User login
Continuing Medical Education Program in
If you wish to receive credit for this activity, which beginson the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which beginson the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
If you wish to receive credit for this activity, which beginson the next page, please refer to the website:
Accreditation and Designation Statement
Blackwell Futura Media Services designates this educational activity for a 1 AMA PRA Category 1 Credit. Physicians should only claim credit commensurate with the extent of their participation in the activity.
Blackwell Futura Media Services is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Educational Objectives
Continuous participation in the Journal of Hospital Medicine CME program will enable learners to be better able to:
-
Interpret clinical guidelines and their applications for higher quality and more efficient care for all hospitalized patients.
-
Describe the standard of care for common illnesses and conditions treated in the hospital; such as pneumonia, COPD exacerbation, acute coronary syndrome, HF exacerbation, glycemic control, venous thromboembolic disease, stroke, etc.
-
Discuss evidence‐based recommendations involving transitions of care, including the hospital discharge process.
-
Gain insights into the roles of hospitalists as medical educators, researchers, medical ethicists, palliative care providers, and hospital‐based geriatricians.
-
Incorporate best practices for hospitalist administration, including quality improvement, patient safety, practice management, leadership, and demonstrating hospitalist value.
-
Identify evidence‐based best practices and trends for both adult and pediatric hospital medicine.
Instructions on Receiving Credit
For information on applicability and acceptance of continuing medical education credit for this activity, please consult your professional licensing board.
This activity is designed to be completed within the time designated on the title page; physicians should claim only those credits that reflect the time actually spent in the activity. To successfully earn credit, participants must complete the activity during the valid credit period that is noted on the title page.
Follow these steps to earn credit:
-
Log on to www.blackwellpublishing.com/cme.
-
Read the target audience, learning objectives, and author disclosures.
-
Read the article in print or online format.
-
Reflect on the article.
-
Access the CME Exam, and choose the best answer to each question.
-
Complete the required evaluation component of the activity.
Management of Ischemic Stroke: Part 2
Inpatient stroke management includes many elements of care, at least as important as the initial portion of the patient's stay, as reviewed in part 1 of this article. The extent of further diagnostic evaluation varies widely depending on apparent risk factors on presentation. Likewise, further therapy, both inpatient and secondary prevention is based on identification of stroke mechanism. Hospitalists are uniquely positioned to have a tremendous impact on both stroke care and the prevention of recurrent disease.
Case Presentation
A 76‐year‐old right‐handed male with a history of hyperlipidemia and myocardial infarction was found at 7 AM with right‐sided paralysis and poor responsiveness on the morning of admission. Upon arrival to the emergency department (ED), with symptoms of partial aphasia, right hemiplegia, and left gaze preference, there was a high suspicion for a left middle cerebral artery (MCA) stroke. Unfortunately, he was excluded from receiving intravenous (IV) tissue plasminogen activator (tPA) or any other acute interventions as the last time he was known to be neurologically intact was the prior evening, which is taken to be the time of onset. Antiplatelet therapy was continued, and the patient admitted for further workup.
Inpatient Care
When an acute ischemic stroke patient is admitted to the hospital, he or she should be placed on a standardized acute stroke protocol (also known as (a.k.a.) a care map, order set, clinical pathway)commonly created by a hospitalist/neurologist and a multidisciplinary team and admitted to a stroke unit. A stroke unit can take many forms, either as a physically separate unit in hospitals with sufficient volume or a floor where a lower volume of stroke patients are always admitted. Multidisciplinary care providers in the stroke unit have special training in stroke, and strong evidence from randomized trials shows that patients cared for in these units have significantly decreased mortality with improved functional outcomes.1 Essentials of the stroke protocol or order set include cardiac telemetry, maintaining euthermia and euglycemia, closely following blood pressure and neurologic status, actively avoiding complications, initiation of secondary prevention treatment, early involvement of rehabilitation services, and patient education.
Euthermia may be assisted by administering scheduled Tylenol to the patient for the first 48 hours, but is not strictly evidence‐based.2 Though euthermia and euglycemia have not been shown to improve outcomes in acute stroke, studies have shown that hyperthermia and hyperglycemia are associated with worsened outcomes for patients with acute strokes.35
Blood Pressure Management
Normally, cerebral vascular autoregulation leads to stable cerebral blood flow over a range of systemic blood pressures. In the setting of an acute stroke, the ability to autoregulate is diminished or absent in regions of and surrounding an acute ischemic stroke; as the area becomes ischemic, autoregulation opens the local vasculature maximally in an effort to drawn in as much blood as possible. Maximally dilated arterioles are perfused in direct correlation with systemic blood pressure, thus any drop in the systemic blood pressure leads to direct decreases in blood flow specifically in the area of ischemia; if there is a penumbra of marginally perfused tissue, such systemic blood pressure drops risk extending the area of fatal ischemia (increasing the size of the ischemic stroke).68 Thus in the acute period of an ischemic stroke, the American Heart Association (AHA)/American Stroke Association (ASA) Guidelines for the Early Management of Adults With Ischemic Stroke (referred to herein as the Guidelines)10 suggest avoid treatment unless systolic blood pressures are >220 or diastolic pressures >105, and review the evidence to support this recommendation (p. 16711672). Those patients who receive tPA have a more stringent blood pressure threshold given their risk of intracranial hemorrhage; systolic blood pressures are accepted up to 180 prior to recommending treatment.
Higher‐quality Inpatient Stroke Care and Harmonized Performance Measures
Beginning in January 2008, a set of 10 performance measures (Table 1) for inpatient acute stroke care have been agreed upon (harmonized) by 3 major stakeholders including the Joint Commission, the ASA's Get with the GuidelinesStroke quality improvement program, and the Center for Disease Control and Prevention's (CDC's) Paul Coverdell Acute stroke registries. These performance measures were selected to help avoid complications (deep vein thrombosis [DVT], aspiration pneumonia), encourage appropriately aggressive care (tPA administration), optimize secondary prevention (antithrombotics, cholesterol lowering, smoking cessation, education), and facilitate functional recovery (early rehabilitation). All 10 measures are appropriate for consideration in every ischemic stroke patient, and 5 are appropriate for the hemorrhagic stroke types.
| Performance measure* | Definition* |
|---|---|
| |
| 1. DVT prophylaxis | Patients who are nonambulatory should start receiving DVT prophylaxis by end of hospital day 2 (can be either compression devices or any low‐dose heparin) |
| 2. Discharged on antithrombotic therapy | Antiplatelet agent(s) or warfarin anticoagulation |
| 3. Patients with atrial fibrillation receiving anticoagulation therapy | A proven approach to secondary prevention in such patients; practice at Harborview varies time of warfarin initiation based on infarct size with larger infarcts waiting up to 2 weeks before initiating warfarin (the best randomized trial showed no benefit for full‐dose low‐molecular‐weight heparin over aspirin in the first 2 weeks)50 |
| 4. Thrombolytic therapy administered | In ischemic stroke patients who arrive at the hospital within 120 minutes (2 hours) of time last known well, for whom IV tPA was initiated at this hospital within 180 minutes (3 hours) of time last known well, and who qualify under strict criteria |
| 5. Antithrombotic therapy by end of hospital day 2 | Usually just antiplatelet agents, a minimal standard of care for ischemic stroke patients; should be started as early as possible, usually in ER |
| 6. Discharged on statin medication | If LDL >100, or not measured or if on a statin drug prior to admission; to reduce risk of subsequent ischemic stroke |
| 7. Dysphagia screening | Prior to any PO food, fluids or medications; to reduce the chances of aspiration pneumonia |
| 8. Stroke education | Including for families if patient unable to participate, must include personal risk factors for stroke, warning signs for stroke, activation of emergency medical system, need for follow‐up after discharge, and medications prescribed |
| 9. Smoking cessation/advice/counseling | For any patient who has smoked in the last year |
| 10. Assessed for rehabilitation | Or received therapy services; to facilitate progress to an optimal function outcome |
Further Workup
After the ischemic stroke patient has had their computed tomography (CT) scan, possibly a computed tomography angiography (CTA), been admitted to the stroke unit, started on an antithrombotic medication, and had their blood pressure appropriately treated, attention then turns to defining the pathophysiology related to the stroke and starting an optimal regimen for secondary prevention. Imaging of the cerebral vasculature including both extracranial and intracranial large vessels is a vital first step in understanding the cause of ischemic stroke. There are multiple potential modalities (magnetic resonance angiography [MRA], CTA, and duplex/transcranial Doppler), the choice of which depends on local availability and expertise as well as the specific clinical situation. Magnetic resonance imaging (MRI) of the brain for all ischemic stroke patients is standard of care at most stroke centers; per the Guidelines, MRI is better at distinguishing acute, small cortical, small deep, and posterior fossa infarcts; at distinguishing acute from chronic ischemia; and at identifying subclinical satellite ischemic lesions that provide information on stroke mechanism (p. 1668). New techniques including magnetic resonance (MR) and CT perfusion scanning can show the ischemic region in the acute setting and may one day help select patients for specific therapies, but are not yet widely available nor have they been shown to alter outcomes.
An electrocardiogram is indicated for all stroke patients, as is admission to a cardiac telemetry bed for at least 24 hours to document any arrhythmias, the most common being atrial fibrillation (Guidelines, p. 1666, 1673). An echocardiographic study (ECHO) of the heart with bubble study should be performed in most cases (although which cases may specifically benefit is unclear) to identify a cardioembolic source for the stroke, such as low cardiac ejection fraction, atrial septal aneurysm, patent foramen ovale (PFO), or a cardiac thrombus. The bubble study increases the sensitivity of detecting a PFO, which could serve as a gateway for venous embolization to the cerebral arteries. Assuming a large PFO is discovered, other studies such as lower extremity Doppler may be warranted to investigate other potential sources of thrombi (ie, DVT).
Regarding laboratory testing, fasting lipids should be checked as hyperlipidemia is a common modifiable risk factor for ischemic stroke. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial included ischemic stroke patients that had low‐density lipoprotein (LDL) cholesterol between 100 mg/dL and 190 mg/dL and randomized them to receive atorvastatin 80 mg/day vs. placebo. Results showed a 16% relative risk reduction in recurrent stroke; however, there was a small increased risk of intracranial hemorrhage.9 As shown in Table 1, use of a statin on discharge is now a national performance measure for ischemic stroke.
Dissection is a common cause of stroke in young patients without traditional risk factors. Other serologies, such as hypercoagulable studies, may be warranted in patients with no other risk factors for strokes, paradoxical embolus, or of young age (eg, 45 years and under). The arterial hypercoagulable panel consists of antiphospholipid antibody panel, homocysteine levels, lupus anticoagulant levels, and prothrombin time/partial thromboplastin time (PT/PTT). The venous hypercoagulable panel consists of the laboratory values checked, with the arterial hypercoagulable and activated protein C (APC) resistance, Factor VIII activity, Factor II DNA, Factor V DNA if the APC resistance is positive, antithrombin III activity, and activity of proteins C and S. If a patient is found to have a hypercoagulable state, long‐term therapy often involves careful consideration of the choice of antiplatelet therapy vs. anticoagulation with warfarin.10
Initiating Secondary Prevention
Upon admission, the clinician faces a variety of treatment choices for secondary stroke prevention. The proper choice depends on the results of the workup and the presumptive pathophysiology.
Noncardioembolic/Atherothrombotic/Lacunar
The Antithrombotic Trialists' Collaboration meta‐analysis found that patients with a prior stroke or transient ischemic attack (TIA) had a highly significant decrease in the rate of subsequent vascular events (over about 3 years) on antiplatelet therapy (17.8% vs. 21.4%, P < 0.0001) and were unable to find a significant difference between low‐dose and high‐dose aspirin for secondary prevention.11 Thus, it is reasonable to place an acute stroke patient naive to antithrombotic therapy on 81 mg of aspirin or 325 mg for long‐term prevention (325 mg is specifically recommended in the acute setting). Several studies such as the WARSS and ESPRIT trials have shown antiplatelet agents to be at least as effective as anticoagulation in noncardioembolic ischemic strokes.12, 13 Guidelines from Europe, the American College of Chest Physicians, and the AHA/ASA all state it is acceptable to choose either aspirin monotherapy, aspirin/extended release dipyridamole combination therapy, or clopidogrel monotherapy as first‐line agents for long‐term secondary prevention in noncardioembolic ischemic stroke.1416 There is no clear evidence that patients who suffer an ischemic stroke while on aspirin will derive additional benefit from increasing the aspirin dose. The newer guidelines go on to recommend aspirin/extended release dipyridamole (ER‐DP) combination therapy or clopidogrel monotherapy over aspirin monotherapy, the former with a stronger level of recommendation based on the results of 2 randomized trials. These recommendations were all published without knowledge of the results of the Prevention Regimen For Effectively Avoiding Second Strokes (PRoFESS) study, which directly compared aspirin/extended release dipyridamole combination therapy to clopidogrel monotherapy for long‐term secondary prevention. The rate of first recurrent stroke was not significantly different between the 2 therapies (9.0% ER‐DP plus aspirin, 8.8% clopidogrel; hazard ratio [HR], 1.01; 95% confidence interval [CI], 0.921.11). Other outcomes also showed few differences, although there were more major hemorrhagic events in the ER‐DP plus aspirin group (4.1% vs. 3.6%; HR, 1.15; 95% CI, 1.001.32; P = 0.06).17
The ASA Stroke Prevention Guideline from 2006 states, with continued relevance, The selection of an antiplatelet agent should be individualized on the basis of patient risk factor profiles, tolerance, and other clinical characteristics.10 Of note, both the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management and Avoidance (CHARISMA) and Management of ATherothrombosis with Clopidogrel in High‐risk patients with recent TIA or ischemic stroke (MATCH) trials found a significant increased risk for hemorrhage complications with long‐term use of the aspirin and clopidogrel combination,18, 19 and the 2008 update to the ASA Stroke prevention guidelines state that the addition of aspirin to clopidogrel increases the risk of hemorrhage. Combination therapy of aspirin and clopidogrel is not routinely recommended for ischemic stroke or TIA patients unless they have a specific indication for this therapy (i.e., coronary stent or acute coronary syndrome).15
Atrial Fibrillation
Though our case patient did not have atrial fibrillation, this condition does deserve mention. About 15% to 20% of ischemic stroke patients have atrial fibrillation. The overall risk for stroke in patients with atrial fibrillation is about 5% per year; however, patients who have a history of stroke increase their risk factors for subsequent strokes to about 12% per year. In most cases, anticoagulation has proven to be the superior agent for primary and secondary stroke prevention with warfarin reducing the risk by 67% compared to aspirin, which only reduces the risk of stroke by 20%. A meta‐analysis from 2002 showed that patients who had a prior stroke or TIA decrease their risk of subsequent strokes to 4%/year on oral anticoagulation therapy, resulting in an 8% absolute risk reduction. Patients on aspirin therapy only decrease their risk to 10%/year, or a 2% reduction in stroke events.20 Unless there is a strong contraindication (eg, bleeding diathesis, history of life threatening gastrointestinal [GI] bleeding, history of fall with subdural hematoma, etc.), virtually all ischemic stroke patients with atrial fibrillation should be anticoagulated for life. Anticoagulation in the setting of atrial fibrillation is seriously underutilized.21 The highest quality study on early anticoagulation for ischemic stroke associated with atrial fibrillation suggested that there was no benefit to starting anticoagulation earlier than 2 weeks after a stroke, and there may actually be a higher complication rate (compared to aspirin).22 Other cardiac indications for anticoagulation include left ventricular thrombus and mechanical valves.
Carotid Stenosis
Significant ipsilateral stenosis of the internal carotid artery in a patient with ischemic stroke is a strong indication for intervention, usually a standard carotid endarterectomy (CEA). Stenosis of 70% to 99% is the strongest indication for CEA, and may be of greatest benefit in men, those 75+ years of age, and if surgery is done <2 weeks after the most recent symptoms.23 In patients with minor stroke or TIA, recent recommendations and our practice is to admit to the hospital and perform endarterectomy as soon as possible (those with major stroke may have a greater risk of complications with early CEA).24 Stenting should only be considered instead of CEA if high risk (for surgical complications) criteria are present. These high risk criteria include patients having significant comorbidities and/or anatomic risk factors (ie, recurrent stenosis and/or previous radical neck dissection), and [who] would be poor candidates for CEA in the opinion of a surgeon.25 For stenoses of 50% to 69%, intervention is not as compelling, and decisions should be individualized based on patient characteristics; in this group, stenting should only be considered in the setting of a clinical trial or if an investigational device exemption (IDE) exists at your institution.26
Dissection of the Carotid or Vertebral Arteries
This is a common cause of stroke in younger adults. It should be suspected in patients without other clear causes of stroke and significant disease of the extracranial arteries. Diagnosis can usually be made with CTA or MRA, though it is suggested that the best modality may be T1‐fat‐saturated MRI images of the neck. Debate exists as to the best approach to treatment of dissections due to the absence of randomized trials. A recent comprehensive review suggested anticoagulation for 3 to 6 months followed by indefinite antiplatelet therapy for symptomatic dissections and antiplatelet therapy alone for asymptomatic dissections.27
PFO‐related Stroke
If the patient is found to have a PFO, its role in comparison to traditional risk factors must be weighed carefully. Epidemiological studies suggest that PFO may be most relevant in younger patients, those with cryptogenic stroke (no obvious cause and lack of traditional risk factors), those with higher risk associations including interatrial septal aneurysm, larger PFOs or history of previous cryptogenic stroke.28, 29 The best medical therapy for seemingly PFO‐related ischemic stroke is also unclear; a reasonable approach might be aspirin if neither high‐risk associations nor a hypercoagulable state is present, and warfarin if either are present. Transcatheter closure of PFO is approved by the U.S. Food and Drug Administration (FDA) only under an IDE for patients who have had a recurrent event on maximally tolerated medical treatment, and requires approval from the human research committee (internal review board [IRB]) at your hospital. It is not known if closure is superior or inferior to best medical therapy, and a practice parameter from the American Academy of Neurology strongly encourages appropriate patients to consider participation in ongoing randomized trials.28 Further information on these trials is available at:
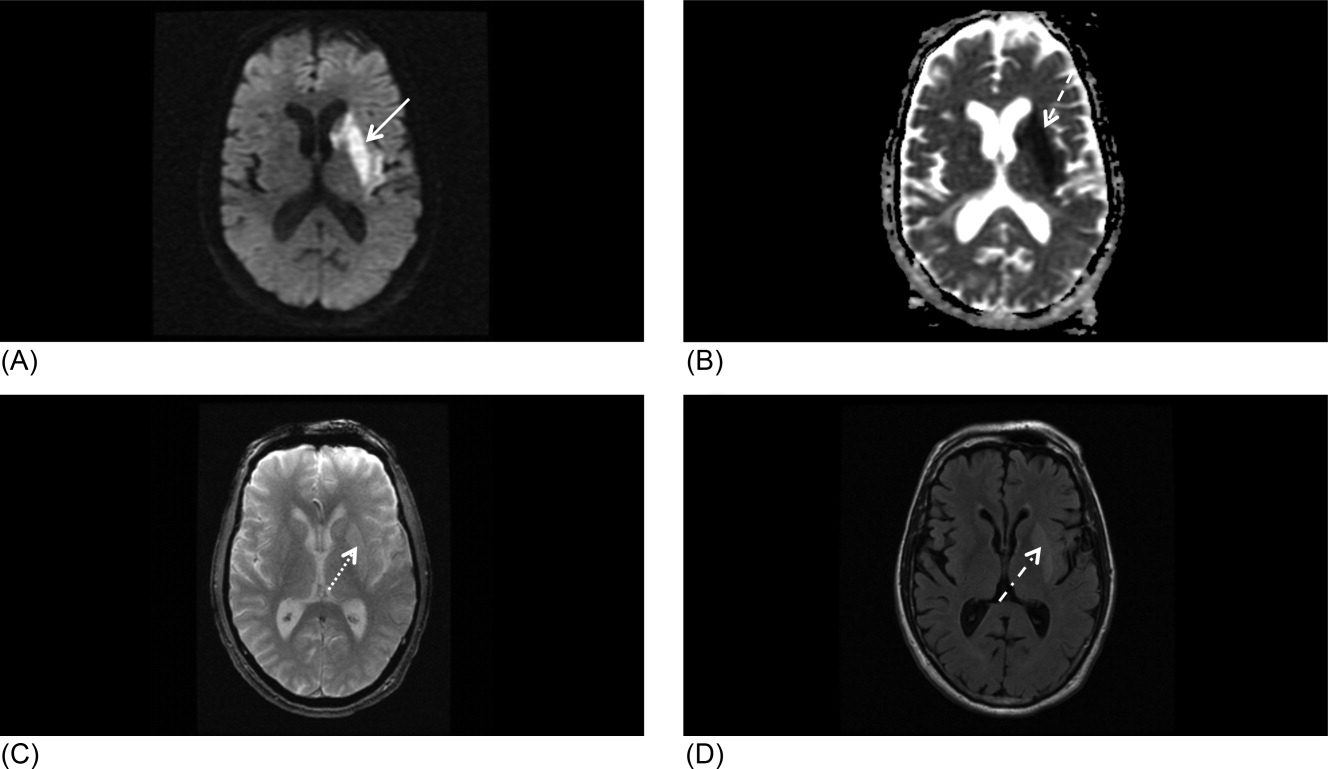
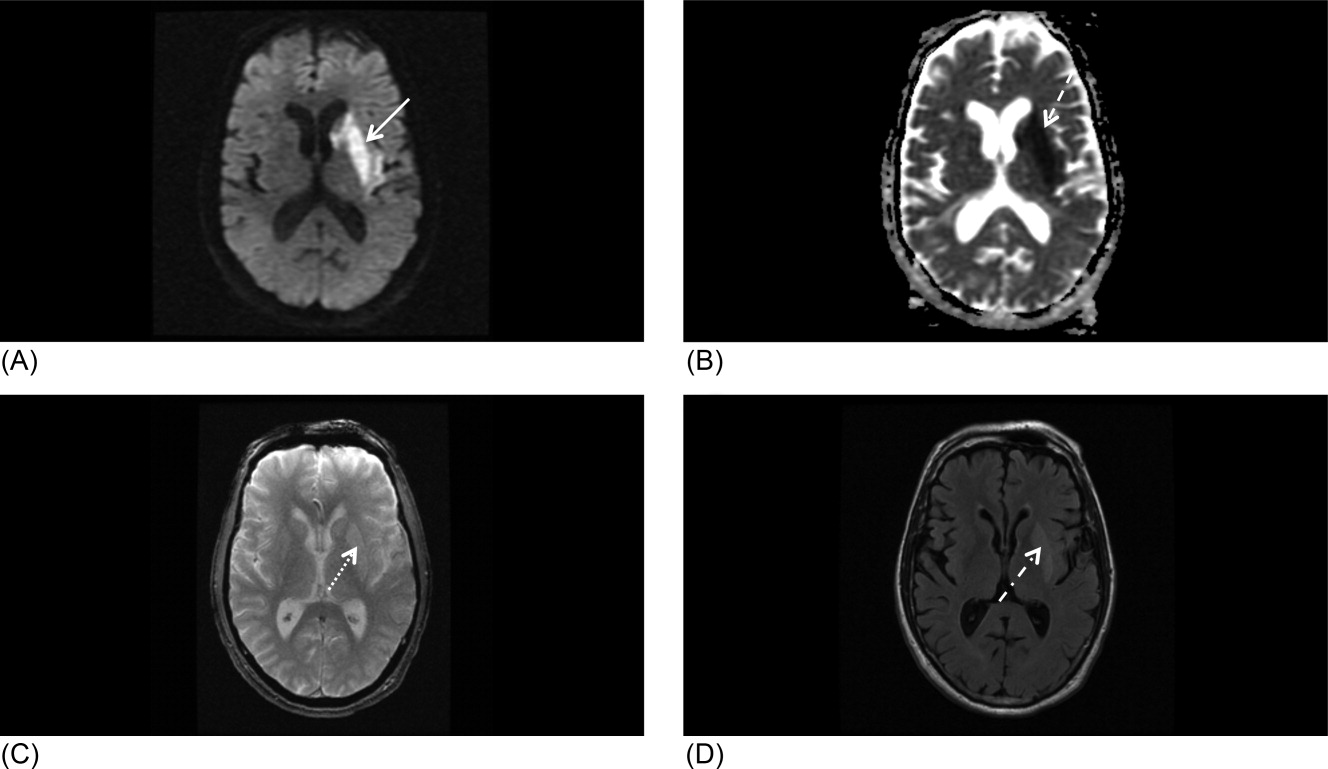
Our patient underwent a CTA of the head and neck in the emergency room to see if he would be a candidate for other interventions; unfortunately, he did not meet the time criteria. CTA showed complete occlusion of the left internal carotid artery at the bifurcation with heterogeneous retrograde filling (Supporting Figure 1). Complete occlusion of the proximal third of the left M1 segment was also seen with relative oligemia in the left MCA distribution, though several small peripheral M3/M4 vessels were opacified in the territory indicating collateralization (Supporting Figure 2). A MRI showed a large area of diffusion‐weighted abnormality (Figure 1). Interestingly, the patient's transthoracic echocardiography (TTE), which did not show evidence of a PFO, did reveal a calcified thrombus in the left ventricle. Though no arrhythmias were captured on telemetry, this thrombus does serve as a potential source of cardioembolic emboli to the cerebral vasculature. It was felt that the most likely source of the patient's acute infarct was from artery‐to‐artery emboli from his internal carotid occlusion given the infarct location and the lack of infarction in other vascular distributions (as one might see from a cardiac embolic source). Therefore, his medical management consisted of an antiplatelet regimen for 2 weeks followed by a transition to warfarin alone 2 weeks after his acute infarct as secondary stroke prevention due to the cardiac thrombus. Given the complete occlusion of the internal carotid artery and M1 segment, there was concern that the penumbra might be at risk of infarction (supporting standard guidelines of permissive hypertension). By the end of his hospitalization, the patient had improved and was transferred to inpatient rehabilitation.
The guidelines for acute stroke management continue to rapidly evolve. Certainly, there are effective treatments for acute ischemic stroke, with variation based on the timing of patient arrival at the hospital, the underlying pathophysiology, and the treatment capabilities of the individual hospital. Secondary stroke prevention is extremely important and has been emphasized during inpatient admissions with the establishment of an appropriate medication regime, given that patients are more likely to stay on treatment that is initiated around the time of a diagnosis.29 Evidence strongly suggests that management of acute stroke is improved by an organized approach to care, including the expertise of a multidisciplinary team in a specialized stroke unit. Hospitals committed to high quality of care for acute stroke patients should strongly consider the Joint Commission certification process or an analogous local certification. Such certification demonstrates a hospital's commitment to providing high‐quality care, what every stroke patient wants and deserves.
- Organised inpatient (stroke unit) care for stroke.Stroke Unit Trialists' Collaboration.Cochrane Database Syst Rev.2000(2):CD000197.
- ,,, et al.Acetaminophen for altering body temperature in acute stroke: a randomized clinical trial.Stroke.2002;33(1):130–134.
- ,,, et al.Fever in acute stroke worsens prognosis. A prospective study.Stroke.1995;26(11):2040–2043.
- ,.Combating hyperthermia in acute stroke: a significant clinical concern.Stroke.1998;29(2):529–534.
- ,,, et al.Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome.Lancet. 171996;347(8999):422–425.
- ,,.Thresholds in cerebral ischemia—the ischemic penumbra.Stroke.1981;12(6):723–725.
- .Ischaemic brain damage of cerebral perfusion failure type after treatment of severe hypertension.Br Med J. 271975;4(5999):739.
- ,,,,.Imaging of acute stroke.Lancet Neurol.2006;5(9):755–768.
- ,,, et al.High‐dose atorvastatin after stroke or transient ischemic attack.N Engl J Med.2006;355(6):549–559.
- ,,, et al.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co‐sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline.Stroke.2006;37(2):577–617.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324(7329):71–86.
- ,,, et al.A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke.N Engl J Med.2001;345(20):1444–1451.
- .Warfarin or aspirin for recurrent ischemic stroke.N Engl J Med.2002;346(15):1169–1171.
- ,,, et al.Prevention. European Stroke Initiative.Cerebrovasc Dis.2004;17(suppl 2):15–29.
- ,,, et al.Update to the AHA/ASA recommendations for the prevention of stroke in patients with stroke and transient ischemic attack.Stroke.2008;39(5):1647–1652.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th edition).Chest.2008;133(suppl):630S–669S.
- ,,, et al.Aspirin and extended‐release dipyridamole versus clopidogrel for recurrent stroke.N Engl J Med.2008;359(12):1238–1251.
- ,,, et al.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354(16):1706–1717.
- ,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364(9431):331–337.
- ,,, et al.Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta‐analysis.JAMA.2002;288(19):2441–2448.
- .Effective anticoagulation therapy: defining the gap between clinical studies and clinical practice.Am J Manag Care.2004;10(suppl):S297–S306; discussionS312–S297.
- ,,,.Low molecular‐weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double‐blind randomised study. HAEST Study Group. Heparin in Acute Embolic Stroke Trial.Lancet.2000;355(9211):1205–1210.
- ,,,,.Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery.Lancet.2004;363(9413):915–924.
- ,,.The timing of carotid endarterectomy post stroke.Neurol Clin.2006;24(4):669–680.
- Centers for Medicare and Medicaid Services (CMS). Department of Health and Human Services (DHHS). CMS Manual System. Pub 100–03 Medicare National Coverage Determinations. Available at: http://www.cms.hhs.gov/Transmittals/Downloads/R64NCD.pdf. Accessed May2009.
- .Current status of carotid endarterectomy and stenting for symptomatic carotid stenosis.Cerebrovasc Dis.2007;24(suppl 1):116–125.
- ,,, et al.Antiplatelets versus anticoagulation in cervical artery dissection.Stroke.2007;38(9):2605–2611.
- ,,, et al.A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke.N Engl J Med2001;345(20):1444–1451.
- .Warfarin or aspirin for recurrent ischemic stroke.N Engl J Med2002;346(15):1169–1171.
- ,,, et al.Practice parameter: recurrent stroke with patent foramen ovale and atrial septal aneurysm: report of the Quality Standards Subcommittee of the American Academy of Neurology.Neurology.2004;62(7):1042–1050.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35(12):2879–2883.
Inpatient stroke management includes many elements of care, at least as important as the initial portion of the patient's stay, as reviewed in part 1 of this article. The extent of further diagnostic evaluation varies widely depending on apparent risk factors on presentation. Likewise, further therapy, both inpatient and secondary prevention is based on identification of stroke mechanism. Hospitalists are uniquely positioned to have a tremendous impact on both stroke care and the prevention of recurrent disease.
Case Presentation
A 76‐year‐old right‐handed male with a history of hyperlipidemia and myocardial infarction was found at 7 AM with right‐sided paralysis and poor responsiveness on the morning of admission. Upon arrival to the emergency department (ED), with symptoms of partial aphasia, right hemiplegia, and left gaze preference, there was a high suspicion for a left middle cerebral artery (MCA) stroke. Unfortunately, he was excluded from receiving intravenous (IV) tissue plasminogen activator (tPA) or any other acute interventions as the last time he was known to be neurologically intact was the prior evening, which is taken to be the time of onset. Antiplatelet therapy was continued, and the patient admitted for further workup.
Inpatient Care
When an acute ischemic stroke patient is admitted to the hospital, he or she should be placed on a standardized acute stroke protocol (also known as (a.k.a.) a care map, order set, clinical pathway)commonly created by a hospitalist/neurologist and a multidisciplinary team and admitted to a stroke unit. A stroke unit can take many forms, either as a physically separate unit in hospitals with sufficient volume or a floor where a lower volume of stroke patients are always admitted. Multidisciplinary care providers in the stroke unit have special training in stroke, and strong evidence from randomized trials shows that patients cared for in these units have significantly decreased mortality with improved functional outcomes.1 Essentials of the stroke protocol or order set include cardiac telemetry, maintaining euthermia and euglycemia, closely following blood pressure and neurologic status, actively avoiding complications, initiation of secondary prevention treatment, early involvement of rehabilitation services, and patient education.
Euthermia may be assisted by administering scheduled Tylenol to the patient for the first 48 hours, but is not strictly evidence‐based.2 Though euthermia and euglycemia have not been shown to improve outcomes in acute stroke, studies have shown that hyperthermia and hyperglycemia are associated with worsened outcomes for patients with acute strokes.35
Blood Pressure Management
Normally, cerebral vascular autoregulation leads to stable cerebral blood flow over a range of systemic blood pressures. In the setting of an acute stroke, the ability to autoregulate is diminished or absent in regions of and surrounding an acute ischemic stroke; as the area becomes ischemic, autoregulation opens the local vasculature maximally in an effort to drawn in as much blood as possible. Maximally dilated arterioles are perfused in direct correlation with systemic blood pressure, thus any drop in the systemic blood pressure leads to direct decreases in blood flow specifically in the area of ischemia; if there is a penumbra of marginally perfused tissue, such systemic blood pressure drops risk extending the area of fatal ischemia (increasing the size of the ischemic stroke).68 Thus in the acute period of an ischemic stroke, the American Heart Association (AHA)/American Stroke Association (ASA) Guidelines for the Early Management of Adults With Ischemic Stroke (referred to herein as the Guidelines)10 suggest avoid treatment unless systolic blood pressures are >220 or diastolic pressures >105, and review the evidence to support this recommendation (p. 16711672). Those patients who receive tPA have a more stringent blood pressure threshold given their risk of intracranial hemorrhage; systolic blood pressures are accepted up to 180 prior to recommending treatment.
Higher‐quality Inpatient Stroke Care and Harmonized Performance Measures
Beginning in January 2008, a set of 10 performance measures (Table 1) for inpatient acute stroke care have been agreed upon (harmonized) by 3 major stakeholders including the Joint Commission, the ASA's Get with the GuidelinesStroke quality improvement program, and the Center for Disease Control and Prevention's (CDC's) Paul Coverdell Acute stroke registries. These performance measures were selected to help avoid complications (deep vein thrombosis [DVT], aspiration pneumonia), encourage appropriately aggressive care (tPA administration), optimize secondary prevention (antithrombotics, cholesterol lowering, smoking cessation, education), and facilitate functional recovery (early rehabilitation). All 10 measures are appropriate for consideration in every ischemic stroke patient, and 5 are appropriate for the hemorrhagic stroke types.
| Performance measure* | Definition* |
|---|---|
| |
| 1. DVT prophylaxis | Patients who are nonambulatory should start receiving DVT prophylaxis by end of hospital day 2 (can be either compression devices or any low‐dose heparin) |
| 2. Discharged on antithrombotic therapy | Antiplatelet agent(s) or warfarin anticoagulation |
| 3. Patients with atrial fibrillation receiving anticoagulation therapy | A proven approach to secondary prevention in such patients; practice at Harborview varies time of warfarin initiation based on infarct size with larger infarcts waiting up to 2 weeks before initiating warfarin (the best randomized trial showed no benefit for full‐dose low‐molecular‐weight heparin over aspirin in the first 2 weeks)50 |
| 4. Thrombolytic therapy administered | In ischemic stroke patients who arrive at the hospital within 120 minutes (2 hours) of time last known well, for whom IV tPA was initiated at this hospital within 180 minutes (3 hours) of time last known well, and who qualify under strict criteria |
| 5. Antithrombotic therapy by end of hospital day 2 | Usually just antiplatelet agents, a minimal standard of care for ischemic stroke patients; should be started as early as possible, usually in ER |
| 6. Discharged on statin medication | If LDL >100, or not measured or if on a statin drug prior to admission; to reduce risk of subsequent ischemic stroke |
| 7. Dysphagia screening | Prior to any PO food, fluids or medications; to reduce the chances of aspiration pneumonia |
| 8. Stroke education | Including for families if patient unable to participate, must include personal risk factors for stroke, warning signs for stroke, activation of emergency medical system, need for follow‐up after discharge, and medications prescribed |
| 9. Smoking cessation/advice/counseling | For any patient who has smoked in the last year |
| 10. Assessed for rehabilitation | Or received therapy services; to facilitate progress to an optimal function outcome |
Further Workup
After the ischemic stroke patient has had their computed tomography (CT) scan, possibly a computed tomography angiography (CTA), been admitted to the stroke unit, started on an antithrombotic medication, and had their blood pressure appropriately treated, attention then turns to defining the pathophysiology related to the stroke and starting an optimal regimen for secondary prevention. Imaging of the cerebral vasculature including both extracranial and intracranial large vessels is a vital first step in understanding the cause of ischemic stroke. There are multiple potential modalities (magnetic resonance angiography [MRA], CTA, and duplex/transcranial Doppler), the choice of which depends on local availability and expertise as well as the specific clinical situation. Magnetic resonance imaging (MRI) of the brain for all ischemic stroke patients is standard of care at most stroke centers; per the Guidelines, MRI is better at distinguishing acute, small cortical, small deep, and posterior fossa infarcts; at distinguishing acute from chronic ischemia; and at identifying subclinical satellite ischemic lesions that provide information on stroke mechanism (p. 1668). New techniques including magnetic resonance (MR) and CT perfusion scanning can show the ischemic region in the acute setting and may one day help select patients for specific therapies, but are not yet widely available nor have they been shown to alter outcomes.
An electrocardiogram is indicated for all stroke patients, as is admission to a cardiac telemetry bed for at least 24 hours to document any arrhythmias, the most common being atrial fibrillation (Guidelines, p. 1666, 1673). An echocardiographic study (ECHO) of the heart with bubble study should be performed in most cases (although which cases may specifically benefit is unclear) to identify a cardioembolic source for the stroke, such as low cardiac ejection fraction, atrial septal aneurysm, patent foramen ovale (PFO), or a cardiac thrombus. The bubble study increases the sensitivity of detecting a PFO, which could serve as a gateway for venous embolization to the cerebral arteries. Assuming a large PFO is discovered, other studies such as lower extremity Doppler may be warranted to investigate other potential sources of thrombi (ie, DVT).
Regarding laboratory testing, fasting lipids should be checked as hyperlipidemia is a common modifiable risk factor for ischemic stroke. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial included ischemic stroke patients that had low‐density lipoprotein (LDL) cholesterol between 100 mg/dL and 190 mg/dL and randomized them to receive atorvastatin 80 mg/day vs. placebo. Results showed a 16% relative risk reduction in recurrent stroke; however, there was a small increased risk of intracranial hemorrhage.9 As shown in Table 1, use of a statin on discharge is now a national performance measure for ischemic stroke.
Dissection is a common cause of stroke in young patients without traditional risk factors. Other serologies, such as hypercoagulable studies, may be warranted in patients with no other risk factors for strokes, paradoxical embolus, or of young age (eg, 45 years and under). The arterial hypercoagulable panel consists of antiphospholipid antibody panel, homocysteine levels, lupus anticoagulant levels, and prothrombin time/partial thromboplastin time (PT/PTT). The venous hypercoagulable panel consists of the laboratory values checked, with the arterial hypercoagulable and activated protein C (APC) resistance, Factor VIII activity, Factor II DNA, Factor V DNA if the APC resistance is positive, antithrombin III activity, and activity of proteins C and S. If a patient is found to have a hypercoagulable state, long‐term therapy often involves careful consideration of the choice of antiplatelet therapy vs. anticoagulation with warfarin.10
Initiating Secondary Prevention
Upon admission, the clinician faces a variety of treatment choices for secondary stroke prevention. The proper choice depends on the results of the workup and the presumptive pathophysiology.
Noncardioembolic/Atherothrombotic/Lacunar
The Antithrombotic Trialists' Collaboration meta‐analysis found that patients with a prior stroke or transient ischemic attack (TIA) had a highly significant decrease in the rate of subsequent vascular events (over about 3 years) on antiplatelet therapy (17.8% vs. 21.4%, P < 0.0001) and were unable to find a significant difference between low‐dose and high‐dose aspirin for secondary prevention.11 Thus, it is reasonable to place an acute stroke patient naive to antithrombotic therapy on 81 mg of aspirin or 325 mg for long‐term prevention (325 mg is specifically recommended in the acute setting). Several studies such as the WARSS and ESPRIT trials have shown antiplatelet agents to be at least as effective as anticoagulation in noncardioembolic ischemic strokes.12, 13 Guidelines from Europe, the American College of Chest Physicians, and the AHA/ASA all state it is acceptable to choose either aspirin monotherapy, aspirin/extended release dipyridamole combination therapy, or clopidogrel monotherapy as first‐line agents for long‐term secondary prevention in noncardioembolic ischemic stroke.1416 There is no clear evidence that patients who suffer an ischemic stroke while on aspirin will derive additional benefit from increasing the aspirin dose. The newer guidelines go on to recommend aspirin/extended release dipyridamole (ER‐DP) combination therapy or clopidogrel monotherapy over aspirin monotherapy, the former with a stronger level of recommendation based on the results of 2 randomized trials. These recommendations were all published without knowledge of the results of the Prevention Regimen For Effectively Avoiding Second Strokes (PRoFESS) study, which directly compared aspirin/extended release dipyridamole combination therapy to clopidogrel monotherapy for long‐term secondary prevention. The rate of first recurrent stroke was not significantly different between the 2 therapies (9.0% ER‐DP plus aspirin, 8.8% clopidogrel; hazard ratio [HR], 1.01; 95% confidence interval [CI], 0.921.11). Other outcomes also showed few differences, although there were more major hemorrhagic events in the ER‐DP plus aspirin group (4.1% vs. 3.6%; HR, 1.15; 95% CI, 1.001.32; P = 0.06).17
The ASA Stroke Prevention Guideline from 2006 states, with continued relevance, The selection of an antiplatelet agent should be individualized on the basis of patient risk factor profiles, tolerance, and other clinical characteristics.10 Of note, both the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management and Avoidance (CHARISMA) and Management of ATherothrombosis with Clopidogrel in High‐risk patients with recent TIA or ischemic stroke (MATCH) trials found a significant increased risk for hemorrhage complications with long‐term use of the aspirin and clopidogrel combination,18, 19 and the 2008 update to the ASA Stroke prevention guidelines state that the addition of aspirin to clopidogrel increases the risk of hemorrhage. Combination therapy of aspirin and clopidogrel is not routinely recommended for ischemic stroke or TIA patients unless they have a specific indication for this therapy (i.e., coronary stent or acute coronary syndrome).15
Atrial Fibrillation
Though our case patient did not have atrial fibrillation, this condition does deserve mention. About 15% to 20% of ischemic stroke patients have atrial fibrillation. The overall risk for stroke in patients with atrial fibrillation is about 5% per year; however, patients who have a history of stroke increase their risk factors for subsequent strokes to about 12% per year. In most cases, anticoagulation has proven to be the superior agent for primary and secondary stroke prevention with warfarin reducing the risk by 67% compared to aspirin, which only reduces the risk of stroke by 20%. A meta‐analysis from 2002 showed that patients who had a prior stroke or TIA decrease their risk of subsequent strokes to 4%/year on oral anticoagulation therapy, resulting in an 8% absolute risk reduction. Patients on aspirin therapy only decrease their risk to 10%/year, or a 2% reduction in stroke events.20 Unless there is a strong contraindication (eg, bleeding diathesis, history of life threatening gastrointestinal [GI] bleeding, history of fall with subdural hematoma, etc.), virtually all ischemic stroke patients with atrial fibrillation should be anticoagulated for life. Anticoagulation in the setting of atrial fibrillation is seriously underutilized.21 The highest quality study on early anticoagulation for ischemic stroke associated with atrial fibrillation suggested that there was no benefit to starting anticoagulation earlier than 2 weeks after a stroke, and there may actually be a higher complication rate (compared to aspirin).22 Other cardiac indications for anticoagulation include left ventricular thrombus and mechanical valves.
Carotid Stenosis
Significant ipsilateral stenosis of the internal carotid artery in a patient with ischemic stroke is a strong indication for intervention, usually a standard carotid endarterectomy (CEA). Stenosis of 70% to 99% is the strongest indication for CEA, and may be of greatest benefit in men, those 75+ years of age, and if surgery is done <2 weeks after the most recent symptoms.23 In patients with minor stroke or TIA, recent recommendations and our practice is to admit to the hospital and perform endarterectomy as soon as possible (those with major stroke may have a greater risk of complications with early CEA).24 Stenting should only be considered instead of CEA if high risk (for surgical complications) criteria are present. These high risk criteria include patients having significant comorbidities and/or anatomic risk factors (ie, recurrent stenosis and/or previous radical neck dissection), and [who] would be poor candidates for CEA in the opinion of a surgeon.25 For stenoses of 50% to 69%, intervention is not as compelling, and decisions should be individualized based on patient characteristics; in this group, stenting should only be considered in the setting of a clinical trial or if an investigational device exemption (IDE) exists at your institution.26
Dissection of the Carotid or Vertebral Arteries
This is a common cause of stroke in younger adults. It should be suspected in patients without other clear causes of stroke and significant disease of the extracranial arteries. Diagnosis can usually be made with CTA or MRA, though it is suggested that the best modality may be T1‐fat‐saturated MRI images of the neck. Debate exists as to the best approach to treatment of dissections due to the absence of randomized trials. A recent comprehensive review suggested anticoagulation for 3 to 6 months followed by indefinite antiplatelet therapy for symptomatic dissections and antiplatelet therapy alone for asymptomatic dissections.27
PFO‐related Stroke
If the patient is found to have a PFO, its role in comparison to traditional risk factors must be weighed carefully. Epidemiological studies suggest that PFO may be most relevant in younger patients, those with cryptogenic stroke (no obvious cause and lack of traditional risk factors), those with higher risk associations including interatrial septal aneurysm, larger PFOs or history of previous cryptogenic stroke.28, 29 The best medical therapy for seemingly PFO‐related ischemic stroke is also unclear; a reasonable approach might be aspirin if neither high‐risk associations nor a hypercoagulable state is present, and warfarin if either are present. Transcatheter closure of PFO is approved by the U.S. Food and Drug Administration (FDA) only under an IDE for patients who have had a recurrent event on maximally tolerated medical treatment, and requires approval from the human research committee (internal review board [IRB]) at your hospital. It is not known if closure is superior or inferior to best medical therapy, and a practice parameter from the American Academy of Neurology strongly encourages appropriate patients to consider participation in ongoing randomized trials.28 Further information on these trials is available at:
Our patient underwent a CTA of the head and neck in the emergency room to see if he would be a candidate for other interventions; unfortunately, he did not meet the time criteria. CTA showed complete occlusion of the left internal carotid artery at the bifurcation with heterogeneous retrograde filling (Supporting Figure 1). Complete occlusion of the proximal third of the left M1 segment was also seen with relative oligemia in the left MCA distribution, though several small peripheral M3/M4 vessels were opacified in the territory indicating collateralization (Supporting Figure 2). A MRI showed a large area of diffusion‐weighted abnormality (Figure 1). Interestingly, the patient's transthoracic echocardiography (TTE), which did not show evidence of a PFO, did reveal a calcified thrombus in the left ventricle. Though no arrhythmias were captured on telemetry, this thrombus does serve as a potential source of cardioembolic emboli to the cerebral vasculature. It was felt that the most likely source of the patient's acute infarct was from artery‐to‐artery emboli from his internal carotid occlusion given the infarct location and the lack of infarction in other vascular distributions (as one might see from a cardiac embolic source). Therefore, his medical management consisted of an antiplatelet regimen for 2 weeks followed by a transition to warfarin alone 2 weeks after his acute infarct as secondary stroke prevention due to the cardiac thrombus. Given the complete occlusion of the internal carotid artery and M1 segment, there was concern that the penumbra might be at risk of infarction (supporting standard guidelines of permissive hypertension). By the end of his hospitalization, the patient had improved and was transferred to inpatient rehabilitation.

The guidelines for acute stroke management continue to rapidly evolve. Certainly, there are effective treatments for acute ischemic stroke, with variation based on the timing of patient arrival at the hospital, the underlying pathophysiology, and the treatment capabilities of the individual hospital. Secondary stroke prevention is extremely important and has been emphasized during inpatient admissions with the establishment of an appropriate medication regime, given that patients are more likely to stay on treatment that is initiated around the time of a diagnosis.29 Evidence strongly suggests that management of acute stroke is improved by an organized approach to care, including the expertise of a multidisciplinary team in a specialized stroke unit. Hospitals committed to high quality of care for acute stroke patients should strongly consider the Joint Commission certification process or an analogous local certification. Such certification demonstrates a hospital's commitment to providing high‐quality care, what every stroke patient wants and deserves.
Inpatient stroke management includes many elements of care, at least as important as the initial portion of the patient's stay, as reviewed in part 1 of this article. The extent of further diagnostic evaluation varies widely depending on apparent risk factors on presentation. Likewise, further therapy, both inpatient and secondary prevention is based on identification of stroke mechanism. Hospitalists are uniquely positioned to have a tremendous impact on both stroke care and the prevention of recurrent disease.
Case Presentation
A 76‐year‐old right‐handed male with a history of hyperlipidemia and myocardial infarction was found at 7 AM with right‐sided paralysis and poor responsiveness on the morning of admission. Upon arrival to the emergency department (ED), with symptoms of partial aphasia, right hemiplegia, and left gaze preference, there was a high suspicion for a left middle cerebral artery (MCA) stroke. Unfortunately, he was excluded from receiving intravenous (IV) tissue plasminogen activator (tPA) or any other acute interventions as the last time he was known to be neurologically intact was the prior evening, which is taken to be the time of onset. Antiplatelet therapy was continued, and the patient admitted for further workup.
Inpatient Care
When an acute ischemic stroke patient is admitted to the hospital, he or she should be placed on a standardized acute stroke protocol (also known as (a.k.a.) a care map, order set, clinical pathway)commonly created by a hospitalist/neurologist and a multidisciplinary team and admitted to a stroke unit. A stroke unit can take many forms, either as a physically separate unit in hospitals with sufficient volume or a floor where a lower volume of stroke patients are always admitted. Multidisciplinary care providers in the stroke unit have special training in stroke, and strong evidence from randomized trials shows that patients cared for in these units have significantly decreased mortality with improved functional outcomes.1 Essentials of the stroke protocol or order set include cardiac telemetry, maintaining euthermia and euglycemia, closely following blood pressure and neurologic status, actively avoiding complications, initiation of secondary prevention treatment, early involvement of rehabilitation services, and patient education.
Euthermia may be assisted by administering scheduled Tylenol to the patient for the first 48 hours, but is not strictly evidence‐based.2 Though euthermia and euglycemia have not been shown to improve outcomes in acute stroke, studies have shown that hyperthermia and hyperglycemia are associated with worsened outcomes for patients with acute strokes.35
Blood Pressure Management
Normally, cerebral vascular autoregulation leads to stable cerebral blood flow over a range of systemic blood pressures. In the setting of an acute stroke, the ability to autoregulate is diminished or absent in regions of and surrounding an acute ischemic stroke; as the area becomes ischemic, autoregulation opens the local vasculature maximally in an effort to drawn in as much blood as possible. Maximally dilated arterioles are perfused in direct correlation with systemic blood pressure, thus any drop in the systemic blood pressure leads to direct decreases in blood flow specifically in the area of ischemia; if there is a penumbra of marginally perfused tissue, such systemic blood pressure drops risk extending the area of fatal ischemia (increasing the size of the ischemic stroke).68 Thus in the acute period of an ischemic stroke, the American Heart Association (AHA)/American Stroke Association (ASA) Guidelines for the Early Management of Adults With Ischemic Stroke (referred to herein as the Guidelines)10 suggest avoid treatment unless systolic blood pressures are >220 or diastolic pressures >105, and review the evidence to support this recommendation (p. 16711672). Those patients who receive tPA have a more stringent blood pressure threshold given their risk of intracranial hemorrhage; systolic blood pressures are accepted up to 180 prior to recommending treatment.
Higher‐quality Inpatient Stroke Care and Harmonized Performance Measures
Beginning in January 2008, a set of 10 performance measures (Table 1) for inpatient acute stroke care have been agreed upon (harmonized) by 3 major stakeholders including the Joint Commission, the ASA's Get with the GuidelinesStroke quality improvement program, and the Center for Disease Control and Prevention's (CDC's) Paul Coverdell Acute stroke registries. These performance measures were selected to help avoid complications (deep vein thrombosis [DVT], aspiration pneumonia), encourage appropriately aggressive care (tPA administration), optimize secondary prevention (antithrombotics, cholesterol lowering, smoking cessation, education), and facilitate functional recovery (early rehabilitation). All 10 measures are appropriate for consideration in every ischemic stroke patient, and 5 are appropriate for the hemorrhagic stroke types.
| Performance measure* | Definition* |
|---|---|
| |
| 1. DVT prophylaxis | Patients who are nonambulatory should start receiving DVT prophylaxis by end of hospital day 2 (can be either compression devices or any low‐dose heparin) |
| 2. Discharged on antithrombotic therapy | Antiplatelet agent(s) or warfarin anticoagulation |
| 3. Patients with atrial fibrillation receiving anticoagulation therapy | A proven approach to secondary prevention in such patients; practice at Harborview varies time of warfarin initiation based on infarct size with larger infarcts waiting up to 2 weeks before initiating warfarin (the best randomized trial showed no benefit for full‐dose low‐molecular‐weight heparin over aspirin in the first 2 weeks)50 |
| 4. Thrombolytic therapy administered | In ischemic stroke patients who arrive at the hospital within 120 minutes (2 hours) of time last known well, for whom IV tPA was initiated at this hospital within 180 minutes (3 hours) of time last known well, and who qualify under strict criteria |
| 5. Antithrombotic therapy by end of hospital day 2 | Usually just antiplatelet agents, a minimal standard of care for ischemic stroke patients; should be started as early as possible, usually in ER |
| 6. Discharged on statin medication | If LDL >100, or not measured or if on a statin drug prior to admission; to reduce risk of subsequent ischemic stroke |
| 7. Dysphagia screening | Prior to any PO food, fluids or medications; to reduce the chances of aspiration pneumonia |
| 8. Stroke education | Including for families if patient unable to participate, must include personal risk factors for stroke, warning signs for stroke, activation of emergency medical system, need for follow‐up after discharge, and medications prescribed |
| 9. Smoking cessation/advice/counseling | For any patient who has smoked in the last year |
| 10. Assessed for rehabilitation | Or received therapy services; to facilitate progress to an optimal function outcome |
Further Workup
After the ischemic stroke patient has had their computed tomography (CT) scan, possibly a computed tomography angiography (CTA), been admitted to the stroke unit, started on an antithrombotic medication, and had their blood pressure appropriately treated, attention then turns to defining the pathophysiology related to the stroke and starting an optimal regimen for secondary prevention. Imaging of the cerebral vasculature including both extracranial and intracranial large vessels is a vital first step in understanding the cause of ischemic stroke. There are multiple potential modalities (magnetic resonance angiography [MRA], CTA, and duplex/transcranial Doppler), the choice of which depends on local availability and expertise as well as the specific clinical situation. Magnetic resonance imaging (MRI) of the brain for all ischemic stroke patients is standard of care at most stroke centers; per the Guidelines, MRI is better at distinguishing acute, small cortical, small deep, and posterior fossa infarcts; at distinguishing acute from chronic ischemia; and at identifying subclinical satellite ischemic lesions that provide information on stroke mechanism (p. 1668). New techniques including magnetic resonance (MR) and CT perfusion scanning can show the ischemic region in the acute setting and may one day help select patients for specific therapies, but are not yet widely available nor have they been shown to alter outcomes.
An electrocardiogram is indicated for all stroke patients, as is admission to a cardiac telemetry bed for at least 24 hours to document any arrhythmias, the most common being atrial fibrillation (Guidelines, p. 1666, 1673). An echocardiographic study (ECHO) of the heart with bubble study should be performed in most cases (although which cases may specifically benefit is unclear) to identify a cardioembolic source for the stroke, such as low cardiac ejection fraction, atrial septal aneurysm, patent foramen ovale (PFO), or a cardiac thrombus. The bubble study increases the sensitivity of detecting a PFO, which could serve as a gateway for venous embolization to the cerebral arteries. Assuming a large PFO is discovered, other studies such as lower extremity Doppler may be warranted to investigate other potential sources of thrombi (ie, DVT).
Regarding laboratory testing, fasting lipids should be checked as hyperlipidemia is a common modifiable risk factor for ischemic stroke. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial included ischemic stroke patients that had low‐density lipoprotein (LDL) cholesterol between 100 mg/dL and 190 mg/dL and randomized them to receive atorvastatin 80 mg/day vs. placebo. Results showed a 16% relative risk reduction in recurrent stroke; however, there was a small increased risk of intracranial hemorrhage.9 As shown in Table 1, use of a statin on discharge is now a national performance measure for ischemic stroke.
Dissection is a common cause of stroke in young patients without traditional risk factors. Other serologies, such as hypercoagulable studies, may be warranted in patients with no other risk factors for strokes, paradoxical embolus, or of young age (eg, 45 years and under). The arterial hypercoagulable panel consists of antiphospholipid antibody panel, homocysteine levels, lupus anticoagulant levels, and prothrombin time/partial thromboplastin time (PT/PTT). The venous hypercoagulable panel consists of the laboratory values checked, with the arterial hypercoagulable and activated protein C (APC) resistance, Factor VIII activity, Factor II DNA, Factor V DNA if the APC resistance is positive, antithrombin III activity, and activity of proteins C and S. If a patient is found to have a hypercoagulable state, long‐term therapy often involves careful consideration of the choice of antiplatelet therapy vs. anticoagulation with warfarin.10
Initiating Secondary Prevention
Upon admission, the clinician faces a variety of treatment choices for secondary stroke prevention. The proper choice depends on the results of the workup and the presumptive pathophysiology.
Noncardioembolic/Atherothrombotic/Lacunar
The Antithrombotic Trialists' Collaboration meta‐analysis found that patients with a prior stroke or transient ischemic attack (TIA) had a highly significant decrease in the rate of subsequent vascular events (over about 3 years) on antiplatelet therapy (17.8% vs. 21.4%, P < 0.0001) and were unable to find a significant difference between low‐dose and high‐dose aspirin for secondary prevention.11 Thus, it is reasonable to place an acute stroke patient naive to antithrombotic therapy on 81 mg of aspirin or 325 mg for long‐term prevention (325 mg is specifically recommended in the acute setting). Several studies such as the WARSS and ESPRIT trials have shown antiplatelet agents to be at least as effective as anticoagulation in noncardioembolic ischemic strokes.12, 13 Guidelines from Europe, the American College of Chest Physicians, and the AHA/ASA all state it is acceptable to choose either aspirin monotherapy, aspirin/extended release dipyridamole combination therapy, or clopidogrel monotherapy as first‐line agents for long‐term secondary prevention in noncardioembolic ischemic stroke.1416 There is no clear evidence that patients who suffer an ischemic stroke while on aspirin will derive additional benefit from increasing the aspirin dose. The newer guidelines go on to recommend aspirin/extended release dipyridamole (ER‐DP) combination therapy or clopidogrel monotherapy over aspirin monotherapy, the former with a stronger level of recommendation based on the results of 2 randomized trials. These recommendations were all published without knowledge of the results of the Prevention Regimen For Effectively Avoiding Second Strokes (PRoFESS) study, which directly compared aspirin/extended release dipyridamole combination therapy to clopidogrel monotherapy for long‐term secondary prevention. The rate of first recurrent stroke was not significantly different between the 2 therapies (9.0% ER‐DP plus aspirin, 8.8% clopidogrel; hazard ratio [HR], 1.01; 95% confidence interval [CI], 0.921.11). Other outcomes also showed few differences, although there were more major hemorrhagic events in the ER‐DP plus aspirin group (4.1% vs. 3.6%; HR, 1.15; 95% CI, 1.001.32; P = 0.06).17
The ASA Stroke Prevention Guideline from 2006 states, with continued relevance, The selection of an antiplatelet agent should be individualized on the basis of patient risk factor profiles, tolerance, and other clinical characteristics.10 Of note, both the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management and Avoidance (CHARISMA) and Management of ATherothrombosis with Clopidogrel in High‐risk patients with recent TIA or ischemic stroke (MATCH) trials found a significant increased risk for hemorrhage complications with long‐term use of the aspirin and clopidogrel combination,18, 19 and the 2008 update to the ASA Stroke prevention guidelines state that the addition of aspirin to clopidogrel increases the risk of hemorrhage. Combination therapy of aspirin and clopidogrel is not routinely recommended for ischemic stroke or TIA patients unless they have a specific indication for this therapy (i.e., coronary stent or acute coronary syndrome).15
Atrial Fibrillation
Though our case patient did not have atrial fibrillation, this condition does deserve mention. About 15% to 20% of ischemic stroke patients have atrial fibrillation. The overall risk for stroke in patients with atrial fibrillation is about 5% per year; however, patients who have a history of stroke increase their risk factors for subsequent strokes to about 12% per year. In most cases, anticoagulation has proven to be the superior agent for primary and secondary stroke prevention with warfarin reducing the risk by 67% compared to aspirin, which only reduces the risk of stroke by 20%. A meta‐analysis from 2002 showed that patients who had a prior stroke or TIA decrease their risk of subsequent strokes to 4%/year on oral anticoagulation therapy, resulting in an 8% absolute risk reduction. Patients on aspirin therapy only decrease their risk to 10%/year, or a 2% reduction in stroke events.20 Unless there is a strong contraindication (eg, bleeding diathesis, history of life threatening gastrointestinal [GI] bleeding, history of fall with subdural hematoma, etc.), virtually all ischemic stroke patients with atrial fibrillation should be anticoagulated for life. Anticoagulation in the setting of atrial fibrillation is seriously underutilized.21 The highest quality study on early anticoagulation for ischemic stroke associated with atrial fibrillation suggested that there was no benefit to starting anticoagulation earlier than 2 weeks after a stroke, and there may actually be a higher complication rate (compared to aspirin).22 Other cardiac indications for anticoagulation include left ventricular thrombus and mechanical valves.
Carotid Stenosis
Significant ipsilateral stenosis of the internal carotid artery in a patient with ischemic stroke is a strong indication for intervention, usually a standard carotid endarterectomy (CEA). Stenosis of 70% to 99% is the strongest indication for CEA, and may be of greatest benefit in men, those 75+ years of age, and if surgery is done <2 weeks after the most recent symptoms.23 In patients with minor stroke or TIA, recent recommendations and our practice is to admit to the hospital and perform endarterectomy as soon as possible (those with major stroke may have a greater risk of complications with early CEA).24 Stenting should only be considered instead of CEA if high risk (for surgical complications) criteria are present. These high risk criteria include patients having significant comorbidities and/or anatomic risk factors (ie, recurrent stenosis and/or previous radical neck dissection), and [who] would be poor candidates for CEA in the opinion of a surgeon.25 For stenoses of 50% to 69%, intervention is not as compelling, and decisions should be individualized based on patient characteristics; in this group, stenting should only be considered in the setting of a clinical trial or if an investigational device exemption (IDE) exists at your institution.26
Dissection of the Carotid or Vertebral Arteries
This is a common cause of stroke in younger adults. It should be suspected in patients without other clear causes of stroke and significant disease of the extracranial arteries. Diagnosis can usually be made with CTA or MRA, though it is suggested that the best modality may be T1‐fat‐saturated MRI images of the neck. Debate exists as to the best approach to treatment of dissections due to the absence of randomized trials. A recent comprehensive review suggested anticoagulation for 3 to 6 months followed by indefinite antiplatelet therapy for symptomatic dissections and antiplatelet therapy alone for asymptomatic dissections.27
PFO‐related Stroke
If the patient is found to have a PFO, its role in comparison to traditional risk factors must be weighed carefully. Epidemiological studies suggest that PFO may be most relevant in younger patients, those with cryptogenic stroke (no obvious cause and lack of traditional risk factors), those with higher risk associations including interatrial septal aneurysm, larger PFOs or history of previous cryptogenic stroke.28, 29 The best medical therapy for seemingly PFO‐related ischemic stroke is also unclear; a reasonable approach might be aspirin if neither high‐risk associations nor a hypercoagulable state is present, and warfarin if either are present. Transcatheter closure of PFO is approved by the U.S. Food and Drug Administration (FDA) only under an IDE for patients who have had a recurrent event on maximally tolerated medical treatment, and requires approval from the human research committee (internal review board [IRB]) at your hospital. It is not known if closure is superior or inferior to best medical therapy, and a practice parameter from the American Academy of Neurology strongly encourages appropriate patients to consider participation in ongoing randomized trials.28 Further information on these trials is available at:
Our patient underwent a CTA of the head and neck in the emergency room to see if he would be a candidate for other interventions; unfortunately, he did not meet the time criteria. CTA showed complete occlusion of the left internal carotid artery at the bifurcation with heterogeneous retrograde filling (Supporting Figure 1). Complete occlusion of the proximal third of the left M1 segment was also seen with relative oligemia in the left MCA distribution, though several small peripheral M3/M4 vessels were opacified in the territory indicating collateralization (Supporting Figure 2). A MRI showed a large area of diffusion‐weighted abnormality (Figure 1). Interestingly, the patient's transthoracic echocardiography (TTE), which did not show evidence of a PFO, did reveal a calcified thrombus in the left ventricle. Though no arrhythmias were captured on telemetry, this thrombus does serve as a potential source of cardioembolic emboli to the cerebral vasculature. It was felt that the most likely source of the patient's acute infarct was from artery‐to‐artery emboli from his internal carotid occlusion given the infarct location and the lack of infarction in other vascular distributions (as one might see from a cardiac embolic source). Therefore, his medical management consisted of an antiplatelet regimen for 2 weeks followed by a transition to warfarin alone 2 weeks after his acute infarct as secondary stroke prevention due to the cardiac thrombus. Given the complete occlusion of the internal carotid artery and M1 segment, there was concern that the penumbra might be at risk of infarction (supporting standard guidelines of permissive hypertension). By the end of his hospitalization, the patient had improved and was transferred to inpatient rehabilitation.

The guidelines for acute stroke management continue to rapidly evolve. Certainly, there are effective treatments for acute ischemic stroke, with variation based on the timing of patient arrival at the hospital, the underlying pathophysiology, and the treatment capabilities of the individual hospital. Secondary stroke prevention is extremely important and has been emphasized during inpatient admissions with the establishment of an appropriate medication regime, given that patients are more likely to stay on treatment that is initiated around the time of a diagnosis.29 Evidence strongly suggests that management of acute stroke is improved by an organized approach to care, including the expertise of a multidisciplinary team in a specialized stroke unit. Hospitals committed to high quality of care for acute stroke patients should strongly consider the Joint Commission certification process or an analogous local certification. Such certification demonstrates a hospital's commitment to providing high‐quality care, what every stroke patient wants and deserves.
- Organised inpatient (stroke unit) care for stroke.Stroke Unit Trialists' Collaboration.Cochrane Database Syst Rev.2000(2):CD000197.
- ,,, et al.Acetaminophen for altering body temperature in acute stroke: a randomized clinical trial.Stroke.2002;33(1):130–134.
- ,,, et al.Fever in acute stroke worsens prognosis. A prospective study.Stroke.1995;26(11):2040–2043.
- ,.Combating hyperthermia in acute stroke: a significant clinical concern.Stroke.1998;29(2):529–534.
- ,,, et al.Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome.Lancet. 171996;347(8999):422–425.
- ,,.Thresholds in cerebral ischemia—the ischemic penumbra.Stroke.1981;12(6):723–725.
- .Ischaemic brain damage of cerebral perfusion failure type after treatment of severe hypertension.Br Med J. 271975;4(5999):739.
- ,,,,.Imaging of acute stroke.Lancet Neurol.2006;5(9):755–768.
- ,,, et al.High‐dose atorvastatin after stroke or transient ischemic attack.N Engl J Med.2006;355(6):549–559.
- ,,, et al.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co‐sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline.Stroke.2006;37(2):577–617.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324(7329):71–86.
- ,,, et al.A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke.N Engl J Med.2001;345(20):1444–1451.
- .Warfarin or aspirin for recurrent ischemic stroke.N Engl J Med.2002;346(15):1169–1171.
- ,,, et al.Prevention. European Stroke Initiative.Cerebrovasc Dis.2004;17(suppl 2):15–29.
- ,,, et al.Update to the AHA/ASA recommendations for the prevention of stroke in patients with stroke and transient ischemic attack.Stroke.2008;39(5):1647–1652.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th edition).Chest.2008;133(suppl):630S–669S.
- ,,, et al.Aspirin and extended‐release dipyridamole versus clopidogrel for recurrent stroke.N Engl J Med.2008;359(12):1238–1251.
- ,,, et al.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354(16):1706–1717.
- ,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364(9431):331–337.
- ,,, et al.Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta‐analysis.JAMA.2002;288(19):2441–2448.
- .Effective anticoagulation therapy: defining the gap between clinical studies and clinical practice.Am J Manag Care.2004;10(suppl):S297–S306; discussionS312–S297.
- ,,,.Low molecular‐weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double‐blind randomised study. HAEST Study Group. Heparin in Acute Embolic Stroke Trial.Lancet.2000;355(9211):1205–1210.
- ,,,,.Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery.Lancet.2004;363(9413):915–924.
- ,,.The timing of carotid endarterectomy post stroke.Neurol Clin.2006;24(4):669–680.
- Centers for Medicare and Medicaid Services (CMS). Department of Health and Human Services (DHHS). CMS Manual System. Pub 100–03 Medicare National Coverage Determinations. Available at: http://www.cms.hhs.gov/Transmittals/Downloads/R64NCD.pdf. Accessed May2009.
- .Current status of carotid endarterectomy and stenting for symptomatic carotid stenosis.Cerebrovasc Dis.2007;24(suppl 1):116–125.
- ,,, et al.Antiplatelets versus anticoagulation in cervical artery dissection.Stroke.2007;38(9):2605–2611.
- ,,, et al.A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke.N Engl J Med2001;345(20):1444–1451.
- .Warfarin or aspirin for recurrent ischemic stroke.N Engl J Med2002;346(15):1169–1171.
- ,,, et al.Practice parameter: recurrent stroke with patent foramen ovale and atrial septal aneurysm: report of the Quality Standards Subcommittee of the American Academy of Neurology.Neurology.2004;62(7):1042–1050.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35(12):2879–2883.
- Organised inpatient (stroke unit) care for stroke.Stroke Unit Trialists' Collaboration.Cochrane Database Syst Rev.2000(2):CD000197.
- ,,, et al.Acetaminophen for altering body temperature in acute stroke: a randomized clinical trial.Stroke.2002;33(1):130–134.
- ,,, et al.Fever in acute stroke worsens prognosis. A prospective study.Stroke.1995;26(11):2040–2043.
- ,.Combating hyperthermia in acute stroke: a significant clinical concern.Stroke.1998;29(2):529–534.
- ,,, et al.Body temperature in acute stroke: relation to stroke severity, infarct size, mortality, and outcome.Lancet. 171996;347(8999):422–425.
- ,,.Thresholds in cerebral ischemia—the ischemic penumbra.Stroke.1981;12(6):723–725.
- .Ischaemic brain damage of cerebral perfusion failure type after treatment of severe hypertension.Br Med J. 271975;4(5999):739.
- ,,,,.Imaging of acute stroke.Lancet Neurol.2006;5(9):755–768.
- ,,, et al.High‐dose atorvastatin after stroke or transient ischemic attack.N Engl J Med.2006;355(6):549–559.
- ,,, et al.Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co‐sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline.Stroke.2006;37(2):577–617.
- Antithrombotic Trialists' Collaboration.Collaborative meta‐analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients.BMJ.2002;324(7329):71–86.
- ,,, et al.A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke.N Engl J Med.2001;345(20):1444–1451.
- .Warfarin or aspirin for recurrent ischemic stroke.N Engl J Med.2002;346(15):1169–1171.
- ,,, et al.Prevention. European Stroke Initiative.Cerebrovasc Dis.2004;17(suppl 2):15–29.
- ,,, et al.Update to the AHA/ASA recommendations for the prevention of stroke in patients with stroke and transient ischemic attack.Stroke.2008;39(5):1647–1652.
- ,,,,.Antithrombotic and thrombolytic therapy for ischemic stroke: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines (8th edition).Chest.2008;133(suppl):630S–669S.
- ,,, et al.Aspirin and extended‐release dipyridamole versus clopidogrel for recurrent stroke.N Engl J Med.2008;359(12):1238–1251.
- ,,, et al.Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events.N Engl J Med.2006;354(16):1706–1717.
- ,,, et al.Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high‐risk patients (MATCH): randomised, double‐blind, placebo‐controlled trial.Lancet.2004;364(9431):331–337.
- ,,, et al.Oral anticoagulants vs aspirin in nonvalvular atrial fibrillation: an individual patient meta‐analysis.JAMA.2002;288(19):2441–2448.
- .Effective anticoagulation therapy: defining the gap between clinical studies and clinical practice.Am J Manag Care.2004;10(suppl):S297–S306; discussionS312–S297.
- ,,,.Low molecular‐weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double‐blind randomised study. HAEST Study Group. Heparin in Acute Embolic Stroke Trial.Lancet.2000;355(9211):1205–1210.
- ,,,,.Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery.Lancet.2004;363(9413):915–924.
- ,,.The timing of carotid endarterectomy post stroke.Neurol Clin.2006;24(4):669–680.
- Centers for Medicare and Medicaid Services (CMS). Department of Health and Human Services (DHHS). CMS Manual System. Pub 100–03 Medicare National Coverage Determinations. Available at: http://www.cms.hhs.gov/Transmittals/Downloads/R64NCD.pdf. Accessed May2009.
- .Current status of carotid endarterectomy and stenting for symptomatic carotid stenosis.Cerebrovasc Dis.2007;24(suppl 1):116–125.
- ,,, et al.Antiplatelets versus anticoagulation in cervical artery dissection.Stroke.2007;38(9):2605–2611.
- ,,, et al.A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke.N Engl J Med2001;345(20):1444–1451.
- .Warfarin or aspirin for recurrent ischemic stroke.N Engl J Med2002;346(15):1169–1171.
- ,,, et al.Practice parameter: recurrent stroke with patent foramen ovale and atrial septal aneurysm: report of the Quality Standards Subcommittee of the American Academy of Neurology.Neurology.2004;62(7):1042–1050.
- ,,, et al.In‐hospital initiation of secondary stroke prevention therapies yields high rates of adherence at follow‐up.Stroke.2004;35(12):2879–2883.
Management of Ischemic Stroke: Part 1
The term stroke is defined by the World Health Organization as rapidly developed clinical signs of focal (or global) disturbance of cerebral function lasting more than 24 hours (unless interrupted by surgery or death), with no apparent cause other than a vascular origin; it includes patients presenting clinical signs and symptoms suggestive of subarachnoid hemorrhage (SAH), intracerebral hemorrhage, or cerebral ischemic necrosis.1 Stroke is 1 of the leading causes of death and the number 1 cause of long‐term disability in the United States, with over 700,000 strokes and over 150,000 stroke deaths each year.2
Given the projections of 30,000 hospitalists nationally by 2010 (
Case Presentation
A 76‐year‐old right‐handed male with a history of hyperlipidemia and myocardial infarction was found at 7 AM with right‐sided paralysis and poor responsiveness on the morning of admission. He seemed to prefer looking to the left and to understand what was being said to him, but had great difficulty speaking. When he went to bed at 9 PM, he was at his neurological baseline. Upon finding him that morning, his wife called 911.
With increased knowledge regarding the pathophysiology of stroke, it has become clear that timeliness is of utmost importance (time is brain) and that acute stroke should be regarded as an acute medical/neurological emergency.
This article reviews the approach in evaluating an acute stroke patient, management strategies, and treatment options. Where not otherwise referenced, data to support our comments come from the recently updated and exhaustive American Heart Association (AHA)/American Stroke Association (ASA) Guidelines for the Early Management of Adults With Ischemic Stroke and will be referred to herein as the Guidelines.4 Harborview Medical Center in Seattle is a Joint Commissioncertified Primary Stroke Center and the home hospital of 2 of the authors (C.L.E., D.L.T.); it is referred to herein as Harborview.
Emergency Room Care (see Acute Stroke Algorithm, Figure 1)
The First 15 Minutes
After assuring stable airway, breathing, and circulation, immediate (STAT) blood draws should be performed, including full complete blood count (CBC) with platelets, international normalized ratio/prothrombin time/partial thromboplastin time (INR/PT/PTT), full electrolytes, and glucose (finger‐stick blood glucose also recommended). Glasgow Coma Scale (GCS) score and NIH Stroke Scale (NIHSS) score should be established via a focused history and physical exam. The GCS is most appropriate for patients with a significantly depressed level of consciousness, while the NIHSS can be scored for any stroke patient (1‐page version of NIHSS used at Harborview is shown in Figure 2). By quantifying stroke severity, the NIHSS score helps both to facilitate communication about neurologic deficit as well as serve as a documented baseline in case of subsequent clinical change. Emergency department (ED) physicians, hospitalists, neurologists, and nursing staff regularly caring for acute stroke patients would be well‐served by obtaining certification in the NIHSS (available free online at
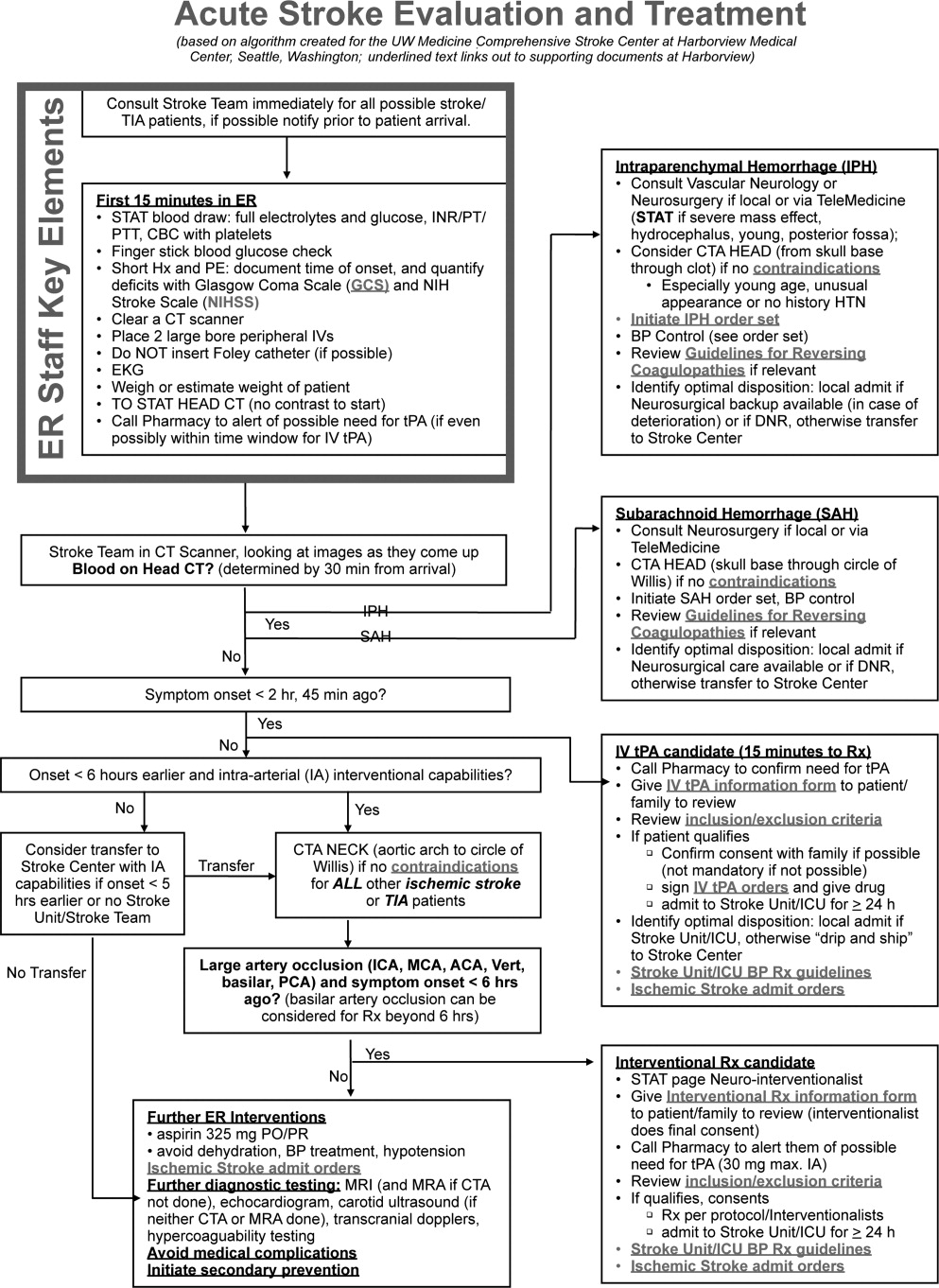
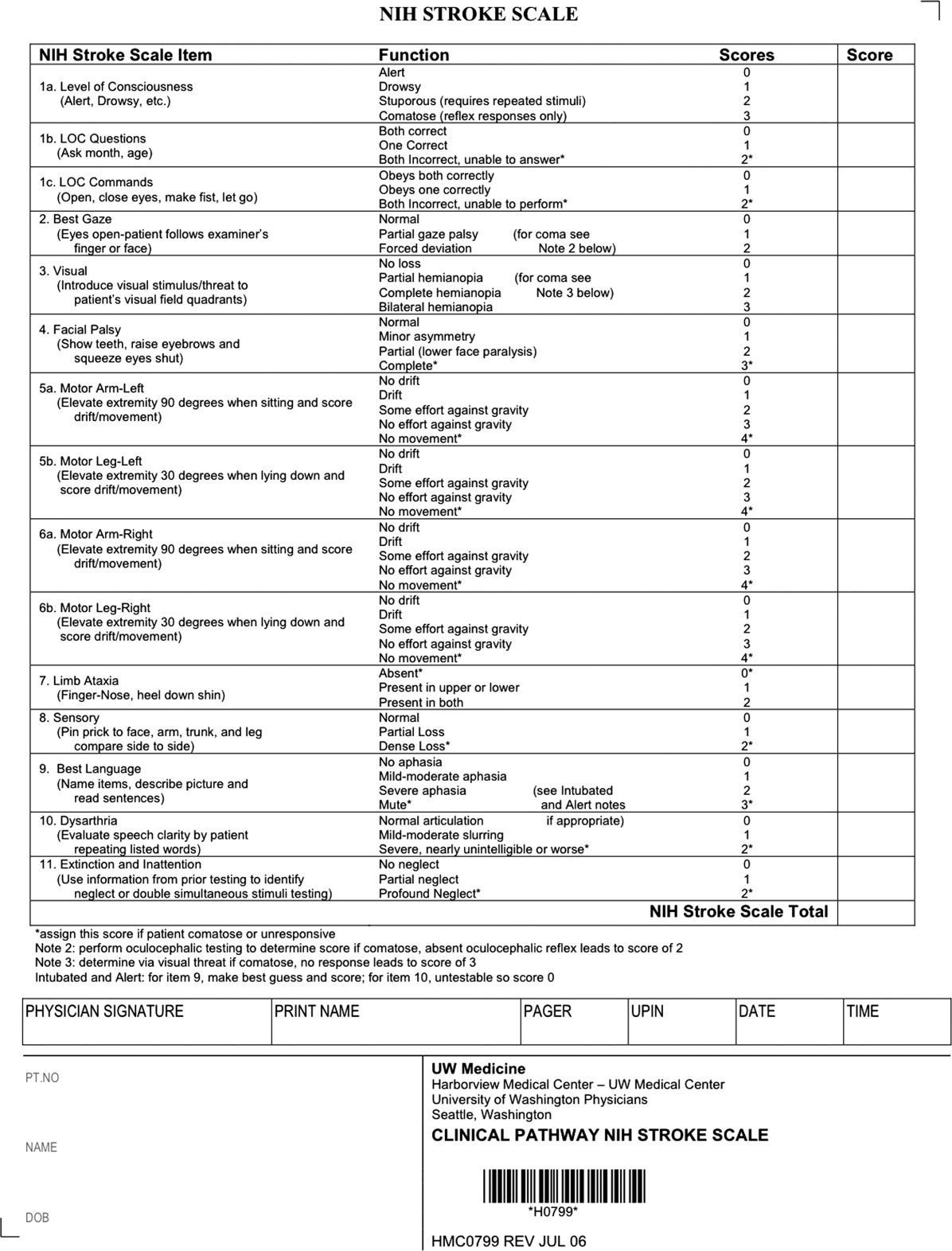
Our case patient's initial NIHSS score was 15, with points given for drowsiness, inability to answer questions, partial facial palsy, no movement in right arm or leg, mild‐moderate aphasia, and mild‐moderate dysarthria (Figure 2).
Differential Diagnosis
Many acute conditions can mimic stroke, and 1 of the goals of the initial emergency room (ER) evaluation is to rule out such stroke mimics. A report of 411 initial ER stroke diagnoses identified 19% as stroke mimics; the most common mimic diagnoses were seizure, systemic infection, brain tumor, and toxic‐metabolic.5 The same study identified decreased level of alertness as associated with a final mimic diagnosis and history of angina as associated with a final diagnosis of stroke. Another study looked at 350 presentations with an initial stroke diagnosis and found 31% stroke mimics; similarly, the main alternative diagnoses were seizure, sepsis, toxic‐metabolic, space‐occupying lesion, and syncope/presyncope.6 Findings associated with a mimic diagnosis included no cognitive impairment and abnormal findings in any other system, while findings associated with a stroke diagnosis were a definite history of focal neurological symptoms, NIHSS score, stroke type classification possible, an exact onset that could be determined, and abnormal vascular findings on imaging.6
Initial Imaging
The patient should receive a STAT noncontrast head CT to evaluate for the presence or absence of blood. At this time, magnetic resonance imaging (MRI) is not essential to confirm the diagnosis of ischemic stroke, as diagnosis is based on clinical suspicion. MRI is more sensitive at imaging acute ischemia (on diffusion‐weighted sequences) and recently has been shown to be equally sensitive in identifying acute blood (previously thought to be a relative advantage of CT).7, 8 Practical and pervasive barriers to emergent MRI include study duration, significant patient cooperation, and that few hospitals are currently set up to perform such rapid MRIS. The Guidelines specifically state that In most instances, CT will provide the information to make decisions about emergency management (p. 1668),4 that vascular imaging should not delay treatment of patients whose symptoms started <3 hours ago and who have acute ischemic stroke, and that emergency treatment of stroke should not be delayed in order to obtain multimodal imaging studies (p. 1669).4
Our case patient's initial imaging, a noncontrast head CT (Supporting Figures 1 and 2), showed subtle clues consistent with the diagnosis of acute ischemic stroke. These include a hyperdense middle cerebral artery (MCA) sign (presumably representing thrombus), possible obscuration of the basal ganglia, and, importantly, no acute intraparenchymal (IPH), SAH, or subdural hemorrhage.
Acute Treatments
After the patient's head CT is completed, the next steps are dependent upon what was seen on the scan and the time from symptom onset.
Blood on the CT Scan
If the initial brain imaging reveals IPH or SAH, further diagnostic testing and early treatments are quite different than for ischemic stroke. New guidelines are available for IPH management,9 and there have been recent review articles of care for SAH.1012 At the authors' institutions, early care of such patients always involves aggressive reversal of any antithrombotic medications the patient was taking prior to presentation. Our approach to warfarin reversal includes vitamin K and fresh frozen plasma (FFP) to achieve an INR 1.4; others have used prothrombin complex concentrate (PCC).13 Blood pressure (BP) treatment goals are generally more aggressive than for ischemic stroke, while supportive care to avoid aspiration, hyperglycemia, fever, and venous thrombosis (here initially with sequential compression devices alone) are similar. Early estimation of prognosis for these patients with IPH and SAH and discussions with families about continued aggressive care are of utmost importance, and should involve providers with sufficient expertise. Care should be taken to avoid overly pessimistic early prognostication, as early do not resuscitate (DNR) decisions in intercranial hemorrhage (ICH) can become a self‐fulfilling prophecy.1416 If the decision is to continue aggressive and supportive care, or if an appropriately expert consultation is not available at the presentation hospital, IPH and SAH patients should be considered for transfer to a hospital with the appropriate resources (including emergency access to neurosurgeons) or be evaluated by such an expert by telemedicine if available.
No Blood on the CT Scan, Results Back in <3 Hours From Symptom Onset
If such a patient is not rapidly resolving their symptoms, and the diagnosis continues to remain clear, inclusion/exclusion criteria for IV tPA should be reviewed (Table 1). Consent should be obtained much like any other procedure with significant risk. As many consider tPA to be standard of care, it is reasonable to proceed in cases of unobtainable consent as one would with any other emergent therapy. This situation is a topic of ongoing debate.17, 18 The Guidelines state that although written consent is not necessary before administration of recombinant tPA (rtPA) for treatment of stroke, a full discussion of the potential risks and benefits of treatment with rtPA with the family and the patient if possible is recommended (p. 1676).4 After tPA is given in the ER, the patient should be admitted to an intensive care unit (ICU) setting for 24 hours for careful monitoring of BP, avoidance of invasive procedures, and no use of antithrombotic medications during that period of time.
| Comments (from the authors) | |
|---|---|
| |
| Inclusion criteria | |
| Diagnosis of ischemic stroke causing measurable neurological deficit | Usually NIHSS > 4 |
| Neurological signs should not be clearing spontaneously | Such a patient may do well without tPA, but there is debate82 |
| Neurological signs should not be minor and isolated. | |
| Onset of symptoms >3 hours before beginning treatment | |
| Patient or family members understand the potential risks and benefits from treatment | Debated, as tPA considered standard of care by many |
| Cautionary criteria | |
| Caution should be exercised in treating a patient with major deficits | Higher risk of hemorrhage, but still may benefit from treatment |
| Exclusion criteria | |
| Symptoms of stroke should not be suggestive of subarachnoid hemorrhage | |
| No head trauma or prior stroke in previous 3 months | |
| No myocardial infarction in the previous 3 months | |
| No gastrointestinal or urinary tract hemorrhage in previous 21 days | |
| No major surgery in the previous 14 days | |
| No arterial puncture at a noncompressible site in the previous 7 days | |
| No history of previous intracranial hemorrhage | |
| Blood pressure not elevated (systolic >185 mm Hg or diastolic 110 mm Hg) | Okay to bring down with labetolol, nitropaste, or nicardipine* |
| No evidence of active bleeding or acute trauma (fracture) on examination | |
| Not taking an oral anticoagulant or, if anticoagulant being taken, INR 1.7 | |
| If receiving heparin in previous 48 hours, aPTT must be in normal range | |
| Platelet count <100,000 mm3 | |
| Blood glucose concentration <50 mg/dL (2.7 mmol/L) | |
| Seizure with postictal residual neurological impairments | Not absolute if treating physician feels stroke also present, or if confirmed by imaging |
| CT does not show a multilobar infarction (hypodensity >1/3 cerebral hemisphere) | Not strictly evidence based, in NINDS trial this finding did not preclude benefit of tPA |
Based mainly on the results of the National Institute of Neurological Disorders and Stroke (NINDS) tPA trial,19 and recently supported by a large Phase IV observational study from the European Union,20 IV tPA for acute ischemic stroke is approved for use in many countries and is endorsed for the treatment of carefully selected ischemic stroke patients in a number of practice guidelines.4 Despite this, the emergency medicine community has been less enthusiastic about the use of IV tPA.21, 22 Although the risk of hemorrhagic complications is greater in certain subgroups of patients (ie, the most severe strokes, significant early CT changes, older age), there is no definitive evidence to suggest that these groups do not still benefit from the treatment.23 It is also clear that if patients are not carefully selected, meeting strict inclusion and exclusion criteria, the rate of complications is increased.24 Thus, as summarized in a practice statement of the American College of Emergency Physicians, There is insufficient evidence at this time to endorse the use of intravenous tPA in clinical practice when systems are not in place to ensure that the inclusion/exclusion criteria established by the NINDS guidelines for tPA use in acute stroke are followed.21 When counseling patients and their families about the benefits and risks of IV tPA, one should keep in mind that the NINDS trial demonstrated increased odds of excellent outcomes despite a significant 10‐fold increase in the risk of symptomatic intracranial hemorrhage (6.4% vs. 0.6%), and did not alter 30‐day mortality. The largest Phase IV cohort study of IV tPA treatment, Safe Implementation of Thrombolysis in Stroke Monitoring Study (SITS‐MOST) was mandated by the European Union upon approval of the medication for use in acute ischemic stroke.20 The results in 6483 patients showed that tPA, when used in strict accordance with published inclusion and exclusion criteria, could perform as well as it did in randomized trials.
The recently published European Cooperative Acute Stroke Study3 (ECASS‐3) trial demonstrated that IV tPA has efficacy with adequate safety up to 4.5 hours after the onset of symptoms. A total of 821 patients were enrolled and 375 received tPA. Exclusion criteria included diabetes being treated with medication with a history of prior stroke, an NIHSS score >25, or treatment with warfarin. The rates of hemorrhage (27.0% vs. 17.6%, P = 0.001) were in line with those of the SITS‐MOST study patients who were treated within the 3‐hour time window. There was no significant difference in mortality (7.7% tPA vs. 8.4% placebo). This study is relatively new; therefore, the data have not been reviewed by guideline committees.25
No Blood on the CT Scan, Results Back in >3 Hours, but 8 Hours, From Symptom Onset
Unfortunately as with our patient, most people do not present to an ER in a timely fashion. Nonetheless, there may be other treatments and interventions possible. If the patient arrives <8 hours from onset of symptoms, intraarterial (IA) interventions are a possibility. In such a case, a CT angiogram (CTA) of the neck from the arch of the aorta to the circle of Willis is recommended (barring any contraindications such as renal failure or iodine allergy). The rationale behind this study is that other treatment options, such as IA tPA or mechanical thrombectomy may be considered if a large arterial occlusion is identified. CTA is preferred over magnetic resonance angiography (MRA) due to the same time and patient cooperation issues mentioned above, though some expert centers may be set up to perform MRI and MRA rapidly in the acute setting. CTA or MRA is of great value early on in the emergent assessment of ischemic stroke patients, as it allows detailed evaluation of the cerebral vasculature; this knowledge helps define the pathophysiology of the ongoing stroke (eg, is there a larger artery occlusion?) and can help inform the approach to subsequent therapies.
The Guidelines (p. 1678)4 recommend IA thrombolysis as a treatment option if it can be started within 6 hours, based on results from the Prolyse in Acute Cerebral Thromboembolism (PROACT) II trial. This study involved angiography with identification of the occluded vessel (the proximal MCA‐M1 in this study) and administration of recombinant pro‐urokinase to the clot with functional outcome as the primary endpoint.26 At 3 months, patients who received the IA thrombolytic had a 40% chance of slight disability; unable to carry out all previous activities, but able to look after own affairs without assistance or better (ie, a modified Rankin Scale score of 2) vs. 25% of those not receiving the IA thrombolytic. Pro‐urokinase is not available in the United States; therefore, many institutions substitute IA tPA. The Guidelines further state that IA thrombolysis can be considered for use in some patients with contraindications to IV tPA (eg, recent surgery), but should not be used instead of IV tPA in patients otherwise eligible (p. 1678).4
There are now two U.S. Food and Drug Administration (FDA)‐approved devices for mechanical cerebral vasculature thrombectomy for use up to 8 hours from symptom onset. The mechanical embolus removal in cerebral ischemia (MERCI) clot retrieval device was originally approved by the FDA in August 2004 for restoring blood flow in the neurovasculature by removing thrombus in patients experiencing ischemic stroke. Modified devices have been approved as recently as January 2007.27 The Penumbra System was FDA‐approved in December 2007 for revascularization of patients with acute ischemic stroke secondary to intracranial large vessel occlusive disease.28 In both cases, the FDA approval was based on demonstration of safety in case series of patients treated with the devices.2931 No randomized trials have shown the use of these devices improves outcomes for stroke patients. The Guidelines state that Although the MERCI device is a reasonable intervention for extraction of IA thrombi in carefully selected patients, the panel also recognizes that the utility of the device in improving outcomes after stroke is unclear (p. 1684);4 this statement applies similarly to the Penumbra device.
More complex imaging techniques, including multimodal CT (CT, CTA, and CT perfusion) and MR (MRI with diffusion, MRA, and MR perfusion) are being used in some stroke centers to make decisions about acute ischemic stroke treatments.32, 33 The theory is that by using these techniques, one can determine the presence or absence of a mismatch, whereby the perfusion imaging suggests more tissue at risk of infarction than is seen as already abnormal on MR diffusion‐weighted images or compared to a clinical assessment. These mismatch patients are then seen as appropriate candidates for the more aggressive interventions (ie, late IV tPA or IA interventions).34 Unfortunately, the 2 largest randomized trials to look at this issue with respect to >3‐hour IV tPA both failed to show a benefit for patients selected in this manner.35, 36 Standardized definitions of mismatch are still needed, and larger randomized trials are needed before this approach can be suggested for routine care.3739
More complex interventions, available only at tertiary or comprehensive stroke centers, include a bridging approach in which IV tPA (at 2/3 standard dose) is followed by IA tPA, IV tPA with transcranial Doppler (TCD)‐enhanced thrombolysis or IA rescue thrombectomy when vascular imaging after IV tPA shows a persistent large artery occlusion. The Guidelines suggests that these more complex combinations of interventions to restore perfusion cannot be recommended outside the setting of clinical trials (p. 1685).4
No Blood on the CT Scan, Results Back in >8 Hours From Symptom Onset (or if Contraindications to Above Interventions)
This time frame takes the more aggressive interventions off the table. Per the Guidelines, 325 mg of aspirin is the default antiplatelet agent for use, and has been shown in 2 very large randomized trials to reduce early death and longer‐term disability vs. placebo after acute ischemic stroke.40, 41 Importantly, all patients who do not qualify for thrombolysis in the 0‐hour to 8‐hour time window should receive aspirin.
Although a number of small or pilot studies suggest a benefit of the addition of clopidogrel to aspirin for a period (13 months) immediately after ischemic stroke,4244 this more aggressive antiplatelet intervention is not an endorsed standard of care. As described below, the long‐term use of this antiplatelet combination has been consistently associated with a higher risk of hemorrhagic complications. There are no published data regarding the use of aspirin plus dipyridamole in the acute stroke setting. A number of randomized trials have now been performed that have consistently failed to show a benefit of heparin, or heparin‐like medications, for the routine treatment of acute ischemic stroke. Despite this, a number of exceptions exist, based more on tradition and theory than on evidence. These exceptions, for which an IV heparin drip will at times still be considered, include acute ischemic stroke due to dissection of the carotid or vertebral arteries, cardioembolic stroke with fresh clot seen on echocardiogram (ECHO), and a clinically progressive syndrome suggestive of basilar artery occlusion (see below).45, 46 Good evidence exists to specifically recommend the use of full‐dose heparin in the setting of cerebral venous sinus thrombosis.47
Basilar Artery Occlusion Syndromes
Basilar artery occlusion syndromes warrant special mention. These may involve patients who present with quadriparesis, altered mental status, vertigo, diplopia, and other brainstem signs. Conventional treatment of basilar artery occlusion has been associated with 40% mortality with 65% of survivors having severe disability.48 If suspected, an urgent CTA can usually confirm the diagnosis, and urge the clinician to expeditiously consider aggressive intervention. Only case series have been reported regarding basilar artery thrombosis and acute treatments. Based on these studies, it is generally agreed upon that patients who appear comatose or quadriplegic for more than 3 hours will likely have a very poor functional outcome regardless of treatment, and interventional treatment is withheld. If a basilar occlusion patient presents within the 3‐hour time window for IV tPA, they are thus treated, with follow‐up vascular imaging, and possible rescue IA mechanical thrombectomy if recanalization from the IV tPA does not occur. However, if the patient still has preserved neurologic function, or is waxing and waning, there is no clear time limit for IA interventions and they may be useful a day or more after presentation. For basilar occlusion patients with severe stenoses not responsive to lysis, or continuing to be symptomatic, angioplasty and stenting has also been used.46 Despite a lack of evidence, many stroke clinicians will use an IV heparin drip for treatment of acute basilar occlusive disease.
Malignant Middle Cerebral Artery (MCA) Infarction
Malignant MCA infarction is another specific clinical syndrome worthy of special consideration. It is most generally defined as a large infarction (1/2 or 2/3) of the MCA territory, somewhat depressed level of consciousness, and high stroke scale scores (ie, severe deficits) that goes on to severe cerebral edema, mass effect, and often herniation with death.49, 50 Associated patient characteristics include younger age, abnormal (incomplete) ipsilateral collateral circulation, and internal carotid artery occlusion.51 Maximal edema occurs 2 to 5 days from stroke onset and, despite best intensive therapy, has been associated with mortality rates of 70% to 80%.49, 50 A recent pooling of 3 small randomized trials of early decompressive hemicraniectomy and durotomy showed a 50% absolute risk reduction for mortality and a 23% absolute benefit in long‐term independence (modified Rankin scale 3).49 This treatment option should be strongly considered in carefully selected patients., Transfer to an appropriately equipped facility should be offered if not available at your hospital.
Returning to our case patient, upon arrival to the ED with symptoms of partial aphasia, right hemiplegia, and left gaze preference, there was a high suspicion for a left MCA stroke. Unfortunately, he was excluded from receiving IV tPA or any other interventions, as the last time he was known to be neurologically intact was the prior evening, which is taken to be the time of onset. Antiplatelet therapy was continued, and the patient was admitted for further workup.
The initial care of the patient with a cerebrovascular event is often quite complicated. Assimilation of a great deal of data must occur and decisions around therapy must be made in a timely fashion. In prior years there was little to offer in the way of therapy, which also meant there was little initial potential for iatrogenic complication. Both diagnostic and therapeutic options are evolving rapidly. We now have much to offer these patients both emergently and in areas of secondary prevention. In part 2 of this article, the patient's inpatient course and therapy will be reviewed.
- Organization WH. MONICA Manual, Part IV: Event Registration. Available at: http://www.ktl.fi/publications/monica/manual/part4/iv‐2.htm#s2. Accessed May2009.
- ,,, et al.Heart disease and stroke statistics 2008 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.200829;117(4):e25–e146.
- .Neurology in the next two decades: report of the Workforce Task Force of the American Academy of Neurology.Neurology.2000;54(4):787–789.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists.Stroke.2007;38(5):1655–1711.
- ,,,.Conditions that mimic stroke in the emergency department. Implications for acute stroke trials.Arch Neurol.1995;52(11):1119–1122.
- ,,,,.Distinguishing between stroke and mimic at the bedside: the brain attack study.Stroke.2006;37(3):769–775.
- ,,, et al.Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: a multicenter study on the validity of stroke imaging.Stroke.2004;35(2):502–506.
- ,,, et al.Comparison of MRI and CT for detection of acute intracerebral hemorrhage.JAMA.2004;292(15):1823–1830.
- ,,, et al.Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group.Stroke.2007;38(6):2001–2023.
- ,,.Aneurysmal subarachnoid hemorrhage.N Engl J Med.2006;354(4):387–396.
- ,,,.Subarachnoid haemorrhage.BMJ.2006;333(7561):235–240.
- ,,.Subarachnoid haemorrhage.Lancet.2007;369(9558):306–318.
- ,,.Intracerebral hemorrhage associated with oral anticoagulant therapy: current practices and unresolved questions.Stroke.2006;37(1):256–262.
- ,,, et al.Withdrawal of support in intracerebral hemorrhage may lead to self‐fulfilling prophecies.Neurology.2001;56(6):766–772.
- ,,,.Hospital usage of early do‐not‐resuscitate orders and outcome after intracerebral hemorrhage.Stroke.2004;35(5):1130–1134.
- ,,, et al.Early care limitations independently predict mortality after intracerebral hemorrhage.Neurology.2007;68(20):1651–1657.
- ,,,.Consent for intravenous thrombolysis in acute stroke: review and future directions.Arch Neurol.2007;64(6):785–792.
- .Thrombolysis (tissue plasminogen activator) in stroke: a medicolegal quagmire.Stroke.2006;37(7):1917–1922.
- Tissue plasminogen activator for acute ischemic stroke.The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group.N Engl J Med.1995;333(24):1581–1587.
- ,,, et al.Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke‐Monitoring Study (SITS‐MOST): an observational study.Lancet.2007;369(9558):275–282.
- American College of Emergency Physicians (ACEP). Use of Intravenous tPA for the Management of Acute Stroke in the Emergency Department. ACEP Policy Statement. February 2002. Available at: http://www.acep.org/practres.aspx?id=29834. Accessed May2009.
- American Academy of Emergency Medicine (AAEM). Position statement on the use of intravenous thrombolytic therapy in the treatment of stroke. January 2002. Available at: http://aaem.org/positionstatements/thrombolytictherapy.php. Accessed May2009.
- ,,, et al.Lack of clinical significance of early ischemic changes on computed tomography in acute stroke.JAMA.2001;286(22):2830–2838.
- ,,,,.Thrombolysis for acute stroke in routine clinical practice.Arch Intern Med.2002;162(17):1994–2001.
- ,,, et al.Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke.N Engl J Med.2008;359:1317–1329,1393–1395.
- ,,, et al.Intra‐arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism.JAMA.1999;282(21):2003–2011.
- Modified MERCI Retriever FDA marketing approval letter. Available at: www.fda.gov/cdrh/pdf6/K062046.pdf. Accessed May2009.
- Penumbra System FDA marketing approval letter. Available at: www.fda.gov/cdrh/pdf7/K072718.pdf. Accessed May2009.
- .Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the multi mechanical embolus removal in cerebral ischemia (MERCI) trial, part I.AJNR Am J Neuroradiol.2006;27(6):1177–1182.
- ,,, et al.Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial.Stroke.2005;36(7):1432–1438.
- ,,, et al.The Penumbra System: a mechanical device for the treatment of acute stroke due to thromboembolism.AJNR Am J Neuroradiol.2008;29(7):1409–1413.
- ,,, et al.Comparison of CT perfusion and angiography and MRI in selecting stroke patients for acute treatment.Neurology.2007;68(9):694–697.
- ,,, et al.Combined intravenous and intraarterial revascularization therapy using MRI perfusion/diffusion mismatch selection for acute ischemic stroke at 3–6 h after symptom onset.Neurocrit Care.2008;8(3):353–359.
- ,,, et al.Refining the perfusion‐diffusion mismatch hypothesis.Stroke.2005;36(6):1153–1159.
- . DIAS‐2: no benefit of desmoteplase in acute ischemic stroke. Available at: www.medscape.com/viewarticle/557663. Accessed May2009.
- ,,, et al.Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo‐controlled randomised trial.Lancet Neurol.2008;7(4):299–309.
- ,,.Magnetic resonance perfusion diffusion mismatch and thrombolysis in acute ischaemic stroke: a systematic review of the evidence to date.J Neurol Neurosurg Psychiatry.2007;78(5):485–491.
- ,,, et al.Optimal definition for PWI/DWI mismatch in acute ischemic stroke patients.J Cereb Blood Flow Metab.2008;28(5):887–891.
- ,,, et al.Rapid assessment of perfusion‐diffusion mismatch.Stroke.2008;39(1):75–81.
- CAST: randomised placebo‐controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke.CAST (Chinese Acute Stroke Trial) Collaborative Group.Lancet.1997;349(9066):1641–1649.
- The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke.International Stroke Trial Collaborative Group.Lancet.1997;349(9065):1569–1581.
- ,,, et al.Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: the clopidogrel and aspirin for reduction of emboli in symptomatic carotid stenosis (CARESS) trial.Circulation.2005;111(17):2233–2240.
- ,,, et al.Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population‐based sequential comparison.Lancet.2007;370(9596):1432–1442.
- ,,,,,.Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial.Lancet Neurol.2007;6(11):961–969.
- ,,, et al.Antiplatelets versus anticoagulation in cervical artery dissection.Stroke.2007;38(9):2605–2611.
- ,,.Basilar artery occlusion.Neurocrit Care.2004;1(3):319–329.
- ,.Cerebral venous thrombosis: an update.Lancet Neurol.2007;6(2):162–170.
- ,,,,.Outcome in patients with basilar artery occlusion treated conventionally.J Neurol Neurosurg Psychiatry.2005;76(9):1238–1241.
- ,,, et al.Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials.Lancet Neurol.2007;6(3):215–222.
- ,,,,,.‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs.Arch Neurol.1996;53(4):309–315.
- ,,,,.Predictors for malignant middle cerebral artery infarctions: a postmortem analysis.Neurology. 282006;66(6):815–820.
- ,,,,,.Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke.Stroke.2005;36(11):2497–2499.
The term stroke is defined by the World Health Organization as rapidly developed clinical signs of focal (or global) disturbance of cerebral function lasting more than 24 hours (unless interrupted by surgery or death), with no apparent cause other than a vascular origin; it includes patients presenting clinical signs and symptoms suggestive of subarachnoid hemorrhage (SAH), intracerebral hemorrhage, or cerebral ischemic necrosis.1 Stroke is 1 of the leading causes of death and the number 1 cause of long‐term disability in the United States, with over 700,000 strokes and over 150,000 stroke deaths each year.2
Given the projections of 30,000 hospitalists nationally by 2010 (
Case Presentation
A 76‐year‐old right‐handed male with a history of hyperlipidemia and myocardial infarction was found at 7 AM with right‐sided paralysis and poor responsiveness on the morning of admission. He seemed to prefer looking to the left and to understand what was being said to him, but had great difficulty speaking. When he went to bed at 9 PM, he was at his neurological baseline. Upon finding him that morning, his wife called 911.
With increased knowledge regarding the pathophysiology of stroke, it has become clear that timeliness is of utmost importance (time is brain) and that acute stroke should be regarded as an acute medical/neurological emergency.
This article reviews the approach in evaluating an acute stroke patient, management strategies, and treatment options. Where not otherwise referenced, data to support our comments come from the recently updated and exhaustive American Heart Association (AHA)/American Stroke Association (ASA) Guidelines for the Early Management of Adults With Ischemic Stroke and will be referred to herein as the Guidelines.4 Harborview Medical Center in Seattle is a Joint Commissioncertified Primary Stroke Center and the home hospital of 2 of the authors (C.L.E., D.L.T.); it is referred to herein as Harborview.
Emergency Room Care (see Acute Stroke Algorithm, Figure 1)
The First 15 Minutes
After assuring stable airway, breathing, and circulation, immediate (STAT) blood draws should be performed, including full complete blood count (CBC) with platelets, international normalized ratio/prothrombin time/partial thromboplastin time (INR/PT/PTT), full electrolytes, and glucose (finger‐stick blood glucose also recommended). Glasgow Coma Scale (GCS) score and NIH Stroke Scale (NIHSS) score should be established via a focused history and physical exam. The GCS is most appropriate for patients with a significantly depressed level of consciousness, while the NIHSS can be scored for any stroke patient (1‐page version of NIHSS used at Harborview is shown in Figure 2). By quantifying stroke severity, the NIHSS score helps both to facilitate communication about neurologic deficit as well as serve as a documented baseline in case of subsequent clinical change. Emergency department (ED) physicians, hospitalists, neurologists, and nursing staff regularly caring for acute stroke patients would be well‐served by obtaining certification in the NIHSS (available free online at


Our case patient's initial NIHSS score was 15, with points given for drowsiness, inability to answer questions, partial facial palsy, no movement in right arm or leg, mild‐moderate aphasia, and mild‐moderate dysarthria (Figure 2).
Differential Diagnosis
Many acute conditions can mimic stroke, and 1 of the goals of the initial emergency room (ER) evaluation is to rule out such stroke mimics. A report of 411 initial ER stroke diagnoses identified 19% as stroke mimics; the most common mimic diagnoses were seizure, systemic infection, brain tumor, and toxic‐metabolic.5 The same study identified decreased level of alertness as associated with a final mimic diagnosis and history of angina as associated with a final diagnosis of stroke. Another study looked at 350 presentations with an initial stroke diagnosis and found 31% stroke mimics; similarly, the main alternative diagnoses were seizure, sepsis, toxic‐metabolic, space‐occupying lesion, and syncope/presyncope.6 Findings associated with a mimic diagnosis included no cognitive impairment and abnormal findings in any other system, while findings associated with a stroke diagnosis were a definite history of focal neurological symptoms, NIHSS score, stroke type classification possible, an exact onset that could be determined, and abnormal vascular findings on imaging.6
Initial Imaging
The patient should receive a STAT noncontrast head CT to evaluate for the presence or absence of blood. At this time, magnetic resonance imaging (MRI) is not essential to confirm the diagnosis of ischemic stroke, as diagnosis is based on clinical suspicion. MRI is more sensitive at imaging acute ischemia (on diffusion‐weighted sequences) and recently has been shown to be equally sensitive in identifying acute blood (previously thought to be a relative advantage of CT).7, 8 Practical and pervasive barriers to emergent MRI include study duration, significant patient cooperation, and that few hospitals are currently set up to perform such rapid MRIS. The Guidelines specifically state that In most instances, CT will provide the information to make decisions about emergency management (p. 1668),4 that vascular imaging should not delay treatment of patients whose symptoms started <3 hours ago and who have acute ischemic stroke, and that emergency treatment of stroke should not be delayed in order to obtain multimodal imaging studies (p. 1669).4
Our case patient's initial imaging, a noncontrast head CT (Supporting Figures 1 and 2), showed subtle clues consistent with the diagnosis of acute ischemic stroke. These include a hyperdense middle cerebral artery (MCA) sign (presumably representing thrombus), possible obscuration of the basal ganglia, and, importantly, no acute intraparenchymal (IPH), SAH, or subdural hemorrhage.
Acute Treatments
After the patient's head CT is completed, the next steps are dependent upon what was seen on the scan and the time from symptom onset.
Blood on the CT Scan
If the initial brain imaging reveals IPH or SAH, further diagnostic testing and early treatments are quite different than for ischemic stroke. New guidelines are available for IPH management,9 and there have been recent review articles of care for SAH.1012 At the authors' institutions, early care of such patients always involves aggressive reversal of any antithrombotic medications the patient was taking prior to presentation. Our approach to warfarin reversal includes vitamin K and fresh frozen plasma (FFP) to achieve an INR 1.4; others have used prothrombin complex concentrate (PCC).13 Blood pressure (BP) treatment goals are generally more aggressive than for ischemic stroke, while supportive care to avoid aspiration, hyperglycemia, fever, and venous thrombosis (here initially with sequential compression devices alone) are similar. Early estimation of prognosis for these patients with IPH and SAH and discussions with families about continued aggressive care are of utmost importance, and should involve providers with sufficient expertise. Care should be taken to avoid overly pessimistic early prognostication, as early do not resuscitate (DNR) decisions in intercranial hemorrhage (ICH) can become a self‐fulfilling prophecy.1416 If the decision is to continue aggressive and supportive care, or if an appropriately expert consultation is not available at the presentation hospital, IPH and SAH patients should be considered for transfer to a hospital with the appropriate resources (including emergency access to neurosurgeons) or be evaluated by such an expert by telemedicine if available.
No Blood on the CT Scan, Results Back in <3 Hours From Symptom Onset
If such a patient is not rapidly resolving their symptoms, and the diagnosis continues to remain clear, inclusion/exclusion criteria for IV tPA should be reviewed (Table 1). Consent should be obtained much like any other procedure with significant risk. As many consider tPA to be standard of care, it is reasonable to proceed in cases of unobtainable consent as one would with any other emergent therapy. This situation is a topic of ongoing debate.17, 18 The Guidelines state that although written consent is not necessary before administration of recombinant tPA (rtPA) for treatment of stroke, a full discussion of the potential risks and benefits of treatment with rtPA with the family and the patient if possible is recommended (p. 1676).4 After tPA is given in the ER, the patient should be admitted to an intensive care unit (ICU) setting for 24 hours for careful monitoring of BP, avoidance of invasive procedures, and no use of antithrombotic medications during that period of time.
| Comments (from the authors) | |
|---|---|
| |
| Inclusion criteria | |
| Diagnosis of ischemic stroke causing measurable neurological deficit | Usually NIHSS > 4 |
| Neurological signs should not be clearing spontaneously | Such a patient may do well without tPA, but there is debate82 |
| Neurological signs should not be minor and isolated. | |
| Onset of symptoms >3 hours before beginning treatment | |
| Patient or family members understand the potential risks and benefits from treatment | Debated, as tPA considered standard of care by many |
| Cautionary criteria | |
| Caution should be exercised in treating a patient with major deficits | Higher risk of hemorrhage, but still may benefit from treatment |
| Exclusion criteria | |
| Symptoms of stroke should not be suggestive of subarachnoid hemorrhage | |
| No head trauma or prior stroke in previous 3 months | |
| No myocardial infarction in the previous 3 months | |
| No gastrointestinal or urinary tract hemorrhage in previous 21 days | |
| No major surgery in the previous 14 days | |
| No arterial puncture at a noncompressible site in the previous 7 days | |
| No history of previous intracranial hemorrhage | |
| Blood pressure not elevated (systolic >185 mm Hg or diastolic 110 mm Hg) | Okay to bring down with labetolol, nitropaste, or nicardipine* |
| No evidence of active bleeding or acute trauma (fracture) on examination | |
| Not taking an oral anticoagulant or, if anticoagulant being taken, INR 1.7 | |
| If receiving heparin in previous 48 hours, aPTT must be in normal range | |
| Platelet count <100,000 mm3 | |
| Blood glucose concentration <50 mg/dL (2.7 mmol/L) | |
| Seizure with postictal residual neurological impairments | Not absolute if treating physician feels stroke also present, or if confirmed by imaging |
| CT does not show a multilobar infarction (hypodensity >1/3 cerebral hemisphere) | Not strictly evidence based, in NINDS trial this finding did not preclude benefit of tPA |
Based mainly on the results of the National Institute of Neurological Disorders and Stroke (NINDS) tPA trial,19 and recently supported by a large Phase IV observational study from the European Union,20 IV tPA for acute ischemic stroke is approved for use in many countries and is endorsed for the treatment of carefully selected ischemic stroke patients in a number of practice guidelines.4 Despite this, the emergency medicine community has been less enthusiastic about the use of IV tPA.21, 22 Although the risk of hemorrhagic complications is greater in certain subgroups of patients (ie, the most severe strokes, significant early CT changes, older age), there is no definitive evidence to suggest that these groups do not still benefit from the treatment.23 It is also clear that if patients are not carefully selected, meeting strict inclusion and exclusion criteria, the rate of complications is increased.24 Thus, as summarized in a practice statement of the American College of Emergency Physicians, There is insufficient evidence at this time to endorse the use of intravenous tPA in clinical practice when systems are not in place to ensure that the inclusion/exclusion criteria established by the NINDS guidelines for tPA use in acute stroke are followed.21 When counseling patients and their families about the benefits and risks of IV tPA, one should keep in mind that the NINDS trial demonstrated increased odds of excellent outcomes despite a significant 10‐fold increase in the risk of symptomatic intracranial hemorrhage (6.4% vs. 0.6%), and did not alter 30‐day mortality. The largest Phase IV cohort study of IV tPA treatment, Safe Implementation of Thrombolysis in Stroke Monitoring Study (SITS‐MOST) was mandated by the European Union upon approval of the medication for use in acute ischemic stroke.20 The results in 6483 patients showed that tPA, when used in strict accordance with published inclusion and exclusion criteria, could perform as well as it did in randomized trials.
The recently published European Cooperative Acute Stroke Study3 (ECASS‐3) trial demonstrated that IV tPA has efficacy with adequate safety up to 4.5 hours after the onset of symptoms. A total of 821 patients were enrolled and 375 received tPA. Exclusion criteria included diabetes being treated with medication with a history of prior stroke, an NIHSS score >25, or treatment with warfarin. The rates of hemorrhage (27.0% vs. 17.6%, P = 0.001) were in line with those of the SITS‐MOST study patients who were treated within the 3‐hour time window. There was no significant difference in mortality (7.7% tPA vs. 8.4% placebo). This study is relatively new; therefore, the data have not been reviewed by guideline committees.25
No Blood on the CT Scan, Results Back in >3 Hours, but 8 Hours, From Symptom Onset
Unfortunately as with our patient, most people do not present to an ER in a timely fashion. Nonetheless, there may be other treatments and interventions possible. If the patient arrives <8 hours from onset of symptoms, intraarterial (IA) interventions are a possibility. In such a case, a CT angiogram (CTA) of the neck from the arch of the aorta to the circle of Willis is recommended (barring any contraindications such as renal failure or iodine allergy). The rationale behind this study is that other treatment options, such as IA tPA or mechanical thrombectomy may be considered if a large arterial occlusion is identified. CTA is preferred over magnetic resonance angiography (MRA) due to the same time and patient cooperation issues mentioned above, though some expert centers may be set up to perform MRI and MRA rapidly in the acute setting. CTA or MRA is of great value early on in the emergent assessment of ischemic stroke patients, as it allows detailed evaluation of the cerebral vasculature; this knowledge helps define the pathophysiology of the ongoing stroke (eg, is there a larger artery occlusion?) and can help inform the approach to subsequent therapies.
The Guidelines (p. 1678)4 recommend IA thrombolysis as a treatment option if it can be started within 6 hours, based on results from the Prolyse in Acute Cerebral Thromboembolism (PROACT) II trial. This study involved angiography with identification of the occluded vessel (the proximal MCA‐M1 in this study) and administration of recombinant pro‐urokinase to the clot with functional outcome as the primary endpoint.26 At 3 months, patients who received the IA thrombolytic had a 40% chance of slight disability; unable to carry out all previous activities, but able to look after own affairs without assistance or better (ie, a modified Rankin Scale score of 2) vs. 25% of those not receiving the IA thrombolytic. Pro‐urokinase is not available in the United States; therefore, many institutions substitute IA tPA. The Guidelines further state that IA thrombolysis can be considered for use in some patients with contraindications to IV tPA (eg, recent surgery), but should not be used instead of IV tPA in patients otherwise eligible (p. 1678).4
There are now two U.S. Food and Drug Administration (FDA)‐approved devices for mechanical cerebral vasculature thrombectomy for use up to 8 hours from symptom onset. The mechanical embolus removal in cerebral ischemia (MERCI) clot retrieval device was originally approved by the FDA in August 2004 for restoring blood flow in the neurovasculature by removing thrombus in patients experiencing ischemic stroke. Modified devices have been approved as recently as January 2007.27 The Penumbra System was FDA‐approved in December 2007 for revascularization of patients with acute ischemic stroke secondary to intracranial large vessel occlusive disease.28 In both cases, the FDA approval was based on demonstration of safety in case series of patients treated with the devices.2931 No randomized trials have shown the use of these devices improves outcomes for stroke patients. The Guidelines state that Although the MERCI device is a reasonable intervention for extraction of IA thrombi in carefully selected patients, the panel also recognizes that the utility of the device in improving outcomes after stroke is unclear (p. 1684);4 this statement applies similarly to the Penumbra device.
More complex imaging techniques, including multimodal CT (CT, CTA, and CT perfusion) and MR (MRI with diffusion, MRA, and MR perfusion) are being used in some stroke centers to make decisions about acute ischemic stroke treatments.32, 33 The theory is that by using these techniques, one can determine the presence or absence of a mismatch, whereby the perfusion imaging suggests more tissue at risk of infarction than is seen as already abnormal on MR diffusion‐weighted images or compared to a clinical assessment. These mismatch patients are then seen as appropriate candidates for the more aggressive interventions (ie, late IV tPA or IA interventions).34 Unfortunately, the 2 largest randomized trials to look at this issue with respect to >3‐hour IV tPA both failed to show a benefit for patients selected in this manner.35, 36 Standardized definitions of mismatch are still needed, and larger randomized trials are needed before this approach can be suggested for routine care.3739
More complex interventions, available only at tertiary or comprehensive stroke centers, include a bridging approach in which IV tPA (at 2/3 standard dose) is followed by IA tPA, IV tPA with transcranial Doppler (TCD)‐enhanced thrombolysis or IA rescue thrombectomy when vascular imaging after IV tPA shows a persistent large artery occlusion. The Guidelines suggests that these more complex combinations of interventions to restore perfusion cannot be recommended outside the setting of clinical trials (p. 1685).4
No Blood on the CT Scan, Results Back in >8 Hours From Symptom Onset (or if Contraindications to Above Interventions)
This time frame takes the more aggressive interventions off the table. Per the Guidelines, 325 mg of aspirin is the default antiplatelet agent for use, and has been shown in 2 very large randomized trials to reduce early death and longer‐term disability vs. placebo after acute ischemic stroke.40, 41 Importantly, all patients who do not qualify for thrombolysis in the 0‐hour to 8‐hour time window should receive aspirin.
Although a number of small or pilot studies suggest a benefit of the addition of clopidogrel to aspirin for a period (13 months) immediately after ischemic stroke,4244 this more aggressive antiplatelet intervention is not an endorsed standard of care. As described below, the long‐term use of this antiplatelet combination has been consistently associated with a higher risk of hemorrhagic complications. There are no published data regarding the use of aspirin plus dipyridamole in the acute stroke setting. A number of randomized trials have now been performed that have consistently failed to show a benefit of heparin, or heparin‐like medications, for the routine treatment of acute ischemic stroke. Despite this, a number of exceptions exist, based more on tradition and theory than on evidence. These exceptions, for which an IV heparin drip will at times still be considered, include acute ischemic stroke due to dissection of the carotid or vertebral arteries, cardioembolic stroke with fresh clot seen on echocardiogram (ECHO), and a clinically progressive syndrome suggestive of basilar artery occlusion (see below).45, 46 Good evidence exists to specifically recommend the use of full‐dose heparin in the setting of cerebral venous sinus thrombosis.47
Basilar Artery Occlusion Syndromes
Basilar artery occlusion syndromes warrant special mention. These may involve patients who present with quadriparesis, altered mental status, vertigo, diplopia, and other brainstem signs. Conventional treatment of basilar artery occlusion has been associated with 40% mortality with 65% of survivors having severe disability.48 If suspected, an urgent CTA can usually confirm the diagnosis, and urge the clinician to expeditiously consider aggressive intervention. Only case series have been reported regarding basilar artery thrombosis and acute treatments. Based on these studies, it is generally agreed upon that patients who appear comatose or quadriplegic for more than 3 hours will likely have a very poor functional outcome regardless of treatment, and interventional treatment is withheld. If a basilar occlusion patient presents within the 3‐hour time window for IV tPA, they are thus treated, with follow‐up vascular imaging, and possible rescue IA mechanical thrombectomy if recanalization from the IV tPA does not occur. However, if the patient still has preserved neurologic function, or is waxing and waning, there is no clear time limit for IA interventions and they may be useful a day or more after presentation. For basilar occlusion patients with severe stenoses not responsive to lysis, or continuing to be symptomatic, angioplasty and stenting has also been used.46 Despite a lack of evidence, many stroke clinicians will use an IV heparin drip for treatment of acute basilar occlusive disease.
Malignant Middle Cerebral Artery (MCA) Infarction
Malignant MCA infarction is another specific clinical syndrome worthy of special consideration. It is most generally defined as a large infarction (1/2 or 2/3) of the MCA territory, somewhat depressed level of consciousness, and high stroke scale scores (ie, severe deficits) that goes on to severe cerebral edema, mass effect, and often herniation with death.49, 50 Associated patient characteristics include younger age, abnormal (incomplete) ipsilateral collateral circulation, and internal carotid artery occlusion.51 Maximal edema occurs 2 to 5 days from stroke onset and, despite best intensive therapy, has been associated with mortality rates of 70% to 80%.49, 50 A recent pooling of 3 small randomized trials of early decompressive hemicraniectomy and durotomy showed a 50% absolute risk reduction for mortality and a 23% absolute benefit in long‐term independence (modified Rankin scale 3).49 This treatment option should be strongly considered in carefully selected patients., Transfer to an appropriately equipped facility should be offered if not available at your hospital.
Returning to our case patient, upon arrival to the ED with symptoms of partial aphasia, right hemiplegia, and left gaze preference, there was a high suspicion for a left MCA stroke. Unfortunately, he was excluded from receiving IV tPA or any other interventions, as the last time he was known to be neurologically intact was the prior evening, which is taken to be the time of onset. Antiplatelet therapy was continued, and the patient was admitted for further workup.
The initial care of the patient with a cerebrovascular event is often quite complicated. Assimilation of a great deal of data must occur and decisions around therapy must be made in a timely fashion. In prior years there was little to offer in the way of therapy, which also meant there was little initial potential for iatrogenic complication. Both diagnostic and therapeutic options are evolving rapidly. We now have much to offer these patients both emergently and in areas of secondary prevention. In part 2 of this article, the patient's inpatient course and therapy will be reviewed.
The term stroke is defined by the World Health Organization as rapidly developed clinical signs of focal (or global) disturbance of cerebral function lasting more than 24 hours (unless interrupted by surgery or death), with no apparent cause other than a vascular origin; it includes patients presenting clinical signs and symptoms suggestive of subarachnoid hemorrhage (SAH), intracerebral hemorrhage, or cerebral ischemic necrosis.1 Stroke is 1 of the leading causes of death and the number 1 cause of long‐term disability in the United States, with over 700,000 strokes and over 150,000 stroke deaths each year.2
Given the projections of 30,000 hospitalists nationally by 2010 (
Case Presentation
A 76‐year‐old right‐handed male with a history of hyperlipidemia and myocardial infarction was found at 7 AM with right‐sided paralysis and poor responsiveness on the morning of admission. He seemed to prefer looking to the left and to understand what was being said to him, but had great difficulty speaking. When he went to bed at 9 PM, he was at his neurological baseline. Upon finding him that morning, his wife called 911.
With increased knowledge regarding the pathophysiology of stroke, it has become clear that timeliness is of utmost importance (time is brain) and that acute stroke should be regarded as an acute medical/neurological emergency.
This article reviews the approach in evaluating an acute stroke patient, management strategies, and treatment options. Where not otherwise referenced, data to support our comments come from the recently updated and exhaustive American Heart Association (AHA)/American Stroke Association (ASA) Guidelines for the Early Management of Adults With Ischemic Stroke and will be referred to herein as the Guidelines.4 Harborview Medical Center in Seattle is a Joint Commissioncertified Primary Stroke Center and the home hospital of 2 of the authors (C.L.E., D.L.T.); it is referred to herein as Harborview.
Emergency Room Care (see Acute Stroke Algorithm, Figure 1)
The First 15 Minutes
After assuring stable airway, breathing, and circulation, immediate (STAT) blood draws should be performed, including full complete blood count (CBC) with platelets, international normalized ratio/prothrombin time/partial thromboplastin time (INR/PT/PTT), full electrolytes, and glucose (finger‐stick blood glucose also recommended). Glasgow Coma Scale (GCS) score and NIH Stroke Scale (NIHSS) score should be established via a focused history and physical exam. The GCS is most appropriate for patients with a significantly depressed level of consciousness, while the NIHSS can be scored for any stroke patient (1‐page version of NIHSS used at Harborview is shown in Figure 2). By quantifying stroke severity, the NIHSS score helps both to facilitate communication about neurologic deficit as well as serve as a documented baseline in case of subsequent clinical change. Emergency department (ED) physicians, hospitalists, neurologists, and nursing staff regularly caring for acute stroke patients would be well‐served by obtaining certification in the NIHSS (available free online at


Our case patient's initial NIHSS score was 15, with points given for drowsiness, inability to answer questions, partial facial palsy, no movement in right arm or leg, mild‐moderate aphasia, and mild‐moderate dysarthria (Figure 2).
Differential Diagnosis
Many acute conditions can mimic stroke, and 1 of the goals of the initial emergency room (ER) evaluation is to rule out such stroke mimics. A report of 411 initial ER stroke diagnoses identified 19% as stroke mimics; the most common mimic diagnoses were seizure, systemic infection, brain tumor, and toxic‐metabolic.5 The same study identified decreased level of alertness as associated with a final mimic diagnosis and history of angina as associated with a final diagnosis of stroke. Another study looked at 350 presentations with an initial stroke diagnosis and found 31% stroke mimics; similarly, the main alternative diagnoses were seizure, sepsis, toxic‐metabolic, space‐occupying lesion, and syncope/presyncope.6 Findings associated with a mimic diagnosis included no cognitive impairment and abnormal findings in any other system, while findings associated with a stroke diagnosis were a definite history of focal neurological symptoms, NIHSS score, stroke type classification possible, an exact onset that could be determined, and abnormal vascular findings on imaging.6
Initial Imaging
The patient should receive a STAT noncontrast head CT to evaluate for the presence or absence of blood. At this time, magnetic resonance imaging (MRI) is not essential to confirm the diagnosis of ischemic stroke, as diagnosis is based on clinical suspicion. MRI is more sensitive at imaging acute ischemia (on diffusion‐weighted sequences) and recently has been shown to be equally sensitive in identifying acute blood (previously thought to be a relative advantage of CT).7, 8 Practical and pervasive barriers to emergent MRI include study duration, significant patient cooperation, and that few hospitals are currently set up to perform such rapid MRIS. The Guidelines specifically state that In most instances, CT will provide the information to make decisions about emergency management (p. 1668),4 that vascular imaging should not delay treatment of patients whose symptoms started <3 hours ago and who have acute ischemic stroke, and that emergency treatment of stroke should not be delayed in order to obtain multimodal imaging studies (p. 1669).4
Our case patient's initial imaging, a noncontrast head CT (Supporting Figures 1 and 2), showed subtle clues consistent with the diagnosis of acute ischemic stroke. These include a hyperdense middle cerebral artery (MCA) sign (presumably representing thrombus), possible obscuration of the basal ganglia, and, importantly, no acute intraparenchymal (IPH), SAH, or subdural hemorrhage.
Acute Treatments
After the patient's head CT is completed, the next steps are dependent upon what was seen on the scan and the time from symptom onset.
Blood on the CT Scan
If the initial brain imaging reveals IPH or SAH, further diagnostic testing and early treatments are quite different than for ischemic stroke. New guidelines are available for IPH management,9 and there have been recent review articles of care for SAH.1012 At the authors' institutions, early care of such patients always involves aggressive reversal of any antithrombotic medications the patient was taking prior to presentation. Our approach to warfarin reversal includes vitamin K and fresh frozen plasma (FFP) to achieve an INR 1.4; others have used prothrombin complex concentrate (PCC).13 Blood pressure (BP) treatment goals are generally more aggressive than for ischemic stroke, while supportive care to avoid aspiration, hyperglycemia, fever, and venous thrombosis (here initially with sequential compression devices alone) are similar. Early estimation of prognosis for these patients with IPH and SAH and discussions with families about continued aggressive care are of utmost importance, and should involve providers with sufficient expertise. Care should be taken to avoid overly pessimistic early prognostication, as early do not resuscitate (DNR) decisions in intercranial hemorrhage (ICH) can become a self‐fulfilling prophecy.1416 If the decision is to continue aggressive and supportive care, or if an appropriately expert consultation is not available at the presentation hospital, IPH and SAH patients should be considered for transfer to a hospital with the appropriate resources (including emergency access to neurosurgeons) or be evaluated by such an expert by telemedicine if available.
No Blood on the CT Scan, Results Back in <3 Hours From Symptom Onset
If such a patient is not rapidly resolving their symptoms, and the diagnosis continues to remain clear, inclusion/exclusion criteria for IV tPA should be reviewed (Table 1). Consent should be obtained much like any other procedure with significant risk. As many consider tPA to be standard of care, it is reasonable to proceed in cases of unobtainable consent as one would with any other emergent therapy. This situation is a topic of ongoing debate.17, 18 The Guidelines state that although written consent is not necessary before administration of recombinant tPA (rtPA) for treatment of stroke, a full discussion of the potential risks and benefits of treatment with rtPA with the family and the patient if possible is recommended (p. 1676).4 After tPA is given in the ER, the patient should be admitted to an intensive care unit (ICU) setting for 24 hours for careful monitoring of BP, avoidance of invasive procedures, and no use of antithrombotic medications during that period of time.
| Comments (from the authors) | |
|---|---|
| |
| Inclusion criteria | |
| Diagnosis of ischemic stroke causing measurable neurological deficit | Usually NIHSS > 4 |
| Neurological signs should not be clearing spontaneously | Such a patient may do well without tPA, but there is debate82 |
| Neurological signs should not be minor and isolated. | |
| Onset of symptoms >3 hours before beginning treatment | |
| Patient or family members understand the potential risks and benefits from treatment | Debated, as tPA considered standard of care by many |
| Cautionary criteria | |
| Caution should be exercised in treating a patient with major deficits | Higher risk of hemorrhage, but still may benefit from treatment |
| Exclusion criteria | |
| Symptoms of stroke should not be suggestive of subarachnoid hemorrhage | |
| No head trauma or prior stroke in previous 3 months | |
| No myocardial infarction in the previous 3 months | |
| No gastrointestinal or urinary tract hemorrhage in previous 21 days | |
| No major surgery in the previous 14 days | |
| No arterial puncture at a noncompressible site in the previous 7 days | |
| No history of previous intracranial hemorrhage | |
| Blood pressure not elevated (systolic >185 mm Hg or diastolic 110 mm Hg) | Okay to bring down with labetolol, nitropaste, or nicardipine* |
| No evidence of active bleeding or acute trauma (fracture) on examination | |
| Not taking an oral anticoagulant or, if anticoagulant being taken, INR 1.7 | |
| If receiving heparin in previous 48 hours, aPTT must be in normal range | |
| Platelet count <100,000 mm3 | |
| Blood glucose concentration <50 mg/dL (2.7 mmol/L) | |
| Seizure with postictal residual neurological impairments | Not absolute if treating physician feels stroke also present, or if confirmed by imaging |
| CT does not show a multilobar infarction (hypodensity >1/3 cerebral hemisphere) | Not strictly evidence based, in NINDS trial this finding did not preclude benefit of tPA |
Based mainly on the results of the National Institute of Neurological Disorders and Stroke (NINDS) tPA trial,19 and recently supported by a large Phase IV observational study from the European Union,20 IV tPA for acute ischemic stroke is approved for use in many countries and is endorsed for the treatment of carefully selected ischemic stroke patients in a number of practice guidelines.4 Despite this, the emergency medicine community has been less enthusiastic about the use of IV tPA.21, 22 Although the risk of hemorrhagic complications is greater in certain subgroups of patients (ie, the most severe strokes, significant early CT changes, older age), there is no definitive evidence to suggest that these groups do not still benefit from the treatment.23 It is also clear that if patients are not carefully selected, meeting strict inclusion and exclusion criteria, the rate of complications is increased.24 Thus, as summarized in a practice statement of the American College of Emergency Physicians, There is insufficient evidence at this time to endorse the use of intravenous tPA in clinical practice when systems are not in place to ensure that the inclusion/exclusion criteria established by the NINDS guidelines for tPA use in acute stroke are followed.21 When counseling patients and their families about the benefits and risks of IV tPA, one should keep in mind that the NINDS trial demonstrated increased odds of excellent outcomes despite a significant 10‐fold increase in the risk of symptomatic intracranial hemorrhage (6.4% vs. 0.6%), and did not alter 30‐day mortality. The largest Phase IV cohort study of IV tPA treatment, Safe Implementation of Thrombolysis in Stroke Monitoring Study (SITS‐MOST) was mandated by the European Union upon approval of the medication for use in acute ischemic stroke.20 The results in 6483 patients showed that tPA, when used in strict accordance with published inclusion and exclusion criteria, could perform as well as it did in randomized trials.
The recently published European Cooperative Acute Stroke Study3 (ECASS‐3) trial demonstrated that IV tPA has efficacy with adequate safety up to 4.5 hours after the onset of symptoms. A total of 821 patients were enrolled and 375 received tPA. Exclusion criteria included diabetes being treated with medication with a history of prior stroke, an NIHSS score >25, or treatment with warfarin. The rates of hemorrhage (27.0% vs. 17.6%, P = 0.001) were in line with those of the SITS‐MOST study patients who were treated within the 3‐hour time window. There was no significant difference in mortality (7.7% tPA vs. 8.4% placebo). This study is relatively new; therefore, the data have not been reviewed by guideline committees.25
No Blood on the CT Scan, Results Back in >3 Hours, but 8 Hours, From Symptom Onset
Unfortunately as with our patient, most people do not present to an ER in a timely fashion. Nonetheless, there may be other treatments and interventions possible. If the patient arrives <8 hours from onset of symptoms, intraarterial (IA) interventions are a possibility. In such a case, a CT angiogram (CTA) of the neck from the arch of the aorta to the circle of Willis is recommended (barring any contraindications such as renal failure or iodine allergy). The rationale behind this study is that other treatment options, such as IA tPA or mechanical thrombectomy may be considered if a large arterial occlusion is identified. CTA is preferred over magnetic resonance angiography (MRA) due to the same time and patient cooperation issues mentioned above, though some expert centers may be set up to perform MRI and MRA rapidly in the acute setting. CTA or MRA is of great value early on in the emergent assessment of ischemic stroke patients, as it allows detailed evaluation of the cerebral vasculature; this knowledge helps define the pathophysiology of the ongoing stroke (eg, is there a larger artery occlusion?) and can help inform the approach to subsequent therapies.
The Guidelines (p. 1678)4 recommend IA thrombolysis as a treatment option if it can be started within 6 hours, based on results from the Prolyse in Acute Cerebral Thromboembolism (PROACT) II trial. This study involved angiography with identification of the occluded vessel (the proximal MCA‐M1 in this study) and administration of recombinant pro‐urokinase to the clot with functional outcome as the primary endpoint.26 At 3 months, patients who received the IA thrombolytic had a 40% chance of slight disability; unable to carry out all previous activities, but able to look after own affairs without assistance or better (ie, a modified Rankin Scale score of 2) vs. 25% of those not receiving the IA thrombolytic. Pro‐urokinase is not available in the United States; therefore, many institutions substitute IA tPA. The Guidelines further state that IA thrombolysis can be considered for use in some patients with contraindications to IV tPA (eg, recent surgery), but should not be used instead of IV tPA in patients otherwise eligible (p. 1678).4
There are now two U.S. Food and Drug Administration (FDA)‐approved devices for mechanical cerebral vasculature thrombectomy for use up to 8 hours from symptom onset. The mechanical embolus removal in cerebral ischemia (MERCI) clot retrieval device was originally approved by the FDA in August 2004 for restoring blood flow in the neurovasculature by removing thrombus in patients experiencing ischemic stroke. Modified devices have been approved as recently as January 2007.27 The Penumbra System was FDA‐approved in December 2007 for revascularization of patients with acute ischemic stroke secondary to intracranial large vessel occlusive disease.28 In both cases, the FDA approval was based on demonstration of safety in case series of patients treated with the devices.2931 No randomized trials have shown the use of these devices improves outcomes for stroke patients. The Guidelines state that Although the MERCI device is a reasonable intervention for extraction of IA thrombi in carefully selected patients, the panel also recognizes that the utility of the device in improving outcomes after stroke is unclear (p. 1684);4 this statement applies similarly to the Penumbra device.
More complex imaging techniques, including multimodal CT (CT, CTA, and CT perfusion) and MR (MRI with diffusion, MRA, and MR perfusion) are being used in some stroke centers to make decisions about acute ischemic stroke treatments.32, 33 The theory is that by using these techniques, one can determine the presence or absence of a mismatch, whereby the perfusion imaging suggests more tissue at risk of infarction than is seen as already abnormal on MR diffusion‐weighted images or compared to a clinical assessment. These mismatch patients are then seen as appropriate candidates for the more aggressive interventions (ie, late IV tPA or IA interventions).34 Unfortunately, the 2 largest randomized trials to look at this issue with respect to >3‐hour IV tPA both failed to show a benefit for patients selected in this manner.35, 36 Standardized definitions of mismatch are still needed, and larger randomized trials are needed before this approach can be suggested for routine care.3739
More complex interventions, available only at tertiary or comprehensive stroke centers, include a bridging approach in which IV tPA (at 2/3 standard dose) is followed by IA tPA, IV tPA with transcranial Doppler (TCD)‐enhanced thrombolysis or IA rescue thrombectomy when vascular imaging after IV tPA shows a persistent large artery occlusion. The Guidelines suggests that these more complex combinations of interventions to restore perfusion cannot be recommended outside the setting of clinical trials (p. 1685).4
No Blood on the CT Scan, Results Back in >8 Hours From Symptom Onset (or if Contraindications to Above Interventions)
This time frame takes the more aggressive interventions off the table. Per the Guidelines, 325 mg of aspirin is the default antiplatelet agent for use, and has been shown in 2 very large randomized trials to reduce early death and longer‐term disability vs. placebo after acute ischemic stroke.40, 41 Importantly, all patients who do not qualify for thrombolysis in the 0‐hour to 8‐hour time window should receive aspirin.
Although a number of small or pilot studies suggest a benefit of the addition of clopidogrel to aspirin for a period (13 months) immediately after ischemic stroke,4244 this more aggressive antiplatelet intervention is not an endorsed standard of care. As described below, the long‐term use of this antiplatelet combination has been consistently associated with a higher risk of hemorrhagic complications. There are no published data regarding the use of aspirin plus dipyridamole in the acute stroke setting. A number of randomized trials have now been performed that have consistently failed to show a benefit of heparin, or heparin‐like medications, for the routine treatment of acute ischemic stroke. Despite this, a number of exceptions exist, based more on tradition and theory than on evidence. These exceptions, for which an IV heparin drip will at times still be considered, include acute ischemic stroke due to dissection of the carotid or vertebral arteries, cardioembolic stroke with fresh clot seen on echocardiogram (ECHO), and a clinically progressive syndrome suggestive of basilar artery occlusion (see below).45, 46 Good evidence exists to specifically recommend the use of full‐dose heparin in the setting of cerebral venous sinus thrombosis.47
Basilar Artery Occlusion Syndromes
Basilar artery occlusion syndromes warrant special mention. These may involve patients who present with quadriparesis, altered mental status, vertigo, diplopia, and other brainstem signs. Conventional treatment of basilar artery occlusion has been associated with 40% mortality with 65% of survivors having severe disability.48 If suspected, an urgent CTA can usually confirm the diagnosis, and urge the clinician to expeditiously consider aggressive intervention. Only case series have been reported regarding basilar artery thrombosis and acute treatments. Based on these studies, it is generally agreed upon that patients who appear comatose or quadriplegic for more than 3 hours will likely have a very poor functional outcome regardless of treatment, and interventional treatment is withheld. If a basilar occlusion patient presents within the 3‐hour time window for IV tPA, they are thus treated, with follow‐up vascular imaging, and possible rescue IA mechanical thrombectomy if recanalization from the IV tPA does not occur. However, if the patient still has preserved neurologic function, or is waxing and waning, there is no clear time limit for IA interventions and they may be useful a day or more after presentation. For basilar occlusion patients with severe stenoses not responsive to lysis, or continuing to be symptomatic, angioplasty and stenting has also been used.46 Despite a lack of evidence, many stroke clinicians will use an IV heparin drip for treatment of acute basilar occlusive disease.
Malignant Middle Cerebral Artery (MCA) Infarction
Malignant MCA infarction is another specific clinical syndrome worthy of special consideration. It is most generally defined as a large infarction (1/2 or 2/3) of the MCA territory, somewhat depressed level of consciousness, and high stroke scale scores (ie, severe deficits) that goes on to severe cerebral edema, mass effect, and often herniation with death.49, 50 Associated patient characteristics include younger age, abnormal (incomplete) ipsilateral collateral circulation, and internal carotid artery occlusion.51 Maximal edema occurs 2 to 5 days from stroke onset and, despite best intensive therapy, has been associated with mortality rates of 70% to 80%.49, 50 A recent pooling of 3 small randomized trials of early decompressive hemicraniectomy and durotomy showed a 50% absolute risk reduction for mortality and a 23% absolute benefit in long‐term independence (modified Rankin scale 3).49 This treatment option should be strongly considered in carefully selected patients., Transfer to an appropriately equipped facility should be offered if not available at your hospital.
Returning to our case patient, upon arrival to the ED with symptoms of partial aphasia, right hemiplegia, and left gaze preference, there was a high suspicion for a left MCA stroke. Unfortunately, he was excluded from receiving IV tPA or any other interventions, as the last time he was known to be neurologically intact was the prior evening, which is taken to be the time of onset. Antiplatelet therapy was continued, and the patient was admitted for further workup.
The initial care of the patient with a cerebrovascular event is often quite complicated. Assimilation of a great deal of data must occur and decisions around therapy must be made in a timely fashion. In prior years there was little to offer in the way of therapy, which also meant there was little initial potential for iatrogenic complication. Both diagnostic and therapeutic options are evolving rapidly. We now have much to offer these patients both emergently and in areas of secondary prevention. In part 2 of this article, the patient's inpatient course and therapy will be reviewed.
- Organization WH. MONICA Manual, Part IV: Event Registration. Available at: http://www.ktl.fi/publications/monica/manual/part4/iv‐2.htm#s2. Accessed May2009.
- ,,, et al.Heart disease and stroke statistics 2008 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.200829;117(4):e25–e146.
- .Neurology in the next two decades: report of the Workforce Task Force of the American Academy of Neurology.Neurology.2000;54(4):787–789.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists.Stroke.2007;38(5):1655–1711.
- ,,,.Conditions that mimic stroke in the emergency department. Implications for acute stroke trials.Arch Neurol.1995;52(11):1119–1122.
- ,,,,.Distinguishing between stroke and mimic at the bedside: the brain attack study.Stroke.2006;37(3):769–775.
- ,,, et al.Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: a multicenter study on the validity of stroke imaging.Stroke.2004;35(2):502–506.
- ,,, et al.Comparison of MRI and CT for detection of acute intracerebral hemorrhage.JAMA.2004;292(15):1823–1830.
- ,,, et al.Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group.Stroke.2007;38(6):2001–2023.
- ,,.Aneurysmal subarachnoid hemorrhage.N Engl J Med.2006;354(4):387–396.
- ,,,.Subarachnoid haemorrhage.BMJ.2006;333(7561):235–240.
- ,,.Subarachnoid haemorrhage.Lancet.2007;369(9558):306–318.
- ,,.Intracerebral hemorrhage associated with oral anticoagulant therapy: current practices and unresolved questions.Stroke.2006;37(1):256–262.
- ,,, et al.Withdrawal of support in intracerebral hemorrhage may lead to self‐fulfilling prophecies.Neurology.2001;56(6):766–772.
- ,,,.Hospital usage of early do‐not‐resuscitate orders and outcome after intracerebral hemorrhage.Stroke.2004;35(5):1130–1134.
- ,,, et al.Early care limitations independently predict mortality after intracerebral hemorrhage.Neurology.2007;68(20):1651–1657.
- ,,,.Consent for intravenous thrombolysis in acute stroke: review and future directions.Arch Neurol.2007;64(6):785–792.
- .Thrombolysis (tissue plasminogen activator) in stroke: a medicolegal quagmire.Stroke.2006;37(7):1917–1922.
- Tissue plasminogen activator for acute ischemic stroke.The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group.N Engl J Med.1995;333(24):1581–1587.
- ,,, et al.Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke‐Monitoring Study (SITS‐MOST): an observational study.Lancet.2007;369(9558):275–282.
- American College of Emergency Physicians (ACEP). Use of Intravenous tPA for the Management of Acute Stroke in the Emergency Department. ACEP Policy Statement. February 2002. Available at: http://www.acep.org/practres.aspx?id=29834. Accessed May2009.
- American Academy of Emergency Medicine (AAEM). Position statement on the use of intravenous thrombolytic therapy in the treatment of stroke. January 2002. Available at: http://aaem.org/positionstatements/thrombolytictherapy.php. Accessed May2009.
- ,,, et al.Lack of clinical significance of early ischemic changes on computed tomography in acute stroke.JAMA.2001;286(22):2830–2838.
- ,,,,.Thrombolysis for acute stroke in routine clinical practice.Arch Intern Med.2002;162(17):1994–2001.
- ,,, et al.Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke.N Engl J Med.2008;359:1317–1329,1393–1395.
- ,,, et al.Intra‐arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism.JAMA.1999;282(21):2003–2011.
- Modified MERCI Retriever FDA marketing approval letter. Available at: www.fda.gov/cdrh/pdf6/K062046.pdf. Accessed May2009.
- Penumbra System FDA marketing approval letter. Available at: www.fda.gov/cdrh/pdf7/K072718.pdf. Accessed May2009.
- .Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the multi mechanical embolus removal in cerebral ischemia (MERCI) trial, part I.AJNR Am J Neuroradiol.2006;27(6):1177–1182.
- ,,, et al.Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial.Stroke.2005;36(7):1432–1438.
- ,,, et al.The Penumbra System: a mechanical device for the treatment of acute stroke due to thromboembolism.AJNR Am J Neuroradiol.2008;29(7):1409–1413.
- ,,, et al.Comparison of CT perfusion and angiography and MRI in selecting stroke patients for acute treatment.Neurology.2007;68(9):694–697.
- ,,, et al.Combined intravenous and intraarterial revascularization therapy using MRI perfusion/diffusion mismatch selection for acute ischemic stroke at 3–6 h after symptom onset.Neurocrit Care.2008;8(3):353–359.
- ,,, et al.Refining the perfusion‐diffusion mismatch hypothesis.Stroke.2005;36(6):1153–1159.
- . DIAS‐2: no benefit of desmoteplase in acute ischemic stroke. Available at: www.medscape.com/viewarticle/557663. Accessed May2009.
- ,,, et al.Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo‐controlled randomised trial.Lancet Neurol.2008;7(4):299–309.
- ,,.Magnetic resonance perfusion diffusion mismatch and thrombolysis in acute ischaemic stroke: a systematic review of the evidence to date.J Neurol Neurosurg Psychiatry.2007;78(5):485–491.
- ,,, et al.Optimal definition for PWI/DWI mismatch in acute ischemic stroke patients.J Cereb Blood Flow Metab.2008;28(5):887–891.
- ,,, et al.Rapid assessment of perfusion‐diffusion mismatch.Stroke.2008;39(1):75–81.
- CAST: randomised placebo‐controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke.CAST (Chinese Acute Stroke Trial) Collaborative Group.Lancet.1997;349(9066):1641–1649.
- The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke.International Stroke Trial Collaborative Group.Lancet.1997;349(9065):1569–1581.
- ,,, et al.Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: the clopidogrel and aspirin for reduction of emboli in symptomatic carotid stenosis (CARESS) trial.Circulation.2005;111(17):2233–2240.
- ,,, et al.Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population‐based sequential comparison.Lancet.2007;370(9596):1432–1442.
- ,,,,,.Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial.Lancet Neurol.2007;6(11):961–969.
- ,,, et al.Antiplatelets versus anticoagulation in cervical artery dissection.Stroke.2007;38(9):2605–2611.
- ,,.Basilar artery occlusion.Neurocrit Care.2004;1(3):319–329.
- ,.Cerebral venous thrombosis: an update.Lancet Neurol.2007;6(2):162–170.
- ,,,,.Outcome in patients with basilar artery occlusion treated conventionally.J Neurol Neurosurg Psychiatry.2005;76(9):1238–1241.
- ,,, et al.Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials.Lancet Neurol.2007;6(3):215–222.
- ,,,,,.‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs.Arch Neurol.1996;53(4):309–315.
- ,,,,.Predictors for malignant middle cerebral artery infarctions: a postmortem analysis.Neurology. 282006;66(6):815–820.
- ,,,,,.Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke.Stroke.2005;36(11):2497–2499.
- Organization WH. MONICA Manual, Part IV: Event Registration. Available at: http://www.ktl.fi/publications/monica/manual/part4/iv‐2.htm#s2. Accessed May2009.
- ,,, et al.Heart disease and stroke statistics 2008 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.200829;117(4):e25–e146.
- .Neurology in the next two decades: report of the Workforce Task Force of the American Academy of Neurology.Neurology.2000;54(4):787–789.
- ,,, et al.Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists.Stroke.2007;38(5):1655–1711.
- ,,,.Conditions that mimic stroke in the emergency department. Implications for acute stroke trials.Arch Neurol.1995;52(11):1119–1122.
- ,,,,.Distinguishing between stroke and mimic at the bedside: the brain attack study.Stroke.2006;37(3):769–775.
- ,,, et al.Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: a multicenter study on the validity of stroke imaging.Stroke.2004;35(2):502–506.
- ,,, et al.Comparison of MRI and CT for detection of acute intracerebral hemorrhage.JAMA.2004;292(15):1823–1830.
- ,,, et al.Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group.Stroke.2007;38(6):2001–2023.
- ,,.Aneurysmal subarachnoid hemorrhage.N Engl J Med.2006;354(4):387–396.
- ,,,.Subarachnoid haemorrhage.BMJ.2006;333(7561):235–240.
- ,,.Subarachnoid haemorrhage.Lancet.2007;369(9558):306–318.
- ,,.Intracerebral hemorrhage associated with oral anticoagulant therapy: current practices and unresolved questions.Stroke.2006;37(1):256–262.
- ,,, et al.Withdrawal of support in intracerebral hemorrhage may lead to self‐fulfilling prophecies.Neurology.2001;56(6):766–772.
- ,,,.Hospital usage of early do‐not‐resuscitate orders and outcome after intracerebral hemorrhage.Stroke.2004;35(5):1130–1134.
- ,,, et al.Early care limitations independently predict mortality after intracerebral hemorrhage.Neurology.2007;68(20):1651–1657.
- ,,,.Consent for intravenous thrombolysis in acute stroke: review and future directions.Arch Neurol.2007;64(6):785–792.
- .Thrombolysis (tissue plasminogen activator) in stroke: a medicolegal quagmire.Stroke.2006;37(7):1917–1922.
- Tissue plasminogen activator for acute ischemic stroke.The National Institute of Neurological Disorders and Stroke rt‐PA Stroke Study Group.N Engl J Med.1995;333(24):1581–1587.
- ,,, et al.Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke‐Monitoring Study (SITS‐MOST): an observational study.Lancet.2007;369(9558):275–282.
- American College of Emergency Physicians (ACEP). Use of Intravenous tPA for the Management of Acute Stroke in the Emergency Department. ACEP Policy Statement. February 2002. Available at: http://www.acep.org/practres.aspx?id=29834. Accessed May2009.
- American Academy of Emergency Medicine (AAEM). Position statement on the use of intravenous thrombolytic therapy in the treatment of stroke. January 2002. Available at: http://aaem.org/positionstatements/thrombolytictherapy.php. Accessed May2009.
- ,,, et al.Lack of clinical significance of early ischemic changes on computed tomography in acute stroke.JAMA.2001;286(22):2830–2838.
- ,,,,.Thrombolysis for acute stroke in routine clinical practice.Arch Intern Med.2002;162(17):1994–2001.
- ,,, et al.Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke.N Engl J Med.2008;359:1317–1329,1393–1395.
- ,,, et al.Intra‐arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism.JAMA.1999;282(21):2003–2011.
- Modified MERCI Retriever FDA marketing approval letter. Available at: www.fda.gov/cdrh/pdf6/K062046.pdf. Accessed May2009.
- Penumbra System FDA marketing approval letter. Available at: www.fda.gov/cdrh/pdf7/K072718.pdf. Accessed May2009.
- .Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the multi mechanical embolus removal in cerebral ischemia (MERCI) trial, part I.AJNR Am J Neuroradiol.2006;27(6):1177–1182.
- ,,, et al.Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial.Stroke.2005;36(7):1432–1438.
- ,,, et al.The Penumbra System: a mechanical device for the treatment of acute stroke due to thromboembolism.AJNR Am J Neuroradiol.2008;29(7):1409–1413.
- ,,, et al.Comparison of CT perfusion and angiography and MRI in selecting stroke patients for acute treatment.Neurology.2007;68(9):694–697.
- ,,, et al.Combined intravenous and intraarterial revascularization therapy using MRI perfusion/diffusion mismatch selection for acute ischemic stroke at 3–6 h after symptom onset.Neurocrit Care.2008;8(3):353–359.
- ,,, et al.Refining the perfusion‐diffusion mismatch hypothesis.Stroke.2005;36(6):1153–1159.
- . DIAS‐2: no benefit of desmoteplase in acute ischemic stroke. Available at: www.medscape.com/viewarticle/557663. Accessed May2009.
- ,,, et al.Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo‐controlled randomised trial.Lancet Neurol.2008;7(4):299–309.
- ,,.Magnetic resonance perfusion diffusion mismatch and thrombolysis in acute ischaemic stroke: a systematic review of the evidence to date.J Neurol Neurosurg Psychiatry.2007;78(5):485–491.
- ,,, et al.Optimal definition for PWI/DWI mismatch in acute ischemic stroke patients.J Cereb Blood Flow Metab.2008;28(5):887–891.
- ,,, et al.Rapid assessment of perfusion‐diffusion mismatch.Stroke.2008;39(1):75–81.
- CAST: randomised placebo‐controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke.CAST (Chinese Acute Stroke Trial) Collaborative Group.Lancet.1997;349(9066):1641–1649.
- The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke.International Stroke Trial Collaborative Group.Lancet.1997;349(9065):1569–1581.
- ,,, et al.Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: the clopidogrel and aspirin for reduction of emboli in symptomatic carotid stenosis (CARESS) trial.Circulation.2005;111(17):2233–2240.
- ,,, et al.Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population‐based sequential comparison.Lancet.2007;370(9596):1432–1442.
- ,,,,,.Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial.Lancet Neurol.2007;6(11):961–969.
- ,,, et al.Antiplatelets versus anticoagulation in cervical artery dissection.Stroke.2007;38(9):2605–2611.
- ,,.Basilar artery occlusion.Neurocrit Care.2004;1(3):319–329.
- ,.Cerebral venous thrombosis: an update.Lancet Neurol.2007;6(2):162–170.
- ,,,,.Outcome in patients with basilar artery occlusion treated conventionally.J Neurol Neurosurg Psychiatry.2005;76(9):1238–1241.
- ,,, et al.Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials.Lancet Neurol.2007;6(3):215–222.
- ,,,,,.‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs.Arch Neurol.1996;53(4):309–315.
- ,,,,.Predictors for malignant middle cerebral artery infarctions: a postmortem analysis.Neurology. 282006;66(6):815–820.
- ,,,,,.Poor outcomes in patients who do not receive intravenous tissue plasminogen activator because of mild or improving ischemic stroke.Stroke.2005;36(11):2497–2499.
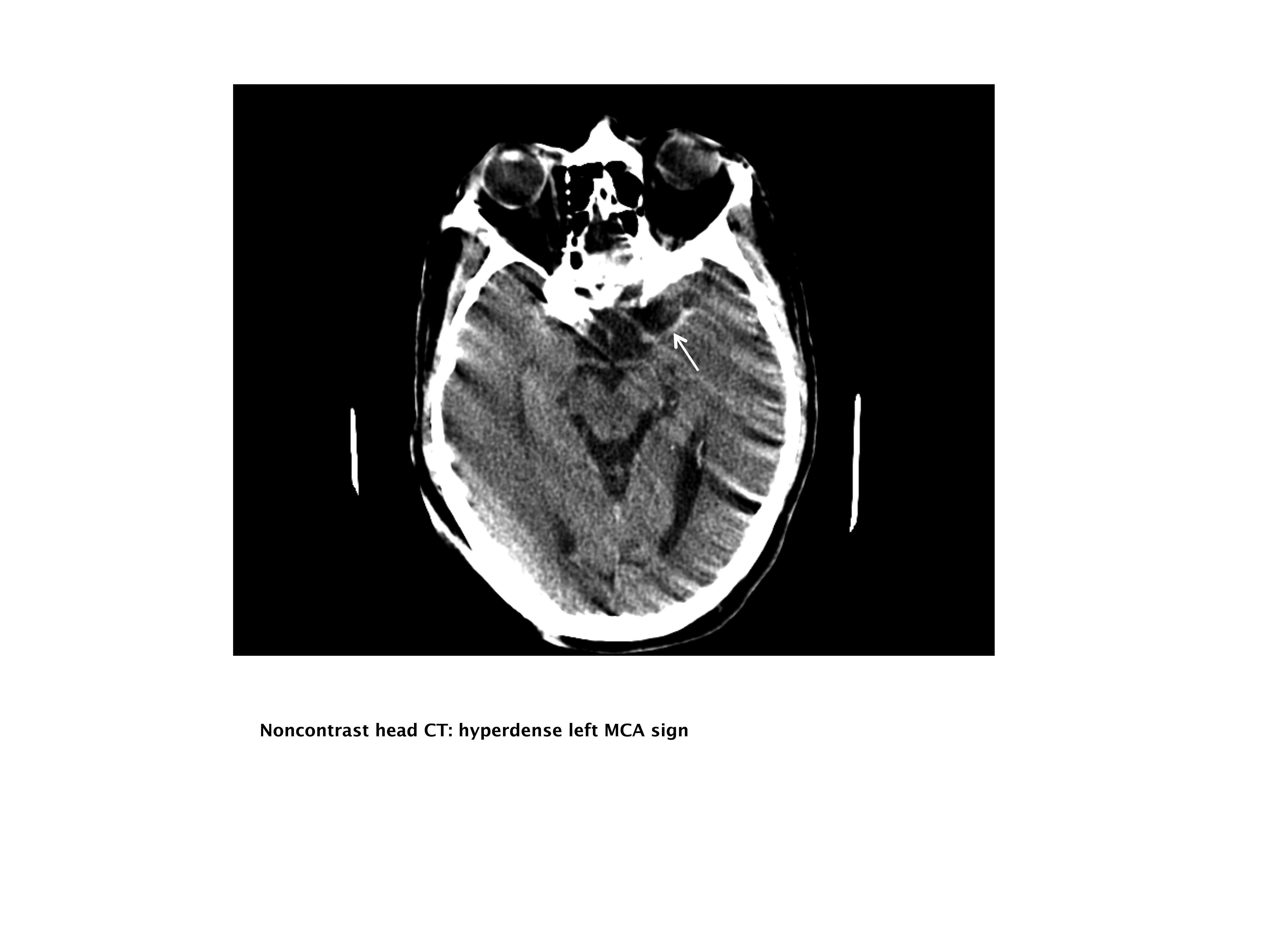
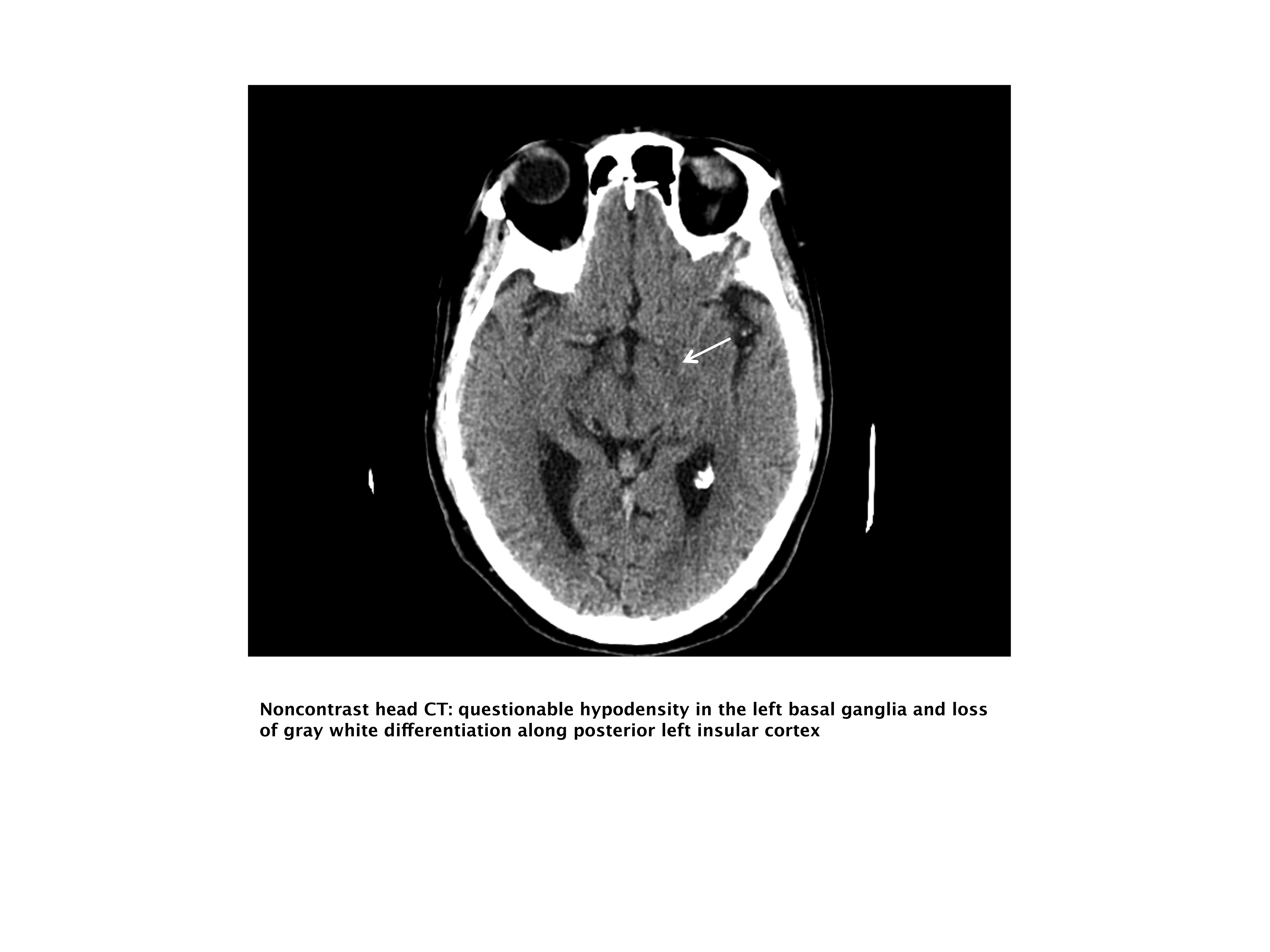
Recognition, Risk, and Treatment of ADHF
Currently, acute decompensated heart failure (ADHF) accounts for 3% of all hospitalizations in the United States and is the second most common indication for hospitalization in individuals 65 years of age.1 These hospitalizations are costly and frequently have limited sustained benefits. The total direct medical cost attributable to ADHF hospitalization in the United States is estimated to be $18.8 billion annually.2 Furthermore, 50% of all patients hospitalized for ADHF are readmitted within 6 months of discharge.3 Clearly, the hospital management of these patients requires reevaluation.
The purpose of this article is to review the recognition, risk stratification, and treatment of ADHF and to discuss the role hospitalists can play in improving this treatment.
RECOGNITION OF ADHF
The American College of Cardiology/American Heart Association guidelines classify patients with heart failure into 1 of 4 stages, A through D.4 Patients with heart failure risk factors who do not have evidence of structural heart disease are classified as Stage A. Patients with evidence of structural heart disease who have never been symptomatic are classified as Stage B. Patients who are presently or previously symptomatic and responsive to standard therapies are classified as Stage C. Finally, patients are classified as Stage D if they are refractory to standard therapies and require specialized advanced treatment such as mechanical circulatory support, continuous inotropic infusions, or cardiac transplantation. By definition, patients with ADHF have either Stage C or Stage D heart failure.
Early recognition and appropriate treatment are key components in improving the management of these patients.57 Hospitalization is recommended for patients with evidence of severely decompensated heart failure, dyspnea at rest, hemodynamically significant arrhythmias, and acute coronary syndromes and should be considered in patients with worsening congestion, major electrolyte abnormalities, associated comorbid conditions, and repeated implantable cardioverter‐defibrillator firings.8 However, correctly identifying ADHF at the time of hospital presentation can be challenging.9 The diagnosis of ADHF is based on signs and symptoms, supported by radiographic findings, biomarkers, and echocardiography.8, 10 Unfortunately, the typical signs and symptoms of ADHFfor example, rales, peripheral edema, dyspnea at rest, and fatiguemay be missing at hospital presentation. In an early evaluation, rales, edema, and elevated mean jugular venous pressure were absent in 18 of 43 patients with documented pulmonary capillary wedge pressures (PCWP) 22 mm Hg.11 These findings have recently been confirmed using data from 2 large registries, the Acute Decompensated Heart Failure National Registry (ADHERE) and the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF) registry. In these registries, 32%36% of patients admitted with ADHF did not have rales, 33%35% did not have peripheral edema, 56%64% did not have dyspnea at rest, and approximately 67% did not have fatigue (Figure 1).12, 13 Furthermore, even when these signs and symptoms are present, they are nondiagnostic, because they can be produced by a variety of disorders, including hepatic, renal, and pulmonary dysfunction.8, 14
Similarly, radiographic and echocardiographic features of ADHF are not always present. Overall, 26% of patients in ADHERE did not have evidence of pulmonary congestion on their initial chest radiograph, and 50%55% of patients in both registries had preserved systolic function.13, 1517 Consequently, attention has turned to biomarkers as a means of rapidly and accurately identifying ADHF. Serum Btype natriuretic peptide (BNP) and its N‐terminal prohormone (NT‐proBNP) have proven to be both diagnostic and prognostic indicators in ADHF.14, 1825 In the Breathing Not Properly Multinational Study, a BNP level 100 pg/mL was found to have a 90% sensitivity (95% confidence interval [CI]: 88%92%) and a 76% specificity (95% CI: 73%79%) for heart failure in patients presenting to the emergency department with dyspnea.21 In addition, BNP levels have been shown to correlate with heart failure severity18 and to be a more accurate reflection of this severity than clinical judgment.23 In a prospective randomized evaluation, the addition of BNP assessment to a standard diagnostic evaluation resulted in fewer patients being hospitalized (75% vs. 85%; P = .008), more rapid initiation of appropriate therapy (63 vs. 90 minutes; P = .03), and a shorter median duration of hospitalization (8 vs. 11 days; P = .001).26 As a result, the American College of Emergency Physicians guidelines now state that measurement of BNP or NT‐proBNP can improve diagnostic accuracy in acute heart failure syndrome when compared with standard clinical judgment alone.27
It is important to remember, however, that BNP levels cannot be interpreted in isolation; clinical judgment still plays a vital role. Obesity decreases BNP levels due to the expression of natriuretic peptide clearance receptors in adipose tissue.9, 28, 29 In contrast, BNP levels increase with age and are higher in women than in men.29 In addition, pulmonary embolism, an important diagnostic consideration in patients presenting with dyspnea, has been shown to increase serum BNP levels above the diagnostic threshold for ADHF.9, 29 Likewise, renal dysfunction, a common comorbidity in patients with heart failure (cardiorenal syndrome), increases serum BNP levels.30 As a result, the BNP threshold value for the diagnosis of ADHF rises from 100 pg/mL in patients with normal renal function to 200 pg/mL in patients with an estimated glomerular filtration rate <60 mL/min/1.73 m2.30 Finally, it is now well recognized that BNP production is up‐regulated by numerous physiologic conditions in addition to heart failure, including cardiac hypertrophy, endothelial dysfunction, and arrhythmia.31 Consequently, an elevated BNP level may indicate one of these conditions instead of ADHF. For example, recent data demonstrate that BNP levels are increased in patients with acute coronary syndromes and also serve as a significant prognostic factor in these patients.32, 33
RISK STRATIFICATION
Risk stratification, another important component in improving the management of patients with ADHF, helps determine the appropriate location (eg, outpatient, hospital ward, intensive care unit) for and intensity of initial monitoring and treatment.13, 25, 3452 Univariate analyses have identified several morbidity and/or mortality risk factors, including age,3540 blood pressure,13, 34, 37, 3941 respiratory rate,37 left ventricular ejection fraction (LVEF),36, 41, 48 renal function,34, 36, 37, 39, 40, 42, 43 anemia,25, 44, 45 hyponatremia,37, 39, 46 BNP level,36, 49, 50 cardiac troponin level,48 diuretic dose,36, 49, 50 previous heart failure hospitalization,44, 51, 52 and comorbid conditions.35, 37, 39 Unfortunately, these univariate factors are not very helpful in and of themselves, as they regularly occur in conjunction with each other. True risk assessment requires multivariate analyses of large datasets.
Multivariate risk factors for short‐term mortality in patients admitted for ADHF have been evaluated in 3 separate studies. Lee et al used multiple logistic regression to analyze data from 4031 hospitalization episodes at 34 centers in Canada,37 Fonarow et al used both classification and regression tree and multivariate regression models to analyze data from 65,275 hospitalization episodes at 263 centers in the United States,34 and Rohde et al used stepwise logistic regression to analyze data from 779 consecutive hospitalization episodes at a single center in Brazil.39 Despite these differences in statistical methodology and geographic location, the findings of these 3 analyses are remarkably similar. All 3 evaluations identified advanced age, lower systolic blood pressure, and renal dysfunction (cardiorenal syndrome) as significant and independent risk factors for short‐term mortality, and 2 of the 3 identified hyponatremia and comorbid cancer as additional risk factors (Table 1).34 Of note, lower systolic blood pressure did not mean hypotension in these evaluations. Mortality risk was significantly increased in patients with systolic blood pressure <115‐124 mm Hg. In the largest of these studies, a simple risk tree utilizing admission blood pressure, serum creatinine concentration, and blood urea nitrogen level stratified patients into groups with in‐hospital mortality risk ranging from 2.1%21.9% in the derivation and 2.3%19.8% in the validation cohorts (Figure 2).34 Taken together, these studies underscore the substantial role age, blood pressure, renal function, serum sodium concentration, and comorbidities play in increasing mortality risk, and these factors should always be considered in determining the intensity and location of ADHF treatment and degree of monitoring employed therein.
| Parameter | Study | ||
|---|---|---|---|
| Lee et al37 | Fonarow et al34 | Rohde et al39 | |
| |||
| Data source | 34 Hospitals | 263 Hospitals | Single center |
| Admissions evaluated | 4031 | 65,275 | 779 |
| Outcome parameter | 30‐Day mortality | In‐hospital mortality | In‐hospital mortality |
| Independent risk factors | |||
| Older age | Yes | Yes | Yes (>70 years) |
| Lower SBP | Yes | Yes (<115 mm Hg) | Yes (124 mm Hg) |
| Renal dysfunction | Yes | Yes | Yes |
| Elevated BUN | Yes | Yes (>43 mg/dL) | Yes (>37 mg/dL) |
| Elevated serum creatinine | Yes (>2.75 mg/dL) | Yes (>1.4 mg/dL) | |
| Hyponatremia | Yes | Yes (<136 mEq/L) | |
| Elevated heart rate | Yes | ||
| Elevated respiratory rate | Yes | ||
| Comorbid conditions | Yes | Yes | |
| Cancer | Yes | Yes | |
| Cerebrovascular disease | Yes | ||
| COPD | Yes | ||
| Dementia | Yes | ||
Although BNP and cardiac troponin level were not significant risk factors in the multivariate models, these levels were not routinely assessed in patients admitted for ADHF 5 to 10 years ago. For example, admission BNP was available in only 18% of patients in the Fonarow analysis,34 and this lack of data may explain the absence of these parameters in these multivariate analyses. In a recent analysis limited to patients with admission BNP and cardiac troponin data, in‐hospital mortality was significantly increased when BNP was 840 pg/mL (odds ratio [OR]: 1.60; 95% CI: 1.431.80; P < .001), cardiac troponin was positive (OR: 1.85; 95% CI: 1.572.18; P < .001) or both (OR: 3.00; 95% CI: 2.473.66; P < .001) even after adjusting for differences in age, gender, blood urea nitrogen, systolic blood pressure, serum creatinine concentration, serum sodium concentration, heart rate, and dyspnea at rest.4
THERAPY
Ideally, treatment should be rooted in evidence‐based guidelines. However, relatively few randomized, controlled clinical trials have been completed in patients with ADHF, and consequently there are minimal data available to construct these guidelines. The American College of Cardiology and the American Heart Association have jointly published guidelines since 1995 on the management of heart failure.4, 53 However, these guidelines, which were last updated in 2005, discuss only the management of chronic heart failure, not the management of ADHF.4 In fact, the most recent version of these guidelines specifically states, The committee elected to focus this document on the diagnosis and management of chronic heart failure It specifically did not consider acute heart failure, which might merit a separate set of guidelines.4
The first guideline to specifically address the management of ADHF was published in 2004.5 These guidelines, a consensus statement based on expert panel review of the available literature, were created to improve treatment at member hospitals of a national group purchasing organization and focused only on the initial 24 hours of care. They had 2 important components. The first was a timeline emphasizing rapid assessment and institution of therapy, followed by serial reevaluations every couple of hours thereafter.5 The second was a flow chart detailing recommended initial therapies based on the current clinical findings and the patient's chronic outpatient pharmacotherapy, followed by modifications to this initial therapy based on the response observed during the serial reevaluations. Treatment recommendations were as follows: for patients with mild volume overload, an intravenous diuretic; for patients with moderate to severe volume overload, an intravenous diuretic plus an intravenous vasodilator (nitroglycerin or nesiritide); and for patients with low cardiac output, an inotropic agent with or without a subsequent intravenous vasodilator.
In 2005, the European Society of Cardiology published its guidelines for the treatment of ADHF.10 These guidelines state that the immediate goal of ADHF therapy is to improve symptoms and stabilize hemodynamics, but these short‐term benefits must be accompanied by favorable effects on long‐term outcomes.10 Recommended treatment consists of fluid loading, diuretics, vasodilators (glyceryl trinitrate, isosorbide dinitrate, nitroprusside, or nesiritide), and/or inotropic agents (dopamine, dobutamine, milrinone, enoximone, levosimendan, norepinephrine, or epinephrine), depending on the patient's clinical status and hemodynamics.10 In general, the guidelines recommend fluid loading in patients with low cardiac output and low PCWP; a vasodilator or inotropic agent, depending on systolic blood pressure, in patients with low cardiac output and normal to high PCWP; and an intravenous diuretic in patients with normal cardiac output and high PCWP pressure. Finally, respiratory support, eg, continuous positive airway pressure (CPAP), noninvasive positive pressure ventilation, or endotracheal intubation and mechanical ventilation, may be necessary in some patients with left‐heart failure.
In 2006, the Heart Failure Society of America published comprehensive heart failure practice guidelines.8 These guidelines expand the goals of ADHF therapy to include improving symptoms, optimizing volume status, identifying precipitating factors, enhancing chronic oral therapy, and minimizing side effects. They provide the most detailed recommendations yet with respect to monitoring patents admitted for ADHF.8 According to these guidelines, this monitoring should include more than daily assessment of vital signs, including orthostatic blood pressure, and at least daily assessment of heart failure signs and symptoms, fluid intake and output, weight, electrolytes, and renal function. Treatment recommendations are similar to those in preceding guidelines. Intravenous loop diuretics are recommended as first‐line therapy in patients with volume overload.8 In the absence of systemic hypotension, the addition of an intravenous vasodilator (nitroglycerin, nitroprusside, or nesiritide) should be considered to achieve rapid symptomatic improvement.8 Intravenous inotropic therapy may be considered to improve symptoms and end‐organ function in patients with low‐output syndrome (left ventricular dilation, reduced LVEF, and diminished peripheral perfusion), especially if systolic blood pressure is <90 mm Hg or there is symptomatic hypotension despite adequate filling pressures.54 Outside of this small select group of patients, there is no rationale for the use of inotropic agents.8 Patients with ADHF who received an inotropic agent in the absence of a clearly defined clinical indication had an increased risk of adverse events without any evidence of clinical benefit in the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME‐CHF) trial.54 Ultrafiltration may be considered in patients who fail to respond adequately to diuretic therapy,6, 8 and an implantable left ventricular assist device (LVAD) should be considered as a bridge to cardiac transplantation in patients with severe heart failure (Stage D) who have become refractory to all means of medical circulatory support and may be considered in highly selected nontransplantation candidates who cannot be weaned from intravenous inotropic support.8, 52
Whether to continue or temporarily stop chronic oral heart failure medications during treatment of an acute decompensation is not addressed in any of the evidence‐based guidelines and ultimately, this decision must be based on the patient's clinical status. In general, guideline‐recommended intravenous diuretic therapy temporarily replaces the patient's chronic oral diuretic regimen. Oral ‐blocker therapy should be continued whenever possible, as long as the patient's blood pressure and clinical status can tolerate it. In an analysis of data from the OPTIMIZE‐HF registry, patients with ADHF who had withdrawal of ‐blocker had significantly greater risk‐adjusted mortality compared to those in whom this therapy was continued (hazard ratio: 2.3; 95% CI: 1.2‐4.6; P = .013).55, 56 Finally, it is recommended that patients receiving an angiotensin‐converting enzyme inhibitor be continued on this agent as long as they are not in cardiogenic shock and do not have significantly deteriorating renal function.8
ROLE OF THE HOSPITALIST
Despite the presence of treatment guidelines, significant variation in the treatment of patients with ADHF persists.8, 58 Treatment of these patients is frequently contrary to the recommendations in published guidelines and can adversely impact both the cost of hospitalization and the ultimate clinical outcome. Low adherence to accepted standards of medical care has been shown to be a significant and independent risk factor for early hospital readmission.58 Furthermore, the main determinant of inotrope use in the ESCAPE trial was not the patient's cardiac output, blood pressure, or PCWP, but instead was the hospital to which the patient was admitted.59
Hospitalists are positioned to play a key role in improving both inpatient care of ADHF patients and the transition to long‐term patient management.60, 61 However, specific core competencies are required before hospitalists can effectively undertake this role. Table 2 highlights some of these core competencies.57
| Domain | Competencies |
|---|---|
| |
| Knowledge | Underlying causes of heart failure (eg, ischemia, cardiomyopathy, arrhythmia, drugs, alcohol) |
| Precipitating factors leading to exacerbation (eg, fluid overload) | |
| Indicated tests to evaluate heart failure (eg, chest x‐ray, echocardiography, B‐type natriuretic peptide levels) | |
| Risk factors for the development of heart failure (eg, hypertension, hyperlipidemia, coronary artery disease, diabetes, obesity) | |
| Risk stratification in patients admitted with heart failure | |
| Evidence‐based therapeutic options for management of both acute and chronic heart failure | |
| Indications, contraindications, and mechanisms of action of drugs used to treat heart failure | |
| Skills | Identify signs of low perfusion (eg, capillary refill, end‐organ dysfunction) |
| Attitudes | Recognize indications for cardiac consultation (eg, ischemia, atypical presentation, unresponsive to usual therapy) |
| Recognize indications for transplantation evaluation (eg, uncontrollable severe heart failure) | |
| System organization and improvement | Advocate establishment and support of outpatient heart failure management teams |
Data indicate that hospitalists are more likely than nonhospitalists to implement evidence‐based assessments and treatment.62 Lindenauer et al conducted a retrospective review of medical records from patients admitted for ADHF at a community‐based teaching hospital who were not managed by cardiologists and found that the assessment of left ventricular function was significantly greater when the patient's care was managed by a hospitalist (94%) compared to a nonhospitalist (87%; P = .04).61 Similarly, Roytman et al performed a retrospective review of medical records from another community‐based teaching hospital and found that patients admitted for ADHF who were managed by hospitalists were more likely than patients managed by community physicians (55% cardiologists) to receive intravenous diuretics (90% vs. 73%; P < .001) and to have angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker therapy initiated or resumed within 24 hours of hospital admission (86% vs. 72%; P = .003).62
Hospitalist care has also been shown to significantly reduce the duration of hospitalization. In the evaluation by Lindenauer et al, the risk‐adjusted length of stay was significantly shorter in patients whose care was managed by a hospitalist (P = .03). This benefit was greatest for patients in the major severity category.61 Similarly, in the review by Roytman et al, hospitalist care was associated with a 13%40% reduction in adjusted length of stay (P = .002), depending on disease severity.62 These reductions appear to be directly related to the greater experience of hospitalists in managing this and other acute disorders. In a retrospective review of data from an urban teaching hospital, care by a hospitalist, when compared with that by a nonhospitalist, was associated with a 15% reduction in overall length of stay (5.0 vs. 5.9 days; P < .02), with the greatest benefit observed in those patients whose disorders required close clinical monitoring (ie, heart failure, stroke, asthma, or pneumonia) or complex discharge planning.63 Moreover, there was a significant inverse correlation between the mean duration of hospitalization and the number of months of inpatient care provided by the attending physician each year ( = 0.19 day per month of inpatient care; P < .002).63
Finally, hospitalists are uniquely situated to influence medical care. Hospitalists have the ability to closely interact with patients over the course of several days. This exposure enhances opportunities to provide and reinforce patient education and information on lifestyle modifications, which have been shown to reduce the frequency of rehospitalization.60 In one evaluation, initiation of a care‐management program that included increased patient education reduced rehospitalizations for heart failure by 85% (P < .001).64 In another, an intensive, targeted education program significantly decreased the 1‐year risk‐adjusted probability of readmission or death (hazard ratio: 0.56; 95% CI: 0.32‐0.96; P = .03).65 Finally, it is important to remember that hospitalists also play a key role in the education of medical students and residents.60 This opportunity permits hospitalists to promote the adoption of standardized treatment algorithms that hopefully will be retained and propagated by these students long after their initial exposure to the hospitalist, thereby magnifying the effects of this education.
- ,,.National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data.Vital Health Stat 13.2007; (165):1–209.
- ,,, et al.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2008;117(4):e25–e146.
- .Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department.Rev Cardiovasc Med.2002;3(suppl 4):S3–S9.
- ,,, et al.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure).Circulation.2005;112(12):1825–1852.
- ,,, et al.Guidelines for acute decompensated heart failure treatment.Ann Pharmacother.2004;38(4):649–660.
- ,,,.Early ultrafiltration in patients with decompensated heart failure and diuretic resistance.J Am Coll Cardiol.2005;46(11):2047–2051.
- ,,,,.Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE.Cardiology.2007;107(1):44–51.
- Heart Failure Society of America.Executive Summary: HFSA 2006 Comprehensive Heart Failure Practice Guideline.J Card Fail.2006;12(1):10–38.
- ,,, et al.BNP Consensus Panel 2004: a clinical approach for the diagnostic, prognostic, screening, treatment monitoring, and therapeutic roles of natriuretic peptides in cardiovascular diseases.Congest Heart Fail.2004;10(5 suppl 3):1–30.
- ,,, et al.Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology.Eur Heart J.2005;26(4):384–416.
- ,.The limited reliability of physical signs for estimating hemodynamics in chronic heart failure.JAMA.1989;261(6):884–888.
- ,,, et al.In‐hospital mortality in patients with acute decompensated heart failure treated with intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE).J Am Coll Cardiol.2005;46(1):57–64.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296(18):2217–2226.
- ,,,,,.Utility of a rapid B‐natriuretic peptide assay in differentiating congestive heart failure from lung disease in patients presenting with dyspnea.J Am Coll Cardiol.2002;39(2):202–209.
- .Overview of acutely decompensated congestive heart failure (ADHF): a report from the ADHERE registry.Heart Fail Rev.2004;9(3):179–185.
- ,,, et al.The safety of intravenous diuretics alone versus diuretics plus parenteral vasoactive therapies in hospitalized patients with acutely decompensated heart failure: a propensity score and instrumental variable analysis using the Acutely Decompensated Heart Failure National Registry (ADHERE) database.Am Heart J.2007;154(2):267–277.
- ,,, et al.Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE‐HF registry.J Am Coll Cardiol.2007;50(8):768–777.
- ,,, et al.Utility of B‐type natriuretic peptide in the diagnosis of congestive heart failure in an urgent‐care setting.J Am Coll Cardiol.2001;37(2):379–385.
- ,,, et al.B‐type natriuretic peptide predicts future cardiac events in patients presenting to the emergency department with dyspnea.Ann Emerg Med.2002;39(2):131–138.
- .B‐type natriuretic peptide levels: diagnostic and prognostic in congestive heart failure: what's next?Circulation.2002;105(20):2328–2331.
- ,,, et al.Rapid measurement of B‐type natriuretic peptide in the emergency diagnosis of heart failure.N Engl J Med.2002;347(3):161–167.
- ,,, et al.Bedside B‐type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction: results from the Breathing Not Properly Multinational Study.J Am Coll Cardiol.2003;41(11):2010–2017.
- ,,, et al.Primary results of the Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT): a multicenter study of B‐type natriuretic peptide levels, emergency department decision making, and outcomes in patients presenting with shortness of breath.J Am Coll Cardiol.2004;44(6):1328–1333.
- ,,, et al.Application of NT‐proBNP and BNP measurements in cardiac care: a more discerning marker for the detection and evaluation of heart failure.Eur J Heart Fail.2004;6(3):295–300.
- ,,, et al.Hemoglobin and N‐terminal pro‐brain natriuretic peptide: independent and synergistic predictors of mortality in patients with acute heart failure. Results from the International Collaborative of NT‐proBNP (ICON) Study.Clin Chim Acta.2007;381(2):145–150.
- ,,, et al.Use of B‐type natriuretic peptide in the evaluation and management of acute dyspnea.N Engl J Med.2004;350(7):647–654.
- American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Acute Heart Failure Syndromes,,,,,.Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute heart failure syndromes.Ann Emerg Med.2007;49(5):627–669.
- ,,,,.Expression of natriuretic peptide receptors in human adipose and other tissues.J Endocrinol Invest.1996;19(9):581–585.
- ,,, et al.Clinical applications of B‐type natriuretic peptide (BNP) testing.Eur Heart J.2003;24(19):1710–1718.
- ,,, et al.B‐type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study.Am J Kidney Dis.2003;41(3):571–579.
- ,.B‐type natriuretic peptide as a biomarker beyond heart failure: speculations and opportunities.Mayo Clin Proc.2005;80(8):1029–1036.
- ,,.Natriuretic peptides for risk stratification of patients with acute coronary syndromes.Eur J Heart Fail.2004;6(3):327–333.
- ,,,,.Predicting outcome in patients with acute coronary syndrome: evaluation of B‐type natriuretic peptide and the global registry of acute coronary events (GRACE) risk score.Scott Med J.2007;52(3):8–13.
- ,,,,.Risk stratification for in‐hospital mortality in acutely decompensated heart failure: classification and regression tree analysis.JAMA.2005;293(5):572–580.
- ,,.Prognosis for patients newly admitted to hospital with heart failure: survival trends in 12 220 index admissions in Leicestershire 1993–2001.Heart.2003;89(6):615–620.
- ,,,.Predictors of mortality in younger and older patients with heart failure and preserved or reduced left ventricular ejection fraction.Am Heart J.2003;146(2):286–290.
- ,,,,,.Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model.JAMA.2003;290(19):2581–2587.
- Clinical Quality Improvement Network Investigators.Mortality risk and patterns of practice in 4606 acute care patients with congestive heart failure: the relative importance of age, sex, and medical therapy.Arch Intern Med.1996;156(15):1669–1673.
- ,,, et al.A simple clinically based predictive rule for heart failure in‐hospital mortality.J Card Fail.2006;12(8):587–593.
- ,,,,,.Characteristics, outcomes, and predictors of 1‐year mortality in patients hospitalized for acute heart failure.Eur Heart J.2006;27(24):3011–3017.
- ,,, et al.Ejection fraction and blood pressure are important and interactive predictors of 4‐week mortality in severe acute heart failure.Eur J Heart Fail.2007;9(9):935–941.
- ,,, et al.Worsening renal function: what is a clinically meaningful change in creatinine during hospitalization with heart failure?J Card Fail.2003;9(1):13–25.
- ,,, et al.Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure.J Am Coll Cardiol.2004;43(1):61–67.
- ,,, et al.Usefulness of anemia as a predictor of death and rehospitalization in patients with decompensated heart failure.Am J Cardiol.2003;92(5):625–628.
- ,,, et al.Relation of low hemoglobin and anemia to morbidity and mortality in patients hospitalized with heart failure (insight from the OPTIMIZE‐HF registry).Am J Cardiol.2008;101(2):223–230.
- ,,, et al.Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE‐HF registry.Eur Heart J.2007;28(8):980–988.
- ,,, et al.Prognostic value of combination of cardiac troponin T and B‐type natriuretic peptide after initiation of treatment in patients with chronic heart failure.Clin Chem.2003;49(12):2020–2026.
- ,,, et al.Usefulness of B‐type natriuretic peptide and cardiac troponin levels to predict in‐hospital mortality from ADHERE.Am J Cardiol.2008;101(2):231–237.
- ,,.Relation of loop diuretic dose to mortality in advanced heart failure.Am J Cardiol.2006;97(12):1759–1764.
- ,,, et al.Relation between diuretic dose and outcome in a heart failure population: results of the ESCAPE trial [Abstract 250].J Card Fail.2005;11(6 Suppl):S157.
- ,,.Repeated hospitalizations predict mortality in the community population with heart failure.Am Heart J.2007;154(2):260–266.
- ,,.Characteristics of “Stage D” heart failure: insights from the Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM).Am Heart J.2008;155(2):339–347.
- American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Guidelines for the evaluation and management of heart failure. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Evaluation and Management of Heart Failure).J Am Coll Cardiol.1995;26(5):1376–1398.
- ,,, et al.Short‐term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial.JAMA.2002;287(12):1541–1547.
- ,,, et al.Influence of beta‐blocker continuation or withdrawal on outcomes in patients hospitalized with heart failure: findings from the OPTIMIZE‐HF program.J Am Coll Cardiol.2008;52(3):190–199.
- ,,, et al.Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF): rationale and design.Am Heart J.2004;148(1):43–51.
- ,,,,, eds.The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine.J Hosp Med.2006;1(Suppl 1):2–95.
- ,,,,.The association between the quality of inpatient care and early readmission.Ann Intern Med.1995;122(6):415–421.
- ,,, et al.Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure.Am Heart J.2007;153(1):98–104.
- .Improving the management of patients after myocardial infarction, from admission to discharge.Clin Ther.2006;28(10):1509–1539.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162(11):1251–1256.
- ,,.Comparison of practice patterns of hospitalists and community physicians in the care of patients with congestive heart failure.J Hosp Med.2008;3(1):35–41.
- ,,,,.Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring.Arch Intern Med.2007;167(17):1869–1874.
- ,,, et al.Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure.J Am Coll Cardiol.1997;30(3):725–732.
- ,,, et al.Randomized trial of an education and support intervention to prevent readmission of patients with heart failure.J Am Coll Cardiol.2002;39(1):83–89.
Currently, acute decompensated heart failure (ADHF) accounts for 3% of all hospitalizations in the United States and is the second most common indication for hospitalization in individuals 65 years of age.1 These hospitalizations are costly and frequently have limited sustained benefits. The total direct medical cost attributable to ADHF hospitalization in the United States is estimated to be $18.8 billion annually.2 Furthermore, 50% of all patients hospitalized for ADHF are readmitted within 6 months of discharge.3 Clearly, the hospital management of these patients requires reevaluation.
The purpose of this article is to review the recognition, risk stratification, and treatment of ADHF and to discuss the role hospitalists can play in improving this treatment.
RECOGNITION OF ADHF
The American College of Cardiology/American Heart Association guidelines classify patients with heart failure into 1 of 4 stages, A through D.4 Patients with heart failure risk factors who do not have evidence of structural heart disease are classified as Stage A. Patients with evidence of structural heart disease who have never been symptomatic are classified as Stage B. Patients who are presently or previously symptomatic and responsive to standard therapies are classified as Stage C. Finally, patients are classified as Stage D if they are refractory to standard therapies and require specialized advanced treatment such as mechanical circulatory support, continuous inotropic infusions, or cardiac transplantation. By definition, patients with ADHF have either Stage C or Stage D heart failure.
Early recognition and appropriate treatment are key components in improving the management of these patients.57 Hospitalization is recommended for patients with evidence of severely decompensated heart failure, dyspnea at rest, hemodynamically significant arrhythmias, and acute coronary syndromes and should be considered in patients with worsening congestion, major electrolyte abnormalities, associated comorbid conditions, and repeated implantable cardioverter‐defibrillator firings.8 However, correctly identifying ADHF at the time of hospital presentation can be challenging.9 The diagnosis of ADHF is based on signs and symptoms, supported by radiographic findings, biomarkers, and echocardiography.8, 10 Unfortunately, the typical signs and symptoms of ADHFfor example, rales, peripheral edema, dyspnea at rest, and fatiguemay be missing at hospital presentation. In an early evaluation, rales, edema, and elevated mean jugular venous pressure were absent in 18 of 43 patients with documented pulmonary capillary wedge pressures (PCWP) 22 mm Hg.11 These findings have recently been confirmed using data from 2 large registries, the Acute Decompensated Heart Failure National Registry (ADHERE) and the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF) registry. In these registries, 32%36% of patients admitted with ADHF did not have rales, 33%35% did not have peripheral edema, 56%64% did not have dyspnea at rest, and approximately 67% did not have fatigue (Figure 1).12, 13 Furthermore, even when these signs and symptoms are present, they are nondiagnostic, because they can be produced by a variety of disorders, including hepatic, renal, and pulmonary dysfunction.8, 14

Similarly, radiographic and echocardiographic features of ADHF are not always present. Overall, 26% of patients in ADHERE did not have evidence of pulmonary congestion on their initial chest radiograph, and 50%55% of patients in both registries had preserved systolic function.13, 1517 Consequently, attention has turned to biomarkers as a means of rapidly and accurately identifying ADHF. Serum Btype natriuretic peptide (BNP) and its N‐terminal prohormone (NT‐proBNP) have proven to be both diagnostic and prognostic indicators in ADHF.14, 1825 In the Breathing Not Properly Multinational Study, a BNP level 100 pg/mL was found to have a 90% sensitivity (95% confidence interval [CI]: 88%92%) and a 76% specificity (95% CI: 73%79%) for heart failure in patients presenting to the emergency department with dyspnea.21 In addition, BNP levels have been shown to correlate with heart failure severity18 and to be a more accurate reflection of this severity than clinical judgment.23 In a prospective randomized evaluation, the addition of BNP assessment to a standard diagnostic evaluation resulted in fewer patients being hospitalized (75% vs. 85%; P = .008), more rapid initiation of appropriate therapy (63 vs. 90 minutes; P = .03), and a shorter median duration of hospitalization (8 vs. 11 days; P = .001).26 As a result, the American College of Emergency Physicians guidelines now state that measurement of BNP or NT‐proBNP can improve diagnostic accuracy in acute heart failure syndrome when compared with standard clinical judgment alone.27
It is important to remember, however, that BNP levels cannot be interpreted in isolation; clinical judgment still plays a vital role. Obesity decreases BNP levels due to the expression of natriuretic peptide clearance receptors in adipose tissue.9, 28, 29 In contrast, BNP levels increase with age and are higher in women than in men.29 In addition, pulmonary embolism, an important diagnostic consideration in patients presenting with dyspnea, has been shown to increase serum BNP levels above the diagnostic threshold for ADHF.9, 29 Likewise, renal dysfunction, a common comorbidity in patients with heart failure (cardiorenal syndrome), increases serum BNP levels.30 As a result, the BNP threshold value for the diagnosis of ADHF rises from 100 pg/mL in patients with normal renal function to 200 pg/mL in patients with an estimated glomerular filtration rate <60 mL/min/1.73 m2.30 Finally, it is now well recognized that BNP production is up‐regulated by numerous physiologic conditions in addition to heart failure, including cardiac hypertrophy, endothelial dysfunction, and arrhythmia.31 Consequently, an elevated BNP level may indicate one of these conditions instead of ADHF. For example, recent data demonstrate that BNP levels are increased in patients with acute coronary syndromes and also serve as a significant prognostic factor in these patients.32, 33
RISK STRATIFICATION
Risk stratification, another important component in improving the management of patients with ADHF, helps determine the appropriate location (eg, outpatient, hospital ward, intensive care unit) for and intensity of initial monitoring and treatment.13, 25, 3452 Univariate analyses have identified several morbidity and/or mortality risk factors, including age,3540 blood pressure,13, 34, 37, 3941 respiratory rate,37 left ventricular ejection fraction (LVEF),36, 41, 48 renal function,34, 36, 37, 39, 40, 42, 43 anemia,25, 44, 45 hyponatremia,37, 39, 46 BNP level,36, 49, 50 cardiac troponin level,48 diuretic dose,36, 49, 50 previous heart failure hospitalization,44, 51, 52 and comorbid conditions.35, 37, 39 Unfortunately, these univariate factors are not very helpful in and of themselves, as they regularly occur in conjunction with each other. True risk assessment requires multivariate analyses of large datasets.
Multivariate risk factors for short‐term mortality in patients admitted for ADHF have been evaluated in 3 separate studies. Lee et al used multiple logistic regression to analyze data from 4031 hospitalization episodes at 34 centers in Canada,37 Fonarow et al used both classification and regression tree and multivariate regression models to analyze data from 65,275 hospitalization episodes at 263 centers in the United States,34 and Rohde et al used stepwise logistic regression to analyze data from 779 consecutive hospitalization episodes at a single center in Brazil.39 Despite these differences in statistical methodology and geographic location, the findings of these 3 analyses are remarkably similar. All 3 evaluations identified advanced age, lower systolic blood pressure, and renal dysfunction (cardiorenal syndrome) as significant and independent risk factors for short‐term mortality, and 2 of the 3 identified hyponatremia and comorbid cancer as additional risk factors (Table 1).34 Of note, lower systolic blood pressure did not mean hypotension in these evaluations. Mortality risk was significantly increased in patients with systolic blood pressure <115‐124 mm Hg. In the largest of these studies, a simple risk tree utilizing admission blood pressure, serum creatinine concentration, and blood urea nitrogen level stratified patients into groups with in‐hospital mortality risk ranging from 2.1%21.9% in the derivation and 2.3%19.8% in the validation cohorts (Figure 2).34 Taken together, these studies underscore the substantial role age, blood pressure, renal function, serum sodium concentration, and comorbidities play in increasing mortality risk, and these factors should always be considered in determining the intensity and location of ADHF treatment and degree of monitoring employed therein.

| Parameter | Study | ||
|---|---|---|---|
| Lee et al37 | Fonarow et al34 | Rohde et al39 | |
| |||
| Data source | 34 Hospitals | 263 Hospitals | Single center |
| Admissions evaluated | 4031 | 65,275 | 779 |
| Outcome parameter | 30‐Day mortality | In‐hospital mortality | In‐hospital mortality |
| Independent risk factors | |||
| Older age | Yes | Yes | Yes (>70 years) |
| Lower SBP | Yes | Yes (<115 mm Hg) | Yes (124 mm Hg) |
| Renal dysfunction | Yes | Yes | Yes |
| Elevated BUN | Yes | Yes (>43 mg/dL) | Yes (>37 mg/dL) |
| Elevated serum creatinine | Yes (>2.75 mg/dL) | Yes (>1.4 mg/dL) | |
| Hyponatremia | Yes | Yes (<136 mEq/L) | |
| Elevated heart rate | Yes | ||
| Elevated respiratory rate | Yes | ||
| Comorbid conditions | Yes | Yes | |
| Cancer | Yes | Yes | |
| Cerebrovascular disease | Yes | ||
| COPD | Yes | ||
| Dementia | Yes | ||
Although BNP and cardiac troponin level were not significant risk factors in the multivariate models, these levels were not routinely assessed in patients admitted for ADHF 5 to 10 years ago. For example, admission BNP was available in only 18% of patients in the Fonarow analysis,34 and this lack of data may explain the absence of these parameters in these multivariate analyses. In a recent analysis limited to patients with admission BNP and cardiac troponin data, in‐hospital mortality was significantly increased when BNP was 840 pg/mL (odds ratio [OR]: 1.60; 95% CI: 1.431.80; P < .001), cardiac troponin was positive (OR: 1.85; 95% CI: 1.572.18; P < .001) or both (OR: 3.00; 95% CI: 2.473.66; P < .001) even after adjusting for differences in age, gender, blood urea nitrogen, systolic blood pressure, serum creatinine concentration, serum sodium concentration, heart rate, and dyspnea at rest.4
THERAPY
Ideally, treatment should be rooted in evidence‐based guidelines. However, relatively few randomized, controlled clinical trials have been completed in patients with ADHF, and consequently there are minimal data available to construct these guidelines. The American College of Cardiology and the American Heart Association have jointly published guidelines since 1995 on the management of heart failure.4, 53 However, these guidelines, which were last updated in 2005, discuss only the management of chronic heart failure, not the management of ADHF.4 In fact, the most recent version of these guidelines specifically states, The committee elected to focus this document on the diagnosis and management of chronic heart failure It specifically did not consider acute heart failure, which might merit a separate set of guidelines.4
The first guideline to specifically address the management of ADHF was published in 2004.5 These guidelines, a consensus statement based on expert panel review of the available literature, were created to improve treatment at member hospitals of a national group purchasing organization and focused only on the initial 24 hours of care. They had 2 important components. The first was a timeline emphasizing rapid assessment and institution of therapy, followed by serial reevaluations every couple of hours thereafter.5 The second was a flow chart detailing recommended initial therapies based on the current clinical findings and the patient's chronic outpatient pharmacotherapy, followed by modifications to this initial therapy based on the response observed during the serial reevaluations. Treatment recommendations were as follows: for patients with mild volume overload, an intravenous diuretic; for patients with moderate to severe volume overload, an intravenous diuretic plus an intravenous vasodilator (nitroglycerin or nesiritide); and for patients with low cardiac output, an inotropic agent with or without a subsequent intravenous vasodilator.
In 2005, the European Society of Cardiology published its guidelines for the treatment of ADHF.10 These guidelines state that the immediate goal of ADHF therapy is to improve symptoms and stabilize hemodynamics, but these short‐term benefits must be accompanied by favorable effects on long‐term outcomes.10 Recommended treatment consists of fluid loading, diuretics, vasodilators (glyceryl trinitrate, isosorbide dinitrate, nitroprusside, or nesiritide), and/or inotropic agents (dopamine, dobutamine, milrinone, enoximone, levosimendan, norepinephrine, or epinephrine), depending on the patient's clinical status and hemodynamics.10 In general, the guidelines recommend fluid loading in patients with low cardiac output and low PCWP; a vasodilator or inotropic agent, depending on systolic blood pressure, in patients with low cardiac output and normal to high PCWP; and an intravenous diuretic in patients with normal cardiac output and high PCWP pressure. Finally, respiratory support, eg, continuous positive airway pressure (CPAP), noninvasive positive pressure ventilation, or endotracheal intubation and mechanical ventilation, may be necessary in some patients with left‐heart failure.
In 2006, the Heart Failure Society of America published comprehensive heart failure practice guidelines.8 These guidelines expand the goals of ADHF therapy to include improving symptoms, optimizing volume status, identifying precipitating factors, enhancing chronic oral therapy, and minimizing side effects. They provide the most detailed recommendations yet with respect to monitoring patents admitted for ADHF.8 According to these guidelines, this monitoring should include more than daily assessment of vital signs, including orthostatic blood pressure, and at least daily assessment of heart failure signs and symptoms, fluid intake and output, weight, electrolytes, and renal function. Treatment recommendations are similar to those in preceding guidelines. Intravenous loop diuretics are recommended as first‐line therapy in patients with volume overload.8 In the absence of systemic hypotension, the addition of an intravenous vasodilator (nitroglycerin, nitroprusside, or nesiritide) should be considered to achieve rapid symptomatic improvement.8 Intravenous inotropic therapy may be considered to improve symptoms and end‐organ function in patients with low‐output syndrome (left ventricular dilation, reduced LVEF, and diminished peripheral perfusion), especially if systolic blood pressure is <90 mm Hg or there is symptomatic hypotension despite adequate filling pressures.54 Outside of this small select group of patients, there is no rationale for the use of inotropic agents.8 Patients with ADHF who received an inotropic agent in the absence of a clearly defined clinical indication had an increased risk of adverse events without any evidence of clinical benefit in the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME‐CHF) trial.54 Ultrafiltration may be considered in patients who fail to respond adequately to diuretic therapy,6, 8 and an implantable left ventricular assist device (LVAD) should be considered as a bridge to cardiac transplantation in patients with severe heart failure (Stage D) who have become refractory to all means of medical circulatory support and may be considered in highly selected nontransplantation candidates who cannot be weaned from intravenous inotropic support.8, 52
Whether to continue or temporarily stop chronic oral heart failure medications during treatment of an acute decompensation is not addressed in any of the evidence‐based guidelines and ultimately, this decision must be based on the patient's clinical status. In general, guideline‐recommended intravenous diuretic therapy temporarily replaces the patient's chronic oral diuretic regimen. Oral ‐blocker therapy should be continued whenever possible, as long as the patient's blood pressure and clinical status can tolerate it. In an analysis of data from the OPTIMIZE‐HF registry, patients with ADHF who had withdrawal of ‐blocker had significantly greater risk‐adjusted mortality compared to those in whom this therapy was continued (hazard ratio: 2.3; 95% CI: 1.2‐4.6; P = .013).55, 56 Finally, it is recommended that patients receiving an angiotensin‐converting enzyme inhibitor be continued on this agent as long as they are not in cardiogenic shock and do not have significantly deteriorating renal function.8
ROLE OF THE HOSPITALIST
Despite the presence of treatment guidelines, significant variation in the treatment of patients with ADHF persists.8, 58 Treatment of these patients is frequently contrary to the recommendations in published guidelines and can adversely impact both the cost of hospitalization and the ultimate clinical outcome. Low adherence to accepted standards of medical care has been shown to be a significant and independent risk factor for early hospital readmission.58 Furthermore, the main determinant of inotrope use in the ESCAPE trial was not the patient's cardiac output, blood pressure, or PCWP, but instead was the hospital to which the patient was admitted.59
Hospitalists are positioned to play a key role in improving both inpatient care of ADHF patients and the transition to long‐term patient management.60, 61 However, specific core competencies are required before hospitalists can effectively undertake this role. Table 2 highlights some of these core competencies.57
| Domain | Competencies |
|---|---|
| |
| Knowledge | Underlying causes of heart failure (eg, ischemia, cardiomyopathy, arrhythmia, drugs, alcohol) |
| Precipitating factors leading to exacerbation (eg, fluid overload) | |
| Indicated tests to evaluate heart failure (eg, chest x‐ray, echocardiography, B‐type natriuretic peptide levels) | |
| Risk factors for the development of heart failure (eg, hypertension, hyperlipidemia, coronary artery disease, diabetes, obesity) | |
| Risk stratification in patients admitted with heart failure | |
| Evidence‐based therapeutic options for management of both acute and chronic heart failure | |
| Indications, contraindications, and mechanisms of action of drugs used to treat heart failure | |
| Skills | Identify signs of low perfusion (eg, capillary refill, end‐organ dysfunction) |
| Attitudes | Recognize indications for cardiac consultation (eg, ischemia, atypical presentation, unresponsive to usual therapy) |
| Recognize indications for transplantation evaluation (eg, uncontrollable severe heart failure) | |
| System organization and improvement | Advocate establishment and support of outpatient heart failure management teams |
Data indicate that hospitalists are more likely than nonhospitalists to implement evidence‐based assessments and treatment.62 Lindenauer et al conducted a retrospective review of medical records from patients admitted for ADHF at a community‐based teaching hospital who were not managed by cardiologists and found that the assessment of left ventricular function was significantly greater when the patient's care was managed by a hospitalist (94%) compared to a nonhospitalist (87%; P = .04).61 Similarly, Roytman et al performed a retrospective review of medical records from another community‐based teaching hospital and found that patients admitted for ADHF who were managed by hospitalists were more likely than patients managed by community physicians (55% cardiologists) to receive intravenous diuretics (90% vs. 73%; P < .001) and to have angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker therapy initiated or resumed within 24 hours of hospital admission (86% vs. 72%; P = .003).62
Hospitalist care has also been shown to significantly reduce the duration of hospitalization. In the evaluation by Lindenauer et al, the risk‐adjusted length of stay was significantly shorter in patients whose care was managed by a hospitalist (P = .03). This benefit was greatest for patients in the major severity category.61 Similarly, in the review by Roytman et al, hospitalist care was associated with a 13%40% reduction in adjusted length of stay (P = .002), depending on disease severity.62 These reductions appear to be directly related to the greater experience of hospitalists in managing this and other acute disorders. In a retrospective review of data from an urban teaching hospital, care by a hospitalist, when compared with that by a nonhospitalist, was associated with a 15% reduction in overall length of stay (5.0 vs. 5.9 days; P < .02), with the greatest benefit observed in those patients whose disorders required close clinical monitoring (ie, heart failure, stroke, asthma, or pneumonia) or complex discharge planning.63 Moreover, there was a significant inverse correlation between the mean duration of hospitalization and the number of months of inpatient care provided by the attending physician each year ( = 0.19 day per month of inpatient care; P < .002).63
Finally, hospitalists are uniquely situated to influence medical care. Hospitalists have the ability to closely interact with patients over the course of several days. This exposure enhances opportunities to provide and reinforce patient education and information on lifestyle modifications, which have been shown to reduce the frequency of rehospitalization.60 In one evaluation, initiation of a care‐management program that included increased patient education reduced rehospitalizations for heart failure by 85% (P < .001).64 In another, an intensive, targeted education program significantly decreased the 1‐year risk‐adjusted probability of readmission or death (hazard ratio: 0.56; 95% CI: 0.32‐0.96; P = .03).65 Finally, it is important to remember that hospitalists also play a key role in the education of medical students and residents.60 This opportunity permits hospitalists to promote the adoption of standardized treatment algorithms that hopefully will be retained and propagated by these students long after their initial exposure to the hospitalist, thereby magnifying the effects of this education.
Currently, acute decompensated heart failure (ADHF) accounts for 3% of all hospitalizations in the United States and is the second most common indication for hospitalization in individuals 65 years of age.1 These hospitalizations are costly and frequently have limited sustained benefits. The total direct medical cost attributable to ADHF hospitalization in the United States is estimated to be $18.8 billion annually.2 Furthermore, 50% of all patients hospitalized for ADHF are readmitted within 6 months of discharge.3 Clearly, the hospital management of these patients requires reevaluation.
The purpose of this article is to review the recognition, risk stratification, and treatment of ADHF and to discuss the role hospitalists can play in improving this treatment.
RECOGNITION OF ADHF
The American College of Cardiology/American Heart Association guidelines classify patients with heart failure into 1 of 4 stages, A through D.4 Patients with heart failure risk factors who do not have evidence of structural heart disease are classified as Stage A. Patients with evidence of structural heart disease who have never been symptomatic are classified as Stage B. Patients who are presently or previously symptomatic and responsive to standard therapies are classified as Stage C. Finally, patients are classified as Stage D if they are refractory to standard therapies and require specialized advanced treatment such as mechanical circulatory support, continuous inotropic infusions, or cardiac transplantation. By definition, patients with ADHF have either Stage C or Stage D heart failure.
Early recognition and appropriate treatment are key components in improving the management of these patients.57 Hospitalization is recommended for patients with evidence of severely decompensated heart failure, dyspnea at rest, hemodynamically significant arrhythmias, and acute coronary syndromes and should be considered in patients with worsening congestion, major electrolyte abnormalities, associated comorbid conditions, and repeated implantable cardioverter‐defibrillator firings.8 However, correctly identifying ADHF at the time of hospital presentation can be challenging.9 The diagnosis of ADHF is based on signs and symptoms, supported by radiographic findings, biomarkers, and echocardiography.8, 10 Unfortunately, the typical signs and symptoms of ADHFfor example, rales, peripheral edema, dyspnea at rest, and fatiguemay be missing at hospital presentation. In an early evaluation, rales, edema, and elevated mean jugular venous pressure were absent in 18 of 43 patients with documented pulmonary capillary wedge pressures (PCWP) 22 mm Hg.11 These findings have recently been confirmed using data from 2 large registries, the Acute Decompensated Heart Failure National Registry (ADHERE) and the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF) registry. In these registries, 32%36% of patients admitted with ADHF did not have rales, 33%35% did not have peripheral edema, 56%64% did not have dyspnea at rest, and approximately 67% did not have fatigue (Figure 1).12, 13 Furthermore, even when these signs and symptoms are present, they are nondiagnostic, because they can be produced by a variety of disorders, including hepatic, renal, and pulmonary dysfunction.8, 14

Similarly, radiographic and echocardiographic features of ADHF are not always present. Overall, 26% of patients in ADHERE did not have evidence of pulmonary congestion on their initial chest radiograph, and 50%55% of patients in both registries had preserved systolic function.13, 1517 Consequently, attention has turned to biomarkers as a means of rapidly and accurately identifying ADHF. Serum Btype natriuretic peptide (BNP) and its N‐terminal prohormone (NT‐proBNP) have proven to be both diagnostic and prognostic indicators in ADHF.14, 1825 In the Breathing Not Properly Multinational Study, a BNP level 100 pg/mL was found to have a 90% sensitivity (95% confidence interval [CI]: 88%92%) and a 76% specificity (95% CI: 73%79%) for heart failure in patients presenting to the emergency department with dyspnea.21 In addition, BNP levels have been shown to correlate with heart failure severity18 and to be a more accurate reflection of this severity than clinical judgment.23 In a prospective randomized evaluation, the addition of BNP assessment to a standard diagnostic evaluation resulted in fewer patients being hospitalized (75% vs. 85%; P = .008), more rapid initiation of appropriate therapy (63 vs. 90 minutes; P = .03), and a shorter median duration of hospitalization (8 vs. 11 days; P = .001).26 As a result, the American College of Emergency Physicians guidelines now state that measurement of BNP or NT‐proBNP can improve diagnostic accuracy in acute heart failure syndrome when compared with standard clinical judgment alone.27
It is important to remember, however, that BNP levels cannot be interpreted in isolation; clinical judgment still plays a vital role. Obesity decreases BNP levels due to the expression of natriuretic peptide clearance receptors in adipose tissue.9, 28, 29 In contrast, BNP levels increase with age and are higher in women than in men.29 In addition, pulmonary embolism, an important diagnostic consideration in patients presenting with dyspnea, has been shown to increase serum BNP levels above the diagnostic threshold for ADHF.9, 29 Likewise, renal dysfunction, a common comorbidity in patients with heart failure (cardiorenal syndrome), increases serum BNP levels.30 As a result, the BNP threshold value for the diagnosis of ADHF rises from 100 pg/mL in patients with normal renal function to 200 pg/mL in patients with an estimated glomerular filtration rate <60 mL/min/1.73 m2.30 Finally, it is now well recognized that BNP production is up‐regulated by numerous physiologic conditions in addition to heart failure, including cardiac hypertrophy, endothelial dysfunction, and arrhythmia.31 Consequently, an elevated BNP level may indicate one of these conditions instead of ADHF. For example, recent data demonstrate that BNP levels are increased in patients with acute coronary syndromes and also serve as a significant prognostic factor in these patients.32, 33
RISK STRATIFICATION
Risk stratification, another important component in improving the management of patients with ADHF, helps determine the appropriate location (eg, outpatient, hospital ward, intensive care unit) for and intensity of initial monitoring and treatment.13, 25, 3452 Univariate analyses have identified several morbidity and/or mortality risk factors, including age,3540 blood pressure,13, 34, 37, 3941 respiratory rate,37 left ventricular ejection fraction (LVEF),36, 41, 48 renal function,34, 36, 37, 39, 40, 42, 43 anemia,25, 44, 45 hyponatremia,37, 39, 46 BNP level,36, 49, 50 cardiac troponin level,48 diuretic dose,36, 49, 50 previous heart failure hospitalization,44, 51, 52 and comorbid conditions.35, 37, 39 Unfortunately, these univariate factors are not very helpful in and of themselves, as they regularly occur in conjunction with each other. True risk assessment requires multivariate analyses of large datasets.
Multivariate risk factors for short‐term mortality in patients admitted for ADHF have been evaluated in 3 separate studies. Lee et al used multiple logistic regression to analyze data from 4031 hospitalization episodes at 34 centers in Canada,37 Fonarow et al used both classification and regression tree and multivariate regression models to analyze data from 65,275 hospitalization episodes at 263 centers in the United States,34 and Rohde et al used stepwise logistic regression to analyze data from 779 consecutive hospitalization episodes at a single center in Brazil.39 Despite these differences in statistical methodology and geographic location, the findings of these 3 analyses are remarkably similar. All 3 evaluations identified advanced age, lower systolic blood pressure, and renal dysfunction (cardiorenal syndrome) as significant and independent risk factors for short‐term mortality, and 2 of the 3 identified hyponatremia and comorbid cancer as additional risk factors (Table 1).34 Of note, lower systolic blood pressure did not mean hypotension in these evaluations. Mortality risk was significantly increased in patients with systolic blood pressure <115‐124 mm Hg. In the largest of these studies, a simple risk tree utilizing admission blood pressure, serum creatinine concentration, and blood urea nitrogen level stratified patients into groups with in‐hospital mortality risk ranging from 2.1%21.9% in the derivation and 2.3%19.8% in the validation cohorts (Figure 2).34 Taken together, these studies underscore the substantial role age, blood pressure, renal function, serum sodium concentration, and comorbidities play in increasing mortality risk, and these factors should always be considered in determining the intensity and location of ADHF treatment and degree of monitoring employed therein.

| Parameter | Study | ||
|---|---|---|---|
| Lee et al37 | Fonarow et al34 | Rohde et al39 | |
| |||
| Data source | 34 Hospitals | 263 Hospitals | Single center |
| Admissions evaluated | 4031 | 65,275 | 779 |
| Outcome parameter | 30‐Day mortality | In‐hospital mortality | In‐hospital mortality |
| Independent risk factors | |||
| Older age | Yes | Yes | Yes (>70 years) |
| Lower SBP | Yes | Yes (<115 mm Hg) | Yes (124 mm Hg) |
| Renal dysfunction | Yes | Yes | Yes |
| Elevated BUN | Yes | Yes (>43 mg/dL) | Yes (>37 mg/dL) |
| Elevated serum creatinine | Yes (>2.75 mg/dL) | Yes (>1.4 mg/dL) | |
| Hyponatremia | Yes | Yes (<136 mEq/L) | |
| Elevated heart rate | Yes | ||
| Elevated respiratory rate | Yes | ||
| Comorbid conditions | Yes | Yes | |
| Cancer | Yes | Yes | |
| Cerebrovascular disease | Yes | ||
| COPD | Yes | ||
| Dementia | Yes | ||
Although BNP and cardiac troponin level were not significant risk factors in the multivariate models, these levels were not routinely assessed in patients admitted for ADHF 5 to 10 years ago. For example, admission BNP was available in only 18% of patients in the Fonarow analysis,34 and this lack of data may explain the absence of these parameters in these multivariate analyses. In a recent analysis limited to patients with admission BNP and cardiac troponin data, in‐hospital mortality was significantly increased when BNP was 840 pg/mL (odds ratio [OR]: 1.60; 95% CI: 1.431.80; P < .001), cardiac troponin was positive (OR: 1.85; 95% CI: 1.572.18; P < .001) or both (OR: 3.00; 95% CI: 2.473.66; P < .001) even after adjusting for differences in age, gender, blood urea nitrogen, systolic blood pressure, serum creatinine concentration, serum sodium concentration, heart rate, and dyspnea at rest.4
THERAPY
Ideally, treatment should be rooted in evidence‐based guidelines. However, relatively few randomized, controlled clinical trials have been completed in patients with ADHF, and consequently there are minimal data available to construct these guidelines. The American College of Cardiology and the American Heart Association have jointly published guidelines since 1995 on the management of heart failure.4, 53 However, these guidelines, which were last updated in 2005, discuss only the management of chronic heart failure, not the management of ADHF.4 In fact, the most recent version of these guidelines specifically states, The committee elected to focus this document on the diagnosis and management of chronic heart failure It specifically did not consider acute heart failure, which might merit a separate set of guidelines.4
The first guideline to specifically address the management of ADHF was published in 2004.5 These guidelines, a consensus statement based on expert panel review of the available literature, were created to improve treatment at member hospitals of a national group purchasing organization and focused only on the initial 24 hours of care. They had 2 important components. The first was a timeline emphasizing rapid assessment and institution of therapy, followed by serial reevaluations every couple of hours thereafter.5 The second was a flow chart detailing recommended initial therapies based on the current clinical findings and the patient's chronic outpatient pharmacotherapy, followed by modifications to this initial therapy based on the response observed during the serial reevaluations. Treatment recommendations were as follows: for patients with mild volume overload, an intravenous diuretic; for patients with moderate to severe volume overload, an intravenous diuretic plus an intravenous vasodilator (nitroglycerin or nesiritide); and for patients with low cardiac output, an inotropic agent with or without a subsequent intravenous vasodilator.
In 2005, the European Society of Cardiology published its guidelines for the treatment of ADHF.10 These guidelines state that the immediate goal of ADHF therapy is to improve symptoms and stabilize hemodynamics, but these short‐term benefits must be accompanied by favorable effects on long‐term outcomes.10 Recommended treatment consists of fluid loading, diuretics, vasodilators (glyceryl trinitrate, isosorbide dinitrate, nitroprusside, or nesiritide), and/or inotropic agents (dopamine, dobutamine, milrinone, enoximone, levosimendan, norepinephrine, or epinephrine), depending on the patient's clinical status and hemodynamics.10 In general, the guidelines recommend fluid loading in patients with low cardiac output and low PCWP; a vasodilator or inotropic agent, depending on systolic blood pressure, in patients with low cardiac output and normal to high PCWP; and an intravenous diuretic in patients with normal cardiac output and high PCWP pressure. Finally, respiratory support, eg, continuous positive airway pressure (CPAP), noninvasive positive pressure ventilation, or endotracheal intubation and mechanical ventilation, may be necessary in some patients with left‐heart failure.
In 2006, the Heart Failure Society of America published comprehensive heart failure practice guidelines.8 These guidelines expand the goals of ADHF therapy to include improving symptoms, optimizing volume status, identifying precipitating factors, enhancing chronic oral therapy, and minimizing side effects. They provide the most detailed recommendations yet with respect to monitoring patents admitted for ADHF.8 According to these guidelines, this monitoring should include more than daily assessment of vital signs, including orthostatic blood pressure, and at least daily assessment of heart failure signs and symptoms, fluid intake and output, weight, electrolytes, and renal function. Treatment recommendations are similar to those in preceding guidelines. Intravenous loop diuretics are recommended as first‐line therapy in patients with volume overload.8 In the absence of systemic hypotension, the addition of an intravenous vasodilator (nitroglycerin, nitroprusside, or nesiritide) should be considered to achieve rapid symptomatic improvement.8 Intravenous inotropic therapy may be considered to improve symptoms and end‐organ function in patients with low‐output syndrome (left ventricular dilation, reduced LVEF, and diminished peripheral perfusion), especially if systolic blood pressure is <90 mm Hg or there is symptomatic hypotension despite adequate filling pressures.54 Outside of this small select group of patients, there is no rationale for the use of inotropic agents.8 Patients with ADHF who received an inotropic agent in the absence of a clearly defined clinical indication had an increased risk of adverse events without any evidence of clinical benefit in the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME‐CHF) trial.54 Ultrafiltration may be considered in patients who fail to respond adequately to diuretic therapy,6, 8 and an implantable left ventricular assist device (LVAD) should be considered as a bridge to cardiac transplantation in patients with severe heart failure (Stage D) who have become refractory to all means of medical circulatory support and may be considered in highly selected nontransplantation candidates who cannot be weaned from intravenous inotropic support.8, 52
Whether to continue or temporarily stop chronic oral heart failure medications during treatment of an acute decompensation is not addressed in any of the evidence‐based guidelines and ultimately, this decision must be based on the patient's clinical status. In general, guideline‐recommended intravenous diuretic therapy temporarily replaces the patient's chronic oral diuretic regimen. Oral ‐blocker therapy should be continued whenever possible, as long as the patient's blood pressure and clinical status can tolerate it. In an analysis of data from the OPTIMIZE‐HF registry, patients with ADHF who had withdrawal of ‐blocker had significantly greater risk‐adjusted mortality compared to those in whom this therapy was continued (hazard ratio: 2.3; 95% CI: 1.2‐4.6; P = .013).55, 56 Finally, it is recommended that patients receiving an angiotensin‐converting enzyme inhibitor be continued on this agent as long as they are not in cardiogenic shock and do not have significantly deteriorating renal function.8
ROLE OF THE HOSPITALIST
Despite the presence of treatment guidelines, significant variation in the treatment of patients with ADHF persists.8, 58 Treatment of these patients is frequently contrary to the recommendations in published guidelines and can adversely impact both the cost of hospitalization and the ultimate clinical outcome. Low adherence to accepted standards of medical care has been shown to be a significant and independent risk factor for early hospital readmission.58 Furthermore, the main determinant of inotrope use in the ESCAPE trial was not the patient's cardiac output, blood pressure, or PCWP, but instead was the hospital to which the patient was admitted.59
Hospitalists are positioned to play a key role in improving both inpatient care of ADHF patients and the transition to long‐term patient management.60, 61 However, specific core competencies are required before hospitalists can effectively undertake this role. Table 2 highlights some of these core competencies.57
| Domain | Competencies |
|---|---|
| |
| Knowledge | Underlying causes of heart failure (eg, ischemia, cardiomyopathy, arrhythmia, drugs, alcohol) |
| Precipitating factors leading to exacerbation (eg, fluid overload) | |
| Indicated tests to evaluate heart failure (eg, chest x‐ray, echocardiography, B‐type natriuretic peptide levels) | |
| Risk factors for the development of heart failure (eg, hypertension, hyperlipidemia, coronary artery disease, diabetes, obesity) | |
| Risk stratification in patients admitted with heart failure | |
| Evidence‐based therapeutic options for management of both acute and chronic heart failure | |
| Indications, contraindications, and mechanisms of action of drugs used to treat heart failure | |
| Skills | Identify signs of low perfusion (eg, capillary refill, end‐organ dysfunction) |
| Attitudes | Recognize indications for cardiac consultation (eg, ischemia, atypical presentation, unresponsive to usual therapy) |
| Recognize indications for transplantation evaluation (eg, uncontrollable severe heart failure) | |
| System organization and improvement | Advocate establishment and support of outpatient heart failure management teams |
Data indicate that hospitalists are more likely than nonhospitalists to implement evidence‐based assessments and treatment.62 Lindenauer et al conducted a retrospective review of medical records from patients admitted for ADHF at a community‐based teaching hospital who were not managed by cardiologists and found that the assessment of left ventricular function was significantly greater when the patient's care was managed by a hospitalist (94%) compared to a nonhospitalist (87%; P = .04).61 Similarly, Roytman et al performed a retrospective review of medical records from another community‐based teaching hospital and found that patients admitted for ADHF who were managed by hospitalists were more likely than patients managed by community physicians (55% cardiologists) to receive intravenous diuretics (90% vs. 73%; P < .001) and to have angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker therapy initiated or resumed within 24 hours of hospital admission (86% vs. 72%; P = .003).62
Hospitalist care has also been shown to significantly reduce the duration of hospitalization. In the evaluation by Lindenauer et al, the risk‐adjusted length of stay was significantly shorter in patients whose care was managed by a hospitalist (P = .03). This benefit was greatest for patients in the major severity category.61 Similarly, in the review by Roytman et al, hospitalist care was associated with a 13%40% reduction in adjusted length of stay (P = .002), depending on disease severity.62 These reductions appear to be directly related to the greater experience of hospitalists in managing this and other acute disorders. In a retrospective review of data from an urban teaching hospital, care by a hospitalist, when compared with that by a nonhospitalist, was associated with a 15% reduction in overall length of stay (5.0 vs. 5.9 days; P < .02), with the greatest benefit observed in those patients whose disorders required close clinical monitoring (ie, heart failure, stroke, asthma, or pneumonia) or complex discharge planning.63 Moreover, there was a significant inverse correlation between the mean duration of hospitalization and the number of months of inpatient care provided by the attending physician each year ( = 0.19 day per month of inpatient care; P < .002).63
Finally, hospitalists are uniquely situated to influence medical care. Hospitalists have the ability to closely interact with patients over the course of several days. This exposure enhances opportunities to provide and reinforce patient education and information on lifestyle modifications, which have been shown to reduce the frequency of rehospitalization.60 In one evaluation, initiation of a care‐management program that included increased patient education reduced rehospitalizations for heart failure by 85% (P < .001).64 In another, an intensive, targeted education program significantly decreased the 1‐year risk‐adjusted probability of readmission or death (hazard ratio: 0.56; 95% CI: 0.32‐0.96; P = .03).65 Finally, it is important to remember that hospitalists also play a key role in the education of medical students and residents.60 This opportunity permits hospitalists to promote the adoption of standardized treatment algorithms that hopefully will be retained and propagated by these students long after their initial exposure to the hospitalist, thereby magnifying the effects of this education.
- ,,.National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data.Vital Health Stat 13.2007; (165):1–209.
- ,,, et al.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2008;117(4):e25–e146.
- .Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department.Rev Cardiovasc Med.2002;3(suppl 4):S3–S9.
- ,,, et al.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure).Circulation.2005;112(12):1825–1852.
- ,,, et al.Guidelines for acute decompensated heart failure treatment.Ann Pharmacother.2004;38(4):649–660.
- ,,,.Early ultrafiltration in patients with decompensated heart failure and diuretic resistance.J Am Coll Cardiol.2005;46(11):2047–2051.
- ,,,,.Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE.Cardiology.2007;107(1):44–51.
- Heart Failure Society of America.Executive Summary: HFSA 2006 Comprehensive Heart Failure Practice Guideline.J Card Fail.2006;12(1):10–38.
- ,,, et al.BNP Consensus Panel 2004: a clinical approach for the diagnostic, prognostic, screening, treatment monitoring, and therapeutic roles of natriuretic peptides in cardiovascular diseases.Congest Heart Fail.2004;10(5 suppl 3):1–30.
- ,,, et al.Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology.Eur Heart J.2005;26(4):384–416.
- ,.The limited reliability of physical signs for estimating hemodynamics in chronic heart failure.JAMA.1989;261(6):884–888.
- ,,, et al.In‐hospital mortality in patients with acute decompensated heart failure treated with intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE).J Am Coll Cardiol.2005;46(1):57–64.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296(18):2217–2226.
- ,,,,,.Utility of a rapid B‐natriuretic peptide assay in differentiating congestive heart failure from lung disease in patients presenting with dyspnea.J Am Coll Cardiol.2002;39(2):202–209.
- .Overview of acutely decompensated congestive heart failure (ADHF): a report from the ADHERE registry.Heart Fail Rev.2004;9(3):179–185.
- ,,, et al.The safety of intravenous diuretics alone versus diuretics plus parenteral vasoactive therapies in hospitalized patients with acutely decompensated heart failure: a propensity score and instrumental variable analysis using the Acutely Decompensated Heart Failure National Registry (ADHERE) database.Am Heart J.2007;154(2):267–277.
- ,,, et al.Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE‐HF registry.J Am Coll Cardiol.2007;50(8):768–777.
- ,,, et al.Utility of B‐type natriuretic peptide in the diagnosis of congestive heart failure in an urgent‐care setting.J Am Coll Cardiol.2001;37(2):379–385.
- ,,, et al.B‐type natriuretic peptide predicts future cardiac events in patients presenting to the emergency department with dyspnea.Ann Emerg Med.2002;39(2):131–138.
- .B‐type natriuretic peptide levels: diagnostic and prognostic in congestive heart failure: what's next?Circulation.2002;105(20):2328–2331.
- ,,, et al.Rapid measurement of B‐type natriuretic peptide in the emergency diagnosis of heart failure.N Engl J Med.2002;347(3):161–167.
- ,,, et al.Bedside B‐type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction: results from the Breathing Not Properly Multinational Study.J Am Coll Cardiol.2003;41(11):2010–2017.
- ,,, et al.Primary results of the Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT): a multicenter study of B‐type natriuretic peptide levels, emergency department decision making, and outcomes in patients presenting with shortness of breath.J Am Coll Cardiol.2004;44(6):1328–1333.
- ,,, et al.Application of NT‐proBNP and BNP measurements in cardiac care: a more discerning marker for the detection and evaluation of heart failure.Eur J Heart Fail.2004;6(3):295–300.
- ,,, et al.Hemoglobin and N‐terminal pro‐brain natriuretic peptide: independent and synergistic predictors of mortality in patients with acute heart failure. Results from the International Collaborative of NT‐proBNP (ICON) Study.Clin Chim Acta.2007;381(2):145–150.
- ,,, et al.Use of B‐type natriuretic peptide in the evaluation and management of acute dyspnea.N Engl J Med.2004;350(7):647–654.
- American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Acute Heart Failure Syndromes,,,,,.Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute heart failure syndromes.Ann Emerg Med.2007;49(5):627–669.
- ,,,,.Expression of natriuretic peptide receptors in human adipose and other tissues.J Endocrinol Invest.1996;19(9):581–585.
- ,,, et al.Clinical applications of B‐type natriuretic peptide (BNP) testing.Eur Heart J.2003;24(19):1710–1718.
- ,,, et al.B‐type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study.Am J Kidney Dis.2003;41(3):571–579.
- ,.B‐type natriuretic peptide as a biomarker beyond heart failure: speculations and opportunities.Mayo Clin Proc.2005;80(8):1029–1036.
- ,,.Natriuretic peptides for risk stratification of patients with acute coronary syndromes.Eur J Heart Fail.2004;6(3):327–333.
- ,,,,.Predicting outcome in patients with acute coronary syndrome: evaluation of B‐type natriuretic peptide and the global registry of acute coronary events (GRACE) risk score.Scott Med J.2007;52(3):8–13.
- ,,,,.Risk stratification for in‐hospital mortality in acutely decompensated heart failure: classification and regression tree analysis.JAMA.2005;293(5):572–580.
- ,,.Prognosis for patients newly admitted to hospital with heart failure: survival trends in 12 220 index admissions in Leicestershire 1993–2001.Heart.2003;89(6):615–620.
- ,,,.Predictors of mortality in younger and older patients with heart failure and preserved or reduced left ventricular ejection fraction.Am Heart J.2003;146(2):286–290.
- ,,,,,.Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model.JAMA.2003;290(19):2581–2587.
- Clinical Quality Improvement Network Investigators.Mortality risk and patterns of practice in 4606 acute care patients with congestive heart failure: the relative importance of age, sex, and medical therapy.Arch Intern Med.1996;156(15):1669–1673.
- ,,, et al.A simple clinically based predictive rule for heart failure in‐hospital mortality.J Card Fail.2006;12(8):587–593.
- ,,,,,.Characteristics, outcomes, and predictors of 1‐year mortality in patients hospitalized for acute heart failure.Eur Heart J.2006;27(24):3011–3017.
- ,,, et al.Ejection fraction and blood pressure are important and interactive predictors of 4‐week mortality in severe acute heart failure.Eur J Heart Fail.2007;9(9):935–941.
- ,,, et al.Worsening renal function: what is a clinically meaningful change in creatinine during hospitalization with heart failure?J Card Fail.2003;9(1):13–25.
- ,,, et al.Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure.J Am Coll Cardiol.2004;43(1):61–67.
- ,,, et al.Usefulness of anemia as a predictor of death and rehospitalization in patients with decompensated heart failure.Am J Cardiol.2003;92(5):625–628.
- ,,, et al.Relation of low hemoglobin and anemia to morbidity and mortality in patients hospitalized with heart failure (insight from the OPTIMIZE‐HF registry).Am J Cardiol.2008;101(2):223–230.
- ,,, et al.Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE‐HF registry.Eur Heart J.2007;28(8):980–988.
- ,,, et al.Prognostic value of combination of cardiac troponin T and B‐type natriuretic peptide after initiation of treatment in patients with chronic heart failure.Clin Chem.2003;49(12):2020–2026.
- ,,, et al.Usefulness of B‐type natriuretic peptide and cardiac troponin levels to predict in‐hospital mortality from ADHERE.Am J Cardiol.2008;101(2):231–237.
- ,,.Relation of loop diuretic dose to mortality in advanced heart failure.Am J Cardiol.2006;97(12):1759–1764.
- ,,, et al.Relation between diuretic dose and outcome in a heart failure population: results of the ESCAPE trial [Abstract 250].J Card Fail.2005;11(6 Suppl):S157.
- ,,.Repeated hospitalizations predict mortality in the community population with heart failure.Am Heart J.2007;154(2):260–266.
- ,,.Characteristics of “Stage D” heart failure: insights from the Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM).Am Heart J.2008;155(2):339–347.
- American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Guidelines for the evaluation and management of heart failure. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Evaluation and Management of Heart Failure).J Am Coll Cardiol.1995;26(5):1376–1398.
- ,,, et al.Short‐term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial.JAMA.2002;287(12):1541–1547.
- ,,, et al.Influence of beta‐blocker continuation or withdrawal on outcomes in patients hospitalized with heart failure: findings from the OPTIMIZE‐HF program.J Am Coll Cardiol.2008;52(3):190–199.
- ,,, et al.Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF): rationale and design.Am Heart J.2004;148(1):43–51.
- ,,,,, eds.The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine.J Hosp Med.2006;1(Suppl 1):2–95.
- ,,,,.The association between the quality of inpatient care and early readmission.Ann Intern Med.1995;122(6):415–421.
- ,,, et al.Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure.Am Heart J.2007;153(1):98–104.
- .Improving the management of patients after myocardial infarction, from admission to discharge.Clin Ther.2006;28(10):1509–1539.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162(11):1251–1256.
- ,,.Comparison of practice patterns of hospitalists and community physicians in the care of patients with congestive heart failure.J Hosp Med.2008;3(1):35–41.
- ,,,,.Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring.Arch Intern Med.2007;167(17):1869–1874.
- ,,, et al.Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure.J Am Coll Cardiol.1997;30(3):725–732.
- ,,, et al.Randomized trial of an education and support intervention to prevent readmission of patients with heart failure.J Am Coll Cardiol.2002;39(1):83–89.
- ,,.National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data.Vital Health Stat 13.2007; (165):1–209.
- ,,, et al.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2008;117(4):e25–e146.
- .Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department.Rev Cardiovasc Med.2002;3(suppl 4):S3–S9.
- ,,, et al.ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult—summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure).Circulation.2005;112(12):1825–1852.
- ,,, et al.Guidelines for acute decompensated heart failure treatment.Ann Pharmacother.2004;38(4):649–660.
- ,,,.Early ultrafiltration in patients with decompensated heart failure and diuretic resistance.J Am Coll Cardiol.2005;46(11):2047–2051.
- ,,,,.Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE.Cardiology.2007;107(1):44–51.
- Heart Failure Society of America.Executive Summary: HFSA 2006 Comprehensive Heart Failure Practice Guideline.J Card Fail.2006;12(1):10–38.
- ,,, et al.BNP Consensus Panel 2004: a clinical approach for the diagnostic, prognostic, screening, treatment monitoring, and therapeutic roles of natriuretic peptides in cardiovascular diseases.Congest Heart Fail.2004;10(5 suppl 3):1–30.
- ,,, et al.Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology.Eur Heart J.2005;26(4):384–416.
- ,.The limited reliability of physical signs for estimating hemodynamics in chronic heart failure.JAMA.1989;261(6):884–888.
- ,,, et al.In‐hospital mortality in patients with acute decompensated heart failure treated with intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE).J Am Coll Cardiol.2005;46(1):57–64.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296(18):2217–2226.
- ,,,,,.Utility of a rapid B‐natriuretic peptide assay in differentiating congestive heart failure from lung disease in patients presenting with dyspnea.J Am Coll Cardiol.2002;39(2):202–209.
- .Overview of acutely decompensated congestive heart failure (ADHF): a report from the ADHERE registry.Heart Fail Rev.2004;9(3):179–185.
- ,,, et al.The safety of intravenous diuretics alone versus diuretics plus parenteral vasoactive therapies in hospitalized patients with acutely decompensated heart failure: a propensity score and instrumental variable analysis using the Acutely Decompensated Heart Failure National Registry (ADHERE) database.Am Heart J.2007;154(2):267–277.
- ,,, et al.Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE‐HF registry.J Am Coll Cardiol.2007;50(8):768–777.
- ,,, et al.Utility of B‐type natriuretic peptide in the diagnosis of congestive heart failure in an urgent‐care setting.J Am Coll Cardiol.2001;37(2):379–385.
- ,,, et al.B‐type natriuretic peptide predicts future cardiac events in patients presenting to the emergency department with dyspnea.Ann Emerg Med.2002;39(2):131–138.
- .B‐type natriuretic peptide levels: diagnostic and prognostic in congestive heart failure: what's next?Circulation.2002;105(20):2328–2331.
- ,,, et al.Rapid measurement of B‐type natriuretic peptide in the emergency diagnosis of heart failure.N Engl J Med.2002;347(3):161–167.
- ,,, et al.Bedside B‐type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction: results from the Breathing Not Properly Multinational Study.J Am Coll Cardiol.2003;41(11):2010–2017.
- ,,, et al.Primary results of the Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT): a multicenter study of B‐type natriuretic peptide levels, emergency department decision making, and outcomes in patients presenting with shortness of breath.J Am Coll Cardiol.2004;44(6):1328–1333.
- ,,, et al.Application of NT‐proBNP and BNP measurements in cardiac care: a more discerning marker for the detection and evaluation of heart failure.Eur J Heart Fail.2004;6(3):295–300.
- ,,, et al.Hemoglobin and N‐terminal pro‐brain natriuretic peptide: independent and synergistic predictors of mortality in patients with acute heart failure. Results from the International Collaborative of NT‐proBNP (ICON) Study.Clin Chim Acta.2007;381(2):145–150.
- ,,, et al.Use of B‐type natriuretic peptide in the evaluation and management of acute dyspnea.N Engl J Med.2004;350(7):647–654.
- American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Acute Heart Failure Syndromes,,,,,.Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute heart failure syndromes.Ann Emerg Med.2007;49(5):627–669.
- ,,,,.Expression of natriuretic peptide receptors in human adipose and other tissues.J Endocrinol Invest.1996;19(9):581–585.
- ,,, et al.Clinical applications of B‐type natriuretic peptide (BNP) testing.Eur Heart J.2003;24(19):1710–1718.
- ,,, et al.B‐type natriuretic peptide and renal function in the diagnosis of heart failure: an analysis from the Breathing Not Properly Multinational Study.Am J Kidney Dis.2003;41(3):571–579.
- ,.B‐type natriuretic peptide as a biomarker beyond heart failure: speculations and opportunities.Mayo Clin Proc.2005;80(8):1029–1036.
- ,,.Natriuretic peptides for risk stratification of patients with acute coronary syndromes.Eur J Heart Fail.2004;6(3):327–333.
- ,,,,.Predicting outcome in patients with acute coronary syndrome: evaluation of B‐type natriuretic peptide and the global registry of acute coronary events (GRACE) risk score.Scott Med J.2007;52(3):8–13.
- ,,,,.Risk stratification for in‐hospital mortality in acutely decompensated heart failure: classification and regression tree analysis.JAMA.2005;293(5):572–580.
- ,,.Prognosis for patients newly admitted to hospital with heart failure: survival trends in 12 220 index admissions in Leicestershire 1993–2001.Heart.2003;89(6):615–620.
- ,,,.Predictors of mortality in younger and older patients with heart failure and preserved or reduced left ventricular ejection fraction.Am Heart J.2003;146(2):286–290.
- ,,,,,.Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model.JAMA.2003;290(19):2581–2587.
- Clinical Quality Improvement Network Investigators.Mortality risk and patterns of practice in 4606 acute care patients with congestive heart failure: the relative importance of age, sex, and medical therapy.Arch Intern Med.1996;156(15):1669–1673.
- ,,, et al.A simple clinically based predictive rule for heart failure in‐hospital mortality.J Card Fail.2006;12(8):587–593.
- ,,,,,.Characteristics, outcomes, and predictors of 1‐year mortality in patients hospitalized for acute heart failure.Eur Heart J.2006;27(24):3011–3017.
- ,,, et al.Ejection fraction and blood pressure are important and interactive predictors of 4‐week mortality in severe acute heart failure.Eur J Heart Fail.2007;9(9):935–941.
- ,,, et al.Worsening renal function: what is a clinically meaningful change in creatinine during hospitalization with heart failure?J Card Fail.2003;9(1):13–25.
- ,,, et al.Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure.J Am Coll Cardiol.2004;43(1):61–67.
- ,,, et al.Usefulness of anemia as a predictor of death and rehospitalization in patients with decompensated heart failure.Am J Cardiol.2003;92(5):625–628.
- ,,, et al.Relation of low hemoglobin and anemia to morbidity and mortality in patients hospitalized with heart failure (insight from the OPTIMIZE‐HF registry).Am J Cardiol.2008;101(2):223–230.
- ,,, et al.Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE‐HF registry.Eur Heart J.2007;28(8):980–988.
- ,,, et al.Prognostic value of combination of cardiac troponin T and B‐type natriuretic peptide after initiation of treatment in patients with chronic heart failure.Clin Chem.2003;49(12):2020–2026.
- ,,, et al.Usefulness of B‐type natriuretic peptide and cardiac troponin levels to predict in‐hospital mortality from ADHERE.Am J Cardiol.2008;101(2):231–237.
- ,,.Relation of loop diuretic dose to mortality in advanced heart failure.Am J Cardiol.2006;97(12):1759–1764.
- ,,, et al.Relation between diuretic dose and outcome in a heart failure population: results of the ESCAPE trial [Abstract 250].J Card Fail.2005;11(6 Suppl):S157.
- ,,.Repeated hospitalizations predict mortality in the community population with heart failure.Am Heart J.2007;154(2):260–266.
- ,,.Characteristics of “Stage D” heart failure: insights from the Acute Decompensated Heart Failure National Registry Longitudinal Module (ADHERE LM).Am Heart J.2008;155(2):339–347.
- American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Guidelines for the evaluation and management of heart failure. Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Evaluation and Management of Heart Failure).J Am Coll Cardiol.1995;26(5):1376–1398.
- ,,, et al.Short‐term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial.JAMA.2002;287(12):1541–1547.
- ,,, et al.Influence of beta‐blocker continuation or withdrawal on outcomes in patients hospitalized with heart failure: findings from the OPTIMIZE‐HF program.J Am Coll Cardiol.2008;52(3):190–199.
- ,,, et al.Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF): rationale and design.Am Heart J.2004;148(1):43–51.
- ,,,,, eds.The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine.J Hosp Med.2006;1(Suppl 1):2–95.
- ,,,,.The association between the quality of inpatient care and early readmission.Ann Intern Med.1995;122(6):415–421.
- ,,, et al.Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure.Am Heart J.2007;153(1):98–104.
- .Improving the management of patients after myocardial infarction, from admission to discharge.Clin Ther.2006;28(10):1509–1539.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162(11):1251–1256.
- ,,.Comparison of practice patterns of hospitalists and community physicians in the care of patients with congestive heart failure.J Hosp Med.2008;3(1):35–41.
- ,,,,.Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring.Arch Intern Med.2007;167(17):1869–1874.
- ,,, et al.Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure.J Am Coll Cardiol.1997;30(3):725–732.
- ,,, et al.Randomized trial of an education and support intervention to prevent readmission of patients with heart failure.J Am Coll Cardiol.2002;39(1):83–89.
Multidisciplinary Management of ADHF
Acute decompensated heart failure (ADHF) is a common disorder that is frequently managed by hospitalists. This management is expected to expand over the next several years because of a continuing increase in the number of ADHF admissions coupled with a plateau or possible decline in the number of practicing cardiologists (Figure 1).114 In addition, 12% of fellowship training positions in cardiology were eliminated between 1995 and 2001, and the fact that the current number of training positions is inadequate to meet future demands is not recognized.15, 16 Given the severity of this disorder, the limited data from randomized, controlled clinical trials,17 and the limitations of current treatment, this management can be both challenging and rewarding. The goal of this special supplement of the Journal of Hospital Medicine is to assist hospitalists in this endeavor by summarizing the currently available data and treatment options and presenting a rational evidence‐based algorithm for the management of ADHF.
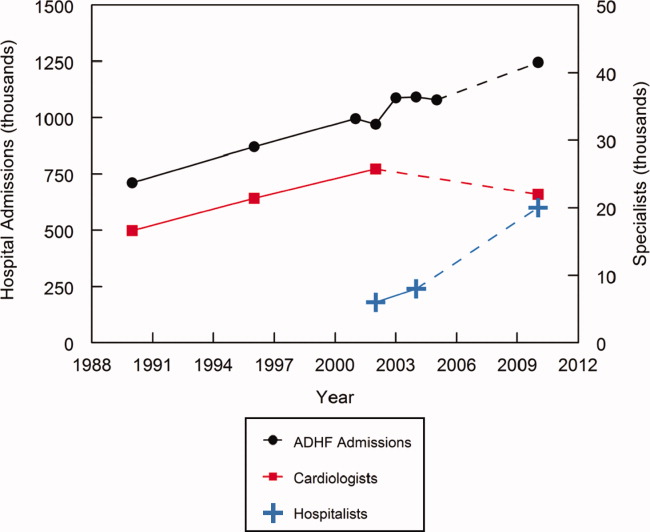
A multidisciplinary approach to heart failure has been shown to reduce cost, decrease length of stay, curtail readmissions, and improve compliance.1820 By leading and coordinating teams of physicians, pharmacists, nurses, nutritionists, physical therapists, and case managers and by developing and implementing indications for cardiology consultation, hospitalists can facilitate this multidisciplinary approach.21, 22 However, it is important to remember that hospitalists do not replace cardiologists, who remain a valuable and key component of this multidisciplinary team. Their input is vital in developing care pathways and criteria for consultation, and they, along with primary care physicians, will be the primary source of patient care following hospital discharge. Good communication between hospitalists and cardiologists is essential to optimize the care of patients with ADHF.
Maximizing the efficacy of ADHF care requires a thorough understanding of (1) the causes and potential treatments for the patient's acute decompensation, (2) the management of the patient's chronic heart failure, and (3) potential future therapies. Strategies to improve the continuum of heart failure care have been employed to help improve patient outcomes.23 For example, hospital‐based disease management programs have consistently been shown to optimize care and reduce rehospitalization rates in patients with heart failure.24 These programs involve a multidisciplinary, multifaceted approach to care in order to provide a continuum of care extending from hospitalization and into a patient's home environment.
Because of their practice location and experience, hospitalists are uniquely suited to influence acute inpatient care.25 They see patients in a variety of hospital settings and consequently tend to think of the entire system and not just an isolated component or patient.14 In addition, they have a vested interest in hospital quality improvement measures and are frequently involved in evaluating policies and procedures and developing and implementing clinical pathways, guidelines, and decision‐support tools.26 Data demonstrate that compliance is greater with evidence‐based guidelines and core performance measures when inpatient care is directed by a hospitalist.2730 Improved compliance with selected quality measures in patients with acute myocardial infarction and congestive heart failure has been observed when hospitals implement standardized admission and discharge orders.31, 32
Numerous transitions, such as outpatient to inpatient, intensive care unit to ward, and ward to home, occur during hospitalization, and these transitions are frequently associated with changes in the patient's medication regimen. During an acute illness, chronic medications may be held or discontinued, long‐acting medications may be changed to short‐acting ones to better titrate dose and achieve tighter control, and closed formularies may necessitate substituting 1 medication for another.33 A breakdown in communication during hospitalization‐associated transitions commonly affects medication regimens and can adversely impact patient care.3436 In a prospective evaluation, 53.6% [95% confidence interval (CI): 45.7%61.6%] of patients admitted to the hospital had at least 1 unintended discrepancy between their admission medication orders and their chronic outpatient regimen; 38.6% of these discrepancies were considered a potential threat to the patient.34 Likewise, 49% of patients being discharged from the hospital in another evaluation had an unexplained discrepancy between their preadmission and discharge medications.36 As a result, the Joint Commission on Accreditation of Healthcare Organizations now requires accredited facilities to perform medication reconciliation whenever a patient changes service, setting, provider, or level of care and new medication orders are written.37 This reconciliation is especially important in patients with heart failure, for whom polypharmacy is common and noncompliance with appropriate treatment regimens substantially increases readmission rates.3842
During these transition periods, hospitalists can play an important role in bridging the communication gap and providing this medication reconciliation.33 For example, actively involving hospitalists in all aspects of the reconciliation process at 1 institution resulted in a 4‐fold increase in consistency with preadmission medications.43 Similarly, because of the number of discharge summaries that they write, hospitalists are well suited to lead implementation of new policies and procedures to ensure compliance with recent changes in the Joint Commission on Accreditation of Healthcare Organizations requirements regarding these summaries.
In addition to playing an active role in acute patient management, hospitalists can substantially influence long‐term care and outcomes. Consequently, hospitalists must be well versed in the management of chronic heart failure. Patients are intensely focused on their illness during the hospitalization period, and this focus enhances opportunities for meaningful education and behavior modification. Numerous studies have demonstrated that adherence to long‐term therapy is improved when this therapy is initiated before or at hospital discharge.4446 In an evaluation of data from the Organized Program To Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure Registry (OPTIMIZE‐HF), the prescription of a ‐blocker at discharge was associated with a significant reduction in 60‐ to 90‐day mortality [hazard ratio (HR): 0.48; 95% CI: 0.30‐0.79], and prescription of an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker at discharge was associated with a significant reduction in 60‐ to 90‐day mortality and/or rehospitalization (HR: 0.51; 95% CI: 0.34‐0.78).47 In the Cardiac Hospitalization Atherosclerosis Management Program (CHAMP), emphasizing initiation of chronic therapy prior to hospital discharge was associated with 3.0‐fold greater angiotensin‐converting enzyme inhibitor use and 3.2‐fold greater ‐blocker use at 1 year (both P < 0.01).46 Similarly, in patients surviving acute myocardial infarction, the strongest predictor of ‐blocker use at 30 days following discharge was receipt of a ‐blocker prescription at the time of discharge (HR: 15.8; 95% CI: 10.8‐23.3), and this beneficial effect was sustained for up to a year (Figure 2).44 Likewise, in patients with ADHF, the prevalence of ‐blocker therapy at 60 days was significantly increased when this therapy was initiated before discharge (91%) versus after discharge (73%; P < 0.001).45 This predischarge initiation of chronic therapy has been shown to reduce morbidity and mortality.
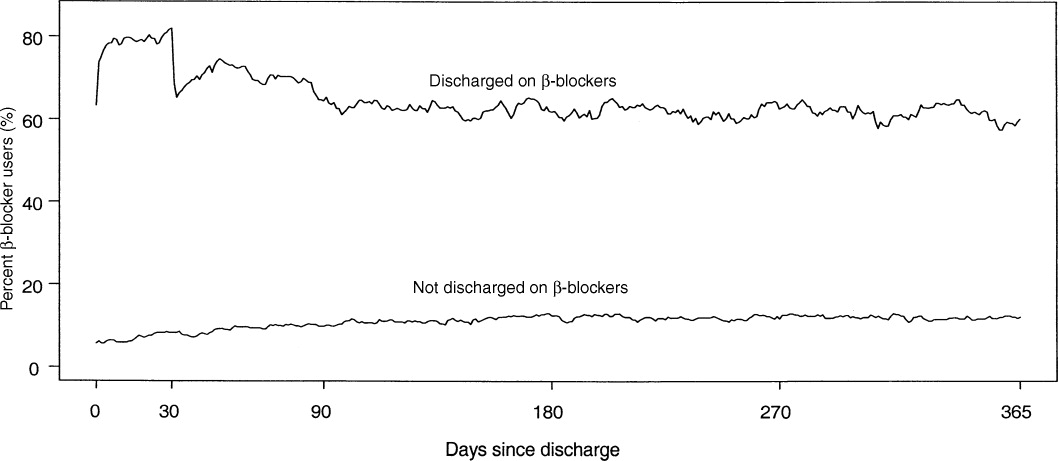
An awareness of new therapies for ADHF that are in late stages of clinical development can improve understanding of the complex pathophysiology of ADHF and enhance appropriate adaptation of these therapies once they become clinically available. These new therapies represent an attempt to improve on existing therapies, and consequently, they fall into the same 3 general categories as current therapies: diuretics, vasodilators, and inotropic agents.48, 49 Vasopressin receptor antagonists and adenosine receptor antagonists represent an attempt to stimulate aquaresis without inducing hyponatremia, hypokalemia, diminished glomerular filtration, or adverse neurohormonal activation;4854 endothelin receptor antagonists and newer natriuretic peptides represent an attempt to stimulate vasodilation and improve cardiac output without diminishing renal function;49, 55 and myosin activators and sodium‐potassium adenosine triphosphatase inhibitors represent an attempt to enhance contractility without inducing arrhythmogenicity or increasing mortality risk4859 (Table 1).
| Class/MOA | Agent(s) | Advantages/Disadvantages | References |
|---|---|---|---|
| |||
| Vasopressin receptor antagonists | Tolvaptan | Induce aquaresis without natriuresis | deGoma et al.48 |
| Conivaptan | Potentially avoid hyponatremia and hypokalemia | Tang and Hobbs49 | |
| Lixivaptan | Konstam et al.50 | ||
| SR‐121463b | Schrier et al.51 | ||
| Schweiger and Zdanowicz52 | |||
| Adenosine A1 receptor antagonists | Rolofylline | Increase renal blood flow | Tang and Hobbs49 |
| BG‐9719 | Increase intraglomerular hydraulic pressure | deGoma et al.48 | |
| BG‐9928 | May produce diuresis without adversely affecting glomerular filtration and renal function | Givertz et al.53 Greenberg et al.54 | |
| Endothelin receptor antagonists | Tezosentan | Potent vasodilator | Tang and Hobbs49 |
| Improves cardiac output | McMurray et al.55 | ||
| Hemodynamic effects have not translated into an improvement in heart failure symptoms or risk of death. | |||
| Natriuretic peptides | Ularitide | Resists inactivation by neutral endopeptidase | deGoma et al.48 |
| Improves filling pressures and dyspnea scores | Mitrovic et al.59 | ||
| No apparent deleterious effect on short‐term renal function | |||
| Myosin activators | CK‐1827452 | Tries to dissociate inotropy from arrhythmogenicity | deGoma et al.48 |
| Enhances contractility by targeting myocardial myosin, the force generating cardiac enzymes | Cytokinetics56 | ||
| Still very early in clinical development (just entered phase 2) | |||
| Sodium‐potassium ATPase inhibitors | Istaroxime | Tries to dissociate inotropy from arrhythmogenicity | deGoma et al.48 |
| Enhances contractility by stimulating calcium entry into the sarcolemmal Na/Ca exchanger | Blair et al.57 | ||
| Lusitropic | Cleland et al.58 | ||
| Still very early in clinical development (just completed first phase 2 trial) | |||
Finally, although major advancements in the medical therapy of heart failure patients have substantially improved outcomes,60 technological advances in mechanical devices,61 including automatic implantable cardioverter defibrillators, cardiac resynchronization therapy, and ventricular assist devices, as well as advances in the surgical treatment of heart failure,62 have also been used to support the failing heart. Heart failure patients being treated with mechanical devices, as well as those following cardiac transplant, require unique care. As more mechanical and surgical innovations emerge, nonpharmacologic therapy will continue to evolve as a cornerstone of the management strategy in heart failure patients. Hospitalists will need to rely on care pathways, criteria for consultation, and good communication with cardiologists to optimize the care of these patients. Hospitalists should work with their cardiology colleagues in their local institution to develop appropriate criteria for cardiology consultation, and everyone should be educated on these criteria.
The subsequent discussions in this supplement expand on these topics. First, I review the presentation and early recognition, risk stratification, and treatment of patients with ADHF and the role of the hospitalist in this assessment and treatment process. Next, Dr. Khan and Dr. Heywood review the role of diuretics, vasodilators, and ultrafiltration in the management of patients with volume overload and high filling pressures and conclude with a discussion of potential future pharmacologic treatment options, such as tolvaptan and rolofylline, and nonpharmacologic modalities, such as wireless hemodynamic monitoring through implanted devices. Finally, Dr. Michota and I discuss bridging the gap between evidence and practice in the management of patients with ADHF. We review the evidence‐based guidelines that are currently available; discuss the appropriate location for treatment based on the patient's initial history and physical, radiographic, and laboratory findings; provide a practical algorithm for this treatment; and discuss means to transition care from the inpatient setting to the outpatient setting in a manner that enhances compliance with long‐term therapy and reduces recidivism. Given the anticipated growth in ADHF and the need for hospitalists to manage this disease together with cardiologists and others, we believe that the provided information will be helpful in the management of ADHF.
- ,.National Hospital Discharge Survey: annual summary, 1996.Vital Health Stat.1999;13(140):1–46.
- ,,,.Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995.Am Heart J.1999;137(2):352–360.
- ,,.National Hospital Discharge Survey: 2001 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2004;13(156):1–198.
- ,,.National Hospital Discharge Survey: 2002 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2005;13(158):1–199.
- ,,.National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2007;13(165):1–209.
- ,,.National Hospital Discharge Survey: 2004 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2006;13(162):1–209.
- ,,.National Hospital Discharge Survey: 2003 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2006;13(160):1–206.
- Division for Heart Disease and Stroke Prevention. Heart failure fact sheet. Available at: http://www.cdc.gov/dhdsp/library/fs_heart_failure_longdesc.htm. Accessed September2008.
- US Census Bureau. Projected population of the United States, by age and sex: 2000 to 2050. Available at: http://www.census.gov/population/www/projections/usinterimproj/natprojtab02a.pdf. Accessed September2008.
- ,,,.Demographics and cardiology, 1950–2050.J Am Coll Cardiol.2000;35(4):1067–1081.
- 35th Bethesda Conference.Cardiology's workforce crisis: a pragmatic approach. Bethesda, Maryland, 17–18 October 2003.J Am Coll Cardiol.2004;44(2):216–275.
- ,.The evolution of the hospitalist movement in the USA.Clin Med.2002;2(4):327–330.
- .Hospitalists in the United States—mission accomplished or work in progress?N Engl J Med.2004;350(19):1935–1936.
- .Identifying strategies to improve outcomes and reduce costs—a role for the hospitalist.Curr Opin Pulm Med.2004;10(suppl):S19–S22.
- ,.Cardiovascular manpower: the looming crisis.Circulation.2004;109(7):817–820.
- ,.The United States cardiovascular care deficit.Circulation.2004;109(7):821–823.
- Heart Failure Society of America.Evaluation and management of patients with acute decompensated heart failure.J Card Fail.2006;12(1):e86–e103.
- ,,,.Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team. Results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) study.Arch Intern Med.1999;159(16):1939–1945.
- ,,,,.Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial.CMAJ.2005;173(1):40–45.
- ,.Implementing a congestive heart failure disease management program to decrease length of stay and cost.J Cardiovasc Nurs.1999;14(1):55–74.
- ,,,,.The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine.J Hosp Med.2006;1(suppl 1):2–95.
- ,,, et al.ASHP‐SHM joint statement on hospitalist‐pharmacist collaboration.J Hosp Med.2008;3(suppl 3). doi://10.1002/jhm.315. Available at: http://www3.interscience.wiley.com.
- ,,,,,.Heart failure: improving the continuum of care.Care Manag J.2006;7(2):58–63.
- ,,.Strategies to reduce hospitalization in the management of heart failure.Lippincotts Case Manag.2005;10(6 suppl):S1–S15.
- .Improving the management of patients after myocardial infarction, from admission to discharge.Clin Ther.2006;28(10):1509–1539.
- .The role of hospitalists in the management of acute decompensated heart failure.Am Heart Hosp J.2005;3(2):111–117.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162(11):1251–1256.
- ,.Productive interdisciplinary team relationships: the hospitalist and the case manager.Lippincotts Case Manag.2006;11(3):160–164.
- .Use of pay for performance in a community hospital private hospitalist group: a preliminary report.Trans Am Clin Climatol Assoc.2007;118:263–272.
- ,,.Comparison of practice patterns of hospitalists and community physicians in the care of patients with congestive heart failure.J Hosp Med.2008;3(1):35–41.
- ,,,,,.Integrating “best of care” protocols into clinicians' workflow via care provider order entry: impact on quality‐of‐care indicators for acute myocardial infarction.J Am Med Inform Assoc.2006;13(2):188–196.
- ,,, et al.Improved compliance with quality measures at hospital discharge with a computerized physician order entry system.Am Heart J.2006;151(3):643–653.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,,, et al.Unintended medication discrepancies at the time of hospital admission.Arch Intern Med.2005;165(4):424–429.
- ,,,,,.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166(5):565–571.
- Joint Commission on Accreditation of Healthcare Organizations. Using medication reconciliation to prevent errors. Sentinel Event Alert #35. Available at: http://www.jointcommission.org/sentinelevents/sentineleventalert/sea_35.htm. Accessed September2008.
- ,,,.Precipitating factors leading to decompensation of heart failure: traits among urban blacks.Arch Intern Med.1988;148(9):2013–2016.
- ,,,,.Early readmission of elderly patients with congestive heart failure.J Am Geriatr Soc.1990;38(12):1290–1295.
- ,.Observation unit management of heart failure.Emerg Med Clin North Am.2001;19(1):209–232.
- ,,, et al.Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Failure National Registry (ADHERE).Am Heart J.2005;149(2):209–216.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296(18):2217–2226.
- . Eliminating drug errors: hospitals adopt medication reconciliation to improve patient safety. Available at: http://www.acponline.org/clinical_information/journals_publications/acp_hospitalist/may07/drug_errors.htm. Accessed September2008.
- ,,, et al.Outpatient adherence to beta‐blocker therapy after acute myocardial infarction.JAm Coll Cardiol.2002;40(9):1589–1595.
- ,.Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure.Am J Cardiol.2004;93(9A):74B–76B.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol.2001;87(7):819–822.
- ,,, et al.Association between performance measures and clinical outcomes for patients hospitalized with heart failure.JAMA.2007;297(1):61–70.
- ,,,.Emerging therapies for the management of decompensated heart failure: from bench to bedside.J Am Coll Cardiol.2006;48(12):2397–2409.
- ,.Novel strategies for the management of acute decompensated heart failure.Curr Cardiol Rev.2005;1(1):1–5.
- ,,, et al.Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial.JAMA.2007;297(12):1319–1331.
- ,,, et al.Tolvaptan, a selective oral vasopressin V2‐receptor antagonist, for hyponatremia.N Engl J Med.2006;355(20):2099–2112.
- ,.Vasopressin‐receptor antagonists in heart failure.Am J Health Syst Pharm.2008;65(9):807–817.
- ,,,,.The effects of KW‐3902, an adenosine A1‐receptor antagonist, on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance.J Am Coll Cardiol.2007;50(16):1551–1560.
- ,,, et al.Effects of multiple oral doses of an A1 adenosine antagonist, BG9928, in patients with heart failure: results of a placebo‐controlled, dose‐escalation study.J Am Coll Cardiol.2007;50(7):600–606.
- ,,, et al.Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials.JAMA.2007;298(17):2009–2019.
- CK‐1827452. Cytokinetics Web site. Available at: http://www.cytokinetics.com/ck_1827452. Accessed September2008.
- ,,, et al.Rationale and design of the hemodynamic, echocardiographic and neurohormonal effects of istaroxime, a novel intravenous inotropic and lusitropic agent: a randomized controlled trial in patients hospitalized with heart failure (HORIZON‐HF) trial.Am J Ther.2008;15(3):231–240.
- ,,, et al.Clinical trials update from the American College of Cardiology 2008: CARISMA, TRENDS, meta‐analysis of Cox‐2 studies, HAT, ON‐TARGET, HYVET, ACCOMPLISH, MOMENTUM, PROTECT, HORIZON‐HF and REVERSE.Eur J Heart Fail.2008;10(6):614–620.
- ,,, et al.Haemodynamic and clinical effects of ularitide in decompensated heart failure.Eur Heart J.2006;27(23):2823–2832.
- ,,.Mechanical support in acute and chronic heart failure.Curr Cardiol Rep.2008;10(3):168–175.
- ,.Devices in acute heart failure.Crit Care Med.2008;36(1 suppl):S121–S128.
- ,.Advances in the surgical treatment of heart failure.Curr Opin Cardiol.2008;23(3):249–253.
Acute decompensated heart failure (ADHF) is a common disorder that is frequently managed by hospitalists. This management is expected to expand over the next several years because of a continuing increase in the number of ADHF admissions coupled with a plateau or possible decline in the number of practicing cardiologists (Figure 1).114 In addition, 12% of fellowship training positions in cardiology were eliminated between 1995 and 2001, and the fact that the current number of training positions is inadequate to meet future demands is not recognized.15, 16 Given the severity of this disorder, the limited data from randomized, controlled clinical trials,17 and the limitations of current treatment, this management can be both challenging and rewarding. The goal of this special supplement of the Journal of Hospital Medicine is to assist hospitalists in this endeavor by summarizing the currently available data and treatment options and presenting a rational evidence‐based algorithm for the management of ADHF.

A multidisciplinary approach to heart failure has been shown to reduce cost, decrease length of stay, curtail readmissions, and improve compliance.1820 By leading and coordinating teams of physicians, pharmacists, nurses, nutritionists, physical therapists, and case managers and by developing and implementing indications for cardiology consultation, hospitalists can facilitate this multidisciplinary approach.21, 22 However, it is important to remember that hospitalists do not replace cardiologists, who remain a valuable and key component of this multidisciplinary team. Their input is vital in developing care pathways and criteria for consultation, and they, along with primary care physicians, will be the primary source of patient care following hospital discharge. Good communication between hospitalists and cardiologists is essential to optimize the care of patients with ADHF.
Maximizing the efficacy of ADHF care requires a thorough understanding of (1) the causes and potential treatments for the patient's acute decompensation, (2) the management of the patient's chronic heart failure, and (3) potential future therapies. Strategies to improve the continuum of heart failure care have been employed to help improve patient outcomes.23 For example, hospital‐based disease management programs have consistently been shown to optimize care and reduce rehospitalization rates in patients with heart failure.24 These programs involve a multidisciplinary, multifaceted approach to care in order to provide a continuum of care extending from hospitalization and into a patient's home environment.
Because of their practice location and experience, hospitalists are uniquely suited to influence acute inpatient care.25 They see patients in a variety of hospital settings and consequently tend to think of the entire system and not just an isolated component or patient.14 In addition, they have a vested interest in hospital quality improvement measures and are frequently involved in evaluating policies and procedures and developing and implementing clinical pathways, guidelines, and decision‐support tools.26 Data demonstrate that compliance is greater with evidence‐based guidelines and core performance measures when inpatient care is directed by a hospitalist.2730 Improved compliance with selected quality measures in patients with acute myocardial infarction and congestive heart failure has been observed when hospitals implement standardized admission and discharge orders.31, 32
Numerous transitions, such as outpatient to inpatient, intensive care unit to ward, and ward to home, occur during hospitalization, and these transitions are frequently associated with changes in the patient's medication regimen. During an acute illness, chronic medications may be held or discontinued, long‐acting medications may be changed to short‐acting ones to better titrate dose and achieve tighter control, and closed formularies may necessitate substituting 1 medication for another.33 A breakdown in communication during hospitalization‐associated transitions commonly affects medication regimens and can adversely impact patient care.3436 In a prospective evaluation, 53.6% [95% confidence interval (CI): 45.7%61.6%] of patients admitted to the hospital had at least 1 unintended discrepancy between their admission medication orders and their chronic outpatient regimen; 38.6% of these discrepancies were considered a potential threat to the patient.34 Likewise, 49% of patients being discharged from the hospital in another evaluation had an unexplained discrepancy between their preadmission and discharge medications.36 As a result, the Joint Commission on Accreditation of Healthcare Organizations now requires accredited facilities to perform medication reconciliation whenever a patient changes service, setting, provider, or level of care and new medication orders are written.37 This reconciliation is especially important in patients with heart failure, for whom polypharmacy is common and noncompliance with appropriate treatment regimens substantially increases readmission rates.3842
During these transition periods, hospitalists can play an important role in bridging the communication gap and providing this medication reconciliation.33 For example, actively involving hospitalists in all aspects of the reconciliation process at 1 institution resulted in a 4‐fold increase in consistency with preadmission medications.43 Similarly, because of the number of discharge summaries that they write, hospitalists are well suited to lead implementation of new policies and procedures to ensure compliance with recent changes in the Joint Commission on Accreditation of Healthcare Organizations requirements regarding these summaries.
In addition to playing an active role in acute patient management, hospitalists can substantially influence long‐term care and outcomes. Consequently, hospitalists must be well versed in the management of chronic heart failure. Patients are intensely focused on their illness during the hospitalization period, and this focus enhances opportunities for meaningful education and behavior modification. Numerous studies have demonstrated that adherence to long‐term therapy is improved when this therapy is initiated before or at hospital discharge.4446 In an evaluation of data from the Organized Program To Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure Registry (OPTIMIZE‐HF), the prescription of a ‐blocker at discharge was associated with a significant reduction in 60‐ to 90‐day mortality [hazard ratio (HR): 0.48; 95% CI: 0.30‐0.79], and prescription of an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker at discharge was associated with a significant reduction in 60‐ to 90‐day mortality and/or rehospitalization (HR: 0.51; 95% CI: 0.34‐0.78).47 In the Cardiac Hospitalization Atherosclerosis Management Program (CHAMP), emphasizing initiation of chronic therapy prior to hospital discharge was associated with 3.0‐fold greater angiotensin‐converting enzyme inhibitor use and 3.2‐fold greater ‐blocker use at 1 year (both P < 0.01).46 Similarly, in patients surviving acute myocardial infarction, the strongest predictor of ‐blocker use at 30 days following discharge was receipt of a ‐blocker prescription at the time of discharge (HR: 15.8; 95% CI: 10.8‐23.3), and this beneficial effect was sustained for up to a year (Figure 2).44 Likewise, in patients with ADHF, the prevalence of ‐blocker therapy at 60 days was significantly increased when this therapy was initiated before discharge (91%) versus after discharge (73%; P < 0.001).45 This predischarge initiation of chronic therapy has been shown to reduce morbidity and mortality.

An awareness of new therapies for ADHF that are in late stages of clinical development can improve understanding of the complex pathophysiology of ADHF and enhance appropriate adaptation of these therapies once they become clinically available. These new therapies represent an attempt to improve on existing therapies, and consequently, they fall into the same 3 general categories as current therapies: diuretics, vasodilators, and inotropic agents.48, 49 Vasopressin receptor antagonists and adenosine receptor antagonists represent an attempt to stimulate aquaresis without inducing hyponatremia, hypokalemia, diminished glomerular filtration, or adverse neurohormonal activation;4854 endothelin receptor antagonists and newer natriuretic peptides represent an attempt to stimulate vasodilation and improve cardiac output without diminishing renal function;49, 55 and myosin activators and sodium‐potassium adenosine triphosphatase inhibitors represent an attempt to enhance contractility without inducing arrhythmogenicity or increasing mortality risk4859 (Table 1).
| Class/MOA | Agent(s) | Advantages/Disadvantages | References |
|---|---|---|---|
| |||
| Vasopressin receptor antagonists | Tolvaptan | Induce aquaresis without natriuresis | deGoma et al.48 |
| Conivaptan | Potentially avoid hyponatremia and hypokalemia | Tang and Hobbs49 | |
| Lixivaptan | Konstam et al.50 | ||
| SR‐121463b | Schrier et al.51 | ||
| Schweiger and Zdanowicz52 | |||
| Adenosine A1 receptor antagonists | Rolofylline | Increase renal blood flow | Tang and Hobbs49 |
| BG‐9719 | Increase intraglomerular hydraulic pressure | deGoma et al.48 | |
| BG‐9928 | May produce diuresis without adversely affecting glomerular filtration and renal function | Givertz et al.53 Greenberg et al.54 | |
| Endothelin receptor antagonists | Tezosentan | Potent vasodilator | Tang and Hobbs49 |
| Improves cardiac output | McMurray et al.55 | ||
| Hemodynamic effects have not translated into an improvement in heart failure symptoms or risk of death. | |||
| Natriuretic peptides | Ularitide | Resists inactivation by neutral endopeptidase | deGoma et al.48 |
| Improves filling pressures and dyspnea scores | Mitrovic et al.59 | ||
| No apparent deleterious effect on short‐term renal function | |||
| Myosin activators | CK‐1827452 | Tries to dissociate inotropy from arrhythmogenicity | deGoma et al.48 |
| Enhances contractility by targeting myocardial myosin, the force generating cardiac enzymes | Cytokinetics56 | ||
| Still very early in clinical development (just entered phase 2) | |||
| Sodium‐potassium ATPase inhibitors | Istaroxime | Tries to dissociate inotropy from arrhythmogenicity | deGoma et al.48 |
| Enhances contractility by stimulating calcium entry into the sarcolemmal Na/Ca exchanger | Blair et al.57 | ||
| Lusitropic | Cleland et al.58 | ||
| Still very early in clinical development (just completed first phase 2 trial) | |||
Finally, although major advancements in the medical therapy of heart failure patients have substantially improved outcomes,60 technological advances in mechanical devices,61 including automatic implantable cardioverter defibrillators, cardiac resynchronization therapy, and ventricular assist devices, as well as advances in the surgical treatment of heart failure,62 have also been used to support the failing heart. Heart failure patients being treated with mechanical devices, as well as those following cardiac transplant, require unique care. As more mechanical and surgical innovations emerge, nonpharmacologic therapy will continue to evolve as a cornerstone of the management strategy in heart failure patients. Hospitalists will need to rely on care pathways, criteria for consultation, and good communication with cardiologists to optimize the care of these patients. Hospitalists should work with their cardiology colleagues in their local institution to develop appropriate criteria for cardiology consultation, and everyone should be educated on these criteria.
The subsequent discussions in this supplement expand on these topics. First, I review the presentation and early recognition, risk stratification, and treatment of patients with ADHF and the role of the hospitalist in this assessment and treatment process. Next, Dr. Khan and Dr. Heywood review the role of diuretics, vasodilators, and ultrafiltration in the management of patients with volume overload and high filling pressures and conclude with a discussion of potential future pharmacologic treatment options, such as tolvaptan and rolofylline, and nonpharmacologic modalities, such as wireless hemodynamic monitoring through implanted devices. Finally, Dr. Michota and I discuss bridging the gap between evidence and practice in the management of patients with ADHF. We review the evidence‐based guidelines that are currently available; discuss the appropriate location for treatment based on the patient's initial history and physical, radiographic, and laboratory findings; provide a practical algorithm for this treatment; and discuss means to transition care from the inpatient setting to the outpatient setting in a manner that enhances compliance with long‐term therapy and reduces recidivism. Given the anticipated growth in ADHF and the need for hospitalists to manage this disease together with cardiologists and others, we believe that the provided information will be helpful in the management of ADHF.
Acute decompensated heart failure (ADHF) is a common disorder that is frequently managed by hospitalists. This management is expected to expand over the next several years because of a continuing increase in the number of ADHF admissions coupled with a plateau or possible decline in the number of practicing cardiologists (Figure 1).114 In addition, 12% of fellowship training positions in cardiology were eliminated between 1995 and 2001, and the fact that the current number of training positions is inadequate to meet future demands is not recognized.15, 16 Given the severity of this disorder, the limited data from randomized, controlled clinical trials,17 and the limitations of current treatment, this management can be both challenging and rewarding. The goal of this special supplement of the Journal of Hospital Medicine is to assist hospitalists in this endeavor by summarizing the currently available data and treatment options and presenting a rational evidence‐based algorithm for the management of ADHF.

A multidisciplinary approach to heart failure has been shown to reduce cost, decrease length of stay, curtail readmissions, and improve compliance.1820 By leading and coordinating teams of physicians, pharmacists, nurses, nutritionists, physical therapists, and case managers and by developing and implementing indications for cardiology consultation, hospitalists can facilitate this multidisciplinary approach.21, 22 However, it is important to remember that hospitalists do not replace cardiologists, who remain a valuable and key component of this multidisciplinary team. Their input is vital in developing care pathways and criteria for consultation, and they, along with primary care physicians, will be the primary source of patient care following hospital discharge. Good communication between hospitalists and cardiologists is essential to optimize the care of patients with ADHF.
Maximizing the efficacy of ADHF care requires a thorough understanding of (1) the causes and potential treatments for the patient's acute decompensation, (2) the management of the patient's chronic heart failure, and (3) potential future therapies. Strategies to improve the continuum of heart failure care have been employed to help improve patient outcomes.23 For example, hospital‐based disease management programs have consistently been shown to optimize care and reduce rehospitalization rates in patients with heart failure.24 These programs involve a multidisciplinary, multifaceted approach to care in order to provide a continuum of care extending from hospitalization and into a patient's home environment.
Because of their practice location and experience, hospitalists are uniquely suited to influence acute inpatient care.25 They see patients in a variety of hospital settings and consequently tend to think of the entire system and not just an isolated component or patient.14 In addition, they have a vested interest in hospital quality improvement measures and are frequently involved in evaluating policies and procedures and developing and implementing clinical pathways, guidelines, and decision‐support tools.26 Data demonstrate that compliance is greater with evidence‐based guidelines and core performance measures when inpatient care is directed by a hospitalist.2730 Improved compliance with selected quality measures in patients with acute myocardial infarction and congestive heart failure has been observed when hospitals implement standardized admission and discharge orders.31, 32
Numerous transitions, such as outpatient to inpatient, intensive care unit to ward, and ward to home, occur during hospitalization, and these transitions are frequently associated with changes in the patient's medication regimen. During an acute illness, chronic medications may be held or discontinued, long‐acting medications may be changed to short‐acting ones to better titrate dose and achieve tighter control, and closed formularies may necessitate substituting 1 medication for another.33 A breakdown in communication during hospitalization‐associated transitions commonly affects medication regimens and can adversely impact patient care.3436 In a prospective evaluation, 53.6% [95% confidence interval (CI): 45.7%61.6%] of patients admitted to the hospital had at least 1 unintended discrepancy between their admission medication orders and their chronic outpatient regimen; 38.6% of these discrepancies were considered a potential threat to the patient.34 Likewise, 49% of patients being discharged from the hospital in another evaluation had an unexplained discrepancy between their preadmission and discharge medications.36 As a result, the Joint Commission on Accreditation of Healthcare Organizations now requires accredited facilities to perform medication reconciliation whenever a patient changes service, setting, provider, or level of care and new medication orders are written.37 This reconciliation is especially important in patients with heart failure, for whom polypharmacy is common and noncompliance with appropriate treatment regimens substantially increases readmission rates.3842
During these transition periods, hospitalists can play an important role in bridging the communication gap and providing this medication reconciliation.33 For example, actively involving hospitalists in all aspects of the reconciliation process at 1 institution resulted in a 4‐fold increase in consistency with preadmission medications.43 Similarly, because of the number of discharge summaries that they write, hospitalists are well suited to lead implementation of new policies and procedures to ensure compliance with recent changes in the Joint Commission on Accreditation of Healthcare Organizations requirements regarding these summaries.
In addition to playing an active role in acute patient management, hospitalists can substantially influence long‐term care and outcomes. Consequently, hospitalists must be well versed in the management of chronic heart failure. Patients are intensely focused on their illness during the hospitalization period, and this focus enhances opportunities for meaningful education and behavior modification. Numerous studies have demonstrated that adherence to long‐term therapy is improved when this therapy is initiated before or at hospital discharge.4446 In an evaluation of data from the Organized Program To Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure Registry (OPTIMIZE‐HF), the prescription of a ‐blocker at discharge was associated with a significant reduction in 60‐ to 90‐day mortality [hazard ratio (HR): 0.48; 95% CI: 0.30‐0.79], and prescription of an angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker at discharge was associated with a significant reduction in 60‐ to 90‐day mortality and/or rehospitalization (HR: 0.51; 95% CI: 0.34‐0.78).47 In the Cardiac Hospitalization Atherosclerosis Management Program (CHAMP), emphasizing initiation of chronic therapy prior to hospital discharge was associated with 3.0‐fold greater angiotensin‐converting enzyme inhibitor use and 3.2‐fold greater ‐blocker use at 1 year (both P < 0.01).46 Similarly, in patients surviving acute myocardial infarction, the strongest predictor of ‐blocker use at 30 days following discharge was receipt of a ‐blocker prescription at the time of discharge (HR: 15.8; 95% CI: 10.8‐23.3), and this beneficial effect was sustained for up to a year (Figure 2).44 Likewise, in patients with ADHF, the prevalence of ‐blocker therapy at 60 days was significantly increased when this therapy was initiated before discharge (91%) versus after discharge (73%; P < 0.001).45 This predischarge initiation of chronic therapy has been shown to reduce morbidity and mortality.

An awareness of new therapies for ADHF that are in late stages of clinical development can improve understanding of the complex pathophysiology of ADHF and enhance appropriate adaptation of these therapies once they become clinically available. These new therapies represent an attempt to improve on existing therapies, and consequently, they fall into the same 3 general categories as current therapies: diuretics, vasodilators, and inotropic agents.48, 49 Vasopressin receptor antagonists and adenosine receptor antagonists represent an attempt to stimulate aquaresis without inducing hyponatremia, hypokalemia, diminished glomerular filtration, or adverse neurohormonal activation;4854 endothelin receptor antagonists and newer natriuretic peptides represent an attempt to stimulate vasodilation and improve cardiac output without diminishing renal function;49, 55 and myosin activators and sodium‐potassium adenosine triphosphatase inhibitors represent an attempt to enhance contractility without inducing arrhythmogenicity or increasing mortality risk4859 (Table 1).
| Class/MOA | Agent(s) | Advantages/Disadvantages | References |
|---|---|---|---|
| |||
| Vasopressin receptor antagonists | Tolvaptan | Induce aquaresis without natriuresis | deGoma et al.48 |
| Conivaptan | Potentially avoid hyponatremia and hypokalemia | Tang and Hobbs49 | |
| Lixivaptan | Konstam et al.50 | ||
| SR‐121463b | Schrier et al.51 | ||
| Schweiger and Zdanowicz52 | |||
| Adenosine A1 receptor antagonists | Rolofylline | Increase renal blood flow | Tang and Hobbs49 |
| BG‐9719 | Increase intraglomerular hydraulic pressure | deGoma et al.48 | |
| BG‐9928 | May produce diuresis without adversely affecting glomerular filtration and renal function | Givertz et al.53 Greenberg et al.54 | |
| Endothelin receptor antagonists | Tezosentan | Potent vasodilator | Tang and Hobbs49 |
| Improves cardiac output | McMurray et al.55 | ||
| Hemodynamic effects have not translated into an improvement in heart failure symptoms or risk of death. | |||
| Natriuretic peptides | Ularitide | Resists inactivation by neutral endopeptidase | deGoma et al.48 |
| Improves filling pressures and dyspnea scores | Mitrovic et al.59 | ||
| No apparent deleterious effect on short‐term renal function | |||
| Myosin activators | CK‐1827452 | Tries to dissociate inotropy from arrhythmogenicity | deGoma et al.48 |
| Enhances contractility by targeting myocardial myosin, the force generating cardiac enzymes | Cytokinetics56 | ||
| Still very early in clinical development (just entered phase 2) | |||
| Sodium‐potassium ATPase inhibitors | Istaroxime | Tries to dissociate inotropy from arrhythmogenicity | deGoma et al.48 |
| Enhances contractility by stimulating calcium entry into the sarcolemmal Na/Ca exchanger | Blair et al.57 | ||
| Lusitropic | Cleland et al.58 | ||
| Still very early in clinical development (just completed first phase 2 trial) | |||
Finally, although major advancements in the medical therapy of heart failure patients have substantially improved outcomes,60 technological advances in mechanical devices,61 including automatic implantable cardioverter defibrillators, cardiac resynchronization therapy, and ventricular assist devices, as well as advances in the surgical treatment of heart failure,62 have also been used to support the failing heart. Heart failure patients being treated with mechanical devices, as well as those following cardiac transplant, require unique care. As more mechanical and surgical innovations emerge, nonpharmacologic therapy will continue to evolve as a cornerstone of the management strategy in heart failure patients. Hospitalists will need to rely on care pathways, criteria for consultation, and good communication with cardiologists to optimize the care of these patients. Hospitalists should work with their cardiology colleagues in their local institution to develop appropriate criteria for cardiology consultation, and everyone should be educated on these criteria.
The subsequent discussions in this supplement expand on these topics. First, I review the presentation and early recognition, risk stratification, and treatment of patients with ADHF and the role of the hospitalist in this assessment and treatment process. Next, Dr. Khan and Dr. Heywood review the role of diuretics, vasodilators, and ultrafiltration in the management of patients with volume overload and high filling pressures and conclude with a discussion of potential future pharmacologic treatment options, such as tolvaptan and rolofylline, and nonpharmacologic modalities, such as wireless hemodynamic monitoring through implanted devices. Finally, Dr. Michota and I discuss bridging the gap between evidence and practice in the management of patients with ADHF. We review the evidence‐based guidelines that are currently available; discuss the appropriate location for treatment based on the patient's initial history and physical, radiographic, and laboratory findings; provide a practical algorithm for this treatment; and discuss means to transition care from the inpatient setting to the outpatient setting in a manner that enhances compliance with long‐term therapy and reduces recidivism. Given the anticipated growth in ADHF and the need for hospitalists to manage this disease together with cardiologists and others, we believe that the provided information will be helpful in the management of ADHF.
- ,.National Hospital Discharge Survey: annual summary, 1996.Vital Health Stat.1999;13(140):1–46.
- ,,,.Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995.Am Heart J.1999;137(2):352–360.
- ,,.National Hospital Discharge Survey: 2001 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2004;13(156):1–198.
- ,,.National Hospital Discharge Survey: 2002 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2005;13(158):1–199.
- ,,.National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2007;13(165):1–209.
- ,,.National Hospital Discharge Survey: 2004 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2006;13(162):1–209.
- ,,.National Hospital Discharge Survey: 2003 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2006;13(160):1–206.
- Division for Heart Disease and Stroke Prevention. Heart failure fact sheet. Available at: http://www.cdc.gov/dhdsp/library/fs_heart_failure_longdesc.htm. Accessed September2008.
- US Census Bureau. Projected population of the United States, by age and sex: 2000 to 2050. Available at: http://www.census.gov/population/www/projections/usinterimproj/natprojtab02a.pdf. Accessed September2008.
- ,,,.Demographics and cardiology, 1950–2050.J Am Coll Cardiol.2000;35(4):1067–1081.
- 35th Bethesda Conference.Cardiology's workforce crisis: a pragmatic approach. Bethesda, Maryland, 17–18 October 2003.J Am Coll Cardiol.2004;44(2):216–275.
- ,.The evolution of the hospitalist movement in the USA.Clin Med.2002;2(4):327–330.
- .Hospitalists in the United States—mission accomplished or work in progress?N Engl J Med.2004;350(19):1935–1936.
- .Identifying strategies to improve outcomes and reduce costs—a role for the hospitalist.Curr Opin Pulm Med.2004;10(suppl):S19–S22.
- ,.Cardiovascular manpower: the looming crisis.Circulation.2004;109(7):817–820.
- ,.The United States cardiovascular care deficit.Circulation.2004;109(7):821–823.
- Heart Failure Society of America.Evaluation and management of patients with acute decompensated heart failure.J Card Fail.2006;12(1):e86–e103.
- ,,,.Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team. Results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) study.Arch Intern Med.1999;159(16):1939–1945.
- ,,,,.Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial.CMAJ.2005;173(1):40–45.
- ,.Implementing a congestive heart failure disease management program to decrease length of stay and cost.J Cardiovasc Nurs.1999;14(1):55–74.
- ,,,,.The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine.J Hosp Med.2006;1(suppl 1):2–95.
- ,,, et al.ASHP‐SHM joint statement on hospitalist‐pharmacist collaboration.J Hosp Med.2008;3(suppl 3). doi://10.1002/jhm.315. Available at: http://www3.interscience.wiley.com.
- ,,,,,.Heart failure: improving the continuum of care.Care Manag J.2006;7(2):58–63.
- ,,.Strategies to reduce hospitalization in the management of heart failure.Lippincotts Case Manag.2005;10(6 suppl):S1–S15.
- .Improving the management of patients after myocardial infarction, from admission to discharge.Clin Ther.2006;28(10):1509–1539.
- .The role of hospitalists in the management of acute decompensated heart failure.Am Heart Hosp J.2005;3(2):111–117.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162(11):1251–1256.
- ,.Productive interdisciplinary team relationships: the hospitalist and the case manager.Lippincotts Case Manag.2006;11(3):160–164.
- .Use of pay for performance in a community hospital private hospitalist group: a preliminary report.Trans Am Clin Climatol Assoc.2007;118:263–272.
- ,,.Comparison of practice patterns of hospitalists and community physicians in the care of patients with congestive heart failure.J Hosp Med.2008;3(1):35–41.
- ,,,,,.Integrating “best of care” protocols into clinicians' workflow via care provider order entry: impact on quality‐of‐care indicators for acute myocardial infarction.J Am Med Inform Assoc.2006;13(2):188–196.
- ,,, et al.Improved compliance with quality measures at hospital discharge with a computerized physician order entry system.Am Heart J.2006;151(3):643–653.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,,, et al.Unintended medication discrepancies at the time of hospital admission.Arch Intern Med.2005;165(4):424–429.
- ,,,,,.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166(5):565–571.
- Joint Commission on Accreditation of Healthcare Organizations. Using medication reconciliation to prevent errors. Sentinel Event Alert #35. Available at: http://www.jointcommission.org/sentinelevents/sentineleventalert/sea_35.htm. Accessed September2008.
- ,,,.Precipitating factors leading to decompensation of heart failure: traits among urban blacks.Arch Intern Med.1988;148(9):2013–2016.
- ,,,,.Early readmission of elderly patients with congestive heart failure.J Am Geriatr Soc.1990;38(12):1290–1295.
- ,.Observation unit management of heart failure.Emerg Med Clin North Am.2001;19(1):209–232.
- ,,, et al.Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Failure National Registry (ADHERE).Am Heart J.2005;149(2):209–216.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296(18):2217–2226.
- . Eliminating drug errors: hospitals adopt medication reconciliation to improve patient safety. Available at: http://www.acponline.org/clinical_information/journals_publications/acp_hospitalist/may07/drug_errors.htm. Accessed September2008.
- ,,, et al.Outpatient adherence to beta‐blocker therapy after acute myocardial infarction.JAm Coll Cardiol.2002;40(9):1589–1595.
- ,.Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure.Am J Cardiol.2004;93(9A):74B–76B.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol.2001;87(7):819–822.
- ,,, et al.Association between performance measures and clinical outcomes for patients hospitalized with heart failure.JAMA.2007;297(1):61–70.
- ,,,.Emerging therapies for the management of decompensated heart failure: from bench to bedside.J Am Coll Cardiol.2006;48(12):2397–2409.
- ,.Novel strategies for the management of acute decompensated heart failure.Curr Cardiol Rev.2005;1(1):1–5.
- ,,, et al.Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial.JAMA.2007;297(12):1319–1331.
- ,,, et al.Tolvaptan, a selective oral vasopressin V2‐receptor antagonist, for hyponatremia.N Engl J Med.2006;355(20):2099–2112.
- ,.Vasopressin‐receptor antagonists in heart failure.Am J Health Syst Pharm.2008;65(9):807–817.
- ,,,,.The effects of KW‐3902, an adenosine A1‐receptor antagonist, on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance.J Am Coll Cardiol.2007;50(16):1551–1560.
- ,,, et al.Effects of multiple oral doses of an A1 adenosine antagonist, BG9928, in patients with heart failure: results of a placebo‐controlled, dose‐escalation study.J Am Coll Cardiol.2007;50(7):600–606.
- ,,, et al.Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials.JAMA.2007;298(17):2009–2019.
- CK‐1827452. Cytokinetics Web site. Available at: http://www.cytokinetics.com/ck_1827452. Accessed September2008.
- ,,, et al.Rationale and design of the hemodynamic, echocardiographic and neurohormonal effects of istaroxime, a novel intravenous inotropic and lusitropic agent: a randomized controlled trial in patients hospitalized with heart failure (HORIZON‐HF) trial.Am J Ther.2008;15(3):231–240.
- ,,, et al.Clinical trials update from the American College of Cardiology 2008: CARISMA, TRENDS, meta‐analysis of Cox‐2 studies, HAT, ON‐TARGET, HYVET, ACCOMPLISH, MOMENTUM, PROTECT, HORIZON‐HF and REVERSE.Eur J Heart Fail.2008;10(6):614–620.
- ,,, et al.Haemodynamic and clinical effects of ularitide in decompensated heart failure.Eur Heart J.2006;27(23):2823–2832.
- ,,.Mechanical support in acute and chronic heart failure.Curr Cardiol Rep.2008;10(3):168–175.
- ,.Devices in acute heart failure.Crit Care Med.2008;36(1 suppl):S121–S128.
- ,.Advances in the surgical treatment of heart failure.Curr Opin Cardiol.2008;23(3):249–253.
- ,.National Hospital Discharge Survey: annual summary, 1996.Vital Health Stat.1999;13(140):1–46.
- ,,,.Hospitalization of patients with heart failure: National Hospital Discharge Survey, 1985 to 1995.Am Heart J.1999;137(2):352–360.
- ,,.National Hospital Discharge Survey: 2001 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2004;13(156):1–198.
- ,,.National Hospital Discharge Survey: 2002 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2005;13(158):1–199.
- ,,.National Hospital Discharge Survey: 2005 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2007;13(165):1–209.
- ,,.National Hospital Discharge Survey: 2004 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2006;13(162):1–209.
- ,,.National Hospital Discharge Survey: 2003 annual summary with detailed diagnosis and procedure data.Vital Health Stat.2006;13(160):1–206.
- Division for Heart Disease and Stroke Prevention. Heart failure fact sheet. Available at: http://www.cdc.gov/dhdsp/library/fs_heart_failure_longdesc.htm. Accessed September2008.
- US Census Bureau. Projected population of the United States, by age and sex: 2000 to 2050. Available at: http://www.census.gov/population/www/projections/usinterimproj/natprojtab02a.pdf. Accessed September2008.
- ,,,.Demographics and cardiology, 1950–2050.J Am Coll Cardiol.2000;35(4):1067–1081.
- 35th Bethesda Conference.Cardiology's workforce crisis: a pragmatic approach. Bethesda, Maryland, 17–18 October 2003.J Am Coll Cardiol.2004;44(2):216–275.
- ,.The evolution of the hospitalist movement in the USA.Clin Med.2002;2(4):327–330.
- .Hospitalists in the United States—mission accomplished or work in progress?N Engl J Med.2004;350(19):1935–1936.
- .Identifying strategies to improve outcomes and reduce costs—a role for the hospitalist.Curr Opin Pulm Med.2004;10(suppl):S19–S22.
- ,.Cardiovascular manpower: the looming crisis.Circulation.2004;109(7):817–820.
- ,.The United States cardiovascular care deficit.Circulation.2004;109(7):821–823.
- Heart Failure Society of America.Evaluation and management of patients with acute decompensated heart failure.J Card Fail.2006;12(1):e86–e103.
- ,,,.Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team. Results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) study.Arch Intern Med.1999;159(16):1939–1945.
- ,,,,.Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial.CMAJ.2005;173(1):40–45.
- ,.Implementing a congestive heart failure disease management program to decrease length of stay and cost.J Cardiovasc Nurs.1999;14(1):55–74.
- ,,,,.The core competencies in hospital medicine: a framework for curriculum development by the Society of Hospital Medicine.J Hosp Med.2006;1(suppl 1):2–95.
- ,,, et al.ASHP‐SHM joint statement on hospitalist‐pharmacist collaboration.J Hosp Med.2008;3(suppl 3). doi://10.1002/jhm.315. Available at: http://www3.interscience.wiley.com.
- ,,,,,.Heart failure: improving the continuum of care.Care Manag J.2006;7(2):58–63.
- ,,.Strategies to reduce hospitalization in the management of heart failure.Lippincotts Case Manag.2005;10(6 suppl):S1–S15.
- .Improving the management of patients after myocardial infarction, from admission to discharge.Clin Ther.2006;28(10):1509–1539.
- .The role of hospitalists in the management of acute decompensated heart failure.Am Heart Hosp J.2005;3(2):111–117.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162(11):1251–1256.
- ,.Productive interdisciplinary team relationships: the hospitalist and the case manager.Lippincotts Case Manag.2006;11(3):160–164.
- .Use of pay for performance in a community hospital private hospitalist group: a preliminary report.Trans Am Clin Climatol Assoc.2007;118:263–272.
- ,,.Comparison of practice patterns of hospitalists and community physicians in the care of patients with congestive heart failure.J Hosp Med.2008;3(1):35–41.
- ,,,,,.Integrating “best of care” protocols into clinicians' workflow via care provider order entry: impact on quality‐of‐care indicators for acute myocardial infarction.J Am Med Inform Assoc.2006;13(2):188–196.
- ,,, et al.Improved compliance with quality measures at hospital discharge with a computerized physician order entry system.Am Heart J.2006;151(3):643–653.
- ,,,.Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists.J Hosp Med.2007;2(5):314–323.
- ,,, et al.Unintended medication discrepancies at the time of hospital admission.Arch Intern Med.2005;165(4):424–429.
- ,,,,,.Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients.Am J Health Syst Pharm.2004;61(16):1689–1695.
- ,,, et al.Role of pharmacist counseling in preventing adverse drug events after hospitalization.Arch Intern Med.2006;166(5):565–571.
- Joint Commission on Accreditation of Healthcare Organizations. Using medication reconciliation to prevent errors. Sentinel Event Alert #35. Available at: http://www.jointcommission.org/sentinelevents/sentineleventalert/sea_35.htm. Accessed September2008.
- ,,,.Precipitating factors leading to decompensation of heart failure: traits among urban blacks.Arch Intern Med.1988;148(9):2013–2016.
- ,,,,.Early readmission of elderly patients with congestive heart failure.J Am Geriatr Soc.1990;38(12):1290–1295.
- ,.Observation unit management of heart failure.Emerg Med Clin North Am.2001;19(1):209–232.
- ,,, et al.Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Failure National Registry (ADHERE).Am Heart J.2005;149(2):209–216.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296(18):2217–2226.
- . Eliminating drug errors: hospitals adopt medication reconciliation to improve patient safety. Available at: http://www.acponline.org/clinical_information/journals_publications/acp_hospitalist/may07/drug_errors.htm. Accessed September2008.
- ,,, et al.Outpatient adherence to beta‐blocker therapy after acute myocardial infarction.JAm Coll Cardiol.2002;40(9):1589–1595.
- ,.Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure.Am J Cardiol.2004;93(9A):74B–76B.
- ,,,.Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP).Am J Cardiol.2001;87(7):819–822.
- ,,, et al.Association between performance measures and clinical outcomes for patients hospitalized with heart failure.JAMA.2007;297(1):61–70.
- ,,,.Emerging therapies for the management of decompensated heart failure: from bench to bedside.J Am Coll Cardiol.2006;48(12):2397–2409.
- ,.Novel strategies for the management of acute decompensated heart failure.Curr Cardiol Rev.2005;1(1):1–5.
- ,,, et al.Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial.JAMA.2007;297(12):1319–1331.
- ,,, et al.Tolvaptan, a selective oral vasopressin V2‐receptor antagonist, for hyponatremia.N Engl J Med.2006;355(20):2099–2112.
- ,.Vasopressin‐receptor antagonists in heart failure.Am J Health Syst Pharm.2008;65(9):807–817.
- ,,,,.The effects of KW‐3902, an adenosine A1‐receptor antagonist, on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance.J Am Coll Cardiol.2007;50(16):1551–1560.
- ,,, et al.Effects of multiple oral doses of an A1 adenosine antagonist, BG9928, in patients with heart failure: results of a placebo‐controlled, dose‐escalation study.J Am Coll Cardiol.2007;50(7):600–606.
- ,,, et al.Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: the VERITAS randomized controlled trials.JAMA.2007;298(17):2009–2019.
- CK‐1827452. Cytokinetics Web site. Available at: http://www.cytokinetics.com/ck_1827452. Accessed September2008.
- ,,, et al.Rationale and design of the hemodynamic, echocardiographic and neurohormonal effects of istaroxime, a novel intravenous inotropic and lusitropic agent: a randomized controlled trial in patients hospitalized with heart failure (HORIZON‐HF) trial.Am J Ther.2008;15(3):231–240.
- ,,, et al.Clinical trials update from the American College of Cardiology 2008: CARISMA, TRENDS, meta‐analysis of Cox‐2 studies, HAT, ON‐TARGET, HYVET, ACCOMPLISH, MOMENTUM, PROTECT, HORIZON‐HF and REVERSE.Eur J Heart Fail.2008;10(6):614–620.
- ,,, et al.Haemodynamic and clinical effects of ularitide in decompensated heart failure.Eur Heart J.2006;27(23):2823–2832.
- ,,.Mechanical support in acute and chronic heart failure.Curr Cardiol Rep.2008;10(3):168–175.
- ,.Devices in acute heart failure.Crit Care Med.2008;36(1 suppl):S121–S128.
- ,.Advances in the surgical treatment of heart failure.Curr Opin Cardiol.2008;23(3):249–253.
Bridging the Evidence/Practice Gap
Optimizing quality of care in patients with acute decompensated heart failure (ADHF) is crucial, given both the frequency and cost of hospitalization for this disorder. Several quality improvement strategies have been identified, including provider education; provider reminder systems and decision support; audit and feedback; patient education; organizational change; and financial incentives, regulation, and policy.1
To assist hospitalists in implementing these strategies, this article briefly reviews evidence‐based guidelines for the treatment of ADHF, presents a practical algorithm for patient assessment and treatment derived from these guidelines and personal experience, and discusses systems to enhance the ultimate transition of patient care from the inpatient to outpatient setting.
EVIDENCE‐BASED GUIDELINES
Evidence‐based guidelines are created in an attempt to promote optimal management of a condition or disorder based on expert analysis of all available relevant scientific data. Current guidelines for the assessment and treatment of ADHF have been developed by a national group purchasing organization,2 the European Society of Cardiology,3 the Heart Failure Society of America,4 and the American College of Emergency Physicians.5 Relevant components of these guidelines will be discussed in the patient assessment and treatment section below.
Publication of guidelines, in and of itself, however, is inadequate to ensure their acceptance and use.1 Data from the American Heart Association (AHA)/American Stroke Association (ASA) Get With The GuidelinesHeart Failure (GWTG‐HF) program continue to demonstrate a substantial gap between guideline recommendations and current care of patients with ADHF.6, 7 One way to promote systemwide adherence with published guidelines is to directly involve healthcare professionals in the implementation process. Consequently, development of local, hospital‐based procedures derived from national or international guidelines may be more effective than the simple dissemination of the guidelines themselves.1 Hospitalists have a unique insight into both patient care and the hospital setting and are frequently involved in evaluating hospital policies and procedures and implementing clinical pathways and guidelines.8 In addition, hospitalist care has been associated with greater compliance with disease‐specific guidelines compared to nonhospitalist care.9 As a result, hospitalists are uniquely suited to play a key role in the development of these procedures.
PATIENT ASSESSMENT AND TREATMENT
The differential diagnosis of any individual presenting to the emergency department (ED) with signs of systemic or pulmonary edema should include ADHF (Figure 1).25 These individuals require a rapid initial assessment to (1) establish the diagnosis, (2) determine the best location for subsequent treatment, and (3) institute the most appropriate initial therapy.
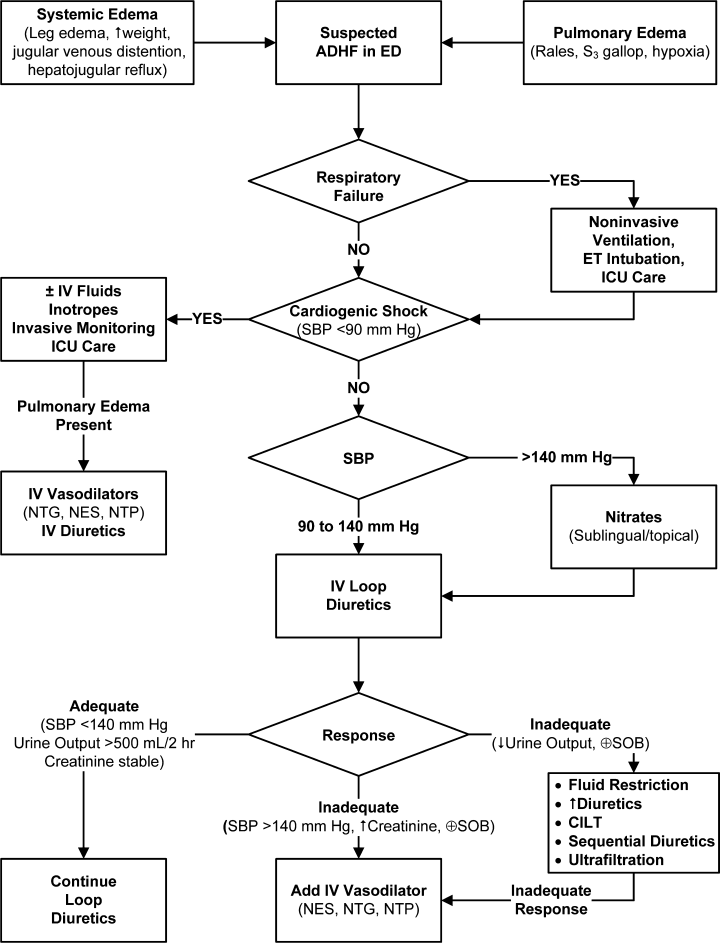
Treatment Location
Effective and efficient management of ADHF requires determining proper treatment location. Inpatient management of ADHF is expensive, accounting for approximately 60% of the $31.7 billion spent annually on heart failure care in the United States.10 Clearly, patients with impending respiratory failure requiring ventilation assistance and patients with cardiogenic shock requiring inotropic agents and invasive monitoring are best cared for in an intensive care unit (ICU) setting. However, these patients constitute the minority of patients with ADHF. For example, systolic blood pressure (SBP) <90 mm Hg was present in only 2.3% of patients in the Acute Decompensated Heart Failure National Registry (ADHERE), a registry designed to study characteristics, management, and outcomes in a broad sample of patients hospitalized with ADHF.11
Most patients with ADHF present with congestion, not respiratory failure or cardiogenic shock,11, 12 and a select subgroup of these patients will respond to treatment within 1224 hours.13 Although this may be an inordinate amount of time to keep patients in an ED, it is not long enough to generally require full hospital admission. Instead, these patients can be effectively managed in an observation unit (OU).14 The goal of these units is to provide the required level of care over a 12‐ to 24‐hour period while simultaneously reducing costs by eliminating the need for hospital admission. Selecting patients who will respond to therapy during this time frame is a critical component in instituting effective OU management of ADHF. Key entry and exclusion criteria are listed in Table 1.14 In patients who meet these criteria, management in an OU has been shown to yield outcomes comparable to inpatient care, but at a lower cost.1416
| Entry criteria |
|---|
|
| History (at least one of the following) |
| Dyspnea on exertion |
| Paroxysmal nocturnal dyspnea |
| Shortness of breath |
| Edema of legs or abdomen |
| Weight gain |
| Physical examination (at least one of the following) |
| Jugular venous distention or elevation in pulsation |
| Positive abdominal jugular reflux |
| S3/S4 gallop |
| Inspiratory rales |
| Peripheral edema |
| Chest x‐ray (at least one of the following) |
| Cardiomegaly |
| Pulmonary vascular congestion |
| Kerley B lines |
| Pulmonary edema |
| Pleural effusion |
| Exclusion criteria |
| Unstable vital signs (BP >220/120 mm Hg, respiratory rate >25 breath/min, heart rate >130 beats/min) |
| Temperature >38.5C |
| Unstable airway or need for >4 L/min supplemental O2 to keep O2 saturation >90% |
| Peak flow <50% of predicted with wheezing |
| Clinically significant arrhythmia or sustained ventricular tachycardia |
| Any ECG with diagnostic criteria for AMI or ischemia |
| Chest x‐ray with pulmonary infiltrates |
| Any CK‐MB >8.8 ng/mL |
| Any troponin T >0.1 g/L (>0.5 g/L if creatinine >2.0 mg/dL) |
| Requirement for continuous vasoactive medication to stabilize hemodynamics |
| Complex decompensation: concomitant end‐organ hypoperfusion, volume overload, and systemic vasoconstriction |
| Requirement for care guided by pulmonary artery catheter |
| Severe electrolyte imbalance |
| Chronic renal failure requiring dialysis |
| Acute mental status abnormality |
Early Initiation of Therapy
Early institution of effective therapy has been shown to improve outcomes. Consequently, selection of initial therapy should occur concurrently with determination of proper treatment location. In the Prospective Randomized Outcomes Study of Acutely Decompensated Congestive Heart Failure Treated Initially as Outpatients with Nesiritide (PROACTION) trial, initiation of nesiritide in the ED/OU was associated with an 11% reduction in hospital admissions at the index visit (P = .436), a 57% reduction in hospitalizations within 30 days after discharge from the index hospitalization (P = .058), and a 62% reduction in median duration of rehospitalization (P = .032).17 The incidence of symptomatic hypotension was low and did not differ between the groups.17 Likewise, in separate analyses of data from ADHERE, ED initiation of intravenous (IV) vasoactive therapy, including nitroglycerin, nesiritide, milrinone, or dobutamine significantly reduced the risk of requiring transfer to an ICU and reduced ICU length of stay and total hospital length of stay compared with inpatient initiation of these same therapies.18, 19
Treatment Algorithm
Treatment of ADHF should proceed along a logical care pathway governed by both clinical status and response to prior therapies (Figure 1). One must first consider whether there is evidence of respiratory failure or impending respiratory failure.20 If so, patients should receive immediate ventilatory support via continuous positive airway pressure (CPAP), bilevel positive airway pressure (BiPAP), or endotracheal intubation, depending on the degree of respiratory impairment.3, 5 In prospective controlled evaluations, patients with acute respiratory failure secondary to pulmonary edema who were randomized to treatment with CPAP demonstrated significant improvement in cardiopulmonary indices21, 22 and significant reductions in need for endotracheal intubation21 and short‐term mortality22 when compared with similar patients who received standard therapy without CPAP.
Once potential respiratory issues have been addressed, the next items for consideration are circulation and perfusion. Patients with low cardiac output and hypotension (cardiogenic shock) are at risk for developing critical end‐organ dysfunction. In these patients, insertion of a pulmonary artery catheter may aid in assessment of hemodynamic status and response to therapy.4 Patients with low cardiac output and low filling pressures should receive IV fluid loading.3 In contrast, for patients with low cardiac output and high filling pressures, inotropic agents should be considered.3, 4 Also, these patients may require IV vasodilators and/or IV diuretics to treat pulmonary edema once blood pressure (BP) and cardiac output have been stabilized.2, 3, 20
For patients with ADHF who present with symptoms of congestion, but not respiratory failure or cardiogenic shock, the initial therapeutic decision is governed by their BP. Approximately 50% of patients with ADHF will have an SBP > 140 mm Hg.12, 23 These patients tend to be older and to have diastolic rather than systolic dysfunction.12, 20, 23 Symptoms typically have been present for only a short period of time (2448 hours) and are more often due to maldistribution of fluid producing pulmonary edema than total body fluid overload. Consequently, initial treatment should focus on aggressive BP control to relieve this edema. Sublingual or topical nitrates are recommended as a first step, and initial diuretic use should be minimal to avoid intravascular volume depletion leading to renal dysfunction.20 In contrast, patients presenting with SBP between 90 mm Hg and 140 mm Hg are more likely to have some degree of systolic dysfunction, leading to a gradual worsening of their heart failure symptoms and total body fluid overload over a period of weeks.20 These patients require aggressive diuresis. Although the efficacy of IV loop diuretics has not been established in randomized, controlled clinical trials, observational experience demonstrates that they can effectively reduce filling pressures, relieve volume overload, and decrease symptoms of congestion.4 They are currently the mainstay of therapy for ADHF secondary to fluid retention, and their use is recommended in all 4 guidelines.25
This initial therapeutic choice, however, is only the starting point, and it is important not to stop at this stage. No single definitive therapy for ADHF exists, and not all patients will respond to initial treatment. Optimal management requires early recognition and addressing of both an inadequate response to therapy and any adverse affects induced by this therapy. Frequent reevaluations are an essential component of treating patients with ADHF. For example, the timeline in one of the guidelines calls for assessing the patient's response at 2 and 4 hours after initiation of IV therapy and adjusting treatment as indicated based on these assessments (Figure 2).2
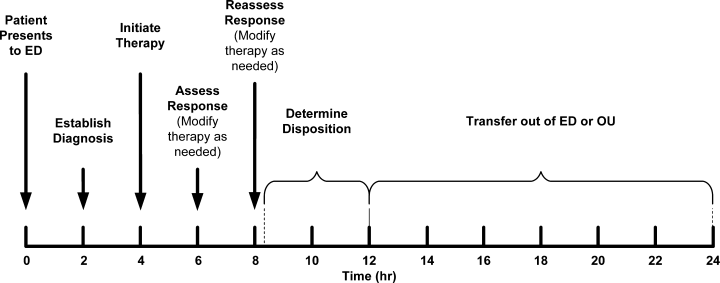
If the patient has an adequate response to initial therapy, defined as SBP <140 mm Hg, stable renal function, and urine output >500 mL over 2 hours (>250 mL if serum creatinine >2.5 mg/dL), this therapy can continue unchanged, and focus shifts to long‐term management issues.2, 4, 14 However, if the response is inadequate, it is important to identify and treat the cause of this inadequate response.
Inadequate urine output secondary to diuretic resistance is common in patients with ADHF, especially in those on long‐term diuretic therapy.3 Despite a 90% prevalence of IV diuretic use in ADHERE, 70% of patients either gained weight or lost fewer than 5 pounds during hospitalization, and 42% were discharged with unresolved symptoms.24 Clearly, diuretic therapy did not produce the desired effect in many of these patients. This inadequate response to loop diuretics is a direct result of their pharmacologic properties, especially as they relate to patients with heart failure. The physiologic effects of loop diuretics are directly related to their concentration in the lumen of the nephron. This concentration depends on both the patient's renal function and the dose and half‐life of the administered diuretic.25 Comorbid renal dysfunction is common in patients with ADHF.26 In addition, even in the absence of this dysfunction, the short half‐life of loop diuretics limits the amount of time that their luminal concentration is in the effective range, and rebound sodium retention can occur whenever the diuretic concentration is below this range.25 Furthermore, the dose‐response curve of loop diuretics is S‐shaped. As a result, a threshold concentration exists beyond which no further augmentation in urine output occurs; ie, there is a maximum physiologic response that is reduced in patients with heart failure.25 Guideline recommendations for patients with diuretic resistance attempt to address these physiologic and pharmacologic limitations. These recommendations include fluid restriction to decrease the overall volume of diuresis necessary, increasing diuretic dose or instituting continuous infusion loop diuretic therapy to increase the amount of time during which the luminal concentration is within the effective range, sequential diuretic blockade to take advantage of the different mechanisms of action of the various diuretic classes to affect different components of the nephron, bypassing the kidney through the use of ultrafiltration, and when these are inadequate, adding a vasodilator in an attempt to augment cardiac output and renal perfusion.3, 4, 24, 25, 2729
Addition of an IV vasodilator is the primary means of addressing an inadequate response typified by hypertension, worsening renal function, and/or persistent symptoms, rather than diuretic resistance. Approximately 25% of patients with ADHF receive IV vasoactive therapy, including nitroglycerin, nesiritide, milrinone, or dobutamine, although predominantly vasodilators, at some point during their hospitalization.11, 12 These agents improve hemodynamics and reduce symptoms of ADHF.5, 3032 Their use, in combination with low‐dose diuretics, has been shown to be more efficacious than high‐dose diuretics alone.3, 27 Adding a vasodilator may reduce adverse, diuretic‐induced, neurohormonal activation. In an animal model, combining nesiritide with IV furosemide significantly attenuated the rise in plasma aldosterone produced by IV furosemide alone,33 and this finding has been subsequently confirmed in patients with heart failure.34 Finally, vasodilators have proven to be a safer alternative than inotropes in patients with ADHF. In an analysis of data from ADHERE, covariate‐adjusted and propensity‐adjusted mortality risk was >50% lower for patients receiving nitroglycerin or nesiritide compared with those receiving dobutamine,11 and in an analysis of data from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial, which evaluated patients with advanced heart failure, the risk‐adjusted mortality hazard ratio (HR) was significantly increased for inotropes (HR: 2.14; 95% confidence interval [CI]: 1.104.15) but not for vasodilators in the absence of inotropes (HR: 1.39; 95% CI: 0.643.00).35
Performance Measures
In addition to instituting effective therapy for the acute decompensation, it is important to implement measures that may improve long‐term outcomes. The Joint Commission on Accreditation of Healthcare Organizations and the AHA/ASA have identified a series of 5 core performance measures that should be completed during hospitalization for AHDF: discharge instructions relevant to patient's education, documentation of left ventricular systolic function evaluation, prescription of angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) at discharge in patients with left ventricular systolic dysfunction, adult smoking cessation advice/counseling, and prescription of ‐blocker at discharge (Table 2).36, 37 In an analysis of data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF) registry, prescription of a ‐blocker at discharge significantly reduced the risk‐adjusted odds ratio (OR) for mortality (OR: 0.48; 95% CI: 0.300.79), and prescription of an ACE inhibitor or ARB at discharge significantly reduced the risk‐adjusted OR for rehospitalization or death (OR: 0.51; 95% CI: 0.340.78) at 6090 days.38 Although no correlation was detected between outcomes and the other 3 core performance measures in this evaluation, the 60‐day to 90‐day time frame may have been too short to identify the full effects of smoking cessation counseling and left ventricular function assessment. Failure to detect a beneficial effect of discharge instructions is disappointing, especially given the proven benefit of disease management programs (see below) and may reflect a limitation of this measure, as currently implemented, to determine the thoroughness and patient understanding of the instructions provided.38, 39
| Measure | Description | Source |
|---|---|---|
| HF‐1 | Discharge instructions relevant to patient education | JCAHO; AHA/ASA |
| HF‐2 | Documentation of left ventricular systolic function evaluation | JCAHO; AHA/ASA |
| HF‐3 | Prescription of ACE inhibitor or ARB at discharge in patients with left ventricular systolic dysfunction | JCAHO; AHA/ASA |
| HF‐4 | Adult smoking cessation advice/counseling | JCAHO; AHA/ASA |
| HF‐5 | Prescription of ‐blocker at discharge | AHA/ASA |
TRANSITION OF CARE
Lastly, optimal management of ADHF requires successful transition of care from an inpatient to an outpatient setting. Recidivism is both common and costly. Approximately 2% of patients with ADHF are readmitted within 2 days, 20% within 1 month, and 50% within 6 months of hospital discharge.40 Frequently, these readmissions are caused by nonadherence to the therapeutic regimen following discharge.41 In an evaluation of patients hospitalized for ADHF at a large urban medical center, noncompliance with prescribed diet and/or drugs was the most common precipitating factor for admission (64% of patients), followed by uncontrolled hypertension (44%), cardiac arrhythmia (29%), environmental factors (19%), and inadequate therapy (17%).42 Similarly, in a prospective evaluation of elderly patients hospitalized for ADHF, 53% of readmissions occurring within 90 days of discharge were deemed to be preventable, with the most common contributing factors being noncompliance with medications and/or diet (33%), inadequate discharge planning (15%), inadequate follow‐up (20%), insufficient support system (21%), and failure to seek medical attention promptly when symptoms recurred (20%).43 Consequently, patient education and arrangement for appropriate follow‐up are crucial components of successfully transitioning care to an outpatient setting.
Effective patient education is time‐consuming. Patients must be taught when, how, and why to take their medication. They need to understand their dietary guidelines and the reasons for these guidelines. They need to know how to use daily weigh‐ins as a means of monitoring their fluid status and what to do in response to a change in weight or symptoms. Finally, they need to be cognizant of what constitutes appropriate exercise and the need for this exercise.4447 To enhance understanding and retention, this information should be presented to the patient over the course of the hospitalization. Comprehension should be tested continually and education repeated until appropriate understanding is ensured. Patient education provided in a rushed or perfunctory manner at the moment of discharge is unlikely to be retained or effective.38, 39
Ideally, the patient should be referred to a comprehensive heart failure disease management program for postdischarge care. Numerous evaluations have established the effectiveness of these programs in enhancing use of appropriate medications, improving functional status, reducing readmissions and mortality, and decreasing costs.4454 For example, in separate evaluations, the prevalences of appropriate vasodilator use (93% vs. 61%; P < .001),51 ‐blocker use (71% vs. 40%; P < .001),50 and ACE inhibitor use (84% vs. 59%; P < .001)52 were significantly greater for disease management program participants compared with nonparticipants. In addition, participation in a disease management program was associated with a 52% reduction in the risk of hospitalization for cardiovascular causes (P < .001) and a 72% reduction in ED visits (P < .01) in 1 evaluation,45 a 36% reduction (95% CI: 16.7%50.9%) in the risk of heart failure admission or death in another,53 and a 67% reduction (95% CI: 41%82%) in the adjusted risk of death in yet another evaluation.52 Unfortunately, recent data indicate that these programs must be ongoing to sustain these benefits. In a prospective evaluation, patients with heart failure were randomized to either standard care or a multidisciplinary disease management program for 6 months followed by standard care.55, 56 Significantly fewer patients in the disease management group required readmission to the hospital (HR: 0.55; 95% CI: 0.350.88) during the 6‐month period in which they actively participated in the disease management program.56 However, by the end of follow‐up (mean 2.8 years), there was no significant difference between treatment groups in all‐cause mortality (HR: 1.09; 95% CI: 0.691.72) or the composite endpoint of death, ED visit, or hospitalization (HR: 1.01; 95% CI: 0.751.37).55
CONCLUSION
A gap between evidence‐based guidelines and current management of patients with ADHF exists. Multiple strategies to bridge this gap in patient management can be employed. Patients with ADHF require rapid assessment to determine appropriate treatment location and initial therapy. Clinical status should guide treatment selection. Once effective acute therapy has been established, strategies to improve long‐term outcomes should be implemented. These strategies include ensuring that care complies with established core performance measures, providing patient education in a manner suited to ensure comprehension and retention, and arranging for appropriate outpatient follow‐up, ideally in a comprehensive heart failure disease management program. Increasing the awareness of the gap between evidence‐based guidelines and current management, as well as strategies to bridge this gap, is crucial to improving the outcomes of patients with ADHF.
- .Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improvement process.J Gen Intern Med.2007;22(12):1762–1770.
- ,,, et al.Guidelines for acute decompensated heart failure treatment.Ann Pharmacother.2004;38(4):649–660.
- ,,, et al.Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology.Eur Heart J.2005;26(4):384–416.
- Heart Failure Society of America.Executive summary: HFSA 2006 comprehensive heart failure practice guideline.J Card Fail.2006;12(1):10–38.
- ,,,,,American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute heart failure syndromes.Ann Emerg Med.2007;49(5):627–669.
- ,,, et al.Influence of the American Heart Association's Get With The Guidelines‐Heart Failure (GWTG‐HF) program on emerging performance measures for patients hospitalized with heart failure.Circulation.2006;114:II–572. Abstract 2740.
- ,,, et al.Quality of care for heart failure patients with concomitant kidney disease in the American Heart Association's Get With The Guidelines‐Heart Failure (GWTG‐HF) program.Circulation.2006;114:II–859. Abstract 3993.
- .The role of hospitalists in the management of acute decompensated heart failure.Am Heart Hosp J.2005;3(2):111–117.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162(11):1251–1256.
- ,,, et al.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2008;117(4):e25–e146.
- ,,, et al.In‐hospital mortality in patients with acute decompensated heart failure treated with intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE).J Am Coll Cardiol.2005;46(1):57–64.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296(18):2217–2226.
- ,,,.Preliminary experience with an emergency department observation unit protocol for heart failure.Acad Emerg Med.2000;7(10):1171. Abstract 33.
- ,,,,,.Effective observation unit treatment of decompensated heart failure.Congest Heart Fail.2002;8(2):68–73.
- ,.Patient outcome and costs following an acute heart failure (HF) management program in an emergency department (ED) observation unit (OU).J Heart Lung Transplant.1999;18(1):92. Abstract 240.
- ,,,,,.Inpatient versus emergency department observation unit management of heart failure.Ann Emerg Med.1998;32(3 part 2):S46–S47. Abstract 180.
- ,,, et al.Observation unit treatment of heart failure with nesiritide: results from the proaction trial.J Emerg Med.2005;29(3):243–252.
- ,,,.Early initiation of IV vasoactive therapy improves heart failure outcomes: an analysis from the ADHERE™ registry database.Ann Emerg Med.2003;42(4 suppl):S26. Abstract 92.
- ,,, et al.Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE.Cardiology.2007;107(1):44–51.
- ,,,,,.Beyond pulmonary edema: diagnostic, risk stratification, and treatment challenges of acute heart failure management in the emergency department.Ann Emerg Med.2008;51(1):45–57.
- ,,,,,.Reappraisal of continuous positive airway pressure therapy in acute cardiogenic pulmonary edema. Short‐term results and long‐term follow‐up.Chest.1995;107(5):1379–1386.
- ,,, et al.Noninvasive continuous positive airway pressure in elderly cardiogenic pulmonary edema patients.Intensive Care Med.2004;30(5):882–888.
- ,,,,.Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database.J Am Coll Cardiol.2006;47(1):76–84.
- ,,,.Early ultrafiltration in patients with decompensated heart failure and diuretic resistance.J Am Coll Cardiol.2005;46(11):2047–2051.
- .Diuretic therapy in congestive heart failure.Congest Heart Fail.2000;6(4):197–201.
- ,,, et al.High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database.J Card Fail.2007;13(6):422–430.
- ,,, et al.Randomised trial of high‐dose isosorbide dinitrate plus low‐dose furosemide versus high‐dose furosemide plus low‐dose isosorbide dinitrate in severe pulmonary oedema.Lancet.1998;351(9100):389–393.
- .Diuretic therapy and resistance in congestive heart failure.Cardiology.2001;96(3–4):132–143.
- .Management of diuretic‐refractory, volume‐overloaded patients with acutely decompensated heart failure.Curr Cardiol Rep.2005;7(3):204–210.
- ,,, et al.Sustained hemodynamic effects of an infusion of nesiritide (human b‐type natriuretic peptide) in heart failure: a randomized, double‐blind, placebo‐controlled clinical trial.J Am Coll Cardiol.1999;34(1):155–162.
- ,,, et al.Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure.N Engl J Med.2000;343(4):246–253.
- Publication Committee for the VMAC Investigators.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial.JAMA.2002;287(12):1531–1540.
- ,,, et al.Brain natriuretic peptide enhances renal actions of furosemide and suppresses furosemide‐induced aldosterone activation in experimental heart failure.Circulation.2004;109(13):1680–1685.
- ,,.Nesiritide appears to inhibit the rise in plasma aldosterone associated with furosemide diuresis.J Card Fail.2006;12(6 suppl 1):S85–S86. Abstract 275.
- ,,, et al.Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure.Am Heart J.2007;153(1):98–104.
- Joint Commission on Accreditation of Healthcare Organizations. Specifications Manual for National Implementation of Hospital Quality Measures. Version 2.3b. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Historical+NHQM+manuals.htm. Accessed October 10,2008.
- American Heart Association/American Stroke Association. Get with the guidelines–heart failure. Fact sheet. http://www.americanheart.org/downloadable/heart/1163802072170HFFactSheet.pdf. Accessed October 10,2008.
- ,,, et al.Association between performance measures and clinical outcomes for patients hospitalized with heart failure.JAMA.2007;297(1):61–70.
- ,,, et al.Evaluating quality of care for patients with heart failure.Circulation.2000;101(12):e122–e140.
- .Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department.Rev Cardiovasc Med.2002;3(suppl 4):S3–S9.
- ,.Observation unit management of heart failure.Emerg Med Clin North Am.2001;19(1):209–232.
- ,,,.Precipitating factors leading to decompensation of heart failure. Traits among urban blacks.Arch Intern Med.1988;148(9):2013–2016.
- ,,,,.Early readmission of elderly patients with congestive heart failure.J Am Geriatr Soc.1990;38(12):1290–1295.
- ,,, et al.Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure.J Am Coll Cardiol.1997;30(3):725–732.
- ,,, et al.Prospective evaluation of an outpatient heart failure management program.J Card Fail.2001;7(1):64–74.
- ,,, et al.Improved outcomes from a comprehensive management system for heart failure.Eur J Heart Fail.2001;3(5):619–625.
- ,,, et al.The benefit of implementing a heart failure disease management program.Arch Intern Med.2001;161(18):2223–2228.
- ,,,,,.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333(18):1190–1195.
- ,,.Heart failure disease management in an indigent population.Am Heart J.2001;141(2):254–258.
- ,,, et al.Cost/utility ratio in chronic heart failure: comparison between heart failure management program delivered by day‐hospital and usual care.J Am Coll Cardiol.2002;40(7):1259–1266.
- ,,, et al.A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission.J Am Coll Cardiol.2002;39(3):471–480.
- ,,, et al.Mortality benefit of a comprehensive heart failure disease management program in indigent patients.Am Heart J.2006;151(2):478–483.
- ,,, et al.Two‐year outcome of a prospective, controlled study of a disease management programme for elderly patients with heart failure.J Cardiovasc Med (Hagerstown).2007;8(5):324–329.
- ,,,,.Impact of the implementation of telemanagement on a disease management program in an elderly heart failure cohort.Prog Cardiovasc Nurs.2007;22(4):196–200.
- ,,, et al.Lack of long‐term benefits of a 6‐month heart failure disease management program.J Card Fail.2007;13(4):287–293.
- ,,,,.Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial.CMAJ.2005;173(1):40–45.
Optimizing quality of care in patients with acute decompensated heart failure (ADHF) is crucial, given both the frequency and cost of hospitalization for this disorder. Several quality improvement strategies have been identified, including provider education; provider reminder systems and decision support; audit and feedback; patient education; organizational change; and financial incentives, regulation, and policy.1
To assist hospitalists in implementing these strategies, this article briefly reviews evidence‐based guidelines for the treatment of ADHF, presents a practical algorithm for patient assessment and treatment derived from these guidelines and personal experience, and discusses systems to enhance the ultimate transition of patient care from the inpatient to outpatient setting.
EVIDENCE‐BASED GUIDELINES
Evidence‐based guidelines are created in an attempt to promote optimal management of a condition or disorder based on expert analysis of all available relevant scientific data. Current guidelines for the assessment and treatment of ADHF have been developed by a national group purchasing organization,2 the European Society of Cardiology,3 the Heart Failure Society of America,4 and the American College of Emergency Physicians.5 Relevant components of these guidelines will be discussed in the patient assessment and treatment section below.
Publication of guidelines, in and of itself, however, is inadequate to ensure their acceptance and use.1 Data from the American Heart Association (AHA)/American Stroke Association (ASA) Get With The GuidelinesHeart Failure (GWTG‐HF) program continue to demonstrate a substantial gap between guideline recommendations and current care of patients with ADHF.6, 7 One way to promote systemwide adherence with published guidelines is to directly involve healthcare professionals in the implementation process. Consequently, development of local, hospital‐based procedures derived from national or international guidelines may be more effective than the simple dissemination of the guidelines themselves.1 Hospitalists have a unique insight into both patient care and the hospital setting and are frequently involved in evaluating hospital policies and procedures and implementing clinical pathways and guidelines.8 In addition, hospitalist care has been associated with greater compliance with disease‐specific guidelines compared to nonhospitalist care.9 As a result, hospitalists are uniquely suited to play a key role in the development of these procedures.
PATIENT ASSESSMENT AND TREATMENT
The differential diagnosis of any individual presenting to the emergency department (ED) with signs of systemic or pulmonary edema should include ADHF (Figure 1).25 These individuals require a rapid initial assessment to (1) establish the diagnosis, (2) determine the best location for subsequent treatment, and (3) institute the most appropriate initial therapy.

Treatment Location
Effective and efficient management of ADHF requires determining proper treatment location. Inpatient management of ADHF is expensive, accounting for approximately 60% of the $31.7 billion spent annually on heart failure care in the United States.10 Clearly, patients with impending respiratory failure requiring ventilation assistance and patients with cardiogenic shock requiring inotropic agents and invasive monitoring are best cared for in an intensive care unit (ICU) setting. However, these patients constitute the minority of patients with ADHF. For example, systolic blood pressure (SBP) <90 mm Hg was present in only 2.3% of patients in the Acute Decompensated Heart Failure National Registry (ADHERE), a registry designed to study characteristics, management, and outcomes in a broad sample of patients hospitalized with ADHF.11
Most patients with ADHF present with congestion, not respiratory failure or cardiogenic shock,11, 12 and a select subgroup of these patients will respond to treatment within 1224 hours.13 Although this may be an inordinate amount of time to keep patients in an ED, it is not long enough to generally require full hospital admission. Instead, these patients can be effectively managed in an observation unit (OU).14 The goal of these units is to provide the required level of care over a 12‐ to 24‐hour period while simultaneously reducing costs by eliminating the need for hospital admission. Selecting patients who will respond to therapy during this time frame is a critical component in instituting effective OU management of ADHF. Key entry and exclusion criteria are listed in Table 1.14 In patients who meet these criteria, management in an OU has been shown to yield outcomes comparable to inpatient care, but at a lower cost.1416
| Entry criteria |
|---|
|
| History (at least one of the following) |
| Dyspnea on exertion |
| Paroxysmal nocturnal dyspnea |
| Shortness of breath |
| Edema of legs or abdomen |
| Weight gain |
| Physical examination (at least one of the following) |
| Jugular venous distention or elevation in pulsation |
| Positive abdominal jugular reflux |
| S3/S4 gallop |
| Inspiratory rales |
| Peripheral edema |
| Chest x‐ray (at least one of the following) |
| Cardiomegaly |
| Pulmonary vascular congestion |
| Kerley B lines |
| Pulmonary edema |
| Pleural effusion |
| Exclusion criteria |
| Unstable vital signs (BP >220/120 mm Hg, respiratory rate >25 breath/min, heart rate >130 beats/min) |
| Temperature >38.5C |
| Unstable airway or need for >4 L/min supplemental O2 to keep O2 saturation >90% |
| Peak flow <50% of predicted with wheezing |
| Clinically significant arrhythmia or sustained ventricular tachycardia |
| Any ECG with diagnostic criteria for AMI or ischemia |
| Chest x‐ray with pulmonary infiltrates |
| Any CK‐MB >8.8 ng/mL |
| Any troponin T >0.1 g/L (>0.5 g/L if creatinine >2.0 mg/dL) |
| Requirement for continuous vasoactive medication to stabilize hemodynamics |
| Complex decompensation: concomitant end‐organ hypoperfusion, volume overload, and systemic vasoconstriction |
| Requirement for care guided by pulmonary artery catheter |
| Severe electrolyte imbalance |
| Chronic renal failure requiring dialysis |
| Acute mental status abnormality |
Early Initiation of Therapy
Early institution of effective therapy has been shown to improve outcomes. Consequently, selection of initial therapy should occur concurrently with determination of proper treatment location. In the Prospective Randomized Outcomes Study of Acutely Decompensated Congestive Heart Failure Treated Initially as Outpatients with Nesiritide (PROACTION) trial, initiation of nesiritide in the ED/OU was associated with an 11% reduction in hospital admissions at the index visit (P = .436), a 57% reduction in hospitalizations within 30 days after discharge from the index hospitalization (P = .058), and a 62% reduction in median duration of rehospitalization (P = .032).17 The incidence of symptomatic hypotension was low and did not differ between the groups.17 Likewise, in separate analyses of data from ADHERE, ED initiation of intravenous (IV) vasoactive therapy, including nitroglycerin, nesiritide, milrinone, or dobutamine significantly reduced the risk of requiring transfer to an ICU and reduced ICU length of stay and total hospital length of stay compared with inpatient initiation of these same therapies.18, 19
Treatment Algorithm
Treatment of ADHF should proceed along a logical care pathway governed by both clinical status and response to prior therapies (Figure 1). One must first consider whether there is evidence of respiratory failure or impending respiratory failure.20 If so, patients should receive immediate ventilatory support via continuous positive airway pressure (CPAP), bilevel positive airway pressure (BiPAP), or endotracheal intubation, depending on the degree of respiratory impairment.3, 5 In prospective controlled evaluations, patients with acute respiratory failure secondary to pulmonary edema who were randomized to treatment with CPAP demonstrated significant improvement in cardiopulmonary indices21, 22 and significant reductions in need for endotracheal intubation21 and short‐term mortality22 when compared with similar patients who received standard therapy without CPAP.
Once potential respiratory issues have been addressed, the next items for consideration are circulation and perfusion. Patients with low cardiac output and hypotension (cardiogenic shock) are at risk for developing critical end‐organ dysfunction. In these patients, insertion of a pulmonary artery catheter may aid in assessment of hemodynamic status and response to therapy.4 Patients with low cardiac output and low filling pressures should receive IV fluid loading.3 In contrast, for patients with low cardiac output and high filling pressures, inotropic agents should be considered.3, 4 Also, these patients may require IV vasodilators and/or IV diuretics to treat pulmonary edema once blood pressure (BP) and cardiac output have been stabilized.2, 3, 20
For patients with ADHF who present with symptoms of congestion, but not respiratory failure or cardiogenic shock, the initial therapeutic decision is governed by their BP. Approximately 50% of patients with ADHF will have an SBP > 140 mm Hg.12, 23 These patients tend to be older and to have diastolic rather than systolic dysfunction.12, 20, 23 Symptoms typically have been present for only a short period of time (2448 hours) and are more often due to maldistribution of fluid producing pulmonary edema than total body fluid overload. Consequently, initial treatment should focus on aggressive BP control to relieve this edema. Sublingual or topical nitrates are recommended as a first step, and initial diuretic use should be minimal to avoid intravascular volume depletion leading to renal dysfunction.20 In contrast, patients presenting with SBP between 90 mm Hg and 140 mm Hg are more likely to have some degree of systolic dysfunction, leading to a gradual worsening of their heart failure symptoms and total body fluid overload over a period of weeks.20 These patients require aggressive diuresis. Although the efficacy of IV loop diuretics has not been established in randomized, controlled clinical trials, observational experience demonstrates that they can effectively reduce filling pressures, relieve volume overload, and decrease symptoms of congestion.4 They are currently the mainstay of therapy for ADHF secondary to fluid retention, and their use is recommended in all 4 guidelines.25
This initial therapeutic choice, however, is only the starting point, and it is important not to stop at this stage. No single definitive therapy for ADHF exists, and not all patients will respond to initial treatment. Optimal management requires early recognition and addressing of both an inadequate response to therapy and any adverse affects induced by this therapy. Frequent reevaluations are an essential component of treating patients with ADHF. For example, the timeline in one of the guidelines calls for assessing the patient's response at 2 and 4 hours after initiation of IV therapy and adjusting treatment as indicated based on these assessments (Figure 2).2

If the patient has an adequate response to initial therapy, defined as SBP <140 mm Hg, stable renal function, and urine output >500 mL over 2 hours (>250 mL if serum creatinine >2.5 mg/dL), this therapy can continue unchanged, and focus shifts to long‐term management issues.2, 4, 14 However, if the response is inadequate, it is important to identify and treat the cause of this inadequate response.
Inadequate urine output secondary to diuretic resistance is common in patients with ADHF, especially in those on long‐term diuretic therapy.3 Despite a 90% prevalence of IV diuretic use in ADHERE, 70% of patients either gained weight or lost fewer than 5 pounds during hospitalization, and 42% were discharged with unresolved symptoms.24 Clearly, diuretic therapy did not produce the desired effect in many of these patients. This inadequate response to loop diuretics is a direct result of their pharmacologic properties, especially as they relate to patients with heart failure. The physiologic effects of loop diuretics are directly related to their concentration in the lumen of the nephron. This concentration depends on both the patient's renal function and the dose and half‐life of the administered diuretic.25 Comorbid renal dysfunction is common in patients with ADHF.26 In addition, even in the absence of this dysfunction, the short half‐life of loop diuretics limits the amount of time that their luminal concentration is in the effective range, and rebound sodium retention can occur whenever the diuretic concentration is below this range.25 Furthermore, the dose‐response curve of loop diuretics is S‐shaped. As a result, a threshold concentration exists beyond which no further augmentation in urine output occurs; ie, there is a maximum physiologic response that is reduced in patients with heart failure.25 Guideline recommendations for patients with diuretic resistance attempt to address these physiologic and pharmacologic limitations. These recommendations include fluid restriction to decrease the overall volume of diuresis necessary, increasing diuretic dose or instituting continuous infusion loop diuretic therapy to increase the amount of time during which the luminal concentration is within the effective range, sequential diuretic blockade to take advantage of the different mechanisms of action of the various diuretic classes to affect different components of the nephron, bypassing the kidney through the use of ultrafiltration, and when these are inadequate, adding a vasodilator in an attempt to augment cardiac output and renal perfusion.3, 4, 24, 25, 2729
Addition of an IV vasodilator is the primary means of addressing an inadequate response typified by hypertension, worsening renal function, and/or persistent symptoms, rather than diuretic resistance. Approximately 25% of patients with ADHF receive IV vasoactive therapy, including nitroglycerin, nesiritide, milrinone, or dobutamine, although predominantly vasodilators, at some point during their hospitalization.11, 12 These agents improve hemodynamics and reduce symptoms of ADHF.5, 3032 Their use, in combination with low‐dose diuretics, has been shown to be more efficacious than high‐dose diuretics alone.3, 27 Adding a vasodilator may reduce adverse, diuretic‐induced, neurohormonal activation. In an animal model, combining nesiritide with IV furosemide significantly attenuated the rise in plasma aldosterone produced by IV furosemide alone,33 and this finding has been subsequently confirmed in patients with heart failure.34 Finally, vasodilators have proven to be a safer alternative than inotropes in patients with ADHF. In an analysis of data from ADHERE, covariate‐adjusted and propensity‐adjusted mortality risk was >50% lower for patients receiving nitroglycerin or nesiritide compared with those receiving dobutamine,11 and in an analysis of data from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial, which evaluated patients with advanced heart failure, the risk‐adjusted mortality hazard ratio (HR) was significantly increased for inotropes (HR: 2.14; 95% confidence interval [CI]: 1.104.15) but not for vasodilators in the absence of inotropes (HR: 1.39; 95% CI: 0.643.00).35
Performance Measures
In addition to instituting effective therapy for the acute decompensation, it is important to implement measures that may improve long‐term outcomes. The Joint Commission on Accreditation of Healthcare Organizations and the AHA/ASA have identified a series of 5 core performance measures that should be completed during hospitalization for AHDF: discharge instructions relevant to patient's education, documentation of left ventricular systolic function evaluation, prescription of angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) at discharge in patients with left ventricular systolic dysfunction, adult smoking cessation advice/counseling, and prescription of ‐blocker at discharge (Table 2).36, 37 In an analysis of data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF) registry, prescription of a ‐blocker at discharge significantly reduced the risk‐adjusted odds ratio (OR) for mortality (OR: 0.48; 95% CI: 0.300.79), and prescription of an ACE inhibitor or ARB at discharge significantly reduced the risk‐adjusted OR for rehospitalization or death (OR: 0.51; 95% CI: 0.340.78) at 6090 days.38 Although no correlation was detected between outcomes and the other 3 core performance measures in this evaluation, the 60‐day to 90‐day time frame may have been too short to identify the full effects of smoking cessation counseling and left ventricular function assessment. Failure to detect a beneficial effect of discharge instructions is disappointing, especially given the proven benefit of disease management programs (see below) and may reflect a limitation of this measure, as currently implemented, to determine the thoroughness and patient understanding of the instructions provided.38, 39
| Measure | Description | Source |
|---|---|---|
| HF‐1 | Discharge instructions relevant to patient education | JCAHO; AHA/ASA |
| HF‐2 | Documentation of left ventricular systolic function evaluation | JCAHO; AHA/ASA |
| HF‐3 | Prescription of ACE inhibitor or ARB at discharge in patients with left ventricular systolic dysfunction | JCAHO; AHA/ASA |
| HF‐4 | Adult smoking cessation advice/counseling | JCAHO; AHA/ASA |
| HF‐5 | Prescription of ‐blocker at discharge | AHA/ASA |
TRANSITION OF CARE
Lastly, optimal management of ADHF requires successful transition of care from an inpatient to an outpatient setting. Recidivism is both common and costly. Approximately 2% of patients with ADHF are readmitted within 2 days, 20% within 1 month, and 50% within 6 months of hospital discharge.40 Frequently, these readmissions are caused by nonadherence to the therapeutic regimen following discharge.41 In an evaluation of patients hospitalized for ADHF at a large urban medical center, noncompliance with prescribed diet and/or drugs was the most common precipitating factor for admission (64% of patients), followed by uncontrolled hypertension (44%), cardiac arrhythmia (29%), environmental factors (19%), and inadequate therapy (17%).42 Similarly, in a prospective evaluation of elderly patients hospitalized for ADHF, 53% of readmissions occurring within 90 days of discharge were deemed to be preventable, with the most common contributing factors being noncompliance with medications and/or diet (33%), inadequate discharge planning (15%), inadequate follow‐up (20%), insufficient support system (21%), and failure to seek medical attention promptly when symptoms recurred (20%).43 Consequently, patient education and arrangement for appropriate follow‐up are crucial components of successfully transitioning care to an outpatient setting.
Effective patient education is time‐consuming. Patients must be taught when, how, and why to take their medication. They need to understand their dietary guidelines and the reasons for these guidelines. They need to know how to use daily weigh‐ins as a means of monitoring their fluid status and what to do in response to a change in weight or symptoms. Finally, they need to be cognizant of what constitutes appropriate exercise and the need for this exercise.4447 To enhance understanding and retention, this information should be presented to the patient over the course of the hospitalization. Comprehension should be tested continually and education repeated until appropriate understanding is ensured. Patient education provided in a rushed or perfunctory manner at the moment of discharge is unlikely to be retained or effective.38, 39
Ideally, the patient should be referred to a comprehensive heart failure disease management program for postdischarge care. Numerous evaluations have established the effectiveness of these programs in enhancing use of appropriate medications, improving functional status, reducing readmissions and mortality, and decreasing costs.4454 For example, in separate evaluations, the prevalences of appropriate vasodilator use (93% vs. 61%; P < .001),51 ‐blocker use (71% vs. 40%; P < .001),50 and ACE inhibitor use (84% vs. 59%; P < .001)52 were significantly greater for disease management program participants compared with nonparticipants. In addition, participation in a disease management program was associated with a 52% reduction in the risk of hospitalization for cardiovascular causes (P < .001) and a 72% reduction in ED visits (P < .01) in 1 evaluation,45 a 36% reduction (95% CI: 16.7%50.9%) in the risk of heart failure admission or death in another,53 and a 67% reduction (95% CI: 41%82%) in the adjusted risk of death in yet another evaluation.52 Unfortunately, recent data indicate that these programs must be ongoing to sustain these benefits. In a prospective evaluation, patients with heart failure were randomized to either standard care or a multidisciplinary disease management program for 6 months followed by standard care.55, 56 Significantly fewer patients in the disease management group required readmission to the hospital (HR: 0.55; 95% CI: 0.350.88) during the 6‐month period in which they actively participated in the disease management program.56 However, by the end of follow‐up (mean 2.8 years), there was no significant difference between treatment groups in all‐cause mortality (HR: 1.09; 95% CI: 0.691.72) or the composite endpoint of death, ED visit, or hospitalization (HR: 1.01; 95% CI: 0.751.37).55
CONCLUSION
A gap between evidence‐based guidelines and current management of patients with ADHF exists. Multiple strategies to bridge this gap in patient management can be employed. Patients with ADHF require rapid assessment to determine appropriate treatment location and initial therapy. Clinical status should guide treatment selection. Once effective acute therapy has been established, strategies to improve long‐term outcomes should be implemented. These strategies include ensuring that care complies with established core performance measures, providing patient education in a manner suited to ensure comprehension and retention, and arranging for appropriate outpatient follow‐up, ideally in a comprehensive heart failure disease management program. Increasing the awareness of the gap between evidence‐based guidelines and current management, as well as strategies to bridge this gap, is crucial to improving the outcomes of patients with ADHF.
Optimizing quality of care in patients with acute decompensated heart failure (ADHF) is crucial, given both the frequency and cost of hospitalization for this disorder. Several quality improvement strategies have been identified, including provider education; provider reminder systems and decision support; audit and feedback; patient education; organizational change; and financial incentives, regulation, and policy.1
To assist hospitalists in implementing these strategies, this article briefly reviews evidence‐based guidelines for the treatment of ADHF, presents a practical algorithm for patient assessment and treatment derived from these guidelines and personal experience, and discusses systems to enhance the ultimate transition of patient care from the inpatient to outpatient setting.
EVIDENCE‐BASED GUIDELINES
Evidence‐based guidelines are created in an attempt to promote optimal management of a condition or disorder based on expert analysis of all available relevant scientific data. Current guidelines for the assessment and treatment of ADHF have been developed by a national group purchasing organization,2 the European Society of Cardiology,3 the Heart Failure Society of America,4 and the American College of Emergency Physicians.5 Relevant components of these guidelines will be discussed in the patient assessment and treatment section below.
Publication of guidelines, in and of itself, however, is inadequate to ensure their acceptance and use.1 Data from the American Heart Association (AHA)/American Stroke Association (ASA) Get With The GuidelinesHeart Failure (GWTG‐HF) program continue to demonstrate a substantial gap between guideline recommendations and current care of patients with ADHF.6, 7 One way to promote systemwide adherence with published guidelines is to directly involve healthcare professionals in the implementation process. Consequently, development of local, hospital‐based procedures derived from national or international guidelines may be more effective than the simple dissemination of the guidelines themselves.1 Hospitalists have a unique insight into both patient care and the hospital setting and are frequently involved in evaluating hospital policies and procedures and implementing clinical pathways and guidelines.8 In addition, hospitalist care has been associated with greater compliance with disease‐specific guidelines compared to nonhospitalist care.9 As a result, hospitalists are uniquely suited to play a key role in the development of these procedures.
PATIENT ASSESSMENT AND TREATMENT
The differential diagnosis of any individual presenting to the emergency department (ED) with signs of systemic or pulmonary edema should include ADHF (Figure 1).25 These individuals require a rapid initial assessment to (1) establish the diagnosis, (2) determine the best location for subsequent treatment, and (3) institute the most appropriate initial therapy.

Treatment Location
Effective and efficient management of ADHF requires determining proper treatment location. Inpatient management of ADHF is expensive, accounting for approximately 60% of the $31.7 billion spent annually on heart failure care in the United States.10 Clearly, patients with impending respiratory failure requiring ventilation assistance and patients with cardiogenic shock requiring inotropic agents and invasive monitoring are best cared for in an intensive care unit (ICU) setting. However, these patients constitute the minority of patients with ADHF. For example, systolic blood pressure (SBP) <90 mm Hg was present in only 2.3% of patients in the Acute Decompensated Heart Failure National Registry (ADHERE), a registry designed to study characteristics, management, and outcomes in a broad sample of patients hospitalized with ADHF.11
Most patients with ADHF present with congestion, not respiratory failure or cardiogenic shock,11, 12 and a select subgroup of these patients will respond to treatment within 1224 hours.13 Although this may be an inordinate amount of time to keep patients in an ED, it is not long enough to generally require full hospital admission. Instead, these patients can be effectively managed in an observation unit (OU).14 The goal of these units is to provide the required level of care over a 12‐ to 24‐hour period while simultaneously reducing costs by eliminating the need for hospital admission. Selecting patients who will respond to therapy during this time frame is a critical component in instituting effective OU management of ADHF. Key entry and exclusion criteria are listed in Table 1.14 In patients who meet these criteria, management in an OU has been shown to yield outcomes comparable to inpatient care, but at a lower cost.1416
| Entry criteria |
|---|
|
| History (at least one of the following) |
| Dyspnea on exertion |
| Paroxysmal nocturnal dyspnea |
| Shortness of breath |
| Edema of legs or abdomen |
| Weight gain |
| Physical examination (at least one of the following) |
| Jugular venous distention or elevation in pulsation |
| Positive abdominal jugular reflux |
| S3/S4 gallop |
| Inspiratory rales |
| Peripheral edema |
| Chest x‐ray (at least one of the following) |
| Cardiomegaly |
| Pulmonary vascular congestion |
| Kerley B lines |
| Pulmonary edema |
| Pleural effusion |
| Exclusion criteria |
| Unstable vital signs (BP >220/120 mm Hg, respiratory rate >25 breath/min, heart rate >130 beats/min) |
| Temperature >38.5C |
| Unstable airway or need for >4 L/min supplemental O2 to keep O2 saturation >90% |
| Peak flow <50% of predicted with wheezing |
| Clinically significant arrhythmia or sustained ventricular tachycardia |
| Any ECG with diagnostic criteria for AMI or ischemia |
| Chest x‐ray with pulmonary infiltrates |
| Any CK‐MB >8.8 ng/mL |
| Any troponin T >0.1 g/L (>0.5 g/L if creatinine >2.0 mg/dL) |
| Requirement for continuous vasoactive medication to stabilize hemodynamics |
| Complex decompensation: concomitant end‐organ hypoperfusion, volume overload, and systemic vasoconstriction |
| Requirement for care guided by pulmonary artery catheter |
| Severe electrolyte imbalance |
| Chronic renal failure requiring dialysis |
| Acute mental status abnormality |
Early Initiation of Therapy
Early institution of effective therapy has been shown to improve outcomes. Consequently, selection of initial therapy should occur concurrently with determination of proper treatment location. In the Prospective Randomized Outcomes Study of Acutely Decompensated Congestive Heart Failure Treated Initially as Outpatients with Nesiritide (PROACTION) trial, initiation of nesiritide in the ED/OU was associated with an 11% reduction in hospital admissions at the index visit (P = .436), a 57% reduction in hospitalizations within 30 days after discharge from the index hospitalization (P = .058), and a 62% reduction in median duration of rehospitalization (P = .032).17 The incidence of symptomatic hypotension was low and did not differ between the groups.17 Likewise, in separate analyses of data from ADHERE, ED initiation of intravenous (IV) vasoactive therapy, including nitroglycerin, nesiritide, milrinone, or dobutamine significantly reduced the risk of requiring transfer to an ICU and reduced ICU length of stay and total hospital length of stay compared with inpatient initiation of these same therapies.18, 19
Treatment Algorithm
Treatment of ADHF should proceed along a logical care pathway governed by both clinical status and response to prior therapies (Figure 1). One must first consider whether there is evidence of respiratory failure or impending respiratory failure.20 If so, patients should receive immediate ventilatory support via continuous positive airway pressure (CPAP), bilevel positive airway pressure (BiPAP), or endotracheal intubation, depending on the degree of respiratory impairment.3, 5 In prospective controlled evaluations, patients with acute respiratory failure secondary to pulmonary edema who were randomized to treatment with CPAP demonstrated significant improvement in cardiopulmonary indices21, 22 and significant reductions in need for endotracheal intubation21 and short‐term mortality22 when compared with similar patients who received standard therapy without CPAP.
Once potential respiratory issues have been addressed, the next items for consideration are circulation and perfusion. Patients with low cardiac output and hypotension (cardiogenic shock) are at risk for developing critical end‐organ dysfunction. In these patients, insertion of a pulmonary artery catheter may aid in assessment of hemodynamic status and response to therapy.4 Patients with low cardiac output and low filling pressures should receive IV fluid loading.3 In contrast, for patients with low cardiac output and high filling pressures, inotropic agents should be considered.3, 4 Also, these patients may require IV vasodilators and/or IV diuretics to treat pulmonary edema once blood pressure (BP) and cardiac output have been stabilized.2, 3, 20
For patients with ADHF who present with symptoms of congestion, but not respiratory failure or cardiogenic shock, the initial therapeutic decision is governed by their BP. Approximately 50% of patients with ADHF will have an SBP > 140 mm Hg.12, 23 These patients tend to be older and to have diastolic rather than systolic dysfunction.12, 20, 23 Symptoms typically have been present for only a short period of time (2448 hours) and are more often due to maldistribution of fluid producing pulmonary edema than total body fluid overload. Consequently, initial treatment should focus on aggressive BP control to relieve this edema. Sublingual or topical nitrates are recommended as a first step, and initial diuretic use should be minimal to avoid intravascular volume depletion leading to renal dysfunction.20 In contrast, patients presenting with SBP between 90 mm Hg and 140 mm Hg are more likely to have some degree of systolic dysfunction, leading to a gradual worsening of their heart failure symptoms and total body fluid overload over a period of weeks.20 These patients require aggressive diuresis. Although the efficacy of IV loop diuretics has not been established in randomized, controlled clinical trials, observational experience demonstrates that they can effectively reduce filling pressures, relieve volume overload, and decrease symptoms of congestion.4 They are currently the mainstay of therapy for ADHF secondary to fluid retention, and their use is recommended in all 4 guidelines.25
This initial therapeutic choice, however, is only the starting point, and it is important not to stop at this stage. No single definitive therapy for ADHF exists, and not all patients will respond to initial treatment. Optimal management requires early recognition and addressing of both an inadequate response to therapy and any adverse affects induced by this therapy. Frequent reevaluations are an essential component of treating patients with ADHF. For example, the timeline in one of the guidelines calls for assessing the patient's response at 2 and 4 hours after initiation of IV therapy and adjusting treatment as indicated based on these assessments (Figure 2).2

If the patient has an adequate response to initial therapy, defined as SBP <140 mm Hg, stable renal function, and urine output >500 mL over 2 hours (>250 mL if serum creatinine >2.5 mg/dL), this therapy can continue unchanged, and focus shifts to long‐term management issues.2, 4, 14 However, if the response is inadequate, it is important to identify and treat the cause of this inadequate response.
Inadequate urine output secondary to diuretic resistance is common in patients with ADHF, especially in those on long‐term diuretic therapy.3 Despite a 90% prevalence of IV diuretic use in ADHERE, 70% of patients either gained weight or lost fewer than 5 pounds during hospitalization, and 42% were discharged with unresolved symptoms.24 Clearly, diuretic therapy did not produce the desired effect in many of these patients. This inadequate response to loop diuretics is a direct result of their pharmacologic properties, especially as they relate to patients with heart failure. The physiologic effects of loop diuretics are directly related to their concentration in the lumen of the nephron. This concentration depends on both the patient's renal function and the dose and half‐life of the administered diuretic.25 Comorbid renal dysfunction is common in patients with ADHF.26 In addition, even in the absence of this dysfunction, the short half‐life of loop diuretics limits the amount of time that their luminal concentration is in the effective range, and rebound sodium retention can occur whenever the diuretic concentration is below this range.25 Furthermore, the dose‐response curve of loop diuretics is S‐shaped. As a result, a threshold concentration exists beyond which no further augmentation in urine output occurs; ie, there is a maximum physiologic response that is reduced in patients with heart failure.25 Guideline recommendations for patients with diuretic resistance attempt to address these physiologic and pharmacologic limitations. These recommendations include fluid restriction to decrease the overall volume of diuresis necessary, increasing diuretic dose or instituting continuous infusion loop diuretic therapy to increase the amount of time during which the luminal concentration is within the effective range, sequential diuretic blockade to take advantage of the different mechanisms of action of the various diuretic classes to affect different components of the nephron, bypassing the kidney through the use of ultrafiltration, and when these are inadequate, adding a vasodilator in an attempt to augment cardiac output and renal perfusion.3, 4, 24, 25, 2729
Addition of an IV vasodilator is the primary means of addressing an inadequate response typified by hypertension, worsening renal function, and/or persistent symptoms, rather than diuretic resistance. Approximately 25% of patients with ADHF receive IV vasoactive therapy, including nitroglycerin, nesiritide, milrinone, or dobutamine, although predominantly vasodilators, at some point during their hospitalization.11, 12 These agents improve hemodynamics and reduce symptoms of ADHF.5, 3032 Their use, in combination with low‐dose diuretics, has been shown to be more efficacious than high‐dose diuretics alone.3, 27 Adding a vasodilator may reduce adverse, diuretic‐induced, neurohormonal activation. In an animal model, combining nesiritide with IV furosemide significantly attenuated the rise in plasma aldosterone produced by IV furosemide alone,33 and this finding has been subsequently confirmed in patients with heart failure.34 Finally, vasodilators have proven to be a safer alternative than inotropes in patients with ADHF. In an analysis of data from ADHERE, covariate‐adjusted and propensity‐adjusted mortality risk was >50% lower for patients receiving nitroglycerin or nesiritide compared with those receiving dobutamine,11 and in an analysis of data from the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial, which evaluated patients with advanced heart failure, the risk‐adjusted mortality hazard ratio (HR) was significantly increased for inotropes (HR: 2.14; 95% confidence interval [CI]: 1.104.15) but not for vasodilators in the absence of inotropes (HR: 1.39; 95% CI: 0.643.00).35
Performance Measures
In addition to instituting effective therapy for the acute decompensation, it is important to implement measures that may improve long‐term outcomes. The Joint Commission on Accreditation of Healthcare Organizations and the AHA/ASA have identified a series of 5 core performance measures that should be completed during hospitalization for AHDF: discharge instructions relevant to patient's education, documentation of left ventricular systolic function evaluation, prescription of angiotensin‐converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) at discharge in patients with left ventricular systolic dysfunction, adult smoking cessation advice/counseling, and prescription of ‐blocker at discharge (Table 2).36, 37 In an analysis of data from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE‐HF) registry, prescription of a ‐blocker at discharge significantly reduced the risk‐adjusted odds ratio (OR) for mortality (OR: 0.48; 95% CI: 0.300.79), and prescription of an ACE inhibitor or ARB at discharge significantly reduced the risk‐adjusted OR for rehospitalization or death (OR: 0.51; 95% CI: 0.340.78) at 6090 days.38 Although no correlation was detected between outcomes and the other 3 core performance measures in this evaluation, the 60‐day to 90‐day time frame may have been too short to identify the full effects of smoking cessation counseling and left ventricular function assessment. Failure to detect a beneficial effect of discharge instructions is disappointing, especially given the proven benefit of disease management programs (see below) and may reflect a limitation of this measure, as currently implemented, to determine the thoroughness and patient understanding of the instructions provided.38, 39
| Measure | Description | Source |
|---|---|---|
| HF‐1 | Discharge instructions relevant to patient education | JCAHO; AHA/ASA |
| HF‐2 | Documentation of left ventricular systolic function evaluation | JCAHO; AHA/ASA |
| HF‐3 | Prescription of ACE inhibitor or ARB at discharge in patients with left ventricular systolic dysfunction | JCAHO; AHA/ASA |
| HF‐4 | Adult smoking cessation advice/counseling | JCAHO; AHA/ASA |
| HF‐5 | Prescription of ‐blocker at discharge | AHA/ASA |
TRANSITION OF CARE
Lastly, optimal management of ADHF requires successful transition of care from an inpatient to an outpatient setting. Recidivism is both common and costly. Approximately 2% of patients with ADHF are readmitted within 2 days, 20% within 1 month, and 50% within 6 months of hospital discharge.40 Frequently, these readmissions are caused by nonadherence to the therapeutic regimen following discharge.41 In an evaluation of patients hospitalized for ADHF at a large urban medical center, noncompliance with prescribed diet and/or drugs was the most common precipitating factor for admission (64% of patients), followed by uncontrolled hypertension (44%), cardiac arrhythmia (29%), environmental factors (19%), and inadequate therapy (17%).42 Similarly, in a prospective evaluation of elderly patients hospitalized for ADHF, 53% of readmissions occurring within 90 days of discharge were deemed to be preventable, with the most common contributing factors being noncompliance with medications and/or diet (33%), inadequate discharge planning (15%), inadequate follow‐up (20%), insufficient support system (21%), and failure to seek medical attention promptly when symptoms recurred (20%).43 Consequently, patient education and arrangement for appropriate follow‐up are crucial components of successfully transitioning care to an outpatient setting.
Effective patient education is time‐consuming. Patients must be taught when, how, and why to take their medication. They need to understand their dietary guidelines and the reasons for these guidelines. They need to know how to use daily weigh‐ins as a means of monitoring their fluid status and what to do in response to a change in weight or symptoms. Finally, they need to be cognizant of what constitutes appropriate exercise and the need for this exercise.4447 To enhance understanding and retention, this information should be presented to the patient over the course of the hospitalization. Comprehension should be tested continually and education repeated until appropriate understanding is ensured. Patient education provided in a rushed or perfunctory manner at the moment of discharge is unlikely to be retained or effective.38, 39
Ideally, the patient should be referred to a comprehensive heart failure disease management program for postdischarge care. Numerous evaluations have established the effectiveness of these programs in enhancing use of appropriate medications, improving functional status, reducing readmissions and mortality, and decreasing costs.4454 For example, in separate evaluations, the prevalences of appropriate vasodilator use (93% vs. 61%; P < .001),51 ‐blocker use (71% vs. 40%; P < .001),50 and ACE inhibitor use (84% vs. 59%; P < .001)52 were significantly greater for disease management program participants compared with nonparticipants. In addition, participation in a disease management program was associated with a 52% reduction in the risk of hospitalization for cardiovascular causes (P < .001) and a 72% reduction in ED visits (P < .01) in 1 evaluation,45 a 36% reduction (95% CI: 16.7%50.9%) in the risk of heart failure admission or death in another,53 and a 67% reduction (95% CI: 41%82%) in the adjusted risk of death in yet another evaluation.52 Unfortunately, recent data indicate that these programs must be ongoing to sustain these benefits. In a prospective evaluation, patients with heart failure were randomized to either standard care or a multidisciplinary disease management program for 6 months followed by standard care.55, 56 Significantly fewer patients in the disease management group required readmission to the hospital (HR: 0.55; 95% CI: 0.350.88) during the 6‐month period in which they actively participated in the disease management program.56 However, by the end of follow‐up (mean 2.8 years), there was no significant difference between treatment groups in all‐cause mortality (HR: 1.09; 95% CI: 0.691.72) or the composite endpoint of death, ED visit, or hospitalization (HR: 1.01; 95% CI: 0.751.37).55
CONCLUSION
A gap between evidence‐based guidelines and current management of patients with ADHF exists. Multiple strategies to bridge this gap in patient management can be employed. Patients with ADHF require rapid assessment to determine appropriate treatment location and initial therapy. Clinical status should guide treatment selection. Once effective acute therapy has been established, strategies to improve long‐term outcomes should be implemented. These strategies include ensuring that care complies with established core performance measures, providing patient education in a manner suited to ensure comprehension and retention, and arranging for appropriate outpatient follow‐up, ideally in a comprehensive heart failure disease management program. Increasing the awareness of the gap between evidence‐based guidelines and current management, as well as strategies to bridge this gap, is crucial to improving the outcomes of patients with ADHF.
- .Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improvement process.J Gen Intern Med.2007;22(12):1762–1770.
- ,,, et al.Guidelines for acute decompensated heart failure treatment.Ann Pharmacother.2004;38(4):649–660.
- ,,, et al.Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology.Eur Heart J.2005;26(4):384–416.
- Heart Failure Society of America.Executive summary: HFSA 2006 comprehensive heart failure practice guideline.J Card Fail.2006;12(1):10–38.
- ,,,,,American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute heart failure syndromes.Ann Emerg Med.2007;49(5):627–669.
- ,,, et al.Influence of the American Heart Association's Get With The Guidelines‐Heart Failure (GWTG‐HF) program on emerging performance measures for patients hospitalized with heart failure.Circulation.2006;114:II–572. Abstract 2740.
- ,,, et al.Quality of care for heart failure patients with concomitant kidney disease in the American Heart Association's Get With The Guidelines‐Heart Failure (GWTG‐HF) program.Circulation.2006;114:II–859. Abstract 3993.
- .The role of hospitalists in the management of acute decompensated heart failure.Am Heart Hosp J.2005;3(2):111–117.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162(11):1251–1256.
- ,,, et al.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2008;117(4):e25–e146.
- ,,, et al.In‐hospital mortality in patients with acute decompensated heart failure treated with intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE).J Am Coll Cardiol.2005;46(1):57–64.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296(18):2217–2226.
- ,,,.Preliminary experience with an emergency department observation unit protocol for heart failure.Acad Emerg Med.2000;7(10):1171. Abstract 33.
- ,,,,,.Effective observation unit treatment of decompensated heart failure.Congest Heart Fail.2002;8(2):68–73.
- ,.Patient outcome and costs following an acute heart failure (HF) management program in an emergency department (ED) observation unit (OU).J Heart Lung Transplant.1999;18(1):92. Abstract 240.
- ,,,,,.Inpatient versus emergency department observation unit management of heart failure.Ann Emerg Med.1998;32(3 part 2):S46–S47. Abstract 180.
- ,,, et al.Observation unit treatment of heart failure with nesiritide: results from the proaction trial.J Emerg Med.2005;29(3):243–252.
- ,,,.Early initiation of IV vasoactive therapy improves heart failure outcomes: an analysis from the ADHERE™ registry database.Ann Emerg Med.2003;42(4 suppl):S26. Abstract 92.
- ,,, et al.Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE.Cardiology.2007;107(1):44–51.
- ,,,,,.Beyond pulmonary edema: diagnostic, risk stratification, and treatment challenges of acute heart failure management in the emergency department.Ann Emerg Med.2008;51(1):45–57.
- ,,,,,.Reappraisal of continuous positive airway pressure therapy in acute cardiogenic pulmonary edema. Short‐term results and long‐term follow‐up.Chest.1995;107(5):1379–1386.
- ,,, et al.Noninvasive continuous positive airway pressure in elderly cardiogenic pulmonary edema patients.Intensive Care Med.2004;30(5):882–888.
- ,,,,.Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database.J Am Coll Cardiol.2006;47(1):76–84.
- ,,,.Early ultrafiltration in patients with decompensated heart failure and diuretic resistance.J Am Coll Cardiol.2005;46(11):2047–2051.
- .Diuretic therapy in congestive heart failure.Congest Heart Fail.2000;6(4):197–201.
- ,,, et al.High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database.J Card Fail.2007;13(6):422–430.
- ,,, et al.Randomised trial of high‐dose isosorbide dinitrate plus low‐dose furosemide versus high‐dose furosemide plus low‐dose isosorbide dinitrate in severe pulmonary oedema.Lancet.1998;351(9100):389–393.
- .Diuretic therapy and resistance in congestive heart failure.Cardiology.2001;96(3–4):132–143.
- .Management of diuretic‐refractory, volume‐overloaded patients with acutely decompensated heart failure.Curr Cardiol Rep.2005;7(3):204–210.
- ,,, et al.Sustained hemodynamic effects of an infusion of nesiritide (human b‐type natriuretic peptide) in heart failure: a randomized, double‐blind, placebo‐controlled clinical trial.J Am Coll Cardiol.1999;34(1):155–162.
- ,,, et al.Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure.N Engl J Med.2000;343(4):246–253.
- Publication Committee for the VMAC Investigators.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial.JAMA.2002;287(12):1531–1540.
- ,,, et al.Brain natriuretic peptide enhances renal actions of furosemide and suppresses furosemide‐induced aldosterone activation in experimental heart failure.Circulation.2004;109(13):1680–1685.
- ,,.Nesiritide appears to inhibit the rise in plasma aldosterone associated with furosemide diuresis.J Card Fail.2006;12(6 suppl 1):S85–S86. Abstract 275.
- ,,, et al.Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure.Am Heart J.2007;153(1):98–104.
- Joint Commission on Accreditation of Healthcare Organizations. Specifications Manual for National Implementation of Hospital Quality Measures. Version 2.3b. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Historical+NHQM+manuals.htm. Accessed October 10,2008.
- American Heart Association/American Stroke Association. Get with the guidelines–heart failure. Fact sheet. http://www.americanheart.org/downloadable/heart/1163802072170HFFactSheet.pdf. Accessed October 10,2008.
- ,,, et al.Association between performance measures and clinical outcomes for patients hospitalized with heart failure.JAMA.2007;297(1):61–70.
- ,,, et al.Evaluating quality of care for patients with heart failure.Circulation.2000;101(12):e122–e140.
- .Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department.Rev Cardiovasc Med.2002;3(suppl 4):S3–S9.
- ,.Observation unit management of heart failure.Emerg Med Clin North Am.2001;19(1):209–232.
- ,,,.Precipitating factors leading to decompensation of heart failure. Traits among urban blacks.Arch Intern Med.1988;148(9):2013–2016.
- ,,,,.Early readmission of elderly patients with congestive heart failure.J Am Geriatr Soc.1990;38(12):1290–1295.
- ,,, et al.Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure.J Am Coll Cardiol.1997;30(3):725–732.
- ,,, et al.Prospective evaluation of an outpatient heart failure management program.J Card Fail.2001;7(1):64–74.
- ,,, et al.Improved outcomes from a comprehensive management system for heart failure.Eur J Heart Fail.2001;3(5):619–625.
- ,,, et al.The benefit of implementing a heart failure disease management program.Arch Intern Med.2001;161(18):2223–2228.
- ,,,,,.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333(18):1190–1195.
- ,,.Heart failure disease management in an indigent population.Am Heart J.2001;141(2):254–258.
- ,,, et al.Cost/utility ratio in chronic heart failure: comparison between heart failure management program delivered by day‐hospital and usual care.J Am Coll Cardiol.2002;40(7):1259–1266.
- ,,, et al.A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission.J Am Coll Cardiol.2002;39(3):471–480.
- ,,, et al.Mortality benefit of a comprehensive heart failure disease management program in indigent patients.Am Heart J.2006;151(2):478–483.
- ,,, et al.Two‐year outcome of a prospective, controlled study of a disease management programme for elderly patients with heart failure.J Cardiovasc Med (Hagerstown).2007;8(5):324–329.
- ,,,,.Impact of the implementation of telemanagement on a disease management program in an elderly heart failure cohort.Prog Cardiovasc Nurs.2007;22(4):196–200.
- ,,, et al.Lack of long‐term benefits of a 6‐month heart failure disease management program.J Card Fail.2007;13(4):287–293.
- ,,,,.Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial.CMAJ.2005;173(1):40–45.
- .Bridging the gap between evidence and practice in venous thromboembolism prophylaxis: the quality improvement process.J Gen Intern Med.2007;22(12):1762–1770.
- ,,, et al.Guidelines for acute decompensated heart failure treatment.Ann Pharmacother.2004;38(4):649–660.
- ,,, et al.Executive summary of the guidelines on the diagnosis and treatment of acute heart failure: the Task Force on Acute Heart Failure of the European Society of Cardiology.Eur Heart J.2005;26(4):384–416.
- Heart Failure Society of America.Executive summary: HFSA 2006 comprehensive heart failure practice guideline.J Card Fail.2006;12(1):10–38.
- ,,,,,American College of Emergency Physicians. Clinical policy: critical issues in the evaluation and management of adult patients presenting to the emergency department with acute heart failure syndromes.Ann Emerg Med.2007;49(5):627–669.
- ,,, et al.Influence of the American Heart Association's Get With The Guidelines‐Heart Failure (GWTG‐HF) program on emerging performance measures for patients hospitalized with heart failure.Circulation.2006;114:II–572. Abstract 2740.
- ,,, et al.Quality of care for heart failure patients with concomitant kidney disease in the American Heart Association's Get With The Guidelines‐Heart Failure (GWTG‐HF) program.Circulation.2006;114:II–859. Abstract 3993.
- .The role of hospitalists in the management of acute decompensated heart failure.Am Heart Hosp J.2005;3(2):111–117.
- ,,,,.Quality of care for patients hospitalized with heart failure: assessing the impact of hospitalists.Arch Intern Med.2002;162(11):1251–1256.
- ,,, et al.Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee.Circulation.2008;117(4):e25–e146.
- ,,, et al.In‐hospital mortality in patients with acute decompensated heart failure treated with intravenous vasoactive medications: an analysis from the Acute Decompensated Heart Failure National Registry (ADHERE).J Am Coll Cardiol.2005;46(1):57–64.
- ,,, et al.Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure.JAMA.2006;296(18):2217–2226.
- ,,,.Preliminary experience with an emergency department observation unit protocol for heart failure.Acad Emerg Med.2000;7(10):1171. Abstract 33.
- ,,,,,.Effective observation unit treatment of decompensated heart failure.Congest Heart Fail.2002;8(2):68–73.
- ,.Patient outcome and costs following an acute heart failure (HF) management program in an emergency department (ED) observation unit (OU).J Heart Lung Transplant.1999;18(1):92. Abstract 240.
- ,,,,,.Inpatient versus emergency department observation unit management of heart failure.Ann Emerg Med.1998;32(3 part 2):S46–S47. Abstract 180.
- ,,, et al.Observation unit treatment of heart failure with nesiritide: results from the proaction trial.J Emerg Med.2005;29(3):243–252.
- ,,,.Early initiation of IV vasoactive therapy improves heart failure outcomes: an analysis from the ADHERE™ registry database.Ann Emerg Med.2003;42(4 suppl):S26. Abstract 92.
- ,,, et al.Impact of early initiation of intravenous therapy for acute decompensated heart failure on outcomes in ADHERE.Cardiology.2007;107(1):44–51.
- ,,,,,.Beyond pulmonary edema: diagnostic, risk stratification, and treatment challenges of acute heart failure management in the emergency department.Ann Emerg Med.2008;51(1):45–57.
- ,,,,,.Reappraisal of continuous positive airway pressure therapy in acute cardiogenic pulmonary edema. Short‐term results and long‐term follow‐up.Chest.1995;107(5):1379–1386.
- ,,, et al.Noninvasive continuous positive airway pressure in elderly cardiogenic pulmonary edema patients.Intensive Care Med.2004;30(5):882–888.
- ,,,,.Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database.J Am Coll Cardiol.2006;47(1):76–84.
- ,,,.Early ultrafiltration in patients with decompensated heart failure and diuretic resistance.J Am Coll Cardiol.2005;46(11):2047–2051.
- .Diuretic therapy in congestive heart failure.Congest Heart Fail.2000;6(4):197–201.
- ,,, et al.High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database.J Card Fail.2007;13(6):422–430.
- ,,, et al.Randomised trial of high‐dose isosorbide dinitrate plus low‐dose furosemide versus high‐dose furosemide plus low‐dose isosorbide dinitrate in severe pulmonary oedema.Lancet.1998;351(9100):389–393.
- .Diuretic therapy and resistance in congestive heart failure.Cardiology.2001;96(3–4):132–143.
- .Management of diuretic‐refractory, volume‐overloaded patients with acutely decompensated heart failure.Curr Cardiol Rep.2005;7(3):204–210.
- ,,, et al.Sustained hemodynamic effects of an infusion of nesiritide (human b‐type natriuretic peptide) in heart failure: a randomized, double‐blind, placebo‐controlled clinical trial.J Am Coll Cardiol.1999;34(1):155–162.
- ,,, et al.Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure.N Engl J Med.2000;343(4):246–253.
- Publication Committee for the VMAC Investigators.Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial.JAMA.2002;287(12):1531–1540.
- ,,, et al.Brain natriuretic peptide enhances renal actions of furosemide and suppresses furosemide‐induced aldosterone activation in experimental heart failure.Circulation.2004;109(13):1680–1685.
- ,,.Nesiritide appears to inhibit the rise in plasma aldosterone associated with furosemide diuresis.J Card Fail.2006;12(6 suppl 1):S85–S86. Abstract 275.
- ,,, et al.Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure.Am Heart J.2007;153(1):98–104.
- Joint Commission on Accreditation of Healthcare Organizations. Specifications Manual for National Implementation of Hospital Quality Measures. Version 2.3b. http://www.jointcommission.org/PerformanceMeasurement/PerformanceMeasurement/Historical+NHQM+manuals.htm. Accessed October 10,2008.
- American Heart Association/American Stroke Association. Get with the guidelines–heart failure. Fact sheet. http://www.americanheart.org/downloadable/heart/1163802072170HFFactSheet.pdf. Accessed October 10,2008.
- ,,, et al.Association between performance measures and clinical outcomes for patients hospitalized with heart failure.JAMA.2007;297(1):61–70.
- ,,, et al.Evaluating quality of care for patients with heart failure.Circulation.2000;101(12):e122–e140.
- .Acutely decompensated heart failure: opportunities to improve care and outcomes in the emergency department.Rev Cardiovasc Med.2002;3(suppl 4):S3–S9.
- ,.Observation unit management of heart failure.Emerg Med Clin North Am.2001;19(1):209–232.
- ,,,.Precipitating factors leading to decompensation of heart failure. Traits among urban blacks.Arch Intern Med.1988;148(9):2013–2016.
- ,,,,.Early readmission of elderly patients with congestive heart failure.J Am Geriatr Soc.1990;38(12):1290–1295.
- ,,, et al.Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure.J Am Coll Cardiol.1997;30(3):725–732.
- ,,, et al.Prospective evaluation of an outpatient heart failure management program.J Card Fail.2001;7(1):64–74.
- ,,, et al.Improved outcomes from a comprehensive management system for heart failure.Eur J Heart Fail.2001;3(5):619–625.
- ,,, et al.The benefit of implementing a heart failure disease management program.Arch Intern Med.2001;161(18):2223–2228.
- ,,,,,.A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure.N Engl J Med.1995;333(18):1190–1195.
- ,,.Heart failure disease management in an indigent population.Am Heart J.2001;141(2):254–258.
- ,,, et al.Cost/utility ratio in chronic heart failure: comparison between heart failure management program delivered by day‐hospital and usual care.J Am Coll Cardiol.2002;40(7):1259–1266.
- ,,, et al.A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission.J Am Coll Cardiol.2002;39(3):471–480.
- ,,, et al.Mortality benefit of a comprehensive heart failure disease management program in indigent patients.Am Heart J.2006;151(2):478–483.
- ,,, et al.Two‐year outcome of a prospective, controlled study of a disease management programme for elderly patients with heart failure.J Cardiovasc Med (Hagerstown).2007;8(5):324–329.
- ,,,,.Impact of the implementation of telemanagement on a disease management program in an elderly heart failure cohort.Prog Cardiovasc Nurs.2007;22(4):196–200.
- ,,, et al.Lack of long‐term benefits of a 6‐month heart failure disease management program.J Card Fail.2007;13(4):287–293.
- ,,,,.Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial.CMAJ.2005;173(1):40–45.
A State-of-the-Art Report on Hospital Medicine Education
The Society of Hospital Medicine (SHM) is committed to improving the quality of in-patient care through the provision of educational programs, tools, and resources to its membership. In 2002, the SHM Education Committee and leadership met to develop its first strategic plan for education. Long-range and short-term goals were determined, establishing a strategic vision and direction for education for the organization. Long-range goals included defining and developing a core curriculum in hospital medicine; developing a variety of modalities to deliver education; promoting collaborative efforts with other organizations; promoting original research in hospital medicine; and establishing a recognition program for completion of the core curriculum.
To help achieve its short-term goals, the Education Committee formed three task forces: the Core Curriculum Task Force, Leadership Task Force, and Geriatrics Task Force. As a result of the work of committees, task forces, and members, an impressive array of educational programs and products has been developed for membership, and much progress has been made in reaching SHM’s long-term educational goals. SHM would like to acknowledge and thank all of the members who have contributed countless hours and tireless effort to the educational initiatives of the organization.
The Education Committee formed two Core Curriculum Task Forces, an Adult and a Pediatric task force. Each task force has been defining and developing the core curriculum, which will identify the expected proficiencies of members and establish the distinctive differences between hospital medicine and other related medical specialties. The Adult Core Curriculum Task Force executive committee includes Mike Pistoria, Alpesh Amin, Tina Budnitz, Dan Dressler, and Sylvia McKean. An Adult Core Curriculum Guide is expected to be published and released in the spring of 2005. The Guide will contain three sections, each with multiple chapters: Clinical Conditions, Systems, and Procedures. Each chapter begins with an introduction articulating its importance to the practice of hospital medicine, followed by competencies categorized into four areas: knowledge, skills, attitudes, and systems organization and improvement. The core curriculum will be the basis for all future SHM educational activities. It is anticipated that the Core Curriculum Guide will standardize efforts to educate hospitalists across the continuum of medical education. The guide will be provided to internal medicine clerkship and residency program directors and hospitalist fellowship directors. Educators responsible for developing continuing medical education activities for physicians will find the guide to be a valuable resource.
The Pediatric Core Curriculum Guide will be similar in structure to the Adult Guide and is a work in progress. The Pediatric Core Curriculum Task Force executive committee includes Tim Cornell, Dan Rauch, and Alpesh Amin.
The Society of Hospital Medicine’s annual meeting has become the premier meeting for health care professionals who specialize in hospital medicine. The annual meeting provides a wide range of opportunities for learning. Participants can select from a variety of tracks, which include adult and pediatric clinical topics, operational and organizational issues, and challenges for academic hospitalists. The diversity of the annual meeting allows for more personal and individualized learning and tremendous opportunities for networking with colleagues. Preetha Basaviah is the course director of the 2005 annual meeting, which will be held April 28-30, in Chicago, Illinois. The theme for this 8th annual meeting is, “Hospital Medicine in 2005: Strategies for Success.”
In 2004, Regional meetings were held in the Northeast, Western, and Southern regions with much success. Forty-three chapters across the country are also developing educational sessions for their local meetings. Several courses focusing on specific topics of interest to hospitalists have been developed and are offered the day prior to the annual or regional meetings. Pre-courses include Practice Management, Leadership Survival, Perioperative and Consultative Medicine, and Critical Care for the Hospitalist, offering additional opportunities for members to enhance their skills and knowledge.
The Society of Hospital Medicine continues to develop educational tools and resources for the Web site (www.hospitalmedicine.org). The first resource room, addressing the prevention of antimicrobial resistance, was created. Resource rooms provide links to guidelines and relevant CME on-line courses, reviews of pertinent literature, methods to create quality-improvement programs, fact sheets, slide sets and other important information to assist hospitalists in their daily practice. Several unrestricted educational grants have been secured through pharmaceutical companies to support the creation of additional resource rooms to be launched in the near future on topics such as DVT awareness, stroke, and geriatric inpatient care.
The Centers for Disease Control and Prevention (CDC) has extended their Cooperative Agreement with SHM for an additional year. As a part of this agreement, SHM will evaluate and revise the applied learning workshop, “Implementing Quality Improvement Programs to Reduce Antimicrobial Resistance,” by Dan Dressler. This revised workshop will be conducted at three different chapter or other local meetings. If you are interested in bringing this great workshop to your chapter meeting, contact Tina Budnitz at TBudnitz@hospitalmedicine.org.
SHM has also received a substantial grant from the John A. Hartford Foundation to assist in the creation of educational symposia and enduring materials related to improving outcomes in older adults. The grant also supports the development of a discharge planning tool for hospitalists, and a demonstration project at three sites to implement quality improvement programs to educate members about important issues affecting outcomes in older patients. Members interested in participating in the creation of a discharge planning tool and supporting guidelines should plan to attend the discharge planning workshop at the 2005 Annual Meeting.
An outgrowth of the Hartford grant has been the establishment of the Leadership Academy. A Leadership pre-course for 100 hospitalist leaders was successfully included in the 2004 Annual Meeting. The next intensive workshop is scheduled for January 2005 under the direction of course directors Mark Williams and Russ Holman in Arizona, with a subsequent session scheduled for September 2005 in Vail, CO. The Leadership Academy was developed to provide the skills and resources required to successfully lead and manage a hospital medicine program now and in the future. In-depth training is provided on strategic planning, conflict resolution and negotiation, understanding critical hospital performance metrics, and leading and managing change.
SHM’s vision for hospital medicine education is forwarded through the development of strategic collaboration with organizations such as the American Board of Internal Medicine, Society of General Internal Medicine, JCAHO, the American Hospital Association, and other specialty societies such as the American College of Chest Physicians and the American Academy of Pediatrics. SHM continues its strong affiliation and relationship with the American College of Physicians. Relationships with these key organizations will enable SHM to refine its recognition program for members completing the core curriculum and further the recognition of hospital medicine as a distinct specialty.
In 2006, the Society of Hospital Medicine will launch its official journal, The Journal of Hospital Medicine. The journal will provide a vehicle for the dissemination of research and innovations in hospital medicine.
To further signify its commitment to education, SHM hired a Director of Education, Jane Mihelic, to establish a Division of Education in July of 2004. As the development phase of the core curriculum concludes, SHM will hold the second education summit meeting early in 2005 to develop the next phase of the strategic plan for education and establish new goals and objectives. Future plans will include implementing the core curriculum, becoming an accredited provider of continuing medical education, and developing additional interactive self-directed learning materials.
Care of the hospitalized patient necessitates mastery and continued maintenance of sophisticated knowledge, skills, attitudes, and systems organization. The Society of Hospital Medicine is poised to foster, promote, and support hospitalists in meeting their life-long learning needs.
Please feel free to contact Alpesh Amin, MD, (anamin@uci.edu) Chair, SHM Education Committee, or Jane Mihelic (jmihelic@hospitalmedicine.org), SHM Director of Education, regarding thoughts or ideas on hospital medicine education.
The Society of Hospital Medicine (SHM) is committed to improving the quality of in-patient care through the provision of educational programs, tools, and resources to its membership. In 2002, the SHM Education Committee and leadership met to develop its first strategic plan for education. Long-range and short-term goals were determined, establishing a strategic vision and direction for education for the organization. Long-range goals included defining and developing a core curriculum in hospital medicine; developing a variety of modalities to deliver education; promoting collaborative efforts with other organizations; promoting original research in hospital medicine; and establishing a recognition program for completion of the core curriculum.
To help achieve its short-term goals, the Education Committee formed three task forces: the Core Curriculum Task Force, Leadership Task Force, and Geriatrics Task Force. As a result of the work of committees, task forces, and members, an impressive array of educational programs and products has been developed for membership, and much progress has been made in reaching SHM’s long-term educational goals. SHM would like to acknowledge and thank all of the members who have contributed countless hours and tireless effort to the educational initiatives of the organization.
The Education Committee formed two Core Curriculum Task Forces, an Adult and a Pediatric task force. Each task force has been defining and developing the core curriculum, which will identify the expected proficiencies of members and establish the distinctive differences between hospital medicine and other related medical specialties. The Adult Core Curriculum Task Force executive committee includes Mike Pistoria, Alpesh Amin, Tina Budnitz, Dan Dressler, and Sylvia McKean. An Adult Core Curriculum Guide is expected to be published and released in the spring of 2005. The Guide will contain three sections, each with multiple chapters: Clinical Conditions, Systems, and Procedures. Each chapter begins with an introduction articulating its importance to the practice of hospital medicine, followed by competencies categorized into four areas: knowledge, skills, attitudes, and systems organization and improvement. The core curriculum will be the basis for all future SHM educational activities. It is anticipated that the Core Curriculum Guide will standardize efforts to educate hospitalists across the continuum of medical education. The guide will be provided to internal medicine clerkship and residency program directors and hospitalist fellowship directors. Educators responsible for developing continuing medical education activities for physicians will find the guide to be a valuable resource.
The Pediatric Core Curriculum Guide will be similar in structure to the Adult Guide and is a work in progress. The Pediatric Core Curriculum Task Force executive committee includes Tim Cornell, Dan Rauch, and Alpesh Amin.
The Society of Hospital Medicine’s annual meeting has become the premier meeting for health care professionals who specialize in hospital medicine. The annual meeting provides a wide range of opportunities for learning. Participants can select from a variety of tracks, which include adult and pediatric clinical topics, operational and organizational issues, and challenges for academic hospitalists. The diversity of the annual meeting allows for more personal and individualized learning and tremendous opportunities for networking with colleagues. Preetha Basaviah is the course director of the 2005 annual meeting, which will be held April 28-30, in Chicago, Illinois. The theme for this 8th annual meeting is, “Hospital Medicine in 2005: Strategies for Success.”
In 2004, Regional meetings were held in the Northeast, Western, and Southern regions with much success. Forty-three chapters across the country are also developing educational sessions for their local meetings. Several courses focusing on specific topics of interest to hospitalists have been developed and are offered the day prior to the annual or regional meetings. Pre-courses include Practice Management, Leadership Survival, Perioperative and Consultative Medicine, and Critical Care for the Hospitalist, offering additional opportunities for members to enhance their skills and knowledge.
The Society of Hospital Medicine continues to develop educational tools and resources for the Web site (www.hospitalmedicine.org). The first resource room, addressing the prevention of antimicrobial resistance, was created. Resource rooms provide links to guidelines and relevant CME on-line courses, reviews of pertinent literature, methods to create quality-improvement programs, fact sheets, slide sets and other important information to assist hospitalists in their daily practice. Several unrestricted educational grants have been secured through pharmaceutical companies to support the creation of additional resource rooms to be launched in the near future on topics such as DVT awareness, stroke, and geriatric inpatient care.
The Centers for Disease Control and Prevention (CDC) has extended their Cooperative Agreement with SHM for an additional year. As a part of this agreement, SHM will evaluate and revise the applied learning workshop, “Implementing Quality Improvement Programs to Reduce Antimicrobial Resistance,” by Dan Dressler. This revised workshop will be conducted at three different chapter or other local meetings. If you are interested in bringing this great workshop to your chapter meeting, contact Tina Budnitz at TBudnitz@hospitalmedicine.org.
SHM has also received a substantial grant from the John A. Hartford Foundation to assist in the creation of educational symposia and enduring materials related to improving outcomes in older adults. The grant also supports the development of a discharge planning tool for hospitalists, and a demonstration project at three sites to implement quality improvement programs to educate members about important issues affecting outcomes in older patients. Members interested in participating in the creation of a discharge planning tool and supporting guidelines should plan to attend the discharge planning workshop at the 2005 Annual Meeting.
An outgrowth of the Hartford grant has been the establishment of the Leadership Academy. A Leadership pre-course for 100 hospitalist leaders was successfully included in the 2004 Annual Meeting. The next intensive workshop is scheduled for January 2005 under the direction of course directors Mark Williams and Russ Holman in Arizona, with a subsequent session scheduled for September 2005 in Vail, CO. The Leadership Academy was developed to provide the skills and resources required to successfully lead and manage a hospital medicine program now and in the future. In-depth training is provided on strategic planning, conflict resolution and negotiation, understanding critical hospital performance metrics, and leading and managing change.
SHM’s vision for hospital medicine education is forwarded through the development of strategic collaboration with organizations such as the American Board of Internal Medicine, Society of General Internal Medicine, JCAHO, the American Hospital Association, and other specialty societies such as the American College of Chest Physicians and the American Academy of Pediatrics. SHM continues its strong affiliation and relationship with the American College of Physicians. Relationships with these key organizations will enable SHM to refine its recognition program for members completing the core curriculum and further the recognition of hospital medicine as a distinct specialty.
In 2006, the Society of Hospital Medicine will launch its official journal, The Journal of Hospital Medicine. The journal will provide a vehicle for the dissemination of research and innovations in hospital medicine.
To further signify its commitment to education, SHM hired a Director of Education, Jane Mihelic, to establish a Division of Education in July of 2004. As the development phase of the core curriculum concludes, SHM will hold the second education summit meeting early in 2005 to develop the next phase of the strategic plan for education and establish new goals and objectives. Future plans will include implementing the core curriculum, becoming an accredited provider of continuing medical education, and developing additional interactive self-directed learning materials.
Care of the hospitalized patient necessitates mastery and continued maintenance of sophisticated knowledge, skills, attitudes, and systems organization. The Society of Hospital Medicine is poised to foster, promote, and support hospitalists in meeting their life-long learning needs.
Please feel free to contact Alpesh Amin, MD, (anamin@uci.edu) Chair, SHM Education Committee, or Jane Mihelic (jmihelic@hospitalmedicine.org), SHM Director of Education, regarding thoughts or ideas on hospital medicine education.
The Society of Hospital Medicine (SHM) is committed to improving the quality of in-patient care through the provision of educational programs, tools, and resources to its membership. In 2002, the SHM Education Committee and leadership met to develop its first strategic plan for education. Long-range and short-term goals were determined, establishing a strategic vision and direction for education for the organization. Long-range goals included defining and developing a core curriculum in hospital medicine; developing a variety of modalities to deliver education; promoting collaborative efforts with other organizations; promoting original research in hospital medicine; and establishing a recognition program for completion of the core curriculum.
To help achieve its short-term goals, the Education Committee formed three task forces: the Core Curriculum Task Force, Leadership Task Force, and Geriatrics Task Force. As a result of the work of committees, task forces, and members, an impressive array of educational programs and products has been developed for membership, and much progress has been made in reaching SHM’s long-term educational goals. SHM would like to acknowledge and thank all of the members who have contributed countless hours and tireless effort to the educational initiatives of the organization.
The Education Committee formed two Core Curriculum Task Forces, an Adult and a Pediatric task force. Each task force has been defining and developing the core curriculum, which will identify the expected proficiencies of members and establish the distinctive differences between hospital medicine and other related medical specialties. The Adult Core Curriculum Task Force executive committee includes Mike Pistoria, Alpesh Amin, Tina Budnitz, Dan Dressler, and Sylvia McKean. An Adult Core Curriculum Guide is expected to be published and released in the spring of 2005. The Guide will contain three sections, each with multiple chapters: Clinical Conditions, Systems, and Procedures. Each chapter begins with an introduction articulating its importance to the practice of hospital medicine, followed by competencies categorized into four areas: knowledge, skills, attitudes, and systems organization and improvement. The core curriculum will be the basis for all future SHM educational activities. It is anticipated that the Core Curriculum Guide will standardize efforts to educate hospitalists across the continuum of medical education. The guide will be provided to internal medicine clerkship and residency program directors and hospitalist fellowship directors. Educators responsible for developing continuing medical education activities for physicians will find the guide to be a valuable resource.
The Pediatric Core Curriculum Guide will be similar in structure to the Adult Guide and is a work in progress. The Pediatric Core Curriculum Task Force executive committee includes Tim Cornell, Dan Rauch, and Alpesh Amin.
The Society of Hospital Medicine’s annual meeting has become the premier meeting for health care professionals who specialize in hospital medicine. The annual meeting provides a wide range of opportunities for learning. Participants can select from a variety of tracks, which include adult and pediatric clinical topics, operational and organizational issues, and challenges for academic hospitalists. The diversity of the annual meeting allows for more personal and individualized learning and tremendous opportunities for networking with colleagues. Preetha Basaviah is the course director of the 2005 annual meeting, which will be held April 28-30, in Chicago, Illinois. The theme for this 8th annual meeting is, “Hospital Medicine in 2005: Strategies for Success.”
In 2004, Regional meetings were held in the Northeast, Western, and Southern regions with much success. Forty-three chapters across the country are also developing educational sessions for their local meetings. Several courses focusing on specific topics of interest to hospitalists have been developed and are offered the day prior to the annual or regional meetings. Pre-courses include Practice Management, Leadership Survival, Perioperative and Consultative Medicine, and Critical Care for the Hospitalist, offering additional opportunities for members to enhance their skills and knowledge.
The Society of Hospital Medicine continues to develop educational tools and resources for the Web site (www.hospitalmedicine.org). The first resource room, addressing the prevention of antimicrobial resistance, was created. Resource rooms provide links to guidelines and relevant CME on-line courses, reviews of pertinent literature, methods to create quality-improvement programs, fact sheets, slide sets and other important information to assist hospitalists in their daily practice. Several unrestricted educational grants have been secured through pharmaceutical companies to support the creation of additional resource rooms to be launched in the near future on topics such as DVT awareness, stroke, and geriatric inpatient care.
The Centers for Disease Control and Prevention (CDC) has extended their Cooperative Agreement with SHM for an additional year. As a part of this agreement, SHM will evaluate and revise the applied learning workshop, “Implementing Quality Improvement Programs to Reduce Antimicrobial Resistance,” by Dan Dressler. This revised workshop will be conducted at three different chapter or other local meetings. If you are interested in bringing this great workshop to your chapter meeting, contact Tina Budnitz at TBudnitz@hospitalmedicine.org.
SHM has also received a substantial grant from the John A. Hartford Foundation to assist in the creation of educational symposia and enduring materials related to improving outcomes in older adults. The grant also supports the development of a discharge planning tool for hospitalists, and a demonstration project at three sites to implement quality improvement programs to educate members about important issues affecting outcomes in older patients. Members interested in participating in the creation of a discharge planning tool and supporting guidelines should plan to attend the discharge planning workshop at the 2005 Annual Meeting.
An outgrowth of the Hartford grant has been the establishment of the Leadership Academy. A Leadership pre-course for 100 hospitalist leaders was successfully included in the 2004 Annual Meeting. The next intensive workshop is scheduled for January 2005 under the direction of course directors Mark Williams and Russ Holman in Arizona, with a subsequent session scheduled for September 2005 in Vail, CO. The Leadership Academy was developed to provide the skills and resources required to successfully lead and manage a hospital medicine program now and in the future. In-depth training is provided on strategic planning, conflict resolution and negotiation, understanding critical hospital performance metrics, and leading and managing change.
SHM’s vision for hospital medicine education is forwarded through the development of strategic collaboration with organizations such as the American Board of Internal Medicine, Society of General Internal Medicine, JCAHO, the American Hospital Association, and other specialty societies such as the American College of Chest Physicians and the American Academy of Pediatrics. SHM continues its strong affiliation and relationship with the American College of Physicians. Relationships with these key organizations will enable SHM to refine its recognition program for members completing the core curriculum and further the recognition of hospital medicine as a distinct specialty.
In 2006, the Society of Hospital Medicine will launch its official journal, The Journal of Hospital Medicine. The journal will provide a vehicle for the dissemination of research and innovations in hospital medicine.
To further signify its commitment to education, SHM hired a Director of Education, Jane Mihelic, to establish a Division of Education in July of 2004. As the development phase of the core curriculum concludes, SHM will hold the second education summit meeting early in 2005 to develop the next phase of the strategic plan for education and establish new goals and objectives. Future plans will include implementing the core curriculum, becoming an accredited provider of continuing medical education, and developing additional interactive self-directed learning materials.
Care of the hospitalized patient necessitates mastery and continued maintenance of sophisticated knowledge, skills, attitudes, and systems organization. The Society of Hospital Medicine is poised to foster, promote, and support hospitalists in meeting their life-long learning needs.
Please feel free to contact Alpesh Amin, MD, (anamin@uci.edu) Chair, SHM Education Committee, or Jane Mihelic (jmihelic@hospitalmedicine.org), SHM Director of Education, regarding thoughts or ideas on hospital medicine education.