User login
Heart failure risk reduced with higher levels of physical activity
Physical activity reduces heart failure risk, and the more exertion, the stronger the effect, according to a meta-analysis published online Oct. 5 in Circulation.
“Walking 30 minutes a day as recommended in the U.S. physical activity guidelines, may not be good enough – significantly more physical activity may be necessary to reduce the risk of heart failure,” Dr. Jarett D. Berry, senior author of the study, said in a press release.
Although physical inactivity has been implicated as a contributing factor in cardiovascular disease and coronary heart disease, a comprehensive analysis of the literature on the relationship of physical activity and heart failure risk has not previously been conducted, said Dr. Berry of the University of Texas Southwest Medical School in Dallas, and his colleagues. They conducted a meta-analysis of prospective cohort studies on the relationship of physical activity and heart failure in participants 18 years or older. They presented results by category of physical activity, using a random effects model, and by quantity of metabolic equivalent (MET)-minimum per week.
Twelve cohort studies with 20,203 heart failure events and 370,460 study participants during an average follow-up of 13 years were included in the meta-analysis.
Heart failure risk was 30% lower in the highest category of physical activity versus the lowest level of physical activity in a pooled analysis of hazard ratios. That risk was also reduced by 22% and 15% in moderate and light categories of physical activity, compared with the lowest, respectively (Circulation. 2015 Oct 5. doi: 10.1161/circulationaha.115.015853). All differences were statistically significant.
Subgroup analysis showed similar correlations between high levels of physical activity and reduced heart failure risk even with different ages, sex, and geographic locations.
As measured quantitatively, participants who engaged in the minimal recommended level of physical activity (500 MET mins/week) had a 10% lower risk of heart failure, compared with no physical activity, a 19% lower risk with twice the recommended level of physical activity, and a 35% lower risk with four times the recommended level of physical activity), all statistically significant differences.
Dr. Berry continued in the press release, “If you look at the general population, we’ve had tremendous success in reducing coronary heart disease over the last 30 years. But heart failure rates have not declined enough. The findings from the present study suggest that higher levels of physical activity may help combat this growing burden of heart failure.”
The authors report no conflicts of interest. Dr. Berry received funding from the Dedman Family Scholar in Clinical Care endowment at the University of Texas Southwestern Medical Center from the American Heart Association prevention network.
Dr. Berry received funding from the Dedman Family Scholar in Clinical Care endowment at the University of Texas Southwestern Medical Center from the American Heart Association prevention network. The authors report no conflicts of interest.
Physical activity reduces heart failure risk, and the more exertion, the stronger the effect, according to a meta-analysis published online Oct. 5 in Circulation.
“Walking 30 minutes a day as recommended in the U.S. physical activity guidelines, may not be good enough – significantly more physical activity may be necessary to reduce the risk of heart failure,” Dr. Jarett D. Berry, senior author of the study, said in a press release.
Although physical inactivity has been implicated as a contributing factor in cardiovascular disease and coronary heart disease, a comprehensive analysis of the literature on the relationship of physical activity and heart failure risk has not previously been conducted, said Dr. Berry of the University of Texas Southwest Medical School in Dallas, and his colleagues. They conducted a meta-analysis of prospective cohort studies on the relationship of physical activity and heart failure in participants 18 years or older. They presented results by category of physical activity, using a random effects model, and by quantity of metabolic equivalent (MET)-minimum per week.
Twelve cohort studies with 20,203 heart failure events and 370,460 study participants during an average follow-up of 13 years were included in the meta-analysis.
Heart failure risk was 30% lower in the highest category of physical activity versus the lowest level of physical activity in a pooled analysis of hazard ratios. That risk was also reduced by 22% and 15% in moderate and light categories of physical activity, compared with the lowest, respectively (Circulation. 2015 Oct 5. doi: 10.1161/circulationaha.115.015853). All differences were statistically significant.
Subgroup analysis showed similar correlations between high levels of physical activity and reduced heart failure risk even with different ages, sex, and geographic locations.
As measured quantitatively, participants who engaged in the minimal recommended level of physical activity (500 MET mins/week) had a 10% lower risk of heart failure, compared with no physical activity, a 19% lower risk with twice the recommended level of physical activity, and a 35% lower risk with four times the recommended level of physical activity), all statistically significant differences.
Dr. Berry continued in the press release, “If you look at the general population, we’ve had tremendous success in reducing coronary heart disease over the last 30 years. But heart failure rates have not declined enough. The findings from the present study suggest that higher levels of physical activity may help combat this growing burden of heart failure.”
The authors report no conflicts of interest. Dr. Berry received funding from the Dedman Family Scholar in Clinical Care endowment at the University of Texas Southwestern Medical Center from the American Heart Association prevention network.
Dr. Berry received funding from the Dedman Family Scholar in Clinical Care endowment at the University of Texas Southwestern Medical Center from the American Heart Association prevention network. The authors report no conflicts of interest.
Physical activity reduces heart failure risk, and the more exertion, the stronger the effect, according to a meta-analysis published online Oct. 5 in Circulation.
“Walking 30 minutes a day as recommended in the U.S. physical activity guidelines, may not be good enough – significantly more physical activity may be necessary to reduce the risk of heart failure,” Dr. Jarett D. Berry, senior author of the study, said in a press release.
Although physical inactivity has been implicated as a contributing factor in cardiovascular disease and coronary heart disease, a comprehensive analysis of the literature on the relationship of physical activity and heart failure risk has not previously been conducted, said Dr. Berry of the University of Texas Southwest Medical School in Dallas, and his colleagues. They conducted a meta-analysis of prospective cohort studies on the relationship of physical activity and heart failure in participants 18 years or older. They presented results by category of physical activity, using a random effects model, and by quantity of metabolic equivalent (MET)-minimum per week.
Twelve cohort studies with 20,203 heart failure events and 370,460 study participants during an average follow-up of 13 years were included in the meta-analysis.
Heart failure risk was 30% lower in the highest category of physical activity versus the lowest level of physical activity in a pooled analysis of hazard ratios. That risk was also reduced by 22% and 15% in moderate and light categories of physical activity, compared with the lowest, respectively (Circulation. 2015 Oct 5. doi: 10.1161/circulationaha.115.015853). All differences were statistically significant.
Subgroup analysis showed similar correlations between high levels of physical activity and reduced heart failure risk even with different ages, sex, and geographic locations.
As measured quantitatively, participants who engaged in the minimal recommended level of physical activity (500 MET mins/week) had a 10% lower risk of heart failure, compared with no physical activity, a 19% lower risk with twice the recommended level of physical activity, and a 35% lower risk with four times the recommended level of physical activity), all statistically significant differences.
Dr. Berry continued in the press release, “If you look at the general population, we’ve had tremendous success in reducing coronary heart disease over the last 30 years. But heart failure rates have not declined enough. The findings from the present study suggest that higher levels of physical activity may help combat this growing burden of heart failure.”
The authors report no conflicts of interest. Dr. Berry received funding from the Dedman Family Scholar in Clinical Care endowment at the University of Texas Southwestern Medical Center from the American Heart Association prevention network.
Dr. Berry received funding from the Dedman Family Scholar in Clinical Care endowment at the University of Texas Southwestern Medical Center from the American Heart Association prevention network. The authors report no conflicts of interest.
FROM CIRCULATION
Key clinical point: Heart failure risk and physical activity have an inverse, dose-response relationship.
Major finding: Participants who engaged in the minimal recommended level of physical activity had a 10% lower risk of heart failure, compared with no physical activity, a 19% lower risk with two times that level, and 35% lower risk with four times the level.
Data source: A meta-analysis of prospective cohort studies on the relationship of physical activity and heart failure in participants 18 years or older.
Disclosures: Dr. Berry received funding from the Dedman Family Scholar in Clinical Care endowment at the University of Texas Southwestern Medical Center from the American Heart Association prevention network. The authors report no conflicts of interest.
BLOG: Cardiologists quickly adopt SPRINT’s 120-mm Hg blood pressure target
Heart failure physicians were quick to embrace the sub–120 mm Hg systolic blood pressure target for both patients and at-risk people fewer than 3 weeks after the early-release news came out from the SPRINT trial that treating to this level produced a significant cut in cardiovascular disease events, compared with a less stringent systolic blood-pressure target of below 140 mm Hg.
At the annual meeting of the Heart Failure Society of America this week – one of the first cardiology gatherings held since the SPRINT announcement on Sept. 11 – at least 10 heart failure specialists offered their opinion at various times during the sessions on what the results have already meant for their practice. What was most striking was their unanimity in accepting this new blood pressure treatment target for heart failure patients and for people at increased risk for developing heart failure, and the rapidity at which they had come to their decision despite the absence so far of details about the trial’s results.
The unifying theme was that these physicians constituted a highly receptive audience for the SPRINT message, several of these specialists said in interviews. They appeared poised to pounce on a scientific rationale as soon as it became available to adopt a more aggressive blood pressure goal. It’s certainly no coincidence that most (if not all) these physicians now see uncontrolled hypertension as one of the top drivers of both heart failure onset and progression.
“We’ve all had some misgivings” about the systolic blood pressure targets set by the JNC 8 panel members last year of less than 150 mm Hg or less than 140 mm Hg (depending on age), and as a result of those misgivings, “I think people were ready to do a reverse and accept a new, lower target,” said Dr. Margaret M. Redfield, a heart failure specialist who was among those at the meeting who voiced endorsement for a new, sub–120 mm Hg target. “We don’t have the [SPRINT] data yet, but it’s very unusual for the NHLBI to release trial information ahead of time, so it must be very dramatic,” she added.
“The data and safety monitoring board had strict rules to govern early stopping,” said Dr. Clyde W. Yancy, a heart failure cardiologist and a member of the SPRINT data and safety monitoring panel. While careful not to prematurely provide any unreleased details of the SPRINT results, Dr. Yancy tried to frame what the September announcement meant in objective terms, while also rationalizing the quick uptake of the finding by so many of his colleagues.
“The decision to stop early must have been driven by some high level of evidence,” he told me, “You can infer a significant scientific rational” for a new target of less than 120 mm Hg “and you have to also surmise that there was no signal of harm.”
And, of course, some cardiologists had decided even before the SPRINT results came out in September that a lower target was better even without any evidence to back up that instinct.
“I love the 120s; a 140-mm Hg target has always been too high for me,” said Dr. Ileana L. Piña, a heart failure specialist at Montefiore Medical Center, Bronx, N.Y.
Another question, following so many endorsements of the 120-mm Hg goal, was whether these physicians believed people stood a good chance to reach this ambitious target.
“It’s a target; it’s not that everyone will get to it, but we need to apply the same rigor for blood pressure control as we use in heart failure clinics,” Dr. Redfield said. “We need to use a system” for patient support and monitoring and to promote adherence to treatment, “and keep pushing” patients to work toward their goal, she said.
Dr. Yancy predicted that a more aggressive blood pressure goal will help generate a stronger and more innovative infrastructure of drugs and monitoring tools to help patients get there.
“If a target blood pressure of 120/80 mm Hg gains credibility in guidelines, I think that industry will respond” with more combined drug formulations, new drugs, and innovative methods for easier blood pressure measurement, he said.
On Twitter @mitchelzoler
Heart failure physicians were quick to embrace the sub–120 mm Hg systolic blood pressure target for both patients and at-risk people fewer than 3 weeks after the early-release news came out from the SPRINT trial that treating to this level produced a significant cut in cardiovascular disease events, compared with a less stringent systolic blood-pressure target of below 140 mm Hg.
At the annual meeting of the Heart Failure Society of America this week – one of the first cardiology gatherings held since the SPRINT announcement on Sept. 11 – at least 10 heart failure specialists offered their opinion at various times during the sessions on what the results have already meant for their practice. What was most striking was their unanimity in accepting this new blood pressure treatment target for heart failure patients and for people at increased risk for developing heart failure, and the rapidity at which they had come to their decision despite the absence so far of details about the trial’s results.
The unifying theme was that these physicians constituted a highly receptive audience for the SPRINT message, several of these specialists said in interviews. They appeared poised to pounce on a scientific rationale as soon as it became available to adopt a more aggressive blood pressure goal. It’s certainly no coincidence that most (if not all) these physicians now see uncontrolled hypertension as one of the top drivers of both heart failure onset and progression.
“We’ve all had some misgivings” about the systolic blood pressure targets set by the JNC 8 panel members last year of less than 150 mm Hg or less than 140 mm Hg (depending on age), and as a result of those misgivings, “I think people were ready to do a reverse and accept a new, lower target,” said Dr. Margaret M. Redfield, a heart failure specialist who was among those at the meeting who voiced endorsement for a new, sub–120 mm Hg target. “We don’t have the [SPRINT] data yet, but it’s very unusual for the NHLBI to release trial information ahead of time, so it must be very dramatic,” she added.
“The data and safety monitoring board had strict rules to govern early stopping,” said Dr. Clyde W. Yancy, a heart failure cardiologist and a member of the SPRINT data and safety monitoring panel. While careful not to prematurely provide any unreleased details of the SPRINT results, Dr. Yancy tried to frame what the September announcement meant in objective terms, while also rationalizing the quick uptake of the finding by so many of his colleagues.
“The decision to stop early must have been driven by some high level of evidence,” he told me, “You can infer a significant scientific rational” for a new target of less than 120 mm Hg “and you have to also surmise that there was no signal of harm.”
And, of course, some cardiologists had decided even before the SPRINT results came out in September that a lower target was better even without any evidence to back up that instinct.
“I love the 120s; a 140-mm Hg target has always been too high for me,” said Dr. Ileana L. Piña, a heart failure specialist at Montefiore Medical Center, Bronx, N.Y.
Another question, following so many endorsements of the 120-mm Hg goal, was whether these physicians believed people stood a good chance to reach this ambitious target.
“It’s a target; it’s not that everyone will get to it, but we need to apply the same rigor for blood pressure control as we use in heart failure clinics,” Dr. Redfield said. “We need to use a system” for patient support and monitoring and to promote adherence to treatment, “and keep pushing” patients to work toward their goal, she said.
Dr. Yancy predicted that a more aggressive blood pressure goal will help generate a stronger and more innovative infrastructure of drugs and monitoring tools to help patients get there.
“If a target blood pressure of 120/80 mm Hg gains credibility in guidelines, I think that industry will respond” with more combined drug formulations, new drugs, and innovative methods for easier blood pressure measurement, he said.
On Twitter @mitchelzoler
Heart failure physicians were quick to embrace the sub–120 mm Hg systolic blood pressure target for both patients and at-risk people fewer than 3 weeks after the early-release news came out from the SPRINT trial that treating to this level produced a significant cut in cardiovascular disease events, compared with a less stringent systolic blood-pressure target of below 140 mm Hg.
At the annual meeting of the Heart Failure Society of America this week – one of the first cardiology gatherings held since the SPRINT announcement on Sept. 11 – at least 10 heart failure specialists offered their opinion at various times during the sessions on what the results have already meant for their practice. What was most striking was their unanimity in accepting this new blood pressure treatment target for heart failure patients and for people at increased risk for developing heart failure, and the rapidity at which they had come to their decision despite the absence so far of details about the trial’s results.
The unifying theme was that these physicians constituted a highly receptive audience for the SPRINT message, several of these specialists said in interviews. They appeared poised to pounce on a scientific rationale as soon as it became available to adopt a more aggressive blood pressure goal. It’s certainly no coincidence that most (if not all) these physicians now see uncontrolled hypertension as one of the top drivers of both heart failure onset and progression.
“We’ve all had some misgivings” about the systolic blood pressure targets set by the JNC 8 panel members last year of less than 150 mm Hg or less than 140 mm Hg (depending on age), and as a result of those misgivings, “I think people were ready to do a reverse and accept a new, lower target,” said Dr. Margaret M. Redfield, a heart failure specialist who was among those at the meeting who voiced endorsement for a new, sub–120 mm Hg target. “We don’t have the [SPRINT] data yet, but it’s very unusual for the NHLBI to release trial information ahead of time, so it must be very dramatic,” she added.
“The data and safety monitoring board had strict rules to govern early stopping,” said Dr. Clyde W. Yancy, a heart failure cardiologist and a member of the SPRINT data and safety monitoring panel. While careful not to prematurely provide any unreleased details of the SPRINT results, Dr. Yancy tried to frame what the September announcement meant in objective terms, while also rationalizing the quick uptake of the finding by so many of his colleagues.
“The decision to stop early must have been driven by some high level of evidence,” he told me, “You can infer a significant scientific rational” for a new target of less than 120 mm Hg “and you have to also surmise that there was no signal of harm.”
And, of course, some cardiologists had decided even before the SPRINT results came out in September that a lower target was better even without any evidence to back up that instinct.
“I love the 120s; a 140-mm Hg target has always been too high for me,” said Dr. Ileana L. Piña, a heart failure specialist at Montefiore Medical Center, Bronx, N.Y.
Another question, following so many endorsements of the 120-mm Hg goal, was whether these physicians believed people stood a good chance to reach this ambitious target.
“It’s a target; it’s not that everyone will get to it, but we need to apply the same rigor for blood pressure control as we use in heart failure clinics,” Dr. Redfield said. “We need to use a system” for patient support and monitoring and to promote adherence to treatment, “and keep pushing” patients to work toward their goal, she said.
Dr. Yancy predicted that a more aggressive blood pressure goal will help generate a stronger and more innovative infrastructure of drugs and monitoring tools to help patients get there.
“If a target blood pressure of 120/80 mm Hg gains credibility in guidelines, I think that industry will respond” with more combined drug formulations, new drugs, and innovative methods for easier blood pressure measurement, he said.
On Twitter @mitchelzoler
ESC: Mechanical dyssynchrony in narrow-QRS heart failure spells trouble
LONDON – Persistent mechanical dyssynchrony in patients with heart failure with reduced ejection fraction and a narrow QRS width appears to be a new marker of heightened risk.
That’s the key take-away message of an update of the EchoCRT trial presented by Dr. John Gorcsan III at the annual congress of the European Society of Cardiology.
EchoCRT was a large, multicenter randomized trial of cardiac resynchronization therapy (CRT) in patients with severely symptomatic heart failure with a QRS width of less than 130 ms, a left ventricular ejection fraction of 35% or less, and echocardiographic evidence of dyssynchrony. It was a negative study, with no improvement in rates of death or heart failure hospitalizations noted with CRT turned on versus off (N Engl J Med. 2013 Oct 10;369[15]:1395-405).
However, this still left open the question of the clinical significance of mechanical dyssynchrony in such patients. Dr. Gorcsan and coinvestigators conducted a secondary subgroup analysis of 614 EchoCRT participants with baseline and 6-month echocardiograms in order to provide the answer.
“Our hypothesis was that persistent or worsening dyssynchrony is associated with unfavorable outcomes,” explained Dr. Gorcsan, professor of medicine and director of echocardiography at the University of Pittsburgh.
This indeed turned out to be the case. Three-quarters of patients experienced persistent or worsening dyssynchrony as measured by tissue Doppler or speckle-tracking radial strain delay during 6 months of follow-up, and they were 1.54-fold more likely to experience the combined primary endpoint of death or heart failure hospitalization, compared with the 25% of patients who experienced improvement in their dyssynchrony.
Moreover, even after statistical adjustment for potential confounders including baseline QRS width, ejection fraction, and left ventricular end-diastolic diameter, persistent or worsening dyssynchrony at 6 months remained associated with a 1.57-fold increased likelihood of heart failure hospitalization.
CRT being turned on or off had no impact on whether a patient’s dyssynchrony improved or not during follow-up.
“We hypothesize that a reason for improvement in dyssynchrony may be in part due to favorable left ventricular reverse remodeling – 97% of patients were on a beta-blocker and 95% were on an ACE inhibitor or angiotensin receptor blocker – but the precise mechanism remains uncertain,” Dr. Gorcsan observed.
Simultaneous with Dr. Gorcsan’s presentation, the clinical update of the EchoCRT trial was published online (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv418).
In an accompanying editorial, Dr. Amil M. Shah and Dr. Scott D. Solomon of Brigham and Women’s Hospital, Boston, said the new EchoCRT findings suggest mechanical dyssynchrony is a risk factor – a marker of progressive contractile dysfunction – but not a viable treatment target. That’s worth bearing in mind because mechanical dyssynchrony is now under consideration as a potential therapeutic target in patients with heart failure with preserved ejection fraction and other populations with conditions other than those addressed in EchoCRT (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv458).
The EchoCRT trial was sponsored by Biotronik. Dr. Gorcsan reported receiving research grants from Biotronik, GE, Medtronic, and St. Jude.
LONDON – Persistent mechanical dyssynchrony in patients with heart failure with reduced ejection fraction and a narrow QRS width appears to be a new marker of heightened risk.
That’s the key take-away message of an update of the EchoCRT trial presented by Dr. John Gorcsan III at the annual congress of the European Society of Cardiology.
EchoCRT was a large, multicenter randomized trial of cardiac resynchronization therapy (CRT) in patients with severely symptomatic heart failure with a QRS width of less than 130 ms, a left ventricular ejection fraction of 35% or less, and echocardiographic evidence of dyssynchrony. It was a negative study, with no improvement in rates of death or heart failure hospitalizations noted with CRT turned on versus off (N Engl J Med. 2013 Oct 10;369[15]:1395-405).
However, this still left open the question of the clinical significance of mechanical dyssynchrony in such patients. Dr. Gorcsan and coinvestigators conducted a secondary subgroup analysis of 614 EchoCRT participants with baseline and 6-month echocardiograms in order to provide the answer.
“Our hypothesis was that persistent or worsening dyssynchrony is associated with unfavorable outcomes,” explained Dr. Gorcsan, professor of medicine and director of echocardiography at the University of Pittsburgh.
This indeed turned out to be the case. Three-quarters of patients experienced persistent or worsening dyssynchrony as measured by tissue Doppler or speckle-tracking radial strain delay during 6 months of follow-up, and they were 1.54-fold more likely to experience the combined primary endpoint of death or heart failure hospitalization, compared with the 25% of patients who experienced improvement in their dyssynchrony.
Moreover, even after statistical adjustment for potential confounders including baseline QRS width, ejection fraction, and left ventricular end-diastolic diameter, persistent or worsening dyssynchrony at 6 months remained associated with a 1.57-fold increased likelihood of heart failure hospitalization.
CRT being turned on or off had no impact on whether a patient’s dyssynchrony improved or not during follow-up.
“We hypothesize that a reason for improvement in dyssynchrony may be in part due to favorable left ventricular reverse remodeling – 97% of patients were on a beta-blocker and 95% were on an ACE inhibitor or angiotensin receptor blocker – but the precise mechanism remains uncertain,” Dr. Gorcsan observed.
Simultaneous with Dr. Gorcsan’s presentation, the clinical update of the EchoCRT trial was published online (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv418).
In an accompanying editorial, Dr. Amil M. Shah and Dr. Scott D. Solomon of Brigham and Women’s Hospital, Boston, said the new EchoCRT findings suggest mechanical dyssynchrony is a risk factor – a marker of progressive contractile dysfunction – but not a viable treatment target. That’s worth bearing in mind because mechanical dyssynchrony is now under consideration as a potential therapeutic target in patients with heart failure with preserved ejection fraction and other populations with conditions other than those addressed in EchoCRT (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv458).
The EchoCRT trial was sponsored by Biotronik. Dr. Gorcsan reported receiving research grants from Biotronik, GE, Medtronic, and St. Jude.
LONDON – Persistent mechanical dyssynchrony in patients with heart failure with reduced ejection fraction and a narrow QRS width appears to be a new marker of heightened risk.
That’s the key take-away message of an update of the EchoCRT trial presented by Dr. John Gorcsan III at the annual congress of the European Society of Cardiology.
EchoCRT was a large, multicenter randomized trial of cardiac resynchronization therapy (CRT) in patients with severely symptomatic heart failure with a QRS width of less than 130 ms, a left ventricular ejection fraction of 35% or less, and echocardiographic evidence of dyssynchrony. It was a negative study, with no improvement in rates of death or heart failure hospitalizations noted with CRT turned on versus off (N Engl J Med. 2013 Oct 10;369[15]:1395-405).
However, this still left open the question of the clinical significance of mechanical dyssynchrony in such patients. Dr. Gorcsan and coinvestigators conducted a secondary subgroup analysis of 614 EchoCRT participants with baseline and 6-month echocardiograms in order to provide the answer.
“Our hypothesis was that persistent or worsening dyssynchrony is associated with unfavorable outcomes,” explained Dr. Gorcsan, professor of medicine and director of echocardiography at the University of Pittsburgh.
This indeed turned out to be the case. Three-quarters of patients experienced persistent or worsening dyssynchrony as measured by tissue Doppler or speckle-tracking radial strain delay during 6 months of follow-up, and they were 1.54-fold more likely to experience the combined primary endpoint of death or heart failure hospitalization, compared with the 25% of patients who experienced improvement in their dyssynchrony.
Moreover, even after statistical adjustment for potential confounders including baseline QRS width, ejection fraction, and left ventricular end-diastolic diameter, persistent or worsening dyssynchrony at 6 months remained associated with a 1.57-fold increased likelihood of heart failure hospitalization.
CRT being turned on or off had no impact on whether a patient’s dyssynchrony improved or not during follow-up.
“We hypothesize that a reason for improvement in dyssynchrony may be in part due to favorable left ventricular reverse remodeling – 97% of patients were on a beta-blocker and 95% were on an ACE inhibitor or angiotensin receptor blocker – but the precise mechanism remains uncertain,” Dr. Gorcsan observed.
Simultaneous with Dr. Gorcsan’s presentation, the clinical update of the EchoCRT trial was published online (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv418).
In an accompanying editorial, Dr. Amil M. Shah and Dr. Scott D. Solomon of Brigham and Women’s Hospital, Boston, said the new EchoCRT findings suggest mechanical dyssynchrony is a risk factor – a marker of progressive contractile dysfunction – but not a viable treatment target. That’s worth bearing in mind because mechanical dyssynchrony is now under consideration as a potential therapeutic target in patients with heart failure with preserved ejection fraction and other populations with conditions other than those addressed in EchoCRT (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv458).
The EchoCRT trial was sponsored by Biotronik. Dr. Gorcsan reported receiving research grants from Biotronik, GE, Medtronic, and St. Jude.
AT THE ESC CONGRESS 2015
Key clinical point: Persistent mechanical dyssynchrony in patients with heart failure and a narrow QRS interval appears to be a risk factor for poor outcomes rather than a modifiable therapeutic target.
Major finding: Patients with heart failure, narrow QRS width, and mechanical dyssynchrony whose dyssynchrony persisted or worsened during 6 months of follow-up were 54% more likely to experience death or heart failure hospitalization.
Data source: A secondary analysis of the large, multicenter, prospective, EchoCRT trial, which randomized patients to cardiac resynchronization therapy turned on or off.
Disclosures: The EchoCRT trial was sponsored by Biotronik. The study presenter reported receiving research grants from Biotronik, GE, Medtronic, and St. Jude.
ESC: Mechanical dyssynchrony in narrow-QRS heart failure spells trouble
LONDON – Persistent mechanical dyssynchrony in patients with heart failure with reduced ejection fraction and a narrow QRS width appears to be a new marker of heightened risk.
That’s the key take-away message of an update of the EchoCRT trial presented by Dr. John Gorcsan III at the annual congress of the European Society of Cardiology.
EchoCRT was a large, multicenter randomized trial of cardiac resynchronization therapy (CRT) in patients with severely symptomatic heart failure with a QRS width of less than 130 ms, a left ventricular ejection fraction of 35% or less, and echocardiographic evidence of dyssynchrony. It was a negative study, with no improvement in rates of death or heart failure hospitalizations noted with CRT turned on versus off (N Engl J Med. 2013 Oct 10;369[15]:1395-405).
However, this still left open the question of the clinical significance of mechanical dyssynchrony in such patients. Dr. Gorcsan and coinvestigators conducted a secondary subgroup analysis of 614 EchoCRT participants with baseline and 6-month echocardiograms in order to provide the answer.
“Our hypothesis was that persistent or worsening dyssynchrony is associated with unfavorable outcomes,” explained Dr. Gorcsan, professor of medicine and director of echocardiography at the University of Pittsburgh.
This indeed turned out to be the case. Three-quarters of patients experienced persistent or worsening dyssynchrony as measured by tissue Doppler or speckle-tracking radial strain delay during 6 months of follow-up, and they were 1.54-fold more likely to experience the combined primary endpoint of death or heart failure hospitalization, compared with the 25% of patients who experienced improvement in their dyssynchrony.
Moreover, even after statistical adjustment for potential confounders including baseline QRS width, ejection fraction, and left ventricular end-diastolic diameter, persistent or worsening dyssynchrony at 6 months remained associated with a 1.57-fold increased likelihood of heart failure hospitalization.
CRT being turned on or off had no impact on whether a patient’s dyssynchrony improved or not during follow-up.
“We hypothesize that a reason for improvement in dyssynchrony may be in part due to favorable left ventricular reverse remodeling – 97% of patients were on a beta-blocker and 95% were on an ACE inhibitor or angiotensin receptor blocker – but the precise mechanism remains uncertain,” Dr. Gorcsan observed.
Simultaneous with Dr. Gorcsan’s presentation, the clinical update of the EchoCRT trial was published online (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv418).
In an accompanying editorial, Dr. Amil M. Shah and Dr. Scott D. Solomon of Brigham and Women’s Hospital, Boston, said the new EchoCRT findings suggest mechanical dyssynchrony is a risk factor – a marker of progressive contractile dysfunction – but not a viable treatment target. That’s worth bearing in mind because mechanical dyssynchrony is now under consideration as a potential therapeutic target in patients with heart failure with preserved ejection fraction and other populations with conditions other than those addressed in EchoCRT (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv458).
The EchoCRT trial was sponsored by Biotronik. Dr. Gorcsan reported receiving research grants from Biotronik, GE, Medtronic, and St. Jude.
LONDON – Persistent mechanical dyssynchrony in patients with heart failure with reduced ejection fraction and a narrow QRS width appears to be a new marker of heightened risk.
That’s the key take-away message of an update of the EchoCRT trial presented by Dr. John Gorcsan III at the annual congress of the European Society of Cardiology.
EchoCRT was a large, multicenter randomized trial of cardiac resynchronization therapy (CRT) in patients with severely symptomatic heart failure with a QRS width of less than 130 ms, a left ventricular ejection fraction of 35% or less, and echocardiographic evidence of dyssynchrony. It was a negative study, with no improvement in rates of death or heart failure hospitalizations noted with CRT turned on versus off (N Engl J Med. 2013 Oct 10;369[15]:1395-405).
However, this still left open the question of the clinical significance of mechanical dyssynchrony in such patients. Dr. Gorcsan and coinvestigators conducted a secondary subgroup analysis of 614 EchoCRT participants with baseline and 6-month echocardiograms in order to provide the answer.
“Our hypothesis was that persistent or worsening dyssynchrony is associated with unfavorable outcomes,” explained Dr. Gorcsan, professor of medicine and director of echocardiography at the University of Pittsburgh.
This indeed turned out to be the case. Three-quarters of patients experienced persistent or worsening dyssynchrony as measured by tissue Doppler or speckle-tracking radial strain delay during 6 months of follow-up, and they were 1.54-fold more likely to experience the combined primary endpoint of death or heart failure hospitalization, compared with the 25% of patients who experienced improvement in their dyssynchrony.
Moreover, even after statistical adjustment for potential confounders including baseline QRS width, ejection fraction, and left ventricular end-diastolic diameter, persistent or worsening dyssynchrony at 6 months remained associated with a 1.57-fold increased likelihood of heart failure hospitalization.
CRT being turned on or off had no impact on whether a patient’s dyssynchrony improved or not during follow-up.
“We hypothesize that a reason for improvement in dyssynchrony may be in part due to favorable left ventricular reverse remodeling – 97% of patients were on a beta-blocker and 95% were on an ACE inhibitor or angiotensin receptor blocker – but the precise mechanism remains uncertain,” Dr. Gorcsan observed.
Simultaneous with Dr. Gorcsan’s presentation, the clinical update of the EchoCRT trial was published online (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv418).
In an accompanying editorial, Dr. Amil M. Shah and Dr. Scott D. Solomon of Brigham and Women’s Hospital, Boston, said the new EchoCRT findings suggest mechanical dyssynchrony is a risk factor – a marker of progressive contractile dysfunction – but not a viable treatment target. That’s worth bearing in mind because mechanical dyssynchrony is now under consideration as a potential therapeutic target in patients with heart failure with preserved ejection fraction and other populations with conditions other than those addressed in EchoCRT (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv458).
The EchoCRT trial was sponsored by Biotronik. Dr. Gorcsan reported receiving research grants from Biotronik, GE, Medtronic, and St. Jude.
LONDON – Persistent mechanical dyssynchrony in patients with heart failure with reduced ejection fraction and a narrow QRS width appears to be a new marker of heightened risk.
That’s the key take-away message of an update of the EchoCRT trial presented by Dr. John Gorcsan III at the annual congress of the European Society of Cardiology.
EchoCRT was a large, multicenter randomized trial of cardiac resynchronization therapy (CRT) in patients with severely symptomatic heart failure with a QRS width of less than 130 ms, a left ventricular ejection fraction of 35% or less, and echocardiographic evidence of dyssynchrony. It was a negative study, with no improvement in rates of death or heart failure hospitalizations noted with CRT turned on versus off (N Engl J Med. 2013 Oct 10;369[15]:1395-405).
However, this still left open the question of the clinical significance of mechanical dyssynchrony in such patients. Dr. Gorcsan and coinvestigators conducted a secondary subgroup analysis of 614 EchoCRT participants with baseline and 6-month echocardiograms in order to provide the answer.
“Our hypothesis was that persistent or worsening dyssynchrony is associated with unfavorable outcomes,” explained Dr. Gorcsan, professor of medicine and director of echocardiography at the University of Pittsburgh.
This indeed turned out to be the case. Three-quarters of patients experienced persistent or worsening dyssynchrony as measured by tissue Doppler or speckle-tracking radial strain delay during 6 months of follow-up, and they were 1.54-fold more likely to experience the combined primary endpoint of death or heart failure hospitalization, compared with the 25% of patients who experienced improvement in their dyssynchrony.
Moreover, even after statistical adjustment for potential confounders including baseline QRS width, ejection fraction, and left ventricular end-diastolic diameter, persistent or worsening dyssynchrony at 6 months remained associated with a 1.57-fold increased likelihood of heart failure hospitalization.
CRT being turned on or off had no impact on whether a patient’s dyssynchrony improved or not during follow-up.
“We hypothesize that a reason for improvement in dyssynchrony may be in part due to favorable left ventricular reverse remodeling – 97% of patients were on a beta-blocker and 95% were on an ACE inhibitor or angiotensin receptor blocker – but the precise mechanism remains uncertain,” Dr. Gorcsan observed.
Simultaneous with Dr. Gorcsan’s presentation, the clinical update of the EchoCRT trial was published online (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv418).
In an accompanying editorial, Dr. Amil M. Shah and Dr. Scott D. Solomon of Brigham and Women’s Hospital, Boston, said the new EchoCRT findings suggest mechanical dyssynchrony is a risk factor – a marker of progressive contractile dysfunction – but not a viable treatment target. That’s worth bearing in mind because mechanical dyssynchrony is now under consideration as a potential therapeutic target in patients with heart failure with preserved ejection fraction and other populations with conditions other than those addressed in EchoCRT (Eur Heart J. 2015 Aug 30. doi: 10.1093/eurheartj/ehv458).
The EchoCRT trial was sponsored by Biotronik. Dr. Gorcsan reported receiving research grants from Biotronik, GE, Medtronic, and St. Jude.
AT THE ESC CONGRESS 2015
Key clinical point: Persistent mechanical dyssynchrony in patients with heart failure and a narrow QRS interval appears to be a risk factor for poor outcomes rather than a modifiable therapeutic target.
Major finding: Patients with heart failure, narrow QRS width, and mechanical dyssynchrony whose dyssynchrony persisted or worsened during 6 months of follow-up were 54% more likely to experience death or heart failure hospitalization.
Data source: A secondary analysis of the large, multicenter, prospective, EchoCRT trial, which randomized patients to cardiac resynchronization therapy turned on or off.
Disclosures: The EchoCRT trial was sponsored by Biotronik. The study presenter reported receiving research grants from Biotronik, GE, Medtronic, and St. Jude.
ESC: Heart failure patients have increased cancer incidence
LONDON – Adult patients with chronic heart failure had an average 51% increased incidence of all forms of cancer, compared with the general population in an analysis of more than 9,000 Danish patients treated for heart failure during 2002-2009.
Although the study did not address causality, the elevated risk for new-onset cancers in heart failure patients may stem from shared risk factors for the two diseases, Dr. Ann Banke said at the annual congress of the European Society of Cardiology. Smoking, for example, is a potential etiologic factor for both heart failure and certain cancers. Other potential shared risk factors could include other types of unhealthy lifestyle choices, and a heightened inflammatory state, suggested Dr. Banke, a researcher in the department of cardiology at Odense (Denmark) University Hospital.
“Increased awareness of the risk for development of cancer in heart failure patients is warranted,” especially given the relatively long life expectancy now seen among many patients with chronic heart failure, Dr. Banke said.
She and her associates reviewed 9,307 Danish patients with chronic heart failure and no history of cancer who entered a national heart failure cohort during 2002-2009. Their average age was 68 years, 73% were men, 89% had heart failure with reduced ejection fraction, 80% had New York Heart Association class I or II heart failure, and 15% had diabetes. During follow-up through 2013, a total of 975 of these patients received a diagnosis of cancer. The researchers compared the incidence rate of cancer among these heart failure patients with the rate in the general Danish adult population in the same 2002-2013 period, during which the general population developed 330,843 cancers.
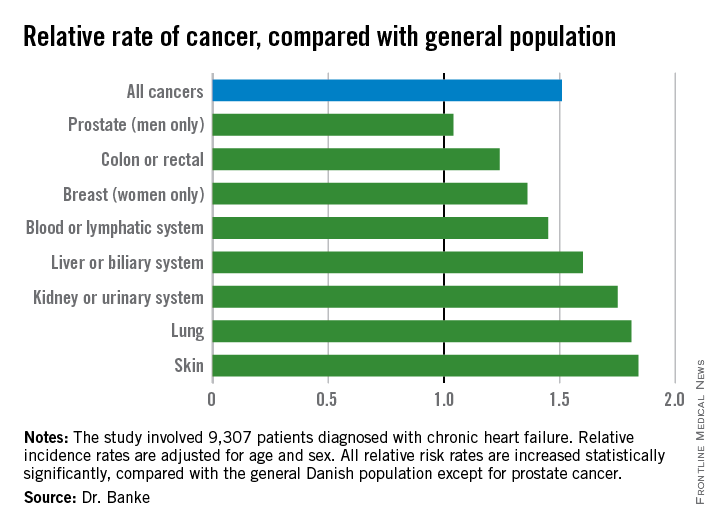
In an analysis that adjusted for age and sex, the heart failure patients had a statistically significant 51% increased rate of incidence cancers of all types (P less than .0001). When broken down by cancer site, the heart failure patients showed significantly increased rates for all types of cancers except prostate cancer (see chart). Dr. Banke speculated that the absence of an increased incidence rate in prostate cancer among heart failure patients may reflect the age of the heart failure patients, or perhaps because prostate cancer does not share as many risk factors with heart failure as do the other cancer types.
On Twitter @mitchelzoler
LONDON – Adult patients with chronic heart failure had an average 51% increased incidence of all forms of cancer, compared with the general population in an analysis of more than 9,000 Danish patients treated for heart failure during 2002-2009.
Although the study did not address causality, the elevated risk for new-onset cancers in heart failure patients may stem from shared risk factors for the two diseases, Dr. Ann Banke said at the annual congress of the European Society of Cardiology. Smoking, for example, is a potential etiologic factor for both heart failure and certain cancers. Other potential shared risk factors could include other types of unhealthy lifestyle choices, and a heightened inflammatory state, suggested Dr. Banke, a researcher in the department of cardiology at Odense (Denmark) University Hospital.
“Increased awareness of the risk for development of cancer in heart failure patients is warranted,” especially given the relatively long life expectancy now seen among many patients with chronic heart failure, Dr. Banke said.
She and her associates reviewed 9,307 Danish patients with chronic heart failure and no history of cancer who entered a national heart failure cohort during 2002-2009. Their average age was 68 years, 73% were men, 89% had heart failure with reduced ejection fraction, 80% had New York Heart Association class I or II heart failure, and 15% had diabetes. During follow-up through 2013, a total of 975 of these patients received a diagnosis of cancer. The researchers compared the incidence rate of cancer among these heart failure patients with the rate in the general Danish adult population in the same 2002-2013 period, during which the general population developed 330,843 cancers.
In an analysis that adjusted for age and sex, the heart failure patients had a statistically significant 51% increased rate of incidence cancers of all types (P less than .0001). When broken down by cancer site, the heart failure patients showed significantly increased rates for all types of cancers except prostate cancer (see chart). Dr. Banke speculated that the absence of an increased incidence rate in prostate cancer among heart failure patients may reflect the age of the heart failure patients, or perhaps because prostate cancer does not share as many risk factors with heart failure as do the other cancer types.

On Twitter @mitchelzoler
LONDON – Adult patients with chronic heart failure had an average 51% increased incidence of all forms of cancer, compared with the general population in an analysis of more than 9,000 Danish patients treated for heart failure during 2002-2009.
Although the study did not address causality, the elevated risk for new-onset cancers in heart failure patients may stem from shared risk factors for the two diseases, Dr. Ann Banke said at the annual congress of the European Society of Cardiology. Smoking, for example, is a potential etiologic factor for both heart failure and certain cancers. Other potential shared risk factors could include other types of unhealthy lifestyle choices, and a heightened inflammatory state, suggested Dr. Banke, a researcher in the department of cardiology at Odense (Denmark) University Hospital.
“Increased awareness of the risk for development of cancer in heart failure patients is warranted,” especially given the relatively long life expectancy now seen among many patients with chronic heart failure, Dr. Banke said.
She and her associates reviewed 9,307 Danish patients with chronic heart failure and no history of cancer who entered a national heart failure cohort during 2002-2009. Their average age was 68 years, 73% were men, 89% had heart failure with reduced ejection fraction, 80% had New York Heart Association class I or II heart failure, and 15% had diabetes. During follow-up through 2013, a total of 975 of these patients received a diagnosis of cancer. The researchers compared the incidence rate of cancer among these heart failure patients with the rate in the general Danish adult population in the same 2002-2013 period, during which the general population developed 330,843 cancers.
In an analysis that adjusted for age and sex, the heart failure patients had a statistically significant 51% increased rate of incidence cancers of all types (P less than .0001). When broken down by cancer site, the heart failure patients showed significantly increased rates for all types of cancers except prostate cancer (see chart). Dr. Banke speculated that the absence of an increased incidence rate in prostate cancer among heart failure patients may reflect the age of the heart failure patients, or perhaps because prostate cancer does not share as many risk factors with heart failure as do the other cancer types.

On Twitter @mitchelzoler
AT THE ESC CONGRESS 2015
Key clinical point: Adults with chronic heart failure had a significantly increased incidence of cancer, compared with the general population.
Major finding: Patients with chronic heart failure had an average 51% higher incidence of cancer, compared with the general population.
Data source: A nationwide Danish cohort with 9,307 patients diagnosed with chronic heart failure.
Disclosures: Dr. Banke had no disclosures.
HFSA: In heart failure, beta-blocker dosage trumps heart rate
NATIONAL HARBOR, MD. – In beta-blocker treatment of heart failure, dose matters; heart rate, not so much.
When using beta-blockers to treat patients with heart failure and reduced ejection fraction, administering a high, evidence-backed dosage of the drug had much more beneficial impact than did getting patients to a low heart rate, according to a post hoc analysis of data from more than 2,000 patients.
The finding provides new evidence supporting “titrating the beta-blocker to evidence-based, high dosages of a beta-blocker and not holding back,” Mona Fiuzat, Ph.D., said in an interview at the annual scientific meeting of the Heart Failure Society of America. “It’s not just lowering the heart rate; there is something else, other mechanisms [of beta-blocker effects] that may be of greater benefit” to patients beyond heart-rate reduction when patients they receive the full dosage of beta-blocker treatment that had been shown effective in the randomized trials run about 2 decades ago.
“The point of our report is that if you have [heart failure with reduced ejection fraction] patients on a beta-blocker, they will be better off by increasing the dose” to the target level rather than by adding an additional, non–beta-blocker drug, such as ivabradine (Corlanor), to further reduce heart rate, said Dr. Fiuzat, a clinical pharmacologist and heart-failure researcher at Duke University in Durham, N.C. In the trial that enrolled the 2,320 patients included in the current analysis, half the patients were not on their target beta-blocker dosage.
Some clinicians are too heart-rate focused, she said. “Our results show that there is added benefit from using the full beta-blocker dosage even when the patient’s heart rate is already low.”
The analysis run by Dr. Fiuzat and her associates used data collected in HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training), a randomized, controlled study that assessed the impact of exercise training on patients with heart failure with reduced ejection fraction (JAMA 2009 Apr 8;301[14]:1439-50). All patients received usual care (and the intervention group also received exercise training), which meant they were on whichever beta-blocker and dosage their individual physician prescribed.
The current analysis converted these baseline dosages into carvedilol (Coreg) equivalents and identified the dosage levels as either low, defined as a daily carvedilol equivalent of less than 25 mg/day, or high, a daily equivalent of at least 25 mg/day. The analysis also categorized heart rate at baseline into a high rate defined as at least 70 beats/min, and a low rate of less than 70 beats/min. At entry, 71% of patients were on a high beta-blocker dosage (although in many cases not their full, ideal dosage), and 52% of patients had a high heart rate.
The new analysis showed that after a median follow-up of 2.5 years, the rate of all-cause death and all-cause hospitalization was significantly higher among patients who entered the study on a low beta-blocker dosage and with a high heart rate compared with patients in the other three groups: low dosage/low heart rate, high dosage/high heart rate, and high dosage/low heart rate. In addition, the results showed that a high beta-blocker dosage at baseline mitigated the impact of a high heart rate; patients in this subgroup had outcomes comparable to those of patients on a high beta-blocker dosage with a low heart rate.
In an analysis that adjusted for baseline differences in several clinical and demographic variables, patients on a high beta-blocker dosage had a statistically significant 13% reduced rate of all-cause death or all-cause hospitalization compared with patients on a low-dosage beta-blocker. In contrast, baseline heart rate had no statistically significant impact on outcomes, Dr. Fiuzat and her associates reported in an article published online concurrently with the presentation of the results at the meeting by one of Dr. Fiuzat’s colleagues (JACC: Heart Failure 2015. doi: 10.1016/j.jchf.2015.09.002]).
On Twitter @mitchelzoler
NATIONAL HARBOR, MD. – In beta-blocker treatment of heart failure, dose matters; heart rate, not so much.
When using beta-blockers to treat patients with heart failure and reduced ejection fraction, administering a high, evidence-backed dosage of the drug had much more beneficial impact than did getting patients to a low heart rate, according to a post hoc analysis of data from more than 2,000 patients.
The finding provides new evidence supporting “titrating the beta-blocker to evidence-based, high dosages of a beta-blocker and not holding back,” Mona Fiuzat, Ph.D., said in an interview at the annual scientific meeting of the Heart Failure Society of America. “It’s not just lowering the heart rate; there is something else, other mechanisms [of beta-blocker effects] that may be of greater benefit” to patients beyond heart-rate reduction when patients they receive the full dosage of beta-blocker treatment that had been shown effective in the randomized trials run about 2 decades ago.
“The point of our report is that if you have [heart failure with reduced ejection fraction] patients on a beta-blocker, they will be better off by increasing the dose” to the target level rather than by adding an additional, non–beta-blocker drug, such as ivabradine (Corlanor), to further reduce heart rate, said Dr. Fiuzat, a clinical pharmacologist and heart-failure researcher at Duke University in Durham, N.C. In the trial that enrolled the 2,320 patients included in the current analysis, half the patients were not on their target beta-blocker dosage.
Some clinicians are too heart-rate focused, she said. “Our results show that there is added benefit from using the full beta-blocker dosage even when the patient’s heart rate is already low.”
The analysis run by Dr. Fiuzat and her associates used data collected in HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training), a randomized, controlled study that assessed the impact of exercise training on patients with heart failure with reduced ejection fraction (JAMA 2009 Apr 8;301[14]:1439-50). All patients received usual care (and the intervention group also received exercise training), which meant they were on whichever beta-blocker and dosage their individual physician prescribed.
The current analysis converted these baseline dosages into carvedilol (Coreg) equivalents and identified the dosage levels as either low, defined as a daily carvedilol equivalent of less than 25 mg/day, or high, a daily equivalent of at least 25 mg/day. The analysis also categorized heart rate at baseline into a high rate defined as at least 70 beats/min, and a low rate of less than 70 beats/min. At entry, 71% of patients were on a high beta-blocker dosage (although in many cases not their full, ideal dosage), and 52% of patients had a high heart rate.
The new analysis showed that after a median follow-up of 2.5 years, the rate of all-cause death and all-cause hospitalization was significantly higher among patients who entered the study on a low beta-blocker dosage and with a high heart rate compared with patients in the other three groups: low dosage/low heart rate, high dosage/high heart rate, and high dosage/low heart rate. In addition, the results showed that a high beta-blocker dosage at baseline mitigated the impact of a high heart rate; patients in this subgroup had outcomes comparable to those of patients on a high beta-blocker dosage with a low heart rate.
In an analysis that adjusted for baseline differences in several clinical and demographic variables, patients on a high beta-blocker dosage had a statistically significant 13% reduced rate of all-cause death or all-cause hospitalization compared with patients on a low-dosage beta-blocker. In contrast, baseline heart rate had no statistically significant impact on outcomes, Dr. Fiuzat and her associates reported in an article published online concurrently with the presentation of the results at the meeting by one of Dr. Fiuzat’s colleagues (JACC: Heart Failure 2015. doi: 10.1016/j.jchf.2015.09.002]).
On Twitter @mitchelzoler
NATIONAL HARBOR, MD. – In beta-blocker treatment of heart failure, dose matters; heart rate, not so much.
When using beta-blockers to treat patients with heart failure and reduced ejection fraction, administering a high, evidence-backed dosage of the drug had much more beneficial impact than did getting patients to a low heart rate, according to a post hoc analysis of data from more than 2,000 patients.
The finding provides new evidence supporting “titrating the beta-blocker to evidence-based, high dosages of a beta-blocker and not holding back,” Mona Fiuzat, Ph.D., said in an interview at the annual scientific meeting of the Heart Failure Society of America. “It’s not just lowering the heart rate; there is something else, other mechanisms [of beta-blocker effects] that may be of greater benefit” to patients beyond heart-rate reduction when patients they receive the full dosage of beta-blocker treatment that had been shown effective in the randomized trials run about 2 decades ago.
“The point of our report is that if you have [heart failure with reduced ejection fraction] patients on a beta-blocker, they will be better off by increasing the dose” to the target level rather than by adding an additional, non–beta-blocker drug, such as ivabradine (Corlanor), to further reduce heart rate, said Dr. Fiuzat, a clinical pharmacologist and heart-failure researcher at Duke University in Durham, N.C. In the trial that enrolled the 2,320 patients included in the current analysis, half the patients were not on their target beta-blocker dosage.
Some clinicians are too heart-rate focused, she said. “Our results show that there is added benefit from using the full beta-blocker dosage even when the patient’s heart rate is already low.”
The analysis run by Dr. Fiuzat and her associates used data collected in HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training), a randomized, controlled study that assessed the impact of exercise training on patients with heart failure with reduced ejection fraction (JAMA 2009 Apr 8;301[14]:1439-50). All patients received usual care (and the intervention group also received exercise training), which meant they were on whichever beta-blocker and dosage their individual physician prescribed.
The current analysis converted these baseline dosages into carvedilol (Coreg) equivalents and identified the dosage levels as either low, defined as a daily carvedilol equivalent of less than 25 mg/day, or high, a daily equivalent of at least 25 mg/day. The analysis also categorized heart rate at baseline into a high rate defined as at least 70 beats/min, and a low rate of less than 70 beats/min. At entry, 71% of patients were on a high beta-blocker dosage (although in many cases not their full, ideal dosage), and 52% of patients had a high heart rate.
The new analysis showed that after a median follow-up of 2.5 years, the rate of all-cause death and all-cause hospitalization was significantly higher among patients who entered the study on a low beta-blocker dosage and with a high heart rate compared with patients in the other three groups: low dosage/low heart rate, high dosage/high heart rate, and high dosage/low heart rate. In addition, the results showed that a high beta-blocker dosage at baseline mitigated the impact of a high heart rate; patients in this subgroup had outcomes comparable to those of patients on a high beta-blocker dosage with a low heart rate.
In an analysis that adjusted for baseline differences in several clinical and demographic variables, patients on a high beta-blocker dosage had a statistically significant 13% reduced rate of all-cause death or all-cause hospitalization compared with patients on a low-dosage beta-blocker. In contrast, baseline heart rate had no statistically significant impact on outcomes, Dr. Fiuzat and her associates reported in an article published online concurrently with the presentation of the results at the meeting by one of Dr. Fiuzat’s colleagues (JACC: Heart Failure 2015. doi: 10.1016/j.jchf.2015.09.002]).
On Twitter @mitchelzoler
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point: High-dose beta-blockers produced significantly better outcomes than did low-dose beta-blockers, while heart rate had no significant impact.
Major finding: Patients on high beta-blocker dosage had a significant 13% reduced rate of bad outcomes compared with low-dosage patients.
Data source: A post hoc analysis of data from the HF-ACTION trial, which enrolled 2,331 patients with heart failure and reduced ejection fraction.
Disclosures: Dr. Fiuzat had no disclosures.
HFSA: Next-generation LVAD meets survival goal
NATIONAL HARBOR, MD. – A next-generation left-ventricular assist device, the HeartMate 3, gave a solid debut performance in an uncontrolled series of the first 50 recipients, which was designed to gain the device CE mark approval in Europe.
In this study, run at 10 sites in Australia, Austria, Canada, Czech Republic, Germany, and Kazakhstan, the new-design left ventricular assist device (LVAD) numerically surpassed the study’s prespecified primary endpoint with 6-month recipient survival of 92%. This bested the target survival rate of 88% that the trial’s designers derived from the survival rate among 50 matched patients who had received a LVAD during 2012-2014 and had entered the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), Dr. Ivan Netuka said at the annual meeting of the Heart Failure Society of America.
Other notable findings of the HeartMate 3’s performance in the first 50 patients followed for 6 months were no pump malfunctions, no thrombosis within the pump, and no evidence of hemolysis, said Dr. Netuka, deputy director of cardiovascular surgery at IKEM hospital in Prague.
HeartMate 3 features several improvements over the HeartMate II model, such as a fully magnetically levitated rotor designed to eliminate friction and wear within the pump. The device also is engineered to produce an artificial pulse of 30 beats per minute, and it can deliver a wide blood-flow range of 2-10 L/min. Larger and consistent pump gaps are designed to reduced shear stress on blood components.
The study enrolled patients during June-November 2014 with NYHA class IIIB or IV heart failure and stage D heart failure, with a left ventricular ejection fraction of 25% or less. The 50 patients averaged 59 years of age and 90% were men; they were divided about equally between patients who received the device as a bridge to transplant and those who received the LVAD as destination therapy.
During 6 months of follow-up, two patients received a heart transplant. Twenty-one (42%) of the enrolled patients classified as INTERMACS patient profile 3, 20 (40%) as profile 4, and 5 (10%) as profile 2 patients, with the remaining four patients falling into other profile levels. Twenty-one patients had concomitant heart surgery when they received their LVAD, usually valve replacement. All patients received warfarin treatment and aspirin following device placement. Dr. Netuka and his associates calculated an expected 6-month survival of 78% for the enrolled patients without LVAD intervention.
The four deaths included a patient who died from cardiac arrest following a stroke on day 19 – a complication judged attributable to the device-placement procedure, a patient with circulatory failure on day 48, a suicide on day 113, and a patient with multiorgan failure on day 144.
After 6 months of follow-up, notable adverse events included bleeding in 19 patients (38%) – including gastrointestinal bleeds in 4 patients (8%) – strokes in 6 patients (12%), and infections in 18 patients (36%). Most of the adverse events occurred in the first 7 days following LVAD placement. Three of the six strokes were judged procedure associated, Dr. Netuka said.
Following device placement, patients showed improvements in their NYHA class and quality of life; their 6-minute walk distance improved by an average of 231 m.
The HeartMate 3 device is currently undergoing U.S. assessment in comparison to HeartMate II prior to submission to the Food and Drug Administration. The randomized trial, known as MOMENTUM 3, plans to enroll 1,028 patients with completion scheduled for 2018.
The study was sponsored by Thoratec, which is developing the HeartMate 3 device. Dr. Netuka is a speaker for and consultant to Thoratec.
On Twitter @mitchelzoler
It is extremely exciting to see this next-generation left ventricular assist device move forward, but it is important not to overinterpret the findings because the number of patients treated was relatively small and, as a result, the findings are limited by very wide confidence limits.
 |
| Mitchel L. Zoler/Frontline Medical News Dr. Marvin A. Konstam |
The HeartMate 3 device probably represents an important advance beyond currently available technology. Its attractive features include full magnetic levitation of the rotor, production of an artificial pulse, and the ability to deliver a wide range of blood-flow rates. These features may improve performance and could have favorable effects on thrombus and stroke rates.
The device clearly achieved its primary performance goal of 88% 6-month survival. The INTERMACS profiles of the enrolled patients included 40% of patients with profile 4 and 10% with profile 2. This does not exactly mimic the typical U.S. population receiving these devices, which recently had 15% of patients with a level 4 profile and 36% of patients with more severe disease at level 2. I applaud the decision to include patients who received their devices as destination therapy as well as patients who received it as a bridge to transplant.
The technologic advances that this new device represents are a step in the right direction, and the results provide a green light for further assessment. I look forward to seeing results from the U.S. randomized trial.
Dr. Marvin A. Konstam is professor and chief physician executive of the CardioVascular Center at Tufts Medical Center in Boston. He made these comments as designated discussant for Dr. Netuka’s report. Dr. Konstam has been a consultant to Merck, Novartis, Amgen, Johnson & Johnson, Arbor, Mast, and Cardioxyl.
It is extremely exciting to see this next-generation left ventricular assist device move forward, but it is important not to overinterpret the findings because the number of patients treated was relatively small and, as a result, the findings are limited by very wide confidence limits.
 |
| Mitchel L. Zoler/Frontline Medical News Dr. Marvin A. Konstam |
The HeartMate 3 device probably represents an important advance beyond currently available technology. Its attractive features include full magnetic levitation of the rotor, production of an artificial pulse, and the ability to deliver a wide range of blood-flow rates. These features may improve performance and could have favorable effects on thrombus and stroke rates.
The device clearly achieved its primary performance goal of 88% 6-month survival. The INTERMACS profiles of the enrolled patients included 40% of patients with profile 4 and 10% with profile 2. This does not exactly mimic the typical U.S. population receiving these devices, which recently had 15% of patients with a level 4 profile and 36% of patients with more severe disease at level 2. I applaud the decision to include patients who received their devices as destination therapy as well as patients who received it as a bridge to transplant.
The technologic advances that this new device represents are a step in the right direction, and the results provide a green light for further assessment. I look forward to seeing results from the U.S. randomized trial.
Dr. Marvin A. Konstam is professor and chief physician executive of the CardioVascular Center at Tufts Medical Center in Boston. He made these comments as designated discussant for Dr. Netuka’s report. Dr. Konstam has been a consultant to Merck, Novartis, Amgen, Johnson & Johnson, Arbor, Mast, and Cardioxyl.
It is extremely exciting to see this next-generation left ventricular assist device move forward, but it is important not to overinterpret the findings because the number of patients treated was relatively small and, as a result, the findings are limited by very wide confidence limits.
 |
| Mitchel L. Zoler/Frontline Medical News Dr. Marvin A. Konstam |
The HeartMate 3 device probably represents an important advance beyond currently available technology. Its attractive features include full magnetic levitation of the rotor, production of an artificial pulse, and the ability to deliver a wide range of blood-flow rates. These features may improve performance and could have favorable effects on thrombus and stroke rates.
The device clearly achieved its primary performance goal of 88% 6-month survival. The INTERMACS profiles of the enrolled patients included 40% of patients with profile 4 and 10% with profile 2. This does not exactly mimic the typical U.S. population receiving these devices, which recently had 15% of patients with a level 4 profile and 36% of patients with more severe disease at level 2. I applaud the decision to include patients who received their devices as destination therapy as well as patients who received it as a bridge to transplant.
The technologic advances that this new device represents are a step in the right direction, and the results provide a green light for further assessment. I look forward to seeing results from the U.S. randomized trial.
Dr. Marvin A. Konstam is professor and chief physician executive of the CardioVascular Center at Tufts Medical Center in Boston. He made these comments as designated discussant for Dr. Netuka’s report. Dr. Konstam has been a consultant to Merck, Novartis, Amgen, Johnson & Johnson, Arbor, Mast, and Cardioxyl.
NATIONAL HARBOR, MD. – A next-generation left-ventricular assist device, the HeartMate 3, gave a solid debut performance in an uncontrolled series of the first 50 recipients, which was designed to gain the device CE mark approval in Europe.
In this study, run at 10 sites in Australia, Austria, Canada, Czech Republic, Germany, and Kazakhstan, the new-design left ventricular assist device (LVAD) numerically surpassed the study’s prespecified primary endpoint with 6-month recipient survival of 92%. This bested the target survival rate of 88% that the trial’s designers derived from the survival rate among 50 matched patients who had received a LVAD during 2012-2014 and had entered the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), Dr. Ivan Netuka said at the annual meeting of the Heart Failure Society of America.
Other notable findings of the HeartMate 3’s performance in the first 50 patients followed for 6 months were no pump malfunctions, no thrombosis within the pump, and no evidence of hemolysis, said Dr. Netuka, deputy director of cardiovascular surgery at IKEM hospital in Prague.
HeartMate 3 features several improvements over the HeartMate II model, such as a fully magnetically levitated rotor designed to eliminate friction and wear within the pump. The device also is engineered to produce an artificial pulse of 30 beats per minute, and it can deliver a wide blood-flow range of 2-10 L/min. Larger and consistent pump gaps are designed to reduced shear stress on blood components.
The study enrolled patients during June-November 2014 with NYHA class IIIB or IV heart failure and stage D heart failure, with a left ventricular ejection fraction of 25% or less. The 50 patients averaged 59 years of age and 90% were men; they were divided about equally between patients who received the device as a bridge to transplant and those who received the LVAD as destination therapy.
During 6 months of follow-up, two patients received a heart transplant. Twenty-one (42%) of the enrolled patients classified as INTERMACS patient profile 3, 20 (40%) as profile 4, and 5 (10%) as profile 2 patients, with the remaining four patients falling into other profile levels. Twenty-one patients had concomitant heart surgery when they received their LVAD, usually valve replacement. All patients received warfarin treatment and aspirin following device placement. Dr. Netuka and his associates calculated an expected 6-month survival of 78% for the enrolled patients without LVAD intervention.
The four deaths included a patient who died from cardiac arrest following a stroke on day 19 – a complication judged attributable to the device-placement procedure, a patient with circulatory failure on day 48, a suicide on day 113, and a patient with multiorgan failure on day 144.
After 6 months of follow-up, notable adverse events included bleeding in 19 patients (38%) – including gastrointestinal bleeds in 4 patients (8%) – strokes in 6 patients (12%), and infections in 18 patients (36%). Most of the adverse events occurred in the first 7 days following LVAD placement. Three of the six strokes were judged procedure associated, Dr. Netuka said.
Following device placement, patients showed improvements in their NYHA class and quality of life; their 6-minute walk distance improved by an average of 231 m.
The HeartMate 3 device is currently undergoing U.S. assessment in comparison to HeartMate II prior to submission to the Food and Drug Administration. The randomized trial, known as MOMENTUM 3, plans to enroll 1,028 patients with completion scheduled for 2018.
The study was sponsored by Thoratec, which is developing the HeartMate 3 device. Dr. Netuka is a speaker for and consultant to Thoratec.
On Twitter @mitchelzoler
NATIONAL HARBOR, MD. – A next-generation left-ventricular assist device, the HeartMate 3, gave a solid debut performance in an uncontrolled series of the first 50 recipients, which was designed to gain the device CE mark approval in Europe.
In this study, run at 10 sites in Australia, Austria, Canada, Czech Republic, Germany, and Kazakhstan, the new-design left ventricular assist device (LVAD) numerically surpassed the study’s prespecified primary endpoint with 6-month recipient survival of 92%. This bested the target survival rate of 88% that the trial’s designers derived from the survival rate among 50 matched patients who had received a LVAD during 2012-2014 and had entered the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), Dr. Ivan Netuka said at the annual meeting of the Heart Failure Society of America.
Other notable findings of the HeartMate 3’s performance in the first 50 patients followed for 6 months were no pump malfunctions, no thrombosis within the pump, and no evidence of hemolysis, said Dr. Netuka, deputy director of cardiovascular surgery at IKEM hospital in Prague.
HeartMate 3 features several improvements over the HeartMate II model, such as a fully magnetically levitated rotor designed to eliminate friction and wear within the pump. The device also is engineered to produce an artificial pulse of 30 beats per minute, and it can deliver a wide blood-flow range of 2-10 L/min. Larger and consistent pump gaps are designed to reduced shear stress on blood components.
The study enrolled patients during June-November 2014 with NYHA class IIIB or IV heart failure and stage D heart failure, with a left ventricular ejection fraction of 25% or less. The 50 patients averaged 59 years of age and 90% were men; they were divided about equally between patients who received the device as a bridge to transplant and those who received the LVAD as destination therapy.
During 6 months of follow-up, two patients received a heart transplant. Twenty-one (42%) of the enrolled patients classified as INTERMACS patient profile 3, 20 (40%) as profile 4, and 5 (10%) as profile 2 patients, with the remaining four patients falling into other profile levels. Twenty-one patients had concomitant heart surgery when they received their LVAD, usually valve replacement. All patients received warfarin treatment and aspirin following device placement. Dr. Netuka and his associates calculated an expected 6-month survival of 78% for the enrolled patients without LVAD intervention.
The four deaths included a patient who died from cardiac arrest following a stroke on day 19 – a complication judged attributable to the device-placement procedure, a patient with circulatory failure on day 48, a suicide on day 113, and a patient with multiorgan failure on day 144.
After 6 months of follow-up, notable adverse events included bleeding in 19 patients (38%) – including gastrointestinal bleeds in 4 patients (8%) – strokes in 6 patients (12%), and infections in 18 patients (36%). Most of the adverse events occurred in the first 7 days following LVAD placement. Three of the six strokes were judged procedure associated, Dr. Netuka said.
Following device placement, patients showed improvements in their NYHA class and quality of life; their 6-minute walk distance improved by an average of 231 m.
The HeartMate 3 device is currently undergoing U.S. assessment in comparison to HeartMate II prior to submission to the Food and Drug Administration. The randomized trial, known as MOMENTUM 3, plans to enroll 1,028 patients with completion scheduled for 2018.
The study was sponsored by Thoratec, which is developing the HeartMate 3 device. Dr. Netuka is a speaker for and consultant to Thoratec.
On Twitter @mitchelzoler
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point: A next-generation left ventricular assist device, HeartMate 3, met its 6-month survival goal to receive CE mark approval in Europe.
Major finding: The advanced heart failure patients who received the HeartMate 3 LVAD had a 92% survival rate after 6 months.
Data source: A prospective series of 50 patients enrolled at 10 centers in six countries.
Disclosures: The study was sponsored by Thoratec, which is developing the HeartMate 3 device. Dr. Netuka is a speaker for and consultant to Thoratec.
CBT improves depression but not self-care in heart failure patients
Cognitive behavioral therapy significantly improved major depression but did not improve self-care by heart failure patients, investigators reported online in JAMA Internal Medicine.
“The results suggest that CBT is superior to usual care for depression in patients with heart failure,” said Dr. Kenneth Freedland and his associates at Washington University in St. Louis. They called the findings “especially encouraging” in light of recent negative results from the SADHART-CHF and MOOD-HF trials of selective serotonin reuptake inhibitors in this population.Patients in heart failure often have major depression, which increases their chances of poor self-care, hospitalization, and mortality, the researchers noted. Their single-blind, randomized trial included 158 patients who were in New York Heart Association class I, II, or III heart failure and met criteria for major depression. Patients in the intervention group received standard medical care, plus up to 6 months of CBT designed for cardiac patients.Patients received CBT weekly, then biweekly, and then monthly as they reached their treatment goals, but they also received telephone follow-up to help prevent relapse. The control group received standard medical care plus consultation with a cardiac nurse, written materials on heart failure self-care, and three follow-up phone calls with the nurse (JAMA Intern Med. 2015 Sept. 28. doi:10.1001/jamainternmed.2015.5220). At 6 months, the CBT group scored significantly lower on the BDI-II than did controls (mean score, 12.8 [standard deviation, 10.6] vs. 17.3 [10.7]; P = .008), the researchers said. Remission rates with CBT were 46% based on the BDI-II and 51% based on the Hamilton Depression Scale, both of which significantly exceeded remission rates of 19-20% for controls. The CBT group also improved significantly more than did controls on standard measures for anxiety, heart failure-related quality of life, mental health–related quality of life, fatigue, and social functioning, but not on measures of physical functioning, the researchers reported.
The National Heart, Lung, and Blood Institute partially funded the study. The researchers declared no competing interests.
When depression occurs in patients with heart failure, which is often, the illness burden and management complexity increase multifold. Freedland et al. tested the hypothesis that the effective treatment of comorbid depression with cognitive behavioral therapy (CBT) would also lead to improvements in heart failure self-care and physical functioning and found that it did not. The good news is that CBT did significantly improve emotional health and overall quality of life, and the improvement in depressive symptoms associated with CBT was larger than observed in pharmacotherapy trials for depression in patients with heart disease. This supports evidence for a shift in practice away from so much pharmacotherapy and more use of psychotherapy to achieve better mental health and overall quality of life outcomes in patients with heart failure. In reframing how we think about the management of depression in patients with heart failure, we should be talking more and prescribing less.
Dr. Patrick G. O’Malley is deputy editor of JAMA Internal Medicine. He declared no competing interests. These comments were taken from his accompanying editorial (JAMA Intern Med. 2015 Sept. 28).
When depression occurs in patients with heart failure, which is often, the illness burden and management complexity increase multifold. Freedland et al. tested the hypothesis that the effective treatment of comorbid depression with cognitive behavioral therapy (CBT) would also lead to improvements in heart failure self-care and physical functioning and found that it did not. The good news is that CBT did significantly improve emotional health and overall quality of life, and the improvement in depressive symptoms associated with CBT was larger than observed in pharmacotherapy trials for depression in patients with heart disease. This supports evidence for a shift in practice away from so much pharmacotherapy and more use of psychotherapy to achieve better mental health and overall quality of life outcomes in patients with heart failure. In reframing how we think about the management of depression in patients with heart failure, we should be talking more and prescribing less.
Dr. Patrick G. O’Malley is deputy editor of JAMA Internal Medicine. He declared no competing interests. These comments were taken from his accompanying editorial (JAMA Intern Med. 2015 Sept. 28).
When depression occurs in patients with heart failure, which is often, the illness burden and management complexity increase multifold. Freedland et al. tested the hypothesis that the effective treatment of comorbid depression with cognitive behavioral therapy (CBT) would also lead to improvements in heart failure self-care and physical functioning and found that it did not. The good news is that CBT did significantly improve emotional health and overall quality of life, and the improvement in depressive symptoms associated with CBT was larger than observed in pharmacotherapy trials for depression in patients with heart disease. This supports evidence for a shift in practice away from so much pharmacotherapy and more use of psychotherapy to achieve better mental health and overall quality of life outcomes in patients with heart failure. In reframing how we think about the management of depression in patients with heart failure, we should be talking more and prescribing less.
Dr. Patrick G. O’Malley is deputy editor of JAMA Internal Medicine. He declared no competing interests. These comments were taken from his accompanying editorial (JAMA Intern Med. 2015 Sept. 28).
Cognitive behavioral therapy significantly improved major depression but did not improve self-care by heart failure patients, investigators reported online in JAMA Internal Medicine.
“The results suggest that CBT is superior to usual care for depression in patients with heart failure,” said Dr. Kenneth Freedland and his associates at Washington University in St. Louis. They called the findings “especially encouraging” in light of recent negative results from the SADHART-CHF and MOOD-HF trials of selective serotonin reuptake inhibitors in this population.Patients in heart failure often have major depression, which increases their chances of poor self-care, hospitalization, and mortality, the researchers noted. Their single-blind, randomized trial included 158 patients who were in New York Heart Association class I, II, or III heart failure and met criteria for major depression. Patients in the intervention group received standard medical care, plus up to 6 months of CBT designed for cardiac patients.Patients received CBT weekly, then biweekly, and then monthly as they reached their treatment goals, but they also received telephone follow-up to help prevent relapse. The control group received standard medical care plus consultation with a cardiac nurse, written materials on heart failure self-care, and three follow-up phone calls with the nurse (JAMA Intern Med. 2015 Sept. 28. doi:10.1001/jamainternmed.2015.5220). At 6 months, the CBT group scored significantly lower on the BDI-II than did controls (mean score, 12.8 [standard deviation, 10.6] vs. 17.3 [10.7]; P = .008), the researchers said. Remission rates with CBT were 46% based on the BDI-II and 51% based on the Hamilton Depression Scale, both of which significantly exceeded remission rates of 19-20% for controls. The CBT group also improved significantly more than did controls on standard measures for anxiety, heart failure-related quality of life, mental health–related quality of life, fatigue, and social functioning, but not on measures of physical functioning, the researchers reported.
The National Heart, Lung, and Blood Institute partially funded the study. The researchers declared no competing interests.
Cognitive behavioral therapy significantly improved major depression but did not improve self-care by heart failure patients, investigators reported online in JAMA Internal Medicine.
“The results suggest that CBT is superior to usual care for depression in patients with heart failure,” said Dr. Kenneth Freedland and his associates at Washington University in St. Louis. They called the findings “especially encouraging” in light of recent negative results from the SADHART-CHF and MOOD-HF trials of selective serotonin reuptake inhibitors in this population.Patients in heart failure often have major depression, which increases their chances of poor self-care, hospitalization, and mortality, the researchers noted. Their single-blind, randomized trial included 158 patients who were in New York Heart Association class I, II, or III heart failure and met criteria for major depression. Patients in the intervention group received standard medical care, plus up to 6 months of CBT designed for cardiac patients.Patients received CBT weekly, then biweekly, and then monthly as they reached their treatment goals, but they also received telephone follow-up to help prevent relapse. The control group received standard medical care plus consultation with a cardiac nurse, written materials on heart failure self-care, and three follow-up phone calls with the nurse (JAMA Intern Med. 2015 Sept. 28. doi:10.1001/jamainternmed.2015.5220). At 6 months, the CBT group scored significantly lower on the BDI-II than did controls (mean score, 12.8 [standard deviation, 10.6] vs. 17.3 [10.7]; P = .008), the researchers said. Remission rates with CBT were 46% based on the BDI-II and 51% based on the Hamilton Depression Scale, both of which significantly exceeded remission rates of 19-20% for controls. The CBT group also improved significantly more than did controls on standard measures for anxiety, heart failure-related quality of life, mental health–related quality of life, fatigue, and social functioning, but not on measures of physical functioning, the researchers reported.
The National Heart, Lung, and Blood Institute partially funded the study. The researchers declared no competing interests.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Cognitive behavior therapy significantly improved major depression but not self-care among patients with heart failure.
Major finding: At 6 months, mean BDI-II scores were 12.8 for CBT vs. 17.3 for enhanced usual care (P = .008).
Data source: Single-blind, randomized trial of 158 patients.
Disclosures: The National Heart, Lung, and Blood Institute partially funded the study. The researchers declared no conflicts of interest.
VIDEO: CardioMEMS early adopters must sort out data use
Use of CardioMEMS by U.S. clinicians to measure pulmonary artery pressures daily in more advanced heart failure patients is currently quite variable around the country, with some enthusiastic, early adopters of the technology while many other programs have so far opted not to invest in the technology
Among the early adopters of CardioMEMS many programs are “trying to sort through” how to use the data they collect from patients using the implanted device, Dr. Mary Norine Walsh said during an interview. “Each clinical teams needs to work out not only how the data will be interpreted but also who will act on it, and how will the patient get the information,” said Dr. Walsh, medical director of the heart failure and transplantation program at the St. Vincent Heart Center in Indianapolis. “We need to get the data to patients quickly,” she said.
Despite this uncertainty over how to best use and disseminate the pulmonary artery pressure data collected using CardioMEMS, Dr. Walsh voiced confidence that it will ultimately make a difference for how advanced heart failure patients are managed and their rehospitalization rates. But for the time being “how much CardioMEMS and other technologies can have an impact on avoidable readmissions is not yet clear” for real-world practice, she said.
Dr. Walsh had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
Use of CardioMEMS by U.S. clinicians to measure pulmonary artery pressures daily in more advanced heart failure patients is currently quite variable around the country, with some enthusiastic, early adopters of the technology while many other programs have so far opted not to invest in the technology
Among the early adopters of CardioMEMS many programs are “trying to sort through” how to use the data they collect from patients using the implanted device, Dr. Mary Norine Walsh said during an interview. “Each clinical teams needs to work out not only how the data will be interpreted but also who will act on it, and how will the patient get the information,” said Dr. Walsh, medical director of the heart failure and transplantation program at the St. Vincent Heart Center in Indianapolis. “We need to get the data to patients quickly,” she said.
Despite this uncertainty over how to best use and disseminate the pulmonary artery pressure data collected using CardioMEMS, Dr. Walsh voiced confidence that it will ultimately make a difference for how advanced heart failure patients are managed and their rehospitalization rates. But for the time being “how much CardioMEMS and other technologies can have an impact on avoidable readmissions is not yet clear” for real-world practice, she said.
Dr. Walsh had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
Use of CardioMEMS by U.S. clinicians to measure pulmonary artery pressures daily in more advanced heart failure patients is currently quite variable around the country, with some enthusiastic, early adopters of the technology while many other programs have so far opted not to invest in the technology
Among the early adopters of CardioMEMS many programs are “trying to sort through” how to use the data they collect from patients using the implanted device, Dr. Mary Norine Walsh said during an interview. “Each clinical teams needs to work out not only how the data will be interpreted but also who will act on it, and how will the patient get the information,” said Dr. Walsh, medical director of the heart failure and transplantation program at the St. Vincent Heart Center in Indianapolis. “We need to get the data to patients quickly,” she said.
Despite this uncertainty over how to best use and disseminate the pulmonary artery pressure data collected using CardioMEMS, Dr. Walsh voiced confidence that it will ultimately make a difference for how advanced heart failure patients are managed and their rehospitalization rates. But for the time being “how much CardioMEMS and other technologies can have an impact on avoidable readmissions is not yet clear” for real-world practice, she said.
Dr. Walsh had no disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
Empagliflozin’s triumph respins FDA’s diabetes drug mandate
What a difference a few weeks and the unexpected results from a home run–hitting trial made in the medical community’s take on the Food and Drug Administration’s demand to assess the cardiovascular safety of drugs for type 2 diabetes.
On August 31, the relatively ho-hum, noninferiority results from the two latest, large outcomes trials to assess the cardiovascular safety of new oral hypoglycemic drugs led to bashing of these trials by several cardiologists speaking from the main stage of the European Society of Cardiology’s (ESC) annual congress in London. On Sept. 17, less than 3 weeks later, the remarkably beneficial survival effect seen with another new oral hypoglycemic, empagliflozin (Jardiance) in a cardiovascular safety study and reported at the European Association for the Study of Diabetes (EASD) annual meeting in Stockholm and in a simultaneously-published article resulted in researchers calling the results “amazing” and audience members hailing the trial a “landmark.”
Is the biomedical field really so fickle, or did physicians just need proof of the serendipitous possibilities when running a large outcomes clinical trial using a potent drug with underexplored potential?
The back story to this attitudinal change began in 2008, when the FDA issued guidance that called on companies to collect evidence for the cardiovascular safety of new drugs that treat type 2 diabetes, a stand that launched a fleet of big studies. Reports of the results from the first two trials conducted to meet this mandate came out 2 years ago from studies of new dipeptidyl peptidase 4 (DPP-4) inhibitors, the SAVOR-TIMI-53 trial of saxagliptin (Onglyza), and the EXAMINE trial of alogliptin (Nesina). Results from the two studies were notable not only for generally showing cardiovascular safety but also for showing a small but apparently real uptick in the rate of hospitalization for heart failure associated with saxagliptin treatment.
Findings from the next two trials in the series came out in reports in June at the American Diabetes Association annual meeting in Boston, with follow-up reports on the same two studies presented at the ESC meeting on August 31. Results from the TECOS study of yet a third new DDP-4 inhibitor, sitagliptin (Januvia), showed no cardiovascular safety signals with notably no suggestion of causing any sort of heart failure problem. The fourth study, ELIXA, was the first of the FDA mandates to report on a drug from a different class, lixisenatide (Lyxumia), a glucagonlike–peptide 1 receptor agonist, and it too provided a clean outcome with no excess of cardiovascular events, compared with the control arm.
It was this steady drumbeat of neutral results showing no evidence of cardiovascular harm – aside from the problem of heart-failure exacerbation with saxagliptin – that triggered criticism of the FDA’s mandate and its consequences at ESC, specifically in remarks by Dr. Philippe Gabriel Steg, the ESC’s designated discussant for the ELIXA report.
“Are these trials a waste of resources?” asked Dr. Steg in his comments. He questioned how representative and generalizable the studies are, by enrolling patients at very high cardiovascular risk, usually patients with a recent acute coronary syndrome event. He also critiqued the trials’ relatively short follow-up, on the order of 2-3 years, saying that this is generally too brief to demonstrate a potential benefit. Most of all, he questioned launching a series of safety trials that have enrolled a total of roughly 150,000 patients in randomized, controlled trials designed to test noninferiority for cardiovascular safety against standard-treatment control arms, studies that he posited divert money and resources from investigations focused on finding new treatments and could be accomplished in a different way for a lot less money.
Dr. Steg further complained that the series of neutral results have fostered misleading beliefs about their implications. The noninferiority results “have been mistakenly interpreted as lack of efficacy,” resulting in “greater skepticism among nonspecialists about treatment of diabetes and the need to control glycemia,” he said. He also said that the noninferiority design shortchanged enrolled patients, inconveniencing them by the demands of the trial when the best they could expect was to fare no worse than control patients.
Even more striking, Dr. Steg’s critique received immediate support from the next two speakers at the meeting, Dr. Jaakko Tuomilehto, designated discussant for the TECOS sidy, and then Dr. Thomas M. MacDonald, who followed at the podium to report results from an entirely different study. “I fully agree. I think it’s a waste of resources,” said Dr. Tuomilehto.
Was it reasonable for these trialists to anticipate anything more from studies designed to confirm cardiovascular safety? “Some think the results [from these four trials] have been disappointing, others think it’s what you would expect,” commented Dr. Bernard Zinman a few weeks later in September at the EASD meeting.
I spoke with Dr. Steg soon after he lambasted the FDA-mandated cardiovascular safety trials at ESC, and he further explained to me that while he didn’t dispute the importance of better evaluating the cardiovascular safety of these oral hypoglycemic drugs, he believed this could be more efficiently assessed with a more comprehensive approach to postmarketing surveillance. He also highlighted the importance of examining cardiovascular safety in type 2 diabetes patients who better resemble real-world patients instead of in the extreme high-risk patients who have enrolled in the trials. Finally, Dr. Steg told me that he wasn’t nearly as skeptical of these trials when the FDA first announced its guidance 7 years ago, but grew increasingly dismayed as he saw how the laudable goal of collecting data on cardiovascular safety led to such profligate and questionable studies.
Attitudes shifted dramatically fewer than 3 weeks later at EASD when the empagliflozin results came out from the EMPA-REG OUTCOME study. Randomizing 7,028 patients, the study showed that treatment with either of two dosages of empagliflozin, a blocker of the sodium glucose cotransporter 2 (SGLT-2) protein in the kidney, on top of standard oral hypoglycemic and other standard lipid-lowering and antihypertensive therapies produced a “wonderful and quite profound” 38% relative risk reduction in the rate of cardiovascular death, a 32% drop in all-cause death, and a 35% cut in heart failure hospitalizations, noted trial discussant Dr. Hertzel C. Gerstein. The “unexpected” results “will open new research,” Dr. Gerstein added when speaking at EASD. “The claims that people have made that large, randomized clinical trials in patients with diabetes are not needed appear unfounded. Here is a perfect case; we need to do these trials to find lifesaving therapies.”
The empagliflozin results were so dramatic and surprising that you can’t help wondering what would have happened if the FDA had not issued its mandate for cardiovascular safety studies in 2008. Would a trial like EMPA-REG OUTCOME ever have been done? Would this effect, which the researchers suggested was likely a class effect for all SGLT-2 inhibitors, ever have been found?
The empagliflozin findings also raise doubts about the unwavering reliance cardiovascular studies have had on a primary combined outcome that marries patient survival with the incidence of nonfatal ischemic events, strokes, and MIs. The EMPA-REG OUTCOME showed a major survival benefit while simultaneously having no discernible impact on nonfatal ischemic events.
The question of what aside from the FDA’s mandate might have gotten a trial like EMPA-REG OUTCOME off the ground is especially important because the findings hint that SGLT-2 inhibitors may have treatment implications that go beyond patients with type 2 diabetes. The rapid onset of the mortality benefit seemed to point to the diuretic effect of the drug as a major mediating factor, said several at the EASD session. Might SGLT-2 inhibitors be the eagerly sought new option for managing fluid congestion in the organs of patients with acute heart failure? Aside from survival it was heart failure hospitalization where the treatment showed benefit. Better fluid management is currently a desperate need as heart failure physicians increasingly recognize that diuretic treatment alone often is inadequate for purging excess fluid from affected organs in patients with acute heart failure episodes.
Some critics of the FDA mandate weren’t willing to accept that it made EMPA-REG OUTCOME possible. A New York Times news article published on September 18 quoted Dr. Robert E. Ratner from the American Diabetes Association as saying that studies like EMPA-REG OUTCOME get launched through the initiative of drug companies acting on their own, without FDA prodding. Perhaps Dr. Ratner is correct, but which studies out there now reflect this? It certainly doesn’t seem like companies have been motivated to organize trials that assess the cardiovascular impact of oral hypoglycemic drugs outside of meeting the FDA’s requirements.
A comment that nicely summed up the game-changing impact of the empagliflozin study came from a member of the EASD audience in Stockholm, the last attendee to pose a comment from the floor as the session wrapped up on Sept. 17. “I hope everyone in the audience understands what has happened, how important this is, a real landmark study,” commented Dr. Klas Malmberg, a cardiologist and diabetes researcher at the Karolinska Institute in Stockholm.
This unexpected finding from a large outcomes study looks like it may substantially alter the way type 2 diabetes and perhaps other diseases will get treated in the future. The finding also singlehandedly morphed the FDA’s safety study mandate from “a waste of resources” to the heroic driver behind a landmark study.
On Twitter @mitchelzoler
What a difference a few weeks and the unexpected results from a home run–hitting trial made in the medical community’s take on the Food and Drug Administration’s demand to assess the cardiovascular safety of drugs for type 2 diabetes.
On August 31, the relatively ho-hum, noninferiority results from the two latest, large outcomes trials to assess the cardiovascular safety of new oral hypoglycemic drugs led to bashing of these trials by several cardiologists speaking from the main stage of the European Society of Cardiology’s (ESC) annual congress in London. On Sept. 17, less than 3 weeks later, the remarkably beneficial survival effect seen with another new oral hypoglycemic, empagliflozin (Jardiance) in a cardiovascular safety study and reported at the European Association for the Study of Diabetes (EASD) annual meeting in Stockholm and in a simultaneously-published article resulted in researchers calling the results “amazing” and audience members hailing the trial a “landmark.”
Is the biomedical field really so fickle, or did physicians just need proof of the serendipitous possibilities when running a large outcomes clinical trial using a potent drug with underexplored potential?
The back story to this attitudinal change began in 2008, when the FDA issued guidance that called on companies to collect evidence for the cardiovascular safety of new drugs that treat type 2 diabetes, a stand that launched a fleet of big studies. Reports of the results from the first two trials conducted to meet this mandate came out 2 years ago from studies of new dipeptidyl peptidase 4 (DPP-4) inhibitors, the SAVOR-TIMI-53 trial of saxagliptin (Onglyza), and the EXAMINE trial of alogliptin (Nesina). Results from the two studies were notable not only for generally showing cardiovascular safety but also for showing a small but apparently real uptick in the rate of hospitalization for heart failure associated with saxagliptin treatment.
Findings from the next two trials in the series came out in reports in June at the American Diabetes Association annual meeting in Boston, with follow-up reports on the same two studies presented at the ESC meeting on August 31. Results from the TECOS study of yet a third new DDP-4 inhibitor, sitagliptin (Januvia), showed no cardiovascular safety signals with notably no suggestion of causing any sort of heart failure problem. The fourth study, ELIXA, was the first of the FDA mandates to report on a drug from a different class, lixisenatide (Lyxumia), a glucagonlike–peptide 1 receptor agonist, and it too provided a clean outcome with no excess of cardiovascular events, compared with the control arm.
It was this steady drumbeat of neutral results showing no evidence of cardiovascular harm – aside from the problem of heart-failure exacerbation with saxagliptin – that triggered criticism of the FDA’s mandate and its consequences at ESC, specifically in remarks by Dr. Philippe Gabriel Steg, the ESC’s designated discussant for the ELIXA report.
“Are these trials a waste of resources?” asked Dr. Steg in his comments. He questioned how representative and generalizable the studies are, by enrolling patients at very high cardiovascular risk, usually patients with a recent acute coronary syndrome event. He also critiqued the trials’ relatively short follow-up, on the order of 2-3 years, saying that this is generally too brief to demonstrate a potential benefit. Most of all, he questioned launching a series of safety trials that have enrolled a total of roughly 150,000 patients in randomized, controlled trials designed to test noninferiority for cardiovascular safety against standard-treatment control arms, studies that he posited divert money and resources from investigations focused on finding new treatments and could be accomplished in a different way for a lot less money.
Dr. Steg further complained that the series of neutral results have fostered misleading beliefs about their implications. The noninferiority results “have been mistakenly interpreted as lack of efficacy,” resulting in “greater skepticism among nonspecialists about treatment of diabetes and the need to control glycemia,” he said. He also said that the noninferiority design shortchanged enrolled patients, inconveniencing them by the demands of the trial when the best they could expect was to fare no worse than control patients.
Even more striking, Dr. Steg’s critique received immediate support from the next two speakers at the meeting, Dr. Jaakko Tuomilehto, designated discussant for the TECOS sidy, and then Dr. Thomas M. MacDonald, who followed at the podium to report results from an entirely different study. “I fully agree. I think it’s a waste of resources,” said Dr. Tuomilehto.
Was it reasonable for these trialists to anticipate anything more from studies designed to confirm cardiovascular safety? “Some think the results [from these four trials] have been disappointing, others think it’s what you would expect,” commented Dr. Bernard Zinman a few weeks later in September at the EASD meeting.
I spoke with Dr. Steg soon after he lambasted the FDA-mandated cardiovascular safety trials at ESC, and he further explained to me that while he didn’t dispute the importance of better evaluating the cardiovascular safety of these oral hypoglycemic drugs, he believed this could be more efficiently assessed with a more comprehensive approach to postmarketing surveillance. He also highlighted the importance of examining cardiovascular safety in type 2 diabetes patients who better resemble real-world patients instead of in the extreme high-risk patients who have enrolled in the trials. Finally, Dr. Steg told me that he wasn’t nearly as skeptical of these trials when the FDA first announced its guidance 7 years ago, but grew increasingly dismayed as he saw how the laudable goal of collecting data on cardiovascular safety led to such profligate and questionable studies.
Attitudes shifted dramatically fewer than 3 weeks later at EASD when the empagliflozin results came out from the EMPA-REG OUTCOME study. Randomizing 7,028 patients, the study showed that treatment with either of two dosages of empagliflozin, a blocker of the sodium glucose cotransporter 2 (SGLT-2) protein in the kidney, on top of standard oral hypoglycemic and other standard lipid-lowering and antihypertensive therapies produced a “wonderful and quite profound” 38% relative risk reduction in the rate of cardiovascular death, a 32% drop in all-cause death, and a 35% cut in heart failure hospitalizations, noted trial discussant Dr. Hertzel C. Gerstein. The “unexpected” results “will open new research,” Dr. Gerstein added when speaking at EASD. “The claims that people have made that large, randomized clinical trials in patients with diabetes are not needed appear unfounded. Here is a perfect case; we need to do these trials to find lifesaving therapies.”
The empagliflozin results were so dramatic and surprising that you can’t help wondering what would have happened if the FDA had not issued its mandate for cardiovascular safety studies in 2008. Would a trial like EMPA-REG OUTCOME ever have been done? Would this effect, which the researchers suggested was likely a class effect for all SGLT-2 inhibitors, ever have been found?
The empagliflozin findings also raise doubts about the unwavering reliance cardiovascular studies have had on a primary combined outcome that marries patient survival with the incidence of nonfatal ischemic events, strokes, and MIs. The EMPA-REG OUTCOME showed a major survival benefit while simultaneously having no discernible impact on nonfatal ischemic events.
The question of what aside from the FDA’s mandate might have gotten a trial like EMPA-REG OUTCOME off the ground is especially important because the findings hint that SGLT-2 inhibitors may have treatment implications that go beyond patients with type 2 diabetes. The rapid onset of the mortality benefit seemed to point to the diuretic effect of the drug as a major mediating factor, said several at the EASD session. Might SGLT-2 inhibitors be the eagerly sought new option for managing fluid congestion in the organs of patients with acute heart failure? Aside from survival it was heart failure hospitalization where the treatment showed benefit. Better fluid management is currently a desperate need as heart failure physicians increasingly recognize that diuretic treatment alone often is inadequate for purging excess fluid from affected organs in patients with acute heart failure episodes.
Some critics of the FDA mandate weren’t willing to accept that it made EMPA-REG OUTCOME possible. A New York Times news article published on September 18 quoted Dr. Robert E. Ratner from the American Diabetes Association as saying that studies like EMPA-REG OUTCOME get launched through the initiative of drug companies acting on their own, without FDA prodding. Perhaps Dr. Ratner is correct, but which studies out there now reflect this? It certainly doesn’t seem like companies have been motivated to organize trials that assess the cardiovascular impact of oral hypoglycemic drugs outside of meeting the FDA’s requirements.
A comment that nicely summed up the game-changing impact of the empagliflozin study came from a member of the EASD audience in Stockholm, the last attendee to pose a comment from the floor as the session wrapped up on Sept. 17. “I hope everyone in the audience understands what has happened, how important this is, a real landmark study,” commented Dr. Klas Malmberg, a cardiologist and diabetes researcher at the Karolinska Institute in Stockholm.
This unexpected finding from a large outcomes study looks like it may substantially alter the way type 2 diabetes and perhaps other diseases will get treated in the future. The finding also singlehandedly morphed the FDA’s safety study mandate from “a waste of resources” to the heroic driver behind a landmark study.
On Twitter @mitchelzoler
What a difference a few weeks and the unexpected results from a home run–hitting trial made in the medical community’s take on the Food and Drug Administration’s demand to assess the cardiovascular safety of drugs for type 2 diabetes.
On August 31, the relatively ho-hum, noninferiority results from the two latest, large outcomes trials to assess the cardiovascular safety of new oral hypoglycemic drugs led to bashing of these trials by several cardiologists speaking from the main stage of the European Society of Cardiology’s (ESC) annual congress in London. On Sept. 17, less than 3 weeks later, the remarkably beneficial survival effect seen with another new oral hypoglycemic, empagliflozin (Jardiance) in a cardiovascular safety study and reported at the European Association for the Study of Diabetes (EASD) annual meeting in Stockholm and in a simultaneously-published article resulted in researchers calling the results “amazing” and audience members hailing the trial a “landmark.”
Is the biomedical field really so fickle, or did physicians just need proof of the serendipitous possibilities when running a large outcomes clinical trial using a potent drug with underexplored potential?
The back story to this attitudinal change began in 2008, when the FDA issued guidance that called on companies to collect evidence for the cardiovascular safety of new drugs that treat type 2 diabetes, a stand that launched a fleet of big studies. Reports of the results from the first two trials conducted to meet this mandate came out 2 years ago from studies of new dipeptidyl peptidase 4 (DPP-4) inhibitors, the SAVOR-TIMI-53 trial of saxagliptin (Onglyza), and the EXAMINE trial of alogliptin (Nesina). Results from the two studies were notable not only for generally showing cardiovascular safety but also for showing a small but apparently real uptick in the rate of hospitalization for heart failure associated with saxagliptin treatment.
Findings from the next two trials in the series came out in reports in June at the American Diabetes Association annual meeting in Boston, with follow-up reports on the same two studies presented at the ESC meeting on August 31. Results from the TECOS study of yet a third new DDP-4 inhibitor, sitagliptin (Januvia), showed no cardiovascular safety signals with notably no suggestion of causing any sort of heart failure problem. The fourth study, ELIXA, was the first of the FDA mandates to report on a drug from a different class, lixisenatide (Lyxumia), a glucagonlike–peptide 1 receptor agonist, and it too provided a clean outcome with no excess of cardiovascular events, compared with the control arm.
It was this steady drumbeat of neutral results showing no evidence of cardiovascular harm – aside from the problem of heart-failure exacerbation with saxagliptin – that triggered criticism of the FDA’s mandate and its consequences at ESC, specifically in remarks by Dr. Philippe Gabriel Steg, the ESC’s designated discussant for the ELIXA report.
“Are these trials a waste of resources?” asked Dr. Steg in his comments. He questioned how representative and generalizable the studies are, by enrolling patients at very high cardiovascular risk, usually patients with a recent acute coronary syndrome event. He also critiqued the trials’ relatively short follow-up, on the order of 2-3 years, saying that this is generally too brief to demonstrate a potential benefit. Most of all, he questioned launching a series of safety trials that have enrolled a total of roughly 150,000 patients in randomized, controlled trials designed to test noninferiority for cardiovascular safety against standard-treatment control arms, studies that he posited divert money and resources from investigations focused on finding new treatments and could be accomplished in a different way for a lot less money.
Dr. Steg further complained that the series of neutral results have fostered misleading beliefs about their implications. The noninferiority results “have been mistakenly interpreted as lack of efficacy,” resulting in “greater skepticism among nonspecialists about treatment of diabetes and the need to control glycemia,” he said. He also said that the noninferiority design shortchanged enrolled patients, inconveniencing them by the demands of the trial when the best they could expect was to fare no worse than control patients.
Even more striking, Dr. Steg’s critique received immediate support from the next two speakers at the meeting, Dr. Jaakko Tuomilehto, designated discussant for the TECOS sidy, and then Dr. Thomas M. MacDonald, who followed at the podium to report results from an entirely different study. “I fully agree. I think it’s a waste of resources,” said Dr. Tuomilehto.
Was it reasonable for these trialists to anticipate anything more from studies designed to confirm cardiovascular safety? “Some think the results [from these four trials] have been disappointing, others think it’s what you would expect,” commented Dr. Bernard Zinman a few weeks later in September at the EASD meeting.
I spoke with Dr. Steg soon after he lambasted the FDA-mandated cardiovascular safety trials at ESC, and he further explained to me that while he didn’t dispute the importance of better evaluating the cardiovascular safety of these oral hypoglycemic drugs, he believed this could be more efficiently assessed with a more comprehensive approach to postmarketing surveillance. He also highlighted the importance of examining cardiovascular safety in type 2 diabetes patients who better resemble real-world patients instead of in the extreme high-risk patients who have enrolled in the trials. Finally, Dr. Steg told me that he wasn’t nearly as skeptical of these trials when the FDA first announced its guidance 7 years ago, but grew increasingly dismayed as he saw how the laudable goal of collecting data on cardiovascular safety led to such profligate and questionable studies.
Attitudes shifted dramatically fewer than 3 weeks later at EASD when the empagliflozin results came out from the EMPA-REG OUTCOME study. Randomizing 7,028 patients, the study showed that treatment with either of two dosages of empagliflozin, a blocker of the sodium glucose cotransporter 2 (SGLT-2) protein in the kidney, on top of standard oral hypoglycemic and other standard lipid-lowering and antihypertensive therapies produced a “wonderful and quite profound” 38% relative risk reduction in the rate of cardiovascular death, a 32% drop in all-cause death, and a 35% cut in heart failure hospitalizations, noted trial discussant Dr. Hertzel C. Gerstein. The “unexpected” results “will open new research,” Dr. Gerstein added when speaking at EASD. “The claims that people have made that large, randomized clinical trials in patients with diabetes are not needed appear unfounded. Here is a perfect case; we need to do these trials to find lifesaving therapies.”
The empagliflozin results were so dramatic and surprising that you can’t help wondering what would have happened if the FDA had not issued its mandate for cardiovascular safety studies in 2008. Would a trial like EMPA-REG OUTCOME ever have been done? Would this effect, which the researchers suggested was likely a class effect for all SGLT-2 inhibitors, ever have been found?
The empagliflozin findings also raise doubts about the unwavering reliance cardiovascular studies have had on a primary combined outcome that marries patient survival with the incidence of nonfatal ischemic events, strokes, and MIs. The EMPA-REG OUTCOME showed a major survival benefit while simultaneously having no discernible impact on nonfatal ischemic events.
The question of what aside from the FDA’s mandate might have gotten a trial like EMPA-REG OUTCOME off the ground is especially important because the findings hint that SGLT-2 inhibitors may have treatment implications that go beyond patients with type 2 diabetes. The rapid onset of the mortality benefit seemed to point to the diuretic effect of the drug as a major mediating factor, said several at the EASD session. Might SGLT-2 inhibitors be the eagerly sought new option for managing fluid congestion in the organs of patients with acute heart failure? Aside from survival it was heart failure hospitalization where the treatment showed benefit. Better fluid management is currently a desperate need as heart failure physicians increasingly recognize that diuretic treatment alone often is inadequate for purging excess fluid from affected organs in patients with acute heart failure episodes.
Some critics of the FDA mandate weren’t willing to accept that it made EMPA-REG OUTCOME possible. A New York Times news article published on September 18 quoted Dr. Robert E. Ratner from the American Diabetes Association as saying that studies like EMPA-REG OUTCOME get launched through the initiative of drug companies acting on their own, without FDA prodding. Perhaps Dr. Ratner is correct, but which studies out there now reflect this? It certainly doesn’t seem like companies have been motivated to organize trials that assess the cardiovascular impact of oral hypoglycemic drugs outside of meeting the FDA’s requirements.
A comment that nicely summed up the game-changing impact of the empagliflozin study came from a member of the EASD audience in Stockholm, the last attendee to pose a comment from the floor as the session wrapped up on Sept. 17. “I hope everyone in the audience understands what has happened, how important this is, a real landmark study,” commented Dr. Klas Malmberg, a cardiologist and diabetes researcher at the Karolinska Institute in Stockholm.
This unexpected finding from a large outcomes study looks like it may substantially alter the way type 2 diabetes and perhaps other diseases will get treated in the future. The finding also singlehandedly morphed the FDA’s safety study mandate from “a waste of resources” to the heroic driver behind a landmark study.
On Twitter @mitchelzoler