User login
Genotype, need for transfusion predict death in VEXAS syndrome
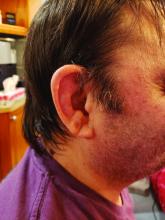
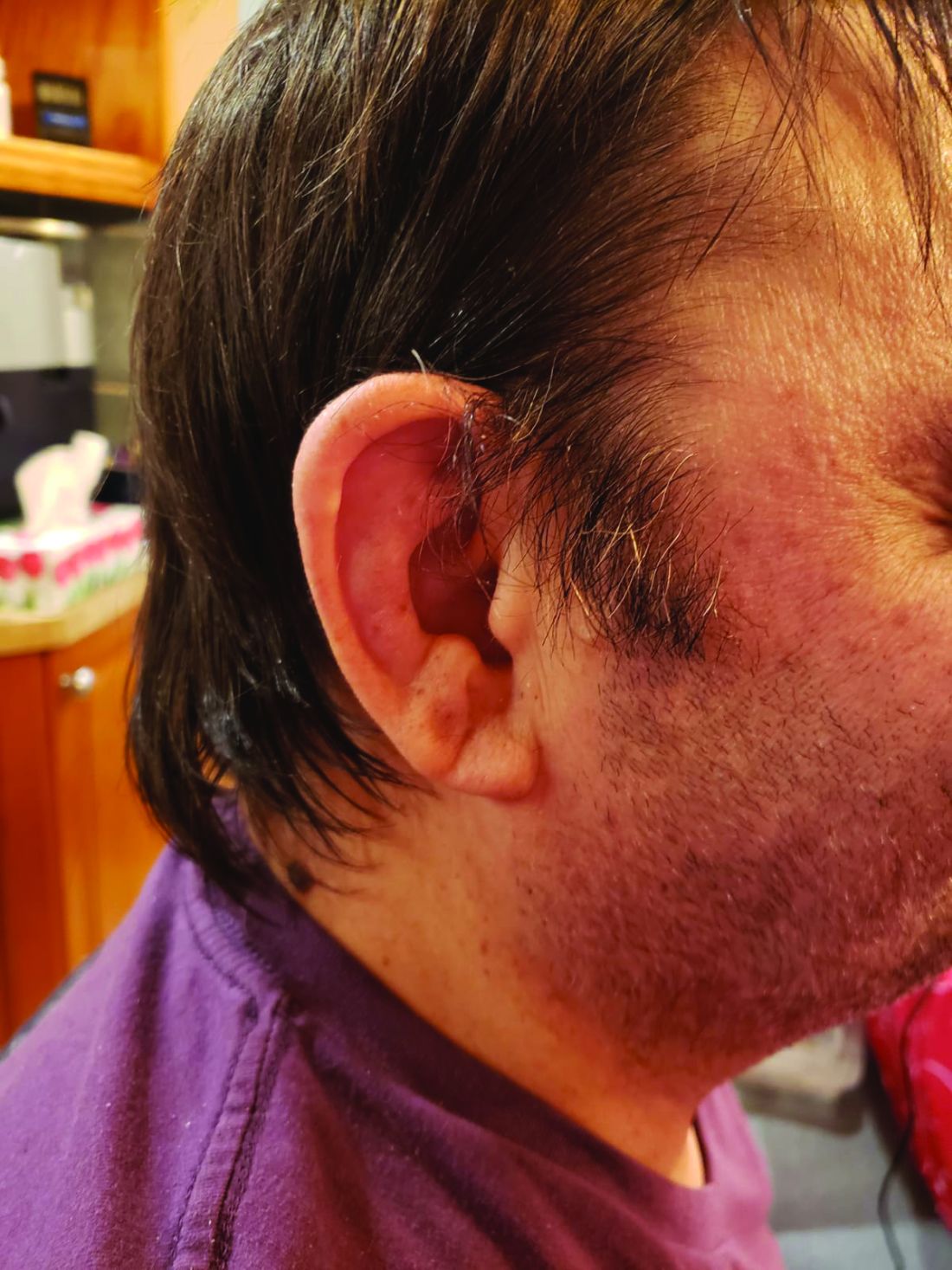
Among patients with the recently defined severe autoinflammatory syndrome VEXAS, those who are transfusion dependent or have a specific amino acid substitution are at highest risk for death, whereas those with ear chondritis are at significantly lower risk, a multinational team of investigators has found.
Their study of mortality and predictors of survival among patients with genetically confirmed VEXAS showed that patients with a VEXAS variant resulting in an amino acid substitution of a methionine for a valine had a 3.5-fold higher risk for death, compared with patients with either a methionine-to-threonine substitution or a methionine-to-leucine swap.
Transfusion dependence was an independent predictor of mortality. Patients who became dependent on transfusions after symptom onset had a nearly threefold higher risk for death, reported Marcela A. Ferrada, MD, a clinical fellow at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
“These findings should inform risk assessment and clinical management in patients with VEXAS syndrome,” she said in an oral abstract presentation during the virtual annual meeting of the American College of Rheumatology.
“These genetic findings have proven right now to be not only diagnostic, but we have shown that they’re also prognostic, and we hope that this is going to help us identify patients who could have more aggressive treatment,” Dr. Ferrada said.
She also discussed her findings in a media briefing held 2 days prior to her plenary presentation. At that briefing, this news organization asked participating clinicians whether they had patients who they suspected may have had undiagnosed VEXAS.
“My answer to that is interesting,” replied moderator Vaneet Sandhu, MD, from Loma Linda (Calif.) University and Riverside University Health System.
“In the last couple of days, I’ve been reading about VEXAS, and actually texted one of my colleagues yesterday and said, ‘Hey, you know these patients we’ve been seeing who have these strange rashes and chondritis and have maybe a diagnosis of leukocytoclastic vasculitis or something else – are we not diagnosing these patients?’ ” she said.
“I think we are looking at every patient with chondritis and reexamining their phenotype. We had dismissed certain symptoms because they didn’t fit the archetype for relapsing polychondritis, for example, but it could be VEXAS,” said Alfred Kim, MD, PhD, of Washington University in St. Louis, who also presented data during the briefing.
Three variants
VEXAS is caused by somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation.
The syndrome’s name is an acronym descriptive of the major features:
- Vacuoles in bone marrow cells.
- E-1 activating enzyme that UBA1 encodes for.
- X-linked.
- Autoinflammatory.
- Somatic mutation featuring hematologic mosaicism.
VEXAS results in rheumatologic, dermatologic, and hematologic symptoms that are often misdiagnosed as being caused by treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, giant cell arteritis, or myelodysplastic syndrome (MDS).
VEXAS was identified as a distinct syndrome within the past year by Dr. Ferrada and other investigators at NIAMS, the National Human Genome Research Institute, and other institutions.
In the study reported at ACR 2021, Dr. Ferrada and colleagues assessed 83 men who had been referred for genetic testing for VEXAS at the National Institutes of Health, in Bethesda, Md., and at Leeds (England) Teaching Hospitals NHS Trust.
All patients were confirmed to have VEXAS-defining genetic mutations in UBA1 by Sanger sequencing of peripheral blood samples. Only those patients with mutations at codon p.Met41 were included in the investigators’ analysis. Mutations at that site account for nearly all cases of VEXAS that have been identified to date.
The most common clinical manifestation of VEXAS was skin involvement, which occurred in all but one of the 83 patients. Other common manifestations included arthritis (58 patients), pulmonary infiltrates (57 patients), and ear chondritis (54 patients).
Fifteen patients were found to have the leucine variant, 18 had the valine variant, and 50 had the threonine variant. The median age at disease onset was 66 years in the leucine and threonine variant groups and 65 in the valine variant group.
The clinical diagnosis differed according to genotype: 4 of 18 patients (22%) with the valine variant were diagnosed with relapsing polychondritis, compared with 8 of 15 (53%) with the leucine variant and 31 of 50 (62%) with the threonine variant (P = .01).
In contrast, 55% of patients with valine genotype were diagnosed with undifferentiated fever, compared with 6% of those with the leucine and 16% with the threonine genotypes (P = .001). More patients with the leucine variant (60%) were diagnosed with Sweet syndrome, compared with 11% and 14% of patients with the valine and threonine variants, respectively (P = .001).
There was no significant difference among the three genotypes in the percentage of patients diagnosed with MDS.
The follow-up period ranged from 1 to 18 years (median, 4.7 years). The median survival time from disease onset for all patients was 10 years.
Among patients with the valine variant, median survival was 9 years, which was significantly less than among patients with the other two variants (P = .01).
In univariable analysis, independent predictors of mortality were ear chondritis (hazard ratio, 0.26; P = .005), transfusion dependence, a time-dependent variable (HR, 2.59; P = .03), and the valine variant (HR, 3.5; P = .008).
The association between VEXAS genotype and phenotype could be explained by the finding that, among patients with the valine variant, there was significantly less translation of the catalytically proficient UBA1b isoform than in patients with the other two variants, Dr. Ferrada said.
Therapeutic options
Dr. Ferrada noted that to date no drugs have been shown to provide consistent therapeutic benefits for patients with VEXAS, but evidence as to the etiology of the syndrome points to possible treatment approaches.
“All of these findings I think are extremely important to help us guide management of these patients, as we know that the mutation is located in the stem cells in the bone marrow. So we suspect that doing a bone marrow transplant in these patients is going to be curative,” Dr. Ferrada said during the briefing.
Investigators are planning a phase 2 trial of allogeneic hematopoietic stem cell transplant for patients with VEXAS.
The study was supported by the National Institutes of Health. Dr. Ferrada, Dr. Sandhu, and Dr. Kim have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among patients with the recently defined severe autoinflammatory syndrome VEXAS, those who are transfusion dependent or have a specific amino acid substitution are at highest risk for death, whereas those with ear chondritis are at significantly lower risk, a multinational team of investigators has found.
Their study of mortality and predictors of survival among patients with genetically confirmed VEXAS showed that patients with a VEXAS variant resulting in an amino acid substitution of a methionine for a valine had a 3.5-fold higher risk for death, compared with patients with either a methionine-to-threonine substitution or a methionine-to-leucine swap.
Transfusion dependence was an independent predictor of mortality. Patients who became dependent on transfusions after symptom onset had a nearly threefold higher risk for death, reported Marcela A. Ferrada, MD, a clinical fellow at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
“These findings should inform risk assessment and clinical management in patients with VEXAS syndrome,” she said in an oral abstract presentation during the virtual annual meeting of the American College of Rheumatology.
“These genetic findings have proven right now to be not only diagnostic, but we have shown that they’re also prognostic, and we hope that this is going to help us identify patients who could have more aggressive treatment,” Dr. Ferrada said.
She also discussed her findings in a media briefing held 2 days prior to her plenary presentation. At that briefing, this news organization asked participating clinicians whether they had patients who they suspected may have had undiagnosed VEXAS.
“My answer to that is interesting,” replied moderator Vaneet Sandhu, MD, from Loma Linda (Calif.) University and Riverside University Health System.
“In the last couple of days, I’ve been reading about VEXAS, and actually texted one of my colleagues yesterday and said, ‘Hey, you know these patients we’ve been seeing who have these strange rashes and chondritis and have maybe a diagnosis of leukocytoclastic vasculitis or something else – are we not diagnosing these patients?’ ” she said.
“I think we are looking at every patient with chondritis and reexamining their phenotype. We had dismissed certain symptoms because they didn’t fit the archetype for relapsing polychondritis, for example, but it could be VEXAS,” said Alfred Kim, MD, PhD, of Washington University in St. Louis, who also presented data during the briefing.
Three variants
VEXAS is caused by somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation.
The syndrome’s name is an acronym descriptive of the major features:
- Vacuoles in bone marrow cells.
- E-1 activating enzyme that UBA1 encodes for.
- X-linked.
- Autoinflammatory.
- Somatic mutation featuring hematologic mosaicism.
VEXAS results in rheumatologic, dermatologic, and hematologic symptoms that are often misdiagnosed as being caused by treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, giant cell arteritis, or myelodysplastic syndrome (MDS).
VEXAS was identified as a distinct syndrome within the past year by Dr. Ferrada and other investigators at NIAMS, the National Human Genome Research Institute, and other institutions.
In the study reported at ACR 2021, Dr. Ferrada and colleagues assessed 83 men who had been referred for genetic testing for VEXAS at the National Institutes of Health, in Bethesda, Md., and at Leeds (England) Teaching Hospitals NHS Trust.
All patients were confirmed to have VEXAS-defining genetic mutations in UBA1 by Sanger sequencing of peripheral blood samples. Only those patients with mutations at codon p.Met41 were included in the investigators’ analysis. Mutations at that site account for nearly all cases of VEXAS that have been identified to date.
The most common clinical manifestation of VEXAS was skin involvement, which occurred in all but one of the 83 patients. Other common manifestations included arthritis (58 patients), pulmonary infiltrates (57 patients), and ear chondritis (54 patients).
Fifteen patients were found to have the leucine variant, 18 had the valine variant, and 50 had the threonine variant. The median age at disease onset was 66 years in the leucine and threonine variant groups and 65 in the valine variant group.
The clinical diagnosis differed according to genotype: 4 of 18 patients (22%) with the valine variant were diagnosed with relapsing polychondritis, compared with 8 of 15 (53%) with the leucine variant and 31 of 50 (62%) with the threonine variant (P = .01).
In contrast, 55% of patients with valine genotype were diagnosed with undifferentiated fever, compared with 6% of those with the leucine and 16% with the threonine genotypes (P = .001). More patients with the leucine variant (60%) were diagnosed with Sweet syndrome, compared with 11% and 14% of patients with the valine and threonine variants, respectively (P = .001).
There was no significant difference among the three genotypes in the percentage of patients diagnosed with MDS.
The follow-up period ranged from 1 to 18 years (median, 4.7 years). The median survival time from disease onset for all patients was 10 years.
Among patients with the valine variant, median survival was 9 years, which was significantly less than among patients with the other two variants (P = .01).
In univariable analysis, independent predictors of mortality were ear chondritis (hazard ratio, 0.26; P = .005), transfusion dependence, a time-dependent variable (HR, 2.59; P = .03), and the valine variant (HR, 3.5; P = .008).
The association between VEXAS genotype and phenotype could be explained by the finding that, among patients with the valine variant, there was significantly less translation of the catalytically proficient UBA1b isoform than in patients with the other two variants, Dr. Ferrada said.
Therapeutic options
Dr. Ferrada noted that to date no drugs have been shown to provide consistent therapeutic benefits for patients with VEXAS, but evidence as to the etiology of the syndrome points to possible treatment approaches.
“All of these findings I think are extremely important to help us guide management of these patients, as we know that the mutation is located in the stem cells in the bone marrow. So we suspect that doing a bone marrow transplant in these patients is going to be curative,” Dr. Ferrada said during the briefing.
Investigators are planning a phase 2 trial of allogeneic hematopoietic stem cell transplant for patients with VEXAS.
The study was supported by the National Institutes of Health. Dr. Ferrada, Dr. Sandhu, and Dr. Kim have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among patients with the recently defined severe autoinflammatory syndrome VEXAS, those who are transfusion dependent or have a specific amino acid substitution are at highest risk for death, whereas those with ear chondritis are at significantly lower risk, a multinational team of investigators has found.
Their study of mortality and predictors of survival among patients with genetically confirmed VEXAS showed that patients with a VEXAS variant resulting in an amino acid substitution of a methionine for a valine had a 3.5-fold higher risk for death, compared with patients with either a methionine-to-threonine substitution or a methionine-to-leucine swap.
Transfusion dependence was an independent predictor of mortality. Patients who became dependent on transfusions after symptom onset had a nearly threefold higher risk for death, reported Marcela A. Ferrada, MD, a clinical fellow at the National Institute of Arthritis and Musculoskeletal and Skin Diseases.
“These findings should inform risk assessment and clinical management in patients with VEXAS syndrome,” she said in an oral abstract presentation during the virtual annual meeting of the American College of Rheumatology.
“These genetic findings have proven right now to be not only diagnostic, but we have shown that they’re also prognostic, and we hope that this is going to help us identify patients who could have more aggressive treatment,” Dr. Ferrada said.
She also discussed her findings in a media briefing held 2 days prior to her plenary presentation. At that briefing, this news organization asked participating clinicians whether they had patients who they suspected may have had undiagnosed VEXAS.
“My answer to that is interesting,” replied moderator Vaneet Sandhu, MD, from Loma Linda (Calif.) University and Riverside University Health System.
“In the last couple of days, I’ve been reading about VEXAS, and actually texted one of my colleagues yesterday and said, ‘Hey, you know these patients we’ve been seeing who have these strange rashes and chondritis and have maybe a diagnosis of leukocytoclastic vasculitis or something else – are we not diagnosing these patients?’ ” she said.
“I think we are looking at every patient with chondritis and reexamining their phenotype. We had dismissed certain symptoms because they didn’t fit the archetype for relapsing polychondritis, for example, but it could be VEXAS,” said Alfred Kim, MD, PhD, of Washington University in St. Louis, who also presented data during the briefing.
Three variants
VEXAS is caused by somatic mutations in UBA1, a gene that initiates cytoplasmic ubiquitylation, a process by which misfolded proteins are tagged for degradation.
The syndrome’s name is an acronym descriptive of the major features:
- Vacuoles in bone marrow cells.
- E-1 activating enzyme that UBA1 encodes for.
- X-linked.
- Autoinflammatory.
- Somatic mutation featuring hematologic mosaicism.
VEXAS results in rheumatologic, dermatologic, and hematologic symptoms that are often misdiagnosed as being caused by treatment-refractory relapsing polychondritis, polyarteritis nodosa, Sweet syndrome, giant cell arteritis, or myelodysplastic syndrome (MDS).
VEXAS was identified as a distinct syndrome within the past year by Dr. Ferrada and other investigators at NIAMS, the National Human Genome Research Institute, and other institutions.
In the study reported at ACR 2021, Dr. Ferrada and colleagues assessed 83 men who had been referred for genetic testing for VEXAS at the National Institutes of Health, in Bethesda, Md., and at Leeds (England) Teaching Hospitals NHS Trust.
All patients were confirmed to have VEXAS-defining genetic mutations in UBA1 by Sanger sequencing of peripheral blood samples. Only those patients with mutations at codon p.Met41 were included in the investigators’ analysis. Mutations at that site account for nearly all cases of VEXAS that have been identified to date.
The most common clinical manifestation of VEXAS was skin involvement, which occurred in all but one of the 83 patients. Other common manifestations included arthritis (58 patients), pulmonary infiltrates (57 patients), and ear chondritis (54 patients).
Fifteen patients were found to have the leucine variant, 18 had the valine variant, and 50 had the threonine variant. The median age at disease onset was 66 years in the leucine and threonine variant groups and 65 in the valine variant group.
The clinical diagnosis differed according to genotype: 4 of 18 patients (22%) with the valine variant were diagnosed with relapsing polychondritis, compared with 8 of 15 (53%) with the leucine variant and 31 of 50 (62%) with the threonine variant (P = .01).
In contrast, 55% of patients with valine genotype were diagnosed with undifferentiated fever, compared with 6% of those with the leucine and 16% with the threonine genotypes (P = .001). More patients with the leucine variant (60%) were diagnosed with Sweet syndrome, compared with 11% and 14% of patients with the valine and threonine variants, respectively (P = .001).
There was no significant difference among the three genotypes in the percentage of patients diagnosed with MDS.
The follow-up period ranged from 1 to 18 years (median, 4.7 years). The median survival time from disease onset for all patients was 10 years.
Among patients with the valine variant, median survival was 9 years, which was significantly less than among patients with the other two variants (P = .01).
In univariable analysis, independent predictors of mortality were ear chondritis (hazard ratio, 0.26; P = .005), transfusion dependence, a time-dependent variable (HR, 2.59; P = .03), and the valine variant (HR, 3.5; P = .008).
The association between VEXAS genotype and phenotype could be explained by the finding that, among patients with the valine variant, there was significantly less translation of the catalytically proficient UBA1b isoform than in patients with the other two variants, Dr. Ferrada said.
Therapeutic options
Dr. Ferrada noted that to date no drugs have been shown to provide consistent therapeutic benefits for patients with VEXAS, but evidence as to the etiology of the syndrome points to possible treatment approaches.
“All of these findings I think are extremely important to help us guide management of these patients, as we know that the mutation is located in the stem cells in the bone marrow. So we suspect that doing a bone marrow transplant in these patients is going to be curative,” Dr. Ferrada said during the briefing.
Investigators are planning a phase 2 trial of allogeneic hematopoietic stem cell transplant for patients with VEXAS.
The study was supported by the National Institutes of Health. Dr. Ferrada, Dr. Sandhu, and Dr. Kim have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ACR 2021
MDS: Elevated mature monocytes in bone marrow can supplement IPSS-R as a prognostic indicator
Key clinical point: Increased percentage of mature monocyte in bone marrow (PMMBM) may assist the Revised International Prognostic Scoring System (IPSS-R) to predict poor prognosis in patients with myelodysplastic syndromes (MDS).
Major finding: Elevated (>6%) vs. normal PMMBM was associated with shorter overall survival (24 months vs. 37 months; P = .026) along with higher risk distribution in terms of IPSS-R (P = .025) and higher frequency of IDH2 mutation (P = .007).
Study details: The data come from a retrospective analysis of 216 MDS patients, categorized into elevated and normal PMMBM groups.
Disclosures: The study was supported by the Zhejiang Provincial Natural Science Foundation of China, Medical and Health Science and Technology Projects of Zhejiang Province, National Science Foundation of Ningbo, and Chinese Medicine Science and Technology Plan Project of Zhejiang Province. The authors declared no conflicts of interest.
Source: Wu A et al. BMC Cancer. 2021 May 13. doi: 10.1186/s12885-021-08303-8.
Key clinical point: Increased percentage of mature monocyte in bone marrow (PMMBM) may assist the Revised International Prognostic Scoring System (IPSS-R) to predict poor prognosis in patients with myelodysplastic syndromes (MDS).
Major finding: Elevated (>6%) vs. normal PMMBM was associated with shorter overall survival (24 months vs. 37 months; P = .026) along with higher risk distribution in terms of IPSS-R (P = .025) and higher frequency of IDH2 mutation (P = .007).
Study details: The data come from a retrospective analysis of 216 MDS patients, categorized into elevated and normal PMMBM groups.
Disclosures: The study was supported by the Zhejiang Provincial Natural Science Foundation of China, Medical and Health Science and Technology Projects of Zhejiang Province, National Science Foundation of Ningbo, and Chinese Medicine Science and Technology Plan Project of Zhejiang Province. The authors declared no conflicts of interest.
Source: Wu A et al. BMC Cancer. 2021 May 13. doi: 10.1186/s12885-021-08303-8.
Key clinical point: Increased percentage of mature monocyte in bone marrow (PMMBM) may assist the Revised International Prognostic Scoring System (IPSS-R) to predict poor prognosis in patients with myelodysplastic syndromes (MDS).
Major finding: Elevated (>6%) vs. normal PMMBM was associated with shorter overall survival (24 months vs. 37 months; P = .026) along with higher risk distribution in terms of IPSS-R (P = .025) and higher frequency of IDH2 mutation (P = .007).
Study details: The data come from a retrospective analysis of 216 MDS patients, categorized into elevated and normal PMMBM groups.
Disclosures: The study was supported by the Zhejiang Provincial Natural Science Foundation of China, Medical and Health Science and Technology Projects of Zhejiang Province, National Science Foundation of Ningbo, and Chinese Medicine Science and Technology Plan Project of Zhejiang Province. The authors declared no conflicts of interest.
Source: Wu A et al. BMC Cancer. 2021 May 13. doi: 10.1186/s12885-021-08303-8.
MDS: Antibiotics can be stopped after 3 days in patients with febrile neutropenia after chemotherapy
Key clinical point: During remission induction chemotherapy in patients with myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML), antibiotics can be safely stopped after 3 days of febrile neutropenia in the absence of infection.
Major finding: Serious medical complication (SMC) was seen in 12.5% of patients receiving the 3-day empirical broad-spectrum antibiotic therapy (EBAT) vs. 8.9% of patients receiving the prolonged regimen (P = .17). After adjustment for confounders, there was no significant difference between both strategies in the number of SMCs (hazard ratio, 1.357; P = .297).
Study details: AML or MDS patients who received chemotherapy were treated with either 3-day EBAT or a prolonged antibiotic regimen (until neutrophil recovery).
Disclosures: The study did not receive any specific funding. A Schauwvlieghe, J Maertens, and T Mercier reported relationships with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Schauwvlieghe A et al. EClinicalMedicine. 2021 Apr 25. doi: 10.1016/j.eclinm.2021.100855.
Key clinical point: During remission induction chemotherapy in patients with myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML), antibiotics can be safely stopped after 3 days of febrile neutropenia in the absence of infection.
Major finding: Serious medical complication (SMC) was seen in 12.5% of patients receiving the 3-day empirical broad-spectrum antibiotic therapy (EBAT) vs. 8.9% of patients receiving the prolonged regimen (P = .17). After adjustment for confounders, there was no significant difference between both strategies in the number of SMCs (hazard ratio, 1.357; P = .297).
Study details: AML or MDS patients who received chemotherapy were treated with either 3-day EBAT or a prolonged antibiotic regimen (until neutrophil recovery).
Disclosures: The study did not receive any specific funding. A Schauwvlieghe, J Maertens, and T Mercier reported relationships with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Schauwvlieghe A et al. EClinicalMedicine. 2021 Apr 25. doi: 10.1016/j.eclinm.2021.100855.
Key clinical point: During remission induction chemotherapy in patients with myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML), antibiotics can be safely stopped after 3 days of febrile neutropenia in the absence of infection.
Major finding: Serious medical complication (SMC) was seen in 12.5% of patients receiving the 3-day empirical broad-spectrum antibiotic therapy (EBAT) vs. 8.9% of patients receiving the prolonged regimen (P = .17). After adjustment for confounders, there was no significant difference between both strategies in the number of SMCs (hazard ratio, 1.357; P = .297).
Study details: AML or MDS patients who received chemotherapy were treated with either 3-day EBAT or a prolonged antibiotic regimen (until neutrophil recovery).
Disclosures: The study did not receive any specific funding. A Schauwvlieghe, J Maertens, and T Mercier reported relationships with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Schauwvlieghe A et al. EClinicalMedicine. 2021 Apr 25. doi: 10.1016/j.eclinm.2021.100855.
De novo MDS: Pretransplant RBC and platelet transfusion burden tied to poor survival outcomes
Key clinical point: Higher pretransplant red blood cell (RBC) and platelet transfusion burden has an independent association with higher overall and relapse-related mortality following allogeneic hematopoietic stem cell transplantation (allo-HSCT) for de novo myelodysplastic syndrome (MDS).
Major finding: A higher pretransplant RBC transfusion burden was significantly associated with lower overall survival (OS; P less than .001) and higher relapse-related mortality (P less than .001). Similarly, a higher pretransplant platelet transfusion burden was associated with lower OS (P less than .001) and higher relapse-related mortality (P = .001).
Study details: A retrospective study examined the effects of pretransplant RBC and platelet transfusion burden on outcomes after allo-HSCT in 1,007 adults with de novo MDS.
Disclosures: This study was supported in part by the Practical Research Project for Allergic Diseases and Immunology (Research Technology of Medical Transplantation) from the Japan Agency for Medical Research and Development. The authors declared no conflicts of interest.
Source: Konuma T et al. Transplant Cell Ther. 2021 May 12. doi: 10.1016/j.jtct.2021.05.003.
Key clinical point: Higher pretransplant red blood cell (RBC) and platelet transfusion burden has an independent association with higher overall and relapse-related mortality following allogeneic hematopoietic stem cell transplantation (allo-HSCT) for de novo myelodysplastic syndrome (MDS).
Major finding: A higher pretransplant RBC transfusion burden was significantly associated with lower overall survival (OS; P less than .001) and higher relapse-related mortality (P less than .001). Similarly, a higher pretransplant platelet transfusion burden was associated with lower OS (P less than .001) and higher relapse-related mortality (P = .001).
Study details: A retrospective study examined the effects of pretransplant RBC and platelet transfusion burden on outcomes after allo-HSCT in 1,007 adults with de novo MDS.
Disclosures: This study was supported in part by the Practical Research Project for Allergic Diseases and Immunology (Research Technology of Medical Transplantation) from the Japan Agency for Medical Research and Development. The authors declared no conflicts of interest.
Source: Konuma T et al. Transplant Cell Ther. 2021 May 12. doi: 10.1016/j.jtct.2021.05.003.
Key clinical point: Higher pretransplant red blood cell (RBC) and platelet transfusion burden has an independent association with higher overall and relapse-related mortality following allogeneic hematopoietic stem cell transplantation (allo-HSCT) for de novo myelodysplastic syndrome (MDS).
Major finding: A higher pretransplant RBC transfusion burden was significantly associated with lower overall survival (OS; P less than .001) and higher relapse-related mortality (P less than .001). Similarly, a higher pretransplant platelet transfusion burden was associated with lower OS (P less than .001) and higher relapse-related mortality (P = .001).
Study details: A retrospective study examined the effects of pretransplant RBC and platelet transfusion burden on outcomes after allo-HSCT in 1,007 adults with de novo MDS.
Disclosures: This study was supported in part by the Practical Research Project for Allergic Diseases and Immunology (Research Technology of Medical Transplantation) from the Japan Agency for Medical Research and Development. The authors declared no conflicts of interest.
Source: Konuma T et al. Transplant Cell Ther. 2021 May 12. doi: 10.1016/j.jtct.2021.05.003.
Less restrictive enrollment criteria warranted for MDS clinical trials
Key clinical point: Less restrictive inclusion and exclusion criteria are warranted to improve the participation of patients with myelodysplastic syndrome (MDS) in clinical trials.
Major finding: Each trial was suitable for ~18% of patients in the cohort, whereas 34% of the patients were eligible for at least 1 of the 9 trials. Pharma-initiated trials excluded more than twice the fraction of patients vs. investigator-initiated trials (inclusion, 10% vs. 21%). Key reasons for exclusion included karyotype (average exclusion rate, 58%), comorbidities (40%), and previous therapies (55%)
Study details: A simulation exercise was performed to estimate the average proportion of MDS patients eligible for participation in a clinical trial. A total of 1,809 patients were included in the cohort.
Disclosures: This study did not receive any funding. K Nachtkamp, T Schroeder, E Schuler, J Kaivers, A Giagounidis, C Rautenberg, N Gattermann, and U Germing reported relationships with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Nachtkamp K et al. Leuk Res. 2021 May 11. doi: 10.1016/j.leukres.2021.106611.
Key clinical point: Less restrictive inclusion and exclusion criteria are warranted to improve the participation of patients with myelodysplastic syndrome (MDS) in clinical trials.
Major finding: Each trial was suitable for ~18% of patients in the cohort, whereas 34% of the patients were eligible for at least 1 of the 9 trials. Pharma-initiated trials excluded more than twice the fraction of patients vs. investigator-initiated trials (inclusion, 10% vs. 21%). Key reasons for exclusion included karyotype (average exclusion rate, 58%), comorbidities (40%), and previous therapies (55%)
Study details: A simulation exercise was performed to estimate the average proportion of MDS patients eligible for participation in a clinical trial. A total of 1,809 patients were included in the cohort.
Disclosures: This study did not receive any funding. K Nachtkamp, T Schroeder, E Schuler, J Kaivers, A Giagounidis, C Rautenberg, N Gattermann, and U Germing reported relationships with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Nachtkamp K et al. Leuk Res. 2021 May 11. doi: 10.1016/j.leukres.2021.106611.
Key clinical point: Less restrictive inclusion and exclusion criteria are warranted to improve the participation of patients with myelodysplastic syndrome (MDS) in clinical trials.
Major finding: Each trial was suitable for ~18% of patients in the cohort, whereas 34% of the patients were eligible for at least 1 of the 9 trials. Pharma-initiated trials excluded more than twice the fraction of patients vs. investigator-initiated trials (inclusion, 10% vs. 21%). Key reasons for exclusion included karyotype (average exclusion rate, 58%), comorbidities (40%), and previous therapies (55%)
Study details: A simulation exercise was performed to estimate the average proportion of MDS patients eligible for participation in a clinical trial. A total of 1,809 patients were included in the cohort.
Disclosures: This study did not receive any funding. K Nachtkamp, T Schroeder, E Schuler, J Kaivers, A Giagounidis, C Rautenberg, N Gattermann, and U Germing reported relationships with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Nachtkamp K et al. Leuk Res. 2021 May 11. doi: 10.1016/j.leukres.2021.106611.
T-cell inhibition by PD-L1-expressing stem cells may play a role in MDS development
Key clinical point: Inhibition of T cells by programmed death-ligand 1 (PD-L1)-expressing hematopoietic stem cells could be an underlying mechanism in the development of myelodysplastic syndrome (MDS). The findings support the potential use of immune checkpoint inhibitors in the treatment of suitable MDS patients.
Major finding: Significantly increased proportions of PD-L1+CD34+ stem cells were seen in MDS patients compared with hematopoietic stem cell transplantation (HSCT) recipients in remission for both the CD38− subset (P = .0127) and CD38+ subset (P = .0336).
Study details: The study included 7 MDS and 9 acute myeloid leukemia samples. Six HSCT recipients who remained in remission for more than 6 months were considered controls.
Disclosures: The study was supported by the Düsseldorf School of Oncology (funded by the Comprehensive Cancer Center Düsseldorf/Deutsche Krebshilfe and the Medical Faculty HHU Düsseldorf). The authors declared no conflicts of interest.
Source: Moskorz W et al. Br J Haematol. 2021 May 6. doi: 10.1111/bjh.17461.
Key clinical point: Inhibition of T cells by programmed death-ligand 1 (PD-L1)-expressing hematopoietic stem cells could be an underlying mechanism in the development of myelodysplastic syndrome (MDS). The findings support the potential use of immune checkpoint inhibitors in the treatment of suitable MDS patients.
Major finding: Significantly increased proportions of PD-L1+CD34+ stem cells were seen in MDS patients compared with hematopoietic stem cell transplantation (HSCT) recipients in remission for both the CD38− subset (P = .0127) and CD38+ subset (P = .0336).
Study details: The study included 7 MDS and 9 acute myeloid leukemia samples. Six HSCT recipients who remained in remission for more than 6 months were considered controls.
Disclosures: The study was supported by the Düsseldorf School of Oncology (funded by the Comprehensive Cancer Center Düsseldorf/Deutsche Krebshilfe and the Medical Faculty HHU Düsseldorf). The authors declared no conflicts of interest.
Source: Moskorz W et al. Br J Haematol. 2021 May 6. doi: 10.1111/bjh.17461.
Key clinical point: Inhibition of T cells by programmed death-ligand 1 (PD-L1)-expressing hematopoietic stem cells could be an underlying mechanism in the development of myelodysplastic syndrome (MDS). The findings support the potential use of immune checkpoint inhibitors in the treatment of suitable MDS patients.
Major finding: Significantly increased proportions of PD-L1+CD34+ stem cells were seen in MDS patients compared with hematopoietic stem cell transplantation (HSCT) recipients in remission for both the CD38− subset (P = .0127) and CD38+ subset (P = .0336).
Study details: The study included 7 MDS and 9 acute myeloid leukemia samples. Six HSCT recipients who remained in remission for more than 6 months were considered controls.
Disclosures: The study was supported by the Düsseldorf School of Oncology (funded by the Comprehensive Cancer Center Düsseldorf/Deutsche Krebshilfe and the Medical Faculty HHU Düsseldorf). The authors declared no conflicts of interest.
Source: Moskorz W et al. Br J Haematol. 2021 May 6. doi: 10.1111/bjh.17461.
Increased cumulative exposure to melphalan in multiple myeloma patients increases MDS risk
Key clinical point: Increased cumulative exposure to the alkylating agent melphalan increases the subsequent risk for developing acute myeloid leukemia/myelodysplastic syndromes (AML/MDS) in patients with multiple myeloma (MM).
Major finding: Cumulative exposure to melphalan was significantly higher (odds ratio, 2.8; P less than .001) among patients with MM and AML/MDS (median, 988 mg) than control participants (median, 578 mg). The median time to development of AML/MDS was 3.8 years.
Study details: The study included 26,627 patients diagnosed with MM between 1985 and 2011, of which 124 (0.5%) patients developed subsequent AML/MDS. Each patient with MM and AML/MDS diagnosis was matched with a control MM patient without AML/MDS.
Disclosures: The study was supported by grants from the Asrun Einarsdottir Foundation in Iceland, University of Iceland Research Fund, Icelandic Centre for Research, Landspitali University Hospital Research Fund, Thorman’s foundation, and Sylvester Comprehensive Cancer Center NCI Core Grant. O Landgren and M Björkholm reported ties with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Jonsdottir G et al. Eur J Haematol. 2021 May 9. doi: 10.1111/ejh.13650.
Key clinical point: Increased cumulative exposure to the alkylating agent melphalan increases the subsequent risk for developing acute myeloid leukemia/myelodysplastic syndromes (AML/MDS) in patients with multiple myeloma (MM).
Major finding: Cumulative exposure to melphalan was significantly higher (odds ratio, 2.8; P less than .001) among patients with MM and AML/MDS (median, 988 mg) than control participants (median, 578 mg). The median time to development of AML/MDS was 3.8 years.
Study details: The study included 26,627 patients diagnosed with MM between 1985 and 2011, of which 124 (0.5%) patients developed subsequent AML/MDS. Each patient with MM and AML/MDS diagnosis was matched with a control MM patient without AML/MDS.
Disclosures: The study was supported by grants from the Asrun Einarsdottir Foundation in Iceland, University of Iceland Research Fund, Icelandic Centre for Research, Landspitali University Hospital Research Fund, Thorman’s foundation, and Sylvester Comprehensive Cancer Center NCI Core Grant. O Landgren and M Björkholm reported ties with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Jonsdottir G et al. Eur J Haematol. 2021 May 9. doi: 10.1111/ejh.13650.
Key clinical point: Increased cumulative exposure to the alkylating agent melphalan increases the subsequent risk for developing acute myeloid leukemia/myelodysplastic syndromes (AML/MDS) in patients with multiple myeloma (MM).
Major finding: Cumulative exposure to melphalan was significantly higher (odds ratio, 2.8; P less than .001) among patients with MM and AML/MDS (median, 988 mg) than control participants (median, 578 mg). The median time to development of AML/MDS was 3.8 years.
Study details: The study included 26,627 patients diagnosed with MM between 1985 and 2011, of which 124 (0.5%) patients developed subsequent AML/MDS. Each patient with MM and AML/MDS diagnosis was matched with a control MM patient without AML/MDS.
Disclosures: The study was supported by grants from the Asrun Einarsdottir Foundation in Iceland, University of Iceland Research Fund, Icelandic Centre for Research, Landspitali University Hospital Research Fund, Thorman’s foundation, and Sylvester Comprehensive Cancer Center NCI Core Grant. O Landgren and M Björkholm reported ties with various pharmaceutical companies. The remaining authors declared no conflicts of interest.
Source: Jonsdottir G et al. Eur J Haematol. 2021 May 9. doi: 10.1111/ejh.13650.
MDS: Adolescents and young adults have a favorable survival with allo-HSCT
Key clinical point: Adolescent and young adult (AYA) patients with myelodysplastic syndrome (MDS) receiving allogeneic hematopoietic stem cell transplantation (allo-HSCT) generally exhibit better survival than their non-AYA counterparts.
Major finding: The 3-year overall survival (OS) in AYA patients was 71.2% (95% confidence interval, 67.4%-74.6%). Predictors of poor 3-year OS were active disease status (adjusted hazard ratio [aHR], 1.54; P = .016), poor cytogenetic risk (aHR, 1.62; P = .011), poor performance status (aHR, 2.01; P = .016), human leukocyte antigen (HLA)-matched unrelated donors (aHR, 2.23; P less than .001), HLA-mismatched unrelated donors (aHR, 2.16; P = .027), and cord blood transplantation (aHR, 2.44; P = 0.001).
Study details: The study included 645 AYA patients with MDS, aged 16-39 years, who received first allo-HSCT.
Disclosures: No funding source was identified. The authors declared no conflicts of interest.
Source: Shimomura Y et al. Bone Marrow Transplant. 2021 May 15. doi: 10.1038/s41409-021-01324-8.
Key clinical point: Adolescent and young adult (AYA) patients with myelodysplastic syndrome (MDS) receiving allogeneic hematopoietic stem cell transplantation (allo-HSCT) generally exhibit better survival than their non-AYA counterparts.
Major finding: The 3-year overall survival (OS) in AYA patients was 71.2% (95% confidence interval, 67.4%-74.6%). Predictors of poor 3-year OS were active disease status (adjusted hazard ratio [aHR], 1.54; P = .016), poor cytogenetic risk (aHR, 1.62; P = .011), poor performance status (aHR, 2.01; P = .016), human leukocyte antigen (HLA)-matched unrelated donors (aHR, 2.23; P less than .001), HLA-mismatched unrelated donors (aHR, 2.16; P = .027), and cord blood transplantation (aHR, 2.44; P = 0.001).
Study details: The study included 645 AYA patients with MDS, aged 16-39 years, who received first allo-HSCT.
Disclosures: No funding source was identified. The authors declared no conflicts of interest.
Source: Shimomura Y et al. Bone Marrow Transplant. 2021 May 15. doi: 10.1038/s41409-021-01324-8.
Key clinical point: Adolescent and young adult (AYA) patients with myelodysplastic syndrome (MDS) receiving allogeneic hematopoietic stem cell transplantation (allo-HSCT) generally exhibit better survival than their non-AYA counterparts.
Major finding: The 3-year overall survival (OS) in AYA patients was 71.2% (95% confidence interval, 67.4%-74.6%). Predictors of poor 3-year OS were active disease status (adjusted hazard ratio [aHR], 1.54; P = .016), poor cytogenetic risk (aHR, 1.62; P = .011), poor performance status (aHR, 2.01; P = .016), human leukocyte antigen (HLA)-matched unrelated donors (aHR, 2.23; P less than .001), HLA-mismatched unrelated donors (aHR, 2.16; P = .027), and cord blood transplantation (aHR, 2.44; P = 0.001).
Study details: The study included 645 AYA patients with MDS, aged 16-39 years, who received first allo-HSCT.
Disclosures: No funding source was identified. The authors declared no conflicts of interest.
Source: Shimomura Y et al. Bone Marrow Transplant. 2021 May 15. doi: 10.1038/s41409-021-01324-8.
MDS del5q: Could DNA methylation patterns predict response to lenalidomide?
Key clinical point: Lenalidomide had no relevant effect on DNA methylation status in patients with myelodysplastic syndrome with isolated deletion of chromosome 5q (MDS del5q).
Major finding: Lenalidomide treatment did not have a relevant impact on genome-wide DNA methylation in patients with MDS del5q. However, methylation analysis before treatment could identify a distinct subgroup of patients (27%) with a trend toward inferior overall survival but not inferior progression-free survival.
Study details: DNA methylation analysis was performed on 51 MDS del5q patients treated with lenalidomide. Direct effects of lenalidomide on DNA methylation were studied using 17 paired samples pre- and posttreatment.
Disclosures: Open access funding by Projekt DEAL. This study was supported by funds from the Deutsche Forschungsgemeinschaft, “Deutsche Krebshilfe,” Gutermuth Foundation, H.W. & J. Hector fund, Baden Wuerttemberg, and Dr Rolf M Schwiete Fund. Mannheim DN is an endowed Professor of the German José-Carreras-Stiftung. The study was partly funded by a research grant from Celgene Inc.
Source: Hecht A et al. Ann Hematol. 2021 April 27. doi: 10.1007/s00277-021-04492-1.
Key clinical point: Lenalidomide had no relevant effect on DNA methylation status in patients with myelodysplastic syndrome with isolated deletion of chromosome 5q (MDS del5q).
Major finding: Lenalidomide treatment did not have a relevant impact on genome-wide DNA methylation in patients with MDS del5q. However, methylation analysis before treatment could identify a distinct subgroup of patients (27%) with a trend toward inferior overall survival but not inferior progression-free survival.
Study details: DNA methylation analysis was performed on 51 MDS del5q patients treated with lenalidomide. Direct effects of lenalidomide on DNA methylation were studied using 17 paired samples pre- and posttreatment.
Disclosures: Open access funding by Projekt DEAL. This study was supported by funds from the Deutsche Forschungsgemeinschaft, “Deutsche Krebshilfe,” Gutermuth Foundation, H.W. & J. Hector fund, Baden Wuerttemberg, and Dr Rolf M Schwiete Fund. Mannheim DN is an endowed Professor of the German José-Carreras-Stiftung. The study was partly funded by a research grant from Celgene Inc.
Source: Hecht A et al. Ann Hematol. 2021 April 27. doi: 10.1007/s00277-021-04492-1.
Key clinical point: Lenalidomide had no relevant effect on DNA methylation status in patients with myelodysplastic syndrome with isolated deletion of chromosome 5q (MDS del5q).
Major finding: Lenalidomide treatment did not have a relevant impact on genome-wide DNA methylation in patients with MDS del5q. However, methylation analysis before treatment could identify a distinct subgroup of patients (27%) with a trend toward inferior overall survival but not inferior progression-free survival.
Study details: DNA methylation analysis was performed on 51 MDS del5q patients treated with lenalidomide. Direct effects of lenalidomide on DNA methylation were studied using 17 paired samples pre- and posttreatment.
Disclosures: Open access funding by Projekt DEAL. This study was supported by funds from the Deutsche Forschungsgemeinschaft, “Deutsche Krebshilfe,” Gutermuth Foundation, H.W. & J. Hector fund, Baden Wuerttemberg, and Dr Rolf M Schwiete Fund. Mannheim DN is an endowed Professor of the German José-Carreras-Stiftung. The study was partly funded by a research grant from Celgene Inc.
Source: Hecht A et al. Ann Hematol. 2021 April 27. doi: 10.1007/s00277-021-04492-1.
Upfront allo-HSCT preferable for MDS
Key clinical point: Pretransplant cytoreductive therapy is not associated with improved outcomes in patients with myelodysplastic syndromes (MDS) who have undergone allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Major finding: Five-year overall survival after diagnosis was 73.6% (95% confidence interval [CI], 70.3%-76.9%), 43.4% (95% CI, 39.2%-47.6%), and 46.9% (95% CI, 44.7%-49.1%) in the upfront transplantation (upfront), induction chemotherapy (CT), and hypomethylating agents alone (HMA) groups, respectively (P = .033). Treatment-related mortality was 13.0% (95% CI, 11.4%-15.1%), 32.4% (95% CI, 30.1%-35.3%), and 28.4% (95% CI, 26.2%-30.3%) in the 3 groups, respectively (P = .028).
Study details: A total of 157 MDS patients were categorized into 3 groups based on the pretransplantation therapy: upfront (n=54), CT (n=66), and HMA (n=37). In addition, 124 patients underwent allo-HSCT.
Disclosures: This study was supported by the National Natural Science Foundation of China, Research and Development Program in Key Areas of Guangdong Province, Science and Technology Program of Guangzhou, and Clinical Research Start-up Project of Southern Medical University. The authors declared no conflicts of interest.
Source: Chen Y et al. Int J Cancer. 2021 Apr 25. doi: 10.1002/ijc.33608.
Key clinical point: Pretransplant cytoreductive therapy is not associated with improved outcomes in patients with myelodysplastic syndromes (MDS) who have undergone allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Major finding: Five-year overall survival after diagnosis was 73.6% (95% confidence interval [CI], 70.3%-76.9%), 43.4% (95% CI, 39.2%-47.6%), and 46.9% (95% CI, 44.7%-49.1%) in the upfront transplantation (upfront), induction chemotherapy (CT), and hypomethylating agents alone (HMA) groups, respectively (P = .033). Treatment-related mortality was 13.0% (95% CI, 11.4%-15.1%), 32.4% (95% CI, 30.1%-35.3%), and 28.4% (95% CI, 26.2%-30.3%) in the 3 groups, respectively (P = .028).
Study details: A total of 157 MDS patients were categorized into 3 groups based on the pretransplantation therapy: upfront (n=54), CT (n=66), and HMA (n=37). In addition, 124 patients underwent allo-HSCT.
Disclosures: This study was supported by the National Natural Science Foundation of China, Research and Development Program in Key Areas of Guangdong Province, Science and Technology Program of Guangzhou, and Clinical Research Start-up Project of Southern Medical University. The authors declared no conflicts of interest.
Source: Chen Y et al. Int J Cancer. 2021 Apr 25. doi: 10.1002/ijc.33608.
Key clinical point: Pretransplant cytoreductive therapy is not associated with improved outcomes in patients with myelodysplastic syndromes (MDS) who have undergone allogeneic hematopoietic stem cell transplantation (allo-HSCT).
Major finding: Five-year overall survival after diagnosis was 73.6% (95% confidence interval [CI], 70.3%-76.9%), 43.4% (95% CI, 39.2%-47.6%), and 46.9% (95% CI, 44.7%-49.1%) in the upfront transplantation (upfront), induction chemotherapy (CT), and hypomethylating agents alone (HMA) groups, respectively (P = .033). Treatment-related mortality was 13.0% (95% CI, 11.4%-15.1%), 32.4% (95% CI, 30.1%-35.3%), and 28.4% (95% CI, 26.2%-30.3%) in the 3 groups, respectively (P = .028).
Study details: A total of 157 MDS patients were categorized into 3 groups based on the pretransplantation therapy: upfront (n=54), CT (n=66), and HMA (n=37). In addition, 124 patients underwent allo-HSCT.
Disclosures: This study was supported by the National Natural Science Foundation of China, Research and Development Program in Key Areas of Guangdong Province, Science and Technology Program of Guangzhou, and Clinical Research Start-up Project of Southern Medical University. The authors declared no conflicts of interest.
Source: Chen Y et al. Int J Cancer. 2021 Apr 25. doi: 10.1002/ijc.33608.