News
Stress-related disorders linked to later neurodegenerative diseases
- Author:
- Andrew D. Bowser
Individuals with PTSD, acute stress reaction, adjustment disorder, or other stress reactions had an 80% increased risk of vascular...
News
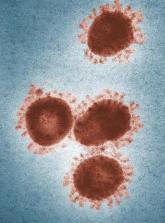
Novel coronavirus may cause environmental contamination through fecal shedding
- Author:
- Andrew D. Bowser
Beyond respiratory droplets, fecal shedding could also be a potential route of transmission.
News

No sedation fails to improve mortality in mechanically ventilated patients
- Author:
- Andrew D. Bowser
ORLANDO – The lack of sedation did significantly improve certain secondary endpoints, including a reduced number of thromboembolic events and...
News

Guidance defines vaping-related respiratory syndrome
- Author:
- Andrew D. Bowser
ORLANDO – The guidance is designed to help critical care professionals efficiently identify those individuals at high risk of respiratory failure...
News
Medicare beneficiaries get few home health visits after ICU stay
- Author:
- Andrew D. Bowser
ORLANDO – The beneficiaries received an average of less than one visit per week in the month after ICU discharge, while a third received no visits...
News

Opioid use disorder up in sepsis hospitalizations
- Author:
- Andrew D. Bowser
ORLANDO – “While we don’t necessarily screen every sepsis patient for endocarditis, if it’s an opioid use disorder patient – particularly one with...
News
Zilucoplan improved efficacy outcomes in myasthenia gravis
- Author:
- Andrew D. Bowser
The complement C5 inhibitor had a similar magnitude of clinical effect as that seen in eculizumab studies.
News

Critical care admissions up for pediatric opioid poisonings
- Author:
- Andrew D. Bowser
ORLANDO – Attempted suicide has represented an increasingly large proportion of these severe poisonings.
News

As novel coronavirus outbreak evolves, critical care providers need to be prepared
- Author:
- Andrew D. Bowser
Despite “substantial uncertainty” over impact outside China, any influx of seriously ill patients could strain...
News
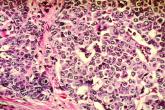
Adding pembrolizumab to chemo doubled pCR rates in early-stage breast cancer
- Author:
- Andrew D. Bowser
The doubling of pathologic complete response rates was seen in all three biomarker signatures studied.
News
Glioma trials should track living well, not just longer
- Author:
- Andrew D. Bowser
“Patients want to live longer, but they also want to continue to function as well as possible for as long as possible,“ said Terri S. Armstrong,...
News

Value of very early etanercept plus methotrexate not confirmed in real-world RA trial
- Author:
- Andrew D. Bowser
In the open-label VEDERA trial, differences in remission rates were not in line with an outsized effect suggested by exploratory analysis of an...
News
Walk test may predict complications after lung cancer surgery
- Author:
- Andrew D. Bowser
Findings suggest role for curative resection in patients with moderately decreased lung function but a longer 6-minute walk distance,...
News

Dietary flavonol intake linked to reduced risk of Alzheimer’s
- Author:
- Andrew D. Bowser
A class of polyphenols found in fruits and vegetables was associated with lower rates of Alzheimer’s in an observational study, setting the stage...
News
Genetic factor linked to impaired memory after heading many soccer balls
- Author:
- Andrew D. Bowser
Findings, while preliminary, may be evidence of “early subclinical effects” in those with high levels of ball-heading exposure.
