User login
Peripheral T-cell lymphoma incidence and survival varies significantly by race
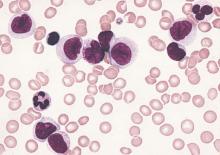
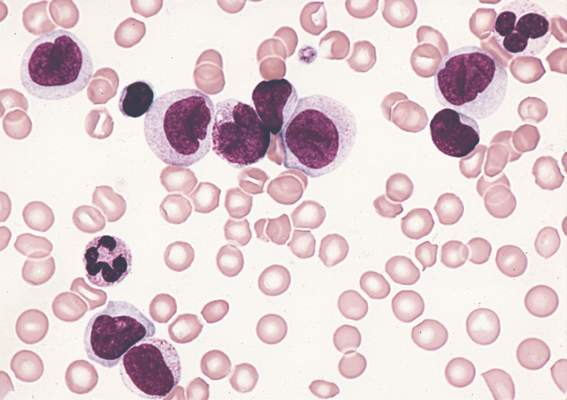
Peripheral T-cell lymphoma (PTCL) incidence, proportions of subtypes, and survival differed markedly among racial/ethnic subpopulations in the United States, according to analysis of SEER cancer registries.
Compared with non-Hispanic whites, blacks had higher incidence rates overall of PTCL, due to higher rates of PTCL not otherwise specified (PTCL-NOS) (incidence rate ratio [IRR], 1.67; 95% CI, 1.53-1.82) and adult T-cell leukemia/lymphoma (ATLL) (IRR, 4.37; 3.42-5.56). By contrast, blacks had lower incidence rates of angioimmunoblastic T-cell lymphoma (AITL) and extranodal NK/T-cell lymphoma and natural killer–cell leukemia (ENKCL) than non-Hispanic whites. Underlying causes for these differences are not understood.
“Our findings also highlight the need for stronger efforts to increase recruitment of blacks into clinical trials of PTCL therapies,” wrote Dr. Scott Adams of Fred Hutchinson Cancer Research Center, Seattle, and colleagues (J Clin Oncol. 2016 Jan 25. doi: 10.1200/JCO.2015.635540).
Asians/Pacific Islanders and Hispanic whites had higher rates of ENKCL (IRR, 3.61; 2.93-4.42), and ENKCL comprised a larger proportion of PTCL (14%) in these populations than for non-Hispanic whites (3%), findings which reflect global variations. High rates of ENKCL may be influenced by both genetic and environmental factors, as 35% of this subpopulation was born outside the U.S., compared with 16% of all patients with PTCL. Epstein-Barre virus infection is associated with ENKCL.
Similar to global incidence patterns, Asians/Pacific Islanders had lower rates of anaplastic large-cell lymphoma (ALCL).
Higher incidence of ATLL among blacks and Asians/Pacific Islanders corresponded to a higher prevalence of human T-lymphotropic virus type 1 in these populations. Substantial differences in survival were also observed. Compared with non-Hispanic whites, survival with PTCL-NOS was shorter for every minority group, and blacks had worse prognoses for almost every histology. For patients with any PTCL, median survival time varied by race: 49 months for non-Hispanic whites, 24 for blacks, 22 for Asians/Pacific Islanders, 28 for Hispanic whites, and 36 for American Indians/Alaskan natives.
Reasons for survival disparities are not known, but contributing factors may include racial variations in pharmacokinetics or pharmacodynamics of therapeutic agents and genomic variations of the tumors, as well as healthcare disparities and socioeconomic factors.
The analysis used data from SEER cancer registries of 13,107 patients 15 years and older who had PTCL diagnosed in the U.S. between 2000 and 2012.
For non-Hispanic whites, the annual incidence rate of all PTCLs was 1.56 (95% CI; 1.52-1.59) per 100,000. Compared with non-Hispanic whites, incidence rate ratios were 1.32 (1.25-1.39; P less than .001) for blacks, 0.89 (0.83-0.95; P less than .001) for Asians/Pacific Islanders, 0.63 (0.49-0.79; P less than .001) for American Indians/Alaskan natives, and 0.96 (0.90-1.01; P = .13) for Hispanic whites.
Peripheral T-cell lymphoma (PTCL) incidence, proportions of subtypes, and survival differed markedly among racial/ethnic subpopulations in the United States, according to analysis of SEER cancer registries.
Compared with non-Hispanic whites, blacks had higher incidence rates overall of PTCL, due to higher rates of PTCL not otherwise specified (PTCL-NOS) (incidence rate ratio [IRR], 1.67; 95% CI, 1.53-1.82) and adult T-cell leukemia/lymphoma (ATLL) (IRR, 4.37; 3.42-5.56). By contrast, blacks had lower incidence rates of angioimmunoblastic T-cell lymphoma (AITL) and extranodal NK/T-cell lymphoma and natural killer–cell leukemia (ENKCL) than non-Hispanic whites. Underlying causes for these differences are not understood.
“Our findings also highlight the need for stronger efforts to increase recruitment of blacks into clinical trials of PTCL therapies,” wrote Dr. Scott Adams of Fred Hutchinson Cancer Research Center, Seattle, and colleagues (J Clin Oncol. 2016 Jan 25. doi: 10.1200/JCO.2015.635540).
Asians/Pacific Islanders and Hispanic whites had higher rates of ENKCL (IRR, 3.61; 2.93-4.42), and ENKCL comprised a larger proportion of PTCL (14%) in these populations than for non-Hispanic whites (3%), findings which reflect global variations. High rates of ENKCL may be influenced by both genetic and environmental factors, as 35% of this subpopulation was born outside the U.S., compared with 16% of all patients with PTCL. Epstein-Barre virus infection is associated with ENKCL.
Similar to global incidence patterns, Asians/Pacific Islanders had lower rates of anaplastic large-cell lymphoma (ALCL).
Higher incidence of ATLL among blacks and Asians/Pacific Islanders corresponded to a higher prevalence of human T-lymphotropic virus type 1 in these populations. Substantial differences in survival were also observed. Compared with non-Hispanic whites, survival with PTCL-NOS was shorter for every minority group, and blacks had worse prognoses for almost every histology. For patients with any PTCL, median survival time varied by race: 49 months for non-Hispanic whites, 24 for blacks, 22 for Asians/Pacific Islanders, 28 for Hispanic whites, and 36 for American Indians/Alaskan natives.
Reasons for survival disparities are not known, but contributing factors may include racial variations in pharmacokinetics or pharmacodynamics of therapeutic agents and genomic variations of the tumors, as well as healthcare disparities and socioeconomic factors.
The analysis used data from SEER cancer registries of 13,107 patients 15 years and older who had PTCL diagnosed in the U.S. between 2000 and 2012.
For non-Hispanic whites, the annual incidence rate of all PTCLs was 1.56 (95% CI; 1.52-1.59) per 100,000. Compared with non-Hispanic whites, incidence rate ratios were 1.32 (1.25-1.39; P less than .001) for blacks, 0.89 (0.83-0.95; P less than .001) for Asians/Pacific Islanders, 0.63 (0.49-0.79; P less than .001) for American Indians/Alaskan natives, and 0.96 (0.90-1.01; P = .13) for Hispanic whites.
Peripheral T-cell lymphoma (PTCL) incidence, proportions of subtypes, and survival differed markedly among racial/ethnic subpopulations in the United States, according to analysis of SEER cancer registries.
Compared with non-Hispanic whites, blacks had higher incidence rates overall of PTCL, due to higher rates of PTCL not otherwise specified (PTCL-NOS) (incidence rate ratio [IRR], 1.67; 95% CI, 1.53-1.82) and adult T-cell leukemia/lymphoma (ATLL) (IRR, 4.37; 3.42-5.56). By contrast, blacks had lower incidence rates of angioimmunoblastic T-cell lymphoma (AITL) and extranodal NK/T-cell lymphoma and natural killer–cell leukemia (ENKCL) than non-Hispanic whites. Underlying causes for these differences are not understood.
“Our findings also highlight the need for stronger efforts to increase recruitment of blacks into clinical trials of PTCL therapies,” wrote Dr. Scott Adams of Fred Hutchinson Cancer Research Center, Seattle, and colleagues (J Clin Oncol. 2016 Jan 25. doi: 10.1200/JCO.2015.635540).
Asians/Pacific Islanders and Hispanic whites had higher rates of ENKCL (IRR, 3.61; 2.93-4.42), and ENKCL comprised a larger proportion of PTCL (14%) in these populations than for non-Hispanic whites (3%), findings which reflect global variations. High rates of ENKCL may be influenced by both genetic and environmental factors, as 35% of this subpopulation was born outside the U.S., compared with 16% of all patients with PTCL. Epstein-Barre virus infection is associated with ENKCL.
Similar to global incidence patterns, Asians/Pacific Islanders had lower rates of anaplastic large-cell lymphoma (ALCL).
Higher incidence of ATLL among blacks and Asians/Pacific Islanders corresponded to a higher prevalence of human T-lymphotropic virus type 1 in these populations. Substantial differences in survival were also observed. Compared with non-Hispanic whites, survival with PTCL-NOS was shorter for every minority group, and blacks had worse prognoses for almost every histology. For patients with any PTCL, median survival time varied by race: 49 months for non-Hispanic whites, 24 for blacks, 22 for Asians/Pacific Islanders, 28 for Hispanic whites, and 36 for American Indians/Alaskan natives.
Reasons for survival disparities are not known, but contributing factors may include racial variations in pharmacokinetics or pharmacodynamics of therapeutic agents and genomic variations of the tumors, as well as healthcare disparities and socioeconomic factors.
The analysis used data from SEER cancer registries of 13,107 patients 15 years and older who had PTCL diagnosed in the U.S. between 2000 and 2012.
For non-Hispanic whites, the annual incidence rate of all PTCLs was 1.56 (95% CI; 1.52-1.59) per 100,000. Compared with non-Hispanic whites, incidence rate ratios were 1.32 (1.25-1.39; P less than .001) for blacks, 0.89 (0.83-0.95; P less than .001) for Asians/Pacific Islanders, 0.63 (0.49-0.79; P less than .001) for American Indians/Alaskan natives, and 0.96 (0.90-1.01; P = .13) for Hispanic whites.
Key clinical point: Peripheral T-cell lymphoma incidence, proportions of subtypes, and survival differed markedly among racial/ethnic subpopulations in the United States.
Major finding: Compared with non-Hispanic whites, blacks had higher incidence rates of PTCL not otherwise specified (PTCL-NOS) and adult T-cell leukemia/lymphoma (ATLL), and lower incidence rates of angioimmunoblastic T-cell lymphoma (AITL) and extranodal NK/T-cell lymphoma and natural killer–cell leukemia (ENKCL); Asians/Pacific Islanders and Hispanic whites had higher rates of ENKCL; Asians/Pacific Islanders had lower rates of anaplastic large-cell lymphoma (ALCL); survival with PTCL-NOS was shorter for every minority group compared with non-Hispanic whites.
Data source: SEER cancer registries of 13,107 patients 15 years and older who had PTCL diagnosed in the U.S. between 2000 and 2012.
Disclosures: Dr. Adams reported having no disclosures. Dr. Shustov, a coauthor, reported financial ties to Celgene, Spectrum Pharmaceuticals, Bristol-Myers Squibb, Millennium, Gilead Sciences, Seattle Genetics, and Pfizer.
Antilymphocyte globulin curbs chronic graft-versus-host disease
Antilymphocyte globulin (ATG) added to the myeloablative conditioning regimen of patients undergoing allogeneic stem cell transplantation resulted in a lower incidence of chronic graft-versus-host disease (GVHD) compared with conditioning regimens without ATG.
At two years, the cumulative incidence of chronic GVHD was 32.2% (95% CI, 22.1–46.7) for the ATG group vs 68.7% (58.4-80.7) for the non-ATG group (P < .001). At one year, 91% of the ATG group had discontinued cyclosporine, compared with 39% in the non-ATG group. The difference between groups was most pronounced in rates of the clinical extensive form of chronic GVHD: 7.6% (3.0-19.6) with ATG compared with 52.4% (39.3-69.9) without ATG.
T-cell depletion may cause a loss of graft-versus-leukemia effects, which is cause for concern. This study showed similar rates of relapse-free and overall survival for the ATG and non-ATG groups. Two-year relapse-free survival was 59.4% (47.8-69.2) in the ATG group and 64.6% (50.9-75.3) in the non-ATG group. The composite 2-year survival, free from chronic GVHD and relapse, was 36.6% (25.2-48.0) for the ATG group vs 16.8% (9.2-26.4) for the non-ATG group, according to the study reported in the January 7 issue of the New England Journal of Medicine (2016;374:43-53).
Chronic GVHD is the leading cause of later illness and death after allogeneic hematopoietic stem-cell transplantation, according to the authors. “Even if a modest increase in the rate of relapse in the ATG group cannot be ruled out, the significantly lower incidence of chronic GVHD with ATG resulted in a significantly higher rate of 2-year survival free from chronic GVD among patients who received ATG than among those who did not receive ATG (50% vs. 23%),” wrote Dr. Nicolaus Kröger, Professor and Medical Director of the Department of Stem Cell Transplantation at the University Hospital Hamburg-Eppendorf, Germany, and colleagues.
The prospective, open-label, randomized phase 3 trial evaluated 155 patients at 27 centers from 2006 to 2012. Patients with acute leukemia undergoing allogeneic stem cell transplantation with peripheral blood stem cells were randomized 1:1 to receive myeloablative conditioning with or without ATG.
Post-transplantation lymphoproliferative disorder was not observed in either group. The ATG and non-ATG groups had similar rates of infectious complications (57.8% and 54.2%, respectively), and nonrelapse-related death at 2 years (14.0% and 12.0%, respectively).
The study was funded by the Neovii Biotech and the European Society for Blood and Marrow Transplantation; ClinicalTrials.gov number, NCT00678275. Dr. Kröger reported grant support from Neovii Biotech. Several of his coauthors reported ties to industry.
Antilymphocyte globulin (ATG) added to the myeloablative conditioning regimen of patients undergoing allogeneic stem cell transplantation resulted in a lower incidence of chronic graft-versus-host disease (GVHD) compared with conditioning regimens without ATG.
At two years, the cumulative incidence of chronic GVHD was 32.2% (95% CI, 22.1–46.7) for the ATG group vs 68.7% (58.4-80.7) for the non-ATG group (P < .001). At one year, 91% of the ATG group had discontinued cyclosporine, compared with 39% in the non-ATG group. The difference between groups was most pronounced in rates of the clinical extensive form of chronic GVHD: 7.6% (3.0-19.6) with ATG compared with 52.4% (39.3-69.9) without ATG.
T-cell depletion may cause a loss of graft-versus-leukemia effects, which is cause for concern. This study showed similar rates of relapse-free and overall survival for the ATG and non-ATG groups. Two-year relapse-free survival was 59.4% (47.8-69.2) in the ATG group and 64.6% (50.9-75.3) in the non-ATG group. The composite 2-year survival, free from chronic GVHD and relapse, was 36.6% (25.2-48.0) for the ATG group vs 16.8% (9.2-26.4) for the non-ATG group, according to the study reported in the January 7 issue of the New England Journal of Medicine (2016;374:43-53).
Chronic GVHD is the leading cause of later illness and death after allogeneic hematopoietic stem-cell transplantation, according to the authors. “Even if a modest increase in the rate of relapse in the ATG group cannot be ruled out, the significantly lower incidence of chronic GVHD with ATG resulted in a significantly higher rate of 2-year survival free from chronic GVD among patients who received ATG than among those who did not receive ATG (50% vs. 23%),” wrote Dr. Nicolaus Kröger, Professor and Medical Director of the Department of Stem Cell Transplantation at the University Hospital Hamburg-Eppendorf, Germany, and colleagues.
The prospective, open-label, randomized phase 3 trial evaluated 155 patients at 27 centers from 2006 to 2012. Patients with acute leukemia undergoing allogeneic stem cell transplantation with peripheral blood stem cells were randomized 1:1 to receive myeloablative conditioning with or without ATG.
Post-transplantation lymphoproliferative disorder was not observed in either group. The ATG and non-ATG groups had similar rates of infectious complications (57.8% and 54.2%, respectively), and nonrelapse-related death at 2 years (14.0% and 12.0%, respectively).
The study was funded by the Neovii Biotech and the European Society for Blood and Marrow Transplantation; ClinicalTrials.gov number, NCT00678275. Dr. Kröger reported grant support from Neovii Biotech. Several of his coauthors reported ties to industry.
Antilymphocyte globulin (ATG) added to the myeloablative conditioning regimen of patients undergoing allogeneic stem cell transplantation resulted in a lower incidence of chronic graft-versus-host disease (GVHD) compared with conditioning regimens without ATG.
At two years, the cumulative incidence of chronic GVHD was 32.2% (95% CI, 22.1–46.7) for the ATG group vs 68.7% (58.4-80.7) for the non-ATG group (P < .001). At one year, 91% of the ATG group had discontinued cyclosporine, compared with 39% in the non-ATG group. The difference between groups was most pronounced in rates of the clinical extensive form of chronic GVHD: 7.6% (3.0-19.6) with ATG compared with 52.4% (39.3-69.9) without ATG.
T-cell depletion may cause a loss of graft-versus-leukemia effects, which is cause for concern. This study showed similar rates of relapse-free and overall survival for the ATG and non-ATG groups. Two-year relapse-free survival was 59.4% (47.8-69.2) in the ATG group and 64.6% (50.9-75.3) in the non-ATG group. The composite 2-year survival, free from chronic GVHD and relapse, was 36.6% (25.2-48.0) for the ATG group vs 16.8% (9.2-26.4) for the non-ATG group, according to the study reported in the January 7 issue of the New England Journal of Medicine (2016;374:43-53).
Chronic GVHD is the leading cause of later illness and death after allogeneic hematopoietic stem-cell transplantation, according to the authors. “Even if a modest increase in the rate of relapse in the ATG group cannot be ruled out, the significantly lower incidence of chronic GVHD with ATG resulted in a significantly higher rate of 2-year survival free from chronic GVD among patients who received ATG than among those who did not receive ATG (50% vs. 23%),” wrote Dr. Nicolaus Kröger, Professor and Medical Director of the Department of Stem Cell Transplantation at the University Hospital Hamburg-Eppendorf, Germany, and colleagues.
The prospective, open-label, randomized phase 3 trial evaluated 155 patients at 27 centers from 2006 to 2012. Patients with acute leukemia undergoing allogeneic stem cell transplantation with peripheral blood stem cells were randomized 1:1 to receive myeloablative conditioning with or without ATG.
Post-transplantation lymphoproliferative disorder was not observed in either group. The ATG and non-ATG groups had similar rates of infectious complications (57.8% and 54.2%, respectively), and nonrelapse-related death at 2 years (14.0% and 12.0%, respectively).
The study was funded by the Neovii Biotech and the European Society for Blood and Marrow Transplantation; ClinicalTrials.gov number, NCT00678275. Dr. Kröger reported grant support from Neovii Biotech. Several of his coauthors reported ties to industry.
FROM NEJM
Key clinical point: Chronic graft-versus-host disease (GVHD) after allogeneic stem cell transplantation with peripheral blood stem cells was less than half as frequent with antilymphocyte globulin added to the conditioning regimen.
Major finding: Cumulative incidence of chronic GVHD at 2 years was 32.2% with antilymphocyte globulin vs 68.7% without it.
Data source: The prospective, open-label, randomized phase 3 trial evaluated 155 patients at 27 centers from 2006 to 2012.
Disclosures: Research was supported in part by Neovii Biotech. Dr. Kröger reported grant support from Neovii Biotech. Several of his coauthors reported ties to industry.
Medical Roundtable: Peripheral T-Cell Lymphomas: A Practical Approach to Newly Diagnosed and Relapsed Patients
Moderator: Steven M. Horwitz, MD1
Discussants: Alison Moskowitz, MD1; Michelle Fanale, MD2; Andrei Shustov, MD3
From Memorial Sloan Kettering Cancer Center, New York, NY1; MD Anderson Cancer Center, Houston, TX2; University of Washington Medical Center, Seattle, WA3
Address for correspondence: Steven M. Horwitz, MD, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065
E-mail: horwitzs@mskcc.org
DR. HORWITZ: My name is Dr. Steven Horwitz from Memorial Sloan-Kettering Cancer Center. I’m joined today by Drs. Alison Moskowitz, Memorial Sloan-Kettering, Michelle Fanale of MD Anderson, and Andrei Shustov, University of Washington in Seattle. Thank you all for joining us for this conversation on T-cell lymphoma. My colleagues are all well known as experts in T-cell lymphomas. Those of you who treat these diseases recognize the systemic T-cell lymphomas as one of our greater challenges in hematologic malignancies in terms of the treatment options for patients and the frequent lack of definitive data to guide our decisions.
I thought what we would do today is have a very practical discussion about the way we think about these diseases, the decisions we make, and the way we make those decisions. I'll start off by asking when you get a referral of a patient with a new diagnosis of peripheral T-cell lymphoma (PTCL), what are some of the basic things that you first think about in terms of approaching a new patient?
DR. SHUSTOV: I think one of the biggest challenges in T-cell lymphomas still remains making the proper diagnosis. In general, pathologists in the United States have a pretty good idea when they see T-cell lymphomas, however, subclassification remains a challenge even for expert hematopathologists due to frequent histologic overlap between the subtypes of PTCL, and even with non-malignant autoimmune disorders. I frequently see patients who are diagnosed with or misdiagnosed with a different subtype of T-cell lymphoma. The most challenging is differentiation between angioimmunoblastic T-cell lymphoma (AITL), anaplastic large cell T-cell lymphoma (ALCL), and PTCL not otherwise specified (PTCL-NOS), especially when the latter patients have high expression of CD30 and/or bear features resembling AITL.
Sometimes they are a slam-dunk diagnosis, but frequently our hematopathologists reverse the diagnosis after doing additional studies on the biopsy material. The most recent case I've seen in my clinic for consultation was a patient with a diagnosis of extranodal NK-cell lymphoma that was reclassified as a gamma-delta T-cell lymphoma after additional work-up. I truly believe that it is advisable that majority, if not all PTCL cases are reviewed by expert hematopathology teams at academic centers that see large volumes of these cases.
I think it's very important to educate community physicians and patients about a proper PTCL diagnosis, especially now that more targeted therapies are being developed and the gene expression profiling techniques will probably lead to identification of specific pathways that are amenable to therapy with specific biologic agents.
DR. FANALE: I'd like to expand upon what Andrei just said. For me the next step after confirming the pathology diagnosis is to think about two things. To think about whether or not this patient is a patient who might be eligible for an ongoing front-line trial, typically if the patient meets eligibility criteria for one of our ongoing front-line trials I would really recommend to the patient to consider being enrolled in that trial, and I also think about whether the patient, if he or she enters into remission with front-line therapy, can be considered for a consolidative autologous stem cell transplant.
I think it's very important to educate community physicians and patients about a proper PTCL diagnosis. Right now, our ongoing front-line trial is the ECHELON-2 trial which is evaluating brentuximab vedotin (BV) plus cyclophosphamide, doxorubicin, prednisone (CHP [BV-CHP]) chemotherapy compared to standard of care cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) chemotherapy, and that trial is based on the promising data that we've seen in the initial phase I trial that combined BV plus CHP chemotherapy followed by maintenance BV which demonstrated both high and durable complete remission (CR) rates.1 BV is an antibody drug conjugate that's targeted at CD30 and carries initial US Food and Drug Administration approval for patients who have relapsed or refractory ALCL which has a 100% level of CD30 positivity and then also has a National Comprehensive Cancer Network listing for treating other types of relapsed or refractory CD30 positive PTCL as well.
Also there is another upcoming front-line trial, which is to combine pralatrexate plus CHOP. If a patient isn't eligible for a clinical trial or it's just not feasible for that patient to enroll in a clinical trial, I will then look further at what would be potentially the best standard of care option for that patient.
I'll look at the patient's age and performance status and if they are generally less than age 65 or so and if otherwise well, I'll preferentially treat that patient with CHOEP which is CHOP plus etoposide. And then, for a patient who's either older than this or has multiple other comorbidities, I would treat that patient typically with CHOP alone.
DR. HORWITZ: Thank you, we'll go further into the selection of initial therapies but first circle back, I was curious as to how often your center comes to a different diagnosis than the referring center, and are there pitfalls you see that alert you to be suspicious of diagnosis.
DR. SHUSTOV: I'd say probably 10% of cases that we see at our center will be reclassified by our hematopathologists. In most cases, they do not necessarily reverse the diagnosis, but provide further clarification. It is occurring to me that in the community, pathologists are less likely to call the subtype of T-cell lymphoma and limit the report to the general description of T-cell lymphoprolipherative disorder, or state something like “consistent with T-cell lymphoma with features of AITL, or with features of anaplastic lymphoma.” I would admit though, that sometimes it's very difficult to identify the specific subtype of PTCL even in the expert hands; but I'd say these cases would constitute no more than 5% of PTCL patients.
DR. HORWITZ: And in your experience is it mostly fine tuning a T-cell lymphoma diagnosis, or do you see totally different diagnoses?
DR. MOSKOWITZ: Usually review by expert hematopathologists simply leads to fine-tuning the T-cell lymphoma diagnosis, however, I occasionally see significant changes in diagnosis. Often, alterations or clarification of a diagnosis are made possible only after we provide the pathologist with clinical history. For example, a lymph node biopsy may be interpreted as ALCL, however the knowledge that the particular patient has a history of mycosis fungoides would lead the pathologist to consider the diagnosis of large cell transformation of mycosis fungoides rather than ALCL. In such a case, molecular studies are helpful in confirming that the lymph node findings originate from the same clone as that in the original mycosis fungoides lesions, rather than representing a second primary.
DR. FANALE: Very occasionally, I've seen patients truly “with a misdiagnosis and a complete revision of diagnosis” and, usually, those pitfalls that I've seen them occasionally have been the young patients—the young patients who generally have disease in the thorax and neck, who are treated as though they have classical Hodgkin lymphoma, who have very significant progression of disease on standard of care treatment. So it's important that not all cases that have CD30 positivity are classical Hodgkin lymphoma even if the patient is young.
DR. HORWITZ: I often think the systemic T-cell lymphomas usually behave in an aggressive fashion so when the clinical picture and the diagnosis don't really fit, I think about getting a second biopsy before we finalize the diagnosis. Of course, when people are ill with obviously progressing disease, you may need to move more quickly.
I often think the systemic T-cell lymphomas usually behave in an aggressive fashion so when the clinical picture and the diagnosis don't really fit, I think about getting a second biopsy before we finalize the diagnosis. To move on, when you first see a patient, what is the decision tree in your mind in terms of picking therapy or planning therapy, or what kind of things do you consider?
DR. MOSKOWITZ: The first thing I think about when deciding upon treatment for a patient with T-cell lymphoma is whether or not I plan to use a curative approach to therapy. As was mentioned by Michelle, this decision is partly based upon the patients’ comorbidities and age. For patients who are eligible for curative therapy, our frontline approach for the most common types of T-cell lymphoma is to use CHOP with or without etoposide followed by consideration for autologous stem cell transplant in first remission. There are certainly individuals for whom such an aggressive strategy would not be appropriate due to age or co-morbidities. In these individuals, we may consider CHOP-based therapy alone or sometimes even milder approaches aimed at disease control.
DR. HORWITZ: Andrei, are you similar in the CHOP/CHOEP paradigm? And if so, what do you think of those data? How do you decide between the two and when do you do that regimen versus something different?
DR. SHUSTOV: I think I will double or triple what Michelle and Alison just said; for me, the two most important decisions that I have to make in the first encounter with patients with newly diagnosed PTCL are: 1) whether we're going to pursue curative approach strategy; and 2) whether the patient can tolerate the intensity of treatments that would provide him/her with the best chance of cure or long term remission. Patients who are elderly and have high risk disease would be very hard to cure, especially considering that the consolidative transplant might carry high rates of morbidity or mortality; more conservative strategies might be appropriate in these cases. On the other hand, younger and more fit patients might benefit more from intensified initial regimens—ie, CHOEP—followed by high-dose therapy and either autologous or allogeneic stem cell transplant (ALLO).
I usually have a long initial discussion with patients and families during which we decide on the intent of treatment and what to expect from certain regimens in terms of toxicities. I typically choose CHOEP regimen (or infusional version, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin [EPOCH]) for younger patients based on recent German data, even though this was a retrospective study and benefit of adding etoposide only approached statistical significance for the majority of PTCL subtypes.2 In older patients, I try to avoid anthracyclines, especially in the palliative intent setting, based on the retrospective analysis by the International T-Cell Lymphoma Project, and frequently use CEOP regimen (CHOEP minus anthracycline).3 I find that the majority of older patients tolerate this treatment somewhat better than anthracycline-containing combinations like CHOP.
In the very elderly and frail patients, I try to avoid combination chemotherapy all together. It is a somewhat easier decision for patients with AITL. Some of them are more indolent than other sub-types, and I would treat such patients with immunomodulatory approach, ie with a combination of prednisone and cyclosporine; then, I would consider single agent therapy with one of the recently approved agents for relapsed and refractory PTCL. I would also double what Michelle said in regard to making the best attempt to enroll patients into open clinical trials, because the current standards are not really satisfactory for many T-cell lymphoma patients.
DR. HORWITZ: It sounds like we all approach a new patient similarly. However, several of our up front trials are randomized against CHOP as opposed to CHOEP. When you're consenting a patient to those trials, how do you explain it to a patient or how important do you think the etoposide is?
DR. SHUSTOV: I share your frustration with quite a few of the current study designs for that exact reason. Some of these are confirmatory trials after conditional approvals of the new agents and are important. However, as often happens, in the confirmatory trials, we use controls that are a default from a lot of historical data, or the “common” way that the majority of patients are being treated. It is really a challenge when you consent patients to studies where a control arm is something that you think might not be quite adequate.
Having said that, the CHOEP study that was mentioned several times is a retrospective subgroup analysis and the addition of etoposide had marginal benefit that approached significance, but certainly was not a home run. I don't think we are ready to say that etoposide provides survival advantage in T-cell lymphoma patients and dismiss clinical trials in favor of just giving a patient the CHOEP, even though CHOP is admittedly not the best comparator. I discuss this controversy with patients and tell them frankly that the data that we base addition of etoposide on are not the best evidence one may have. Then, I ask patients to decide which approach sounds more reasonable to them and they make their choice.
DR. FANALE: To expand just slightly upon what Andrei said, I do emphasize to the patient and to the referring doctors that if you look at National Comprehensive Cancer Network guidelines, CHOP, CHOEP, EPOCH, all of these are potential options. So there's really not yet one trial that would say that one is clearly superior to the other at this time. I also emphasize to the patient and to the referring clinician that the only way, let's say, the patient could get the targeted agent plus the chemotherapy and have that 50% chance of, potentially, receiving that combination is really through the randomized clinical trial.
DR. HORWITZ: That was excellent, thank you. So you have alluded to this a bit. How much does subtype specific treatment come into play?
DR. MOSKOWITZ: At this point in time, outside of a clinical trial, for PTCL-NOS, AITL, and ALCL, the front-line approach would typically be CHOEP followed by autologous stem cell transplant. As Michelle mentioned, for a patient with ALCL, I would offer enrollment on the ECHELON-2 study in which patients are randomized to CHOP or BV-CHP, in order to give patients the option of potentially receiving BV.
For AITL, we are now obtaining molecular testing for certain mutations, particularly IDH2, because we currently have a study open with an IDH2 inhibitor that specifically enrolls patients with IDH2-mutant AITL. Because the testing takes some time to get back, we typically test patients’ biopsies at the time of diagnosis, so that we know if they're eligible for future clinical trials in case their disease does not respond to upfront therapy.
There are T-cell lymphoma subtypes that we treat quite differently from the entities discussed so far. For human T-lymphotropic virus-1 associated adult T-cell lymphoma, for example, the data with CHOP are really not good and we typically used EPOCH with the aim to consolidate with an allogeneic stem cell transplant.
Likewise, for hepatosplenic T-cell lymphoma, we have not observed good responses with the CHOP-based therapy and therefore we typically use platinum-based or ifosfamide-based therapy upfront with the aim to consolidate with allogeneic stem cell transplant. Extranodal NK/T-cell lymphoma is another entity for which CHOP is typically not effective and we have adopted asparaginase-based regimens, such as dexamethasone, methotrexate, ifosfamide, L-asparaginase, etoposide (SMILE) for this disease.
DR. SHUSTOV: I completely agree with Alison; for more common nodal lymphomas it is really hard at this point to base a treatment decision on histology, at least, in the front-line setting. However, some of the rare and unique subtypes, we generally treat with a completely different approach (ie, extranodal NK-cell lymphomas, enteropathy-associated T-cell lymphomas, adult T-cell leukemia/lymphomas).
DR. HORWITZ: Excellent. I’m curious, in a patient with ALCL who refused randomization of the ECHELON-2 trial, would you give them BV-CHP off study or would you discuss that as an alternative were they not to participate in the trial?
DR. FANALE: No. I don't urge treatment that's not approved for a particular line of therapy off clinical protocol with the exception that, let's say, if a patient is not a candidate for chemotherapy in second line setting for a particular lymphoma such as Hodgkin lymphoma—then, I would reference published data like Alison's data for a second line use of BV say for classical Hodgkin lymphoma and work with the insurance company to get the drug approved for that patient population, but if a patient just doesn't want to go on trial because they don't like the 50/50 chance of receiving BV-CHP compared to CHOP and they say, “I really want the 100% chance. Why don't you just contact my insurance?” I will explain to them the rationale of why the trial is being conducted including the hope that if the endpoints are met and the regimen is approved for front-line therapy then patients in the future can get BV-CHP at any oncology clinic, but I tell them for now it is a protocol based treatment option.
DR. HORWITZ: That's a very good answer. Can we now touch on the idea of transplant vs no transplant in first remission. Always, sometimes, never; how do you all think about that?
DR. SHUSTOV: I think that the idea of consolidative transplant is very heavily debated and discussed at the majority of specialized PTCL meetings. The reason for that is controversial nature of current clinical evidence. In the absence of randomized trials it is very hard to compare prospective phase II trial data to historical controls. You can interpret this in two ways; you might say, well, these are good data, outcome numbers look better than historical controls, and all patients with PTCL should have a consolidative transplant; or you can say, well, I don't have randomized data, and only a randomized study would really tell us whether post-induction consolidative transplant improves survival.
That is why it is such a controversial subject, and we agree that the phase II perspective studies of transplantation are hampered by significant bias; patients who are able and willing to travel to academic centers, were more robust and able to tolerate high dose therapy. They also have chemosensitive disease and that puts them in a completely different category than those you see in populational or retrospective studies, or other historical control trials.
Having said that, when I talk to patients, and I think that this is where I involve patients into treatment decision very heavily, I present them with the data and say that it is not wrong to do or not do the transplant in first CR. I make patients and family a big part of the decision. When they ask me what would give the patient the best chance being in remission five years from today, my answer is transplant, however, the data are not perfect, and the curative potential of autologous transplant in PTCL is not known.
DR. FANALE: So typically here, we would refer most patients on for front-line consolidative autologous stem cell transplant, I think, some exceptions are the ones that Andrei already touched on. If we're seeing a patient who has PTCL-NOS and this patient is the very rare patient who has early stage disease, with really no significant risk factors, that patient, unless there were pathologic features that showed a higher level of aggressiveness as Alison commented on, this patient might be one where we might defer doing the consolidative stem cell transplant for particularly if the patient’s disease entered into remission very quickly, but this still is controversial.
I'm not sure how you practice within each of your centers, but another patient, or where we would potentially defer, is a patient with fairly limited nasal NK/T-cell lymphoma, a patient that we’ve treated, let's say, with the dexamethasone, etoposide, ifosfamide, carboplatin (DeVIC) chemotherapy regimen plus a concomitant high-dose radiation, we typically historically here have deferred doing consolidative stem cell transplant for that patient population.
In terms of what Andrei commented on for when we might consider an ALLO and for a frontline consolidation, here we typically would not. The exception to that rule is that I've had a couple of patients here who have PTCL with a secondary hemophagocytic lymphohistiocytosis syndrome and then, for those patients because the concern is that the autologous stem cell transplant probably wouldn't be enough and for those patients given that they have such a dismal prognosis from the secondary hemophagocytic lymphohistiocytosis, we send those patients on for an ALLO.
DR. MOSKOWITZ: To expand upon early stage extranodal NK/T-cell lymphoma, I agree with Michelle that we wouldn't use an autologous stem cell transplant in first remission. Typically, for patients with localized disease, we recommend 2 cycles of SMILE followed by involved field radiation. Our patients treated with this approach have typically done quite well without needing a transplant.
DR. HORWITZ: That was great. If there are no further comments on upfront therapy, we can move over to relapse setting. When you see a patient at relapse, what are your strategies and how do you approach that patient?
DR. MOSKOWITZ: The first question in my mind when a patient is either not responding to or relapsing following front-line therapy is: what is my ultimate goal? For a patient who is fit and refractory to CHOP or has relapsed after CHOP or autologous stem cell transplant, my ultimate goal would be to try to get them to an ALLO. My choice of treatment following front-line therapy depends upon whether or not we are aiming for an ALLO as well as whether a donor has been identified for the patient.
Usually, if a patient just relapsed following front-line therapy, we are not necessarily ready to proceed quickly to ALLO. This may be because a donor has not yet been identified or because the patients’ eligibility for an ALLO is not clear. In this situation, my choice of treatment would be something that can be continued, potentially for several months, while we get things in place for the transplant. Treatment options in this situation would include the approved single agents for T-cell lymphoma, such as romidepsin, belinostat, pralatrexate, as well as BV (which would be specific for ALCL). In addition, I would consider enrollment on a promising clinical trial. Among these options, my choice of treatment typically depends upon the side effect profile and/or schedule, which is individualized for the patient. The one caveat would be that I typically will aim to use a histone deacetylase inhibitor (HDAC) inhibitor as my first choice for a patient with AITL.
Usually, if a patient just relapsed following front-line therapy, we are not necessarily ready to proceed quickly to ALLO. This may be because a donor has not yet been identified or because the patients’ eligibility for an ALLO is not clear. If my patient has a donor available and we are ready to move quickly to transplant, I would use one of the multi-agent regimens, such as ifosfamide, carboplatin, etoposide (ICE) or cisplatin, cytosine arabinoside, dexamethasone (DHAP), with the aim to try to get them into remission and relatively quickly proceed to transplant. The problem with using one of these types of regimens for everyone in the second-line setting is that the treatment cannot be continued indefinitely due to cumulative toxicity; therefore we need to know that we are ready to proceed to transplant if we are going to use one of the more intense regimens.
DR. HORWITZ: How do the others approach relapse?
DR. FANALE: Generally, a lot of similarities with what Alison discussed. Typically, it's if we're going to be sending the patient on for consideration for ALLO, I think the favor would be to try to use a regimen that has a high CR rate knowing that there is a trend toward improved progression free survival for the patient who enters into CR when compared to those who did not. I think the only slight difference might be just the timing and the selection, so, typically, here, even if the patient doesn't actually have a definite established donor quite yet we'll typically favor a combination type of a treatment approach, so whether or not that would be a platinum-based regimen such as ICE, as Alison mentioned, or gemcitabine-based chemotherapy regimen or similar.
I would generally favor one of those options, usually, over a single-agent therapeutic approach just knowing that the CR rates, generally, with exception of BV for ALCL are, generally, ranging about the 10%–15% range. And then, of course, first priority would be if there is a clinical trial available whether that's with a doublet of targeted agents or with the targeted agent plus chemotherapy in the relapse setting, we would typically favor that approach for a patient being considered for a stem cell transplant, ALLO approach.
DR. SHUSTOV: I'll consolidate what Michelle and Alison just said. I think we're on the same page. To me, the most important decision (even more important in the relapse setting) is to figure out whether we are still going to try to cure the lymphoma or we will pursue a palliative approach for which now we have several treatment options. So again, I’d have a long discussion with the patient, whether we're going to try and cure the disease, or we will pursue a palliative approach.
If this is a curative approach strategy and we're heading toward ALLO, we would start searching for the donor immediately. The choice of salvage therapy depends on duration of first remission. Primary refractory disease patients who failed CHOP, CHOEP, or EPOCH outright or within 3 months, I typically consider using the single agent approach, because even ICE and DHAP type regimens don’t work well for these patients. I put my “money” into HDAC inhibitors or antifolates, or other targeted therapy, if this is anaplastic lymphoma.
For patients who had at least 6 months remission from the initial therapy, younger patients who are still fit, I agree, I would consider standard salvage regimens like DHAP, etoposide, methylprednisolone, high-dosecytarabine, cisplatin (ESHAP) and ICE, and then, follow this with allograft.
DR. HORWITZ: You have all talked about allografting, I have two questions. What is your best guess in terms of percentage of patients you see with relapsed T-cell lymphoma who actually undergo ALLO at some point and do you ever consider autologous transplantation in those who didn't have it as part of their initial therapy?
DR. SHUSTOV: It certainly depends on the type of the academic center that patients are referred to, and we all practice at academic institutions with very strong ALLO programs. In patients who we select for a curative approach (one half to two thirds) we will at least make the best effort to take them to allogeneic grafting. Patients might fall off this track if they don't respond to salvage therapies or develop significant treatment/disease-related complications, ie fungal infections, organ toxicities, poor performance status, etc. Overall, I'd say that we successfully take about half of the patients with relapsed PTCL that are willing to pursue curative approach to allograft.
In addition, I consider autologous stem cell transplant in relapse setting for three situations. One is relapsed ALK-positive ALCL, some patients with ALK-negative ALCL who have had long initial remission and after relapse, and achieved second remission with BV or other salvage therapy. Finally, I’d consider auto-transplant in patients with AITL who achieved second CR with salvage therapy.
DR. HORWITZ: In terms of the relapse setting—some of you talked about angioimmunoblastic and HDAC inhibitors or BV for ALCL—when you have a relapse patient with PTCL-NOS, how do you pick a second line agent if you're not going directly to transplant, and are there new drugs that you're particularly excited about?
DR. FANALE: To answer your questions in terms of how I might choose a targeted agent for a patient who has PTCL-NOS and doesn't have AITL or ALCL, if the patient isn't eligible for trial or trial isn't feasible for that patient, I'll typically look at schedule, and then I'll look at side effect profile. For instance, for comparison, there are two HDAC inhibitor drugs right now approved, romidepsin and belinostat, so I think that potentially in a private practice setting, belinostat might be one when you look at the efficacy, being generally, around equivalent on where a patient might prefer that regimen because of the fact that they come in for five days in a row and then, their treatment is done for that cycle.
At a larger referral center to which patients are often traveling back and forth, it can be quite difficult for a patient to stay in town to do five days of treatment in a row. Often, these patients will prefer the romidepsin schedule because of the fact that they're coming back in once per week for three weeks in a row per cycle. The other way that I'll look at it is side effect profile, so, let's say, pralatrexate compared to the HDAC inhibitors, I will usually favor the HDAC inhibitors because of the potential side effect of mucositis with pralatrexate, although I have been able to manage through the mucositis by giving the patients more of a cutaneous T-cell lymphoma dosing format.
There had been a high level of interest in new emerging agents such as the aurora A-kinase inhibitors, alisertib, and that trial is now complete and the data are to be presented at The American Society of Hematology’s Annual Meeting this year.4 Other new therapies that are emerging are the PI3K inhibitors such as duvelisib and others. One advantage for many of the drugs in current trials is that they are oral agents.
My interest also lies in the combination approach, such as two or three targeted agent combinations, with the goal that if you see favorable CR rates and progression-free survival rates in the relapsed setting that you can then potentially move these treatments to the front-line setting to potentially move beyond the CHOP-based platform of therapies.
DR. SHUSTOV: For patients outside anaplastic lymphoma where, obviously, most of us would likely use BV, given the response rate to this agent, the decision is really based on convenience of the schedule, toxicity profile, and—sometimes—patient’s preference. So, toxicity and quality of life become kind of more decision-driving factors than disease biology. I’d have a discussion with patients about all three drugs, how they are administered, and what toxicities can be expected. Four-hour infusion of romidepsin might not be acceptable for some patients who have to travel long distances to treatment centers. They may prefer more rapid infusion (they would favor a trial of pralatrexate; or, as Alison mentioned, patient prefers a five-day/daily versus weekly administration.
It's kind of a coin toss decision outside clinical trials. Certainly, participation in studies in relapse setting is the high priority. We are all really looking forward to developing doublet combinations of novel agents, ie studies like the one Michelle is running at MD Anderson with a combination of alisertib and romidepsin.
DR. MOSKOWITZ: I'll echo what both Andrei and Michelle said that our choice of treatment is individualized and depends upon side-effect profile and the schedule. With regard to novel agents, I am also very excited about the studies that will be opening with the PI-3 kinase inhibitor, IPI-145. There have been promising results seen with single-agent IPI-1455 and we will be opening a study evaluating it in combination with bortezomib or romidepsin.
In addition, I mentioned earlier the IDH2 inhibitor, which we're studying right now in patients with AITL that is characterized by the presence of the IDH2 mutation. It would be exciting to see if targeting a specific mutation in this disease translates into responses.
DR. HORWITZ: Great, I think that was fantastic. I would like to thank Drs. Moskowitz, Shustov, and Fanale for a very thorough impractical discussion on how they approach and manage patients with T-cell lymphoma. I’m impressed that there is significant consistency among these experts in terms of how they manage these uncommon and often challenging lymphomas in terms of upfront combination chemotherapy approaches, combination considerations for the use of transplantation, as well as their enthusiasm for and dedication to incorporating clinical trials as part of everyday management.
References
1. Fanale MA, Horwitz SM, Forero-Torres A, et al. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: results of a phase I study. J Clin Oncol. 2014;32(28):3137–3143.
2. Schmitz N, Trümper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418–3425. doi: 10.1182/blood-2010-02-270785. Epub 2010 Jul 21.
3. Vose J, Armitage J, Weisenburger D, for the International T-Cell Lymphoma Project. J Clin Oncol. 2008;26(25):4124–4130. doi: 10.1200/JCO.2008.16.4558. Epub 2008 Jul 14.
4. O’Connor OA, Ozcan M, Jacobsen ED, et al. First multicenter, randomized phase 3 study in patients with relapsed/refractory peripheral T-cell lymphoma: alisertib versus investigator’s choice. Paper to be presented at: American Society of Hematology 57th Annual Meeting & Exposition; December 5–8, 2015; Orlando, FL.
5. Horwitz SM, Porcu P, Flinn I, et al. Duvelisib (IPI-145), a phosphoinositide-3-kinsae-γδ inhibitor, shows activity in patients with relapsed/refractory T-cell lymphoma. Paper presented at: American Society of Hematology 56th Annual Meeting & Exposition; December 6–9, 2014; San Francisco, CA.
Moderator: Steven M. Horwitz, MD1
Discussants: Alison Moskowitz, MD1; Michelle Fanale, MD2; Andrei Shustov, MD3
From Memorial Sloan Kettering Cancer Center, New York, NY1; MD Anderson Cancer Center, Houston, TX2; University of Washington Medical Center, Seattle, WA3
Address for correspondence: Steven M. Horwitz, MD, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065
E-mail: horwitzs@mskcc.org
DR. HORWITZ: My name is Dr. Steven Horwitz from Memorial Sloan-Kettering Cancer Center. I’m joined today by Drs. Alison Moskowitz, Memorial Sloan-Kettering, Michelle Fanale of MD Anderson, and Andrei Shustov, University of Washington in Seattle. Thank you all for joining us for this conversation on T-cell lymphoma. My colleagues are all well known as experts in T-cell lymphomas. Those of you who treat these diseases recognize the systemic T-cell lymphomas as one of our greater challenges in hematologic malignancies in terms of the treatment options for patients and the frequent lack of definitive data to guide our decisions.
I thought what we would do today is have a very practical discussion about the way we think about these diseases, the decisions we make, and the way we make those decisions. I'll start off by asking when you get a referral of a patient with a new diagnosis of peripheral T-cell lymphoma (PTCL), what are some of the basic things that you first think about in terms of approaching a new patient?
DR. SHUSTOV: I think one of the biggest challenges in T-cell lymphomas still remains making the proper diagnosis. In general, pathologists in the United States have a pretty good idea when they see T-cell lymphomas, however, subclassification remains a challenge even for expert hematopathologists due to frequent histologic overlap between the subtypes of PTCL, and even with non-malignant autoimmune disorders. I frequently see patients who are diagnosed with or misdiagnosed with a different subtype of T-cell lymphoma. The most challenging is differentiation between angioimmunoblastic T-cell lymphoma (AITL), anaplastic large cell T-cell lymphoma (ALCL), and PTCL not otherwise specified (PTCL-NOS), especially when the latter patients have high expression of CD30 and/or bear features resembling AITL.
Sometimes they are a slam-dunk diagnosis, but frequently our hematopathologists reverse the diagnosis after doing additional studies on the biopsy material. The most recent case I've seen in my clinic for consultation was a patient with a diagnosis of extranodal NK-cell lymphoma that was reclassified as a gamma-delta T-cell lymphoma after additional work-up. I truly believe that it is advisable that majority, if not all PTCL cases are reviewed by expert hematopathology teams at academic centers that see large volumes of these cases.
I think it's very important to educate community physicians and patients about a proper PTCL diagnosis, especially now that more targeted therapies are being developed and the gene expression profiling techniques will probably lead to identification of specific pathways that are amenable to therapy with specific biologic agents.
DR. FANALE: I'd like to expand upon what Andrei just said. For me the next step after confirming the pathology diagnosis is to think about two things. To think about whether or not this patient is a patient who might be eligible for an ongoing front-line trial, typically if the patient meets eligibility criteria for one of our ongoing front-line trials I would really recommend to the patient to consider being enrolled in that trial, and I also think about whether the patient, if he or she enters into remission with front-line therapy, can be considered for a consolidative autologous stem cell transplant.
I think it's very important to educate community physicians and patients about a proper PTCL diagnosis. Right now, our ongoing front-line trial is the ECHELON-2 trial which is evaluating brentuximab vedotin (BV) plus cyclophosphamide, doxorubicin, prednisone (CHP [BV-CHP]) chemotherapy compared to standard of care cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) chemotherapy, and that trial is based on the promising data that we've seen in the initial phase I trial that combined BV plus CHP chemotherapy followed by maintenance BV which demonstrated both high and durable complete remission (CR) rates.1 BV is an antibody drug conjugate that's targeted at CD30 and carries initial US Food and Drug Administration approval for patients who have relapsed or refractory ALCL which has a 100% level of CD30 positivity and then also has a National Comprehensive Cancer Network listing for treating other types of relapsed or refractory CD30 positive PTCL as well.
Also there is another upcoming front-line trial, which is to combine pralatrexate plus CHOP. If a patient isn't eligible for a clinical trial or it's just not feasible for that patient to enroll in a clinical trial, I will then look further at what would be potentially the best standard of care option for that patient.
I'll look at the patient's age and performance status and if they are generally less than age 65 or so and if otherwise well, I'll preferentially treat that patient with CHOEP which is CHOP plus etoposide. And then, for a patient who's either older than this or has multiple other comorbidities, I would treat that patient typically with CHOP alone.
DR. HORWITZ: Thank you, we'll go further into the selection of initial therapies but first circle back, I was curious as to how often your center comes to a different diagnosis than the referring center, and are there pitfalls you see that alert you to be suspicious of diagnosis.
DR. SHUSTOV: I'd say probably 10% of cases that we see at our center will be reclassified by our hematopathologists. In most cases, they do not necessarily reverse the diagnosis, but provide further clarification. It is occurring to me that in the community, pathologists are less likely to call the subtype of T-cell lymphoma and limit the report to the general description of T-cell lymphoprolipherative disorder, or state something like “consistent with T-cell lymphoma with features of AITL, or with features of anaplastic lymphoma.” I would admit though, that sometimes it's very difficult to identify the specific subtype of PTCL even in the expert hands; but I'd say these cases would constitute no more than 5% of PTCL patients.
DR. HORWITZ: And in your experience is it mostly fine tuning a T-cell lymphoma diagnosis, or do you see totally different diagnoses?
DR. MOSKOWITZ: Usually review by expert hematopathologists simply leads to fine-tuning the T-cell lymphoma diagnosis, however, I occasionally see significant changes in diagnosis. Often, alterations or clarification of a diagnosis are made possible only after we provide the pathologist with clinical history. For example, a lymph node biopsy may be interpreted as ALCL, however the knowledge that the particular patient has a history of mycosis fungoides would lead the pathologist to consider the diagnosis of large cell transformation of mycosis fungoides rather than ALCL. In such a case, molecular studies are helpful in confirming that the lymph node findings originate from the same clone as that in the original mycosis fungoides lesions, rather than representing a second primary.
DR. FANALE: Very occasionally, I've seen patients truly “with a misdiagnosis and a complete revision of diagnosis” and, usually, those pitfalls that I've seen them occasionally have been the young patients—the young patients who generally have disease in the thorax and neck, who are treated as though they have classical Hodgkin lymphoma, who have very significant progression of disease on standard of care treatment. So it's important that not all cases that have CD30 positivity are classical Hodgkin lymphoma even if the patient is young.
DR. HORWITZ: I often think the systemic T-cell lymphomas usually behave in an aggressive fashion so when the clinical picture and the diagnosis don't really fit, I think about getting a second biopsy before we finalize the diagnosis. Of course, when people are ill with obviously progressing disease, you may need to move more quickly.
I often think the systemic T-cell lymphomas usually behave in an aggressive fashion so when the clinical picture and the diagnosis don't really fit, I think about getting a second biopsy before we finalize the diagnosis. To move on, when you first see a patient, what is the decision tree in your mind in terms of picking therapy or planning therapy, or what kind of things do you consider?
DR. MOSKOWITZ: The first thing I think about when deciding upon treatment for a patient with T-cell lymphoma is whether or not I plan to use a curative approach to therapy. As was mentioned by Michelle, this decision is partly based upon the patients’ comorbidities and age. For patients who are eligible for curative therapy, our frontline approach for the most common types of T-cell lymphoma is to use CHOP with or without etoposide followed by consideration for autologous stem cell transplant in first remission. There are certainly individuals for whom such an aggressive strategy would not be appropriate due to age or co-morbidities. In these individuals, we may consider CHOP-based therapy alone or sometimes even milder approaches aimed at disease control.
DR. HORWITZ: Andrei, are you similar in the CHOP/CHOEP paradigm? And if so, what do you think of those data? How do you decide between the two and when do you do that regimen versus something different?
DR. SHUSTOV: I think I will double or triple what Michelle and Alison just said; for me, the two most important decisions that I have to make in the first encounter with patients with newly diagnosed PTCL are: 1) whether we're going to pursue curative approach strategy; and 2) whether the patient can tolerate the intensity of treatments that would provide him/her with the best chance of cure or long term remission. Patients who are elderly and have high risk disease would be very hard to cure, especially considering that the consolidative transplant might carry high rates of morbidity or mortality; more conservative strategies might be appropriate in these cases. On the other hand, younger and more fit patients might benefit more from intensified initial regimens—ie, CHOEP—followed by high-dose therapy and either autologous or allogeneic stem cell transplant (ALLO).
I usually have a long initial discussion with patients and families during which we decide on the intent of treatment and what to expect from certain regimens in terms of toxicities. I typically choose CHOEP regimen (or infusional version, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin [EPOCH]) for younger patients based on recent German data, even though this was a retrospective study and benefit of adding etoposide only approached statistical significance for the majority of PTCL subtypes.2 In older patients, I try to avoid anthracyclines, especially in the palliative intent setting, based on the retrospective analysis by the International T-Cell Lymphoma Project, and frequently use CEOP regimen (CHOEP minus anthracycline).3 I find that the majority of older patients tolerate this treatment somewhat better than anthracycline-containing combinations like CHOP.
In the very elderly and frail patients, I try to avoid combination chemotherapy all together. It is a somewhat easier decision for patients with AITL. Some of them are more indolent than other sub-types, and I would treat such patients with immunomodulatory approach, ie with a combination of prednisone and cyclosporine; then, I would consider single agent therapy with one of the recently approved agents for relapsed and refractory PTCL. I would also double what Michelle said in regard to making the best attempt to enroll patients into open clinical trials, because the current standards are not really satisfactory for many T-cell lymphoma patients.
DR. HORWITZ: It sounds like we all approach a new patient similarly. However, several of our up front trials are randomized against CHOP as opposed to CHOEP. When you're consenting a patient to those trials, how do you explain it to a patient or how important do you think the etoposide is?
DR. SHUSTOV: I share your frustration with quite a few of the current study designs for that exact reason. Some of these are confirmatory trials after conditional approvals of the new agents and are important. However, as often happens, in the confirmatory trials, we use controls that are a default from a lot of historical data, or the “common” way that the majority of patients are being treated. It is really a challenge when you consent patients to studies where a control arm is something that you think might not be quite adequate.
Having said that, the CHOEP study that was mentioned several times is a retrospective subgroup analysis and the addition of etoposide had marginal benefit that approached significance, but certainly was not a home run. I don't think we are ready to say that etoposide provides survival advantage in T-cell lymphoma patients and dismiss clinical trials in favor of just giving a patient the CHOEP, even though CHOP is admittedly not the best comparator. I discuss this controversy with patients and tell them frankly that the data that we base addition of etoposide on are not the best evidence one may have. Then, I ask patients to decide which approach sounds more reasonable to them and they make their choice.
DR. FANALE: To expand just slightly upon what Andrei said, I do emphasize to the patient and to the referring doctors that if you look at National Comprehensive Cancer Network guidelines, CHOP, CHOEP, EPOCH, all of these are potential options. So there's really not yet one trial that would say that one is clearly superior to the other at this time. I also emphasize to the patient and to the referring clinician that the only way, let's say, the patient could get the targeted agent plus the chemotherapy and have that 50% chance of, potentially, receiving that combination is really through the randomized clinical trial.
DR. HORWITZ: That was excellent, thank you. So you have alluded to this a bit. How much does subtype specific treatment come into play?
DR. MOSKOWITZ: At this point in time, outside of a clinical trial, for PTCL-NOS, AITL, and ALCL, the front-line approach would typically be CHOEP followed by autologous stem cell transplant. As Michelle mentioned, for a patient with ALCL, I would offer enrollment on the ECHELON-2 study in which patients are randomized to CHOP or BV-CHP, in order to give patients the option of potentially receiving BV.
For AITL, we are now obtaining molecular testing for certain mutations, particularly IDH2, because we currently have a study open with an IDH2 inhibitor that specifically enrolls patients with IDH2-mutant AITL. Because the testing takes some time to get back, we typically test patients’ biopsies at the time of diagnosis, so that we know if they're eligible for future clinical trials in case their disease does not respond to upfront therapy.
There are T-cell lymphoma subtypes that we treat quite differently from the entities discussed so far. For human T-lymphotropic virus-1 associated adult T-cell lymphoma, for example, the data with CHOP are really not good and we typically used EPOCH with the aim to consolidate with an allogeneic stem cell transplant.
Likewise, for hepatosplenic T-cell lymphoma, we have not observed good responses with the CHOP-based therapy and therefore we typically use platinum-based or ifosfamide-based therapy upfront with the aim to consolidate with allogeneic stem cell transplant. Extranodal NK/T-cell lymphoma is another entity for which CHOP is typically not effective and we have adopted asparaginase-based regimens, such as dexamethasone, methotrexate, ifosfamide, L-asparaginase, etoposide (SMILE) for this disease.
DR. SHUSTOV: I completely agree with Alison; for more common nodal lymphomas it is really hard at this point to base a treatment decision on histology, at least, in the front-line setting. However, some of the rare and unique subtypes, we generally treat with a completely different approach (ie, extranodal NK-cell lymphomas, enteropathy-associated T-cell lymphomas, adult T-cell leukemia/lymphomas).
DR. HORWITZ: Excellent. I’m curious, in a patient with ALCL who refused randomization of the ECHELON-2 trial, would you give them BV-CHP off study or would you discuss that as an alternative were they not to participate in the trial?
DR. FANALE: No. I don't urge treatment that's not approved for a particular line of therapy off clinical protocol with the exception that, let's say, if a patient is not a candidate for chemotherapy in second line setting for a particular lymphoma such as Hodgkin lymphoma—then, I would reference published data like Alison's data for a second line use of BV say for classical Hodgkin lymphoma and work with the insurance company to get the drug approved for that patient population, but if a patient just doesn't want to go on trial because they don't like the 50/50 chance of receiving BV-CHP compared to CHOP and they say, “I really want the 100% chance. Why don't you just contact my insurance?” I will explain to them the rationale of why the trial is being conducted including the hope that if the endpoints are met and the regimen is approved for front-line therapy then patients in the future can get BV-CHP at any oncology clinic, but I tell them for now it is a protocol based treatment option.
DR. HORWITZ: That's a very good answer. Can we now touch on the idea of transplant vs no transplant in first remission. Always, sometimes, never; how do you all think about that?
DR. SHUSTOV: I think that the idea of consolidative transplant is very heavily debated and discussed at the majority of specialized PTCL meetings. The reason for that is controversial nature of current clinical evidence. In the absence of randomized trials it is very hard to compare prospective phase II trial data to historical controls. You can interpret this in two ways; you might say, well, these are good data, outcome numbers look better than historical controls, and all patients with PTCL should have a consolidative transplant; or you can say, well, I don't have randomized data, and only a randomized study would really tell us whether post-induction consolidative transplant improves survival.
That is why it is such a controversial subject, and we agree that the phase II perspective studies of transplantation are hampered by significant bias; patients who are able and willing to travel to academic centers, were more robust and able to tolerate high dose therapy. They also have chemosensitive disease and that puts them in a completely different category than those you see in populational or retrospective studies, or other historical control trials.
Having said that, when I talk to patients, and I think that this is where I involve patients into treatment decision very heavily, I present them with the data and say that it is not wrong to do or not do the transplant in first CR. I make patients and family a big part of the decision. When they ask me what would give the patient the best chance being in remission five years from today, my answer is transplant, however, the data are not perfect, and the curative potential of autologous transplant in PTCL is not known.
DR. FANALE: So typically here, we would refer most patients on for front-line consolidative autologous stem cell transplant, I think, some exceptions are the ones that Andrei already touched on. If we're seeing a patient who has PTCL-NOS and this patient is the very rare patient who has early stage disease, with really no significant risk factors, that patient, unless there were pathologic features that showed a higher level of aggressiveness as Alison commented on, this patient might be one where we might defer doing the consolidative stem cell transplant for particularly if the patient’s disease entered into remission very quickly, but this still is controversial.
I'm not sure how you practice within each of your centers, but another patient, or where we would potentially defer, is a patient with fairly limited nasal NK/T-cell lymphoma, a patient that we’ve treated, let's say, with the dexamethasone, etoposide, ifosfamide, carboplatin (DeVIC) chemotherapy regimen plus a concomitant high-dose radiation, we typically historically here have deferred doing consolidative stem cell transplant for that patient population.
In terms of what Andrei commented on for when we might consider an ALLO and for a frontline consolidation, here we typically would not. The exception to that rule is that I've had a couple of patients here who have PTCL with a secondary hemophagocytic lymphohistiocytosis syndrome and then, for those patients because the concern is that the autologous stem cell transplant probably wouldn't be enough and for those patients given that they have such a dismal prognosis from the secondary hemophagocytic lymphohistiocytosis, we send those patients on for an ALLO.
DR. MOSKOWITZ: To expand upon early stage extranodal NK/T-cell lymphoma, I agree with Michelle that we wouldn't use an autologous stem cell transplant in first remission. Typically, for patients with localized disease, we recommend 2 cycles of SMILE followed by involved field radiation. Our patients treated with this approach have typically done quite well without needing a transplant.
DR. HORWITZ: That was great. If there are no further comments on upfront therapy, we can move over to relapse setting. When you see a patient at relapse, what are your strategies and how do you approach that patient?
DR. MOSKOWITZ: The first question in my mind when a patient is either not responding to or relapsing following front-line therapy is: what is my ultimate goal? For a patient who is fit and refractory to CHOP or has relapsed after CHOP or autologous stem cell transplant, my ultimate goal would be to try to get them to an ALLO. My choice of treatment following front-line therapy depends upon whether or not we are aiming for an ALLO as well as whether a donor has been identified for the patient.
Usually, if a patient just relapsed following front-line therapy, we are not necessarily ready to proceed quickly to ALLO. This may be because a donor has not yet been identified or because the patients’ eligibility for an ALLO is not clear. In this situation, my choice of treatment would be something that can be continued, potentially for several months, while we get things in place for the transplant. Treatment options in this situation would include the approved single agents for T-cell lymphoma, such as romidepsin, belinostat, pralatrexate, as well as BV (which would be specific for ALCL). In addition, I would consider enrollment on a promising clinical trial. Among these options, my choice of treatment typically depends upon the side effect profile and/or schedule, which is individualized for the patient. The one caveat would be that I typically will aim to use a histone deacetylase inhibitor (HDAC) inhibitor as my first choice for a patient with AITL.
Usually, if a patient just relapsed following front-line therapy, we are not necessarily ready to proceed quickly to ALLO. This may be because a donor has not yet been identified or because the patients’ eligibility for an ALLO is not clear. If my patient has a donor available and we are ready to move quickly to transplant, I would use one of the multi-agent regimens, such as ifosfamide, carboplatin, etoposide (ICE) or cisplatin, cytosine arabinoside, dexamethasone (DHAP), with the aim to try to get them into remission and relatively quickly proceed to transplant. The problem with using one of these types of regimens for everyone in the second-line setting is that the treatment cannot be continued indefinitely due to cumulative toxicity; therefore we need to know that we are ready to proceed to transplant if we are going to use one of the more intense regimens.
DR. HORWITZ: How do the others approach relapse?
DR. FANALE: Generally, a lot of similarities with what Alison discussed. Typically, it's if we're going to be sending the patient on for consideration for ALLO, I think the favor would be to try to use a regimen that has a high CR rate knowing that there is a trend toward improved progression free survival for the patient who enters into CR when compared to those who did not. I think the only slight difference might be just the timing and the selection, so, typically, here, even if the patient doesn't actually have a definite established donor quite yet we'll typically favor a combination type of a treatment approach, so whether or not that would be a platinum-based regimen such as ICE, as Alison mentioned, or gemcitabine-based chemotherapy regimen or similar.
I would generally favor one of those options, usually, over a single-agent therapeutic approach just knowing that the CR rates, generally, with exception of BV for ALCL are, generally, ranging about the 10%–15% range. And then, of course, first priority would be if there is a clinical trial available whether that's with a doublet of targeted agents or with the targeted agent plus chemotherapy in the relapse setting, we would typically favor that approach for a patient being considered for a stem cell transplant, ALLO approach.
DR. SHUSTOV: I'll consolidate what Michelle and Alison just said. I think we're on the same page. To me, the most important decision (even more important in the relapse setting) is to figure out whether we are still going to try to cure the lymphoma or we will pursue a palliative approach for which now we have several treatment options. So again, I’d have a long discussion with the patient, whether we're going to try and cure the disease, or we will pursue a palliative approach.
If this is a curative approach strategy and we're heading toward ALLO, we would start searching for the donor immediately. The choice of salvage therapy depends on duration of first remission. Primary refractory disease patients who failed CHOP, CHOEP, or EPOCH outright or within 3 months, I typically consider using the single agent approach, because even ICE and DHAP type regimens don’t work well for these patients. I put my “money” into HDAC inhibitors or antifolates, or other targeted therapy, if this is anaplastic lymphoma.
For patients who had at least 6 months remission from the initial therapy, younger patients who are still fit, I agree, I would consider standard salvage regimens like DHAP, etoposide, methylprednisolone, high-dosecytarabine, cisplatin (ESHAP) and ICE, and then, follow this with allograft.
DR. HORWITZ: You have all talked about allografting, I have two questions. What is your best guess in terms of percentage of patients you see with relapsed T-cell lymphoma who actually undergo ALLO at some point and do you ever consider autologous transplantation in those who didn't have it as part of their initial therapy?
DR. SHUSTOV: It certainly depends on the type of the academic center that patients are referred to, and we all practice at academic institutions with very strong ALLO programs. In patients who we select for a curative approach (one half to two thirds) we will at least make the best effort to take them to allogeneic grafting. Patients might fall off this track if they don't respond to salvage therapies or develop significant treatment/disease-related complications, ie fungal infections, organ toxicities, poor performance status, etc. Overall, I'd say that we successfully take about half of the patients with relapsed PTCL that are willing to pursue curative approach to allograft.
In addition, I consider autologous stem cell transplant in relapse setting for three situations. One is relapsed ALK-positive ALCL, some patients with ALK-negative ALCL who have had long initial remission and after relapse, and achieved second remission with BV or other salvage therapy. Finally, I’d consider auto-transplant in patients with AITL who achieved second CR with salvage therapy.
DR. HORWITZ: In terms of the relapse setting—some of you talked about angioimmunoblastic and HDAC inhibitors or BV for ALCL—when you have a relapse patient with PTCL-NOS, how do you pick a second line agent if you're not going directly to transplant, and are there new drugs that you're particularly excited about?
DR. FANALE: To answer your questions in terms of how I might choose a targeted agent for a patient who has PTCL-NOS and doesn't have AITL or ALCL, if the patient isn't eligible for trial or trial isn't feasible for that patient, I'll typically look at schedule, and then I'll look at side effect profile. For instance, for comparison, there are two HDAC inhibitor drugs right now approved, romidepsin and belinostat, so I think that potentially in a private practice setting, belinostat might be one when you look at the efficacy, being generally, around equivalent on where a patient might prefer that regimen because of the fact that they come in for five days in a row and then, their treatment is done for that cycle.
At a larger referral center to which patients are often traveling back and forth, it can be quite difficult for a patient to stay in town to do five days of treatment in a row. Often, these patients will prefer the romidepsin schedule because of the fact that they're coming back in once per week for three weeks in a row per cycle. The other way that I'll look at it is side effect profile, so, let's say, pralatrexate compared to the HDAC inhibitors, I will usually favor the HDAC inhibitors because of the potential side effect of mucositis with pralatrexate, although I have been able to manage through the mucositis by giving the patients more of a cutaneous T-cell lymphoma dosing format.
There had been a high level of interest in new emerging agents such as the aurora A-kinase inhibitors, alisertib, and that trial is now complete and the data are to be presented at The American Society of Hematology’s Annual Meeting this year.4 Other new therapies that are emerging are the PI3K inhibitors such as duvelisib and others. One advantage for many of the drugs in current trials is that they are oral agents.
My interest also lies in the combination approach, such as two or three targeted agent combinations, with the goal that if you see favorable CR rates and progression-free survival rates in the relapsed setting that you can then potentially move these treatments to the front-line setting to potentially move beyond the CHOP-based platform of therapies.
DR. SHUSTOV: For patients outside anaplastic lymphoma where, obviously, most of us would likely use BV, given the response rate to this agent, the decision is really based on convenience of the schedule, toxicity profile, and—sometimes—patient’s preference. So, toxicity and quality of life become kind of more decision-driving factors than disease biology. I’d have a discussion with patients about all three drugs, how they are administered, and what toxicities can be expected. Four-hour infusion of romidepsin might not be acceptable for some patients who have to travel long distances to treatment centers. They may prefer more rapid infusion (they would favor a trial of pralatrexate; or, as Alison mentioned, patient prefers a five-day/daily versus weekly administration.
It's kind of a coin toss decision outside clinical trials. Certainly, participation in studies in relapse setting is the high priority. We are all really looking forward to developing doublet combinations of novel agents, ie studies like the one Michelle is running at MD Anderson with a combination of alisertib and romidepsin.
DR. MOSKOWITZ: I'll echo what both Andrei and Michelle said that our choice of treatment is individualized and depends upon side-effect profile and the schedule. With regard to novel agents, I am also very excited about the studies that will be opening with the PI-3 kinase inhibitor, IPI-145. There have been promising results seen with single-agent IPI-1455 and we will be opening a study evaluating it in combination with bortezomib or romidepsin.
In addition, I mentioned earlier the IDH2 inhibitor, which we're studying right now in patients with AITL that is characterized by the presence of the IDH2 mutation. It would be exciting to see if targeting a specific mutation in this disease translates into responses.
DR. HORWITZ: Great, I think that was fantastic. I would like to thank Drs. Moskowitz, Shustov, and Fanale for a very thorough impractical discussion on how they approach and manage patients with T-cell lymphoma. I’m impressed that there is significant consistency among these experts in terms of how they manage these uncommon and often challenging lymphomas in terms of upfront combination chemotherapy approaches, combination considerations for the use of transplantation, as well as their enthusiasm for and dedication to incorporating clinical trials as part of everyday management.
References
1. Fanale MA, Horwitz SM, Forero-Torres A, et al. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: results of a phase I study. J Clin Oncol. 2014;32(28):3137–3143.
2. Schmitz N, Trümper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418–3425. doi: 10.1182/blood-2010-02-270785. Epub 2010 Jul 21.
3. Vose J, Armitage J, Weisenburger D, for the International T-Cell Lymphoma Project. J Clin Oncol. 2008;26(25):4124–4130. doi: 10.1200/JCO.2008.16.4558. Epub 2008 Jul 14.
4. O’Connor OA, Ozcan M, Jacobsen ED, et al. First multicenter, randomized phase 3 study in patients with relapsed/refractory peripheral T-cell lymphoma: alisertib versus investigator’s choice. Paper to be presented at: American Society of Hematology 57th Annual Meeting & Exposition; December 5–8, 2015; Orlando, FL.
5. Horwitz SM, Porcu P, Flinn I, et al. Duvelisib (IPI-145), a phosphoinositide-3-kinsae-γδ inhibitor, shows activity in patients with relapsed/refractory T-cell lymphoma. Paper presented at: American Society of Hematology 56th Annual Meeting & Exposition; December 6–9, 2014; San Francisco, CA.
Moderator: Steven M. Horwitz, MD1
Discussants: Alison Moskowitz, MD1; Michelle Fanale, MD2; Andrei Shustov, MD3
From Memorial Sloan Kettering Cancer Center, New York, NY1; MD Anderson Cancer Center, Houston, TX2; University of Washington Medical Center, Seattle, WA3
Address for correspondence: Steven M. Horwitz, MD, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065
E-mail: horwitzs@mskcc.org
DR. HORWITZ: My name is Dr. Steven Horwitz from Memorial Sloan-Kettering Cancer Center. I’m joined today by Drs. Alison Moskowitz, Memorial Sloan-Kettering, Michelle Fanale of MD Anderson, and Andrei Shustov, University of Washington in Seattle. Thank you all for joining us for this conversation on T-cell lymphoma. My colleagues are all well known as experts in T-cell lymphomas. Those of you who treat these diseases recognize the systemic T-cell lymphomas as one of our greater challenges in hematologic malignancies in terms of the treatment options for patients and the frequent lack of definitive data to guide our decisions.
I thought what we would do today is have a very practical discussion about the way we think about these diseases, the decisions we make, and the way we make those decisions. I'll start off by asking when you get a referral of a patient with a new diagnosis of peripheral T-cell lymphoma (PTCL), what are some of the basic things that you first think about in terms of approaching a new patient?
DR. SHUSTOV: I think one of the biggest challenges in T-cell lymphomas still remains making the proper diagnosis. In general, pathologists in the United States have a pretty good idea when they see T-cell lymphomas, however, subclassification remains a challenge even for expert hematopathologists due to frequent histologic overlap between the subtypes of PTCL, and even with non-malignant autoimmune disorders. I frequently see patients who are diagnosed with or misdiagnosed with a different subtype of T-cell lymphoma. The most challenging is differentiation between angioimmunoblastic T-cell lymphoma (AITL), anaplastic large cell T-cell lymphoma (ALCL), and PTCL not otherwise specified (PTCL-NOS), especially when the latter patients have high expression of CD30 and/or bear features resembling AITL.
Sometimes they are a slam-dunk diagnosis, but frequently our hematopathologists reverse the diagnosis after doing additional studies on the biopsy material. The most recent case I've seen in my clinic for consultation was a patient with a diagnosis of extranodal NK-cell lymphoma that was reclassified as a gamma-delta T-cell lymphoma after additional work-up. I truly believe that it is advisable that majority, if not all PTCL cases are reviewed by expert hematopathology teams at academic centers that see large volumes of these cases.
I think it's very important to educate community physicians and patients about a proper PTCL diagnosis, especially now that more targeted therapies are being developed and the gene expression profiling techniques will probably lead to identification of specific pathways that are amenable to therapy with specific biologic agents.
DR. FANALE: I'd like to expand upon what Andrei just said. For me the next step after confirming the pathology diagnosis is to think about two things. To think about whether or not this patient is a patient who might be eligible for an ongoing front-line trial, typically if the patient meets eligibility criteria for one of our ongoing front-line trials I would really recommend to the patient to consider being enrolled in that trial, and I also think about whether the patient, if he or she enters into remission with front-line therapy, can be considered for a consolidative autologous stem cell transplant.
I think it's very important to educate community physicians and patients about a proper PTCL diagnosis. Right now, our ongoing front-line trial is the ECHELON-2 trial which is evaluating brentuximab vedotin (BV) plus cyclophosphamide, doxorubicin, prednisone (CHP [BV-CHP]) chemotherapy compared to standard of care cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) chemotherapy, and that trial is based on the promising data that we've seen in the initial phase I trial that combined BV plus CHP chemotherapy followed by maintenance BV which demonstrated both high and durable complete remission (CR) rates.1 BV is an antibody drug conjugate that's targeted at CD30 and carries initial US Food and Drug Administration approval for patients who have relapsed or refractory ALCL which has a 100% level of CD30 positivity and then also has a National Comprehensive Cancer Network listing for treating other types of relapsed or refractory CD30 positive PTCL as well.
Also there is another upcoming front-line trial, which is to combine pralatrexate plus CHOP. If a patient isn't eligible for a clinical trial or it's just not feasible for that patient to enroll in a clinical trial, I will then look further at what would be potentially the best standard of care option for that patient.
I'll look at the patient's age and performance status and if they are generally less than age 65 or so and if otherwise well, I'll preferentially treat that patient with CHOEP which is CHOP plus etoposide. And then, for a patient who's either older than this or has multiple other comorbidities, I would treat that patient typically with CHOP alone.
DR. HORWITZ: Thank you, we'll go further into the selection of initial therapies but first circle back, I was curious as to how often your center comes to a different diagnosis than the referring center, and are there pitfalls you see that alert you to be suspicious of diagnosis.
DR. SHUSTOV: I'd say probably 10% of cases that we see at our center will be reclassified by our hematopathologists. In most cases, they do not necessarily reverse the diagnosis, but provide further clarification. It is occurring to me that in the community, pathologists are less likely to call the subtype of T-cell lymphoma and limit the report to the general description of T-cell lymphoprolipherative disorder, or state something like “consistent with T-cell lymphoma with features of AITL, or with features of anaplastic lymphoma.” I would admit though, that sometimes it's very difficult to identify the specific subtype of PTCL even in the expert hands; but I'd say these cases would constitute no more than 5% of PTCL patients.
DR. HORWITZ: And in your experience is it mostly fine tuning a T-cell lymphoma diagnosis, or do you see totally different diagnoses?
DR. MOSKOWITZ: Usually review by expert hematopathologists simply leads to fine-tuning the T-cell lymphoma diagnosis, however, I occasionally see significant changes in diagnosis. Often, alterations or clarification of a diagnosis are made possible only after we provide the pathologist with clinical history. For example, a lymph node biopsy may be interpreted as ALCL, however the knowledge that the particular patient has a history of mycosis fungoides would lead the pathologist to consider the diagnosis of large cell transformation of mycosis fungoides rather than ALCL. In such a case, molecular studies are helpful in confirming that the lymph node findings originate from the same clone as that in the original mycosis fungoides lesions, rather than representing a second primary.
DR. FANALE: Very occasionally, I've seen patients truly “with a misdiagnosis and a complete revision of diagnosis” and, usually, those pitfalls that I've seen them occasionally have been the young patients—the young patients who generally have disease in the thorax and neck, who are treated as though they have classical Hodgkin lymphoma, who have very significant progression of disease on standard of care treatment. So it's important that not all cases that have CD30 positivity are classical Hodgkin lymphoma even if the patient is young.
DR. HORWITZ: I often think the systemic T-cell lymphomas usually behave in an aggressive fashion so when the clinical picture and the diagnosis don't really fit, I think about getting a second biopsy before we finalize the diagnosis. Of course, when people are ill with obviously progressing disease, you may need to move more quickly.
I often think the systemic T-cell lymphomas usually behave in an aggressive fashion so when the clinical picture and the diagnosis don't really fit, I think about getting a second biopsy before we finalize the diagnosis. To move on, when you first see a patient, what is the decision tree in your mind in terms of picking therapy or planning therapy, or what kind of things do you consider?
DR. MOSKOWITZ: The first thing I think about when deciding upon treatment for a patient with T-cell lymphoma is whether or not I plan to use a curative approach to therapy. As was mentioned by Michelle, this decision is partly based upon the patients’ comorbidities and age. For patients who are eligible for curative therapy, our frontline approach for the most common types of T-cell lymphoma is to use CHOP with or without etoposide followed by consideration for autologous stem cell transplant in first remission. There are certainly individuals for whom such an aggressive strategy would not be appropriate due to age or co-morbidities. In these individuals, we may consider CHOP-based therapy alone or sometimes even milder approaches aimed at disease control.
DR. HORWITZ: Andrei, are you similar in the CHOP/CHOEP paradigm? And if so, what do you think of those data? How do you decide between the two and when do you do that regimen versus something different?
DR. SHUSTOV: I think I will double or triple what Michelle and Alison just said; for me, the two most important decisions that I have to make in the first encounter with patients with newly diagnosed PTCL are: 1) whether we're going to pursue curative approach strategy; and 2) whether the patient can tolerate the intensity of treatments that would provide him/her with the best chance of cure or long term remission. Patients who are elderly and have high risk disease would be very hard to cure, especially considering that the consolidative transplant might carry high rates of morbidity or mortality; more conservative strategies might be appropriate in these cases. On the other hand, younger and more fit patients might benefit more from intensified initial regimens—ie, CHOEP—followed by high-dose therapy and either autologous or allogeneic stem cell transplant (ALLO).
I usually have a long initial discussion with patients and families during which we decide on the intent of treatment and what to expect from certain regimens in terms of toxicities. I typically choose CHOEP regimen (or infusional version, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin [EPOCH]) for younger patients based on recent German data, even though this was a retrospective study and benefit of adding etoposide only approached statistical significance for the majority of PTCL subtypes.2 In older patients, I try to avoid anthracyclines, especially in the palliative intent setting, based on the retrospective analysis by the International T-Cell Lymphoma Project, and frequently use CEOP regimen (CHOEP minus anthracycline).3 I find that the majority of older patients tolerate this treatment somewhat better than anthracycline-containing combinations like CHOP.
In the very elderly and frail patients, I try to avoid combination chemotherapy all together. It is a somewhat easier decision for patients with AITL. Some of them are more indolent than other sub-types, and I would treat such patients with immunomodulatory approach, ie with a combination of prednisone and cyclosporine; then, I would consider single agent therapy with one of the recently approved agents for relapsed and refractory PTCL. I would also double what Michelle said in regard to making the best attempt to enroll patients into open clinical trials, because the current standards are not really satisfactory for many T-cell lymphoma patients.
DR. HORWITZ: It sounds like we all approach a new patient similarly. However, several of our up front trials are randomized against CHOP as opposed to CHOEP. When you're consenting a patient to those trials, how do you explain it to a patient or how important do you think the etoposide is?
DR. SHUSTOV: I share your frustration with quite a few of the current study designs for that exact reason. Some of these are confirmatory trials after conditional approvals of the new agents and are important. However, as often happens, in the confirmatory trials, we use controls that are a default from a lot of historical data, or the “common” way that the majority of patients are being treated. It is really a challenge when you consent patients to studies where a control arm is something that you think might not be quite adequate.
Having said that, the CHOEP study that was mentioned several times is a retrospective subgroup analysis and the addition of etoposide had marginal benefit that approached significance, but certainly was not a home run. I don't think we are ready to say that etoposide provides survival advantage in T-cell lymphoma patients and dismiss clinical trials in favor of just giving a patient the CHOEP, even though CHOP is admittedly not the best comparator. I discuss this controversy with patients and tell them frankly that the data that we base addition of etoposide on are not the best evidence one may have. Then, I ask patients to decide which approach sounds more reasonable to them and they make their choice.
DR. FANALE: To expand just slightly upon what Andrei said, I do emphasize to the patient and to the referring doctors that if you look at National Comprehensive Cancer Network guidelines, CHOP, CHOEP, EPOCH, all of these are potential options. So there's really not yet one trial that would say that one is clearly superior to the other at this time. I also emphasize to the patient and to the referring clinician that the only way, let's say, the patient could get the targeted agent plus the chemotherapy and have that 50% chance of, potentially, receiving that combination is really through the randomized clinical trial.
DR. HORWITZ: That was excellent, thank you. So you have alluded to this a bit. How much does subtype specific treatment come into play?
DR. MOSKOWITZ: At this point in time, outside of a clinical trial, for PTCL-NOS, AITL, and ALCL, the front-line approach would typically be CHOEP followed by autologous stem cell transplant. As Michelle mentioned, for a patient with ALCL, I would offer enrollment on the ECHELON-2 study in which patients are randomized to CHOP or BV-CHP, in order to give patients the option of potentially receiving BV.
For AITL, we are now obtaining molecular testing for certain mutations, particularly IDH2, because we currently have a study open with an IDH2 inhibitor that specifically enrolls patients with IDH2-mutant AITL. Because the testing takes some time to get back, we typically test patients’ biopsies at the time of diagnosis, so that we know if they're eligible for future clinical trials in case their disease does not respond to upfront therapy.
There are T-cell lymphoma subtypes that we treat quite differently from the entities discussed so far. For human T-lymphotropic virus-1 associated adult T-cell lymphoma, for example, the data with CHOP are really not good and we typically used EPOCH with the aim to consolidate with an allogeneic stem cell transplant.
Likewise, for hepatosplenic T-cell lymphoma, we have not observed good responses with the CHOP-based therapy and therefore we typically use platinum-based or ifosfamide-based therapy upfront with the aim to consolidate with allogeneic stem cell transplant. Extranodal NK/T-cell lymphoma is another entity for which CHOP is typically not effective and we have adopted asparaginase-based regimens, such as dexamethasone, methotrexate, ifosfamide, L-asparaginase, etoposide (SMILE) for this disease.
DR. SHUSTOV: I completely agree with Alison; for more common nodal lymphomas it is really hard at this point to base a treatment decision on histology, at least, in the front-line setting. However, some of the rare and unique subtypes, we generally treat with a completely different approach (ie, extranodal NK-cell lymphomas, enteropathy-associated T-cell lymphomas, adult T-cell leukemia/lymphomas).
DR. HORWITZ: Excellent. I’m curious, in a patient with ALCL who refused randomization of the ECHELON-2 trial, would you give them BV-CHP off study or would you discuss that as an alternative were they not to participate in the trial?
DR. FANALE: No. I don't urge treatment that's not approved for a particular line of therapy off clinical protocol with the exception that, let's say, if a patient is not a candidate for chemotherapy in second line setting for a particular lymphoma such as Hodgkin lymphoma—then, I would reference published data like Alison's data for a second line use of BV say for classical Hodgkin lymphoma and work with the insurance company to get the drug approved for that patient population, but if a patient just doesn't want to go on trial because they don't like the 50/50 chance of receiving BV-CHP compared to CHOP and they say, “I really want the 100% chance. Why don't you just contact my insurance?” I will explain to them the rationale of why the trial is being conducted including the hope that if the endpoints are met and the regimen is approved for front-line therapy then patients in the future can get BV-CHP at any oncology clinic, but I tell them for now it is a protocol based treatment option.
DR. HORWITZ: That's a very good answer. Can we now touch on the idea of transplant vs no transplant in first remission. Always, sometimes, never; how do you all think about that?
DR. SHUSTOV: I think that the idea of consolidative transplant is very heavily debated and discussed at the majority of specialized PTCL meetings. The reason for that is controversial nature of current clinical evidence. In the absence of randomized trials it is very hard to compare prospective phase II trial data to historical controls. You can interpret this in two ways; you might say, well, these are good data, outcome numbers look better than historical controls, and all patients with PTCL should have a consolidative transplant; or you can say, well, I don't have randomized data, and only a randomized study would really tell us whether post-induction consolidative transplant improves survival.
That is why it is such a controversial subject, and we agree that the phase II perspective studies of transplantation are hampered by significant bias; patients who are able and willing to travel to academic centers, were more robust and able to tolerate high dose therapy. They also have chemosensitive disease and that puts them in a completely different category than those you see in populational or retrospective studies, or other historical control trials.
Having said that, when I talk to patients, and I think that this is where I involve patients into treatment decision very heavily, I present them with the data and say that it is not wrong to do or not do the transplant in first CR. I make patients and family a big part of the decision. When they ask me what would give the patient the best chance being in remission five years from today, my answer is transplant, however, the data are not perfect, and the curative potential of autologous transplant in PTCL is not known.
DR. FANALE: So typically here, we would refer most patients on for front-line consolidative autologous stem cell transplant, I think, some exceptions are the ones that Andrei already touched on. If we're seeing a patient who has PTCL-NOS and this patient is the very rare patient who has early stage disease, with really no significant risk factors, that patient, unless there were pathologic features that showed a higher level of aggressiveness as Alison commented on, this patient might be one where we might defer doing the consolidative stem cell transplant for particularly if the patient’s disease entered into remission very quickly, but this still is controversial.
I'm not sure how you practice within each of your centers, but another patient, or where we would potentially defer, is a patient with fairly limited nasal NK/T-cell lymphoma, a patient that we’ve treated, let's say, with the dexamethasone, etoposide, ifosfamide, carboplatin (DeVIC) chemotherapy regimen plus a concomitant high-dose radiation, we typically historically here have deferred doing consolidative stem cell transplant for that patient population.
In terms of what Andrei commented on for when we might consider an ALLO and for a frontline consolidation, here we typically would not. The exception to that rule is that I've had a couple of patients here who have PTCL with a secondary hemophagocytic lymphohistiocytosis syndrome and then, for those patients because the concern is that the autologous stem cell transplant probably wouldn't be enough and for those patients given that they have such a dismal prognosis from the secondary hemophagocytic lymphohistiocytosis, we send those patients on for an ALLO.
DR. MOSKOWITZ: To expand upon early stage extranodal NK/T-cell lymphoma, I agree with Michelle that we wouldn't use an autologous stem cell transplant in first remission. Typically, for patients with localized disease, we recommend 2 cycles of SMILE followed by involved field radiation. Our patients treated with this approach have typically done quite well without needing a transplant.
DR. HORWITZ: That was great. If there are no further comments on upfront therapy, we can move over to relapse setting. When you see a patient at relapse, what are your strategies and how do you approach that patient?
DR. MOSKOWITZ: The first question in my mind when a patient is either not responding to or relapsing following front-line therapy is: what is my ultimate goal? For a patient who is fit and refractory to CHOP or has relapsed after CHOP or autologous stem cell transplant, my ultimate goal would be to try to get them to an ALLO. My choice of treatment following front-line therapy depends upon whether or not we are aiming for an ALLO as well as whether a donor has been identified for the patient.
Usually, if a patient just relapsed following front-line therapy, we are not necessarily ready to proceed quickly to ALLO. This may be because a donor has not yet been identified or because the patients’ eligibility for an ALLO is not clear. In this situation, my choice of treatment would be something that can be continued, potentially for several months, while we get things in place for the transplant. Treatment options in this situation would include the approved single agents for T-cell lymphoma, such as romidepsin, belinostat, pralatrexate, as well as BV (which would be specific for ALCL). In addition, I would consider enrollment on a promising clinical trial. Among these options, my choice of treatment typically depends upon the side effect profile and/or schedule, which is individualized for the patient. The one caveat would be that I typically will aim to use a histone deacetylase inhibitor (HDAC) inhibitor as my first choice for a patient with AITL.
Usually, if a patient just relapsed following front-line therapy, we are not necessarily ready to proceed quickly to ALLO. This may be because a donor has not yet been identified or because the patients’ eligibility for an ALLO is not clear. If my patient has a donor available and we are ready to move quickly to transplant, I would use one of the multi-agent regimens, such as ifosfamide, carboplatin, etoposide (ICE) or cisplatin, cytosine arabinoside, dexamethasone (DHAP), with the aim to try to get them into remission and relatively quickly proceed to transplant. The problem with using one of these types of regimens for everyone in the second-line setting is that the treatment cannot be continued indefinitely due to cumulative toxicity; therefore we need to know that we are ready to proceed to transplant if we are going to use one of the more intense regimens.
DR. HORWITZ: How do the others approach relapse?
DR. FANALE: Generally, a lot of similarities with what Alison discussed. Typically, it's if we're going to be sending the patient on for consideration for ALLO, I think the favor would be to try to use a regimen that has a high CR rate knowing that there is a trend toward improved progression free survival for the patient who enters into CR when compared to those who did not. I think the only slight difference might be just the timing and the selection, so, typically, here, even if the patient doesn't actually have a definite established donor quite yet we'll typically favor a combination type of a treatment approach, so whether or not that would be a platinum-based regimen such as ICE, as Alison mentioned, or gemcitabine-based chemotherapy regimen or similar.
I would generally favor one of those options, usually, over a single-agent therapeutic approach just knowing that the CR rates, generally, with exception of BV for ALCL are, generally, ranging about the 10%–15% range. And then, of course, first priority would be if there is a clinical trial available whether that's with a doublet of targeted agents or with the targeted agent plus chemotherapy in the relapse setting, we would typically favor that approach for a patient being considered for a stem cell transplant, ALLO approach.
DR. SHUSTOV: I'll consolidate what Michelle and Alison just said. I think we're on the same page. To me, the most important decision (even more important in the relapse setting) is to figure out whether we are still going to try to cure the lymphoma or we will pursue a palliative approach for which now we have several treatment options. So again, I’d have a long discussion with the patient, whether we're going to try and cure the disease, or we will pursue a palliative approach.
If this is a curative approach strategy and we're heading toward ALLO, we would start searching for the donor immediately. The choice of salvage therapy depends on duration of first remission. Primary refractory disease patients who failed CHOP, CHOEP, or EPOCH outright or within 3 months, I typically consider using the single agent approach, because even ICE and DHAP type regimens don’t work well for these patients. I put my “money” into HDAC inhibitors or antifolates, or other targeted therapy, if this is anaplastic lymphoma.
For patients who had at least 6 months remission from the initial therapy, younger patients who are still fit, I agree, I would consider standard salvage regimens like DHAP, etoposide, methylprednisolone, high-dosecytarabine, cisplatin (ESHAP) and ICE, and then, follow this with allograft.
DR. HORWITZ: You have all talked about allografting, I have two questions. What is your best guess in terms of percentage of patients you see with relapsed T-cell lymphoma who actually undergo ALLO at some point and do you ever consider autologous transplantation in those who didn't have it as part of their initial therapy?
DR. SHUSTOV: It certainly depends on the type of the academic center that patients are referred to, and we all practice at academic institutions with very strong ALLO programs. In patients who we select for a curative approach (one half to two thirds) we will at least make the best effort to take them to allogeneic grafting. Patients might fall off this track if they don't respond to salvage therapies or develop significant treatment/disease-related complications, ie fungal infections, organ toxicities, poor performance status, etc. Overall, I'd say that we successfully take about half of the patients with relapsed PTCL that are willing to pursue curative approach to allograft.
In addition, I consider autologous stem cell transplant in relapse setting for three situations. One is relapsed ALK-positive ALCL, some patients with ALK-negative ALCL who have had long initial remission and after relapse, and achieved second remission with BV or other salvage therapy. Finally, I’d consider auto-transplant in patients with AITL who achieved second CR with salvage therapy.
DR. HORWITZ: In terms of the relapse setting—some of you talked about angioimmunoblastic and HDAC inhibitors or BV for ALCL—when you have a relapse patient with PTCL-NOS, how do you pick a second line agent if you're not going directly to transplant, and are there new drugs that you're particularly excited about?
DR. FANALE: To answer your questions in terms of how I might choose a targeted agent for a patient who has PTCL-NOS and doesn't have AITL or ALCL, if the patient isn't eligible for trial or trial isn't feasible for that patient, I'll typically look at schedule, and then I'll look at side effect profile. For instance, for comparison, there are two HDAC inhibitor drugs right now approved, romidepsin and belinostat, so I think that potentially in a private practice setting, belinostat might be one when you look at the efficacy, being generally, around equivalent on where a patient might prefer that regimen because of the fact that they come in for five days in a row and then, their treatment is done for that cycle.
At a larger referral center to which patients are often traveling back and forth, it can be quite difficult for a patient to stay in town to do five days of treatment in a row. Often, these patients will prefer the romidepsin schedule because of the fact that they're coming back in once per week for three weeks in a row per cycle. The other way that I'll look at it is side effect profile, so, let's say, pralatrexate compared to the HDAC inhibitors, I will usually favor the HDAC inhibitors because of the potential side effect of mucositis with pralatrexate, although I have been able to manage through the mucositis by giving the patients more of a cutaneous T-cell lymphoma dosing format.
There had been a high level of interest in new emerging agents such as the aurora A-kinase inhibitors, alisertib, and that trial is now complete and the data are to be presented at The American Society of Hematology’s Annual Meeting this year.4 Other new therapies that are emerging are the PI3K inhibitors such as duvelisib and others. One advantage for many of the drugs in current trials is that they are oral agents.
My interest also lies in the combination approach, such as two or three targeted agent combinations, with the goal that if you see favorable CR rates and progression-free survival rates in the relapsed setting that you can then potentially move these treatments to the front-line setting to potentially move beyond the CHOP-based platform of therapies.
DR. SHUSTOV: For patients outside anaplastic lymphoma where, obviously, most of us would likely use BV, given the response rate to this agent, the decision is really based on convenience of the schedule, toxicity profile, and—sometimes—patient’s preference. So, toxicity and quality of life become kind of more decision-driving factors than disease biology. I’d have a discussion with patients about all three drugs, how they are administered, and what toxicities can be expected. Four-hour infusion of romidepsin might not be acceptable for some patients who have to travel long distances to treatment centers. They may prefer more rapid infusion (they would favor a trial of pralatrexate; or, as Alison mentioned, patient prefers a five-day/daily versus weekly administration.
It's kind of a coin toss decision outside clinical trials. Certainly, participation in studies in relapse setting is the high priority. We are all really looking forward to developing doublet combinations of novel agents, ie studies like the one Michelle is running at MD Anderson with a combination of alisertib and romidepsin.
DR. MOSKOWITZ: I'll echo what both Andrei and Michelle said that our choice of treatment is individualized and depends upon side-effect profile and the schedule. With regard to novel agents, I am also very excited about the studies that will be opening with the PI-3 kinase inhibitor, IPI-145. There have been promising results seen with single-agent IPI-1455 and we will be opening a study evaluating it in combination with bortezomib or romidepsin.
In addition, I mentioned earlier the IDH2 inhibitor, which we're studying right now in patients with AITL that is characterized by the presence of the IDH2 mutation. It would be exciting to see if targeting a specific mutation in this disease translates into responses.
DR. HORWITZ: Great, I think that was fantastic. I would like to thank Drs. Moskowitz, Shustov, and Fanale for a very thorough impractical discussion on how they approach and manage patients with T-cell lymphoma. I’m impressed that there is significant consistency among these experts in terms of how they manage these uncommon and often challenging lymphomas in terms of upfront combination chemotherapy approaches, combination considerations for the use of transplantation, as well as their enthusiasm for and dedication to incorporating clinical trials as part of everyday management.
References
1. Fanale MA, Horwitz SM, Forero-Torres A, et al. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: results of a phase I study. J Clin Oncol. 2014;32(28):3137–3143.
2. Schmitz N, Trümper L, Ziepert M, et al. Treatment and prognosis of mature T-cell and NK-cell lymphoma: an analysis of patients with T-cell lymphoma treated in studies of the German High-Grade Non-Hodgkin Lymphoma Study Group. Blood. 2010;116(18):3418–3425. doi: 10.1182/blood-2010-02-270785. Epub 2010 Jul 21.
3. Vose J, Armitage J, Weisenburger D, for the International T-Cell Lymphoma Project. J Clin Oncol. 2008;26(25):4124–4130. doi: 10.1200/JCO.2008.16.4558. Epub 2008 Jul 14.
4. O’Connor OA, Ozcan M, Jacobsen ED, et al. First multicenter, randomized phase 3 study in patients with relapsed/refractory peripheral T-cell lymphoma: alisertib versus investigator’s choice. Paper to be presented at: American Society of Hematology 57th Annual Meeting & Exposition; December 5–8, 2015; Orlando, FL.
5. Horwitz SM, Porcu P, Flinn I, et al. Duvelisib (IPI-145), a phosphoinositide-3-kinsae-γδ inhibitor, shows activity in patients with relapsed/refractory T-cell lymphoma. Paper presented at: American Society of Hematology 56th Annual Meeting & Exposition; December 6–9, 2014; San Francisco, CA.
ACR: Years of TNF blockers did not increase risk of lymphoma in RA
SAN FRANCISCO – Rheumatoid arthritis conferred a doubling of the risk of lymphoma when compared against the general population in a large Swedish registry study, regardless of the patients’ experience with biological disease-modifying antirheumatic drugs.
But patients who took biological disease-modifying antirheumatic drugs (bDMARDs) had a sixfold greater risk of natural killer or T-cell lymphoma than did the general population, and that association was about four times stronger than for patients who had never taken biologics, reported Dr. Karin Hellgren, who led the study at the Karolinska Institute in Stockholm. That finding in particular shows the need to keep studying the links between bDMARDs and specific lymphoma subtypes, he said.
Severe rheumatoid arthritis seems to strongly increase the risk of lymphoma (Arthritis Rheum. 2006;54[3]:692-701), but researchers have debated whether the reason relates to bDMARDs or RA itself. Clinical trials have produced conflicting results; observational studies have reported no overall link between bDMARDs and lymphoma, but have raised questions about long-term exposure, the effects of individual agents, and lymphoma subtypes, Dr. Hellgren said at the annual meeting of the American College of Rheumatology.
To delve deeper into these issues, he and his associates compared 15 years of data for 13,240 RA patients on bDMARDs from the Swedish Biologics (ARTIS), Patient, and Cancer Registers and a national cohort of 46,568 bDMARD-naive patients. The researchers also compared both groups with 458,846 age- and gender-matched adults from the Swedish Population Register, following individuals until the end of 2012 or until lymphoma diagnosis, death, emigration, or bDMARD initiation, in the case of the naive patients.
Overall, patients with RA averaged one diagnosis of lymphoma per 1,000 population, compared with 0.5 cases per 1,000 for the overall population of Sweden, Dr. Hellgren said. In terms of absolute numbers, there were 241 cases of lymphoma among bDMARD-naive patients, 1,413 cases in the general population, and 69 cases among patients on bDMARDs, including 68 who were taking TNF inhibitors. The average age of the latter group of patients was 57 years. They had been diagnosed with RA at about age 50, had a mean 28-joint Disease Activity Score score of 5.3, and averaged 5.9 years of exposure to TNF inhibitors. Their risk of any type of malignant lymphoma was about 20% higher than for bDMARD-naive patients, but the difference was insignificant overall and in subgroups stratified by gender, age, and year starting treatment. Likewise, although patients were at greater risk of lymphoma if they had been on bDMARDs for 5-15 years (hazard ratio, 1.9) than for less time (HRs, about 1.0), there was no significant difference in risk compared with bDMARD-naive patients.
“There also were no statistically significant differences between drugs,” including infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira), Dr. Hellgren said. “In terms of the newer TNF inhibitors and other biologics, data are still too scarce to evaluate,” he added. Both exposed and bDMARD-naive patients were at especially high risk of Hodgkin lymphoma and diffuse large B-cell lymphoma, compared with the general population, but the strongest association of all was between bDMARD exposure and natural killer or T-cell lymphoma (HR, 6.0; 95% CI, 2.7-13.3). “The distribution of lymphoma subtypes warrants further assessment,” Dr. Hellgren concluded.
The ARTIS registry is funded by AbbVie, Bristol-Myers Squibb, Roche, Merck, Pfizer, Sobi, Lilly, and UCB. Dr. Hellgren had no disclosures. One coauthor reported financial relationships with AstraZeneca, Pfizer, UCB, Roche, Merck, Bristol-Myers Squibb, and AbbVie.
SAN FRANCISCO – Rheumatoid arthritis conferred a doubling of the risk of lymphoma when compared against the general population in a large Swedish registry study, regardless of the patients’ experience with biological disease-modifying antirheumatic drugs.
But patients who took biological disease-modifying antirheumatic drugs (bDMARDs) had a sixfold greater risk of natural killer or T-cell lymphoma than did the general population, and that association was about four times stronger than for patients who had never taken biologics, reported Dr. Karin Hellgren, who led the study at the Karolinska Institute in Stockholm. That finding in particular shows the need to keep studying the links between bDMARDs and specific lymphoma subtypes, he said.
Severe rheumatoid arthritis seems to strongly increase the risk of lymphoma (Arthritis Rheum. 2006;54[3]:692-701), but researchers have debated whether the reason relates to bDMARDs or RA itself. Clinical trials have produced conflicting results; observational studies have reported no overall link between bDMARDs and lymphoma, but have raised questions about long-term exposure, the effects of individual agents, and lymphoma subtypes, Dr. Hellgren said at the annual meeting of the American College of Rheumatology.
To delve deeper into these issues, he and his associates compared 15 years of data for 13,240 RA patients on bDMARDs from the Swedish Biologics (ARTIS), Patient, and Cancer Registers and a national cohort of 46,568 bDMARD-naive patients. The researchers also compared both groups with 458,846 age- and gender-matched adults from the Swedish Population Register, following individuals until the end of 2012 or until lymphoma diagnosis, death, emigration, or bDMARD initiation, in the case of the naive patients.
Overall, patients with RA averaged one diagnosis of lymphoma per 1,000 population, compared with 0.5 cases per 1,000 for the overall population of Sweden, Dr. Hellgren said. In terms of absolute numbers, there were 241 cases of lymphoma among bDMARD-naive patients, 1,413 cases in the general population, and 69 cases among patients on bDMARDs, including 68 who were taking TNF inhibitors. The average age of the latter group of patients was 57 years. They had been diagnosed with RA at about age 50, had a mean 28-joint Disease Activity Score score of 5.3, and averaged 5.9 years of exposure to TNF inhibitors. Their risk of any type of malignant lymphoma was about 20% higher than for bDMARD-naive patients, but the difference was insignificant overall and in subgroups stratified by gender, age, and year starting treatment. Likewise, although patients were at greater risk of lymphoma if they had been on bDMARDs for 5-15 years (hazard ratio, 1.9) than for less time (HRs, about 1.0), there was no significant difference in risk compared with bDMARD-naive patients.
“There also were no statistically significant differences between drugs,” including infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira), Dr. Hellgren said. “In terms of the newer TNF inhibitors and other biologics, data are still too scarce to evaluate,” he added. Both exposed and bDMARD-naive patients were at especially high risk of Hodgkin lymphoma and diffuse large B-cell lymphoma, compared with the general population, but the strongest association of all was between bDMARD exposure and natural killer or T-cell lymphoma (HR, 6.0; 95% CI, 2.7-13.3). “The distribution of lymphoma subtypes warrants further assessment,” Dr. Hellgren concluded.
The ARTIS registry is funded by AbbVie, Bristol-Myers Squibb, Roche, Merck, Pfizer, Sobi, Lilly, and UCB. Dr. Hellgren had no disclosures. One coauthor reported financial relationships with AstraZeneca, Pfizer, UCB, Roche, Merck, Bristol-Myers Squibb, and AbbVie.
SAN FRANCISCO – Rheumatoid arthritis conferred a doubling of the risk of lymphoma when compared against the general population in a large Swedish registry study, regardless of the patients’ experience with biological disease-modifying antirheumatic drugs.
But patients who took biological disease-modifying antirheumatic drugs (bDMARDs) had a sixfold greater risk of natural killer or T-cell lymphoma than did the general population, and that association was about four times stronger than for patients who had never taken biologics, reported Dr. Karin Hellgren, who led the study at the Karolinska Institute in Stockholm. That finding in particular shows the need to keep studying the links between bDMARDs and specific lymphoma subtypes, he said.
Severe rheumatoid arthritis seems to strongly increase the risk of lymphoma (Arthritis Rheum. 2006;54[3]:692-701), but researchers have debated whether the reason relates to bDMARDs or RA itself. Clinical trials have produced conflicting results; observational studies have reported no overall link between bDMARDs and lymphoma, but have raised questions about long-term exposure, the effects of individual agents, and lymphoma subtypes, Dr. Hellgren said at the annual meeting of the American College of Rheumatology.
To delve deeper into these issues, he and his associates compared 15 years of data for 13,240 RA patients on bDMARDs from the Swedish Biologics (ARTIS), Patient, and Cancer Registers and a national cohort of 46,568 bDMARD-naive patients. The researchers also compared both groups with 458,846 age- and gender-matched adults from the Swedish Population Register, following individuals until the end of 2012 or until lymphoma diagnosis, death, emigration, or bDMARD initiation, in the case of the naive patients.
Overall, patients with RA averaged one diagnosis of lymphoma per 1,000 population, compared with 0.5 cases per 1,000 for the overall population of Sweden, Dr. Hellgren said. In terms of absolute numbers, there were 241 cases of lymphoma among bDMARD-naive patients, 1,413 cases in the general population, and 69 cases among patients on bDMARDs, including 68 who were taking TNF inhibitors. The average age of the latter group of patients was 57 years. They had been diagnosed with RA at about age 50, had a mean 28-joint Disease Activity Score score of 5.3, and averaged 5.9 years of exposure to TNF inhibitors. Their risk of any type of malignant lymphoma was about 20% higher than for bDMARD-naive patients, but the difference was insignificant overall and in subgroups stratified by gender, age, and year starting treatment. Likewise, although patients were at greater risk of lymphoma if they had been on bDMARDs for 5-15 years (hazard ratio, 1.9) than for less time (HRs, about 1.0), there was no significant difference in risk compared with bDMARD-naive patients.
“There also were no statistically significant differences between drugs,” including infliximab (Remicade), etanercept (Enbrel), and adalimumab (Humira), Dr. Hellgren said. “In terms of the newer TNF inhibitors and other biologics, data are still too scarce to evaluate,” he added. Both exposed and bDMARD-naive patients were at especially high risk of Hodgkin lymphoma and diffuse large B-cell lymphoma, compared with the general population, but the strongest association of all was between bDMARD exposure and natural killer or T-cell lymphoma (HR, 6.0; 95% CI, 2.7-13.3). “The distribution of lymphoma subtypes warrants further assessment,” Dr. Hellgren concluded.
The ARTIS registry is funded by AbbVie, Bristol-Myers Squibb, Roche, Merck, Pfizer, Sobi, Lilly, and UCB. Dr. Hellgren had no disclosures. One coauthor reported financial relationships with AstraZeneca, Pfizer, UCB, Roche, Merck, Bristol-Myers Squibb, and AbbVie.
AT THE ACR ANNUAL MEETING
Key clinical point: Exposure to biological disease-modifying antirheumatic drugs did not increase the overall risk of lymphoma among patients with rheumatoid arthritis.
Major finding: Patients were at greater risk of lymphoma if they had been on bDMARDs for 5-15 years (hazard ratio, 1.9) than for less time (HRs, about 1.0), but there was no significant difference in risk compared with bDMARD-naive patients. However, patients on bDMARDs had about a fourfold greater risk of NK/T-cell lymphoma compared with bDMARD-naive patients.
Data source: A matched registry analysis of 13,240 patients from the Swedish Biologics (ARTIS), Patient, and Cancer Registers; 46,568 bio-naive patients; and 458,846 members of the general population.
Disclosures: The ARTIS registry is funded by AbbVie, Bristol-Myers Squibb, Roche, Merck, Pfizer, Sobi, Lilly, and UCB. Dr. Hellgren had no disclosures. One coauthor reported financial relationships with AstraZeneca, Pfizer, UCB, Roche, Merck, Bristol-Myers Squibb, and AbbVie.
What’s on tap at ASH 2015
FROM A TELECONFERENCE – The American Society of Hematology’s (ASH) 57th annual meeting in Orlando is chock-full of much-anticipated results in cancer immunotherapies such as CAR T cell therapies and checkpoint inhibitors, advances in sickle cell disease, and practical advice on managing the latest drugs in the clinic, ASH officials said in a teleconference. Here are some of the day-by-day picks selected by ASH president Dr. David Williams and ASH secretary Dr. Stephanie J. Lee, who gave their recommendations during a conference call for the press. Meeting abstracts are now available online.
Saturday, Dec. 5
Clinical applications of newly approved drugs
The popular special education session on clinical applications of newly approved drugs returns on Saturday, Dec. 5 at 9:30 a.m., with didactic presentations that address issues clinicians may face such as drug-drug interactions, side effects, and adverse events. The three drugs to be discussed this year are: idarucizumab (Praxbind), the first specific reversal agent approved for dabigatran reversal; blinatumomab (Blincyto), approved for second-line treatment of Philadelphia chromosomenegative acute lymphoblastic leukemia; and the histone deacetylase (HDAC) inhibitor panobinostat (Farydak), approved for the treatment of multiple myeloma.
Adoptive immunotherapy
One presentation to look out for next month is abstract 99at 12:30 p.m. on Saturday, Dec. 5 in the adoptive immunotherapy session, Dr. Williams told reporters. The chimeric antigen receptor (CAR)-T-cell approach has relied on genetically engineering the patient’s own T cells to rev up the immune system. This group’s approach is to treat B-cell malignancies after allogeneic hematopoietic stem cell transplantation using a single infusion of anti-CD19 CAR-T cells from the patient’s transplant donor.
Eight of 20 patients treated with this strategy achieved remission, including six complete remissions and two partial remissions. Importantly, none of these patients developed acute graft-versus-host disease, a potential consequence of using allogeneic rather than autologous T cells, he said. The authors also noted that patients who responded and went into remission were marked by higher numbers of these infused CAR-T cells in their circulation, suggesting a biomarker of response.
Checkpoint, please?
Immunotherapy is a “very hot area,” so ASH has put together a special session at 4 p.m. Saturday called “Checkpoint, Please?” Dr. Williams said. Topics include the role of programmed death (PD)-1 and PD-ligand 1 in acute and chronic graft-versus-host disease, checkpoint blockade with neoantigen cancer vaccines, and insights into the mechanisms of action of anti-CTLA-4 (cytotoxic T-lymphocyte–associated protein 4) antibody therapy.
Sunday, Dec. 6
Precision medicine
Sunday’s plenary scientific session will include several noteworthy personalized medicine abstracts featuring emerging therapies targeted to specific genetic subtypes, Dr. Lee, from the University of Washington, Seattle, said.
Plenary abstract 6 is a large, multinational study looking at whether adding the multikinase inhibitor midostaurin to standard induction therapy and carried through 1 year of maintenance would improve outcomes in newly diagnosed acute myeloid leukemia with FLT3 mutations. Patients with these deleterious mutations do enter remission with chemotherapy, but often relapse.
Overall and event-free survival were better at 5 years by about 7% to 8% in the experimental arm using midostaurin, she said. Caveats are that complete response rates were similar in both arms and lower than reported in other trials.
“Because we know that patients with this FLT3 mutation have a very poor prognosis with standard chemotherapy, more than half of the patients in this trial received an allogeneic transplant,” Dr. Lee noted. “But the abstract does say that the results are similar if you censor at the time of the transplant.”
In this same vein of precision medicine is plenary abstract 1, testing whether adding rituximab to standard chemotherapy improves outcomes in adults with CD-20–positive, Philadelphia chromosome–negative, B-cell precursor acute lymphoblastic leukemia (ALL). Rituximab (Rituxan) binds to CD-20, which is found in about 30% to 50% of adult B-cell ALL, she said.
At 2 years, patients treated with rituximab had longer event-free survival than controls (65% vs. 52%; P = .038), but similar overall survival (71% vs. 64%; P = .09), according to the abstract. The rituximab arm also received more allogeneic transplants, but again, after censoring the data, the abstract states that both event-free and overall survival were longer with rituximab, Dr. Lee said.
Sickle cell anemia
Sunday’s plenary session will also feature the very important TWiTCH (TCD with Transfusions Changing to Hydroxyurea) study evaluating hydroxyurea therapy as an alternative to chronic blood transfusions to prevent stroke. Stroke is one of the most dreaded complications of sickle cell disease, occurring in up to 10% of children, Dr. Williams said. Though transfusions are effective, they have to be continued indefinitely and lead to iron overload. Hydroxyurea increases the amount of fetal hemoglobin and fetal red blood cells and has become a standard therapy to attenuate the complications of sickle cell.
The phase III noninferiority study, which used Transcranial Doppler (TCD) screening to identify children at elevated risk for stroke, showed that hydroxyurea “was as good as current therapy with red cell transfusions and there was some indication, although not significant, that it might even be superior in lowering the TCD levels,” Dr. Williams said. An added benefit of the hydroxyurea was that it improved the patients’ iron overload status. There were no strokes in either group.
Sunday’s abstract 202 is another presentation “that I’m sure will get a lot of attention,” Dr. Williams said. It offers updated details on outcomes from patients with sickle cell disease (SCD) treated with a novel gene therapy transduced with the LentiGlobin BB305 (Bluebird Bio) lentiviral vector. Patients with beta thalassemia major have remained transfusion-independent for more than a year after this treatment, with results now available from four patients with SCD. One patient with a severe phenotype has had no sickle cell complications and has been able to stop his transfusion therapy, while two of the other four patients are also transfusion-independent.
“This is an early study showing what appears to be efficacy of the gene therapy approach not in thalassemia, but in sickle cell disease,” Dr. Williams said, noting that abstract 3233 will also feature results using LentiGlobin gene therapy in severe SCD.
ASH/EHA joint symposium
Also noteworthy is a special joint ASH/European Hematology Association symposium looking at how well genomic data are being incorporated into practice in the U.S. and Europe.
Monday, Dec. 7
ASH/FDA joint symposium
A joint ASH/FDA symposium on late-breaking drug approvals is new this year and features drugs that gained approval in November 2015. FDA product-reviewers will discuss safety and efficacy issues in the clinical approval trials and toxicity studies, while clinicians will share their experiences in the real-world use of these drugs.
“This is information that is really going to be very hot off the press and presented in conjunction with the FDA,” Dr. Lee said.
Dr. Williams reported research funding from Bluebird Bio. Dr. Lee reported having no conflicts of interest.
FROM A TELECONFERENCE – The American Society of Hematology’s (ASH) 57th annual meeting in Orlando is chock-full of much-anticipated results in cancer immunotherapies such as CAR T cell therapies and checkpoint inhibitors, advances in sickle cell disease, and practical advice on managing the latest drugs in the clinic, ASH officials said in a teleconference. Here are some of the day-by-day picks selected by ASH president Dr. David Williams and ASH secretary Dr. Stephanie J. Lee, who gave their recommendations during a conference call for the press. Meeting abstracts are now available online.
Saturday, Dec. 5
Clinical applications of newly approved drugs
The popular special education session on clinical applications of newly approved drugs returns on Saturday, Dec. 5 at 9:30 a.m., with didactic presentations that address issues clinicians may face such as drug-drug interactions, side effects, and adverse events. The three drugs to be discussed this year are: idarucizumab (Praxbind), the first specific reversal agent approved for dabigatran reversal; blinatumomab (Blincyto), approved for second-line treatment of Philadelphia chromosomenegative acute lymphoblastic leukemia; and the histone deacetylase (HDAC) inhibitor panobinostat (Farydak), approved for the treatment of multiple myeloma.
Adoptive immunotherapy
One presentation to look out for next month is abstract 99at 12:30 p.m. on Saturday, Dec. 5 in the adoptive immunotherapy session, Dr. Williams told reporters. The chimeric antigen receptor (CAR)-T-cell approach has relied on genetically engineering the patient’s own T cells to rev up the immune system. This group’s approach is to treat B-cell malignancies after allogeneic hematopoietic stem cell transplantation using a single infusion of anti-CD19 CAR-T cells from the patient’s transplant donor.
Eight of 20 patients treated with this strategy achieved remission, including six complete remissions and two partial remissions. Importantly, none of these patients developed acute graft-versus-host disease, a potential consequence of using allogeneic rather than autologous T cells, he said. The authors also noted that patients who responded and went into remission were marked by higher numbers of these infused CAR-T cells in their circulation, suggesting a biomarker of response.
Checkpoint, please?
Immunotherapy is a “very hot area,” so ASH has put together a special session at 4 p.m. Saturday called “Checkpoint, Please?” Dr. Williams said. Topics include the role of programmed death (PD)-1 and PD-ligand 1 in acute and chronic graft-versus-host disease, checkpoint blockade with neoantigen cancer vaccines, and insights into the mechanisms of action of anti-CTLA-4 (cytotoxic T-lymphocyte–associated protein 4) antibody therapy.
Sunday, Dec. 6
Precision medicine
Sunday’s plenary scientific session will include several noteworthy personalized medicine abstracts featuring emerging therapies targeted to specific genetic subtypes, Dr. Lee, from the University of Washington, Seattle, said.
Plenary abstract 6 is a large, multinational study looking at whether adding the multikinase inhibitor midostaurin to standard induction therapy and carried through 1 year of maintenance would improve outcomes in newly diagnosed acute myeloid leukemia with FLT3 mutations. Patients with these deleterious mutations do enter remission with chemotherapy, but often relapse.
Overall and event-free survival were better at 5 years by about 7% to 8% in the experimental arm using midostaurin, she said. Caveats are that complete response rates were similar in both arms and lower than reported in other trials.
“Because we know that patients with this FLT3 mutation have a very poor prognosis with standard chemotherapy, more than half of the patients in this trial received an allogeneic transplant,” Dr. Lee noted. “But the abstract does say that the results are similar if you censor at the time of the transplant.”
In this same vein of precision medicine is plenary abstract 1, testing whether adding rituximab to standard chemotherapy improves outcomes in adults with CD-20–positive, Philadelphia chromosome–negative, B-cell precursor acute lymphoblastic leukemia (ALL). Rituximab (Rituxan) binds to CD-20, which is found in about 30% to 50% of adult B-cell ALL, she said.
At 2 years, patients treated with rituximab had longer event-free survival than controls (65% vs. 52%; P = .038), but similar overall survival (71% vs. 64%; P = .09), according to the abstract. The rituximab arm also received more allogeneic transplants, but again, after censoring the data, the abstract states that both event-free and overall survival were longer with rituximab, Dr. Lee said.
Sickle cell anemia
Sunday’s plenary session will also feature the very important TWiTCH (TCD with Transfusions Changing to Hydroxyurea) study evaluating hydroxyurea therapy as an alternative to chronic blood transfusions to prevent stroke. Stroke is one of the most dreaded complications of sickle cell disease, occurring in up to 10% of children, Dr. Williams said. Though transfusions are effective, they have to be continued indefinitely and lead to iron overload. Hydroxyurea increases the amount of fetal hemoglobin and fetal red blood cells and has become a standard therapy to attenuate the complications of sickle cell.
The phase III noninferiority study, which used Transcranial Doppler (TCD) screening to identify children at elevated risk for stroke, showed that hydroxyurea “was as good as current therapy with red cell transfusions and there was some indication, although not significant, that it might even be superior in lowering the TCD levels,” Dr. Williams said. An added benefit of the hydroxyurea was that it improved the patients’ iron overload status. There were no strokes in either group.
Sunday’s abstract 202 is another presentation “that I’m sure will get a lot of attention,” Dr. Williams said. It offers updated details on outcomes from patients with sickle cell disease (SCD) treated with a novel gene therapy transduced with the LentiGlobin BB305 (Bluebird Bio) lentiviral vector. Patients with beta thalassemia major have remained transfusion-independent for more than a year after this treatment, with results now available from four patients with SCD. One patient with a severe phenotype has had no sickle cell complications and has been able to stop his transfusion therapy, while two of the other four patients are also transfusion-independent.
“This is an early study showing what appears to be efficacy of the gene therapy approach not in thalassemia, but in sickle cell disease,” Dr. Williams said, noting that abstract 3233 will also feature results using LentiGlobin gene therapy in severe SCD.
ASH/EHA joint symposium
Also noteworthy is a special joint ASH/European Hematology Association symposium looking at how well genomic data are being incorporated into practice in the U.S. and Europe.
Monday, Dec. 7
ASH/FDA joint symposium
A joint ASH/FDA symposium on late-breaking drug approvals is new this year and features drugs that gained approval in November 2015. FDA product-reviewers will discuss safety and efficacy issues in the clinical approval trials and toxicity studies, while clinicians will share their experiences in the real-world use of these drugs.
“This is information that is really going to be very hot off the press and presented in conjunction with the FDA,” Dr. Lee said.
Dr. Williams reported research funding from Bluebird Bio. Dr. Lee reported having no conflicts of interest.
FROM A TELECONFERENCE – The American Society of Hematology’s (ASH) 57th annual meeting in Orlando is chock-full of much-anticipated results in cancer immunotherapies such as CAR T cell therapies and checkpoint inhibitors, advances in sickle cell disease, and practical advice on managing the latest drugs in the clinic, ASH officials said in a teleconference. Here are some of the day-by-day picks selected by ASH president Dr. David Williams and ASH secretary Dr. Stephanie J. Lee, who gave their recommendations during a conference call for the press. Meeting abstracts are now available online.
Saturday, Dec. 5
Clinical applications of newly approved drugs
The popular special education session on clinical applications of newly approved drugs returns on Saturday, Dec. 5 at 9:30 a.m., with didactic presentations that address issues clinicians may face such as drug-drug interactions, side effects, and adverse events. The three drugs to be discussed this year are: idarucizumab (Praxbind), the first specific reversal agent approved for dabigatran reversal; blinatumomab (Blincyto), approved for second-line treatment of Philadelphia chromosomenegative acute lymphoblastic leukemia; and the histone deacetylase (HDAC) inhibitor panobinostat (Farydak), approved for the treatment of multiple myeloma.
Adoptive immunotherapy
One presentation to look out for next month is abstract 99at 12:30 p.m. on Saturday, Dec. 5 in the adoptive immunotherapy session, Dr. Williams told reporters. The chimeric antigen receptor (CAR)-T-cell approach has relied on genetically engineering the patient’s own T cells to rev up the immune system. This group’s approach is to treat B-cell malignancies after allogeneic hematopoietic stem cell transplantation using a single infusion of anti-CD19 CAR-T cells from the patient’s transplant donor.
Eight of 20 patients treated with this strategy achieved remission, including six complete remissions and two partial remissions. Importantly, none of these patients developed acute graft-versus-host disease, a potential consequence of using allogeneic rather than autologous T cells, he said. The authors also noted that patients who responded and went into remission were marked by higher numbers of these infused CAR-T cells in their circulation, suggesting a biomarker of response.
Checkpoint, please?
Immunotherapy is a “very hot area,” so ASH has put together a special session at 4 p.m. Saturday called “Checkpoint, Please?” Dr. Williams said. Topics include the role of programmed death (PD)-1 and PD-ligand 1 in acute and chronic graft-versus-host disease, checkpoint blockade with neoantigen cancer vaccines, and insights into the mechanisms of action of anti-CTLA-4 (cytotoxic T-lymphocyte–associated protein 4) antibody therapy.
Sunday, Dec. 6
Precision medicine
Sunday’s plenary scientific session will include several noteworthy personalized medicine abstracts featuring emerging therapies targeted to specific genetic subtypes, Dr. Lee, from the University of Washington, Seattle, said.
Plenary abstract 6 is a large, multinational study looking at whether adding the multikinase inhibitor midostaurin to standard induction therapy and carried through 1 year of maintenance would improve outcomes in newly diagnosed acute myeloid leukemia with FLT3 mutations. Patients with these deleterious mutations do enter remission with chemotherapy, but often relapse.
Overall and event-free survival were better at 5 years by about 7% to 8% in the experimental arm using midostaurin, she said. Caveats are that complete response rates were similar in both arms and lower than reported in other trials.
“Because we know that patients with this FLT3 mutation have a very poor prognosis with standard chemotherapy, more than half of the patients in this trial received an allogeneic transplant,” Dr. Lee noted. “But the abstract does say that the results are similar if you censor at the time of the transplant.”
In this same vein of precision medicine is plenary abstract 1, testing whether adding rituximab to standard chemotherapy improves outcomes in adults with CD-20–positive, Philadelphia chromosome–negative, B-cell precursor acute lymphoblastic leukemia (ALL). Rituximab (Rituxan) binds to CD-20, which is found in about 30% to 50% of adult B-cell ALL, she said.
At 2 years, patients treated with rituximab had longer event-free survival than controls (65% vs. 52%; P = .038), but similar overall survival (71% vs. 64%; P = .09), according to the abstract. The rituximab arm also received more allogeneic transplants, but again, after censoring the data, the abstract states that both event-free and overall survival were longer with rituximab, Dr. Lee said.
Sickle cell anemia
Sunday’s plenary session will also feature the very important TWiTCH (TCD with Transfusions Changing to Hydroxyurea) study evaluating hydroxyurea therapy as an alternative to chronic blood transfusions to prevent stroke. Stroke is one of the most dreaded complications of sickle cell disease, occurring in up to 10% of children, Dr. Williams said. Though transfusions are effective, they have to be continued indefinitely and lead to iron overload. Hydroxyurea increases the amount of fetal hemoglobin and fetal red blood cells and has become a standard therapy to attenuate the complications of sickle cell.
The phase III noninferiority study, which used Transcranial Doppler (TCD) screening to identify children at elevated risk for stroke, showed that hydroxyurea “was as good as current therapy with red cell transfusions and there was some indication, although not significant, that it might even be superior in lowering the TCD levels,” Dr. Williams said. An added benefit of the hydroxyurea was that it improved the patients’ iron overload status. There were no strokes in either group.
Sunday’s abstract 202 is another presentation “that I’m sure will get a lot of attention,” Dr. Williams said. It offers updated details on outcomes from patients with sickle cell disease (SCD) treated with a novel gene therapy transduced with the LentiGlobin BB305 (Bluebird Bio) lentiviral vector. Patients with beta thalassemia major have remained transfusion-independent for more than a year after this treatment, with results now available from four patients with SCD. One patient with a severe phenotype has had no sickle cell complications and has been able to stop his transfusion therapy, while two of the other four patients are also transfusion-independent.
“This is an early study showing what appears to be efficacy of the gene therapy approach not in thalassemia, but in sickle cell disease,” Dr. Williams said, noting that abstract 3233 will also feature results using LentiGlobin gene therapy in severe SCD.
ASH/EHA joint symposium
Also noteworthy is a special joint ASH/European Hematology Association symposium looking at how well genomic data are being incorporated into practice in the U.S. and Europe.
Monday, Dec. 7
ASH/FDA joint symposium
A joint ASH/FDA symposium on late-breaking drug approvals is new this year and features drugs that gained approval in November 2015. FDA product-reviewers will discuss safety and efficacy issues in the clinical approval trials and toxicity studies, while clinicians will share their experiences in the real-world use of these drugs.
“This is information that is really going to be very hot off the press and presented in conjunction with the FDA,” Dr. Lee said.
Dr. Williams reported research funding from Bluebird Bio. Dr. Lee reported having no conflicts of interest.
Topical resiquimod effective for early-stage cutaneous T-cell lymphoma
Topical resiquimod was effective and well tolerated in patients with early-stage cutaneous T-cell lymphoma (CTCL), in some cases inducing regression in both treated and untreated lesions, according to researchers.
The mean number of prior unsuccessful therapies among the patients was 6, yet the majority of patients (11 of 12) experienced significant improvement, and 2 patients had complete clinical responses with no evidence of disease after treatment. One patient, despite a 15-year history of disease and 11 unsuccessful treatments, experienced a complete resolution of both treated and untreated skin lesions.
The open-label, phase I trial evaluated 12 patients with early-stage CTCL. Patients experienced minor adverse effects (all grade 1), which were primarily skin irritation. The trial evaluated 0.03% and 0.06% resiquimod, with complete and more rapid responses occurring at the higher dose. Both doses were equally well tolerated.
“These studies support further trials of this medication in early-stage, skin-limited CTCL and suggest resiquimod might also be useful as an adjuvant therapy in the treatment of more advanced CTCL,” wrote Dr. Alain Rook of the Department of Dermatology and the Center for Clinical Biostatistics and Epidemiology, Perelman School of Medicine, Philadelphia, and colleagues.
Arising from T cells that traffic to the skin, CTCLs are non-Hodgkin lymphomas whose only potential cure is stem cell transplantation. Studies suggest that host antitumor immunity plays an important role in the disease, and in this study, high responders showed recruitment and expansion of benign T-cell clones and activation of T cells and natural killer cells in the skin.
In the absence of cell-surface markers to distinguish malignant from benign T cells in the lesion, the team used high throughput screening of the T-cell receptor–beta gene to quantify malignant cells and monitor response to therapy. Of the 10 patients with identified malignant cells, biopsied lesions showed that most had reduction of malignant T-cell clones and 3 had complete eradication. The results may not reflect responses in nonbiopsied lesions.
Resiquimod recruits T cells and other immune cells to the skin, causing inflammation that the researchers observed persisted after complete or nearly complete malignant T-cell eradication. Study results suggested that activation of CD4+ cells and expansion of tumor-specific T cells is critical for effectiveness of resiquimod.
Topical resiquimod was effective and well tolerated in patients with early-stage cutaneous T-cell lymphoma (CTCL), in some cases inducing regression in both treated and untreated lesions, according to researchers.
The mean number of prior unsuccessful therapies among the patients was 6, yet the majority of patients (11 of 12) experienced significant improvement, and 2 patients had complete clinical responses with no evidence of disease after treatment. One patient, despite a 15-year history of disease and 11 unsuccessful treatments, experienced a complete resolution of both treated and untreated skin lesions.
The open-label, phase I trial evaluated 12 patients with early-stage CTCL. Patients experienced minor adverse effects (all grade 1), which were primarily skin irritation. The trial evaluated 0.03% and 0.06% resiquimod, with complete and more rapid responses occurring at the higher dose. Both doses were equally well tolerated.
“These studies support further trials of this medication in early-stage, skin-limited CTCL and suggest resiquimod might also be useful as an adjuvant therapy in the treatment of more advanced CTCL,” wrote Dr. Alain Rook of the Department of Dermatology and the Center for Clinical Biostatistics and Epidemiology, Perelman School of Medicine, Philadelphia, and colleagues.
Arising from T cells that traffic to the skin, CTCLs are non-Hodgkin lymphomas whose only potential cure is stem cell transplantation. Studies suggest that host antitumor immunity plays an important role in the disease, and in this study, high responders showed recruitment and expansion of benign T-cell clones and activation of T cells and natural killer cells in the skin.
In the absence of cell-surface markers to distinguish malignant from benign T cells in the lesion, the team used high throughput screening of the T-cell receptor–beta gene to quantify malignant cells and monitor response to therapy. Of the 10 patients with identified malignant cells, biopsied lesions showed that most had reduction of malignant T-cell clones and 3 had complete eradication. The results may not reflect responses in nonbiopsied lesions.
Resiquimod recruits T cells and other immune cells to the skin, causing inflammation that the researchers observed persisted after complete or nearly complete malignant T-cell eradication. Study results suggested that activation of CD4+ cells and expansion of tumor-specific T cells is critical for effectiveness of resiquimod.
Topical resiquimod was effective and well tolerated in patients with early-stage cutaneous T-cell lymphoma (CTCL), in some cases inducing regression in both treated and untreated lesions, according to researchers.
The mean number of prior unsuccessful therapies among the patients was 6, yet the majority of patients (11 of 12) experienced significant improvement, and 2 patients had complete clinical responses with no evidence of disease after treatment. One patient, despite a 15-year history of disease and 11 unsuccessful treatments, experienced a complete resolution of both treated and untreated skin lesions.
The open-label, phase I trial evaluated 12 patients with early-stage CTCL. Patients experienced minor adverse effects (all grade 1), which were primarily skin irritation. The trial evaluated 0.03% and 0.06% resiquimod, with complete and more rapid responses occurring at the higher dose. Both doses were equally well tolerated.
“These studies support further trials of this medication in early-stage, skin-limited CTCL and suggest resiquimod might also be useful as an adjuvant therapy in the treatment of more advanced CTCL,” wrote Dr. Alain Rook of the Department of Dermatology and the Center for Clinical Biostatistics and Epidemiology, Perelman School of Medicine, Philadelphia, and colleagues.
Arising from T cells that traffic to the skin, CTCLs are non-Hodgkin lymphomas whose only potential cure is stem cell transplantation. Studies suggest that host antitumor immunity plays an important role in the disease, and in this study, high responders showed recruitment and expansion of benign T-cell clones and activation of T cells and natural killer cells in the skin.
In the absence of cell-surface markers to distinguish malignant from benign T cells in the lesion, the team used high throughput screening of the T-cell receptor–beta gene to quantify malignant cells and monitor response to therapy. Of the 10 patients with identified malignant cells, biopsied lesions showed that most had reduction of malignant T-cell clones and 3 had complete eradication. The results may not reflect responses in nonbiopsied lesions.
Resiquimod recruits T cells and other immune cells to the skin, causing inflammation that the researchers observed persisted after complete or nearly complete malignant T-cell eradication. Study results suggested that activation of CD4+ cells and expansion of tumor-specific T cells is critical for effectiveness of resiquimod.
FROM BLOOD
Key clinical point: Topical resiquimod was effective and well tolerated in patients with early-stage cutaneous T-cell lymphoma (CTCL).
Major finding: In total, 11 of 12 patients had significant improvement: 2 had resolution of all evidence of disease, and 9 experienced improvement greater than or equal to 50%.
Data source: The open-label, phase I trial evaluated 12 patients with early stage CTCL.
Disclosures: Dr. Rook and one coauthor have patents pending on HTS in cutaneous lymphoma. His coauthor is employed by Adaptive Biotechnologies.
Immunotherapy: Inject locally, treat globally?
NEW YORK – “Inject locally, treat globally” may become a new mantra for cancer immunotherapy. Dr. Ronald Levy, professor and chief of the department of oncology at Stanford (Calif.) University, discussed impressive and durable systemic results from local treatment of tumors, reviewing his group’s recent work and discussing ongoing early stage clinical trials.
In the hope of triggering an immune response that induces a systemic CD8 T-cell response, Dr. Levy and other investigators began experimenting with intralesional injections of immunotherapies, with and without adjunctive radiation or chemotherapy. The principle has been evaluated in a mouse model, in which up to 80% of mice receiving intratumoral immunotherapy were cured. Now, human trials are underway for solid tumors as well as lymphoma.
An early clinical trial involving 15 patients with recurrent low-grade B-cell lymphoma combined targeted low-dose radiation of a single tumor site with injection of CpG, immune-boosting snippets of DNA, into the same site. Looking for partial or complete regression, investigators saw promising results, though some patients who were responders didn’t see full effect until 24 weeks after injection (J Clin Oncol. 2010 Oct 1;28[28]:4324-32). The overall objective response rate was 27%, although 80% of patients (12 of 15) had stable disease or partial or complete response through a median follow-up of 33.7 months. Some individual patients had marked regression or disappearance of bulky tumors at distant sites.
CpG – motifs of cytosines and guanines – were strung together, said Dr. Levy, with a sulfur rather than a phosphate backbone to make them more stable for injection. These bits of DNA, which are present in both bacteria and vertebrates, are an agonist for toll-like receptor 9, activating B cells and dendritic cells, and then tumor-specific T-cells.
Dr. Levy and his collaborators used a two-tumor mouse model to track intralesional injection effectiveness for a variety of immunotherapies. Mice seeded with tumor cells bilaterally over the abdomen were injected with CpG and two other immune therapies at a single abdominal tumor site, and bilateral regression, if any, was tracked in comparison to systemic immunotherapy (J Clin Invest. 2013;123[6]:2447-63).
Though both treatment arms had good initial response, 70% of the systemically treated mice relapsed by 150 days after injection, compared with just 10% of those receiving intratumoral therapy (P = .002).
Of the 23 mice whose therapy consisted of intratumoral administration of CpG together with anti-CTLA4 and anti-OX40 antibodies (aCTLA4 and aOX40), 21 (91%) were alive 50 days after treatment. These mice fared better than did those receiving any other intratumoral treatment combination (P = .004, compared with CpG+aOX40; P = .03, compared with aCTLA4), suggesting a synergistic benefit to the triple combination.
Somewhat surprisingly, even murine models that also had tumor seeding into brain tissue saw marked reduction or even resolution of brain tumors, showing that the blood-brain barrier does not impede the effect within the CNS.
What didn’t work? PD-1 inhibitors were not particularly effective at provoking a systemic effect when injected into tumors. Peritumoral injection, though theoretically taking advantage of some aspects of the tumor microenvironment, also did not show global effect.
Based on the human CpG + radiation trials and the mouse experiments, the research group is proceeding with phase I and II clinical trials of CpG in combinations with aCTLA4 and aOX40. These, Dr. Levy said, were more likely to be available clinically, and human intratumoral T regulatory cells are known to express both CTLA4 and OX40.
All agents and combinations had an enhancing effect, said Dr. Levy. “We like this treatment and trial design,” he said, noting that everyone sees benefit at the local injection site, and some see global results.
Dr. Levy discussed multiple studies, and he disclosed receiving grant support from Pfizer, Dynavax, and Bristol-Myers Squibb. He also has served as a consultant to Five Prime, Kite, BeiGene, Innate Pharma, Bullet Biotech, and Immune Design.
NEW YORK – “Inject locally, treat globally” may become a new mantra for cancer immunotherapy. Dr. Ronald Levy, professor and chief of the department of oncology at Stanford (Calif.) University, discussed impressive and durable systemic results from local treatment of tumors, reviewing his group’s recent work and discussing ongoing early stage clinical trials.
In the hope of triggering an immune response that induces a systemic CD8 T-cell response, Dr. Levy and other investigators began experimenting with intralesional injections of immunotherapies, with and without adjunctive radiation or chemotherapy. The principle has been evaluated in a mouse model, in which up to 80% of mice receiving intratumoral immunotherapy were cured. Now, human trials are underway for solid tumors as well as lymphoma.
An early clinical trial involving 15 patients with recurrent low-grade B-cell lymphoma combined targeted low-dose radiation of a single tumor site with injection of CpG, immune-boosting snippets of DNA, into the same site. Looking for partial or complete regression, investigators saw promising results, though some patients who were responders didn’t see full effect until 24 weeks after injection (J Clin Oncol. 2010 Oct 1;28[28]:4324-32). The overall objective response rate was 27%, although 80% of patients (12 of 15) had stable disease or partial or complete response through a median follow-up of 33.7 months. Some individual patients had marked regression or disappearance of bulky tumors at distant sites.
CpG – motifs of cytosines and guanines – were strung together, said Dr. Levy, with a sulfur rather than a phosphate backbone to make them more stable for injection. These bits of DNA, which are present in both bacteria and vertebrates, are an agonist for toll-like receptor 9, activating B cells and dendritic cells, and then tumor-specific T-cells.
Dr. Levy and his collaborators used a two-tumor mouse model to track intralesional injection effectiveness for a variety of immunotherapies. Mice seeded with tumor cells bilaterally over the abdomen were injected with CpG and two other immune therapies at a single abdominal tumor site, and bilateral regression, if any, was tracked in comparison to systemic immunotherapy (J Clin Invest. 2013;123[6]:2447-63).
Though both treatment arms had good initial response, 70% of the systemically treated mice relapsed by 150 days after injection, compared with just 10% of those receiving intratumoral therapy (P = .002).
Of the 23 mice whose therapy consisted of intratumoral administration of CpG together with anti-CTLA4 and anti-OX40 antibodies (aCTLA4 and aOX40), 21 (91%) were alive 50 days after treatment. These mice fared better than did those receiving any other intratumoral treatment combination (P = .004, compared with CpG+aOX40; P = .03, compared with aCTLA4), suggesting a synergistic benefit to the triple combination.
Somewhat surprisingly, even murine models that also had tumor seeding into brain tissue saw marked reduction or even resolution of brain tumors, showing that the blood-brain barrier does not impede the effect within the CNS.
What didn’t work? PD-1 inhibitors were not particularly effective at provoking a systemic effect when injected into tumors. Peritumoral injection, though theoretically taking advantage of some aspects of the tumor microenvironment, also did not show global effect.
Based on the human CpG + radiation trials and the mouse experiments, the research group is proceeding with phase I and II clinical trials of CpG in combinations with aCTLA4 and aOX40. These, Dr. Levy said, were more likely to be available clinically, and human intratumoral T regulatory cells are known to express both CTLA4 and OX40.
All agents and combinations had an enhancing effect, said Dr. Levy. “We like this treatment and trial design,” he said, noting that everyone sees benefit at the local injection site, and some see global results.
Dr. Levy discussed multiple studies, and he disclosed receiving grant support from Pfizer, Dynavax, and Bristol-Myers Squibb. He also has served as a consultant to Five Prime, Kite, BeiGene, Innate Pharma, Bullet Biotech, and Immune Design.
NEW YORK – “Inject locally, treat globally” may become a new mantra for cancer immunotherapy. Dr. Ronald Levy, professor and chief of the department of oncology at Stanford (Calif.) University, discussed impressive and durable systemic results from local treatment of tumors, reviewing his group’s recent work and discussing ongoing early stage clinical trials.
In the hope of triggering an immune response that induces a systemic CD8 T-cell response, Dr. Levy and other investigators began experimenting with intralesional injections of immunotherapies, with and without adjunctive radiation or chemotherapy. The principle has been evaluated in a mouse model, in which up to 80% of mice receiving intratumoral immunotherapy were cured. Now, human trials are underway for solid tumors as well as lymphoma.
An early clinical trial involving 15 patients with recurrent low-grade B-cell lymphoma combined targeted low-dose radiation of a single tumor site with injection of CpG, immune-boosting snippets of DNA, into the same site. Looking for partial or complete regression, investigators saw promising results, though some patients who were responders didn’t see full effect until 24 weeks after injection (J Clin Oncol. 2010 Oct 1;28[28]:4324-32). The overall objective response rate was 27%, although 80% of patients (12 of 15) had stable disease or partial or complete response through a median follow-up of 33.7 months. Some individual patients had marked regression or disappearance of bulky tumors at distant sites.
CpG – motifs of cytosines and guanines – were strung together, said Dr. Levy, with a sulfur rather than a phosphate backbone to make them more stable for injection. These bits of DNA, which are present in both bacteria and vertebrates, are an agonist for toll-like receptor 9, activating B cells and dendritic cells, and then tumor-specific T-cells.
Dr. Levy and his collaborators used a two-tumor mouse model to track intralesional injection effectiveness for a variety of immunotherapies. Mice seeded with tumor cells bilaterally over the abdomen were injected with CpG and two other immune therapies at a single abdominal tumor site, and bilateral regression, if any, was tracked in comparison to systemic immunotherapy (J Clin Invest. 2013;123[6]:2447-63).
Though both treatment arms had good initial response, 70% of the systemically treated mice relapsed by 150 days after injection, compared with just 10% of those receiving intratumoral therapy (P = .002).
Of the 23 mice whose therapy consisted of intratumoral administration of CpG together with anti-CTLA4 and anti-OX40 antibodies (aCTLA4 and aOX40), 21 (91%) were alive 50 days after treatment. These mice fared better than did those receiving any other intratumoral treatment combination (P = .004, compared with CpG+aOX40; P = .03, compared with aCTLA4), suggesting a synergistic benefit to the triple combination.
Somewhat surprisingly, even murine models that also had tumor seeding into brain tissue saw marked reduction or even resolution of brain tumors, showing that the blood-brain barrier does not impede the effect within the CNS.
What didn’t work? PD-1 inhibitors were not particularly effective at provoking a systemic effect when injected into tumors. Peritumoral injection, though theoretically taking advantage of some aspects of the tumor microenvironment, also did not show global effect.
Based on the human CpG + radiation trials and the mouse experiments, the research group is proceeding with phase I and II clinical trials of CpG in combinations with aCTLA4 and aOX40. These, Dr. Levy said, were more likely to be available clinically, and human intratumoral T regulatory cells are known to express both CTLA4 and OX40.
All agents and combinations had an enhancing effect, said Dr. Levy. “We like this treatment and trial design,” he said, noting that everyone sees benefit at the local injection site, and some see global results.
Dr. Levy discussed multiple studies, and he disclosed receiving grant support from Pfizer, Dynavax, and Bristol-Myers Squibb. He also has served as a consultant to Five Prime, Kite, BeiGene, Innate Pharma, Bullet Biotech, and Immune Design.
EXPERT ANALYSIS FROM THE FIRST INTERNATIONAL CANCER IMMUNOTHERAPY CONFERENCE
No signal for the superiority of autologous versus allogenic stem-cell transplants in T-cell lymphoma
A randomized trial designed to compare autologous to allogeneic stem cell transplantation as first-line therapy in younger patients with peripheral T-cell lymphoma was discontinued early because nearly 40% of the patients had early disease progression and did not undergo transplantation.
Peripheral T-cell lymphoma generally yields a poor prognosis when treated with conventional chemotherapy, but autologous or allogeneic stem cell transplants were thought to be an option for patients with relapsing or refractory disease. Based on this hypothesis, the AATT (Autologous or Allogeneic Transplantation in T-Cell Lymphoma) study explored stem cell transplant as a first-line therapy, enrolling 104 patients aged 18-60 between 2011 and 2014.
All patients received four courses of chemotherapy with CHOEP-14 (cyclophosphamide, adriamycin, vincristine, etoposide, and prednisone).
Those in the autologous stem cell group and those without a suitable donor proceeded to one course of DHAP (high-dose ara-C, cis-platinum, and dexamethasone) and stem cell collection. Patients randomized to autologous transplantation received high dose therapy (BCNU, etoposide, cytarabine, melphalan: BEAM) followed 4-6 weeks later by transplantation of autologous stem cells.
Patients randomized to allogeneic transplantation received high dose therapy (fludarabine, busulfan, cyclophosphamide: FBC) followed by transplantation of allogeneic stem cells. GvHD prophylaxis included antithymocyte globuline (ATG), cyclosporine A, and mycophenolate mofetil.
Among the 58 patients eligible for the interim analysis, the mean age was 50 and 64% were male. Thirteen of the 28 patients randomized for allogeneic transplants underwent transplants; the others were not allografted because of progressive disease or lack of a donor. Of the 30 patients randomized to autologous SCT, 19 had the procedure; 11 did not receive transplants because of progressive disease or infection, Dr. Norbert Schmitz of Asklepios Klinik St. Georg, Hamburg, Germany, reported at the International Congress on Malignant Lymphoma in Lugano, Switzerland.
The primary outcome, 1-year event-free survival (EFS), was 41% in the intent-to-treat population (95% CI, 27%–54%).
Causes of death included lymphoma (seven autologous, five allogeneic), salvage therapy (two), early or late infections (four), and graft vs. host disease (two).
Survival rates did not significantly differ in the two stem cell transplant groups, but the findings lend themselves to limited interpretation as more than 30% of patients did not receive the procedure. Based on the low probability of meeting the primary outcome, the data safety monitoring board decided to stop patient accrual and discontinue the trial.
*This article was updated 7/8/2015.
As outcomes for patients with PTCL are suboptimal with standard therapy, usually CHOP/CHOEP, young and fit patients are commonly offered high dose chemotherapy with stem cell support (SCT) to consolidate 1st remission, though no firm data support this approach. As a trial of SCT vs observation would be difficult to accomplish, the AATT trail was undertaken to compare autologous vs allogeneic transplantation. The trial was not able to answer this question as it was halted early due to the high proportion of patients unable to proceed to SCT. One lesson here is that data reported for PTCL patients who receive SCT in 1st remission suffers from selection bias, unless accompanied by an intent-to-treat analysis. There is a clear need for improved induction therapy for PTCL.
As outcomes for patients with PTCL are suboptimal with standard therapy, usually CHOP/CHOEP, young and fit patients are commonly offered high dose chemotherapy with stem cell support (SCT) to consolidate 1st remission, though no firm data support this approach. As a trial of SCT vs observation would be difficult to accomplish, the AATT trail was undertaken to compare autologous vs allogeneic transplantation. The trial was not able to answer this question as it was halted early due to the high proportion of patients unable to proceed to SCT. One lesson here is that data reported for PTCL patients who receive SCT in 1st remission suffers from selection bias, unless accompanied by an intent-to-treat analysis. There is a clear need for improved induction therapy for PTCL.
As outcomes for patients with PTCL are suboptimal with standard therapy, usually CHOP/CHOEP, young and fit patients are commonly offered high dose chemotherapy with stem cell support (SCT) to consolidate 1st remission, though no firm data support this approach. As a trial of SCT vs observation would be difficult to accomplish, the AATT trail was undertaken to compare autologous vs allogeneic transplantation. The trial was not able to answer this question as it was halted early due to the high proportion of patients unable to proceed to SCT. One lesson here is that data reported for PTCL patients who receive SCT in 1st remission suffers from selection bias, unless accompanied by an intent-to-treat analysis. There is a clear need for improved induction therapy for PTCL.
A randomized trial designed to compare autologous to allogeneic stem cell transplantation as first-line therapy in younger patients with peripheral T-cell lymphoma was discontinued early because nearly 40% of the patients had early disease progression and did not undergo transplantation.
Peripheral T-cell lymphoma generally yields a poor prognosis when treated with conventional chemotherapy, but autologous or allogeneic stem cell transplants were thought to be an option for patients with relapsing or refractory disease. Based on this hypothesis, the AATT (Autologous or Allogeneic Transplantation in T-Cell Lymphoma) study explored stem cell transplant as a first-line therapy, enrolling 104 patients aged 18-60 between 2011 and 2014.
All patients received four courses of chemotherapy with CHOEP-14 (cyclophosphamide, adriamycin, vincristine, etoposide, and prednisone).
Those in the autologous stem cell group and those without a suitable donor proceeded to one course of DHAP (high-dose ara-C, cis-platinum, and dexamethasone) and stem cell collection. Patients randomized to autologous transplantation received high dose therapy (BCNU, etoposide, cytarabine, melphalan: BEAM) followed 4-6 weeks later by transplantation of autologous stem cells.
Patients randomized to allogeneic transplantation received high dose therapy (fludarabine, busulfan, cyclophosphamide: FBC) followed by transplantation of allogeneic stem cells. GvHD prophylaxis included antithymocyte globuline (ATG), cyclosporine A, and mycophenolate mofetil.
Among the 58 patients eligible for the interim analysis, the mean age was 50 and 64% were male. Thirteen of the 28 patients randomized for allogeneic transplants underwent transplants; the others were not allografted because of progressive disease or lack of a donor. Of the 30 patients randomized to autologous SCT, 19 had the procedure; 11 did not receive transplants because of progressive disease or infection, Dr. Norbert Schmitz of Asklepios Klinik St. Georg, Hamburg, Germany, reported at the International Congress on Malignant Lymphoma in Lugano, Switzerland.
The primary outcome, 1-year event-free survival (EFS), was 41% in the intent-to-treat population (95% CI, 27%–54%).
Causes of death included lymphoma (seven autologous, five allogeneic), salvage therapy (two), early or late infections (four), and graft vs. host disease (two).
Survival rates did not significantly differ in the two stem cell transplant groups, but the findings lend themselves to limited interpretation as more than 30% of patients did not receive the procedure. Based on the low probability of meeting the primary outcome, the data safety monitoring board decided to stop patient accrual and discontinue the trial.
*This article was updated 7/8/2015.
A randomized trial designed to compare autologous to allogeneic stem cell transplantation as first-line therapy in younger patients with peripheral T-cell lymphoma was discontinued early because nearly 40% of the patients had early disease progression and did not undergo transplantation.
Peripheral T-cell lymphoma generally yields a poor prognosis when treated with conventional chemotherapy, but autologous or allogeneic stem cell transplants were thought to be an option for patients with relapsing or refractory disease. Based on this hypothesis, the AATT (Autologous or Allogeneic Transplantation in T-Cell Lymphoma) study explored stem cell transplant as a first-line therapy, enrolling 104 patients aged 18-60 between 2011 and 2014.
All patients received four courses of chemotherapy with CHOEP-14 (cyclophosphamide, adriamycin, vincristine, etoposide, and prednisone).
Those in the autologous stem cell group and those without a suitable donor proceeded to one course of DHAP (high-dose ara-C, cis-platinum, and dexamethasone) and stem cell collection. Patients randomized to autologous transplantation received high dose therapy (BCNU, etoposide, cytarabine, melphalan: BEAM) followed 4-6 weeks later by transplantation of autologous stem cells.
Patients randomized to allogeneic transplantation received high dose therapy (fludarabine, busulfan, cyclophosphamide: FBC) followed by transplantation of allogeneic stem cells. GvHD prophylaxis included antithymocyte globuline (ATG), cyclosporine A, and mycophenolate mofetil.
Among the 58 patients eligible for the interim analysis, the mean age was 50 and 64% were male. Thirteen of the 28 patients randomized for allogeneic transplants underwent transplants; the others were not allografted because of progressive disease or lack of a donor. Of the 30 patients randomized to autologous SCT, 19 had the procedure; 11 did not receive transplants because of progressive disease or infection, Dr. Norbert Schmitz of Asklepios Klinik St. Georg, Hamburg, Germany, reported at the International Congress on Malignant Lymphoma in Lugano, Switzerland.
The primary outcome, 1-year event-free survival (EFS), was 41% in the intent-to-treat population (95% CI, 27%–54%).
Causes of death included lymphoma (seven autologous, five allogeneic), salvage therapy (two), early or late infections (four), and graft vs. host disease (two).
Survival rates did not significantly differ in the two stem cell transplant groups, but the findings lend themselves to limited interpretation as more than 30% of patients did not receive the procedure. Based on the low probability of meeting the primary outcome, the data safety monitoring board decided to stop patient accrual and discontinue the trial.
*This article was updated 7/8/2015.
FROM 13-ICML
Key clinical point: Survival rates did not significantly differ for autologous versus allogenic stem cell transplant in patients with peripheral T-cell lymphoma, but the findings lend themselves to limited interpretation as more than 30% of patients did not receive the procedures.
Major finding: Early disease progression led to the discontinuation of a randomized trial comparing autologous to allogeneic stem cell transplantation in younger patients with peripheral T-cell lymphoma.
Data source: Results from 58 patients eligible for the interim analysis.
Disclosures: There were no relevant financial disclosures.
Naloxone lotion improves disabling itch in CTCL
VANCOUVER, B.C. – Naloxone lotion appears to be a safe and effective treatment for the severe chronic itching that occurs in most patients with cutaneous T-cell lymphoma, Dr. Madeleine Duvic reported at the World Congress of Dermatology.
A major unmet need exists for better treatments for pruritis in CTCL. Antihistamines are generally ineffective. Chemotherapeutic agents provide little relief. Moreover, it has been estimated that up to half of all patients with CTCL die as a result of systemic infections arising secondary to pruritic skin excoriations, according to Dr. Duvic, professor of medicine and dermatology at the University of Texas and MD Anderson Cancer Center, Houston.
Naloxone is a pure opiate antagonist with no agonist effects. Naloxone lotion is an investigational agent that has received orphan drug status for treatment of pruritis in CTCL and a fast-track evaluation designation from the Food and Drug Administration.
Dr. Duvic presented a double-blind, vehicle-controlled, multicenter, crossover study involving 15 CTCL patients with severe itching. They were assigned to apply naloxone lotion 0.5% or its vehicle four times daily for 8 days and then cross over to the other regimen for another 8 days following a washout period.
After 8 days of naloxone lotion, patients reported a mean 66% reduction in itch severity from baseline on a visual analog scale, a significantly better result than the 45% reduction on vehicle.
The study suffered from small numbers, as four patients withdrew during part 1 while on vehicle, two dropped out while on naloxone lotion, and one was excluded for a concomitant medication violation. Of the nine patients available for Physician Global Assessment, seven were rated better or much better. Seven of the nine patients also rated themselves as globally better or much better while on naloxone lotion. These ratings were numerically better than while patients were on vehicle.
Adverse events were limited to two cases of mild or moderate application-site erythema.
The study was funded by Elorac. Dr. Duvic reported having no financial conflicts. She noted that the University of Texas received a research grant to conduct the study.
VANCOUVER, B.C. – Naloxone lotion appears to be a safe and effective treatment for the severe chronic itching that occurs in most patients with cutaneous T-cell lymphoma, Dr. Madeleine Duvic reported at the World Congress of Dermatology.
A major unmet need exists for better treatments for pruritis in CTCL. Antihistamines are generally ineffective. Chemotherapeutic agents provide little relief. Moreover, it has been estimated that up to half of all patients with CTCL die as a result of systemic infections arising secondary to pruritic skin excoriations, according to Dr. Duvic, professor of medicine and dermatology at the University of Texas and MD Anderson Cancer Center, Houston.
Naloxone is a pure opiate antagonist with no agonist effects. Naloxone lotion is an investigational agent that has received orphan drug status for treatment of pruritis in CTCL and a fast-track evaluation designation from the Food and Drug Administration.
Dr. Duvic presented a double-blind, vehicle-controlled, multicenter, crossover study involving 15 CTCL patients with severe itching. They were assigned to apply naloxone lotion 0.5% or its vehicle four times daily for 8 days and then cross over to the other regimen for another 8 days following a washout period.
After 8 days of naloxone lotion, patients reported a mean 66% reduction in itch severity from baseline on a visual analog scale, a significantly better result than the 45% reduction on vehicle.
The study suffered from small numbers, as four patients withdrew during part 1 while on vehicle, two dropped out while on naloxone lotion, and one was excluded for a concomitant medication violation. Of the nine patients available for Physician Global Assessment, seven were rated better or much better. Seven of the nine patients also rated themselves as globally better or much better while on naloxone lotion. These ratings were numerically better than while patients were on vehicle.
Adverse events were limited to two cases of mild or moderate application-site erythema.
The study was funded by Elorac. Dr. Duvic reported having no financial conflicts. She noted that the University of Texas received a research grant to conduct the study.
VANCOUVER, B.C. – Naloxone lotion appears to be a safe and effective treatment for the severe chronic itching that occurs in most patients with cutaneous T-cell lymphoma, Dr. Madeleine Duvic reported at the World Congress of Dermatology.
A major unmet need exists for better treatments for pruritis in CTCL. Antihistamines are generally ineffective. Chemotherapeutic agents provide little relief. Moreover, it has been estimated that up to half of all patients with CTCL die as a result of systemic infections arising secondary to pruritic skin excoriations, according to Dr. Duvic, professor of medicine and dermatology at the University of Texas and MD Anderson Cancer Center, Houston.
Naloxone is a pure opiate antagonist with no agonist effects. Naloxone lotion is an investigational agent that has received orphan drug status for treatment of pruritis in CTCL and a fast-track evaluation designation from the Food and Drug Administration.
Dr. Duvic presented a double-blind, vehicle-controlled, multicenter, crossover study involving 15 CTCL patients with severe itching. They were assigned to apply naloxone lotion 0.5% or its vehicle four times daily for 8 days and then cross over to the other regimen for another 8 days following a washout period.
After 8 days of naloxone lotion, patients reported a mean 66% reduction in itch severity from baseline on a visual analog scale, a significantly better result than the 45% reduction on vehicle.
The study suffered from small numbers, as four patients withdrew during part 1 while on vehicle, two dropped out while on naloxone lotion, and one was excluded for a concomitant medication violation. Of the nine patients available for Physician Global Assessment, seven were rated better or much better. Seven of the nine patients also rated themselves as globally better or much better while on naloxone lotion. These ratings were numerically better than while patients were on vehicle.
Adverse events were limited to two cases of mild or moderate application-site erythema.
The study was funded by Elorac. Dr. Duvic reported having no financial conflicts. She noted that the University of Texas received a research grant to conduct the study.
AT WCD 2015
Key clinical point: Naloxone lotion shows promise for the severe pruritis that accompanies cutaneous T-cell lymphoma.
Major finding: Patients with cutaneous T-cell lymphoma reported an absolute 21% greater reduction in pruritis with naloxone lotion than with its vehicle.
Data source: This was a 15-patient, multicenter, double-blind, crossover study.
Disclosures: The study was funded by Elorac. The presenter reported having no financial conflicts.
FDA approves belinostat for peripheral T-cell lymphoma
Belinostat, a histone deacetylase inhibitor, has been approved for treating peripheral T-cell lymphoma, based on the results of the BELIEF study that found an overall response rate of nearly 26% among treated patients.
This is the third drug approved for this rare, aggressive form of non-Hodgkin’s lymphoma (NHL) since 2009, according to the Food and Drug Administration statement announcing the approval on July 3.
The other two drugs are pralatrexate injection (Folotyn), a folate analogue metabolic inhibitor approved in 2009 for treating relapsed or refractory peripheral T-cell lymphoma (PTCL); and romidepsin (Istodax), a histone deacetylase (HDAC) inhibitor approved in 2011 for treating PTCL in patients who have received at least one previous treatment.
This is an accelerated approval, which is based on surrogate or intermediate endpoints considered by the FDA as "reasonably likely to predict clinical benefit for patients with serious conditions with unmet medical needs." Confirmatory trials that verify the clinical benefit are required for full approval; otherwise, the approval can be withdrawn by the FDA. Belinostat will be marketed as Beleodaq by Spectrum Pharmaceuticals, which also markets Folotyn.
HDAC inhibitors "catalyze the removal of acetyl groups from the lysine residues of histones and some nonhistone proteins," and in vitro, belinostat "caused the accumulation of acetylated histones and other proteins, inducing cell cycle arrest and/or apoptosis of some transformed cells," according to a statement on the approval, issued by Spectrum on July 7. "Belinostat shows preferential cytotoxicity towards tumor cells compared to normal cells," and it "inhibited the enzymatic activity of histone deacetylases at nanomolar concentrations," the statement said.
In the BELIEF study, an open-label, single-arm, nonrandomized study, 129 patients with relapsed or refractory PTCL were treated with belinostat, administered via an IV infusion, once a day on days 1-5 of a 21-day cycle, repeated every 3 weeks until the disease progressed or adverse effects became unacceptable. The overall response rate (complete and partial responses), the primary efficacy endpoint, was 25.8%. Nausea, vomiting, fatigue, pyrexia, and anemia were the most common adverse events associated with treatment, according to the FDA.
The company said that the drug is expected to be available in less than 3 weeks of approval (before July 24). The confirmatory trial is a phase III study that will evaluate belinostat plus CHOP (cyclophosphamide, vincristine, doxorubicin, prednisone), compared with CHOP alone.
PTCL accounts for about 10%-15% of NHL cases in North America, according to the FDA, which cites National Cancer Institute estimates that 70,800 Americans will be diagnosed with NHL and 18,990 will die of NHL in 2014.
The prescribing information for belinostat is available here.
Belinostat, a histone deacetylase inhibitor, has been approved for treating peripheral T-cell lymphoma, based on the results of the BELIEF study that found an overall response rate of nearly 26% among treated patients.
This is the third drug approved for this rare, aggressive form of non-Hodgkin’s lymphoma (NHL) since 2009, according to the Food and Drug Administration statement announcing the approval on July 3.
The other two drugs are pralatrexate injection (Folotyn), a folate analogue metabolic inhibitor approved in 2009 for treating relapsed or refractory peripheral T-cell lymphoma (PTCL); and romidepsin (Istodax), a histone deacetylase (HDAC) inhibitor approved in 2011 for treating PTCL in patients who have received at least one previous treatment.
This is an accelerated approval, which is based on surrogate or intermediate endpoints considered by the FDA as "reasonably likely to predict clinical benefit for patients with serious conditions with unmet medical needs." Confirmatory trials that verify the clinical benefit are required for full approval; otherwise, the approval can be withdrawn by the FDA. Belinostat will be marketed as Beleodaq by Spectrum Pharmaceuticals, which also markets Folotyn.
HDAC inhibitors "catalyze the removal of acetyl groups from the lysine residues of histones and some nonhistone proteins," and in vitro, belinostat "caused the accumulation of acetylated histones and other proteins, inducing cell cycle arrest and/or apoptosis of some transformed cells," according to a statement on the approval, issued by Spectrum on July 7. "Belinostat shows preferential cytotoxicity towards tumor cells compared to normal cells," and it "inhibited the enzymatic activity of histone deacetylases at nanomolar concentrations," the statement said.
In the BELIEF study, an open-label, single-arm, nonrandomized study, 129 patients with relapsed or refractory PTCL were treated with belinostat, administered via an IV infusion, once a day on days 1-5 of a 21-day cycle, repeated every 3 weeks until the disease progressed or adverse effects became unacceptable. The overall response rate (complete and partial responses), the primary efficacy endpoint, was 25.8%. Nausea, vomiting, fatigue, pyrexia, and anemia were the most common adverse events associated with treatment, according to the FDA.
The company said that the drug is expected to be available in less than 3 weeks of approval (before July 24). The confirmatory trial is a phase III study that will evaluate belinostat plus CHOP (cyclophosphamide, vincristine, doxorubicin, prednisone), compared with CHOP alone.
PTCL accounts for about 10%-15% of NHL cases in North America, according to the FDA, which cites National Cancer Institute estimates that 70,800 Americans will be diagnosed with NHL and 18,990 will die of NHL in 2014.
The prescribing information for belinostat is available here.
Belinostat, a histone deacetylase inhibitor, has been approved for treating peripheral T-cell lymphoma, based on the results of the BELIEF study that found an overall response rate of nearly 26% among treated patients.
This is the third drug approved for this rare, aggressive form of non-Hodgkin’s lymphoma (NHL) since 2009, according to the Food and Drug Administration statement announcing the approval on July 3.
The other two drugs are pralatrexate injection (Folotyn), a folate analogue metabolic inhibitor approved in 2009 for treating relapsed or refractory peripheral T-cell lymphoma (PTCL); and romidepsin (Istodax), a histone deacetylase (HDAC) inhibitor approved in 2011 for treating PTCL in patients who have received at least one previous treatment.
This is an accelerated approval, which is based on surrogate or intermediate endpoints considered by the FDA as "reasonably likely to predict clinical benefit for patients with serious conditions with unmet medical needs." Confirmatory trials that verify the clinical benefit are required for full approval; otherwise, the approval can be withdrawn by the FDA. Belinostat will be marketed as Beleodaq by Spectrum Pharmaceuticals, which also markets Folotyn.
HDAC inhibitors "catalyze the removal of acetyl groups from the lysine residues of histones and some nonhistone proteins," and in vitro, belinostat "caused the accumulation of acetylated histones and other proteins, inducing cell cycle arrest and/or apoptosis of some transformed cells," according to a statement on the approval, issued by Spectrum on July 7. "Belinostat shows preferential cytotoxicity towards tumor cells compared to normal cells," and it "inhibited the enzymatic activity of histone deacetylases at nanomolar concentrations," the statement said.
In the BELIEF study, an open-label, single-arm, nonrandomized study, 129 patients with relapsed or refractory PTCL were treated with belinostat, administered via an IV infusion, once a day on days 1-5 of a 21-day cycle, repeated every 3 weeks until the disease progressed or adverse effects became unacceptable. The overall response rate (complete and partial responses), the primary efficacy endpoint, was 25.8%. Nausea, vomiting, fatigue, pyrexia, and anemia were the most common adverse events associated with treatment, according to the FDA.
The company said that the drug is expected to be available in less than 3 weeks of approval (before July 24). The confirmatory trial is a phase III study that will evaluate belinostat plus CHOP (cyclophosphamide, vincristine, doxorubicin, prednisone), compared with CHOP alone.
PTCL accounts for about 10%-15% of NHL cases in North America, according to the FDA, which cites National Cancer Institute estimates that 70,800 Americans will be diagnosed with NHL and 18,990 will die of NHL in 2014.
The prescribing information for belinostat is available here.