User login
HFSA Roundtable, part 1: Beta-blockers remain heart failure management linchpin
NATIONAL HARBOR, MD. – More than 20 years ago, treating chronic heart failure patients with a beta-blocker drug seemed counterintuitive, but it turned out to be a landmark step, both for clinical efficacy and for improved understanding of the role neurohormonal drivers play in chronic heart failure.
The Heart Failure Society of America awarded its annual Lifetime Achievement Award to Dr. Sidney Goldstein during the its annual meeting in September. On the occasion of that award, we gathered Dr. Goldstein and some of his associates to discuss the beta-blocker legacy and other aspects of how heart failure treatments developed and where they stand today. Joining this round table were Dr. Jay N. Cohn, Hani N. Sabbah, Ph.D., and Dr. Prakash Deedwania.
In this first segment of the interview, the panel reminisced about how a rationale gradually developed supporting the principle behind beta-blockers and other neurohormonal interventions for heart failure, and they discussed how the explanation of beta-blocker activity in heart failure patients remains controversial even today.
Despite a track record of consistent efficacy across drugs in the beta-blocker class that goes back some 2 decades, getting patients to their appropriate beta-blocker dosage remains a challenge, noted Dr. Goldstein, a cardiologist at Henry Ford Hospital and a professor of medicine at Wayne State University in Detroit. “Most heart failure patients whom I see on a beta-blocker are on far too low a dosage,” he said. Clinicians continue to believe that maximum dosing with a beta-blocker is nearly impossible, while in reality “if you advance the treatment, patients tolerate it quite well. There has always been this mystique about the tolerability of beta-blockers, but that’s pure fantasy,” Dr. Goldstein said.
Dr. Goldstein had no disclosures. Dr. Deedwania had no disclosures. Dr. Cohn receives royalties from Arbor Pharmaceuticals related to his work on hydralazine and isosorbide dinitrate. Dr. Sabbah is a consultant to Boston Scientific and an adviser to BioControl Medical, and he has received research grants from both companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
NATIONAL HARBOR, MD. – More than 20 years ago, treating chronic heart failure patients with a beta-blocker drug seemed counterintuitive, but it turned out to be a landmark step, both for clinical efficacy and for improved understanding of the role neurohormonal drivers play in chronic heart failure.
The Heart Failure Society of America awarded its annual Lifetime Achievement Award to Dr. Sidney Goldstein during the its annual meeting in September. On the occasion of that award, we gathered Dr. Goldstein and some of his associates to discuss the beta-blocker legacy and other aspects of how heart failure treatments developed and where they stand today. Joining this round table were Dr. Jay N. Cohn, Hani N. Sabbah, Ph.D., and Dr. Prakash Deedwania.
In this first segment of the interview, the panel reminisced about how a rationale gradually developed supporting the principle behind beta-blockers and other neurohormonal interventions for heart failure, and they discussed how the explanation of beta-blocker activity in heart failure patients remains controversial even today.
Despite a track record of consistent efficacy across drugs in the beta-blocker class that goes back some 2 decades, getting patients to their appropriate beta-blocker dosage remains a challenge, noted Dr. Goldstein, a cardiologist at Henry Ford Hospital and a professor of medicine at Wayne State University in Detroit. “Most heart failure patients whom I see on a beta-blocker are on far too low a dosage,” he said. Clinicians continue to believe that maximum dosing with a beta-blocker is nearly impossible, while in reality “if you advance the treatment, patients tolerate it quite well. There has always been this mystique about the tolerability of beta-blockers, but that’s pure fantasy,” Dr. Goldstein said.
Dr. Goldstein had no disclosures. Dr. Deedwania had no disclosures. Dr. Cohn receives royalties from Arbor Pharmaceuticals related to his work on hydralazine and isosorbide dinitrate. Dr. Sabbah is a consultant to Boston Scientific and an adviser to BioControl Medical, and he has received research grants from both companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
NATIONAL HARBOR, MD. – More than 20 years ago, treating chronic heart failure patients with a beta-blocker drug seemed counterintuitive, but it turned out to be a landmark step, both for clinical efficacy and for improved understanding of the role neurohormonal drivers play in chronic heart failure.
The Heart Failure Society of America awarded its annual Lifetime Achievement Award to Dr. Sidney Goldstein during the its annual meeting in September. On the occasion of that award, we gathered Dr. Goldstein and some of his associates to discuss the beta-blocker legacy and other aspects of how heart failure treatments developed and where they stand today. Joining this round table were Dr. Jay N. Cohn, Hani N. Sabbah, Ph.D., and Dr. Prakash Deedwania.
In this first segment of the interview, the panel reminisced about how a rationale gradually developed supporting the principle behind beta-blockers and other neurohormonal interventions for heart failure, and they discussed how the explanation of beta-blocker activity in heart failure patients remains controversial even today.
Despite a track record of consistent efficacy across drugs in the beta-blocker class that goes back some 2 decades, getting patients to their appropriate beta-blocker dosage remains a challenge, noted Dr. Goldstein, a cardiologist at Henry Ford Hospital and a professor of medicine at Wayne State University in Detroit. “Most heart failure patients whom I see on a beta-blocker are on far too low a dosage,” he said. Clinicians continue to believe that maximum dosing with a beta-blocker is nearly impossible, while in reality “if you advance the treatment, patients tolerate it quite well. There has always been this mystique about the tolerability of beta-blockers, but that’s pure fantasy,” Dr. Goldstein said.
Dr. Goldstein had no disclosures. Dr. Deedwania had no disclosures. Dr. Cohn receives royalties from Arbor Pharmaceuticals related to his work on hydralazine and isosorbide dinitrate. Dr. Sabbah is a consultant to Boston Scientific and an adviser to BioControl Medical, and he has received research grants from both companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM HFSA
Beta-blockers remain a key part of treatment for chronic heart failure, and control of neurohormonal activation remains a central principle of treatment.
HFSA: Emphasizing "acute" in acute decompensated heart failure
NATIONAL HARBOR, MD. – Acute decompensated heart failure is becoming more of an emergency.
Traditionally, it has been seen as a lumbering event that could be treated at a relatively leisurely pace, but heart failure physicians increasingly see the moment when patients arrive in the hospital with an episode of acute decompensated heart failure as a time-sensitive event that requires rapid intervention in a manner much more akin to an acute MI than to chronic heart failure.
While the tide is slowly shifting to put a premium on rapid treatment to try to decongest acute heart failure patients, the treatment options clinicians have available for these patients often remain inadequate.
“Development of drugs for acutely decompensated heart failure has been extremely difficult. We have done a really horrible job treating this disease,” Dr. Milton Packer said at the HFSA annual scientific meeting.
Treatment of acute heart failure patients has generally focused on relieving dyspnea, but part of the new appreciation of this state as an emergency event involves understanding that the pathology patients have when they reach the hospital is much more global and has profoundly morbid consequences.
“We want more from treatment than for patients to feel a little bit better an hour or two sooner” by relieving dyspnea, said Dr. Packer, professor of medicine and a heart failure specialist at the University of Texas Southwestern Medical Center in Dallas. “In the first 6 hours [of acute heart failure hospitalization], many patients are spilling troponin. We don’t know what this means, but the patients who spill troponin have a markedly increased risk for a more complicated hospital course.” About 10%-25% of patients hospitalized with acute heart failure have recurrent worsening heart failure, and many of these are also the patients who have a spike in their troponin level during initial hospitalization, he noted.
The troponin release in many patients and its association with worse outcomes is a clue that these patients are experiencing an ischemic myocardial event similar to an acute MI, possibly caused by myocardial-wall stretch, Dr. Packer said in an interview.
“If we can reduce this early acute cardiac dilatation, maybe we can reduce myocardial injury, reduce troponin release, and have favorable effects on clinically relevant events both short-term and long-term,” he said. “That’s why in trials [of investigational drugs for acute heart failure] we are treating patients earlier. Before we said we could enroll patients [into acute-treatment trials] within 48 hours of hospital admission. Now we enroll within 16 hours, or within 12 hours. We’ve learned that early intervention is important. That makes acute heart failure a lot more similar to an acute MI.”
European Society recommends faster acute heart failure management
European heart failure specialists have also become convinced that acute heart failure is an emergency that needs a rapid response. In June, the Heart Failure Association of the European Society of Cardiology published new recommendations on the in-hospital management of patients with acute heart failure (Eur J Heart Fail 2015 June;17[6];544-58). In the document, the association’s writing panel said, “The potentially greater benefit of early treatment is of conceptual importance in many cardiovascular presentations (e.g., myocardial infarction). Unfortunately, acute heart failure has not been considered with this regard until recently.” Breaking with the past, the association’s new recommendations now say that “all acute heart failure patients should receive appropriate therapy as early as possible,” an approach that involves starting acute management in the prehospital setting.
One member of the writing group for these recommendations put it more succinctly while speaking at the annual congress of the European Society of Cardiology in London in August: “Time is muscle in acute heart failure,” said Dr. Piotr Ponikowski, professor and heart failure specialist at the Medical University in Wroclaw, Poland. “When a patient has acute coronary syndrome everyone rushes, but we have patients with acute heart failure and no one rushes. We give furosemide, maybe something else, and then we wait and see.” He recommended adhering to a schedule that would have a patient assessed and initially treated within the first hour of hospitalization, and even sooner if treatment could start at the prehospital stage.
Like Dr. Packer, Dr. Ponikowski also lamented the inadequate tools now available for treating acute heart failure and the pressing need to identify better approaches to treatment, especially for selected acute heart failure patients.
“It is too simple to think that one drug or one treatment will help the entire spectrum of acute heart failure patients,” he said. “Our hypothesis is that profiling patients at every step of acute heart failure is crucial.”
He itemized five distinct types of acute heart failure patients based on their precipitating triggers of decompensation:
• Rapid arrhythmia or rhythm disturbance.
• Hypertension emergency.
• Pulmonary embolism.
• Pulmonary infection.
• Mechanical cause of acute heart failure.
“We need to clinically profile” patients into these subgroups to better tailor management, he said.
Another important aspect of patient heterogeneity is that fluid congestion may be less important in many patients compared with fluid redistribution from the splanchnic circulation. This distinction is important because fluid redistribution may be better treated with a vasodilator than with a diuretic, he noted. He voiced hope that two phase III trials now in progress with two unique vasodilator drugs, the TRUE-AHF trial of ularitide, and the RELAX-AHF-2 trial of serelaxin, may identify two new vasodilators with “unique effects” that could potentially launch a new era in management of selected patients with acute heart failure. Dr. Packer, the principal investigator for the ularitide trial, offered similar hope.
The responsiveness of acute heart failure patients to in-hospital treatment may vary depending on what end-organ damage they experience, Dr. Ponikowski said.
This end-organ damage is often an acute process occurring during hospitalization caused by the fluid congestion and redistribution that occurs during acute heart failure, said Dr. Alexandre Mebazaa, professor of anesthesiology and critical care medicine at Lariboisière Hospital in Paris.
“Fluid overload leads to organ dysfunction. In the past, we thought that kidney dysfunction [occurring during acute heart failure] was due to low cardiac output, but we know that dysfunction in the kidney and liver is due to congestion, and diuretics do not remove water from the liver and kidney,” Dr. Mebazaa said in an interview. “Diuretics may remove fluid from vessels, but not from organs. We need new approaches to remove fluid from organs – from the kidney, liver, and lungs” – during acute heart failure. This is another reason why heart failure physicians are excited about the possibility of finding new vasodilators, such a ularitide and serelaxin, that might address the issue of venous congestion in peripheral organs.
Faster management endorsed by U.S. clinicians, too
“We used to think that the reason why patients with acute heart failure were not voiding well and became diuretic resistant was because of poor cardiac output. Now we know that there is a lot of venous congestion with an impact on the liver and kidneys,” agreed Dr. Mariell L. Jessup, professor and medical director of the Penn Heart and Vascular Center at the University of Pennsylvania in Philadelphia. “We’ve begun to appreciate how important venous congestion is in causing high pressures on the right side” of the circulatory system, she said in an interview.
Other U.S. physicians echo the call by Dr. Packer and the European cardiologists for faster treatment of acute heart failure. “I collected data at U.S. hospitals and found it took an average of 22 hours for decompensated heart failure patients to receive treatment,” said Dr. Maria Rosa Costanzo, medical director of the heart failure and pulmonary hypertension program at Advocate Heart Institute in Naperville, Ill. “I have tried to convey the message that these patients must be treated early, and this is associated with better outcomes,” she said in an interview during the HFSA meeting.
“Early treatment means at least two doses of intravenous diuretic in the emergency department. We’ve seen that the two immediate doses can make a big difference, producing shorter lengths of stay in the intensive care unit, fewer rehospitalizations, and fewer deaths,” according to data collected in the ADHERE (Acute Decompensated Heart Failure National Registry), she said. “But this has not yet been picked up in a lot of U.S. practice.” Although the hemodynamic abnormalities that lead up to an acute decompensation event can take several weeks of steady worsening before severe symptoms drive a patient to the hospital, once the patient requires hospitalization “it should be treated as an emergency,” she said.
Dr. Costanzo is a major advocate for using ultrafiltration as a second-line treatment for acute decompensated heart failure patients who do not adequately respond to diuretic treatment, but for the time being, ultrafiltration remains a controversial option that at least some other heart failure physicians do not endorse, and it can involve reimbursement issues as many insurers consider it investigational.
“Try to get the patient decongested within the first 6 hours [after arrival at the hospital] or even sooner, within the first 1-2 hours,” recommended Dr. Christopher M. O’Connor, chief executive officer of Inova Heart and Vascular Institute in Falls Church, Va. He suggested treating patients with a combination of diuretics and vasodilators. “Some people are talking about instituting a performance measure for treating acute heart failure within the first 6 hours,” Dr. O’Connor said in an interview.
Currently, vasodilator treatment is limited to standard agents such as intravenous nitroglycerin, but Dr. O’Connor shared the hope that sometime soon a new vasodilator may be shown effective for acutely decompensated patients. He is a coinvestigator on the TRUE-AHF study of ularitide. “We hope that these new vasodilators, ularitide and serelaxin, will be good complements to diuretics, he said. Dr. O’Connor also recommended that clinicians shy away from using ultrafiltration as a back-up therapy, believing that it was shown ineffective and potentially harmful in results from the CARRESS-HF trial (N Engl J Med 2012 Dec 13;367[24]:2296-304).
But not all heart failure specialists see acute heart failure as a new frontier for early treatment and new drug discovery.
“So much energy has already been spent on acute heart failure with very little return,” said Dr. Clyde W. Yancy, professor and chief of cardiology at Northwestern University in Chicago. “I think that our best opportunities in heart failure are in prevention and in better chronic care. The hospitalized patient is so broad and complex; if we’re looking at how to best spend our resources I think it’s best to focus on prevention,” he said in an interview.
“The hospital experience needs to shift toward better use of systems of care and focus less on the biology. The biggest challenge is how to coordinate all the systems to make sure that patients have access to the resources and can obtain [existing] medications. Patients don’t often have the literacy to understand discharge instructions, and our systems are overwhelmed by trying to have 7-day follow-up visits. Focusing on management of the hospitalized patient does not give us a good return on the investment. There is no question that acute heart failure is an unmet need, but the greater unmet need is prevention and improved chronic care. No single intervention will dramatically change the acute heart failure experience. Focusing on the hospitalization does not offer us management opportunities that are as robust as we once thought,” Dr. Yancy said.
Dr. Packer has been a consultant to 22 companies. Dr. Ponikowski has been a consultant to, speaker for, or has received research grants from 11 companies. Dr. Mebazaa has received speaking honoraria and consulting fees from 11 companies. Dr. Jessup, Dr. Costanzo, and Dr. Yancy had no disclosures. Dr. O’Connor has been a consultant to ResMed, Roche Diagnostics, Cardiorentis, Bayer, and Actelion and has received research grants from Otsuka, ResMed, and Roche Diagnostics.
On Twitter@mitchelzoler
NATIONAL HARBOR, MD. – Acute decompensated heart failure is becoming more of an emergency.
Traditionally, it has been seen as a lumbering event that could be treated at a relatively leisurely pace, but heart failure physicians increasingly see the moment when patients arrive in the hospital with an episode of acute decompensated heart failure as a time-sensitive event that requires rapid intervention in a manner much more akin to an acute MI than to chronic heart failure.
While the tide is slowly shifting to put a premium on rapid treatment to try to decongest acute heart failure patients, the treatment options clinicians have available for these patients often remain inadequate.
“Development of drugs for acutely decompensated heart failure has been extremely difficult. We have done a really horrible job treating this disease,” Dr. Milton Packer said at the HFSA annual scientific meeting.
Treatment of acute heart failure patients has generally focused on relieving dyspnea, but part of the new appreciation of this state as an emergency event involves understanding that the pathology patients have when they reach the hospital is much more global and has profoundly morbid consequences.
“We want more from treatment than for patients to feel a little bit better an hour or two sooner” by relieving dyspnea, said Dr. Packer, professor of medicine and a heart failure specialist at the University of Texas Southwestern Medical Center in Dallas. “In the first 6 hours [of acute heart failure hospitalization], many patients are spilling troponin. We don’t know what this means, but the patients who spill troponin have a markedly increased risk for a more complicated hospital course.” About 10%-25% of patients hospitalized with acute heart failure have recurrent worsening heart failure, and many of these are also the patients who have a spike in their troponin level during initial hospitalization, he noted.
The troponin release in many patients and its association with worse outcomes is a clue that these patients are experiencing an ischemic myocardial event similar to an acute MI, possibly caused by myocardial-wall stretch, Dr. Packer said in an interview.
“If we can reduce this early acute cardiac dilatation, maybe we can reduce myocardial injury, reduce troponin release, and have favorable effects on clinically relevant events both short-term and long-term,” he said. “That’s why in trials [of investigational drugs for acute heart failure] we are treating patients earlier. Before we said we could enroll patients [into acute-treatment trials] within 48 hours of hospital admission. Now we enroll within 16 hours, or within 12 hours. We’ve learned that early intervention is important. That makes acute heart failure a lot more similar to an acute MI.”
European Society recommends faster acute heart failure management
European heart failure specialists have also become convinced that acute heart failure is an emergency that needs a rapid response. In June, the Heart Failure Association of the European Society of Cardiology published new recommendations on the in-hospital management of patients with acute heart failure (Eur J Heart Fail 2015 June;17[6];544-58). In the document, the association’s writing panel said, “The potentially greater benefit of early treatment is of conceptual importance in many cardiovascular presentations (e.g., myocardial infarction). Unfortunately, acute heart failure has not been considered with this regard until recently.” Breaking with the past, the association’s new recommendations now say that “all acute heart failure patients should receive appropriate therapy as early as possible,” an approach that involves starting acute management in the prehospital setting.
One member of the writing group for these recommendations put it more succinctly while speaking at the annual congress of the European Society of Cardiology in London in August: “Time is muscle in acute heart failure,” said Dr. Piotr Ponikowski, professor and heart failure specialist at the Medical University in Wroclaw, Poland. “When a patient has acute coronary syndrome everyone rushes, but we have patients with acute heart failure and no one rushes. We give furosemide, maybe something else, and then we wait and see.” He recommended adhering to a schedule that would have a patient assessed and initially treated within the first hour of hospitalization, and even sooner if treatment could start at the prehospital stage.
Like Dr. Packer, Dr. Ponikowski also lamented the inadequate tools now available for treating acute heart failure and the pressing need to identify better approaches to treatment, especially for selected acute heart failure patients.
“It is too simple to think that one drug or one treatment will help the entire spectrum of acute heart failure patients,” he said. “Our hypothesis is that profiling patients at every step of acute heart failure is crucial.”
He itemized five distinct types of acute heart failure patients based on their precipitating triggers of decompensation:
• Rapid arrhythmia or rhythm disturbance.
• Hypertension emergency.
• Pulmonary embolism.
• Pulmonary infection.
• Mechanical cause of acute heart failure.
“We need to clinically profile” patients into these subgroups to better tailor management, he said.
Another important aspect of patient heterogeneity is that fluid congestion may be less important in many patients compared with fluid redistribution from the splanchnic circulation. This distinction is important because fluid redistribution may be better treated with a vasodilator than with a diuretic, he noted. He voiced hope that two phase III trials now in progress with two unique vasodilator drugs, the TRUE-AHF trial of ularitide, and the RELAX-AHF-2 trial of serelaxin, may identify two new vasodilators with “unique effects” that could potentially launch a new era in management of selected patients with acute heart failure. Dr. Packer, the principal investigator for the ularitide trial, offered similar hope.
The responsiveness of acute heart failure patients to in-hospital treatment may vary depending on what end-organ damage they experience, Dr. Ponikowski said.
This end-organ damage is often an acute process occurring during hospitalization caused by the fluid congestion and redistribution that occurs during acute heart failure, said Dr. Alexandre Mebazaa, professor of anesthesiology and critical care medicine at Lariboisière Hospital in Paris.
“Fluid overload leads to organ dysfunction. In the past, we thought that kidney dysfunction [occurring during acute heart failure] was due to low cardiac output, but we know that dysfunction in the kidney and liver is due to congestion, and diuretics do not remove water from the liver and kidney,” Dr. Mebazaa said in an interview. “Diuretics may remove fluid from vessels, but not from organs. We need new approaches to remove fluid from organs – from the kidney, liver, and lungs” – during acute heart failure. This is another reason why heart failure physicians are excited about the possibility of finding new vasodilators, such a ularitide and serelaxin, that might address the issue of venous congestion in peripheral organs.
Faster management endorsed by U.S. clinicians, too
“We used to think that the reason why patients with acute heart failure were not voiding well and became diuretic resistant was because of poor cardiac output. Now we know that there is a lot of venous congestion with an impact on the liver and kidneys,” agreed Dr. Mariell L. Jessup, professor and medical director of the Penn Heart and Vascular Center at the University of Pennsylvania in Philadelphia. “We’ve begun to appreciate how important venous congestion is in causing high pressures on the right side” of the circulatory system, she said in an interview.
Other U.S. physicians echo the call by Dr. Packer and the European cardiologists for faster treatment of acute heart failure. “I collected data at U.S. hospitals and found it took an average of 22 hours for decompensated heart failure patients to receive treatment,” said Dr. Maria Rosa Costanzo, medical director of the heart failure and pulmonary hypertension program at Advocate Heart Institute in Naperville, Ill. “I have tried to convey the message that these patients must be treated early, and this is associated with better outcomes,” she said in an interview during the HFSA meeting.
“Early treatment means at least two doses of intravenous diuretic in the emergency department. We’ve seen that the two immediate doses can make a big difference, producing shorter lengths of stay in the intensive care unit, fewer rehospitalizations, and fewer deaths,” according to data collected in the ADHERE (Acute Decompensated Heart Failure National Registry), she said. “But this has not yet been picked up in a lot of U.S. practice.” Although the hemodynamic abnormalities that lead up to an acute decompensation event can take several weeks of steady worsening before severe symptoms drive a patient to the hospital, once the patient requires hospitalization “it should be treated as an emergency,” she said.
Dr. Costanzo is a major advocate for using ultrafiltration as a second-line treatment for acute decompensated heart failure patients who do not adequately respond to diuretic treatment, but for the time being, ultrafiltration remains a controversial option that at least some other heart failure physicians do not endorse, and it can involve reimbursement issues as many insurers consider it investigational.
“Try to get the patient decongested within the first 6 hours [after arrival at the hospital] or even sooner, within the first 1-2 hours,” recommended Dr. Christopher M. O’Connor, chief executive officer of Inova Heart and Vascular Institute in Falls Church, Va. He suggested treating patients with a combination of diuretics and vasodilators. “Some people are talking about instituting a performance measure for treating acute heart failure within the first 6 hours,” Dr. O’Connor said in an interview.
Currently, vasodilator treatment is limited to standard agents such as intravenous nitroglycerin, but Dr. O’Connor shared the hope that sometime soon a new vasodilator may be shown effective for acutely decompensated patients. He is a coinvestigator on the TRUE-AHF study of ularitide. “We hope that these new vasodilators, ularitide and serelaxin, will be good complements to diuretics, he said. Dr. O’Connor also recommended that clinicians shy away from using ultrafiltration as a back-up therapy, believing that it was shown ineffective and potentially harmful in results from the CARRESS-HF trial (N Engl J Med 2012 Dec 13;367[24]:2296-304).
But not all heart failure specialists see acute heart failure as a new frontier for early treatment and new drug discovery.
“So much energy has already been spent on acute heart failure with very little return,” said Dr. Clyde W. Yancy, professor and chief of cardiology at Northwestern University in Chicago. “I think that our best opportunities in heart failure are in prevention and in better chronic care. The hospitalized patient is so broad and complex; if we’re looking at how to best spend our resources I think it’s best to focus on prevention,” he said in an interview.
“The hospital experience needs to shift toward better use of systems of care and focus less on the biology. The biggest challenge is how to coordinate all the systems to make sure that patients have access to the resources and can obtain [existing] medications. Patients don’t often have the literacy to understand discharge instructions, and our systems are overwhelmed by trying to have 7-day follow-up visits. Focusing on management of the hospitalized patient does not give us a good return on the investment. There is no question that acute heart failure is an unmet need, but the greater unmet need is prevention and improved chronic care. No single intervention will dramatically change the acute heart failure experience. Focusing on the hospitalization does not offer us management opportunities that are as robust as we once thought,” Dr. Yancy said.
Dr. Packer has been a consultant to 22 companies. Dr. Ponikowski has been a consultant to, speaker for, or has received research grants from 11 companies. Dr. Mebazaa has received speaking honoraria and consulting fees from 11 companies. Dr. Jessup, Dr. Costanzo, and Dr. Yancy had no disclosures. Dr. O’Connor has been a consultant to ResMed, Roche Diagnostics, Cardiorentis, Bayer, and Actelion and has received research grants from Otsuka, ResMed, and Roche Diagnostics.
On Twitter@mitchelzoler
NATIONAL HARBOR, MD. – Acute decompensated heart failure is becoming more of an emergency.
Traditionally, it has been seen as a lumbering event that could be treated at a relatively leisurely pace, but heart failure physicians increasingly see the moment when patients arrive in the hospital with an episode of acute decompensated heart failure as a time-sensitive event that requires rapid intervention in a manner much more akin to an acute MI than to chronic heart failure.
While the tide is slowly shifting to put a premium on rapid treatment to try to decongest acute heart failure patients, the treatment options clinicians have available for these patients often remain inadequate.
“Development of drugs for acutely decompensated heart failure has been extremely difficult. We have done a really horrible job treating this disease,” Dr. Milton Packer said at the HFSA annual scientific meeting.
Treatment of acute heart failure patients has generally focused on relieving dyspnea, but part of the new appreciation of this state as an emergency event involves understanding that the pathology patients have when they reach the hospital is much more global and has profoundly morbid consequences.
“We want more from treatment than for patients to feel a little bit better an hour or two sooner” by relieving dyspnea, said Dr. Packer, professor of medicine and a heart failure specialist at the University of Texas Southwestern Medical Center in Dallas. “In the first 6 hours [of acute heart failure hospitalization], many patients are spilling troponin. We don’t know what this means, but the patients who spill troponin have a markedly increased risk for a more complicated hospital course.” About 10%-25% of patients hospitalized with acute heart failure have recurrent worsening heart failure, and many of these are also the patients who have a spike in their troponin level during initial hospitalization, he noted.
The troponin release in many patients and its association with worse outcomes is a clue that these patients are experiencing an ischemic myocardial event similar to an acute MI, possibly caused by myocardial-wall stretch, Dr. Packer said in an interview.
“If we can reduce this early acute cardiac dilatation, maybe we can reduce myocardial injury, reduce troponin release, and have favorable effects on clinically relevant events both short-term and long-term,” he said. “That’s why in trials [of investigational drugs for acute heart failure] we are treating patients earlier. Before we said we could enroll patients [into acute-treatment trials] within 48 hours of hospital admission. Now we enroll within 16 hours, or within 12 hours. We’ve learned that early intervention is important. That makes acute heart failure a lot more similar to an acute MI.”
European Society recommends faster acute heart failure management
European heart failure specialists have also become convinced that acute heart failure is an emergency that needs a rapid response. In June, the Heart Failure Association of the European Society of Cardiology published new recommendations on the in-hospital management of patients with acute heart failure (Eur J Heart Fail 2015 June;17[6];544-58). In the document, the association’s writing panel said, “The potentially greater benefit of early treatment is of conceptual importance in many cardiovascular presentations (e.g., myocardial infarction). Unfortunately, acute heart failure has not been considered with this regard until recently.” Breaking with the past, the association’s new recommendations now say that “all acute heart failure patients should receive appropriate therapy as early as possible,” an approach that involves starting acute management in the prehospital setting.
One member of the writing group for these recommendations put it more succinctly while speaking at the annual congress of the European Society of Cardiology in London in August: “Time is muscle in acute heart failure,” said Dr. Piotr Ponikowski, professor and heart failure specialist at the Medical University in Wroclaw, Poland. “When a patient has acute coronary syndrome everyone rushes, but we have patients with acute heart failure and no one rushes. We give furosemide, maybe something else, and then we wait and see.” He recommended adhering to a schedule that would have a patient assessed and initially treated within the first hour of hospitalization, and even sooner if treatment could start at the prehospital stage.
Like Dr. Packer, Dr. Ponikowski also lamented the inadequate tools now available for treating acute heart failure and the pressing need to identify better approaches to treatment, especially for selected acute heart failure patients.
“It is too simple to think that one drug or one treatment will help the entire spectrum of acute heart failure patients,” he said. “Our hypothesis is that profiling patients at every step of acute heart failure is crucial.”
He itemized five distinct types of acute heart failure patients based on their precipitating triggers of decompensation:
• Rapid arrhythmia or rhythm disturbance.
• Hypertension emergency.
• Pulmonary embolism.
• Pulmonary infection.
• Mechanical cause of acute heart failure.
“We need to clinically profile” patients into these subgroups to better tailor management, he said.
Another important aspect of patient heterogeneity is that fluid congestion may be less important in many patients compared with fluid redistribution from the splanchnic circulation. This distinction is important because fluid redistribution may be better treated with a vasodilator than with a diuretic, he noted. He voiced hope that two phase III trials now in progress with two unique vasodilator drugs, the TRUE-AHF trial of ularitide, and the RELAX-AHF-2 trial of serelaxin, may identify two new vasodilators with “unique effects” that could potentially launch a new era in management of selected patients with acute heart failure. Dr. Packer, the principal investigator for the ularitide trial, offered similar hope.
The responsiveness of acute heart failure patients to in-hospital treatment may vary depending on what end-organ damage they experience, Dr. Ponikowski said.
This end-organ damage is often an acute process occurring during hospitalization caused by the fluid congestion and redistribution that occurs during acute heart failure, said Dr. Alexandre Mebazaa, professor of anesthesiology and critical care medicine at Lariboisière Hospital in Paris.
“Fluid overload leads to organ dysfunction. In the past, we thought that kidney dysfunction [occurring during acute heart failure] was due to low cardiac output, but we know that dysfunction in the kidney and liver is due to congestion, and diuretics do not remove water from the liver and kidney,” Dr. Mebazaa said in an interview. “Diuretics may remove fluid from vessels, but not from organs. We need new approaches to remove fluid from organs – from the kidney, liver, and lungs” – during acute heart failure. This is another reason why heart failure physicians are excited about the possibility of finding new vasodilators, such a ularitide and serelaxin, that might address the issue of venous congestion in peripheral organs.
Faster management endorsed by U.S. clinicians, too
“We used to think that the reason why patients with acute heart failure were not voiding well and became diuretic resistant was because of poor cardiac output. Now we know that there is a lot of venous congestion with an impact on the liver and kidneys,” agreed Dr. Mariell L. Jessup, professor and medical director of the Penn Heart and Vascular Center at the University of Pennsylvania in Philadelphia. “We’ve begun to appreciate how important venous congestion is in causing high pressures on the right side” of the circulatory system, she said in an interview.
Other U.S. physicians echo the call by Dr. Packer and the European cardiologists for faster treatment of acute heart failure. “I collected data at U.S. hospitals and found it took an average of 22 hours for decompensated heart failure patients to receive treatment,” said Dr. Maria Rosa Costanzo, medical director of the heart failure and pulmonary hypertension program at Advocate Heart Institute in Naperville, Ill. “I have tried to convey the message that these patients must be treated early, and this is associated with better outcomes,” she said in an interview during the HFSA meeting.
“Early treatment means at least two doses of intravenous diuretic in the emergency department. We’ve seen that the two immediate doses can make a big difference, producing shorter lengths of stay in the intensive care unit, fewer rehospitalizations, and fewer deaths,” according to data collected in the ADHERE (Acute Decompensated Heart Failure National Registry), she said. “But this has not yet been picked up in a lot of U.S. practice.” Although the hemodynamic abnormalities that lead up to an acute decompensation event can take several weeks of steady worsening before severe symptoms drive a patient to the hospital, once the patient requires hospitalization “it should be treated as an emergency,” she said.
Dr. Costanzo is a major advocate for using ultrafiltration as a second-line treatment for acute decompensated heart failure patients who do not adequately respond to diuretic treatment, but for the time being, ultrafiltration remains a controversial option that at least some other heart failure physicians do not endorse, and it can involve reimbursement issues as many insurers consider it investigational.
“Try to get the patient decongested within the first 6 hours [after arrival at the hospital] or even sooner, within the first 1-2 hours,” recommended Dr. Christopher M. O’Connor, chief executive officer of Inova Heart and Vascular Institute in Falls Church, Va. He suggested treating patients with a combination of diuretics and vasodilators. “Some people are talking about instituting a performance measure for treating acute heart failure within the first 6 hours,” Dr. O’Connor said in an interview.
Currently, vasodilator treatment is limited to standard agents such as intravenous nitroglycerin, but Dr. O’Connor shared the hope that sometime soon a new vasodilator may be shown effective for acutely decompensated patients. He is a coinvestigator on the TRUE-AHF study of ularitide. “We hope that these new vasodilators, ularitide and serelaxin, will be good complements to diuretics, he said. Dr. O’Connor also recommended that clinicians shy away from using ultrafiltration as a back-up therapy, believing that it was shown ineffective and potentially harmful in results from the CARRESS-HF trial (N Engl J Med 2012 Dec 13;367[24]:2296-304).
But not all heart failure specialists see acute heart failure as a new frontier for early treatment and new drug discovery.
“So much energy has already been spent on acute heart failure with very little return,” said Dr. Clyde W. Yancy, professor and chief of cardiology at Northwestern University in Chicago. “I think that our best opportunities in heart failure are in prevention and in better chronic care. The hospitalized patient is so broad and complex; if we’re looking at how to best spend our resources I think it’s best to focus on prevention,” he said in an interview.
“The hospital experience needs to shift toward better use of systems of care and focus less on the biology. The biggest challenge is how to coordinate all the systems to make sure that patients have access to the resources and can obtain [existing] medications. Patients don’t often have the literacy to understand discharge instructions, and our systems are overwhelmed by trying to have 7-day follow-up visits. Focusing on management of the hospitalized patient does not give us a good return on the investment. There is no question that acute heart failure is an unmet need, but the greater unmet need is prevention and improved chronic care. No single intervention will dramatically change the acute heart failure experience. Focusing on the hospitalization does not offer us management opportunities that are as robust as we once thought,” Dr. Yancy said.
Dr. Packer has been a consultant to 22 companies. Dr. Ponikowski has been a consultant to, speaker for, or has received research grants from 11 companies. Dr. Mebazaa has received speaking honoraria and consulting fees from 11 companies. Dr. Jessup, Dr. Costanzo, and Dr. Yancy had no disclosures. Dr. O’Connor has been a consultant to ResMed, Roche Diagnostics, Cardiorentis, Bayer, and Actelion and has received research grants from Otsuka, ResMed, and Roche Diagnostics.
On Twitter@mitchelzoler
EXPERT ANALYSIS FROM THE HFSA ANNUAL SCIENTIFIC MEETING
TCT: FORMA system tested in severe tricuspid regurgitation
The investigational FORMA system seems safe and may be effective in patients with NYHA Class III/IV heart failure and severe tricuspid valve regurgitation, based on 13 first-in-human cases.
A Canadian surgical team employed the FORMA system (Edwards Lifesciences) as compassionate use therapy for a set of patients with inoperable tricuspid regurgitation. The device was successfully deployed in 12 of the 13 patients, according to data presented at the Transcatheter Cardiovascular Therapeutics annual meeting. There were no deaths or major clinical complications in any of the patients.
A report on seven of these patients was simultaneously published in the Journal of the American College of Cardiology. All of the patients had severe tricuspid regurgitation and heart failure; before surgery, six had a New York Heart Association (NYHA) Functional Classification of III/IV. By 30 days after the procedure, all had improved to NYHA II, wrote Dr. Francisco Campelo-Parada of the Quebec Heart and Lung Institute, the paper’s primary author. Peripheral edema declined and all patients experienced functional improvement, as well.
According to Edwards Lifesciences, the FORMA device uses a foam-filled polymer balloon spacer to reduce tricuspid regurgitation by occupying the regurgitant orifice area and providing a surface for the coaptation of the valve’s native leaflets. Implantation is performed via the left axillary vein.
Patients in the series were a mean of 76 years old. All had severe tricuspid regurgitation. The mean maximal vena contracta was 15.5 mm.
Six had coronary artery disease and five had previously undergone open heart surgery. Additionally, two had previously undergone mitral valve surgery and two had undergone aortic valve surgery. Pulmonary hypertension was present in five. Five patients also had persistent atrial fibrillation. Six had renal insufficiency, with one patient on dialysis. The baseline furosemide dose was 80 mg/day.
All procedures were performed under general sedation and fluoroscopic guidance, with postprocedural positioning checked by cardiac-CT and/or a chest x-ray. The mean postop stay was 4 days.
Tricuspid regurgitation was reduced by at least 1 degree in all patients during the operation; four patients had an immediate 2-degree reduction, reclassifying their regurgitation as mild. Two experienced new-onset atrial fibrillation, and one had several episodes of nonsustained ventricular tachycardia that was managed with beta-blockers.
At the first clinical follow-up 30 days after surgery, all but one patient had an improvement to Class II NYHA status.
Two patients were able to reduce their diuretic dosage; there were no other medication changes. Peripheral edema declined in the entire cohort. Tricuspid regurgitation was graded as moderate in all patients.
There were also associated improvements in quality of life, based on scores on the Kansas City Cardiomyopathy Questionnaire, which increased from 59 before surgery to 86 after surgery. Exercise capacity as measured by the 6-Minute Walk Test improved from 297 meters to 326 meters.
The authors suggested that the 15-mm spacer used in the FORMA device was not well-matched with the mean 15.5-mm vena contracta size in the cohort. Better outcomes might be possible if a larger spacer were available.
“Despite good device positioning, complete coaptation was not achieved, resulting in significant residual degree of postprocedural tricuspid regurgitation,” they said. “Also, the very advanced stage of the disease in most patients may have played a role in the mild reduction at 30 days.”
Despite the rather mild, 1-degree improvement, patients did make considerable improvements in heart failure and functional status. Therefore, the team recommended further study for FORMA, with an eye toward optimizing patient selection.
“Specific criteria for quantifying right ventricular dysfunction and pulmonary hypertension, along with novel quantitative echocardiographic imaging criteria may be required,” they said. “It is conceivable that larger than the currently available spacer sizes may be required to improve echocardiographic results in patients with large noncoaptation defects and vena contracta.”
Dr. Campelo-Parada had no financial disclosures with regard to the device.
The investigational FORMA system seems safe and may be effective in patients with NYHA Class III/IV heart failure and severe tricuspid valve regurgitation, based on 13 first-in-human cases.
A Canadian surgical team employed the FORMA system (Edwards Lifesciences) as compassionate use therapy for a set of patients with inoperable tricuspid regurgitation. The device was successfully deployed in 12 of the 13 patients, according to data presented at the Transcatheter Cardiovascular Therapeutics annual meeting. There were no deaths or major clinical complications in any of the patients.
A report on seven of these patients was simultaneously published in the Journal of the American College of Cardiology. All of the patients had severe tricuspid regurgitation and heart failure; before surgery, six had a New York Heart Association (NYHA) Functional Classification of III/IV. By 30 days after the procedure, all had improved to NYHA II, wrote Dr. Francisco Campelo-Parada of the Quebec Heart and Lung Institute, the paper’s primary author. Peripheral edema declined and all patients experienced functional improvement, as well.
According to Edwards Lifesciences, the FORMA device uses a foam-filled polymer balloon spacer to reduce tricuspid regurgitation by occupying the regurgitant orifice area and providing a surface for the coaptation of the valve’s native leaflets. Implantation is performed via the left axillary vein.
Patients in the series were a mean of 76 years old. All had severe tricuspid regurgitation. The mean maximal vena contracta was 15.5 mm.
Six had coronary artery disease and five had previously undergone open heart surgery. Additionally, two had previously undergone mitral valve surgery and two had undergone aortic valve surgery. Pulmonary hypertension was present in five. Five patients also had persistent atrial fibrillation. Six had renal insufficiency, with one patient on dialysis. The baseline furosemide dose was 80 mg/day.
All procedures were performed under general sedation and fluoroscopic guidance, with postprocedural positioning checked by cardiac-CT and/or a chest x-ray. The mean postop stay was 4 days.
Tricuspid regurgitation was reduced by at least 1 degree in all patients during the operation; four patients had an immediate 2-degree reduction, reclassifying their regurgitation as mild. Two experienced new-onset atrial fibrillation, and one had several episodes of nonsustained ventricular tachycardia that was managed with beta-blockers.
At the first clinical follow-up 30 days after surgery, all but one patient had an improvement to Class II NYHA status.
Two patients were able to reduce their diuretic dosage; there were no other medication changes. Peripheral edema declined in the entire cohort. Tricuspid regurgitation was graded as moderate in all patients.
There were also associated improvements in quality of life, based on scores on the Kansas City Cardiomyopathy Questionnaire, which increased from 59 before surgery to 86 after surgery. Exercise capacity as measured by the 6-Minute Walk Test improved from 297 meters to 326 meters.
The authors suggested that the 15-mm spacer used in the FORMA device was not well-matched with the mean 15.5-mm vena contracta size in the cohort. Better outcomes might be possible if a larger spacer were available.
“Despite good device positioning, complete coaptation was not achieved, resulting in significant residual degree of postprocedural tricuspid regurgitation,” they said. “Also, the very advanced stage of the disease in most patients may have played a role in the mild reduction at 30 days.”
Despite the rather mild, 1-degree improvement, patients did make considerable improvements in heart failure and functional status. Therefore, the team recommended further study for FORMA, with an eye toward optimizing patient selection.
“Specific criteria for quantifying right ventricular dysfunction and pulmonary hypertension, along with novel quantitative echocardiographic imaging criteria may be required,” they said. “It is conceivable that larger than the currently available spacer sizes may be required to improve echocardiographic results in patients with large noncoaptation defects and vena contracta.”
Dr. Campelo-Parada had no financial disclosures with regard to the device.
The investigational FORMA system seems safe and may be effective in patients with NYHA Class III/IV heart failure and severe tricuspid valve regurgitation, based on 13 first-in-human cases.
A Canadian surgical team employed the FORMA system (Edwards Lifesciences) as compassionate use therapy for a set of patients with inoperable tricuspid regurgitation. The device was successfully deployed in 12 of the 13 patients, according to data presented at the Transcatheter Cardiovascular Therapeutics annual meeting. There were no deaths or major clinical complications in any of the patients.
A report on seven of these patients was simultaneously published in the Journal of the American College of Cardiology. All of the patients had severe tricuspid regurgitation and heart failure; before surgery, six had a New York Heart Association (NYHA) Functional Classification of III/IV. By 30 days after the procedure, all had improved to NYHA II, wrote Dr. Francisco Campelo-Parada of the Quebec Heart and Lung Institute, the paper’s primary author. Peripheral edema declined and all patients experienced functional improvement, as well.
According to Edwards Lifesciences, the FORMA device uses a foam-filled polymer balloon spacer to reduce tricuspid regurgitation by occupying the regurgitant orifice area and providing a surface for the coaptation of the valve’s native leaflets. Implantation is performed via the left axillary vein.
Patients in the series were a mean of 76 years old. All had severe tricuspid regurgitation. The mean maximal vena contracta was 15.5 mm.
Six had coronary artery disease and five had previously undergone open heart surgery. Additionally, two had previously undergone mitral valve surgery and two had undergone aortic valve surgery. Pulmonary hypertension was present in five. Five patients also had persistent atrial fibrillation. Six had renal insufficiency, with one patient on dialysis. The baseline furosemide dose was 80 mg/day.
All procedures were performed under general sedation and fluoroscopic guidance, with postprocedural positioning checked by cardiac-CT and/or a chest x-ray. The mean postop stay was 4 days.
Tricuspid regurgitation was reduced by at least 1 degree in all patients during the operation; four patients had an immediate 2-degree reduction, reclassifying their regurgitation as mild. Two experienced new-onset atrial fibrillation, and one had several episodes of nonsustained ventricular tachycardia that was managed with beta-blockers.
At the first clinical follow-up 30 days after surgery, all but one patient had an improvement to Class II NYHA status.
Two patients were able to reduce their diuretic dosage; there were no other medication changes. Peripheral edema declined in the entire cohort. Tricuspid regurgitation was graded as moderate in all patients.
There were also associated improvements in quality of life, based on scores on the Kansas City Cardiomyopathy Questionnaire, which increased from 59 before surgery to 86 after surgery. Exercise capacity as measured by the 6-Minute Walk Test improved from 297 meters to 326 meters.
The authors suggested that the 15-mm spacer used in the FORMA device was not well-matched with the mean 15.5-mm vena contracta size in the cohort. Better outcomes might be possible if a larger spacer were available.
“Despite good device positioning, complete coaptation was not achieved, resulting in significant residual degree of postprocedural tricuspid regurgitation,” they said. “Also, the very advanced stage of the disease in most patients may have played a role in the mild reduction at 30 days.”
Despite the rather mild, 1-degree improvement, patients did make considerable improvements in heart failure and functional status. Therefore, the team recommended further study for FORMA, with an eye toward optimizing patient selection.
“Specific criteria for quantifying right ventricular dysfunction and pulmonary hypertension, along with novel quantitative echocardiographic imaging criteria may be required,” they said. “It is conceivable that larger than the currently available spacer sizes may be required to improve echocardiographic results in patients with large noncoaptation defects and vena contracta.”
Dr. Campelo-Parada had no financial disclosures with regard to the device.
FROM TCT 2015
Key clinical point: The investigational FORMA system seems safe and may be effective in patients with NYHA Class III/IV heart failure and severe tricuspid valve regurgitation.
Major finding: The improved heart failure from NYHA Class III/IV to Class II in six of seven patients with severe tricuspid valve regurgitation.
Data source: The device has been used in 13 patients thus far, under compassionate use allowance.
Disclosures: Edwards Lifesciences manufactures and is investigating the device. Dr. Campelo-Parada had no disclosures.
TCT: SAPIEN XT TAVR system gains valve-in-valve indication
SAN FRANCISCO – Food and Drug Administration approval of the SAPIEN XT transcatheter aortic valve for the valve-in-valve indication in high–surgical risk patients means that, officially, there are now two options for transcatheter replacement of a faulty prosthetic aortic valve.
CoreValve, the competing device for transcatheter aortic valve replacement (TAVR) in U.S. practice, received FDA approval for the valve-in-valve indication in March.
Researchers reported the data behind SAPIEN XT’s approval from two U.S. registries with a total of 197 high-risk patients at the Transcatheter Cardiovascular Therapeutics annual meeting on Oct. 16, the same day the FDA approval was announced by Edwards Lifesciences. The mortality rate was 4% after 30 days and 13% after 1 year, and the 1-year stroke rate was 4%, Dr. Danny Dvir said at the meeting.
One feature of the 1-year survival data that Dr. Dvir called “quite amazing” was the absence of any deaths during the first 30 days post TAVR with the SAPIEN XT system, and 7% at 1 year among the 100 patients treated during the final 8 months of the registry, who comprised the second half of patients enrolled in the registry. “I think we learned how to choose the right patients for this procedure,” said Dr. Dvir, an interventional cardiologist at St. Paul’s Hospital in Vancouver, B.C.
Given the risk from replacing a failed bioprosthetic valve by open surgery, “TAVR is becoming the preferred approach to valve-in-valve,” commented Dr. Ajay J. Kirtane, director of the cardiac catheterization laboratories at Columbia University in New York.
The registry results also highlighted a key limitation of the SAPIEN XT valve: patients with a bioprosthetic valve with a relatively narrow inner diameter of 21 mm or less.
Because the SAPIEN series of valves are balloon expandable and designed to sit directly in the aortic-valve annulus, the inner diameter of an existing bioprothesis can limit the TAVR options. The CoreValve, self-expanding valves sit in a supravalvular location and better fit through a narrow-frame existing valve.
This geometric limitation means that SAPIEN XT cannot work in patients with existing valves less than 21 mm in inner diameter, and the registry excluded such patients, who generally comprise about 10% of all patients with severe aortic stenosis.
In addition, among the 28% of patients enrolled in the registry who had an existing valve diameter of 21 mm, the 1-year mortality rate was strikingly high – 20% – compared with an 11% mortality rate in patients with existing valve diameters of 23 or 25 mm, Dr. Dvir reported.
“If a patient had a smaller inner diameter I’d definitely go with the supravalvular valve. For a bigger valve you have more choices,” commented Dr. Axel H.P. Linke, codirector of cardiology at the Heart Center at the University of Leipzig (Germany).
“It’s not fair to extrapolate these findings with SAPIEN XT to smaller [existing bioprosthetic] valves. There is the ability to go to lower inner diameters” using CoreValve, commented Dr. Jeffrey J. Popma, who led the U.S. CoreValve studies and is professor at Harvard Medical School and director of interventional cardiology at Beth Israel Deaconess Medical Center in Boston.
The two sequential valve-in-valve U.S. registries for SAPIEN XT ran as part of the PARTNER 2 trial, designed to test the safety and efficacy of SAPIEN XT in patients undergoing TAVR for a de novo aortic valve. The 197 patients in the full registry averaged 79 years of age, 60% were men, their average Society of Thoracic Surgeons mortality risk score was 9.7%, 50% had atrial fibrillation, 95% had New York Heart Association class III or IV symptoms, and baseline screening identified one-third of the patients as frail.
The PARTNER 2 trial valve-in-valve registry and extended registry are sponsored by Edwards Lifesciences, which markets the SAPIEN XT system. Dr. Dvir has been a consultant to Edwards and to Medtronic, the company that markets the CoreValve systems. Dr. Kirtane has received research grants from Boston Scientific, St. Jude, Eli Lilly, GlaxoSmithKline, Abiomed, and Abbott Vascular. Dr. Linke has been a consultant to Boston Scientific, St. Jude, Bard, Edwards, and Medtronic and owns equity in Claret. Dr. Popma has been principal investigator of the CoreValve studies, has been a consultant to Abbott Vascular, Boston Scientific, and Director Flow; he owns equity in Direct Flow, and he has received research grants from Abbott Vascular, Medtronic, Boston Scientific, and Cook Medical.
On Twitter @mitchelzoler
Transcatheter aortic valve replacement is on track to be the preferred treatment for aortic valve-in-valve replacement. How many surgeons are eager to do a reoperation on an aortic valve?
 |
Dr. David L. Brown |
Transcatheter valve replacement is the best way to treat aortic valve-in-valve disease. The results reported by Dr. Dvir further support that notion. One attraction of the balloon-expandable TAVR valve is that it greatly reduces the risk of annular rupture. But it is important to use a TAVR valve that is right sized. If the existing bioprosthesis is narrow then you have no choice but to use a self-expanding valve.
We had already been placing the SAPIEN XT, as well as SAPIEN 3 valves before XT received the valve-in-valve indication. In the past, when we did this we were never sure if the procedure would receive insurance coverage, but now XT should be covered because of the FDA approval, so this is a huge deal for us. It eliminates our economic uncertainty.
Dr. David L. Brown is director of interventional cardiology at the Heart Hospital Baylor Plano, Texas. He had no disclosures. He made these comments as a discussant at the meeting and in an interview.
Transcatheter aortic valve replacement is on track to be the preferred treatment for aortic valve-in-valve replacement. How many surgeons are eager to do a reoperation on an aortic valve?
 |
Dr. David L. Brown |
Transcatheter valve replacement is the best way to treat aortic valve-in-valve disease. The results reported by Dr. Dvir further support that notion. One attraction of the balloon-expandable TAVR valve is that it greatly reduces the risk of annular rupture. But it is important to use a TAVR valve that is right sized. If the existing bioprosthesis is narrow then you have no choice but to use a self-expanding valve.
We had already been placing the SAPIEN XT, as well as SAPIEN 3 valves before XT received the valve-in-valve indication. In the past, when we did this we were never sure if the procedure would receive insurance coverage, but now XT should be covered because of the FDA approval, so this is a huge deal for us. It eliminates our economic uncertainty.
Dr. David L. Brown is director of interventional cardiology at the Heart Hospital Baylor Plano, Texas. He had no disclosures. He made these comments as a discussant at the meeting and in an interview.
Transcatheter aortic valve replacement is on track to be the preferred treatment for aortic valve-in-valve replacement. How many surgeons are eager to do a reoperation on an aortic valve?
 |
Dr. David L. Brown |
Transcatheter valve replacement is the best way to treat aortic valve-in-valve disease. The results reported by Dr. Dvir further support that notion. One attraction of the balloon-expandable TAVR valve is that it greatly reduces the risk of annular rupture. But it is important to use a TAVR valve that is right sized. If the existing bioprosthesis is narrow then you have no choice but to use a self-expanding valve.
We had already been placing the SAPIEN XT, as well as SAPIEN 3 valves before XT received the valve-in-valve indication. In the past, when we did this we were never sure if the procedure would receive insurance coverage, but now XT should be covered because of the FDA approval, so this is a huge deal for us. It eliminates our economic uncertainty.
Dr. David L. Brown is director of interventional cardiology at the Heart Hospital Baylor Plano, Texas. He had no disclosures. He made these comments as a discussant at the meeting and in an interview.
SAN FRANCISCO – Food and Drug Administration approval of the SAPIEN XT transcatheter aortic valve for the valve-in-valve indication in high–surgical risk patients means that, officially, there are now two options for transcatheter replacement of a faulty prosthetic aortic valve.
CoreValve, the competing device for transcatheter aortic valve replacement (TAVR) in U.S. practice, received FDA approval for the valve-in-valve indication in March.
Researchers reported the data behind SAPIEN XT’s approval from two U.S. registries with a total of 197 high-risk patients at the Transcatheter Cardiovascular Therapeutics annual meeting on Oct. 16, the same day the FDA approval was announced by Edwards Lifesciences. The mortality rate was 4% after 30 days and 13% after 1 year, and the 1-year stroke rate was 4%, Dr. Danny Dvir said at the meeting.
One feature of the 1-year survival data that Dr. Dvir called “quite amazing” was the absence of any deaths during the first 30 days post TAVR with the SAPIEN XT system, and 7% at 1 year among the 100 patients treated during the final 8 months of the registry, who comprised the second half of patients enrolled in the registry. “I think we learned how to choose the right patients for this procedure,” said Dr. Dvir, an interventional cardiologist at St. Paul’s Hospital in Vancouver, B.C.
Given the risk from replacing a failed bioprosthetic valve by open surgery, “TAVR is becoming the preferred approach to valve-in-valve,” commented Dr. Ajay J. Kirtane, director of the cardiac catheterization laboratories at Columbia University in New York.
The registry results also highlighted a key limitation of the SAPIEN XT valve: patients with a bioprosthetic valve with a relatively narrow inner diameter of 21 mm or less.
Because the SAPIEN series of valves are balloon expandable and designed to sit directly in the aortic-valve annulus, the inner diameter of an existing bioprothesis can limit the TAVR options. The CoreValve, self-expanding valves sit in a supravalvular location and better fit through a narrow-frame existing valve.
This geometric limitation means that SAPIEN XT cannot work in patients with existing valves less than 21 mm in inner diameter, and the registry excluded such patients, who generally comprise about 10% of all patients with severe aortic stenosis.
In addition, among the 28% of patients enrolled in the registry who had an existing valve diameter of 21 mm, the 1-year mortality rate was strikingly high – 20% – compared with an 11% mortality rate in patients with existing valve diameters of 23 or 25 mm, Dr. Dvir reported.
“If a patient had a smaller inner diameter I’d definitely go with the supravalvular valve. For a bigger valve you have more choices,” commented Dr. Axel H.P. Linke, codirector of cardiology at the Heart Center at the University of Leipzig (Germany).
“It’s not fair to extrapolate these findings with SAPIEN XT to smaller [existing bioprosthetic] valves. There is the ability to go to lower inner diameters” using CoreValve, commented Dr. Jeffrey J. Popma, who led the U.S. CoreValve studies and is professor at Harvard Medical School and director of interventional cardiology at Beth Israel Deaconess Medical Center in Boston.
The two sequential valve-in-valve U.S. registries for SAPIEN XT ran as part of the PARTNER 2 trial, designed to test the safety and efficacy of SAPIEN XT in patients undergoing TAVR for a de novo aortic valve. The 197 patients in the full registry averaged 79 years of age, 60% were men, their average Society of Thoracic Surgeons mortality risk score was 9.7%, 50% had atrial fibrillation, 95% had New York Heart Association class III or IV symptoms, and baseline screening identified one-third of the patients as frail.
The PARTNER 2 trial valve-in-valve registry and extended registry are sponsored by Edwards Lifesciences, which markets the SAPIEN XT system. Dr. Dvir has been a consultant to Edwards and to Medtronic, the company that markets the CoreValve systems. Dr. Kirtane has received research grants from Boston Scientific, St. Jude, Eli Lilly, GlaxoSmithKline, Abiomed, and Abbott Vascular. Dr. Linke has been a consultant to Boston Scientific, St. Jude, Bard, Edwards, and Medtronic and owns equity in Claret. Dr. Popma has been principal investigator of the CoreValve studies, has been a consultant to Abbott Vascular, Boston Scientific, and Director Flow; he owns equity in Direct Flow, and he has received research grants from Abbott Vascular, Medtronic, Boston Scientific, and Cook Medical.
On Twitter @mitchelzoler
SAN FRANCISCO – Food and Drug Administration approval of the SAPIEN XT transcatheter aortic valve for the valve-in-valve indication in high–surgical risk patients means that, officially, there are now two options for transcatheter replacement of a faulty prosthetic aortic valve.
CoreValve, the competing device for transcatheter aortic valve replacement (TAVR) in U.S. practice, received FDA approval for the valve-in-valve indication in March.
Researchers reported the data behind SAPIEN XT’s approval from two U.S. registries with a total of 197 high-risk patients at the Transcatheter Cardiovascular Therapeutics annual meeting on Oct. 16, the same day the FDA approval was announced by Edwards Lifesciences. The mortality rate was 4% after 30 days and 13% after 1 year, and the 1-year stroke rate was 4%, Dr. Danny Dvir said at the meeting.
One feature of the 1-year survival data that Dr. Dvir called “quite amazing” was the absence of any deaths during the first 30 days post TAVR with the SAPIEN XT system, and 7% at 1 year among the 100 patients treated during the final 8 months of the registry, who comprised the second half of patients enrolled in the registry. “I think we learned how to choose the right patients for this procedure,” said Dr. Dvir, an interventional cardiologist at St. Paul’s Hospital in Vancouver, B.C.
Given the risk from replacing a failed bioprosthetic valve by open surgery, “TAVR is becoming the preferred approach to valve-in-valve,” commented Dr. Ajay J. Kirtane, director of the cardiac catheterization laboratories at Columbia University in New York.
The registry results also highlighted a key limitation of the SAPIEN XT valve: patients with a bioprosthetic valve with a relatively narrow inner diameter of 21 mm or less.
Because the SAPIEN series of valves are balloon expandable and designed to sit directly in the aortic-valve annulus, the inner diameter of an existing bioprothesis can limit the TAVR options. The CoreValve, self-expanding valves sit in a supravalvular location and better fit through a narrow-frame existing valve.
This geometric limitation means that SAPIEN XT cannot work in patients with existing valves less than 21 mm in inner diameter, and the registry excluded such patients, who generally comprise about 10% of all patients with severe aortic stenosis.
In addition, among the 28% of patients enrolled in the registry who had an existing valve diameter of 21 mm, the 1-year mortality rate was strikingly high – 20% – compared with an 11% mortality rate in patients with existing valve diameters of 23 or 25 mm, Dr. Dvir reported.
“If a patient had a smaller inner diameter I’d definitely go with the supravalvular valve. For a bigger valve you have more choices,” commented Dr. Axel H.P. Linke, codirector of cardiology at the Heart Center at the University of Leipzig (Germany).
“It’s not fair to extrapolate these findings with SAPIEN XT to smaller [existing bioprosthetic] valves. There is the ability to go to lower inner diameters” using CoreValve, commented Dr. Jeffrey J. Popma, who led the U.S. CoreValve studies and is professor at Harvard Medical School and director of interventional cardiology at Beth Israel Deaconess Medical Center in Boston.
The two sequential valve-in-valve U.S. registries for SAPIEN XT ran as part of the PARTNER 2 trial, designed to test the safety and efficacy of SAPIEN XT in patients undergoing TAVR for a de novo aortic valve. The 197 patients in the full registry averaged 79 years of age, 60% were men, their average Society of Thoracic Surgeons mortality risk score was 9.7%, 50% had atrial fibrillation, 95% had New York Heart Association class III or IV symptoms, and baseline screening identified one-third of the patients as frail.
The PARTNER 2 trial valve-in-valve registry and extended registry are sponsored by Edwards Lifesciences, which markets the SAPIEN XT system. Dr. Dvir has been a consultant to Edwards and to Medtronic, the company that markets the CoreValve systems. Dr. Kirtane has received research grants from Boston Scientific, St. Jude, Eli Lilly, GlaxoSmithKline, Abiomed, and Abbott Vascular. Dr. Linke has been a consultant to Boston Scientific, St. Jude, Bard, Edwards, and Medtronic and owns equity in Claret. Dr. Popma has been principal investigator of the CoreValve studies, has been a consultant to Abbott Vascular, Boston Scientific, and Director Flow; he owns equity in Direct Flow, and he has received research grants from Abbott Vascular, Medtronic, Boston Scientific, and Cook Medical.
On Twitter @mitchelzoler
AT TCT 2015
Key clinical point:The SAPIEN XT balloon-expandable system for transcatheter aortic valve replacement received FDA approval for valve-in-valve use in high-risk patients.
Major finding: High-risk patients treated for aortic valve-in-replacement with SAPIEN XT had a 13% 1-year mortality rate.
Data source: The PARTNER 2 trial valve-in-valve registry and extended registry, a multicenter center based primarily in the United States with 197 high-risk patients.
Disclosures: The PARTNER 2 trial valve-in-valve registry and extended registry are sponsored by Edwards Lifesciences, which markets the SAPIEN XT system. Dr. Dvir has been a consultant to Edwards and to Medtronic, the company that markets the CoreValve systems.
Depression, hypertension combo compounds cardiovascular risk
LONDON – The combination of depression and very high systolic blood pressure increased the risk for a major cardiovascular event by 83%, compared with normal blood pressure and no depression, in patients with existing heart disease, diabetes, or stroke.
In a large family practice–based study, the hazard ratio for first hospital admission due to myocardial infarction, stroke or heart failure, or cardiovascular death in patients with high (at least 160 mm Hg) systolic blood pressure who were also depressed was 1.83, a highly significant difference compared with individuals with normal BP and no depression.
The presence of depression also significantly increased the risk for these cardiovascular events by 36% in patients with lower systolic BPs, compared with those with normal BP and no depression.
“Patients with existing heart disease, diabetes, or stroke are more likely to have another heart attack or another stroke or die from heart problems than the general population,” Dr. Bhautesh Jani of the Institute of Health and Wellbeing, University of Glasgow (Scotland), said at the annual congress of the European Society of Cardiology.
“In particular, those who have extremes of blood pressure or those who have depressive symptoms are at higher risk, which has been shown by previous evidence. What is relatively unknown is the combined effect of having extremes of blood pressure and depressive symptoms together, and our study tried to address that problem,” Dr. Jani explained.
The study involved more than 35,500 individuals with existing heart disease, diabetes, or stroke living in Scotland, of whom 3,939 (11%) had at least one major cardiovascular event that needed hospital admission during a 4-year period. Patients had been divided into groups based on their systolic BP, and depressive symptoms were assessed using the Hospital Anxiety and Depression Scale (HADS) during 2008-2009. A HADS score of more than 7 was considered indicative of depression.
Dr. Jani noted that all the results were adjusted for multiple confounding factors, including age, gender, socioeconomic status, number of comorbid conditions, total cholesterol values, body mass index, and the initiation of antidepressant medication.
“The key implications I would suggest are for secondary prevention and to perhaps focus our resources on monitoring blood pressure and providing treatment in patients with associated depressive symptoms,” Dr. Jani proposed.
Dr. Christi Deaton, professor of clinical nursing research at the University of Cambridge (England), who commented on the study findings, noted that it highlighted the importance of raising awareness among both clinicians and patients of the potential risk associated with a combination of hypertension and depression, particularly among patients with a high level of other risk factors for further cardiovascular events.
“Screening patients for depression is very simple to do and you can start with a couple of questions asking about whether or not the patient felt sad or had low mood, and then go on to use other screening tools,” she observed. “All providers should be screening patients and thinking about the synergy of these two risk factors,” she said.
Other research presented at the ESC Congress by cardiologist Dr. Salim Hayek of Emory University in Atlanta also highlighted the importance of screening for depression, this time in patients with angina. In a study of 5,202 adults enrolled in the university’s biobank between 2004 and 2013 and who underwent left heart catheterization, patients with frequent chest pain were found to have more depressive symptoms than those without angina. Depressive symptoms in this study were assessed using the Patient Health Questionnaire (PHQ-9).
“Chest pain was more frequent in patients with mild depression with and without coronary artery disease, regardless of sex or history of myocardial infarction,” Dr. Hayek said. After multivariate analysis, depression was the most important predictor of frequent chest pain, he added. Other factors independently associated with chest pain frequency were female sex, coronary artery disease severity, history of MI, body mass index, and high blood lipid levels.
“At follow-up, a decrease in depressive symptoms was associated with improvement in chest pain,” Dr. Hayek said, but patients with depression who were revascularized did not show an improvement in chest pain.
The findings suggest that the association between chest pain and depression was independent of the underlying arterial disease and further studies are needed to look at the effect of revascularization and angina relief on depressive symptoms, and conversely if antidepressant medications could help alleviate chest pain.
The study Dr. Jani presented was funded by the Scottish Government. Dr. Jani and Dr. Hayek both reported having no disclosures.
This article was updated February 3, 2016.
LONDON – The combination of depression and very high systolic blood pressure increased the risk for a major cardiovascular event by 83%, compared with normal blood pressure and no depression, in patients with existing heart disease, diabetes, or stroke.
In a large family practice–based study, the hazard ratio for first hospital admission due to myocardial infarction, stroke or heart failure, or cardiovascular death in patients with high (at least 160 mm Hg) systolic blood pressure who were also depressed was 1.83, a highly significant difference compared with individuals with normal BP and no depression.
The presence of depression also significantly increased the risk for these cardiovascular events by 36% in patients with lower systolic BPs, compared with those with normal BP and no depression.
“Patients with existing heart disease, diabetes, or stroke are more likely to have another heart attack or another stroke or die from heart problems than the general population,” Dr. Bhautesh Jani of the Institute of Health and Wellbeing, University of Glasgow (Scotland), said at the annual congress of the European Society of Cardiology.
“In particular, those who have extremes of blood pressure or those who have depressive symptoms are at higher risk, which has been shown by previous evidence. What is relatively unknown is the combined effect of having extremes of blood pressure and depressive symptoms together, and our study tried to address that problem,” Dr. Jani explained.
The study involved more than 35,500 individuals with existing heart disease, diabetes, or stroke living in Scotland, of whom 3,939 (11%) had at least one major cardiovascular event that needed hospital admission during a 4-year period. Patients had been divided into groups based on their systolic BP, and depressive symptoms were assessed using the Hospital Anxiety and Depression Scale (HADS) during 2008-2009. A HADS score of more than 7 was considered indicative of depression.
Dr. Jani noted that all the results were adjusted for multiple confounding factors, including age, gender, socioeconomic status, number of comorbid conditions, total cholesterol values, body mass index, and the initiation of antidepressant medication.
“The key implications I would suggest are for secondary prevention and to perhaps focus our resources on monitoring blood pressure and providing treatment in patients with associated depressive symptoms,” Dr. Jani proposed.
Dr. Christi Deaton, professor of clinical nursing research at the University of Cambridge (England), who commented on the study findings, noted that it highlighted the importance of raising awareness among both clinicians and patients of the potential risk associated with a combination of hypertension and depression, particularly among patients with a high level of other risk factors for further cardiovascular events.
“Screening patients for depression is very simple to do and you can start with a couple of questions asking about whether or not the patient felt sad or had low mood, and then go on to use other screening tools,” she observed. “All providers should be screening patients and thinking about the synergy of these two risk factors,” she said.
Other research presented at the ESC Congress by cardiologist Dr. Salim Hayek of Emory University in Atlanta also highlighted the importance of screening for depression, this time in patients with angina. In a study of 5,202 adults enrolled in the university’s biobank between 2004 and 2013 and who underwent left heart catheterization, patients with frequent chest pain were found to have more depressive symptoms than those without angina. Depressive symptoms in this study were assessed using the Patient Health Questionnaire (PHQ-9).
“Chest pain was more frequent in patients with mild depression with and without coronary artery disease, regardless of sex or history of myocardial infarction,” Dr. Hayek said. After multivariate analysis, depression was the most important predictor of frequent chest pain, he added. Other factors independently associated with chest pain frequency were female sex, coronary artery disease severity, history of MI, body mass index, and high blood lipid levels.
“At follow-up, a decrease in depressive symptoms was associated with improvement in chest pain,” Dr. Hayek said, but patients with depression who were revascularized did not show an improvement in chest pain.
The findings suggest that the association between chest pain and depression was independent of the underlying arterial disease and further studies are needed to look at the effect of revascularization and angina relief on depressive symptoms, and conversely if antidepressant medications could help alleviate chest pain.
The study Dr. Jani presented was funded by the Scottish Government. Dr. Jani and Dr. Hayek both reported having no disclosures.
This article was updated February 3, 2016.
LONDON – The combination of depression and very high systolic blood pressure increased the risk for a major cardiovascular event by 83%, compared with normal blood pressure and no depression, in patients with existing heart disease, diabetes, or stroke.
In a large family practice–based study, the hazard ratio for first hospital admission due to myocardial infarction, stroke or heart failure, or cardiovascular death in patients with high (at least 160 mm Hg) systolic blood pressure who were also depressed was 1.83, a highly significant difference compared with individuals with normal BP and no depression.
The presence of depression also significantly increased the risk for these cardiovascular events by 36% in patients with lower systolic BPs, compared with those with normal BP and no depression.
“Patients with existing heart disease, diabetes, or stroke are more likely to have another heart attack or another stroke or die from heart problems than the general population,” Dr. Bhautesh Jani of the Institute of Health and Wellbeing, University of Glasgow (Scotland), said at the annual congress of the European Society of Cardiology.
“In particular, those who have extremes of blood pressure or those who have depressive symptoms are at higher risk, which has been shown by previous evidence. What is relatively unknown is the combined effect of having extremes of blood pressure and depressive symptoms together, and our study tried to address that problem,” Dr. Jani explained.
The study involved more than 35,500 individuals with existing heart disease, diabetes, or stroke living in Scotland, of whom 3,939 (11%) had at least one major cardiovascular event that needed hospital admission during a 4-year period. Patients had been divided into groups based on their systolic BP, and depressive symptoms were assessed using the Hospital Anxiety and Depression Scale (HADS) during 2008-2009. A HADS score of more than 7 was considered indicative of depression.
Dr. Jani noted that all the results were adjusted for multiple confounding factors, including age, gender, socioeconomic status, number of comorbid conditions, total cholesterol values, body mass index, and the initiation of antidepressant medication.
“The key implications I would suggest are for secondary prevention and to perhaps focus our resources on monitoring blood pressure and providing treatment in patients with associated depressive symptoms,” Dr. Jani proposed.
Dr. Christi Deaton, professor of clinical nursing research at the University of Cambridge (England), who commented on the study findings, noted that it highlighted the importance of raising awareness among both clinicians and patients of the potential risk associated with a combination of hypertension and depression, particularly among patients with a high level of other risk factors for further cardiovascular events.
“Screening patients for depression is very simple to do and you can start with a couple of questions asking about whether or not the patient felt sad or had low mood, and then go on to use other screening tools,” she observed. “All providers should be screening patients and thinking about the synergy of these two risk factors,” she said.
Other research presented at the ESC Congress by cardiologist Dr. Salim Hayek of Emory University in Atlanta also highlighted the importance of screening for depression, this time in patients with angina. In a study of 5,202 adults enrolled in the university’s biobank between 2004 and 2013 and who underwent left heart catheterization, patients with frequent chest pain were found to have more depressive symptoms than those without angina. Depressive symptoms in this study were assessed using the Patient Health Questionnaire (PHQ-9).
“Chest pain was more frequent in patients with mild depression with and without coronary artery disease, regardless of sex or history of myocardial infarction,” Dr. Hayek said. After multivariate analysis, depression was the most important predictor of frequent chest pain, he added. Other factors independently associated with chest pain frequency were female sex, coronary artery disease severity, history of MI, body mass index, and high blood lipid levels.
“At follow-up, a decrease in depressive symptoms was associated with improvement in chest pain,” Dr. Hayek said, but patients with depression who were revascularized did not show an improvement in chest pain.
The findings suggest that the association between chest pain and depression was independent of the underlying arterial disease and further studies are needed to look at the effect of revascularization and angina relief on depressive symptoms, and conversely if antidepressant medications could help alleviate chest pain.
The study Dr. Jani presented was funded by the Scottish Government. Dr. Jani and Dr. Hayek both reported having no disclosures.
This article was updated February 3, 2016.
AT THE ESC CONGRESS 2015
Key clinical point: Better monitoring and management of both hypertension and depression could help reduce further cardiovascular morbidity and mortality.
Major finding: The rate of major cardiovascular events was 83% higher in depressed patients with very high (at least 160 mm Hg) systolic BP, compared with individuals with normal pressure and no depression.
Data source: Large family practice study of 35,537 patients with existing coronary heart disease, diabetes, or stroke followed up for 4 years.
Disclosures: The study Dr. Jani presented was funded by the Scottish Government. Dr. Jani had no disclosures.
VIDEO: Ultrafiltration’s role for acute heart failure remains uncertain
NATIONAL HARBOR, MD. – The trial designed to definitively test the safety and efficacy of ultrafiltration as an alternative to intravenous diuretics for patients hospitalized with acute decompensated heart failure got cut to about a quarter of its planned enrollment by the company that recently acquired the ultrafiltration technology. This outcomes means that ultrafiltration’s role in acute heart failure remains uncertain and will stay that way for the foreseeable future.
As a consequence, ultrafiltration (also known as aquapheresis), approved for use in the United States by the Food and Drug Administration in 2002, will be used by believers in the treatment on some of the estimated 200,000 or more U.S. heart failure patients who become hospitalized each year with acute, severe congestion that is unresponsive to diuretic treatment. Other clinicians who remain skeptical of ultrafiltration’s safety and efficacy will not use it, and currently no prospect remains to resolve this uncertainty. Also limiting ultrafiltration’s use is its designation as investigational by U.S. health insurers.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“Early termination off the AVOID-HF [Aquapheresis Vs. Intravenous Diuretics and Hospitalization for Heart Failure] trial was a tragedy because it is unlikely we will ever see a trial of its size again,” commented Dr. G. Michael Felker during a discussion of treatment for acute heart failure at the at the annual meeting of the Heart Failure Society of America. “We still have no definitive answer” about ultrafiltration,” said Dr. Felker, professor of medicine and a cardiologist specializing in acute heart failure at Duke University in Durham, N.C.
Opinions about ultrafiltration and its role in treating patients with acute decompensated heart failure remain sharply split, although at this point, even proponents of the treatment concede that its use is limited to severely congested decompensated heart failure patients who prove unresponsive to intravenous diuretic therapy.
Ultrafiltration is for patients with “recurrent decompensation (not the first episode) and a large amount of fluid overload (more than 10 pounds) who also have elevated central venous pressure and elevated abdominal pressure that reduces blood flow to the kidneys,” Dr. Maria Rosa Costanzo said in a video interview. Used in these patients at a low rate of 20-50 cc/hour to “gently remove fluid,” the treatment reduces central venous and intra-abdominal pressures, leading to increased urine output and restoration of diuretic sensitivity, said Dr. Costanzo, lead investigator for the AVOID-HF trial and medical director of the heart failure and pulmonary hypertension program at the Advocate Heart Institute in Naperville, Ill.
A dramatically opposing view of ultrafiltration came from Dr. Christopher M. O’Connor, a heart failure cardiologist and CEO of the Inova Heart and Vascular Institute in Falls Church, Va.
“Even in diuretic-resistant, patients I would not use ultrafiltration. It does not anymore fit into management” of acute decompensated heart failure patients, Dr. O’Connor said in an interview. The CARRESS-HF [Cardiorenal Rescue Study in Acute Decompensated Heart Failure] trial targeted patients with diuretic resistance, the very population we would need to treat, and we did not see an advantage to ultrafiltration,” said Dr. O’Connor, a coinvestigator on CARRESS-HF, an earlier trial that compared the two treatment methods (N Engl J Med. 2012 Dec 13;367[24]:2296-304).
“We need to understand who is the right patient” for ultrafiltration. “We need to continue to investigate [ultrafiltration] to know where it best fits,” commented Dr. Clyde W. Yancy, professor and chief of cardiology at Northwestern University in Chicago.
AVOID-HF enrolled and randomized 224 patients with acute decompensated heart failure out of an anticipated enrollment of 810 patients. Analysis of the study’s primary endpoint, time to first heart failure event within the first 90 days following hospital discharge, occurred after an average of 34 days in 108 loop-diuretic treated patients and after an average 62 days in 105 ultrafiltration-treated patients, a difference that did not achieve statistical significance. A heart failure event within the first 90 days occurred in 25% of the ultrafiltration patients and in 35% of those on diuretic treatment, also a nonsignificant difference, Dr. Costanzo reported. Concurrent with her report at the meeting an article appeared online with the results (JACC Heart Fail. 2015;doi: 10.1016/j.jchf.2015.08.005).
Increases in serum creatinine were modest following ultrafiltration and similar to changes seen in the patients who received loop diuretic treatment. The biggest rise in average serum creatinine level occurred at 30 days after randomization, with an average 0.37 mg/dL rise among the ultrafiltration patients.
The abrupt shutdown in the AVOID-HF trial occurred following a change in ownership of the ultrafiltration technology used in the study. Ultrafiltration had been initially developed by CHF Solutions of Brooklyn Park, Minn. In 2010, Gambro, a Swedish company, acquired CHF Solutions and rights to the ultrafiltration system, and Gambro initiated the AVOID-HF trial. Baxter, in Deerfield, Ill., acquired Gambro in September 2013, and in April 2014, Baxter stopped the AVOID-HF trial because of slow projected enrollment, said Dr. Costanzo. But she strongly disagreed with this projection, and said that the trial showed no signs of futility or safety concerns when it came to a stop. Getting the data from Baxter to allow her and her associates to write their report and deliver their findings at the meeting took a lot of persuasion, Dr. Costanzo added.
Dr. Felker has been a consultant to and received research grants from 10 drug or device companies. He was a coinvestigator on the CARRESS-HF trial. Dr. Costanzo, lead investigator of AVOID-HF, said she had no relevant disclosures. Dr. O’Connor has been a consultant to ResMed, Roche Diagnostics, Cardiorentis, Bayer, and Actelion and has received research grants from Otsuka, ResMed, Roche Diagnostics. He was a coinvestigator on the CARESS-HF trial. Dr. Yancy had no disclosures.
On Twitter @mitchelzoler
Patients hospitalized with vascular congestion caused by an acute exacerbation of heart failure universally receive loop diuretics, but most patients continue to have persistent congestion and poor outcomes following treatment. In one recent trial, fewer than 20% of patients left the hospital with adequate decongestion regardless of whether they received high- or low-dose furosemide and whether they received it as a bolus or as a continuous infusion (N Engl J Med. 2011 Mar 3;364[9]:797-805). We need an alternative to decongestion by loop-diuretic treatment.
Ultrafiltration offers a number of potential advantages for treating acute congestion, especially for patients unresponsive to diuretic treatment. It provides a way to quickly and predictably remove isotonic fluid with no effect on electrolytes, no neurohormonal stimulation, and in several trials, no significant effect on renal function. It also can restore diuretic responsiveness,
 |
Dr. Bradley A. Bart |
Critics of ultrafiltration often cite the results of the CARRESS-HF [Cardiorenal Rescue Study in Acute Decompensated Heart Failure] trial, which I led (N Engl J Med 2012 Dec 13;367[24]:2296-304). I’m not convinced that the CARRESS-HF results apply to the large majority of patients with acute heart failure. All of the patients enrolled in this trial had acute kidney injury. Also, in CARRESS-HF, we used a control arm that received diuretic treatment in a stepped pharmacologic way, which is not the dosing strategy we usually see in community practice. In addition, CARRESS-HF did not use variable dosing in the ultrafiltration arm. A better comparison of diuretic treatment and ultrafiltration would use variable dosing in both arms.
That’s the design used in AVOID-HF. In this trial, clinicians adjusted both diuretic and ultrafiltration dosages based on the renal function and hemodynamics of each enrolled patient. Unfortunately, AVOID-HF ended too soon, after enrolling just a quarter of the patients calculated as necessary to produce a statistically significant difference in outcomes between the two treatment arms. This appeared to result in a nonsignificant trend in favor of ultrafiltration for the study’s primary endpoint. Despite being extremely underpowered, the results showed significant benefits for ultrafiltration for several secondary endpoints. The results also showed no deleterious effects on renal function with ultrafiltration.
If AVOID-HF had shown no signal of benefit from ultrafiltration then I think that would have meant to end of ultrafiltration, but that is not what happened. The strong trends in favor of ultrafiltration make the case to keep studying it for patients who have become unresponsive to diuretics. In these patients ultrafiltration is a good alternative. Several questions remain unanswered about the use of ultrafiltration, such as exactly when a patient has become too unresponsive to diuretic treatment to warrant using ultrafiltration, and how to optimally dose ultrafiltration. Despite this lingering uncertainty ultrafiltration remains a viable option that deserves more study.
Dr. Bradley A. Bart is professor of medicine at the University of Minnesota and chief of cardiology at Hennepin County Medical Center, both in Minneapolis. He had no financial disclosures. Dr. Bart was lead investigator for the CARRESS-HF trial and a coinvestigator on the AVOID-HF trial. He made these comments in a talk at the meeting and during an interview.
Patients hospitalized with vascular congestion caused by an acute exacerbation of heart failure universally receive loop diuretics, but most patients continue to have persistent congestion and poor outcomes following treatment. In one recent trial, fewer than 20% of patients left the hospital with adequate decongestion regardless of whether they received high- or low-dose furosemide and whether they received it as a bolus or as a continuous infusion (N Engl J Med. 2011 Mar 3;364[9]:797-805). We need an alternative to decongestion by loop-diuretic treatment.
Ultrafiltration offers a number of potential advantages for treating acute congestion, especially for patients unresponsive to diuretic treatment. It provides a way to quickly and predictably remove isotonic fluid with no effect on electrolytes, no neurohormonal stimulation, and in several trials, no significant effect on renal function. It also can restore diuretic responsiveness,
 |
Dr. Bradley A. Bart |
Critics of ultrafiltration often cite the results of the CARRESS-HF [Cardiorenal Rescue Study in Acute Decompensated Heart Failure] trial, which I led (N Engl J Med 2012 Dec 13;367[24]:2296-304). I’m not convinced that the CARRESS-HF results apply to the large majority of patients with acute heart failure. All of the patients enrolled in this trial had acute kidney injury. Also, in CARRESS-HF, we used a control arm that received diuretic treatment in a stepped pharmacologic way, which is not the dosing strategy we usually see in community practice. In addition, CARRESS-HF did not use variable dosing in the ultrafiltration arm. A better comparison of diuretic treatment and ultrafiltration would use variable dosing in both arms.
That’s the design used in AVOID-HF. In this trial, clinicians adjusted both diuretic and ultrafiltration dosages based on the renal function and hemodynamics of each enrolled patient. Unfortunately, AVOID-HF ended too soon, after enrolling just a quarter of the patients calculated as necessary to produce a statistically significant difference in outcomes between the two treatment arms. This appeared to result in a nonsignificant trend in favor of ultrafiltration for the study’s primary endpoint. Despite being extremely underpowered, the results showed significant benefits for ultrafiltration for several secondary endpoints. The results also showed no deleterious effects on renal function with ultrafiltration.
If AVOID-HF had shown no signal of benefit from ultrafiltration then I think that would have meant to end of ultrafiltration, but that is not what happened. The strong trends in favor of ultrafiltration make the case to keep studying it for patients who have become unresponsive to diuretics. In these patients ultrafiltration is a good alternative. Several questions remain unanswered about the use of ultrafiltration, such as exactly when a patient has become too unresponsive to diuretic treatment to warrant using ultrafiltration, and how to optimally dose ultrafiltration. Despite this lingering uncertainty ultrafiltration remains a viable option that deserves more study.
Dr. Bradley A. Bart is professor of medicine at the University of Minnesota and chief of cardiology at Hennepin County Medical Center, both in Minneapolis. He had no financial disclosures. Dr. Bart was lead investigator for the CARRESS-HF trial and a coinvestigator on the AVOID-HF trial. He made these comments in a talk at the meeting and during an interview.
Patients hospitalized with vascular congestion caused by an acute exacerbation of heart failure universally receive loop diuretics, but most patients continue to have persistent congestion and poor outcomes following treatment. In one recent trial, fewer than 20% of patients left the hospital with adequate decongestion regardless of whether they received high- or low-dose furosemide and whether they received it as a bolus or as a continuous infusion (N Engl J Med. 2011 Mar 3;364[9]:797-805). We need an alternative to decongestion by loop-diuretic treatment.
Ultrafiltration offers a number of potential advantages for treating acute congestion, especially for patients unresponsive to diuretic treatment. It provides a way to quickly and predictably remove isotonic fluid with no effect on electrolytes, no neurohormonal stimulation, and in several trials, no significant effect on renal function. It also can restore diuretic responsiveness,
 |
Dr. Bradley A. Bart |
Critics of ultrafiltration often cite the results of the CARRESS-HF [Cardiorenal Rescue Study in Acute Decompensated Heart Failure] trial, which I led (N Engl J Med 2012 Dec 13;367[24]:2296-304). I’m not convinced that the CARRESS-HF results apply to the large majority of patients with acute heart failure. All of the patients enrolled in this trial had acute kidney injury. Also, in CARRESS-HF, we used a control arm that received diuretic treatment in a stepped pharmacologic way, which is not the dosing strategy we usually see in community practice. In addition, CARRESS-HF did not use variable dosing in the ultrafiltration arm. A better comparison of diuretic treatment and ultrafiltration would use variable dosing in both arms.
That’s the design used in AVOID-HF. In this trial, clinicians adjusted both diuretic and ultrafiltration dosages based on the renal function and hemodynamics of each enrolled patient. Unfortunately, AVOID-HF ended too soon, after enrolling just a quarter of the patients calculated as necessary to produce a statistically significant difference in outcomes between the two treatment arms. This appeared to result in a nonsignificant trend in favor of ultrafiltration for the study’s primary endpoint. Despite being extremely underpowered, the results showed significant benefits for ultrafiltration for several secondary endpoints. The results also showed no deleterious effects on renal function with ultrafiltration.
If AVOID-HF had shown no signal of benefit from ultrafiltration then I think that would have meant to end of ultrafiltration, but that is not what happened. The strong trends in favor of ultrafiltration make the case to keep studying it for patients who have become unresponsive to diuretics. In these patients ultrafiltration is a good alternative. Several questions remain unanswered about the use of ultrafiltration, such as exactly when a patient has become too unresponsive to diuretic treatment to warrant using ultrafiltration, and how to optimally dose ultrafiltration. Despite this lingering uncertainty ultrafiltration remains a viable option that deserves more study.
Dr. Bradley A. Bart is professor of medicine at the University of Minnesota and chief of cardiology at Hennepin County Medical Center, both in Minneapolis. He had no financial disclosures. Dr. Bart was lead investigator for the CARRESS-HF trial and a coinvestigator on the AVOID-HF trial. He made these comments in a talk at the meeting and during an interview.
NATIONAL HARBOR, MD. – The trial designed to definitively test the safety and efficacy of ultrafiltration as an alternative to intravenous diuretics for patients hospitalized with acute decompensated heart failure got cut to about a quarter of its planned enrollment by the company that recently acquired the ultrafiltration technology. This outcomes means that ultrafiltration’s role in acute heart failure remains uncertain and will stay that way for the foreseeable future.
As a consequence, ultrafiltration (also known as aquapheresis), approved for use in the United States by the Food and Drug Administration in 2002, will be used by believers in the treatment on some of the estimated 200,000 or more U.S. heart failure patients who become hospitalized each year with acute, severe congestion that is unresponsive to diuretic treatment. Other clinicians who remain skeptical of ultrafiltration’s safety and efficacy will not use it, and currently no prospect remains to resolve this uncertainty. Also limiting ultrafiltration’s use is its designation as investigational by U.S. health insurers.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“Early termination off the AVOID-HF [Aquapheresis Vs. Intravenous Diuretics and Hospitalization for Heart Failure] trial was a tragedy because it is unlikely we will ever see a trial of its size again,” commented Dr. G. Michael Felker during a discussion of treatment for acute heart failure at the at the annual meeting of the Heart Failure Society of America. “We still have no definitive answer” about ultrafiltration,” said Dr. Felker, professor of medicine and a cardiologist specializing in acute heart failure at Duke University in Durham, N.C.
Opinions about ultrafiltration and its role in treating patients with acute decompensated heart failure remain sharply split, although at this point, even proponents of the treatment concede that its use is limited to severely congested decompensated heart failure patients who prove unresponsive to intravenous diuretic therapy.
Ultrafiltration is for patients with “recurrent decompensation (not the first episode) and a large amount of fluid overload (more than 10 pounds) who also have elevated central venous pressure and elevated abdominal pressure that reduces blood flow to the kidneys,” Dr. Maria Rosa Costanzo said in a video interview. Used in these patients at a low rate of 20-50 cc/hour to “gently remove fluid,” the treatment reduces central venous and intra-abdominal pressures, leading to increased urine output and restoration of diuretic sensitivity, said Dr. Costanzo, lead investigator for the AVOID-HF trial and medical director of the heart failure and pulmonary hypertension program at the Advocate Heart Institute in Naperville, Ill.
A dramatically opposing view of ultrafiltration came from Dr. Christopher M. O’Connor, a heart failure cardiologist and CEO of the Inova Heart and Vascular Institute in Falls Church, Va.
“Even in diuretic-resistant, patients I would not use ultrafiltration. It does not anymore fit into management” of acute decompensated heart failure patients, Dr. O’Connor said in an interview. The CARRESS-HF [Cardiorenal Rescue Study in Acute Decompensated Heart Failure] trial targeted patients with diuretic resistance, the very population we would need to treat, and we did not see an advantage to ultrafiltration,” said Dr. O’Connor, a coinvestigator on CARRESS-HF, an earlier trial that compared the two treatment methods (N Engl J Med. 2012 Dec 13;367[24]:2296-304).
“We need to understand who is the right patient” for ultrafiltration. “We need to continue to investigate [ultrafiltration] to know where it best fits,” commented Dr. Clyde W. Yancy, professor and chief of cardiology at Northwestern University in Chicago.
AVOID-HF enrolled and randomized 224 patients with acute decompensated heart failure out of an anticipated enrollment of 810 patients. Analysis of the study’s primary endpoint, time to first heart failure event within the first 90 days following hospital discharge, occurred after an average of 34 days in 108 loop-diuretic treated patients and after an average 62 days in 105 ultrafiltration-treated patients, a difference that did not achieve statistical significance. A heart failure event within the first 90 days occurred in 25% of the ultrafiltration patients and in 35% of those on diuretic treatment, also a nonsignificant difference, Dr. Costanzo reported. Concurrent with her report at the meeting an article appeared online with the results (JACC Heart Fail. 2015;doi: 10.1016/j.jchf.2015.08.005).
Increases in serum creatinine were modest following ultrafiltration and similar to changes seen in the patients who received loop diuretic treatment. The biggest rise in average serum creatinine level occurred at 30 days after randomization, with an average 0.37 mg/dL rise among the ultrafiltration patients.
The abrupt shutdown in the AVOID-HF trial occurred following a change in ownership of the ultrafiltration technology used in the study. Ultrafiltration had been initially developed by CHF Solutions of Brooklyn Park, Minn. In 2010, Gambro, a Swedish company, acquired CHF Solutions and rights to the ultrafiltration system, and Gambro initiated the AVOID-HF trial. Baxter, in Deerfield, Ill., acquired Gambro in September 2013, and in April 2014, Baxter stopped the AVOID-HF trial because of slow projected enrollment, said Dr. Costanzo. But she strongly disagreed with this projection, and said that the trial showed no signs of futility or safety concerns when it came to a stop. Getting the data from Baxter to allow her and her associates to write their report and deliver their findings at the meeting took a lot of persuasion, Dr. Costanzo added.
Dr. Felker has been a consultant to and received research grants from 10 drug or device companies. He was a coinvestigator on the CARRESS-HF trial. Dr. Costanzo, lead investigator of AVOID-HF, said she had no relevant disclosures. Dr. O’Connor has been a consultant to ResMed, Roche Diagnostics, Cardiorentis, Bayer, and Actelion and has received research grants from Otsuka, ResMed, Roche Diagnostics. He was a coinvestigator on the CARESS-HF trial. Dr. Yancy had no disclosures.
On Twitter @mitchelzoler
NATIONAL HARBOR, MD. – The trial designed to definitively test the safety and efficacy of ultrafiltration as an alternative to intravenous diuretics for patients hospitalized with acute decompensated heart failure got cut to about a quarter of its planned enrollment by the company that recently acquired the ultrafiltration technology. This outcomes means that ultrafiltration’s role in acute heart failure remains uncertain and will stay that way for the foreseeable future.
As a consequence, ultrafiltration (also known as aquapheresis), approved for use in the United States by the Food and Drug Administration in 2002, will be used by believers in the treatment on some of the estimated 200,000 or more U.S. heart failure patients who become hospitalized each year with acute, severe congestion that is unresponsive to diuretic treatment. Other clinicians who remain skeptical of ultrafiltration’s safety and efficacy will not use it, and currently no prospect remains to resolve this uncertainty. Also limiting ultrafiltration’s use is its designation as investigational by U.S. health insurers.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
“Early termination off the AVOID-HF [Aquapheresis Vs. Intravenous Diuretics and Hospitalization for Heart Failure] trial was a tragedy because it is unlikely we will ever see a trial of its size again,” commented Dr. G. Michael Felker during a discussion of treatment for acute heart failure at the at the annual meeting of the Heart Failure Society of America. “We still have no definitive answer” about ultrafiltration,” said Dr. Felker, professor of medicine and a cardiologist specializing in acute heart failure at Duke University in Durham, N.C.
Opinions about ultrafiltration and its role in treating patients with acute decompensated heart failure remain sharply split, although at this point, even proponents of the treatment concede that its use is limited to severely congested decompensated heart failure patients who prove unresponsive to intravenous diuretic therapy.
Ultrafiltration is for patients with “recurrent decompensation (not the first episode) and a large amount of fluid overload (more than 10 pounds) who also have elevated central venous pressure and elevated abdominal pressure that reduces blood flow to the kidneys,” Dr. Maria Rosa Costanzo said in a video interview. Used in these patients at a low rate of 20-50 cc/hour to “gently remove fluid,” the treatment reduces central venous and intra-abdominal pressures, leading to increased urine output and restoration of diuretic sensitivity, said Dr. Costanzo, lead investigator for the AVOID-HF trial and medical director of the heart failure and pulmonary hypertension program at the Advocate Heart Institute in Naperville, Ill.
A dramatically opposing view of ultrafiltration came from Dr. Christopher M. O’Connor, a heart failure cardiologist and CEO of the Inova Heart and Vascular Institute in Falls Church, Va.
“Even in diuretic-resistant, patients I would not use ultrafiltration. It does not anymore fit into management” of acute decompensated heart failure patients, Dr. O’Connor said in an interview. The CARRESS-HF [Cardiorenal Rescue Study in Acute Decompensated Heart Failure] trial targeted patients with diuretic resistance, the very population we would need to treat, and we did not see an advantage to ultrafiltration,” said Dr. O’Connor, a coinvestigator on CARRESS-HF, an earlier trial that compared the two treatment methods (N Engl J Med. 2012 Dec 13;367[24]:2296-304).
“We need to understand who is the right patient” for ultrafiltration. “We need to continue to investigate [ultrafiltration] to know where it best fits,” commented Dr. Clyde W. Yancy, professor and chief of cardiology at Northwestern University in Chicago.
AVOID-HF enrolled and randomized 224 patients with acute decompensated heart failure out of an anticipated enrollment of 810 patients. Analysis of the study’s primary endpoint, time to first heart failure event within the first 90 days following hospital discharge, occurred after an average of 34 days in 108 loop-diuretic treated patients and after an average 62 days in 105 ultrafiltration-treated patients, a difference that did not achieve statistical significance. A heart failure event within the first 90 days occurred in 25% of the ultrafiltration patients and in 35% of those on diuretic treatment, also a nonsignificant difference, Dr. Costanzo reported. Concurrent with her report at the meeting an article appeared online with the results (JACC Heart Fail. 2015;doi: 10.1016/j.jchf.2015.08.005).
Increases in serum creatinine were modest following ultrafiltration and similar to changes seen in the patients who received loop diuretic treatment. The biggest rise in average serum creatinine level occurred at 30 days after randomization, with an average 0.37 mg/dL rise among the ultrafiltration patients.
The abrupt shutdown in the AVOID-HF trial occurred following a change in ownership of the ultrafiltration technology used in the study. Ultrafiltration had been initially developed by CHF Solutions of Brooklyn Park, Minn. In 2010, Gambro, a Swedish company, acquired CHF Solutions and rights to the ultrafiltration system, and Gambro initiated the AVOID-HF trial. Baxter, in Deerfield, Ill., acquired Gambro in September 2013, and in April 2014, Baxter stopped the AVOID-HF trial because of slow projected enrollment, said Dr. Costanzo. But she strongly disagreed with this projection, and said that the trial showed no signs of futility or safety concerns when it came to a stop. Getting the data from Baxter to allow her and her associates to write their report and deliver their findings at the meeting took a lot of persuasion, Dr. Costanzo added.
Dr. Felker has been a consultant to and received research grants from 10 drug or device companies. He was a coinvestigator on the CARRESS-HF trial. Dr. Costanzo, lead investigator of AVOID-HF, said she had no relevant disclosures. Dr. O’Connor has been a consultant to ResMed, Roche Diagnostics, Cardiorentis, Bayer, and Actelion and has received research grants from Otsuka, ResMed, Roche Diagnostics. He was a coinvestigator on the CARESS-HF trial. Dr. Yancy had no disclosures.
On Twitter @mitchelzoler
AT THE HFSA ANNUAL SCIENTIFIC MEETING
Key clinical point: Early halt to the AVOID-HF trial prevented the study from definitively comparing ultrafiltration with intravenous diuretic treatment in patients with acute decompensated heart failure.
Major finding: Average time to first heart-failure event was 62 days with ultrafiltration and 34 days with diuretic treatment, a nonsignificant difference.
Data source: AVOID-HF, a randomized trial that enrolled 224 patients at 30 U.S. centers.
Disclosures: AVOID-HF was initially sponsored by Gambro, which was later acquired by Baxter. Dr. Costanzo had no relevant disclosures.
TCT: CTO treatment after MI doesn’t benefit LV function
SAN FRANCISCO – Recanalization of a chronic total occlusion in a noninfarct-related artery within a week after primary percutaneous coronary intervention was safe and feasible but did not improve overall left ventricular ejection fraction or LV end diastolic volume in the randomized, prospective EXPLORE trial.
At 4 months after primary percutaneous coronary intervention (pPCI), cardiac magnetic resonance imaging showed that left ventricular ejection fraction (LVEF) was similar in 136 patients who underwent chronic total occlusion percutaneous coronary intervention (CTO-PCI) and 144 who did not undergo CTO-PCI (44.1 and 44.8, respectively) within 1 week after the pPCI, Dr. Jose P.S. Henriques reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
LV end diastolic volume also was similar in the two groups (215.6 and 212.8, respectively), Dr. Henriques of the Academic Medical Center, Amsterdam, said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
A subgroup analysis, however, showed that LVEF did improve significantly with CTO-PCI in 69 patients whose CTOs were located in the left anterior descending (LAD) artery, compared with 211 patients with non-LAD CTOs (treatment effect, 6.8 vs. –3.2), Dr. Henriques said.
Patients enrolled in the EXPLORE trial had a mean age of 60 years, and most were men (89% in the CTO-PCI group and 82% in the non-CTO-PCI group). The two groups were well balanced with respect to clinical and demographic characteristics. Of note, both groups had a high rate of triple-vessel disease with greater than 70% stenosis and high rates of multiple CTOs (9% and 14%), he said.
Of those who underwent CTO-PCI, 6 had multiple CTO arteries treated, 124 were treated using an antegrade-only technique, 23 were treated using a retrograde technique, and 5 were treated using Crossboss/Stingray. The self-reported PCI success rates was 80%, but this was downgraded to 72% based on core lab adjudication.
About 10% of ST-segment–elevation myocardial infarction (STEMI) patients have a noninfarct-related artery CTO, but randomized controlled data on management are lacking.
“We don’t know how to treat them or what to do with these patients. What we do know is that the observed mortality in multivessel-disease patients vs. single vessel–disease patients is mainly driven by confirmed total occlusion. Also, the observed reduced LV function in multivessel-disease patients vs. single vessel–disease patients is also mainly driven by chronic total occlusion,” Dr. Henriques said.
“The EXPLORE trial is the first randomized controlled trial on the impact of PCI of CTO on LV function and clinical outcome in post-STEMI patients,” he said.
The findings suggest that CTO-PCI for a post-STEMI CTO located in the LAD may improve LV function and potentially improve clinical outcome in the long term, he concluded.
Dr. Henriques reported receiving grant or research support from Abbott Vascular, Abiomed, Biotronik, and B.Braun.
SAN FRANCISCO – Recanalization of a chronic total occlusion in a noninfarct-related artery within a week after primary percutaneous coronary intervention was safe and feasible but did not improve overall left ventricular ejection fraction or LV end diastolic volume in the randomized, prospective EXPLORE trial.
At 4 months after primary percutaneous coronary intervention (pPCI), cardiac magnetic resonance imaging showed that left ventricular ejection fraction (LVEF) was similar in 136 patients who underwent chronic total occlusion percutaneous coronary intervention (CTO-PCI) and 144 who did not undergo CTO-PCI (44.1 and 44.8, respectively) within 1 week after the pPCI, Dr. Jose P.S. Henriques reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
LV end diastolic volume also was similar in the two groups (215.6 and 212.8, respectively), Dr. Henriques of the Academic Medical Center, Amsterdam, said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
A subgroup analysis, however, showed that LVEF did improve significantly with CTO-PCI in 69 patients whose CTOs were located in the left anterior descending (LAD) artery, compared with 211 patients with non-LAD CTOs (treatment effect, 6.8 vs. –3.2), Dr. Henriques said.
Patients enrolled in the EXPLORE trial had a mean age of 60 years, and most were men (89% in the CTO-PCI group and 82% in the non-CTO-PCI group). The two groups were well balanced with respect to clinical and demographic characteristics. Of note, both groups had a high rate of triple-vessel disease with greater than 70% stenosis and high rates of multiple CTOs (9% and 14%), he said.
Of those who underwent CTO-PCI, 6 had multiple CTO arteries treated, 124 were treated using an antegrade-only technique, 23 were treated using a retrograde technique, and 5 were treated using Crossboss/Stingray. The self-reported PCI success rates was 80%, but this was downgraded to 72% based on core lab adjudication.
About 10% of ST-segment–elevation myocardial infarction (STEMI) patients have a noninfarct-related artery CTO, but randomized controlled data on management are lacking.
“We don’t know how to treat them or what to do with these patients. What we do know is that the observed mortality in multivessel-disease patients vs. single vessel–disease patients is mainly driven by confirmed total occlusion. Also, the observed reduced LV function in multivessel-disease patients vs. single vessel–disease patients is also mainly driven by chronic total occlusion,” Dr. Henriques said.
“The EXPLORE trial is the first randomized controlled trial on the impact of PCI of CTO on LV function and clinical outcome in post-STEMI patients,” he said.
The findings suggest that CTO-PCI for a post-STEMI CTO located in the LAD may improve LV function and potentially improve clinical outcome in the long term, he concluded.
Dr. Henriques reported receiving grant or research support from Abbott Vascular, Abiomed, Biotronik, and B.Braun.
SAN FRANCISCO – Recanalization of a chronic total occlusion in a noninfarct-related artery within a week after primary percutaneous coronary intervention was safe and feasible but did not improve overall left ventricular ejection fraction or LV end diastolic volume in the randomized, prospective EXPLORE trial.
At 4 months after primary percutaneous coronary intervention (pPCI), cardiac magnetic resonance imaging showed that left ventricular ejection fraction (LVEF) was similar in 136 patients who underwent chronic total occlusion percutaneous coronary intervention (CTO-PCI) and 144 who did not undergo CTO-PCI (44.1 and 44.8, respectively) within 1 week after the pPCI, Dr. Jose P.S. Henriques reported at the Transcatheter Cardiovascular Therapeutics annual meeting.
LV end diastolic volume also was similar in the two groups (215.6 and 212.8, respectively), Dr. Henriques of the Academic Medical Center, Amsterdam, said at the meeting, which was sponsored by the Cardiovascular Research Foundation.
A subgroup analysis, however, showed that LVEF did improve significantly with CTO-PCI in 69 patients whose CTOs were located in the left anterior descending (LAD) artery, compared with 211 patients with non-LAD CTOs (treatment effect, 6.8 vs. –3.2), Dr. Henriques said.
Patients enrolled in the EXPLORE trial had a mean age of 60 years, and most were men (89% in the CTO-PCI group and 82% in the non-CTO-PCI group). The two groups were well balanced with respect to clinical and demographic characteristics. Of note, both groups had a high rate of triple-vessel disease with greater than 70% stenosis and high rates of multiple CTOs (9% and 14%), he said.
Of those who underwent CTO-PCI, 6 had multiple CTO arteries treated, 124 were treated using an antegrade-only technique, 23 were treated using a retrograde technique, and 5 were treated using Crossboss/Stingray. The self-reported PCI success rates was 80%, but this was downgraded to 72% based on core lab adjudication.
About 10% of ST-segment–elevation myocardial infarction (STEMI) patients have a noninfarct-related artery CTO, but randomized controlled data on management are lacking.
“We don’t know how to treat them or what to do with these patients. What we do know is that the observed mortality in multivessel-disease patients vs. single vessel–disease patients is mainly driven by confirmed total occlusion. Also, the observed reduced LV function in multivessel-disease patients vs. single vessel–disease patients is also mainly driven by chronic total occlusion,” Dr. Henriques said.
“The EXPLORE trial is the first randomized controlled trial on the impact of PCI of CTO on LV function and clinical outcome in post-STEMI patients,” he said.
The findings suggest that CTO-PCI for a post-STEMI CTO located in the LAD may improve LV function and potentially improve clinical outcome in the long term, he concluded.
Dr. Henriques reported receiving grant or research support from Abbott Vascular, Abiomed, Biotronik, and B.Braun.
AT TCT 2015
Key clinical point: Recanalization of a chronic total occlusion in a noninfarct-related artery within a week after primary percutaneous coronary intervention was safe and feasible.
Major finding: LVEF improved significantly with CTO-PCI in patients with LAD artery CTOs vs. non-LAD CTOs (treatment effect, 6.8 vs. –3.2).
Data source: The randomized, prospective EXPLORE Trial of 280 patients.
Disclosures: Dr. Henriques reported receiving grant or research support from Abbott Vascular, Abiomed, Biotronik, and B.Braun.
ESC: What’s the hottest recent advance in cardiology? And the winner is …
LONDON – What was the top development in all of cardiology during the past year, the advance that holds the most far-reaching implications for clinical practice?
At the annual congress of the European Society of Cardiology, six experts each made a case for the biggest game changer in their discipline – risk prevention, electrophysiology, imaging, heart failure, percutaneous coronary intervention, and acute cardiac care. And when the audience of perhaps 400-strong had cast their votes, the winner was … the novel angiotensin receptor neprilysin inhibitor (ARNI) known as LCZ696 or valsartan/sacubitril. In the landmark PARADIGM-HF trial, the drug reduced the risk of cardiovascular death by 20% and heart failure hospitalization by 21% over and above what’s achieved with enalapril plus the other current guideline-recommended heart failure medications. “I’m a device person, but I’ve decided a device is not the most important recent innovation in heart failure,” Dr. Cecilia Linde said in her winning argument.
“This ARNI is the first new drug in years with a very clear impact on morbidity and mortality. This is why I believe PARADIGM-HF is the most important study result of the last year in heart failure. It will directly impact treatment and will change the ESC guidelines for heart failure therapy. The PARADIGM-HF results suggest that the ARNI should be given as first-line therapy instead of an ACE inhibitor or angiotensin receptor blocker,” said Dr. Linde, professor and head of cardiology at the Karolinska Institute, Stockholm.
In the double-blind, randomized 8,399-patient PARADIGM-HF trial (N Engl J Med. 2014 Sep 11;371[11]:993-1004), the number needed to treat with LCZ696 instead of enalapril for 27 months in order to avoid one cardiovascular death or heart failure hospitalization was 21. The number needed to treat to avoid one cardiovascular death was 32.
Electrophysiology
The big news here is the concept of the autonomic nervous system as the master controller of atrial fibrillation, governing both the firing of arrhythmic triggers and the change in the arrhythmogenic substrate over time, according to Dr. Sabine Ernst of the National Heart and Lung Institute at Imperial College, London.
“There is a new recognition of how the sympathetic and parasympathetic nervous systems interact to initiate and maintain arrhythmias. This will change the electrophysiology world forever,” she predicted.
Indeed, the future of antiarrhythmic therapy lies in neuromodulation of the autonomic nervous system, and it’s a lot closer than most cardiologists realize, according to the electrophysiologist.
She pointed to a recent study in which investigators at the University of Oklahoma Heart Rhythm Institute randomized 40 patients with paroxysmal atrial fibrillation to noninvasive low-level electrical stimulation of the vagus nerve or to sham treatment. The stimulation at 20 Hz suppressed atrial fibrillation and reduced levels of inflammatory cytokines (J Am Coll Cardiol. 2015 Mar 10;65[9]:867-75).
Vagus nerve stimulation was accomplished using a pair of clips attached to the external ear in order to access the tragus nerve. At just 20 Hz, participants felt no discomfort.
“This is just the very first step. It’s probably not the right frequency or intensity yet. But maybe – and I just want you to start to dream about this – just maybe this could be easily implanted in something we put in our ears. How nice it would be if we could add it to a hearing aid for a patient with atrial fibrillation; we would not need to bother with rate control anymore. Or to prevent atrial fibrillation, [we could put] a low-level vagus stimulator in the headphones for a smartphone,” Dr. Ernst said.
The noninvasiveness of this novel approach is what she finds most appealing.
“I want to stop putting catheters in other people’s hearts. I want to use a method I can ideally apply in the outpatient setting. I think we’ve got to move away from just destroying myocardium in patients with arrhythmias,” she said.
Cardiovascular prevention
Dr. Joep Perk nominated as the most important development of the past year in this field a new set of refined ECG screening criteria for asymptomatic hypertrophic cardiomyopathy (HCM) in athletes. Previous criteria – both the 2010 ESC criteria and the recently published Seattle criteria developed by an international collaborative group (Br J Sports Med. 2013 Feb;47[3]:122-4) – have unacceptably high false-positive rates, which lead to further testing, particularly in black athletes.
“In my personal experience, these young athletes start to think there is something wrong with their heart. They’ll be worried and might be erroneously disqualified. So even though we mean well, it does a lot of psychological harm,” said Dr. Perk, head of the department of internal medicine at Oskarshamn (Sweden) Hospital.
The so-called refined criteria (Circulation. 2014 Apr 22;129[16]:1637-49) were designed to improve upon the specificity of the ESC and Seattle criteria by excluding several isolated ECG patterns that have been shown not relevant in black athletes.
When the developers of the refined criteria applied all three sets of criteria to a large population of black and white athletes, including 103 young athletes with HCM, all three showed 98% sensitivity for the detection of HCM. However, the false-positive ECG rate in black athletes improved from 40.4% using the ESC criteria, to 18.4% with the Seattle criteria, to 11.5% using the refined criteria. Among white athletes, the false-positive rates using the three sets of criteria were 16.2%, 7.1%, and 5.3%.
“These new refined criteria should be incorporated into guidelines for the screening of athletes. They provide a 71% reduction in positive ECGs in black athletes, compared with the ESC recommendations,” Dr. Perk said.
Cardiac imaging
“I really think 3-D printing is going to revolutionize every aspect of medicine,” asserted Dr. Luigi Badano of the University of Padua (Italy).
At the ESC congress, his research group presented a study in which they used custom software to create an exact model of a real patient’s tricuspid valve out of liquid resin based on transthoracic echo images. It took 90 minutes.
“This technology allows us to hold the physical structure of the heart in our hands,” he noted. “We can use it to teach anatomy to medical students without a corpse, plan surgical interventions, and communicate with patients, showing them exact structures and revolutionizing the concept of informed consent.”
And that’s just scratching the surface. He noted that investigators at Wake Forest Baptist Medical Center Institute for Regenerative Medicine in North Carolina recently utilized 3-D printing with bio-ink and bio-paper to print 3-D beating cardiac cells clustered into “organoids.” It’s the first step toward creating a prototype beating heart.
“Can you dream about that? The donor heart shortage could in the future be solved by printing a beating heart for insertion into the patient. The investigators predict they’ll have a functional beating heart within 20 years,” Dr. Badano said.
Acute cardiac care
Dr. Maddalena Lettino and her fellow leaders of the European Acute Cardiac Care Association agreed that the breakthrough of the year in their field was validation of a novel 1-hour rule-in/rule-out algorithm using high-sensitivity cardiac troponin T to accelerate management of patients who present to the emergency department with chest pain. According to studies totaling more than 3,000 patients with more than 600 MIs in which the assay and algorithm were tested, roughly 75% of patients can safely and accurately have acute MI ruled out or ruled in within 1 hour.
Given that close to 10% of all emergency department visits are for chest pain, adoption of this algorithm will reduce ED overcrowding, speed physician workflow, save health care systems money, and spare patients and families the anxiety that comes with a delayed diagnosis, said Dr. Lettino, director of the clinical cardiology unit at Humanitas Research Hospital in Milan.
Coronary intervention
The 15%-20% of coronary stent recipients who are at high bleeding risk constitute “the forgotten patient population,” said Dr. Philippe Garot of the Paris South Cardiovascular Institute.
He noted that the key question of whether such patients can be managed safely with a mere 1-month course of dual antiplatelet therapy will finally be answered this fall with the release of the LEADERS FREE trial results. This large, randomized double-blind trial compares safety and efficacy outcomes in patients assigned to a bare metal stent or the novel drug-eluting BioFreedom stent.
Stay tuned, because LEADERS FREE could be a game changer in interventional cardiology, he said.
The six presenters indicated they had no relevant financial conflicts.
LONDON – What was the top development in all of cardiology during the past year, the advance that holds the most far-reaching implications for clinical practice?
At the annual congress of the European Society of Cardiology, six experts each made a case for the biggest game changer in their discipline – risk prevention, electrophysiology, imaging, heart failure, percutaneous coronary intervention, and acute cardiac care. And when the audience of perhaps 400-strong had cast their votes, the winner was … the novel angiotensin receptor neprilysin inhibitor (ARNI) known as LCZ696 or valsartan/sacubitril. In the landmark PARADIGM-HF trial, the drug reduced the risk of cardiovascular death by 20% and heart failure hospitalization by 21% over and above what’s achieved with enalapril plus the other current guideline-recommended heart failure medications. “I’m a device person, but I’ve decided a device is not the most important recent innovation in heart failure,” Dr. Cecilia Linde said in her winning argument.
“This ARNI is the first new drug in years with a very clear impact on morbidity and mortality. This is why I believe PARADIGM-HF is the most important study result of the last year in heart failure. It will directly impact treatment and will change the ESC guidelines for heart failure therapy. The PARADIGM-HF results suggest that the ARNI should be given as first-line therapy instead of an ACE inhibitor or angiotensin receptor blocker,” said Dr. Linde, professor and head of cardiology at the Karolinska Institute, Stockholm.
In the double-blind, randomized 8,399-patient PARADIGM-HF trial (N Engl J Med. 2014 Sep 11;371[11]:993-1004), the number needed to treat with LCZ696 instead of enalapril for 27 months in order to avoid one cardiovascular death or heart failure hospitalization was 21. The number needed to treat to avoid one cardiovascular death was 32.
Electrophysiology
The big news here is the concept of the autonomic nervous system as the master controller of atrial fibrillation, governing both the firing of arrhythmic triggers and the change in the arrhythmogenic substrate over time, according to Dr. Sabine Ernst of the National Heart and Lung Institute at Imperial College, London.
“There is a new recognition of how the sympathetic and parasympathetic nervous systems interact to initiate and maintain arrhythmias. This will change the electrophysiology world forever,” she predicted.
Indeed, the future of antiarrhythmic therapy lies in neuromodulation of the autonomic nervous system, and it’s a lot closer than most cardiologists realize, according to the electrophysiologist.
She pointed to a recent study in which investigators at the University of Oklahoma Heart Rhythm Institute randomized 40 patients with paroxysmal atrial fibrillation to noninvasive low-level electrical stimulation of the vagus nerve or to sham treatment. The stimulation at 20 Hz suppressed atrial fibrillation and reduced levels of inflammatory cytokines (J Am Coll Cardiol. 2015 Mar 10;65[9]:867-75).
Vagus nerve stimulation was accomplished using a pair of clips attached to the external ear in order to access the tragus nerve. At just 20 Hz, participants felt no discomfort.
“This is just the very first step. It’s probably not the right frequency or intensity yet. But maybe – and I just want you to start to dream about this – just maybe this could be easily implanted in something we put in our ears. How nice it would be if we could add it to a hearing aid for a patient with atrial fibrillation; we would not need to bother with rate control anymore. Or to prevent atrial fibrillation, [we could put] a low-level vagus stimulator in the headphones for a smartphone,” Dr. Ernst said.
The noninvasiveness of this novel approach is what she finds most appealing.
“I want to stop putting catheters in other people’s hearts. I want to use a method I can ideally apply in the outpatient setting. I think we’ve got to move away from just destroying myocardium in patients with arrhythmias,” she said.
Cardiovascular prevention
Dr. Joep Perk nominated as the most important development of the past year in this field a new set of refined ECG screening criteria for asymptomatic hypertrophic cardiomyopathy (HCM) in athletes. Previous criteria – both the 2010 ESC criteria and the recently published Seattle criteria developed by an international collaborative group (Br J Sports Med. 2013 Feb;47[3]:122-4) – have unacceptably high false-positive rates, which lead to further testing, particularly in black athletes.
“In my personal experience, these young athletes start to think there is something wrong with their heart. They’ll be worried and might be erroneously disqualified. So even though we mean well, it does a lot of psychological harm,” said Dr. Perk, head of the department of internal medicine at Oskarshamn (Sweden) Hospital.
The so-called refined criteria (Circulation. 2014 Apr 22;129[16]:1637-49) were designed to improve upon the specificity of the ESC and Seattle criteria by excluding several isolated ECG patterns that have been shown not relevant in black athletes.
When the developers of the refined criteria applied all three sets of criteria to a large population of black and white athletes, including 103 young athletes with HCM, all three showed 98% sensitivity for the detection of HCM. However, the false-positive ECG rate in black athletes improved from 40.4% using the ESC criteria, to 18.4% with the Seattle criteria, to 11.5% using the refined criteria. Among white athletes, the false-positive rates using the three sets of criteria were 16.2%, 7.1%, and 5.3%.
“These new refined criteria should be incorporated into guidelines for the screening of athletes. They provide a 71% reduction in positive ECGs in black athletes, compared with the ESC recommendations,” Dr. Perk said.
Cardiac imaging
“I really think 3-D printing is going to revolutionize every aspect of medicine,” asserted Dr. Luigi Badano of the University of Padua (Italy).
At the ESC congress, his research group presented a study in which they used custom software to create an exact model of a real patient’s tricuspid valve out of liquid resin based on transthoracic echo images. It took 90 minutes.
“This technology allows us to hold the physical structure of the heart in our hands,” he noted. “We can use it to teach anatomy to medical students without a corpse, plan surgical interventions, and communicate with patients, showing them exact structures and revolutionizing the concept of informed consent.”
And that’s just scratching the surface. He noted that investigators at Wake Forest Baptist Medical Center Institute for Regenerative Medicine in North Carolina recently utilized 3-D printing with bio-ink and bio-paper to print 3-D beating cardiac cells clustered into “organoids.” It’s the first step toward creating a prototype beating heart.
“Can you dream about that? The donor heart shortage could in the future be solved by printing a beating heart for insertion into the patient. The investigators predict they’ll have a functional beating heart within 20 years,” Dr. Badano said.
Acute cardiac care
Dr. Maddalena Lettino and her fellow leaders of the European Acute Cardiac Care Association agreed that the breakthrough of the year in their field was validation of a novel 1-hour rule-in/rule-out algorithm using high-sensitivity cardiac troponin T to accelerate management of patients who present to the emergency department with chest pain. According to studies totaling more than 3,000 patients with more than 600 MIs in which the assay and algorithm were tested, roughly 75% of patients can safely and accurately have acute MI ruled out or ruled in within 1 hour.
Given that close to 10% of all emergency department visits are for chest pain, adoption of this algorithm will reduce ED overcrowding, speed physician workflow, save health care systems money, and spare patients and families the anxiety that comes with a delayed diagnosis, said Dr. Lettino, director of the clinical cardiology unit at Humanitas Research Hospital in Milan.
Coronary intervention
The 15%-20% of coronary stent recipients who are at high bleeding risk constitute “the forgotten patient population,” said Dr. Philippe Garot of the Paris South Cardiovascular Institute.
He noted that the key question of whether such patients can be managed safely with a mere 1-month course of dual antiplatelet therapy will finally be answered this fall with the release of the LEADERS FREE trial results. This large, randomized double-blind trial compares safety and efficacy outcomes in patients assigned to a bare metal stent or the novel drug-eluting BioFreedom stent.
Stay tuned, because LEADERS FREE could be a game changer in interventional cardiology, he said.
The six presenters indicated they had no relevant financial conflicts.
LONDON – What was the top development in all of cardiology during the past year, the advance that holds the most far-reaching implications for clinical practice?
At the annual congress of the European Society of Cardiology, six experts each made a case for the biggest game changer in their discipline – risk prevention, electrophysiology, imaging, heart failure, percutaneous coronary intervention, and acute cardiac care. And when the audience of perhaps 400-strong had cast their votes, the winner was … the novel angiotensin receptor neprilysin inhibitor (ARNI) known as LCZ696 or valsartan/sacubitril. In the landmark PARADIGM-HF trial, the drug reduced the risk of cardiovascular death by 20% and heart failure hospitalization by 21% over and above what’s achieved with enalapril plus the other current guideline-recommended heart failure medications. “I’m a device person, but I’ve decided a device is not the most important recent innovation in heart failure,” Dr. Cecilia Linde said in her winning argument.
“This ARNI is the first new drug in years with a very clear impact on morbidity and mortality. This is why I believe PARADIGM-HF is the most important study result of the last year in heart failure. It will directly impact treatment and will change the ESC guidelines for heart failure therapy. The PARADIGM-HF results suggest that the ARNI should be given as first-line therapy instead of an ACE inhibitor or angiotensin receptor blocker,” said Dr. Linde, professor and head of cardiology at the Karolinska Institute, Stockholm.
In the double-blind, randomized 8,399-patient PARADIGM-HF trial (N Engl J Med. 2014 Sep 11;371[11]:993-1004), the number needed to treat with LCZ696 instead of enalapril for 27 months in order to avoid one cardiovascular death or heart failure hospitalization was 21. The number needed to treat to avoid one cardiovascular death was 32.
Electrophysiology
The big news here is the concept of the autonomic nervous system as the master controller of atrial fibrillation, governing both the firing of arrhythmic triggers and the change in the arrhythmogenic substrate over time, according to Dr. Sabine Ernst of the National Heart and Lung Institute at Imperial College, London.
“There is a new recognition of how the sympathetic and parasympathetic nervous systems interact to initiate and maintain arrhythmias. This will change the electrophysiology world forever,” she predicted.
Indeed, the future of antiarrhythmic therapy lies in neuromodulation of the autonomic nervous system, and it’s a lot closer than most cardiologists realize, according to the electrophysiologist.
She pointed to a recent study in which investigators at the University of Oklahoma Heart Rhythm Institute randomized 40 patients with paroxysmal atrial fibrillation to noninvasive low-level electrical stimulation of the vagus nerve or to sham treatment. The stimulation at 20 Hz suppressed atrial fibrillation and reduced levels of inflammatory cytokines (J Am Coll Cardiol. 2015 Mar 10;65[9]:867-75).
Vagus nerve stimulation was accomplished using a pair of clips attached to the external ear in order to access the tragus nerve. At just 20 Hz, participants felt no discomfort.
“This is just the very first step. It’s probably not the right frequency or intensity yet. But maybe – and I just want you to start to dream about this – just maybe this could be easily implanted in something we put in our ears. How nice it would be if we could add it to a hearing aid for a patient with atrial fibrillation; we would not need to bother with rate control anymore. Or to prevent atrial fibrillation, [we could put] a low-level vagus stimulator in the headphones for a smartphone,” Dr. Ernst said.
The noninvasiveness of this novel approach is what she finds most appealing.
“I want to stop putting catheters in other people’s hearts. I want to use a method I can ideally apply in the outpatient setting. I think we’ve got to move away from just destroying myocardium in patients with arrhythmias,” she said.
Cardiovascular prevention
Dr. Joep Perk nominated as the most important development of the past year in this field a new set of refined ECG screening criteria for asymptomatic hypertrophic cardiomyopathy (HCM) in athletes. Previous criteria – both the 2010 ESC criteria and the recently published Seattle criteria developed by an international collaborative group (Br J Sports Med. 2013 Feb;47[3]:122-4) – have unacceptably high false-positive rates, which lead to further testing, particularly in black athletes.
“In my personal experience, these young athletes start to think there is something wrong with their heart. They’ll be worried and might be erroneously disqualified. So even though we mean well, it does a lot of psychological harm,” said Dr. Perk, head of the department of internal medicine at Oskarshamn (Sweden) Hospital.
The so-called refined criteria (Circulation. 2014 Apr 22;129[16]:1637-49) were designed to improve upon the specificity of the ESC and Seattle criteria by excluding several isolated ECG patterns that have been shown not relevant in black athletes.
When the developers of the refined criteria applied all three sets of criteria to a large population of black and white athletes, including 103 young athletes with HCM, all three showed 98% sensitivity for the detection of HCM. However, the false-positive ECG rate in black athletes improved from 40.4% using the ESC criteria, to 18.4% with the Seattle criteria, to 11.5% using the refined criteria. Among white athletes, the false-positive rates using the three sets of criteria were 16.2%, 7.1%, and 5.3%.
“These new refined criteria should be incorporated into guidelines for the screening of athletes. They provide a 71% reduction in positive ECGs in black athletes, compared with the ESC recommendations,” Dr. Perk said.
Cardiac imaging
“I really think 3-D printing is going to revolutionize every aspect of medicine,” asserted Dr. Luigi Badano of the University of Padua (Italy).
At the ESC congress, his research group presented a study in which they used custom software to create an exact model of a real patient’s tricuspid valve out of liquid resin based on transthoracic echo images. It took 90 minutes.
“This technology allows us to hold the physical structure of the heart in our hands,” he noted. “We can use it to teach anatomy to medical students without a corpse, plan surgical interventions, and communicate with patients, showing them exact structures and revolutionizing the concept of informed consent.”
And that’s just scratching the surface. He noted that investigators at Wake Forest Baptist Medical Center Institute for Regenerative Medicine in North Carolina recently utilized 3-D printing with bio-ink and bio-paper to print 3-D beating cardiac cells clustered into “organoids.” It’s the first step toward creating a prototype beating heart.
“Can you dream about that? The donor heart shortage could in the future be solved by printing a beating heart for insertion into the patient. The investigators predict they’ll have a functional beating heart within 20 years,” Dr. Badano said.
Acute cardiac care
Dr. Maddalena Lettino and her fellow leaders of the European Acute Cardiac Care Association agreed that the breakthrough of the year in their field was validation of a novel 1-hour rule-in/rule-out algorithm using high-sensitivity cardiac troponin T to accelerate management of patients who present to the emergency department with chest pain. According to studies totaling more than 3,000 patients with more than 600 MIs in which the assay and algorithm were tested, roughly 75% of patients can safely and accurately have acute MI ruled out or ruled in within 1 hour.
Given that close to 10% of all emergency department visits are for chest pain, adoption of this algorithm will reduce ED overcrowding, speed physician workflow, save health care systems money, and spare patients and families the anxiety that comes with a delayed diagnosis, said Dr. Lettino, director of the clinical cardiology unit at Humanitas Research Hospital in Milan.
Coronary intervention
The 15%-20% of coronary stent recipients who are at high bleeding risk constitute “the forgotten patient population,” said Dr. Philippe Garot of the Paris South Cardiovascular Institute.
He noted that the key question of whether such patients can be managed safely with a mere 1-month course of dual antiplatelet therapy will finally be answered this fall with the release of the LEADERS FREE trial results. This large, randomized double-blind trial compares safety and efficacy outcomes in patients assigned to a bare metal stent or the novel drug-eluting BioFreedom stent.
Stay tuned, because LEADERS FREE could be a game changer in interventional cardiology, he said.
The six presenters indicated they had no relevant financial conflicts.
EXPERT ANALYSIS FROM THE ESC CONGRESS 2015
ESC: Cancer itself may cause cardiotoxicity
LONDON – Cancer itself has cardiotoxic effects independent of those caused by chemotherapy, Dr. Stephan von Haehling said at the annual congress of the European Society of Cardiology.
Evidence from both animal and human studies indicates that the malignancy itself may be exerting adverse cardiac effects even before chemotherapy provides an additional hit to the heart, according to Dr. von Haehling, who is a cardiologist at Charity Medical School, Berlin.
“In patients with advanced cancer, significant alterations exist in several markers of cardiovascular perturbation independent of high-dose chemotherapy. So it looks like the cancer is doing something that’s further worsened when chemotherapy starts,” he explained.
Dr. von Haehling and his coinvestigators first demonstrated this phenomenon in a rat model of liver cancer (Eur Heart J. 2014 Apr;35[14]:932-41). The tumor-bearing rats had the classic symptoms of cancer cachexia, including fatigue, impaired exercise capacity, loss of body weight, and dyspnea, as well as progressive wasting of left ventricular mass, even before exposure to chemotherapy. Strikingly, administration of the cardioselective beta-blocker bisoprolol and the aldosterone inhibitor spironolactone reduced left ventricular wasting, curbed cardiac dysfunction, improved a validated measure of rat quality of life, and significantly prolonged rat survival, compared with placebo.
Further exploration of these findings in clinical trials deserves to be a priority in light of the potential quality-of-life benefits for cancer patients, Dr. von Haehling observed.
He and his coworkers followed up the rat study with a prospective study of 50 patients with colorectal cancer, 51 with heart failure, and 51 healthy controls. Of the colorectal cancer patients, 24 underwent echocardiography and other cardiovascular function studies before they went on chemotherapy, while the other 26 did so after starting chemotherapy.
The colorectal cancer patients had a mildly elevated heart rate: an average of 73 beats per minute, compared with 65 bpm in controls and in heart failure patients on beta-blocker therapy. “This is something I see quite often. These patients usually have a mildly elevated heart rate in the range of 80-90 [bpms] or even slightly above,” he said.
Heart rate variability, exercise capacity as measured by treadmill VO2 max testing, and left ventricular ejection fraction were significantly lower in cancer patients than controls, and lower still in the heart failure patients. More interesting were the differences between chemotherapy-naive and on-treatment colorectal cancer patients. Several major determinants of cardiovascular function were impaired in chemotherapy-naive cancer patients, compared with controls, and even more severely impaired in cancer patients on chemotherapy.
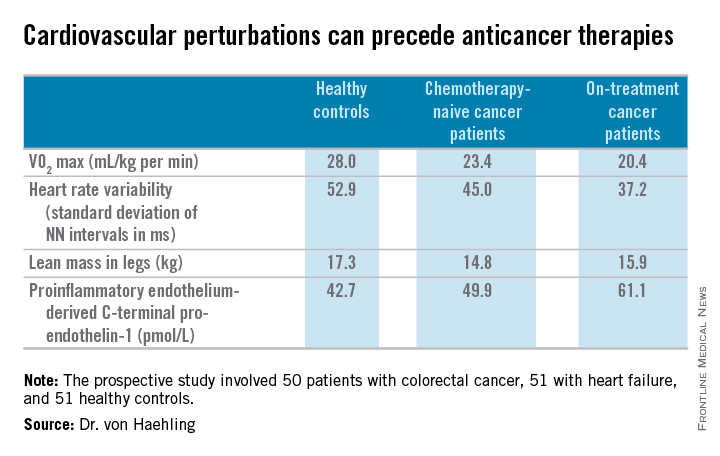
For more about current thinking regarding the prevention, monitoring, and treatment of cardiac side effects of anticancer therapies, Dr. von Haehling recommended the multidisciplinary clinical practice guidelines developed by the European Society for Medical Oncology (Ann Oncol. 2012 Oct;23 Suppl 7:vii155-66).
He reported having no financial conflicts regarding his cardio-oncology studies.
LONDON – Cancer itself has cardiotoxic effects independent of those caused by chemotherapy, Dr. Stephan von Haehling said at the annual congress of the European Society of Cardiology.
Evidence from both animal and human studies indicates that the malignancy itself may be exerting adverse cardiac effects even before chemotherapy provides an additional hit to the heart, according to Dr. von Haehling, who is a cardiologist at Charity Medical School, Berlin.
“In patients with advanced cancer, significant alterations exist in several markers of cardiovascular perturbation independent of high-dose chemotherapy. So it looks like the cancer is doing something that’s further worsened when chemotherapy starts,” he explained.
Dr. von Haehling and his coinvestigators first demonstrated this phenomenon in a rat model of liver cancer (Eur Heart J. 2014 Apr;35[14]:932-41). The tumor-bearing rats had the classic symptoms of cancer cachexia, including fatigue, impaired exercise capacity, loss of body weight, and dyspnea, as well as progressive wasting of left ventricular mass, even before exposure to chemotherapy. Strikingly, administration of the cardioselective beta-blocker bisoprolol and the aldosterone inhibitor spironolactone reduced left ventricular wasting, curbed cardiac dysfunction, improved a validated measure of rat quality of life, and significantly prolonged rat survival, compared with placebo.
Further exploration of these findings in clinical trials deserves to be a priority in light of the potential quality-of-life benefits for cancer patients, Dr. von Haehling observed.
He and his coworkers followed up the rat study with a prospective study of 50 patients with colorectal cancer, 51 with heart failure, and 51 healthy controls. Of the colorectal cancer patients, 24 underwent echocardiography and other cardiovascular function studies before they went on chemotherapy, while the other 26 did so after starting chemotherapy.
The colorectal cancer patients had a mildly elevated heart rate: an average of 73 beats per minute, compared with 65 bpm in controls and in heart failure patients on beta-blocker therapy. “This is something I see quite often. These patients usually have a mildly elevated heart rate in the range of 80-90 [bpms] or even slightly above,” he said.
Heart rate variability, exercise capacity as measured by treadmill VO2 max testing, and left ventricular ejection fraction were significantly lower in cancer patients than controls, and lower still in the heart failure patients. More interesting were the differences between chemotherapy-naive and on-treatment colorectal cancer patients. Several major determinants of cardiovascular function were impaired in chemotherapy-naive cancer patients, compared with controls, and even more severely impaired in cancer patients on chemotherapy.

For more about current thinking regarding the prevention, monitoring, and treatment of cardiac side effects of anticancer therapies, Dr. von Haehling recommended the multidisciplinary clinical practice guidelines developed by the European Society for Medical Oncology (Ann Oncol. 2012 Oct;23 Suppl 7:vii155-66).
He reported having no financial conflicts regarding his cardio-oncology studies.
LONDON – Cancer itself has cardiotoxic effects independent of those caused by chemotherapy, Dr. Stephan von Haehling said at the annual congress of the European Society of Cardiology.
Evidence from both animal and human studies indicates that the malignancy itself may be exerting adverse cardiac effects even before chemotherapy provides an additional hit to the heart, according to Dr. von Haehling, who is a cardiologist at Charity Medical School, Berlin.
“In patients with advanced cancer, significant alterations exist in several markers of cardiovascular perturbation independent of high-dose chemotherapy. So it looks like the cancer is doing something that’s further worsened when chemotherapy starts,” he explained.
Dr. von Haehling and his coinvestigators first demonstrated this phenomenon in a rat model of liver cancer (Eur Heart J. 2014 Apr;35[14]:932-41). The tumor-bearing rats had the classic symptoms of cancer cachexia, including fatigue, impaired exercise capacity, loss of body weight, and dyspnea, as well as progressive wasting of left ventricular mass, even before exposure to chemotherapy. Strikingly, administration of the cardioselective beta-blocker bisoprolol and the aldosterone inhibitor spironolactone reduced left ventricular wasting, curbed cardiac dysfunction, improved a validated measure of rat quality of life, and significantly prolonged rat survival, compared with placebo.
Further exploration of these findings in clinical trials deserves to be a priority in light of the potential quality-of-life benefits for cancer patients, Dr. von Haehling observed.
He and his coworkers followed up the rat study with a prospective study of 50 patients with colorectal cancer, 51 with heart failure, and 51 healthy controls. Of the colorectal cancer patients, 24 underwent echocardiography and other cardiovascular function studies before they went on chemotherapy, while the other 26 did so after starting chemotherapy.
The colorectal cancer patients had a mildly elevated heart rate: an average of 73 beats per minute, compared with 65 bpm in controls and in heart failure patients on beta-blocker therapy. “This is something I see quite often. These patients usually have a mildly elevated heart rate in the range of 80-90 [bpms] or even slightly above,” he said.
Heart rate variability, exercise capacity as measured by treadmill VO2 max testing, and left ventricular ejection fraction were significantly lower in cancer patients than controls, and lower still in the heart failure patients. More interesting were the differences between chemotherapy-naive and on-treatment colorectal cancer patients. Several major determinants of cardiovascular function were impaired in chemotherapy-naive cancer patients, compared with controls, and even more severely impaired in cancer patients on chemotherapy.

For more about current thinking regarding the prevention, monitoring, and treatment of cardiac side effects of anticancer therapies, Dr. von Haehling recommended the multidisciplinary clinical practice guidelines developed by the European Society for Medical Oncology (Ann Oncol. 2012 Oct;23 Suppl 7:vii155-66).
He reported having no financial conflicts regarding his cardio-oncology studies.
EXPERT ANALYSIS FROM THE ESC CONGRESS 2015
FDA approves combo therapy for pulmonary hypertension
The Food and Drug Administration has approved the combined use of ambrisentan (Letairis) and tadalafil for the treatment of pulmonary arterial hypertension based on positive results from the AMBITION trial, according to Gilead Sciences.
In the trial, 605 PAH patients with World Health Organization functional class II or III symptoms were randomly assigned to receive either ambrisentan, tadalafil, or a combination of the two. One-month outcomes were significantly better in the combination group, with only 8% of patients needing to be hospitalized for worsening PAH, compared with 22% in the ambrisentan group and 15% in the tadalafil group (N Engl J Med. 2015 Aug 27. doi: 10.1056/NEJMoa1413687).
Improvement from baseline in 6-minute walk distance after 24 weeks was also higher in the combination group, where patients walked a median of 24 meters farther than did the ambrisentan group and 20 meters more than did the tadalafil group.
Side effects tended to be more common in the combination group than in either of the single-drug groups, with the most common side effect, peripheral edema, affecting 45% of the combination group, 38% of the ambrisentan group, and 28% of the tadalafil group. Other common side effects included headache, nasal congestion, cough, anemia, dyspepsia, and bronchitis.
“Patients receiving ambrisentan and tadalafil up front are less likely to experience disease progression or be hospitalized, and have more improvement in exercise ability than patients receiving either effective therapy alone. As such, this combination represents a new treatment strategy for patients living with this debilitating and life-threatening disease,” Dr. Ronald J. Ortiz, professor of medicine at the University of California, Los Angeles, and an AMBITION investigator, said in a statement.
Ambrisentan, an endothelin receptor antagonist, was approved in 2007 as monotherapy for PAH to improve exercise ability and delay clinical worsening. Tadalafil, a phosphodiesterase type 5 inhibitor, was approved in 2009 to improve exercise ability in PAH patients.
Find the full press release on the Gilead website.
The Food and Drug Administration has approved the combined use of ambrisentan (Letairis) and tadalafil for the treatment of pulmonary arterial hypertension based on positive results from the AMBITION trial, according to Gilead Sciences.
In the trial, 605 PAH patients with World Health Organization functional class II or III symptoms were randomly assigned to receive either ambrisentan, tadalafil, or a combination of the two. One-month outcomes were significantly better in the combination group, with only 8% of patients needing to be hospitalized for worsening PAH, compared with 22% in the ambrisentan group and 15% in the tadalafil group (N Engl J Med. 2015 Aug 27. doi: 10.1056/NEJMoa1413687).
Improvement from baseline in 6-minute walk distance after 24 weeks was also higher in the combination group, where patients walked a median of 24 meters farther than did the ambrisentan group and 20 meters more than did the tadalafil group.
Side effects tended to be more common in the combination group than in either of the single-drug groups, with the most common side effect, peripheral edema, affecting 45% of the combination group, 38% of the ambrisentan group, and 28% of the tadalafil group. Other common side effects included headache, nasal congestion, cough, anemia, dyspepsia, and bronchitis.
“Patients receiving ambrisentan and tadalafil up front are less likely to experience disease progression or be hospitalized, and have more improvement in exercise ability than patients receiving either effective therapy alone. As such, this combination represents a new treatment strategy for patients living with this debilitating and life-threatening disease,” Dr. Ronald J. Ortiz, professor of medicine at the University of California, Los Angeles, and an AMBITION investigator, said in a statement.
Ambrisentan, an endothelin receptor antagonist, was approved in 2007 as monotherapy for PAH to improve exercise ability and delay clinical worsening. Tadalafil, a phosphodiesterase type 5 inhibitor, was approved in 2009 to improve exercise ability in PAH patients.
Find the full press release on the Gilead website.
The Food and Drug Administration has approved the combined use of ambrisentan (Letairis) and tadalafil for the treatment of pulmonary arterial hypertension based on positive results from the AMBITION trial, according to Gilead Sciences.
In the trial, 605 PAH patients with World Health Organization functional class II or III symptoms were randomly assigned to receive either ambrisentan, tadalafil, or a combination of the two. One-month outcomes were significantly better in the combination group, with only 8% of patients needing to be hospitalized for worsening PAH, compared with 22% in the ambrisentan group and 15% in the tadalafil group (N Engl J Med. 2015 Aug 27. doi: 10.1056/NEJMoa1413687).
Improvement from baseline in 6-minute walk distance after 24 weeks was also higher in the combination group, where patients walked a median of 24 meters farther than did the ambrisentan group and 20 meters more than did the tadalafil group.
Side effects tended to be more common in the combination group than in either of the single-drug groups, with the most common side effect, peripheral edema, affecting 45% of the combination group, 38% of the ambrisentan group, and 28% of the tadalafil group. Other common side effects included headache, nasal congestion, cough, anemia, dyspepsia, and bronchitis.
“Patients receiving ambrisentan and tadalafil up front are less likely to experience disease progression or be hospitalized, and have more improvement in exercise ability than patients receiving either effective therapy alone. As such, this combination represents a new treatment strategy for patients living with this debilitating and life-threatening disease,” Dr. Ronald J. Ortiz, professor of medicine at the University of California, Los Angeles, and an AMBITION investigator, said in a statement.
Ambrisentan, an endothelin receptor antagonist, was approved in 2007 as monotherapy for PAH to improve exercise ability and delay clinical worsening. Tadalafil, a phosphodiesterase type 5 inhibitor, was approved in 2009 to improve exercise ability in PAH patients.
Find the full press release on the Gilead website.




























