User login
ASH: Oral drug offers alternative to lifelong transfusions in sickle cell
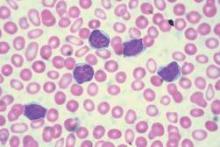
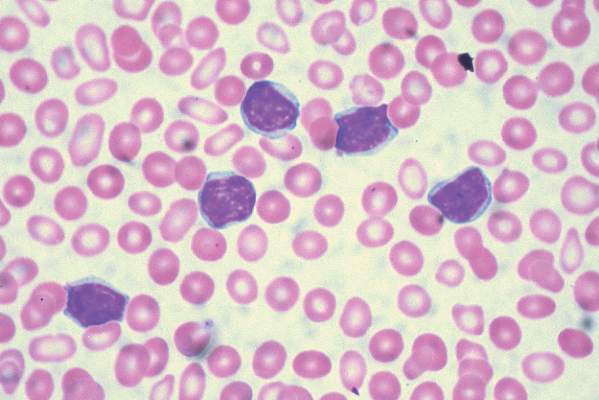
ORLANDO – Oral hydroxyurea is as good as chronic red blood cell transfusions for prevention of primary stroke in children at high-risk for this devastating complication of sickle cell disease, results of the TWiTCH study show.
No child suffered a stroke with either hydroxyurea or monthly transfusions and transcranial doppler (TCD) velocities were maintained in both arms.
The study was stopped early, however, after noninferiority was shown for the primary end point of TCD mean velocities on the index side at 24 months, with a post-hoc analysis suggesting hydroxyurea may even be superior, study author Dr. Russell E. Ware, director of hematology at Cincinnati (Ohio) Children’s Hospital Medical Center, reported during the plenary session at the annual meeting of the American Society of Hematology.
“Hydroxyurea therapy can substitute for chronic transfusions to maintain TCD velocities and help prevent primary stroke,” he said during a press briefing.
The final mean TCD velocities were 143 cm/second in the transfusion arm and 138 cm/sec in the hydroxyurea arm, resulting in P values of 8.82 x 10-16 for non-inferiority by intention-to-treat analysis and 0.023 for superiority in a post-hoc analysis.
Hydroxyurea also had the added benefit of improving iron overload status more than monthly transfusions based on a greater average change in serum ferritin (-1,085 ng/mL vs. -38 ng/mL; P less than .001) and liver iron concentrations (-1.9 mg/g vs. +2.4 mg/g; P = .001), according to their report.
Press briefing moderator Dr. Alexis Thompson, of the Ann & Robert H. Lurie Children’s Hospital of Chicago, commented, “This truly is one of the abstracts that are being presenting at the meeting today that can be defined as practice changing. There are many families who have great difficulty accepting the reality, prior to the TWiTCH study, of their children having to be transfused lifelong.”
Strokes occur in up to 10% of children with sickle cell disease (SCD). Transfusions are effective for stroke prophylaxis in this setting, but have to be continued lifelong and can lead to iron overload and other complications.
Based on the participating sites, at least 80% of children with abnormal TCD velocities currently on blood transfusions to prevent stroke would be eligible for treatment with hydroxyurea, Dr. Ware said.
Hydroxyurea increases the amount of fetal hemoglobin and fetal red blood cells and was approved more than a decade ago to ameliorate the acute and chronic complications of SCD. Its use could provide dramatic cost savings for families since a transfusion costs about $1,000 to $2,000 every month, whereas hydroxyurea costs less than a dollar a day, Dr. Ware said in an interview.
TWiTCH (TCD with Transfusions Changing to Hydroxyurea) was conducted at 26 pediatric programs and used TCD to identify 121 children with SCD who were at elevated risk of stroke based on abnormally high cerebral artery flow velocities of at least 200 cm/sec. The children were evenly randomized to 24 months of treatment. TCD velocities were obtained every 12 weeks and reviewed centrally, with local investigators blinded to the results. All children had received transfusions for at least 12 months, but had not developed severe vasculopathy.
The transfusion arm was maintained at a target hemoglobin S level of less than 30% and chelation used to manage elevated liver concentrations. Transfusions were allowed in the hydroxyurea arm until a stable maximum tolerated dose (MTD) of hydroxyurea was reached, and were then replaced by serial phlebotomy to reduce iron overload. The MTD was reached after 6 months at an average dose of about 25 mg/kg/day.
The transfusion overlap with hydroxyurea was designed as a safety measure to avoid strokes if monthly transfusions were abruptly discontinued in the hydroxyurea arm before the children had time to achieve MTD.
“The fact that that overlap was about 6 months and the fact we had about 24 months of follow-up tracking the TCD velocities over time, we feel that doesn’t affect the end statistical analysis,” Dr. Ware said.
As for whether hydroxyurea is superior to monthly transfusions, he noted that the trial was not designed for superiority and superiority was seen in a post-hoc analysis. “What we can say with certainty is that it’s non-inferior to the standard treatment,” Dr. Ware said.
The final analysis was based on 42 patients randomized to the transfusion arm who completed all 24 months of treatment, 11 with truncated treatment, and 8 withdrawals, and 41 patients assigned to the hydroxyurea arm who completed all treatment, 13 with truncated treatment and 6 withdrawals.
Sickle cell-related serious adverse events were more common in the hydroxyurea arm than the transfusion arm (23 vs. 15), but none were related to the study drug or procedures.
There were 29 new centrally adjudicated neurological events including 3 transient ischemic attacks in each arm. In the transfusion arm, one child was withdrawn per protocol after developing TCD velocities exceeding 240 cm/sec and a second developed new vasculopathy. The safety of long-term hydroxyurea has been established in multiple pediatric studies, Dr. Ware said.
TWiTCH was funded by the National Heart, Lung and Blood Institute and sponsored by Cincinnati Children’s Hospital Medical Center. Dr. Ware reported serving as a data safety monitoring board member for Eli Lilly, consultancy for Bayer, and research funding from Bristol Myers Squibb. Dr. Thompson disclosed research funding from Amgen, Baxalta, bluebird bio, Celgene, Eli Lilly, GlaxoSmithKline, Mast, and Novartis, and consultancy for ApoPharma, bluebird bio, and Novartis.
Abnormal transcranial Doppler findings in sickle cell disease indicates that the pediatric patient is at high risk of a primary stroke. To date, the standard of care has been lifelong transfusion for these patients.
This study indicates there may be a viable alternative to transfusions with less invasive therapy and reduced risk of iron loading. What is not addressed, however, is whether we use hydroxyurea as front line therapy for children with abnormal findings on transcranial Doppler. The study patients had been on transfusions for at least 12 months when they were enrolled in the study and had normalized velocities on transcranial Doppler.
Do we still need to screen sickle cell disease patient patients with transcranial Doppler for stroke risk? The answer is 'yes.' What if they are already on hydroxyurea? Still the answer is 'yes.'
Should transcranial Doppler screening also be linked with MRI/MRA to look at vessels? This study refers to a select group of patients with no significant vasculopathy noted on brain imaging.
So how does the community provider interpret these finding for the real-world care of a patient? How do we not over interpret or under interpret the data? In partnership with a hematologist each child should continue to get transcranial Doppler screening annually and transfusions initiated for abnormal transcranial Doppler and study methods should be followed with crossing over to hydroxyurea for patients who normalize their transcranial Doppler results at 12 months and have no evidence of significant vasculopathy on brain imaging.
Transcranial Doppler screening should continue annually per guidelines even if the patient is already on hydroxyurea for other indications or was switched to hydroxyurea from transfusions. This is a situation that is more likely to occur in the real world clinical setting as hydroxyurea use is advocated for children as early as 9 months of age.
What happens then if transcranial Doppler findings become abnormal while on hydroxyurea? If compliance is assured and the hydroxyurea dose is optimized, then we are back to square one. Primary stroke prevention with lifelong transfusion is currently the standard of care. At least until another randomized study is done to prove non inferiority.
Dr. Ifeyinwa Osunkwo is the medical director for the sickle cell program at the Levine Cancer Institute, Carolinas Healthcare Systems, Charlotte, NC. and a member of the editorial board for Hematology News.
Abnormal transcranial Doppler findings in sickle cell disease indicates that the pediatric patient is at high risk of a primary stroke. To date, the standard of care has been lifelong transfusion for these patients.
This study indicates there may be a viable alternative to transfusions with less invasive therapy and reduced risk of iron loading. What is not addressed, however, is whether we use hydroxyurea as front line therapy for children with abnormal findings on transcranial Doppler. The study patients had been on transfusions for at least 12 months when they were enrolled in the study and had normalized velocities on transcranial Doppler.
Do we still need to screen sickle cell disease patient patients with transcranial Doppler for stroke risk? The answer is 'yes.' What if they are already on hydroxyurea? Still the answer is 'yes.'
Should transcranial Doppler screening also be linked with MRI/MRA to look at vessels? This study refers to a select group of patients with no significant vasculopathy noted on brain imaging.
So how does the community provider interpret these finding for the real-world care of a patient? How do we not over interpret or under interpret the data? In partnership with a hematologist each child should continue to get transcranial Doppler screening annually and transfusions initiated for abnormal transcranial Doppler and study methods should be followed with crossing over to hydroxyurea for patients who normalize their transcranial Doppler results at 12 months and have no evidence of significant vasculopathy on brain imaging.
Transcranial Doppler screening should continue annually per guidelines even if the patient is already on hydroxyurea for other indications or was switched to hydroxyurea from transfusions. This is a situation that is more likely to occur in the real world clinical setting as hydroxyurea use is advocated for children as early as 9 months of age.
What happens then if transcranial Doppler findings become abnormal while on hydroxyurea? If compliance is assured and the hydroxyurea dose is optimized, then we are back to square one. Primary stroke prevention with lifelong transfusion is currently the standard of care. At least until another randomized study is done to prove non inferiority.
Dr. Ifeyinwa Osunkwo is the medical director for the sickle cell program at the Levine Cancer Institute, Carolinas Healthcare Systems, Charlotte, NC. and a member of the editorial board for Hematology News.
Abnormal transcranial Doppler findings in sickle cell disease indicates that the pediatric patient is at high risk of a primary stroke. To date, the standard of care has been lifelong transfusion for these patients.
This study indicates there may be a viable alternative to transfusions with less invasive therapy and reduced risk of iron loading. What is not addressed, however, is whether we use hydroxyurea as front line therapy for children with abnormal findings on transcranial Doppler. The study patients had been on transfusions for at least 12 months when they were enrolled in the study and had normalized velocities on transcranial Doppler.
Do we still need to screen sickle cell disease patient patients with transcranial Doppler for stroke risk? The answer is 'yes.' What if they are already on hydroxyurea? Still the answer is 'yes.'
Should transcranial Doppler screening also be linked with MRI/MRA to look at vessels? This study refers to a select group of patients with no significant vasculopathy noted on brain imaging.
So how does the community provider interpret these finding for the real-world care of a patient? How do we not over interpret or under interpret the data? In partnership with a hematologist each child should continue to get transcranial Doppler screening annually and transfusions initiated for abnormal transcranial Doppler and study methods should be followed with crossing over to hydroxyurea for patients who normalize their transcranial Doppler results at 12 months and have no evidence of significant vasculopathy on brain imaging.
Transcranial Doppler screening should continue annually per guidelines even if the patient is already on hydroxyurea for other indications or was switched to hydroxyurea from transfusions. This is a situation that is more likely to occur in the real world clinical setting as hydroxyurea use is advocated for children as early as 9 months of age.
What happens then if transcranial Doppler findings become abnormal while on hydroxyurea? If compliance is assured and the hydroxyurea dose is optimized, then we are back to square one. Primary stroke prevention with lifelong transfusion is currently the standard of care. At least until another randomized study is done to prove non inferiority.
Dr. Ifeyinwa Osunkwo is the medical director for the sickle cell program at the Levine Cancer Institute, Carolinas Healthcare Systems, Charlotte, NC. and a member of the editorial board for Hematology News.
ORLANDO – Oral hydroxyurea is as good as chronic red blood cell transfusions for prevention of primary stroke in children at high-risk for this devastating complication of sickle cell disease, results of the TWiTCH study show.
No child suffered a stroke with either hydroxyurea or monthly transfusions and transcranial doppler (TCD) velocities were maintained in both arms.
The study was stopped early, however, after noninferiority was shown for the primary end point of TCD mean velocities on the index side at 24 months, with a post-hoc analysis suggesting hydroxyurea may even be superior, study author Dr. Russell E. Ware, director of hematology at Cincinnati (Ohio) Children’s Hospital Medical Center, reported during the plenary session at the annual meeting of the American Society of Hematology.
“Hydroxyurea therapy can substitute for chronic transfusions to maintain TCD velocities and help prevent primary stroke,” he said during a press briefing.
The final mean TCD velocities were 143 cm/second in the transfusion arm and 138 cm/sec in the hydroxyurea arm, resulting in P values of 8.82 x 10-16 for non-inferiority by intention-to-treat analysis and 0.023 for superiority in a post-hoc analysis.
Hydroxyurea also had the added benefit of improving iron overload status more than monthly transfusions based on a greater average change in serum ferritin (-1,085 ng/mL vs. -38 ng/mL; P less than .001) and liver iron concentrations (-1.9 mg/g vs. +2.4 mg/g; P = .001), according to their report.
Press briefing moderator Dr. Alexis Thompson, of the Ann & Robert H. Lurie Children’s Hospital of Chicago, commented, “This truly is one of the abstracts that are being presenting at the meeting today that can be defined as practice changing. There are many families who have great difficulty accepting the reality, prior to the TWiTCH study, of their children having to be transfused lifelong.”
Strokes occur in up to 10% of children with sickle cell disease (SCD). Transfusions are effective for stroke prophylaxis in this setting, but have to be continued lifelong and can lead to iron overload and other complications.
Based on the participating sites, at least 80% of children with abnormal TCD velocities currently on blood transfusions to prevent stroke would be eligible for treatment with hydroxyurea, Dr. Ware said.
Hydroxyurea increases the amount of fetal hemoglobin and fetal red blood cells and was approved more than a decade ago to ameliorate the acute and chronic complications of SCD. Its use could provide dramatic cost savings for families since a transfusion costs about $1,000 to $2,000 every month, whereas hydroxyurea costs less than a dollar a day, Dr. Ware said in an interview.
TWiTCH (TCD with Transfusions Changing to Hydroxyurea) was conducted at 26 pediatric programs and used TCD to identify 121 children with SCD who were at elevated risk of stroke based on abnormally high cerebral artery flow velocities of at least 200 cm/sec. The children were evenly randomized to 24 months of treatment. TCD velocities were obtained every 12 weeks and reviewed centrally, with local investigators blinded to the results. All children had received transfusions for at least 12 months, but had not developed severe vasculopathy.
The transfusion arm was maintained at a target hemoglobin S level of less than 30% and chelation used to manage elevated liver concentrations. Transfusions were allowed in the hydroxyurea arm until a stable maximum tolerated dose (MTD) of hydroxyurea was reached, and were then replaced by serial phlebotomy to reduce iron overload. The MTD was reached after 6 months at an average dose of about 25 mg/kg/day.
The transfusion overlap with hydroxyurea was designed as a safety measure to avoid strokes if monthly transfusions were abruptly discontinued in the hydroxyurea arm before the children had time to achieve MTD.
“The fact that that overlap was about 6 months and the fact we had about 24 months of follow-up tracking the TCD velocities over time, we feel that doesn’t affect the end statistical analysis,” Dr. Ware said.
As for whether hydroxyurea is superior to monthly transfusions, he noted that the trial was not designed for superiority and superiority was seen in a post-hoc analysis. “What we can say with certainty is that it’s non-inferior to the standard treatment,” Dr. Ware said.
The final analysis was based on 42 patients randomized to the transfusion arm who completed all 24 months of treatment, 11 with truncated treatment, and 8 withdrawals, and 41 patients assigned to the hydroxyurea arm who completed all treatment, 13 with truncated treatment and 6 withdrawals.
Sickle cell-related serious adverse events were more common in the hydroxyurea arm than the transfusion arm (23 vs. 15), but none were related to the study drug or procedures.
There were 29 new centrally adjudicated neurological events including 3 transient ischemic attacks in each arm. In the transfusion arm, one child was withdrawn per protocol after developing TCD velocities exceeding 240 cm/sec and a second developed new vasculopathy. The safety of long-term hydroxyurea has been established in multiple pediatric studies, Dr. Ware said.
TWiTCH was funded by the National Heart, Lung and Blood Institute and sponsored by Cincinnati Children’s Hospital Medical Center. Dr. Ware reported serving as a data safety monitoring board member for Eli Lilly, consultancy for Bayer, and research funding from Bristol Myers Squibb. Dr. Thompson disclosed research funding from Amgen, Baxalta, bluebird bio, Celgene, Eli Lilly, GlaxoSmithKline, Mast, and Novartis, and consultancy for ApoPharma, bluebird bio, and Novartis.
ORLANDO – Oral hydroxyurea is as good as chronic red blood cell transfusions for prevention of primary stroke in children at high-risk for this devastating complication of sickle cell disease, results of the TWiTCH study show.
No child suffered a stroke with either hydroxyurea or monthly transfusions and transcranial doppler (TCD) velocities were maintained in both arms.
The study was stopped early, however, after noninferiority was shown for the primary end point of TCD mean velocities on the index side at 24 months, with a post-hoc analysis suggesting hydroxyurea may even be superior, study author Dr. Russell E. Ware, director of hematology at Cincinnati (Ohio) Children’s Hospital Medical Center, reported during the plenary session at the annual meeting of the American Society of Hematology.
“Hydroxyurea therapy can substitute for chronic transfusions to maintain TCD velocities and help prevent primary stroke,” he said during a press briefing.
The final mean TCD velocities were 143 cm/second in the transfusion arm and 138 cm/sec in the hydroxyurea arm, resulting in P values of 8.82 x 10-16 for non-inferiority by intention-to-treat analysis and 0.023 for superiority in a post-hoc analysis.
Hydroxyurea also had the added benefit of improving iron overload status more than monthly transfusions based on a greater average change in serum ferritin (-1,085 ng/mL vs. -38 ng/mL; P less than .001) and liver iron concentrations (-1.9 mg/g vs. +2.4 mg/g; P = .001), according to their report.
Press briefing moderator Dr. Alexis Thompson, of the Ann & Robert H. Lurie Children’s Hospital of Chicago, commented, “This truly is one of the abstracts that are being presenting at the meeting today that can be defined as practice changing. There are many families who have great difficulty accepting the reality, prior to the TWiTCH study, of their children having to be transfused lifelong.”
Strokes occur in up to 10% of children with sickle cell disease (SCD). Transfusions are effective for stroke prophylaxis in this setting, but have to be continued lifelong and can lead to iron overload and other complications.
Based on the participating sites, at least 80% of children with abnormal TCD velocities currently on blood transfusions to prevent stroke would be eligible for treatment with hydroxyurea, Dr. Ware said.
Hydroxyurea increases the amount of fetal hemoglobin and fetal red blood cells and was approved more than a decade ago to ameliorate the acute and chronic complications of SCD. Its use could provide dramatic cost savings for families since a transfusion costs about $1,000 to $2,000 every month, whereas hydroxyurea costs less than a dollar a day, Dr. Ware said in an interview.
TWiTCH (TCD with Transfusions Changing to Hydroxyurea) was conducted at 26 pediatric programs and used TCD to identify 121 children with SCD who were at elevated risk of stroke based on abnormally high cerebral artery flow velocities of at least 200 cm/sec. The children were evenly randomized to 24 months of treatment. TCD velocities were obtained every 12 weeks and reviewed centrally, with local investigators blinded to the results. All children had received transfusions for at least 12 months, but had not developed severe vasculopathy.
The transfusion arm was maintained at a target hemoglobin S level of less than 30% and chelation used to manage elevated liver concentrations. Transfusions were allowed in the hydroxyurea arm until a stable maximum tolerated dose (MTD) of hydroxyurea was reached, and were then replaced by serial phlebotomy to reduce iron overload. The MTD was reached after 6 months at an average dose of about 25 mg/kg/day.
The transfusion overlap with hydroxyurea was designed as a safety measure to avoid strokes if monthly transfusions were abruptly discontinued in the hydroxyurea arm before the children had time to achieve MTD.
“The fact that that overlap was about 6 months and the fact we had about 24 months of follow-up tracking the TCD velocities over time, we feel that doesn’t affect the end statistical analysis,” Dr. Ware said.
As for whether hydroxyurea is superior to monthly transfusions, he noted that the trial was not designed for superiority and superiority was seen in a post-hoc analysis. “What we can say with certainty is that it’s non-inferior to the standard treatment,” Dr. Ware said.
The final analysis was based on 42 patients randomized to the transfusion arm who completed all 24 months of treatment, 11 with truncated treatment, and 8 withdrawals, and 41 patients assigned to the hydroxyurea arm who completed all treatment, 13 with truncated treatment and 6 withdrawals.
Sickle cell-related serious adverse events were more common in the hydroxyurea arm than the transfusion arm (23 vs. 15), but none were related to the study drug or procedures.
There were 29 new centrally adjudicated neurological events including 3 transient ischemic attacks in each arm. In the transfusion arm, one child was withdrawn per protocol after developing TCD velocities exceeding 240 cm/sec and a second developed new vasculopathy. The safety of long-term hydroxyurea has been established in multiple pediatric studies, Dr. Ware said.
TWiTCH was funded by the National Heart, Lung and Blood Institute and sponsored by Cincinnati Children’s Hospital Medical Center. Dr. Ware reported serving as a data safety monitoring board member for Eli Lilly, consultancy for Bayer, and research funding from Bristol Myers Squibb. Dr. Thompson disclosed research funding from Amgen, Baxalta, bluebird bio, Celgene, Eli Lilly, GlaxoSmithKline, Mast, and Novartis, and consultancy for ApoPharma, bluebird bio, and Novartis.
AT ASH 2015
Key clinical point: Hydroxyurea can substitute for chronic transfusions to prevent primary stroke in children with sickle cell disease and abnormal transcranial doppler velocities.
Major finding: Mean TCD velocities were 143 cm/sec with transfusions vs. 138 cm/sec with hydroxyurea (P less than 8.82 x 10-16 for noninferiority; P = .023 for superiority).
Data source: Phase III, noninferiority study in 121 children with sickle cell disease.
Disclosures: TWiTCH was funded by the National Heart, Lung and Blood Institute and sponsored by Cincinnati Children’s Hospital Medical Center. Dr. Ware reported serving as a data safety monitoring board member for Eli Lilly, consultancy for Bayer, and research funding from Bristol Myers Squibb. Dr. Thompson disclosed research funding from Amgen, Baxalta, bluebird bio, Celgene, Eli Lilly, GlaxoSmithKline, Mast, and Novartis, and consultancy for ApoPharma, bluebird bio, and Novartis.
VIDEO: Gene therapy repairs immune function in older patients with SCID-XI
ORLANDO – Older patients with x-linked severe combined immunodeficiency syndrome (SCID-X1) treated initially with haploidentical stem cell transplantation without conditioning have impaired immunity and experience severe, life-long morbidities. Dr. Harry L. Malech, chief of the Laboratory of Host Defenses and Chief, Genetic Immunotherapy Section, U.S. National Institute of Allergy and Infectious Diseases, Bethesda, Md., discusses how investigators at the U.S. NAID are treating autologous CD34 cells from patients with SCID-X1 with a lentiviral vector that transduced the cells with normal copies of the gene which in its mutated form causes SCID-X1. In early trials, they have been able to significantly improve immune function in some patients without an apparent increase in risk for malignancies, a common problem with earlier gene therapies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Older patients with x-linked severe combined immunodeficiency syndrome (SCID-X1) treated initially with haploidentical stem cell transplantation without conditioning have impaired immunity and experience severe, life-long morbidities. Dr. Harry L. Malech, chief of the Laboratory of Host Defenses and Chief, Genetic Immunotherapy Section, U.S. National Institute of Allergy and Infectious Diseases, Bethesda, Md., discusses how investigators at the U.S. NAID are treating autologous CD34 cells from patients with SCID-X1 with a lentiviral vector that transduced the cells with normal copies of the gene which in its mutated form causes SCID-X1. In early trials, they have been able to significantly improve immune function in some patients without an apparent increase in risk for malignancies, a common problem with earlier gene therapies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ORLANDO – Older patients with x-linked severe combined immunodeficiency syndrome (SCID-X1) treated initially with haploidentical stem cell transplantation without conditioning have impaired immunity and experience severe, life-long morbidities. Dr. Harry L. Malech, chief of the Laboratory of Host Defenses and Chief, Genetic Immunotherapy Section, U.S. National Institute of Allergy and Infectious Diseases, Bethesda, Md., discusses how investigators at the U.S. NAID are treating autologous CD34 cells from patients with SCID-X1 with a lentiviral vector that transduced the cells with normal copies of the gene which in its mutated form causes SCID-X1. In early trials, they have been able to significantly improve immune function in some patients without an apparent increase in risk for malignancies, a common problem with earlier gene therapies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT ASH 2015
ASH: Longer-stored RBCs equivalent to shorter for children with severe anemia
ORLANDO – Stored red blood cells continue to do their primary job of tissue reoxygenation for more than a month after being collected, a finding that has profound positive implications for countries where blood products are in perennially short supply and demand is high, said investigators from Africa and North America.
In a randomized controlled clinical trial, red blood cells (RBCs) stored for 25 to 35 days were not-inferior to RBCs stored for 1 to 10 days for tissue reox-ygenation in African children with severe anemia with lactic acidosis.
“We found no justification to shorten the current storage duration for RBCs judged by their fundamental role to deliver oxygen,” said Dr. Christine M. Cserti-Gazdewich from the University of Toronto, Ontario, Canada.
Their findings suggest that stored RBCs should be evaluated by their ability to effectively deliver oxygen to tissues rather than by the cell survival measures and in vitro markers of hemolysis used as the current standard for viability by regulatory agencies, Dr. Cserti-Gazdewich said at the American Society of Hematology annual meeting.
The study clinicaltrials.gov ID NCT01586923 is also published online in JAMA.
“This study won’t change our clinical practice, but it will substantiate what we have been doing,” said co-author Dr. Walter H “Sunny” Dzik from Mas-sachusetts General Hospital in Boston, in an interview.
“We didn’t go into this business to harm people, yet there’s a lot of lab data that has been pointing fingers at us, suggesting rather forcefully that by try-ing to manage blood inventories using older blood that we’re harming people,” he said.
Concerns about the shelf life of RBCs derive from evidence that the cells undergo cumulative changes in structure, biochemistry, and enzymatic active that ultimately could degrade their ability to deliver oxygen to target tissues. But those studies were based largely on laboratory findings and not on clinical practice, she noted.
To see whether RBCs stored for up to 5 weeks could be as effective at tissue oxygenation as more recently packaged cells, the investigators conducted a randomized clinical trial at a university hospital urgent-care facility in Kampala, Uganda, where diseases such as malaria and sickle-cell anemia, as well as malnutrion, cause severe anemia on a scale seldom seen in the developed world.
They enrolled 290 children from the ages of 6 to 60 months who presented with lactic acidosis due to severe anemia in the absence of shock, trauma, impaired cardiac function, refractory hypoxia, liver disease, or tissue injury. The patients all had hemoglobin levels of 5 g/dL or5 less, and serum lactate levels of 5 mM or greater.
The patients were randomly assigned, 145 to each study arm, to receive leukoreduced RBCs stored for either 1-10 days or from 25 to 35 days. All patients received 10 mL/kg of RBCs over 2 hours at the start of the study and, if indicated per protocol, an additional 10 mL/kg during hours 4-6. Blood lactate levels were measured at baseline and at 2, 4, 6, 8 and 24 hours.
The mean hemoglobin level at presentation was 3.7 ±1.3 g/dL and mean lactate was 9.3 ±3.4 mM.
The investigators found that the proportion of patients achieving a lactate ≤ 3 mM at 8 hours was 58% among patients who received the short-storage RBCs, compared with 61% among patients who received long-storage cells (P = 0.72). This result met the primary endpoint of non-inferiority for long-er-stored RBCs.
In fact, there were no significant differences between the groups in mean lactate levels at any of the time points beyond baseline.
There were no significant differences in lactate clearance, and there were similar degrees of improvement in clinical assessments, serial measurements of hemoglobin concentration, cerebral tissue oxygen saturation, and electrolyte abnormalities following RBC transfusions.
Adverse events, 30-day recovery, and survival were also comparable between the groups.
The authors also found in a pre-specified sub-group analysis that there were no significant between group differences in those patients who received a total of 20 mL/kg RBCs, Dr. Cserti-Gazdewich said.
“This is a crushingly urgent problem, in that there have been belief systems developed over the last couple of years that older blood is not as good as newer blood, without any real evidence to support that. The evidence that Christine is [presenting], I think, is pretty compelling evidence that that hy-pothesis is incorrect,” commented Dr. Mark Crowther, Professor and Chair in the Department of Pathology and Molecular Medicine of McMaster Univer-sity in Hamilton, Ontario, Canada. Dr. Crowther moderated a briefing where Dr. Cserti-Gazdewich presented the data.
The study was funded by the National Institutes of Health. Dr. Cserti-Gazdewich, Dr. Dzik, and Dr. Crowther reported having no relevant conflicts of interest.
ORLANDO – Stored red blood cells continue to do their primary job of tissue reoxygenation for more than a month after being collected, a finding that has profound positive implications for countries where blood products are in perennially short supply and demand is high, said investigators from Africa and North America.
In a randomized controlled clinical trial, red blood cells (RBCs) stored for 25 to 35 days were not-inferior to RBCs stored for 1 to 10 days for tissue reox-ygenation in African children with severe anemia with lactic acidosis.
“We found no justification to shorten the current storage duration for RBCs judged by their fundamental role to deliver oxygen,” said Dr. Christine M. Cserti-Gazdewich from the University of Toronto, Ontario, Canada.
Their findings suggest that stored RBCs should be evaluated by their ability to effectively deliver oxygen to tissues rather than by the cell survival measures and in vitro markers of hemolysis used as the current standard for viability by regulatory agencies, Dr. Cserti-Gazdewich said at the American Society of Hematology annual meeting.
The study clinicaltrials.gov ID NCT01586923 is also published online in JAMA.
“This study won’t change our clinical practice, but it will substantiate what we have been doing,” said co-author Dr. Walter H “Sunny” Dzik from Mas-sachusetts General Hospital in Boston, in an interview.
“We didn’t go into this business to harm people, yet there’s a lot of lab data that has been pointing fingers at us, suggesting rather forcefully that by try-ing to manage blood inventories using older blood that we’re harming people,” he said.
Concerns about the shelf life of RBCs derive from evidence that the cells undergo cumulative changes in structure, biochemistry, and enzymatic active that ultimately could degrade their ability to deliver oxygen to target tissues. But those studies were based largely on laboratory findings and not on clinical practice, she noted.
To see whether RBCs stored for up to 5 weeks could be as effective at tissue oxygenation as more recently packaged cells, the investigators conducted a randomized clinical trial at a university hospital urgent-care facility in Kampala, Uganda, where diseases such as malaria and sickle-cell anemia, as well as malnutrion, cause severe anemia on a scale seldom seen in the developed world.
They enrolled 290 children from the ages of 6 to 60 months who presented with lactic acidosis due to severe anemia in the absence of shock, trauma, impaired cardiac function, refractory hypoxia, liver disease, or tissue injury. The patients all had hemoglobin levels of 5 g/dL or5 less, and serum lactate levels of 5 mM or greater.
The patients were randomly assigned, 145 to each study arm, to receive leukoreduced RBCs stored for either 1-10 days or from 25 to 35 days. All patients received 10 mL/kg of RBCs over 2 hours at the start of the study and, if indicated per protocol, an additional 10 mL/kg during hours 4-6. Blood lactate levels were measured at baseline and at 2, 4, 6, 8 and 24 hours.
The mean hemoglobin level at presentation was 3.7 ±1.3 g/dL and mean lactate was 9.3 ±3.4 mM.
The investigators found that the proportion of patients achieving a lactate ≤ 3 mM at 8 hours was 58% among patients who received the short-storage RBCs, compared with 61% among patients who received long-storage cells (P = 0.72). This result met the primary endpoint of non-inferiority for long-er-stored RBCs.
In fact, there were no significant differences between the groups in mean lactate levels at any of the time points beyond baseline.
There were no significant differences in lactate clearance, and there were similar degrees of improvement in clinical assessments, serial measurements of hemoglobin concentration, cerebral tissue oxygen saturation, and electrolyte abnormalities following RBC transfusions.
Adverse events, 30-day recovery, and survival were also comparable between the groups.
The authors also found in a pre-specified sub-group analysis that there were no significant between group differences in those patients who received a total of 20 mL/kg RBCs, Dr. Cserti-Gazdewich said.
“This is a crushingly urgent problem, in that there have been belief systems developed over the last couple of years that older blood is not as good as newer blood, without any real evidence to support that. The evidence that Christine is [presenting], I think, is pretty compelling evidence that that hy-pothesis is incorrect,” commented Dr. Mark Crowther, Professor and Chair in the Department of Pathology and Molecular Medicine of McMaster Univer-sity in Hamilton, Ontario, Canada. Dr. Crowther moderated a briefing where Dr. Cserti-Gazdewich presented the data.
The study was funded by the National Institutes of Health. Dr. Cserti-Gazdewich, Dr. Dzik, and Dr. Crowther reported having no relevant conflicts of interest.
ORLANDO – Stored red blood cells continue to do their primary job of tissue reoxygenation for more than a month after being collected, a finding that has profound positive implications for countries where blood products are in perennially short supply and demand is high, said investigators from Africa and North America.
In a randomized controlled clinical trial, red blood cells (RBCs) stored for 25 to 35 days were not-inferior to RBCs stored for 1 to 10 days for tissue reox-ygenation in African children with severe anemia with lactic acidosis.
“We found no justification to shorten the current storage duration for RBCs judged by their fundamental role to deliver oxygen,” said Dr. Christine M. Cserti-Gazdewich from the University of Toronto, Ontario, Canada.
Their findings suggest that stored RBCs should be evaluated by their ability to effectively deliver oxygen to tissues rather than by the cell survival measures and in vitro markers of hemolysis used as the current standard for viability by regulatory agencies, Dr. Cserti-Gazdewich said at the American Society of Hematology annual meeting.
The study clinicaltrials.gov ID NCT01586923 is also published online in JAMA.
“This study won’t change our clinical practice, but it will substantiate what we have been doing,” said co-author Dr. Walter H “Sunny” Dzik from Mas-sachusetts General Hospital in Boston, in an interview.
“We didn’t go into this business to harm people, yet there’s a lot of lab data that has been pointing fingers at us, suggesting rather forcefully that by try-ing to manage blood inventories using older blood that we’re harming people,” he said.
Concerns about the shelf life of RBCs derive from evidence that the cells undergo cumulative changes in structure, biochemistry, and enzymatic active that ultimately could degrade their ability to deliver oxygen to target tissues. But those studies were based largely on laboratory findings and not on clinical practice, she noted.
To see whether RBCs stored for up to 5 weeks could be as effective at tissue oxygenation as more recently packaged cells, the investigators conducted a randomized clinical trial at a university hospital urgent-care facility in Kampala, Uganda, where diseases such as malaria and sickle-cell anemia, as well as malnutrion, cause severe anemia on a scale seldom seen in the developed world.
They enrolled 290 children from the ages of 6 to 60 months who presented with lactic acidosis due to severe anemia in the absence of shock, trauma, impaired cardiac function, refractory hypoxia, liver disease, or tissue injury. The patients all had hemoglobin levels of 5 g/dL or5 less, and serum lactate levels of 5 mM or greater.
The patients were randomly assigned, 145 to each study arm, to receive leukoreduced RBCs stored for either 1-10 days or from 25 to 35 days. All patients received 10 mL/kg of RBCs over 2 hours at the start of the study and, if indicated per protocol, an additional 10 mL/kg during hours 4-6. Blood lactate levels were measured at baseline and at 2, 4, 6, 8 and 24 hours.
The mean hemoglobin level at presentation was 3.7 ±1.3 g/dL and mean lactate was 9.3 ±3.4 mM.
The investigators found that the proportion of patients achieving a lactate ≤ 3 mM at 8 hours was 58% among patients who received the short-storage RBCs, compared with 61% among patients who received long-storage cells (P = 0.72). This result met the primary endpoint of non-inferiority for long-er-stored RBCs.
In fact, there were no significant differences between the groups in mean lactate levels at any of the time points beyond baseline.
There were no significant differences in lactate clearance, and there were similar degrees of improvement in clinical assessments, serial measurements of hemoglobin concentration, cerebral tissue oxygen saturation, and electrolyte abnormalities following RBC transfusions.
Adverse events, 30-day recovery, and survival were also comparable between the groups.
The authors also found in a pre-specified sub-group analysis that there were no significant between group differences in those patients who received a total of 20 mL/kg RBCs, Dr. Cserti-Gazdewich said.
“This is a crushingly urgent problem, in that there have been belief systems developed over the last couple of years that older blood is not as good as newer blood, without any real evidence to support that. The evidence that Christine is [presenting], I think, is pretty compelling evidence that that hy-pothesis is incorrect,” commented Dr. Mark Crowther, Professor and Chair in the Department of Pathology and Molecular Medicine of McMaster Univer-sity in Hamilton, Ontario, Canada. Dr. Crowther moderated a briefing where Dr. Cserti-Gazdewich presented the data.
The study was funded by the National Institutes of Health. Dr. Cserti-Gazdewich, Dr. Dzik, and Dr. Crowther reported having no relevant conflicts of interest.
AT ASH 2015
Key clinical point: Red blood cells stored for up to 35 days were not inferior to more recently collected RBCs for tissue reoxygenation.
Major finding: The proportion of children with severe anemia achieving a lactate ≤ 3 mM at 8 hours was 58% among patients who received the short-storage RBCs, compared with 61% among patients who received long-storage cells.
Data source: Randomized controlled clinical trial in 290 children with lactic acidosis due to severe anemia.
Disclosures: The study was funded by the National Institutes of Health. Dr. Cserti-Gazdewich, Dr. Dzik, and Dr. Crowther reported having no relevant conflicts of interest.
ASH: Donor CAR-T cells elicit responses in mixture of progressive B-cell cancers
ORLANDO – A single infusion of donor-derived chimeric antigen receptor (CAR)-modified T cells targeting CD19 achieved remission in 9 of 20 patients with B-cell malignancies that progressed after allogeneic stem cell transplant, a study shows.
The seven complete remissions and two partial remissions occurred without any chemotherapy and without causing acute graft-versus-host disease (GVHD).
The experimental anti-CD 19 CAR T-cells seem particularly effective against acute lymphoid leukemia (ALL) and chronic lymphocytic leukemia (CLL), but responses also occurred in lymphoma, Dr. James Kochenderfer of the Center for Cancer Research, National Cancer Institute, in Bethesda, Md., reported at the annual meeting of the American Society of Hematology.
B-cell malignancies that persist after transplantation are often treated with unmanipulated donor lymphocytes, but these infusions are often ineffective and associated with significant morbidity and mortality from GVHD.
To improve on this approach, 20 patients were infused with T cells obtained from the original stem cell donor and transduced with a CD19-directed CAR that was encoded by a gamma-retroviral vector and included a CD28 co-stimulatory domain. The highest dose reached in the phase I study was 107 total cell/kg. Production of the anti-CD19 CAR T cells took only eight days for each patient, Dr. Kochenderfer said at a press briefing.
The patients had received at least one standard donor-leukocyte infusion, had to have minimal or no GVHD, and could not be receiving systemic immunosuppressive drugs.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The highest response rates were in ALL, where four of five patients obtained complete remission (CR) with no detectable minimal residual disease by multi-color flow cytometry, Dr. Kochenderfer said. Two of these patients later relapsed, one is in ongoing CR at 18 months, and one went on to a second allogeneic transplant and continues in complete remission.
The longest ongoing CR at 36 months occurred in a patient treated for CLL. Another patient achieved a partial remission (PR) ongoing at 18 months, two patients progressed, and one has stable disease.
In five patients treated for Mantle cell lymphoma, there is one CR ongoing at 31 months, one PR, and three stable diseases.
Three of the five patients treated for diffuse large B-cell lymphoma experienced stable disease, one progressive disease, and one obtained a CR, but is no longer evaluable because she received other therapies for chronic GVHD. Dr. Kochenderfer went on describe an impressive response in this patient, who had large lymphoma masses at the back of her head and in her eye socket before infusion.
“Amazingly, the tumor masses completely disappeared within five days of CAR T-cell infusion,” he said.
Patients with high tumor burdens, however, experienced severe cytokine-release syndrome with fever, tachycardia, and hypertension that was treated with the interleukin-6 receptor antagonist tocilizumab (Actemra).
Only one case of mild aphasia occurred, which contrasts with other CAR T-cell therapies where neurotoxicity is common, Dr. Kochenderfer said.
One patient had continued worsening of pre-existing chronic GVHD after CAR T-cell therapy, and one patient developed very mild chronic eye GVHD more than a year after infusion.
The press corps was not fully convinced by the findings, however, asking Dr. Kochenderfer why they should be excited by the 40% remission rate when other CAR T-cell therapies have yielded remission rates as high as 90%.
Dr. Kochenderfer pointed out that four of the five ALL patients (80%) achieved a MRD-negative complete response, which compares favorably with other protocols. The remaining patients had far more advanced, treatment-resident disease of varying histologies than evaluated in other trials and, unlike most trials, all patients had received an allogeneic transplant. Further, the investigators used no chemotherapy whatsoever, whereas other CAR T-cells trials have used chemotherapy, sometimes in huge does, he said.
ORLANDO – A single infusion of donor-derived chimeric antigen receptor (CAR)-modified T cells targeting CD19 achieved remission in 9 of 20 patients with B-cell malignancies that progressed after allogeneic stem cell transplant, a study shows.
The seven complete remissions and two partial remissions occurred without any chemotherapy and without causing acute graft-versus-host disease (GVHD).
The experimental anti-CD 19 CAR T-cells seem particularly effective against acute lymphoid leukemia (ALL) and chronic lymphocytic leukemia (CLL), but responses also occurred in lymphoma, Dr. James Kochenderfer of the Center for Cancer Research, National Cancer Institute, in Bethesda, Md., reported at the annual meeting of the American Society of Hematology.
B-cell malignancies that persist after transplantation are often treated with unmanipulated donor lymphocytes, but these infusions are often ineffective and associated with significant morbidity and mortality from GVHD.
To improve on this approach, 20 patients were infused with T cells obtained from the original stem cell donor and transduced with a CD19-directed CAR that was encoded by a gamma-retroviral vector and included a CD28 co-stimulatory domain. The highest dose reached in the phase I study was 107 total cell/kg. Production of the anti-CD19 CAR T cells took only eight days for each patient, Dr. Kochenderfer said at a press briefing.
The patients had received at least one standard donor-leukocyte infusion, had to have minimal or no GVHD, and could not be receiving systemic immunosuppressive drugs.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The highest response rates were in ALL, where four of five patients obtained complete remission (CR) with no detectable minimal residual disease by multi-color flow cytometry, Dr. Kochenderfer said. Two of these patients later relapsed, one is in ongoing CR at 18 months, and one went on to a second allogeneic transplant and continues in complete remission.
The longest ongoing CR at 36 months occurred in a patient treated for CLL. Another patient achieved a partial remission (PR) ongoing at 18 months, two patients progressed, and one has stable disease.
In five patients treated for Mantle cell lymphoma, there is one CR ongoing at 31 months, one PR, and three stable diseases.
Three of the five patients treated for diffuse large B-cell lymphoma experienced stable disease, one progressive disease, and one obtained a CR, but is no longer evaluable because she received other therapies for chronic GVHD. Dr. Kochenderfer went on describe an impressive response in this patient, who had large lymphoma masses at the back of her head and in her eye socket before infusion.
“Amazingly, the tumor masses completely disappeared within five days of CAR T-cell infusion,” he said.
Patients with high tumor burdens, however, experienced severe cytokine-release syndrome with fever, tachycardia, and hypertension that was treated with the interleukin-6 receptor antagonist tocilizumab (Actemra).
Only one case of mild aphasia occurred, which contrasts with other CAR T-cell therapies where neurotoxicity is common, Dr. Kochenderfer said.
One patient had continued worsening of pre-existing chronic GVHD after CAR T-cell therapy, and one patient developed very mild chronic eye GVHD more than a year after infusion.
The press corps was not fully convinced by the findings, however, asking Dr. Kochenderfer why they should be excited by the 40% remission rate when other CAR T-cell therapies have yielded remission rates as high as 90%.
Dr. Kochenderfer pointed out that four of the five ALL patients (80%) achieved a MRD-negative complete response, which compares favorably with other protocols. The remaining patients had far more advanced, treatment-resident disease of varying histologies than evaluated in other trials and, unlike most trials, all patients had received an allogeneic transplant. Further, the investigators used no chemotherapy whatsoever, whereas other CAR T-cells trials have used chemotherapy, sometimes in huge does, he said.
ORLANDO – A single infusion of donor-derived chimeric antigen receptor (CAR)-modified T cells targeting CD19 achieved remission in 9 of 20 patients with B-cell malignancies that progressed after allogeneic stem cell transplant, a study shows.
The seven complete remissions and two partial remissions occurred without any chemotherapy and without causing acute graft-versus-host disease (GVHD).
The experimental anti-CD 19 CAR T-cells seem particularly effective against acute lymphoid leukemia (ALL) and chronic lymphocytic leukemia (CLL), but responses also occurred in lymphoma, Dr. James Kochenderfer of the Center for Cancer Research, National Cancer Institute, in Bethesda, Md., reported at the annual meeting of the American Society of Hematology.
B-cell malignancies that persist after transplantation are often treated with unmanipulated donor lymphocytes, but these infusions are often ineffective and associated with significant morbidity and mortality from GVHD.
To improve on this approach, 20 patients were infused with T cells obtained from the original stem cell donor and transduced with a CD19-directed CAR that was encoded by a gamma-retroviral vector and included a CD28 co-stimulatory domain. The highest dose reached in the phase I study was 107 total cell/kg. Production of the anti-CD19 CAR T cells took only eight days for each patient, Dr. Kochenderfer said at a press briefing.
The patients had received at least one standard donor-leukocyte infusion, had to have minimal or no GVHD, and could not be receiving systemic immunosuppressive drugs.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The highest response rates were in ALL, where four of five patients obtained complete remission (CR) with no detectable minimal residual disease by multi-color flow cytometry, Dr. Kochenderfer said. Two of these patients later relapsed, one is in ongoing CR at 18 months, and one went on to a second allogeneic transplant and continues in complete remission.
The longest ongoing CR at 36 months occurred in a patient treated for CLL. Another patient achieved a partial remission (PR) ongoing at 18 months, two patients progressed, and one has stable disease.
In five patients treated for Mantle cell lymphoma, there is one CR ongoing at 31 months, one PR, and three stable diseases.
Three of the five patients treated for diffuse large B-cell lymphoma experienced stable disease, one progressive disease, and one obtained a CR, but is no longer evaluable because she received other therapies for chronic GVHD. Dr. Kochenderfer went on describe an impressive response in this patient, who had large lymphoma masses at the back of her head and in her eye socket before infusion.
“Amazingly, the tumor masses completely disappeared within five days of CAR T-cell infusion,” he said.
Patients with high tumor burdens, however, experienced severe cytokine-release syndrome with fever, tachycardia, and hypertension that was treated with the interleukin-6 receptor antagonist tocilizumab (Actemra).
Only one case of mild aphasia occurred, which contrasts with other CAR T-cell therapies where neurotoxicity is common, Dr. Kochenderfer said.
One patient had continued worsening of pre-existing chronic GVHD after CAR T-cell therapy, and one patient developed very mild chronic eye GVHD more than a year after infusion.
The press corps was not fully convinced by the findings, however, asking Dr. Kochenderfer why they should be excited by the 40% remission rate when other CAR T-cell therapies have yielded remission rates as high as 90%.
Dr. Kochenderfer pointed out that four of the five ALL patients (80%) achieved a MRD-negative complete response, which compares favorably with other protocols. The remaining patients had far more advanced, treatment-resident disease of varying histologies than evaluated in other trials and, unlike most trials, all patients had received an allogeneic transplant. Further, the investigators used no chemotherapy whatsoever, whereas other CAR T-cells trials have used chemotherapy, sometimes in huge does, he said.
AT ASH 2015
Key clinical point: Allogeneic anti-CD19 CAR T-cell therapy showed promise in a treatment approach for B-cell malignancies persisting after allogeneic transplantation.
Major finding: Nine of 20 patients achieved remission with anti-CD19 CAR T-cell therapy.
Data source: Phase I study in 20 patients with CD19-positive B-cell malignancies progressing after allogeneic transplant.
Disclosures: Dr. Kochenderfer reported research funding from Bluebird bio, the study sponsor.
Heme Themes: Transplant timing for myelofibrosis in the era of JAK2 inhibitors
ALEXANDRIA, VA. – How are mutation analysis gene panels affecting risk stratification and decision making regarding stem cell transplants in myelofibrosis patients? How are the results of the Dynamic International Prognostic Scoring System (DIPSS) and performance status improvements seen with JAK2 inhibitors influencing who is a candidate for transplant and the timing of transplants?
Watch the conversation between Dr. Vikas Gupta of the leukemia and bone marrow transplant programs at the Princess Margaret Cancer Centre, Toronto, and associate professor of medicine at the University of Toronto, and Dr. Rami S. Komrokji of the Moffitt Cancer Center, Tampa, Fla., as they discuss their individual approaches that consider patient wishes and goals, type of donor, and disease risk in their decisions to perform stem cell transplants upfront or to delay them until after JAK2 inhibitor therapy.
Have an insight to share? Post a comment.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ALEXANDRIA, VA. – How are mutation analysis gene panels affecting risk stratification and decision making regarding stem cell transplants in myelofibrosis patients? How are the results of the Dynamic International Prognostic Scoring System (DIPSS) and performance status improvements seen with JAK2 inhibitors influencing who is a candidate for transplant and the timing of transplants?
Watch the conversation between Dr. Vikas Gupta of the leukemia and bone marrow transplant programs at the Princess Margaret Cancer Centre, Toronto, and associate professor of medicine at the University of Toronto, and Dr. Rami S. Komrokji of the Moffitt Cancer Center, Tampa, Fla., as they discuss their individual approaches that consider patient wishes and goals, type of donor, and disease risk in their decisions to perform stem cell transplants upfront or to delay them until after JAK2 inhibitor therapy.
Have an insight to share? Post a comment.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ALEXANDRIA, VA. – How are mutation analysis gene panels affecting risk stratification and decision making regarding stem cell transplants in myelofibrosis patients? How are the results of the Dynamic International Prognostic Scoring System (DIPSS) and performance status improvements seen with JAK2 inhibitors influencing who is a candidate for transplant and the timing of transplants?
Watch the conversation between Dr. Vikas Gupta of the leukemia and bone marrow transplant programs at the Princess Margaret Cancer Centre, Toronto, and associate professor of medicine at the University of Toronto, and Dr. Rami S. Komrokji of the Moffitt Cancer Center, Tampa, Fla., as they discuss their individual approaches that consider patient wishes and goals, type of donor, and disease risk in their decisions to perform stem cell transplants upfront or to delay them until after JAK2 inhibitor therapy.
Have an insight to share? Post a comment.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT U.S. FOCUS ON MPN AND MDS
CLL patients achieve remission with CAR-modified T-cells
Treatment with chimeric antigen receptor (CAR)-modified T cells targeting CD19 achieved a response in 8 of 14 patients (57%) with advanced chronic lymphocytic leukemia (CLL), of whom 4 experienced a complete remission without relapse, based on the mature results of a small pilot study.
Of these four patients, two have remained free of their disease for up to 4 years after they received treatment. An analysis of blood samples also showed that these modified T cells can multiply and persist in the body for a period of years, the researchers report in a study published Sept. 2 in Science Translational Medicine
“Both patients remain alive and cancer free and just passed the 5-year anniversary of their treatment this summer,” said Dr. David L. Porter, the Jodi Fisher Horowitz Professor in Leukemia Care Excellence and director of blood and marrow transplantation at the University of Pennsylvania’s Abramson Cancer Center in Philadelphia. “A third patient in remission just passed the 3-year anniversary with no signs of leukemia” (Sci Transl Med. 2015;7:303ra139).
The current study indicates the mature results from this trial, which began in the summer of 2010. In 2011, preliminary findings from the first three patients to enroll in the study were published and showed that two of them had experienced a complete response. Their disease currently remains in remission more than 4 years after beginning treatment. The first patient to receive the therapy has been cancer free for 5 years.
In the current trial, 14 patients with relapsed or refractory CLL received at least one infusion of autologous T cells transduced with a CD19-directed CAR (CTL019) lentiviral vector. All of the patients had active disease at the time they received the experimental treatment, and had received a median of 5 previous therapies (range, 1-11). One participant had undergone two previous autologous stem cell transplants and one had progressed on ibrutinib therapy.
In addition to those who achieved a complete remission, four other patients (29%) had partial responses to the therapy with responses that persisted for a median of 7 months. Two died of disease progression at 10 and 27 months after receiving CTL019, and one died from a pulmonary embolism; the remaining patient remains alive after CLL progressed at 13 months, and is receiving other therapies.
Overall, the CTL019 infusions were well tolerated, with grade less than 2 toxicities that included primarily low-grade fevers and chills. The most frequent related events were associated with complications of neutropenia and delayed cytokine release syndrome, which correlated with in vivo CTL019 expansion. There were two cases of tumor lysis syndrome, and one patient died in remission 21 months after T cell infusion, after developing ecthyma gangrenosum after pseudomonas infection at a skin biopsy site.
Six subjects (43%) had no response and all six progressed within 1-9 months (median, 4 months) of CTL019 therapy. “We are working hard to determine why this therapy may be appropriate for some patients and not others, and trying to optimize either treatment conditions or patient-specific factors so that this might be more effective for more patients,” Dr. Porter wrote.
Minimal residual disease was not detectable in patients who achieved a complete response, suggesting that disease eradication may be possible in some patients with advanced CLL. The activity of CTLO19 seemed to be on par with results achieved with allogeneic stem cell transplantation, suggesting that this therapy could possibly cure CLL. But Dr. Porter pointed out that this study was conducted with a small number of patients and for CLL, a relatively short follow-up.
“However, these patients all had heavily pretreated resistant disease,” he said. “Though we do not know if patients are indeed cured, it is certainly our goal to find a cure for CLL and without the toxicities and limitations of allogeneic stem cell transplantation. Indeed, longer follow-up will be needed but we are quite excited about the results to date.”
Dr. Porter said he and his team have ongoing trials in CLL in progress, where they are working on trying to identify the optimal dose of T cells for this approach. Also, “this research has led to expansion of this approach to other B cell malignancies such as acute lymphocytic leukemia.”
Novartis, the Leukemia and Lymphoma Society (Specialized Center of Research Award), and the National Institutes of Health funded the study. The University of Pennsylvania has licensed technologies involved in this trial to Novartis. Some scientists involved in these trials, including Dr. Porter, are inventors of these technologies. As a result of the licensing relationship with Novartis, the University of Pennsylvania receives significant financial benefit, and these inventors have benefited financially and/or may benefit financially in the future.
Treatment with chimeric antigen receptor (CAR)-modified T cells targeting CD19 achieved a response in 8 of 14 patients (57%) with advanced chronic lymphocytic leukemia (CLL), of whom 4 experienced a complete remission without relapse, based on the mature results of a small pilot study.
Of these four patients, two have remained free of their disease for up to 4 years after they received treatment. An analysis of blood samples also showed that these modified T cells can multiply and persist in the body for a period of years, the researchers report in a study published Sept. 2 in Science Translational Medicine
“Both patients remain alive and cancer free and just passed the 5-year anniversary of their treatment this summer,” said Dr. David L. Porter, the Jodi Fisher Horowitz Professor in Leukemia Care Excellence and director of blood and marrow transplantation at the University of Pennsylvania’s Abramson Cancer Center in Philadelphia. “A third patient in remission just passed the 3-year anniversary with no signs of leukemia” (Sci Transl Med. 2015;7:303ra139).
The current study indicates the mature results from this trial, which began in the summer of 2010. In 2011, preliminary findings from the first three patients to enroll in the study were published and showed that two of them had experienced a complete response. Their disease currently remains in remission more than 4 years after beginning treatment. The first patient to receive the therapy has been cancer free for 5 years.
In the current trial, 14 patients with relapsed or refractory CLL received at least one infusion of autologous T cells transduced with a CD19-directed CAR (CTL019) lentiviral vector. All of the patients had active disease at the time they received the experimental treatment, and had received a median of 5 previous therapies (range, 1-11). One participant had undergone two previous autologous stem cell transplants and one had progressed on ibrutinib therapy.
In addition to those who achieved a complete remission, four other patients (29%) had partial responses to the therapy with responses that persisted for a median of 7 months. Two died of disease progression at 10 and 27 months after receiving CTL019, and one died from a pulmonary embolism; the remaining patient remains alive after CLL progressed at 13 months, and is receiving other therapies.
Overall, the CTL019 infusions were well tolerated, with grade less than 2 toxicities that included primarily low-grade fevers and chills. The most frequent related events were associated with complications of neutropenia and delayed cytokine release syndrome, which correlated with in vivo CTL019 expansion. There were two cases of tumor lysis syndrome, and one patient died in remission 21 months after T cell infusion, after developing ecthyma gangrenosum after pseudomonas infection at a skin biopsy site.
Six subjects (43%) had no response and all six progressed within 1-9 months (median, 4 months) of CTL019 therapy. “We are working hard to determine why this therapy may be appropriate for some patients and not others, and trying to optimize either treatment conditions or patient-specific factors so that this might be more effective for more patients,” Dr. Porter wrote.
Minimal residual disease was not detectable in patients who achieved a complete response, suggesting that disease eradication may be possible in some patients with advanced CLL. The activity of CTLO19 seemed to be on par with results achieved with allogeneic stem cell transplantation, suggesting that this therapy could possibly cure CLL. But Dr. Porter pointed out that this study was conducted with a small number of patients and for CLL, a relatively short follow-up.
“However, these patients all had heavily pretreated resistant disease,” he said. “Though we do not know if patients are indeed cured, it is certainly our goal to find a cure for CLL and without the toxicities and limitations of allogeneic stem cell transplantation. Indeed, longer follow-up will be needed but we are quite excited about the results to date.”
Dr. Porter said he and his team have ongoing trials in CLL in progress, where they are working on trying to identify the optimal dose of T cells for this approach. Also, “this research has led to expansion of this approach to other B cell malignancies such as acute lymphocytic leukemia.”
Novartis, the Leukemia and Lymphoma Society (Specialized Center of Research Award), and the National Institutes of Health funded the study. The University of Pennsylvania has licensed technologies involved in this trial to Novartis. Some scientists involved in these trials, including Dr. Porter, are inventors of these technologies. As a result of the licensing relationship with Novartis, the University of Pennsylvania receives significant financial benefit, and these inventors have benefited financially and/or may benefit financially in the future.
Treatment with chimeric antigen receptor (CAR)-modified T cells targeting CD19 achieved a response in 8 of 14 patients (57%) with advanced chronic lymphocytic leukemia (CLL), of whom 4 experienced a complete remission without relapse, based on the mature results of a small pilot study.
Of these four patients, two have remained free of their disease for up to 4 years after they received treatment. An analysis of blood samples also showed that these modified T cells can multiply and persist in the body for a period of years, the researchers report in a study published Sept. 2 in Science Translational Medicine
“Both patients remain alive and cancer free and just passed the 5-year anniversary of their treatment this summer,” said Dr. David L. Porter, the Jodi Fisher Horowitz Professor in Leukemia Care Excellence and director of blood and marrow transplantation at the University of Pennsylvania’s Abramson Cancer Center in Philadelphia. “A third patient in remission just passed the 3-year anniversary with no signs of leukemia” (Sci Transl Med. 2015;7:303ra139).
The current study indicates the mature results from this trial, which began in the summer of 2010. In 2011, preliminary findings from the first three patients to enroll in the study were published and showed that two of them had experienced a complete response. Their disease currently remains in remission more than 4 years after beginning treatment. The first patient to receive the therapy has been cancer free for 5 years.
In the current trial, 14 patients with relapsed or refractory CLL received at least one infusion of autologous T cells transduced with a CD19-directed CAR (CTL019) lentiviral vector. All of the patients had active disease at the time they received the experimental treatment, and had received a median of 5 previous therapies (range, 1-11). One participant had undergone two previous autologous stem cell transplants and one had progressed on ibrutinib therapy.
In addition to those who achieved a complete remission, four other patients (29%) had partial responses to the therapy with responses that persisted for a median of 7 months. Two died of disease progression at 10 and 27 months after receiving CTL019, and one died from a pulmonary embolism; the remaining patient remains alive after CLL progressed at 13 months, and is receiving other therapies.
Overall, the CTL019 infusions were well tolerated, with grade less than 2 toxicities that included primarily low-grade fevers and chills. The most frequent related events were associated with complications of neutropenia and delayed cytokine release syndrome, which correlated with in vivo CTL019 expansion. There were two cases of tumor lysis syndrome, and one patient died in remission 21 months after T cell infusion, after developing ecthyma gangrenosum after pseudomonas infection at a skin biopsy site.
Six subjects (43%) had no response and all six progressed within 1-9 months (median, 4 months) of CTL019 therapy. “We are working hard to determine why this therapy may be appropriate for some patients and not others, and trying to optimize either treatment conditions or patient-specific factors so that this might be more effective for more patients,” Dr. Porter wrote.
Minimal residual disease was not detectable in patients who achieved a complete response, suggesting that disease eradication may be possible in some patients with advanced CLL. The activity of CTLO19 seemed to be on par with results achieved with allogeneic stem cell transplantation, suggesting that this therapy could possibly cure CLL. But Dr. Porter pointed out that this study was conducted with a small number of patients and for CLL, a relatively short follow-up.
“However, these patients all had heavily pretreated resistant disease,” he said. “Though we do not know if patients are indeed cured, it is certainly our goal to find a cure for CLL and without the toxicities and limitations of allogeneic stem cell transplantation. Indeed, longer follow-up will be needed but we are quite excited about the results to date.”
Dr. Porter said he and his team have ongoing trials in CLL in progress, where they are working on trying to identify the optimal dose of T cells for this approach. Also, “this research has led to expansion of this approach to other B cell malignancies such as acute lymphocytic leukemia.”
Novartis, the Leukemia and Lymphoma Society (Specialized Center of Research Award), and the National Institutes of Health funded the study. The University of Pennsylvania has licensed technologies involved in this trial to Novartis. Some scientists involved in these trials, including Dr. Porter, are inventors of these technologies. As a result of the licensing relationship with Novartis, the University of Pennsylvania receives significant financial benefit, and these inventors have benefited financially and/or may benefit financially in the future.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: CAR-modified T cell therapy lacks the toxicities and limitations of allogeneic stem cell transplantation and may be an effective treatment for chronic lymphocytic leukemia.
Major finding: CAR-modified T cell therapy elicited a response in 8 of 14 patients (57%) with relapsed and refractory chronic lymphocytic leukemia, and 4 patients (29%) achieved a complete remission.
Data source: Mature results from a pilot clinical trial.
Disclosures: Novartis, the Leukemia and Lymphoma Society (Specialized Center of Research Award), and the National Institutes of Health funded the study. The University of Pennsylvania has licensed technologies involved in this trial to Novartis. Some scientists involved in these trials, including Dr. Porter, are inventors of these technologies. As a result of the licensing relationship with Novartis, the University of Pennsylvania receives significant financial benefit, and these inventors have benefited financially and/or may benefit financially in the future.
HLA-matched sibling transplants provide best outcomes in infantile osteopetrosis
Long-term survival after hematopoietic stem cell transplantation for infantile osteopetrosis was highest when grafts were taken from human leukocyte antigen (HLA)-matched siblings, according to the largest cohort of patients with the disease that has been compiled to date.
For HLA-matched sibling transplants, 5- and 10-year survival probabilities were both 62%, whereas the combined average survival probability for HLA-mismatched relative donors, HLA-matched unrelated donors, and HLA-unmatched unrelated donors was 42% after 5 years and 39% after 10 years, Dr. Paul J. Orchard of the University of Minnesota, Minneapolis, and his colleagues reported.
Of surviving patients, 70% have visual impairment and 10% have auditory impairment and motor delay. Despite this, most survivors are attending a public or specialized school, and 65% of survivors reported performance scores of 90 or 100 at last contact, the investigators said.
Graft failure was the most common cause of death, occurring in 50% of HLA-matched sibling transplant patients and in 43% of alternative HLA transplant patients. Veno-occlusive disease and interstitial pneumonitis rates were also high, both at about 20%.
“There is an urgent need to improve engraftment by developing novel strategies that target the microenvironment and study the association between genetic variants of osteopetrosis and transplantation outcomes,” the researchers said.
Find the full article in the July 9 issue of Blood (Blood 2015;126:270-6).
Long-term survival after hematopoietic stem cell transplantation for infantile osteopetrosis was highest when grafts were taken from human leukocyte antigen (HLA)-matched siblings, according to the largest cohort of patients with the disease that has been compiled to date.
For HLA-matched sibling transplants, 5- and 10-year survival probabilities were both 62%, whereas the combined average survival probability for HLA-mismatched relative donors, HLA-matched unrelated donors, and HLA-unmatched unrelated donors was 42% after 5 years and 39% after 10 years, Dr. Paul J. Orchard of the University of Minnesota, Minneapolis, and his colleagues reported.
Of surviving patients, 70% have visual impairment and 10% have auditory impairment and motor delay. Despite this, most survivors are attending a public or specialized school, and 65% of survivors reported performance scores of 90 or 100 at last contact, the investigators said.
Graft failure was the most common cause of death, occurring in 50% of HLA-matched sibling transplant patients and in 43% of alternative HLA transplant patients. Veno-occlusive disease and interstitial pneumonitis rates were also high, both at about 20%.
“There is an urgent need to improve engraftment by developing novel strategies that target the microenvironment and study the association between genetic variants of osteopetrosis and transplantation outcomes,” the researchers said.
Find the full article in the July 9 issue of Blood (Blood 2015;126:270-6).
Long-term survival after hematopoietic stem cell transplantation for infantile osteopetrosis was highest when grafts were taken from human leukocyte antigen (HLA)-matched siblings, according to the largest cohort of patients with the disease that has been compiled to date.
For HLA-matched sibling transplants, 5- and 10-year survival probabilities were both 62%, whereas the combined average survival probability for HLA-mismatched relative donors, HLA-matched unrelated donors, and HLA-unmatched unrelated donors was 42% after 5 years and 39% after 10 years, Dr. Paul J. Orchard of the University of Minnesota, Minneapolis, and his colleagues reported.
Of surviving patients, 70% have visual impairment and 10% have auditory impairment and motor delay. Despite this, most survivors are attending a public or specialized school, and 65% of survivors reported performance scores of 90 or 100 at last contact, the investigators said.
Graft failure was the most common cause of death, occurring in 50% of HLA-matched sibling transplant patients and in 43% of alternative HLA transplant patients. Veno-occlusive disease and interstitial pneumonitis rates were also high, both at about 20%.
“There is an urgent need to improve engraftment by developing novel strategies that target the microenvironment and study the association between genetic variants of osteopetrosis and transplantation outcomes,” the researchers said.
Find the full article in the July 9 issue of Blood (Blood 2015;126:270-6).
Doxorubicin, radiation doses predict heart risk in lymphoma survivors
Adult lymphoma survivors who were treated with autologous hematopoietic stem-cell transplantation had a greater than sixfold increased risk of left ventricular systolic dysfunction compared with controls, according to a study published online in the Journal of Clinical Oncology.
Among 274 adult survivors of Hodgkin or non-Hodgkin lymphoma, 16% had left ventricular systolic dysfunction (LVSD): 11% had overt heart failure (HF) and 5% had asymptomatic LVSD, defined as a left ventricular ejection fraction of less than 50%.Heart symptoms were significantly associated with exposure to doxorubicin at a cumulative dose of 300 mg/m2 or more and with cardiac radiation therapy of more than 30 Gy. Recognizing these patient risk factors allows for more intensive follow-up with the goal of “identification and early treatment of asymptomatic LVSD [which] may prevent the development of HF,” wrote Dr. Klaus Murbraech of Oslo University Hospital and his colleagues (J. Clin. Oncol. 2015 July 13 [doi:10.1200/JCO.2015.60.8125]).
The investigators observed no association between lower-dose cardiac radiation therapy and LVSD. There was only a marginally significant association between the presence of two or more traditional cardiovascular disease risk factors and LVSD.
The cross-sectional multicenter cohort study is the first to assess the prevalence of LVSD, according to Dr. Murbraech and his colleagues. The study included adult survivors of Hodgkin or non-Hodgkin lymphoma, median age 56 years, who underwent autologous stem-cell transplants in Norway from 1987 to 2008. The median observation time was 13 years (range, 4-34 years). The control group consisted of initially healthy patients in an echocardiographic follow-up study. Controls were matched to patients based on age, sex, systolic blood pressure, and body mass index.
The study was supported by the South-Eastern Norway Regional Health Authority and Extrastiftelsen. Dr. Murbraech reported having no disclosures.
Adult lymphoma survivors who were treated with autologous hematopoietic stem-cell transplantation had a greater than sixfold increased risk of left ventricular systolic dysfunction compared with controls, according to a study published online in the Journal of Clinical Oncology.
Among 274 adult survivors of Hodgkin or non-Hodgkin lymphoma, 16% had left ventricular systolic dysfunction (LVSD): 11% had overt heart failure (HF) and 5% had asymptomatic LVSD, defined as a left ventricular ejection fraction of less than 50%.Heart symptoms were significantly associated with exposure to doxorubicin at a cumulative dose of 300 mg/m2 or more and with cardiac radiation therapy of more than 30 Gy. Recognizing these patient risk factors allows for more intensive follow-up with the goal of “identification and early treatment of asymptomatic LVSD [which] may prevent the development of HF,” wrote Dr. Klaus Murbraech of Oslo University Hospital and his colleagues (J. Clin. Oncol. 2015 July 13 [doi:10.1200/JCO.2015.60.8125]).
The investigators observed no association between lower-dose cardiac radiation therapy and LVSD. There was only a marginally significant association between the presence of two or more traditional cardiovascular disease risk factors and LVSD.
The cross-sectional multicenter cohort study is the first to assess the prevalence of LVSD, according to Dr. Murbraech and his colleagues. The study included adult survivors of Hodgkin or non-Hodgkin lymphoma, median age 56 years, who underwent autologous stem-cell transplants in Norway from 1987 to 2008. The median observation time was 13 years (range, 4-34 years). The control group consisted of initially healthy patients in an echocardiographic follow-up study. Controls were matched to patients based on age, sex, systolic blood pressure, and body mass index.
The study was supported by the South-Eastern Norway Regional Health Authority and Extrastiftelsen. Dr. Murbraech reported having no disclosures.
Adult lymphoma survivors who were treated with autologous hematopoietic stem-cell transplantation had a greater than sixfold increased risk of left ventricular systolic dysfunction compared with controls, according to a study published online in the Journal of Clinical Oncology.
Among 274 adult survivors of Hodgkin or non-Hodgkin lymphoma, 16% had left ventricular systolic dysfunction (LVSD): 11% had overt heart failure (HF) and 5% had asymptomatic LVSD, defined as a left ventricular ejection fraction of less than 50%.Heart symptoms were significantly associated with exposure to doxorubicin at a cumulative dose of 300 mg/m2 or more and with cardiac radiation therapy of more than 30 Gy. Recognizing these patient risk factors allows for more intensive follow-up with the goal of “identification and early treatment of asymptomatic LVSD [which] may prevent the development of HF,” wrote Dr. Klaus Murbraech of Oslo University Hospital and his colleagues (J. Clin. Oncol. 2015 July 13 [doi:10.1200/JCO.2015.60.8125]).
The investigators observed no association between lower-dose cardiac radiation therapy and LVSD. There was only a marginally significant association between the presence of two or more traditional cardiovascular disease risk factors and LVSD.
The cross-sectional multicenter cohort study is the first to assess the prevalence of LVSD, according to Dr. Murbraech and his colleagues. The study included adult survivors of Hodgkin or non-Hodgkin lymphoma, median age 56 years, who underwent autologous stem-cell transplants in Norway from 1987 to 2008. The median observation time was 13 years (range, 4-34 years). The control group consisted of initially healthy patients in an echocardiographic follow-up study. Controls were matched to patients based on age, sex, systolic blood pressure, and body mass index.
The study was supported by the South-Eastern Norway Regional Health Authority and Extrastiftelsen. Dr. Murbraech reported having no disclosures.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Lymphoma survivors treated with autologous hematopoietic stem-cell transplantation (auto-HSC) had a significantly higher risk of left ventricular systolic dysfunction than did controls.
Major finding: Treatment with at least 300 mg/m2 cumulative of doxorubicin and with over 30 Gy of cardiac radiation therapy were independent risk factors for LVSD.
Data source: A cross-sectional multicenter cohort study of 274 Hodgkin or non-Hodgkin lymphoma survivors.
Disclosures: Supported by the South-Eastern Norway Regional Health Authority and Extrastiftelsen. Dr. Murbraech reported having no disclosures.
Chronic lymphocytic leukemia prognosis relatively good after transplantation failure
Unlike for those with acute leukemia, patients with chronic lymphocytic leukemia who had disease progression after undergoing transplantation had a relatively good prognosis, with 2- and 5-year overall survival rates of 67% and 38%, investigators reported online April 6 in the Journal of Clinical Oncology.*
Patients with chronic lymphocytic leukemia (CLL) who experienced acute or chronic graft-versus-host disease (GVHD) had significantly longer overall survival (OS) than those who did not have GVHD (P = .04 and P = .05, respectively). “Nearly half our patients with active CLL after transplantation had chronic GVHD, and the association of chronic GVHD with achieving cure and its power to predict OS among patients for whom transplantation failed suggests that the GVL (graft-versus-leukemia) effect contributes to prolonged survival even in patients with a high burden of disease,” wrote Dr. Uri Rozovski and associates at the University of Texas MD Anderson Cancer Center, Houston (J. Clin. Oncol. 2015 Apr. 6 [doi:10.1200/JCO.2014.58.6750]).
A matched-pair analysis showed that patients who underwent allogeneic stem cell transplant (SCT) about the same time and did not relapse also had higher rates of acute and chronic GVHD (P = .004 and P = .011, respectively).
The retrospective review of the Bone Marrow Transplantation Program database identified 358 patients with CLL or RT who underwent SCT from 1998 to 2011. The study evaluated 72 patients who had disease progression at a median 74 months after SCT, most of whom received one to eight lines of treatment after relapse and had a median OS of almost 3 years from the time of progression. Multivariate analysis showed that low hemoglobin levels and the presence of Richter’s transformation (RT) were associated with shorter OS; chronic GVHD and response to the first post-SCT treatment predicted longer OS.
Patients with RT had a worse prognosis, with a median OS of 15 months (95% confidence interval, 2-28 months) and 2- and 5-year survival rates of 36% and 0%. Transplantation carried a significant risk for transformation: 16 (30%) patients with CLL developed RT after allogeneic SCT. Conversely, four patients with RT developed CLL after transplantation.
The authors note that even in patients who did not maintain a durable response, SCT was beneficial. The study cohort received a variety of salvage treatments, and patients who received ibrutinib responded well. “Because of the favorable outcomes with ibrutinib in relapsed/refractory CLL, we believe that ibrutinib might have a role in the treatment of disease progression following transplantation failure,” they wrote.
*Correction, 4/8/2015: A previous version of this article misstated the type of leukemia referenced in the study.
Unlike for those with acute leukemia, patients with chronic lymphocytic leukemia who had disease progression after undergoing transplantation had a relatively good prognosis, with 2- and 5-year overall survival rates of 67% and 38%, investigators reported online April 6 in the Journal of Clinical Oncology.*
Patients with chronic lymphocytic leukemia (CLL) who experienced acute or chronic graft-versus-host disease (GVHD) had significantly longer overall survival (OS) than those who did not have GVHD (P = .04 and P = .05, respectively). “Nearly half our patients with active CLL after transplantation had chronic GVHD, and the association of chronic GVHD with achieving cure and its power to predict OS among patients for whom transplantation failed suggests that the GVL (graft-versus-leukemia) effect contributes to prolonged survival even in patients with a high burden of disease,” wrote Dr. Uri Rozovski and associates at the University of Texas MD Anderson Cancer Center, Houston (J. Clin. Oncol. 2015 Apr. 6 [doi:10.1200/JCO.2014.58.6750]).
A matched-pair analysis showed that patients who underwent allogeneic stem cell transplant (SCT) about the same time and did not relapse also had higher rates of acute and chronic GVHD (P = .004 and P = .011, respectively).
The retrospective review of the Bone Marrow Transplantation Program database identified 358 patients with CLL or RT who underwent SCT from 1998 to 2011. The study evaluated 72 patients who had disease progression at a median 74 months after SCT, most of whom received one to eight lines of treatment after relapse and had a median OS of almost 3 years from the time of progression. Multivariate analysis showed that low hemoglobin levels and the presence of Richter’s transformation (RT) were associated with shorter OS; chronic GVHD and response to the first post-SCT treatment predicted longer OS.
Patients with RT had a worse prognosis, with a median OS of 15 months (95% confidence interval, 2-28 months) and 2- and 5-year survival rates of 36% and 0%. Transplantation carried a significant risk for transformation: 16 (30%) patients with CLL developed RT after allogeneic SCT. Conversely, four patients with RT developed CLL after transplantation.
The authors note that even in patients who did not maintain a durable response, SCT was beneficial. The study cohort received a variety of salvage treatments, and patients who received ibrutinib responded well. “Because of the favorable outcomes with ibrutinib in relapsed/refractory CLL, we believe that ibrutinib might have a role in the treatment of disease progression following transplantation failure,” they wrote.
*Correction, 4/8/2015: A previous version of this article misstated the type of leukemia referenced in the study.
Unlike for those with acute leukemia, patients with chronic lymphocytic leukemia who had disease progression after undergoing transplantation had a relatively good prognosis, with 2- and 5-year overall survival rates of 67% and 38%, investigators reported online April 6 in the Journal of Clinical Oncology.*
Patients with chronic lymphocytic leukemia (CLL) who experienced acute or chronic graft-versus-host disease (GVHD) had significantly longer overall survival (OS) than those who did not have GVHD (P = .04 and P = .05, respectively). “Nearly half our patients with active CLL after transplantation had chronic GVHD, and the association of chronic GVHD with achieving cure and its power to predict OS among patients for whom transplantation failed suggests that the GVL (graft-versus-leukemia) effect contributes to prolonged survival even in patients with a high burden of disease,” wrote Dr. Uri Rozovski and associates at the University of Texas MD Anderson Cancer Center, Houston (J. Clin. Oncol. 2015 Apr. 6 [doi:10.1200/JCO.2014.58.6750]).
A matched-pair analysis showed that patients who underwent allogeneic stem cell transplant (SCT) about the same time and did not relapse also had higher rates of acute and chronic GVHD (P = .004 and P = .011, respectively).
The retrospective review of the Bone Marrow Transplantation Program database identified 358 patients with CLL or RT who underwent SCT from 1998 to 2011. The study evaluated 72 patients who had disease progression at a median 74 months after SCT, most of whom received one to eight lines of treatment after relapse and had a median OS of almost 3 years from the time of progression. Multivariate analysis showed that low hemoglobin levels and the presence of Richter’s transformation (RT) were associated with shorter OS; chronic GVHD and response to the first post-SCT treatment predicted longer OS.
Patients with RT had a worse prognosis, with a median OS of 15 months (95% confidence interval, 2-28 months) and 2- and 5-year survival rates of 36% and 0%. Transplantation carried a significant risk for transformation: 16 (30%) patients with CLL developed RT after allogeneic SCT. Conversely, four patients with RT developed CLL after transplantation.
The authors note that even in patients who did not maintain a durable response, SCT was beneficial. The study cohort received a variety of salvage treatments, and patients who received ibrutinib responded well. “Because of the favorable outcomes with ibrutinib in relapsed/refractory CLL, we believe that ibrutinib might have a role in the treatment of disease progression following transplantation failure,” they wrote.
*Correction, 4/8/2015: A previous version of this article misstated the type of leukemia referenced in the study.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: The prognosis for patients with chronic lymphocytic* leukemia who have undergone failed stem cell transplantation (SCT) is relatively good.
Major finding: From the time of progression after SCT, median OS was 36 months (95% CI, 24-48) for patients with CLL, and 38% survived 5 or more years.
Data source: The retrospective database review identified 72 patients with CLL or Richter’s transformation who underwent allogenic SCT from 1998 to 2011 and progressed after transplantation.
Disclosures: Dr. Rozovski reported having no disclosures. Two of the coauthors reported ties to several industry sources.
CAR-T cell therapy rolls on in pediatric ALL
SAN FRANCISCO – CAR-T cell therapy drove relapsed, refractory acute lymphoblastic leukemia into complete remission in 92% or all but three of 39 children in a phase I/IIa study.
Complete responses were seen within 28 days of receiving a chimeric antigen receptor (CAR)-T cell infusion and have persisted in 15 patients for a year or more, Dr. Stephan A. Grupp reported at the annual meeting of the American Society of Hematology.
Ten relapses have occurred during follow-up of up to 31 months (median 6 months). Half were due to disappearance of the T cells, resulting in CD19-positive relapse, and half were related to antigen escape, resulting in CD19-negative relapse.
Five of the relapsed patients died. No events have been seen in patients who remain in remission after 12 months.
Importantly, CAR-T cell therapy was not used as a bridge to transplant, with only three patients subsequently going on to stem cell transplantation, Dr. Grupp, a pediatric oncologist at the Children’s Hospital of Philadelphia (CHOP) and professor of pediatrics at the University of Pennsylvania, said during a press briefing.
When asked whether CAR-T therapy could be a replacement for transplantation in the future, Dr. Grupp responded, “That would be my fondest hope. We’re not quite there yet, but we’re a lot closer than we used to be.”
The novel immunotherapy first hit the front pages in 2011 after researchers at CHOP and the University of Pennsylvania reported breakthrough results in a handful of children treated with CTL019 cells. T cells are collected from the patient and then genetically reengineered with a CAR directed against tumor B cells expressing the CD19 surface antigen.
More than 130 patients have now been treated by the Pennsylvania team with the CTL019 approach, which received breakthrough therapy status from the Food and Drug Administration in July 2014.
The updated results presented by Dr. Grupp build on those reported earlier this year (N. Engl. J. Med. 2014;371:1507-17) and involve 39 children and young adults. This includes the first 30 pediatric patients with relapsed, refractory ALL treated in the pilot trial. Their median age was 10 years and most were refractory to multiple prior therapies.
At 6 months, the duration of response was 76% and event-free survival was 70%.
The ability of patients to retain their T cells for 6 months or longer was observed in about two-thirds of patients and “is a key point in maintaining remission in these patients,” Dr. Grupp said.
Response rates were independent of disease burden at the time of infusion: 82% response in patients with more than 50% leukemia blast cells, 88% in those with more than 5% blasts, and 100% in those with 0.01%-5% blasts or less than 0.01% blasts.
Patients with higher baseline disease burden (more than 50% blasts), however, were significantly more likely to experience severe cytokine release syndrome (CRS), compared with those with lower disease burden (P < .002).
CRS has been seen across CAR-T cell studies, but there are insufficient data to determine whether this toxicity differs between adult and pediatric patients.
“The key to the cytokine release syndrome, and I believe this carries across platforms and actually may also apply to blinatumomab, is the amplification of the macrophage system through interleukin-6,” Dr. Grupp explained. “This is a classical feedback loop that is actually druggable” using the IL-6 receptor blocker tocilizumab (Actemra).
This strategy produced “remarkable control” of the CRS toxicity, with many of the severe CRS cases experiencing resolution within hours and all cases resolving within 2-3 days, he said.
B-cell aplasia was observed in all responding patients to date and was managed with intravenous immunoglobulin replacement therapy.
The two key questions for the future of CTL019 therapy are toxicity and the logistics of collecting a cell sample and sending it to a centralized manufacturing facility, Dr. Grupp said. This process has already been done on a small scale at CHOP because their cells are made at the University of Pennsylvania. Novartis, which licensed the technology, has built a cell-manufacturing facility and an ongoing phase II study is evaluating whether the technology can be safely rolled out to eight or nine pediatric centers across the country. An adult study will follow, he said.
SAN FRANCISCO – CAR-T cell therapy drove relapsed, refractory acute lymphoblastic leukemia into complete remission in 92% or all but three of 39 children in a phase I/IIa study.
Complete responses were seen within 28 days of receiving a chimeric antigen receptor (CAR)-T cell infusion and have persisted in 15 patients for a year or more, Dr. Stephan A. Grupp reported at the annual meeting of the American Society of Hematology.
Ten relapses have occurred during follow-up of up to 31 months (median 6 months). Half were due to disappearance of the T cells, resulting in CD19-positive relapse, and half were related to antigen escape, resulting in CD19-negative relapse.
Five of the relapsed patients died. No events have been seen in patients who remain in remission after 12 months.
Importantly, CAR-T cell therapy was not used as a bridge to transplant, with only three patients subsequently going on to stem cell transplantation, Dr. Grupp, a pediatric oncologist at the Children’s Hospital of Philadelphia (CHOP) and professor of pediatrics at the University of Pennsylvania, said during a press briefing.
When asked whether CAR-T therapy could be a replacement for transplantation in the future, Dr. Grupp responded, “That would be my fondest hope. We’re not quite there yet, but we’re a lot closer than we used to be.”
The novel immunotherapy first hit the front pages in 2011 after researchers at CHOP and the University of Pennsylvania reported breakthrough results in a handful of children treated with CTL019 cells. T cells are collected from the patient and then genetically reengineered with a CAR directed against tumor B cells expressing the CD19 surface antigen.
More than 130 patients have now been treated by the Pennsylvania team with the CTL019 approach, which received breakthrough therapy status from the Food and Drug Administration in July 2014.
The updated results presented by Dr. Grupp build on those reported earlier this year (N. Engl. J. Med. 2014;371:1507-17) and involve 39 children and young adults. This includes the first 30 pediatric patients with relapsed, refractory ALL treated in the pilot trial. Their median age was 10 years and most were refractory to multiple prior therapies.
At 6 months, the duration of response was 76% and event-free survival was 70%.
The ability of patients to retain their T cells for 6 months or longer was observed in about two-thirds of patients and “is a key point in maintaining remission in these patients,” Dr. Grupp said.
Response rates were independent of disease burden at the time of infusion: 82% response in patients with more than 50% leukemia blast cells, 88% in those with more than 5% blasts, and 100% in those with 0.01%-5% blasts or less than 0.01% blasts.
Patients with higher baseline disease burden (more than 50% blasts), however, were significantly more likely to experience severe cytokine release syndrome (CRS), compared with those with lower disease burden (P < .002).
CRS has been seen across CAR-T cell studies, but there are insufficient data to determine whether this toxicity differs between adult and pediatric patients.
“The key to the cytokine release syndrome, and I believe this carries across platforms and actually may also apply to blinatumomab, is the amplification of the macrophage system through interleukin-6,” Dr. Grupp explained. “This is a classical feedback loop that is actually druggable” using the IL-6 receptor blocker tocilizumab (Actemra).
This strategy produced “remarkable control” of the CRS toxicity, with many of the severe CRS cases experiencing resolution within hours and all cases resolving within 2-3 days, he said.
B-cell aplasia was observed in all responding patients to date and was managed with intravenous immunoglobulin replacement therapy.
The two key questions for the future of CTL019 therapy are toxicity and the logistics of collecting a cell sample and sending it to a centralized manufacturing facility, Dr. Grupp said. This process has already been done on a small scale at CHOP because their cells are made at the University of Pennsylvania. Novartis, which licensed the technology, has built a cell-manufacturing facility and an ongoing phase II study is evaluating whether the technology can be safely rolled out to eight or nine pediatric centers across the country. An adult study will follow, he said.
SAN FRANCISCO – CAR-T cell therapy drove relapsed, refractory acute lymphoblastic leukemia into complete remission in 92% or all but three of 39 children in a phase I/IIa study.
Complete responses were seen within 28 days of receiving a chimeric antigen receptor (CAR)-T cell infusion and have persisted in 15 patients for a year or more, Dr. Stephan A. Grupp reported at the annual meeting of the American Society of Hematology.
Ten relapses have occurred during follow-up of up to 31 months (median 6 months). Half were due to disappearance of the T cells, resulting in CD19-positive relapse, and half were related to antigen escape, resulting in CD19-negative relapse.
Five of the relapsed patients died. No events have been seen in patients who remain in remission after 12 months.
Importantly, CAR-T cell therapy was not used as a bridge to transplant, with only three patients subsequently going on to stem cell transplantation, Dr. Grupp, a pediatric oncologist at the Children’s Hospital of Philadelphia (CHOP) and professor of pediatrics at the University of Pennsylvania, said during a press briefing.
When asked whether CAR-T therapy could be a replacement for transplantation in the future, Dr. Grupp responded, “That would be my fondest hope. We’re not quite there yet, but we’re a lot closer than we used to be.”
The novel immunotherapy first hit the front pages in 2011 after researchers at CHOP and the University of Pennsylvania reported breakthrough results in a handful of children treated with CTL019 cells. T cells are collected from the patient and then genetically reengineered with a CAR directed against tumor B cells expressing the CD19 surface antigen.
More than 130 patients have now been treated by the Pennsylvania team with the CTL019 approach, which received breakthrough therapy status from the Food and Drug Administration in July 2014.
The updated results presented by Dr. Grupp build on those reported earlier this year (N. Engl. J. Med. 2014;371:1507-17) and involve 39 children and young adults. This includes the first 30 pediatric patients with relapsed, refractory ALL treated in the pilot trial. Their median age was 10 years and most were refractory to multiple prior therapies.
At 6 months, the duration of response was 76% and event-free survival was 70%.
The ability of patients to retain their T cells for 6 months or longer was observed in about two-thirds of patients and “is a key point in maintaining remission in these patients,” Dr. Grupp said.
Response rates were independent of disease burden at the time of infusion: 82% response in patients with more than 50% leukemia blast cells, 88% in those with more than 5% blasts, and 100% in those with 0.01%-5% blasts or less than 0.01% blasts.
Patients with higher baseline disease burden (more than 50% blasts), however, were significantly more likely to experience severe cytokine release syndrome (CRS), compared with those with lower disease burden (P < .002).
CRS has been seen across CAR-T cell studies, but there are insufficient data to determine whether this toxicity differs between adult and pediatric patients.
“The key to the cytokine release syndrome, and I believe this carries across platforms and actually may also apply to blinatumomab, is the amplification of the macrophage system through interleukin-6,” Dr. Grupp explained. “This is a classical feedback loop that is actually druggable” using the IL-6 receptor blocker tocilizumab (Actemra).
This strategy produced “remarkable control” of the CRS toxicity, with many of the severe CRS cases experiencing resolution within hours and all cases resolving within 2-3 days, he said.
B-cell aplasia was observed in all responding patients to date and was managed with intravenous immunoglobulin replacement therapy.
The two key questions for the future of CTL019 therapy are toxicity and the logistics of collecting a cell sample and sending it to a centralized manufacturing facility, Dr. Grupp said. This process has already been done on a small scale at CHOP because their cells are made at the University of Pennsylvania. Novartis, which licensed the technology, has built a cell-manufacturing facility and an ongoing phase II study is evaluating whether the technology can be safely rolled out to eight or nine pediatric centers across the country. An adult study will follow, he said.
AT ASH 2014
Key clinical point: CAR-T cell therapy continues to provide durable responses in early studies of children with refractory ALL.
Major finding: Complete remission occurred in 36 of 39 patients (92%) treated with CAR-T cell therapy.
Data source: Phase I/IIa a study in 39 children and young adults with relapsed, refractory acute lymphoblastic leukemia.
Disclosures: The authors reported financial ties with Novartis, the study sponsor.