User login
Albuminuria reduction fuels finerenone’s kidney benefits
PHILADELPHIA – Reducing albuminuria is a key mediator of the way finerenone (Kerendia, Bayer) reduces adverse renal and cardiovascular events in people with type 2 diabetes and chronic kidney disease (CKD), based on findings from two novel mediation analyses run on data from more than 12,000 people included in the two finerenone pivotal trials.
Results from these analyses showed that that finerenone treatment produced in the FIDELIO-DKD and FIGARO-DKD phase 3 trials. FIDELIO-DKD, which had protection against adverse kidney outcomes as its primary endpoint, supplied the data that led to finerenone’s approval in 2021 by the U.S. Food and Drug Administration for treating people with type 2 diabetes and CKD.
The findings of the mediation analyses underscore the important role that albuminuria plays in the nephropathy and related comorbidities associated with type 2 diabetes and CKD and highlight the importance of ongoing monitoring of albuminuria to guide treatments aimed at minimizing this pathology, said Rajiv Agarwal, MD, who presented a poster on the mediation analyses at Kidney Week 2023, organized by the American Society of Nephrology.
“My hope is that this [report] heightens awareness of UACR” as an important marker of both CKD and of the response by patients with CKD to their treatment, said Dr. Agarwal, a nephrologist and professor at Indiana University in Indianapolis.
“Only about half of people with type 2 diabetes get their UACR measured even though every guideline says measure UACR in people with diabetes. Our findings say that UACR is important not just for CKD diagnosis but also to give feedback” on whether management is working, Dr. Agarwal said in an interview.
Incorporate UACR into clinical decision-making
“My hope is that clinicians will look at UACR as something they should incorporate into clinical decision-making. I measure UACR in my patients [with CKD and type 2 diabetes] at every visit; it’s so inexpensive. Albuminuria is not a good sign. If it’s not reduced in a patient by at least 30% [the recommended minimum reduction by the American Diabetes Association for people who start with a UACR of at least 300 mg/g] clinicians should think of what else they could do to lower albuminuria”: Reduce salt intake, improve blood pressure control, make sure the patient is adherent to treatments, and add additional treatments, Dr. Agarwal advised.
Multiple efforts are now underway or will soon start to boost the rate at which at-risk people get their UACR measured, noted Leslie A. Inker, MD, in a separate talk during Kidney Week. These efforts include the National Kidney Foundation’s CKD Learning Collaborative, which aims to improve clinician awareness of CKD and improve routine testing for CKD. Early results during 2023 from this program in Missouri showed a nearly 8–percentage point increase in the screening rate for UACR levels in at-risk people, said Dr. Inker, professor and director of the Kidney and Blood Pressure Center at Tufts Medical Center in Boston.
A second advance was introduction in 2018 of the “kidney profile” lab order by the American College of Clinical Pathology that allows clinicians to order as a single test both an estimated glomerular filtration rate (eGFR) and a UACR.
Also, the Centers for Medicare & Medicaid Services and the National Committee for Quality Assurance have both taken steps to encourage UACR ordering. The NCQA established a new Healthcare Effectiveness Data and Information Set performance measure for U.S. physicians starting in 2023 that will track measurement of UACR and eGFR in people with diabetes. CMS also has made assessment of kidney health a measure of care quality in programs effective in 2023 and 2024, Dr. Inker noted.
Most subjects had elevated UACRs
The study run by Dr. Agarwal and his associates used data from 12,512 of the more than 13,000 people enrolled in either FIDELITY-DKD or FIGARO-DKD who had UACR measurements recorded at baseline, at 4 months into either study, or both. Their median UACR at the time they began on finerenone or placebo was 514 mg/g, with 67% having a UACR of at least 300 mg/g (macroalbuminuria) and 31% having a UACR of 30-299 mg/g (microalbuminuria). By design, virtually all patients in these two trials were on a renin-angiotensin system inhibitor (either an angiotensin-converting enzyme inhibitor or an angiotensin-receptor blocker), but given the time period when the two trials enrolled participants (during 2015-2018) only 7% of those enrolled were on a sodium-glucose cotransporter 2 inhibitor and only 7% were on a glucagonlike peptide–1 receptor agonist.
Four months after treatment began, 53% of those randomized to finerenone treatment and 27% of those in the placebo arm had their UACR reduced by at least 30% from baseline, the cutpoint chosen by Dr. Agarwal based on the American Diabetes Association guideline.
Kaplan-Meier analyses showed that the incidence of the primary kidney outcome – kidney failure, a sustained ≥ 57% decrease in eGFR from baseline, or kidney death – showed close correlation with at least a 30% reduction in UACR regardless of whether the patients in this subgroup received finerenone or placebo.
A different correlation was found in those with a less than 30% reduction in their UACR from baseline to 4 months, regardless of whether this happened on finerenone or placebo. People in the two finerenone trials who had a lesser reduction from baseline in their UACR also had a significantly higher rate of adverse kidney outcomes whether they received finerenone or placebo.
84% of finerenone’s kidney benefit linked to lowering of UACR
The causal-mediation analysis run by Dr. Agarwal quantified this observation, showing that 84% of finerenone’s effect on the kidney outcome was mediated by the reduction in UACR.
“It seems like the kidney benefit [from finerenone] travels through the level of albuminuria. This has broad implications for treatment of people with type 2 diabetes and CKD,” he said.
The link with reduction in albuminuria was weaker for the primary cardiovascular disease outcome: CV death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure. The strongest effect on this outcome was only seen in Kaplan-Meier analysis in those on finerenone who had at least a 30% reduction in their UACR. Those on placebo and with a similarly robust 4-month reduction in UACR showed a much more modest cardiovascular benefit that resembled those on either finerenone or placebo who had a smaller, less than 30% UACR reduction. The mediation analysis of these data showed that UACR reduction accounted for about 37% of the observed cardiovascular benefit seen during the trials.
“The effect of UACR is much stronger for the kidney outcomes,” summed up Dr. Agarwal. The results suggest that for cardiovascular outcomes finerenone works through factors other than lowering of UACR, but he admitted that no one currently knows what those other factors might be.
Treat aggressively to lower UACR by 30%
“I wouldn’t stop finerenone treatment in people who do not get a 30% reduction in their UACR” because these analyses suggest that a portion of the overall benefits from finerenone occurs via other mechanisms, he said. But in patients whose UACR is not reduced by at least 30% “be more aggressive on other measures to reduce UACR,” he advised.
The mediation analyses he ran are “the first time this has been done in nephrology,” producing a “groundbreaking” analysis and finding, Dr. Agarwal said. He also highlighted that the findings primarily relate to the importance of controlling UACR rather than an endorsement of finerenone as the best way to achieve this.
“All I care about is that people think about UACR as a modifiable risk factor. It doesn’t have to be treated with finerenone. It could be a renin-angiotensin system inhibitor, it could be chlorthalidone [a thiazide diuretic]. It just happened that we had a large dataset of people treated with finerenone or placebo.”
He said that future mediation analyses should look at the link between outcomes and UACR reductions produced by agents from the classes of sodium-glucose cotransporter 2 inhibitors and the glucagonlike peptide–1 receptor agonists.
FIDELIO-DKD and FIGARO-DKD were both sponsored by Bayer, the company that markets finerenone. Dr. Agarwal has received personal fees and nonfinancial support from Bayer. He has also received personal fees and nonfinancial support from Akebia Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Vifor Pharma, and he is a member of data safety monitoring committees for Chinook and Vertex. Dr. Inker is a consultant to Diamtrix, and her department receives research funding from Chinook, Omeros, Reata, and Tricida.
PHILADELPHIA – Reducing albuminuria is a key mediator of the way finerenone (Kerendia, Bayer) reduces adverse renal and cardiovascular events in people with type 2 diabetes and chronic kidney disease (CKD), based on findings from two novel mediation analyses run on data from more than 12,000 people included in the two finerenone pivotal trials.
Results from these analyses showed that that finerenone treatment produced in the FIDELIO-DKD and FIGARO-DKD phase 3 trials. FIDELIO-DKD, which had protection against adverse kidney outcomes as its primary endpoint, supplied the data that led to finerenone’s approval in 2021 by the U.S. Food and Drug Administration for treating people with type 2 diabetes and CKD.
The findings of the mediation analyses underscore the important role that albuminuria plays in the nephropathy and related comorbidities associated with type 2 diabetes and CKD and highlight the importance of ongoing monitoring of albuminuria to guide treatments aimed at minimizing this pathology, said Rajiv Agarwal, MD, who presented a poster on the mediation analyses at Kidney Week 2023, organized by the American Society of Nephrology.
“My hope is that this [report] heightens awareness of UACR” as an important marker of both CKD and of the response by patients with CKD to their treatment, said Dr. Agarwal, a nephrologist and professor at Indiana University in Indianapolis.
“Only about half of people with type 2 diabetes get their UACR measured even though every guideline says measure UACR in people with diabetes. Our findings say that UACR is important not just for CKD diagnosis but also to give feedback” on whether management is working, Dr. Agarwal said in an interview.
Incorporate UACR into clinical decision-making
“My hope is that clinicians will look at UACR as something they should incorporate into clinical decision-making. I measure UACR in my patients [with CKD and type 2 diabetes] at every visit; it’s so inexpensive. Albuminuria is not a good sign. If it’s not reduced in a patient by at least 30% [the recommended minimum reduction by the American Diabetes Association for people who start with a UACR of at least 300 mg/g] clinicians should think of what else they could do to lower albuminuria”: Reduce salt intake, improve blood pressure control, make sure the patient is adherent to treatments, and add additional treatments, Dr. Agarwal advised.
Multiple efforts are now underway or will soon start to boost the rate at which at-risk people get their UACR measured, noted Leslie A. Inker, MD, in a separate talk during Kidney Week. These efforts include the National Kidney Foundation’s CKD Learning Collaborative, which aims to improve clinician awareness of CKD and improve routine testing for CKD. Early results during 2023 from this program in Missouri showed a nearly 8–percentage point increase in the screening rate for UACR levels in at-risk people, said Dr. Inker, professor and director of the Kidney and Blood Pressure Center at Tufts Medical Center in Boston.
A second advance was introduction in 2018 of the “kidney profile” lab order by the American College of Clinical Pathology that allows clinicians to order as a single test both an estimated glomerular filtration rate (eGFR) and a UACR.
Also, the Centers for Medicare & Medicaid Services and the National Committee for Quality Assurance have both taken steps to encourage UACR ordering. The NCQA established a new Healthcare Effectiveness Data and Information Set performance measure for U.S. physicians starting in 2023 that will track measurement of UACR and eGFR in people with diabetes. CMS also has made assessment of kidney health a measure of care quality in programs effective in 2023 and 2024, Dr. Inker noted.
Most subjects had elevated UACRs
The study run by Dr. Agarwal and his associates used data from 12,512 of the more than 13,000 people enrolled in either FIDELITY-DKD or FIGARO-DKD who had UACR measurements recorded at baseline, at 4 months into either study, or both. Their median UACR at the time they began on finerenone or placebo was 514 mg/g, with 67% having a UACR of at least 300 mg/g (macroalbuminuria) and 31% having a UACR of 30-299 mg/g (microalbuminuria). By design, virtually all patients in these two trials were on a renin-angiotensin system inhibitor (either an angiotensin-converting enzyme inhibitor or an angiotensin-receptor blocker), but given the time period when the two trials enrolled participants (during 2015-2018) only 7% of those enrolled were on a sodium-glucose cotransporter 2 inhibitor and only 7% were on a glucagonlike peptide–1 receptor agonist.
Four months after treatment began, 53% of those randomized to finerenone treatment and 27% of those in the placebo arm had their UACR reduced by at least 30% from baseline, the cutpoint chosen by Dr. Agarwal based on the American Diabetes Association guideline.
Kaplan-Meier analyses showed that the incidence of the primary kidney outcome – kidney failure, a sustained ≥ 57% decrease in eGFR from baseline, or kidney death – showed close correlation with at least a 30% reduction in UACR regardless of whether the patients in this subgroup received finerenone or placebo.
A different correlation was found in those with a less than 30% reduction in their UACR from baseline to 4 months, regardless of whether this happened on finerenone or placebo. People in the two finerenone trials who had a lesser reduction from baseline in their UACR also had a significantly higher rate of adverse kidney outcomes whether they received finerenone or placebo.
84% of finerenone’s kidney benefit linked to lowering of UACR
The causal-mediation analysis run by Dr. Agarwal quantified this observation, showing that 84% of finerenone’s effect on the kidney outcome was mediated by the reduction in UACR.
“It seems like the kidney benefit [from finerenone] travels through the level of albuminuria. This has broad implications for treatment of people with type 2 diabetes and CKD,” he said.
The link with reduction in albuminuria was weaker for the primary cardiovascular disease outcome: CV death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure. The strongest effect on this outcome was only seen in Kaplan-Meier analysis in those on finerenone who had at least a 30% reduction in their UACR. Those on placebo and with a similarly robust 4-month reduction in UACR showed a much more modest cardiovascular benefit that resembled those on either finerenone or placebo who had a smaller, less than 30% UACR reduction. The mediation analysis of these data showed that UACR reduction accounted for about 37% of the observed cardiovascular benefit seen during the trials.
“The effect of UACR is much stronger for the kidney outcomes,” summed up Dr. Agarwal. The results suggest that for cardiovascular outcomes finerenone works through factors other than lowering of UACR, but he admitted that no one currently knows what those other factors might be.
Treat aggressively to lower UACR by 30%
“I wouldn’t stop finerenone treatment in people who do not get a 30% reduction in their UACR” because these analyses suggest that a portion of the overall benefits from finerenone occurs via other mechanisms, he said. But in patients whose UACR is not reduced by at least 30% “be more aggressive on other measures to reduce UACR,” he advised.
The mediation analyses he ran are “the first time this has been done in nephrology,” producing a “groundbreaking” analysis and finding, Dr. Agarwal said. He also highlighted that the findings primarily relate to the importance of controlling UACR rather than an endorsement of finerenone as the best way to achieve this.
“All I care about is that people think about UACR as a modifiable risk factor. It doesn’t have to be treated with finerenone. It could be a renin-angiotensin system inhibitor, it could be chlorthalidone [a thiazide diuretic]. It just happened that we had a large dataset of people treated with finerenone or placebo.”
He said that future mediation analyses should look at the link between outcomes and UACR reductions produced by agents from the classes of sodium-glucose cotransporter 2 inhibitors and the glucagonlike peptide–1 receptor agonists.
FIDELIO-DKD and FIGARO-DKD were both sponsored by Bayer, the company that markets finerenone. Dr. Agarwal has received personal fees and nonfinancial support from Bayer. He has also received personal fees and nonfinancial support from Akebia Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Vifor Pharma, and he is a member of data safety monitoring committees for Chinook and Vertex. Dr. Inker is a consultant to Diamtrix, and her department receives research funding from Chinook, Omeros, Reata, and Tricida.
PHILADELPHIA – Reducing albuminuria is a key mediator of the way finerenone (Kerendia, Bayer) reduces adverse renal and cardiovascular events in people with type 2 diabetes and chronic kidney disease (CKD), based on findings from two novel mediation analyses run on data from more than 12,000 people included in the two finerenone pivotal trials.
Results from these analyses showed that that finerenone treatment produced in the FIDELIO-DKD and FIGARO-DKD phase 3 trials. FIDELIO-DKD, which had protection against adverse kidney outcomes as its primary endpoint, supplied the data that led to finerenone’s approval in 2021 by the U.S. Food and Drug Administration for treating people with type 2 diabetes and CKD.
The findings of the mediation analyses underscore the important role that albuminuria plays in the nephropathy and related comorbidities associated with type 2 diabetes and CKD and highlight the importance of ongoing monitoring of albuminuria to guide treatments aimed at minimizing this pathology, said Rajiv Agarwal, MD, who presented a poster on the mediation analyses at Kidney Week 2023, organized by the American Society of Nephrology.
“My hope is that this [report] heightens awareness of UACR” as an important marker of both CKD and of the response by patients with CKD to their treatment, said Dr. Agarwal, a nephrologist and professor at Indiana University in Indianapolis.
“Only about half of people with type 2 diabetes get their UACR measured even though every guideline says measure UACR in people with diabetes. Our findings say that UACR is important not just for CKD diagnosis but also to give feedback” on whether management is working, Dr. Agarwal said in an interview.
Incorporate UACR into clinical decision-making
“My hope is that clinicians will look at UACR as something they should incorporate into clinical decision-making. I measure UACR in my patients [with CKD and type 2 diabetes] at every visit; it’s so inexpensive. Albuminuria is not a good sign. If it’s not reduced in a patient by at least 30% [the recommended minimum reduction by the American Diabetes Association for people who start with a UACR of at least 300 mg/g] clinicians should think of what else they could do to lower albuminuria”: Reduce salt intake, improve blood pressure control, make sure the patient is adherent to treatments, and add additional treatments, Dr. Agarwal advised.
Multiple efforts are now underway or will soon start to boost the rate at which at-risk people get their UACR measured, noted Leslie A. Inker, MD, in a separate talk during Kidney Week. These efforts include the National Kidney Foundation’s CKD Learning Collaborative, which aims to improve clinician awareness of CKD and improve routine testing for CKD. Early results during 2023 from this program in Missouri showed a nearly 8–percentage point increase in the screening rate for UACR levels in at-risk people, said Dr. Inker, professor and director of the Kidney and Blood Pressure Center at Tufts Medical Center in Boston.
A second advance was introduction in 2018 of the “kidney profile” lab order by the American College of Clinical Pathology that allows clinicians to order as a single test both an estimated glomerular filtration rate (eGFR) and a UACR.
Also, the Centers for Medicare & Medicaid Services and the National Committee for Quality Assurance have both taken steps to encourage UACR ordering. The NCQA established a new Healthcare Effectiveness Data and Information Set performance measure for U.S. physicians starting in 2023 that will track measurement of UACR and eGFR in people with diabetes. CMS also has made assessment of kidney health a measure of care quality in programs effective in 2023 and 2024, Dr. Inker noted.
Most subjects had elevated UACRs
The study run by Dr. Agarwal and his associates used data from 12,512 of the more than 13,000 people enrolled in either FIDELITY-DKD or FIGARO-DKD who had UACR measurements recorded at baseline, at 4 months into either study, or both. Their median UACR at the time they began on finerenone or placebo was 514 mg/g, with 67% having a UACR of at least 300 mg/g (macroalbuminuria) and 31% having a UACR of 30-299 mg/g (microalbuminuria). By design, virtually all patients in these two trials were on a renin-angiotensin system inhibitor (either an angiotensin-converting enzyme inhibitor or an angiotensin-receptor blocker), but given the time period when the two trials enrolled participants (during 2015-2018) only 7% of those enrolled were on a sodium-glucose cotransporter 2 inhibitor and only 7% were on a glucagonlike peptide–1 receptor agonist.
Four months after treatment began, 53% of those randomized to finerenone treatment and 27% of those in the placebo arm had their UACR reduced by at least 30% from baseline, the cutpoint chosen by Dr. Agarwal based on the American Diabetes Association guideline.
Kaplan-Meier analyses showed that the incidence of the primary kidney outcome – kidney failure, a sustained ≥ 57% decrease in eGFR from baseline, or kidney death – showed close correlation with at least a 30% reduction in UACR regardless of whether the patients in this subgroup received finerenone or placebo.
A different correlation was found in those with a less than 30% reduction in their UACR from baseline to 4 months, regardless of whether this happened on finerenone or placebo. People in the two finerenone trials who had a lesser reduction from baseline in their UACR also had a significantly higher rate of adverse kidney outcomes whether they received finerenone or placebo.
84% of finerenone’s kidney benefit linked to lowering of UACR
The causal-mediation analysis run by Dr. Agarwal quantified this observation, showing that 84% of finerenone’s effect on the kidney outcome was mediated by the reduction in UACR.
“It seems like the kidney benefit [from finerenone] travels through the level of albuminuria. This has broad implications for treatment of people with type 2 diabetes and CKD,” he said.
The link with reduction in albuminuria was weaker for the primary cardiovascular disease outcome: CV death, nonfatal myocardial infarction, nonfatal stroke, or hospitalization for heart failure. The strongest effect on this outcome was only seen in Kaplan-Meier analysis in those on finerenone who had at least a 30% reduction in their UACR. Those on placebo and with a similarly robust 4-month reduction in UACR showed a much more modest cardiovascular benefit that resembled those on either finerenone or placebo who had a smaller, less than 30% UACR reduction. The mediation analysis of these data showed that UACR reduction accounted for about 37% of the observed cardiovascular benefit seen during the trials.
“The effect of UACR is much stronger for the kidney outcomes,” summed up Dr. Agarwal. The results suggest that for cardiovascular outcomes finerenone works through factors other than lowering of UACR, but he admitted that no one currently knows what those other factors might be.
Treat aggressively to lower UACR by 30%
“I wouldn’t stop finerenone treatment in people who do not get a 30% reduction in their UACR” because these analyses suggest that a portion of the overall benefits from finerenone occurs via other mechanisms, he said. But in patients whose UACR is not reduced by at least 30% “be more aggressive on other measures to reduce UACR,” he advised.
The mediation analyses he ran are “the first time this has been done in nephrology,” producing a “groundbreaking” analysis and finding, Dr. Agarwal said. He also highlighted that the findings primarily relate to the importance of controlling UACR rather than an endorsement of finerenone as the best way to achieve this.
“All I care about is that people think about UACR as a modifiable risk factor. It doesn’t have to be treated with finerenone. It could be a renin-angiotensin system inhibitor, it could be chlorthalidone [a thiazide diuretic]. It just happened that we had a large dataset of people treated with finerenone or placebo.”
He said that future mediation analyses should look at the link between outcomes and UACR reductions produced by agents from the classes of sodium-glucose cotransporter 2 inhibitors and the glucagonlike peptide–1 receptor agonists.
FIDELIO-DKD and FIGARO-DKD were both sponsored by Bayer, the company that markets finerenone. Dr. Agarwal has received personal fees and nonfinancial support from Bayer. He has also received personal fees and nonfinancial support from Akebia Therapeutics, AstraZeneca, Boehringer Ingelheim, Eli Lilly, and Vifor Pharma, and he is a member of data safety monitoring committees for Chinook and Vertex. Dr. Inker is a consultant to Diamtrix, and her department receives research funding from Chinook, Omeros, Reata, and Tricida.
AT KIDNEY WEEK 2023
Dropping aspirin cuts bleeding in LVAD patients: ARIES-HM3
PHILADELPHIA – particularly if it’s a newer device that does not use the centrifugal- or continuous-flow pump technology of conventional LVADs, new randomized results suggest.
“We’ve always thought that somehow aspirin prevents stroke and prevents clotting and that it’s anti-inflammatory, and what we found in ARIES was the exact opposite,” said Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center and Harvard Medical School, both in Boston, who reported results of the ARIES-HM3 trial of the HeartMate 3 LVAD, a device that uses a fully magnetically levitated rotor to maintain blood flow.
ARIES-HM3 randomly assigned 589 patients who received the HeartMate 3 device to vitamin K therapy with aspirin or to placebo. Dr. Mehra said it was the first international trial to conclusively evaluate medical therapy in patients who get an LVAD.
Unexpected findings
“To be honest with you, we set this up as a safety study to see if we could eliminate aspirin,” Dr. Mehra said in an interview. “We didn’t expect that the bleeding rates would decrease by 34% and that gastrointestinal bleeding in particular would decrease by 40%. We didn’t expect that it would nearly halve the days spent in the hospital, and we didn’t expect that the cost of care would decrease by 40%.”
Dr. Mehra reported the results at the annual scientific sessions of the American Heart Association. They were published simultaneously online in JAMA.
The researchers found that 74% of patients in the placebo group met the primary endpoint of being alive and not having any hemocompatibility events at 12 months vs 68% of the aspirin patients. The rate of nonsurgical bleeding events was 30% in the placebo group versus 42.4% in the aspirin patients. The rates of GI bleeding were 13% and 21.6% in the respective groups.
In his talk, Dr. Mehra noted the placebo group spent 47% fewer days in the hospital for bleeding, with hospitalization costs 41% lower than the aspirin group.
“We are very quick to throw things as deemed medical therapy at patients and this study outcome should give us pause that not everything we do may be right, and that we need to start building a stronger evidence base in medical therapy for what we do with patients that are on device support,” Dr. Mehra said.
Shift of focus to therapy
The study’s focus on aspirin therapy may be as significant as its evaluation of the HeartMate 3 LVAD, discussant Eric David Adler, MD, a cardiologist and section head of heart transplant at the University of California, San Diego, said in an interview.
“We focus so much on the device,” he said. “It’s like a set-it-and-forget-it kind of thing and we’re surprised that we see complications because we haven’t put a lot of effort into the medical therapy component.”
But he credited this study for doing just that, adding that it can serve as a model for future studies of LVADs, although such studies can face hurdles. “These studies are not trivial to accomplish,” he said. “Placebo medical therapy studies are very expensive, but I think this is a mandate for doing more studies. This is just the tip of the iceberg.”
Additionally, evaluating hospital stays in LVAD studies “is a really important endpoint,” Dr. Adler said.
“For me, one of the key things that we don’t think about enough is that lowering days in the hospital is a really big deal,” he said. “No one wants to spend time in the hospital, so anything we can do to lower the amount of hospital days is real impactful.”
Abbott funded and sponsored the ARIES-HM3 trial. Dr. Mehra disclosed relationships with Abbott, Moderna, Natera, Transmedics, Paragonix, NupulseCV, FineHeart, and Leviticus. Dr. Adler has disclosed no relevant financial relationships.
PHILADELPHIA – particularly if it’s a newer device that does not use the centrifugal- or continuous-flow pump technology of conventional LVADs, new randomized results suggest.
“We’ve always thought that somehow aspirin prevents stroke and prevents clotting and that it’s anti-inflammatory, and what we found in ARIES was the exact opposite,” said Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center and Harvard Medical School, both in Boston, who reported results of the ARIES-HM3 trial of the HeartMate 3 LVAD, a device that uses a fully magnetically levitated rotor to maintain blood flow.
ARIES-HM3 randomly assigned 589 patients who received the HeartMate 3 device to vitamin K therapy with aspirin or to placebo. Dr. Mehra said it was the first international trial to conclusively evaluate medical therapy in patients who get an LVAD.
Unexpected findings
“To be honest with you, we set this up as a safety study to see if we could eliminate aspirin,” Dr. Mehra said in an interview. “We didn’t expect that the bleeding rates would decrease by 34% and that gastrointestinal bleeding in particular would decrease by 40%. We didn’t expect that it would nearly halve the days spent in the hospital, and we didn’t expect that the cost of care would decrease by 40%.”
Dr. Mehra reported the results at the annual scientific sessions of the American Heart Association. They were published simultaneously online in JAMA.
The researchers found that 74% of patients in the placebo group met the primary endpoint of being alive and not having any hemocompatibility events at 12 months vs 68% of the aspirin patients. The rate of nonsurgical bleeding events was 30% in the placebo group versus 42.4% in the aspirin patients. The rates of GI bleeding were 13% and 21.6% in the respective groups.
In his talk, Dr. Mehra noted the placebo group spent 47% fewer days in the hospital for bleeding, with hospitalization costs 41% lower than the aspirin group.
“We are very quick to throw things as deemed medical therapy at patients and this study outcome should give us pause that not everything we do may be right, and that we need to start building a stronger evidence base in medical therapy for what we do with patients that are on device support,” Dr. Mehra said.
Shift of focus to therapy
The study’s focus on aspirin therapy may be as significant as its evaluation of the HeartMate 3 LVAD, discussant Eric David Adler, MD, a cardiologist and section head of heart transplant at the University of California, San Diego, said in an interview.
“We focus so much on the device,” he said. “It’s like a set-it-and-forget-it kind of thing and we’re surprised that we see complications because we haven’t put a lot of effort into the medical therapy component.”
But he credited this study for doing just that, adding that it can serve as a model for future studies of LVADs, although such studies can face hurdles. “These studies are not trivial to accomplish,” he said. “Placebo medical therapy studies are very expensive, but I think this is a mandate for doing more studies. This is just the tip of the iceberg.”
Additionally, evaluating hospital stays in LVAD studies “is a really important endpoint,” Dr. Adler said.
“For me, one of the key things that we don’t think about enough is that lowering days in the hospital is a really big deal,” he said. “No one wants to spend time in the hospital, so anything we can do to lower the amount of hospital days is real impactful.”
Abbott funded and sponsored the ARIES-HM3 trial. Dr. Mehra disclosed relationships with Abbott, Moderna, Natera, Transmedics, Paragonix, NupulseCV, FineHeart, and Leviticus. Dr. Adler has disclosed no relevant financial relationships.
PHILADELPHIA – particularly if it’s a newer device that does not use the centrifugal- or continuous-flow pump technology of conventional LVADs, new randomized results suggest.
“We’ve always thought that somehow aspirin prevents stroke and prevents clotting and that it’s anti-inflammatory, and what we found in ARIES was the exact opposite,” said Mandeep Mehra, MD, of Brigham and Women’s Hospital Heart and Vascular Center and Harvard Medical School, both in Boston, who reported results of the ARIES-HM3 trial of the HeartMate 3 LVAD, a device that uses a fully magnetically levitated rotor to maintain blood flow.
ARIES-HM3 randomly assigned 589 patients who received the HeartMate 3 device to vitamin K therapy with aspirin or to placebo. Dr. Mehra said it was the first international trial to conclusively evaluate medical therapy in patients who get an LVAD.
Unexpected findings
“To be honest with you, we set this up as a safety study to see if we could eliminate aspirin,” Dr. Mehra said in an interview. “We didn’t expect that the bleeding rates would decrease by 34% and that gastrointestinal bleeding in particular would decrease by 40%. We didn’t expect that it would nearly halve the days spent in the hospital, and we didn’t expect that the cost of care would decrease by 40%.”
Dr. Mehra reported the results at the annual scientific sessions of the American Heart Association. They were published simultaneously online in JAMA.
The researchers found that 74% of patients in the placebo group met the primary endpoint of being alive and not having any hemocompatibility events at 12 months vs 68% of the aspirin patients. The rate of nonsurgical bleeding events was 30% in the placebo group versus 42.4% in the aspirin patients. The rates of GI bleeding were 13% and 21.6% in the respective groups.
In his talk, Dr. Mehra noted the placebo group spent 47% fewer days in the hospital for bleeding, with hospitalization costs 41% lower than the aspirin group.
“We are very quick to throw things as deemed medical therapy at patients and this study outcome should give us pause that not everything we do may be right, and that we need to start building a stronger evidence base in medical therapy for what we do with patients that are on device support,” Dr. Mehra said.
Shift of focus to therapy
The study’s focus on aspirin therapy may be as significant as its evaluation of the HeartMate 3 LVAD, discussant Eric David Adler, MD, a cardiologist and section head of heart transplant at the University of California, San Diego, said in an interview.
“We focus so much on the device,” he said. “It’s like a set-it-and-forget-it kind of thing and we’re surprised that we see complications because we haven’t put a lot of effort into the medical therapy component.”
But he credited this study for doing just that, adding that it can serve as a model for future studies of LVADs, although such studies can face hurdles. “These studies are not trivial to accomplish,” he said. “Placebo medical therapy studies are very expensive, but I think this is a mandate for doing more studies. This is just the tip of the iceberg.”
Additionally, evaluating hospital stays in LVAD studies “is a really important endpoint,” Dr. Adler said.
“For me, one of the key things that we don’t think about enough is that lowering days in the hospital is a really big deal,” he said. “No one wants to spend time in the hospital, so anything we can do to lower the amount of hospital days is real impactful.”
Abbott funded and sponsored the ARIES-HM3 trial. Dr. Mehra disclosed relationships with Abbott, Moderna, Natera, Transmedics, Paragonix, NupulseCV, FineHeart, and Leviticus. Dr. Adler has disclosed no relevant financial relationships.
AT AHA 2023
Women have worse outcomes in cardiogenic shock
“These data identify the need for us to continue working to identify barriers in terms of diagnosis, management, and technological innovations for women in cardiogenic shock to resolve these issues and improve outcomes,” the senior author of the study, Navin Kapur, MD, Tufts Medical Center, Boston, said in an interview.
The study is said to be the one of the largest contemporary analyses of real-world registry data on the characteristics and outcomes of women in comparison with men with cardiogenic shock.
It showed sex-specific differences in outcomes that were primarily driven by differences in heart failure–related cardiogenic shock. Women with heart failure–related cardiogenic shock had more severe cardiogenic shock, worse survival at discharge, and more vascular complications than men. Outcomes in cardiogenic shock related to MI were similar for men and women.
The study, which will be presented at the upcoming annual meeting of the American Heart Association, was published online in JACC: Heart Failure.
Dr. Kapur founded the Cardiogenic Shock Working Group in 2017 to collect quality data on the condition.
“We realized our patients were dying, and we didn’t have enough data on how best to manage them. So, we started this registry, and now have detailed data on close to 9,000 patients with cardiogenic shock from 45 hospitals in the U.S., Mexico, Australia, and Japan,” he explained.
“The primary goal is to try to investigate the questions related to cardiogenic shock that can inform management, and one of the key questions that came up was differences in how men and women present with cardiogenic shock and what their outcomes may be. This is what we are reporting in this paper,” he added.
Cardiogenic shock is defined as having a low cardiac output most commonly because of MI or an episode of acute heart failure, Dr. Kapur said. Patients with cardiogenic shock are identified by their low blood pressure or hypoperfusion evidenced by clinical exam or biomarkers, such as elevated lactate levels.
“In this analysis, we’re looking at patients presenting with cardiogenic shock, so were not looking at the incidence of the condition in men versus women,” Dr. Kapur noted. “However, we believe that cardiogenic shock is probably more underrepresented in women, who may present with an MI or acute heart failure and may or may not be identified as having low cardiac output states until quite late. The likelihood is that the incidence is similar in men and women, but women are more often undiagnosed.”
For the current study, the authors analyzed data on 5,083 patients with cardiogenic shock in the registry, of whom 1,522 (30%) were women. Compared with men, women had slightly higher body mass index (BMI) and smaller body surface area.
Results showed that women with heart failure–related cardiogenic shock had worse survival at discharge than men (69.9% vs. 74.4%) and a higher rate of refractory shock (SCAI stage E; 26% vs. 21%). Women were also less likely to undergo pulmonary artery catheterization (52.9% vs. 54.6%), heart transplantation (6.5% vs. 10.3%), or left ventricular assist device implantation (7.8% vs. 10%).
Regardless of cardiogenic shock etiology, women had more vascular complications (8.8% vs. 5.7%), bleeding (7.1% vs. 5.2%), and limb ischemia (6.8% vs. 4.5%).
“This analysis is quite revealing. We identified some important distinctions between men and women,” Dr. Kapur commented.
For many patients who present with MI-related cardiogenic shock, many of the baseline characteristics in men and women were quite similar, he said. “But in heart failure–related cardiogenic shock, we saw more differences, with typical comorbidities associated with cardiogenic shock [e.g., diabetes, chronic kidney disease, hypertension] being less common in women than in men. This suggests there may be phenotypic differences as to why women present with heart failure shock versus men.”
Dr. Kapur pointed out that differences in BMI or body surface area between men and women may play into some of the management decision-making.
“Women having a smaller stature may lead to a selection bias where we don’t want to use large-bore pumps or devices because we’re worried about causing complications. We found in the analysis that vascular complications such as bleeding or ischemia of the lower extremity where these devices typically go were more frequent in women,” he noted.
“We also found that women were less likely to receive invasive therapies in general, including pulmonary artery catheters, temporary mechanical support, and heart replacements, such as LVAD or transplants,” he added.
Further results showed that, after propensity score matching, some of the gender differences disappeared, but women continued to have a higher rate of vascular complications (10.4% women vs. 7.4% men).
But Dr. Kapur warned that the propensity-matched analysis had some caveats.
“Essentially what we are doing with propensity matching is creating two populations that are as similar as possible, and this reduced the number of patients in the analysis down to 25% of the original population,” he said. “One of the things we had to match was body surface area, and in doing this, we are taking out one of the most important differences between men and women, and as a result, a lot of the differences in outcomes go away.
“In this respect, propensity matching can be a bit of a double-edge sword,” he added. “I think the non–propensity-matched results are more interesting, as they are more of a reflection of the real world.”
Dr. Kapur concluded that these findings are compelling enough to suggest that there are important differences between women and men with cardiogenic shock in terms of outcomes as well as complication rates.
“Our decision-making around women seems to be different to that around men. I think this paper should start to trigger more awareness of that.”
Dr. Kapur also emphasized the importance of paying attention to vascular complications in women.
“The higher rates of bleeding and limb ischemia issues in women may explain the rationale for being less aggressive with invasive therapies in women,” he said. “But we need to come up with better solutions or technologies so they can be used more effectively in women. This could include adapting technology for smaller vascular sizes, which should lead to better outcome and fewer complications in women.”
He added that further granular data on this issue are needed. “We have very limited datasets in cardiogenic shock. There are few randomized controlled trials, and women are not well represented in such trials. We need to make sure we enroll women in randomized trials.”
Dr. Kapur said more women physicians who treat cardiogenic shock are also required, which would include cardiologists, critical care specialists, cardiac surgeons, and anesthesia personnel.
He pointed out that the two first authors of the current study are women – Van-Khue Ton, MD, Massachusetts General Hospital, Boston, and Manreet Kanwar, MD, Allegheny Health Network, Pittsburgh.
“We worked hard to involve women as principal investigators. They led the effort. These are investigations led by women, on women, to advance the care of women,” he commented.
Gender-related inequality
In an editorial accompanying publication of the study, Sara Kalantari, MD, and Jonathan Grinstein, MD, University of Chicago, and Robert O. Roswell, MD, Hofstra University, Hempstead, N.Y., said these results “provide valuable information about gender-related inequality in care and outcomes in the management of cardiogenic shock, although the exact mechanisms driving these observed differences still need to be elucidated.
“Broadly speaking, barriers in the care of women with heart failure and cardiogenic shock include a reduced awareness among both patients and providers, a deficiency of sex-specific objective criteria for guiding therapy, and unfavorable temporary mechanical circulatory support devices with higher rates of hemocompatibility-related complications in women,” they added.
“In the era of the multidisciplinary shock team and shock pathways with protocolized management algorithms, it is imperative that we still allow for personalization of care to match the physiologic needs of the patient in order for us to continue to close the gender gap in the care of patients presenting with cardiogenic shock,” the editorialists concluded.
A version of this article appeared on Medscape.com.
“These data identify the need for us to continue working to identify barriers in terms of diagnosis, management, and technological innovations for women in cardiogenic shock to resolve these issues and improve outcomes,” the senior author of the study, Navin Kapur, MD, Tufts Medical Center, Boston, said in an interview.
The study is said to be the one of the largest contemporary analyses of real-world registry data on the characteristics and outcomes of women in comparison with men with cardiogenic shock.
It showed sex-specific differences in outcomes that were primarily driven by differences in heart failure–related cardiogenic shock. Women with heart failure–related cardiogenic shock had more severe cardiogenic shock, worse survival at discharge, and more vascular complications than men. Outcomes in cardiogenic shock related to MI were similar for men and women.
The study, which will be presented at the upcoming annual meeting of the American Heart Association, was published online in JACC: Heart Failure.
Dr. Kapur founded the Cardiogenic Shock Working Group in 2017 to collect quality data on the condition.
“We realized our patients were dying, and we didn’t have enough data on how best to manage them. So, we started this registry, and now have detailed data on close to 9,000 patients with cardiogenic shock from 45 hospitals in the U.S., Mexico, Australia, and Japan,” he explained.
“The primary goal is to try to investigate the questions related to cardiogenic shock that can inform management, and one of the key questions that came up was differences in how men and women present with cardiogenic shock and what their outcomes may be. This is what we are reporting in this paper,” he added.
Cardiogenic shock is defined as having a low cardiac output most commonly because of MI or an episode of acute heart failure, Dr. Kapur said. Patients with cardiogenic shock are identified by their low blood pressure or hypoperfusion evidenced by clinical exam or biomarkers, such as elevated lactate levels.
“In this analysis, we’re looking at patients presenting with cardiogenic shock, so were not looking at the incidence of the condition in men versus women,” Dr. Kapur noted. “However, we believe that cardiogenic shock is probably more underrepresented in women, who may present with an MI or acute heart failure and may or may not be identified as having low cardiac output states until quite late. The likelihood is that the incidence is similar in men and women, but women are more often undiagnosed.”
For the current study, the authors analyzed data on 5,083 patients with cardiogenic shock in the registry, of whom 1,522 (30%) were women. Compared with men, women had slightly higher body mass index (BMI) and smaller body surface area.
Results showed that women with heart failure–related cardiogenic shock had worse survival at discharge than men (69.9% vs. 74.4%) and a higher rate of refractory shock (SCAI stage E; 26% vs. 21%). Women were also less likely to undergo pulmonary artery catheterization (52.9% vs. 54.6%), heart transplantation (6.5% vs. 10.3%), or left ventricular assist device implantation (7.8% vs. 10%).
Regardless of cardiogenic shock etiology, women had more vascular complications (8.8% vs. 5.7%), bleeding (7.1% vs. 5.2%), and limb ischemia (6.8% vs. 4.5%).
“This analysis is quite revealing. We identified some important distinctions between men and women,” Dr. Kapur commented.
For many patients who present with MI-related cardiogenic shock, many of the baseline characteristics in men and women were quite similar, he said. “But in heart failure–related cardiogenic shock, we saw more differences, with typical comorbidities associated with cardiogenic shock [e.g., diabetes, chronic kidney disease, hypertension] being less common in women than in men. This suggests there may be phenotypic differences as to why women present with heart failure shock versus men.”
Dr. Kapur pointed out that differences in BMI or body surface area between men and women may play into some of the management decision-making.
“Women having a smaller stature may lead to a selection bias where we don’t want to use large-bore pumps or devices because we’re worried about causing complications. We found in the analysis that vascular complications such as bleeding or ischemia of the lower extremity where these devices typically go were more frequent in women,” he noted.
“We also found that women were less likely to receive invasive therapies in general, including pulmonary artery catheters, temporary mechanical support, and heart replacements, such as LVAD or transplants,” he added.
Further results showed that, after propensity score matching, some of the gender differences disappeared, but women continued to have a higher rate of vascular complications (10.4% women vs. 7.4% men).
But Dr. Kapur warned that the propensity-matched analysis had some caveats.
“Essentially what we are doing with propensity matching is creating two populations that are as similar as possible, and this reduced the number of patients in the analysis down to 25% of the original population,” he said. “One of the things we had to match was body surface area, and in doing this, we are taking out one of the most important differences between men and women, and as a result, a lot of the differences in outcomes go away.
“In this respect, propensity matching can be a bit of a double-edge sword,” he added. “I think the non–propensity-matched results are more interesting, as they are more of a reflection of the real world.”
Dr. Kapur concluded that these findings are compelling enough to suggest that there are important differences between women and men with cardiogenic shock in terms of outcomes as well as complication rates.
“Our decision-making around women seems to be different to that around men. I think this paper should start to trigger more awareness of that.”
Dr. Kapur also emphasized the importance of paying attention to vascular complications in women.
“The higher rates of bleeding and limb ischemia issues in women may explain the rationale for being less aggressive with invasive therapies in women,” he said. “But we need to come up with better solutions or technologies so they can be used more effectively in women. This could include adapting technology for smaller vascular sizes, which should lead to better outcome and fewer complications in women.”
He added that further granular data on this issue are needed. “We have very limited datasets in cardiogenic shock. There are few randomized controlled trials, and women are not well represented in such trials. We need to make sure we enroll women in randomized trials.”
Dr. Kapur said more women physicians who treat cardiogenic shock are also required, which would include cardiologists, critical care specialists, cardiac surgeons, and anesthesia personnel.
He pointed out that the two first authors of the current study are women – Van-Khue Ton, MD, Massachusetts General Hospital, Boston, and Manreet Kanwar, MD, Allegheny Health Network, Pittsburgh.
“We worked hard to involve women as principal investigators. They led the effort. These are investigations led by women, on women, to advance the care of women,” he commented.
Gender-related inequality
In an editorial accompanying publication of the study, Sara Kalantari, MD, and Jonathan Grinstein, MD, University of Chicago, and Robert O. Roswell, MD, Hofstra University, Hempstead, N.Y., said these results “provide valuable information about gender-related inequality in care and outcomes in the management of cardiogenic shock, although the exact mechanisms driving these observed differences still need to be elucidated.
“Broadly speaking, barriers in the care of women with heart failure and cardiogenic shock include a reduced awareness among both patients and providers, a deficiency of sex-specific objective criteria for guiding therapy, and unfavorable temporary mechanical circulatory support devices with higher rates of hemocompatibility-related complications in women,” they added.
“In the era of the multidisciplinary shock team and shock pathways with protocolized management algorithms, it is imperative that we still allow for personalization of care to match the physiologic needs of the patient in order for us to continue to close the gender gap in the care of patients presenting with cardiogenic shock,” the editorialists concluded.
A version of this article appeared on Medscape.com.
“These data identify the need for us to continue working to identify barriers in terms of diagnosis, management, and technological innovations for women in cardiogenic shock to resolve these issues and improve outcomes,” the senior author of the study, Navin Kapur, MD, Tufts Medical Center, Boston, said in an interview.
The study is said to be the one of the largest contemporary analyses of real-world registry data on the characteristics and outcomes of women in comparison with men with cardiogenic shock.
It showed sex-specific differences in outcomes that were primarily driven by differences in heart failure–related cardiogenic shock. Women with heart failure–related cardiogenic shock had more severe cardiogenic shock, worse survival at discharge, and more vascular complications than men. Outcomes in cardiogenic shock related to MI were similar for men and women.
The study, which will be presented at the upcoming annual meeting of the American Heart Association, was published online in JACC: Heart Failure.
Dr. Kapur founded the Cardiogenic Shock Working Group in 2017 to collect quality data on the condition.
“We realized our patients were dying, and we didn’t have enough data on how best to manage them. So, we started this registry, and now have detailed data on close to 9,000 patients with cardiogenic shock from 45 hospitals in the U.S., Mexico, Australia, and Japan,” he explained.
“The primary goal is to try to investigate the questions related to cardiogenic shock that can inform management, and one of the key questions that came up was differences in how men and women present with cardiogenic shock and what their outcomes may be. This is what we are reporting in this paper,” he added.
Cardiogenic shock is defined as having a low cardiac output most commonly because of MI or an episode of acute heart failure, Dr. Kapur said. Patients with cardiogenic shock are identified by their low blood pressure or hypoperfusion evidenced by clinical exam or biomarkers, such as elevated lactate levels.
“In this analysis, we’re looking at patients presenting with cardiogenic shock, so were not looking at the incidence of the condition in men versus women,” Dr. Kapur noted. “However, we believe that cardiogenic shock is probably more underrepresented in women, who may present with an MI or acute heart failure and may or may not be identified as having low cardiac output states until quite late. The likelihood is that the incidence is similar in men and women, but women are more often undiagnosed.”
For the current study, the authors analyzed data on 5,083 patients with cardiogenic shock in the registry, of whom 1,522 (30%) were women. Compared with men, women had slightly higher body mass index (BMI) and smaller body surface area.
Results showed that women with heart failure–related cardiogenic shock had worse survival at discharge than men (69.9% vs. 74.4%) and a higher rate of refractory shock (SCAI stage E; 26% vs. 21%). Women were also less likely to undergo pulmonary artery catheterization (52.9% vs. 54.6%), heart transplantation (6.5% vs. 10.3%), or left ventricular assist device implantation (7.8% vs. 10%).
Regardless of cardiogenic shock etiology, women had more vascular complications (8.8% vs. 5.7%), bleeding (7.1% vs. 5.2%), and limb ischemia (6.8% vs. 4.5%).
“This analysis is quite revealing. We identified some important distinctions between men and women,” Dr. Kapur commented.
For many patients who present with MI-related cardiogenic shock, many of the baseline characteristics in men and women were quite similar, he said. “But in heart failure–related cardiogenic shock, we saw more differences, with typical comorbidities associated with cardiogenic shock [e.g., diabetes, chronic kidney disease, hypertension] being less common in women than in men. This suggests there may be phenotypic differences as to why women present with heart failure shock versus men.”
Dr. Kapur pointed out that differences in BMI or body surface area between men and women may play into some of the management decision-making.
“Women having a smaller stature may lead to a selection bias where we don’t want to use large-bore pumps or devices because we’re worried about causing complications. We found in the analysis that vascular complications such as bleeding or ischemia of the lower extremity where these devices typically go were more frequent in women,” he noted.
“We also found that women were less likely to receive invasive therapies in general, including pulmonary artery catheters, temporary mechanical support, and heart replacements, such as LVAD or transplants,” he added.
Further results showed that, after propensity score matching, some of the gender differences disappeared, but women continued to have a higher rate of vascular complications (10.4% women vs. 7.4% men).
But Dr. Kapur warned that the propensity-matched analysis had some caveats.
“Essentially what we are doing with propensity matching is creating two populations that are as similar as possible, and this reduced the number of patients in the analysis down to 25% of the original population,” he said. “One of the things we had to match was body surface area, and in doing this, we are taking out one of the most important differences between men and women, and as a result, a lot of the differences in outcomes go away.
“In this respect, propensity matching can be a bit of a double-edge sword,” he added. “I think the non–propensity-matched results are more interesting, as they are more of a reflection of the real world.”
Dr. Kapur concluded that these findings are compelling enough to suggest that there are important differences between women and men with cardiogenic shock in terms of outcomes as well as complication rates.
“Our decision-making around women seems to be different to that around men. I think this paper should start to trigger more awareness of that.”
Dr. Kapur also emphasized the importance of paying attention to vascular complications in women.
“The higher rates of bleeding and limb ischemia issues in women may explain the rationale for being less aggressive with invasive therapies in women,” he said. “But we need to come up with better solutions or technologies so they can be used more effectively in women. This could include adapting technology for smaller vascular sizes, which should lead to better outcome and fewer complications in women.”
He added that further granular data on this issue are needed. “We have very limited datasets in cardiogenic shock. There are few randomized controlled trials, and women are not well represented in such trials. We need to make sure we enroll women in randomized trials.”
Dr. Kapur said more women physicians who treat cardiogenic shock are also required, which would include cardiologists, critical care specialists, cardiac surgeons, and anesthesia personnel.
He pointed out that the two first authors of the current study are women – Van-Khue Ton, MD, Massachusetts General Hospital, Boston, and Manreet Kanwar, MD, Allegheny Health Network, Pittsburgh.
“We worked hard to involve women as principal investigators. They led the effort. These are investigations led by women, on women, to advance the care of women,” he commented.
Gender-related inequality
In an editorial accompanying publication of the study, Sara Kalantari, MD, and Jonathan Grinstein, MD, University of Chicago, and Robert O. Roswell, MD, Hofstra University, Hempstead, N.Y., said these results “provide valuable information about gender-related inequality in care and outcomes in the management of cardiogenic shock, although the exact mechanisms driving these observed differences still need to be elucidated.
“Broadly speaking, barriers in the care of women with heart failure and cardiogenic shock include a reduced awareness among both patients and providers, a deficiency of sex-specific objective criteria for guiding therapy, and unfavorable temporary mechanical circulatory support devices with higher rates of hemocompatibility-related complications in women,” they added.
“In the era of the multidisciplinary shock team and shock pathways with protocolized management algorithms, it is imperative that we still allow for personalization of care to match the physiologic needs of the patient in order for us to continue to close the gender gap in the care of patients presenting with cardiogenic shock,” the editorialists concluded.
A version of this article appeared on Medscape.com.
FROM AHA 2023
Aprocitentan reduces resistant hypertension in CKD
PHILADELPHIA – (CKD). The results come from a prespecified subgroup analysis of data collected in the drug’s pivotal trial, PRECISION.
The findings provide support for potentially using aprocitentan, if approved for U.S. marketing in 2024, in patients with blood pressure that remains elevated despite treatment with three established antihypertensive drug classes and with stage 3 CKD with an estimated glomerular filtration rate of 30-59 mL/min per 1.73 m2. This is a key group of patients because “chronic kidney disease is the most common comorbidity in patients with resistant hypertension,” said George Bakris, MD, who presented the subgroup analysis at Kidney Week 2023, organized by the American Society of Nephrology.
The CKD subgroup analysis showed “good evidence for safety and evidence in stage 3 CKD,” a subgroup of 141 patients among the total 730 enrolled in PRECISION, said Dr. Bakris. Professor and director of the Comprehensive Hypertension Center at the University of Chicago, he acknowledged that while the results also showed a signal for safety and efficacy in the 21 enrolled patients with stage 4 hypertension, 15-29 mL/min per 1.73m2, this number of stage 4 patients was too small to allow definitive conclusions.
Nephrologist Nishigandha Pradhan, MD, who cochaired the session with this report, agreed. “Resistant hypertension is a particularly intractable problem in patients with CKD, and the risk is greatest with stage 4 CKD. If studies could show that aprocitentan is safe in people with stage 4 CKD, that would be a big plus, but we need more data,” commented Dr. Pradhan in an interview.
Incremental blood pressure reductions
The parallel-group, phase 3 PRECISION trial investigated the safety and short-term antihypertensive effect of aprocitentan in patients with resistant hypertension. The study’s primary efficacy endpoint was blood pressure reduction from baseline in 730 randomized people with persistent systolic hypertension despite treatment with three established antihypertensive agents including a diuretic. The study ran during June 2018–April 2022 at 191 sites in 22 countries.
The primary outcome after 4 weeks on treatment was a least-square mean reduction in office-measured systolic blood pressure, compared with placebo, of 3.8 mm Hg with a 12.5-mg daily oral dose of aprocitentan and 3.7 mm Hg with a 25-mg daily oral dose. Both significant differences were first reported in 2022. Twenty-four–hour ambulatory systolic blood pressures after 4 weeks of treatment fell by an average of 4.2 mm Hg on the lower dose compared with placebo and by an average of 5.9 mm Hg on the higher daily dose, compared with placebo.
Consistent blood pressure reductions occurred in the CKD subgroups. Among people with stage 3 CKD, daytime ambulatory blood pressure at 4 weeks fell by about 10 mm Hg on both the 12.5-mg daily and 25-mg daily doses, compared with placebo.
Among the small number of people with stage 4 CKD, the incremental nighttime systolic blood pressure on aprocitentan, compared with placebo, was even greater, with about a 15–mm Hg incremental reduction on 12.5 mg daily and about a 17–mm Hg incremental reduction on the higher dose.
“This is the first evidence for a change in nocturnal blood pressure in people with stage 4 CKD [and treatment-resistant hypertension], but it was just 21 patients so not yet a big deal,” Dr. Bakris noted.
Increased rates of fluid retention
Although aprocitentan was generally well tolerated, the most common adverse effect was edema or fluid retention, mainly during the first 4 weeks of treatment. In the full PRECISION cohort, this adverse event occurred in 2.1% of people treated with placebo, 9.1% of those on the 12.5-mg daily dose, and in 18.4% of those on the higher dose during the initial 4-week phase of treatment.
Among all stage 3 and 4 CKD patients on aprocitentan, edema or fluid retention occurred in 21% during the first 4 weeks, and in 27% during an additional 32 weeks of treatment with 25 mg aprocitentan daily. A majority of these patients started a diuretic to address their excess fluid, with only two discontinuing aprocitentan treatment.
“Fluid retention is an issue with aprocitentan,” Dr. Bakris acknowledged. But he also highlighted than only 6 of the 162 patients with CKD required hospitalization for heart failure during the study, and one of these cases had placebo treatment. Among the five with acute heart failure while on aprocitentan, none had to stop their treatment, and two had a clear prior history of heart failure.
The companies developing aprocitentan, Janssen and Idorsia, used the PRECISION results as the centerpiece in filing for a new drug approval to the FDA, with a March 2024 goal for the FDA‘s decision. Dr. Bakris called the application “a solid case for approval.” But he added that approval will likely require that all treatment candidates first undergo testing of their heart function or fluid volume, such as a measure of their blood level of N-terminal pro-B-type natriuretic peptide, with treatment withheld when the level is too high.
The upside of aprocitentan compared with current drug options for treating resistant hypertension is that it has not appeared to cause any increase in blood potassium levels, which is an issue with the current top agent for resistant hypertension, spironolactone.
“The problem with spironolactone is the risk for hyperkalemia, which keeps us looking for something with lower risk,” commented Dr. Pradhan, a nephrologist with University Hospitals in Cleveland. Hyperkalemia is an even greater risk for people with CKD. Although the PRECISION trial identified the issue of fluid retention with aprocitentan, titrating an effective dose of a loop diuretic for treated patients may effectively blunt the edema risk, Dr. Pradhan said.
Endothelin has a potent vasoconstrictive effect and is “implicated in the pathogenesis of hypertension,” Dr. Bakris explained. Aprocitentan antagonizes both the endothelin A and B receptors. The subgroup analyses also showed that in people with CKD, treatment with aprocitentan led to roughly a halving of the baseline level of urine albumin-to-creatinine ratio, a small and stable decrease in estimated glomerular filtration rate, and a modest and stable increase in blood levels of N-terminal pro-B-type natriuretic hormone.
The PRECISION trial was sponsored by Janssen Pharmaceuticals and Idorsia Pharmaceuticals, the companies jointly developing aprocitentan. Dr. Bakris has been a consultant to Janssen, and also a consultant to or honoraria recipient of Alnylam, AstraZeneca, Bayer, Dia Medica Therapeutics, Ionis, inREGEN, KBP Biosciences, Merck, Novo Nordisk, and Quantum Genomics. Dr. Pradhan had no disclosures.
PHILADELPHIA – (CKD). The results come from a prespecified subgroup analysis of data collected in the drug’s pivotal trial, PRECISION.
The findings provide support for potentially using aprocitentan, if approved for U.S. marketing in 2024, in patients with blood pressure that remains elevated despite treatment with three established antihypertensive drug classes and with stage 3 CKD with an estimated glomerular filtration rate of 30-59 mL/min per 1.73 m2. This is a key group of patients because “chronic kidney disease is the most common comorbidity in patients with resistant hypertension,” said George Bakris, MD, who presented the subgroup analysis at Kidney Week 2023, organized by the American Society of Nephrology.
The CKD subgroup analysis showed “good evidence for safety and evidence in stage 3 CKD,” a subgroup of 141 patients among the total 730 enrolled in PRECISION, said Dr. Bakris. Professor and director of the Comprehensive Hypertension Center at the University of Chicago, he acknowledged that while the results also showed a signal for safety and efficacy in the 21 enrolled patients with stage 4 hypertension, 15-29 mL/min per 1.73m2, this number of stage 4 patients was too small to allow definitive conclusions.
Nephrologist Nishigandha Pradhan, MD, who cochaired the session with this report, agreed. “Resistant hypertension is a particularly intractable problem in patients with CKD, and the risk is greatest with stage 4 CKD. If studies could show that aprocitentan is safe in people with stage 4 CKD, that would be a big plus, but we need more data,” commented Dr. Pradhan in an interview.
Incremental blood pressure reductions
The parallel-group, phase 3 PRECISION trial investigated the safety and short-term antihypertensive effect of aprocitentan in patients with resistant hypertension. The study’s primary efficacy endpoint was blood pressure reduction from baseline in 730 randomized people with persistent systolic hypertension despite treatment with three established antihypertensive agents including a diuretic. The study ran during June 2018–April 2022 at 191 sites in 22 countries.
The primary outcome after 4 weeks on treatment was a least-square mean reduction in office-measured systolic blood pressure, compared with placebo, of 3.8 mm Hg with a 12.5-mg daily oral dose of aprocitentan and 3.7 mm Hg with a 25-mg daily oral dose. Both significant differences were first reported in 2022. Twenty-four–hour ambulatory systolic blood pressures after 4 weeks of treatment fell by an average of 4.2 mm Hg on the lower dose compared with placebo and by an average of 5.9 mm Hg on the higher daily dose, compared with placebo.
Consistent blood pressure reductions occurred in the CKD subgroups. Among people with stage 3 CKD, daytime ambulatory blood pressure at 4 weeks fell by about 10 mm Hg on both the 12.5-mg daily and 25-mg daily doses, compared with placebo.
Among the small number of people with stage 4 CKD, the incremental nighttime systolic blood pressure on aprocitentan, compared with placebo, was even greater, with about a 15–mm Hg incremental reduction on 12.5 mg daily and about a 17–mm Hg incremental reduction on the higher dose.
“This is the first evidence for a change in nocturnal blood pressure in people with stage 4 CKD [and treatment-resistant hypertension], but it was just 21 patients so not yet a big deal,” Dr. Bakris noted.
Increased rates of fluid retention
Although aprocitentan was generally well tolerated, the most common adverse effect was edema or fluid retention, mainly during the first 4 weeks of treatment. In the full PRECISION cohort, this adverse event occurred in 2.1% of people treated with placebo, 9.1% of those on the 12.5-mg daily dose, and in 18.4% of those on the higher dose during the initial 4-week phase of treatment.
Among all stage 3 and 4 CKD patients on aprocitentan, edema or fluid retention occurred in 21% during the first 4 weeks, and in 27% during an additional 32 weeks of treatment with 25 mg aprocitentan daily. A majority of these patients started a diuretic to address their excess fluid, with only two discontinuing aprocitentan treatment.
“Fluid retention is an issue with aprocitentan,” Dr. Bakris acknowledged. But he also highlighted than only 6 of the 162 patients with CKD required hospitalization for heart failure during the study, and one of these cases had placebo treatment. Among the five with acute heart failure while on aprocitentan, none had to stop their treatment, and two had a clear prior history of heart failure.
The companies developing aprocitentan, Janssen and Idorsia, used the PRECISION results as the centerpiece in filing for a new drug approval to the FDA, with a March 2024 goal for the FDA‘s decision. Dr. Bakris called the application “a solid case for approval.” But he added that approval will likely require that all treatment candidates first undergo testing of their heart function or fluid volume, such as a measure of their blood level of N-terminal pro-B-type natriuretic peptide, with treatment withheld when the level is too high.
The upside of aprocitentan compared with current drug options for treating resistant hypertension is that it has not appeared to cause any increase in blood potassium levels, which is an issue with the current top agent for resistant hypertension, spironolactone.
“The problem with spironolactone is the risk for hyperkalemia, which keeps us looking for something with lower risk,” commented Dr. Pradhan, a nephrologist with University Hospitals in Cleveland. Hyperkalemia is an even greater risk for people with CKD. Although the PRECISION trial identified the issue of fluid retention with aprocitentan, titrating an effective dose of a loop diuretic for treated patients may effectively blunt the edema risk, Dr. Pradhan said.
Endothelin has a potent vasoconstrictive effect and is “implicated in the pathogenesis of hypertension,” Dr. Bakris explained. Aprocitentan antagonizes both the endothelin A and B receptors. The subgroup analyses also showed that in people with CKD, treatment with aprocitentan led to roughly a halving of the baseline level of urine albumin-to-creatinine ratio, a small and stable decrease in estimated glomerular filtration rate, and a modest and stable increase in blood levels of N-terminal pro-B-type natriuretic hormone.
The PRECISION trial was sponsored by Janssen Pharmaceuticals and Idorsia Pharmaceuticals, the companies jointly developing aprocitentan. Dr. Bakris has been a consultant to Janssen, and also a consultant to or honoraria recipient of Alnylam, AstraZeneca, Bayer, Dia Medica Therapeutics, Ionis, inREGEN, KBP Biosciences, Merck, Novo Nordisk, and Quantum Genomics. Dr. Pradhan had no disclosures.
PHILADELPHIA – (CKD). The results come from a prespecified subgroup analysis of data collected in the drug’s pivotal trial, PRECISION.
The findings provide support for potentially using aprocitentan, if approved for U.S. marketing in 2024, in patients with blood pressure that remains elevated despite treatment with three established antihypertensive drug classes and with stage 3 CKD with an estimated glomerular filtration rate of 30-59 mL/min per 1.73 m2. This is a key group of patients because “chronic kidney disease is the most common comorbidity in patients with resistant hypertension,” said George Bakris, MD, who presented the subgroup analysis at Kidney Week 2023, organized by the American Society of Nephrology.
The CKD subgroup analysis showed “good evidence for safety and evidence in stage 3 CKD,” a subgroup of 141 patients among the total 730 enrolled in PRECISION, said Dr. Bakris. Professor and director of the Comprehensive Hypertension Center at the University of Chicago, he acknowledged that while the results also showed a signal for safety and efficacy in the 21 enrolled patients with stage 4 hypertension, 15-29 mL/min per 1.73m2, this number of stage 4 patients was too small to allow definitive conclusions.
Nephrologist Nishigandha Pradhan, MD, who cochaired the session with this report, agreed. “Resistant hypertension is a particularly intractable problem in patients with CKD, and the risk is greatest with stage 4 CKD. If studies could show that aprocitentan is safe in people with stage 4 CKD, that would be a big plus, but we need more data,” commented Dr. Pradhan in an interview.
Incremental blood pressure reductions
The parallel-group, phase 3 PRECISION trial investigated the safety and short-term antihypertensive effect of aprocitentan in patients with resistant hypertension. The study’s primary efficacy endpoint was blood pressure reduction from baseline in 730 randomized people with persistent systolic hypertension despite treatment with three established antihypertensive agents including a diuretic. The study ran during June 2018–April 2022 at 191 sites in 22 countries.
The primary outcome after 4 weeks on treatment was a least-square mean reduction in office-measured systolic blood pressure, compared with placebo, of 3.8 mm Hg with a 12.5-mg daily oral dose of aprocitentan and 3.7 mm Hg with a 25-mg daily oral dose. Both significant differences were first reported in 2022. Twenty-four–hour ambulatory systolic blood pressures after 4 weeks of treatment fell by an average of 4.2 mm Hg on the lower dose compared with placebo and by an average of 5.9 mm Hg on the higher daily dose, compared with placebo.
Consistent blood pressure reductions occurred in the CKD subgroups. Among people with stage 3 CKD, daytime ambulatory blood pressure at 4 weeks fell by about 10 mm Hg on both the 12.5-mg daily and 25-mg daily doses, compared with placebo.
Among the small number of people with stage 4 CKD, the incremental nighttime systolic blood pressure on aprocitentan, compared with placebo, was even greater, with about a 15–mm Hg incremental reduction on 12.5 mg daily and about a 17–mm Hg incremental reduction on the higher dose.
“This is the first evidence for a change in nocturnal blood pressure in people with stage 4 CKD [and treatment-resistant hypertension], but it was just 21 patients so not yet a big deal,” Dr. Bakris noted.
Increased rates of fluid retention
Although aprocitentan was generally well tolerated, the most common adverse effect was edema or fluid retention, mainly during the first 4 weeks of treatment. In the full PRECISION cohort, this adverse event occurred in 2.1% of people treated with placebo, 9.1% of those on the 12.5-mg daily dose, and in 18.4% of those on the higher dose during the initial 4-week phase of treatment.
Among all stage 3 and 4 CKD patients on aprocitentan, edema or fluid retention occurred in 21% during the first 4 weeks, and in 27% during an additional 32 weeks of treatment with 25 mg aprocitentan daily. A majority of these patients started a diuretic to address their excess fluid, with only two discontinuing aprocitentan treatment.
“Fluid retention is an issue with aprocitentan,” Dr. Bakris acknowledged. But he also highlighted than only 6 of the 162 patients with CKD required hospitalization for heart failure during the study, and one of these cases had placebo treatment. Among the five with acute heart failure while on aprocitentan, none had to stop their treatment, and two had a clear prior history of heart failure.
The companies developing aprocitentan, Janssen and Idorsia, used the PRECISION results as the centerpiece in filing for a new drug approval to the FDA, with a March 2024 goal for the FDA‘s decision. Dr. Bakris called the application “a solid case for approval.” But he added that approval will likely require that all treatment candidates first undergo testing of their heart function or fluid volume, such as a measure of their blood level of N-terminal pro-B-type natriuretic peptide, with treatment withheld when the level is too high.
The upside of aprocitentan compared with current drug options for treating resistant hypertension is that it has not appeared to cause any increase in blood potassium levels, which is an issue with the current top agent for resistant hypertension, spironolactone.
“The problem with spironolactone is the risk for hyperkalemia, which keeps us looking for something with lower risk,” commented Dr. Pradhan, a nephrologist with University Hospitals in Cleveland. Hyperkalemia is an even greater risk for people with CKD. Although the PRECISION trial identified the issue of fluid retention with aprocitentan, titrating an effective dose of a loop diuretic for treated patients may effectively blunt the edema risk, Dr. Pradhan said.
Endothelin has a potent vasoconstrictive effect and is “implicated in the pathogenesis of hypertension,” Dr. Bakris explained. Aprocitentan antagonizes both the endothelin A and B receptors. The subgroup analyses also showed that in people with CKD, treatment with aprocitentan led to roughly a halving of the baseline level of urine albumin-to-creatinine ratio, a small and stable decrease in estimated glomerular filtration rate, and a modest and stable increase in blood levels of N-terminal pro-B-type natriuretic hormone.
The PRECISION trial was sponsored by Janssen Pharmaceuticals and Idorsia Pharmaceuticals, the companies jointly developing aprocitentan. Dr. Bakris has been a consultant to Janssen, and also a consultant to or honoraria recipient of Alnylam, AstraZeneca, Bayer, Dia Medica Therapeutics, Ionis, inREGEN, KBP Biosciences, Merck, Novo Nordisk, and Quantum Genomics. Dr. Pradhan had no disclosures.
AT KIDNEY WEEK 2023
Durable LVAD for advanced HF still underutilized
The prognosis for patients with advanced heart failure (HF) who fail guideline-directed medical therapy is poor, but
Those are the key takeaways from a scientific statement on durable mechanical circulatory support, published online in the Journal of the American College of Cardiology.
“I think it is important to highlight this issue because of the sheer impact that heart failure has on American citizens,” corresponding author Jennifer Cowger, MD, MS, advanced heart failure specialist, Henry Ford Health, Detroit, said in an interview.
“End-stage heart failure has no medication that has shown a gain in survival, and most are dead by 1 year,” she said.
This scientific statement highlights the “amazing evolution of LVAD support and associated improvement in outcomes,” Dr. Cowger said.
Yet because LVADs are only implanted at roughly 170 U.S. centers, “many cardiologists are not aware of the amazing survival improvement with modern LVAD technology, and patients are under-referred,” Dr. Cowger noted.
Contemporary outcomes on par with heart transplant
The authors note that survival with durable LVAD (dLVAD) has markedly improved over the years. Current survival is approximately 87% at 1 year for patients supported with a contemporary LVAD.
Average patient survival is now similar to that of heart transplantation at 2 years, with 5-year dLVAD survival now approaching 60%, they point out.
Contemporary dLVAD yields significant and sustained improvements in functional capacity. Data show that roughly 80% of patients improve to NYHA functional class I and II, with significant improvements in 6-minute walk distances and health-related quality of life, the authors note.
In addition, innovations in dLVAD technology have reduced the risk of several adverse events, including pump thrombosis, stroke, and bleeding.
“Novel devices are on the horizon of clinical investigation, offering smaller size, permitting less invasive surgical implantation, and eliminating the percutaneous lead for power supply,” the authors note.
“Unfortunately, greater adoption of dLVAD therapy has not been realized due to delayed referral of patients to advanced HF centers, insufficient clinician knowledge of contemporary dLVAD outcomes (including gains in quality of life), and deprioritization of patients with dLVAD support waiting for heart transplantation,” they write.
In addition to highlighting contemporary outcomes with dLVAD support, the 18-page statement also includes sections on:
- Current indications and timing of referral
- Surgical considerations (device selection, surgical techniques and approach to concomitant valvular disease, and management of acute right ventricular dysfunction)
- Unique patient populations (women, children, and adult congenital heart disease)
- Summary, gaps, and future directions
A recent workshop held by the National Heart, Lung, and Blood Institute (NHLBI) identified critical gaps in the field of advanced HF.
One of the major gaps identified was the need to improve mechanical circulatory support use as a “complement or alternative” therapy to heart transplantation. The workshop also emphasized the need to “synergize” LVAD and heart transplant in the same patient to maximize health-related quality of life and survival benefit.
The NHLBI workshop also highlighted the need to model how different patient subset characteristics may affect mechanical circulatory support outcomes to inform bridge-to-transplantation or bridge-to-decision/candidacy opportunities more appropriately.
This research had no commercial funding. A number of study authors disclosed relationships with industry. The full list is available with the original article.
A version of this article first appeared on Medscape.com.
The prognosis for patients with advanced heart failure (HF) who fail guideline-directed medical therapy is poor, but
Those are the key takeaways from a scientific statement on durable mechanical circulatory support, published online in the Journal of the American College of Cardiology.
“I think it is important to highlight this issue because of the sheer impact that heart failure has on American citizens,” corresponding author Jennifer Cowger, MD, MS, advanced heart failure specialist, Henry Ford Health, Detroit, said in an interview.
“End-stage heart failure has no medication that has shown a gain in survival, and most are dead by 1 year,” she said.
This scientific statement highlights the “amazing evolution of LVAD support and associated improvement in outcomes,” Dr. Cowger said.
Yet because LVADs are only implanted at roughly 170 U.S. centers, “many cardiologists are not aware of the amazing survival improvement with modern LVAD technology, and patients are under-referred,” Dr. Cowger noted.
Contemporary outcomes on par with heart transplant
The authors note that survival with durable LVAD (dLVAD) has markedly improved over the years. Current survival is approximately 87% at 1 year for patients supported with a contemporary LVAD.
Average patient survival is now similar to that of heart transplantation at 2 years, with 5-year dLVAD survival now approaching 60%, they point out.
Contemporary dLVAD yields significant and sustained improvements in functional capacity. Data show that roughly 80% of patients improve to NYHA functional class I and II, with significant improvements in 6-minute walk distances and health-related quality of life, the authors note.
In addition, innovations in dLVAD technology have reduced the risk of several adverse events, including pump thrombosis, stroke, and bleeding.
“Novel devices are on the horizon of clinical investigation, offering smaller size, permitting less invasive surgical implantation, and eliminating the percutaneous lead for power supply,” the authors note.
“Unfortunately, greater adoption of dLVAD therapy has not been realized due to delayed referral of patients to advanced HF centers, insufficient clinician knowledge of contemporary dLVAD outcomes (including gains in quality of life), and deprioritization of patients with dLVAD support waiting for heart transplantation,” they write.
In addition to highlighting contemporary outcomes with dLVAD support, the 18-page statement also includes sections on:
- Current indications and timing of referral
- Surgical considerations (device selection, surgical techniques and approach to concomitant valvular disease, and management of acute right ventricular dysfunction)
- Unique patient populations (women, children, and adult congenital heart disease)
- Summary, gaps, and future directions
A recent workshop held by the National Heart, Lung, and Blood Institute (NHLBI) identified critical gaps in the field of advanced HF.
One of the major gaps identified was the need to improve mechanical circulatory support use as a “complement or alternative” therapy to heart transplantation. The workshop also emphasized the need to “synergize” LVAD and heart transplant in the same patient to maximize health-related quality of life and survival benefit.
The NHLBI workshop also highlighted the need to model how different patient subset characteristics may affect mechanical circulatory support outcomes to inform bridge-to-transplantation or bridge-to-decision/candidacy opportunities more appropriately.
This research had no commercial funding. A number of study authors disclosed relationships with industry. The full list is available with the original article.
A version of this article first appeared on Medscape.com.
The prognosis for patients with advanced heart failure (HF) who fail guideline-directed medical therapy is poor, but
Those are the key takeaways from a scientific statement on durable mechanical circulatory support, published online in the Journal of the American College of Cardiology.
“I think it is important to highlight this issue because of the sheer impact that heart failure has on American citizens,” corresponding author Jennifer Cowger, MD, MS, advanced heart failure specialist, Henry Ford Health, Detroit, said in an interview.
“End-stage heart failure has no medication that has shown a gain in survival, and most are dead by 1 year,” she said.
This scientific statement highlights the “amazing evolution of LVAD support and associated improvement in outcomes,” Dr. Cowger said.
Yet because LVADs are only implanted at roughly 170 U.S. centers, “many cardiologists are not aware of the amazing survival improvement with modern LVAD technology, and patients are under-referred,” Dr. Cowger noted.
Contemporary outcomes on par with heart transplant
The authors note that survival with durable LVAD (dLVAD) has markedly improved over the years. Current survival is approximately 87% at 1 year for patients supported with a contemporary LVAD.
Average patient survival is now similar to that of heart transplantation at 2 years, with 5-year dLVAD survival now approaching 60%, they point out.
Contemporary dLVAD yields significant and sustained improvements in functional capacity. Data show that roughly 80% of patients improve to NYHA functional class I and II, with significant improvements in 6-minute walk distances and health-related quality of life, the authors note.
In addition, innovations in dLVAD technology have reduced the risk of several adverse events, including pump thrombosis, stroke, and bleeding.
“Novel devices are on the horizon of clinical investigation, offering smaller size, permitting less invasive surgical implantation, and eliminating the percutaneous lead for power supply,” the authors note.
“Unfortunately, greater adoption of dLVAD therapy has not been realized due to delayed referral of patients to advanced HF centers, insufficient clinician knowledge of contemporary dLVAD outcomes (including gains in quality of life), and deprioritization of patients with dLVAD support waiting for heart transplantation,” they write.
In addition to highlighting contemporary outcomes with dLVAD support, the 18-page statement also includes sections on:
- Current indications and timing of referral
- Surgical considerations (device selection, surgical techniques and approach to concomitant valvular disease, and management of acute right ventricular dysfunction)
- Unique patient populations (women, children, and adult congenital heart disease)
- Summary, gaps, and future directions
A recent workshop held by the National Heart, Lung, and Blood Institute (NHLBI) identified critical gaps in the field of advanced HF.
One of the major gaps identified was the need to improve mechanical circulatory support use as a “complement or alternative” therapy to heart transplantation. The workshop also emphasized the need to “synergize” LVAD and heart transplant in the same patient to maximize health-related quality of life and survival benefit.
The NHLBI workshop also highlighted the need to model how different patient subset characteristics may affect mechanical circulatory support outcomes to inform bridge-to-transplantation or bridge-to-decision/candidacy opportunities more appropriately.
This research had no commercial funding. A number of study authors disclosed relationships with industry. The full list is available with the original article.
A version of this article first appeared on Medscape.com.
FROM JACC
Trials say start sacubitril-valsartan in hospital in HF with ‘below normal’ LVEF
CLEVELAND – suggests a combined analysis of two major studies.
Short-term risk for cardiovascular (CV) death or HF hospitalization fell 30% for such patients put on the angiotensin-receptor/neprilysin inhibitor (ARNI) at that early stage, compared with those assigned to receive an ACE inhibitor or angiotensin receptor blocker (ARB).
Of note, the risk-reduction benefit reached 41% among the overwhelming majority of patients with a left ventricular ejection fraction (LVEF) of 60% or lower across the two trials, PIONEER-HF and PARAGLIDE-HF. No such significant benefit was seen in patients with higher LVEF.
The prespecified analysis of 1,347 patients medically stabilized after a “worsening-HF event” was reported by Robert J. Mentz, MD, Duke Clinical Research Institute, Durham, N.C., at the annual scientific meeting of the Heart Failure Society of America.
Across both studies, levels of the prognostically telling biomarker NT-proBNP dropped further in the ARNI group, by almost a fourth, compared with those getting an ACE inhibitor or ARB. The difference emerged within a week and was “similar and consistent” throughout at least 8 weeks of follow-up, Dr. Mentz said.
Sacubitril-valsartan is approved in the United States for chronic HF, broadly but with labeling suggesting clearer efficacy at lower LVEF levels, based on the PARADIGM-HF and PARAGON-HF trials.
The PIONEER-HF and PARAGLIDE-HF trials lending patients to the current analysis demonstrated superiority for the drug vs. an ACE inhibitor or ARB when started in hospital in stabilized patients with HF.
Cautions about starting sacubitril-valsartan
In the pooled analysis, patients on sacubitril-valsartan were more likely to experience symptomatic hypotension, with a relative risk for drug’s known potential side effect reaching 1.35 (95% confidence interval, 1.05-1.72), compared with ACE inhibitor or ARB recipients.
But the hypotension risk when starting the drug is manageable to some extent, observed Dr. Mentz. “We can safely start sacubitril-valsartan in the hospital or early post discharge, but we need to make sure their volume status is okay” and keep track of their blood pressure trajectory, he told this news organization.
Those with initially low BP, unsurprisingly, seem more susceptible to the problem, Dr. Mentz said. In such patients “on antihypertensives or other therapies that aren’t going to give them a clinical outcome benefit,” those meds can be withdrawn or their dosages reduced before sacubitril-valsartan is added.
Such cautions are an “important take-home message” of the analysis, observed invited discussant Carolyn S. P. Lam, MBBS, PhD, National Heart Centre, Singapore, after the Dr. Mentz presentation.
Sacubitril-valsartan should be started only in stabilized patients, she emphasized. It should be delayed in those “with ongoing adjustments of antihypertensives, diuretics, and so on,” in whom premature initiation of the drug may promote symptomatic hypotension. Should that happen, Dr. Lam cautioned, there’s a risk that such patients would be “mislabeled as intolerant” of the ARNI and so wouldn’t be started on it later.
The pooled PIONEER-HF and PARAGLIDE-HF analysis, Dr. Lam proposed, might also help overcome the “clinical inertia and fear” that is slowing the uptake of early guideline-directed drug therapy initiation in patients hospitalized with HF.
LVEF spectrum across two studies
As Dr. Mentz reported, the analysis included 881 and 466 patients from PIONEER-HF and PARAGLIDE-HF, respectively. Of the total, 673 were assigned to receive valsartan and 674 to receive either enalapril or valsartan. Overall, 36% of the population were women.
Patients in PIONEER-HF, with an LVEF 40% or lower, were started on their assigned drug during an acute-HF hospitalization and followed a median of 8 weeks. PARAGLIDE-HF patients, with LVEF higher than 40%, started therapy either in hospital (in 70% of cases) or within 30 days of their HF event; they were followed a median of 6 months.
Hazard ratios for outcomes in the sacubitril-valsartan group vs. those on ACE inhibitors or ARBs were 0.76; 95% CI, 0.69-0.83; P < .0001 for change in NT-proBNP levels. For the composite of CV death or HF hospitalization, HRs were as follows:
- 0.70 (95% CI, 0.54-0.91; P = .0077) overall.
- 0.59 (95% CI, 0.44-0.79) for LVEF < 60%.
- 1.53 (95% CI, 0.80-2.91) for LVEF > 60%.
Current guidelines, Dr. Mentz noted, recommend that sacubitril-valsartan “be initiated de novo” predischarge in patients without contraindications who are hospitalized with acute HF with reduced LVEF. The combined analysis of PIONEER-HF and PARAGLIDE-HF, he said, potentially extends the recommendation “across the ejection fraction spectrum.”
Dr. Mentz has received research support and honoraria from Abbott, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Fast BioMedical, Gilead, Innolife, Eli Lilly, Medtronic, Medable, Merck, Novartis, Novo Nordisk, Pfizer, Pharmacosmos, Relypsa, Respicardia, Roche, Sanofi, Vifor, Windtree Therapeutics, and Zoll. Dr. Lam has reported financial relationships “with more than 25 pharmaceutical or device manufacturers, many of which produce therapies for heart failure,” as well as with Medscape/WebMD Global LLC.
A version of this article first appeared on Medscape.com.
CLEVELAND – suggests a combined analysis of two major studies.
Short-term risk for cardiovascular (CV) death or HF hospitalization fell 30% for such patients put on the angiotensin-receptor/neprilysin inhibitor (ARNI) at that early stage, compared with those assigned to receive an ACE inhibitor or angiotensin receptor blocker (ARB).
Of note, the risk-reduction benefit reached 41% among the overwhelming majority of patients with a left ventricular ejection fraction (LVEF) of 60% or lower across the two trials, PIONEER-HF and PARAGLIDE-HF. No such significant benefit was seen in patients with higher LVEF.
The prespecified analysis of 1,347 patients medically stabilized after a “worsening-HF event” was reported by Robert J. Mentz, MD, Duke Clinical Research Institute, Durham, N.C., at the annual scientific meeting of the Heart Failure Society of America.
Across both studies, levels of the prognostically telling biomarker NT-proBNP dropped further in the ARNI group, by almost a fourth, compared with those getting an ACE inhibitor or ARB. The difference emerged within a week and was “similar and consistent” throughout at least 8 weeks of follow-up, Dr. Mentz said.
Sacubitril-valsartan is approved in the United States for chronic HF, broadly but with labeling suggesting clearer efficacy at lower LVEF levels, based on the PARADIGM-HF and PARAGON-HF trials.
The PIONEER-HF and PARAGLIDE-HF trials lending patients to the current analysis demonstrated superiority for the drug vs. an ACE inhibitor or ARB when started in hospital in stabilized patients with HF.
Cautions about starting sacubitril-valsartan
In the pooled analysis, patients on sacubitril-valsartan were more likely to experience symptomatic hypotension, with a relative risk for drug’s known potential side effect reaching 1.35 (95% confidence interval, 1.05-1.72), compared with ACE inhibitor or ARB recipients.
But the hypotension risk when starting the drug is manageable to some extent, observed Dr. Mentz. “We can safely start sacubitril-valsartan in the hospital or early post discharge, but we need to make sure their volume status is okay” and keep track of their blood pressure trajectory, he told this news organization.
Those with initially low BP, unsurprisingly, seem more susceptible to the problem, Dr. Mentz said. In such patients “on antihypertensives or other therapies that aren’t going to give them a clinical outcome benefit,” those meds can be withdrawn or their dosages reduced before sacubitril-valsartan is added.
Such cautions are an “important take-home message” of the analysis, observed invited discussant Carolyn S. P. Lam, MBBS, PhD, National Heart Centre, Singapore, after the Dr. Mentz presentation.
Sacubitril-valsartan should be started only in stabilized patients, she emphasized. It should be delayed in those “with ongoing adjustments of antihypertensives, diuretics, and so on,” in whom premature initiation of the drug may promote symptomatic hypotension. Should that happen, Dr. Lam cautioned, there’s a risk that such patients would be “mislabeled as intolerant” of the ARNI and so wouldn’t be started on it later.
The pooled PIONEER-HF and PARAGLIDE-HF analysis, Dr. Lam proposed, might also help overcome the “clinical inertia and fear” that is slowing the uptake of early guideline-directed drug therapy initiation in patients hospitalized with HF.
LVEF spectrum across two studies
As Dr. Mentz reported, the analysis included 881 and 466 patients from PIONEER-HF and PARAGLIDE-HF, respectively. Of the total, 673 were assigned to receive valsartan and 674 to receive either enalapril or valsartan. Overall, 36% of the population were women.
Patients in PIONEER-HF, with an LVEF 40% or lower, were started on their assigned drug during an acute-HF hospitalization and followed a median of 8 weeks. PARAGLIDE-HF patients, with LVEF higher than 40%, started therapy either in hospital (in 70% of cases) or within 30 days of their HF event; they were followed a median of 6 months.
Hazard ratios for outcomes in the sacubitril-valsartan group vs. those on ACE inhibitors or ARBs were 0.76; 95% CI, 0.69-0.83; P < .0001 for change in NT-proBNP levels. For the composite of CV death or HF hospitalization, HRs were as follows:
- 0.70 (95% CI, 0.54-0.91; P = .0077) overall.
- 0.59 (95% CI, 0.44-0.79) for LVEF < 60%.
- 1.53 (95% CI, 0.80-2.91) for LVEF > 60%.
Current guidelines, Dr. Mentz noted, recommend that sacubitril-valsartan “be initiated de novo” predischarge in patients without contraindications who are hospitalized with acute HF with reduced LVEF. The combined analysis of PIONEER-HF and PARAGLIDE-HF, he said, potentially extends the recommendation “across the ejection fraction spectrum.”
Dr. Mentz has received research support and honoraria from Abbott, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Fast BioMedical, Gilead, Innolife, Eli Lilly, Medtronic, Medable, Merck, Novartis, Novo Nordisk, Pfizer, Pharmacosmos, Relypsa, Respicardia, Roche, Sanofi, Vifor, Windtree Therapeutics, and Zoll. Dr. Lam has reported financial relationships “with more than 25 pharmaceutical or device manufacturers, many of which produce therapies for heart failure,” as well as with Medscape/WebMD Global LLC.
A version of this article first appeared on Medscape.com.
CLEVELAND – suggests a combined analysis of two major studies.
Short-term risk for cardiovascular (CV) death or HF hospitalization fell 30% for such patients put on the angiotensin-receptor/neprilysin inhibitor (ARNI) at that early stage, compared with those assigned to receive an ACE inhibitor or angiotensin receptor blocker (ARB).
Of note, the risk-reduction benefit reached 41% among the overwhelming majority of patients with a left ventricular ejection fraction (LVEF) of 60% or lower across the two trials, PIONEER-HF and PARAGLIDE-HF. No such significant benefit was seen in patients with higher LVEF.
The prespecified analysis of 1,347 patients medically stabilized after a “worsening-HF event” was reported by Robert J. Mentz, MD, Duke Clinical Research Institute, Durham, N.C., at the annual scientific meeting of the Heart Failure Society of America.
Across both studies, levels of the prognostically telling biomarker NT-proBNP dropped further in the ARNI group, by almost a fourth, compared with those getting an ACE inhibitor or ARB. The difference emerged within a week and was “similar and consistent” throughout at least 8 weeks of follow-up, Dr. Mentz said.
Sacubitril-valsartan is approved in the United States for chronic HF, broadly but with labeling suggesting clearer efficacy at lower LVEF levels, based on the PARADIGM-HF and PARAGON-HF trials.
The PIONEER-HF and PARAGLIDE-HF trials lending patients to the current analysis demonstrated superiority for the drug vs. an ACE inhibitor or ARB when started in hospital in stabilized patients with HF.
Cautions about starting sacubitril-valsartan
In the pooled analysis, patients on sacubitril-valsartan were more likely to experience symptomatic hypotension, with a relative risk for drug’s known potential side effect reaching 1.35 (95% confidence interval, 1.05-1.72), compared with ACE inhibitor or ARB recipients.
But the hypotension risk when starting the drug is manageable to some extent, observed Dr. Mentz. “We can safely start sacubitril-valsartan in the hospital or early post discharge, but we need to make sure their volume status is okay” and keep track of their blood pressure trajectory, he told this news organization.
Those with initially low BP, unsurprisingly, seem more susceptible to the problem, Dr. Mentz said. In such patients “on antihypertensives or other therapies that aren’t going to give them a clinical outcome benefit,” those meds can be withdrawn or their dosages reduced before sacubitril-valsartan is added.
Such cautions are an “important take-home message” of the analysis, observed invited discussant Carolyn S. P. Lam, MBBS, PhD, National Heart Centre, Singapore, after the Dr. Mentz presentation.
Sacubitril-valsartan should be started only in stabilized patients, she emphasized. It should be delayed in those “with ongoing adjustments of antihypertensives, diuretics, and so on,” in whom premature initiation of the drug may promote symptomatic hypotension. Should that happen, Dr. Lam cautioned, there’s a risk that such patients would be “mislabeled as intolerant” of the ARNI and so wouldn’t be started on it later.
The pooled PIONEER-HF and PARAGLIDE-HF analysis, Dr. Lam proposed, might also help overcome the “clinical inertia and fear” that is slowing the uptake of early guideline-directed drug therapy initiation in patients hospitalized with HF.
LVEF spectrum across two studies
As Dr. Mentz reported, the analysis included 881 and 466 patients from PIONEER-HF and PARAGLIDE-HF, respectively. Of the total, 673 were assigned to receive valsartan and 674 to receive either enalapril or valsartan. Overall, 36% of the population were women.
Patients in PIONEER-HF, with an LVEF 40% or lower, were started on their assigned drug during an acute-HF hospitalization and followed a median of 8 weeks. PARAGLIDE-HF patients, with LVEF higher than 40%, started therapy either in hospital (in 70% of cases) or within 30 days of their HF event; they were followed a median of 6 months.
Hazard ratios for outcomes in the sacubitril-valsartan group vs. those on ACE inhibitors or ARBs were 0.76; 95% CI, 0.69-0.83; P < .0001 for change in NT-proBNP levels. For the composite of CV death or HF hospitalization, HRs were as follows:
- 0.70 (95% CI, 0.54-0.91; P = .0077) overall.
- 0.59 (95% CI, 0.44-0.79) for LVEF < 60%.
- 1.53 (95% CI, 0.80-2.91) for LVEF > 60%.
Current guidelines, Dr. Mentz noted, recommend that sacubitril-valsartan “be initiated de novo” predischarge in patients without contraindications who are hospitalized with acute HF with reduced LVEF. The combined analysis of PIONEER-HF and PARAGLIDE-HF, he said, potentially extends the recommendation “across the ejection fraction spectrum.”
Dr. Mentz has received research support and honoraria from Abbott, American Regent, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Fast BioMedical, Gilead, Innolife, Eli Lilly, Medtronic, Medable, Merck, Novartis, Novo Nordisk, Pfizer, Pharmacosmos, Relypsa, Respicardia, Roche, Sanofi, Vifor, Windtree Therapeutics, and Zoll. Dr. Lam has reported financial relationships “with more than 25 pharmaceutical or device manufacturers, many of which produce therapies for heart failure,” as well as with Medscape/WebMD Global LLC.
A version of this article first appeared on Medscape.com.
AT HFSA 2023
Hopeful insights, no overall HFpEF gains from splanchnic nerve ablation: REBALANCE-HF
It’s still early days for a potential transcatheter technique that tones down sympathetic activation mediating blood volume shifts to the heart and lungs. Such volume transfers can contribute to congestion and acute decompensation in some patients with heart failure. But a randomized trial with negative overall results still may have moved the novel procedure a modest step forward.
The procedure, right-sided splanchnic-nerve ablation for volume management (SAVM), failed to show significant effects on hemodynamics, exercise capacity, natriuretic peptides, or quality of life in a trial covering a broad population of patients with heart failure with preserved ejection fraction (HFpEF).
The study, called REBALANCE-HF, compared ablation of the right greater splanchnic nerve with a sham version of the procedure for any effects on hemodynamic or functional outcomes.
Among such “potential responders,” those undergoing SAVM trended better than patients receiving the sham procedure with respect to hemodynamic, functional, natriuretic peptide, and quality of life endpoints.
The potential predictors of SAVM success included elevated or preserved cardiac output and pulse pressure with exercise or on standing up; appropriate heart-rate exercise responses; and little or no echocardiographic evidence of diastolic dysfunction.
The panel of features might potentially identify patients more likely to respond to the procedure and perhaps sharpen entry criteria in future clinical trials, Marat Fudim, MD, MHS, Duke University Medical Center, Durham, N.C., said in an interview.
Dr. Fudim presented the REBALANCE-HF findings at the annual meeting of the Heart Failure Society of America.
How SAVM works
Sympathetic activation can lead to acute or chronic constriction of vessels in the splanchnic bed within the upper and lower abdomen, one of the body’s largest blood reservoirs, Dr. Fudim explained. Resulting volume shifts to the general circulation, and therefore the heart and lungs, are a normal exercise response that, in HF, can fall out of balance and excessively raise cardiac filling pressure.
Lessened sympathetic tone after unilateral GNS ablation can promote splanchnic venous dilation that reduces intrathoracic blood volume, potentially averting congestion, and decompensation, observed Kavita Sharma, MD, invited discussant for Dr. Fudim’s presentation.
The trial’s potential-responder cohort “seemed able to augment cardiac output in response to stress” and to “maintain or augment their orthostatic pulse pressure,” more effectively than the other participants, said Dr. Sharma, of Johns Hopkins University, Baltimore.
Although the trial was overall negative for 1-month change in pulmonary capillary wedge pressure (PCWP), the primary efficacy endpoint, Dr. Sharma said, it confirmed SAVM as a safe procedure in HFpEF and “ensured its replicability and technical success.”
Future studies should explore ways to characterize unlikely SAVM responders, she proposed. “I would argue these patients are probably more important than even the responders.”
Yet it’s unknown why, for example, cardiac output wouldn’t increase with exercise in a patient with HFpEF. “Is it related to preload insufficiency, right ventricular failure, atrial myopathy, perhaps more restrictive physiology, chronotropic incompetence, or medications – or a combination of the above?”
REBALANCE-HF assigned 90 patients with HFpEF to either the active or sham SAVM groups, 44 and 46 patients, respectively. To be eligible, patients were stable on HF meds and had either elevated natriuretic peptides or, within the past year, at least one HF hospitalization or escalation of intravenous diuretics for worsening HF.
The active and sham control groups fared similarly for the primary PCWP endpoint and for the secondary endpoints of Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score, 6-minute walk distance (6MWD), and natriuretic peptide levels at 6 and 12 months.
Predicting SAVM response
In analysis limited to potential responders, PCWP, KCCQ, 6MWD, and natriuretic peptide outcomes for patients were combined into z scores, a single metric that reflects multiple outcomes, Dr. Fudim explained.
The z scores were derived for tertiles of patients in subgroups defined by a range of parameters that included demographics, medical history, and hemodynamic and echocardiographic variables.
Four such variables were found to interact across tertiles in a way that suggested their value as SAVM outcome predictors and were then used to select the cohort of potential responders. The variables were exertion-related changes in cardiac index, pulse pressure, and heart rate, and mitral E/A ratio – the latter a measure of diastolic dysfunction.
Among potential responders, those who underwent SAVM showed a 2.9–mm Hg steeper drop in peak PCWP at 1 month (P = .02), compared with patients getting the sham procedure.
They also bested control patients at both 6 and 12 months for KCCQ score, 6MWD, and natriuretic peptide levels, the latter of which fell in the SAVM group and climbed in control patients at both follow-ups.
“Hypothetically, it makes sense” to target the splanchnic nerve in HFpEF, and indeed in HF with reduced ejection fraction, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, said in an interview.
And should SAVM enter the mainstream, it would definitely be important to identify “the right” patients for such an invasive procedure, those likely to show “efficacy with a good safety margin,” said Dr. Bozkurt, who was not associated with REBALANCE-HF.
But the trial, she said, “unfortunately did not give real signals of outcome benefit.”
REBALANCE-HF was supported by Axon Therapies. Dr. Fudim disclosed consulting, receiving royalties, or having ownership or equity in Axon Therapies. Dr. Sharma disclosed receiving honoraria for speaking from Novartis and Janssen and serving on an advisory board or consulting for Novartis, Janssen, and Bayer. Dr. Bozkurt disclosed receiving honoraria from AstraZeneca, Baxter Health Care, and Sanofi Aventis and having other relationships with Renovacor, Respicardia, Abbott Vascular, Liva Nova, Vifor, and Cardurion.
A version of this article first appeared on Medscape.com.
It’s still early days for a potential transcatheter technique that tones down sympathetic activation mediating blood volume shifts to the heart and lungs. Such volume transfers can contribute to congestion and acute decompensation in some patients with heart failure. But a randomized trial with negative overall results still may have moved the novel procedure a modest step forward.
The procedure, right-sided splanchnic-nerve ablation for volume management (SAVM), failed to show significant effects on hemodynamics, exercise capacity, natriuretic peptides, or quality of life in a trial covering a broad population of patients with heart failure with preserved ejection fraction (HFpEF).
The study, called REBALANCE-HF, compared ablation of the right greater splanchnic nerve with a sham version of the procedure for any effects on hemodynamic or functional outcomes.
Among such “potential responders,” those undergoing SAVM trended better than patients receiving the sham procedure with respect to hemodynamic, functional, natriuretic peptide, and quality of life endpoints.
The potential predictors of SAVM success included elevated or preserved cardiac output and pulse pressure with exercise or on standing up; appropriate heart-rate exercise responses; and little or no echocardiographic evidence of diastolic dysfunction.
The panel of features might potentially identify patients more likely to respond to the procedure and perhaps sharpen entry criteria in future clinical trials, Marat Fudim, MD, MHS, Duke University Medical Center, Durham, N.C., said in an interview.
Dr. Fudim presented the REBALANCE-HF findings at the annual meeting of the Heart Failure Society of America.
How SAVM works
Sympathetic activation can lead to acute or chronic constriction of vessels in the splanchnic bed within the upper and lower abdomen, one of the body’s largest blood reservoirs, Dr. Fudim explained. Resulting volume shifts to the general circulation, and therefore the heart and lungs, are a normal exercise response that, in HF, can fall out of balance and excessively raise cardiac filling pressure.
Lessened sympathetic tone after unilateral GNS ablation can promote splanchnic venous dilation that reduces intrathoracic blood volume, potentially averting congestion, and decompensation, observed Kavita Sharma, MD, invited discussant for Dr. Fudim’s presentation.
The trial’s potential-responder cohort “seemed able to augment cardiac output in response to stress” and to “maintain or augment their orthostatic pulse pressure,” more effectively than the other participants, said Dr. Sharma, of Johns Hopkins University, Baltimore.
Although the trial was overall negative for 1-month change in pulmonary capillary wedge pressure (PCWP), the primary efficacy endpoint, Dr. Sharma said, it confirmed SAVM as a safe procedure in HFpEF and “ensured its replicability and technical success.”
Future studies should explore ways to characterize unlikely SAVM responders, she proposed. “I would argue these patients are probably more important than even the responders.”
Yet it’s unknown why, for example, cardiac output wouldn’t increase with exercise in a patient with HFpEF. “Is it related to preload insufficiency, right ventricular failure, atrial myopathy, perhaps more restrictive physiology, chronotropic incompetence, or medications – or a combination of the above?”
REBALANCE-HF assigned 90 patients with HFpEF to either the active or sham SAVM groups, 44 and 46 patients, respectively. To be eligible, patients were stable on HF meds and had either elevated natriuretic peptides or, within the past year, at least one HF hospitalization or escalation of intravenous diuretics for worsening HF.
The active and sham control groups fared similarly for the primary PCWP endpoint and for the secondary endpoints of Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score, 6-minute walk distance (6MWD), and natriuretic peptide levels at 6 and 12 months.
Predicting SAVM response
In analysis limited to potential responders, PCWP, KCCQ, 6MWD, and natriuretic peptide outcomes for patients were combined into z scores, a single metric that reflects multiple outcomes, Dr. Fudim explained.
The z scores were derived for tertiles of patients in subgroups defined by a range of parameters that included demographics, medical history, and hemodynamic and echocardiographic variables.
Four such variables were found to interact across tertiles in a way that suggested their value as SAVM outcome predictors and were then used to select the cohort of potential responders. The variables were exertion-related changes in cardiac index, pulse pressure, and heart rate, and mitral E/A ratio – the latter a measure of diastolic dysfunction.
Among potential responders, those who underwent SAVM showed a 2.9–mm Hg steeper drop in peak PCWP at 1 month (P = .02), compared with patients getting the sham procedure.
They also bested control patients at both 6 and 12 months for KCCQ score, 6MWD, and natriuretic peptide levels, the latter of which fell in the SAVM group and climbed in control patients at both follow-ups.
“Hypothetically, it makes sense” to target the splanchnic nerve in HFpEF, and indeed in HF with reduced ejection fraction, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, said in an interview.
And should SAVM enter the mainstream, it would definitely be important to identify “the right” patients for such an invasive procedure, those likely to show “efficacy with a good safety margin,” said Dr. Bozkurt, who was not associated with REBALANCE-HF.
But the trial, she said, “unfortunately did not give real signals of outcome benefit.”
REBALANCE-HF was supported by Axon Therapies. Dr. Fudim disclosed consulting, receiving royalties, or having ownership or equity in Axon Therapies. Dr. Sharma disclosed receiving honoraria for speaking from Novartis and Janssen and serving on an advisory board or consulting for Novartis, Janssen, and Bayer. Dr. Bozkurt disclosed receiving honoraria from AstraZeneca, Baxter Health Care, and Sanofi Aventis and having other relationships with Renovacor, Respicardia, Abbott Vascular, Liva Nova, Vifor, and Cardurion.
A version of this article first appeared on Medscape.com.
It’s still early days for a potential transcatheter technique that tones down sympathetic activation mediating blood volume shifts to the heart and lungs. Such volume transfers can contribute to congestion and acute decompensation in some patients with heart failure. But a randomized trial with negative overall results still may have moved the novel procedure a modest step forward.
The procedure, right-sided splanchnic-nerve ablation for volume management (SAVM), failed to show significant effects on hemodynamics, exercise capacity, natriuretic peptides, or quality of life in a trial covering a broad population of patients with heart failure with preserved ejection fraction (HFpEF).
The study, called REBALANCE-HF, compared ablation of the right greater splanchnic nerve with a sham version of the procedure for any effects on hemodynamic or functional outcomes.
Among such “potential responders,” those undergoing SAVM trended better than patients receiving the sham procedure with respect to hemodynamic, functional, natriuretic peptide, and quality of life endpoints.
The potential predictors of SAVM success included elevated or preserved cardiac output and pulse pressure with exercise or on standing up; appropriate heart-rate exercise responses; and little or no echocardiographic evidence of diastolic dysfunction.
The panel of features might potentially identify patients more likely to respond to the procedure and perhaps sharpen entry criteria in future clinical trials, Marat Fudim, MD, MHS, Duke University Medical Center, Durham, N.C., said in an interview.
Dr. Fudim presented the REBALANCE-HF findings at the annual meeting of the Heart Failure Society of America.
How SAVM works
Sympathetic activation can lead to acute or chronic constriction of vessels in the splanchnic bed within the upper and lower abdomen, one of the body’s largest blood reservoirs, Dr. Fudim explained. Resulting volume shifts to the general circulation, and therefore the heart and lungs, are a normal exercise response that, in HF, can fall out of balance and excessively raise cardiac filling pressure.
Lessened sympathetic tone after unilateral GNS ablation can promote splanchnic venous dilation that reduces intrathoracic blood volume, potentially averting congestion, and decompensation, observed Kavita Sharma, MD, invited discussant for Dr. Fudim’s presentation.
The trial’s potential-responder cohort “seemed able to augment cardiac output in response to stress” and to “maintain or augment their orthostatic pulse pressure,” more effectively than the other participants, said Dr. Sharma, of Johns Hopkins University, Baltimore.
Although the trial was overall negative for 1-month change in pulmonary capillary wedge pressure (PCWP), the primary efficacy endpoint, Dr. Sharma said, it confirmed SAVM as a safe procedure in HFpEF and “ensured its replicability and technical success.”
Future studies should explore ways to characterize unlikely SAVM responders, she proposed. “I would argue these patients are probably more important than even the responders.”
Yet it’s unknown why, for example, cardiac output wouldn’t increase with exercise in a patient with HFpEF. “Is it related to preload insufficiency, right ventricular failure, atrial myopathy, perhaps more restrictive physiology, chronotropic incompetence, or medications – or a combination of the above?”
REBALANCE-HF assigned 90 patients with HFpEF to either the active or sham SAVM groups, 44 and 46 patients, respectively. To be eligible, patients were stable on HF meds and had either elevated natriuretic peptides or, within the past year, at least one HF hospitalization or escalation of intravenous diuretics for worsening HF.
The active and sham control groups fared similarly for the primary PCWP endpoint and for the secondary endpoints of Kansas City Cardiomyopathy Questionnaire (KCCQ) overall summary score, 6-minute walk distance (6MWD), and natriuretic peptide levels at 6 and 12 months.
Predicting SAVM response
In analysis limited to potential responders, PCWP, KCCQ, 6MWD, and natriuretic peptide outcomes for patients were combined into z scores, a single metric that reflects multiple outcomes, Dr. Fudim explained.
The z scores were derived for tertiles of patients in subgroups defined by a range of parameters that included demographics, medical history, and hemodynamic and echocardiographic variables.
Four such variables were found to interact across tertiles in a way that suggested their value as SAVM outcome predictors and were then used to select the cohort of potential responders. The variables were exertion-related changes in cardiac index, pulse pressure, and heart rate, and mitral E/A ratio – the latter a measure of diastolic dysfunction.
Among potential responders, those who underwent SAVM showed a 2.9–mm Hg steeper drop in peak PCWP at 1 month (P = .02), compared with patients getting the sham procedure.
They also bested control patients at both 6 and 12 months for KCCQ score, 6MWD, and natriuretic peptide levels, the latter of which fell in the SAVM group and climbed in control patients at both follow-ups.
“Hypothetically, it makes sense” to target the splanchnic nerve in HFpEF, and indeed in HF with reduced ejection fraction, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, said in an interview.
And should SAVM enter the mainstream, it would definitely be important to identify “the right” patients for such an invasive procedure, those likely to show “efficacy with a good safety margin,” said Dr. Bozkurt, who was not associated with REBALANCE-HF.
But the trial, she said, “unfortunately did not give real signals of outcome benefit.”
REBALANCE-HF was supported by Axon Therapies. Dr. Fudim disclosed consulting, receiving royalties, or having ownership or equity in Axon Therapies. Dr. Sharma disclosed receiving honoraria for speaking from Novartis and Janssen and serving on an advisory board or consulting for Novartis, Janssen, and Bayer. Dr. Bozkurt disclosed receiving honoraria from AstraZeneca, Baxter Health Care, and Sanofi Aventis and having other relationships with Renovacor, Respicardia, Abbott Vascular, Liva Nova, Vifor, and Cardurion.
A version of this article first appeared on Medscape.com.
FROM HFSA 2023
Spreading out daily meals and snacks may boost heart failure survival
CLEVELAND – , an observational study suggests.
The new findings, based primarily on 15 years of data from the National Health and Nutrition Examination Survey (NHANES), may argue against time-restricted diet interventions like intermittent fasting for patients with HF, researchers say.
The study’s nearly 1,000 participants on medical therapy for HF reported a mean daily eating window of 11 hours and daily average of four “eating occasions,” defined as meals or snacks of at least 50 kcal.
A daily eating window of 11 or more hours, compared with less than 11 hours, corresponded to a greater than 40% drop in risk for CV mortality (P = .013) over 5-6 years, reported Hayley E. Billingsley, RD, CEP, Virginia Commonwealth University, Richmond, Va,, at the annual scientific meeting of the Heart Failure Society of America.
The analysis adjusted for caloric intake, daily number of eating occasions, body mass index (BMI), history of CV disease and cancer, diabetes, and a slew of other potential confounders.
Prior evidence, mostly from healthy people, has suggested that extended fasting during the day is associated with less physical activity, Ms. Billingsley said in an interview. So it may be that people with HF who spread out their calorie intake are more active throughout the day.
A longer time window for eating, therefore, may have indirect metabolic benefits and help preserve their lean body mass, possibly reducing CV risk in a patient group at risk for muscle wasting.
The findings add to earlier evidence from Ms. Billingsley’s center that suggests that expanded daily time windows for eating, especially later final food rather than earlier first food, may help boost CV fitness for patients with obesity and HF with preserved ejection fraction.
Intermittent fasting and other practices involving the timing of food intake have been studied for weight loss and metabolic health in mostly healthy people and patients with diabetes, she noted. “But it’s really underexplored in people with established cardiovascular disease.”
On the basis of admittedly “very preliminary” findings, it may be that some patients should not shorten their daily time windows for eating or engage in intermittent fasting, Ms. Billingsley said. It’s probably worth considering, before the approach is recommended, “what their risk is for malnutrition or sarcopenia.”
The current study included 991 persons who entered the NHANES database from 2003 to 2018. The patients self-identified as having HF, reported taking medications commonly prescribed in HF, and provided at least two “reliable” dietary recalls.
The average age of the patients was 68 years, and they had had HF for a mean of 9.5 years; 47% were women, three-fourths were White persons, two thirds had dyslipidemia, and a quarter had a history of cancer.
On average, their first eating occasion of the day was at about 8:30 a.m., and the last occasion was at about 7:30 p.m., for a time window of about 11 hours; daily calorie consumption averaged about 1,830 kcal.
About 52% died over the mean follow-up of 69 months; about 44% of deaths were from CV causes.
In a model adjusted for demographics, BMI, smoking status, times of eating occasions, CV disease, diabetes, and cancer history, the all-cause mortality hazard ratio for time windows ≥ 11 hours vs. < 11 hours was 0.236 (95% confidence interval, 0.07-0.715; P = .011).
The reduction was no longer significant on further adjustment for duration of HF, a score reflecting difficulty walking, nightly hours of sleep (which averaged 7.2 hours), daily number of eating occasions, and caloric intake, Ms. Billingsley reported.
But in the fully adjusted analysis, the HR for CV mortality for the longer vs. shorter time window was 0.368 (95% CI, 0.169-0.803; P = .013).
The issue deserves further exploration in a randomized trial, Ms. Billingsley proposed, perhaps one in which patients with HF wear accelerometers to track daily activity levels. “We’d love to do a pilot study of extending their eating window that really digs into what the mechanism of any benefit might be if we assign them to a longer time window and whether it’s related to physical activity.”
Ms. Billingsley reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
CLEVELAND – , an observational study suggests.
The new findings, based primarily on 15 years of data from the National Health and Nutrition Examination Survey (NHANES), may argue against time-restricted diet interventions like intermittent fasting for patients with HF, researchers say.
The study’s nearly 1,000 participants on medical therapy for HF reported a mean daily eating window of 11 hours and daily average of four “eating occasions,” defined as meals or snacks of at least 50 kcal.
A daily eating window of 11 or more hours, compared with less than 11 hours, corresponded to a greater than 40% drop in risk for CV mortality (P = .013) over 5-6 years, reported Hayley E. Billingsley, RD, CEP, Virginia Commonwealth University, Richmond, Va,, at the annual scientific meeting of the Heart Failure Society of America.
The analysis adjusted for caloric intake, daily number of eating occasions, body mass index (BMI), history of CV disease and cancer, diabetes, and a slew of other potential confounders.
Prior evidence, mostly from healthy people, has suggested that extended fasting during the day is associated with less physical activity, Ms. Billingsley said in an interview. So it may be that people with HF who spread out their calorie intake are more active throughout the day.
A longer time window for eating, therefore, may have indirect metabolic benefits and help preserve their lean body mass, possibly reducing CV risk in a patient group at risk for muscle wasting.
The findings add to earlier evidence from Ms. Billingsley’s center that suggests that expanded daily time windows for eating, especially later final food rather than earlier first food, may help boost CV fitness for patients with obesity and HF with preserved ejection fraction.
Intermittent fasting and other practices involving the timing of food intake have been studied for weight loss and metabolic health in mostly healthy people and patients with diabetes, she noted. “But it’s really underexplored in people with established cardiovascular disease.”
On the basis of admittedly “very preliminary” findings, it may be that some patients should not shorten their daily time windows for eating or engage in intermittent fasting, Ms. Billingsley said. It’s probably worth considering, before the approach is recommended, “what their risk is for malnutrition or sarcopenia.”
The current study included 991 persons who entered the NHANES database from 2003 to 2018. The patients self-identified as having HF, reported taking medications commonly prescribed in HF, and provided at least two “reliable” dietary recalls.
The average age of the patients was 68 years, and they had had HF for a mean of 9.5 years; 47% were women, three-fourths were White persons, two thirds had dyslipidemia, and a quarter had a history of cancer.
On average, their first eating occasion of the day was at about 8:30 a.m., and the last occasion was at about 7:30 p.m., for a time window of about 11 hours; daily calorie consumption averaged about 1,830 kcal.
About 52% died over the mean follow-up of 69 months; about 44% of deaths were from CV causes.
In a model adjusted for demographics, BMI, smoking status, times of eating occasions, CV disease, diabetes, and cancer history, the all-cause mortality hazard ratio for time windows ≥ 11 hours vs. < 11 hours was 0.236 (95% confidence interval, 0.07-0.715; P = .011).
The reduction was no longer significant on further adjustment for duration of HF, a score reflecting difficulty walking, nightly hours of sleep (which averaged 7.2 hours), daily number of eating occasions, and caloric intake, Ms. Billingsley reported.
But in the fully adjusted analysis, the HR for CV mortality for the longer vs. shorter time window was 0.368 (95% CI, 0.169-0.803; P = .013).
The issue deserves further exploration in a randomized trial, Ms. Billingsley proposed, perhaps one in which patients with HF wear accelerometers to track daily activity levels. “We’d love to do a pilot study of extending their eating window that really digs into what the mechanism of any benefit might be if we assign them to a longer time window and whether it’s related to physical activity.”
Ms. Billingsley reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
CLEVELAND – , an observational study suggests.
The new findings, based primarily on 15 years of data from the National Health and Nutrition Examination Survey (NHANES), may argue against time-restricted diet interventions like intermittent fasting for patients with HF, researchers say.
The study’s nearly 1,000 participants on medical therapy for HF reported a mean daily eating window of 11 hours and daily average of four “eating occasions,” defined as meals or snacks of at least 50 kcal.
A daily eating window of 11 or more hours, compared with less than 11 hours, corresponded to a greater than 40% drop in risk for CV mortality (P = .013) over 5-6 years, reported Hayley E. Billingsley, RD, CEP, Virginia Commonwealth University, Richmond, Va,, at the annual scientific meeting of the Heart Failure Society of America.
The analysis adjusted for caloric intake, daily number of eating occasions, body mass index (BMI), history of CV disease and cancer, diabetes, and a slew of other potential confounders.
Prior evidence, mostly from healthy people, has suggested that extended fasting during the day is associated with less physical activity, Ms. Billingsley said in an interview. So it may be that people with HF who spread out their calorie intake are more active throughout the day.
A longer time window for eating, therefore, may have indirect metabolic benefits and help preserve their lean body mass, possibly reducing CV risk in a patient group at risk for muscle wasting.
The findings add to earlier evidence from Ms. Billingsley’s center that suggests that expanded daily time windows for eating, especially later final food rather than earlier first food, may help boost CV fitness for patients with obesity and HF with preserved ejection fraction.
Intermittent fasting and other practices involving the timing of food intake have been studied for weight loss and metabolic health in mostly healthy people and patients with diabetes, she noted. “But it’s really underexplored in people with established cardiovascular disease.”
On the basis of admittedly “very preliminary” findings, it may be that some patients should not shorten their daily time windows for eating or engage in intermittent fasting, Ms. Billingsley said. It’s probably worth considering, before the approach is recommended, “what their risk is for malnutrition or sarcopenia.”
The current study included 991 persons who entered the NHANES database from 2003 to 2018. The patients self-identified as having HF, reported taking medications commonly prescribed in HF, and provided at least two “reliable” dietary recalls.
The average age of the patients was 68 years, and they had had HF for a mean of 9.5 years; 47% were women, three-fourths were White persons, two thirds had dyslipidemia, and a quarter had a history of cancer.
On average, their first eating occasion of the day was at about 8:30 a.m., and the last occasion was at about 7:30 p.m., for a time window of about 11 hours; daily calorie consumption averaged about 1,830 kcal.
About 52% died over the mean follow-up of 69 months; about 44% of deaths were from CV causes.
In a model adjusted for demographics, BMI, smoking status, times of eating occasions, CV disease, diabetes, and cancer history, the all-cause mortality hazard ratio for time windows ≥ 11 hours vs. < 11 hours was 0.236 (95% confidence interval, 0.07-0.715; P = .011).
The reduction was no longer significant on further adjustment for duration of HF, a score reflecting difficulty walking, nightly hours of sleep (which averaged 7.2 hours), daily number of eating occasions, and caloric intake, Ms. Billingsley reported.
But in the fully adjusted analysis, the HR for CV mortality for the longer vs. shorter time window was 0.368 (95% CI, 0.169-0.803; P = .013).
The issue deserves further exploration in a randomized trial, Ms. Billingsley proposed, perhaps one in which patients with HF wear accelerometers to track daily activity levels. “We’d love to do a pilot study of extending their eating window that really digs into what the mechanism of any benefit might be if we assign them to a longer time window and whether it’s related to physical activity.”
Ms. Billingsley reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
AT HFSA 2023
Semaglutide win in HFpEF with obesity regardless of ejection fraction: STEP-HFpEF
CLEVELAND – independently of baseline left-ventricular ejection fraction (LVEF).
The finding comes from a prespecified secondary analysis of the STEP-HFpEF trial of more than 500 nondiabetic patients with obesity and HF with an initial LVEF of 45% or greater.
They suggest that for patients with the obesity phenotype of HFpEF, semaglutide (Wegovy) could potentially join SGLT2 inhibitors on the short list of meds with consistent treatment effects whether LVEF is mildly reduced, preserved, or in the normal range.
That would distinguish the drug, a glucagon-like peptide-1 (GLP-1) receptor agonist, from mineralocorticoid receptor antagonists (MRA), sacubitril-valsartan (Entresto), and other renin-angiotensin-system inhibitors (RASi), whose benefits tend to taper off with rising LVEF.
The patients assigned to semaglutide showed significant improvement in both primary endpoints – change in Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) and change in body weight at 52 weeks – whether their baseline LVEF was 45%-49%, 50%-59%, or 60% or greater.
Results were similar for improvements in 6-minute walk distance (6MWD) and levels of NT-terminal pro–brain natriuretic peptide (NT-proBNP) and C-reactive protein, observed Javed Butler, MD, when presenting the analysis at the annual meeting of the Heart Failure Society of America, Cleveland.
Dr. Butler, of Baylor Scott and White Research Institute, Dallas, and the University of Mississippi, Jackson, is also lead author of the study, which was published on the same day in the Journal of the American College of Cardiology.
In his presentation, Dr. Butler singled out the NT-proBNP finding as “very meaningful” with respect to understanding potential mechanisms of the drug effects observed in the trial.
For example, people with obesity tend to have lower than average natriuretic peptide levels that “actually go up a bit” when they lose weight, he observed. But in the trial, “we saw a reduction in NT-proBNP in spite of the weight loss,” regardless of LVEF category.
John McMurray, MD, University of Glasgow, the invited discussant for Dr. Butler’s presentation, agreed that it raises the question whether weight loss was the sole semaglutide effect responsible for the improvement in heart failure status and biomarkers. The accompanying NT-proBNP reductions – when the opposite might otherwise have been expected – may point to a possible mechanism of action that is “something more than just weight loss,” he said. “If that were the case, it becomes very important, because it means that this treatment might do good things in non-obese patients or might do good things in patients with other types of heart failure.”
‘Vital reassurance’
More definitive trials are needed “to clarify safety and efficacy of obesity-targeted therapeutics in HF across the ejection fraction spectrum,” according to an accompanying editorial).
Still, the STEP-HFpEF analysis “strengthens the role of GLP-1 [receptor agonists] to ameliorate health status” for patients with obesity and HF with mildly reduced or preserved ejection fraction, write Muthiah Vaduganathan, MD, MPH, and John W. Ostrominski, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
Its findings “provide vital reassurance” on semaglutide safety and efficacy in HF with below-normal LVEF and “tentatively support the existence of a more general, LVEF-independent, obesity-related HF phenotype capable of favorable modification with incretin-based therapies.”
The lack of heterogeneity in treatment effects across LVEF subgroups “is not surprising,” but “the findings reinforce that the benefits of this therapy in those meeting trial criteria do not vary by left ventricular ejection fraction,” Gregg C. Fonarow, MD, University of California, Los Angeles, Medical Center, said in an interview.
It remains unknown, however, “whether the improvement in health status, functional status, and reduced inflammation” will translate to reduced risk of cardiovascular death or HF hospitalization, said Dr. Fonarow, who isn’t connected to STEP-HFpEF.
It’s a question for future studies, he agreed, whether semaglutide would confer similar benefits for patients with obesity and HF with LVEF less than 45% or in non-obese HF patients.
Dr. McMurray proposed that future GLP-1 receptor agonist heart-failure trials should include non-obese patients to determine whether the effects seen in STEP-HFpEF were due to something more than weight loss. Trials in patients with obesity and HF with reduced LVEF would also be important.
“If it turns out just to be about weight loss, then we need to think about the alternatives,” including diet, exercise, and bariatric surgery but also, potentially, weight-loss drugs other than semaglutide, he said.
No heterogeneity by LVEF
STEP-HFpEF randomly assigned 529 patients free of diabetes with an LVEF greater than or equal to 45%, a body mass index (BMI) of at least 30 kg/m2, and NYHA functional status of 2-4 to either a placebo injection or 2.4-mg semaglutide subcutaneously once a week (the dose used for weight reduction) atop standard care.
As previously reported, those assigned to semaglutide showed significant improvements at 1 year in symptoms and in physical limitation, per changes in KCCQ-CSS, and weight loss, compared with the control group. Their exercise capacity, as measured by 6MWD, also improved.
The more weight patients lost while taking semaglutide, the better their KCCQ-CSS and 6MWD outcomes, a prior secondary analysis suggested. But the STEP-HFpEF researchers said weight loss did not appear to explain all of their gains, compared with usual care.
For the current analysis, the 263 patients assigned to receive semaglutide and 266 control patients were divided into three groups by baseline LVEF and compared for the same outcomes.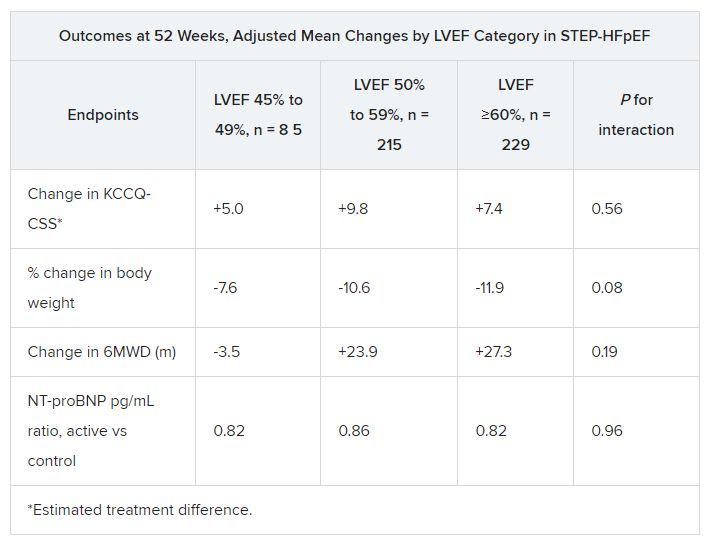
The semaglutide group, compared with control patients, also showed a significantly increased hierarchical composite win ratio, 1.72 (95% CI, 1.37-2.15; P < .001), that was consistent across LVEF categories and that accounted for all-cause mortality, HF events, KCCQ-CSS and 6MWD changes, and change in CRP.
Limitations make it hard to generalize the results, the authors caution. Well over 90% of the participants were White patients, for example, and the overall trial was not powered to show subgroup differences.
Given the many patients with HFpEF who have a cardiometabolic phenotype and are with overweight or obesity, write Dr. Butler and colleagues, their treatment approach “may ultimately include combination therapy with SGLT2 inhibitors and GLP-1 receptor agonists, given their non-overlapping and complementary mechanisms of action.”
Dr. Fonarow noted that both MRAs and sacubitril-valsartan offer clinical benefits for patients with HF and LVEF “in the 41%-60% range” that are evident “across BMI categories.”
So it’s likely, he said, that those medications as well as SGLT2 inhibitors will be used along with GLP-1 receptor agonists for patients with HFpEF and obesity.
STEP-HFpEF was funded by Novo Nordisk. Dr. Butler and the other authors disclose consulting for many companies, a list of which can be found in the report. Dr. Fonarow reports consulting for multiple companies. Dr. McMurray discloses consulting for AstraZeneca. Dr. Ostrominski reports no relevant disclosures. Dr. Vaduganathan discloses receiving grant support, serving on advisory boards, or speaking for multiple companies and serving on committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article appeared on Medscape.com.
CLEVELAND – independently of baseline left-ventricular ejection fraction (LVEF).
The finding comes from a prespecified secondary analysis of the STEP-HFpEF trial of more than 500 nondiabetic patients with obesity and HF with an initial LVEF of 45% or greater.
They suggest that for patients with the obesity phenotype of HFpEF, semaglutide (Wegovy) could potentially join SGLT2 inhibitors on the short list of meds with consistent treatment effects whether LVEF is mildly reduced, preserved, or in the normal range.
That would distinguish the drug, a glucagon-like peptide-1 (GLP-1) receptor agonist, from mineralocorticoid receptor antagonists (MRA), sacubitril-valsartan (Entresto), and other renin-angiotensin-system inhibitors (RASi), whose benefits tend to taper off with rising LVEF.
The patients assigned to semaglutide showed significant improvement in both primary endpoints – change in Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) and change in body weight at 52 weeks – whether their baseline LVEF was 45%-49%, 50%-59%, or 60% or greater.
Results were similar for improvements in 6-minute walk distance (6MWD) and levels of NT-terminal pro–brain natriuretic peptide (NT-proBNP) and C-reactive protein, observed Javed Butler, MD, when presenting the analysis at the annual meeting of the Heart Failure Society of America, Cleveland.
Dr. Butler, of Baylor Scott and White Research Institute, Dallas, and the University of Mississippi, Jackson, is also lead author of the study, which was published on the same day in the Journal of the American College of Cardiology.
In his presentation, Dr. Butler singled out the NT-proBNP finding as “very meaningful” with respect to understanding potential mechanisms of the drug effects observed in the trial.
For example, people with obesity tend to have lower than average natriuretic peptide levels that “actually go up a bit” when they lose weight, he observed. But in the trial, “we saw a reduction in NT-proBNP in spite of the weight loss,” regardless of LVEF category.
John McMurray, MD, University of Glasgow, the invited discussant for Dr. Butler’s presentation, agreed that it raises the question whether weight loss was the sole semaglutide effect responsible for the improvement in heart failure status and biomarkers. The accompanying NT-proBNP reductions – when the opposite might otherwise have been expected – may point to a possible mechanism of action that is “something more than just weight loss,” he said. “If that were the case, it becomes very important, because it means that this treatment might do good things in non-obese patients or might do good things in patients with other types of heart failure.”
‘Vital reassurance’
More definitive trials are needed “to clarify safety and efficacy of obesity-targeted therapeutics in HF across the ejection fraction spectrum,” according to an accompanying editorial).
Still, the STEP-HFpEF analysis “strengthens the role of GLP-1 [receptor agonists] to ameliorate health status” for patients with obesity and HF with mildly reduced or preserved ejection fraction, write Muthiah Vaduganathan, MD, MPH, and John W. Ostrominski, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
Its findings “provide vital reassurance” on semaglutide safety and efficacy in HF with below-normal LVEF and “tentatively support the existence of a more general, LVEF-independent, obesity-related HF phenotype capable of favorable modification with incretin-based therapies.”
The lack of heterogeneity in treatment effects across LVEF subgroups “is not surprising,” but “the findings reinforce that the benefits of this therapy in those meeting trial criteria do not vary by left ventricular ejection fraction,” Gregg C. Fonarow, MD, University of California, Los Angeles, Medical Center, said in an interview.
It remains unknown, however, “whether the improvement in health status, functional status, and reduced inflammation” will translate to reduced risk of cardiovascular death or HF hospitalization, said Dr. Fonarow, who isn’t connected to STEP-HFpEF.
It’s a question for future studies, he agreed, whether semaglutide would confer similar benefits for patients with obesity and HF with LVEF less than 45% or in non-obese HF patients.
Dr. McMurray proposed that future GLP-1 receptor agonist heart-failure trials should include non-obese patients to determine whether the effects seen in STEP-HFpEF were due to something more than weight loss. Trials in patients with obesity and HF with reduced LVEF would also be important.
“If it turns out just to be about weight loss, then we need to think about the alternatives,” including diet, exercise, and bariatric surgery but also, potentially, weight-loss drugs other than semaglutide, he said.
No heterogeneity by LVEF
STEP-HFpEF randomly assigned 529 patients free of diabetes with an LVEF greater than or equal to 45%, a body mass index (BMI) of at least 30 kg/m2, and NYHA functional status of 2-4 to either a placebo injection or 2.4-mg semaglutide subcutaneously once a week (the dose used for weight reduction) atop standard care.
As previously reported, those assigned to semaglutide showed significant improvements at 1 year in symptoms and in physical limitation, per changes in KCCQ-CSS, and weight loss, compared with the control group. Their exercise capacity, as measured by 6MWD, also improved.
The more weight patients lost while taking semaglutide, the better their KCCQ-CSS and 6MWD outcomes, a prior secondary analysis suggested. But the STEP-HFpEF researchers said weight loss did not appear to explain all of their gains, compared with usual care.
For the current analysis, the 263 patients assigned to receive semaglutide and 266 control patients were divided into three groups by baseline LVEF and compared for the same outcomes.
The semaglutide group, compared with control patients, also showed a significantly increased hierarchical composite win ratio, 1.72 (95% CI, 1.37-2.15; P < .001), that was consistent across LVEF categories and that accounted for all-cause mortality, HF events, KCCQ-CSS and 6MWD changes, and change in CRP.
Limitations make it hard to generalize the results, the authors caution. Well over 90% of the participants were White patients, for example, and the overall trial was not powered to show subgroup differences.
Given the many patients with HFpEF who have a cardiometabolic phenotype and are with overweight or obesity, write Dr. Butler and colleagues, their treatment approach “may ultimately include combination therapy with SGLT2 inhibitors and GLP-1 receptor agonists, given their non-overlapping and complementary mechanisms of action.”
Dr. Fonarow noted that both MRAs and sacubitril-valsartan offer clinical benefits for patients with HF and LVEF “in the 41%-60% range” that are evident “across BMI categories.”
So it’s likely, he said, that those medications as well as SGLT2 inhibitors will be used along with GLP-1 receptor agonists for patients with HFpEF and obesity.
STEP-HFpEF was funded by Novo Nordisk. Dr. Butler and the other authors disclose consulting for many companies, a list of which can be found in the report. Dr. Fonarow reports consulting for multiple companies. Dr. McMurray discloses consulting for AstraZeneca. Dr. Ostrominski reports no relevant disclosures. Dr. Vaduganathan discloses receiving grant support, serving on advisory boards, or speaking for multiple companies and serving on committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article appeared on Medscape.com.
CLEVELAND – independently of baseline left-ventricular ejection fraction (LVEF).
The finding comes from a prespecified secondary analysis of the STEP-HFpEF trial of more than 500 nondiabetic patients with obesity and HF with an initial LVEF of 45% or greater.
They suggest that for patients with the obesity phenotype of HFpEF, semaglutide (Wegovy) could potentially join SGLT2 inhibitors on the short list of meds with consistent treatment effects whether LVEF is mildly reduced, preserved, or in the normal range.
That would distinguish the drug, a glucagon-like peptide-1 (GLP-1) receptor agonist, from mineralocorticoid receptor antagonists (MRA), sacubitril-valsartan (Entresto), and other renin-angiotensin-system inhibitors (RASi), whose benefits tend to taper off with rising LVEF.
The patients assigned to semaglutide showed significant improvement in both primary endpoints – change in Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) and change in body weight at 52 weeks – whether their baseline LVEF was 45%-49%, 50%-59%, or 60% or greater.
Results were similar for improvements in 6-minute walk distance (6MWD) and levels of NT-terminal pro–brain natriuretic peptide (NT-proBNP) and C-reactive protein, observed Javed Butler, MD, when presenting the analysis at the annual meeting of the Heart Failure Society of America, Cleveland.
Dr. Butler, of Baylor Scott and White Research Institute, Dallas, and the University of Mississippi, Jackson, is also lead author of the study, which was published on the same day in the Journal of the American College of Cardiology.
In his presentation, Dr. Butler singled out the NT-proBNP finding as “very meaningful” with respect to understanding potential mechanisms of the drug effects observed in the trial.
For example, people with obesity tend to have lower than average natriuretic peptide levels that “actually go up a bit” when they lose weight, he observed. But in the trial, “we saw a reduction in NT-proBNP in spite of the weight loss,” regardless of LVEF category.
John McMurray, MD, University of Glasgow, the invited discussant for Dr. Butler’s presentation, agreed that it raises the question whether weight loss was the sole semaglutide effect responsible for the improvement in heart failure status and biomarkers. The accompanying NT-proBNP reductions – when the opposite might otherwise have been expected – may point to a possible mechanism of action that is “something more than just weight loss,” he said. “If that were the case, it becomes very important, because it means that this treatment might do good things in non-obese patients or might do good things in patients with other types of heart failure.”
‘Vital reassurance’
More definitive trials are needed “to clarify safety and efficacy of obesity-targeted therapeutics in HF across the ejection fraction spectrum,” according to an accompanying editorial).
Still, the STEP-HFpEF analysis “strengthens the role of GLP-1 [receptor agonists] to ameliorate health status” for patients with obesity and HF with mildly reduced or preserved ejection fraction, write Muthiah Vaduganathan, MD, MPH, and John W. Ostrominski, MD, Brigham and Women’s Hospital and Harvard Medical School, both in Boston.
Its findings “provide vital reassurance” on semaglutide safety and efficacy in HF with below-normal LVEF and “tentatively support the existence of a more general, LVEF-independent, obesity-related HF phenotype capable of favorable modification with incretin-based therapies.”
The lack of heterogeneity in treatment effects across LVEF subgroups “is not surprising,” but “the findings reinforce that the benefits of this therapy in those meeting trial criteria do not vary by left ventricular ejection fraction,” Gregg C. Fonarow, MD, University of California, Los Angeles, Medical Center, said in an interview.
It remains unknown, however, “whether the improvement in health status, functional status, and reduced inflammation” will translate to reduced risk of cardiovascular death or HF hospitalization, said Dr. Fonarow, who isn’t connected to STEP-HFpEF.
It’s a question for future studies, he agreed, whether semaglutide would confer similar benefits for patients with obesity and HF with LVEF less than 45% or in non-obese HF patients.
Dr. McMurray proposed that future GLP-1 receptor agonist heart-failure trials should include non-obese patients to determine whether the effects seen in STEP-HFpEF were due to something more than weight loss. Trials in patients with obesity and HF with reduced LVEF would also be important.
“If it turns out just to be about weight loss, then we need to think about the alternatives,” including diet, exercise, and bariatric surgery but also, potentially, weight-loss drugs other than semaglutide, he said.
No heterogeneity by LVEF
STEP-HFpEF randomly assigned 529 patients free of diabetes with an LVEF greater than or equal to 45%, a body mass index (BMI) of at least 30 kg/m2, and NYHA functional status of 2-4 to either a placebo injection or 2.4-mg semaglutide subcutaneously once a week (the dose used for weight reduction) atop standard care.
As previously reported, those assigned to semaglutide showed significant improvements at 1 year in symptoms and in physical limitation, per changes in KCCQ-CSS, and weight loss, compared with the control group. Their exercise capacity, as measured by 6MWD, also improved.
The more weight patients lost while taking semaglutide, the better their KCCQ-CSS and 6MWD outcomes, a prior secondary analysis suggested. But the STEP-HFpEF researchers said weight loss did not appear to explain all of their gains, compared with usual care.
For the current analysis, the 263 patients assigned to receive semaglutide and 266 control patients were divided into three groups by baseline LVEF and compared for the same outcomes.
The semaglutide group, compared with control patients, also showed a significantly increased hierarchical composite win ratio, 1.72 (95% CI, 1.37-2.15; P < .001), that was consistent across LVEF categories and that accounted for all-cause mortality, HF events, KCCQ-CSS and 6MWD changes, and change in CRP.
Limitations make it hard to generalize the results, the authors caution. Well over 90% of the participants were White patients, for example, and the overall trial was not powered to show subgroup differences.
Given the many patients with HFpEF who have a cardiometabolic phenotype and are with overweight or obesity, write Dr. Butler and colleagues, their treatment approach “may ultimately include combination therapy with SGLT2 inhibitors and GLP-1 receptor agonists, given their non-overlapping and complementary mechanisms of action.”
Dr. Fonarow noted that both MRAs and sacubitril-valsartan offer clinical benefits for patients with HF and LVEF “in the 41%-60% range” that are evident “across BMI categories.”
So it’s likely, he said, that those medications as well as SGLT2 inhibitors will be used along with GLP-1 receptor agonists for patients with HFpEF and obesity.
STEP-HFpEF was funded by Novo Nordisk. Dr. Butler and the other authors disclose consulting for many companies, a list of which can be found in the report. Dr. Fonarow reports consulting for multiple companies. Dr. McMurray discloses consulting for AstraZeneca. Dr. Ostrominski reports no relevant disclosures. Dr. Vaduganathan discloses receiving grant support, serving on advisory boards, or speaking for multiple companies and serving on committees for studies sponsored by AstraZeneca, Galmed, Novartis, Bayer AG, Occlutech, and Impulse Dynamics.
A version of this article appeared on Medscape.com.
AT HFSA 2023
The how and why of quad therapy in reduced-EF heart failure
It’s as if hospitals, clinicians, and the health care system itself were unprepared for such success as a powerful multiple-drug regimen emerged for hospitalized patients with heart failure with reduced ejection fraction (HFrEF).
Uptake in practice has been sluggish for the management strategy driven by a quartet of medications, each with its own mechanisms of action, started in the hospital simultaneously or in rapid succession over a few days. Key to the regimen, dosages are at least partly uptitrated in the hospital then optimized during close postdischarge follow-up.
The so-called four pillars of medical therapy for HFrEF, defined by a left ventricular ejection fraction (LVEF) of 40% or lower, include an SGLT2 inhibitor, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin-system (RAS) inhibitor – preferably sacubitril-valsartan (Entresto) or, as a backup, an ACE inhibitor or angiotensin receptor blocker (ARB).
Academic consensus on the strategy is strong. The approach is consistent with heart failure (HF) guidelines on both sides of the Atlantic and is backed by solid trial evidence suggesting striking improvements in survival, readmission risk, and quality of life.
Gregg C. Fonarow, MD, University of California, Los Angeles, said in an interview.
“Yet, when we look at their actual implementation in clinical practice, we’ve seen this slow and variable uptake.”
So, why is that?
The STRONG-HF trial tested a version of the multiple-drug strategy and demonstrated what it could achieve even without a contribution from SGLT2 inhibitors, which weren’t yet indicated for HF. Eligibility for the trial, with more than 1,000 patients, wasn’t dependent on their LVEF.
Patients assigned to early and rapidly sequential initiation of a beta-blocker, an MRA, and a RAS inhibitor, compared with a standard-care control group, benefited with a 34% drop (P = .002) in risk for death or HF readmission over the next 6 months.
Few doubt – and the bulk of evidence suggests – that adding an SGLT2 inhibitor to round out the four-pillar strategy would safely boost its clinical potential in HFrEF.
The strategy’s smooth adoption in practice likely has multiple confounders that include clinical inertia, perceptions of HF medical management as a long-term outpatient process, and the onerous and Kafkaesque systems of care and reimbursement in the United States.
For example, the drug initiation and uptitration process may seem too complex for integration into slow-to-change hospital practices. And there could be a misguided sense that the regimen and follow-up must abide by the same exacting detail and standards set forth in, for example, the STRONG-HF protocol.
But starting hospitalized patients with HFrEF on the quartet of drugs and optimizing their dosages in hospital and after discharge can be simpler and more straightforward than that, Dr. Fonarow and other experts explain.
The academic community’s buy-in is a first step, but broader acceptance is frustrated by an “overwhelming culture of clinical care for heart failure” that encourages a more drawn-out process for adding medications, said Stephen J. Greene, MD, Duke Clinical Research Institute, Durham, N.C. “We need to turn our thinking on its head about heart failure in clinical practice.”
The “dramatic” underuse of the four pillars in the hospital stems in part from “outmoded” treatment algorithms that clinicians are following, Dr. Fonarow said. And they have “no sense of urgency,” sometimes wrongly believing “that it takes months for these medications to ultimately kick in.”
For hospitalized patients with HFrEF, “there is an imperative to overcome these timid algorithms and timid thinking,” he said. They should be on “full quadruple therapy” before discharge.
“And for newly diagnosed outpatients, you should essentially give yourself 7 days to get these drugs on board,” he added, either simultaneously or in “very rapid sequence.”
What’s needed is a “cultural shift” in medicine that “elevates heart failure to the same level of urgency that we have in the care of some other disease states,” agreed Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital and Harvard Medical School, Boston.
Hospital as opportunity
The patient’s 4-7 days in the hospital typically represent a “wonderful opportunity” to initiate all four drug classes in rapid succession and start uptitrations. But most hospitals and other health care settings, Dr. Vaduganathan observed, lack the structure and systems to support the process. Broad application will require “buy-in from multiple parties – from the clinician, from the patient, their caregivers, and their partners as well as the health system.”
Physician awareness and support for the strategy, suggests at least one of these experts, is probably much less of a challenge to its broad adoption than the bewildering mechanics of health care delivery and reimbursement.
“The problem is not education. The problem is the way that our health care system is structured,” said Milton Packer, MD, Baylor Heart and Vascular Institute, Dallas.
For example, sacubitril-valsartan and the SGLT2 inhibitors are still under patent and are far more expensive than longtime generic beta-blockers and MRAs. That means physicians typically spend valuable time pursuing prior authorizations for the brand-name drugs under pressure to eventually discharge the patient because of limits on hospital reimbursement.
Clinicians in the hospital are “almost disincentivized by the system” to implement management plans that call for early and rapid initiation of multiple drugs, Dr. Vaduganathan pointed out.
One change per day
There’s no one formula for carrying out the quadruple drug strategy, Dr. Vaduganathan noted. “I make only a single change per day” to the regimen, such as uptitration or addition of a single agent. That way, tolerability can be evaluated one drug at a time, “and then the following day, I can make the next therapeutic change.”
The order in which the drugs are started mostly does not matter, in contrast to a traditional approach that might have added new drugs in the sequence of their approval for HFrEF or adoption in guidelines. Under that scenario, each successive agent might be fully uptitrated before the next could be brought on board.
Historically, Dr. Packer observed, “you would start with an ACE inhibitor, add a beta-blocker, add an MRA, switch to sacubitril-valsartan, add an SGLT2 inhibitor – and it would take 8 months.” Any prescribed sequence is pointless given the short time frame that is ideal for initiating all the drugs, he said.
Hypothetically, however, there is some rationale for starting them in an order that leverages their unique actions and side effects. For example, Dr. Vaduganathan and others observed, it may be helpful to start an SGLT2 inhibitor and sacubitril-valsartan early in the process, because they can mitigate any hyperkalemia from the subsequent addition of an MRA.
That being said, “I don’t think we have firm evidence that any particular order is more efficacious than another,” Dr. Vaduganathan said. “It’s really about getting patients on all four drugs as quickly as possible, regardless of the sequence.”
Discussions about sequencing the drugs are “a distraction for our field,” Dr. Greene said. In trials, clinical benefit from the multiple-drug regimen has emerged almost right away once the drugs were on board. “The data clearly show that initiating all four, at least at low doses, gives the best bang for your buck and would be a high-yield strategy.”
Best evidence suggests that once all four agents have been started, attention can turn to uptitration, “with the beta-blocker as the higher priority,” Dr. Greene said. “The bottom line is to keep it simple: four drugs, simultaneously or within 1 week, and prioritize initiation at low doses to maximize tolerability.”
The four-drug approach yields survival and rehospitalization benefits even when uptitrations don’t reach prespecified goals, Dr. Fonarow observed. The SGLT2 inhibitors are started and maintained at the same dosage. But for the other three agents, uptitration should aim for the highest well-tolerated level, up to the target, even if the highest tolerated is the initial dosage.
‘Challenging to generalize’
The goal in STRONG-HF was to start and at least partly uptitrate a beta-blocker, an MRA, and sacubitril-valsartan in the hospital and fully optimize their dosages within 2 weeks after discharge. Symptoms and laboratory biomarkers, including natriuretic peptides, were closely monitored at four in-person evaluations during the first 6 outpatient weeks.
But few believe the trial’s intensive drug regimen and postdischarge follow-up, as stipulated in the protocol, would be tolerated by current systems of care and reimbursement.
STRONG-HF “affirms the strategy in a rigorous, well conducted way,” Dr. Vaduganathan said, but would be “challenging to generalize to all health care systems.”
As a result, some in the field are “quick to almost disregard STRONG-HF in its entirety” and consider it “wishful thinking,” Dr. Greene said. Better that providers not become distracted by the precise details of its protocol.
At Duke, he said, “we see all our patients within 1 week of discharge to ensure they’re doing okay in terms of volume status and look for opportunities to escalate their guideline-directed medical therapy.”
But that can be done without in-person visits. A lot of the follow-up and uptitrations, Dr. Greene said, can be achieved by telephone or at virtual appointments in conjunction with regular laboratory testing. “That, I think, really is the path for the future, in this age when clinics are overwhelmed by in-person visits.”
Mildly reduced and preserved EF
STRONG-HF, in which patients were enrolled without regard to ejection fraction, suggests that its rapidly sequential drug regimen and intensive management protocol improves outcomes for patients with HF at any level of LVEF.
Those findings and others, along with DELIVER, EMPEROR-Preserved and other studies, make a tantalizing case for the quadruple drug approach in patients with HF and LVEF >40% – that is, those with mildly reduced (LVEF > 40% to < 50%, HFmrEF) or preserved LVEF > 50%, HFpEF) ejection fraction.
But the case isn’t solid enough to declare the four agents as core therapy for HF and LVEF > 40%, observed Dr. Vaduganathan. Currently, SGLT2 inhibitors “are the only drug class that we are routinely implementing” in HFmrEF and HFpEF.
There have been suggestions of clinical benefit for such patients with sacubitril-valsartan and MRAs, especially in PARAGON-HF and TOPCAT, respectively. The evidence is stronger in HFmrEF than in HFpEF, but in either case it’s weaker than the clear-cut trial support for SGLT2 inhibitors in those HF categories.
Trials also suggest that in HF with LVEF > 40%, clinical benefits from RAS inhibitors and MRAs taper off with increasing ejection fraction, especially into the > 60% range.
In both HFmrEF and HFpEF, “I routinely try to get the patient on an SGLT2 inhibitor rapidly and then treat with some of the other agents on a more individual basis,” Dr. Vaduganathan said. An LVEF in the HFmrEF range, for example, would likely call for the addition of an MRA and sacubitril-valsartan.
Dr. Packer said he would likely recommend all four agents for patients with HF and LVEF up to 60%, which he considers a more appropriate definition of HFrEF. Their clinical benefits appear consistent across that LVEF range, he said, although they thin out somewhat at the higher end.
Evidence supporting the four pillars in HF with LV > 40% and < 60% is weakest for beta-blockers, Dr. Packer noted, so arguably those drugs could be left out of the mix for patients with ejection fractions in that range.
Dr. Fonarow reported ties with Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Eli Lilly, Johnson & Johnson, Medtronic, Merck, Novartis, and Pfizer. Dr. Greene disclosed ties with Amgen, AstraZeneca, Bayer, Boehringer Ingelheim/Lilly, Bristol-Myers Squibb, Corteria, CSL Vifor, Cytokinetics, Lexicon Merck, Novartis, Pfizer, PharmaIN, Roche Diagnostics, Sanofi, scPharmaceuticals, Tricog Health, and Urovant Pharmaceuticals. Dr. Vaduganathan disclosed ties with American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Chiesi, Cytokinetics, Galmed, Impulse Dynamics, Lexicon Pharmaceuticals, Merck, Novartis, Novo Nordisk, Occlutech, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health. Dr. Packer disclosed relationships with 89bio, AbbVie, Actavis, Amarin, Amgen, AstraZeneca, Attralus, Boehringer Ingelheim, Caladrius, Casana, CSL Behring, Cytokinetics, Imara, Lilly, Medtronic, Moderna, Novartis, Pharmacosmos, Reata, Regeneron, Relypsa, and Salamandra.
A version of this article first appeared on Medscape.com.
It’s as if hospitals, clinicians, and the health care system itself were unprepared for such success as a powerful multiple-drug regimen emerged for hospitalized patients with heart failure with reduced ejection fraction (HFrEF).
Uptake in practice has been sluggish for the management strategy driven by a quartet of medications, each with its own mechanisms of action, started in the hospital simultaneously or in rapid succession over a few days. Key to the regimen, dosages are at least partly uptitrated in the hospital then optimized during close postdischarge follow-up.
The so-called four pillars of medical therapy for HFrEF, defined by a left ventricular ejection fraction (LVEF) of 40% or lower, include an SGLT2 inhibitor, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin-system (RAS) inhibitor – preferably sacubitril-valsartan (Entresto) or, as a backup, an ACE inhibitor or angiotensin receptor blocker (ARB).
Academic consensus on the strategy is strong. The approach is consistent with heart failure (HF) guidelines on both sides of the Atlantic and is backed by solid trial evidence suggesting striking improvements in survival, readmission risk, and quality of life.
Gregg C. Fonarow, MD, University of California, Los Angeles, said in an interview.
“Yet, when we look at their actual implementation in clinical practice, we’ve seen this slow and variable uptake.”
So, why is that?
The STRONG-HF trial tested a version of the multiple-drug strategy and demonstrated what it could achieve even without a contribution from SGLT2 inhibitors, which weren’t yet indicated for HF. Eligibility for the trial, with more than 1,000 patients, wasn’t dependent on their LVEF.
Patients assigned to early and rapidly sequential initiation of a beta-blocker, an MRA, and a RAS inhibitor, compared with a standard-care control group, benefited with a 34% drop (P = .002) in risk for death or HF readmission over the next 6 months.
Few doubt – and the bulk of evidence suggests – that adding an SGLT2 inhibitor to round out the four-pillar strategy would safely boost its clinical potential in HFrEF.
The strategy’s smooth adoption in practice likely has multiple confounders that include clinical inertia, perceptions of HF medical management as a long-term outpatient process, and the onerous and Kafkaesque systems of care and reimbursement in the United States.
For example, the drug initiation and uptitration process may seem too complex for integration into slow-to-change hospital practices. And there could be a misguided sense that the regimen and follow-up must abide by the same exacting detail and standards set forth in, for example, the STRONG-HF protocol.
But starting hospitalized patients with HFrEF on the quartet of drugs and optimizing their dosages in hospital and after discharge can be simpler and more straightforward than that, Dr. Fonarow and other experts explain.
The academic community’s buy-in is a first step, but broader acceptance is frustrated by an “overwhelming culture of clinical care for heart failure” that encourages a more drawn-out process for adding medications, said Stephen J. Greene, MD, Duke Clinical Research Institute, Durham, N.C. “We need to turn our thinking on its head about heart failure in clinical practice.”
The “dramatic” underuse of the four pillars in the hospital stems in part from “outmoded” treatment algorithms that clinicians are following, Dr. Fonarow said. And they have “no sense of urgency,” sometimes wrongly believing “that it takes months for these medications to ultimately kick in.”
For hospitalized patients with HFrEF, “there is an imperative to overcome these timid algorithms and timid thinking,” he said. They should be on “full quadruple therapy” before discharge.
“And for newly diagnosed outpatients, you should essentially give yourself 7 days to get these drugs on board,” he added, either simultaneously or in “very rapid sequence.”
What’s needed is a “cultural shift” in medicine that “elevates heart failure to the same level of urgency that we have in the care of some other disease states,” agreed Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital and Harvard Medical School, Boston.
Hospital as opportunity
The patient’s 4-7 days in the hospital typically represent a “wonderful opportunity” to initiate all four drug classes in rapid succession and start uptitrations. But most hospitals and other health care settings, Dr. Vaduganathan observed, lack the structure and systems to support the process. Broad application will require “buy-in from multiple parties – from the clinician, from the patient, their caregivers, and their partners as well as the health system.”
Physician awareness and support for the strategy, suggests at least one of these experts, is probably much less of a challenge to its broad adoption than the bewildering mechanics of health care delivery and reimbursement.
“The problem is not education. The problem is the way that our health care system is structured,” said Milton Packer, MD, Baylor Heart and Vascular Institute, Dallas.
For example, sacubitril-valsartan and the SGLT2 inhibitors are still under patent and are far more expensive than longtime generic beta-blockers and MRAs. That means physicians typically spend valuable time pursuing prior authorizations for the brand-name drugs under pressure to eventually discharge the patient because of limits on hospital reimbursement.
Clinicians in the hospital are “almost disincentivized by the system” to implement management plans that call for early and rapid initiation of multiple drugs, Dr. Vaduganathan pointed out.
One change per day
There’s no one formula for carrying out the quadruple drug strategy, Dr. Vaduganathan noted. “I make only a single change per day” to the regimen, such as uptitration or addition of a single agent. That way, tolerability can be evaluated one drug at a time, “and then the following day, I can make the next therapeutic change.”
The order in which the drugs are started mostly does not matter, in contrast to a traditional approach that might have added new drugs in the sequence of their approval for HFrEF or adoption in guidelines. Under that scenario, each successive agent might be fully uptitrated before the next could be brought on board.
Historically, Dr. Packer observed, “you would start with an ACE inhibitor, add a beta-blocker, add an MRA, switch to sacubitril-valsartan, add an SGLT2 inhibitor – and it would take 8 months.” Any prescribed sequence is pointless given the short time frame that is ideal for initiating all the drugs, he said.
Hypothetically, however, there is some rationale for starting them in an order that leverages their unique actions and side effects. For example, Dr. Vaduganathan and others observed, it may be helpful to start an SGLT2 inhibitor and sacubitril-valsartan early in the process, because they can mitigate any hyperkalemia from the subsequent addition of an MRA.
That being said, “I don’t think we have firm evidence that any particular order is more efficacious than another,” Dr. Vaduganathan said. “It’s really about getting patients on all four drugs as quickly as possible, regardless of the sequence.”
Discussions about sequencing the drugs are “a distraction for our field,” Dr. Greene said. In trials, clinical benefit from the multiple-drug regimen has emerged almost right away once the drugs were on board. “The data clearly show that initiating all four, at least at low doses, gives the best bang for your buck and would be a high-yield strategy.”
Best evidence suggests that once all four agents have been started, attention can turn to uptitration, “with the beta-blocker as the higher priority,” Dr. Greene said. “The bottom line is to keep it simple: four drugs, simultaneously or within 1 week, and prioritize initiation at low doses to maximize tolerability.”
The four-drug approach yields survival and rehospitalization benefits even when uptitrations don’t reach prespecified goals, Dr. Fonarow observed. The SGLT2 inhibitors are started and maintained at the same dosage. But for the other three agents, uptitration should aim for the highest well-tolerated level, up to the target, even if the highest tolerated is the initial dosage.
‘Challenging to generalize’
The goal in STRONG-HF was to start and at least partly uptitrate a beta-blocker, an MRA, and sacubitril-valsartan in the hospital and fully optimize their dosages within 2 weeks after discharge. Symptoms and laboratory biomarkers, including natriuretic peptides, were closely monitored at four in-person evaluations during the first 6 outpatient weeks.
But few believe the trial’s intensive drug regimen and postdischarge follow-up, as stipulated in the protocol, would be tolerated by current systems of care and reimbursement.
STRONG-HF “affirms the strategy in a rigorous, well conducted way,” Dr. Vaduganathan said, but would be “challenging to generalize to all health care systems.”
As a result, some in the field are “quick to almost disregard STRONG-HF in its entirety” and consider it “wishful thinking,” Dr. Greene said. Better that providers not become distracted by the precise details of its protocol.
At Duke, he said, “we see all our patients within 1 week of discharge to ensure they’re doing okay in terms of volume status and look for opportunities to escalate their guideline-directed medical therapy.”
But that can be done without in-person visits. A lot of the follow-up and uptitrations, Dr. Greene said, can be achieved by telephone or at virtual appointments in conjunction with regular laboratory testing. “That, I think, really is the path for the future, in this age when clinics are overwhelmed by in-person visits.”
Mildly reduced and preserved EF
STRONG-HF, in which patients were enrolled without regard to ejection fraction, suggests that its rapidly sequential drug regimen and intensive management protocol improves outcomes for patients with HF at any level of LVEF.
Those findings and others, along with DELIVER, EMPEROR-Preserved and other studies, make a tantalizing case for the quadruple drug approach in patients with HF and LVEF >40% – that is, those with mildly reduced (LVEF > 40% to < 50%, HFmrEF) or preserved LVEF > 50%, HFpEF) ejection fraction.
But the case isn’t solid enough to declare the four agents as core therapy for HF and LVEF > 40%, observed Dr. Vaduganathan. Currently, SGLT2 inhibitors “are the only drug class that we are routinely implementing” in HFmrEF and HFpEF.
There have been suggestions of clinical benefit for such patients with sacubitril-valsartan and MRAs, especially in PARAGON-HF and TOPCAT, respectively. The evidence is stronger in HFmrEF than in HFpEF, but in either case it’s weaker than the clear-cut trial support for SGLT2 inhibitors in those HF categories.
Trials also suggest that in HF with LVEF > 40%, clinical benefits from RAS inhibitors and MRAs taper off with increasing ejection fraction, especially into the > 60% range.
In both HFmrEF and HFpEF, “I routinely try to get the patient on an SGLT2 inhibitor rapidly and then treat with some of the other agents on a more individual basis,” Dr. Vaduganathan said. An LVEF in the HFmrEF range, for example, would likely call for the addition of an MRA and sacubitril-valsartan.
Dr. Packer said he would likely recommend all four agents for patients with HF and LVEF up to 60%, which he considers a more appropriate definition of HFrEF. Their clinical benefits appear consistent across that LVEF range, he said, although they thin out somewhat at the higher end.
Evidence supporting the four pillars in HF with LV > 40% and < 60% is weakest for beta-blockers, Dr. Packer noted, so arguably those drugs could be left out of the mix for patients with ejection fractions in that range.
Dr. Fonarow reported ties with Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Eli Lilly, Johnson & Johnson, Medtronic, Merck, Novartis, and Pfizer. Dr. Greene disclosed ties with Amgen, AstraZeneca, Bayer, Boehringer Ingelheim/Lilly, Bristol-Myers Squibb, Corteria, CSL Vifor, Cytokinetics, Lexicon Merck, Novartis, Pfizer, PharmaIN, Roche Diagnostics, Sanofi, scPharmaceuticals, Tricog Health, and Urovant Pharmaceuticals. Dr. Vaduganathan disclosed ties with American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Chiesi, Cytokinetics, Galmed, Impulse Dynamics, Lexicon Pharmaceuticals, Merck, Novartis, Novo Nordisk, Occlutech, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health. Dr. Packer disclosed relationships with 89bio, AbbVie, Actavis, Amarin, Amgen, AstraZeneca, Attralus, Boehringer Ingelheim, Caladrius, Casana, CSL Behring, Cytokinetics, Imara, Lilly, Medtronic, Moderna, Novartis, Pharmacosmos, Reata, Regeneron, Relypsa, and Salamandra.
A version of this article first appeared on Medscape.com.
It’s as if hospitals, clinicians, and the health care system itself were unprepared for such success as a powerful multiple-drug regimen emerged for hospitalized patients with heart failure with reduced ejection fraction (HFrEF).
Uptake in practice has been sluggish for the management strategy driven by a quartet of medications, each with its own mechanisms of action, started in the hospital simultaneously or in rapid succession over a few days. Key to the regimen, dosages are at least partly uptitrated in the hospital then optimized during close postdischarge follow-up.
The so-called four pillars of medical therapy for HFrEF, defined by a left ventricular ejection fraction (LVEF) of 40% or lower, include an SGLT2 inhibitor, a beta-blocker, a mineralocorticoid receptor antagonist (MRA), and a renin-angiotensin-system (RAS) inhibitor – preferably sacubitril-valsartan (Entresto) or, as a backup, an ACE inhibitor or angiotensin receptor blocker (ARB).
Academic consensus on the strategy is strong. The approach is consistent with heart failure (HF) guidelines on both sides of the Atlantic and is backed by solid trial evidence suggesting striking improvements in survival, readmission risk, and quality of life.
Gregg C. Fonarow, MD, University of California, Los Angeles, said in an interview.
“Yet, when we look at their actual implementation in clinical practice, we’ve seen this slow and variable uptake.”
So, why is that?
The STRONG-HF trial tested a version of the multiple-drug strategy and demonstrated what it could achieve even without a contribution from SGLT2 inhibitors, which weren’t yet indicated for HF. Eligibility for the trial, with more than 1,000 patients, wasn’t dependent on their LVEF.
Patients assigned to early and rapidly sequential initiation of a beta-blocker, an MRA, and a RAS inhibitor, compared with a standard-care control group, benefited with a 34% drop (P = .002) in risk for death or HF readmission over the next 6 months.
Few doubt – and the bulk of evidence suggests – that adding an SGLT2 inhibitor to round out the four-pillar strategy would safely boost its clinical potential in HFrEF.
The strategy’s smooth adoption in practice likely has multiple confounders that include clinical inertia, perceptions of HF medical management as a long-term outpatient process, and the onerous and Kafkaesque systems of care and reimbursement in the United States.
For example, the drug initiation and uptitration process may seem too complex for integration into slow-to-change hospital practices. And there could be a misguided sense that the regimen and follow-up must abide by the same exacting detail and standards set forth in, for example, the STRONG-HF protocol.
But starting hospitalized patients with HFrEF on the quartet of drugs and optimizing their dosages in hospital and after discharge can be simpler and more straightforward than that, Dr. Fonarow and other experts explain.
The academic community’s buy-in is a first step, but broader acceptance is frustrated by an “overwhelming culture of clinical care for heart failure” that encourages a more drawn-out process for adding medications, said Stephen J. Greene, MD, Duke Clinical Research Institute, Durham, N.C. “We need to turn our thinking on its head about heart failure in clinical practice.”
The “dramatic” underuse of the four pillars in the hospital stems in part from “outmoded” treatment algorithms that clinicians are following, Dr. Fonarow said. And they have “no sense of urgency,” sometimes wrongly believing “that it takes months for these medications to ultimately kick in.”
For hospitalized patients with HFrEF, “there is an imperative to overcome these timid algorithms and timid thinking,” he said. They should be on “full quadruple therapy” before discharge.
“And for newly diagnosed outpatients, you should essentially give yourself 7 days to get these drugs on board,” he added, either simultaneously or in “very rapid sequence.”
What’s needed is a “cultural shift” in medicine that “elevates heart failure to the same level of urgency that we have in the care of some other disease states,” agreed Muthiah Vaduganathan, MD, MPH, Brigham and Women’s Hospital and Harvard Medical School, Boston.
Hospital as opportunity
The patient’s 4-7 days in the hospital typically represent a “wonderful opportunity” to initiate all four drug classes in rapid succession and start uptitrations. But most hospitals and other health care settings, Dr. Vaduganathan observed, lack the structure and systems to support the process. Broad application will require “buy-in from multiple parties – from the clinician, from the patient, their caregivers, and their partners as well as the health system.”
Physician awareness and support for the strategy, suggests at least one of these experts, is probably much less of a challenge to its broad adoption than the bewildering mechanics of health care delivery and reimbursement.
“The problem is not education. The problem is the way that our health care system is structured,” said Milton Packer, MD, Baylor Heart and Vascular Institute, Dallas.
For example, sacubitril-valsartan and the SGLT2 inhibitors are still under patent and are far more expensive than longtime generic beta-blockers and MRAs. That means physicians typically spend valuable time pursuing prior authorizations for the brand-name drugs under pressure to eventually discharge the patient because of limits on hospital reimbursement.
Clinicians in the hospital are “almost disincentivized by the system” to implement management plans that call for early and rapid initiation of multiple drugs, Dr. Vaduganathan pointed out.
One change per day
There’s no one formula for carrying out the quadruple drug strategy, Dr. Vaduganathan noted. “I make only a single change per day” to the regimen, such as uptitration or addition of a single agent. That way, tolerability can be evaluated one drug at a time, “and then the following day, I can make the next therapeutic change.”
The order in which the drugs are started mostly does not matter, in contrast to a traditional approach that might have added new drugs in the sequence of their approval for HFrEF or adoption in guidelines. Under that scenario, each successive agent might be fully uptitrated before the next could be brought on board.
Historically, Dr. Packer observed, “you would start with an ACE inhibitor, add a beta-blocker, add an MRA, switch to sacubitril-valsartan, add an SGLT2 inhibitor – and it would take 8 months.” Any prescribed sequence is pointless given the short time frame that is ideal for initiating all the drugs, he said.
Hypothetically, however, there is some rationale for starting them in an order that leverages their unique actions and side effects. For example, Dr. Vaduganathan and others observed, it may be helpful to start an SGLT2 inhibitor and sacubitril-valsartan early in the process, because they can mitigate any hyperkalemia from the subsequent addition of an MRA.
That being said, “I don’t think we have firm evidence that any particular order is more efficacious than another,” Dr. Vaduganathan said. “It’s really about getting patients on all four drugs as quickly as possible, regardless of the sequence.”
Discussions about sequencing the drugs are “a distraction for our field,” Dr. Greene said. In trials, clinical benefit from the multiple-drug regimen has emerged almost right away once the drugs were on board. “The data clearly show that initiating all four, at least at low doses, gives the best bang for your buck and would be a high-yield strategy.”
Best evidence suggests that once all four agents have been started, attention can turn to uptitration, “with the beta-blocker as the higher priority,” Dr. Greene said. “The bottom line is to keep it simple: four drugs, simultaneously or within 1 week, and prioritize initiation at low doses to maximize tolerability.”
The four-drug approach yields survival and rehospitalization benefits even when uptitrations don’t reach prespecified goals, Dr. Fonarow observed. The SGLT2 inhibitors are started and maintained at the same dosage. But for the other three agents, uptitration should aim for the highest well-tolerated level, up to the target, even if the highest tolerated is the initial dosage.
‘Challenging to generalize’
The goal in STRONG-HF was to start and at least partly uptitrate a beta-blocker, an MRA, and sacubitril-valsartan in the hospital and fully optimize their dosages within 2 weeks after discharge. Symptoms and laboratory biomarkers, including natriuretic peptides, were closely monitored at four in-person evaluations during the first 6 outpatient weeks.
But few believe the trial’s intensive drug regimen and postdischarge follow-up, as stipulated in the protocol, would be tolerated by current systems of care and reimbursement.
STRONG-HF “affirms the strategy in a rigorous, well conducted way,” Dr. Vaduganathan said, but would be “challenging to generalize to all health care systems.”
As a result, some in the field are “quick to almost disregard STRONG-HF in its entirety” and consider it “wishful thinking,” Dr. Greene said. Better that providers not become distracted by the precise details of its protocol.
At Duke, he said, “we see all our patients within 1 week of discharge to ensure they’re doing okay in terms of volume status and look for opportunities to escalate their guideline-directed medical therapy.”
But that can be done without in-person visits. A lot of the follow-up and uptitrations, Dr. Greene said, can be achieved by telephone or at virtual appointments in conjunction with regular laboratory testing. “That, I think, really is the path for the future, in this age when clinics are overwhelmed by in-person visits.”
Mildly reduced and preserved EF
STRONG-HF, in which patients were enrolled without regard to ejection fraction, suggests that its rapidly sequential drug regimen and intensive management protocol improves outcomes for patients with HF at any level of LVEF.
Those findings and others, along with DELIVER, EMPEROR-Preserved and other studies, make a tantalizing case for the quadruple drug approach in patients with HF and LVEF >40% – that is, those with mildly reduced (LVEF > 40% to < 50%, HFmrEF) or preserved LVEF > 50%, HFpEF) ejection fraction.
But the case isn’t solid enough to declare the four agents as core therapy for HF and LVEF > 40%, observed Dr. Vaduganathan. Currently, SGLT2 inhibitors “are the only drug class that we are routinely implementing” in HFmrEF and HFpEF.
There have been suggestions of clinical benefit for such patients with sacubitril-valsartan and MRAs, especially in PARAGON-HF and TOPCAT, respectively. The evidence is stronger in HFmrEF than in HFpEF, but in either case it’s weaker than the clear-cut trial support for SGLT2 inhibitors in those HF categories.
Trials also suggest that in HF with LVEF > 40%, clinical benefits from RAS inhibitors and MRAs taper off with increasing ejection fraction, especially into the > 60% range.
In both HFmrEF and HFpEF, “I routinely try to get the patient on an SGLT2 inhibitor rapidly and then treat with some of the other agents on a more individual basis,” Dr. Vaduganathan said. An LVEF in the HFmrEF range, for example, would likely call for the addition of an MRA and sacubitril-valsartan.
Dr. Packer said he would likely recommend all four agents for patients with HF and LVEF up to 60%, which he considers a more appropriate definition of HFrEF. Their clinical benefits appear consistent across that LVEF range, he said, although they thin out somewhat at the higher end.
Evidence supporting the four pillars in HF with LV > 40% and < 60% is weakest for beta-blockers, Dr. Packer noted, so arguably those drugs could be left out of the mix for patients with ejection fractions in that range.
Dr. Fonarow reported ties with Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Eli Lilly, Johnson & Johnson, Medtronic, Merck, Novartis, and Pfizer. Dr. Greene disclosed ties with Amgen, AstraZeneca, Bayer, Boehringer Ingelheim/Lilly, Bristol-Myers Squibb, Corteria, CSL Vifor, Cytokinetics, Lexicon Merck, Novartis, Pfizer, PharmaIN, Roche Diagnostics, Sanofi, scPharmaceuticals, Tricog Health, and Urovant Pharmaceuticals. Dr. Vaduganathan disclosed ties with American Regent, Amgen, AstraZeneca, Bayer AG, Baxter Healthcare, Boehringer Ingelheim, Chiesi, Cytokinetics, Galmed, Impulse Dynamics, Lexicon Pharmaceuticals, Merck, Novartis, Novo Nordisk, Occlutech, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health. Dr. Packer disclosed relationships with 89bio, AbbVie, Actavis, Amarin, Amgen, AstraZeneca, Attralus, Boehringer Ingelheim, Caladrius, Casana, CSL Behring, Cytokinetics, Imara, Lilly, Medtronic, Moderna, Novartis, Pharmacosmos, Reata, Regeneron, Relypsa, and Salamandra.
A version of this article first appeared on Medscape.com.




