User login
Vaccines committee approves recommended influenza strains for 2016-2017 vaccine
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee unanimously approved recommendations regarding the trivalent and quadrivalent influenza vaccines to be distributed during the 2016-2017 flu season.
The 14-member committee voted that the components of the trivalent influenza vaccine for the upcoming flu season should include an A/California/7/2009 (H1N1) pdm09-like virus; an A/Hong Kong/4801/2014 (H3N2)-like virus; and a B/Brisbane/60/2008-like virus of the B/Victoria lineage.
Additionally, the quadrivalent influenza vaccine should include a B/Phuket/3073/2013-like virus of the B/Yamagata lineage as “the second influenza B strain in the vaccine.”
All four components correspond with the recommendations of the World Health Organization, which announced its proposed components for influenza vaccines in the Northern Hemisphere on Feb. 25.
“I’m comfortable trying to follow the footprint of the virus we’ve seen today, quite elegantly put out in front of us,” said committee member Dr. Sarah Long, professor of pediatrics at Drexel University in Philadelphia, adding that she was “very pleased with what happened in the last year” regarding the predictions of dominant virus strains and the effectiveness of the eventual vaccine.
The proposed vaccine for next season differs from the one distributed during the 2015-16 flu season. While both vaccines contain the identical California strain of influenza A and the Phuket strain of influenza B, the 2015-2016 vaccine included an A/Switzerland/9715293/2013 (H3N2)-like virus in its trivalent form, and a B/Brisbane/60/2008-like virus of the B/Victoria lineage in its quadrivalent form.
“Timely vaccine supply requires close collaboration and communication between multiple stakeholders to ensure sufficient provision of [a] well-matched vaccine,” said Matthew Downham, Ph.D., associate director of biopharmaceutical development, AstraZeneca. Timely strain selection will ensure “vaccine availability and usage” for the most widespread and effective coverage.
While the FDA is not obligated to follow the recommendations of the Vaccines and Related Biological Products Advisory Committee, it generally does.
None of the committee members reported any relevant financial disclosures, nor were there any waivers for conflicts of interest.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee unanimously approved recommendations regarding the trivalent and quadrivalent influenza vaccines to be distributed during the 2016-2017 flu season.
The 14-member committee voted that the components of the trivalent influenza vaccine for the upcoming flu season should include an A/California/7/2009 (H1N1) pdm09-like virus; an A/Hong Kong/4801/2014 (H3N2)-like virus; and a B/Brisbane/60/2008-like virus of the B/Victoria lineage.
Additionally, the quadrivalent influenza vaccine should include a B/Phuket/3073/2013-like virus of the B/Yamagata lineage as “the second influenza B strain in the vaccine.”
All four components correspond with the recommendations of the World Health Organization, which announced its proposed components for influenza vaccines in the Northern Hemisphere on Feb. 25.
“I’m comfortable trying to follow the footprint of the virus we’ve seen today, quite elegantly put out in front of us,” said committee member Dr. Sarah Long, professor of pediatrics at Drexel University in Philadelphia, adding that she was “very pleased with what happened in the last year” regarding the predictions of dominant virus strains and the effectiveness of the eventual vaccine.
The proposed vaccine for next season differs from the one distributed during the 2015-16 flu season. While both vaccines contain the identical California strain of influenza A and the Phuket strain of influenza B, the 2015-2016 vaccine included an A/Switzerland/9715293/2013 (H3N2)-like virus in its trivalent form, and a B/Brisbane/60/2008-like virus of the B/Victoria lineage in its quadrivalent form.
“Timely vaccine supply requires close collaboration and communication between multiple stakeholders to ensure sufficient provision of [a] well-matched vaccine,” said Matthew Downham, Ph.D., associate director of biopharmaceutical development, AstraZeneca. Timely strain selection will ensure “vaccine availability and usage” for the most widespread and effective coverage.
While the FDA is not obligated to follow the recommendations of the Vaccines and Related Biological Products Advisory Committee, it generally does.
None of the committee members reported any relevant financial disclosures, nor were there any waivers for conflicts of interest.
The Food and Drug Administration’s Vaccines and Related Biological Products Advisory Committee unanimously approved recommendations regarding the trivalent and quadrivalent influenza vaccines to be distributed during the 2016-2017 flu season.
The 14-member committee voted that the components of the trivalent influenza vaccine for the upcoming flu season should include an A/California/7/2009 (H1N1) pdm09-like virus; an A/Hong Kong/4801/2014 (H3N2)-like virus; and a B/Brisbane/60/2008-like virus of the B/Victoria lineage.
Additionally, the quadrivalent influenza vaccine should include a B/Phuket/3073/2013-like virus of the B/Yamagata lineage as “the second influenza B strain in the vaccine.”
All four components correspond with the recommendations of the World Health Organization, which announced its proposed components for influenza vaccines in the Northern Hemisphere on Feb. 25.
“I’m comfortable trying to follow the footprint of the virus we’ve seen today, quite elegantly put out in front of us,” said committee member Dr. Sarah Long, professor of pediatrics at Drexel University in Philadelphia, adding that she was “very pleased with what happened in the last year” regarding the predictions of dominant virus strains and the effectiveness of the eventual vaccine.
The proposed vaccine for next season differs from the one distributed during the 2015-16 flu season. While both vaccines contain the identical California strain of influenza A and the Phuket strain of influenza B, the 2015-2016 vaccine included an A/Switzerland/9715293/2013 (H3N2)-like virus in its trivalent form, and a B/Brisbane/60/2008-like virus of the B/Victoria lineage in its quadrivalent form.
“Timely vaccine supply requires close collaboration and communication between multiple stakeholders to ensure sufficient provision of [a] well-matched vaccine,” said Matthew Downham, Ph.D., associate director of biopharmaceutical development, AstraZeneca. Timely strain selection will ensure “vaccine availability and usage” for the most widespread and effective coverage.
While the FDA is not obligated to follow the recommendations of the Vaccines and Related Biological Products Advisory Committee, it generally does.
None of the committee members reported any relevant financial disclosures, nor were there any waivers for conflicts of interest.
FROM AN FDA ADVISORY COMMITTEE MEETING
U.S. flu activity continues steady climb
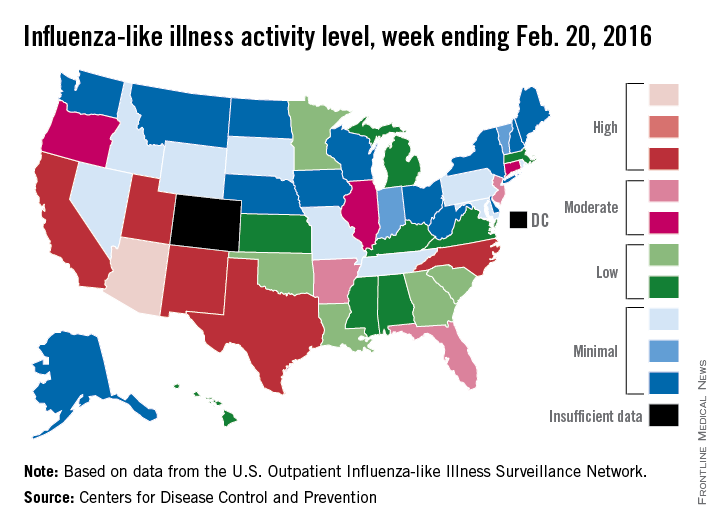
Influenza-like illness (ILI) activity reached a new national high for the 2015-2016 flu season during the week ending Feb. 20, according to the Centers for Disease Control and Prevention.
Nationwide, the proportion of outpatient visits for ILI was 3.2%, up from 3.1% the week before and well over the national baseline of 2.1%. Arizona and Puerto Rico were still at level 10 on the CDC’s 1-10 scale for ILI activity, and they were joined in the “high” range of activity by California, New Mexico, North Carolina, Texas, and Utah, which were all at level 8, the CDC reported Feb. 26.
States in the “moderate” range of activity for week 19 of the 2015-2016 flu season (week 7 of calendar year 2016) were Arkansas, Florida, and New Jersey at level 7 and Connecticut, Illinois, and Oregon at level 6. There were 13 states in the “low” range of activity – five at level 5 and eight states at level 4 – and 24 states in the “minimal” range, of which 13 were at level 1. Colorado and the District of Columbia had insufficient data to determine activity level, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
For the week, one flu-related pediatric death, associated with an influenza B virus, was reported to the CDC, bringing the total to 14 for the season. The only states reporting more that one death are California (two) and Florida (three), the CDC said. The average number of deaths for the three previous flu seasons is over 143.
During week 19, a total of 18,844 respiratory specimens were tested, 13.8% of which were positive: 76.1% for influenza A and 23.9% for influenza B. Since Oct. 1, 2015, 4.2% of specimens have tested positive for influenza, with a 70% to 30% split between influenza A and B, the CDC report showed.
Influenza-like illness (ILI) activity reached a new national high for the 2015-2016 flu season during the week ending Feb. 20, according to the Centers for Disease Control and Prevention.
Nationwide, the proportion of outpatient visits for ILI was 3.2%, up from 3.1% the week before and well over the national baseline of 2.1%. Arizona and Puerto Rico were still at level 10 on the CDC’s 1-10 scale for ILI activity, and they were joined in the “high” range of activity by California, New Mexico, North Carolina, Texas, and Utah, which were all at level 8, the CDC reported Feb. 26.
States in the “moderate” range of activity for week 19 of the 2015-2016 flu season (week 7 of calendar year 2016) were Arkansas, Florida, and New Jersey at level 7 and Connecticut, Illinois, and Oregon at level 6. There were 13 states in the “low” range of activity – five at level 5 and eight states at level 4 – and 24 states in the “minimal” range, of which 13 were at level 1. Colorado and the District of Columbia had insufficient data to determine activity level, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
For the week, one flu-related pediatric death, associated with an influenza B virus, was reported to the CDC, bringing the total to 14 for the season. The only states reporting more that one death are California (two) and Florida (three), the CDC said. The average number of deaths for the three previous flu seasons is over 143.
During week 19, a total of 18,844 respiratory specimens were tested, 13.8% of which were positive: 76.1% for influenza A and 23.9% for influenza B. Since Oct. 1, 2015, 4.2% of specimens have tested positive for influenza, with a 70% to 30% split between influenza A and B, the CDC report showed.
Influenza-like illness (ILI) activity reached a new national high for the 2015-2016 flu season during the week ending Feb. 20, according to the Centers for Disease Control and Prevention.
Nationwide, the proportion of outpatient visits for ILI was 3.2%, up from 3.1% the week before and well over the national baseline of 2.1%. Arizona and Puerto Rico were still at level 10 on the CDC’s 1-10 scale for ILI activity, and they were joined in the “high” range of activity by California, New Mexico, North Carolina, Texas, and Utah, which were all at level 8, the CDC reported Feb. 26.
States in the “moderate” range of activity for week 19 of the 2015-2016 flu season (week 7 of calendar year 2016) were Arkansas, Florida, and New Jersey at level 7 and Connecticut, Illinois, and Oregon at level 6. There were 13 states in the “low” range of activity – five at level 5 and eight states at level 4 – and 24 states in the “minimal” range, of which 13 were at level 1. Colorado and the District of Columbia had insufficient data to determine activity level, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
For the week, one flu-related pediatric death, associated with an influenza B virus, was reported to the CDC, bringing the total to 14 for the season. The only states reporting more that one death are California (two) and Florida (three), the CDC said. The average number of deaths for the three previous flu seasons is over 143.
During week 19, a total of 18,844 respiratory specimens were tested, 13.8% of which were positive: 76.1% for influenza A and 23.9% for influenza B. Since Oct. 1, 2015, 4.2% of specimens have tested positive for influenza, with a 70% to 30% split between influenza A and B, the CDC report showed.
2015-2016 flu vaccine 59% effective, CDC says
The overall rate of effectiveness for the 2015-2016 season’s influenza vaccine is 59%, according to preliminary data shared at a Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices meeting.
“This means that getting a flu vaccine this season reduced the risk of having to go to the doctor because of flu by nearly 60%,” Dr. Joseph Bresee – who heads the CDC’s Epidemiology and Prevention Branch – stated in a press release. “It’s good news and underscores the importance and the benefit of both annual and ongoing vaccination efforts this season.”
These findings are based on data collected by the U.S. Flu Vaccine Effectiveness Network between November 2, 2015, and February 12, 2016. A 59% effectiveness rate would be roughly in line with what previous years’ vaccines have rated when similar strains of influenza have been prevalent, according to the CDC.
Against the H1N1 strain, which was “responsible for most flu illness this season,” the influenza vaccine was 51% effective. Dr. Bresee noted that the CDC has received reports of unvaccinated individuals this season dying or falling seriously ill because of infection from the H1N1 strain.
The vaccine was 76% effective against all influenza B virus strains, and 79% effective against the B/Yamagata virus strains. There are not enough data at this time, however, to determine the vaccine’s effectiveness against B/Victoria or H3N2 strains.
The CDC cautions that, because the current influenza season is still weeks away from being over, the final rate of vaccine effectiveness for 2015-2016 may change once all the data are analyzed. On average, influenza seasons last 13 weeks.
“Flu activity this season started a bit later and has been lower so far than we’ve seen during the previous three seasons, but activity is still on the upswing and expected to continue for several weeks,” Dr. Bresee stated.
The overall rate of effectiveness for the 2015-2016 season’s influenza vaccine is 59%, according to preliminary data shared at a Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices meeting.
“This means that getting a flu vaccine this season reduced the risk of having to go to the doctor because of flu by nearly 60%,” Dr. Joseph Bresee – who heads the CDC’s Epidemiology and Prevention Branch – stated in a press release. “It’s good news and underscores the importance and the benefit of both annual and ongoing vaccination efforts this season.”
These findings are based on data collected by the U.S. Flu Vaccine Effectiveness Network between November 2, 2015, and February 12, 2016. A 59% effectiveness rate would be roughly in line with what previous years’ vaccines have rated when similar strains of influenza have been prevalent, according to the CDC.
Against the H1N1 strain, which was “responsible for most flu illness this season,” the influenza vaccine was 51% effective. Dr. Bresee noted that the CDC has received reports of unvaccinated individuals this season dying or falling seriously ill because of infection from the H1N1 strain.
The vaccine was 76% effective against all influenza B virus strains, and 79% effective against the B/Yamagata virus strains. There are not enough data at this time, however, to determine the vaccine’s effectiveness against B/Victoria or H3N2 strains.
The CDC cautions that, because the current influenza season is still weeks away from being over, the final rate of vaccine effectiveness for 2015-2016 may change once all the data are analyzed. On average, influenza seasons last 13 weeks.
“Flu activity this season started a bit later and has been lower so far than we’ve seen during the previous three seasons, but activity is still on the upswing and expected to continue for several weeks,” Dr. Bresee stated.
The overall rate of effectiveness for the 2015-2016 season’s influenza vaccine is 59%, according to preliminary data shared at a Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices meeting.
“This means that getting a flu vaccine this season reduced the risk of having to go to the doctor because of flu by nearly 60%,” Dr. Joseph Bresee – who heads the CDC’s Epidemiology and Prevention Branch – stated in a press release. “It’s good news and underscores the importance and the benefit of both annual and ongoing vaccination efforts this season.”
These findings are based on data collected by the U.S. Flu Vaccine Effectiveness Network between November 2, 2015, and February 12, 2016. A 59% effectiveness rate would be roughly in line with what previous years’ vaccines have rated when similar strains of influenza have been prevalent, according to the CDC.
Against the H1N1 strain, which was “responsible for most flu illness this season,” the influenza vaccine was 51% effective. Dr. Bresee noted that the CDC has received reports of unvaccinated individuals this season dying or falling seriously ill because of infection from the H1N1 strain.
The vaccine was 76% effective against all influenza B virus strains, and 79% effective against the B/Yamagata virus strains. There are not enough data at this time, however, to determine the vaccine’s effectiveness against B/Victoria or H3N2 strains.
The CDC cautions that, because the current influenza season is still weeks away from being over, the final rate of vaccine effectiveness for 2015-2016 may change once all the data are analyzed. On average, influenza seasons last 13 weeks.
“Flu activity this season started a bit later and has been lower so far than we’ve seen during the previous three seasons, but activity is still on the upswing and expected to continue for several weeks,” Dr. Bresee stated.
FROM AN ACIP MEETING
ACIP recommends LAIV as an option for all people with egg allergies
Live attenuated influenza vaccine (LAIV) is likely to be an option for all individuals with egg allergies, regardless of allergy severity, because the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted to approve proposed amendments to the existing recommendations regarding administration of LAIV in individuals with egg allergies.
With 11 members voting “yes” and three choosing to abstain, the committee agreed to leave sections 1, 2, and 3 as they currently are, with a slight change to section 1 so that it will now include the latter half of recommendations that were previously placed under section 4. Therefore, section 1 of the recommendations for influenza vaccination of persons with egg allergy – which already states that “Regardless of a recipient’s allergy history, all vaccines should be administered in settings in which personnel and equipment for rapid recognition and treatment of anaphylaxis are available” – will now also include text stating that the vaccine should be administered in a medical setting and supervised by a health care provider with “experience in the recognition and management of severe allergic conditions,” or that such a medical professional should be “immediately available.”
Sections 2 and 3 will remain the same. Section 2 states that a previous allergic reaction to the influenza vaccine is a contraindication to ever receiving the vaccine again in the future. Section 3 states that individuals with egg allergy who have only experienced hives due to eggs still should be administered the influenza vaccine.
The committee voted to strike section 5 of the existing recommendations, which calls for a 30-minute postvaccination observation period. However, the committee still advises that health care providers monitor all patients, particularly adolescents, for 15 minutes immediately after vaccination, and that individuals should be seated or lying down “to decrease the risk for injury should syncopy occur.”
The CDC generally follows ACIP’s recommendations.
Live attenuated influenza vaccine (LAIV) is likely to be an option for all individuals with egg allergies, regardless of allergy severity, because the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted to approve proposed amendments to the existing recommendations regarding administration of LAIV in individuals with egg allergies.
With 11 members voting “yes” and three choosing to abstain, the committee agreed to leave sections 1, 2, and 3 as they currently are, with a slight change to section 1 so that it will now include the latter half of recommendations that were previously placed under section 4. Therefore, section 1 of the recommendations for influenza vaccination of persons with egg allergy – which already states that “Regardless of a recipient’s allergy history, all vaccines should be administered in settings in which personnel and equipment for rapid recognition and treatment of anaphylaxis are available” – will now also include text stating that the vaccine should be administered in a medical setting and supervised by a health care provider with “experience in the recognition and management of severe allergic conditions,” or that such a medical professional should be “immediately available.”
Sections 2 and 3 will remain the same. Section 2 states that a previous allergic reaction to the influenza vaccine is a contraindication to ever receiving the vaccine again in the future. Section 3 states that individuals with egg allergy who have only experienced hives due to eggs still should be administered the influenza vaccine.
The committee voted to strike section 5 of the existing recommendations, which calls for a 30-minute postvaccination observation period. However, the committee still advises that health care providers monitor all patients, particularly adolescents, for 15 minutes immediately after vaccination, and that individuals should be seated or lying down “to decrease the risk for injury should syncopy occur.”
The CDC generally follows ACIP’s recommendations.
Live attenuated influenza vaccine (LAIV) is likely to be an option for all individuals with egg allergies, regardless of allergy severity, because the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted to approve proposed amendments to the existing recommendations regarding administration of LAIV in individuals with egg allergies.
With 11 members voting “yes” and three choosing to abstain, the committee agreed to leave sections 1, 2, and 3 as they currently are, with a slight change to section 1 so that it will now include the latter half of recommendations that were previously placed under section 4. Therefore, section 1 of the recommendations for influenza vaccination of persons with egg allergy – which already states that “Regardless of a recipient’s allergy history, all vaccines should be administered in settings in which personnel and equipment for rapid recognition and treatment of anaphylaxis are available” – will now also include text stating that the vaccine should be administered in a medical setting and supervised by a health care provider with “experience in the recognition and management of severe allergic conditions,” or that such a medical professional should be “immediately available.”
Sections 2 and 3 will remain the same. Section 2 states that a previous allergic reaction to the influenza vaccine is a contraindication to ever receiving the vaccine again in the future. Section 3 states that individuals with egg allergy who have only experienced hives due to eggs still should be administered the influenza vaccine.
The committee voted to strike section 5 of the existing recommendations, which calls for a 30-minute postvaccination observation period. However, the committee still advises that health care providers monitor all patients, particularly adolescents, for 15 minutes immediately after vaccination, and that individuals should be seated or lying down “to decrease the risk for injury should syncopy occur.”
The CDC generally follows ACIP’s recommendations.
FROM AN ACIP MEETING
Treating influenza: A guide to antiviral safety in pregnancy
Oseltamivir and zanamivir are competitive inhibitors for the neuraminidase enzyme for the influenza virus. They block the surface receptor enzyme and prevent release of virus from the host cell, thus limiting propagation of the infection. These medications can be given as prophylaxis after exposure to influenza or can be given therapeutically for a suspected or confirmed infection. Oseltamivir is recommended for treatment of suspected or confirmed influenza infection in the special population of pregnant women, as the risk for complications of influenza is increased in this group.
Safety evidence
However, there are limited data on the safety and efficacy of the neuraminidase inhibitors in pregnancy. With respect to safety, there have been seven publications in the literature addressing the risk for major birth defects following treatment or prophylaxis with one or both of these products, with the majority of the published data relating to oseltamivir exposure.
In a review by Tanaka et al. in 2009, 90 pregnancies treated therapeutically with oseltamivir in the first trimester were reported to two teratogen information services in Japan; one major birth defect (1.1%) was reported (CMAJ. 2009 Jul 7;181[1-2]:55-8). A year later, Greer et al. published a retrospective chart review at a Texas hospital between 2003 and 2008. During that period, 137 pregnancies that involved a pharmacy record of dispensing of oseltamivir were identified. Of these, 18 were dispensed in the first trimester, and none were linked to a major birth defect outcome (Obstet Gynecol. 2010 Apr;115[4]:711-6).
A 2011 record linkage study in Sweden identified 86 pregnant women for whom oseltamivir (n=81) or zanamivir had been prescribed. Of these, four were linked to a major birth defect in the infant; however, only one of the four prescriptions had been filled in the first trimester (Pharmacoepidemiol Drug Saf. 2011 Oct;20[10]:1030-4). In 2013, Saito et al. reported on a case series gathered from 157 obstetric facilities in Japan. Among 156 infants born to women exposed to oseltamivir in the first trimester, 2 (1.3%) were reported to have a major congenital anomaly; there were no congenital malformations reported in the 15 first-trimester exposures to zanamivir (Am J Obstet Gynecol. 2013 Aug;209[2[:130.e1-9).
In 2014, a teratogen information service in the United Kingdom reported on eight first-trimester exposures to oseltamivir and 37 to zanamivir, with no major birth defects noted in either group (BJOG. 2014 Jun;121[7]:901-6). Additionally, a French prescription database study identified 49 pregnancies thought to be exposed to oseltamivir in the first trimester with one reported congenital anomaly (BJOG. 2014 Jun;121[7]:895-900).
Finally, the manufacturer of oseltamivir published a summary of pregnancies from global pharmacovigilance data accumulated through spontaneous reports and other studies between 2000 and 2012 (Pharmacoepidemiol Drug Saf. 2014 Oct;23[10]:1035-42). Outcomes were available for 1,875 infants. Among these, 81 (4.3%) had major birth defects. However, following case review, the authors indicated that only 11 of the defects (occurring in 9 infants) were biologically plausible based on the timing of the exposure to oseltamivir.
Efficacy examined
With respect to efficacy, two small studies have addressed the pharmacokinetics of oseltamivir in pregnancy to determine if the recommended dosages for nonpregnant individuals are appropriate for pregnancy.
In the earlier of the two studies, Greer et al. looked at the pharmacokinetics of oseltamivir in 30 pregnant women, 10 in each of the three trimesters, who were taking 75 mg of the drug either once or twice daily. Maternal samples were drawn before and after the first dose of oseltamivir. They found little evidence of differences across the three trimesters and concluded that the parent drug values were in the pharmacologic range for clinical efficacy (Am J Obstet Gynecol. 2011 Jun;204[6 Suppl 1]:S89-93).
In contrast, Pillai et al. enrolled a small sample of women being treated with oseltamivir; they evaluated pharmacokinetics for the active metabolite of oseltamivir following 48 or more hours of treatment in 29 pregnant and 35 nonpregnant women (Br J Clin Pharmacol. 2015 Nov;80[5]:1042-50). Significantly lower levels of the active metabolite were noted in the pregnant women, compared with nonpregnant women. The authors suggested that the physiologic changes of pregnancy, correlated with increased renal clearance, produced an approximate 30% lower exposure to the drug in the pregnant state. While they were not able to relate this to maternal or infant outcomes, this finding suggested that further work is needed to determine if dosing recommendations should be adjusted in pregnancy.
The current recommendation is that pregnant women or women within 2 weeks post partum be given oseltamivir for treatment of suspected or confirmed influenza regardless of trimester of pregnancy. The limited safety data that are currently available have not suggested an increased risk for major birth defects following treatment with this product. However, the data are sparse for oseltamivir and even more so for zanamivir. Larger studies focused on these treatments are needed.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She reported having no financial disclosures relevant to this column, but has received research funding Roche-Genentech and GlaxoSmithKline unrelated to antiviral medications. Email her at obnews@frontlinemedcom.com.
Oseltamivir and zanamivir are competitive inhibitors for the neuraminidase enzyme for the influenza virus. They block the surface receptor enzyme and prevent release of virus from the host cell, thus limiting propagation of the infection. These medications can be given as prophylaxis after exposure to influenza or can be given therapeutically for a suspected or confirmed infection. Oseltamivir is recommended for treatment of suspected or confirmed influenza infection in the special population of pregnant women, as the risk for complications of influenza is increased in this group.
Safety evidence
However, there are limited data on the safety and efficacy of the neuraminidase inhibitors in pregnancy. With respect to safety, there have been seven publications in the literature addressing the risk for major birth defects following treatment or prophylaxis with one or both of these products, with the majority of the published data relating to oseltamivir exposure.
In a review by Tanaka et al. in 2009, 90 pregnancies treated therapeutically with oseltamivir in the first trimester were reported to two teratogen information services in Japan; one major birth defect (1.1%) was reported (CMAJ. 2009 Jul 7;181[1-2]:55-8). A year later, Greer et al. published a retrospective chart review at a Texas hospital between 2003 and 2008. During that period, 137 pregnancies that involved a pharmacy record of dispensing of oseltamivir were identified. Of these, 18 were dispensed in the first trimester, and none were linked to a major birth defect outcome (Obstet Gynecol. 2010 Apr;115[4]:711-6).
A 2011 record linkage study in Sweden identified 86 pregnant women for whom oseltamivir (n=81) or zanamivir had been prescribed. Of these, four were linked to a major birth defect in the infant; however, only one of the four prescriptions had been filled in the first trimester (Pharmacoepidemiol Drug Saf. 2011 Oct;20[10]:1030-4). In 2013, Saito et al. reported on a case series gathered from 157 obstetric facilities in Japan. Among 156 infants born to women exposed to oseltamivir in the first trimester, 2 (1.3%) were reported to have a major congenital anomaly; there were no congenital malformations reported in the 15 first-trimester exposures to zanamivir (Am J Obstet Gynecol. 2013 Aug;209[2[:130.e1-9).
In 2014, a teratogen information service in the United Kingdom reported on eight first-trimester exposures to oseltamivir and 37 to zanamivir, with no major birth defects noted in either group (BJOG. 2014 Jun;121[7]:901-6). Additionally, a French prescription database study identified 49 pregnancies thought to be exposed to oseltamivir in the first trimester with one reported congenital anomaly (BJOG. 2014 Jun;121[7]:895-900).
Finally, the manufacturer of oseltamivir published a summary of pregnancies from global pharmacovigilance data accumulated through spontaneous reports and other studies between 2000 and 2012 (Pharmacoepidemiol Drug Saf. 2014 Oct;23[10]:1035-42). Outcomes were available for 1,875 infants. Among these, 81 (4.3%) had major birth defects. However, following case review, the authors indicated that only 11 of the defects (occurring in 9 infants) were biologically plausible based on the timing of the exposure to oseltamivir.
Efficacy examined
With respect to efficacy, two small studies have addressed the pharmacokinetics of oseltamivir in pregnancy to determine if the recommended dosages for nonpregnant individuals are appropriate for pregnancy.
In the earlier of the two studies, Greer et al. looked at the pharmacokinetics of oseltamivir in 30 pregnant women, 10 in each of the three trimesters, who were taking 75 mg of the drug either once or twice daily. Maternal samples were drawn before and after the first dose of oseltamivir. They found little evidence of differences across the three trimesters and concluded that the parent drug values were in the pharmacologic range for clinical efficacy (Am J Obstet Gynecol. 2011 Jun;204[6 Suppl 1]:S89-93).
In contrast, Pillai et al. enrolled a small sample of women being treated with oseltamivir; they evaluated pharmacokinetics for the active metabolite of oseltamivir following 48 or more hours of treatment in 29 pregnant and 35 nonpregnant women (Br J Clin Pharmacol. 2015 Nov;80[5]:1042-50). Significantly lower levels of the active metabolite were noted in the pregnant women, compared with nonpregnant women. The authors suggested that the physiologic changes of pregnancy, correlated with increased renal clearance, produced an approximate 30% lower exposure to the drug in the pregnant state. While they were not able to relate this to maternal or infant outcomes, this finding suggested that further work is needed to determine if dosing recommendations should be adjusted in pregnancy.
The current recommendation is that pregnant women or women within 2 weeks post partum be given oseltamivir for treatment of suspected or confirmed influenza regardless of trimester of pregnancy. The limited safety data that are currently available have not suggested an increased risk for major birth defects following treatment with this product. However, the data are sparse for oseltamivir and even more so for zanamivir. Larger studies focused on these treatments are needed.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She reported having no financial disclosures relevant to this column, but has received research funding Roche-Genentech and GlaxoSmithKline unrelated to antiviral medications. Email her at obnews@frontlinemedcom.com.
Oseltamivir and zanamivir are competitive inhibitors for the neuraminidase enzyme for the influenza virus. They block the surface receptor enzyme and prevent release of virus from the host cell, thus limiting propagation of the infection. These medications can be given as prophylaxis after exposure to influenza or can be given therapeutically for a suspected or confirmed infection. Oseltamivir is recommended for treatment of suspected or confirmed influenza infection in the special population of pregnant women, as the risk for complications of influenza is increased in this group.
Safety evidence
However, there are limited data on the safety and efficacy of the neuraminidase inhibitors in pregnancy. With respect to safety, there have been seven publications in the literature addressing the risk for major birth defects following treatment or prophylaxis with one or both of these products, with the majority of the published data relating to oseltamivir exposure.
In a review by Tanaka et al. in 2009, 90 pregnancies treated therapeutically with oseltamivir in the first trimester were reported to two teratogen information services in Japan; one major birth defect (1.1%) was reported (CMAJ. 2009 Jul 7;181[1-2]:55-8). A year later, Greer et al. published a retrospective chart review at a Texas hospital between 2003 and 2008. During that period, 137 pregnancies that involved a pharmacy record of dispensing of oseltamivir were identified. Of these, 18 were dispensed in the first trimester, and none were linked to a major birth defect outcome (Obstet Gynecol. 2010 Apr;115[4]:711-6).
A 2011 record linkage study in Sweden identified 86 pregnant women for whom oseltamivir (n=81) or zanamivir had been prescribed. Of these, four were linked to a major birth defect in the infant; however, only one of the four prescriptions had been filled in the first trimester (Pharmacoepidemiol Drug Saf. 2011 Oct;20[10]:1030-4). In 2013, Saito et al. reported on a case series gathered from 157 obstetric facilities in Japan. Among 156 infants born to women exposed to oseltamivir in the first trimester, 2 (1.3%) were reported to have a major congenital anomaly; there were no congenital malformations reported in the 15 first-trimester exposures to zanamivir (Am J Obstet Gynecol. 2013 Aug;209[2[:130.e1-9).
In 2014, a teratogen information service in the United Kingdom reported on eight first-trimester exposures to oseltamivir and 37 to zanamivir, with no major birth defects noted in either group (BJOG. 2014 Jun;121[7]:901-6). Additionally, a French prescription database study identified 49 pregnancies thought to be exposed to oseltamivir in the first trimester with one reported congenital anomaly (BJOG. 2014 Jun;121[7]:895-900).
Finally, the manufacturer of oseltamivir published a summary of pregnancies from global pharmacovigilance data accumulated through spontaneous reports and other studies between 2000 and 2012 (Pharmacoepidemiol Drug Saf. 2014 Oct;23[10]:1035-42). Outcomes were available for 1,875 infants. Among these, 81 (4.3%) had major birth defects. However, following case review, the authors indicated that only 11 of the defects (occurring in 9 infants) were biologically plausible based on the timing of the exposure to oseltamivir.
Efficacy examined
With respect to efficacy, two small studies have addressed the pharmacokinetics of oseltamivir in pregnancy to determine if the recommended dosages for nonpregnant individuals are appropriate for pregnancy.
In the earlier of the two studies, Greer et al. looked at the pharmacokinetics of oseltamivir in 30 pregnant women, 10 in each of the three trimesters, who were taking 75 mg of the drug either once or twice daily. Maternal samples were drawn before and after the first dose of oseltamivir. They found little evidence of differences across the three trimesters and concluded that the parent drug values were in the pharmacologic range for clinical efficacy (Am J Obstet Gynecol. 2011 Jun;204[6 Suppl 1]:S89-93).
In contrast, Pillai et al. enrolled a small sample of women being treated with oseltamivir; they evaluated pharmacokinetics for the active metabolite of oseltamivir following 48 or more hours of treatment in 29 pregnant and 35 nonpregnant women (Br J Clin Pharmacol. 2015 Nov;80[5]:1042-50). Significantly lower levels of the active metabolite were noted in the pregnant women, compared with nonpregnant women. The authors suggested that the physiologic changes of pregnancy, correlated with increased renal clearance, produced an approximate 30% lower exposure to the drug in the pregnant state. While they were not able to relate this to maternal or infant outcomes, this finding suggested that further work is needed to determine if dosing recommendations should be adjusted in pregnancy.
The current recommendation is that pregnant women or women within 2 weeks post partum be given oseltamivir for treatment of suspected or confirmed influenza regardless of trimester of pregnancy. The limited safety data that are currently available have not suggested an increased risk for major birth defects following treatment with this product. However, the data are sparse for oseltamivir and even more so for zanamivir. Larger studies focused on these treatments are needed.
Dr. Chambers is professor of pediatrics and director of clinical research at Rady Children’s Hospital, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She reported having no financial disclosures relevant to this column, but has received research funding Roche-Genentech and GlaxoSmithKline unrelated to antiviral medications. Email her at obnews@frontlinemedcom.com.
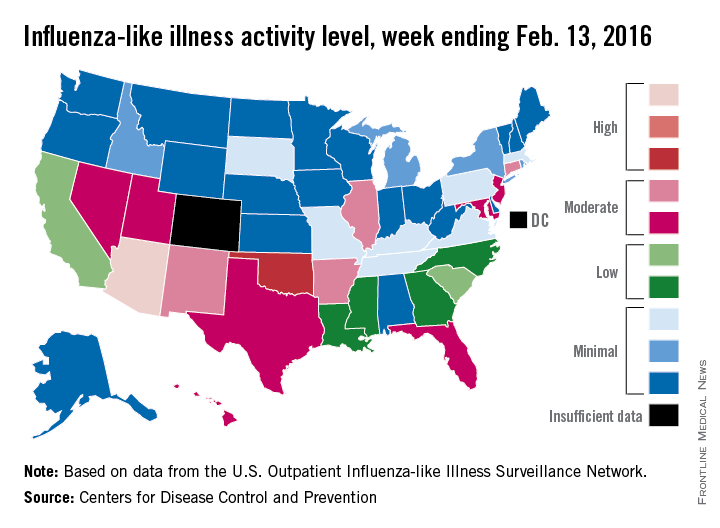
U.S. flu activity at its highest level yet
Activity of influenza-like illness (ILI) was at its highest level so far for week 18 of the 2015-2016 flu season, according to the Centers for Disease Control and Prevention.
That increased activity put Arizona and Puerto Rico at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending Feb. 13. Joining them in the “high” range of activity was Oklahoma at level 8, the CDC reported Feb. 19.
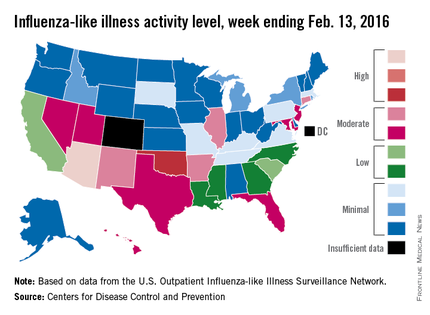
The percentage of visits for ILI in week 18 was 3.1%, higher than the national baseline of 2.1% and the highest level of the flu season. For the week, 30 states were at level 2 or higher on the CDC’s 1-10 scale, which is the highest number for the season. States in the “moderate” range were Arkansas, Connecticut, Illinois, and New Mexico at level 7 and Florida, Hawaii, Maryland, Nevada, New Jersey, Texas, and Utah at level 6. California and South Carolina (level 5) and Georgia, Louisiana, Mississippi, and North Carolina (level 4) took their place in the “low” range, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network show.
Two ILI-related pediatric deaths were reported for the week – one of which occurred during the week ending Jan. 16 – bringing the total to 13 for the season. Three of those deaths occurred in Florida and two in California, with Arizona, Illinois, Louisiana, Michigan, Nevada, Puerto Rico, Tennessee, and Washington each reporting one death, the CDC said.
Since Oct. 1, 2015, the overall ILI-related hospitalization rate is 4.1 per 100,000 population, with 1,147 laboratory-confirmed flu hospitalizations reported. Among all hospitalizations, 71.1% were associated with influenza A, 26.4% with influenza B, 1.7% with influenza A and B coinfection, and 0.8% had no virus type information. Hospitalization data come from the Influenza Hospitalization Surveillance Network, which covers more than 70 counties in 10 states as well as 3 additional states.
Activity of influenza-like illness (ILI) was at its highest level so far for week 18 of the 2015-2016 flu season, according to the Centers for Disease Control and Prevention.
That increased activity put Arizona and Puerto Rico at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending Feb. 13. Joining them in the “high” range of activity was Oklahoma at level 8, the CDC reported Feb. 19.

The percentage of visits for ILI in week 18 was 3.1%, higher than the national baseline of 2.1% and the highest level of the flu season. For the week, 30 states were at level 2 or higher on the CDC’s 1-10 scale, which is the highest number for the season. States in the “moderate” range were Arkansas, Connecticut, Illinois, and New Mexico at level 7 and Florida, Hawaii, Maryland, Nevada, New Jersey, Texas, and Utah at level 6. California and South Carolina (level 5) and Georgia, Louisiana, Mississippi, and North Carolina (level 4) took their place in the “low” range, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network show.
Two ILI-related pediatric deaths were reported for the week – one of which occurred during the week ending Jan. 16 – bringing the total to 13 for the season. Three of those deaths occurred in Florida and two in California, with Arizona, Illinois, Louisiana, Michigan, Nevada, Puerto Rico, Tennessee, and Washington each reporting one death, the CDC said.
Since Oct. 1, 2015, the overall ILI-related hospitalization rate is 4.1 per 100,000 population, with 1,147 laboratory-confirmed flu hospitalizations reported. Among all hospitalizations, 71.1% were associated with influenza A, 26.4% with influenza B, 1.7% with influenza A and B coinfection, and 0.8% had no virus type information. Hospitalization data come from the Influenza Hospitalization Surveillance Network, which covers more than 70 counties in 10 states as well as 3 additional states.
Activity of influenza-like illness (ILI) was at its highest level so far for week 18 of the 2015-2016 flu season, according to the Centers for Disease Control and Prevention.
That increased activity put Arizona and Puerto Rico at level 10 on the CDC’s 1-10 scale of ILI activity for the week ending Feb. 13. Joining them in the “high” range of activity was Oklahoma at level 8, the CDC reported Feb. 19.

The percentage of visits for ILI in week 18 was 3.1%, higher than the national baseline of 2.1% and the highest level of the flu season. For the week, 30 states were at level 2 or higher on the CDC’s 1-10 scale, which is the highest number for the season. States in the “moderate” range were Arkansas, Connecticut, Illinois, and New Mexico at level 7 and Florida, Hawaii, Maryland, Nevada, New Jersey, Texas, and Utah at level 6. California and South Carolina (level 5) and Georgia, Louisiana, Mississippi, and North Carolina (level 4) took their place in the “low” range, data from the CDC’s Outpatient Influenza-like Illness Surveillance Network show.
Two ILI-related pediatric deaths were reported for the week – one of which occurred during the week ending Jan. 16 – bringing the total to 13 for the season. Three of those deaths occurred in Florida and two in California, with Arizona, Illinois, Louisiana, Michigan, Nevada, Puerto Rico, Tennessee, and Washington each reporting one death, the CDC said.
Since Oct. 1, 2015, the overall ILI-related hospitalization rate is 4.1 per 100,000 population, with 1,147 laboratory-confirmed flu hospitalizations reported. Among all hospitalizations, 71.1% were associated with influenza A, 26.4% with influenza B, 1.7% with influenza A and B coinfection, and 0.8% had no virus type information. Hospitalization data come from the Influenza Hospitalization Surveillance Network, which covers more than 70 counties in 10 states as well as 3 additional states.
Poverty promotes flu hospitalizations
Influenza-related hospitalizations were approximately twice as high among residents of areas where at least 20% of the population lived below the federal poverty level, compared with areas of less poverty, based on data from more than 27 million individuals in the United States.
Census and hospitalization data included 14 states and spanned two flu seasons (2010-2011 and 2011-2012). Overall, the incidence of flu-related hospitalizations was approximately 21 per 100,000 person-years in high-poverty areas, compared with approximately 11 per 100,000 person-years in census areas where less than 5% of the population lived below the poverty level. The data were consistent across all age groups and ethnicities.
Flu vaccination rates were inversely associated with poverty level, ranging from a high of 48% in high-income areas to a low of 35% in areas with the most poverty. This finding, however, was probably caused by lower vaccination rates among adults aged 65 years and older in higher-poverty areas, compared with high-income areas (80% vs. 94%) noted Dr. James L. Hadler of the Yale School of Public Health in New Haven, Conn., and colleagues.
“Enhanced influenza outreach to improve influenza vaccination coverage for persons living in poorer neighborhoods and efforts to increase use of antivirals by clinicians serving these neighborhoods could reduce poverty-related disparities in severe influenza outcomes,” the researchers noted.
The findings were published in the Morbidity and Mortality Weekly Report (MMWR 2016;65[5]:101-5). Read the full article here.
Influenza-related hospitalizations were approximately twice as high among residents of areas where at least 20% of the population lived below the federal poverty level, compared with areas of less poverty, based on data from more than 27 million individuals in the United States.
Census and hospitalization data included 14 states and spanned two flu seasons (2010-2011 and 2011-2012). Overall, the incidence of flu-related hospitalizations was approximately 21 per 100,000 person-years in high-poverty areas, compared with approximately 11 per 100,000 person-years in census areas where less than 5% of the population lived below the poverty level. The data were consistent across all age groups and ethnicities.
Flu vaccination rates were inversely associated with poverty level, ranging from a high of 48% in high-income areas to a low of 35% in areas with the most poverty. This finding, however, was probably caused by lower vaccination rates among adults aged 65 years and older in higher-poverty areas, compared with high-income areas (80% vs. 94%) noted Dr. James L. Hadler of the Yale School of Public Health in New Haven, Conn., and colleagues.
“Enhanced influenza outreach to improve influenza vaccination coverage for persons living in poorer neighborhoods and efforts to increase use of antivirals by clinicians serving these neighborhoods could reduce poverty-related disparities in severe influenza outcomes,” the researchers noted.
The findings were published in the Morbidity and Mortality Weekly Report (MMWR 2016;65[5]:101-5). Read the full article here.
Influenza-related hospitalizations were approximately twice as high among residents of areas where at least 20% of the population lived below the federal poverty level, compared with areas of less poverty, based on data from more than 27 million individuals in the United States.
Census and hospitalization data included 14 states and spanned two flu seasons (2010-2011 and 2011-2012). Overall, the incidence of flu-related hospitalizations was approximately 21 per 100,000 person-years in high-poverty areas, compared with approximately 11 per 100,000 person-years in census areas where less than 5% of the population lived below the poverty level. The data were consistent across all age groups and ethnicities.
Flu vaccination rates were inversely associated with poverty level, ranging from a high of 48% in high-income areas to a low of 35% in areas with the most poverty. This finding, however, was probably caused by lower vaccination rates among adults aged 65 years and older in higher-poverty areas, compared with high-income areas (80% vs. 94%) noted Dr. James L. Hadler of the Yale School of Public Health in New Haven, Conn., and colleagues.
“Enhanced influenza outreach to improve influenza vaccination coverage for persons living in poorer neighborhoods and efforts to increase use of antivirals by clinicians serving these neighborhoods could reduce poverty-related disparities in severe influenza outcomes,” the researchers noted.
The findings were published in the Morbidity and Mortality Weekly Report (MMWR 2016;65[5]:101-5). Read the full article here.
FROM MMWR
Influenza linked to atrial fibrillation in large observational study
A diagnosis of influenza increased the risk of subsequent atrial fibrillation by about 18%, investigators reported online in Heart Rhythm.
Clinicians therefore should consider atrial fibrillation (AF) in patients with influenza-like symptoms who report palpitations or experience an ischemic stroke, said Dr. Ting-Yung Chang of Taipei Veterans General Hospital in Taiwan and his associates. Influenza vaccination might help prevent AF, and high-risk patients should be encouraged to receive the vaccination annually, they said. However, a large prospective study is needed to clarify whether influenza vaccination reduces the risk of AF and subsequent ischemic stroke and systemic thromboembolic events, they added.
Atrial fibrillation increases the risk of stroke about fivefold, triples the risk of heart failure, and doubles the chances of dementia and death, the researchers noted. Mounting evidence implicates inflammation and sympathetic nervous system dysregulation in the pathogenesis of AF, raising questions about whether influenza might underlie or contribute to some cases of AF. To explore relationships among AF, influenza, and influenza vaccination, the investigators analyzed data for 11,374 patients with AF who were enrolled in the Taiwan National Health Insurance Research Database between 2000 and 2010. They matched each patient with AF to four controls based on age, sex, enrollment date, and the Charlson comorbidity index (
Heart Rhythm. 2016 Feb. doi: 10.1016/j.hrthm.2016.01.026
).
Unvaccinated patients with influenza were 18% more likely to develop AF than unvaccinated patients without influenza (odds ratio, 1.18; 95% confidence interval, 1.01-1.38; P = .032), even after adjusting for demographic factors, medical history, and use of relevant health care services, the researchers reported. In contrast, vaccinated patients who later developed influenza were about as likely to develop AF as unvaccinated patients who did not develop influenza, both in the overall analysis and in subgroups stratified by age, sex, and comorbidities. Moreover, vaccinated patients without influenza were even less likely to develop AF than unvaccinated patients without influenza (OR, 0.88; 95% CI, 0.84-0.93; P less than .001).
The registry database excluded relevant data on smoking history, body mass index, and physical activity level, the researchers said. “Influenza infection was diagnosed using ICD-9 codes with concomitant use of antiviral agents, and was not further confirmed based on the results of viral culture with throat swab,” they added. “The diagnostic accuracy of influenza infection cannot be fully ascertained.”
The National Science Council and the Taipei Veterans General Hospital funded the study. The researchers had no disclosures.
The authors readily acknowledge the limitations of [this] large, observational study using an insurance database. Despite these admitted limitations, the authors should be commended on adding to the literature regarding modifiable risk factor reduction for the prevention of AF. Recently, a growing body of literature has examined this topic with several straightforward yet promising interventions identified. Weight loss, moderate exercise, and treatment for underlying obstructive sleep apnea have all been shown to reduce the risk of atrial fibrillation incidence or recurrence. Influenza vaccination could represent another simple, cost‐effective intervention to prevent AF. Although the flu vaccine is already recommended for many patient groups, this study suggests that there are even more potential public health benefits of the vaccine.
Dr. Bradley P. Knight is with the division of cardiology, department of medicine, at Northwestern University, Chicago. He had no disclosures. These comments were adapted from his editorial (Heart Rhythm. 2016 Feb. doi: 10.1016/j.hrthm.2016.01.025).
The authors readily acknowledge the limitations of [this] large, observational study using an insurance database. Despite these admitted limitations, the authors should be commended on adding to the literature regarding modifiable risk factor reduction for the prevention of AF. Recently, a growing body of literature has examined this topic with several straightforward yet promising interventions identified. Weight loss, moderate exercise, and treatment for underlying obstructive sleep apnea have all been shown to reduce the risk of atrial fibrillation incidence or recurrence. Influenza vaccination could represent another simple, cost‐effective intervention to prevent AF. Although the flu vaccine is already recommended for many patient groups, this study suggests that there are even more potential public health benefits of the vaccine.
Dr. Bradley P. Knight is with the division of cardiology, department of medicine, at Northwestern University, Chicago. He had no disclosures. These comments were adapted from his editorial (Heart Rhythm. 2016 Feb. doi: 10.1016/j.hrthm.2016.01.025).
The authors readily acknowledge the limitations of [this] large, observational study using an insurance database. Despite these admitted limitations, the authors should be commended on adding to the literature regarding modifiable risk factor reduction for the prevention of AF. Recently, a growing body of literature has examined this topic with several straightforward yet promising interventions identified. Weight loss, moderate exercise, and treatment for underlying obstructive sleep apnea have all been shown to reduce the risk of atrial fibrillation incidence or recurrence. Influenza vaccination could represent another simple, cost‐effective intervention to prevent AF. Although the flu vaccine is already recommended for many patient groups, this study suggests that there are even more potential public health benefits of the vaccine.
Dr. Bradley P. Knight is with the division of cardiology, department of medicine, at Northwestern University, Chicago. He had no disclosures. These comments were adapted from his editorial (Heart Rhythm. 2016 Feb. doi: 10.1016/j.hrthm.2016.01.025).
A diagnosis of influenza increased the risk of subsequent atrial fibrillation by about 18%, investigators reported online in Heart Rhythm.
Clinicians therefore should consider atrial fibrillation (AF) in patients with influenza-like symptoms who report palpitations or experience an ischemic stroke, said Dr. Ting-Yung Chang of Taipei Veterans General Hospital in Taiwan and his associates. Influenza vaccination might help prevent AF, and high-risk patients should be encouraged to receive the vaccination annually, they said. However, a large prospective study is needed to clarify whether influenza vaccination reduces the risk of AF and subsequent ischemic stroke and systemic thromboembolic events, they added.
Atrial fibrillation increases the risk of stroke about fivefold, triples the risk of heart failure, and doubles the chances of dementia and death, the researchers noted. Mounting evidence implicates inflammation and sympathetic nervous system dysregulation in the pathogenesis of AF, raising questions about whether influenza might underlie or contribute to some cases of AF. To explore relationships among AF, influenza, and influenza vaccination, the investigators analyzed data for 11,374 patients with AF who were enrolled in the Taiwan National Health Insurance Research Database between 2000 and 2010. They matched each patient with AF to four controls based on age, sex, enrollment date, and the Charlson comorbidity index (
Heart Rhythm. 2016 Feb. doi: 10.1016/j.hrthm.2016.01.026
).
Unvaccinated patients with influenza were 18% more likely to develop AF than unvaccinated patients without influenza (odds ratio, 1.18; 95% confidence interval, 1.01-1.38; P = .032), even after adjusting for demographic factors, medical history, and use of relevant health care services, the researchers reported. In contrast, vaccinated patients who later developed influenza were about as likely to develop AF as unvaccinated patients who did not develop influenza, both in the overall analysis and in subgroups stratified by age, sex, and comorbidities. Moreover, vaccinated patients without influenza were even less likely to develop AF than unvaccinated patients without influenza (OR, 0.88; 95% CI, 0.84-0.93; P less than .001).
The registry database excluded relevant data on smoking history, body mass index, and physical activity level, the researchers said. “Influenza infection was diagnosed using ICD-9 codes with concomitant use of antiviral agents, and was not further confirmed based on the results of viral culture with throat swab,” they added. “The diagnostic accuracy of influenza infection cannot be fully ascertained.”
The National Science Council and the Taipei Veterans General Hospital funded the study. The researchers had no disclosures.
A diagnosis of influenza increased the risk of subsequent atrial fibrillation by about 18%, investigators reported online in Heart Rhythm.
Clinicians therefore should consider atrial fibrillation (AF) in patients with influenza-like symptoms who report palpitations or experience an ischemic stroke, said Dr. Ting-Yung Chang of Taipei Veterans General Hospital in Taiwan and his associates. Influenza vaccination might help prevent AF, and high-risk patients should be encouraged to receive the vaccination annually, they said. However, a large prospective study is needed to clarify whether influenza vaccination reduces the risk of AF and subsequent ischemic stroke and systemic thromboembolic events, they added.
Atrial fibrillation increases the risk of stroke about fivefold, triples the risk of heart failure, and doubles the chances of dementia and death, the researchers noted. Mounting evidence implicates inflammation and sympathetic nervous system dysregulation in the pathogenesis of AF, raising questions about whether influenza might underlie or contribute to some cases of AF. To explore relationships among AF, influenza, and influenza vaccination, the investigators analyzed data for 11,374 patients with AF who were enrolled in the Taiwan National Health Insurance Research Database between 2000 and 2010. They matched each patient with AF to four controls based on age, sex, enrollment date, and the Charlson comorbidity index (
Heart Rhythm. 2016 Feb. doi: 10.1016/j.hrthm.2016.01.026
).
Unvaccinated patients with influenza were 18% more likely to develop AF than unvaccinated patients without influenza (odds ratio, 1.18; 95% confidence interval, 1.01-1.38; P = .032), even after adjusting for demographic factors, medical history, and use of relevant health care services, the researchers reported. In contrast, vaccinated patients who later developed influenza were about as likely to develop AF as unvaccinated patients who did not develop influenza, both in the overall analysis and in subgroups stratified by age, sex, and comorbidities. Moreover, vaccinated patients without influenza were even less likely to develop AF than unvaccinated patients without influenza (OR, 0.88; 95% CI, 0.84-0.93; P less than .001).
The registry database excluded relevant data on smoking history, body mass index, and physical activity level, the researchers said. “Influenza infection was diagnosed using ICD-9 codes with concomitant use of antiviral agents, and was not further confirmed based on the results of viral culture with throat swab,” they added. “The diagnostic accuracy of influenza infection cannot be fully ascertained.”
The National Science Council and the Taipei Veterans General Hospital funded the study. The researchers had no disclosures.
Key clinical point: Influenza might underlie some cases of atrial fibrillation.
Major finding: Among unvaccinated patients, an influenza diagnosis increased the odds of atrial fibrillation by 18% (OR, 1.18; P = .03).
Data source: An observational registry study of 11,374 patients with atrial fibrillation and 45,496 healthy controls.
Disclosures: The National Science Council and the Taipei Veterans General Hospital funded the study. The researchers had no disclosures.
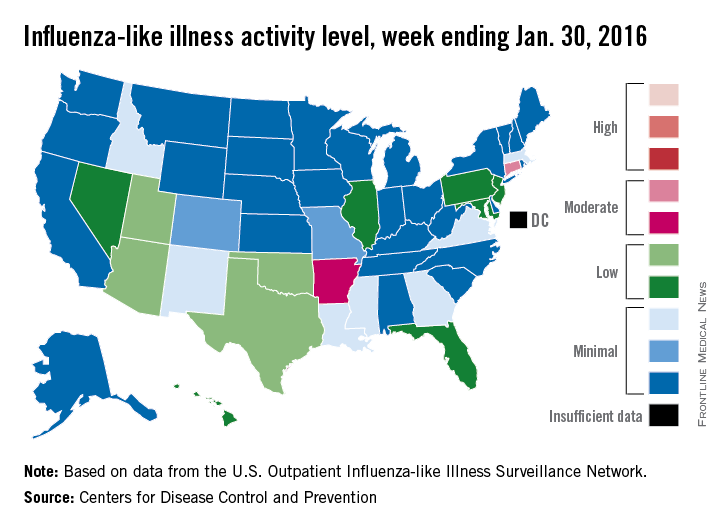
U.S. flu activity steady in late January
Overall influenza-like illness (ILI) activity underwent a small increase in the United States during week 16 of the 2015-2016 flu season, but Puerto Rico took a significant turn for the worse as activity there rose to the highest possible level, the Centers for Disease Control and Prevention reported Feb. 5.
Puerto Rico, which had been at level 8 on the CDC’s 1-10 scale of flu activity for the previous 3 weeks, jumped up to level 10 for the week ending Jan. 30, 2016. Meanwhile, the main measure for national flu activity, the proportion of outpatient visits for ILI, was 2.2% for the second week in a row, just above the national baseline of 2.1%, the CDC said.
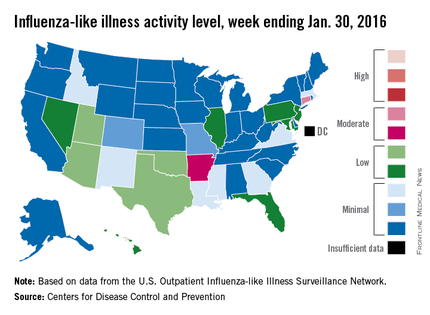
The state with the highest level of flu activity (level 7) for the week was Connecticut, with Arkansas the only other state in the “moderate” range at level 6. States in the “low” range were Florida, Hawaii, Illinois, Maryland, Nevada, New Jersey, and Pennsylvania at level 4 and Georgia, Idaho, Louisiana, Massachusetts, Mississippi, New Mexico, and Virginia at level 3. All told, there were 22 states with activity above level 1, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
There were two influenza-associated pediatric deaths reported during week 16, although one death actually occurred during the week ending Jan. 16. There have been nine flu-associated pediatric deaths reported so far during the 2015-2016 season.
The hospitalization rate for the season so far is 2.6/100,000 population, up from 2.1/100,000 last week. Adults aged 65 years and older had the highest rate at 8.5/100,000, with children aged 0-4 years next at 3.8/100,000.
Overall influenza-like illness (ILI) activity underwent a small increase in the United States during week 16 of the 2015-2016 flu season, but Puerto Rico took a significant turn for the worse as activity there rose to the highest possible level, the Centers for Disease Control and Prevention reported Feb. 5.
Puerto Rico, which had been at level 8 on the CDC’s 1-10 scale of flu activity for the previous 3 weeks, jumped up to level 10 for the week ending Jan. 30, 2016. Meanwhile, the main measure for national flu activity, the proportion of outpatient visits for ILI, was 2.2% for the second week in a row, just above the national baseline of 2.1%, the CDC said.

The state with the highest level of flu activity (level 7) for the week was Connecticut, with Arkansas the only other state in the “moderate” range at level 6. States in the “low” range were Florida, Hawaii, Illinois, Maryland, Nevada, New Jersey, and Pennsylvania at level 4 and Georgia, Idaho, Louisiana, Massachusetts, Mississippi, New Mexico, and Virginia at level 3. All told, there were 22 states with activity above level 1, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
There were two influenza-associated pediatric deaths reported during week 16, although one death actually occurred during the week ending Jan. 16. There have been nine flu-associated pediatric deaths reported so far during the 2015-2016 season.
The hospitalization rate for the season so far is 2.6/100,000 population, up from 2.1/100,000 last week. Adults aged 65 years and older had the highest rate at 8.5/100,000, with children aged 0-4 years next at 3.8/100,000.
Overall influenza-like illness (ILI) activity underwent a small increase in the United States during week 16 of the 2015-2016 flu season, but Puerto Rico took a significant turn for the worse as activity there rose to the highest possible level, the Centers for Disease Control and Prevention reported Feb. 5.
Puerto Rico, which had been at level 8 on the CDC’s 1-10 scale of flu activity for the previous 3 weeks, jumped up to level 10 for the week ending Jan. 30, 2016. Meanwhile, the main measure for national flu activity, the proportion of outpatient visits for ILI, was 2.2% for the second week in a row, just above the national baseline of 2.1%, the CDC said.

The state with the highest level of flu activity (level 7) for the week was Connecticut, with Arkansas the only other state in the “moderate” range at level 6. States in the “low” range were Florida, Hawaii, Illinois, Maryland, Nevada, New Jersey, and Pennsylvania at level 4 and Georgia, Idaho, Louisiana, Massachusetts, Mississippi, New Mexico, and Virginia at level 3. All told, there were 22 states with activity above level 1, according to data from the CDC’s Outpatient Influenza-like Illness Surveillance Network.
There were two influenza-associated pediatric deaths reported during week 16, although one death actually occurred during the week ending Jan. 16. There have been nine flu-associated pediatric deaths reported so far during the 2015-2016 season.
The hospitalization rate for the season so far is 2.6/100,000 population, up from 2.1/100,000 last week. Adults aged 65 years and older had the highest rate at 8.5/100,000, with children aged 0-4 years next at 3.8/100,000.
Majority of children aged 6-23 months are not vaccinated for flu
Less than half of children aged 6-23 months are vaccinated for influenza in the United States, according to an analysis of data obtained via the 2003-2013 National Immunization Survey.
The researchers analyzed providers’ reports of influenza vaccinations, received as one or two doses by children aged 6-23 months. The age group studied is at highest risk of influenza-related complications and was the first group of children for which the Advisory Committee on Immunization Practices recommended influenza vaccination, regardless of an individual’s medical condition.
A child’s age was defined by his or her age on Nov. 1 of each influenza season under study. Two full calendar years of data files were combined to enable analysis of full influenza seasons, which cover parts of 2 consecutive calendar years. The percentages of children requiring two doses to be considered fully vaccinated were based on the dosage recommendations for each flu season.
Overall, flu vaccination coverage increased, reaching 45% in the 2011-2012 flu season, up from 5% during the 2002-2003 flu season. Within each racial/ethnic group examined, influenza vaccination coverage also grew; however, lower percentages of non-Hispanic black children and Hispanic children were vaccinated than of non-Hispanic white children during all 10 of the flu seasons studied. Coverage ranged from 24% in Mississippi to 72% in Massachusetts.
“Despite the increase, the majority of children 6-23 months in the United States were not fully vaccinated against influenza,” said Tammy A. Santibanez, Ph.D., of the Centers for Disease Control and Prevention, and her colleagues.
Among other findings consistent throughout each flu season examined was that full influenza vaccination coverage was higher among children requiring only one dose of a flu vaccine, compared with those requiring two doses of a flu vaccine.
“Prevention of influenza among infants and young children is a public health priority because of their high risk for influenza-related complications,” wrote Dr. Santibanez and her colleagues. “Appropriate implementation of evidence-based strategies is needed to increase the percentage of children who are fully vaccinated.”
Read the study in Pediatrics (doi: 10.1542/peds.2015.3280).
Less than half of children aged 6-23 months are vaccinated for influenza in the United States, according to an analysis of data obtained via the 2003-2013 National Immunization Survey.
The researchers analyzed providers’ reports of influenza vaccinations, received as one or two doses by children aged 6-23 months. The age group studied is at highest risk of influenza-related complications and was the first group of children for which the Advisory Committee on Immunization Practices recommended influenza vaccination, regardless of an individual’s medical condition.
A child’s age was defined by his or her age on Nov. 1 of each influenza season under study. Two full calendar years of data files were combined to enable analysis of full influenza seasons, which cover parts of 2 consecutive calendar years. The percentages of children requiring two doses to be considered fully vaccinated were based on the dosage recommendations for each flu season.
Overall, flu vaccination coverage increased, reaching 45% in the 2011-2012 flu season, up from 5% during the 2002-2003 flu season. Within each racial/ethnic group examined, influenza vaccination coverage also grew; however, lower percentages of non-Hispanic black children and Hispanic children were vaccinated than of non-Hispanic white children during all 10 of the flu seasons studied. Coverage ranged from 24% in Mississippi to 72% in Massachusetts.
“Despite the increase, the majority of children 6-23 months in the United States were not fully vaccinated against influenza,” said Tammy A. Santibanez, Ph.D., of the Centers for Disease Control and Prevention, and her colleagues.
Among other findings consistent throughout each flu season examined was that full influenza vaccination coverage was higher among children requiring only one dose of a flu vaccine, compared with those requiring two doses of a flu vaccine.
“Prevention of influenza among infants and young children is a public health priority because of their high risk for influenza-related complications,” wrote Dr. Santibanez and her colleagues. “Appropriate implementation of evidence-based strategies is needed to increase the percentage of children who are fully vaccinated.”
Read the study in Pediatrics (doi: 10.1542/peds.2015.3280).
Less than half of children aged 6-23 months are vaccinated for influenza in the United States, according to an analysis of data obtained via the 2003-2013 National Immunization Survey.
The researchers analyzed providers’ reports of influenza vaccinations, received as one or two doses by children aged 6-23 months. The age group studied is at highest risk of influenza-related complications and was the first group of children for which the Advisory Committee on Immunization Practices recommended influenza vaccination, regardless of an individual’s medical condition.
A child’s age was defined by his or her age on Nov. 1 of each influenza season under study. Two full calendar years of data files were combined to enable analysis of full influenza seasons, which cover parts of 2 consecutive calendar years. The percentages of children requiring two doses to be considered fully vaccinated were based on the dosage recommendations for each flu season.
Overall, flu vaccination coverage increased, reaching 45% in the 2011-2012 flu season, up from 5% during the 2002-2003 flu season. Within each racial/ethnic group examined, influenza vaccination coverage also grew; however, lower percentages of non-Hispanic black children and Hispanic children were vaccinated than of non-Hispanic white children during all 10 of the flu seasons studied. Coverage ranged from 24% in Mississippi to 72% in Massachusetts.
“Despite the increase, the majority of children 6-23 months in the United States were not fully vaccinated against influenza,” said Tammy A. Santibanez, Ph.D., of the Centers for Disease Control and Prevention, and her colleagues.
Among other findings consistent throughout each flu season examined was that full influenza vaccination coverage was higher among children requiring only one dose of a flu vaccine, compared with those requiring two doses of a flu vaccine.
“Prevention of influenza among infants and young children is a public health priority because of their high risk for influenza-related complications,” wrote Dr. Santibanez and her colleagues. “Appropriate implementation of evidence-based strategies is needed to increase the percentage of children who are fully vaccinated.”
Read the study in Pediatrics (doi: 10.1542/peds.2015.3280).
FROM PEDIATRICS