User login
Hormonal contraceptives protective against suicide?
Contrary to previous analyses, new research suggests.
In a study of more than 800 women younger than age 50 who attempted suicide and more than 3,000 age-matched peers, results showed those who took hormonal contraceptives had a 27% reduced risk for attempted suicide.
Further analysis showed this was confined to women without a history of psychiatric illness and the reduction in risk rose to 43% among those who took combined hormonal contraceptives rather than progestin-only versions.
The protective effect against attempted suicide increased further to 46% if ethinyl estradiol (EE)–containing preparations were used. Moreover, the beneficial effect of contraceptive use increased over time.
The main message is the “current use of hormonal contraceptives is not associated with an increased risk of attempted suicide in our population,” study presenter Elena Toffol, MD, PhD, department of public health, University of Helsinki, told meeting attendees at the European Psychiatric Association 2022 Congress.
Age range differences
Dr. Toffol said there could be “several reasons” why the results are different from those in previous studies, including that the researchers included a “larger age range.” She noted it is known that “older women have a lower rate of attempted suicide and use different types of contraceptives.”
Dr. Toffol said in an interview that, although it’s “hard to estimate any causality” because this is an observational study, it is “tempting to speculate, and it is plausible, that hormones partly play a role with some, but not all, women being more sensitive to hormonal influences.”
However, the results “may also reflect life choices or a protective life status; for example, more stable relationships or more conscious and health-focused behaviors,” she said.
“It may also be that the underlying characteristics of women who are prescribed or opt for certain types of contraceptives are somehow related to their suicidal risk,” she added.
In 2019, the global age-standardized suicide rate was 9.0 per 100,000, which translates into more than 700,000 deaths every year, Dr. Toffol noted.
However, she emphasized the World Health Organization has calculated that, for every adult who dies by suicide, more than 20 people attempt suicide. In addition, data from the U.S. Centers for Disease Control and Prevention indicate that attempted suicides are three times more common among young women than in men.
“What are the reasons for this gender gap?” Dr. Toffol asked during her presentation.
“It is known that the major risk factor for suicidal behavior is a psychiatric disorder, and in particular depression and mood disorders. And depression and mood disorders are more common in women than in men,” she said.
However, there is also “growing interest into the role of biological factors” in the risk for suicide, including hormones and hormonal contraception. Some studies have also suggested that there is an increased risk for depression and “both completed and attempted suicide” after starting hormonal contraception.
Dr. Toffol added that about 70% of European women use some form of contraception and, among Finnish women, 40% choose a hormonal contraceptive.
Nested analysis
The researchers conducted a nested case-control analysis combining 2017 national prescription data on 587,823 women aged 15-49 years with information from general and primary healthcare registers for the years 2018 to 2019.
They were able to identify 818 cases of attempted suicide among the women. These were matched 4:1 with 3,272 age-matched healthy women who acted as the control group. Use of hormonal contraceptives in the previous 180 days was determined for the whole cohort.
Among users of hormonal contraceptives, there were 344 attempted suicides in 2017, at an incidence rate of 0.59 per 1,000 person-years. This compared with 474 attempted suicides among nonusers, at an incidence rate of 0.81 per 1000 person-years.
Kaplan-Meier analysis showed there was a significant difference in rates for attempted suicide among hormonal contraceptive users versus nonusers, at an incidence rate ratio of 0.73 (P < .0001) – and the difference increased over time.
In addition, the incidence of attempted suicide decreased with increasing age, with the highest incidence rate in women aged 15-19 years (1.62 per 1,000 person-years).
Conditional logistic regression analysis that controlled for education, marital status, chronic disease, recent psychiatric hospitalization, and current use of psychotropic medication showed hormonal contraceptive use was not linked to an increased risk of attempted suicide overall, at an odds ratio of 0.79 (95% confidence interval, 0.56-1.11).
However, when they looked specifically at women without a history of psychiatric illness, the association became significant, at an OR of 0.73 for attempted suicide among hormonal contraceptive users (95% CI, 0.58-0.91), while the relationship remained nonsignificant in women with a history of psychiatric disorders.
Further analysis suggested the significant association was confined to women taking combined hormonal contraceptives, at an OR of 0.57 for suicide attempt versus nonusers (95% CI, 0.44-0.75), and those use EE-containing preparations (OR, 0.54; 95% CI, 0.40-0.73).
There was a suggestion in the data that hormonal contraceptives containing desogestrel or drospirenone alongside EE may offer the greatest reduction in attempted suicide risk, but that did not survive multivariate analysis.
Dr. Toffol also noted that they were not able to capture data on use of intrauterine devices in their analysis.
“There is a growing number of municipalities in Finland that are providing free-of-charge contraception to young women” that is often an intrauterine device, she said. The researchers hope to include these women in a future analysis.
‘Age matters’
Commenting on the findings, Alexis C. Edwards, PhD, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond, said the current study’s findings “made a lot of sense.” Dr. Edwards wasn’t involved with this study but conducted a previous study of 216,702 Swedish women aged 15-22 years that showed use of combination or progestin-only oral contraceptives was associated with an increased risk for suicidal behavior.
She agreed with Dr. Toffol that the “much larger age range” in the new study may have played a role in showing the opposite result.
“The trajectory that we saw if we had been able to continue following the women for longer – which we couldn’t, due to limitations of the registries – [was that] using hormonal contraceptives was going to end up being protective, so I do think that it matters what age you’re looking at,” she said.
Dr. Edwards noted the takeaway from both studies “is that, even if there is a slight increase in risk from using hormonal contraceptives, it’s short lived and it’s probably specific to young women, which is important.”
She suggested the hormonal benefit from extended contraceptive use could come from the regulation of mood, as it offers a “more stable hormonal course than what their body might be putting them through in the absence of using the pill.”
Overall, it is “really lovely to see very well-executed studies on this, providing more empirical evidence on this question, because it is something that’s relevant to anyone who’s potentially going to be using hormonal contraception,” Dr. Edwards said.
Clinical implications?
Andrea Fiorillo, MD, PhD, department of psychiatry, University of Campania “Luigi Vanvitelli,” Naples, Italy, said in a press release that the “striking” findings of the current study need “careful evaluation.”
They also need to be replicated in “different cohorts of women and controlled for the impact of several psychosocial stressors, such as economic upheavals, social insecurity, and uncertainty due to the COVID pandemic,” said Dr. Fiorillo, who was not involved with the research.
Nevertheless, she believes the “clinical implications of the study are obvious and may help to destigmatize the use of hormonal contraceptives.”
The study was funded by the Jane and Aatos Erkko Foundation, the Avohoidon Tsukimis äätiö (Foundation for Primary Care Research), the Yrj ö Jahnsson Foundation, and the Finnish Cultural Foundation. No relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
Contrary to previous analyses, new research suggests.
In a study of more than 800 women younger than age 50 who attempted suicide and more than 3,000 age-matched peers, results showed those who took hormonal contraceptives had a 27% reduced risk for attempted suicide.
Further analysis showed this was confined to women without a history of psychiatric illness and the reduction in risk rose to 43% among those who took combined hormonal contraceptives rather than progestin-only versions.
The protective effect against attempted suicide increased further to 46% if ethinyl estradiol (EE)–containing preparations were used. Moreover, the beneficial effect of contraceptive use increased over time.
The main message is the “current use of hormonal contraceptives is not associated with an increased risk of attempted suicide in our population,” study presenter Elena Toffol, MD, PhD, department of public health, University of Helsinki, told meeting attendees at the European Psychiatric Association 2022 Congress.
Age range differences
Dr. Toffol said there could be “several reasons” why the results are different from those in previous studies, including that the researchers included a “larger age range.” She noted it is known that “older women have a lower rate of attempted suicide and use different types of contraceptives.”
Dr. Toffol said in an interview that, although it’s “hard to estimate any causality” because this is an observational study, it is “tempting to speculate, and it is plausible, that hormones partly play a role with some, but not all, women being more sensitive to hormonal influences.”
However, the results “may also reflect life choices or a protective life status; for example, more stable relationships or more conscious and health-focused behaviors,” she said.
“It may also be that the underlying characteristics of women who are prescribed or opt for certain types of contraceptives are somehow related to their suicidal risk,” she added.
In 2019, the global age-standardized suicide rate was 9.0 per 100,000, which translates into more than 700,000 deaths every year, Dr. Toffol noted.
However, she emphasized the World Health Organization has calculated that, for every adult who dies by suicide, more than 20 people attempt suicide. In addition, data from the U.S. Centers for Disease Control and Prevention indicate that attempted suicides are three times more common among young women than in men.
“What are the reasons for this gender gap?” Dr. Toffol asked during her presentation.
“It is known that the major risk factor for suicidal behavior is a psychiatric disorder, and in particular depression and mood disorders. And depression and mood disorders are more common in women than in men,” she said.
However, there is also “growing interest into the role of biological factors” in the risk for suicide, including hormones and hormonal contraception. Some studies have also suggested that there is an increased risk for depression and “both completed and attempted suicide” after starting hormonal contraception.
Dr. Toffol added that about 70% of European women use some form of contraception and, among Finnish women, 40% choose a hormonal contraceptive.
Nested analysis
The researchers conducted a nested case-control analysis combining 2017 national prescription data on 587,823 women aged 15-49 years with information from general and primary healthcare registers for the years 2018 to 2019.
They were able to identify 818 cases of attempted suicide among the women. These were matched 4:1 with 3,272 age-matched healthy women who acted as the control group. Use of hormonal contraceptives in the previous 180 days was determined for the whole cohort.
Among users of hormonal contraceptives, there were 344 attempted suicides in 2017, at an incidence rate of 0.59 per 1,000 person-years. This compared with 474 attempted suicides among nonusers, at an incidence rate of 0.81 per 1000 person-years.
Kaplan-Meier analysis showed there was a significant difference in rates for attempted suicide among hormonal contraceptive users versus nonusers, at an incidence rate ratio of 0.73 (P < .0001) – and the difference increased over time.
In addition, the incidence of attempted suicide decreased with increasing age, with the highest incidence rate in women aged 15-19 years (1.62 per 1,000 person-years).
Conditional logistic regression analysis that controlled for education, marital status, chronic disease, recent psychiatric hospitalization, and current use of psychotropic medication showed hormonal contraceptive use was not linked to an increased risk of attempted suicide overall, at an odds ratio of 0.79 (95% confidence interval, 0.56-1.11).
However, when they looked specifically at women without a history of psychiatric illness, the association became significant, at an OR of 0.73 for attempted suicide among hormonal contraceptive users (95% CI, 0.58-0.91), while the relationship remained nonsignificant in women with a history of psychiatric disorders.
Further analysis suggested the significant association was confined to women taking combined hormonal contraceptives, at an OR of 0.57 for suicide attempt versus nonusers (95% CI, 0.44-0.75), and those use EE-containing preparations (OR, 0.54; 95% CI, 0.40-0.73).
There was a suggestion in the data that hormonal contraceptives containing desogestrel or drospirenone alongside EE may offer the greatest reduction in attempted suicide risk, but that did not survive multivariate analysis.
Dr. Toffol also noted that they were not able to capture data on use of intrauterine devices in their analysis.
“There is a growing number of municipalities in Finland that are providing free-of-charge contraception to young women” that is often an intrauterine device, she said. The researchers hope to include these women in a future analysis.
‘Age matters’
Commenting on the findings, Alexis C. Edwards, PhD, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond, said the current study’s findings “made a lot of sense.” Dr. Edwards wasn’t involved with this study but conducted a previous study of 216,702 Swedish women aged 15-22 years that showed use of combination or progestin-only oral contraceptives was associated with an increased risk for suicidal behavior.
She agreed with Dr. Toffol that the “much larger age range” in the new study may have played a role in showing the opposite result.
“The trajectory that we saw if we had been able to continue following the women for longer – which we couldn’t, due to limitations of the registries – [was that] using hormonal contraceptives was going to end up being protective, so I do think that it matters what age you’re looking at,” she said.
Dr. Edwards noted the takeaway from both studies “is that, even if there is a slight increase in risk from using hormonal contraceptives, it’s short lived and it’s probably specific to young women, which is important.”
She suggested the hormonal benefit from extended contraceptive use could come from the regulation of mood, as it offers a “more stable hormonal course than what their body might be putting them through in the absence of using the pill.”
Overall, it is “really lovely to see very well-executed studies on this, providing more empirical evidence on this question, because it is something that’s relevant to anyone who’s potentially going to be using hormonal contraception,” Dr. Edwards said.
Clinical implications?
Andrea Fiorillo, MD, PhD, department of psychiatry, University of Campania “Luigi Vanvitelli,” Naples, Italy, said in a press release that the “striking” findings of the current study need “careful evaluation.”
They also need to be replicated in “different cohorts of women and controlled for the impact of several psychosocial stressors, such as economic upheavals, social insecurity, and uncertainty due to the COVID pandemic,” said Dr. Fiorillo, who was not involved with the research.
Nevertheless, she believes the “clinical implications of the study are obvious and may help to destigmatize the use of hormonal contraceptives.”
The study was funded by the Jane and Aatos Erkko Foundation, the Avohoidon Tsukimis äätiö (Foundation for Primary Care Research), the Yrj ö Jahnsson Foundation, and the Finnish Cultural Foundation. No relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
Contrary to previous analyses, new research suggests.
In a study of more than 800 women younger than age 50 who attempted suicide and more than 3,000 age-matched peers, results showed those who took hormonal contraceptives had a 27% reduced risk for attempted suicide.
Further analysis showed this was confined to women without a history of psychiatric illness and the reduction in risk rose to 43% among those who took combined hormonal contraceptives rather than progestin-only versions.
The protective effect against attempted suicide increased further to 46% if ethinyl estradiol (EE)–containing preparations were used. Moreover, the beneficial effect of contraceptive use increased over time.
The main message is the “current use of hormonal contraceptives is not associated with an increased risk of attempted suicide in our population,” study presenter Elena Toffol, MD, PhD, department of public health, University of Helsinki, told meeting attendees at the European Psychiatric Association 2022 Congress.
Age range differences
Dr. Toffol said there could be “several reasons” why the results are different from those in previous studies, including that the researchers included a “larger age range.” She noted it is known that “older women have a lower rate of attempted suicide and use different types of contraceptives.”
Dr. Toffol said in an interview that, although it’s “hard to estimate any causality” because this is an observational study, it is “tempting to speculate, and it is plausible, that hormones partly play a role with some, but not all, women being more sensitive to hormonal influences.”
However, the results “may also reflect life choices or a protective life status; for example, more stable relationships or more conscious and health-focused behaviors,” she said.
“It may also be that the underlying characteristics of women who are prescribed or opt for certain types of contraceptives are somehow related to their suicidal risk,” she added.
In 2019, the global age-standardized suicide rate was 9.0 per 100,000, which translates into more than 700,000 deaths every year, Dr. Toffol noted.
However, she emphasized the World Health Organization has calculated that, for every adult who dies by suicide, more than 20 people attempt suicide. In addition, data from the U.S. Centers for Disease Control and Prevention indicate that attempted suicides are three times more common among young women than in men.
“What are the reasons for this gender gap?” Dr. Toffol asked during her presentation.
“It is known that the major risk factor for suicidal behavior is a psychiatric disorder, and in particular depression and mood disorders. And depression and mood disorders are more common in women than in men,” she said.
However, there is also “growing interest into the role of biological factors” in the risk for suicide, including hormones and hormonal contraception. Some studies have also suggested that there is an increased risk for depression and “both completed and attempted suicide” after starting hormonal contraception.
Dr. Toffol added that about 70% of European women use some form of contraception and, among Finnish women, 40% choose a hormonal contraceptive.
Nested analysis
The researchers conducted a nested case-control analysis combining 2017 national prescription data on 587,823 women aged 15-49 years with information from general and primary healthcare registers for the years 2018 to 2019.
They were able to identify 818 cases of attempted suicide among the women. These were matched 4:1 with 3,272 age-matched healthy women who acted as the control group. Use of hormonal contraceptives in the previous 180 days was determined for the whole cohort.
Among users of hormonal contraceptives, there were 344 attempted suicides in 2017, at an incidence rate of 0.59 per 1,000 person-years. This compared with 474 attempted suicides among nonusers, at an incidence rate of 0.81 per 1000 person-years.
Kaplan-Meier analysis showed there was a significant difference in rates for attempted suicide among hormonal contraceptive users versus nonusers, at an incidence rate ratio of 0.73 (P < .0001) – and the difference increased over time.
In addition, the incidence of attempted suicide decreased with increasing age, with the highest incidence rate in women aged 15-19 years (1.62 per 1,000 person-years).
Conditional logistic regression analysis that controlled for education, marital status, chronic disease, recent psychiatric hospitalization, and current use of psychotropic medication showed hormonal contraceptive use was not linked to an increased risk of attempted suicide overall, at an odds ratio of 0.79 (95% confidence interval, 0.56-1.11).
However, when they looked specifically at women without a history of psychiatric illness, the association became significant, at an OR of 0.73 for attempted suicide among hormonal contraceptive users (95% CI, 0.58-0.91), while the relationship remained nonsignificant in women with a history of psychiatric disorders.
Further analysis suggested the significant association was confined to women taking combined hormonal contraceptives, at an OR of 0.57 for suicide attempt versus nonusers (95% CI, 0.44-0.75), and those use EE-containing preparations (OR, 0.54; 95% CI, 0.40-0.73).
There was a suggestion in the data that hormonal contraceptives containing desogestrel or drospirenone alongside EE may offer the greatest reduction in attempted suicide risk, but that did not survive multivariate analysis.
Dr. Toffol also noted that they were not able to capture data on use of intrauterine devices in their analysis.
“There is a growing number of municipalities in Finland that are providing free-of-charge contraception to young women” that is often an intrauterine device, she said. The researchers hope to include these women in a future analysis.
‘Age matters’
Commenting on the findings, Alexis C. Edwards, PhD, Virginia Institute for Psychiatric and Behavioral Genetics, Virginia Commonwealth University, Richmond, said the current study’s findings “made a lot of sense.” Dr. Edwards wasn’t involved with this study but conducted a previous study of 216,702 Swedish women aged 15-22 years that showed use of combination or progestin-only oral contraceptives was associated with an increased risk for suicidal behavior.
She agreed with Dr. Toffol that the “much larger age range” in the new study may have played a role in showing the opposite result.
“The trajectory that we saw if we had been able to continue following the women for longer – which we couldn’t, due to limitations of the registries – [was that] using hormonal contraceptives was going to end up being protective, so I do think that it matters what age you’re looking at,” she said.
Dr. Edwards noted the takeaway from both studies “is that, even if there is a slight increase in risk from using hormonal contraceptives, it’s short lived and it’s probably specific to young women, which is important.”
She suggested the hormonal benefit from extended contraceptive use could come from the regulation of mood, as it offers a “more stable hormonal course than what their body might be putting them through in the absence of using the pill.”
Overall, it is “really lovely to see very well-executed studies on this, providing more empirical evidence on this question, because it is something that’s relevant to anyone who’s potentially going to be using hormonal contraception,” Dr. Edwards said.
Clinical implications?
Andrea Fiorillo, MD, PhD, department of psychiatry, University of Campania “Luigi Vanvitelli,” Naples, Italy, said in a press release that the “striking” findings of the current study need “careful evaluation.”
They also need to be replicated in “different cohorts of women and controlled for the impact of several psychosocial stressors, such as economic upheavals, social insecurity, and uncertainty due to the COVID pandemic,” said Dr. Fiorillo, who was not involved with the research.
Nevertheless, she believes the “clinical implications of the study are obvious and may help to destigmatize the use of hormonal contraceptives.”
The study was funded by the Jane and Aatos Erkko Foundation, the Avohoidon Tsukimis äätiö (Foundation for Primary Care Research), the Yrj ö Jahnsson Foundation, and the Finnish Cultural Foundation. No relevant financial relationships were reported.
A version of this article first appeared on Medscape.com.
FROM EPA 2022
Male contraceptive pill appears feasible in very early trials
ATLANTA – Potential once-daily male oral contraceptives have passed a first clinical hurdle, showing a degree of testosterone suppression that should be sufficient for a contraceptive effect without causing symptomatic hypogonadism, according to phase 1 study results to be presented at the annual meeting of the Endocrine Society.

Credit: Flickr/Marco Verch Professional Photographer/CC by 2.0
There are two pills in development and the studies so far suggest that both or a combination might be able to provide an acceptable balance of efficacy and tolerability, according to Tamar Jacobsohn, a researcher in the Contraceptive Development Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md.
The two drugs evaluated in this study are dimethandrolone undecanoate (DMAU) and 11b-methyl-19-nortestosterone-17b-dodecylcarbonate (11b-MNTDC). Both are bifunctional prodrugs with androgenic and progestogenic effects. The prodrugs are designed to be cleaved after ingestion so that the active hormones are released over 24 hours, permitting once-daily dosing.
“As potent androgens, these steroids suppress gonadotropin secretion, leading to markedly decreased serum testosterone production,” explained Ms. Jacobsohn in an interview.
However, she noted that there is still a long way to go on this research path. While the phase 1 studies have shown tolerability, the biology involved in suppressing sperm production suggests that men would need to take these pills daily for about 3 months at the very beginning of contraceptive treatment, until adequate sperm suppression is achieved to prevent pregnancy.
“We are working toward a phase 2 trial that will include a contraceptive efficacy endpoint, but there are lots of steps to get there, including more early phase studies,” she noted.
“There is a huge unmet need in terms of male contraceptive methods,” said Arthi Thirumalai, MBBS, an endocrinologist and assistant professor of medicine at the University of Washington School of Medicine in Seattle.
Senior author of a 2020 review article on male contraception, Dr. Thirumalai said in an interview that prodrugs and other hormonal methods to lower testosterone and suppress sperm production are attractive because of convenience, efficacy, and reversibility,
“We hope that oral formulations can be used to address this need,” said Dr. Thirumalai, who has participated in several experimental and clinical studies of male contraception methods. She is, in fact, one of the many coauthors of the data presented by Ms. Jacobsohn.
Ms. Jacobsohn emphasized: “Development of an effective, reversible male contraceptive method will improve reproductive options for men and women, have a major impact on public health by decreasing unintended pregnancy, and allow men to have an increasingly active role in family planning.”
Phase 1 results with DMAU and MNTDC
The work that led to phase 1 studies suggested that each of the drugs — DMAU and MNTDC — might provide adequate hormone suppression to reduce sperm counts without inducing unacceptable symptoms of hypogonadism. To test this potential, dose-ranging phase 1 studies with an endpoint of testosterone suppression were conducted with each one.
In the two placebo-controlled phase 1a studies, which are to be presented in a poster on June 13, healthy male subjects were randomly assigned to two pills of active therapy, four pills of active therapy, or placebo. In the two studies combined, 39 subjects received DMAU, 30 received 11b-MNTDC, and 28 received placebo.
Efficacy was evaluated by measuring testosterone levels. Tolerability was largely based on patient questionnaires.
At the end of 7 days, testosterone levels remained at reference levels (400 to 600 ng/dL) in those who received placebo. The levels fell to less than 100 ng/dL in all subjects assigned to an active agent regardless of which agent or dose was used.
From day 7 to 28, there was less median suppression of testosterone on 200 mg than 400 mg daily (92.7 ng/dL vs. 49.6 ng/dL; P < .001), but both remained below the target of 100 ng/dL, Ms. Jacobsohn reported.
The difference in degree of testosterone suppression did not appear to influence tolerability.
Subjects on four vs. two daily pills “did not report a significant difference in general satisfaction or their willingness to use the pills in the future or recommend them to other men,” said Ms. Jacobson, presenting P values for these outcomes among subjects on active therapy relative to placebo that were not significant, ranging from 0.48 to 0.85.
Overall, there were no serious adverse events. Mild side effects associated with hypogonadism did occur, but “all resolved by the end of the study,” she said.
Zero sperm production is not the goal. Lowering it sufficiently is
Dr. Thirumalai said the need for a male contraceptive is strong. While condoms have a substantial failure rate, vasectomy is not reliably reversible even though the majority of men agree that the responsibility for preventing pregnancy should be shared, she said.
Dr. Thirumalai’s earlier review article found that clinical trials of hormonal suppression to provide male contraception have been conducted for at least 30 years. The challenge has been finding an effective therapy that is well tolerated.
Drugs that combine both androgenic and progestogenic activity might be the answer. By manipulating hormones that lower testosterone, sperm production is reduced without eliminating a man’s ability to ejaculate. Zero sperm production is not the goal, according to data in Dr. Thirumalai’s review article.
Rather, studies suggest that when ejaculate contains less than 1 million sperm per mL (levels typically range from 15 to 200 million sperm/mL), the antipregnancy efficacy is similar to that achieved with female oral contraceptives.
However, clinical trials to demonstrate that this can be achieved safely have yet to be conducted.
Ms. Jacobsohn said that sperm half-life is about 3 months. This means that patients would need to be on hormonal therapy for a period of about this duration before reliable contraception is achieved.
In other words, the efficacy endpoint used in this current study [of 28 days duration] does not ensure effective contraception, but Ms. Jacobsohn suggested this is nevertheless an important step forward in clinical development.
Ms. Jacobsohn and Dr. Thirumalai report no relevant financial relationships.
A version of this article first appeared on Medscape.com .
ATLANTA – Potential once-daily male oral contraceptives have passed a first clinical hurdle, showing a degree of testosterone suppression that should be sufficient for a contraceptive effect without causing symptomatic hypogonadism, according to phase 1 study results to be presented at the annual meeting of the Endocrine Society.

Credit: Flickr/Marco Verch Professional Photographer/CC by 2.0
There are two pills in development and the studies so far suggest that both or a combination might be able to provide an acceptable balance of efficacy and tolerability, according to Tamar Jacobsohn, a researcher in the Contraceptive Development Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md.
The two drugs evaluated in this study are dimethandrolone undecanoate (DMAU) and 11b-methyl-19-nortestosterone-17b-dodecylcarbonate (11b-MNTDC). Both are bifunctional prodrugs with androgenic and progestogenic effects. The prodrugs are designed to be cleaved after ingestion so that the active hormones are released over 24 hours, permitting once-daily dosing.
“As potent androgens, these steroids suppress gonadotropin secretion, leading to markedly decreased serum testosterone production,” explained Ms. Jacobsohn in an interview.
However, she noted that there is still a long way to go on this research path. While the phase 1 studies have shown tolerability, the biology involved in suppressing sperm production suggests that men would need to take these pills daily for about 3 months at the very beginning of contraceptive treatment, until adequate sperm suppression is achieved to prevent pregnancy.
“We are working toward a phase 2 trial that will include a contraceptive efficacy endpoint, but there are lots of steps to get there, including more early phase studies,” she noted.
“There is a huge unmet need in terms of male contraceptive methods,” said Arthi Thirumalai, MBBS, an endocrinologist and assistant professor of medicine at the University of Washington School of Medicine in Seattle.
Senior author of a 2020 review article on male contraception, Dr. Thirumalai said in an interview that prodrugs and other hormonal methods to lower testosterone and suppress sperm production are attractive because of convenience, efficacy, and reversibility,
“We hope that oral formulations can be used to address this need,” said Dr. Thirumalai, who has participated in several experimental and clinical studies of male contraception methods. She is, in fact, one of the many coauthors of the data presented by Ms. Jacobsohn.
Ms. Jacobsohn emphasized: “Development of an effective, reversible male contraceptive method will improve reproductive options for men and women, have a major impact on public health by decreasing unintended pregnancy, and allow men to have an increasingly active role in family planning.”
Phase 1 results with DMAU and MNTDC
The work that led to phase 1 studies suggested that each of the drugs — DMAU and MNTDC — might provide adequate hormone suppression to reduce sperm counts without inducing unacceptable symptoms of hypogonadism. To test this potential, dose-ranging phase 1 studies with an endpoint of testosterone suppression were conducted with each one.
In the two placebo-controlled phase 1a studies, which are to be presented in a poster on June 13, healthy male subjects were randomly assigned to two pills of active therapy, four pills of active therapy, or placebo. In the two studies combined, 39 subjects received DMAU, 30 received 11b-MNTDC, and 28 received placebo.
Efficacy was evaluated by measuring testosterone levels. Tolerability was largely based on patient questionnaires.
At the end of 7 days, testosterone levels remained at reference levels (400 to 600 ng/dL) in those who received placebo. The levels fell to less than 100 ng/dL in all subjects assigned to an active agent regardless of which agent or dose was used.
From day 7 to 28, there was less median suppression of testosterone on 200 mg than 400 mg daily (92.7 ng/dL vs. 49.6 ng/dL; P < .001), but both remained below the target of 100 ng/dL, Ms. Jacobsohn reported.
The difference in degree of testosterone suppression did not appear to influence tolerability.
Subjects on four vs. two daily pills “did not report a significant difference in general satisfaction or their willingness to use the pills in the future or recommend them to other men,” said Ms. Jacobson, presenting P values for these outcomes among subjects on active therapy relative to placebo that were not significant, ranging from 0.48 to 0.85.
Overall, there were no serious adverse events. Mild side effects associated with hypogonadism did occur, but “all resolved by the end of the study,” she said.
Zero sperm production is not the goal. Lowering it sufficiently is
Dr. Thirumalai said the need for a male contraceptive is strong. While condoms have a substantial failure rate, vasectomy is not reliably reversible even though the majority of men agree that the responsibility for preventing pregnancy should be shared, she said.
Dr. Thirumalai’s earlier review article found that clinical trials of hormonal suppression to provide male contraception have been conducted for at least 30 years. The challenge has been finding an effective therapy that is well tolerated.
Drugs that combine both androgenic and progestogenic activity might be the answer. By manipulating hormones that lower testosterone, sperm production is reduced without eliminating a man’s ability to ejaculate. Zero sperm production is not the goal, according to data in Dr. Thirumalai’s review article.
Rather, studies suggest that when ejaculate contains less than 1 million sperm per mL (levels typically range from 15 to 200 million sperm/mL), the antipregnancy efficacy is similar to that achieved with female oral contraceptives.
However, clinical trials to demonstrate that this can be achieved safely have yet to be conducted.
Ms. Jacobsohn said that sperm half-life is about 3 months. This means that patients would need to be on hormonal therapy for a period of about this duration before reliable contraception is achieved.
In other words, the efficacy endpoint used in this current study [of 28 days duration] does not ensure effective contraception, but Ms. Jacobsohn suggested this is nevertheless an important step forward in clinical development.
Ms. Jacobsohn and Dr. Thirumalai report no relevant financial relationships.
A version of this article first appeared on Medscape.com .
ATLANTA – Potential once-daily male oral contraceptives have passed a first clinical hurdle, showing a degree of testosterone suppression that should be sufficient for a contraceptive effect without causing symptomatic hypogonadism, according to phase 1 study results to be presented at the annual meeting of the Endocrine Society.

Credit: Flickr/Marco Verch Professional Photographer/CC by 2.0
There are two pills in development and the studies so far suggest that both or a combination might be able to provide an acceptable balance of efficacy and tolerability, according to Tamar Jacobsohn, a researcher in the Contraceptive Development Program, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Md.
The two drugs evaluated in this study are dimethandrolone undecanoate (DMAU) and 11b-methyl-19-nortestosterone-17b-dodecylcarbonate (11b-MNTDC). Both are bifunctional prodrugs with androgenic and progestogenic effects. The prodrugs are designed to be cleaved after ingestion so that the active hormones are released over 24 hours, permitting once-daily dosing.
“As potent androgens, these steroids suppress gonadotropin secretion, leading to markedly decreased serum testosterone production,” explained Ms. Jacobsohn in an interview.
However, she noted that there is still a long way to go on this research path. While the phase 1 studies have shown tolerability, the biology involved in suppressing sperm production suggests that men would need to take these pills daily for about 3 months at the very beginning of contraceptive treatment, until adequate sperm suppression is achieved to prevent pregnancy.
“We are working toward a phase 2 trial that will include a contraceptive efficacy endpoint, but there are lots of steps to get there, including more early phase studies,” she noted.
“There is a huge unmet need in terms of male contraceptive methods,” said Arthi Thirumalai, MBBS, an endocrinologist and assistant professor of medicine at the University of Washington School of Medicine in Seattle.
Senior author of a 2020 review article on male contraception, Dr. Thirumalai said in an interview that prodrugs and other hormonal methods to lower testosterone and suppress sperm production are attractive because of convenience, efficacy, and reversibility,
“We hope that oral formulations can be used to address this need,” said Dr. Thirumalai, who has participated in several experimental and clinical studies of male contraception methods. She is, in fact, one of the many coauthors of the data presented by Ms. Jacobsohn.
Ms. Jacobsohn emphasized: “Development of an effective, reversible male contraceptive method will improve reproductive options for men and women, have a major impact on public health by decreasing unintended pregnancy, and allow men to have an increasingly active role in family planning.”
Phase 1 results with DMAU and MNTDC
The work that led to phase 1 studies suggested that each of the drugs — DMAU and MNTDC — might provide adequate hormone suppression to reduce sperm counts without inducing unacceptable symptoms of hypogonadism. To test this potential, dose-ranging phase 1 studies with an endpoint of testosterone suppression were conducted with each one.
In the two placebo-controlled phase 1a studies, which are to be presented in a poster on June 13, healthy male subjects were randomly assigned to two pills of active therapy, four pills of active therapy, or placebo. In the two studies combined, 39 subjects received DMAU, 30 received 11b-MNTDC, and 28 received placebo.
Efficacy was evaluated by measuring testosterone levels. Tolerability was largely based on patient questionnaires.
At the end of 7 days, testosterone levels remained at reference levels (400 to 600 ng/dL) in those who received placebo. The levels fell to less than 100 ng/dL in all subjects assigned to an active agent regardless of which agent or dose was used.
From day 7 to 28, there was less median suppression of testosterone on 200 mg than 400 mg daily (92.7 ng/dL vs. 49.6 ng/dL; P < .001), but both remained below the target of 100 ng/dL, Ms. Jacobsohn reported.
The difference in degree of testosterone suppression did not appear to influence tolerability.
Subjects on four vs. two daily pills “did not report a significant difference in general satisfaction or their willingness to use the pills in the future or recommend them to other men,” said Ms. Jacobson, presenting P values for these outcomes among subjects on active therapy relative to placebo that were not significant, ranging from 0.48 to 0.85.
Overall, there were no serious adverse events. Mild side effects associated with hypogonadism did occur, but “all resolved by the end of the study,” she said.
Zero sperm production is not the goal. Lowering it sufficiently is
Dr. Thirumalai said the need for a male contraceptive is strong. While condoms have a substantial failure rate, vasectomy is not reliably reversible even though the majority of men agree that the responsibility for preventing pregnancy should be shared, she said.
Dr. Thirumalai’s earlier review article found that clinical trials of hormonal suppression to provide male contraception have been conducted for at least 30 years. The challenge has been finding an effective therapy that is well tolerated.
Drugs that combine both androgenic and progestogenic activity might be the answer. By manipulating hormones that lower testosterone, sperm production is reduced without eliminating a man’s ability to ejaculate. Zero sperm production is not the goal, according to data in Dr. Thirumalai’s review article.
Rather, studies suggest that when ejaculate contains less than 1 million sperm per mL (levels typically range from 15 to 200 million sperm/mL), the antipregnancy efficacy is similar to that achieved with female oral contraceptives.
However, clinical trials to demonstrate that this can be achieved safely have yet to be conducted.
Ms. Jacobsohn said that sperm half-life is about 3 months. This means that patients would need to be on hormonal therapy for a period of about this duration before reliable contraception is achieved.
In other words, the efficacy endpoint used in this current study [of 28 days duration] does not ensure effective contraception, but Ms. Jacobsohn suggested this is nevertheless an important step forward in clinical development.
Ms. Jacobsohn and Dr. Thirumalai report no relevant financial relationships.
A version of this article first appeared on Medscape.com .
AT ENDO 2022
Jury still out on cardiovascular safety of testosterone
Despite a new meta-analysis claiming to show that testosterone replacement therapy for men with hypogonadism does not increase the risk of cardiovascular outcomes such as myocardial infarction or stroke, experts say the jury is still out.
A more definitive answer for cardiovascular safety of testosterone therapy will come from the TRAVERSE dedicated cardiovascular outcome trial, sponsored by AbbVie, which will have up to 5 years of follow-up, with results expected later this year.
The current meta-analysis by Jemma Hudson of Aberdeen (Scotland) University and colleagues was published online in The Lancet Healthy Longevity. The work will also be presented June 13 at ENDO 2022, the Endocrine Society’s annual meeting in Atlanta, Georgia, by senior author Channa Y. Jayasena, MD, PhD.
In 2014, the U.S. Food and Drug Administration mandated a label on testosterone products warning of possible increased cardiovascular risks and to reserve the therapy for symptomatic hypogonadism only. In contrast, the European Medicines Agency concluded that when hypogonadism is properly diagnosed and managed, there is currently no clear, consistent evidence that testosterone therapy causes increased cardiovascular risk.
To address this uncertainty, Dr. Hudson and colleagues formed a global collaborative to obtain individual patient data on cardiovascular outcomes from randomized controlled trials of testosterone therapy for men with hypogonadism.
They pooled data from 35 trials published from 1992 to Aug. 27, 2018, including 17 trials (3,431 patients) for which the researchers obtained patient-level data. The individual trials were 3-12 months long, except for one 3-year trial.
During a mean follow-up of 9.5 months, there was no significant increase in cardiovascular outcomes in men randomized to testosterone therapy versus placebo (odds ratio, 1.07; P = .62), nor were there any significantly increased risks of death, stroke, or different types of cardiovascular outcome, although those numbers were small.
This is “the most comprehensive study to date investigating the safety of testosterone treatment of hypogonadism,” according to the researchers. “The current results provide some reassurance about the short-term to medium-term safety of testosterone to treat male hypogonadism,” they conclude.
However, they also acknowledge that “long-term data are needed to fully evaluate the safety of testosterone.”
Erin D. Michos, MD, coauthor of an accompanying editorial, told this news organization, “This study doesn’t say to me that low testosterone necessarily needs to be treated. It’s still not indicated in people just for a low number [for blood testosterone] with less-severe symptoms. It really comes down to each individual person, how symptomatic they are, and their cardiovascular risk.”
‘Trial is not definitive’
Dr. Michos is not the only person to be skeptical. Together with Steven Nissen, MD, an investigator for the TRAVERSE trial, she agrees that this new evidence is not yet decisive, largely because the individual trials in the meta-analysis were short and not designed as cardiovascular outcome trials.
Dr. Nissen, a cardiologist at Cleveland Clinic, added that the individual trials were heterogeneous, with “very few real cardiovascular events,” so the meta-analysis “is not definitive,” he said in an interview.
While this meta-analysis “that pooled together a lot of smaller studies is reassuring that there’s no signal of harm, it’s really inconclusive because the follow-up was really short – a mean of only 9.5 months – and you really need a larger study with longer follow up to be more conclusive,” Dr. Michos noted.
“We should have more data soon” from TRAVERSE, said Dr. Michos, from the division of cardiology, Johns Hopkins University, Baltimore, who is not involved with that study.
Meanwhile, “I don’t think [this analysis] changes the current recommendations,” she said.
“We should continue to use caution as indicated by the FDA label and only use testosterone therapy selectively in people who have true symptoms of hypogonadism,” and be cautious about using it particularly in men at higher cardiovascular risk because of family history or known personal heart disease.
On the other hand, the meta-analysis did not show harm, she noted, “so we don’t necessarily need to pull patients off therapy if they are already taking it. But I wouldn’t right now just start new patients on it unless they had a strong indication.”
“Certainly, great caution is advised regarding the use of testosterone replacement therapy in people with established atherosclerosis due to the findings of plaque progression in the testosterone trials and the excess cardiovascular events observed in the TOM trial, write Dr. Michos and fellow editorialist Matthew J. Budoff, MD, of University of California, Los Angeles, in their editorial.
Earlier data inconclusive
Testosterone concentrations progressively decline in men with advancing age, at about 2% per year, Dr. Michos and Dr. Budoff write. In addition, men with obesity or with diabetes have low levels of testosterone, Dr. Michos noted.
Low testosterone blood levels have been associated with insulin resistance, inflammation, dyslipidemia, and atherosclerosis. Testosterone replacement therapy has been used to increase libido, improve erectile dysfunction, and boost energy levels, mood, and muscle strength.
But it is well known that testosterone increases hematocrit, which has the potential to increase the risk of venous thromboembolism.
Two large observational studies have reported increased risks of myocardial infarction, stroke, and death in men taking testosterone, compared with nonusers, but the study designs have been widely criticized, Dr. Hudson and coauthors say in their article.
A placebo-controlled trial was stopped early by its data- and safety-monitoring board following increased cardiovascular events in men aged 65 and older who received 6 months of testosterone. Other controlled trials have not observed these effects, but none was sufficiently powered.
Meta-analysis results
Dr. Hudson and colleagues performed a meta-analysis of 35 trials in 5,601 men aged 18 years and older with low baseline testosterone (≤ 350 nmol/dL) who had been randomized to testosterone replacement therapy or placebo for at least 3 months, for which there were data on mortality, stroke, and cardiovascular outcomes.
The men were a mean age of 65, had a mean body mass index of 30 kg/m2, and most (88%) were White. A quarter had angina, 8% had a previous myocardial infarction, and 27% had diabetes.
Cardiovascular and cerebrovascular outcomes were not primary outcomes.
During a mean follow-up of 9.5 months, in the 13 trials that provided this information, the rate of cardiovascular events was similar in the men who received testosterone (120/1,601, 7.5%) compared with those who received placebo (110/1,519, 7.2%).
In the 14 trials that provided this information, fewer deaths were reported during testosterone treatment (6/1,621, 0.4%) than during placebo treatment (12/1,537, 0.8%), but these numbers were too small to establish whether testosterone reduced mortality risk.
The most common cardiovascular events were arrhythmia, followed by coronary heart disease, heart failure, and myocardial infarction.
Patient age, baseline testosterone, smoking status, or diabetes status were not associated with cardiovascular risk.
The only detected adverse effects were edema and a modest lowering of HDL cholesterol.
“Men who develop sexual dysfunction, unexplained anemia, or osteoporosis should be tested for low testosterone,” senior author of the meta-analysis Dr. Jayasena said in an email to this news organization.
However, Dr. Jayasena added, “Mass screening for testosterone has no benefit in asymptomatic men.”
“Older men may still benefit from testosterone, but only if they have the clinical features [of hypogonadism] and low testosterone levels,” he concluded.
The current study is supported by the Health Technology Assessment program of the National Institute for Health Research. The TRAVERSE trial is sponsored by AbbVie. Dr. Jayasena has reported receiving research grants from LogixX Pharma. Dr. Hudson has reported no relevant financial relationships. Disclosures for the other authors are listed in the article. Dr. Michos has reported receiving support from the Amato Fund in Women’s Cardiovascular Health at Johns Hopkins School of Medicine and serving on medical advisory boards for Novartis, Esperion, Amarin, and AstraZeneca outside the submitted work. Dr. Budoff has reported receiving grant support from General Electric.
A version of this article first appeared on Medscape.com.
Despite a new meta-analysis claiming to show that testosterone replacement therapy for men with hypogonadism does not increase the risk of cardiovascular outcomes such as myocardial infarction or stroke, experts say the jury is still out.
A more definitive answer for cardiovascular safety of testosterone therapy will come from the TRAVERSE dedicated cardiovascular outcome trial, sponsored by AbbVie, which will have up to 5 years of follow-up, with results expected later this year.
The current meta-analysis by Jemma Hudson of Aberdeen (Scotland) University and colleagues was published online in The Lancet Healthy Longevity. The work will also be presented June 13 at ENDO 2022, the Endocrine Society’s annual meeting in Atlanta, Georgia, by senior author Channa Y. Jayasena, MD, PhD.
In 2014, the U.S. Food and Drug Administration mandated a label on testosterone products warning of possible increased cardiovascular risks and to reserve the therapy for symptomatic hypogonadism only. In contrast, the European Medicines Agency concluded that when hypogonadism is properly diagnosed and managed, there is currently no clear, consistent evidence that testosterone therapy causes increased cardiovascular risk.
To address this uncertainty, Dr. Hudson and colleagues formed a global collaborative to obtain individual patient data on cardiovascular outcomes from randomized controlled trials of testosterone therapy for men with hypogonadism.
They pooled data from 35 trials published from 1992 to Aug. 27, 2018, including 17 trials (3,431 patients) for which the researchers obtained patient-level data. The individual trials were 3-12 months long, except for one 3-year trial.
During a mean follow-up of 9.5 months, there was no significant increase in cardiovascular outcomes in men randomized to testosterone therapy versus placebo (odds ratio, 1.07; P = .62), nor were there any significantly increased risks of death, stroke, or different types of cardiovascular outcome, although those numbers were small.
This is “the most comprehensive study to date investigating the safety of testosterone treatment of hypogonadism,” according to the researchers. “The current results provide some reassurance about the short-term to medium-term safety of testosterone to treat male hypogonadism,” they conclude.
However, they also acknowledge that “long-term data are needed to fully evaluate the safety of testosterone.”
Erin D. Michos, MD, coauthor of an accompanying editorial, told this news organization, “This study doesn’t say to me that low testosterone necessarily needs to be treated. It’s still not indicated in people just for a low number [for blood testosterone] with less-severe symptoms. It really comes down to each individual person, how symptomatic they are, and their cardiovascular risk.”
‘Trial is not definitive’
Dr. Michos is not the only person to be skeptical. Together with Steven Nissen, MD, an investigator for the TRAVERSE trial, she agrees that this new evidence is not yet decisive, largely because the individual trials in the meta-analysis were short and not designed as cardiovascular outcome trials.
Dr. Nissen, a cardiologist at Cleveland Clinic, added that the individual trials were heterogeneous, with “very few real cardiovascular events,” so the meta-analysis “is not definitive,” he said in an interview.
While this meta-analysis “that pooled together a lot of smaller studies is reassuring that there’s no signal of harm, it’s really inconclusive because the follow-up was really short – a mean of only 9.5 months – and you really need a larger study with longer follow up to be more conclusive,” Dr. Michos noted.
“We should have more data soon” from TRAVERSE, said Dr. Michos, from the division of cardiology, Johns Hopkins University, Baltimore, who is not involved with that study.
Meanwhile, “I don’t think [this analysis] changes the current recommendations,” she said.
“We should continue to use caution as indicated by the FDA label and only use testosterone therapy selectively in people who have true symptoms of hypogonadism,” and be cautious about using it particularly in men at higher cardiovascular risk because of family history or known personal heart disease.
On the other hand, the meta-analysis did not show harm, she noted, “so we don’t necessarily need to pull patients off therapy if they are already taking it. But I wouldn’t right now just start new patients on it unless they had a strong indication.”
“Certainly, great caution is advised regarding the use of testosterone replacement therapy in people with established atherosclerosis due to the findings of plaque progression in the testosterone trials and the excess cardiovascular events observed in the TOM trial, write Dr. Michos and fellow editorialist Matthew J. Budoff, MD, of University of California, Los Angeles, in their editorial.
Earlier data inconclusive
Testosterone concentrations progressively decline in men with advancing age, at about 2% per year, Dr. Michos and Dr. Budoff write. In addition, men with obesity or with diabetes have low levels of testosterone, Dr. Michos noted.
Low testosterone blood levels have been associated with insulin resistance, inflammation, dyslipidemia, and atherosclerosis. Testosterone replacement therapy has been used to increase libido, improve erectile dysfunction, and boost energy levels, mood, and muscle strength.
But it is well known that testosterone increases hematocrit, which has the potential to increase the risk of venous thromboembolism.
Two large observational studies have reported increased risks of myocardial infarction, stroke, and death in men taking testosterone, compared with nonusers, but the study designs have been widely criticized, Dr. Hudson and coauthors say in their article.
A placebo-controlled trial was stopped early by its data- and safety-monitoring board following increased cardiovascular events in men aged 65 and older who received 6 months of testosterone. Other controlled trials have not observed these effects, but none was sufficiently powered.
Meta-analysis results
Dr. Hudson and colleagues performed a meta-analysis of 35 trials in 5,601 men aged 18 years and older with low baseline testosterone (≤ 350 nmol/dL) who had been randomized to testosterone replacement therapy or placebo for at least 3 months, for which there were data on mortality, stroke, and cardiovascular outcomes.
The men were a mean age of 65, had a mean body mass index of 30 kg/m2, and most (88%) were White. A quarter had angina, 8% had a previous myocardial infarction, and 27% had diabetes.
Cardiovascular and cerebrovascular outcomes were not primary outcomes.
During a mean follow-up of 9.5 months, in the 13 trials that provided this information, the rate of cardiovascular events was similar in the men who received testosterone (120/1,601, 7.5%) compared with those who received placebo (110/1,519, 7.2%).
In the 14 trials that provided this information, fewer deaths were reported during testosterone treatment (6/1,621, 0.4%) than during placebo treatment (12/1,537, 0.8%), but these numbers were too small to establish whether testosterone reduced mortality risk.
The most common cardiovascular events were arrhythmia, followed by coronary heart disease, heart failure, and myocardial infarction.
Patient age, baseline testosterone, smoking status, or diabetes status were not associated with cardiovascular risk.
The only detected adverse effects were edema and a modest lowering of HDL cholesterol.
“Men who develop sexual dysfunction, unexplained anemia, or osteoporosis should be tested for low testosterone,” senior author of the meta-analysis Dr. Jayasena said in an email to this news organization.
However, Dr. Jayasena added, “Mass screening for testosterone has no benefit in asymptomatic men.”
“Older men may still benefit from testosterone, but only if they have the clinical features [of hypogonadism] and low testosterone levels,” he concluded.
The current study is supported by the Health Technology Assessment program of the National Institute for Health Research. The TRAVERSE trial is sponsored by AbbVie. Dr. Jayasena has reported receiving research grants from LogixX Pharma. Dr. Hudson has reported no relevant financial relationships. Disclosures for the other authors are listed in the article. Dr. Michos has reported receiving support from the Amato Fund in Women’s Cardiovascular Health at Johns Hopkins School of Medicine and serving on medical advisory boards for Novartis, Esperion, Amarin, and AstraZeneca outside the submitted work. Dr. Budoff has reported receiving grant support from General Electric.
A version of this article first appeared on Medscape.com.
Despite a new meta-analysis claiming to show that testosterone replacement therapy for men with hypogonadism does not increase the risk of cardiovascular outcomes such as myocardial infarction or stroke, experts say the jury is still out.
A more definitive answer for cardiovascular safety of testosterone therapy will come from the TRAVERSE dedicated cardiovascular outcome trial, sponsored by AbbVie, which will have up to 5 years of follow-up, with results expected later this year.
The current meta-analysis by Jemma Hudson of Aberdeen (Scotland) University and colleagues was published online in The Lancet Healthy Longevity. The work will also be presented June 13 at ENDO 2022, the Endocrine Society’s annual meeting in Atlanta, Georgia, by senior author Channa Y. Jayasena, MD, PhD.
In 2014, the U.S. Food and Drug Administration mandated a label on testosterone products warning of possible increased cardiovascular risks and to reserve the therapy for symptomatic hypogonadism only. In contrast, the European Medicines Agency concluded that when hypogonadism is properly diagnosed and managed, there is currently no clear, consistent evidence that testosterone therapy causes increased cardiovascular risk.
To address this uncertainty, Dr. Hudson and colleagues formed a global collaborative to obtain individual patient data on cardiovascular outcomes from randomized controlled trials of testosterone therapy for men with hypogonadism.
They pooled data from 35 trials published from 1992 to Aug. 27, 2018, including 17 trials (3,431 patients) for which the researchers obtained patient-level data. The individual trials were 3-12 months long, except for one 3-year trial.
During a mean follow-up of 9.5 months, there was no significant increase in cardiovascular outcomes in men randomized to testosterone therapy versus placebo (odds ratio, 1.07; P = .62), nor were there any significantly increased risks of death, stroke, or different types of cardiovascular outcome, although those numbers were small.
This is “the most comprehensive study to date investigating the safety of testosterone treatment of hypogonadism,” according to the researchers. “The current results provide some reassurance about the short-term to medium-term safety of testosterone to treat male hypogonadism,” they conclude.
However, they also acknowledge that “long-term data are needed to fully evaluate the safety of testosterone.”
Erin D. Michos, MD, coauthor of an accompanying editorial, told this news organization, “This study doesn’t say to me that low testosterone necessarily needs to be treated. It’s still not indicated in people just for a low number [for blood testosterone] with less-severe symptoms. It really comes down to each individual person, how symptomatic they are, and their cardiovascular risk.”
‘Trial is not definitive’
Dr. Michos is not the only person to be skeptical. Together with Steven Nissen, MD, an investigator for the TRAVERSE trial, she agrees that this new evidence is not yet decisive, largely because the individual trials in the meta-analysis were short and not designed as cardiovascular outcome trials.
Dr. Nissen, a cardiologist at Cleveland Clinic, added that the individual trials were heterogeneous, with “very few real cardiovascular events,” so the meta-analysis “is not definitive,” he said in an interview.
While this meta-analysis “that pooled together a lot of smaller studies is reassuring that there’s no signal of harm, it’s really inconclusive because the follow-up was really short – a mean of only 9.5 months – and you really need a larger study with longer follow up to be more conclusive,” Dr. Michos noted.
“We should have more data soon” from TRAVERSE, said Dr. Michos, from the division of cardiology, Johns Hopkins University, Baltimore, who is not involved with that study.
Meanwhile, “I don’t think [this analysis] changes the current recommendations,” she said.
“We should continue to use caution as indicated by the FDA label and only use testosterone therapy selectively in people who have true symptoms of hypogonadism,” and be cautious about using it particularly in men at higher cardiovascular risk because of family history or known personal heart disease.
On the other hand, the meta-analysis did not show harm, she noted, “so we don’t necessarily need to pull patients off therapy if they are already taking it. But I wouldn’t right now just start new patients on it unless they had a strong indication.”
“Certainly, great caution is advised regarding the use of testosterone replacement therapy in people with established atherosclerosis due to the findings of plaque progression in the testosterone trials and the excess cardiovascular events observed in the TOM trial, write Dr. Michos and fellow editorialist Matthew J. Budoff, MD, of University of California, Los Angeles, in their editorial.
Earlier data inconclusive
Testosterone concentrations progressively decline in men with advancing age, at about 2% per year, Dr. Michos and Dr. Budoff write. In addition, men with obesity or with diabetes have low levels of testosterone, Dr. Michos noted.
Low testosterone blood levels have been associated with insulin resistance, inflammation, dyslipidemia, and atherosclerosis. Testosterone replacement therapy has been used to increase libido, improve erectile dysfunction, and boost energy levels, mood, and muscle strength.
But it is well known that testosterone increases hematocrit, which has the potential to increase the risk of venous thromboembolism.
Two large observational studies have reported increased risks of myocardial infarction, stroke, and death in men taking testosterone, compared with nonusers, but the study designs have been widely criticized, Dr. Hudson and coauthors say in their article.
A placebo-controlled trial was stopped early by its data- and safety-monitoring board following increased cardiovascular events in men aged 65 and older who received 6 months of testosterone. Other controlled trials have not observed these effects, but none was sufficiently powered.
Meta-analysis results
Dr. Hudson and colleagues performed a meta-analysis of 35 trials in 5,601 men aged 18 years and older with low baseline testosterone (≤ 350 nmol/dL) who had been randomized to testosterone replacement therapy or placebo for at least 3 months, for which there were data on mortality, stroke, and cardiovascular outcomes.
The men were a mean age of 65, had a mean body mass index of 30 kg/m2, and most (88%) were White. A quarter had angina, 8% had a previous myocardial infarction, and 27% had diabetes.
Cardiovascular and cerebrovascular outcomes were not primary outcomes.
During a mean follow-up of 9.5 months, in the 13 trials that provided this information, the rate of cardiovascular events was similar in the men who received testosterone (120/1,601, 7.5%) compared with those who received placebo (110/1,519, 7.2%).
In the 14 trials that provided this information, fewer deaths were reported during testosterone treatment (6/1,621, 0.4%) than during placebo treatment (12/1,537, 0.8%), but these numbers were too small to establish whether testosterone reduced mortality risk.
The most common cardiovascular events were arrhythmia, followed by coronary heart disease, heart failure, and myocardial infarction.
Patient age, baseline testosterone, smoking status, or diabetes status were not associated with cardiovascular risk.
The only detected adverse effects were edema and a modest lowering of HDL cholesterol.
“Men who develop sexual dysfunction, unexplained anemia, or osteoporosis should be tested for low testosterone,” senior author of the meta-analysis Dr. Jayasena said in an email to this news organization.
However, Dr. Jayasena added, “Mass screening for testosterone has no benefit in asymptomatic men.”
“Older men may still benefit from testosterone, but only if they have the clinical features [of hypogonadism] and low testosterone levels,” he concluded.
The current study is supported by the Health Technology Assessment program of the National Institute for Health Research. The TRAVERSE trial is sponsored by AbbVie. Dr. Jayasena has reported receiving research grants from LogixX Pharma. Dr. Hudson has reported no relevant financial relationships. Disclosures for the other authors are listed in the article. Dr. Michos has reported receiving support from the Amato Fund in Women’s Cardiovascular Health at Johns Hopkins School of Medicine and serving on medical advisory boards for Novartis, Esperion, Amarin, and AstraZeneca outside the submitted work. Dr. Budoff has reported receiving grant support from General Electric.
A version of this article first appeared on Medscape.com.
FROM THE LANCET HEALTHY LONGEVITY
When is the ideal time to try for a baby after bariatric surgery?
Doctors are advising women who have had bariatric surgery to wait at least 2 years before trying to conceive to reduce the risk of a small-for-gestational-age baby.
In fact, babies conceived less than 2 years post bariatric surgery are 15 times more likely to be small for gestational age as those conceived after this cut-off point, new study findings indicate.
Ana Carreira, MD, Coimbra Hospital and University Centre, Portugal, presented the findings as a poster at the European Congress on Obesity (ECO) 2022.
“The prevalence of small-for-gestational-age babies was similar across the different types of bariatric surgery, and we calculated that the cut-off for the bariatric-surgery-to-conception interval for a lower risk of small for gestational age babies was 24.5 months,” Dr. Carreira reported.
The study also found that for each additional month after the 2-year time point from bariatric surgery to conception, there was a 4.2-g (0.15-oz) increase in birth weight, and there was a 5% lower risk for a small-for-gestational-age neonate.
“Clinically, this is very significant,” she told this news organization.
“While it may be possible to slightly adjust this on an individual basis, it is important that women who are undergoing bariatric surgery are aware of the risk of early conception and of the benefits of delaying pregnancy,” she added.
Asked to comment, Kari Johansson, PhD, of the Karolinska Institute, Stockholm, who has worked in the field, said: “These increased risks have been hypothesized to potentially be attributed to the inadequate in utero availability of nutrients to the fetus, especially during the first year post bariatric surgery when the rapid and largest weight loss occurs. This is why many clinical guidelines recommend women wait 12-24 months until getting pregnant.”
Indeed, the American College of Obstetricians and Gynecologists recommends women wait 12-24 months post bariatric surgery before trying to conceive.
Dr. Johansson also noted, however, that there were no significant increased risks of adverse outcomes between pregnancies with a surgery-to-conception interval of 12 months or less versus over 12 months in a recent meta-analysis. But those authors also concluded that large cohorts with sufficient power are needed “before any definite conclusions can be made on the optimal surgery-to-conception interval,” she cautioned.
All types of bariatric surgery investigated
Bariatric surgery, which is increasingly popular in women of reproductive age, involves rapid weight loss, which can trigger improved fertility, Dr. Carreira explained. Currently, clinics generally advise women to wait at least 1 year before trying for a baby post-surgery.
Dr. Carreira and colleagues conducted the study because “the optimal bariatric-surgery-to-conception interval has yet to be determined,” and they wanted to examine the issue of small-for-gestational-age babies in particular, she noted. They also examined outcomes after a number of different bariatric procedures.
They retrospectively reviewed a cohort of 48 post surgery pregnancies (in 2008-2020) with a minimum follow-up of 30 weeks and determined the proportion of small-for-gestational-age neonates, defined as having a birth weight less than the 10th percentile according to National Center for Health Statistics growth charts.
Mean maternal age was 34.3 years, mean body mass index at conception was 30.9 kg/m2, and 70.8% had a bariatric-surgery-to-conception interval of over 24 months, 14.6% of 12-24 months, and 14.6% of less than 12 months.
Bariatric surgeries included adjustable gastric banding (22.9%), sleeve gastrectomy (35.4%), Roux-en-Y gastric bypass (37.5%), and biliopancreatic diversion (4.2%).
Overall, mean birth weight was 2.98 kg (6.6 lb) and the prevalence of small-for-gestational-age babies was 26.3%.
“For an interval of less than 24 months, around 60% of babies were small for gestational age,” Dr. Carreira noted.
Most babies who were small for gestational age were conceived at 18 months (median), and those who were not were conceived at 59 months (median).
And, after adjustment for maternal comorbidities, the odds ratio for a small-for-gestational-age neonate was 15.1 (95% confidence interval, 2.4-93.1) for a baby conceived less than 24 months after surgery.
“Some people think the interval can change according to the type of bariatric surgery, but we found no difference in findings according to [surgery] type,” added Dr. Carreira.
She pointed out that after discharge from their endocrinology clinic (after bariatric surgery), the women are cared for by their family doctor, “and we find that when they return to us in pregnancy their nutrient deficiencies have not been properly addressed. They need to be addressed at least 6 months prior to conception.”
“We recommend that women wait at least 2 years after bariatric surgery before trying to conceive, irrespective of the type of surgery,” she reiterated.
Dr. Carreira and Dr. Johansson have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Doctors are advising women who have had bariatric surgery to wait at least 2 years before trying to conceive to reduce the risk of a small-for-gestational-age baby.
In fact, babies conceived less than 2 years post bariatric surgery are 15 times more likely to be small for gestational age as those conceived after this cut-off point, new study findings indicate.
Ana Carreira, MD, Coimbra Hospital and University Centre, Portugal, presented the findings as a poster at the European Congress on Obesity (ECO) 2022.
“The prevalence of small-for-gestational-age babies was similar across the different types of bariatric surgery, and we calculated that the cut-off for the bariatric-surgery-to-conception interval for a lower risk of small for gestational age babies was 24.5 months,” Dr. Carreira reported.
The study also found that for each additional month after the 2-year time point from bariatric surgery to conception, there was a 4.2-g (0.15-oz) increase in birth weight, and there was a 5% lower risk for a small-for-gestational-age neonate.
“Clinically, this is very significant,” she told this news organization.
“While it may be possible to slightly adjust this on an individual basis, it is important that women who are undergoing bariatric surgery are aware of the risk of early conception and of the benefits of delaying pregnancy,” she added.
Asked to comment, Kari Johansson, PhD, of the Karolinska Institute, Stockholm, who has worked in the field, said: “These increased risks have been hypothesized to potentially be attributed to the inadequate in utero availability of nutrients to the fetus, especially during the first year post bariatric surgery when the rapid and largest weight loss occurs. This is why many clinical guidelines recommend women wait 12-24 months until getting pregnant.”
Indeed, the American College of Obstetricians and Gynecologists recommends women wait 12-24 months post bariatric surgery before trying to conceive.
Dr. Johansson also noted, however, that there were no significant increased risks of adverse outcomes between pregnancies with a surgery-to-conception interval of 12 months or less versus over 12 months in a recent meta-analysis. But those authors also concluded that large cohorts with sufficient power are needed “before any definite conclusions can be made on the optimal surgery-to-conception interval,” she cautioned.
All types of bariatric surgery investigated
Bariatric surgery, which is increasingly popular in women of reproductive age, involves rapid weight loss, which can trigger improved fertility, Dr. Carreira explained. Currently, clinics generally advise women to wait at least 1 year before trying for a baby post-surgery.
Dr. Carreira and colleagues conducted the study because “the optimal bariatric-surgery-to-conception interval has yet to be determined,” and they wanted to examine the issue of small-for-gestational-age babies in particular, she noted. They also examined outcomes after a number of different bariatric procedures.
They retrospectively reviewed a cohort of 48 post surgery pregnancies (in 2008-2020) with a minimum follow-up of 30 weeks and determined the proportion of small-for-gestational-age neonates, defined as having a birth weight less than the 10th percentile according to National Center for Health Statistics growth charts.
Mean maternal age was 34.3 years, mean body mass index at conception was 30.9 kg/m2, and 70.8% had a bariatric-surgery-to-conception interval of over 24 months, 14.6% of 12-24 months, and 14.6% of less than 12 months.
Bariatric surgeries included adjustable gastric banding (22.9%), sleeve gastrectomy (35.4%), Roux-en-Y gastric bypass (37.5%), and biliopancreatic diversion (4.2%).
Overall, mean birth weight was 2.98 kg (6.6 lb) and the prevalence of small-for-gestational-age babies was 26.3%.
“For an interval of less than 24 months, around 60% of babies were small for gestational age,” Dr. Carreira noted.
Most babies who were small for gestational age were conceived at 18 months (median), and those who were not were conceived at 59 months (median).
And, after adjustment for maternal comorbidities, the odds ratio for a small-for-gestational-age neonate was 15.1 (95% confidence interval, 2.4-93.1) for a baby conceived less than 24 months after surgery.
“Some people think the interval can change according to the type of bariatric surgery, but we found no difference in findings according to [surgery] type,” added Dr. Carreira.
She pointed out that after discharge from their endocrinology clinic (after bariatric surgery), the women are cared for by their family doctor, “and we find that when they return to us in pregnancy their nutrient deficiencies have not been properly addressed. They need to be addressed at least 6 months prior to conception.”
“We recommend that women wait at least 2 years after bariatric surgery before trying to conceive, irrespective of the type of surgery,” she reiterated.
Dr. Carreira and Dr. Johansson have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Doctors are advising women who have had bariatric surgery to wait at least 2 years before trying to conceive to reduce the risk of a small-for-gestational-age baby.
In fact, babies conceived less than 2 years post bariatric surgery are 15 times more likely to be small for gestational age as those conceived after this cut-off point, new study findings indicate.
Ana Carreira, MD, Coimbra Hospital and University Centre, Portugal, presented the findings as a poster at the European Congress on Obesity (ECO) 2022.
“The prevalence of small-for-gestational-age babies was similar across the different types of bariatric surgery, and we calculated that the cut-off for the bariatric-surgery-to-conception interval for a lower risk of small for gestational age babies was 24.5 months,” Dr. Carreira reported.
The study also found that for each additional month after the 2-year time point from bariatric surgery to conception, there was a 4.2-g (0.15-oz) increase in birth weight, and there was a 5% lower risk for a small-for-gestational-age neonate.
“Clinically, this is very significant,” she told this news organization.
“While it may be possible to slightly adjust this on an individual basis, it is important that women who are undergoing bariatric surgery are aware of the risk of early conception and of the benefits of delaying pregnancy,” she added.
Asked to comment, Kari Johansson, PhD, of the Karolinska Institute, Stockholm, who has worked in the field, said: “These increased risks have been hypothesized to potentially be attributed to the inadequate in utero availability of nutrients to the fetus, especially during the first year post bariatric surgery when the rapid and largest weight loss occurs. This is why many clinical guidelines recommend women wait 12-24 months until getting pregnant.”
Indeed, the American College of Obstetricians and Gynecologists recommends women wait 12-24 months post bariatric surgery before trying to conceive.
Dr. Johansson also noted, however, that there were no significant increased risks of adverse outcomes between pregnancies with a surgery-to-conception interval of 12 months or less versus over 12 months in a recent meta-analysis. But those authors also concluded that large cohorts with sufficient power are needed “before any definite conclusions can be made on the optimal surgery-to-conception interval,” she cautioned.
All types of bariatric surgery investigated
Bariatric surgery, which is increasingly popular in women of reproductive age, involves rapid weight loss, which can trigger improved fertility, Dr. Carreira explained. Currently, clinics generally advise women to wait at least 1 year before trying for a baby post-surgery.
Dr. Carreira and colleagues conducted the study because “the optimal bariatric-surgery-to-conception interval has yet to be determined,” and they wanted to examine the issue of small-for-gestational-age babies in particular, she noted. They also examined outcomes after a number of different bariatric procedures.
They retrospectively reviewed a cohort of 48 post surgery pregnancies (in 2008-2020) with a minimum follow-up of 30 weeks and determined the proportion of small-for-gestational-age neonates, defined as having a birth weight less than the 10th percentile according to National Center for Health Statistics growth charts.
Mean maternal age was 34.3 years, mean body mass index at conception was 30.9 kg/m2, and 70.8% had a bariatric-surgery-to-conception interval of over 24 months, 14.6% of 12-24 months, and 14.6% of less than 12 months.
Bariatric surgeries included adjustable gastric banding (22.9%), sleeve gastrectomy (35.4%), Roux-en-Y gastric bypass (37.5%), and biliopancreatic diversion (4.2%).
Overall, mean birth weight was 2.98 kg (6.6 lb) and the prevalence of small-for-gestational-age babies was 26.3%.
“For an interval of less than 24 months, around 60% of babies were small for gestational age,” Dr. Carreira noted.
Most babies who were small for gestational age were conceived at 18 months (median), and those who were not were conceived at 59 months (median).
And, after adjustment for maternal comorbidities, the odds ratio for a small-for-gestational-age neonate was 15.1 (95% confidence interval, 2.4-93.1) for a baby conceived less than 24 months after surgery.
“Some people think the interval can change according to the type of bariatric surgery, but we found no difference in findings according to [surgery] type,” added Dr. Carreira.
She pointed out that after discharge from their endocrinology clinic (after bariatric surgery), the women are cared for by their family doctor, “and we find that when they return to us in pregnancy their nutrient deficiencies have not been properly addressed. They need to be addressed at least 6 months prior to conception.”
“We recommend that women wait at least 2 years after bariatric surgery before trying to conceive, irrespective of the type of surgery,” she reiterated.
Dr. Carreira and Dr. Johansson have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Three-parent IVF now legal in two countries
: the United Kingdom and Australia.
Australia’s senate passed a bill on March 30 amending pre-existing laws to allow the procedure in certain circumstances.
The goal of this procedure is to prevent genetic disorders caused by defective mitochondria, the power plants inside our cells that provide energy for normal growth and development. When mitochondria don’t produce any energy at all, the resulting genetic disorders are quickly fatal. When mitochondria make only a little energy, children can have severe illnesses and disabilities.
“The outcomes from this problem are really severe, and it’s highly likely that the baby will be very sick or die,” says Arthur Caplan, PhD, head of the division of medical ethics at the New York University Grossman School of Medicine.
Mitochondria have a little bit of DNA, and children inherit them from their mother. To avoid children inheriting this damaged genetic material, mitochondrial donation, also known as three-parent in vitro fertilization (IVF), takes the nucleus, which contains most of the DNA that makes us who we are, from an egg of the mother and puts it into a donated egg from a woman with healthy mitochondria.
The egg is then fertilized with sperm through IVF, and the resulting embryo has genetic material from two women and one man.
One ethical conundrum about mitochondrial donation is that any child conceived this way would inherit modified DNA and pass that along to their own children.
“I think it’s likely that we are going to go down this road to repair disease,” Dr. Caplan says. “I don’t think all genetic engineering of embryos is wrong, but we have to draw the line between enhancement versus treating disease.”
For couples who want a child that shares at least some of their own DNA, there are other ways to have a child without damaged mitochondria. One option would be genetic screening of their embryos to find healthy embryos without this defect, which would work for some women who have relatively few mitochondrial mutations. Another alternative is using a donor egg from a woman with healthy mitochondria.
Mitochondrial donation may appeal to couples who want their children to have a genetic connection to both parents, Dr. Caplan says. But prospective parents also need to be aware that this procedure is relatively new and, unlike egg donation, doesn’t have a long track record of success.
“It looks promising, but we don’t have the full safety picture yet, and we’re not going to start to get it for another decade or so,” Dr. Caplan cautions. “I do think it’s worth offering as one option, but you also have to get people to think about how important it is to have a biological child together and make sure that they understand that even if we try this technique, we don’t know the long-term outcomes for children yet.”
A version of this article first appeared on WebMD.com.
: the United Kingdom and Australia.
Australia’s senate passed a bill on March 30 amending pre-existing laws to allow the procedure in certain circumstances.
The goal of this procedure is to prevent genetic disorders caused by defective mitochondria, the power plants inside our cells that provide energy for normal growth and development. When mitochondria don’t produce any energy at all, the resulting genetic disorders are quickly fatal. When mitochondria make only a little energy, children can have severe illnesses and disabilities.
“The outcomes from this problem are really severe, and it’s highly likely that the baby will be very sick or die,” says Arthur Caplan, PhD, head of the division of medical ethics at the New York University Grossman School of Medicine.
Mitochondria have a little bit of DNA, and children inherit them from their mother. To avoid children inheriting this damaged genetic material, mitochondrial donation, also known as three-parent in vitro fertilization (IVF), takes the nucleus, which contains most of the DNA that makes us who we are, from an egg of the mother and puts it into a donated egg from a woman with healthy mitochondria.
The egg is then fertilized with sperm through IVF, and the resulting embryo has genetic material from two women and one man.
One ethical conundrum about mitochondrial donation is that any child conceived this way would inherit modified DNA and pass that along to their own children.
“I think it’s likely that we are going to go down this road to repair disease,” Dr. Caplan says. “I don’t think all genetic engineering of embryos is wrong, but we have to draw the line between enhancement versus treating disease.”
For couples who want a child that shares at least some of their own DNA, there are other ways to have a child without damaged mitochondria. One option would be genetic screening of their embryos to find healthy embryos without this defect, which would work for some women who have relatively few mitochondrial mutations. Another alternative is using a donor egg from a woman with healthy mitochondria.
Mitochondrial donation may appeal to couples who want their children to have a genetic connection to both parents, Dr. Caplan says. But prospective parents also need to be aware that this procedure is relatively new and, unlike egg donation, doesn’t have a long track record of success.
“It looks promising, but we don’t have the full safety picture yet, and we’re not going to start to get it for another decade or so,” Dr. Caplan cautions. “I do think it’s worth offering as one option, but you also have to get people to think about how important it is to have a biological child together and make sure that they understand that even if we try this technique, we don’t know the long-term outcomes for children yet.”
A version of this article first appeared on WebMD.com.
: the United Kingdom and Australia.
Australia’s senate passed a bill on March 30 amending pre-existing laws to allow the procedure in certain circumstances.
The goal of this procedure is to prevent genetic disorders caused by defective mitochondria, the power plants inside our cells that provide energy for normal growth and development. When mitochondria don’t produce any energy at all, the resulting genetic disorders are quickly fatal. When mitochondria make only a little energy, children can have severe illnesses and disabilities.
“The outcomes from this problem are really severe, and it’s highly likely that the baby will be very sick or die,” says Arthur Caplan, PhD, head of the division of medical ethics at the New York University Grossman School of Medicine.
Mitochondria have a little bit of DNA, and children inherit them from their mother. To avoid children inheriting this damaged genetic material, mitochondrial donation, also known as three-parent in vitro fertilization (IVF), takes the nucleus, which contains most of the DNA that makes us who we are, from an egg of the mother and puts it into a donated egg from a woman with healthy mitochondria.
The egg is then fertilized with sperm through IVF, and the resulting embryo has genetic material from two women and one man.
One ethical conundrum about mitochondrial donation is that any child conceived this way would inherit modified DNA and pass that along to their own children.
“I think it’s likely that we are going to go down this road to repair disease,” Dr. Caplan says. “I don’t think all genetic engineering of embryos is wrong, but we have to draw the line between enhancement versus treating disease.”
For couples who want a child that shares at least some of their own DNA, there are other ways to have a child without damaged mitochondria. One option would be genetic screening of their embryos to find healthy embryos without this defect, which would work for some women who have relatively few mitochondrial mutations. Another alternative is using a donor egg from a woman with healthy mitochondria.
Mitochondrial donation may appeal to couples who want their children to have a genetic connection to both parents, Dr. Caplan says. But prospective parents also need to be aware that this procedure is relatively new and, unlike egg donation, doesn’t have a long track record of success.
“It looks promising, but we don’t have the full safety picture yet, and we’re not going to start to get it for another decade or so,” Dr. Caplan cautions. “I do think it’s worth offering as one option, but you also have to get people to think about how important it is to have a biological child together and make sure that they understand that even if we try this technique, we don’t know the long-term outcomes for children yet.”
A version of this article first appeared on WebMD.com.
Mediterranean diet linked to lower risk for preeclampsia
Pregnant women who had a higher adherence to a Mediterranean-style diet had a lower risk of preeclampsia, according to the results of a new study.
“As an observational study, it obviously has limitations that need to be considered, but these results build on other evidence that Mediterranean diet reduces cardiovascular risk and extends those findings to pregnancy as preeclampsia is a cardiovascular outcome,” senior author Noel T. Mueller, PhD, associate professor at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in an interview.
The study was published online April 20 in the Journal of the American Heart Association.
The authors noted that preeclampsia, characterized by a range of symptoms including hypertension, proteinuria, and end-organ dysfunction, is a disorder that occurs in up to 5%-10% of all pregnant women worldwide, and is more common in Black women. It is a major cause of maternal and fetal morbidity and raises the risk for long-term cardiovascular disease (CVD), including chronic hypertension, coronary artery disease, ischemic stroke, and heart failure.
Children born to mothers with preeclampsia are at an elevated risk of having higher blood pressure and other abnormal cardiometabolic parameters.
The authors noted that multiple studies have demonstrated the benefit of the Mediterranean diet – characterized primarily by high intake of vegetables, fruits, and unsaturated fats – in reducing cardiovascular risk in the nonpregnant population. The current study was conducted to investigate whether benefits could also be seen in pregnant women in the form of a reduced risk of preeclampsia.
For the study, which used data from the Boston Birth Cohort, maternal sociodemographic and dietary data were obtained from 8,507 women via interview and food frequency questionnaire within 24-72 hours of giving birth. A Mediterranean-style diet score was calculated from the food frequency questionnaire. Additional clinical information, including physician diagnoses of preexisting conditions and preeclampsia, were extracted from medical records.
Of the women in the sample, 848 developed preeclampsia, of whom 47% were Black, and 28% were Hispanic.
After multivariable adjustment, the greatest adherence to a Mediterranean-style diet was associated with lower odds of developing preeclampsia (adjusted odds ratio comparing tertile 3 to tertile 1, 0.78; 95% confidence interval [CI], 0.64-0.96).
A subgroup analysis of Black women demonstrated a similar benefit with an adjusted odds ratio comparing tertile 3 to tertile 1 of 0.74 (95% CI, 0.76-0.96).
“In this racially and ethnically diverse cohort, women who had greater adherence to a Mediterranean-style diet during pregnancy had a greater than 20% lower odds of developing preeclampsia, after [adjustment] for potential confounders. In addition, the evidence for the protective effect of a Mediterranean-style diet against the odds of developing preeclampsia remained present in a subgroup analysis of Black women,” the researchers concluded.
Asked whether this would be enough evidence to recommend a Mediterranean diet to pregnant women, Dr. Mueller said that the organizations that issue dietary guidelines would probably require replication of these results and also possibly a randomized trial in a diverse population group before advocating such a diet.
“That is something we would like to do but this will take time and money,” he added.
Lead study author Anum Minhas, MD, Johns Hopkins University, Baltimore, said that in the meantime she would be recommending a Mediterranean diet to her pregnant patients.
“The Mediterranean diet is a very healthy way of eating. I can’t see any downside of following such a diet in pregnancy, especially for high-risk women – those with obesity, hypertension or gestational diabetes, and there are likely other potential benefits such as reduced weight gain and reduced gestational diabetes,” she said.
Dr. Mueller said he appreciated this pragmatic approach. “Sometimes there can be hesitation on making recommendations from observational studies, but the alternative to recommending this diet is either no recommendations on diet or recommending an alternative diet,” he said. “The Mediterranean diet or the DASH diet, which is quite similar, have shown by far the most evidence of cardioprotection of any diets. They have been shown to reduce blood pressure and lipids and improve cardiovascular risk, and I think we can now assume that that likely extends to pregnancy. I feel comfortable for this diet to be recommended to pregnant women.”
But he added: “Having said that, there is still a need for a randomized trial in pregnancy. We think it works but until we have a randomized trial we won’t know for sure, and we won’t know how much of a benefit we can get.”
Commenting on the study, JoAnn Manson, MD, chief of the division of preventive medicine at Brigham and Women’s Hospital, Boston, pointed out that this type of observational study is important for hypothesis generation but cannot prove cause and effect relationships.
“The evidence is promising enough,” said Dr. Manson, who was not involved with this study. But she added that to move forward, a randomized trial in women at elevated risk of preeclampsia would be needed, beginning in early pregnancy, if not earlier.
“In the meantime,” she noted, “several large-scale cohorts could be leveraged to look at diet assessed before or during pregnancy to see if this dietary pattern is prospectively related to lower risk of preeclampsia.
“With additional supportive data, and in view of the diet’s safety and general cardiovascular benefits, it could become a major tool for preventing adverse pregnancy outcomes.”
The Boston Birth Cohort study was supported in part by grants from the March of Dimes, the National Institutes of Health, and the Health Resources and Services Administration of the U.S. Department of Health and Human Services. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women who had a higher adherence to a Mediterranean-style diet had a lower risk of preeclampsia, according to the results of a new study.
“As an observational study, it obviously has limitations that need to be considered, but these results build on other evidence that Mediterranean diet reduces cardiovascular risk and extends those findings to pregnancy as preeclampsia is a cardiovascular outcome,” senior author Noel T. Mueller, PhD, associate professor at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in an interview.
The study was published online April 20 in the Journal of the American Heart Association.
The authors noted that preeclampsia, characterized by a range of symptoms including hypertension, proteinuria, and end-organ dysfunction, is a disorder that occurs in up to 5%-10% of all pregnant women worldwide, and is more common in Black women. It is a major cause of maternal and fetal morbidity and raises the risk for long-term cardiovascular disease (CVD), including chronic hypertension, coronary artery disease, ischemic stroke, and heart failure.
Children born to mothers with preeclampsia are at an elevated risk of having higher blood pressure and other abnormal cardiometabolic parameters.
The authors noted that multiple studies have demonstrated the benefit of the Mediterranean diet – characterized primarily by high intake of vegetables, fruits, and unsaturated fats – in reducing cardiovascular risk in the nonpregnant population. The current study was conducted to investigate whether benefits could also be seen in pregnant women in the form of a reduced risk of preeclampsia.
For the study, which used data from the Boston Birth Cohort, maternal sociodemographic and dietary data were obtained from 8,507 women via interview and food frequency questionnaire within 24-72 hours of giving birth. A Mediterranean-style diet score was calculated from the food frequency questionnaire. Additional clinical information, including physician diagnoses of preexisting conditions and preeclampsia, were extracted from medical records.
Of the women in the sample, 848 developed preeclampsia, of whom 47% were Black, and 28% were Hispanic.
After multivariable adjustment, the greatest adherence to a Mediterranean-style diet was associated with lower odds of developing preeclampsia (adjusted odds ratio comparing tertile 3 to tertile 1, 0.78; 95% confidence interval [CI], 0.64-0.96).
A subgroup analysis of Black women demonstrated a similar benefit with an adjusted odds ratio comparing tertile 3 to tertile 1 of 0.74 (95% CI, 0.76-0.96).
“In this racially and ethnically diverse cohort, women who had greater adherence to a Mediterranean-style diet during pregnancy had a greater than 20% lower odds of developing preeclampsia, after [adjustment] for potential confounders. In addition, the evidence for the protective effect of a Mediterranean-style diet against the odds of developing preeclampsia remained present in a subgroup analysis of Black women,” the researchers concluded.
Asked whether this would be enough evidence to recommend a Mediterranean diet to pregnant women, Dr. Mueller said that the organizations that issue dietary guidelines would probably require replication of these results and also possibly a randomized trial in a diverse population group before advocating such a diet.
“That is something we would like to do but this will take time and money,” he added.
Lead study author Anum Minhas, MD, Johns Hopkins University, Baltimore, said that in the meantime she would be recommending a Mediterranean diet to her pregnant patients.
“The Mediterranean diet is a very healthy way of eating. I can’t see any downside of following such a diet in pregnancy, especially for high-risk women – those with obesity, hypertension or gestational diabetes, and there are likely other potential benefits such as reduced weight gain and reduced gestational diabetes,” she said.
Dr. Mueller said he appreciated this pragmatic approach. “Sometimes there can be hesitation on making recommendations from observational studies, but the alternative to recommending this diet is either no recommendations on diet or recommending an alternative diet,” he said. “The Mediterranean diet or the DASH diet, which is quite similar, have shown by far the most evidence of cardioprotection of any diets. They have been shown to reduce blood pressure and lipids and improve cardiovascular risk, and I think we can now assume that that likely extends to pregnancy. I feel comfortable for this diet to be recommended to pregnant women.”
But he added: “Having said that, there is still a need for a randomized trial in pregnancy. We think it works but until we have a randomized trial we won’t know for sure, and we won’t know how much of a benefit we can get.”
Commenting on the study, JoAnn Manson, MD, chief of the division of preventive medicine at Brigham and Women’s Hospital, Boston, pointed out that this type of observational study is important for hypothesis generation but cannot prove cause and effect relationships.
“The evidence is promising enough,” said Dr. Manson, who was not involved with this study. But she added that to move forward, a randomized trial in women at elevated risk of preeclampsia would be needed, beginning in early pregnancy, if not earlier.
“In the meantime,” she noted, “several large-scale cohorts could be leveraged to look at diet assessed before or during pregnancy to see if this dietary pattern is prospectively related to lower risk of preeclampsia.
“With additional supportive data, and in view of the diet’s safety and general cardiovascular benefits, it could become a major tool for preventing adverse pregnancy outcomes.”
The Boston Birth Cohort study was supported in part by grants from the March of Dimes, the National Institutes of Health, and the Health Resources and Services Administration of the U.S. Department of Health and Human Services. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pregnant women who had a higher adherence to a Mediterranean-style diet had a lower risk of preeclampsia, according to the results of a new study.
“As an observational study, it obviously has limitations that need to be considered, but these results build on other evidence that Mediterranean diet reduces cardiovascular risk and extends those findings to pregnancy as preeclampsia is a cardiovascular outcome,” senior author Noel T. Mueller, PhD, associate professor at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in an interview.
The study was published online April 20 in the Journal of the American Heart Association.
The authors noted that preeclampsia, characterized by a range of symptoms including hypertension, proteinuria, and end-organ dysfunction, is a disorder that occurs in up to 5%-10% of all pregnant women worldwide, and is more common in Black women. It is a major cause of maternal and fetal morbidity and raises the risk for long-term cardiovascular disease (CVD), including chronic hypertension, coronary artery disease, ischemic stroke, and heart failure.
Children born to mothers with preeclampsia are at an elevated risk of having higher blood pressure and other abnormal cardiometabolic parameters.
The authors noted that multiple studies have demonstrated the benefit of the Mediterranean diet – characterized primarily by high intake of vegetables, fruits, and unsaturated fats – in reducing cardiovascular risk in the nonpregnant population. The current study was conducted to investigate whether benefits could also be seen in pregnant women in the form of a reduced risk of preeclampsia.
For the study, which used data from the Boston Birth Cohort, maternal sociodemographic and dietary data were obtained from 8,507 women via interview and food frequency questionnaire within 24-72 hours of giving birth. A Mediterranean-style diet score was calculated from the food frequency questionnaire. Additional clinical information, including physician diagnoses of preexisting conditions and preeclampsia, were extracted from medical records.
Of the women in the sample, 848 developed preeclampsia, of whom 47% were Black, and 28% were Hispanic.
After multivariable adjustment, the greatest adherence to a Mediterranean-style diet was associated with lower odds of developing preeclampsia (adjusted odds ratio comparing tertile 3 to tertile 1, 0.78; 95% confidence interval [CI], 0.64-0.96).
A subgroup analysis of Black women demonstrated a similar benefit with an adjusted odds ratio comparing tertile 3 to tertile 1 of 0.74 (95% CI, 0.76-0.96).
“In this racially and ethnically diverse cohort, women who had greater adherence to a Mediterranean-style diet during pregnancy had a greater than 20% lower odds of developing preeclampsia, after [adjustment] for potential confounders. In addition, the evidence for the protective effect of a Mediterranean-style diet against the odds of developing preeclampsia remained present in a subgroup analysis of Black women,” the researchers concluded.
Asked whether this would be enough evidence to recommend a Mediterranean diet to pregnant women, Dr. Mueller said that the organizations that issue dietary guidelines would probably require replication of these results and also possibly a randomized trial in a diverse population group before advocating such a diet.
“That is something we would like to do but this will take time and money,” he added.
Lead study author Anum Minhas, MD, Johns Hopkins University, Baltimore, said that in the meantime she would be recommending a Mediterranean diet to her pregnant patients.
“The Mediterranean diet is a very healthy way of eating. I can’t see any downside of following such a diet in pregnancy, especially for high-risk women – those with obesity, hypertension or gestational diabetes, and there are likely other potential benefits such as reduced weight gain and reduced gestational diabetes,” she said.
Dr. Mueller said he appreciated this pragmatic approach. “Sometimes there can be hesitation on making recommendations from observational studies, but the alternative to recommending this diet is either no recommendations on diet or recommending an alternative diet,” he said. “The Mediterranean diet or the DASH diet, which is quite similar, have shown by far the most evidence of cardioprotection of any diets. They have been shown to reduce blood pressure and lipids and improve cardiovascular risk, and I think we can now assume that that likely extends to pregnancy. I feel comfortable for this diet to be recommended to pregnant women.”
But he added: “Having said that, there is still a need for a randomized trial in pregnancy. We think it works but until we have a randomized trial we won’t know for sure, and we won’t know how much of a benefit we can get.”
Commenting on the study, JoAnn Manson, MD, chief of the division of preventive medicine at Brigham and Women’s Hospital, Boston, pointed out that this type of observational study is important for hypothesis generation but cannot prove cause and effect relationships.
“The evidence is promising enough,” said Dr. Manson, who was not involved with this study. But she added that to move forward, a randomized trial in women at elevated risk of preeclampsia would be needed, beginning in early pregnancy, if not earlier.
“In the meantime,” she noted, “several large-scale cohorts could be leveraged to look at diet assessed before or during pregnancy to see if this dietary pattern is prospectively related to lower risk of preeclampsia.
“With additional supportive data, and in view of the diet’s safety and general cardiovascular benefits, it could become a major tool for preventing adverse pregnancy outcomes.”
The Boston Birth Cohort study was supported in part by grants from the March of Dimes, the National Institutes of Health, and the Health Resources and Services Administration of the U.S. Department of Health and Human Services. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN HEART ASSOCIATION
Hormones after cancer: Are they safe?
The impact of a gynecologic cancer diagnosis reaches beyond the obvious side effects of surgery, chemotherapy, and radiation. Many of our patients experience the quality-of-life–limiting side effects of abrupt hormone withdrawal as a consequence of our treatments. Assumptions are common, by both patients and providers, that hormonal therapy is unsafe after a gynecologic cancer diagnosis and that it is associated with an increased risk for recurrence. This sentiment likely originates from the fallout of the Womens’ Health Initiative (WHI) studies which showed an increased risk of breast cancer among users of combined estrogen and progesterone therapy.1 While this may be true for breast cancer risk, when initiated early, hormonal therapy is safe, even beneficial, for many patients with a history of gynecologic cancer, and can significantly improve their quality of life in addition to reducing all-cause mortality and incidence of osteoporosis, dementia, and cardiovascular disease.2
Premenopausal women undergoing surgery for endometrial cancer or preinvasive hyperplasia should be considered for ovarian preservation at the time of surgery. This strategy has been shown to be safe and not associated with an increased risk of recurrence. If oophorectomy is performed, hormonal therapy has been shown to be a safe remedy to the side effects of surgical menopause and the deleterious acceleration of bone loss and cardiovascular aging. The safety of hormone therapy for early-stage endometrial cancer has been thoroughly studied, including in a randomized controlled trial of more than 1,200 patients.3 This study showed no difference in the recurrence rate in users when compared with nonusers.
While hormone therapy is safe, from an oncologic standpoint, for women with a history of early-stage endometrial cancer other risks must also be considered. Given the association between endometrial cancer and obesity, these patients are at higher risk for venous thromboembolic (VTE) events, more so with the addition of exogenous hormone therapy. While not an overt contraindication to hormone prescription, obese patients who are prescribed these agents should be counseled regarding their risks for VTE.
The subgroup of patients with endometrial cancer in whom hormones should not be prescribed are those with advanced or recurrent disease. It is common for these tumors to express estrogen receptors, as evidenced by the responsiveness of these tumors to progesterone and antiestrogen treatments. Therefore, there is a theoretical risk for progression while using estrogen. In addition, as stated above, the risk of VTE is particularly elevated for women with metastatic malignancy receiving systemic therapies.
Cervical cancer commonly affects women of premenopausal age; therefore, early ovarian failure is particularly deleterious for this group of patients. Early-stage cervical cancer is most commonly treated with radical or extrafascial hysterectomy. Oophorectomy is not obligatory for the majority of these cases, and can be omitted in pre-, or perimenopausal patients to prevent surgical menopause. Ovarian metastases have been reported in cases of cervical adenocarcinoma, which led to the concern that ovarian preservation was not safe for this histology. However, recent data dispute this concern. A contemporary retrospective series of 105 patients with cervical adenocarcinoma identified no significant difference in overall survival when comparing those who had undergone ovarian preservation versus bilateral salpingo-oophorectomy.4
Ovarian preservation during cervical cancer surgery may not be enough to prevent early menopause. Approximately 20% of cervical cancer patients may require postoperative radiation for high- or intermediate-risk disease (such as positive lymph nodes, or adverse features in the tumor). For these women, ovarian ablation results, even if the ovaries were preserved at the time of surgery. Transposition of the ovaries to a location outside of the potential radiation fields is a strategy to mitigate this risk. To achieve this, the preserved ovaries and their vascular pedicles are skeletonized. The ovaries are then sutured to the paracolic gutter peritoneum or similar location above the pelvic brim, taking care to ensure that the vascular pedicle is not compromised or twisted. Placement of radio-opaque surgical clips on the caudad aspect of the transposed ovary aids in their identification by radiation oncologists when planning their treatment fields.
Ovarian transposition is most commonly used for women who are undergoing definitive surgery for cervical cancer. However, this strategy can also be used as a lead-in procedure for young women with advanced cervical cancer in whom definitive chemoradiation is planned. If the ovaries cannot be spared or moved out of “harm’s way” for premenopausal women undergoing treatment with definitive radiation, hormone therapy may be necessary and is safe for patients with cervical cancer, including those with adenocarcinoma. If the patient has not undergone hysterectomy, a regimen that includes a combination of estrogen and progesterone is necessary to avoid carcinogenic effects of unopposed estrogen on an intact endometrium, even after radiation has ablated those tissues.
When ovarian and fallopian cancers arise in premenopausal patients and appear confined to a single adnexa, contralateral ovarian preservation can be considered. However, for advanced disease, this is usually not possible or appropriate. Given that most ovarian cancers arise in a postmenopausal population, these patients may be preexisting users of hormone therapy. The data, including a randomized controlled trial, would suggest that it is safe to continue to use hormone therapy during or following a diagnosis of ovarian, fallopian tube, or primary peritoneal cancer and that it is not associated with worse outcomes from their cancer.5
Once again, patients should be carefully counseled about the additive risks for VTE that come from metastatic ovarian cancer, surgery via laparotomy, and exogenous hormonal therapy. However, these patients need not be subjected to an abrupt transition to menopause, because level I evidence suggests that these therapies are not associated with worse oncologic outcomes. All patients with ovarian, fallopian tube, and primary peritoneal cancer should receive genetic testing, and if deleterious mutations are found in BRCA 1 or 2 genes indicating an elevated risk for breast cancer, decision making regarding continued exogenous hormonal therapy is complicated. The most contemporary data, including long-term follow-up from the Women’s Health Initiative clinical trials, do not suggest an increased risk for breast cancer with estrogen-only preparations of hormone therapy.6 Given that most women with gynecologic cancers have undergone hysterectomy as part of their treatment, these estrogen-only preparations are appropriate for most.
For patients with rare tumors, such as endometrial stromal tumors or uterine leiomyosarcoma, the safety of exogenous hormone therapy should be dictated by the receptor profile of their particular cancer. Many of these cancers express estrogen receptors; therefore, current guidelines recommend against the use of hormones after these diagnoses when estrogen receptors are expressed.
Gynecologic cancer treatments induce many toxicities with long-term deleterious effects on quality of life. Use of hormones to mitigate the symptoms of menopause is an important tool in the toolkit for gynecologists. Assumptions should not be made that hormonal therapies are always unsafe for all of these patients. It is important to closely evaluate the patient’s tumor and other risk factors before withholding potentially valuable therapies.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Chlebowski R et al. JAMA. 2010 Oct 20;304(15):1684-92.
2. Sinno AK et al. Gynecol Oncol. 2020;157(2):303-6.
3. Barakat et al. J Clin Oncol. 2006;24(4):587-92.
4. Hu Jun et al. J Obstet Gynaecol. 2017 Nov;37(8):1065-9.
5. Eeles R et al. J Clin Oncol. 2015 Dec 10;33(35):4138-44.
6. Chlebowski R et al. JAMA Jul 28 2020;324(4):369-80.
The impact of a gynecologic cancer diagnosis reaches beyond the obvious side effects of surgery, chemotherapy, and radiation. Many of our patients experience the quality-of-life–limiting side effects of abrupt hormone withdrawal as a consequence of our treatments. Assumptions are common, by both patients and providers, that hormonal therapy is unsafe after a gynecologic cancer diagnosis and that it is associated with an increased risk for recurrence. This sentiment likely originates from the fallout of the Womens’ Health Initiative (WHI) studies which showed an increased risk of breast cancer among users of combined estrogen and progesterone therapy.1 While this may be true for breast cancer risk, when initiated early, hormonal therapy is safe, even beneficial, for many patients with a history of gynecologic cancer, and can significantly improve their quality of life in addition to reducing all-cause mortality and incidence of osteoporosis, dementia, and cardiovascular disease.2
Premenopausal women undergoing surgery for endometrial cancer or preinvasive hyperplasia should be considered for ovarian preservation at the time of surgery. This strategy has been shown to be safe and not associated with an increased risk of recurrence. If oophorectomy is performed, hormonal therapy has been shown to be a safe remedy to the side effects of surgical menopause and the deleterious acceleration of bone loss and cardiovascular aging. The safety of hormone therapy for early-stage endometrial cancer has been thoroughly studied, including in a randomized controlled trial of more than 1,200 patients.3 This study showed no difference in the recurrence rate in users when compared with nonusers.
While hormone therapy is safe, from an oncologic standpoint, for women with a history of early-stage endometrial cancer other risks must also be considered. Given the association between endometrial cancer and obesity, these patients are at higher risk for venous thromboembolic (VTE) events, more so with the addition of exogenous hormone therapy. While not an overt contraindication to hormone prescription, obese patients who are prescribed these agents should be counseled regarding their risks for VTE.
The subgroup of patients with endometrial cancer in whom hormones should not be prescribed are those with advanced or recurrent disease. It is common for these tumors to express estrogen receptors, as evidenced by the responsiveness of these tumors to progesterone and antiestrogen treatments. Therefore, there is a theoretical risk for progression while using estrogen. In addition, as stated above, the risk of VTE is particularly elevated for women with metastatic malignancy receiving systemic therapies.
Cervical cancer commonly affects women of premenopausal age; therefore, early ovarian failure is particularly deleterious for this group of patients. Early-stage cervical cancer is most commonly treated with radical or extrafascial hysterectomy. Oophorectomy is not obligatory for the majority of these cases, and can be omitted in pre-, or perimenopausal patients to prevent surgical menopause. Ovarian metastases have been reported in cases of cervical adenocarcinoma, which led to the concern that ovarian preservation was not safe for this histology. However, recent data dispute this concern. A contemporary retrospective series of 105 patients with cervical adenocarcinoma identified no significant difference in overall survival when comparing those who had undergone ovarian preservation versus bilateral salpingo-oophorectomy.4
Ovarian preservation during cervical cancer surgery may not be enough to prevent early menopause. Approximately 20% of cervical cancer patients may require postoperative radiation for high- or intermediate-risk disease (such as positive lymph nodes, or adverse features in the tumor). For these women, ovarian ablation results, even if the ovaries were preserved at the time of surgery. Transposition of the ovaries to a location outside of the potential radiation fields is a strategy to mitigate this risk. To achieve this, the preserved ovaries and their vascular pedicles are skeletonized. The ovaries are then sutured to the paracolic gutter peritoneum or similar location above the pelvic brim, taking care to ensure that the vascular pedicle is not compromised or twisted. Placement of radio-opaque surgical clips on the caudad aspect of the transposed ovary aids in their identification by radiation oncologists when planning their treatment fields.
Ovarian transposition is most commonly used for women who are undergoing definitive surgery for cervical cancer. However, this strategy can also be used as a lead-in procedure for young women with advanced cervical cancer in whom definitive chemoradiation is planned. If the ovaries cannot be spared or moved out of “harm’s way” for premenopausal women undergoing treatment with definitive radiation, hormone therapy may be necessary and is safe for patients with cervical cancer, including those with adenocarcinoma. If the patient has not undergone hysterectomy, a regimen that includes a combination of estrogen and progesterone is necessary to avoid carcinogenic effects of unopposed estrogen on an intact endometrium, even after radiation has ablated those tissues.
When ovarian and fallopian cancers arise in premenopausal patients and appear confined to a single adnexa, contralateral ovarian preservation can be considered. However, for advanced disease, this is usually not possible or appropriate. Given that most ovarian cancers arise in a postmenopausal population, these patients may be preexisting users of hormone therapy. The data, including a randomized controlled trial, would suggest that it is safe to continue to use hormone therapy during or following a diagnosis of ovarian, fallopian tube, or primary peritoneal cancer and that it is not associated with worse outcomes from their cancer.5
Once again, patients should be carefully counseled about the additive risks for VTE that come from metastatic ovarian cancer, surgery via laparotomy, and exogenous hormonal therapy. However, these patients need not be subjected to an abrupt transition to menopause, because level I evidence suggests that these therapies are not associated with worse oncologic outcomes. All patients with ovarian, fallopian tube, and primary peritoneal cancer should receive genetic testing, and if deleterious mutations are found in BRCA 1 or 2 genes indicating an elevated risk for breast cancer, decision making regarding continued exogenous hormonal therapy is complicated. The most contemporary data, including long-term follow-up from the Women’s Health Initiative clinical trials, do not suggest an increased risk for breast cancer with estrogen-only preparations of hormone therapy.6 Given that most women with gynecologic cancers have undergone hysterectomy as part of their treatment, these estrogen-only preparations are appropriate for most.
For patients with rare tumors, such as endometrial stromal tumors or uterine leiomyosarcoma, the safety of exogenous hormone therapy should be dictated by the receptor profile of their particular cancer. Many of these cancers express estrogen receptors; therefore, current guidelines recommend against the use of hormones after these diagnoses when estrogen receptors are expressed.
Gynecologic cancer treatments induce many toxicities with long-term deleterious effects on quality of life. Use of hormones to mitigate the symptoms of menopause is an important tool in the toolkit for gynecologists. Assumptions should not be made that hormonal therapies are always unsafe for all of these patients. It is important to closely evaluate the patient’s tumor and other risk factors before withholding potentially valuable therapies.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Chlebowski R et al. JAMA. 2010 Oct 20;304(15):1684-92.
2. Sinno AK et al. Gynecol Oncol. 2020;157(2):303-6.
3. Barakat et al. J Clin Oncol. 2006;24(4):587-92.
4. Hu Jun et al. J Obstet Gynaecol. 2017 Nov;37(8):1065-9.
5. Eeles R et al. J Clin Oncol. 2015 Dec 10;33(35):4138-44.
6. Chlebowski R et al. JAMA Jul 28 2020;324(4):369-80.
The impact of a gynecologic cancer diagnosis reaches beyond the obvious side effects of surgery, chemotherapy, and radiation. Many of our patients experience the quality-of-life–limiting side effects of abrupt hormone withdrawal as a consequence of our treatments. Assumptions are common, by both patients and providers, that hormonal therapy is unsafe after a gynecologic cancer diagnosis and that it is associated with an increased risk for recurrence. This sentiment likely originates from the fallout of the Womens’ Health Initiative (WHI) studies which showed an increased risk of breast cancer among users of combined estrogen and progesterone therapy.1 While this may be true for breast cancer risk, when initiated early, hormonal therapy is safe, even beneficial, for many patients with a history of gynecologic cancer, and can significantly improve their quality of life in addition to reducing all-cause mortality and incidence of osteoporosis, dementia, and cardiovascular disease.2
Premenopausal women undergoing surgery for endometrial cancer or preinvasive hyperplasia should be considered for ovarian preservation at the time of surgery. This strategy has been shown to be safe and not associated with an increased risk of recurrence. If oophorectomy is performed, hormonal therapy has been shown to be a safe remedy to the side effects of surgical menopause and the deleterious acceleration of bone loss and cardiovascular aging. The safety of hormone therapy for early-stage endometrial cancer has been thoroughly studied, including in a randomized controlled trial of more than 1,200 patients.3 This study showed no difference in the recurrence rate in users when compared with nonusers.
While hormone therapy is safe, from an oncologic standpoint, for women with a history of early-stage endometrial cancer other risks must also be considered. Given the association between endometrial cancer and obesity, these patients are at higher risk for venous thromboembolic (VTE) events, more so with the addition of exogenous hormone therapy. While not an overt contraindication to hormone prescription, obese patients who are prescribed these agents should be counseled regarding their risks for VTE.
The subgroup of patients with endometrial cancer in whom hormones should not be prescribed are those with advanced or recurrent disease. It is common for these tumors to express estrogen receptors, as evidenced by the responsiveness of these tumors to progesterone and antiestrogen treatments. Therefore, there is a theoretical risk for progression while using estrogen. In addition, as stated above, the risk of VTE is particularly elevated for women with metastatic malignancy receiving systemic therapies.
Cervical cancer commonly affects women of premenopausal age; therefore, early ovarian failure is particularly deleterious for this group of patients. Early-stage cervical cancer is most commonly treated with radical or extrafascial hysterectomy. Oophorectomy is not obligatory for the majority of these cases, and can be omitted in pre-, or perimenopausal patients to prevent surgical menopause. Ovarian metastases have been reported in cases of cervical adenocarcinoma, which led to the concern that ovarian preservation was not safe for this histology. However, recent data dispute this concern. A contemporary retrospective series of 105 patients with cervical adenocarcinoma identified no significant difference in overall survival when comparing those who had undergone ovarian preservation versus bilateral salpingo-oophorectomy.4
Ovarian preservation during cervical cancer surgery may not be enough to prevent early menopause. Approximately 20% of cervical cancer patients may require postoperative radiation for high- or intermediate-risk disease (such as positive lymph nodes, or adverse features in the tumor). For these women, ovarian ablation results, even if the ovaries were preserved at the time of surgery. Transposition of the ovaries to a location outside of the potential radiation fields is a strategy to mitigate this risk. To achieve this, the preserved ovaries and their vascular pedicles are skeletonized. The ovaries are then sutured to the paracolic gutter peritoneum or similar location above the pelvic brim, taking care to ensure that the vascular pedicle is not compromised or twisted. Placement of radio-opaque surgical clips on the caudad aspect of the transposed ovary aids in their identification by radiation oncologists when planning their treatment fields.
Ovarian transposition is most commonly used for women who are undergoing definitive surgery for cervical cancer. However, this strategy can also be used as a lead-in procedure for young women with advanced cervical cancer in whom definitive chemoradiation is planned. If the ovaries cannot be spared or moved out of “harm’s way” for premenopausal women undergoing treatment with definitive radiation, hormone therapy may be necessary and is safe for patients with cervical cancer, including those with adenocarcinoma. If the patient has not undergone hysterectomy, a regimen that includes a combination of estrogen and progesterone is necessary to avoid carcinogenic effects of unopposed estrogen on an intact endometrium, even after radiation has ablated those tissues.
When ovarian and fallopian cancers arise in premenopausal patients and appear confined to a single adnexa, contralateral ovarian preservation can be considered. However, for advanced disease, this is usually not possible or appropriate. Given that most ovarian cancers arise in a postmenopausal population, these patients may be preexisting users of hormone therapy. The data, including a randomized controlled trial, would suggest that it is safe to continue to use hormone therapy during or following a diagnosis of ovarian, fallopian tube, or primary peritoneal cancer and that it is not associated with worse outcomes from their cancer.5
Once again, patients should be carefully counseled about the additive risks for VTE that come from metastatic ovarian cancer, surgery via laparotomy, and exogenous hormonal therapy. However, these patients need not be subjected to an abrupt transition to menopause, because level I evidence suggests that these therapies are not associated with worse oncologic outcomes. All patients with ovarian, fallopian tube, and primary peritoneal cancer should receive genetic testing, and if deleterious mutations are found in BRCA 1 or 2 genes indicating an elevated risk for breast cancer, decision making regarding continued exogenous hormonal therapy is complicated. The most contemporary data, including long-term follow-up from the Women’s Health Initiative clinical trials, do not suggest an increased risk for breast cancer with estrogen-only preparations of hormone therapy.6 Given that most women with gynecologic cancers have undergone hysterectomy as part of their treatment, these estrogen-only preparations are appropriate for most.
For patients with rare tumors, such as endometrial stromal tumors or uterine leiomyosarcoma, the safety of exogenous hormone therapy should be dictated by the receptor profile of their particular cancer. Many of these cancers express estrogen receptors; therefore, current guidelines recommend against the use of hormones after these diagnoses when estrogen receptors are expressed.
Gynecologic cancer treatments induce many toxicities with long-term deleterious effects on quality of life. Use of hormones to mitigate the symptoms of menopause is an important tool in the toolkit for gynecologists. Assumptions should not be made that hormonal therapies are always unsafe for all of these patients. It is important to closely evaluate the patient’s tumor and other risk factors before withholding potentially valuable therapies.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She has no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Chlebowski R et al. JAMA. 2010 Oct 20;304(15):1684-92.
2. Sinno AK et al. Gynecol Oncol. 2020;157(2):303-6.
3. Barakat et al. J Clin Oncol. 2006;24(4):587-92.
4. Hu Jun et al. J Obstet Gynaecol. 2017 Nov;37(8):1065-9.
5. Eeles R et al. J Clin Oncol. 2015 Dec 10;33(35):4138-44.
6. Chlebowski R et al. JAMA Jul 28 2020;324(4):369-80.
Unraveling primary ovarian insufficiency
In the presentation of secondary amenorrhea, pregnancy is the No. 1 differential diagnosis. Once this has been excluded, an algorithm is initiated to determine the etiology, including an assessment of the hypothalamic-pituitary-ovarian axis. While the early onset of ovarian failure can be physically and psychologically disrupting, the effect on fertility is an especially devastating event. Previously identified by terms including premature ovarian failure and premature menopause, “primary ovarian insufficiency” (POI) is now the preferred designation. This month’s article will address the diagnosis, evaluation, and management of POI.
The definition of POI is the development of primary hypogonadism before the age of 40 years. Spontaneous POI occurs in approximately 1 in 250 women by age 35 years and 1 in 100 by age 40 years. After excluding pregnancy, the clinician should determine signs and symptoms that can lead to expedited and cost-efficient testing.
Consequences
POI is an important risk factor for bone loss and osteoporosis, especially in young women who develop ovarian dysfunction before they achieve peak adult bone mass. At the time of diagnosis of POI, a bone density test (dual-energy x-ray absorptiometry) should be obtained. Women with POI may also develop depression and anxiety as well as experience an increased risk for cardiovascular morbidity and mortality, possibly related to endothelial dysfunction.
Young women with spontaneous POI are at increased risk of developing autoimmune adrenal insufficiency (AAI), a potentially fatal disorder. Consequently, to diagnose AAI, serum adrenal cortical and 21-hydroxylase antibodies should be measured in all women who have a karyotype of 46,XX and experience spontaneous POI. Women with AAI have a 50% risk of developing adrenal insufficiency. Despite initial normal adrenal function, women with positive adrenal cortical antibodies should be followed annually.
Causes (see table for a more complete list)
Iatrogenic
Known causes of POI include chemotherapy/radiation often in the setting of cancer treatment. The three most commonly used drugs, cyclophosphamide, cisplatin, and doxorubicin, cause POI by inducing death and/or accelerated activation of primordial follicles and increased atresia of growing follicles. The most damaging agents are alkylating drugs. A cyclophosphamide equivalent dose calculator has been established for ovarian failure risk stratification from chemotherapy based on the cumulative dose of alkylating agents received.
One study estimated the radiosensitivity of the oocyte to be less than 2 Gy. Based upon this estimate, the authors calculated the dose of radiotherapy that would result in immediate and permanent ovarian failure in 97.5% of patients as follows:
- 20.3 Gy at birth
- 18.4 Gy at age 10 years
- 16.5 Gy at age 20 years
- 14.3 Gy at age 30 years
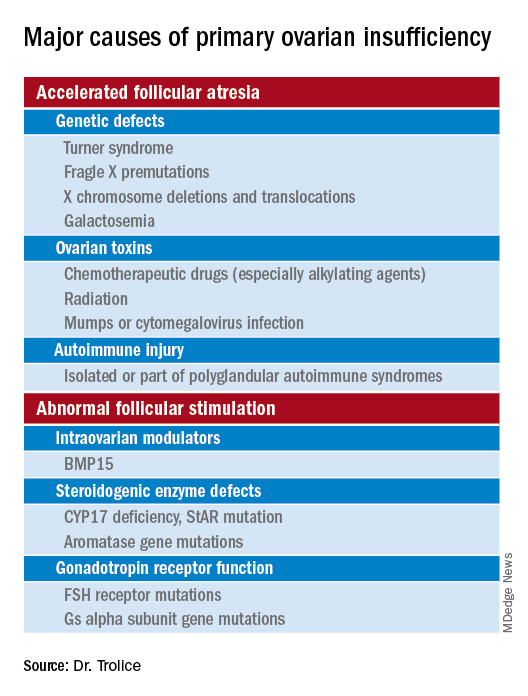
Genetic
Approximately 10% of cases are familial. A family history of POI raises concern for a fragile X premutation. Fragile X syndrome is an X-linked form of intellectual disability that is one of the most common causes of mental retardation worldwide. There is a strong relationship between age at menopause, including POI, and premutations for fragile X syndrome. The American College of Obstetricians and Gynecologists recommends that women with POI or an elevated follicle-stimulating hormone (FSH) level before age 40 years without known cause be screened for FMR1 premutations. Approximately 6% of cases of POI are associated with premutations in the FMR1 gene.
Turner syndrome is one of the most common causes of POI and results from the lack of a second X chromosome. The most common chromosomal defect in humans, TS occurs in up to 1.5% of conceptions, 10% of spontaneous abortions, and 1 of 2,500 live births.
Serum antiadrenal and/or anti–21-hydroxylase antibodies and antithyroid antiperoxidase antibodies, can aid in the diagnosis of adrenal gland, ovary, and thyroid autoimmune causes, which is found in 4% of women with spontaneous POI. Testing for the presence of 21-hydroxylase autoantibodies or adrenal autoantibodies is sufficient to make the diagnosis of autoimmune oophoritis in women with proven spontaneous POI.
The etiology of POI remains unknown in approximately 75%-90% of cases. However, studies using whole exome or whole genome sequencing have identified genetic variants in approximately 30%-35% of these patients.
Risk factors
Factors that are thought to play a role in determining the age of menopause, include genetics (e.g., FMR1 premutation and mosaic Turner syndrome), ethnicity (earlier among Hispanic women and later in Japanese American women when compared with White women), and smoking (reduced by approximately 2 years ).
Regarding ovarian aging, the holy grail of the reproductive life span is to predict menopause. While the definitive age eludes us, anti-Müllerian hormone levels appear to show promise. An ultrasensitive anti-Müllerian hormone assay (< 0.01 ng/mL) predicted a 79% probability of menopause within 12 months for women aged 51 and above; the probability was 51% for women below age 48.
Diagnosis
The three P’s of secondary amenorrhea are physiological, pharmacological, or pathological and can guide the clinician to a targeted evaluation. Physiological causes are pregnancy, the first 6 months of continuous breastfeeding (from elevated prolactin), and natural menopause. Pharmacological etiologies, excluding hormonal treatment that suppresses ovulation (combined oral contraceptives, gonadotropin-releasing hormone agonist/antagonist, or danazol), include agents that inhibit dopamine thereby increasing serum prolactin, such as metoclopramide; phenothiazine antipsychotics, such as haloperidol; and tardive dystonia dopamine-depleting medications, such as reserpine. Pathological causes include pituitary adenomas, thyroid disease, functional hypothalamic amenorrhea from changes in weight, exercise regimen, and stress.
Management
About 50%-75% of women with 46,XX spontaneous POI experience intermittent ovarian function and 5%-10% of women remain able to conceive. Anecdotally, a 32-year-old woman presented to me with primary infertility, secondary amenorrhea, and suspected POI based on vasomotor symptoms and elevated FSH levels. Pelvic ultrasound showed a hemorrhagic cyst, suspicious for a corpus luteum. Two weeks thereafter she reported a positive home urine human chorionic gonadotropin test and ultimately delivered twins. Her diagnosis of POI with amenorrhea remained postpartum.
Unless there is an absolute contraindication, estrogen therapy should be prescribed to women with POI to reduce the risk of osteoporosis, cardiovascular disease, and urogenital atrophy as well as to maintain sexual health and quality of life. For those with an intact uterus, women should receive progesterone because of the risk of endometrial hyperplasia from unopposed estrogen. Rather than oral estrogen, the use of transdermal or vaginal delivery of estrogen is a more physiological approach and provides lower risks of venous thromboembolism and gallbladder disease. Of note, standard postmenopausal hormone therapy, which has a much lower dose of estrogen than combined estrogen-progestin contraceptives, does not provide effective contraception. Per ACOG, systemic hormone treatment should be prescribed until age 50-51 years to all women with POI.
For fertility, women with spontaneous POI can be offered oocyte or embryo donation. The uterus does not age reproductively, unlike oocytes, therefore women can achieve reasonable pregnancy success rates through egg donation despite experiencing menopause.
Future potential options
Female germline stem cells have been isolated from neonatal mice and transplanted into sterile adult mice, who then were able to produce offspring. In a second study, oogonial stem cells were isolated from neonatal and adult mouse ovaries; pups were subsequently born from the oocytes. Further experiments are needed before the implications for humans can be determined.
Emotionally traumatic for most women, POI disrupts life plans, hopes, and dreams of raising a family. The approach to the patient with POI involves the above evidence-based testing along with empathy from the health care provider.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
In the presentation of secondary amenorrhea, pregnancy is the No. 1 differential diagnosis. Once this has been excluded, an algorithm is initiated to determine the etiology, including an assessment of the hypothalamic-pituitary-ovarian axis. While the early onset of ovarian failure can be physically and psychologically disrupting, the effect on fertility is an especially devastating event. Previously identified by terms including premature ovarian failure and premature menopause, “primary ovarian insufficiency” (POI) is now the preferred designation. This month’s article will address the diagnosis, evaluation, and management of POI.
The definition of POI is the development of primary hypogonadism before the age of 40 years. Spontaneous POI occurs in approximately 1 in 250 women by age 35 years and 1 in 100 by age 40 years. After excluding pregnancy, the clinician should determine signs and symptoms that can lead to expedited and cost-efficient testing.
Consequences
POI is an important risk factor for bone loss and osteoporosis, especially in young women who develop ovarian dysfunction before they achieve peak adult bone mass. At the time of diagnosis of POI, a bone density test (dual-energy x-ray absorptiometry) should be obtained. Women with POI may also develop depression and anxiety as well as experience an increased risk for cardiovascular morbidity and mortality, possibly related to endothelial dysfunction.
Young women with spontaneous POI are at increased risk of developing autoimmune adrenal insufficiency (AAI), a potentially fatal disorder. Consequently, to diagnose AAI, serum adrenal cortical and 21-hydroxylase antibodies should be measured in all women who have a karyotype of 46,XX and experience spontaneous POI. Women with AAI have a 50% risk of developing adrenal insufficiency. Despite initial normal adrenal function, women with positive adrenal cortical antibodies should be followed annually.
Causes (see table for a more complete list)
Iatrogenic
Known causes of POI include chemotherapy/radiation often in the setting of cancer treatment. The three most commonly used drugs, cyclophosphamide, cisplatin, and doxorubicin, cause POI by inducing death and/or accelerated activation of primordial follicles and increased atresia of growing follicles. The most damaging agents are alkylating drugs. A cyclophosphamide equivalent dose calculator has been established for ovarian failure risk stratification from chemotherapy based on the cumulative dose of alkylating agents received.
One study estimated the radiosensitivity of the oocyte to be less than 2 Gy. Based upon this estimate, the authors calculated the dose of radiotherapy that would result in immediate and permanent ovarian failure in 97.5% of patients as follows:
- 20.3 Gy at birth
- 18.4 Gy at age 10 years
- 16.5 Gy at age 20 years
- 14.3 Gy at age 30 years

Genetic
Approximately 10% of cases are familial. A family history of POI raises concern for a fragile X premutation. Fragile X syndrome is an X-linked form of intellectual disability that is one of the most common causes of mental retardation worldwide. There is a strong relationship between age at menopause, including POI, and premutations for fragile X syndrome. The American College of Obstetricians and Gynecologists recommends that women with POI or an elevated follicle-stimulating hormone (FSH) level before age 40 years without known cause be screened for FMR1 premutations. Approximately 6% of cases of POI are associated with premutations in the FMR1 gene.
Turner syndrome is one of the most common causes of POI and results from the lack of a second X chromosome. The most common chromosomal defect in humans, TS occurs in up to 1.5% of conceptions, 10% of spontaneous abortions, and 1 of 2,500 live births.
Serum antiadrenal and/or anti–21-hydroxylase antibodies and antithyroid antiperoxidase antibodies, can aid in the diagnosis of adrenal gland, ovary, and thyroid autoimmune causes, which is found in 4% of women with spontaneous POI. Testing for the presence of 21-hydroxylase autoantibodies or adrenal autoantibodies is sufficient to make the diagnosis of autoimmune oophoritis in women with proven spontaneous POI.
The etiology of POI remains unknown in approximately 75%-90% of cases. However, studies using whole exome or whole genome sequencing have identified genetic variants in approximately 30%-35% of these patients.
Risk factors
Factors that are thought to play a role in determining the age of menopause, include genetics (e.g., FMR1 premutation and mosaic Turner syndrome), ethnicity (earlier among Hispanic women and later in Japanese American women when compared with White women), and smoking (reduced by approximately 2 years ).
Regarding ovarian aging, the holy grail of the reproductive life span is to predict menopause. While the definitive age eludes us, anti-Müllerian hormone levels appear to show promise. An ultrasensitive anti-Müllerian hormone assay (< 0.01 ng/mL) predicted a 79% probability of menopause within 12 months for women aged 51 and above; the probability was 51% for women below age 48.
Diagnosis
The three P’s of secondary amenorrhea are physiological, pharmacological, or pathological and can guide the clinician to a targeted evaluation. Physiological causes are pregnancy, the first 6 months of continuous breastfeeding (from elevated prolactin), and natural menopause. Pharmacological etiologies, excluding hormonal treatment that suppresses ovulation (combined oral contraceptives, gonadotropin-releasing hormone agonist/antagonist, or danazol), include agents that inhibit dopamine thereby increasing serum prolactin, such as metoclopramide; phenothiazine antipsychotics, such as haloperidol; and tardive dystonia dopamine-depleting medications, such as reserpine. Pathological causes include pituitary adenomas, thyroid disease, functional hypothalamic amenorrhea from changes in weight, exercise regimen, and stress.
Management
About 50%-75% of women with 46,XX spontaneous POI experience intermittent ovarian function and 5%-10% of women remain able to conceive. Anecdotally, a 32-year-old woman presented to me with primary infertility, secondary amenorrhea, and suspected POI based on vasomotor symptoms and elevated FSH levels. Pelvic ultrasound showed a hemorrhagic cyst, suspicious for a corpus luteum. Two weeks thereafter she reported a positive home urine human chorionic gonadotropin test and ultimately delivered twins. Her diagnosis of POI with amenorrhea remained postpartum.
Unless there is an absolute contraindication, estrogen therapy should be prescribed to women with POI to reduce the risk of osteoporosis, cardiovascular disease, and urogenital atrophy as well as to maintain sexual health and quality of life. For those with an intact uterus, women should receive progesterone because of the risk of endometrial hyperplasia from unopposed estrogen. Rather than oral estrogen, the use of transdermal or vaginal delivery of estrogen is a more physiological approach and provides lower risks of venous thromboembolism and gallbladder disease. Of note, standard postmenopausal hormone therapy, which has a much lower dose of estrogen than combined estrogen-progestin contraceptives, does not provide effective contraception. Per ACOG, systemic hormone treatment should be prescribed until age 50-51 years to all women with POI.
For fertility, women with spontaneous POI can be offered oocyte or embryo donation. The uterus does not age reproductively, unlike oocytes, therefore women can achieve reasonable pregnancy success rates through egg donation despite experiencing menopause.
Future potential options
Female germline stem cells have been isolated from neonatal mice and transplanted into sterile adult mice, who then were able to produce offspring. In a second study, oogonial stem cells were isolated from neonatal and adult mouse ovaries; pups were subsequently born from the oocytes. Further experiments are needed before the implications for humans can be determined.
Emotionally traumatic for most women, POI disrupts life plans, hopes, and dreams of raising a family. The approach to the patient with POI involves the above evidence-based testing along with empathy from the health care provider.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
In the presentation of secondary amenorrhea, pregnancy is the No. 1 differential diagnosis. Once this has been excluded, an algorithm is initiated to determine the etiology, including an assessment of the hypothalamic-pituitary-ovarian axis. While the early onset of ovarian failure can be physically and psychologically disrupting, the effect on fertility is an especially devastating event. Previously identified by terms including premature ovarian failure and premature menopause, “primary ovarian insufficiency” (POI) is now the preferred designation. This month’s article will address the diagnosis, evaluation, and management of POI.
The definition of POI is the development of primary hypogonadism before the age of 40 years. Spontaneous POI occurs in approximately 1 in 250 women by age 35 years and 1 in 100 by age 40 years. After excluding pregnancy, the clinician should determine signs and symptoms that can lead to expedited and cost-efficient testing.
Consequences
POI is an important risk factor for bone loss and osteoporosis, especially in young women who develop ovarian dysfunction before they achieve peak adult bone mass. At the time of diagnosis of POI, a bone density test (dual-energy x-ray absorptiometry) should be obtained. Women with POI may also develop depression and anxiety as well as experience an increased risk for cardiovascular morbidity and mortality, possibly related to endothelial dysfunction.
Young women with spontaneous POI are at increased risk of developing autoimmune adrenal insufficiency (AAI), a potentially fatal disorder. Consequently, to diagnose AAI, serum adrenal cortical and 21-hydroxylase antibodies should be measured in all women who have a karyotype of 46,XX and experience spontaneous POI. Women with AAI have a 50% risk of developing adrenal insufficiency. Despite initial normal adrenal function, women with positive adrenal cortical antibodies should be followed annually.
Causes (see table for a more complete list)
Iatrogenic
Known causes of POI include chemotherapy/radiation often in the setting of cancer treatment. The three most commonly used drugs, cyclophosphamide, cisplatin, and doxorubicin, cause POI by inducing death and/or accelerated activation of primordial follicles and increased atresia of growing follicles. The most damaging agents are alkylating drugs. A cyclophosphamide equivalent dose calculator has been established for ovarian failure risk stratification from chemotherapy based on the cumulative dose of alkylating agents received.
One study estimated the radiosensitivity of the oocyte to be less than 2 Gy. Based upon this estimate, the authors calculated the dose of radiotherapy that would result in immediate and permanent ovarian failure in 97.5% of patients as follows:
- 20.3 Gy at birth
- 18.4 Gy at age 10 years
- 16.5 Gy at age 20 years
- 14.3 Gy at age 30 years

Genetic
Approximately 10% of cases are familial. A family history of POI raises concern for a fragile X premutation. Fragile X syndrome is an X-linked form of intellectual disability that is one of the most common causes of mental retardation worldwide. There is a strong relationship between age at menopause, including POI, and premutations for fragile X syndrome. The American College of Obstetricians and Gynecologists recommends that women with POI or an elevated follicle-stimulating hormone (FSH) level before age 40 years without known cause be screened for FMR1 premutations. Approximately 6% of cases of POI are associated with premutations in the FMR1 gene.
Turner syndrome is one of the most common causes of POI and results from the lack of a second X chromosome. The most common chromosomal defect in humans, TS occurs in up to 1.5% of conceptions, 10% of spontaneous abortions, and 1 of 2,500 live births.
Serum antiadrenal and/or anti–21-hydroxylase antibodies and antithyroid antiperoxidase antibodies, can aid in the diagnosis of adrenal gland, ovary, and thyroid autoimmune causes, which is found in 4% of women with spontaneous POI. Testing for the presence of 21-hydroxylase autoantibodies or adrenal autoantibodies is sufficient to make the diagnosis of autoimmune oophoritis in women with proven spontaneous POI.
The etiology of POI remains unknown in approximately 75%-90% of cases. However, studies using whole exome or whole genome sequencing have identified genetic variants in approximately 30%-35% of these patients.
Risk factors
Factors that are thought to play a role in determining the age of menopause, include genetics (e.g., FMR1 premutation and mosaic Turner syndrome), ethnicity (earlier among Hispanic women and later in Japanese American women when compared with White women), and smoking (reduced by approximately 2 years ).
Regarding ovarian aging, the holy grail of the reproductive life span is to predict menopause. While the definitive age eludes us, anti-Müllerian hormone levels appear to show promise. An ultrasensitive anti-Müllerian hormone assay (< 0.01 ng/mL) predicted a 79% probability of menopause within 12 months for women aged 51 and above; the probability was 51% for women below age 48.
Diagnosis
The three P’s of secondary amenorrhea are physiological, pharmacological, or pathological and can guide the clinician to a targeted evaluation. Physiological causes are pregnancy, the first 6 months of continuous breastfeeding (from elevated prolactin), and natural menopause. Pharmacological etiologies, excluding hormonal treatment that suppresses ovulation (combined oral contraceptives, gonadotropin-releasing hormone agonist/antagonist, or danazol), include agents that inhibit dopamine thereby increasing serum prolactin, such as metoclopramide; phenothiazine antipsychotics, such as haloperidol; and tardive dystonia dopamine-depleting medications, such as reserpine. Pathological causes include pituitary adenomas, thyroid disease, functional hypothalamic amenorrhea from changes in weight, exercise regimen, and stress.
Management
About 50%-75% of women with 46,XX spontaneous POI experience intermittent ovarian function and 5%-10% of women remain able to conceive. Anecdotally, a 32-year-old woman presented to me with primary infertility, secondary amenorrhea, and suspected POI based on vasomotor symptoms and elevated FSH levels. Pelvic ultrasound showed a hemorrhagic cyst, suspicious for a corpus luteum. Two weeks thereafter she reported a positive home urine human chorionic gonadotropin test and ultimately delivered twins. Her diagnosis of POI with amenorrhea remained postpartum.
Unless there is an absolute contraindication, estrogen therapy should be prescribed to women with POI to reduce the risk of osteoporosis, cardiovascular disease, and urogenital atrophy as well as to maintain sexual health and quality of life. For those with an intact uterus, women should receive progesterone because of the risk of endometrial hyperplasia from unopposed estrogen. Rather than oral estrogen, the use of transdermal or vaginal delivery of estrogen is a more physiological approach and provides lower risks of venous thromboembolism and gallbladder disease. Of note, standard postmenopausal hormone therapy, which has a much lower dose of estrogen than combined estrogen-progestin contraceptives, does not provide effective contraception. Per ACOG, systemic hormone treatment should be prescribed until age 50-51 years to all women with POI.
For fertility, women with spontaneous POI can be offered oocyte or embryo donation. The uterus does not age reproductively, unlike oocytes, therefore women can achieve reasonable pregnancy success rates through egg donation despite experiencing menopause.
Future potential options
Female germline stem cells have been isolated from neonatal mice and transplanted into sterile adult mice, who then were able to produce offspring. In a second study, oogonial stem cells were isolated from neonatal and adult mouse ovaries; pups were subsequently born from the oocytes. Further experiments are needed before the implications for humans can be determined.
Emotionally traumatic for most women, POI disrupts life plans, hopes, and dreams of raising a family. The approach to the patient with POI involves the above evidence-based testing along with empathy from the health care provider.
Dr. Trolice is director of The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
Racial and ethnic disparities persist in pregnant women with gestational diabetes
Between 2014 and 2020, the frequency of adverse pregnancy outcomes in the United States increased among women with gestational diabetes, with persisting differences in adverse outcomes by race and ethnicity, according to a report in JAMA
“[Previous] population-based studies on racial and ethnic disparities in gestational diabetes have focused on differences in the rate of diagnosis, rather than adverse pregnancy outcomes,” lead author Kartik K. Venkatesh, MD, PhD, of Ohio State University, Columbus, and colleagues explained.
The researchers conducted a cross-sectional, descriptive study to evaluate whether the frequency of adverse pregnancy outcomes with gestational diabetes changed over time and whether the risk of these outcomes differed by maternal race and ethnicity.
The data were obtained from the Centers for Disease Control and Prevention’s National Center for Health Statistics Natality Files. Exposures of interest were year of delivery, as well as race and ethnicity.
Results
The study cohort included 1,560,822 pregnant women with gestational diabetes aged 15-44 years. Among the study participants the mean age was 31 years (standard deviation, 5.5 years) and the majority were White (48%), followed by Hispanic/Latina (27%), Asian/Pacific Islander (13%), and Black (12%).
There was a significant increase in the overall frequency of transfusion (8.0%; 95% confidence interval, 3.8%-12.4%), preeclampsia or gestational hypertension (4.2%; 95% CI, 3.3%-5.2%), NICU admission (1.0%; 95% CI, 0.3%-1.7%), and preterm birth at less than 37 weeks (0.9%; 95% CI, 0.3%-1.5%) from 2014 to 2020 for these women and their infants.
In addition, there was a significant decrease in the following outcomes: macrosomia (–4.7%; 95% CI, –5.3% to –4.0%), cesarean delivery (–1.4%; 95% CI, –1.7% to –1.1%), primary cesarean delivery (–1.2%; 95% CI, –1.5% to –0.9%), and large for gestational age (–2.3%; 95% CI, –2.8% to –1.8%), but there was no significant differences in maternal ICU admission and small-for-gestational-age infants.
From 2014 through 2020, differences in adverse outcomes by race and ethnicity persisted; in comparison with Whites, Black participants were at significantly higher risk of all evaluated outcomes, except for macrosomia and large for gestational age.
Hispanic/Latina and Asian/Pacific Islander individuals were also at significantly higher risk of preterm birth, NICU admission, maternal ICU admission, and small for gestational age. Furthermore, American Indian participants were at significantly higher risk of all evaluated outcomes, except for cesarean delivery and small for gestational age.
Results in context
Health policy researcher Felicia Hill-Briggs, PhD, at the Feinstein Institutes for Medical Research in Manhasset, N.Y. commented: “Two alarming trends highlighted by this study: 1) Racial and ethnic inequities in adverse gestational diabetes outcomes; and 2) the rising rates of gestational diabetes overall – both must and can be halted.”
“Optimizing medical management of gestational diabetes, whether through improved access to diabetes care in pregnancy, behavioral interventions, and pharmacotherapy can decrease the risk of adverse pregnancy outcomes,” Dr. Venkatesh commented. “It is possible that the equitable delivery of these interventions to address glycemic control could decrease racial and ethnic disparities in adverse pregnancy outcomes among individuals with gestational diabetes.”
Dr. Venkatesh and his colleagues acknowledged that a key limitation of the study was the use of administrative data; thus, inferences on maternal care improvements could not be determined.
“Further research could focus on greater understanding of racial and ethnic differences in the management of gestational diabetes,” the researchers concluded.
This study was supported by the Care Innovation and Community Improvement Program at Ohio State University. One author reported receiving grants from the National Institutes of Health outside of this study. The other authors reported no relevant disclosures. Dr. Hill-Briggs had no relevant disclosures.
Between 2014 and 2020, the frequency of adverse pregnancy outcomes in the United States increased among women with gestational diabetes, with persisting differences in adverse outcomes by race and ethnicity, according to a report in JAMA
“[Previous] population-based studies on racial and ethnic disparities in gestational diabetes have focused on differences in the rate of diagnosis, rather than adverse pregnancy outcomes,” lead author Kartik K. Venkatesh, MD, PhD, of Ohio State University, Columbus, and colleagues explained.
The researchers conducted a cross-sectional, descriptive study to evaluate whether the frequency of adverse pregnancy outcomes with gestational diabetes changed over time and whether the risk of these outcomes differed by maternal race and ethnicity.
The data were obtained from the Centers for Disease Control and Prevention’s National Center for Health Statistics Natality Files. Exposures of interest were year of delivery, as well as race and ethnicity.
Results
The study cohort included 1,560,822 pregnant women with gestational diabetes aged 15-44 years. Among the study participants the mean age was 31 years (standard deviation, 5.5 years) and the majority were White (48%), followed by Hispanic/Latina (27%), Asian/Pacific Islander (13%), and Black (12%).
There was a significant increase in the overall frequency of transfusion (8.0%; 95% confidence interval, 3.8%-12.4%), preeclampsia or gestational hypertension (4.2%; 95% CI, 3.3%-5.2%), NICU admission (1.0%; 95% CI, 0.3%-1.7%), and preterm birth at less than 37 weeks (0.9%; 95% CI, 0.3%-1.5%) from 2014 to 2020 for these women and their infants.
In addition, there was a significant decrease in the following outcomes: macrosomia (–4.7%; 95% CI, –5.3% to –4.0%), cesarean delivery (–1.4%; 95% CI, –1.7% to –1.1%), primary cesarean delivery (–1.2%; 95% CI, –1.5% to –0.9%), and large for gestational age (–2.3%; 95% CI, –2.8% to –1.8%), but there was no significant differences in maternal ICU admission and small-for-gestational-age infants.
From 2014 through 2020, differences in adverse outcomes by race and ethnicity persisted; in comparison with Whites, Black participants were at significantly higher risk of all evaluated outcomes, except for macrosomia and large for gestational age.
Hispanic/Latina and Asian/Pacific Islander individuals were also at significantly higher risk of preterm birth, NICU admission, maternal ICU admission, and small for gestational age. Furthermore, American Indian participants were at significantly higher risk of all evaluated outcomes, except for cesarean delivery and small for gestational age.
Results in context
Health policy researcher Felicia Hill-Briggs, PhD, at the Feinstein Institutes for Medical Research in Manhasset, N.Y. commented: “Two alarming trends highlighted by this study: 1) Racial and ethnic inequities in adverse gestational diabetes outcomes; and 2) the rising rates of gestational diabetes overall – both must and can be halted.”
“Optimizing medical management of gestational diabetes, whether through improved access to diabetes care in pregnancy, behavioral interventions, and pharmacotherapy can decrease the risk of adverse pregnancy outcomes,” Dr. Venkatesh commented. “It is possible that the equitable delivery of these interventions to address glycemic control could decrease racial and ethnic disparities in adverse pregnancy outcomes among individuals with gestational diabetes.”
Dr. Venkatesh and his colleagues acknowledged that a key limitation of the study was the use of administrative data; thus, inferences on maternal care improvements could not be determined.
“Further research could focus on greater understanding of racial and ethnic differences in the management of gestational diabetes,” the researchers concluded.
This study was supported by the Care Innovation and Community Improvement Program at Ohio State University. One author reported receiving grants from the National Institutes of Health outside of this study. The other authors reported no relevant disclosures. Dr. Hill-Briggs had no relevant disclosures.
Between 2014 and 2020, the frequency of adverse pregnancy outcomes in the United States increased among women with gestational diabetes, with persisting differences in adverse outcomes by race and ethnicity, according to a report in JAMA
“[Previous] population-based studies on racial and ethnic disparities in gestational diabetes have focused on differences in the rate of diagnosis, rather than adverse pregnancy outcomes,” lead author Kartik K. Venkatesh, MD, PhD, of Ohio State University, Columbus, and colleagues explained.
The researchers conducted a cross-sectional, descriptive study to evaluate whether the frequency of adverse pregnancy outcomes with gestational diabetes changed over time and whether the risk of these outcomes differed by maternal race and ethnicity.
The data were obtained from the Centers for Disease Control and Prevention’s National Center for Health Statistics Natality Files. Exposures of interest were year of delivery, as well as race and ethnicity.
Results
The study cohort included 1,560,822 pregnant women with gestational diabetes aged 15-44 years. Among the study participants the mean age was 31 years (standard deviation, 5.5 years) and the majority were White (48%), followed by Hispanic/Latina (27%), Asian/Pacific Islander (13%), and Black (12%).
There was a significant increase in the overall frequency of transfusion (8.0%; 95% confidence interval, 3.8%-12.4%), preeclampsia or gestational hypertension (4.2%; 95% CI, 3.3%-5.2%), NICU admission (1.0%; 95% CI, 0.3%-1.7%), and preterm birth at less than 37 weeks (0.9%; 95% CI, 0.3%-1.5%) from 2014 to 2020 for these women and their infants.
In addition, there was a significant decrease in the following outcomes: macrosomia (–4.7%; 95% CI, –5.3% to –4.0%), cesarean delivery (–1.4%; 95% CI, –1.7% to –1.1%), primary cesarean delivery (–1.2%; 95% CI, –1.5% to –0.9%), and large for gestational age (–2.3%; 95% CI, –2.8% to –1.8%), but there was no significant differences in maternal ICU admission and small-for-gestational-age infants.
From 2014 through 2020, differences in adverse outcomes by race and ethnicity persisted; in comparison with Whites, Black participants were at significantly higher risk of all evaluated outcomes, except for macrosomia and large for gestational age.
Hispanic/Latina and Asian/Pacific Islander individuals were also at significantly higher risk of preterm birth, NICU admission, maternal ICU admission, and small for gestational age. Furthermore, American Indian participants were at significantly higher risk of all evaluated outcomes, except for cesarean delivery and small for gestational age.
Results in context
Health policy researcher Felicia Hill-Briggs, PhD, at the Feinstein Institutes for Medical Research in Manhasset, N.Y. commented: “Two alarming trends highlighted by this study: 1) Racial and ethnic inequities in adverse gestational diabetes outcomes; and 2) the rising rates of gestational diabetes overall – both must and can be halted.”
“Optimizing medical management of gestational diabetes, whether through improved access to diabetes care in pregnancy, behavioral interventions, and pharmacotherapy can decrease the risk of adverse pregnancy outcomes,” Dr. Venkatesh commented. “It is possible that the equitable delivery of these interventions to address glycemic control could decrease racial and ethnic disparities in adverse pregnancy outcomes among individuals with gestational diabetes.”
Dr. Venkatesh and his colleagues acknowledged that a key limitation of the study was the use of administrative data; thus, inferences on maternal care improvements could not be determined.
“Further research could focus on greater understanding of racial and ethnic differences in the management of gestational diabetes,” the researchers concluded.
This study was supported by the Care Innovation and Community Improvement Program at Ohio State University. One author reported receiving grants from the National Institutes of Health outside of this study. The other authors reported no relevant disclosures. Dr. Hill-Briggs had no relevant disclosures.
FROM JAMA
First comprehensive guidelines for managing anorexia in pregnancy
The first comprehensive guidelines to manage pregnant women with anorexia nervosa (AN) have been released.
Pregnant women with AN are at greater risk of poor outcomes, including stillbirth, underweight infant, or pre-term birth, yet there are no clear guidelines on the management of the condition.
“Anorexia in pregnancy has been an overlooked area of clinical care, as many believed only women in remission become pregnant, and it is clear that is not the case,” lead author Megan Galbally, MBBS, PhD, professor and director, Centre of Women’s and Children’s Mental Health at Monash University School of Clinical Sciences, Melbourne, told this news organization.
“There are great opportunities to support women in their mental health and give them and their babies a healthier start to parenthood and life,” said Dr. Galbally.
“For instance, reducing the likelihood of prematurity or low birth weight at birth that can be associated with anorexia in pregnancy has extraordinary benefits for that child for lifelong health and well-being,” she added.
The guidelines were published online in Lancet Psychiatry.
Spike in cases
Dr. Galbally noted that during her 20 years of working in perinatal mental health within tertiary maternity services, she only ever saw an occasional pregnant woman with current AN.
In contrast, over the last 3 to 4 years, there has been a “steep increase in women presenting in pregnancy with very low body mass index (BMI) and current anorexia nervosa requiring treatment in pregnancy,” Dr. Galbally said.
Despite the complexity of managing AN in pregnancy, few studies are available to guide care. In a systematic literature review, the researchers identified only eight studies that addressed the management of AN in pregnancy. These studies were case studies or case reports examining narrow aspects of management.
Digging deeper, the researchers conducted a state-of-the-art research review in relevant disciplines and areas of expertise for managing anorexia nervosa in pregnancy. They synthesized their findings into “recommendations and principles” for multidisciplinary care of pregnant women with AN.
The researchers note that AN in pregnancy is associated with increased risks of pregnancy complications and poorer outcomes for infants, and measures such as BMI are less accurate in pregnancy for assessing severity or change in anorexia nervosa.
Anorexia affects pregnancy and neonatal outcomes through low calorie intake, nutritional and vitamin deficiencies, stress, fasting, low body mass, and poor placentation and uteroplacental function.
The authors note that managing AN in pregnancy requires multidisciplinary care that considers the substantial physiological changes for women and requirements for monitoring fetal growth and development.
At a minimum, they recommend monitoring the following:
- Sodium, potassium, magnesium, phosphate, and chloride concentration
- Iron status, vitamin D and bone mineral density, blood sugar concentration (fasting or random), and A1c
- Liver function (including bilirubin, aspartate transaminase, alanine aminotransferase, and gamma-glutamyl transferase) and bone marrow function (including full blood examination, white cell count, neutrophil count, platelets, and hemoglobin)
- Inflammatory markers (C-reactive protein and erythrocyte sedimentation rate)
- Cardiac function (electrocardiogram and echocardiogram)
- Blood pressure and heart rate (lying and standing) and body temperature
“There are considerable risks for women and their unborn child in managing moderate to severe AN in pregnancy,” said Dr. Galbally.
“While we have provided some recommendations, it still requires considerable adaptation to individual presentations and circumstances, and this is best done with a maternity service that manages other high-risk pregnancies such as through maternal-fetal medicine teams,” she said.
“While this area of clinical care can be new to high-risk pregnancy teams, it is clearly important that high-risk pregnancy services and mental health work together to improve care for women with anorexia in pregnancy,” Dr. Galbally added.
A nightmare, a dream come true
Reached for comment, Kamryn T. Eddy, PhD, co-director, Eating Disorders Clinical and Research Program, Massachusetts General Hospital, said, “for many with anorexia nervosa, pregnancy realizes their greatest nightmare and dream come true, both at once.”
“The physical demands of pregnancy can be taxing, and for those with anorexia nervosa, closer clinical management makes sense and may help to support patients who are at risk for return to or worsening of symptoms with the increased nutritional needs and weight gain that occur in pregnancy,” Dr. Eddy, associate professor, department of psychiatry, Harvard Medical School, Boston, told this news organization.
“At the same time, the desire to have a child can be a strong motivator for patients to make the changes needed to recover, and for some, the transition to mother can also help in recovery by broadening the range of things that influence their self-worth,” Dr. Eddy added.
This research had no specific funding. Dr. Galbally and Dr. Eddy report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The first comprehensive guidelines to manage pregnant women with anorexia nervosa (AN) have been released.
Pregnant women with AN are at greater risk of poor outcomes, including stillbirth, underweight infant, or pre-term birth, yet there are no clear guidelines on the management of the condition.
“Anorexia in pregnancy has been an overlooked area of clinical care, as many believed only women in remission become pregnant, and it is clear that is not the case,” lead author Megan Galbally, MBBS, PhD, professor and director, Centre of Women’s and Children’s Mental Health at Monash University School of Clinical Sciences, Melbourne, told this news organization.
“There are great opportunities to support women in their mental health and give them and their babies a healthier start to parenthood and life,” said Dr. Galbally.
“For instance, reducing the likelihood of prematurity or low birth weight at birth that can be associated with anorexia in pregnancy has extraordinary benefits for that child for lifelong health and well-being,” she added.
The guidelines were published online in Lancet Psychiatry.
Spike in cases
Dr. Galbally noted that during her 20 years of working in perinatal mental health within tertiary maternity services, she only ever saw an occasional pregnant woman with current AN.
In contrast, over the last 3 to 4 years, there has been a “steep increase in women presenting in pregnancy with very low body mass index (BMI) and current anorexia nervosa requiring treatment in pregnancy,” Dr. Galbally said.
Despite the complexity of managing AN in pregnancy, few studies are available to guide care. In a systematic literature review, the researchers identified only eight studies that addressed the management of AN in pregnancy. These studies were case studies or case reports examining narrow aspects of management.
Digging deeper, the researchers conducted a state-of-the-art research review in relevant disciplines and areas of expertise for managing anorexia nervosa in pregnancy. They synthesized their findings into “recommendations and principles” for multidisciplinary care of pregnant women with AN.
The researchers note that AN in pregnancy is associated with increased risks of pregnancy complications and poorer outcomes for infants, and measures such as BMI are less accurate in pregnancy for assessing severity or change in anorexia nervosa.
Anorexia affects pregnancy and neonatal outcomes through low calorie intake, nutritional and vitamin deficiencies, stress, fasting, low body mass, and poor placentation and uteroplacental function.
The authors note that managing AN in pregnancy requires multidisciplinary care that considers the substantial physiological changes for women and requirements for monitoring fetal growth and development.
At a minimum, they recommend monitoring the following:
- Sodium, potassium, magnesium, phosphate, and chloride concentration
- Iron status, vitamin D and bone mineral density, blood sugar concentration (fasting or random), and A1c
- Liver function (including bilirubin, aspartate transaminase, alanine aminotransferase, and gamma-glutamyl transferase) and bone marrow function (including full blood examination, white cell count, neutrophil count, platelets, and hemoglobin)
- Inflammatory markers (C-reactive protein and erythrocyte sedimentation rate)
- Cardiac function (electrocardiogram and echocardiogram)
- Blood pressure and heart rate (lying and standing) and body temperature
“There are considerable risks for women and their unborn child in managing moderate to severe AN in pregnancy,” said Dr. Galbally.
“While we have provided some recommendations, it still requires considerable adaptation to individual presentations and circumstances, and this is best done with a maternity service that manages other high-risk pregnancies such as through maternal-fetal medicine teams,” she said.
“While this area of clinical care can be new to high-risk pregnancy teams, it is clearly important that high-risk pregnancy services and mental health work together to improve care for women with anorexia in pregnancy,” Dr. Galbally added.
A nightmare, a dream come true
Reached for comment, Kamryn T. Eddy, PhD, co-director, Eating Disorders Clinical and Research Program, Massachusetts General Hospital, said, “for many with anorexia nervosa, pregnancy realizes their greatest nightmare and dream come true, both at once.”
“The physical demands of pregnancy can be taxing, and for those with anorexia nervosa, closer clinical management makes sense and may help to support patients who are at risk for return to or worsening of symptoms with the increased nutritional needs and weight gain that occur in pregnancy,” Dr. Eddy, associate professor, department of psychiatry, Harvard Medical School, Boston, told this news organization.
“At the same time, the desire to have a child can be a strong motivator for patients to make the changes needed to recover, and for some, the transition to mother can also help in recovery by broadening the range of things that influence their self-worth,” Dr. Eddy added.
This research had no specific funding. Dr. Galbally and Dr. Eddy report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The first comprehensive guidelines to manage pregnant women with anorexia nervosa (AN) have been released.
Pregnant women with AN are at greater risk of poor outcomes, including stillbirth, underweight infant, or pre-term birth, yet there are no clear guidelines on the management of the condition.
“Anorexia in pregnancy has been an overlooked area of clinical care, as many believed only women in remission become pregnant, and it is clear that is not the case,” lead author Megan Galbally, MBBS, PhD, professor and director, Centre of Women’s and Children’s Mental Health at Monash University School of Clinical Sciences, Melbourne, told this news organization.
“There are great opportunities to support women in their mental health and give them and their babies a healthier start to parenthood and life,” said Dr. Galbally.
“For instance, reducing the likelihood of prematurity or low birth weight at birth that can be associated with anorexia in pregnancy has extraordinary benefits for that child for lifelong health and well-being,” she added.
The guidelines were published online in Lancet Psychiatry.
Spike in cases
Dr. Galbally noted that during her 20 years of working in perinatal mental health within tertiary maternity services, she only ever saw an occasional pregnant woman with current AN.
In contrast, over the last 3 to 4 years, there has been a “steep increase in women presenting in pregnancy with very low body mass index (BMI) and current anorexia nervosa requiring treatment in pregnancy,” Dr. Galbally said.
Despite the complexity of managing AN in pregnancy, few studies are available to guide care. In a systematic literature review, the researchers identified only eight studies that addressed the management of AN in pregnancy. These studies were case studies or case reports examining narrow aspects of management.
Digging deeper, the researchers conducted a state-of-the-art research review in relevant disciplines and areas of expertise for managing anorexia nervosa in pregnancy. They synthesized their findings into “recommendations and principles” for multidisciplinary care of pregnant women with AN.
The researchers note that AN in pregnancy is associated with increased risks of pregnancy complications and poorer outcomes for infants, and measures such as BMI are less accurate in pregnancy for assessing severity or change in anorexia nervosa.
Anorexia affects pregnancy and neonatal outcomes through low calorie intake, nutritional and vitamin deficiencies, stress, fasting, low body mass, and poor placentation and uteroplacental function.
The authors note that managing AN in pregnancy requires multidisciplinary care that considers the substantial physiological changes for women and requirements for monitoring fetal growth and development.
At a minimum, they recommend monitoring the following:
- Sodium, potassium, magnesium, phosphate, and chloride concentration
- Iron status, vitamin D and bone mineral density, blood sugar concentration (fasting or random), and A1c
- Liver function (including bilirubin, aspartate transaminase, alanine aminotransferase, and gamma-glutamyl transferase) and bone marrow function (including full blood examination, white cell count, neutrophil count, platelets, and hemoglobin)
- Inflammatory markers (C-reactive protein and erythrocyte sedimentation rate)
- Cardiac function (electrocardiogram and echocardiogram)
- Blood pressure and heart rate (lying and standing) and body temperature
“There are considerable risks for women and their unborn child in managing moderate to severe AN in pregnancy,” said Dr. Galbally.
“While we have provided some recommendations, it still requires considerable adaptation to individual presentations and circumstances, and this is best done with a maternity service that manages other high-risk pregnancies such as through maternal-fetal medicine teams,” she said.
“While this area of clinical care can be new to high-risk pregnancy teams, it is clearly important that high-risk pregnancy services and mental health work together to improve care for women with anorexia in pregnancy,” Dr. Galbally added.
A nightmare, a dream come true
Reached for comment, Kamryn T. Eddy, PhD, co-director, Eating Disorders Clinical and Research Program, Massachusetts General Hospital, said, “for many with anorexia nervosa, pregnancy realizes their greatest nightmare and dream come true, both at once.”
“The physical demands of pregnancy can be taxing, and for those with anorexia nervosa, closer clinical management makes sense and may help to support patients who are at risk for return to or worsening of symptoms with the increased nutritional needs and weight gain that occur in pregnancy,” Dr. Eddy, associate professor, department of psychiatry, Harvard Medical School, Boston, told this news organization.
“At the same time, the desire to have a child can be a strong motivator for patients to make the changes needed to recover, and for some, the transition to mother can also help in recovery by broadening the range of things that influence their self-worth,” Dr. Eddy added.
This research had no specific funding. Dr. Galbally and Dr. Eddy report no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.










