriately treated,” said Dr. Bozkurt, of Baylor College of Medicine, Houston.
The consensus statement was a group effort led by the HFSA, the Heart Failure Association of the European Society of Cardiology, and the Japanese Heart Failure Society, with endorsements from the Canadian Heart Failure Society, the Heart Failure Association of India, the Cardiac Society of Australia and New Zealand, and the Chinese Heart Failure Association.
The article was published March 1 in the Journal of Cardiac Failure and the European Journal of Heart Failure, authored by a writing committee of 38 individuals with domain expertise in HF, cardiomyopathy, and cardiovascular disease.
“This is a very thorough and very carefully written document that I think will be helpful for clinicians because they’ve tapped into important changes in the field that have occurred over the past 10 years and that now allow us to do more for patients than we could before,” Eugene Braunwald, MD, said in an interview.
Dr. Braunwald and Elliott M. Antman, MD, both from TIMI Study Group at Brigham and Women’s Hospital and Harvard Medical School in Boston, wrote an editorial that accompanied the European Journal of Heart Failure article.
A new universal definition
“[Heart failure] is a clinical syndrome with symptoms and or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion.”
This proposed definition, said the authors, is designed to be contemporary and simple “but conceptually comprehensive, with near universal applicability, prognostic and therapeutic viability, and acceptable sensitivity and specificity.”
Both left and right HF qualifies under this definition, said the authors, but conditions that result in marked volume overload, such as chronic kidney disease, which may present with signs and symptoms of HF, do not.
“Although some of these patients may have concomitant HF, these patients have a primary abnormality that may require a specific treatment beyond that for HF,” said the consensus statement authors.
For his part, Douglas L. Mann, MD, is happy to see what he considers a more accurate and practical definition for heart failure.
“We’ve had some wacky definitions in heart failure that haven’t made sense for 30 years, the principal of which is the definition of heart failure that says it’s the inability of the heart to meet the metabolic demands of the body,” Dr. Mann, of Washington University, St. Louis, said in an interview.
“I think this description was developed thinking about people with end-stage heart failure, but it makes no sense in clinical practice. Does it make sense to say about someone with New York Heart Association class I heart failure that their heart can’t meet the metabolic demands of the body?” said Dr. Mann, who was not involved with the writing of the consensus statement.
Proposed revised stages of the HF continuum
Overall, minimal changes have been made to the HF stages, with tweaks intended to enhance understanding and address the evolving role of biomarkers.
The authors proposed an approach to staging of HF:
- At-risk for HF (stage A), for patients at risk for HF but without current or prior symptoms or signs of HF and without structural or biomarkers evidence of heart disease.
- Pre-HF (stage B), for patients without current or prior symptoms or signs of HF, but evidence of structural heart disease or abnormal cardiac function, or elevated natriuretic peptide levels.
- HF (stage C), for patients with current or prior symptoms and/or signs of HF caused by a structural and/or functional cardiac abnormality.
- Advanced HF (stage D), for patients with severe symptoms and/or signs of HF at rest, recurrent hospitalizations despite guideline-directed management and therapy (GDMT), refractory or intolerant to GDMT, requiring advanced therapies such as consideration for transplant, mechanical circulatory support, or palliative care.

One notable change to the staging scheme is stage B, which the authors have reframed as “pre–heart failure.”
“Pre-cancer is a term widely understood and considered actionable and we wanted to tap into this successful messaging and embrace the pre–heart failure concept as something that is treatable and preventable,” said Dr. Bozkurt.
“We want patients and clinicians to understand that there are things we can do to prevent heart failure, strategies we didn’t have before, like SGLT2 inhibitors in patients with diabetes at risk for HF,” she added.
The revision also avoids the stigma of HF before the symptoms are manifest.
“Not calling it stage A and stage B heart failure you might say is semantics, but it’s important semantics,” said Dr. Braunwald. “When you’re talking to a patient or a relative and tell them they have stage A heart failure, it’s scares them unnecessarily. They don’t hear the stage A or B part, just the heart failure part.”
New classifications according to LVEF
And finally, in what some might consider the most obviously needed modification, the document proposes a new and revised classification of HF according to left ventricular ejection fraction (LVEF). Most agree on how to classify heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF), but although the middle range has long been understood to be a clinically relevant, it has no proper name or clear delineation.
“For standardization across practice guidelines, to recognize clinical trajectories in HF, and to facilitate the recognition of different heart failure entities in a sensitive and specific manner that can guide therapy, we want to formalize the heart failure categories according to ejection fraction,” said Dr. Bozkurt.
To this end, the authors propose the following four classifications of EF:
- HF with reduced EF (HFrEF): LVEF of up to 40%.
- HF with mildly reduced EF (HFmrEF): LVEF of 41-49%.
- HF with preserved EF (HFpEF)HF with an LVEF of at least 50%.
- HF with improved EF (HFimpEF): HF with a baseline LVEF of 40% or less, an increase of at least 10 points from baseline LVEF, and a second measurement of LVEF of greater than 40%.
HFmrEF is usually a transition period, noted Dr. Bozkurt. “Patients with HF in this range may represent a population whose EF is likely to change, either increase or decrease over time and it’s important to be cognizant of that trajectory. Understanding where your patient is headed is crucial for prognosis and optimization of guideline-directed treatment,” she said.
Improved, not recovered, HF
The last classification of heart failure with improved ejection fraction (HFimpEF) represents an important change to the current classification scheme.
“We want to clarify what terms to use but also which not to use. For example, we don’t want people to use recovered heart failure or heart failure in remission, partly because we don’t want the medication to be stopped. We don’t want to give the false message that there has been full recovery,” said Dr. Bozkurt.
As seen in the TRED-HF trial, guideline-directed medical therapy should be continued in patients with HF with improved EF regardless of whether it has improved to a normal range of above 50% in subsequent measurements.
“This is a distinct group of people, and for a while the guidelines were lumping them in with HFpEF, which I think is totally wrong,” said Dr. Mann.
“I think it’s very important that we emphasize heart failure as a continuum, rather than a one-way street of [inevitable] progression. Because we do see improvements in ejection fraction and we do see that we can prevent heart failure if we do the right things, and this should be reflected in the terminology we use,” he added.
Dr. Bozkurt stressed that HFimpEF only applies if the EF improves to above 40%. A move from an EF of 10%-20% would still see the patient classified as having HFrEF, but a patient whose EF improved from, say, 30% to 45% would be classified as HFimpEF.
“The reason for this, again, is because a transition from, say an EF of 10%-20% does not change therapy, but a move upward over 40% might, especially regarding decisions for device therapies, so the trajectory as well as the absolute EF is important,” she added.
“Particularly in the early stages, people are responsive to therapy and it’s possible in some cases to reverse heart failure, so I think this change helps us understand when that’s happened,” said Dr. Braunwald.
One step toward universality
“The implementation of this terminology and nomenclature into practice will require a variety of tactics,” said Dr. Bozkurt. “For example, the current ICD 10 codes need to incorporate the at-risk and pre–heart failure categories, as well as the mid-range EF, preserved, and improved EF classifications, because the treatment differs between those three domains.”
In terms of how these proposed changes will be worked into practice guidelines, Dr. Bozkurt declined to comment on this to avoid any perception of conflict of interest as she is the cochair of the American College of Cardiology/American Heart Association HF guideline writing committee.
Dr. Braunwald and Dr. Antman suggest it may be premature to call the new terminology and classifications “universal.” In an interview, Dr. Braunwald lamented the absence of the World Heart Federation, the ACC, and the AHA as active participants in this effort and suggested this paper is only the first step of a multistep process that requires input from many stakeholders.
“It’s important that these organizations be involved, not just to bless it, but to contribute their expertise to the process,” he said.
For his part, Dr. Mann hopes these changes will gain widespread acceptance and clinical traction. “The problem sometimes with guidelines is that they’re so data driven that you just can’t come out and say the obvious, so making a position statement is a good first step. And they got good international representation on this, so I think these changes will be accepted in the next heart failure guidelines.”
To encourage further discussion and acceptance, Robert J. Mentz, MD, and Anuradha Lala, MD, editor-in-chief and deputy editor of the Journal of Cardiac Failure, respectively, announced a series of multidisciplinary perspective pieces to be published in the journal monthly, starting in May with editorials from Dr. Clyde W Yancy, MD, MSc, and Carolyn S.P. Lam, MBBS, PhD, both of whom were authors of the consensus statement.
Dr. Bozkurt reports being a consultant for Abbott, Amgen, Baxter, Bristol Myers Squibb, Liva Nova Relypsa/Vifor Pharma, Respicardia, and being on the registry steering committee for Sanofi-Aventis. Dr. Braunwald reports research grant support through Brigham and Women’s Hospital from AstraZeneca, Daiichi Sankyo, Merck, and Novartis; and consulting for Amgen, Boehringer-Ingelheim/Lilly, Cardurion, MyoKardia, Novo Nordisk, and Verve. Dr. Mann has been a consultant to Novartis, is on the steering committee for the PARADISE trial, and is on the scientific advisory board for MyoKardia/Bristol Myers Squibb.
The terminology and classification scheme for heart failure (HF) is changing in ways that experts hope will directly impact patient outcomes.
In a new consensus statement, a multisociety group of experts proposed a new universal definition of heart failure and made substantial revisions to the way in which the disease is staged and classified.
The authors of the statement, led by writing committee chair and immediate past president of the Heart Failure Society of America Biykem Bozkurt, MD, PhD, hope their efforts will go far to improve standardization of terminology, but more importantly will facilitate better management of the disease in ways that keep pace with current knowledge and advances in the field.
“There is a great need for reframing and standardizing the terminology across societies and different stakeholders, and importantly for patients because a lot of the terminology we were using was understood by academicians, but were not being translated in important ways to ensure patients are being appropriately treated,” said Dr. Bozkurt, of Baylor College of Medicine, Houston.
The consensus statement was a group effort led by the HFSA, the Heart Failure Association of the European Society of Cardiology, and the Japanese Heart Failure Society, with endorsements from the Canadian Heart Failure Society, the Heart Failure Association of India, the Cardiac Society of Australia and New Zealand, and the Chinese Heart Failure Association.
The article was published March 1 in the Journal of Cardiac Failure and the European Journal of Heart Failure, authored by a writing committee of 38 individuals with domain expertise in HF, cardiomyopathy, and cardiovascular disease.
“This is a very thorough and very carefully written document that I think will be helpful for clinicians because they’ve tapped into important changes in the field that have occurred over the past 10 years and that now allow us to do more for patients than we could before,” Eugene Braunwald, MD, said in an interview.
Dr. Braunwald and Elliott M. Antman, MD, both from TIMI Study Group at Brigham and Women’s Hospital and Harvard Medical School in Boston, wrote an editorial that accompanied the European Journal of Heart Failure article.
A new universal definition
“[Heart failure] is a clinical syndrome with symptoms and or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion.”
This proposed definition, said the authors, is designed to be contemporary and simple “but conceptually comprehensive, with near universal applicability, prognostic and therapeutic viability, and acceptable sensitivity and specificity.”
Both left and right HF qualifies under this definition, said the authors, but conditions that result in marked volume overload, such as chronic kidney disease, which may present with signs and symptoms of HF, do not.
“Although some of these patients may have concomitant HF, these patients have a primary abnormality that may require a specific treatment beyond that for HF,” said the consensus statement authors.
For his part, Douglas L. Mann, MD, is happy to see what he considers a more accurate and practical definition for heart failure.
“We’ve had some wacky definitions in heart failure that haven’t made sense for 30 years, the principal of which is the definition of heart failure that says it’s the inability of the heart to meet the metabolic demands of the body,” Dr. Mann, of Washington University, St. Louis, said in an interview.
“I think this description was developed thinking about people with end-stage heart failure, but it makes no sense in clinical practice. Does it make sense to say about someone with New York Heart Association class I heart failure that their heart can’t meet the metabolic demands of the body?” said Dr. Mann, who was not involved with the writing of the consensus statement.
Proposed revised stages of the HF continuum
Overall, minimal changes have been made to the HF stages, with tweaks intended to enhance understanding and address the evolving role of biomarkers.
The authors proposed an approach to staging of HF:
- At-risk for HF (stage A), for patients at risk for HF but without current or prior symptoms or signs of HF and without structural or biomarkers evidence of heart disease.
- Pre-HF (stage B), for patients without current or prior symptoms or signs of HF, but evidence of structural heart disease or abnormal cardiac function, or elevated natriuretic peptide levels.
- HF (stage C), for patients with current or prior symptoms and/or signs of HF caused by a structural and/or functional cardiac abnormality.
- Advanced HF (stage D), for patients with severe symptoms and/or signs of HF at rest, recurrent hospitalizations despite guideline-directed management and therapy (GDMT), refractory or intolerant to GDMT, requiring advanced therapies such as consideration for transplant, mechanical circulatory support, or palliative care.

One notable change to the staging scheme is stage B, which the authors have reframed as “pre–heart failure.”
“Pre-cancer is a term widely understood and considered actionable and we wanted to tap into this successful messaging and embrace the pre–heart failure concept as something that is treatable and preventable,” said Dr. Bozkurt.
“We want patients and clinicians to understand that there are things we can do to prevent heart failure, strategies we didn’t have before, like SGLT2 inhibitors in patients with diabetes at risk for HF,” she added.
The revision also avoids the stigma of HF before the symptoms are manifest.
“Not calling it stage A and stage B heart failure you might say is semantics, but it’s important semantics,” said Dr. Braunwald. “When you’re talking to a patient or a relative and tell them they have stage A heart failure, it’s scares them unnecessarily. They don’t hear the stage A or B part, just the heart failure part.”
New classifications according to LVEF
And finally, in what some might consider the most obviously needed modification, the document proposes a new and revised classification of HF according to left ventricular ejection fraction (LVEF). Most agree on how to classify heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF), but although the middle range has long been understood to be a clinically relevant, it has no proper name or clear delineation.
“For standardization across practice guidelines, to recognize clinical trajectories in HF, and to facilitate the recognition of different heart failure entities in a sensitive and specific manner that can guide therapy, we want to formalize the heart failure categories according to ejection fraction,” said Dr. Bozkurt.
To this end, the authors propose the following four classifications of EF:
- HF with reduced EF (HFrEF): LVEF of up to 40%.
- HF with mildly reduced EF (HFmrEF): LVEF of 41-49%.
- HF with preserved EF (HFpEF)HF with an LVEF of at least 50%.
- HF with improved EF (HFimpEF): HF with a baseline LVEF of 40% or less, an increase of at least 10 points from baseline LVEF, and a second measurement of LVEF of greater than 40%.
HFmrEF is usually a transition period, noted Dr. Bozkurt. “Patients with HF in this range may represent a population whose EF is likely to change, either increase or decrease over time and it’s important to be cognizant of that trajectory. Understanding where your patient is headed is crucial for prognosis and optimization of guideline-directed treatment,” she said.
Improved, not recovered, HF
The last classification of heart failure with improved ejection fraction (HFimpEF) represents an important change to the current classification scheme.
“We want to clarify what terms to use but also which not to use. For example, we don’t want people to use recovered heart failure or heart failure in remission, partly because we don’t want the medication to be stopped. We don’t want to give the false message that there has been full recovery,” said Dr. Bozkurt.
As seen in the TRED-HF trial, guideline-directed medical therapy should be continued in patients with HF with improved EF regardless of whether it has improved to a normal range of above 50% in subsequent measurements.
“This is a distinct group of people, and for a while the guidelines were lumping them in with HFpEF, which I think is totally wrong,” said Dr. Mann.
“I think it’s very important that we emphasize heart failure as a continuum, rather than a one-way street of [inevitable] progression. Because we do see improvements in ejection fraction and we do see that we can prevent heart failure if we do the right things, and this should be reflected in the terminology we use,” he added.
Dr. Bozkurt stressed that HFimpEF only applies if the EF improves to above 40%. A move from an EF of 10%-20% would still see the patient classified as having HFrEF, but a patient whose EF improved from, say, 30% to 45% would be classified as HFimpEF.
“The reason for this, again, is because a transition from, say an EF of 10%-20% does not change therapy, but a move upward over 40% might, especially regarding decisions for device therapies, so the trajectory as well as the absolute EF is important,” she added.
“Particularly in the early stages, people are responsive to therapy and it’s possible in some cases to reverse heart failure, so I think this change helps us understand when that’s happened,” said Dr. Braunwald.
One step toward universality
“The implementation of this terminology and nomenclature into practice will require a variety of tactics,” said Dr. Bozkurt. “For example, the current ICD 10 codes need to incorporate the at-risk and pre–heart failure categories, as well as the mid-range EF, preserved, and improved EF classifications, because the treatment differs between those three domains.”
In terms of how these proposed changes will be worked into practice guidelines, Dr. Bozkurt declined to comment on this to avoid any perception of conflict of interest as she is the cochair of the American College of Cardiology/American Heart Association HF guideline writing committee.
Dr. Braunwald and Dr. Antman suggest it may be premature to call the new terminology and classifications “universal.” In an interview, Dr. Braunwald lamented the absence of the World Heart Federation, the ACC, and the AHA as active participants in this effort and suggested this paper is only the first step of a multistep process that requires input from many stakeholders.
“It’s important that these organizations be involved, not just to bless it, but to contribute their expertise to the process,” he said.
For his part, Dr. Mann hopes these changes will gain widespread acceptance and clinical traction. “The problem sometimes with guidelines is that they’re so data driven that you just can’t come out and say the obvious, so making a position statement is a good first step. And they got good international representation on this, so I think these changes will be accepted in the next heart failure guidelines.”
To encourage further discussion and acceptance, Robert J. Mentz, MD, and Anuradha Lala, MD, editor-in-chief and deputy editor of the Journal of Cardiac Failure, respectively, announced a series of multidisciplinary perspective pieces to be published in the journal monthly, starting in May with editorials from Dr. Clyde W Yancy, MD, MSc, and Carolyn S.P. Lam, MBBS, PhD, both of whom were authors of the consensus statement.
Dr. Bozkurt reports being a consultant for Abbott, Amgen, Baxter, Bristol Myers Squibb, Liva Nova Relypsa/Vifor Pharma, Respicardia, and being on the registry steering committee for Sanofi-Aventis. Dr. Braunwald reports research grant support through Brigham and Women’s Hospital from AstraZeneca, Daiichi Sankyo, Merck, and Novartis; and consulting for Amgen, Boehringer-Ingelheim/Lilly, Cardurion, MyoKardia, Novo Nordisk, and Verve. Dr. Mann has been a consultant to Novartis, is on the steering committee for the PARADISE trial, and is on the scientific advisory board for MyoKardia/Bristol Myers Squibb.
FROM THE JOURNAL OF CARDIAC FAILURE
Heart health in pregnancy tied to CV risk in adolescent offspring
Children born to mothers in poor cardiovascular health during pregnancy had an almost eight times higher risk for landing in the poorest cardiovascular health category in early adolescence than children born to mothers who had ideal cardiovascular health during pregnancy.
In an observational cohort study that involved 2,302 mother-child dyads, 6.0% of mothers and 2.6% of children were considered to be in the poorest category of cardiovascular health on the basis of specific risk factors.
The children of mothers with any “intermediate” cardiovascular health metrics in pregnancy – for example, being overweight but not obese – were at just more than two times higher risk for poor cardiovascular health in early adolescence.
Although acknowledging the limitations of observational data, Amanda M. Perak, MD, Northwestern University, Chicago, suggested that focusing on whether or not the relationships seen in this study are causal might be throwing the baby out with the bathwater.
“I would suggest that it may not actually matter whether there is causality or correlation here, because if you can identify newborns at birth who have an eight times higher risk for poor cardiovascular health in childhood based on mom’s health during pregnancy, that’s valuable information either way,” said Dr. Perak.
“Even if you don’t know why their risk is elevated, you might be able to target those children for more intensive preventative efforts throughout childhood to help them hold on to their cardiovascular health for longer.”
That said, she thinks it’s possible that the intrauterine environment might actually directly affect offspring health, either through epigenetics modifications to cardiometabolic regulatory genes or possibly through actual organ development. Her group is collecting epigenetic data to study this further.
“We also need to do a study to see if intervening during pregnancy with mothers leads to better cardiovascular health in offspring, and that’s a question we can answer with a clinical trial,” said Dr. Perak.
This study was published on Feb. 16, 2021, in JAMA.
Equal footing
“We’ve always talked about cardiovascular health as if everyone is born with ideal cardiovascular health and loses it from there, and I think what this article points out is that not everybody starts on equal footing,” said Stephen R. Daniels, MD, PhD, University of Colorado at Denver, Aurora, who wrote an editorial accompanying the study.
“We need to start upstream, working with mothers before and during pregnancy, but it’s also important to understand, from a pediatric standpoint, that with some of these kids the horse is kind of already out of the barn very early.”
Dr. Daniels is pediatrician in chief and chair of pediatrics at Children’s Hospital Colorado in Aurora.
This study is the first to examine the relevance of maternal gestational cardiovascular health to offspring cardiovascular health and an important first step toward developing new approaches to address the concept of primordial prevention, he said.
“If primary prevention is identifying risk factors and treating them, I think of primordial prevention as preventing the development of those risk factors in the first place,” said Dr. Daniels.
Future trials, he added, should focus on the various mechanistic pathways – biological effects, shared genetics, and lifestyle being the options – to better understand opportunities for intervention.
Mother-child pairs
Dr. Perak and colleagues used data from the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study and the HAPO Follow-up Study.
Participants were 2,302 mother-child pairs from nine field centers in Barbados, Canada, China, Thailand, United Kingdom, and the United States, and represented a racially and ethnically diverse cohort.
The mean ages were 29.6 years for pregnant mothers and 11.3 years for children. The pregnancies occurred between 2000 and 2006, and the children were examined from 2013 to 2016, when the children were aged 10-14 years.
Using the American Heart Association’s definition of cardiovascular health, the scientists categorized pregnancy health for mothers based on their measures of body mass index, blood pressure, total cholesterol, glucose level, and smoking status at 28 weeks’ gestation. These five metrics of gestational cardiovascular health have been significantly associated with adverse pregnancy outcomes.
They categorized cardiovascular health for offspring at age 10-14 years based on four of these five metrics: body mass index, blood pressure, cholesterol, and glucose.
Only 32.8% of mothers and 42.2% of children had ideal cardiovascular health.
In analyses adjusted for pregnancy and birth outcomes, the associations seen between poor gestational maternal health and offspring cardiovascular health persisted but were attenuated.
Dr. Perak reported receiving grants from the Woman’s Board of Northwestern Memorial Hospital; the Dixon Family; the American Heart Association; and the National Heart, Lung, and Blood Institute. Dr. Daniels reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Children born to mothers in poor cardiovascular health during pregnancy had an almost eight times higher risk for landing in the poorest cardiovascular health category in early adolescence than children born to mothers who had ideal cardiovascular health during pregnancy.
In an observational cohort study that involved 2,302 mother-child dyads, 6.0% of mothers and 2.6% of children were considered to be in the poorest category of cardiovascular health on the basis of specific risk factors.
The children of mothers with any “intermediate” cardiovascular health metrics in pregnancy – for example, being overweight but not obese – were at just more than two times higher risk for poor cardiovascular health in early adolescence.
Although acknowledging the limitations of observational data, Amanda M. Perak, MD, Northwestern University, Chicago, suggested that focusing on whether or not the relationships seen in this study are causal might be throwing the baby out with the bathwater.
“I would suggest that it may not actually matter whether there is causality or correlation here, because if you can identify newborns at birth who have an eight times higher risk for poor cardiovascular health in childhood based on mom’s health during pregnancy, that’s valuable information either way,” said Dr. Perak.
“Even if you don’t know why their risk is elevated, you might be able to target those children for more intensive preventative efforts throughout childhood to help them hold on to their cardiovascular health for longer.”
That said, she thinks it’s possible that the intrauterine environment might actually directly affect offspring health, either through epigenetics modifications to cardiometabolic regulatory genes or possibly through actual organ development. Her group is collecting epigenetic data to study this further.
“We also need to do a study to see if intervening during pregnancy with mothers leads to better cardiovascular health in offspring, and that’s a question we can answer with a clinical trial,” said Dr. Perak.
This study was published on Feb. 16, 2021, in JAMA.
Equal footing
“We’ve always talked about cardiovascular health as if everyone is born with ideal cardiovascular health and loses it from there, and I think what this article points out is that not everybody starts on equal footing,” said Stephen R. Daniels, MD, PhD, University of Colorado at Denver, Aurora, who wrote an editorial accompanying the study.
“We need to start upstream, working with mothers before and during pregnancy, but it’s also important to understand, from a pediatric standpoint, that with some of these kids the horse is kind of already out of the barn very early.”
Dr. Daniels is pediatrician in chief and chair of pediatrics at Children’s Hospital Colorado in Aurora.
This study is the first to examine the relevance of maternal gestational cardiovascular health to offspring cardiovascular health and an important first step toward developing new approaches to address the concept of primordial prevention, he said.
“If primary prevention is identifying risk factors and treating them, I think of primordial prevention as preventing the development of those risk factors in the first place,” said Dr. Daniels.
Future trials, he added, should focus on the various mechanistic pathways – biological effects, shared genetics, and lifestyle being the options – to better understand opportunities for intervention.
Mother-child pairs
Dr. Perak and colleagues used data from the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study and the HAPO Follow-up Study.
Participants were 2,302 mother-child pairs from nine field centers in Barbados, Canada, China, Thailand, United Kingdom, and the United States, and represented a racially and ethnically diverse cohort.
The mean ages were 29.6 years for pregnant mothers and 11.3 years for children. The pregnancies occurred between 2000 and 2006, and the children were examined from 2013 to 2016, when the children were aged 10-14 years.
Using the American Heart Association’s definition of cardiovascular health, the scientists categorized pregnancy health for mothers based on their measures of body mass index, blood pressure, total cholesterol, glucose level, and smoking status at 28 weeks’ gestation. These five metrics of gestational cardiovascular health have been significantly associated with adverse pregnancy outcomes.
They categorized cardiovascular health for offspring at age 10-14 years based on four of these five metrics: body mass index, blood pressure, cholesterol, and glucose.
Only 32.8% of mothers and 42.2% of children had ideal cardiovascular health.
In analyses adjusted for pregnancy and birth outcomes, the associations seen between poor gestational maternal health and offspring cardiovascular health persisted but were attenuated.
Dr. Perak reported receiving grants from the Woman’s Board of Northwestern Memorial Hospital; the Dixon Family; the American Heart Association; and the National Heart, Lung, and Blood Institute. Dr. Daniels reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Children born to mothers in poor cardiovascular health during pregnancy had an almost eight times higher risk for landing in the poorest cardiovascular health category in early adolescence than children born to mothers who had ideal cardiovascular health during pregnancy.
In an observational cohort study that involved 2,302 mother-child dyads, 6.0% of mothers and 2.6% of children were considered to be in the poorest category of cardiovascular health on the basis of specific risk factors.
The children of mothers with any “intermediate” cardiovascular health metrics in pregnancy – for example, being overweight but not obese – were at just more than two times higher risk for poor cardiovascular health in early adolescence.
Although acknowledging the limitations of observational data, Amanda M. Perak, MD, Northwestern University, Chicago, suggested that focusing on whether or not the relationships seen in this study are causal might be throwing the baby out with the bathwater.
“I would suggest that it may not actually matter whether there is causality or correlation here, because if you can identify newborns at birth who have an eight times higher risk for poor cardiovascular health in childhood based on mom’s health during pregnancy, that’s valuable information either way,” said Dr. Perak.
“Even if you don’t know why their risk is elevated, you might be able to target those children for more intensive preventative efforts throughout childhood to help them hold on to their cardiovascular health for longer.”
That said, she thinks it’s possible that the intrauterine environment might actually directly affect offspring health, either through epigenetics modifications to cardiometabolic regulatory genes or possibly through actual organ development. Her group is collecting epigenetic data to study this further.
“We also need to do a study to see if intervening during pregnancy with mothers leads to better cardiovascular health in offspring, and that’s a question we can answer with a clinical trial,” said Dr. Perak.
This study was published on Feb. 16, 2021, in JAMA.
Equal footing
“We’ve always talked about cardiovascular health as if everyone is born with ideal cardiovascular health and loses it from there, and I think what this article points out is that not everybody starts on equal footing,” said Stephen R. Daniels, MD, PhD, University of Colorado at Denver, Aurora, who wrote an editorial accompanying the study.
“We need to start upstream, working with mothers before and during pregnancy, but it’s also important to understand, from a pediatric standpoint, that with some of these kids the horse is kind of already out of the barn very early.”
Dr. Daniels is pediatrician in chief and chair of pediatrics at Children’s Hospital Colorado in Aurora.
This study is the first to examine the relevance of maternal gestational cardiovascular health to offspring cardiovascular health and an important first step toward developing new approaches to address the concept of primordial prevention, he said.
“If primary prevention is identifying risk factors and treating them, I think of primordial prevention as preventing the development of those risk factors in the first place,” said Dr. Daniels.
Future trials, he added, should focus on the various mechanistic pathways – biological effects, shared genetics, and lifestyle being the options – to better understand opportunities for intervention.
Mother-child pairs
Dr. Perak and colleagues used data from the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study and the HAPO Follow-up Study.
Participants were 2,302 mother-child pairs from nine field centers in Barbados, Canada, China, Thailand, United Kingdom, and the United States, and represented a racially and ethnically diverse cohort.
The mean ages were 29.6 years for pregnant mothers and 11.3 years for children. The pregnancies occurred between 2000 and 2006, and the children were examined from 2013 to 2016, when the children were aged 10-14 years.
Using the American Heart Association’s definition of cardiovascular health, the scientists categorized pregnancy health for mothers based on their measures of body mass index, blood pressure, total cholesterol, glucose level, and smoking status at 28 weeks’ gestation. These five metrics of gestational cardiovascular health have been significantly associated with adverse pregnancy outcomes.
They categorized cardiovascular health for offspring at age 10-14 years based on four of these five metrics: body mass index, blood pressure, cholesterol, and glucose.
Only 32.8% of mothers and 42.2% of children had ideal cardiovascular health.
In analyses adjusted for pregnancy and birth outcomes, the associations seen between poor gestational maternal health and offspring cardiovascular health persisted but were attenuated.
Dr. Perak reported receiving grants from the Woman’s Board of Northwestern Memorial Hospital; the Dixon Family; the American Heart Association; and the National Heart, Lung, and Blood Institute. Dr. Daniels reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
New-onset arrhythmias low in COVID-19 and flu
Among 3,970 patients treated during the early months of the pandemic, new onset AF/AFL was seen in 4%, matching the 4% incidence found in a historic cohort of patients hospitalized with influenza.
On the other hand, mortality was similarly high in both groups of patients studied with AF/AFL, showing a 77% increased risk of death in COVID-19 and a 78% increased risk in influenza, a team from Icahn School of Medicine at Mount Sinai in New York reported.
“We saw new onset Afib and flutter in a minority of patients and it was associated with much higher mortality, but the point is that this increase is basically the same as what you see in influenza, which we feel is an indication that this is more of a generalized response to the inflammatory milieu of such a severe viral illness, as opposed to something specific to COVID,” Vivek Y. Reddy, MD, said in the report, published online Feb. 25 in JACC: Clinical Electrophysiology.
“Here we see, with a similar respiratory virus used as controls, that the results are exactly what I would have expected to see, which is that where there is a lot of inflammation, we see Afib,” said John Mandrola, MD, of Baptist Medical Associates, Louisville, Ky., who was not involved with the study.
“We need more studies like this one because we know SARS-CoV-2 is a bad virus that may have important effects on the heart, but all the of research done so far has been problematic because it didn’t include controls.”
Atrial arrhythmias in COVID and flu
Dr. Reddy and coinvestigators performed a retrospective analysis of a large cohort of patients admitted with laboratory-confirmed COVID-19 during Feb. 4-April 22, 2020, to one of five hospitals within the Mount Sinai Health System.
Their comparator arm included 1,420 patients with confirmed influenza A or B hospitalized between Jan. 1, 2017, and Jan. 1, 2020. For both cohorts, automated electronic record abstraction was used and all patient data were de-identified prior to analysis. In the COVID-19 cohort, a manual review of 1,110 charts was also performed.
Compared with those who did not develop AF/AFL, COVID-19 patients with newly detected AF/AFL and COVID-19 were older (74 vs. 66 years; P < .01) and had higher levels of inflammatory markers, including C-reactive protein and interleukin-6, and higher troponin and D-dimer levels (all P < .01).
Overall, including those with a history of atrial arrhythmias, 10% of patients with hospitalized COVID-19 (13% in the manual review) and 12% of those with influenza had AF/AFL detected during their hospitalization.
Mortality at 30 days was higher in COVID-19 patients with AF/AFL compared to those without (46% vs. 26%; P < .01), as were the rates of intubation (27% vs. 15%; relative risk, 1.8; P < .01), and stroke (1.6% vs. 0.6%, RR, 2.7; P = .05).
Despite having more comorbidities, in-hospital mortality was significantly lower in the influenza cohort overall, compared to the COVID-19 cohort (9% vs. 29%; P < .01), reflecting the higher case fatality rate in COVID-19, Dr. Reddy, director of cardiac arrhythmia services at Mount Sinai Hospital, said in an interview.
But as with COVID-19, those influenza patients who had in-hospital AF/AFL were more likely to require intubation (14% vs. 7%; P = .004) or die (16% vs. 10%; P = .003).
“The data are not perfect and there are always limitations when doing an observational study using historic controls, but my guess would be that if we looked at other databases and other populations hospitalized for severe illness, we’d likely see something similar because when the body is inflamed, you’re more likely to see Afib,” said Dr. Mandrola.
Dr. Reddy concurred, noting that they considered comparing other populations to COVID-19 patients, including those with “just generalized severe illness,” but in the end felt there were many similarities between influenza and COVID-19, even though mortality in the latter is higher.
“It would be interesting for people to look at other illnesses and see if they find the same thing,” he said.
Dr. Reddy reported having no disclosures relevant to COVID-19. Dr. Mandrola is chief cardiology correspondent for Medscape.com. He reported having no relevant disclosures. MDedge is a member of the Medscape Professional Network.
Among 3,970 patients treated during the early months of the pandemic, new onset AF/AFL was seen in 4%, matching the 4% incidence found in a historic cohort of patients hospitalized with influenza.
On the other hand, mortality was similarly high in both groups of patients studied with AF/AFL, showing a 77% increased risk of death in COVID-19 and a 78% increased risk in influenza, a team from Icahn School of Medicine at Mount Sinai in New York reported.
“We saw new onset Afib and flutter in a minority of patients and it was associated with much higher mortality, but the point is that this increase is basically the same as what you see in influenza, which we feel is an indication that this is more of a generalized response to the inflammatory milieu of such a severe viral illness, as opposed to something specific to COVID,” Vivek Y. Reddy, MD, said in the report, published online Feb. 25 in JACC: Clinical Electrophysiology.
“Here we see, with a similar respiratory virus used as controls, that the results are exactly what I would have expected to see, which is that where there is a lot of inflammation, we see Afib,” said John Mandrola, MD, of Baptist Medical Associates, Louisville, Ky., who was not involved with the study.
“We need more studies like this one because we know SARS-CoV-2 is a bad virus that may have important effects on the heart, but all the of research done so far has been problematic because it didn’t include controls.”
Atrial arrhythmias in COVID and flu
Dr. Reddy and coinvestigators performed a retrospective analysis of a large cohort of patients admitted with laboratory-confirmed COVID-19 during Feb. 4-April 22, 2020, to one of five hospitals within the Mount Sinai Health System.
Their comparator arm included 1,420 patients with confirmed influenza A or B hospitalized between Jan. 1, 2017, and Jan. 1, 2020. For both cohorts, automated electronic record abstraction was used and all patient data were de-identified prior to analysis. In the COVID-19 cohort, a manual review of 1,110 charts was also performed.
Compared with those who did not develop AF/AFL, COVID-19 patients with newly detected AF/AFL and COVID-19 were older (74 vs. 66 years; P < .01) and had higher levels of inflammatory markers, including C-reactive protein and interleukin-6, and higher troponin and D-dimer levels (all P < .01).
Overall, including those with a history of atrial arrhythmias, 10% of patients with hospitalized COVID-19 (13% in the manual review) and 12% of those with influenza had AF/AFL detected during their hospitalization.
Mortality at 30 days was higher in COVID-19 patients with AF/AFL compared to those without (46% vs. 26%; P < .01), as were the rates of intubation (27% vs. 15%; relative risk, 1.8; P < .01), and stroke (1.6% vs. 0.6%, RR, 2.7; P = .05).
Despite having more comorbidities, in-hospital mortality was significantly lower in the influenza cohort overall, compared to the COVID-19 cohort (9% vs. 29%; P < .01), reflecting the higher case fatality rate in COVID-19, Dr. Reddy, director of cardiac arrhythmia services at Mount Sinai Hospital, said in an interview.
But as with COVID-19, those influenza patients who had in-hospital AF/AFL were more likely to require intubation (14% vs. 7%; P = .004) or die (16% vs. 10%; P = .003).
“The data are not perfect and there are always limitations when doing an observational study using historic controls, but my guess would be that if we looked at other databases and other populations hospitalized for severe illness, we’d likely see something similar because when the body is inflamed, you’re more likely to see Afib,” said Dr. Mandrola.
Dr. Reddy concurred, noting that they considered comparing other populations to COVID-19 patients, including those with “just generalized severe illness,” but in the end felt there were many similarities between influenza and COVID-19, even though mortality in the latter is higher.
“It would be interesting for people to look at other illnesses and see if they find the same thing,” he said.
Dr. Reddy reported having no disclosures relevant to COVID-19. Dr. Mandrola is chief cardiology correspondent for Medscape.com. He reported having no relevant disclosures. MDedge is a member of the Medscape Professional Network.
Among 3,970 patients treated during the early months of the pandemic, new onset AF/AFL was seen in 4%, matching the 4% incidence found in a historic cohort of patients hospitalized with influenza.
On the other hand, mortality was similarly high in both groups of patients studied with AF/AFL, showing a 77% increased risk of death in COVID-19 and a 78% increased risk in influenza, a team from Icahn School of Medicine at Mount Sinai in New York reported.
“We saw new onset Afib and flutter in a minority of patients and it was associated with much higher mortality, but the point is that this increase is basically the same as what you see in influenza, which we feel is an indication that this is more of a generalized response to the inflammatory milieu of such a severe viral illness, as opposed to something specific to COVID,” Vivek Y. Reddy, MD, said in the report, published online Feb. 25 in JACC: Clinical Electrophysiology.
“Here we see, with a similar respiratory virus used as controls, that the results are exactly what I would have expected to see, which is that where there is a lot of inflammation, we see Afib,” said John Mandrola, MD, of Baptist Medical Associates, Louisville, Ky., who was not involved with the study.
“We need more studies like this one because we know SARS-CoV-2 is a bad virus that may have important effects on the heart, but all the of research done so far has been problematic because it didn’t include controls.”
Atrial arrhythmias in COVID and flu
Dr. Reddy and coinvestigators performed a retrospective analysis of a large cohort of patients admitted with laboratory-confirmed COVID-19 during Feb. 4-April 22, 2020, to one of five hospitals within the Mount Sinai Health System.
Their comparator arm included 1,420 patients with confirmed influenza A or B hospitalized between Jan. 1, 2017, and Jan. 1, 2020. For both cohorts, automated electronic record abstraction was used and all patient data were de-identified prior to analysis. In the COVID-19 cohort, a manual review of 1,110 charts was also performed.
Compared with those who did not develop AF/AFL, COVID-19 patients with newly detected AF/AFL and COVID-19 were older (74 vs. 66 years; P < .01) and had higher levels of inflammatory markers, including C-reactive protein and interleukin-6, and higher troponin and D-dimer levels (all P < .01).
Overall, including those with a history of atrial arrhythmias, 10% of patients with hospitalized COVID-19 (13% in the manual review) and 12% of those with influenza had AF/AFL detected during their hospitalization.
Mortality at 30 days was higher in COVID-19 patients with AF/AFL compared to those without (46% vs. 26%; P < .01), as were the rates of intubation (27% vs. 15%; relative risk, 1.8; P < .01), and stroke (1.6% vs. 0.6%, RR, 2.7; P = .05).
Despite having more comorbidities, in-hospital mortality was significantly lower in the influenza cohort overall, compared to the COVID-19 cohort (9% vs. 29%; P < .01), reflecting the higher case fatality rate in COVID-19, Dr. Reddy, director of cardiac arrhythmia services at Mount Sinai Hospital, said in an interview.
But as with COVID-19, those influenza patients who had in-hospital AF/AFL were more likely to require intubation (14% vs. 7%; P = .004) or die (16% vs. 10%; P = .003).
“The data are not perfect and there are always limitations when doing an observational study using historic controls, but my guess would be that if we looked at other databases and other populations hospitalized for severe illness, we’d likely see something similar because when the body is inflamed, you’re more likely to see Afib,” said Dr. Mandrola.
Dr. Reddy concurred, noting that they considered comparing other populations to COVID-19 patients, including those with “just generalized severe illness,” but in the end felt there were many similarities between influenza and COVID-19, even though mortality in the latter is higher.
“It would be interesting for people to look at other illnesses and see if they find the same thing,” he said.
Dr. Reddy reported having no disclosures relevant to COVID-19. Dr. Mandrola is chief cardiology correspondent for Medscape.com. He reported having no relevant disclosures. MDedge is a member of the Medscape Professional Network.
FROM JACC: CLINICAL ELECTROPHYSIOLOGY
Myocardial injury seen on MRI in 54% of recovered COVID-19 patients
About half of 148 patients hospitalized with COVID-19 infection and elevated troponin levels had at least some evidence of myocardial injury on cardiac magnetic resonance (CMR) imaging 2 months later, a new study shows.
“Our results demonstrate that in this subset of patients surviving severe COVID-19 and with troponin elevation, ongoing localized myocardial inflammation, whilst less frequent than previously reported, remains present in a proportion of patients and may represent an emerging issue of clinical relevance,” wrote Marianna Fontana, MD, PhD, of University College London, and colleagues.
The cardiac abnormalities identified were classified as nonischemic (including “myocarditis-like” late gadolinium enhancement [LGE]) in 26% of the cohort; as related to ischemic heart disease (infarction or inducible ischemia) in 22%; and as dual pathology in 6%.
Left ventricular (LV) function was normal in 89% of the 148 patients. In the 17 patients (11%) with LV dysfunction, only four had an ejection fraction below 35%. Of the nine patients whose LV dysfunction was related to myocardial infarction, six had a known history of ischemic heart disease.
No patients with “myocarditis-pattern” LGE had regional wall motion abnormalities, and neither admission nor peak troponin values were predictive of the diagnosis of myocarditis.
The results were published online Feb. 18 in the European Heart Journal.
Glass half full
Taking a “glass half full” approach, co–senior author Graham D. Cole, MD, PhD, noted on Twitter that nearly half the patients had no major cardiac abnormalities on CMR just 2 months after a bout with troponin-positive COVID-19.
“We think this is important: Even in a group who had been very sick with raised troponin, it was common to find no evidence of heart damage,” said Dr. Cole, of the Royal Free London NHS Foundation Trust.
“We believe our data challenge the hypothesis that chronic inflammation, diffuse fibrosis, or long-term LV dysfunction is a dominant feature in those surviving COVID-19,” the investigators concluded in their report.
In an interview, Dr. Fontana explained further: “It has been reported in an early ‘pathfinder’ study that two-thirds of patients recovered from COVID-19 had CMR evidence of abnormal findings with a high incidence of elevated T1 and T2 in keeping with diffuse fibrosis and edema. Our findings with a larger, multicenter study and better controls show low rates of heart impairment and much less ongoing inflammation, which is reassuring.”
She also noted that the different patterns of injury suggest that different mechanisms are at play, including the possibility that “at least some of the found damage might have been preexisting, because people with heart damage are more likely to get severe disease.”
The investigators, including first author Tushar Kotecha, MBChB, PhD, of the Royal Free London NHS Foundation Trust, also noted that myocarditis-like injury was limited to three or fewer myocardial segments in 88% of cases with no associated ventricular dysfunction, and that biventricular function was no different than in those without myocarditis.
“We use the word ‘myocarditis-like’ but we don’t have histology,” Dr. Fontana said. “Our group actually suspects a lot of this will be microvascular clotting (microangiopathic thrombosis). This is exciting, as newer anticoagulation strategies – for example, those being tried in RECOVERY – may have benefit.”
Aloke V. Finn, MD, of the CVPath Institute in Gaithersburg, Md., wishes researchers would stop using the term myocarditis altogether to describe clinical or imaging findings in COVID-19.
“MRI can’t diagnose myocarditis. It is a specific diagnosis that requires, ideally, histology, as the investigators acknowledged,” Dr. Finn said in an interview.
His group at CVPath recently published data showing pathologic evidence of myocarditis after SARS-CoV-2 infection, as reported by theheart.org | Medscape Cardiology.
“As a clinician, when I think of myocarditis, I look at the echo and an LV gram, and I see if there is a wall motion abnormality and troponin elevation, but with normal coronary arteries. And if all that is there, then I think about myocarditis in my differential diagnosis,” he said. “But in most of these cases, as the authors rightly point out, most patients did not have what is necessary to really entertain a diagnosis of myocarditis.”
He agreed with Dr. Fontana’s suggestion that what the CMR might be picking up in these survivors is microthrombi, as his group saw in their recent autopsy study.
“It’s very possible these findings are concordant with the recent autopsy studies done by my group and others in terms of detecting the presence of microthrombi, but we don’t know this for certain because no one has ever studied this entity before in the clinic and we don’t really know how microthrombi might appear on CMR.”
Largest study to date
The 148 participants (mean age, 64 years; 70% male) in the largest study to date to investigate convalescing COVID-19 patients who had elevated troponins – something identified early in the pandemic as a risk factor for worse outcomes in COVID-19 – were treated at one of six hospitals in London.
Patients who had abnormal troponin levels were offered an MRI scan of the heart after discharge and were compared with those from a control group of patients who had not had COVID-19 and with 40 healthy volunteers.
Median length of stay was 9 days, and 32% of patients required ventilatory support in the intensive care unit.
Just over half the patients (57%) had hypertension, 7% had had a previous myocardial infarction, 34% had diabetes, 46% had hypercholesterolemia, and 24% were smokers. Mean body mass index was 28.5 kg/m2.
CMR follow-up was conducted a median of 68 days after confirmation of a COVID-19 diagnosis.
On Twitter, Dr. Cole noted that the findings are subject to both survivor bias and referral bias. “We didn’t scan frail patients where the clinician felt [CMR] was unlikely to inform management.”
The findings, said Dr. Fontana, “say nothing about what happens to people who are not hospitalized with COVID, or those who are hospitalized but without elevated troponin.”
What they do offer, particularly if replicated, is a way forward in identifying patients at higher or lower risk for long-term sequelae and inform strategies that could improve outcomes, she added.
A version of this article first appeared on Medscape.com.
About half of 148 patients hospitalized with COVID-19 infection and elevated troponin levels had at least some evidence of myocardial injury on cardiac magnetic resonance (CMR) imaging 2 months later, a new study shows.
“Our results demonstrate that in this subset of patients surviving severe COVID-19 and with troponin elevation, ongoing localized myocardial inflammation, whilst less frequent than previously reported, remains present in a proportion of patients and may represent an emerging issue of clinical relevance,” wrote Marianna Fontana, MD, PhD, of University College London, and colleagues.
The cardiac abnormalities identified were classified as nonischemic (including “myocarditis-like” late gadolinium enhancement [LGE]) in 26% of the cohort; as related to ischemic heart disease (infarction or inducible ischemia) in 22%; and as dual pathology in 6%.
Left ventricular (LV) function was normal in 89% of the 148 patients. In the 17 patients (11%) with LV dysfunction, only four had an ejection fraction below 35%. Of the nine patients whose LV dysfunction was related to myocardial infarction, six had a known history of ischemic heart disease.

No patients with “myocarditis-pattern” LGE had regional wall motion abnormalities, and neither admission nor peak troponin values were predictive of the diagnosis of myocarditis.
The results were published online Feb. 18 in the European Heart Journal.
Glass half full
Taking a “glass half full” approach, co–senior author Graham D. Cole, MD, PhD, noted on Twitter that nearly half the patients had no major cardiac abnormalities on CMR just 2 months after a bout with troponin-positive COVID-19.
“We think this is important: Even in a group who had been very sick with raised troponin, it was common to find no evidence of heart damage,” said Dr. Cole, of the Royal Free London NHS Foundation Trust.
“We believe our data challenge the hypothesis that chronic inflammation, diffuse fibrosis, or long-term LV dysfunction is a dominant feature in those surviving COVID-19,” the investigators concluded in their report.
In an interview, Dr. Fontana explained further: “It has been reported in an early ‘pathfinder’ study that two-thirds of patients recovered from COVID-19 had CMR evidence of abnormal findings with a high incidence of elevated T1 and T2 in keeping with diffuse fibrosis and edema. Our findings with a larger, multicenter study and better controls show low rates of heart impairment and much less ongoing inflammation, which is reassuring.”
She also noted that the different patterns of injury suggest that different mechanisms are at play, including the possibility that “at least some of the found damage might have been preexisting, because people with heart damage are more likely to get severe disease.”
The investigators, including first author Tushar Kotecha, MBChB, PhD, of the Royal Free London NHS Foundation Trust, also noted that myocarditis-like injury was limited to three or fewer myocardial segments in 88% of cases with no associated ventricular dysfunction, and that biventricular function was no different than in those without myocarditis.
“We use the word ‘myocarditis-like’ but we don’t have histology,” Dr. Fontana said. “Our group actually suspects a lot of this will be microvascular clotting (microangiopathic thrombosis). This is exciting, as newer anticoagulation strategies – for example, those being tried in RECOVERY – may have benefit.”
Aloke V. Finn, MD, of the CVPath Institute in Gaithersburg, Md., wishes researchers would stop using the term myocarditis altogether to describe clinical or imaging findings in COVID-19.
“MRI can’t diagnose myocarditis. It is a specific diagnosis that requires, ideally, histology, as the investigators acknowledged,” Dr. Finn said in an interview.
His group at CVPath recently published data showing pathologic evidence of myocarditis after SARS-CoV-2 infection, as reported by theheart.org | Medscape Cardiology.
“As a clinician, when I think of myocarditis, I look at the echo and an LV gram, and I see if there is a wall motion abnormality and troponin elevation, but with normal coronary arteries. And if all that is there, then I think about myocarditis in my differential diagnosis,” he said. “But in most of these cases, as the authors rightly point out, most patients did not have what is necessary to really entertain a diagnosis of myocarditis.”
He agreed with Dr. Fontana’s suggestion that what the CMR might be picking up in these survivors is microthrombi, as his group saw in their recent autopsy study.
“It’s very possible these findings are concordant with the recent autopsy studies done by my group and others in terms of detecting the presence of microthrombi, but we don’t know this for certain because no one has ever studied this entity before in the clinic and we don’t really know how microthrombi might appear on CMR.”
Largest study to date
The 148 participants (mean age, 64 years; 70% male) in the largest study to date to investigate convalescing COVID-19 patients who had elevated troponins – something identified early in the pandemic as a risk factor for worse outcomes in COVID-19 – were treated at one of six hospitals in London.
Patients who had abnormal troponin levels were offered an MRI scan of the heart after discharge and were compared with those from a control group of patients who had not had COVID-19 and with 40 healthy volunteers.
Median length of stay was 9 days, and 32% of patients required ventilatory support in the intensive care unit.
Just over half the patients (57%) had hypertension, 7% had had a previous myocardial infarction, 34% had diabetes, 46% had hypercholesterolemia, and 24% were smokers. Mean body mass index was 28.5 kg/m2.
CMR follow-up was conducted a median of 68 days after confirmation of a COVID-19 diagnosis.
On Twitter, Dr. Cole noted that the findings are subject to both survivor bias and referral bias. “We didn’t scan frail patients where the clinician felt [CMR] was unlikely to inform management.”
The findings, said Dr. Fontana, “say nothing about what happens to people who are not hospitalized with COVID, or those who are hospitalized but without elevated troponin.”
What they do offer, particularly if replicated, is a way forward in identifying patients at higher or lower risk for long-term sequelae and inform strategies that could improve outcomes, she added.
A version of this article first appeared on Medscape.com.
About half of 148 patients hospitalized with COVID-19 infection and elevated troponin levels had at least some evidence of myocardial injury on cardiac magnetic resonance (CMR) imaging 2 months later, a new study shows.
“Our results demonstrate that in this subset of patients surviving severe COVID-19 and with troponin elevation, ongoing localized myocardial inflammation, whilst less frequent than previously reported, remains present in a proportion of patients and may represent an emerging issue of clinical relevance,” wrote Marianna Fontana, MD, PhD, of University College London, and colleagues.
The cardiac abnormalities identified were classified as nonischemic (including “myocarditis-like” late gadolinium enhancement [LGE]) in 26% of the cohort; as related to ischemic heart disease (infarction or inducible ischemia) in 22%; and as dual pathology in 6%.
Left ventricular (LV) function was normal in 89% of the 148 patients. In the 17 patients (11%) with LV dysfunction, only four had an ejection fraction below 35%. Of the nine patients whose LV dysfunction was related to myocardial infarction, six had a known history of ischemic heart disease.

No patients with “myocarditis-pattern” LGE had regional wall motion abnormalities, and neither admission nor peak troponin values were predictive of the diagnosis of myocarditis.
The results were published online Feb. 18 in the European Heart Journal.
Glass half full
Taking a “glass half full” approach, co–senior author Graham D. Cole, MD, PhD, noted on Twitter that nearly half the patients had no major cardiac abnormalities on CMR just 2 months after a bout with troponin-positive COVID-19.
“We think this is important: Even in a group who had been very sick with raised troponin, it was common to find no evidence of heart damage,” said Dr. Cole, of the Royal Free London NHS Foundation Trust.
“We believe our data challenge the hypothesis that chronic inflammation, diffuse fibrosis, or long-term LV dysfunction is a dominant feature in those surviving COVID-19,” the investigators concluded in their report.
In an interview, Dr. Fontana explained further: “It has been reported in an early ‘pathfinder’ study that two-thirds of patients recovered from COVID-19 had CMR evidence of abnormal findings with a high incidence of elevated T1 and T2 in keeping with diffuse fibrosis and edema. Our findings with a larger, multicenter study and better controls show low rates of heart impairment and much less ongoing inflammation, which is reassuring.”
She also noted that the different patterns of injury suggest that different mechanisms are at play, including the possibility that “at least some of the found damage might have been preexisting, because people with heart damage are more likely to get severe disease.”
The investigators, including first author Tushar Kotecha, MBChB, PhD, of the Royal Free London NHS Foundation Trust, also noted that myocarditis-like injury was limited to three or fewer myocardial segments in 88% of cases with no associated ventricular dysfunction, and that biventricular function was no different than in those without myocarditis.
“We use the word ‘myocarditis-like’ but we don’t have histology,” Dr. Fontana said. “Our group actually suspects a lot of this will be microvascular clotting (microangiopathic thrombosis). This is exciting, as newer anticoagulation strategies – for example, those being tried in RECOVERY – may have benefit.”
Aloke V. Finn, MD, of the CVPath Institute in Gaithersburg, Md., wishes researchers would stop using the term myocarditis altogether to describe clinical or imaging findings in COVID-19.
“MRI can’t diagnose myocarditis. It is a specific diagnosis that requires, ideally, histology, as the investigators acknowledged,” Dr. Finn said in an interview.
His group at CVPath recently published data showing pathologic evidence of myocarditis after SARS-CoV-2 infection, as reported by theheart.org | Medscape Cardiology.
“As a clinician, when I think of myocarditis, I look at the echo and an LV gram, and I see if there is a wall motion abnormality and troponin elevation, but with normal coronary arteries. And if all that is there, then I think about myocarditis in my differential diagnosis,” he said. “But in most of these cases, as the authors rightly point out, most patients did not have what is necessary to really entertain a diagnosis of myocarditis.”
He agreed with Dr. Fontana’s suggestion that what the CMR might be picking up in these survivors is microthrombi, as his group saw in their recent autopsy study.
“It’s very possible these findings are concordant with the recent autopsy studies done by my group and others in terms of detecting the presence of microthrombi, but we don’t know this for certain because no one has ever studied this entity before in the clinic and we don’t really know how microthrombi might appear on CMR.”
Largest study to date
The 148 participants (mean age, 64 years; 70% male) in the largest study to date to investigate convalescing COVID-19 patients who had elevated troponins – something identified early in the pandemic as a risk factor for worse outcomes in COVID-19 – were treated at one of six hospitals in London.
Patients who had abnormal troponin levels were offered an MRI scan of the heart after discharge and were compared with those from a control group of patients who had not had COVID-19 and with 40 healthy volunteers.
Median length of stay was 9 days, and 32% of patients required ventilatory support in the intensive care unit.
Just over half the patients (57%) had hypertension, 7% had had a previous myocardial infarction, 34% had diabetes, 46% had hypercholesterolemia, and 24% were smokers. Mean body mass index was 28.5 kg/m2.
CMR follow-up was conducted a median of 68 days after confirmation of a COVID-19 diagnosis.
On Twitter, Dr. Cole noted that the findings are subject to both survivor bias and referral bias. “We didn’t scan frail patients where the clinician felt [CMR] was unlikely to inform management.”
The findings, said Dr. Fontana, “say nothing about what happens to people who are not hospitalized with COVID, or those who are hospitalized but without elevated troponin.”
What they do offer, particularly if replicated, is a way forward in identifying patients at higher or lower risk for long-term sequelae and inform strategies that could improve outcomes, she added.
A version of this article first appeared on Medscape.com.
Microthrombi, necrosis seen in COVID-19 hearts on autopsy
Autopsies on patients who died from COVID-19 are providing important clues on how to treat the disease. In an analysis of 40 hearts from COVID-19 patients who died early in the pandemic, myocyte necrosis was seen in 14 hearts, or 35%.
In the majority of these hearts, pathologists found both small areas of focal necrosis and cardiac thrombi, most of which were microthrombi in myocardial capillaries, arterioles, and small muscular cells.
In an interview, senior author Aloke V. Finn, MD, CVPath Institute, Gaithersburg, Md., stressed the importance of understanding what they saw, but also what they didn’t see.
“What we saw in the majority of patients with myocardial injury were these small areas of infarct and microthrombi in small vessels. What we didn’t see was any evidence of myocarditis and or huge infarcts in, like, the LAD artery,” he said.
“What we’re seeing here is not clinically detectable. ... There is no test that will tell you there are microthrombi and no imaging tests that will show these focal areas of necrosis, but that doesn’t mean it’s not there,” he added.
The finding of myocyte necrosis in about one-third of samples is consistent with another study that showed that 30%-40% of patients hospitalized with COVID-19 have elevated troponins, noted Dr. Finn. The investigators were unable to obtain troponin levels on their patients, which could limit the clinical translation of myocardial necrosis detected at autopsy.
Dr. Finn and colleagues, including first author Dario Pellegrini, MD, from Ospedale Papa Giovanni XXIII in Bergamo, Italy, published their findings online in Circulation on Jan. 22, 2020.
The report is a follow-up to another just published by Dr. Finn’s group in the Journal of the American College of Cardiology, which showed that myocarditis is a very rare finding in COVID-19 autopsies.
Only three of 14 individuals (21.4%) with evidence of myocyte necrosis showed evidence of acute MI, which Dr. Finn and colleagues define as an area of necrosis at least 1 cm2 in size. The remaining 11 (78.6%) had only discrete areas of myocyte necrosis (>20 necrotic myocytes with an area of ≥0.05 mm2, but <1 cm2).
“This makes sense when we saw what type of thrombus there was in these cases; it wasn’t thrombus in major epicardial vessels but microthombi in small vessels,” said Dr. Finn.
In those with necrosis, cardiac thrombi were present in 11 of 14 (78.6%) cases, with 2 of 14 (14.2%) having epicardial coronary artery thrombi and 0 of 14 (64.3%) having microthrombi in myocardial capillaries, arterioles, and small muscular arteries.
Further supporting the role of COVID-19–related hypercoagulability as the cause of myocardial injury in many patients, the investigators noted that the incidence of severe coronary artery disease (defined as >75% cross sectional narrowing) did not differ significantly between those with and without necrosis.
COVID-19 vs. non–COVID-19 thrombi
Going one step further, Dr. Finn’s team compared cardiac microthrombi from their COVID-19–positive autopsy cases with intramyocardial thromboemboli from COVID-19 cases. They also compared the samples with aspirated thrombi obtained during primary percutaneous coronary intervention from uninfected and COVID-19–infected patients presenting with ST-segment elevation MI (STEMI).
The autopsy-obtained microthrombi had significantly more fibrin and terminal complement C5b-9 immunostaining than intramyocardial thromboemboli from COVID-19–negative subjects and than aspirated thrombi from either COVID-positive or COVID-negative STEMI patients.
“Basically, what we’re seeing in these thrombi is evidence of an immune-mediated reaction,” said Dr. Finn, explaining that complement C5b-9 is an innate immune system protein that circulates in the blood in response to any kind of activation of the immune system. “It is nonspecific but can also lead to coagulation problems,” he said.
Anticoagulation, yes, but dose unclear
These findings clearly support the use of anticoagulation in hospitalized COVID patients, said Jeffrey Weitz, MD, director of the Thrombosis & Atherosclerosis Research Institute, McMaster University, Hamilton, Ont. But the details of how much anticoagulation, what kind, and for whom are still a moving target.
“I think what we can say at this point is that these autopsy findings fit with previous studies that have shown microthrombi in the lungs and thrombi in the legs and gut, and support the notion that these patients should receive prophylactic doses of anticoagulants if they’re sick enough to be hospitalized,” said Dr. Weitz.
“But it’s not as simple as to say that this study shows clots form in the heart of COVID patients and therefore more anticoagulation is going to be better than less anticoagulation,” he said in an interview.
Recent top-line findings from three linked clinical trials – REMAP-CAP, ACTIV-4, and ATTACC – show that full-dose anticoagulation was beneficial in moderately ill patients hospitalized for COVID-19 and reduced the need for mechanical ventilation.
Moderately ill patients are those not in intensive care and who did not require organ support, such as mechanical ventilation, at the time of enrollment.
However, the same group reported findings in December that showed that routine use of full-dose anticoagulation when started in the ICU in critically ill patients was not beneficial and possibly harmful.
Dr. Weitz was only a little bit surprised by this finding of potential harm in the sickest patients. “I figured everybody should get prophylaxis but I wasn’t sure that everybody should get intensified anticoagulant. But my assumption was that if anybody is going to benefit from it, it would be the ICU patients.”
It was notable, said Dr. Weitz, that levels of D-dimer, a fibrin degradation product, were not associated with outcomes. “So, it doesn’t seem to be that patients with evidence of more clotting are more likely to benefit, which might indicate that it’s not the anticoagulant effect of the heparin that’s helping, but maybe the anti-inflammatory effect. At this point, we just don’t know.”
All three studies have paused enrollment of the critically ill subgroup, but are continuing to enroll patients with moderate illness and expect to publish results in the coming months, according to previous coverage from this news organization.
The study was funded by CVPath, a nonprofit institute that receives funding from a number of different industry entities. Dr. Finn and Dr. Weitz reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Autopsies on patients who died from COVID-19 are providing important clues on how to treat the disease. In an analysis of 40 hearts from COVID-19 patients who died early in the pandemic, myocyte necrosis was seen in 14 hearts, or 35%.
In the majority of these hearts, pathologists found both small areas of focal necrosis and cardiac thrombi, most of which were microthrombi in myocardial capillaries, arterioles, and small muscular cells.
In an interview, senior author Aloke V. Finn, MD, CVPath Institute, Gaithersburg, Md., stressed the importance of understanding what they saw, but also what they didn’t see.
“What we saw in the majority of patients with myocardial injury were these small areas of infarct and microthrombi in small vessels. What we didn’t see was any evidence of myocarditis and or huge infarcts in, like, the LAD artery,” he said.
“What we’re seeing here is not clinically detectable. ... There is no test that will tell you there are microthrombi and no imaging tests that will show these focal areas of necrosis, but that doesn’t mean it’s not there,” he added.
The finding of myocyte necrosis in about one-third of samples is consistent with another study that showed that 30%-40% of patients hospitalized with COVID-19 have elevated troponins, noted Dr. Finn. The investigators were unable to obtain troponin levels on their patients, which could limit the clinical translation of myocardial necrosis detected at autopsy.
Dr. Finn and colleagues, including first author Dario Pellegrini, MD, from Ospedale Papa Giovanni XXIII in Bergamo, Italy, published their findings online in Circulation on Jan. 22, 2020.
The report is a follow-up to another just published by Dr. Finn’s group in the Journal of the American College of Cardiology, which showed that myocarditis is a very rare finding in COVID-19 autopsies.
Only three of 14 individuals (21.4%) with evidence of myocyte necrosis showed evidence of acute MI, which Dr. Finn and colleagues define as an area of necrosis at least 1 cm2 in size. The remaining 11 (78.6%) had only discrete areas of myocyte necrosis (>20 necrotic myocytes with an area of ≥0.05 mm2, but <1 cm2).
“This makes sense when we saw what type of thrombus there was in these cases; it wasn’t thrombus in major epicardial vessels but microthombi in small vessels,” said Dr. Finn.
In those with necrosis, cardiac thrombi were present in 11 of 14 (78.6%) cases, with 2 of 14 (14.2%) having epicardial coronary artery thrombi and 0 of 14 (64.3%) having microthrombi in myocardial capillaries, arterioles, and small muscular arteries.
Further supporting the role of COVID-19–related hypercoagulability as the cause of myocardial injury in many patients, the investigators noted that the incidence of severe coronary artery disease (defined as >75% cross sectional narrowing) did not differ significantly between those with and without necrosis.
COVID-19 vs. non–COVID-19 thrombi
Going one step further, Dr. Finn’s team compared cardiac microthrombi from their COVID-19–positive autopsy cases with intramyocardial thromboemboli from COVID-19 cases. They also compared the samples with aspirated thrombi obtained during primary percutaneous coronary intervention from uninfected and COVID-19–infected patients presenting with ST-segment elevation MI (STEMI).
The autopsy-obtained microthrombi had significantly more fibrin and terminal complement C5b-9 immunostaining than intramyocardial thromboemboli from COVID-19–negative subjects and than aspirated thrombi from either COVID-positive or COVID-negative STEMI patients.
“Basically, what we’re seeing in these thrombi is evidence of an immune-mediated reaction,” said Dr. Finn, explaining that complement C5b-9 is an innate immune system protein that circulates in the blood in response to any kind of activation of the immune system. “It is nonspecific but can also lead to coagulation problems,” he said.
Anticoagulation, yes, but dose unclear
These findings clearly support the use of anticoagulation in hospitalized COVID patients, said Jeffrey Weitz, MD, director of the Thrombosis & Atherosclerosis Research Institute, McMaster University, Hamilton, Ont. But the details of how much anticoagulation, what kind, and for whom are still a moving target.
“I think what we can say at this point is that these autopsy findings fit with previous studies that have shown microthrombi in the lungs and thrombi in the legs and gut, and support the notion that these patients should receive prophylactic doses of anticoagulants if they’re sick enough to be hospitalized,” said Dr. Weitz.
“But it’s not as simple as to say that this study shows clots form in the heart of COVID patients and therefore more anticoagulation is going to be better than less anticoagulation,” he said in an interview.
Recent top-line findings from three linked clinical trials – REMAP-CAP, ACTIV-4, and ATTACC – show that full-dose anticoagulation was beneficial in moderately ill patients hospitalized for COVID-19 and reduced the need for mechanical ventilation.
Moderately ill patients are those not in intensive care and who did not require organ support, such as mechanical ventilation, at the time of enrollment.
However, the same group reported findings in December that showed that routine use of full-dose anticoagulation when started in the ICU in critically ill patients was not beneficial and possibly harmful.
Dr. Weitz was only a little bit surprised by this finding of potential harm in the sickest patients. “I figured everybody should get prophylaxis but I wasn’t sure that everybody should get intensified anticoagulant. But my assumption was that if anybody is going to benefit from it, it would be the ICU patients.”
It was notable, said Dr. Weitz, that levels of D-dimer, a fibrin degradation product, were not associated with outcomes. “So, it doesn’t seem to be that patients with evidence of more clotting are more likely to benefit, which might indicate that it’s not the anticoagulant effect of the heparin that’s helping, but maybe the anti-inflammatory effect. At this point, we just don’t know.”
All three studies have paused enrollment of the critically ill subgroup, but are continuing to enroll patients with moderate illness and expect to publish results in the coming months, according to previous coverage from this news organization.
The study was funded by CVPath, a nonprofit institute that receives funding from a number of different industry entities. Dr. Finn and Dr. Weitz reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Autopsies on patients who died from COVID-19 are providing important clues on how to treat the disease. In an analysis of 40 hearts from COVID-19 patients who died early in the pandemic, myocyte necrosis was seen in 14 hearts, or 35%.
In the majority of these hearts, pathologists found both small areas of focal necrosis and cardiac thrombi, most of which were microthrombi in myocardial capillaries, arterioles, and small muscular cells.
In an interview, senior author Aloke V. Finn, MD, CVPath Institute, Gaithersburg, Md., stressed the importance of understanding what they saw, but also what they didn’t see.
“What we saw in the majority of patients with myocardial injury were these small areas of infarct and microthrombi in small vessels. What we didn’t see was any evidence of myocarditis and or huge infarcts in, like, the LAD artery,” he said.
“What we’re seeing here is not clinically detectable. ... There is no test that will tell you there are microthrombi and no imaging tests that will show these focal areas of necrosis, but that doesn’t mean it’s not there,” he added.
The finding of myocyte necrosis in about one-third of samples is consistent with another study that showed that 30%-40% of patients hospitalized with COVID-19 have elevated troponins, noted Dr. Finn. The investigators were unable to obtain troponin levels on their patients, which could limit the clinical translation of myocardial necrosis detected at autopsy.
Dr. Finn and colleagues, including first author Dario Pellegrini, MD, from Ospedale Papa Giovanni XXIII in Bergamo, Italy, published their findings online in Circulation on Jan. 22, 2020.
The report is a follow-up to another just published by Dr. Finn’s group in the Journal of the American College of Cardiology, which showed that myocarditis is a very rare finding in COVID-19 autopsies.
Only three of 14 individuals (21.4%) with evidence of myocyte necrosis showed evidence of acute MI, which Dr. Finn and colleagues define as an area of necrosis at least 1 cm2 in size. The remaining 11 (78.6%) had only discrete areas of myocyte necrosis (>20 necrotic myocytes with an area of ≥0.05 mm2, but <1 cm2).
“This makes sense when we saw what type of thrombus there was in these cases; it wasn’t thrombus in major epicardial vessels but microthombi in small vessels,” said Dr. Finn.
In those with necrosis, cardiac thrombi were present in 11 of 14 (78.6%) cases, with 2 of 14 (14.2%) having epicardial coronary artery thrombi and 0 of 14 (64.3%) having microthrombi in myocardial capillaries, arterioles, and small muscular arteries.
Further supporting the role of COVID-19–related hypercoagulability as the cause of myocardial injury in many patients, the investigators noted that the incidence of severe coronary artery disease (defined as >75% cross sectional narrowing) did not differ significantly between those with and without necrosis.
COVID-19 vs. non–COVID-19 thrombi
Going one step further, Dr. Finn’s team compared cardiac microthrombi from their COVID-19–positive autopsy cases with intramyocardial thromboemboli from COVID-19 cases. They also compared the samples with aspirated thrombi obtained during primary percutaneous coronary intervention from uninfected and COVID-19–infected patients presenting with ST-segment elevation MI (STEMI).
The autopsy-obtained microthrombi had significantly more fibrin and terminal complement C5b-9 immunostaining than intramyocardial thromboemboli from COVID-19–negative subjects and than aspirated thrombi from either COVID-positive or COVID-negative STEMI patients.
“Basically, what we’re seeing in these thrombi is evidence of an immune-mediated reaction,” said Dr. Finn, explaining that complement C5b-9 is an innate immune system protein that circulates in the blood in response to any kind of activation of the immune system. “It is nonspecific but can also lead to coagulation problems,” he said.
Anticoagulation, yes, but dose unclear
These findings clearly support the use of anticoagulation in hospitalized COVID patients, said Jeffrey Weitz, MD, director of the Thrombosis & Atherosclerosis Research Institute, McMaster University, Hamilton, Ont. But the details of how much anticoagulation, what kind, and for whom are still a moving target.
“I think what we can say at this point is that these autopsy findings fit with previous studies that have shown microthrombi in the lungs and thrombi in the legs and gut, and support the notion that these patients should receive prophylactic doses of anticoagulants if they’re sick enough to be hospitalized,” said Dr. Weitz.
“But it’s not as simple as to say that this study shows clots form in the heart of COVID patients and therefore more anticoagulation is going to be better than less anticoagulation,” he said in an interview.
Recent top-line findings from three linked clinical trials – REMAP-CAP, ACTIV-4, and ATTACC – show that full-dose anticoagulation was beneficial in moderately ill patients hospitalized for COVID-19 and reduced the need for mechanical ventilation.
Moderately ill patients are those not in intensive care and who did not require organ support, such as mechanical ventilation, at the time of enrollment.
However, the same group reported findings in December that showed that routine use of full-dose anticoagulation when started in the ICU in critically ill patients was not beneficial and possibly harmful.
Dr. Weitz was only a little bit surprised by this finding of potential harm in the sickest patients. “I figured everybody should get prophylaxis but I wasn’t sure that everybody should get intensified anticoagulant. But my assumption was that if anybody is going to benefit from it, it would be the ICU patients.”
It was notable, said Dr. Weitz, that levels of D-dimer, a fibrin degradation product, were not associated with outcomes. “So, it doesn’t seem to be that patients with evidence of more clotting are more likely to benefit, which might indicate that it’s not the anticoagulant effect of the heparin that’s helping, but maybe the anti-inflammatory effect. At this point, we just don’t know.”
All three studies have paused enrollment of the critically ill subgroup, but are continuing to enroll patients with moderate illness and expect to publish results in the coming months, according to previous coverage from this news organization.
The study was funded by CVPath, a nonprofit institute that receives funding from a number of different industry entities. Dr. Finn and Dr. Weitz reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
CVD deaths rose, imaging declined during pandemic
While the direct toll of the COVID-19 pandemic is being tallied and shared on the nightly news, the indirect effects will undoubtedly take years to fully measure.
In two papers published online Jan. 11 in the Journal of the American College of Cardiology, researchers have started the process of quantifying the impact of the pandemic on the care of patients with cardiovascular disease (CVD).
In the first study, Rishi Wadhera, MD, MPP, MPhil, and colleagues from the Beth Israel Deaconess Medical Center and Harvard Medical School in Boston examined population-level data to determine how deaths from cardiovascular causes changed in the United States in the early months of the pandemic relative to the same periods in 2019.
In a second paper, Andrew J. Einstein, MD, PhD, from Columbia University Irving Medical Center/New York–Presbyterian Hospital and colleagues looked at the pandemic’s international impact on the diagnosis of heart disease.
Using data from the National Center for Health Statistics, Dr. Wadhera and colleagues compared death rates from cardiovascular causes in the United States from March 18, 2020, to June 2, 2020, (the first wave of the pandemic) and from Jan. 1, 2020, to March 17, 2020, (the period just before the pandemic started) and compared them to the same periods in 2019. ICD codes were used to identify underlying causes of death.
Relative to 2019, they found a significant increase in deaths from ischemic heart disease nationally (1.11; 95% confidence interval, 1.04-1.18), as well as an increase in deaths caused by hypertensive disease (1.17; 95% CI, 1.09-1.26). There was no apparent increase in deaths from heart failure, cerebrovascular disease, or other diseases of the circulatory system.
When they looked just at New York City, the area hit hardest during the early part of the pandemic, the relative increases in deaths from ischemic heart disease were more pronounced.
Deaths from ischemic heart disease or hypertensive diseases jumped 139% and 164%, respectively, between March 18, 2020, and June 2, 2020.
More modest increases in deaths were seen in the remainder of New York state, New Jersey, Michigan and Illinois, while Massachusetts and Louisiana did not see a change in cardiovascular deaths.
Several studies from different parts of the world have indicated a 40%-50% drop in hospitalization for myocardial infarction in the initial months of the pandemic, said Dr. Wadhera in an interview.
“We wanted to understand where did all the heart attacks go? And we worried that patients with urgent heart conditions were not seeking the medical care they needed. I think our data suggest that this may have been the case,” reported Dr. Wadhera.
“This very much reflects the reality of what we’re seeing on the ground,” he told this news organization. “After the initial surge ended, when hospital volumes began to return to normal, we saw patients come into the hospital who clearly had a heart attack during the surge months – and were now experiencing complications of that event – because they had initially not come into the hospital due to concerns about exposure to the virus.”
A limitation of their data, he stressed, is whether some deaths coded as CVD deaths were really deaths from undiagnosed COVID-19. “It’s possible that some portion of the increased deaths we observed really reflect the cardiovascular complications of undiagnosed COVID-19, because we know that testing was quite limited during the early first surge of cases.”
“I think that basically three factors – patients avoiding the health care system because of fear of getting COVID, health care systems being strained and overwhelmed leading to the deferral of cardiovascular care and semi-elective procedures, and the cardiovascular complications of COVID-19 itself – all probably collectively contributed to the rise in cardiovascular deaths that we observed,” said Dr. Wadhera.
In an accompanying editorial, Michael N. Young, MD, Geisel School of Medicine at Dartmouth, Lebanon, N.H., and colleagues write that these data, taken together with an earlier study showing an increase in out-of-hospital cardiac arrests at the pandemic peak in New York City, “support the notion of excess fatalities due to unattended comorbid illnesses.” That said, attribution of death in the COVID era “remains problematic.”
In the second article, Andrew Einstein, MD, PhD, and the INCAPS COVID Investigators Group took a broader approach and looked at the impact of COVID-19 on cardiac diagnostic procedures in over 100 countries.
The INCAPS (International Atomic Energy Agency Noninvasive Cardiology Protocols Study) group has for the past decade conducted numerous studies addressing the use of best practices and worldwide practice variation in CVD diagnosis.
For this effort, they sent a survey link to INCAPS participants worldwide, ultimately including 909 survey responses from 108 countries in the final analysis.
Compared with March 2019, overall procedure volume decreased 42% in March 2020 and 64% in April 2020.
The greatest decreases were seen in stress testing (78%) and transesophageal echocardiography (76%), both procedures, noted Dr. Einstein, associated with a greater risk of aerosolization.
“Whether as we reset after COVID we return to the same place in terms of the use of cardiovascular diagnostic testing remains to be seen, but it certainly poses an opportunity to improve our utilization of various modes of testing,” said Dr. Einstein.
Using regression analysis, Dr. Einstein and colleagues were able to see that sites located in low-income and lower-middle-income countries saw an additional 22% reduction in cardiac procedures and less availability of personal protective equipment (PPE) and telehealth.
Fifty-two percent of survey respondents reported significant shortages of N95 masks early in the pandemic, with fewer issues in supplies of gloves, gowns, and face shields. Lower-income countries were more likely to face significant PPE shortages and less likely to be able to implement telehealth strategies to make up for reduced in-person care. PPE shortage itself, however, was not related to lower procedural volume on multivariable regression.
“It all really begs the question of whether there is more that the world can do to help out the developing world in terms of managing the pandemic in all its facets,” said Dr. Einstein in an interview, adding he was “shocked” to learn how difficult it was for some lower-income countries to get sufficient PPE.
Did shutdowns go too far?
Calling this a “remarkable study,” an editorial written by Darryl P. Leong, MBBS, PhD, John W. Eikelboom, MBBS, and Salim Yusuf, MBBS, DPhil, all from McMaster University, Hamilton, Ont., suggests that perhaps health systems in some places went too far in closing down during the first wave of the pandemic, naming specifically Canada, Eastern Europe, and Saudi Arabia as examples.
“Although these measures were taken to prepare for the worst, overwhelming numbers of patients with COVID-19 did not materialize during the first wave of the pandemic in these countries. It is possible that delaying so-called nonessential services may have been unnecessary and potentially harmful, because it likely led to delays in providing care for the treatment of serious non–COVID-19 illnesses.”
Since then, more experience and more data have largely allowed hospital systems to “tackle the ebb and flow” of COVID-19 cases in ways that limit shutdowns of important health services, they said.
Given the more pronounced effect in low- and middle-income countries, they stressed the need to focus resources on ways to promote prevention and treatment that do not rely on diagnostic procedures.
“This calls for more emphasis on developing efficient systems of telehealth, especially in poorer countries or in remote settings in all countries,” Dr. Leong and colleagues conclude.
Dr. Wadhera has reported research support from the National Heart, Lung, and Blood Institute, along with fellow senior author Robert W. Yeh, MD, MBA, who has also received personal fees and grants from several companies not related to the submitted work. Dr. Einstein, Dr. Leong, Dr. Eikelboom, and Dr. Yusuf have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
While the direct toll of the COVID-19 pandemic is being tallied and shared on the nightly news, the indirect effects will undoubtedly take years to fully measure.
In two papers published online Jan. 11 in the Journal of the American College of Cardiology, researchers have started the process of quantifying the impact of the pandemic on the care of patients with cardiovascular disease (CVD).
In the first study, Rishi Wadhera, MD, MPP, MPhil, and colleagues from the Beth Israel Deaconess Medical Center and Harvard Medical School in Boston examined population-level data to determine how deaths from cardiovascular causes changed in the United States in the early months of the pandemic relative to the same periods in 2019.
In a second paper, Andrew J. Einstein, MD, PhD, from Columbia University Irving Medical Center/New York–Presbyterian Hospital and colleagues looked at the pandemic’s international impact on the diagnosis of heart disease.
Using data from the National Center for Health Statistics, Dr. Wadhera and colleagues compared death rates from cardiovascular causes in the United States from March 18, 2020, to June 2, 2020, (the first wave of the pandemic) and from Jan. 1, 2020, to March 17, 2020, (the period just before the pandemic started) and compared them to the same periods in 2019. ICD codes were used to identify underlying causes of death.
Relative to 2019, they found a significant increase in deaths from ischemic heart disease nationally (1.11; 95% confidence interval, 1.04-1.18), as well as an increase in deaths caused by hypertensive disease (1.17; 95% CI, 1.09-1.26). There was no apparent increase in deaths from heart failure, cerebrovascular disease, or other diseases of the circulatory system.
When they looked just at New York City, the area hit hardest during the early part of the pandemic, the relative increases in deaths from ischemic heart disease were more pronounced.
Deaths from ischemic heart disease or hypertensive diseases jumped 139% and 164%, respectively, between March 18, 2020, and June 2, 2020.
More modest increases in deaths were seen in the remainder of New York state, New Jersey, Michigan and Illinois, while Massachusetts and Louisiana did not see a change in cardiovascular deaths.
Several studies from different parts of the world have indicated a 40%-50% drop in hospitalization for myocardial infarction in the initial months of the pandemic, said Dr. Wadhera in an interview.
“We wanted to understand where did all the heart attacks go? And we worried that patients with urgent heart conditions were not seeking the medical care they needed. I think our data suggest that this may have been the case,” reported Dr. Wadhera.
“This very much reflects the reality of what we’re seeing on the ground,” he told this news organization. “After the initial surge ended, when hospital volumes began to return to normal, we saw patients come into the hospital who clearly had a heart attack during the surge months – and were now experiencing complications of that event – because they had initially not come into the hospital due to concerns about exposure to the virus.”
A limitation of their data, he stressed, is whether some deaths coded as CVD deaths were really deaths from undiagnosed COVID-19. “It’s possible that some portion of the increased deaths we observed really reflect the cardiovascular complications of undiagnosed COVID-19, because we know that testing was quite limited during the early first surge of cases.”
“I think that basically three factors – patients avoiding the health care system because of fear of getting COVID, health care systems being strained and overwhelmed leading to the deferral of cardiovascular care and semi-elective procedures, and the cardiovascular complications of COVID-19 itself – all probably collectively contributed to the rise in cardiovascular deaths that we observed,” said Dr. Wadhera.
In an accompanying editorial, Michael N. Young, MD, Geisel School of Medicine at Dartmouth, Lebanon, N.H., and colleagues write that these data, taken together with an earlier study showing an increase in out-of-hospital cardiac arrests at the pandemic peak in New York City, “support the notion of excess fatalities due to unattended comorbid illnesses.” That said, attribution of death in the COVID era “remains problematic.”
In the second article, Andrew Einstein, MD, PhD, and the INCAPS COVID Investigators Group took a broader approach and looked at the impact of COVID-19 on cardiac diagnostic procedures in over 100 countries.
The INCAPS (International Atomic Energy Agency Noninvasive Cardiology Protocols Study) group has for the past decade conducted numerous studies addressing the use of best practices and worldwide practice variation in CVD diagnosis.
For this effort, they sent a survey link to INCAPS participants worldwide, ultimately including 909 survey responses from 108 countries in the final analysis.
Compared with March 2019, overall procedure volume decreased 42% in March 2020 and 64% in April 2020.
The greatest decreases were seen in stress testing (78%) and transesophageal echocardiography (76%), both procedures, noted Dr. Einstein, associated with a greater risk of aerosolization.
“Whether as we reset after COVID we return to the same place in terms of the use of cardiovascular diagnostic testing remains to be seen, but it certainly poses an opportunity to improve our utilization of various modes of testing,” said Dr. Einstein.
Using regression analysis, Dr. Einstein and colleagues were able to see that sites located in low-income and lower-middle-income countries saw an additional 22% reduction in cardiac procedures and less availability of personal protective equipment (PPE) and telehealth.
Fifty-two percent of survey respondents reported significant shortages of N95 masks early in the pandemic, with fewer issues in supplies of gloves, gowns, and face shields. Lower-income countries were more likely to face significant PPE shortages and less likely to be able to implement telehealth strategies to make up for reduced in-person care. PPE shortage itself, however, was not related to lower procedural volume on multivariable regression.
“It all really begs the question of whether there is more that the world can do to help out the developing world in terms of managing the pandemic in all its facets,” said Dr. Einstein in an interview, adding he was “shocked” to learn how difficult it was for some lower-income countries to get sufficient PPE.
Did shutdowns go too far?
Calling this a “remarkable study,” an editorial written by Darryl P. Leong, MBBS, PhD, John W. Eikelboom, MBBS, and Salim Yusuf, MBBS, DPhil, all from McMaster University, Hamilton, Ont., suggests that perhaps health systems in some places went too far in closing down during the first wave of the pandemic, naming specifically Canada, Eastern Europe, and Saudi Arabia as examples.
“Although these measures were taken to prepare for the worst, overwhelming numbers of patients with COVID-19 did not materialize during the first wave of the pandemic in these countries. It is possible that delaying so-called nonessential services may have been unnecessary and potentially harmful, because it likely led to delays in providing care for the treatment of serious non–COVID-19 illnesses.”
Since then, more experience and more data have largely allowed hospital systems to “tackle the ebb and flow” of COVID-19 cases in ways that limit shutdowns of important health services, they said.
Given the more pronounced effect in low- and middle-income countries, they stressed the need to focus resources on ways to promote prevention and treatment that do not rely on diagnostic procedures.
“This calls for more emphasis on developing efficient systems of telehealth, especially in poorer countries or in remote settings in all countries,” Dr. Leong and colleagues conclude.
Dr. Wadhera has reported research support from the National Heart, Lung, and Blood Institute, along with fellow senior author Robert W. Yeh, MD, MBA, who has also received personal fees and grants from several companies not related to the submitted work. Dr. Einstein, Dr. Leong, Dr. Eikelboom, and Dr. Yusuf have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
While the direct toll of the COVID-19 pandemic is being tallied and shared on the nightly news, the indirect effects will undoubtedly take years to fully measure.
In two papers published online Jan. 11 in the Journal of the American College of Cardiology, researchers have started the process of quantifying the impact of the pandemic on the care of patients with cardiovascular disease (CVD).
In the first study, Rishi Wadhera, MD, MPP, MPhil, and colleagues from the Beth Israel Deaconess Medical Center and Harvard Medical School in Boston examined population-level data to determine how deaths from cardiovascular causes changed in the United States in the early months of the pandemic relative to the same periods in 2019.
In a second paper, Andrew J. Einstein, MD, PhD, from Columbia University Irving Medical Center/New York–Presbyterian Hospital and colleagues looked at the pandemic’s international impact on the diagnosis of heart disease.
Using data from the National Center for Health Statistics, Dr. Wadhera and colleagues compared death rates from cardiovascular causes in the United States from March 18, 2020, to June 2, 2020, (the first wave of the pandemic) and from Jan. 1, 2020, to March 17, 2020, (the period just before the pandemic started) and compared them to the same periods in 2019. ICD codes were used to identify underlying causes of death.
Relative to 2019, they found a significant increase in deaths from ischemic heart disease nationally (1.11; 95% confidence interval, 1.04-1.18), as well as an increase in deaths caused by hypertensive disease (1.17; 95% CI, 1.09-1.26). There was no apparent increase in deaths from heart failure, cerebrovascular disease, or other diseases of the circulatory system.
When they looked just at New York City, the area hit hardest during the early part of the pandemic, the relative increases in deaths from ischemic heart disease were more pronounced.
Deaths from ischemic heart disease or hypertensive diseases jumped 139% and 164%, respectively, between March 18, 2020, and June 2, 2020.
More modest increases in deaths were seen in the remainder of New York state, New Jersey, Michigan and Illinois, while Massachusetts and Louisiana did not see a change in cardiovascular deaths.
Several studies from different parts of the world have indicated a 40%-50% drop in hospitalization for myocardial infarction in the initial months of the pandemic, said Dr. Wadhera in an interview.
“We wanted to understand where did all the heart attacks go? And we worried that patients with urgent heart conditions were not seeking the medical care they needed. I think our data suggest that this may have been the case,” reported Dr. Wadhera.
“This very much reflects the reality of what we’re seeing on the ground,” he told this news organization. “After the initial surge ended, when hospital volumes began to return to normal, we saw patients come into the hospital who clearly had a heart attack during the surge months – and were now experiencing complications of that event – because they had initially not come into the hospital due to concerns about exposure to the virus.”
A limitation of their data, he stressed, is whether some deaths coded as CVD deaths were really deaths from undiagnosed COVID-19. “It’s possible that some portion of the increased deaths we observed really reflect the cardiovascular complications of undiagnosed COVID-19, because we know that testing was quite limited during the early first surge of cases.”
“I think that basically three factors – patients avoiding the health care system because of fear of getting COVID, health care systems being strained and overwhelmed leading to the deferral of cardiovascular care and semi-elective procedures, and the cardiovascular complications of COVID-19 itself – all probably collectively contributed to the rise in cardiovascular deaths that we observed,” said Dr. Wadhera.
In an accompanying editorial, Michael N. Young, MD, Geisel School of Medicine at Dartmouth, Lebanon, N.H., and colleagues write that these data, taken together with an earlier study showing an increase in out-of-hospital cardiac arrests at the pandemic peak in New York City, “support the notion of excess fatalities due to unattended comorbid illnesses.” That said, attribution of death in the COVID era “remains problematic.”
In the second article, Andrew Einstein, MD, PhD, and the INCAPS COVID Investigators Group took a broader approach and looked at the impact of COVID-19 on cardiac diagnostic procedures in over 100 countries.
The INCAPS (International Atomic Energy Agency Noninvasive Cardiology Protocols Study) group has for the past decade conducted numerous studies addressing the use of best practices and worldwide practice variation in CVD diagnosis.
For this effort, they sent a survey link to INCAPS participants worldwide, ultimately including 909 survey responses from 108 countries in the final analysis.
Compared with March 2019, overall procedure volume decreased 42% in March 2020 and 64% in April 2020.
The greatest decreases were seen in stress testing (78%) and transesophageal echocardiography (76%), both procedures, noted Dr. Einstein, associated with a greater risk of aerosolization.
“Whether as we reset after COVID we return to the same place in terms of the use of cardiovascular diagnostic testing remains to be seen, but it certainly poses an opportunity to improve our utilization of various modes of testing,” said Dr. Einstein.
Using regression analysis, Dr. Einstein and colleagues were able to see that sites located in low-income and lower-middle-income countries saw an additional 22% reduction in cardiac procedures and less availability of personal protective equipment (PPE) and telehealth.
Fifty-two percent of survey respondents reported significant shortages of N95 masks early in the pandemic, with fewer issues in supplies of gloves, gowns, and face shields. Lower-income countries were more likely to face significant PPE shortages and less likely to be able to implement telehealth strategies to make up for reduced in-person care. PPE shortage itself, however, was not related to lower procedural volume on multivariable regression.
“It all really begs the question of whether there is more that the world can do to help out the developing world in terms of managing the pandemic in all its facets,” said Dr. Einstein in an interview, adding he was “shocked” to learn how difficult it was for some lower-income countries to get sufficient PPE.
Did shutdowns go too far?
Calling this a “remarkable study,” an editorial written by Darryl P. Leong, MBBS, PhD, John W. Eikelboom, MBBS, and Salim Yusuf, MBBS, DPhil, all from McMaster University, Hamilton, Ont., suggests that perhaps health systems in some places went too far in closing down during the first wave of the pandemic, naming specifically Canada, Eastern Europe, and Saudi Arabia as examples.
“Although these measures were taken to prepare for the worst, overwhelming numbers of patients with COVID-19 did not materialize during the first wave of the pandemic in these countries. It is possible that delaying so-called nonessential services may have been unnecessary and potentially harmful, because it likely led to delays in providing care for the treatment of serious non–COVID-19 illnesses.”
Since then, more experience and more data have largely allowed hospital systems to “tackle the ebb and flow” of COVID-19 cases in ways that limit shutdowns of important health services, they said.
Given the more pronounced effect in low- and middle-income countries, they stressed the need to focus resources on ways to promote prevention and treatment that do not rely on diagnostic procedures.
“This calls for more emphasis on developing efficient systems of telehealth, especially in poorer countries or in remote settings in all countries,” Dr. Leong and colleagues conclude.
Dr. Wadhera has reported research support from the National Heart, Lung, and Blood Institute, along with fellow senior author Robert W. Yeh, MD, MBA, who has also received personal fees and grants from several companies not related to the submitted work. Dr. Einstein, Dr. Leong, Dr. Eikelboom, and Dr. Yusuf have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 mortality in hospitalized HF patients: Nearly 1 in 4
Patients with heart failure who are infected with SARS-CoV-2 are at high risk for complications, with nearly 1 in 4 dying during hospitalization, according to a large database analysis that included more than 8,000 patients who had heart failure and COVID-19.
In-hospital mortality was 24.2% for patients who had a history of heart failure and were hospitalized with COVID-19, as compared with 14.2% for individuals without heart failure who were hospitalized with COVID-19.
For perspective, the researchers compared the patients with heart failure and COVID-19 with patients who had a history of heart failure and were hospitalized for an acute worsening episode: the risk for death was about 10-fold higher with COVID-19.
“These patients really face remarkably high risk, and when we compare that to the risk of in-hospital death with something we are a lot more familiar with – acute heart failure – we see that the risk was about 10-fold greater,” said first author Ankeet S. Bhatt, MD, MBA, from Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
In an article published online in JACC Heart Failure on Dec. 28, a group led by Dr. Bhatt and senior author Scott D. Solomon, MD, reported an analysis of administrative data on a total of 2,041,855 incident hospitalizations logged in the Premier Healthcare Database between April 1, 2020, and Sept. 30, 2020.
The Premier Healthcare Database comprises data from more than 1 billion patient encounters, which equates to approximately 1 in every 5 of all inpatient discharges in the United States.
Of 132,312 hospitalizations of patients with a history of heart failure, 23,843 (18.0%) were hospitalized with acute heart failure, 8,383 patients (6.4%) were hospitalized with COVID-19, and 100,068 (75.6%) were hospitalized for other reasons.
Outcomes and resource utilization were compared with 141,895 COVID-19 hospitalizations of patients who did not have heart failure.
Patients were deemed to have a history of heart failure if they were hospitalized at least once for heart failure from Jan. 1, 2019, to March 21, 2020, or had at least two heart failure outpatient visits during that period.
In a comment, Dr. Solomon noted some of the pros and cons of the data used in this study.
“Premier is a huge database, encompassing about one-quarter of all the health care facilities in the United States and one-fifth of all inpatient visits, so for that reason we’re able to look at things that are very difficult to look at in smaller hospital systems, but the data are also limited in that you don’t have as much granular detail as you might in smaller datasets,” said Dr. Solomon.
“One thing to recognize is that our data start at the point of hospital admission, so were looking only at individuals who have crossed the threshold in terms of their illness and been admitted,” he added.
Use of in-hospital resources was significantly greater for patients with heart failure hospitalized for COVID-19, compared with patients hospitalized for acute heart failure or for other reasons. This included “multifold” higher rates of ICU care (29% vs. 15%), mechanical ventilation (17% vs. 6%), and central venous catheter insertion (19% vs. 7%; P < .001 for all).
The proportion of patients who required mechanical ventilation and care in the ICU in the group with COVID-19 but who did not have no heart failure was similar to those who had both conditions.
The greater odds of in-hospital mortality among patients with both heart failure and COVID-19, compared with individuals with heart failure hospitalized for other reasons, was strongest in April, with an adjusted odds ratio of 14.48, compared with subsequent months (adjusted OR for May-September, 10.11; P for interaction < .001).
“We’re obviously not able to say with certainty what was happening in April, but I think that maybe the patients who were most vulnerable to COVID-19 may be more represented in that population, so the patients with comorbidities or who are immunosuppressed or otherwise,” said Dr. Bhatt in an interview.
“The other thing we think is that there may be a learning curve in terms of how to care for patients with acute severe respiratory illness. That includes increased institutional knowledge – like the use of prone ventilation – but also therapies that were subsequently shown to have benefit in randomized clinical trials, such as dexamethasone,” he added.
“These results should remind us to be innovative and thoughtful in our management of patients with heart failure while trying to maintain equity and good health for all,” wrote Nasrien E. Ibrahim, MD, from Massachusetts General Hospital, Boston; Ersilia DeFillipis, MD, Columbia University, New York; and Mitchel Psotka, MD, PhD, Innova Heart and Vascular Institute, Falls Church, Va., in an editorial accompanying the study.
The data emphasize the importance of ensuring equal access to services such as telemedicine, virtual visits, home nursing visits, and remote monitoring, they noted.
“As the COVID-19 pandemic rages on and disproportionately ravages socioeconomically disadvantaged communities, we should focus our efforts on strategies that minimize these inequities,” the editorialists wrote.
Dr. Solomon noted that, although Black and Hispanic patients were overrepresented in the population of heart failure patients hospitalized with COVID-19, once in the hospital, race was not a predictor of in-hospital mortality or the need for mechanical ventilation.
Dr. Bhatt has received speaker fees from Sanofi Pasteur and is supported by a National Institutes of Health/National Heart, Lung, and Blood Institute postdoctoral training grant. Dr. Solomon has received grant support and/or speaking fees from a number of companies and from the NIH/NHLBI. The editorialists disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with heart failure who are infected with SARS-CoV-2 are at high risk for complications, with nearly 1 in 4 dying during hospitalization, according to a large database analysis that included more than 8,000 patients who had heart failure and COVID-19.
In-hospital mortality was 24.2% for patients who had a history of heart failure and were hospitalized with COVID-19, as compared with 14.2% for individuals without heart failure who were hospitalized with COVID-19.
For perspective, the researchers compared the patients with heart failure and COVID-19 with patients who had a history of heart failure and were hospitalized for an acute worsening episode: the risk for death was about 10-fold higher with COVID-19.
“These patients really face remarkably high risk, and when we compare that to the risk of in-hospital death with something we are a lot more familiar with – acute heart failure – we see that the risk was about 10-fold greater,” said first author Ankeet S. Bhatt, MD, MBA, from Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
In an article published online in JACC Heart Failure on Dec. 28, a group led by Dr. Bhatt and senior author Scott D. Solomon, MD, reported an analysis of administrative data on a total of 2,041,855 incident hospitalizations logged in the Premier Healthcare Database between April 1, 2020, and Sept. 30, 2020.
The Premier Healthcare Database comprises data from more than 1 billion patient encounters, which equates to approximately 1 in every 5 of all inpatient discharges in the United States.
Of 132,312 hospitalizations of patients with a history of heart failure, 23,843 (18.0%) were hospitalized with acute heart failure, 8,383 patients (6.4%) were hospitalized with COVID-19, and 100,068 (75.6%) were hospitalized for other reasons.
Outcomes and resource utilization were compared with 141,895 COVID-19 hospitalizations of patients who did not have heart failure.
Patients were deemed to have a history of heart failure if they were hospitalized at least once for heart failure from Jan. 1, 2019, to March 21, 2020, or had at least two heart failure outpatient visits during that period.
In a comment, Dr. Solomon noted some of the pros and cons of the data used in this study.
“Premier is a huge database, encompassing about one-quarter of all the health care facilities in the United States and one-fifth of all inpatient visits, so for that reason we’re able to look at things that are very difficult to look at in smaller hospital systems, but the data are also limited in that you don’t have as much granular detail as you might in smaller datasets,” said Dr. Solomon.
“One thing to recognize is that our data start at the point of hospital admission, so were looking only at individuals who have crossed the threshold in terms of their illness and been admitted,” he added.
Use of in-hospital resources was significantly greater for patients with heart failure hospitalized for COVID-19, compared with patients hospitalized for acute heart failure or for other reasons. This included “multifold” higher rates of ICU care (29% vs. 15%), mechanical ventilation (17% vs. 6%), and central venous catheter insertion (19% vs. 7%; P < .001 for all).
The proportion of patients who required mechanical ventilation and care in the ICU in the group with COVID-19 but who did not have no heart failure was similar to those who had both conditions.
The greater odds of in-hospital mortality among patients with both heart failure and COVID-19, compared with individuals with heart failure hospitalized for other reasons, was strongest in April, with an adjusted odds ratio of 14.48, compared with subsequent months (adjusted OR for May-September, 10.11; P for interaction < .001).
“We’re obviously not able to say with certainty what was happening in April, but I think that maybe the patients who were most vulnerable to COVID-19 may be more represented in that population, so the patients with comorbidities or who are immunosuppressed or otherwise,” said Dr. Bhatt in an interview.
“The other thing we think is that there may be a learning curve in terms of how to care for patients with acute severe respiratory illness. That includes increased institutional knowledge – like the use of prone ventilation – but also therapies that were subsequently shown to have benefit in randomized clinical trials, such as dexamethasone,” he added.
“These results should remind us to be innovative and thoughtful in our management of patients with heart failure while trying to maintain equity and good health for all,” wrote Nasrien E. Ibrahim, MD, from Massachusetts General Hospital, Boston; Ersilia DeFillipis, MD, Columbia University, New York; and Mitchel Psotka, MD, PhD, Innova Heart and Vascular Institute, Falls Church, Va., in an editorial accompanying the study.
The data emphasize the importance of ensuring equal access to services such as telemedicine, virtual visits, home nursing visits, and remote monitoring, they noted.
“As the COVID-19 pandemic rages on and disproportionately ravages socioeconomically disadvantaged communities, we should focus our efforts on strategies that minimize these inequities,” the editorialists wrote.
Dr. Solomon noted that, although Black and Hispanic patients were overrepresented in the population of heart failure patients hospitalized with COVID-19, once in the hospital, race was not a predictor of in-hospital mortality or the need for mechanical ventilation.
Dr. Bhatt has received speaker fees from Sanofi Pasteur and is supported by a National Institutes of Health/National Heart, Lung, and Blood Institute postdoctoral training grant. Dr. Solomon has received grant support and/or speaking fees from a number of companies and from the NIH/NHLBI. The editorialists disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients with heart failure who are infected with SARS-CoV-2 are at high risk for complications, with nearly 1 in 4 dying during hospitalization, according to a large database analysis that included more than 8,000 patients who had heart failure and COVID-19.
In-hospital mortality was 24.2% for patients who had a history of heart failure and were hospitalized with COVID-19, as compared with 14.2% for individuals without heart failure who were hospitalized with COVID-19.
For perspective, the researchers compared the patients with heart failure and COVID-19 with patients who had a history of heart failure and were hospitalized for an acute worsening episode: the risk for death was about 10-fold higher with COVID-19.
“These patients really face remarkably high risk, and when we compare that to the risk of in-hospital death with something we are a lot more familiar with – acute heart failure – we see that the risk was about 10-fold greater,” said first author Ankeet S. Bhatt, MD, MBA, from Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
In an article published online in JACC Heart Failure on Dec. 28, a group led by Dr. Bhatt and senior author Scott D. Solomon, MD, reported an analysis of administrative data on a total of 2,041,855 incident hospitalizations logged in the Premier Healthcare Database between April 1, 2020, and Sept. 30, 2020.
The Premier Healthcare Database comprises data from more than 1 billion patient encounters, which equates to approximately 1 in every 5 of all inpatient discharges in the United States.
Of 132,312 hospitalizations of patients with a history of heart failure, 23,843 (18.0%) were hospitalized with acute heart failure, 8,383 patients (6.4%) were hospitalized with COVID-19, and 100,068 (75.6%) were hospitalized for other reasons.
Outcomes and resource utilization were compared with 141,895 COVID-19 hospitalizations of patients who did not have heart failure.
Patients were deemed to have a history of heart failure if they were hospitalized at least once for heart failure from Jan. 1, 2019, to March 21, 2020, or had at least two heart failure outpatient visits during that period.
In a comment, Dr. Solomon noted some of the pros and cons of the data used in this study.
“Premier is a huge database, encompassing about one-quarter of all the health care facilities in the United States and one-fifth of all inpatient visits, so for that reason we’re able to look at things that are very difficult to look at in smaller hospital systems, but the data are also limited in that you don’t have as much granular detail as you might in smaller datasets,” said Dr. Solomon.
“One thing to recognize is that our data start at the point of hospital admission, so were looking only at individuals who have crossed the threshold in terms of their illness and been admitted,” he added.
Use of in-hospital resources was significantly greater for patients with heart failure hospitalized for COVID-19, compared with patients hospitalized for acute heart failure or for other reasons. This included “multifold” higher rates of ICU care (29% vs. 15%), mechanical ventilation (17% vs. 6%), and central venous catheter insertion (19% vs. 7%; P < .001 for all).
The proportion of patients who required mechanical ventilation and care in the ICU in the group with COVID-19 but who did not have no heart failure was similar to those who had both conditions.
The greater odds of in-hospital mortality among patients with both heart failure and COVID-19, compared with individuals with heart failure hospitalized for other reasons, was strongest in April, with an adjusted odds ratio of 14.48, compared with subsequent months (adjusted OR for May-September, 10.11; P for interaction < .001).
“We’re obviously not able to say with certainty what was happening in April, but I think that maybe the patients who were most vulnerable to COVID-19 may be more represented in that population, so the patients with comorbidities or who are immunosuppressed or otherwise,” said Dr. Bhatt in an interview.
“The other thing we think is that there may be a learning curve in terms of how to care for patients with acute severe respiratory illness. That includes increased institutional knowledge – like the use of prone ventilation – but also therapies that were subsequently shown to have benefit in randomized clinical trials, such as dexamethasone,” he added.
“These results should remind us to be innovative and thoughtful in our management of patients with heart failure while trying to maintain equity and good health for all,” wrote Nasrien E. Ibrahim, MD, from Massachusetts General Hospital, Boston; Ersilia DeFillipis, MD, Columbia University, New York; and Mitchel Psotka, MD, PhD, Innova Heart and Vascular Institute, Falls Church, Va., in an editorial accompanying the study.
The data emphasize the importance of ensuring equal access to services such as telemedicine, virtual visits, home nursing visits, and remote monitoring, they noted.
“As the COVID-19 pandemic rages on and disproportionately ravages socioeconomically disadvantaged communities, we should focus our efforts on strategies that minimize these inequities,” the editorialists wrote.
Dr. Solomon noted that, although Black and Hispanic patients were overrepresented in the population of heart failure patients hospitalized with COVID-19, once in the hospital, race was not a predictor of in-hospital mortality or the need for mechanical ventilation.
Dr. Bhatt has received speaker fees from Sanofi Pasteur and is supported by a National Institutes of Health/National Heart, Lung, and Blood Institute postdoctoral training grant. Dr. Solomon has received grant support and/or speaking fees from a number of companies and from the NIH/NHLBI. The editorialists disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NETs a possible therapeutic target for COVID-19 thrombosis?
Researchers in Madrid may have found a clue to the pathogenesis of ST-segment elevation myocardial infarction (STEMI) in patients with COVID-19; it might also offer a therapeutic target to counter the hypercoagulability seen with COVID-19.
In a case series of five patients with COVID-19 who had an STEMI, neutrophil extracellular traps (NETs) were detected in coronary thrombi of all five patients. The median density was 66%, which is significantly higher than that seen in a historical series of patients with STEMI. In that series, NETs were found in only two-thirds of patients; in that series, the median density was 19%.
In the patients with COVID-19 and STEMI and in the patients reported in the prepandemic historical series from 2015, intracoronary aspirates were obtained during percutaneous coronary intervention using a thrombus aspiration device.
Histologically, findings in the patients from 2015 differed from those of patients with COVID-19. In the patients with COVID, thrombi were composed mostly of fibrin and polymorphonuclear cells. None showed fragments of atherosclerotic plaque or iron deposits indicative of previous episodes of plaque rupture. In contrast, 65% of thrombi from the 2015 series contained plaque fragments.
Ana Blasco, MD, PhD, Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, and colleagues report their findings in an article published online Dec. 29 in JAMA Cardiology.
Commenting on the findings in an interview, Irene Lang, MD, from the Medical University of Vienna said, “This is really a very small series, purely observational, and suffering from the problem that acute STEMI is uncommon in COVID-19, but it does serve to demonstrate once more the abundance of NETs in acute myocardial infarction.”
“NETs are very much at the cutting edge of thrombosis research, and NET formation provides yet another link between inflammation and clot formation,” added Peter Libby, MD, from Harvard Medical School and Brigham and Women’s Hospital, Boston.
“Multiple observations have shown thrombosis of arteries large and small, microvessels, and veins in COVID-19. The observations of Blasco et al. add to the growing literature about NETs as contributors to the havoc wrought in multiple organs in advanced COVID-19,” he added in an email exchange with this news organization.
Neither Dr. Lang nor Dr. Libby were involved in this research; both have been actively studying NETs and their contribution to cardiothrombotic disease in recent years.
NETs are newly recognized contributors to venous and arterial thrombosis. These weblike DNA strands are extruded by activated or dying neutrophils and have protein mediators that ensnare pathogens while minimizing damage to the host cell.
First described in 2004, exaggerated NET formation has also been linked to the initiation and accretion of inflammation and thrombosis.
“NETs thus furnish a previously unsuspected link between inflammation, innate immunity, thrombosis, oxidative stress, and cardiovascular diseases,” Dr. Libby and his coauthors wrote in an article on the topic published in Circulation Research earlier this year.
Limiting NET formation or “dissolving” existing NETs could provide a therapeutic avenue not just for patients with COVID-19 but for all patients with thrombotic disease.
“The concept of NETs as a therapeutic target is appealing, in and out of COVID times,” said Dr. Lang.
“I personally believe that the work helps to raise awareness for the potential use of deoxyribonuclease (DNase), an enzyme that acts to clear NETs by dissolving the DNA strands, in the acute treatment of STEMI. Rapid injection of engineered recombinant DNases could potentially wipe away coronary obstructions, ideally before they may cause damage to the myocardium,” she added.
Dr. Blasco and colleagues and Dr. Lang have disclosed no relevant financial relationships. Dr. Libby is an unpaid consultant or member of the advisory board for a number of companies.
A version of this article first appeared on Medscape.com.
Researchers in Madrid may have found a clue to the pathogenesis of ST-segment elevation myocardial infarction (STEMI) in patients with COVID-19; it might also offer a therapeutic target to counter the hypercoagulability seen with COVID-19.
In a case series of five patients with COVID-19 who had an STEMI, neutrophil extracellular traps (NETs) were detected in coronary thrombi of all five patients. The median density was 66%, which is significantly higher than that seen in a historical series of patients with STEMI. In that series, NETs were found in only two-thirds of patients; in that series, the median density was 19%.
In the patients with COVID-19 and STEMI and in the patients reported in the prepandemic historical series from 2015, intracoronary aspirates were obtained during percutaneous coronary intervention using a thrombus aspiration device.
Histologically, findings in the patients from 2015 differed from those of patients with COVID-19. In the patients with COVID, thrombi were composed mostly of fibrin and polymorphonuclear cells. None showed fragments of atherosclerotic plaque or iron deposits indicative of previous episodes of plaque rupture. In contrast, 65% of thrombi from the 2015 series contained plaque fragments.
Ana Blasco, MD, PhD, Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, and colleagues report their findings in an article published online Dec. 29 in JAMA Cardiology.
Commenting on the findings in an interview, Irene Lang, MD, from the Medical University of Vienna said, “This is really a very small series, purely observational, and suffering from the problem that acute STEMI is uncommon in COVID-19, but it does serve to demonstrate once more the abundance of NETs in acute myocardial infarction.”
“NETs are very much at the cutting edge of thrombosis research, and NET formation provides yet another link between inflammation and clot formation,” added Peter Libby, MD, from Harvard Medical School and Brigham and Women’s Hospital, Boston.
“Multiple observations have shown thrombosis of arteries large and small, microvessels, and veins in COVID-19. The observations of Blasco et al. add to the growing literature about NETs as contributors to the havoc wrought in multiple organs in advanced COVID-19,” he added in an email exchange with this news organization.
Neither Dr. Lang nor Dr. Libby were involved in this research; both have been actively studying NETs and their contribution to cardiothrombotic disease in recent years.
NETs are newly recognized contributors to venous and arterial thrombosis. These weblike DNA strands are extruded by activated or dying neutrophils and have protein mediators that ensnare pathogens while minimizing damage to the host cell.
First described in 2004, exaggerated NET formation has also been linked to the initiation and accretion of inflammation and thrombosis.
“NETs thus furnish a previously unsuspected link between inflammation, innate immunity, thrombosis, oxidative stress, and cardiovascular diseases,” Dr. Libby and his coauthors wrote in an article on the topic published in Circulation Research earlier this year.
Limiting NET formation or “dissolving” existing NETs could provide a therapeutic avenue not just for patients with COVID-19 but for all patients with thrombotic disease.
“The concept of NETs as a therapeutic target is appealing, in and out of COVID times,” said Dr. Lang.
“I personally believe that the work helps to raise awareness for the potential use of deoxyribonuclease (DNase), an enzyme that acts to clear NETs by dissolving the DNA strands, in the acute treatment of STEMI. Rapid injection of engineered recombinant DNases could potentially wipe away coronary obstructions, ideally before they may cause damage to the myocardium,” she added.
Dr. Blasco and colleagues and Dr. Lang have disclosed no relevant financial relationships. Dr. Libby is an unpaid consultant or member of the advisory board for a number of companies.
A version of this article first appeared on Medscape.com.
Researchers in Madrid may have found a clue to the pathogenesis of ST-segment elevation myocardial infarction (STEMI) in patients with COVID-19; it might also offer a therapeutic target to counter the hypercoagulability seen with COVID-19.
In a case series of five patients with COVID-19 who had an STEMI, neutrophil extracellular traps (NETs) were detected in coronary thrombi of all five patients. The median density was 66%, which is significantly higher than that seen in a historical series of patients with STEMI. In that series, NETs were found in only two-thirds of patients; in that series, the median density was 19%.
In the patients with COVID-19 and STEMI and in the patients reported in the prepandemic historical series from 2015, intracoronary aspirates were obtained during percutaneous coronary intervention using a thrombus aspiration device.
Histologically, findings in the patients from 2015 differed from those of patients with COVID-19. In the patients with COVID, thrombi were composed mostly of fibrin and polymorphonuclear cells. None showed fragments of atherosclerotic plaque or iron deposits indicative of previous episodes of plaque rupture. In contrast, 65% of thrombi from the 2015 series contained plaque fragments.
Ana Blasco, MD, PhD, Hospital Universitario Puerta de Hierro-Majadahonda, Madrid, and colleagues report their findings in an article published online Dec. 29 in JAMA Cardiology.
Commenting on the findings in an interview, Irene Lang, MD, from the Medical University of Vienna said, “This is really a very small series, purely observational, and suffering from the problem that acute STEMI is uncommon in COVID-19, but it does serve to demonstrate once more the abundance of NETs in acute myocardial infarction.”
“NETs are very much at the cutting edge of thrombosis research, and NET formation provides yet another link between inflammation and clot formation,” added Peter Libby, MD, from Harvard Medical School and Brigham and Women’s Hospital, Boston.
“Multiple observations have shown thrombosis of arteries large and small, microvessels, and veins in COVID-19. The observations of Blasco et al. add to the growing literature about NETs as contributors to the havoc wrought in multiple organs in advanced COVID-19,” he added in an email exchange with this news organization.
Neither Dr. Lang nor Dr. Libby were involved in this research; both have been actively studying NETs and their contribution to cardiothrombotic disease in recent years.
NETs are newly recognized contributors to venous and arterial thrombosis. These weblike DNA strands are extruded by activated or dying neutrophils and have protein mediators that ensnare pathogens while minimizing damage to the host cell.
First described in 2004, exaggerated NET formation has also been linked to the initiation and accretion of inflammation and thrombosis.
“NETs thus furnish a previously unsuspected link between inflammation, innate immunity, thrombosis, oxidative stress, and cardiovascular diseases,” Dr. Libby and his coauthors wrote in an article on the topic published in Circulation Research earlier this year.
Limiting NET formation or “dissolving” existing NETs could provide a therapeutic avenue not just for patients with COVID-19 but for all patients with thrombotic disease.
“The concept of NETs as a therapeutic target is appealing, in and out of COVID times,” said Dr. Lang.
“I personally believe that the work helps to raise awareness for the potential use of deoxyribonuclease (DNase), an enzyme that acts to clear NETs by dissolving the DNA strands, in the acute treatment of STEMI. Rapid injection of engineered recombinant DNases could potentially wipe away coronary obstructions, ideally before they may cause damage to the myocardium,” she added.
Dr. Blasco and colleagues and Dr. Lang have disclosed no relevant financial relationships. Dr. Libby is an unpaid consultant or member of the advisory board for a number of companies.
A version of this article first appeared on Medscape.com.













