User login
For MD-IQ on Family Practice News, but a regular topic for Rheumatology News
Osteoporosis, osteoarthritis risk high among cerebral palsy patients
compared with adults without the disorder, according to a study published in Bone.
Neil E. O’Connell, PhD, of Brunel University London, and colleagues assessed the risks of osteoporosis, osteoarthritis, and inflammatory musculoskeletal diseases in a population-based cohort study that used data collected by the U.K. Clinical Practice Research Datalink during 1987-2015. The study included 1,705 patients with CP and 5,115 patients matched for age, sex, and general practices; data on smoking status and alcohol consumption for many of the patients also were gathered.
After adjustment for smoking status, alcohol consumption, and mean yearly general practice visits, investigators found evidence of significantly increased risk for osteoarthritis (hazard ratio, 1.54; 95% confidence interval, 1.17-2.02; P = .002) and osteoporosis (HR, 6.19; 95% CI, 3.37-11.39; P less than .001); they did not see increased risk for inflammatory musculoskeletal diseases (HR, 0.89; 95% CI, 0.45-1.75; P = .731).
One limitation of the study is the risk for residual confounding given the investigators could not account for mobility status or physical activity. Other limitations include potential incompleteness of diagnostic code lists, how identification of cases is depending on quality of original recording in the database, and that data regarding smoking status and alcohol consumption were missing for a substantial proportion of patients.
“Despite previous studies identifying a high prevalence of joint pain and functional deterioration among people with CP, there is a dearth of literature on the burden of musculoskeletal disorders in this population,” they wrote. “Further research is required into effective management of these conditions in adults with CP.”
This study was supported by an interdisciplinary award from Brunel University London’s Research Catalyst Fund. The authors declared no competing interests.
SOURCE: O’Connell NE et al. Bone. 2019 Aug;125:30-5.
compared with adults without the disorder, according to a study published in Bone.
Neil E. O’Connell, PhD, of Brunel University London, and colleagues assessed the risks of osteoporosis, osteoarthritis, and inflammatory musculoskeletal diseases in a population-based cohort study that used data collected by the U.K. Clinical Practice Research Datalink during 1987-2015. The study included 1,705 patients with CP and 5,115 patients matched for age, sex, and general practices; data on smoking status and alcohol consumption for many of the patients also were gathered.
After adjustment for smoking status, alcohol consumption, and mean yearly general practice visits, investigators found evidence of significantly increased risk for osteoarthritis (hazard ratio, 1.54; 95% confidence interval, 1.17-2.02; P = .002) and osteoporosis (HR, 6.19; 95% CI, 3.37-11.39; P less than .001); they did not see increased risk for inflammatory musculoskeletal diseases (HR, 0.89; 95% CI, 0.45-1.75; P = .731).
One limitation of the study is the risk for residual confounding given the investigators could not account for mobility status or physical activity. Other limitations include potential incompleteness of diagnostic code lists, how identification of cases is depending on quality of original recording in the database, and that data regarding smoking status and alcohol consumption were missing for a substantial proportion of patients.
“Despite previous studies identifying a high prevalence of joint pain and functional deterioration among people with CP, there is a dearth of literature on the burden of musculoskeletal disorders in this population,” they wrote. “Further research is required into effective management of these conditions in adults with CP.”
This study was supported by an interdisciplinary award from Brunel University London’s Research Catalyst Fund. The authors declared no competing interests.
SOURCE: O’Connell NE et al. Bone. 2019 Aug;125:30-5.
compared with adults without the disorder, according to a study published in Bone.
Neil E. O’Connell, PhD, of Brunel University London, and colleagues assessed the risks of osteoporosis, osteoarthritis, and inflammatory musculoskeletal diseases in a population-based cohort study that used data collected by the U.K. Clinical Practice Research Datalink during 1987-2015. The study included 1,705 patients with CP and 5,115 patients matched for age, sex, and general practices; data on smoking status and alcohol consumption for many of the patients also were gathered.
After adjustment for smoking status, alcohol consumption, and mean yearly general practice visits, investigators found evidence of significantly increased risk for osteoarthritis (hazard ratio, 1.54; 95% confidence interval, 1.17-2.02; P = .002) and osteoporosis (HR, 6.19; 95% CI, 3.37-11.39; P less than .001); they did not see increased risk for inflammatory musculoskeletal diseases (HR, 0.89; 95% CI, 0.45-1.75; P = .731).
One limitation of the study is the risk for residual confounding given the investigators could not account for mobility status or physical activity. Other limitations include potential incompleteness of diagnostic code lists, how identification of cases is depending on quality of original recording in the database, and that data regarding smoking status and alcohol consumption were missing for a substantial proportion of patients.
“Despite previous studies identifying a high prevalence of joint pain and functional deterioration among people with CP, there is a dearth of literature on the burden of musculoskeletal disorders in this population,” they wrote. “Further research is required into effective management of these conditions in adults with CP.”
This study was supported by an interdisciplinary award from Brunel University London’s Research Catalyst Fund. The authors declared no competing interests.
SOURCE: O’Connell NE et al. Bone. 2019 Aug;125:30-5.
FROM BONE
Tanezumab improves osteoarthritis pain, function in phase 3 trial
MADRID – Tanezumab, an investigational monoclonal antibody directed against nerve growth factor that is under development to treat osteoarthritis pain, met most of the coprimary efficacy endpoints set for the drug in a randomized, double-blind, parallel-group, placebo-controlled phase 3 study.
At the end of a 24-week, double-blind treatment period, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain and WOMAC physical function subscale scores were significantly improved, compared with placebo in the two tanezumab (2.5 mg and 5 mg) dose groups.
The least squares (ls) mean change from baseline in WOMAC pain scores were –2.24 for placebo, –2.70 for tanezumab 2.5 mg, and –2.85 for tanezumab 5 mg (P less than or equal to .01 and P less than or equal to .001 vs. placebo).
The ls mean change from baseline in WOMAC physical function scores were a respective –2.11, –2.70, and –2.82 (P less than or equal to .001 for both vs. placebo).
The coprimary endpoint of patients’ global assessment of OA (PGA-OA) was also significantly improved with tanezumab 5 mg (–0.90; P less than or equal to .05) but not 2.5 mg (–0.82) versus placebo (–0.72).
As the 2.5-mg dose of tanezumab didn’t meet one of the three coprimary endpoints, further hypothesis testing was not possible, but exploratory findings suggested that tanezumab at 2.5 mg or 5 mg yielded higher proportions of patients with reductions from baseline in WOMAC pain scores when compared against placebo. This was the case for reductions of at least 30% (65.6%, 68.7%, 56.6%, respectively), 50% (45.4%, 47.9%, 33.8%), or 70% (21.3%, 23.2%, 17.8%).
“I think that we have now a lot of studies with tanezumab showing a significant effect on hip and knee OA pain and function, so we have the studies in order to have the drug on the market,” study first author Francis Berenbaum, MD, PhD, of Saint-Antoine Hospital, Sorbonne Université in Paris, said in an interview at the European Congress of Rheumatology.
“Of course, because of the safety issue with rapid progressive osteoarthritis (RPOA), what we are discussing now is: ‘For which patients will there be an optimal benefit-to-risk?’ So, it’s now more a discussion around the population of patients who can benefit the most with the drug,” Dr. Berenbaum added.
A possible link between the use of tanezumab and a risk for developing RPOA was first suggested by preclinical and early clinical trial data, prompting the U.S. Food and Drug Administration to place partial holds on its clinical development in 2010, and again in 2012.
However, Dr. Berenbaum noted that a “mitigation plan” had been put in place for the phase 3 program to try to lower the likelihood of RPOA. This included: lowering the dose of the drug used and delivering it subcutaneously rather than intravenously; not prescribing it with NSAIDs and testing its possible effects and safety in a difficult-to-treat population of patients with no known risk factors for the potentially very serious adverse event.
“Based on this mitigation plan, the risk of rapid progressive osteoarthritis has considerably decreased,” Dr. Berenbaum observed. Indeed, in the phase 3 study he presented at the meeting, he said that around 2% of patients developed RPOA, which is “exactly in line with what has already been shown.” RPOA was reported in none of the placebo-treated patients, in 1.4% of those treated with tanezumab 2.5 mg, and in 2.8% in those treated with tanezumab 5 mg.
However, a “striking” finding of the current study was that despite the small increase in RPOA seen, there was no difference between the tanezumab and placebo groups in the number of patients needing total joint replacement (TJR). The percentages of patients undergoing at least one TJR was 6.7% in the placebo group, 7.8% in the tanezumab 2.5-mg group, and 7.0% in the tanezumab 5-mg group.
The joint safety events seen in the study, including TJRs, were adjudicated as being part of the normal progression of OA in the majority (73.4%) of cases. Other joint events of note were one case of subchondral insufficiency fracture occurring in a patient treated with tanezumab 2.5 mg and one case of primary osteonecrosis in a patient treated with tanezumab 5 mg.
During his presentation of the findings in a late-breaking oral abstract session, Dr. Berenbaum noted that this was a difficult-to-treat population of patients. All 849 patients who had been recruited had moderate to severe OA pain of the knee or hip and had a history of insufficient pain relief or intolerance to treatment with acetaminophen, oral NSAIDs, and tramadol and were also not responding to, or unwilling to take, opioid painkillers. Patients had to have no radiographic evidence of specified bone conditions, including RPOA.
Patients had been treated with subcutaneous tanezumab 2.5 mg (n = 283) or 5 mg (n = 284) or placebo (n = 282) at baseline, week 8, and week 16, with the three coprimary efficacy endpoints assessed at week 24.
Discussing the risk-to-benefit ratio of the drug after his presentation, Dr. Berenbaum said: “You have to keep in mind that, first, it was in very difficult-to-treat patients, compared to the other trials in the field of OA symptoms.”
He added: “Second, is that compared to the other trials, this one was able to include patients with Kellgren-Lawrence grade 4, meaning that this is a more serious population,” and third, “when you look at the responders – WOMAC 30%, 50%, 70% – there is a strong difference in terms of responders.”
Dr. Berenbaum and his coauthors noted on the poster that accompanied the late-breaking oral presentation that “an active-controlled study will provide data to further characterize the risk-benefit of tanezumab in patients with OA.”
The study was sponsored by Pfizer and Eli Lilly. Dr. Berenbaum disclosed receiving research funding through his institution from Pfizer and acting as a consultant to, and speaker for, the company as well as multiple other pharmaceutical companies. Coauthors of the study also disclosed research funding or consultancy agreements with Pfizer or Eli Lilly or were employees of the companies.
SOURCE: Berenbaum F et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):262-4. Abstract LB0007, doi: 10.1136/annrheumdis-2019-eular.8660
MADRID – Tanezumab, an investigational monoclonal antibody directed against nerve growth factor that is under development to treat osteoarthritis pain, met most of the coprimary efficacy endpoints set for the drug in a randomized, double-blind, parallel-group, placebo-controlled phase 3 study.
At the end of a 24-week, double-blind treatment period, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain and WOMAC physical function subscale scores were significantly improved, compared with placebo in the two tanezumab (2.5 mg and 5 mg) dose groups.
The least squares (ls) mean change from baseline in WOMAC pain scores were –2.24 for placebo, –2.70 for tanezumab 2.5 mg, and –2.85 for tanezumab 5 mg (P less than or equal to .01 and P less than or equal to .001 vs. placebo).
The ls mean change from baseline in WOMAC physical function scores were a respective –2.11, –2.70, and –2.82 (P less than or equal to .001 for both vs. placebo).
The coprimary endpoint of patients’ global assessment of OA (PGA-OA) was also significantly improved with tanezumab 5 mg (–0.90; P less than or equal to .05) but not 2.5 mg (–0.82) versus placebo (–0.72).
As the 2.5-mg dose of tanezumab didn’t meet one of the three coprimary endpoints, further hypothesis testing was not possible, but exploratory findings suggested that tanezumab at 2.5 mg or 5 mg yielded higher proportions of patients with reductions from baseline in WOMAC pain scores when compared against placebo. This was the case for reductions of at least 30% (65.6%, 68.7%, 56.6%, respectively), 50% (45.4%, 47.9%, 33.8%), or 70% (21.3%, 23.2%, 17.8%).
“I think that we have now a lot of studies with tanezumab showing a significant effect on hip and knee OA pain and function, so we have the studies in order to have the drug on the market,” study first author Francis Berenbaum, MD, PhD, of Saint-Antoine Hospital, Sorbonne Université in Paris, said in an interview at the European Congress of Rheumatology.
“Of course, because of the safety issue with rapid progressive osteoarthritis (RPOA), what we are discussing now is: ‘For which patients will there be an optimal benefit-to-risk?’ So, it’s now more a discussion around the population of patients who can benefit the most with the drug,” Dr. Berenbaum added.
A possible link between the use of tanezumab and a risk for developing RPOA was first suggested by preclinical and early clinical trial data, prompting the U.S. Food and Drug Administration to place partial holds on its clinical development in 2010, and again in 2012.
However, Dr. Berenbaum noted that a “mitigation plan” had been put in place for the phase 3 program to try to lower the likelihood of RPOA. This included: lowering the dose of the drug used and delivering it subcutaneously rather than intravenously; not prescribing it with NSAIDs and testing its possible effects and safety in a difficult-to-treat population of patients with no known risk factors for the potentially very serious adverse event.
“Based on this mitigation plan, the risk of rapid progressive osteoarthritis has considerably decreased,” Dr. Berenbaum observed. Indeed, in the phase 3 study he presented at the meeting, he said that around 2% of patients developed RPOA, which is “exactly in line with what has already been shown.” RPOA was reported in none of the placebo-treated patients, in 1.4% of those treated with tanezumab 2.5 mg, and in 2.8% in those treated with tanezumab 5 mg.
However, a “striking” finding of the current study was that despite the small increase in RPOA seen, there was no difference between the tanezumab and placebo groups in the number of patients needing total joint replacement (TJR). The percentages of patients undergoing at least one TJR was 6.7% in the placebo group, 7.8% in the tanezumab 2.5-mg group, and 7.0% in the tanezumab 5-mg group.
The joint safety events seen in the study, including TJRs, were adjudicated as being part of the normal progression of OA in the majority (73.4%) of cases. Other joint events of note were one case of subchondral insufficiency fracture occurring in a patient treated with tanezumab 2.5 mg and one case of primary osteonecrosis in a patient treated with tanezumab 5 mg.
During his presentation of the findings in a late-breaking oral abstract session, Dr. Berenbaum noted that this was a difficult-to-treat population of patients. All 849 patients who had been recruited had moderate to severe OA pain of the knee or hip and had a history of insufficient pain relief or intolerance to treatment with acetaminophen, oral NSAIDs, and tramadol and were also not responding to, or unwilling to take, opioid painkillers. Patients had to have no radiographic evidence of specified bone conditions, including RPOA.
Patients had been treated with subcutaneous tanezumab 2.5 mg (n = 283) or 5 mg (n = 284) or placebo (n = 282) at baseline, week 8, and week 16, with the three coprimary efficacy endpoints assessed at week 24.
Discussing the risk-to-benefit ratio of the drug after his presentation, Dr. Berenbaum said: “You have to keep in mind that, first, it was in very difficult-to-treat patients, compared to the other trials in the field of OA symptoms.”
He added: “Second, is that compared to the other trials, this one was able to include patients with Kellgren-Lawrence grade 4, meaning that this is a more serious population,” and third, “when you look at the responders – WOMAC 30%, 50%, 70% – there is a strong difference in terms of responders.”
Dr. Berenbaum and his coauthors noted on the poster that accompanied the late-breaking oral presentation that “an active-controlled study will provide data to further characterize the risk-benefit of tanezumab in patients with OA.”
The study was sponsored by Pfizer and Eli Lilly. Dr. Berenbaum disclosed receiving research funding through his institution from Pfizer and acting as a consultant to, and speaker for, the company as well as multiple other pharmaceutical companies. Coauthors of the study also disclosed research funding or consultancy agreements with Pfizer or Eli Lilly or were employees of the companies.
SOURCE: Berenbaum F et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):262-4. Abstract LB0007, doi: 10.1136/annrheumdis-2019-eular.8660
MADRID – Tanezumab, an investigational monoclonal antibody directed against nerve growth factor that is under development to treat osteoarthritis pain, met most of the coprimary efficacy endpoints set for the drug in a randomized, double-blind, parallel-group, placebo-controlled phase 3 study.
At the end of a 24-week, double-blind treatment period, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain and WOMAC physical function subscale scores were significantly improved, compared with placebo in the two tanezumab (2.5 mg and 5 mg) dose groups.
The least squares (ls) mean change from baseline in WOMAC pain scores were –2.24 for placebo, –2.70 for tanezumab 2.5 mg, and –2.85 for tanezumab 5 mg (P less than or equal to .01 and P less than or equal to .001 vs. placebo).
The ls mean change from baseline in WOMAC physical function scores were a respective –2.11, –2.70, and –2.82 (P less than or equal to .001 for both vs. placebo).
The coprimary endpoint of patients’ global assessment of OA (PGA-OA) was also significantly improved with tanezumab 5 mg (–0.90; P less than or equal to .05) but not 2.5 mg (–0.82) versus placebo (–0.72).
As the 2.5-mg dose of tanezumab didn’t meet one of the three coprimary endpoints, further hypothesis testing was not possible, but exploratory findings suggested that tanezumab at 2.5 mg or 5 mg yielded higher proportions of patients with reductions from baseline in WOMAC pain scores when compared against placebo. This was the case for reductions of at least 30% (65.6%, 68.7%, 56.6%, respectively), 50% (45.4%, 47.9%, 33.8%), or 70% (21.3%, 23.2%, 17.8%).
“I think that we have now a lot of studies with tanezumab showing a significant effect on hip and knee OA pain and function, so we have the studies in order to have the drug on the market,” study first author Francis Berenbaum, MD, PhD, of Saint-Antoine Hospital, Sorbonne Université in Paris, said in an interview at the European Congress of Rheumatology.
“Of course, because of the safety issue with rapid progressive osteoarthritis (RPOA), what we are discussing now is: ‘For which patients will there be an optimal benefit-to-risk?’ So, it’s now more a discussion around the population of patients who can benefit the most with the drug,” Dr. Berenbaum added.
A possible link between the use of tanezumab and a risk for developing RPOA was first suggested by preclinical and early clinical trial data, prompting the U.S. Food and Drug Administration to place partial holds on its clinical development in 2010, and again in 2012.
However, Dr. Berenbaum noted that a “mitigation plan” had been put in place for the phase 3 program to try to lower the likelihood of RPOA. This included: lowering the dose of the drug used and delivering it subcutaneously rather than intravenously; not prescribing it with NSAIDs and testing its possible effects and safety in a difficult-to-treat population of patients with no known risk factors for the potentially very serious adverse event.
“Based on this mitigation plan, the risk of rapid progressive osteoarthritis has considerably decreased,” Dr. Berenbaum observed. Indeed, in the phase 3 study he presented at the meeting, he said that around 2% of patients developed RPOA, which is “exactly in line with what has already been shown.” RPOA was reported in none of the placebo-treated patients, in 1.4% of those treated with tanezumab 2.5 mg, and in 2.8% in those treated with tanezumab 5 mg.
However, a “striking” finding of the current study was that despite the small increase in RPOA seen, there was no difference between the tanezumab and placebo groups in the number of patients needing total joint replacement (TJR). The percentages of patients undergoing at least one TJR was 6.7% in the placebo group, 7.8% in the tanezumab 2.5-mg group, and 7.0% in the tanezumab 5-mg group.
The joint safety events seen in the study, including TJRs, were adjudicated as being part of the normal progression of OA in the majority (73.4%) of cases. Other joint events of note were one case of subchondral insufficiency fracture occurring in a patient treated with tanezumab 2.5 mg and one case of primary osteonecrosis in a patient treated with tanezumab 5 mg.
During his presentation of the findings in a late-breaking oral abstract session, Dr. Berenbaum noted that this was a difficult-to-treat population of patients. All 849 patients who had been recruited had moderate to severe OA pain of the knee or hip and had a history of insufficient pain relief or intolerance to treatment with acetaminophen, oral NSAIDs, and tramadol and were also not responding to, or unwilling to take, opioid painkillers. Patients had to have no radiographic evidence of specified bone conditions, including RPOA.
Patients had been treated with subcutaneous tanezumab 2.5 mg (n = 283) or 5 mg (n = 284) or placebo (n = 282) at baseline, week 8, and week 16, with the three coprimary efficacy endpoints assessed at week 24.
Discussing the risk-to-benefit ratio of the drug after his presentation, Dr. Berenbaum said: “You have to keep in mind that, first, it was in very difficult-to-treat patients, compared to the other trials in the field of OA symptoms.”
He added: “Second, is that compared to the other trials, this one was able to include patients with Kellgren-Lawrence grade 4, meaning that this is a more serious population,” and third, “when you look at the responders – WOMAC 30%, 50%, 70% – there is a strong difference in terms of responders.”
Dr. Berenbaum and his coauthors noted on the poster that accompanied the late-breaking oral presentation that “an active-controlled study will provide data to further characterize the risk-benefit of tanezumab in patients with OA.”
The study was sponsored by Pfizer and Eli Lilly. Dr. Berenbaum disclosed receiving research funding through his institution from Pfizer and acting as a consultant to, and speaker for, the company as well as multiple other pharmaceutical companies. Coauthors of the study also disclosed research funding or consultancy agreements with Pfizer or Eli Lilly or were employees of the companies.
SOURCE: Berenbaum F et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):262-4. Abstract LB0007, doi: 10.1136/annrheumdis-2019-eular.8660
REPORTING FROM EULAR 2019 CONGRESS
Foot OA: Forgotten no longer
TORONTO – Foot osteoarthritis has been a relatively neglected topic by researchers – but that’s finally changing, Michelle Marshall, PhD, observed at the OARSI 2019 World Congress.
She was a coinvestigator in the groundbreaking Clinical Assessment of the Foot (CASF), a large prospective study that has brought new insights into the prevalence of foot osteoarthritis (OA), its risk factors, the sizable disease burden, and foot OA’s diverse phenotypes. She shared study highlights at the meeting, which was sponsored by the Osteoarthritis Research Society International.
Elsewhere at OARSI 2019, Lucy S. Gates, PhD, presented the eagerly awaited results of the Chingford 1000 Women Study of the progression pattern of symptomatic radiographic OA of the first metatarsophalangeal joint (MTPJ). With 19 years of follow-up, Chingford is far and away the largest and longest longitudinal study of first MTP joint OA.
The prospective, population-based, observational cohort CASF study was carried out by Dr. Marshall and her coinvestigators at Keele University in Staffordshire, England. They surveyed Staffordshire residents aged 50 and older regarding whether they had experienced foot pain within the last 12 months. Those who answered affirmatively were invited to come in for a more detailed assessment and get weight-bearing x-rays of both feet. Among the 557 symptomatic participants with foot x-rays, the prevalence of radiographic OA of the foot was 16.7%, or roughly one in six – underscoring that it’s a common condition. The first MTP joint was the most commonly affected site, with a prevalence of 7.8%, followed by the second cuneometatarsal joint (CMJ) at 6.8%, the talonavicular joint (TNJ) at 5.2%, the navicular first cuneiform joint (NCJ) at 5.2%, and the first CMJ at 3.9%. Three-quarters of those who had symptomatic radiographic foot OA reported disabling symptoms, an established risk factor for falls (Ann Rheum Dis. 2015 Jan;74[1]:156-63).
With an eye toward identification of potential distinct phenotypes of foot OA, the CASF investigators conducted a separate analysis of those study participants with symptomatic radiographic midfoot OA – that is, OA of the TNJ, NCJ, and/or first or second CMJs, but not the first MTP joint. The prevalence in the Staffordshire population over age 50 with a history of foot pain was 12%. Independent risk factors for midfoot OA included obesity, with an adjusted odds ratio of 2.0; pain in other weight-bearing lower limb joints, with an adjusted odds ratio of 8.5; diabetes, odds ratio of 1.9; and previous foot injury, with an associated 1.6-fold increased risk. Midfoot OA was most prevalent in women older than 75 years; however, contrary to the conventional wisdom, a history of frequently wearing high-heeled shoes posed no increased risk.
The burden associated with midfoot OA was reflected in affected individuals’ frequent use of health care resources: During the past year, 46% of them had consulted their primary care physicians about their foot pain, 48% had been to a podiatrist, and 19% had seen a physical therapist (Arthritis Res Ther. 2015 Jul 13;17:178. doi: 10.1186/s13075-015-0693-3).
In a separate analysis, the investigators compiled additional evidence from CASF pointing to the existence of two phenotypes of foot OA: isolated first MTP OA and polyarticular foot OA, with distinct risk factors and symptom profiles (Arthritis Care Res [Hoboken]. 2016 Feb;68[2]:217-27).
“We found that OA affected both feet significantly more than was expected by chance, and we identified strong symmetrical patterns. This mirrors findings in hand OA and implies involvement of systemic components within a foot,” Dr. Marshall said.
The course of foot OA
During 18 months of prospective follow-up in CASF, subjects with isolated first MTP joint or polyarticular foot OA showed no clinically meaningful change in symptoms (Arthritis Care Res [Hoboken]. 2018 Jul;70[7]:1107-12).
But that finding may have been a function of the relatively brief follow-up, as the Chingford 1000 Women Study, with its 19 years of prospective follow-up, told a different story. Dr. Gates, of the University of Southampton (England), reported that among the 193 patients with foot x-rays at both baseline and follow-up, by which point they averaged nearly 76 years in age, 33.2% had OA of the first MTP joint of either foot at baseline as defined by at least a grade 2 score on the LaTrobe foot atlas, and 13% had prevalent involvement of both feet. During 19 years of follow-up of the women from Chingford, an area in northeast London, the incidence of new-onset radiographic first MTP joint OA was 7% in the left foot and 17% in the right. Meanwhile, progression to grade 3 radiographic OA occurred in the left foot of 28% of those with grade 2 disease at baseline and in 35% of those with baseline first MTP joint OA of the right foot. Twenty-eight percent of patients with unilateral first MTP joint OA at baseline progressed to bilateral involvement within 19 years.
Dr. Gates reported having no financial conflicts regarding the Chingford study, funded primarily by Arthritis Research UK, which merged with Arthritis Care in 2018 to form Versus Arthritis.
Similarly, Dr. Marshall reported no financial conflicts regarding CASF, also funded by Arthritis Research UK.
SOURCES: Marshall M. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S16, Abstract I-8 and Magnusson K et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S260-S261, Abstract 367.
TORONTO – Foot osteoarthritis has been a relatively neglected topic by researchers – but that’s finally changing, Michelle Marshall, PhD, observed at the OARSI 2019 World Congress.
She was a coinvestigator in the groundbreaking Clinical Assessment of the Foot (CASF), a large prospective study that has brought new insights into the prevalence of foot osteoarthritis (OA), its risk factors, the sizable disease burden, and foot OA’s diverse phenotypes. She shared study highlights at the meeting, which was sponsored by the Osteoarthritis Research Society International.
Elsewhere at OARSI 2019, Lucy S. Gates, PhD, presented the eagerly awaited results of the Chingford 1000 Women Study of the progression pattern of symptomatic radiographic OA of the first metatarsophalangeal joint (MTPJ). With 19 years of follow-up, Chingford is far and away the largest and longest longitudinal study of first MTP joint OA.
The prospective, population-based, observational cohort CASF study was carried out by Dr. Marshall and her coinvestigators at Keele University in Staffordshire, England. They surveyed Staffordshire residents aged 50 and older regarding whether they had experienced foot pain within the last 12 months. Those who answered affirmatively were invited to come in for a more detailed assessment and get weight-bearing x-rays of both feet. Among the 557 symptomatic participants with foot x-rays, the prevalence of radiographic OA of the foot was 16.7%, or roughly one in six – underscoring that it’s a common condition. The first MTP joint was the most commonly affected site, with a prevalence of 7.8%, followed by the second cuneometatarsal joint (CMJ) at 6.8%, the talonavicular joint (TNJ) at 5.2%, the navicular first cuneiform joint (NCJ) at 5.2%, and the first CMJ at 3.9%. Three-quarters of those who had symptomatic radiographic foot OA reported disabling symptoms, an established risk factor for falls (Ann Rheum Dis. 2015 Jan;74[1]:156-63).
With an eye toward identification of potential distinct phenotypes of foot OA, the CASF investigators conducted a separate analysis of those study participants with symptomatic radiographic midfoot OA – that is, OA of the TNJ, NCJ, and/or first or second CMJs, but not the first MTP joint. The prevalence in the Staffordshire population over age 50 with a history of foot pain was 12%. Independent risk factors for midfoot OA included obesity, with an adjusted odds ratio of 2.0; pain in other weight-bearing lower limb joints, with an adjusted odds ratio of 8.5; diabetes, odds ratio of 1.9; and previous foot injury, with an associated 1.6-fold increased risk. Midfoot OA was most prevalent in women older than 75 years; however, contrary to the conventional wisdom, a history of frequently wearing high-heeled shoes posed no increased risk.
The burden associated with midfoot OA was reflected in affected individuals’ frequent use of health care resources: During the past year, 46% of them had consulted their primary care physicians about their foot pain, 48% had been to a podiatrist, and 19% had seen a physical therapist (Arthritis Res Ther. 2015 Jul 13;17:178. doi: 10.1186/s13075-015-0693-3).
In a separate analysis, the investigators compiled additional evidence from CASF pointing to the existence of two phenotypes of foot OA: isolated first MTP OA and polyarticular foot OA, with distinct risk factors and symptom profiles (Arthritis Care Res [Hoboken]. 2016 Feb;68[2]:217-27).
“We found that OA affected both feet significantly more than was expected by chance, and we identified strong symmetrical patterns. This mirrors findings in hand OA and implies involvement of systemic components within a foot,” Dr. Marshall said.
The course of foot OA
During 18 months of prospective follow-up in CASF, subjects with isolated first MTP joint or polyarticular foot OA showed no clinically meaningful change in symptoms (Arthritis Care Res [Hoboken]. 2018 Jul;70[7]:1107-12).
But that finding may have been a function of the relatively brief follow-up, as the Chingford 1000 Women Study, with its 19 years of prospective follow-up, told a different story. Dr. Gates, of the University of Southampton (England), reported that among the 193 patients with foot x-rays at both baseline and follow-up, by which point they averaged nearly 76 years in age, 33.2% had OA of the first MTP joint of either foot at baseline as defined by at least a grade 2 score on the LaTrobe foot atlas, and 13% had prevalent involvement of both feet. During 19 years of follow-up of the women from Chingford, an area in northeast London, the incidence of new-onset radiographic first MTP joint OA was 7% in the left foot and 17% in the right. Meanwhile, progression to grade 3 radiographic OA occurred in the left foot of 28% of those with grade 2 disease at baseline and in 35% of those with baseline first MTP joint OA of the right foot. Twenty-eight percent of patients with unilateral first MTP joint OA at baseline progressed to bilateral involvement within 19 years.
Dr. Gates reported having no financial conflicts regarding the Chingford study, funded primarily by Arthritis Research UK, which merged with Arthritis Care in 2018 to form Versus Arthritis.
Similarly, Dr. Marshall reported no financial conflicts regarding CASF, also funded by Arthritis Research UK.
SOURCES: Marshall M. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S16, Abstract I-8 and Magnusson K et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S260-S261, Abstract 367.
TORONTO – Foot osteoarthritis has been a relatively neglected topic by researchers – but that’s finally changing, Michelle Marshall, PhD, observed at the OARSI 2019 World Congress.
She was a coinvestigator in the groundbreaking Clinical Assessment of the Foot (CASF), a large prospective study that has brought new insights into the prevalence of foot osteoarthritis (OA), its risk factors, the sizable disease burden, and foot OA’s diverse phenotypes. She shared study highlights at the meeting, which was sponsored by the Osteoarthritis Research Society International.
Elsewhere at OARSI 2019, Lucy S. Gates, PhD, presented the eagerly awaited results of the Chingford 1000 Women Study of the progression pattern of symptomatic radiographic OA of the first metatarsophalangeal joint (MTPJ). With 19 years of follow-up, Chingford is far and away the largest and longest longitudinal study of first MTP joint OA.
The prospective, population-based, observational cohort CASF study was carried out by Dr. Marshall and her coinvestigators at Keele University in Staffordshire, England. They surveyed Staffordshire residents aged 50 and older regarding whether they had experienced foot pain within the last 12 months. Those who answered affirmatively were invited to come in for a more detailed assessment and get weight-bearing x-rays of both feet. Among the 557 symptomatic participants with foot x-rays, the prevalence of radiographic OA of the foot was 16.7%, or roughly one in six – underscoring that it’s a common condition. The first MTP joint was the most commonly affected site, with a prevalence of 7.8%, followed by the second cuneometatarsal joint (CMJ) at 6.8%, the talonavicular joint (TNJ) at 5.2%, the navicular first cuneiform joint (NCJ) at 5.2%, and the first CMJ at 3.9%. Three-quarters of those who had symptomatic radiographic foot OA reported disabling symptoms, an established risk factor for falls (Ann Rheum Dis. 2015 Jan;74[1]:156-63).
With an eye toward identification of potential distinct phenotypes of foot OA, the CASF investigators conducted a separate analysis of those study participants with symptomatic radiographic midfoot OA – that is, OA of the TNJ, NCJ, and/or first or second CMJs, but not the first MTP joint. The prevalence in the Staffordshire population over age 50 with a history of foot pain was 12%. Independent risk factors for midfoot OA included obesity, with an adjusted odds ratio of 2.0; pain in other weight-bearing lower limb joints, with an adjusted odds ratio of 8.5; diabetes, odds ratio of 1.9; and previous foot injury, with an associated 1.6-fold increased risk. Midfoot OA was most prevalent in women older than 75 years; however, contrary to the conventional wisdom, a history of frequently wearing high-heeled shoes posed no increased risk.
The burden associated with midfoot OA was reflected in affected individuals’ frequent use of health care resources: During the past year, 46% of them had consulted their primary care physicians about their foot pain, 48% had been to a podiatrist, and 19% had seen a physical therapist (Arthritis Res Ther. 2015 Jul 13;17:178. doi: 10.1186/s13075-015-0693-3).
In a separate analysis, the investigators compiled additional evidence from CASF pointing to the existence of two phenotypes of foot OA: isolated first MTP OA and polyarticular foot OA, with distinct risk factors and symptom profiles (Arthritis Care Res [Hoboken]. 2016 Feb;68[2]:217-27).
“We found that OA affected both feet significantly more than was expected by chance, and we identified strong symmetrical patterns. This mirrors findings in hand OA and implies involvement of systemic components within a foot,” Dr. Marshall said.
The course of foot OA
During 18 months of prospective follow-up in CASF, subjects with isolated first MTP joint or polyarticular foot OA showed no clinically meaningful change in symptoms (Arthritis Care Res [Hoboken]. 2018 Jul;70[7]:1107-12).
But that finding may have been a function of the relatively brief follow-up, as the Chingford 1000 Women Study, with its 19 years of prospective follow-up, told a different story. Dr. Gates, of the University of Southampton (England), reported that among the 193 patients with foot x-rays at both baseline and follow-up, by which point they averaged nearly 76 years in age, 33.2% had OA of the first MTP joint of either foot at baseline as defined by at least a grade 2 score on the LaTrobe foot atlas, and 13% had prevalent involvement of both feet. During 19 years of follow-up of the women from Chingford, an area in northeast London, the incidence of new-onset radiographic first MTP joint OA was 7% in the left foot and 17% in the right. Meanwhile, progression to grade 3 radiographic OA occurred in the left foot of 28% of those with grade 2 disease at baseline and in 35% of those with baseline first MTP joint OA of the right foot. Twenty-eight percent of patients with unilateral first MTP joint OA at baseline progressed to bilateral involvement within 19 years.
Dr. Gates reported having no financial conflicts regarding the Chingford study, funded primarily by Arthritis Research UK, which merged with Arthritis Care in 2018 to form Versus Arthritis.
Similarly, Dr. Marshall reported no financial conflicts regarding CASF, also funded by Arthritis Research UK.
SOURCES: Marshall M. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S16, Abstract I-8 and Magnusson K et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S260-S261, Abstract 367.
REPORTING FROM OARSI 2019
Weight loss in knee OA patients sustained with liraglutide over 1 year
MADRID – The glucagonlike peptide–1 receptor agonist liraglutide appears to be effective for keeping weight off following an intensive weight-loss program in patients with knee osteoarthritis, according to a randomized, double-blind, placebo-controlled trial presented at the European Congress of Rheumatology.
However, even though the 8-week intensive dietary program led to substantial weight loss and significant improvement in pain, additional weight loss of nearly 2.5 kg over 52 weeks of daily liraglutide treatment did not translate into more pain control.
According to study author Lars Erik Kristensen, MD, PhD, this is the first randomized trial to test the ability of liraglutide to provide a sustained weight loss in OA patients. The Food and Drug Administration indication for liraglutide is as an adjunct to diet and exercise for glycemic control in type 2 diabetes mellitus.
The study compared liraglutide against placebo in patients who had completed an intensive weight-control program in which the median loss was 12.46 kg. They were followed for 52 weeks.
At the end of follow-up, patients in the placebo group had gained a mean of 1.17 kg while those randomized to liraglutide lost an additional 2.76 kg. The between-group difference of 3.93 kg was statistically significant (P = .008).
“We believe that liraglutide is a promising agent for sustained weight loss in OA patients,” concluded Dr. Kristensen, a clinical researcher in rheumatology in the Parker Institute at Bispebjerg-Frederiksberg Hospital in Copenhagen.
In the single-center study, 156 patients were enrolled and randomized. In an initial 8-week diet intervention undertaken by both groups, an intensive program for weight loss included average daily calorie intakes of less than 800 kcal along with dietetic counseling. Patients were monitored for daily activities.
The majority of patients achieved a 10% or greater loss of total body weight during the intensive program before initiating 3 mg of once-daily liraglutide or a placebo.
Over the course of 52 weeks, the attrition from the study was relatively low. Among the 80 patients randomized to liraglutide, only 2 were lost because of noncompliance. Another 12 participants left the study before completion, 10 of whom did so for treatment-associated adverse effects. In the placebo arm, four patients were noncompliant, four left for treatment-associated adverse effects, and five left for other reasons.
Following the 8-week intensive dietary program, there was 11.86-point improvement in the pain subscale of the Knee and Osteoarthritis Outcome Score, confirming a substantial symptomatic benefit from this degree of weight loss. While this improvement in pain score was sustained at 52 weeks in both groups, the additional weight loss in the liraglutide arm did not lead to additional pain control.
The lack of additional pain control in the liraglutide group was disappointing, and the reason is unclear, but Dr. Kristensen emphasized that the persistent improvement in pain control was a positive result. In patients who are overweight or obese, regardless of whether they have concomitant OA, weight loss is not only difficult to achieve but difficult to sustain even after a successful intervention.
Dr. Kristensen reported financial relationships with multiple pharmaceutical companies. The trial received funding from Novo Nordisk.
SOURCE: Kristensen LE et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):71-2. Abstract OP0011. doi: 10.1136/annrheumdis-2019-eular.1375.
MADRID – The glucagonlike peptide–1 receptor agonist liraglutide appears to be effective for keeping weight off following an intensive weight-loss program in patients with knee osteoarthritis, according to a randomized, double-blind, placebo-controlled trial presented at the European Congress of Rheumatology.
However, even though the 8-week intensive dietary program led to substantial weight loss and significant improvement in pain, additional weight loss of nearly 2.5 kg over 52 weeks of daily liraglutide treatment did not translate into more pain control.
According to study author Lars Erik Kristensen, MD, PhD, this is the first randomized trial to test the ability of liraglutide to provide a sustained weight loss in OA patients. The Food and Drug Administration indication for liraglutide is as an adjunct to diet and exercise for glycemic control in type 2 diabetes mellitus.
The study compared liraglutide against placebo in patients who had completed an intensive weight-control program in which the median loss was 12.46 kg. They were followed for 52 weeks.
At the end of follow-up, patients in the placebo group had gained a mean of 1.17 kg while those randomized to liraglutide lost an additional 2.76 kg. The between-group difference of 3.93 kg was statistically significant (P = .008).
“We believe that liraglutide is a promising agent for sustained weight loss in OA patients,” concluded Dr. Kristensen, a clinical researcher in rheumatology in the Parker Institute at Bispebjerg-Frederiksberg Hospital in Copenhagen.
In the single-center study, 156 patients were enrolled and randomized. In an initial 8-week diet intervention undertaken by both groups, an intensive program for weight loss included average daily calorie intakes of less than 800 kcal along with dietetic counseling. Patients were monitored for daily activities.
The majority of patients achieved a 10% or greater loss of total body weight during the intensive program before initiating 3 mg of once-daily liraglutide or a placebo.
Over the course of 52 weeks, the attrition from the study was relatively low. Among the 80 patients randomized to liraglutide, only 2 were lost because of noncompliance. Another 12 participants left the study before completion, 10 of whom did so for treatment-associated adverse effects. In the placebo arm, four patients were noncompliant, four left for treatment-associated adverse effects, and five left for other reasons.
Following the 8-week intensive dietary program, there was 11.86-point improvement in the pain subscale of the Knee and Osteoarthritis Outcome Score, confirming a substantial symptomatic benefit from this degree of weight loss. While this improvement in pain score was sustained at 52 weeks in both groups, the additional weight loss in the liraglutide arm did not lead to additional pain control.
The lack of additional pain control in the liraglutide group was disappointing, and the reason is unclear, but Dr. Kristensen emphasized that the persistent improvement in pain control was a positive result. In patients who are overweight or obese, regardless of whether they have concomitant OA, weight loss is not only difficult to achieve but difficult to sustain even after a successful intervention.
Dr. Kristensen reported financial relationships with multiple pharmaceutical companies. The trial received funding from Novo Nordisk.
SOURCE: Kristensen LE et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):71-2. Abstract OP0011. doi: 10.1136/annrheumdis-2019-eular.1375.
MADRID – The glucagonlike peptide–1 receptor agonist liraglutide appears to be effective for keeping weight off following an intensive weight-loss program in patients with knee osteoarthritis, according to a randomized, double-blind, placebo-controlled trial presented at the European Congress of Rheumatology.
However, even though the 8-week intensive dietary program led to substantial weight loss and significant improvement in pain, additional weight loss of nearly 2.5 kg over 52 weeks of daily liraglutide treatment did not translate into more pain control.
According to study author Lars Erik Kristensen, MD, PhD, this is the first randomized trial to test the ability of liraglutide to provide a sustained weight loss in OA patients. The Food and Drug Administration indication for liraglutide is as an adjunct to diet and exercise for glycemic control in type 2 diabetes mellitus.
The study compared liraglutide against placebo in patients who had completed an intensive weight-control program in which the median loss was 12.46 kg. They were followed for 52 weeks.
At the end of follow-up, patients in the placebo group had gained a mean of 1.17 kg while those randomized to liraglutide lost an additional 2.76 kg. The between-group difference of 3.93 kg was statistically significant (P = .008).
“We believe that liraglutide is a promising agent for sustained weight loss in OA patients,” concluded Dr. Kristensen, a clinical researcher in rheumatology in the Parker Institute at Bispebjerg-Frederiksberg Hospital in Copenhagen.
In the single-center study, 156 patients were enrolled and randomized. In an initial 8-week diet intervention undertaken by both groups, an intensive program for weight loss included average daily calorie intakes of less than 800 kcal along with dietetic counseling. Patients were monitored for daily activities.
The majority of patients achieved a 10% or greater loss of total body weight during the intensive program before initiating 3 mg of once-daily liraglutide or a placebo.
Over the course of 52 weeks, the attrition from the study was relatively low. Among the 80 patients randomized to liraglutide, only 2 were lost because of noncompliance. Another 12 participants left the study before completion, 10 of whom did so for treatment-associated adverse effects. In the placebo arm, four patients were noncompliant, four left for treatment-associated adverse effects, and five left for other reasons.
Following the 8-week intensive dietary program, there was 11.86-point improvement in the pain subscale of the Knee and Osteoarthritis Outcome Score, confirming a substantial symptomatic benefit from this degree of weight loss. While this improvement in pain score was sustained at 52 weeks in both groups, the additional weight loss in the liraglutide arm did not lead to additional pain control.
The lack of additional pain control in the liraglutide group was disappointing, and the reason is unclear, but Dr. Kristensen emphasized that the persistent improvement in pain control was a positive result. In patients who are overweight or obese, regardless of whether they have concomitant OA, weight loss is not only difficult to achieve but difficult to sustain even after a successful intervention.
Dr. Kristensen reported financial relationships with multiple pharmaceutical companies. The trial received funding from Novo Nordisk.
SOURCE: Kristensen LE et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):71-2. Abstract OP0011. doi: 10.1136/annrheumdis-2019-eular.1375.
REPORTING FROM EULAR 2019 CONGRESS
Patient selection important for osteoarthritis structural and symptom endpoints
MADRID – To achieve positive trials with new agents in osteoarthritis, patient selection should be considered in the context of the primary endpoints, according to Philip G. Conaghan, MBBS, PhD, chair of musculoskeletal medicine at the University of Leeds (England).
In an interview, Dr. Conaghan explained that the issue has arisen with emerging agents that are designed for structural improvements with the expectation that symptom improvements will follow. Recapping a presentation he made at the European Congress of Rheumatology, he cautioned that the key aspects of trial design for these novel agents, including patient and endpoint selection, are particularly challenging.
As an example, Dr. Conaghan referred to the experience so far with the ongoing phase 2 FORWARD trial with sprifermin, a recombinant form of human fibroblast growth factor. In this study, sprifermin has already shown promise for growing cartilage, but the benefit accrues slowly, and there is no symptomatic improvement early in the course of treatment.
Based on the experience with FORWARD, much has been learned about a potential tension between structural and symptomatic endpoints in osteoarthritis, according to Dr. Conaghan. For one, it appears to be important to select patients most likely to achieve measurable structural improvements quickly to achieve a positive result in a reasonable period of time.
For another, it may be necessary to select symptom endpoints that reflect structural change while cautioning patients about the potential for a long delay before a clinical benefit is experienced.
In osteoarthritis, clinical benefit has been traditionally captured with relief of pain. Although an improvement in joint structure might be the best way to produce this result, this has to be proved. Reasonable and achievable endpoints are needed for emerging drugs with the potential to rebuild the joint not just to control pain, he said.
SOURCE: Gühring H et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):70-1. Abstract OP0010. doi: 10.1136/annrheumdis-2019-eular.1216.
MADRID – To achieve positive trials with new agents in osteoarthritis, patient selection should be considered in the context of the primary endpoints, according to Philip G. Conaghan, MBBS, PhD, chair of musculoskeletal medicine at the University of Leeds (England).
In an interview, Dr. Conaghan explained that the issue has arisen with emerging agents that are designed for structural improvements with the expectation that symptom improvements will follow. Recapping a presentation he made at the European Congress of Rheumatology, he cautioned that the key aspects of trial design for these novel agents, including patient and endpoint selection, are particularly challenging.
As an example, Dr. Conaghan referred to the experience so far with the ongoing phase 2 FORWARD trial with sprifermin, a recombinant form of human fibroblast growth factor. In this study, sprifermin has already shown promise for growing cartilage, but the benefit accrues slowly, and there is no symptomatic improvement early in the course of treatment.
Based on the experience with FORWARD, much has been learned about a potential tension between structural and symptomatic endpoints in osteoarthritis, according to Dr. Conaghan. For one, it appears to be important to select patients most likely to achieve measurable structural improvements quickly to achieve a positive result in a reasonable period of time.
For another, it may be necessary to select symptom endpoints that reflect structural change while cautioning patients about the potential for a long delay before a clinical benefit is experienced.
In osteoarthritis, clinical benefit has been traditionally captured with relief of pain. Although an improvement in joint structure might be the best way to produce this result, this has to be proved. Reasonable and achievable endpoints are needed for emerging drugs with the potential to rebuild the joint not just to control pain, he said.
SOURCE: Gühring H et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):70-1. Abstract OP0010. doi: 10.1136/annrheumdis-2019-eular.1216.
MADRID – To achieve positive trials with new agents in osteoarthritis, patient selection should be considered in the context of the primary endpoints, according to Philip G. Conaghan, MBBS, PhD, chair of musculoskeletal medicine at the University of Leeds (England).
In an interview, Dr. Conaghan explained that the issue has arisen with emerging agents that are designed for structural improvements with the expectation that symptom improvements will follow. Recapping a presentation he made at the European Congress of Rheumatology, he cautioned that the key aspects of trial design for these novel agents, including patient and endpoint selection, are particularly challenging.
As an example, Dr. Conaghan referred to the experience so far with the ongoing phase 2 FORWARD trial with sprifermin, a recombinant form of human fibroblast growth factor. In this study, sprifermin has already shown promise for growing cartilage, but the benefit accrues slowly, and there is no symptomatic improvement early in the course of treatment.
Based on the experience with FORWARD, much has been learned about a potential tension between structural and symptomatic endpoints in osteoarthritis, according to Dr. Conaghan. For one, it appears to be important to select patients most likely to achieve measurable structural improvements quickly to achieve a positive result in a reasonable period of time.
For another, it may be necessary to select symptom endpoints that reflect structural change while cautioning patients about the potential for a long delay before a clinical benefit is experienced.
In osteoarthritis, clinical benefit has been traditionally captured with relief of pain. Although an improvement in joint structure might be the best way to produce this result, this has to be proved. Reasonable and achievable endpoints are needed for emerging drugs with the potential to rebuild the joint not just to control pain, he said.
SOURCE: Gühring H et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):70-1. Abstract OP0010. doi: 10.1136/annrheumdis-2019-eular.1216.
REPORTING FROM EULAR 2019 CONGRESS
Swedish OA self-management program earns high marks
TORONTO – A in an observational registry study of 47,035 participants, Therese S. Jönsson reported at the OARSI 2019 World Congress.
“The BOA [Better management of patients with Osteoarthritis] program is feasible and demonstrates positive results in a large clinical context. Our results indicate that offering this intervention as the first-line treatment for patients with hip and knee osteoarthritis may reduce the burden of disease,” she said at the meeting, sponsored by the Osteoarthritis Research Society International.
Indeed, the results of the Swedish BOA program for nonsurgical treatment of OA played an influential role in the new draft of OARSI guidelines for management of knee osteoarthritis. The program could serve as a template for implementation of a similar approach in other health care settings. The BOA program has been rolled out to more than 700 Swedish primary care practice sites, according to Ms. Jönsson, a PhD student at Lund (Sweden) University.
The program was created to meet a defined national goal that, as early as possible in the course of the disease, every Swedish patient with knee or hip OA should receive education about their disease and the importance of exercise as a means of improving their quality of life. The impetus for BOA was a widespread concern that, in Sweden and elsewhere, far too many OA patients were being referred for joint surgery without ever having tried the evidence-based core nonsurgical treatments.
The BOA intervention
Following patient referral by a primary care physician, the Swedish BOA program starts off with individual assessment and biomechanical testing by a physical therapist. This is followed by two small-group education sessions of about 90 minutes led by a physical therapist or occupational therapist. Session one includes information about the pathology of OA, risk factors, symptoms, and the available treatments. Session two focuses on coping skills, self-management strategies to reduce pain and symptoms, the central role of exercise as a core treatment in OA, and ways to incorporate physical activity into daily living.
Then comes a decision point. Having listened to a motivational message extolling the benefits of exercise as a means of empowering self-management of their chronic disease, participants next have three choices: They can attend supervised group exercise classes twice weekly for 6 weeks to kick-start a more physically active lifestyle, they can start an individually adapted home exercise program, or they can decline exercise.
Giving patients a choice in this matter is a strategy rooted in the psychological concept of motivational stages of change, which recognizes that some patients with a chronic illness whose course is modifiable through lifestyle change are initially in a precontemplation stage of change. And pushing them hard at that point is counterproductive. The home exercise option, which permits patients to take a low-and-slow approach to exercise, is based upon the BOA program developers’ stated philosophy that 5 minutes of exercise daily, performed as part of everyday life, has a bigger impact upon function than does a 30-minute exercise program that’s abandoned after a few weeks. The goal of the BOA program is for patients to eventually build up to at least 150 minutes of moderate-intensity exercise per week.
The results
Roughly 15% of patients enrolled in the registry declined the exercise option. Ms. Jönsson’s analysis focused on those who opted to participate in an exercise program, 40% of whom selected the home exercise option. This analysis included 30,682 patients with knee OA and 16,363 with hip OA. They returned to the physical therapist for a face-to-face reassessment after 3 months, and they completed a mailed outcome-oriented questionnaire at 12 months.
The BOA intervention was more effective in reducing pain in the knee OA group than in those with hip OA. A statistically significant reduction in self-assessed pain scores on a 0-10 scale was seen in both the knee and hip OA groups at 3 and 12 months; however, only the knee OA patients achieved a clinically important decrease in pain, defined as at least a 15% drop in pain scores. Their pain scores improved from 5.24 at baseline to 4.07 at 3 months and 4.23 at 12 months. In the hip OA patients, pain scores went from 5.39 at baseline to 4.56 at 3 months and 4.7 at 12 months.
However, at 3 and 12 months, significantly fewer patients in both the hip and knee OA groups reported experiencing pain more than once per week, compared with baseline. They also took fewer pain-killing medications, reported less avoidance behavior involving fear of movement, were less willing to undergo joint surgery, and scored significantly higher on the five-level EQ-5D quality-of-life measure than at baseline. Moreover, fewer patients were on sick leave at the 12-month follow-up than at baseline, an outcome that wasn’t assessed at 3 months.
Adherence to the group exercise classes was “quite low,” according to Ms. Jönsson, and poor adherence was reflected in smaller reductions in pain scores. Only 30% of patients who elected the supervised group exercise option attended 10 of the 12 sessions, she noted.
Ms. Jönsson reported having no financial conflicts regarding her study. The BOA program is funded by the Swedish government.
SOURCE: Jönsson TS et al. Osteoarthritis Cartilage. 2019 Apr;27(suppl 1):S497, Abstract 717.
TORONTO – A in an observational registry study of 47,035 participants, Therese S. Jönsson reported at the OARSI 2019 World Congress.
“The BOA [Better management of patients with Osteoarthritis] program is feasible and demonstrates positive results in a large clinical context. Our results indicate that offering this intervention as the first-line treatment for patients with hip and knee osteoarthritis may reduce the burden of disease,” she said at the meeting, sponsored by the Osteoarthritis Research Society International.
Indeed, the results of the Swedish BOA program for nonsurgical treatment of OA played an influential role in the new draft of OARSI guidelines for management of knee osteoarthritis. The program could serve as a template for implementation of a similar approach in other health care settings. The BOA program has been rolled out to more than 700 Swedish primary care practice sites, according to Ms. Jönsson, a PhD student at Lund (Sweden) University.
The program was created to meet a defined national goal that, as early as possible in the course of the disease, every Swedish patient with knee or hip OA should receive education about their disease and the importance of exercise as a means of improving their quality of life. The impetus for BOA was a widespread concern that, in Sweden and elsewhere, far too many OA patients were being referred for joint surgery without ever having tried the evidence-based core nonsurgical treatments.
The BOA intervention
Following patient referral by a primary care physician, the Swedish BOA program starts off with individual assessment and biomechanical testing by a physical therapist. This is followed by two small-group education sessions of about 90 minutes led by a physical therapist or occupational therapist. Session one includes information about the pathology of OA, risk factors, symptoms, and the available treatments. Session two focuses on coping skills, self-management strategies to reduce pain and symptoms, the central role of exercise as a core treatment in OA, and ways to incorporate physical activity into daily living.
Then comes a decision point. Having listened to a motivational message extolling the benefits of exercise as a means of empowering self-management of their chronic disease, participants next have three choices: They can attend supervised group exercise classes twice weekly for 6 weeks to kick-start a more physically active lifestyle, they can start an individually adapted home exercise program, or they can decline exercise.
Giving patients a choice in this matter is a strategy rooted in the psychological concept of motivational stages of change, which recognizes that some patients with a chronic illness whose course is modifiable through lifestyle change are initially in a precontemplation stage of change. And pushing them hard at that point is counterproductive. The home exercise option, which permits patients to take a low-and-slow approach to exercise, is based upon the BOA program developers’ stated philosophy that 5 minutes of exercise daily, performed as part of everyday life, has a bigger impact upon function than does a 30-minute exercise program that’s abandoned after a few weeks. The goal of the BOA program is for patients to eventually build up to at least 150 minutes of moderate-intensity exercise per week.
The results
Roughly 15% of patients enrolled in the registry declined the exercise option. Ms. Jönsson’s analysis focused on those who opted to participate in an exercise program, 40% of whom selected the home exercise option. This analysis included 30,682 patients with knee OA and 16,363 with hip OA. They returned to the physical therapist for a face-to-face reassessment after 3 months, and they completed a mailed outcome-oriented questionnaire at 12 months.
The BOA intervention was more effective in reducing pain in the knee OA group than in those with hip OA. A statistically significant reduction in self-assessed pain scores on a 0-10 scale was seen in both the knee and hip OA groups at 3 and 12 months; however, only the knee OA patients achieved a clinically important decrease in pain, defined as at least a 15% drop in pain scores. Their pain scores improved from 5.24 at baseline to 4.07 at 3 months and 4.23 at 12 months. In the hip OA patients, pain scores went from 5.39 at baseline to 4.56 at 3 months and 4.7 at 12 months.
However, at 3 and 12 months, significantly fewer patients in both the hip and knee OA groups reported experiencing pain more than once per week, compared with baseline. They also took fewer pain-killing medications, reported less avoidance behavior involving fear of movement, were less willing to undergo joint surgery, and scored significantly higher on the five-level EQ-5D quality-of-life measure than at baseline. Moreover, fewer patients were on sick leave at the 12-month follow-up than at baseline, an outcome that wasn’t assessed at 3 months.
Adherence to the group exercise classes was “quite low,” according to Ms. Jönsson, and poor adherence was reflected in smaller reductions in pain scores. Only 30% of patients who elected the supervised group exercise option attended 10 of the 12 sessions, she noted.
Ms. Jönsson reported having no financial conflicts regarding her study. The BOA program is funded by the Swedish government.
SOURCE: Jönsson TS et al. Osteoarthritis Cartilage. 2019 Apr;27(suppl 1):S497, Abstract 717.
TORONTO – A in an observational registry study of 47,035 participants, Therese S. Jönsson reported at the OARSI 2019 World Congress.
“The BOA [Better management of patients with Osteoarthritis] program is feasible and demonstrates positive results in a large clinical context. Our results indicate that offering this intervention as the first-line treatment for patients with hip and knee osteoarthritis may reduce the burden of disease,” she said at the meeting, sponsored by the Osteoarthritis Research Society International.
Indeed, the results of the Swedish BOA program for nonsurgical treatment of OA played an influential role in the new draft of OARSI guidelines for management of knee osteoarthritis. The program could serve as a template for implementation of a similar approach in other health care settings. The BOA program has been rolled out to more than 700 Swedish primary care practice sites, according to Ms. Jönsson, a PhD student at Lund (Sweden) University.
The program was created to meet a defined national goal that, as early as possible in the course of the disease, every Swedish patient with knee or hip OA should receive education about their disease and the importance of exercise as a means of improving their quality of life. The impetus for BOA was a widespread concern that, in Sweden and elsewhere, far too many OA patients were being referred for joint surgery without ever having tried the evidence-based core nonsurgical treatments.
The BOA intervention
Following patient referral by a primary care physician, the Swedish BOA program starts off with individual assessment and biomechanical testing by a physical therapist. This is followed by two small-group education sessions of about 90 minutes led by a physical therapist or occupational therapist. Session one includes information about the pathology of OA, risk factors, symptoms, and the available treatments. Session two focuses on coping skills, self-management strategies to reduce pain and symptoms, the central role of exercise as a core treatment in OA, and ways to incorporate physical activity into daily living.
Then comes a decision point. Having listened to a motivational message extolling the benefits of exercise as a means of empowering self-management of their chronic disease, participants next have three choices: They can attend supervised group exercise classes twice weekly for 6 weeks to kick-start a more physically active lifestyle, they can start an individually adapted home exercise program, or they can decline exercise.
Giving patients a choice in this matter is a strategy rooted in the psychological concept of motivational stages of change, which recognizes that some patients with a chronic illness whose course is modifiable through lifestyle change are initially in a precontemplation stage of change. And pushing them hard at that point is counterproductive. The home exercise option, which permits patients to take a low-and-slow approach to exercise, is based upon the BOA program developers’ stated philosophy that 5 minutes of exercise daily, performed as part of everyday life, has a bigger impact upon function than does a 30-minute exercise program that’s abandoned after a few weeks. The goal of the BOA program is for patients to eventually build up to at least 150 minutes of moderate-intensity exercise per week.
The results
Roughly 15% of patients enrolled in the registry declined the exercise option. Ms. Jönsson’s analysis focused on those who opted to participate in an exercise program, 40% of whom selected the home exercise option. This analysis included 30,682 patients with knee OA and 16,363 with hip OA. They returned to the physical therapist for a face-to-face reassessment after 3 months, and they completed a mailed outcome-oriented questionnaire at 12 months.
The BOA intervention was more effective in reducing pain in the knee OA group than in those with hip OA. A statistically significant reduction in self-assessed pain scores on a 0-10 scale was seen in both the knee and hip OA groups at 3 and 12 months; however, only the knee OA patients achieved a clinically important decrease in pain, defined as at least a 15% drop in pain scores. Their pain scores improved from 5.24 at baseline to 4.07 at 3 months and 4.23 at 12 months. In the hip OA patients, pain scores went from 5.39 at baseline to 4.56 at 3 months and 4.7 at 12 months.
However, at 3 and 12 months, significantly fewer patients in both the hip and knee OA groups reported experiencing pain more than once per week, compared with baseline. They also took fewer pain-killing medications, reported less avoidance behavior involving fear of movement, were less willing to undergo joint surgery, and scored significantly higher on the five-level EQ-5D quality-of-life measure than at baseline. Moreover, fewer patients were on sick leave at the 12-month follow-up than at baseline, an outcome that wasn’t assessed at 3 months.
Adherence to the group exercise classes was “quite low,” according to Ms. Jönsson, and poor adherence was reflected in smaller reductions in pain scores. Only 30% of patients who elected the supervised group exercise option attended 10 of the 12 sessions, she noted.
Ms. Jönsson reported having no financial conflicts regarding her study. The BOA program is funded by the Swedish government.
SOURCE: Jönsson TS et al. Osteoarthritis Cartilage. 2019 Apr;27(suppl 1):S497, Abstract 717.
REPORTING FROM OARSI 2019
OA is underrepresented in the medical literature
TORONTO – Osteoarthritis research doesn’t get nearly the respect it deserves in the medical literature, Elizabeth M. Badley, PhD, asserted at the OARSI 2019 World Congress.
“Osteoarthritis is by far the most common type of arthritis. There are easily 10 times more people who have osteoarthritis than any other joint disease, but when you look at the literature, the situation is kind of reversed. Osteoarthritis is brushed off by society to a degree,” she said at the meeting, sponsored by the Osteoarthritis Research Society International.
Dr. Bradley and colleagues performed a search of MEDLINE for 2007-2016, which turned up a total of 1,625 publications in 2016 on osteoarthritis, excluding those with an orthopedic surgery focus, compared with 10,904 results regarding the broader topic of joint diseases and 28,932 on musculoskeletal diseases.
The bottom line: “Progress is slow, and at this rate osteoarthritis will not be receiving the attention it deserves in our lifetime,” said Dr. Badley, of the department of epidemiology at the University of Toronto and a senior scientist at the Krembil Research Institute, also in Toronto.
The number of publications per year devoted to OA rose by a robust 88% during 2007-2016, while the number on OA not focused on orthopedic procedures grew by 65%. Both of these increases were greater than those for publications on musculoskeletal diseases and joint diseases overall, which were 41% and 51%, respectively. But the absolute number of OA publications was dwarfed by the numbers of those in the other search categories. For example, the number of publications on OA without an orthopedic surgery thrust was 985 in 2007, compared with 7,204 on joint diseases overall.
Among the striking findings of the investigators’ study of the medical literature was the disconnect between the amount of attention devoted to some of the joint-specific manifestations of OA and the actual prevalence of these conditions in the population. For example, the prevalence of hand OA in people living with OA was 52% according to the 2009 Survey on Living with Chronic Diseases in Canada, conducted by Statistics Canada, yet only 6.5% of the publications on OA in 2016 were devoted to hand/thumb OA. Similarly, the prevalence of spine OA was 52% among Canadians with OA, but only 4.3% of OA publications in 2016 focused on that topic. And while the number of publications devoted to elbow OA soared by a seemingly impressive 233% during the study period, the actual numbers were 3 publications in 2007 and 10 in 2016.
“Also, the average number of affected joints in people with osteoarthritis is four. Yet very, very few papers are about multijoint osteoarthritis. And when they do talk about multijoint osteoarthritis, they’re still only talking about hand/hip/knee. So we’re missing the bigger picture of osteoarthritis as a multijoint disease. We’re missing the spine, largely, as a part of osteoarthritis, and we’re missing the peripheral joints,” she said.
Dr. Badley reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Badley EM et al. Osteoarthritis Cartilage. 2019 Apr;27(Suppl 1):S278, Abstract 393.
TORONTO – Osteoarthritis research doesn’t get nearly the respect it deserves in the medical literature, Elizabeth M. Badley, PhD, asserted at the OARSI 2019 World Congress.
“Osteoarthritis is by far the most common type of arthritis. There are easily 10 times more people who have osteoarthritis than any other joint disease, but when you look at the literature, the situation is kind of reversed. Osteoarthritis is brushed off by society to a degree,” she said at the meeting, sponsored by the Osteoarthritis Research Society International.
Dr. Bradley and colleagues performed a search of MEDLINE for 2007-2016, which turned up a total of 1,625 publications in 2016 on osteoarthritis, excluding those with an orthopedic surgery focus, compared with 10,904 results regarding the broader topic of joint diseases and 28,932 on musculoskeletal diseases.
The bottom line: “Progress is slow, and at this rate osteoarthritis will not be receiving the attention it deserves in our lifetime,” said Dr. Badley, of the department of epidemiology at the University of Toronto and a senior scientist at the Krembil Research Institute, also in Toronto.
The number of publications per year devoted to OA rose by a robust 88% during 2007-2016, while the number on OA not focused on orthopedic procedures grew by 65%. Both of these increases were greater than those for publications on musculoskeletal diseases and joint diseases overall, which were 41% and 51%, respectively. But the absolute number of OA publications was dwarfed by the numbers of those in the other search categories. For example, the number of publications on OA without an orthopedic surgery thrust was 985 in 2007, compared with 7,204 on joint diseases overall.
Among the striking findings of the investigators’ study of the medical literature was the disconnect between the amount of attention devoted to some of the joint-specific manifestations of OA and the actual prevalence of these conditions in the population. For example, the prevalence of hand OA in people living with OA was 52% according to the 2009 Survey on Living with Chronic Diseases in Canada, conducted by Statistics Canada, yet only 6.5% of the publications on OA in 2016 were devoted to hand/thumb OA. Similarly, the prevalence of spine OA was 52% among Canadians with OA, but only 4.3% of OA publications in 2016 focused on that topic. And while the number of publications devoted to elbow OA soared by a seemingly impressive 233% during the study period, the actual numbers were 3 publications in 2007 and 10 in 2016.
“Also, the average number of affected joints in people with osteoarthritis is four. Yet very, very few papers are about multijoint osteoarthritis. And when they do talk about multijoint osteoarthritis, they’re still only talking about hand/hip/knee. So we’re missing the bigger picture of osteoarthritis as a multijoint disease. We’re missing the spine, largely, as a part of osteoarthritis, and we’re missing the peripheral joints,” she said.
Dr. Badley reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Badley EM et al. Osteoarthritis Cartilage. 2019 Apr;27(Suppl 1):S278, Abstract 393.
TORONTO – Osteoarthritis research doesn’t get nearly the respect it deserves in the medical literature, Elizabeth M. Badley, PhD, asserted at the OARSI 2019 World Congress.
“Osteoarthritis is by far the most common type of arthritis. There are easily 10 times more people who have osteoarthritis than any other joint disease, but when you look at the literature, the situation is kind of reversed. Osteoarthritis is brushed off by society to a degree,” she said at the meeting, sponsored by the Osteoarthritis Research Society International.
Dr. Bradley and colleagues performed a search of MEDLINE for 2007-2016, which turned up a total of 1,625 publications in 2016 on osteoarthritis, excluding those with an orthopedic surgery focus, compared with 10,904 results regarding the broader topic of joint diseases and 28,932 on musculoskeletal diseases.
The bottom line: “Progress is slow, and at this rate osteoarthritis will not be receiving the attention it deserves in our lifetime,” said Dr. Badley, of the department of epidemiology at the University of Toronto and a senior scientist at the Krembil Research Institute, also in Toronto.
The number of publications per year devoted to OA rose by a robust 88% during 2007-2016, while the number on OA not focused on orthopedic procedures grew by 65%. Both of these increases were greater than those for publications on musculoskeletal diseases and joint diseases overall, which were 41% and 51%, respectively. But the absolute number of OA publications was dwarfed by the numbers of those in the other search categories. For example, the number of publications on OA without an orthopedic surgery thrust was 985 in 2007, compared with 7,204 on joint diseases overall.
Among the striking findings of the investigators’ study of the medical literature was the disconnect between the amount of attention devoted to some of the joint-specific manifestations of OA and the actual prevalence of these conditions in the population. For example, the prevalence of hand OA in people living with OA was 52% according to the 2009 Survey on Living with Chronic Diseases in Canada, conducted by Statistics Canada, yet only 6.5% of the publications on OA in 2016 were devoted to hand/thumb OA. Similarly, the prevalence of spine OA was 52% among Canadians with OA, but only 4.3% of OA publications in 2016 focused on that topic. And while the number of publications devoted to elbow OA soared by a seemingly impressive 233% during the study period, the actual numbers were 3 publications in 2007 and 10 in 2016.
“Also, the average number of affected joints in people with osteoarthritis is four. Yet very, very few papers are about multijoint osteoarthritis. And when they do talk about multijoint osteoarthritis, they’re still only talking about hand/hip/knee. So we’re missing the bigger picture of osteoarthritis as a multijoint disease. We’re missing the spine, largely, as a part of osteoarthritis, and we’re missing the peripheral joints,” she said.
Dr. Badley reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Badley EM et al. Osteoarthritis Cartilage. 2019 Apr;27(Suppl 1):S278, Abstract 393.
REPORTING FROM OARSI 2019
Methotrexate significantly reduced knee OA pain
TORONTO – Philip G. Conaghan, MD, PhD, reported at the OARSI 2019 World Congress.
There is, however, an asterisk attached to these findings. “Despite a moderate standard effect size, the treatment effect was smaller than some of the thresholds for what is considered clinically meaningful,” he noted at the meeting sponsored by the Osteoarthritis Research Society International.
That being said, the rheumatologist is convinced further investigation of methotrexate in osteoarthritis is warranted.
“I have to say that, unlike our earlier hydroxychloroquine trial, which was robustly negative with nothing more to say, I think there is a signal in this study. I need to understand the results of this trial better to understand if there is a subgroup we could treat with methotrexate. It’s a cheap drug, it’s readily available, and we’ve got a lot of experience with it,” noted Dr. Conaghan, professor of musculoskeletal medicine at the University of Leeds (England) and director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The rationale for the 15-center PROMOTE trial is that synovitis is common in OA. Synovitis is associated with pain, methotrexate is the gold-standard treatment for synovitis in inflammatory forms of arthritis, and current treatments for OA are, to say the least, severely limited. Also, an earlier 30-patient, open-label pilot study of methotrexate in patients with painful knee OA conducted by Dr. Conaghan and coworkers suggested the drug was promising (Rheumatology [Oxford]. 2013 May;52[5]:888-92).
PROMOTE included 134 patients with symptomatic and radiographic knee OA who were randomized in double-blind fashion to 6 months of oral methotrexate at 10 mg titrated to a target dose of 25 mg/week or to placebo. All patients also received usual care with oral NSAIDs and/or acetaminophen. Their mean baseline knee pain on a 0-10 numeric rating scale was 6.6.
The primary endpoint, assessed at 6 months, was the difference between the two study arms in average knee pain during the previous week on a 0-10 scale. The score was 5.1 in the methotrexate group and 6.2 in the placebo arm, for a baseline-adjusted treatment difference of 0.83 points, which works out to a standard effect size of 0.36. When the data were reanalyzed after excluding the 15 patients who missed more than four doses of medication within any 3-month period, the between-group difference in pain scores increased to 0.95 points in favor of the methotrexate group.
A significant difference in favor of the methotrexate group was documented in the OARSI-OMERACT response rate at 6 months: 45% in the methotrexate group and 26% in the controls. Some secondary endpoints were positive as well, with statistically significant differences seen at 6 months in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) stiffness, WOMAC physical function, and several other endpoints. But there were no significant differences in WOMAC pain, SF-12 physical component or SF-12 mental component scores, or in an OA quality of life measure.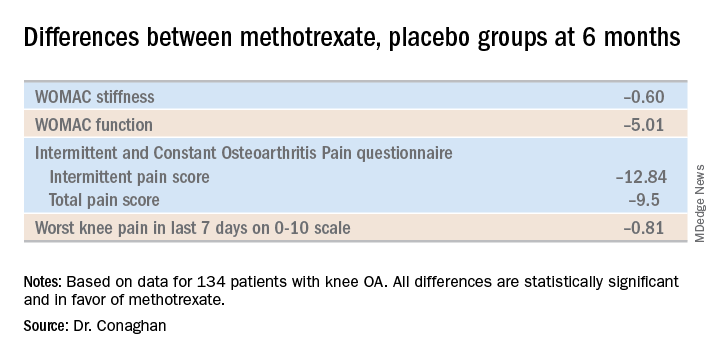
The mean dose of methotrexate used in the study was about 17 mg/week. Dr. Conaghan said that if he could do the trial over again, he would have used subcutaneous methotrexate.
“It’s a more reliable way of getting a dose into people and probably of getting a slightly higher dose into people. In the rheumatoid arthritis world, we use a lot more subcutaneous methotrexate now than we did 10 years ago because it gets around a lot of the minor side effects and helps compliance,” he said.
One audience member suggested that one potentially useful way to zero in on a subgroup of knee OA patients likely to derive the most benefit from methotrexate would be to have screened potential study participants for comorbid fibromyalgia and exclude those with the disorder. Dr. Conaghan replied that the PROMOTE investigators did gather data on participants’ pain at sites other than the knee. That data can be used to identify those at increased likelihood of fibromyalgia, and he agreed that’s worth looking into.
Dr. Conaghan reported having no financial conflicts regarding PROMOTE, which was funded by the U.K. National Institute for Health Research and Versus Arthritis.
SOURCE: Conaghan PG et al. OARSI 2019, Abstract 86.
TORONTO – Philip G. Conaghan, MD, PhD, reported at the OARSI 2019 World Congress.
There is, however, an asterisk attached to these findings. “Despite a moderate standard effect size, the treatment effect was smaller than some of the thresholds for what is considered clinically meaningful,” he noted at the meeting sponsored by the Osteoarthritis Research Society International.
That being said, the rheumatologist is convinced further investigation of methotrexate in osteoarthritis is warranted.
“I have to say that, unlike our earlier hydroxychloroquine trial, which was robustly negative with nothing more to say, I think there is a signal in this study. I need to understand the results of this trial better to understand if there is a subgroup we could treat with methotrexate. It’s a cheap drug, it’s readily available, and we’ve got a lot of experience with it,” noted Dr. Conaghan, professor of musculoskeletal medicine at the University of Leeds (England) and director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The rationale for the 15-center PROMOTE trial is that synovitis is common in OA. Synovitis is associated with pain, methotrexate is the gold-standard treatment for synovitis in inflammatory forms of arthritis, and current treatments for OA are, to say the least, severely limited. Also, an earlier 30-patient, open-label pilot study of methotrexate in patients with painful knee OA conducted by Dr. Conaghan and coworkers suggested the drug was promising (Rheumatology [Oxford]. 2013 May;52[5]:888-92).
PROMOTE included 134 patients with symptomatic and radiographic knee OA who were randomized in double-blind fashion to 6 months of oral methotrexate at 10 mg titrated to a target dose of 25 mg/week or to placebo. All patients also received usual care with oral NSAIDs and/or acetaminophen. Their mean baseline knee pain on a 0-10 numeric rating scale was 6.6.
The primary endpoint, assessed at 6 months, was the difference between the two study arms in average knee pain during the previous week on a 0-10 scale. The score was 5.1 in the methotrexate group and 6.2 in the placebo arm, for a baseline-adjusted treatment difference of 0.83 points, which works out to a standard effect size of 0.36. When the data were reanalyzed after excluding the 15 patients who missed more than four doses of medication within any 3-month period, the between-group difference in pain scores increased to 0.95 points in favor of the methotrexate group.
A significant difference in favor of the methotrexate group was documented in the OARSI-OMERACT response rate at 6 months: 45% in the methotrexate group and 26% in the controls. Some secondary endpoints were positive as well, with statistically significant differences seen at 6 months in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) stiffness, WOMAC physical function, and several other endpoints. But there were no significant differences in WOMAC pain, SF-12 physical component or SF-12 mental component scores, or in an OA quality of life measure.
The mean dose of methotrexate used in the study was about 17 mg/week. Dr. Conaghan said that if he could do the trial over again, he would have used subcutaneous methotrexate.
“It’s a more reliable way of getting a dose into people and probably of getting a slightly higher dose into people. In the rheumatoid arthritis world, we use a lot more subcutaneous methotrexate now than we did 10 years ago because it gets around a lot of the minor side effects and helps compliance,” he said.
One audience member suggested that one potentially useful way to zero in on a subgroup of knee OA patients likely to derive the most benefit from methotrexate would be to have screened potential study participants for comorbid fibromyalgia and exclude those with the disorder. Dr. Conaghan replied that the PROMOTE investigators did gather data on participants’ pain at sites other than the knee. That data can be used to identify those at increased likelihood of fibromyalgia, and he agreed that’s worth looking into.
Dr. Conaghan reported having no financial conflicts regarding PROMOTE, which was funded by the U.K. National Institute for Health Research and Versus Arthritis.
SOURCE: Conaghan PG et al. OARSI 2019, Abstract 86.
TORONTO – Philip G. Conaghan, MD, PhD, reported at the OARSI 2019 World Congress.
There is, however, an asterisk attached to these findings. “Despite a moderate standard effect size, the treatment effect was smaller than some of the thresholds for what is considered clinically meaningful,” he noted at the meeting sponsored by the Osteoarthritis Research Society International.
That being said, the rheumatologist is convinced further investigation of methotrexate in osteoarthritis is warranted.
“I have to say that, unlike our earlier hydroxychloroquine trial, which was robustly negative with nothing more to say, I think there is a signal in this study. I need to understand the results of this trial better to understand if there is a subgroup we could treat with methotrexate. It’s a cheap drug, it’s readily available, and we’ve got a lot of experience with it,” noted Dr. Conaghan, professor of musculoskeletal medicine at the University of Leeds (England) and director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine.
The rationale for the 15-center PROMOTE trial is that synovitis is common in OA. Synovitis is associated with pain, methotrexate is the gold-standard treatment for synovitis in inflammatory forms of arthritis, and current treatments for OA are, to say the least, severely limited. Also, an earlier 30-patient, open-label pilot study of methotrexate in patients with painful knee OA conducted by Dr. Conaghan and coworkers suggested the drug was promising (Rheumatology [Oxford]. 2013 May;52[5]:888-92).
PROMOTE included 134 patients with symptomatic and radiographic knee OA who were randomized in double-blind fashion to 6 months of oral methotrexate at 10 mg titrated to a target dose of 25 mg/week or to placebo. All patients also received usual care with oral NSAIDs and/or acetaminophen. Their mean baseline knee pain on a 0-10 numeric rating scale was 6.6.
The primary endpoint, assessed at 6 months, was the difference between the two study arms in average knee pain during the previous week on a 0-10 scale. The score was 5.1 in the methotrexate group and 6.2 in the placebo arm, for a baseline-adjusted treatment difference of 0.83 points, which works out to a standard effect size of 0.36. When the data were reanalyzed after excluding the 15 patients who missed more than four doses of medication within any 3-month period, the between-group difference in pain scores increased to 0.95 points in favor of the methotrexate group.
A significant difference in favor of the methotrexate group was documented in the OARSI-OMERACT response rate at 6 months: 45% in the methotrexate group and 26% in the controls. Some secondary endpoints were positive as well, with statistically significant differences seen at 6 months in Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) stiffness, WOMAC physical function, and several other endpoints. But there were no significant differences in WOMAC pain, SF-12 physical component or SF-12 mental component scores, or in an OA quality of life measure.
The mean dose of methotrexate used in the study was about 17 mg/week. Dr. Conaghan said that if he could do the trial over again, he would have used subcutaneous methotrexate.
“It’s a more reliable way of getting a dose into people and probably of getting a slightly higher dose into people. In the rheumatoid arthritis world, we use a lot more subcutaneous methotrexate now than we did 10 years ago because it gets around a lot of the minor side effects and helps compliance,” he said.
One audience member suggested that one potentially useful way to zero in on a subgroup of knee OA patients likely to derive the most benefit from methotrexate would be to have screened potential study participants for comorbid fibromyalgia and exclude those with the disorder. Dr. Conaghan replied that the PROMOTE investigators did gather data on participants’ pain at sites other than the knee. That data can be used to identify those at increased likelihood of fibromyalgia, and he agreed that’s worth looking into.
Dr. Conaghan reported having no financial conflicts regarding PROMOTE, which was funded by the U.K. National Institute for Health Research and Versus Arthritis.
SOURCE: Conaghan PG et al. OARSI 2019, Abstract 86.
REPORTING FROM OARSI 2019
Scandinavian studies shed light on OA inheritance
TORONTO – Patients with osteoarthritis often want to know if their debilitating disease is likely to be passed on to their children. Karin Magnusson, PhD, believes she can answer that question based upon an analysis of two large Nordic studies.
“OA in the mother, but not in the father, increases the risk of surgical and clinically defined hip, knee, and hand OA in the offspring, and particularly in daughters,” she reported at the OARSI 2019 World Congress.
Dr. Magnusson, an epidemiologist at Lund (Sweden) University, and her coinvestigators, turned to the Musculoskeletal Pain in Ullensaker Study (MUST) of 630 individuals aged 40-79 with rheumatologist-diagnosed hand, hip, or knee OA by American College of Rheumatology clinical criteria and their offspring, as well as the Nor-Twin OA Study of 7,184 twins, aged 30-75, and their children. Linkage with a national registry that records virtually all joint arthroplasties performed in Norway enabled the investigators to identify which subjects in the two studies had joint surgery for OA, she explained at the meeting, sponsored by the Osteoarthritis Research Society International.
The main outcome in this analysis was the relative risk of hip, knee, or hand OA in the sons and daughters of families in which a parent had OA at those sites, compared with the rate when neither parent had OA. The key finding: If the mother had OA, her daughters had a 13% increased risk of OA in MUST and a 44% increased risk in the Nor-Twin OA Study when compared with daughters of women without OA. In contrast, the sons of a mother with OA had no significant increase in risk of OA. And when OA was present in the father, there was no increased risk of OA at any site in his daughters or sons.
“The implication is the heredity of OA is linked to maternal genes and/or maternal-specific factors, such as the fetal environment,” according to Dr. Magnusson.
And for clinical practice, the implication is that it’s important to ask about family history of OA, and in which parent, to better predict future risk of disease transmission to the children, she added.
These Norwegian study results open the door to exploration of the possible role of mitochondrial DNA in familial clustering of OA, since mitochondrial DNA is inherited only from the mother, Dr. Magnusson noted.
David T. Felson, MD, rose from the audience to say, “I’m a little bit worried” about the fact that when he and other Framingham Heart Study investigators looked specifically for possible mother/daughter, mother/son, father/daughter, and father/son associations for knee and hip OA, “we really didn’t find any.
“You can go through all of the explanations that you want about maternal inheritance, but I’m not sure that’s the best explanation. It might just be that what’s going on here is you’re seeing guys who are relatively young and who got their OA through injury or sports, which is fairly common in young men, and not through inheritance,” said Dr. Felson, professor of medicine and epidemiology at Boston University.
So a third observational study in an independent cohort might be needed as a tie breaker regarding the issue of OA inheritance.
Dr. Magnusson reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Magnusson K et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S47, Abstract 33
TORONTO – Patients with osteoarthritis often want to know if their debilitating disease is likely to be passed on to their children. Karin Magnusson, PhD, believes she can answer that question based upon an analysis of two large Nordic studies.
“OA in the mother, but not in the father, increases the risk of surgical and clinically defined hip, knee, and hand OA in the offspring, and particularly in daughters,” she reported at the OARSI 2019 World Congress.
Dr. Magnusson, an epidemiologist at Lund (Sweden) University, and her coinvestigators, turned to the Musculoskeletal Pain in Ullensaker Study (MUST) of 630 individuals aged 40-79 with rheumatologist-diagnosed hand, hip, or knee OA by American College of Rheumatology clinical criteria and their offspring, as well as the Nor-Twin OA Study of 7,184 twins, aged 30-75, and their children. Linkage with a national registry that records virtually all joint arthroplasties performed in Norway enabled the investigators to identify which subjects in the two studies had joint surgery for OA, she explained at the meeting, sponsored by the Osteoarthritis Research Society International.
The main outcome in this analysis was the relative risk of hip, knee, or hand OA in the sons and daughters of families in which a parent had OA at those sites, compared with the rate when neither parent had OA. The key finding: If the mother had OA, her daughters had a 13% increased risk of OA in MUST and a 44% increased risk in the Nor-Twin OA Study when compared with daughters of women without OA. In contrast, the sons of a mother with OA had no significant increase in risk of OA. And when OA was present in the father, there was no increased risk of OA at any site in his daughters or sons.
“The implication is the heredity of OA is linked to maternal genes and/or maternal-specific factors, such as the fetal environment,” according to Dr. Magnusson.
And for clinical practice, the implication is that it’s important to ask about family history of OA, and in which parent, to better predict future risk of disease transmission to the children, she added.
These Norwegian study results open the door to exploration of the possible role of mitochondrial DNA in familial clustering of OA, since mitochondrial DNA is inherited only from the mother, Dr. Magnusson noted.
David T. Felson, MD, rose from the audience to say, “I’m a little bit worried” about the fact that when he and other Framingham Heart Study investigators looked specifically for possible mother/daughter, mother/son, father/daughter, and father/son associations for knee and hip OA, “we really didn’t find any.
“You can go through all of the explanations that you want about maternal inheritance, but I’m not sure that’s the best explanation. It might just be that what’s going on here is you’re seeing guys who are relatively young and who got their OA through injury or sports, which is fairly common in young men, and not through inheritance,” said Dr. Felson, professor of medicine and epidemiology at Boston University.
So a third observational study in an independent cohort might be needed as a tie breaker regarding the issue of OA inheritance.
Dr. Magnusson reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Magnusson K et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S47, Abstract 33
TORONTO – Patients with osteoarthritis often want to know if their debilitating disease is likely to be passed on to their children. Karin Magnusson, PhD, believes she can answer that question based upon an analysis of two large Nordic studies.
“OA in the mother, but not in the father, increases the risk of surgical and clinically defined hip, knee, and hand OA in the offspring, and particularly in daughters,” she reported at the OARSI 2019 World Congress.
Dr. Magnusson, an epidemiologist at Lund (Sweden) University, and her coinvestigators, turned to the Musculoskeletal Pain in Ullensaker Study (MUST) of 630 individuals aged 40-79 with rheumatologist-diagnosed hand, hip, or knee OA by American College of Rheumatology clinical criteria and their offspring, as well as the Nor-Twin OA Study of 7,184 twins, aged 30-75, and their children. Linkage with a national registry that records virtually all joint arthroplasties performed in Norway enabled the investigators to identify which subjects in the two studies had joint surgery for OA, she explained at the meeting, sponsored by the Osteoarthritis Research Society International.
The main outcome in this analysis was the relative risk of hip, knee, or hand OA in the sons and daughters of families in which a parent had OA at those sites, compared with the rate when neither parent had OA. The key finding: If the mother had OA, her daughters had a 13% increased risk of OA in MUST and a 44% increased risk in the Nor-Twin OA Study when compared with daughters of women without OA. In contrast, the sons of a mother with OA had no significant increase in risk of OA. And when OA was present in the father, there was no increased risk of OA at any site in his daughters or sons.
“The implication is the heredity of OA is linked to maternal genes and/or maternal-specific factors, such as the fetal environment,” according to Dr. Magnusson.
And for clinical practice, the implication is that it’s important to ask about family history of OA, and in which parent, to better predict future risk of disease transmission to the children, she added.
These Norwegian study results open the door to exploration of the possible role of mitochondrial DNA in familial clustering of OA, since mitochondrial DNA is inherited only from the mother, Dr. Magnusson noted.
David T. Felson, MD, rose from the audience to say, “I’m a little bit worried” about the fact that when he and other Framingham Heart Study investigators looked specifically for possible mother/daughter, mother/son, father/daughter, and father/son associations for knee and hip OA, “we really didn’t find any.
“You can go through all of the explanations that you want about maternal inheritance, but I’m not sure that’s the best explanation. It might just be that what’s going on here is you’re seeing guys who are relatively young and who got their OA through injury or sports, which is fairly common in young men, and not through inheritance,” said Dr. Felson, professor of medicine and epidemiology at Boston University.
So a third observational study in an independent cohort might be needed as a tie breaker regarding the issue of OA inheritance.
Dr. Magnusson reported having no financial conflicts regarding her study, conducted free of commercial support.
SOURCE: Magnusson K et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S47, Abstract 33
REPORTING FROM OARSI 2019
Tanezumab acts fast for OA pain relief
TORONTO – , according to a secondary analysis of a phase 3 randomized trial.
“The onset is relatively quick. It’s a monoclonal antibody, so it doesn’t work overnight, but by 3-5 days you see a significant difference,” Thomas J. Schnitzer, MD, PhD, reported at the OARSI 2019 World Congress.
He had previously presented the primary outcomes of this 696-patient, phase 3, randomized trial at the 2018 annual meeting of the American College of Rheumatology. At OARSI 2019, the rheumatologist presented new data focusing on the speed and durability of the pain relief provided by tanezumab, a humanized monoclonal antibody designed to help keep pain signals produced in the periphery from reaching the CNS.
The double-blind trial included U.S. patients with an average 9.3-year disease duration who were randomized to either two 2.5-mg subcutaneous injections of the nerve growth factor inhibitor 8 weeks apart, a 2.5-mg dose followed 8 weeks later by a 5-mg dose, or two placebo injections. Eighty-five percent of subjects had knee OA, and the rest had hip OA. The patients had fairly severe pain, with average baseline Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scores of 7.1-7.4. Notably, all study participants had to have a documented history of previous failure to respond to at least three pain relievers: acetaminophen, oral NSAIDs, and tramadol or opioids, according to Dr. Schnitzer, a rheumatologist who is professor of physical medicine and rehabilitation, anesthesiology, and medicine at Northwestern University in Chicago.
As previously reported, the co–primary endpoint of change from baseline to week 16 in WOMAC pain was –3.22 points with the 2.5-mg tanezumab regimen and –3.45 with the 2.5/5–mg strategy, both significantly better than the 2.56-point improvement with placebo. Improvement in WOMAC physical function followed suit, he said at the meeting sponsored by the Osteoarthritis Research Society International.
Assessments were made at office visits every 2 weeks during the study. By the first visit at week 2, tanezumab was significantly better than placebo on both WOMAC measures, an advantage maintained for the rest of the 16 weeks. Pain relief in the tanezumab-treated groups was maximum at weeks 4 and 12; that is, 4 weeks following the first and second injections.
“This suggests that there’s an immediate effect of the antibody, which then tends to wane as the antibody begins to get cleared,” Dr. Schnitzer observed.
Study participants kept a structured daily pain diary, which enabled investigators to zero in on the timing of pain relief. Statistically significant separation from placebo was documented by day 3 in one group on tanezumab and by day 5 in the other.
An increased rate of rapidly progressive OA was a concern years ago in earlier studies of a now-abandoned intravenous formulation of tanezumab. However, in the phase 3 trial of the subcutaneous humanized monoclonal antibody, rapidly progressive OA occurred in only six patients, or 1.3%, during the 24-week safety follow-up period. Interestingly, the phenomenon was not dose related, as five of the six cases occurred in patients on the twin 2.5-mg regimen, and only one in the 2.5/5-mg group. No cases of osteonecrosis occurred in the trial.
One audience member rose to say she and her fellow rheumatologists are very excited about the prospect of possible access to a novel and more effective OA therapy. But she took issue with the trial’s reliance on WOMAC pain and physical function scores as primary endpoints, noting that OARSI experts have developed and validated several more comprehensive and globally informative assessment tools. Dr. Schnitzer readily agreed. The investigators utilized WOMAC pain and physical function because that’s what the U.S. and European regulatory agencies insist upon, he explained.
Clinicians should stay tuned because the results of much larger, longer-term phase 3 trials of tanezumab are due to be presented soon, he added.
Dr. Schnitzer reported serving as a consultant to Pfizer and Eli Lilly, which are jointly developing tanezumab and sponsored the trial, as well as to a handful of other pharmaceutical companies.
SOURCE: Bessette L et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S85-6, Abstract 88.
TORONTO – , according to a secondary analysis of a phase 3 randomized trial.
“The onset is relatively quick. It’s a monoclonal antibody, so it doesn’t work overnight, but by 3-5 days you see a significant difference,” Thomas J. Schnitzer, MD, PhD, reported at the OARSI 2019 World Congress.
He had previously presented the primary outcomes of this 696-patient, phase 3, randomized trial at the 2018 annual meeting of the American College of Rheumatology. At OARSI 2019, the rheumatologist presented new data focusing on the speed and durability of the pain relief provided by tanezumab, a humanized monoclonal antibody designed to help keep pain signals produced in the periphery from reaching the CNS.
The double-blind trial included U.S. patients with an average 9.3-year disease duration who were randomized to either two 2.5-mg subcutaneous injections of the nerve growth factor inhibitor 8 weeks apart, a 2.5-mg dose followed 8 weeks later by a 5-mg dose, or two placebo injections. Eighty-five percent of subjects had knee OA, and the rest had hip OA. The patients had fairly severe pain, with average baseline Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scores of 7.1-7.4. Notably, all study participants had to have a documented history of previous failure to respond to at least three pain relievers: acetaminophen, oral NSAIDs, and tramadol or opioids, according to Dr. Schnitzer, a rheumatologist who is professor of physical medicine and rehabilitation, anesthesiology, and medicine at Northwestern University in Chicago.
As previously reported, the co–primary endpoint of change from baseline to week 16 in WOMAC pain was –3.22 points with the 2.5-mg tanezumab regimen and –3.45 with the 2.5/5–mg strategy, both significantly better than the 2.56-point improvement with placebo. Improvement in WOMAC physical function followed suit, he said at the meeting sponsored by the Osteoarthritis Research Society International.
Assessments were made at office visits every 2 weeks during the study. By the first visit at week 2, tanezumab was significantly better than placebo on both WOMAC measures, an advantage maintained for the rest of the 16 weeks. Pain relief in the tanezumab-treated groups was maximum at weeks 4 and 12; that is, 4 weeks following the first and second injections.
“This suggests that there’s an immediate effect of the antibody, which then tends to wane as the antibody begins to get cleared,” Dr. Schnitzer observed.
Study participants kept a structured daily pain diary, which enabled investigators to zero in on the timing of pain relief. Statistically significant separation from placebo was documented by day 3 in one group on tanezumab and by day 5 in the other.
An increased rate of rapidly progressive OA was a concern years ago in earlier studies of a now-abandoned intravenous formulation of tanezumab. However, in the phase 3 trial of the subcutaneous humanized monoclonal antibody, rapidly progressive OA occurred in only six patients, or 1.3%, during the 24-week safety follow-up period. Interestingly, the phenomenon was not dose related, as five of the six cases occurred in patients on the twin 2.5-mg regimen, and only one in the 2.5/5-mg group. No cases of osteonecrosis occurred in the trial.
One audience member rose to say she and her fellow rheumatologists are very excited about the prospect of possible access to a novel and more effective OA therapy. But she took issue with the trial’s reliance on WOMAC pain and physical function scores as primary endpoints, noting that OARSI experts have developed and validated several more comprehensive and globally informative assessment tools. Dr. Schnitzer readily agreed. The investigators utilized WOMAC pain and physical function because that’s what the U.S. and European regulatory agencies insist upon, he explained.
Clinicians should stay tuned because the results of much larger, longer-term phase 3 trials of tanezumab are due to be presented soon, he added.
Dr. Schnitzer reported serving as a consultant to Pfizer and Eli Lilly, which are jointly developing tanezumab and sponsored the trial, as well as to a handful of other pharmaceutical companies.
SOURCE: Bessette L et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S85-6, Abstract 88.
TORONTO – , according to a secondary analysis of a phase 3 randomized trial.
“The onset is relatively quick. It’s a monoclonal antibody, so it doesn’t work overnight, but by 3-5 days you see a significant difference,” Thomas J. Schnitzer, MD, PhD, reported at the OARSI 2019 World Congress.
He had previously presented the primary outcomes of this 696-patient, phase 3, randomized trial at the 2018 annual meeting of the American College of Rheumatology. At OARSI 2019, the rheumatologist presented new data focusing on the speed and durability of the pain relief provided by tanezumab, a humanized monoclonal antibody designed to help keep pain signals produced in the periphery from reaching the CNS.
The double-blind trial included U.S. patients with an average 9.3-year disease duration who were randomized to either two 2.5-mg subcutaneous injections of the nerve growth factor inhibitor 8 weeks apart, a 2.5-mg dose followed 8 weeks later by a 5-mg dose, or two placebo injections. Eighty-five percent of subjects had knee OA, and the rest had hip OA. The patients had fairly severe pain, with average baseline Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scores of 7.1-7.4. Notably, all study participants had to have a documented history of previous failure to respond to at least three pain relievers: acetaminophen, oral NSAIDs, and tramadol or opioids, according to Dr. Schnitzer, a rheumatologist who is professor of physical medicine and rehabilitation, anesthesiology, and medicine at Northwestern University in Chicago.
As previously reported, the co–primary endpoint of change from baseline to week 16 in WOMAC pain was –3.22 points with the 2.5-mg tanezumab regimen and –3.45 with the 2.5/5–mg strategy, both significantly better than the 2.56-point improvement with placebo. Improvement in WOMAC physical function followed suit, he said at the meeting sponsored by the Osteoarthritis Research Society International.
Assessments were made at office visits every 2 weeks during the study. By the first visit at week 2, tanezumab was significantly better than placebo on both WOMAC measures, an advantage maintained for the rest of the 16 weeks. Pain relief in the tanezumab-treated groups was maximum at weeks 4 and 12; that is, 4 weeks following the first and second injections.
“This suggests that there’s an immediate effect of the antibody, which then tends to wane as the antibody begins to get cleared,” Dr. Schnitzer observed.
Study participants kept a structured daily pain diary, which enabled investigators to zero in on the timing of pain relief. Statistically significant separation from placebo was documented by day 3 in one group on tanezumab and by day 5 in the other.
An increased rate of rapidly progressive OA was a concern years ago in earlier studies of a now-abandoned intravenous formulation of tanezumab. However, in the phase 3 trial of the subcutaneous humanized monoclonal antibody, rapidly progressive OA occurred in only six patients, or 1.3%, during the 24-week safety follow-up period. Interestingly, the phenomenon was not dose related, as five of the six cases occurred in patients on the twin 2.5-mg regimen, and only one in the 2.5/5-mg group. No cases of osteonecrosis occurred in the trial.
One audience member rose to say she and her fellow rheumatologists are very excited about the prospect of possible access to a novel and more effective OA therapy. But she took issue with the trial’s reliance on WOMAC pain and physical function scores as primary endpoints, noting that OARSI experts have developed and validated several more comprehensive and globally informative assessment tools. Dr. Schnitzer readily agreed. The investigators utilized WOMAC pain and physical function because that’s what the U.S. and European regulatory agencies insist upon, he explained.
Clinicians should stay tuned because the results of much larger, longer-term phase 3 trials of tanezumab are due to be presented soon, he added.
Dr. Schnitzer reported serving as a consultant to Pfizer and Eli Lilly, which are jointly developing tanezumab and sponsored the trial, as well as to a handful of other pharmaceutical companies.
SOURCE: Bessette L et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S85-6, Abstract 88.
REPORTING FROM OARSI 2019
Key clinical point: The nerve growth factor inhibitor tanezumab brings rapid improvement in pain.
Major finding: Tanezumab-treated patients experienced significant pain reduction within 3-5 days after their first dose.
Study details: This was a phase 3, prospective, multicenter, double-blind, placebo-controlled trial in 696 patients with refractory pain attributable to knee or hip OA.
Disclosures: The presenter reported serving as a consultant to Pfizer and Eli Lilly, which cosponsored the phase 3 trial.
Source: Bessette L et al. Osteoarthritis Cartilage. 2019 Apr;27[suppl 1]:S85-6, Abstract 88.















