User login
Glioma trials should track living well, not just longer
Neuro-oncology working group backs focus on how patients feel, function
Glioma treatment goals traditionally have focused on tumor shrinkage or prolonging survival, but it’s time for those endpoints to be supplemented by clinical outcomes that are meaningful to the patient, according to a recently published report from a neuro-oncology working group.
The group, which includes representatives of previous oncology working groups, the Food and Drug Administration, and observers from the European Medicines Agency, has established a core set of symptoms and functional points that they say could be used in clinical trials and clinical care for patients with high-grade gliomas.
“Patients want to live longer, but they also want to continue to function as well as possible for as long as possible,” said Terri S. Armstrong, PhD, of the National Cancer Institute (NCI), and coauthors in a report that sums up the work to date of the Fast Track COA Group.
That work, while specific to gliomas, echoes results from broader initiatives that seek to standardize patient-reported outcomes in oncology trials, Dr. Armstrong and coauthors wrote. The report was published in the Lancet Oncology.
The core set of symptom constructs and functional issues identified by the work group are represented already in patient-reported outcome measures, according to the authors.
The symptoms worth measuring fall into five categories, including pain, difficulty communicating, perceived cognition, seizures, and symptomatic adverse events. The functional issues were divided into two categories, physical functioning, including weakness or walking, and role functioning, which they defined as the ability to work or participate in social or leisure activities.
Some of those outcomes can be challenging or cumbersome to track, Dr. Armstrong and coauthors said.
Pain has “many dimensions“ and is important to track, the group wrote. Likewise, patients’ concerns related to language function also are important, but are very “noisy“ as a variable and can be specific to tumor location.
Collecting data on seizure frequency and severity is important yet complicated, because of the variability in seizures and considerable difference between focal and generalized seizures. Assessment of cognitive functioning can be lengthy and burdensome to patients.
Adverse events of relevance will vary, depending on the drug used, its mechanism of action, and available data, though some allowance needs to be made for the possibility of “overlap“ with disease-related symptoms, the report said.
Physical functioning, including walking and weakness, should be evaluated. It also would be useful to distinguish the duration of time that patients have deficits in physical functioning in the later stages of their disease progression, authors said.
Role and social functioning should be assessed in most patients with high-grade gliomas, who will have symptoms and deficits that prevent returning to a job. “Patients might spend a substantial portion of their lives feeling ill, unable to do usual activities, or meet occupational, social, financial, and family obligations,” said Dr. Armstrong and coauthors in the report.
The scales and tools used to measure symptoms and functional concerns need to be those that best fit a particular clinical trial or clinical practice scenario. Several instruments that would be appropriate are discussed in the report, including the NCI Patient-Reported Outcome of the Common Toxicity Criteria Adverse Events (NCI PRO-CTCAE) and symptom and function scales or items in the Patient-Reported Outcomes Measurement System (PROMIS).
, such as time to recurrence or survival.
“Strategies for introducing these constructs to clinical trial cooperative groups and sponsors will be necessary,” they concluded.
Dr. Armstrong reported employment as a senior investigator and deputy chief of the neuro-oncology branch of the Center for Cancer Research at the NCI. His coauthors reported disclosures related to several companies and interests, including AbbVie, AstraZeneca, Bristol-Myers Squibb, Genentech, Merck, Taiho, and Tocagen.
SOURCE: Armstrong TS et al. Lancet Oncol. 2020;21(2):e97-103.
Neuro-oncology working group backs focus on how patients feel, function
Neuro-oncology working group backs focus on how patients feel, function
Glioma treatment goals traditionally have focused on tumor shrinkage or prolonging survival, but it’s time for those endpoints to be supplemented by clinical outcomes that are meaningful to the patient, according to a recently published report from a neuro-oncology working group.
The group, which includes representatives of previous oncology working groups, the Food and Drug Administration, and observers from the European Medicines Agency, has established a core set of symptoms and functional points that they say could be used in clinical trials and clinical care for patients with high-grade gliomas.
“Patients want to live longer, but they also want to continue to function as well as possible for as long as possible,” said Terri S. Armstrong, PhD, of the National Cancer Institute (NCI), and coauthors in a report that sums up the work to date of the Fast Track COA Group.
That work, while specific to gliomas, echoes results from broader initiatives that seek to standardize patient-reported outcomes in oncology trials, Dr. Armstrong and coauthors wrote. The report was published in the Lancet Oncology.
The core set of symptom constructs and functional issues identified by the work group are represented already in patient-reported outcome measures, according to the authors.
The symptoms worth measuring fall into five categories, including pain, difficulty communicating, perceived cognition, seizures, and symptomatic adverse events. The functional issues were divided into two categories, physical functioning, including weakness or walking, and role functioning, which they defined as the ability to work or participate in social or leisure activities.
Some of those outcomes can be challenging or cumbersome to track, Dr. Armstrong and coauthors said.
Pain has “many dimensions“ and is important to track, the group wrote. Likewise, patients’ concerns related to language function also are important, but are very “noisy“ as a variable and can be specific to tumor location.
Collecting data on seizure frequency and severity is important yet complicated, because of the variability in seizures and considerable difference between focal and generalized seizures. Assessment of cognitive functioning can be lengthy and burdensome to patients.
Adverse events of relevance will vary, depending on the drug used, its mechanism of action, and available data, though some allowance needs to be made for the possibility of “overlap“ with disease-related symptoms, the report said.
Physical functioning, including walking and weakness, should be evaluated. It also would be useful to distinguish the duration of time that patients have deficits in physical functioning in the later stages of their disease progression, authors said.
Role and social functioning should be assessed in most patients with high-grade gliomas, who will have symptoms and deficits that prevent returning to a job. “Patients might spend a substantial portion of their lives feeling ill, unable to do usual activities, or meet occupational, social, financial, and family obligations,” said Dr. Armstrong and coauthors in the report.
The scales and tools used to measure symptoms and functional concerns need to be those that best fit a particular clinical trial or clinical practice scenario. Several instruments that would be appropriate are discussed in the report, including the NCI Patient-Reported Outcome of the Common Toxicity Criteria Adverse Events (NCI PRO-CTCAE) and symptom and function scales or items in the Patient-Reported Outcomes Measurement System (PROMIS).
, such as time to recurrence or survival.
“Strategies for introducing these constructs to clinical trial cooperative groups and sponsors will be necessary,” they concluded.
Dr. Armstrong reported employment as a senior investigator and deputy chief of the neuro-oncology branch of the Center for Cancer Research at the NCI. His coauthors reported disclosures related to several companies and interests, including AbbVie, AstraZeneca, Bristol-Myers Squibb, Genentech, Merck, Taiho, and Tocagen.
SOURCE: Armstrong TS et al. Lancet Oncol. 2020;21(2):e97-103.
Glioma treatment goals traditionally have focused on tumor shrinkage or prolonging survival, but it’s time for those endpoints to be supplemented by clinical outcomes that are meaningful to the patient, according to a recently published report from a neuro-oncology working group.
The group, which includes representatives of previous oncology working groups, the Food and Drug Administration, and observers from the European Medicines Agency, has established a core set of symptoms and functional points that they say could be used in clinical trials and clinical care for patients with high-grade gliomas.
“Patients want to live longer, but they also want to continue to function as well as possible for as long as possible,” said Terri S. Armstrong, PhD, of the National Cancer Institute (NCI), and coauthors in a report that sums up the work to date of the Fast Track COA Group.
That work, while specific to gliomas, echoes results from broader initiatives that seek to standardize patient-reported outcomes in oncology trials, Dr. Armstrong and coauthors wrote. The report was published in the Lancet Oncology.
The core set of symptom constructs and functional issues identified by the work group are represented already in patient-reported outcome measures, according to the authors.
The symptoms worth measuring fall into five categories, including pain, difficulty communicating, perceived cognition, seizures, and symptomatic adverse events. The functional issues were divided into two categories, physical functioning, including weakness or walking, and role functioning, which they defined as the ability to work or participate in social or leisure activities.
Some of those outcomes can be challenging or cumbersome to track, Dr. Armstrong and coauthors said.
Pain has “many dimensions“ and is important to track, the group wrote. Likewise, patients’ concerns related to language function also are important, but are very “noisy“ as a variable and can be specific to tumor location.
Collecting data on seizure frequency and severity is important yet complicated, because of the variability in seizures and considerable difference between focal and generalized seizures. Assessment of cognitive functioning can be lengthy and burdensome to patients.
Adverse events of relevance will vary, depending on the drug used, its mechanism of action, and available data, though some allowance needs to be made for the possibility of “overlap“ with disease-related symptoms, the report said.
Physical functioning, including walking and weakness, should be evaluated. It also would be useful to distinguish the duration of time that patients have deficits in physical functioning in the later stages of their disease progression, authors said.
Role and social functioning should be assessed in most patients with high-grade gliomas, who will have symptoms and deficits that prevent returning to a job. “Patients might spend a substantial portion of their lives feeling ill, unable to do usual activities, or meet occupational, social, financial, and family obligations,” said Dr. Armstrong and coauthors in the report.
The scales and tools used to measure symptoms and functional concerns need to be those that best fit a particular clinical trial or clinical practice scenario. Several instruments that would be appropriate are discussed in the report, including the NCI Patient-Reported Outcome of the Common Toxicity Criteria Adverse Events (NCI PRO-CTCAE) and symptom and function scales or items in the Patient-Reported Outcomes Measurement System (PROMIS).
, such as time to recurrence or survival.
“Strategies for introducing these constructs to clinical trial cooperative groups and sponsors will be necessary,” they concluded.
Dr. Armstrong reported employment as a senior investigator and deputy chief of the neuro-oncology branch of the Center for Cancer Research at the NCI. His coauthors reported disclosures related to several companies and interests, including AbbVie, AstraZeneca, Bristol-Myers Squibb, Genentech, Merck, Taiho, and Tocagen.
SOURCE: Armstrong TS et al. Lancet Oncol. 2020;21(2):e97-103.
FROM THE LANCET ONCOLOGY
Value of very early etanercept plus methotrexate not confirmed in real-world RA trial
, investigators say.
A remission rate of 52% was seen with first-line etanercept plus methotrexate, compared with 38% for a strategy of methotrexate escalated to add etanercept in patients not in remission at 24 weeks in the study, known as VEDERA (Very Early Versus Delayed Etanercept in Patients With RA).
Investigators said a difference of 14 percentage points between remission rates was comparable to what was seen among patients with early RA in an earlier randomized trial of etanercept plus methotrexate versus methotrexate monotherapy.
However, the difference was not on par with the “larger than standard” effect of about 30% seen in an exploratory analysis of the very early RA subset in that previous study, according to VEDERA study authors, led by Paul Emery, MD, of the University of Leeds (England).
Taken together, the results highlight a “ceiling effect” in achieving remission in this real-life, treatment-naive cohort, Dr. Emery and coauthors noted in their report, which appears in Annals of the Rheumatic Diseases.
The study population aligned with real-world clinical practice, according to the investigators, who noted that half the cohort had at least one comorbidity.
“This may have partly driven the generally poorer than expected performance, the exact mechanisms for which are unclear,” they wrote in their discussion of results.
Delaying etanercept until failure of methotrexate, instead of giving both drugs up front, was linked to poorer etanercept response in an exploratory analysis of VEDERA. However, Dr. Emery and coinvestigators noted that this finding “requires validation and further investigation.”
While first-line etanercept plus methotrexate is a “clinically appropriate approach” in early RA, results of the VEDERA study don’t help to inform clinicians as to when it would be prudent to select that therapeutic approach, said Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles.
“Whether you’re treating with methotrexate or methotrexate plus etanercept, they do pretty well,” Dr. Furst said in an interview. “What that says to me is when you have patients with very early RA and the disease is moderately active, you should really try methotrexate before you add expensive other drugs.”
The phase 4, open-label, randomized VEDERA trial included 120 adult patients with new-onset early RA with symptom duration of less than 12 months. All patients in VEDERA had a 28-joint disease activity score based on erythrocyte sedimentation rate (DAS28-ESR) of 3.2 or greater and clinical evidence of synovitis. They were all positive for anti-citrullinated peptide antibody or rheumatoid factor, or had evidence of disease activity in at least one joint by power Doppler ultrasonography.
The patients were randomized to 48 weeks of treatment with either first-line etanercept plus methotrexate, or with methotrexate in a treat-to-target strategy that called for the addition of etanercept if the DAS28-ESR was still 2.6 or greater at 24 weeks.
Based on results of the earlier trial, known as COMET, a confirmatory remission rate in the etanercept plus methotrexate arm was anticipated to be 70%, versus 40% for the methotrexate treat-to-target arm, investigators said in a discussion of their statistical methods.
Study results did not confirm a large effect size, according to the investigators. By week 48, DAS28-ESR remission was achieved in 52% of patients in the first-line etanercept plus methotrexate arm, versus 38% in the methotrexate treat-to-target arm, for an absolute difference of 14 percentage points (odds ratio, 1.73; 95% confidence interval, 0.81-3.70; P = .160).
In early, new-onset RA, remission is the goal, Dr. Emery and coauthors said. The proportions of patients in remission in both arms are “suboptimal rates for the contemporary era,” they wrote.
The escalation to etanercept at week 24 did not improve remission rates appreciably, with about 60% still failing to achieve that endpoint. However, an exploratory analysis suggested the subsequent 24 weeks of etanercept exposure in those escalated patients was associated with a lower rate of remission, compared with 24 weeks of etanercept in the front-line approach, investigators said.
In that analysis, the adjusted odds ratio of achieving DAS28-ESR remission was 2.84 (95% CI, 0.84-9.60) in favor of the first-line etanercept approach.
Dr. Furst said in the interview that the VEDERA results are subject to the inherent biases of an open-label study. He also suggested that further investigations could compare the two first-line treatment approaches specifically in very early RA patients with markers of more severe disease.
“That’s the only way I would think we might gain a little bit more, but so far, these data don’t support getting terribly aggressive,” he said.
The study was funded through an investigator-sponsored research grant provided by Pfizer. Three authors disclosed financial relationships with multiple pharmaceutical companies that market drugs for RA, including Pfizer.
SOURCE: Emery P et al. Ann Rheum Dis. 2020 Jan 29. doi: 10.1136/annrheumdis-2019-216539.
, investigators say.
A remission rate of 52% was seen with first-line etanercept plus methotrexate, compared with 38% for a strategy of methotrexate escalated to add etanercept in patients not in remission at 24 weeks in the study, known as VEDERA (Very Early Versus Delayed Etanercept in Patients With RA).
Investigators said a difference of 14 percentage points between remission rates was comparable to what was seen among patients with early RA in an earlier randomized trial of etanercept plus methotrexate versus methotrexate monotherapy.
However, the difference was not on par with the “larger than standard” effect of about 30% seen in an exploratory analysis of the very early RA subset in that previous study, according to VEDERA study authors, led by Paul Emery, MD, of the University of Leeds (England).
Taken together, the results highlight a “ceiling effect” in achieving remission in this real-life, treatment-naive cohort, Dr. Emery and coauthors noted in their report, which appears in Annals of the Rheumatic Diseases.
The study population aligned with real-world clinical practice, according to the investigators, who noted that half the cohort had at least one comorbidity.
“This may have partly driven the generally poorer than expected performance, the exact mechanisms for which are unclear,” they wrote in their discussion of results.
Delaying etanercept until failure of methotrexate, instead of giving both drugs up front, was linked to poorer etanercept response in an exploratory analysis of VEDERA. However, Dr. Emery and coinvestigators noted that this finding “requires validation and further investigation.”
While first-line etanercept plus methotrexate is a “clinically appropriate approach” in early RA, results of the VEDERA study don’t help to inform clinicians as to when it would be prudent to select that therapeutic approach, said Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles.
“Whether you’re treating with methotrexate or methotrexate plus etanercept, they do pretty well,” Dr. Furst said in an interview. “What that says to me is when you have patients with very early RA and the disease is moderately active, you should really try methotrexate before you add expensive other drugs.”
The phase 4, open-label, randomized VEDERA trial included 120 adult patients with new-onset early RA with symptom duration of less than 12 months. All patients in VEDERA had a 28-joint disease activity score based on erythrocyte sedimentation rate (DAS28-ESR) of 3.2 or greater and clinical evidence of synovitis. They were all positive for anti-citrullinated peptide antibody or rheumatoid factor, or had evidence of disease activity in at least one joint by power Doppler ultrasonography.
The patients were randomized to 48 weeks of treatment with either first-line etanercept plus methotrexate, or with methotrexate in a treat-to-target strategy that called for the addition of etanercept if the DAS28-ESR was still 2.6 or greater at 24 weeks.
Based on results of the earlier trial, known as COMET, a confirmatory remission rate in the etanercept plus methotrexate arm was anticipated to be 70%, versus 40% for the methotrexate treat-to-target arm, investigators said in a discussion of their statistical methods.
Study results did not confirm a large effect size, according to the investigators. By week 48, DAS28-ESR remission was achieved in 52% of patients in the first-line etanercept plus methotrexate arm, versus 38% in the methotrexate treat-to-target arm, for an absolute difference of 14 percentage points (odds ratio, 1.73; 95% confidence interval, 0.81-3.70; P = .160).
In early, new-onset RA, remission is the goal, Dr. Emery and coauthors said. The proportions of patients in remission in both arms are “suboptimal rates for the contemporary era,” they wrote.
The escalation to etanercept at week 24 did not improve remission rates appreciably, with about 60% still failing to achieve that endpoint. However, an exploratory analysis suggested the subsequent 24 weeks of etanercept exposure in those escalated patients was associated with a lower rate of remission, compared with 24 weeks of etanercept in the front-line approach, investigators said.
In that analysis, the adjusted odds ratio of achieving DAS28-ESR remission was 2.84 (95% CI, 0.84-9.60) in favor of the first-line etanercept approach.
Dr. Furst said in the interview that the VEDERA results are subject to the inherent biases of an open-label study. He also suggested that further investigations could compare the two first-line treatment approaches specifically in very early RA patients with markers of more severe disease.
“That’s the only way I would think we might gain a little bit more, but so far, these data don’t support getting terribly aggressive,” he said.
The study was funded through an investigator-sponsored research grant provided by Pfizer. Three authors disclosed financial relationships with multiple pharmaceutical companies that market drugs for RA, including Pfizer.
SOURCE: Emery P et al. Ann Rheum Dis. 2020 Jan 29. doi: 10.1136/annrheumdis-2019-216539.
, investigators say.
A remission rate of 52% was seen with first-line etanercept plus methotrexate, compared with 38% for a strategy of methotrexate escalated to add etanercept in patients not in remission at 24 weeks in the study, known as VEDERA (Very Early Versus Delayed Etanercept in Patients With RA).
Investigators said a difference of 14 percentage points between remission rates was comparable to what was seen among patients with early RA in an earlier randomized trial of etanercept plus methotrexate versus methotrexate monotherapy.
However, the difference was not on par with the “larger than standard” effect of about 30% seen in an exploratory analysis of the very early RA subset in that previous study, according to VEDERA study authors, led by Paul Emery, MD, of the University of Leeds (England).
Taken together, the results highlight a “ceiling effect” in achieving remission in this real-life, treatment-naive cohort, Dr. Emery and coauthors noted in their report, which appears in Annals of the Rheumatic Diseases.
The study population aligned with real-world clinical practice, according to the investigators, who noted that half the cohort had at least one comorbidity.
“This may have partly driven the generally poorer than expected performance, the exact mechanisms for which are unclear,” they wrote in their discussion of results.
Delaying etanercept until failure of methotrexate, instead of giving both drugs up front, was linked to poorer etanercept response in an exploratory analysis of VEDERA. However, Dr. Emery and coinvestigators noted that this finding “requires validation and further investigation.”
While first-line etanercept plus methotrexate is a “clinically appropriate approach” in early RA, results of the VEDERA study don’t help to inform clinicians as to when it would be prudent to select that therapeutic approach, said Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles.
“Whether you’re treating with methotrexate or methotrexate plus etanercept, they do pretty well,” Dr. Furst said in an interview. “What that says to me is when you have patients with very early RA and the disease is moderately active, you should really try methotrexate before you add expensive other drugs.”
The phase 4, open-label, randomized VEDERA trial included 120 adult patients with new-onset early RA with symptom duration of less than 12 months. All patients in VEDERA had a 28-joint disease activity score based on erythrocyte sedimentation rate (DAS28-ESR) of 3.2 or greater and clinical evidence of synovitis. They were all positive for anti-citrullinated peptide antibody or rheumatoid factor, or had evidence of disease activity in at least one joint by power Doppler ultrasonography.
The patients were randomized to 48 weeks of treatment with either first-line etanercept plus methotrexate, or with methotrexate in a treat-to-target strategy that called for the addition of etanercept if the DAS28-ESR was still 2.6 or greater at 24 weeks.
Based on results of the earlier trial, known as COMET, a confirmatory remission rate in the etanercept plus methotrexate arm was anticipated to be 70%, versus 40% for the methotrexate treat-to-target arm, investigators said in a discussion of their statistical methods.
Study results did not confirm a large effect size, according to the investigators. By week 48, DAS28-ESR remission was achieved in 52% of patients in the first-line etanercept plus methotrexate arm, versus 38% in the methotrexate treat-to-target arm, for an absolute difference of 14 percentage points (odds ratio, 1.73; 95% confidence interval, 0.81-3.70; P = .160).
In early, new-onset RA, remission is the goal, Dr. Emery and coauthors said. The proportions of patients in remission in both arms are “suboptimal rates for the contemporary era,” they wrote.
The escalation to etanercept at week 24 did not improve remission rates appreciably, with about 60% still failing to achieve that endpoint. However, an exploratory analysis suggested the subsequent 24 weeks of etanercept exposure in those escalated patients was associated with a lower rate of remission, compared with 24 weeks of etanercept in the front-line approach, investigators said.
In that analysis, the adjusted odds ratio of achieving DAS28-ESR remission was 2.84 (95% CI, 0.84-9.60) in favor of the first-line etanercept approach.
Dr. Furst said in the interview that the VEDERA results are subject to the inherent biases of an open-label study. He also suggested that further investigations could compare the two first-line treatment approaches specifically in very early RA patients with markers of more severe disease.
“That’s the only way I would think we might gain a little bit more, but so far, these data don’t support getting terribly aggressive,” he said.
The study was funded through an investigator-sponsored research grant provided by Pfizer. Three authors disclosed financial relationships with multiple pharmaceutical companies that market drugs for RA, including Pfizer.
SOURCE: Emery P et al. Ann Rheum Dis. 2020 Jan 29. doi: 10.1136/annrheumdis-2019-216539.
FROM ANNALS OF THE RHEUMATIC DISEASES
Walk test may predict complications after lung cancer surgery
Risk of cardiopulmonary complications increased nearly eightfold in patients with moderate lung function decreases who failed to walk 400 m or more, according to the study, which included data on 416 patients with non–small cell lung cancer (NSCLC) who underwent lobectomy.
This is believed to be the first large study evaluating the utility of the 6-minute walk test (6MWT) to predict postoperative cardiopulmonary complications in this surgical setting, according to researchers led by Hyun Lee, MD, of Hanyang University in Seoul, South Korea.
“Our findings suggest that 6-minute walk distance would provide additional information in lung cancer patients with moderately decreased lung function who plan to undergo surgical resection,” said Dr. Lee and coauthors of the study report, which appears in CHEST.
More specifically, the option of curative resection should be considered in those lung cancer patients with moderately decreased lung function but a longer 6-minute walk distance, they added.
Exercise testing is currently recommended to further stratify risk of postoperative complications among patient with moderately decreased lung function, according to the researchers. The 6-minute walk test might be a good tool to evaluate feasibility for moderate risk patients, according to one recent review. However, studies so far have been limited by small numbers of patients, and larger studies have not specifically looked at predicted postoperative lung function status, they said.
Accordingly, the researchers evaluated data from patients expected to undergo curative lung cancer surgery who were enrolled in a prospective cohort study in Korea. They were classified as low or moderate risk based on pulmonary function tests, and further classified into short distance (less than 400 m) and long distance (400 m or more) groups based on their performance on the 6-minute walk test.
Postoperative cardiopulmonary complications were seen in 42.9% of the moderate-risk, short-distance group, versus 14.4% of patients in the moderate-risk, long-distance group. In the low-risk patients, those complications were seen in 9.5% and 8.3% of those in the long and short distance groups.
Odds for postoperative cardiopulmonary complications were significantly increased in the moderate-risk, short-distance group, compared with the low-risk, long-distance group (adjusted odds ratio, 7.84; 95% confidence interval, 2.24-27.46).
By contrast, odds for complications were not significantly increased in the moderate-risk, long-distance group, nor in the low-risk, short-distance groups, investigators said.
Dr. Lee and coauthors said they had no conflicts of interest to disclose.
SOURCE: Lee H et al. CHEST. 2020. doi: 10.1016/j.chest.2019.12.039.
Risk of cardiopulmonary complications increased nearly eightfold in patients with moderate lung function decreases who failed to walk 400 m or more, according to the study, which included data on 416 patients with non–small cell lung cancer (NSCLC) who underwent lobectomy.
This is believed to be the first large study evaluating the utility of the 6-minute walk test (6MWT) to predict postoperative cardiopulmonary complications in this surgical setting, according to researchers led by Hyun Lee, MD, of Hanyang University in Seoul, South Korea.
“Our findings suggest that 6-minute walk distance would provide additional information in lung cancer patients with moderately decreased lung function who plan to undergo surgical resection,” said Dr. Lee and coauthors of the study report, which appears in CHEST.
More specifically, the option of curative resection should be considered in those lung cancer patients with moderately decreased lung function but a longer 6-minute walk distance, they added.
Exercise testing is currently recommended to further stratify risk of postoperative complications among patient with moderately decreased lung function, according to the researchers. The 6-minute walk test might be a good tool to evaluate feasibility for moderate risk patients, according to one recent review. However, studies so far have been limited by small numbers of patients, and larger studies have not specifically looked at predicted postoperative lung function status, they said.
Accordingly, the researchers evaluated data from patients expected to undergo curative lung cancer surgery who were enrolled in a prospective cohort study in Korea. They were classified as low or moderate risk based on pulmonary function tests, and further classified into short distance (less than 400 m) and long distance (400 m or more) groups based on their performance on the 6-minute walk test.
Postoperative cardiopulmonary complications were seen in 42.9% of the moderate-risk, short-distance group, versus 14.4% of patients in the moderate-risk, long-distance group. In the low-risk patients, those complications were seen in 9.5% and 8.3% of those in the long and short distance groups.
Odds for postoperative cardiopulmonary complications were significantly increased in the moderate-risk, short-distance group, compared with the low-risk, long-distance group (adjusted odds ratio, 7.84; 95% confidence interval, 2.24-27.46).
By contrast, odds for complications were not significantly increased in the moderate-risk, long-distance group, nor in the low-risk, short-distance groups, investigators said.
Dr. Lee and coauthors said they had no conflicts of interest to disclose.
SOURCE: Lee H et al. CHEST. 2020. doi: 10.1016/j.chest.2019.12.039.
Risk of cardiopulmonary complications increased nearly eightfold in patients with moderate lung function decreases who failed to walk 400 m or more, according to the study, which included data on 416 patients with non–small cell lung cancer (NSCLC) who underwent lobectomy.
This is believed to be the first large study evaluating the utility of the 6-minute walk test (6MWT) to predict postoperative cardiopulmonary complications in this surgical setting, according to researchers led by Hyun Lee, MD, of Hanyang University in Seoul, South Korea.
“Our findings suggest that 6-minute walk distance would provide additional information in lung cancer patients with moderately decreased lung function who plan to undergo surgical resection,” said Dr. Lee and coauthors of the study report, which appears in CHEST.
More specifically, the option of curative resection should be considered in those lung cancer patients with moderately decreased lung function but a longer 6-minute walk distance, they added.
Exercise testing is currently recommended to further stratify risk of postoperative complications among patient with moderately decreased lung function, according to the researchers. The 6-minute walk test might be a good tool to evaluate feasibility for moderate risk patients, according to one recent review. However, studies so far have been limited by small numbers of patients, and larger studies have not specifically looked at predicted postoperative lung function status, they said.
Accordingly, the researchers evaluated data from patients expected to undergo curative lung cancer surgery who were enrolled in a prospective cohort study in Korea. They were classified as low or moderate risk based on pulmonary function tests, and further classified into short distance (less than 400 m) and long distance (400 m or more) groups based on their performance on the 6-minute walk test.
Postoperative cardiopulmonary complications were seen in 42.9% of the moderate-risk, short-distance group, versus 14.4% of patients in the moderate-risk, long-distance group. In the low-risk patients, those complications were seen in 9.5% and 8.3% of those in the long and short distance groups.
Odds for postoperative cardiopulmonary complications were significantly increased in the moderate-risk, short-distance group, compared with the low-risk, long-distance group (adjusted odds ratio, 7.84; 95% confidence interval, 2.24-27.46).
By contrast, odds for complications were not significantly increased in the moderate-risk, long-distance group, nor in the low-risk, short-distance groups, investigators said.
Dr. Lee and coauthors said they had no conflicts of interest to disclose.
SOURCE: Lee H et al. CHEST. 2020. doi: 10.1016/j.chest.2019.12.039.
FROM CHEST
Dietary flavonol intake linked to reduced risk of Alzheimer’s
Onset of Alzheimer’s disease (AD) was inversely associated with intake of flavonols, a subclass of flavonoids with antioxidant and anti-inflammatory properties, according to the study authors.
The rate of developing AD was reduced by 50% among individuals reporting high intake of kaempferol, a flavonol plentiful in leafy green vegetables, and by 38% for high intake of the flavonols myricetin and isorhamnetin, researchers said in a report published in Neurology.
The findings are from the Rush Memory and Aging Project (MAP), a large, prospective study of older individuals in retirement communities and public housing in the Chicago area that has been ongoing since 1997.
“Although there is more work to be done, the associations that we observed are promising and deserve further study,” said Thomas M. Holland, MD, of the Rush Institute for Healthy Aging in Chicago, and coauthors.
Those associations between flavonol intake and AD help set the stage for U.S. POINTER and other randomized, controlled trials that seek to evaluate the effects of dietary interventions in a more rigorous way, according to Laura D. Baker, PhD, associate professor of internal medicine at Wake Forest University, Winston-Salem, N.C.
“This kind of data helps us feel like we are looking in the right direction in the randomized, controlled trials,” Dr. Baker said in an interview.
Dr. Baker is an investigator in the U.S. POINTER study, which will in part evaluate the impact of the MIND diet, which has been shown to slow cognitive decline with age in a previously published MAP study.
However, in the absence of randomized, controlled trial data, Dr. Baker cautioned against “prematurely advocating” for specific dietary approaches when speaking to patients and caregivers now.
“What I say is, we know for sure that the standard American Heart Association diet has been shown in clinical trials to reduce the risk of heart disease, and in terms of brain health, if you can reduce risk of heart disease, you are protecting your brain,” she said in the interview.
The present MAP study linking a reduced rate of AD to flavonol consumption is believed to be the first of its kind, though two previous studies from the early 2000s did find inverse associations between incident AD and intake of flavonoids, of which flavonoids are just one subclass, said Dr. Holland and coinvestigators in their report.
Moreover, in a MAP study published in 2018, Martha Clare Morris, ScD, and coauthors concluded that consuming about a serving per day of green leafy vegetables and foods rich in kaempferol, among other nutrients and bioactive compounds, may help slow cognitive decline associated with aging.
To more specifically study the relationship between kaempferol and other flavonols and the development of AD, Dr. Holland and colleagues evaluated data for MAP participants who had completed a comprehensive food frequency questionnaire and underwent at least two evaluations to assess incidence of disease.
The mean age of the 921 individuals in the present analysis was 81 years, three-quarters were female, and over approximately 6 years of follow-up, 220 developed AD.
The rate of developing AD was 48% lower among participants reporting the highest total dietary intake of flavonols, compared with those reporting the lowest intake, Dr. Holland and coauthors reported.
Intake of the specific flavonols kaempferol, myricetin, and isorhamnetin were associated with incident AD reductions of 50%, 38%, and 38%, respectively. Another flavonol, quercetin, was by contrast not inversely associated with incident AD, according to the report.
Kaempferol was independently associated with AD in subsequent analyses, while there was no such independent association for myricetin, isorhamnetin, or quercetin, according to Dr. Holland and coinvestigators.
Further analyses of the data suggested the linkages between flavonols and AD were independent of lifestyle factors, dietary intakes, or cardiovascular conditions, they said in their report.
“Confirmation of these findings is warranted through other longitudinal epidemiologic studies and clinical trials, in addition to further elucidation of the biologic mechanisms,” they concluded.
The study was funded by grants from the National Institutes of Health and the USDA Agricultural Research Service. Dr. Holland and coauthors said that they had no disclosures relevant to their report.
SOURCE: Holland TM et al. Neurology. 2020 Jan 29. doi: 10.1212/WNL.0000000000008981.
Onset of Alzheimer’s disease (AD) was inversely associated with intake of flavonols, a subclass of flavonoids with antioxidant and anti-inflammatory properties, according to the study authors.
The rate of developing AD was reduced by 50% among individuals reporting high intake of kaempferol, a flavonol plentiful in leafy green vegetables, and by 38% for high intake of the flavonols myricetin and isorhamnetin, researchers said in a report published in Neurology.
The findings are from the Rush Memory and Aging Project (MAP), a large, prospective study of older individuals in retirement communities and public housing in the Chicago area that has been ongoing since 1997.
“Although there is more work to be done, the associations that we observed are promising and deserve further study,” said Thomas M. Holland, MD, of the Rush Institute for Healthy Aging in Chicago, and coauthors.
Those associations between flavonol intake and AD help set the stage for U.S. POINTER and other randomized, controlled trials that seek to evaluate the effects of dietary interventions in a more rigorous way, according to Laura D. Baker, PhD, associate professor of internal medicine at Wake Forest University, Winston-Salem, N.C.
“This kind of data helps us feel like we are looking in the right direction in the randomized, controlled trials,” Dr. Baker said in an interview.
Dr. Baker is an investigator in the U.S. POINTER study, which will in part evaluate the impact of the MIND diet, which has been shown to slow cognitive decline with age in a previously published MAP study.
However, in the absence of randomized, controlled trial data, Dr. Baker cautioned against “prematurely advocating” for specific dietary approaches when speaking to patients and caregivers now.
“What I say is, we know for sure that the standard American Heart Association diet has been shown in clinical trials to reduce the risk of heart disease, and in terms of brain health, if you can reduce risk of heart disease, you are protecting your brain,” she said in the interview.
The present MAP study linking a reduced rate of AD to flavonol consumption is believed to be the first of its kind, though two previous studies from the early 2000s did find inverse associations between incident AD and intake of flavonoids, of which flavonoids are just one subclass, said Dr. Holland and coinvestigators in their report.
Moreover, in a MAP study published in 2018, Martha Clare Morris, ScD, and coauthors concluded that consuming about a serving per day of green leafy vegetables and foods rich in kaempferol, among other nutrients and bioactive compounds, may help slow cognitive decline associated with aging.
To more specifically study the relationship between kaempferol and other flavonols and the development of AD, Dr. Holland and colleagues evaluated data for MAP participants who had completed a comprehensive food frequency questionnaire and underwent at least two evaluations to assess incidence of disease.
The mean age of the 921 individuals in the present analysis was 81 years, three-quarters were female, and over approximately 6 years of follow-up, 220 developed AD.
The rate of developing AD was 48% lower among participants reporting the highest total dietary intake of flavonols, compared with those reporting the lowest intake, Dr. Holland and coauthors reported.
Intake of the specific flavonols kaempferol, myricetin, and isorhamnetin were associated with incident AD reductions of 50%, 38%, and 38%, respectively. Another flavonol, quercetin, was by contrast not inversely associated with incident AD, according to the report.
Kaempferol was independently associated with AD in subsequent analyses, while there was no such independent association for myricetin, isorhamnetin, or quercetin, according to Dr. Holland and coinvestigators.
Further analyses of the data suggested the linkages between flavonols and AD were independent of lifestyle factors, dietary intakes, or cardiovascular conditions, they said in their report.
“Confirmation of these findings is warranted through other longitudinal epidemiologic studies and clinical trials, in addition to further elucidation of the biologic mechanisms,” they concluded.
The study was funded by grants from the National Institutes of Health and the USDA Agricultural Research Service. Dr. Holland and coauthors said that they had no disclosures relevant to their report.
SOURCE: Holland TM et al. Neurology. 2020 Jan 29. doi: 10.1212/WNL.0000000000008981.
Onset of Alzheimer’s disease (AD) was inversely associated with intake of flavonols, a subclass of flavonoids with antioxidant and anti-inflammatory properties, according to the study authors.
The rate of developing AD was reduced by 50% among individuals reporting high intake of kaempferol, a flavonol plentiful in leafy green vegetables, and by 38% for high intake of the flavonols myricetin and isorhamnetin, researchers said in a report published in Neurology.
The findings are from the Rush Memory and Aging Project (MAP), a large, prospective study of older individuals in retirement communities and public housing in the Chicago area that has been ongoing since 1997.
“Although there is more work to be done, the associations that we observed are promising and deserve further study,” said Thomas M. Holland, MD, of the Rush Institute for Healthy Aging in Chicago, and coauthors.
Those associations between flavonol intake and AD help set the stage for U.S. POINTER and other randomized, controlled trials that seek to evaluate the effects of dietary interventions in a more rigorous way, according to Laura D. Baker, PhD, associate professor of internal medicine at Wake Forest University, Winston-Salem, N.C.
“This kind of data helps us feel like we are looking in the right direction in the randomized, controlled trials,” Dr. Baker said in an interview.
Dr. Baker is an investigator in the U.S. POINTER study, which will in part evaluate the impact of the MIND diet, which has been shown to slow cognitive decline with age in a previously published MAP study.
However, in the absence of randomized, controlled trial data, Dr. Baker cautioned against “prematurely advocating” for specific dietary approaches when speaking to patients and caregivers now.
“What I say is, we know for sure that the standard American Heart Association diet has been shown in clinical trials to reduce the risk of heart disease, and in terms of brain health, if you can reduce risk of heart disease, you are protecting your brain,” she said in the interview.
The present MAP study linking a reduced rate of AD to flavonol consumption is believed to be the first of its kind, though two previous studies from the early 2000s did find inverse associations between incident AD and intake of flavonoids, of which flavonoids are just one subclass, said Dr. Holland and coinvestigators in their report.
Moreover, in a MAP study published in 2018, Martha Clare Morris, ScD, and coauthors concluded that consuming about a serving per day of green leafy vegetables and foods rich in kaempferol, among other nutrients and bioactive compounds, may help slow cognitive decline associated with aging.
To more specifically study the relationship between kaempferol and other flavonols and the development of AD, Dr. Holland and colleagues evaluated data for MAP participants who had completed a comprehensive food frequency questionnaire and underwent at least two evaluations to assess incidence of disease.
The mean age of the 921 individuals in the present analysis was 81 years, three-quarters were female, and over approximately 6 years of follow-up, 220 developed AD.
The rate of developing AD was 48% lower among participants reporting the highest total dietary intake of flavonols, compared with those reporting the lowest intake, Dr. Holland and coauthors reported.
Intake of the specific flavonols kaempferol, myricetin, and isorhamnetin were associated with incident AD reductions of 50%, 38%, and 38%, respectively. Another flavonol, quercetin, was by contrast not inversely associated with incident AD, according to the report.
Kaempferol was independently associated with AD in subsequent analyses, while there was no such independent association for myricetin, isorhamnetin, or quercetin, according to Dr. Holland and coinvestigators.
Further analyses of the data suggested the linkages between flavonols and AD were independent of lifestyle factors, dietary intakes, or cardiovascular conditions, they said in their report.
“Confirmation of these findings is warranted through other longitudinal epidemiologic studies and clinical trials, in addition to further elucidation of the biologic mechanisms,” they concluded.
The study was funded by grants from the National Institutes of Health and the USDA Agricultural Research Service. Dr. Holland and coauthors said that they had no disclosures relevant to their report.
SOURCE: Holland TM et al. Neurology. 2020 Jan 29. doi: 10.1212/WNL.0000000000008981.
FROM NEUROLOGY
Genetic factor linked to impaired memory after heading many soccer balls
according to authors of a recent longitudinal study. Worse verbal memory was linked to high levels of ball heading among those players who were APOE e4–positive, compared with those who were APOE e4–negative, according to the authors, led by Liane E. Hunter, PhD, of the Gruss Magnetic Resonance Imaging Center at Albert Einstein College of Medicine, New York.
These findings, while preliminary, do raise the possibility that “safe levels for soccer heading” could be proposed to protect players from harm or that APOE e4-positive players might be advised to limit their exposure to head impacts, Dr. Hunter and coauthors wrote in a report in JAMA Neurology.
However, the findings should “in no way” be used to justify APOE testing to make clinical decisions regarding the safety of playing soccer, said Sarah J. Banks, PhD, of the University of California, San Diego, and Jesse Mez, MD, of Boston University in a related editorial (doi: 10.1001/jamaneurol.2019.4451). “Like most good science, the study provides an important, but incremental, step to understanding gene-environment interactions in sports,” Dr. Banks and Dr. Mez wrote in their editorial.
While there are some studies tying APOE e4 to poorer neuropsychiatric performance in boxers and U.S. football players, there are no such studies looking at the role of APOE e4 in soccer players exposed to repetitive “subconcussive” ball heading, according to Dr. Hunter and coresearchers. Accordingly, they sought to analyze APOE e4 and neuropsychological performance in relation to ball heading in 352 adult amateur soccer players enrolled in the Einstein Soccer Study between November 2013 and January 2018. About three-quarters of the players were male, and the median age at enrollment was 23 years.
The players completed a computer-based questionnaire designed to estimate their exposure to soccer heading at enrollment and at follow-up visits every 3-6 months. To test verbal memory at each visit, players were asked to memorize a 12-item grocery list, and then asked to recall the items 20 minutes later.
High levels of heading were linked to poorer performance on the verbal memory task, similar to one previously reported study, investigators said.
There was no association overall of APOE e4 and heading with performance on the shopping list task, according to investigators. By contrast, there was a 4.1-fold increased deficit in verbal memory for APOE e4–positive players with high heading exposure, compared with those with low exposure, investigators reported. Likewise, there was an 8.5-fold increased deficit in verbal memory for APOE e4–positive players with high versus moderate heading exposure.
That said, the absolute difference in performance was “subtle” and difficult to interpret in the context of a cross-sectional study, Dr. Banks and Dr. Mez said in their editorial.
In absolute terms, the mean decrease in scores on the 13-point shopping list task between the high and low heading exposure was 1.13 points greater for the APOE e4–positive group, compared with the APOE e4–negative group, and the decrease between the high and moderate heading exposure groups was 0.98 points greater, according to the report.
“The effect size of our interaction is relatively small,” Dr. Hunter and colleagues acknowledged in their report. “However, similar to the widely cited model of disease evolution in Alzheimer disease, our findings may be evidence of early subclinical effects, which could accumulate in APOE e4–positive players over a protracted time frame and ultimately be associated with overt clinical dysfunction.”
Several study authors said they had received grants from the National Institutes of Health and affiliated institutes, the Migraine Research Foundation, and the National Headache Foundation. They reported disclosures related to Amgen, Avanir, Biohaven Holdings, Biovision, Boston Scientific, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, and Pfizer, among others.
SOURCE: Hunter LE et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4828.
according to authors of a recent longitudinal study. Worse verbal memory was linked to high levels of ball heading among those players who were APOE e4–positive, compared with those who were APOE e4–negative, according to the authors, led by Liane E. Hunter, PhD, of the Gruss Magnetic Resonance Imaging Center at Albert Einstein College of Medicine, New York.
These findings, while preliminary, do raise the possibility that “safe levels for soccer heading” could be proposed to protect players from harm or that APOE e4-positive players might be advised to limit their exposure to head impacts, Dr. Hunter and coauthors wrote in a report in JAMA Neurology.
However, the findings should “in no way” be used to justify APOE testing to make clinical decisions regarding the safety of playing soccer, said Sarah J. Banks, PhD, of the University of California, San Diego, and Jesse Mez, MD, of Boston University in a related editorial (doi: 10.1001/jamaneurol.2019.4451). “Like most good science, the study provides an important, but incremental, step to understanding gene-environment interactions in sports,” Dr. Banks and Dr. Mez wrote in their editorial.
While there are some studies tying APOE e4 to poorer neuropsychiatric performance in boxers and U.S. football players, there are no such studies looking at the role of APOE e4 in soccer players exposed to repetitive “subconcussive” ball heading, according to Dr. Hunter and coresearchers. Accordingly, they sought to analyze APOE e4 and neuropsychological performance in relation to ball heading in 352 adult amateur soccer players enrolled in the Einstein Soccer Study between November 2013 and January 2018. About three-quarters of the players were male, and the median age at enrollment was 23 years.
The players completed a computer-based questionnaire designed to estimate their exposure to soccer heading at enrollment and at follow-up visits every 3-6 months. To test verbal memory at each visit, players were asked to memorize a 12-item grocery list, and then asked to recall the items 20 minutes later.
High levels of heading were linked to poorer performance on the verbal memory task, similar to one previously reported study, investigators said.
There was no association overall of APOE e4 and heading with performance on the shopping list task, according to investigators. By contrast, there was a 4.1-fold increased deficit in verbal memory for APOE e4–positive players with high heading exposure, compared with those with low exposure, investigators reported. Likewise, there was an 8.5-fold increased deficit in verbal memory for APOE e4–positive players with high versus moderate heading exposure.
That said, the absolute difference in performance was “subtle” and difficult to interpret in the context of a cross-sectional study, Dr. Banks and Dr. Mez said in their editorial.
In absolute terms, the mean decrease in scores on the 13-point shopping list task between the high and low heading exposure was 1.13 points greater for the APOE e4–positive group, compared with the APOE e4–negative group, and the decrease between the high and moderate heading exposure groups was 0.98 points greater, according to the report.
“The effect size of our interaction is relatively small,” Dr. Hunter and colleagues acknowledged in their report. “However, similar to the widely cited model of disease evolution in Alzheimer disease, our findings may be evidence of early subclinical effects, which could accumulate in APOE e4–positive players over a protracted time frame and ultimately be associated with overt clinical dysfunction.”
Several study authors said they had received grants from the National Institutes of Health and affiliated institutes, the Migraine Research Foundation, and the National Headache Foundation. They reported disclosures related to Amgen, Avanir, Biohaven Holdings, Biovision, Boston Scientific, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, and Pfizer, among others.
SOURCE: Hunter LE et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4828.
according to authors of a recent longitudinal study. Worse verbal memory was linked to high levels of ball heading among those players who were APOE e4–positive, compared with those who were APOE e4–negative, according to the authors, led by Liane E. Hunter, PhD, of the Gruss Magnetic Resonance Imaging Center at Albert Einstein College of Medicine, New York.
These findings, while preliminary, do raise the possibility that “safe levels for soccer heading” could be proposed to protect players from harm or that APOE e4-positive players might be advised to limit their exposure to head impacts, Dr. Hunter and coauthors wrote in a report in JAMA Neurology.
However, the findings should “in no way” be used to justify APOE testing to make clinical decisions regarding the safety of playing soccer, said Sarah J. Banks, PhD, of the University of California, San Diego, and Jesse Mez, MD, of Boston University in a related editorial (doi: 10.1001/jamaneurol.2019.4451). “Like most good science, the study provides an important, but incremental, step to understanding gene-environment interactions in sports,” Dr. Banks and Dr. Mez wrote in their editorial.
While there are some studies tying APOE e4 to poorer neuropsychiatric performance in boxers and U.S. football players, there are no such studies looking at the role of APOE e4 in soccer players exposed to repetitive “subconcussive” ball heading, according to Dr. Hunter and coresearchers. Accordingly, they sought to analyze APOE e4 and neuropsychological performance in relation to ball heading in 352 adult amateur soccer players enrolled in the Einstein Soccer Study between November 2013 and January 2018. About three-quarters of the players were male, and the median age at enrollment was 23 years.
The players completed a computer-based questionnaire designed to estimate their exposure to soccer heading at enrollment and at follow-up visits every 3-6 months. To test verbal memory at each visit, players were asked to memorize a 12-item grocery list, and then asked to recall the items 20 minutes later.
High levels of heading were linked to poorer performance on the verbal memory task, similar to one previously reported study, investigators said.
There was no association overall of APOE e4 and heading with performance on the shopping list task, according to investigators. By contrast, there was a 4.1-fold increased deficit in verbal memory for APOE e4–positive players with high heading exposure, compared with those with low exposure, investigators reported. Likewise, there was an 8.5-fold increased deficit in verbal memory for APOE e4–positive players with high versus moderate heading exposure.
That said, the absolute difference in performance was “subtle” and difficult to interpret in the context of a cross-sectional study, Dr. Banks and Dr. Mez said in their editorial.
In absolute terms, the mean decrease in scores on the 13-point shopping list task between the high and low heading exposure was 1.13 points greater for the APOE e4–positive group, compared with the APOE e4–negative group, and the decrease between the high and moderate heading exposure groups was 0.98 points greater, according to the report.
“The effect size of our interaction is relatively small,” Dr. Hunter and colleagues acknowledged in their report. “However, similar to the widely cited model of disease evolution in Alzheimer disease, our findings may be evidence of early subclinical effects, which could accumulate in APOE e4–positive players over a protracted time frame and ultimately be associated with overt clinical dysfunction.”
Several study authors said they had received grants from the National Institutes of Health and affiliated institutes, the Migraine Research Foundation, and the National Headache Foundation. They reported disclosures related to Amgen, Avanir, Biohaven Holdings, Biovision, Boston Scientific, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, and Pfizer, among others.
SOURCE: Hunter LE et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4828.
FROM JAMA Neurology
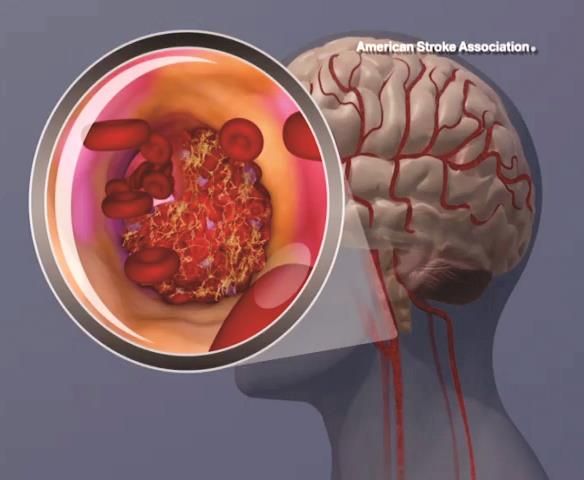
BP levels during endovascular stroke therapy affect neurologic outcomes
For patients with acute ischemic stroke, prolonged durations of blood pressure above or below certain thresholds during endovascular therapy may be linked to poor functional outcome, results of a retrospective study suggest.
Mean arterial blood pressure (MABP) lower than 70 mm Hg for 10 minutes or more, or higher than 90 mm Hg for 45 minutes or more, represented “critical thresholds” associated with worse neurologic outcomes, the study authors wrote in JAMA Neurology.
“These results suggest MABP may be a modifiable therapeutic target to prevent or reduce poor functional outcome in patients undergoing endovascular therapy for acute ischemic stroke, and that MABP should possibly be maintained within such narrow limits, wrote the authors, led by Mads Rasmussen, MD, PhD, of the department of anesthesia at Aarhus (Denmark) University Hospital.
The findings come from an analysis of BP data from 365 patients with acute ischemic stroke enrolled in three randomized trials evaluating different strategies for anesthesia. Among those patients, the mean age was approximately 71 years, and about 45% were women.
The investigators looked at a variety of BP-related variables during endovascular therapy to assess their impact on functional outcome, based on modified Rankin Scale (mRS) scores at 90 days.
Having an MABP below 70 mm Hg for a cumulative time of at least 10 minutes substantially increased odds of higher 90-day mRS scores (odds ratio, 1.51; 95% confidence interval, 1.02-2.22), according to Dr. Rasmussen and colleagues. The number needed to harm (NNH) at this threshold was 10; in other words, to harm 1 patient, 10 patients are needed with procedural MABP below 70 mm Hg for at least 10 minutes.
Likewise, having an MABP above 90 mm Hg for a cumulated time of at least 45 minutes significantly increased odds of higher 90-day mRS scores, with an OR of 1.49 (95% CI, 1.11-2.02) and a number needed to harm of 10.
Odds of shifting toward a worse neurologic outcome increased by 62% for every continuous 10 minutes of MABP below 70 mm Hg, and by 8% for every continuous 10 minutes above 90 mm Hg.
The maximum MABP during the procedure was significantly associated with neurologic outcomes in the study, while by contrast, maximum procedural systolic BP was not, according to the investigators.
In general, the study findings suggest that MABP is “more sensitive” than systolic BP when assessing hypotension and hypertension in these patients. However, these findings are subject to a number of limitations, the investigators wrote, including the retrospective nature of the analysis and the selected group of patients enrolled in studies designed to evaluate anesthesia strategies, not hemodynamic management.
“Randomized studies are needed to determine the optimal blood pressure management strategy during endovascular therapy,” the investigators wrote.
Dr. Rasmussen reported grant support from the Health Research Foundation of Central Denmark Region and the National Helicopter Emergency Medical Service Foundation. Coauthors reported receiving grant support from the Novo Nordisk Foundation; a research award from the Patient-Centered Outcomes Research Institute; and personal fees from Abbott Medical Sweden, I4L Innovation for Life, Boehringer Ingelheim, Medtronic, and Zoll.
SOURCE: Rasmussen M et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4838.
For patients with acute ischemic stroke, prolonged durations of blood pressure above or below certain thresholds during endovascular therapy may be linked to poor functional outcome, results of a retrospective study suggest.
Mean arterial blood pressure (MABP) lower than 70 mm Hg for 10 minutes or more, or higher than 90 mm Hg for 45 minutes or more, represented “critical thresholds” associated with worse neurologic outcomes, the study authors wrote in JAMA Neurology.
“These results suggest MABP may be a modifiable therapeutic target to prevent or reduce poor functional outcome in patients undergoing endovascular therapy for acute ischemic stroke, and that MABP should possibly be maintained within such narrow limits, wrote the authors, led by Mads Rasmussen, MD, PhD, of the department of anesthesia at Aarhus (Denmark) University Hospital.
The findings come from an analysis of BP data from 365 patients with acute ischemic stroke enrolled in three randomized trials evaluating different strategies for anesthesia. Among those patients, the mean age was approximately 71 years, and about 45% were women.
The investigators looked at a variety of BP-related variables during endovascular therapy to assess their impact on functional outcome, based on modified Rankin Scale (mRS) scores at 90 days.
Having an MABP below 70 mm Hg for a cumulative time of at least 10 minutes substantially increased odds of higher 90-day mRS scores (odds ratio, 1.51; 95% confidence interval, 1.02-2.22), according to Dr. Rasmussen and colleagues. The number needed to harm (NNH) at this threshold was 10; in other words, to harm 1 patient, 10 patients are needed with procedural MABP below 70 mm Hg for at least 10 minutes.
Likewise, having an MABP above 90 mm Hg for a cumulated time of at least 45 minutes significantly increased odds of higher 90-day mRS scores, with an OR of 1.49 (95% CI, 1.11-2.02) and a number needed to harm of 10.
Odds of shifting toward a worse neurologic outcome increased by 62% for every continuous 10 minutes of MABP below 70 mm Hg, and by 8% for every continuous 10 minutes above 90 mm Hg.
The maximum MABP during the procedure was significantly associated with neurologic outcomes in the study, while by contrast, maximum procedural systolic BP was not, according to the investigators.
In general, the study findings suggest that MABP is “more sensitive” than systolic BP when assessing hypotension and hypertension in these patients. However, these findings are subject to a number of limitations, the investigators wrote, including the retrospective nature of the analysis and the selected group of patients enrolled in studies designed to evaluate anesthesia strategies, not hemodynamic management.
“Randomized studies are needed to determine the optimal blood pressure management strategy during endovascular therapy,” the investigators wrote.
Dr. Rasmussen reported grant support from the Health Research Foundation of Central Denmark Region and the National Helicopter Emergency Medical Service Foundation. Coauthors reported receiving grant support from the Novo Nordisk Foundation; a research award from the Patient-Centered Outcomes Research Institute; and personal fees from Abbott Medical Sweden, I4L Innovation for Life, Boehringer Ingelheim, Medtronic, and Zoll.
SOURCE: Rasmussen M et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4838.
For patients with acute ischemic stroke, prolonged durations of blood pressure above or below certain thresholds during endovascular therapy may be linked to poor functional outcome, results of a retrospective study suggest.
Mean arterial blood pressure (MABP) lower than 70 mm Hg for 10 minutes or more, or higher than 90 mm Hg for 45 minutes or more, represented “critical thresholds” associated with worse neurologic outcomes, the study authors wrote in JAMA Neurology.
“These results suggest MABP may be a modifiable therapeutic target to prevent or reduce poor functional outcome in patients undergoing endovascular therapy for acute ischemic stroke, and that MABP should possibly be maintained within such narrow limits, wrote the authors, led by Mads Rasmussen, MD, PhD, of the department of anesthesia at Aarhus (Denmark) University Hospital.
The findings come from an analysis of BP data from 365 patients with acute ischemic stroke enrolled in three randomized trials evaluating different strategies for anesthesia. Among those patients, the mean age was approximately 71 years, and about 45% were women.
The investigators looked at a variety of BP-related variables during endovascular therapy to assess their impact on functional outcome, based on modified Rankin Scale (mRS) scores at 90 days.
Having an MABP below 70 mm Hg for a cumulative time of at least 10 minutes substantially increased odds of higher 90-day mRS scores (odds ratio, 1.51; 95% confidence interval, 1.02-2.22), according to Dr. Rasmussen and colleagues. The number needed to harm (NNH) at this threshold was 10; in other words, to harm 1 patient, 10 patients are needed with procedural MABP below 70 mm Hg for at least 10 minutes.
Likewise, having an MABP above 90 mm Hg for a cumulated time of at least 45 minutes significantly increased odds of higher 90-day mRS scores, with an OR of 1.49 (95% CI, 1.11-2.02) and a number needed to harm of 10.
Odds of shifting toward a worse neurologic outcome increased by 62% for every continuous 10 minutes of MABP below 70 mm Hg, and by 8% for every continuous 10 minutes above 90 mm Hg.
The maximum MABP during the procedure was significantly associated with neurologic outcomes in the study, while by contrast, maximum procedural systolic BP was not, according to the investigators.
In general, the study findings suggest that MABP is “more sensitive” than systolic BP when assessing hypotension and hypertension in these patients. However, these findings are subject to a number of limitations, the investigators wrote, including the retrospective nature of the analysis and the selected group of patients enrolled in studies designed to evaluate anesthesia strategies, not hemodynamic management.
“Randomized studies are needed to determine the optimal blood pressure management strategy during endovascular therapy,” the investigators wrote.
Dr. Rasmussen reported grant support from the Health Research Foundation of Central Denmark Region and the National Helicopter Emergency Medical Service Foundation. Coauthors reported receiving grant support from the Novo Nordisk Foundation; a research award from the Patient-Centered Outcomes Research Institute; and personal fees from Abbott Medical Sweden, I4L Innovation for Life, Boehringer Ingelheim, Medtronic, and Zoll.
SOURCE: Rasmussen M et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4838.
FROM JAMA NEUROLOGY
T-VEC plus pembrolizumab yields promising response rate in phase 2 sarcoma study
The combination of oncolytic immunotherapy and an immune checkpoint inhibitor had promising activity in a phase 2 study including patients with previously treated advanced sarcoma, investigators report.
Responses were seen in about one-third of the patients (30%) at 24 weeks after starting treatment with talimogene laherparepvec (T-VEC) and pembrolizumab, according to the investigators, led by Ciara M. Kelly, MBBChBAO, MD, of the department of sarcoma oncology at Memorial Sloan Kettering Cancer Center, New York.
“To our knowledge, this is one of the highest ORRs [objective response rates] reported in an unselected sarcoma-specific study population evaluating the efficacy of combination immunotherapy,” Dr. Kelly and coauthors wrote in JAMA Oncology.
Two of the patients who responded to the combination had previously failed regimens that included an immune checkpoint inhibitor, suggesting T-VEC might be augmenting the efficacy of pembrolizumab, according to the authors.
Among responders, the mean number of prior treatments was one, while by contrast, most of the study cohort (60%) had three or more prior treatments. “This finding supports the rationale to enroll patients with sarcoma in immunotherapy trials earlier in their treatment course,” Dr. Kelly and colleagues wrote.
In studies of immunotherapy in Merkel cell carcinoma and other cancer types, higher response rates have been seen in patients who were naive to chemotherapy, as opposed to those who were refractory to it.
The present single-institution, phase 2 study by Dr. Kelly and colleagues included 20 adult patients with histologically confirmed locally advanced or metastatic sarcoma. The patients received a fixed dose of pembrolizumab administered intravenously, and had T-VEC injected into palpable lesions, on day 1 of 21-day cycles.
While the ORR was 30% at 24 weeks (7 of 35 patients), an additional patient had a response at 32 weeks, bringing the ORR to 35%, according to the report.
“Maximal response to therapy may take a prolonged period to achieve,” the investigators wrote, noting that the median time to response was 14.4 weeks.
Median duration of response was 56.1 weeks, suggesting the combination provided durable disease control, they added.
Safety was consistent with what was seen in a previous study of T-VEC and pembrolizumab in melanoma. In this sarcoma study, the incidence of grade 3 treatment-related adverse events was 20%, while in studies of conventional chemotherapy in this setting, the rate of those events has been “comparable but generally higher,” the investigators wrote.
Dr. Kelly and colleagues wrote that a randomized clinical trial is the “ideal next step” in the development of the regimen, adding that an expansion of the present phase 2 study is in progress.
The study was funded by Amgen and Merck, with additional grant support from Cycle for Survival and the National Institutes of Health/National Cancer Institute. Dr. Kelly reported disclosures related to Merck, Amgen, Agios Pharmaceuticals, and Exicure. Her coauthors reported disclosures with Regeneron, Novartis, Takeda, and Nektar, among others.
SOURCE: Kelly CM et al. JAMA Oncol. 2020 Jan 23. doi: 10.1001/jamaoncol.2019.6152.
The combination of oncolytic immunotherapy and an immune checkpoint inhibitor had promising activity in a phase 2 study including patients with previously treated advanced sarcoma, investigators report.
Responses were seen in about one-third of the patients (30%) at 24 weeks after starting treatment with talimogene laherparepvec (T-VEC) and pembrolizumab, according to the investigators, led by Ciara M. Kelly, MBBChBAO, MD, of the department of sarcoma oncology at Memorial Sloan Kettering Cancer Center, New York.
“To our knowledge, this is one of the highest ORRs [objective response rates] reported in an unselected sarcoma-specific study population evaluating the efficacy of combination immunotherapy,” Dr. Kelly and coauthors wrote in JAMA Oncology.
Two of the patients who responded to the combination had previously failed regimens that included an immune checkpoint inhibitor, suggesting T-VEC might be augmenting the efficacy of pembrolizumab, according to the authors.
Among responders, the mean number of prior treatments was one, while by contrast, most of the study cohort (60%) had three or more prior treatments. “This finding supports the rationale to enroll patients with sarcoma in immunotherapy trials earlier in their treatment course,” Dr. Kelly and colleagues wrote.
In studies of immunotherapy in Merkel cell carcinoma and other cancer types, higher response rates have been seen in patients who were naive to chemotherapy, as opposed to those who were refractory to it.
The present single-institution, phase 2 study by Dr. Kelly and colleagues included 20 adult patients with histologically confirmed locally advanced or metastatic sarcoma. The patients received a fixed dose of pembrolizumab administered intravenously, and had T-VEC injected into palpable lesions, on day 1 of 21-day cycles.
While the ORR was 30% at 24 weeks (7 of 35 patients), an additional patient had a response at 32 weeks, bringing the ORR to 35%, according to the report.
“Maximal response to therapy may take a prolonged period to achieve,” the investigators wrote, noting that the median time to response was 14.4 weeks.
Median duration of response was 56.1 weeks, suggesting the combination provided durable disease control, they added.
Safety was consistent with what was seen in a previous study of T-VEC and pembrolizumab in melanoma. In this sarcoma study, the incidence of grade 3 treatment-related adverse events was 20%, while in studies of conventional chemotherapy in this setting, the rate of those events has been “comparable but generally higher,” the investigators wrote.
Dr. Kelly and colleagues wrote that a randomized clinical trial is the “ideal next step” in the development of the regimen, adding that an expansion of the present phase 2 study is in progress.
The study was funded by Amgen and Merck, with additional grant support from Cycle for Survival and the National Institutes of Health/National Cancer Institute. Dr. Kelly reported disclosures related to Merck, Amgen, Agios Pharmaceuticals, and Exicure. Her coauthors reported disclosures with Regeneron, Novartis, Takeda, and Nektar, among others.
SOURCE: Kelly CM et al. JAMA Oncol. 2020 Jan 23. doi: 10.1001/jamaoncol.2019.6152.
The combination of oncolytic immunotherapy and an immune checkpoint inhibitor had promising activity in a phase 2 study including patients with previously treated advanced sarcoma, investigators report.
Responses were seen in about one-third of the patients (30%) at 24 weeks after starting treatment with talimogene laherparepvec (T-VEC) and pembrolizumab, according to the investigators, led by Ciara M. Kelly, MBBChBAO, MD, of the department of sarcoma oncology at Memorial Sloan Kettering Cancer Center, New York.
“To our knowledge, this is one of the highest ORRs [objective response rates] reported in an unselected sarcoma-specific study population evaluating the efficacy of combination immunotherapy,” Dr. Kelly and coauthors wrote in JAMA Oncology.
Two of the patients who responded to the combination had previously failed regimens that included an immune checkpoint inhibitor, suggesting T-VEC might be augmenting the efficacy of pembrolizumab, according to the authors.
Among responders, the mean number of prior treatments was one, while by contrast, most of the study cohort (60%) had three or more prior treatments. “This finding supports the rationale to enroll patients with sarcoma in immunotherapy trials earlier in their treatment course,” Dr. Kelly and colleagues wrote.
In studies of immunotherapy in Merkel cell carcinoma and other cancer types, higher response rates have been seen in patients who were naive to chemotherapy, as opposed to those who were refractory to it.
The present single-institution, phase 2 study by Dr. Kelly and colleagues included 20 adult patients with histologically confirmed locally advanced or metastatic sarcoma. The patients received a fixed dose of pembrolizumab administered intravenously, and had T-VEC injected into palpable lesions, on day 1 of 21-day cycles.
While the ORR was 30% at 24 weeks (7 of 35 patients), an additional patient had a response at 32 weeks, bringing the ORR to 35%, according to the report.
“Maximal response to therapy may take a prolonged period to achieve,” the investigators wrote, noting that the median time to response was 14.4 weeks.
Median duration of response was 56.1 weeks, suggesting the combination provided durable disease control, they added.
Safety was consistent with what was seen in a previous study of T-VEC and pembrolizumab in melanoma. In this sarcoma study, the incidence of grade 3 treatment-related adverse events was 20%, while in studies of conventional chemotherapy in this setting, the rate of those events has been “comparable but generally higher,” the investigators wrote.
Dr. Kelly and colleagues wrote that a randomized clinical trial is the “ideal next step” in the development of the regimen, adding that an expansion of the present phase 2 study is in progress.
The study was funded by Amgen and Merck, with additional grant support from Cycle for Survival and the National Institutes of Health/National Cancer Institute. Dr. Kelly reported disclosures related to Merck, Amgen, Agios Pharmaceuticals, and Exicure. Her coauthors reported disclosures with Regeneron, Novartis, Takeda, and Nektar, among others.
SOURCE: Kelly CM et al. JAMA Oncol. 2020 Jan 23. doi: 10.1001/jamaoncol.2019.6152.
FROM JAMA ONCOLOGY
Start of myeloma therapy may be delayed for women, minorities
Women and racial minorities with multiple myeloma may be at increased risk of delayed treatment, a situation that should be addressed urgently, according to authors of a recent analysis of a clinical oncology database.
By contrast, patients receiving myeloma treatment sooner after diagnosis included patients who were over 80 years of age, had multiple comorbidities, were treated at specialized cancer programs or in areas other than the Northeast, and had Medicaid or did not have private insurance, the authors reported.
Contrary to what was expected, levels of education and income did not significantly affect the timeliness of treatment in this analysis by Vivek Kumar, MD, of Dana-Farber Cancer Institute in Boston and coinvestigators.
While results of studies to date are “conflicting” as to whether timeliness of myeloma therapy will affect patient outcomes, recent studies in breast cancer and other tumor types suggest earlier treatment intervention may reduce morbidity, improve quality of life, and possibly prolong survival, according to Dr. Kumar and colleagues.
Moreover, the focus of myeloma treatment has shifted toward earlier treatment in light of the superiority of today’s treatment options, which was demonstrated in the 2014 update of the International Myeloma Working Group (IMWG) diagnostic criteria, according to the investigators.
“The definition of active MM [multiple myeloma] has been updated so that patients who may have been considered to have smoldering MM previously are now treated sooner to prevent end-organ damage whenever possible,” said Dr. Kumar and coauthors in their report in JCO Oncology Practice.
The analysis of timely myeloma treatment was based on for 74,722 patients in the National Cancer Database who received a diagnosis of multiple myeloma between 2004 and 2015 and went on to receive systemic treatment within the first year of diagnosis.
Delay in treatment, defined as receiving antimyeloma therapy 40 or more days after diagnosis, occurred in 18,375 of those patients, or about one-quarter of the study cohort. The mean time from diagnosis to start of treatment in that group was 63 days.
Compared with patients who received treatment within 7 days of diagnosis, patients with delays in treatment were more likely to be women (odds ratio, 1.15; 95% confidence interval, 1.1-1.2) and more likely to be non-Hispanic black (OR, 1.21; 95% CI, 1.14-1.28), the investigators reported.
A previous analysis of the SEER-Medicare database suggested that certain antimyeloma agents are used later in racial and ethnic minorities, including Hispanic patients, who had the highest median time to first dose of bortezomib, Dr. Kumar and colleagues noted.
However, no report before the present one had looked at the time to overall initial treatment in racial and ethnic minorities, they added.
Patients diagnosed in more recent years had higher odds of treatment delay, though this could have been caused by an increase in the number of patients diagnosed early; prior to the 2014 IMWG diagnostic criteria revision, many would have been offered therapy only when signs of end-organ damage were present, while patients without end-organ damage would have been said to have smoldering disease, authors said.
Patients 80 years of age and older and those with a higher Charlson comorbidity score had a lower likelihood of treatment delay in this analysis, possibly reflecting the frailty of those patients and an urgent need for treatment, according to investigators.
Uninsured patients and those with Medicaid were less likely than insured patients to experience treatment delay, according to the report.
“This may be associated with the fact that, for these insurances, prior authorization is typically not required before initiating treatment,” said Dr. Kumar and colleagues. “However, this could also depend on several other possible factors, including availability of caregiver support and seeking medical care later.”
Dr. Kumar reported no conflicts of interest related to the analysis. Coauthors reported disclosures with Takeda, Guardant Health, and other pharmaceutical companies.
SOURCE: Kumar V et al. JCO Oncology Practice. 2020 Jan 21. doi: 10.1200/JOP.19.00309.
Women and racial minorities with multiple myeloma may be at increased risk of delayed treatment, a situation that should be addressed urgently, according to authors of a recent analysis of a clinical oncology database.
By contrast, patients receiving myeloma treatment sooner after diagnosis included patients who were over 80 years of age, had multiple comorbidities, were treated at specialized cancer programs or in areas other than the Northeast, and had Medicaid or did not have private insurance, the authors reported.
Contrary to what was expected, levels of education and income did not significantly affect the timeliness of treatment in this analysis by Vivek Kumar, MD, of Dana-Farber Cancer Institute in Boston and coinvestigators.
While results of studies to date are “conflicting” as to whether timeliness of myeloma therapy will affect patient outcomes, recent studies in breast cancer and other tumor types suggest earlier treatment intervention may reduce morbidity, improve quality of life, and possibly prolong survival, according to Dr. Kumar and colleagues.
Moreover, the focus of myeloma treatment has shifted toward earlier treatment in light of the superiority of today’s treatment options, which was demonstrated in the 2014 update of the International Myeloma Working Group (IMWG) diagnostic criteria, according to the investigators.
“The definition of active MM [multiple myeloma] has been updated so that patients who may have been considered to have smoldering MM previously are now treated sooner to prevent end-organ damage whenever possible,” said Dr. Kumar and coauthors in their report in JCO Oncology Practice.
The analysis of timely myeloma treatment was based on for 74,722 patients in the National Cancer Database who received a diagnosis of multiple myeloma between 2004 and 2015 and went on to receive systemic treatment within the first year of diagnosis.
Delay in treatment, defined as receiving antimyeloma therapy 40 or more days after diagnosis, occurred in 18,375 of those patients, or about one-quarter of the study cohort. The mean time from diagnosis to start of treatment in that group was 63 days.
Compared with patients who received treatment within 7 days of diagnosis, patients with delays in treatment were more likely to be women (odds ratio, 1.15; 95% confidence interval, 1.1-1.2) and more likely to be non-Hispanic black (OR, 1.21; 95% CI, 1.14-1.28), the investigators reported.
A previous analysis of the SEER-Medicare database suggested that certain antimyeloma agents are used later in racial and ethnic minorities, including Hispanic patients, who had the highest median time to first dose of bortezomib, Dr. Kumar and colleagues noted.
However, no report before the present one had looked at the time to overall initial treatment in racial and ethnic minorities, they added.
Patients diagnosed in more recent years had higher odds of treatment delay, though this could have been caused by an increase in the number of patients diagnosed early; prior to the 2014 IMWG diagnostic criteria revision, many would have been offered therapy only when signs of end-organ damage were present, while patients without end-organ damage would have been said to have smoldering disease, authors said.
Patients 80 years of age and older and those with a higher Charlson comorbidity score had a lower likelihood of treatment delay in this analysis, possibly reflecting the frailty of those patients and an urgent need for treatment, according to investigators.
Uninsured patients and those with Medicaid were less likely than insured patients to experience treatment delay, according to the report.
“This may be associated with the fact that, for these insurances, prior authorization is typically not required before initiating treatment,” said Dr. Kumar and colleagues. “However, this could also depend on several other possible factors, including availability of caregiver support and seeking medical care later.”
Dr. Kumar reported no conflicts of interest related to the analysis. Coauthors reported disclosures with Takeda, Guardant Health, and other pharmaceutical companies.
SOURCE: Kumar V et al. JCO Oncology Practice. 2020 Jan 21. doi: 10.1200/JOP.19.00309.
Women and racial minorities with multiple myeloma may be at increased risk of delayed treatment, a situation that should be addressed urgently, according to authors of a recent analysis of a clinical oncology database.
By contrast, patients receiving myeloma treatment sooner after diagnosis included patients who were over 80 years of age, had multiple comorbidities, were treated at specialized cancer programs or in areas other than the Northeast, and had Medicaid or did not have private insurance, the authors reported.
Contrary to what was expected, levels of education and income did not significantly affect the timeliness of treatment in this analysis by Vivek Kumar, MD, of Dana-Farber Cancer Institute in Boston and coinvestigators.
While results of studies to date are “conflicting” as to whether timeliness of myeloma therapy will affect patient outcomes, recent studies in breast cancer and other tumor types suggest earlier treatment intervention may reduce morbidity, improve quality of life, and possibly prolong survival, according to Dr. Kumar and colleagues.
Moreover, the focus of myeloma treatment has shifted toward earlier treatment in light of the superiority of today’s treatment options, which was demonstrated in the 2014 update of the International Myeloma Working Group (IMWG) diagnostic criteria, according to the investigators.
“The definition of active MM [multiple myeloma] has been updated so that patients who may have been considered to have smoldering MM previously are now treated sooner to prevent end-organ damage whenever possible,” said Dr. Kumar and coauthors in their report in JCO Oncology Practice.
The analysis of timely myeloma treatment was based on for 74,722 patients in the National Cancer Database who received a diagnosis of multiple myeloma between 2004 and 2015 and went on to receive systemic treatment within the first year of diagnosis.
Delay in treatment, defined as receiving antimyeloma therapy 40 or more days after diagnosis, occurred in 18,375 of those patients, or about one-quarter of the study cohort. The mean time from diagnosis to start of treatment in that group was 63 days.
Compared with patients who received treatment within 7 days of diagnosis, patients with delays in treatment were more likely to be women (odds ratio, 1.15; 95% confidence interval, 1.1-1.2) and more likely to be non-Hispanic black (OR, 1.21; 95% CI, 1.14-1.28), the investigators reported.
A previous analysis of the SEER-Medicare database suggested that certain antimyeloma agents are used later in racial and ethnic minorities, including Hispanic patients, who had the highest median time to first dose of bortezomib, Dr. Kumar and colleagues noted.
However, no report before the present one had looked at the time to overall initial treatment in racial and ethnic minorities, they added.
Patients diagnosed in more recent years had higher odds of treatment delay, though this could have been caused by an increase in the number of patients diagnosed early; prior to the 2014 IMWG diagnostic criteria revision, many would have been offered therapy only when signs of end-organ damage were present, while patients without end-organ damage would have been said to have smoldering disease, authors said.
Patients 80 years of age and older and those with a higher Charlson comorbidity score had a lower likelihood of treatment delay in this analysis, possibly reflecting the frailty of those patients and an urgent need for treatment, according to investigators.
Uninsured patients and those with Medicaid were less likely than insured patients to experience treatment delay, according to the report.
“This may be associated with the fact that, for these insurances, prior authorization is typically not required before initiating treatment,” said Dr. Kumar and colleagues. “However, this could also depend on several other possible factors, including availability of caregiver support and seeking medical care later.”
Dr. Kumar reported no conflicts of interest related to the analysis. Coauthors reported disclosures with Takeda, Guardant Health, and other pharmaceutical companies.
SOURCE: Kumar V et al. JCO Oncology Practice. 2020 Jan 21. doi: 10.1200/JOP.19.00309.
FROM JCO ONCOLOGY PRACTICE
Key clinical point:
Major finding: Patients with delays in treatment were more likely to be women (odds ratio, 1.15) and more likely to be non-Hispanic blacks (OR, 1.21).
Study details: Retrospective analysis of 74,722 patients in the National Cancer Database diagnosed with multiple myeloma between 2004 and 2015.
Disclosures: Dr. Kumar reported no conflicts of interest related to the analysis. Coauthors reported disclosures with Takeda, Guardant Health, and other pharmaceutical companies.
Source: Kumar V et al. JCO Oncology Practice. 2020 Jan 21. doi: 10.1200/JOP.19.00309.
‘Simple’ model predicts severe neutropenia risk in lung cancer patients
A new and simple model for predicting risk of severe neutropenia in advanced lung cancer could ease the evaluation process and help inform patient management decisions, according to investigators.
The model, based on 10 pretreatment variables, appears to overcome limitations of a comprehensive risk prediction model that is not specific to lung cancer, according to Xiaowen Cao of Duke University, Durham, N.C., and coinvestigators.
“We believe that this model, once validated, will help oncologists accurately identify those patients with lung cancer who are at a high risk of developing severe neutropenia, based on simple, readily available information,” the researchers said in a report on their work, which appears in the journal Lung Cancer.
Oncologists could then make “proactive” decisions about monitoring of high-risk patients, modifying the dose of chemotherapy, and using prophylactic growth factors, the authors added.
Accurate, lung cancer–specific prediction models would be useful to estimate risk of neutropenia, which the investigators acknowledged as a serious chemotherapy-induced toxicity linked to life-threatening infections, dose delays, and reductions that can compromise treatment efficacy, and reduced health-related quality of life.
There are other, previously developed models to predict chemotherapy-induced neutropenia, but those have significant limitations, including development based on small patient sample sizes, according to the researchers.
A comprehensive risk model for neutropenic complications has been developed by Gary H. Lyman, MD, and colleagues, based on a large, prospective cohort including nearly 3,800 patients. That model performs well and had a 90% sensitivity and 96% predictive value; however, it’s not lung cancer specific, and hasn’t been externally validated, according to Ms. Cao and coauthors.
Accordingly, they set out to develop a new risk prediction model based on a lung cancer data set encompassing 11,352 patients from 67 phase 2 or 3 cooperative group studies conducted between 1991 and 2010.
The Lyman model in this data set had an area under the curve (AUC) of 0.8772 in patients with small cell lung cancer (SCLC), but an AUC of just 0.6787 in non–small cell lung cancer (NSCLC), suggesting “much better predictive performance in the SCLC,” the researchers noted.
They used stepwise logistic regression and lasso regression to develop a new model, which was derived based on about two-thirds of the patients, randomly selected, while the validation was conducted using the remaining third.
Variables included in the final model included age, gender, weight, BMI, insurance status, disease stage, number of metastatic sites, chemotherapy agents used, number of chemotherapy agents, planned growth factor use, duration of planned therapy, pleural effusion, presence of symptoms, and performance status.
That model had a good AUC, according to investigators, in both the training set and the testing set (0.8348 and 0.8234, respectively).
“It is worth noticing that our final model compensated for the deficiency of Lyman’s risk model in NSCLC patients,” the researchers noted in a discussion of their results.
The study was supported in part by grants from the National Institutes of Health, the National Center for Advancing Translational Sciences, and the Health and Medical Research Fund of Hong Kong. One study coauthor reported a conflict of interest outside the submitted work related to Genentech.
SOURCE: Cao X et al. Lung Cancer 2020 Jan 5. doi: 10.1016/j.lungcan.2020.01.004.
A new and simple model for predicting risk of severe neutropenia in advanced lung cancer could ease the evaluation process and help inform patient management decisions, according to investigators.
The model, based on 10 pretreatment variables, appears to overcome limitations of a comprehensive risk prediction model that is not specific to lung cancer, according to Xiaowen Cao of Duke University, Durham, N.C., and coinvestigators.
“We believe that this model, once validated, will help oncologists accurately identify those patients with lung cancer who are at a high risk of developing severe neutropenia, based on simple, readily available information,” the researchers said in a report on their work, which appears in the journal Lung Cancer.
Oncologists could then make “proactive” decisions about monitoring of high-risk patients, modifying the dose of chemotherapy, and using prophylactic growth factors, the authors added.
Accurate, lung cancer–specific prediction models would be useful to estimate risk of neutropenia, which the investigators acknowledged as a serious chemotherapy-induced toxicity linked to life-threatening infections, dose delays, and reductions that can compromise treatment efficacy, and reduced health-related quality of life.
There are other, previously developed models to predict chemotherapy-induced neutropenia, but those have significant limitations, including development based on small patient sample sizes, according to the researchers.
A comprehensive risk model for neutropenic complications has been developed by Gary H. Lyman, MD, and colleagues, based on a large, prospective cohort including nearly 3,800 patients. That model performs well and had a 90% sensitivity and 96% predictive value; however, it’s not lung cancer specific, and hasn’t been externally validated, according to Ms. Cao and coauthors.
Accordingly, they set out to develop a new risk prediction model based on a lung cancer data set encompassing 11,352 patients from 67 phase 2 or 3 cooperative group studies conducted between 1991 and 2010.
The Lyman model in this data set had an area under the curve (AUC) of 0.8772 in patients with small cell lung cancer (SCLC), but an AUC of just 0.6787 in non–small cell lung cancer (NSCLC), suggesting “much better predictive performance in the SCLC,” the researchers noted.
They used stepwise logistic regression and lasso regression to develop a new model, which was derived based on about two-thirds of the patients, randomly selected, while the validation was conducted using the remaining third.
Variables included in the final model included age, gender, weight, BMI, insurance status, disease stage, number of metastatic sites, chemotherapy agents used, number of chemotherapy agents, planned growth factor use, duration of planned therapy, pleural effusion, presence of symptoms, and performance status.
That model had a good AUC, according to investigators, in both the training set and the testing set (0.8348 and 0.8234, respectively).
“It is worth noticing that our final model compensated for the deficiency of Lyman’s risk model in NSCLC patients,” the researchers noted in a discussion of their results.
The study was supported in part by grants from the National Institutes of Health, the National Center for Advancing Translational Sciences, and the Health and Medical Research Fund of Hong Kong. One study coauthor reported a conflict of interest outside the submitted work related to Genentech.
SOURCE: Cao X et al. Lung Cancer 2020 Jan 5. doi: 10.1016/j.lungcan.2020.01.004.
A new and simple model for predicting risk of severe neutropenia in advanced lung cancer could ease the evaluation process and help inform patient management decisions, according to investigators.
The model, based on 10 pretreatment variables, appears to overcome limitations of a comprehensive risk prediction model that is not specific to lung cancer, according to Xiaowen Cao of Duke University, Durham, N.C., and coinvestigators.
“We believe that this model, once validated, will help oncologists accurately identify those patients with lung cancer who are at a high risk of developing severe neutropenia, based on simple, readily available information,” the researchers said in a report on their work, which appears in the journal Lung Cancer.
Oncologists could then make “proactive” decisions about monitoring of high-risk patients, modifying the dose of chemotherapy, and using prophylactic growth factors, the authors added.
Accurate, lung cancer–specific prediction models would be useful to estimate risk of neutropenia, which the investigators acknowledged as a serious chemotherapy-induced toxicity linked to life-threatening infections, dose delays, and reductions that can compromise treatment efficacy, and reduced health-related quality of life.
There are other, previously developed models to predict chemotherapy-induced neutropenia, but those have significant limitations, including development based on small patient sample sizes, according to the researchers.
A comprehensive risk model for neutropenic complications has been developed by Gary H. Lyman, MD, and colleagues, based on a large, prospective cohort including nearly 3,800 patients. That model performs well and had a 90% sensitivity and 96% predictive value; however, it’s not lung cancer specific, and hasn’t been externally validated, according to Ms. Cao and coauthors.
Accordingly, they set out to develop a new risk prediction model based on a lung cancer data set encompassing 11,352 patients from 67 phase 2 or 3 cooperative group studies conducted between 1991 and 2010.
The Lyman model in this data set had an area under the curve (AUC) of 0.8772 in patients with small cell lung cancer (SCLC), but an AUC of just 0.6787 in non–small cell lung cancer (NSCLC), suggesting “much better predictive performance in the SCLC,” the researchers noted.
They used stepwise logistic regression and lasso regression to develop a new model, which was derived based on about two-thirds of the patients, randomly selected, while the validation was conducted using the remaining third.
Variables included in the final model included age, gender, weight, BMI, insurance status, disease stage, number of metastatic sites, chemotherapy agents used, number of chemotherapy agents, planned growth factor use, duration of planned therapy, pleural effusion, presence of symptoms, and performance status.
That model had a good AUC, according to investigators, in both the training set and the testing set (0.8348 and 0.8234, respectively).
“It is worth noticing that our final model compensated for the deficiency of Lyman’s risk model in NSCLC patients,” the researchers noted in a discussion of their results.
The study was supported in part by grants from the National Institutes of Health, the National Center for Advancing Translational Sciences, and the Health and Medical Research Fund of Hong Kong. One study coauthor reported a conflict of interest outside the submitted work related to Genentech.
SOURCE: Cao X et al. Lung Cancer 2020 Jan 5. doi: 10.1016/j.lungcan.2020.01.004.
FROM LUNG CANCER
Decreased incidence, survival in low-grade serous ovarian cancer illustrate ‘diagnostic shift’
Relative to serous borderline ovarian tumors, the proportion of low-grade serous ovarian cancers has decreased in recent years, paralleled by a trend toward decreased survival, results of a large, population-based study suggest.
The number of low-grade serous ovarian cancer (LGSOC) cases declined by almost 26% in the retrospective analysis of registry data from 1998 to 2000, with a relative decrease of survival by nearly 29% over that time period.
Those decreases in LGSOC incidence and survival may be explained by a “diagnosis shift” toward versus serous borderline ovarian tumors (SBOTs) over time, though it could also be due to earlier detection of SBOT, which may be a precursor lesion of LGSOC, said authors of the study, led by oncology researcher Koji Matsuo, MD, PhD, of the University of Southern California, Los Angeles.
Taken together, the findings support the need to distinguish between LGSOC and SBOT, given their “distinctly different” oncologic outcomes and treatment approaches, according to Dr. Matsuo and colleagues.
“Making a proper diagnosis for LGSOC versus SBOT is paramount, as it impacts surgical and postoperative management,” the authors wrote in their report on the study, which appears in Gynecologic Oncology.
The retrospective, population-based, observational study, based on data from the Surveillance, Epidemiology, and End Results program of the National Cancer Institute, included women with LGSOC or SBOT diagnosed between 1988 and 2000 in the United States. A total of 4,712 cases were analyzed.
The number of LGSOC from 23.2% in 1988 down to 17.2% by 2000, or a relative decrease of 25.9%, Dr. Matsuo and coauthors reported. The decrease was even more pronounced in stage I disease, with a relative decrease of 37.6% versus 21.1% for stage II-IV.
As compared with women with SBOT, women with LGSOC were more likely to be older, more likely to have stage II-IV disease at diagnosis, more likely to have undergone hysterectomy, and less likely to be residents of the western United States, results of a multivariable analysis showed.
There was a downward trend in 15-year overall survival among women with LGSOC, from 53.3% to 38.0% by 2000, representing a relative decrease of 28.7%, according to the investigators. By contrast, overall survival was unchanged in the SBOT cohort, at 76.7% in 1988 and 78.7% by 2000, for a relative increase of about 2.5%.
Gradual changes in diagnostic classification over time may explain these trends, according to the investigators.
That shift would have started with the recognition of borderline ovarian tumors as a separate entity by the World Health Organization until 1971, leading to an increasing number of SBOTs over time as diagnostic practice patterns gradually changed. Later, it would have been fueled by the recognition of LGSOC as a new classification in the mid-1980s, which may have accelerated the shift by enhanced discrimination of SBOT from other serous tumors, the investigators wrote.
On the other hand, since SBOT may be a precursor lesion to LGSOC, the shift in diagnosis over time could be caused at least in part by increasing use of transvaginal ultrasonography, leading to more early detection of SBOT before they could progress to LGGOC. “More study is necessary to identify if SBOT can progress to LGSOC,” Dr. Matsuo and coauthors wrote.
The study was funded by the Ensign Endowment for Gynecologic Cancer Research. Dr. Matsuo reported disclosures related to Chugai, Springer, and VBL Therapeutics. His coauthors provided additional disclosures related to Kiyatec, Merck, Biopath, M-Trap, Quantgene, Tesaro, GlaxoSmithKline, Celgene, Johnson & Johnson, Biogin, Clovis Oncology, Novartis, Elsevier, and UpToDate.
SOURCE: Matsuo K et al. Gynecol Oncol. 2020 Jan 15. doi: 10.1016/j.ygyno.2019.08.030.
Relative to serous borderline ovarian tumors, the proportion of low-grade serous ovarian cancers has decreased in recent years, paralleled by a trend toward decreased survival, results of a large, population-based study suggest.
The number of low-grade serous ovarian cancer (LGSOC) cases declined by almost 26% in the retrospective analysis of registry data from 1998 to 2000, with a relative decrease of survival by nearly 29% over that time period.
Those decreases in LGSOC incidence and survival may be explained by a “diagnosis shift” toward versus serous borderline ovarian tumors (SBOTs) over time, though it could also be due to earlier detection of SBOT, which may be a precursor lesion of LGSOC, said authors of the study, led by oncology researcher Koji Matsuo, MD, PhD, of the University of Southern California, Los Angeles.
Taken together, the findings support the need to distinguish between LGSOC and SBOT, given their “distinctly different” oncologic outcomes and treatment approaches, according to Dr. Matsuo and colleagues.
“Making a proper diagnosis for LGSOC versus SBOT is paramount, as it impacts surgical and postoperative management,” the authors wrote in their report on the study, which appears in Gynecologic Oncology.
The retrospective, population-based, observational study, based on data from the Surveillance, Epidemiology, and End Results program of the National Cancer Institute, included women with LGSOC or SBOT diagnosed between 1988 and 2000 in the United States. A total of 4,712 cases were analyzed.
The number of LGSOC from 23.2% in 1988 down to 17.2% by 2000, or a relative decrease of 25.9%, Dr. Matsuo and coauthors reported. The decrease was even more pronounced in stage I disease, with a relative decrease of 37.6% versus 21.1% for stage II-IV.
As compared with women with SBOT, women with LGSOC were more likely to be older, more likely to have stage II-IV disease at diagnosis, more likely to have undergone hysterectomy, and less likely to be residents of the western United States, results of a multivariable analysis showed.
There was a downward trend in 15-year overall survival among women with LGSOC, from 53.3% to 38.0% by 2000, representing a relative decrease of 28.7%, according to the investigators. By contrast, overall survival was unchanged in the SBOT cohort, at 76.7% in 1988 and 78.7% by 2000, for a relative increase of about 2.5%.
Gradual changes in diagnostic classification over time may explain these trends, according to the investigators.
That shift would have started with the recognition of borderline ovarian tumors as a separate entity by the World Health Organization until 1971, leading to an increasing number of SBOTs over time as diagnostic practice patterns gradually changed. Later, it would have been fueled by the recognition of LGSOC as a new classification in the mid-1980s, which may have accelerated the shift by enhanced discrimination of SBOT from other serous tumors, the investigators wrote.
On the other hand, since SBOT may be a precursor lesion to LGSOC, the shift in diagnosis over time could be caused at least in part by increasing use of transvaginal ultrasonography, leading to more early detection of SBOT before they could progress to LGGOC. “More study is necessary to identify if SBOT can progress to LGSOC,” Dr. Matsuo and coauthors wrote.
The study was funded by the Ensign Endowment for Gynecologic Cancer Research. Dr. Matsuo reported disclosures related to Chugai, Springer, and VBL Therapeutics. His coauthors provided additional disclosures related to Kiyatec, Merck, Biopath, M-Trap, Quantgene, Tesaro, GlaxoSmithKline, Celgene, Johnson & Johnson, Biogin, Clovis Oncology, Novartis, Elsevier, and UpToDate.
SOURCE: Matsuo K et al. Gynecol Oncol. 2020 Jan 15. doi: 10.1016/j.ygyno.2019.08.030.
Relative to serous borderline ovarian tumors, the proportion of low-grade serous ovarian cancers has decreased in recent years, paralleled by a trend toward decreased survival, results of a large, population-based study suggest.
The number of low-grade serous ovarian cancer (LGSOC) cases declined by almost 26% in the retrospective analysis of registry data from 1998 to 2000, with a relative decrease of survival by nearly 29% over that time period.
Those decreases in LGSOC incidence and survival may be explained by a “diagnosis shift” toward versus serous borderline ovarian tumors (SBOTs) over time, though it could also be due to earlier detection of SBOT, which may be a precursor lesion of LGSOC, said authors of the study, led by oncology researcher Koji Matsuo, MD, PhD, of the University of Southern California, Los Angeles.
Taken together, the findings support the need to distinguish between LGSOC and SBOT, given their “distinctly different” oncologic outcomes and treatment approaches, according to Dr. Matsuo and colleagues.
“Making a proper diagnosis for LGSOC versus SBOT is paramount, as it impacts surgical and postoperative management,” the authors wrote in their report on the study, which appears in Gynecologic Oncology.
The retrospective, population-based, observational study, based on data from the Surveillance, Epidemiology, and End Results program of the National Cancer Institute, included women with LGSOC or SBOT diagnosed between 1988 and 2000 in the United States. A total of 4,712 cases were analyzed.
The number of LGSOC from 23.2% in 1988 down to 17.2% by 2000, or a relative decrease of 25.9%, Dr. Matsuo and coauthors reported. The decrease was even more pronounced in stage I disease, with a relative decrease of 37.6% versus 21.1% for stage II-IV.
As compared with women with SBOT, women with LGSOC were more likely to be older, more likely to have stage II-IV disease at diagnosis, more likely to have undergone hysterectomy, and less likely to be residents of the western United States, results of a multivariable analysis showed.
There was a downward trend in 15-year overall survival among women with LGSOC, from 53.3% to 38.0% by 2000, representing a relative decrease of 28.7%, according to the investigators. By contrast, overall survival was unchanged in the SBOT cohort, at 76.7% in 1988 and 78.7% by 2000, for a relative increase of about 2.5%.
Gradual changes in diagnostic classification over time may explain these trends, according to the investigators.
That shift would have started with the recognition of borderline ovarian tumors as a separate entity by the World Health Organization until 1971, leading to an increasing number of SBOTs over time as diagnostic practice patterns gradually changed. Later, it would have been fueled by the recognition of LGSOC as a new classification in the mid-1980s, which may have accelerated the shift by enhanced discrimination of SBOT from other serous tumors, the investigators wrote.
On the other hand, since SBOT may be a precursor lesion to LGSOC, the shift in diagnosis over time could be caused at least in part by increasing use of transvaginal ultrasonography, leading to more early detection of SBOT before they could progress to LGGOC. “More study is necessary to identify if SBOT can progress to LGSOC,” Dr. Matsuo and coauthors wrote.
The study was funded by the Ensign Endowment for Gynecologic Cancer Research. Dr. Matsuo reported disclosures related to Chugai, Springer, and VBL Therapeutics. His coauthors provided additional disclosures related to Kiyatec, Merck, Biopath, M-Trap, Quantgene, Tesaro, GlaxoSmithKline, Celgene, Johnson & Johnson, Biogin, Clovis Oncology, Novartis, Elsevier, and UpToDate.
SOURCE: Matsuo K et al. Gynecol Oncol. 2020 Jan 15. doi: 10.1016/j.ygyno.2019.08.030.
FROM GYNECOLOGIC ONCOLOGY