User login
Irradiation safe, effective as chemotherapy before cell transplant in mantle cell lymphoma
As preparation for autologous stem cell transplantation, total-body irradiation was as safe and effective as chemotherapy conditioning, in a retrospective analysis of mantle cell lymphoma patient records.
“Our data suggest that both TBI [total-body irradiation] and BEAM [carmustine, etoposide, cytarabine, and melphalan]-based conditioning regimens remain viable conditioning options for MCL patients undergoing ASCT [autologous stem cell transplantation],” wrote Yolanda D. Tseng, MD, of the University of Washington, Seattle, and her coinvestigators.
Progression-free survival, the primary outcome, was greater at 5 years’ follow-up in 43 TBI patients (66%) than in the 32 chemotherapy-conditioned patients (52%), but the difference did not reach statistical significance. Overall survival followed a similar pattern: TBI patients’ overall survival at 5 years was 82%, compared with 68% in the TBI patients, but this difference also lacked significance (Biol Blood Marrow Transplant. 2017 Oct 20; doi: 10.1016/j.bbmt.2017.10.029).
Safety outcomes, including early toxicity, nonrelapse mortality, and secondary malignancies, also were similar between groups.
For the analysis, Dr. Tseng and her colleagues reviewed the records of 75 consecutive adult patients treated during 2001-2011 with ASCT at the Fred Hutchinson Cancer Research Center in Seattle. All underwent conditioning with either myeloablative TBI or a BEAM-based regimen.
Most of the patients had chemosensitive disease (97%), and nearly all received rituximab prior to ASCT.
Prior studies have shown that TBI can be at least as effective as chemotherapy conditioning, but none have looked at toxicity. These results indicate that either approach remains viable for patients undergoing ASCT for MCL, Dr. Tseng and her colleagues concluded.
No disclosures were reported.
As preparation for autologous stem cell transplantation, total-body irradiation was as safe and effective as chemotherapy conditioning, in a retrospective analysis of mantle cell lymphoma patient records.
“Our data suggest that both TBI [total-body irradiation] and BEAM [carmustine, etoposide, cytarabine, and melphalan]-based conditioning regimens remain viable conditioning options for MCL patients undergoing ASCT [autologous stem cell transplantation],” wrote Yolanda D. Tseng, MD, of the University of Washington, Seattle, and her coinvestigators.
Progression-free survival, the primary outcome, was greater at 5 years’ follow-up in 43 TBI patients (66%) than in the 32 chemotherapy-conditioned patients (52%), but the difference did not reach statistical significance. Overall survival followed a similar pattern: TBI patients’ overall survival at 5 years was 82%, compared with 68% in the TBI patients, but this difference also lacked significance (Biol Blood Marrow Transplant. 2017 Oct 20; doi: 10.1016/j.bbmt.2017.10.029).
Safety outcomes, including early toxicity, nonrelapse mortality, and secondary malignancies, also were similar between groups.
For the analysis, Dr. Tseng and her colleagues reviewed the records of 75 consecutive adult patients treated during 2001-2011 with ASCT at the Fred Hutchinson Cancer Research Center in Seattle. All underwent conditioning with either myeloablative TBI or a BEAM-based regimen.
Most of the patients had chemosensitive disease (97%), and nearly all received rituximab prior to ASCT.
Prior studies have shown that TBI can be at least as effective as chemotherapy conditioning, but none have looked at toxicity. These results indicate that either approach remains viable for patients undergoing ASCT for MCL, Dr. Tseng and her colleagues concluded.
No disclosures were reported.
As preparation for autologous stem cell transplantation, total-body irradiation was as safe and effective as chemotherapy conditioning, in a retrospective analysis of mantle cell lymphoma patient records.
“Our data suggest that both TBI [total-body irradiation] and BEAM [carmustine, etoposide, cytarabine, and melphalan]-based conditioning regimens remain viable conditioning options for MCL patients undergoing ASCT [autologous stem cell transplantation],” wrote Yolanda D. Tseng, MD, of the University of Washington, Seattle, and her coinvestigators.
Progression-free survival, the primary outcome, was greater at 5 years’ follow-up in 43 TBI patients (66%) than in the 32 chemotherapy-conditioned patients (52%), but the difference did not reach statistical significance. Overall survival followed a similar pattern: TBI patients’ overall survival at 5 years was 82%, compared with 68% in the TBI patients, but this difference also lacked significance (Biol Blood Marrow Transplant. 2017 Oct 20; doi: 10.1016/j.bbmt.2017.10.029).
Safety outcomes, including early toxicity, nonrelapse mortality, and secondary malignancies, also were similar between groups.
For the analysis, Dr. Tseng and her colleagues reviewed the records of 75 consecutive adult patients treated during 2001-2011 with ASCT at the Fred Hutchinson Cancer Research Center in Seattle. All underwent conditioning with either myeloablative TBI or a BEAM-based regimen.
Most of the patients had chemosensitive disease (97%), and nearly all received rituximab prior to ASCT.
Prior studies have shown that TBI can be at least as effective as chemotherapy conditioning, but none have looked at toxicity. These results indicate that either approach remains viable for patients undergoing ASCT for MCL, Dr. Tseng and her colleagues concluded.
No disclosures were reported.
FROM BIOLOGY OF BLOOD AND MARROW TRANSPLANTATION
Key clinical point:
Major finding: Five-year progression-free survival was 66% in MCL patients who underwent TBI and 52% in chemotherapy-conditioned patients, a nonsignificant difference.
Data source: An analysis of 75 MCL patients undergoing ASCT in a single institution.
Disclosures: No disclosures were reported.
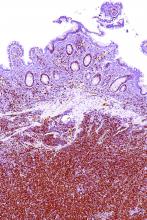
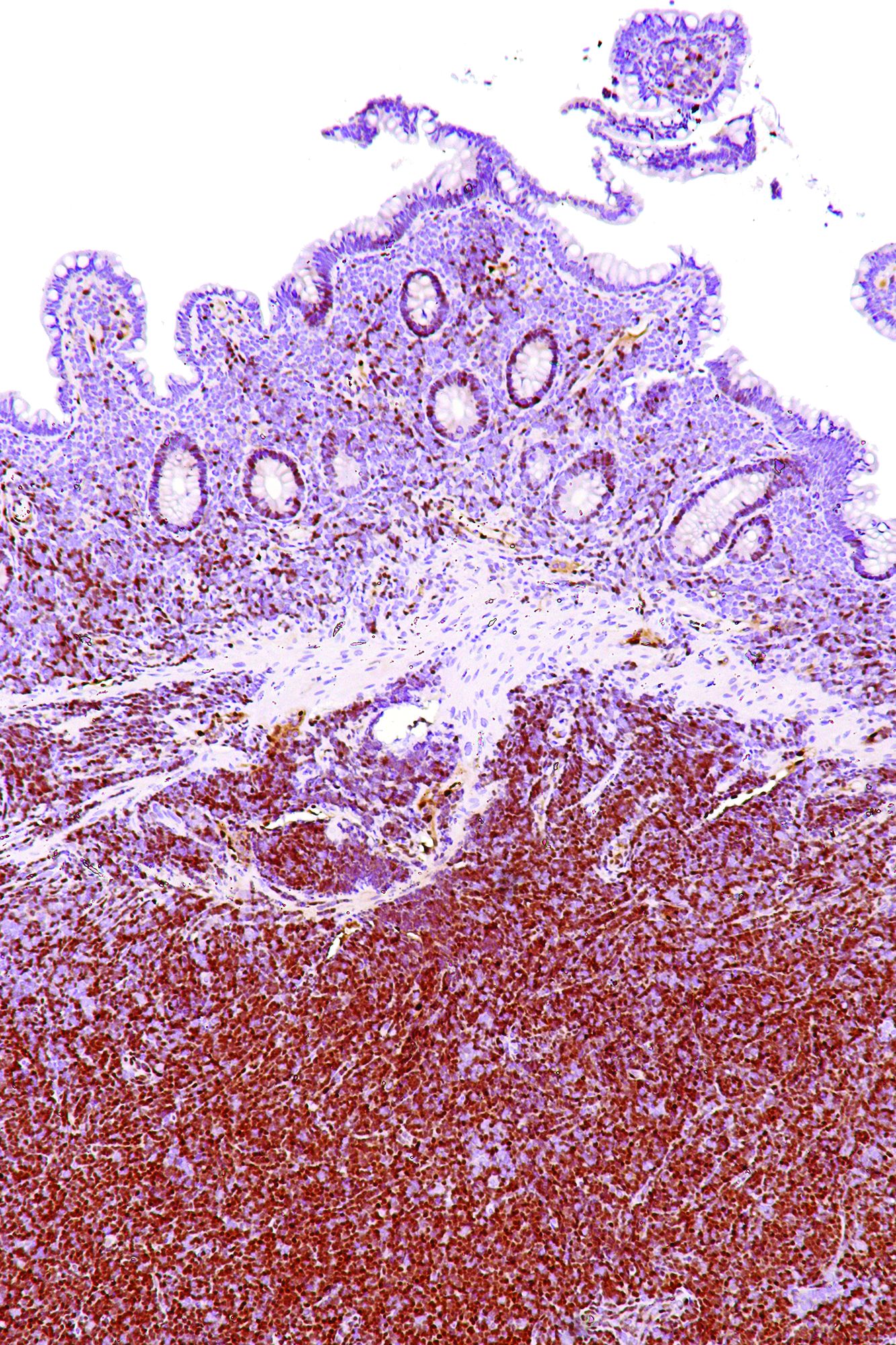
Rare type of MCL mimics Castleman disease
A rare type of mantle cell lymphoma (MCL) has features that are similar to those of Castleman disease, according to a recent report based on three patient cases.
Lymph node biopsies for these patients initially indicated histologic features consistent with those of plasma cell (PC)-type Castleman disease, reported Takuro Igawa, MD, PhD, of Okayama (Japan) University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, and his coauthors. Further work-up, including flow cytometric analysis and cyclin D1 immunostaining, showed features consistent with those of MCL.
“This rare type of MCL can mimic Castleman disease in the clinical setting and upon histological examination,” Dr. Igawa and his colleagues wrote (Pathol Res Pract. 2017 Sep 18. pii: S0344-0338[17]30684-2. doi: 10.1016/j.prp.2017.09.015). “These confusing characteristics can make the diagnosis challenging, and careful flow cytometric analysis is recommended when a histopathological diagnosis is made.”
The patients in the study, all male, were 51, 74, and 81 years of age. Each presented with systemic lymphadenopathy, along with abnormal laboratory findings that according to the investigators are not usually associated with B-cell lymphomas such as MCL, including anemia, polyclonal IgG hypergammaglobulinemia, and elevated levels of C-reactive protein.
In lymph node biopsy specimens, the MCL component was “masked by histological features of PC-type Castleman disease” such as interfollicular plasmacytosis and atrophic germinal centers, the researchers wrote.
However, further pathologic investigations revealed features that were “essential to distinguish these 3 cases of MCL from PC-type Castleman disease,” they added.
In particular, an abnormal B-cell population was found using flow cytometric analysis, while subsequent cyclin D1 immunostaining in all three cases showed abnormal B-cells primarily in the mantle zone that were positive for CD20 and CD5, both typically expressed by MCL, along with SOX11, which is an “excellent diagnostic marker for MCL, including atypical MCL,” the investigators wrote.
These case reports also provide some evidence that interleukin-6 (IL-6), which is thought to be a driver of Castleman disease, might also be implicated in the pathogenesis of this rare MCL variant. the researchers found that all three cases had positive IL-6 staining in the interfollicular areas.
If further studies confirm the role of IL-6 in this rare setting, “specific treatments other than chemotherapy could potentially be used for patients with MCL with features of Castleman disease, such as an anti-IL-6 receptor antibody (tocilizumab), which is already used for patients with Castleman disease,” they said.
A rare type of mantle cell lymphoma (MCL) has features that are similar to those of Castleman disease, according to a recent report based on three patient cases.
Lymph node biopsies for these patients initially indicated histologic features consistent with those of plasma cell (PC)-type Castleman disease, reported Takuro Igawa, MD, PhD, of Okayama (Japan) University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, and his coauthors. Further work-up, including flow cytometric analysis and cyclin D1 immunostaining, showed features consistent with those of MCL.
“This rare type of MCL can mimic Castleman disease in the clinical setting and upon histological examination,” Dr. Igawa and his colleagues wrote (Pathol Res Pract. 2017 Sep 18. pii: S0344-0338[17]30684-2. doi: 10.1016/j.prp.2017.09.015). “These confusing characteristics can make the diagnosis challenging, and careful flow cytometric analysis is recommended when a histopathological diagnosis is made.”
The patients in the study, all male, were 51, 74, and 81 years of age. Each presented with systemic lymphadenopathy, along with abnormal laboratory findings that according to the investigators are not usually associated with B-cell lymphomas such as MCL, including anemia, polyclonal IgG hypergammaglobulinemia, and elevated levels of C-reactive protein.
In lymph node biopsy specimens, the MCL component was “masked by histological features of PC-type Castleman disease” such as interfollicular plasmacytosis and atrophic germinal centers, the researchers wrote.
However, further pathologic investigations revealed features that were “essential to distinguish these 3 cases of MCL from PC-type Castleman disease,” they added.
In particular, an abnormal B-cell population was found using flow cytometric analysis, while subsequent cyclin D1 immunostaining in all three cases showed abnormal B-cells primarily in the mantle zone that were positive for CD20 and CD5, both typically expressed by MCL, along with SOX11, which is an “excellent diagnostic marker for MCL, including atypical MCL,” the investigators wrote.
These case reports also provide some evidence that interleukin-6 (IL-6), which is thought to be a driver of Castleman disease, might also be implicated in the pathogenesis of this rare MCL variant. the researchers found that all three cases had positive IL-6 staining in the interfollicular areas.
If further studies confirm the role of IL-6 in this rare setting, “specific treatments other than chemotherapy could potentially be used for patients with MCL with features of Castleman disease, such as an anti-IL-6 receptor antibody (tocilizumab), which is already used for patients with Castleman disease,” they said.
A rare type of mantle cell lymphoma (MCL) has features that are similar to those of Castleman disease, according to a recent report based on three patient cases.
Lymph node biopsies for these patients initially indicated histologic features consistent with those of plasma cell (PC)-type Castleman disease, reported Takuro Igawa, MD, PhD, of Okayama (Japan) University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, and his coauthors. Further work-up, including flow cytometric analysis and cyclin D1 immunostaining, showed features consistent with those of MCL.
“This rare type of MCL can mimic Castleman disease in the clinical setting and upon histological examination,” Dr. Igawa and his colleagues wrote (Pathol Res Pract. 2017 Sep 18. pii: S0344-0338[17]30684-2. doi: 10.1016/j.prp.2017.09.015). “These confusing characteristics can make the diagnosis challenging, and careful flow cytometric analysis is recommended when a histopathological diagnosis is made.”
The patients in the study, all male, were 51, 74, and 81 years of age. Each presented with systemic lymphadenopathy, along with abnormal laboratory findings that according to the investigators are not usually associated with B-cell lymphomas such as MCL, including anemia, polyclonal IgG hypergammaglobulinemia, and elevated levels of C-reactive protein.
In lymph node biopsy specimens, the MCL component was “masked by histological features of PC-type Castleman disease” such as interfollicular plasmacytosis and atrophic germinal centers, the researchers wrote.
However, further pathologic investigations revealed features that were “essential to distinguish these 3 cases of MCL from PC-type Castleman disease,” they added.
In particular, an abnormal B-cell population was found using flow cytometric analysis, while subsequent cyclin D1 immunostaining in all three cases showed abnormal B-cells primarily in the mantle zone that were positive for CD20 and CD5, both typically expressed by MCL, along with SOX11, which is an “excellent diagnostic marker for MCL, including atypical MCL,” the investigators wrote.
These case reports also provide some evidence that interleukin-6 (IL-6), which is thought to be a driver of Castleman disease, might also be implicated in the pathogenesis of this rare MCL variant. the researchers found that all three cases had positive IL-6 staining in the interfollicular areas.
If further studies confirm the role of IL-6 in this rare setting, “specific treatments other than chemotherapy could potentially be used for patients with MCL with features of Castleman disease, such as an anti-IL-6 receptor antibody (tocilizumab), which is already used for patients with Castleman disease,” they said.
FROM PATHOLOGY – RESEARCH AND PRACTICE
Key clinical point:
Major finding: Lymph node biopsy revealed histologic features consistent with plasma cell (PC)-type Castleman disease, but cyclin D1 immunostaining and flow cytometric analysis showed features consistent with a diagnosis of MCL.
Data source: A report on three patient cases of MCL with features of PC-type Castleman disease retrieved from surgical pathology consultation files.
Disclosures: The authors reported no conflicts of interest.
VCR regimen showed efficacy in mantle cell and indolent lymphomas
The combination of bortezomib, cladribine, and rituximab (VCR) was an effective treatment regimen for patients with CD20-positive mantle cell lymphoma (MCL) and indolent non-Hodgkin’s lymphoma (iNHL), based on results of a recent phase 2, open-label study.
The overall response rate was 92% in the single-center, 24-patient study. The 2-year progression-free survival (PFS) was 82% and 54%, respectively, for MCL and iNHL patients; PFS was 80% for treatment-naive patients and 57% for those with refractory/recalcitrant disease, according to Soham D. Puvvada, MD, of the University of Arizona Cancer Center in Tucson, and her associates.
Two-year overall survival was 91% for MCL and 69% for iNHL patients. Median time to progression was 34.5 months, and median PFS had not been reached at 2 years, according to the researchers.
While the study (NCT00980395) was small and limited by its single-center design, the VCR combination “has encouraging activity in both MCL and iNHL and could be compared to standard therapies in future studies,” the researchers wrote. “For MCL in particular, we believe a noninferiority comparison to standard therapies would be justified by our results.”
Adverse events were most commonly hematologic, and three patients experienced febrile neutropenia, data show.
“Although hematological toxicity can be an issue, the regimen provides an alternative option in transplant ineligible relapsed/refractory MCL and iNHL,” wrote Dr. Puvvada and her colleagues. The study was published in Clinical Lymphoma, Myeloma & Leukemia (doi: 10.1016/j.clml.2017.09.001).
The researchers studied the combination of bortezomib, the proteasome inhibitor initially approved for relapsed/refractory MCL, cladribine, which has shown activity and promising response rates in patients with indolent lymphomas, and rituximab in patients with CD20-positive mantle cell or indolent lymphoma.
Patients with follicular lymphomas were eligible to be included in the study if they had received at least one previous line of therapy. All other participants could be treatment naive or have relapsed after previous treatment.
Of the 24 patients enrolled, 11 had MCL, 5 had follicular lymphoma, 4 had marginal zone lymphoma, 3 had lymphoplasmacytic lymphoma, and 1 had small lymphocytic lymphoma.
The VCR regimen, given every 28 days for no more than six cycles, included rituximab at 375 mg/m2 given intravenously on day 1 of each cycle, cladribine 4 mg/m2 given intravenously over 2 hours on days 1 through 5, and bortezomib 1.3 mg/m2 given intravenously on days 1 and 4. Patients received a median of five cycles of therapy.
Adverse events of grade 3 or greater occurred in 14 patients (58%); 8 patients had leukopenia, 6 had thrombocytopenia, 5 had fatigue, and 5 had neutropenia, which included febrile neutropenia in 3 patients.
With a median follow-up of 38.5 months, overall response rate for VCR was 96%. Complete responses occurred in 8 of 23 evaluable patients (35%) and partial responses in 14 more patients (61%).
The combination of bortezomib, cladribine, and rituximab (VCR) was an effective treatment regimen for patients with CD20-positive mantle cell lymphoma (MCL) and indolent non-Hodgkin’s lymphoma (iNHL), based on results of a recent phase 2, open-label study.
The overall response rate was 92% in the single-center, 24-patient study. The 2-year progression-free survival (PFS) was 82% and 54%, respectively, for MCL and iNHL patients; PFS was 80% for treatment-naive patients and 57% for those with refractory/recalcitrant disease, according to Soham D. Puvvada, MD, of the University of Arizona Cancer Center in Tucson, and her associates.
Two-year overall survival was 91% for MCL and 69% for iNHL patients. Median time to progression was 34.5 months, and median PFS had not been reached at 2 years, according to the researchers.
While the study (NCT00980395) was small and limited by its single-center design, the VCR combination “has encouraging activity in both MCL and iNHL and could be compared to standard therapies in future studies,” the researchers wrote. “For MCL in particular, we believe a noninferiority comparison to standard therapies would be justified by our results.”
Adverse events were most commonly hematologic, and three patients experienced febrile neutropenia, data show.
“Although hematological toxicity can be an issue, the regimen provides an alternative option in transplant ineligible relapsed/refractory MCL and iNHL,” wrote Dr. Puvvada and her colleagues. The study was published in Clinical Lymphoma, Myeloma & Leukemia (doi: 10.1016/j.clml.2017.09.001).
The researchers studied the combination of bortezomib, the proteasome inhibitor initially approved for relapsed/refractory MCL, cladribine, which has shown activity and promising response rates in patients with indolent lymphomas, and rituximab in patients with CD20-positive mantle cell or indolent lymphoma.
Patients with follicular lymphomas were eligible to be included in the study if they had received at least one previous line of therapy. All other participants could be treatment naive or have relapsed after previous treatment.
Of the 24 patients enrolled, 11 had MCL, 5 had follicular lymphoma, 4 had marginal zone lymphoma, 3 had lymphoplasmacytic lymphoma, and 1 had small lymphocytic lymphoma.
The VCR regimen, given every 28 days for no more than six cycles, included rituximab at 375 mg/m2 given intravenously on day 1 of each cycle, cladribine 4 mg/m2 given intravenously over 2 hours on days 1 through 5, and bortezomib 1.3 mg/m2 given intravenously on days 1 and 4. Patients received a median of five cycles of therapy.
Adverse events of grade 3 or greater occurred in 14 patients (58%); 8 patients had leukopenia, 6 had thrombocytopenia, 5 had fatigue, and 5 had neutropenia, which included febrile neutropenia in 3 patients.
With a median follow-up of 38.5 months, overall response rate for VCR was 96%. Complete responses occurred in 8 of 23 evaluable patients (35%) and partial responses in 14 more patients (61%).
The combination of bortezomib, cladribine, and rituximab (VCR) was an effective treatment regimen for patients with CD20-positive mantle cell lymphoma (MCL) and indolent non-Hodgkin’s lymphoma (iNHL), based on results of a recent phase 2, open-label study.
The overall response rate was 92% in the single-center, 24-patient study. The 2-year progression-free survival (PFS) was 82% and 54%, respectively, for MCL and iNHL patients; PFS was 80% for treatment-naive patients and 57% for those with refractory/recalcitrant disease, according to Soham D. Puvvada, MD, of the University of Arizona Cancer Center in Tucson, and her associates.
Two-year overall survival was 91% for MCL and 69% for iNHL patients. Median time to progression was 34.5 months, and median PFS had not been reached at 2 years, according to the researchers.
While the study (NCT00980395) was small and limited by its single-center design, the VCR combination “has encouraging activity in both MCL and iNHL and could be compared to standard therapies in future studies,” the researchers wrote. “For MCL in particular, we believe a noninferiority comparison to standard therapies would be justified by our results.”
Adverse events were most commonly hematologic, and three patients experienced febrile neutropenia, data show.
“Although hematological toxicity can be an issue, the regimen provides an alternative option in transplant ineligible relapsed/refractory MCL and iNHL,” wrote Dr. Puvvada and her colleagues. The study was published in Clinical Lymphoma, Myeloma & Leukemia (doi: 10.1016/j.clml.2017.09.001).
The researchers studied the combination of bortezomib, the proteasome inhibitor initially approved for relapsed/refractory MCL, cladribine, which has shown activity and promising response rates in patients with indolent lymphomas, and rituximab in patients with CD20-positive mantle cell or indolent lymphoma.
Patients with follicular lymphomas were eligible to be included in the study if they had received at least one previous line of therapy. All other participants could be treatment naive or have relapsed after previous treatment.
Of the 24 patients enrolled, 11 had MCL, 5 had follicular lymphoma, 4 had marginal zone lymphoma, 3 had lymphoplasmacytic lymphoma, and 1 had small lymphocytic lymphoma.
The VCR regimen, given every 28 days for no more than six cycles, included rituximab at 375 mg/m2 given intravenously on day 1 of each cycle, cladribine 4 mg/m2 given intravenously over 2 hours on days 1 through 5, and bortezomib 1.3 mg/m2 given intravenously on days 1 and 4. Patients received a median of five cycles of therapy.
Adverse events of grade 3 or greater occurred in 14 patients (58%); 8 patients had leukopenia, 6 had thrombocytopenia, 5 had fatigue, and 5 had neutropenia, which included febrile neutropenia in 3 patients.
With a median follow-up of 38.5 months, overall response rate for VCR was 96%. Complete responses occurred in 8 of 23 evaluable patients (35%) and partial responses in 14 more patients (61%).
FROM LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: The overall response rate was 92%, with a 2-year PFS of 82% and 54% for patients with mantle cell lymphoma (MCL) and indolent non-Hodgkin’s lymphoma (iNHL), respectively. Adverse events were most commonly hematologic, and three patients experienced febrile neutropenia.
Data source: A phase 2, open-label study including 24 patients with mantle cell or indolent lymphomas.
Disclosures: No disclosures were reported in the accepted manuscript.
Rituximab maintenance halves MCL death risk after ASCT
, results of a phase 3 trial show.
After 50.2 months median follow-up, the overall survival rate for patients aged 65 or younger randomized to rituximab maintenance after four cycles of induction chemotherapy with rituximab, dexamethasone, cytarabine, and a platinum derivative (R-DHAP) followed by ASCT was 89%, compared with 80% for patients randomized to observation (P = .004), reported Steven Le Gouill, MD, PhD, of University Hospital Hotel-Dieu, in Nantes, Frances, and colleagues.
In an unadjusted regression analysis, the difference translated into a hazard ratio for death within 4 years of 0.50 (P = .004) favoring rituximab, they wrote in the Sept. 28, 2017 issue of The New England Journal of Medicine.
“[A]n induction regimen with four courses of R-DHAP followed by transplantation without total-body irradiation resulted in a high rate of complete response. A 3-year course of rituximab maintenance therapy administered every 2 months prolonged overall survival among young patients with mantle cell lymphoma,” the investigators wrote (N Engl J Med. 2017;377:1250-60).
Dr. Le Gouill and his colleagues hypothesized that relapses following treatment for MCL may be caused by residual malignant cells that chemotherapy and ASCT fail to eradicate, suggesting that maintenance therapy with rituximab could help to suppress residual disease, prolong the duration of responses, and extend both progression-free and overall survival.
They cited an earlier study by members of the European Mantle Cell Lymphoma Network showing that among patients aged 60 and older who had a response to eight cycles of chemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), maintenance therapy with rituximab was associated with an 87% 4-year overall survival rate vs. 63% for patients maintained on interferon alfa (P = .005) (N Engl J Med. 2012;367:520-31).
For the current study, the researchers enrolled 299 patients, of whom 257 went on to ASCT, and 240 of whom were randomized and were included in an intention-to-treat (ITT) analysis.
Patients received induction with four cycles of R-DHAP. Those patients who had partial responses or tumor mass shrinkage of less than 75% on CT received a rescue induction with four cycles of R-CHOP.
Those patients with complete or partial responses could then go on to transplantation after a conditioning regimen of R-BEAM (rituximab, carmustine, etoposide, cytarabine, and melphalan).
Patients randomized after ASCT to rituximab received it every 2 months for 3 years in an intravenous infusion at a dose of 375 mg/m2.
After a median of 50.2 months from randomization, the rate of 4-year event-free survival (no disease progression, relapse, death, or severe infection), the primary endpoint, was 79% for patients maintained on rituximab vs. 61% for those on observation alone (P = .001).
The 4-year progression-free survival rate also favored rituximab at 83% vs. 64%, respectively (P less than .001), with respective overall survival rates of 89% and 80%.
The median event-free survival, progression-free survival, and overall survival was not reached in either study arm.
For the 59 patients who for various reasons did not undergo randomization, the median progression-free survival was 11.0 months, and the median overall survival was 30.6 months.
In all, 83 of the 120 patients randomized to rituximab completed the scheduled 3 years of therapy. Maintenance therapy was stopped for disease progression in 16 patients and because of neutropenia in 9. There were 13 deaths in the rituximab arm, including 3 deaths from second malignancies.
Of the 120 patients assigned to observation, 37 had disease progression during the study period, and 24 died, one from a second malignancy.
Four patients in each study arm had serious infections after ASCT, including one case each of spondylitis, pyelonephritis, septicemia, and varicella pneumonia in the rituximab group, and septicemia, cellulitis, meningitis, and severe pneumonia in the observation group.
Lymphoma was the cause of death in 8 patients assigned to rituximab, and in 16 assigned to observation.
The investigators noted that although some centers use total-body irradiation for conditioning prior to transplant, this modality is not available in all centers and is associated with both short- and long-term toxicities. The progression-free survival results seen in this trial, where only ablative drug regimens were used “suggest that total-body irradiation–based conditioning regimens may not be superior to chemotherapy alone when an effective regimen is used during induction,” they wrote.
The study was supported by Roche and Amgen. Dr. Le Gouill disclosed fees for consulting and honoraria from Roche, Janssen-Cilag, and Celgene. Multiple coauthors disclosed similar relationships with industry.
, results of a phase 3 trial show.
After 50.2 months median follow-up, the overall survival rate for patients aged 65 or younger randomized to rituximab maintenance after four cycles of induction chemotherapy with rituximab, dexamethasone, cytarabine, and a platinum derivative (R-DHAP) followed by ASCT was 89%, compared with 80% for patients randomized to observation (P = .004), reported Steven Le Gouill, MD, PhD, of University Hospital Hotel-Dieu, in Nantes, Frances, and colleagues.
In an unadjusted regression analysis, the difference translated into a hazard ratio for death within 4 years of 0.50 (P = .004) favoring rituximab, they wrote in the Sept. 28, 2017 issue of The New England Journal of Medicine.
“[A]n induction regimen with four courses of R-DHAP followed by transplantation without total-body irradiation resulted in a high rate of complete response. A 3-year course of rituximab maintenance therapy administered every 2 months prolonged overall survival among young patients with mantle cell lymphoma,” the investigators wrote (N Engl J Med. 2017;377:1250-60).
Dr. Le Gouill and his colleagues hypothesized that relapses following treatment for MCL may be caused by residual malignant cells that chemotherapy and ASCT fail to eradicate, suggesting that maintenance therapy with rituximab could help to suppress residual disease, prolong the duration of responses, and extend both progression-free and overall survival.
They cited an earlier study by members of the European Mantle Cell Lymphoma Network showing that among patients aged 60 and older who had a response to eight cycles of chemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), maintenance therapy with rituximab was associated with an 87% 4-year overall survival rate vs. 63% for patients maintained on interferon alfa (P = .005) (N Engl J Med. 2012;367:520-31).
For the current study, the researchers enrolled 299 patients, of whom 257 went on to ASCT, and 240 of whom were randomized and were included in an intention-to-treat (ITT) analysis.
Patients received induction with four cycles of R-DHAP. Those patients who had partial responses or tumor mass shrinkage of less than 75% on CT received a rescue induction with four cycles of R-CHOP.
Those patients with complete or partial responses could then go on to transplantation after a conditioning regimen of R-BEAM (rituximab, carmustine, etoposide, cytarabine, and melphalan).
Patients randomized after ASCT to rituximab received it every 2 months for 3 years in an intravenous infusion at a dose of 375 mg/m2.
After a median of 50.2 months from randomization, the rate of 4-year event-free survival (no disease progression, relapse, death, or severe infection), the primary endpoint, was 79% for patients maintained on rituximab vs. 61% for those on observation alone (P = .001).
The 4-year progression-free survival rate also favored rituximab at 83% vs. 64%, respectively (P less than .001), with respective overall survival rates of 89% and 80%.
The median event-free survival, progression-free survival, and overall survival was not reached in either study arm.
For the 59 patients who for various reasons did not undergo randomization, the median progression-free survival was 11.0 months, and the median overall survival was 30.6 months.
In all, 83 of the 120 patients randomized to rituximab completed the scheduled 3 years of therapy. Maintenance therapy was stopped for disease progression in 16 patients and because of neutropenia in 9. There were 13 deaths in the rituximab arm, including 3 deaths from second malignancies.
Of the 120 patients assigned to observation, 37 had disease progression during the study period, and 24 died, one from a second malignancy.
Four patients in each study arm had serious infections after ASCT, including one case each of spondylitis, pyelonephritis, septicemia, and varicella pneumonia in the rituximab group, and septicemia, cellulitis, meningitis, and severe pneumonia in the observation group.
Lymphoma was the cause of death in 8 patients assigned to rituximab, and in 16 assigned to observation.
The investigators noted that although some centers use total-body irradiation for conditioning prior to transplant, this modality is not available in all centers and is associated with both short- and long-term toxicities. The progression-free survival results seen in this trial, where only ablative drug regimens were used “suggest that total-body irradiation–based conditioning regimens may not be superior to chemotherapy alone when an effective regimen is used during induction,” they wrote.
The study was supported by Roche and Amgen. Dr. Le Gouill disclosed fees for consulting and honoraria from Roche, Janssen-Cilag, and Celgene. Multiple coauthors disclosed similar relationships with industry.
, results of a phase 3 trial show.
After 50.2 months median follow-up, the overall survival rate for patients aged 65 or younger randomized to rituximab maintenance after four cycles of induction chemotherapy with rituximab, dexamethasone, cytarabine, and a platinum derivative (R-DHAP) followed by ASCT was 89%, compared with 80% for patients randomized to observation (P = .004), reported Steven Le Gouill, MD, PhD, of University Hospital Hotel-Dieu, in Nantes, Frances, and colleagues.
In an unadjusted regression analysis, the difference translated into a hazard ratio for death within 4 years of 0.50 (P = .004) favoring rituximab, they wrote in the Sept. 28, 2017 issue of The New England Journal of Medicine.
“[A]n induction regimen with four courses of R-DHAP followed by transplantation without total-body irradiation resulted in a high rate of complete response. A 3-year course of rituximab maintenance therapy administered every 2 months prolonged overall survival among young patients with mantle cell lymphoma,” the investigators wrote (N Engl J Med. 2017;377:1250-60).
Dr. Le Gouill and his colleagues hypothesized that relapses following treatment for MCL may be caused by residual malignant cells that chemotherapy and ASCT fail to eradicate, suggesting that maintenance therapy with rituximab could help to suppress residual disease, prolong the duration of responses, and extend both progression-free and overall survival.
They cited an earlier study by members of the European Mantle Cell Lymphoma Network showing that among patients aged 60 and older who had a response to eight cycles of chemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), maintenance therapy with rituximab was associated with an 87% 4-year overall survival rate vs. 63% for patients maintained on interferon alfa (P = .005) (N Engl J Med. 2012;367:520-31).
For the current study, the researchers enrolled 299 patients, of whom 257 went on to ASCT, and 240 of whom were randomized and were included in an intention-to-treat (ITT) analysis.
Patients received induction with four cycles of R-DHAP. Those patients who had partial responses or tumor mass shrinkage of less than 75% on CT received a rescue induction with four cycles of R-CHOP.
Those patients with complete or partial responses could then go on to transplantation after a conditioning regimen of R-BEAM (rituximab, carmustine, etoposide, cytarabine, and melphalan).
Patients randomized after ASCT to rituximab received it every 2 months for 3 years in an intravenous infusion at a dose of 375 mg/m2.
After a median of 50.2 months from randomization, the rate of 4-year event-free survival (no disease progression, relapse, death, or severe infection), the primary endpoint, was 79% for patients maintained on rituximab vs. 61% for those on observation alone (P = .001).
The 4-year progression-free survival rate also favored rituximab at 83% vs. 64%, respectively (P less than .001), with respective overall survival rates of 89% and 80%.
The median event-free survival, progression-free survival, and overall survival was not reached in either study arm.
For the 59 patients who for various reasons did not undergo randomization, the median progression-free survival was 11.0 months, and the median overall survival was 30.6 months.
In all, 83 of the 120 patients randomized to rituximab completed the scheduled 3 years of therapy. Maintenance therapy was stopped for disease progression in 16 patients and because of neutropenia in 9. There were 13 deaths in the rituximab arm, including 3 deaths from second malignancies.
Of the 120 patients assigned to observation, 37 had disease progression during the study period, and 24 died, one from a second malignancy.
Four patients in each study arm had serious infections after ASCT, including one case each of spondylitis, pyelonephritis, septicemia, and varicella pneumonia in the rituximab group, and septicemia, cellulitis, meningitis, and severe pneumonia in the observation group.
Lymphoma was the cause of death in 8 patients assigned to rituximab, and in 16 assigned to observation.
The investigators noted that although some centers use total-body irradiation for conditioning prior to transplant, this modality is not available in all centers and is associated with both short- and long-term toxicities. The progression-free survival results seen in this trial, where only ablative drug regimens were used “suggest that total-body irradiation–based conditioning regimens may not be superior to chemotherapy alone when an effective regimen is used during induction,” they wrote.
The study was supported by Roche and Amgen. Dr. Le Gouill disclosed fees for consulting and honoraria from Roche, Janssen-Cilag, and Celgene. Multiple coauthors disclosed similar relationships with industry.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Following stem cell transplantation, rituximab maintenance cut in half the risk for death in patients with mantle cell lymphoma.
Major finding: Four-year overall survival was 89% with rituximab maintenance vs. 80% for observation alone.
Data source: Randomized phase 3 trial in 240 patients aged 65 and younger at diagnosis of mantle cell lymphoma.
Disclosures: The study was supported by Roche and Amgen. Dr. Le Gouill disclosed fees for consulting and honoraria from Roche, Janssen-Cilag, and Celgene. Multiple coauthors disclosed similar relationships with industry.
Plerixafor doesn’t overcome HPC failure in R-hyperCVAD for mantle cell lymphoma
A commonly-used intensive induction regimen was associated with higher rates of hematopoietic progenitor cell mobilization failure in patients with mantle cell lymphoma, even when plerixafor rescue was attempted, based on a study by Amandeep Salhotra, MD, and his colleagues at City of Hope, Duarte, Calif.
Patients who received rituximab and hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (R-hyperCVAD) in the era after plerixafor came into use experienced significantly higher rates of peripheral blood stem cell (PBSC) collection failure than did patients receiving other induction regimens (17% vs. 4% failure rate, P = .04).
“Plerixafor does not overcome the negative impact of R-hyperCVAD on PBSC mobilization, and caution is warranted in using R-hyperCVAD in patients with newly diagnosed MCL who are candidates for ASCT (autologous stem cell transplant),” wrote Dr. Salhotra and his colleagues.
The higher rate of hematopoietic progenitor cell collection failure for R-hyperCVAD patients could not be attributed to their age at time of mantle cell lymphoma diagnosis or to the amount of time between diagnosis and collection.
Treatment records for 181 consecutive mantle cell lymphoma patients were examined for a 10 year period in the retrospective single-site study. Plerixafor, a C-X-C chemokine receptor agonist that reduces hematopoietic progenitor cells’ ability to bind to bone marrow stroma, was introduced on August 16, 2009; a total of 71 patients were treated before this point, and 110 were treated afterward.
The R-hyperCVAD regimen was received by 34 pre-plerixafor patients (45%) and by 42 of the post-plerixafor era patients (55%). Other regimens were received by 37 (35%) and 68 (65%) of the pre- and post-plerixafor era patients, respectively.
Before plerixafor came into use, Dr. Salhotra, of City of Hope’s department of hematology and hematopoietic cell transplantation, and his coinvestigators saw no significant difference among their study population in the rates of PBSC collection failure between those receiving R-hyperCVAD (11%) and those receiving other regimens (12%). The findings were reported in Biology of Blood and Marrow Transplantation.
The study was conducted in the context of other recent work that showed higher rates of PBSC collection failure and fewer CD34+ cells collected with the use of an R-hyperCVAD conditioning regimen. The fact that PBSC mobilization rates were significantly lower in R-hyperCVAD patients post-plerixafor surprised the investigators, who had hypothesized that the use of plerixafor would overcome PBSC mobilization failures without regard to the conditioning regimen used.
“It may be worthwhile to consider using a more aggressive strategy for [hematopoetic progenitor cell] mobilization in patients who have received R-hyperCVAD chemotherapy upfront or as salvage for aggressive lymphomas,” the researchers wrote. This might include the use of plerixafor upfront when patients have low CD34 counts before apheresis.
The researchers plan to examine their data to see how the choice of induction regimen and plerixafor usage impact patient survival.
The study authors reported no conflicts of interest.
Source: Amandeep Salhotra, et al. Hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone chemotherapy in mantle cell lymphoma patients is associated with higher rates of hematopoietic progenitor cell mobilization failure despite plerixafor rescue. Biol Blood Marrow Transplant 2017; 23:1264-1268.
koakes@frontlinemedcom.com
SOURCE: Biol Blood Marrow Transplant 2017; 23:1264-1268. http://dx.doi.org/10.1016/j.bbmt.2017.04.011
A commonly-used intensive induction regimen was associated with higher rates of hematopoietic progenitor cell mobilization failure in patients with mantle cell lymphoma, even when plerixafor rescue was attempted, based on a study by Amandeep Salhotra, MD, and his colleagues at City of Hope, Duarte, Calif.
Patients who received rituximab and hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (R-hyperCVAD) in the era after plerixafor came into use experienced significantly higher rates of peripheral blood stem cell (PBSC) collection failure than did patients receiving other induction regimens (17% vs. 4% failure rate, P = .04).
“Plerixafor does not overcome the negative impact of R-hyperCVAD on PBSC mobilization, and caution is warranted in using R-hyperCVAD in patients with newly diagnosed MCL who are candidates for ASCT (autologous stem cell transplant),” wrote Dr. Salhotra and his colleagues.
The higher rate of hematopoietic progenitor cell collection failure for R-hyperCVAD patients could not be attributed to their age at time of mantle cell lymphoma diagnosis or to the amount of time between diagnosis and collection.
Treatment records for 181 consecutive mantle cell lymphoma patients were examined for a 10 year period in the retrospective single-site study. Plerixafor, a C-X-C chemokine receptor agonist that reduces hematopoietic progenitor cells’ ability to bind to bone marrow stroma, was introduced on August 16, 2009; a total of 71 patients were treated before this point, and 110 were treated afterward.
The R-hyperCVAD regimen was received by 34 pre-plerixafor patients (45%) and by 42 of the post-plerixafor era patients (55%). Other regimens were received by 37 (35%) and 68 (65%) of the pre- and post-plerixafor era patients, respectively.
Before plerixafor came into use, Dr. Salhotra, of City of Hope’s department of hematology and hematopoietic cell transplantation, and his coinvestigators saw no significant difference among their study population in the rates of PBSC collection failure between those receiving R-hyperCVAD (11%) and those receiving other regimens (12%). The findings were reported in Biology of Blood and Marrow Transplantation.
The study was conducted in the context of other recent work that showed higher rates of PBSC collection failure and fewer CD34+ cells collected with the use of an R-hyperCVAD conditioning regimen. The fact that PBSC mobilization rates were significantly lower in R-hyperCVAD patients post-plerixafor surprised the investigators, who had hypothesized that the use of plerixafor would overcome PBSC mobilization failures without regard to the conditioning regimen used.
“It may be worthwhile to consider using a more aggressive strategy for [hematopoetic progenitor cell] mobilization in patients who have received R-hyperCVAD chemotherapy upfront or as salvage for aggressive lymphomas,” the researchers wrote. This might include the use of plerixafor upfront when patients have low CD34 counts before apheresis.
The researchers plan to examine their data to see how the choice of induction regimen and plerixafor usage impact patient survival.
The study authors reported no conflicts of interest.
Source: Amandeep Salhotra, et al. Hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone chemotherapy in mantle cell lymphoma patients is associated with higher rates of hematopoietic progenitor cell mobilization failure despite plerixafor rescue. Biol Blood Marrow Transplant 2017; 23:1264-1268.
koakes@frontlinemedcom.com
SOURCE: Biol Blood Marrow Transplant 2017; 23:1264-1268. http://dx.doi.org/10.1016/j.bbmt.2017.04.011
A commonly-used intensive induction regimen was associated with higher rates of hematopoietic progenitor cell mobilization failure in patients with mantle cell lymphoma, even when plerixafor rescue was attempted, based on a study by Amandeep Salhotra, MD, and his colleagues at City of Hope, Duarte, Calif.
Patients who received rituximab and hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (R-hyperCVAD) in the era after plerixafor came into use experienced significantly higher rates of peripheral blood stem cell (PBSC) collection failure than did patients receiving other induction regimens (17% vs. 4% failure rate, P = .04).
“Plerixafor does not overcome the negative impact of R-hyperCVAD on PBSC mobilization, and caution is warranted in using R-hyperCVAD in patients with newly diagnosed MCL who are candidates for ASCT (autologous stem cell transplant),” wrote Dr. Salhotra and his colleagues.
The higher rate of hematopoietic progenitor cell collection failure for R-hyperCVAD patients could not be attributed to their age at time of mantle cell lymphoma diagnosis or to the amount of time between diagnosis and collection.
Treatment records for 181 consecutive mantle cell lymphoma patients were examined for a 10 year period in the retrospective single-site study. Plerixafor, a C-X-C chemokine receptor agonist that reduces hematopoietic progenitor cells’ ability to bind to bone marrow stroma, was introduced on August 16, 2009; a total of 71 patients were treated before this point, and 110 were treated afterward.
The R-hyperCVAD regimen was received by 34 pre-plerixafor patients (45%) and by 42 of the post-plerixafor era patients (55%). Other regimens were received by 37 (35%) and 68 (65%) of the pre- and post-plerixafor era patients, respectively.
Before plerixafor came into use, Dr. Salhotra, of City of Hope’s department of hematology and hematopoietic cell transplantation, and his coinvestigators saw no significant difference among their study population in the rates of PBSC collection failure between those receiving R-hyperCVAD (11%) and those receiving other regimens (12%). The findings were reported in Biology of Blood and Marrow Transplantation.
The study was conducted in the context of other recent work that showed higher rates of PBSC collection failure and fewer CD34+ cells collected with the use of an R-hyperCVAD conditioning regimen. The fact that PBSC mobilization rates were significantly lower in R-hyperCVAD patients post-plerixafor surprised the investigators, who had hypothesized that the use of plerixafor would overcome PBSC mobilization failures without regard to the conditioning regimen used.
“It may be worthwhile to consider using a more aggressive strategy for [hematopoetic progenitor cell] mobilization in patients who have received R-hyperCVAD chemotherapy upfront or as salvage for aggressive lymphomas,” the researchers wrote. This might include the use of plerixafor upfront when patients have low CD34 counts before apheresis.
The researchers plan to examine their data to see how the choice of induction regimen and plerixafor usage impact patient survival.
The study authors reported no conflicts of interest.
Source: Amandeep Salhotra, et al. Hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone chemotherapy in mantle cell lymphoma patients is associated with higher rates of hematopoietic progenitor cell mobilization failure despite plerixafor rescue. Biol Blood Marrow Transplant 2017; 23:1264-1268.
koakes@frontlinemedcom.com
SOURCE: Biol Blood Marrow Transplant 2017; 23:1264-1268. http://dx.doi.org/10.1016/j.bbmt.2017.04.011
FROM BIOLOGY OF BLOOD AND MARROW TRANSPLANTATION
Key clinical point: R-hyperCVAD was associated with increased peripheral blood stem cell (PBSC) collection failure in the post-plerixafor era.
Major finding: Patients receiving R-hyperCVAD in the post-plerixafor era had a 17% PBSC collection failure rate, compared to a 4% rate for those receiving other chemotherapy (P = 0.04).
Study details: Single-center retrospective study of 181 consecutive patients with mantle cell lymphoma over a 10-year period spanning the introduction of plerixafor.
Disclosures: The study was sponsored by City of Hope and the National Cancer Institute; the authors reported no conflicts of interest.
Source: Amandeep Salhotra, et al. Hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone chemotherapy in mantle cell lymphoma patients is associated with higher rates of hematopoietic progenitor cell mobilization failure despite plerixafor rescue. Biol Blood Marrow Transplant 2017; 23:1264-1268.
FDA grants priority review of acalabrutinib for second-line treatment of MCL
The Food and Drug Administration has granted a priority review for acalabrutinib, a Bruton tyrosine kinase inhibitor, for the treatment of patients with mantle cell lymphoma (MCL) who have received at least one prior therapy.
The new drug application is based on results from the phase 2 ACE-LY-004 trial, which evaluated the safety and efficacy of acalabrutinib in patients with relapsed/refractory MCL who had received at least one prior therapy.
The Food and Drug Administration has granted a priority review for acalabrutinib, a Bruton tyrosine kinase inhibitor, for the treatment of patients with mantle cell lymphoma (MCL) who have received at least one prior therapy.
The new drug application is based on results from the phase 2 ACE-LY-004 trial, which evaluated the safety and efficacy of acalabrutinib in patients with relapsed/refractory MCL who had received at least one prior therapy.
The Food and Drug Administration has granted a priority review for acalabrutinib, a Bruton tyrosine kinase inhibitor, for the treatment of patients with mantle cell lymphoma (MCL) who have received at least one prior therapy.
The new drug application is based on results from the phase 2 ACE-LY-004 trial, which evaluated the safety and efficacy of acalabrutinib in patients with relapsed/refractory MCL who had received at least one prior therapy.
IMiD/Anti-CD20 combo induces complete responses in r/r NHL
Lugano, Switzerland – A combination of obinutuzumab (Gazyva) and the experimental immunomodulatory agent CC-122 showed “clinically meaningful” activity against relapsed/refractory diffuse large B cell lymphoma (DLBCL) and indolent non-Hodgkin lymphoma (NHL) in a phase 1b study.
Among 38 patients with heavily pretreated, relapsed/refractory DLBCL, follicular lymphoma (FL), or marginal zone lymphoma (MZL), the overall response rate was 66%, including 12 patients (32%) with a complete response (CR), reported Jean-Marie Michot, MD, from the Goustave-Roussy Cancer Center in Villejuif, France.
CC-122 is a thalidomide analog that shares a molecular target with its cousin lenalidomide (Revlimid). Both molecules bind to the protein cereblon to cause degradation of the lymphoid transcription factors Aiolos and Ikaros.
As a single agent, CC-122 has been shown to have immunomodulatory effects on T-cell and natural killer (NK)–cell functions and has shown clinical activity in heavily pretreated patients with relapsed refractory NHL, including various cell-of-origin–based DLBCL subtypes, Dr, Michot said.
In preclinical studies, the combination of CC-122 and obinutuzumab, an anti-CD20 monoclonal antibody, has shown synergistic effects against FL and greater antilymphoma effects against DLBCL than either agent alone, he added.
In a multicenter, open-label, phase 1b dose-escalation and expansion study, investigators enrolled 19 patients with FL or MZL for whom at least one prior regimen had failed and 19 patients with relapsed/refractory DLBCL following at least two prior regimens and failed autologous stem cell transplant.
The patients received oral CC-122 at different dose levels for 5 of 7 days in each 28 day treatment cycle, plus intravenous obinutuzumab 1000 mg on days 2, 8, and 15 of cycle 1 and day 1 of cycles 2 through 8.
Responses were assessed according to International Working Group 2007 revised response criteria for malignant lymphoma.
Among all 38 patients, 25 (66%) had a response. Responses consisted of 12 CR (3 in patients with DLBCL, and 9 in patients with FL/MZL) and 13 partial responses (six and seven patients, respectively),
The median time to best response was 57 days. Responses were seen in 23 of the 30 patients who received CC-122 at dose level of 3 mg or higher.
“To date, patients receiving CC-122 at a dose of 3 mg and higher have the best and more durable responses to CC-122 plus obinutuzumab,” Dr. Michot said.
Patients generally tolerated the combination well. The most common grade 3 or 4 adverse events were hematologic and included grade 4 febrile neutropenia in two patients. Two patients discontinued treatment because of adverse events.
There was a dose-limiting toxicity, grade 4 neutropenia in one patient who received CC-122 at the 3 mg dose level, and one death from a tumor flare reaction in a patient treated at the 4 mg dose level.
The dose-escalation arm of the study has completed, and investigators are enrolling patients in a dose expansion phase at the 3 mg level.
The study was sponsored by Celgene. Hoffman La-Roche contributed obinutuzumab for the study. Dr. Michot reported serving as an advisor to Bristol-Myers Squibb and receiving travel grants from BMS, Pfizer, and Roche. Seven coauthors are Celgene employees and stockholders.
Lugano, Switzerland – A combination of obinutuzumab (Gazyva) and the experimental immunomodulatory agent CC-122 showed “clinically meaningful” activity against relapsed/refractory diffuse large B cell lymphoma (DLBCL) and indolent non-Hodgkin lymphoma (NHL) in a phase 1b study.
Among 38 patients with heavily pretreated, relapsed/refractory DLBCL, follicular lymphoma (FL), or marginal zone lymphoma (MZL), the overall response rate was 66%, including 12 patients (32%) with a complete response (CR), reported Jean-Marie Michot, MD, from the Goustave-Roussy Cancer Center in Villejuif, France.
CC-122 is a thalidomide analog that shares a molecular target with its cousin lenalidomide (Revlimid). Both molecules bind to the protein cereblon to cause degradation of the lymphoid transcription factors Aiolos and Ikaros.
As a single agent, CC-122 has been shown to have immunomodulatory effects on T-cell and natural killer (NK)–cell functions and has shown clinical activity in heavily pretreated patients with relapsed refractory NHL, including various cell-of-origin–based DLBCL subtypes, Dr, Michot said.
In preclinical studies, the combination of CC-122 and obinutuzumab, an anti-CD20 monoclonal antibody, has shown synergistic effects against FL and greater antilymphoma effects against DLBCL than either agent alone, he added.
In a multicenter, open-label, phase 1b dose-escalation and expansion study, investigators enrolled 19 patients with FL or MZL for whom at least one prior regimen had failed and 19 patients with relapsed/refractory DLBCL following at least two prior regimens and failed autologous stem cell transplant.
The patients received oral CC-122 at different dose levels for 5 of 7 days in each 28 day treatment cycle, plus intravenous obinutuzumab 1000 mg on days 2, 8, and 15 of cycle 1 and day 1 of cycles 2 through 8.
Responses were assessed according to International Working Group 2007 revised response criteria for malignant lymphoma.
Among all 38 patients, 25 (66%) had a response. Responses consisted of 12 CR (3 in patients with DLBCL, and 9 in patients with FL/MZL) and 13 partial responses (six and seven patients, respectively),
The median time to best response was 57 days. Responses were seen in 23 of the 30 patients who received CC-122 at dose level of 3 mg or higher.
“To date, patients receiving CC-122 at a dose of 3 mg and higher have the best and more durable responses to CC-122 plus obinutuzumab,” Dr. Michot said.
Patients generally tolerated the combination well. The most common grade 3 or 4 adverse events were hematologic and included grade 4 febrile neutropenia in two patients. Two patients discontinued treatment because of adverse events.
There was a dose-limiting toxicity, grade 4 neutropenia in one patient who received CC-122 at the 3 mg dose level, and one death from a tumor flare reaction in a patient treated at the 4 mg dose level.
The dose-escalation arm of the study has completed, and investigators are enrolling patients in a dose expansion phase at the 3 mg level.
The study was sponsored by Celgene. Hoffman La-Roche contributed obinutuzumab for the study. Dr. Michot reported serving as an advisor to Bristol-Myers Squibb and receiving travel grants from BMS, Pfizer, and Roche. Seven coauthors are Celgene employees and stockholders.
Lugano, Switzerland – A combination of obinutuzumab (Gazyva) and the experimental immunomodulatory agent CC-122 showed “clinically meaningful” activity against relapsed/refractory diffuse large B cell lymphoma (DLBCL) and indolent non-Hodgkin lymphoma (NHL) in a phase 1b study.
Among 38 patients with heavily pretreated, relapsed/refractory DLBCL, follicular lymphoma (FL), or marginal zone lymphoma (MZL), the overall response rate was 66%, including 12 patients (32%) with a complete response (CR), reported Jean-Marie Michot, MD, from the Goustave-Roussy Cancer Center in Villejuif, France.
CC-122 is a thalidomide analog that shares a molecular target with its cousin lenalidomide (Revlimid). Both molecules bind to the protein cereblon to cause degradation of the lymphoid transcription factors Aiolos and Ikaros.
As a single agent, CC-122 has been shown to have immunomodulatory effects on T-cell and natural killer (NK)–cell functions and has shown clinical activity in heavily pretreated patients with relapsed refractory NHL, including various cell-of-origin–based DLBCL subtypes, Dr, Michot said.
In preclinical studies, the combination of CC-122 and obinutuzumab, an anti-CD20 monoclonal antibody, has shown synergistic effects against FL and greater antilymphoma effects against DLBCL than either agent alone, he added.
In a multicenter, open-label, phase 1b dose-escalation and expansion study, investigators enrolled 19 patients with FL or MZL for whom at least one prior regimen had failed and 19 patients with relapsed/refractory DLBCL following at least two prior regimens and failed autologous stem cell transplant.
The patients received oral CC-122 at different dose levels for 5 of 7 days in each 28 day treatment cycle, plus intravenous obinutuzumab 1000 mg on days 2, 8, and 15 of cycle 1 and day 1 of cycles 2 through 8.
Responses were assessed according to International Working Group 2007 revised response criteria for malignant lymphoma.
Among all 38 patients, 25 (66%) had a response. Responses consisted of 12 CR (3 in patients with DLBCL, and 9 in patients with FL/MZL) and 13 partial responses (six and seven patients, respectively),
The median time to best response was 57 days. Responses were seen in 23 of the 30 patients who received CC-122 at dose level of 3 mg or higher.
“To date, patients receiving CC-122 at a dose of 3 mg and higher have the best and more durable responses to CC-122 plus obinutuzumab,” Dr. Michot said.
Patients generally tolerated the combination well. The most common grade 3 or 4 adverse events were hematologic and included grade 4 febrile neutropenia in two patients. Two patients discontinued treatment because of adverse events.
There was a dose-limiting toxicity, grade 4 neutropenia in one patient who received CC-122 at the 3 mg dose level, and one death from a tumor flare reaction in a patient treated at the 4 mg dose level.
The dose-escalation arm of the study has completed, and investigators are enrolling patients in a dose expansion phase at the 3 mg level.
The study was sponsored by Celgene. Hoffman La-Roche contributed obinutuzumab for the study. Dr. Michot reported serving as an advisor to Bristol-Myers Squibb and receiving travel grants from BMS, Pfizer, and Roche. Seven coauthors are Celgene employees and stockholders.
AT 14-ICML
Key clinical point: A combination of the experimental immunomodulator CC-122 and obinutuzumab showed significant activity against relapsed/refractory non-Hodgkin lymphoma.
Major finding: The overall response rate was 66%, including 32% complete responses.
Data source: A multicenter open-label phase 1b dose-escalation study in 19 patients with DLBCL and 19 with follicular lymphoma or marginal zone lymphoma.
Disclosures: The study was sponsored by Celgene. Hoffman La-Roche contributed obinutuzumab for the study. Dr. Michot reported serving as an advisor to Bristol-Myers Squibb and receiving travel grants from BMS, Pfizer, and Roche. Seven coauthors are Celgene employees and stockholders.
Chemo-free induction in MCL keeps getting better
Lugano, Switzerland – It’s not the end of chemotherapy for young patients with newly diagnosed mantle cell lymphoma (MCL), but it’s a start.
For these patients, induction with a combination of ibrutinib and rituximab, followed by shorter cycles of chemoimmunotherapy, was associated in an early study with an objective response rate of 100%, including 90% complete responses (CR), reported Michael Wang, MD, of the University of Texas MD Anderson Cancer Center in Houston.
“This is the first time for a chemo-free therapy – ibrutinib/rituximab – to achieve an overall response rate of 100%. This has an unprecedented efficacy in the frontline in young patients with mantle-cell lymphoma,” he said at the 14th International Congress on Malignant Lymphoma.
In patients with relapsed or refractory MCL, the combination of ibrutinib and rituximab has been associated with durable responses in 88% of patients. The success of the combination suggests that fit patients younger than age 65 years with newly diagnosed MCL might benefit from a chemotherapy-free induction regimen with ibrutinib and rituximab, followed by consolidation with a short but intense course of chemoimmunotherapy, Dr. Wang said.
He presented updated results from the phase II Window I study, first results of which were reported at the 2016 meeting of the American Society of Hematology.
“Frontline therapy is the most important therapy for mantle cell lymphoma, because mantle cell lymphoma cells are most vulnerable to frontline attack. If the frontline therapy is good enough, it could kill all the mantle cell lymphoma cells, therefore leaving no chance for secondary resistance, and thereby (resulting in) long-term survival. And it is really my belief that if we ideally optimized the frontline therapy, that would be a shortcut to a cure,” he said.
To test this idea, Dr. Wang and MD Anderson colleagues initiated a phase II trial at their institution with 50 patients age 65 years or under with newly diagnosed, CD20-positive and Cyclin D1-positive MCL.
A total of 50 patients age 65 years or younger (median age 54) with newly diagnosed, untreated MCL underwent induction with continuous daily ibrutinib 560 mg, plus rituximab 375 mg/m2 administered weekly for 4 weeks during cycle 1 and on day 1 of cycles 3-12. Consolidation consisted of rituximab plus hyper-CVAD (hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone), alternating every 28 days with rituximab plus high-dose methotrexate–cytarabine.
Patients who had complete responses to induction received four cycles of chemoimmunotherapy, while those who experienced disease progression and those who had partial responses received chemoimmunotherapy for two cycles beyond the point of complete remissions.
At the time of the presentation, all 50 patients were evaluable for the induction phase (part 2), and 47 were evaluable for both induction and consolidation (part 2) .Of the evaluable patients, the overall response rate (ORR) to chemotherapy-free induction therapy alone (Part 1 ) was 100% (50), with CR in 90% of patients and partial responses (PR) in 10%. Of the 47 patients evaluable for part 2 (chemoimmunotherapy), all had CRs, for an ORR of 100%.
Dr. Wang noted that one patient had a dramatic radiographic reduction in spleen size following just two cycles of chemotherapy-free induction, and two other patients had similar reductions after four and six cycles, respectively.
After a median follow-up of 15.9 months, neither the median duration of response, progression-free survival, nor overall survival have been reached. There have been no deaths and only one case of disease progression after one year of therapy.
The patients generally tolerated the regimen very well, Dr. Wang said. There were no cases of lymphocytosis, bleeding, or atrial fibrillation after 332 combined cycles.
Nonhematological adverse events were primarily grade 1 or 2. Grade 3 fatigue was reported in approximately 10% of patients. There were no grade 4 adverse events.
“This study may provide a window of opportunity to reduce the frontline therapies and reduce the long-term toxicities such as secondary malignancies.” Dr. Wang said.
He acknowledged that four cycles of intensive chemotherapy is still toxic and that further efforts to reduce these toxicities are needed. The investigators are currently planning the Window II study, in which a fraction of patients will be treated with no chemotherapy at all, he said.
The study was supported by Pharmacyclics and Janssen. Dr. Wang disclosed receiving research grants and honoraria and serving as a consultant for the companies.
Lugano, Switzerland – It’s not the end of chemotherapy for young patients with newly diagnosed mantle cell lymphoma (MCL), but it’s a start.
For these patients, induction with a combination of ibrutinib and rituximab, followed by shorter cycles of chemoimmunotherapy, was associated in an early study with an objective response rate of 100%, including 90% complete responses (CR), reported Michael Wang, MD, of the University of Texas MD Anderson Cancer Center in Houston.
“This is the first time for a chemo-free therapy – ibrutinib/rituximab – to achieve an overall response rate of 100%. This has an unprecedented efficacy in the frontline in young patients with mantle-cell lymphoma,” he said at the 14th International Congress on Malignant Lymphoma.
In patients with relapsed or refractory MCL, the combination of ibrutinib and rituximab has been associated with durable responses in 88% of patients. The success of the combination suggests that fit patients younger than age 65 years with newly diagnosed MCL might benefit from a chemotherapy-free induction regimen with ibrutinib and rituximab, followed by consolidation with a short but intense course of chemoimmunotherapy, Dr. Wang said.
He presented updated results from the phase II Window I study, first results of which were reported at the 2016 meeting of the American Society of Hematology.
“Frontline therapy is the most important therapy for mantle cell lymphoma, because mantle cell lymphoma cells are most vulnerable to frontline attack. If the frontline therapy is good enough, it could kill all the mantle cell lymphoma cells, therefore leaving no chance for secondary resistance, and thereby (resulting in) long-term survival. And it is really my belief that if we ideally optimized the frontline therapy, that would be a shortcut to a cure,” he said.
To test this idea, Dr. Wang and MD Anderson colleagues initiated a phase II trial at their institution with 50 patients age 65 years or under with newly diagnosed, CD20-positive and Cyclin D1-positive MCL.
A total of 50 patients age 65 years or younger (median age 54) with newly diagnosed, untreated MCL underwent induction with continuous daily ibrutinib 560 mg, plus rituximab 375 mg/m2 administered weekly for 4 weeks during cycle 1 and on day 1 of cycles 3-12. Consolidation consisted of rituximab plus hyper-CVAD (hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone), alternating every 28 days with rituximab plus high-dose methotrexate–cytarabine.
Patients who had complete responses to induction received four cycles of chemoimmunotherapy, while those who experienced disease progression and those who had partial responses received chemoimmunotherapy for two cycles beyond the point of complete remissions.
At the time of the presentation, all 50 patients were evaluable for the induction phase (part 2), and 47 were evaluable for both induction and consolidation (part 2) .Of the evaluable patients, the overall response rate (ORR) to chemotherapy-free induction therapy alone (Part 1 ) was 100% (50), with CR in 90% of patients and partial responses (PR) in 10%. Of the 47 patients evaluable for part 2 (chemoimmunotherapy), all had CRs, for an ORR of 100%.
Dr. Wang noted that one patient had a dramatic radiographic reduction in spleen size following just two cycles of chemotherapy-free induction, and two other patients had similar reductions after four and six cycles, respectively.
After a median follow-up of 15.9 months, neither the median duration of response, progression-free survival, nor overall survival have been reached. There have been no deaths and only one case of disease progression after one year of therapy.
The patients generally tolerated the regimen very well, Dr. Wang said. There were no cases of lymphocytosis, bleeding, or atrial fibrillation after 332 combined cycles.
Nonhematological adverse events were primarily grade 1 or 2. Grade 3 fatigue was reported in approximately 10% of patients. There were no grade 4 adverse events.
“This study may provide a window of opportunity to reduce the frontline therapies and reduce the long-term toxicities such as secondary malignancies.” Dr. Wang said.
He acknowledged that four cycles of intensive chemotherapy is still toxic and that further efforts to reduce these toxicities are needed. The investigators are currently planning the Window II study, in which a fraction of patients will be treated with no chemotherapy at all, he said.
The study was supported by Pharmacyclics and Janssen. Dr. Wang disclosed receiving research grants and honoraria and serving as a consultant for the companies.
Lugano, Switzerland – It’s not the end of chemotherapy for young patients with newly diagnosed mantle cell lymphoma (MCL), but it’s a start.
For these patients, induction with a combination of ibrutinib and rituximab, followed by shorter cycles of chemoimmunotherapy, was associated in an early study with an objective response rate of 100%, including 90% complete responses (CR), reported Michael Wang, MD, of the University of Texas MD Anderson Cancer Center in Houston.
“This is the first time for a chemo-free therapy – ibrutinib/rituximab – to achieve an overall response rate of 100%. This has an unprecedented efficacy in the frontline in young patients with mantle-cell lymphoma,” he said at the 14th International Congress on Malignant Lymphoma.
In patients with relapsed or refractory MCL, the combination of ibrutinib and rituximab has been associated with durable responses in 88% of patients. The success of the combination suggests that fit patients younger than age 65 years with newly diagnosed MCL might benefit from a chemotherapy-free induction regimen with ibrutinib and rituximab, followed by consolidation with a short but intense course of chemoimmunotherapy, Dr. Wang said.
He presented updated results from the phase II Window I study, first results of which were reported at the 2016 meeting of the American Society of Hematology.
“Frontline therapy is the most important therapy for mantle cell lymphoma, because mantle cell lymphoma cells are most vulnerable to frontline attack. If the frontline therapy is good enough, it could kill all the mantle cell lymphoma cells, therefore leaving no chance for secondary resistance, and thereby (resulting in) long-term survival. And it is really my belief that if we ideally optimized the frontline therapy, that would be a shortcut to a cure,” he said.
To test this idea, Dr. Wang and MD Anderson colleagues initiated a phase II trial at their institution with 50 patients age 65 years or under with newly diagnosed, CD20-positive and Cyclin D1-positive MCL.
A total of 50 patients age 65 years or younger (median age 54) with newly diagnosed, untreated MCL underwent induction with continuous daily ibrutinib 560 mg, plus rituximab 375 mg/m2 administered weekly for 4 weeks during cycle 1 and on day 1 of cycles 3-12. Consolidation consisted of rituximab plus hyper-CVAD (hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone), alternating every 28 days with rituximab plus high-dose methotrexate–cytarabine.
Patients who had complete responses to induction received four cycles of chemoimmunotherapy, while those who experienced disease progression and those who had partial responses received chemoimmunotherapy for two cycles beyond the point of complete remissions.
At the time of the presentation, all 50 patients were evaluable for the induction phase (part 2), and 47 were evaluable for both induction and consolidation (part 2) .Of the evaluable patients, the overall response rate (ORR) to chemotherapy-free induction therapy alone (Part 1 ) was 100% (50), with CR in 90% of patients and partial responses (PR) in 10%. Of the 47 patients evaluable for part 2 (chemoimmunotherapy), all had CRs, for an ORR of 100%.
Dr. Wang noted that one patient had a dramatic radiographic reduction in spleen size following just two cycles of chemotherapy-free induction, and two other patients had similar reductions after four and six cycles, respectively.
After a median follow-up of 15.9 months, neither the median duration of response, progression-free survival, nor overall survival have been reached. There have been no deaths and only one case of disease progression after one year of therapy.
The patients generally tolerated the regimen very well, Dr. Wang said. There were no cases of lymphocytosis, bleeding, or atrial fibrillation after 332 combined cycles.
Nonhematological adverse events were primarily grade 1 or 2. Grade 3 fatigue was reported in approximately 10% of patients. There were no grade 4 adverse events.
“This study may provide a window of opportunity to reduce the frontline therapies and reduce the long-term toxicities such as secondary malignancies.” Dr. Wang said.
He acknowledged that four cycles of intensive chemotherapy is still toxic and that further efforts to reduce these toxicities are needed. The investigators are currently planning the Window II study, in which a fraction of patients will be treated with no chemotherapy at all, he said.
The study was supported by Pharmacyclics and Janssen. Dr. Wang disclosed receiving research grants and honoraria and serving as a consultant for the companies.
AT14-ICML
Key clinical point: A chemotherapy-free induction regimen with ibrutinib and rituximab was associated with high response rates in patients with newly diagnosed mantle cell lymphoma (MCL).
Major finding: The overall response rate after induction was 100%, including 90% complete responses.
Data source: Update results from phase II investigator-initiated study in 50 patients aged 65 years and younger with MCL.
Disclosures: The study was supported by Pharmacyclics and Janssen. Dr. Wang disclosed receiving research grants and honoraria and serving as a consultant for the companies.
Bendamustine plus rituximab may have edge for treating indolent NHL, MCL
CHICAGO – Overall survival was comparable at 5 years of follow up for three regimens in treatment-naive patients with indolent non-Hodgkin lymphoma (NHL) or mantle cell lymphoma (MCL), based on long-term results from the BRIGHT study.
While progression-free survival, event-free survival, and duration of response were significantly better with bendamustine plus rituximab (BR), overall survival at 5 years did not significantly differ in patients given this regimen and compared to patients given rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or rituximab with cyclophosphamide, vincristine and prednisone (R-CVP), Ian Flinn, MD, of Tennessee Oncology, Nashville, reported at the annual meeting of the American Society of Clinical Oncology.
Quality of life was somewhat better for the patients given BR, but those patients were also at higher risk for secondary malignancies (42 vs. 24), most of which were squamous cell carcinomas, observed Dr. Kahl, professor of medicine at Washington University, St. Louis.
In BRIGHT, 224 treatment-naive patients with indolent NHL or MCL were randomized to receive BR and were compared to 223 similar patients who received either R-CHOP (104 patients) or R-CVP (119 patients). At least six cycles of therapy were completed by 203 patients in the BR group and by 196 in the R-CHOP/R-CVP group. Rituximab maintenance therapy was given to 43% of the BR group and to 45% of the R-CHOP/R-CVP group.
For BR and R-CHOP/R-CVP, the 5-year progression-free survival rate was 65.5% (95% CI, 58.5-71.6) and 55.8% (95% CI, 48.4-62.5), respectively. The overall survival rate for the entire patient group was 81.7% (75.7-86.3) and 85% (79.3-89.3) respectively. Comparing BR and R-CHOP/R-CVP, the hazard ratio (95% CI) for progression-free survival was 0.61 (0.45-0.85; P = .0025), the HR for event-free survival was 0.63 (0.46-0.84; P = .0020), the HR for duration of response was 0.66 (0.47-0.92; P = .0134), and the HR for overall survival was 1.15 (0.72-1.84; P = .5461).
Similar results were found in indolent NHL (progression-free survival 0.70 [0.49-1.01; P = .0582]) and MCL (progression-free survival 0.40 [0.21-0.75; P = .0035]), with the strongest effect in MCL, Dr. Flinn said.
Dr. Kahl noted that the advantages for the BR regimen include that it is not associated with alopecia, neuropathy, or steroid issues, and that it may extend progression-free survival and time to next treatment. On the other hand, R-CHOP is associated with less GI toxicity, rash, opportunistic infections, and prolonged cytopenia. Also, the BR regimen was associated with a higher risk of secondary cancers, primarily squamous cell carcinomas.
There were 42 secondary malignancies in the BR group and 24 in the R-CHOP/R-CVP group, Dr. Flinn reported.
It is theoretically possible that BR equals R-CHOP plus maintenance therapy from an efficacy perspective, Dr. Kahl said.
As virtually all excess adverse event fatalities occurred during maintenance therapy, it is possible that maintenance therapy after BR “does more harm than good.” This high priority issue “should be evaluated in the BRIGHT data set,” Dr. Kahl recommended.
Teva Branded Pharmaceutical Products R&D sponsored the study. Dr. Flinn had no relationships to disclose; two of his fellow researchers are Teva employees. Dr. Kahl disclosed serving as an adviser or consultant to Abbvie, Acerta Pharma, Celgene, Cell Therapeutics, Genentech/Roche, Incyte, Infinity Pharmaceuticals, Juno Therapeutics, Millennium, Pharmacyclics, Sandoz, and Seattle Genetics.
mdales@frontlinemedcom.com
On Twitter @maryjodales
CHICAGO – Overall survival was comparable at 5 years of follow up for three regimens in treatment-naive patients with indolent non-Hodgkin lymphoma (NHL) or mantle cell lymphoma (MCL), based on long-term results from the BRIGHT study.
While progression-free survival, event-free survival, and duration of response were significantly better with bendamustine plus rituximab (BR), overall survival at 5 years did not significantly differ in patients given this regimen and compared to patients given rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or rituximab with cyclophosphamide, vincristine and prednisone (R-CVP), Ian Flinn, MD, of Tennessee Oncology, Nashville, reported at the annual meeting of the American Society of Clinical Oncology.
Quality of life was somewhat better for the patients given BR, but those patients were also at higher risk for secondary malignancies (42 vs. 24), most of which were squamous cell carcinomas, observed Dr. Kahl, professor of medicine at Washington University, St. Louis.
In BRIGHT, 224 treatment-naive patients with indolent NHL or MCL were randomized to receive BR and were compared to 223 similar patients who received either R-CHOP (104 patients) or R-CVP (119 patients). At least six cycles of therapy were completed by 203 patients in the BR group and by 196 in the R-CHOP/R-CVP group. Rituximab maintenance therapy was given to 43% of the BR group and to 45% of the R-CHOP/R-CVP group.
For BR and R-CHOP/R-CVP, the 5-year progression-free survival rate was 65.5% (95% CI, 58.5-71.6) and 55.8% (95% CI, 48.4-62.5), respectively. The overall survival rate for the entire patient group was 81.7% (75.7-86.3) and 85% (79.3-89.3) respectively. Comparing BR and R-CHOP/R-CVP, the hazard ratio (95% CI) for progression-free survival was 0.61 (0.45-0.85; P = .0025), the HR for event-free survival was 0.63 (0.46-0.84; P = .0020), the HR for duration of response was 0.66 (0.47-0.92; P = .0134), and the HR for overall survival was 1.15 (0.72-1.84; P = .5461).
Similar results were found in indolent NHL (progression-free survival 0.70 [0.49-1.01; P = .0582]) and MCL (progression-free survival 0.40 [0.21-0.75; P = .0035]), with the strongest effect in MCL, Dr. Flinn said.
Dr. Kahl noted that the advantages for the BR regimen include that it is not associated with alopecia, neuropathy, or steroid issues, and that it may extend progression-free survival and time to next treatment. On the other hand, R-CHOP is associated with less GI toxicity, rash, opportunistic infections, and prolonged cytopenia. Also, the BR regimen was associated with a higher risk of secondary cancers, primarily squamous cell carcinomas.
There were 42 secondary malignancies in the BR group and 24 in the R-CHOP/R-CVP group, Dr. Flinn reported.
It is theoretically possible that BR equals R-CHOP plus maintenance therapy from an efficacy perspective, Dr. Kahl said.
As virtually all excess adverse event fatalities occurred during maintenance therapy, it is possible that maintenance therapy after BR “does more harm than good.” This high priority issue “should be evaluated in the BRIGHT data set,” Dr. Kahl recommended.
Teva Branded Pharmaceutical Products R&D sponsored the study. Dr. Flinn had no relationships to disclose; two of his fellow researchers are Teva employees. Dr. Kahl disclosed serving as an adviser or consultant to Abbvie, Acerta Pharma, Celgene, Cell Therapeutics, Genentech/Roche, Incyte, Infinity Pharmaceuticals, Juno Therapeutics, Millennium, Pharmacyclics, Sandoz, and Seattle Genetics.
mdales@frontlinemedcom.com
On Twitter @maryjodales
CHICAGO – Overall survival was comparable at 5 years of follow up for three regimens in treatment-naive patients with indolent non-Hodgkin lymphoma (NHL) or mantle cell lymphoma (MCL), based on long-term results from the BRIGHT study.
While progression-free survival, event-free survival, and duration of response were significantly better with bendamustine plus rituximab (BR), overall survival at 5 years did not significantly differ in patients given this regimen and compared to patients given rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) or rituximab with cyclophosphamide, vincristine and prednisone (R-CVP), Ian Flinn, MD, of Tennessee Oncology, Nashville, reported at the annual meeting of the American Society of Clinical Oncology.
Quality of life was somewhat better for the patients given BR, but those patients were also at higher risk for secondary malignancies (42 vs. 24), most of which were squamous cell carcinomas, observed Dr. Kahl, professor of medicine at Washington University, St. Louis.
In BRIGHT, 224 treatment-naive patients with indolent NHL or MCL were randomized to receive BR and were compared to 223 similar patients who received either R-CHOP (104 patients) or R-CVP (119 patients). At least six cycles of therapy were completed by 203 patients in the BR group and by 196 in the R-CHOP/R-CVP group. Rituximab maintenance therapy was given to 43% of the BR group and to 45% of the R-CHOP/R-CVP group.
For BR and R-CHOP/R-CVP, the 5-year progression-free survival rate was 65.5% (95% CI, 58.5-71.6) and 55.8% (95% CI, 48.4-62.5), respectively. The overall survival rate for the entire patient group was 81.7% (75.7-86.3) and 85% (79.3-89.3) respectively. Comparing BR and R-CHOP/R-CVP, the hazard ratio (95% CI) for progression-free survival was 0.61 (0.45-0.85; P = .0025), the HR for event-free survival was 0.63 (0.46-0.84; P = .0020), the HR for duration of response was 0.66 (0.47-0.92; P = .0134), and the HR for overall survival was 1.15 (0.72-1.84; P = .5461).
Similar results were found in indolent NHL (progression-free survival 0.70 [0.49-1.01; P = .0582]) and MCL (progression-free survival 0.40 [0.21-0.75; P = .0035]), with the strongest effect in MCL, Dr. Flinn said.
Dr. Kahl noted that the advantages for the BR regimen include that it is not associated with alopecia, neuropathy, or steroid issues, and that it may extend progression-free survival and time to next treatment. On the other hand, R-CHOP is associated with less GI toxicity, rash, opportunistic infections, and prolonged cytopenia. Also, the BR regimen was associated with a higher risk of secondary cancers, primarily squamous cell carcinomas.
There were 42 secondary malignancies in the BR group and 24 in the R-CHOP/R-CVP group, Dr. Flinn reported.
It is theoretically possible that BR equals R-CHOP plus maintenance therapy from an efficacy perspective, Dr. Kahl said.
As virtually all excess adverse event fatalities occurred during maintenance therapy, it is possible that maintenance therapy after BR “does more harm than good.” This high priority issue “should be evaluated in the BRIGHT data set,” Dr. Kahl recommended.
Teva Branded Pharmaceutical Products R&D sponsored the study. Dr. Flinn had no relationships to disclose; two of his fellow researchers are Teva employees. Dr. Kahl disclosed serving as an adviser or consultant to Abbvie, Acerta Pharma, Celgene, Cell Therapeutics, Genentech/Roche, Incyte, Infinity Pharmaceuticals, Juno Therapeutics, Millennium, Pharmacyclics, Sandoz, and Seattle Genetics.
mdales@frontlinemedcom.com
On Twitter @maryjodales
AT ASCO 2017
Key clinical point:
Major finding: For BR and R-CHOP/R-CVP, the 5-year progression-free survival rate was 65.5% (95% CI, 58.5-71.6) and 55.8% (95% CI, 48.4-62.5), respectively.
Data source: In BRIGHT, 224 treatment-naive patients with indolent non-Hodgkin lymphoma or mantle cell lymphoma were randomized to receive BR and were compared to 223 similar patients who received either R-CHOP (104 patients) or R-CVP (119 patients).
Disclosures: Teva Branded Pharmaceutical Products R&D sponsored the study. Dr. Flinn had no relationships to disclose; two of his fellow researchers are Teva employees. Dr. Kahl disclosed serving as an adviser or consultant to Abbvie, Acerta Pharma, Celgene, Cell Therapeutics, Genentech/Roche, Incyte, Infinity Pharmaceuticals, Juno Therapeutics, Millennium, Pharmacyclics, Sandoz, and Seattle Genetics.
Hitting BTK, PI3K pays off in B-cell malignancies
LUGANO, SWITZERLAND – A combination of ibrutinib and umbralisib, an investigational inhibitor of phosphatidylinostiol 3-kinase (PI3K), induced high response rates in patients with relapsed/refractory B-cell malignancies, with no dose-limiting toxicities, based on updated early efficacy results from a phase I/IB dose-escalation study.
One-year progression-free survival (PFS) was 88% for patients with chronic lymphocytic leukemia (CLL), and 1-year overall survival (OS) was 94%, reported Matthew S. Davids, MD, MMSc, of the Dana-Farber Cancer Institute in Boston.
Single-agent ibrutinib (Imbruvica), an inhibitor of Bruton’s tyrosine kinase, is effective in patients with high-risk CLL or MCL, but the depth and durability of response are limited, he said. Umbralisib (TGR-1202) is a second-generation PI3K inhibitor with a high degree of specificity for the delta isoform of the kinase. It was designed to have a better safety profile than the first-in-class agent idelalisib (Zydelig).
“We hypothesized that inhibiting multiple BCR [B-cell receptor] pathways with kinase inhibitors may both deepen and prolong response and potentially overcome resistance mutations,” he said at the International Conference on Malignant Lymphoma.
In an ongoing, investigator-initiated phase I/IB trial, Dr. Davids and his colleagues enrolled 14 patients with MCL and 18 with CLL into parallel dose-escalation arms. Data were insufficient for the preliminary efficacy analysis.
Among patients with CLL, the objective response rate was 94% (16 of 17 patients). Of the 17 patients, 15 had a partial response or a partial response with lymphocytosis. One patient had a complete response, and three had radiographic complete responses, but these were not included in the objective response rate.
All three patients who had prior exposure to a PI3K inhibitor had responses, as did one of two patients with prior ibrutinib exposure.
For the patients with MCL, the objective response rate was 79% (11 of 14 patients); 10 had a partial response and 1 had a complete response. One other patient with a radiographic complete response was not included in the objective response rate.
Median follow-up among survivors was 14 months. As noted, the 1-year PFS and OS for patients with CLL were 88% and 94%, and the median PFS and OS for patients with MCL were 8.4 and 11.6 months.
One patient with CLL and five with MCL died of disease progression. A sixth patient with MCL did not have an adequate response to ibrutinib/umbralisib and died of toxicities related to the next line of therapy.
The safety analysis showed no dose-limiting toxicities, and the maximum tolerated dose was not identified with umbralisib at doses of 400 mg, 600 mg, or 800 mg daily in patients with either CLL or MCL.
The most common hematologic adverse events were grade 3/4 neutropenia in approximately 37% of patients in each arm, thrombocytopenia in 11% of CLL patients and 36% of MCL patients, and anemia in 15% and 29%, respectively.
The MCL arm of the study is still accruing patients, and correlative studies are in progress, Dr. Davids said.
The study is supported by TG Therapeutics, BCRP/LLS TAP, and grants from ASCO and the National Institutes of Health. Dr. Davids disclosed honoraria from Janssen and research funding to his institution from Phamarcyclics.
LUGANO, SWITZERLAND – A combination of ibrutinib and umbralisib, an investigational inhibitor of phosphatidylinostiol 3-kinase (PI3K), induced high response rates in patients with relapsed/refractory B-cell malignancies, with no dose-limiting toxicities, based on updated early efficacy results from a phase I/IB dose-escalation study.
One-year progression-free survival (PFS) was 88% for patients with chronic lymphocytic leukemia (CLL), and 1-year overall survival (OS) was 94%, reported Matthew S. Davids, MD, MMSc, of the Dana-Farber Cancer Institute in Boston.
Single-agent ibrutinib (Imbruvica), an inhibitor of Bruton’s tyrosine kinase, is effective in patients with high-risk CLL or MCL, but the depth and durability of response are limited, he said. Umbralisib (TGR-1202) is a second-generation PI3K inhibitor with a high degree of specificity for the delta isoform of the kinase. It was designed to have a better safety profile than the first-in-class agent idelalisib (Zydelig).
“We hypothesized that inhibiting multiple BCR [B-cell receptor] pathways with kinase inhibitors may both deepen and prolong response and potentially overcome resistance mutations,” he said at the International Conference on Malignant Lymphoma.
In an ongoing, investigator-initiated phase I/IB trial, Dr. Davids and his colleagues enrolled 14 patients with MCL and 18 with CLL into parallel dose-escalation arms. Data were insufficient for the preliminary efficacy analysis.
Among patients with CLL, the objective response rate was 94% (16 of 17 patients). Of the 17 patients, 15 had a partial response or a partial response with lymphocytosis. One patient had a complete response, and three had radiographic complete responses, but these were not included in the objective response rate.
All three patients who had prior exposure to a PI3K inhibitor had responses, as did one of two patients with prior ibrutinib exposure.
For the patients with MCL, the objective response rate was 79% (11 of 14 patients); 10 had a partial response and 1 had a complete response. One other patient with a radiographic complete response was not included in the objective response rate.
Median follow-up among survivors was 14 months. As noted, the 1-year PFS and OS for patients with CLL were 88% and 94%, and the median PFS and OS for patients with MCL were 8.4 and 11.6 months.
One patient with CLL and five with MCL died of disease progression. A sixth patient with MCL did not have an adequate response to ibrutinib/umbralisib and died of toxicities related to the next line of therapy.
The safety analysis showed no dose-limiting toxicities, and the maximum tolerated dose was not identified with umbralisib at doses of 400 mg, 600 mg, or 800 mg daily in patients with either CLL or MCL.
The most common hematologic adverse events were grade 3/4 neutropenia in approximately 37% of patients in each arm, thrombocytopenia in 11% of CLL patients and 36% of MCL patients, and anemia in 15% and 29%, respectively.
The MCL arm of the study is still accruing patients, and correlative studies are in progress, Dr. Davids said.
The study is supported by TG Therapeutics, BCRP/LLS TAP, and grants from ASCO and the National Institutes of Health. Dr. Davids disclosed honoraria from Janssen and research funding to his institution from Phamarcyclics.
LUGANO, SWITZERLAND – A combination of ibrutinib and umbralisib, an investigational inhibitor of phosphatidylinostiol 3-kinase (PI3K), induced high response rates in patients with relapsed/refractory B-cell malignancies, with no dose-limiting toxicities, based on updated early efficacy results from a phase I/IB dose-escalation study.
One-year progression-free survival (PFS) was 88% for patients with chronic lymphocytic leukemia (CLL), and 1-year overall survival (OS) was 94%, reported Matthew S. Davids, MD, MMSc, of the Dana-Farber Cancer Institute in Boston.
Single-agent ibrutinib (Imbruvica), an inhibitor of Bruton’s tyrosine kinase, is effective in patients with high-risk CLL or MCL, but the depth and durability of response are limited, he said. Umbralisib (TGR-1202) is a second-generation PI3K inhibitor with a high degree of specificity for the delta isoform of the kinase. It was designed to have a better safety profile than the first-in-class agent idelalisib (Zydelig).
“We hypothesized that inhibiting multiple BCR [B-cell receptor] pathways with kinase inhibitors may both deepen and prolong response and potentially overcome resistance mutations,” he said at the International Conference on Malignant Lymphoma.
In an ongoing, investigator-initiated phase I/IB trial, Dr. Davids and his colleagues enrolled 14 patients with MCL and 18 with CLL into parallel dose-escalation arms. Data were insufficient for the preliminary efficacy analysis.
Among patients with CLL, the objective response rate was 94% (16 of 17 patients). Of the 17 patients, 15 had a partial response or a partial response with lymphocytosis. One patient had a complete response, and three had radiographic complete responses, but these were not included in the objective response rate.
All three patients who had prior exposure to a PI3K inhibitor had responses, as did one of two patients with prior ibrutinib exposure.
For the patients with MCL, the objective response rate was 79% (11 of 14 patients); 10 had a partial response and 1 had a complete response. One other patient with a radiographic complete response was not included in the objective response rate.
Median follow-up among survivors was 14 months. As noted, the 1-year PFS and OS for patients with CLL were 88% and 94%, and the median PFS and OS for patients with MCL were 8.4 and 11.6 months.
One patient with CLL and five with MCL died of disease progression. A sixth patient with MCL did not have an adequate response to ibrutinib/umbralisib and died of toxicities related to the next line of therapy.
The safety analysis showed no dose-limiting toxicities, and the maximum tolerated dose was not identified with umbralisib at doses of 400 mg, 600 mg, or 800 mg daily in patients with either CLL or MCL.
The most common hematologic adverse events were grade 3/4 neutropenia in approximately 37% of patients in each arm, thrombocytopenia in 11% of CLL patients and 36% of MCL patients, and anemia in 15% and 29%, respectively.
The MCL arm of the study is still accruing patients, and correlative studies are in progress, Dr. Davids said.
The study is supported by TG Therapeutics, BCRP/LLS TAP, and grants from ASCO and the National Institutes of Health. Dr. Davids disclosed honoraria from Janssen and research funding to his institution from Phamarcyclics.
AT 14-ICML
Key clinical point:
Major finding: The objective response rate to the combination was 94% in 18 patients with chronic lymphocytic leukemia and 79% in 14 patients with mantle cell lymphoma.
Data source: A phase I/IB dose-escalation study.
Disclosures: The study is supported by TG Therapeutics, BCRP/LLS TAP, and grants from ASCO and the National Institutes of Health. Dr. Davids disclosed honoraria from Janssen and research funding to his institution from Phamarcyclics.