User login
Sorafenib extends PFS for refractory desmoid tumors
For patients with progressive, refractory, or symptomatic desmoid tumors – also known as aggressive fibromatosis – treatment with daily sorafenib (Nexavar) was associated with durable responses and a significant improvement in progression-free survival.
After a median follow-up of 27.2 months, the 2-year progression-free survival (PFS) rate for patients randomly assigned to receive 400 mg sorafenib daily was 81%, compared with 36% for patients assigned to placebo (P less than .001), reported Mrinal M. Gounder, MD, from Memorial Sloan Kettering Cancer Center in New York City, and his colleagues.
“Other agents that are used to treat these tumors include anthracyclines [e.g., pegylated liposomal doxorubicin], vinca alkaloids, and pazopanib. On the basis of the predictable toxic-effects profile and substantial progression-free survival advantage conferred by sorafenib, the drug has antitumor activity as first-line therapy or as subsequent therapy for desmoid tumors,” they wrote in the New England Journal of Medicine.
There is no accepted standard of care for the systemic treatment for desmoid tumors, with options ranging from hormonal blockade, cytotoxic chemotherapy, and targeted agents such as tyrosine kinase inhibitors (TKIs).
Based on a retrospective study showing that the multitargeting oral TKI sorafenib was associated with a 25% response rate and acceptable safety in patients with desmoid tumors, the investigators initiated a phase 3, randomized trial to evaluate the efficacy and safety of sorafenib in this population.
They enrolled 87 patients aged 18 years or older with a histologically documented desmoid tumor that showed clinical and radiographic progression of at least 10% in maximum unidimensional measurement within the last 6 months, symptomatic disease, or recurrent or primary disease that was either inoperable or deemed to require extensive surgery.
The patients were randomized in double-blinded fashion on a 2:1 basis to receive either sorafenib 400 mg daily or placebo until progression. Crossover to sorafenib was allowed for patients assigned to placebo who experienced disease progressions.
As noted before, investigator-assessed PFS, the primary endpoint, clearly favored sorafenib.
Objective response rates before crossover were 33% in the sorafenib arm, consisting of 1 complete and 15 partial responses, and 20% in the placebo arm, consisting of 7 partial responses. The respective median times to objective response were 9.6 months versus 13.3 months. The earliest response, defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, occurred at 2.2 months in the sorafenib arm versus 8.8 months in the placebo arm.
The authors also performed an exploratory analysis looking at MRI as a measure of response evaluation and found that “changes in T2-weighted signal intensity and volumetric measurements may be better measures of treatment effect than RECIST. This is particularly evident when the best response according to RECIST is stable disease.”
The most frequently reported adverse events among patients treated with sorafenib were grade 1 or 2 rash in 73%, fatigue in 67%, hypertension in 55%, and diarrhea in 51%. The most frequent treatment-emergent adverse events in the placebo group were rash of any kind in 42% and palmar-plantar erythrodysesthesia syndrome in 22%.
The investigators acknowledged that the mechanism of action of sorafenib in desmoid tumors is unknown, but noted that they are looking for clues in 25 sets of paired biopsy samples.
The study was supported by grants from the National Cancer Institute, Bayer, Memorial Sloan Kettering Cancer Center, the American Society of Clinical Oncology, Desmoid Tumor Research Foundation, and an Orphan Products Clinical Trials Grant from the Food and Drug Administration. Dr. Gounder reported fees for advisory board activities/consulting for Bayer, Epizyme, Karyopharm Therapeutics, Daiichi Sankyo, TRACON Pharmaceuticals, and Amgen, and travel expenses from Epizyme.
SOURCE: Gounder MM et al. N Engl J Med. 2018 Dec 19. doi: 10.1056/NEJMoa1805052.
For patients with progressive, refractory, or symptomatic desmoid tumors – also known as aggressive fibromatosis – treatment with daily sorafenib (Nexavar) was associated with durable responses and a significant improvement in progression-free survival.
After a median follow-up of 27.2 months, the 2-year progression-free survival (PFS) rate for patients randomly assigned to receive 400 mg sorafenib daily was 81%, compared with 36% for patients assigned to placebo (P less than .001), reported Mrinal M. Gounder, MD, from Memorial Sloan Kettering Cancer Center in New York City, and his colleagues.
“Other agents that are used to treat these tumors include anthracyclines [e.g., pegylated liposomal doxorubicin], vinca alkaloids, and pazopanib. On the basis of the predictable toxic-effects profile and substantial progression-free survival advantage conferred by sorafenib, the drug has antitumor activity as first-line therapy or as subsequent therapy for desmoid tumors,” they wrote in the New England Journal of Medicine.
There is no accepted standard of care for the systemic treatment for desmoid tumors, with options ranging from hormonal blockade, cytotoxic chemotherapy, and targeted agents such as tyrosine kinase inhibitors (TKIs).
Based on a retrospective study showing that the multitargeting oral TKI sorafenib was associated with a 25% response rate and acceptable safety in patients with desmoid tumors, the investigators initiated a phase 3, randomized trial to evaluate the efficacy and safety of sorafenib in this population.
They enrolled 87 patients aged 18 years or older with a histologically documented desmoid tumor that showed clinical and radiographic progression of at least 10% in maximum unidimensional measurement within the last 6 months, symptomatic disease, or recurrent or primary disease that was either inoperable or deemed to require extensive surgery.
The patients were randomized in double-blinded fashion on a 2:1 basis to receive either sorafenib 400 mg daily or placebo until progression. Crossover to sorafenib was allowed for patients assigned to placebo who experienced disease progressions.
As noted before, investigator-assessed PFS, the primary endpoint, clearly favored sorafenib.
Objective response rates before crossover were 33% in the sorafenib arm, consisting of 1 complete and 15 partial responses, and 20% in the placebo arm, consisting of 7 partial responses. The respective median times to objective response were 9.6 months versus 13.3 months. The earliest response, defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, occurred at 2.2 months in the sorafenib arm versus 8.8 months in the placebo arm.
The authors also performed an exploratory analysis looking at MRI as a measure of response evaluation and found that “changes in T2-weighted signal intensity and volumetric measurements may be better measures of treatment effect than RECIST. This is particularly evident when the best response according to RECIST is stable disease.”
The most frequently reported adverse events among patients treated with sorafenib were grade 1 or 2 rash in 73%, fatigue in 67%, hypertension in 55%, and diarrhea in 51%. The most frequent treatment-emergent adverse events in the placebo group were rash of any kind in 42% and palmar-plantar erythrodysesthesia syndrome in 22%.
The investigators acknowledged that the mechanism of action of sorafenib in desmoid tumors is unknown, but noted that they are looking for clues in 25 sets of paired biopsy samples.
The study was supported by grants from the National Cancer Institute, Bayer, Memorial Sloan Kettering Cancer Center, the American Society of Clinical Oncology, Desmoid Tumor Research Foundation, and an Orphan Products Clinical Trials Grant from the Food and Drug Administration. Dr. Gounder reported fees for advisory board activities/consulting for Bayer, Epizyme, Karyopharm Therapeutics, Daiichi Sankyo, TRACON Pharmaceuticals, and Amgen, and travel expenses from Epizyme.
SOURCE: Gounder MM et al. N Engl J Med. 2018 Dec 19. doi: 10.1056/NEJMoa1805052.
For patients with progressive, refractory, or symptomatic desmoid tumors – also known as aggressive fibromatosis – treatment with daily sorafenib (Nexavar) was associated with durable responses and a significant improvement in progression-free survival.
After a median follow-up of 27.2 months, the 2-year progression-free survival (PFS) rate for patients randomly assigned to receive 400 mg sorafenib daily was 81%, compared with 36% for patients assigned to placebo (P less than .001), reported Mrinal M. Gounder, MD, from Memorial Sloan Kettering Cancer Center in New York City, and his colleagues.
“Other agents that are used to treat these tumors include anthracyclines [e.g., pegylated liposomal doxorubicin], vinca alkaloids, and pazopanib. On the basis of the predictable toxic-effects profile and substantial progression-free survival advantage conferred by sorafenib, the drug has antitumor activity as first-line therapy or as subsequent therapy for desmoid tumors,” they wrote in the New England Journal of Medicine.
There is no accepted standard of care for the systemic treatment for desmoid tumors, with options ranging from hormonal blockade, cytotoxic chemotherapy, and targeted agents such as tyrosine kinase inhibitors (TKIs).
Based on a retrospective study showing that the multitargeting oral TKI sorafenib was associated with a 25% response rate and acceptable safety in patients with desmoid tumors, the investigators initiated a phase 3, randomized trial to evaluate the efficacy and safety of sorafenib in this population.
They enrolled 87 patients aged 18 years or older with a histologically documented desmoid tumor that showed clinical and radiographic progression of at least 10% in maximum unidimensional measurement within the last 6 months, symptomatic disease, or recurrent or primary disease that was either inoperable or deemed to require extensive surgery.
The patients were randomized in double-blinded fashion on a 2:1 basis to receive either sorafenib 400 mg daily or placebo until progression. Crossover to sorafenib was allowed for patients assigned to placebo who experienced disease progressions.
As noted before, investigator-assessed PFS, the primary endpoint, clearly favored sorafenib.
Objective response rates before crossover were 33% in the sorafenib arm, consisting of 1 complete and 15 partial responses, and 20% in the placebo arm, consisting of 7 partial responses. The respective median times to objective response were 9.6 months versus 13.3 months. The earliest response, defined by Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, occurred at 2.2 months in the sorafenib arm versus 8.8 months in the placebo arm.
The authors also performed an exploratory analysis looking at MRI as a measure of response evaluation and found that “changes in T2-weighted signal intensity and volumetric measurements may be better measures of treatment effect than RECIST. This is particularly evident when the best response according to RECIST is stable disease.”
The most frequently reported adverse events among patients treated with sorafenib were grade 1 or 2 rash in 73%, fatigue in 67%, hypertension in 55%, and diarrhea in 51%. The most frequent treatment-emergent adverse events in the placebo group were rash of any kind in 42% and palmar-plantar erythrodysesthesia syndrome in 22%.
The investigators acknowledged that the mechanism of action of sorafenib in desmoid tumors is unknown, but noted that they are looking for clues in 25 sets of paired biopsy samples.
The study was supported by grants from the National Cancer Institute, Bayer, Memorial Sloan Kettering Cancer Center, the American Society of Clinical Oncology, Desmoid Tumor Research Foundation, and an Orphan Products Clinical Trials Grant from the Food and Drug Administration. Dr. Gounder reported fees for advisory board activities/consulting for Bayer, Epizyme, Karyopharm Therapeutics, Daiichi Sankyo, TRACON Pharmaceuticals, and Amgen, and travel expenses from Epizyme.
SOURCE: Gounder MM et al. N Engl J Med. 2018 Dec 19. doi: 10.1056/NEJMoa1805052.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: There is no accepted standard of systemic therapy for recurrent, refractory, or symptomatic desmoid tumors.
Major finding: Median progression-free survival with sorafenib after a median follow-up of 27.2 months was 81% versus 36% for placebo.
Study details: A double-blind, phase 3 trial with 2:1 randomization of sorafenib to placebo in 87 patients.
Disclosures: The study was supported by grants from the National Cancer Institute, Bayer, Memorial Sloan Kettering Cancer Center, the American Society of Clinical Oncology, Desmoid Tumor Research Foundation, and an Orphan Products Clinical Trials Grant from the Food and Drug Administration. Dr. Gounder reported fees for advisory board activities/consulting for Bayer, Epizyme, Karyopharm Therapeutics, Daiichi Sankyo, TRACON Pharmaceuticals, and Amgen, and travel expenses from Epizyme.
Source: Gounder MM et al. N Engl J Med. 2018 Dec 19. doi: 10.1056/NEJMoa1805052.
Reports from the annual meeting of The Connective Tissue Oncology Society held in Rome, November 14-17, 2018 Sarcoma of the Year: Intimal Sarcoma
This year’s annual meeting of The Connective Tissue Oncology Society brought new insights on intimal sarcoma. Four studies in a featured session at the meeting examined both current and novel treatments for this rare and aggressive cancer, and emphasized the need for new therapies.
Anthracycline-based regimens as preferred first-line therapies
Anthracycline-based regimens were the preferred first-line therapies used in 83 adults with intimal sarcomas in a retrospective study of data from the World Sarcoma Network, reported by Anna Maria Frezza, MD, of the, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy, and her colleagues. The researchers described the experience with anthracycline-based regimens as well as gemcitabine-based regimens and pazopanib among MDM2-positive patients with intimal sarcomas treated at 16 sarcoma reference centers in Europe, the United States, and Japan. Their findings speak to the need for new active drugs, which they said should target the MDM2 and CDK4 overexpression seen in patients with this rare sarcoma.Of the 83 patients studied, nearly all (76 patients) initially received an anthracycline-based regimen. Gemcitabine-based regimens were used in 29 patients and pazopanib in 10 patients; 20 of the 39 patients received more than one treatment.
Anthracycline-based regimens were associated with a 12-month progression-free survival rate of 38% in 76 patients with intimal sarcomas. All of the 76 patients received anthracycline regimens as their initial systemic therapy; 27 were treated for localized disease with a curative intent and the remaining 49 had advanced disease. The researchers also noted that anthracycline regimens were safely used in 22 patients with cardiac intimal sarcomas, as none of them died of cardiotoxicity.
Based on RECIST 1.1 measures, the overall response rate was 37% in 57 evaluable patients: 3 patients had a complete response, 18 had a partial response, 27 had stable disease, and 9 had progressive disease. For those with localized disease, the median time to progression was 14 months, and overall survival time was 51. For patients with advanced disease, the median time to progression was 8 months and overall survival was 22 months.
Outcomes were less favorable when patients were treated with gemcitabine regimens or pazopanib. In most of these cases, however, patients were either on their second (gemcitabine) or third (pazopanib) lines of therapy.
In the gemcitabine group, 2 patients were treated for localized disease with curative intent and 27 for advanced disease. Of 28 evaluable patients, best response was partial remission in 3, stable disease in 8, and progressive disease in 17. In the 27 patients with advanced disease, the median progression free survival time was 3 months and overall survival was 13 months.
All 10 patients in the pazopanib group had advanced disease and had undergone a median of two prior lines of therapy. One patient had a partial remission, 3 had stable disease, and 6 had progressive disease. The median progression free survival was 4 months and median overall survival was 12 months.
Rarest of the rare: Primary malignant sarcoma of the heart
Luke Smith, of the School of Clinical Medicine, University of Cambridge, U.K., detailed the experience of 28 patients diagnosed with sarcomas of the heart or great vessels at the university’s Royal Papworth Hospital and Addenbrooke’s Hospital between 2000 and 2018.
Based on this retrospective review, surgery offers the best chance for long-term survival for these patients, who would otherwise experience progressive heart failure and die. Adjuvant chemotherapy and radiation therapy might be able to extend their survival and improve symptomatic relief, he said, but these outcomes have not been prospectively studied.
Typically, the patients in this series, 20 with pulmonary artery sarcoma and 8 with cardiac sarcoma, presented with symptoms mimicking heart failure, pulmonary hypertension, or thromboembolic disease. Nearly all, 24 patients reported breathlessness. Eight patients had chest pain or tightness, 6 had cough, 6 had peripheral edema, 6 had constitutional symptoms, 3 had hemoptysis, and 1 had a TIA. Only 1 patient had a seriously impaired left ventricular ejection fraction of less than 30%. LVEF was normal at 55% or more in 16 patients, and moderately impaired at 30% or more in 10 patients.
Median overall survival was 17 months. The 19 patients who underwent surgical resection of their primary tumor survived much longer than the 10 patients who did not--median overall survival of 20 months vs. 9 months--but this finding may simply reflect more advanced disease in patients with inoperable disease. There were 3 perioperative deaths among the 19 patients who underwent surgery: 14 with pulmonary artery sarcomas had pulmonary endarterectomy and 4 with cardiac sarcomas underwent resection or maximal debulking of their tumors.
Based on the retrospective study, adjuvant chemotherapy and radiation were safe and may lead to better outcomes for these patients. Active chemotherapy regimens in the palliative setting included paclitaxel (angiosarcoma) and anthracycline ± ifosfamide.
Nine patients received post-surgical chemotherapy, and after completion five also had radiotherapy. The 3 cardiac sarcoma patients who had surgical resection with curative intent were treated with adjuvant ifosfamide-based chemotherapy (with close monitoring of fluid balance), and showed no evidence of disease on last follow-ups. One patient received post-operative paclitaxel following maximal debulking of a cardiac angiosarcoma.
Post-surgical anthracycline with and without ifosfamide were used in patients with pulmonary artery sarcomas with no clinical cardiotoxicity. Although the median overall survival for patients who received post-operative chemo- and radio-therapy was 28 months and the median overall survival with surgery alone was 9 months, the difference was not statistically significant.
In the palliative setting, partial responses were observed with paclitaxel and anthracycline (including liposomal doxorubicin) in patients with cardiac angiosarcoma. For pulmonary artery intimal sarcomas, partial responses were achieved with anthracycline with and without ifosfamide. Radiotherapy provided good local control.
The longest surviving pulmonary artery sarcoma patient, at 103 months, had pulmonary artery endarterectomy, followed by adjuvant epirubicin and radiotherapy. She developed lung metastases 7 years later and was treated with radiofrequency ablation. The longest surviving cardiac sarcoma patient, at 24 months, remains disease free. He had surgery to resect a high-grade undifferentiated sarcoma with involved margins, followed by adjuvant ifosfamide and radiotherapy to the right atrium.
Therapeutically exploitable genetic aberrations in intimal sarcomas
Imatinib and olaratumab might prove to be therapeutic approaches for some patients with intimal sarcomas, based on a retrospective evaluation of genetic aberrations in 11 patients with intimal sarcomas, Jason Roszik, PhD, MBA, reported at the meeting.
Dr. Roszik and his colleagues at the University of Texas MD Anderson Cancer Center, Houston, analyzed information on 11 patients with intimal sarcomas in the American Association for Cancer Research (AACR) project, Genomics Evidence Neoplasia Information Exchange (GENIE). Sampling was taken from the primary tumor in 8 patients and from the metastatic site in the other 3.
MDM2 amplifications were seen in 8 of 10 patients with available copy number alterations. Amplifications in the CDK pathway were present in 5, PDGFRA gain was seen in 4, and CDKN2A copy number loss was present in 3. Mutations that could be targeted with drugs included ALK, ATM/ATR, PTCH1 and PDGFRB, he said.
Unique genomic rearrangement events included PDE4DIP-NOTCH2 and MRPS30-ARID2 fusions. Co-occurring alterations included a NOTCH2 copy number gain in the PDE4DIP-NOTCH2 fusion tumor, and PDGFRB mutations in both fusion-positive cases.
The researchers also drew on the published findings of whole-exome sequencing and array-comparative genomic hybridization from an autopsy case of cardiac intimal sarcoma (Virchows Arch. 2017 Sep;471(3):423-428). That study identified concurrent PDGFRA amplification and PDGFRB mutation.
The researchers additionally examined clinical trial enrollments and could find no patient with intimal sarcoma among 406 sarcoma enrolled patients. Intimal sarcomas were not eligible for any clinical trial given the location of the tumors in major blood vessels.
“The somatic mutations and DNA copy number alterations in the PDGFR pathway relevant to the pathogenesis and potential targeted therapy of cardiac intimal sarcoma may be targeted by imatinib or olaratumab. Inclusion of such rare tumors in targeted therapy basket trials with a waiver for inclusion criteria is warranted,” Dr. Roszik and his colleagues concluded in the abstract of their presentation.
The promise of combination therapy
The “largest experience using multimodality therapy with proton based local therapy” for sarcomas involving the pericardium, myocardium, valves, pulmonary veins, or pulmonary arteries was reported by Yen-Lin E. Chen, MD, and her colleagues at Massachusetts General Hospital, Boston.
They examined an institutional sarcoma data repository of 13,950 patients and found 37 patients with sarcomas arising from the pericardium, myocardium, valves, pulmonary veins, or pulmonary arteries. These included 9 with unclassified pleomorphic sarcoma/malignant fibrous histiocytoma, 8 with angiosarcoma, 4 with spindle cell sarcoma, 4 with sarcoma not otherwise specified, 3 with leiomyosarcoma, 2 with osteosarcoma, 2 with Ewing sarcoma, and 1 each with chondrosarcoma, malignant peripheral nerve sheath tumor, rhabdomyosarcoma, synovial sarcoma, and intimal sarcoma.
Two-thirds of the patients had induction chemotherapy with or without maintenance therapy. Adriamycin, ifosfamide, and taxol therapies were most common. Two-thirds received proton based radiotherapy. Of the 23 patients who underwent resection, 11 were R2 (macroscopic positive margins), 3 were R1 (microscopic positive margins), and 9 were R0 (clear margins).
The 1-year overall survival rate was 64%, which fell to 37% at 3 years and to 28% at 5 years. Median survival was 28 months, twice that typically seen in the literature, Dr. Chen said.
For patients receiving proton based radiotherapy to a median dose of 64.8 GyRBE (range 63-72 GyRBE, 3 with additional intraoperative electrons), local failure free survivals were 80%, 64%, and 52% at 1, 3, and 5 years, respectively. For patients who did not receive radiotherapy, local failure free survival rates were 13%, 10%, 10%, respectively.
Overall, the 1, 3, and 5 year metastatic free survival rates were 25%, 14%, and 14%.
Survival rate was significantly better for patients with tumors smaller than 5 cm ( P =0.036), those over 40 years old ( P =0.028), those able to have surgery ( P =0.011), and those with non-angiosarcoma histologies ( P = 0.002).
This year’s annual meeting of The Connective Tissue Oncology Society brought new insights on intimal sarcoma. Four studies in a featured session at the meeting examined both current and novel treatments for this rare and aggressive cancer, and emphasized the need for new therapies.
Anthracycline-based regimens as preferred first-line therapies
Anthracycline-based regimens were the preferred first-line therapies used in 83 adults with intimal sarcomas in a retrospective study of data from the World Sarcoma Network, reported by Anna Maria Frezza, MD, of the, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy, and her colleagues. The researchers described the experience with anthracycline-based regimens as well as gemcitabine-based regimens and pazopanib among MDM2-positive patients with intimal sarcomas treated at 16 sarcoma reference centers in Europe, the United States, and Japan. Their findings speak to the need for new active drugs, which they said should target the MDM2 and CDK4 overexpression seen in patients with this rare sarcoma.Of the 83 patients studied, nearly all (76 patients) initially received an anthracycline-based regimen. Gemcitabine-based regimens were used in 29 patients and pazopanib in 10 patients; 20 of the 39 patients received more than one treatment.
Anthracycline-based regimens were associated with a 12-month progression-free survival rate of 38% in 76 patients with intimal sarcomas. All of the 76 patients received anthracycline regimens as their initial systemic therapy; 27 were treated for localized disease with a curative intent and the remaining 49 had advanced disease. The researchers also noted that anthracycline regimens were safely used in 22 patients with cardiac intimal sarcomas, as none of them died of cardiotoxicity.
Based on RECIST 1.1 measures, the overall response rate was 37% in 57 evaluable patients: 3 patients had a complete response, 18 had a partial response, 27 had stable disease, and 9 had progressive disease. For those with localized disease, the median time to progression was 14 months, and overall survival time was 51. For patients with advanced disease, the median time to progression was 8 months and overall survival was 22 months.
Outcomes were less favorable when patients were treated with gemcitabine regimens or pazopanib. In most of these cases, however, patients were either on their second (gemcitabine) or third (pazopanib) lines of therapy.
In the gemcitabine group, 2 patients were treated for localized disease with curative intent and 27 for advanced disease. Of 28 evaluable patients, best response was partial remission in 3, stable disease in 8, and progressive disease in 17. In the 27 patients with advanced disease, the median progression free survival time was 3 months and overall survival was 13 months.
All 10 patients in the pazopanib group had advanced disease and had undergone a median of two prior lines of therapy. One patient had a partial remission, 3 had stable disease, and 6 had progressive disease. The median progression free survival was 4 months and median overall survival was 12 months.
Rarest of the rare: Primary malignant sarcoma of the heart
Luke Smith, of the School of Clinical Medicine, University of Cambridge, U.K., detailed the experience of 28 patients diagnosed with sarcomas of the heart or great vessels at the university’s Royal Papworth Hospital and Addenbrooke’s Hospital between 2000 and 2018.
Based on this retrospective review, surgery offers the best chance for long-term survival for these patients, who would otherwise experience progressive heart failure and die. Adjuvant chemotherapy and radiation therapy might be able to extend their survival and improve symptomatic relief, he said, but these outcomes have not been prospectively studied.
Typically, the patients in this series, 20 with pulmonary artery sarcoma and 8 with cardiac sarcoma, presented with symptoms mimicking heart failure, pulmonary hypertension, or thromboembolic disease. Nearly all, 24 patients reported breathlessness. Eight patients had chest pain or tightness, 6 had cough, 6 had peripheral edema, 6 had constitutional symptoms, 3 had hemoptysis, and 1 had a TIA. Only 1 patient had a seriously impaired left ventricular ejection fraction of less than 30%. LVEF was normal at 55% or more in 16 patients, and moderately impaired at 30% or more in 10 patients.
Median overall survival was 17 months. The 19 patients who underwent surgical resection of their primary tumor survived much longer than the 10 patients who did not--median overall survival of 20 months vs. 9 months--but this finding may simply reflect more advanced disease in patients with inoperable disease. There were 3 perioperative deaths among the 19 patients who underwent surgery: 14 with pulmonary artery sarcomas had pulmonary endarterectomy and 4 with cardiac sarcomas underwent resection or maximal debulking of their tumors.
Based on the retrospective study, adjuvant chemotherapy and radiation were safe and may lead to better outcomes for these patients. Active chemotherapy regimens in the palliative setting included paclitaxel (angiosarcoma) and anthracycline ± ifosfamide.
Nine patients received post-surgical chemotherapy, and after completion five also had radiotherapy. The 3 cardiac sarcoma patients who had surgical resection with curative intent were treated with adjuvant ifosfamide-based chemotherapy (with close monitoring of fluid balance), and showed no evidence of disease on last follow-ups. One patient received post-operative paclitaxel following maximal debulking of a cardiac angiosarcoma.
Post-surgical anthracycline with and without ifosfamide were used in patients with pulmonary artery sarcomas with no clinical cardiotoxicity. Although the median overall survival for patients who received post-operative chemo- and radio-therapy was 28 months and the median overall survival with surgery alone was 9 months, the difference was not statistically significant.
In the palliative setting, partial responses were observed with paclitaxel and anthracycline (including liposomal doxorubicin) in patients with cardiac angiosarcoma. For pulmonary artery intimal sarcomas, partial responses were achieved with anthracycline with and without ifosfamide. Radiotherapy provided good local control.
The longest surviving pulmonary artery sarcoma patient, at 103 months, had pulmonary artery endarterectomy, followed by adjuvant epirubicin and radiotherapy. She developed lung metastases 7 years later and was treated with radiofrequency ablation. The longest surviving cardiac sarcoma patient, at 24 months, remains disease free. He had surgery to resect a high-grade undifferentiated sarcoma with involved margins, followed by adjuvant ifosfamide and radiotherapy to the right atrium.
Therapeutically exploitable genetic aberrations in intimal sarcomas
Imatinib and olaratumab might prove to be therapeutic approaches for some patients with intimal sarcomas, based on a retrospective evaluation of genetic aberrations in 11 patients with intimal sarcomas, Jason Roszik, PhD, MBA, reported at the meeting.
Dr. Roszik and his colleagues at the University of Texas MD Anderson Cancer Center, Houston, analyzed information on 11 patients with intimal sarcomas in the American Association for Cancer Research (AACR) project, Genomics Evidence Neoplasia Information Exchange (GENIE). Sampling was taken from the primary tumor in 8 patients and from the metastatic site in the other 3.
MDM2 amplifications were seen in 8 of 10 patients with available copy number alterations. Amplifications in the CDK pathway were present in 5, PDGFRA gain was seen in 4, and CDKN2A copy number loss was present in 3. Mutations that could be targeted with drugs included ALK, ATM/ATR, PTCH1 and PDGFRB, he said.
Unique genomic rearrangement events included PDE4DIP-NOTCH2 and MRPS30-ARID2 fusions. Co-occurring alterations included a NOTCH2 copy number gain in the PDE4DIP-NOTCH2 fusion tumor, and PDGFRB mutations in both fusion-positive cases.
The researchers also drew on the published findings of whole-exome sequencing and array-comparative genomic hybridization from an autopsy case of cardiac intimal sarcoma (Virchows Arch. 2017 Sep;471(3):423-428). That study identified concurrent PDGFRA amplification and PDGFRB mutation.
The researchers additionally examined clinical trial enrollments and could find no patient with intimal sarcoma among 406 sarcoma enrolled patients. Intimal sarcomas were not eligible for any clinical trial given the location of the tumors in major blood vessels.
“The somatic mutations and DNA copy number alterations in the PDGFR pathway relevant to the pathogenesis and potential targeted therapy of cardiac intimal sarcoma may be targeted by imatinib or olaratumab. Inclusion of such rare tumors in targeted therapy basket trials with a waiver for inclusion criteria is warranted,” Dr. Roszik and his colleagues concluded in the abstract of their presentation.
The promise of combination therapy
The “largest experience using multimodality therapy with proton based local therapy” for sarcomas involving the pericardium, myocardium, valves, pulmonary veins, or pulmonary arteries was reported by Yen-Lin E. Chen, MD, and her colleagues at Massachusetts General Hospital, Boston.
They examined an institutional sarcoma data repository of 13,950 patients and found 37 patients with sarcomas arising from the pericardium, myocardium, valves, pulmonary veins, or pulmonary arteries. These included 9 with unclassified pleomorphic sarcoma/malignant fibrous histiocytoma, 8 with angiosarcoma, 4 with spindle cell sarcoma, 4 with sarcoma not otherwise specified, 3 with leiomyosarcoma, 2 with osteosarcoma, 2 with Ewing sarcoma, and 1 each with chondrosarcoma, malignant peripheral nerve sheath tumor, rhabdomyosarcoma, synovial sarcoma, and intimal sarcoma.
Two-thirds of the patients had induction chemotherapy with or without maintenance therapy. Adriamycin, ifosfamide, and taxol therapies were most common. Two-thirds received proton based radiotherapy. Of the 23 patients who underwent resection, 11 were R2 (macroscopic positive margins), 3 were R1 (microscopic positive margins), and 9 were R0 (clear margins).
The 1-year overall survival rate was 64%, which fell to 37% at 3 years and to 28% at 5 years. Median survival was 28 months, twice that typically seen in the literature, Dr. Chen said.
For patients receiving proton based radiotherapy to a median dose of 64.8 GyRBE (range 63-72 GyRBE, 3 with additional intraoperative electrons), local failure free survivals were 80%, 64%, and 52% at 1, 3, and 5 years, respectively. For patients who did not receive radiotherapy, local failure free survival rates were 13%, 10%, 10%, respectively.
Overall, the 1, 3, and 5 year metastatic free survival rates were 25%, 14%, and 14%.
Survival rate was significantly better for patients with tumors smaller than 5 cm ( P =0.036), those over 40 years old ( P =0.028), those able to have surgery ( P =0.011), and those with non-angiosarcoma histologies ( P = 0.002).
This year’s annual meeting of The Connective Tissue Oncology Society brought new insights on intimal sarcoma. Four studies in a featured session at the meeting examined both current and novel treatments for this rare and aggressive cancer, and emphasized the need for new therapies.
Anthracycline-based regimens as preferred first-line therapies
Anthracycline-based regimens were the preferred first-line therapies used in 83 adults with intimal sarcomas in a retrospective study of data from the World Sarcoma Network, reported by Anna Maria Frezza, MD, of the, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy, and her colleagues. The researchers described the experience with anthracycline-based regimens as well as gemcitabine-based regimens and pazopanib among MDM2-positive patients with intimal sarcomas treated at 16 sarcoma reference centers in Europe, the United States, and Japan. Their findings speak to the need for new active drugs, which they said should target the MDM2 and CDK4 overexpression seen in patients with this rare sarcoma.Of the 83 patients studied, nearly all (76 patients) initially received an anthracycline-based regimen. Gemcitabine-based regimens were used in 29 patients and pazopanib in 10 patients; 20 of the 39 patients received more than one treatment.
Anthracycline-based regimens were associated with a 12-month progression-free survival rate of 38% in 76 patients with intimal sarcomas. All of the 76 patients received anthracycline regimens as their initial systemic therapy; 27 were treated for localized disease with a curative intent and the remaining 49 had advanced disease. The researchers also noted that anthracycline regimens were safely used in 22 patients with cardiac intimal sarcomas, as none of them died of cardiotoxicity.
Based on RECIST 1.1 measures, the overall response rate was 37% in 57 evaluable patients: 3 patients had a complete response, 18 had a partial response, 27 had stable disease, and 9 had progressive disease. For those with localized disease, the median time to progression was 14 months, and overall survival time was 51. For patients with advanced disease, the median time to progression was 8 months and overall survival was 22 months.
Outcomes were less favorable when patients were treated with gemcitabine regimens or pazopanib. In most of these cases, however, patients were either on their second (gemcitabine) or third (pazopanib) lines of therapy.
In the gemcitabine group, 2 patients were treated for localized disease with curative intent and 27 for advanced disease. Of 28 evaluable patients, best response was partial remission in 3, stable disease in 8, and progressive disease in 17. In the 27 patients with advanced disease, the median progression free survival time was 3 months and overall survival was 13 months.
All 10 patients in the pazopanib group had advanced disease and had undergone a median of two prior lines of therapy. One patient had a partial remission, 3 had stable disease, and 6 had progressive disease. The median progression free survival was 4 months and median overall survival was 12 months.
Rarest of the rare: Primary malignant sarcoma of the heart
Luke Smith, of the School of Clinical Medicine, University of Cambridge, U.K., detailed the experience of 28 patients diagnosed with sarcomas of the heart or great vessels at the university’s Royal Papworth Hospital and Addenbrooke’s Hospital between 2000 and 2018.
Based on this retrospective review, surgery offers the best chance for long-term survival for these patients, who would otherwise experience progressive heart failure and die. Adjuvant chemotherapy and radiation therapy might be able to extend their survival and improve symptomatic relief, he said, but these outcomes have not been prospectively studied.
Typically, the patients in this series, 20 with pulmonary artery sarcoma and 8 with cardiac sarcoma, presented with symptoms mimicking heart failure, pulmonary hypertension, or thromboembolic disease. Nearly all, 24 patients reported breathlessness. Eight patients had chest pain or tightness, 6 had cough, 6 had peripheral edema, 6 had constitutional symptoms, 3 had hemoptysis, and 1 had a TIA. Only 1 patient had a seriously impaired left ventricular ejection fraction of less than 30%. LVEF was normal at 55% or more in 16 patients, and moderately impaired at 30% or more in 10 patients.
Median overall survival was 17 months. The 19 patients who underwent surgical resection of their primary tumor survived much longer than the 10 patients who did not--median overall survival of 20 months vs. 9 months--but this finding may simply reflect more advanced disease in patients with inoperable disease. There were 3 perioperative deaths among the 19 patients who underwent surgery: 14 with pulmonary artery sarcomas had pulmonary endarterectomy and 4 with cardiac sarcomas underwent resection or maximal debulking of their tumors.
Based on the retrospective study, adjuvant chemotherapy and radiation were safe and may lead to better outcomes for these patients. Active chemotherapy regimens in the palliative setting included paclitaxel (angiosarcoma) and anthracycline ± ifosfamide.
Nine patients received post-surgical chemotherapy, and after completion five also had radiotherapy. The 3 cardiac sarcoma patients who had surgical resection with curative intent were treated with adjuvant ifosfamide-based chemotherapy (with close monitoring of fluid balance), and showed no evidence of disease on last follow-ups. One patient received post-operative paclitaxel following maximal debulking of a cardiac angiosarcoma.
Post-surgical anthracycline with and without ifosfamide were used in patients with pulmonary artery sarcomas with no clinical cardiotoxicity. Although the median overall survival for patients who received post-operative chemo- and radio-therapy was 28 months and the median overall survival with surgery alone was 9 months, the difference was not statistically significant.
In the palliative setting, partial responses were observed with paclitaxel and anthracycline (including liposomal doxorubicin) in patients with cardiac angiosarcoma. For pulmonary artery intimal sarcomas, partial responses were achieved with anthracycline with and without ifosfamide. Radiotherapy provided good local control.
The longest surviving pulmonary artery sarcoma patient, at 103 months, had pulmonary artery endarterectomy, followed by adjuvant epirubicin and radiotherapy. She developed lung metastases 7 years later and was treated with radiofrequency ablation. The longest surviving cardiac sarcoma patient, at 24 months, remains disease free. He had surgery to resect a high-grade undifferentiated sarcoma with involved margins, followed by adjuvant ifosfamide and radiotherapy to the right atrium.
Therapeutically exploitable genetic aberrations in intimal sarcomas
Imatinib and olaratumab might prove to be therapeutic approaches for some patients with intimal sarcomas, based on a retrospective evaluation of genetic aberrations in 11 patients with intimal sarcomas, Jason Roszik, PhD, MBA, reported at the meeting.
Dr. Roszik and his colleagues at the University of Texas MD Anderson Cancer Center, Houston, analyzed information on 11 patients with intimal sarcomas in the American Association for Cancer Research (AACR) project, Genomics Evidence Neoplasia Information Exchange (GENIE). Sampling was taken from the primary tumor in 8 patients and from the metastatic site in the other 3.
MDM2 amplifications were seen in 8 of 10 patients with available copy number alterations. Amplifications in the CDK pathway were present in 5, PDGFRA gain was seen in 4, and CDKN2A copy number loss was present in 3. Mutations that could be targeted with drugs included ALK, ATM/ATR, PTCH1 and PDGFRB, he said.
Unique genomic rearrangement events included PDE4DIP-NOTCH2 and MRPS30-ARID2 fusions. Co-occurring alterations included a NOTCH2 copy number gain in the PDE4DIP-NOTCH2 fusion tumor, and PDGFRB mutations in both fusion-positive cases.
The researchers also drew on the published findings of whole-exome sequencing and array-comparative genomic hybridization from an autopsy case of cardiac intimal sarcoma (Virchows Arch. 2017 Sep;471(3):423-428). That study identified concurrent PDGFRA amplification and PDGFRB mutation.
The researchers additionally examined clinical trial enrollments and could find no patient with intimal sarcoma among 406 sarcoma enrolled patients. Intimal sarcomas were not eligible for any clinical trial given the location of the tumors in major blood vessels.
“The somatic mutations and DNA copy number alterations in the PDGFR pathway relevant to the pathogenesis and potential targeted therapy of cardiac intimal sarcoma may be targeted by imatinib or olaratumab. Inclusion of such rare tumors in targeted therapy basket trials with a waiver for inclusion criteria is warranted,” Dr. Roszik and his colleagues concluded in the abstract of their presentation.
The promise of combination therapy
The “largest experience using multimodality therapy with proton based local therapy” for sarcomas involving the pericardium, myocardium, valves, pulmonary veins, or pulmonary arteries was reported by Yen-Lin E. Chen, MD, and her colleagues at Massachusetts General Hospital, Boston.
They examined an institutional sarcoma data repository of 13,950 patients and found 37 patients with sarcomas arising from the pericardium, myocardium, valves, pulmonary veins, or pulmonary arteries. These included 9 with unclassified pleomorphic sarcoma/malignant fibrous histiocytoma, 8 with angiosarcoma, 4 with spindle cell sarcoma, 4 with sarcoma not otherwise specified, 3 with leiomyosarcoma, 2 with osteosarcoma, 2 with Ewing sarcoma, and 1 each with chondrosarcoma, malignant peripheral nerve sheath tumor, rhabdomyosarcoma, synovial sarcoma, and intimal sarcoma.
Two-thirds of the patients had induction chemotherapy with or without maintenance therapy. Adriamycin, ifosfamide, and taxol therapies were most common. Two-thirds received proton based radiotherapy. Of the 23 patients who underwent resection, 11 were R2 (macroscopic positive margins), 3 were R1 (microscopic positive margins), and 9 were R0 (clear margins).
The 1-year overall survival rate was 64%, which fell to 37% at 3 years and to 28% at 5 years. Median survival was 28 months, twice that typically seen in the literature, Dr. Chen said.
For patients receiving proton based radiotherapy to a median dose of 64.8 GyRBE (range 63-72 GyRBE, 3 with additional intraoperative electrons), local failure free survivals were 80%, 64%, and 52% at 1, 3, and 5 years, respectively. For patients who did not receive radiotherapy, local failure free survival rates were 13%, 10%, 10%, respectively.
Overall, the 1, 3, and 5 year metastatic free survival rates were 25%, 14%, and 14%.
Survival rate was significantly better for patients with tumors smaller than 5 cm ( P =0.036), those over 40 years old ( P =0.028), those able to have surgery ( P =0.011), and those with non-angiosarcoma histologies ( P = 0.002).
Primary renal synovial sarcoma – a diagnostic dilemma
Soft tissue sarcomas are rare mesenchymal tumors that comprise 1% of all malignancies. Synovial sarcoma accounts for 5% to 10% of adult soft tissue sarcomas and usually occurs in close association with joint capsules, tendon sheaths, and bursa in the extremities of young and middle-aged adults.1 Synovial sarcomas have been reported in other unusual sites, including the head and neck, thoracic and abdominal wall, retroperitoneum, bone, pleura, and visceral organs such as the lung, prostate, or kidney.2 Primary renal synovial sarcoma is an extremely rare tumor accounting for <2% of all malignant renal tumors.3 To the best of our knowledge, fewer than 50 cases of primary renal synovial sarcoma have been described in the English literature.4 It presents as a diagnostic dilemma because of the dearth of specific clinical and imaging findings and is often confused with benign and malignant tumors. The differential diagnosis includes angiomyolipoma, renal cell carcinoma with sarcomatoid differentiation, metastatic sarcoma, hemangiopericytoma, malignant solitary fibrous tumor, Wilms tumor, and malignant peripheral nerve sheath tumor. Hence, a combination of histomorphologic, immunohistochemical, cytogenetic, and molecular studies that show a unique chromosomal translocation t(X;18) (p11;q11) is imperative in the diagnosis of primary renal synovial sarcoma.4 In the present report, we present the case of a 38-year-old man who was diagnosed with primary renal synovial sarcoma.
Case presentation and summary
A 38-year-old man with a medical history of gastroesophageal reflux disease and Barrett’s esophagus presented to our hospital for the first time with persistent and progressive right-sided flank and abdominal pain that was aggravated after a minor trauma to the back. There was no associated hematuria or dysuria.
Of note is that he had experienced intermittent flank pain for 2 years before this transfer. He had initially been diagnosed at his local hospital close to his home by ultrasound with an angiomyolipoma of 2 × 3 cm arising from the upper pole of his right kidney, which remained stable on repeat sonograms. About 22 months after his initial presentation at his local hospital, the flank pain increased, and a computed-tomographic (CT) scan revealed a perinephric hematoma that was thought to originate from a ruptured angiomyolipoma. He subsequently underwent embolization, but his symptoms recurred soon after. He presented again to his local hospital where CT imaging revealed a significant increase in the size of the retroperitoneal mass, and findings were suggestive of a hematoma. Subsequent angiogram did not reveal active extravasation, so a biopsy was performed.
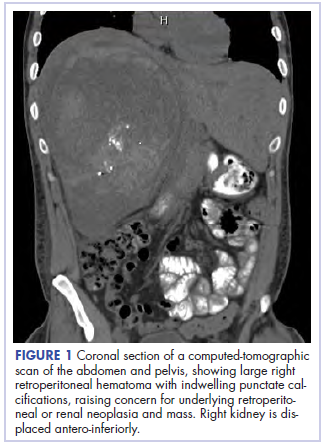
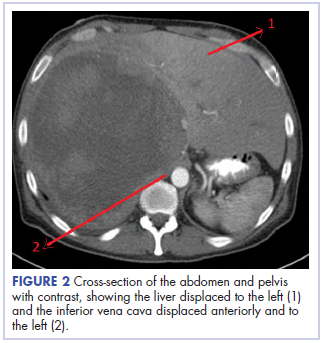
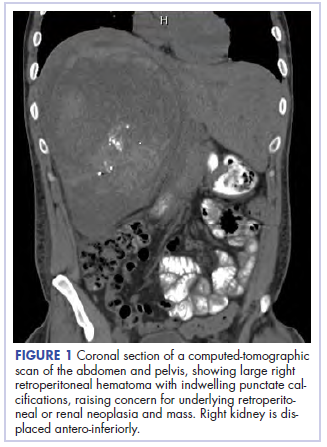
Before confirmatory pathologic evaluation could be completed, the patient presented to his local hospital again in excruciating pain. A CT scan of his abdomen and pelvis demonstrated a massive subacute on chronic hematoma in the right retroperitoneum measuring 22 × 19 × 18 cm, with calcifications originating from an upper pole right renal neoplasm. The right kidney was displaced antero-inferiorly, and the inferior vena cava was displaced anteriorly and to the left. The preliminary pathology returned with findings suggestive of sarcoma (Figures 1 and 2).
The patient was then transferred to our institution, where he was evaluated by medical and surgical oncology. A CT scan of the chest and magnetic-resonance imaging (MRI) of the brain did not reveal metastatic disease. He underwent exploratory laparotomy that involved the resection of a 22-cm retroperitoneal mass, right nephrectomy, right adrenalectomy, partial right hepatectomy, and a full thickness resection of the right postero-inferior diaphragm followed by mesh repair because of involvement by the tumor.
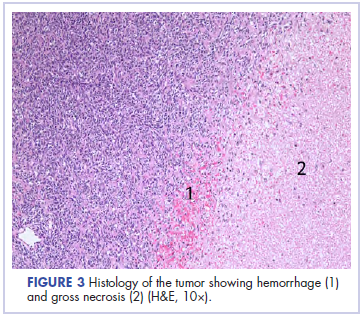
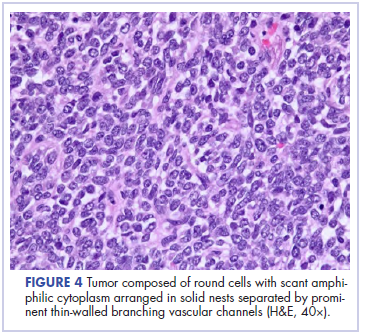
In its entirety, the specimen was a mass of 26 × 24 × 14 cm. It was sectioned to show extensively necrotic and hemorrhagic variegated white to tan-red parenchyma (Figure 3). Histology revealed a poorly differentiated malignant neoplasm composed of round cells with scant amphophilic cytoplasm arranged in solid, variably sized nests separated by prominent thin-walled branching vascular channels (Figure 4). The mitotic rate was high. It was determined to be a histologically ungraded sarcoma according to the French Federation of Comprehensive Cancer Centers system of grading soft tissue sarcomas; the margins were indeterminate. Immunohistochemistry was positive for EMA, TLE1, and negative for AE1/AE3, S100, STAT6, and Nkx2.2. Molecular pathology fluorescent in situ hybridization (FISH) analysis demonstrated positivity for SS18 gene rearrangement (SS18-SSX1 fusion).
After recovering from surgery, the patient received adjuvant chemotherapy with doxorubicin and ifosfamide. It has been almost 16 months since we first saw this patient. He was started on doxorubicin 20 mg/m2 on days 1 to 4, ifosfamide 2,500 mg on days 1 to 4, and mesna 800 mg on days 1 to 4, for a total of 6 cycles. He did well for the first 5 months, after which he developed disease recurrence in the postoperative nephrectomy bed (a biopsy showed it to be recurrent synovial sarcoma) as well as pulmonary nodules, for which he was started on trabectedin 1.5 mg/m2 every 3 weeks. Two months later, a CT scan showed an increase in the size of his retroperitoneal mass, and the treatment was changed to pazopanib 400 mg daily orally, on which he remained at the time of publication.
Discussion
Synovial sarcoma is the fourth most common type of soft tissue sarcoma, accounting for 2.5% to 10.5% of all primary soft tissue malignancies worldwide. It occurs most frequently in adolescents and young adults, with most patients presenting between the ages of 15 and 40 years. Median age of presentation is 36 years. Despite the nomenclature, synovial sarcoma does not arise in intra-articular locations but typically occurs in proximity to joints in the extremities. Synovial sarcomas are less commonly described in other sites, including the head and neck, mediastinum, intraperitoneum, retroperitoneum, lung, pleura, and kidney.4,5 Renal synovial sarcoma was first described in a published article by Argani and colleagues in 2000.5
Adult renal mesenchymal tumors are classified into benign and malignant tumors on the basis of the histologic features and clinicobiologic behavior.6,7 The benign esenchymal renal tumors include angiomyolipoma, leiomyoma, hemangioma, lymphangioma, juxtaglomerular cell tumor, renomedullary interstitial cell tumor (medullary fibroma), lipoma, solitary fibrous tumor, and schwannoma. Malignant renal tumors of mesenchymal origin include leiomyosarcoma, rhabdomyosarcoma, angiosarcoma, osteosarcoma, fibrosarcoma, malignant fibrous histiocytoma, solitary fibrous tumor, and synovial sarcoma.
Most of these tumor types cause the same nonspecific symptoms in patients – abdominal pain, flank pain, abdominal fullness, a palpable mass, and hematuria – although they can be clinically silent. The average duration of symptoms in synovial sarcoma is 2 to 4 years.8 The long duration of symptoms and initial slow growth of synovial sarcomas may give a false impression of a benign process.
A preoperative radiological diagnosis of primary renal synovial sarcoma may be suspected by analyzing the tumor’s growth patterns on CT scans.9 Renal synovial sarcomas often appear as large, well-defined soft tissue masses that can extend into the renal pelvis or into the perinephric region.9 A CT scan may identify soft tissue calcifications, especially subtle ones in areas where the tumor anatomy is complex. A CT scan may also reveal areas of hemorrhage, necrosis, or cyst formation within the tumor, and can easily confirm bone involvement. Intravenous contrast may help in differentiating the mass from adjacent muscle and neurovascular complex.9,10 On MRI, renal synovial sarcomas are often described as nonspecific heterogeneous masses, although they may also exhibit heterogeneous enhancement of hemorrhagic areas, calcifications, and air-fluid levels (known as “triple sign”) as well as septae. The triple sign may be identified as areas of low, intermediate, and high signal intensity, correlating with areas of hemorrhage, calcification, and air-fluid level.9,10 Signal intensity is about equal to that of skeletal muscle on T1-weighted MRI and higher than that of subcutaneous fat on T2-weighted MRI.
In the present case, the tumor was initially misdiagnosed as an angiomyolipoma, the most common benign tumor of the kidney. Angiomyolipomas are usually solid triphasic tumors arising from the renal cortex and are composed of 3 major elements: dysmorphic blood vessels, smooth muscle components, and adipose tissue. When angiomyolipomas are large enough, they are readily recognized by the identification of macroscopic fat within the tumor, either by CT scan or MRI.11 When they are small, they may be difficult to distinguish from a small cyst on CT because of volume averaging.
On pathology, synovial sarcoma has dual epithelial and mesenchymal differentiation. They are frequently multi-lobulated, and areas of necrosis, hemorrhage, and cyst formation are also common. There are 3 main histologic subtypes of synovial sarcoma: biphasic (20%-30%), monophasic (50%-60%), and poorly differentiated (15%-25%). Poorly differentiated synovial sarcomas are generally epithelioid in morphology, have high mitotic activity (usually 10-20 mitoses/10 high-power field; range is <5 for well differentiated, low-grade tumors), and can be confused with round cell tumors such as Ewing sarcoma. Poorly differentiated synovial sarcomas are high-grade tumors.
Immunohistochemical studies can confirm the pathological diagnosis. Synovial sarcomas usually stain positive for Bcl2, CD99/Mic2, CD56, Vim, and focally for EMA but negatively for desmin, actin, WT1, S-100, CD34, and CD31.5 Currently, the gold standard for diagnosis and hallmark for synovial sarcomas are the t (X;18) translocation and SYT-SSX gene fusion products (SYT-SSX1 in 67% and SYT-SSX2 in 33% of cases). These can be detected either by FISH or reverse-transcription polymerase chain reaction. This genetic alteration is identified in more than 90% of synovial sarcomas and is highly specific.
The role of SYT-SSX gene fusion in the pathogenesis of synovial sarcoma is an active area of investigation. The fusion of SYT with SSX translates into a fusion protein that binds to the transcription activator SMARCA4 that is involved in chromatin remodeling, thus displacing both the wildtype SYT and the tumor suppressor gene SMARCB1. The modified protein complex then binds at several super-enhancer loci, unlocking suppressed genes such as Sox2, which is known to be necessary for synovial sarcoma proliferation. Alterations in SMARCB1 are involved in several cancer types, implicating this event as a driver of these malignancies.12 This results in a global alteration in chromatin remodeling that needs to be better understood to design targeted therapies.
The clinical course of synovial sarcoma, regardless of the tissue of origin, is typically poor. Multiple clinical and pathologic factors, including tumor size, location, patient age, and presence of poorly differentiated areas, are thought to have prognostic significance. A tumor size of more than 5 cm at presentation has the greatest impact on prognosis, with studies showing 5-year survival rates of 64% for patients with tumors smaller than 5 cm and 26% for patients with masses greater than 5 cm.13,14 High-grade synovial sarcoma is favored in tumors that have cystic components, hemorrhage, and fluid levels and the triple sign.
Patients with tumors in the extremities have a more favorable prognosis than those with lesions in the head and neck area or axially, a feature that likely reflects better surgical control available for extremity lesions. Patient age of less than 15 to 20 years is also associated with a better long-term prognosis.15,16 Varela-Duran and Enzinger17 reported that the presence of extensive calcifications suggests improved long-term survival, with 5-year survival rates of 82% and decreased rates of local recurrence (32%) and metastatic disease (29%). The poorly differentiated subtype is associated with a worsened prognosis, with a 5-year survival rate of 20% through 30%.18,19 Other pathologic factors associated with worsened prognosis include presence of rhabdoid cells, extensive tumor necrosis, high nuclear grade, p53 mutations, and high mitotic rate (>10 mitoses/10 high-power field). More recently, the gene fusion type SYT-SSX2 (more common in monophasic lesions) has been associated with an improved prognosis, compared with that for SYT-SSX1, and an 89% metastasis-free survival.20
Although there are no guidelines for the treatment of primary renal synovial sarcoma because of the limited number of cases reported, surgery is considered the first choice. Adjuvant chemotherapy with an anthracycline (doxorubicin or epirubicin) combined with ifosfamide has been the most frequently used regimen in published cases, especially in those in which patients have poor prognostic factors as mentioned above.
Overall, the 5-year survival rate ranges from 36% to 76%.14 The clinical course of synovial sarcoma is characterized by a high rate of local recurrence (30%-50%) and metastatic disease (41%). Most metastases occur within the first 2 to 5 years after treatment cessation. Metastases are present in 16% to 25% of patients at their initial presentation, with the most frequent metastatic site being the lung, followed by the lymph nodes (4%-18%) and bone (8%-11%).
Conclusion
Primary renal synovial sarcoma is extremely rare, and preoperative diagnosis is difficult in the absence of specific clinical or imaging findings. A high index of suspicion combined with pathologic, immunohistochemical, cytogenetic, and molecular studies is essential for accurate diagnosis and subsequent treatment planning. The differential diagnosis of renal synovial sarcoma can be extensive, and our experience with this patient illustrates the diagnostic dilemma associated with renal synovial sarcoma.
1. Majumder A, Dey S, Khandakar B, Medda S, Chandra Paul P. Primary renal synovial sarcoma: a rare tumor with an atypical presentation. Arch Iran Med. 2014;17(10):726-728.
2. Fetsch JF, Meis JM. Synovial sarcoma of the abdominal wall. Cancer. 1993;72(2):469 477.
3. Wang Z, Zhong Z, Zhu L, et al. Primary synovial sarcoma of the kidney: a case report. Oncol Lett. 2015;10(6):3542-3544.
4. Abbas M, Dämmrich ME, Braubach P, et al. Synovial sarcoma of the kidney in a young patient with a review of the literature. Rare tumors. 2014;6(2):5393
5. Argani P, Faria PA, Epstein JI, et al. Primary renal synovial sarcoma: molecular and morphologic delineation of an entity previously included among embryonal sarcomas of the kidney. Am J Surg Pathol. 2000;24(8):1087-1096.
6. Eble JN, Sauter G, Epstein JI, Sesterhenn IA, eds. World Health Organization classification of tumours: pathology and genetics of tumours of the urinary system and male genital organs. Lyon, France: IARC; 2004.
7. Tamboli P, Ro JY, Amin MB, Ligato S, Ayala AG. Benign tumors and tumor-like lesions of the adult kidney. Part II: benign mesenchymal and mixed neoplasms, and tumor-like lesions. Adv Anat Pathol. 2000;7(1):47-66.
8. Weiss SW, Goldblum JR. Malignant soft tissue tumors of uncertain type. In: Weiss SW, Goldblum JR, eds. Enzinger and Weiss’s soft tissue tumors. 4th ed. St. Louis, MO: Mosby, 2001; 1483-1565.
9. Lacovelli R, Altavilla A, Ciardi A, et al. Clinical and pathological features of primary renal synovial sarcoma: analysis of 64 cases from 11 years of medical literature. BJU Int. 2012;110(10):1449-1454.
10. Alhazzani AR, El-Sharkawy MS, Hassan H. Primary retroperitoneal synovial sarcoma in CT and MRI. Urol Ann. 2010;2(1):39-41.
11. Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO, Prasad SR. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010;30(6):1525-1540.
12. Sápi Z, Papp G, Szendrői M, et al. Epigenetic regulation of SMARCB1 by miR-206, -381 and -671- 5p is evident in a variety of SMARCB1 immunonegative soft tissue sarcomas, while miR-765 appears specific for epithelioid sarcoma. A miRNA study of 223 soft tissue sarcomas. Genes Chromosomes Cancer. 2016;55(10):786-802.
13. Ferrari A, Gronchi A, Casanova M, et al. Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer. 2004;101(3):627-634.
14. Rangheard AS, Vanel D, Viala J, Schwaab G, Casiraghi O, Sigal R. Synovial sarcomas of the head and neck: CT and MR imaging findings of eight patients. Am J Neuroradiol. 2001;22(5):851-857.
15. Oda Y, Hashimoto H, Tsuneyoshi M, Takeshita S. Survival in synovial sarcoma: a multivariate study of prognostic factors with special emphasis on the comparison between early death and long-term survival. Am J Surg Pathol. 1993;17(1):35-44.
16. Raney RB. Synovial sarcoma in young people: background, prognostic factors and therapeutic questions. J Pediatr Hematol Oncol. 2005;27(4):207-211.
17. Varela-Duran J, Enzinger FM. Calcifying synovial sarcoma. Cancer. 1982;50(2):345-352.
18. Cagle LA, Mirra JM, Storm FK, Roe DJ, Eilber FR. Histologic features relating to prognosis in synovial sarcoma. Cancer. 1987;59(10):1810-1814.
19. Skytting B, Meis-Kindblom JM, Larsson O, et al. Synovial sarcoma – identification of favorable and unfavorable histologic types: a Scandinavian sarcoma group study of 104 cases. Acta Orthop Scand. 1999:70(6):543-554.
20. Murphey MD, Gibson MS, Jennings BT, Crespo-Rodríguez AM, Fanburg-Smith J, Gajewski DA. Imaging of synovial sarcoma with radiologic-pathologic correlation. Radiographics. 2006;26(5):1543-1565.
Soft tissue sarcomas are rare mesenchymal tumors that comprise 1% of all malignancies. Synovial sarcoma accounts for 5% to 10% of adult soft tissue sarcomas and usually occurs in close association with joint capsules, tendon sheaths, and bursa in the extremities of young and middle-aged adults.1 Synovial sarcomas have been reported in other unusual sites, including the head and neck, thoracic and abdominal wall, retroperitoneum, bone, pleura, and visceral organs such as the lung, prostate, or kidney.2 Primary renal synovial sarcoma is an extremely rare tumor accounting for <2% of all malignant renal tumors.3 To the best of our knowledge, fewer than 50 cases of primary renal synovial sarcoma have been described in the English literature.4 It presents as a diagnostic dilemma because of the dearth of specific clinical and imaging findings and is often confused with benign and malignant tumors. The differential diagnosis includes angiomyolipoma, renal cell carcinoma with sarcomatoid differentiation, metastatic sarcoma, hemangiopericytoma, malignant solitary fibrous tumor, Wilms tumor, and malignant peripheral nerve sheath tumor. Hence, a combination of histomorphologic, immunohistochemical, cytogenetic, and molecular studies that show a unique chromosomal translocation t(X;18) (p11;q11) is imperative in the diagnosis of primary renal synovial sarcoma.4 In the present report, we present the case of a 38-year-old man who was diagnosed with primary renal synovial sarcoma.
Case presentation and summary
A 38-year-old man with a medical history of gastroesophageal reflux disease and Barrett’s esophagus presented to our hospital for the first time with persistent and progressive right-sided flank and abdominal pain that was aggravated after a minor trauma to the back. There was no associated hematuria or dysuria.
Of note is that he had experienced intermittent flank pain for 2 years before this transfer. He had initially been diagnosed at his local hospital close to his home by ultrasound with an angiomyolipoma of 2 × 3 cm arising from the upper pole of his right kidney, which remained stable on repeat sonograms. About 22 months after his initial presentation at his local hospital, the flank pain increased, and a computed-tomographic (CT) scan revealed a perinephric hematoma that was thought to originate from a ruptured angiomyolipoma. He subsequently underwent embolization, but his symptoms recurred soon after. He presented again to his local hospital where CT imaging revealed a significant increase in the size of the retroperitoneal mass, and findings were suggestive of a hematoma. Subsequent angiogram did not reveal active extravasation, so a biopsy was performed.
Before confirmatory pathologic evaluation could be completed, the patient presented to his local hospital again in excruciating pain. A CT scan of his abdomen and pelvis demonstrated a massive subacute on chronic hematoma in the right retroperitoneum measuring 22 × 19 × 18 cm, with calcifications originating from an upper pole right renal neoplasm. The right kidney was displaced antero-inferiorly, and the inferior vena cava was displaced anteriorly and to the left. The preliminary pathology returned with findings suggestive of sarcoma (Figures 1 and 2).
The patient was then transferred to our institution, where he was evaluated by medical and surgical oncology. A CT scan of the chest and magnetic-resonance imaging (MRI) of the brain did not reveal metastatic disease. He underwent exploratory laparotomy that involved the resection of a 22-cm retroperitoneal mass, right nephrectomy, right adrenalectomy, partial right hepatectomy, and a full thickness resection of the right postero-inferior diaphragm followed by mesh repair because of involvement by the tumor.
In its entirety, the specimen was a mass of 26 × 24 × 14 cm. It was sectioned to show extensively necrotic and hemorrhagic variegated white to tan-red parenchyma (Figure 3). Histology revealed a poorly differentiated malignant neoplasm composed of round cells with scant amphophilic cytoplasm arranged in solid, variably sized nests separated by prominent thin-walled branching vascular channels (Figure 4). The mitotic rate was high. It was determined to be a histologically ungraded sarcoma according to the French Federation of Comprehensive Cancer Centers system of grading soft tissue sarcomas; the margins were indeterminate. Immunohistochemistry was positive for EMA, TLE1, and negative for AE1/AE3, S100, STAT6, and Nkx2.2. Molecular pathology fluorescent in situ hybridization (FISH) analysis demonstrated positivity for SS18 gene rearrangement (SS18-SSX1 fusion).
After recovering from surgery, the patient received adjuvant chemotherapy with doxorubicin and ifosfamide. It has been almost 16 months since we first saw this patient. He was started on doxorubicin 20 mg/m2 on days 1 to 4, ifosfamide 2,500 mg on days 1 to 4, and mesna 800 mg on days 1 to 4, for a total of 6 cycles. He did well for the first 5 months, after which he developed disease recurrence in the postoperative nephrectomy bed (a biopsy showed it to be recurrent synovial sarcoma) as well as pulmonary nodules, for which he was started on trabectedin 1.5 mg/m2 every 3 weeks. Two months later, a CT scan showed an increase in the size of his retroperitoneal mass, and the treatment was changed to pazopanib 400 mg daily orally, on which he remained at the time of publication.
Discussion
Synovial sarcoma is the fourth most common type of soft tissue sarcoma, accounting for 2.5% to 10.5% of all primary soft tissue malignancies worldwide. It occurs most frequently in adolescents and young adults, with most patients presenting between the ages of 15 and 40 years. Median age of presentation is 36 years. Despite the nomenclature, synovial sarcoma does not arise in intra-articular locations but typically occurs in proximity to joints in the extremities. Synovial sarcomas are less commonly described in other sites, including the head and neck, mediastinum, intraperitoneum, retroperitoneum, lung, pleura, and kidney.4,5 Renal synovial sarcoma was first described in a published article by Argani and colleagues in 2000.5
Adult renal mesenchymal tumors are classified into benign and malignant tumors on the basis of the histologic features and clinicobiologic behavior.6,7 The benign esenchymal renal tumors include angiomyolipoma, leiomyoma, hemangioma, lymphangioma, juxtaglomerular cell tumor, renomedullary interstitial cell tumor (medullary fibroma), lipoma, solitary fibrous tumor, and schwannoma. Malignant renal tumors of mesenchymal origin include leiomyosarcoma, rhabdomyosarcoma, angiosarcoma, osteosarcoma, fibrosarcoma, malignant fibrous histiocytoma, solitary fibrous tumor, and synovial sarcoma.
Most of these tumor types cause the same nonspecific symptoms in patients – abdominal pain, flank pain, abdominal fullness, a palpable mass, and hematuria – although they can be clinically silent. The average duration of symptoms in synovial sarcoma is 2 to 4 years.8 The long duration of symptoms and initial slow growth of synovial sarcomas may give a false impression of a benign process.
A preoperative radiological diagnosis of primary renal synovial sarcoma may be suspected by analyzing the tumor’s growth patterns on CT scans.9 Renal synovial sarcomas often appear as large, well-defined soft tissue masses that can extend into the renal pelvis or into the perinephric region.9 A CT scan may identify soft tissue calcifications, especially subtle ones in areas where the tumor anatomy is complex. A CT scan may also reveal areas of hemorrhage, necrosis, or cyst formation within the tumor, and can easily confirm bone involvement. Intravenous contrast may help in differentiating the mass from adjacent muscle and neurovascular complex.9,10 On MRI, renal synovial sarcomas are often described as nonspecific heterogeneous masses, although they may also exhibit heterogeneous enhancement of hemorrhagic areas, calcifications, and air-fluid levels (known as “triple sign”) as well as septae. The triple sign may be identified as areas of low, intermediate, and high signal intensity, correlating with areas of hemorrhage, calcification, and air-fluid level.9,10 Signal intensity is about equal to that of skeletal muscle on T1-weighted MRI and higher than that of subcutaneous fat on T2-weighted MRI.
In the present case, the tumor was initially misdiagnosed as an angiomyolipoma, the most common benign tumor of the kidney. Angiomyolipomas are usually solid triphasic tumors arising from the renal cortex and are composed of 3 major elements: dysmorphic blood vessels, smooth muscle components, and adipose tissue. When angiomyolipomas are large enough, they are readily recognized by the identification of macroscopic fat within the tumor, either by CT scan or MRI.11 When they are small, they may be difficult to distinguish from a small cyst on CT because of volume averaging.
On pathology, synovial sarcoma has dual epithelial and mesenchymal differentiation. They are frequently multi-lobulated, and areas of necrosis, hemorrhage, and cyst formation are also common. There are 3 main histologic subtypes of synovial sarcoma: biphasic (20%-30%), monophasic (50%-60%), and poorly differentiated (15%-25%). Poorly differentiated synovial sarcomas are generally epithelioid in morphology, have high mitotic activity (usually 10-20 mitoses/10 high-power field; range is <5 for well differentiated, low-grade tumors), and can be confused with round cell tumors such as Ewing sarcoma. Poorly differentiated synovial sarcomas are high-grade tumors.
Immunohistochemical studies can confirm the pathological diagnosis. Synovial sarcomas usually stain positive for Bcl2, CD99/Mic2, CD56, Vim, and focally for EMA but negatively for desmin, actin, WT1, S-100, CD34, and CD31.5 Currently, the gold standard for diagnosis and hallmark for synovial sarcomas are the t (X;18) translocation and SYT-SSX gene fusion products (SYT-SSX1 in 67% and SYT-SSX2 in 33% of cases). These can be detected either by FISH or reverse-transcription polymerase chain reaction. This genetic alteration is identified in more than 90% of synovial sarcomas and is highly specific.
The role of SYT-SSX gene fusion in the pathogenesis of synovial sarcoma is an active area of investigation. The fusion of SYT with SSX translates into a fusion protein that binds to the transcription activator SMARCA4 that is involved in chromatin remodeling, thus displacing both the wildtype SYT and the tumor suppressor gene SMARCB1. The modified protein complex then binds at several super-enhancer loci, unlocking suppressed genes such as Sox2, which is known to be necessary for synovial sarcoma proliferation. Alterations in SMARCB1 are involved in several cancer types, implicating this event as a driver of these malignancies.12 This results in a global alteration in chromatin remodeling that needs to be better understood to design targeted therapies.
The clinical course of synovial sarcoma, regardless of the tissue of origin, is typically poor. Multiple clinical and pathologic factors, including tumor size, location, patient age, and presence of poorly differentiated areas, are thought to have prognostic significance. A tumor size of more than 5 cm at presentation has the greatest impact on prognosis, with studies showing 5-year survival rates of 64% for patients with tumors smaller than 5 cm and 26% for patients with masses greater than 5 cm.13,14 High-grade synovial sarcoma is favored in tumors that have cystic components, hemorrhage, and fluid levels and the triple sign.
Patients with tumors in the extremities have a more favorable prognosis than those with lesions in the head and neck area or axially, a feature that likely reflects better surgical control available for extremity lesions. Patient age of less than 15 to 20 years is also associated with a better long-term prognosis.15,16 Varela-Duran and Enzinger17 reported that the presence of extensive calcifications suggests improved long-term survival, with 5-year survival rates of 82% and decreased rates of local recurrence (32%) and metastatic disease (29%). The poorly differentiated subtype is associated with a worsened prognosis, with a 5-year survival rate of 20% through 30%.18,19 Other pathologic factors associated with worsened prognosis include presence of rhabdoid cells, extensive tumor necrosis, high nuclear grade, p53 mutations, and high mitotic rate (>10 mitoses/10 high-power field). More recently, the gene fusion type SYT-SSX2 (more common in monophasic lesions) has been associated with an improved prognosis, compared with that for SYT-SSX1, and an 89% metastasis-free survival.20
Although there are no guidelines for the treatment of primary renal synovial sarcoma because of the limited number of cases reported, surgery is considered the first choice. Adjuvant chemotherapy with an anthracycline (doxorubicin or epirubicin) combined with ifosfamide has been the most frequently used regimen in published cases, especially in those in which patients have poor prognostic factors as mentioned above.
Overall, the 5-year survival rate ranges from 36% to 76%.14 The clinical course of synovial sarcoma is characterized by a high rate of local recurrence (30%-50%) and metastatic disease (41%). Most metastases occur within the first 2 to 5 years after treatment cessation. Metastases are present in 16% to 25% of patients at their initial presentation, with the most frequent metastatic site being the lung, followed by the lymph nodes (4%-18%) and bone (8%-11%).
Conclusion
Primary renal synovial sarcoma is extremely rare, and preoperative diagnosis is difficult in the absence of specific clinical or imaging findings. A high index of suspicion combined with pathologic, immunohistochemical, cytogenetic, and molecular studies is essential for accurate diagnosis and subsequent treatment planning. The differential diagnosis of renal synovial sarcoma can be extensive, and our experience with this patient illustrates the diagnostic dilemma associated with renal synovial sarcoma.
Soft tissue sarcomas are rare mesenchymal tumors that comprise 1% of all malignancies. Synovial sarcoma accounts for 5% to 10% of adult soft tissue sarcomas and usually occurs in close association with joint capsules, tendon sheaths, and bursa in the extremities of young and middle-aged adults.1 Synovial sarcomas have been reported in other unusual sites, including the head and neck, thoracic and abdominal wall, retroperitoneum, bone, pleura, and visceral organs such as the lung, prostate, or kidney.2 Primary renal synovial sarcoma is an extremely rare tumor accounting for <2% of all malignant renal tumors.3 To the best of our knowledge, fewer than 50 cases of primary renal synovial sarcoma have been described in the English literature.4 It presents as a diagnostic dilemma because of the dearth of specific clinical and imaging findings and is often confused with benign and malignant tumors. The differential diagnosis includes angiomyolipoma, renal cell carcinoma with sarcomatoid differentiation, metastatic sarcoma, hemangiopericytoma, malignant solitary fibrous tumor, Wilms tumor, and malignant peripheral nerve sheath tumor. Hence, a combination of histomorphologic, immunohistochemical, cytogenetic, and molecular studies that show a unique chromosomal translocation t(X;18) (p11;q11) is imperative in the diagnosis of primary renal synovial sarcoma.4 In the present report, we present the case of a 38-year-old man who was diagnosed with primary renal synovial sarcoma.
Case presentation and summary
A 38-year-old man with a medical history of gastroesophageal reflux disease and Barrett’s esophagus presented to our hospital for the first time with persistent and progressive right-sided flank and abdominal pain that was aggravated after a minor trauma to the back. There was no associated hematuria or dysuria.
Of note is that he had experienced intermittent flank pain for 2 years before this transfer. He had initially been diagnosed at his local hospital close to his home by ultrasound with an angiomyolipoma of 2 × 3 cm arising from the upper pole of his right kidney, which remained stable on repeat sonograms. About 22 months after his initial presentation at his local hospital, the flank pain increased, and a computed-tomographic (CT) scan revealed a perinephric hematoma that was thought to originate from a ruptured angiomyolipoma. He subsequently underwent embolization, but his symptoms recurred soon after. He presented again to his local hospital where CT imaging revealed a significant increase in the size of the retroperitoneal mass, and findings were suggestive of a hematoma. Subsequent angiogram did not reveal active extravasation, so a biopsy was performed.
Before confirmatory pathologic evaluation could be completed, the patient presented to his local hospital again in excruciating pain. A CT scan of his abdomen and pelvis demonstrated a massive subacute on chronic hematoma in the right retroperitoneum measuring 22 × 19 × 18 cm, with calcifications originating from an upper pole right renal neoplasm. The right kidney was displaced antero-inferiorly, and the inferior vena cava was displaced anteriorly and to the left. The preliminary pathology returned with findings suggestive of sarcoma (Figures 1 and 2).
The patient was then transferred to our institution, where he was evaluated by medical and surgical oncology. A CT scan of the chest and magnetic-resonance imaging (MRI) of the brain did not reveal metastatic disease. He underwent exploratory laparotomy that involved the resection of a 22-cm retroperitoneal mass, right nephrectomy, right adrenalectomy, partial right hepatectomy, and a full thickness resection of the right postero-inferior diaphragm followed by mesh repair because of involvement by the tumor.
In its entirety, the specimen was a mass of 26 × 24 × 14 cm. It was sectioned to show extensively necrotic and hemorrhagic variegated white to tan-red parenchyma (Figure 3). Histology revealed a poorly differentiated malignant neoplasm composed of round cells with scant amphophilic cytoplasm arranged in solid, variably sized nests separated by prominent thin-walled branching vascular channels (Figure 4). The mitotic rate was high. It was determined to be a histologically ungraded sarcoma according to the French Federation of Comprehensive Cancer Centers system of grading soft tissue sarcomas; the margins were indeterminate. Immunohistochemistry was positive for EMA, TLE1, and negative for AE1/AE3, S100, STAT6, and Nkx2.2. Molecular pathology fluorescent in situ hybridization (FISH) analysis demonstrated positivity for SS18 gene rearrangement (SS18-SSX1 fusion).
After recovering from surgery, the patient received adjuvant chemotherapy with doxorubicin and ifosfamide. It has been almost 16 months since we first saw this patient. He was started on doxorubicin 20 mg/m2 on days 1 to 4, ifosfamide 2,500 mg on days 1 to 4, and mesna 800 mg on days 1 to 4, for a total of 6 cycles. He did well for the first 5 months, after which he developed disease recurrence in the postoperative nephrectomy bed (a biopsy showed it to be recurrent synovial sarcoma) as well as pulmonary nodules, for which he was started on trabectedin 1.5 mg/m2 every 3 weeks. Two months later, a CT scan showed an increase in the size of his retroperitoneal mass, and the treatment was changed to pazopanib 400 mg daily orally, on which he remained at the time of publication.
Discussion
Synovial sarcoma is the fourth most common type of soft tissue sarcoma, accounting for 2.5% to 10.5% of all primary soft tissue malignancies worldwide. It occurs most frequently in adolescents and young adults, with most patients presenting between the ages of 15 and 40 years. Median age of presentation is 36 years. Despite the nomenclature, synovial sarcoma does not arise in intra-articular locations but typically occurs in proximity to joints in the extremities. Synovial sarcomas are less commonly described in other sites, including the head and neck, mediastinum, intraperitoneum, retroperitoneum, lung, pleura, and kidney.4,5 Renal synovial sarcoma was first described in a published article by Argani and colleagues in 2000.5
Adult renal mesenchymal tumors are classified into benign and malignant tumors on the basis of the histologic features and clinicobiologic behavior.6,7 The benign esenchymal renal tumors include angiomyolipoma, leiomyoma, hemangioma, lymphangioma, juxtaglomerular cell tumor, renomedullary interstitial cell tumor (medullary fibroma), lipoma, solitary fibrous tumor, and schwannoma. Malignant renal tumors of mesenchymal origin include leiomyosarcoma, rhabdomyosarcoma, angiosarcoma, osteosarcoma, fibrosarcoma, malignant fibrous histiocytoma, solitary fibrous tumor, and synovial sarcoma.
Most of these tumor types cause the same nonspecific symptoms in patients – abdominal pain, flank pain, abdominal fullness, a palpable mass, and hematuria – although they can be clinically silent. The average duration of symptoms in synovial sarcoma is 2 to 4 years.8 The long duration of symptoms and initial slow growth of synovial sarcomas may give a false impression of a benign process.
A preoperative radiological diagnosis of primary renal synovial sarcoma may be suspected by analyzing the tumor’s growth patterns on CT scans.9 Renal synovial sarcomas often appear as large, well-defined soft tissue masses that can extend into the renal pelvis or into the perinephric region.9 A CT scan may identify soft tissue calcifications, especially subtle ones in areas where the tumor anatomy is complex. A CT scan may also reveal areas of hemorrhage, necrosis, or cyst formation within the tumor, and can easily confirm bone involvement. Intravenous contrast may help in differentiating the mass from adjacent muscle and neurovascular complex.9,10 On MRI, renal synovial sarcomas are often described as nonspecific heterogeneous masses, although they may also exhibit heterogeneous enhancement of hemorrhagic areas, calcifications, and air-fluid levels (known as “triple sign”) as well as septae. The triple sign may be identified as areas of low, intermediate, and high signal intensity, correlating with areas of hemorrhage, calcification, and air-fluid level.9,10 Signal intensity is about equal to that of skeletal muscle on T1-weighted MRI and higher than that of subcutaneous fat on T2-weighted MRI.
In the present case, the tumor was initially misdiagnosed as an angiomyolipoma, the most common benign tumor of the kidney. Angiomyolipomas are usually solid triphasic tumors arising from the renal cortex and are composed of 3 major elements: dysmorphic blood vessels, smooth muscle components, and adipose tissue. When angiomyolipomas are large enough, they are readily recognized by the identification of macroscopic fat within the tumor, either by CT scan or MRI.11 When they are small, they may be difficult to distinguish from a small cyst on CT because of volume averaging.
On pathology, synovial sarcoma has dual epithelial and mesenchymal differentiation. They are frequently multi-lobulated, and areas of necrosis, hemorrhage, and cyst formation are also common. There are 3 main histologic subtypes of synovial sarcoma: biphasic (20%-30%), monophasic (50%-60%), and poorly differentiated (15%-25%). Poorly differentiated synovial sarcomas are generally epithelioid in morphology, have high mitotic activity (usually 10-20 mitoses/10 high-power field; range is <5 for well differentiated, low-grade tumors), and can be confused with round cell tumors such as Ewing sarcoma. Poorly differentiated synovial sarcomas are high-grade tumors.
Immunohistochemical studies can confirm the pathological diagnosis. Synovial sarcomas usually stain positive for Bcl2, CD99/Mic2, CD56, Vim, and focally for EMA but negatively for desmin, actin, WT1, S-100, CD34, and CD31.5 Currently, the gold standard for diagnosis and hallmark for synovial sarcomas are the t (X;18) translocation and SYT-SSX gene fusion products (SYT-SSX1 in 67% and SYT-SSX2 in 33% of cases). These can be detected either by FISH or reverse-transcription polymerase chain reaction. This genetic alteration is identified in more than 90% of synovial sarcomas and is highly specific.
The role of SYT-SSX gene fusion in the pathogenesis of synovial sarcoma is an active area of investigation. The fusion of SYT with SSX translates into a fusion protein that binds to the transcription activator SMARCA4 that is involved in chromatin remodeling, thus displacing both the wildtype SYT and the tumor suppressor gene SMARCB1. The modified protein complex then binds at several super-enhancer loci, unlocking suppressed genes such as Sox2, which is known to be necessary for synovial sarcoma proliferation. Alterations in SMARCB1 are involved in several cancer types, implicating this event as a driver of these malignancies.12 This results in a global alteration in chromatin remodeling that needs to be better understood to design targeted therapies.
The clinical course of synovial sarcoma, regardless of the tissue of origin, is typically poor. Multiple clinical and pathologic factors, including tumor size, location, patient age, and presence of poorly differentiated areas, are thought to have prognostic significance. A tumor size of more than 5 cm at presentation has the greatest impact on prognosis, with studies showing 5-year survival rates of 64% for patients with tumors smaller than 5 cm and 26% for patients with masses greater than 5 cm.13,14 High-grade synovial sarcoma is favored in tumors that have cystic components, hemorrhage, and fluid levels and the triple sign.
Patients with tumors in the extremities have a more favorable prognosis than those with lesions in the head and neck area or axially, a feature that likely reflects better surgical control available for extremity lesions. Patient age of less than 15 to 20 years is also associated with a better long-term prognosis.15,16 Varela-Duran and Enzinger17 reported that the presence of extensive calcifications suggests improved long-term survival, with 5-year survival rates of 82% and decreased rates of local recurrence (32%) and metastatic disease (29%). The poorly differentiated subtype is associated with a worsened prognosis, with a 5-year survival rate of 20% through 30%.18,19 Other pathologic factors associated with worsened prognosis include presence of rhabdoid cells, extensive tumor necrosis, high nuclear grade, p53 mutations, and high mitotic rate (>10 mitoses/10 high-power field). More recently, the gene fusion type SYT-SSX2 (more common in monophasic lesions) has been associated with an improved prognosis, compared with that for SYT-SSX1, and an 89% metastasis-free survival.20
Although there are no guidelines for the treatment of primary renal synovial sarcoma because of the limited number of cases reported, surgery is considered the first choice. Adjuvant chemotherapy with an anthracycline (doxorubicin or epirubicin) combined with ifosfamide has been the most frequently used regimen in published cases, especially in those in which patients have poor prognostic factors as mentioned above.
Overall, the 5-year survival rate ranges from 36% to 76%.14 The clinical course of synovial sarcoma is characterized by a high rate of local recurrence (30%-50%) and metastatic disease (41%). Most metastases occur within the first 2 to 5 years after treatment cessation. Metastases are present in 16% to 25% of patients at their initial presentation, with the most frequent metastatic site being the lung, followed by the lymph nodes (4%-18%) and bone (8%-11%).
Conclusion
Primary renal synovial sarcoma is extremely rare, and preoperative diagnosis is difficult in the absence of specific clinical or imaging findings. A high index of suspicion combined with pathologic, immunohistochemical, cytogenetic, and molecular studies is essential for accurate diagnosis and subsequent treatment planning. The differential diagnosis of renal synovial sarcoma can be extensive, and our experience with this patient illustrates the diagnostic dilemma associated with renal synovial sarcoma.
1. Majumder A, Dey S, Khandakar B, Medda S, Chandra Paul P. Primary renal synovial sarcoma: a rare tumor with an atypical presentation. Arch Iran Med. 2014;17(10):726-728.
2. Fetsch JF, Meis JM. Synovial sarcoma of the abdominal wall. Cancer. 1993;72(2):469 477.
3. Wang Z, Zhong Z, Zhu L, et al. Primary synovial sarcoma of the kidney: a case report. Oncol Lett. 2015;10(6):3542-3544.
4. Abbas M, Dämmrich ME, Braubach P, et al. Synovial sarcoma of the kidney in a young patient with a review of the literature. Rare tumors. 2014;6(2):5393
5. Argani P, Faria PA, Epstein JI, et al. Primary renal synovial sarcoma: molecular and morphologic delineation of an entity previously included among embryonal sarcomas of the kidney. Am J Surg Pathol. 2000;24(8):1087-1096.
6. Eble JN, Sauter G, Epstein JI, Sesterhenn IA, eds. World Health Organization classification of tumours: pathology and genetics of tumours of the urinary system and male genital organs. Lyon, France: IARC; 2004.
7. Tamboli P, Ro JY, Amin MB, Ligato S, Ayala AG. Benign tumors and tumor-like lesions of the adult kidney. Part II: benign mesenchymal and mixed neoplasms, and tumor-like lesions. Adv Anat Pathol. 2000;7(1):47-66.
8. Weiss SW, Goldblum JR. Malignant soft tissue tumors of uncertain type. In: Weiss SW, Goldblum JR, eds. Enzinger and Weiss’s soft tissue tumors. 4th ed. St. Louis, MO: Mosby, 2001; 1483-1565.
9. Lacovelli R, Altavilla A, Ciardi A, et al. Clinical and pathological features of primary renal synovial sarcoma: analysis of 64 cases from 11 years of medical literature. BJU Int. 2012;110(10):1449-1454.
10. Alhazzani AR, El-Sharkawy MS, Hassan H. Primary retroperitoneal synovial sarcoma in CT and MRI. Urol Ann. 2010;2(1):39-41.
11. Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO, Prasad SR. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010;30(6):1525-1540.
12. Sápi Z, Papp G, Szendrői M, et al. Epigenetic regulation of SMARCB1 by miR-206, -381 and -671- 5p is evident in a variety of SMARCB1 immunonegative soft tissue sarcomas, while miR-765 appears specific for epithelioid sarcoma. A miRNA study of 223 soft tissue sarcomas. Genes Chromosomes Cancer. 2016;55(10):786-802.
13. Ferrari A, Gronchi A, Casanova M, et al. Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer. 2004;101(3):627-634.
14. Rangheard AS, Vanel D, Viala J, Schwaab G, Casiraghi O, Sigal R. Synovial sarcomas of the head and neck: CT and MR imaging findings of eight patients. Am J Neuroradiol. 2001;22(5):851-857.
15. Oda Y, Hashimoto H, Tsuneyoshi M, Takeshita S. Survival in synovial sarcoma: a multivariate study of prognostic factors with special emphasis on the comparison between early death and long-term survival. Am J Surg Pathol. 1993;17(1):35-44.
16. Raney RB. Synovial sarcoma in young people: background, prognostic factors and therapeutic questions. J Pediatr Hematol Oncol. 2005;27(4):207-211.
17. Varela-Duran J, Enzinger FM. Calcifying synovial sarcoma. Cancer. 1982;50(2):345-352.
18. Cagle LA, Mirra JM, Storm FK, Roe DJ, Eilber FR. Histologic features relating to prognosis in synovial sarcoma. Cancer. 1987;59(10):1810-1814.
19. Skytting B, Meis-Kindblom JM, Larsson O, et al. Synovial sarcoma – identification of favorable and unfavorable histologic types: a Scandinavian sarcoma group study of 104 cases. Acta Orthop Scand. 1999:70(6):543-554.
20. Murphey MD, Gibson MS, Jennings BT, Crespo-Rodríguez AM, Fanburg-Smith J, Gajewski DA. Imaging of synovial sarcoma with radiologic-pathologic correlation. Radiographics. 2006;26(5):1543-1565.
1. Majumder A, Dey S, Khandakar B, Medda S, Chandra Paul P. Primary renal synovial sarcoma: a rare tumor with an atypical presentation. Arch Iran Med. 2014;17(10):726-728.
2. Fetsch JF, Meis JM. Synovial sarcoma of the abdominal wall. Cancer. 1993;72(2):469 477.
3. Wang Z, Zhong Z, Zhu L, et al. Primary synovial sarcoma of the kidney: a case report. Oncol Lett. 2015;10(6):3542-3544.
4. Abbas M, Dämmrich ME, Braubach P, et al. Synovial sarcoma of the kidney in a young patient with a review of the literature. Rare tumors. 2014;6(2):5393
5. Argani P, Faria PA, Epstein JI, et al. Primary renal synovial sarcoma: molecular and morphologic delineation of an entity previously included among embryonal sarcomas of the kidney. Am J Surg Pathol. 2000;24(8):1087-1096.
6. Eble JN, Sauter G, Epstein JI, Sesterhenn IA, eds. World Health Organization classification of tumours: pathology and genetics of tumours of the urinary system and male genital organs. Lyon, France: IARC; 2004.
7. Tamboli P, Ro JY, Amin MB, Ligato S, Ayala AG. Benign tumors and tumor-like lesions of the adult kidney. Part II: benign mesenchymal and mixed neoplasms, and tumor-like lesions. Adv Anat Pathol. 2000;7(1):47-66.
8. Weiss SW, Goldblum JR. Malignant soft tissue tumors of uncertain type. In: Weiss SW, Goldblum JR, eds. Enzinger and Weiss’s soft tissue tumors. 4th ed. St. Louis, MO: Mosby, 2001; 1483-1565.
9. Lacovelli R, Altavilla A, Ciardi A, et al. Clinical and pathological features of primary renal synovial sarcoma: analysis of 64 cases from 11 years of medical literature. BJU Int. 2012;110(10):1449-1454.
10. Alhazzani AR, El-Sharkawy MS, Hassan H. Primary retroperitoneal synovial sarcoma in CT and MRI. Urol Ann. 2010;2(1):39-41.
11. Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO, Prasad SR. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010;30(6):1525-1540.
12. Sápi Z, Papp G, Szendrői M, et al. Epigenetic regulation of SMARCB1 by miR-206, -381 and -671- 5p is evident in a variety of SMARCB1 immunonegative soft tissue sarcomas, while miR-765 appears specific for epithelioid sarcoma. A miRNA study of 223 soft tissue sarcomas. Genes Chromosomes Cancer. 2016;55(10):786-802.
13. Ferrari A, Gronchi A, Casanova M, et al. Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer. 2004;101(3):627-634.
14. Rangheard AS, Vanel D, Viala J, Schwaab G, Casiraghi O, Sigal R. Synovial sarcomas of the head and neck: CT and MR imaging findings of eight patients. Am J Neuroradiol. 2001;22(5):851-857.
15. Oda Y, Hashimoto H, Tsuneyoshi M, Takeshita S. Survival in synovial sarcoma: a multivariate study of prognostic factors with special emphasis on the comparison between early death and long-term survival. Am J Surg Pathol. 1993;17(1):35-44.
16. Raney RB. Synovial sarcoma in young people: background, prognostic factors and therapeutic questions. J Pediatr Hematol Oncol. 2005;27(4):207-211.
17. Varela-Duran J, Enzinger FM. Calcifying synovial sarcoma. Cancer. 1982;50(2):345-352.
18. Cagle LA, Mirra JM, Storm FK, Roe DJ, Eilber FR. Histologic features relating to prognosis in synovial sarcoma. Cancer. 1987;59(10):1810-1814.
19. Skytting B, Meis-Kindblom JM, Larsson O, et al. Synovial sarcoma – identification of favorable and unfavorable histologic types: a Scandinavian sarcoma group study of 104 cases. Acta Orthop Scand. 1999:70(6):543-554.
20. Murphey MD, Gibson MS, Jennings BT, Crespo-Rodríguez AM, Fanburg-Smith J, Gajewski DA. Imaging of synovial sarcoma with radiologic-pathologic correlation. Radiographics. 2006;26(5):1543-1565.
TKIs and immunotherapy hold promise for alveolar soft part sarcoma
Alveolar soft part sarcoma (ASPS) has often proven to be resistant to conventional doxorubicin-based chemotherapy, but tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs) may provide new treatment strategies for this rare type of sarcoma, according to a literature review.
A rare, translocation-driven sarcoma of the soft tissues, ASPS often affects young adults and is characterized by indolent behavior and early metastasis. Despite its resistance to chemotherapy, studies indicate that survival is often prolonged in patients with metastatic disease. The literature has shown 5-year survival rates at about 60%, and this percentage has remained fairly consistent for the past 3 decades.
Luca Paoluzzi, MD, of New York University, and Robert G. Maki, MD, PhD, of Hofstra University, Hempstead, N.Y., reviewed the literature from 1952 to March 2018, in order to gain a better understanding of ASPS and the opportunities “for the translation of such knowledge into clinical practice,” they wrote in JAMA.
From a therapeutic standpoint, ASPS is characterized by sensitivity to vascular endothelial growth factor receptor–predominant TKIs, compared with other soft tissue sarcomas (STS), and recent data have emphasized that it is responsive to new immunotherapy regimens including ICIs. Pazopanib is currently the only agent that has received regulatory approval for use in STS refractory to other treatments and it appears to have consistent activity in metastatic ASPS. Management of ASPS generally also involves surgical resection and/or systemic treatment for metastatic disease. Conventional agents such as anthracycline-based chemotherapy have demonstrated a poor response rate lower than 10%, and while a complete resection may be curative, metastases are common and can occur years after resection of the primary tumor.
Conversely, ICIs “represent a promising area of drug development in ASPS; the data to date are limited but encouraging,” wrote Dr. Paoluzzi and Dr. Maki.
They pointed to one study that included 50 patients with sarcoma with 14 different subtypes of STS who were enrolled in immunotherapy trials conducted at the University of Texas MD Anderson Cancer Center, Houston. There were two pretreated patients with ASPS (two to four prior lines) in the cohort who received antiprogrammed death-ligand 1–based therapy, and achieved a partial response bordering on a complete response that lasted 8 and 12 months. An additional two patients achieved stable disease.
Another paper, presented at the 2017 Connective Tissue Oncology Society annual meeting, presented preliminary data from a phase 2 study that showed four of nine evaluable patients with ASPS treated with the TKI axitinib, combined with pembrolizumab, achieved a partial response. Three others had stable disease.
“Pathway-driven basket trials facilitate the enrollment of patients with such uncommon cancers and should provide valuable information regarding a second type of immune responsiveness to ICIs, one that is not a function of high tumor mutational burden,” the authors concluded.
No outside funding sources were reported. Dr. Maki reported receiving consultant fees from numerous sources and research support to New York University from Immune Design, Immunocore, Eli Lilly, Presage Biosciences, TRACON Pharmaceuticals, SARC, Regeneron, and Genentech. No other conflicts were reported.
SOURCE: doi: 10.1001/jamaoncol.2018.4490.
Alveolar soft part sarcoma (ASPS) has often proven to be resistant to conventional doxorubicin-based chemotherapy, but tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs) may provide new treatment strategies for this rare type of sarcoma, according to a literature review.
A rare, translocation-driven sarcoma of the soft tissues, ASPS often affects young adults and is characterized by indolent behavior and early metastasis. Despite its resistance to chemotherapy, studies indicate that survival is often prolonged in patients with metastatic disease. The literature has shown 5-year survival rates at about 60%, and this percentage has remained fairly consistent for the past 3 decades.
Luca Paoluzzi, MD, of New York University, and Robert G. Maki, MD, PhD, of Hofstra University, Hempstead, N.Y., reviewed the literature from 1952 to March 2018, in order to gain a better understanding of ASPS and the opportunities “for the translation of such knowledge into clinical practice,” they wrote in JAMA.
From a therapeutic standpoint, ASPS is characterized by sensitivity to vascular endothelial growth factor receptor–predominant TKIs, compared with other soft tissue sarcomas (STS), and recent data have emphasized that it is responsive to new immunotherapy regimens including ICIs. Pazopanib is currently the only agent that has received regulatory approval for use in STS refractory to other treatments and it appears to have consistent activity in metastatic ASPS. Management of ASPS generally also involves surgical resection and/or systemic treatment for metastatic disease. Conventional agents such as anthracycline-based chemotherapy have demonstrated a poor response rate lower than 10%, and while a complete resection may be curative, metastases are common and can occur years after resection of the primary tumor.
Conversely, ICIs “represent a promising area of drug development in ASPS; the data to date are limited but encouraging,” wrote Dr. Paoluzzi and Dr. Maki.
They pointed to one study that included 50 patients with sarcoma with 14 different subtypes of STS who were enrolled in immunotherapy trials conducted at the University of Texas MD Anderson Cancer Center, Houston. There were two pretreated patients with ASPS (two to four prior lines) in the cohort who received antiprogrammed death-ligand 1–based therapy, and achieved a partial response bordering on a complete response that lasted 8 and 12 months. An additional two patients achieved stable disease.
Another paper, presented at the 2017 Connective Tissue Oncology Society annual meeting, presented preliminary data from a phase 2 study that showed four of nine evaluable patients with ASPS treated with the TKI axitinib, combined with pembrolizumab, achieved a partial response. Three others had stable disease.
“Pathway-driven basket trials facilitate the enrollment of patients with such uncommon cancers and should provide valuable information regarding a second type of immune responsiveness to ICIs, one that is not a function of high tumor mutational burden,” the authors concluded.
No outside funding sources were reported. Dr. Maki reported receiving consultant fees from numerous sources and research support to New York University from Immune Design, Immunocore, Eli Lilly, Presage Biosciences, TRACON Pharmaceuticals, SARC, Regeneron, and Genentech. No other conflicts were reported.
SOURCE: doi: 10.1001/jamaoncol.2018.4490.
Alveolar soft part sarcoma (ASPS) has often proven to be resistant to conventional doxorubicin-based chemotherapy, but tyrosine kinase inhibitors (TKIs) and immune checkpoint inhibitors (ICIs) may provide new treatment strategies for this rare type of sarcoma, according to a literature review.
A rare, translocation-driven sarcoma of the soft tissues, ASPS often affects young adults and is characterized by indolent behavior and early metastasis. Despite its resistance to chemotherapy, studies indicate that survival is often prolonged in patients with metastatic disease. The literature has shown 5-year survival rates at about 60%, and this percentage has remained fairly consistent for the past 3 decades.
Luca Paoluzzi, MD, of New York University, and Robert G. Maki, MD, PhD, of Hofstra University, Hempstead, N.Y., reviewed the literature from 1952 to March 2018, in order to gain a better understanding of ASPS and the opportunities “for the translation of such knowledge into clinical practice,” they wrote in JAMA.
From a therapeutic standpoint, ASPS is characterized by sensitivity to vascular endothelial growth factor receptor–predominant TKIs, compared with other soft tissue sarcomas (STS), and recent data have emphasized that it is responsive to new immunotherapy regimens including ICIs. Pazopanib is currently the only agent that has received regulatory approval for use in STS refractory to other treatments and it appears to have consistent activity in metastatic ASPS. Management of ASPS generally also involves surgical resection and/or systemic treatment for metastatic disease. Conventional agents such as anthracycline-based chemotherapy have demonstrated a poor response rate lower than 10%, and while a complete resection may be curative, metastases are common and can occur years after resection of the primary tumor.
Conversely, ICIs “represent a promising area of drug development in ASPS; the data to date are limited but encouraging,” wrote Dr. Paoluzzi and Dr. Maki.
They pointed to one study that included 50 patients with sarcoma with 14 different subtypes of STS who were enrolled in immunotherapy trials conducted at the University of Texas MD Anderson Cancer Center, Houston. There were two pretreated patients with ASPS (two to four prior lines) in the cohort who received antiprogrammed death-ligand 1–based therapy, and achieved a partial response bordering on a complete response that lasted 8 and 12 months. An additional two patients achieved stable disease.
Another paper, presented at the 2017 Connective Tissue Oncology Society annual meeting, presented preliminary data from a phase 2 study that showed four of nine evaluable patients with ASPS treated with the TKI axitinib, combined with pembrolizumab, achieved a partial response. Three others had stable disease.
“Pathway-driven basket trials facilitate the enrollment of patients with such uncommon cancers and should provide valuable information regarding a second type of immune responsiveness to ICIs, one that is not a function of high tumor mutational burden,” the authors concluded.
No outside funding sources were reported. Dr. Maki reported receiving consultant fees from numerous sources and research support to New York University from Immune Design, Immunocore, Eli Lilly, Presage Biosciences, TRACON Pharmaceuticals, SARC, Regeneron, and Genentech. No other conflicts were reported.
SOURCE: doi: 10.1001/jamaoncol.2018.4490.
FROM JAMA
Key clinical point: Alveolar soft part sarcoma has often proven to be resistant to conventional doxorubicin-based chemotherapy, tyrosine kinase inhibitors and immune checkpoint inhibitors may provide new treatment strategies.
Major finding: In one study of sarcoma patients enrolled in immunotherapy trials, two pretreated patients with alveolar soft part sarcoma (two to four prior lines) who received antiprogrammed death-ligand 1–based therapy achieved partial responses, bordering on a complete response, that lasted 8 and 12 months.
Study details: A review of literature concerning treatment for alveolar soft part sarcoma.
Disclosures: No outside funding sources were reported. Dr. Maki reported receiving consultant fees from numerous sources and research support to New York University from Immune Design, Immunocore, Eli Lilly, Presage Biosciences, TRACON Pharmaceuticals, SARC, Regeneron, and Genentech. No other conflicts were reported.
Source:
Sorafenib boosts PFS in desmoid tumor patients
Sorafenib was well tolerated with significantly improved progression-free survival in select patients with desmoid tumors, reported Mrinal M. Gounder, MD, of Memorial Sloan-Kettering Cancer Center, New York.
“The study exceeded its primary endpoint for progression-free survival ... Sorafenib may represent a new, first-line or subsequent-line standard of care in select patients with desmoid tumors,” Dr. Gounder said at the annual meeting of the American Society of Clinical Oncology.
For this international prospective study of progression-free survival response to sorafenib, 87 patients were enrolled over 17 months at 25 sites. Patients had unresectable progressive or symptomatic desmoid tumors. Patients were stratified by pain level and disease site and randomized 2:1 to sorafenib 400 mg/day or placebo. Placebo-treated patients were crossed over to sorafenib if they reached RECIST 1.1.
After a median follow up for 26 months, disease had progressed in 22 of 32 patients on placebo and in 7 of 43 patients on sorafenib. One sorafenib-treated patient died. Durable partial responses were seen in 14 of 43 on sorafenib and in 7 of 32 on placebo. At one year, progression-free survival was 43% with placebo (median PFS 9.4 months) and 87% with sorafenib (median PFS not reached [HR = 0.14 (95% CI 0.06-0.33), P less than 0.0001)].
The authors disclosed funding from a wide range of drug companies. Several authors received funding from Bayer, the maker of sorafenib (Nexavar). Clinical trial information: NCT02066181.
SOURCE: Gounder M et al. ASCO 2018 (the annual meeting of the American Society of Clinical Oncology), Abstract 11500.
Sorafenib was well tolerated with significantly improved progression-free survival in select patients with desmoid tumors, reported Mrinal M. Gounder, MD, of Memorial Sloan-Kettering Cancer Center, New York.
“The study exceeded its primary endpoint for progression-free survival ... Sorafenib may represent a new, first-line or subsequent-line standard of care in select patients with desmoid tumors,” Dr. Gounder said at the annual meeting of the American Society of Clinical Oncology.
For this international prospective study of progression-free survival response to sorafenib, 87 patients were enrolled over 17 months at 25 sites. Patients had unresectable progressive or symptomatic desmoid tumors. Patients were stratified by pain level and disease site and randomized 2:1 to sorafenib 400 mg/day or placebo. Placebo-treated patients were crossed over to sorafenib if they reached RECIST 1.1.
After a median follow up for 26 months, disease had progressed in 22 of 32 patients on placebo and in 7 of 43 patients on sorafenib. One sorafenib-treated patient died. Durable partial responses were seen in 14 of 43 on sorafenib and in 7 of 32 on placebo. At one year, progression-free survival was 43% with placebo (median PFS 9.4 months) and 87% with sorafenib (median PFS not reached [HR = 0.14 (95% CI 0.06-0.33), P less than 0.0001)].
The authors disclosed funding from a wide range of drug companies. Several authors received funding from Bayer, the maker of sorafenib (Nexavar). Clinical trial information: NCT02066181.
SOURCE: Gounder M et al. ASCO 2018 (the annual meeting of the American Society of Clinical Oncology), Abstract 11500.
Sorafenib was well tolerated with significantly improved progression-free survival in select patients with desmoid tumors, reported Mrinal M. Gounder, MD, of Memorial Sloan-Kettering Cancer Center, New York.
“The study exceeded its primary endpoint for progression-free survival ... Sorafenib may represent a new, first-line or subsequent-line standard of care in select patients with desmoid tumors,” Dr. Gounder said at the annual meeting of the American Society of Clinical Oncology.
For this international prospective study of progression-free survival response to sorafenib, 87 patients were enrolled over 17 months at 25 sites. Patients had unresectable progressive or symptomatic desmoid tumors. Patients were stratified by pain level and disease site and randomized 2:1 to sorafenib 400 mg/day or placebo. Placebo-treated patients were crossed over to sorafenib if they reached RECIST 1.1.
After a median follow up for 26 months, disease had progressed in 22 of 32 patients on placebo and in 7 of 43 patients on sorafenib. One sorafenib-treated patient died. Durable partial responses were seen in 14 of 43 on sorafenib and in 7 of 32 on placebo. At one year, progression-free survival was 43% with placebo (median PFS 9.4 months) and 87% with sorafenib (median PFS not reached [HR = 0.14 (95% CI 0.06-0.33), P less than 0.0001)].
The authors disclosed funding from a wide range of drug companies. Several authors received funding from Bayer, the maker of sorafenib (Nexavar). Clinical trial information: NCT02066181.
SOURCE: Gounder M et al. ASCO 2018 (the annual meeting of the American Society of Clinical Oncology), Abstract 11500.
FROM ASCO 2018
Childhood soft tissue sarcoma: Professional resources from the National Cancer Institute
Childhood Soft Tissue Sarcoma Treatment (PDQ®)–Health Professional Version
General Information About Childhood Soft Tissue Sarcoma
Dramatic improvements in survival have been achieved for children and adolescents with cancer. Between 1975 and 2010, childhood cancer mortality decreased by more than 50%.[1] Childhood and adolescent cancer survivors require close monitoring because cancer therapy side effects may persist or develop months or years after treatment. (Refer to the PDQ summary on Late Effects of Treatment for Childhood Cancer for specific information about the incidence, type, and monitoring of late effects in childhood and adolescent cancer survivors.)
Rhabdomyosarcoma, a tumor of striated muscle, is the most common soft tissue sarcoma in children aged 0 to 14 years and accounts for 50% of tumors in this age group.[2] (Refer to the PDQ summary on Childhood Rhabdomyosarcoma Treatment for more information.) In pediatrics, the remaining soft tissue sarcomas are commonly referred to as nonrhabdomyosarcomatous soft tissue sarcomas and account for approximately 3% of all childhood tumors.[3] This heterogeneous group of tumors includes the following neoplasms:[4]
- Connective tissue (e.g., desmoid-type fibromatosis).
- Peripheral nervous system (e.g., malignant peripheral nerve sheath tumor).
- Smooth muscle (e.g., leiomyosarcoma).
- Vascular tissue (blood and lymphatic vessels, e.g., angiosarcoma). (Refer to the PDQ summary on Childhood Vascular Tumors Treatment for more information about childhood vascular tumors.)
Distribution of Soft Tissue Sarcoma by Age and Histology
Pediatric soft tissue sarcomas are a heterogenous group of malignant tumors that originate from primitive mesenchymal tissue and account for 7% of all childhood tumors.[5]
The distribution of soft tissue sarcomas by histology and age, based on the Surveillance, Epidemiology, and End Results (SEER) information from 1975 to 2012, is depicted in Table 1. The distribution of histologic subtypes by age is also shown in Figure 2.
| Age <5 y | Age 5–9 y | Age 10–14 y | Age 15–19 y | % of the Total Number of STS Cases <20 y | |||
|---|---|---|---|---|---|---|---|
| pPNET = peripheral primitive neuroectodermal tumors; SEER = Surveillance, Epidemiology, and End Results; STS = soft tissue sarcoma. | |||||||
| aSEER data is available at http://seer.cancer.gov. | |||||||
| bDermatofibrosarcoma accounts for 75% of these cases. | |||||||
| All soft tissue and other extraosseous sarcomas | 923 | 631 | 946 | 1,267 | 100 | ||
| Rhabdomyosarcomas | 551 | 348 | 312 | 270 | 39 | ||
| Fibrosarcomas, peripheral nerve, and other fibrous neoplasms | 116 | 50 | 88 | 141 | 10 | ||
| Fibroblastic and myofibroblastic tumors | 97 | 24 | 31 | 62 | 6 | ||
| Nerve sheath tumors | 19 | 26 | 56 | 77 | 5 | ||
| Other fibromatous neoplasms | 0 | 0 | 1 | 2 | 0.1 | ||
| Kaposi sarcoma | 2 | 1 | 1 | 9 | 0.3 | ||
| Other specified soft tissue sarcomas | 194 | 190 | 424 | 708 | 40 | ||
| Ewing tumor and Askin tumor of soft tissue | 27 | 30 | 62 | 92 | 6 | ||
| pPNET of soft tissue | 21 | 18 | 36 | 46 | 3.2 | ||
| Extrarenal rhabdoid tumor | 61 | 3 | 7 | 3 | 2 | ||
| Liposarcomas | 3 | 5 | 22 | 57 | 2.3 | ||
| Fibrohistiocytic tumors b | 34 | 54 | 108 | 188 | 10 | ||
| Leiomyosarcomas | 9 | 14 | 15 | 36 | 2 | ||
| Synovial sarcomas | 10 | 34 | 111 | 175 | 9 | ||
| Blood vessel tumors | 11 | 7 | 8 | 25 | 1.4 | ||
| Osseous and chondromatous neoplasms of soft tissue | 1 | 6 | 13 | 10 | 0.8 | ||
| Alveolar soft parts sarcoma | 4 | 3 | 16 | 29 | 1.4 | ||
| Miscellaneous soft tissue sarcomas | 13 | 16 | 36 | 47 | 3 | ||
| Unspecified soft tissue sarcomas | 60 | 40 | 111 | 139 | 9.3 | ||
Nonrhabdomyosarcomatous soft tissue sarcomas are more common in adolescents and adults,[4] and most of the information regarding treatment and natural history of the disease in younger patients has been based on adult studies. The distributions of these tumors by age according to stage, histologic subtype, and tumor site are shown in Figures 1, 2, and 3, respectively.[6]



Risk Factors
Some genetic and environmental factors have been associated with the development of nonrhabdomyosarcomatous soft tissue sarcoma, including the following:
- Genetic factors:
- Li-Fraumeni syndrome: Patients with Li-Fraumeni syndrome (usually due to heritable cancer-associated changes of the TP53 tumor suppressor gene) have an increased risk of developing soft tissue tumors (mostly nonrhabdomyosarcomatous soft tissue sarcomas), bone sarcomas, breast cancer, brain tumors, and acute leukemia.[7,8]
- Familial adenomatous polyposis: Patients with familial adenomatous polyposis are at increased risk of developing desmoid-type fibromatosis.[9]
- Retinoblastoma (RB1) gene: Germline mutations of the retinoblastoma gene have been associated with an increased risk of developing soft tissue sarcomas, particularly leiomyosarcoma.[10]
- SMARCB1 gene: Germline mutations or deletions of the SMARCB1 (INI1) gene are associated with an increased risk of developing extrarenal rhabdoid tumors.[11]
- Neurofibromatosis type 1: Approximately 4% of patients with neurofibromatosis type 1 develop malignant peripheral nerve sheath tumors, which usually develop after a long latency; some patients develop multiple lesions.[12-14]
- Werner syndrome: Werner syndrome is characterized by spontaneous chromosomal instability, resulting in increased susceptibility to cancer and premature aging. An excess of soft tissue sarcomas has been reported in patients with Werner syndrome.[15]
- Environmental factors:
- Radiation: Some nonrhabdomyosarcomatous soft tissue sarcomas (particularly malignant fibrous histiocytoma) can develop within a previously irradiated site.[3,16]
- Epstein-Barr virus infection in patients with AIDS: Some nonrhabdomyosarcomatous soft tissue sarcomas (e.g., leiomyosarcoma) have been linked to Epstein-Barr virus infection in patients with AIDS.[3,17]
Clinical Presentation
Although nonrhabdomyosarcomatous soft tissue sarcomas can develop in any part of the body, they arise most commonly in the trunk and extremities.[18-20] These neoplasms can present initially as an asymptomatic solid mass, or they may be symptomatic because of local invasion of adjacent anatomical structures. Although rare, these tumors can arise primarily in brain tissue and are treated according to the histotype.[21]
Systemic symptoms (e.g., fever, weight loss, and night sweats) are rare. Hypoglycemia and hypophosphatemic rickets have been reported in cases of hemangiopericytoma, whereas hyperglycemia has been noted in patients with fibrosarcoma of the lung.[22]
Diagnostic and Staging Evaluation
When a suspicious lesion is identified, it is crucial that a complete workup, followed by adequate biopsy be performed. It is best to image the lesion using the following procedures before initiating any intervention:
- Plain films. Plain films can be used to rule out bone involvement and detect calcifications that may be seen in soft tissue tumors such as extraskeletal osteosarcoma or synovial sarcoma.
- Chest computed tomography (CT). Chest CT is essential to assess the presence of metastases.
- Abdominal CT or magnetic resonance imaging (MRI). Abdominal CT or MRI can be used to image intra-abdominal tumors, such as liposarcoma.
- Extremity MRI. MRI is essential for extremity lesions.
- Positron emission tomography (PET) scan and bone scan. In children with rhabdomyosarcoma, PET-CT performed better than conventional imaging in identifying nodal, bone, bone marrow, and soft tissue disease. The authors of an imaging comparison study suggest that bone scans with technetium Tc 99m might be eliminated as a staging procedure.[23] The use of this modality in pediatric nonrhabdomyosarcomatous soft tissue sarcoma has not been studied extensively. However, a small study of nine patients with nonrhabdomyosarcomatous soft tissue sarcoma suggests that PET-CT is more accurate and cost effective than either modality alone in identifying distant metastatic disease.[24]
The imaging characteristics of some tumors can be highly suggestive of this diagnosis. For example, the imaging characteristics of pediatric low-grade fibromyxoid sarcoma and alveolar soft part sarcoma have been described and can aid in the diagnosis of these rare neoplasms.[25]
Biopsy strategies
Although nonrhabdomyosarcomatous soft tissue tumors are fairly readily distinguished pathologically from rhabdomyosarcoma and Ewing sarcoma, the classification of childhood nonrhabdomyosarcomatous soft tissue sarcoma type is often difficult. Core-needle biopsy, incisional biopsy, or excisional biopsy can be used to diagnose a nonrhabdomyosarcomatous soft tissue sarcoma. If possible, the surgeon who will perform the definitive resection needs to be involved in the biopsy decision. Poorly placed incisional or needle biopsies may adversely affect the performance of the primary resection.
Considerations related to the selection of a biopsy procedure are as follows:
- Given the diagnostic importance of translocations, a core-needle biopsy or small incisional biopsy that obtains adequate tumor tissue is crucial to allow for conventional histology, immunocytochemical analysis, and other studies such as light and electron microscopy, cytogenetics, fluorescence in situ hybridization, and molecular pathology.[26,27] Core-needle biopsy for a deep-seated tumor can lead to formation of a hematoma, which affects subsequent resection and/or radiation; in these cases, incisional biopsy is the preferred procedure.
- Fine-needle biopsy is usually not recommended because it is difficult to determine the accurate histologic diagnosis and grade of the tumor in this heterogeneous group of tumors.
- Image guidance using ultrasound, CT scan, or MRI may be necessary to ensure a representative biopsy.[28]
- Needle biopsy techniques must ensure adequate tissue sampling. The acquisition of multiple cores of tissue may be required.
- Incisional biopsies must not compromise subsequent wide local excision.
- Excisional biopsy of the lesion is only appropriate for small superficial lesions (<3 cm in size) and are discouraged.[29,30] If an excisional biopsy is contemplated, then MRI of the area is recommended to define the area of involvement as subsequent surgery or radiation therapy is likely.
- Various institutional series have demonstrated the feasibility and effectiveness of sentinel node biopsy as a staging procedure in pediatric patients with soft tissue sarcomas.[31-36]
- Transverse extremity incisions are avoided to reduce skin loss and because they require a greater cross-sectional volume of tissue to be covered in the radiation field. Other extensive surgical procedures are also avoided before definitive diagnosis. For these reasons, open biopsy or multiple core-needle biopsies are strongly encouraged so that adequate tumor tissue can be obtained to allow crucial studies to be performed and to avoid limiting future treatment options.
Unplanned resection
In children with unplanned resection of nonrhabdomyosarcomatous soft tissue sarcomas, primary re-excision is frequently recommended because many patients will have tumor present in the re-excision specimen.[37,38] A single-institution analysis of adolescents and adults compared patients with unplanned excision of soft tissue sarcoma to stage-matched controls. In this retrospective analysis, unplanned initial excision of soft tissue sarcoma resulted in increased risk of local recurrence, metastasis, and death; this increase was greatest for high-grade tumors.[39][Level of evidence: 3iiA]
Chromosomal abnormalities
Many nonrhabdomyosarcomatous soft tissue sarcomas are characterized by chromosomal abnormalities. Some of these chromosomal translocations lead to a fusion of two disparate genes. The resulting fusion transcript can be readily detected by using polymerase chain reaction-based techniques, thus facilitating the diagnosis of those neoplasms that have translocations.
Some of the most frequent aberrations seen in nonrhabdomyosarcomatous soft tissue tumors are listed in Table 2.
| Histology | Chromosomal Aberrations | Genes Involved |
|---|---|---|
| aAdapted from Sandberg,[40] Slater et al.,[41] Mertens et al.,[42] and Romeo.[43] | ||
| Alveolar soft part sarcoma | t(x;17)(p11.2;q25) | ASPL/TFE3 [44-46] |
| Angiomatoid fibrous histiocytoma | t(12;16)(q13;p11), t(2;22)(q33;q12), t(12;22)(q13;q12) | FUS/ATF1, EWSR1/CREB1,[47] EWS/ATF1 |
| Clear cell sarcoma | t(12;22)(q13;q12), t(2;22)(q33;q12) | ATF1/EWS, EWSR1/CREB1 |
| Congenital (infantile) fibrosarcoma/mesoblastic nephroma | t(12;15)(p13;q25) | ETV-NTRK3 |
| Dermatofibrosarcoma protuberans | t(17;22)(q22;q13) | COL1A1/PDGFB |
| Desmoid fibromatosis | Trisomy 8 or 20, loss of 5q21 | CTNNB1 or APC mutations |
| Desmoplastic small round cell tumors | t(11;22)(p13;q12) | EWS/WT1 [48,49] |
| Epithelioid hemangioendothelioma | t(1;3)(p36;q25) [50] | WWTR1/CAMTA1 |
| Epithelioid sarcoma | Inactivation SMARCB1 | SMARCB1 |
| Extraskeletal myxoid chondrosarcoma | t(9;22)(q22;q12), t(9;17)(q22;q11), t(9;15)(q22;q21), t(3;9)(q11;q22) | EWSR1/NR4A3, TAF2N/NR4A3, TCF12/NR4A3, TGF/NR4A3 |
| Hemangiopericytoma | t(12;19)(q13;q13.3) and t(13;22)(q22;q13.3) | |
| Infantile fibrosarcoma | t(12;15)(p13;q25) | ETV6/NTRK3 |
| Inflammatory myofibroblastic tumor | t(1;2)(q23;q23), t(2;19)(q23;q13), t(2;17)(q23;q23), t(2;2)(p23;q13), t(2;11)(p23;p15) [51] | TPM3/ALK, TPM4/ALK, CLTC/ALK, RANBP2/ALK, CARS/ALK, RAS |
| Low-grade fibromyxoid sarcoma | t(7;16)(q33;p11), t(11;16)(p11;p11) | FUS/CREB3L2, FUS/CREB3L1 |
| Malignant peripheral nerve sheath tumor | 17q11.2, loss or rearrangement 10p, 11q, 17q, 22q | NF1 |
| Mesenchymal chondrosarcoma | Del(8)(q13.3q21.1) | HEY1/NCOA2 |
| Myoepithelioma | t(19;22)(q13;q12), t(1;22)(q23;q12), t(6;22)(p21;q12) | EWSR/ZNF44, EWSR/PBX1, EWSR/POU5F1 |
| Myxoid/round cell liposarcoma | t(12;16)(q13;p11), t(12;22)(q13;q12) | FUS/DD1T3, EWSR/DD1T3 |
| Rhabdoid tumor | Inactivation SMARCB1 | SMARCB1 |
| Solitary fibrous tumor | Inv(12)(q13q13) | NAB2/STAT6 |
| Synovial sarcoma | t(x;18)(p11.2;q11.2) | SYT/SSX |
| Tenosynovial giant cell tumor | t(1;2)(p13;q35) | COL6A3/CSF1 |
Prognosis
The prognosis of nonrhabdomyosarcomatous soft tissue sarcoma varies greatly depending on the following factors:[52-54]
- Site of the primary tumor.
- Tumor size.
- Tumor grade. (Refer to the Prognostic Significance of Tumor Grading section of this summary for more information.)
- Tumor histology.
- Depth of tumor invasion.
- Presence of metastases.
- Resectability of the tumor.
- Use of radiation therapy.
Several adult and pediatric series have shown that patients with large or invasive tumors have a significantly worse prognosis than do those with small, noninvasive tumors. A retrospective review of soft tissue sarcomas in children and adolescents suggests that the 5 cm cutoff used for adults with soft tissue sarcoma may not be ideal for smaller children, especially infants. The review identified an interaction between tumor diameter and body surface area.[55] This relationship requires further study to determine the therapeutic implications of the observation.
In a review of a large adult series of nonrhabdomyosarcomatous soft tissue sarcomas, superficial extremity sarcomas had a better prognosis than did deep tumors. Thus, in addition to grade and size, the depth of invasion of the tumor should be considered.[56]
Some pediatric nonrhabdomyosarcomatous soft tissue sarcomas are associated with a better outcome. For instance, infantile fibrosarcoma, presenting in infants and children younger than 5 years, has an excellent prognosis given that surgery alone can cure a significant number of these patients and the tumor is highly chemosensitive.[3]
Soft tissue sarcomas in older children and adolescents often behave similarly to those in adult patients.[3,26] A large, prospective, multinational Children's Oncology Group study (ARST0332 [NCT00346164]) enrolled newly diagnosed patients younger than 30 years. Patients were assigned to treatment on the basis of their risk group (refer to Figure 4).[57][Level of evidence: 2A]

- Arm A (grossly excised low-grade tumor and ≤5 cm widely excised high-grade tumor): Surgery only.
- Arm B (≤5 cm marginally resected high-grade tumor): 55.8 Gy of radiation therapy.
- Arm C (>5 cm grossly resected tumor ± metastases): Ifosfamide/doxorubicin chemotherapy and 55.8 Gy of radiation therapy.
- Arm D (>5 cm unresected tumor ± metastases): Preoperative ifosfamide/doxorubicin chemotherapy and 45 Gy of radiation therapy, and then surgery and a radiation boost that was based on margins.
Of 551 patients enrolled, at a median follow-up of 2.6 years, the preliminary analysis estimated the following 3-year survival rates:[57]
- Arm A: 91% event-free survival (EFS); 99% overall survival (OS).
- Arm B: 79% EFS; 100% OS.
- Arm C: 68% EFS; 81% OS.
- Arm D: 52% EFS; 66% OS.
Pediatric patients with unresected localized nonrhabdomyosarcomatous soft tissue sarcomas have a poor outcome. Only about one-third of patients treated with multimodality therapy remain disease free.[52,58]; [59,60][Level of evidence: 3iiiA] In a review of 30 Italian patients with nonrhabdomyosarcomatous soft tissue sarcoma at visceral sites, only ten patients survived at 5 years. Unfavorable prognostic factors included inability to achieve complete resection, large tumor size, tumor invasion, histologic subtype, and lung-pleura sites.[61][Level of evidence: 3iiB]
In a pooled analysis from U.S. and European pediatric centers, outcome was better for patients whose tumor removal procedure was deemed complete than for patients whose tumor removal was incomplete. Outcome was better for patients who received radiation therapy than for patients who did not.[59][Level of evidence: 3iiiA]
Because long-term related morbidity must be minimized while disease-free survival is maximized, the ideal therapy for each patient must be carefully and individually determined utilizing these prognostic factors before initiating therapy.[19,62-66]
Related Summaries
Refer to the following PDQ summaries for information about other types of sarcoma:
- Childhood Rhabdomyosarcoma Treatment.
- Childhood Vascular Tumors Treatment.
- Ewing Sarcoma Treatment (extraosseous Ewing, peripheral neuroepithelioma, and Askin tumors).
- Unusual Cancers of Childhood Treatment (gastrointestinal stromal tumors).
- Adult Soft Tissue Sarcoma Treatment.
References
- Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014. [PUBMED Abstract]
- Ries LA, Smith MA, Gurney JG, et al., eds.: Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. Bethesda, Md: National Cancer Institute, SEER Program, 1999. NIH Pub.No. 99-4649. Also available online. Last accessed January 24, 2018.
- Spunt SL, Million L, Coffin C: The nonrhabdomyosarcoma soft tissue sarcoma. In: Pizzo PA, Poplack DG, eds.: Principles and Practice of Pediatric Oncology. 7th ed. Philadelphia, Pa: Lippincott Williams and Wilkins, 2015, pp 827-54.
- Weiss SW, Goldblum JR: General considerations. In: Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 5th ed. St. Louis, Mo: Mosby, 2008, pp 1-14.
- Pappo AS, Pratt CB: Soft tissue sarcomas in children. Cancer Treat Res 91: 205-22, 1997. [PUBMED Abstract]
- Ferrari A, Sultan I, Huang TT, et al.: Soft tissue sarcoma across the age spectrum: a population-based study from the Surveillance Epidemiology and End Results database. Pediatr Blood Cancer 57 (6): 943-9, 2011. [PUBMED Abstract]
- Chang F, Syrjänen S, Syrjänen K: Implications of the p53 tumor-suppressor gene in clinical oncology. J Clin Oncol 13 (4): 1009-22, 1995. [PUBMED Abstract]
- Plon SE, Malkin D: Childhood cancer and hereditary. In: Pizzo PA, Poplack DG, eds.: Principles and Practice of Pediatric Oncology. 7th ed. Philadelphia, Pa: Lippincott Williams and Wilkins, 2015, pp 13-31.
- Groen EJ, Roos A, Muntinghe FL, et al.: Extra-intestinal manifestations of familial adenomatous polyposis. Ann Surg Oncol 15 (9): 2439-50, 2008. [PUBMED Abstract]
- Kleinerman RA, Tucker MA, Abramson DH, et al.: Risk of soft tissue sarcomas by individual subtype in survivors of hereditary retinoblastoma. J Natl Cancer Inst 99 (1): 24-31, 2007. [PUBMED Abstract]
- Eaton KW, Tooke LS, Wainwright LM, et al.: Spectrum of SMARCB1/INI1 mutations in familial and sporadic rhabdoid tumors. Pediatr Blood Cancer 56 (1): 7-15, 2011. [PUBMED Abstract]
- Weiss SW, Goldblum JR: Benign tumors of peripheral nerves. In: Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 5th ed. St. Louis, Mo: Mosby, 2008, pp 825-901.
- deCou JM, Rao BN, Parham DM, et al.: Malignant peripheral nerve sheath tumors: the St. Jude Children's Research Hospital experience. Ann Surg Oncol 2 (6): 524-9, 1995. [PUBMED Abstract]
- Stark AM, Buhl R, Hugo HH, et al.: Malignant peripheral nerve sheath tumours--report of 8 cases and review of the literature. Acta Neurochir (Wien) 143 (4): 357-63; discussion 363-4, 2001. [PUBMED Abstract]
- Goto M, Miller RW, Ishikawa Y, et al.: Excess of rare cancers in Werner syndrome (adult progeria). Cancer Epidemiol Biomarkers Prev 5 (4): 239-46, 1996. [PUBMED Abstract]
- Weiss SW, Goldblum JR: Malignant fibrous histiocytoma (pleomorphic undifferentiated sarcoma). In: Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 5th ed. St. Louis, Mo: Mosby, 2008, pp 403-27.
- McClain KL, Leach CT, Jenson HB, et al.: Association of Epstein-Barr virus with leiomyosarcomas in children with AIDS. N Engl J Med 332 (1): 12-8, 1995. [PUBMED Abstract]
- Dillon P, Maurer H, Jenkins J, et al.: A prospective study of nonrhabdomyosarcoma soft tissue sarcomas in the pediatric age group. J Pediatr Surg 27 (2): 241-4; discussion 244-5, 1992. [PUBMED Abstract]
- Rao BN: Nonrhabdomyosarcoma in children: prognostic factors influencing survival. Semin Surg Oncol 9 (6): 524-31, 1993 Nov-Dec. [PUBMED Abstract]
- Zeytoonjian T, Mankin HJ, Gebhardt MC, et al.: Distal lower extremity sarcomas: frequency of occurrence and patient survival rate. Foot Ankle Int 25 (5): 325-30, 2004. [PUBMED Abstract]
- Benesch M, von Bueren AO, Dantonello T, et al.: Primary intracranial soft tissue sarcoma in children and adolescents: a cooperative analysis of the European CWS and HIT study groups. J Neurooncol 111 (3): 337-45, 2013. [PUBMED Abstract]
- Weiss SW, Goldblum JR: Miscellaneous tumors of intermediate malignancy. In: Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 5th ed. St. Louis, Mo: Mosby, 2008, pp 1093-1160.
- Federico SM, Spunt SL, Krasin MJ, et al.: Comparison of PET-CT and conventional imaging in staging pediatric rhabdomyosarcoma. Pediatr Blood Cancer 60 (7): 1128-34, 2013. [PUBMED Abstract]
- Tateishi U, Hosono A, Makimoto A, et al.: Accuracy of 18F fluorodeoxyglucose positron emission tomography/computed tomography in staging of pediatric sarcomas. J Pediatr Hematol Oncol 29 (9): 608-12, 2007. [PUBMED Abstract]
- Sargar K, Kao SC, Spunt SL, et al.: MRI and CT of Low-Grade Fibromyxoid Sarcoma in Children: A Report From Children's Oncology Group Study ARST0332. AJR Am J Roentgenol 205 (2): 414-20, 2015. [PUBMED Abstract]
- Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 4th ed. St. Louis, Mo: Mosby, 2001.
- Recommendations for the reporting of soft tissue sarcomas. Association of Directors of Anatomic and Surgical Pathology. Mod Pathol 11 (12): 1257-61, 1998. [PUBMED Abstract]
- Chowdhury T, Barnacle A, Haque S, et al.: Ultrasound-guided core needle biopsy for the diagnosis of rhabdomyosarcoma in childhood. Pediatr Blood Cancer 53 (3): 356-60, 2009. [PUBMED Abstract]
- Coffin CM, Dehner LP, O'Shea PA: Pediatric Soft Tissue Tumors: A Clinical, Pathological, and Therapeutic Approach. Baltimore, Md: Williams and Wilkins, 1997.
- Smith LM, Watterson J, Scott SM: Medical and surgical management of pediatric soft tissue tumors. In: Coffin CM, Dehner LP, O'Shea PA: Pediatric Soft Tissue Tumors: A Clinical, Pathological, and Therapeutic Approach. Baltimore, Md: Williams and Wilkins, 1997, pp 360-71.
- Neville HL, Andrassy RJ, Lally KP, et al.: Lymphatic mapping with sentinel node biopsy in pediatric patients. J Pediatr Surg 35 (6): 961-4, 2000. [PUBMED Abstract]
- Neville HL, Raney RB, Andrassy RJ, et al.: Multidisciplinary management of pediatric soft-tissue sarcoma. Oncology (Huntingt) 14 (10): 1471-81; discussion 1482-6, 1489-90, 2000. [PUBMED Abstract]
- Kayton ML, Delgado R, Busam K, et al.: Experience with 31 sentinel lymph node biopsies for sarcomas and carcinomas in pediatric patients. Cancer 112 (9): 2052-9, 2008. [PUBMED Abstract]
- Dall'Igna P, De Corti F, Alaggio R, et al.: Sentinel node biopsy in pediatric patients: the experience in a single institution. Eur J Pediatr Surg 24 (6): 482-7, 2014. [PUBMED Abstract]
- Parida L, Morrisson GT, Shammas A, et al.: Role of lymphoscintigraphy and sentinel lymph node biopsy in the management of pediatric melanoma and sarcoma. Pediatr Surg Int 28 (6): 571-8, 2012. [PUBMED Abstract]
- Alcorn KM, Deans KJ, Congeni A, et al.: Sentinel lymph node biopsy in pediatric soft tissue sarcoma patients: utility and concordance with imaging. J Pediatr Surg 48 (9): 1903-6, 2013. [PUBMED Abstract]
- Chui CH, Spunt SL, Liu T, et al.: Is reexcision in pediatric nonrhabdomyosarcoma soft tissue sarcoma necessary after an initial unplanned resection? J Pediatr Surg 37 (10): 1424-9, 2002. [PUBMED Abstract]
- Cecchetto G, Guglielmi M, Inserra A, et al.: Primary re-excision: the Italian experience in patients with localized soft-tissue sarcomas. Pediatr Surg Int 17 (7): 532-4, 2001. [PUBMED Abstract]
- Qureshi YA, Huddy JR, Miller JD, et al.: Unplanned excision of soft tissue sarcoma results in increased rates of local recurrence despite full further oncological treatment. Ann Surg Oncol 19 (3): 871-7, 2012. [PUBMED Abstract]
- Sandberg AA: Translocations in malignant tumors. Am J Pathol 159 (6): 1979-80, 2001. [PUBMED Abstract]
- Slater O, Shipley J: Clinical relevance of molecular genetics to paediatric sarcomas. J Clin Pathol 60 (11): 1187-94, 2007. [PUBMED Abstract]
- Mertens F, Antonescu CR, Hohenberger P, et al.: Translocation-related sarcomas. Semin Oncol 36 (4): 312-23, 2009. [PUBMED Abstract]
- Romeo S, Dei Tos AP: Clinical application of molecular pathology in sarcomas. Curr Opin Oncol 23 (4): 379-84, 2011. [PUBMED Abstract]
- Ladanyi M, Lui MY, Antonescu CR, et al.: The der(17)t(X;17)(p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene 20 (1): 48-57, 2001. [PUBMED Abstract]
- Ladanyi M: The emerging molecular genetics of sarcoma translocations. Diagn Mol Pathol 4 (3): 162-73, 1995. [PUBMED Abstract]
- Williams A, Bartle G, Sumathi VP, et al.: Detection of ASPL/TFE3 fusion transcripts and the TFE3 antigen in formalin-fixed, paraffin-embedded tissue in a series of 18 cases of alveolar soft part sarcoma: useful diagnostic tools in cases with unusual histological features. Virchows Arch 458 (3): 291-300, 2011. [PUBMED Abstract]
- Antonescu CR, Dal Cin P, Nafa K, et al.: EWSR1-CREB1 is the predominant gene fusion in angiomatoid fibrous histiocytoma. Genes Chromosomes Cancer 46 (12): 1051-60, 2007. [PUBMED Abstract]
- Barnoud R, Sabourin JC, Pasquier D, et al.: Immunohistochemical expression of WT1 by desmoplastic small round cell tumor: a comparative study with other small round cell tumors. Am J Surg Pathol 24 (6): 830-6, 2000. [PUBMED Abstract]
- Wang LL, Perlman EJ, Vujanic GM, et al.: Desmoplastic small round cell tumor of the kidney in childhood. Am J Surg Pathol 31 (4): 576-84, 2007. [PUBMED Abstract]
- Errani C, Zhang L, Sung YS, et al.: A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer 50 (8): 644-53, 2011. [PUBMED Abstract]
- Jain S, Xu R, Prieto VG, et al.: Molecular classification of soft tissue sarcomas and its clinical applications. Int J Clin Exp Pathol 3 (4): 416-28, 2010. [PUBMED Abstract]
- Spunt SL, Hill DA, Motosue AM, et al.: Clinical features and outcome of initially unresected nonmetastatic pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Clin Oncol 20 (15): 3225-35, 2002. [PUBMED Abstract]
- Spunt SL, Poquette CA, Hurt YS, et al.: Prognostic factors for children and adolescents with surgically resected nonrhabdomyosarcoma soft tissue sarcoma: an analysis of 121 patients treated at St Jude Children's Research Hospital. J Clin Oncol 17 (12): 3697-705, 1999. [PUBMED Abstract]
- Ferrari A, Casanova M, Collini P, et al.: Adult-type soft tissue sarcomas in pediatric-age patients: experience at the Istituto Nazionale Tumori in Milan. J Clin Oncol 23 (18): 4021-30, 2005. [PUBMED Abstract]
- Ferrari A, Miceli R, Meazza C, et al.: Soft tissue sarcomas of childhood and adolescence: the prognostic role of tumor size in relation to patient body size. J Clin Oncol 27 (3): 371-6, 2009. [PUBMED Abstract]
- Brooks AD, Heslin MJ, Leung DH, et al.: Superficial extremity soft tissue sarcoma: an analysis of prognostic factors. Ann Surg Oncol 5 (1): 41-7, 1998 Jan-Feb. [PUBMED Abstract]
- Spunt SL, Million L, Anderson JR, et al.: Risk-based treatment for nonrhabdomyosarcoma soft tissue sarcomas (NRSTS) in patients under 30 years of age: Children’s Oncology Group study ARST0332. [Abstract] J Clin Oncol 32 (Suppl 15): A-10008, 2014. Also available online. Last accessed April 02, 2018.
- O'Sullivan B, Davis AM, Turcotte R, et al.: Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359 (9325): 2235-41, 2002. [PUBMED Abstract]
- Ferrari A, Miceli R, Rey A, et al.: Non-metastatic unresected paediatric non-rhabdomyosarcoma soft tissue sarcomas: results of a pooled analysis from United States and European groups. Eur J Cancer 47 (5): 724-31, 2011. [PUBMED Abstract]
- Smith KB, Indelicato DJ, Knapik JA, et al.: Definitive radiotherapy for unresectable pediatric and young adult nonrhabdomyosarcoma soft tissue sarcoma. Pediatr Blood Cancer 57 (2): 247-51, 2011. [PUBMED Abstract]
- Ferrari A, Magni C, Bergamaschi L, et al.: Pediatric nonrhabdomyosarcoma soft tissue sarcomas arising at visceral sites. Pediatr Blood Cancer 64 (9): , 2017. [PUBMED Abstract]
- Dillon PW, Whalen TV, Azizkhan RG, et al.: Neonatal soft tissue sarcomas: the influence of pathology on treatment and survival. Children's Cancer Group Surgical Committee. J Pediatr Surg 30 (7): 1038-41, 1995. [PUBMED Abstract]
- Pappo AS, Fontanesi J, Luo X, et al.: Synovial sarcoma in children and adolescents: the St Jude Children's Research Hospital experience. J Clin Oncol 12 (11): 2360-6, 1994. [PUBMED Abstract]
- Marcus KC, Grier HE, Shamberger RC, et al.: Childhood soft tissue sarcoma: a 20-year experience. J Pediatr 131 (4): 603-7, 1997. [PUBMED Abstract]
- Pratt CB, Pappo AS, Gieser P, et al.: Role of adjuvant chemotherapy in the treatment of surgically resected pediatric nonrhabdomyosarcomatous soft tissue sarcomas: A Pediatric Oncology Group Study. J Clin Oncol 17 (4): 1219, 1999. [PUBMED Abstract]
- Pratt CB, Maurer HM, Gieser P, et al.: Treatment of unresectable or metastatic pediatric soft tissue sarcomas with surgery, irradiation, and chemotherapy: a Pediatric Oncology Group study. Med Pediatr Oncol 30 (4): 201-9, 1998. [PUBMED Abstract]
Histopathological Classification of Childhood Soft Tissue Sarcoma
World Health Organization (WHO) Classification of Soft Tissue Sarcomas
The WHO lists the following cell types in its classification of soft tissue sarcomas:[1,2]
- Adipocytic tumors.
- Intermediate (locally aggressive).
- Malignant.
- Chondro-osseous tumors.
- Extraskeletal mesenchymal chondrosarcoma.[3]
- Extraskeletal osteosarcoma.
- Soft tissue chondroma.
- Fibroblastic/myofibroblastic tumors.
- Intermediate-grade (locally aggressive).
- Desmoid-type fibromatosis (previously called desmoid tumor or aggressive fibromatoses).
- Giant cell fibroblastoma.
- Lipofibromatosis.
- Palmar/plantar fibromatosis.
- Intermediate-grade (rarely metastasizing).
- Dermatofibrosarcoma protuberans.
- Infantile fibrosarcoma.[4]
- Inflammatory myofibroblastic tumor.
- Low-grade myofibroblastic tumor.
- Myxoinflammatory fibroblastic sarcoma.
- Solitary fibrous tumor.
- Malignant.
- Intermediate-grade (locally aggressive).
- Skeletal muscle tumors.
- Rhabdomyosarcoma (embryonal, alveolar, and pleomorphic forms). (Refer to the PDQ summary on Childhood Rhabdomyosarcoma Treatment for more information.)
- Smooth muscle tumors.
- So-called fibrohistiocytic tumors (intermediate, rarely metastasizing).
- Giant cell tumors of soft tissue.
- Plexiform fibrohistiocytic tumor.
- Tumors of peripheral nerves.
- Pericytic (perivascular) tumors.
- Malignant glomus tumor and variants.
- Myopericytoma.
- Angioleiomyoma.
- Myofibroma.
- Infantile myofibroma (previously called hemangiopericytoma [infantile]).
- Myofibromatosis.
- Infantile myofibromatosis.
- Tumors of uncertain differentiation.
- Alveolar soft part sarcoma.
- Clear cell sarcoma of soft tissue.
- Desmoplastic small round cell tumor.
- Epithelioid sarcoma.
- Extrarenal rhabdoid tumor.
- Extraskeletal myxoid chondrosarcoma.
- Neoplasms with perivascular epithelioid cell differentiation (PEComa NOS, malignant).
- Primitive neuroectodermal tumor/extraskeletal Ewing tumor.
- Synovial sarcoma (NOS, spindle cell, and biphasic varieties).
- Undifferentiated/unclassified sarcomas.
- Undifferentiated epithelial sarcoma.
- Undifferentiated pleomorphic sarcoma.
- Undifferentiated round cell sarcoma.
- Undifferentiated sarcoma; sarcoma, NOS.[6]
- Undifferentiated spindle cell sarcoma.
- Vascular tumors.
References
- Soft tissue sarcoma. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 291-6.
- Brodowicz T, Schwameis E, Widder J, et al.: Intensified Adjuvant IFADIC Chemotherapy for Adult Soft Tissue Sarcoma: A Prospective Randomized Feasibility Trial. Sarcoma 4 (4): 151-60, 2000. [PUBMED Abstract]
- Dantonello TM, Int-Veen C, Leuschner I, et al.: Mesenchymal chondrosarcoma of soft tissues and bone in children, adolescents, and young adults: experiences of the CWS and COSS study groups. Cancer 112 (11): 2424-31, 2008. [PUBMED Abstract]
- Steelman C, Katzenstein H, Parham D, et al.: Unusual presentation of congenital infantile fibrosarcoma in seven infants with molecular-genetic analysis. Fetal Pediatr Pathol 30 (5): 329-37, 2011. [PUBMED Abstract]
- Evans HL: Low-grade fibromyxoid sarcoma: a clinicopathologic study of 33 cases with long-term follow-up. Am J Surg Pathol 35 (10): 1450-62, 2011. [PUBMED Abstract]
- Alaggio R, Collini P, Randall RL, et al.: Undifferentiated high-grade pleomorphic sarcomas in children: a clinicopathologic study of 10 cases and review of literature. Pediatr Dev Pathol 13 (3): 209-17, 2010 May-Jun. [PUBMED Abstract]
Staging and Grading Systems for Childhood Soft Tissue Sarcoma
Clinical staging has an important role in predicting the clinical outcome and determining the most effective therapy for pediatric soft tissue sarcomas. As yet, there is no well-accepted staging system that is applicable to all childhood sarcomas. The system from the American Joint Committee on Cancer (AJCC) that is used for adults has not been validated in pediatric studies. Although a standardized staging system for pediatric nonrhabdomyosarcomatous soft tissue sarcoma does not exist, two systems are currently in use for staging pediatric nonrhabdomyosarcomatous soft tissue sarcoma.[1]
- Surgico-pathologic staging system: The surgico-pathologic staging system used by the Intergroup Rhabdomyosarcoma Study (see below) is based on the amount, or extent, of tumor that remains after initial surgery and whether the disease has metastasized. This staging system was used in early pediatric trials.[2]
- TNM staging system: The TNM staging system is a collaborative effort between the AJCC (United States) and the International Union Against Cancer (worldwide). Staging is based on the extent of the tumor (T), the extent of spread to the lymph nodes (N), and the presence of metastasis (M). Refer to Tables 3, 4, 5, and 6 for the staging of soft tissue sarcoma from the eighth edition of the AJCC Cancer Staging Manual.[3-7] The last Children's Oncology Group trial used the sixth edition AJCC Cancer Staging Manual for soft tissue sarcoma (with central pathology review).[1] A review of children with non-rhabdomyosarcoma soft tissue sarcomas was performed with data from the Surveillance, Epidemiology, and End Results (SEER) program and identified 941 patients between 1988 and 2007.[8] The COG risk stratification was validated in this cohort.
Intergroup Rhabdomyosarcoma Study Staging System
Nonmetastatic disease
- Group I: Localized tumor completely resected with histologically negative margins.
- Group II: Grossly resected tumor with microscopic residual tumor at the margin(s) and/or extension into regional lymph nodes.
- IIA: Localized, grossly resected tumor with microscopic residual disease.
- IIB: Regional disease with involved nodes completely resected with no microscopic disease. The most proximal (to the patient, most distal to the tumor) regional lymph node must be negative.
- IIC: Regional disease with involved nodes grossly resected but with evidence of residual microscopic disease at the primary site and/or histologic involvement of the most proximal regional lymph node in the dissection.
- Group III: Localized tumor, incompletely resected, or biopsy only, with gross residual tumor.
Metastatic disease
- Group IV: Any localized or regional tumor with distant metastases present at the time of diagnosis. This includes the presence of malignant cells in effusions (pleural, peritoneal) and/or cerebrospinal fluid (rare).
Recurrent/progressive disease
- Any soft tissue sarcoma that recurs after initial treatment or progresses after radiation therapy, chemotherapy, or initial surgery.
TNM Staging System
The eighth edition of the AJCC Cancer Staging Manual has designated staging by the four criteria of tumor size, nodal status, histologic grade, and metastasis and by anatomic primary tumor site (head and neck; trunk and extremities; abdomen and thoracic visceral organs; retroperitoneum; and unusual histologies and sites) (refer to Tables 3, 4, 5, and 6).[3-7] For information on unusual histologies and sites, refer to the AJCC Cancer Staging Manual.[7]
| T Category | Soft Tissue Sarcoma of the Trunk, Extremities, and Retroperitoneum | Soft Tissue Sarcoma of the Head and Neck | Soft Tissue Sarcoma of the Abdomen and Thoracic Visceral Organs |
|---|---|---|---|
| aAdapted from O'Sullivan et al.,[3] Yoon et al.,[4] Raut et al.,[5] and Pollock et al.[6] | |||
| TX | Primary tumor cannot be assessed. | Primary tumor cannot be assessed. | Primary tumor cannot be assessed. |
| T0 | No evidence of primary tumor. | ||
| T1 | Tumor ≤5 cm in greatest dimension. | Tumor ≤2 cm. | Organ confined. |
| T2 | Tumor >5 cm and ≤10 cm in greatest dimension. | Tumor >2 to ≤4 cm. | Tumor extension into tissue beyond organ. |
| T2a | Invades serosa or visceral peritoneum. | ||
| T2b | Extension beyond serosa (mesentery). | ||
| T3 | Tumor >10 cm and ≤15 cm in greatest dimension. | Tumor >4 cm. | Invades another organ. |
| T4 | Tumor >15 cm in greatest dimension. | Tumor with invasion of adjoining structures. | Multifocal involvement. |
| T4a | Tumor with orbital invasion, skull base/dural invasion, invasion of central compartment viscera, involvement of facial skeleton, or invasion of pterygoid muscles. | Multifocal (2 sites). | |
| T4b | Tumor with brain parenchymal invasion, carotid artery encasement, prevertebral muscle invasion, or central nervous system involvement via perineural spread. | Multifocal (3–5 sites). | |
| T4c | Multifocal (>5 sites). | ||
| aAdapted from O'Sullivan et al.,[3] Yoon et al.,[4] Raut et al.,[5] and Pollock et al.[6] | |
| bFor soft tissue sarcoma of the abdomen and thoracic visceral organs, N0 = no lymph node involvement or unknown lymph node status and N1 = lymph node involvement present. | |
| N0 | No regional lymph node metastasis or unknown lymph node status.b |
| N1 | Regional lymph node metastasis.b |
| aAdapted from O'Sullivan et al.,[3] Yoon et al.,[4] Raut et al.,[5] and Pollock et al.[6] | |
| bFor soft tissue sarcoma of the abdomen and thoracic visceral organs, M0 = no metastases and M1 = metastases present. | |
| M0 | No distant metastasis.b |
| M1 | Distant metastasis.b |
| Stage | T | N | M | Grade |
|---|---|---|---|---|
| aAdapted from Yoon et al. [4] and Pollock et al.[6] | ||||
| bStage IIIB for soft tissue sarcoma of the retroperitoneum; stage IV for soft tissue sarcoma of the trunk and extremities. | ||||
| IA | T1 | N0 | M0 | G1, GX |
| IB | T2, T3, T4 | N0 | M0 | G1, GX |
| II | T1 | N0 | M0 | G2, G3 |
| IIIA | T2 | N0 | M0 | G2, G3 |
| IIIB | T3, T4 | N0 | M0 | G2, G3 |
| IIIB/IVb | Any T | N1 | M0 | Any G |
| IV | Any T | Any N | M1 | Any G |
Soft Tissue Sarcoma Tumor Pathological Grading System
In most cases, accurate histopathologic classification alone of soft tissue sarcomas does not yield optimal information about their clinical behavior. Therefore, several histologic parameters are evaluated in the grading process, including the following:
- Degree of cellularity.
- Cellular pleomorphism.
- Mitotic activity.
- Degree of necrosis.
- Invasive growth.
This process is used to improve the correlation between histologic findings and clinical outcome.[9] In children, grading of soft tissue sarcoma is compromised by the good prognosis of certain tumors, such as infantile fibrosarcoma and hemangiopericytoma, which have a good prognosis in children younger than 4 years, and also angiomatoid fibrous histiocytoma and dermatofibrosarcoma protuberans, which may recur locally if incompletely excised, but usually do not metastasize.
Testing the validity of a grading system within the pediatric population is difficult because of the rarity of these neoplasms. In March 1986, the Pediatric Oncology Group (POG) conducted a prospective study on pediatric soft tissue sarcomas other than rhabdomyosarcoma and devised the POG grading system. Analysis of outcome for patients with localized soft tissue sarcomas other than rhabdomyosarcoma demonstrated that patients with grade 3 tumors fared significantly worse than those with grade 1 or grade 2 lesions. This finding suggests that this system can accurately predict the clinical behavior of nonrhabdomyosarcomatous soft tissue sarcoma.[9-11]
The grading systems developed by the POG and the French Federation of Comprehensive Cancer Centers (Fédération Nationale des Centres de Lutte Contre Le Cancer [FNCLCC]) Sarcoma Group are described below. These grading systems are being compared by the central review pathologists on the COG-ARST0332 study. The study has closed and results are pending.
POG grading system
The POG grading system is described below.[9] It is an older grading system of historical value that is no longer being used for treatment.
Grade I
Grade I lesions are based on histologic type, well-differentiated cytohistologic features, and/or age of the patient.
- Angiomatoid fibrous histiocytoma.
- Dermatofibrosarcoma protuberans.
- Liposarcoma–myxoid or well-differentiated.
- Myxoid chondrosarcoma.
- Well-differentiated malignant peripheral nerve sheath tumor.
- Well-differentiated or infantile (aged ≤4 years) fibrosarcoma.
- Well-differentiated or infantile (aged ≤4 years) hemangiopericytoma.
Grade II
Grade II lesions are soft tissue sarcomas not included in grade I or III by histologic diagnosis (with <5 mitoses/10 high-power fields or <15% necrosis):
- 15% or less of the surface area shows necrosis (primary criteria).
- The mitotic count is <5 mitotic figures per 10 high-power fields (40X objective) (primary criteria).
- Nuclear atypia is not marked (secondary criteria).
- The tumor is not markedly cellular (secondary criteria).
Grade III
Grade III lesions are similar to grade II lesions and include certain tumors known to be clinically aggressive by virtue of histologic diagnosis and non-grade I tumors (with >4 mitoses per 10 high-power fields or >15% necrosis):
- Alveolar soft part sarcoma.
- Extraskeletal osteogenic sarcoma.
- Malignant triton tumor.
- Mesenchymal chondrosarcoma.
- Pleomorphic or round-cell liposarcoma.
- Any other sarcoma not in grade I with >15% necrosis and/or ≥5 mitotic figures per 10 high-power fields (40X objective). Marked atypia and cellularity are less predictive but may assist in placing tumors in this category.
FNCLCC grading system
The FNCLCC histologic grading system was developed for adults with soft tissue sarcoma. The purpose of the grading system is to predict which patients will develop metastasis and subsequently benefit from postoperative chemotherapy.[12,13] The system is described in Table 7 and Table 8.
| FNCLCC = Fédération Nationale des Centres de Lutte Contre Le Cancer; HPF = high-power field. | |
| Tumor Differentiation | |
| Score 1 | Sarcoma closely resembling normal adult mesenchymal tissue (e.g., well-differentiated liposarcoma) |
| Score 2 | Sarcomas for which histologic typing is certain (e.g., myxoid liposarcoma) |
| Score 3 | Embryonal and undifferentiated sarcomas, sarcomas of doubtful type, and synovial sarcomas |
| Mitotic Count | |
| Score 1 | 0–9 mitoses per 10 HPF |
| Score 2 | 10–19 mitoses per 10 HPF |
| Score 3 | ≥20 mitoses per 10 HPF |
| Tumor Necrosis | |
| Score 0 | No necrosis |
| Score 1 | <50% tumor necrosis |
| Score 2 | ≥50% tumor necrosis |
| Total Score | Histologic Grade |
|---|---|
| 2–3 | Grade I |
| 4–5 | Grade II |
| 6–8 | Grade III |
Prognostic Significance of Tumor Grading
The POG and FNCLCC grading systems have proven to be of prognostic value in pediatric and adult nonrhabdomyosarcomatous soft tissue sarcomas.[14-18] In a study of 130 tumors from children and adolescents with nonrhabdomyosarcomatous soft tissue sarcoma enrolled in three prospective clinical trials, a correlation was found between the POG-assigned grade and the FNCLCC-assigned grade. However, grading did not correlate in all cases; 44 patients whose tumors received discrepant grades (POG grade 3, FNCLCC grade 1 or 2) had outcomes between concurrent grade 3 and grades 1 and 2. A mitotic index of 10 or greater emerged as an important prognostic factor.[19] The recently completed COG-ARST0332 trial will analyze data comparing the POG and FNCLCC pathologic grading systems to determine which system better correlates with clinical outcomes. The current open trial (ARST1321 [NCT02180867]) uses the FNCLCC system to assign histological grade.
References
- American Joint Committee on Cancer: AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer, 2002.
- Maurer HM, Beltangady M, Gehan EA, et al.: The Intergroup Rhabdomyosarcoma Study-I. A final report. Cancer 61 (2): 209-20, 1988. [PUBMED Abstract]
- O'Sullivan B, Maki RG, Agulnik M, et al.: Soft tissue sarcoma of the head and neck. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 499-505.
- Yoon SS, Maki RG, Asare EA, et al.: Soft tissue sarcoma of the trunk and extremities. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 507-15.
- Raut CP, Maki RG, Baldini EH, et al.: Soft tissue sarcoma of the abdomen and thoracic visceral organs. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 517-21.
- Pollock RE, Maki RG, Baldini EH, et al.: Soft tissue sarcoma of the retroperitoneum. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 531-7.
- Maki RG, Folpe AL, Guadagnolo BA, et al.: Soft tissue sarcoma - unusual histologies and sites. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 539-45.
- Waxweiler TV, Rusthoven CG, Proper MS, et al.: Non-Rhabdomyosarcoma Soft Tissue Sarcomas in Children: A Surveillance, Epidemiology, and End Results Analysis Validating COG Risk Stratifications. Int J Radiat Oncol Biol Phys 92 (2): 339-48, 2015. [PUBMED Abstract]
- Parham DM, Webber BL, Jenkins JJ 3rd, et al.: Nonrhabdomyosarcomatous soft tissue sarcomas of childhood: formulation of a simplified system for grading. Mod Pathol 8 (7): 705-10, 1995. [PUBMED Abstract]
- Recommendations for the reporting of soft tissue sarcomas. Association of Directors of Anatomic and Surgical Pathology. Mod Pathol 11 (12): 1257-61, 1998. [PUBMED Abstract]
- Skytting B, Meis-Kindblom JM, Larsson O, et al.: Synovial sarcoma--identification of favorable and unfavorable histologic types: a Scandinavian sarcoma group study of 104 cases. Acta Orthop Scand 70 (6): 543-54, 1999. [PUBMED Abstract]
- Coindre JM, Terrier P, Guillou L, et al.: Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 91 (10): 1914-26, 2001. [PUBMED Abstract]
- Guillou L, Coindre JM, Bonichon F, et al.: Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol 15 (1): 350-62, 1997. [PUBMED Abstract]
- Rao BN: Nonrhabdomyosarcoma in children: prognostic factors influencing survival. Semin Surg Oncol 9 (6): 524-31, 1993 Nov-Dec. [PUBMED Abstract]
- Pisters PW, Leung DH, Woodruff J, et al.: Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 14 (5): 1679-89, 1996. [PUBMED Abstract]
- Coindre JM, Terrier P, Bui NB, et al.: Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol 14 (3): 869-77, 1996. [PUBMED Abstract]
- Pappo AS, Fontanesi J, Luo X, et al.: Synovial sarcoma in children and adolescents: the St Jude Children's Research Hospital experience. J Clin Oncol 12 (11): 2360-6, 1994. [PUBMED Abstract]
- Pratt CB, Maurer HM, Gieser P, et al.: Treatment of unresectable or metastatic pediatric soft tissue sarcomas with surgery, irradiation, and chemotherapy: a Pediatric Oncology Group study. Med Pediatr Oncol 30 (4): 201-9, 1998. [PUBMED Abstract]
- Khoury JD, Coffin CM, Spunt SL, et al.: Grading of nonrhabdomyosarcoma soft tissue sarcoma in children and adolescents: a comparison of parameters used for the Fédération Nationale des Centers de Lutte Contre le Cancer and Pediatric Oncology Group Systems. Cancer 116 (9): 2266-74, 2010. [PUBMED Abstract]
Treatment Option Overview for Childhood Soft Tissue Sarcoma
Because of the rarity of pediatric nonrhabdomyosarcomatous soft tissue sarcomas, coordination of treatment by a multidisciplinary team comprising oncologists (pediatric or medical), pathologists, surgeons, and radiation oncologists should be considered for all children, adolescents, and young adults with these tumors. In addition, to better define the tumors' natural history and response to therapy, entry into national or institutional treatment protocols should be considered for children with rare neoplasms. Information about ongoing clinical trials is available from the NCI website.
Surgery
After an appropriate biopsy and pathologic diagnosis, every attempt is made to resect the primary tumor with negative margins before or after chemotherapy and/or radiation therapy. Involvement of a surgeon with special expertise in the resection of soft tissue sarcomas in the decision is highly desirable.
The timing of surgery depends on an assessment of the feasibility and morbidity of surgery. If the initial operation fails to achieve pathologically negative tissue margins or if the initial surgery was done without the knowledge that cancer was present, a re-excision of the affected area is performed to obtain clear, but not necessarily wide, margins.[1-4] This surgical tenet is true even if no mass is detected by magnetic resonance imaging after initial surgery.[5]; [6][Level of evidence: 3iiA]
Regional lymph node metastases at diagnosis are unusual and are most often seen in patients with epithelioid and clear cell sarcomas.[7,8] Various institutional series have demonstrated the feasibility and effectiveness of sentinel node biopsy as a staging procedure in pediatric patients with soft tissue sarcomas.[9-14]
Radiation Therapy
Considerations for radiation therapy are based on the potential for surgery, with or without chemotherapy, to obtain local control without loss of critical organs or significant functional, cosmetic, or psychological impairment. This will vary according to the following:
- Patient variables (e.g., age and sex).
- Tumor variables (e.g., histopathology, site, size, and grade).
- Surgical margin status.
- Expectations for radiation-induced morbidities (e.g., impaired bone or muscle development, organ damage, or second malignancy).
Radiation therapy can be given preoperatively. Radiation field size and dose will be based on patient and tumor variables and the operability of the tumor. Preoperative radiation therapy has been associated with excellent local control rates.[15,16] This approach has the advantage of treating smaller tissue volumes because it does not necessitate treating a postsurgical bed; it also has the advantage of somewhat lower radiation doses because relative hypoxia from surgical disruption of vasculature and scarring is not present. Preoperative radiation therapy has been associated with an increased rate of wound complications in adults, primarily in lower extremity tumors, but the degree of this is questionable.[17] Conversely, preoperative radiation therapy may lead to less fibrosis than with postoperative approaches, perhaps due to the smaller treatment volume and dose.[18]
Retroperitoneal sarcomas are unique in that radiosensitivity of the bowel to injury makes postoperative radiation therapy less desirable.[19,20] Postoperative adhesions and bowel immobility can increase the risk of damage from any given radiation dose. This contrasts with the preoperative approach in which the tumor often displaces bowel outside of the radiation field, and any exposed bowel is more mobile, which decreases exposure to specific bowel segments.
Radiation therapy can also be given postoperatively. In general, radiation is indicated for patients with inadequate surgical margins and for larger, high-grade tumors.[21,22] This is particularly important in high-grade tumors with tumor margins smaller than 1 cm.[23,24]; [25][Level of evidence: 3iiDiv] With combined surgery and radiation therapy, local control of the primary tumor can be achieved in more than 80% of patients.[26,27]
Brachytherapy and intraoperative radiation may be applicable in select situations.[27-29]; [30][Level of evidence: 3iiiDii]
Radiation volume and dose depend on the patient, tumor, and surgical variables noted above, as well as the following:
- Patient age and growth potential.
- Ability to avoid critical organs, epiphyseal plates, and lymphatics (but not the neurovascular bundles that are relatively radiation tolerant).
- Functional/cosmetic outcome.
Radiation doses are typically 45 Gy to 50 Gy preoperatively, with consideration for postoperative boost of 10 Gy to 20 Gy if resection margins are microscopically or grossly positive, or planned brachytherapy if the resection is predicted to be subtotal. However, data documenting the efficacy of a postoperative boost are lacking.[31] The postoperative radiation dose is 55 Gy to 60 Gy, or rarely, higher when unresectable gross residual disease exists.
Radiation margins are typically 2 cm to 4 cm longitudinally and encompass fascial planes axially.[32,33]
Chemotherapy
The role of postoperative chemotherapy remains unclear as evidenced by the following studies:[34]
- A meta-analysis of data from all randomized trials of adults with soft tissue sarcoma concluded that recurrence-free survival was better with postoperative chemotherapy for patients with high-grade tumors larger than 5 cm.[35]
- In a European trial, adults with completely resected soft tissue sarcoma were randomly assigned to observation or postoperative chemotherapy with ifosfamide and doxorubicin. Postoperative chemotherapy was not associated with improved event-free survival (EFS) or overall survival (OS). It is difficult to extrapolate this trial to pediatric patients because the trial included 1) a wide variety of histologies; 2) a relatively low dose of ifosfamide; 3) patients assigned to chemotherapy had definitive radiation delayed until completion of chemotherapy; and 4) almost one-half of the patients in the trial had intermediate-grade tumors. In the discussion section, the authors merged their patients with previously published series, including those from the European meta-analysis, and concluded that the results suggested a benefit for postoperative chemotherapy.[36][Level of evidence: 1iiA]
- The largest prospective pediatric trial failed to demonstrate any benefit with postoperative vincristine, dactinomycin, cyclophosphamide, and doxorubicin.[26]
- Doxorubicin and ifosfamide were used in the risk-based COG ARST0332 (NCT00346164) trial. Although this was not a randomized study, results at 2.6 years show that patients with high-risk (>5 cm and high grade), grossly resected, nonmetastatic tumors who were treated with radiation therapy and postoperative doxorubicin and ifosfamide had a 3-year EFS of 68% and OS of 81%. In patients with metastatic disease treated with preoperative chemotherapy and radiation therapy, the estimated 3-year failure-free survival was 52% and OS was 66%.[37][Level of evidence: 3iiiA]
Targeted Therapy
The use of angiogenesis and mammalian target of rapamycin (mTOR) inhibitors has been explored in the treatment of adult soft tissue sarcomas but not in pediatrics.
- In a trial of 711 randomly assigned adult patients who achieved a response or stable disease after chemotherapy, the administration of ridaforolimus was associated with a 3-week improvement in progression-free survival (PFS) when compared with placebo.[38]
- In another trial of 371 randomly assigned adult patients with metastatic soft tissue sarcoma that progressed after chemotherapy, pazopanib was compared with placebo. The median PFS for the pazopanib arm was 4.6 months compared with 1.6 months for the placebo arm. OS was not different between the two arms.[39]
- In a randomized study of 182 previously treated adult patients with recurrent liposarcoma, leiomyosarcoma, synovial sarcoma, and other sarcomas, patients with nonadipocytic tumors who were treated with regorafenib had significant improvements in progression-free survival when compared with patients who were treated with placebo.[40]
Special Considerations for the Treatment of Children With Soft Tissue Sarcoma
Cancer in children and adolescents is rare, although the overall incidence of childhood cancer has been slowly increasing since 1975.[41] Children and adolescents with cancer should be referred to medical centers that have a multidisciplinary team of cancer specialists with experience treating the cancers that occur during childhood and adolescence. This multidisciplinary team approach incorporates the skills of the following health care professionals and others to ensure that children receive treatment, supportive care, and rehabilitation that will achieve optimal survival and quality of life:
- Primary care physicians.
- Pediatric surgical specialists.
- Pediatric radiation oncologists.
- Pediatric medical oncologists/hematologists.
- Rehabilitation specialists.
- Pediatric nurse specialists.
- Social workers.
- Child life professionals.
- Psychologists.
(Refer to the PDQ Supportive and Palliative Care summaries for specific information about supportive care for children and adolescents with cancer.)
Guidelines for pediatric cancer centers and their role in the treatment of pediatric patients with cancer have been outlined by the American Academy of Pediatrics.[42] At these pediatric cancer centers, clinical trials are available for most types of cancer that occur in children and adolescents, and the opportunity to participate in these trials is offered to most patients/families. Multidisciplinary evaluation in pediatric cancer centers that have surgical and radiotherapeutic expertise is of critical importance to ensure the best clinical outcome for these patients. Although surgery with or without radiation therapy can be curative for a significant proportion of patients, the addition of chemotherapy might benefit subsets of children with the disease; therefore, enrollment into clinical trials is encouraged. Clinical trials for children and adolescents with cancer are generally designed to compare potentially better therapy with therapy that is currently accepted as standard. Most of the progress made in identifying curative therapies for childhood cancers has been achieved through clinical trials. Information about ongoing clinical trials is available from the NCI website.
Many therapeutic strategies for children and adolescents with soft tissue tumors are similar to those for adult patients, although there are important differences. For example, the biology of the neoplasm in pediatric patients may differ dramatically from that of the adult lesion. Additionally, limb-sparing procedures are more difficult to perform in pediatric patients. The morbidity associated with radiation therapy, particularly in infants and young children, may be much greater than that observed in adults.[43]
Improved outcomes with multimodality therapy in adults and children with soft tissue sarcomas over the past 20 years has caused increasing concern about the potential long-term side effects of this therapy in children, especially when considering the expected longer life span of children versus adults. Therefore, to maximize tumor control and minimize long-term morbidity, treatment must be individualized for children and adolescents with nonrhabdomyosarcomatous soft tissue sarcoma. These patients should be enrolled in prospective studies that accurately assess any potential complications.[44]
References
- Sugiura H, Takahashi M, Katagiri H, et al.: Additional wide resection of malignant soft tissue tumors. Clin Orthop (394): 201-10, 2002. [PUBMED Abstract]
- Cecchetto G, Guglielmi M, Inserra A, et al.: Primary re-excision: the Italian experience in patients with localized soft-tissue sarcomas. Pediatr Surg Int 17 (7): 532-4, 2001. [PUBMED Abstract]
- Chui CH, Spunt SL, Liu T, et al.: Is reexcision in pediatric nonrhabdomyosarcoma soft tissue sarcoma necessary after an initial unplanned resection? J Pediatr Surg 37 (10): 1424-9, 2002. [PUBMED Abstract]
- Paulino AC, Ritchie J, Wen BC: The value of postoperative radiotherapy in childhood nonrhabdomyosarcoma soft tissue sarcoma. Pediatr Blood Cancer 43 (5): 587-93, 2004. [PUBMED Abstract]
- Kaste SC, Hill A, Conley L, et al.: Magnetic resonance imaging after incomplete resection of soft tissue sarcoma. Clin Orthop (397): 204-11, 2002. [PUBMED Abstract]
- Chandrasekar CR, Wafa H, Grimer RJ, et al.: The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br 90 (2): 203-8, 2008. [PUBMED Abstract]
- Daigeler A, Kuhnen C, Moritz R, et al.: Lymph node metastases in soft tissue sarcomas: a single center analysis of 1,597 patients. Langenbecks Arch Surg 394 (2): 321-9, 2009. [PUBMED Abstract]
- Mazeron JJ, Suit HD: Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer 60 (8): 1800-8, 1987. [PUBMED Abstract]
- Neville HL, Andrassy RJ, Lally KP, et al.: Lymphatic mapping with sentinel node biopsy in pediatric patients. J Pediatr Surg 35 (6): 961-4, 2000. [PUBMED Abstract]
- Neville HL, Raney RB, Andrassy RJ, et al.: Multidisciplinary management of pediatric soft-tissue sarcoma. Oncology (Huntingt) 14 (10): 1471-81; discussion 1482-6, 1489-90, 2000. [PUBMED Abstract]
- Kayton ML, Delgado R, Busam K, et al.: Experience with 31 sentinel lymph node biopsies for sarcomas and carcinomas in pediatric patients. Cancer 112 (9): 2052-9, 2008. [PUBMED Abstract]
- Dall'Igna P, De Corti F, Alaggio R, et al.: Sentinel node biopsy in pediatric patients: the experience in a single institution. Eur J Pediatr Surg 24 (6): 482-7, 2014. [PUBMED Abstract]
- Parida L, Morrisson GT, Shammas A, et al.: Role of lymphoscintigraphy and sentinel lymph node biopsy in the management of pediatric melanoma and sarcoma. Pediatr Surg Int 28 (6): 571-8, 2012. [PUBMED Abstract]
- Alcorn KM, Deans KJ, Congeni A, et al.: Sentinel lymph node biopsy in pediatric soft tissue sarcoma patients: utility and concordance with imaging. J Pediatr Surg 48 (9): 1903-6, 2013. [PUBMED Abstract]
- Virkus WW, Mollabashy A, Reith JD, et al.: Preoperative radiotherapy in the treatment of soft tissue sarcomas. Clin Orthop (397): 177-89, 2002. [PUBMED Abstract]
- Zagars GK, Ballo MT, Pisters PW, et al.: Preoperative vs. postoperative radiation therapy for soft tissue sarcoma: a retrospective comparative evaluation of disease outcome. Int J Radiat Oncol Biol Phys 56 (2): 482-8, 2003. [PUBMED Abstract]
- O'Sullivan B, Davis AM, Turcotte R, et al.: Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359 (9325): 2235-41, 2002. [PUBMED Abstract]
- Davis AM, O'Sullivan B, Turcotte R, et al.: Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 75 (1): 48-53, 2005. [PUBMED Abstract]
- Baldini EH, Wang D, Haas RL, et al.: Treatment Guidelines for Preoperative Radiation Therapy for Retroperitoneal Sarcoma: Preliminary Consensus of an International Expert Panel. Int J Radiat Oncol Biol Phys 92 (3): 602-12, 2015. [PUBMED Abstract]
- Bishop AJ, Zagars GK, Torres KE, et al.: Combined Modality Management of Retroperitoneal Sarcomas: A Single-Institution Series of 121 Patients. Int J Radiat Oncol Biol Phys 93 (1): 158-65, 2015. [PUBMED Abstract]
- Marcus KC, Grier HE, Shamberger RC, et al.: Childhood soft tissue sarcoma: a 20-year experience. J Pediatr 131 (4): 603-7, 1997. [PUBMED Abstract]
- Delaney TF, Kepka L, Goldberg SI, et al.: Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys 67 (5): 1460-9, 2007. [PUBMED Abstract]
- Blakely ML, Spurbeck WW, Pappo AS, et al.: The impact of margin of resection on outcome in pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Pediatr Surg 34 (5): 672-5, 1999. [PUBMED Abstract]
- Skytting B: Synovial sarcoma. A Scandinavian Sarcoma Group project. Acta Orthop Scand Suppl 291: 1-28, 2000. [PUBMED Abstract]
- Hua C, Gray JM, Merchant TE, et al.: Treatment planning and delivery of external beam radiotherapy for pediatric sarcoma: the St. Jude Children's Research Hospital experience. Int J Radiat Oncol Biol Phys 70 (5): 1598-606, 2008. [PUBMED Abstract]
- Pratt CB, Pappo AS, Gieser P, et al.: Role of adjuvant chemotherapy in the treatment of surgically resected pediatric nonrhabdomyosarcomatous soft tissue sarcomas: A Pediatric Oncology Group Study. J Clin Oncol 17 (4): 1219, 1999. [PUBMED Abstract]
- Merchant TE, Parsh N, del Valle PL, et al.: Brachytherapy for pediatric soft-tissue sarcoma. Int J Radiat Oncol Biol Phys 46 (2): 427-32, 2000. [PUBMED Abstract]
- Schomberg PJ, Gunderson LL, Moir CR, et al.: Intraoperative electron irradiation in the management of pediatric malignancies. Cancer 79 (11): 2251-6, 1997. [PUBMED Abstract]
- Nag S, Shasha D, Janjan N, et al.: The American Brachytherapy Society recommendations for brachytherapy of soft tissue sarcomas. Int J Radiat Oncol Biol Phys 49 (4): 1033-43, 2001. [PUBMED Abstract]
- Viani GA, Novaes PE, Jacinto AA, et al.: High-dose-rate brachytherapy for soft tissue sarcoma in children: a single institution experience. Radiat Oncol 3: 9, 2008. [PUBMED Abstract]
- Al Yami A, Griffin AM, Ferguson PC, et al.: Positive surgical margins in soft tissue sarcoma treated with preoperative radiation: is a postoperative boost necessary? Int J Radiat Oncol Biol Phys 77 (4): 1191-7, 2010. [PUBMED Abstract]
- Wang D, Bosch W, Kirsch DG, et al.: Variation in the gross tumor volume and clinical target volume for preoperative radiotherapy of primary large high-grade soft tissue sarcoma of the extremity among RTOG sarcoma radiation oncologists. Int J Radiat Oncol Biol Phys 81 (5): e775-80, 2011. [PUBMED Abstract]
- Bahig H, Roberge D, Bosch W, et al.: Agreement among RTOG sarcoma radiation oncologists in contouring suspicious peritumoral edema for preoperative radiation therapy of soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys 86 (2): 298-303, 2013. [PUBMED Abstract]
- Ferrari A: Role of chemotherapy in pediatric nonrhabdomyosarcoma soft-tissue sarcomas. Expert Rev Anticancer Ther 8 (6): 929-38, 2008. [PUBMED Abstract]
- Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet 350 (9092): 1647-54, 1997. [PUBMED Abstract]
- Woll PJ, Reichardt P, Le Cesne A, et al.: Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol 13 (10): 1045-54, 2012. [PUBMED Abstract]
- Spunt SL, Million L, Anderson JR, et al.: Risk-based treatment for nonrhabdomyosarcoma soft tissue sarcomas (NRSTS) in patients under 30 years of age: Children’s Oncology Group study ARST0332. [Abstract] J Clin Oncol 32 (Suppl 15): A-10008, 2014. Also available online. Last accessed April 02, 2018.
- Demetri GD, Chawla SP, Ray-Coquard I, et al.: Results of an international randomized phase III trial of the mammalian target of rapamycin inhibitor ridaforolimus versus placebo to control metastatic sarcomas in patients after benefit from prior chemotherapy. J Clin Oncol 31 (19): 2485-92, 2013. [PUBMED Abstract]
- van der Graaf WT, Blay JY, Chawla SP, et al.: Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 379 (9829): 1879-86, 2012. [PUBMED Abstract]
- Mir O, Brodowicz T, Italiano A, et al.: Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 17 (12): 1732-1742, 2016. [PUBMED Abstract]
- Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014. [PUBMED Abstract]
- Corrigan JJ, Feig SA; American Academy of Pediatrics: Guidelines for pediatric cancer centers. Pediatrics 113 (6): 1833-5, 2004. [PUBMED Abstract]
- Suit H, Spiro I: Radiation as a therapeutic modality in sarcomas of the soft tissue. Hematol Oncol Clin North Am 9 (4): 733-46, 1995. [PUBMED Abstract]
- Spunt SL, Million L, Coffin C: The nonrhabdomyosarcoma soft tissue sarcoma. In: Pizzo PA, Poplack DG, eds.: Principles and Practice of Pediatric Oncology. 7th ed. Philadelphia, Pa: Lippincott Williams and Wilkins, 2015, pp 827-54.
Treatment of Newly Diagnosed Childhood Soft Tissue Sarcoma
Adipocytic Tumors
Liposarcoma
Liposarcoma accounts for 3% of soft tissue sarcoma in patients younger than 20 years (refer to Table 1).
Liposarcoma is rare in the pediatric population. In a review of 182 pediatric patients with adult-type sarcomas, only 14 had a diagnosis of liposarcoma.[1] One retrospective study identified 34 patients younger than 22 years from 1960 to 2011.[2] There were roughly equal numbers of male and female patients and the median age was 18 years. In an international clinicopathological review, the characteristics of 82 cases of pediatric liposarcoma were reported. The median age was 15.5 years and females were more commonly affected.[3] In both reports, the great majority of patients had myxoid liposarcoma.
Histopathologic classification
The World Health Organization (WHO) classification for liposarcoma is as follows:
- Intermediate grade (rarely metastasizing).
- Atypical lipomatous neoplasm/well-differentiated liposarcoma. These tumors do not metastasize unless they undergo dedifferentiation.
- Malignant.
- Liposarcoma, not otherwise specified (NOS).
- Myxoid liposarcoma. Pure myxoid liposarcomas are characterized by a t(12;16)(q13;p11) translocation and can metastasize but usually have an excellent outcome in the absence of a round cell component.[4]
- Dedifferentiated liposarcoma.
- Pleomorphic liposarcoma.
Clinical presentation
The majority of liposarcomas in the pediatric and adolescent age range are low grade and located subcutaneously. Metastasis to lymph nodes is very uncommon, and the great majority of metastases are pulmonary. Tumors arising in the periphery are more likely to be low grade and myxoid. Tumors arising centrally are more likely to be high grade, pleomorphic, and present with metastasis or recur with metastasis.
Prognosis
Higher grade or central tumors are associated with a significantly higher risk of death. In a retrospective review, 5-year survival for central tumors was 42%. In the international review, seven of ten patients with pleomorphic myxoid liposarcoma died because of their disease.[3] In a retrospective study of 14 patients, 5-year survival was 78% and tumor grade, histologic subtype, and primary location correlated with survival.[2]
Treatment
Treatment options for liposarcoma include the following:
Surgery is the most important treatment for liposarcoma. After surgical resection of myxoid liposarcoma, event-free survival (EFS) and overall survival (OS) are roughly 90%. If initial surgery is incomplete, re-excision should be performed to achieve a wide margin of resection. Local recurrences have been seen and are controlled with a second resection of the tumor.
There are reports of the use of chemotherapy to decrease the size of liposarcoma before surgery to facilitate complete resection, particularly in central tumors.[10,11] The role of postoperative chemotherapy for liposarcoma is poorly defined. There does not appear to be a need for any postoperative therapy for completely resected myxoid liposarcoma. Even with the use of postoperative chemotherapy, the survival of pleomorphic liposarcoma remains poor.[12]
Trabectedin has produced encouraging responses in adults with advanced myxoid liposarcoma.[13] In one study, adult patients with recurrent liposarcoma and leiomyosarcoma were randomly assigned to treatment with either trabectedin or dacarbazine. Patients treated with trabectedin had a 45% reduction in disease progression.[14][Level of evidence: 1iiDiii] There are very limited data to support the use of trabectedin in pediatric patients.[15]
Treatment options under clinical evaluation
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma, excluding myxoid liposarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with liposarcoma are eligible for this trial.
Chondro-osseous Tumors
Chondro-osseous tumors include the following tumor subtypes:
- Extraskeletal mesenchymal chondrosarcoma.
- Extraskeletal osteosarcoma.
- Soft tissue chondroma.
Extraskeletal mesenchymal chondrosarcoma
Osseous and chondromatous neoplasms account for 0.8% of soft tissue sarcoma in patients younger than 20 years (refer to Table 1).
Histopathology and molecular features
Mesenchymal chondrosarcoma is a rare tumor characterized by small round cells and hyaline cartilage that more commonly affects young adults and has a predilection for involving the head and neck region.
Mesenchymal chondrosarcoma has been associated with consistent chromosomal rearrangement. A retrospective analysis of cases of mesenchymal chondrosarcoma identified a HEY1-NCOA2 fusion in 10 of 15 tested specimens.[16] This gene fusion was not associated with chromosomal changes that could be detected by karyotyping. In one instance, translocation t(1;5)(q42;q32) was identified in a case of mesenchymal chondrosarcoma and shown to be associated with a novel IRF2BP-CDX1 fusion gene.[17]
Prognosis
A retrospective survey of European institutions identified 113 children and adults with mesenchymal chondrosarcoma. Factors associated with better outcome included the following:[18][Level of evidence: 3iiiA]
- Lack of metastatic disease at initial presentation.
- Clear resection margins.
- Administration of postoperative chemotherapy following resection for patients with initially localized disease.
Treatment
Treatment options for extraskeletal mesenchymal chondrosarcoma include the following:
A review of 15 patients younger than 26 years from the German Cooperative Soft Tissue Sarcoma Study Group (11 with soft-tissue lesions) and the German-Austrian-Swiss Cooperative Osteosarcoma Study Group (four with primary bone lesions) protocols suggests that complete surgical removal, or incomplete resection followed by radiation therapy, is necessary for local control.[19][Level of evidence: 3iiA]
A single-institution, retrospective review identified 12 pediatric patients with mesenchymal chondrosarcoma.[20] The presence of the NCOA2 rearrangement in tumors was documented in these patients. It was also confirmed that surgical resection is necessary for cure. Eleven patients presented with localized disease and one presented with pulmonary nodules. All patients received chemotherapy—six patients before and after surgical resection and six patients only after resection. All patients received postoperative chemotherapy (most commonly ifosfamide/doxorubicin) with or without radiation therapy (median dose, 59.4 Gy). At a median follow-up of 4.8 years, 5-year disease-free survival (DFS) was 68.2% (95% CI, 39.8%–96.6%) and OS was 88.9% (95% CI, 66.9%–100%).
Extraskeletal osteosarcoma
Osseous and chondromatous neoplasms account for 0.8% of soft tissue sarcomas in patients younger than 20 years (refer to Table 1).
Extraskeletal osteosarcoma is extremely rare in the pediatric and adolescent age range. A 2003 review identified only ten case reports in the medical literature.[21]
Prognosis
Extraskeletal osteosarcoma is associated with a high risk of local recurrence and pulmonary metastasis.[22]
Treatment
Treatment options for extraskeletal osteosarcoma include the following:
- Surgery followed by chemotherapy.
(Refer to the PDQ summary on Osteosarcoma and Malignant Fibrous Histiocytoma of Bone Treatment for more information.)
Treatment options under clinical evaluation
Information about National Cancer Institute NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with extraskeletal mesenchymal chondrosarcoma and extraskeletal osteosarcoma are eligible for this trial.
Fibroblastic/Myofibroblastic Tumors
Fibroblastic/myofibroblastic tumors include the following tumor subtypes:
- Fibroblastic/myofibroblastic tumors.
- Intermediate grade (locally aggressive).
- Desmoid-type fibromatosis (previously called desmoid tumor or aggressive fibromatoses).
- Giant cell fibroblastoma.
- Lipofibromatosis.
- Palmar/plantar fibromatosis.
- Intermediate grade (rarely metastasizing).
- Dermatofibrosarcoma protuberans.
- Infantile fibrosarcoma.
- Inflammatory myofibroblastic tumor.
- Low-grade myofibroblastic sarcoma.
- Myxoinflammatory fibroblastic sarcoma.
- Solitary fibrous tumor.
- Malignant.
- Intermediate grade (locally aggressive).
Desmoid-type fibromatosis
Desmoid-type fibromatosis has previously been called desmoid tumors or aggressive fibromatoses.
Risk factors
A small number of desmoid-type fibromatosis tumors may occur in association with a mutation in the adenomatous polyposis coli (APC) gene (associated with intestinal polyps and a high incidence of colon cancer). In a study of 519 patients older than 10 years with a diagnosis of desmoid-type fibromatosis, 39 (7.5%, a possible underestimation) were found to have familial adenomatous polyposis (FAP).[23] The patients with FAP and desmoid-type fibromatosis were younger, more often male, and had more abdominal wall or mesenteric tumors than did patients with desmoid-type fibromatosis without FAP.
A family history of colon cancer, the presence of congenital hyperplasia of the retinal pigment epithelium,[24,25] or location of the desmoid-type fibromatosis in the abdomen or abdominal wall [23] should prompt referral to a genetic counselor. Currently, there are no general recommendations for genetic testing in children with desmoid-type fibromatosis. Pathology and molecular characteristics of the tumor only provide guidance for screening. If the tumor has a somatic CTNNB1 mutation, screening is not necessary, because the APC gene mutation has not been described in this setting. If a CTNNB1 mutation is not identified, screening for the APC mutation may be warranted.[26,27] (Refer to the Familial Adenomatous Polyposis (FAP) section of the PDQ summary on Genetics of Colorectal Cancer for more information.)
Prognosis
Desmoid-type fibromatosis has an extremely low potential to metastasize. The tumors are locally infiltrating, and surgical control can be difficult because of the need to preserve normal structures.
These tumors have a high potential for local recurrence. Desmoid-type fibromatosis has a highly variable natural history, including well documented examples of spontaneous regression.[28] Mutations in exon 3 of the beta-catenin gene are seen in over 80% of desmoid-type fibromatosis and the mutation 45F has been associated with an increased risk of disease recurrence.[29] Repeated surgical resection can sometimes bring recurrent lesions under control.[30]
Treatment
Evaluation of the benefit of interventions for treatment of desmoid-type fibromatosis has been extremely difficult, because desmoid-type fibromatosis has a highly variable natural history. Large adult series and smaller pediatric series have reported long periods of disease stabilization and even regression without systemic therapy.[30,31]; [32][Level of evidence: 3iiiDi]
Treatment options for desmoid-type fibromatosis include the following:
- Surgery.
- Observation, for tumors that are incompletely resected or recurrent that do not pose a danger to vital organs, if other treatment options are not available.[30,33-39] Whenever possible, however, the treatment of choice is complete resection.
- Chemotherapy, for unresectable or recurrent tumors.
- Other drug therapy, such as nonsteroidal anti-inflammatory drugs (NSAIDs) or antiestrogen therapy.
- Surgery preceded or followed by radiation therapy, for incompletely resected tumors or to avoid recurrence and subsequent surgery that may result in functional or cosmetic compromise.
- Radiation therapy alone, for unresectable tumors.
The treatment of choice is resection to achieve clear margins. However, a retrospective review of children who underwent surgery for desmoid-type fibromatosis at the St. Jude Children’s Research Hospital (SJCRH) reported no correlation between surgical margins and risk of recurrence.[39]
When the diagnosis is known and complete surgical excision is not feasible, and if the tumor poses significant potential for mortality or morbidity, preoperative strategies may include the following:[40,41]
- Observation.
- Chemotherapy.
- Anti-estrogen therapy.
- NSAID therapy.
- External-beam radiation therapy.
Desmoid-type fibromatosis often behaves in a nonaggressive manner. In a study that included mostly adults with extra-abdominal primary fibromatosis, nonsurgical approaches (medical and observation) had similar 3-year EFS compared with surgery.[34] In a subsequent study of adolescents and adults with abdominal wall aggressive fibromatosis, 102 patients were treated with a watch and wait approach, of which 65 patients required no further treatment at 3 years. Approximately one-third of patients had regression of the tumor.[33]
Chemotherapy regimens may include the following:
- Combination chemotherapy using vinblastine and methotrexate produced objective responses in about one-third of patients with unresectable or recurrent desmoid-type fibromatosis.[40]
- A series of mainly adult patients with FAP and unresectable desmoid-type fibromatosis that were unresponsive to hormone therapy showed that doxorubicin plus dacarbazine followed by meloxicam (an NSAID) can be safely administered and can induce responses.[42]
- Pegylated liposomal doxorubicin has been used with some responses.[43] In a series of five patients, a median progression-free interval of 29 months was reported.[44]
- Tyrosine kinase inhibitors: A small retrospective study of adults with desmoid-type fibromatosis showed objective responses to the multi-targeted kinase inhibitor sorafenib.[45][Level of evidence: 3iiiDiv] Previous studies with imatinib did not support its use.[46,47] A small series reported symptomatic improvement and stable disease in seven patients with desmoid-type fibromatosis who were treated with pazopanib.[48]
- The NOTCH pathway has been implicated in the development of desmoid tumors.[49] Partial responses to the gamma secretase inhibitor PF-03084014 have been noted in adults with desmoid-type fibromatosis.[50][Level of evidence: 3iiiDiv]
- Hydroxyurea has been used successfully to treat a few patients after other treatments, but more data are needed.[51-53]
Other drug therapy may include the following:
- NSAIDs such as sulindac have been used in single cases for desmoid-type fibromatosis; the responses seen were usually disease stabilization.[54]
- Antiestrogen treatment, usually tamoxifen, plus sulindac has also resulted in disease stabilization.[55] A prospective trial of the combination of tamoxifen and sulindac reported few side effects, although asymptomatic ovarian cysts were common in girls. This combination showed relatively little activity, as measured by rates of response and progression-free survival (PFS).[56][Level of evidence: 2Diii]
Postoperative radiation therapy is a consideration when progression would entail additional surgery that might cause functional or cosmetic compromise and if radiation is considered acceptable in terms of morbidities.
Radiation has been used for unresectable desmoid-type fibromatosis or postoperatively for tumors with inadequate resections. The potential long-term complications of radiation therapy, especially subsequent neoplasms, make using this modality less appealing in a young population.[57]
Dermatofibrosarcoma protuberans
Dermatofibrosarcoma is a rare tumor that can be present in all age groups, but many of the reported cases arise in children.[58-60] A review of 451 cases in children younger than 20 years in the SEER database found that the incidence was 1 case per 1 million, highest among black patients aged 15 to 19 years. The most common sites were trunk and extremities, which is similar to what is found in adults. Ninety-five percent of patients underwent surgery. OS was 100% at 5 years, 98% at 15 years, and 97% at 30 years. Males had decreased survival compared with females (P < .05).[61][Level of evidence: 3iA]
Molecular features
The tumor has a consistent chromosomal translocation t(17;22)(q22;q13) that juxtaposes the COL1A1 gene with the PDGF-beta gene.
Treatment
Treatment of dermatofibrosarcoma protuberans includes the following:
- Surgery.
- Surgery preceded or followed by radiation therapy.
- Radiation therapy and imatinib therapy, for unresectable or recurrent tumors.
Most dermatofibrosarcoma tumors can be cured by complete surgical resection. Wide excision with negative margins or Mohs or modified Mohs surgery will prevent most tumors from recurring.[62] Despite the locally aggressive behavior of the tumor, lymph node or visceral metastasis rarely occurs.
In retrospective reviews, postoperative radiation therapy after incomplete excision may have decreased the likelihood of recurrence.[63,64]
When surgical resection cannot be accomplished or the tumor is recurrent, treatment with imatinib has been effective.[65-67] Because metastatic disease is more likely after multiple recurrences, radiation or other adjuvant therapy should be considered in patients with recurrence that cannot be managed surgically.[59,61]
Guidelines for workup and management of dermatofibrosarcoma protuberans have been published.[68]
Infantile fibrosarcoma
There are two distinct types of fibrosarcoma in children and adolescents: infantile fibrosarcoma (also called congenital fibrosarcoma) and fibrosarcoma that is indistinguishable from fibrosarcoma seen in adults. These are two distinct pathologic diagnoses and require different treatments. Adult-type fibrosarcoma is addressed below.
Infantile fibrosarcoma usually occurs in children younger than 1 year. It occasionally occurs in children up to age 4 years. A tumor with similar morphology has been identified in older children; in these older children, the tumors do not have the t(12;15)(ETV-NTRK3) translocation that is characteristic of the younger patients.[69] In several of these patients, BRAF gene fusions have been identified.
Clinical presentation
Infantile fibrosarcoma usually presents with a rapidly growing mass, often noted at birth or even seen in prenatal ultrasound. The tumors are often quite large at the time of presentation.[70]
Molecular features
The tumor usually has a characteristic cytogenetic translocation t(12;15)(ETV-NTRK3). Infantile fibrosarcoma shares this translocation and a virtually identical histologic appearance with mesoblastic nephroma.
Prognosis
These tumors have a low incidence of metastases at diagnosis.
Treatment
Treatment options for infantile fibrosarcoma include the following:
- Surgery followed by observation.
- Surgery followed by chemotherapy.
- Chemotherapy followed by surgery.
Complete resection is curative in the majority of patients with infantile fibrosarcoma. However, the large size of the lesion frequently makes resection without major functional consequences impossible (for instance, tumors of the extremities often require amputation for complete excision). The European pediatric group has reported that observation may also be an option in patients with group II disease after surgery.[71] Twelve patients with group II disease received no further therapy and two patients relapsed. One patient obtained a complete remission after chemotherapy. Postoperative chemotherapy was administered to patients with higher group disease and those who progressed. In a subsequent study, only one of seven patients with group II disease progressed during observation; that patient achieved complete remission with chemotherapy.[72][Level of evidence: 3iiA]
Preoperative chemotherapy has made a more conservative surgical approach possible; agents active in this setting include vincristine, dactinomycin, cyclophosphamide, and ifosfamide.[73,74]; [72,75][Level of evidence: 3iiA]; [76][Level of evidence: 3iiB]
Three studies of patients with infantile fibrosarcoma suggest that an alkylator-free regimen is effective and should be used as the first treatment choice in patients with macroscopic disease.[71,72,77] Two cases with variant LMNA/NTRK1 fusions responded to crizotinib.[78,79]
A pediatric patient (aged 16 months) with refractory infantile fibrosarcoma with constitutive activation of the tropomyosin-related kinase signaling pathway from an ETS variant gene 6–neurotrophin 3 receptor gene fusion (ETV6-NTRK3) responded to LOXO-101, with a 90% reduction in tumor size after 2 months of treatment.[80]
A patient aged 2 months with infantile fibrosarcoma was initially treated with chemotherapy. At disease progression, a response was seen with pazopanib.[81]
A rare case of spontaneous regression without treatment has been reported.[82][Level of evidence: 3iiiDiv]
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- LOXO-TRK-15003 (NCT02637687) (Oral TRK Inhibitor LOXO-101 for Treatment of Advanced Pediatric Solid or Primary Central Nervous System [CNS] Tumors): A phase I trial of the pan-TRK inhibitor LOXO-101 is being conducted for children with solid tumors or brain tumors whose disease has progressed or was nonresponsive to available therapies, and for which no standard or available curative therapy exists. LOXO-101 is a highly selective inhibitor of all three TRK family kinases.
- RXDX-101-03 (NCT02650401) (Study of RXDX-101 in Children With Recurrent or Refractory Solid Tumors and Primary CNS Tumors): This is a four-part, open-label, phase I/Ib, dose-escalation study in pediatric patients with: 1) relapsed or refractory solid tumors; 2) primary CNS tumors; 3) neuroblastoma; and 4) non-neuroblastoma, extracranial solid tumors with NTRK1/2/3, ROS1 or ALK gene rearrangements. The study is designed to explore the safety, maximum tolerated dose or recommended phase II dose, pharmacokinetics, and antitumor activity of entrectinib (RXDX-101).
Inflammatory myofibroblastic tumor
Inflammatory myofibroblastic tumor is a rare mesenchymal tumor that has a predilection for children and adolescents.[83-85]
Clinical presentation
Inflammatory myofibroblastic tumors are rare tumors that affect soft tissues and visceral organs of children and young adults.[86] They rarely metastasize but tend to be locally invasive. Usual anatomical sites of disease include soft tissue, lungs, spleen, colon, and breast.[83] A review of 42 cases of pediatric inflammatory myofibroblastic tumor of the bladder was published in 2015.[87]
Molecular features
Roughly half of inflammatory myofibroblastic tumors exhibit a clonal mutation that activates the anaplastic lymphoma kinase (ALK)-receptor tyrosine kinase gene at chromosome 2p23.[88] ROS1 and PDGFR-beta kinase fusions have been identified in 8 of 11 cases (73%) who are negative for ALK by immunohistochemistry.[89][Level of evidence: 3iiiDiv]
Prognosis
Inflammatory myofibroblastic tumor recurs frequently but is rarely metastatic.[83-85]
Treatment
Treatment options for inflammatory myofibroblastic tumor include the following:
- Surgery.
- Chemotherapy.
- Steroid therapy.
- NSAID therapy.
- Targeted therapy (ALK inhibitors).
Complete surgical removal, when feasible, is the mainstay of therapy.[90] In a series of nine patients, four patients achieved continuous remission after complete resection, three patients with residual disease recurred but later achieved continuous remission, and one patient with metastatic disease responded to multiagent chemotherapy.[91][Level of evidence: 3iiA] The benefit of chemotherapy has been noted in case reports.[92] There are case reports of response to either steroids or NSAIDs.[93,94] A series of 32 patients aged 18 years and younger found that complete excision was the mainstay of therapy, although some patients were treated with steroids or cytotoxic chemotherapy. OS was 94%; three patients relapsed and two of them died of the disease. With complete excision, with or without other treatments such as steroids, there was a high survival rate for patients with this disease.[95][Level of evidence: 3iiA]
Inflammatory myofibroblastic tumors respond to crizotinib. Two adults with ALK-rearranged inflammatory myofibroblastic tumor achieved partial response with crizotinib.[96][Level of evidence: 3iiiDiv] For pediatric patients with measurable disease, the use of crizotinib achieved partial tumor responses in three of six patients with ALK-translocated inflammatory myofibroblastic tumors.[97] A case report of a patient aged 16 years with metastatic/multifocal ALK-positive inflammatory myofibroblastic tumor demonstrated a complete response and a 3-year disease-free interval with crizotinib therapy.[98] In a phase I trial of ceritinib for adult patients previously treated with ALK inhibitors, one patient with inflammatory myofibroblastic tumor had a partial response.[99] Finally, one study included 14 patients with inflammatory myofibroblastic tumor who were treated with crizotinib. With crizotinib therapy, five patients had a complete response, seven had a partial response, and the remaining two had stable disease; no patient had relapsed at the time the article was published.[100][Level of evidence: 3iiDiv]
Adult-type fibrosarcoma
These tumors lack the translocation seen in infantile fibrosarcomas. They present like the great majority of nonrhabdomyosarcomas and the management approach is similar.
Low-grade fibromyxoid sarcoma
Low-grade fibromyxoid sarcoma is a histologically deceptive soft tissue neoplasm that most commonly affects young and middle-aged adults, is commonly located deep within the extremities, and is characterized by a FUS/CREB3L3 translocation.[101,102]
Prognosis
In a review of 33 patients (three were younger than 18 years) with low grade fibromyxoid sarcoma, 21 of 33 patients developed a local recurrence after intervals of up to 15 years (median, 3.5 years) and 15 developed metastases up to 45 years (median, 5 years) from diagnosis, most commonly to the lungs and pleura, emphasizing the need for continued follow-up of these patients.[101] Even after metastases occur, the course may be indolent.[103]
In another report, 14 of 73 cases were younger than 18 years of age. In this series with a relatively short follow up (median of 24 months), only 8 of 54 patients with adequate follow up developed local (9%) or distant (6%) recurrence. This report suggests that the behavior of this tumor might be significantly better than previously reported.[104] However, because of the occurrence of late metastases, careful monitoring of these patients is warranted.
The most recent Children's Oncology Group (COG) trial (ARST0332 [NCT00346164]) enrolled 11 patients with this tumor entity. The median age at diagnosis was 13 years and males were more commonly affected. The most common sites were the lower and upper extremity (n = 9) and none of the patients had developed local or distant disease recurrence at a median follow up of 2.7 years.[105]
Treatment
Treatment options for low-grade fibromyxoid sarcoma include the following:
- Surgery.
The limited treatment information for low-grade fibromyxoid sarcoma suggest that surgery is the treatment of choice as the tumor is not very chemosensitive.[103] There are little data regarding the use of chemotherapy and/or radiation therapy in this disease. One report suggests that trabectedin may be effective in the treatment of low-grade fibromyxoid sarcoma.[106]
Myxofibrosarcoma
Myxofibrosarcoma is a rare lesion, especially in childhood. It is typically treated with complete surgical resection.
Sclerosing epithelioid fibrosarcoma
Sclerosing epithelioid fibrosarcoma is a rare malignant sarcoma that commonly harbors EWSR1 gene rearrangements and has an aggressive clinical course.[107] It is typically treated with complete surgical excision. Long-term follow-up is recommended because local recurrence and metastases can occur late.
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with infantile fibrosarcoma, inflammatory myofibroblastic tumor, low-grade myofibroblastic tumor, myxoinflammatory fibroblastic sarcoma, solitary fibrous tumor, adult-type fibrosarcoma, low-grade fibromyxoid sarcoma, myxofibrosarcoma, and sclerosing epithelioid fibrosarcoma are eligible for this trial.
Skeletal Muscle Tumors
Rhabdomyosarcoma
Refer to the PDQ summary on Childhood Rhabdomyosarcoma Treatment for more information.
Smooth Muscle Tumors
Leiomyosarcoma
Leiomyosarcoma accounts for 2% of soft tissue sarcoma in patients younger than 20 years (refer to Table 1).
Risk factors
Among 43 children with HIV/AIDS who developed tumors, eight developed Epstein-Barr virus–associated leiomyosarcoma.[108] Survivors of hereditary retinoblastoma have a statistically significant increased risk of developing leiomyosarcoma and 78% of these were diagnosed 30 or more years after the initial diagnosis of retinoblastoma.[109]
Treatment
Treatment options for leiomyosarcoma include the following:
- Chemotherapy (trabectedin).
In an open-label study of trabectedin in adult patients with recurrent sarcomas, the best overall response rate (complete remission and partial remission) was seen in patients with leiomyosarcoma (7.5%).[110] The clinical benefit rate (includes stable disease) for leiomyosarcoma was 54%. In another adult study, patients with recurrent liposarcoma and leiomyosarcoma were randomly assigned to receive treatment with either trabectedin or dacarbazine. Patients treated with trabectedin had a 45% reduction in disease progression.[14] There are no data to support the use of trabectedin in pediatric patients.
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with leiomyosarcoma are eligible for this trial.
So-called Fibrohistiocytic Tumors
So-called fibrohistiocytic tumors include the following tumor subtypes:
- Giant cell tumor of soft tissue.
- Plexiform fibrohistiocytic tumor.
Plexiform fibrohistiocytic tumor
Plexiform histiocytic tumor is a rare, low- to intermediate-grade tumor that most commonly affects children and young adults. Depending on the series, the median age at presentation ranges from 8 to 14.5 years; however, the tumor has been described in patients as young as 3 months.[111,112]
Clinical presentation
The tumor commonly arises as a painless mass in the skin or subcutaneous tissue and most often involves the upper extremities, including the fingers, hand, and wrist.[113-115] There are rare reports of spread to regional lymph nodes or the lungs.[111,115,116]
Molecular features
No consistent chromosomal anomalies have been detected but a t(4;15)(q21;q15) translocation has been reported.[117]
Prognosis
Plexiform fibrohistiocytic tumor is an intermediate-grade tumor that rarely metastasizes.
Treatment
Surgery is the treatment of choice but local recurrence has been reported in 12% to 50% of cases.[118]
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with giant cell tumors of soft tissue and plexiform fibrohistiocytic tumor are eligible for this trial.
Tumors of Peripheral Nerves
Ectomesenchymoma
Ectomesenchymoma is a rare nerve sheath tumor that mainly occurs in children. It is a biphenotypic soft tissue sarcoma with both mesenchymal and ectodermal components. Elements similar to rhabdomyosarcoma have been identified.
The German Soft Tissue Sarcoma Group (Cooperative Weichteilsarkom Studiengruppe [CWS]) reported on six patients (ages 0.2–13.5 years) registered over 14 years.[119][Level of evidence: 3iiA] The tumors were located in various sites including the extremities, abdomen, and orbit. All six patients were treated with surgery and chemotherapy directed at rhabdomyosarcoma. Two patients received radiation therapy. Three patients recurred with rhabdomyosarcoma features. Although data are scant, it appears that the tumor may respond to chemotherapy.[119]
Malignant peripheral nerve sheath tumor
Malignant peripheral nerve sheath tumors account for 5% of soft tissue sarcoma in patients younger than 20 years (refer to Table 1).
Risk factors
Malignant peripheral nerve sheath tumor can arise sporadically and in children with type 1 neurofibromatosis (NF1).[120]
Molecular features
Inactivating mutations of SUZ12 have been described in these tumors and are absent in neurofibromas.[121]
Prognosis
Features associated with a favorable prognosis include the following:[120,122-124]
- Smaller tumor size. In a multivariate analysis, only tumor size and nuclear p53 expression were found to be independent predictors of disease-specific survival.[123]
- Male sex and non-Hispanic white race.[125]
- No metastasis at presentation. A retrospective review of 140 patients with malignant peripheral nerve sheath tumor from the MD Anderson Cancer Center included children and adolescents. The disease-specific survival at 10 years was 32%. In this series, presence of metastatic disease was associated with a much worse prognosis.[123]
- Lower stage.
- Lower histologic grade.
- Extremity as the primary site.
Features associated with an unfavorable prognosis include the following:[126]
- High grade.
- Deep tumor location.
- Locally advanced stage at diagnosis.
- Macroscopically incomplete resection (R2).
For patients with localized disease in the MD Anderson Cancer Center study, there was no significant difference in outcome between patients with and without NF1.[123] In other studies, it was not clear whether the absence of NF1 is a favorable prognostic factor as it has been associated with both favorable [122] and unfavorable outcomes.[120,122,124] In the French Sarcoma Group study, NF1 was associated with other adverse prognostic features, but was not an independent predictor of poor outcome.[126] The Italian Sarcoma Group reported on outcomes after recurrence in 73 children and adolescents with malignant peripheral nerve sheath tumor.[127][Level of evidence: 3iiiA] The median overall survival after first relapse was 11 months, and the survival rates were 39.2% at 1 year and 15.8% at 5 years. The factors associated with a better prognosis for these patients who relapsed were less initial tumor invasiveness, longer time to relapse, and the achievement of a secondary complete remission (which was related to the feasibility of radical surgery).
Treatment
Treatment options for malignant peripheral nerve sheath tumor include the following:
Complete surgical removal of the tumor, whenever possible, is the mainstay of treatment.
The role of radiation therapy is difficult to assess, but durable local control of known postoperative microscopic residual tumor is not assured after radiation therapy.
Chemotherapy has achieved objective responses in childhood malignant peripheral nerve sheath tumor. A large retrospective analysis of the German and Italian experience with malignant peripheral nerve sheath tumor reported that 65% of measurable tumors had objective responses to ifosfamide-containing chemotherapy regimens, but the analysis did not conclusively demonstrate improved survival for chemotherapy.[120] This retrospective analysis also noted a trend toward improved outcome with postoperative radiation therapy.[120] A series of 37 young patients with malignant peripheral nerve sheath tumor and NF1 showed that most patients had large invasive tumors that were poorly responsive to chemotherapy; PFS was 19% and 5-year OS was 28%.[128]
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with malignant peripheral nerve sheath tumor are eligible for this trial.
- SARC023 (NCT02008877) (Ganetespib and Sirolimus in Patients With Malignant Peripheral Nerve Sheath Tumors): This trial is testing the combination of ganetespib, the heat shock protein inhibitor, and sirolimus, the mammalian target of rapamycin (mTOR) inhibitor, for the treatment of patients with unresectable or metastatic malignant peripheral nerve sheath tumors. Patients with unresectable soft tissue or bone sarcomas are eligible for phase I of the trial. Patients with unresectable malignant peripheral nerve sheath tumors are eligible for phase II of the trial. Eligibility is restricted to patients aged 18 years and older.
- NCT02601937 (A Phase 1 Study of the EZH2 Inhibitor Tazemetostat in Pediatric Subjects With Relapsed or Refractory INI1-Negative Tumors or Synovial Sarcoma): Patients with INI1-negative tumors are eligible for targeted treatment with an EZH2 inhibitor. This is a phase I, open-label, dose-escalation, and dose-expansion study with a twice-daily oral dose of tazemetostat.
- ADVL1522 (NCT02452554) (Lorvotuzumab Mertansine in Treating Younger Patients with Relapsed or Refractory Wilms Tumor, Rhabdomyosarcoma, Neuroblastoma, Pleuropulmonary Blastoma, Malignant Peripheral Nerve Sheath Tumor, or Synovial Sarcoma): This is a phase II study of IMGN901 (lorvotuzumab mertansine) in children with relapsed or refractory Wilms tumor, rhabdomyosarcoma, neuroblastoma, pleuropulmonary blastoma, malignant peripheral nerve sheath tumor, and synovial sarcoma. This trial is studying the effects of IMGN901, an antibody-drug conjugate that links a potent antimitotic to antibodies that target CD56.
Malignant triton tumor
Malignant triton tumors are a variant of malignant peripheral nerve sheath tumors. They occur most often in patients with neurofibromatosis type I and consist of neurogenic and rhabdomyoblastic components. Malignant triton tumors are high-grade malignancies. They usually occur before age 35 years and are very rare in children (case reports only).[129]
Malignant triton tumors are not usually responsive to chemotherapy and radiation therapy but have been treated with rhabdomyosarcoma therapy.[129][Level of evidence: 3iiiA] (Refer to the PDQ summary on Childhood Rhabdomyosarcoma Treatment for more information.)
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with malignant triton tumor are eligible for this trial.
Pericytic (Perivascular) Tumors
Myopericytoma
Infantile hemangiopericytoma is a subtype of myopericytoma.
Hemangiopericytoma is a highly vascularized tumor of uncertain origin.
Histology
Histologically, hemangiopericytomas are composed of packed round or fusiform cells that are arranged around a complex vasculature, forming many branch-like structures. Hyalinization is often present. Infantile hemangiopericytomas have similar histology but many are multilobular with vasculature outside the tumor mass.[130]
Treatment and outcome
Treatment of infantile hemangiopericytomas includes the following:
- Chemotherapy.
In a series of 17 children, the differences in metastatic potential and response to treatment were clearly demonstrated for adult and infantile hemangiopericytomas.[131] Eleven children were older than 1 year. Several of these patients had disease in the lymph nodes or lungs. Six patients with stage II or III disease progressed and died. Three patients with stage I disease survived, although one had recurrence in the lungs. Six patients had infantile hemangiopericytoma, most were greater than stage I (5 of 6). All six patients survived and three had good responses to vincristine, actinomycin, and cyclophosphamide. Hemangiopericytoma in children younger than 1 year seems to have a better prognosis than in children older than 1 year.[132-134]
Infantile myofibromatosis
This entity is a fibrous tumor of infancy and childhood that most commonly presents in the first 2 years of life.[135] The lesion can present as a single subcutaneous nodule (myofibroma) most commonly involving the head and neck region or lesions can affect multiple skin areas, muscle, and bone (myofibromatosis).[136-139]
An autosomal dominant form of the disease has been described and it is associated with germline mutations of the PDGFRB gene.[140]
Treatment
These lesions have an excellent prognosis and can regress spontaneously.
About one-third of cases with multicentric involvement will also have visceral involvement, and the prognosis for these patients is poor.[138,139,141] The use of combination therapy with vincristine/dactinomycin and vinblastine/methotrexate have proven effective in cases of multicentric disease with visceral involvement and in cases in which the disease has progressed and has threatened the life of the patient (e.g., upper airway obstruction).[138,139,142]
Tumors of Uncertain Differentiation
Tumors of uncertain differentiation include the following tumor subtypes:
- Alveolar soft part sarcoma.
- Clear cell sarcoma of soft tissue.
- Desmoplastic small round cell tumor.
- Epithelioid sarcoma.
- Extrarenal rhabdoid tumor.
- Extraskeletal myxoid chondrosarcoma.
- Neoplasms with perivascular epithelioid cell differentiation (PEComa NOS, malignant)
- Primitive neuroectodermal tumor (PNET)/extraskeletal Ewing tumor.
- Synovial sarcoma (NOS, spindle cell, and biphasic varieties).
Alveolar soft part sarcoma
Alveolar soft parts sarcomas account for 1.4% of soft tissue sarcomas in patients younger than 20 years (refer to Table 1).
Clinical presentation
The median age at presentation is 25 years, and alveolar soft part sarcoma most commonly arises in the extremities but can occur in the oral and maxillofacial region.[143-145] Alveolar soft part sarcoma in children can present with evidence of metastatic disease.[146]
Molecular features
This tumor of uncertain histogenesis is characterized by a consistent chromosomal translocation t(X;17)(p11.2;q25) that fuses the ASPSCR1 gene with the TFE3 gene.[147,148]
Prognosis
Alveolar soft part sarcoma in children may have an indolent course.[146] Patients with alveolar soft part sarcoma may relapse several years after a prolonged period of apparent remission.[149] Because these tumors are rare, all children with alveolar soft part sarcoma should be considered for enrollment in prospective clinical trials.
In a series of 19 treated patients, one group reported a 5-year OS rate of 80%, a 91% OS rate for patients with localized disease, a 100% OS rate for patients with tumors 5 cm or smaller, and a 31% OS rate for patients with tumors larger than 5 cm.[150] In another series of 33 patients, OS was 68% at 5 years from diagnosis and 53% at 10 years from diagnosis. Survival was better for smaller tumors (≤5 cm) and completely resected tumors.[151][Level of evidence: 3iiA] Delayed metastases to the brain and lung are uncommon.[143] A retrospective review of children and young adults younger than 30 years (median age, 17 years; range, 1.5–30 years) from four institutions identified 69 patients treated primarily with surgery between 1980 and 2014.[152][Level of evidence: 3iiA] The ASPL-TFE3 translocation was present in all 26 patients tested. There were 19 patients with Intergroup Rhabdomyosarcoma Study (IRS) postsurgical staging group I tumors (28%), 7 patients with IRS group II tumors (10%), 5 patients with IRS group III tumors (7%), and 38 patients with IRS group IV tumors (55%). The 5-year EFS was 80% and the OS was 87% for the 31 patients with localized tumors (IRS postsurgical groups I, II, and III). The 5-year EFS was 7% and the OS was 61% for the 38 patients with metastatic tumors (IRS postsurgical group IV).
Treatment
Treatment options for alveolar soft part sarcoma include the following:
The standard approach is complete resection of the primary lesion.[150] If complete excision is not feasible, radiation therapy should be administered. A study from China reported on 18 patients with alveolar soft part sarcoma of the oral and maxillofacial region; 15 patients were younger than 30 years.[145][Level of evidence: 3iiDii] Surgical removal with negative margins was the primary treatment. All patients survived, and only one patient had metastatic disease recurrence.
A series of 51 pediatric patients aged 0 to 21 years with alveolar soft part sarcoma found an OS rate at 10 years of 78% and an EFS rate of about 63%. Patients with localized disease (n = 37) had a 10-year OS of 87%, and the 14 patients with metastases at diagnosis had a 10-year OS of 44%, partly resulting from surgical removal of primary tumor and lung metastases in some patients. Only 3 of 18 patients (17%) with measurable disease had a response to conventional antisarcoma chemotherapy, but two of four patients treated with sunitinib had a partial response.[143][Level of evidence: 3iiiA] There have been sporadic reports of objective responses to interferon-alpha and bevacizumab.[143,153,154]
A small retrospective study of nine adult patients with metastatic alveolar soft part sarcoma treated with sunitinib reported partial response in five patients and stable disease in two patients.[155][Level of evidence: 3iiiDiv] In a phase II trial of cediranib, an inhibitor of all three known vascular epidermal growth factor receptors, 15 of 43 adult patients (35%) with metastatic alveolar soft part sarcoma had a partial response.[156][Level of evidence: 3iiDiv]
There have been no open trials for patients with metastatic alveolar soft part sarcoma.
Treatment options under clinical evaluation for alveolar soft part sarcoma
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- NCT00942877 (Phase II Study of Cediranib [AZD2171] in Patients With Alveolar Soft Part Sarcoma): A phase II study of cediranib in patients with alveolar soft part sarcoma is being conducted in patients younger than 16 years at the Clinical Center of the National Institutes of Health.
- NCT01391962 (Sunitinib or Cediranib for Alveolar Soft Part Sarcoma): A phase II trial in which patients with metastatic alveolar soft part sarcoma are randomly assigned to either sunitinib or cediranib monotherapy, with crossover at disease progression. Patients aged 16 years and older are eligible. This study is being conducted at the Clinical Center of the National Institutes of Health.
Clear cell sarcoma of soft tissue
Clear cell sarcoma (formerly and inappropriately called malignant melanoma of soft parts) is a rare soft tissue sarcoma that typically involves the deep soft tissues of the extremities. It is also called clear cell sarcoma of tendons and aponeuroses. The tumor often affects adolescents and young adults.
Patients who have small, localized tumors with low mitotic rate and intermediate histologic grade fare best.[157]
Clinical presentation
The tumor most commonly affects the lower extremity, particularly the foot, heel, and ankle.[158,159] It has a high propensity for nodal dissemination, especially metastases to regional lymph nodes (12%–43%).[159,160] The tumor typically has an indolent clinical course.
Molecular features
Clear cell sarcoma of soft tissue is characterized by an EWS-ATF1 fusion.[161]
Treatment
Treatment options for clear cell sarcoma of soft tissue include the following:
In a series of 28 pediatric patients reported by the Italian and German Soft Tissue Cooperative Studies, the median age at diagnosis was 14 years and the lower extremity was the most common primary site (50%). Surgery with or without radiotherapy is the treatment of choice and offers the best chance for cure. In this series, 12 of 13 patients with completely resected tumors were cured. For patients with more advanced disease the outcome is poor and chemotherapy is rarely effective.[162]; [163][Level of evidence: 3iiDii]
Desmoplastic small round cell tumor
Desmoplastic small round cell tumor is a rare primitive sarcoma.
Clinical presentation
Desmoplastic small round cell tumor most frequently involves the abdomen, pelvis, or tissues around the testes, but it may occur in the kidney.[164-167] The tumor occurs more commonly in males and may spread to the lungs and elsewhere. Peritoneal and pelvic lesions frequently have widespread peritoneal implants.[168]
In a large, single-institution series of 65 patients, a correlation was made between computed tomography (CT) scans in most patients and positron-emission tomography (PET)/CT scans in 11 patients. PET/CT scans had very few false-negative results and detected metastatic sites missed on conventional CT scans.[168]
Molecular features
Cytogenetic studies of these tumors have demonstrated the recurrent translocation t(11;22)(p13;q12), which has been characterized as a fusion of the WT1 and EWS genes.[167,169] The WT1-EWS fusion confirms the diagnosis of desmoplastic small round cell tumor.
Prognosis
The overall prognosis for desmoplastic small round cell tumor remains extremely poor, with reported rates of death at 90%. Greater than 90% tumor resection either at presentation or after preoperative chemotherapy may be a favorable prognostic factor for OS.[170,171]; [172][Level of evidence: 3iiiA]
Treatment
There is no standard approach to the treatment of desmoplastic small round cell tumor.
Treatment options for desmoplastic small round cell tumor include the following:
- Surgery.
- Chemotherapy followed by surgery.
- Radiation therapy.
Complete surgical resections are rare, and the overall prognosis for desmoplastic small round cell tumor remains extremely poor, with reported rates of death at 90%. Treatment may include chemotherapy, surgery, and radiation therapy. Multiagent chemotherapy analogous to that used for sarcomas has been used, as well as total abdominal radiation therapy.[164,165,170,173-176]
A single-institution study reported that five of five patients with recurrent desmoplastic small round cell tumor had partial responses to treatment with the combination of vinorelbine, cyclophosphamide, and temsirolimus.[177]
The Center for International Blood and Marrow Transplant Research (CIBMTR) analyzed patients with desmoplastic small round cell tumor in their registry who received consolidation with high dose chemotherapy and autologous stem cell reconstitution.[178] While this retrospective registry analysis suggested some benefit for this approach, other investigators have abandoned the approach because of excessive toxicity and lack of efficacy.[170]
Epithelioid sarcoma
Epithelioid sarcoma is a rare mesenchymal tumor of uncertain histogenesis which displays multilineage differentiation.[179]
Clinical presentation
Epithelioid sarcoma commonly presents as a slowly growing firm nodule based in the deep soft tissue; the proximal type predominantly affects adults and involves the axial skeleton and proximal sites. The tumor is highly aggressive and has a propensity for lymph node metastases.
Molecular features
Epithelioid sarcoma is characterized by inactivation of the SMARCB1 gene, which is present in both conventional and proximal types of epithelioid sarcoma.[180] This abnormality leads to increased dependence on EZH2 and tumor formation.[181]
Treatment
Treatment options for epithelioid sarcoma include the following
- Chemotherapy.
- Surgery.
- Surgery preceded or followed by radiation therapy.
Patients should be carefully evaluated for the presence of involved lymph nodes; suspicious lymph nodes should be biopsied. Surgical removal of primary and recurrent tumor(s) is the most effective treatment.[182][Level of evidence: 3iiiA]
In a review of 30 pediatric patients with epithelioid sarcoma (median age at presentation, 12 years), responses to chemotherapy were reported in 40% of patients using sarcoma-based regimens, and 60% of patients were alive at 5 years after initial diagnosis.[183] A single-institution retrospective review of 20 patients, including children and adults (median age, 27.3 years), found no difference in the probability of recurrence between patients who received chemotherapy and those who did not receive chemotherapy and suggested that radiation therapy may be useful.[182]
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- NCT02601937 (A Phase 1 Study of the EZH2 Inhibitor Tazemetostat in Pediatric Subjects With Relapsed or Refractory INI1-Negative Tumors or Synovial Sarcoma): Patients with INI1-negative tumors are eligible for targeted treatment with an EZH2 inhibitor. This is a phase I, open-label, dose-escalation, and dose-expansion study with a twice-daily oral dose of tazemetostat.
Extrarenal (extracranial) rhabdoid tumor
Malignant rhabdoid tumors were first described in children with renal tumors in 1981 (refer to the PDQ summary on Wilms Tumor and Other Childhood Kidney Tumors Treatment for more information) and were later found in a variety of extrarenal sites. These tumors are uncommon and highly malignant, especially in children younger than 2 years.
Extrarenal (extracranial) rhabdoid tumors account for 2% of soft tissue sarcoma in patients younger than 20 years (refer to Table 1).
Molecular features
The first sizeable series of 26 children with extrarenal extracranial malignant rhabdoid tumor of soft tissues came from patients enrolled on the Intergroup Rhabdomyosarcoma Studies I through III during a review of pathology material. Only five patients (19%) were alive without disease.[184] Later, investigation of children with atypical teratoid/rhabdoid tumors of the brain, as well as those with renal and extrarenal malignant rhabdoid tumors, found germline and acquired mutations of the SMARCB1 gene in all 29 tumors tested.[185] Rhabdoid tumors may be associated with germline mutations of the SMARCB1 gene and may be inherited from an apparently unaffected parent.[186] This observation was extended to 32 malignant rhabdoid tumors at all sites in patients whose mean age at diagnosis was 12 months.[187]
Prognosis
In a Surveillance, Epidemiology, and End Results (SEER) study of 229 patients with renal, central nervous system, and extrarenal malignant rhabdoid tumor, patients aged 2 to 18 years, limited extent of tumor, and delivery of radiation therapy were shown to affect the outcome favorably compared with other patients (P < .002 for each comparison). Site of the primary tumor was not prognostically significant. OS at 5 years was 33%.[188]
Treatment
Treatment includes surgical removal when possible, chemotherapy as used for soft tissue sarcomas (but no single regimen is currently accepted as best), and radiation therapy.[189][Level of evidence: 3iA]; [190,191][Level of evidence: 3iiiB]
Responses to alisertib have been documented in four patients with central nervous system (CNS) atypical teratoid/rhabdoid tumors.[192] (Refer to the PDQ summary on Childhood Central Nervous System Atypical Teratoid/Rhabdoid Tumor Treatment summary for more information about CNS atypical teratoid/rhabdoid tumors.)
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- NCT02601937 (A Phase 1 Study of the EZH2 Inhibitor Tazemetostat in Pediatric Subjects With Relapsed or Refractory INI1-Negative Tumors or Synovial Sarcoma): Patients with INI1-negative tumors are eligible for targeted treatment with an EZH2 inhibitor. This is a phase I, open-label, dose-escalation, and dose-expansion study with a twice-daily oral dose of tazemetostat.
Extraskeletal myxoid chondrosarcoma
Extraskeletal myxoid chondrosarcoma is relatively rare among soft tissue sarcomas, representing only 2.3% of all soft tissue sarcoma.[193] It has been reported in children and adolescents.[194]
Molecular features
Extraskeletal myxoid chondrosarcoma is a multinodular neoplasm. The rounded cells are arranged in cords and strands in a chondroitin sulfate myxoid background. Several cytogenetic abnormalities have been identified (refer to Table 2), with the most frequent being the translocation t(9;22)(q22;q12), involving the EWSR1/NR4A3 genes.[195]
Prognosis
The tumor has traditionally been considered of low-grade malignant potential.[196] However, recent reports from large institutions showed that extraskeletal myxoid chondrosarcoma has significant malignant potential, especially if patients are followed for a long time.[197,198] Patients tend to have slow protracted courses. Nodal involvement has been well described. Local recurrence (57%) and metastatic spread to lungs (26%) have been reported.[198]
Treatment
Treatment options for extraskeletal myxoid chondrosarcoma include the following:
- Surgery.
- Radiation therapy.
The therapeutic benefit of chemotherapy has not been established. Aggressive local control and resection of metastases led to OS of 87% at 5 years and 63% at 10 years. Tumors were relatively resistant to radiation therapy.[197]
There may be potential genetic targets for small molecules, but these should be studied as part of a clinical trial. In an adult study, six of ten patients who received sunitinib achieved a partial response.[199]
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- NCT02601937 (A Phase 1 Study of the EZH2 Inhibitor Tazemetostat in Pediatric Subjects With Relapsed or Refractory INI1-Negative Tumors or Synovial Sarcoma): Patients with INI1-negative tumors are eligible for targeted treatment with an EZH2 inhibitor. This is a phase I, open-label, dose-escalation, and dose-expansion study with a twice-daily oral dose of tazemetostat.
Neoplasms with perivascular epithelioid cell differentiation (PEComas)
Risk factors and molecular features
Benign PEComas are common in tuberous sclerosis, an autosomal dominant syndrome that also predisposes to renal cell cancer and brain tumors. Tuberous sclerosis is caused by germline inactivation of either TSC1 (9q34) or TSC2 (16p13.3), and the same tumor suppressor genes are inactivated somatically in sporadic PEComas.[200] Inactivation of either gene results in stimulation of the mTOR pathway, providing the basis for the treatment of nonsurgically curable PEComas with mTOR inhibitors.[201,202] A small proportion of PEComas have TFE3 rearrangements with fusions involving various genes including SFPQ/PSF and RAD51B.[203]
Clinical presentation
PEComas occur in various rare gastrointestinal, pulmonary, gynecologic, and genitourinary sites. Soft tissue, visceral, and gynecologic PEComas are more commonly seen in middle-aged female patients and are usually not associated with the tuberous sclerosis complex.[204] The disease course may be indolent.
Prognosis
Most PEComas have a benign clinical course, but malignant behavior has been reported and can be predicted based on the size of the tumor, mitotic rate, and presence of necrosis.[205]
Treatment
Treatment options have not been defined. Treatment may include surgery or observation followed by surgery when the tumor is large.[206]
Clinical activity with mTOR inhibitors, such as sirolimus, in tumors with evidence of mTORC1 activation and TSC loss has been well documented.[207]
Primitive neuroectodermal tumor (PNET)/extraskeletal Ewing tumor
(Refer to the PDQ summary on Ewing Sarcoma Treatment for more information.)
Synovial sarcoma
Synovial sarcoma accounts for 9% of soft tissue sarcomas in patients younger than 20 years (refer to Table 1).
Synovial sarcoma is one of the most common nonrhabdomyosarcomatous soft tissue sarcomas in children and adolescents. In a 1973 to 2005 SEER review, 1,268 patients with synovial sarcoma were identified. Approximately 17% of these patients were children and adolescents and the median age at diagnosis was 34 years.[208]
Histologic classification
Synovial sarcoma can be subclassified as the following types:
- Synovial sarcoma, NOS.
- Synovial sarcoma, spindle cell.
- Synovial sarcoma, biphasic.
Clinical presentation
The most common tumor location is the extremities, followed by trunk and head and neck.[208] Rarely, a synovial sarcoma may arise in the heart or pericardium.[209]
The most common site of metastasis is the lung.[210,211] The risk of metastases is highly influenced by tumor size; it is estimated that patients with tumors that are larger than 5 cm have a 32-fold risk of developing metastases when compared with other patients.
Diagnostic evaluation
The diagnosis of synovial sarcoma is made by immunohistochemical analysis, ultrastructural findings, and demonstration of the specific chromosomal translocation t(x;18)(p11.2;q11.2). This abnormality is specific for synovial sarcoma and is found in all morphologic subtypes. Synovial sarcoma results in rearrangement of the SYT gene on chromosome 18 with one of the subtypes (1, 2, or 4) of the SSX gene on chromosome X.[212,213] It is thought that the SYT/SSX18 transcript promotes epigenetic silencing of key tumor suppressor genes.[214]
In one report, reduced INI1 nuclear reactivity on immunohistochemical staining was seen in 49 cases of synovial sarcoma, suggesting that this pattern may help distinguish synovial sarcoma from other histologies.[215]
Prognosis
Patients younger than 10 years have more favorable outcomes and clinical features, including extremity primaries, smaller tumors, and localized disease, than do older patients.[208,216] A meta-analysis also suggested that response to chemotherapy was correlated with improved survival.[217]
The following studies have reported multiple factors associated with unfavorable outcomes:
- In a retrospective analysis of synovial sarcoma in children and adolescents who were treated in Germany and Italy, tumor size (>5 cm or ≤5 cm in greatest dimension) was an important predictor of EFS.[218] In this analysis, local invasiveness conferred an inferior probability of EFS, but surgical margins were not associated with clinical outcome.
- In a single-institution retrospective analysis of 111 patients with synovial sarcoma who were younger than 22 years at diagnosis, larger tumor size, greater depth in tissue, greater local invasiveness, and more proximal tumor location were associated with poorer OS.[219][Level of evidence: 3iiA]
- A multicenter analysis of 219 children from various treating centers including Germany, SJCRH, Instituto Tumori, and MD Anderson Cancer Center reported an estimated 5-year OS of 80% and EFS rate of 72%.[217] In this analysis, an interaction between tumor size and invasiveness was observed; in multivariate analysis, patients with large or invasive tumors or with Intergroup Rhabdomyosarcoma Study Clinical Group III disease (localized, incompletely resected or with biopsy only) and IV (metastases at diagnosis) had decreased OS. Treatment with radiation therapy was related to improved OS (hazard ratio, 0.4; 95% confidence interval, 0.2–0.7). In Intergroup Rhabdomyosarcoma Study Group III patients, objective response to chemotherapy (18 of 30 [60%]) correlated with improved survival. In adults, factors such as International Union Against Cancer/American Joint Committee on Cancer stage III and stage IVA, tumor necrosis, truncal location, elevated mitotic rate, age, and histologic grade have been associated with a worse prognosis.[220-222]
- Expression and genomic index prognostic signatures have been studied in synovial sarcoma. Complex genomic profiles, with greater rearrangement of the genome, are more common in adults than in younger patients with synovial sarcoma and are associated with a higher risk of metastasis.[223]
- A review of 84 patients with localized synovial sarcoma who had information on fusion status (SYT-SSX) and histologic grading found no difference in OS according to these criteria. However, for tumor size at diagnosis, the study showed that patients with tumors between 5 cm and 10 cm had a worse prognosis than those with smaller tumors (P = .02), and patients with tumors larger than 10 cm had even worse OS (P = .0003).[224][Level of evidence: 3iiiA]
- The German CWS group reviewed 27 evaluable patients younger than 21 years with pulmonary metastases among 296 patients with synovial sarcoma. Metastases involved the lungs in all patients. The 5-year EFS rate was 26%, and the OS rate was 30%. The most important prognostic factor at presentation was that the metastases were limited to one lesion in one lung or one lesion in both lungs (a group they termed oligometastatic). Treatment elements associated with superior survival were adequate local therapy of the primary tumor and, if feasible, for the metastases. The use of whole-lung irradiation did not correlate with better outcomes.[225][Level of evidence: 3iiA]
Survival after relapse is poor (30% at 5 years). Factors associated with outcome after relapse include duration of first remission (> or ≤ 18 months) and lack of a second remission.[226]
Treatment
Treatment options for synovial sarcoma include the following:
The COG and the European Pediatric Soft Tissue Sarcoma Study Group reported a combined analysis of 60 patients younger than 21 years with localized synovial sarcoma prospectively assigned to surgery without adjuvant radiation therapy or chemotherapy.[227] Enrollment was limited to patients with initial complete resection with histologically free margins, with a grade 2 tumor of any size or a grade 3 tumor 5 cm or smaller. The 3-year EFS was 90% (median follow-up, 5.2 years; range, 1.9–9.1). All eight events were local tumor recurrence; no metastatic recurrences were seen. All patients with recurrent disease were effectively treated with salvage therapy, resulting in 100% OS.
Synovial sarcoma appears to be more sensitive to chemotherapy than many other soft tissue sarcomas, and children with synovial sarcoma seem to have a better prognosis when compared with adults.[11,211,222,228-232] The most commonly used regimens for the treatment of synovial sarcoma incorporate ifosfamide and doxorubicin.[217,231,233] Response rates to the ifosfamide and doxorubicin regimen are higher than in other nonrhabdomyosarcomatous soft tissue sarcomas.[234]
Several studies have reported the following chemotherapy-associated treatment findings:
- Several treatment centers advocate postoperative chemotherapy after resection and radiation therapy of synovial sarcoma in children and young adults.[217,218,235-237]
- The International Society of Pediatric Oncology-Malignant Mesenchymal Tumors studies showed that select patients (young age, <5 cm resected tumors) with nonmetastatic synovial sarcoma can have excellent outcome in the absence of radiation, but it is still unclear whether that approach obviates an advantage of radiation for local or regional control.[236]
- A German trial suggested a benefit for postoperative chemotherapy in children with synovial sarcoma.[237]
- A meta-analysis also suggested that chemotherapy may provide benefit.[217]
- In the most recent COG ARST0332 (NCT00346164) study, 129 patients with synovial sarcoma were prospectively treated using a risk-based therapy program (as detailed in the prognosis section), of which 43 were categorized as low risk, 66 as intermediate risk, and 20 as high risk. At a median follow-up of 2.6 years, 3-year EFS for low-, intermediate-, and high-risk groups were 83%, 79%, and 16%, respectively. The use of risk factor–directed therapy accurately predicted outcomes.[238]
- The European Pediatric Soft Tissue Sarcoma Study Group performed a prospective study of patients younger than 21 years with synovial sarcoma (CCLG-EPSSG-NRSTS-2005 [NCT00334854]).[239][Level of evidence: 3iiA] Patients were stratified into the following three risks groups and nonrandomly assigned to treatment by risk group:
- Low-risk patients had Intergroup Rhabdomyosarcoma Study (IRS) group I tumors less than 5 cm in size and nonaxial primary tumors.
- Intermediate-risk patients had no axial primary tumors and IRS group I tumors greater than 5 cm or IRS group II tumors.
- High-risk patients included all patients with axial primary sites (head and neck, lung and pleura, trunk, retroperitoneal), IRS group III tumors, or N1 tumors.
Outcomes for patients treated on the CCLG-EPSSG-NRSTS-2005 trial are described in Table 9.
Table 9. Event-Free Survival (EFS) and Overall Survival (OS) in Patients With Low-, Intermediate-, and High-Risk Synovial Sarcoma Treated on the CCLG-EPSSG-NRSTS-2005 Trial Risk Group Treatment 3-Year EFS (%) 3-Year OS (%) IRS = Intergroup Rhabdomyosarcoma Study; RT = radiation therapy. aChemotherapy was ifosfamide/doxorubicin, with doxorubicin omitted during radiation therapy. b59.4 Gy in cases without the option of secondary resection; 50.4 Gy as preoperative radiation therapy; 50.4, 54, and 59.4 Gy as postoperative radiation therapy, in the case of R0, R1, and R2 resections, respectively (no additional radiation therapy in the case of secondary complete resections with free margins, in children younger than 6 years). Low Surgery alone 92 100 Intermediate Surgery, 3–6 cycles chemotherapya ± RTb 91 100 High (IRS group III) 3 cycles of chemotherapya surgery, 3 additional cycles of chemotherapy, ± RTb 77 94 High (axial primary sites) Surgery, 6 cycles of chemotherapya, RTb 78 100
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- ADP 04511 (NCT01343043) (A Pilot Study of Genetically Engineered NY-ESO-1 Specific [c259] T Cells in HLA-A2+ Patients With Synovial Sarcoma): Patients with unresectable, metastatic, or recurrent synovial sarcoma undergo apheresis. Cells are shipped to a central laboratory where they undergo NY-ESO-1 transduction, expansion, and cryopreservation. Patients undergo lymphodepletion with fludarabine and cyclophosphamide, followed by an infusion of autologous transfected cells. Eligibility is restricted to patients with HLA type A2+, age older than 4 years, and weight greater than 18 kg.
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with alveolar soft part sarcoma, clear cell sarcoma of soft tissue, epithelioid sarcoma, extraskeletal myxoid chondrosarcoma, PEComa, and synovial sarcoma are eligible for this trial.
- NCT02601937 (A Phase 1 Study of the EZH2 Inhibitor Tazemetostat in Pediatric Subjects With Relapsed or Refractory INI1-Negative Tumors or Synovial Sarcoma): Patients with INI1-negative tumors are eligible for targeted treatment with an EZH2 inhibitor. This is a phase I, open-label, dose-escalation, and dose-expansion study with a twice-daily oral dose of tazemetostat.
- ADVL1522 (NCT02452554) (Lorvotuzumab Mertansine in Treating Younger Patients with Relapsed or Refractory Wilms Tumor, Rhabdomyosarcoma, Neuroblastoma, Pleuropulmonary Blastoma, Malignant Peripheral Nerve Sheath Tumor, or Synovial Sarcoma): This is a phase II study of IMGN901 (lorvotuzumab mertansine) in children with relapsed or refractory Wilms tumor, rhabdomyosarcoma, neuroblastoma, pleuropulmonary blastoma, malignant peripheral nerve sheath tumor, and synovial sarcoma. This trial is studying the effects of IMGN901, an antibody-drug conjugate that links a potent antimitotic to antibodies that target CD56.
Undifferentiated/unclassified sarcoma
Patients with undifferentiated soft tissue sarcoma had been eligible for participation in rhabdomyosarcoma trials coordinated by the Intergroup Rhabdomyosarcoma Study Group and the COG from 1972 to 2006. The rationale was the observation that patients with undifferentiated soft tissue sarcoma had similar sites of disease and outcome as those with alveolar rhabdomyosarcoma. Therapeutic trials for adults with soft tissue sarcoma include patients with undifferentiated soft tissue sarcoma and other histologies, which are treated similarly, using ifosfamide and doxorubicin, and sometimes with other chemotherapy agents, surgery, and radiation therapy.
In the COG ARST0332 (NCT00346164) trial, patients with high-grade undifferentiated sarcoma were treated with an ifosfamide and doxorubicin-based regimen and were treated with rhabdomyosarcoma-directed therapies in previous Intergroup Rhabdomyosarcoma Study Group studies with a 5-year survival estimate for nonmetastatic patients of 72%.[240][Level of evidence: 3iiA] Currently, these patients are eligible for the COG open ARST1321 (NCT02180867) trial for patients with nonrhabdomyosarcomatous soft tissue sarcoma.
Undifferentiated pleomorphic sarcoma/malignant fibrous histiocytoma (high-grade)
At one time, malignant fibrous histiocytoma was the single most common histotype among adults with soft tissue sarcomas. Since it was first recognized in the early 1960s, malignant fibrous histiocytoma has been plagued by controversy in terms of both its histogenesis and its validity as a clinicopathologic entity. The latest WHO classification no longer includes malignant fibrous histiocytoma as a distinct diagnostic category but rather as a subtype of an undifferentiated pleomorphic sarcoma.[241]
This entity accounts for 2% to 6% of all childhood soft tissue sarcomas.[242] These tumors can arise in previously irradiated sites or as a second malignancy in patients with retinoblastoma.
These tumors occur mainly in the second decade of life. In a series of ten patients, the median age was 10 years and the tumor was most commonly located in the extremities. In this series, all tumors were localized and five of nine (for whom follow-up was available) were alive and in first remission.[242] In another series of 17 pediatric patients with malignant fibrous histiocytoma, the median age at diagnosis was 5 years and the extremities were involved in eight cases.[243] All patients with metastatic disease died and two patients experienced a clinical response to a doxorubicin-based regimen.
(Refer to the PDQ summary on Osteosarcoma and Malignant Fibrous Histiocytoma of Bone Treatment for more information about the treatment of malignant fibrous histiocytoma of bone.)
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with undifferentiated epithelial sarcoma, undifferentiated pleomorphic sarcoma, undifferentiated round cell sarcoma, and undifferentiated spindle cell sarcoma are eligible for this trial.
Vascular Tumors
Vascular tumors vary from hemangiomas, which are always considered benign, to angiosarcomas, which are highly malignant.[244] Vascular tumors include the following tumor subtypes:
Angiosarcoma of the soft tissue
Incidence
Angiosarcoma is a rare (accounting for 2% of sarcomas), aggressive, vascular tumor that can arise in any part of the body, but is more common in the soft tissue. Angiosarcoma has an estimated incidence of 2 cases per 1 million; in the United States, it annually affects approximately 600 people who are typically aged 60 to 70 years.[245]
Angiosarcomas are extremely rare in children and it is unclear if the pathophysiology of this tumor is different in the pediatric population. Cases have been reported in neonates and toddlers, with presentation of multiple cutaneous lesions and liver lesions, some of which are GLUT1 positive.[246-249] Most angiosarcomas involve the skin and superficial soft tissue, although the liver, spleen, and lung can be affected; bone is rarely affected.
Risk factors
Established risk factors include vinyl chloride exposure, radiation exposure, and chronic lymphedema from any cause, including Stewart-Treves syndrome.[250]
Pathology and biology
Angiosarcomas are largely aneuploid tumors. The rare cases of angiosarcoma that arise from benign lesions such as hemangiomas have a distinct pathway that needs to be investigated. MYC amplification is seen in radiation-induced angiosarcoma. KDR-VEGFR2 mutations and FLT4-VEGFR3 amplifications have been seen with a frequency of less than 50%.[250]
Histopathologic diagnosis can be very difficult because there can be areas of varied atypia. The common feature is an irregular network of channels in a dissective pattern along dermal collagen bundles. There is varied cellular shape, size, mitosis, endothelial multilayering, and papillary formation. Epithelioid cells can also be present. Necrosis and hemorrhage are common. Tumors stain for factor VIII, CD31, and CD34. Some liver lesions can mimic infantile hemangiomas and have focal GLUT1 positivity. Nomenclature of these liver lesions has been difficult and confusing with use of terminology from 1971 (e.g., type I hemangioendothelioma: infantile hemangioma; type II hemangioendothelioma: low-grade angiosarcoma; type III hemangioendothelioma: high-grade angiosarcoma).[247]
Treatment of angiosarcoma of the soft tissue
Treatment options for angiosarcoma of the soft tissue include the following:
- Surgery (localized disease).
- Radiation therapy (localized cutaneous disease in adults).
- Surgery, chemotherapy, and radiation therapy (metastatic disease).
Localized disease is cured by aggressive surgery. Complete surgical excision appears to be crucial for angiosarcomas and lymphangiosarcomas despite evidence of tumor shrinkage in some patients who were treated with local or systemic therapy.[248,251-253] A review of 222 patients (median age, 62 years; range, age 15–90 years) showed an overall disease-specific survival (DSS) rate of 38% at 5 years. Five-year DSS was 44% in 138 patients with localized, resected tumors but only 16% in 43 patients with metastases at diagnosis.[253] Data on liver transplantation for localized angiosarcoma are limited.[254][Level of evidence: 3iiA]
Localized disease, especially cutaneous angiosarcoma, can be treated with radiation therapy. Most of these reported cases are in adults.[255]
Multimodal treatment with surgery, systemic chemotherapy, and radiation therapy is used for metastatic disease, although it is rarely curative.[256] Disease control is the objective in metastatic angiosarcoma, with published progression-free survival rates between 3 months and 7 months [257] and a median overall survival (OS) rate of 14 months to 18 months.[258] In both adults and children, 5-year OS rates between 20% and 35% are reported.[248,249,259]
In a child diagnosed with angiosarcoma secondary to malignant transformation from infantile hemangioma, response to treatment with bevacizumab, a monoclonal antibody against vascular endothelial growth factor, combined with systemic chemotherapy, has been reported.[246,256] A report of eight cases of liver angiosarcoma in children highlighted the misuse of the term hemangioendothelioma and the importance of early diagnosis and treatment of these tumors.[260]
Biologic agents that inhibit angiogenesis have shown activity in adults with angiosarcoma.[247,259]
Treatment options under clinical evaluation for angiosarcoma of the soft tissue
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery [PAZNTIS]): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with angiosarcoma of the soft tissue are eligible for this trial.
- NCT01532687 (Gemcitabine Hydrochloride With or Without Pazopanib Hydrochloride in Treating Patients With Refractory Soft Tissue Sarcoma): This randomized phase II trial studies how well gemcitabine hydrochloride works with or without pazopanib hydrochloride in treating patients with refractory soft tissue sarcoma.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Epithelioid hemangioendothelioma
Incidence and outcome
This tumor was first described in soft tissue by Weiss and Enzinger in 1982. Epithelioid hemangioendotheliomas can occur at younger ages, but the peak incidence is in the fourth and fifth decades of life. The tumors can have an indolent or very aggressive course, with overall survival of 73% at 5 years. There are case reports of patients with untreated multiple lesions who have a very benign course compared with other patients who have a very aggressive course. Some pathologists have tried to stratify patients to evaluate risks and adjust treatment, but more research is needed.[261-267]
The presence of effusions, tumor size larger than 3 cm, and a high mitotic index (>3 mitoses/50 high-power fields) have been associated with unfavorable outcomes.[263]
Pathology and biology
A WWTR1-CAMTA1 gene fusion has been found in a large percentage of patients; less commonly, a YAP1-TFE3 gene fusion has been reported.[261] These fusions are not directly targetable with current medicines. Monoclonality has been described in multiple liver lesions, suggesting a metastatic process.
Histologically, these lesions are characterized as epithelioid lesions arranged in nests, strands, and trabecular patterns, with infrequent vascular spaces. Features that may be associated with aggressive clinical behavior include cellular atypia, one or more mitoses per 10 high-power fields, an increased proportion of spindled cells, focal necrosis, and metaplastic bone formation.[263]
The number of pediatric patients reported in the literature is limited.
Clinical presentation and diagnostic evaluation
Common sites of involvement are liver alone (21%), liver plus lung (18%), lung alone (12%), and bone alone (14%).[263,268,269] Clinical presentation depends on site of involvement, as follows:
- Liver: Hepatic nodules have central vascularity on ultrasound, contrast-enhancing lesions by computed tomography, and low T1 signal and moderate T2 signal on magnetic resonance imaging.
- Lung: Pulmonary epithelioid hemangioendothelioma may be an asymptomatic finding on chest x-ray or be associated with pleuritic pain, hemoptysis, anemia, and fibrosis.
- Bone: Bone metastasis may be associated with pathologic fracture. On x-rays, they are well-defined osteolytic lesions and can be multiple or solitary.
- Soft tissue: Thirty percent of soft tissue cases are associated with metastases, and when present, can have a very aggressive course, with limited response to chemotherapy.
- Skin: Cutaneous lesions can be raised and nodular or can be warm red-brown plaques.
Treatment of epithelioid hemangioendothelioma
Treatment options for epithelioid hemangioendothelioma include the following:
- Observation.
- Surgery.
- Immunotherapy.
- Targeted therapy.
- Chemotherapy.
For indolent cases, observation is warranted. For more aggressive cases, multiple medications have been used, including interferon, thalidomide, sorafenib, pazopanib, and sirolimus.[270] The most aggressive cases are treated with angiosarcoma-type chemotherapy. Surgery is used when possible. Liver transplantation has been used with aggressive liver lesions, both with and without metastases.[263,271-274]
Treatment options under clinical evaluation for epithelioid hemangioendothelioma
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- NCT03148275 (Trametinib in Treating Patients with Epithelioid Hemangioendothelioma That Is Metastatic, Locally Advanced, or Cannot Be Removed by Surgery): This is a phase II trial assessing the efficacy of trametinib, with patient-reported outcomes as secondary aims.
- NCT01532687 (Gemcitabine Hydrochloride With or Without Pazopanib Hydrochloride in Treating Patients With Refractory Soft Tissue Sarcoma): This randomized phase II trial studies how well gemcitabine hydrochloride works with or without pazopanib hydrochloride in treating patients with refractory soft tissue sarcoma.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Ferrari A, Casanova M, Collini P, et al.: Adult-type soft tissue sarcomas in pediatric-age patients: experience at the Istituto Nazionale Tumori in Milan. J Clin Oncol 23 (18): 4021-30, 2005. [PUBMED Abstract]
- Stanelle EJ, Christison-Lagay ER, Sidebotham EL, et al.: Prognostic factors and survival in pediatric and adolescent liposarcoma. Sarcoma 2012: 870910, 2012. [PUBMED Abstract]
- Alaggio R, Coffin CM, Weiss SW, et al.: Liposarcomas in young patients: a study of 82 cases occurring in patients younger than 22 years of age. Am J Surg Pathol 33 (5): 645-58, 2009. [PUBMED Abstract]
- Sreekantaiah C, Karakousis CP, Leong SP, et al.: Cytogenetic findings in liposarcoma correlate with histopathologic subtypes. Cancer 69 (10): 2484-95, 1992. [PUBMED Abstract]
- Sugiura H, Takahashi M, Katagiri H, et al.: Additional wide resection of malignant soft tissue tumors. Clin Orthop (394): 201-10, 2002. [PUBMED Abstract]
- Cecchetto G, Guglielmi M, Inserra A, et al.: Primary re-excision: the Italian experience in patients with localized soft-tissue sarcomas. Pediatr Surg Int 17 (7): 532-4, 2001. [PUBMED Abstract]
- Chui CH, Spunt SL, Liu T, et al.: Is reexcision in pediatric nonrhabdomyosarcoma soft tissue sarcoma necessary after an initial unplanned resection? J Pediatr Surg 37 (10): 1424-9, 2002. [PUBMED Abstract]
- Bahig H, Roberge D, Bosch W, et al.: Agreement among RTOG sarcoma radiation oncologists in contouring suspicious peritumoral edema for preoperative radiation therapy of soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys 86 (2): 298-303, 2013. [PUBMED Abstract]
- Baldini EH, Wang D, Haas RL, et al.: Treatment Guidelines for Preoperative Radiation Therapy for Retroperitoneal Sarcoma: Preliminary Consensus of an International Expert Panel. Int J Radiat Oncol Biol Phys 92 (3): 602-12, 2015. [PUBMED Abstract]
- Ferrari A, Casanova M, Spreafico F, et al.: Childhood liposarcoma: a single-institutional twenty-year experience. Pediatr Hematol Oncol 16 (5): 415-21, 1999 Sep-Oct. [PUBMED Abstract]
- Cecchetto G, Alaggio R, Dall'Igna P, et al.: Localized unresectable non-rhabdo soft tissue sarcomas of the extremities in pediatric age: results from the Italian studies. Cancer 104 (9): 2006-12, 2005. [PUBMED Abstract]
- Huh WW, Yuen C, Munsell M, et al.: Liposarcoma in children and young adults: a multi-institutional experience. Pediatr Blood Cancer 57 (7): 1142-6, 2011. [PUBMED Abstract]
- Gronchi A, Bui BN, Bonvalot S, et al.: Phase II clinical trial of neoadjuvant trabectedin in patients with advanced localized myxoid liposarcoma. Ann Oncol 23 (3): 771-6, 2012. [PUBMED Abstract]
- Demetri GD, von Mehren M, Jones RL, et al.: Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J Clin Oncol 34 (8): 786-93, 2016. [PUBMED Abstract]
- Baruchel S, Pappo A, Krailo M, et al.: A phase 2 trial of trabectedin in children with recurrent rhabdomyosarcoma, Ewing sarcoma and non-rhabdomyosarcoma soft tissue sarcomas: a report from the Children's Oncology Group. Eur J Cancer 48 (4): 579-85, 2012. [PUBMED Abstract]
- Wang L, Motoi T, Khanin R, et al.: Identification of a novel, recurrent HEY1-NCOA2 fusion in mesenchymal chondrosarcoma based on a genome-wide screen of exon-level expression data. Genes Chromosomes Cancer 51 (2): 127-39, 2012. [PUBMED Abstract]
- Nyquist KB, Panagopoulos I, Thorsen J, et al.: Whole-transcriptome sequencing identifies novel IRF2BP2-CDX1 fusion gene brought about by translocation t(1;5)(q42;q32) in mesenchymal chondrosarcoma. PLoS One 7 (11): e49705, 2012. [PUBMED Abstract]
- Frezza AM, Cesari M, Baumhoer D, et al.: Mesenchymal chondrosarcoma: prognostic factors and outcome in 113 patients. A European Musculoskeletal Oncology Society study. Eur J Cancer 51 (3): 374-81, 2015. [PUBMED Abstract]
- Dantonello TM, Int-Veen C, Leuschner I, et al.: Mesenchymal chondrosarcoma of soft tissues and bone in children, adolescents, and young adults: experiences of the CWS and COSS study groups. Cancer 112 (11): 2424-31, 2008. [PUBMED Abstract]
- Bishop MW, Somerville JM, Bahrami A, et al.: Mesenchymal Chondrosarcoma in Children and Young Adults: A Single Institution Retrospective Review. Sarcoma 2015: 608279, 2015. [PUBMED Abstract]
- Wodowski K, Hill DA, Pappo AS, et al.: A chemosensitive pediatric extraosseous osteosarcoma: case report and review of the literature. J Pediatr Hematol Oncol 25 (1): 73-7, 2003. [PUBMED Abstract]
- Sordillo PP, Hajdu SI, Magill GB, et al.: Extraosseous osteogenic sarcoma. A review of 48 patients. Cancer 51 (4): 727-34, 1983. [PUBMED Abstract]
- Nieuwenhuis MH, Casparie M, Mathus-Vliegen LM, et al.: A nation-wide study comparing sporadic and familial adenomatous polyposis-related desmoid-type fibromatoses. Int J Cancer 129 (1): 256-61, 2011. [PUBMED Abstract]
- Rossato M, Rigotti M, Grazia M, et al.: Congenital hypertrophy of the retinal pigment epithelium (CHRPE) and familial adenomatous polyposis (FAP). Acta Ophthalmol Scand 74 (4): 338-42, 1996. [PUBMED Abstract]
- Baker RH, Heinemann MH, Miller HH, et al.: Hyperpigmented lesions of the retinal pigment epithelium in familial adenomatous polyposis. Am J Med Genet 31 (2): 427-35, 1988. [PUBMED Abstract]
- Kattentidt Mouravieva AA, Geurts-Giele IR, de Krijger RR, et al.: Identification of Familial Adenomatous Polyposis carriers among children with desmoid tumours. Eur J Cancer 48 (12): 1867-74, 2012. [PUBMED Abstract]
- Wang WL, Nero C, Pappo A, et al.: CTNNB1 genotyping and APC screening in pediatric desmoid tumors: a proposed algorithm. Pediatr Dev Pathol 15 (5): 361-7, 2012 Sep-Oct. [PUBMED Abstract]
- Lewis JJ, Boland PJ, Leung DH, et al.: The enigma of desmoid tumors. Ann Surg 229 (6): 866-72; discussion 872-3, 1999. [PUBMED Abstract]
- Lazar AJ, Tuvin D, Hajibashi S, et al.: Specific mutations in the beta-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol 173 (5): 1518-27, 2008. [PUBMED Abstract]
- Faulkner LB, Hajdu SI, Kher U, et al.: Pediatric desmoid tumor: retrospective analysis of 63 cases. J Clin Oncol 13 (11): 2813-8, 1995. [PUBMED Abstract]
- Merchant NB, Lewis JJ, Woodruff JM, et al.: Extremity and trunk desmoid tumors: a multifactorial analysis of outcome. Cancer 86 (10): 2045-52, 1999. [PUBMED Abstract]
- Honeyman JN, Theilen TM, Knowles MA, et al.: Desmoid fibromatosis in children and adolescents: a conservative approach to management. J Pediatr Surg 48 (1): 62-6, 2013. [PUBMED Abstract]
- Bonvalot S, Ternès N, Fiore M, et al.: Spontaneous regression of primary abdominal wall desmoid tumors: more common than previously thought. Ann Surg Oncol 20 (13): 4096-102, 2013. [PUBMED Abstract]
- Bonvalot S, Eldweny H, Haddad V, et al.: Extra-abdominal primary fibromatosis: Aggressive management could be avoided in a subgroup of patients. Eur J Surg Oncol 34 (4): 462-8, 2008. [PUBMED Abstract]
- Merchant TE, Nguyen D, Walter AW, et al.: Long-term results with radiation therapy for pediatric desmoid tumors. Int J Radiat Oncol Biol Phys 47 (5): 1267-71, 2000. [PUBMED Abstract]
- Zelefsky MJ, Harrison LB, Shiu MH, et al.: Combined surgical resection and iridium 192 implantation for locally advanced and recurrent desmoid tumors. Cancer 67 (2): 380-4, 1991. [PUBMED Abstract]
- Weiss AJ, Lackman RD: Low-dose chemotherapy of desmoid tumors. Cancer 64 (6): 1192-4, 1989. [PUBMED Abstract]
- Klein WA, Miller HH, Anderson M, et al.: The use of indomethacin, sulindac, and tamoxifen for the treatment of desmoid tumors associated with familial polyposis. Cancer 60 (12): 2863-8, 1987. [PUBMED Abstract]
- Soto-Miranda MA, Sandoval JA, Rao B, et al.: Surgical treatment of pediatric desmoid tumors. A 12-year, single-center experience. Ann Surg Oncol 20 (11): 3384-90, 2013. [PUBMED Abstract]
- Skapek SX, Ferguson WS, Granowetter L, et al.: Vinblastine and methotrexate for desmoid fibromatosis in children: results of a Pediatric Oncology Group Phase II Trial. J Clin Oncol 25 (5): 501-6, 2007. [PUBMED Abstract]
- Gandhi MM, Nathan PC, Weitzman S, et al.: Successful treatment of life-threatening generalized infantile myofibromatosis using low-dose chemotherapy. J Pediatr Hematol Oncol 25 (9): 750-4, 2003. [PUBMED Abstract]
- Gega M, Yanagi H, Yoshikawa R, et al.: Successful chemotherapeutic modality of doxorubicin plus dacarbazine for the treatment of desmoid tumors in association with familial adenomatous polyposis. J Clin Oncol 24 (1): 102-5, 2006. [PUBMED Abstract]
- Constantinidou A, Jones RL, Scurr M, et al.: Pegylated liposomal doxorubicin, an effective, well-tolerated treatment for refractory aggressive fibromatosis. Eur J Cancer 45 (17): 2930-4, 2009. [PUBMED Abstract]
- Ananth P, Werger A, Voss S, et al.: Liposomal doxorubicin: Effective treatment for pediatric desmoid fibromatosis. Pediatr Blood Cancer 64 (7): , 2017. [PUBMED Abstract]
- Gounder MM, Lefkowitz RA, Keohan ML, et al.: Activity of Sorafenib against desmoid tumor/deep fibromatosis. Clin Cancer Res 17 (12): 4082-90, 2011. [PUBMED Abstract]
- Heinrich MC, McArthur GA, Demetri GD, et al.: Clinical and molecular studies of the effect of imatinib on advanced aggressive fibromatosis (desmoid tumor). J Clin Oncol 24 (7): 1195-203, 2006. [PUBMED Abstract]
- Chugh R, Wathen JK, Patel SR, et al.: Efficacy of imatinib in aggressive fibromatosis: Results of a phase II multicenter Sarcoma Alliance for Research through Collaboration (SARC) trial. Clin Cancer Res 16 (19): 4884-91, 2010. [PUBMED Abstract]
- Agresta L, Kim H, Turpin BK, et al.: Pazopanib therapy for desmoid tumors in adolescent and young adult patients. Pediatr Blood Cancer : , 2018. [PUBMED Abstract]
- Shang H, Braggio D, Lee YJ, et al.: Targeting the Notch pathway: A potential therapeutic approach for desmoid tumors. Cancer 121 (22): 4088-96, 2015. [PUBMED Abstract]
- Messersmith WA, Shapiro GI, Cleary JM, et al.: A Phase I, dose-finding study in patients with advanced solid malignancies of the oral γ-secretase inhibitor PF-03084014. Clin Cancer Res 21 (1): 60-7, 2015. [PUBMED Abstract]
- Bisogno G, Tagarelli A, Stramare R, et al.: Hydroxyurea treatment can avoid the need for aggressive surgery in pediatric fibromatosis. J Pediatr Hematol Oncol 35 (4): e171-3, 2013. [PUBMED Abstract]
- Meazza C, Casanova M, Trecate G, et al.: Objective response to hydroxyurea in a patient with heavily pre-treated aggressive fibromatosis. Pediatr Blood Cancer 55 (3): 587-8, 2010. [PUBMED Abstract]
- Balamuth NJ, Womer RB: Successful treatment of fibromatosis with hydroxyurea: Analysis of 20 pediatric cases. [Abstract] The Connective Tissue Oncology Society (CTOS) 14th Annual Meeting, 13–15 November 2008, London, United Kingdom A-34852, 2008. Also available online. Last accessed April 02, 2018.
- Meazza C, Bisogno G, Gronchi A, et al.: Aggressive fibromatosis in children and adolescents: the Italian experience. Cancer 116 (1): 233-40, 2010. [PUBMED Abstract]
- Hansmann A, Adolph C, Vogel T, et al.: High-dose tamoxifen and sulindac as first-line treatment for desmoid tumors. Cancer 100 (3): 612-20, 2004. [PUBMED Abstract]
- Skapek SX, Anderson JR, Hill DA, et al.: Safety and efficacy of high-dose tamoxifen and sulindac for desmoid tumor in children: results of a Children's Oncology Group (COG) phase II study. Pediatr Blood Cancer 60 (7): 1108-12, 2013. [PUBMED Abstract]
- Rutenberg MS, Indelicato DJ, Knapik JA, et al.: External-beam radiotherapy for pediatric and young adult desmoid tumors. Pediatr Blood Cancer 57 (3): 435-42, 2011. [PUBMED Abstract]
- Buckley PG, Mantripragada KK, Benetkiewicz M, et al.: A full-coverage, high-resolution human chromosome 22 genomic microarray for clinical and research applications. Hum Mol Genet 11 (25): 3221-9, 2002. [PUBMED Abstract]
- Valdivielso-Ramos M, Torrelo A, Campos M, et al.: Pediatric dermatofibrosarcoma protuberans in Madrid, Spain: multi-institutional outcomes. Pediatr Dermatol 31 (6): 676-82, 2014 Nov-Dec. [PUBMED Abstract]
- Gooskens SL, Oranje AP, van Adrichem LN, et al.: Imatinib mesylate for children with dermatofibrosarcoma protuberans (DFSP). Pediatr Blood Cancer 55 (2): 369-73, 2010. [PUBMED Abstract]
- Rubio GA, Alvarado A, Gerth DJ, et al.: Incidence and Outcomes of Dermatofibrosarcoma Protuberans in the US Pediatric Population. J Craniofac Surg 28 (1): 182-184, 2017. [PUBMED Abstract]
- Meguerditchian AN, Wang J, Lema B, et al.: Wide excision or Mohs micrographic surgery for the treatment of primary dermatofibrosarcoma protuberans. Am J Clin Oncol 33 (3): 300-3, 2010. [PUBMED Abstract]
- Dagan R, Morris CG, Zlotecki RA, et al.: Radiotherapy in the treatment of dermatofibrosarcoma protuberans. Am J Clin Oncol 28 (6): 537-9, 2005. [PUBMED Abstract]
- Sun LM, Wang CJ, Huang CC, et al.: Dermatofibrosarcoma protuberans: treatment results of 35 cases. Radiother Oncol 57 (2): 175-81, 2000. [PUBMED Abstract]
- Price VE, Fletcher JA, Zielenska M, et al.: Imatinib mesylate: an attractive alternative in young children with large, surgically challenging dermatofibrosarcoma protuberans. Pediatr Blood Cancer 44 (5): 511-5, 2005. [PUBMED Abstract]
- McArthur GA, Demetri GD, van Oosterom A, et al.: Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol 23 (4): 866-73, 2005. [PUBMED Abstract]
- Rutkowski P, Van Glabbeke M, Rankin CJ, et al.: Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials. J Clin Oncol 28 (10): 1772-9, 2010. [PUBMED Abstract]
- Miller SJ, Alam M, Andersen JS, et al.: Dermatofibrosarcoma protuberans. J Natl Compr Canc Netw 10 (3): 312-8, 2012. [PUBMED Abstract]
- Kao YC, Fletcher CDM, Alaggio R, et al.: Recurrent BRAF Gene Fusions in a Subset of Pediatric Spindle Cell Sarcomas: Expanding the Genetic Spectrum of Tumors With Overlapping Features With Infantile Fibrosarcoma. Am J Surg Pathol 42 (1): 28-38, 2018. [PUBMED Abstract]
- Sulkowski JP, Raval MV, Browne M: Margin status and multimodal therapy in infantile fibrosarcoma. Pediatr Surg Int 29 (8): 771-6, 2013. [PUBMED Abstract]
- Orbach D, Rey A, Cecchetto G, et al.: Infantile fibrosarcoma: management based on the European experience. J Clin Oncol 28 (2): 318-23, 2010. [PUBMED Abstract]
- Orbach D, Brennan B, De Paoli A, et al.: Conservative strategy in infantile fibrosarcoma is possible: The European paediatric Soft tissue sarcoma Study Group experience. Eur J Cancer 57: 1-9, 2016. [PUBMED Abstract]
- Spunt SL, Million L, Coffin C: The nonrhabdomyosarcoma soft tissue sarcoma. In: Pizzo PA, Poplack DG, eds.: Principles and Practice of Pediatric Oncology. 7th ed. Philadelphia, Pa: Lippincott Williams and Wilkins, 2015, pp 827-54.
- Loh ML, Ahn P, Perez-Atayde AR, et al.: Treatment of infantile fibrosarcoma with chemotherapy and surgery: results from the Dana-Farber Cancer Institute and Children's Hospital, Boston. J Pediatr Hematol Oncol 24 (9): 722-6, 2002. [PUBMED Abstract]
- Akyüz C, Küpeli S, Varan A, et al.: Infantile fibrosarcoma: retrospective analysis of eleven patients. Tumori 97 (2): 166-9, 2011 Mar-Apr. [PUBMED Abstract]
- Gallego S, Pericas N, Barber I, et al.: Infantile fibrosarcoma of the retroperitoneum: a site of unfavorable prognosis? Pediatr Hematol Oncol 28 (5): 451-3, 2011. [PUBMED Abstract]
- Parida L, Fernandez-Pineda I, Uffman JK, et al.: Clinical management of infantile fibrosarcoma: a retrospective single-institution review. Pediatr Surg Int 29 (7): 703-8, 2013. [PUBMED Abstract]
- Mody RJ, Wu YM, Lonigro RJ, et al.: Integrative Clinical Sequencing in the Management of Refractory or Relapsed Cancer in Youth. JAMA 314 (9): 913-25, 2015. [PUBMED Abstract]
- Wong V, Pavlick D, Brennan T, et al.: Evaluation of a Congenital Infantile Fibrosarcoma by Comprehensive Genomic Profiling Reveals an LMNA-NTRK1 Gene Fusion Responsive to Crizotinib. J Natl Cancer Inst 108 (1): , 2016. [PUBMED Abstract]
- Nagasubramanian R, Wei J, Gordon P, et al.: Infantile Fibrosarcoma With NTRK3-ETV6 Fusion Successfully Treated With the Tropomyosin-Related Kinase Inhibitor LOXO-101. Pediatr Blood Cancer 63 (8): 1468-70, 2016. [PUBMED Abstract]
- Yanagisawa R, Noguchi M, Fujita K, et al.: Preoperative Treatment With Pazopanib in a Case of Chemotherapy-Resistant Infantile Fibrosarcoma. Pediatr Blood Cancer 63 (2): 348-51, 2016. [PUBMED Abstract]
- Madden NP, Spicer RD, Allibone EB, et al.: Spontaneous regression of neonatal fibrosarcoma. Br J Cancer Suppl 18: S72-5, 1992. [PUBMED Abstract]
- Kovach SJ, Fischer AC, Katzman PJ, et al.: Inflammatory myofibroblastic tumors. J Surg Oncol 94 (5): 385-91, 2006. [PUBMED Abstract]
- Brodlie M, Barwick SC, Wood KM, et al.: Inflammatory myofibroblastic tumours of the respiratory tract: paediatric case series with varying clinical presentations. J Laryngol Otol 125 (8): 865-8, 2011. [PUBMED Abstract]
- Xiao Y, Zhou S, Ma C, et al.: Radiological and histopathological features of hepatic inflammatory myofibroblastic tumour: analysis of 10 cases. Clin Radiol 68 (11): 1114-20, 2013. [PUBMED Abstract]
- Karnak I, Senocak ME, Ciftci AO, et al.: Inflammatory myofibroblastic tumor in children: diagnosis and treatment. J Pediatr Surg 36 (6): 908-12, 2001. [PUBMED Abstract]
- Collin M, Charles A, Barker A, et al.: Inflammatory myofibroblastic tumour of the bladder in children: a review. J Pediatr Urol 11 (5): 239-45, 2015. [PUBMED Abstract]
- Coffin CM, Hornick JL, Fletcher CD: Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 31 (4): 509-20, 2007. [PUBMED Abstract]
- Lovly CM, Gupta A, Lipson D, et al.: Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov 4 (8): 889-95, 2014. [PUBMED Abstract]
- Devaney KO, Lafeir DJ, Triantafyllou A, et al.: Inflammatory myofibroblastic tumors of the head and neck: evaluation of clinicopathologic and prognostic features. Eur Arch Otorhinolaryngol 269 (12): 2461-5, 2012. [PUBMED Abstract]
- Mehta B, Mascarenhas L, Zhou S, et al.: Inflammatory myofibroblastic tumors in childhood. Pediatr Hematol Oncol 30 (7): 640-5, 2013. [PUBMED Abstract]
- Favini F, Resti AG, Collini P, et al.: Inflammatory myofibroblastic tumor of the conjunctiva: response to chemotherapy with low-dose methotrexate and vinorelbine. Pediatr Blood Cancer 54 (3): 483-5, 2010. [PUBMED Abstract]
- Doski JJ, Priebe CJ Jr, Driessnack M, et al.: Corticosteroids in the management of unresected plasma cell granuloma (inflammatory pseudotumor) of the lung. J Pediatr Surg 26 (9): 1064-6, 1991. [PUBMED Abstract]
- Diop B, Konate I, Ka S, et al.: Mesenteric myofibroblastic tumor: NSAID therapy after incomplete resection. J Visc Surg 148 (4): e311-4, 2011. [PUBMED Abstract]
- Dalton BG, Thomas PG, Sharp NE, et al.: Inflammatory myofibroblastic tumors in children. J Pediatr Surg 51 (4): 541-4, 2016. [PUBMED Abstract]
- Butrynski JE, D'Adamo DR, Hornick JL, et al.: Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med 363 (18): 1727-33, 2010. [PUBMED Abstract]
- Mossé YP, Lim MS, Voss SD, et al.: Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children's Oncology Group phase 1 consortium study. Lancet Oncol 14 (6): 472-80, 2013. [PUBMED Abstract]
- Gaudichon J, Jeanne-Pasquier C, Deparis M, et al.: Complete and Repeated Response of a Metastatic ALK-rearranged Inflammatory Myofibroblastic Tumor to Crizotinib in a Teenage Girl. J Pediatr Hematol Oncol 38 (4): 308-11, 2016. [PUBMED Abstract]
- Nishio M, Murakami H, Horiike A, et al.: Phase I Study of Ceritinib (LDK378) in Japanese Patients with Advanced, Anaplastic Lymphoma Kinase-Rearranged Non-Small-Cell Lung Cancer or Other Tumors. J Thorac Oncol 10 (7): 1058-66, 2015. [PUBMED Abstract]
- Mossé YP, Voss SD, Lim MS, et al.: Targeting ALK With Crizotinib in Pediatric Anaplastic Large Cell Lymphoma and Inflammatory Myofibroblastic Tumor: A Children's Oncology Group Study. J Clin Oncol 35 (28): 3215-3221, 2017. [PUBMED Abstract]
- Evans HL: Low-grade fibromyxoid sarcoma: a clinicopathologic study of 33 cases with long-term follow-up. Am J Surg Pathol 35 (10): 1450-62, 2011. [PUBMED Abstract]
- Guillou L, Benhattar J, Gengler C, et al.: Translocation-positive low-grade fibromyxoid sarcoma: clinicopathologic and molecular analysis of a series expanding the morphologic spectrum and suggesting potential relationship to sclerosing epithelioid fibrosarcoma: a study from the French Sarcoma Group. Am J Surg Pathol 31 (9): 1387-402, 2007. [PUBMED Abstract]
- O'Sullivan MJ, Sirgi KE, Dehner LP: Low-grade fibrosarcoma (hyalinizing spindle cell tumor with giant rosettes) with pulmonary metastases at presentation: case report and review of the literature. Int J Surg Pathol 10 (3): 211-6, 2002. [PUBMED Abstract]
- Folpe AL, Lane KL, Paull G, et al.: Low-grade fibromyxoid sarcoma and hyalinizing spindle cell tumor with giant rosettes: a clinicopathologic study of 73 cases supporting their identity and assessing the impact of high-grade areas. Am J Surg Pathol 24 (10): 1353-60, 2000. [PUBMED Abstract]
- Sargar K, Kao SC, Spunt SL, et al.: MRI and CT of Low-Grade Fibromyxoid Sarcoma in Children: A Report From Children's Oncology Group Study ARST0332. AJR Am J Roentgenol 205 (2): 414-20, 2015. [PUBMED Abstract]
- Maretty-Nielsen K, Baerentzen S, Keller J, et al.: Low-Grade Fibromyxoid Sarcoma: Incidence, Treatment Strategy of Metastases, and Clinical Significance of the FUS Gene. Sarcoma 2013: 256280, 2013. [PUBMED Abstract]
- Prieto-Granada C, Zhang L, Chen HW, et al.: A genetic dichotomy between pure sclerosing epithelioid fibrosarcoma (SEF) and hybrid SEF/low-grade fibromyxoid sarcoma: a pathologic and molecular study of 18 cases. Genes Chromosomes Cancer 54 (1): 28-38, 2015. [PUBMED Abstract]
- Pollock BH, Jenson HB, Leach CT, et al.: Risk factors for pediatric human immunodeficiency virus-related malignancy. JAMA 289 (18): 2393-9, 2003. [PUBMED Abstract]
- Kleinerman RA, Tucker MA, Abramson DH, et al.: Risk of soft tissue sarcomas by individual subtype in survivors of hereditary retinoblastoma. J Natl Cancer Inst 99 (1): 24-31, 2007. [PUBMED Abstract]
- Samuels BL, Chawla S, Patel S, et al.: Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: results of a worldwide expanded access program study. Ann Oncol 24 (6): 1703-9, 2013. [PUBMED Abstract]
- Enzinger FM, Zhang RY: Plexiform fibrohistiocytic tumor presenting in children and young adults. An analysis of 65 cases. Am J Surg Pathol 12 (11): 818-26, 1988. [PUBMED Abstract]
- Black J, Coffin CM, Dehner LP: Fibrohistiocytic tumors and related neoplasms in children and adolescents. Pediatr Dev Pathol 15 (1 Suppl): 181-210, 2012. [PUBMED Abstract]
- Moosavi C, Jha P, Fanburg-Smith JC: An update on plexiform fibrohistiocytic tumor and addition of 66 new cases from the Armed Forces Institute of Pathology, in honor of Franz M. Enzinger, MD. Ann Diagn Pathol 11 (5): 313-9, 2007. [PUBMED Abstract]
- Billings SD, Folpe AL: Cutaneous and subcutaneous fibrohistiocytic tumors of intermediate malignancy: an update. Am J Dermatopathol 26 (2): 141-55, 2004. [PUBMED Abstract]
- Remstein ED, Arndt CA, Nascimento AG: Plexiform fibrohistiocytic tumor: clinicopathologic analysis of 22 cases. Am J Surg Pathol 23 (6): 662-70, 1999. [PUBMED Abstract]
- Salomao DR, Nascimento AG: Plexiform fibrohistiocytic tumor with systemic metastases: a case report. Am J Surg Pathol 21 (4): 469-76, 1997. [PUBMED Abstract]
- Redlich GC, Montgomery KD, Allgood GA, et al.: Plexiform fibrohistiocytic tumor with a clonal cytogenetic anomaly. Cancer Genet Cytogenet 108 (2): 141-3, 1999. [PUBMED Abstract]
- Luzar B, Calonje E: Cutaneous fibrohistiocytic tumours - an update. Histopathology 56 (1): 148-65, 2010. [PUBMED Abstract]
- Dantonello TM, Leuschner I, Vokuhl C, et al.: Malignant ectomesenchymoma in children and adolescents: report from the Cooperative Weichteilsarkom Studiengruppe (CWS). Pediatr Blood Cancer 60 (2): 224-9, 2013. [PUBMED Abstract]
- Carli M, Ferrari A, Mattke A, et al.: Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J Clin Oncol 23 (33): 8422-30, 2005. [PUBMED Abstract]
- Zhang M, Wang Y, Jones S, et al.: Somatic mutations of SUZ12 in malignant peripheral nerve sheath tumors. Nat Genet 46 (11): 1170-2, 2014. [PUBMED Abstract]
- Hagel C, Zils U, Peiper M, et al.: Histopathology and clinical outcome of NF1-associated vs. sporadic malignant peripheral nerve sheath tumors. J Neurooncol 82 (2): 187-92, 2007. [PUBMED Abstract]
- Zou C, Smith KD, Liu J, et al.: Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg 249 (6): 1014-22, 2009. [PUBMED Abstract]
- Okada K, Hasegawa T, Tajino T, et al.: Clinical relevance of pathological grades of malignant peripheral nerve sheath tumor: a multi-institution TMTS study of 56 cases in Northern Japan. Ann Surg Oncol 14 (2): 597-604, 2007. [PUBMED Abstract]
- Amirian ES, Goodman JC, New P, et al.: Pediatric and adult malignant peripheral nerve sheath tumors: an analysis of data from the surveillance, epidemiology, and end results program. J Neurooncol 116 (3): 609-16, 2014. [PUBMED Abstract]
- Valentin T, Le Cesne A, Ray-Coquard I, et al.: Management and prognosis of malignant peripheral nerve sheath tumors: The experience of the French Sarcoma Group (GSF-GETO). Eur J Cancer 56: 77-84, 2016. [PUBMED Abstract]
- Bergamaschi L, Bisogno G, Manzitti C, et al.: Salvage rates and prognostic factors after relapse in children and adolescents with malignant peripheral nerve sheath tumors. Pediatr Blood Cancer 65 (2): , 2018. [PUBMED Abstract]
- Ferrari A, Bisogno G, Macaluso A, et al.: Soft-tissue sarcomas in children and adolescents with neurofibromatosis type 1. Cancer 109 (7): 1406-12, 2007. [PUBMED Abstract]
- Okur FV, Oguz A, Karadeniz C, et al.: Malignant triton tumor of the pelvis in a 2-year-old boy. J Pediatr Hematol Oncol 28 (3): 173-6, 2006. [PUBMED Abstract]
- Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 4th ed. St. Louis, Mo: Mosby, 2001.
- Fernandez-Pineda I, Parida L, Jenkins JJ, et al.: Childhood hemangiopericytoma: review of St Jude Children's Research Hospital. J Pediatr Hematol Oncol 33 (5): 356-9, 2011. [PUBMED Abstract]
- Rodriguez-Galindo C, Ramsey K, Jenkins JJ, et al.: Hemangiopericytoma in children and infants. Cancer 88 (1): 198-204, 2000. [PUBMED Abstract]
- Ferrari A, Casanova M, Bisogno G, et al.: Hemangiopericytoma in pediatric ages: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Cancer 92 (10): 2692-8, 2001. [PUBMED Abstract]
- Bien E, Stachowicz-Stencel T, Godzinski J, et al.: Retrospective multi-institutional study on hemangiopericytoma in Polish children. Pediatr Int 51 (1): 19-24, 2009. [PUBMED Abstract]
- Wiswell TE, Davis J, Cunningham BE, et al.: Infantile myofibromatosis: the most common fibrous tumor of infancy. J Pediatr Surg 23 (4): 315-8, 1988. [PUBMED Abstract]
- Chung EB, Enzinger FM: Infantile myofibromatosis. Cancer 48 (8): 1807-18, 1981. [PUBMED Abstract]
- Modi N: Congenital generalised fibromatosis. Arch Dis Child 57 (11): 881-2, 1982. [PUBMED Abstract]
- Levine E, Fréneaux P, Schleiermacher G, et al.: Risk-adapted therapy for infantile myofibromatosis in children. Pediatr Blood Cancer 59 (1): 115-20, 2012. [PUBMED Abstract]
- Larralde M, Hoffner MV, Boggio P, et al.: Infantile myofibromatosis: report of nine patients. Pediatr Dermatol 27 (1): 29-33, 2010 Jan-Feb. [PUBMED Abstract]
- Cheung YH, Gayden T, Campeau PM, et al.: A recurrent PDGFRB mutation causes familial infantile myofibromatosis. Am J Hum Genet 92 (6): 996-1000, 2013. [PUBMED Abstract]
- Gopal M, Chahal G, Al-Rifai Z, et al.: Infantile myofibromatosis. Pediatr Surg Int 24 (3): 287-91, 2008. [PUBMED Abstract]
- Weaver MS, Navid F, Huppmann A, et al.: Vincristine and Dactinomycin in Infantile Myofibromatosis With a Review of Treatment Options. J Pediatr Hematol Oncol 37 (3): 237-41, 2015. [PUBMED Abstract]
- Orbach D, Brennan B, Casanova M, et al.: Paediatric and adolescent alveolar soft part sarcoma: A joint series from European cooperative groups. Pediatr Blood Cancer 60 (11): 1826-32, 2013. [PUBMED Abstract]
- Ferrari A, Sultan I, Huang TT, et al.: Soft tissue sarcoma across the age spectrum: a population-based study from the Surveillance Epidemiology and End Results database. Pediatr Blood Cancer 57 (6): 943-9, 2011. [PUBMED Abstract]
- Wang HW, Qin XJ, Yang WJ, et al.: Alveolar soft part sarcoma of the oral and maxillofacial region: clinical analysis in a series of 18 patients. Oral Surg Oral Med Oral Pathol Oral Radiol 119 (4): 396-401, 2015. [PUBMED Abstract]
- Kayton ML, Meyers P, Wexler LH, et al.: Clinical presentation, treatment, and outcome of alveolar soft part sarcoma in children, adolescents, and young adults. J Pediatr Surg 41 (1): 187-93, 2006. [PUBMED Abstract]
- Ladanyi M, Lui MY, Antonescu CR, et al.: The der(17)t(X;17)(p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene 20 (1): 48-57, 2001. [PUBMED Abstract]
- Williams A, Bartle G, Sumathi VP, et al.: Detection of ASPL/TFE3 fusion transcripts and the TFE3 antigen in formalin-fixed, paraffin-embedded tissue in a series of 18 cases of alveolar soft part sarcoma: useful diagnostic tools in cases with unusual histological features. Virchows Arch 458 (3): 291-300, 2011. [PUBMED Abstract]
- Lieberman PH, Brennan MF, Kimmel M, et al.: Alveolar soft-part sarcoma. A clinico-pathologic study of half a century. Cancer 63 (1): 1-13, 1989. [PUBMED Abstract]
- Casanova M, Ferrari A, Bisogno G, et al.: Alveolar soft part sarcoma in children and adolescents: A report from the Soft-Tissue Sarcoma Italian Cooperative Group. Ann Oncol 11 (11): 1445-9, 2000. [PUBMED Abstract]
- Pennacchioli E, Fiore M, Collini P, et al.: Alveolar soft part sarcoma: clinical presentation, treatment, and outcome in a series of 33 patients at a single institution. Ann Surg Oncol 17 (12): 3229-33, 2010. [PUBMED Abstract]
- Flores RJ, Harrison DJ, Federman NC, et al.: Alveolar soft part sarcoma in children and young adults: A report of 69 cases. Pediatr Blood Cancer : , 2018. [PUBMED Abstract]
- Roozendaal KJ, de Valk B, ten Velden JJ, et al.: Alveolar soft-part sarcoma responding to interferon alpha-2b. Br J Cancer 89 (2): 243-5, 2003. [PUBMED Abstract]
- Conde N, Cruz O, Albert A, et al.: Antiangiogenic treatment as a pre-operative management of alveolar soft-part sarcoma. Pediatr Blood Cancer 57 (6): 1071-3, 2011. [PUBMED Abstract]
- Stacchiotti S, Negri T, Zaffaroni N, et al.: Sunitinib in advanced alveolar soft part sarcoma: evidence of a direct antitumor effect. Ann Oncol 22 (7): 1682-90, 2011. [PUBMED Abstract]
- Kummar S, Allen D, Monks A, et al.: Cediranib for metastatic alveolar soft part sarcoma. J Clin Oncol 31 (18): 2296-302, 2013. [PUBMED Abstract]
- Coindre JM, Hostein I, Terrier P, et al.: Diagnosis of clear cell sarcoma by real-time reverse transcriptase-polymerase chain reaction analysis of paraffin embedded tissues: clinicopathologic and molecular analysis of 44 patients from the French sarcoma group. Cancer 107 (5): 1055-64, 2006. [PUBMED Abstract]
- Meis-Kindblom JM: Clear cell sarcoma of tendons and aponeuroses: a historical perspective and tribute to the man behind the entity. Adv Anat Pathol 13 (6): 286-92, 2006. [PUBMED Abstract]
- Dim DC, Cooley LD, Miranda RN: Clear cell sarcoma of tendons and aponeuroses: a review. Arch Pathol Lab Med 131 (1): 152-6, 2007. [PUBMED Abstract]
- Blazer DG 3rd, Lazar AJ, Xing Y, et al.: Clinical outcomes of molecularly confirmed clear cell sarcoma from a single institution and in comparison with data from the Surveillance, Epidemiology, and End Results registry. Cancer 115 (13): 2971-9, 2009. [PUBMED Abstract]
- Fujimura Y, Siddique H, Lee L, et al.: EWS-ATF-1 chimeric protein in soft tissue clear cell sarcoma associates with CREB-binding protein and interferes with p53-mediated trans-activation function. Oncogene 20 (46): 6653-9, 2001. [PUBMED Abstract]
- Ferrari A, Casanova M, Bisogno G, et al.: Clear cell sarcoma of tendons and aponeuroses in pediatric patients: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Cancer 94 (12): 3269-76, 2002. [PUBMED Abstract]
- Karita M, Tsuchiya H, Yamamoto N, et al.: Caffeine-potentiated chemotherapy for clear cell sarcoma: a report of five cases. Int J Clin Oncol 18 (1): 33-7, 2013. [PUBMED Abstract]
- Leuschner I, Radig K, Harms D: Desmoplastic small round cell tumor. Semin Diagn Pathol 13 (3): 204-12, 1996. [PUBMED Abstract]
- Kushner BH, LaQuaglia MP, Wollner N, et al.: Desmoplastic small round-cell tumor: prolonged progression-free survival with aggressive multimodality therapy. J Clin Oncol 14 (5): 1526-31, 1996. [PUBMED Abstract]
- Saab R, Khoury JD, Krasin M, et al.: Desmoplastic small round cell tumor in childhood: the St. Jude Children's Research Hospital experience. Pediatr Blood Cancer 49 (3): 274-9, 2007. [PUBMED Abstract]
- Wang LL, Perlman EJ, Vujanic GM, et al.: Desmoplastic small round cell tumor of the kidney in childhood. Am J Surg Pathol 31 (4): 576-84, 2007. [PUBMED Abstract]
- Arora VC, Price AP, Fleming S, et al.: Characteristic imaging features of desmoplastic small round cell tumour. Pediatr Radiol 43 (1): 93-102, 2013. [PUBMED Abstract]
- Gerald WL, Ladanyi M, de Alava E, et al.: Clinical, pathologic, and molecular spectrum of tumors associated with t(11;22)(p13;q12): desmoplastic small round-cell tumor and its variants. J Clin Oncol 16 (9): 3028-36, 1998. [PUBMED Abstract]
- Lal DR, Su WT, Wolden SL, et al.: Results of multimodal treatment for desmoplastic small round cell tumors. J Pediatr Surg 40 (1): 251-5, 2005. [PUBMED Abstract]
- Philippe-Chomette P, Kabbara N, Andre N, et al.: Desmoplastic small round cell tumors with EWS-WT1 fusion transcript in children and young adults. Pediatr Blood Cancer 58 (6): 891-7, 2012. [PUBMED Abstract]
- Sedig L, Geiger J, Mody R, et al.: Paratesticular desmoplastic small round cell tumors: A case report and review of the literature. Pediatr Blood Cancer 64 (12): , 2017. [PUBMED Abstract]
- Schwarz RE, Gerald WL, Kushner BH, et al.: Desmoplastic small round cell tumors: prognostic indicators and results of surgical management. Ann Surg Oncol 5 (5): 416-22, 1998 Jul-Aug. [PUBMED Abstract]
- Goodman KA, Wolden SL, La Quaglia MP, et al.: Whole abdominopelvic radiotherapy for desmoplastic small round-cell tumor. Int J Radiat Oncol Biol Phys 54 (1): 170-6, 2002. [PUBMED Abstract]
- Osborne EM, Briere TM, Hayes-Jordan A, et al.: Survival and toxicity following sequential multimodality treatment including whole abdominopelvic radiotherapy for patients with desmoplastic small round cell tumor. Radiother Oncol 119 (1): 40-4, 2016. [PUBMED Abstract]
- Atallah V, Honore C, Orbach D, et al.: Role of Adjuvant Radiation Therapy After Surgery for Abdominal Desmoplastic Small Round Cell Tumors. Int J Radiat Oncol Biol Phys 95 (4): 1244-53, 2016. [PUBMED Abstract]
- Tarek N, Hayes-Jordan A, Salvador L, et al.: Recurrent desmoplastic small round cell tumor responding to an mTOR inhibitor containing regimen. Pediatr Blood Cancer 65 (1): , 2018. [PUBMED Abstract]
- Cook RJ, Wang Z, Arora M, et al.: Clinical outcomes of patients with desmoplastic small round cell tumor of the peritoneum undergoing autologous HCT: a CIBMTR retrospective analysis. Bone Marrow Transplant 47 (11): 1455-8, 2012. [PUBMED Abstract]
- Chbani L, Guillou L, Terrier P, et al.: Epithelioid sarcoma: a clinicopathologic and immunohistochemical analysis of 106 cases from the French sarcoma group. Am J Clin Pathol 131 (2): 222-7, 2009. [PUBMED Abstract]
- Hornick JL, Dal Cin P, Fletcher CD: Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol 33 (4): 542-50, 2009. [PUBMED Abstract]
- Knutson SK, Warholic NM, Wigle TJ, et al.: Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. Proc Natl Acad Sci U S A 110 (19): 7922-7, 2013. [PUBMED Abstract]
- Guzzetta AA, Montgomery EA, Lyu H, et al.: Epithelioid sarcoma: one institution's experience with a rare sarcoma. J Surg Res 177 (1): 116-22, 2012. [PUBMED Abstract]
- Casanova M, Ferrari A, Collini P, et al.: Epithelioid sarcoma in children and adolescents: a report from the Italian Soft Tissue Sarcoma Committee. Cancer 106 (3): 708-17, 2006. [PUBMED Abstract]
- Kodet R, Newton WA Jr, Sachs N, et al.: Rhabdoid tumors of soft tissues: a clinicopathologic study of 26 cases enrolled on the Intergroup Rhabdomyosarcoma Study. Hum Pathol 22 (7): 674-84, 1991. [PUBMED Abstract]
- Biegel JA, Zhou JY, Rorke LB, et al.: Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res 59 (1): 74-9, 1999. [PUBMED Abstract]
- Eaton KW, Tooke LS, Wainwright LM, et al.: Spectrum of SMARCB1/INI1 mutations in familial and sporadic rhabdoid tumors. Pediatr Blood Cancer 56 (1): 7-15, 2011. [PUBMED Abstract]
- Lee RS, Stewart C, Carter SL, et al.: A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J Clin Invest 122 (8): 2983-8, 2012. [PUBMED Abstract]
- Sultan I, Qaddoumi I, Rodríguez-Galindo C, et al.: Age, stage, and radiotherapy, but not primary tumor site, affects the outcome of patients with malignant rhabdoid tumors. Pediatr Blood Cancer 54 (1): 35-40, 2010. [PUBMED Abstract]
- Puri DR, Meyers PA, Kraus DH, et al.: Radiotherapy in the multimodal treatment of extrarenal extracranial malignant rhabdoid tumors. Pediatr Blood Cancer 50 (1): 167-9, 2008. [PUBMED Abstract]
- Madigan CE, Armenian SH, Malogolowkin MH, et al.: Extracranial malignant rhabdoid tumors in childhood: the Childrens Hospital Los Angeles experience. Cancer 110 (9): 2061-6, 2007. [PUBMED Abstract]
- Bourdeaut F, Fréneaux P, Thuille B, et al.: Extra-renal non-cerebral rhabdoid tumours. Pediatr Blood Cancer 51 (3): 363-8, 2008. [PUBMED Abstract]
- Wetmore C, Boyett J, Li S, et al.: Alisertib is active as single agent in recurrent atypical teratoid rhabdoid tumors in 4 children. Neuro Oncol 17 (6): 882-8, 2015. [PUBMED Abstract]
- Tsuneyoshi M, Enjoji M, Iwasaki H, et al.: Extraskeletal myxoid chondrosarcoma--a clinicopathologic and electron microscopic study. Acta Pathol Jpn 31 (3): 439-47, 1981. [PUBMED Abstract]
- Hachitanda Y, Tsuneyoshi M, Daimaru Y, et al.: Extraskeletal myxoid chondrosarcoma in young children. Cancer 61 (12): 2521-6, 1988. [PUBMED Abstract]
- Hisaoka M, Ishida T, Imamura T, et al.: TFG is a novel fusion partner of NOR1 in extraskeletal myxoid chondrosarcoma. Genes Chromosomes Cancer 40 (4): 325-8, 2004. [PUBMED Abstract]
- Enzinger FM, Shiraki M: Extraskeletal myxoid chondrosarcoma. An analysis of 34 cases. Hum Pathol 3 (3): 421-35, 1972. [PUBMED Abstract]
- McGrory JE, Rock MG, Nascimento AG, et al.: Extraskeletal myxoid chondrosarcoma. Clin Orthop Relat Res (382): 185-90, 2001. [PUBMED Abstract]
- Drilon AD, Popat S, Bhuchar G, et al.: Extraskeletal myxoid chondrosarcoma: a retrospective review from 2 referral centers emphasizing long-term outcomes with surgery and chemotherapy. Cancer 113 (12): 3364-71, 2008. [PUBMED Abstract]
- Stacchiotti S, Pantaleo MA, Astolfi A, et al.: Activity of sunitinib in extraskeletal myxoid chondrosarcoma. Eur J Cancer 50 (9): 1657-64, 2014. [PUBMED Abstract]
- Martignoni G, Pea M, Reghellin D, et al.: Molecular pathology of lymphangioleiomyomatosis and other perivascular epithelioid cell tumors. Arch Pathol Lab Med 134 (1): 33-40, 2010. [PUBMED Abstract]
- Bissler JJ, McCormack FX, Young LR, et al.: Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med 358 (2): 140-51, 2008. [PUBMED Abstract]
- Davies DM, Johnson SR, Tattersfield AE, et al.: Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. N Engl J Med 358 (2): 200-3, 2008. [PUBMED Abstract]
- Agaram NP, Sung YS, Zhang L, et al.: Dichotomy of Genetic Abnormalities in PEComas With Therapeutic Implications. Am J Surg Pathol 39 (6): 813-25, 2015. [PUBMED Abstract]
- Folpe A, Inwards C, eds.: Bone and Soft Tissue Pathology: A Volume in the Foundations in Diagnostic Pathology. Philadelphia, Pa: WB Saunders Co, 2010.
- Armah HB, Parwani AV: Perivascular epithelioid cell tumor. Arch Pathol Lab Med 133 (4): 648-54, 2009. [PUBMED Abstract]
- Alaggio R, Cecchetto G, Martignoni G, et al.: Malignant perivascular epithelioid cell tumor in children: description of a case and review of the literature. J Pediatr Surg 47 (6): e31-40, 2012. [PUBMED Abstract]
- Wagner AJ, Malinowska-Kolodziej I, Morgan JA, et al.: Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol 28 (5): 835-40, 2010. [PUBMED Abstract]
- Sultan I, Rodriguez-Galindo C, Saab R, et al.: Comparing children and adults with synovial sarcoma in the Surveillance, Epidemiology, and End Results program, 1983 to 2005: an analysis of 1268 patients. Cancer 115 (15): 3537-47, 2009. [PUBMED Abstract]
- Wang JG, Li NN: Primary cardiac synovial sarcoma. Ann Thorac Surg 95 (6): 2202-9, 2013. [PUBMED Abstract]
- Pappo AS, Fontanesi J, Luo X, et al.: Synovial sarcoma in children and adolescents: the St Jude Children's Research Hospital experience. J Clin Oncol 12 (11): 2360-6, 1994. [PUBMED Abstract]
- Ferrari A, De Salvo GL, Oberlin O, et al.: Synovial sarcoma in children and adolescents: a critical reappraisal of staging investigations in relation to the rate of metastatic involvement at diagnosis. Eur J Cancer 48 (9): 1370-5, 2012. [PUBMED Abstract]
- van de Rijn M, Barr FG, Collins MH, et al.: Absence of SYT-SSX fusion products in soft tissue tumors other than synovial sarcoma. Am J Clin Pathol 112 (1): 43-9, 1999. [PUBMED Abstract]
- Krsková L, Sumerauer D, Stejskalová E, et al.: A novel variant of SYT-SSX1 fusion gene in a case of spindle cell synovial sarcoma. Diagn Mol Pathol 16 (3): 179-83, 2007. [PUBMED Abstract]
- Su L, Sampaio AV, Jones KB, et al.: Deconstruction of the SS18-SSX fusion oncoprotein complex: insights into disease etiology and therapeutics. Cancer Cell 21 (3): 333-47, 2012. [PUBMED Abstract]
- Arnold MA, Arnold CA, Li G, et al.: A unique pattern of INI1 immunohistochemistry distinguishes synovial sarcoma from its histologic mimics. Hum Pathol 44 (5): 881-7, 2013. [PUBMED Abstract]
- Vlenterie M, Ho VK, Kaal SE, et al.: Age as an independent prognostic factor for survival of localised synovial sarcoma patients. Br J Cancer 113 (11): 1602-6, 2015. [PUBMED Abstract]
- Okcu MF, Munsell M, Treuner J, et al.: Synovial sarcoma of childhood and adolescence: a multicenter, multivariate analysis of outcome. J Clin Oncol 21 (8): 1602-11, 2003. [PUBMED Abstract]
- Brecht IB, Ferrari A, Int-Veen C, et al.: Grossly-resected synovial sarcoma treated by the German and Italian Pediatric Soft Tissue Sarcoma Cooperative Groups: discussion on the role of adjuvant therapies. Pediatr Blood Cancer 46 (1): 11-7, 2006. [PUBMED Abstract]
- Stanelle EJ, Christison-Lagay ER, Healey JH, et al.: Pediatric and adolescent synovial sarcoma: multivariate analysis of prognostic factors and survival outcomes. Ann Surg Oncol 20 (1): 73-9, 2013. [PUBMED Abstract]
- Trassard M, Le Doussal V, Hacène K, et al.: Prognostic factors in localized primary synovial sarcoma: a multicenter study of 128 adult patients. J Clin Oncol 19 (2): 525-34, 2001. [PUBMED Abstract]
- Guillou L, Benhattar J, Bonichon F, et al.: Histologic grade, but not SYT-SSX fusion type, is an important prognostic factor in patients with synovial sarcoma: a multicenter, retrospective analysis. J Clin Oncol 22 (20): 4040-50, 2004. [PUBMED Abstract]
- Ferrari A, Gronchi A, Casanova M, et al.: Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer 101 (3): 627-34, 2004. [PUBMED Abstract]
- Lagarde P, Przybyl J, Brulard C, et al.: Chromosome instability accounts for reverse metastatic outcomes of pediatric and adult synovial sarcomas. J Clin Oncol 31 (5): 608-15, 2013. [PUBMED Abstract]
- Stegmaier S, Leuschner I, Poremba C, et al.: The prognostic impact of SYT-SSX fusion type and histological grade in pediatric patients with synovial sarcoma treated according to the CWS (Cooperative Weichteilsarkom Studie) trials. Pediatr Blood Cancer 64 (1): 89-95, 2017. [PUBMED Abstract]
- Scheer M, Dantonello T, Hallmen E, et al.: Primary Metastatic Synovial Sarcoma: Experience of the CWS Study Group. Pediatr Blood Cancer 63 (7): 1198-206, 2016. [PUBMED Abstract]
- Ferrari A, De Salvo GL, Dall'Igna P, et al.: Salvage rates and prognostic factors after relapse in children and adolescents with initially localised synovial sarcoma. Eur J Cancer 48 (18): 3448-55, 2012. [PUBMED Abstract]
- Ferrari A, Chi YY, De Salvo GL, et al.: Surgery alone is sufficient therapy for children and adolescents with low-risk synovial sarcoma: A joint analysis from the European paediatric soft tissue sarcoma Study Group and the Children's Oncology Group. Eur J Cancer 78: 1-6, 2017. [PUBMED Abstract]
- McGrory JE, Pritchard DJ, Arndt CA, et al.: Nonrhabdomyosarcoma soft tissue sarcomas in children. The Mayo Clinic experience. Clin Orthop (374): 247-58, 2000. [PUBMED Abstract]
- Van Glabbeke M, van Oosterom AT, Oosterhuis JW, et al.: Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens--a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 17 (1): 150-7, 1999. [PUBMED Abstract]
- Koscielniak E, Harms D, Henze G, et al.: Results of treatment for soft tissue sarcoma in childhood and adolescence: a final report of the German Cooperative Soft Tissue Sarcoma Study CWS-86. J Clin Oncol 17 (12): 3706-19, 1999. [PUBMED Abstract]
- Pappo AS, Devidas M, Jenkins J, et al.: Phase II trial of neoadjuvant vincristine, ifosfamide, and doxorubicin with granulocyte colony-stimulating factor support in children and adolescents with advanced-stage nonrhabdomyosarcomatous soft tissue sarcomas: a Pediatric Oncology Group Study. J Clin Oncol 23 (18): 4031-8, 2005. [PUBMED Abstract]
- Pappo AS, Rao BN, Jenkins JJ, et al.: Metastatic nonrhabdomyosarcomatous soft-tissue sarcomas in children and adolescents: the St. Jude Children's Research Hospital experience. Med Pediatr Oncol 33 (2): 76-82, 1999. [PUBMED Abstract]
- Brennan B, Stevens M, Kelsey A, et al.: Synovial sarcoma in childhood and adolescence: a retrospective series of 77 patients registered by the Children's Cancer and Leukaemia Group between 1991 and 2006. Pediatr Blood Cancer 55 (1): 85-90, 2010. [PUBMED Abstract]
- Ferrari A, Miceli R, Rey A, et al.: Non-metastatic unresected paediatric non-rhabdomyosarcoma soft tissue sarcomas: results of a pooled analysis from United States and European groups. Eur J Cancer 47 (5): 724-31, 2011. [PUBMED Abstract]
- Raney RB: Synovial sarcoma in young people: background, prognostic factors, and therapeutic questions. J Pediatr Hematol Oncol 27 (4): 207-11, 2005. [PUBMED Abstract]
- Orbach D, Mc Dowell H, Rey A, et al.: Sparing strategy does not compromise prognosis in pediatric localized synovial sarcoma: experience of the International Society of Pediatric Oncology, Malignant Mesenchymal Tumors (SIOP-MMT) Working Group. Pediatr Blood Cancer 57 (7): 1130-6, 2011. [PUBMED Abstract]
- Ladenstein R, Treuner J, Koscielniak E, et al.: Synovial sarcoma of childhood and adolescence. Report of the German CWS-81 study. Cancer 71 (11): 3647-55, 1993. [PUBMED Abstract]
- Venkatramani R, Anderson JR, Million L, et al.: Risk-based treatment for synovial sarcoma in patients under 30 years of age: Children’s Oncology Group study ARST0332. [Abstract] J Clin Oncol 33 (15 Suppl): A-10012, 2015. Also available online. Last accessed April 02, 2018.
- Ferrari A, De Salvo GL, Brennan B, et al.: Synovial sarcoma in children and adolescents: the European Pediatric Soft Tissue Sarcoma Study Group prospective trial (EpSSG NRSTS 2005). Ann Oncol 26 (3): 567-72, 2015. [PUBMED Abstract]
- Spunt SL, Million L, Anderson JR, et al.: Risk-based treatment for nonrhabdomyosarcoma soft tissue sarcomas (NRSTS) in patients under 30 years of age: Children’s Oncology Group study ARST0332. [Abstract] J Clin Oncol 32 (Suppl 15): A-10008, 2014. Also available online. Last accessed April 02, 2018.
- Randall RL, Albritton KH, Ferney BJ, et al.: Malignant fibrous histiocytoma of soft tissue: an abandoned diagnosis. Am J Orthop 33 (12): 602-8, 2004. [PUBMED Abstract]
- Alaggio R, Collini P, Randall RL, et al.: Undifferentiated high-grade pleomorphic sarcomas in children: a clinicopathologic study of 10 cases and review of literature. Pediatr Dev Pathol 13 (3): 209-17, 2010 May-Jun. [PUBMED Abstract]
- Daw NC, Billups CA, Pappo AS, et al.: Malignant fibrous histiocytoma and other fibrohistiocytic tumors in pediatric patients: the St. Jude Children's Research Hospital experience. Cancer 97 (11): 2839-47, 2003. [PUBMED Abstract]
- Coffin CM, Dehner LP: Vascular tumors in children and adolescents: a clinicopathologic study of 228 tumors in 222 patients. Pathol Annu 28 Pt 1: 97-120, 1993. [PUBMED Abstract]
- Cioffi A, Reichert S, Antonescu CR, et al.: Angiosarcomas and other sarcomas of endothelial origin. Hematol Oncol Clin North Am 27 (5): 975-88, 2013. [PUBMED Abstract]
- Jeng MR, Fuh B, Blatt J, et al.: Malignant transformation of infantile hemangioma to angiosarcoma: response to chemotherapy with bevacizumab. Pediatr Blood Cancer 61 (11): 2115-7, 2014. [PUBMED Abstract]
- Dehner LP, Ishak KG: Vascular tumors of the liver in infants and children. A study of 30 cases and review of the literature. Arch Pathol 92 (2): 101-11, 1971. [PUBMED Abstract]
- Ferrari A, Casanova M, Bisogno G, et al.: Malignant vascular tumors in children and adolescents: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Med Pediatr Oncol 39 (2): 109-14, 2002. [PUBMED Abstract]
- Deyrup AT, Miettinen M, North PE, et al.: Pediatric cutaneous angiosarcomas: a clinicopathologic study of 10 cases. Am J Surg Pathol 35 (1): 70-5, 2011. [PUBMED Abstract]
- Elliott P, Kleinschmidt I: Angiosarcoma of the liver in Great Britain in proximity to vinyl chloride sites. Occup Environ Med 54 (1): 14-8, 1997. [PUBMED Abstract]
- Lezama-del Valle P, Gerald WL, Tsai J, et al.: Malignant vascular tumors in young patients. Cancer 83 (8): 1634-9, 1998. [PUBMED Abstract]
- Fata F, O'Reilly E, Ilson D, et al.: Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer 86 (10): 2034-7, 1999. [PUBMED Abstract]
- Lahat G, Dhuka AR, Hallevi H, et al.: Angiosarcoma: clinical and molecular insights. Ann Surg 251 (6): 1098-106, 2010. [PUBMED Abstract]
- Orlando G, Adam R, Mirza D, et al.: Hepatic hemangiosarcoma: an absolute contraindication to liver transplantation--the European Liver Transplant Registry experience. Transplantation 95 (6): 872-7, 2013. [PUBMED Abstract]
- Sanada T, Nakayama H, Irisawa R, et al.: Clinical outcome and dose volume evaluation in patients who undergo brachytherapy for angiosarcoma of the scalp and face. Mol Clin Oncol 6 (3): 334-340, 2017. [PUBMED Abstract]
- Dickson MA, D'Adamo DR, Keohan ML, et al.: Phase II Trial of Gemcitabine and Docetaxel with Bevacizumab in Soft Tissue Sarcoma. Sarcoma 2015: 532478, 2015. [PUBMED Abstract]
- North PE, Waner M, Mizeracki A, et al.: A unique microvascular phenotype shared by juvenile hemangiomas and human placenta. Arch Dermatol 137 (5): 559-70, 2001. [PUBMED Abstract]
- Boye E, Yu Y, Paranya G, et al.: Clonality and altered behavior of endothelial cells from hemangiomas. J Clin Invest 107 (6): 745-52, 2001. [PUBMED Abstract]
- Ravi V, Patel S: Vascular sarcomas. Curr Oncol Rep 15 (4): 347-55, 2013. [PUBMED Abstract]
- Grassia KL, Peterman CM, Iacobas I, et al.: Clinical case series of pediatric hepatic angiosarcoma. Pediatr Blood Cancer 64 (11): , 2017. [PUBMED Abstract]
- Mehrabi A, Kashfi A, Fonouni H, et al.: Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer 107 (9): 2108-21, 2006. [PUBMED Abstract]
- Haro A, Saitoh G, Tamiya S, et al.: Four-year natural clinical course of pulmonary epithelioid hemangioendothelioma without therapy. Thorac Cancer 6 (4): 544-7, 2015. [PUBMED Abstract]
- Sardaro A, Bardoscia L, Petruzzelli MF, et al.: Epithelioid hemangioendothelioma: an overview and update on a rare vascular tumor. Oncol Rev 8 (2): 259, 2014. [PUBMED Abstract]
- Dong K, Wang XX, Feng JL, et al.: Pathological characteristics of liver biopsies in eight patients with hepatic epithelioid hemangioendothelioma. Int J Clin Exp Pathol 8 (9): 11015-23, 2015. [PUBMED Abstract]
- Adams DM, Hammill A: Other vascular tumors. Semin Pediatr Surg 23 (4): 173-7, 2014. [PUBMED Abstract]
- Xiao Y, Wang C, Song Y, et al.: Primary epithelioid hemangioendothelioma of the kidney: the first case report in a child and literature review. Urology 82 (4): 925-7, 2013. [PUBMED Abstract]
- Reich S, Ringe H, Uhlenberg B, et al.: Epithelioid hemangioendothelioma of the lung presenting with pneumonia and heart rhythm disturbances in a teenage girl. J Pediatr Hematol Oncol 32 (4): 274-6, 2010. [PUBMED Abstract]
- Daller JA, Bueno J, Gutierrez J, et al.: Hepatic hemangioendothelioma: clinical experience and management strategy. J Pediatr Surg 34 (1): 98-105; discussion 105-6, 1999. [PUBMED Abstract]
- Ackermann O, Fabre M, Franchi S, et al.: Widening spectrum of liver angiosarcoma in children. J Pediatr Gastroenterol Nutr 53 (6): 615-9, 2011. [PUBMED Abstract]
- Stacchiotti S, Provenzano S, Dagrada G, et al.: Sirolimus in Advanced Epithelioid Hemangioendothelioma: A Retrospective Case-Series Analysis from the Italian Rare Cancer Network Database. Ann Surg Oncol 23 (9): 2735-44, 2016. [PUBMED Abstract]
- Semenisty V, Naroditsky I, Keidar Z, et al.: Pazopanib for metastatic pulmonary epithelioid hemangioendothelioma-a suitable treatment option: case report and review of anti-angiogenic treatment options. BMC Cancer 15: 402, 2015. [PUBMED Abstract]
- Raheja A, Suri A, Singh S, et al.: Multimodality management of a giant skull base hemangioendothelioma of the sphenopetroclival region. J Clin Neurosci 22 (9): 1495-8, 2015. [PUBMED Abstract]
- Ahmad N, Adams DM, Wang J, et al.: Hepatic epithelioid hemangioendothelioma in a patient with hemochromatosis. J Natl Compr Canc Netw 12 (9): 1203-7, 2014. [PUBMED Abstract]
- Otte JB, Zimmerman A: The role of liver transplantation for pediatric epithelioid hemangioendothelioma. Pediatr Transplant 14 (3): 295-7, 2010. [PUBMED Abstract]
Treatment of Metastatic Childhood Soft Tissue Sarcoma
Standard treatment options for metastatic childhood soft tissue sarcoma include the following:
- Combination therapy using chemotherapy, radiation therapy, and surgical resection of pulmonary metastases.
For treatment options, refer to the individual tumor type sections of the summary.
The prognosis for children with metastatic soft tissue sarcomas is poor,[1-6] and these children should receive combined treatment with chemotherapy, radiation therapy, and surgical resection of pulmonary metastases. In a prospective randomized trial, chemotherapy with vincristine, dactinomycin, doxorubicin, and cyclophosphamide, with or without dacarbazine, led to tumor responses in one-third of patients with unresectable or metastatic disease. The estimated 4-year survival rate, however, was poor, with fewer than one-third of children surviving.[6-8]
Pulmonary Metastases
Generally, children with isolated pulmonary metastases should be considered for a surgical procedure in an attempt to resect all gross disease.[9] For patients with multiple or recurrent pulmonary metastases, additional surgical procedures can be performed if the morbidity is deemed acceptable. In a retrospective review, patients with synovial sarcoma and pulmonary metastases for whom it was possible to completely resect all metastatic lung lesions had better survival than did patients for whom it was not possible to achieve complete resections.[9][Level of evidence: 3iiiA] Formal segmentectomy, lobectomy, and mediastinal lymph node dissection are unnecessary.[10]
An alternative approach is focused radiation therapy (fractionated stereotactic radiation therapy), which has been successfully used in adults to control lesions. The estimated 5-year survival rate after thoracotomy for pulmonary metastasectomy has ranged from 10% to 58% in adult studies. Emerging data suggest a similar outcome after the administration of focused radiation therapy.[11]
References
- Demetri GD, Elias AD: Results of single-agent and combination chemotherapy for advanced soft tissue sarcomas. Implications for decision making in the clinic. Hematol Oncol Clin North Am 9 (4): 765-85, 1995. [PUBMED Abstract]
- Elias A, Ryan L, Sulkes A, et al.: Response to mesna, doxorubicin, ifosfamide, and dacarbazine in 108 patients with metastatic or unresectable sarcoma and no prior chemotherapy. J Clin Oncol 7 (9): 1208-16, 1989. [PUBMED Abstract]
- Edmonson JH, Ryan LM, Blum RH, et al.: Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol 11 (7): 1269-75, 1993. [PUBMED Abstract]
- Rao BN: Nonrhabdomyosarcoma in children: prognostic factors influencing survival. Semin Surg Oncol 9 (6): 524-31, 1993 Nov-Dec. [PUBMED Abstract]
- deCou JM, Rao BN, Parham DM, et al.: Malignant peripheral nerve sheath tumors: the St. Jude Children's Research Hospital experience. Ann Surg Oncol 2 (6): 524-9, 1995. [PUBMED Abstract]
- Pappo AS, Rao BN, Jenkins JJ, et al.: Metastatic nonrhabdomyosarcomatous soft-tissue sarcomas in children and adolescents: the St. Jude Children's Research Hospital experience. Med Pediatr Oncol 33 (2): 76-82, 1999. [PUBMED Abstract]
- Pratt CB, Pappo AS, Gieser P, et al.: Role of adjuvant chemotherapy in the treatment of surgically resected pediatric nonrhabdomyosarcomatous soft tissue sarcomas: A Pediatric Oncology Group Study. J Clin Oncol 17 (4): 1219, 1999. [PUBMED Abstract]
- Pratt CB, Maurer HM, Gieser P, et al.: Treatment of unresectable or metastatic pediatric soft tissue sarcomas with surgery, irradiation, and chemotherapy: a Pediatric Oncology Group study. Med Pediatr Oncol 30 (4): 201-9, 1998. [PUBMED Abstract]
- Stanelle EJ, Christison-Lagay ER, Wolden SL, et al.: Pulmonary metastasectomy in pediatric/adolescent patients with synovial sarcoma: an institutional review. J Pediatr Surg 48 (4): 757-63, 2013. [PUBMED Abstract]
- Putnam JB Jr, Roth JA: Surgical treatment for pulmonary metastases from sarcoma. Hematol Oncol Clin North Am 9 (4): 869-87, 1995. [PUBMED Abstract]
- Dhakal S, Corbin KS, Milano MT, et al.: Stereotactic body radiotherapy for pulmonary metastases from soft-tissue sarcomas: excellent local lesion control and improved patient survival. Int J Radiat Oncol Biol Phys 82 (2): 940-5, 2012. [PUBMED Abstract]
Treatment of Progressive/Recurrent Childhood Soft Tissue Sarcoma
With the possible exception of infants with infantile fibrosarcoma, the prognosis for patients with recurrent or progressive disease is poor. No prospective trial has been able to prove that enhanced local control of pediatric soft tissue sarcomas will ultimately improve survival. Therefore, treatment should be individualized for the site of recurrence, biologic characteristics of the tumor (e.g., grade, invasiveness, and size), previous therapies, and individual patient considerations.
Treatment options for recurrent or progressive disease include the following:
- Surgical excision of local recurrence or isolated pulmonary recurrence.
- An Italian review of 73 patients with recurrent malignant peripheral nerve sheath tumors found that most relapses were local. Multivariate analysis showed that the factors associated with improved survival were no tumor invasiveness at initial diagnosis (T1), time of recurrence more than 12 months after initial diagnosis, and achievement of a second complete response with surgical removal of the recurrence(s). Only 15.8% of patients who had complete surgical excisions of local recurrence(s) were alive at 5 years.[1][Level of evidence: 3iiiA]
- Surgical excision of local recurrence followed by radiation therapy or brachytherapy (if no previous radiation therapy was given).
- Limb amputation (only for some children with extremity sarcomas that have already received radiation therapy).
- Gemcitabine and docetaxel.[2]
- Trabectedin.[3-5]
- Pazopanib. A phase I trial of pazopanib reported one partial response in a patient with desmoplastic small round cell tumor and prolonged disease stabilization in eight patients with recurrent sarcoma.[6][Level of evidence: 2Diii] Pazopanib has been approved for use in recurrent soft tissue sarcoma. The clinical trial that was used to obtain approval was limited to adults and demonstrated disease stabilization and prolonged time to progression; it did not demonstrate improved overall survival.[7] One 13-year-old boy and one 14-year-old girl with multiply recurrent synovial sarcoma and lung metastases had responses to pazopanib for 14 and 15 months, respectively.[8][Level of evidence: 3iiDi]
- A clinical trial of new chemotherapeutic regimens.
Resection is the standard treatment for recurrent pediatric nonrhabdomyosarcomatous soft tissue sarcomas. If the patient has not yet received radiation therapy, postoperative radiation should be considered after local excision of the recurrent tumor. Limb-sparing procedures with postoperative brachytherapy have been evaluated in adults but have not been studied extensively in children. For some children with extremity sarcomas who have received previous radiation therapy, amputation may be the only therapeutic option.
Pulmonary metastasectomy may achieve prolonged disease control for some patients.[9] A large, retrospective analysis of patients with recurrent soft tissue sarcoma showed that isolated local relapse had a better prognosis and that resection of pulmonary metastases improved the probability of survival.[10] In 31 children and adolescents younger than 23 years with pulmonary metastases from synovial sarcoma, complete resection of lung metastases appeared to prolong survival when compared with ten other patients who were not considered candidates for metastasectomy.[11][Level of evidence: 3iiiA] All patients with recurrent tumors should be considered for current clinical trials.
Published results of two studies addressed the outcomes for children with relapsed synovial sarcoma. Most patients in one study had distant relapse (29 of 44 patients),[12] while most patients in the second study had local relapse (27 of 37 patients).[13] Distant recurrence was a poor prognostic variable, while tumor resectability at relapse (as manifested by extremity recurrence) was associated with a better outcome in both studies.
Treatment Options Under Clinical Evaluation
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- APEC1621 (NCT03155620) (Pediatric MATCH: Targeted Therapy Directed by Genetic Testing in Treating Pediatric Patients with Relapsed or Refractory Advanced Solid Tumors, Non-Hodgkin Lymphomas, or Histiocytic Disorders): NCI-COG Pediatric Molecular Analysis for Therapeutic Choice (MATCH), referred to as Pediatric MATCH, will match targeted agents with specific molecular changes identified using a next-generation sequencing targeted assay of more than 3,000 different mutations across more than 160 genes in refractory and recurrent solid tumors. Children and adolescents aged 1 to 21 years are eligible for the trial.
Tumor tissue from progressive or recurrent disease must be available for molecular characterization. Patients with tumors that have molecular variants addressed by treatment arms included in the trial will be offered treatment on Pediatric MATCH. Additional information can be obtained on the ClinicalTrials.gov website for APEC1621 (NCT03155620).
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Bergamaschi L, Bisogno G, Manzitti C, et al.: Salvage rates and prognostic factors after relapse in children and adolescents with malignant peripheral nerve sheath tumors. Pediatr Blood Cancer 65 (2): , 2018. [PUBMED Abstract]
- Maki RG, Wathen JK, Patel SR, et al.: Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 25 (19): 2755-63, 2007. [PUBMED Abstract]
- Le Cesne A, Cresta S, Maki RG, et al.: A retrospective analysis of antitumour activity with trabectedin in translocation-related sarcomas. Eur J Cancer 48 (16): 3036-44, 2012. [PUBMED Abstract]
- Garcia-Carbonero R, Supko JG, Maki RG, et al.: Ecteinascidin-743 (ET-743) for chemotherapy-naive patients with advanced soft tissue sarcomas: multicenter phase II and pharmacokinetic study. J Clin Oncol 23 (24): 5484-92, 2005. [PUBMED Abstract]
- Garcia-Carbonero R, Supko JG, Manola J, et al.: Phase II and pharmacokinetic study of ecteinascidin 743 in patients with progressive sarcomas of soft tissues refractory to chemotherapy. J Clin Oncol 22 (8): 1480-90, 2004. [PUBMED Abstract]
- Glade Bender JL, Lee A, Reid JM, et al.: Phase I pharmacokinetic and pharmacodynamic study of pazopanib in children with soft tissue sarcoma and other refractory solid tumors: a children's oncology group phase I consortium report. J Clin Oncol 31 (24): 3034-43, 2013. [PUBMED Abstract]
- van der Graaf WT, Blay JY, Chawla SP, et al.: Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 379 (9829): 1879-86, 2012. [PUBMED Abstract]
- Casanova M, Basso E, Magni C, et al.: Response to pazopanib in two pediatric patients with pretreated relapsing synovial sarcoma. Tumori 103 (1): e1-e3, 2017. [PUBMED Abstract]
- Belal A, Salah E, Hajjar W, et al.: Pulmonary metastatectomy for soft tissue sarcomas: is it valuable? J Cardiovasc Surg (Torino) 42 (6): 835-40, 2001. [PUBMED Abstract]
- Zagars GK, Ballo MT, Pisters PW, et al.: Prognostic factors for disease-specific survival after first relapse of soft-tissue sarcoma: analysis of 402 patients with disease relapse after initial conservative surgery and radiotherapy. Int J Radiat Oncol Biol Phys 57 (3): 739-47, 2003. [PUBMED Abstract]
- Stanelle EJ, Christison-Lagay ER, Wolden SL, et al.: Pulmonary metastasectomy in pediatric/adolescent patients with synovial sarcoma: an institutional review. J Pediatr Surg 48 (4): 757-63, 2013. [PUBMED Abstract]
- Ferrari A, De Salvo GL, Dall'Igna P, et al.: Salvage rates and prognostic factors after relapse in children and adolescents with initially localised synovial sarcoma. Eur J Cancer 48 (18): 3448-55, 2012. [PUBMED Abstract]
- Soole F, Maupain C, Defachelles AS, et al.: Synovial sarcoma relapses in children and adolescents: prognostic factors, treatment, and outcome. Pediatr Blood Cancer 61 (8): 1387-93, 2014. [PUBMED Abstract]
Changes to This Summary (04/02/2018)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Treatment of Newly Diagnosed Childhood Soft Tissue Sarcoma
Added text to state that a small series reported symptomatic improvement and stable disease in seven patients with desmoid-type fibromatosis who were treated with pazopanib (cited Agresta et al. as reference 48).
Added text to state that a tumor with morphology similar to that of infantile fibrosarcoma has been identified in older children; in these older children, the tumors do not have the t(12;15)(ETV-NTRK3) translocation that is characteristic of the younger patients. In several of these patients, BRAF gene fusions have been identified (cited Kao et al. as reference 69).
Added text about the outcome results of 73 children and adolescents with recurrent malignant peripheral nerve sheath tumor reported by the Italian Sarcoma Group (cited Bergamaschi et al. as reference 127 and level of evidence 3iiiA).
Added text about the patient characteristics and results of a retrospective review of children and young adults younger than 30 years from four institutions, which identified 69 patients with alveolar soft part sarcoma treated primarily with surgery between 1980 and 2014 (cited Flores et al. as reference 152 and level of evidence 3iiA).
Added Sedig et al. as reference 172 and level of evidence 3iiiA.
Treatment of Progressive/Recurrent Childhood Soft Tissue Sarcoma
Added text about the prognostic factors and outcome results reported in an Italian review of 73 children and adolescents with recurrent malignant peripheral nerve sheath tumor (cited Bergamaschi et al. as reference 1 and level of evidence 3iiiA).
This summary is written and maintained by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood soft tissue sarcoma. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Childhood Soft Tissue Sarcoma Treatment are:
- Denise Adams, MD (Children's Hospital Boston)
- Louis S. Constine, MD (James P. Wilmot Cancer Center at University of Rochester Medical Center)
- Holcombe Edwin Grier, MD (Dana-Farber Cancer Institute/Boston Children's Hospital)
- Andrea A. Hayes-Jordan, MD, FACS, FAAP (M.D. Anderson Cancer Center)
- Paul A. Meyers, MD (Memorial Sloan-Kettering Cancer Center)
- Thomas A. Olson, MD (Aflac Cancer and Blood Disorders Center of Children's Healthcare of Atlanta - Egleston Campus)
- Alberto S. Pappo, MD (St. Jude Children's Research Hospital)
- R Beverly Raney, MD (Consultant)
- Stephen J. Shochat, MD (St. Jude Children's Research Hospital)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Soft Tissue Sarcoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/soft-tissue-sarcoma/hp/child-soft-tissue-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389361]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.
Updated: April 2, 2018
Source URL: https://www.cancer.gov/publishedcontent/syndication/3899.htm
Source Agency: National Cancer Institute (NCI)
Captured Date: 2013-09-14 09:01:57.0
Childhood Soft Tissue Sarcoma Treatment (PDQ®)–Health Professional Version
General Information About Childhood Soft Tissue Sarcoma
Dramatic improvements in survival have been achieved for children and adolescents with cancer. Between 1975 and 2010, childhood cancer mortality decreased by more than 50%.[1] Childhood and adolescent cancer survivors require close monitoring because cancer therapy side effects may persist or develop months or years after treatment. (Refer to the PDQ summary on Late Effects of Treatment for Childhood Cancer for specific information about the incidence, type, and monitoring of late effects in childhood and adolescent cancer survivors.)
Rhabdomyosarcoma, a tumor of striated muscle, is the most common soft tissue sarcoma in children aged 0 to 14 years and accounts for 50% of tumors in this age group.[2] (Refer to the PDQ summary on Childhood Rhabdomyosarcoma Treatment for more information.) In pediatrics, the remaining soft tissue sarcomas are commonly referred to as nonrhabdomyosarcomatous soft tissue sarcomas and account for approximately 3% of all childhood tumors.[3] This heterogeneous group of tumors includes the following neoplasms:[4]
- Connective tissue (e.g., desmoid-type fibromatosis).
- Peripheral nervous system (e.g., malignant peripheral nerve sheath tumor).
- Smooth muscle (e.g., leiomyosarcoma).
- Vascular tissue (blood and lymphatic vessels, e.g., angiosarcoma). (Refer to the PDQ summary on Childhood Vascular Tumors Treatment for more information about childhood vascular tumors.)
Distribution of Soft Tissue Sarcoma by Age and Histology
Pediatric soft tissue sarcomas are a heterogenous group of malignant tumors that originate from primitive mesenchymal tissue and account for 7% of all childhood tumors.[5]
The distribution of soft tissue sarcomas by histology and age, based on the Surveillance, Epidemiology, and End Results (SEER) information from 1975 to 2012, is depicted in Table 1. The distribution of histologic subtypes by age is also shown in Figure 2.
| Age <5 y | Age 5–9 y | Age 10–14 y | Age 15–19 y | % of the Total Number of STS Cases <20 y | |||
|---|---|---|---|---|---|---|---|
| pPNET = peripheral primitive neuroectodermal tumors; SEER = Surveillance, Epidemiology, and End Results; STS = soft tissue sarcoma. | |||||||
| aSEER data is available at http://seer.cancer.gov. | |||||||
| bDermatofibrosarcoma accounts for 75% of these cases. | |||||||
| All soft tissue and other extraosseous sarcomas | 923 | 631 | 946 | 1,267 | 100 | ||
| Rhabdomyosarcomas | 551 | 348 | 312 | 270 | 39 | ||
| Fibrosarcomas, peripheral nerve, and other fibrous neoplasms | 116 | 50 | 88 | 141 | 10 | ||
| Fibroblastic and myofibroblastic tumors | 97 | 24 | 31 | 62 | 6 | ||
| Nerve sheath tumors | 19 | 26 | 56 | 77 | 5 | ||
| Other fibromatous neoplasms | 0 | 0 | 1 | 2 | 0.1 | ||
| Kaposi sarcoma | 2 | 1 | 1 | 9 | 0.3 | ||
| Other specified soft tissue sarcomas | 194 | 190 | 424 | 708 | 40 | ||
| Ewing tumor and Askin tumor of soft tissue | 27 | 30 | 62 | 92 | 6 | ||
| pPNET of soft tissue | 21 | 18 | 36 | 46 | 3.2 | ||
| Extrarenal rhabdoid tumor | 61 | 3 | 7 | 3 | 2 | ||
| Liposarcomas | 3 | 5 | 22 | 57 | 2.3 | ||
| Fibrohistiocytic tumors b | 34 | 54 | 108 | 188 | 10 | ||
| Leiomyosarcomas | 9 | 14 | 15 | 36 | 2 | ||
| Synovial sarcomas | 10 | 34 | 111 | 175 | 9 | ||
| Blood vessel tumors | 11 | 7 | 8 | 25 | 1.4 | ||
| Osseous and chondromatous neoplasms of soft tissue | 1 | 6 | 13 | 10 | 0.8 | ||
| Alveolar soft parts sarcoma | 4 | 3 | 16 | 29 | 1.4 | ||
| Miscellaneous soft tissue sarcomas | 13 | 16 | 36 | 47 | 3 | ||
| Unspecified soft tissue sarcomas | 60 | 40 | 111 | 139 | 9.3 | ||
Nonrhabdomyosarcomatous soft tissue sarcomas are more common in adolescents and adults,[4] and most of the information regarding treatment and natural history of the disease in younger patients has been based on adult studies. The distributions of these tumors by age according to stage, histologic subtype, and tumor site are shown in Figures 1, 2, and 3, respectively.[6]



Risk Factors
Some genetic and environmental factors have been associated with the development of nonrhabdomyosarcomatous soft tissue sarcoma, including the following:
- Genetic factors:
- Li-Fraumeni syndrome: Patients with Li-Fraumeni syndrome (usually due to heritable cancer-associated changes of the TP53 tumor suppressor gene) have an increased risk of developing soft tissue tumors (mostly nonrhabdomyosarcomatous soft tissue sarcomas), bone sarcomas, breast cancer, brain tumors, and acute leukemia.[7,8]
- Familial adenomatous polyposis: Patients with familial adenomatous polyposis are at increased risk of developing desmoid-type fibromatosis.[9]
- Retinoblastoma (RB1) gene: Germline mutations of the retinoblastoma gene have been associated with an increased risk of developing soft tissue sarcomas, particularly leiomyosarcoma.[10]
- SMARCB1 gene: Germline mutations or deletions of the SMARCB1 (INI1) gene are associated with an increased risk of developing extrarenal rhabdoid tumors.[11]
- Neurofibromatosis type 1: Approximately 4% of patients with neurofibromatosis type 1 develop malignant peripheral nerve sheath tumors, which usually develop after a long latency; some patients develop multiple lesions.[12-14]
- Werner syndrome: Werner syndrome is characterized by spontaneous chromosomal instability, resulting in increased susceptibility to cancer and premature aging. An excess of soft tissue sarcomas has been reported in patients with Werner syndrome.[15]
- Environmental factors:
- Radiation: Some nonrhabdomyosarcomatous soft tissue sarcomas (particularly malignant fibrous histiocytoma) can develop within a previously irradiated site.[3,16]
- Epstein-Barr virus infection in patients with AIDS: Some nonrhabdomyosarcomatous soft tissue sarcomas (e.g., leiomyosarcoma) have been linked to Epstein-Barr virus infection in patients with AIDS.[3,17]
Clinical Presentation
Although nonrhabdomyosarcomatous soft tissue sarcomas can develop in any part of the body, they arise most commonly in the trunk and extremities.[18-20] These neoplasms can present initially as an asymptomatic solid mass, or they may be symptomatic because of local invasion of adjacent anatomical structures. Although rare, these tumors can arise primarily in brain tissue and are treated according to the histotype.[21]
Systemic symptoms (e.g., fever, weight loss, and night sweats) are rare. Hypoglycemia and hypophosphatemic rickets have been reported in cases of hemangiopericytoma, whereas hyperglycemia has been noted in patients with fibrosarcoma of the lung.[22]
Diagnostic and Staging Evaluation
When a suspicious lesion is identified, it is crucial that a complete workup, followed by adequate biopsy be performed. It is best to image the lesion using the following procedures before initiating any intervention:
- Plain films. Plain films can be used to rule out bone involvement and detect calcifications that may be seen in soft tissue tumors such as extraskeletal osteosarcoma or synovial sarcoma.
- Chest computed tomography (CT). Chest CT is essential to assess the presence of metastases.
- Abdominal CT or magnetic resonance imaging (MRI). Abdominal CT or MRI can be used to image intra-abdominal tumors, such as liposarcoma.
- Extremity MRI. MRI is essential for extremity lesions.
- Positron emission tomography (PET) scan and bone scan. In children with rhabdomyosarcoma, PET-CT performed better than conventional imaging in identifying nodal, bone, bone marrow, and soft tissue disease. The authors of an imaging comparison study suggest that bone scans with technetium Tc 99m might be eliminated as a staging procedure.[23] The use of this modality in pediatric nonrhabdomyosarcomatous soft tissue sarcoma has not been studied extensively. However, a small study of nine patients with nonrhabdomyosarcomatous soft tissue sarcoma suggests that PET-CT is more accurate and cost effective than either modality alone in identifying distant metastatic disease.[24]
The imaging characteristics of some tumors can be highly suggestive of this diagnosis. For example, the imaging characteristics of pediatric low-grade fibromyxoid sarcoma and alveolar soft part sarcoma have been described and can aid in the diagnosis of these rare neoplasms.[25]
Biopsy strategies
Although nonrhabdomyosarcomatous soft tissue tumors are fairly readily distinguished pathologically from rhabdomyosarcoma and Ewing sarcoma, the classification of childhood nonrhabdomyosarcomatous soft tissue sarcoma type is often difficult. Core-needle biopsy, incisional biopsy, or excisional biopsy can be used to diagnose a nonrhabdomyosarcomatous soft tissue sarcoma. If possible, the surgeon who will perform the definitive resection needs to be involved in the biopsy decision. Poorly placed incisional or needle biopsies may adversely affect the performance of the primary resection.
Considerations related to the selection of a biopsy procedure are as follows:
- Given the diagnostic importance of translocations, a core-needle biopsy or small incisional biopsy that obtains adequate tumor tissue is crucial to allow for conventional histology, immunocytochemical analysis, and other studies such as light and electron microscopy, cytogenetics, fluorescence in situ hybridization, and molecular pathology.[26,27] Core-needle biopsy for a deep-seated tumor can lead to formation of a hematoma, which affects subsequent resection and/or radiation; in these cases, incisional biopsy is the preferred procedure.
- Fine-needle biopsy is usually not recommended because it is difficult to determine the accurate histologic diagnosis and grade of the tumor in this heterogeneous group of tumors.
- Image guidance using ultrasound, CT scan, or MRI may be necessary to ensure a representative biopsy.[28]
- Needle biopsy techniques must ensure adequate tissue sampling. The acquisition of multiple cores of tissue may be required.
- Incisional biopsies must not compromise subsequent wide local excision.
- Excisional biopsy of the lesion is only appropriate for small superficial lesions (<3 cm in size) and are discouraged.[29,30] If an excisional biopsy is contemplated, then MRI of the area is recommended to define the area of involvement as subsequent surgery or radiation therapy is likely.
- Various institutional series have demonstrated the feasibility and effectiveness of sentinel node biopsy as a staging procedure in pediatric patients with soft tissue sarcomas.[31-36]
- Transverse extremity incisions are avoided to reduce skin loss and because they require a greater cross-sectional volume of tissue to be covered in the radiation field. Other extensive surgical procedures are also avoided before definitive diagnosis. For these reasons, open biopsy or multiple core-needle biopsies are strongly encouraged so that adequate tumor tissue can be obtained to allow crucial studies to be performed and to avoid limiting future treatment options.
Unplanned resection
In children with unplanned resection of nonrhabdomyosarcomatous soft tissue sarcomas, primary re-excision is frequently recommended because many patients will have tumor present in the re-excision specimen.[37,38] A single-institution analysis of adolescents and adults compared patients with unplanned excision of soft tissue sarcoma to stage-matched controls. In this retrospective analysis, unplanned initial excision of soft tissue sarcoma resulted in increased risk of local recurrence, metastasis, and death; this increase was greatest for high-grade tumors.[39][Level of evidence: 3iiA]
Chromosomal abnormalities
Many nonrhabdomyosarcomatous soft tissue sarcomas are characterized by chromosomal abnormalities. Some of these chromosomal translocations lead to a fusion of two disparate genes. The resulting fusion transcript can be readily detected by using polymerase chain reaction-based techniques, thus facilitating the diagnosis of those neoplasms that have translocations.
Some of the most frequent aberrations seen in nonrhabdomyosarcomatous soft tissue tumors are listed in Table 2.
| Histology | Chromosomal Aberrations | Genes Involved |
|---|---|---|
| aAdapted from Sandberg,[40] Slater et al.,[41] Mertens et al.,[42] and Romeo.[43] | ||
| Alveolar soft part sarcoma | t(x;17)(p11.2;q25) | ASPL/TFE3 [44-46] |
| Angiomatoid fibrous histiocytoma | t(12;16)(q13;p11), t(2;22)(q33;q12), t(12;22)(q13;q12) | FUS/ATF1, EWSR1/CREB1,[47] EWS/ATF1 |
| Clear cell sarcoma | t(12;22)(q13;q12), t(2;22)(q33;q12) | ATF1/EWS, EWSR1/CREB1 |
| Congenital (infantile) fibrosarcoma/mesoblastic nephroma | t(12;15)(p13;q25) | ETV-NTRK3 |
| Dermatofibrosarcoma protuberans | t(17;22)(q22;q13) | COL1A1/PDGFB |
| Desmoid fibromatosis | Trisomy 8 or 20, loss of 5q21 | CTNNB1 or APC mutations |
| Desmoplastic small round cell tumors | t(11;22)(p13;q12) | EWS/WT1 [48,49] |
| Epithelioid hemangioendothelioma | t(1;3)(p36;q25) [50] | WWTR1/CAMTA1 |
| Epithelioid sarcoma | Inactivation SMARCB1 | SMARCB1 |
| Extraskeletal myxoid chondrosarcoma | t(9;22)(q22;q12), t(9;17)(q22;q11), t(9;15)(q22;q21), t(3;9)(q11;q22) | EWSR1/NR4A3, TAF2N/NR4A3, TCF12/NR4A3, TGF/NR4A3 |
| Hemangiopericytoma | t(12;19)(q13;q13.3) and t(13;22)(q22;q13.3) | |
| Infantile fibrosarcoma | t(12;15)(p13;q25) | ETV6/NTRK3 |
| Inflammatory myofibroblastic tumor | t(1;2)(q23;q23), t(2;19)(q23;q13), t(2;17)(q23;q23), t(2;2)(p23;q13), t(2;11)(p23;p15) [51] | TPM3/ALK, TPM4/ALK, CLTC/ALK, RANBP2/ALK, CARS/ALK, RAS |
| Low-grade fibromyxoid sarcoma | t(7;16)(q33;p11), t(11;16)(p11;p11) | FUS/CREB3L2, FUS/CREB3L1 |
| Malignant peripheral nerve sheath tumor | 17q11.2, loss or rearrangement 10p, 11q, 17q, 22q | NF1 |
| Mesenchymal chondrosarcoma | Del(8)(q13.3q21.1) | HEY1/NCOA2 |
| Myoepithelioma | t(19;22)(q13;q12), t(1;22)(q23;q12), t(6;22)(p21;q12) | EWSR/ZNF44, EWSR/PBX1, EWSR/POU5F1 |
| Myxoid/round cell liposarcoma | t(12;16)(q13;p11), t(12;22)(q13;q12) | FUS/DD1T3, EWSR/DD1T3 |
| Rhabdoid tumor | Inactivation SMARCB1 | SMARCB1 |
| Solitary fibrous tumor | Inv(12)(q13q13) | NAB2/STAT6 |
| Synovial sarcoma | t(x;18)(p11.2;q11.2) | SYT/SSX |
| Tenosynovial giant cell tumor | t(1;2)(p13;q35) | COL6A3/CSF1 |
Prognosis
The prognosis of nonrhabdomyosarcomatous soft tissue sarcoma varies greatly depending on the following factors:[52-54]
- Site of the primary tumor.
- Tumor size.
- Tumor grade. (Refer to the Prognostic Significance of Tumor Grading section of this summary for more information.)
- Tumor histology.
- Depth of tumor invasion.
- Presence of metastases.
- Resectability of the tumor.
- Use of radiation therapy.
Several adult and pediatric series have shown that patients with large or invasive tumors have a significantly worse prognosis than do those with small, noninvasive tumors. A retrospective review of soft tissue sarcomas in children and adolescents suggests that the 5 cm cutoff used for adults with soft tissue sarcoma may not be ideal for smaller children, especially infants. The review identified an interaction between tumor diameter and body surface area.[55] This relationship requires further study to determine the therapeutic implications of the observation.
In a review of a large adult series of nonrhabdomyosarcomatous soft tissue sarcomas, superficial extremity sarcomas had a better prognosis than did deep tumors. Thus, in addition to grade and size, the depth of invasion of the tumor should be considered.[56]
Some pediatric nonrhabdomyosarcomatous soft tissue sarcomas are associated with a better outcome. For instance, infantile fibrosarcoma, presenting in infants and children younger than 5 years, has an excellent prognosis given that surgery alone can cure a significant number of these patients and the tumor is highly chemosensitive.[3]
Soft tissue sarcomas in older children and adolescents often behave similarly to those in adult patients.[3,26] A large, prospective, multinational Children's Oncology Group study (ARST0332 [NCT00346164]) enrolled newly diagnosed patients younger than 30 years. Patients were assigned to treatment on the basis of their risk group (refer to Figure 4).[57][Level of evidence: 2A]

- Arm A (grossly excised low-grade tumor and ≤5 cm widely excised high-grade tumor): Surgery only.
- Arm B (≤5 cm marginally resected high-grade tumor): 55.8 Gy of radiation therapy.
- Arm C (>5 cm grossly resected tumor ± metastases): Ifosfamide/doxorubicin chemotherapy and 55.8 Gy of radiation therapy.
- Arm D (>5 cm unresected tumor ± metastases): Preoperative ifosfamide/doxorubicin chemotherapy and 45 Gy of radiation therapy, and then surgery and a radiation boost that was based on margins.
Of 551 patients enrolled, at a median follow-up of 2.6 years, the preliminary analysis estimated the following 3-year survival rates:[57]
- Arm A: 91% event-free survival (EFS); 99% overall survival (OS).
- Arm B: 79% EFS; 100% OS.
- Arm C: 68% EFS; 81% OS.
- Arm D: 52% EFS; 66% OS.
Pediatric patients with unresected localized nonrhabdomyosarcomatous soft tissue sarcomas have a poor outcome. Only about one-third of patients treated with multimodality therapy remain disease free.[52,58]; [59,60][Level of evidence: 3iiiA] In a review of 30 Italian patients with nonrhabdomyosarcomatous soft tissue sarcoma at visceral sites, only ten patients survived at 5 years. Unfavorable prognostic factors included inability to achieve complete resection, large tumor size, tumor invasion, histologic subtype, and lung-pleura sites.[61][Level of evidence: 3iiB]
In a pooled analysis from U.S. and European pediatric centers, outcome was better for patients whose tumor removal procedure was deemed complete than for patients whose tumor removal was incomplete. Outcome was better for patients who received radiation therapy than for patients who did not.[59][Level of evidence: 3iiiA]
Because long-term related morbidity must be minimized while disease-free survival is maximized, the ideal therapy for each patient must be carefully and individually determined utilizing these prognostic factors before initiating therapy.[19,62-66]
Related Summaries
Refer to the following PDQ summaries for information about other types of sarcoma:
- Childhood Rhabdomyosarcoma Treatment.
- Childhood Vascular Tumors Treatment.
- Ewing Sarcoma Treatment (extraosseous Ewing, peripheral neuroepithelioma, and Askin tumors).
- Unusual Cancers of Childhood Treatment (gastrointestinal stromal tumors).
- Adult Soft Tissue Sarcoma Treatment.
References
- Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014. [PUBMED Abstract]
- Ries LA, Smith MA, Gurney JG, et al., eds.: Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. Bethesda, Md: National Cancer Institute, SEER Program, 1999. NIH Pub.No. 99-4649. Also available online. Last accessed January 24, 2018.
- Spunt SL, Million L, Coffin C: The nonrhabdomyosarcoma soft tissue sarcoma. In: Pizzo PA, Poplack DG, eds.: Principles and Practice of Pediatric Oncology. 7th ed. Philadelphia, Pa: Lippincott Williams and Wilkins, 2015, pp 827-54.
- Weiss SW, Goldblum JR: General considerations. In: Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 5th ed. St. Louis, Mo: Mosby, 2008, pp 1-14.
- Pappo AS, Pratt CB: Soft tissue sarcomas in children. Cancer Treat Res 91: 205-22, 1997. [PUBMED Abstract]
- Ferrari A, Sultan I, Huang TT, et al.: Soft tissue sarcoma across the age spectrum: a population-based study from the Surveillance Epidemiology and End Results database. Pediatr Blood Cancer 57 (6): 943-9, 2011. [PUBMED Abstract]
- Chang F, Syrjänen S, Syrjänen K: Implications of the p53 tumor-suppressor gene in clinical oncology. J Clin Oncol 13 (4): 1009-22, 1995. [PUBMED Abstract]
- Plon SE, Malkin D: Childhood cancer and hereditary. In: Pizzo PA, Poplack DG, eds.: Principles and Practice of Pediatric Oncology. 7th ed. Philadelphia, Pa: Lippincott Williams and Wilkins, 2015, pp 13-31.
- Groen EJ, Roos A, Muntinghe FL, et al.: Extra-intestinal manifestations of familial adenomatous polyposis. Ann Surg Oncol 15 (9): 2439-50, 2008. [PUBMED Abstract]
- Kleinerman RA, Tucker MA, Abramson DH, et al.: Risk of soft tissue sarcomas by individual subtype in survivors of hereditary retinoblastoma. J Natl Cancer Inst 99 (1): 24-31, 2007. [PUBMED Abstract]
- Eaton KW, Tooke LS, Wainwright LM, et al.: Spectrum of SMARCB1/INI1 mutations in familial and sporadic rhabdoid tumors. Pediatr Blood Cancer 56 (1): 7-15, 2011. [PUBMED Abstract]
- Weiss SW, Goldblum JR: Benign tumors of peripheral nerves. In: Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 5th ed. St. Louis, Mo: Mosby, 2008, pp 825-901.
- deCou JM, Rao BN, Parham DM, et al.: Malignant peripheral nerve sheath tumors: the St. Jude Children's Research Hospital experience. Ann Surg Oncol 2 (6): 524-9, 1995. [PUBMED Abstract]
- Stark AM, Buhl R, Hugo HH, et al.: Malignant peripheral nerve sheath tumours--report of 8 cases and review of the literature. Acta Neurochir (Wien) 143 (4): 357-63; discussion 363-4, 2001. [PUBMED Abstract]
- Goto M, Miller RW, Ishikawa Y, et al.: Excess of rare cancers in Werner syndrome (adult progeria). Cancer Epidemiol Biomarkers Prev 5 (4): 239-46, 1996. [PUBMED Abstract]
- Weiss SW, Goldblum JR: Malignant fibrous histiocytoma (pleomorphic undifferentiated sarcoma). In: Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 5th ed. St. Louis, Mo: Mosby, 2008, pp 403-27.
- McClain KL, Leach CT, Jenson HB, et al.: Association of Epstein-Barr virus with leiomyosarcomas in children with AIDS. N Engl J Med 332 (1): 12-8, 1995. [PUBMED Abstract]
- Dillon P, Maurer H, Jenkins J, et al.: A prospective study of nonrhabdomyosarcoma soft tissue sarcomas in the pediatric age group. J Pediatr Surg 27 (2): 241-4; discussion 244-5, 1992. [PUBMED Abstract]
- Rao BN: Nonrhabdomyosarcoma in children: prognostic factors influencing survival. Semin Surg Oncol 9 (6): 524-31, 1993 Nov-Dec. [PUBMED Abstract]
- Zeytoonjian T, Mankin HJ, Gebhardt MC, et al.: Distal lower extremity sarcomas: frequency of occurrence and patient survival rate. Foot Ankle Int 25 (5): 325-30, 2004. [PUBMED Abstract]
- Benesch M, von Bueren AO, Dantonello T, et al.: Primary intracranial soft tissue sarcoma in children and adolescents: a cooperative analysis of the European CWS and HIT study groups. J Neurooncol 111 (3): 337-45, 2013. [PUBMED Abstract]
- Weiss SW, Goldblum JR: Miscellaneous tumors of intermediate malignancy. In: Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 5th ed. St. Louis, Mo: Mosby, 2008, pp 1093-1160.
- Federico SM, Spunt SL, Krasin MJ, et al.: Comparison of PET-CT and conventional imaging in staging pediatric rhabdomyosarcoma. Pediatr Blood Cancer 60 (7): 1128-34, 2013. [PUBMED Abstract]
- Tateishi U, Hosono A, Makimoto A, et al.: Accuracy of 18F fluorodeoxyglucose positron emission tomography/computed tomography in staging of pediatric sarcomas. J Pediatr Hematol Oncol 29 (9): 608-12, 2007. [PUBMED Abstract]
- Sargar K, Kao SC, Spunt SL, et al.: MRI and CT of Low-Grade Fibromyxoid Sarcoma in Children: A Report From Children's Oncology Group Study ARST0332. AJR Am J Roentgenol 205 (2): 414-20, 2015. [PUBMED Abstract]
- Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 4th ed. St. Louis, Mo: Mosby, 2001.
- Recommendations for the reporting of soft tissue sarcomas. Association of Directors of Anatomic and Surgical Pathology. Mod Pathol 11 (12): 1257-61, 1998. [PUBMED Abstract]
- Chowdhury T, Barnacle A, Haque S, et al.: Ultrasound-guided core needle biopsy for the diagnosis of rhabdomyosarcoma in childhood. Pediatr Blood Cancer 53 (3): 356-60, 2009. [PUBMED Abstract]
- Coffin CM, Dehner LP, O'Shea PA: Pediatric Soft Tissue Tumors: A Clinical, Pathological, and Therapeutic Approach. Baltimore, Md: Williams and Wilkins, 1997.
- Smith LM, Watterson J, Scott SM: Medical and surgical management of pediatric soft tissue tumors. In: Coffin CM, Dehner LP, O'Shea PA: Pediatric Soft Tissue Tumors: A Clinical, Pathological, and Therapeutic Approach. Baltimore, Md: Williams and Wilkins, 1997, pp 360-71.
- Neville HL, Andrassy RJ, Lally KP, et al.: Lymphatic mapping with sentinel node biopsy in pediatric patients. J Pediatr Surg 35 (6): 961-4, 2000. [PUBMED Abstract]
- Neville HL, Raney RB, Andrassy RJ, et al.: Multidisciplinary management of pediatric soft-tissue sarcoma. Oncology (Huntingt) 14 (10): 1471-81; discussion 1482-6, 1489-90, 2000. [PUBMED Abstract]
- Kayton ML, Delgado R, Busam K, et al.: Experience with 31 sentinel lymph node biopsies for sarcomas and carcinomas in pediatric patients. Cancer 112 (9): 2052-9, 2008. [PUBMED Abstract]
- Dall'Igna P, De Corti F, Alaggio R, et al.: Sentinel node biopsy in pediatric patients: the experience in a single institution. Eur J Pediatr Surg 24 (6): 482-7, 2014. [PUBMED Abstract]
- Parida L, Morrisson GT, Shammas A, et al.: Role of lymphoscintigraphy and sentinel lymph node biopsy in the management of pediatric melanoma and sarcoma. Pediatr Surg Int 28 (6): 571-8, 2012. [PUBMED Abstract]
- Alcorn KM, Deans KJ, Congeni A, et al.: Sentinel lymph node biopsy in pediatric soft tissue sarcoma patients: utility and concordance with imaging. J Pediatr Surg 48 (9): 1903-6, 2013. [PUBMED Abstract]
- Chui CH, Spunt SL, Liu T, et al.: Is reexcision in pediatric nonrhabdomyosarcoma soft tissue sarcoma necessary after an initial unplanned resection? J Pediatr Surg 37 (10): 1424-9, 2002. [PUBMED Abstract]
- Cecchetto G, Guglielmi M, Inserra A, et al.: Primary re-excision: the Italian experience in patients with localized soft-tissue sarcomas. Pediatr Surg Int 17 (7): 532-4, 2001. [PUBMED Abstract]
- Qureshi YA, Huddy JR, Miller JD, et al.: Unplanned excision of soft tissue sarcoma results in increased rates of local recurrence despite full further oncological treatment. Ann Surg Oncol 19 (3): 871-7, 2012. [PUBMED Abstract]
- Sandberg AA: Translocations in malignant tumors. Am J Pathol 159 (6): 1979-80, 2001. [PUBMED Abstract]
- Slater O, Shipley J: Clinical relevance of molecular genetics to paediatric sarcomas. J Clin Pathol 60 (11): 1187-94, 2007. [PUBMED Abstract]
- Mertens F, Antonescu CR, Hohenberger P, et al.: Translocation-related sarcomas. Semin Oncol 36 (4): 312-23, 2009. [PUBMED Abstract]
- Romeo S, Dei Tos AP: Clinical application of molecular pathology in sarcomas. Curr Opin Oncol 23 (4): 379-84, 2011. [PUBMED Abstract]
- Ladanyi M, Lui MY, Antonescu CR, et al.: The der(17)t(X;17)(p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene 20 (1): 48-57, 2001. [PUBMED Abstract]
- Ladanyi M: The emerging molecular genetics of sarcoma translocations. Diagn Mol Pathol 4 (3): 162-73, 1995. [PUBMED Abstract]
- Williams A, Bartle G, Sumathi VP, et al.: Detection of ASPL/TFE3 fusion transcripts and the TFE3 antigen in formalin-fixed, paraffin-embedded tissue in a series of 18 cases of alveolar soft part sarcoma: useful diagnostic tools in cases with unusual histological features. Virchows Arch 458 (3): 291-300, 2011. [PUBMED Abstract]
- Antonescu CR, Dal Cin P, Nafa K, et al.: EWSR1-CREB1 is the predominant gene fusion in angiomatoid fibrous histiocytoma. Genes Chromosomes Cancer 46 (12): 1051-60, 2007. [PUBMED Abstract]
- Barnoud R, Sabourin JC, Pasquier D, et al.: Immunohistochemical expression of WT1 by desmoplastic small round cell tumor: a comparative study with other small round cell tumors. Am J Surg Pathol 24 (6): 830-6, 2000. [PUBMED Abstract]
- Wang LL, Perlman EJ, Vujanic GM, et al.: Desmoplastic small round cell tumor of the kidney in childhood. Am J Surg Pathol 31 (4): 576-84, 2007. [PUBMED Abstract]
- Errani C, Zhang L, Sung YS, et al.: A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer 50 (8): 644-53, 2011. [PUBMED Abstract]
- Jain S, Xu R, Prieto VG, et al.: Molecular classification of soft tissue sarcomas and its clinical applications. Int J Clin Exp Pathol 3 (4): 416-28, 2010. [PUBMED Abstract]
- Spunt SL, Hill DA, Motosue AM, et al.: Clinical features and outcome of initially unresected nonmetastatic pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Clin Oncol 20 (15): 3225-35, 2002. [PUBMED Abstract]
- Spunt SL, Poquette CA, Hurt YS, et al.: Prognostic factors for children and adolescents with surgically resected nonrhabdomyosarcoma soft tissue sarcoma: an analysis of 121 patients treated at St Jude Children's Research Hospital. J Clin Oncol 17 (12): 3697-705, 1999. [PUBMED Abstract]
- Ferrari A, Casanova M, Collini P, et al.: Adult-type soft tissue sarcomas in pediatric-age patients: experience at the Istituto Nazionale Tumori in Milan. J Clin Oncol 23 (18): 4021-30, 2005. [PUBMED Abstract]
- Ferrari A, Miceli R, Meazza C, et al.: Soft tissue sarcomas of childhood and adolescence: the prognostic role of tumor size in relation to patient body size. J Clin Oncol 27 (3): 371-6, 2009. [PUBMED Abstract]
- Brooks AD, Heslin MJ, Leung DH, et al.: Superficial extremity soft tissue sarcoma: an analysis of prognostic factors. Ann Surg Oncol 5 (1): 41-7, 1998 Jan-Feb. [PUBMED Abstract]
- Spunt SL, Million L, Anderson JR, et al.: Risk-based treatment for nonrhabdomyosarcoma soft tissue sarcomas (NRSTS) in patients under 30 years of age: Children’s Oncology Group study ARST0332. [Abstract] J Clin Oncol 32 (Suppl 15): A-10008, 2014. Also available online. Last accessed April 02, 2018.
- O'Sullivan B, Davis AM, Turcotte R, et al.: Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359 (9325): 2235-41, 2002. [PUBMED Abstract]
- Ferrari A, Miceli R, Rey A, et al.: Non-metastatic unresected paediatric non-rhabdomyosarcoma soft tissue sarcomas: results of a pooled analysis from United States and European groups. Eur J Cancer 47 (5): 724-31, 2011. [PUBMED Abstract]
- Smith KB, Indelicato DJ, Knapik JA, et al.: Definitive radiotherapy for unresectable pediatric and young adult nonrhabdomyosarcoma soft tissue sarcoma. Pediatr Blood Cancer 57 (2): 247-51, 2011. [PUBMED Abstract]
- Ferrari A, Magni C, Bergamaschi L, et al.: Pediatric nonrhabdomyosarcoma soft tissue sarcomas arising at visceral sites. Pediatr Blood Cancer 64 (9): , 2017. [PUBMED Abstract]
- Dillon PW, Whalen TV, Azizkhan RG, et al.: Neonatal soft tissue sarcomas: the influence of pathology on treatment and survival. Children's Cancer Group Surgical Committee. J Pediatr Surg 30 (7): 1038-41, 1995. [PUBMED Abstract]
- Pappo AS, Fontanesi J, Luo X, et al.: Synovial sarcoma in children and adolescents: the St Jude Children's Research Hospital experience. J Clin Oncol 12 (11): 2360-6, 1994. [PUBMED Abstract]
- Marcus KC, Grier HE, Shamberger RC, et al.: Childhood soft tissue sarcoma: a 20-year experience. J Pediatr 131 (4): 603-7, 1997. [PUBMED Abstract]
- Pratt CB, Pappo AS, Gieser P, et al.: Role of adjuvant chemotherapy in the treatment of surgically resected pediatric nonrhabdomyosarcomatous soft tissue sarcomas: A Pediatric Oncology Group Study. J Clin Oncol 17 (4): 1219, 1999. [PUBMED Abstract]
- Pratt CB, Maurer HM, Gieser P, et al.: Treatment of unresectable or metastatic pediatric soft tissue sarcomas with surgery, irradiation, and chemotherapy: a Pediatric Oncology Group study. Med Pediatr Oncol 30 (4): 201-9, 1998. [PUBMED Abstract]
Histopathological Classification of Childhood Soft Tissue Sarcoma
World Health Organization (WHO) Classification of Soft Tissue Sarcomas
The WHO lists the following cell types in its classification of soft tissue sarcomas:[1,2]
- Adipocytic tumors.
- Intermediate (locally aggressive).
- Malignant.
- Chondro-osseous tumors.
- Extraskeletal mesenchymal chondrosarcoma.[3]
- Extraskeletal osteosarcoma.
- Soft tissue chondroma.
- Fibroblastic/myofibroblastic tumors.
- Intermediate-grade (locally aggressive).
- Desmoid-type fibromatosis (previously called desmoid tumor or aggressive fibromatoses).
- Giant cell fibroblastoma.
- Lipofibromatosis.
- Palmar/plantar fibromatosis.
- Intermediate-grade (rarely metastasizing).
- Dermatofibrosarcoma protuberans.
- Infantile fibrosarcoma.[4]
- Inflammatory myofibroblastic tumor.
- Low-grade myofibroblastic tumor.
- Myxoinflammatory fibroblastic sarcoma.
- Solitary fibrous tumor.
- Malignant.
- Intermediate-grade (locally aggressive).
- Skeletal muscle tumors.
- Rhabdomyosarcoma (embryonal, alveolar, and pleomorphic forms). (Refer to the PDQ summary on Childhood Rhabdomyosarcoma Treatment for more information.)
- Smooth muscle tumors.
- So-called fibrohistiocytic tumors (intermediate, rarely metastasizing).
- Giant cell tumors of soft tissue.
- Plexiform fibrohistiocytic tumor.
- Tumors of peripheral nerves.
- Pericytic (perivascular) tumors.
- Malignant glomus tumor and variants.
- Myopericytoma.
- Angioleiomyoma.
- Myofibroma.
- Infantile myofibroma (previously called hemangiopericytoma [infantile]).
- Myofibromatosis.
- Infantile myofibromatosis.
- Tumors of uncertain differentiation.
- Alveolar soft part sarcoma.
- Clear cell sarcoma of soft tissue.
- Desmoplastic small round cell tumor.
- Epithelioid sarcoma.
- Extrarenal rhabdoid tumor.
- Extraskeletal myxoid chondrosarcoma.
- Neoplasms with perivascular epithelioid cell differentiation (PEComa NOS, malignant).
- Primitive neuroectodermal tumor/extraskeletal Ewing tumor.
- Synovial sarcoma (NOS, spindle cell, and biphasic varieties).
- Undifferentiated/unclassified sarcomas.
- Undifferentiated epithelial sarcoma.
- Undifferentiated pleomorphic sarcoma.
- Undifferentiated round cell sarcoma.
- Undifferentiated sarcoma; sarcoma, NOS.[6]
- Undifferentiated spindle cell sarcoma.
- Vascular tumors.
References
- Soft tissue sarcoma. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 291-6.
- Brodowicz T, Schwameis E, Widder J, et al.: Intensified Adjuvant IFADIC Chemotherapy for Adult Soft Tissue Sarcoma: A Prospective Randomized Feasibility Trial. Sarcoma 4 (4): 151-60, 2000. [PUBMED Abstract]
- Dantonello TM, Int-Veen C, Leuschner I, et al.: Mesenchymal chondrosarcoma of soft tissues and bone in children, adolescents, and young adults: experiences of the CWS and COSS study groups. Cancer 112 (11): 2424-31, 2008. [PUBMED Abstract]
- Steelman C, Katzenstein H, Parham D, et al.: Unusual presentation of congenital infantile fibrosarcoma in seven infants with molecular-genetic analysis. Fetal Pediatr Pathol 30 (5): 329-37, 2011. [PUBMED Abstract]
- Evans HL: Low-grade fibromyxoid sarcoma: a clinicopathologic study of 33 cases with long-term follow-up. Am J Surg Pathol 35 (10): 1450-62, 2011. [PUBMED Abstract]
- Alaggio R, Collini P, Randall RL, et al.: Undifferentiated high-grade pleomorphic sarcomas in children: a clinicopathologic study of 10 cases and review of literature. Pediatr Dev Pathol 13 (3): 209-17, 2010 May-Jun. [PUBMED Abstract]
Staging and Grading Systems for Childhood Soft Tissue Sarcoma
Clinical staging has an important role in predicting the clinical outcome and determining the most effective therapy for pediatric soft tissue sarcomas. As yet, there is no well-accepted staging system that is applicable to all childhood sarcomas. The system from the American Joint Committee on Cancer (AJCC) that is used for adults has not been validated in pediatric studies. Although a standardized staging system for pediatric nonrhabdomyosarcomatous soft tissue sarcoma does not exist, two systems are currently in use for staging pediatric nonrhabdomyosarcomatous soft tissue sarcoma.[1]
- Surgico-pathologic staging system: The surgico-pathologic staging system used by the Intergroup Rhabdomyosarcoma Study (see below) is based on the amount, or extent, of tumor that remains after initial surgery and whether the disease has metastasized. This staging system was used in early pediatric trials.[2]
- TNM staging system: The TNM staging system is a collaborative effort between the AJCC (United States) and the International Union Against Cancer (worldwide). Staging is based on the extent of the tumor (T), the extent of spread to the lymph nodes (N), and the presence of metastasis (M). Refer to Tables 3, 4, 5, and 6 for the staging of soft tissue sarcoma from the eighth edition of the AJCC Cancer Staging Manual.[3-7] The last Children's Oncology Group trial used the sixth edition AJCC Cancer Staging Manual for soft tissue sarcoma (with central pathology review).[1] A review of children with non-rhabdomyosarcoma soft tissue sarcomas was performed with data from the Surveillance, Epidemiology, and End Results (SEER) program and identified 941 patients between 1988 and 2007.[8] The COG risk stratification was validated in this cohort.
Intergroup Rhabdomyosarcoma Study Staging System
Nonmetastatic disease
- Group I: Localized tumor completely resected with histologically negative margins.
- Group II: Grossly resected tumor with microscopic residual tumor at the margin(s) and/or extension into regional lymph nodes.
- IIA: Localized, grossly resected tumor with microscopic residual disease.
- IIB: Regional disease with involved nodes completely resected with no microscopic disease. The most proximal (to the patient, most distal to the tumor) regional lymph node must be negative.
- IIC: Regional disease with involved nodes grossly resected but with evidence of residual microscopic disease at the primary site and/or histologic involvement of the most proximal regional lymph node in the dissection.
- Group III: Localized tumor, incompletely resected, or biopsy only, with gross residual tumor.
Metastatic disease
- Group IV: Any localized or regional tumor with distant metastases present at the time of diagnosis. This includes the presence of malignant cells in effusions (pleural, peritoneal) and/or cerebrospinal fluid (rare).
Recurrent/progressive disease
- Any soft tissue sarcoma that recurs after initial treatment or progresses after radiation therapy, chemotherapy, or initial surgery.
TNM Staging System
The eighth edition of the AJCC Cancer Staging Manual has designated staging by the four criteria of tumor size, nodal status, histologic grade, and metastasis and by anatomic primary tumor site (head and neck; trunk and extremities; abdomen and thoracic visceral organs; retroperitoneum; and unusual histologies and sites) (refer to Tables 3, 4, 5, and 6).[3-7] For information on unusual histologies and sites, refer to the AJCC Cancer Staging Manual.[7]
| T Category | Soft Tissue Sarcoma of the Trunk, Extremities, and Retroperitoneum | Soft Tissue Sarcoma of the Head and Neck | Soft Tissue Sarcoma of the Abdomen and Thoracic Visceral Organs |
|---|---|---|---|
| aAdapted from O'Sullivan et al.,[3] Yoon et al.,[4] Raut et al.,[5] and Pollock et al.[6] | |||
| TX | Primary tumor cannot be assessed. | Primary tumor cannot be assessed. | Primary tumor cannot be assessed. |
| T0 | No evidence of primary tumor. | ||
| T1 | Tumor ≤5 cm in greatest dimension. | Tumor ≤2 cm. | Organ confined. |
| T2 | Tumor >5 cm and ≤10 cm in greatest dimension. | Tumor >2 to ≤4 cm. | Tumor extension into tissue beyond organ. |
| T2a | Invades serosa or visceral peritoneum. | ||
| T2b | Extension beyond serosa (mesentery). | ||
| T3 | Tumor >10 cm and ≤15 cm in greatest dimension. | Tumor >4 cm. | Invades another organ. |
| T4 | Tumor >15 cm in greatest dimension. | Tumor with invasion of adjoining structures. | Multifocal involvement. |
| T4a | Tumor with orbital invasion, skull base/dural invasion, invasion of central compartment viscera, involvement of facial skeleton, or invasion of pterygoid muscles. | Multifocal (2 sites). | |
| T4b | Tumor with brain parenchymal invasion, carotid artery encasement, prevertebral muscle invasion, or central nervous system involvement via perineural spread. | Multifocal (3–5 sites). | |
| T4c | Multifocal (>5 sites). | ||
| aAdapted from O'Sullivan et al.,[3] Yoon et al.,[4] Raut et al.,[5] and Pollock et al.[6] | |
| bFor soft tissue sarcoma of the abdomen and thoracic visceral organs, N0 = no lymph node involvement or unknown lymph node status and N1 = lymph node involvement present. | |
| N0 | No regional lymph node metastasis or unknown lymph node status.b |
| N1 | Regional lymph node metastasis.b |
| aAdapted from O'Sullivan et al.,[3] Yoon et al.,[4] Raut et al.,[5] and Pollock et al.[6] | |
| bFor soft tissue sarcoma of the abdomen and thoracic visceral organs, M0 = no metastases and M1 = metastases present. | |
| M0 | No distant metastasis.b |
| M1 | Distant metastasis.b |
| Stage | T | N | M | Grade |
|---|---|---|---|---|
| aAdapted from Yoon et al. [4] and Pollock et al.[6] | ||||
| bStage IIIB for soft tissue sarcoma of the retroperitoneum; stage IV for soft tissue sarcoma of the trunk and extremities. | ||||
| IA | T1 | N0 | M0 | G1, GX |
| IB | T2, T3, T4 | N0 | M0 | G1, GX |
| II | T1 | N0 | M0 | G2, G3 |
| IIIA | T2 | N0 | M0 | G2, G3 |
| IIIB | T3, T4 | N0 | M0 | G2, G3 |
| IIIB/IVb | Any T | N1 | M0 | Any G |
| IV | Any T | Any N | M1 | Any G |
Soft Tissue Sarcoma Tumor Pathological Grading System
In most cases, accurate histopathologic classification alone of soft tissue sarcomas does not yield optimal information about their clinical behavior. Therefore, several histologic parameters are evaluated in the grading process, including the following:
- Degree of cellularity.
- Cellular pleomorphism.
- Mitotic activity.
- Degree of necrosis.
- Invasive growth.
This process is used to improve the correlation between histologic findings and clinical outcome.[9] In children, grading of soft tissue sarcoma is compromised by the good prognosis of certain tumors, such as infantile fibrosarcoma and hemangiopericytoma, which have a good prognosis in children younger than 4 years, and also angiomatoid fibrous histiocytoma and dermatofibrosarcoma protuberans, which may recur locally if incompletely excised, but usually do not metastasize.
Testing the validity of a grading system within the pediatric population is difficult because of the rarity of these neoplasms. In March 1986, the Pediatric Oncology Group (POG) conducted a prospective study on pediatric soft tissue sarcomas other than rhabdomyosarcoma and devised the POG grading system. Analysis of outcome for patients with localized soft tissue sarcomas other than rhabdomyosarcoma demonstrated that patients with grade 3 tumors fared significantly worse than those with grade 1 or grade 2 lesions. This finding suggests that this system can accurately predict the clinical behavior of nonrhabdomyosarcomatous soft tissue sarcoma.[9-11]
The grading systems developed by the POG and the French Federation of Comprehensive Cancer Centers (Fédération Nationale des Centres de Lutte Contre Le Cancer [FNCLCC]) Sarcoma Group are described below. These grading systems are being compared by the central review pathologists on the COG-ARST0332 study. The study has closed and results are pending.
POG grading system
The POG grading system is described below.[9] It is an older grading system of historical value that is no longer being used for treatment.
Grade I
Grade I lesions are based on histologic type, well-differentiated cytohistologic features, and/or age of the patient.
- Angiomatoid fibrous histiocytoma.
- Dermatofibrosarcoma protuberans.
- Liposarcoma–myxoid or well-differentiated.
- Myxoid chondrosarcoma.
- Well-differentiated malignant peripheral nerve sheath tumor.
- Well-differentiated or infantile (aged ≤4 years) fibrosarcoma.
- Well-differentiated or infantile (aged ≤4 years) hemangiopericytoma.
Grade II
Grade II lesions are soft tissue sarcomas not included in grade I or III by histologic diagnosis (with <5 mitoses/10 high-power fields or <15% necrosis):
- 15% or less of the surface area shows necrosis (primary criteria).
- The mitotic count is <5 mitotic figures per 10 high-power fields (40X objective) (primary criteria).
- Nuclear atypia is not marked (secondary criteria).
- The tumor is not markedly cellular (secondary criteria).
Grade III
Grade III lesions are similar to grade II lesions and include certain tumors known to be clinically aggressive by virtue of histologic diagnosis and non-grade I tumors (with >4 mitoses per 10 high-power fields or >15% necrosis):
- Alveolar soft part sarcoma.
- Extraskeletal osteogenic sarcoma.
- Malignant triton tumor.
- Mesenchymal chondrosarcoma.
- Pleomorphic or round-cell liposarcoma.
- Any other sarcoma not in grade I with >15% necrosis and/or ≥5 mitotic figures per 10 high-power fields (40X objective). Marked atypia and cellularity are less predictive but may assist in placing tumors in this category.
FNCLCC grading system
The FNCLCC histologic grading system was developed for adults with soft tissue sarcoma. The purpose of the grading system is to predict which patients will develop metastasis and subsequently benefit from postoperative chemotherapy.[12,13] The system is described in Table 7 and Table 8.
| FNCLCC = Fédération Nationale des Centres de Lutte Contre Le Cancer; HPF = high-power field. | |
| Tumor Differentiation | |
| Score 1 | Sarcoma closely resembling normal adult mesenchymal tissue (e.g., well-differentiated liposarcoma) |
| Score 2 | Sarcomas for which histologic typing is certain (e.g., myxoid liposarcoma) |
| Score 3 | Embryonal and undifferentiated sarcomas, sarcomas of doubtful type, and synovial sarcomas |
| Mitotic Count | |
| Score 1 | 0–9 mitoses per 10 HPF |
| Score 2 | 10–19 mitoses per 10 HPF |
| Score 3 | ≥20 mitoses per 10 HPF |
| Tumor Necrosis | |
| Score 0 | No necrosis |
| Score 1 | <50% tumor necrosis |
| Score 2 | ≥50% tumor necrosis |
| Total Score | Histologic Grade |
|---|---|
| 2–3 | Grade I |
| 4–5 | Grade II |
| 6–8 | Grade III |
Prognostic Significance of Tumor Grading
The POG and FNCLCC grading systems have proven to be of prognostic value in pediatric and adult nonrhabdomyosarcomatous soft tissue sarcomas.[14-18] In a study of 130 tumors from children and adolescents with nonrhabdomyosarcomatous soft tissue sarcoma enrolled in three prospective clinical trials, a correlation was found between the POG-assigned grade and the FNCLCC-assigned grade. However, grading did not correlate in all cases; 44 patients whose tumors received discrepant grades (POG grade 3, FNCLCC grade 1 or 2) had outcomes between concurrent grade 3 and grades 1 and 2. A mitotic index of 10 or greater emerged as an important prognostic factor.[19] The recently completed COG-ARST0332 trial will analyze data comparing the POG and FNCLCC pathologic grading systems to determine which system better correlates with clinical outcomes. The current open trial (ARST1321 [NCT02180867]) uses the FNCLCC system to assign histological grade.
References
- American Joint Committee on Cancer: AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer, 2002.
- Maurer HM, Beltangady M, Gehan EA, et al.: The Intergroup Rhabdomyosarcoma Study-I. A final report. Cancer 61 (2): 209-20, 1988. [PUBMED Abstract]
- O'Sullivan B, Maki RG, Agulnik M, et al.: Soft tissue sarcoma of the head and neck. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 499-505.
- Yoon SS, Maki RG, Asare EA, et al.: Soft tissue sarcoma of the trunk and extremities. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 507-15.
- Raut CP, Maki RG, Baldini EH, et al.: Soft tissue sarcoma of the abdomen and thoracic visceral organs. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 517-21.
- Pollock RE, Maki RG, Baldini EH, et al.: Soft tissue sarcoma of the retroperitoneum. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 531-7.
- Maki RG, Folpe AL, Guadagnolo BA, et al.: Soft tissue sarcoma - unusual histologies and sites. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 539-45.
- Waxweiler TV, Rusthoven CG, Proper MS, et al.: Non-Rhabdomyosarcoma Soft Tissue Sarcomas in Children: A Surveillance, Epidemiology, and End Results Analysis Validating COG Risk Stratifications. Int J Radiat Oncol Biol Phys 92 (2): 339-48, 2015. [PUBMED Abstract]
- Parham DM, Webber BL, Jenkins JJ 3rd, et al.: Nonrhabdomyosarcomatous soft tissue sarcomas of childhood: formulation of a simplified system for grading. Mod Pathol 8 (7): 705-10, 1995. [PUBMED Abstract]
- Recommendations for the reporting of soft tissue sarcomas. Association of Directors of Anatomic and Surgical Pathology. Mod Pathol 11 (12): 1257-61, 1998. [PUBMED Abstract]
- Skytting B, Meis-Kindblom JM, Larsson O, et al.: Synovial sarcoma--identification of favorable and unfavorable histologic types: a Scandinavian sarcoma group study of 104 cases. Acta Orthop Scand 70 (6): 543-54, 1999. [PUBMED Abstract]
- Coindre JM, Terrier P, Guillou L, et al.: Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 91 (10): 1914-26, 2001. [PUBMED Abstract]
- Guillou L, Coindre JM, Bonichon F, et al.: Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol 15 (1): 350-62, 1997. [PUBMED Abstract]
- Rao BN: Nonrhabdomyosarcoma in children: prognostic factors influencing survival. Semin Surg Oncol 9 (6): 524-31, 1993 Nov-Dec. [PUBMED Abstract]
- Pisters PW, Leung DH, Woodruff J, et al.: Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 14 (5): 1679-89, 1996. [PUBMED Abstract]
- Coindre JM, Terrier P, Bui NB, et al.: Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol 14 (3): 869-77, 1996. [PUBMED Abstract]
- Pappo AS, Fontanesi J, Luo X, et al.: Synovial sarcoma in children and adolescents: the St Jude Children's Research Hospital experience. J Clin Oncol 12 (11): 2360-6, 1994. [PUBMED Abstract]
- Pratt CB, Maurer HM, Gieser P, et al.: Treatment of unresectable or metastatic pediatric soft tissue sarcomas with surgery, irradiation, and chemotherapy: a Pediatric Oncology Group study. Med Pediatr Oncol 30 (4): 201-9, 1998. [PUBMED Abstract]
- Khoury JD, Coffin CM, Spunt SL, et al.: Grading of nonrhabdomyosarcoma soft tissue sarcoma in children and adolescents: a comparison of parameters used for the Fédération Nationale des Centers de Lutte Contre le Cancer and Pediatric Oncology Group Systems. Cancer 116 (9): 2266-74, 2010. [PUBMED Abstract]
Treatment Option Overview for Childhood Soft Tissue Sarcoma
Because of the rarity of pediatric nonrhabdomyosarcomatous soft tissue sarcomas, coordination of treatment by a multidisciplinary team comprising oncologists (pediatric or medical), pathologists, surgeons, and radiation oncologists should be considered for all children, adolescents, and young adults with these tumors. In addition, to better define the tumors' natural history and response to therapy, entry into national or institutional treatment protocols should be considered for children with rare neoplasms. Information about ongoing clinical trials is available from the NCI website.
Surgery
After an appropriate biopsy and pathologic diagnosis, every attempt is made to resect the primary tumor with negative margins before or after chemotherapy and/or radiation therapy. Involvement of a surgeon with special expertise in the resection of soft tissue sarcomas in the decision is highly desirable.
The timing of surgery depends on an assessment of the feasibility and morbidity of surgery. If the initial operation fails to achieve pathologically negative tissue margins or if the initial surgery was done without the knowledge that cancer was present, a re-excision of the affected area is performed to obtain clear, but not necessarily wide, margins.[1-4] This surgical tenet is true even if no mass is detected by magnetic resonance imaging after initial surgery.[5]; [6][Level of evidence: 3iiA]
Regional lymph node metastases at diagnosis are unusual and are most often seen in patients with epithelioid and clear cell sarcomas.[7,8] Various institutional series have demonstrated the feasibility and effectiveness of sentinel node biopsy as a staging procedure in pediatric patients with soft tissue sarcomas.[9-14]
Radiation Therapy
Considerations for radiation therapy are based on the potential for surgery, with or without chemotherapy, to obtain local control without loss of critical organs or significant functional, cosmetic, or psychological impairment. This will vary according to the following:
- Patient variables (e.g., age and sex).
- Tumor variables (e.g., histopathology, site, size, and grade).
- Surgical margin status.
- Expectations for radiation-induced morbidities (e.g., impaired bone or muscle development, organ damage, or second malignancy).
Radiation therapy can be given preoperatively. Radiation field size and dose will be based on patient and tumor variables and the operability of the tumor. Preoperative radiation therapy has been associated with excellent local control rates.[15,16] This approach has the advantage of treating smaller tissue volumes because it does not necessitate treating a postsurgical bed; it also has the advantage of somewhat lower radiation doses because relative hypoxia from surgical disruption of vasculature and scarring is not present. Preoperative radiation therapy has been associated with an increased rate of wound complications in adults, primarily in lower extremity tumors, but the degree of this is questionable.[17] Conversely, preoperative radiation therapy may lead to less fibrosis than with postoperative approaches, perhaps due to the smaller treatment volume and dose.[18]
Retroperitoneal sarcomas are unique in that radiosensitivity of the bowel to injury makes postoperative radiation therapy less desirable.[19,20] Postoperative adhesions and bowel immobility can increase the risk of damage from any given radiation dose. This contrasts with the preoperative approach in which the tumor often displaces bowel outside of the radiation field, and any exposed bowel is more mobile, which decreases exposure to specific bowel segments.
Radiation therapy can also be given postoperatively. In general, radiation is indicated for patients with inadequate surgical margins and for larger, high-grade tumors.[21,22] This is particularly important in high-grade tumors with tumor margins smaller than 1 cm.[23,24]; [25][Level of evidence: 3iiDiv] With combined surgery and radiation therapy, local control of the primary tumor can be achieved in more than 80% of patients.[26,27]
Brachytherapy and intraoperative radiation may be applicable in select situations.[27-29]; [30][Level of evidence: 3iiiDii]
Radiation volume and dose depend on the patient, tumor, and surgical variables noted above, as well as the following:
- Patient age and growth potential.
- Ability to avoid critical organs, epiphyseal plates, and lymphatics (but not the neurovascular bundles that are relatively radiation tolerant).
- Functional/cosmetic outcome.
Radiation doses are typically 45 Gy to 50 Gy preoperatively, with consideration for postoperative boost of 10 Gy to 20 Gy if resection margins are microscopically or grossly positive, or planned brachytherapy if the resection is predicted to be subtotal. However, data documenting the efficacy of a postoperative boost are lacking.[31] The postoperative radiation dose is 55 Gy to 60 Gy, or rarely, higher when unresectable gross residual disease exists.
Radiation margins are typically 2 cm to 4 cm longitudinally and encompass fascial planes axially.[32,33]
Chemotherapy
The role of postoperative chemotherapy remains unclear as evidenced by the following studies:[34]
- A meta-analysis of data from all randomized trials of adults with soft tissue sarcoma concluded that recurrence-free survival was better with postoperative chemotherapy for patients with high-grade tumors larger than 5 cm.[35]
- In a European trial, adults with completely resected soft tissue sarcoma were randomly assigned to observation or postoperative chemotherapy with ifosfamide and doxorubicin. Postoperative chemotherapy was not associated with improved event-free survival (EFS) or overall survival (OS). It is difficult to extrapolate this trial to pediatric patients because the trial included 1) a wide variety of histologies; 2) a relatively low dose of ifosfamide; 3) patients assigned to chemotherapy had definitive radiation delayed until completion of chemotherapy; and 4) almost one-half of the patients in the trial had intermediate-grade tumors. In the discussion section, the authors merged their patients with previously published series, including those from the European meta-analysis, and concluded that the results suggested a benefit for postoperative chemotherapy.[36][Level of evidence: 1iiA]
- The largest prospective pediatric trial failed to demonstrate any benefit with postoperative vincristine, dactinomycin, cyclophosphamide, and doxorubicin.[26]
- Doxorubicin and ifosfamide were used in the risk-based COG ARST0332 (NCT00346164) trial. Although this was not a randomized study, results at 2.6 years show that patients with high-risk (>5 cm and high grade), grossly resected, nonmetastatic tumors who were treated with radiation therapy and postoperative doxorubicin and ifosfamide had a 3-year EFS of 68% and OS of 81%. In patients with metastatic disease treated with preoperative chemotherapy and radiation therapy, the estimated 3-year failure-free survival was 52% and OS was 66%.[37][Level of evidence: 3iiiA]
Targeted Therapy
The use of angiogenesis and mammalian target of rapamycin (mTOR) inhibitors has been explored in the treatment of adult soft tissue sarcomas but not in pediatrics.
- In a trial of 711 randomly assigned adult patients who achieved a response or stable disease after chemotherapy, the administration of ridaforolimus was associated with a 3-week improvement in progression-free survival (PFS) when compared with placebo.[38]
- In another trial of 371 randomly assigned adult patients with metastatic soft tissue sarcoma that progressed after chemotherapy, pazopanib was compared with placebo. The median PFS for the pazopanib arm was 4.6 months compared with 1.6 months for the placebo arm. OS was not different between the two arms.[39]
- In a randomized study of 182 previously treated adult patients with recurrent liposarcoma, leiomyosarcoma, synovial sarcoma, and other sarcomas, patients with nonadipocytic tumors who were treated with regorafenib had significant improvements in progression-free survival when compared with patients who were treated with placebo.[40]
Special Considerations for the Treatment of Children With Soft Tissue Sarcoma
Cancer in children and adolescents is rare, although the overall incidence of childhood cancer has been slowly increasing since 1975.[41] Children and adolescents with cancer should be referred to medical centers that have a multidisciplinary team of cancer specialists with experience treating the cancers that occur during childhood and adolescence. This multidisciplinary team approach incorporates the skills of the following health care professionals and others to ensure that children receive treatment, supportive care, and rehabilitation that will achieve optimal survival and quality of life:
- Primary care physicians.
- Pediatric surgical specialists.
- Pediatric radiation oncologists.
- Pediatric medical oncologists/hematologists.
- Rehabilitation specialists.
- Pediatric nurse specialists.
- Social workers.
- Child life professionals.
- Psychologists.
(Refer to the PDQ Supportive and Palliative Care summaries for specific information about supportive care for children and adolescents with cancer.)
Guidelines for pediatric cancer centers and their role in the treatment of pediatric patients with cancer have been outlined by the American Academy of Pediatrics.[42] At these pediatric cancer centers, clinical trials are available for most types of cancer that occur in children and adolescents, and the opportunity to participate in these trials is offered to most patients/families. Multidisciplinary evaluation in pediatric cancer centers that have surgical and radiotherapeutic expertise is of critical importance to ensure the best clinical outcome for these patients. Although surgery with or without radiation therapy can be curative for a significant proportion of patients, the addition of chemotherapy might benefit subsets of children with the disease; therefore, enrollment into clinical trials is encouraged. Clinical trials for children and adolescents with cancer are generally designed to compare potentially better therapy with therapy that is currently accepted as standard. Most of the progress made in identifying curative therapies for childhood cancers has been achieved through clinical trials. Information about ongoing clinical trials is available from the NCI website.
Many therapeutic strategies for children and adolescents with soft tissue tumors are similar to those for adult patients, although there are important differences. For example, the biology of the neoplasm in pediatric patients may differ dramatically from that of the adult lesion. Additionally, limb-sparing procedures are more difficult to perform in pediatric patients. The morbidity associated with radiation therapy, particularly in infants and young children, may be much greater than that observed in adults.[43]
Improved outcomes with multimodality therapy in adults and children with soft tissue sarcomas over the past 20 years has caused increasing concern about the potential long-term side effects of this therapy in children, especially when considering the expected longer life span of children versus adults. Therefore, to maximize tumor control and minimize long-term morbidity, treatment must be individualized for children and adolescents with nonrhabdomyosarcomatous soft tissue sarcoma. These patients should be enrolled in prospective studies that accurately assess any potential complications.[44]
References
- Sugiura H, Takahashi M, Katagiri H, et al.: Additional wide resection of malignant soft tissue tumors. Clin Orthop (394): 201-10, 2002. [PUBMED Abstract]
- Cecchetto G, Guglielmi M, Inserra A, et al.: Primary re-excision: the Italian experience in patients with localized soft-tissue sarcomas. Pediatr Surg Int 17 (7): 532-4, 2001. [PUBMED Abstract]
- Chui CH, Spunt SL, Liu T, et al.: Is reexcision in pediatric nonrhabdomyosarcoma soft tissue sarcoma necessary after an initial unplanned resection? J Pediatr Surg 37 (10): 1424-9, 2002. [PUBMED Abstract]
- Paulino AC, Ritchie J, Wen BC: The value of postoperative radiotherapy in childhood nonrhabdomyosarcoma soft tissue sarcoma. Pediatr Blood Cancer 43 (5): 587-93, 2004. [PUBMED Abstract]
- Kaste SC, Hill A, Conley L, et al.: Magnetic resonance imaging after incomplete resection of soft tissue sarcoma. Clin Orthop (397): 204-11, 2002. [PUBMED Abstract]
- Chandrasekar CR, Wafa H, Grimer RJ, et al.: The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br 90 (2): 203-8, 2008. [PUBMED Abstract]
- Daigeler A, Kuhnen C, Moritz R, et al.: Lymph node metastases in soft tissue sarcomas: a single center analysis of 1,597 patients. Langenbecks Arch Surg 394 (2): 321-9, 2009. [PUBMED Abstract]
- Mazeron JJ, Suit HD: Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer 60 (8): 1800-8, 1987. [PUBMED Abstract]
- Neville HL, Andrassy RJ, Lally KP, et al.: Lymphatic mapping with sentinel node biopsy in pediatric patients. J Pediatr Surg 35 (6): 961-4, 2000. [PUBMED Abstract]
- Neville HL, Raney RB, Andrassy RJ, et al.: Multidisciplinary management of pediatric soft-tissue sarcoma. Oncology (Huntingt) 14 (10): 1471-81; discussion 1482-6, 1489-90, 2000. [PUBMED Abstract]
- Kayton ML, Delgado R, Busam K, et al.: Experience with 31 sentinel lymph node biopsies for sarcomas and carcinomas in pediatric patients. Cancer 112 (9): 2052-9, 2008. [PUBMED Abstract]
- Dall'Igna P, De Corti F, Alaggio R, et al.: Sentinel node biopsy in pediatric patients: the experience in a single institution. Eur J Pediatr Surg 24 (6): 482-7, 2014. [PUBMED Abstract]
- Parida L, Morrisson GT, Shammas A, et al.: Role of lymphoscintigraphy and sentinel lymph node biopsy in the management of pediatric melanoma and sarcoma. Pediatr Surg Int 28 (6): 571-8, 2012. [PUBMED Abstract]
- Alcorn KM, Deans KJ, Congeni A, et al.: Sentinel lymph node biopsy in pediatric soft tissue sarcoma patients: utility and concordance with imaging. J Pediatr Surg 48 (9): 1903-6, 2013. [PUBMED Abstract]
- Virkus WW, Mollabashy A, Reith JD, et al.: Preoperative radiotherapy in the treatment of soft tissue sarcomas. Clin Orthop (397): 177-89, 2002. [PUBMED Abstract]
- Zagars GK, Ballo MT, Pisters PW, et al.: Preoperative vs. postoperative radiation therapy for soft tissue sarcoma: a retrospective comparative evaluation of disease outcome. Int J Radiat Oncol Biol Phys 56 (2): 482-8, 2003. [PUBMED Abstract]
- O'Sullivan B, Davis AM, Turcotte R, et al.: Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359 (9325): 2235-41, 2002. [PUBMED Abstract]
- Davis AM, O'Sullivan B, Turcotte R, et al.: Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 75 (1): 48-53, 2005. [PUBMED Abstract]
- Baldini EH, Wang D, Haas RL, et al.: Treatment Guidelines for Preoperative Radiation Therapy for Retroperitoneal Sarcoma: Preliminary Consensus of an International Expert Panel. Int J Radiat Oncol Biol Phys 92 (3): 602-12, 2015. [PUBMED Abstract]
- Bishop AJ, Zagars GK, Torres KE, et al.: Combined Modality Management of Retroperitoneal Sarcomas: A Single-Institution Series of 121 Patients. Int J Radiat Oncol Biol Phys 93 (1): 158-65, 2015. [PUBMED Abstract]
- Marcus KC, Grier HE, Shamberger RC, et al.: Childhood soft tissue sarcoma: a 20-year experience. J Pediatr 131 (4): 603-7, 1997. [PUBMED Abstract]
- Delaney TF, Kepka L, Goldberg SI, et al.: Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys 67 (5): 1460-9, 2007. [PUBMED Abstract]
- Blakely ML, Spurbeck WW, Pappo AS, et al.: The impact of margin of resection on outcome in pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Pediatr Surg 34 (5): 672-5, 1999. [PUBMED Abstract]
- Skytting B: Synovial sarcoma. A Scandinavian Sarcoma Group project. Acta Orthop Scand Suppl 291: 1-28, 2000. [PUBMED Abstract]
- Hua C, Gray JM, Merchant TE, et al.: Treatment planning and delivery of external beam radiotherapy for pediatric sarcoma: the St. Jude Children's Research Hospital experience. Int J Radiat Oncol Biol Phys 70 (5): 1598-606, 2008. [PUBMED Abstract]
- Pratt CB, Pappo AS, Gieser P, et al.: Role of adjuvant chemotherapy in the treatment of surgically resected pediatric nonrhabdomyosarcomatous soft tissue sarcomas: A Pediatric Oncology Group Study. J Clin Oncol 17 (4): 1219, 1999. [PUBMED Abstract]
- Merchant TE, Parsh N, del Valle PL, et al.: Brachytherapy for pediatric soft-tissue sarcoma. Int J Radiat Oncol Biol Phys 46 (2): 427-32, 2000. [PUBMED Abstract]
- Schomberg PJ, Gunderson LL, Moir CR, et al.: Intraoperative electron irradiation in the management of pediatric malignancies. Cancer 79 (11): 2251-6, 1997. [PUBMED Abstract]
- Nag S, Shasha D, Janjan N, et al.: The American Brachytherapy Society recommendations for brachytherapy of soft tissue sarcomas. Int J Radiat Oncol Biol Phys 49 (4): 1033-43, 2001. [PUBMED Abstract]
- Viani GA, Novaes PE, Jacinto AA, et al.: High-dose-rate brachytherapy for soft tissue sarcoma in children: a single institution experience. Radiat Oncol 3: 9, 2008. [PUBMED Abstract]
- Al Yami A, Griffin AM, Ferguson PC, et al.: Positive surgical margins in soft tissue sarcoma treated with preoperative radiation: is a postoperative boost necessary? Int J Radiat Oncol Biol Phys 77 (4): 1191-7, 2010. [PUBMED Abstract]
- Wang D, Bosch W, Kirsch DG, et al.: Variation in the gross tumor volume and clinical target volume for preoperative radiotherapy of primary large high-grade soft tissue sarcoma of the extremity among RTOG sarcoma radiation oncologists. Int J Radiat Oncol Biol Phys 81 (5): e775-80, 2011. [PUBMED Abstract]
- Bahig H, Roberge D, Bosch W, et al.: Agreement among RTOG sarcoma radiation oncologists in contouring suspicious peritumoral edema for preoperative radiation therapy of soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys 86 (2): 298-303, 2013. [PUBMED Abstract]
- Ferrari A: Role of chemotherapy in pediatric nonrhabdomyosarcoma soft-tissue sarcomas. Expert Rev Anticancer Ther 8 (6): 929-38, 2008. [PUBMED Abstract]
- Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet 350 (9092): 1647-54, 1997. [PUBMED Abstract]
- Woll PJ, Reichardt P, Le Cesne A, et al.: Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol 13 (10): 1045-54, 2012. [PUBMED Abstract]
- Spunt SL, Million L, Anderson JR, et al.: Risk-based treatment for nonrhabdomyosarcoma soft tissue sarcomas (NRSTS) in patients under 30 years of age: Children’s Oncology Group study ARST0332. [Abstract] J Clin Oncol 32 (Suppl 15): A-10008, 2014. Also available online. Last accessed April 02, 2018.
- Demetri GD, Chawla SP, Ray-Coquard I, et al.: Results of an international randomized phase III trial of the mammalian target of rapamycin inhibitor ridaforolimus versus placebo to control metastatic sarcomas in patients after benefit from prior chemotherapy. J Clin Oncol 31 (19): 2485-92, 2013. [PUBMED Abstract]
- van der Graaf WT, Blay JY, Chawla SP, et al.: Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 379 (9829): 1879-86, 2012. [PUBMED Abstract]
- Mir O, Brodowicz T, Italiano A, et al.: Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 17 (12): 1732-1742, 2016. [PUBMED Abstract]
- Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014. [PUBMED Abstract]
- Corrigan JJ, Feig SA; American Academy of Pediatrics: Guidelines for pediatric cancer centers. Pediatrics 113 (6): 1833-5, 2004. [PUBMED Abstract]
- Suit H, Spiro I: Radiation as a therapeutic modality in sarcomas of the soft tissue. Hematol Oncol Clin North Am 9 (4): 733-46, 1995. [PUBMED Abstract]
- Spunt SL, Million L, Coffin C: The nonrhabdomyosarcoma soft tissue sarcoma. In: Pizzo PA, Poplack DG, eds.: Principles and Practice of Pediatric Oncology. 7th ed. Philadelphia, Pa: Lippincott Williams and Wilkins, 2015, pp 827-54.
Treatment of Newly Diagnosed Childhood Soft Tissue Sarcoma
Adipocytic Tumors
Liposarcoma
Liposarcoma accounts for 3% of soft tissue sarcoma in patients younger than 20 years (refer to Table 1).
Liposarcoma is rare in the pediatric population. In a review of 182 pediatric patients with adult-type sarcomas, only 14 had a diagnosis of liposarcoma.[1] One retrospective study identified 34 patients younger than 22 years from 1960 to 2011.[2] There were roughly equal numbers of male and female patients and the median age was 18 years. In an international clinicopathological review, the characteristics of 82 cases of pediatric liposarcoma were reported. The median age was 15.5 years and females were more commonly affected.[3] In both reports, the great majority of patients had myxoid liposarcoma.
Histopathologic classification
The World Health Organization (WHO) classification for liposarcoma is as follows:
- Intermediate grade (rarely metastasizing).
- Atypical lipomatous neoplasm/well-differentiated liposarcoma. These tumors do not metastasize unless they undergo dedifferentiation.
- Malignant.
- Liposarcoma, not otherwise specified (NOS).
- Myxoid liposarcoma. Pure myxoid liposarcomas are characterized by a t(12;16)(q13;p11) translocation and can metastasize but usually have an excellent outcome in the absence of a round cell component.[4]
- Dedifferentiated liposarcoma.
- Pleomorphic liposarcoma.
Clinical presentation
The majority of liposarcomas in the pediatric and adolescent age range are low grade and located subcutaneously. Metastasis to lymph nodes is very uncommon, and the great majority of metastases are pulmonary. Tumors arising in the periphery are more likely to be low grade and myxoid. Tumors arising centrally are more likely to be high grade, pleomorphic, and present with metastasis or recur with metastasis.
Prognosis
Higher grade or central tumors are associated with a significantly higher risk of death. In a retrospective review, 5-year survival for central tumors was 42%. In the international review, seven of ten patients with pleomorphic myxoid liposarcoma died because of their disease.[3] In a retrospective study of 14 patients, 5-year survival was 78% and tumor grade, histologic subtype, and primary location correlated with survival.[2]
Treatment
Treatment options for liposarcoma include the following:
Surgery is the most important treatment for liposarcoma. After surgical resection of myxoid liposarcoma, event-free survival (EFS) and overall survival (OS) are roughly 90%. If initial surgery is incomplete, re-excision should be performed to achieve a wide margin of resection. Local recurrences have been seen and are controlled with a second resection of the tumor.
There are reports of the use of chemotherapy to decrease the size of liposarcoma before surgery to facilitate complete resection, particularly in central tumors.[10,11] The role of postoperative chemotherapy for liposarcoma is poorly defined. There does not appear to be a need for any postoperative therapy for completely resected myxoid liposarcoma. Even with the use of postoperative chemotherapy, the survival of pleomorphic liposarcoma remains poor.[12]
Trabectedin has produced encouraging responses in adults with advanced myxoid liposarcoma.[13] In one study, adult patients with recurrent liposarcoma and leiomyosarcoma were randomly assigned to treatment with either trabectedin or dacarbazine. Patients treated with trabectedin had a 45% reduction in disease progression.[14][Level of evidence: 1iiDiii] There are very limited data to support the use of trabectedin in pediatric patients.[15]
Treatment options under clinical evaluation
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma, excluding myxoid liposarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with liposarcoma are eligible for this trial.
Chondro-osseous Tumors
Chondro-osseous tumors include the following tumor subtypes:
- Extraskeletal mesenchymal chondrosarcoma.
- Extraskeletal osteosarcoma.
- Soft tissue chondroma.
Extraskeletal mesenchymal chondrosarcoma
Osseous and chondromatous neoplasms account for 0.8% of soft tissue sarcoma in patients younger than 20 years (refer to Table 1).
Histopathology and molecular features
Mesenchymal chondrosarcoma is a rare tumor characterized by small round cells and hyaline cartilage that more commonly affects young adults and has a predilection for involving the head and neck region.
Mesenchymal chondrosarcoma has been associated with consistent chromosomal rearrangement. A retrospective analysis of cases of mesenchymal chondrosarcoma identified a HEY1-NCOA2 fusion in 10 of 15 tested specimens.[16] This gene fusion was not associated with chromosomal changes that could be detected by karyotyping. In one instance, translocation t(1;5)(q42;q32) was identified in a case of mesenchymal chondrosarcoma and shown to be associated with a novel IRF2BP-CDX1 fusion gene.[17]
Prognosis
A retrospective survey of European institutions identified 113 children and adults with mesenchymal chondrosarcoma. Factors associated with better outcome included the following:[18][Level of evidence: 3iiiA]
- Lack of metastatic disease at initial presentation.
- Clear resection margins.
- Administration of postoperative chemotherapy following resection for patients with initially localized disease.
Treatment
Treatment options for extraskeletal mesenchymal chondrosarcoma include the following:
A review of 15 patients younger than 26 years from the German Cooperative Soft Tissue Sarcoma Study Group (11 with soft-tissue lesions) and the German-Austrian-Swiss Cooperative Osteosarcoma Study Group (four with primary bone lesions) protocols suggests that complete surgical removal, or incomplete resection followed by radiation therapy, is necessary for local control.[19][Level of evidence: 3iiA]
A single-institution, retrospective review identified 12 pediatric patients with mesenchymal chondrosarcoma.[20] The presence of the NCOA2 rearrangement in tumors was documented in these patients. It was also confirmed that surgical resection is necessary for cure. Eleven patients presented with localized disease and one presented with pulmonary nodules. All patients received chemotherapy—six patients before and after surgical resection and six patients only after resection. All patients received postoperative chemotherapy (most commonly ifosfamide/doxorubicin) with or without radiation therapy (median dose, 59.4 Gy). At a median follow-up of 4.8 years, 5-year disease-free survival (DFS) was 68.2% (95% CI, 39.8%–96.6%) and OS was 88.9% (95% CI, 66.9%–100%).
Extraskeletal osteosarcoma
Osseous and chondromatous neoplasms account for 0.8% of soft tissue sarcomas in patients younger than 20 years (refer to Table 1).
Extraskeletal osteosarcoma is extremely rare in the pediatric and adolescent age range. A 2003 review identified only ten case reports in the medical literature.[21]
Prognosis
Extraskeletal osteosarcoma is associated with a high risk of local recurrence and pulmonary metastasis.[22]
Treatment
Treatment options for extraskeletal osteosarcoma include the following:
- Surgery followed by chemotherapy.
(Refer to the PDQ summary on Osteosarcoma and Malignant Fibrous Histiocytoma of Bone Treatment for more information.)
Treatment options under clinical evaluation
Information about National Cancer Institute NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with extraskeletal mesenchymal chondrosarcoma and extraskeletal osteosarcoma are eligible for this trial.
Fibroblastic/Myofibroblastic Tumors
Fibroblastic/myofibroblastic tumors include the following tumor subtypes:
- Fibroblastic/myofibroblastic tumors.
- Intermediate grade (locally aggressive).
- Desmoid-type fibromatosis (previously called desmoid tumor or aggressive fibromatoses).
- Giant cell fibroblastoma.
- Lipofibromatosis.
- Palmar/plantar fibromatosis.
- Intermediate grade (rarely metastasizing).
- Dermatofibrosarcoma protuberans.
- Infantile fibrosarcoma.
- Inflammatory myofibroblastic tumor.
- Low-grade myofibroblastic sarcoma.
- Myxoinflammatory fibroblastic sarcoma.
- Solitary fibrous tumor.
- Malignant.
- Intermediate grade (locally aggressive).
Desmoid-type fibromatosis
Desmoid-type fibromatosis has previously been called desmoid tumors or aggressive fibromatoses.
Risk factors
A small number of desmoid-type fibromatosis tumors may occur in association with a mutation in the adenomatous polyposis coli (APC) gene (associated with intestinal polyps and a high incidence of colon cancer). In a study of 519 patients older than 10 years with a diagnosis of desmoid-type fibromatosis, 39 (7.5%, a possible underestimation) were found to have familial adenomatous polyposis (FAP).[23] The patients with FAP and desmoid-type fibromatosis were younger, more often male, and had more abdominal wall or mesenteric tumors than did patients with desmoid-type fibromatosis without FAP.
A family history of colon cancer, the presence of congenital hyperplasia of the retinal pigment epithelium,[24,25] or location of the desmoid-type fibromatosis in the abdomen or abdominal wall [23] should prompt referral to a genetic counselor. Currently, there are no general recommendations for genetic testing in children with desmoid-type fibromatosis. Pathology and molecular characteristics of the tumor only provide guidance for screening. If the tumor has a somatic CTNNB1 mutation, screening is not necessary, because the APC gene mutation has not been described in this setting. If a CTNNB1 mutation is not identified, screening for the APC mutation may be warranted.[26,27] (Refer to the Familial Adenomatous Polyposis (FAP) section of the PDQ summary on Genetics of Colorectal Cancer for more information.)
Prognosis
Desmoid-type fibromatosis has an extremely low potential to metastasize. The tumors are locally infiltrating, and surgical control can be difficult because of the need to preserve normal structures.
These tumors have a high potential for local recurrence. Desmoid-type fibromatosis has a highly variable natural history, including well documented examples of spontaneous regression.[28] Mutations in exon 3 of the beta-catenin gene are seen in over 80% of desmoid-type fibromatosis and the mutation 45F has been associated with an increased risk of disease recurrence.[29] Repeated surgical resection can sometimes bring recurrent lesions under control.[30]
Treatment
Evaluation of the benefit of interventions for treatment of desmoid-type fibromatosis has been extremely difficult, because desmoid-type fibromatosis has a highly variable natural history. Large adult series and smaller pediatric series have reported long periods of disease stabilization and even regression without systemic therapy.[30,31]; [32][Level of evidence: 3iiiDi]
Treatment options for desmoid-type fibromatosis include the following:
- Surgery.
- Observation, for tumors that are incompletely resected or recurrent that do not pose a danger to vital organs, if other treatment options are not available.[30,33-39] Whenever possible, however, the treatment of choice is complete resection.
- Chemotherapy, for unresectable or recurrent tumors.
- Other drug therapy, such as nonsteroidal anti-inflammatory drugs (NSAIDs) or antiestrogen therapy.
- Surgery preceded or followed by radiation therapy, for incompletely resected tumors or to avoid recurrence and subsequent surgery that may result in functional or cosmetic compromise.
- Radiation therapy alone, for unresectable tumors.
The treatment of choice is resection to achieve clear margins. However, a retrospective review of children who underwent surgery for desmoid-type fibromatosis at the St. Jude Children’s Research Hospital (SJCRH) reported no correlation between surgical margins and risk of recurrence.[39]
When the diagnosis is known and complete surgical excision is not feasible, and if the tumor poses significant potential for mortality or morbidity, preoperative strategies may include the following:[40,41]
- Observation.
- Chemotherapy.
- Anti-estrogen therapy.
- NSAID therapy.
- External-beam radiation therapy.
Desmoid-type fibromatosis often behaves in a nonaggressive manner. In a study that included mostly adults with extra-abdominal primary fibromatosis, nonsurgical approaches (medical and observation) had similar 3-year EFS compared with surgery.[34] In a subsequent study of adolescents and adults with abdominal wall aggressive fibromatosis, 102 patients were treated with a watch and wait approach, of which 65 patients required no further treatment at 3 years. Approximately one-third of patients had regression of the tumor.[33]
Chemotherapy regimens may include the following:
- Combination chemotherapy using vinblastine and methotrexate produced objective responses in about one-third of patients with unresectable or recurrent desmoid-type fibromatosis.[40]
- A series of mainly adult patients with FAP and unresectable desmoid-type fibromatosis that were unresponsive to hormone therapy showed that doxorubicin plus dacarbazine followed by meloxicam (an NSAID) can be safely administered and can induce responses.[42]
- Pegylated liposomal doxorubicin has been used with some responses.[43] In a series of five patients, a median progression-free interval of 29 months was reported.[44]
- Tyrosine kinase inhibitors: A small retrospective study of adults with desmoid-type fibromatosis showed objective responses to the multi-targeted kinase inhibitor sorafenib.[45][Level of evidence: 3iiiDiv] Previous studies with imatinib did not support its use.[46,47] A small series reported symptomatic improvement and stable disease in seven patients with desmoid-type fibromatosis who were treated with pazopanib.[48]
- The NOTCH pathway has been implicated in the development of desmoid tumors.[49] Partial responses to the gamma secretase inhibitor PF-03084014 have been noted in adults with desmoid-type fibromatosis.[50][Level of evidence: 3iiiDiv]
- Hydroxyurea has been used successfully to treat a few patients after other treatments, but more data are needed.[51-53]
Other drug therapy may include the following:
- NSAIDs such as sulindac have been used in single cases for desmoid-type fibromatosis; the responses seen were usually disease stabilization.[54]
- Antiestrogen treatment, usually tamoxifen, plus sulindac has also resulted in disease stabilization.[55] A prospective trial of the combination of tamoxifen and sulindac reported few side effects, although asymptomatic ovarian cysts were common in girls. This combination showed relatively little activity, as measured by rates of response and progression-free survival (PFS).[56][Level of evidence: 2Diii]
Postoperative radiation therapy is a consideration when progression would entail additional surgery that might cause functional or cosmetic compromise and if radiation is considered acceptable in terms of morbidities.
Radiation has been used for unresectable desmoid-type fibromatosis or postoperatively for tumors with inadequate resections. The potential long-term complications of radiation therapy, especially subsequent neoplasms, make using this modality less appealing in a young population.[57]
Dermatofibrosarcoma protuberans
Dermatofibrosarcoma is a rare tumor that can be present in all age groups, but many of the reported cases arise in children.[58-60] A review of 451 cases in children younger than 20 years in the SEER database found that the incidence was 1 case per 1 million, highest among black patients aged 15 to 19 years. The most common sites were trunk and extremities, which is similar to what is found in adults. Ninety-five percent of patients underwent surgery. OS was 100% at 5 years, 98% at 15 years, and 97% at 30 years. Males had decreased survival compared with females (P < .05).[61][Level of evidence: 3iA]
Molecular features
The tumor has a consistent chromosomal translocation t(17;22)(q22;q13) that juxtaposes the COL1A1 gene with the PDGF-beta gene.
Treatment
Treatment of dermatofibrosarcoma protuberans includes the following:
- Surgery.
- Surgery preceded or followed by radiation therapy.
- Radiation therapy and imatinib therapy, for unresectable or recurrent tumors.
Most dermatofibrosarcoma tumors can be cured by complete surgical resection. Wide excision with negative margins or Mohs or modified Mohs surgery will prevent most tumors from recurring.[62] Despite the locally aggressive behavior of the tumor, lymph node or visceral metastasis rarely occurs.
In retrospective reviews, postoperative radiation therapy after incomplete excision may have decreased the likelihood of recurrence.[63,64]
When surgical resection cannot be accomplished or the tumor is recurrent, treatment with imatinib has been effective.[65-67] Because metastatic disease is more likely after multiple recurrences, radiation or other adjuvant therapy should be considered in patients with recurrence that cannot be managed surgically.[59,61]
Guidelines for workup and management of dermatofibrosarcoma protuberans have been published.[68]
Infantile fibrosarcoma
There are two distinct types of fibrosarcoma in children and adolescents: infantile fibrosarcoma (also called congenital fibrosarcoma) and fibrosarcoma that is indistinguishable from fibrosarcoma seen in adults. These are two distinct pathologic diagnoses and require different treatments. Adult-type fibrosarcoma is addressed below.
Infantile fibrosarcoma usually occurs in children younger than 1 year. It occasionally occurs in children up to age 4 years. A tumor with similar morphology has been identified in older children; in these older children, the tumors do not have the t(12;15)(ETV-NTRK3) translocation that is characteristic of the younger patients.[69] In several of these patients, BRAF gene fusions have been identified.
Clinical presentation
Infantile fibrosarcoma usually presents with a rapidly growing mass, often noted at birth or even seen in prenatal ultrasound. The tumors are often quite large at the time of presentation.[70]
Molecular features
The tumor usually has a characteristic cytogenetic translocation t(12;15)(ETV-NTRK3). Infantile fibrosarcoma shares this translocation and a virtually identical histologic appearance with mesoblastic nephroma.
Prognosis
These tumors have a low incidence of metastases at diagnosis.
Treatment
Treatment options for infantile fibrosarcoma include the following:
- Surgery followed by observation.
- Surgery followed by chemotherapy.
- Chemotherapy followed by surgery.
Complete resection is curative in the majority of patients with infantile fibrosarcoma. However, the large size of the lesion frequently makes resection without major functional consequences impossible (for instance, tumors of the extremities often require amputation for complete excision). The European pediatric group has reported that observation may also be an option in patients with group II disease after surgery.[71] Twelve patients with group II disease received no further therapy and two patients relapsed. One patient obtained a complete remission after chemotherapy. Postoperative chemotherapy was administered to patients with higher group disease and those who progressed. In a subsequent study, only one of seven patients with group II disease progressed during observation; that patient achieved complete remission with chemotherapy.[72][Level of evidence: 3iiA]
Preoperative chemotherapy has made a more conservative surgical approach possible; agents active in this setting include vincristine, dactinomycin, cyclophosphamide, and ifosfamide.[73,74]; [72,75][Level of evidence: 3iiA]; [76][Level of evidence: 3iiB]
Three studies of patients with infantile fibrosarcoma suggest that an alkylator-free regimen is effective and should be used as the first treatment choice in patients with macroscopic disease.[71,72,77] Two cases with variant LMNA/NTRK1 fusions responded to crizotinib.[78,79]
A pediatric patient (aged 16 months) with refractory infantile fibrosarcoma with constitutive activation of the tropomyosin-related kinase signaling pathway from an ETS variant gene 6–neurotrophin 3 receptor gene fusion (ETV6-NTRK3) responded to LOXO-101, with a 90% reduction in tumor size after 2 months of treatment.[80]
A patient aged 2 months with infantile fibrosarcoma was initially treated with chemotherapy. At disease progression, a response was seen with pazopanib.[81]
A rare case of spontaneous regression without treatment has been reported.[82][Level of evidence: 3iiiDiv]
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- LOXO-TRK-15003 (NCT02637687) (Oral TRK Inhibitor LOXO-101 for Treatment of Advanced Pediatric Solid or Primary Central Nervous System [CNS] Tumors): A phase I trial of the pan-TRK inhibitor LOXO-101 is being conducted for children with solid tumors or brain tumors whose disease has progressed or was nonresponsive to available therapies, and for which no standard or available curative therapy exists. LOXO-101 is a highly selective inhibitor of all three TRK family kinases.
- RXDX-101-03 (NCT02650401) (Study of RXDX-101 in Children With Recurrent or Refractory Solid Tumors and Primary CNS Tumors): This is a four-part, open-label, phase I/Ib, dose-escalation study in pediatric patients with: 1) relapsed or refractory solid tumors; 2) primary CNS tumors; 3) neuroblastoma; and 4) non-neuroblastoma, extracranial solid tumors with NTRK1/2/3, ROS1 or ALK gene rearrangements. The study is designed to explore the safety, maximum tolerated dose or recommended phase II dose, pharmacokinetics, and antitumor activity of entrectinib (RXDX-101).
Inflammatory myofibroblastic tumor
Inflammatory myofibroblastic tumor is a rare mesenchymal tumor that has a predilection for children and adolescents.[83-85]
Clinical presentation
Inflammatory myofibroblastic tumors are rare tumors that affect soft tissues and visceral organs of children and young adults.[86] They rarely metastasize but tend to be locally invasive. Usual anatomical sites of disease include soft tissue, lungs, spleen, colon, and breast.[83] A review of 42 cases of pediatric inflammatory myofibroblastic tumor of the bladder was published in 2015.[87]
Molecular features
Roughly half of inflammatory myofibroblastic tumors exhibit a clonal mutation that activates the anaplastic lymphoma kinase (ALK)-receptor tyrosine kinase gene at chromosome 2p23.[88] ROS1 and PDGFR-beta kinase fusions have been identified in 8 of 11 cases (73%) who are negative for ALK by immunohistochemistry.[89][Level of evidence: 3iiiDiv]
Prognosis
Inflammatory myofibroblastic tumor recurs frequently but is rarely metastatic.[83-85]
Treatment
Treatment options for inflammatory myofibroblastic tumor include the following:
- Surgery.
- Chemotherapy.
- Steroid therapy.
- NSAID therapy.
- Targeted therapy (ALK inhibitors).
Complete surgical removal, when feasible, is the mainstay of therapy.[90] In a series of nine patients, four patients achieved continuous remission after complete resection, three patients with residual disease recurred but later achieved continuous remission, and one patient with metastatic disease responded to multiagent chemotherapy.[91][Level of evidence: 3iiA] The benefit of chemotherapy has been noted in case reports.[92] There are case reports of response to either steroids or NSAIDs.[93,94] A series of 32 patients aged 18 years and younger found that complete excision was the mainstay of therapy, although some patients were treated with steroids or cytotoxic chemotherapy. OS was 94%; three patients relapsed and two of them died of the disease. With complete excision, with or without other treatments such as steroids, there was a high survival rate for patients with this disease.[95][Level of evidence: 3iiA]
Inflammatory myofibroblastic tumors respond to crizotinib. Two adults with ALK-rearranged inflammatory myofibroblastic tumor achieved partial response with crizotinib.[96][Level of evidence: 3iiiDiv] For pediatric patients with measurable disease, the use of crizotinib achieved partial tumor responses in three of six patients with ALK-translocated inflammatory myofibroblastic tumors.[97] A case report of a patient aged 16 years with metastatic/multifocal ALK-positive inflammatory myofibroblastic tumor demonstrated a complete response and a 3-year disease-free interval with crizotinib therapy.[98] In a phase I trial of ceritinib for adult patients previously treated with ALK inhibitors, one patient with inflammatory myofibroblastic tumor had a partial response.[99] Finally, one study included 14 patients with inflammatory myofibroblastic tumor who were treated with crizotinib. With crizotinib therapy, five patients had a complete response, seven had a partial response, and the remaining two had stable disease; no patient had relapsed at the time the article was published.[100][Level of evidence: 3iiDiv]
Adult-type fibrosarcoma
These tumors lack the translocation seen in infantile fibrosarcomas. They present like the great majority of nonrhabdomyosarcomas and the management approach is similar.
Low-grade fibromyxoid sarcoma
Low-grade fibromyxoid sarcoma is a histologically deceptive soft tissue neoplasm that most commonly affects young and middle-aged adults, is commonly located deep within the extremities, and is characterized by a FUS/CREB3L3 translocation.[101,102]
Prognosis
In a review of 33 patients (three were younger than 18 years) with low grade fibromyxoid sarcoma, 21 of 33 patients developed a local recurrence after intervals of up to 15 years (median, 3.5 years) and 15 developed metastases up to 45 years (median, 5 years) from diagnosis, most commonly to the lungs and pleura, emphasizing the need for continued follow-up of these patients.[101] Even after metastases occur, the course may be indolent.[103]
In another report, 14 of 73 cases were younger than 18 years of age. In this series with a relatively short follow up (median of 24 months), only 8 of 54 patients with adequate follow up developed local (9%) or distant (6%) recurrence. This report suggests that the behavior of this tumor might be significantly better than previously reported.[104] However, because of the occurrence of late metastases, careful monitoring of these patients is warranted.
The most recent Children's Oncology Group (COG) trial (ARST0332 [NCT00346164]) enrolled 11 patients with this tumor entity. The median age at diagnosis was 13 years and males were more commonly affected. The most common sites were the lower and upper extremity (n = 9) and none of the patients had developed local or distant disease recurrence at a median follow up of 2.7 years.[105]
Treatment
Treatment options for low-grade fibromyxoid sarcoma include the following:
- Surgery.
The limited treatment information for low-grade fibromyxoid sarcoma suggest that surgery is the treatment of choice as the tumor is not very chemosensitive.[103] There are little data regarding the use of chemotherapy and/or radiation therapy in this disease. One report suggests that trabectedin may be effective in the treatment of low-grade fibromyxoid sarcoma.[106]
Myxofibrosarcoma
Myxofibrosarcoma is a rare lesion, especially in childhood. It is typically treated with complete surgical resection.
Sclerosing epithelioid fibrosarcoma
Sclerosing epithelioid fibrosarcoma is a rare malignant sarcoma that commonly harbors EWSR1 gene rearrangements and has an aggressive clinical course.[107] It is typically treated with complete surgical excision. Long-term follow-up is recommended because local recurrence and metastases can occur late.
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with infantile fibrosarcoma, inflammatory myofibroblastic tumor, low-grade myofibroblastic tumor, myxoinflammatory fibroblastic sarcoma, solitary fibrous tumor, adult-type fibrosarcoma, low-grade fibromyxoid sarcoma, myxofibrosarcoma, and sclerosing epithelioid fibrosarcoma are eligible for this trial.
Skeletal Muscle Tumors
Rhabdomyosarcoma
Refer to the PDQ summary on Childhood Rhabdomyosarcoma Treatment for more information.
Smooth Muscle Tumors
Leiomyosarcoma
Leiomyosarcoma accounts for 2% of soft tissue sarcoma in patients younger than 20 years (refer to Table 1).
Risk factors
Among 43 children with HIV/AIDS who developed tumors, eight developed Epstein-Barr virus–associated leiomyosarcoma.[108] Survivors of hereditary retinoblastoma have a statistically significant increased risk of developing leiomyosarcoma and 78% of these were diagnosed 30 or more years after the initial diagnosis of retinoblastoma.[109]
Treatment
Treatment options for leiomyosarcoma include the following:
- Chemotherapy (trabectedin).
In an open-label study of trabectedin in adult patients with recurrent sarcomas, the best overall response rate (complete remission and partial remission) was seen in patients with leiomyosarcoma (7.5%).[110] The clinical benefit rate (includes stable disease) for leiomyosarcoma was 54%. In another adult study, patients with recurrent liposarcoma and leiomyosarcoma were randomly assigned to receive treatment with either trabectedin or dacarbazine. Patients treated with trabectedin had a 45% reduction in disease progression.[14] There are no data to support the use of trabectedin in pediatric patients.
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with leiomyosarcoma are eligible for this trial.
So-called Fibrohistiocytic Tumors
So-called fibrohistiocytic tumors include the following tumor subtypes:
- Giant cell tumor of soft tissue.
- Plexiform fibrohistiocytic tumor.
Plexiform fibrohistiocytic tumor
Plexiform histiocytic tumor is a rare, low- to intermediate-grade tumor that most commonly affects children and young adults. Depending on the series, the median age at presentation ranges from 8 to 14.5 years; however, the tumor has been described in patients as young as 3 months.[111,112]
Clinical presentation
The tumor commonly arises as a painless mass in the skin or subcutaneous tissue and most often involves the upper extremities, including the fingers, hand, and wrist.[113-115] There are rare reports of spread to regional lymph nodes or the lungs.[111,115,116]
Molecular features
No consistent chromosomal anomalies have been detected but a t(4;15)(q21;q15) translocation has been reported.[117]
Prognosis
Plexiform fibrohistiocytic tumor is an intermediate-grade tumor that rarely metastasizes.
Treatment
Surgery is the treatment of choice but local recurrence has been reported in 12% to 50% of cases.[118]
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with giant cell tumors of soft tissue and plexiform fibrohistiocytic tumor are eligible for this trial.
Tumors of Peripheral Nerves
Ectomesenchymoma
Ectomesenchymoma is a rare nerve sheath tumor that mainly occurs in children. It is a biphenotypic soft tissue sarcoma with both mesenchymal and ectodermal components. Elements similar to rhabdomyosarcoma have been identified.
The German Soft Tissue Sarcoma Group (Cooperative Weichteilsarkom Studiengruppe [CWS]) reported on six patients (ages 0.2–13.5 years) registered over 14 years.[119][Level of evidence: 3iiA] The tumors were located in various sites including the extremities, abdomen, and orbit. All six patients were treated with surgery and chemotherapy directed at rhabdomyosarcoma. Two patients received radiation therapy. Three patients recurred with rhabdomyosarcoma features. Although data are scant, it appears that the tumor may respond to chemotherapy.[119]
Malignant peripheral nerve sheath tumor
Malignant peripheral nerve sheath tumors account for 5% of soft tissue sarcoma in patients younger than 20 years (refer to Table 1).
Risk factors
Malignant peripheral nerve sheath tumor can arise sporadically and in children with type 1 neurofibromatosis (NF1).[120]
Molecular features
Inactivating mutations of SUZ12 have been described in these tumors and are absent in neurofibromas.[121]
Prognosis
Features associated with a favorable prognosis include the following:[120,122-124]
- Smaller tumor size. In a multivariate analysis, only tumor size and nuclear p53 expression were found to be independent predictors of disease-specific survival.[123]
- Male sex and non-Hispanic white race.[125]
- No metastasis at presentation. A retrospective review of 140 patients with malignant peripheral nerve sheath tumor from the MD Anderson Cancer Center included children and adolescents. The disease-specific survival at 10 years was 32%. In this series, presence of metastatic disease was associated with a much worse prognosis.[123]
- Lower stage.
- Lower histologic grade.
- Extremity as the primary site.
Features associated with an unfavorable prognosis include the following:[126]
- High grade.
- Deep tumor location.
- Locally advanced stage at diagnosis.
- Macroscopically incomplete resection (R2).
For patients with localized disease in the MD Anderson Cancer Center study, there was no significant difference in outcome between patients with and without NF1.[123] In other studies, it was not clear whether the absence of NF1 is a favorable prognostic factor as it has been associated with both favorable [122] and unfavorable outcomes.[120,122,124] In the French Sarcoma Group study, NF1 was associated with other adverse prognostic features, but was not an independent predictor of poor outcome.[126] The Italian Sarcoma Group reported on outcomes after recurrence in 73 children and adolescents with malignant peripheral nerve sheath tumor.[127][Level of evidence: 3iiiA] The median overall survival after first relapse was 11 months, and the survival rates were 39.2% at 1 year and 15.8% at 5 years. The factors associated with a better prognosis for these patients who relapsed were less initial tumor invasiveness, longer time to relapse, and the achievement of a secondary complete remission (which was related to the feasibility of radical surgery).
Treatment
Treatment options for malignant peripheral nerve sheath tumor include the following:
Complete surgical removal of the tumor, whenever possible, is the mainstay of treatment.
The role of radiation therapy is difficult to assess, but durable local control of known postoperative microscopic residual tumor is not assured after radiation therapy.
Chemotherapy has achieved objective responses in childhood malignant peripheral nerve sheath tumor. A large retrospective analysis of the German and Italian experience with malignant peripheral nerve sheath tumor reported that 65% of measurable tumors had objective responses to ifosfamide-containing chemotherapy regimens, but the analysis did not conclusively demonstrate improved survival for chemotherapy.[120] This retrospective analysis also noted a trend toward improved outcome with postoperative radiation therapy.[120] A series of 37 young patients with malignant peripheral nerve sheath tumor and NF1 showed that most patients had large invasive tumors that were poorly responsive to chemotherapy; PFS was 19% and 5-year OS was 28%.[128]
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with malignant peripheral nerve sheath tumor are eligible for this trial.
- SARC023 (NCT02008877) (Ganetespib and Sirolimus in Patients With Malignant Peripheral Nerve Sheath Tumors): This trial is testing the combination of ganetespib, the heat shock protein inhibitor, and sirolimus, the mammalian target of rapamycin (mTOR) inhibitor, for the treatment of patients with unresectable or metastatic malignant peripheral nerve sheath tumors. Patients with unresectable soft tissue or bone sarcomas are eligible for phase I of the trial. Patients with unresectable malignant peripheral nerve sheath tumors are eligible for phase II of the trial. Eligibility is restricted to patients aged 18 years and older.
- NCT02601937 (A Phase 1 Study of the EZH2 Inhibitor Tazemetostat in Pediatric Subjects With Relapsed or Refractory INI1-Negative Tumors or Synovial Sarcoma): Patients with INI1-negative tumors are eligible for targeted treatment with an EZH2 inhibitor. This is a phase I, open-label, dose-escalation, and dose-expansion study with a twice-daily oral dose of tazemetostat.
- ADVL1522 (NCT02452554) (Lorvotuzumab Mertansine in Treating Younger Patients with Relapsed or Refractory Wilms Tumor, Rhabdomyosarcoma, Neuroblastoma, Pleuropulmonary Blastoma, Malignant Peripheral Nerve Sheath Tumor, or Synovial Sarcoma): This is a phase II study of IMGN901 (lorvotuzumab mertansine) in children with relapsed or refractory Wilms tumor, rhabdomyosarcoma, neuroblastoma, pleuropulmonary blastoma, malignant peripheral nerve sheath tumor, and synovial sarcoma. This trial is studying the effects of IMGN901, an antibody-drug conjugate that links a potent antimitotic to antibodies that target CD56.
Malignant triton tumor
Malignant triton tumors are a variant of malignant peripheral nerve sheath tumors. They occur most often in patients with neurofibromatosis type I and consist of neurogenic and rhabdomyoblastic components. Malignant triton tumors are high-grade malignancies. They usually occur before age 35 years and are very rare in children (case reports only).[129]
Malignant triton tumors are not usually responsive to chemotherapy and radiation therapy but have been treated with rhabdomyosarcoma therapy.[129][Level of evidence: 3iiiA] (Refer to the PDQ summary on Childhood Rhabdomyosarcoma Treatment for more information.)
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with malignant triton tumor are eligible for this trial.
Pericytic (Perivascular) Tumors
Myopericytoma
Infantile hemangiopericytoma is a subtype of myopericytoma.
Hemangiopericytoma is a highly vascularized tumor of uncertain origin.
Histology
Histologically, hemangiopericytomas are composed of packed round or fusiform cells that are arranged around a complex vasculature, forming many branch-like structures. Hyalinization is often present. Infantile hemangiopericytomas have similar histology but many are multilobular with vasculature outside the tumor mass.[130]
Treatment and outcome
Treatment of infantile hemangiopericytomas includes the following:
- Chemotherapy.
In a series of 17 children, the differences in metastatic potential and response to treatment were clearly demonstrated for adult and infantile hemangiopericytomas.[131] Eleven children were older than 1 year. Several of these patients had disease in the lymph nodes or lungs. Six patients with stage II or III disease progressed and died. Three patients with stage I disease survived, although one had recurrence in the lungs. Six patients had infantile hemangiopericytoma, most were greater than stage I (5 of 6). All six patients survived and three had good responses to vincristine, actinomycin, and cyclophosphamide. Hemangiopericytoma in children younger than 1 year seems to have a better prognosis than in children older than 1 year.[132-134]
Infantile myofibromatosis
This entity is a fibrous tumor of infancy and childhood that most commonly presents in the first 2 years of life.[135] The lesion can present as a single subcutaneous nodule (myofibroma) most commonly involving the head and neck region or lesions can affect multiple skin areas, muscle, and bone (myofibromatosis).[136-139]
An autosomal dominant form of the disease has been described and it is associated with germline mutations of the PDGFRB gene.[140]
Treatment
These lesions have an excellent prognosis and can regress spontaneously.
About one-third of cases with multicentric involvement will also have visceral involvement, and the prognosis for these patients is poor.[138,139,141] The use of combination therapy with vincristine/dactinomycin and vinblastine/methotrexate have proven effective in cases of multicentric disease with visceral involvement and in cases in which the disease has progressed and has threatened the life of the patient (e.g., upper airway obstruction).[138,139,142]
Tumors of Uncertain Differentiation
Tumors of uncertain differentiation include the following tumor subtypes:
- Alveolar soft part sarcoma.
- Clear cell sarcoma of soft tissue.
- Desmoplastic small round cell tumor.
- Epithelioid sarcoma.
- Extrarenal rhabdoid tumor.
- Extraskeletal myxoid chondrosarcoma.
- Neoplasms with perivascular epithelioid cell differentiation (PEComa NOS, malignant)
- Primitive neuroectodermal tumor (PNET)/extraskeletal Ewing tumor.
- Synovial sarcoma (NOS, spindle cell, and biphasic varieties).
Alveolar soft part sarcoma
Alveolar soft parts sarcomas account for 1.4% of soft tissue sarcomas in patients younger than 20 years (refer to Table 1).
Clinical presentation
The median age at presentation is 25 years, and alveolar soft part sarcoma most commonly arises in the extremities but can occur in the oral and maxillofacial region.[143-145] Alveolar soft part sarcoma in children can present with evidence of metastatic disease.[146]
Molecular features
This tumor of uncertain histogenesis is characterized by a consistent chromosomal translocation t(X;17)(p11.2;q25) that fuses the ASPSCR1 gene with the TFE3 gene.[147,148]
Prognosis
Alveolar soft part sarcoma in children may have an indolent course.[146] Patients with alveolar soft part sarcoma may relapse several years after a prolonged period of apparent remission.[149] Because these tumors are rare, all children with alveolar soft part sarcoma should be considered for enrollment in prospective clinical trials.
In a series of 19 treated patients, one group reported a 5-year OS rate of 80%, a 91% OS rate for patients with localized disease, a 100% OS rate for patients with tumors 5 cm or smaller, and a 31% OS rate for patients with tumors larger than 5 cm.[150] In another series of 33 patients, OS was 68% at 5 years from diagnosis and 53% at 10 years from diagnosis. Survival was better for smaller tumors (≤5 cm) and completely resected tumors.[151][Level of evidence: 3iiA] Delayed metastases to the brain and lung are uncommon.[143] A retrospective review of children and young adults younger than 30 years (median age, 17 years; range, 1.5–30 years) from four institutions identified 69 patients treated primarily with surgery between 1980 and 2014.[152][Level of evidence: 3iiA] The ASPL-TFE3 translocation was present in all 26 patients tested. There were 19 patients with Intergroup Rhabdomyosarcoma Study (IRS) postsurgical staging group I tumors (28%), 7 patients with IRS group II tumors (10%), 5 patients with IRS group III tumors (7%), and 38 patients with IRS group IV tumors (55%). The 5-year EFS was 80% and the OS was 87% for the 31 patients with localized tumors (IRS postsurgical groups I, II, and III). The 5-year EFS was 7% and the OS was 61% for the 38 patients with metastatic tumors (IRS postsurgical group IV).
Treatment
Treatment options for alveolar soft part sarcoma include the following:
The standard approach is complete resection of the primary lesion.[150] If complete excision is not feasible, radiation therapy should be administered. A study from China reported on 18 patients with alveolar soft part sarcoma of the oral and maxillofacial region; 15 patients were younger than 30 years.[145][Level of evidence: 3iiDii] Surgical removal with negative margins was the primary treatment. All patients survived, and only one patient had metastatic disease recurrence.
A series of 51 pediatric patients aged 0 to 21 years with alveolar soft part sarcoma found an OS rate at 10 years of 78% and an EFS rate of about 63%. Patients with localized disease (n = 37) had a 10-year OS of 87%, and the 14 patients with metastases at diagnosis had a 10-year OS of 44%, partly resulting from surgical removal of primary tumor and lung metastases in some patients. Only 3 of 18 patients (17%) with measurable disease had a response to conventional antisarcoma chemotherapy, but two of four patients treated with sunitinib had a partial response.[143][Level of evidence: 3iiiA] There have been sporadic reports of objective responses to interferon-alpha and bevacizumab.[143,153,154]
A small retrospective study of nine adult patients with metastatic alveolar soft part sarcoma treated with sunitinib reported partial response in five patients and stable disease in two patients.[155][Level of evidence: 3iiiDiv] In a phase II trial of cediranib, an inhibitor of all three known vascular epidermal growth factor receptors, 15 of 43 adult patients (35%) with metastatic alveolar soft part sarcoma had a partial response.[156][Level of evidence: 3iiDiv]
There have been no open trials for patients with metastatic alveolar soft part sarcoma.
Treatment options under clinical evaluation for alveolar soft part sarcoma
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- NCT00942877 (Phase II Study of Cediranib [AZD2171] in Patients With Alveolar Soft Part Sarcoma): A phase II study of cediranib in patients with alveolar soft part sarcoma is being conducted in patients younger than 16 years at the Clinical Center of the National Institutes of Health.
- NCT01391962 (Sunitinib or Cediranib for Alveolar Soft Part Sarcoma): A phase II trial in which patients with metastatic alveolar soft part sarcoma are randomly assigned to either sunitinib or cediranib monotherapy, with crossover at disease progression. Patients aged 16 years and older are eligible. This study is being conducted at the Clinical Center of the National Institutes of Health.
Clear cell sarcoma of soft tissue
Clear cell sarcoma (formerly and inappropriately called malignant melanoma of soft parts) is a rare soft tissue sarcoma that typically involves the deep soft tissues of the extremities. It is also called clear cell sarcoma of tendons and aponeuroses. The tumor often affects adolescents and young adults.
Patients who have small, localized tumors with low mitotic rate and intermediate histologic grade fare best.[157]
Clinical presentation
The tumor most commonly affects the lower extremity, particularly the foot, heel, and ankle.[158,159] It has a high propensity for nodal dissemination, especially metastases to regional lymph nodes (12%–43%).[159,160] The tumor typically has an indolent clinical course.
Molecular features
Clear cell sarcoma of soft tissue is characterized by an EWS-ATF1 fusion.[161]
Treatment
Treatment options for clear cell sarcoma of soft tissue include the following:
In a series of 28 pediatric patients reported by the Italian and German Soft Tissue Cooperative Studies, the median age at diagnosis was 14 years and the lower extremity was the most common primary site (50%). Surgery with or without radiotherapy is the treatment of choice and offers the best chance for cure. In this series, 12 of 13 patients with completely resected tumors were cured. For patients with more advanced disease the outcome is poor and chemotherapy is rarely effective.[162]; [163][Level of evidence: 3iiDii]
Desmoplastic small round cell tumor
Desmoplastic small round cell tumor is a rare primitive sarcoma.
Clinical presentation
Desmoplastic small round cell tumor most frequently involves the abdomen, pelvis, or tissues around the testes, but it may occur in the kidney.[164-167] The tumor occurs more commonly in males and may spread to the lungs and elsewhere. Peritoneal and pelvic lesions frequently have widespread peritoneal implants.[168]
In a large, single-institution series of 65 patients, a correlation was made between computed tomography (CT) scans in most patients and positron-emission tomography (PET)/CT scans in 11 patients. PET/CT scans had very few false-negative results and detected metastatic sites missed on conventional CT scans.[168]
Molecular features
Cytogenetic studies of these tumors have demonstrated the recurrent translocation t(11;22)(p13;q12), which has been characterized as a fusion of the WT1 and EWS genes.[167,169] The WT1-EWS fusion confirms the diagnosis of desmoplastic small round cell tumor.
Prognosis
The overall prognosis for desmoplastic small round cell tumor remains extremely poor, with reported rates of death at 90%. Greater than 90% tumor resection either at presentation or after preoperative chemotherapy may be a favorable prognostic factor for OS.[170,171]; [172][Level of evidence: 3iiiA]
Treatment
There is no standard approach to the treatment of desmoplastic small round cell tumor.
Treatment options for desmoplastic small round cell tumor include the following:
- Surgery.
- Chemotherapy followed by surgery.
- Radiation therapy.
Complete surgical resections are rare, and the overall prognosis for desmoplastic small round cell tumor remains extremely poor, with reported rates of death at 90%. Treatment may include chemotherapy, surgery, and radiation therapy. Multiagent chemotherapy analogous to that used for sarcomas has been used, as well as total abdominal radiation therapy.[164,165,170,173-176]
A single-institution study reported that five of five patients with recurrent desmoplastic small round cell tumor had partial responses to treatment with the combination of vinorelbine, cyclophosphamide, and temsirolimus.[177]
The Center for International Blood and Marrow Transplant Research (CIBMTR) analyzed patients with desmoplastic small round cell tumor in their registry who received consolidation with high dose chemotherapy and autologous stem cell reconstitution.[178] While this retrospective registry analysis suggested some benefit for this approach, other investigators have abandoned the approach because of excessive toxicity and lack of efficacy.[170]
Epithelioid sarcoma
Epithelioid sarcoma is a rare mesenchymal tumor of uncertain histogenesis which displays multilineage differentiation.[179]
Clinical presentation
Epithelioid sarcoma commonly presents as a slowly growing firm nodule based in the deep soft tissue; the proximal type predominantly affects adults and involves the axial skeleton and proximal sites. The tumor is highly aggressive and has a propensity for lymph node metastases.
Molecular features
Epithelioid sarcoma is characterized by inactivation of the SMARCB1 gene, which is present in both conventional and proximal types of epithelioid sarcoma.[180] This abnormality leads to increased dependence on EZH2 and tumor formation.[181]
Treatment
Treatment options for epithelioid sarcoma include the following
- Chemotherapy.
- Surgery.
- Surgery preceded or followed by radiation therapy.
Patients should be carefully evaluated for the presence of involved lymph nodes; suspicious lymph nodes should be biopsied. Surgical removal of primary and recurrent tumor(s) is the most effective treatment.[182][Level of evidence: 3iiiA]
In a review of 30 pediatric patients with epithelioid sarcoma (median age at presentation, 12 years), responses to chemotherapy were reported in 40% of patients using sarcoma-based regimens, and 60% of patients were alive at 5 years after initial diagnosis.[183] A single-institution retrospective review of 20 patients, including children and adults (median age, 27.3 years), found no difference in the probability of recurrence between patients who received chemotherapy and those who did not receive chemotherapy and suggested that radiation therapy may be useful.[182]
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- NCT02601937 (A Phase 1 Study of the EZH2 Inhibitor Tazemetostat in Pediatric Subjects With Relapsed or Refractory INI1-Negative Tumors or Synovial Sarcoma): Patients with INI1-negative tumors are eligible for targeted treatment with an EZH2 inhibitor. This is a phase I, open-label, dose-escalation, and dose-expansion study with a twice-daily oral dose of tazemetostat.
Extrarenal (extracranial) rhabdoid tumor
Malignant rhabdoid tumors were first described in children with renal tumors in 1981 (refer to the PDQ summary on Wilms Tumor and Other Childhood Kidney Tumors Treatment for more information) and were later found in a variety of extrarenal sites. These tumors are uncommon and highly malignant, especially in children younger than 2 years.
Extrarenal (extracranial) rhabdoid tumors account for 2% of soft tissue sarcoma in patients younger than 20 years (refer to Table 1).
Molecular features
The first sizeable series of 26 children with extrarenal extracranial malignant rhabdoid tumor of soft tissues came from patients enrolled on the Intergroup Rhabdomyosarcoma Studies I through III during a review of pathology material. Only five patients (19%) were alive without disease.[184] Later, investigation of children with atypical teratoid/rhabdoid tumors of the brain, as well as those with renal and extrarenal malignant rhabdoid tumors, found germline and acquired mutations of the SMARCB1 gene in all 29 tumors tested.[185] Rhabdoid tumors may be associated with germline mutations of the SMARCB1 gene and may be inherited from an apparently unaffected parent.[186] This observation was extended to 32 malignant rhabdoid tumors at all sites in patients whose mean age at diagnosis was 12 months.[187]
Prognosis
In a Surveillance, Epidemiology, and End Results (SEER) study of 229 patients with renal, central nervous system, and extrarenal malignant rhabdoid tumor, patients aged 2 to 18 years, limited extent of tumor, and delivery of radiation therapy were shown to affect the outcome favorably compared with other patients (P < .002 for each comparison). Site of the primary tumor was not prognostically significant. OS at 5 years was 33%.[188]
Treatment
Treatment includes surgical removal when possible, chemotherapy as used for soft tissue sarcomas (but no single regimen is currently accepted as best), and radiation therapy.[189][Level of evidence: 3iA]; [190,191][Level of evidence: 3iiiB]
Responses to alisertib have been documented in four patients with central nervous system (CNS) atypical teratoid/rhabdoid tumors.[192] (Refer to the PDQ summary on Childhood Central Nervous System Atypical Teratoid/Rhabdoid Tumor Treatment summary for more information about CNS atypical teratoid/rhabdoid tumors.)
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- NCT02601937 (A Phase 1 Study of the EZH2 Inhibitor Tazemetostat in Pediatric Subjects With Relapsed or Refractory INI1-Negative Tumors or Synovial Sarcoma): Patients with INI1-negative tumors are eligible for targeted treatment with an EZH2 inhibitor. This is a phase I, open-label, dose-escalation, and dose-expansion study with a twice-daily oral dose of tazemetostat.
Extraskeletal myxoid chondrosarcoma
Extraskeletal myxoid chondrosarcoma is relatively rare among soft tissue sarcomas, representing only 2.3% of all soft tissue sarcoma.[193] It has been reported in children and adolescents.[194]
Molecular features
Extraskeletal myxoid chondrosarcoma is a multinodular neoplasm. The rounded cells are arranged in cords and strands in a chondroitin sulfate myxoid background. Several cytogenetic abnormalities have been identified (refer to Table 2), with the most frequent being the translocation t(9;22)(q22;q12), involving the EWSR1/NR4A3 genes.[195]
Prognosis
The tumor has traditionally been considered of low-grade malignant potential.[196] However, recent reports from large institutions showed that extraskeletal myxoid chondrosarcoma has significant malignant potential, especially if patients are followed for a long time.[197,198] Patients tend to have slow protracted courses. Nodal involvement has been well described. Local recurrence (57%) and metastatic spread to lungs (26%) have been reported.[198]
Treatment
Treatment options for extraskeletal myxoid chondrosarcoma include the following:
- Surgery.
- Radiation therapy.
The therapeutic benefit of chemotherapy has not been established. Aggressive local control and resection of metastases led to OS of 87% at 5 years and 63% at 10 years. Tumors were relatively resistant to radiation therapy.[197]
There may be potential genetic targets for small molecules, but these should be studied as part of a clinical trial. In an adult study, six of ten patients who received sunitinib achieved a partial response.[199]
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- NCT02601937 (A Phase 1 Study of the EZH2 Inhibitor Tazemetostat in Pediatric Subjects With Relapsed or Refractory INI1-Negative Tumors or Synovial Sarcoma): Patients with INI1-negative tumors are eligible for targeted treatment with an EZH2 inhibitor. This is a phase I, open-label, dose-escalation, and dose-expansion study with a twice-daily oral dose of tazemetostat.
Neoplasms with perivascular epithelioid cell differentiation (PEComas)
Risk factors and molecular features
Benign PEComas are common in tuberous sclerosis, an autosomal dominant syndrome that also predisposes to renal cell cancer and brain tumors. Tuberous sclerosis is caused by germline inactivation of either TSC1 (9q34) or TSC2 (16p13.3), and the same tumor suppressor genes are inactivated somatically in sporadic PEComas.[200] Inactivation of either gene results in stimulation of the mTOR pathway, providing the basis for the treatment of nonsurgically curable PEComas with mTOR inhibitors.[201,202] A small proportion of PEComas have TFE3 rearrangements with fusions involving various genes including SFPQ/PSF and RAD51B.[203]
Clinical presentation
PEComas occur in various rare gastrointestinal, pulmonary, gynecologic, and genitourinary sites. Soft tissue, visceral, and gynecologic PEComas are more commonly seen in middle-aged female patients and are usually not associated with the tuberous sclerosis complex.[204] The disease course may be indolent.
Prognosis
Most PEComas have a benign clinical course, but malignant behavior has been reported and can be predicted based on the size of the tumor, mitotic rate, and presence of necrosis.[205]
Treatment
Treatment options have not been defined. Treatment may include surgery or observation followed by surgery when the tumor is large.[206]
Clinical activity with mTOR inhibitors, such as sirolimus, in tumors with evidence of mTORC1 activation and TSC loss has been well documented.[207]
Primitive neuroectodermal tumor (PNET)/extraskeletal Ewing tumor
(Refer to the PDQ summary on Ewing Sarcoma Treatment for more information.)
Synovial sarcoma
Synovial sarcoma accounts for 9% of soft tissue sarcomas in patients younger than 20 years (refer to Table 1).
Synovial sarcoma is one of the most common nonrhabdomyosarcomatous soft tissue sarcomas in children and adolescents. In a 1973 to 2005 SEER review, 1,268 patients with synovial sarcoma were identified. Approximately 17% of these patients were children and adolescents and the median age at diagnosis was 34 years.[208]
Histologic classification
Synovial sarcoma can be subclassified as the following types:
- Synovial sarcoma, NOS.
- Synovial sarcoma, spindle cell.
- Synovial sarcoma, biphasic.
Clinical presentation
The most common tumor location is the extremities, followed by trunk and head and neck.[208] Rarely, a synovial sarcoma may arise in the heart or pericardium.[209]
The most common site of metastasis is the lung.[210,211] The risk of metastases is highly influenced by tumor size; it is estimated that patients with tumors that are larger than 5 cm have a 32-fold risk of developing metastases when compared with other patients.
Diagnostic evaluation
The diagnosis of synovial sarcoma is made by immunohistochemical analysis, ultrastructural findings, and demonstration of the specific chromosomal translocation t(x;18)(p11.2;q11.2). This abnormality is specific for synovial sarcoma and is found in all morphologic subtypes. Synovial sarcoma results in rearrangement of the SYT gene on chromosome 18 with one of the subtypes (1, 2, or 4) of the SSX gene on chromosome X.[212,213] It is thought that the SYT/SSX18 transcript promotes epigenetic silencing of key tumor suppressor genes.[214]
In one report, reduced INI1 nuclear reactivity on immunohistochemical staining was seen in 49 cases of synovial sarcoma, suggesting that this pattern may help distinguish synovial sarcoma from other histologies.[215]
Prognosis
Patients younger than 10 years have more favorable outcomes and clinical features, including extremity primaries, smaller tumors, and localized disease, than do older patients.[208,216] A meta-analysis also suggested that response to chemotherapy was correlated with improved survival.[217]
The following studies have reported multiple factors associated with unfavorable outcomes:
- In a retrospective analysis of synovial sarcoma in children and adolescents who were treated in Germany and Italy, tumor size (>5 cm or ≤5 cm in greatest dimension) was an important predictor of EFS.[218] In this analysis, local invasiveness conferred an inferior probability of EFS, but surgical margins were not associated with clinical outcome.
- In a single-institution retrospective analysis of 111 patients with synovial sarcoma who were younger than 22 years at diagnosis, larger tumor size, greater depth in tissue, greater local invasiveness, and more proximal tumor location were associated with poorer OS.[219][Level of evidence: 3iiA]
- A multicenter analysis of 219 children from various treating centers including Germany, SJCRH, Instituto Tumori, and MD Anderson Cancer Center reported an estimated 5-year OS of 80% and EFS rate of 72%.[217] In this analysis, an interaction between tumor size and invasiveness was observed; in multivariate analysis, patients with large or invasive tumors or with Intergroup Rhabdomyosarcoma Study Clinical Group III disease (localized, incompletely resected or with biopsy only) and IV (metastases at diagnosis) had decreased OS. Treatment with radiation therapy was related to improved OS (hazard ratio, 0.4; 95% confidence interval, 0.2–0.7). In Intergroup Rhabdomyosarcoma Study Group III patients, objective response to chemotherapy (18 of 30 [60%]) correlated with improved survival. In adults, factors such as International Union Against Cancer/American Joint Committee on Cancer stage III and stage IVA, tumor necrosis, truncal location, elevated mitotic rate, age, and histologic grade have been associated with a worse prognosis.[220-222]
- Expression and genomic index prognostic signatures have been studied in synovial sarcoma. Complex genomic profiles, with greater rearrangement of the genome, are more common in adults than in younger patients with synovial sarcoma and are associated with a higher risk of metastasis.[223]
- A review of 84 patients with localized synovial sarcoma who had information on fusion status (SYT-SSX) and histologic grading found no difference in OS according to these criteria. However, for tumor size at diagnosis, the study showed that patients with tumors between 5 cm and 10 cm had a worse prognosis than those with smaller tumors (P = .02), and patients with tumors larger than 10 cm had even worse OS (P = .0003).[224][Level of evidence: 3iiiA]
- The German CWS group reviewed 27 evaluable patients younger than 21 years with pulmonary metastases among 296 patients with synovial sarcoma. Metastases involved the lungs in all patients. The 5-year EFS rate was 26%, and the OS rate was 30%. The most important prognostic factor at presentation was that the metastases were limited to one lesion in one lung or one lesion in both lungs (a group they termed oligometastatic). Treatment elements associated with superior survival were adequate local therapy of the primary tumor and, if feasible, for the metastases. The use of whole-lung irradiation did not correlate with better outcomes.[225][Level of evidence: 3iiA]
Survival after relapse is poor (30% at 5 years). Factors associated with outcome after relapse include duration of first remission (> or ≤ 18 months) and lack of a second remission.[226]
Treatment
Treatment options for synovial sarcoma include the following:
The COG and the European Pediatric Soft Tissue Sarcoma Study Group reported a combined analysis of 60 patients younger than 21 years with localized synovial sarcoma prospectively assigned to surgery without adjuvant radiation therapy or chemotherapy.[227] Enrollment was limited to patients with initial complete resection with histologically free margins, with a grade 2 tumor of any size or a grade 3 tumor 5 cm or smaller. The 3-year EFS was 90% (median follow-up, 5.2 years; range, 1.9–9.1). All eight events were local tumor recurrence; no metastatic recurrences were seen. All patients with recurrent disease were effectively treated with salvage therapy, resulting in 100% OS.
Synovial sarcoma appears to be more sensitive to chemotherapy than many other soft tissue sarcomas, and children with synovial sarcoma seem to have a better prognosis when compared with adults.[11,211,222,228-232] The most commonly used regimens for the treatment of synovial sarcoma incorporate ifosfamide and doxorubicin.[217,231,233] Response rates to the ifosfamide and doxorubicin regimen are higher than in other nonrhabdomyosarcomatous soft tissue sarcomas.[234]
Several studies have reported the following chemotherapy-associated treatment findings:
- Several treatment centers advocate postoperative chemotherapy after resection and radiation therapy of synovial sarcoma in children and young adults.[217,218,235-237]
- The International Society of Pediatric Oncology-Malignant Mesenchymal Tumors studies showed that select patients (young age, <5 cm resected tumors) with nonmetastatic synovial sarcoma can have excellent outcome in the absence of radiation, but it is still unclear whether that approach obviates an advantage of radiation for local or regional control.[236]
- A German trial suggested a benefit for postoperative chemotherapy in children with synovial sarcoma.[237]
- A meta-analysis also suggested that chemotherapy may provide benefit.[217]
- In the most recent COG ARST0332 (NCT00346164) study, 129 patients with synovial sarcoma were prospectively treated using a risk-based therapy program (as detailed in the prognosis section), of which 43 were categorized as low risk, 66 as intermediate risk, and 20 as high risk. At a median follow-up of 2.6 years, 3-year EFS for low-, intermediate-, and high-risk groups were 83%, 79%, and 16%, respectively. The use of risk factor–directed therapy accurately predicted outcomes.[238]
- The European Pediatric Soft Tissue Sarcoma Study Group performed a prospective study of patients younger than 21 years with synovial sarcoma (CCLG-EPSSG-NRSTS-2005 [NCT00334854]).[239][Level of evidence: 3iiA] Patients were stratified into the following three risks groups and nonrandomly assigned to treatment by risk group:
- Low-risk patients had Intergroup Rhabdomyosarcoma Study (IRS) group I tumors less than 5 cm in size and nonaxial primary tumors.
- Intermediate-risk patients had no axial primary tumors and IRS group I tumors greater than 5 cm or IRS group II tumors.
- High-risk patients included all patients with axial primary sites (head and neck, lung and pleura, trunk, retroperitoneal), IRS group III tumors, or N1 tumors.
Outcomes for patients treated on the CCLG-EPSSG-NRSTS-2005 trial are described in Table 9.
Table 9. Event-Free Survival (EFS) and Overall Survival (OS) in Patients With Low-, Intermediate-, and High-Risk Synovial Sarcoma Treated on the CCLG-EPSSG-NRSTS-2005 Trial Risk Group Treatment 3-Year EFS (%) 3-Year OS (%) IRS = Intergroup Rhabdomyosarcoma Study; RT = radiation therapy. aChemotherapy was ifosfamide/doxorubicin, with doxorubicin omitted during radiation therapy. b59.4 Gy in cases without the option of secondary resection; 50.4 Gy as preoperative radiation therapy; 50.4, 54, and 59.4 Gy as postoperative radiation therapy, in the case of R0, R1, and R2 resections, respectively (no additional radiation therapy in the case of secondary complete resections with free margins, in children younger than 6 years). Low Surgery alone 92 100 Intermediate Surgery, 3–6 cycles chemotherapya ± RTb 91 100 High (IRS group III) 3 cycles of chemotherapya surgery, 3 additional cycles of chemotherapy, ± RTb 77 94 High (axial primary sites) Surgery, 6 cycles of chemotherapya, RTb 78 100
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- ADP 04511 (NCT01343043) (A Pilot Study of Genetically Engineered NY-ESO-1 Specific [c259] T Cells in HLA-A2+ Patients With Synovial Sarcoma): Patients with unresectable, metastatic, or recurrent synovial sarcoma undergo apheresis. Cells are shipped to a central laboratory where they undergo NY-ESO-1 transduction, expansion, and cryopreservation. Patients undergo lymphodepletion with fludarabine and cyclophosphamide, followed by an infusion of autologous transfected cells. Eligibility is restricted to patients with HLA type A2+, age older than 4 years, and weight greater than 18 kg.
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with alveolar soft part sarcoma, clear cell sarcoma of soft tissue, epithelioid sarcoma, extraskeletal myxoid chondrosarcoma, PEComa, and synovial sarcoma are eligible for this trial.
- NCT02601937 (A Phase 1 Study of the EZH2 Inhibitor Tazemetostat in Pediatric Subjects With Relapsed or Refractory INI1-Negative Tumors or Synovial Sarcoma): Patients with INI1-negative tumors are eligible for targeted treatment with an EZH2 inhibitor. This is a phase I, open-label, dose-escalation, and dose-expansion study with a twice-daily oral dose of tazemetostat.
- ADVL1522 (NCT02452554) (Lorvotuzumab Mertansine in Treating Younger Patients with Relapsed or Refractory Wilms Tumor, Rhabdomyosarcoma, Neuroblastoma, Pleuropulmonary Blastoma, Malignant Peripheral Nerve Sheath Tumor, or Synovial Sarcoma): This is a phase II study of IMGN901 (lorvotuzumab mertansine) in children with relapsed or refractory Wilms tumor, rhabdomyosarcoma, neuroblastoma, pleuropulmonary blastoma, malignant peripheral nerve sheath tumor, and synovial sarcoma. This trial is studying the effects of IMGN901, an antibody-drug conjugate that links a potent antimitotic to antibodies that target CD56.
Undifferentiated/unclassified sarcoma
Patients with undifferentiated soft tissue sarcoma had been eligible for participation in rhabdomyosarcoma trials coordinated by the Intergroup Rhabdomyosarcoma Study Group and the COG from 1972 to 2006. The rationale was the observation that patients with undifferentiated soft tissue sarcoma had similar sites of disease and outcome as those with alveolar rhabdomyosarcoma. Therapeutic trials for adults with soft tissue sarcoma include patients with undifferentiated soft tissue sarcoma and other histologies, which are treated similarly, using ifosfamide and doxorubicin, and sometimes with other chemotherapy agents, surgery, and radiation therapy.
In the COG ARST0332 (NCT00346164) trial, patients with high-grade undifferentiated sarcoma were treated with an ifosfamide and doxorubicin-based regimen and were treated with rhabdomyosarcoma-directed therapies in previous Intergroup Rhabdomyosarcoma Study Group studies with a 5-year survival estimate for nonmetastatic patients of 72%.[240][Level of evidence: 3iiA] Currently, these patients are eligible for the COG open ARST1321 (NCT02180867) trial for patients with nonrhabdomyosarcomatous soft tissue sarcoma.
Undifferentiated pleomorphic sarcoma/malignant fibrous histiocytoma (high-grade)
At one time, malignant fibrous histiocytoma was the single most common histotype among adults with soft tissue sarcomas. Since it was first recognized in the early 1960s, malignant fibrous histiocytoma has been plagued by controversy in terms of both its histogenesis and its validity as a clinicopathologic entity. The latest WHO classification no longer includes malignant fibrous histiocytoma as a distinct diagnostic category but rather as a subtype of an undifferentiated pleomorphic sarcoma.[241]
This entity accounts for 2% to 6% of all childhood soft tissue sarcomas.[242] These tumors can arise in previously irradiated sites or as a second malignancy in patients with retinoblastoma.
These tumors occur mainly in the second decade of life. In a series of ten patients, the median age was 10 years and the tumor was most commonly located in the extremities. In this series, all tumors were localized and five of nine (for whom follow-up was available) were alive and in first remission.[242] In another series of 17 pediatric patients with malignant fibrous histiocytoma, the median age at diagnosis was 5 years and the extremities were involved in eight cases.[243] All patients with metastatic disease died and two patients experienced a clinical response to a doxorubicin-based regimen.
(Refer to the PDQ summary on Osteosarcoma and Malignant Fibrous Histiocytoma of Bone Treatment for more information about the treatment of malignant fibrous histiocytoma of bone.)
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with undifferentiated epithelial sarcoma, undifferentiated pleomorphic sarcoma, undifferentiated round cell sarcoma, and undifferentiated spindle cell sarcoma are eligible for this trial.
Vascular Tumors
Vascular tumors vary from hemangiomas, which are always considered benign, to angiosarcomas, which are highly malignant.[244] Vascular tumors include the following tumor subtypes:
Angiosarcoma of the soft tissue
Incidence
Angiosarcoma is a rare (accounting for 2% of sarcomas), aggressive, vascular tumor that can arise in any part of the body, but is more common in the soft tissue. Angiosarcoma has an estimated incidence of 2 cases per 1 million; in the United States, it annually affects approximately 600 people who are typically aged 60 to 70 years.[245]
Angiosarcomas are extremely rare in children and it is unclear if the pathophysiology of this tumor is different in the pediatric population. Cases have been reported in neonates and toddlers, with presentation of multiple cutaneous lesions and liver lesions, some of which are GLUT1 positive.[246-249] Most angiosarcomas involve the skin and superficial soft tissue, although the liver, spleen, and lung can be affected; bone is rarely affected.
Risk factors
Established risk factors include vinyl chloride exposure, radiation exposure, and chronic lymphedema from any cause, including Stewart-Treves syndrome.[250]
Pathology and biology
Angiosarcomas are largely aneuploid tumors. The rare cases of angiosarcoma that arise from benign lesions such as hemangiomas have a distinct pathway that needs to be investigated. MYC amplification is seen in radiation-induced angiosarcoma. KDR-VEGFR2 mutations and FLT4-VEGFR3 amplifications have been seen with a frequency of less than 50%.[250]
Histopathologic diagnosis can be very difficult because there can be areas of varied atypia. The common feature is an irregular network of channels in a dissective pattern along dermal collagen bundles. There is varied cellular shape, size, mitosis, endothelial multilayering, and papillary formation. Epithelioid cells can also be present. Necrosis and hemorrhage are common. Tumors stain for factor VIII, CD31, and CD34. Some liver lesions can mimic infantile hemangiomas and have focal GLUT1 positivity. Nomenclature of these liver lesions has been difficult and confusing with use of terminology from 1971 (e.g., type I hemangioendothelioma: infantile hemangioma; type II hemangioendothelioma: low-grade angiosarcoma; type III hemangioendothelioma: high-grade angiosarcoma).[247]
Treatment of angiosarcoma of the soft tissue
Treatment options for angiosarcoma of the soft tissue include the following:
- Surgery (localized disease).
- Radiation therapy (localized cutaneous disease in adults).
- Surgery, chemotherapy, and radiation therapy (metastatic disease).
Localized disease is cured by aggressive surgery. Complete surgical excision appears to be crucial for angiosarcomas and lymphangiosarcomas despite evidence of tumor shrinkage in some patients who were treated with local or systemic therapy.[248,251-253] A review of 222 patients (median age, 62 years; range, age 15–90 years) showed an overall disease-specific survival (DSS) rate of 38% at 5 years. Five-year DSS was 44% in 138 patients with localized, resected tumors but only 16% in 43 patients with metastases at diagnosis.[253] Data on liver transplantation for localized angiosarcoma are limited.[254][Level of evidence: 3iiA]
Localized disease, especially cutaneous angiosarcoma, can be treated with radiation therapy. Most of these reported cases are in adults.[255]
Multimodal treatment with surgery, systemic chemotherapy, and radiation therapy is used for metastatic disease, although it is rarely curative.[256] Disease control is the objective in metastatic angiosarcoma, with published progression-free survival rates between 3 months and 7 months [257] and a median overall survival (OS) rate of 14 months to 18 months.[258] In both adults and children, 5-year OS rates between 20% and 35% are reported.[248,249,259]
In a child diagnosed with angiosarcoma secondary to malignant transformation from infantile hemangioma, response to treatment with bevacizumab, a monoclonal antibody against vascular endothelial growth factor, combined with systemic chemotherapy, has been reported.[246,256] A report of eight cases of liver angiosarcoma in children highlighted the misuse of the term hemangioendothelioma and the importance of early diagnosis and treatment of these tumors.[260]
Biologic agents that inhibit angiogenesis have shown activity in adults with angiosarcoma.[247,259]
Treatment options under clinical evaluation for angiosarcoma of the soft tissue
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery [PAZNTIS]): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with angiosarcoma of the soft tissue are eligible for this trial.
- NCT01532687 (Gemcitabine Hydrochloride With or Without Pazopanib Hydrochloride in Treating Patients With Refractory Soft Tissue Sarcoma): This randomized phase II trial studies how well gemcitabine hydrochloride works with or without pazopanib hydrochloride in treating patients with refractory soft tissue sarcoma.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Epithelioid hemangioendothelioma
Incidence and outcome
This tumor was first described in soft tissue by Weiss and Enzinger in 1982. Epithelioid hemangioendotheliomas can occur at younger ages, but the peak incidence is in the fourth and fifth decades of life. The tumors can have an indolent or very aggressive course, with overall survival of 73% at 5 years. There are case reports of patients with untreated multiple lesions who have a very benign course compared with other patients who have a very aggressive course. Some pathologists have tried to stratify patients to evaluate risks and adjust treatment, but more research is needed.[261-267]
The presence of effusions, tumor size larger than 3 cm, and a high mitotic index (>3 mitoses/50 high-power fields) have been associated with unfavorable outcomes.[263]
Pathology and biology
A WWTR1-CAMTA1 gene fusion has been found in a large percentage of patients; less commonly, a YAP1-TFE3 gene fusion has been reported.[261] These fusions are not directly targetable with current medicines. Monoclonality has been described in multiple liver lesions, suggesting a metastatic process.
Histologically, these lesions are characterized as epithelioid lesions arranged in nests, strands, and trabecular patterns, with infrequent vascular spaces. Features that may be associated with aggressive clinical behavior include cellular atypia, one or more mitoses per 10 high-power fields, an increased proportion of spindled cells, focal necrosis, and metaplastic bone formation.[263]
The number of pediatric patients reported in the literature is limited.
Clinical presentation and diagnostic evaluation
Common sites of involvement are liver alone (21%), liver plus lung (18%), lung alone (12%), and bone alone (14%).[263,268,269] Clinical presentation depends on site of involvement, as follows:
- Liver: Hepatic nodules have central vascularity on ultrasound, contrast-enhancing lesions by computed tomography, and low T1 signal and moderate T2 signal on magnetic resonance imaging.
- Lung: Pulmonary epithelioid hemangioendothelioma may be an asymptomatic finding on chest x-ray or be associated with pleuritic pain, hemoptysis, anemia, and fibrosis.
- Bone: Bone metastasis may be associated with pathologic fracture. On x-rays, they are well-defined osteolytic lesions and can be multiple or solitary.
- Soft tissue: Thirty percent of soft tissue cases are associated with metastases, and when present, can have a very aggressive course, with limited response to chemotherapy.
- Skin: Cutaneous lesions can be raised and nodular or can be warm red-brown plaques.
Treatment of epithelioid hemangioendothelioma
Treatment options for epithelioid hemangioendothelioma include the following:
- Observation.
- Surgery.
- Immunotherapy.
- Targeted therapy.
- Chemotherapy.
For indolent cases, observation is warranted. For more aggressive cases, multiple medications have been used, including interferon, thalidomide, sorafenib, pazopanib, and sirolimus.[270] The most aggressive cases are treated with angiosarcoma-type chemotherapy. Surgery is used when possible. Liver transplantation has been used with aggressive liver lesions, both with and without metastases.[263,271-274]
Treatment options under clinical evaluation for epithelioid hemangioendothelioma
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- NCT03148275 (Trametinib in Treating Patients with Epithelioid Hemangioendothelioma That Is Metastatic, Locally Advanced, or Cannot Be Removed by Surgery): This is a phase II trial assessing the efficacy of trametinib, with patient-reported outcomes as secondary aims.
- NCT01532687 (Gemcitabine Hydrochloride With or Without Pazopanib Hydrochloride in Treating Patients With Refractory Soft Tissue Sarcoma): This randomized phase II trial studies how well gemcitabine hydrochloride works with or without pazopanib hydrochloride in treating patients with refractory soft tissue sarcoma.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Ferrari A, Casanova M, Collini P, et al.: Adult-type soft tissue sarcomas in pediatric-age patients: experience at the Istituto Nazionale Tumori in Milan. J Clin Oncol 23 (18): 4021-30, 2005. [PUBMED Abstract]
- Stanelle EJ, Christison-Lagay ER, Sidebotham EL, et al.: Prognostic factors and survival in pediatric and adolescent liposarcoma. Sarcoma 2012: 870910, 2012. [PUBMED Abstract]
- Alaggio R, Coffin CM, Weiss SW, et al.: Liposarcomas in young patients: a study of 82 cases occurring in patients younger than 22 years of age. Am J Surg Pathol 33 (5): 645-58, 2009. [PUBMED Abstract]
- Sreekantaiah C, Karakousis CP, Leong SP, et al.: Cytogenetic findings in liposarcoma correlate with histopathologic subtypes. Cancer 69 (10): 2484-95, 1992. [PUBMED Abstract]
- Sugiura H, Takahashi M, Katagiri H, et al.: Additional wide resection of malignant soft tissue tumors. Clin Orthop (394): 201-10, 2002. [PUBMED Abstract]
- Cecchetto G, Guglielmi M, Inserra A, et al.: Primary re-excision: the Italian experience in patients with localized soft-tissue sarcomas. Pediatr Surg Int 17 (7): 532-4, 2001. [PUBMED Abstract]
- Chui CH, Spunt SL, Liu T, et al.: Is reexcision in pediatric nonrhabdomyosarcoma soft tissue sarcoma necessary after an initial unplanned resection? J Pediatr Surg 37 (10): 1424-9, 2002. [PUBMED Abstract]
- Bahig H, Roberge D, Bosch W, et al.: Agreement among RTOG sarcoma radiation oncologists in contouring suspicious peritumoral edema for preoperative radiation therapy of soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys 86 (2): 298-303, 2013. [PUBMED Abstract]
- Baldini EH, Wang D, Haas RL, et al.: Treatment Guidelines for Preoperative Radiation Therapy for Retroperitoneal Sarcoma: Preliminary Consensus of an International Expert Panel. Int J Radiat Oncol Biol Phys 92 (3): 602-12, 2015. [PUBMED Abstract]
- Ferrari A, Casanova M, Spreafico F, et al.: Childhood liposarcoma: a single-institutional twenty-year experience. Pediatr Hematol Oncol 16 (5): 415-21, 1999 Sep-Oct. [PUBMED Abstract]
- Cecchetto G, Alaggio R, Dall'Igna P, et al.: Localized unresectable non-rhabdo soft tissue sarcomas of the extremities in pediatric age: results from the Italian studies. Cancer 104 (9): 2006-12, 2005. [PUBMED Abstract]
- Huh WW, Yuen C, Munsell M, et al.: Liposarcoma in children and young adults: a multi-institutional experience. Pediatr Blood Cancer 57 (7): 1142-6, 2011. [PUBMED Abstract]
- Gronchi A, Bui BN, Bonvalot S, et al.: Phase II clinical trial of neoadjuvant trabectedin in patients with advanced localized myxoid liposarcoma. Ann Oncol 23 (3): 771-6, 2012. [PUBMED Abstract]
- Demetri GD, von Mehren M, Jones RL, et al.: Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J Clin Oncol 34 (8): 786-93, 2016. [PUBMED Abstract]
- Baruchel S, Pappo A, Krailo M, et al.: A phase 2 trial of trabectedin in children with recurrent rhabdomyosarcoma, Ewing sarcoma and non-rhabdomyosarcoma soft tissue sarcomas: a report from the Children's Oncology Group. Eur J Cancer 48 (4): 579-85, 2012. [PUBMED Abstract]
- Wang L, Motoi T, Khanin R, et al.: Identification of a novel, recurrent HEY1-NCOA2 fusion in mesenchymal chondrosarcoma based on a genome-wide screen of exon-level expression data. Genes Chromosomes Cancer 51 (2): 127-39, 2012. [PUBMED Abstract]
- Nyquist KB, Panagopoulos I, Thorsen J, et al.: Whole-transcriptome sequencing identifies novel IRF2BP2-CDX1 fusion gene brought about by translocation t(1;5)(q42;q32) in mesenchymal chondrosarcoma. PLoS One 7 (11): e49705, 2012. [PUBMED Abstract]
- Frezza AM, Cesari M, Baumhoer D, et al.: Mesenchymal chondrosarcoma: prognostic factors and outcome in 113 patients. A European Musculoskeletal Oncology Society study. Eur J Cancer 51 (3): 374-81, 2015. [PUBMED Abstract]
- Dantonello TM, Int-Veen C, Leuschner I, et al.: Mesenchymal chondrosarcoma of soft tissues and bone in children, adolescents, and young adults: experiences of the CWS and COSS study groups. Cancer 112 (11): 2424-31, 2008. [PUBMED Abstract]
- Bishop MW, Somerville JM, Bahrami A, et al.: Mesenchymal Chondrosarcoma in Children and Young Adults: A Single Institution Retrospective Review. Sarcoma 2015: 608279, 2015. [PUBMED Abstract]
- Wodowski K, Hill DA, Pappo AS, et al.: A chemosensitive pediatric extraosseous osteosarcoma: case report and review of the literature. J Pediatr Hematol Oncol 25 (1): 73-7, 2003. [PUBMED Abstract]
- Sordillo PP, Hajdu SI, Magill GB, et al.: Extraosseous osteogenic sarcoma. A review of 48 patients. Cancer 51 (4): 727-34, 1983. [PUBMED Abstract]
- Nieuwenhuis MH, Casparie M, Mathus-Vliegen LM, et al.: A nation-wide study comparing sporadic and familial adenomatous polyposis-related desmoid-type fibromatoses. Int J Cancer 129 (1): 256-61, 2011. [PUBMED Abstract]
- Rossato M, Rigotti M, Grazia M, et al.: Congenital hypertrophy of the retinal pigment epithelium (CHRPE) and familial adenomatous polyposis (FAP). Acta Ophthalmol Scand 74 (4): 338-42, 1996. [PUBMED Abstract]
- Baker RH, Heinemann MH, Miller HH, et al.: Hyperpigmented lesions of the retinal pigment epithelium in familial adenomatous polyposis. Am J Med Genet 31 (2): 427-35, 1988. [PUBMED Abstract]
- Kattentidt Mouravieva AA, Geurts-Giele IR, de Krijger RR, et al.: Identification of Familial Adenomatous Polyposis carriers among children with desmoid tumours. Eur J Cancer 48 (12): 1867-74, 2012. [PUBMED Abstract]
- Wang WL, Nero C, Pappo A, et al.: CTNNB1 genotyping and APC screening in pediatric desmoid tumors: a proposed algorithm. Pediatr Dev Pathol 15 (5): 361-7, 2012 Sep-Oct. [PUBMED Abstract]
- Lewis JJ, Boland PJ, Leung DH, et al.: The enigma of desmoid tumors. Ann Surg 229 (6): 866-72; discussion 872-3, 1999. [PUBMED Abstract]
- Lazar AJ, Tuvin D, Hajibashi S, et al.: Specific mutations in the beta-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol 173 (5): 1518-27, 2008. [PUBMED Abstract]
- Faulkner LB, Hajdu SI, Kher U, et al.: Pediatric desmoid tumor: retrospective analysis of 63 cases. J Clin Oncol 13 (11): 2813-8, 1995. [PUBMED Abstract]
- Merchant NB, Lewis JJ, Woodruff JM, et al.: Extremity and trunk desmoid tumors: a multifactorial analysis of outcome. Cancer 86 (10): 2045-52, 1999. [PUBMED Abstract]
- Honeyman JN, Theilen TM, Knowles MA, et al.: Desmoid fibromatosis in children and adolescents: a conservative approach to management. J Pediatr Surg 48 (1): 62-6, 2013. [PUBMED Abstract]
- Bonvalot S, Ternès N, Fiore M, et al.: Spontaneous regression of primary abdominal wall desmoid tumors: more common than previously thought. Ann Surg Oncol 20 (13): 4096-102, 2013. [PUBMED Abstract]
- Bonvalot S, Eldweny H, Haddad V, et al.: Extra-abdominal primary fibromatosis: Aggressive management could be avoided in a subgroup of patients. Eur J Surg Oncol 34 (4): 462-8, 2008. [PUBMED Abstract]
- Merchant TE, Nguyen D, Walter AW, et al.: Long-term results with radiation therapy for pediatric desmoid tumors. Int J Radiat Oncol Biol Phys 47 (5): 1267-71, 2000. [PUBMED Abstract]
- Zelefsky MJ, Harrison LB, Shiu MH, et al.: Combined surgical resection and iridium 192 implantation for locally advanced and recurrent desmoid tumors. Cancer 67 (2): 380-4, 1991. [PUBMED Abstract]
- Weiss AJ, Lackman RD: Low-dose chemotherapy of desmoid tumors. Cancer 64 (6): 1192-4, 1989. [PUBMED Abstract]
- Klein WA, Miller HH, Anderson M, et al.: The use of indomethacin, sulindac, and tamoxifen for the treatment of desmoid tumors associated with familial polyposis. Cancer 60 (12): 2863-8, 1987. [PUBMED Abstract]
- Soto-Miranda MA, Sandoval JA, Rao B, et al.: Surgical treatment of pediatric desmoid tumors. A 12-year, single-center experience. Ann Surg Oncol 20 (11): 3384-90, 2013. [PUBMED Abstract]
- Skapek SX, Ferguson WS, Granowetter L, et al.: Vinblastine and methotrexate for desmoid fibromatosis in children: results of a Pediatric Oncology Group Phase II Trial. J Clin Oncol 25 (5): 501-6, 2007. [PUBMED Abstract]
- Gandhi MM, Nathan PC, Weitzman S, et al.: Successful treatment of life-threatening generalized infantile myofibromatosis using low-dose chemotherapy. J Pediatr Hematol Oncol 25 (9): 750-4, 2003. [PUBMED Abstract]
- Gega M, Yanagi H, Yoshikawa R, et al.: Successful chemotherapeutic modality of doxorubicin plus dacarbazine for the treatment of desmoid tumors in association with familial adenomatous polyposis. J Clin Oncol 24 (1): 102-5, 2006. [PUBMED Abstract]
- Constantinidou A, Jones RL, Scurr M, et al.: Pegylated liposomal doxorubicin, an effective, well-tolerated treatment for refractory aggressive fibromatosis. Eur J Cancer 45 (17): 2930-4, 2009. [PUBMED Abstract]
- Ananth P, Werger A, Voss S, et al.: Liposomal doxorubicin: Effective treatment for pediatric desmoid fibromatosis. Pediatr Blood Cancer 64 (7): , 2017. [PUBMED Abstract]
- Gounder MM, Lefkowitz RA, Keohan ML, et al.: Activity of Sorafenib against desmoid tumor/deep fibromatosis. Clin Cancer Res 17 (12): 4082-90, 2011. [PUBMED Abstract]
- Heinrich MC, McArthur GA, Demetri GD, et al.: Clinical and molecular studies of the effect of imatinib on advanced aggressive fibromatosis (desmoid tumor). J Clin Oncol 24 (7): 1195-203, 2006. [PUBMED Abstract]
- Chugh R, Wathen JK, Patel SR, et al.: Efficacy of imatinib in aggressive fibromatosis: Results of a phase II multicenter Sarcoma Alliance for Research through Collaboration (SARC) trial. Clin Cancer Res 16 (19): 4884-91, 2010. [PUBMED Abstract]
- Agresta L, Kim H, Turpin BK, et al.: Pazopanib therapy for desmoid tumors in adolescent and young adult patients. Pediatr Blood Cancer : , 2018. [PUBMED Abstract]
- Shang H, Braggio D, Lee YJ, et al.: Targeting the Notch pathway: A potential therapeutic approach for desmoid tumors. Cancer 121 (22): 4088-96, 2015. [PUBMED Abstract]
- Messersmith WA, Shapiro GI, Cleary JM, et al.: A Phase I, dose-finding study in patients with advanced solid malignancies of the oral γ-secretase inhibitor PF-03084014. Clin Cancer Res 21 (1): 60-7, 2015. [PUBMED Abstract]
- Bisogno G, Tagarelli A, Stramare R, et al.: Hydroxyurea treatment can avoid the need for aggressive surgery in pediatric fibromatosis. J Pediatr Hematol Oncol 35 (4): e171-3, 2013. [PUBMED Abstract]
- Meazza C, Casanova M, Trecate G, et al.: Objective response to hydroxyurea in a patient with heavily pre-treated aggressive fibromatosis. Pediatr Blood Cancer 55 (3): 587-8, 2010. [PUBMED Abstract]
- Balamuth NJ, Womer RB: Successful treatment of fibromatosis with hydroxyurea: Analysis of 20 pediatric cases. [Abstract] The Connective Tissue Oncology Society (CTOS) 14th Annual Meeting, 13–15 November 2008, London, United Kingdom A-34852, 2008. Also available online. Last accessed April 02, 2018.
- Meazza C, Bisogno G, Gronchi A, et al.: Aggressive fibromatosis in children and adolescents: the Italian experience. Cancer 116 (1): 233-40, 2010. [PUBMED Abstract]
- Hansmann A, Adolph C, Vogel T, et al.: High-dose tamoxifen and sulindac as first-line treatment for desmoid tumors. Cancer 100 (3): 612-20, 2004. [PUBMED Abstract]
- Skapek SX, Anderson JR, Hill DA, et al.: Safety and efficacy of high-dose tamoxifen and sulindac for desmoid tumor in children: results of a Children's Oncology Group (COG) phase II study. Pediatr Blood Cancer 60 (7): 1108-12, 2013. [PUBMED Abstract]
- Rutenberg MS, Indelicato DJ, Knapik JA, et al.: External-beam radiotherapy for pediatric and young adult desmoid tumors. Pediatr Blood Cancer 57 (3): 435-42, 2011. [PUBMED Abstract]
- Buckley PG, Mantripragada KK, Benetkiewicz M, et al.: A full-coverage, high-resolution human chromosome 22 genomic microarray for clinical and research applications. Hum Mol Genet 11 (25): 3221-9, 2002. [PUBMED Abstract]
- Valdivielso-Ramos M, Torrelo A, Campos M, et al.: Pediatric dermatofibrosarcoma protuberans in Madrid, Spain: multi-institutional outcomes. Pediatr Dermatol 31 (6): 676-82, 2014 Nov-Dec. [PUBMED Abstract]
- Gooskens SL, Oranje AP, van Adrichem LN, et al.: Imatinib mesylate for children with dermatofibrosarcoma protuberans (DFSP). Pediatr Blood Cancer 55 (2): 369-73, 2010. [PUBMED Abstract]
- Rubio GA, Alvarado A, Gerth DJ, et al.: Incidence and Outcomes of Dermatofibrosarcoma Protuberans in the US Pediatric Population. J Craniofac Surg 28 (1): 182-184, 2017. [PUBMED Abstract]
- Meguerditchian AN, Wang J, Lema B, et al.: Wide excision or Mohs micrographic surgery for the treatment of primary dermatofibrosarcoma protuberans. Am J Clin Oncol 33 (3): 300-3, 2010. [PUBMED Abstract]
- Dagan R, Morris CG, Zlotecki RA, et al.: Radiotherapy in the treatment of dermatofibrosarcoma protuberans. Am J Clin Oncol 28 (6): 537-9, 2005. [PUBMED Abstract]
- Sun LM, Wang CJ, Huang CC, et al.: Dermatofibrosarcoma protuberans: treatment results of 35 cases. Radiother Oncol 57 (2): 175-81, 2000. [PUBMED Abstract]
- Price VE, Fletcher JA, Zielenska M, et al.: Imatinib mesylate: an attractive alternative in young children with large, surgically challenging dermatofibrosarcoma protuberans. Pediatr Blood Cancer 44 (5): 511-5, 2005. [PUBMED Abstract]
- McArthur GA, Demetri GD, van Oosterom A, et al.: Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol 23 (4): 866-73, 2005. [PUBMED Abstract]
- Rutkowski P, Van Glabbeke M, Rankin CJ, et al.: Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials. J Clin Oncol 28 (10): 1772-9, 2010. [PUBMED Abstract]
- Miller SJ, Alam M, Andersen JS, et al.: Dermatofibrosarcoma protuberans. J Natl Compr Canc Netw 10 (3): 312-8, 2012. [PUBMED Abstract]
- Kao YC, Fletcher CDM, Alaggio R, et al.: Recurrent BRAF Gene Fusions in a Subset of Pediatric Spindle Cell Sarcomas: Expanding the Genetic Spectrum of Tumors With Overlapping Features With Infantile Fibrosarcoma. Am J Surg Pathol 42 (1): 28-38, 2018. [PUBMED Abstract]
- Sulkowski JP, Raval MV, Browne M: Margin status and multimodal therapy in infantile fibrosarcoma. Pediatr Surg Int 29 (8): 771-6, 2013. [PUBMED Abstract]
- Orbach D, Rey A, Cecchetto G, et al.: Infantile fibrosarcoma: management based on the European experience. J Clin Oncol 28 (2): 318-23, 2010. [PUBMED Abstract]
- Orbach D, Brennan B, De Paoli A, et al.: Conservative strategy in infantile fibrosarcoma is possible: The European paediatric Soft tissue sarcoma Study Group experience. Eur J Cancer 57: 1-9, 2016. [PUBMED Abstract]
- Spunt SL, Million L, Coffin C: The nonrhabdomyosarcoma soft tissue sarcoma. In: Pizzo PA, Poplack DG, eds.: Principles and Practice of Pediatric Oncology. 7th ed. Philadelphia, Pa: Lippincott Williams and Wilkins, 2015, pp 827-54.
- Loh ML, Ahn P, Perez-Atayde AR, et al.: Treatment of infantile fibrosarcoma with chemotherapy and surgery: results from the Dana-Farber Cancer Institute and Children's Hospital, Boston. J Pediatr Hematol Oncol 24 (9): 722-6, 2002. [PUBMED Abstract]
- Akyüz C, Küpeli S, Varan A, et al.: Infantile fibrosarcoma: retrospective analysis of eleven patients. Tumori 97 (2): 166-9, 2011 Mar-Apr. [PUBMED Abstract]
- Gallego S, Pericas N, Barber I, et al.: Infantile fibrosarcoma of the retroperitoneum: a site of unfavorable prognosis? Pediatr Hematol Oncol 28 (5): 451-3, 2011. [PUBMED Abstract]
- Parida L, Fernandez-Pineda I, Uffman JK, et al.: Clinical management of infantile fibrosarcoma: a retrospective single-institution review. Pediatr Surg Int 29 (7): 703-8, 2013. [PUBMED Abstract]
- Mody RJ, Wu YM, Lonigro RJ, et al.: Integrative Clinical Sequencing in the Management of Refractory or Relapsed Cancer in Youth. JAMA 314 (9): 913-25, 2015. [PUBMED Abstract]
- Wong V, Pavlick D, Brennan T, et al.: Evaluation of a Congenital Infantile Fibrosarcoma by Comprehensive Genomic Profiling Reveals an LMNA-NTRK1 Gene Fusion Responsive to Crizotinib. J Natl Cancer Inst 108 (1): , 2016. [PUBMED Abstract]
- Nagasubramanian R, Wei J, Gordon P, et al.: Infantile Fibrosarcoma With NTRK3-ETV6 Fusion Successfully Treated With the Tropomyosin-Related Kinase Inhibitor LOXO-101. Pediatr Blood Cancer 63 (8): 1468-70, 2016. [PUBMED Abstract]
- Yanagisawa R, Noguchi M, Fujita K, et al.: Preoperative Treatment With Pazopanib in a Case of Chemotherapy-Resistant Infantile Fibrosarcoma. Pediatr Blood Cancer 63 (2): 348-51, 2016. [PUBMED Abstract]
- Madden NP, Spicer RD, Allibone EB, et al.: Spontaneous regression of neonatal fibrosarcoma. Br J Cancer Suppl 18: S72-5, 1992. [PUBMED Abstract]
- Kovach SJ, Fischer AC, Katzman PJ, et al.: Inflammatory myofibroblastic tumors. J Surg Oncol 94 (5): 385-91, 2006. [PUBMED Abstract]
- Brodlie M, Barwick SC, Wood KM, et al.: Inflammatory myofibroblastic tumours of the respiratory tract: paediatric case series with varying clinical presentations. J Laryngol Otol 125 (8): 865-8, 2011. [PUBMED Abstract]
- Xiao Y, Zhou S, Ma C, et al.: Radiological and histopathological features of hepatic inflammatory myofibroblastic tumour: analysis of 10 cases. Clin Radiol 68 (11): 1114-20, 2013. [PUBMED Abstract]
- Karnak I, Senocak ME, Ciftci AO, et al.: Inflammatory myofibroblastic tumor in children: diagnosis and treatment. J Pediatr Surg 36 (6): 908-12, 2001. [PUBMED Abstract]
- Collin M, Charles A, Barker A, et al.: Inflammatory myofibroblastic tumour of the bladder in children: a review. J Pediatr Urol 11 (5): 239-45, 2015. [PUBMED Abstract]
- Coffin CM, Hornick JL, Fletcher CD: Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 31 (4): 509-20, 2007. [PUBMED Abstract]
- Lovly CM, Gupta A, Lipson D, et al.: Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov 4 (8): 889-95, 2014. [PUBMED Abstract]
- Devaney KO, Lafeir DJ, Triantafyllou A, et al.: Inflammatory myofibroblastic tumors of the head and neck: evaluation of clinicopathologic and prognostic features. Eur Arch Otorhinolaryngol 269 (12): 2461-5, 2012. [PUBMED Abstract]
- Mehta B, Mascarenhas L, Zhou S, et al.: Inflammatory myofibroblastic tumors in childhood. Pediatr Hematol Oncol 30 (7): 640-5, 2013. [PUBMED Abstract]
- Favini F, Resti AG, Collini P, et al.: Inflammatory myofibroblastic tumor of the conjunctiva: response to chemotherapy with low-dose methotrexate and vinorelbine. Pediatr Blood Cancer 54 (3): 483-5, 2010. [PUBMED Abstract]
- Doski JJ, Priebe CJ Jr, Driessnack M, et al.: Corticosteroids in the management of unresected plasma cell granuloma (inflammatory pseudotumor) of the lung. J Pediatr Surg 26 (9): 1064-6, 1991. [PUBMED Abstract]
- Diop B, Konate I, Ka S, et al.: Mesenteric myofibroblastic tumor: NSAID therapy after incomplete resection. J Visc Surg 148 (4): e311-4, 2011. [PUBMED Abstract]
- Dalton BG, Thomas PG, Sharp NE, et al.: Inflammatory myofibroblastic tumors in children. J Pediatr Surg 51 (4): 541-4, 2016. [PUBMED Abstract]
- Butrynski JE, D'Adamo DR, Hornick JL, et al.: Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med 363 (18): 1727-33, 2010. [PUBMED Abstract]
- Mossé YP, Lim MS, Voss SD, et al.: Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children's Oncology Group phase 1 consortium study. Lancet Oncol 14 (6): 472-80, 2013. [PUBMED Abstract]
- Gaudichon J, Jeanne-Pasquier C, Deparis M, et al.: Complete and Repeated Response of a Metastatic ALK-rearranged Inflammatory Myofibroblastic Tumor to Crizotinib in a Teenage Girl. J Pediatr Hematol Oncol 38 (4): 308-11, 2016. [PUBMED Abstract]
- Nishio M, Murakami H, Horiike A, et al.: Phase I Study of Ceritinib (LDK378) in Japanese Patients with Advanced, Anaplastic Lymphoma Kinase-Rearranged Non-Small-Cell Lung Cancer or Other Tumors. J Thorac Oncol 10 (7): 1058-66, 2015. [PUBMED Abstract]
- Mossé YP, Voss SD, Lim MS, et al.: Targeting ALK With Crizotinib in Pediatric Anaplastic Large Cell Lymphoma and Inflammatory Myofibroblastic Tumor: A Children's Oncology Group Study. J Clin Oncol 35 (28): 3215-3221, 2017. [PUBMED Abstract]
- Evans HL: Low-grade fibromyxoid sarcoma: a clinicopathologic study of 33 cases with long-term follow-up. Am J Surg Pathol 35 (10): 1450-62, 2011. [PUBMED Abstract]
- Guillou L, Benhattar J, Gengler C, et al.: Translocation-positive low-grade fibromyxoid sarcoma: clinicopathologic and molecular analysis of a series expanding the morphologic spectrum and suggesting potential relationship to sclerosing epithelioid fibrosarcoma: a study from the French Sarcoma Group. Am J Surg Pathol 31 (9): 1387-402, 2007. [PUBMED Abstract]
- O'Sullivan MJ, Sirgi KE, Dehner LP: Low-grade fibrosarcoma (hyalinizing spindle cell tumor with giant rosettes) with pulmonary metastases at presentation: case report and review of the literature. Int J Surg Pathol 10 (3): 211-6, 2002. [PUBMED Abstract]
- Folpe AL, Lane KL, Paull G, et al.: Low-grade fibromyxoid sarcoma and hyalinizing spindle cell tumor with giant rosettes: a clinicopathologic study of 73 cases supporting their identity and assessing the impact of high-grade areas. Am J Surg Pathol 24 (10): 1353-60, 2000. [PUBMED Abstract]
- Sargar K, Kao SC, Spunt SL, et al.: MRI and CT of Low-Grade Fibromyxoid Sarcoma in Children: A Report From Children's Oncology Group Study ARST0332. AJR Am J Roentgenol 205 (2): 414-20, 2015. [PUBMED Abstract]
- Maretty-Nielsen K, Baerentzen S, Keller J, et al.: Low-Grade Fibromyxoid Sarcoma: Incidence, Treatment Strategy of Metastases, and Clinical Significance of the FUS Gene. Sarcoma 2013: 256280, 2013. [PUBMED Abstract]
- Prieto-Granada C, Zhang L, Chen HW, et al.: A genetic dichotomy between pure sclerosing epithelioid fibrosarcoma (SEF) and hybrid SEF/low-grade fibromyxoid sarcoma: a pathologic and molecular study of 18 cases. Genes Chromosomes Cancer 54 (1): 28-38, 2015. [PUBMED Abstract]
- Pollock BH, Jenson HB, Leach CT, et al.: Risk factors for pediatric human immunodeficiency virus-related malignancy. JAMA 289 (18): 2393-9, 2003. [PUBMED Abstract]
- Kleinerman RA, Tucker MA, Abramson DH, et al.: Risk of soft tissue sarcomas by individual subtype in survivors of hereditary retinoblastoma. J Natl Cancer Inst 99 (1): 24-31, 2007. [PUBMED Abstract]
- Samuels BL, Chawla S, Patel S, et al.: Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: results of a worldwide expanded access program study. Ann Oncol 24 (6): 1703-9, 2013. [PUBMED Abstract]
- Enzinger FM, Zhang RY: Plexiform fibrohistiocytic tumor presenting in children and young adults. An analysis of 65 cases. Am J Surg Pathol 12 (11): 818-26, 1988. [PUBMED Abstract]
- Black J, Coffin CM, Dehner LP: Fibrohistiocytic tumors and related neoplasms in children and adolescents. Pediatr Dev Pathol 15 (1 Suppl): 181-210, 2012. [PUBMED Abstract]
- Moosavi C, Jha P, Fanburg-Smith JC: An update on plexiform fibrohistiocytic tumor and addition of 66 new cases from the Armed Forces Institute of Pathology, in honor of Franz M. Enzinger, MD. Ann Diagn Pathol 11 (5): 313-9, 2007. [PUBMED Abstract]
- Billings SD, Folpe AL: Cutaneous and subcutaneous fibrohistiocytic tumors of intermediate malignancy: an update. Am J Dermatopathol 26 (2): 141-55, 2004. [PUBMED Abstract]
- Remstein ED, Arndt CA, Nascimento AG: Plexiform fibrohistiocytic tumor: clinicopathologic analysis of 22 cases. Am J Surg Pathol 23 (6): 662-70, 1999. [PUBMED Abstract]
- Salomao DR, Nascimento AG: Plexiform fibrohistiocytic tumor with systemic metastases: a case report. Am J Surg Pathol 21 (4): 469-76, 1997. [PUBMED Abstract]
- Redlich GC, Montgomery KD, Allgood GA, et al.: Plexiform fibrohistiocytic tumor with a clonal cytogenetic anomaly. Cancer Genet Cytogenet 108 (2): 141-3, 1999. [PUBMED Abstract]
- Luzar B, Calonje E: Cutaneous fibrohistiocytic tumours - an update. Histopathology 56 (1): 148-65, 2010. [PUBMED Abstract]
- Dantonello TM, Leuschner I, Vokuhl C, et al.: Malignant ectomesenchymoma in children and adolescents: report from the Cooperative Weichteilsarkom Studiengruppe (CWS). Pediatr Blood Cancer 60 (2): 224-9, 2013. [PUBMED Abstract]
- Carli M, Ferrari A, Mattke A, et al.: Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J Clin Oncol 23 (33): 8422-30, 2005. [PUBMED Abstract]
- Zhang M, Wang Y, Jones S, et al.: Somatic mutations of SUZ12 in malignant peripheral nerve sheath tumors. Nat Genet 46 (11): 1170-2, 2014. [PUBMED Abstract]
- Hagel C, Zils U, Peiper M, et al.: Histopathology and clinical outcome of NF1-associated vs. sporadic malignant peripheral nerve sheath tumors. J Neurooncol 82 (2): 187-92, 2007. [PUBMED Abstract]
- Zou C, Smith KD, Liu J, et al.: Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg 249 (6): 1014-22, 2009. [PUBMED Abstract]
- Okada K, Hasegawa T, Tajino T, et al.: Clinical relevance of pathological grades of malignant peripheral nerve sheath tumor: a multi-institution TMTS study of 56 cases in Northern Japan. Ann Surg Oncol 14 (2): 597-604, 2007. [PUBMED Abstract]
- Amirian ES, Goodman JC, New P, et al.: Pediatric and adult malignant peripheral nerve sheath tumors: an analysis of data from the surveillance, epidemiology, and end results program. J Neurooncol 116 (3): 609-16, 2014. [PUBMED Abstract]
- Valentin T, Le Cesne A, Ray-Coquard I, et al.: Management and prognosis of malignant peripheral nerve sheath tumors: The experience of the French Sarcoma Group (GSF-GETO). Eur J Cancer 56: 77-84, 2016. [PUBMED Abstract]
- Bergamaschi L, Bisogno G, Manzitti C, et al.: Salvage rates and prognostic factors after relapse in children and adolescents with malignant peripheral nerve sheath tumors. Pediatr Blood Cancer 65 (2): , 2018. [PUBMED Abstract]
- Ferrari A, Bisogno G, Macaluso A, et al.: Soft-tissue sarcomas in children and adolescents with neurofibromatosis type 1. Cancer 109 (7): 1406-12, 2007. [PUBMED Abstract]
- Okur FV, Oguz A, Karadeniz C, et al.: Malignant triton tumor of the pelvis in a 2-year-old boy. J Pediatr Hematol Oncol 28 (3): 173-6, 2006. [PUBMED Abstract]
- Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 4th ed. St. Louis, Mo: Mosby, 2001.
- Fernandez-Pineda I, Parida L, Jenkins JJ, et al.: Childhood hemangiopericytoma: review of St Jude Children's Research Hospital. J Pediatr Hematol Oncol 33 (5): 356-9, 2011. [PUBMED Abstract]
- Rodriguez-Galindo C, Ramsey K, Jenkins JJ, et al.: Hemangiopericytoma in children and infants. Cancer 88 (1): 198-204, 2000. [PUBMED Abstract]
- Ferrari A, Casanova M, Bisogno G, et al.: Hemangiopericytoma in pediatric ages: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Cancer 92 (10): 2692-8, 2001. [PUBMED Abstract]
- Bien E, Stachowicz-Stencel T, Godzinski J, et al.: Retrospective multi-institutional study on hemangiopericytoma in Polish children. Pediatr Int 51 (1): 19-24, 2009. [PUBMED Abstract]
- Wiswell TE, Davis J, Cunningham BE, et al.: Infantile myofibromatosis: the most common fibrous tumor of infancy. J Pediatr Surg 23 (4): 315-8, 1988. [PUBMED Abstract]
- Chung EB, Enzinger FM: Infantile myofibromatosis. Cancer 48 (8): 1807-18, 1981. [PUBMED Abstract]
- Modi N: Congenital generalised fibromatosis. Arch Dis Child 57 (11): 881-2, 1982. [PUBMED Abstract]
- Levine E, Fréneaux P, Schleiermacher G, et al.: Risk-adapted therapy for infantile myofibromatosis in children. Pediatr Blood Cancer 59 (1): 115-20, 2012. [PUBMED Abstract]
- Larralde M, Hoffner MV, Boggio P, et al.: Infantile myofibromatosis: report of nine patients. Pediatr Dermatol 27 (1): 29-33, 2010 Jan-Feb. [PUBMED Abstract]
- Cheung YH, Gayden T, Campeau PM, et al.: A recurrent PDGFRB mutation causes familial infantile myofibromatosis. Am J Hum Genet 92 (6): 996-1000, 2013. [PUBMED Abstract]
- Gopal M, Chahal G, Al-Rifai Z, et al.: Infantile myofibromatosis. Pediatr Surg Int 24 (3): 287-91, 2008. [PUBMED Abstract]
- Weaver MS, Navid F, Huppmann A, et al.: Vincristine and Dactinomycin in Infantile Myofibromatosis With a Review of Treatment Options. J Pediatr Hematol Oncol 37 (3): 237-41, 2015. [PUBMED Abstract]
- Orbach D, Brennan B, Casanova M, et al.: Paediatric and adolescent alveolar soft part sarcoma: A joint series from European cooperative groups. Pediatr Blood Cancer 60 (11): 1826-32, 2013. [PUBMED Abstract]
- Ferrari A, Sultan I, Huang TT, et al.: Soft tissue sarcoma across the age spectrum: a population-based study from the Surveillance Epidemiology and End Results database. Pediatr Blood Cancer 57 (6): 943-9, 2011. [PUBMED Abstract]
- Wang HW, Qin XJ, Yang WJ, et al.: Alveolar soft part sarcoma of the oral and maxillofacial region: clinical analysis in a series of 18 patients. Oral Surg Oral Med Oral Pathol Oral Radiol 119 (4): 396-401, 2015. [PUBMED Abstract]
- Kayton ML, Meyers P, Wexler LH, et al.: Clinical presentation, treatment, and outcome of alveolar soft part sarcoma in children, adolescents, and young adults. J Pediatr Surg 41 (1): 187-93, 2006. [PUBMED Abstract]
- Ladanyi M, Lui MY, Antonescu CR, et al.: The der(17)t(X;17)(p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene 20 (1): 48-57, 2001. [PUBMED Abstract]
- Williams A, Bartle G, Sumathi VP, et al.: Detection of ASPL/TFE3 fusion transcripts and the TFE3 antigen in formalin-fixed, paraffin-embedded tissue in a series of 18 cases of alveolar soft part sarcoma: useful diagnostic tools in cases with unusual histological features. Virchows Arch 458 (3): 291-300, 2011. [PUBMED Abstract]
- Lieberman PH, Brennan MF, Kimmel M, et al.: Alveolar soft-part sarcoma. A clinico-pathologic study of half a century. Cancer 63 (1): 1-13, 1989. [PUBMED Abstract]
- Casanova M, Ferrari A, Bisogno G, et al.: Alveolar soft part sarcoma in children and adolescents: A report from the Soft-Tissue Sarcoma Italian Cooperative Group. Ann Oncol 11 (11): 1445-9, 2000. [PUBMED Abstract]
- Pennacchioli E, Fiore M, Collini P, et al.: Alveolar soft part sarcoma: clinical presentation, treatment, and outcome in a series of 33 patients at a single institution. Ann Surg Oncol 17 (12): 3229-33, 2010. [PUBMED Abstract]
- Flores RJ, Harrison DJ, Federman NC, et al.: Alveolar soft part sarcoma in children and young adults: A report of 69 cases. Pediatr Blood Cancer : , 2018. [PUBMED Abstract]
- Roozendaal KJ, de Valk B, ten Velden JJ, et al.: Alveolar soft-part sarcoma responding to interferon alpha-2b. Br J Cancer 89 (2): 243-5, 2003. [PUBMED Abstract]
- Conde N, Cruz O, Albert A, et al.: Antiangiogenic treatment as a pre-operative management of alveolar soft-part sarcoma. Pediatr Blood Cancer 57 (6): 1071-3, 2011. [PUBMED Abstract]
- Stacchiotti S, Negri T, Zaffaroni N, et al.: Sunitinib in advanced alveolar soft part sarcoma: evidence of a direct antitumor effect. Ann Oncol 22 (7): 1682-90, 2011. [PUBMED Abstract]
- Kummar S, Allen D, Monks A, et al.: Cediranib for metastatic alveolar soft part sarcoma. J Clin Oncol 31 (18): 2296-302, 2013. [PUBMED Abstract]
- Coindre JM, Hostein I, Terrier P, et al.: Diagnosis of clear cell sarcoma by real-time reverse transcriptase-polymerase chain reaction analysis of paraffin embedded tissues: clinicopathologic and molecular analysis of 44 patients from the French sarcoma group. Cancer 107 (5): 1055-64, 2006. [PUBMED Abstract]
- Meis-Kindblom JM: Clear cell sarcoma of tendons and aponeuroses: a historical perspective and tribute to the man behind the entity. Adv Anat Pathol 13 (6): 286-92, 2006. [PUBMED Abstract]
- Dim DC, Cooley LD, Miranda RN: Clear cell sarcoma of tendons and aponeuroses: a review. Arch Pathol Lab Med 131 (1): 152-6, 2007. [PUBMED Abstract]
- Blazer DG 3rd, Lazar AJ, Xing Y, et al.: Clinical outcomes of molecularly confirmed clear cell sarcoma from a single institution and in comparison with data from the Surveillance, Epidemiology, and End Results registry. Cancer 115 (13): 2971-9, 2009. [PUBMED Abstract]
- Fujimura Y, Siddique H, Lee L, et al.: EWS-ATF-1 chimeric protein in soft tissue clear cell sarcoma associates with CREB-binding protein and interferes with p53-mediated trans-activation function. Oncogene 20 (46): 6653-9, 2001. [PUBMED Abstract]
- Ferrari A, Casanova M, Bisogno G, et al.: Clear cell sarcoma of tendons and aponeuroses in pediatric patients: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Cancer 94 (12): 3269-76, 2002. [PUBMED Abstract]
- Karita M, Tsuchiya H, Yamamoto N, et al.: Caffeine-potentiated chemotherapy for clear cell sarcoma: a report of five cases. Int J Clin Oncol 18 (1): 33-7, 2013. [PUBMED Abstract]
- Leuschner I, Radig K, Harms D: Desmoplastic small round cell tumor. Semin Diagn Pathol 13 (3): 204-12, 1996. [PUBMED Abstract]
- Kushner BH, LaQuaglia MP, Wollner N, et al.: Desmoplastic small round-cell tumor: prolonged progression-free survival with aggressive multimodality therapy. J Clin Oncol 14 (5): 1526-31, 1996. [PUBMED Abstract]
- Saab R, Khoury JD, Krasin M, et al.: Desmoplastic small round cell tumor in childhood: the St. Jude Children's Research Hospital experience. Pediatr Blood Cancer 49 (3): 274-9, 2007. [PUBMED Abstract]
- Wang LL, Perlman EJ, Vujanic GM, et al.: Desmoplastic small round cell tumor of the kidney in childhood. Am J Surg Pathol 31 (4): 576-84, 2007. [PUBMED Abstract]
- Arora VC, Price AP, Fleming S, et al.: Characteristic imaging features of desmoplastic small round cell tumour. Pediatr Radiol 43 (1): 93-102, 2013. [PUBMED Abstract]
- Gerald WL, Ladanyi M, de Alava E, et al.: Clinical, pathologic, and molecular spectrum of tumors associated with t(11;22)(p13;q12): desmoplastic small round-cell tumor and its variants. J Clin Oncol 16 (9): 3028-36, 1998. [PUBMED Abstract]
- Lal DR, Su WT, Wolden SL, et al.: Results of multimodal treatment for desmoplastic small round cell tumors. J Pediatr Surg 40 (1): 251-5, 2005. [PUBMED Abstract]
- Philippe-Chomette P, Kabbara N, Andre N, et al.: Desmoplastic small round cell tumors with EWS-WT1 fusion transcript in children and young adults. Pediatr Blood Cancer 58 (6): 891-7, 2012. [PUBMED Abstract]
- Sedig L, Geiger J, Mody R, et al.: Paratesticular desmoplastic small round cell tumors: A case report and review of the literature. Pediatr Blood Cancer 64 (12): , 2017. [PUBMED Abstract]
- Schwarz RE, Gerald WL, Kushner BH, et al.: Desmoplastic small round cell tumors: prognostic indicators and results of surgical management. Ann Surg Oncol 5 (5): 416-22, 1998 Jul-Aug. [PUBMED Abstract]
- Goodman KA, Wolden SL, La Quaglia MP, et al.: Whole abdominopelvic radiotherapy for desmoplastic small round-cell tumor. Int J Radiat Oncol Biol Phys 54 (1): 170-6, 2002. [PUBMED Abstract]
- Osborne EM, Briere TM, Hayes-Jordan A, et al.: Survival and toxicity following sequential multimodality treatment including whole abdominopelvic radiotherapy for patients with desmoplastic small round cell tumor. Radiother Oncol 119 (1): 40-4, 2016. [PUBMED Abstract]
- Atallah V, Honore C, Orbach D, et al.: Role of Adjuvant Radiation Therapy After Surgery for Abdominal Desmoplastic Small Round Cell Tumors. Int J Radiat Oncol Biol Phys 95 (4): 1244-53, 2016. [PUBMED Abstract]
- Tarek N, Hayes-Jordan A, Salvador L, et al.: Recurrent desmoplastic small round cell tumor responding to an mTOR inhibitor containing regimen. Pediatr Blood Cancer 65 (1): , 2018. [PUBMED Abstract]
- Cook RJ, Wang Z, Arora M, et al.: Clinical outcomes of patients with desmoplastic small round cell tumor of the peritoneum undergoing autologous HCT: a CIBMTR retrospective analysis. Bone Marrow Transplant 47 (11): 1455-8, 2012. [PUBMED Abstract]
- Chbani L, Guillou L, Terrier P, et al.: Epithelioid sarcoma: a clinicopathologic and immunohistochemical analysis of 106 cases from the French sarcoma group. Am J Clin Pathol 131 (2): 222-7, 2009. [PUBMED Abstract]
- Hornick JL, Dal Cin P, Fletcher CD: Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol 33 (4): 542-50, 2009. [PUBMED Abstract]
- Knutson SK, Warholic NM, Wigle TJ, et al.: Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. Proc Natl Acad Sci U S A 110 (19): 7922-7, 2013. [PUBMED Abstract]
- Guzzetta AA, Montgomery EA, Lyu H, et al.: Epithelioid sarcoma: one institution's experience with a rare sarcoma. J Surg Res 177 (1): 116-22, 2012. [PUBMED Abstract]
- Casanova M, Ferrari A, Collini P, et al.: Epithelioid sarcoma in children and adolescents: a report from the Italian Soft Tissue Sarcoma Committee. Cancer 106 (3): 708-17, 2006. [PUBMED Abstract]
- Kodet R, Newton WA Jr, Sachs N, et al.: Rhabdoid tumors of soft tissues: a clinicopathologic study of 26 cases enrolled on the Intergroup Rhabdomyosarcoma Study. Hum Pathol 22 (7): 674-84, 1991. [PUBMED Abstract]
- Biegel JA, Zhou JY, Rorke LB, et al.: Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res 59 (1): 74-9, 1999. [PUBMED Abstract]
- Eaton KW, Tooke LS, Wainwright LM, et al.: Spectrum of SMARCB1/INI1 mutations in familial and sporadic rhabdoid tumors. Pediatr Blood Cancer 56 (1): 7-15, 2011. [PUBMED Abstract]
- Lee RS, Stewart C, Carter SL, et al.: A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J Clin Invest 122 (8): 2983-8, 2012. [PUBMED Abstract]
- Sultan I, Qaddoumi I, Rodríguez-Galindo C, et al.: Age, stage, and radiotherapy, but not primary tumor site, affects the outcome of patients with malignant rhabdoid tumors. Pediatr Blood Cancer 54 (1): 35-40, 2010. [PUBMED Abstract]
- Puri DR, Meyers PA, Kraus DH, et al.: Radiotherapy in the multimodal treatment of extrarenal extracranial malignant rhabdoid tumors. Pediatr Blood Cancer 50 (1): 167-9, 2008. [PUBMED Abstract]
- Madigan CE, Armenian SH, Malogolowkin MH, et al.: Extracranial malignant rhabdoid tumors in childhood: the Childrens Hospital Los Angeles experience. Cancer 110 (9): 2061-6, 2007. [PUBMED Abstract]
- Bourdeaut F, Fréneaux P, Thuille B, et al.: Extra-renal non-cerebral rhabdoid tumours. Pediatr Blood Cancer 51 (3): 363-8, 2008. [PUBMED Abstract]
- Wetmore C, Boyett J, Li S, et al.: Alisertib is active as single agent in recurrent atypical teratoid rhabdoid tumors in 4 children. Neuro Oncol 17 (6): 882-8, 2015. [PUBMED Abstract]
- Tsuneyoshi M, Enjoji M, Iwasaki H, et al.: Extraskeletal myxoid chondrosarcoma--a clinicopathologic and electron microscopic study. Acta Pathol Jpn 31 (3): 439-47, 1981. [PUBMED Abstract]
- Hachitanda Y, Tsuneyoshi M, Daimaru Y, et al.: Extraskeletal myxoid chondrosarcoma in young children. Cancer 61 (12): 2521-6, 1988. [PUBMED Abstract]
- Hisaoka M, Ishida T, Imamura T, et al.: TFG is a novel fusion partner of NOR1 in extraskeletal myxoid chondrosarcoma. Genes Chromosomes Cancer 40 (4): 325-8, 2004. [PUBMED Abstract]
- Enzinger FM, Shiraki M: Extraskeletal myxoid chondrosarcoma. An analysis of 34 cases. Hum Pathol 3 (3): 421-35, 1972. [PUBMED Abstract]
- McGrory JE, Rock MG, Nascimento AG, et al.: Extraskeletal myxoid chondrosarcoma. Clin Orthop Relat Res (382): 185-90, 2001. [PUBMED Abstract]
- Drilon AD, Popat S, Bhuchar G, et al.: Extraskeletal myxoid chondrosarcoma: a retrospective review from 2 referral centers emphasizing long-term outcomes with surgery and chemotherapy. Cancer 113 (12): 3364-71, 2008. [PUBMED Abstract]
- Stacchiotti S, Pantaleo MA, Astolfi A, et al.: Activity of sunitinib in extraskeletal myxoid chondrosarcoma. Eur J Cancer 50 (9): 1657-64, 2014. [PUBMED Abstract]
- Martignoni G, Pea M, Reghellin D, et al.: Molecular pathology of lymphangioleiomyomatosis and other perivascular epithelioid cell tumors. Arch Pathol Lab Med 134 (1): 33-40, 2010. [PUBMED Abstract]
- Bissler JJ, McCormack FX, Young LR, et al.: Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med 358 (2): 140-51, 2008. [PUBMED Abstract]
- Davies DM, Johnson SR, Tattersfield AE, et al.: Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. N Engl J Med 358 (2): 200-3, 2008. [PUBMED Abstract]
- Agaram NP, Sung YS, Zhang L, et al.: Dichotomy of Genetic Abnormalities in PEComas With Therapeutic Implications. Am J Surg Pathol 39 (6): 813-25, 2015. [PUBMED Abstract]
- Folpe A, Inwards C, eds.: Bone and Soft Tissue Pathology: A Volume in the Foundations in Diagnostic Pathology. Philadelphia, Pa: WB Saunders Co, 2010.
- Armah HB, Parwani AV: Perivascular epithelioid cell tumor. Arch Pathol Lab Med 133 (4): 648-54, 2009. [PUBMED Abstract]
- Alaggio R, Cecchetto G, Martignoni G, et al.: Malignant perivascular epithelioid cell tumor in children: description of a case and review of the literature. J Pediatr Surg 47 (6): e31-40, 2012. [PUBMED Abstract]
- Wagner AJ, Malinowska-Kolodziej I, Morgan JA, et al.: Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol 28 (5): 835-40, 2010. [PUBMED Abstract]
- Sultan I, Rodriguez-Galindo C, Saab R, et al.: Comparing children and adults with synovial sarcoma in the Surveillance, Epidemiology, and End Results program, 1983 to 2005: an analysis of 1268 patients. Cancer 115 (15): 3537-47, 2009. [PUBMED Abstract]
- Wang JG, Li NN: Primary cardiac synovial sarcoma. Ann Thorac Surg 95 (6): 2202-9, 2013. [PUBMED Abstract]
- Pappo AS, Fontanesi J, Luo X, et al.: Synovial sarcoma in children and adolescents: the St Jude Children's Research Hospital experience. J Clin Oncol 12 (11): 2360-6, 1994. [PUBMED Abstract]
- Ferrari A, De Salvo GL, Oberlin O, et al.: Synovial sarcoma in children and adolescents: a critical reappraisal of staging investigations in relation to the rate of metastatic involvement at diagnosis. Eur J Cancer 48 (9): 1370-5, 2012. [PUBMED Abstract]
- van de Rijn M, Barr FG, Collins MH, et al.: Absence of SYT-SSX fusion products in soft tissue tumors other than synovial sarcoma. Am J Clin Pathol 112 (1): 43-9, 1999. [PUBMED Abstract]
- Krsková L, Sumerauer D, Stejskalová E, et al.: A novel variant of SYT-SSX1 fusion gene in a case of spindle cell synovial sarcoma. Diagn Mol Pathol 16 (3): 179-83, 2007. [PUBMED Abstract]
- Su L, Sampaio AV, Jones KB, et al.: Deconstruction of the SS18-SSX fusion oncoprotein complex: insights into disease etiology and therapeutics. Cancer Cell 21 (3): 333-47, 2012. [PUBMED Abstract]
- Arnold MA, Arnold CA, Li G, et al.: A unique pattern of INI1 immunohistochemistry distinguishes synovial sarcoma from its histologic mimics. Hum Pathol 44 (5): 881-7, 2013. [PUBMED Abstract]
- Vlenterie M, Ho VK, Kaal SE, et al.: Age as an independent prognostic factor for survival of localised synovial sarcoma patients. Br J Cancer 113 (11): 1602-6, 2015. [PUBMED Abstract]
- Okcu MF, Munsell M, Treuner J, et al.: Synovial sarcoma of childhood and adolescence: a multicenter, multivariate analysis of outcome. J Clin Oncol 21 (8): 1602-11, 2003. [PUBMED Abstract]
- Brecht IB, Ferrari A, Int-Veen C, et al.: Grossly-resected synovial sarcoma treated by the German and Italian Pediatric Soft Tissue Sarcoma Cooperative Groups: discussion on the role of adjuvant therapies. Pediatr Blood Cancer 46 (1): 11-7, 2006. [PUBMED Abstract]
- Stanelle EJ, Christison-Lagay ER, Healey JH, et al.: Pediatric and adolescent synovial sarcoma: multivariate analysis of prognostic factors and survival outcomes. Ann Surg Oncol 20 (1): 73-9, 2013. [PUBMED Abstract]
- Trassard M, Le Doussal V, Hacène K, et al.: Prognostic factors in localized primary synovial sarcoma: a multicenter study of 128 adult patients. J Clin Oncol 19 (2): 525-34, 2001. [PUBMED Abstract]
- Guillou L, Benhattar J, Bonichon F, et al.: Histologic grade, but not SYT-SSX fusion type, is an important prognostic factor in patients with synovial sarcoma: a multicenter, retrospective analysis. J Clin Oncol 22 (20): 4040-50, 2004. [PUBMED Abstract]
- Ferrari A, Gronchi A, Casanova M, et al.: Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer 101 (3): 627-34, 2004. [PUBMED Abstract]
- Lagarde P, Przybyl J, Brulard C, et al.: Chromosome instability accounts for reverse metastatic outcomes of pediatric and adult synovial sarcomas. J Clin Oncol 31 (5): 608-15, 2013. [PUBMED Abstract]
- Stegmaier S, Leuschner I, Poremba C, et al.: The prognostic impact of SYT-SSX fusion type and histological grade in pediatric patients with synovial sarcoma treated according to the CWS (Cooperative Weichteilsarkom Studie) trials. Pediatr Blood Cancer 64 (1): 89-95, 2017. [PUBMED Abstract]
- Scheer M, Dantonello T, Hallmen E, et al.: Primary Metastatic Synovial Sarcoma: Experience of the CWS Study Group. Pediatr Blood Cancer 63 (7): 1198-206, 2016. [PUBMED Abstract]
- Ferrari A, De Salvo GL, Dall'Igna P, et al.: Salvage rates and prognostic factors after relapse in children and adolescents with initially localised synovial sarcoma. Eur J Cancer 48 (18): 3448-55, 2012. [PUBMED Abstract]
- Ferrari A, Chi YY, De Salvo GL, et al.: Surgery alone is sufficient therapy for children and adolescents with low-risk synovial sarcoma: A joint analysis from the European paediatric soft tissue sarcoma Study Group and the Children's Oncology Group. Eur J Cancer 78: 1-6, 2017. [PUBMED Abstract]
- McGrory JE, Pritchard DJ, Arndt CA, et al.: Nonrhabdomyosarcoma soft tissue sarcomas in children. The Mayo Clinic experience. Clin Orthop (374): 247-58, 2000. [PUBMED Abstract]
- Van Glabbeke M, van Oosterom AT, Oosterhuis JW, et al.: Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens--a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 17 (1): 150-7, 1999. [PUBMED Abstract]
- Koscielniak E, Harms D, Henze G, et al.: Results of treatment for soft tissue sarcoma in childhood and adolescence: a final report of the German Cooperative Soft Tissue Sarcoma Study CWS-86. J Clin Oncol 17 (12): 3706-19, 1999. [PUBMED Abstract]
- Pappo AS, Devidas M, Jenkins J, et al.: Phase II trial of neoadjuvant vincristine, ifosfamide, and doxorubicin with granulocyte colony-stimulating factor support in children and adolescents with advanced-stage nonrhabdomyosarcomatous soft tissue sarcomas: a Pediatric Oncology Group Study. J Clin Oncol 23 (18): 4031-8, 2005. [PUBMED Abstract]
- Pappo AS, Rao BN, Jenkins JJ, et al.: Metastatic nonrhabdomyosarcomatous soft-tissue sarcomas in children and adolescents: the St. Jude Children's Research Hospital experience. Med Pediatr Oncol 33 (2): 76-82, 1999. [PUBMED Abstract]
- Brennan B, Stevens M, Kelsey A, et al.: Synovial sarcoma in childhood and adolescence: a retrospective series of 77 patients registered by the Children's Cancer and Leukaemia Group between 1991 and 2006. Pediatr Blood Cancer 55 (1): 85-90, 2010. [PUBMED Abstract]
- Ferrari A, Miceli R, Rey A, et al.: Non-metastatic unresected paediatric non-rhabdomyosarcoma soft tissue sarcomas: results of a pooled analysis from United States and European groups. Eur J Cancer 47 (5): 724-31, 2011. [PUBMED Abstract]
- Raney RB: Synovial sarcoma in young people: background, prognostic factors, and therapeutic questions. J Pediatr Hematol Oncol 27 (4): 207-11, 2005. [PUBMED Abstract]
- Orbach D, Mc Dowell H, Rey A, et al.: Sparing strategy does not compromise prognosis in pediatric localized synovial sarcoma: experience of the International Society of Pediatric Oncology, Malignant Mesenchymal Tumors (SIOP-MMT) Working Group. Pediatr Blood Cancer 57 (7): 1130-6, 2011. [PUBMED Abstract]
- Ladenstein R, Treuner J, Koscielniak E, et al.: Synovial sarcoma of childhood and adolescence. Report of the German CWS-81 study. Cancer 71 (11): 3647-55, 1993. [PUBMED Abstract]
- Venkatramani R, Anderson JR, Million L, et al.: Risk-based treatment for synovial sarcoma in patients under 30 years of age: Children’s Oncology Group study ARST0332. [Abstract] J Clin Oncol 33 (15 Suppl): A-10012, 2015. Also available online. Last accessed April 02, 2018.
- Ferrari A, De Salvo GL, Brennan B, et al.: Synovial sarcoma in children and adolescents: the European Pediatric Soft Tissue Sarcoma Study Group prospective trial (EpSSG NRSTS 2005). Ann Oncol 26 (3): 567-72, 2015. [PUBMED Abstract]
- Spunt SL, Million L, Anderson JR, et al.: Risk-based treatment for nonrhabdomyosarcoma soft tissue sarcomas (NRSTS) in patients under 30 years of age: Children’s Oncology Group study ARST0332. [Abstract] J Clin Oncol 32 (Suppl 15): A-10008, 2014. Also available online. Last accessed April 02, 2018.
- Randall RL, Albritton KH, Ferney BJ, et al.: Malignant fibrous histiocytoma of soft tissue: an abandoned diagnosis. Am J Orthop 33 (12): 602-8, 2004. [PUBMED Abstract]
- Alaggio R, Collini P, Randall RL, et al.: Undifferentiated high-grade pleomorphic sarcomas in children: a clinicopathologic study of 10 cases and review of literature. Pediatr Dev Pathol 13 (3): 209-17, 2010 May-Jun. [PUBMED Abstract]
- Daw NC, Billups CA, Pappo AS, et al.: Malignant fibrous histiocytoma and other fibrohistiocytic tumors in pediatric patients: the St. Jude Children's Research Hospital experience. Cancer 97 (11): 2839-47, 2003. [PUBMED Abstract]
- Coffin CM, Dehner LP: Vascular tumors in children and adolescents: a clinicopathologic study of 228 tumors in 222 patients. Pathol Annu 28 Pt 1: 97-120, 1993. [PUBMED Abstract]
- Cioffi A, Reichert S, Antonescu CR, et al.: Angiosarcomas and other sarcomas of endothelial origin. Hematol Oncol Clin North Am 27 (5): 975-88, 2013. [PUBMED Abstract]
- Jeng MR, Fuh B, Blatt J, et al.: Malignant transformation of infantile hemangioma to angiosarcoma: response to chemotherapy with bevacizumab. Pediatr Blood Cancer 61 (11): 2115-7, 2014. [PUBMED Abstract]
- Dehner LP, Ishak KG: Vascular tumors of the liver in infants and children. A study of 30 cases and review of the literature. Arch Pathol 92 (2): 101-11, 1971. [PUBMED Abstract]
- Ferrari A, Casanova M, Bisogno G, et al.: Malignant vascular tumors in children and adolescents: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Med Pediatr Oncol 39 (2): 109-14, 2002. [PUBMED Abstract]
- Deyrup AT, Miettinen M, North PE, et al.: Pediatric cutaneous angiosarcomas: a clinicopathologic study of 10 cases. Am J Surg Pathol 35 (1): 70-5, 2011. [PUBMED Abstract]
- Elliott P, Kleinschmidt I: Angiosarcoma of the liver in Great Britain in proximity to vinyl chloride sites. Occup Environ Med 54 (1): 14-8, 1997. [PUBMED Abstract]
- Lezama-del Valle P, Gerald WL, Tsai J, et al.: Malignant vascular tumors in young patients. Cancer 83 (8): 1634-9, 1998. [PUBMED Abstract]
- Fata F, O'Reilly E, Ilson D, et al.: Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer 86 (10): 2034-7, 1999. [PUBMED Abstract]
- Lahat G, Dhuka AR, Hallevi H, et al.: Angiosarcoma: clinical and molecular insights. Ann Surg 251 (6): 1098-106, 2010. [PUBMED Abstract]
- Orlando G, Adam R, Mirza D, et al.: Hepatic hemangiosarcoma: an absolute contraindication to liver transplantation--the European Liver Transplant Registry experience. Transplantation 95 (6): 872-7, 2013. [PUBMED Abstract]
- Sanada T, Nakayama H, Irisawa R, et al.: Clinical outcome and dose volume evaluation in patients who undergo brachytherapy for angiosarcoma of the scalp and face. Mol Clin Oncol 6 (3): 334-340, 2017. [PUBMED Abstract]
- Dickson MA, D'Adamo DR, Keohan ML, et al.: Phase II Trial of Gemcitabine and Docetaxel with Bevacizumab in Soft Tissue Sarcoma. Sarcoma 2015: 532478, 2015. [PUBMED Abstract]
- North PE, Waner M, Mizeracki A, et al.: A unique microvascular phenotype shared by juvenile hemangiomas and human placenta. Arch Dermatol 137 (5): 559-70, 2001. [PUBMED Abstract]
- Boye E, Yu Y, Paranya G, et al.: Clonality and altered behavior of endothelial cells from hemangiomas. J Clin Invest 107 (6): 745-52, 2001. [PUBMED Abstract]
- Ravi V, Patel S: Vascular sarcomas. Curr Oncol Rep 15 (4): 347-55, 2013. [PUBMED Abstract]
- Grassia KL, Peterman CM, Iacobas I, et al.: Clinical case series of pediatric hepatic angiosarcoma. Pediatr Blood Cancer 64 (11): , 2017. [PUBMED Abstract]
- Mehrabi A, Kashfi A, Fonouni H, et al.: Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer 107 (9): 2108-21, 2006. [PUBMED Abstract]
- Haro A, Saitoh G, Tamiya S, et al.: Four-year natural clinical course of pulmonary epithelioid hemangioendothelioma without therapy. Thorac Cancer 6 (4): 544-7, 2015. [PUBMED Abstract]
- Sardaro A, Bardoscia L, Petruzzelli MF, et al.: Epithelioid hemangioendothelioma: an overview and update on a rare vascular tumor. Oncol Rev 8 (2): 259, 2014. [PUBMED Abstract]
- Dong K, Wang XX, Feng JL, et al.: Pathological characteristics of liver biopsies in eight patients with hepatic epithelioid hemangioendothelioma. Int J Clin Exp Pathol 8 (9): 11015-23, 2015. [PUBMED Abstract]
- Adams DM, Hammill A: Other vascular tumors. Semin Pediatr Surg 23 (4): 173-7, 2014. [PUBMED Abstract]
- Xiao Y, Wang C, Song Y, et al.: Primary epithelioid hemangioendothelioma of the kidney: the first case report in a child and literature review. Urology 82 (4): 925-7, 2013. [PUBMED Abstract]
- Reich S, Ringe H, Uhlenberg B, et al.: Epithelioid hemangioendothelioma of the lung presenting with pneumonia and heart rhythm disturbances in a teenage girl. J Pediatr Hematol Oncol 32 (4): 274-6, 2010. [PUBMED Abstract]
- Daller JA, Bueno J, Gutierrez J, et al.: Hepatic hemangioendothelioma: clinical experience and management strategy. J Pediatr Surg 34 (1): 98-105; discussion 105-6, 1999. [PUBMED Abstract]
- Ackermann O, Fabre M, Franchi S, et al.: Widening spectrum of liver angiosarcoma in children. J Pediatr Gastroenterol Nutr 53 (6): 615-9, 2011. [PUBMED Abstract]
- Stacchiotti S, Provenzano S, Dagrada G, et al.: Sirolimus in Advanced Epithelioid Hemangioendothelioma: A Retrospective Case-Series Analysis from the Italian Rare Cancer Network Database. Ann Surg Oncol 23 (9): 2735-44, 2016. [PUBMED Abstract]
- Semenisty V, Naroditsky I, Keidar Z, et al.: Pazopanib for metastatic pulmonary epithelioid hemangioendothelioma-a suitable treatment option: case report and review of anti-angiogenic treatment options. BMC Cancer 15: 402, 2015. [PUBMED Abstract]
- Raheja A, Suri A, Singh S, et al.: Multimodality management of a giant skull base hemangioendothelioma of the sphenopetroclival region. J Clin Neurosci 22 (9): 1495-8, 2015. [PUBMED Abstract]
- Ahmad N, Adams DM, Wang J, et al.: Hepatic epithelioid hemangioendothelioma in a patient with hemochromatosis. J Natl Compr Canc Netw 12 (9): 1203-7, 2014. [PUBMED Abstract]
- Otte JB, Zimmerman A: The role of liver transplantation for pediatric epithelioid hemangioendothelioma. Pediatr Transplant 14 (3): 295-7, 2010. [PUBMED Abstract]
Treatment of Metastatic Childhood Soft Tissue Sarcoma
Standard treatment options for metastatic childhood soft tissue sarcoma include the following:
- Combination therapy using chemotherapy, radiation therapy, and surgical resection of pulmonary metastases.
For treatment options, refer to the individual tumor type sections of the summary.
The prognosis for children with metastatic soft tissue sarcomas is poor,[1-6] and these children should receive combined treatment with chemotherapy, radiation therapy, and surgical resection of pulmonary metastases. In a prospective randomized trial, chemotherapy with vincristine, dactinomycin, doxorubicin, and cyclophosphamide, with or without dacarbazine, led to tumor responses in one-third of patients with unresectable or metastatic disease. The estimated 4-year survival rate, however, was poor, with fewer than one-third of children surviving.[6-8]
Pulmonary Metastases
Generally, children with isolated pulmonary metastases should be considered for a surgical procedure in an attempt to resect all gross disease.[9] For patients with multiple or recurrent pulmonary metastases, additional surgical procedures can be performed if the morbidity is deemed acceptable. In a retrospective review, patients with synovial sarcoma and pulmonary metastases for whom it was possible to completely resect all metastatic lung lesions had better survival than did patients for whom it was not possible to achieve complete resections.[9][Level of evidence: 3iiiA] Formal segmentectomy, lobectomy, and mediastinal lymph node dissection are unnecessary.[10]
An alternative approach is focused radiation therapy (fractionated stereotactic radiation therapy), which has been successfully used in adults to control lesions. The estimated 5-year survival rate after thoracotomy for pulmonary metastasectomy has ranged from 10% to 58% in adult studies. Emerging data suggest a similar outcome after the administration of focused radiation therapy.[11]
References
- Demetri GD, Elias AD: Results of single-agent and combination chemotherapy for advanced soft tissue sarcomas. Implications for decision making in the clinic. Hematol Oncol Clin North Am 9 (4): 765-85, 1995. [PUBMED Abstract]
- Elias A, Ryan L, Sulkes A, et al.: Response to mesna, doxorubicin, ifosfamide, and dacarbazine in 108 patients with metastatic or unresectable sarcoma and no prior chemotherapy. J Clin Oncol 7 (9): 1208-16, 1989. [PUBMED Abstract]
- Edmonson JH, Ryan LM, Blum RH, et al.: Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol 11 (7): 1269-75, 1993. [PUBMED Abstract]
- Rao BN: Nonrhabdomyosarcoma in children: prognostic factors influencing survival. Semin Surg Oncol 9 (6): 524-31, 1993 Nov-Dec. [PUBMED Abstract]
- deCou JM, Rao BN, Parham DM, et al.: Malignant peripheral nerve sheath tumors: the St. Jude Children's Research Hospital experience. Ann Surg Oncol 2 (6): 524-9, 1995. [PUBMED Abstract]
- Pappo AS, Rao BN, Jenkins JJ, et al.: Metastatic nonrhabdomyosarcomatous soft-tissue sarcomas in children and adolescents: the St. Jude Children's Research Hospital experience. Med Pediatr Oncol 33 (2): 76-82, 1999. [PUBMED Abstract]
- Pratt CB, Pappo AS, Gieser P, et al.: Role of adjuvant chemotherapy in the treatment of surgically resected pediatric nonrhabdomyosarcomatous soft tissue sarcomas: A Pediatric Oncology Group Study. J Clin Oncol 17 (4): 1219, 1999. [PUBMED Abstract]
- Pratt CB, Maurer HM, Gieser P, et al.: Treatment of unresectable or metastatic pediatric soft tissue sarcomas with surgery, irradiation, and chemotherapy: a Pediatric Oncology Group study. Med Pediatr Oncol 30 (4): 201-9, 1998. [PUBMED Abstract]
- Stanelle EJ, Christison-Lagay ER, Wolden SL, et al.: Pulmonary metastasectomy in pediatric/adolescent patients with synovial sarcoma: an institutional review. J Pediatr Surg 48 (4): 757-63, 2013. [PUBMED Abstract]
- Putnam JB Jr, Roth JA: Surgical treatment for pulmonary metastases from sarcoma. Hematol Oncol Clin North Am 9 (4): 869-87, 1995. [PUBMED Abstract]
- Dhakal S, Corbin KS, Milano MT, et al.: Stereotactic body radiotherapy for pulmonary metastases from soft-tissue sarcomas: excellent local lesion control and improved patient survival. Int J Radiat Oncol Biol Phys 82 (2): 940-5, 2012. [PUBMED Abstract]
Treatment of Progressive/Recurrent Childhood Soft Tissue Sarcoma
With the possible exception of infants with infantile fibrosarcoma, the prognosis for patients with recurrent or progressive disease is poor. No prospective trial has been able to prove that enhanced local control of pediatric soft tissue sarcomas will ultimately improve survival. Therefore, treatment should be individualized for the site of recurrence, biologic characteristics of the tumor (e.g., grade, invasiveness, and size), previous therapies, and individual patient considerations.
Treatment options for recurrent or progressive disease include the following:
- Surgical excision of local recurrence or isolated pulmonary recurrence.
- An Italian review of 73 patients with recurrent malignant peripheral nerve sheath tumors found that most relapses were local. Multivariate analysis showed that the factors associated with improved survival were no tumor invasiveness at initial diagnosis (T1), time of recurrence more than 12 months after initial diagnosis, and achievement of a second complete response with surgical removal of the recurrence(s). Only 15.8% of patients who had complete surgical excisions of local recurrence(s) were alive at 5 years.[1][Level of evidence: 3iiiA]
- Surgical excision of local recurrence followed by radiation therapy or brachytherapy (if no previous radiation therapy was given).
- Limb amputation (only for some children with extremity sarcomas that have already received radiation therapy).
- Gemcitabine and docetaxel.[2]
- Trabectedin.[3-5]
- Pazopanib. A phase I trial of pazopanib reported one partial response in a patient with desmoplastic small round cell tumor and prolonged disease stabilization in eight patients with recurrent sarcoma.[6][Level of evidence: 2Diii] Pazopanib has been approved for use in recurrent soft tissue sarcoma. The clinical trial that was used to obtain approval was limited to adults and demonstrated disease stabilization and prolonged time to progression; it did not demonstrate improved overall survival.[7] One 13-year-old boy and one 14-year-old girl with multiply recurrent synovial sarcoma and lung metastases had responses to pazopanib for 14 and 15 months, respectively.[8][Level of evidence: 3iiDi]
- A clinical trial of new chemotherapeutic regimens.
Resection is the standard treatment for recurrent pediatric nonrhabdomyosarcomatous soft tissue sarcomas. If the patient has not yet received radiation therapy, postoperative radiation should be considered after local excision of the recurrent tumor. Limb-sparing procedures with postoperative brachytherapy have been evaluated in adults but have not been studied extensively in children. For some children with extremity sarcomas who have received previous radiation therapy, amputation may be the only therapeutic option.
Pulmonary metastasectomy may achieve prolonged disease control for some patients.[9] A large, retrospective analysis of patients with recurrent soft tissue sarcoma showed that isolated local relapse had a better prognosis and that resection of pulmonary metastases improved the probability of survival.[10] In 31 children and adolescents younger than 23 years with pulmonary metastases from synovial sarcoma, complete resection of lung metastases appeared to prolong survival when compared with ten other patients who were not considered candidates for metastasectomy.[11][Level of evidence: 3iiiA] All patients with recurrent tumors should be considered for current clinical trials.
Published results of two studies addressed the outcomes for children with relapsed synovial sarcoma. Most patients in one study had distant relapse (29 of 44 patients),[12] while most patients in the second study had local relapse (27 of 37 patients).[13] Distant recurrence was a poor prognostic variable, while tumor resectability at relapse (as manifested by extremity recurrence) was associated with a better outcome in both studies.
Treatment Options Under Clinical Evaluation
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- APEC1621 (NCT03155620) (Pediatric MATCH: Targeted Therapy Directed by Genetic Testing in Treating Pediatric Patients with Relapsed or Refractory Advanced Solid Tumors, Non-Hodgkin Lymphomas, or Histiocytic Disorders): NCI-COG Pediatric Molecular Analysis for Therapeutic Choice (MATCH), referred to as Pediatric MATCH, will match targeted agents with specific molecular changes identified using a next-generation sequencing targeted assay of more than 3,000 different mutations across more than 160 genes in refractory and recurrent solid tumors. Children and adolescents aged 1 to 21 years are eligible for the trial.
Tumor tissue from progressive or recurrent disease must be available for molecular characterization. Patients with tumors that have molecular variants addressed by treatment arms included in the trial will be offered treatment on Pediatric MATCH. Additional information can be obtained on the ClinicalTrials.gov website for APEC1621 (NCT03155620).
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Bergamaschi L, Bisogno G, Manzitti C, et al.: Salvage rates and prognostic factors after relapse in children and adolescents with malignant peripheral nerve sheath tumors. Pediatr Blood Cancer 65 (2): , 2018. [PUBMED Abstract]
- Maki RG, Wathen JK, Patel SR, et al.: Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 25 (19): 2755-63, 2007. [PUBMED Abstract]
- Le Cesne A, Cresta S, Maki RG, et al.: A retrospective analysis of antitumour activity with trabectedin in translocation-related sarcomas. Eur J Cancer 48 (16): 3036-44, 2012. [PUBMED Abstract]
- Garcia-Carbonero R, Supko JG, Maki RG, et al.: Ecteinascidin-743 (ET-743) for chemotherapy-naive patients with advanced soft tissue sarcomas: multicenter phase II and pharmacokinetic study. J Clin Oncol 23 (24): 5484-92, 2005. [PUBMED Abstract]
- Garcia-Carbonero R, Supko JG, Manola J, et al.: Phase II and pharmacokinetic study of ecteinascidin 743 in patients with progressive sarcomas of soft tissues refractory to chemotherapy. J Clin Oncol 22 (8): 1480-90, 2004. [PUBMED Abstract]
- Glade Bender JL, Lee A, Reid JM, et al.: Phase I pharmacokinetic and pharmacodynamic study of pazopanib in children with soft tissue sarcoma and other refractory solid tumors: a children's oncology group phase I consortium report. J Clin Oncol 31 (24): 3034-43, 2013. [PUBMED Abstract]
- van der Graaf WT, Blay JY, Chawla SP, et al.: Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 379 (9829): 1879-86, 2012. [PUBMED Abstract]
- Casanova M, Basso E, Magni C, et al.: Response to pazopanib in two pediatric patients with pretreated relapsing synovial sarcoma. Tumori 103 (1): e1-e3, 2017. [PUBMED Abstract]
- Belal A, Salah E, Hajjar W, et al.: Pulmonary metastatectomy for soft tissue sarcomas: is it valuable? J Cardiovasc Surg (Torino) 42 (6): 835-40, 2001. [PUBMED Abstract]
- Zagars GK, Ballo MT, Pisters PW, et al.: Prognostic factors for disease-specific survival after first relapse of soft-tissue sarcoma: analysis of 402 patients with disease relapse after initial conservative surgery and radiotherapy. Int J Radiat Oncol Biol Phys 57 (3): 739-47, 2003. [PUBMED Abstract]
- Stanelle EJ, Christison-Lagay ER, Wolden SL, et al.: Pulmonary metastasectomy in pediatric/adolescent patients with synovial sarcoma: an institutional review. J Pediatr Surg 48 (4): 757-63, 2013. [PUBMED Abstract]
- Ferrari A, De Salvo GL, Dall'Igna P, et al.: Salvage rates and prognostic factors after relapse in children and adolescents with initially localised synovial sarcoma. Eur J Cancer 48 (18): 3448-55, 2012. [PUBMED Abstract]
- Soole F, Maupain C, Defachelles AS, et al.: Synovial sarcoma relapses in children and adolescents: prognostic factors, treatment, and outcome. Pediatr Blood Cancer 61 (8): 1387-93, 2014. [PUBMED Abstract]
Changes to This Summary (04/02/2018)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Treatment of Newly Diagnosed Childhood Soft Tissue Sarcoma
Added text to state that a small series reported symptomatic improvement and stable disease in seven patients with desmoid-type fibromatosis who were treated with pazopanib (cited Agresta et al. as reference 48).
Added text to state that a tumor with morphology similar to that of infantile fibrosarcoma has been identified in older children; in these older children, the tumors do not have the t(12;15)(ETV-NTRK3) translocation that is characteristic of the younger patients. In several of these patients, BRAF gene fusions have been identified (cited Kao et al. as reference 69).
Added text about the outcome results of 73 children and adolescents with recurrent malignant peripheral nerve sheath tumor reported by the Italian Sarcoma Group (cited Bergamaschi et al. as reference 127 and level of evidence 3iiiA).
Added text about the patient characteristics and results of a retrospective review of children and young adults younger than 30 years from four institutions, which identified 69 patients with alveolar soft part sarcoma treated primarily with surgery between 1980 and 2014 (cited Flores et al. as reference 152 and level of evidence 3iiA).
Added Sedig et al. as reference 172 and level of evidence 3iiiA.
Treatment of Progressive/Recurrent Childhood Soft Tissue Sarcoma
Added text about the prognostic factors and outcome results reported in an Italian review of 73 children and adolescents with recurrent malignant peripheral nerve sheath tumor (cited Bergamaschi et al. as reference 1 and level of evidence 3iiiA).
This summary is written and maintained by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood soft tissue sarcoma. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Childhood Soft Tissue Sarcoma Treatment are:
- Denise Adams, MD (Children's Hospital Boston)
- Louis S. Constine, MD (James P. Wilmot Cancer Center at University of Rochester Medical Center)
- Holcombe Edwin Grier, MD (Dana-Farber Cancer Institute/Boston Children's Hospital)
- Andrea A. Hayes-Jordan, MD, FACS, FAAP (M.D. Anderson Cancer Center)
- Paul A. Meyers, MD (Memorial Sloan-Kettering Cancer Center)
- Thomas A. Olson, MD (Aflac Cancer and Blood Disorders Center of Children's Healthcare of Atlanta - Egleston Campus)
- Alberto S. Pappo, MD (St. Jude Children's Research Hospital)
- R Beverly Raney, MD (Consultant)
- Stephen J. Shochat, MD (St. Jude Children's Research Hospital)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Soft Tissue Sarcoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/soft-tissue-sarcoma/hp/child-soft-tissue-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389361]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.
Updated: April 2, 2018
Source URL: https://www.cancer.gov/publishedcontent/syndication/3899.htm
Source Agency: National Cancer Institute (NCI)
Captured Date: 2013-09-14 09:01:57.0
Childhood Soft Tissue Sarcoma Treatment (PDQ®)–Health Professional Version
General Information About Childhood Soft Tissue Sarcoma
Dramatic improvements in survival have been achieved for children and adolescents with cancer. Between 1975 and 2010, childhood cancer mortality decreased by more than 50%.[1] Childhood and adolescent cancer survivors require close monitoring because cancer therapy side effects may persist or develop months or years after treatment. (Refer to the PDQ summary on Late Effects of Treatment for Childhood Cancer for specific information about the incidence, type, and monitoring of late effects in childhood and adolescent cancer survivors.)
Rhabdomyosarcoma, a tumor of striated muscle, is the most common soft tissue sarcoma in children aged 0 to 14 years and accounts for 50% of tumors in this age group.[2] (Refer to the PDQ summary on Childhood Rhabdomyosarcoma Treatment for more information.) In pediatrics, the remaining soft tissue sarcomas are commonly referred to as nonrhabdomyosarcomatous soft tissue sarcomas and account for approximately 3% of all childhood tumors.[3] This heterogeneous group of tumors includes the following neoplasms:[4]
- Connective tissue (e.g., desmoid-type fibromatosis).
- Peripheral nervous system (e.g., malignant peripheral nerve sheath tumor).
- Smooth muscle (e.g., leiomyosarcoma).
- Vascular tissue (blood and lymphatic vessels, e.g., angiosarcoma). (Refer to the PDQ summary on Childhood Vascular Tumors Treatment for more information about childhood vascular tumors.)
Distribution of Soft Tissue Sarcoma by Age and Histology
Pediatric soft tissue sarcomas are a heterogenous group of malignant tumors that originate from primitive mesenchymal tissue and account for 7% of all childhood tumors.[5]
The distribution of soft tissue sarcomas by histology and age, based on the Surveillance, Epidemiology, and End Results (SEER) information from 1975 to 2012, is depicted in Table 1. The distribution of histologic subtypes by age is also shown in Figure 2.
| Age <5 y | Age 5–9 y | Age 10–14 y | Age 15–19 y | % of the Total Number of STS Cases <20 y | |||
|---|---|---|---|---|---|---|---|
| pPNET = peripheral primitive neuroectodermal tumors; SEER = Surveillance, Epidemiology, and End Results; STS = soft tissue sarcoma. | |||||||
| aSEER data is available at http://seer.cancer.gov. | |||||||
| bDermatofibrosarcoma accounts for 75% of these cases. | |||||||
| All soft tissue and other extraosseous sarcomas | 923 | 631 | 946 | 1,267 | 100 | ||
| Rhabdomyosarcomas | 551 | 348 | 312 | 270 | 39 | ||
| Fibrosarcomas, peripheral nerve, and other fibrous neoplasms | 116 | 50 | 88 | 141 | 10 | ||
| Fibroblastic and myofibroblastic tumors | 97 | 24 | 31 | 62 | 6 | ||
| Nerve sheath tumors | 19 | 26 | 56 | 77 | 5 | ||
| Other fibromatous neoplasms | 0 | 0 | 1 | 2 | 0.1 | ||
| Kaposi sarcoma | 2 | 1 | 1 | 9 | 0.3 | ||
| Other specified soft tissue sarcomas | 194 | 190 | 424 | 708 | 40 | ||
| Ewing tumor and Askin tumor of soft tissue | 27 | 30 | 62 | 92 | 6 | ||
| pPNET of soft tissue | 21 | 18 | 36 | 46 | 3.2 | ||
| Extrarenal rhabdoid tumor | 61 | 3 | 7 | 3 | 2 | ||
| Liposarcomas | 3 | 5 | 22 | 57 | 2.3 | ||
| Fibrohistiocytic tumors b | 34 | 54 | 108 | 188 | 10 | ||
| Leiomyosarcomas | 9 | 14 | 15 | 36 | 2 | ||
| Synovial sarcomas | 10 | 34 | 111 | 175 | 9 | ||
| Blood vessel tumors | 11 | 7 | 8 | 25 | 1.4 | ||
| Osseous and chondromatous neoplasms of soft tissue | 1 | 6 | 13 | 10 | 0.8 | ||
| Alveolar soft parts sarcoma | 4 | 3 | 16 | 29 | 1.4 | ||
| Miscellaneous soft tissue sarcomas | 13 | 16 | 36 | 47 | 3 | ||
| Unspecified soft tissue sarcomas | 60 | 40 | 111 | 139 | 9.3 | ||
Nonrhabdomyosarcomatous soft tissue sarcomas are more common in adolescents and adults,[4] and most of the information regarding treatment and natural history of the disease in younger patients has been based on adult studies. The distributions of these tumors by age according to stage, histologic subtype, and tumor site are shown in Figures 1, 2, and 3, respectively.[6]



Risk Factors
Some genetic and environmental factors have been associated with the development of nonrhabdomyosarcomatous soft tissue sarcoma, including the following:
- Genetic factors:
- Li-Fraumeni syndrome: Patients with Li-Fraumeni syndrome (usually due to heritable cancer-associated changes of the TP53 tumor suppressor gene) have an increased risk of developing soft tissue tumors (mostly nonrhabdomyosarcomatous soft tissue sarcomas), bone sarcomas, breast cancer, brain tumors, and acute leukemia.[7,8]
- Familial adenomatous polyposis: Patients with familial adenomatous polyposis are at increased risk of developing desmoid-type fibromatosis.[9]
- Retinoblastoma (RB1) gene: Germline mutations of the retinoblastoma gene have been associated with an increased risk of developing soft tissue sarcomas, particularly leiomyosarcoma.[10]
- SMARCB1 gene: Germline mutations or deletions of the SMARCB1 (INI1) gene are associated with an increased risk of developing extrarenal rhabdoid tumors.[11]
- Neurofibromatosis type 1: Approximately 4% of patients with neurofibromatosis type 1 develop malignant peripheral nerve sheath tumors, which usually develop after a long latency; some patients develop multiple lesions.[12-14]
- Werner syndrome: Werner syndrome is characterized by spontaneous chromosomal instability, resulting in increased susceptibility to cancer and premature aging. An excess of soft tissue sarcomas has been reported in patients with Werner syndrome.[15]
- Environmental factors:
- Radiation: Some nonrhabdomyosarcomatous soft tissue sarcomas (particularly malignant fibrous histiocytoma) can develop within a previously irradiated site.[3,16]
- Epstein-Barr virus infection in patients with AIDS: Some nonrhabdomyosarcomatous soft tissue sarcomas (e.g., leiomyosarcoma) have been linked to Epstein-Barr virus infection in patients with AIDS.[3,17]
Clinical Presentation
Although nonrhabdomyosarcomatous soft tissue sarcomas can develop in any part of the body, they arise most commonly in the trunk and extremities.[18-20] These neoplasms can present initially as an asymptomatic solid mass, or they may be symptomatic because of local invasion of adjacent anatomical structures. Although rare, these tumors can arise primarily in brain tissue and are treated according to the histotype.[21]
Systemic symptoms (e.g., fever, weight loss, and night sweats) are rare. Hypoglycemia and hypophosphatemic rickets have been reported in cases of hemangiopericytoma, whereas hyperglycemia has been noted in patients with fibrosarcoma of the lung.[22]
Diagnostic and Staging Evaluation
When a suspicious lesion is identified, it is crucial that a complete workup, followed by adequate biopsy be performed. It is best to image the lesion using the following procedures before initiating any intervention:
- Plain films. Plain films can be used to rule out bone involvement and detect calcifications that may be seen in soft tissue tumors such as extraskeletal osteosarcoma or synovial sarcoma.
- Chest computed tomography (CT). Chest CT is essential to assess the presence of metastases.
- Abdominal CT or magnetic resonance imaging (MRI). Abdominal CT or MRI can be used to image intra-abdominal tumors, such as liposarcoma.
- Extremity MRI. MRI is essential for extremity lesions.
- Positron emission tomography (PET) scan and bone scan. In children with rhabdomyosarcoma, PET-CT performed better than conventional imaging in identifying nodal, bone, bone marrow, and soft tissue disease. The authors of an imaging comparison study suggest that bone scans with technetium Tc 99m might be eliminated as a staging procedure.[23] The use of this modality in pediatric nonrhabdomyosarcomatous soft tissue sarcoma has not been studied extensively. However, a small study of nine patients with nonrhabdomyosarcomatous soft tissue sarcoma suggests that PET-CT is more accurate and cost effective than either modality alone in identifying distant metastatic disease.[24]
The imaging characteristics of some tumors can be highly suggestive of this diagnosis. For example, the imaging characteristics of pediatric low-grade fibromyxoid sarcoma and alveolar soft part sarcoma have been described and can aid in the diagnosis of these rare neoplasms.[25]
Biopsy strategies
Although nonrhabdomyosarcomatous soft tissue tumors are fairly readily distinguished pathologically from rhabdomyosarcoma and Ewing sarcoma, the classification of childhood nonrhabdomyosarcomatous soft tissue sarcoma type is often difficult. Core-needle biopsy, incisional biopsy, or excisional biopsy can be used to diagnose a nonrhabdomyosarcomatous soft tissue sarcoma. If possible, the surgeon who will perform the definitive resection needs to be involved in the biopsy decision. Poorly placed incisional or needle biopsies may adversely affect the performance of the primary resection.
Considerations related to the selection of a biopsy procedure are as follows:
- Given the diagnostic importance of translocations, a core-needle biopsy or small incisional biopsy that obtains adequate tumor tissue is crucial to allow for conventional histology, immunocytochemical analysis, and other studies such as light and electron microscopy, cytogenetics, fluorescence in situ hybridization, and molecular pathology.[26,27] Core-needle biopsy for a deep-seated tumor can lead to formation of a hematoma, which affects subsequent resection and/or radiation; in these cases, incisional biopsy is the preferred procedure.
- Fine-needle biopsy is usually not recommended because it is difficult to determine the accurate histologic diagnosis and grade of the tumor in this heterogeneous group of tumors.
- Image guidance using ultrasound, CT scan, or MRI may be necessary to ensure a representative biopsy.[28]
- Needle biopsy techniques must ensure adequate tissue sampling. The acquisition of multiple cores of tissue may be required.
- Incisional biopsies must not compromise subsequent wide local excision.
- Excisional biopsy of the lesion is only appropriate for small superficial lesions (<3 cm in size) and are discouraged.[29,30] If an excisional biopsy is contemplated, then MRI of the area is recommended to define the area of involvement as subsequent surgery or radiation therapy is likely.
- Various institutional series have demonstrated the feasibility and effectiveness of sentinel node biopsy as a staging procedure in pediatric patients with soft tissue sarcomas.[31-36]
- Transverse extremity incisions are avoided to reduce skin loss and because they require a greater cross-sectional volume of tissue to be covered in the radiation field. Other extensive surgical procedures are also avoided before definitive diagnosis. For these reasons, open biopsy or multiple core-needle biopsies are strongly encouraged so that adequate tumor tissue can be obtained to allow crucial studies to be performed and to avoid limiting future treatment options.
Unplanned resection
In children with unplanned resection of nonrhabdomyosarcomatous soft tissue sarcomas, primary re-excision is frequently recommended because many patients will have tumor present in the re-excision specimen.[37,38] A single-institution analysis of adolescents and adults compared patients with unplanned excision of soft tissue sarcoma to stage-matched controls. In this retrospective analysis, unplanned initial excision of soft tissue sarcoma resulted in increased risk of local recurrence, metastasis, and death; this increase was greatest for high-grade tumors.[39][Level of evidence: 3iiA]
Chromosomal abnormalities
Many nonrhabdomyosarcomatous soft tissue sarcomas are characterized by chromosomal abnormalities. Some of these chromosomal translocations lead to a fusion of two disparate genes. The resulting fusion transcript can be readily detected by using polymerase chain reaction-based techniques, thus facilitating the diagnosis of those neoplasms that have translocations.
Some of the most frequent aberrations seen in nonrhabdomyosarcomatous soft tissue tumors are listed in Table 2.
| Histology | Chromosomal Aberrations | Genes Involved |
|---|---|---|
| aAdapted from Sandberg,[40] Slater et al.,[41] Mertens et al.,[42] and Romeo.[43] | ||
| Alveolar soft part sarcoma | t(x;17)(p11.2;q25) | ASPL/TFE3 [44-46] |
| Angiomatoid fibrous histiocytoma | t(12;16)(q13;p11), t(2;22)(q33;q12), t(12;22)(q13;q12) | FUS/ATF1, EWSR1/CREB1,[47] EWS/ATF1 |
| Clear cell sarcoma | t(12;22)(q13;q12), t(2;22)(q33;q12) | ATF1/EWS, EWSR1/CREB1 |
| Congenital (infantile) fibrosarcoma/mesoblastic nephroma | t(12;15)(p13;q25) | ETV-NTRK3 |
| Dermatofibrosarcoma protuberans | t(17;22)(q22;q13) | COL1A1/PDGFB |
| Desmoid fibromatosis | Trisomy 8 or 20, loss of 5q21 | CTNNB1 or APC mutations |
| Desmoplastic small round cell tumors | t(11;22)(p13;q12) | EWS/WT1 [48,49] |
| Epithelioid hemangioendothelioma | t(1;3)(p36;q25) [50] | WWTR1/CAMTA1 |
| Epithelioid sarcoma | Inactivation SMARCB1 | SMARCB1 |
| Extraskeletal myxoid chondrosarcoma | t(9;22)(q22;q12), t(9;17)(q22;q11), t(9;15)(q22;q21), t(3;9)(q11;q22) | EWSR1/NR4A3, TAF2N/NR4A3, TCF12/NR4A3, TGF/NR4A3 |
| Hemangiopericytoma | t(12;19)(q13;q13.3) and t(13;22)(q22;q13.3) | |
| Infantile fibrosarcoma | t(12;15)(p13;q25) | ETV6/NTRK3 |
| Inflammatory myofibroblastic tumor | t(1;2)(q23;q23), t(2;19)(q23;q13), t(2;17)(q23;q23), t(2;2)(p23;q13), t(2;11)(p23;p15) [51] | TPM3/ALK, TPM4/ALK, CLTC/ALK, RANBP2/ALK, CARS/ALK, RAS |
| Low-grade fibromyxoid sarcoma | t(7;16)(q33;p11), t(11;16)(p11;p11) | FUS/CREB3L2, FUS/CREB3L1 |
| Malignant peripheral nerve sheath tumor | 17q11.2, loss or rearrangement 10p, 11q, 17q, 22q | NF1 |
| Mesenchymal chondrosarcoma | Del(8)(q13.3q21.1) | HEY1/NCOA2 |
| Myoepithelioma | t(19;22)(q13;q12), t(1;22)(q23;q12), t(6;22)(p21;q12) | EWSR/ZNF44, EWSR/PBX1, EWSR/POU5F1 |
| Myxoid/round cell liposarcoma | t(12;16)(q13;p11), t(12;22)(q13;q12) | FUS/DD1T3, EWSR/DD1T3 |
| Rhabdoid tumor | Inactivation SMARCB1 | SMARCB1 |
| Solitary fibrous tumor | Inv(12)(q13q13) | NAB2/STAT6 |
| Synovial sarcoma | t(x;18)(p11.2;q11.2) | SYT/SSX |
| Tenosynovial giant cell tumor | t(1;2)(p13;q35) | COL6A3/CSF1 |
Prognosis
The prognosis of nonrhabdomyosarcomatous soft tissue sarcoma varies greatly depending on the following factors:[52-54]
- Site of the primary tumor.
- Tumor size.
- Tumor grade. (Refer to the Prognostic Significance of Tumor Grading section of this summary for more information.)
- Tumor histology.
- Depth of tumor invasion.
- Presence of metastases.
- Resectability of the tumor.
- Use of radiation therapy.
Several adult and pediatric series have shown that patients with large or invasive tumors have a significantly worse prognosis than do those with small, noninvasive tumors. A retrospective review of soft tissue sarcomas in children and adolescents suggests that the 5 cm cutoff used for adults with soft tissue sarcoma may not be ideal for smaller children, especially infants. The review identified an interaction between tumor diameter and body surface area.[55] This relationship requires further study to determine the therapeutic implications of the observation.
In a review of a large adult series of nonrhabdomyosarcomatous soft tissue sarcomas, superficial extremity sarcomas had a better prognosis than did deep tumors. Thus, in addition to grade and size, the depth of invasion of the tumor should be considered.[56]
Some pediatric nonrhabdomyosarcomatous soft tissue sarcomas are associated with a better outcome. For instance, infantile fibrosarcoma, presenting in infants and children younger than 5 years, has an excellent prognosis given that surgery alone can cure a significant number of these patients and the tumor is highly chemosensitive.[3]
Soft tissue sarcomas in older children and adolescents often behave similarly to those in adult patients.[3,26] A large, prospective, multinational Children's Oncology Group study (ARST0332 [NCT00346164]) enrolled newly diagnosed patients younger than 30 years. Patients were assigned to treatment on the basis of their risk group (refer to Figure 4).[57][Level of evidence: 2A]

- Arm A (grossly excised low-grade tumor and ≤5 cm widely excised high-grade tumor): Surgery only.
- Arm B (≤5 cm marginally resected high-grade tumor): 55.8 Gy of radiation therapy.
- Arm C (>5 cm grossly resected tumor ± metastases): Ifosfamide/doxorubicin chemotherapy and 55.8 Gy of radiation therapy.
- Arm D (>5 cm unresected tumor ± metastases): Preoperative ifosfamide/doxorubicin chemotherapy and 45 Gy of radiation therapy, and then surgery and a radiation boost that was based on margins.
Of 551 patients enrolled, at a median follow-up of 2.6 years, the preliminary analysis estimated the following 3-year survival rates:[57]
- Arm A: 91% event-free survival (EFS); 99% overall survival (OS).
- Arm B: 79% EFS; 100% OS.
- Arm C: 68% EFS; 81% OS.
- Arm D: 52% EFS; 66% OS.
Pediatric patients with unresected localized nonrhabdomyosarcomatous soft tissue sarcomas have a poor outcome. Only about one-third of patients treated with multimodality therapy remain disease free.[52,58]; [59,60][Level of evidence: 3iiiA] In a review of 30 Italian patients with nonrhabdomyosarcomatous soft tissue sarcoma at visceral sites, only ten patients survived at 5 years. Unfavorable prognostic factors included inability to achieve complete resection, large tumor size, tumor invasion, histologic subtype, and lung-pleura sites.[61][Level of evidence: 3iiB]
In a pooled analysis from U.S. and European pediatric centers, outcome was better for patients whose tumor removal procedure was deemed complete than for patients whose tumor removal was incomplete. Outcome was better for patients who received radiation therapy than for patients who did not.[59][Level of evidence: 3iiiA]
Because long-term related morbidity must be minimized while disease-free survival is maximized, the ideal therapy for each patient must be carefully and individually determined utilizing these prognostic factors before initiating therapy.[19,62-66]
Related Summaries
Refer to the following PDQ summaries for information about other types of sarcoma:
- Childhood Rhabdomyosarcoma Treatment.
- Childhood Vascular Tumors Treatment.
- Ewing Sarcoma Treatment (extraosseous Ewing, peripheral neuroepithelioma, and Askin tumors).
- Unusual Cancers of Childhood Treatment (gastrointestinal stromal tumors).
- Adult Soft Tissue Sarcoma Treatment.
References
- Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014. [PUBMED Abstract]
- Ries LA, Smith MA, Gurney JG, et al., eds.: Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. Bethesda, Md: National Cancer Institute, SEER Program, 1999. NIH Pub.No. 99-4649. Also available online. Last accessed January 24, 2018.
- Spunt SL, Million L, Coffin C: The nonrhabdomyosarcoma soft tissue sarcoma. In: Pizzo PA, Poplack DG, eds.: Principles and Practice of Pediatric Oncology. 7th ed. Philadelphia, Pa: Lippincott Williams and Wilkins, 2015, pp 827-54.
- Weiss SW, Goldblum JR: General considerations. In: Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 5th ed. St. Louis, Mo: Mosby, 2008, pp 1-14.
- Pappo AS, Pratt CB: Soft tissue sarcomas in children. Cancer Treat Res 91: 205-22, 1997. [PUBMED Abstract]
- Ferrari A, Sultan I, Huang TT, et al.: Soft tissue sarcoma across the age spectrum: a population-based study from the Surveillance Epidemiology and End Results database. Pediatr Blood Cancer 57 (6): 943-9, 2011. [PUBMED Abstract]
- Chang F, Syrjänen S, Syrjänen K: Implications of the p53 tumor-suppressor gene in clinical oncology. J Clin Oncol 13 (4): 1009-22, 1995. [PUBMED Abstract]
- Plon SE, Malkin D: Childhood cancer and hereditary. In: Pizzo PA, Poplack DG, eds.: Principles and Practice of Pediatric Oncology. 7th ed. Philadelphia, Pa: Lippincott Williams and Wilkins, 2015, pp 13-31.
- Groen EJ, Roos A, Muntinghe FL, et al.: Extra-intestinal manifestations of familial adenomatous polyposis. Ann Surg Oncol 15 (9): 2439-50, 2008. [PUBMED Abstract]
- Kleinerman RA, Tucker MA, Abramson DH, et al.: Risk of soft tissue sarcomas by individual subtype in survivors of hereditary retinoblastoma. J Natl Cancer Inst 99 (1): 24-31, 2007. [PUBMED Abstract]
- Eaton KW, Tooke LS, Wainwright LM, et al.: Spectrum of SMARCB1/INI1 mutations in familial and sporadic rhabdoid tumors. Pediatr Blood Cancer 56 (1): 7-15, 2011. [PUBMED Abstract]
- Weiss SW, Goldblum JR: Benign tumors of peripheral nerves. In: Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 5th ed. St. Louis, Mo: Mosby, 2008, pp 825-901.
- deCou JM, Rao BN, Parham DM, et al.: Malignant peripheral nerve sheath tumors: the St. Jude Children's Research Hospital experience. Ann Surg Oncol 2 (6): 524-9, 1995. [PUBMED Abstract]
- Stark AM, Buhl R, Hugo HH, et al.: Malignant peripheral nerve sheath tumours--report of 8 cases and review of the literature. Acta Neurochir (Wien) 143 (4): 357-63; discussion 363-4, 2001. [PUBMED Abstract]
- Goto M, Miller RW, Ishikawa Y, et al.: Excess of rare cancers in Werner syndrome (adult progeria). Cancer Epidemiol Biomarkers Prev 5 (4): 239-46, 1996. [PUBMED Abstract]
- Weiss SW, Goldblum JR: Malignant fibrous histiocytoma (pleomorphic undifferentiated sarcoma). In: Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 5th ed. St. Louis, Mo: Mosby, 2008, pp 403-27.
- McClain KL, Leach CT, Jenson HB, et al.: Association of Epstein-Barr virus with leiomyosarcomas in children with AIDS. N Engl J Med 332 (1): 12-8, 1995. [PUBMED Abstract]
- Dillon P, Maurer H, Jenkins J, et al.: A prospective study of nonrhabdomyosarcoma soft tissue sarcomas in the pediatric age group. J Pediatr Surg 27 (2): 241-4; discussion 244-5, 1992. [PUBMED Abstract]
- Rao BN: Nonrhabdomyosarcoma in children: prognostic factors influencing survival. Semin Surg Oncol 9 (6): 524-31, 1993 Nov-Dec. [PUBMED Abstract]
- Zeytoonjian T, Mankin HJ, Gebhardt MC, et al.: Distal lower extremity sarcomas: frequency of occurrence and patient survival rate. Foot Ankle Int 25 (5): 325-30, 2004. [PUBMED Abstract]
- Benesch M, von Bueren AO, Dantonello T, et al.: Primary intracranial soft tissue sarcoma in children and adolescents: a cooperative analysis of the European CWS and HIT study groups. J Neurooncol 111 (3): 337-45, 2013. [PUBMED Abstract]
- Weiss SW, Goldblum JR: Miscellaneous tumors of intermediate malignancy. In: Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 5th ed. St. Louis, Mo: Mosby, 2008, pp 1093-1160.
- Federico SM, Spunt SL, Krasin MJ, et al.: Comparison of PET-CT and conventional imaging in staging pediatric rhabdomyosarcoma. Pediatr Blood Cancer 60 (7): 1128-34, 2013. [PUBMED Abstract]
- Tateishi U, Hosono A, Makimoto A, et al.: Accuracy of 18F fluorodeoxyglucose positron emission tomography/computed tomography in staging of pediatric sarcomas. J Pediatr Hematol Oncol 29 (9): 608-12, 2007. [PUBMED Abstract]
- Sargar K, Kao SC, Spunt SL, et al.: MRI and CT of Low-Grade Fibromyxoid Sarcoma in Children: A Report From Children's Oncology Group Study ARST0332. AJR Am J Roentgenol 205 (2): 414-20, 2015. [PUBMED Abstract]
- Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 4th ed. St. Louis, Mo: Mosby, 2001.
- Recommendations for the reporting of soft tissue sarcomas. Association of Directors of Anatomic and Surgical Pathology. Mod Pathol 11 (12): 1257-61, 1998. [PUBMED Abstract]
- Chowdhury T, Barnacle A, Haque S, et al.: Ultrasound-guided core needle biopsy for the diagnosis of rhabdomyosarcoma in childhood. Pediatr Blood Cancer 53 (3): 356-60, 2009. [PUBMED Abstract]
- Coffin CM, Dehner LP, O'Shea PA: Pediatric Soft Tissue Tumors: A Clinical, Pathological, and Therapeutic Approach. Baltimore, Md: Williams and Wilkins, 1997.
- Smith LM, Watterson J, Scott SM: Medical and surgical management of pediatric soft tissue tumors. In: Coffin CM, Dehner LP, O'Shea PA: Pediatric Soft Tissue Tumors: A Clinical, Pathological, and Therapeutic Approach. Baltimore, Md: Williams and Wilkins, 1997, pp 360-71.
- Neville HL, Andrassy RJ, Lally KP, et al.: Lymphatic mapping with sentinel node biopsy in pediatric patients. J Pediatr Surg 35 (6): 961-4, 2000. [PUBMED Abstract]
- Neville HL, Raney RB, Andrassy RJ, et al.: Multidisciplinary management of pediatric soft-tissue sarcoma. Oncology (Huntingt) 14 (10): 1471-81; discussion 1482-6, 1489-90, 2000. [PUBMED Abstract]
- Kayton ML, Delgado R, Busam K, et al.: Experience with 31 sentinel lymph node biopsies for sarcomas and carcinomas in pediatric patients. Cancer 112 (9): 2052-9, 2008. [PUBMED Abstract]
- Dall'Igna P, De Corti F, Alaggio R, et al.: Sentinel node biopsy in pediatric patients: the experience in a single institution. Eur J Pediatr Surg 24 (6): 482-7, 2014. [PUBMED Abstract]
- Parida L, Morrisson GT, Shammas A, et al.: Role of lymphoscintigraphy and sentinel lymph node biopsy in the management of pediatric melanoma and sarcoma. Pediatr Surg Int 28 (6): 571-8, 2012. [PUBMED Abstract]
- Alcorn KM, Deans KJ, Congeni A, et al.: Sentinel lymph node biopsy in pediatric soft tissue sarcoma patients: utility and concordance with imaging. J Pediatr Surg 48 (9): 1903-6, 2013. [PUBMED Abstract]
- Chui CH, Spunt SL, Liu T, et al.: Is reexcision in pediatric nonrhabdomyosarcoma soft tissue sarcoma necessary after an initial unplanned resection? J Pediatr Surg 37 (10): 1424-9, 2002. [PUBMED Abstract]
- Cecchetto G, Guglielmi M, Inserra A, et al.: Primary re-excision: the Italian experience in patients with localized soft-tissue sarcomas. Pediatr Surg Int 17 (7): 532-4, 2001. [PUBMED Abstract]
- Qureshi YA, Huddy JR, Miller JD, et al.: Unplanned excision of soft tissue sarcoma results in increased rates of local recurrence despite full further oncological treatment. Ann Surg Oncol 19 (3): 871-7, 2012. [PUBMED Abstract]
- Sandberg AA: Translocations in malignant tumors. Am J Pathol 159 (6): 1979-80, 2001. [PUBMED Abstract]
- Slater O, Shipley J: Clinical relevance of molecular genetics to paediatric sarcomas. J Clin Pathol 60 (11): 1187-94, 2007. [PUBMED Abstract]
- Mertens F, Antonescu CR, Hohenberger P, et al.: Translocation-related sarcomas. Semin Oncol 36 (4): 312-23, 2009. [PUBMED Abstract]
- Romeo S, Dei Tos AP: Clinical application of molecular pathology in sarcomas. Curr Opin Oncol 23 (4): 379-84, 2011. [PUBMED Abstract]
- Ladanyi M, Lui MY, Antonescu CR, et al.: The der(17)t(X;17)(p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene 20 (1): 48-57, 2001. [PUBMED Abstract]
- Ladanyi M: The emerging molecular genetics of sarcoma translocations. Diagn Mol Pathol 4 (3): 162-73, 1995. [PUBMED Abstract]
- Williams A, Bartle G, Sumathi VP, et al.: Detection of ASPL/TFE3 fusion transcripts and the TFE3 antigen in formalin-fixed, paraffin-embedded tissue in a series of 18 cases of alveolar soft part sarcoma: useful diagnostic tools in cases with unusual histological features. Virchows Arch 458 (3): 291-300, 2011. [PUBMED Abstract]
- Antonescu CR, Dal Cin P, Nafa K, et al.: EWSR1-CREB1 is the predominant gene fusion in angiomatoid fibrous histiocytoma. Genes Chromosomes Cancer 46 (12): 1051-60, 2007. [PUBMED Abstract]
- Barnoud R, Sabourin JC, Pasquier D, et al.: Immunohistochemical expression of WT1 by desmoplastic small round cell tumor: a comparative study with other small round cell tumors. Am J Surg Pathol 24 (6): 830-6, 2000. [PUBMED Abstract]
- Wang LL, Perlman EJ, Vujanic GM, et al.: Desmoplastic small round cell tumor of the kidney in childhood. Am J Surg Pathol 31 (4): 576-84, 2007. [PUBMED Abstract]
- Errani C, Zhang L, Sung YS, et al.: A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer 50 (8): 644-53, 2011. [PUBMED Abstract]
- Jain S, Xu R, Prieto VG, et al.: Molecular classification of soft tissue sarcomas and its clinical applications. Int J Clin Exp Pathol 3 (4): 416-28, 2010. [PUBMED Abstract]
- Spunt SL, Hill DA, Motosue AM, et al.: Clinical features and outcome of initially unresected nonmetastatic pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Clin Oncol 20 (15): 3225-35, 2002. [PUBMED Abstract]
- Spunt SL, Poquette CA, Hurt YS, et al.: Prognostic factors for children and adolescents with surgically resected nonrhabdomyosarcoma soft tissue sarcoma: an analysis of 121 patients treated at St Jude Children's Research Hospital. J Clin Oncol 17 (12): 3697-705, 1999. [PUBMED Abstract]
- Ferrari A, Casanova M, Collini P, et al.: Adult-type soft tissue sarcomas in pediatric-age patients: experience at the Istituto Nazionale Tumori in Milan. J Clin Oncol 23 (18): 4021-30, 2005. [PUBMED Abstract]
- Ferrari A, Miceli R, Meazza C, et al.: Soft tissue sarcomas of childhood and adolescence: the prognostic role of tumor size in relation to patient body size. J Clin Oncol 27 (3): 371-6, 2009. [PUBMED Abstract]
- Brooks AD, Heslin MJ, Leung DH, et al.: Superficial extremity soft tissue sarcoma: an analysis of prognostic factors. Ann Surg Oncol 5 (1): 41-7, 1998 Jan-Feb. [PUBMED Abstract]
- Spunt SL, Million L, Anderson JR, et al.: Risk-based treatment for nonrhabdomyosarcoma soft tissue sarcomas (NRSTS) in patients under 30 years of age: Children’s Oncology Group study ARST0332. [Abstract] J Clin Oncol 32 (Suppl 15): A-10008, 2014. Also available online. Last accessed April 02, 2018.
- O'Sullivan B, Davis AM, Turcotte R, et al.: Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359 (9325): 2235-41, 2002. [PUBMED Abstract]
- Ferrari A, Miceli R, Rey A, et al.: Non-metastatic unresected paediatric non-rhabdomyosarcoma soft tissue sarcomas: results of a pooled analysis from United States and European groups. Eur J Cancer 47 (5): 724-31, 2011. [PUBMED Abstract]
- Smith KB, Indelicato DJ, Knapik JA, et al.: Definitive radiotherapy for unresectable pediatric and young adult nonrhabdomyosarcoma soft tissue sarcoma. Pediatr Blood Cancer 57 (2): 247-51, 2011. [PUBMED Abstract]
- Ferrari A, Magni C, Bergamaschi L, et al.: Pediatric nonrhabdomyosarcoma soft tissue sarcomas arising at visceral sites. Pediatr Blood Cancer 64 (9): , 2017. [PUBMED Abstract]
- Dillon PW, Whalen TV, Azizkhan RG, et al.: Neonatal soft tissue sarcomas: the influence of pathology on treatment and survival. Children's Cancer Group Surgical Committee. J Pediatr Surg 30 (7): 1038-41, 1995. [PUBMED Abstract]
- Pappo AS, Fontanesi J, Luo X, et al.: Synovial sarcoma in children and adolescents: the St Jude Children's Research Hospital experience. J Clin Oncol 12 (11): 2360-6, 1994. [PUBMED Abstract]
- Marcus KC, Grier HE, Shamberger RC, et al.: Childhood soft tissue sarcoma: a 20-year experience. J Pediatr 131 (4): 603-7, 1997. [PUBMED Abstract]
- Pratt CB, Pappo AS, Gieser P, et al.: Role of adjuvant chemotherapy in the treatment of surgically resected pediatric nonrhabdomyosarcomatous soft tissue sarcomas: A Pediatric Oncology Group Study. J Clin Oncol 17 (4): 1219, 1999. [PUBMED Abstract]
- Pratt CB, Maurer HM, Gieser P, et al.: Treatment of unresectable or metastatic pediatric soft tissue sarcomas with surgery, irradiation, and chemotherapy: a Pediatric Oncology Group study. Med Pediatr Oncol 30 (4): 201-9, 1998. [PUBMED Abstract]
Histopathological Classification of Childhood Soft Tissue Sarcoma
World Health Organization (WHO) Classification of Soft Tissue Sarcomas
The WHO lists the following cell types in its classification of soft tissue sarcomas:[1,2]
- Adipocytic tumors.
- Intermediate (locally aggressive).
- Malignant.
- Chondro-osseous tumors.
- Extraskeletal mesenchymal chondrosarcoma.[3]
- Extraskeletal osteosarcoma.
- Soft tissue chondroma.
- Fibroblastic/myofibroblastic tumors.
- Intermediate-grade (locally aggressive).
- Desmoid-type fibromatosis (previously called desmoid tumor or aggressive fibromatoses).
- Giant cell fibroblastoma.
- Lipofibromatosis.
- Palmar/plantar fibromatosis.
- Intermediate-grade (rarely metastasizing).
- Dermatofibrosarcoma protuberans.
- Infantile fibrosarcoma.[4]
- Inflammatory myofibroblastic tumor.
- Low-grade myofibroblastic tumor.
- Myxoinflammatory fibroblastic sarcoma.
- Solitary fibrous tumor.
- Malignant.
- Intermediate-grade (locally aggressive).
- Skeletal muscle tumors.
- Rhabdomyosarcoma (embryonal, alveolar, and pleomorphic forms). (Refer to the PDQ summary on Childhood Rhabdomyosarcoma Treatment for more information.)
- Smooth muscle tumors.
- So-called fibrohistiocytic tumors (intermediate, rarely metastasizing).
- Giant cell tumors of soft tissue.
- Plexiform fibrohistiocytic tumor.
- Tumors of peripheral nerves.
- Pericytic (perivascular) tumors.
- Malignant glomus tumor and variants.
- Myopericytoma.
- Angioleiomyoma.
- Myofibroma.
- Infantile myofibroma (previously called hemangiopericytoma [infantile]).
- Myofibromatosis.
- Infantile myofibromatosis.
- Tumors of uncertain differentiation.
- Alveolar soft part sarcoma.
- Clear cell sarcoma of soft tissue.
- Desmoplastic small round cell tumor.
- Epithelioid sarcoma.
- Extrarenal rhabdoid tumor.
- Extraskeletal myxoid chondrosarcoma.
- Neoplasms with perivascular epithelioid cell differentiation (PEComa NOS, malignant).
- Primitive neuroectodermal tumor/extraskeletal Ewing tumor.
- Synovial sarcoma (NOS, spindle cell, and biphasic varieties).
- Undifferentiated/unclassified sarcomas.
- Undifferentiated epithelial sarcoma.
- Undifferentiated pleomorphic sarcoma.
- Undifferentiated round cell sarcoma.
- Undifferentiated sarcoma; sarcoma, NOS.[6]
- Undifferentiated spindle cell sarcoma.
- Vascular tumors.
References
- Soft tissue sarcoma. In: Edge SB, Byrd DR, Compton CC, et al., eds.: AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer, 2010, pp 291-6.
- Brodowicz T, Schwameis E, Widder J, et al.: Intensified Adjuvant IFADIC Chemotherapy for Adult Soft Tissue Sarcoma: A Prospective Randomized Feasibility Trial. Sarcoma 4 (4): 151-60, 2000. [PUBMED Abstract]
- Dantonello TM, Int-Veen C, Leuschner I, et al.: Mesenchymal chondrosarcoma of soft tissues and bone in children, adolescents, and young adults: experiences of the CWS and COSS study groups. Cancer 112 (11): 2424-31, 2008. [PUBMED Abstract]
- Steelman C, Katzenstein H, Parham D, et al.: Unusual presentation of congenital infantile fibrosarcoma in seven infants with molecular-genetic analysis. Fetal Pediatr Pathol 30 (5): 329-37, 2011. [PUBMED Abstract]
- Evans HL: Low-grade fibromyxoid sarcoma: a clinicopathologic study of 33 cases with long-term follow-up. Am J Surg Pathol 35 (10): 1450-62, 2011. [PUBMED Abstract]
- Alaggio R, Collini P, Randall RL, et al.: Undifferentiated high-grade pleomorphic sarcomas in children: a clinicopathologic study of 10 cases and review of literature. Pediatr Dev Pathol 13 (3): 209-17, 2010 May-Jun. [PUBMED Abstract]
Staging and Grading Systems for Childhood Soft Tissue Sarcoma
Clinical staging has an important role in predicting the clinical outcome and determining the most effective therapy for pediatric soft tissue sarcomas. As yet, there is no well-accepted staging system that is applicable to all childhood sarcomas. The system from the American Joint Committee on Cancer (AJCC) that is used for adults has not been validated in pediatric studies. Although a standardized staging system for pediatric nonrhabdomyosarcomatous soft tissue sarcoma does not exist, two systems are currently in use for staging pediatric nonrhabdomyosarcomatous soft tissue sarcoma.[1]
- Surgico-pathologic staging system: The surgico-pathologic staging system used by the Intergroup Rhabdomyosarcoma Study (see below) is based on the amount, or extent, of tumor that remains after initial surgery and whether the disease has metastasized. This staging system was used in early pediatric trials.[2]
- TNM staging system: The TNM staging system is a collaborative effort between the AJCC (United States) and the International Union Against Cancer (worldwide). Staging is based on the extent of the tumor (T), the extent of spread to the lymph nodes (N), and the presence of metastasis (M). Refer to Tables 3, 4, 5, and 6 for the staging of soft tissue sarcoma from the eighth edition of the AJCC Cancer Staging Manual.[3-7] The last Children's Oncology Group trial used the sixth edition AJCC Cancer Staging Manual for soft tissue sarcoma (with central pathology review).[1] A review of children with non-rhabdomyosarcoma soft tissue sarcomas was performed with data from the Surveillance, Epidemiology, and End Results (SEER) program and identified 941 patients between 1988 and 2007.[8] The COG risk stratification was validated in this cohort.
Intergroup Rhabdomyosarcoma Study Staging System
Nonmetastatic disease
- Group I: Localized tumor completely resected with histologically negative margins.
- Group II: Grossly resected tumor with microscopic residual tumor at the margin(s) and/or extension into regional lymph nodes.
- IIA: Localized, grossly resected tumor with microscopic residual disease.
- IIB: Regional disease with involved nodes completely resected with no microscopic disease. The most proximal (to the patient, most distal to the tumor) regional lymph node must be negative.
- IIC: Regional disease with involved nodes grossly resected but with evidence of residual microscopic disease at the primary site and/or histologic involvement of the most proximal regional lymph node in the dissection.
- Group III: Localized tumor, incompletely resected, or biopsy only, with gross residual tumor.
Metastatic disease
- Group IV: Any localized or regional tumor with distant metastases present at the time of diagnosis. This includes the presence of malignant cells in effusions (pleural, peritoneal) and/or cerebrospinal fluid (rare).
Recurrent/progressive disease
- Any soft tissue sarcoma that recurs after initial treatment or progresses after radiation therapy, chemotherapy, or initial surgery.
TNM Staging System
The eighth edition of the AJCC Cancer Staging Manual has designated staging by the four criteria of tumor size, nodal status, histologic grade, and metastasis and by anatomic primary tumor site (head and neck; trunk and extremities; abdomen and thoracic visceral organs; retroperitoneum; and unusual histologies and sites) (refer to Tables 3, 4, 5, and 6).[3-7] For information on unusual histologies and sites, refer to the AJCC Cancer Staging Manual.[7]
| T Category | Soft Tissue Sarcoma of the Trunk, Extremities, and Retroperitoneum | Soft Tissue Sarcoma of the Head and Neck | Soft Tissue Sarcoma of the Abdomen and Thoracic Visceral Organs |
|---|---|---|---|
| aAdapted from O'Sullivan et al.,[3] Yoon et al.,[4] Raut et al.,[5] and Pollock et al.[6] | |||
| TX | Primary tumor cannot be assessed. | Primary tumor cannot be assessed. | Primary tumor cannot be assessed. |
| T0 | No evidence of primary tumor. | ||
| T1 | Tumor ≤5 cm in greatest dimension. | Tumor ≤2 cm. | Organ confined. |
| T2 | Tumor >5 cm and ≤10 cm in greatest dimension. | Tumor >2 to ≤4 cm. | Tumor extension into tissue beyond organ. |
| T2a | Invades serosa or visceral peritoneum. | ||
| T2b | Extension beyond serosa (mesentery). | ||
| T3 | Tumor >10 cm and ≤15 cm in greatest dimension. | Tumor >4 cm. | Invades another organ. |
| T4 | Tumor >15 cm in greatest dimension. | Tumor with invasion of adjoining structures. | Multifocal involvement. |
| T4a | Tumor with orbital invasion, skull base/dural invasion, invasion of central compartment viscera, involvement of facial skeleton, or invasion of pterygoid muscles. | Multifocal (2 sites). | |
| T4b | Tumor with brain parenchymal invasion, carotid artery encasement, prevertebral muscle invasion, or central nervous system involvement via perineural spread. | Multifocal (3–5 sites). | |
| T4c | Multifocal (>5 sites). | ||
| aAdapted from O'Sullivan et al.,[3] Yoon et al.,[4] Raut et al.,[5] and Pollock et al.[6] | |
| bFor soft tissue sarcoma of the abdomen and thoracic visceral organs, N0 = no lymph node involvement or unknown lymph node status and N1 = lymph node involvement present. | |
| N0 | No regional lymph node metastasis or unknown lymph node status.b |
| N1 | Regional lymph node metastasis.b |
| aAdapted from O'Sullivan et al.,[3] Yoon et al.,[4] Raut et al.,[5] and Pollock et al.[6] | |
| bFor soft tissue sarcoma of the abdomen and thoracic visceral organs, M0 = no metastases and M1 = metastases present. | |
| M0 | No distant metastasis.b |
| M1 | Distant metastasis.b |
| Stage | T | N | M | Grade |
|---|---|---|---|---|
| aAdapted from Yoon et al. [4] and Pollock et al.[6] | ||||
| bStage IIIB for soft tissue sarcoma of the retroperitoneum; stage IV for soft tissue sarcoma of the trunk and extremities. | ||||
| IA | T1 | N0 | M0 | G1, GX |
| IB | T2, T3, T4 | N0 | M0 | G1, GX |
| II | T1 | N0 | M0 | G2, G3 |
| IIIA | T2 | N0 | M0 | G2, G3 |
| IIIB | T3, T4 | N0 | M0 | G2, G3 |
| IIIB/IVb | Any T | N1 | M0 | Any G |
| IV | Any T | Any N | M1 | Any G |
Soft Tissue Sarcoma Tumor Pathological Grading System
In most cases, accurate histopathologic classification alone of soft tissue sarcomas does not yield optimal information about their clinical behavior. Therefore, several histologic parameters are evaluated in the grading process, including the following:
- Degree of cellularity.
- Cellular pleomorphism.
- Mitotic activity.
- Degree of necrosis.
- Invasive growth.
This process is used to improve the correlation between histologic findings and clinical outcome.[9] In children, grading of soft tissue sarcoma is compromised by the good prognosis of certain tumors, such as infantile fibrosarcoma and hemangiopericytoma, which have a good prognosis in children younger than 4 years, and also angiomatoid fibrous histiocytoma and dermatofibrosarcoma protuberans, which may recur locally if incompletely excised, but usually do not metastasize.
Testing the validity of a grading system within the pediatric population is difficult because of the rarity of these neoplasms. In March 1986, the Pediatric Oncology Group (POG) conducted a prospective study on pediatric soft tissue sarcomas other than rhabdomyosarcoma and devised the POG grading system. Analysis of outcome for patients with localized soft tissue sarcomas other than rhabdomyosarcoma demonstrated that patients with grade 3 tumors fared significantly worse than those with grade 1 or grade 2 lesions. This finding suggests that this system can accurately predict the clinical behavior of nonrhabdomyosarcomatous soft tissue sarcoma.[9-11]
The grading systems developed by the POG and the French Federation of Comprehensive Cancer Centers (Fédération Nationale des Centres de Lutte Contre Le Cancer [FNCLCC]) Sarcoma Group are described below. These grading systems are being compared by the central review pathologists on the COG-ARST0332 study. The study has closed and results are pending.
POG grading system
The POG grading system is described below.[9] It is an older grading system of historical value that is no longer being used for treatment.
Grade I
Grade I lesions are based on histologic type, well-differentiated cytohistologic features, and/or age of the patient.
- Angiomatoid fibrous histiocytoma.
- Dermatofibrosarcoma protuberans.
- Liposarcoma–myxoid or well-differentiated.
- Myxoid chondrosarcoma.
- Well-differentiated malignant peripheral nerve sheath tumor.
- Well-differentiated or infantile (aged ≤4 years) fibrosarcoma.
- Well-differentiated or infantile (aged ≤4 years) hemangiopericytoma.
Grade II
Grade II lesions are soft tissue sarcomas not included in grade I or III by histologic diagnosis (with <5 mitoses/10 high-power fields or <15% necrosis):
- 15% or less of the surface area shows necrosis (primary criteria).
- The mitotic count is <5 mitotic figures per 10 high-power fields (40X objective) (primary criteria).
- Nuclear atypia is not marked (secondary criteria).
- The tumor is not markedly cellular (secondary criteria).
Grade III
Grade III lesions are similar to grade II lesions and include certain tumors known to be clinically aggressive by virtue of histologic diagnosis and non-grade I tumors (with >4 mitoses per 10 high-power fields or >15% necrosis):
- Alveolar soft part sarcoma.
- Extraskeletal osteogenic sarcoma.
- Malignant triton tumor.
- Mesenchymal chondrosarcoma.
- Pleomorphic or round-cell liposarcoma.
- Any other sarcoma not in grade I with >15% necrosis and/or ≥5 mitotic figures per 10 high-power fields (40X objective). Marked atypia and cellularity are less predictive but may assist in placing tumors in this category.
FNCLCC grading system
The FNCLCC histologic grading system was developed for adults with soft tissue sarcoma. The purpose of the grading system is to predict which patients will develop metastasis and subsequently benefit from postoperative chemotherapy.[12,13] The system is described in Table 7 and Table 8.
| FNCLCC = Fédération Nationale des Centres de Lutte Contre Le Cancer; HPF = high-power field. | |
| Tumor Differentiation | |
| Score 1 | Sarcoma closely resembling normal adult mesenchymal tissue (e.g., well-differentiated liposarcoma) |
| Score 2 | Sarcomas for which histologic typing is certain (e.g., myxoid liposarcoma) |
| Score 3 | Embryonal and undifferentiated sarcomas, sarcomas of doubtful type, and synovial sarcomas |
| Mitotic Count | |
| Score 1 | 0–9 mitoses per 10 HPF |
| Score 2 | 10–19 mitoses per 10 HPF |
| Score 3 | ≥20 mitoses per 10 HPF |
| Tumor Necrosis | |
| Score 0 | No necrosis |
| Score 1 | <50% tumor necrosis |
| Score 2 | ≥50% tumor necrosis |
| Total Score | Histologic Grade |
|---|---|
| 2–3 | Grade I |
| 4–5 | Grade II |
| 6–8 | Grade III |
Prognostic Significance of Tumor Grading
The POG and FNCLCC grading systems have proven to be of prognostic value in pediatric and adult nonrhabdomyosarcomatous soft tissue sarcomas.[14-18] In a study of 130 tumors from children and adolescents with nonrhabdomyosarcomatous soft tissue sarcoma enrolled in three prospective clinical trials, a correlation was found between the POG-assigned grade and the FNCLCC-assigned grade. However, grading did not correlate in all cases; 44 patients whose tumors received discrepant grades (POG grade 3, FNCLCC grade 1 or 2) had outcomes between concurrent grade 3 and grades 1 and 2. A mitotic index of 10 or greater emerged as an important prognostic factor.[19] The recently completed COG-ARST0332 trial will analyze data comparing the POG and FNCLCC pathologic grading systems to determine which system better correlates with clinical outcomes. The current open trial (ARST1321 [NCT02180867]) uses the FNCLCC system to assign histological grade.
References
- American Joint Committee on Cancer: AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer, 2002.
- Maurer HM, Beltangady M, Gehan EA, et al.: The Intergroup Rhabdomyosarcoma Study-I. A final report. Cancer 61 (2): 209-20, 1988. [PUBMED Abstract]
- O'Sullivan B, Maki RG, Agulnik M, et al.: Soft tissue sarcoma of the head and neck. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 499-505.
- Yoon SS, Maki RG, Asare EA, et al.: Soft tissue sarcoma of the trunk and extremities. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 507-15.
- Raut CP, Maki RG, Baldini EH, et al.: Soft tissue sarcoma of the abdomen and thoracic visceral organs. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 517-21.
- Pollock RE, Maki RG, Baldini EH, et al.: Soft tissue sarcoma of the retroperitoneum. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 531-7.
- Maki RG, Folpe AL, Guadagnolo BA, et al.: Soft tissue sarcoma - unusual histologies and sites. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp 539-45.
- Waxweiler TV, Rusthoven CG, Proper MS, et al.: Non-Rhabdomyosarcoma Soft Tissue Sarcomas in Children: A Surveillance, Epidemiology, and End Results Analysis Validating COG Risk Stratifications. Int J Radiat Oncol Biol Phys 92 (2): 339-48, 2015. [PUBMED Abstract]
- Parham DM, Webber BL, Jenkins JJ 3rd, et al.: Nonrhabdomyosarcomatous soft tissue sarcomas of childhood: formulation of a simplified system for grading. Mod Pathol 8 (7): 705-10, 1995. [PUBMED Abstract]
- Recommendations for the reporting of soft tissue sarcomas. Association of Directors of Anatomic and Surgical Pathology. Mod Pathol 11 (12): 1257-61, 1998. [PUBMED Abstract]
- Skytting B, Meis-Kindblom JM, Larsson O, et al.: Synovial sarcoma--identification of favorable and unfavorable histologic types: a Scandinavian sarcoma group study of 104 cases. Acta Orthop Scand 70 (6): 543-54, 1999. [PUBMED Abstract]
- Coindre JM, Terrier P, Guillou L, et al.: Predictive value of grade for metastasis development in the main histologic types of adult soft tissue sarcomas: a study of 1240 patients from the French Federation of Cancer Centers Sarcoma Group. Cancer 91 (10): 1914-26, 2001. [PUBMED Abstract]
- Guillou L, Coindre JM, Bonichon F, et al.: Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J Clin Oncol 15 (1): 350-62, 1997. [PUBMED Abstract]
- Rao BN: Nonrhabdomyosarcoma in children: prognostic factors influencing survival. Semin Surg Oncol 9 (6): 524-31, 1993 Nov-Dec. [PUBMED Abstract]
- Pisters PW, Leung DH, Woodruff J, et al.: Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 14 (5): 1679-89, 1996. [PUBMED Abstract]
- Coindre JM, Terrier P, Bui NB, et al.: Prognostic factors in adult patients with locally controlled soft tissue sarcoma. A study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol 14 (3): 869-77, 1996. [PUBMED Abstract]
- Pappo AS, Fontanesi J, Luo X, et al.: Synovial sarcoma in children and adolescents: the St Jude Children's Research Hospital experience. J Clin Oncol 12 (11): 2360-6, 1994. [PUBMED Abstract]
- Pratt CB, Maurer HM, Gieser P, et al.: Treatment of unresectable or metastatic pediatric soft tissue sarcomas with surgery, irradiation, and chemotherapy: a Pediatric Oncology Group study. Med Pediatr Oncol 30 (4): 201-9, 1998. [PUBMED Abstract]
- Khoury JD, Coffin CM, Spunt SL, et al.: Grading of nonrhabdomyosarcoma soft tissue sarcoma in children and adolescents: a comparison of parameters used for the Fédération Nationale des Centers de Lutte Contre le Cancer and Pediatric Oncology Group Systems. Cancer 116 (9): 2266-74, 2010. [PUBMED Abstract]
Treatment Option Overview for Childhood Soft Tissue Sarcoma
Because of the rarity of pediatric nonrhabdomyosarcomatous soft tissue sarcomas, coordination of treatment by a multidisciplinary team comprising oncologists (pediatric or medical), pathologists, surgeons, and radiation oncologists should be considered for all children, adolescents, and young adults with these tumors. In addition, to better define the tumors' natural history and response to therapy, entry into national or institutional treatment protocols should be considered for children with rare neoplasms. Information about ongoing clinical trials is available from the NCI website.
Surgery
After an appropriate biopsy and pathologic diagnosis, every attempt is made to resect the primary tumor with negative margins before or after chemotherapy and/or radiation therapy. Involvement of a surgeon with special expertise in the resection of soft tissue sarcomas in the decision is highly desirable.
The timing of surgery depends on an assessment of the feasibility and morbidity of surgery. If the initial operation fails to achieve pathologically negative tissue margins or if the initial surgery was done without the knowledge that cancer was present, a re-excision of the affected area is performed to obtain clear, but not necessarily wide, margins.[1-4] This surgical tenet is true even if no mass is detected by magnetic resonance imaging after initial surgery.[5]; [6][Level of evidence: 3iiA]
Regional lymph node metastases at diagnosis are unusual and are most often seen in patients with epithelioid and clear cell sarcomas.[7,8] Various institutional series have demonstrated the feasibility and effectiveness of sentinel node biopsy as a staging procedure in pediatric patients with soft tissue sarcomas.[9-14]
Radiation Therapy
Considerations for radiation therapy are based on the potential for surgery, with or without chemotherapy, to obtain local control without loss of critical organs or significant functional, cosmetic, or psychological impairment. This will vary according to the following:
- Patient variables (e.g., age and sex).
- Tumor variables (e.g., histopathology, site, size, and grade).
- Surgical margin status.
- Expectations for radiation-induced morbidities (e.g., impaired bone or muscle development, organ damage, or second malignancy).
Radiation therapy can be given preoperatively. Radiation field size and dose will be based on patient and tumor variables and the operability of the tumor. Preoperative radiation therapy has been associated with excellent local control rates.[15,16] This approach has the advantage of treating smaller tissue volumes because it does not necessitate treating a postsurgical bed; it also has the advantage of somewhat lower radiation doses because relative hypoxia from surgical disruption of vasculature and scarring is not present. Preoperative radiation therapy has been associated with an increased rate of wound complications in adults, primarily in lower extremity tumors, but the degree of this is questionable.[17] Conversely, preoperative radiation therapy may lead to less fibrosis than with postoperative approaches, perhaps due to the smaller treatment volume and dose.[18]
Retroperitoneal sarcomas are unique in that radiosensitivity of the bowel to injury makes postoperative radiation therapy less desirable.[19,20] Postoperative adhesions and bowel immobility can increase the risk of damage from any given radiation dose. This contrasts with the preoperative approach in which the tumor often displaces bowel outside of the radiation field, and any exposed bowel is more mobile, which decreases exposure to specific bowel segments.
Radiation therapy can also be given postoperatively. In general, radiation is indicated for patients with inadequate surgical margins and for larger, high-grade tumors.[21,22] This is particularly important in high-grade tumors with tumor margins smaller than 1 cm.[23,24]; [25][Level of evidence: 3iiDiv] With combined surgery and radiation therapy, local control of the primary tumor can be achieved in more than 80% of patients.[26,27]
Brachytherapy and intraoperative radiation may be applicable in select situations.[27-29]; [30][Level of evidence: 3iiiDii]
Radiation volume and dose depend on the patient, tumor, and surgical variables noted above, as well as the following:
- Patient age and growth potential.
- Ability to avoid critical organs, epiphyseal plates, and lymphatics (but not the neurovascular bundles that are relatively radiation tolerant).
- Functional/cosmetic outcome.
Radiation doses are typically 45 Gy to 50 Gy preoperatively, with consideration for postoperative boost of 10 Gy to 20 Gy if resection margins are microscopically or grossly positive, or planned brachytherapy if the resection is predicted to be subtotal. However, data documenting the efficacy of a postoperative boost are lacking.[31] The postoperative radiation dose is 55 Gy to 60 Gy, or rarely, higher when unresectable gross residual disease exists.
Radiation margins are typically 2 cm to 4 cm longitudinally and encompass fascial planes axially.[32,33]
Chemotherapy
The role of postoperative chemotherapy remains unclear as evidenced by the following studies:[34]
- A meta-analysis of data from all randomized trials of adults with soft tissue sarcoma concluded that recurrence-free survival was better with postoperative chemotherapy for patients with high-grade tumors larger than 5 cm.[35]
- In a European trial, adults with completely resected soft tissue sarcoma were randomly assigned to observation or postoperative chemotherapy with ifosfamide and doxorubicin. Postoperative chemotherapy was not associated with improved event-free survival (EFS) or overall survival (OS). It is difficult to extrapolate this trial to pediatric patients because the trial included 1) a wide variety of histologies; 2) a relatively low dose of ifosfamide; 3) patients assigned to chemotherapy had definitive radiation delayed until completion of chemotherapy; and 4) almost one-half of the patients in the trial had intermediate-grade tumors. In the discussion section, the authors merged their patients with previously published series, including those from the European meta-analysis, and concluded that the results suggested a benefit for postoperative chemotherapy.[36][Level of evidence: 1iiA]
- The largest prospective pediatric trial failed to demonstrate any benefit with postoperative vincristine, dactinomycin, cyclophosphamide, and doxorubicin.[26]
- Doxorubicin and ifosfamide were used in the risk-based COG ARST0332 (NCT00346164) trial. Although this was not a randomized study, results at 2.6 years show that patients with high-risk (>5 cm and high grade), grossly resected, nonmetastatic tumors who were treated with radiation therapy and postoperative doxorubicin and ifosfamide had a 3-year EFS of 68% and OS of 81%. In patients with metastatic disease treated with preoperative chemotherapy and radiation therapy, the estimated 3-year failure-free survival was 52% and OS was 66%.[37][Level of evidence: 3iiiA]
Targeted Therapy
The use of angiogenesis and mammalian target of rapamycin (mTOR) inhibitors has been explored in the treatment of adult soft tissue sarcomas but not in pediatrics.
- In a trial of 711 randomly assigned adult patients who achieved a response or stable disease after chemotherapy, the administration of ridaforolimus was associated with a 3-week improvement in progression-free survival (PFS) when compared with placebo.[38]
- In another trial of 371 randomly assigned adult patients with metastatic soft tissue sarcoma that progressed after chemotherapy, pazopanib was compared with placebo. The median PFS for the pazopanib arm was 4.6 months compared with 1.6 months for the placebo arm. OS was not different between the two arms.[39]
- In a randomized study of 182 previously treated adult patients with recurrent liposarcoma, leiomyosarcoma, synovial sarcoma, and other sarcomas, patients with nonadipocytic tumors who were treated with regorafenib had significant improvements in progression-free survival when compared with patients who were treated with placebo.[40]
Special Considerations for the Treatment of Children With Soft Tissue Sarcoma
Cancer in children and adolescents is rare, although the overall incidence of childhood cancer has been slowly increasing since 1975.[41] Children and adolescents with cancer should be referred to medical centers that have a multidisciplinary team of cancer specialists with experience treating the cancers that occur during childhood and adolescence. This multidisciplinary team approach incorporates the skills of the following health care professionals and others to ensure that children receive treatment, supportive care, and rehabilitation that will achieve optimal survival and quality of life:
- Primary care physicians.
- Pediatric surgical specialists.
- Pediatric radiation oncologists.
- Pediatric medical oncologists/hematologists.
- Rehabilitation specialists.
- Pediatric nurse specialists.
- Social workers.
- Child life professionals.
- Psychologists.
(Refer to the PDQ Supportive and Palliative Care summaries for specific information about supportive care for children and adolescents with cancer.)
Guidelines for pediatric cancer centers and their role in the treatment of pediatric patients with cancer have been outlined by the American Academy of Pediatrics.[42] At these pediatric cancer centers, clinical trials are available for most types of cancer that occur in children and adolescents, and the opportunity to participate in these trials is offered to most patients/families. Multidisciplinary evaluation in pediatric cancer centers that have surgical and radiotherapeutic expertise is of critical importance to ensure the best clinical outcome for these patients. Although surgery with or without radiation therapy can be curative for a significant proportion of patients, the addition of chemotherapy might benefit subsets of children with the disease; therefore, enrollment into clinical trials is encouraged. Clinical trials for children and adolescents with cancer are generally designed to compare potentially better therapy with therapy that is currently accepted as standard. Most of the progress made in identifying curative therapies for childhood cancers has been achieved through clinical trials. Information about ongoing clinical trials is available from the NCI website.
Many therapeutic strategies for children and adolescents with soft tissue tumors are similar to those for adult patients, although there are important differences. For example, the biology of the neoplasm in pediatric patients may differ dramatically from that of the adult lesion. Additionally, limb-sparing procedures are more difficult to perform in pediatric patients. The morbidity associated with radiation therapy, particularly in infants and young children, may be much greater than that observed in adults.[43]
Improved outcomes with multimodality therapy in adults and children with soft tissue sarcomas over the past 20 years has caused increasing concern about the potential long-term side effects of this therapy in children, especially when considering the expected longer life span of children versus adults. Therefore, to maximize tumor control and minimize long-term morbidity, treatment must be individualized for children and adolescents with nonrhabdomyosarcomatous soft tissue sarcoma. These patients should be enrolled in prospective studies that accurately assess any potential complications.[44]
References
- Sugiura H, Takahashi M, Katagiri H, et al.: Additional wide resection of malignant soft tissue tumors. Clin Orthop (394): 201-10, 2002. [PUBMED Abstract]
- Cecchetto G, Guglielmi M, Inserra A, et al.: Primary re-excision: the Italian experience in patients with localized soft-tissue sarcomas. Pediatr Surg Int 17 (7): 532-4, 2001. [PUBMED Abstract]
- Chui CH, Spunt SL, Liu T, et al.: Is reexcision in pediatric nonrhabdomyosarcoma soft tissue sarcoma necessary after an initial unplanned resection? J Pediatr Surg 37 (10): 1424-9, 2002. [PUBMED Abstract]
- Paulino AC, Ritchie J, Wen BC: The value of postoperative radiotherapy in childhood nonrhabdomyosarcoma soft tissue sarcoma. Pediatr Blood Cancer 43 (5): 587-93, 2004. [PUBMED Abstract]
- Kaste SC, Hill A, Conley L, et al.: Magnetic resonance imaging after incomplete resection of soft tissue sarcoma. Clin Orthop (397): 204-11, 2002. [PUBMED Abstract]
- Chandrasekar CR, Wafa H, Grimer RJ, et al.: The effect of an unplanned excision of a soft-tissue sarcoma on prognosis. J Bone Joint Surg Br 90 (2): 203-8, 2008. [PUBMED Abstract]
- Daigeler A, Kuhnen C, Moritz R, et al.: Lymph node metastases in soft tissue sarcomas: a single center analysis of 1,597 patients. Langenbecks Arch Surg 394 (2): 321-9, 2009. [PUBMED Abstract]
- Mazeron JJ, Suit HD: Lymph nodes as sites of metastases from sarcomas of soft tissue. Cancer 60 (8): 1800-8, 1987. [PUBMED Abstract]
- Neville HL, Andrassy RJ, Lally KP, et al.: Lymphatic mapping with sentinel node biopsy in pediatric patients. J Pediatr Surg 35 (6): 961-4, 2000. [PUBMED Abstract]
- Neville HL, Raney RB, Andrassy RJ, et al.: Multidisciplinary management of pediatric soft-tissue sarcoma. Oncology (Huntingt) 14 (10): 1471-81; discussion 1482-6, 1489-90, 2000. [PUBMED Abstract]
- Kayton ML, Delgado R, Busam K, et al.: Experience with 31 sentinel lymph node biopsies for sarcomas and carcinomas in pediatric patients. Cancer 112 (9): 2052-9, 2008. [PUBMED Abstract]
- Dall'Igna P, De Corti F, Alaggio R, et al.: Sentinel node biopsy in pediatric patients: the experience in a single institution. Eur J Pediatr Surg 24 (6): 482-7, 2014. [PUBMED Abstract]
- Parida L, Morrisson GT, Shammas A, et al.: Role of lymphoscintigraphy and sentinel lymph node biopsy in the management of pediatric melanoma and sarcoma. Pediatr Surg Int 28 (6): 571-8, 2012. [PUBMED Abstract]
- Alcorn KM, Deans KJ, Congeni A, et al.: Sentinel lymph node biopsy in pediatric soft tissue sarcoma patients: utility and concordance with imaging. J Pediatr Surg 48 (9): 1903-6, 2013. [PUBMED Abstract]
- Virkus WW, Mollabashy A, Reith JD, et al.: Preoperative radiotherapy in the treatment of soft tissue sarcomas. Clin Orthop (397): 177-89, 2002. [PUBMED Abstract]
- Zagars GK, Ballo MT, Pisters PW, et al.: Preoperative vs. postoperative radiation therapy for soft tissue sarcoma: a retrospective comparative evaluation of disease outcome. Int J Radiat Oncol Biol Phys 56 (2): 482-8, 2003. [PUBMED Abstract]
- O'Sullivan B, Davis AM, Turcotte R, et al.: Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359 (9325): 2235-41, 2002. [PUBMED Abstract]
- Davis AM, O'Sullivan B, Turcotte R, et al.: Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 75 (1): 48-53, 2005. [PUBMED Abstract]
- Baldini EH, Wang D, Haas RL, et al.: Treatment Guidelines for Preoperative Radiation Therapy for Retroperitoneal Sarcoma: Preliminary Consensus of an International Expert Panel. Int J Radiat Oncol Biol Phys 92 (3): 602-12, 2015. [PUBMED Abstract]
- Bishop AJ, Zagars GK, Torres KE, et al.: Combined Modality Management of Retroperitoneal Sarcomas: A Single-Institution Series of 121 Patients. Int J Radiat Oncol Biol Phys 93 (1): 158-65, 2015. [PUBMED Abstract]
- Marcus KC, Grier HE, Shamberger RC, et al.: Childhood soft tissue sarcoma: a 20-year experience. J Pediatr 131 (4): 603-7, 1997. [PUBMED Abstract]
- Delaney TF, Kepka L, Goldberg SI, et al.: Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys 67 (5): 1460-9, 2007. [PUBMED Abstract]
- Blakely ML, Spurbeck WW, Pappo AS, et al.: The impact of margin of resection on outcome in pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Pediatr Surg 34 (5): 672-5, 1999. [PUBMED Abstract]
- Skytting B: Synovial sarcoma. A Scandinavian Sarcoma Group project. Acta Orthop Scand Suppl 291: 1-28, 2000. [PUBMED Abstract]
- Hua C, Gray JM, Merchant TE, et al.: Treatment planning and delivery of external beam radiotherapy for pediatric sarcoma: the St. Jude Children's Research Hospital experience. Int J Radiat Oncol Biol Phys 70 (5): 1598-606, 2008. [PUBMED Abstract]
- Pratt CB, Pappo AS, Gieser P, et al.: Role of adjuvant chemotherapy in the treatment of surgically resected pediatric nonrhabdomyosarcomatous soft tissue sarcomas: A Pediatric Oncology Group Study. J Clin Oncol 17 (4): 1219, 1999. [PUBMED Abstract]
- Merchant TE, Parsh N, del Valle PL, et al.: Brachytherapy for pediatric soft-tissue sarcoma. Int J Radiat Oncol Biol Phys 46 (2): 427-32, 2000. [PUBMED Abstract]
- Schomberg PJ, Gunderson LL, Moir CR, et al.: Intraoperative electron irradiation in the management of pediatric malignancies. Cancer 79 (11): 2251-6, 1997. [PUBMED Abstract]
- Nag S, Shasha D, Janjan N, et al.: The American Brachytherapy Society recommendations for brachytherapy of soft tissue sarcomas. Int J Radiat Oncol Biol Phys 49 (4): 1033-43, 2001. [PUBMED Abstract]
- Viani GA, Novaes PE, Jacinto AA, et al.: High-dose-rate brachytherapy for soft tissue sarcoma in children: a single institution experience. Radiat Oncol 3: 9, 2008. [PUBMED Abstract]
- Al Yami A, Griffin AM, Ferguson PC, et al.: Positive surgical margins in soft tissue sarcoma treated with preoperative radiation: is a postoperative boost necessary? Int J Radiat Oncol Biol Phys 77 (4): 1191-7, 2010. [PUBMED Abstract]
- Wang D, Bosch W, Kirsch DG, et al.: Variation in the gross tumor volume and clinical target volume for preoperative radiotherapy of primary large high-grade soft tissue sarcoma of the extremity among RTOG sarcoma radiation oncologists. Int J Radiat Oncol Biol Phys 81 (5): e775-80, 2011. [PUBMED Abstract]
- Bahig H, Roberge D, Bosch W, et al.: Agreement among RTOG sarcoma radiation oncologists in contouring suspicious peritumoral edema for preoperative radiation therapy of soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys 86 (2): 298-303, 2013. [PUBMED Abstract]
- Ferrari A: Role of chemotherapy in pediatric nonrhabdomyosarcoma soft-tissue sarcomas. Expert Rev Anticancer Ther 8 (6): 929-38, 2008. [PUBMED Abstract]
- Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet 350 (9092): 1647-54, 1997. [PUBMED Abstract]
- Woll PJ, Reichardt P, Le Cesne A, et al.: Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol 13 (10): 1045-54, 2012. [PUBMED Abstract]
- Spunt SL, Million L, Anderson JR, et al.: Risk-based treatment for nonrhabdomyosarcoma soft tissue sarcomas (NRSTS) in patients under 30 years of age: Children’s Oncology Group study ARST0332. [Abstract] J Clin Oncol 32 (Suppl 15): A-10008, 2014. Also available online. Last accessed April 02, 2018.
- Demetri GD, Chawla SP, Ray-Coquard I, et al.: Results of an international randomized phase III trial of the mammalian target of rapamycin inhibitor ridaforolimus versus placebo to control metastatic sarcomas in patients after benefit from prior chemotherapy. J Clin Oncol 31 (19): 2485-92, 2013. [PUBMED Abstract]
- van der Graaf WT, Blay JY, Chawla SP, et al.: Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 379 (9829): 1879-86, 2012. [PUBMED Abstract]
- Mir O, Brodowicz T, Italiano A, et al.: Safety and efficacy of regorafenib in patients with advanced soft tissue sarcoma (REGOSARC): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 17 (12): 1732-1742, 2016. [PUBMED Abstract]
- Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014. [PUBMED Abstract]
- Corrigan JJ, Feig SA; American Academy of Pediatrics: Guidelines for pediatric cancer centers. Pediatrics 113 (6): 1833-5, 2004. [PUBMED Abstract]
- Suit H, Spiro I: Radiation as a therapeutic modality in sarcomas of the soft tissue. Hematol Oncol Clin North Am 9 (4): 733-46, 1995. [PUBMED Abstract]
- Spunt SL, Million L, Coffin C: The nonrhabdomyosarcoma soft tissue sarcoma. In: Pizzo PA, Poplack DG, eds.: Principles and Practice of Pediatric Oncology. 7th ed. Philadelphia, Pa: Lippincott Williams and Wilkins, 2015, pp 827-54.
Treatment of Newly Diagnosed Childhood Soft Tissue Sarcoma
Adipocytic Tumors
Liposarcoma
Liposarcoma accounts for 3% of soft tissue sarcoma in patients younger than 20 years (refer to Table 1).
Liposarcoma is rare in the pediatric population. In a review of 182 pediatric patients with adult-type sarcomas, only 14 had a diagnosis of liposarcoma.[1] One retrospective study identified 34 patients younger than 22 years from 1960 to 2011.[2] There were roughly equal numbers of male and female patients and the median age was 18 years. In an international clinicopathological review, the characteristics of 82 cases of pediatric liposarcoma were reported. The median age was 15.5 years and females were more commonly affected.[3] In both reports, the great majority of patients had myxoid liposarcoma.
Histopathologic classification
The World Health Organization (WHO) classification for liposarcoma is as follows:
- Intermediate grade (rarely metastasizing).
- Atypical lipomatous neoplasm/well-differentiated liposarcoma. These tumors do not metastasize unless they undergo dedifferentiation.
- Malignant.
- Liposarcoma, not otherwise specified (NOS).
- Myxoid liposarcoma. Pure myxoid liposarcomas are characterized by a t(12;16)(q13;p11) translocation and can metastasize but usually have an excellent outcome in the absence of a round cell component.[4]
- Dedifferentiated liposarcoma.
- Pleomorphic liposarcoma.
Clinical presentation
The majority of liposarcomas in the pediatric and adolescent age range are low grade and located subcutaneously. Metastasis to lymph nodes is very uncommon, and the great majority of metastases are pulmonary. Tumors arising in the periphery are more likely to be low grade and myxoid. Tumors arising centrally are more likely to be high grade, pleomorphic, and present with metastasis or recur with metastasis.
Prognosis
Higher grade or central tumors are associated with a significantly higher risk of death. In a retrospective review, 5-year survival for central tumors was 42%. In the international review, seven of ten patients with pleomorphic myxoid liposarcoma died because of their disease.[3] In a retrospective study of 14 patients, 5-year survival was 78% and tumor grade, histologic subtype, and primary location correlated with survival.[2]
Treatment
Treatment options for liposarcoma include the following:
Surgery is the most important treatment for liposarcoma. After surgical resection of myxoid liposarcoma, event-free survival (EFS) and overall survival (OS) are roughly 90%. If initial surgery is incomplete, re-excision should be performed to achieve a wide margin of resection. Local recurrences have been seen and are controlled with a second resection of the tumor.
There are reports of the use of chemotherapy to decrease the size of liposarcoma before surgery to facilitate complete resection, particularly in central tumors.[10,11] The role of postoperative chemotherapy for liposarcoma is poorly defined. There does not appear to be a need for any postoperative therapy for completely resected myxoid liposarcoma. Even with the use of postoperative chemotherapy, the survival of pleomorphic liposarcoma remains poor.[12]
Trabectedin has produced encouraging responses in adults with advanced myxoid liposarcoma.[13] In one study, adult patients with recurrent liposarcoma and leiomyosarcoma were randomly assigned to treatment with either trabectedin or dacarbazine. Patients treated with trabectedin had a 45% reduction in disease progression.[14][Level of evidence: 1iiDiii] There are very limited data to support the use of trabectedin in pediatric patients.[15]
Treatment options under clinical evaluation
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma, excluding myxoid liposarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with liposarcoma are eligible for this trial.
Chondro-osseous Tumors
Chondro-osseous tumors include the following tumor subtypes:
- Extraskeletal mesenchymal chondrosarcoma.
- Extraskeletal osteosarcoma.
- Soft tissue chondroma.
Extraskeletal mesenchymal chondrosarcoma
Osseous and chondromatous neoplasms account for 0.8% of soft tissue sarcoma in patients younger than 20 years (refer to Table 1).
Histopathology and molecular features
Mesenchymal chondrosarcoma is a rare tumor characterized by small round cells and hyaline cartilage that more commonly affects young adults and has a predilection for involving the head and neck region.
Mesenchymal chondrosarcoma has been associated with consistent chromosomal rearrangement. A retrospective analysis of cases of mesenchymal chondrosarcoma identified a HEY1-NCOA2 fusion in 10 of 15 tested specimens.[16] This gene fusion was not associated with chromosomal changes that could be detected by karyotyping. In one instance, translocation t(1;5)(q42;q32) was identified in a case of mesenchymal chondrosarcoma and shown to be associated with a novel IRF2BP-CDX1 fusion gene.[17]
Prognosis
A retrospective survey of European institutions identified 113 children and adults with mesenchymal chondrosarcoma. Factors associated with better outcome included the following:[18][Level of evidence: 3iiiA]
- Lack of metastatic disease at initial presentation.
- Clear resection margins.
- Administration of postoperative chemotherapy following resection for patients with initially localized disease.
Treatment
Treatment options for extraskeletal mesenchymal chondrosarcoma include the following:
A review of 15 patients younger than 26 years from the German Cooperative Soft Tissue Sarcoma Study Group (11 with soft-tissue lesions) and the German-Austrian-Swiss Cooperative Osteosarcoma Study Group (four with primary bone lesions) protocols suggests that complete surgical removal, or incomplete resection followed by radiation therapy, is necessary for local control.[19][Level of evidence: 3iiA]
A single-institution, retrospective review identified 12 pediatric patients with mesenchymal chondrosarcoma.[20] The presence of the NCOA2 rearrangement in tumors was documented in these patients. It was also confirmed that surgical resection is necessary for cure. Eleven patients presented with localized disease and one presented with pulmonary nodules. All patients received chemotherapy—six patients before and after surgical resection and six patients only after resection. All patients received postoperative chemotherapy (most commonly ifosfamide/doxorubicin) with or without radiation therapy (median dose, 59.4 Gy). At a median follow-up of 4.8 years, 5-year disease-free survival (DFS) was 68.2% (95% CI, 39.8%–96.6%) and OS was 88.9% (95% CI, 66.9%–100%).
Extraskeletal osteosarcoma
Osseous and chondromatous neoplasms account for 0.8% of soft tissue sarcomas in patients younger than 20 years (refer to Table 1).
Extraskeletal osteosarcoma is extremely rare in the pediatric and adolescent age range. A 2003 review identified only ten case reports in the medical literature.[21]
Prognosis
Extraskeletal osteosarcoma is associated with a high risk of local recurrence and pulmonary metastasis.[22]
Treatment
Treatment options for extraskeletal osteosarcoma include the following:
- Surgery followed by chemotherapy.
(Refer to the PDQ summary on Osteosarcoma and Malignant Fibrous Histiocytoma of Bone Treatment for more information.)
Treatment options under clinical evaluation
Information about National Cancer Institute NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with extraskeletal mesenchymal chondrosarcoma and extraskeletal osteosarcoma are eligible for this trial.
Fibroblastic/Myofibroblastic Tumors
Fibroblastic/myofibroblastic tumors include the following tumor subtypes:
- Fibroblastic/myofibroblastic tumors.
- Intermediate grade (locally aggressive).
- Desmoid-type fibromatosis (previously called desmoid tumor or aggressive fibromatoses).
- Giant cell fibroblastoma.
- Lipofibromatosis.
- Palmar/plantar fibromatosis.
- Intermediate grade (rarely metastasizing).
- Dermatofibrosarcoma protuberans.
- Infantile fibrosarcoma.
- Inflammatory myofibroblastic tumor.
- Low-grade myofibroblastic sarcoma.
- Myxoinflammatory fibroblastic sarcoma.
- Solitary fibrous tumor.
- Malignant.
- Intermediate grade (locally aggressive).
Desmoid-type fibromatosis
Desmoid-type fibromatosis has previously been called desmoid tumors or aggressive fibromatoses.
Risk factors
A small number of desmoid-type fibromatosis tumors may occur in association with a mutation in the adenomatous polyposis coli (APC) gene (associated with intestinal polyps and a high incidence of colon cancer). In a study of 519 patients older than 10 years with a diagnosis of desmoid-type fibromatosis, 39 (7.5%, a possible underestimation) were found to have familial adenomatous polyposis (FAP).[23] The patients with FAP and desmoid-type fibromatosis were younger, more often male, and had more abdominal wall or mesenteric tumors than did patients with desmoid-type fibromatosis without FAP.
A family history of colon cancer, the presence of congenital hyperplasia of the retinal pigment epithelium,[24,25] or location of the desmoid-type fibromatosis in the abdomen or abdominal wall [23] should prompt referral to a genetic counselor. Currently, there are no general recommendations for genetic testing in children with desmoid-type fibromatosis. Pathology and molecular characteristics of the tumor only provide guidance for screening. If the tumor has a somatic CTNNB1 mutation, screening is not necessary, because the APC gene mutation has not been described in this setting. If a CTNNB1 mutation is not identified, screening for the APC mutation may be warranted.[26,27] (Refer to the Familial Adenomatous Polyposis (FAP) section of the PDQ summary on Genetics of Colorectal Cancer for more information.)
Prognosis
Desmoid-type fibromatosis has an extremely low potential to metastasize. The tumors are locally infiltrating, and surgical control can be difficult because of the need to preserve normal structures.
These tumors have a high potential for local recurrence. Desmoid-type fibromatosis has a highly variable natural history, including well documented examples of spontaneous regression.[28] Mutations in exon 3 of the beta-catenin gene are seen in over 80% of desmoid-type fibromatosis and the mutation 45F has been associated with an increased risk of disease recurrence.[29] Repeated surgical resection can sometimes bring recurrent lesions under control.[30]
Treatment
Evaluation of the benefit of interventions for treatment of desmoid-type fibromatosis has been extremely difficult, because desmoid-type fibromatosis has a highly variable natural history. Large adult series and smaller pediatric series have reported long periods of disease stabilization and even regression without systemic therapy.[30,31]; [32][Level of evidence: 3iiiDi]
Treatment options for desmoid-type fibromatosis include the following:
- Surgery.
- Observation, for tumors that are incompletely resected or recurrent that do not pose a danger to vital organs, if other treatment options are not available.[30,33-39] Whenever possible, however, the treatment of choice is complete resection.
- Chemotherapy, for unresectable or recurrent tumors.
- Other drug therapy, such as nonsteroidal anti-inflammatory drugs (NSAIDs) or antiestrogen therapy.
- Surgery preceded or followed by radiation therapy, for incompletely resected tumors or to avoid recurrence and subsequent surgery that may result in functional or cosmetic compromise.
- Radiation therapy alone, for unresectable tumors.
The treatment of choice is resection to achieve clear margins. However, a retrospective review of children who underwent surgery for desmoid-type fibromatosis at the St. Jude Children’s Research Hospital (SJCRH) reported no correlation between surgical margins and risk of recurrence.[39]
When the diagnosis is known and complete surgical excision is not feasible, and if the tumor poses significant potential for mortality or morbidity, preoperative strategies may include the following:[40,41]
- Observation.
- Chemotherapy.
- Anti-estrogen therapy.
- NSAID therapy.
- External-beam radiation therapy.
Desmoid-type fibromatosis often behaves in a nonaggressive manner. In a study that included mostly adults with extra-abdominal primary fibromatosis, nonsurgical approaches (medical and observation) had similar 3-year EFS compared with surgery.[34] In a subsequent study of adolescents and adults with abdominal wall aggressive fibromatosis, 102 patients were treated with a watch and wait approach, of which 65 patients required no further treatment at 3 years. Approximately one-third of patients had regression of the tumor.[33]
Chemotherapy regimens may include the following:
- Combination chemotherapy using vinblastine and methotrexate produced objective responses in about one-third of patients with unresectable or recurrent desmoid-type fibromatosis.[40]
- A series of mainly adult patients with FAP and unresectable desmoid-type fibromatosis that were unresponsive to hormone therapy showed that doxorubicin plus dacarbazine followed by meloxicam (an NSAID) can be safely administered and can induce responses.[42]
- Pegylated liposomal doxorubicin has been used with some responses.[43] In a series of five patients, a median progression-free interval of 29 months was reported.[44]
- Tyrosine kinase inhibitors: A small retrospective study of adults with desmoid-type fibromatosis showed objective responses to the multi-targeted kinase inhibitor sorafenib.[45][Level of evidence: 3iiiDiv] Previous studies with imatinib did not support its use.[46,47] A small series reported symptomatic improvement and stable disease in seven patients with desmoid-type fibromatosis who were treated with pazopanib.[48]
- The NOTCH pathway has been implicated in the development of desmoid tumors.[49] Partial responses to the gamma secretase inhibitor PF-03084014 have been noted in adults with desmoid-type fibromatosis.[50][Level of evidence: 3iiiDiv]
- Hydroxyurea has been used successfully to treat a few patients after other treatments, but more data are needed.[51-53]
Other drug therapy may include the following:
- NSAIDs such as sulindac have been used in single cases for desmoid-type fibromatosis; the responses seen were usually disease stabilization.[54]
- Antiestrogen treatment, usually tamoxifen, plus sulindac has also resulted in disease stabilization.[55] A prospective trial of the combination of tamoxifen and sulindac reported few side effects, although asymptomatic ovarian cysts were common in girls. This combination showed relatively little activity, as measured by rates of response and progression-free survival (PFS).[56][Level of evidence: 2Diii]
Postoperative radiation therapy is a consideration when progression would entail additional surgery that might cause functional or cosmetic compromise and if radiation is considered acceptable in terms of morbidities.
Radiation has been used for unresectable desmoid-type fibromatosis or postoperatively for tumors with inadequate resections. The potential long-term complications of radiation therapy, especially subsequent neoplasms, make using this modality less appealing in a young population.[57]
Dermatofibrosarcoma protuberans
Dermatofibrosarcoma is a rare tumor that can be present in all age groups, but many of the reported cases arise in children.[58-60] A review of 451 cases in children younger than 20 years in the SEER database found that the incidence was 1 case per 1 million, highest among black patients aged 15 to 19 years. The most common sites were trunk and extremities, which is similar to what is found in adults. Ninety-five percent of patients underwent surgery. OS was 100% at 5 years, 98% at 15 years, and 97% at 30 years. Males had decreased survival compared with females (P < .05).[61][Level of evidence: 3iA]
Molecular features
The tumor has a consistent chromosomal translocation t(17;22)(q22;q13) that juxtaposes the COL1A1 gene with the PDGF-beta gene.
Treatment
Treatment of dermatofibrosarcoma protuberans includes the following:
- Surgery.
- Surgery preceded or followed by radiation therapy.
- Radiation therapy and imatinib therapy, for unresectable or recurrent tumors.
Most dermatofibrosarcoma tumors can be cured by complete surgical resection. Wide excision with negative margins or Mohs or modified Mohs surgery will prevent most tumors from recurring.[62] Despite the locally aggressive behavior of the tumor, lymph node or visceral metastasis rarely occurs.
In retrospective reviews, postoperative radiation therapy after incomplete excision may have decreased the likelihood of recurrence.[63,64]
When surgical resection cannot be accomplished or the tumor is recurrent, treatment with imatinib has been effective.[65-67] Because metastatic disease is more likely after multiple recurrences, radiation or other adjuvant therapy should be considered in patients with recurrence that cannot be managed surgically.[59,61]
Guidelines for workup and management of dermatofibrosarcoma protuberans have been published.[68]
Infantile fibrosarcoma
There are two distinct types of fibrosarcoma in children and adolescents: infantile fibrosarcoma (also called congenital fibrosarcoma) and fibrosarcoma that is indistinguishable from fibrosarcoma seen in adults. These are two distinct pathologic diagnoses and require different treatments. Adult-type fibrosarcoma is addressed below.
Infantile fibrosarcoma usually occurs in children younger than 1 year. It occasionally occurs in children up to age 4 years. A tumor with similar morphology has been identified in older children; in these older children, the tumors do not have the t(12;15)(ETV-NTRK3) translocation that is characteristic of the younger patients.[69] In several of these patients, BRAF gene fusions have been identified.
Clinical presentation
Infantile fibrosarcoma usually presents with a rapidly growing mass, often noted at birth or even seen in prenatal ultrasound. The tumors are often quite large at the time of presentation.[70]
Molecular features
The tumor usually has a characteristic cytogenetic translocation t(12;15)(ETV-NTRK3). Infantile fibrosarcoma shares this translocation and a virtually identical histologic appearance with mesoblastic nephroma.
Prognosis
These tumors have a low incidence of metastases at diagnosis.
Treatment
Treatment options for infantile fibrosarcoma include the following:
- Surgery followed by observation.
- Surgery followed by chemotherapy.
- Chemotherapy followed by surgery.
Complete resection is curative in the majority of patients with infantile fibrosarcoma. However, the large size of the lesion frequently makes resection without major functional consequences impossible (for instance, tumors of the extremities often require amputation for complete excision). The European pediatric group has reported that observation may also be an option in patients with group II disease after surgery.[71] Twelve patients with group II disease received no further therapy and two patients relapsed. One patient obtained a complete remission after chemotherapy. Postoperative chemotherapy was administered to patients with higher group disease and those who progressed. In a subsequent study, only one of seven patients with group II disease progressed during observation; that patient achieved complete remission with chemotherapy.[72][Level of evidence: 3iiA]
Preoperative chemotherapy has made a more conservative surgical approach possible; agents active in this setting include vincristine, dactinomycin, cyclophosphamide, and ifosfamide.[73,74]; [72,75][Level of evidence: 3iiA]; [76][Level of evidence: 3iiB]
Three studies of patients with infantile fibrosarcoma suggest that an alkylator-free regimen is effective and should be used as the first treatment choice in patients with macroscopic disease.[71,72,77] Two cases with variant LMNA/NTRK1 fusions responded to crizotinib.[78,79]
A pediatric patient (aged 16 months) with refractory infantile fibrosarcoma with constitutive activation of the tropomyosin-related kinase signaling pathway from an ETS variant gene 6–neurotrophin 3 receptor gene fusion (ETV6-NTRK3) responded to LOXO-101, with a 90% reduction in tumor size after 2 months of treatment.[80]
A patient aged 2 months with infantile fibrosarcoma was initially treated with chemotherapy. At disease progression, a response was seen with pazopanib.[81]
A rare case of spontaneous regression without treatment has been reported.[82][Level of evidence: 3iiiDiv]
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- LOXO-TRK-15003 (NCT02637687) (Oral TRK Inhibitor LOXO-101 for Treatment of Advanced Pediatric Solid or Primary Central Nervous System [CNS] Tumors): A phase I trial of the pan-TRK inhibitor LOXO-101 is being conducted for children with solid tumors or brain tumors whose disease has progressed or was nonresponsive to available therapies, and for which no standard or available curative therapy exists. LOXO-101 is a highly selective inhibitor of all three TRK family kinases.
- RXDX-101-03 (NCT02650401) (Study of RXDX-101 in Children With Recurrent or Refractory Solid Tumors and Primary CNS Tumors): This is a four-part, open-label, phase I/Ib, dose-escalation study in pediatric patients with: 1) relapsed or refractory solid tumors; 2) primary CNS tumors; 3) neuroblastoma; and 4) non-neuroblastoma, extracranial solid tumors with NTRK1/2/3, ROS1 or ALK gene rearrangements. The study is designed to explore the safety, maximum tolerated dose or recommended phase II dose, pharmacokinetics, and antitumor activity of entrectinib (RXDX-101).
Inflammatory myofibroblastic tumor
Inflammatory myofibroblastic tumor is a rare mesenchymal tumor that has a predilection for children and adolescents.[83-85]
Clinical presentation
Inflammatory myofibroblastic tumors are rare tumors that affect soft tissues and visceral organs of children and young adults.[86] They rarely metastasize but tend to be locally invasive. Usual anatomical sites of disease include soft tissue, lungs, spleen, colon, and breast.[83] A review of 42 cases of pediatric inflammatory myofibroblastic tumor of the bladder was published in 2015.[87]
Molecular features
Roughly half of inflammatory myofibroblastic tumors exhibit a clonal mutation that activates the anaplastic lymphoma kinase (ALK)-receptor tyrosine kinase gene at chromosome 2p23.[88] ROS1 and PDGFR-beta kinase fusions have been identified in 8 of 11 cases (73%) who are negative for ALK by immunohistochemistry.[89][Level of evidence: 3iiiDiv]
Prognosis
Inflammatory myofibroblastic tumor recurs frequently but is rarely metastatic.[83-85]
Treatment
Treatment options for inflammatory myofibroblastic tumor include the following:
- Surgery.
- Chemotherapy.
- Steroid therapy.
- NSAID therapy.
- Targeted therapy (ALK inhibitors).
Complete surgical removal, when feasible, is the mainstay of therapy.[90] In a series of nine patients, four patients achieved continuous remission after complete resection, three patients with residual disease recurred but later achieved continuous remission, and one patient with metastatic disease responded to multiagent chemotherapy.[91][Level of evidence: 3iiA] The benefit of chemotherapy has been noted in case reports.[92] There are case reports of response to either steroids or NSAIDs.[93,94] A series of 32 patients aged 18 years and younger found that complete excision was the mainstay of therapy, although some patients were treated with steroids or cytotoxic chemotherapy. OS was 94%; three patients relapsed and two of them died of the disease. With complete excision, with or without other treatments such as steroids, there was a high survival rate for patients with this disease.[95][Level of evidence: 3iiA]
Inflammatory myofibroblastic tumors respond to crizotinib. Two adults with ALK-rearranged inflammatory myofibroblastic tumor achieved partial response with crizotinib.[96][Level of evidence: 3iiiDiv] For pediatric patients with measurable disease, the use of crizotinib achieved partial tumor responses in three of six patients with ALK-translocated inflammatory myofibroblastic tumors.[97] A case report of a patient aged 16 years with metastatic/multifocal ALK-positive inflammatory myofibroblastic tumor demonstrated a complete response and a 3-year disease-free interval with crizotinib therapy.[98] In a phase I trial of ceritinib for adult patients previously treated with ALK inhibitors, one patient with inflammatory myofibroblastic tumor had a partial response.[99] Finally, one study included 14 patients with inflammatory myofibroblastic tumor who were treated with crizotinib. With crizotinib therapy, five patients had a complete response, seven had a partial response, and the remaining two had stable disease; no patient had relapsed at the time the article was published.[100][Level of evidence: 3iiDiv]
Adult-type fibrosarcoma
These tumors lack the translocation seen in infantile fibrosarcomas. They present like the great majority of nonrhabdomyosarcomas and the management approach is similar.
Low-grade fibromyxoid sarcoma
Low-grade fibromyxoid sarcoma is a histologically deceptive soft tissue neoplasm that most commonly affects young and middle-aged adults, is commonly located deep within the extremities, and is characterized by a FUS/CREB3L3 translocation.[101,102]
Prognosis
In a review of 33 patients (three were younger than 18 years) with low grade fibromyxoid sarcoma, 21 of 33 patients developed a local recurrence after intervals of up to 15 years (median, 3.5 years) and 15 developed metastases up to 45 years (median, 5 years) from diagnosis, most commonly to the lungs and pleura, emphasizing the need for continued follow-up of these patients.[101] Even after metastases occur, the course may be indolent.[103]
In another report, 14 of 73 cases were younger than 18 years of age. In this series with a relatively short follow up (median of 24 months), only 8 of 54 patients with adequate follow up developed local (9%) or distant (6%) recurrence. This report suggests that the behavior of this tumor might be significantly better than previously reported.[104] However, because of the occurrence of late metastases, careful monitoring of these patients is warranted.
The most recent Children's Oncology Group (COG) trial (ARST0332 [NCT00346164]) enrolled 11 patients with this tumor entity. The median age at diagnosis was 13 years and males were more commonly affected. The most common sites were the lower and upper extremity (n = 9) and none of the patients had developed local or distant disease recurrence at a median follow up of 2.7 years.[105]
Treatment
Treatment options for low-grade fibromyxoid sarcoma include the following:
- Surgery.
The limited treatment information for low-grade fibromyxoid sarcoma suggest that surgery is the treatment of choice as the tumor is not very chemosensitive.[103] There are little data regarding the use of chemotherapy and/or radiation therapy in this disease. One report suggests that trabectedin may be effective in the treatment of low-grade fibromyxoid sarcoma.[106]
Myxofibrosarcoma
Myxofibrosarcoma is a rare lesion, especially in childhood. It is typically treated with complete surgical resection.
Sclerosing epithelioid fibrosarcoma
Sclerosing epithelioid fibrosarcoma is a rare malignant sarcoma that commonly harbors EWSR1 gene rearrangements and has an aggressive clinical course.[107] It is typically treated with complete surgical excision. Long-term follow-up is recommended because local recurrence and metastases can occur late.
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with infantile fibrosarcoma, inflammatory myofibroblastic tumor, low-grade myofibroblastic tumor, myxoinflammatory fibroblastic sarcoma, solitary fibrous tumor, adult-type fibrosarcoma, low-grade fibromyxoid sarcoma, myxofibrosarcoma, and sclerosing epithelioid fibrosarcoma are eligible for this trial.
Skeletal Muscle Tumors
Rhabdomyosarcoma
Refer to the PDQ summary on Childhood Rhabdomyosarcoma Treatment for more information.
Smooth Muscle Tumors
Leiomyosarcoma
Leiomyosarcoma accounts for 2% of soft tissue sarcoma in patients younger than 20 years (refer to Table 1).
Risk factors
Among 43 children with HIV/AIDS who developed tumors, eight developed Epstein-Barr virus–associated leiomyosarcoma.[108] Survivors of hereditary retinoblastoma have a statistically significant increased risk of developing leiomyosarcoma and 78% of these were diagnosed 30 or more years after the initial diagnosis of retinoblastoma.[109]
Treatment
Treatment options for leiomyosarcoma include the following:
- Chemotherapy (trabectedin).
In an open-label study of trabectedin in adult patients with recurrent sarcomas, the best overall response rate (complete remission and partial remission) was seen in patients with leiomyosarcoma (7.5%).[110] The clinical benefit rate (includes stable disease) for leiomyosarcoma was 54%. In another adult study, patients with recurrent liposarcoma and leiomyosarcoma were randomly assigned to receive treatment with either trabectedin or dacarbazine. Patients treated with trabectedin had a 45% reduction in disease progression.[14] There are no data to support the use of trabectedin in pediatric patients.
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with leiomyosarcoma are eligible for this trial.
So-called Fibrohistiocytic Tumors
So-called fibrohistiocytic tumors include the following tumor subtypes:
- Giant cell tumor of soft tissue.
- Plexiform fibrohistiocytic tumor.
Plexiform fibrohistiocytic tumor
Plexiform histiocytic tumor is a rare, low- to intermediate-grade tumor that most commonly affects children and young adults. Depending on the series, the median age at presentation ranges from 8 to 14.5 years; however, the tumor has been described in patients as young as 3 months.[111,112]
Clinical presentation
The tumor commonly arises as a painless mass in the skin or subcutaneous tissue and most often involves the upper extremities, including the fingers, hand, and wrist.[113-115] There are rare reports of spread to regional lymph nodes or the lungs.[111,115,116]
Molecular features
No consistent chromosomal anomalies have been detected but a t(4;15)(q21;q15) translocation has been reported.[117]
Prognosis
Plexiform fibrohistiocytic tumor is an intermediate-grade tumor that rarely metastasizes.
Treatment
Surgery is the treatment of choice but local recurrence has been reported in 12% to 50% of cases.[118]
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with giant cell tumors of soft tissue and plexiform fibrohistiocytic tumor are eligible for this trial.
Tumors of Peripheral Nerves
Ectomesenchymoma
Ectomesenchymoma is a rare nerve sheath tumor that mainly occurs in children. It is a biphenotypic soft tissue sarcoma with both mesenchymal and ectodermal components. Elements similar to rhabdomyosarcoma have been identified.
The German Soft Tissue Sarcoma Group (Cooperative Weichteilsarkom Studiengruppe [CWS]) reported on six patients (ages 0.2–13.5 years) registered over 14 years.[119][Level of evidence: 3iiA] The tumors were located in various sites including the extremities, abdomen, and orbit. All six patients were treated with surgery and chemotherapy directed at rhabdomyosarcoma. Two patients received radiation therapy. Three patients recurred with rhabdomyosarcoma features. Although data are scant, it appears that the tumor may respond to chemotherapy.[119]
Malignant peripheral nerve sheath tumor
Malignant peripheral nerve sheath tumors account for 5% of soft tissue sarcoma in patients younger than 20 years (refer to Table 1).
Risk factors
Malignant peripheral nerve sheath tumor can arise sporadically and in children with type 1 neurofibromatosis (NF1).[120]
Molecular features
Inactivating mutations of SUZ12 have been described in these tumors and are absent in neurofibromas.[121]
Prognosis
Features associated with a favorable prognosis include the following:[120,122-124]
- Smaller tumor size. In a multivariate analysis, only tumor size and nuclear p53 expression were found to be independent predictors of disease-specific survival.[123]
- Male sex and non-Hispanic white race.[125]
- No metastasis at presentation. A retrospective review of 140 patients with malignant peripheral nerve sheath tumor from the MD Anderson Cancer Center included children and adolescents. The disease-specific survival at 10 years was 32%. In this series, presence of metastatic disease was associated with a much worse prognosis.[123]
- Lower stage.
- Lower histologic grade.
- Extremity as the primary site.
Features associated with an unfavorable prognosis include the following:[126]
- High grade.
- Deep tumor location.
- Locally advanced stage at diagnosis.
- Macroscopically incomplete resection (R2).
For patients with localized disease in the MD Anderson Cancer Center study, there was no significant difference in outcome between patients with and without NF1.[123] In other studies, it was not clear whether the absence of NF1 is a favorable prognostic factor as it has been associated with both favorable [122] and unfavorable outcomes.[120,122,124] In the French Sarcoma Group study, NF1 was associated with other adverse prognostic features, but was not an independent predictor of poor outcome.[126] The Italian Sarcoma Group reported on outcomes after recurrence in 73 children and adolescents with malignant peripheral nerve sheath tumor.[127][Level of evidence: 3iiiA] The median overall survival after first relapse was 11 months, and the survival rates were 39.2% at 1 year and 15.8% at 5 years. The factors associated with a better prognosis for these patients who relapsed were less initial tumor invasiveness, longer time to relapse, and the achievement of a secondary complete remission (which was related to the feasibility of radical surgery).
Treatment
Treatment options for malignant peripheral nerve sheath tumor include the following:
Complete surgical removal of the tumor, whenever possible, is the mainstay of treatment.
The role of radiation therapy is difficult to assess, but durable local control of known postoperative microscopic residual tumor is not assured after radiation therapy.
Chemotherapy has achieved objective responses in childhood malignant peripheral nerve sheath tumor. A large retrospective analysis of the German and Italian experience with malignant peripheral nerve sheath tumor reported that 65% of measurable tumors had objective responses to ifosfamide-containing chemotherapy regimens, but the analysis did not conclusively demonstrate improved survival for chemotherapy.[120] This retrospective analysis also noted a trend toward improved outcome with postoperative radiation therapy.[120] A series of 37 young patients with malignant peripheral nerve sheath tumor and NF1 showed that most patients had large invasive tumors that were poorly responsive to chemotherapy; PFS was 19% and 5-year OS was 28%.[128]
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with malignant peripheral nerve sheath tumor are eligible for this trial.
- SARC023 (NCT02008877) (Ganetespib and Sirolimus in Patients With Malignant Peripheral Nerve Sheath Tumors): This trial is testing the combination of ganetespib, the heat shock protein inhibitor, and sirolimus, the mammalian target of rapamycin (mTOR) inhibitor, for the treatment of patients with unresectable or metastatic malignant peripheral nerve sheath tumors. Patients with unresectable soft tissue or bone sarcomas are eligible for phase I of the trial. Patients with unresectable malignant peripheral nerve sheath tumors are eligible for phase II of the trial. Eligibility is restricted to patients aged 18 years and older.
- NCT02601937 (A Phase 1 Study of the EZH2 Inhibitor Tazemetostat in Pediatric Subjects With Relapsed or Refractory INI1-Negative Tumors or Synovial Sarcoma): Patients with INI1-negative tumors are eligible for targeted treatment with an EZH2 inhibitor. This is a phase I, open-label, dose-escalation, and dose-expansion study with a twice-daily oral dose of tazemetostat.
- ADVL1522 (NCT02452554) (Lorvotuzumab Mertansine in Treating Younger Patients with Relapsed or Refractory Wilms Tumor, Rhabdomyosarcoma, Neuroblastoma, Pleuropulmonary Blastoma, Malignant Peripheral Nerve Sheath Tumor, or Synovial Sarcoma): This is a phase II study of IMGN901 (lorvotuzumab mertansine) in children with relapsed or refractory Wilms tumor, rhabdomyosarcoma, neuroblastoma, pleuropulmonary blastoma, malignant peripheral nerve sheath tumor, and synovial sarcoma. This trial is studying the effects of IMGN901, an antibody-drug conjugate that links a potent antimitotic to antibodies that target CD56.
Malignant triton tumor
Malignant triton tumors are a variant of malignant peripheral nerve sheath tumors. They occur most often in patients with neurofibromatosis type I and consist of neurogenic and rhabdomyoblastic components. Malignant triton tumors are high-grade malignancies. They usually occur before age 35 years and are very rare in children (case reports only).[129]
Malignant triton tumors are not usually responsive to chemotherapy and radiation therapy but have been treated with rhabdomyosarcoma therapy.[129][Level of evidence: 3iiiA] (Refer to the PDQ summary on Childhood Rhabdomyosarcoma Treatment for more information.)
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with malignant triton tumor are eligible for this trial.
Pericytic (Perivascular) Tumors
Myopericytoma
Infantile hemangiopericytoma is a subtype of myopericytoma.
Hemangiopericytoma is a highly vascularized tumor of uncertain origin.
Histology
Histologically, hemangiopericytomas are composed of packed round or fusiform cells that are arranged around a complex vasculature, forming many branch-like structures. Hyalinization is often present. Infantile hemangiopericytomas have similar histology but many are multilobular with vasculature outside the tumor mass.[130]
Treatment and outcome
Treatment of infantile hemangiopericytomas includes the following:
- Chemotherapy.
In a series of 17 children, the differences in metastatic potential and response to treatment were clearly demonstrated for adult and infantile hemangiopericytomas.[131] Eleven children were older than 1 year. Several of these patients had disease in the lymph nodes or lungs. Six patients with stage II or III disease progressed and died. Three patients with stage I disease survived, although one had recurrence in the lungs. Six patients had infantile hemangiopericytoma, most were greater than stage I (5 of 6). All six patients survived and three had good responses to vincristine, actinomycin, and cyclophosphamide. Hemangiopericytoma in children younger than 1 year seems to have a better prognosis than in children older than 1 year.[132-134]
Infantile myofibromatosis
This entity is a fibrous tumor of infancy and childhood that most commonly presents in the first 2 years of life.[135] The lesion can present as a single subcutaneous nodule (myofibroma) most commonly involving the head and neck region or lesions can affect multiple skin areas, muscle, and bone (myofibromatosis).[136-139]
An autosomal dominant form of the disease has been described and it is associated with germline mutations of the PDGFRB gene.[140]
Treatment
These lesions have an excellent prognosis and can regress spontaneously.
About one-third of cases with multicentric involvement will also have visceral involvement, and the prognosis for these patients is poor.[138,139,141] The use of combination therapy with vincristine/dactinomycin and vinblastine/methotrexate have proven effective in cases of multicentric disease with visceral involvement and in cases in which the disease has progressed and has threatened the life of the patient (e.g., upper airway obstruction).[138,139,142]
Tumors of Uncertain Differentiation
Tumors of uncertain differentiation include the following tumor subtypes:
- Alveolar soft part sarcoma.
- Clear cell sarcoma of soft tissue.
- Desmoplastic small round cell tumor.
- Epithelioid sarcoma.
- Extrarenal rhabdoid tumor.
- Extraskeletal myxoid chondrosarcoma.
- Neoplasms with perivascular epithelioid cell differentiation (PEComa NOS, malignant)
- Primitive neuroectodermal tumor (PNET)/extraskeletal Ewing tumor.
- Synovial sarcoma (NOS, spindle cell, and biphasic varieties).
Alveolar soft part sarcoma
Alveolar soft parts sarcomas account for 1.4% of soft tissue sarcomas in patients younger than 20 years (refer to Table 1).
Clinical presentation
The median age at presentation is 25 years, and alveolar soft part sarcoma most commonly arises in the extremities but can occur in the oral and maxillofacial region.[143-145] Alveolar soft part sarcoma in children can present with evidence of metastatic disease.[146]
Molecular features
This tumor of uncertain histogenesis is characterized by a consistent chromosomal translocation t(X;17)(p11.2;q25) that fuses the ASPSCR1 gene with the TFE3 gene.[147,148]
Prognosis
Alveolar soft part sarcoma in children may have an indolent course.[146] Patients with alveolar soft part sarcoma may relapse several years after a prolonged period of apparent remission.[149] Because these tumors are rare, all children with alveolar soft part sarcoma should be considered for enrollment in prospective clinical trials.
In a series of 19 treated patients, one group reported a 5-year OS rate of 80%, a 91% OS rate for patients with localized disease, a 100% OS rate for patients with tumors 5 cm or smaller, and a 31% OS rate for patients with tumors larger than 5 cm.[150] In another series of 33 patients, OS was 68% at 5 years from diagnosis and 53% at 10 years from diagnosis. Survival was better for smaller tumors (≤5 cm) and completely resected tumors.[151][Level of evidence: 3iiA] Delayed metastases to the brain and lung are uncommon.[143] A retrospective review of children and young adults younger than 30 years (median age, 17 years; range, 1.5–30 years) from four institutions identified 69 patients treated primarily with surgery between 1980 and 2014.[152][Level of evidence: 3iiA] The ASPL-TFE3 translocation was present in all 26 patients tested. There were 19 patients with Intergroup Rhabdomyosarcoma Study (IRS) postsurgical staging group I tumors (28%), 7 patients with IRS group II tumors (10%), 5 patients with IRS group III tumors (7%), and 38 patients with IRS group IV tumors (55%). The 5-year EFS was 80% and the OS was 87% for the 31 patients with localized tumors (IRS postsurgical groups I, II, and III). The 5-year EFS was 7% and the OS was 61% for the 38 patients with metastatic tumors (IRS postsurgical group IV).
Treatment
Treatment options for alveolar soft part sarcoma include the following:
The standard approach is complete resection of the primary lesion.[150] If complete excision is not feasible, radiation therapy should be administered. A study from China reported on 18 patients with alveolar soft part sarcoma of the oral and maxillofacial region; 15 patients were younger than 30 years.[145][Level of evidence: 3iiDii] Surgical removal with negative margins was the primary treatment. All patients survived, and only one patient had metastatic disease recurrence.
A series of 51 pediatric patients aged 0 to 21 years with alveolar soft part sarcoma found an OS rate at 10 years of 78% and an EFS rate of about 63%. Patients with localized disease (n = 37) had a 10-year OS of 87%, and the 14 patients with metastases at diagnosis had a 10-year OS of 44%, partly resulting from surgical removal of primary tumor and lung metastases in some patients. Only 3 of 18 patients (17%) with measurable disease had a response to conventional antisarcoma chemotherapy, but two of four patients treated with sunitinib had a partial response.[143][Level of evidence: 3iiiA] There have been sporadic reports of objective responses to interferon-alpha and bevacizumab.[143,153,154]
A small retrospective study of nine adult patients with metastatic alveolar soft part sarcoma treated with sunitinib reported partial response in five patients and stable disease in two patients.[155][Level of evidence: 3iiiDiv] In a phase II trial of cediranib, an inhibitor of all three known vascular epidermal growth factor receptors, 15 of 43 adult patients (35%) with metastatic alveolar soft part sarcoma had a partial response.[156][Level of evidence: 3iiDiv]
There have been no open trials for patients with metastatic alveolar soft part sarcoma.
Treatment options under clinical evaluation for alveolar soft part sarcoma
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- NCT00942877 (Phase II Study of Cediranib [AZD2171] in Patients With Alveolar Soft Part Sarcoma): A phase II study of cediranib in patients with alveolar soft part sarcoma is being conducted in patients younger than 16 years at the Clinical Center of the National Institutes of Health.
- NCT01391962 (Sunitinib or Cediranib for Alveolar Soft Part Sarcoma): A phase II trial in which patients with metastatic alveolar soft part sarcoma are randomly assigned to either sunitinib or cediranib monotherapy, with crossover at disease progression. Patients aged 16 years and older are eligible. This study is being conducted at the Clinical Center of the National Institutes of Health.
Clear cell sarcoma of soft tissue
Clear cell sarcoma (formerly and inappropriately called malignant melanoma of soft parts) is a rare soft tissue sarcoma that typically involves the deep soft tissues of the extremities. It is also called clear cell sarcoma of tendons and aponeuroses. The tumor often affects adolescents and young adults.
Patients who have small, localized tumors with low mitotic rate and intermediate histologic grade fare best.[157]
Clinical presentation
The tumor most commonly affects the lower extremity, particularly the foot, heel, and ankle.[158,159] It has a high propensity for nodal dissemination, especially metastases to regional lymph nodes (12%–43%).[159,160] The tumor typically has an indolent clinical course.
Molecular features
Clear cell sarcoma of soft tissue is characterized by an EWS-ATF1 fusion.[161]
Treatment
Treatment options for clear cell sarcoma of soft tissue include the following:
In a series of 28 pediatric patients reported by the Italian and German Soft Tissue Cooperative Studies, the median age at diagnosis was 14 years and the lower extremity was the most common primary site (50%). Surgery with or without radiotherapy is the treatment of choice and offers the best chance for cure. In this series, 12 of 13 patients with completely resected tumors were cured. For patients with more advanced disease the outcome is poor and chemotherapy is rarely effective.[162]; [163][Level of evidence: 3iiDii]
Desmoplastic small round cell tumor
Desmoplastic small round cell tumor is a rare primitive sarcoma.
Clinical presentation
Desmoplastic small round cell tumor most frequently involves the abdomen, pelvis, or tissues around the testes, but it may occur in the kidney.[164-167] The tumor occurs more commonly in males and may spread to the lungs and elsewhere. Peritoneal and pelvic lesions frequently have widespread peritoneal implants.[168]
In a large, single-institution series of 65 patients, a correlation was made between computed tomography (CT) scans in most patients and positron-emission tomography (PET)/CT scans in 11 patients. PET/CT scans had very few false-negative results and detected metastatic sites missed on conventional CT scans.[168]
Molecular features
Cytogenetic studies of these tumors have demonstrated the recurrent translocation t(11;22)(p13;q12), which has been characterized as a fusion of the WT1 and EWS genes.[167,169] The WT1-EWS fusion confirms the diagnosis of desmoplastic small round cell tumor.
Prognosis
The overall prognosis for desmoplastic small round cell tumor remains extremely poor, with reported rates of death at 90%. Greater than 90% tumor resection either at presentation or after preoperative chemotherapy may be a favorable prognostic factor for OS.[170,171]; [172][Level of evidence: 3iiiA]
Treatment
There is no standard approach to the treatment of desmoplastic small round cell tumor.
Treatment options for desmoplastic small round cell tumor include the following:
- Surgery.
- Chemotherapy followed by surgery.
- Radiation therapy.
Complete surgical resections are rare, and the overall prognosis for desmoplastic small round cell tumor remains extremely poor, with reported rates of death at 90%. Treatment may include chemotherapy, surgery, and radiation therapy. Multiagent chemotherapy analogous to that used for sarcomas has been used, as well as total abdominal radiation therapy.[164,165,170,173-176]
A single-institution study reported that five of five patients with recurrent desmoplastic small round cell tumor had partial responses to treatment with the combination of vinorelbine, cyclophosphamide, and temsirolimus.[177]
The Center for International Blood and Marrow Transplant Research (CIBMTR) analyzed patients with desmoplastic small round cell tumor in their registry who received consolidation with high dose chemotherapy and autologous stem cell reconstitution.[178] While this retrospective registry analysis suggested some benefit for this approach, other investigators have abandoned the approach because of excessive toxicity and lack of efficacy.[170]
Epithelioid sarcoma
Epithelioid sarcoma is a rare mesenchymal tumor of uncertain histogenesis which displays multilineage differentiation.[179]
Clinical presentation
Epithelioid sarcoma commonly presents as a slowly growing firm nodule based in the deep soft tissue; the proximal type predominantly affects adults and involves the axial skeleton and proximal sites. The tumor is highly aggressive and has a propensity for lymph node metastases.
Molecular features
Epithelioid sarcoma is characterized by inactivation of the SMARCB1 gene, which is present in both conventional and proximal types of epithelioid sarcoma.[180] This abnormality leads to increased dependence on EZH2 and tumor formation.[181]
Treatment
Treatment options for epithelioid sarcoma include the following
- Chemotherapy.
- Surgery.
- Surgery preceded or followed by radiation therapy.
Patients should be carefully evaluated for the presence of involved lymph nodes; suspicious lymph nodes should be biopsied. Surgical removal of primary and recurrent tumor(s) is the most effective treatment.[182][Level of evidence: 3iiiA]
In a review of 30 pediatric patients with epithelioid sarcoma (median age at presentation, 12 years), responses to chemotherapy were reported in 40% of patients using sarcoma-based regimens, and 60% of patients were alive at 5 years after initial diagnosis.[183] A single-institution retrospective review of 20 patients, including children and adults (median age, 27.3 years), found no difference in the probability of recurrence between patients who received chemotherapy and those who did not receive chemotherapy and suggested that radiation therapy may be useful.[182]
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- NCT02601937 (A Phase 1 Study of the EZH2 Inhibitor Tazemetostat in Pediatric Subjects With Relapsed or Refractory INI1-Negative Tumors or Synovial Sarcoma): Patients with INI1-negative tumors are eligible for targeted treatment with an EZH2 inhibitor. This is a phase I, open-label, dose-escalation, and dose-expansion study with a twice-daily oral dose of tazemetostat.
Extrarenal (extracranial) rhabdoid tumor
Malignant rhabdoid tumors were first described in children with renal tumors in 1981 (refer to the PDQ summary on Wilms Tumor and Other Childhood Kidney Tumors Treatment for more information) and were later found in a variety of extrarenal sites. These tumors are uncommon and highly malignant, especially in children younger than 2 years.
Extrarenal (extracranial) rhabdoid tumors account for 2% of soft tissue sarcoma in patients younger than 20 years (refer to Table 1).
Molecular features
The first sizeable series of 26 children with extrarenal extracranial malignant rhabdoid tumor of soft tissues came from patients enrolled on the Intergroup Rhabdomyosarcoma Studies I through III during a review of pathology material. Only five patients (19%) were alive without disease.[184] Later, investigation of children with atypical teratoid/rhabdoid tumors of the brain, as well as those with renal and extrarenal malignant rhabdoid tumors, found germline and acquired mutations of the SMARCB1 gene in all 29 tumors tested.[185] Rhabdoid tumors may be associated with germline mutations of the SMARCB1 gene and may be inherited from an apparently unaffected parent.[186] This observation was extended to 32 malignant rhabdoid tumors at all sites in patients whose mean age at diagnosis was 12 months.[187]
Prognosis
In a Surveillance, Epidemiology, and End Results (SEER) study of 229 patients with renal, central nervous system, and extrarenal malignant rhabdoid tumor, patients aged 2 to 18 years, limited extent of tumor, and delivery of radiation therapy were shown to affect the outcome favorably compared with other patients (P < .002 for each comparison). Site of the primary tumor was not prognostically significant. OS at 5 years was 33%.[188]
Treatment
Treatment includes surgical removal when possible, chemotherapy as used for soft tissue sarcomas (but no single regimen is currently accepted as best), and radiation therapy.[189][Level of evidence: 3iA]; [190,191][Level of evidence: 3iiiB]
Responses to alisertib have been documented in four patients with central nervous system (CNS) atypical teratoid/rhabdoid tumors.[192] (Refer to the PDQ summary on Childhood Central Nervous System Atypical Teratoid/Rhabdoid Tumor Treatment summary for more information about CNS atypical teratoid/rhabdoid tumors.)
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- NCT02601937 (A Phase 1 Study of the EZH2 Inhibitor Tazemetostat in Pediatric Subjects With Relapsed or Refractory INI1-Negative Tumors or Synovial Sarcoma): Patients with INI1-negative tumors are eligible for targeted treatment with an EZH2 inhibitor. This is a phase I, open-label, dose-escalation, and dose-expansion study with a twice-daily oral dose of tazemetostat.
Extraskeletal myxoid chondrosarcoma
Extraskeletal myxoid chondrosarcoma is relatively rare among soft tissue sarcomas, representing only 2.3% of all soft tissue sarcoma.[193] It has been reported in children and adolescents.[194]
Molecular features
Extraskeletal myxoid chondrosarcoma is a multinodular neoplasm. The rounded cells are arranged in cords and strands in a chondroitin sulfate myxoid background. Several cytogenetic abnormalities have been identified (refer to Table 2), with the most frequent being the translocation t(9;22)(q22;q12), involving the EWSR1/NR4A3 genes.[195]
Prognosis
The tumor has traditionally been considered of low-grade malignant potential.[196] However, recent reports from large institutions showed that extraskeletal myxoid chondrosarcoma has significant malignant potential, especially if patients are followed for a long time.[197,198] Patients tend to have slow protracted courses. Nodal involvement has been well described. Local recurrence (57%) and metastatic spread to lungs (26%) have been reported.[198]
Treatment
Treatment options for extraskeletal myxoid chondrosarcoma include the following:
- Surgery.
- Radiation therapy.
The therapeutic benefit of chemotherapy has not been established. Aggressive local control and resection of metastases led to OS of 87% at 5 years and 63% at 10 years. Tumors were relatively resistant to radiation therapy.[197]
There may be potential genetic targets for small molecules, but these should be studied as part of a clinical trial. In an adult study, six of ten patients who received sunitinib achieved a partial response.[199]
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- NCT02601937 (A Phase 1 Study of the EZH2 Inhibitor Tazemetostat in Pediatric Subjects With Relapsed or Refractory INI1-Negative Tumors or Synovial Sarcoma): Patients with INI1-negative tumors are eligible for targeted treatment with an EZH2 inhibitor. This is a phase I, open-label, dose-escalation, and dose-expansion study with a twice-daily oral dose of tazemetostat.
Neoplasms with perivascular epithelioid cell differentiation (PEComas)
Risk factors and molecular features
Benign PEComas are common in tuberous sclerosis, an autosomal dominant syndrome that also predisposes to renal cell cancer and brain tumors. Tuberous sclerosis is caused by germline inactivation of either TSC1 (9q34) or TSC2 (16p13.3), and the same tumor suppressor genes are inactivated somatically in sporadic PEComas.[200] Inactivation of either gene results in stimulation of the mTOR pathway, providing the basis for the treatment of nonsurgically curable PEComas with mTOR inhibitors.[201,202] A small proportion of PEComas have TFE3 rearrangements with fusions involving various genes including SFPQ/PSF and RAD51B.[203]
Clinical presentation
PEComas occur in various rare gastrointestinal, pulmonary, gynecologic, and genitourinary sites. Soft tissue, visceral, and gynecologic PEComas are more commonly seen in middle-aged female patients and are usually not associated with the tuberous sclerosis complex.[204] The disease course may be indolent.
Prognosis
Most PEComas have a benign clinical course, but malignant behavior has been reported and can be predicted based on the size of the tumor, mitotic rate, and presence of necrosis.[205]
Treatment
Treatment options have not been defined. Treatment may include surgery or observation followed by surgery when the tumor is large.[206]
Clinical activity with mTOR inhibitors, such as sirolimus, in tumors with evidence of mTORC1 activation and TSC loss has been well documented.[207]
Primitive neuroectodermal tumor (PNET)/extraskeletal Ewing tumor
(Refer to the PDQ summary on Ewing Sarcoma Treatment for more information.)
Synovial sarcoma
Synovial sarcoma accounts for 9% of soft tissue sarcomas in patients younger than 20 years (refer to Table 1).
Synovial sarcoma is one of the most common nonrhabdomyosarcomatous soft tissue sarcomas in children and adolescents. In a 1973 to 2005 SEER review, 1,268 patients with synovial sarcoma were identified. Approximately 17% of these patients were children and adolescents and the median age at diagnosis was 34 years.[208]
Histologic classification
Synovial sarcoma can be subclassified as the following types:
- Synovial sarcoma, NOS.
- Synovial sarcoma, spindle cell.
- Synovial sarcoma, biphasic.
Clinical presentation
The most common tumor location is the extremities, followed by trunk and head and neck.[208] Rarely, a synovial sarcoma may arise in the heart or pericardium.[209]
The most common site of metastasis is the lung.[210,211] The risk of metastases is highly influenced by tumor size; it is estimated that patients with tumors that are larger than 5 cm have a 32-fold risk of developing metastases when compared with other patients.
Diagnostic evaluation
The diagnosis of synovial sarcoma is made by immunohistochemical analysis, ultrastructural findings, and demonstration of the specific chromosomal translocation t(x;18)(p11.2;q11.2). This abnormality is specific for synovial sarcoma and is found in all morphologic subtypes. Synovial sarcoma results in rearrangement of the SYT gene on chromosome 18 with one of the subtypes (1, 2, or 4) of the SSX gene on chromosome X.[212,213] It is thought that the SYT/SSX18 transcript promotes epigenetic silencing of key tumor suppressor genes.[214]
In one report, reduced INI1 nuclear reactivity on immunohistochemical staining was seen in 49 cases of synovial sarcoma, suggesting that this pattern may help distinguish synovial sarcoma from other histologies.[215]
Prognosis
Patients younger than 10 years have more favorable outcomes and clinical features, including extremity primaries, smaller tumors, and localized disease, than do older patients.[208,216] A meta-analysis also suggested that response to chemotherapy was correlated with improved survival.[217]
The following studies have reported multiple factors associated with unfavorable outcomes:
- In a retrospective analysis of synovial sarcoma in children and adolescents who were treated in Germany and Italy, tumor size (>5 cm or ≤5 cm in greatest dimension) was an important predictor of EFS.[218] In this analysis, local invasiveness conferred an inferior probability of EFS, but surgical margins were not associated with clinical outcome.
- In a single-institution retrospective analysis of 111 patients with synovial sarcoma who were younger than 22 years at diagnosis, larger tumor size, greater depth in tissue, greater local invasiveness, and more proximal tumor location were associated with poorer OS.[219][Level of evidence: 3iiA]
- A multicenter analysis of 219 children from various treating centers including Germany, SJCRH, Instituto Tumori, and MD Anderson Cancer Center reported an estimated 5-year OS of 80% and EFS rate of 72%.[217] In this analysis, an interaction between tumor size and invasiveness was observed; in multivariate analysis, patients with large or invasive tumors or with Intergroup Rhabdomyosarcoma Study Clinical Group III disease (localized, incompletely resected or with biopsy only) and IV (metastases at diagnosis) had decreased OS. Treatment with radiation therapy was related to improved OS (hazard ratio, 0.4; 95% confidence interval, 0.2–0.7). In Intergroup Rhabdomyosarcoma Study Group III patients, objective response to chemotherapy (18 of 30 [60%]) correlated with improved survival. In adults, factors such as International Union Against Cancer/American Joint Committee on Cancer stage III and stage IVA, tumor necrosis, truncal location, elevated mitotic rate, age, and histologic grade have been associated with a worse prognosis.[220-222]
- Expression and genomic index prognostic signatures have been studied in synovial sarcoma. Complex genomic profiles, with greater rearrangement of the genome, are more common in adults than in younger patients with synovial sarcoma and are associated with a higher risk of metastasis.[223]
- A review of 84 patients with localized synovial sarcoma who had information on fusion status (SYT-SSX) and histologic grading found no difference in OS according to these criteria. However, for tumor size at diagnosis, the study showed that patients with tumors between 5 cm and 10 cm had a worse prognosis than those with smaller tumors (P = .02), and patients with tumors larger than 10 cm had even worse OS (P = .0003).[224][Level of evidence: 3iiiA]
- The German CWS group reviewed 27 evaluable patients younger than 21 years with pulmonary metastases among 296 patients with synovial sarcoma. Metastases involved the lungs in all patients. The 5-year EFS rate was 26%, and the OS rate was 30%. The most important prognostic factor at presentation was that the metastases were limited to one lesion in one lung or one lesion in both lungs (a group they termed oligometastatic). Treatment elements associated with superior survival were adequate local therapy of the primary tumor and, if feasible, for the metastases. The use of whole-lung irradiation did not correlate with better outcomes.[225][Level of evidence: 3iiA]
Survival after relapse is poor (30% at 5 years). Factors associated with outcome after relapse include duration of first remission (> or ≤ 18 months) and lack of a second remission.[226]
Treatment
Treatment options for synovial sarcoma include the following:
The COG and the European Pediatric Soft Tissue Sarcoma Study Group reported a combined analysis of 60 patients younger than 21 years with localized synovial sarcoma prospectively assigned to surgery without adjuvant radiation therapy or chemotherapy.[227] Enrollment was limited to patients with initial complete resection with histologically free margins, with a grade 2 tumor of any size or a grade 3 tumor 5 cm or smaller. The 3-year EFS was 90% (median follow-up, 5.2 years; range, 1.9–9.1). All eight events were local tumor recurrence; no metastatic recurrences were seen. All patients with recurrent disease were effectively treated with salvage therapy, resulting in 100% OS.
Synovial sarcoma appears to be more sensitive to chemotherapy than many other soft tissue sarcomas, and children with synovial sarcoma seem to have a better prognosis when compared with adults.[11,211,222,228-232] The most commonly used regimens for the treatment of synovial sarcoma incorporate ifosfamide and doxorubicin.[217,231,233] Response rates to the ifosfamide and doxorubicin regimen are higher than in other nonrhabdomyosarcomatous soft tissue sarcomas.[234]
Several studies have reported the following chemotherapy-associated treatment findings:
- Several treatment centers advocate postoperative chemotherapy after resection and radiation therapy of synovial sarcoma in children and young adults.[217,218,235-237]
- The International Society of Pediatric Oncology-Malignant Mesenchymal Tumors studies showed that select patients (young age, <5 cm resected tumors) with nonmetastatic synovial sarcoma can have excellent outcome in the absence of radiation, but it is still unclear whether that approach obviates an advantage of radiation for local or regional control.[236]
- A German trial suggested a benefit for postoperative chemotherapy in children with synovial sarcoma.[237]
- A meta-analysis also suggested that chemotherapy may provide benefit.[217]
- In the most recent COG ARST0332 (NCT00346164) study, 129 patients with synovial sarcoma were prospectively treated using a risk-based therapy program (as detailed in the prognosis section), of which 43 were categorized as low risk, 66 as intermediate risk, and 20 as high risk. At a median follow-up of 2.6 years, 3-year EFS for low-, intermediate-, and high-risk groups were 83%, 79%, and 16%, respectively. The use of risk factor–directed therapy accurately predicted outcomes.[238]
- The European Pediatric Soft Tissue Sarcoma Study Group performed a prospective study of patients younger than 21 years with synovial sarcoma (CCLG-EPSSG-NRSTS-2005 [NCT00334854]).[239][Level of evidence: 3iiA] Patients were stratified into the following three risks groups and nonrandomly assigned to treatment by risk group:
- Low-risk patients had Intergroup Rhabdomyosarcoma Study (IRS) group I tumors less than 5 cm in size and nonaxial primary tumors.
- Intermediate-risk patients had no axial primary tumors and IRS group I tumors greater than 5 cm or IRS group II tumors.
- High-risk patients included all patients with axial primary sites (head and neck, lung and pleura, trunk, retroperitoneal), IRS group III tumors, or N1 tumors.
Outcomes for patients treated on the CCLG-EPSSG-NRSTS-2005 trial are described in Table 9.
Table 9. Event-Free Survival (EFS) and Overall Survival (OS) in Patients With Low-, Intermediate-, and High-Risk Synovial Sarcoma Treated on the CCLG-EPSSG-NRSTS-2005 Trial Risk Group Treatment 3-Year EFS (%) 3-Year OS (%) IRS = Intergroup Rhabdomyosarcoma Study; RT = radiation therapy. aChemotherapy was ifosfamide/doxorubicin, with doxorubicin omitted during radiation therapy. b59.4 Gy in cases without the option of secondary resection; 50.4 Gy as preoperative radiation therapy; 50.4, 54, and 59.4 Gy as postoperative radiation therapy, in the case of R0, R1, and R2 resections, respectively (no additional radiation therapy in the case of secondary complete resections with free margins, in children younger than 6 years). Low Surgery alone 92 100 Intermediate Surgery, 3–6 cycles chemotherapya ± RTb 91 100 High (IRS group III) 3 cycles of chemotherapya surgery, 3 additional cycles of chemotherapy, ± RTb 77 94 High (axial primary sites) Surgery, 6 cycles of chemotherapya, RTb 78 100
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- ADP 04511 (NCT01343043) (A Pilot Study of Genetically Engineered NY-ESO-1 Specific [c259] T Cells in HLA-A2+ Patients With Synovial Sarcoma): Patients with unresectable, metastatic, or recurrent synovial sarcoma undergo apheresis. Cells are shipped to a central laboratory where they undergo NY-ESO-1 transduction, expansion, and cryopreservation. Patients undergo lymphodepletion with fludarabine and cyclophosphamide, followed by an infusion of autologous transfected cells. Eligibility is restricted to patients with HLA type A2+, age older than 4 years, and weight greater than 18 kg.
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with alveolar soft part sarcoma, clear cell sarcoma of soft tissue, epithelioid sarcoma, extraskeletal myxoid chondrosarcoma, PEComa, and synovial sarcoma are eligible for this trial.
- NCT02601937 (A Phase 1 Study of the EZH2 Inhibitor Tazemetostat in Pediatric Subjects With Relapsed or Refractory INI1-Negative Tumors or Synovial Sarcoma): Patients with INI1-negative tumors are eligible for targeted treatment with an EZH2 inhibitor. This is a phase I, open-label, dose-escalation, and dose-expansion study with a twice-daily oral dose of tazemetostat.
- ADVL1522 (NCT02452554) (Lorvotuzumab Mertansine in Treating Younger Patients with Relapsed or Refractory Wilms Tumor, Rhabdomyosarcoma, Neuroblastoma, Pleuropulmonary Blastoma, Malignant Peripheral Nerve Sheath Tumor, or Synovial Sarcoma): This is a phase II study of IMGN901 (lorvotuzumab mertansine) in children with relapsed or refractory Wilms tumor, rhabdomyosarcoma, neuroblastoma, pleuropulmonary blastoma, malignant peripheral nerve sheath tumor, and synovial sarcoma. This trial is studying the effects of IMGN901, an antibody-drug conjugate that links a potent antimitotic to antibodies that target CD56.
Undifferentiated/unclassified sarcoma
Patients with undifferentiated soft tissue sarcoma had been eligible for participation in rhabdomyosarcoma trials coordinated by the Intergroup Rhabdomyosarcoma Study Group and the COG from 1972 to 2006. The rationale was the observation that patients with undifferentiated soft tissue sarcoma had similar sites of disease and outcome as those with alveolar rhabdomyosarcoma. Therapeutic trials for adults with soft tissue sarcoma include patients with undifferentiated soft tissue sarcoma and other histologies, which are treated similarly, using ifosfamide and doxorubicin, and sometimes with other chemotherapy agents, surgery, and radiation therapy.
In the COG ARST0332 (NCT00346164) trial, patients with high-grade undifferentiated sarcoma were treated with an ifosfamide and doxorubicin-based regimen and were treated with rhabdomyosarcoma-directed therapies in previous Intergroup Rhabdomyosarcoma Study Group studies with a 5-year survival estimate for nonmetastatic patients of 72%.[240][Level of evidence: 3iiA] Currently, these patients are eligible for the COG open ARST1321 (NCT02180867) trial for patients with nonrhabdomyosarcomatous soft tissue sarcoma.
Undifferentiated pleomorphic sarcoma/malignant fibrous histiocytoma (high-grade)
At one time, malignant fibrous histiocytoma was the single most common histotype among adults with soft tissue sarcomas. Since it was first recognized in the early 1960s, malignant fibrous histiocytoma has been plagued by controversy in terms of both its histogenesis and its validity as a clinicopathologic entity. The latest WHO classification no longer includes malignant fibrous histiocytoma as a distinct diagnostic category but rather as a subtype of an undifferentiated pleomorphic sarcoma.[241]
This entity accounts for 2% to 6% of all childhood soft tissue sarcomas.[242] These tumors can arise in previously irradiated sites or as a second malignancy in patients with retinoblastoma.
These tumors occur mainly in the second decade of life. In a series of ten patients, the median age was 10 years and the tumor was most commonly located in the extremities. In this series, all tumors were localized and five of nine (for whom follow-up was available) were alive and in first remission.[242] In another series of 17 pediatric patients with malignant fibrous histiocytoma, the median age at diagnosis was 5 years and the extremities were involved in eight cases.[243] All patients with metastatic disease died and two patients experienced a clinical response to a doxorubicin-based regimen.
(Refer to the PDQ summary on Osteosarcoma and Malignant Fibrous Histiocytoma of Bone Treatment for more information about the treatment of malignant fibrous histiocytoma of bone.)
Treatment options under clinical evaluation
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable, intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with undifferentiated epithelial sarcoma, undifferentiated pleomorphic sarcoma, undifferentiated round cell sarcoma, and undifferentiated spindle cell sarcoma are eligible for this trial.
Vascular Tumors
Vascular tumors vary from hemangiomas, which are always considered benign, to angiosarcomas, which are highly malignant.[244] Vascular tumors include the following tumor subtypes:
Angiosarcoma of the soft tissue
Incidence
Angiosarcoma is a rare (accounting for 2% of sarcomas), aggressive, vascular tumor that can arise in any part of the body, but is more common in the soft tissue. Angiosarcoma has an estimated incidence of 2 cases per 1 million; in the United States, it annually affects approximately 600 people who are typically aged 60 to 70 years.[245]
Angiosarcomas are extremely rare in children and it is unclear if the pathophysiology of this tumor is different in the pediatric population. Cases have been reported in neonates and toddlers, with presentation of multiple cutaneous lesions and liver lesions, some of which are GLUT1 positive.[246-249] Most angiosarcomas involve the skin and superficial soft tissue, although the liver, spleen, and lung can be affected; bone is rarely affected.
Risk factors
Established risk factors include vinyl chloride exposure, radiation exposure, and chronic lymphedema from any cause, including Stewart-Treves syndrome.[250]
Pathology and biology
Angiosarcomas are largely aneuploid tumors. The rare cases of angiosarcoma that arise from benign lesions such as hemangiomas have a distinct pathway that needs to be investigated. MYC amplification is seen in radiation-induced angiosarcoma. KDR-VEGFR2 mutations and FLT4-VEGFR3 amplifications have been seen with a frequency of less than 50%.[250]
Histopathologic diagnosis can be very difficult because there can be areas of varied atypia. The common feature is an irregular network of channels in a dissective pattern along dermal collagen bundles. There is varied cellular shape, size, mitosis, endothelial multilayering, and papillary formation. Epithelioid cells can also be present. Necrosis and hemorrhage are common. Tumors stain for factor VIII, CD31, and CD34. Some liver lesions can mimic infantile hemangiomas and have focal GLUT1 positivity. Nomenclature of these liver lesions has been difficult and confusing with use of terminology from 1971 (e.g., type I hemangioendothelioma: infantile hemangioma; type II hemangioendothelioma: low-grade angiosarcoma; type III hemangioendothelioma: high-grade angiosarcoma).[247]
Treatment of angiosarcoma of the soft tissue
Treatment options for angiosarcoma of the soft tissue include the following:
- Surgery (localized disease).
- Radiation therapy (localized cutaneous disease in adults).
- Surgery, chemotherapy, and radiation therapy (metastatic disease).
Localized disease is cured by aggressive surgery. Complete surgical excision appears to be crucial for angiosarcomas and lymphangiosarcomas despite evidence of tumor shrinkage in some patients who were treated with local or systemic therapy.[248,251-253] A review of 222 patients (median age, 62 years; range, age 15–90 years) showed an overall disease-specific survival (DSS) rate of 38% at 5 years. Five-year DSS was 44% in 138 patients with localized, resected tumors but only 16% in 43 patients with metastases at diagnosis.[253] Data on liver transplantation for localized angiosarcoma are limited.[254][Level of evidence: 3iiA]
Localized disease, especially cutaneous angiosarcoma, can be treated with radiation therapy. Most of these reported cases are in adults.[255]
Multimodal treatment with surgery, systemic chemotherapy, and radiation therapy is used for metastatic disease, although it is rarely curative.[256] Disease control is the objective in metastatic angiosarcoma, with published progression-free survival rates between 3 months and 7 months [257] and a median overall survival (OS) rate of 14 months to 18 months.[258] In both adults and children, 5-year OS rates between 20% and 35% are reported.[248,249,259]
In a child diagnosed with angiosarcoma secondary to malignant transformation from infantile hemangioma, response to treatment with bevacizumab, a monoclonal antibody against vascular endothelial growth factor, combined with systemic chemotherapy, has been reported.[246,256] A report of eight cases of liver angiosarcoma in children highlighted the misuse of the term hemangioendothelioma and the importance of early diagnosis and treatment of these tumors.[260]
Biologic agents that inhibit angiogenesis have shown activity in adults with angiosarcoma.[247,259]
Treatment options under clinical evaluation for angiosarcoma of the soft tissue
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- ARST1321 (NCT02180867) (Radiation Therapy With or Without Combination Chemotherapy or Pazopanib Hydrochloride Before Surgery in Treating Patients With Newly Diagnosed Nonrhabdomyosarcoma Soft Tissue Sarcomas That Can be Removed by Surgery [PAZNTIS]): This study will first determine the feasibility of adding a tyrosine kinase inhibitor in combination with radiation therapy or chemotherapy (ifosfamide/etoposide) and radiation therapy in pediatric and adult patients newly diagnosed with unresected intermediate-risk and high-risk nonrhabdomyosarcomatous soft tissue sarcoma. Subsequently, this trial will compare the rates of near-complete pathologic response (>90% necrosis) of: 1) preoperative pazopanib plus chemoradiation therapy versus preoperative chemoradiation therapy alone for potentially resectable (>5 cm), grade 3 intermediate-risk to high-risk chemotherapy-sensitive adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma; and 2) pazopanib plus preoperative radiation therapy versus preoperative radiation therapy alone for potentially resectable intermediate-risk to high-risk adult and pediatric nonrhabdomyosarcomatous soft tissue sarcoma. Patients with angiosarcoma of the soft tissue are eligible for this trial.
- NCT01532687 (Gemcitabine Hydrochloride With or Without Pazopanib Hydrochloride in Treating Patients With Refractory Soft Tissue Sarcoma): This randomized phase II trial studies how well gemcitabine hydrochloride works with or without pazopanib hydrochloride in treating patients with refractory soft tissue sarcoma.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Epithelioid hemangioendothelioma
Incidence and outcome
This tumor was first described in soft tissue by Weiss and Enzinger in 1982. Epithelioid hemangioendotheliomas can occur at younger ages, but the peak incidence is in the fourth and fifth decades of life. The tumors can have an indolent or very aggressive course, with overall survival of 73% at 5 years. There are case reports of patients with untreated multiple lesions who have a very benign course compared with other patients who have a very aggressive course. Some pathologists have tried to stratify patients to evaluate risks and adjust treatment, but more research is needed.[261-267]
The presence of effusions, tumor size larger than 3 cm, and a high mitotic index (>3 mitoses/50 high-power fields) have been associated with unfavorable outcomes.[263]
Pathology and biology
A WWTR1-CAMTA1 gene fusion has been found in a large percentage of patients; less commonly, a YAP1-TFE3 gene fusion has been reported.[261] These fusions are not directly targetable with current medicines. Monoclonality has been described in multiple liver lesions, suggesting a metastatic process.
Histologically, these lesions are characterized as epithelioid lesions arranged in nests, strands, and trabecular patterns, with infrequent vascular spaces. Features that may be associated with aggressive clinical behavior include cellular atypia, one or more mitoses per 10 high-power fields, an increased proportion of spindled cells, focal necrosis, and metaplastic bone formation.[263]
The number of pediatric patients reported in the literature is limited.
Clinical presentation and diagnostic evaluation
Common sites of involvement are liver alone (21%), liver plus lung (18%), lung alone (12%), and bone alone (14%).[263,268,269] Clinical presentation depends on site of involvement, as follows:
- Liver: Hepatic nodules have central vascularity on ultrasound, contrast-enhancing lesions by computed tomography, and low T1 signal and moderate T2 signal on magnetic resonance imaging.
- Lung: Pulmonary epithelioid hemangioendothelioma may be an asymptomatic finding on chest x-ray or be associated with pleuritic pain, hemoptysis, anemia, and fibrosis.
- Bone: Bone metastasis may be associated with pathologic fracture. On x-rays, they are well-defined osteolytic lesions and can be multiple or solitary.
- Soft tissue: Thirty percent of soft tissue cases are associated with metastases, and when present, can have a very aggressive course, with limited response to chemotherapy.
- Skin: Cutaneous lesions can be raised and nodular or can be warm red-brown plaques.
Treatment of epithelioid hemangioendothelioma
Treatment options for epithelioid hemangioendothelioma include the following:
- Observation.
- Surgery.
- Immunotherapy.
- Targeted therapy.
- Chemotherapy.
For indolent cases, observation is warranted. For more aggressive cases, multiple medications have been used, including interferon, thalidomide, sorafenib, pazopanib, and sirolimus.[270] The most aggressive cases are treated with angiosarcoma-type chemotherapy. Surgery is used when possible. Liver transplantation has been used with aggressive liver lesions, both with and without metastases.[263,271-274]
Treatment options under clinical evaluation for epithelioid hemangioendothelioma
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
- NCT03148275 (Trametinib in Treating Patients with Epithelioid Hemangioendothelioma That Is Metastatic, Locally Advanced, or Cannot Be Removed by Surgery): This is a phase II trial assessing the efficacy of trametinib, with patient-reported outcomes as secondary aims.
- NCT01532687 (Gemcitabine Hydrochloride With or Without Pazopanib Hydrochloride in Treating Patients With Refractory Soft Tissue Sarcoma): This randomized phase II trial studies how well gemcitabine hydrochloride works with or without pazopanib hydrochloride in treating patients with refractory soft tissue sarcoma.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Ferrari A, Casanova M, Collini P, et al.: Adult-type soft tissue sarcomas in pediatric-age patients: experience at the Istituto Nazionale Tumori in Milan. J Clin Oncol 23 (18): 4021-30, 2005. [PUBMED Abstract]
- Stanelle EJ, Christison-Lagay ER, Sidebotham EL, et al.: Prognostic factors and survival in pediatric and adolescent liposarcoma. Sarcoma 2012: 870910, 2012. [PUBMED Abstract]
- Alaggio R, Coffin CM, Weiss SW, et al.: Liposarcomas in young patients: a study of 82 cases occurring in patients younger than 22 years of age. Am J Surg Pathol 33 (5): 645-58, 2009. [PUBMED Abstract]
- Sreekantaiah C, Karakousis CP, Leong SP, et al.: Cytogenetic findings in liposarcoma correlate with histopathologic subtypes. Cancer 69 (10): 2484-95, 1992. [PUBMED Abstract]
- Sugiura H, Takahashi M, Katagiri H, et al.: Additional wide resection of malignant soft tissue tumors. Clin Orthop (394): 201-10, 2002. [PUBMED Abstract]
- Cecchetto G, Guglielmi M, Inserra A, et al.: Primary re-excision: the Italian experience in patients with localized soft-tissue sarcomas. Pediatr Surg Int 17 (7): 532-4, 2001. [PUBMED Abstract]
- Chui CH, Spunt SL, Liu T, et al.: Is reexcision in pediatric nonrhabdomyosarcoma soft tissue sarcoma necessary after an initial unplanned resection? J Pediatr Surg 37 (10): 1424-9, 2002. [PUBMED Abstract]
- Bahig H, Roberge D, Bosch W, et al.: Agreement among RTOG sarcoma radiation oncologists in contouring suspicious peritumoral edema for preoperative radiation therapy of soft tissue sarcoma of the extremity. Int J Radiat Oncol Biol Phys 86 (2): 298-303, 2013. [PUBMED Abstract]
- Baldini EH, Wang D, Haas RL, et al.: Treatment Guidelines for Preoperative Radiation Therapy for Retroperitoneal Sarcoma: Preliminary Consensus of an International Expert Panel. Int J Radiat Oncol Biol Phys 92 (3): 602-12, 2015. [PUBMED Abstract]
- Ferrari A, Casanova M, Spreafico F, et al.: Childhood liposarcoma: a single-institutional twenty-year experience. Pediatr Hematol Oncol 16 (5): 415-21, 1999 Sep-Oct. [PUBMED Abstract]
- Cecchetto G, Alaggio R, Dall'Igna P, et al.: Localized unresectable non-rhabdo soft tissue sarcomas of the extremities in pediatric age: results from the Italian studies. Cancer 104 (9): 2006-12, 2005. [PUBMED Abstract]
- Huh WW, Yuen C, Munsell M, et al.: Liposarcoma in children and young adults: a multi-institutional experience. Pediatr Blood Cancer 57 (7): 1142-6, 2011. [PUBMED Abstract]
- Gronchi A, Bui BN, Bonvalot S, et al.: Phase II clinical trial of neoadjuvant trabectedin in patients with advanced localized myxoid liposarcoma. Ann Oncol 23 (3): 771-6, 2012. [PUBMED Abstract]
- Demetri GD, von Mehren M, Jones RL, et al.: Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J Clin Oncol 34 (8): 786-93, 2016. [PUBMED Abstract]
- Baruchel S, Pappo A, Krailo M, et al.: A phase 2 trial of trabectedin in children with recurrent rhabdomyosarcoma, Ewing sarcoma and non-rhabdomyosarcoma soft tissue sarcomas: a report from the Children's Oncology Group. Eur J Cancer 48 (4): 579-85, 2012. [PUBMED Abstract]
- Wang L, Motoi T, Khanin R, et al.: Identification of a novel, recurrent HEY1-NCOA2 fusion in mesenchymal chondrosarcoma based on a genome-wide screen of exon-level expression data. Genes Chromosomes Cancer 51 (2): 127-39, 2012. [PUBMED Abstract]
- Nyquist KB, Panagopoulos I, Thorsen J, et al.: Whole-transcriptome sequencing identifies novel IRF2BP2-CDX1 fusion gene brought about by translocation t(1;5)(q42;q32) in mesenchymal chondrosarcoma. PLoS One 7 (11): e49705, 2012. [PUBMED Abstract]
- Frezza AM, Cesari M, Baumhoer D, et al.: Mesenchymal chondrosarcoma: prognostic factors and outcome in 113 patients. A European Musculoskeletal Oncology Society study. Eur J Cancer 51 (3): 374-81, 2015. [PUBMED Abstract]
- Dantonello TM, Int-Veen C, Leuschner I, et al.: Mesenchymal chondrosarcoma of soft tissues and bone in children, adolescents, and young adults: experiences of the CWS and COSS study groups. Cancer 112 (11): 2424-31, 2008. [PUBMED Abstract]
- Bishop MW, Somerville JM, Bahrami A, et al.: Mesenchymal Chondrosarcoma in Children and Young Adults: A Single Institution Retrospective Review. Sarcoma 2015: 608279, 2015. [PUBMED Abstract]
- Wodowski K, Hill DA, Pappo AS, et al.: A chemosensitive pediatric extraosseous osteosarcoma: case report and review of the literature. J Pediatr Hematol Oncol 25 (1): 73-7, 2003. [PUBMED Abstract]
- Sordillo PP, Hajdu SI, Magill GB, et al.: Extraosseous osteogenic sarcoma. A review of 48 patients. Cancer 51 (4): 727-34, 1983. [PUBMED Abstract]
- Nieuwenhuis MH, Casparie M, Mathus-Vliegen LM, et al.: A nation-wide study comparing sporadic and familial adenomatous polyposis-related desmoid-type fibromatoses. Int J Cancer 129 (1): 256-61, 2011. [PUBMED Abstract]
- Rossato M, Rigotti M, Grazia M, et al.: Congenital hypertrophy of the retinal pigment epithelium (CHRPE) and familial adenomatous polyposis (FAP). Acta Ophthalmol Scand 74 (4): 338-42, 1996. [PUBMED Abstract]
- Baker RH, Heinemann MH, Miller HH, et al.: Hyperpigmented lesions of the retinal pigment epithelium in familial adenomatous polyposis. Am J Med Genet 31 (2): 427-35, 1988. [PUBMED Abstract]
- Kattentidt Mouravieva AA, Geurts-Giele IR, de Krijger RR, et al.: Identification of Familial Adenomatous Polyposis carriers among children with desmoid tumours. Eur J Cancer 48 (12): 1867-74, 2012. [PUBMED Abstract]
- Wang WL, Nero C, Pappo A, et al.: CTNNB1 genotyping and APC screening in pediatric desmoid tumors: a proposed algorithm. Pediatr Dev Pathol 15 (5): 361-7, 2012 Sep-Oct. [PUBMED Abstract]
- Lewis JJ, Boland PJ, Leung DH, et al.: The enigma of desmoid tumors. Ann Surg 229 (6): 866-72; discussion 872-3, 1999. [PUBMED Abstract]
- Lazar AJ, Tuvin D, Hajibashi S, et al.: Specific mutations in the beta-catenin gene (CTNNB1) correlate with local recurrence in sporadic desmoid tumors. Am J Pathol 173 (5): 1518-27, 2008. [PUBMED Abstract]
- Faulkner LB, Hajdu SI, Kher U, et al.: Pediatric desmoid tumor: retrospective analysis of 63 cases. J Clin Oncol 13 (11): 2813-8, 1995. [PUBMED Abstract]
- Merchant NB, Lewis JJ, Woodruff JM, et al.: Extremity and trunk desmoid tumors: a multifactorial analysis of outcome. Cancer 86 (10): 2045-52, 1999. [PUBMED Abstract]
- Honeyman JN, Theilen TM, Knowles MA, et al.: Desmoid fibromatosis in children and adolescents: a conservative approach to management. J Pediatr Surg 48 (1): 62-6, 2013. [PUBMED Abstract]
- Bonvalot S, Ternès N, Fiore M, et al.: Spontaneous regression of primary abdominal wall desmoid tumors: more common than previously thought. Ann Surg Oncol 20 (13): 4096-102, 2013. [PUBMED Abstract]
- Bonvalot S, Eldweny H, Haddad V, et al.: Extra-abdominal primary fibromatosis: Aggressive management could be avoided in a subgroup of patients. Eur J Surg Oncol 34 (4): 462-8, 2008. [PUBMED Abstract]
- Merchant TE, Nguyen D, Walter AW, et al.: Long-term results with radiation therapy for pediatric desmoid tumors. Int J Radiat Oncol Biol Phys 47 (5): 1267-71, 2000. [PUBMED Abstract]
- Zelefsky MJ, Harrison LB, Shiu MH, et al.: Combined surgical resection and iridium 192 implantation for locally advanced and recurrent desmoid tumors. Cancer 67 (2): 380-4, 1991. [PUBMED Abstract]
- Weiss AJ, Lackman RD: Low-dose chemotherapy of desmoid tumors. Cancer 64 (6): 1192-4, 1989. [PUBMED Abstract]
- Klein WA, Miller HH, Anderson M, et al.: The use of indomethacin, sulindac, and tamoxifen for the treatment of desmoid tumors associated with familial polyposis. Cancer 60 (12): 2863-8, 1987. [PUBMED Abstract]
- Soto-Miranda MA, Sandoval JA, Rao B, et al.: Surgical treatment of pediatric desmoid tumors. A 12-year, single-center experience. Ann Surg Oncol 20 (11): 3384-90, 2013. [PUBMED Abstract]
- Skapek SX, Ferguson WS, Granowetter L, et al.: Vinblastine and methotrexate for desmoid fibromatosis in children: results of a Pediatric Oncology Group Phase II Trial. J Clin Oncol 25 (5): 501-6, 2007. [PUBMED Abstract]
- Gandhi MM, Nathan PC, Weitzman S, et al.: Successful treatment of life-threatening generalized infantile myofibromatosis using low-dose chemotherapy. J Pediatr Hematol Oncol 25 (9): 750-4, 2003. [PUBMED Abstract]
- Gega M, Yanagi H, Yoshikawa R, et al.: Successful chemotherapeutic modality of doxorubicin plus dacarbazine for the treatment of desmoid tumors in association with familial adenomatous polyposis. J Clin Oncol 24 (1): 102-5, 2006. [PUBMED Abstract]
- Constantinidou A, Jones RL, Scurr M, et al.: Pegylated liposomal doxorubicin, an effective, well-tolerated treatment for refractory aggressive fibromatosis. Eur J Cancer 45 (17): 2930-4, 2009. [PUBMED Abstract]
- Ananth P, Werger A, Voss S, et al.: Liposomal doxorubicin: Effective treatment for pediatric desmoid fibromatosis. Pediatr Blood Cancer 64 (7): , 2017. [PUBMED Abstract]
- Gounder MM, Lefkowitz RA, Keohan ML, et al.: Activity of Sorafenib against desmoid tumor/deep fibromatosis. Clin Cancer Res 17 (12): 4082-90, 2011. [PUBMED Abstract]
- Heinrich MC, McArthur GA, Demetri GD, et al.: Clinical and molecular studies of the effect of imatinib on advanced aggressive fibromatosis (desmoid tumor). J Clin Oncol 24 (7): 1195-203, 2006. [PUBMED Abstract]
- Chugh R, Wathen JK, Patel SR, et al.: Efficacy of imatinib in aggressive fibromatosis: Results of a phase II multicenter Sarcoma Alliance for Research through Collaboration (SARC) trial. Clin Cancer Res 16 (19): 4884-91, 2010. [PUBMED Abstract]
- Agresta L, Kim H, Turpin BK, et al.: Pazopanib therapy for desmoid tumors in adolescent and young adult patients. Pediatr Blood Cancer : , 2018. [PUBMED Abstract]
- Shang H, Braggio D, Lee YJ, et al.: Targeting the Notch pathway: A potential therapeutic approach for desmoid tumors. Cancer 121 (22): 4088-96, 2015. [PUBMED Abstract]
- Messersmith WA, Shapiro GI, Cleary JM, et al.: A Phase I, dose-finding study in patients with advanced solid malignancies of the oral γ-secretase inhibitor PF-03084014. Clin Cancer Res 21 (1): 60-7, 2015. [PUBMED Abstract]
- Bisogno G, Tagarelli A, Stramare R, et al.: Hydroxyurea treatment can avoid the need for aggressive surgery in pediatric fibromatosis. J Pediatr Hematol Oncol 35 (4): e171-3, 2013. [PUBMED Abstract]
- Meazza C, Casanova M, Trecate G, et al.: Objective response to hydroxyurea in a patient with heavily pre-treated aggressive fibromatosis. Pediatr Blood Cancer 55 (3): 587-8, 2010. [PUBMED Abstract]
- Balamuth NJ, Womer RB: Successful treatment of fibromatosis with hydroxyurea: Analysis of 20 pediatric cases. [Abstract] The Connective Tissue Oncology Society (CTOS) 14th Annual Meeting, 13–15 November 2008, London, United Kingdom A-34852, 2008. Also available online. Last accessed April 02, 2018.
- Meazza C, Bisogno G, Gronchi A, et al.: Aggressive fibromatosis in children and adolescents: the Italian experience. Cancer 116 (1): 233-40, 2010. [PUBMED Abstract]
- Hansmann A, Adolph C, Vogel T, et al.: High-dose tamoxifen and sulindac as first-line treatment for desmoid tumors. Cancer 100 (3): 612-20, 2004. [PUBMED Abstract]
- Skapek SX, Anderson JR, Hill DA, et al.: Safety and efficacy of high-dose tamoxifen and sulindac for desmoid tumor in children: results of a Children's Oncology Group (COG) phase II study. Pediatr Blood Cancer 60 (7): 1108-12, 2013. [PUBMED Abstract]
- Rutenberg MS, Indelicato DJ, Knapik JA, et al.: External-beam radiotherapy for pediatric and young adult desmoid tumors. Pediatr Blood Cancer 57 (3): 435-42, 2011. [PUBMED Abstract]
- Buckley PG, Mantripragada KK, Benetkiewicz M, et al.: A full-coverage, high-resolution human chromosome 22 genomic microarray for clinical and research applications. Hum Mol Genet 11 (25): 3221-9, 2002. [PUBMED Abstract]
- Valdivielso-Ramos M, Torrelo A, Campos M, et al.: Pediatric dermatofibrosarcoma protuberans in Madrid, Spain: multi-institutional outcomes. Pediatr Dermatol 31 (6): 676-82, 2014 Nov-Dec. [PUBMED Abstract]
- Gooskens SL, Oranje AP, van Adrichem LN, et al.: Imatinib mesylate for children with dermatofibrosarcoma protuberans (DFSP). Pediatr Blood Cancer 55 (2): 369-73, 2010. [PUBMED Abstract]
- Rubio GA, Alvarado A, Gerth DJ, et al.: Incidence and Outcomes of Dermatofibrosarcoma Protuberans in the US Pediatric Population. J Craniofac Surg 28 (1): 182-184, 2017. [PUBMED Abstract]
- Meguerditchian AN, Wang J, Lema B, et al.: Wide excision or Mohs micrographic surgery for the treatment of primary dermatofibrosarcoma protuberans. Am J Clin Oncol 33 (3): 300-3, 2010. [PUBMED Abstract]
- Dagan R, Morris CG, Zlotecki RA, et al.: Radiotherapy in the treatment of dermatofibrosarcoma protuberans. Am J Clin Oncol 28 (6): 537-9, 2005. [PUBMED Abstract]
- Sun LM, Wang CJ, Huang CC, et al.: Dermatofibrosarcoma protuberans: treatment results of 35 cases. Radiother Oncol 57 (2): 175-81, 2000. [PUBMED Abstract]
- Price VE, Fletcher JA, Zielenska M, et al.: Imatinib mesylate: an attractive alternative in young children with large, surgically challenging dermatofibrosarcoma protuberans. Pediatr Blood Cancer 44 (5): 511-5, 2005. [PUBMED Abstract]
- McArthur GA, Demetri GD, van Oosterom A, et al.: Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib Target Exploration Consortium Study B2225. J Clin Oncol 23 (4): 866-73, 2005. [PUBMED Abstract]
- Rutkowski P, Van Glabbeke M, Rankin CJ, et al.: Imatinib mesylate in advanced dermatofibrosarcoma protuberans: pooled analysis of two phase II clinical trials. J Clin Oncol 28 (10): 1772-9, 2010. [PUBMED Abstract]
- Miller SJ, Alam M, Andersen JS, et al.: Dermatofibrosarcoma protuberans. J Natl Compr Canc Netw 10 (3): 312-8, 2012. [PUBMED Abstract]
- Kao YC, Fletcher CDM, Alaggio R, et al.: Recurrent BRAF Gene Fusions in a Subset of Pediatric Spindle Cell Sarcomas: Expanding the Genetic Spectrum of Tumors With Overlapping Features With Infantile Fibrosarcoma. Am J Surg Pathol 42 (1): 28-38, 2018. [PUBMED Abstract]
- Sulkowski JP, Raval MV, Browne M: Margin status and multimodal therapy in infantile fibrosarcoma. Pediatr Surg Int 29 (8): 771-6, 2013. [PUBMED Abstract]
- Orbach D, Rey A, Cecchetto G, et al.: Infantile fibrosarcoma: management based on the European experience. J Clin Oncol 28 (2): 318-23, 2010. [PUBMED Abstract]
- Orbach D, Brennan B, De Paoli A, et al.: Conservative strategy in infantile fibrosarcoma is possible: The European paediatric Soft tissue sarcoma Study Group experience. Eur J Cancer 57: 1-9, 2016. [PUBMED Abstract]
- Spunt SL, Million L, Coffin C: The nonrhabdomyosarcoma soft tissue sarcoma. In: Pizzo PA, Poplack DG, eds.: Principles and Practice of Pediatric Oncology. 7th ed. Philadelphia, Pa: Lippincott Williams and Wilkins, 2015, pp 827-54.
- Loh ML, Ahn P, Perez-Atayde AR, et al.: Treatment of infantile fibrosarcoma with chemotherapy and surgery: results from the Dana-Farber Cancer Institute and Children's Hospital, Boston. J Pediatr Hematol Oncol 24 (9): 722-6, 2002. [PUBMED Abstract]
- Akyüz C, Küpeli S, Varan A, et al.: Infantile fibrosarcoma: retrospective analysis of eleven patients. Tumori 97 (2): 166-9, 2011 Mar-Apr. [PUBMED Abstract]
- Gallego S, Pericas N, Barber I, et al.: Infantile fibrosarcoma of the retroperitoneum: a site of unfavorable prognosis? Pediatr Hematol Oncol 28 (5): 451-3, 2011. [PUBMED Abstract]
- Parida L, Fernandez-Pineda I, Uffman JK, et al.: Clinical management of infantile fibrosarcoma: a retrospective single-institution review. Pediatr Surg Int 29 (7): 703-8, 2013. [PUBMED Abstract]
- Mody RJ, Wu YM, Lonigro RJ, et al.: Integrative Clinical Sequencing in the Management of Refractory or Relapsed Cancer in Youth. JAMA 314 (9): 913-25, 2015. [PUBMED Abstract]
- Wong V, Pavlick D, Brennan T, et al.: Evaluation of a Congenital Infantile Fibrosarcoma by Comprehensive Genomic Profiling Reveals an LMNA-NTRK1 Gene Fusion Responsive to Crizotinib. J Natl Cancer Inst 108 (1): , 2016. [PUBMED Abstract]
- Nagasubramanian R, Wei J, Gordon P, et al.: Infantile Fibrosarcoma With NTRK3-ETV6 Fusion Successfully Treated With the Tropomyosin-Related Kinase Inhibitor LOXO-101. Pediatr Blood Cancer 63 (8): 1468-70, 2016. [PUBMED Abstract]
- Yanagisawa R, Noguchi M, Fujita K, et al.: Preoperative Treatment With Pazopanib in a Case of Chemotherapy-Resistant Infantile Fibrosarcoma. Pediatr Blood Cancer 63 (2): 348-51, 2016. [PUBMED Abstract]
- Madden NP, Spicer RD, Allibone EB, et al.: Spontaneous regression of neonatal fibrosarcoma. Br J Cancer Suppl 18: S72-5, 1992. [PUBMED Abstract]
- Kovach SJ, Fischer AC, Katzman PJ, et al.: Inflammatory myofibroblastic tumors. J Surg Oncol 94 (5): 385-91, 2006. [PUBMED Abstract]
- Brodlie M, Barwick SC, Wood KM, et al.: Inflammatory myofibroblastic tumours of the respiratory tract: paediatric case series with varying clinical presentations. J Laryngol Otol 125 (8): 865-8, 2011. [PUBMED Abstract]
- Xiao Y, Zhou S, Ma C, et al.: Radiological and histopathological features of hepatic inflammatory myofibroblastic tumour: analysis of 10 cases. Clin Radiol 68 (11): 1114-20, 2013. [PUBMED Abstract]
- Karnak I, Senocak ME, Ciftci AO, et al.: Inflammatory myofibroblastic tumor in children: diagnosis and treatment. J Pediatr Surg 36 (6): 908-12, 2001. [PUBMED Abstract]
- Collin M, Charles A, Barker A, et al.: Inflammatory myofibroblastic tumour of the bladder in children: a review. J Pediatr Urol 11 (5): 239-45, 2015. [PUBMED Abstract]
- Coffin CM, Hornick JL, Fletcher CD: Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 31 (4): 509-20, 2007. [PUBMED Abstract]
- Lovly CM, Gupta A, Lipson D, et al.: Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov 4 (8): 889-95, 2014. [PUBMED Abstract]
- Devaney KO, Lafeir DJ, Triantafyllou A, et al.: Inflammatory myofibroblastic tumors of the head and neck: evaluation of clinicopathologic and prognostic features. Eur Arch Otorhinolaryngol 269 (12): 2461-5, 2012. [PUBMED Abstract]
- Mehta B, Mascarenhas L, Zhou S, et al.: Inflammatory myofibroblastic tumors in childhood. Pediatr Hematol Oncol 30 (7): 640-5, 2013. [PUBMED Abstract]
- Favini F, Resti AG, Collini P, et al.: Inflammatory myofibroblastic tumor of the conjunctiva: response to chemotherapy with low-dose methotrexate and vinorelbine. Pediatr Blood Cancer 54 (3): 483-5, 2010. [PUBMED Abstract]
- Doski JJ, Priebe CJ Jr, Driessnack M, et al.: Corticosteroids in the management of unresected plasma cell granuloma (inflammatory pseudotumor) of the lung. J Pediatr Surg 26 (9): 1064-6, 1991. [PUBMED Abstract]
- Diop B, Konate I, Ka S, et al.: Mesenteric myofibroblastic tumor: NSAID therapy after incomplete resection. J Visc Surg 148 (4): e311-4, 2011. [PUBMED Abstract]
- Dalton BG, Thomas PG, Sharp NE, et al.: Inflammatory myofibroblastic tumors in children. J Pediatr Surg 51 (4): 541-4, 2016. [PUBMED Abstract]
- Butrynski JE, D'Adamo DR, Hornick JL, et al.: Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med 363 (18): 1727-33, 2010. [PUBMED Abstract]
- Mossé YP, Lim MS, Voss SD, et al.: Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: a Children's Oncology Group phase 1 consortium study. Lancet Oncol 14 (6): 472-80, 2013. [PUBMED Abstract]
- Gaudichon J, Jeanne-Pasquier C, Deparis M, et al.: Complete and Repeated Response of a Metastatic ALK-rearranged Inflammatory Myofibroblastic Tumor to Crizotinib in a Teenage Girl. J Pediatr Hematol Oncol 38 (4): 308-11, 2016. [PUBMED Abstract]
- Nishio M, Murakami H, Horiike A, et al.: Phase I Study of Ceritinib (LDK378) in Japanese Patients with Advanced, Anaplastic Lymphoma Kinase-Rearranged Non-Small-Cell Lung Cancer or Other Tumors. J Thorac Oncol 10 (7): 1058-66, 2015. [PUBMED Abstract]
- Mossé YP, Voss SD, Lim MS, et al.: Targeting ALK With Crizotinib in Pediatric Anaplastic Large Cell Lymphoma and Inflammatory Myofibroblastic Tumor: A Children's Oncology Group Study. J Clin Oncol 35 (28): 3215-3221, 2017. [PUBMED Abstract]
- Evans HL: Low-grade fibromyxoid sarcoma: a clinicopathologic study of 33 cases with long-term follow-up. Am J Surg Pathol 35 (10): 1450-62, 2011. [PUBMED Abstract]
- Guillou L, Benhattar J, Gengler C, et al.: Translocation-positive low-grade fibromyxoid sarcoma: clinicopathologic and molecular analysis of a series expanding the morphologic spectrum and suggesting potential relationship to sclerosing epithelioid fibrosarcoma: a study from the French Sarcoma Group. Am J Surg Pathol 31 (9): 1387-402, 2007. [PUBMED Abstract]
- O'Sullivan MJ, Sirgi KE, Dehner LP: Low-grade fibrosarcoma (hyalinizing spindle cell tumor with giant rosettes) with pulmonary metastases at presentation: case report and review of the literature. Int J Surg Pathol 10 (3): 211-6, 2002. [PUBMED Abstract]
- Folpe AL, Lane KL, Paull G, et al.: Low-grade fibromyxoid sarcoma and hyalinizing spindle cell tumor with giant rosettes: a clinicopathologic study of 73 cases supporting their identity and assessing the impact of high-grade areas. Am J Surg Pathol 24 (10): 1353-60, 2000. [PUBMED Abstract]
- Sargar K, Kao SC, Spunt SL, et al.: MRI and CT of Low-Grade Fibromyxoid Sarcoma in Children: A Report From Children's Oncology Group Study ARST0332. AJR Am J Roentgenol 205 (2): 414-20, 2015. [PUBMED Abstract]
- Maretty-Nielsen K, Baerentzen S, Keller J, et al.: Low-Grade Fibromyxoid Sarcoma: Incidence, Treatment Strategy of Metastases, and Clinical Significance of the FUS Gene. Sarcoma 2013: 256280, 2013. [PUBMED Abstract]
- Prieto-Granada C, Zhang L, Chen HW, et al.: A genetic dichotomy between pure sclerosing epithelioid fibrosarcoma (SEF) and hybrid SEF/low-grade fibromyxoid sarcoma: a pathologic and molecular study of 18 cases. Genes Chromosomes Cancer 54 (1): 28-38, 2015. [PUBMED Abstract]
- Pollock BH, Jenson HB, Leach CT, et al.: Risk factors for pediatric human immunodeficiency virus-related malignancy. JAMA 289 (18): 2393-9, 2003. [PUBMED Abstract]
- Kleinerman RA, Tucker MA, Abramson DH, et al.: Risk of soft tissue sarcomas by individual subtype in survivors of hereditary retinoblastoma. J Natl Cancer Inst 99 (1): 24-31, 2007. [PUBMED Abstract]
- Samuels BL, Chawla S, Patel S, et al.: Clinical outcomes and safety with trabectedin therapy in patients with advanced soft tissue sarcomas following failure of prior chemotherapy: results of a worldwide expanded access program study. Ann Oncol 24 (6): 1703-9, 2013. [PUBMED Abstract]
- Enzinger FM, Zhang RY: Plexiform fibrohistiocytic tumor presenting in children and young adults. An analysis of 65 cases. Am J Surg Pathol 12 (11): 818-26, 1988. [PUBMED Abstract]
- Black J, Coffin CM, Dehner LP: Fibrohistiocytic tumors and related neoplasms in children and adolescents. Pediatr Dev Pathol 15 (1 Suppl): 181-210, 2012. [PUBMED Abstract]
- Moosavi C, Jha P, Fanburg-Smith JC: An update on plexiform fibrohistiocytic tumor and addition of 66 new cases from the Armed Forces Institute of Pathology, in honor of Franz M. Enzinger, MD. Ann Diagn Pathol 11 (5): 313-9, 2007. [PUBMED Abstract]
- Billings SD, Folpe AL: Cutaneous and subcutaneous fibrohistiocytic tumors of intermediate malignancy: an update. Am J Dermatopathol 26 (2): 141-55, 2004. [PUBMED Abstract]
- Remstein ED, Arndt CA, Nascimento AG: Plexiform fibrohistiocytic tumor: clinicopathologic analysis of 22 cases. Am J Surg Pathol 23 (6): 662-70, 1999. [PUBMED Abstract]
- Salomao DR, Nascimento AG: Plexiform fibrohistiocytic tumor with systemic metastases: a case report. Am J Surg Pathol 21 (4): 469-76, 1997. [PUBMED Abstract]
- Redlich GC, Montgomery KD, Allgood GA, et al.: Plexiform fibrohistiocytic tumor with a clonal cytogenetic anomaly. Cancer Genet Cytogenet 108 (2): 141-3, 1999. [PUBMED Abstract]
- Luzar B, Calonje E: Cutaneous fibrohistiocytic tumours - an update. Histopathology 56 (1): 148-65, 2010. [PUBMED Abstract]
- Dantonello TM, Leuschner I, Vokuhl C, et al.: Malignant ectomesenchymoma in children and adolescents: report from the Cooperative Weichteilsarkom Studiengruppe (CWS). Pediatr Blood Cancer 60 (2): 224-9, 2013. [PUBMED Abstract]
- Carli M, Ferrari A, Mattke A, et al.: Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J Clin Oncol 23 (33): 8422-30, 2005. [PUBMED Abstract]
- Zhang M, Wang Y, Jones S, et al.: Somatic mutations of SUZ12 in malignant peripheral nerve sheath tumors. Nat Genet 46 (11): 1170-2, 2014. [PUBMED Abstract]
- Hagel C, Zils U, Peiper M, et al.: Histopathology and clinical outcome of NF1-associated vs. sporadic malignant peripheral nerve sheath tumors. J Neurooncol 82 (2): 187-92, 2007. [PUBMED Abstract]
- Zou C, Smith KD, Liu J, et al.: Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg 249 (6): 1014-22, 2009. [PUBMED Abstract]
- Okada K, Hasegawa T, Tajino T, et al.: Clinical relevance of pathological grades of malignant peripheral nerve sheath tumor: a multi-institution TMTS study of 56 cases in Northern Japan. Ann Surg Oncol 14 (2): 597-604, 2007. [PUBMED Abstract]
- Amirian ES, Goodman JC, New P, et al.: Pediatric and adult malignant peripheral nerve sheath tumors: an analysis of data from the surveillance, epidemiology, and end results program. J Neurooncol 116 (3): 609-16, 2014. [PUBMED Abstract]
- Valentin T, Le Cesne A, Ray-Coquard I, et al.: Management and prognosis of malignant peripheral nerve sheath tumors: The experience of the French Sarcoma Group (GSF-GETO). Eur J Cancer 56: 77-84, 2016. [PUBMED Abstract]
- Bergamaschi L, Bisogno G, Manzitti C, et al.: Salvage rates and prognostic factors after relapse in children and adolescents with malignant peripheral nerve sheath tumors. Pediatr Blood Cancer 65 (2): , 2018. [PUBMED Abstract]
- Ferrari A, Bisogno G, Macaluso A, et al.: Soft-tissue sarcomas in children and adolescents with neurofibromatosis type 1. Cancer 109 (7): 1406-12, 2007. [PUBMED Abstract]
- Okur FV, Oguz A, Karadeniz C, et al.: Malignant triton tumor of the pelvis in a 2-year-old boy. J Pediatr Hematol Oncol 28 (3): 173-6, 2006. [PUBMED Abstract]
- Weiss SW, Goldblum JR: Enzinger and Weiss's Soft Tissue Tumors. 4th ed. St. Louis, Mo: Mosby, 2001.
- Fernandez-Pineda I, Parida L, Jenkins JJ, et al.: Childhood hemangiopericytoma: review of St Jude Children's Research Hospital. J Pediatr Hematol Oncol 33 (5): 356-9, 2011. [PUBMED Abstract]
- Rodriguez-Galindo C, Ramsey K, Jenkins JJ, et al.: Hemangiopericytoma in children and infants. Cancer 88 (1): 198-204, 2000. [PUBMED Abstract]
- Ferrari A, Casanova M, Bisogno G, et al.: Hemangiopericytoma in pediatric ages: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Cancer 92 (10): 2692-8, 2001. [PUBMED Abstract]
- Bien E, Stachowicz-Stencel T, Godzinski J, et al.: Retrospective multi-institutional study on hemangiopericytoma in Polish children. Pediatr Int 51 (1): 19-24, 2009. [PUBMED Abstract]
- Wiswell TE, Davis J, Cunningham BE, et al.: Infantile myofibromatosis: the most common fibrous tumor of infancy. J Pediatr Surg 23 (4): 315-8, 1988. [PUBMED Abstract]
- Chung EB, Enzinger FM: Infantile myofibromatosis. Cancer 48 (8): 1807-18, 1981. [PUBMED Abstract]
- Modi N: Congenital generalised fibromatosis. Arch Dis Child 57 (11): 881-2, 1982. [PUBMED Abstract]
- Levine E, Fréneaux P, Schleiermacher G, et al.: Risk-adapted therapy for infantile myofibromatosis in children. Pediatr Blood Cancer 59 (1): 115-20, 2012. [PUBMED Abstract]
- Larralde M, Hoffner MV, Boggio P, et al.: Infantile myofibromatosis: report of nine patients. Pediatr Dermatol 27 (1): 29-33, 2010 Jan-Feb. [PUBMED Abstract]
- Cheung YH, Gayden T, Campeau PM, et al.: A recurrent PDGFRB mutation causes familial infantile myofibromatosis. Am J Hum Genet 92 (6): 996-1000, 2013. [PUBMED Abstract]
- Gopal M, Chahal G, Al-Rifai Z, et al.: Infantile myofibromatosis. Pediatr Surg Int 24 (3): 287-91, 2008. [PUBMED Abstract]
- Weaver MS, Navid F, Huppmann A, et al.: Vincristine and Dactinomycin in Infantile Myofibromatosis With a Review of Treatment Options. J Pediatr Hematol Oncol 37 (3): 237-41, 2015. [PUBMED Abstract]
- Orbach D, Brennan B, Casanova M, et al.: Paediatric and adolescent alveolar soft part sarcoma: A joint series from European cooperative groups. Pediatr Blood Cancer 60 (11): 1826-32, 2013. [PUBMED Abstract]
- Ferrari A, Sultan I, Huang TT, et al.: Soft tissue sarcoma across the age spectrum: a population-based study from the Surveillance Epidemiology and End Results database. Pediatr Blood Cancer 57 (6): 943-9, 2011. [PUBMED Abstract]
- Wang HW, Qin XJ, Yang WJ, et al.: Alveolar soft part sarcoma of the oral and maxillofacial region: clinical analysis in a series of 18 patients. Oral Surg Oral Med Oral Pathol Oral Radiol 119 (4): 396-401, 2015. [PUBMED Abstract]
- Kayton ML, Meyers P, Wexler LH, et al.: Clinical presentation, treatment, and outcome of alveolar soft part sarcoma in children, adolescents, and young adults. J Pediatr Surg 41 (1): 187-93, 2006. [PUBMED Abstract]
- Ladanyi M, Lui MY, Antonescu CR, et al.: The der(17)t(X;17)(p11;q25) of human alveolar soft part sarcoma fuses the TFE3 transcription factor gene to ASPL, a novel gene at 17q25. Oncogene 20 (1): 48-57, 2001. [PUBMED Abstract]
- Williams A, Bartle G, Sumathi VP, et al.: Detection of ASPL/TFE3 fusion transcripts and the TFE3 antigen in formalin-fixed, paraffin-embedded tissue in a series of 18 cases of alveolar soft part sarcoma: useful diagnostic tools in cases with unusual histological features. Virchows Arch 458 (3): 291-300, 2011. [PUBMED Abstract]
- Lieberman PH, Brennan MF, Kimmel M, et al.: Alveolar soft-part sarcoma. A clinico-pathologic study of half a century. Cancer 63 (1): 1-13, 1989. [PUBMED Abstract]
- Casanova M, Ferrari A, Bisogno G, et al.: Alveolar soft part sarcoma in children and adolescents: A report from the Soft-Tissue Sarcoma Italian Cooperative Group. Ann Oncol 11 (11): 1445-9, 2000. [PUBMED Abstract]
- Pennacchioli E, Fiore M, Collini P, et al.: Alveolar soft part sarcoma: clinical presentation, treatment, and outcome in a series of 33 patients at a single institution. Ann Surg Oncol 17 (12): 3229-33, 2010. [PUBMED Abstract]
- Flores RJ, Harrison DJ, Federman NC, et al.: Alveolar soft part sarcoma in children and young adults: A report of 69 cases. Pediatr Blood Cancer : , 2018. [PUBMED Abstract]
- Roozendaal KJ, de Valk B, ten Velden JJ, et al.: Alveolar soft-part sarcoma responding to interferon alpha-2b. Br J Cancer 89 (2): 243-5, 2003. [PUBMED Abstract]
- Conde N, Cruz O, Albert A, et al.: Antiangiogenic treatment as a pre-operative management of alveolar soft-part sarcoma. Pediatr Blood Cancer 57 (6): 1071-3, 2011. [PUBMED Abstract]
- Stacchiotti S, Negri T, Zaffaroni N, et al.: Sunitinib in advanced alveolar soft part sarcoma: evidence of a direct antitumor effect. Ann Oncol 22 (7): 1682-90, 2011. [PUBMED Abstract]
- Kummar S, Allen D, Monks A, et al.: Cediranib for metastatic alveolar soft part sarcoma. J Clin Oncol 31 (18): 2296-302, 2013. [PUBMED Abstract]
- Coindre JM, Hostein I, Terrier P, et al.: Diagnosis of clear cell sarcoma by real-time reverse transcriptase-polymerase chain reaction analysis of paraffin embedded tissues: clinicopathologic and molecular analysis of 44 patients from the French sarcoma group. Cancer 107 (5): 1055-64, 2006. [PUBMED Abstract]
- Meis-Kindblom JM: Clear cell sarcoma of tendons and aponeuroses: a historical perspective and tribute to the man behind the entity. Adv Anat Pathol 13 (6): 286-92, 2006. [PUBMED Abstract]
- Dim DC, Cooley LD, Miranda RN: Clear cell sarcoma of tendons and aponeuroses: a review. Arch Pathol Lab Med 131 (1): 152-6, 2007. [PUBMED Abstract]
- Blazer DG 3rd, Lazar AJ, Xing Y, et al.: Clinical outcomes of molecularly confirmed clear cell sarcoma from a single institution and in comparison with data from the Surveillance, Epidemiology, and End Results registry. Cancer 115 (13): 2971-9, 2009. [PUBMED Abstract]
- Fujimura Y, Siddique H, Lee L, et al.: EWS-ATF-1 chimeric protein in soft tissue clear cell sarcoma associates with CREB-binding protein and interferes with p53-mediated trans-activation function. Oncogene 20 (46): 6653-9, 2001. [PUBMED Abstract]
- Ferrari A, Casanova M, Bisogno G, et al.: Clear cell sarcoma of tendons and aponeuroses in pediatric patients: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Cancer 94 (12): 3269-76, 2002. [PUBMED Abstract]
- Karita M, Tsuchiya H, Yamamoto N, et al.: Caffeine-potentiated chemotherapy for clear cell sarcoma: a report of five cases. Int J Clin Oncol 18 (1): 33-7, 2013. [PUBMED Abstract]
- Leuschner I, Radig K, Harms D: Desmoplastic small round cell tumor. Semin Diagn Pathol 13 (3): 204-12, 1996. [PUBMED Abstract]
- Kushner BH, LaQuaglia MP, Wollner N, et al.: Desmoplastic small round-cell tumor: prolonged progression-free survival with aggressive multimodality therapy. J Clin Oncol 14 (5): 1526-31, 1996. [PUBMED Abstract]
- Saab R, Khoury JD, Krasin M, et al.: Desmoplastic small round cell tumor in childhood: the St. Jude Children's Research Hospital experience. Pediatr Blood Cancer 49 (3): 274-9, 2007. [PUBMED Abstract]
- Wang LL, Perlman EJ, Vujanic GM, et al.: Desmoplastic small round cell tumor of the kidney in childhood. Am J Surg Pathol 31 (4): 576-84, 2007. [PUBMED Abstract]
- Arora VC, Price AP, Fleming S, et al.: Characteristic imaging features of desmoplastic small round cell tumour. Pediatr Radiol 43 (1): 93-102, 2013. [PUBMED Abstract]
- Gerald WL, Ladanyi M, de Alava E, et al.: Clinical, pathologic, and molecular spectrum of tumors associated with t(11;22)(p13;q12): desmoplastic small round-cell tumor and its variants. J Clin Oncol 16 (9): 3028-36, 1998. [PUBMED Abstract]
- Lal DR, Su WT, Wolden SL, et al.: Results of multimodal treatment for desmoplastic small round cell tumors. J Pediatr Surg 40 (1): 251-5, 2005. [PUBMED Abstract]
- Philippe-Chomette P, Kabbara N, Andre N, et al.: Desmoplastic small round cell tumors with EWS-WT1 fusion transcript in children and young adults. Pediatr Blood Cancer 58 (6): 891-7, 2012. [PUBMED Abstract]
- Sedig L, Geiger J, Mody R, et al.: Paratesticular desmoplastic small round cell tumors: A case report and review of the literature. Pediatr Blood Cancer 64 (12): , 2017. [PUBMED Abstract]
- Schwarz RE, Gerald WL, Kushner BH, et al.: Desmoplastic small round cell tumors: prognostic indicators and results of surgical management. Ann Surg Oncol 5 (5): 416-22, 1998 Jul-Aug. [PUBMED Abstract]
- Goodman KA, Wolden SL, La Quaglia MP, et al.: Whole abdominopelvic radiotherapy for desmoplastic small round-cell tumor. Int J Radiat Oncol Biol Phys 54 (1): 170-6, 2002. [PUBMED Abstract]
- Osborne EM, Briere TM, Hayes-Jordan A, et al.: Survival and toxicity following sequential multimodality treatment including whole abdominopelvic radiotherapy for patients with desmoplastic small round cell tumor. Radiother Oncol 119 (1): 40-4, 2016. [PUBMED Abstract]
- Atallah V, Honore C, Orbach D, et al.: Role of Adjuvant Radiation Therapy After Surgery for Abdominal Desmoplastic Small Round Cell Tumors. Int J Radiat Oncol Biol Phys 95 (4): 1244-53, 2016. [PUBMED Abstract]
- Tarek N, Hayes-Jordan A, Salvador L, et al.: Recurrent desmoplastic small round cell tumor responding to an mTOR inhibitor containing regimen. Pediatr Blood Cancer 65 (1): , 2018. [PUBMED Abstract]
- Cook RJ, Wang Z, Arora M, et al.: Clinical outcomes of patients with desmoplastic small round cell tumor of the peritoneum undergoing autologous HCT: a CIBMTR retrospective analysis. Bone Marrow Transplant 47 (11): 1455-8, 2012. [PUBMED Abstract]
- Chbani L, Guillou L, Terrier P, et al.: Epithelioid sarcoma: a clinicopathologic and immunohistochemical analysis of 106 cases from the French sarcoma group. Am J Clin Pathol 131 (2): 222-7, 2009. [PUBMED Abstract]
- Hornick JL, Dal Cin P, Fletcher CD: Loss of INI1 expression is characteristic of both conventional and proximal-type epithelioid sarcoma. Am J Surg Pathol 33 (4): 542-50, 2009. [PUBMED Abstract]
- Knutson SK, Warholic NM, Wigle TJ, et al.: Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. Proc Natl Acad Sci U S A 110 (19): 7922-7, 2013. [PUBMED Abstract]
- Guzzetta AA, Montgomery EA, Lyu H, et al.: Epithelioid sarcoma: one institution's experience with a rare sarcoma. J Surg Res 177 (1): 116-22, 2012. [PUBMED Abstract]
- Casanova M, Ferrari A, Collini P, et al.: Epithelioid sarcoma in children and adolescents: a report from the Italian Soft Tissue Sarcoma Committee. Cancer 106 (3): 708-17, 2006. [PUBMED Abstract]
- Kodet R, Newton WA Jr, Sachs N, et al.: Rhabdoid tumors of soft tissues: a clinicopathologic study of 26 cases enrolled on the Intergroup Rhabdomyosarcoma Study. Hum Pathol 22 (7): 674-84, 1991. [PUBMED Abstract]
- Biegel JA, Zhou JY, Rorke LB, et al.: Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res 59 (1): 74-9, 1999. [PUBMED Abstract]
- Eaton KW, Tooke LS, Wainwright LM, et al.: Spectrum of SMARCB1/INI1 mutations in familial and sporadic rhabdoid tumors. Pediatr Blood Cancer 56 (1): 7-15, 2011. [PUBMED Abstract]
- Lee RS, Stewart C, Carter SL, et al.: A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J Clin Invest 122 (8): 2983-8, 2012. [PUBMED Abstract]
- Sultan I, Qaddoumi I, Rodríguez-Galindo C, et al.: Age, stage, and radiotherapy, but not primary tumor site, affects the outcome of patients with malignant rhabdoid tumors. Pediatr Blood Cancer 54 (1): 35-40, 2010. [PUBMED Abstract]
- Puri DR, Meyers PA, Kraus DH, et al.: Radiotherapy in the multimodal treatment of extrarenal extracranial malignant rhabdoid tumors. Pediatr Blood Cancer 50 (1): 167-9, 2008. [PUBMED Abstract]
- Madigan CE, Armenian SH, Malogolowkin MH, et al.: Extracranial malignant rhabdoid tumors in childhood: the Childrens Hospital Los Angeles experience. Cancer 110 (9): 2061-6, 2007. [PUBMED Abstract]
- Bourdeaut F, Fréneaux P, Thuille B, et al.: Extra-renal non-cerebral rhabdoid tumours. Pediatr Blood Cancer 51 (3): 363-8, 2008. [PUBMED Abstract]
- Wetmore C, Boyett J, Li S, et al.: Alisertib is active as single agent in recurrent atypical teratoid rhabdoid tumors in 4 children. Neuro Oncol 17 (6): 882-8, 2015. [PUBMED Abstract]
- Tsuneyoshi M, Enjoji M, Iwasaki H, et al.: Extraskeletal myxoid chondrosarcoma--a clinicopathologic and electron microscopic study. Acta Pathol Jpn 31 (3): 439-47, 1981. [PUBMED Abstract]
- Hachitanda Y, Tsuneyoshi M, Daimaru Y, et al.: Extraskeletal myxoid chondrosarcoma in young children. Cancer 61 (12): 2521-6, 1988. [PUBMED Abstract]
- Hisaoka M, Ishida T, Imamura T, et al.: TFG is a novel fusion partner of NOR1 in extraskeletal myxoid chondrosarcoma. Genes Chromosomes Cancer 40 (4): 325-8, 2004. [PUBMED Abstract]
- Enzinger FM, Shiraki M: Extraskeletal myxoid chondrosarcoma. An analysis of 34 cases. Hum Pathol 3 (3): 421-35, 1972. [PUBMED Abstract]
- McGrory JE, Rock MG, Nascimento AG, et al.: Extraskeletal myxoid chondrosarcoma. Clin Orthop Relat Res (382): 185-90, 2001. [PUBMED Abstract]
- Drilon AD, Popat S, Bhuchar G, et al.: Extraskeletal myxoid chondrosarcoma: a retrospective review from 2 referral centers emphasizing long-term outcomes with surgery and chemotherapy. Cancer 113 (12): 3364-71, 2008. [PUBMED Abstract]
- Stacchiotti S, Pantaleo MA, Astolfi A, et al.: Activity of sunitinib in extraskeletal myxoid chondrosarcoma. Eur J Cancer 50 (9): 1657-64, 2014. [PUBMED Abstract]
- Martignoni G, Pea M, Reghellin D, et al.: Molecular pathology of lymphangioleiomyomatosis and other perivascular epithelioid cell tumors. Arch Pathol Lab Med 134 (1): 33-40, 2010. [PUBMED Abstract]
- Bissler JJ, McCormack FX, Young LR, et al.: Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med 358 (2): 140-51, 2008. [PUBMED Abstract]
- Davies DM, Johnson SR, Tattersfield AE, et al.: Sirolimus therapy in tuberous sclerosis or sporadic lymphangioleiomyomatosis. N Engl J Med 358 (2): 200-3, 2008. [PUBMED Abstract]
- Agaram NP, Sung YS, Zhang L, et al.: Dichotomy of Genetic Abnormalities in PEComas With Therapeutic Implications. Am J Surg Pathol 39 (6): 813-25, 2015. [PUBMED Abstract]
- Folpe A, Inwards C, eds.: Bone and Soft Tissue Pathology: A Volume in the Foundations in Diagnostic Pathology. Philadelphia, Pa: WB Saunders Co, 2010.
- Armah HB, Parwani AV: Perivascular epithelioid cell tumor. Arch Pathol Lab Med 133 (4): 648-54, 2009. [PUBMED Abstract]
- Alaggio R, Cecchetto G, Martignoni G, et al.: Malignant perivascular epithelioid cell tumor in children: description of a case and review of the literature. J Pediatr Surg 47 (6): e31-40, 2012. [PUBMED Abstract]
- Wagner AJ, Malinowska-Kolodziej I, Morgan JA, et al.: Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol 28 (5): 835-40, 2010. [PUBMED Abstract]
- Sultan I, Rodriguez-Galindo C, Saab R, et al.: Comparing children and adults with synovial sarcoma in the Surveillance, Epidemiology, and End Results program, 1983 to 2005: an analysis of 1268 patients. Cancer 115 (15): 3537-47, 2009. [PUBMED Abstract]
- Wang JG, Li NN: Primary cardiac synovial sarcoma. Ann Thorac Surg 95 (6): 2202-9, 2013. [PUBMED Abstract]
- Pappo AS, Fontanesi J, Luo X, et al.: Synovial sarcoma in children and adolescents: the St Jude Children's Research Hospital experience. J Clin Oncol 12 (11): 2360-6, 1994. [PUBMED Abstract]
- Ferrari A, De Salvo GL, Oberlin O, et al.: Synovial sarcoma in children and adolescents: a critical reappraisal of staging investigations in relation to the rate of metastatic involvement at diagnosis. Eur J Cancer 48 (9): 1370-5, 2012. [PUBMED Abstract]
- van de Rijn M, Barr FG, Collins MH, et al.: Absence of SYT-SSX fusion products in soft tissue tumors other than synovial sarcoma. Am J Clin Pathol 112 (1): 43-9, 1999. [PUBMED Abstract]
- Krsková L, Sumerauer D, Stejskalová E, et al.: A novel variant of SYT-SSX1 fusion gene in a case of spindle cell synovial sarcoma. Diagn Mol Pathol 16 (3): 179-83, 2007. [PUBMED Abstract]
- Su L, Sampaio AV, Jones KB, et al.: Deconstruction of the SS18-SSX fusion oncoprotein complex: insights into disease etiology and therapeutics. Cancer Cell 21 (3): 333-47, 2012. [PUBMED Abstract]
- Arnold MA, Arnold CA, Li G, et al.: A unique pattern of INI1 immunohistochemistry distinguishes synovial sarcoma from its histologic mimics. Hum Pathol 44 (5): 881-7, 2013. [PUBMED Abstract]
- Vlenterie M, Ho VK, Kaal SE, et al.: Age as an independent prognostic factor for survival of localised synovial sarcoma patients. Br J Cancer 113 (11): 1602-6, 2015. [PUBMED Abstract]
- Okcu MF, Munsell M, Treuner J, et al.: Synovial sarcoma of childhood and adolescence: a multicenter, multivariate analysis of outcome. J Clin Oncol 21 (8): 1602-11, 2003. [PUBMED Abstract]
- Brecht IB, Ferrari A, Int-Veen C, et al.: Grossly-resected synovial sarcoma treated by the German and Italian Pediatric Soft Tissue Sarcoma Cooperative Groups: discussion on the role of adjuvant therapies. Pediatr Blood Cancer 46 (1): 11-7, 2006. [PUBMED Abstract]
- Stanelle EJ, Christison-Lagay ER, Healey JH, et al.: Pediatric and adolescent synovial sarcoma: multivariate analysis of prognostic factors and survival outcomes. Ann Surg Oncol 20 (1): 73-9, 2013. [PUBMED Abstract]
- Trassard M, Le Doussal V, Hacène K, et al.: Prognostic factors in localized primary synovial sarcoma: a multicenter study of 128 adult patients. J Clin Oncol 19 (2): 525-34, 2001. [PUBMED Abstract]
- Guillou L, Benhattar J, Bonichon F, et al.: Histologic grade, but not SYT-SSX fusion type, is an important prognostic factor in patients with synovial sarcoma: a multicenter, retrospective analysis. J Clin Oncol 22 (20): 4040-50, 2004. [PUBMED Abstract]
- Ferrari A, Gronchi A, Casanova M, et al.: Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer 101 (3): 627-34, 2004. [PUBMED Abstract]
- Lagarde P, Przybyl J, Brulard C, et al.: Chromosome instability accounts for reverse metastatic outcomes of pediatric and adult synovial sarcomas. J Clin Oncol 31 (5): 608-15, 2013. [PUBMED Abstract]
- Stegmaier S, Leuschner I, Poremba C, et al.: The prognostic impact of SYT-SSX fusion type and histological grade in pediatric patients with synovial sarcoma treated according to the CWS (Cooperative Weichteilsarkom Studie) trials. Pediatr Blood Cancer 64 (1): 89-95, 2017. [PUBMED Abstract]
- Scheer M, Dantonello T, Hallmen E, et al.: Primary Metastatic Synovial Sarcoma: Experience of the CWS Study Group. Pediatr Blood Cancer 63 (7): 1198-206, 2016. [PUBMED Abstract]
- Ferrari A, De Salvo GL, Dall'Igna P, et al.: Salvage rates and prognostic factors after relapse in children and adolescents with initially localised synovial sarcoma. Eur J Cancer 48 (18): 3448-55, 2012. [PUBMED Abstract]
- Ferrari A, Chi YY, De Salvo GL, et al.: Surgery alone is sufficient therapy for children and adolescents with low-risk synovial sarcoma: A joint analysis from the European paediatric soft tissue sarcoma Study Group and the Children's Oncology Group. Eur J Cancer 78: 1-6, 2017. [PUBMED Abstract]
- McGrory JE, Pritchard DJ, Arndt CA, et al.: Nonrhabdomyosarcoma soft tissue sarcomas in children. The Mayo Clinic experience. Clin Orthop (374): 247-58, 2000. [PUBMED Abstract]
- Van Glabbeke M, van Oosterom AT, Oosterhuis JW, et al.: Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens--a European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study. J Clin Oncol 17 (1): 150-7, 1999. [PUBMED Abstract]
- Koscielniak E, Harms D, Henze G, et al.: Results of treatment for soft tissue sarcoma in childhood and adolescence: a final report of the German Cooperative Soft Tissue Sarcoma Study CWS-86. J Clin Oncol 17 (12): 3706-19, 1999. [PUBMED Abstract]
- Pappo AS, Devidas M, Jenkins J, et al.: Phase II trial of neoadjuvant vincristine, ifosfamide, and doxorubicin with granulocyte colony-stimulating factor support in children and adolescents with advanced-stage nonrhabdomyosarcomatous soft tissue sarcomas: a Pediatric Oncology Group Study. J Clin Oncol 23 (18): 4031-8, 2005. [PUBMED Abstract]
- Pappo AS, Rao BN, Jenkins JJ, et al.: Metastatic nonrhabdomyosarcomatous soft-tissue sarcomas in children and adolescents: the St. Jude Children's Research Hospital experience. Med Pediatr Oncol 33 (2): 76-82, 1999. [PUBMED Abstract]
- Brennan B, Stevens M, Kelsey A, et al.: Synovial sarcoma in childhood and adolescence: a retrospective series of 77 patients registered by the Children's Cancer and Leukaemia Group between 1991 and 2006. Pediatr Blood Cancer 55 (1): 85-90, 2010. [PUBMED Abstract]
- Ferrari A, Miceli R, Rey A, et al.: Non-metastatic unresected paediatric non-rhabdomyosarcoma soft tissue sarcomas: results of a pooled analysis from United States and European groups. Eur J Cancer 47 (5): 724-31, 2011. [PUBMED Abstract]
- Raney RB: Synovial sarcoma in young people: background, prognostic factors, and therapeutic questions. J Pediatr Hematol Oncol 27 (4): 207-11, 2005. [PUBMED Abstract]
- Orbach D, Mc Dowell H, Rey A, et al.: Sparing strategy does not compromise prognosis in pediatric localized synovial sarcoma: experience of the International Society of Pediatric Oncology, Malignant Mesenchymal Tumors (SIOP-MMT) Working Group. Pediatr Blood Cancer 57 (7): 1130-6, 2011. [PUBMED Abstract]
- Ladenstein R, Treuner J, Koscielniak E, et al.: Synovial sarcoma of childhood and adolescence. Report of the German CWS-81 study. Cancer 71 (11): 3647-55, 1993. [PUBMED Abstract]
- Venkatramani R, Anderson JR, Million L, et al.: Risk-based treatment for synovial sarcoma in patients under 30 years of age: Children’s Oncology Group study ARST0332. [Abstract] J Clin Oncol 33 (15 Suppl): A-10012, 2015. Also available online. Last accessed April 02, 2018.
- Ferrari A, De Salvo GL, Brennan B, et al.: Synovial sarcoma in children and adolescents: the European Pediatric Soft Tissue Sarcoma Study Group prospective trial (EpSSG NRSTS 2005). Ann Oncol 26 (3): 567-72, 2015. [PUBMED Abstract]
- Spunt SL, Million L, Anderson JR, et al.: Risk-based treatment for nonrhabdomyosarcoma soft tissue sarcomas (NRSTS) in patients under 30 years of age: Children’s Oncology Group study ARST0332. [Abstract] J Clin Oncol 32 (Suppl 15): A-10008, 2014. Also available online. Last accessed April 02, 2018.
- Randall RL, Albritton KH, Ferney BJ, et al.: Malignant fibrous histiocytoma of soft tissue: an abandoned diagnosis. Am J Orthop 33 (12): 602-8, 2004. [PUBMED Abstract]
- Alaggio R, Collini P, Randall RL, et al.: Undifferentiated high-grade pleomorphic sarcomas in children: a clinicopathologic study of 10 cases and review of literature. Pediatr Dev Pathol 13 (3): 209-17, 2010 May-Jun. [PUBMED Abstract]
- Daw NC, Billups CA, Pappo AS, et al.: Malignant fibrous histiocytoma and other fibrohistiocytic tumors in pediatric patients: the St. Jude Children's Research Hospital experience. Cancer 97 (11): 2839-47, 2003. [PUBMED Abstract]
- Coffin CM, Dehner LP: Vascular tumors in children and adolescents: a clinicopathologic study of 228 tumors in 222 patients. Pathol Annu 28 Pt 1: 97-120, 1993. [PUBMED Abstract]
- Cioffi A, Reichert S, Antonescu CR, et al.: Angiosarcomas and other sarcomas of endothelial origin. Hematol Oncol Clin North Am 27 (5): 975-88, 2013. [PUBMED Abstract]
- Jeng MR, Fuh B, Blatt J, et al.: Malignant transformation of infantile hemangioma to angiosarcoma: response to chemotherapy with bevacizumab. Pediatr Blood Cancer 61 (11): 2115-7, 2014. [PUBMED Abstract]
- Dehner LP, Ishak KG: Vascular tumors of the liver in infants and children. A study of 30 cases and review of the literature. Arch Pathol 92 (2): 101-11, 1971. [PUBMED Abstract]
- Ferrari A, Casanova M, Bisogno G, et al.: Malignant vascular tumors in children and adolescents: a report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Med Pediatr Oncol 39 (2): 109-14, 2002. [PUBMED Abstract]
- Deyrup AT, Miettinen M, North PE, et al.: Pediatric cutaneous angiosarcomas: a clinicopathologic study of 10 cases. Am J Surg Pathol 35 (1): 70-5, 2011. [PUBMED Abstract]
- Elliott P, Kleinschmidt I: Angiosarcoma of the liver in Great Britain in proximity to vinyl chloride sites. Occup Environ Med 54 (1): 14-8, 1997. [PUBMED Abstract]
- Lezama-del Valle P, Gerald WL, Tsai J, et al.: Malignant vascular tumors in young patients. Cancer 83 (8): 1634-9, 1998. [PUBMED Abstract]
- Fata F, O'Reilly E, Ilson D, et al.: Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer 86 (10): 2034-7, 1999. [PUBMED Abstract]
- Lahat G, Dhuka AR, Hallevi H, et al.: Angiosarcoma: clinical and molecular insights. Ann Surg 251 (6): 1098-106, 2010. [PUBMED Abstract]
- Orlando G, Adam R, Mirza D, et al.: Hepatic hemangiosarcoma: an absolute contraindication to liver transplantation--the European Liver Transplant Registry experience. Transplantation 95 (6): 872-7, 2013. [PUBMED Abstract]
- Sanada T, Nakayama H, Irisawa R, et al.: Clinical outcome and dose volume evaluation in patients who undergo brachytherapy for angiosarcoma of the scalp and face. Mol Clin Oncol 6 (3): 334-340, 2017. [PUBMED Abstract]
- Dickson MA, D'Adamo DR, Keohan ML, et al.: Phase II Trial of Gemcitabine and Docetaxel with Bevacizumab in Soft Tissue Sarcoma. Sarcoma 2015: 532478, 2015. [PUBMED Abstract]
- North PE, Waner M, Mizeracki A, et al.: A unique microvascular phenotype shared by juvenile hemangiomas and human placenta. Arch Dermatol 137 (5): 559-70, 2001. [PUBMED Abstract]
- Boye E, Yu Y, Paranya G, et al.: Clonality and altered behavior of endothelial cells from hemangiomas. J Clin Invest 107 (6): 745-52, 2001. [PUBMED Abstract]
- Ravi V, Patel S: Vascular sarcomas. Curr Oncol Rep 15 (4): 347-55, 2013. [PUBMED Abstract]
- Grassia KL, Peterman CM, Iacobas I, et al.: Clinical case series of pediatric hepatic angiosarcoma. Pediatr Blood Cancer 64 (11): , 2017. [PUBMED Abstract]
- Mehrabi A, Kashfi A, Fonouni H, et al.: Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer 107 (9): 2108-21, 2006. [PUBMED Abstract]
- Haro A, Saitoh G, Tamiya S, et al.: Four-year natural clinical course of pulmonary epithelioid hemangioendothelioma without therapy. Thorac Cancer 6 (4): 544-7, 2015. [PUBMED Abstract]
- Sardaro A, Bardoscia L, Petruzzelli MF, et al.: Epithelioid hemangioendothelioma: an overview and update on a rare vascular tumor. Oncol Rev 8 (2): 259, 2014. [PUBMED Abstract]
- Dong K, Wang XX, Feng JL, et al.: Pathological characteristics of liver biopsies in eight patients with hepatic epithelioid hemangioendothelioma. Int J Clin Exp Pathol 8 (9): 11015-23, 2015. [PUBMED Abstract]
- Adams DM, Hammill A: Other vascular tumors. Semin Pediatr Surg 23 (4): 173-7, 2014. [PUBMED Abstract]
- Xiao Y, Wang C, Song Y, et al.: Primary epithelioid hemangioendothelioma of the kidney: the first case report in a child and literature review. Urology 82 (4): 925-7, 2013. [PUBMED Abstract]
- Reich S, Ringe H, Uhlenberg B, et al.: Epithelioid hemangioendothelioma of the lung presenting with pneumonia and heart rhythm disturbances in a teenage girl. J Pediatr Hematol Oncol 32 (4): 274-6, 2010. [PUBMED Abstract]
- Daller JA, Bueno J, Gutierrez J, et al.: Hepatic hemangioendothelioma: clinical experience and management strategy. J Pediatr Surg 34 (1): 98-105; discussion 105-6, 1999. [PUBMED Abstract]
- Ackermann O, Fabre M, Franchi S, et al.: Widening spectrum of liver angiosarcoma in children. J Pediatr Gastroenterol Nutr 53 (6): 615-9, 2011. [PUBMED Abstract]
- Stacchiotti S, Provenzano S, Dagrada G, et al.: Sirolimus in Advanced Epithelioid Hemangioendothelioma: A Retrospective Case-Series Analysis from the Italian Rare Cancer Network Database. Ann Surg Oncol 23 (9): 2735-44, 2016. [PUBMED Abstract]
- Semenisty V, Naroditsky I, Keidar Z, et al.: Pazopanib for metastatic pulmonary epithelioid hemangioendothelioma-a suitable treatment option: case report and review of anti-angiogenic treatment options. BMC Cancer 15: 402, 2015. [PUBMED Abstract]
- Raheja A, Suri A, Singh S, et al.: Multimodality management of a giant skull base hemangioendothelioma of the sphenopetroclival region. J Clin Neurosci 22 (9): 1495-8, 2015. [PUBMED Abstract]
- Ahmad N, Adams DM, Wang J, et al.: Hepatic epithelioid hemangioendothelioma in a patient with hemochromatosis. J Natl Compr Canc Netw 12 (9): 1203-7, 2014. [PUBMED Abstract]
- Otte JB, Zimmerman A: The role of liver transplantation for pediatric epithelioid hemangioendothelioma. Pediatr Transplant 14 (3): 295-7, 2010. [PUBMED Abstract]
Treatment of Metastatic Childhood Soft Tissue Sarcoma
Standard treatment options for metastatic childhood soft tissue sarcoma include the following:
- Combination therapy using chemotherapy, radiation therapy, and surgical resection of pulmonary metastases.
For treatment options, refer to the individual tumor type sections of the summary.
The prognosis for children with metastatic soft tissue sarcomas is poor,[1-6] and these children should receive combined treatment with chemotherapy, radiation therapy, and surgical resection of pulmonary metastases. In a prospective randomized trial, chemotherapy with vincristine, dactinomycin, doxorubicin, and cyclophosphamide, with or without dacarbazine, led to tumor responses in one-third of patients with unresectable or metastatic disease. The estimated 4-year survival rate, however, was poor, with fewer than one-third of children surviving.[6-8]
Pulmonary Metastases
Generally, children with isolated pulmonary metastases should be considered for a surgical procedure in an attempt to resect all gross disease.[9] For patients with multiple or recurrent pulmonary metastases, additional surgical procedures can be performed if the morbidity is deemed acceptable. In a retrospective review, patients with synovial sarcoma and pulmonary metastases for whom it was possible to completely resect all metastatic lung lesions had better survival than did patients for whom it was not possible to achieve complete resections.[9][Level of evidence: 3iiiA] Formal segmentectomy, lobectomy, and mediastinal lymph node dissection are unnecessary.[10]
An alternative approach is focused radiation therapy (fractionated stereotactic radiation therapy), which has been successfully used in adults to control lesions. The estimated 5-year survival rate after thoracotomy for pulmonary metastasectomy has ranged from 10% to 58% in adult studies. Emerging data suggest a similar outcome after the administration of focused radiation therapy.[11]
References
- Demetri GD, Elias AD: Results of single-agent and combination chemotherapy for advanced soft tissue sarcomas. Implications for decision making in the clinic. Hematol Oncol Clin North Am 9 (4): 765-85, 1995. [PUBMED Abstract]
- Elias A, Ryan L, Sulkes A, et al.: Response to mesna, doxorubicin, ifosfamide, and dacarbazine in 108 patients with metastatic or unresectable sarcoma and no prior chemotherapy. J Clin Oncol 7 (9): 1208-16, 1989. [PUBMED Abstract]
- Edmonson JH, Ryan LM, Blum RH, et al.: Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol 11 (7): 1269-75, 1993. [PUBMED Abstract]
- Rao BN: Nonrhabdomyosarcoma in children: prognostic factors influencing survival. Semin Surg Oncol 9 (6): 524-31, 1993 Nov-Dec. [PUBMED Abstract]
- deCou JM, Rao BN, Parham DM, et al.: Malignant peripheral nerve sheath tumors: the St. Jude Children's Research Hospital experience. Ann Surg Oncol 2 (6): 524-9, 1995. [PUBMED Abstract]
- Pappo AS, Rao BN, Jenkins JJ, et al.: Metastatic nonrhabdomyosarcomatous soft-tissue sarcomas in children and adolescents: the St. Jude Children's Research Hospital experience. Med Pediatr Oncol 33 (2): 76-82, 1999. [PUBMED Abstract]
- Pratt CB, Pappo AS, Gieser P, et al.: Role of adjuvant chemotherapy in the treatment of surgically resected pediatric nonrhabdomyosarcomatous soft tissue sarcomas: A Pediatric Oncology Group Study. J Clin Oncol 17 (4): 1219, 1999. [PUBMED Abstract]
- Pratt CB, Maurer HM, Gieser P, et al.: Treatment of unresectable or metastatic pediatric soft tissue sarcomas with surgery, irradiation, and chemotherapy: a Pediatric Oncology Group study. Med Pediatr Oncol 30 (4): 201-9, 1998. [PUBMED Abstract]
- Stanelle EJ, Christison-Lagay ER, Wolden SL, et al.: Pulmonary metastasectomy in pediatric/adolescent patients with synovial sarcoma: an institutional review. J Pediatr Surg 48 (4): 757-63, 2013. [PUBMED Abstract]
- Putnam JB Jr, Roth JA: Surgical treatment for pulmonary metastases from sarcoma. Hematol Oncol Clin North Am 9 (4): 869-87, 1995. [PUBMED Abstract]
- Dhakal S, Corbin KS, Milano MT, et al.: Stereotactic body radiotherapy for pulmonary metastases from soft-tissue sarcomas: excellent local lesion control and improved patient survival. Int J Radiat Oncol Biol Phys 82 (2): 940-5, 2012. [PUBMED Abstract]
Treatment of Progressive/Recurrent Childhood Soft Tissue Sarcoma
With the possible exception of infants with infantile fibrosarcoma, the prognosis for patients with recurrent or progressive disease is poor. No prospective trial has been able to prove that enhanced local control of pediatric soft tissue sarcomas will ultimately improve survival. Therefore, treatment should be individualized for the site of recurrence, biologic characteristics of the tumor (e.g., grade, invasiveness, and size), previous therapies, and individual patient considerations.
Treatment options for recurrent or progressive disease include the following:
- Surgical excision of local recurrence or isolated pulmonary recurrence.
- An Italian review of 73 patients with recurrent malignant peripheral nerve sheath tumors found that most relapses were local. Multivariate analysis showed that the factors associated with improved survival were no tumor invasiveness at initial diagnosis (T1), time of recurrence more than 12 months after initial diagnosis, and achievement of a second complete response with surgical removal of the recurrence(s). Only 15.8% of patients who had complete surgical excisions of local recurrence(s) were alive at 5 years.[1][Level of evidence: 3iiiA]
- Surgical excision of local recurrence followed by radiation therapy or brachytherapy (if no previous radiation therapy was given).
- Limb amputation (only for some children with extremity sarcomas that have already received radiation therapy).
- Gemcitabine and docetaxel.[2]
- Trabectedin.[3-5]
- Pazopanib. A phase I trial of pazopanib reported one partial response in a patient with desmoplastic small round cell tumor and prolonged disease stabilization in eight patients with recurrent sarcoma.[6][Level of evidence: 2Diii] Pazopanib has been approved for use in recurrent soft tissue sarcoma. The clinical trial that was used to obtain approval was limited to adults and demonstrated disease stabilization and prolonged time to progression; it did not demonstrate improved overall survival.[7] One 13-year-old boy and one 14-year-old girl with multiply recurrent synovial sarcoma and lung metastases had responses to pazopanib for 14 and 15 months, respectively.[8][Level of evidence: 3iiDi]
- A clinical trial of new chemotherapeutic regimens.
Resection is the standard treatment for recurrent pediatric nonrhabdomyosarcomatous soft tissue sarcomas. If the patient has not yet received radiation therapy, postoperative radiation should be considered after local excision of the recurrent tumor. Limb-sparing procedures with postoperative brachytherapy have been evaluated in adults but have not been studied extensively in children. For some children with extremity sarcomas who have received previous radiation therapy, amputation may be the only therapeutic option.
Pulmonary metastasectomy may achieve prolonged disease control for some patients.[9] A large, retrospective analysis of patients with recurrent soft tissue sarcoma showed that isolated local relapse had a better prognosis and that resection of pulmonary metastases improved the probability of survival.[10] In 31 children and adolescents younger than 23 years with pulmonary metastases from synovial sarcoma, complete resection of lung metastases appeared to prolong survival when compared with ten other patients who were not considered candidates for metastasectomy.[11][Level of evidence: 3iiiA] All patients with recurrent tumors should be considered for current clinical trials.
Published results of two studies addressed the outcomes for children with relapsed synovial sarcoma. Most patients in one study had distant relapse (29 of 44 patients),[12] while most patients in the second study had local relapse (27 of 37 patients).[13] Distant recurrence was a poor prognostic variable, while tumor resectability at relapse (as manifested by extremity recurrence) was associated with a better outcome in both studies.
Treatment Options Under Clinical Evaluation
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- APEC1621 (NCT03155620) (Pediatric MATCH: Targeted Therapy Directed by Genetic Testing in Treating Pediatric Patients with Relapsed or Refractory Advanced Solid Tumors, Non-Hodgkin Lymphomas, or Histiocytic Disorders): NCI-COG Pediatric Molecular Analysis for Therapeutic Choice (MATCH), referred to as Pediatric MATCH, will match targeted agents with specific molecular changes identified using a next-generation sequencing targeted assay of more than 3,000 different mutations across more than 160 genes in refractory and recurrent solid tumors. Children and adolescents aged 1 to 21 years are eligible for the trial.
Tumor tissue from progressive or recurrent disease must be available for molecular characterization. Patients with tumors that have molecular variants addressed by treatment arms included in the trial will be offered treatment on Pediatric MATCH. Additional information can be obtained on the ClinicalTrials.gov website for APEC1621 (NCT03155620).
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Bergamaschi L, Bisogno G, Manzitti C, et al.: Salvage rates and prognostic factors after relapse in children and adolescents with malignant peripheral nerve sheath tumors. Pediatr Blood Cancer 65 (2): , 2018. [PUBMED Abstract]
- Maki RG, Wathen JK, Patel SR, et al.: Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected]. J Clin Oncol 25 (19): 2755-63, 2007. [PUBMED Abstract]
- Le Cesne A, Cresta S, Maki RG, et al.: A retrospective analysis of antitumour activity with trabectedin in translocation-related sarcomas. Eur J Cancer 48 (16): 3036-44, 2012. [PUBMED Abstract]
- Garcia-Carbonero R, Supko JG, Maki RG, et al.: Ecteinascidin-743 (ET-743) for chemotherapy-naive patients with advanced soft tissue sarcomas: multicenter phase II and pharmacokinetic study. J Clin Oncol 23 (24): 5484-92, 2005. [PUBMED Abstract]
- Garcia-Carbonero R, Supko JG, Manola J, et al.: Phase II and pharmacokinetic study of ecteinascidin 743 in patients with progressive sarcomas of soft tissues refractory to chemotherapy. J Clin Oncol 22 (8): 1480-90, 2004. [PUBMED Abstract]
- Glade Bender JL, Lee A, Reid JM, et al.: Phase I pharmacokinetic and pharmacodynamic study of pazopanib in children with soft tissue sarcoma and other refractory solid tumors: a children's oncology group phase I consortium report. J Clin Oncol 31 (24): 3034-43, 2013. [PUBMED Abstract]
- van der Graaf WT, Blay JY, Chawla SP, et al.: Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 379 (9829): 1879-86, 2012. [PUBMED Abstract]
- Casanova M, Basso E, Magni C, et al.: Response to pazopanib in two pediatric patients with pretreated relapsing synovial sarcoma. Tumori 103 (1): e1-e3, 2017. [PUBMED Abstract]
- Belal A, Salah E, Hajjar W, et al.: Pulmonary metastatectomy for soft tissue sarcomas: is it valuable? J Cardiovasc Surg (Torino) 42 (6): 835-40, 2001. [PUBMED Abstract]
- Zagars GK, Ballo MT, Pisters PW, et al.: Prognostic factors for disease-specific survival after first relapse of soft-tissue sarcoma: analysis of 402 patients with disease relapse after initial conservative surgery and radiotherapy. Int J Radiat Oncol Biol Phys 57 (3): 739-47, 2003. [PUBMED Abstract]
- Stanelle EJ, Christison-Lagay ER, Wolden SL, et al.: Pulmonary metastasectomy in pediatric/adolescent patients with synovial sarcoma: an institutional review. J Pediatr Surg 48 (4): 757-63, 2013. [PUBMED Abstract]
- Ferrari A, De Salvo GL, Dall'Igna P, et al.: Salvage rates and prognostic factors after relapse in children and adolescents with initially localised synovial sarcoma. Eur J Cancer 48 (18): 3448-55, 2012. [PUBMED Abstract]
- Soole F, Maupain C, Defachelles AS, et al.: Synovial sarcoma relapses in children and adolescents: prognostic factors, treatment, and outcome. Pediatr Blood Cancer 61 (8): 1387-93, 2014. [PUBMED Abstract]
Changes to This Summary (04/02/2018)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Treatment of Newly Diagnosed Childhood Soft Tissue Sarcoma
Added text to state that a small series reported symptomatic improvement and stable disease in seven patients with desmoid-type fibromatosis who were treated with pazopanib (cited Agresta et al. as reference 48).
Added text to state that a tumor with morphology similar to that of infantile fibrosarcoma has been identified in older children; in these older children, the tumors do not have the t(12;15)(ETV-NTRK3) translocation that is characteristic of the younger patients. In several of these patients, BRAF gene fusions have been identified (cited Kao et al. as reference 69).
Added text about the outcome results of 73 children and adolescents with recurrent malignant peripheral nerve sheath tumor reported by the Italian Sarcoma Group (cited Bergamaschi et al. as reference 127 and level of evidence 3iiiA).
Added text about the patient characteristics and results of a retrospective review of children and young adults younger than 30 years from four institutions, which identified 69 patients with alveolar soft part sarcoma treated primarily with surgery between 1980 and 2014 (cited Flores et al. as reference 152 and level of evidence 3iiA).
Added Sedig et al. as reference 172 and level of evidence 3iiiA.
Treatment of Progressive/Recurrent Childhood Soft Tissue Sarcoma
Added text about the prognostic factors and outcome results reported in an Italian review of 73 children and adolescents with recurrent malignant peripheral nerve sheath tumor (cited Bergamaschi et al. as reference 1 and level of evidence 3iiiA).
This summary is written and maintained by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood soft tissue sarcoma. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Childhood Soft Tissue Sarcoma Treatment are:
- Denise Adams, MD (Children's Hospital Boston)
- Louis S. Constine, MD (James P. Wilmot Cancer Center at University of Rochester Medical Center)
- Holcombe Edwin Grier, MD (Dana-Farber Cancer Institute/Boston Children's Hospital)
- Andrea A. Hayes-Jordan, MD, FACS, FAAP (M.D. Anderson Cancer Center)
- Paul A. Meyers, MD (Memorial Sloan-Kettering Cancer Center)
- Thomas A. Olson, MD (Aflac Cancer and Blood Disorders Center of Children's Healthcare of Atlanta - Egleston Campus)
- Alberto S. Pappo, MD (St. Jude Children's Research Hospital)
- R Beverly Raney, MD (Consultant)
- Stephen J. Shochat, MD (St. Jude Children's Research Hospital)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Soft Tissue Sarcoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/soft-tissue-sarcoma/hp/child-soft-tissue-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389361]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.
Updated: April 2, 2018
Source URL: https://www.cancer.gov/publishedcontent/syndication/3899.htm
Source Agency: National Cancer Institute (NCI)
Captured Date: 2013-09-14 09:01:57.0
Kaposi sarcoma: Professional resources from the National Cancer Institute
Kaposi Sarcoma Treatment (PDQ®)–Health Professional Version
General Information About Kaposi Sarcoma
Epidemiology
KS was first described in 1872 by the Hungarian dermatologist, Moritz Kaposi. From that time until the current human immunodeficiency virus (HIV) disease epidemic identified with the Acquired Immunodeficiency Syndrome (AIDS), KS remained a rare tumor. While most of the cases seen in Europe and North America have occurred in elderly men of Italian or Eastern European Jewish ancestry, the neoplasm also occurs in several other distinct populations: young black African adult males, prepubescent children, renal allograft recipients, and other patients receiving immunosuppressive therapy. The disseminated, fulminant form of KS associated with HIV disease is referred to as epidemic KS to distinguish it from the classic, African, and transplant-related varieties of the neoplasm. In addition, KS has been identified in homosexual men apart from the HIV disease epidemic.[1]
Histopathology
Although the histopathology of the different types of the Kaposi tumor is essentially identical in all of these groups, the clinical manifestations and course of the disease differ dramatically.[2] A key piece to the puzzle of KS pathogenesis was the 1994 discovery of a gamma herpes virus, human herpes virus type 8 (HHV-8), also known as Kaposi sarcoma herpes virus.[3] HHV-8 was identified in KS tissue biopsies from virtually all patients with classic, African, transplant-related, and AIDS-associated KS but was absent from noninvolved tissue.[4-7]
Classic Kaposi Sarcoma
Considered a rare disease, classic KS occurs more often in males, with a ratio of approximately 10 to 15 males to 1 female. In North Americans and Europeans, the usual age at onset is between 50 and 70 years. Classic KS tumors usually present with one or more asymptomatic red, purple, or brown patches, plaques, or nodular skin lesions. The disease is often limited to single or multiple lesions usually localized to one or both lower extremities, especially involving the ankles and soles.
Classic KS most commonly runs a relatively benign, indolent course for 10 to 15 years or more, with slow enlargement of the original tumors and the gradual development of additional lesions. Venous stasis and lymphedema of the involved lower extremity are frequent complications. In long-standing cases, systemic lesions can develop along the gastrointestinal tract, in lymph nodes, and in other organs. The visceral lesions are generally asymptomatic and are most often discovered only at autopsy, though clinically, gastrointestinal bleeding can occur. As many as 33% of the patients with classic KS develop a second primary malignancy, which is most often non-Hodgkin lymphoma.[8-10]
African Kaposi Sarcoma
In the 1950s, KS was recognized as a relatively common neoplasm endemic in native populations in equatorial Africa and comprised approximately 9% of all cancers seen in Ugandan males. African KS is seen as either an indolent neoplasm identical to the classic disease seen in Europe and North America or as an aggressive disease with fungating and exophytic tumors that may invade the subcutaneous and surrounding tissue including the underlying bone. In Africa, both the indolent and locally more aggressive forms of KS occur with a male-to-female ratio comparable to that observed with the classic KS tumor seen in North America and Europe. In general, however, patients in Africa are significantly younger than their European counterparts. A lymphadenopathic form of KS is also seen in Africa, primarily in prepubescent children (male:female ratio, 3:1). In these cases, the generalized lymphadenopathy is frequently associated with visceral organ involvement. The prognosis is very poor with a 100% fatality rate within 3 years.[11,12]
Immunosuppressive Treatment–Related Kaposi Sarcoma
In 1969, the first case of KS in association with immunosuppression in a renal transplant patient was described. Since that time, a number of renal and other organ allograft recipients who received prednisone and azathioprine developed KS shortly after the onset of immunosuppressive therapy.[13] Estimates of the incidence of KS in immunosuppressed renal transplant recipients are between 150 and 200 times the expected incidence of the tumor in the general population. The average time to develop KS after transplantation is 16 months. Although the KS tumor in iatrogenically immunosuppressed patients often remains localized to the skin, widespread dissemination with mucocutaneous or visceral organ involvement is common. In some cases, the KS tumors have regressed as a result of reduction or changes in immunosuppressive therapy. Clinical management of renal transplant patients who develop KS is difficult and requires a balance between the risk of death from generalized KS and the risk of graft rejection and complications of renal failure that may occur if the immunosuppressive therapy is discontinued.
Epidemic Kaposi Sarcoma
In 1981, a fulminant and disseminated form of KS in young homosexual or bisexual men was first reported as part of an epidemic now known as AIDS.[14] The etiology of AIDS is a T-cell lymphotropic retrovirus known as HIV. The underlying immunologic deficiency that characterizes HIV disease is an acquired profound disorder of cell-mediated immune functions. This immunologic deficiency and immune dysregulation predisposes the host to a variety of opportunistic infections and unusual neoplasms, especially KS. HIV may play an indirect role in the development of KS.[15]
Approximately 95% of all the cases of epidemic KS in the United States have been diagnosed in homosexual or bisexual men. In the past, approximately 26% of all homosexual males with HIV disease presented with, or eventually developed, KS during the course of their illness. By comparison, fewer than 3% of all heterosexual intravenous drug users with HIV disease developed KS. The proportion of HIV disease patients with KS has steadily decreased since the epidemic was first identified in 1981.[16] About 48% of AIDS patients in 1981 had KS as their presenting AIDS diagnosis. By August 1987, the cumulative proportion of AIDS patients with KS had diminished to fewer than 20%. The introduction of combined antiretroviral therapy (cART) has delayed or prevented the emergence of drug-resistant HIV strains, profoundly decreased viral load, led to increased survival, and lessened the risk of opportunistic infections.[17-19] The use of cART has been associated with a sustained and substantial decline in KS incidence in multiple large cohorts.[20-25]
The lesions that develop may involve the skin; oral mucosa; lymph nodes; and visceral organs, such as the gastrointestinal tract, lung, liver, and spleen. Most patients with HIV disease who present with the mucocutaneous lesions of KS feel healthy and are usually free of systemic symptoms, as compared with HIV patients who first develop an opportunistic infection. The sites of disease at presentation of epidemic KS are much more varied than the sites seen in other types of this neoplasm. In an early report on the clinical manifestations of the disease, 49 patients were described.[26] Of these patients, 8% had no skin involvement, 27% had localized or fewer than five skin lesions, and 63% had innumerable skin lesions widely distributed over the skin surface area. Of these patients, 61% had generalized lymphadenopathy at the time of the first examination. Four of these patients, who had generalized lymphadenopathy in the absence of skin lesions or detectable visceral organ involvement at the time of presentation, were found to have biopsy-proven KS localized to the lymph nodes. In 45% of the patients studied, KS lesions were found in one or more sites along the gastrointestinal tract. Of these patients, 29% had either unexplained fever or unexplained weight loss when first seen. While most patients present with skin disease, KS involvement of lymph nodes or the gastrointestinal tract may occasionally precede the appearance of the cutaneous lesions.
Eventually, most patients with epidemic KS develop disseminated disease. The disease often progresses in an orderly fashion from a few localized or widespread mucocutaneous lesions to more numerous lesions and generalized skin disease with lymph node, gastrointestinal tract disease, and other organ involvement. Pleuropulmonary KS is an ominous sign usually occurring late in the course of the disease, especially in those patients whose death is directly attributed to KS.[27] Most patients with epidemic KS die of one or more complicating opportunistic infections.
References
- Friedman-Kien AE, Saltzman BR, Cao YZ, et al.: Kaposi's sarcoma in HIV-negative homosexual men. Lancet 335 (8682): 168-9, 1990. [PUBMED Abstract]
- Safai B: Kaposi's sarcoma and acquired immunodeficiency syndrome. In: DeVita VT, Hellman S, Rosenberg S, eds.: AIDS: Etiology, Diagnosis, Treatment and Prevention. 4th ed. Philadelphia, Pa: Lippincott-Raven Publishers, 1997, pp 295-318.
- Chang Y, Cesarman E, Pessin MS, et al.: Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266 (5192): 1865-9, 1994. [PUBMED Abstract]
- Moore PS, Chang Y: Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and without HIV infection. N Engl J Med 332 (18): 1181-5, 1995. [PUBMED Abstract]
- Su IJ, Hsu YS, Chang YC, et al.: Herpesvirus-like DNA sequence in Kaposi's sarcoma from AIDS and non-AIDS patients in Taiwan. Lancet 345 (8951): 722-3, 1995. [PUBMED Abstract]
- Gao SJ, Kingsley L, Li M, et al.: KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nat Med 2 (8): 925-8, 1996. [PUBMED Abstract]
- Chang Y, Ziegler J, Wabinga H, et al.: Kaposi's sarcoma-associated herpesvirus and Kaposi's sarcoma in Africa. Uganda Kaposi's Sarcoma Study Group. Arch Intern Med 156 (2): 202-4, 1996. [PUBMED Abstract]
- Safai B, Good RA: Kaposi's sarcoma: a review and recent developments. Clin Bull 10 (2): 62-9, 1980. [PUBMED Abstract]
- Reynolds WA, Winkelmann RK, Soule EH: Kaposi's sarcoma: a clinicopathologic study with particular reference to its relationship to the reticuloendothelial system. Medicine (Baltimore) 44 (5): 419-43, 1965. [PUBMED Abstract]
- Safai B, Miké V, Giraldo G, et al.: Association of Kaposi's sarcoma with second primary malignancies: possible etiopathogenic implications. Cancer 45 (6): 1472-9, 1980. [PUBMED Abstract]
- Taylor JF, Templeton AC, Vogel CL, et al.: Kaposi's sarcoma in Uganda: a clinico-pathological study. Int J Cancer 8 (1): 122-35, 1971. [PUBMED Abstract]
- Templeton AC, Bhana D: Prognosis in Kaposi's sarcoma. J Natl Cancer Inst 55 (6): 1301-4, 1975. [PUBMED Abstract]
- Penn I: Kaposi's sarcoma in organ transplant recipients: report of 20 cases. Transplantation 27 (1): 8-11, 1979. [PUBMED Abstract]
- Kaposi's sarcoma and Pneumocystis pneumonia among homosexual men--New York City and California. MMWR Morb Mortal Wkly Rep 30 (25): 305-8, 1981. [PUBMED Abstract]
- Vogel J, Hinrichs SH, Reynolds RK, et al.: The HIV tat gene induces dermal lesions resembling Kaposi's sarcoma in transgenic mice. Nature 335 (6191): 606-11, 1988. [PUBMED Abstract]
- Selik RM, Starcher ET, Curran JW: Opportunistic diseases reported in AIDS patients: frequencies, associations, and trends. AIDS 1 (3): 175-82, 1987. [PUBMED Abstract]
- Flexner C: HIV-protease inhibitors. N Engl J Med 338 (18): 1281-92, 1998. [PUBMED Abstract]
- Palella FJ Jr, Delaney KM, Moorman AC, et al.: Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338 (13): 853-60, 1998. [PUBMED Abstract]
- Lodi S, Guiguet M, Costagliola D, et al.: Kaposi sarcoma incidence and survival among HIV-infected homosexual men after HIV seroconversion. J Natl Cancer Inst 102 (11): 784-92, 2010. [PUBMED Abstract]
- Portsmouth S, Stebbing J, Gill J, et al.: A comparison of regimens based on non-nucleoside reverse transcriptase inhibitors or protease inhibitors in preventing Kaposi's sarcoma. AIDS 17 (11): F17-22, 2003. [PUBMED Abstract]
- International Collaboration on HIV and Cancer: Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst 92 (22): 1823-30, 2000. [PUBMED Abstract]
- Dupont C, Vasseur E, Beauchet A, et al.: Long-term efficacy on Kaposi's sarcoma of highly active antiretroviral therapy in a cohort of HIV-positive patients. CISIH 92. Centre d'information et de soins de l'immunodéficience humaine. AIDS 14 (8): 987-93, 2000. [PUBMED Abstract]
- Tam HK, Zhang ZF, Jacobson LP, et al.: Effect of highly active antiretroviral therapy on survival among HIV-infected men with Kaposi sarcoma or non-Hodgkin lymphoma. Int J Cancer 98 (6): 916-22, 2002. [PUBMED Abstract]
- Carrieri MP, Pradier C, Piselli P, et al.: Reduced incidence of Kaposi's sarcoma and of systemic non-hodgkin's lymphoma in HIV-infected individuals treated with highly active antiretroviral therapy. Int J Cancer 103 (1): 142-4, 2003. [PUBMED Abstract]
- Grabar S, Abraham B, Mahamat A, et al.: Differential impact of combination antiretroviral therapy in preventing Kaposi's sarcoma with and without visceral involvement. J Clin Oncol 24 (21): 3408-14, 2006. [PUBMED Abstract]
- Krigel RL, Laubenstein LJ, Muggia FM: Kaposi's sarcoma: a new staging classification. Cancer Treat Rep 67 (6): 531-4, 1983. [PUBMED Abstract]
- Gill PS, Akil B, Colletti P, et al.: Pulmonary Kaposi's sarcoma: clinical findings and results of therapy. Am J Med 87 (1): 57-61, 1989. [PUBMED Abstract]
Stage Information for Kaposi Sarcoma
The staging evaluation of patients with classic Kaposi sarcoma (KS) should be individualized. The advanced age of most of the patients, localized nature of the tumor, rarity of visceral involvement, and usually indolent course of the disease should temper the extent of the evaluation. A careful examination of the skin and lymph nodes is sufficient in most cases. For the rare patient with rapidly progressive tumor or signs or symptoms of visceral involvement, appropriate evaluation is indicated. No universally accepted classification is available for epidemic KS. Staging schemes that incorporate laboratory parameters as well as clinical features have been proposed. Since most patients with epidemic KS do not die from the disease, factors besides tumor burden are apparently involved in survival.
The conventions used to stage KS and the methods used to evaluate the benefits of KS treatment continue to evolve because of changes in the treatment of human immunodeficiency virus (HIV) and in recognition of deficiencies in standard tumor assessment. The clinical course of KS, the selection of treatment, and the response to treatment are heavily influenced by the degree of underlying immune dysfunction and opportunistic infections.
The AIDS Clinical Trials Group (ACTG) Oncology Committee has published criteria for the evaluation of epidemic KS.[1] The staging system incorporates measures of extent of disease, severity of immunodeficiency, and presence of systemic symptoms. As shown in Table 1 below, the ACTG criteria categorizes the extent of the tumor as localized or disseminated, the CD4 cell number as high or low, and a systemic illness as absent or present.
A subsequent prospective analysis of 294 patients entered on ACTG trials for KS between 1989 and 1995 showed that each of the tumor, immune system, and systemic illness variables was independently associated with survival.[2] Multivariate analysis showed that immune system impairment was the most important single predictor of survival. In patients with relatively high CD4 counts, tumor stage was predictive. A CD4 count of 150 cells/mm³ may be a better discriminator than the published cutoff of 200 cells/mm³. A study is in progress to determine if viral load adds predictive information. None of the prior studies were conducted at a time when combined antiretroviral therapy (cART) was readily available. The impact of cART on survival in KS requires continued assessment.
| Good Risk (0) | Poor Risk (1) | |
|---|---|---|
| (Any of the following) | (Any of the following) | |
| Tumor (T) | Confined to skin and/or lymph nodes and/or minimal oral disease[Note: Minimal oral disease is non-nodular KS confined to the palate.] | Tumor-associated edema or ulceration |
| Extensive oral KS | ||
| Gastrointestinal KS | ||
| KS in other non-nodal viscera | ||
| Immune system (I) | CD4 cells ≥ = 200/µL | CD4 cells <200 per cubic mm |
| Systemic illness (S) | No history of OIs or thrush[Note: OIs are opportunistic infections.] | History of OIs and/or thrush |
| No “B” symptoms[Note: “B” symptoms are unexplained fever, night sweats, >10% involuntary weight loss, or diarrhea persisting >2 weeks.] | “B” symptoms present | |
| Performance status ≥70 (Karnofsky) | Performance status <70 | |
| Other HIV-related illness (e.g., neurological disease or lymphoma) |
References
- Krown SE, Metroka C, Wernz JC: Kaposi's sarcoma in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol 7 (9): 1201-7, 1989. [PUBMED Abstract]
- Krown SE, Testa MA, Huang J: AIDS-related Kaposi's sarcoma: prospective validation of the AIDS Clinical Trials Group staging classification. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol 15 (9): 3085-92, 1997. [PUBMED Abstract]
Classic Kaposi Sarcoma Treatment
Classic Kaposi sarcoma (KS) usually is limited to the skin and has an indolent course. Patients with this tumor are predisposed to the development of a second primary malignancy, and the treating physician should consider this factor when arranging a schedule of follow-up treatment for the patient.
Equivalent standard treatment options:
Solitary lesions:
- Radiation therapy: For solitary lesions or lesions of limited extent, modest doses of radiation applied to the lesions with a limited margin provide excellent control of disease in the treated area. Usually, superficial radiation beams such as electron beams are used. Some authors believe disease recurrence in adjacent, untreated skin is common if only involved-field radiation therapy is used and claim better cure rates when extended-field radiation therapy is used.[1,2]
- Low-voltage (100 kv) photon radiation: 8 Gy to 10 Gy given as a single dose or 15 Gy to 20 Gy given over 1 week because solitary lesions control nearly 100% of local disease, but recurrence in adjacent areas is common.
- Electron-beam radiation therapy (EBRT): 4 Gy given once weekly for 6 to 8 consecutive weeks with a 4-MeV to 6-MeV electron beam. Ports should include the entire skin surface 15 cm above the lesion.
- Surgical excision may be of benefit in some patients with small superficial lesions, but local recurrence is likely to be a problem. However, over the years, multiple small excisions can be performed to achieve good disease control.
Widespread skin disease:
- Radiation therapy: Modest doses are effective in controlling disease. The type of radiation (i.e., photon vs. electron) and fields used must be tailored to suit the distribution of disease in the individual patient.[2]
- Extended-field EBRT.
- For disease limited to areas distal to the knee, subtotal-skin EBRT directed to skin below the umbilicus.
- For disease that extends above the knee, total-skin EBRT.
EBRT used in this manner gave long-term results that were superior to those obtained with radiation therapy administered to successive individual lesions as they appeared.[2]
- EBRT: 4 Gy given once weekly for 6 to 8 consecutive weeks, and subtotal- or total-skin radiation therapy given for extensive disease.
- Chemotherapy: Because classic KS is such a rare disease in the United States and is usually treated initially with radiation therapy, few patients have been treated with chemotherapy, and no randomized prospective trials have compared one agent to another. Several authors have used single-agent vinblastine given as a weekly dose of approximately 0.1 mg/kg.[3-6] Almost all of the patients had good to excellent response. In most cases, patients required prolonged courses of therapy, for several years, to maintain a partial response. Doses of vinblastine were titrated in individual patients to maintain a white blood count of more than 3,000 leukocytes. Follow-up after completion of therapy was not presented. In a multicenter trial of 55 patients who were treated over a decade, a 71% overall response rate was seen using pegylated liposomal doxorubicin.[7][Level of evidence: 3iiiDiv] In addition to the positive response rates of pegylated liposomal doxorubicin and the vinca alkaloids, response rates showing a greater than 50% decrease in lesions have also been reported in small, uncontrolled series for etoposide, taxanes, gemcitabine, and interferon alfa.[8][Level of evidence: 3iiiDiv]
One patient was treated repeatedly with intralesional injections of 0.25 to 0.50 mg of vincristine, which resulted in complete disappearance of the treated lesion.[9] Multiple courses of therapy were required because of the recurrence of disease in untreated areas.
Electroporation of the skin lesions was combined with intravenous bleomycin for 19 patients with classical KS. Most patients responded after one application, the rest after two or three applications, with a median duration of response of 16 months.[10][Level of evidence: 3iiiDiv]
Lymph node and gastrointestinal tract involvement:
- Chemotherapy: Several patients who had widespread skin disease and were treated with chemotherapy also had lymph node and gastrointestinal tract involvement. The disease in these sites also responded to vinblastine. Trials are required to define therapy. One such trial, MSKCC-04055 (NCT00096538), has been completed.
- Local radiation therapy may be added to chemotherapy if individual lesions require urgent therapy.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Hamilton CR, Cummings BJ, Harwood AR: Radiotherapy of Kaposi's sarcoma. Int J Radiat Oncol Biol Phys 12 (11): 1931-5, 1986. [PUBMED Abstract]
- Nisce LZ, Safai B, Poussin-Rosillo H: Once weekly total and subtotal skin electron beam therapy for Kaposi's sarcoma. Cancer 47 (4): 640-4, 1981. [PUBMED Abstract]
- Solan AJ, Greenwald ES, Silvay O: Long-term complete remissions of Kaposi's sarcoma with vinblastine therapy. Cancer 47 (4): 637-9, 1981. [PUBMED Abstract]
- Tucker SB, Winkelmann RK: Treatment of Kaposi sarcoma with vinblastine. Arch Dermatol 112 (7): 958-61, 1976. [PUBMED Abstract]
- Scott WP, Voight JA: Kaposi's sarcoma. Management with vincaleucoblastine. Cancer 19 (4): 557-64, 1966. [PUBMED Abstract]
- Klein E, Schwartz RA, Laor Y, et al.: Treatment of Kaposi's sarcoma with vinblastine. Cancer 45 (3): 427-31, 1980. [PUBMED Abstract]
- Di Lorenzo G, Kreuter A, Di Trolio R, et al.: Activity and safety of pegylated liposomal doxorubicin as first-line therapy in the treatment of non-visceral classic Kaposi's sarcoma: a multicenter study. J Invest Dermatol 128 (6): 1578-80, 2008. [PUBMED Abstract]
- Régnier-Rosencher E, Guillot B, Dupin N: Treatments for classic Kaposi sarcoma: a systematic review of the literature. J Am Acad Dermatol 68 (2): 313-31, 2013. [PUBMED Abstract]
- Odom RB, Goette DK: Treatment of cutaneous Kaposi's sarcoma with intralesional vincristine. Arch Dermatol 114 (11): 1693-4, 1978. [PUBMED Abstract]
- Di Monta G, Caracò C, Benedetto L, et al.: Electrochemotherapy as "new standard of care" treatment for cutaneous Kaposi's sarcoma. Eur J Surg Oncol 40 (1): 61-6, 2014. [PUBMED Abstract]
Immunosuppressive Therapy–Related Kaposi Sarcoma Treatment
Some patients with Kaposi Sarcoma (KS) have noted spontaneous and lasting remissions following discontinuation of immunosuppressive therapy. In managing these patients, if immunosuppressive therapy is not critical, its discontinuation is a reasonable first step.
Standard treatment options:
- Discontinue immunosuppressive therapy (often results in tumor regression). This option is critically important in patients who are receiving immunosuppressive drugs, as in the case of certain transplant patients.
- Radiation therapy (for disease limited to skin).[1-4]
- Chemotherapy (single or multiple drug): Most systemic chemotherapy trials in KS patients have been carried out in the African and epidemic varieties. See the section on the treatment of Epidemic Kaposi Sarcoma. The applicability of the results of these trials to KS in immunosuppressed patients is unknown.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Cohen L: Dose, time, and volume parameters in irradiation therapy of Kaposi's sarcoma. Br J Radiol 35 (415): 485-488, 1962.
- Hamilton CR, Cummings BJ, Harwood AR: Radiotherapy of Kaposi's sarcoma. Int J Radiat Oncol Biol Phys 12 (11): 1931-5, 1986. [PUBMED Abstract]
- Lo TC, Salzman FA, Smedal MI, et al.: Radiotherapy for Kaposi's sarcoma. Cancer 45 (4): 684-7, 1980. [PUBMED Abstract]
- Nisce LZ, Safai B, Poussin-Rosillo H: Once weekly total and subtotal skin electron beam therapy for Kaposi's sarcoma. Cancer 47 (4): 640-4, 1981. [PUBMED Abstract]
Epidemic Kaposi Sarcoma Treatment
Treatment may result:
- In a disappearance or reduction in size of specific skin lesions, thereby alleviating the discomfort associated with the chronic edema and ulcerations that often accompany multiple skin tumors seen on the lower extremities.
- In control of symptoms associated with mucosal or visceral lesions.
No data are available, however, to show that treatment improves survival.[1] In addition to antitumor treatment, essential components of an optimal Kaposi sarcoma (KS) treatment strategy include combined antiretroviral treatment (cART), prophylaxis for opportunistic infections, and rapid recognition and treatment of intercurrent infections.
Most good-risk patients, defined by the AIDS Clinical Trials Group as T0, show tumor regression with cART alone.[2-4] Poor-risk patients, defined as T1, usually require a combination of cART and chemotherapy with discontinuation of the chemotherapy after disappearance of the skin lesion.[2-4] The combination of cART and liposomal doxorubicin resulted in a 5-year overall survival (OS) rate of 85% in 140 patients with T1 disease.[3][Level of evidence: 3iiiDiv]
Local modalities
Small localized lesions of KS may be treated by electrodesiccation and curettage, cryotherapy, or by surgical excision. KS tumors are also generally very responsive to local radiation therapy, and excellent palliation has been obtained with doses at 20 Gy or slightly higher.[5-7] One report demonstrated a response rate higher than 90%, with a median time to progression of 21 months. Although no difference in response was noted with a variety of fractionation regimens, a single fraction of 8 Gy is indicated for cutaneous lesions and is associated with significantly fewer severe reactions.[8] Radiation therapy is generally reserved to treat localized areas of the skin and oral cavity. It is less often used to control pulmonary, gastrointestinal tract, or other sites of KS lesions. Localized KS lesions have also been effectively treated with intralesional injections of vinblastine.[9] Alitretinoin 0.1% gel provided local control in a randomized prospective multicenter trial.[10][Level of evidence: 1iiDiv]
Chemotherapy
In epidemic KS, the already profoundly depressed immunologic status of the host limits the therapeutic usefulness of systemic chemotherapy. Systemic chemotherapy studies in epidemic KS have used as single agents or in combinations doxorubicin, bleomycin, vinblastine, vincristine, etoposide, paclitaxel, and docetaxel.[11-15][Level of evidence: 3iiiDiv] The combination of cART and liposomal doxorubicin resulted in a 5-year OS of 85% in 140 patients with T1 disease.[3][Level of evidence: 3iiiDiv]
Randomized multicenter trials showed an improvement in response rate (45%–60% vs. 20%–25%) and a more favorable toxic effects profile for pegylated liposomal doxorubicin or liposomal daunorubicin, compared to the combination of doxorubicin, bleomycin, and vincristine or bleomycin and vincristine.[16-18][Level of evidence: 1iiDiv] During cART, both pegylated liposomal doxorubicin and paclitaxel are active single agents with response rates close to 50%.[19][Level of evidence: 1iiDiv]
Biologic and targeted therapy
The interferon alphas have also been widely studied and show a 40% objective response rate in patients with epidemic KS.[20,21] In these reports, the responses differed significantly according to the prognostic factors of extent of disease, prior or coexistent opportunistic infections, prior treatment with chemotherapy, CD4 lymphocyte counts lower than 200 cells/mm³, the presence of circulating acid-labile interferon alpha, and an increase in beta-2-microglobulin. Several treatment studies have combined interferon alpha with other chemotherapeutic agents. Overall, these trials have shown no benefit with the interferon-chemotherapy combinations as compared to the single-agent activities.
Recombinant interferon alpha-2a and interferon alpha-2b were the first agents approved for the treatment of KS. Approval was based on single-agent studies performed in the 1980s before the advent of antiretroviral therapy. The early studies demonstrated improved efficacy at relatively high doses. High-dose monotherapy is rarely used today, and instead, interferon is given in combination with other anti-HIV drugs in doses of 4 to 18 million units. Neutropenia is dose limiting, and trials of doses of 1 to 10 million units combined with less myelosuppressive antiretrovirals are in progress. Response to interferon is slow, and the maximum effect is seen after 6 or more months. Interferon should probably not be used in the treatment of patients with rapidly progressive, symptomatic KS.
Imatinib, a c-kit/PDGF (platelet-derived growth factor) receptor inhibitor, resulted in partial responses in 10 of 30 previously treated patients (cART + chemotherapy).[22]
Bevacizumab, the humanized, antivascular, endothelial growth–factor monoclonal antibody, had a response rate in 5 of 16 patients who did not improve after the institution of cART and chemotherapy.[23][Level of evidence: 3iiiDiv]
Interleukin-12 had a response rate of 71% (95% confidence interval, 48%–89%) among 24 evaluable patients in a phase I and phase II trial.[24][Level of evidence: 3iiiDiv]
Treatment options under clinical evaluation:
- Patients with epidemic KS are appropriate candidates for clinical trials evaluating new drugs or biologicals.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Safai B: Kaposi's sarcoma and acquired immunodeficiency syndrome. In: DeVita VT, Hellman S, Rosenberg S, eds.: AIDS: Etiology, Diagnosis, Treatment and Prevention. 4th ed. Philadelphia, Pa: Lippincott-Raven Publishers, 1997, pp 295-318.
- Krown SE: Highly active antiretroviral therapy in AIDS-associated Kaposi's sarcoma: implications for the design of therapeutic trials in patients with advanced, symptomatic Kaposi's sarcoma. J Clin Oncol 22 (3): 399-402, 2004. [PUBMED Abstract]
- Bower M, Dalla Pria A, Coyle C, et al.: Prospective stage-stratified approach to AIDS-related Kaposi's sarcoma. J Clin Oncol 32 (5): 409-14, 2014. [PUBMED Abstract]
- Krell J, Stebbing J: Broader implications of a stage-guided stratified therapeutic approach for AIDS-related Kaposi's sarcoma. J Clin Oncol 32 (5): 373-5, 2014. [PUBMED Abstract]
- Cooper JS, Steinfeld AD, Lerch I: Intentions and outcomes in the radiotherapeutic management of epidemic Kaposi's sarcoma. Int J Radiat Oncol Biol Phys 20 (3): 419-22, 1991. [PUBMED Abstract]
- Nobler MP, Leddy ME, Huh SH: The impact of palliative irradiation on the management of patients with acquired immune deficiency syndrome. J Clin Oncol 5 (1): 107-12, 1987. [PUBMED Abstract]
- Singh NB, Lakier RH, Donde B: Hypofractionated radiation therapy in the treatment of epidemic Kaposi sarcoma--a prospective randomized trial. Radiother Oncol 88 (2): 211-6, 2008. [PUBMED Abstract]
- Berson AM, Quivey JM, Harris JW, et al.: Radiation therapy for AIDS-related Kaposi's Sarcoma. Int J Radiat Oncol Biol Phys 19 (3): 569-75, 1990. [PUBMED Abstract]
- Epstein JB, Lozada-Nur F, McLeod WA, et al.: Oral Kaposi's sarcoma in acquired immunodeficiency syndrome. Review of management and report of the efficacy of intralesional vinblastine. Cancer 64 (12): 2424-30, 1989. [PUBMED Abstract]
- Bodsworth NJ, Bloch M, Bower M, et al.: Phase III vehicle-controlled, multi-centered study of topical alitretinoin gel 0.1% in cutaneous AIDS-related Kaposi's sarcoma. Am J Clin Dermatol 2 (2): 77-87, 2001. [PUBMED Abstract]
- Evans SR, Krown SE, Testa MA, et al.: Phase II evaluation of low-dose oral etoposide for the treatment of relapsed or progressive AIDS-related Kaposi's sarcoma: an AIDS Clinical Trials Group clinical study. J Clin Oncol 20 (15): 3236-41, 2002. [PUBMED Abstract]
- Saville MW, Lietzau J, Pluda JM, et al.: Treatment of HIV-associated Kaposi's sarcoma with paclitaxel. Lancet 346 (8966): 26-8, 1995. [PUBMED Abstract]
- Lim ST, Tupule A, Espina BM, et al.: Weekly docetaxel is safe and effective in the treatment of advanced-stage acquired immunodeficiency syndrome-related Kaposi sarcoma. Cancer 103 (2): 417-21, 2005. [PUBMED Abstract]
- Gill PS, Tulpule A, Espina BM, et al.: Paclitaxel is safe and effective in the treatment of advanced AIDS-related Kaposi's sarcoma. J Clin Oncol 17 (6): 1876-83, 1999. [PUBMED Abstract]
- Di Lorenzo G, Konstantinopoulos PA, Pantanowitz L, et al.: Management of AIDS-related Kaposi's sarcoma. Lancet Oncol 8 (2): 167-76, 2007. [PUBMED Abstract]
- Stewart S, Jablonowski H, Goebel FD, et al.: Randomized comparative trial of pegylated liposomal doxorubicin versus bleomycin and vincristine in the treatment of AIDS-related Kaposi's sarcoma. International Pegylated Liposomal Doxorubicin Study Group. J Clin Oncol 16 (2): 683-91, 1998. [PUBMED Abstract]
- Northfelt DW, Dezube BJ, Thommes JA, et al.: Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi's sarcoma: results of a randomized phase III clinical trial. J Clin Oncol 16 (7): 2445-51, 1998. [PUBMED Abstract]
- Gill PS, Wernz J, Scadden DT, et al.: Randomized phase III trial of liposomal daunorubicin versus doxorubicin, bleomycin, and vincristine in AIDS-related Kaposi's sarcoma. J Clin Oncol 14 (8): 2353-64, 1996. [PUBMED Abstract]
- Cianfrocca M, Lee S, Von Roenn J, et al.: Randomized trial of paclitaxel versus pegylated liposomal doxorubicin for advanced human immunodeficiency virus-associated Kaposi sarcoma: evidence of symptom palliation from chemotherapy. Cancer 116 (16): 3969-77, 2010. [PUBMED Abstract]
- Real FX, Oettgen HF, Krown SE: Kaposi's sarcoma and the acquired immunodeficiency syndrome: treatment with high and low doses of recombinant leukocyte A interferon. J Clin Oncol 4 (4): 544-51, 1986. [PUBMED Abstract]
- Groopman JE, Gottlieb MS, Goodman J, et al.: Recombinant alpha-2 interferon therapy for Kaposi's sarcoma associated with the acquired immunodeficiency syndrome. Ann Intern Med 100 (5): 671-6, 1984. [PUBMED Abstract]
- Koon HB, Krown SE, Lee JY, et al.: Phase II trial of imatinib in AIDS-associated Kaposi's sarcoma: AIDS Malignancy Consortium Protocol 042. J Clin Oncol 32 (5): 402-8, 2014. [PUBMED Abstract]
- Uldrick TS, Wyvill KM, Kumar P, et al.: Phase II study of bevacizumab in patients with HIV-associated Kaposi's sarcoma receiving antiretroviral therapy. J Clin Oncol 30 (13): 1476-83, 2012. [PUBMED Abstract]
- Little RF, Pluda JM, Wyvill KM, et al.: Activity of subcutaneous interleukin-12 in AIDS-related Kaposi sarcoma. Blood 107 (12): 4650-7, 2006. [PUBMED Abstract]
Recurrent Kaposi Sarcoma Treatment
The prognosis for any treated Kaposi sarcoma patient with progressing, recurring, or relapsing disease is highly variable. Deciding on further treatment depends on many factors, most importantly the clinical setting (i.e., classic, immunosuppressive treatment, or AIDS) in which the tumor arises as well as individual patient considerations.
Clinical trials are appropriate and should be considered when possible.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Changes to This Summary (01/30/2018)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Editorial changes were made to this summary.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of Kaposi sarcoma. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Kaposi Sarcoma Treatment are:
- Eric J. Seifter, MD (Johns Hopkins University)
- Minh Tam Truong, MD (Boston University Medical Center)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Kaposi Sarcoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/soft-tissue-sarcoma/hp/kaposi-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389335]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.
Updated: January 30, 2018
Source URL: https://www.cancer.gov/publishedcontent/syndication/3524.htm
Source Agency: National Cancer Institute (NCI)
Captured Date: 2013-09-14 09:01:43.0
Kaposi Sarcoma Treatment (PDQ®)–Health Professional Version
General Information About Kaposi Sarcoma
Epidemiology
KS was first described in 1872 by the Hungarian dermatologist, Moritz Kaposi. From that time until the current human immunodeficiency virus (HIV) disease epidemic identified with the Acquired Immunodeficiency Syndrome (AIDS), KS remained a rare tumor. While most of the cases seen in Europe and North America have occurred in elderly men of Italian or Eastern European Jewish ancestry, the neoplasm also occurs in several other distinct populations: young black African adult males, prepubescent children, renal allograft recipients, and other patients receiving immunosuppressive therapy. The disseminated, fulminant form of KS associated with HIV disease is referred to as epidemic KS to distinguish it from the classic, African, and transplant-related varieties of the neoplasm. In addition, KS has been identified in homosexual men apart from the HIV disease epidemic.[1]
Histopathology
Although the histopathology of the different types of the Kaposi tumor is essentially identical in all of these groups, the clinical manifestations and course of the disease differ dramatically.[2] A key piece to the puzzle of KS pathogenesis was the 1994 discovery of a gamma herpes virus, human herpes virus type 8 (HHV-8), also known as Kaposi sarcoma herpes virus.[3] HHV-8 was identified in KS tissue biopsies from virtually all patients with classic, African, transplant-related, and AIDS-associated KS but was absent from noninvolved tissue.[4-7]
Classic Kaposi Sarcoma
Considered a rare disease, classic KS occurs more often in males, with a ratio of approximately 10 to 15 males to 1 female. In North Americans and Europeans, the usual age at onset is between 50 and 70 years. Classic KS tumors usually present with one or more asymptomatic red, purple, or brown patches, plaques, or nodular skin lesions. The disease is often limited to single or multiple lesions usually localized to one or both lower extremities, especially involving the ankles and soles.
Classic KS most commonly runs a relatively benign, indolent course for 10 to 15 years or more, with slow enlargement of the original tumors and the gradual development of additional lesions. Venous stasis and lymphedema of the involved lower extremity are frequent complications. In long-standing cases, systemic lesions can develop along the gastrointestinal tract, in lymph nodes, and in other organs. The visceral lesions are generally asymptomatic and are most often discovered only at autopsy, though clinically, gastrointestinal bleeding can occur. As many as 33% of the patients with classic KS develop a second primary malignancy, which is most often non-Hodgkin lymphoma.[8-10]
African Kaposi Sarcoma
In the 1950s, KS was recognized as a relatively common neoplasm endemic in native populations in equatorial Africa and comprised approximately 9% of all cancers seen in Ugandan males. African KS is seen as either an indolent neoplasm identical to the classic disease seen in Europe and North America or as an aggressive disease with fungating and exophytic tumors that may invade the subcutaneous and surrounding tissue including the underlying bone. In Africa, both the indolent and locally more aggressive forms of KS occur with a male-to-female ratio comparable to that observed with the classic KS tumor seen in North America and Europe. In general, however, patients in Africa are significantly younger than their European counterparts. A lymphadenopathic form of KS is also seen in Africa, primarily in prepubescent children (male:female ratio, 3:1). In these cases, the generalized lymphadenopathy is frequently associated with visceral organ involvement. The prognosis is very poor with a 100% fatality rate within 3 years.[11,12]
Immunosuppressive Treatment–Related Kaposi Sarcoma
In 1969, the first case of KS in association with immunosuppression in a renal transplant patient was described. Since that time, a number of renal and other organ allograft recipients who received prednisone and azathioprine developed KS shortly after the onset of immunosuppressive therapy.[13] Estimates of the incidence of KS in immunosuppressed renal transplant recipients are between 150 and 200 times the expected incidence of the tumor in the general population. The average time to develop KS after transplantation is 16 months. Although the KS tumor in iatrogenically immunosuppressed patients often remains localized to the skin, widespread dissemination with mucocutaneous or visceral organ involvement is common. In some cases, the KS tumors have regressed as a result of reduction or changes in immunosuppressive therapy. Clinical management of renal transplant patients who develop KS is difficult and requires a balance between the risk of death from generalized KS and the risk of graft rejection and complications of renal failure that may occur if the immunosuppressive therapy is discontinued.
Epidemic Kaposi Sarcoma
In 1981, a fulminant and disseminated form of KS in young homosexual or bisexual men was first reported as part of an epidemic now known as AIDS.[14] The etiology of AIDS is a T-cell lymphotropic retrovirus known as HIV. The underlying immunologic deficiency that characterizes HIV disease is an acquired profound disorder of cell-mediated immune functions. This immunologic deficiency and immune dysregulation predisposes the host to a variety of opportunistic infections and unusual neoplasms, especially KS. HIV may play an indirect role in the development of KS.[15]
Approximately 95% of all the cases of epidemic KS in the United States have been diagnosed in homosexual or bisexual men. In the past, approximately 26% of all homosexual males with HIV disease presented with, or eventually developed, KS during the course of their illness. By comparison, fewer than 3% of all heterosexual intravenous drug users with HIV disease developed KS. The proportion of HIV disease patients with KS has steadily decreased since the epidemic was first identified in 1981.[16] About 48% of AIDS patients in 1981 had KS as their presenting AIDS diagnosis. By August 1987, the cumulative proportion of AIDS patients with KS had diminished to fewer than 20%. The introduction of combined antiretroviral therapy (cART) has delayed or prevented the emergence of drug-resistant HIV strains, profoundly decreased viral load, led to increased survival, and lessened the risk of opportunistic infections.[17-19] The use of cART has been associated with a sustained and substantial decline in KS incidence in multiple large cohorts.[20-25]
The lesions that develop may involve the skin; oral mucosa; lymph nodes; and visceral organs, such as the gastrointestinal tract, lung, liver, and spleen. Most patients with HIV disease who present with the mucocutaneous lesions of KS feel healthy and are usually free of systemic symptoms, as compared with HIV patients who first develop an opportunistic infection. The sites of disease at presentation of epidemic KS are much more varied than the sites seen in other types of this neoplasm. In an early report on the clinical manifestations of the disease, 49 patients were described.[26] Of these patients, 8% had no skin involvement, 27% had localized or fewer than five skin lesions, and 63% had innumerable skin lesions widely distributed over the skin surface area. Of these patients, 61% had generalized lymphadenopathy at the time of the first examination. Four of these patients, who had generalized lymphadenopathy in the absence of skin lesions or detectable visceral organ involvement at the time of presentation, were found to have biopsy-proven KS localized to the lymph nodes. In 45% of the patients studied, KS lesions were found in one or more sites along the gastrointestinal tract. Of these patients, 29% had either unexplained fever or unexplained weight loss when first seen. While most patients present with skin disease, KS involvement of lymph nodes or the gastrointestinal tract may occasionally precede the appearance of the cutaneous lesions.
Eventually, most patients with epidemic KS develop disseminated disease. The disease often progresses in an orderly fashion from a few localized or widespread mucocutaneous lesions to more numerous lesions and generalized skin disease with lymph node, gastrointestinal tract disease, and other organ involvement. Pleuropulmonary KS is an ominous sign usually occurring late in the course of the disease, especially in those patients whose death is directly attributed to KS.[27] Most patients with epidemic KS die of one or more complicating opportunistic infections.
References
- Friedman-Kien AE, Saltzman BR, Cao YZ, et al.: Kaposi's sarcoma in HIV-negative homosexual men. Lancet 335 (8682): 168-9, 1990. [PUBMED Abstract]
- Safai B: Kaposi's sarcoma and acquired immunodeficiency syndrome. In: DeVita VT, Hellman S, Rosenberg S, eds.: AIDS: Etiology, Diagnosis, Treatment and Prevention. 4th ed. Philadelphia, Pa: Lippincott-Raven Publishers, 1997, pp 295-318.
- Chang Y, Cesarman E, Pessin MS, et al.: Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266 (5192): 1865-9, 1994. [PUBMED Abstract]
- Moore PS, Chang Y: Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and without HIV infection. N Engl J Med 332 (18): 1181-5, 1995. [PUBMED Abstract]
- Su IJ, Hsu YS, Chang YC, et al.: Herpesvirus-like DNA sequence in Kaposi's sarcoma from AIDS and non-AIDS patients in Taiwan. Lancet 345 (8951): 722-3, 1995. [PUBMED Abstract]
- Gao SJ, Kingsley L, Li M, et al.: KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nat Med 2 (8): 925-8, 1996. [PUBMED Abstract]
- Chang Y, Ziegler J, Wabinga H, et al.: Kaposi's sarcoma-associated herpesvirus and Kaposi's sarcoma in Africa. Uganda Kaposi's Sarcoma Study Group. Arch Intern Med 156 (2): 202-4, 1996. [PUBMED Abstract]
- Safai B, Good RA: Kaposi's sarcoma: a review and recent developments. Clin Bull 10 (2): 62-9, 1980. [PUBMED Abstract]
- Reynolds WA, Winkelmann RK, Soule EH: Kaposi's sarcoma: a clinicopathologic study with particular reference to its relationship to the reticuloendothelial system. Medicine (Baltimore) 44 (5): 419-43, 1965. [PUBMED Abstract]
- Safai B, Miké V, Giraldo G, et al.: Association of Kaposi's sarcoma with second primary malignancies: possible etiopathogenic implications. Cancer 45 (6): 1472-9, 1980. [PUBMED Abstract]
- Taylor JF, Templeton AC, Vogel CL, et al.: Kaposi's sarcoma in Uganda: a clinico-pathological study. Int J Cancer 8 (1): 122-35, 1971. [PUBMED Abstract]
- Templeton AC, Bhana D: Prognosis in Kaposi's sarcoma. J Natl Cancer Inst 55 (6): 1301-4, 1975. [PUBMED Abstract]
- Penn I: Kaposi's sarcoma in organ transplant recipients: report of 20 cases. Transplantation 27 (1): 8-11, 1979. [PUBMED Abstract]
- Kaposi's sarcoma and Pneumocystis pneumonia among homosexual men--New York City and California. MMWR Morb Mortal Wkly Rep 30 (25): 305-8, 1981. [PUBMED Abstract]
- Vogel J, Hinrichs SH, Reynolds RK, et al.: The HIV tat gene induces dermal lesions resembling Kaposi's sarcoma in transgenic mice. Nature 335 (6191): 606-11, 1988. [PUBMED Abstract]
- Selik RM, Starcher ET, Curran JW: Opportunistic diseases reported in AIDS patients: frequencies, associations, and trends. AIDS 1 (3): 175-82, 1987. [PUBMED Abstract]
- Flexner C: HIV-protease inhibitors. N Engl J Med 338 (18): 1281-92, 1998. [PUBMED Abstract]
- Palella FJ Jr, Delaney KM, Moorman AC, et al.: Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338 (13): 853-60, 1998. [PUBMED Abstract]
- Lodi S, Guiguet M, Costagliola D, et al.: Kaposi sarcoma incidence and survival among HIV-infected homosexual men after HIV seroconversion. J Natl Cancer Inst 102 (11): 784-92, 2010. [PUBMED Abstract]
- Portsmouth S, Stebbing J, Gill J, et al.: A comparison of regimens based on non-nucleoside reverse transcriptase inhibitors or protease inhibitors in preventing Kaposi's sarcoma. AIDS 17 (11): F17-22, 2003. [PUBMED Abstract]
- International Collaboration on HIV and Cancer: Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst 92 (22): 1823-30, 2000. [PUBMED Abstract]
- Dupont C, Vasseur E, Beauchet A, et al.: Long-term efficacy on Kaposi's sarcoma of highly active antiretroviral therapy in a cohort of HIV-positive patients. CISIH 92. Centre d'information et de soins de l'immunodéficience humaine. AIDS 14 (8): 987-93, 2000. [PUBMED Abstract]
- Tam HK, Zhang ZF, Jacobson LP, et al.: Effect of highly active antiretroviral therapy on survival among HIV-infected men with Kaposi sarcoma or non-Hodgkin lymphoma. Int J Cancer 98 (6): 916-22, 2002. [PUBMED Abstract]
- Carrieri MP, Pradier C, Piselli P, et al.: Reduced incidence of Kaposi's sarcoma and of systemic non-hodgkin's lymphoma in HIV-infected individuals treated with highly active antiretroviral therapy. Int J Cancer 103 (1): 142-4, 2003. [PUBMED Abstract]
- Grabar S, Abraham B, Mahamat A, et al.: Differential impact of combination antiretroviral therapy in preventing Kaposi's sarcoma with and without visceral involvement. J Clin Oncol 24 (21): 3408-14, 2006. [PUBMED Abstract]
- Krigel RL, Laubenstein LJ, Muggia FM: Kaposi's sarcoma: a new staging classification. Cancer Treat Rep 67 (6): 531-4, 1983. [PUBMED Abstract]
- Gill PS, Akil B, Colletti P, et al.: Pulmonary Kaposi's sarcoma: clinical findings and results of therapy. Am J Med 87 (1): 57-61, 1989. [PUBMED Abstract]
Stage Information for Kaposi Sarcoma
The staging evaluation of patients with classic Kaposi sarcoma (KS) should be individualized. The advanced age of most of the patients, localized nature of the tumor, rarity of visceral involvement, and usually indolent course of the disease should temper the extent of the evaluation. A careful examination of the skin and lymph nodes is sufficient in most cases. For the rare patient with rapidly progressive tumor or signs or symptoms of visceral involvement, appropriate evaluation is indicated. No universally accepted classification is available for epidemic KS. Staging schemes that incorporate laboratory parameters as well as clinical features have been proposed. Since most patients with epidemic KS do not die from the disease, factors besides tumor burden are apparently involved in survival.
The conventions used to stage KS and the methods used to evaluate the benefits of KS treatment continue to evolve because of changes in the treatment of human immunodeficiency virus (HIV) and in recognition of deficiencies in standard tumor assessment. The clinical course of KS, the selection of treatment, and the response to treatment are heavily influenced by the degree of underlying immune dysfunction and opportunistic infections.
The AIDS Clinical Trials Group (ACTG) Oncology Committee has published criteria for the evaluation of epidemic KS.[1] The staging system incorporates measures of extent of disease, severity of immunodeficiency, and presence of systemic symptoms. As shown in Table 1 below, the ACTG criteria categorizes the extent of the tumor as localized or disseminated, the CD4 cell number as high or low, and a systemic illness as absent or present.
A subsequent prospective analysis of 294 patients entered on ACTG trials for KS between 1989 and 1995 showed that each of the tumor, immune system, and systemic illness variables was independently associated with survival.[2] Multivariate analysis showed that immune system impairment was the most important single predictor of survival. In patients with relatively high CD4 counts, tumor stage was predictive. A CD4 count of 150 cells/mm³ may be a better discriminator than the published cutoff of 200 cells/mm³. A study is in progress to determine if viral load adds predictive information. None of the prior studies were conducted at a time when combined antiretroviral therapy (cART) was readily available. The impact of cART on survival in KS requires continued assessment.
| Good Risk (0) | Poor Risk (1) | |
|---|---|---|
| (Any of the following) | (Any of the following) | |
| Tumor (T) | Confined to skin and/or lymph nodes and/or minimal oral disease[Note: Minimal oral disease is non-nodular KS confined to the palate.] | Tumor-associated edema or ulceration |
| Extensive oral KS | ||
| Gastrointestinal KS | ||
| KS in other non-nodal viscera | ||
| Immune system (I) | CD4 cells ≥ = 200/µL | CD4 cells <200 per cubic mm |
| Systemic illness (S) | No history of OIs or thrush[Note: OIs are opportunistic infections.] | History of OIs and/or thrush |
| No “B” symptoms[Note: “B” symptoms are unexplained fever, night sweats, >10% involuntary weight loss, or diarrhea persisting >2 weeks.] | “B” symptoms present | |
| Performance status ≥70 (Karnofsky) | Performance status <70 | |
| Other HIV-related illness (e.g., neurological disease or lymphoma) |
References
- Krown SE, Metroka C, Wernz JC: Kaposi's sarcoma in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol 7 (9): 1201-7, 1989. [PUBMED Abstract]
- Krown SE, Testa MA, Huang J: AIDS-related Kaposi's sarcoma: prospective validation of the AIDS Clinical Trials Group staging classification. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol 15 (9): 3085-92, 1997. [PUBMED Abstract]
Classic Kaposi Sarcoma Treatment
Classic Kaposi sarcoma (KS) usually is limited to the skin and has an indolent course. Patients with this tumor are predisposed to the development of a second primary malignancy, and the treating physician should consider this factor when arranging a schedule of follow-up treatment for the patient.
Equivalent standard treatment options:
Solitary lesions:
- Radiation therapy: For solitary lesions or lesions of limited extent, modest doses of radiation applied to the lesions with a limited margin provide excellent control of disease in the treated area. Usually, superficial radiation beams such as electron beams are used. Some authors believe disease recurrence in adjacent, untreated skin is common if only involved-field radiation therapy is used and claim better cure rates when extended-field radiation therapy is used.[1,2]
- Low-voltage (100 kv) photon radiation: 8 Gy to 10 Gy given as a single dose or 15 Gy to 20 Gy given over 1 week because solitary lesions control nearly 100% of local disease, but recurrence in adjacent areas is common.
- Electron-beam radiation therapy (EBRT): 4 Gy given once weekly for 6 to 8 consecutive weeks with a 4-MeV to 6-MeV electron beam. Ports should include the entire skin surface 15 cm above the lesion.
- Surgical excision may be of benefit in some patients with small superficial lesions, but local recurrence is likely to be a problem. However, over the years, multiple small excisions can be performed to achieve good disease control.
Widespread skin disease:
- Radiation therapy: Modest doses are effective in controlling disease. The type of radiation (i.e., photon vs. electron) and fields used must be tailored to suit the distribution of disease in the individual patient.[2]
- Extended-field EBRT.
- For disease limited to areas distal to the knee, subtotal-skin EBRT directed to skin below the umbilicus.
- For disease that extends above the knee, total-skin EBRT.
EBRT used in this manner gave long-term results that were superior to those obtained with radiation therapy administered to successive individual lesions as they appeared.[2]
- EBRT: 4 Gy given once weekly for 6 to 8 consecutive weeks, and subtotal- or total-skin radiation therapy given for extensive disease.
- Chemotherapy: Because classic KS is such a rare disease in the United States and is usually treated initially with radiation therapy, few patients have been treated with chemotherapy, and no randomized prospective trials have compared one agent to another. Several authors have used single-agent vinblastine given as a weekly dose of approximately 0.1 mg/kg.[3-6] Almost all of the patients had good to excellent response. In most cases, patients required prolonged courses of therapy, for several years, to maintain a partial response. Doses of vinblastine were titrated in individual patients to maintain a white blood count of more than 3,000 leukocytes. Follow-up after completion of therapy was not presented. In a multicenter trial of 55 patients who were treated over a decade, a 71% overall response rate was seen using pegylated liposomal doxorubicin.[7][Level of evidence: 3iiiDiv] In addition to the positive response rates of pegylated liposomal doxorubicin and the vinca alkaloids, response rates showing a greater than 50% decrease in lesions have also been reported in small, uncontrolled series for etoposide, taxanes, gemcitabine, and interferon alfa.[8][Level of evidence: 3iiiDiv]
One patient was treated repeatedly with intralesional injections of 0.25 to 0.50 mg of vincristine, which resulted in complete disappearance of the treated lesion.[9] Multiple courses of therapy were required because of the recurrence of disease in untreated areas.
Electroporation of the skin lesions was combined with intravenous bleomycin for 19 patients with classical KS. Most patients responded after one application, the rest after two or three applications, with a median duration of response of 16 months.[10][Level of evidence: 3iiiDiv]
Lymph node and gastrointestinal tract involvement:
- Chemotherapy: Several patients who had widespread skin disease and were treated with chemotherapy also had lymph node and gastrointestinal tract involvement. The disease in these sites also responded to vinblastine. Trials are required to define therapy. One such trial, MSKCC-04055 (NCT00096538), has been completed.
- Local radiation therapy may be added to chemotherapy if individual lesions require urgent therapy.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Hamilton CR, Cummings BJ, Harwood AR: Radiotherapy of Kaposi's sarcoma. Int J Radiat Oncol Biol Phys 12 (11): 1931-5, 1986. [PUBMED Abstract]
- Nisce LZ, Safai B, Poussin-Rosillo H: Once weekly total and subtotal skin electron beam therapy for Kaposi's sarcoma. Cancer 47 (4): 640-4, 1981. [PUBMED Abstract]
- Solan AJ, Greenwald ES, Silvay O: Long-term complete remissions of Kaposi's sarcoma with vinblastine therapy. Cancer 47 (4): 637-9, 1981. [PUBMED Abstract]
- Tucker SB, Winkelmann RK: Treatment of Kaposi sarcoma with vinblastine. Arch Dermatol 112 (7): 958-61, 1976. [PUBMED Abstract]
- Scott WP, Voight JA: Kaposi's sarcoma. Management with vincaleucoblastine. Cancer 19 (4): 557-64, 1966. [PUBMED Abstract]
- Klein E, Schwartz RA, Laor Y, et al.: Treatment of Kaposi's sarcoma with vinblastine. Cancer 45 (3): 427-31, 1980. [PUBMED Abstract]
- Di Lorenzo G, Kreuter A, Di Trolio R, et al.: Activity and safety of pegylated liposomal doxorubicin as first-line therapy in the treatment of non-visceral classic Kaposi's sarcoma: a multicenter study. J Invest Dermatol 128 (6): 1578-80, 2008. [PUBMED Abstract]
- Régnier-Rosencher E, Guillot B, Dupin N: Treatments for classic Kaposi sarcoma: a systematic review of the literature. J Am Acad Dermatol 68 (2): 313-31, 2013. [PUBMED Abstract]
- Odom RB, Goette DK: Treatment of cutaneous Kaposi's sarcoma with intralesional vincristine. Arch Dermatol 114 (11): 1693-4, 1978. [PUBMED Abstract]
- Di Monta G, Caracò C, Benedetto L, et al.: Electrochemotherapy as "new standard of care" treatment for cutaneous Kaposi's sarcoma. Eur J Surg Oncol 40 (1): 61-6, 2014. [PUBMED Abstract]
Immunosuppressive Therapy–Related Kaposi Sarcoma Treatment
Some patients with Kaposi Sarcoma (KS) have noted spontaneous and lasting remissions following discontinuation of immunosuppressive therapy. In managing these patients, if immunosuppressive therapy is not critical, its discontinuation is a reasonable first step.
Standard treatment options:
- Discontinue immunosuppressive therapy (often results in tumor regression). This option is critically important in patients who are receiving immunosuppressive drugs, as in the case of certain transplant patients.
- Radiation therapy (for disease limited to skin).[1-4]
- Chemotherapy (single or multiple drug): Most systemic chemotherapy trials in KS patients have been carried out in the African and epidemic varieties. See the section on the treatment of Epidemic Kaposi Sarcoma. The applicability of the results of these trials to KS in immunosuppressed patients is unknown.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Cohen L: Dose, time, and volume parameters in irradiation therapy of Kaposi's sarcoma. Br J Radiol 35 (415): 485-488, 1962.
- Hamilton CR, Cummings BJ, Harwood AR: Radiotherapy of Kaposi's sarcoma. Int J Radiat Oncol Biol Phys 12 (11): 1931-5, 1986. [PUBMED Abstract]
- Lo TC, Salzman FA, Smedal MI, et al.: Radiotherapy for Kaposi's sarcoma. Cancer 45 (4): 684-7, 1980. [PUBMED Abstract]
- Nisce LZ, Safai B, Poussin-Rosillo H: Once weekly total and subtotal skin electron beam therapy for Kaposi's sarcoma. Cancer 47 (4): 640-4, 1981. [PUBMED Abstract]
Epidemic Kaposi Sarcoma Treatment
Treatment may result:
- In a disappearance or reduction in size of specific skin lesions, thereby alleviating the discomfort associated with the chronic edema and ulcerations that often accompany multiple skin tumors seen on the lower extremities.
- In control of symptoms associated with mucosal or visceral lesions.
No data are available, however, to show that treatment improves survival.[1] In addition to antitumor treatment, essential components of an optimal Kaposi sarcoma (KS) treatment strategy include combined antiretroviral treatment (cART), prophylaxis for opportunistic infections, and rapid recognition and treatment of intercurrent infections.
Most good-risk patients, defined by the AIDS Clinical Trials Group as T0, show tumor regression with cART alone.[2-4] Poor-risk patients, defined as T1, usually require a combination of cART and chemotherapy with discontinuation of the chemotherapy after disappearance of the skin lesion.[2-4] The combination of cART and liposomal doxorubicin resulted in a 5-year overall survival (OS) rate of 85% in 140 patients with T1 disease.[3][Level of evidence: 3iiiDiv]
Local modalities
Small localized lesions of KS may be treated by electrodesiccation and curettage, cryotherapy, or by surgical excision. KS tumors are also generally very responsive to local radiation therapy, and excellent palliation has been obtained with doses at 20 Gy or slightly higher.[5-7] One report demonstrated a response rate higher than 90%, with a median time to progression of 21 months. Although no difference in response was noted with a variety of fractionation regimens, a single fraction of 8 Gy is indicated for cutaneous lesions and is associated with significantly fewer severe reactions.[8] Radiation therapy is generally reserved to treat localized areas of the skin and oral cavity. It is less often used to control pulmonary, gastrointestinal tract, or other sites of KS lesions. Localized KS lesions have also been effectively treated with intralesional injections of vinblastine.[9] Alitretinoin 0.1% gel provided local control in a randomized prospective multicenter trial.[10][Level of evidence: 1iiDiv]
Chemotherapy
In epidemic KS, the already profoundly depressed immunologic status of the host limits the therapeutic usefulness of systemic chemotherapy. Systemic chemotherapy studies in epidemic KS have used as single agents or in combinations doxorubicin, bleomycin, vinblastine, vincristine, etoposide, paclitaxel, and docetaxel.[11-15][Level of evidence: 3iiiDiv] The combination of cART and liposomal doxorubicin resulted in a 5-year OS of 85% in 140 patients with T1 disease.[3][Level of evidence: 3iiiDiv]
Randomized multicenter trials showed an improvement in response rate (45%–60% vs. 20%–25%) and a more favorable toxic effects profile for pegylated liposomal doxorubicin or liposomal daunorubicin, compared to the combination of doxorubicin, bleomycin, and vincristine or bleomycin and vincristine.[16-18][Level of evidence: 1iiDiv] During cART, both pegylated liposomal doxorubicin and paclitaxel are active single agents with response rates close to 50%.[19][Level of evidence: 1iiDiv]
Biologic and targeted therapy
The interferon alphas have also been widely studied and show a 40% objective response rate in patients with epidemic KS.[20,21] In these reports, the responses differed significantly according to the prognostic factors of extent of disease, prior or coexistent opportunistic infections, prior treatment with chemotherapy, CD4 lymphocyte counts lower than 200 cells/mm³, the presence of circulating acid-labile interferon alpha, and an increase in beta-2-microglobulin. Several treatment studies have combined interferon alpha with other chemotherapeutic agents. Overall, these trials have shown no benefit with the interferon-chemotherapy combinations as compared to the single-agent activities.
Recombinant interferon alpha-2a and interferon alpha-2b were the first agents approved for the treatment of KS. Approval was based on single-agent studies performed in the 1980s before the advent of antiretroviral therapy. The early studies demonstrated improved efficacy at relatively high doses. High-dose monotherapy is rarely used today, and instead, interferon is given in combination with other anti-HIV drugs in doses of 4 to 18 million units. Neutropenia is dose limiting, and trials of doses of 1 to 10 million units combined with less myelosuppressive antiretrovirals are in progress. Response to interferon is slow, and the maximum effect is seen after 6 or more months. Interferon should probably not be used in the treatment of patients with rapidly progressive, symptomatic KS.
Imatinib, a c-kit/PDGF (platelet-derived growth factor) receptor inhibitor, resulted in partial responses in 10 of 30 previously treated patients (cART + chemotherapy).[22]
Bevacizumab, the humanized, antivascular, endothelial growth–factor monoclonal antibody, had a response rate in 5 of 16 patients who did not improve after the institution of cART and chemotherapy.[23][Level of evidence: 3iiiDiv]
Interleukin-12 had a response rate of 71% (95% confidence interval, 48%–89%) among 24 evaluable patients in a phase I and phase II trial.[24][Level of evidence: 3iiiDiv]
Treatment options under clinical evaluation:
- Patients with epidemic KS are appropriate candidates for clinical trials evaluating new drugs or biologicals.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Safai B: Kaposi's sarcoma and acquired immunodeficiency syndrome. In: DeVita VT, Hellman S, Rosenberg S, eds.: AIDS: Etiology, Diagnosis, Treatment and Prevention. 4th ed. Philadelphia, Pa: Lippincott-Raven Publishers, 1997, pp 295-318.
- Krown SE: Highly active antiretroviral therapy in AIDS-associated Kaposi's sarcoma: implications for the design of therapeutic trials in patients with advanced, symptomatic Kaposi's sarcoma. J Clin Oncol 22 (3): 399-402, 2004. [PUBMED Abstract]
- Bower M, Dalla Pria A, Coyle C, et al.: Prospective stage-stratified approach to AIDS-related Kaposi's sarcoma. J Clin Oncol 32 (5): 409-14, 2014. [PUBMED Abstract]
- Krell J, Stebbing J: Broader implications of a stage-guided stratified therapeutic approach for AIDS-related Kaposi's sarcoma. J Clin Oncol 32 (5): 373-5, 2014. [PUBMED Abstract]
- Cooper JS, Steinfeld AD, Lerch I: Intentions and outcomes in the radiotherapeutic management of epidemic Kaposi's sarcoma. Int J Radiat Oncol Biol Phys 20 (3): 419-22, 1991. [PUBMED Abstract]
- Nobler MP, Leddy ME, Huh SH: The impact of palliative irradiation on the management of patients with acquired immune deficiency syndrome. J Clin Oncol 5 (1): 107-12, 1987. [PUBMED Abstract]
- Singh NB, Lakier RH, Donde B: Hypofractionated radiation therapy in the treatment of epidemic Kaposi sarcoma--a prospective randomized trial. Radiother Oncol 88 (2): 211-6, 2008. [PUBMED Abstract]
- Berson AM, Quivey JM, Harris JW, et al.: Radiation therapy for AIDS-related Kaposi's Sarcoma. Int J Radiat Oncol Biol Phys 19 (3): 569-75, 1990. [PUBMED Abstract]
- Epstein JB, Lozada-Nur F, McLeod WA, et al.: Oral Kaposi's sarcoma in acquired immunodeficiency syndrome. Review of management and report of the efficacy of intralesional vinblastine. Cancer 64 (12): 2424-30, 1989. [PUBMED Abstract]
- Bodsworth NJ, Bloch M, Bower M, et al.: Phase III vehicle-controlled, multi-centered study of topical alitretinoin gel 0.1% in cutaneous AIDS-related Kaposi's sarcoma. Am J Clin Dermatol 2 (2): 77-87, 2001. [PUBMED Abstract]
- Evans SR, Krown SE, Testa MA, et al.: Phase II evaluation of low-dose oral etoposide for the treatment of relapsed or progressive AIDS-related Kaposi's sarcoma: an AIDS Clinical Trials Group clinical study. J Clin Oncol 20 (15): 3236-41, 2002. [PUBMED Abstract]
- Saville MW, Lietzau J, Pluda JM, et al.: Treatment of HIV-associated Kaposi's sarcoma with paclitaxel. Lancet 346 (8966): 26-8, 1995. [PUBMED Abstract]
- Lim ST, Tupule A, Espina BM, et al.: Weekly docetaxel is safe and effective in the treatment of advanced-stage acquired immunodeficiency syndrome-related Kaposi sarcoma. Cancer 103 (2): 417-21, 2005. [PUBMED Abstract]
- Gill PS, Tulpule A, Espina BM, et al.: Paclitaxel is safe and effective in the treatment of advanced AIDS-related Kaposi's sarcoma. J Clin Oncol 17 (6): 1876-83, 1999. [PUBMED Abstract]
- Di Lorenzo G, Konstantinopoulos PA, Pantanowitz L, et al.: Management of AIDS-related Kaposi's sarcoma. Lancet Oncol 8 (2): 167-76, 2007. [PUBMED Abstract]
- Stewart S, Jablonowski H, Goebel FD, et al.: Randomized comparative trial of pegylated liposomal doxorubicin versus bleomycin and vincristine in the treatment of AIDS-related Kaposi's sarcoma. International Pegylated Liposomal Doxorubicin Study Group. J Clin Oncol 16 (2): 683-91, 1998. [PUBMED Abstract]
- Northfelt DW, Dezube BJ, Thommes JA, et al.: Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi's sarcoma: results of a randomized phase III clinical trial. J Clin Oncol 16 (7): 2445-51, 1998. [PUBMED Abstract]
- Gill PS, Wernz J, Scadden DT, et al.: Randomized phase III trial of liposomal daunorubicin versus doxorubicin, bleomycin, and vincristine in AIDS-related Kaposi's sarcoma. J Clin Oncol 14 (8): 2353-64, 1996. [PUBMED Abstract]
- Cianfrocca M, Lee S, Von Roenn J, et al.: Randomized trial of paclitaxel versus pegylated liposomal doxorubicin for advanced human immunodeficiency virus-associated Kaposi sarcoma: evidence of symptom palliation from chemotherapy. Cancer 116 (16): 3969-77, 2010. [PUBMED Abstract]
- Real FX, Oettgen HF, Krown SE: Kaposi's sarcoma and the acquired immunodeficiency syndrome: treatment with high and low doses of recombinant leukocyte A interferon. J Clin Oncol 4 (4): 544-51, 1986. [PUBMED Abstract]
- Groopman JE, Gottlieb MS, Goodman J, et al.: Recombinant alpha-2 interferon therapy for Kaposi's sarcoma associated with the acquired immunodeficiency syndrome. Ann Intern Med 100 (5): 671-6, 1984. [PUBMED Abstract]
- Koon HB, Krown SE, Lee JY, et al.: Phase II trial of imatinib in AIDS-associated Kaposi's sarcoma: AIDS Malignancy Consortium Protocol 042. J Clin Oncol 32 (5): 402-8, 2014. [PUBMED Abstract]
- Uldrick TS, Wyvill KM, Kumar P, et al.: Phase II study of bevacizumab in patients with HIV-associated Kaposi's sarcoma receiving antiretroviral therapy. J Clin Oncol 30 (13): 1476-83, 2012. [PUBMED Abstract]
- Little RF, Pluda JM, Wyvill KM, et al.: Activity of subcutaneous interleukin-12 in AIDS-related Kaposi sarcoma. Blood 107 (12): 4650-7, 2006. [PUBMED Abstract]
Recurrent Kaposi Sarcoma Treatment
The prognosis for any treated Kaposi sarcoma patient with progressing, recurring, or relapsing disease is highly variable. Deciding on further treatment depends on many factors, most importantly the clinical setting (i.e., classic, immunosuppressive treatment, or AIDS) in which the tumor arises as well as individual patient considerations.
Clinical trials are appropriate and should be considered when possible.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Changes to This Summary (01/30/2018)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Editorial changes were made to this summary.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of Kaposi sarcoma. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Kaposi Sarcoma Treatment are:
- Eric J. Seifter, MD (Johns Hopkins University)
- Minh Tam Truong, MD (Boston University Medical Center)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Kaposi Sarcoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/soft-tissue-sarcoma/hp/kaposi-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389335]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.
Updated: January 30, 2018
Source URL: https://www.cancer.gov/publishedcontent/syndication/3524.htm
Source Agency: National Cancer Institute (NCI)
Captured Date: 2013-09-14 09:01:43.0
Kaposi Sarcoma Treatment (PDQ®)–Health Professional Version
General Information About Kaposi Sarcoma
Epidemiology
KS was first described in 1872 by the Hungarian dermatologist, Moritz Kaposi. From that time until the current human immunodeficiency virus (HIV) disease epidemic identified with the Acquired Immunodeficiency Syndrome (AIDS), KS remained a rare tumor. While most of the cases seen in Europe and North America have occurred in elderly men of Italian or Eastern European Jewish ancestry, the neoplasm also occurs in several other distinct populations: young black African adult males, prepubescent children, renal allograft recipients, and other patients receiving immunosuppressive therapy. The disseminated, fulminant form of KS associated with HIV disease is referred to as epidemic KS to distinguish it from the classic, African, and transplant-related varieties of the neoplasm. In addition, KS has been identified in homosexual men apart from the HIV disease epidemic.[1]
Histopathology
Although the histopathology of the different types of the Kaposi tumor is essentially identical in all of these groups, the clinical manifestations and course of the disease differ dramatically.[2] A key piece to the puzzle of KS pathogenesis was the 1994 discovery of a gamma herpes virus, human herpes virus type 8 (HHV-8), also known as Kaposi sarcoma herpes virus.[3] HHV-8 was identified in KS tissue biopsies from virtually all patients with classic, African, transplant-related, and AIDS-associated KS but was absent from noninvolved tissue.[4-7]
Classic Kaposi Sarcoma
Considered a rare disease, classic KS occurs more often in males, with a ratio of approximately 10 to 15 males to 1 female. In North Americans and Europeans, the usual age at onset is between 50 and 70 years. Classic KS tumors usually present with one or more asymptomatic red, purple, or brown patches, plaques, or nodular skin lesions. The disease is often limited to single or multiple lesions usually localized to one or both lower extremities, especially involving the ankles and soles.
Classic KS most commonly runs a relatively benign, indolent course for 10 to 15 years or more, with slow enlargement of the original tumors and the gradual development of additional lesions. Venous stasis and lymphedema of the involved lower extremity are frequent complications. In long-standing cases, systemic lesions can develop along the gastrointestinal tract, in lymph nodes, and in other organs. The visceral lesions are generally asymptomatic and are most often discovered only at autopsy, though clinically, gastrointestinal bleeding can occur. As many as 33% of the patients with classic KS develop a second primary malignancy, which is most often non-Hodgkin lymphoma.[8-10]
African Kaposi Sarcoma
In the 1950s, KS was recognized as a relatively common neoplasm endemic in native populations in equatorial Africa and comprised approximately 9% of all cancers seen in Ugandan males. African KS is seen as either an indolent neoplasm identical to the classic disease seen in Europe and North America or as an aggressive disease with fungating and exophytic tumors that may invade the subcutaneous and surrounding tissue including the underlying bone. In Africa, both the indolent and locally more aggressive forms of KS occur with a male-to-female ratio comparable to that observed with the classic KS tumor seen in North America and Europe. In general, however, patients in Africa are significantly younger than their European counterparts. A lymphadenopathic form of KS is also seen in Africa, primarily in prepubescent children (male:female ratio, 3:1). In these cases, the generalized lymphadenopathy is frequently associated with visceral organ involvement. The prognosis is very poor with a 100% fatality rate within 3 years.[11,12]
Immunosuppressive Treatment–Related Kaposi Sarcoma
In 1969, the first case of KS in association with immunosuppression in a renal transplant patient was described. Since that time, a number of renal and other organ allograft recipients who received prednisone and azathioprine developed KS shortly after the onset of immunosuppressive therapy.[13] Estimates of the incidence of KS in immunosuppressed renal transplant recipients are between 150 and 200 times the expected incidence of the tumor in the general population. The average time to develop KS after transplantation is 16 months. Although the KS tumor in iatrogenically immunosuppressed patients often remains localized to the skin, widespread dissemination with mucocutaneous or visceral organ involvement is common. In some cases, the KS tumors have regressed as a result of reduction or changes in immunosuppressive therapy. Clinical management of renal transplant patients who develop KS is difficult and requires a balance between the risk of death from generalized KS and the risk of graft rejection and complications of renal failure that may occur if the immunosuppressive therapy is discontinued.
Epidemic Kaposi Sarcoma
In 1981, a fulminant and disseminated form of KS in young homosexual or bisexual men was first reported as part of an epidemic now known as AIDS.[14] The etiology of AIDS is a T-cell lymphotropic retrovirus known as HIV. The underlying immunologic deficiency that characterizes HIV disease is an acquired profound disorder of cell-mediated immune functions. This immunologic deficiency and immune dysregulation predisposes the host to a variety of opportunistic infections and unusual neoplasms, especially KS. HIV may play an indirect role in the development of KS.[15]
Approximately 95% of all the cases of epidemic KS in the United States have been diagnosed in homosexual or bisexual men. In the past, approximately 26% of all homosexual males with HIV disease presented with, or eventually developed, KS during the course of their illness. By comparison, fewer than 3% of all heterosexual intravenous drug users with HIV disease developed KS. The proportion of HIV disease patients with KS has steadily decreased since the epidemic was first identified in 1981.[16] About 48% of AIDS patients in 1981 had KS as their presenting AIDS diagnosis. By August 1987, the cumulative proportion of AIDS patients with KS had diminished to fewer than 20%. The introduction of combined antiretroviral therapy (cART) has delayed or prevented the emergence of drug-resistant HIV strains, profoundly decreased viral load, led to increased survival, and lessened the risk of opportunistic infections.[17-19] The use of cART has been associated with a sustained and substantial decline in KS incidence in multiple large cohorts.[20-25]
The lesions that develop may involve the skin; oral mucosa; lymph nodes; and visceral organs, such as the gastrointestinal tract, lung, liver, and spleen. Most patients with HIV disease who present with the mucocutaneous lesions of KS feel healthy and are usually free of systemic symptoms, as compared with HIV patients who first develop an opportunistic infection. The sites of disease at presentation of epidemic KS are much more varied than the sites seen in other types of this neoplasm. In an early report on the clinical manifestations of the disease, 49 patients were described.[26] Of these patients, 8% had no skin involvement, 27% had localized or fewer than five skin lesions, and 63% had innumerable skin lesions widely distributed over the skin surface area. Of these patients, 61% had generalized lymphadenopathy at the time of the first examination. Four of these patients, who had generalized lymphadenopathy in the absence of skin lesions or detectable visceral organ involvement at the time of presentation, were found to have biopsy-proven KS localized to the lymph nodes. In 45% of the patients studied, KS lesions were found in one or more sites along the gastrointestinal tract. Of these patients, 29% had either unexplained fever or unexplained weight loss when first seen. While most patients present with skin disease, KS involvement of lymph nodes or the gastrointestinal tract may occasionally precede the appearance of the cutaneous lesions.
Eventually, most patients with epidemic KS develop disseminated disease. The disease often progresses in an orderly fashion from a few localized or widespread mucocutaneous lesions to more numerous lesions and generalized skin disease with lymph node, gastrointestinal tract disease, and other organ involvement. Pleuropulmonary KS is an ominous sign usually occurring late in the course of the disease, especially in those patients whose death is directly attributed to KS.[27] Most patients with epidemic KS die of one or more complicating opportunistic infections.
References
- Friedman-Kien AE, Saltzman BR, Cao YZ, et al.: Kaposi's sarcoma in HIV-negative homosexual men. Lancet 335 (8682): 168-9, 1990. [PUBMED Abstract]
- Safai B: Kaposi's sarcoma and acquired immunodeficiency syndrome. In: DeVita VT, Hellman S, Rosenberg S, eds.: AIDS: Etiology, Diagnosis, Treatment and Prevention. 4th ed. Philadelphia, Pa: Lippincott-Raven Publishers, 1997, pp 295-318.
- Chang Y, Cesarman E, Pessin MS, et al.: Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266 (5192): 1865-9, 1994. [PUBMED Abstract]
- Moore PS, Chang Y: Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and without HIV infection. N Engl J Med 332 (18): 1181-5, 1995. [PUBMED Abstract]
- Su IJ, Hsu YS, Chang YC, et al.: Herpesvirus-like DNA sequence in Kaposi's sarcoma from AIDS and non-AIDS patients in Taiwan. Lancet 345 (8951): 722-3, 1995. [PUBMED Abstract]
- Gao SJ, Kingsley L, Li M, et al.: KSHV antibodies among Americans, Italians and Ugandans with and without Kaposi's sarcoma. Nat Med 2 (8): 925-8, 1996. [PUBMED Abstract]
- Chang Y, Ziegler J, Wabinga H, et al.: Kaposi's sarcoma-associated herpesvirus and Kaposi's sarcoma in Africa. Uganda Kaposi's Sarcoma Study Group. Arch Intern Med 156 (2): 202-4, 1996. [PUBMED Abstract]
- Safai B, Good RA: Kaposi's sarcoma: a review and recent developments. Clin Bull 10 (2): 62-9, 1980. [PUBMED Abstract]
- Reynolds WA, Winkelmann RK, Soule EH: Kaposi's sarcoma: a clinicopathologic study with particular reference to its relationship to the reticuloendothelial system. Medicine (Baltimore) 44 (5): 419-43, 1965. [PUBMED Abstract]
- Safai B, Miké V, Giraldo G, et al.: Association of Kaposi's sarcoma with second primary malignancies: possible etiopathogenic implications. Cancer 45 (6): 1472-9, 1980. [PUBMED Abstract]
- Taylor JF, Templeton AC, Vogel CL, et al.: Kaposi's sarcoma in Uganda: a clinico-pathological study. Int J Cancer 8 (1): 122-35, 1971. [PUBMED Abstract]
- Templeton AC, Bhana D: Prognosis in Kaposi's sarcoma. J Natl Cancer Inst 55 (6): 1301-4, 1975. [PUBMED Abstract]
- Penn I: Kaposi's sarcoma in organ transplant recipients: report of 20 cases. Transplantation 27 (1): 8-11, 1979. [PUBMED Abstract]
- Kaposi's sarcoma and Pneumocystis pneumonia among homosexual men--New York City and California. MMWR Morb Mortal Wkly Rep 30 (25): 305-8, 1981. [PUBMED Abstract]
- Vogel J, Hinrichs SH, Reynolds RK, et al.: The HIV tat gene induces dermal lesions resembling Kaposi's sarcoma in transgenic mice. Nature 335 (6191): 606-11, 1988. [PUBMED Abstract]
- Selik RM, Starcher ET, Curran JW: Opportunistic diseases reported in AIDS patients: frequencies, associations, and trends. AIDS 1 (3): 175-82, 1987. [PUBMED Abstract]
- Flexner C: HIV-protease inhibitors. N Engl J Med 338 (18): 1281-92, 1998. [PUBMED Abstract]
- Palella FJ Jr, Delaney KM, Moorman AC, et al.: Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338 (13): 853-60, 1998. [PUBMED Abstract]
- Lodi S, Guiguet M, Costagliola D, et al.: Kaposi sarcoma incidence and survival among HIV-infected homosexual men after HIV seroconversion. J Natl Cancer Inst 102 (11): 784-92, 2010. [PUBMED Abstract]
- Portsmouth S, Stebbing J, Gill J, et al.: A comparison of regimens based on non-nucleoside reverse transcriptase inhibitors or protease inhibitors in preventing Kaposi's sarcoma. AIDS 17 (11): F17-22, 2003. [PUBMED Abstract]
- International Collaboration on HIV and Cancer: Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst 92 (22): 1823-30, 2000. [PUBMED Abstract]
- Dupont C, Vasseur E, Beauchet A, et al.: Long-term efficacy on Kaposi's sarcoma of highly active antiretroviral therapy in a cohort of HIV-positive patients. CISIH 92. Centre d'information et de soins de l'immunodéficience humaine. AIDS 14 (8): 987-93, 2000. [PUBMED Abstract]
- Tam HK, Zhang ZF, Jacobson LP, et al.: Effect of highly active antiretroviral therapy on survival among HIV-infected men with Kaposi sarcoma or non-Hodgkin lymphoma. Int J Cancer 98 (6): 916-22, 2002. [PUBMED Abstract]
- Carrieri MP, Pradier C, Piselli P, et al.: Reduced incidence of Kaposi's sarcoma and of systemic non-hodgkin's lymphoma in HIV-infected individuals treated with highly active antiretroviral therapy. Int J Cancer 103 (1): 142-4, 2003. [PUBMED Abstract]
- Grabar S, Abraham B, Mahamat A, et al.: Differential impact of combination antiretroviral therapy in preventing Kaposi's sarcoma with and without visceral involvement. J Clin Oncol 24 (21): 3408-14, 2006. [PUBMED Abstract]
- Krigel RL, Laubenstein LJ, Muggia FM: Kaposi's sarcoma: a new staging classification. Cancer Treat Rep 67 (6): 531-4, 1983. [PUBMED Abstract]
- Gill PS, Akil B, Colletti P, et al.: Pulmonary Kaposi's sarcoma: clinical findings and results of therapy. Am J Med 87 (1): 57-61, 1989. [PUBMED Abstract]
Stage Information for Kaposi Sarcoma
The staging evaluation of patients with classic Kaposi sarcoma (KS) should be individualized. The advanced age of most of the patients, localized nature of the tumor, rarity of visceral involvement, and usually indolent course of the disease should temper the extent of the evaluation. A careful examination of the skin and lymph nodes is sufficient in most cases. For the rare patient with rapidly progressive tumor or signs or symptoms of visceral involvement, appropriate evaluation is indicated. No universally accepted classification is available for epidemic KS. Staging schemes that incorporate laboratory parameters as well as clinical features have been proposed. Since most patients with epidemic KS do not die from the disease, factors besides tumor burden are apparently involved in survival.
The conventions used to stage KS and the methods used to evaluate the benefits of KS treatment continue to evolve because of changes in the treatment of human immunodeficiency virus (HIV) and in recognition of deficiencies in standard tumor assessment. The clinical course of KS, the selection of treatment, and the response to treatment are heavily influenced by the degree of underlying immune dysfunction and opportunistic infections.
The AIDS Clinical Trials Group (ACTG) Oncology Committee has published criteria for the evaluation of epidemic KS.[1] The staging system incorporates measures of extent of disease, severity of immunodeficiency, and presence of systemic symptoms. As shown in Table 1 below, the ACTG criteria categorizes the extent of the tumor as localized or disseminated, the CD4 cell number as high or low, and a systemic illness as absent or present.
A subsequent prospective analysis of 294 patients entered on ACTG trials for KS between 1989 and 1995 showed that each of the tumor, immune system, and systemic illness variables was independently associated with survival.[2] Multivariate analysis showed that immune system impairment was the most important single predictor of survival. In patients with relatively high CD4 counts, tumor stage was predictive. A CD4 count of 150 cells/mm³ may be a better discriminator than the published cutoff of 200 cells/mm³. A study is in progress to determine if viral load adds predictive information. None of the prior studies were conducted at a time when combined antiretroviral therapy (cART) was readily available. The impact of cART on survival in KS requires continued assessment.
| Good Risk (0) | Poor Risk (1) | |
|---|---|---|
| (Any of the following) | (Any of the following) | |
| Tumor (T) | Confined to skin and/or lymph nodes and/or minimal oral disease[Note: Minimal oral disease is non-nodular KS confined to the palate.] | Tumor-associated edema or ulceration |
| Extensive oral KS | ||
| Gastrointestinal KS | ||
| KS in other non-nodal viscera | ||
| Immune system (I) | CD4 cells ≥ = 200/µL | CD4 cells <200 per cubic mm |
| Systemic illness (S) | No history of OIs or thrush[Note: OIs are opportunistic infections.] | History of OIs and/or thrush |
| No “B” symptoms[Note: “B” symptoms are unexplained fever, night sweats, >10% involuntary weight loss, or diarrhea persisting >2 weeks.] | “B” symptoms present | |
| Performance status ≥70 (Karnofsky) | Performance status <70 | |
| Other HIV-related illness (e.g., neurological disease or lymphoma) |
References
- Krown SE, Metroka C, Wernz JC: Kaposi's sarcoma in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol 7 (9): 1201-7, 1989. [PUBMED Abstract]
- Krown SE, Testa MA, Huang J: AIDS-related Kaposi's sarcoma: prospective validation of the AIDS Clinical Trials Group staging classification. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol 15 (9): 3085-92, 1997. [PUBMED Abstract]
Classic Kaposi Sarcoma Treatment
Classic Kaposi sarcoma (KS) usually is limited to the skin and has an indolent course. Patients with this tumor are predisposed to the development of a second primary malignancy, and the treating physician should consider this factor when arranging a schedule of follow-up treatment for the patient.
Equivalent standard treatment options:
Solitary lesions:
- Radiation therapy: For solitary lesions or lesions of limited extent, modest doses of radiation applied to the lesions with a limited margin provide excellent control of disease in the treated area. Usually, superficial radiation beams such as electron beams are used. Some authors believe disease recurrence in adjacent, untreated skin is common if only involved-field radiation therapy is used and claim better cure rates when extended-field radiation therapy is used.[1,2]
- Low-voltage (100 kv) photon radiation: 8 Gy to 10 Gy given as a single dose or 15 Gy to 20 Gy given over 1 week because solitary lesions control nearly 100% of local disease, but recurrence in adjacent areas is common.
- Electron-beam radiation therapy (EBRT): 4 Gy given once weekly for 6 to 8 consecutive weeks with a 4-MeV to 6-MeV electron beam. Ports should include the entire skin surface 15 cm above the lesion.
- Surgical excision may be of benefit in some patients with small superficial lesions, but local recurrence is likely to be a problem. However, over the years, multiple small excisions can be performed to achieve good disease control.
Widespread skin disease:
- Radiation therapy: Modest doses are effective in controlling disease. The type of radiation (i.e., photon vs. electron) and fields used must be tailored to suit the distribution of disease in the individual patient.[2]
- Extended-field EBRT.
- For disease limited to areas distal to the knee, subtotal-skin EBRT directed to skin below the umbilicus.
- For disease that extends above the knee, total-skin EBRT.
EBRT used in this manner gave long-term results that were superior to those obtained with radiation therapy administered to successive individual lesions as they appeared.[2]
- EBRT: 4 Gy given once weekly for 6 to 8 consecutive weeks, and subtotal- or total-skin radiation therapy given for extensive disease.
- Chemotherapy: Because classic KS is such a rare disease in the United States and is usually treated initially with radiation therapy, few patients have been treated with chemotherapy, and no randomized prospective trials have compared one agent to another. Several authors have used single-agent vinblastine given as a weekly dose of approximately 0.1 mg/kg.[3-6] Almost all of the patients had good to excellent response. In most cases, patients required prolonged courses of therapy, for several years, to maintain a partial response. Doses of vinblastine were titrated in individual patients to maintain a white blood count of more than 3,000 leukocytes. Follow-up after completion of therapy was not presented. In a multicenter trial of 55 patients who were treated over a decade, a 71% overall response rate was seen using pegylated liposomal doxorubicin.[7][Level of evidence: 3iiiDiv] In addition to the positive response rates of pegylated liposomal doxorubicin and the vinca alkaloids, response rates showing a greater than 50% decrease in lesions have also been reported in small, uncontrolled series for etoposide, taxanes, gemcitabine, and interferon alfa.[8][Level of evidence: 3iiiDiv]
One patient was treated repeatedly with intralesional injections of 0.25 to 0.50 mg of vincristine, which resulted in complete disappearance of the treated lesion.[9] Multiple courses of therapy were required because of the recurrence of disease in untreated areas.
Electroporation of the skin lesions was combined with intravenous bleomycin for 19 patients with classical KS. Most patients responded after one application, the rest after two or three applications, with a median duration of response of 16 months.[10][Level of evidence: 3iiiDiv]
Lymph node and gastrointestinal tract involvement:
- Chemotherapy: Several patients who had widespread skin disease and were treated with chemotherapy also had lymph node and gastrointestinal tract involvement. The disease in these sites also responded to vinblastine. Trials are required to define therapy. One such trial, MSKCC-04055 (NCT00096538), has been completed.
- Local radiation therapy may be added to chemotherapy if individual lesions require urgent therapy.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Hamilton CR, Cummings BJ, Harwood AR: Radiotherapy of Kaposi's sarcoma. Int J Radiat Oncol Biol Phys 12 (11): 1931-5, 1986. [PUBMED Abstract]
- Nisce LZ, Safai B, Poussin-Rosillo H: Once weekly total and subtotal skin electron beam therapy for Kaposi's sarcoma. Cancer 47 (4): 640-4, 1981. [PUBMED Abstract]
- Solan AJ, Greenwald ES, Silvay O: Long-term complete remissions of Kaposi's sarcoma with vinblastine therapy. Cancer 47 (4): 637-9, 1981. [PUBMED Abstract]
- Tucker SB, Winkelmann RK: Treatment of Kaposi sarcoma with vinblastine. Arch Dermatol 112 (7): 958-61, 1976. [PUBMED Abstract]
- Scott WP, Voight JA: Kaposi's sarcoma. Management with vincaleucoblastine. Cancer 19 (4): 557-64, 1966. [PUBMED Abstract]
- Klein E, Schwartz RA, Laor Y, et al.: Treatment of Kaposi's sarcoma with vinblastine. Cancer 45 (3): 427-31, 1980. [PUBMED Abstract]
- Di Lorenzo G, Kreuter A, Di Trolio R, et al.: Activity and safety of pegylated liposomal doxorubicin as first-line therapy in the treatment of non-visceral classic Kaposi's sarcoma: a multicenter study. J Invest Dermatol 128 (6): 1578-80, 2008. [PUBMED Abstract]
- Régnier-Rosencher E, Guillot B, Dupin N: Treatments for classic Kaposi sarcoma: a systematic review of the literature. J Am Acad Dermatol 68 (2): 313-31, 2013. [PUBMED Abstract]
- Odom RB, Goette DK: Treatment of cutaneous Kaposi's sarcoma with intralesional vincristine. Arch Dermatol 114 (11): 1693-4, 1978. [PUBMED Abstract]
- Di Monta G, Caracò C, Benedetto L, et al.: Electrochemotherapy as "new standard of care" treatment for cutaneous Kaposi's sarcoma. Eur J Surg Oncol 40 (1): 61-6, 2014. [PUBMED Abstract]
Immunosuppressive Therapy–Related Kaposi Sarcoma Treatment
Some patients with Kaposi Sarcoma (KS) have noted spontaneous and lasting remissions following discontinuation of immunosuppressive therapy. In managing these patients, if immunosuppressive therapy is not critical, its discontinuation is a reasonable first step.
Standard treatment options:
- Discontinue immunosuppressive therapy (often results in tumor regression). This option is critically important in patients who are receiving immunosuppressive drugs, as in the case of certain transplant patients.
- Radiation therapy (for disease limited to skin).[1-4]
- Chemotherapy (single or multiple drug): Most systemic chemotherapy trials in KS patients have been carried out in the African and epidemic varieties. See the section on the treatment of Epidemic Kaposi Sarcoma. The applicability of the results of these trials to KS in immunosuppressed patients is unknown.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Cohen L: Dose, time, and volume parameters in irradiation therapy of Kaposi's sarcoma. Br J Radiol 35 (415): 485-488, 1962.
- Hamilton CR, Cummings BJ, Harwood AR: Radiotherapy of Kaposi's sarcoma. Int J Radiat Oncol Biol Phys 12 (11): 1931-5, 1986. [PUBMED Abstract]
- Lo TC, Salzman FA, Smedal MI, et al.: Radiotherapy for Kaposi's sarcoma. Cancer 45 (4): 684-7, 1980. [PUBMED Abstract]
- Nisce LZ, Safai B, Poussin-Rosillo H: Once weekly total and subtotal skin electron beam therapy for Kaposi's sarcoma. Cancer 47 (4): 640-4, 1981. [PUBMED Abstract]
Epidemic Kaposi Sarcoma Treatment
Treatment may result:
- In a disappearance or reduction in size of specific skin lesions, thereby alleviating the discomfort associated with the chronic edema and ulcerations that often accompany multiple skin tumors seen on the lower extremities.
- In control of symptoms associated with mucosal or visceral lesions.
No data are available, however, to show that treatment improves survival.[1] In addition to antitumor treatment, essential components of an optimal Kaposi sarcoma (KS) treatment strategy include combined antiretroviral treatment (cART), prophylaxis for opportunistic infections, and rapid recognition and treatment of intercurrent infections.
Most good-risk patients, defined by the AIDS Clinical Trials Group as T0, show tumor regression with cART alone.[2-4] Poor-risk patients, defined as T1, usually require a combination of cART and chemotherapy with discontinuation of the chemotherapy after disappearance of the skin lesion.[2-4] The combination of cART and liposomal doxorubicin resulted in a 5-year overall survival (OS) rate of 85% in 140 patients with T1 disease.[3][Level of evidence: 3iiiDiv]
Local modalities
Small localized lesions of KS may be treated by electrodesiccation and curettage, cryotherapy, or by surgical excision. KS tumors are also generally very responsive to local radiation therapy, and excellent palliation has been obtained with doses at 20 Gy or slightly higher.[5-7] One report demonstrated a response rate higher than 90%, with a median time to progression of 21 months. Although no difference in response was noted with a variety of fractionation regimens, a single fraction of 8 Gy is indicated for cutaneous lesions and is associated with significantly fewer severe reactions.[8] Radiation therapy is generally reserved to treat localized areas of the skin and oral cavity. It is less often used to control pulmonary, gastrointestinal tract, or other sites of KS lesions. Localized KS lesions have also been effectively treated with intralesional injections of vinblastine.[9] Alitretinoin 0.1% gel provided local control in a randomized prospective multicenter trial.[10][Level of evidence: 1iiDiv]
Chemotherapy
In epidemic KS, the already profoundly depressed immunologic status of the host limits the therapeutic usefulness of systemic chemotherapy. Systemic chemotherapy studies in epidemic KS have used as single agents or in combinations doxorubicin, bleomycin, vinblastine, vincristine, etoposide, paclitaxel, and docetaxel.[11-15][Level of evidence: 3iiiDiv] The combination of cART and liposomal doxorubicin resulted in a 5-year OS of 85% in 140 patients with T1 disease.[3][Level of evidence: 3iiiDiv]
Randomized multicenter trials showed an improvement in response rate (45%–60% vs. 20%–25%) and a more favorable toxic effects profile for pegylated liposomal doxorubicin or liposomal daunorubicin, compared to the combination of doxorubicin, bleomycin, and vincristine or bleomycin and vincristine.[16-18][Level of evidence: 1iiDiv] During cART, both pegylated liposomal doxorubicin and paclitaxel are active single agents with response rates close to 50%.[19][Level of evidence: 1iiDiv]
Biologic and targeted therapy
The interferon alphas have also been widely studied and show a 40% objective response rate in patients with epidemic KS.[20,21] In these reports, the responses differed significantly according to the prognostic factors of extent of disease, prior or coexistent opportunistic infections, prior treatment with chemotherapy, CD4 lymphocyte counts lower than 200 cells/mm³, the presence of circulating acid-labile interferon alpha, and an increase in beta-2-microglobulin. Several treatment studies have combined interferon alpha with other chemotherapeutic agents. Overall, these trials have shown no benefit with the interferon-chemotherapy combinations as compared to the single-agent activities.
Recombinant interferon alpha-2a and interferon alpha-2b were the first agents approved for the treatment of KS. Approval was based on single-agent studies performed in the 1980s before the advent of antiretroviral therapy. The early studies demonstrated improved efficacy at relatively high doses. High-dose monotherapy is rarely used today, and instead, interferon is given in combination with other anti-HIV drugs in doses of 4 to 18 million units. Neutropenia is dose limiting, and trials of doses of 1 to 10 million units combined with less myelosuppressive antiretrovirals are in progress. Response to interferon is slow, and the maximum effect is seen after 6 or more months. Interferon should probably not be used in the treatment of patients with rapidly progressive, symptomatic KS.
Imatinib, a c-kit/PDGF (platelet-derived growth factor) receptor inhibitor, resulted in partial responses in 10 of 30 previously treated patients (cART + chemotherapy).[22]
Bevacizumab, the humanized, antivascular, endothelial growth–factor monoclonal antibody, had a response rate in 5 of 16 patients who did not improve after the institution of cART and chemotherapy.[23][Level of evidence: 3iiiDiv]
Interleukin-12 had a response rate of 71% (95% confidence interval, 48%–89%) among 24 evaluable patients in a phase I and phase II trial.[24][Level of evidence: 3iiiDiv]
Treatment options under clinical evaluation:
- Patients with epidemic KS are appropriate candidates for clinical trials evaluating new drugs or biologicals.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
References
- Safai B: Kaposi's sarcoma and acquired immunodeficiency syndrome. In: DeVita VT, Hellman S, Rosenberg S, eds.: AIDS: Etiology, Diagnosis, Treatment and Prevention. 4th ed. Philadelphia, Pa: Lippincott-Raven Publishers, 1997, pp 295-318.
- Krown SE: Highly active antiretroviral therapy in AIDS-associated Kaposi's sarcoma: implications for the design of therapeutic trials in patients with advanced, symptomatic Kaposi's sarcoma. J Clin Oncol 22 (3): 399-402, 2004. [PUBMED Abstract]
- Bower M, Dalla Pria A, Coyle C, et al.: Prospective stage-stratified approach to AIDS-related Kaposi's sarcoma. J Clin Oncol 32 (5): 409-14, 2014. [PUBMED Abstract]
- Krell J, Stebbing J: Broader implications of a stage-guided stratified therapeutic approach for AIDS-related Kaposi's sarcoma. J Clin Oncol 32 (5): 373-5, 2014. [PUBMED Abstract]
- Cooper JS, Steinfeld AD, Lerch I: Intentions and outcomes in the radiotherapeutic management of epidemic Kaposi's sarcoma. Int J Radiat Oncol Biol Phys 20 (3): 419-22, 1991. [PUBMED Abstract]
- Nobler MP, Leddy ME, Huh SH: The impact of palliative irradiation on the management of patients with acquired immune deficiency syndrome. J Clin Oncol 5 (1): 107-12, 1987. [PUBMED Abstract]
- Singh NB, Lakier RH, Donde B: Hypofractionated radiation therapy in the treatment of epidemic Kaposi sarcoma--a prospective randomized trial. Radiother Oncol 88 (2): 211-6, 2008. [PUBMED Abstract]
- Berson AM, Quivey JM, Harris JW, et al.: Radiation therapy for AIDS-related Kaposi's Sarcoma. Int J Radiat Oncol Biol Phys 19 (3): 569-75, 1990. [PUBMED Abstract]
- Epstein JB, Lozada-Nur F, McLeod WA, et al.: Oral Kaposi's sarcoma in acquired immunodeficiency syndrome. Review of management and report of the efficacy of intralesional vinblastine. Cancer 64 (12): 2424-30, 1989. [PUBMED Abstract]
- Bodsworth NJ, Bloch M, Bower M, et al.: Phase III vehicle-controlled, multi-centered study of topical alitretinoin gel 0.1% in cutaneous AIDS-related Kaposi's sarcoma. Am J Clin Dermatol 2 (2): 77-87, 2001. [PUBMED Abstract]
- Evans SR, Krown SE, Testa MA, et al.: Phase II evaluation of low-dose oral etoposide for the treatment of relapsed or progressive AIDS-related Kaposi's sarcoma: an AIDS Clinical Trials Group clinical study. J Clin Oncol 20 (15): 3236-41, 2002. [PUBMED Abstract]
- Saville MW, Lietzau J, Pluda JM, et al.: Treatment of HIV-associated Kaposi's sarcoma with paclitaxel. Lancet 346 (8966): 26-8, 1995. [PUBMED Abstract]
- Lim ST, Tupule A, Espina BM, et al.: Weekly docetaxel is safe and effective in the treatment of advanced-stage acquired immunodeficiency syndrome-related Kaposi sarcoma. Cancer 103 (2): 417-21, 2005. [PUBMED Abstract]
- Gill PS, Tulpule A, Espina BM, et al.: Paclitaxel is safe and effective in the treatment of advanced AIDS-related Kaposi's sarcoma. J Clin Oncol 17 (6): 1876-83, 1999. [PUBMED Abstract]
- Di Lorenzo G, Konstantinopoulos PA, Pantanowitz L, et al.: Management of AIDS-related Kaposi's sarcoma. Lancet Oncol 8 (2): 167-76, 2007. [PUBMED Abstract]
- Stewart S, Jablonowski H, Goebel FD, et al.: Randomized comparative trial of pegylated liposomal doxorubicin versus bleomycin and vincristine in the treatment of AIDS-related Kaposi's sarcoma. International Pegylated Liposomal Doxorubicin Study Group. J Clin Oncol 16 (2): 683-91, 1998. [PUBMED Abstract]
- Northfelt DW, Dezube BJ, Thommes JA, et al.: Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi's sarcoma: results of a randomized phase III clinical trial. J Clin Oncol 16 (7): 2445-51, 1998. [PUBMED Abstract]
- Gill PS, Wernz J, Scadden DT, et al.: Randomized phase III trial of liposomal daunorubicin versus doxorubicin, bleomycin, and vincristine in AIDS-related Kaposi's sarcoma. J Clin Oncol 14 (8): 2353-64, 1996. [PUBMED Abstract]
- Cianfrocca M, Lee S, Von Roenn J, et al.: Randomized trial of paclitaxel versus pegylated liposomal doxorubicin for advanced human immunodeficiency virus-associated Kaposi sarcoma: evidence of symptom palliation from chemotherapy. Cancer 116 (16): 3969-77, 2010. [PUBMED Abstract]
- Real FX, Oettgen HF, Krown SE: Kaposi's sarcoma and the acquired immunodeficiency syndrome: treatment with high and low doses of recombinant leukocyte A interferon. J Clin Oncol 4 (4): 544-51, 1986. [PUBMED Abstract]
- Groopman JE, Gottlieb MS, Goodman J, et al.: Recombinant alpha-2 interferon therapy for Kaposi's sarcoma associated with the acquired immunodeficiency syndrome. Ann Intern Med 100 (5): 671-6, 1984. [PUBMED Abstract]
- Koon HB, Krown SE, Lee JY, et al.: Phase II trial of imatinib in AIDS-associated Kaposi's sarcoma: AIDS Malignancy Consortium Protocol 042. J Clin Oncol 32 (5): 402-8, 2014. [PUBMED Abstract]
- Uldrick TS, Wyvill KM, Kumar P, et al.: Phase II study of bevacizumab in patients with HIV-associated Kaposi's sarcoma receiving antiretroviral therapy. J Clin Oncol 30 (13): 1476-83, 2012. [PUBMED Abstract]
- Little RF, Pluda JM, Wyvill KM, et al.: Activity of subcutaneous interleukin-12 in AIDS-related Kaposi sarcoma. Blood 107 (12): 4650-7, 2006. [PUBMED Abstract]
Recurrent Kaposi Sarcoma Treatment
The prognosis for any treated Kaposi sarcoma patient with progressing, recurring, or relapsing disease is highly variable. Deciding on further treatment depends on many factors, most importantly the clinical setting (i.e., classic, immunosuppressive treatment, or AIDS) in which the tumor arises as well as individual patient considerations.
Clinical trials are appropriate and should be considered when possible.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Changes to This Summary (01/30/2018)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Editorial changes were made to this summary.
This summary is written and maintained by the PDQ Adult Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of Kaposi sarcoma. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Adult Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Kaposi Sarcoma Treatment are:
- Eric J. Seifter, MD (Johns Hopkins University)
- Minh Tam Truong, MD (Boston University Medical Center)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Adult Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Kaposi Sarcoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/soft-tissue-sarcoma/hp/kaposi-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389335]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.
Updated: January 30, 2018
Source URL: https://www.cancer.gov/publishedcontent/syndication/3524.htm
Source Agency: National Cancer Institute (NCI)
Captured Date: 2013-09-14 09:01:43.0
Anti-PD-1 therapy with nivolumab in the treatment of metastatic malignant PEComa
Perivascular epithelioid cell neoplasms (PEComas) are an uncommon class of tumors consisting on histology of perivascular epithelioid cells occurring in both localized and metastatic forms at various body sites. The approach to treatment of these tumors generally involves a combination of surgical resection, chemotherapy, and/or radiation therapy.1
Case presentation and summary
A 46-year-old man presented to our institution with a non-tender, slowly enlarging, 8.3 cm mass in his right popliteal fossa. Upon biopsy, the pathologic findings were consistent with an epithelioid malignancy with melanocytic differentiation most consistent with a PEComa. Discussion of the pathologic diagnosis of our patient has been reported by the pathology group at our institution in a separate case report.2
Our patient was initially offered and refused amputation. He was started on therapy with the mechanistic Target of Rapamycin (mTOR) inhibitor everolimus, but was unable to tolerate the side effects after the first week of treatment. He then elected to monitor his symptoms clinically.
Approximately one year after his initial diagnosis, he presented to our facility with sepsis and bleeding from a now fungating tumor on his right knee. At this time, emergent above-knee amputation was performed. Re-staging images now showed the presence of multiple pulmonary nodules in his right lung as well as a lytic rib lesion, a concerning finding for metastatic disease. Video-Assisted Thorascopic Surgery (VATS) and right lower lobe wedge resection were performed and findings confirmed metastatic PEComa.
Given the patient’s intolerance to everolimus, he was started on the growth factor inhibitor, pazopanib. His disease did not progress on pazopanib, and improvement was noted in the dominant pulmonary nodule. Subsequently, however, he developed significant skin irritation and discontinued pazopanib. Repeat imaging approximately 2 months after stopping pazopanib showed significant disease progression.
We elected to start the patient on a non-standard approach to therapy with nivolumab infusions once every 2 weeks and concurrent radiation therapy to the rib lesion. At 2 and 5 months after initiating this treatment approach, CT imaging showed improvement in disease. At 12 months, significant disease response was noted (Figure 1).
The patient is now at 12 months of nivolumab therapy with progression free survival and no new identifiable metastatic lesions. He has been tolerating the medication with minimal side effects and has had an overall improvement in his pain and functional status. He continues to work full time.
Discussion
Our patient’s response presents a unique opportunity to talk about the role of immunotherapy as a treatment modality in patients with PEComa. The efficacy of check-point blockade in soft tissue sarcoma is still unclear predominantly because it is difficult to assess the degree of expression of immunogenic cell surface markers such as programmed cell death protein 1 (PD-1).1,3 Nivolumab has been tried in small cohorts for treatment of soft tissue sarcomas that express PD-1 and results showed some clinical benefit in about half of patients.4 Further, the expression of PD-1 has been assessed in soft tissue sarcomas and has been reported to suggest a negative prognostic role.5
To our knowledge, there has not yet been another reported case of PEComa that has been treated with immunotherapy and achieved a sustained response. Further clinical studies need to be done to assess response to agents such as nivolumab in the treatment of PEComa to bolster our observation that nivolumab is a viable treatment option that may lead to lasting remission. Our patient’s case also brings to light the need for further inquiry into assessing the immune tumor microenvironments, particularly looking at the expression of cell surface proteins such as PD-1, as it ultimately affects treatment options. TSJ
Correspondence
REFERENCES
1. Burgess, Melissa, et al. “Immunotherapy in Sarcoma: Future Horizons.” Current Oncology Reports, vol. 17, no. 11, 2015, doi:10.1007/s11912-015-0476-7.
2. Alnajar, Hussein, et al. “Metastatic Malignant PEComa of the Leg with Identification of ATRX Mutation by next-Generation Sequencing.” Virchows Archiv (2017). https://doi:10.1007/s004280172208-x.
3. Ghosn, Marwan, et al. “Immunotherapies in Sarcoma: Updates and Future Perspectives.” World Journal of Clinical Oncology, vol. 8, no. 2, 2017, p. 145., doi:10.5306/wjco.v8.i2.145.
4. Paoluzzi, L., et al. “Response to Anti-PD1 Therapy with Nivolumab in Metastatic Sarcomas.” Clinical Sarcoma Research, vol. 6, no. 1, 2016, doi:10.1186/s13569-016 0064-0.
5. Kim, Chan, et al. “Prognostic Implications of PD-L1 Expression in Patients with Soft Tissue Sarcoma.” BMC Cancer, BioMed Central 8 July 2016.
Perivascular epithelioid cell neoplasms (PEComas) are an uncommon class of tumors consisting on histology of perivascular epithelioid cells occurring in both localized and metastatic forms at various body sites. The approach to treatment of these tumors generally involves a combination of surgical resection, chemotherapy, and/or radiation therapy.1
Case presentation and summary
A 46-year-old man presented to our institution with a non-tender, slowly enlarging, 8.3 cm mass in his right popliteal fossa. Upon biopsy, the pathologic findings were consistent with an epithelioid malignancy with melanocytic differentiation most consistent with a PEComa. Discussion of the pathologic diagnosis of our patient has been reported by the pathology group at our institution in a separate case report.2
Our patient was initially offered and refused amputation. He was started on therapy with the mechanistic Target of Rapamycin (mTOR) inhibitor everolimus, but was unable to tolerate the side effects after the first week of treatment. He then elected to monitor his symptoms clinically.
Approximately one year after his initial diagnosis, he presented to our facility with sepsis and bleeding from a now fungating tumor on his right knee. At this time, emergent above-knee amputation was performed. Re-staging images now showed the presence of multiple pulmonary nodules in his right lung as well as a lytic rib lesion, a concerning finding for metastatic disease. Video-Assisted Thorascopic Surgery (VATS) and right lower lobe wedge resection were performed and findings confirmed metastatic PEComa.
Given the patient’s intolerance to everolimus, he was started on the growth factor inhibitor, pazopanib. His disease did not progress on pazopanib, and improvement was noted in the dominant pulmonary nodule. Subsequently, however, he developed significant skin irritation and discontinued pazopanib. Repeat imaging approximately 2 months after stopping pazopanib showed significant disease progression.
We elected to start the patient on a non-standard approach to therapy with nivolumab infusions once every 2 weeks and concurrent radiation therapy to the rib lesion. At 2 and 5 months after initiating this treatment approach, CT imaging showed improvement in disease. At 12 months, significant disease response was noted (Figure 1).
The patient is now at 12 months of nivolumab therapy with progression free survival and no new identifiable metastatic lesions. He has been tolerating the medication with minimal side effects and has had an overall improvement in his pain and functional status. He continues to work full time.
Discussion
Our patient’s response presents a unique opportunity to talk about the role of immunotherapy as a treatment modality in patients with PEComa. The efficacy of check-point blockade in soft tissue sarcoma is still unclear predominantly because it is difficult to assess the degree of expression of immunogenic cell surface markers such as programmed cell death protein 1 (PD-1).1,3 Nivolumab has been tried in small cohorts for treatment of soft tissue sarcomas that express PD-1 and results showed some clinical benefit in about half of patients.4 Further, the expression of PD-1 has been assessed in soft tissue sarcomas and has been reported to suggest a negative prognostic role.5
To our knowledge, there has not yet been another reported case of PEComa that has been treated with immunotherapy and achieved a sustained response. Further clinical studies need to be done to assess response to agents such as nivolumab in the treatment of PEComa to bolster our observation that nivolumab is a viable treatment option that may lead to lasting remission. Our patient’s case also brings to light the need for further inquiry into assessing the immune tumor microenvironments, particularly looking at the expression of cell surface proteins such as PD-1, as it ultimately affects treatment options. TSJ
Correspondence
REFERENCES
1. Burgess, Melissa, et al. “Immunotherapy in Sarcoma: Future Horizons.” Current Oncology Reports, vol. 17, no. 11, 2015, doi:10.1007/s11912-015-0476-7.
2. Alnajar, Hussein, et al. “Metastatic Malignant PEComa of the Leg with Identification of ATRX Mutation by next-Generation Sequencing.” Virchows Archiv (2017). https://doi:10.1007/s004280172208-x.
3. Ghosn, Marwan, et al. “Immunotherapies in Sarcoma: Updates and Future Perspectives.” World Journal of Clinical Oncology, vol. 8, no. 2, 2017, p. 145., doi:10.5306/wjco.v8.i2.145.
4. Paoluzzi, L., et al. “Response to Anti-PD1 Therapy with Nivolumab in Metastatic Sarcomas.” Clinical Sarcoma Research, vol. 6, no. 1, 2016, doi:10.1186/s13569-016 0064-0.
5. Kim, Chan, et al. “Prognostic Implications of PD-L1 Expression in Patients with Soft Tissue Sarcoma.” BMC Cancer, BioMed Central 8 July 2016.
Perivascular epithelioid cell neoplasms (PEComas) are an uncommon class of tumors consisting on histology of perivascular epithelioid cells occurring in both localized and metastatic forms at various body sites. The approach to treatment of these tumors generally involves a combination of surgical resection, chemotherapy, and/or radiation therapy.1
Case presentation and summary
A 46-year-old man presented to our institution with a non-tender, slowly enlarging, 8.3 cm mass in his right popliteal fossa. Upon biopsy, the pathologic findings were consistent with an epithelioid malignancy with melanocytic differentiation most consistent with a PEComa. Discussion of the pathologic diagnosis of our patient has been reported by the pathology group at our institution in a separate case report.2
Our patient was initially offered and refused amputation. He was started on therapy with the mechanistic Target of Rapamycin (mTOR) inhibitor everolimus, but was unable to tolerate the side effects after the first week of treatment. He then elected to monitor his symptoms clinically.
Approximately one year after his initial diagnosis, he presented to our facility with sepsis and bleeding from a now fungating tumor on his right knee. At this time, emergent above-knee amputation was performed. Re-staging images now showed the presence of multiple pulmonary nodules in his right lung as well as a lytic rib lesion, a concerning finding for metastatic disease. Video-Assisted Thorascopic Surgery (VATS) and right lower lobe wedge resection were performed and findings confirmed metastatic PEComa.
Given the patient’s intolerance to everolimus, he was started on the growth factor inhibitor, pazopanib. His disease did not progress on pazopanib, and improvement was noted in the dominant pulmonary nodule. Subsequently, however, he developed significant skin irritation and discontinued pazopanib. Repeat imaging approximately 2 months after stopping pazopanib showed significant disease progression.
We elected to start the patient on a non-standard approach to therapy with nivolumab infusions once every 2 weeks and concurrent radiation therapy to the rib lesion. At 2 and 5 months after initiating this treatment approach, CT imaging showed improvement in disease. At 12 months, significant disease response was noted (Figure 1).
The patient is now at 12 months of nivolumab therapy with progression free survival and no new identifiable metastatic lesions. He has been tolerating the medication with minimal side effects and has had an overall improvement in his pain and functional status. He continues to work full time.
Discussion
Our patient’s response presents a unique opportunity to talk about the role of immunotherapy as a treatment modality in patients with PEComa. The efficacy of check-point blockade in soft tissue sarcoma is still unclear predominantly because it is difficult to assess the degree of expression of immunogenic cell surface markers such as programmed cell death protein 1 (PD-1).1,3 Nivolumab has been tried in small cohorts for treatment of soft tissue sarcomas that express PD-1 and results showed some clinical benefit in about half of patients.4 Further, the expression of PD-1 has been assessed in soft tissue sarcomas and has been reported to suggest a negative prognostic role.5
To our knowledge, there has not yet been another reported case of PEComa that has been treated with immunotherapy and achieved a sustained response. Further clinical studies need to be done to assess response to agents such as nivolumab in the treatment of PEComa to bolster our observation that nivolumab is a viable treatment option that may lead to lasting remission. Our patient’s case also brings to light the need for further inquiry into assessing the immune tumor microenvironments, particularly looking at the expression of cell surface proteins such as PD-1, as it ultimately affects treatment options. TSJ
Correspondence
REFERENCES
1. Burgess, Melissa, et al. “Immunotherapy in Sarcoma: Future Horizons.” Current Oncology Reports, vol. 17, no. 11, 2015, doi:10.1007/s11912-015-0476-7.
2. Alnajar, Hussein, et al. “Metastatic Malignant PEComa of the Leg with Identification of ATRX Mutation by next-Generation Sequencing.” Virchows Archiv (2017). https://doi:10.1007/s004280172208-x.
3. Ghosn, Marwan, et al. “Immunotherapies in Sarcoma: Updates and Future Perspectives.” World Journal of Clinical Oncology, vol. 8, no. 2, 2017, p. 145., doi:10.5306/wjco.v8.i2.145.
4. Paoluzzi, L., et al. “Response to Anti-PD1 Therapy with Nivolumab in Metastatic Sarcomas.” Clinical Sarcoma Research, vol. 6, no. 1, 2016, doi:10.1186/s13569-016 0064-0.
5. Kim, Chan, et al. “Prognostic Implications of PD-L1 Expression in Patients with Soft Tissue Sarcoma.” BMC Cancer, BioMed Central 8 July 2016.
Pulmonary sarcomatoid carcinoma presenting as a necrotizing cavitary lung lesion: diagnostic dilemma
Pulmonary sarcomatoid carcinoma (PSC) is a rare histological subtype that has an aggressive course with average survival of 11-13 months.1 In clinical practice, the possible presentations of this rare cancer are not widely known, resulting in a misdiagnosis. That is what happened with our patient, who presented with necrotizing cavitary lung lesion and soft tissue necrotizing lymphadenitis. The clinical picture was reminiscent of tuberculosis or granulomatosis with polyangiitis and was further confounded by negative computed-tomography (CT)-guided biopsy and bronchoscopy findings, which added to the delay in diagnosis. With the currently available knowledge, the diagnosis of PSC depends largely on evaluation of the surgically resected specimen, which in most cases is avoided until there is a high suspicion of PSC. Biopsy is not useful due to extensive necrosis, as will be seen in our case. Consequently, most of the data in the literature is based on case series of autopsy specimen, and the clinical characteristics of PSC remain unclear. The rarity of PSC has prevented its characterization in literature. We report here a rare presentation of PSC with necrotizing lung lesion, to add to the paucity of the current data.
Case presentation and summary
A 58-year-old homeless man presented to the Upstate University Hospital, Syracuse, New York, with a 25-pound weight loss during the previous month and associated productive cough and hemoptysis for a week and a painful mass in the nape of his neck. He denied any fever, chest pain, sick contacts, or joint pain. He had a history of about 40 pack-years of smoking, and his brother had recently been diagnosed with lung cancer. A tender fluctuant mass was detected in the nape of his neck on examination (Figure 1).
The patient had presented 9 months earlier with persistent cough and hemoptysis, and at that visit was found to have a cavitary lesion in the right lung measuring 2 cm (0.8 in). He had undergone a computed-tomograpghy (CT)-guided biopsy of the lesion, which had shown acute and chronic inflammation with fibrosis, and he had negative bronchoscopy findings. The patient tested negative for tuberculosis during the first visit but he left the hospital against the medical advice of the physicians and he was lost to follow-up until his re-presentation.
On physical examination at his re-presentation, the patient seemed cachectic, with a blood pressure of 94/62 mm of Hg. The mass in the nape of his neck was about 3 cm (1.2 in) long, with erythema of the surrounding skin (Figure 1). Bronchial breath sounds were heard in the right upper lobe of the lung, likely due to the underlying cavitary lesion (Figure 2B). Relevant lab findings included a negative HIV test and repeat AFB (acid-fast bacilli) sputum cultures. A CT-guided biopsy with contrast of the thorax showed an interval increase in the size of the cavitary lesion in the patient’s right upper lobe, now measuring about 10 cm (4 in). Also seen were multiple nodules elsewhere in both lungs, with the largest measuring 8 mm (0.3 in). A CT scan of the neck showed 3 cm cystic mass within the posterior subcutaneous soft tissue of the C3 level, confirming the examination finding of the neck mass (Figure 2A) with peripheral enhancement and surrounding infiltrative changes, likely abscess or malignant lymph node versus necrotic infection. He underwent bronchoscopy, which again failed to reveal any endobronchial lesions. Bronchoalveolar lavage was sent for microbiological analysis, including AFB and fungus, but came back negative. Transbronchial biopsy cytology revealed fragments of tumor composed of large pleomorphic cells without glandular or squamous differentiation, within large areas of necrosis (Figure 3). Immunohistochemical studies showed strong reactivity with cytokeratin CAM5.2 (Figure 4), weak and focal reactivity with cytokeratin AE1/AE3 (Figure 5), and lack of reactivity with CD20, CD3, CD30, S-100, MART-1, TTF-1 and p63, all findings consistent with sarcomatoid carcinoma.
The patient underwent fine-needle aspiration and drainage of the neck lesion and the culture grew mixed organisms The results of a bone scan, which was done within a week, showed multiple foci of uptake in the ribs and cervical spine. Given the patient’s advanced disease, he was started on palliative radiotherapy with radiosensitizing chemotherapy with carboplatin (target AUC 6) and paclitaxel (135 mg/m2 over 24 hours). His symptoms of hemoptysis improved transiently after the first cycle, but he became hypotensive and drowsy during the second cycle of therapy, and the family decided to make the patient comfort care and withdraw all further treatment. He was discharged to hospice.
Discussion
PSC is a rare variant of non-small-cell carcinoma lung cancer, accounting for up to 0.4% of lung malignancy.1 It was
recently subtyped by the World Health Organization as a non-small cell lung carcinoma with certain amount of differentiation resembling sarcoma or containing elements of sarcoma.2-4 It is not known why both elements co-exist in the tumor, but Franks and colleagues some theories have been postulated in the literature, including possible origin from a single, aberrant stem cell with progenies differentiating in two separate pathways.3
Sarcomatoid carcinoma consists of spectrum of tumors including pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, carcinosarcoma, and blastoma.3,4 It usually shows male preponderance, and association with smoking.3 The diagnosis commonly occurs in the sixth decade of life, except for pulmonary blastoma, which is more common in the fourth decade andnwith equal gender distribution.4
The presenting symptoms can be variable and nonspecific, but predominantly include chest pain, cough, hemoptysis, and/or weight loss.5 Radiologically, pulmonary sarcomatoid cancer presenting as a necrotizing cavitary lesion in the lung is a rare finding, seldom reported in the past.6,7 The presentation in our case, with necrotizing lymphadenitis, was reminiscent of an infectious or autoimmune etiology such as tuberculosis or granulomatosis with polyangiitis. The presence of extensive necrosis in the lesion and the characteristic heterogeneity of the tumor had resulted in inconclusive biopsy findings during the previous presentation. In clinical practice, there is over-reliance on biopsy findings to make the distinction between cancer and other mimicking conditions. This is especially true for rare tumors such as PSC, which often results in misdiagnosis and a delay in administering the proper treatment. Transbronchial biopsy in cases such as the present case, carries little benefit because the diagnosis depends on the site from which the biopsy is taken and whether the biopsied tissue is representative of the entire mass. The diagnosis can be suspected based on the clinical and radiological findings but confirmation requires a surgical resection to delineate the accurate cytology and architecture.5,6,8 Huang and colleagues showed a misdiagnosis rate of PSC of >70% preoperatively.4 Resective surgery is feasible only in patients with high index of suspicion for a malignancy, which in most cases requires previous confirmation with a biopsy. The rarity of this cancer, its unusual presentations, and the lack of specific testing preclude early diagnosis and timely treatment of this fatal condition.
Initial treatment options for localized or with limited spread disease is resective surgery. The role of chemo- or radiation therapy is not known, but they have not previously shown promising results,6,8 except in some cases when they are used as postoperative adjuvant chemotherapy4 or in bulky, locally invasive tumors.1 The recurrence rate after surgery is very high, resulting in a poor 5-year survival rate.1,8 Experimental therapies, such as antibodies that target epidermal growth factor receptor mutations, have not shown much success either.8 In conclusion, the outlook for patients with PSC with the current available knowledge and treatment protocols, is dismal.
Most of the current knowledge and data in the literature is based on cases from autopsy or early-stage surgical resections rather than on patients with advanced cancer.5 Moreover, the role of surgical resection in PSC is questionable, given the high recurrence rate. Subsequently, the clinical and pathological manifestations have yet to be well characterized.4 There has been advance with the publication of more studies recently. Cytokeratin markers such as CAM 5.2 and AE1/AE3 are commonly useful to support the diagnosis when suspected.3 Other markers, including the carcinoembryonic antigen, CD15, and thyroid transcription factor-1 may be variably positive, based on the differentiation of the cancer. Other exciting prospects in the study of PSC include the suggestion of a modified vimentin histologic score for better characterization of the cancer and the discovery of high plateletderived growth factor receptor beta immunohistochemistry expression in PSC as a potential target for future therapy.
Conclusion
Pulmonary sarcomatoid lung cancer can present with a predominant necrotizing picture that mimics diseases such as tuberculosis. In such case, transbronchial biopsy carries little benefit because the diagnosis depends on whether the biopsied tissue is representative of the entire mass, often confounded by the extensive necrosis. More data is needed to determine prognostic factors and appropriate therapeutic strategies. TSJ
Correspondence
Gaurang Nandkishor Vaidya, MD
References
1. Martin LW, Correa AM, Ordonez NG, et al. Sarcomatoid carcinoma of the lung: a predictor of poor prognosis. Ann Thorac Surg. 2007;84(3):973-980.
2. Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J. 2001;18(6):1059-1068.
3. Franks TJ, Galvin JR. Sarcomatoid carcinoma of the lung: histologic criteria and common lesions in the differential diagnosis. Arch Pathol Lab Med. 2010;134(1):49-54.
4. Huang SY, Shen SJ, Li XY. Pulmonary sarcomatoid carcinoma: a clinicopathologic study and prognostic analysis of 51 cases. http://wjso. biomedcentral.com/articles/10.1186/1477-7819-11-252. Published 2013. Accessed March 12, 2017.
5. Travis WD. Sarcomatoid neoplasms of the lung and pleura. Arch Pathol Lab Med. 2010;134(11):1645-1658.
6. Pelosi G, Sonzogni A, De Pas T, et al. Review article: pulmonary sarcomatoid carcinomas: a practical overview. Int J Surg Pathol. 2010;18(2):103-120.
7. Chang YL, Lee YC, Shih JY, Wu CT. Pulmonary pleomorphic (spindle) cell carcinoma: peculiar clinicopathologic manifestations different from ordinary non-small cell carcinoma. Lung Cancer. 2001;34(1):91-97.
8. Park JS, Lee Y, Han J, et al. Clinicopathologic outcomes of curative resection for sarcomatoid carcinoma of the lung. Oncology. 2011;81(3-4):206-213.
Pulmonary sarcomatoid carcinoma (PSC) is a rare histological subtype that has an aggressive course with average survival of 11-13 months.1 In clinical practice, the possible presentations of this rare cancer are not widely known, resulting in a misdiagnosis. That is what happened with our patient, who presented with necrotizing cavitary lung lesion and soft tissue necrotizing lymphadenitis. The clinical picture was reminiscent of tuberculosis or granulomatosis with polyangiitis and was further confounded by negative computed-tomography (CT)-guided biopsy and bronchoscopy findings, which added to the delay in diagnosis. With the currently available knowledge, the diagnosis of PSC depends largely on evaluation of the surgically resected specimen, which in most cases is avoided until there is a high suspicion of PSC. Biopsy is not useful due to extensive necrosis, as will be seen in our case. Consequently, most of the data in the literature is based on case series of autopsy specimen, and the clinical characteristics of PSC remain unclear. The rarity of PSC has prevented its characterization in literature. We report here a rare presentation of PSC with necrotizing lung lesion, to add to the paucity of the current data.
Case presentation and summary
A 58-year-old homeless man presented to the Upstate University Hospital, Syracuse, New York, with a 25-pound weight loss during the previous month and associated productive cough and hemoptysis for a week and a painful mass in the nape of his neck. He denied any fever, chest pain, sick contacts, or joint pain. He had a history of about 40 pack-years of smoking, and his brother had recently been diagnosed with lung cancer. A tender fluctuant mass was detected in the nape of his neck on examination (Figure 1).
The patient had presented 9 months earlier with persistent cough and hemoptysis, and at that visit was found to have a cavitary lesion in the right lung measuring 2 cm (0.8 in). He had undergone a computed-tomograpghy (CT)-guided biopsy of the lesion, which had shown acute and chronic inflammation with fibrosis, and he had negative bronchoscopy findings. The patient tested negative for tuberculosis during the first visit but he left the hospital against the medical advice of the physicians and he was lost to follow-up until his re-presentation.
On physical examination at his re-presentation, the patient seemed cachectic, with a blood pressure of 94/62 mm of Hg. The mass in the nape of his neck was about 3 cm (1.2 in) long, with erythema of the surrounding skin (Figure 1). Bronchial breath sounds were heard in the right upper lobe of the lung, likely due to the underlying cavitary lesion (Figure 2B). Relevant lab findings included a negative HIV test and repeat AFB (acid-fast bacilli) sputum cultures. A CT-guided biopsy with contrast of the thorax showed an interval increase in the size of the cavitary lesion in the patient’s right upper lobe, now measuring about 10 cm (4 in). Also seen were multiple nodules elsewhere in both lungs, with the largest measuring 8 mm (0.3 in). A CT scan of the neck showed 3 cm cystic mass within the posterior subcutaneous soft tissue of the C3 level, confirming the examination finding of the neck mass (Figure 2A) with peripheral enhancement and surrounding infiltrative changes, likely abscess or malignant lymph node versus necrotic infection. He underwent bronchoscopy, which again failed to reveal any endobronchial lesions. Bronchoalveolar lavage was sent for microbiological analysis, including AFB and fungus, but came back negative. Transbronchial biopsy cytology revealed fragments of tumor composed of large pleomorphic cells without glandular or squamous differentiation, within large areas of necrosis (Figure 3). Immunohistochemical studies showed strong reactivity with cytokeratin CAM5.2 (Figure 4), weak and focal reactivity with cytokeratin AE1/AE3 (Figure 5), and lack of reactivity with CD20, CD3, CD30, S-100, MART-1, TTF-1 and p63, all findings consistent with sarcomatoid carcinoma.
The patient underwent fine-needle aspiration and drainage of the neck lesion and the culture grew mixed organisms The results of a bone scan, which was done within a week, showed multiple foci of uptake in the ribs and cervical spine. Given the patient’s advanced disease, he was started on palliative radiotherapy with radiosensitizing chemotherapy with carboplatin (target AUC 6) and paclitaxel (135 mg/m2 over 24 hours). His symptoms of hemoptysis improved transiently after the first cycle, but he became hypotensive and drowsy during the second cycle of therapy, and the family decided to make the patient comfort care and withdraw all further treatment. He was discharged to hospice.
Discussion
PSC is a rare variant of non-small-cell carcinoma lung cancer, accounting for up to 0.4% of lung malignancy.1 It was
recently subtyped by the World Health Organization as a non-small cell lung carcinoma with certain amount of differentiation resembling sarcoma or containing elements of sarcoma.2-4 It is not known why both elements co-exist in the tumor, but Franks and colleagues some theories have been postulated in the literature, including possible origin from a single, aberrant stem cell with progenies differentiating in two separate pathways.3
Sarcomatoid carcinoma consists of spectrum of tumors including pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, carcinosarcoma, and blastoma.3,4 It usually shows male preponderance, and association with smoking.3 The diagnosis commonly occurs in the sixth decade of life, except for pulmonary blastoma, which is more common in the fourth decade andnwith equal gender distribution.4
The presenting symptoms can be variable and nonspecific, but predominantly include chest pain, cough, hemoptysis, and/or weight loss.5 Radiologically, pulmonary sarcomatoid cancer presenting as a necrotizing cavitary lesion in the lung is a rare finding, seldom reported in the past.6,7 The presentation in our case, with necrotizing lymphadenitis, was reminiscent of an infectious or autoimmune etiology such as tuberculosis or granulomatosis with polyangiitis. The presence of extensive necrosis in the lesion and the characteristic heterogeneity of the tumor had resulted in inconclusive biopsy findings during the previous presentation. In clinical practice, there is over-reliance on biopsy findings to make the distinction between cancer and other mimicking conditions. This is especially true for rare tumors such as PSC, which often results in misdiagnosis and a delay in administering the proper treatment. Transbronchial biopsy in cases such as the present case, carries little benefit because the diagnosis depends on the site from which the biopsy is taken and whether the biopsied tissue is representative of the entire mass. The diagnosis can be suspected based on the clinical and radiological findings but confirmation requires a surgical resection to delineate the accurate cytology and architecture.5,6,8 Huang and colleagues showed a misdiagnosis rate of PSC of >70% preoperatively.4 Resective surgery is feasible only in patients with high index of suspicion for a malignancy, which in most cases requires previous confirmation with a biopsy. The rarity of this cancer, its unusual presentations, and the lack of specific testing preclude early diagnosis and timely treatment of this fatal condition.
Initial treatment options for localized or with limited spread disease is resective surgery. The role of chemo- or radiation therapy is not known, but they have not previously shown promising results,6,8 except in some cases when they are used as postoperative adjuvant chemotherapy4 or in bulky, locally invasive tumors.1 The recurrence rate after surgery is very high, resulting in a poor 5-year survival rate.1,8 Experimental therapies, such as antibodies that target epidermal growth factor receptor mutations, have not shown much success either.8 In conclusion, the outlook for patients with PSC with the current available knowledge and treatment protocols, is dismal.
Most of the current knowledge and data in the literature is based on cases from autopsy or early-stage surgical resections rather than on patients with advanced cancer.5 Moreover, the role of surgical resection in PSC is questionable, given the high recurrence rate. Subsequently, the clinical and pathological manifestations have yet to be well characterized.4 There has been advance with the publication of more studies recently. Cytokeratin markers such as CAM 5.2 and AE1/AE3 are commonly useful to support the diagnosis when suspected.3 Other markers, including the carcinoembryonic antigen, CD15, and thyroid transcription factor-1 may be variably positive, based on the differentiation of the cancer. Other exciting prospects in the study of PSC include the suggestion of a modified vimentin histologic score for better characterization of the cancer and the discovery of high plateletderived growth factor receptor beta immunohistochemistry expression in PSC as a potential target for future therapy.
Conclusion
Pulmonary sarcomatoid lung cancer can present with a predominant necrotizing picture that mimics diseases such as tuberculosis. In such case, transbronchial biopsy carries little benefit because the diagnosis depends on whether the biopsied tissue is representative of the entire mass, often confounded by the extensive necrosis. More data is needed to determine prognostic factors and appropriate therapeutic strategies. TSJ
Correspondence
Gaurang Nandkishor Vaidya, MD
References
1. Martin LW, Correa AM, Ordonez NG, et al. Sarcomatoid carcinoma of the lung: a predictor of poor prognosis. Ann Thorac Surg. 2007;84(3):973-980.
2. Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J. 2001;18(6):1059-1068.
3. Franks TJ, Galvin JR. Sarcomatoid carcinoma of the lung: histologic criteria and common lesions in the differential diagnosis. Arch Pathol Lab Med. 2010;134(1):49-54.
4. Huang SY, Shen SJ, Li XY. Pulmonary sarcomatoid carcinoma: a clinicopathologic study and prognostic analysis of 51 cases. http://wjso. biomedcentral.com/articles/10.1186/1477-7819-11-252. Published 2013. Accessed March 12, 2017.
5. Travis WD. Sarcomatoid neoplasms of the lung and pleura. Arch Pathol Lab Med. 2010;134(11):1645-1658.
6. Pelosi G, Sonzogni A, De Pas T, et al. Review article: pulmonary sarcomatoid carcinomas: a practical overview. Int J Surg Pathol. 2010;18(2):103-120.
7. Chang YL, Lee YC, Shih JY, Wu CT. Pulmonary pleomorphic (spindle) cell carcinoma: peculiar clinicopathologic manifestations different from ordinary non-small cell carcinoma. Lung Cancer. 2001;34(1):91-97.
8. Park JS, Lee Y, Han J, et al. Clinicopathologic outcomes of curative resection for sarcomatoid carcinoma of the lung. Oncology. 2011;81(3-4):206-213.
Pulmonary sarcomatoid carcinoma (PSC) is a rare histological subtype that has an aggressive course with average survival of 11-13 months.1 In clinical practice, the possible presentations of this rare cancer are not widely known, resulting in a misdiagnosis. That is what happened with our patient, who presented with necrotizing cavitary lung lesion and soft tissue necrotizing lymphadenitis. The clinical picture was reminiscent of tuberculosis or granulomatosis with polyangiitis and was further confounded by negative computed-tomography (CT)-guided biopsy and bronchoscopy findings, which added to the delay in diagnosis. With the currently available knowledge, the diagnosis of PSC depends largely on evaluation of the surgically resected specimen, which in most cases is avoided until there is a high suspicion of PSC. Biopsy is not useful due to extensive necrosis, as will be seen in our case. Consequently, most of the data in the literature is based on case series of autopsy specimen, and the clinical characteristics of PSC remain unclear. The rarity of PSC has prevented its characterization in literature. We report here a rare presentation of PSC with necrotizing lung lesion, to add to the paucity of the current data.
Case presentation and summary
A 58-year-old homeless man presented to the Upstate University Hospital, Syracuse, New York, with a 25-pound weight loss during the previous month and associated productive cough and hemoptysis for a week and a painful mass in the nape of his neck. He denied any fever, chest pain, sick contacts, or joint pain. He had a history of about 40 pack-years of smoking, and his brother had recently been diagnosed with lung cancer. A tender fluctuant mass was detected in the nape of his neck on examination (Figure 1).
The patient had presented 9 months earlier with persistent cough and hemoptysis, and at that visit was found to have a cavitary lesion in the right lung measuring 2 cm (0.8 in). He had undergone a computed-tomograpghy (CT)-guided biopsy of the lesion, which had shown acute and chronic inflammation with fibrosis, and he had negative bronchoscopy findings. The patient tested negative for tuberculosis during the first visit but he left the hospital against the medical advice of the physicians and he was lost to follow-up until his re-presentation.
On physical examination at his re-presentation, the patient seemed cachectic, with a blood pressure of 94/62 mm of Hg. The mass in the nape of his neck was about 3 cm (1.2 in) long, with erythema of the surrounding skin (Figure 1). Bronchial breath sounds were heard in the right upper lobe of the lung, likely due to the underlying cavitary lesion (Figure 2B). Relevant lab findings included a negative HIV test and repeat AFB (acid-fast bacilli) sputum cultures. A CT-guided biopsy with contrast of the thorax showed an interval increase in the size of the cavitary lesion in the patient’s right upper lobe, now measuring about 10 cm (4 in). Also seen were multiple nodules elsewhere in both lungs, with the largest measuring 8 mm (0.3 in). A CT scan of the neck showed 3 cm cystic mass within the posterior subcutaneous soft tissue of the C3 level, confirming the examination finding of the neck mass (Figure 2A) with peripheral enhancement and surrounding infiltrative changes, likely abscess or malignant lymph node versus necrotic infection. He underwent bronchoscopy, which again failed to reveal any endobronchial lesions. Bronchoalveolar lavage was sent for microbiological analysis, including AFB and fungus, but came back negative. Transbronchial biopsy cytology revealed fragments of tumor composed of large pleomorphic cells without glandular or squamous differentiation, within large areas of necrosis (Figure 3). Immunohistochemical studies showed strong reactivity with cytokeratin CAM5.2 (Figure 4), weak and focal reactivity with cytokeratin AE1/AE3 (Figure 5), and lack of reactivity with CD20, CD3, CD30, S-100, MART-1, TTF-1 and p63, all findings consistent with sarcomatoid carcinoma.
The patient underwent fine-needle aspiration and drainage of the neck lesion and the culture grew mixed organisms The results of a bone scan, which was done within a week, showed multiple foci of uptake in the ribs and cervical spine. Given the patient’s advanced disease, he was started on palliative radiotherapy with radiosensitizing chemotherapy with carboplatin (target AUC 6) and paclitaxel (135 mg/m2 over 24 hours). His symptoms of hemoptysis improved transiently after the first cycle, but he became hypotensive and drowsy during the second cycle of therapy, and the family decided to make the patient comfort care and withdraw all further treatment. He was discharged to hospice.
Discussion
PSC is a rare variant of non-small-cell carcinoma lung cancer, accounting for up to 0.4% of lung malignancy.1 It was
recently subtyped by the World Health Organization as a non-small cell lung carcinoma with certain amount of differentiation resembling sarcoma or containing elements of sarcoma.2-4 It is not known why both elements co-exist in the tumor, but Franks and colleagues some theories have been postulated in the literature, including possible origin from a single, aberrant stem cell with progenies differentiating in two separate pathways.3
Sarcomatoid carcinoma consists of spectrum of tumors including pleomorphic carcinoma, spindle cell carcinoma, giant cell carcinoma, carcinosarcoma, and blastoma.3,4 It usually shows male preponderance, and association with smoking.3 The diagnosis commonly occurs in the sixth decade of life, except for pulmonary blastoma, which is more common in the fourth decade andnwith equal gender distribution.4
The presenting symptoms can be variable and nonspecific, but predominantly include chest pain, cough, hemoptysis, and/or weight loss.5 Radiologically, pulmonary sarcomatoid cancer presenting as a necrotizing cavitary lesion in the lung is a rare finding, seldom reported in the past.6,7 The presentation in our case, with necrotizing lymphadenitis, was reminiscent of an infectious or autoimmune etiology such as tuberculosis or granulomatosis with polyangiitis. The presence of extensive necrosis in the lesion and the characteristic heterogeneity of the tumor had resulted in inconclusive biopsy findings during the previous presentation. In clinical practice, there is over-reliance on biopsy findings to make the distinction between cancer and other mimicking conditions. This is especially true for rare tumors such as PSC, which often results in misdiagnosis and a delay in administering the proper treatment. Transbronchial biopsy in cases such as the present case, carries little benefit because the diagnosis depends on the site from which the biopsy is taken and whether the biopsied tissue is representative of the entire mass. The diagnosis can be suspected based on the clinical and radiological findings but confirmation requires a surgical resection to delineate the accurate cytology and architecture.5,6,8 Huang and colleagues showed a misdiagnosis rate of PSC of >70% preoperatively.4 Resective surgery is feasible only in patients with high index of suspicion for a malignancy, which in most cases requires previous confirmation with a biopsy. The rarity of this cancer, its unusual presentations, and the lack of specific testing preclude early diagnosis and timely treatment of this fatal condition.
Initial treatment options for localized or with limited spread disease is resective surgery. The role of chemo- or radiation therapy is not known, but they have not previously shown promising results,6,8 except in some cases when they are used as postoperative adjuvant chemotherapy4 or in bulky, locally invasive tumors.1 The recurrence rate after surgery is very high, resulting in a poor 5-year survival rate.1,8 Experimental therapies, such as antibodies that target epidermal growth factor receptor mutations, have not shown much success either.8 In conclusion, the outlook for patients with PSC with the current available knowledge and treatment protocols, is dismal.
Most of the current knowledge and data in the literature is based on cases from autopsy or early-stage surgical resections rather than on patients with advanced cancer.5 Moreover, the role of surgical resection in PSC is questionable, given the high recurrence rate. Subsequently, the clinical and pathological manifestations have yet to be well characterized.4 There has been advance with the publication of more studies recently. Cytokeratin markers such as CAM 5.2 and AE1/AE3 are commonly useful to support the diagnosis when suspected.3 Other markers, including the carcinoembryonic antigen, CD15, and thyroid transcription factor-1 may be variably positive, based on the differentiation of the cancer. Other exciting prospects in the study of PSC include the suggestion of a modified vimentin histologic score for better characterization of the cancer and the discovery of high plateletderived growth factor receptor beta immunohistochemistry expression in PSC as a potential target for future therapy.
Conclusion
Pulmonary sarcomatoid lung cancer can present with a predominant necrotizing picture that mimics diseases such as tuberculosis. In such case, transbronchial biopsy carries little benefit because the diagnosis depends on whether the biopsied tissue is representative of the entire mass, often confounded by the extensive necrosis. More data is needed to determine prognostic factors and appropriate therapeutic strategies. TSJ
Correspondence
Gaurang Nandkishor Vaidya, MD
References
1. Martin LW, Correa AM, Ordonez NG, et al. Sarcomatoid carcinoma of the lung: a predictor of poor prognosis. Ann Thorac Surg. 2007;84(3):973-980.
2. Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J. 2001;18(6):1059-1068.
3. Franks TJ, Galvin JR. Sarcomatoid carcinoma of the lung: histologic criteria and common lesions in the differential diagnosis. Arch Pathol Lab Med. 2010;134(1):49-54.
4. Huang SY, Shen SJ, Li XY. Pulmonary sarcomatoid carcinoma: a clinicopathologic study and prognostic analysis of 51 cases. http://wjso. biomedcentral.com/articles/10.1186/1477-7819-11-252. Published 2013. Accessed March 12, 2017.
5. Travis WD. Sarcomatoid neoplasms of the lung and pleura. Arch Pathol Lab Med. 2010;134(11):1645-1658.
6. Pelosi G, Sonzogni A, De Pas T, et al. Review article: pulmonary sarcomatoid carcinomas: a practical overview. Int J Surg Pathol. 2010;18(2):103-120.
7. Chang YL, Lee YC, Shih JY, Wu CT. Pulmonary pleomorphic (spindle) cell carcinoma: peculiar clinicopathologic manifestations different from ordinary non-small cell carcinoma. Lung Cancer. 2001;34(1):91-97.
8. Park JS, Lee Y, Han J, et al. Clinicopathologic outcomes of curative resection for sarcomatoid carcinoma of the lung. Oncology. 2011;81(3-4):206-213.
This article was originally published in the Journal of Community and Supportive Oncology (JCSO 2017;15(2):103-105). doi: https://doi.org/10.12788/jcso.0259. It is reproduced here with permission of the copyright owner. Further reproduction is prohibited without permission.
Bilateral chylothorax in an AIDS patient with newly diagnosed Kaposi sarcoma
Kaposi sarcoma is an angioproliferative tumor that is associated with human herpes virus-B (HIV-B). Mucocutaneous disease is the most common site for manifestation of AIDS-related Kaposi sarcoma, commonly affecting the lower extremeties, oral mucosa, face, and genitalia. Pleural effusions can occur in 36%-60% of patients with Kaposi sarcoma, and it has been documented that chylothorax is a rare, but plausible presentation in patients with Kaposi sarcoma.1 We present here a case of bilateral chylothorax in a patient with AIDS-related Kaposi sarcoma.
Case presentation and summary
A 52-year-old MSM male with AIDS (CD4, <20 mm3 ; viral load, 58 copies/ml) presented to the emergency department with complaints of shortness of breath, productive cough, and diarrhea for 2 days prior to presentation. His medical history also included chronic obstructive pulmonary disease, coronary artery disease, and hyperlipidemia.The patient was not on HAART because of his history of noncompliance. The results of a chest X-ray and computed-tomography (CT) scan showed that the patient had bilateral pleural effusion and a spiculated 14-mm nodule in the left upper lobe.
The patient underwent ultrasound-guided placement of a 12-French left-sided chest catheter, and a milky white fluid was aspirated from the left pleural space. Laboratory analysis of the pleural fluid confirmed an exudate with an elevated triglyceride level of 120 mg/dL (chylous, >110 mg/dL) indicating chylothorax.
On close physical examination, the patient was found to have multiple irregular plaques on the back and lower extremities. As described by dermatology, there was a violaceous indurated plaque on the left axillae, violaceous indurated plaques with superficial scale grouped on the left midlateral back, and hyperpigmented lichenified plaques and papules on bilateral shins, with some with plate-like scale. Two punch biopsies were taken of the skin lesions, which confirmed Kaposi sarcoma, plaque stage from the lesion biopsied on the back, and patch stage from the lesion biopsied in the left axilla. Cytology of the pleural fluid was negative for malignant cells. On review by the radiologist of the CT scan of the chest, there was no indication of gross distention of the thoracic duct. Treatment options were offered to the patient, and the patient was considering options for chemotherapy and home hospice given his advanced disease state at the time of discharge.
Discussion
Chylothorax occurs with a thoracic duct obstruction, which results in leakage of lymphatic fluid into the pleural cavity. The two leading causes of chylothorax are trauma and malignancy, with lymphoma being the most common cause of chylothorax among those with malignancy.2 Chylothorax, however, is a rare but documented complication of Kaposi sarcoma. Marais and colleagues reported the case of a 3-year-old HIV-positive patient with newly diagnosed Kaposi sarcoma who was found to have tumor infiltration in the thoracic duct leading to bilateral chylothorax.3 Maradona and colleagues described a 40-year-old man with AIDS-related Kaposi sarcoma who was found to have pleural and pericardial Kaposi sarcoma with chylothorax.4 Priest and colleagues wrote about a 32-year-old patient with AIDS with biopsy-proven Kaposi sarcoma who required multiple therapeutic thoracenteses for rapidly recurrent left chylothorax effusions.5
There are two leading discussions as to the pathophysiology of chylothorax that is related to Kaposi sarcoma: chylothorax developing secondary to metastatic disease or the development of chylothorax secondary to primary Kaposi sarcoma arising from the pleural region.6 One case report examined pleural and lung biopsies in a 34-year-old patient with AIDS-related Kaposi sarcoma that showed immunohistochemical staining that was suggestive of early-stage Kaposi sarcoma of lymphatic endothelial origin. The authors were attempting to illustrate that Kaposi sarcoma may have a stem-cell origin which can differentiate into lymph cells. Kontantinopoulos and colleagues postulated that in situ Kaposi sarcoma can arise from the lymphatic system with a resultant clinical presentation of chylothorax.7 The more mainstream thought however, is that chylothorax has been found to develop secondary to metastatic disease. The present case, therefore, illustrates an unusual presentation of cytology negative chylothorax in a patient with AIDS-related Kaposi sarcoma. TSJ
Rebecca E Neril, MD; Department of Internal Medicine, SBH Health System, Bronx, New York.
References
1. Sridar S, Garza EG, Cox J, Rumbak MJ. Serosanguineous pleural effusions in a patient with HIV and Kaposi sarcoma: pleuroscopic findings. J Bronchology Interv Pulmonol. 2011;18(4):337-339.
2. Light RW. Chylothorax and pseudochylothorax. In: Light RW, ed. Pleural diseases. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2013:412-426.
3. Marais BJ, Pienaar J, Gie RP. Kaposi sarcoma with upper airway obstruction and bilateral chylothoraces. Pediatr Infect Dis J. 2003;22:926-928.
4. Maradona JA, Carton JA, Asensi V, Rodriguez-Guardado A. AIDSrelated Kaposi sarcoma with chylothorax and pericardial involvement satisfactorily treated with liposomal doxorubicin. AIDS. 2002;16(5):806.
5. Priest ER, Weiss R. Chylothorax with Kaposi sarcoma. South Med J. 1991;84:806-807.
6. Pantanowitz L, Dezube BJ. Kaposi sarcoma in unusual locations. BMC Cancer. 2008;8:190.
7. Konstantinopoulos PA, Dezube BJ, Pantanowitz L. Morphologic and immunophenotypic evidence of in situ Kaposi sarcoma. BMC Clin Pathol. 2006;30:6:7.
Kaposi sarcoma is an angioproliferative tumor that is associated with human herpes virus-B (HIV-B). Mucocutaneous disease is the most common site for manifestation of AIDS-related Kaposi sarcoma, commonly affecting the lower extremeties, oral mucosa, face, and genitalia. Pleural effusions can occur in 36%-60% of patients with Kaposi sarcoma, and it has been documented that chylothorax is a rare, but plausible presentation in patients with Kaposi sarcoma.1 We present here a case of bilateral chylothorax in a patient with AIDS-related Kaposi sarcoma.
Case presentation and summary
A 52-year-old MSM male with AIDS (CD4, <20 mm3 ; viral load, 58 copies/ml) presented to the emergency department with complaints of shortness of breath, productive cough, and diarrhea for 2 days prior to presentation. His medical history also included chronic obstructive pulmonary disease, coronary artery disease, and hyperlipidemia.The patient was not on HAART because of his history of noncompliance. The results of a chest X-ray and computed-tomography (CT) scan showed that the patient had bilateral pleural effusion and a spiculated 14-mm nodule in the left upper lobe.
The patient underwent ultrasound-guided placement of a 12-French left-sided chest catheter, and a milky white fluid was aspirated from the left pleural space. Laboratory analysis of the pleural fluid confirmed an exudate with an elevated triglyceride level of 120 mg/dL (chylous, >110 mg/dL) indicating chylothorax.
On close physical examination, the patient was found to have multiple irregular plaques on the back and lower extremities. As described by dermatology, there was a violaceous indurated plaque on the left axillae, violaceous indurated plaques with superficial scale grouped on the left midlateral back, and hyperpigmented lichenified plaques and papules on bilateral shins, with some with plate-like scale. Two punch biopsies were taken of the skin lesions, which confirmed Kaposi sarcoma, plaque stage from the lesion biopsied on the back, and patch stage from the lesion biopsied in the left axilla. Cytology of the pleural fluid was negative for malignant cells. On review by the radiologist of the CT scan of the chest, there was no indication of gross distention of the thoracic duct. Treatment options were offered to the patient, and the patient was considering options for chemotherapy and home hospice given his advanced disease state at the time of discharge.
Discussion
Chylothorax occurs with a thoracic duct obstruction, which results in leakage of lymphatic fluid into the pleural cavity. The two leading causes of chylothorax are trauma and malignancy, with lymphoma being the most common cause of chylothorax among those with malignancy.2 Chylothorax, however, is a rare but documented complication of Kaposi sarcoma. Marais and colleagues reported the case of a 3-year-old HIV-positive patient with newly diagnosed Kaposi sarcoma who was found to have tumor infiltration in the thoracic duct leading to bilateral chylothorax.3 Maradona and colleagues described a 40-year-old man with AIDS-related Kaposi sarcoma who was found to have pleural and pericardial Kaposi sarcoma with chylothorax.4 Priest and colleagues wrote about a 32-year-old patient with AIDS with biopsy-proven Kaposi sarcoma who required multiple therapeutic thoracenteses for rapidly recurrent left chylothorax effusions.5
There are two leading discussions as to the pathophysiology of chylothorax that is related to Kaposi sarcoma: chylothorax developing secondary to metastatic disease or the development of chylothorax secondary to primary Kaposi sarcoma arising from the pleural region.6 One case report examined pleural and lung biopsies in a 34-year-old patient with AIDS-related Kaposi sarcoma that showed immunohistochemical staining that was suggestive of early-stage Kaposi sarcoma of lymphatic endothelial origin. The authors were attempting to illustrate that Kaposi sarcoma may have a stem-cell origin which can differentiate into lymph cells. Kontantinopoulos and colleagues postulated that in situ Kaposi sarcoma can arise from the lymphatic system with a resultant clinical presentation of chylothorax.7 The more mainstream thought however, is that chylothorax has been found to develop secondary to metastatic disease. The present case, therefore, illustrates an unusual presentation of cytology negative chylothorax in a patient with AIDS-related Kaposi sarcoma. TSJ
Rebecca E Neril, MD; Department of Internal Medicine, SBH Health System, Bronx, New York.
Kaposi sarcoma is an angioproliferative tumor that is associated with human herpes virus-B (HIV-B). Mucocutaneous disease is the most common site for manifestation of AIDS-related Kaposi sarcoma, commonly affecting the lower extremeties, oral mucosa, face, and genitalia. Pleural effusions can occur in 36%-60% of patients with Kaposi sarcoma, and it has been documented that chylothorax is a rare, but plausible presentation in patients with Kaposi sarcoma.1 We present here a case of bilateral chylothorax in a patient with AIDS-related Kaposi sarcoma.
Case presentation and summary
A 52-year-old MSM male with AIDS (CD4, <20 mm3 ; viral load, 58 copies/ml) presented to the emergency department with complaints of shortness of breath, productive cough, and diarrhea for 2 days prior to presentation. His medical history also included chronic obstructive pulmonary disease, coronary artery disease, and hyperlipidemia.The patient was not on HAART because of his history of noncompliance. The results of a chest X-ray and computed-tomography (CT) scan showed that the patient had bilateral pleural effusion and a spiculated 14-mm nodule in the left upper lobe.
The patient underwent ultrasound-guided placement of a 12-French left-sided chest catheter, and a milky white fluid was aspirated from the left pleural space. Laboratory analysis of the pleural fluid confirmed an exudate with an elevated triglyceride level of 120 mg/dL (chylous, >110 mg/dL) indicating chylothorax.
On close physical examination, the patient was found to have multiple irregular plaques on the back and lower extremities. As described by dermatology, there was a violaceous indurated plaque on the left axillae, violaceous indurated plaques with superficial scale grouped on the left midlateral back, and hyperpigmented lichenified plaques and papules on bilateral shins, with some with plate-like scale. Two punch biopsies were taken of the skin lesions, which confirmed Kaposi sarcoma, plaque stage from the lesion biopsied on the back, and patch stage from the lesion biopsied in the left axilla. Cytology of the pleural fluid was negative for malignant cells. On review by the radiologist of the CT scan of the chest, there was no indication of gross distention of the thoracic duct. Treatment options were offered to the patient, and the patient was considering options for chemotherapy and home hospice given his advanced disease state at the time of discharge.
Discussion
Chylothorax occurs with a thoracic duct obstruction, which results in leakage of lymphatic fluid into the pleural cavity. The two leading causes of chylothorax are trauma and malignancy, with lymphoma being the most common cause of chylothorax among those with malignancy.2 Chylothorax, however, is a rare but documented complication of Kaposi sarcoma. Marais and colleagues reported the case of a 3-year-old HIV-positive patient with newly diagnosed Kaposi sarcoma who was found to have tumor infiltration in the thoracic duct leading to bilateral chylothorax.3 Maradona and colleagues described a 40-year-old man with AIDS-related Kaposi sarcoma who was found to have pleural and pericardial Kaposi sarcoma with chylothorax.4 Priest and colleagues wrote about a 32-year-old patient with AIDS with biopsy-proven Kaposi sarcoma who required multiple therapeutic thoracenteses for rapidly recurrent left chylothorax effusions.5
There are two leading discussions as to the pathophysiology of chylothorax that is related to Kaposi sarcoma: chylothorax developing secondary to metastatic disease or the development of chylothorax secondary to primary Kaposi sarcoma arising from the pleural region.6 One case report examined pleural and lung biopsies in a 34-year-old patient with AIDS-related Kaposi sarcoma that showed immunohistochemical staining that was suggestive of early-stage Kaposi sarcoma of lymphatic endothelial origin. The authors were attempting to illustrate that Kaposi sarcoma may have a stem-cell origin which can differentiate into lymph cells. Kontantinopoulos and colleagues postulated that in situ Kaposi sarcoma can arise from the lymphatic system with a resultant clinical presentation of chylothorax.7 The more mainstream thought however, is that chylothorax has been found to develop secondary to metastatic disease. The present case, therefore, illustrates an unusual presentation of cytology negative chylothorax in a patient with AIDS-related Kaposi sarcoma. TSJ
Rebecca E Neril, MD; Department of Internal Medicine, SBH Health System, Bronx, New York.
References
1. Sridar S, Garza EG, Cox J, Rumbak MJ. Serosanguineous pleural effusions in a patient with HIV and Kaposi sarcoma: pleuroscopic findings. J Bronchology Interv Pulmonol. 2011;18(4):337-339.
2. Light RW. Chylothorax and pseudochylothorax. In: Light RW, ed. Pleural diseases. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2013:412-426.
3. Marais BJ, Pienaar J, Gie RP. Kaposi sarcoma with upper airway obstruction and bilateral chylothoraces. Pediatr Infect Dis J. 2003;22:926-928.
4. Maradona JA, Carton JA, Asensi V, Rodriguez-Guardado A. AIDSrelated Kaposi sarcoma with chylothorax and pericardial involvement satisfactorily treated with liposomal doxorubicin. AIDS. 2002;16(5):806.
5. Priest ER, Weiss R. Chylothorax with Kaposi sarcoma. South Med J. 1991;84:806-807.
6. Pantanowitz L, Dezube BJ. Kaposi sarcoma in unusual locations. BMC Cancer. 2008;8:190.
7. Konstantinopoulos PA, Dezube BJ, Pantanowitz L. Morphologic and immunophenotypic evidence of in situ Kaposi sarcoma. BMC Clin Pathol. 2006;30:6:7.
References
1. Sridar S, Garza EG, Cox J, Rumbak MJ. Serosanguineous pleural effusions in a patient with HIV and Kaposi sarcoma: pleuroscopic findings. J Bronchology Interv Pulmonol. 2011;18(4):337-339.
2. Light RW. Chylothorax and pseudochylothorax. In: Light RW, ed. Pleural diseases. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2013:412-426.
3. Marais BJ, Pienaar J, Gie RP. Kaposi sarcoma with upper airway obstruction and bilateral chylothoraces. Pediatr Infect Dis J. 2003;22:926-928.
4. Maradona JA, Carton JA, Asensi V, Rodriguez-Guardado A. AIDSrelated Kaposi sarcoma with chylothorax and pericardial involvement satisfactorily treated with liposomal doxorubicin. AIDS. 2002;16(5):806.
5. Priest ER, Weiss R. Chylothorax with Kaposi sarcoma. South Med J. 1991;84:806-807.
6. Pantanowitz L, Dezube BJ. Kaposi sarcoma in unusual locations. BMC Cancer. 2008;8:190.
7. Konstantinopoulos PA, Dezube BJ, Pantanowitz L. Morphologic and immunophenotypic evidence of in situ Kaposi sarcoma. BMC Clin Pathol. 2006;30:6:7.
This article was originally published in the Journal of Community and Supportive Oncology (JCSO 2017;15(3):e174-e175). doi: https://doi.org/10.12788/jcso.0261. It is reproduced here with permission of the copyright owner. Further reproduction is prohibited without permission.