News

Targeted CLL treatments found effective in managing associated autoimmune cytopenias
- Author:
- Andrew D. Bowser
Preexisting autoimmune cytopenias frequently improved or resolved on ibrutinib, idelalisib, or venetoclax, while...
News

‘Praise Diabetes’: Support programs in Black churches yield lasting A1c changes
- Author:
- Andrew D. Bowser
Statistically significant reductions in A1c and diabetes distress were seen in the Praise Diabetes Project, a 33-month randomized trial, an...
News
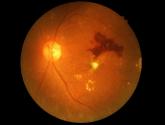
Anti-VEGF injections for diabetic retinopathy linked to mortality risk
- Author:
- Andrew D. Bowser
Results suggest a need to be “very careful” with treatment choice in certain patients, investigator says, though much more research is needed to...
News

'Full throttle': 'Diabetes Garage' workshops boost Mexican American men's self-management
- Author:
- Andrew D. Bowser
Infusing diabetes education with concepts and lingo from car culture is a promising approach to improving self-management behaviors, and might...
News

Intervention opens access to care for minority youths with type 1 diabetes
- Author:
- Andrew D. Bowser
Program adds support for children with diabetes and their families who are struggling because of lack of resources or marginalization in the...
News
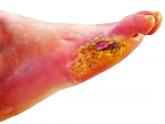
Type 1 diabetes amputation rates fall in Sweden, rise in U.S.
- Author:
- Andrew D. Bowser
Coupled with renal and A1c data, the findings suggest improved prognosis for these patients, Swedish investigators say.
News

Unmanaged diabetes, high blood glucose tied to COVID-19 severity
- Author:
- Andrew D. Bowser
Findings suggest in part that hospitalized COVID-19 patients need aggressive treatment of hyperglycemia, regardless of the diagnosis of diabetes...
News

FDA approves OTC antihistamine nasal spray
- Author:
- Andrew D. Bowser
Nasal antihistamines “work quickly, whereas the nasal steroids don’t, and I think a lot of people who go to the drugstore looking for allergy...
News
HER3-targeted treatment demonstrates efficacy and safety in phase 1 lung cancer study
- Author:
- Andrew D. Bowser
Responses with patritumab deruxtecan were seen across a spectrum of resistance mutations among patients...
News
Surgical outcomes favor addition of nivolumab to neoadjuvant chemo in resectable lung cancers
- Author:
- Andrew D. Bowser
Combined with pathologic complete response data reported previously, findings of CheckMate 816 build a case for...
News
Vinorelbine survival benefit in mesothelioma overshadowed by advances in immuno-oncology
- Author:
- Andrew D. Bowser
The increasing use of immuno-oncology approaches and new clinical trials are pushing cytotoxic chemotherapy to...
News
KRAS inhibitor improved survival in phase 2 lung cancer trial
- Author:
- Andrew D. Bowser
Subgroup analyses suggest benefit even in patients with co-occurring mutations usually associated with poor...
News
Community practice lung cancer patients insufficiently tested for treatment-related biomarkers
- Author:
- Andrew D. Bowser
Patients are missing the opportunity to be considered for FDA-approved therapies and clinical trials, the...
News
NSCLC: Immune-related AEs during checkpoint inhibitor therapy may predict outcomes
- Author:
- Andrew D. Bowser
Results of the exploratory analyses are hypothesis generating but suggest a link between treatment-emergent...
News

GI symptoms and chronic fatigue may persist months after COVID-19
- Author:
- Andrew D. Bowser
Loose stools, somatization, and chronic fatigue risks increased at a mean of 5 months after SARS-CoV-2 infection, suggesting a common...
