User login
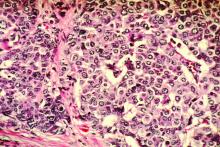
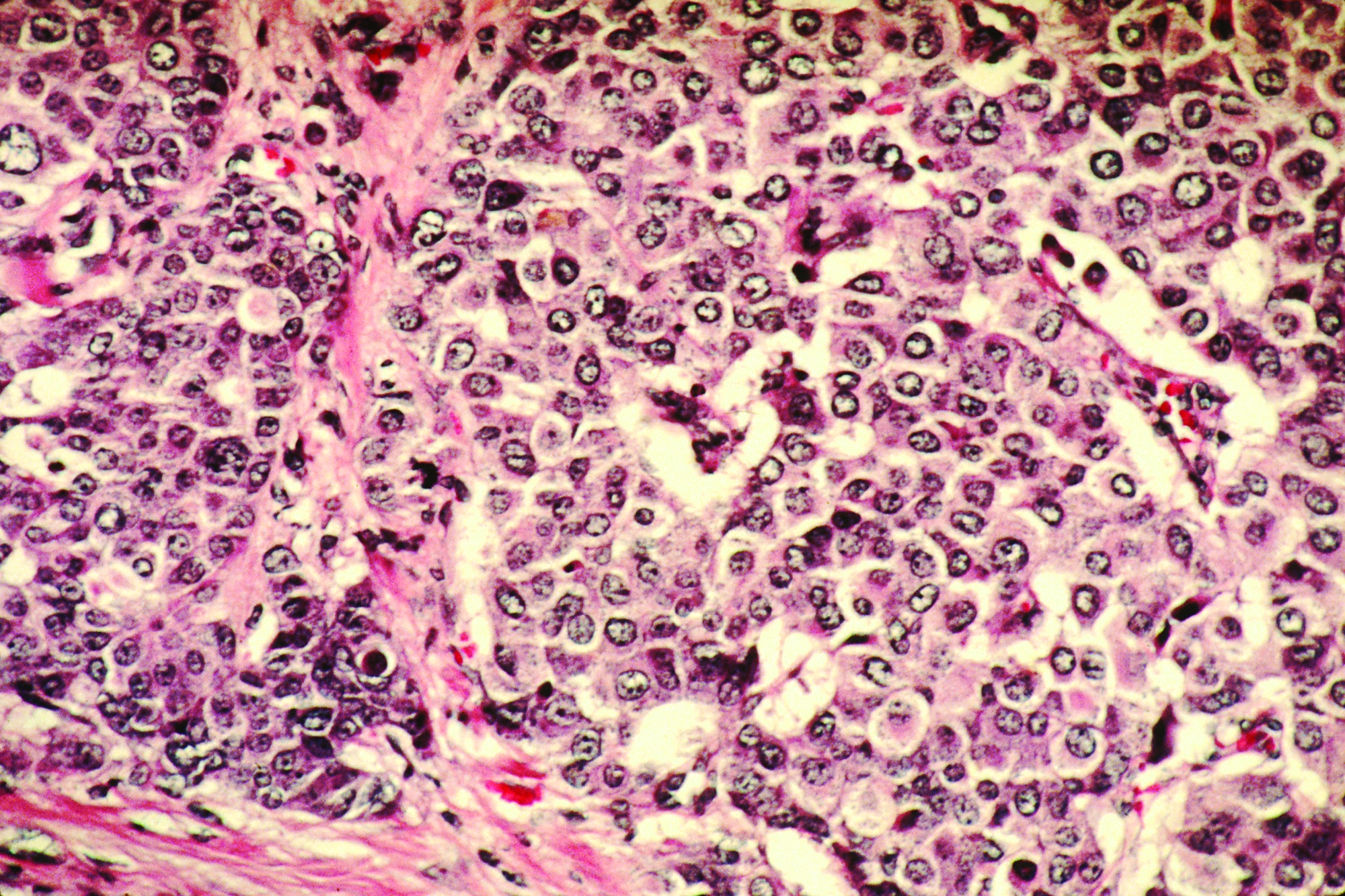
Stress-related disorders linked to later neurodegenerative diseases
Individuals with posttraumatic stress disorder (PTSD), acute stress reaction, adjustment disorder, or other stress reactions had an 80% increased risk of vascular neurodegenerative diseases, according to results of the study, which was based on Swedish population registry data.
Risk of primary neurodegenerative diseases was increased as well in people with those conditions, but only by 31%, according to lead author Huan Song, MD, PhD, of Sichuan University in Chengdu, China.
“The stronger association observed for neurodegenerative diseases with a vascular component, compared with primary neurodegenerative diseases, suggested a considerable role of a possible cerebrovascular pathway,” Dr. Song and coauthors said in a report on the study appearing in JAMA Neurology.
While some previous studies have linked stress-related disorders to neurodegenerative diseases – particularly PTSD and dementia – this is believed to be the first, according to the investigators, to comprehensively evaluate all stress-related disorders in relation to the most common neurodegenerative conditions.
When considering neurodegenerative conditions separately, they found a statistically significant association between stress-related disorders and Alzheimer’s disease, while linkages with Parkinson’s disease and amyotrophic lateral sclerosis (ALS) were “comparable” but associations did not reach statistical significance, according to investigators.
Based on these findings, stress reduction should be recommended in addition to daily physical activity, mental activity, and a heart-healthy diet to potentially reduce risk of onset or worsening of cognitive decline, according to Chun Lim, MD, PhD, medical director of the cognitive neurology unit at Beth Israel Deaconess Medical Center in Boston.
“We don’t really have great evidence that anything slows down the progression of Alzheimer’s disease, but there are some suggestions that for people who lead heart-healthy lifestyles or adhere to a Mediterranean diet, fewer develop cognitive issues over 5-10 years,” Dr. Lim said in an interview. “Because of this paper, stress reduction may be one additional way to hopefully help these patients these patients that have or are concerned about cognitive issues.”
The population-matched cohort of the study included 61,748 individuals with stress-related disorders and 595,335 matched individuals without those disorders, while the sibling-matched cohort included 44,839 individuals with those disorders and 78,482 without. The median age at the start of follow-up was 47 years and 39.4% of those with stress-related disorders were male.
During follow-up, the incidence of neurodegenerative diseases per 1,000 person-years was 1.50 for individuals with stress-related disorders, versus 0.82 for those without stress-related disorders, according to the report. Risk of primary neurodegenerative diseases was increased among those with stress-related disorders, compared with those without, with a hazard ratio of 1.31 (95% confidence interval, 1.15-1.48). However, the risk of vascular neurodegenerative diseases was significantly higher, with an HR of 1.80 (95% CI, 1.40-2.31; P = .03 for the difference between hazard ratios).
Results of the matched sibling cohort supported results of the population-matched cohort, though the elevated risk of vascular neurodegenerative diseases among those with stress-related disorders was “slightly lower” than in the population-based cohort, Dr. Song and coauthors wrote in their report.
Beyond causing a host of hormonal and medical issues, stress can lead to sleep issues that may have long-term consequences, Dr. Lim noted in the interview.
“There’s some thought that quality sleep is important for memory formation, and if people are under a fair amount of stress and they have really poor sleep, that can also lead to cognitive issues including memory impairment,” he said.
“There are these multiple avenues that may be contributing to the accelerated development of these kinds of issues,” he added, “so I think this paper suggests more ways to counsel the patients about using lifestyle modifications to slow down the development of these cognitive impairments.”
Funding for the study came from the Swedish Research Council, Icelandic Research Fund; ,European Research Council the Karolinska Institutet, Swedish Research Council, and West China Hospital. Authors of the study provided disclosures related to those organizations as well as Shire/Takeda and Evolan.
SOURCE: Song H et al. JAMA Neurol. 2020 Mar 9. doi: 10.1001/jamaneurol.2020.0117.
Individuals with posttraumatic stress disorder (PTSD), acute stress reaction, adjustment disorder, or other stress reactions had an 80% increased risk of vascular neurodegenerative diseases, according to results of the study, which was based on Swedish population registry data.
Risk of primary neurodegenerative diseases was increased as well in people with those conditions, but only by 31%, according to lead author Huan Song, MD, PhD, of Sichuan University in Chengdu, China.
“The stronger association observed for neurodegenerative diseases with a vascular component, compared with primary neurodegenerative diseases, suggested a considerable role of a possible cerebrovascular pathway,” Dr. Song and coauthors said in a report on the study appearing in JAMA Neurology.
While some previous studies have linked stress-related disorders to neurodegenerative diseases – particularly PTSD and dementia – this is believed to be the first, according to the investigators, to comprehensively evaluate all stress-related disorders in relation to the most common neurodegenerative conditions.
When considering neurodegenerative conditions separately, they found a statistically significant association between stress-related disorders and Alzheimer’s disease, while linkages with Parkinson’s disease and amyotrophic lateral sclerosis (ALS) were “comparable” but associations did not reach statistical significance, according to investigators.
Based on these findings, stress reduction should be recommended in addition to daily physical activity, mental activity, and a heart-healthy diet to potentially reduce risk of onset or worsening of cognitive decline, according to Chun Lim, MD, PhD, medical director of the cognitive neurology unit at Beth Israel Deaconess Medical Center in Boston.
“We don’t really have great evidence that anything slows down the progression of Alzheimer’s disease, but there are some suggestions that for people who lead heart-healthy lifestyles or adhere to a Mediterranean diet, fewer develop cognitive issues over 5-10 years,” Dr. Lim said in an interview. “Because of this paper, stress reduction may be one additional way to hopefully help these patients these patients that have or are concerned about cognitive issues.”
The population-matched cohort of the study included 61,748 individuals with stress-related disorders and 595,335 matched individuals without those disorders, while the sibling-matched cohort included 44,839 individuals with those disorders and 78,482 without. The median age at the start of follow-up was 47 years and 39.4% of those with stress-related disorders were male.
During follow-up, the incidence of neurodegenerative diseases per 1,000 person-years was 1.50 for individuals with stress-related disorders, versus 0.82 for those without stress-related disorders, according to the report. Risk of primary neurodegenerative diseases was increased among those with stress-related disorders, compared with those without, with a hazard ratio of 1.31 (95% confidence interval, 1.15-1.48). However, the risk of vascular neurodegenerative diseases was significantly higher, with an HR of 1.80 (95% CI, 1.40-2.31; P = .03 for the difference between hazard ratios).
Results of the matched sibling cohort supported results of the population-matched cohort, though the elevated risk of vascular neurodegenerative diseases among those with stress-related disorders was “slightly lower” than in the population-based cohort, Dr. Song and coauthors wrote in their report.
Beyond causing a host of hormonal and medical issues, stress can lead to sleep issues that may have long-term consequences, Dr. Lim noted in the interview.
“There’s some thought that quality sleep is important for memory formation, and if people are under a fair amount of stress and they have really poor sleep, that can also lead to cognitive issues including memory impairment,” he said.
“There are these multiple avenues that may be contributing to the accelerated development of these kinds of issues,” he added, “so I think this paper suggests more ways to counsel the patients about using lifestyle modifications to slow down the development of these cognitive impairments.”
Funding for the study came from the Swedish Research Council, Icelandic Research Fund; ,European Research Council the Karolinska Institutet, Swedish Research Council, and West China Hospital. Authors of the study provided disclosures related to those organizations as well as Shire/Takeda and Evolan.
SOURCE: Song H et al. JAMA Neurol. 2020 Mar 9. doi: 10.1001/jamaneurol.2020.0117.
Individuals with posttraumatic stress disorder (PTSD), acute stress reaction, adjustment disorder, or other stress reactions had an 80% increased risk of vascular neurodegenerative diseases, according to results of the study, which was based on Swedish population registry data.
Risk of primary neurodegenerative diseases was increased as well in people with those conditions, but only by 31%, according to lead author Huan Song, MD, PhD, of Sichuan University in Chengdu, China.
“The stronger association observed for neurodegenerative diseases with a vascular component, compared with primary neurodegenerative diseases, suggested a considerable role of a possible cerebrovascular pathway,” Dr. Song and coauthors said in a report on the study appearing in JAMA Neurology.
While some previous studies have linked stress-related disorders to neurodegenerative diseases – particularly PTSD and dementia – this is believed to be the first, according to the investigators, to comprehensively evaluate all stress-related disorders in relation to the most common neurodegenerative conditions.
When considering neurodegenerative conditions separately, they found a statistically significant association between stress-related disorders and Alzheimer’s disease, while linkages with Parkinson’s disease and amyotrophic lateral sclerosis (ALS) were “comparable” but associations did not reach statistical significance, according to investigators.
Based on these findings, stress reduction should be recommended in addition to daily physical activity, mental activity, and a heart-healthy diet to potentially reduce risk of onset or worsening of cognitive decline, according to Chun Lim, MD, PhD, medical director of the cognitive neurology unit at Beth Israel Deaconess Medical Center in Boston.
“We don’t really have great evidence that anything slows down the progression of Alzheimer’s disease, but there are some suggestions that for people who lead heart-healthy lifestyles or adhere to a Mediterranean diet, fewer develop cognitive issues over 5-10 years,” Dr. Lim said in an interview. “Because of this paper, stress reduction may be one additional way to hopefully help these patients these patients that have or are concerned about cognitive issues.”
The population-matched cohort of the study included 61,748 individuals with stress-related disorders and 595,335 matched individuals without those disorders, while the sibling-matched cohort included 44,839 individuals with those disorders and 78,482 without. The median age at the start of follow-up was 47 years and 39.4% of those with stress-related disorders were male.
During follow-up, the incidence of neurodegenerative diseases per 1,000 person-years was 1.50 for individuals with stress-related disorders, versus 0.82 for those without stress-related disorders, according to the report. Risk of primary neurodegenerative diseases was increased among those with stress-related disorders, compared with those without, with a hazard ratio of 1.31 (95% confidence interval, 1.15-1.48). However, the risk of vascular neurodegenerative diseases was significantly higher, with an HR of 1.80 (95% CI, 1.40-2.31; P = .03 for the difference between hazard ratios).
Results of the matched sibling cohort supported results of the population-matched cohort, though the elevated risk of vascular neurodegenerative diseases among those with stress-related disorders was “slightly lower” than in the population-based cohort, Dr. Song and coauthors wrote in their report.
Beyond causing a host of hormonal and medical issues, stress can lead to sleep issues that may have long-term consequences, Dr. Lim noted in the interview.
“There’s some thought that quality sleep is important for memory formation, and if people are under a fair amount of stress and they have really poor sleep, that can also lead to cognitive issues including memory impairment,” he said.
“There are these multiple avenues that may be contributing to the accelerated development of these kinds of issues,” he added, “so I think this paper suggests more ways to counsel the patients about using lifestyle modifications to slow down the development of these cognitive impairments.”
Funding for the study came from the Swedish Research Council, Icelandic Research Fund; ,European Research Council the Karolinska Institutet, Swedish Research Council, and West China Hospital. Authors of the study provided disclosures related to those organizations as well as Shire/Takeda and Evolan.
SOURCE: Song H et al. JAMA Neurol. 2020 Mar 9. doi: 10.1001/jamaneurol.2020.0117.
FROM JAMA NEUROLOGY
Novel coronavirus may cause environmental contamination through fecal shedding
The toilet bowl, sink, and bathroom door handle of an isolation room housing a patient with the novel coronavirus tested positive for the virus, raising the possibility that viral shedding in the stool could represent another route of transmission, investigators reported.
Air outlet fans and other room sites also tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), though an anteroom, a corridor, and most personal protective equipment (PPE) worn by health care providers tested negative, according to the researchers, led by Sean Wei Xiang Ong, MBBS, of the National Centre for Infectious Diseases, Singapore.
Taken together, these findings suggest a “need for strict adherence to environmental and hand hygiene” to combat significant environmental contamination through respiratory droplets and fecal shedding, Dr. Ong and colleagues wrote in JAMA.
Aaron Eli Glatt, MD, chair of medicine at Mount Sinai South Nassau in New York, said these results demonstrate that SARS-CoV-2 is “clearly capable” of contaminating bathroom sinks and toilets.
“That wouldn’t have been the first place I would have thought of, before this study,” he said in an interview. “You need to pay attention to cleaning the bathrooms, which we obviously do, but that’s an important reminder.”
The report by Dr. Ong and coauthors included a total of three patients housed in airborne infection isolation rooms in a dedicated SARS-CoV-2 outbreak center in Singapore. For each patient, surface samples were taken from 26 sites in the isolation room, an anteroom, and a bathroom. Samples were also taken from PPE on physicians as they left the patient rooms.
Samples for the first patient, taken right after routine cleaning, were all negative, according to researchers. That room was sampled twice, on days 4 and 10 of the illness, while the patient was still symptomatic. Likewise, for the second patient, postcleaning samples were negative; those samples were taken 2 days after cleaning.
However, for the third patient, samples were taken before routine cleaning. In this case, Dr. Ong and colleagues said 13 of 15 room sites (87%) were positive, including air outlet fans, while 3 of 5 toilet sites (60%) were positive as well, though no contamination was found in the anteroom, corridor, or in air samples.
That patient had two stool samples that were positive for SARS-CoV-2, but no diarrhea, authors said, and had upper respiratory tract involvement without pneumonia.
The fact that swabs of the air exhaust outlets tested positive suggests that virus-laden droplets could be “displaced by airflows” and end up on vents or other equipment, Dr. Ong and coauthors reported.
All PPE samples tested negative, except for the front of one shoe.
“The risk of transmission from contaminated footwear is likely low, as evidenced by negative results in the anteroom and corridor,” they wrote.
While this study included only a small number of patients, Dr. Glatt said the findings represent an important and useful contribution to the literature on coronavirus disease 2019 (COVID-19).
“Every day we’re getting more information, and each little piece of the puzzle helps us in the overall management of individuals with COVID-19,” he said in the interview. “They’re adding to our ability to manage, control, and mitigate further spread of the disease.”
Funding for the study came from the National Medical Research Council in Singapore and DSO National Laboratories. Dr. Ong and colleagues reported no conflicts of interest.
SOURCE: Ong SWX et al. JAMA. 2020 Mar 4. doi: 10.1001/jama.2020.3227.
The toilet bowl, sink, and bathroom door handle of an isolation room housing a patient with the novel coronavirus tested positive for the virus, raising the possibility that viral shedding in the stool could represent another route of transmission, investigators reported.
Air outlet fans and other room sites also tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), though an anteroom, a corridor, and most personal protective equipment (PPE) worn by health care providers tested negative, according to the researchers, led by Sean Wei Xiang Ong, MBBS, of the National Centre for Infectious Diseases, Singapore.
Taken together, these findings suggest a “need for strict adherence to environmental and hand hygiene” to combat significant environmental contamination through respiratory droplets and fecal shedding, Dr. Ong and colleagues wrote in JAMA.
Aaron Eli Glatt, MD, chair of medicine at Mount Sinai South Nassau in New York, said these results demonstrate that SARS-CoV-2 is “clearly capable” of contaminating bathroom sinks and toilets.
“That wouldn’t have been the first place I would have thought of, before this study,” he said in an interview. “You need to pay attention to cleaning the bathrooms, which we obviously do, but that’s an important reminder.”
The report by Dr. Ong and coauthors included a total of three patients housed in airborne infection isolation rooms in a dedicated SARS-CoV-2 outbreak center in Singapore. For each patient, surface samples were taken from 26 sites in the isolation room, an anteroom, and a bathroom. Samples were also taken from PPE on physicians as they left the patient rooms.
Samples for the first patient, taken right after routine cleaning, were all negative, according to researchers. That room was sampled twice, on days 4 and 10 of the illness, while the patient was still symptomatic. Likewise, for the second patient, postcleaning samples were negative; those samples were taken 2 days after cleaning.
However, for the third patient, samples were taken before routine cleaning. In this case, Dr. Ong and colleagues said 13 of 15 room sites (87%) were positive, including air outlet fans, while 3 of 5 toilet sites (60%) were positive as well, though no contamination was found in the anteroom, corridor, or in air samples.
That patient had two stool samples that were positive for SARS-CoV-2, but no diarrhea, authors said, and had upper respiratory tract involvement without pneumonia.
The fact that swabs of the air exhaust outlets tested positive suggests that virus-laden droplets could be “displaced by airflows” and end up on vents or other equipment, Dr. Ong and coauthors reported.
All PPE samples tested negative, except for the front of one shoe.
“The risk of transmission from contaminated footwear is likely low, as evidenced by negative results in the anteroom and corridor,” they wrote.
While this study included only a small number of patients, Dr. Glatt said the findings represent an important and useful contribution to the literature on coronavirus disease 2019 (COVID-19).
“Every day we’re getting more information, and each little piece of the puzzle helps us in the overall management of individuals with COVID-19,” he said in the interview. “They’re adding to our ability to manage, control, and mitigate further spread of the disease.”
Funding for the study came from the National Medical Research Council in Singapore and DSO National Laboratories. Dr. Ong and colleagues reported no conflicts of interest.
SOURCE: Ong SWX et al. JAMA. 2020 Mar 4. doi: 10.1001/jama.2020.3227.
The toilet bowl, sink, and bathroom door handle of an isolation room housing a patient with the novel coronavirus tested positive for the virus, raising the possibility that viral shedding in the stool could represent another route of transmission, investigators reported.
Air outlet fans and other room sites also tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), though an anteroom, a corridor, and most personal protective equipment (PPE) worn by health care providers tested negative, according to the researchers, led by Sean Wei Xiang Ong, MBBS, of the National Centre for Infectious Diseases, Singapore.
Taken together, these findings suggest a “need for strict adherence to environmental and hand hygiene” to combat significant environmental contamination through respiratory droplets and fecal shedding, Dr. Ong and colleagues wrote in JAMA.
Aaron Eli Glatt, MD, chair of medicine at Mount Sinai South Nassau in New York, said these results demonstrate that SARS-CoV-2 is “clearly capable” of contaminating bathroom sinks and toilets.
“That wouldn’t have been the first place I would have thought of, before this study,” he said in an interview. “You need to pay attention to cleaning the bathrooms, which we obviously do, but that’s an important reminder.”
The report by Dr. Ong and coauthors included a total of three patients housed in airborne infection isolation rooms in a dedicated SARS-CoV-2 outbreak center in Singapore. For each patient, surface samples were taken from 26 sites in the isolation room, an anteroom, and a bathroom. Samples were also taken from PPE on physicians as they left the patient rooms.
Samples for the first patient, taken right after routine cleaning, were all negative, according to researchers. That room was sampled twice, on days 4 and 10 of the illness, while the patient was still symptomatic. Likewise, for the second patient, postcleaning samples were negative; those samples were taken 2 days after cleaning.
However, for the third patient, samples were taken before routine cleaning. In this case, Dr. Ong and colleagues said 13 of 15 room sites (87%) were positive, including air outlet fans, while 3 of 5 toilet sites (60%) were positive as well, though no contamination was found in the anteroom, corridor, or in air samples.
That patient had two stool samples that were positive for SARS-CoV-2, but no diarrhea, authors said, and had upper respiratory tract involvement without pneumonia.
The fact that swabs of the air exhaust outlets tested positive suggests that virus-laden droplets could be “displaced by airflows” and end up on vents or other equipment, Dr. Ong and coauthors reported.
All PPE samples tested negative, except for the front of one shoe.
“The risk of transmission from contaminated footwear is likely low, as evidenced by negative results in the anteroom and corridor,” they wrote.
While this study included only a small number of patients, Dr. Glatt said the findings represent an important and useful contribution to the literature on coronavirus disease 2019 (COVID-19).
“Every day we’re getting more information, and each little piece of the puzzle helps us in the overall management of individuals with COVID-19,” he said in the interview. “They’re adding to our ability to manage, control, and mitigate further spread of the disease.”
Funding for the study came from the National Medical Research Council in Singapore and DSO National Laboratories. Dr. Ong and colleagues reported no conflicts of interest.
SOURCE: Ong SWX et al. JAMA. 2020 Mar 4. doi: 10.1001/jama.2020.3227.
FROM JAMA
No sedation fails to improve mortality in mechanically ventilated patients
ORLANDO – For critically ill, according to results of a multicenter, randomized trial.
The lack of sedation did significantly improve certain secondary endpoints, including a reduced number of thromboembolic events and preservation of physical function, according to Palle Toft, PhD, DMSc, of Odense (Denmark) University Hospital.
However, the 90-day mortality rate was 42.4% in the no-sedation group versus 37.0% in the sedation group in the NONSEDA study, which was intended to test the hypothesis that mortality would be lower in the no-sedation group.
That 5.4 percentage point difference between arms in NONSEDA was not statistically significant (P = .65) in results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine and concurrently published in the New England Journal of Medicine.
Yet that mortality trend is in the “opposite direction” of an earlier, single-center trial by Dr. Toft and colleagues, noted Claude Guérin, MD, PhD, in a related editorial that also appeared in the journal. In that earlier study, the reported hospital mortality rates were 36% for no sedation and 47% for sedation with daily interruption.
“The results from this trial [NONSEDA] are important because they arouse concern about omitting sedation in mechanically ventilated patients and reinforce the need to monitor sedation clinically, with the aim of discontinuing it as early as possible or at least interrupting it daily,” Dr. Guérin wrote in his editorial.
That said, the earlier, single-center trial was not statistically powered to show between-group differences in mortality, Dr. Toft and coauthors wrote in their journal article.
In his presentation, Dr. Toft emphasized that light sedation with a wake-up trial was “comparable” with no sedation with regard to mortality.
“I think my main message is that we have to individualize patient treatment,” Dr. Toft told attendees at a late-breaking literature session. “Many patients would benefit from nonsedation, and some would benefit by light sedation with a daily wake-up trial. We have to respect patient autonomy, and try to establish a two-way communication with patients in 2020.”
Sandra L. Kane-Gill, PharmD, treasurer of SCCM and assistant professor of pharmacy and therapeutics at the University of Pittsburgh, said that current SCCM guidelines recommend using light sedation in critically ill, mechanically ventilated adults.
“I think we should stay consistent with what the guidelines are saying,” Dr. Kane-Gill said in an interview. “How you do that may vary, but targeting light sedation is consistent with what the evidence is suggesting in those guidelines.”
The depth of sedation between the no-sedation group in the light sedation group in the present study was not as great as the investigators had anticipated, which may explain the lack of statistically significant difference in mortality, according to Dr. Kane-Gill.
According to the report, 38.4% of patients in the no-sedation group received medication for sedation during their ICU stay, while Richmond Agitation and Sedation Scores increased in both groups, indicating a more alert state in both groups.
The multicenter NONSEDA trial included 700 mechanically ventilated ICU patients randomized either to no sedation or to light sedation, such that the patient was arousable, with daily interruption.
Previous studies have shown that daily interruption of sedation reduced mechanical ventilation duration, ICU stay length, and mortality in comparison with no interruption, the investigators noted.
While mortality at 90 days did not differ significantly between the no-sedation and light-sedation approaches, no sedation reduced thromboembolic events, Dr. Toft said at the meeting. The number of thrombolic events within 90 days was 10 (5%) in the sedation group and 1 (0.5%) in the no-sedation group (P less than .05), according to the reported data.
Likewise, several measures of physical function significantly improved in an a prior defined subgroup of 200 patients, he said. Those measures included hand grip at extubation and ICU discharge, as well as scores on the Barthel Index for Activities of Daily Living.
Nonsedation might improve kidney function, based on other reported outcomes of the study, Dr. Toft said. The number of coma- and delirium-free days was 3.0 in the no-sedation group versus 1.0 in the sedation group (P less than .01), he added.
The benefits of no sedation may extend beyond objective changes in health outcomes, according to Dr. Toft. “The patients are able to communicate with the staff, they might be able to enjoy food, in the evening they can look at the television instead of being sedated – and they can be mobilized and they can write their opinion about the treatments to the doctor, and in this way, you have two-way communication,” he explained in his presentation.
Dr. Toft reported that he had no financial relationships to disclose.
SOURCE: Toft P et al. N Engl J Med. 2019 Feb 16. doi: 10.1056/NEJMoa1906759.
ORLANDO – For critically ill, according to results of a multicenter, randomized trial.
The lack of sedation did significantly improve certain secondary endpoints, including a reduced number of thromboembolic events and preservation of physical function, according to Palle Toft, PhD, DMSc, of Odense (Denmark) University Hospital.
However, the 90-day mortality rate was 42.4% in the no-sedation group versus 37.0% in the sedation group in the NONSEDA study, which was intended to test the hypothesis that mortality would be lower in the no-sedation group.
That 5.4 percentage point difference between arms in NONSEDA was not statistically significant (P = .65) in results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine and concurrently published in the New England Journal of Medicine.
Yet that mortality trend is in the “opposite direction” of an earlier, single-center trial by Dr. Toft and colleagues, noted Claude Guérin, MD, PhD, in a related editorial that also appeared in the journal. In that earlier study, the reported hospital mortality rates were 36% for no sedation and 47% for sedation with daily interruption.
“The results from this trial [NONSEDA] are important because they arouse concern about omitting sedation in mechanically ventilated patients and reinforce the need to monitor sedation clinically, with the aim of discontinuing it as early as possible or at least interrupting it daily,” Dr. Guérin wrote in his editorial.
That said, the earlier, single-center trial was not statistically powered to show between-group differences in mortality, Dr. Toft and coauthors wrote in their journal article.
In his presentation, Dr. Toft emphasized that light sedation with a wake-up trial was “comparable” with no sedation with regard to mortality.
“I think my main message is that we have to individualize patient treatment,” Dr. Toft told attendees at a late-breaking literature session. “Many patients would benefit from nonsedation, and some would benefit by light sedation with a daily wake-up trial. We have to respect patient autonomy, and try to establish a two-way communication with patients in 2020.”
Sandra L. Kane-Gill, PharmD, treasurer of SCCM and assistant professor of pharmacy and therapeutics at the University of Pittsburgh, said that current SCCM guidelines recommend using light sedation in critically ill, mechanically ventilated adults.
“I think we should stay consistent with what the guidelines are saying,” Dr. Kane-Gill said in an interview. “How you do that may vary, but targeting light sedation is consistent with what the evidence is suggesting in those guidelines.”
The depth of sedation between the no-sedation group in the light sedation group in the present study was not as great as the investigators had anticipated, which may explain the lack of statistically significant difference in mortality, according to Dr. Kane-Gill.
According to the report, 38.4% of patients in the no-sedation group received medication for sedation during their ICU stay, while Richmond Agitation and Sedation Scores increased in both groups, indicating a more alert state in both groups.
The multicenter NONSEDA trial included 700 mechanically ventilated ICU patients randomized either to no sedation or to light sedation, such that the patient was arousable, with daily interruption.
Previous studies have shown that daily interruption of sedation reduced mechanical ventilation duration, ICU stay length, and mortality in comparison with no interruption, the investigators noted.
While mortality at 90 days did not differ significantly between the no-sedation and light-sedation approaches, no sedation reduced thromboembolic events, Dr. Toft said at the meeting. The number of thrombolic events within 90 days was 10 (5%) in the sedation group and 1 (0.5%) in the no-sedation group (P less than .05), according to the reported data.
Likewise, several measures of physical function significantly improved in an a prior defined subgroup of 200 patients, he said. Those measures included hand grip at extubation and ICU discharge, as well as scores on the Barthel Index for Activities of Daily Living.
Nonsedation might improve kidney function, based on other reported outcomes of the study, Dr. Toft said. The number of coma- and delirium-free days was 3.0 in the no-sedation group versus 1.0 in the sedation group (P less than .01), he added.
The benefits of no sedation may extend beyond objective changes in health outcomes, according to Dr. Toft. “The patients are able to communicate with the staff, they might be able to enjoy food, in the evening they can look at the television instead of being sedated – and they can be mobilized and they can write their opinion about the treatments to the doctor, and in this way, you have two-way communication,” he explained in his presentation.
Dr. Toft reported that he had no financial relationships to disclose.
SOURCE: Toft P et al. N Engl J Med. 2019 Feb 16. doi: 10.1056/NEJMoa1906759.
ORLANDO – For critically ill, according to results of a multicenter, randomized trial.
The lack of sedation did significantly improve certain secondary endpoints, including a reduced number of thromboembolic events and preservation of physical function, according to Palle Toft, PhD, DMSc, of Odense (Denmark) University Hospital.
However, the 90-day mortality rate was 42.4% in the no-sedation group versus 37.0% in the sedation group in the NONSEDA study, which was intended to test the hypothesis that mortality would be lower in the no-sedation group.
That 5.4 percentage point difference between arms in NONSEDA was not statistically significant (P = .65) in results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine and concurrently published in the New England Journal of Medicine.
Yet that mortality trend is in the “opposite direction” of an earlier, single-center trial by Dr. Toft and colleagues, noted Claude Guérin, MD, PhD, in a related editorial that also appeared in the journal. In that earlier study, the reported hospital mortality rates were 36% for no sedation and 47% for sedation with daily interruption.
“The results from this trial [NONSEDA] are important because they arouse concern about omitting sedation in mechanically ventilated patients and reinforce the need to monitor sedation clinically, with the aim of discontinuing it as early as possible or at least interrupting it daily,” Dr. Guérin wrote in his editorial.
That said, the earlier, single-center trial was not statistically powered to show between-group differences in mortality, Dr. Toft and coauthors wrote in their journal article.
In his presentation, Dr. Toft emphasized that light sedation with a wake-up trial was “comparable” with no sedation with regard to mortality.
“I think my main message is that we have to individualize patient treatment,” Dr. Toft told attendees at a late-breaking literature session. “Many patients would benefit from nonsedation, and some would benefit by light sedation with a daily wake-up trial. We have to respect patient autonomy, and try to establish a two-way communication with patients in 2020.”
Sandra L. Kane-Gill, PharmD, treasurer of SCCM and assistant professor of pharmacy and therapeutics at the University of Pittsburgh, said that current SCCM guidelines recommend using light sedation in critically ill, mechanically ventilated adults.
“I think we should stay consistent with what the guidelines are saying,” Dr. Kane-Gill said in an interview. “How you do that may vary, but targeting light sedation is consistent with what the evidence is suggesting in those guidelines.”
The depth of sedation between the no-sedation group in the light sedation group in the present study was not as great as the investigators had anticipated, which may explain the lack of statistically significant difference in mortality, according to Dr. Kane-Gill.
According to the report, 38.4% of patients in the no-sedation group received medication for sedation during their ICU stay, while Richmond Agitation and Sedation Scores increased in both groups, indicating a more alert state in both groups.
The multicenter NONSEDA trial included 700 mechanically ventilated ICU patients randomized either to no sedation or to light sedation, such that the patient was arousable, with daily interruption.
Previous studies have shown that daily interruption of sedation reduced mechanical ventilation duration, ICU stay length, and mortality in comparison with no interruption, the investigators noted.
While mortality at 90 days did not differ significantly between the no-sedation and light-sedation approaches, no sedation reduced thromboembolic events, Dr. Toft said at the meeting. The number of thrombolic events within 90 days was 10 (5%) in the sedation group and 1 (0.5%) in the no-sedation group (P less than .05), according to the reported data.
Likewise, several measures of physical function significantly improved in an a prior defined subgroup of 200 patients, he said. Those measures included hand grip at extubation and ICU discharge, as well as scores on the Barthel Index for Activities of Daily Living.
Nonsedation might improve kidney function, based on other reported outcomes of the study, Dr. Toft said. The number of coma- and delirium-free days was 3.0 in the no-sedation group versus 1.0 in the sedation group (P less than .01), he added.
The benefits of no sedation may extend beyond objective changes in health outcomes, according to Dr. Toft. “The patients are able to communicate with the staff, they might be able to enjoy food, in the evening they can look at the television instead of being sedated – and they can be mobilized and they can write their opinion about the treatments to the doctor, and in this way, you have two-way communication,” he explained in his presentation.
Dr. Toft reported that he had no financial relationships to disclose.
SOURCE: Toft P et al. N Engl J Med. 2019 Feb 16. doi: 10.1056/NEJMoa1906759.
REPORTING FROM CCC49
Guidance defines vaping-related respiratory syndrome
ORLANDO – Knowledge of vaping devices, familiarity with terminology, and the ability to quickly pinpoint individuals at risk of lung injury are just a few skills that can help critical care professionals confronted with patients who may have vaping-associated lung disease, according to a new guidance document.
The guidance offers a risk-stratification system that classifies patients into groups based on exposure, symptoms, and imaging results, and provides specific evaluation needs and management strategies for each. The guidance is designed to help critical care professionals efficiently identify those at high risk of respiratory failure.
Physicians also need to communicate with patients to identify what substances are being vaped and develop effective methods to encourage abstinence, according to the authors, led by Craig M. Lilly, MD, FCCP, professor of medicine, anesthesiology, and surgery at the University of Massachusetts, Worcester.
“I would encourage every intensivist, when they leave their intensive care unit at night, [to ask], ‘have I advised against vaping today?’ ” Dr. Lilly said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The guidelines, concurrently published as a review article in Critical Care Explorations, propose the term vaping-associated respiratory distress syndrome (VARDS), which the authors say constitutes an acute and progressive respiratory syndrome marked by pathologic changes of lung injury and potentially life-threatening hypoxemic respiratory failure.
They also introduce the three-group Worcester classification system, which is intended to triage vaping-exposed individuals for risk of VARDS based on the presence or absence of vaping-related symptoms and infiltrates, and normal or abnormal oxygen saturation.
“It’s very simple,” said Dr. Lilly, who added that the risk stratification model was developed at the request of Massachusetts public health officials.
Patients with vaping exposure but no symptoms attributable to vaping, such as cough, chest pain, or weight loss, are classified as Worcester Low Risk and testing is not recommended, he said.
By contrast, individuals are considered Worcester Medium Risk if they have vaping exposure, symptoms, and a vaping-associated abnormal pattern on imaging, but no hypoxemia; the presence of hypoxemia would tip the scale toward Worcester High Risk.
“Most patients that have died from vaping have been sent out of emergency rooms when they were noted to be hypoxic,” Dr. Lilly told meeting attendees.
Louella B. Amos, MD, a pediatric pulmonologist at Children’s Hospital of Wisconsin in Milwaukee, said she expects the guidance and risk stratification system will be useful not only for critical care specialists, but for other health care providers as well.
“It’s important to make decisions relatively quickly, depending on the severity of symptoms, and I think this is nice and simple,” Dr. Amos said in an interview.
“We always triage when we see patients, either at the door or in our clinic, or behind that, even in the hospital,” she said. “So I think this can be a great tool for everybody, not only the intensivist, but people who are triaging at the front.”
Management of individuals at low risk of VARDS begins with encouragement of abstinence. “We think that every vaping patient should be advised to quit vaping,” Dr. Lilly said. Patients who are interested in quitting who have not yet worked with someone in their health care team whom they trust can be referred to their primary care physicians for counseling, he added, while those struggling with addiction, unable to quit, and unable to partner with a primary care physician can be referred to an addiction medicine specialist.
For moderate-risk patients, vaping cessation is “absolutely mandatory,” said Dr. Lilly, who recommended monitoring of vaping abstinence, outpatient evaluation based on imaging studies, and adequate follow-up to ensure symptoms resolve, tests normalize, and daily activities bounce back to baseline levels.
The guidance offers more extensive recommendations for the VARDS high-risk group, including supervised vaping abstinence, continuous pulse oximetry, and early intervention with noninvasive ventilation, and mechanical ventilation if required, Dr. Lilly said.
Judging vaping exposure is challenging, requiring clinicians to have a familiarity with the many different devices that are available.
Beyond device type, he added, it’s important to know the various terms for devices and lingo that patients may use to describe them, what solutions are vaped, whether those solutions are commercially prepared or off the street, the dose the device delivers, and a number of other factors, he said.
Clinical evaluation typically comes down to unexplained cough, chest pain, weight loss, fatigue, or dyspnea, though one other clue is whether there are gastrointestinal symptoms: “The same way that aerosols can go down to the lungs, they also go into the GI tract, and when nausea, vomiting, or cramping abdominal pain is tightly associated with vaping exposure, one should assume that the patient has been toxin exposed,” he explained.
Dr. Lilly said he had no financial relationships to disclose.
ORLANDO – Knowledge of vaping devices, familiarity with terminology, and the ability to quickly pinpoint individuals at risk of lung injury are just a few skills that can help critical care professionals confronted with patients who may have vaping-associated lung disease, according to a new guidance document.
The guidance offers a risk-stratification system that classifies patients into groups based on exposure, symptoms, and imaging results, and provides specific evaluation needs and management strategies for each. The guidance is designed to help critical care professionals efficiently identify those at high risk of respiratory failure.
Physicians also need to communicate with patients to identify what substances are being vaped and develop effective methods to encourage abstinence, according to the authors, led by Craig M. Lilly, MD, FCCP, professor of medicine, anesthesiology, and surgery at the University of Massachusetts, Worcester.
“I would encourage every intensivist, when they leave their intensive care unit at night, [to ask], ‘have I advised against vaping today?’ ” Dr. Lilly said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The guidelines, concurrently published as a review article in Critical Care Explorations, propose the term vaping-associated respiratory distress syndrome (VARDS), which the authors say constitutes an acute and progressive respiratory syndrome marked by pathologic changes of lung injury and potentially life-threatening hypoxemic respiratory failure.
They also introduce the three-group Worcester classification system, which is intended to triage vaping-exposed individuals for risk of VARDS based on the presence or absence of vaping-related symptoms and infiltrates, and normal or abnormal oxygen saturation.
“It’s very simple,” said Dr. Lilly, who added that the risk stratification model was developed at the request of Massachusetts public health officials.
Patients with vaping exposure but no symptoms attributable to vaping, such as cough, chest pain, or weight loss, are classified as Worcester Low Risk and testing is not recommended, he said.
By contrast, individuals are considered Worcester Medium Risk if they have vaping exposure, symptoms, and a vaping-associated abnormal pattern on imaging, but no hypoxemia; the presence of hypoxemia would tip the scale toward Worcester High Risk.
“Most patients that have died from vaping have been sent out of emergency rooms when they were noted to be hypoxic,” Dr. Lilly told meeting attendees.
Louella B. Amos, MD, a pediatric pulmonologist at Children’s Hospital of Wisconsin in Milwaukee, said she expects the guidance and risk stratification system will be useful not only for critical care specialists, but for other health care providers as well.
“It’s important to make decisions relatively quickly, depending on the severity of symptoms, and I think this is nice and simple,” Dr. Amos said in an interview.
“We always triage when we see patients, either at the door or in our clinic, or behind that, even in the hospital,” she said. “So I think this can be a great tool for everybody, not only the intensivist, but people who are triaging at the front.”
Management of individuals at low risk of VARDS begins with encouragement of abstinence. “We think that every vaping patient should be advised to quit vaping,” Dr. Lilly said. Patients who are interested in quitting who have not yet worked with someone in their health care team whom they trust can be referred to their primary care physicians for counseling, he added, while those struggling with addiction, unable to quit, and unable to partner with a primary care physician can be referred to an addiction medicine specialist.
For moderate-risk patients, vaping cessation is “absolutely mandatory,” said Dr. Lilly, who recommended monitoring of vaping abstinence, outpatient evaluation based on imaging studies, and adequate follow-up to ensure symptoms resolve, tests normalize, and daily activities bounce back to baseline levels.
The guidance offers more extensive recommendations for the VARDS high-risk group, including supervised vaping abstinence, continuous pulse oximetry, and early intervention with noninvasive ventilation, and mechanical ventilation if required, Dr. Lilly said.
Judging vaping exposure is challenging, requiring clinicians to have a familiarity with the many different devices that are available.
Beyond device type, he added, it’s important to know the various terms for devices and lingo that patients may use to describe them, what solutions are vaped, whether those solutions are commercially prepared or off the street, the dose the device delivers, and a number of other factors, he said.
Clinical evaluation typically comes down to unexplained cough, chest pain, weight loss, fatigue, or dyspnea, though one other clue is whether there are gastrointestinal symptoms: “The same way that aerosols can go down to the lungs, they also go into the GI tract, and when nausea, vomiting, or cramping abdominal pain is tightly associated with vaping exposure, one should assume that the patient has been toxin exposed,” he explained.
Dr. Lilly said he had no financial relationships to disclose.
ORLANDO – Knowledge of vaping devices, familiarity with terminology, and the ability to quickly pinpoint individuals at risk of lung injury are just a few skills that can help critical care professionals confronted with patients who may have vaping-associated lung disease, according to a new guidance document.
The guidance offers a risk-stratification system that classifies patients into groups based on exposure, symptoms, and imaging results, and provides specific evaluation needs and management strategies for each. The guidance is designed to help critical care professionals efficiently identify those at high risk of respiratory failure.
Physicians also need to communicate with patients to identify what substances are being vaped and develop effective methods to encourage abstinence, according to the authors, led by Craig M. Lilly, MD, FCCP, professor of medicine, anesthesiology, and surgery at the University of Massachusetts, Worcester.
“I would encourage every intensivist, when they leave their intensive care unit at night, [to ask], ‘have I advised against vaping today?’ ” Dr. Lilly said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
The guidelines, concurrently published as a review article in Critical Care Explorations, propose the term vaping-associated respiratory distress syndrome (VARDS), which the authors say constitutes an acute and progressive respiratory syndrome marked by pathologic changes of lung injury and potentially life-threatening hypoxemic respiratory failure.
They also introduce the three-group Worcester classification system, which is intended to triage vaping-exposed individuals for risk of VARDS based on the presence or absence of vaping-related symptoms and infiltrates, and normal or abnormal oxygen saturation.
“It’s very simple,” said Dr. Lilly, who added that the risk stratification model was developed at the request of Massachusetts public health officials.
Patients with vaping exposure but no symptoms attributable to vaping, such as cough, chest pain, or weight loss, are classified as Worcester Low Risk and testing is not recommended, he said.
By contrast, individuals are considered Worcester Medium Risk if they have vaping exposure, symptoms, and a vaping-associated abnormal pattern on imaging, but no hypoxemia; the presence of hypoxemia would tip the scale toward Worcester High Risk.
“Most patients that have died from vaping have been sent out of emergency rooms when they were noted to be hypoxic,” Dr. Lilly told meeting attendees.
Louella B. Amos, MD, a pediatric pulmonologist at Children’s Hospital of Wisconsin in Milwaukee, said she expects the guidance and risk stratification system will be useful not only for critical care specialists, but for other health care providers as well.
“It’s important to make decisions relatively quickly, depending on the severity of symptoms, and I think this is nice and simple,” Dr. Amos said in an interview.
“We always triage when we see patients, either at the door or in our clinic, or behind that, even in the hospital,” she said. “So I think this can be a great tool for everybody, not only the intensivist, but people who are triaging at the front.”
Management of individuals at low risk of VARDS begins with encouragement of abstinence. “We think that every vaping patient should be advised to quit vaping,” Dr. Lilly said. Patients who are interested in quitting who have not yet worked with someone in their health care team whom they trust can be referred to their primary care physicians for counseling, he added, while those struggling with addiction, unable to quit, and unable to partner with a primary care physician can be referred to an addiction medicine specialist.
For moderate-risk patients, vaping cessation is “absolutely mandatory,” said Dr. Lilly, who recommended monitoring of vaping abstinence, outpatient evaluation based on imaging studies, and adequate follow-up to ensure symptoms resolve, tests normalize, and daily activities bounce back to baseline levels.
The guidance offers more extensive recommendations for the VARDS high-risk group, including supervised vaping abstinence, continuous pulse oximetry, and early intervention with noninvasive ventilation, and mechanical ventilation if required, Dr. Lilly said.
Judging vaping exposure is challenging, requiring clinicians to have a familiarity with the many different devices that are available.
Beyond device type, he added, it’s important to know the various terms for devices and lingo that patients may use to describe them, what solutions are vaped, whether those solutions are commercially prepared or off the street, the dose the device delivers, and a number of other factors, he said.
Clinical evaluation typically comes down to unexplained cough, chest pain, weight loss, fatigue, or dyspnea, though one other clue is whether there are gastrointestinal symptoms: “The same way that aerosols can go down to the lungs, they also go into the GI tract, and when nausea, vomiting, or cramping abdominal pain is tightly associated with vaping exposure, one should assume that the patient has been toxin exposed,” he explained.
Dr. Lilly said he had no financial relationships to disclose.
REPORTING FROM CCC49
Medicare beneficiaries get few home health visits after ICU stay
ORLANDO – , an analysis of hospital and home health claims data suggests.
The beneficiaries, all discharged directly to home health after an intensive care unit stay, received an average of less than one visit per week in the ensuing month, while a full third received no visits at all, according to authors of the analysis, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Living alone and living in a rural area were associated with significantly fewer home health rehabilitation visits, according to investigator Jason Raymond Falvey, PT, DPT, PhD, of Yale University, New Haven, Conn.
“We identified that these patients are receiving low doses of rehabilitation in home health care settings, and perhaps doses low enough to not be physiologically adequate to overcome the deconditioning and aerobic capacity concerns that these patients have,” Dr. Falvey said.
These findings reflect an “underrecognition” of the importance of rehabilitation both outside and inside the hospital setting, according to Patricia J. Posa, RN, of Saint Joseph Mercy Hospital, Northville, Mich.
“We even struggle to provide sufficient rehabilitation while they’re in the hospital,” Ms. Posa said in an interview. “So I think that we still have a major gap in providing rehab services across the continuum, and part of that is recognizing the deficits that patients, especially our elderly patients, might be leaving the hospital with.”
Medicare beneficiaries who survive a critical illness are often discharged with referrals for physical, occupational, or speech therapy, yet there are not much data on the delivery of that care or how many visits actually take place, according to Dr. Falvey.
He and coinvestigators analyzed data on 3,176 Medicare beneficiaries discharged to home health right after an acute hospitalization with an ICU stay of at least 24 hours. To do this, they linked 2012 Medicare hospital and home health claims data with Medicare demographic and patient assessment data.
They found that the beneficiaries received just 3.5 home rehabilitation visits in 30 days, while 33% had no visits on record.
The factors most strongly associated with receiving fewer rehabilitation visits, in adjusted models, included living in a rural setting, with a rate ratio (RR) of 0.87 and living alone, with an RR of 0.88.
Higher comorbidity count also was associated with fewer visits (RR, 0.98), according to the investigators.
On the other hand, Medicare beneficiaries who received more visits were more likely to be older (RR, 1.03; 1.01-1.04; for every 5 years), more likely to have higher disability scores (RR, 1.03; 1.02-1.04; per point on the Elixhauser Comorbidity Index), and more likely to have reported severe dyspnea (RR, 1.12; 1.04-1.21), according to the report.
More research will be needed to determine the appropriate number of home health rehabilitation visits for older hospitalized patients, according to Ms. Pena, a member of the Society of Critical Care Medicine’s ICU Liberation initiative, which aims to free patients from the harmful effects of pain, agitation/sedation, delirium, immobility, and sleep disruption in the ICU, as well as improve patient outcomes after an ICU stay.
The literature is already fairly robust, she said, on how frequently visits are warranted following specific scenarios such as postsurgical hip or knee replacement or stroke.
“For the general hospitalized patients that are just losing function because they were sick and didn’t get out of bed enough, we don’t really have good data to say, ‘you know, they need three visits a week, or they need two visits a week for an hour in order to improve,’ ” she said, “so the science is still not caught up with the frequency.”
In the absence of data, the number of visits may be left up to an individual clinician’s knowledge and past experience as well as what insurance will pay for, Ms. Pena said.
Dr. Falvey reported royalties related to an online continuing education course on hospital readmissions. No other disclosures were reported.
SOURCE: Falvey J et al. Crit Care Med. 2020 Jan;48(1):28.
ORLANDO – , an analysis of hospital and home health claims data suggests.
The beneficiaries, all discharged directly to home health after an intensive care unit stay, received an average of less than one visit per week in the ensuing month, while a full third received no visits at all, according to authors of the analysis, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Living alone and living in a rural area were associated with significantly fewer home health rehabilitation visits, according to investigator Jason Raymond Falvey, PT, DPT, PhD, of Yale University, New Haven, Conn.
“We identified that these patients are receiving low doses of rehabilitation in home health care settings, and perhaps doses low enough to not be physiologically adequate to overcome the deconditioning and aerobic capacity concerns that these patients have,” Dr. Falvey said.
These findings reflect an “underrecognition” of the importance of rehabilitation both outside and inside the hospital setting, according to Patricia J. Posa, RN, of Saint Joseph Mercy Hospital, Northville, Mich.
“We even struggle to provide sufficient rehabilitation while they’re in the hospital,” Ms. Posa said in an interview. “So I think that we still have a major gap in providing rehab services across the continuum, and part of that is recognizing the deficits that patients, especially our elderly patients, might be leaving the hospital with.”
Medicare beneficiaries who survive a critical illness are often discharged with referrals for physical, occupational, or speech therapy, yet there are not much data on the delivery of that care or how many visits actually take place, according to Dr. Falvey.
He and coinvestigators analyzed data on 3,176 Medicare beneficiaries discharged to home health right after an acute hospitalization with an ICU stay of at least 24 hours. To do this, they linked 2012 Medicare hospital and home health claims data with Medicare demographic and patient assessment data.
They found that the beneficiaries received just 3.5 home rehabilitation visits in 30 days, while 33% had no visits on record.
The factors most strongly associated with receiving fewer rehabilitation visits, in adjusted models, included living in a rural setting, with a rate ratio (RR) of 0.87 and living alone, with an RR of 0.88.
Higher comorbidity count also was associated with fewer visits (RR, 0.98), according to the investigators.
On the other hand, Medicare beneficiaries who received more visits were more likely to be older (RR, 1.03; 1.01-1.04; for every 5 years), more likely to have higher disability scores (RR, 1.03; 1.02-1.04; per point on the Elixhauser Comorbidity Index), and more likely to have reported severe dyspnea (RR, 1.12; 1.04-1.21), according to the report.
More research will be needed to determine the appropriate number of home health rehabilitation visits for older hospitalized patients, according to Ms. Pena, a member of the Society of Critical Care Medicine’s ICU Liberation initiative, which aims to free patients from the harmful effects of pain, agitation/sedation, delirium, immobility, and sleep disruption in the ICU, as well as improve patient outcomes after an ICU stay.
The literature is already fairly robust, she said, on how frequently visits are warranted following specific scenarios such as postsurgical hip or knee replacement or stroke.
“For the general hospitalized patients that are just losing function because they were sick and didn’t get out of bed enough, we don’t really have good data to say, ‘you know, they need three visits a week, or they need two visits a week for an hour in order to improve,’ ” she said, “so the science is still not caught up with the frequency.”
In the absence of data, the number of visits may be left up to an individual clinician’s knowledge and past experience as well as what insurance will pay for, Ms. Pena said.
Dr. Falvey reported royalties related to an online continuing education course on hospital readmissions. No other disclosures were reported.
SOURCE: Falvey J et al. Crit Care Med. 2020 Jan;48(1):28.
ORLANDO – , an analysis of hospital and home health claims data suggests.
The beneficiaries, all discharged directly to home health after an intensive care unit stay, received an average of less than one visit per week in the ensuing month, while a full third received no visits at all, according to authors of the analysis, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
Living alone and living in a rural area were associated with significantly fewer home health rehabilitation visits, according to investigator Jason Raymond Falvey, PT, DPT, PhD, of Yale University, New Haven, Conn.
“We identified that these patients are receiving low doses of rehabilitation in home health care settings, and perhaps doses low enough to not be physiologically adequate to overcome the deconditioning and aerobic capacity concerns that these patients have,” Dr. Falvey said.
These findings reflect an “underrecognition” of the importance of rehabilitation both outside and inside the hospital setting, according to Patricia J. Posa, RN, of Saint Joseph Mercy Hospital, Northville, Mich.
“We even struggle to provide sufficient rehabilitation while they’re in the hospital,” Ms. Posa said in an interview. “So I think that we still have a major gap in providing rehab services across the continuum, and part of that is recognizing the deficits that patients, especially our elderly patients, might be leaving the hospital with.”
Medicare beneficiaries who survive a critical illness are often discharged with referrals for physical, occupational, or speech therapy, yet there are not much data on the delivery of that care or how many visits actually take place, according to Dr. Falvey.
He and coinvestigators analyzed data on 3,176 Medicare beneficiaries discharged to home health right after an acute hospitalization with an ICU stay of at least 24 hours. To do this, they linked 2012 Medicare hospital and home health claims data with Medicare demographic and patient assessment data.
They found that the beneficiaries received just 3.5 home rehabilitation visits in 30 days, while 33% had no visits on record.
The factors most strongly associated with receiving fewer rehabilitation visits, in adjusted models, included living in a rural setting, with a rate ratio (RR) of 0.87 and living alone, with an RR of 0.88.
Higher comorbidity count also was associated with fewer visits (RR, 0.98), according to the investigators.
On the other hand, Medicare beneficiaries who received more visits were more likely to be older (RR, 1.03; 1.01-1.04; for every 5 years), more likely to have higher disability scores (RR, 1.03; 1.02-1.04; per point on the Elixhauser Comorbidity Index), and more likely to have reported severe dyspnea (RR, 1.12; 1.04-1.21), according to the report.
More research will be needed to determine the appropriate number of home health rehabilitation visits for older hospitalized patients, according to Ms. Pena, a member of the Society of Critical Care Medicine’s ICU Liberation initiative, which aims to free patients from the harmful effects of pain, agitation/sedation, delirium, immobility, and sleep disruption in the ICU, as well as improve patient outcomes after an ICU stay.
The literature is already fairly robust, she said, on how frequently visits are warranted following specific scenarios such as postsurgical hip or knee replacement or stroke.
“For the general hospitalized patients that are just losing function because they were sick and didn’t get out of bed enough, we don’t really have good data to say, ‘you know, they need three visits a week, or they need two visits a week for an hour in order to improve,’ ” she said, “so the science is still not caught up with the frequency.”
In the absence of data, the number of visits may be left up to an individual clinician’s knowledge and past experience as well as what insurance will pay for, Ms. Pena said.
Dr. Falvey reported royalties related to an online continuing education course on hospital readmissions. No other disclosures were reported.
SOURCE: Falvey J et al. Crit Care Med. 2020 Jan;48(1):28.
REPORTING FROM CCC49
Opioid use disorder up in sepsis hospitalizations
ORLANDO –
The prevalence of opioid use disorder (OUD) has significantly increased over the past 15 years, the analysis further shows.
Results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine, further suggested that OUD disproportionately contributes to sepsis deaths in younger, healthier patients.
Together, these findings underscore the importance of ongoing efforts to address the opioid epidemic in the United States, according to researcher Mohammad Alrawashdeh, PhD, MSN, a postdoctoral research fellow with Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston.
“In addition to ongoing efforts to combat the opioid crisis, future public health interventions should focus on increasing awareness, recognition, and aggressive treatment of sepsis in this population,” Dr. Alrawashdeh said in an oral presentation of the study.
This study fills an important knowledge gap regarding the connection between OUD and sepsis, according to Greg S. Martin, MD, MS, FCCM, professor of medicine in pulmonary critical care at Emory University, Atlanta, and secretary for the Society of Critical Care Medicine.
“We’ve not really ever been able to piece together the relationship between opioid use disorders and sepsis,” Dr. Martin said in an interview. “It’s not that people wouldn’t suspect that there’s a connection – it’s more that we have simply not been able to get the kind of data that you can use, like they’ve done here, that really helps you to answer that question.”
The study suggests not only that OUD and sepsis are linked, Dr. Martin added, but that health care providers need to be prepared to potentially see further increases in the number of patients with OUD seen in the intensive care unit.
“Both of those are things that we certainly need to be aware of, both from the individual practitioner perspective and also the public health planning perspective,” he said.
The retrospective study by Dr. Alrawashdeh and coinvestigators focused on electronic health record data for adults admitted to 373 hospitals in the United States between 2009 and 2015, including 375,479 who had sepsis.
Over time, there was a significant increase in the prevalence of OUD among those hospitalized for sepsis, from less than 2.0% in 2009 to more than 3% in 2015, representing a significant 77.3% increase. In general, the prevalence of sepsis was significantly higher among hospitalized patients with OUD compared with patients without the disorder, at 7.2% and 5.6%, respectively.
The sepsis patients with OUD tended to be younger, healthier, and more likely to be white compared with patients without OUD, according to the report. Moreover, the sepsis patients with OUD more often had endocarditis and gram-positive and fungal bloodstream infections. They also required more mechanical ventilation and had more ICU admissions, with longer stays in both the ICU and hospital.
The OUD patients accounted for 2.1% of sepsis-associated deaths overall, but 3.3% of those deaths in healthy patients, and 7.1% of deaths among younger patients, according to the report.
Those findings provide some clues that could help guide clinical practice, according to Dr. Martin. For example, the data show a nearly fivefold increased risk of endocarditis with OUD (3.9% versus 0.7%), which may inform screening practices.
“While we don’t necessarily screen every sepsis patient for endocarditis, if it’s an opioid use disorder patient – particularly one with a bloodstream infection – then that’s almost certainly something you should be doing,” Dr. Martin said.
The data suggest gram-positive bacterial and fungal infections will more likely be encountered among these patients, which could guide empiric treatment, he said.
Providers specializing in OUD should have a heightened awareness of the potential for infection and sepsis among those patients, and perhaps be more attuned to fever and other signs of infection that might warrant a referral or additional care, Dr. Martin added.
Dr. Alrawashdeh reported no disclosures related to the study.
SOURCE: Alrawashdeh M et al. Crit Care Med. 2020 Jan;48(1):28. Abstract 56.
ORLANDO –
The prevalence of opioid use disorder (OUD) has significantly increased over the past 15 years, the analysis further shows.
Results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine, further suggested that OUD disproportionately contributes to sepsis deaths in younger, healthier patients.
Together, these findings underscore the importance of ongoing efforts to address the opioid epidemic in the United States, according to researcher Mohammad Alrawashdeh, PhD, MSN, a postdoctoral research fellow with Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston.
“In addition to ongoing efforts to combat the opioid crisis, future public health interventions should focus on increasing awareness, recognition, and aggressive treatment of sepsis in this population,” Dr. Alrawashdeh said in an oral presentation of the study.
This study fills an important knowledge gap regarding the connection between OUD and sepsis, according to Greg S. Martin, MD, MS, FCCM, professor of medicine in pulmonary critical care at Emory University, Atlanta, and secretary for the Society of Critical Care Medicine.
“We’ve not really ever been able to piece together the relationship between opioid use disorders and sepsis,” Dr. Martin said in an interview. “It’s not that people wouldn’t suspect that there’s a connection – it’s more that we have simply not been able to get the kind of data that you can use, like they’ve done here, that really helps you to answer that question.”
The study suggests not only that OUD and sepsis are linked, Dr. Martin added, but that health care providers need to be prepared to potentially see further increases in the number of patients with OUD seen in the intensive care unit.
“Both of those are things that we certainly need to be aware of, both from the individual practitioner perspective and also the public health planning perspective,” he said.
The retrospective study by Dr. Alrawashdeh and coinvestigators focused on electronic health record data for adults admitted to 373 hospitals in the United States between 2009 and 2015, including 375,479 who had sepsis.
Over time, there was a significant increase in the prevalence of OUD among those hospitalized for sepsis, from less than 2.0% in 2009 to more than 3% in 2015, representing a significant 77.3% increase. In general, the prevalence of sepsis was significantly higher among hospitalized patients with OUD compared with patients without the disorder, at 7.2% and 5.6%, respectively.
The sepsis patients with OUD tended to be younger, healthier, and more likely to be white compared with patients without OUD, according to the report. Moreover, the sepsis patients with OUD more often had endocarditis and gram-positive and fungal bloodstream infections. They also required more mechanical ventilation and had more ICU admissions, with longer stays in both the ICU and hospital.
The OUD patients accounted for 2.1% of sepsis-associated deaths overall, but 3.3% of those deaths in healthy patients, and 7.1% of deaths among younger patients, according to the report.
Those findings provide some clues that could help guide clinical practice, according to Dr. Martin. For example, the data show a nearly fivefold increased risk of endocarditis with OUD (3.9% versus 0.7%), which may inform screening practices.
“While we don’t necessarily screen every sepsis patient for endocarditis, if it’s an opioid use disorder patient – particularly one with a bloodstream infection – then that’s almost certainly something you should be doing,” Dr. Martin said.
The data suggest gram-positive bacterial and fungal infections will more likely be encountered among these patients, which could guide empiric treatment, he said.
Providers specializing in OUD should have a heightened awareness of the potential for infection and sepsis among those patients, and perhaps be more attuned to fever and other signs of infection that might warrant a referral or additional care, Dr. Martin added.
Dr. Alrawashdeh reported no disclosures related to the study.
SOURCE: Alrawashdeh M et al. Crit Care Med. 2020 Jan;48(1):28. Abstract 56.
ORLANDO –
The prevalence of opioid use disorder (OUD) has significantly increased over the past 15 years, the analysis further shows.
Results of the study, presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine, further suggested that OUD disproportionately contributes to sepsis deaths in younger, healthier patients.
Together, these findings underscore the importance of ongoing efforts to address the opioid epidemic in the United States, according to researcher Mohammad Alrawashdeh, PhD, MSN, a postdoctoral research fellow with Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston.
“In addition to ongoing efforts to combat the opioid crisis, future public health interventions should focus on increasing awareness, recognition, and aggressive treatment of sepsis in this population,” Dr. Alrawashdeh said in an oral presentation of the study.
This study fills an important knowledge gap regarding the connection between OUD and sepsis, according to Greg S. Martin, MD, MS, FCCM, professor of medicine in pulmonary critical care at Emory University, Atlanta, and secretary for the Society of Critical Care Medicine.
“We’ve not really ever been able to piece together the relationship between opioid use disorders and sepsis,” Dr. Martin said in an interview. “It’s not that people wouldn’t suspect that there’s a connection – it’s more that we have simply not been able to get the kind of data that you can use, like they’ve done here, that really helps you to answer that question.”
The study suggests not only that OUD and sepsis are linked, Dr. Martin added, but that health care providers need to be prepared to potentially see further increases in the number of patients with OUD seen in the intensive care unit.
“Both of those are things that we certainly need to be aware of, both from the individual practitioner perspective and also the public health planning perspective,” he said.
The retrospective study by Dr. Alrawashdeh and coinvestigators focused on electronic health record data for adults admitted to 373 hospitals in the United States between 2009 and 2015, including 375,479 who had sepsis.
Over time, there was a significant increase in the prevalence of OUD among those hospitalized for sepsis, from less than 2.0% in 2009 to more than 3% in 2015, representing a significant 77.3% increase. In general, the prevalence of sepsis was significantly higher among hospitalized patients with OUD compared with patients without the disorder, at 7.2% and 5.6%, respectively.
The sepsis patients with OUD tended to be younger, healthier, and more likely to be white compared with patients without OUD, according to the report. Moreover, the sepsis patients with OUD more often had endocarditis and gram-positive and fungal bloodstream infections. They also required more mechanical ventilation and had more ICU admissions, with longer stays in both the ICU and hospital.
The OUD patients accounted for 2.1% of sepsis-associated deaths overall, but 3.3% of those deaths in healthy patients, and 7.1% of deaths among younger patients, according to the report.
Those findings provide some clues that could help guide clinical practice, according to Dr. Martin. For example, the data show a nearly fivefold increased risk of endocarditis with OUD (3.9% versus 0.7%), which may inform screening practices.
“While we don’t necessarily screen every sepsis patient for endocarditis, if it’s an opioid use disorder patient – particularly one with a bloodstream infection – then that’s almost certainly something you should be doing,” Dr. Martin said.
The data suggest gram-positive bacterial and fungal infections will more likely be encountered among these patients, which could guide empiric treatment, he said.
Providers specializing in OUD should have a heightened awareness of the potential for infection and sepsis among those patients, and perhaps be more attuned to fever and other signs of infection that might warrant a referral or additional care, Dr. Martin added.
Dr. Alrawashdeh reported no disclosures related to the study.
SOURCE: Alrawashdeh M et al. Crit Care Med. 2020 Jan;48(1):28. Abstract 56.
REPORTING FROM CCC49
Zilucoplan improved efficacy outcomes in myasthenia gravis
The clinical effect of the self-administered macrocyclic peptide inhibitor was “similar,” the investigators wrote, to what was seen in studies of the intravenously administered complement inhibitor eculizumab, which is approved by the Food and Drug Administration for treatment of gMG.
While eculizumab studies were restricted to patients with refractory gMG, the investigators wrote that their study of zilucoplan included a broader population, including patients who had not failed prior therapies, who were earlier in their disease course, and who had a history of thymoma.
“This observation is important because in gMG, disease severity frequently peaks within the first few years after diagnosis, before all treatment options have been exhausted, and before patients may be formally declared treatment refractory,” wrote James F. Howard Jr, MD, of the University of North Carolina in Chapel Hill, and coauthors.
Complement inhibition is a “targeted approach” that addresses the primary mechanism of tissue damage in gMG, the investigators wrote.
That stands in contrast to conventional gMG treatments including pyridostigmine, corticosteroids, and other immunosuppressants. “These treatments lack strong evidence from clinical trials to support their efficacy, are often poorly tolerated, and can be associated with considerable long-term toxicities,” Dr. Howard and colleagues wrote in their report, which was published in JAMA Neurology.
A total of 44 adult patients with gMG were randomized to receive daily zilucoplan 0.1 mg/kg, 0.3 mg/kg, or placebo for 12 weeks in this 25-center North American study. All patients had acetylcholine receptor autoantibody–positive disease and a Quantitative Myasthenia Gravis (QMG) score of 12 or higher. The QMG score ranges from 0, indicating no muscle weakness, to 39, or severe weakness.
Per the study protocol, patients had to keep taking their current gMG medication without changing the dose.
Change in QMG score from baseline to 12 weeks, the primary efficacy endpoint of the study, showed a significant and clinically meaningful difference favoring zilucoplan 0.3 mg/kg over placebo, according to the investigators.
The mean change was –6.0 points for zilucoplan 0.3 mg/kg and –3.2 for placebo (P = .05), according to their report, which indicated a rapid onset of action apparent 1 week after starting treatment.
Zilucoplan 0.1 mg/kg also yielded a significant and clinically meaningful improvement versus placebo, but its magnitude was smaller and took 4 weeks to become apparent.
Treatment with zilucoplan also significantly improved MG Activities of Daily Living scores versus placebo, a key secondary endpoint of the trial, according to the researchers.
Treatment-emergent adverse events, which included local injection-site reactions, were mild and judged to be unrelated to the study treatment, according to the report.
Ra Pharmaceuticals funded the study. Dr. Howard reported disclosures related to Ra Pharmaceuticals, Alexion Pharmaceuticals, argenx, Viela Bio, and others.
SOURCE: Howard Jr JF et al. JAMA Neurol. 2020 Feb 17. doi: 10.1001/jamaneurol.2019.5125.
The clinical effect of the self-administered macrocyclic peptide inhibitor was “similar,” the investigators wrote, to what was seen in studies of the intravenously administered complement inhibitor eculizumab, which is approved by the Food and Drug Administration for treatment of gMG.
While eculizumab studies were restricted to patients with refractory gMG, the investigators wrote that their study of zilucoplan included a broader population, including patients who had not failed prior therapies, who were earlier in their disease course, and who had a history of thymoma.
“This observation is important because in gMG, disease severity frequently peaks within the first few years after diagnosis, before all treatment options have been exhausted, and before patients may be formally declared treatment refractory,” wrote James F. Howard Jr, MD, of the University of North Carolina in Chapel Hill, and coauthors.
Complement inhibition is a “targeted approach” that addresses the primary mechanism of tissue damage in gMG, the investigators wrote.
That stands in contrast to conventional gMG treatments including pyridostigmine, corticosteroids, and other immunosuppressants. “These treatments lack strong evidence from clinical trials to support their efficacy, are often poorly tolerated, and can be associated with considerable long-term toxicities,” Dr. Howard and colleagues wrote in their report, which was published in JAMA Neurology.
A total of 44 adult patients with gMG were randomized to receive daily zilucoplan 0.1 mg/kg, 0.3 mg/kg, or placebo for 12 weeks in this 25-center North American study. All patients had acetylcholine receptor autoantibody–positive disease and a Quantitative Myasthenia Gravis (QMG) score of 12 or higher. The QMG score ranges from 0, indicating no muscle weakness, to 39, or severe weakness.
Per the study protocol, patients had to keep taking their current gMG medication without changing the dose.
Change in QMG score from baseline to 12 weeks, the primary efficacy endpoint of the study, showed a significant and clinically meaningful difference favoring zilucoplan 0.3 mg/kg over placebo, according to the investigators.
The mean change was –6.0 points for zilucoplan 0.3 mg/kg and –3.2 for placebo (P = .05), according to their report, which indicated a rapid onset of action apparent 1 week after starting treatment.
Zilucoplan 0.1 mg/kg also yielded a significant and clinically meaningful improvement versus placebo, but its magnitude was smaller and took 4 weeks to become apparent.
Treatment with zilucoplan also significantly improved MG Activities of Daily Living scores versus placebo, a key secondary endpoint of the trial, according to the researchers.
Treatment-emergent adverse events, which included local injection-site reactions, were mild and judged to be unrelated to the study treatment, according to the report.
Ra Pharmaceuticals funded the study. Dr. Howard reported disclosures related to Ra Pharmaceuticals, Alexion Pharmaceuticals, argenx, Viela Bio, and others.
SOURCE: Howard Jr JF et al. JAMA Neurol. 2020 Feb 17. doi: 10.1001/jamaneurol.2019.5125.
The clinical effect of the self-administered macrocyclic peptide inhibitor was “similar,” the investigators wrote, to what was seen in studies of the intravenously administered complement inhibitor eculizumab, which is approved by the Food and Drug Administration for treatment of gMG.
While eculizumab studies were restricted to patients with refractory gMG, the investigators wrote that their study of zilucoplan included a broader population, including patients who had not failed prior therapies, who were earlier in their disease course, and who had a history of thymoma.
“This observation is important because in gMG, disease severity frequently peaks within the first few years after diagnosis, before all treatment options have been exhausted, and before patients may be formally declared treatment refractory,” wrote James F. Howard Jr, MD, of the University of North Carolina in Chapel Hill, and coauthors.
Complement inhibition is a “targeted approach” that addresses the primary mechanism of tissue damage in gMG, the investigators wrote.
That stands in contrast to conventional gMG treatments including pyridostigmine, corticosteroids, and other immunosuppressants. “These treatments lack strong evidence from clinical trials to support their efficacy, are often poorly tolerated, and can be associated with considerable long-term toxicities,” Dr. Howard and colleagues wrote in their report, which was published in JAMA Neurology.
A total of 44 adult patients with gMG were randomized to receive daily zilucoplan 0.1 mg/kg, 0.3 mg/kg, or placebo for 12 weeks in this 25-center North American study. All patients had acetylcholine receptor autoantibody–positive disease and a Quantitative Myasthenia Gravis (QMG) score of 12 or higher. The QMG score ranges from 0, indicating no muscle weakness, to 39, or severe weakness.
Per the study protocol, patients had to keep taking their current gMG medication without changing the dose.
Change in QMG score from baseline to 12 weeks, the primary efficacy endpoint of the study, showed a significant and clinically meaningful difference favoring zilucoplan 0.3 mg/kg over placebo, according to the investigators.
The mean change was –6.0 points for zilucoplan 0.3 mg/kg and –3.2 for placebo (P = .05), according to their report, which indicated a rapid onset of action apparent 1 week after starting treatment.
Zilucoplan 0.1 mg/kg also yielded a significant and clinically meaningful improvement versus placebo, but its magnitude was smaller and took 4 weeks to become apparent.
Treatment with zilucoplan also significantly improved MG Activities of Daily Living scores versus placebo, a key secondary endpoint of the trial, according to the researchers.
Treatment-emergent adverse events, which included local injection-site reactions, were mild and judged to be unrelated to the study treatment, according to the report.
Ra Pharmaceuticals funded the study. Dr. Howard reported disclosures related to Ra Pharmaceuticals, Alexion Pharmaceuticals, argenx, Viela Bio, and others.
SOURCE: Howard Jr JF et al. JAMA Neurol. 2020 Feb 17. doi: 10.1001/jamaneurol.2019.5125.
FROM JAMA NEUROLOGY
Critical care admissions up for pediatric opioid poisonings
ORLANDO – The proportion of children and adolescents admitted to critical care for serious poisonings has increased in recent years, according to authors of a study of more than 750,000 reported opioid exposures.
Critical care units were involved in 10% of pediatric opioid poisoning cases registered in 2015-2018, up from 7% in 2005-2009, reported Megan E. Land, MD, of Emory University, Atlanta, and coinvestigators.
Attempted suicide has represented an increasingly large proportion of pediatric opioid poisonings from 2005 to 2018, according to the researchers, based on retrospective analysis of cases reported to U.S. poison centers.
Mortality related to these pediatric poisonings increased over time, and among children and adolescents admitted to a pediatric ICU, CPR and naloxone use also increased over time, Dr. Land and associates noted.
These said Dr. Land, who presented the findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“I think that this really requires a two-pronged approach,” she explained. “One is that we need to increase mental health resources for kids to address adolescent suicidality, and secondly, we need to decrease access to opioids in the hands of pediatric patients by decreasing prescribing and then also getting those that are unused out of the homes.”
Jeffrey Zimmerman, MD, past president of SCCM, said these findings on pediatric opioid poisonings represent the “iceberg tip” of a much larger societal issue that has impacts well beyond critical care.
“I think acutely, we’re well equipped to deal with the situation in terms of interventions,” Dr. Zimmerman said in an interview. “The bigger issue is dealing with what happens afterward, when the patient leaves the ICU in the hospital.”
When the issue is chronic opioid use among adolescents or children, critical care specialists can help by initiating opioid tapering in the hospital setting, rather than allowing the complete weaning process to play out at home, he said.
All clinicians can help prevent future injury by asking questions of the child and family to ensure that any opiates and other prescription medications at home are locked up, he added.
“These aren’t very glamorous things, but they’re common sense, and there’s more need for this common sense now than there ever has been,” Dr. Zimmerman concluded.
The study by Dr. Land and colleagues included data on primary opioid ingestions registered at 55 poison control centers in the United States. They assessed trends over three time periods: 2005-2009, 2010-2014, and 2015-2018.
They found that children under 19 years of age accounted for 28% of the 753,592 opioid poisonings reported over that time period.
The overall number of reported opioid poisonings among children declined somewhat since about 2010. However, the proportion admitted to a critical care unit increased from 7% in the 2005-2009 period to 10% in the 2015-2018 period, said Dr. Land, who added that the probability of a moderate or major effect increased by 0.55% and 0.11% per year, respectively, over the 14 years studied.
Mortality – 0.21% overall – increased from 0.18% in the earliest era to 0.28% in the most recent era, according to the investigators.
Suicidal intent increased from 14% in the earliest era to 21% in the most recent era, and was linked to near tenfold odds of undergoing a pediatric ICU procedure, Dr. Land and colleagues reported.
Among those children admitted to a pediatric ICU, use of CPR increased from 1% to 3% in the earliest and latest time periods, respectively; likewise, naloxone administration increased from 42% to 51% over those two time periods. By contrast, there was no change in use of mechanical ventilation (12%) or vasopressors (3%) over time, they added.
The opioids most commonly linked to pediatric ICU procedures were fentanyl (odds ratio, 12), heroin (OR, 11), and methadone (OR, 15).
Some funding for the study came from the Georgia Poison Center. Dr. Land had no disclosures relevant to the research.
SOURCE: Land M et al. Crit Care Med. 2020 doi: 10.1097/01.ccm.0000618708.38414.ea.
ORLANDO – The proportion of children and adolescents admitted to critical care for serious poisonings has increased in recent years, according to authors of a study of more than 750,000 reported opioid exposures.
Critical care units were involved in 10% of pediatric opioid poisoning cases registered in 2015-2018, up from 7% in 2005-2009, reported Megan E. Land, MD, of Emory University, Atlanta, and coinvestigators.
Attempted suicide has represented an increasingly large proportion of pediatric opioid poisonings from 2005 to 2018, according to the researchers, based on retrospective analysis of cases reported to U.S. poison centers.
Mortality related to these pediatric poisonings increased over time, and among children and adolescents admitted to a pediatric ICU, CPR and naloxone use also increased over time, Dr. Land and associates noted.
These said Dr. Land, who presented the findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“I think that this really requires a two-pronged approach,” she explained. “One is that we need to increase mental health resources for kids to address adolescent suicidality, and secondly, we need to decrease access to opioids in the hands of pediatric patients by decreasing prescribing and then also getting those that are unused out of the homes.”
Jeffrey Zimmerman, MD, past president of SCCM, said these findings on pediatric opioid poisonings represent the “iceberg tip” of a much larger societal issue that has impacts well beyond critical care.
“I think acutely, we’re well equipped to deal with the situation in terms of interventions,” Dr. Zimmerman said in an interview. “The bigger issue is dealing with what happens afterward, when the patient leaves the ICU in the hospital.”
When the issue is chronic opioid use among adolescents or children, critical care specialists can help by initiating opioid tapering in the hospital setting, rather than allowing the complete weaning process to play out at home, he said.
All clinicians can help prevent future injury by asking questions of the child and family to ensure that any opiates and other prescription medications at home are locked up, he added.
“These aren’t very glamorous things, but they’re common sense, and there’s more need for this common sense now than there ever has been,” Dr. Zimmerman concluded.
The study by Dr. Land and colleagues included data on primary opioid ingestions registered at 55 poison control centers in the United States. They assessed trends over three time periods: 2005-2009, 2010-2014, and 2015-2018.
They found that children under 19 years of age accounted for 28% of the 753,592 opioid poisonings reported over that time period.
The overall number of reported opioid poisonings among children declined somewhat since about 2010. However, the proportion admitted to a critical care unit increased from 7% in the 2005-2009 period to 10% in the 2015-2018 period, said Dr. Land, who added that the probability of a moderate or major effect increased by 0.55% and 0.11% per year, respectively, over the 14 years studied.
Mortality – 0.21% overall – increased from 0.18% in the earliest era to 0.28% in the most recent era, according to the investigators.
Suicidal intent increased from 14% in the earliest era to 21% in the most recent era, and was linked to near tenfold odds of undergoing a pediatric ICU procedure, Dr. Land and colleagues reported.
Among those children admitted to a pediatric ICU, use of CPR increased from 1% to 3% in the earliest and latest time periods, respectively; likewise, naloxone administration increased from 42% to 51% over those two time periods. By contrast, there was no change in use of mechanical ventilation (12%) or vasopressors (3%) over time, they added.
The opioids most commonly linked to pediatric ICU procedures were fentanyl (odds ratio, 12), heroin (OR, 11), and methadone (OR, 15).
Some funding for the study came from the Georgia Poison Center. Dr. Land had no disclosures relevant to the research.
SOURCE: Land M et al. Crit Care Med. 2020 doi: 10.1097/01.ccm.0000618708.38414.ea.
ORLANDO – The proportion of children and adolescents admitted to critical care for serious poisonings has increased in recent years, according to authors of a study of more than 750,000 reported opioid exposures.
Critical care units were involved in 10% of pediatric opioid poisoning cases registered in 2015-2018, up from 7% in 2005-2009, reported Megan E. Land, MD, of Emory University, Atlanta, and coinvestigators.
Attempted suicide has represented an increasingly large proportion of pediatric opioid poisonings from 2005 to 2018, according to the researchers, based on retrospective analysis of cases reported to U.S. poison centers.
Mortality related to these pediatric poisonings increased over time, and among children and adolescents admitted to a pediatric ICU, CPR and naloxone use also increased over time, Dr. Land and associates noted.
These said Dr. Land, who presented the findings at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“I think that this really requires a two-pronged approach,” she explained. “One is that we need to increase mental health resources for kids to address adolescent suicidality, and secondly, we need to decrease access to opioids in the hands of pediatric patients by decreasing prescribing and then also getting those that are unused out of the homes.”
Jeffrey Zimmerman, MD, past president of SCCM, said these findings on pediatric opioid poisonings represent the “iceberg tip” of a much larger societal issue that has impacts well beyond critical care.
“I think acutely, we’re well equipped to deal with the situation in terms of interventions,” Dr. Zimmerman said in an interview. “The bigger issue is dealing with what happens afterward, when the patient leaves the ICU in the hospital.”
When the issue is chronic opioid use among adolescents or children, critical care specialists can help by initiating opioid tapering in the hospital setting, rather than allowing the complete weaning process to play out at home, he said.
All clinicians can help prevent future injury by asking questions of the child and family to ensure that any opiates and other prescription medications at home are locked up, he added.
“These aren’t very glamorous things, but they’re common sense, and there’s more need for this common sense now than there ever has been,” Dr. Zimmerman concluded.
The study by Dr. Land and colleagues included data on primary opioid ingestions registered at 55 poison control centers in the United States. They assessed trends over three time periods: 2005-2009, 2010-2014, and 2015-2018.
They found that children under 19 years of age accounted for 28% of the 753,592 opioid poisonings reported over that time period.
The overall number of reported opioid poisonings among children declined somewhat since about 2010. However, the proportion admitted to a critical care unit increased from 7% in the 2005-2009 period to 10% in the 2015-2018 period, said Dr. Land, who added that the probability of a moderate or major effect increased by 0.55% and 0.11% per year, respectively, over the 14 years studied.
Mortality – 0.21% overall – increased from 0.18% in the earliest era to 0.28% in the most recent era, according to the investigators.
Suicidal intent increased from 14% in the earliest era to 21% in the most recent era, and was linked to near tenfold odds of undergoing a pediatric ICU procedure, Dr. Land and colleagues reported.
Among those children admitted to a pediatric ICU, use of CPR increased from 1% to 3% in the earliest and latest time periods, respectively; likewise, naloxone administration increased from 42% to 51% over those two time periods. By contrast, there was no change in use of mechanical ventilation (12%) or vasopressors (3%) over time, they added.
The opioids most commonly linked to pediatric ICU procedures were fentanyl (odds ratio, 12), heroin (OR, 11), and methadone (OR, 15).
Some funding for the study came from the Georgia Poison Center. Dr. Land had no disclosures relevant to the research.
SOURCE: Land M et al. Crit Care Med. 2020 doi: 10.1097/01.ccm.0000618708.38414.ea.
REPORTING FROM CCC49
As novel coronavirus outbreak evolves, critical care providers need to be prepared
ORLANDO – While the impact of the 2019 novel coronavirus outbreak on hospitals outside of China remains to be determined, there are several practical points critical care professionals need to know to be prepared in the face of this dynamic and rapidly evolving outbreak, speakers said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“Priorities for us in our hospitals are early detection, infection prevention, staff safety, and obviously, taking care of sick people,” said Ryan C. Maves, MD, of the Naval Medical Center San Diego in a special session on the 2019 Novel Coronavirus outbreak.*
Approximately 72,000 cases of coronavirus disease 2019 (COVID-19) had been reported as of Feb. 17, 2020, the day of Dr. Maves’ talk, according to statistics from Johns Hopkins Center for Science and Engineering in Baltimore. A total of 1,775 deaths had been recorded, nearly all of which were in Hubei Province, the central point of the outbreak. In the United States, the number of cases stood at 15, with no deaths reported.
While the dynamics of the 2019 novel coronavirus are still being learned, the estimated range of spread for droplet transmission is 2 meters, according to Dr. Maves. The duration of environmental persistence is not yet known, but he said that other coronaviruses persist in low-humidity conditions for up to 4 days.
The number of secondary cases that arise from a primary infection, or R0, is estimated to be between 1.5 and 3, though it can change as exposure evolves; by comparison, the R0 for H1N1 influenza has been reported as 1.5, while measles is 12-18, indicating that it is “very contagious,” said Dr. Maves. Severe acute respiratory syndrome had an initial R0 of about 3.5, which he said declined rapidly to 0.7 as environmental and policy controls were put into place.
Critical care professionals need to know how to identify patients at risk of having COVID-19 and determine whether they need further work-up, according to Dr. Maves, who highlighted recent criteria released by the Centers for Disease Control and Prevention.
The highest-risk category, he said, are individuals exposed to a laboratory-confirmed coronavirus case, which along with fever or signs and symptoms of a lower respiratory illness would be sufficient to classify them as a “person of interest” requiring further evaluation for disease. A history of travel from Hubei Province plus fever and signs/symptoms of lower respiratory illness would also meet criteria for evaluation, according to the CDC, while travel to mainland China would also meet the threshold, if those symptoms required hospitalization.
The CDC also published a step-wise flowchart to evaluate patients who may have been exposed to the 2019 novel coronavirus. According to that flowchart, if an individual has traveled to China or had close contact with someone infected with the 2019 Novel Coronavirus within 14 days of symptoms, and that individual has fever or symptoms of lower respiratory illness such as cough or shortness of breath, then providers should isolate that individual and assess clinical status, in addition to contacting the local health department.
Laura E. Evans, MD, MS, FCCM, of New York University, said she might recommend providers “flip the script” on that CDC algorithm when it comes to identifying patients who may have been exposed.
“I think perhaps what we should be doing at sites of entry is not talking about travel as the first question, but rather fever or symptoms of lower respiratory illnesses as the first question, and use that as the opportunity to implement risk mitigation at that stage,” Dr. Evans said in a presentation on preparing for COVID-19.
Even with “substantial uncertainty” about the potential impact of the 2019 Novel Coronavirus, a significant influx of seriously ill patients would put strain the U.S. health care delivery system, she added.
“None of us have tons of extra capacity in our emergency departments, inpatient units, or ICUs, and I think we need to be prepared for that,” she added. “We need to know what our process is for ‘identify, isolate, and inform,’ and we need to be testing that now.”
Dr. Maves and Dr. Evans both reported that they had no financial conflicts of interest to report. Dr. Maves indicated that the views expressed in his presentation did not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States government.
*Correction, 2/19/20: An earlier version of this article misstated the location of the naval center.
ORLANDO – While the impact of the 2019 novel coronavirus outbreak on hospitals outside of China remains to be determined, there are several practical points critical care professionals need to know to be prepared in the face of this dynamic and rapidly evolving outbreak, speakers said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“Priorities for us in our hospitals are early detection, infection prevention, staff safety, and obviously, taking care of sick people,” said Ryan C. Maves, MD, of the Naval Medical Center San Diego in a special session on the 2019 Novel Coronavirus outbreak.*
Approximately 72,000 cases of coronavirus disease 2019 (COVID-19) had been reported as of Feb. 17, 2020, the day of Dr. Maves’ talk, according to statistics from Johns Hopkins Center for Science and Engineering in Baltimore. A total of 1,775 deaths had been recorded, nearly all of which were in Hubei Province, the central point of the outbreak. In the United States, the number of cases stood at 15, with no deaths reported.
While the dynamics of the 2019 novel coronavirus are still being learned, the estimated range of spread for droplet transmission is 2 meters, according to Dr. Maves. The duration of environmental persistence is not yet known, but he said that other coronaviruses persist in low-humidity conditions for up to 4 days.
The number of secondary cases that arise from a primary infection, or R0, is estimated to be between 1.5 and 3, though it can change as exposure evolves; by comparison, the R0 for H1N1 influenza has been reported as 1.5, while measles is 12-18, indicating that it is “very contagious,” said Dr. Maves. Severe acute respiratory syndrome had an initial R0 of about 3.5, which he said declined rapidly to 0.7 as environmental and policy controls were put into place.
Critical care professionals need to know how to identify patients at risk of having COVID-19 and determine whether they need further work-up, according to Dr. Maves, who highlighted recent criteria released by the Centers for Disease Control and Prevention.
The highest-risk category, he said, are individuals exposed to a laboratory-confirmed coronavirus case, which along with fever or signs and symptoms of a lower respiratory illness would be sufficient to classify them as a “person of interest” requiring further evaluation for disease. A history of travel from Hubei Province plus fever and signs/symptoms of lower respiratory illness would also meet criteria for evaluation, according to the CDC, while travel to mainland China would also meet the threshold, if those symptoms required hospitalization.
The CDC also published a step-wise flowchart to evaluate patients who may have been exposed to the 2019 novel coronavirus. According to that flowchart, if an individual has traveled to China or had close contact with someone infected with the 2019 Novel Coronavirus within 14 days of symptoms, and that individual has fever or symptoms of lower respiratory illness such as cough or shortness of breath, then providers should isolate that individual and assess clinical status, in addition to contacting the local health department.
Laura E. Evans, MD, MS, FCCM, of New York University, said she might recommend providers “flip the script” on that CDC algorithm when it comes to identifying patients who may have been exposed.
“I think perhaps what we should be doing at sites of entry is not talking about travel as the first question, but rather fever or symptoms of lower respiratory illnesses as the first question, and use that as the opportunity to implement risk mitigation at that stage,” Dr. Evans said in a presentation on preparing for COVID-19.
Even with “substantial uncertainty” about the potential impact of the 2019 Novel Coronavirus, a significant influx of seriously ill patients would put strain the U.S. health care delivery system, she added.
“None of us have tons of extra capacity in our emergency departments, inpatient units, or ICUs, and I think we need to be prepared for that,” she added. “We need to know what our process is for ‘identify, isolate, and inform,’ and we need to be testing that now.”
Dr. Maves and Dr. Evans both reported that they had no financial conflicts of interest to report. Dr. Maves indicated that the views expressed in his presentation did not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States government.
*Correction, 2/19/20: An earlier version of this article misstated the location of the naval center.
ORLANDO – While the impact of the 2019 novel coronavirus outbreak on hospitals outside of China remains to be determined, there are several practical points critical care professionals need to know to be prepared in the face of this dynamic and rapidly evolving outbreak, speakers said at the Critical Care Congress sponsored by the Society of Critical Care Medicine.
“Priorities for us in our hospitals are early detection, infection prevention, staff safety, and obviously, taking care of sick people,” said Ryan C. Maves, MD, of the Naval Medical Center San Diego in a special session on the 2019 Novel Coronavirus outbreak.*
Approximately 72,000 cases of coronavirus disease 2019 (COVID-19) had been reported as of Feb. 17, 2020, the day of Dr. Maves’ talk, according to statistics from Johns Hopkins Center for Science and Engineering in Baltimore. A total of 1,775 deaths had been recorded, nearly all of which were in Hubei Province, the central point of the outbreak. In the United States, the number of cases stood at 15, with no deaths reported.
While the dynamics of the 2019 novel coronavirus are still being learned, the estimated range of spread for droplet transmission is 2 meters, according to Dr. Maves. The duration of environmental persistence is not yet known, but he said that other coronaviruses persist in low-humidity conditions for up to 4 days.
The number of secondary cases that arise from a primary infection, or R0, is estimated to be between 1.5 and 3, though it can change as exposure evolves; by comparison, the R0 for H1N1 influenza has been reported as 1.5, while measles is 12-18, indicating that it is “very contagious,” said Dr. Maves. Severe acute respiratory syndrome had an initial R0 of about 3.5, which he said declined rapidly to 0.7 as environmental and policy controls were put into place.
Critical care professionals need to know how to identify patients at risk of having COVID-19 and determine whether they need further work-up, according to Dr. Maves, who highlighted recent criteria released by the Centers for Disease Control and Prevention.
The highest-risk category, he said, are individuals exposed to a laboratory-confirmed coronavirus case, which along with fever or signs and symptoms of a lower respiratory illness would be sufficient to classify them as a “person of interest” requiring further evaluation for disease. A history of travel from Hubei Province plus fever and signs/symptoms of lower respiratory illness would also meet criteria for evaluation, according to the CDC, while travel to mainland China would also meet the threshold, if those symptoms required hospitalization.
The CDC also published a step-wise flowchart to evaluate patients who may have been exposed to the 2019 novel coronavirus. According to that flowchart, if an individual has traveled to China or had close contact with someone infected with the 2019 Novel Coronavirus within 14 days of symptoms, and that individual has fever or symptoms of lower respiratory illness such as cough or shortness of breath, then providers should isolate that individual and assess clinical status, in addition to contacting the local health department.
Laura E. Evans, MD, MS, FCCM, of New York University, said she might recommend providers “flip the script” on that CDC algorithm when it comes to identifying patients who may have been exposed.
“I think perhaps what we should be doing at sites of entry is not talking about travel as the first question, but rather fever or symptoms of lower respiratory illnesses as the first question, and use that as the opportunity to implement risk mitigation at that stage,” Dr. Evans said in a presentation on preparing for COVID-19.
Even with “substantial uncertainty” about the potential impact of the 2019 Novel Coronavirus, a significant influx of seriously ill patients would put strain the U.S. health care delivery system, she added.
“None of us have tons of extra capacity in our emergency departments, inpatient units, or ICUs, and I think we need to be prepared for that,” she added. “We need to know what our process is for ‘identify, isolate, and inform,’ and we need to be testing that now.”
Dr. Maves and Dr. Evans both reported that they had no financial conflicts of interest to report. Dr. Maves indicated that the views expressed in his presentation did not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States government.
*Correction, 2/19/20: An earlier version of this article misstated the location of the naval center.
EXPERT ANALYSIS FROM CCC49
Adding pembrolizumab to chemo doubled pCR rates in early-stage breast cancer
Adding pembrolizumab to neoadjuvant chemotherapy more than doubled the rate of pathologic complete response, compared with chemotherapy alone, in women with early-stage breast cancer enrolled in the phase 2 I-SPY2 trial.
Pathologic complete response (pCR) rates up to 60% were reported for patients with high-risk, stage II/III breast cancer who received pembrolizumab plus chemotherapy in I-SPY2, an ongoing platform trial designed to rapidly screen multiple agents and pinpoint those with a high probability of success.
The doubling of pCR rates was seen in all three biomarker signatures studied, including ERBB2(HER2)-negative, hormone receptor (HR)-positive/ERBB2-negative, or triple-negative breast cancer.
These results mean that pembrolizumab can “graduate” from I-SPY2 and suggest a greater than 99% predictive probability that the pembrolizumab-plus-chemotherapy approach will be superior to chemotherapy alone in a phase 3 trial, according to Rita Nanda, MD, of the University of Chicago, and colleagues.
“Notably, pembrolizumab was the first agent of 10 studied to graduate in the HR-positive/ERBB2-negative signature since I-SPY2 opened in 2010,” Dr. Nanda and colleagues wrote in JAMA Oncology.
The I-SPY2 study has enrolled adult women with stage II/III breast cancer at high risk of recurrence. The control arm included 181 patients randomized to receive standard neoadjuvant paclitaxel followed by doxorubicin plus cyclophosphamide. The pembrolizumab arm included 69 patients who received the same chemotherapy regimen plus pembrolizumab given concurrently with paclitaxel.
In ERBB2-negative patients, the estimated pCR rates were 44% in the pembrolizumab arm and 17% in the control arm. In HR-positive/ERBB2-negative patients, the estimated pCR rates were 30% and 13%, respectively. In triple-negative patients, the estimated pCR rates were 60% and 22%, respectively.
Residual cancer burden classified as extensive was less often seen in the pembrolizumab-treated patients, the investigators noted.
Event-free survival was qualitatively similar between the pembrolizumab and control arms, although the investigators cautioned against drawing conclusions based on this exploratory analysis in a small number of patients.
“Patients who achieved pCR had excellent outcomes regardless of arm,” the investigators wrote.
Immune-related adverse events (irAEs) were seen in the pembrolizumab-treated patients, although most were grade 1 or 2 and managed with dose interruption or corticosteroid therapy.
The most common irAE was thyroid dysfunction in 13% of patients, which was on par with what was seen in previously published reports. By contrast, adrenal insufficiency was observed in about 9% of patients, which is higher than in published reports for reasons that are unclear.
“Future work to characterize the risk factors for developing irAEs is warranted to improve the therapeutic index of these agents,” Dr. Nanda and colleagues wrote.
Pembrolizumab plus standard neoadjuvant chemotherapy is being evaluated in two ongoing, randomized phase 3 trials – KEYNOTE 522, which is evaluating patients with triple-negative breast cancer, and KEYNOTE 756, which is focused on high-risk, HR-positive/ERBB2-negative breast cancer.
The ongoing I-SPY2 study is supported by a grant from the National Cancer Institute as well as funding from charitable organizations, pharmaceutical companies, and private individuals. The investigators disclosed relationships with a range of pharmaceutical companies.
SOURCE: Nanda R et al. JAMA Oncol. 2020 Feb 13. doi: 10.1001/jamaoncol.2019.6650.
Adding pembrolizumab to neoadjuvant chemotherapy more than doubled the rate of pathologic complete response, compared with chemotherapy alone, in women with early-stage breast cancer enrolled in the phase 2 I-SPY2 trial.
Pathologic complete response (pCR) rates up to 60% were reported for patients with high-risk, stage II/III breast cancer who received pembrolizumab plus chemotherapy in I-SPY2, an ongoing platform trial designed to rapidly screen multiple agents and pinpoint those with a high probability of success.
The doubling of pCR rates was seen in all three biomarker signatures studied, including ERBB2(HER2)-negative, hormone receptor (HR)-positive/ERBB2-negative, or triple-negative breast cancer.
These results mean that pembrolizumab can “graduate” from I-SPY2 and suggest a greater than 99% predictive probability that the pembrolizumab-plus-chemotherapy approach will be superior to chemotherapy alone in a phase 3 trial, according to Rita Nanda, MD, of the University of Chicago, and colleagues.
“Notably, pembrolizumab was the first agent of 10 studied to graduate in the HR-positive/ERBB2-negative signature since I-SPY2 opened in 2010,” Dr. Nanda and colleagues wrote in JAMA Oncology.
The I-SPY2 study has enrolled adult women with stage II/III breast cancer at high risk of recurrence. The control arm included 181 patients randomized to receive standard neoadjuvant paclitaxel followed by doxorubicin plus cyclophosphamide. The pembrolizumab arm included 69 patients who received the same chemotherapy regimen plus pembrolizumab given concurrently with paclitaxel.
In ERBB2-negative patients, the estimated pCR rates were 44% in the pembrolizumab arm and 17% in the control arm. In HR-positive/ERBB2-negative patients, the estimated pCR rates were 30% and 13%, respectively. In triple-negative patients, the estimated pCR rates were 60% and 22%, respectively.
Residual cancer burden classified as extensive was less often seen in the pembrolizumab-treated patients, the investigators noted.
Event-free survival was qualitatively similar between the pembrolizumab and control arms, although the investigators cautioned against drawing conclusions based on this exploratory analysis in a small number of patients.
“Patients who achieved pCR had excellent outcomes regardless of arm,” the investigators wrote.
Immune-related adverse events (irAEs) were seen in the pembrolizumab-treated patients, although most were grade 1 or 2 and managed with dose interruption or corticosteroid therapy.
The most common irAE was thyroid dysfunction in 13% of patients, which was on par with what was seen in previously published reports. By contrast, adrenal insufficiency was observed in about 9% of patients, which is higher than in published reports for reasons that are unclear.
“Future work to characterize the risk factors for developing irAEs is warranted to improve the therapeutic index of these agents,” Dr. Nanda and colleagues wrote.
Pembrolizumab plus standard neoadjuvant chemotherapy is being evaluated in two ongoing, randomized phase 3 trials – KEYNOTE 522, which is evaluating patients with triple-negative breast cancer, and KEYNOTE 756, which is focused on high-risk, HR-positive/ERBB2-negative breast cancer.
The ongoing I-SPY2 study is supported by a grant from the National Cancer Institute as well as funding from charitable organizations, pharmaceutical companies, and private individuals. The investigators disclosed relationships with a range of pharmaceutical companies.
SOURCE: Nanda R et al. JAMA Oncol. 2020 Feb 13. doi: 10.1001/jamaoncol.2019.6650.
Adding pembrolizumab to neoadjuvant chemotherapy more than doubled the rate of pathologic complete response, compared with chemotherapy alone, in women with early-stage breast cancer enrolled in the phase 2 I-SPY2 trial.
Pathologic complete response (pCR) rates up to 60% were reported for patients with high-risk, stage II/III breast cancer who received pembrolizumab plus chemotherapy in I-SPY2, an ongoing platform trial designed to rapidly screen multiple agents and pinpoint those with a high probability of success.
The doubling of pCR rates was seen in all three biomarker signatures studied, including ERBB2(HER2)-negative, hormone receptor (HR)-positive/ERBB2-negative, or triple-negative breast cancer.
These results mean that pembrolizumab can “graduate” from I-SPY2 and suggest a greater than 99% predictive probability that the pembrolizumab-plus-chemotherapy approach will be superior to chemotherapy alone in a phase 3 trial, according to Rita Nanda, MD, of the University of Chicago, and colleagues.
“Notably, pembrolizumab was the first agent of 10 studied to graduate in the HR-positive/ERBB2-negative signature since I-SPY2 opened in 2010,” Dr. Nanda and colleagues wrote in JAMA Oncology.
The I-SPY2 study has enrolled adult women with stage II/III breast cancer at high risk of recurrence. The control arm included 181 patients randomized to receive standard neoadjuvant paclitaxel followed by doxorubicin plus cyclophosphamide. The pembrolizumab arm included 69 patients who received the same chemotherapy regimen plus pembrolizumab given concurrently with paclitaxel.
In ERBB2-negative patients, the estimated pCR rates were 44% in the pembrolizumab arm and 17% in the control arm. In HR-positive/ERBB2-negative patients, the estimated pCR rates were 30% and 13%, respectively. In triple-negative patients, the estimated pCR rates were 60% and 22%, respectively.
Residual cancer burden classified as extensive was less often seen in the pembrolizumab-treated patients, the investigators noted.
Event-free survival was qualitatively similar between the pembrolizumab and control arms, although the investigators cautioned against drawing conclusions based on this exploratory analysis in a small number of patients.
“Patients who achieved pCR had excellent outcomes regardless of arm,” the investigators wrote.
Immune-related adverse events (irAEs) were seen in the pembrolizumab-treated patients, although most were grade 1 or 2 and managed with dose interruption or corticosteroid therapy.
The most common irAE was thyroid dysfunction in 13% of patients, which was on par with what was seen in previously published reports. By contrast, adrenal insufficiency was observed in about 9% of patients, which is higher than in published reports for reasons that are unclear.
“Future work to characterize the risk factors for developing irAEs is warranted to improve the therapeutic index of these agents,” Dr. Nanda and colleagues wrote.
Pembrolizumab plus standard neoadjuvant chemotherapy is being evaluated in two ongoing, randomized phase 3 trials – KEYNOTE 522, which is evaluating patients with triple-negative breast cancer, and KEYNOTE 756, which is focused on high-risk, HR-positive/ERBB2-negative breast cancer.
The ongoing I-SPY2 study is supported by a grant from the National Cancer Institute as well as funding from charitable organizations, pharmaceutical companies, and private individuals. The investigators disclosed relationships with a range of pharmaceutical companies.
SOURCE: Nanda R et al. JAMA Oncol. 2020 Feb 13. doi: 10.1001/jamaoncol.2019.6650.
FROM JAMA ONCOLOGY
Key clinical point: Adding pembrolizumab to neoadjuvant chemotherapy more than doubled the rate of pathologic complete response (pCR) in women with early-stage breast cancer.
Major finding: In ERBB2-negative patients, the estimated pCR rates were 44% in the pembrolizumab arm and 17% in the control arm. In HR-positive/ERBB2-negative patients, the estimated pCR rates were 30% and 13%, respectively. In triple-negative patients, the estimated pCR rates were 60% and 22%, respectively.
Study details: Phase 2 trial of 69 patients treated with pembrolizumab and chemotherapy, compared with 181 chemotherapy-treated control subjects.
Disclosures: The trial is supported by a grant from the National Cancer Institute as well as funding from charitable organizations, pharmaceutical companies, and private individuals. The investigators disclosed relationships with a range of pharmaceutical companies.
Source: Nanda R et al. JAMA Oncol. 2020 Feb 13.