User login
Meet Argireline, the neurotoxinlike cosmeceutical
Acetyl hexapeptide-8 (or -3), better known by its brand name, Argireline (Lubrizol; Wickliffe, Ohio), is a synthetic peptide gaining popularity in cosmeceutical products for its antiaging benefits. Argireline was developed by the company Lipotec in 2001. Media, beauty bloggers, and product claims have likened this product to a “Botox [or other neurotoxin] alternative,” or “Botox mimicker.”
Mechanism of action
Understanding how Argireline works requires a brief refresher on the mechanism of action of botulinum neurotoxin (BoNT). BoNT relaxes facial muscles and smooths expression lines by inhibiting acetylcholine release at the neuromuscular junction.1 More specifically, the various serotypes of BoNT are single-chain polypeptides that target members of the SNARE complex: SNAP-25, syntaxin, and Vamp. The proteins within the SNARE complex are involved in the docking and fusion of presynaptic vesicles to the presynaptic membrane, necessary steps for acetylcholine release into the neuromuscular junction and muscle contraction. By blocking the action of the SNARE complex proteins, BoNT inhibits release of acetylcholine in the neuromuscular junction and prevents muscle contraction.
Argireline is a synthetic peptide with the sequence Ac-EEMQRR-NH2.2 It is patterned after the N-terminal domain of SNAP-25, one of the members of the SNARE complex targeted by BoNT, and functions to interfere with the assembly of the SNARE complex. In this manner, Argireline would theoretically inhibit fusion of presynaptic vesicles and release of acetylcholine into the neuromuscular junction, thus impeding muscle movement. For this reason, it has been likened to topical Botox. Unlike Botox and other neurotoxins, Argireline was developed for topical application rather than injection.
Preclinical studies
In vitro work done 20 years ago demonstrated that Argireline can prevent assembly of the SNARE complex and inhibit neurotransmitter release with a potency similar to that of BoNT A (Botox).2
In 2013, Wang et al. evaluated the histologic effects of Argireline in aged mouse skin induced by D-galactose. For 6 weeks, Argireline was applied twice daily, and histological changes were assessed using hematoxylin and eosin (H&E) and picrosirius–polarization (PSP) stains. The researchers found elevated levels of type I collagen (P < .01) and reduced type III collagen (P < .05) with the Argireline treatment. These results demonstrated that Argireline could histologically enhance collagen in a manner consistent with skin rejuvenation.3
Clinical studies
In 2002, Blanes et al. assessed the antiwrinkle activity of Argireline by measuring skin topography from silicone implants in the lateral periorbital region of an oil/water (O/W) emulsion containing 10% of the acetyl-hexapeptide in 10 healthy women volunteers. The hexapeptide emulsion was applied twice daily in one lateral periorbital area, and the emulsion vehicle alone was applied twice daily on the contralateral side. Over 30 days of treatment, wrinkle depth was found to have decreased by 30%. The investigators also found that Argireline significantly hindered neurotransmitter release in vitro as robustly as BoNT A, though with notably lower efficacy. No toxicity or irritation was associated with this treatment.2 However, it should be noted that this small study conducted 2 decades ago evaluated only silicone implants with confocal microscopy to evaluate wrinkle depth. There was no subjective clinical assessment of dynamic facial wrinkles. As such, their study is an insufficient basis for drawing conclusions that Argireline is a BoNT mimic. Botox and other types of BoNT affect dynamic facial wrinkles mostly (i.e., wrinkles created by moving muscles of facial expression). This study primarily considers static wrinkles on periorbital skin. While static wrinkles may result from longstanding dynamic wrinkles, BoNT mainly targets dynamic wrinkles, again not comparing apples to apples.
At the same time that Wang et al. conducted their experiment on the skin of aged mice as noted above, they performed a multicenter clinical trial in 60 human subjects who received a randomized treatment of Argireline or placebo in a ratio of 3:1 to assess its safety and efficacy. For 4 weeks, the test product or placebo was applied to periorbital wrinkles twice daily. The researchers found the total antiwrinkle efficacy in the Argireline group to be 48.9% based on the subjective evaluation, compared with 0% in the placebo group. The objective evaluation indicated that all parameters of roughness were diminished in the Argireline group (P < .01), with no reduction observed in the placebo group (P < .05).4 There was a little more to appreciate from this study compared with the one reported by Blanes et al., insofar as subjective evaluations and objective evaluations with silica replicas were done. However, this study was not blinded, so the 48.9% wrinkle reduction in the Argireline group vs. 0% in the control group seems suspicious. Additionally, there was a greater focus on static rather than dynamic wrinkles.
In 2017, Raikou et al. conducted a prospective, randomized controlled study to assess the effects of acetyl hexapeptide-3 (Argireline) and tripeptide-10 citrulline in 24 healthy female volunteers (aged 30-60 years) and determine if there was any synergistic action between the peptides. Subjects were randomized to receive a combination of the peptides, tripeptide-10 citrulline only, acetyl hexapeptide-3 only, or neither peptide for 60 days. The researchers found a significant reduction in transepidermal water loss (TEWL) in the Argireline group, compared with the placebo group.5 The result of this study makes me question if the decrease in depth of the wrinkles measured in the former studies is really just a measure of increased skin hydration from the Argireline, rather than a neurotoxic effect of Argireline.
Formulation and penetration: Can Argireline get through your skin?
One of the fundamental questions regarding Argireline is whether it can penetrate through the stratum corneum and find its target – the facial muscles – where it is intended to function. Argireline is a charged, hydrophilic, and large–molecular weight peptide, and each of these factors impairs penetration through the stratum corneum. Therefore, studies assessing penetration are particularly important.
In 2015, Kraeling et al. conducted an in vitro evaluation of the skin penetration of acetyl hexapeptide-8 in hairless guinea pig and human cadaver skin. An oil-in-water (O/W) emulsion containing 10% acetyl hexapeptide-8 was applied (2 mg/cm2) and penetration was quantified in skin layers via hydrophilic interaction liquid chromatography with tandem mass spectrometry. Most of the acetyl hexapeptide-8 was found to have been washed from human cadaver, as well as guinea pig, skin. Less than 1% of the peptide penetrated the guinea pig or human skin. Of this small amount that penetrated the skin, most stayed in the stratum corneum of guinea pigs (0.54%) and human cadavers (0.22%). The levels of acetyl hexapeptide-8 declined further with each layer of tape stripping removal. Epidermal levels of the peptide in tested skin were similar at 0.01%, and none of the peptide was found in the dermis.6 These results indicate negligible penetration by this highly touted peptide ingredient.
Some studies have shown that altering the formulation of acetyl hexapeptide-8 can enhance penetration. Hoppel et al. demonstrated that formulations of the peptide, especially in a water-oil-water (W/O/W emulsion [as compared with O/W and W/O emulsions] can increase penetration into the stratum corneum in porcine skin.7 Notably, this is still very superficial relative to the dermis and muscles. Irrespective of formulation, studies have shown that Argireline barely penetrates the stratum corneum, let alone the dermis. Therefore, I would give pause to attributing any clinical impact or benefit of Argireline to its neurotoxinlike effects measured in vitro.
Conclusion
Despite the growing popularity of this ingredient in cosmeceuticals and the praise it gets in media for acting as a topical neurotoxin, there are no rigorous clinical trials or data demonstrating its efficacy in suppressing dynamic facial wrinkles like BoNT does. Most importantly, without penetration into the stratum corneum and deeper layers of the skin, it seems unlikely that Argireline’s clinical benefit derives from a neurotoxiclike mechanism of action. It seems more likely that the Argireline-containing product enhances hydration or imparts some other quality to the skin surface. While there is certainly great appeal for a neurotoxinlike product without injections, I do not believe this ingredient will replace injections of BoNT in the foreseeable future, or at least until scientists can figure out how to enable these products to penetrate into the deeper layers of the skin.
Dr. Goldman is a dermatologist in private practice in Miami and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a general dermatology practice. Dr. Goldman has no relevant disclosures. Write to her at dermnews@mdedge.com or message her on Instagram @DrChloeGoldman.
References
1. Reddy BY et al. Exp Dermatol. 2012 Aug;21(8):569-75.
2. Blanes-Mira C et al. Int J Cosmet Sci. 2002 Oct;24(5):303-10.
3. Wang Y et al. J Cosmet Laser Ther. 2013 Aug;15(4):237-41.
4. Wang Y et al. J Cosmet Laser Ther. 2013;14(2):147-53.
5. Raikou V et al. J Cosmet Dermatol. 2017 Jun;16(2):271-8.
6. Kraeling ME et al. Cutan Ocul Toxicol. 2015 Mar;34(1):46-52.
7. Hoppel M et al. Eur J Pharm Sci. 2015 Feb 20;68:27-35.
Acetyl hexapeptide-8 (or -3), better known by its brand name, Argireline (Lubrizol; Wickliffe, Ohio), is a synthetic peptide gaining popularity in cosmeceutical products for its antiaging benefits. Argireline was developed by the company Lipotec in 2001. Media, beauty bloggers, and product claims have likened this product to a “Botox [or other neurotoxin] alternative,” or “Botox mimicker.”
Mechanism of action
Understanding how Argireline works requires a brief refresher on the mechanism of action of botulinum neurotoxin (BoNT). BoNT relaxes facial muscles and smooths expression lines by inhibiting acetylcholine release at the neuromuscular junction.1 More specifically, the various serotypes of BoNT are single-chain polypeptides that target members of the SNARE complex: SNAP-25, syntaxin, and Vamp. The proteins within the SNARE complex are involved in the docking and fusion of presynaptic vesicles to the presynaptic membrane, necessary steps for acetylcholine release into the neuromuscular junction and muscle contraction. By blocking the action of the SNARE complex proteins, BoNT inhibits release of acetylcholine in the neuromuscular junction and prevents muscle contraction.
Argireline is a synthetic peptide with the sequence Ac-EEMQRR-NH2.2 It is patterned after the N-terminal domain of SNAP-25, one of the members of the SNARE complex targeted by BoNT, and functions to interfere with the assembly of the SNARE complex. In this manner, Argireline would theoretically inhibit fusion of presynaptic vesicles and release of acetylcholine into the neuromuscular junction, thus impeding muscle movement. For this reason, it has been likened to topical Botox. Unlike Botox and other neurotoxins, Argireline was developed for topical application rather than injection.
Preclinical studies
In vitro work done 20 years ago demonstrated that Argireline can prevent assembly of the SNARE complex and inhibit neurotransmitter release with a potency similar to that of BoNT A (Botox).2
In 2013, Wang et al. evaluated the histologic effects of Argireline in aged mouse skin induced by D-galactose. For 6 weeks, Argireline was applied twice daily, and histological changes were assessed using hematoxylin and eosin (H&E) and picrosirius–polarization (PSP) stains. The researchers found elevated levels of type I collagen (P < .01) and reduced type III collagen (P < .05) with the Argireline treatment. These results demonstrated that Argireline could histologically enhance collagen in a manner consistent with skin rejuvenation.3
Clinical studies
In 2002, Blanes et al. assessed the antiwrinkle activity of Argireline by measuring skin topography from silicone implants in the lateral periorbital region of an oil/water (O/W) emulsion containing 10% of the acetyl-hexapeptide in 10 healthy women volunteers. The hexapeptide emulsion was applied twice daily in one lateral periorbital area, and the emulsion vehicle alone was applied twice daily on the contralateral side. Over 30 days of treatment, wrinkle depth was found to have decreased by 30%. The investigators also found that Argireline significantly hindered neurotransmitter release in vitro as robustly as BoNT A, though with notably lower efficacy. No toxicity or irritation was associated with this treatment.2 However, it should be noted that this small study conducted 2 decades ago evaluated only silicone implants with confocal microscopy to evaluate wrinkle depth. There was no subjective clinical assessment of dynamic facial wrinkles. As such, their study is an insufficient basis for drawing conclusions that Argireline is a BoNT mimic. Botox and other types of BoNT affect dynamic facial wrinkles mostly (i.e., wrinkles created by moving muscles of facial expression). This study primarily considers static wrinkles on periorbital skin. While static wrinkles may result from longstanding dynamic wrinkles, BoNT mainly targets dynamic wrinkles, again not comparing apples to apples.
At the same time that Wang et al. conducted their experiment on the skin of aged mice as noted above, they performed a multicenter clinical trial in 60 human subjects who received a randomized treatment of Argireline or placebo in a ratio of 3:1 to assess its safety and efficacy. For 4 weeks, the test product or placebo was applied to periorbital wrinkles twice daily. The researchers found the total antiwrinkle efficacy in the Argireline group to be 48.9% based on the subjective evaluation, compared with 0% in the placebo group. The objective evaluation indicated that all parameters of roughness were diminished in the Argireline group (P < .01), with no reduction observed in the placebo group (P < .05).4 There was a little more to appreciate from this study compared with the one reported by Blanes et al., insofar as subjective evaluations and objective evaluations with silica replicas were done. However, this study was not blinded, so the 48.9% wrinkle reduction in the Argireline group vs. 0% in the control group seems suspicious. Additionally, there was a greater focus on static rather than dynamic wrinkles.
In 2017, Raikou et al. conducted a prospective, randomized controlled study to assess the effects of acetyl hexapeptide-3 (Argireline) and tripeptide-10 citrulline in 24 healthy female volunteers (aged 30-60 years) and determine if there was any synergistic action between the peptides. Subjects were randomized to receive a combination of the peptides, tripeptide-10 citrulline only, acetyl hexapeptide-3 only, or neither peptide for 60 days. The researchers found a significant reduction in transepidermal water loss (TEWL) in the Argireline group, compared with the placebo group.5 The result of this study makes me question if the decrease in depth of the wrinkles measured in the former studies is really just a measure of increased skin hydration from the Argireline, rather than a neurotoxic effect of Argireline.
Formulation and penetration: Can Argireline get through your skin?
One of the fundamental questions regarding Argireline is whether it can penetrate through the stratum corneum and find its target – the facial muscles – where it is intended to function. Argireline is a charged, hydrophilic, and large–molecular weight peptide, and each of these factors impairs penetration through the stratum corneum. Therefore, studies assessing penetration are particularly important.
In 2015, Kraeling et al. conducted an in vitro evaluation of the skin penetration of acetyl hexapeptide-8 in hairless guinea pig and human cadaver skin. An oil-in-water (O/W) emulsion containing 10% acetyl hexapeptide-8 was applied (2 mg/cm2) and penetration was quantified in skin layers via hydrophilic interaction liquid chromatography with tandem mass spectrometry. Most of the acetyl hexapeptide-8 was found to have been washed from human cadaver, as well as guinea pig, skin. Less than 1% of the peptide penetrated the guinea pig or human skin. Of this small amount that penetrated the skin, most stayed in the stratum corneum of guinea pigs (0.54%) and human cadavers (0.22%). The levels of acetyl hexapeptide-8 declined further with each layer of tape stripping removal. Epidermal levels of the peptide in tested skin were similar at 0.01%, and none of the peptide was found in the dermis.6 These results indicate negligible penetration by this highly touted peptide ingredient.
Some studies have shown that altering the formulation of acetyl hexapeptide-8 can enhance penetration. Hoppel et al. demonstrated that formulations of the peptide, especially in a water-oil-water (W/O/W emulsion [as compared with O/W and W/O emulsions] can increase penetration into the stratum corneum in porcine skin.7 Notably, this is still very superficial relative to the dermis and muscles. Irrespective of formulation, studies have shown that Argireline barely penetrates the stratum corneum, let alone the dermis. Therefore, I would give pause to attributing any clinical impact or benefit of Argireline to its neurotoxinlike effects measured in vitro.
Conclusion
Despite the growing popularity of this ingredient in cosmeceuticals and the praise it gets in media for acting as a topical neurotoxin, there are no rigorous clinical trials or data demonstrating its efficacy in suppressing dynamic facial wrinkles like BoNT does. Most importantly, without penetration into the stratum corneum and deeper layers of the skin, it seems unlikely that Argireline’s clinical benefit derives from a neurotoxiclike mechanism of action. It seems more likely that the Argireline-containing product enhances hydration or imparts some other quality to the skin surface. While there is certainly great appeal for a neurotoxinlike product without injections, I do not believe this ingredient will replace injections of BoNT in the foreseeable future, or at least until scientists can figure out how to enable these products to penetrate into the deeper layers of the skin.
Dr. Goldman is a dermatologist in private practice in Miami and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a general dermatology practice. Dr. Goldman has no relevant disclosures. Write to her at dermnews@mdedge.com or message her on Instagram @DrChloeGoldman.
References
1. Reddy BY et al. Exp Dermatol. 2012 Aug;21(8):569-75.
2. Blanes-Mira C et al. Int J Cosmet Sci. 2002 Oct;24(5):303-10.
3. Wang Y et al. J Cosmet Laser Ther. 2013 Aug;15(4):237-41.
4. Wang Y et al. J Cosmet Laser Ther. 2013;14(2):147-53.
5. Raikou V et al. J Cosmet Dermatol. 2017 Jun;16(2):271-8.
6. Kraeling ME et al. Cutan Ocul Toxicol. 2015 Mar;34(1):46-52.
7. Hoppel M et al. Eur J Pharm Sci. 2015 Feb 20;68:27-35.
Acetyl hexapeptide-8 (or -3), better known by its brand name, Argireline (Lubrizol; Wickliffe, Ohio), is a synthetic peptide gaining popularity in cosmeceutical products for its antiaging benefits. Argireline was developed by the company Lipotec in 2001. Media, beauty bloggers, and product claims have likened this product to a “Botox [or other neurotoxin] alternative,” or “Botox mimicker.”
Mechanism of action
Understanding how Argireline works requires a brief refresher on the mechanism of action of botulinum neurotoxin (BoNT). BoNT relaxes facial muscles and smooths expression lines by inhibiting acetylcholine release at the neuromuscular junction.1 More specifically, the various serotypes of BoNT are single-chain polypeptides that target members of the SNARE complex: SNAP-25, syntaxin, and Vamp. The proteins within the SNARE complex are involved in the docking and fusion of presynaptic vesicles to the presynaptic membrane, necessary steps for acetylcholine release into the neuromuscular junction and muscle contraction. By blocking the action of the SNARE complex proteins, BoNT inhibits release of acetylcholine in the neuromuscular junction and prevents muscle contraction.
Argireline is a synthetic peptide with the sequence Ac-EEMQRR-NH2.2 It is patterned after the N-terminal domain of SNAP-25, one of the members of the SNARE complex targeted by BoNT, and functions to interfere with the assembly of the SNARE complex. In this manner, Argireline would theoretically inhibit fusion of presynaptic vesicles and release of acetylcholine into the neuromuscular junction, thus impeding muscle movement. For this reason, it has been likened to topical Botox. Unlike Botox and other neurotoxins, Argireline was developed for topical application rather than injection.
Preclinical studies
In vitro work done 20 years ago demonstrated that Argireline can prevent assembly of the SNARE complex and inhibit neurotransmitter release with a potency similar to that of BoNT A (Botox).2
In 2013, Wang et al. evaluated the histologic effects of Argireline in aged mouse skin induced by D-galactose. For 6 weeks, Argireline was applied twice daily, and histological changes were assessed using hematoxylin and eosin (H&E) and picrosirius–polarization (PSP) stains. The researchers found elevated levels of type I collagen (P < .01) and reduced type III collagen (P < .05) with the Argireline treatment. These results demonstrated that Argireline could histologically enhance collagen in a manner consistent with skin rejuvenation.3
Clinical studies
In 2002, Blanes et al. assessed the antiwrinkle activity of Argireline by measuring skin topography from silicone implants in the lateral periorbital region of an oil/water (O/W) emulsion containing 10% of the acetyl-hexapeptide in 10 healthy women volunteers. The hexapeptide emulsion was applied twice daily in one lateral periorbital area, and the emulsion vehicle alone was applied twice daily on the contralateral side. Over 30 days of treatment, wrinkle depth was found to have decreased by 30%. The investigators also found that Argireline significantly hindered neurotransmitter release in vitro as robustly as BoNT A, though with notably lower efficacy. No toxicity or irritation was associated with this treatment.2 However, it should be noted that this small study conducted 2 decades ago evaluated only silicone implants with confocal microscopy to evaluate wrinkle depth. There was no subjective clinical assessment of dynamic facial wrinkles. As such, their study is an insufficient basis for drawing conclusions that Argireline is a BoNT mimic. Botox and other types of BoNT affect dynamic facial wrinkles mostly (i.e., wrinkles created by moving muscles of facial expression). This study primarily considers static wrinkles on periorbital skin. While static wrinkles may result from longstanding dynamic wrinkles, BoNT mainly targets dynamic wrinkles, again not comparing apples to apples.
At the same time that Wang et al. conducted their experiment on the skin of aged mice as noted above, they performed a multicenter clinical trial in 60 human subjects who received a randomized treatment of Argireline or placebo in a ratio of 3:1 to assess its safety and efficacy. For 4 weeks, the test product or placebo was applied to periorbital wrinkles twice daily. The researchers found the total antiwrinkle efficacy in the Argireline group to be 48.9% based on the subjective evaluation, compared with 0% in the placebo group. The objective evaluation indicated that all parameters of roughness were diminished in the Argireline group (P < .01), with no reduction observed in the placebo group (P < .05).4 There was a little more to appreciate from this study compared with the one reported by Blanes et al., insofar as subjective evaluations and objective evaluations with silica replicas were done. However, this study was not blinded, so the 48.9% wrinkle reduction in the Argireline group vs. 0% in the control group seems suspicious. Additionally, there was a greater focus on static rather than dynamic wrinkles.
In 2017, Raikou et al. conducted a prospective, randomized controlled study to assess the effects of acetyl hexapeptide-3 (Argireline) and tripeptide-10 citrulline in 24 healthy female volunteers (aged 30-60 years) and determine if there was any synergistic action between the peptides. Subjects were randomized to receive a combination of the peptides, tripeptide-10 citrulline only, acetyl hexapeptide-3 only, or neither peptide for 60 days. The researchers found a significant reduction in transepidermal water loss (TEWL) in the Argireline group, compared with the placebo group.5 The result of this study makes me question if the decrease in depth of the wrinkles measured in the former studies is really just a measure of increased skin hydration from the Argireline, rather than a neurotoxic effect of Argireline.
Formulation and penetration: Can Argireline get through your skin?
One of the fundamental questions regarding Argireline is whether it can penetrate through the stratum corneum and find its target – the facial muscles – where it is intended to function. Argireline is a charged, hydrophilic, and large–molecular weight peptide, and each of these factors impairs penetration through the stratum corneum. Therefore, studies assessing penetration are particularly important.
In 2015, Kraeling et al. conducted an in vitro evaluation of the skin penetration of acetyl hexapeptide-8 in hairless guinea pig and human cadaver skin. An oil-in-water (O/W) emulsion containing 10% acetyl hexapeptide-8 was applied (2 mg/cm2) and penetration was quantified in skin layers via hydrophilic interaction liquid chromatography with tandem mass spectrometry. Most of the acetyl hexapeptide-8 was found to have been washed from human cadaver, as well as guinea pig, skin. Less than 1% of the peptide penetrated the guinea pig or human skin. Of this small amount that penetrated the skin, most stayed in the stratum corneum of guinea pigs (0.54%) and human cadavers (0.22%). The levels of acetyl hexapeptide-8 declined further with each layer of tape stripping removal. Epidermal levels of the peptide in tested skin were similar at 0.01%, and none of the peptide was found in the dermis.6 These results indicate negligible penetration by this highly touted peptide ingredient.
Some studies have shown that altering the formulation of acetyl hexapeptide-8 can enhance penetration. Hoppel et al. demonstrated that formulations of the peptide, especially in a water-oil-water (W/O/W emulsion [as compared with O/W and W/O emulsions] can increase penetration into the stratum corneum in porcine skin.7 Notably, this is still very superficial relative to the dermis and muscles. Irrespective of formulation, studies have shown that Argireline barely penetrates the stratum corneum, let alone the dermis. Therefore, I would give pause to attributing any clinical impact or benefit of Argireline to its neurotoxinlike effects measured in vitro.
Conclusion
Despite the growing popularity of this ingredient in cosmeceuticals and the praise it gets in media for acting as a topical neurotoxin, there are no rigorous clinical trials or data demonstrating its efficacy in suppressing dynamic facial wrinkles like BoNT does. Most importantly, without penetration into the stratum corneum and deeper layers of the skin, it seems unlikely that Argireline’s clinical benefit derives from a neurotoxiclike mechanism of action. It seems more likely that the Argireline-containing product enhances hydration or imparts some other quality to the skin surface. While there is certainly great appeal for a neurotoxinlike product without injections, I do not believe this ingredient will replace injections of BoNT in the foreseeable future, or at least until scientists can figure out how to enable these products to penetrate into the deeper layers of the skin.
Dr. Goldman is a dermatologist in private practice in Miami and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a general dermatology practice. Dr. Goldman has no relevant disclosures. Write to her at dermnews@mdedge.com or message her on Instagram @DrChloeGoldman.
References
1. Reddy BY et al. Exp Dermatol. 2012 Aug;21(8):569-75.
2. Blanes-Mira C et al. Int J Cosmet Sci. 2002 Oct;24(5):303-10.
3. Wang Y et al. J Cosmet Laser Ther. 2013 Aug;15(4):237-41.
4. Wang Y et al. J Cosmet Laser Ther. 2013;14(2):147-53.
5. Raikou V et al. J Cosmet Dermatol. 2017 Jun;16(2):271-8.
6. Kraeling ME et al. Cutan Ocul Toxicol. 2015 Mar;34(1):46-52.
7. Hoppel M et al. Eur J Pharm Sci. 2015 Feb 20;68:27-35.
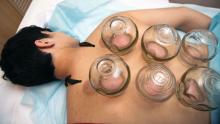
Cupping in dermatology
My inspiration to write about cupping this month stems from the perception that everyone seems to be talking about it, from a facialist who suggested it for me to a coworker who swears by cupping to treat her allergies. Cupping is by no means a novel procedure. Its use as a health therapy dates back thousands of years to ancient Egypt (1500 BCE), ancient Greece (described by Hippocrates), ancient Rome (described by the Greek physician Galen), China (during the Han dynasty, 206 BCE to 220 CE) and traditional Islamic culture.1 Over the past decade, the popularity of this ancient procedure has been increasing in the United States.1 Cupping has been applied as a remedy for various dermatologic and medical conditions, including herpes zoster, headaches, diminished appetite, maldigestion, abscess evacuation, narcolepsy, pain, fever, dysmenorrhea, and gout.1,2
Theories on the mechanism(s) of action
The practice of cupping is differentiated into dry and wet cupping.1,2 Traditionally, with dry cupping, a flame is applied to heat the air inside a thick glass cup (rather than the cup itself).1 The cup is placed on the skin surface, and negative pressure suctions the skin into the cup. Wet cupping differs mainly from dry cupping in that it involves blood-letting. Cups made of either silicone or glass of varying size and shapes are used. Modern adaptations to cupping include needle, herbal, and pulsatile cupping, as well as a “moving cupping” technique (vs. traditionally stationary cups).1
There are several theories, many of which are derived from the nondermatologic literature (that is, pain management), as to how cupping may deliver a clinical benefit. Some theories are based in scientific and medical principles, whereas other theories are more whimsical – specifically, that cupping draws out evil spirits.2 Studies of dry cupping have suggested that the procedure results in increased oxygenation of muscles via a local increase in oxygenated hemoglobin, which may help improve muscular activity and reduce pain.1 As theorized by Lowe in 2017, negative pressure exerted by dry cupping leads to stretching and dilation of capillaries, which increases blood flow.3 Wet cupping has been shown to increase heat shock protein 70 (HSP70) and beta-endorphin expression in rat models, which is thought to facilitate pain management.1 Removal of oxidants and reduction of reactive oxygen species in the blood is believed to be among the benefits of wet cupping.1
Cupping in general dermatology
While , as well as various inflammatory conditions.
Herpes zoster
In 2010, Cao et al. reported on their systematic review of wet cupping after completing searches of multiple databases (that is, PubMed, the Cochrane Library [Issue 3, 2008], China Network Knowledge Infrastructure, Chinese Scientific Journal Database, and Wan Fang Database). They identified eight randomized controlled trials involving 651 patients, with meta-analyses revealing that wet cupping performed better than medications in terms of the number of “cured” patients, number of patients with improved symptoms, and a lower incidence of postherpetic neuralgia. Wet cupping, in addition to medication, was also found to be superior to medication alone in multiple patients. The researchers concluded that wet cupping appears to effectively treat herpes zoster.4 However, the study failed to identify which medications were used to treat herpes zoster. In the United States, common medications for herpes zoster include acyclovir, valacyclovir, steroids, gabapentin, and other neuromodulators. Without knowing which medications were used, it is difficult to compare cupping to medication in terms of efficacy in treating herpes zoster.
Urticaria
Urticaria (hives) is an inflammatory skin condition that can be very uncomfortable for patients but often resolves without intervention within several months after onset. In 2001, Li and Ding reported on the treatment with cupping of 40 patients with urticaria. The cure rate among the treatment group was cited as 55%, compared with 30% in the control group, who were treated with a traditional Chinese remedy and an unidentified first-generation antihistamine.1,5 In 2020, Xiao et al. conducted a systematic review and meta-analysis of cupping therapy for patients with chronic urticaria. They identified 13 comparisons from 12 randomized controlled trials involving 842 subjects. The investigators found no significant differences between wet cupping and medication usage. They also found that cupping combined with antihistamine treatment was superior to antihistamines alone, and cupping therapy with acupuncture was more effective than acupuncture alone. The investigators did call for caution, citing the poor quality of the studies reviewed.6
It is important to note that it is difficult to attribute resolution of urticaria to the use of cupping given the self-resolution often associated with this condition. Antihistamines are the mainstay of therapy for urticaria, but in my personal experience, patients are not entirely satisfied with the level of symptom control with antihistamines alone and often search for alternative therapies to control the pesky hives and associated itch. In 2014, omalizumab (Xolair) was approved for treating chronic idiopathic urticaria, which has helped patients control symptoms of chronic idiopathic urticaria without needing to take antihistamines. There was no indication that the studies reviewed by Xiao et al. compared cupping against this new effective treatment. Therefore, these studies comparing cupping to medical management are outdated.
Acne, eczema, and psoriasis
Soliman’s 2018 review of cupping in dermatology included a few studies on these common cutaneous conditions. For instance, a 2013 single-blind prospective study by Xu et al. reported on the results of patients with moderate acne who received wet cupping (in the form of prickling bloodletting) twice weekly for 6 weeks.7 They reported that patients demonstrated improvement in the global acne grading system (GAGS) score by the end of the trial.1,7 Unfortunately, cupping was not compared with standard acne treatments (that is, benzoyl peroxide, topical and oral antibiotics, isotretinoin, topical retinoids, spironolactone).
In evaluating cupping for acute eczema, wet cupping was compared with oral loratadine and topical ointments in a 2007 study by Yao and Li. They divided 88 cases into treatment and control groups, with the former group (n = 46) receiving bloodletting puncturing and cupping and the control group (n = 42) receiving oral loratadine and topical Pairuisong (an herbal ointment used in Chinese medicine). The investigators observed no significant difference in total effective rates but a superior difference in the rates of responses that were considered “cured” and “markedly effective” in favor of the cupping treatment.1,8 However, a case report by Hon et al. has indicated that cupping therapy may be associated with more harm than benefit when used as an eczema treatment.1,9
In addition, it is important to note that the past 5 years have been gamechanging in the management of chronic eczema in terms of the array of novel and effective therapies (e.g., dupilumab and JAK inhibitors) and chronic moderate-to-severe eczema has become very treatable. Similarly, acute eczema is often successfully managed with topical steroids, calcineurin inhibitors, and emollients. As such, there is no compelling reason to consider an unproven treatment such as cupping.
In 2020, Xing et al. reviewed 16 randomized controlled trials assessing the use of “moving cupping” for plaque psoriasis, with 1,164 patients meeting inclusion criteria. Moving cupping was found to be significantly more effective than “no-moving” cupping therapy, and moving cupping, combined with medications, performed better than medications alone.10 None of the trials evaluated in this study included randomized controlled trials that compared patients using any of the more modern psoriasis medications, specifically biologics. And, again, the studies evaluated were not of the highest quality.
The data that support cupping, as summarized above, are based mostly on case reports, and strong double-blind prospective studies are lacking. Additionally, most of the studies cited gauged the efficacy of cupping using qualitative endpoints, rather than standardized quantitative endpoints and scales. Moreover, spontaneous remission of various dermatoses can occur, or they can improve over time, including acute eczema, psoriasis, and, especially, urticaria.
Adverse effects of cupping
Often alternative therapies are seen as “benign” and without adverse effects. However, complications can result from cupping. Trauma can be induced from the cupping itself by damaging superficial blood vessels and causing bruising.1,11 Blistering can also occur secondary to the suction effect, and the epidermal and dermal layers of the skin can be separated.1,11 Further, burns and discoloration have also been noted secondary to heat, trauma, and post inflammatory pigmentary changes.1,11 Another risk of cupping is the Koebner phenomenon, which occurs with psoriasis, with new lesions appearing in traumatized skin.12 Other adverse outcomes that have been reported with cupping include reactivation of herpes simplex virus secondary to skin trauma, iron deficiency anemia (secondary to blood loss), panniculitis, infections, and residual marks mistaken for signs of child abuse.1,11
Cupping in aesthetic dermatology
Facial cupping, a distinct practice from body cupping used to treat general dermatology conditions described previously, is also increasing in popularity. This practice is usually conducted in association with a facial or facial acupuncture by an aesthetician or other licensed professional. It can also be performed using at-home kits. The marketing claims for facial cupping cite improved tightening and contouring of facial skin, increased facial microcirculation and collagen synthesis, and enhanced lymphatic flow to aid with facial puffiness or swelling. One supposed mechanism for these benefits is that cupping increases blood flow. Interestingly, there was a 2020 animal study in which photoacoustic imaging of a mouse ear revealed increased temporary blood flow in the cupping microenvironment.13 Currently, however, there is no evidence in the English scientific literature that supports facial cupping. The benefits attributed to facial cupping for aesthetic purposes have emerged only in personal anecdotes. The temporary increase in blood flow may induce inflammation and swelling that adds volume to the face and temporarily diminishes wrinkles. However, this temporary plumpness may be associated with adverse effects, such as local trauma, irritation, bruising, postinflammatory pigmentary alteration, or even herpes reactivation. In my opinion, the possible adverse effects of cupping outweigh any potential benefit, especially given the insufficient evidence supporting the utility of cupping for cosmetic enhancement.
Summary
There is increasing interest among patients to incorporate complementary and alternative medicine – including the ancient tradition of cupping – in managing medical dermatologic conditions. However, current evidence supporting cupping as an effective therapeutic strategy is not strong, with most studies to date appearing to be of poor quality or not sufficiently convincing to displace standard therapies. Our medical strategies for managing chronic dermatologic conditions, particularly inflammatory disorders, continue to improve from both a safety and a proven efficacy standpoint. Therefore, I would not forgo medical management in favor of cupping. While cupping can be used as an adjunct therapy, I would caution patients about possible adverse side effects. In the aesthetic world, cupping is also gaining popularity, but this trend is also not supported by current evidence or studies, at least in the Western literature.
Dr. Goldman is a dermatologist in private practice in Miami and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a general dermatology practice. Write to her at dermnews@mdedge.com or message her on Instragram @DrChloeGoldman. Dr. Goldman receives compensation to create social media content for Replenix, a skin care company. She has no other disclosures.
References
1. Soliman Y et al. Acta Dermatovenerol Alp Pannonica Adriat. 2018 Jun;27(2):103-7.
2. França K and Lotti T. Advances in Integrative Dermatology. John Wiley & Sons, 2019.
3. Lowe DT. Complement Ther Clin Pract. 2017 Nov;29:162-8.
4.Cao H et al. Altern Ther Health Med. 2010 Nov-Dec;16(6):48-54.
5. Li L and Ding J. J Tradit Chin Med. 2001 Mar;21(1):37-8.
6. Xiao XJ et al. J Integr Med. 2020 Jul;18(4):303-12.
7. Xu J et al. J Tradit Chin Med. 2013 Dec;33(6):752-6.
8. Yao J et al. Zhongguo Zhen Jiu. 2007; Jun;27(6):424-6.
9. Hon KL et al. Case Rep Pediatr. 2013;2013:605829.
10. Xing M et al. Medicine (Baltimore). 2020 Oct 9;99(41):e22539.
11. Kim TH et al. Eur J Integr Med. 2014 Aug 1;6(4):434-40.
12. Vender R and Vender R. J Cutan Med Surg. 2015 May-Jun;19(3):320-2.
13. Zhou Y et al. Biomed Opt Express. 2020 Apr 6;11(5):2394-401.
This article was updated 4/25/22.
My inspiration to write about cupping this month stems from the perception that everyone seems to be talking about it, from a facialist who suggested it for me to a coworker who swears by cupping to treat her allergies. Cupping is by no means a novel procedure. Its use as a health therapy dates back thousands of years to ancient Egypt (1500 BCE), ancient Greece (described by Hippocrates), ancient Rome (described by the Greek physician Galen), China (during the Han dynasty, 206 BCE to 220 CE) and traditional Islamic culture.1 Over the past decade, the popularity of this ancient procedure has been increasing in the United States.1 Cupping has been applied as a remedy for various dermatologic and medical conditions, including herpes zoster, headaches, diminished appetite, maldigestion, abscess evacuation, narcolepsy, pain, fever, dysmenorrhea, and gout.1,2
Theories on the mechanism(s) of action
The practice of cupping is differentiated into dry and wet cupping.1,2 Traditionally, with dry cupping, a flame is applied to heat the air inside a thick glass cup (rather than the cup itself).1 The cup is placed on the skin surface, and negative pressure suctions the skin into the cup. Wet cupping differs mainly from dry cupping in that it involves blood-letting. Cups made of either silicone or glass of varying size and shapes are used. Modern adaptations to cupping include needle, herbal, and pulsatile cupping, as well as a “moving cupping” technique (vs. traditionally stationary cups).1
There are several theories, many of which are derived from the nondermatologic literature (that is, pain management), as to how cupping may deliver a clinical benefit. Some theories are based in scientific and medical principles, whereas other theories are more whimsical – specifically, that cupping draws out evil spirits.2 Studies of dry cupping have suggested that the procedure results in increased oxygenation of muscles via a local increase in oxygenated hemoglobin, which may help improve muscular activity and reduce pain.1 As theorized by Lowe in 2017, negative pressure exerted by dry cupping leads to stretching and dilation of capillaries, which increases blood flow.3 Wet cupping has been shown to increase heat shock protein 70 (HSP70) and beta-endorphin expression in rat models, which is thought to facilitate pain management.1 Removal of oxidants and reduction of reactive oxygen species in the blood is believed to be among the benefits of wet cupping.1
Cupping in general dermatology
While , as well as various inflammatory conditions.
Herpes zoster
In 2010, Cao et al. reported on their systematic review of wet cupping after completing searches of multiple databases (that is, PubMed, the Cochrane Library [Issue 3, 2008], China Network Knowledge Infrastructure, Chinese Scientific Journal Database, and Wan Fang Database). They identified eight randomized controlled trials involving 651 patients, with meta-analyses revealing that wet cupping performed better than medications in terms of the number of “cured” patients, number of patients with improved symptoms, and a lower incidence of postherpetic neuralgia. Wet cupping, in addition to medication, was also found to be superior to medication alone in multiple patients. The researchers concluded that wet cupping appears to effectively treat herpes zoster.4 However, the study failed to identify which medications were used to treat herpes zoster. In the United States, common medications for herpes zoster include acyclovir, valacyclovir, steroids, gabapentin, and other neuromodulators. Without knowing which medications were used, it is difficult to compare cupping to medication in terms of efficacy in treating herpes zoster.
Urticaria
Urticaria (hives) is an inflammatory skin condition that can be very uncomfortable for patients but often resolves without intervention within several months after onset. In 2001, Li and Ding reported on the treatment with cupping of 40 patients with urticaria. The cure rate among the treatment group was cited as 55%, compared with 30% in the control group, who were treated with a traditional Chinese remedy and an unidentified first-generation antihistamine.1,5 In 2020, Xiao et al. conducted a systematic review and meta-analysis of cupping therapy for patients with chronic urticaria. They identified 13 comparisons from 12 randomized controlled trials involving 842 subjects. The investigators found no significant differences between wet cupping and medication usage. They also found that cupping combined with antihistamine treatment was superior to antihistamines alone, and cupping therapy with acupuncture was more effective than acupuncture alone. The investigators did call for caution, citing the poor quality of the studies reviewed.6
It is important to note that it is difficult to attribute resolution of urticaria to the use of cupping given the self-resolution often associated with this condition. Antihistamines are the mainstay of therapy for urticaria, but in my personal experience, patients are not entirely satisfied with the level of symptom control with antihistamines alone and often search for alternative therapies to control the pesky hives and associated itch. In 2014, omalizumab (Xolair) was approved for treating chronic idiopathic urticaria, which has helped patients control symptoms of chronic idiopathic urticaria without needing to take antihistamines. There was no indication that the studies reviewed by Xiao et al. compared cupping against this new effective treatment. Therefore, these studies comparing cupping to medical management are outdated.
Acne, eczema, and psoriasis
Soliman’s 2018 review of cupping in dermatology included a few studies on these common cutaneous conditions. For instance, a 2013 single-blind prospective study by Xu et al. reported on the results of patients with moderate acne who received wet cupping (in the form of prickling bloodletting) twice weekly for 6 weeks.7 They reported that patients demonstrated improvement in the global acne grading system (GAGS) score by the end of the trial.1,7 Unfortunately, cupping was not compared with standard acne treatments (that is, benzoyl peroxide, topical and oral antibiotics, isotretinoin, topical retinoids, spironolactone).
In evaluating cupping for acute eczema, wet cupping was compared with oral loratadine and topical ointments in a 2007 study by Yao and Li. They divided 88 cases into treatment and control groups, with the former group (n = 46) receiving bloodletting puncturing and cupping and the control group (n = 42) receiving oral loratadine and topical Pairuisong (an herbal ointment used in Chinese medicine). The investigators observed no significant difference in total effective rates but a superior difference in the rates of responses that were considered “cured” and “markedly effective” in favor of the cupping treatment.1,8 However, a case report by Hon et al. has indicated that cupping therapy may be associated with more harm than benefit when used as an eczema treatment.1,9
In addition, it is important to note that the past 5 years have been gamechanging in the management of chronic eczema in terms of the array of novel and effective therapies (e.g., dupilumab and JAK inhibitors) and chronic moderate-to-severe eczema has become very treatable. Similarly, acute eczema is often successfully managed with topical steroids, calcineurin inhibitors, and emollients. As such, there is no compelling reason to consider an unproven treatment such as cupping.
In 2020, Xing et al. reviewed 16 randomized controlled trials assessing the use of “moving cupping” for plaque psoriasis, with 1,164 patients meeting inclusion criteria. Moving cupping was found to be significantly more effective than “no-moving” cupping therapy, and moving cupping, combined with medications, performed better than medications alone.10 None of the trials evaluated in this study included randomized controlled trials that compared patients using any of the more modern psoriasis medications, specifically biologics. And, again, the studies evaluated were not of the highest quality.
The data that support cupping, as summarized above, are based mostly on case reports, and strong double-blind prospective studies are lacking. Additionally, most of the studies cited gauged the efficacy of cupping using qualitative endpoints, rather than standardized quantitative endpoints and scales. Moreover, spontaneous remission of various dermatoses can occur, or they can improve over time, including acute eczema, psoriasis, and, especially, urticaria.
Adverse effects of cupping
Often alternative therapies are seen as “benign” and without adverse effects. However, complications can result from cupping. Trauma can be induced from the cupping itself by damaging superficial blood vessels and causing bruising.1,11 Blistering can also occur secondary to the suction effect, and the epidermal and dermal layers of the skin can be separated.1,11 Further, burns and discoloration have also been noted secondary to heat, trauma, and post inflammatory pigmentary changes.1,11 Another risk of cupping is the Koebner phenomenon, which occurs with psoriasis, with new lesions appearing in traumatized skin.12 Other adverse outcomes that have been reported with cupping include reactivation of herpes simplex virus secondary to skin trauma, iron deficiency anemia (secondary to blood loss), panniculitis, infections, and residual marks mistaken for signs of child abuse.1,11
Cupping in aesthetic dermatology
Facial cupping, a distinct practice from body cupping used to treat general dermatology conditions described previously, is also increasing in popularity. This practice is usually conducted in association with a facial or facial acupuncture by an aesthetician or other licensed professional. It can also be performed using at-home kits. The marketing claims for facial cupping cite improved tightening and contouring of facial skin, increased facial microcirculation and collagen synthesis, and enhanced lymphatic flow to aid with facial puffiness or swelling. One supposed mechanism for these benefits is that cupping increases blood flow. Interestingly, there was a 2020 animal study in which photoacoustic imaging of a mouse ear revealed increased temporary blood flow in the cupping microenvironment.13 Currently, however, there is no evidence in the English scientific literature that supports facial cupping. The benefits attributed to facial cupping for aesthetic purposes have emerged only in personal anecdotes. The temporary increase in blood flow may induce inflammation and swelling that adds volume to the face and temporarily diminishes wrinkles. However, this temporary plumpness may be associated with adverse effects, such as local trauma, irritation, bruising, postinflammatory pigmentary alteration, or even herpes reactivation. In my opinion, the possible adverse effects of cupping outweigh any potential benefit, especially given the insufficient evidence supporting the utility of cupping for cosmetic enhancement.
Summary
There is increasing interest among patients to incorporate complementary and alternative medicine – including the ancient tradition of cupping – in managing medical dermatologic conditions. However, current evidence supporting cupping as an effective therapeutic strategy is not strong, with most studies to date appearing to be of poor quality or not sufficiently convincing to displace standard therapies. Our medical strategies for managing chronic dermatologic conditions, particularly inflammatory disorders, continue to improve from both a safety and a proven efficacy standpoint. Therefore, I would not forgo medical management in favor of cupping. While cupping can be used as an adjunct therapy, I would caution patients about possible adverse side effects. In the aesthetic world, cupping is also gaining popularity, but this trend is also not supported by current evidence or studies, at least in the Western literature.
Dr. Goldman is a dermatologist in private practice in Miami and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a general dermatology practice. Write to her at dermnews@mdedge.com or message her on Instragram @DrChloeGoldman. Dr. Goldman receives compensation to create social media content for Replenix, a skin care company. She has no other disclosures.
References
1. Soliman Y et al. Acta Dermatovenerol Alp Pannonica Adriat. 2018 Jun;27(2):103-7.
2. França K and Lotti T. Advances in Integrative Dermatology. John Wiley & Sons, 2019.
3. Lowe DT. Complement Ther Clin Pract. 2017 Nov;29:162-8.
4.Cao H et al. Altern Ther Health Med. 2010 Nov-Dec;16(6):48-54.
5. Li L and Ding J. J Tradit Chin Med. 2001 Mar;21(1):37-8.
6. Xiao XJ et al. J Integr Med. 2020 Jul;18(4):303-12.
7. Xu J et al. J Tradit Chin Med. 2013 Dec;33(6):752-6.
8. Yao J et al. Zhongguo Zhen Jiu. 2007; Jun;27(6):424-6.
9. Hon KL et al. Case Rep Pediatr. 2013;2013:605829.
10. Xing M et al. Medicine (Baltimore). 2020 Oct 9;99(41):e22539.
11. Kim TH et al. Eur J Integr Med. 2014 Aug 1;6(4):434-40.
12. Vender R and Vender R. J Cutan Med Surg. 2015 May-Jun;19(3):320-2.
13. Zhou Y et al. Biomed Opt Express. 2020 Apr 6;11(5):2394-401.
This article was updated 4/25/22.
My inspiration to write about cupping this month stems from the perception that everyone seems to be talking about it, from a facialist who suggested it for me to a coworker who swears by cupping to treat her allergies. Cupping is by no means a novel procedure. Its use as a health therapy dates back thousands of years to ancient Egypt (1500 BCE), ancient Greece (described by Hippocrates), ancient Rome (described by the Greek physician Galen), China (during the Han dynasty, 206 BCE to 220 CE) and traditional Islamic culture.1 Over the past decade, the popularity of this ancient procedure has been increasing in the United States.1 Cupping has been applied as a remedy for various dermatologic and medical conditions, including herpes zoster, headaches, diminished appetite, maldigestion, abscess evacuation, narcolepsy, pain, fever, dysmenorrhea, and gout.1,2
Theories on the mechanism(s) of action
The practice of cupping is differentiated into dry and wet cupping.1,2 Traditionally, with dry cupping, a flame is applied to heat the air inside a thick glass cup (rather than the cup itself).1 The cup is placed on the skin surface, and negative pressure suctions the skin into the cup. Wet cupping differs mainly from dry cupping in that it involves blood-letting. Cups made of either silicone or glass of varying size and shapes are used. Modern adaptations to cupping include needle, herbal, and pulsatile cupping, as well as a “moving cupping” technique (vs. traditionally stationary cups).1
There are several theories, many of which are derived from the nondermatologic literature (that is, pain management), as to how cupping may deliver a clinical benefit. Some theories are based in scientific and medical principles, whereas other theories are more whimsical – specifically, that cupping draws out evil spirits.2 Studies of dry cupping have suggested that the procedure results in increased oxygenation of muscles via a local increase in oxygenated hemoglobin, which may help improve muscular activity and reduce pain.1 As theorized by Lowe in 2017, negative pressure exerted by dry cupping leads to stretching and dilation of capillaries, which increases blood flow.3 Wet cupping has been shown to increase heat shock protein 70 (HSP70) and beta-endorphin expression in rat models, which is thought to facilitate pain management.1 Removal of oxidants and reduction of reactive oxygen species in the blood is believed to be among the benefits of wet cupping.1
Cupping in general dermatology
While , as well as various inflammatory conditions.
Herpes zoster
In 2010, Cao et al. reported on their systematic review of wet cupping after completing searches of multiple databases (that is, PubMed, the Cochrane Library [Issue 3, 2008], China Network Knowledge Infrastructure, Chinese Scientific Journal Database, and Wan Fang Database). They identified eight randomized controlled trials involving 651 patients, with meta-analyses revealing that wet cupping performed better than medications in terms of the number of “cured” patients, number of patients with improved symptoms, and a lower incidence of postherpetic neuralgia. Wet cupping, in addition to medication, was also found to be superior to medication alone in multiple patients. The researchers concluded that wet cupping appears to effectively treat herpes zoster.4 However, the study failed to identify which medications were used to treat herpes zoster. In the United States, common medications for herpes zoster include acyclovir, valacyclovir, steroids, gabapentin, and other neuromodulators. Without knowing which medications were used, it is difficult to compare cupping to medication in terms of efficacy in treating herpes zoster.
Urticaria
Urticaria (hives) is an inflammatory skin condition that can be very uncomfortable for patients but often resolves without intervention within several months after onset. In 2001, Li and Ding reported on the treatment with cupping of 40 patients with urticaria. The cure rate among the treatment group was cited as 55%, compared with 30% in the control group, who were treated with a traditional Chinese remedy and an unidentified first-generation antihistamine.1,5 In 2020, Xiao et al. conducted a systematic review and meta-analysis of cupping therapy for patients with chronic urticaria. They identified 13 comparisons from 12 randomized controlled trials involving 842 subjects. The investigators found no significant differences between wet cupping and medication usage. They also found that cupping combined with antihistamine treatment was superior to antihistamines alone, and cupping therapy with acupuncture was more effective than acupuncture alone. The investigators did call for caution, citing the poor quality of the studies reviewed.6
It is important to note that it is difficult to attribute resolution of urticaria to the use of cupping given the self-resolution often associated with this condition. Antihistamines are the mainstay of therapy for urticaria, but in my personal experience, patients are not entirely satisfied with the level of symptom control with antihistamines alone and often search for alternative therapies to control the pesky hives and associated itch. In 2014, omalizumab (Xolair) was approved for treating chronic idiopathic urticaria, which has helped patients control symptoms of chronic idiopathic urticaria without needing to take antihistamines. There was no indication that the studies reviewed by Xiao et al. compared cupping against this new effective treatment. Therefore, these studies comparing cupping to medical management are outdated.
Acne, eczema, and psoriasis
Soliman’s 2018 review of cupping in dermatology included a few studies on these common cutaneous conditions. For instance, a 2013 single-blind prospective study by Xu et al. reported on the results of patients with moderate acne who received wet cupping (in the form of prickling bloodletting) twice weekly for 6 weeks.7 They reported that patients demonstrated improvement in the global acne grading system (GAGS) score by the end of the trial.1,7 Unfortunately, cupping was not compared with standard acne treatments (that is, benzoyl peroxide, topical and oral antibiotics, isotretinoin, topical retinoids, spironolactone).
In evaluating cupping for acute eczema, wet cupping was compared with oral loratadine and topical ointments in a 2007 study by Yao and Li. They divided 88 cases into treatment and control groups, with the former group (n = 46) receiving bloodletting puncturing and cupping and the control group (n = 42) receiving oral loratadine and topical Pairuisong (an herbal ointment used in Chinese medicine). The investigators observed no significant difference in total effective rates but a superior difference in the rates of responses that were considered “cured” and “markedly effective” in favor of the cupping treatment.1,8 However, a case report by Hon et al. has indicated that cupping therapy may be associated with more harm than benefit when used as an eczema treatment.1,9
In addition, it is important to note that the past 5 years have been gamechanging in the management of chronic eczema in terms of the array of novel and effective therapies (e.g., dupilumab and JAK inhibitors) and chronic moderate-to-severe eczema has become very treatable. Similarly, acute eczema is often successfully managed with topical steroids, calcineurin inhibitors, and emollients. As such, there is no compelling reason to consider an unproven treatment such as cupping.
In 2020, Xing et al. reviewed 16 randomized controlled trials assessing the use of “moving cupping” for plaque psoriasis, with 1,164 patients meeting inclusion criteria. Moving cupping was found to be significantly more effective than “no-moving” cupping therapy, and moving cupping, combined with medications, performed better than medications alone.10 None of the trials evaluated in this study included randomized controlled trials that compared patients using any of the more modern psoriasis medications, specifically biologics. And, again, the studies evaluated were not of the highest quality.
The data that support cupping, as summarized above, are based mostly on case reports, and strong double-blind prospective studies are lacking. Additionally, most of the studies cited gauged the efficacy of cupping using qualitative endpoints, rather than standardized quantitative endpoints and scales. Moreover, spontaneous remission of various dermatoses can occur, or they can improve over time, including acute eczema, psoriasis, and, especially, urticaria.
Adverse effects of cupping
Often alternative therapies are seen as “benign” and without adverse effects. However, complications can result from cupping. Trauma can be induced from the cupping itself by damaging superficial blood vessels and causing bruising.1,11 Blistering can also occur secondary to the suction effect, and the epidermal and dermal layers of the skin can be separated.1,11 Further, burns and discoloration have also been noted secondary to heat, trauma, and post inflammatory pigmentary changes.1,11 Another risk of cupping is the Koebner phenomenon, which occurs with psoriasis, with new lesions appearing in traumatized skin.12 Other adverse outcomes that have been reported with cupping include reactivation of herpes simplex virus secondary to skin trauma, iron deficiency anemia (secondary to blood loss), panniculitis, infections, and residual marks mistaken for signs of child abuse.1,11
Cupping in aesthetic dermatology
Facial cupping, a distinct practice from body cupping used to treat general dermatology conditions described previously, is also increasing in popularity. This practice is usually conducted in association with a facial or facial acupuncture by an aesthetician or other licensed professional. It can also be performed using at-home kits. The marketing claims for facial cupping cite improved tightening and contouring of facial skin, increased facial microcirculation and collagen synthesis, and enhanced lymphatic flow to aid with facial puffiness or swelling. One supposed mechanism for these benefits is that cupping increases blood flow. Interestingly, there was a 2020 animal study in which photoacoustic imaging of a mouse ear revealed increased temporary blood flow in the cupping microenvironment.13 Currently, however, there is no evidence in the English scientific literature that supports facial cupping. The benefits attributed to facial cupping for aesthetic purposes have emerged only in personal anecdotes. The temporary increase in blood flow may induce inflammation and swelling that adds volume to the face and temporarily diminishes wrinkles. However, this temporary plumpness may be associated with adverse effects, such as local trauma, irritation, bruising, postinflammatory pigmentary alteration, or even herpes reactivation. In my opinion, the possible adverse effects of cupping outweigh any potential benefit, especially given the insufficient evidence supporting the utility of cupping for cosmetic enhancement.
Summary
There is increasing interest among patients to incorporate complementary and alternative medicine – including the ancient tradition of cupping – in managing medical dermatologic conditions. However, current evidence supporting cupping as an effective therapeutic strategy is not strong, with most studies to date appearing to be of poor quality or not sufficiently convincing to displace standard therapies. Our medical strategies for managing chronic dermatologic conditions, particularly inflammatory disorders, continue to improve from both a safety and a proven efficacy standpoint. Therefore, I would not forgo medical management in favor of cupping. While cupping can be used as an adjunct therapy, I would caution patients about possible adverse side effects. In the aesthetic world, cupping is also gaining popularity, but this trend is also not supported by current evidence or studies, at least in the Western literature.
Dr. Goldman is a dermatologist in private practice in Miami and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a general dermatology practice. Write to her at dermnews@mdedge.com or message her on Instragram @DrChloeGoldman. Dr. Goldman receives compensation to create social media content for Replenix, a skin care company. She has no other disclosures.
References
1. Soliman Y et al. Acta Dermatovenerol Alp Pannonica Adriat. 2018 Jun;27(2):103-7.
2. França K and Lotti T. Advances in Integrative Dermatology. John Wiley & Sons, 2019.
3. Lowe DT. Complement Ther Clin Pract. 2017 Nov;29:162-8.
4.Cao H et al. Altern Ther Health Med. 2010 Nov-Dec;16(6):48-54.
5. Li L and Ding J. J Tradit Chin Med. 2001 Mar;21(1):37-8.
6. Xiao XJ et al. J Integr Med. 2020 Jul;18(4):303-12.
7. Xu J et al. J Tradit Chin Med. 2013 Dec;33(6):752-6.
8. Yao J et al. Zhongguo Zhen Jiu. 2007; Jun;27(6):424-6.
9. Hon KL et al. Case Rep Pediatr. 2013;2013:605829.
10. Xing M et al. Medicine (Baltimore). 2020 Oct 9;99(41):e22539.
11. Kim TH et al. Eur J Integr Med. 2014 Aug 1;6(4):434-40.
12. Vender R and Vender R. J Cutan Med Surg. 2015 May-Jun;19(3):320-2.
13. Zhou Y et al. Biomed Opt Express. 2020 Apr 6;11(5):2394-401.
This article was updated 4/25/22.
The gap in cosmeceuticals education
Starting this month, I will be joining Dr. Leslie S. Baumann as a cocontributor to the Cosmeceutical Critique column, and since this is my first column, I would like to formally introduce myself. I am a cosmetic and general dermatologist in private practice in Miami and a longtime skin care enthusiast. My path toward becoming a dermatologist began when I was working in New York City, my hometown, as a scientific researcher, fulfilling my passion for scientific inquiry. After realizing that I most enjoyed applying discoveries made in the lab directly to patient care, I decided to pursue medical school at New York University before completing a dermatology residency at the University of Miami, serving as Chief Resident during my final year. Although I was born and raised in New York, staying in Miami was an obvious decision for me. In addition to the tropical weather and amazing lifestyle, the medical community in Miami supports adventure, creativity, and innovation, which are key aspects that drew me to the University of Miami and continue to drive my personal evolution in private practice.
I now practice at Baumann Cosmetic & Research Institute alongside my mentor, Dr. Baumann. I truly have my dream job – I get to talk skin care and do a wide array of cosmetics procedures, perform skin surgeries, and solve complex medical dermatology cases all in a day’s work. My career sits at the intersection of my passions for science, critical thinking, beauty, aesthetics, and most importantly, engaging with patients.
For my first column, I want to , and I will provide a simple framework to approach the design of skin care regimens and utilization of cosmeceuticals in practice.
The focus of a dermatology residency is on medical and surgical skills. We become experts in diagnosing and treating conditions ranging from life-threatening drug reactions like Stevens-Johnson Syndrome to complex diseases like dermatomyositis, utilizing medications and treatments ranging from cyclosporine and methotrexate to biologics and intravenous immunoglobulin, and performing advanced skin surgeries utilizing flaps and grafts to repair defects.
The discipline of cosmetic dermatology, let alone cosmeceuticals, accounts for a fraction of our didactic and hands-on training. I completed a top dermatology residency program that prepared me to treat any dermatologic condition; however, I honestly felt like I didn’t have a strong understanding of cosmeceuticals and skin care and how to integrate them with prescription therapies when I completed residency, which is a sentiment shared by residents across the country. I remember a study break while preparing for my final board exam when I went into a tailspin for an entire day trying to decode an ingredient list of a new “antiaging serum” and researching its mechanisms of action and the clinical data supporting the active ingredients in the serum, which included bakuchiol and a blend of peptides. As a dermatologist who likes to treat and provide recommendations based on scientific rationale and data to deliver the highest level of care, I admit that I felt insecure not being as knowledgeable about cosmeceuticals as I was about more complex dermatology treatments. As both a cosmetic and general dermatologist, discussing skin care and cosmeceuticals independent of or in conjunction with medical management occurs daily, and I recognized that becoming an expert in this area is essential to becoming a top, well-rounded dermatologist.
A gap in cosmeceutical education in dermatology residency
Multiple studies have established that the field of cosmetic dermatology comprises a fraction of dermatology residency training. In 2013, Kirby et al. published a survey of dermatology instructors and chief residents across the country and found that only 67% of responders reported having received formal lectures on cosmetic dermatology.1 In 2014, Bauer et al. published a survey of dermatology program directors assessing attitudes toward cosmetic dermatology and reported that only 38% of program directors believed that cosmetic dermatology should be a necessary aspect of residency training.2 A survey sent to dermatology residents published in 2012 found that among respondents, more than 58% of residency programs have an “encouraging or somewhat encouraging” attitude toward teaching cosmetic dermatology, yet 22% of programs had a “somewhat discouraging” or “discouraging” attitude.3 While these noted studies have focused on procedural aspects of cosmetic dermatology training, Feetham et al. surveyed dermatology residents and faculty to assess attitudes toward and training on skin care and cosmeceuticals specifically. Among resident respondents, most (74.5%) reported their education on skin care and cosmeceuticals has been “too little or nonexistent” during residency and 76.5% “agree or strongly agree” that it should be part of their education.4 In contrast, 60% of faculty reported resident education on skin care and cosmeceuticals is “just the right amount or too much” (P < .001).
In my personal experience as a resident, discussing skin care was emphasized when treating patients with eczema, contact dermatitis, acne, and hair disorders, but otherwise, the majority of skin care discussions relied on having a stock list of recommended cleansers, moisturizers, and sunscreens. In regards to cosmeceuticals for facial skin specifically, there were only a handful of instances in which alternative ingredients, such as vitamin C for hyperpigmentation, were discussed and specific brands were mentioned. Upon reflection, I wish I had more opportunity to see the clinical benefits of cosmeceuticals first hand, just like when I observe dupilumab clear patients with severe atopic dermatitis, rather than reading about it in textbooks and journals.
While one hypothesis for programs’ limited attention given to cosmetic training may be that it detracts from medical training, the survey by Bauer et al. found that residents did not feel less prepared (94.9%) or less interested (97.4%) in medical dermatology as a result of their cosmetic training.2 In addition, providers in an academic dermatology residency may limit discussions of skin care because of the high patient volume and because extensive skin care discussions will not impact insurance billings. Academic dermatology programs often service patients with more financial constraints, which further limits OTC cosmeceutical discussions. In my residency experience, I had the opportunity to regularly treat more severe and rare dermatologic cases than those I encounter in private practice; therefore, I spent more time focusing on systemic therapies, with fewer opportunities to dedicate time to cosmeceuticals.
Why skin care and cosmeceuticals should be an essential aspect of residency training
Discussing skin care and cosmeceuticals is a valuable aspect of medical and general dermatology, not just aesthetic dermatology. When treating general dermatologic conditions, guidance on proper skin care can improve both adherence and efficacy of medical treatments. For example, an acne study by de Lucas et al. demonstrated that adherence to adjuvant treatment of acne (such as the use of moisturizers) was associated not only with a 2.4-fold increase in the probability of adherence to pharmacological treatment, but also with a significant reduction in acne severity.5 Aside from skin care, cosmeceuticals themselves have efficacy in treating general dermatologic conditions. In the treatment of acne, topical niacinamide, a popular cosmeceutical ingredient, has been shown to have sebosuppressive and anti-inflammatory effects, addressing key aspects of acne pathogenesis.6 A double-blind study by Draelos et al. reported topical 2% niacinamide was effective in reducing the rate of sebum excretion in 50 Japanese patients over 4 weeks.6 In several double-blind studies that have compared twice daily application of 4% nicotinamide gel with the same application of 1% clindamycin gel in moderate inflammatory acne over 8 weeks, nicotinamide gel reduced the number of inflammatory papules and acne lesions to a level comparable with clindamycin gel.6 These studies support the use of niacinamide cosmeceutical products as an adjunctive treatment for acne.
With increased clinical data supporting cosmeceuticals, it can be expected that some cosmeceuticals will substitute traditional prescription medications in the dermatologists’ arsenal. For example, hydroquinone – both prescription strength and OTC 2% – is a workhorse in treating melasma; however, there is increasing interest in hydroquinone-free treatments, especially since OTC cosmeceuticals containing 2% hydroquinone were banned in 2020 because of safety concerns. Dermatologists will therefore need to provide guidance about hydroquinone alternatives for skin lightening, including soy, licorice extracts, kojic acid, arbutin, niacinamide, N-acetylglucosamine, and vitamin C, among others.7 Utilizing knowledge of a cosmeceutical’s mechanisms of action and clinical data, the dermatologist is in the best position to guide patients toward optimal ingredients and dispel cosmeceutical myths. Given that cosmeceuticals are not regulated by the Food and Drug Administration, it is even more important that the dermatologist serves as an authority on cosmeceuticals.
How to become a master skin care and cosmeceutical prescriber
A common pitfall I have observed among practitioners less experienced with aesthetic-focused skin care and cosmeceuticals is adapting a one-size-fits-all approach. In the one-size-fits-all approach, every patient concerned about aging gets the same vitamin C serum and retinoid, and every patient with hyperpigmentation gets the same hydroquinone prescription, for example. This approach, however, does not take into account unique differences in patients’ skin. Below
is the basic skin care framework that I follow, taught to me by Dr. Baumann. It utilizes an individualized approach based on the patient’s skin qualities to achieve optimal results.
Determine the patient’s skin type (dry vs. oily; sensitive vs. not sensitive; pigmentation issues vs. no hyperpigmentation; wrinkled and mature vs. nonwrinkled) and identify concerns (e.g., dark spots, redness, acne, dehydration).
Separate products into categories of cleansers, eye creams, moisturizers, sun protection, and treatments. Treatments refers to any additional products in a skin care regimen intended to ameliorate a particular condition (e.g., vitamin C for hyperpigmentation, retinoids for fine lines).
Choose products for each category in step 2 (cleansers, eye creams, moisturizers, sun protection, treatments) that are complementary to the patient’s skin type (determined in step 1) and aid the patient in meeting their particular skin goals. For example, a salicylic acid cleanser would be beneficial for a patient with oily skin and acne, but this same cleanser may be too drying and irritating for an acne patient with dry skin.
Ensure that chosen ingredients and products work together harmoniously. For example, while the acne patient may benefit from a salicylic acid cleanser and retinoid cream, using them in succession initially may be overly drying for some patients.
Spend the time to make sure patients understand the appropriate order of application and recognize when efficacy of a product is impacted by another product in the regimen. For example, a low pH cleanser can increase penetration of an ascorbic acid product that follows it in the regimen.
After establishing a basic skin care framework, the next step for beginners is learning about ingredients and their mechanisms of action and familiarizing themselves with scientific and clinical studies. Until cosmeceuticals become an integral part of the training curriculum, dermatologists can gain knowledge independently by reading literature and studies on cosmeceutical active ingredients and experimenting with consumer products. I look forward to regularly contributing to this column to further our awareness and understanding of the mechanisms of and data supporting cosmeceuticals so that we can better guide our patients.
Please feel free to email me at chloe@derm.net or message me on Instagram @DrChloeGoldman with ideas that you would like me to address in this column.
Dr. Goldman is a dermatologist in private practice in Miami, and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a new general dermatology practice. Dr. Goldman receives compensation to create social media content for Replenix, a skin care company. She has no other relevant disclosures.
References
1. Kirby JS et al. J Am Acad Dermatol. 2013;68(2):e23-8.
2. Bauer et al. JAMA Dermatol. 2014;150(2):125-9.
3. Group A et al. Dermatol Surg. 2012;38(12):1975-80.
4. Feetham HJ et al. J Cosmet Dermatol. 2018;17(2):220-6.
5. de Lucas R et al. BMC Dermatol. 2015;15:17.
6. Araviiskaia E and Dreno BJ. Eur Acad Dermatol Venereol. 2016;30(6):926-35.
7. Leyden JJ et al. J Eur Acad Dermatol Venereol. 2011;25(10):1140-5.
Starting this month, I will be joining Dr. Leslie S. Baumann as a cocontributor to the Cosmeceutical Critique column, and since this is my first column, I would like to formally introduce myself. I am a cosmetic and general dermatologist in private practice in Miami and a longtime skin care enthusiast. My path toward becoming a dermatologist began when I was working in New York City, my hometown, as a scientific researcher, fulfilling my passion for scientific inquiry. After realizing that I most enjoyed applying discoveries made in the lab directly to patient care, I decided to pursue medical school at New York University before completing a dermatology residency at the University of Miami, serving as Chief Resident during my final year. Although I was born and raised in New York, staying in Miami was an obvious decision for me. In addition to the tropical weather and amazing lifestyle, the medical community in Miami supports adventure, creativity, and innovation, which are key aspects that drew me to the University of Miami and continue to drive my personal evolution in private practice.
I now practice at Baumann Cosmetic & Research Institute alongside my mentor, Dr. Baumann. I truly have my dream job – I get to talk skin care and do a wide array of cosmetics procedures, perform skin surgeries, and solve complex medical dermatology cases all in a day’s work. My career sits at the intersection of my passions for science, critical thinking, beauty, aesthetics, and most importantly, engaging with patients.
For my first column, I want to , and I will provide a simple framework to approach the design of skin care regimens and utilization of cosmeceuticals in practice.
The focus of a dermatology residency is on medical and surgical skills. We become experts in diagnosing and treating conditions ranging from life-threatening drug reactions like Stevens-Johnson Syndrome to complex diseases like dermatomyositis, utilizing medications and treatments ranging from cyclosporine and methotrexate to biologics and intravenous immunoglobulin, and performing advanced skin surgeries utilizing flaps and grafts to repair defects.
The discipline of cosmetic dermatology, let alone cosmeceuticals, accounts for a fraction of our didactic and hands-on training. I completed a top dermatology residency program that prepared me to treat any dermatologic condition; however, I honestly felt like I didn’t have a strong understanding of cosmeceuticals and skin care and how to integrate them with prescription therapies when I completed residency, which is a sentiment shared by residents across the country. I remember a study break while preparing for my final board exam when I went into a tailspin for an entire day trying to decode an ingredient list of a new “antiaging serum” and researching its mechanisms of action and the clinical data supporting the active ingredients in the serum, which included bakuchiol and a blend of peptides. As a dermatologist who likes to treat and provide recommendations based on scientific rationale and data to deliver the highest level of care, I admit that I felt insecure not being as knowledgeable about cosmeceuticals as I was about more complex dermatology treatments. As both a cosmetic and general dermatologist, discussing skin care and cosmeceuticals independent of or in conjunction with medical management occurs daily, and I recognized that becoming an expert in this area is essential to becoming a top, well-rounded dermatologist.
A gap in cosmeceutical education in dermatology residency
Multiple studies have established that the field of cosmetic dermatology comprises a fraction of dermatology residency training. In 2013, Kirby et al. published a survey of dermatology instructors and chief residents across the country and found that only 67% of responders reported having received formal lectures on cosmetic dermatology.1 In 2014, Bauer et al. published a survey of dermatology program directors assessing attitudes toward cosmetic dermatology and reported that only 38% of program directors believed that cosmetic dermatology should be a necessary aspect of residency training.2 A survey sent to dermatology residents published in 2012 found that among respondents, more than 58% of residency programs have an “encouraging or somewhat encouraging” attitude toward teaching cosmetic dermatology, yet 22% of programs had a “somewhat discouraging” or “discouraging” attitude.3 While these noted studies have focused on procedural aspects of cosmetic dermatology training, Feetham et al. surveyed dermatology residents and faculty to assess attitudes toward and training on skin care and cosmeceuticals specifically. Among resident respondents, most (74.5%) reported their education on skin care and cosmeceuticals has been “too little or nonexistent” during residency and 76.5% “agree or strongly agree” that it should be part of their education.4 In contrast, 60% of faculty reported resident education on skin care and cosmeceuticals is “just the right amount or too much” (P < .001).
In my personal experience as a resident, discussing skin care was emphasized when treating patients with eczema, contact dermatitis, acne, and hair disorders, but otherwise, the majority of skin care discussions relied on having a stock list of recommended cleansers, moisturizers, and sunscreens. In regards to cosmeceuticals for facial skin specifically, there were only a handful of instances in which alternative ingredients, such as vitamin C for hyperpigmentation, were discussed and specific brands were mentioned. Upon reflection, I wish I had more opportunity to see the clinical benefits of cosmeceuticals first hand, just like when I observe dupilumab clear patients with severe atopic dermatitis, rather than reading about it in textbooks and journals.
While one hypothesis for programs’ limited attention given to cosmetic training may be that it detracts from medical training, the survey by Bauer et al. found that residents did not feel less prepared (94.9%) or less interested (97.4%) in medical dermatology as a result of their cosmetic training.2 In addition, providers in an academic dermatology residency may limit discussions of skin care because of the high patient volume and because extensive skin care discussions will not impact insurance billings. Academic dermatology programs often service patients with more financial constraints, which further limits OTC cosmeceutical discussions. In my residency experience, I had the opportunity to regularly treat more severe and rare dermatologic cases than those I encounter in private practice; therefore, I spent more time focusing on systemic therapies, with fewer opportunities to dedicate time to cosmeceuticals.
Why skin care and cosmeceuticals should be an essential aspect of residency training
Discussing skin care and cosmeceuticals is a valuable aspect of medical and general dermatology, not just aesthetic dermatology. When treating general dermatologic conditions, guidance on proper skin care can improve both adherence and efficacy of medical treatments. For example, an acne study by de Lucas et al. demonstrated that adherence to adjuvant treatment of acne (such as the use of moisturizers) was associated not only with a 2.4-fold increase in the probability of adherence to pharmacological treatment, but also with a significant reduction in acne severity.5 Aside from skin care, cosmeceuticals themselves have efficacy in treating general dermatologic conditions. In the treatment of acne, topical niacinamide, a popular cosmeceutical ingredient, has been shown to have sebosuppressive and anti-inflammatory effects, addressing key aspects of acne pathogenesis.6 A double-blind study by Draelos et al. reported topical 2% niacinamide was effective in reducing the rate of sebum excretion in 50 Japanese patients over 4 weeks.6 In several double-blind studies that have compared twice daily application of 4% nicotinamide gel with the same application of 1% clindamycin gel in moderate inflammatory acne over 8 weeks, nicotinamide gel reduced the number of inflammatory papules and acne lesions to a level comparable with clindamycin gel.6 These studies support the use of niacinamide cosmeceutical products as an adjunctive treatment for acne.
With increased clinical data supporting cosmeceuticals, it can be expected that some cosmeceuticals will substitute traditional prescription medications in the dermatologists’ arsenal. For example, hydroquinone – both prescription strength and OTC 2% – is a workhorse in treating melasma; however, there is increasing interest in hydroquinone-free treatments, especially since OTC cosmeceuticals containing 2% hydroquinone were banned in 2020 because of safety concerns. Dermatologists will therefore need to provide guidance about hydroquinone alternatives for skin lightening, including soy, licorice extracts, kojic acid, arbutin, niacinamide, N-acetylglucosamine, and vitamin C, among others.7 Utilizing knowledge of a cosmeceutical’s mechanisms of action and clinical data, the dermatologist is in the best position to guide patients toward optimal ingredients and dispel cosmeceutical myths. Given that cosmeceuticals are not regulated by the Food and Drug Administration, it is even more important that the dermatologist serves as an authority on cosmeceuticals.
How to become a master skin care and cosmeceutical prescriber
A common pitfall I have observed among practitioners less experienced with aesthetic-focused skin care and cosmeceuticals is adapting a one-size-fits-all approach. In the one-size-fits-all approach, every patient concerned about aging gets the same vitamin C serum and retinoid, and every patient with hyperpigmentation gets the same hydroquinone prescription, for example. This approach, however, does not take into account unique differences in patients’ skin. Below
is the basic skin care framework that I follow, taught to me by Dr. Baumann. It utilizes an individualized approach based on the patient’s skin qualities to achieve optimal results.
Determine the patient’s skin type (dry vs. oily; sensitive vs. not sensitive; pigmentation issues vs. no hyperpigmentation; wrinkled and mature vs. nonwrinkled) and identify concerns (e.g., dark spots, redness, acne, dehydration).
Separate products into categories of cleansers, eye creams, moisturizers, sun protection, and treatments. Treatments refers to any additional products in a skin care regimen intended to ameliorate a particular condition (e.g., vitamin C for hyperpigmentation, retinoids for fine lines).
Choose products for each category in step 2 (cleansers, eye creams, moisturizers, sun protection, treatments) that are complementary to the patient’s skin type (determined in step 1) and aid the patient in meeting their particular skin goals. For example, a salicylic acid cleanser would be beneficial for a patient with oily skin and acne, but this same cleanser may be too drying and irritating for an acne patient with dry skin.
Ensure that chosen ingredients and products work together harmoniously. For example, while the acne patient may benefit from a salicylic acid cleanser and retinoid cream, using them in succession initially may be overly drying for some patients.
Spend the time to make sure patients understand the appropriate order of application and recognize when efficacy of a product is impacted by another product in the regimen. For example, a low pH cleanser can increase penetration of an ascorbic acid product that follows it in the regimen.
After establishing a basic skin care framework, the next step for beginners is learning about ingredients and their mechanisms of action and familiarizing themselves with scientific and clinical studies. Until cosmeceuticals become an integral part of the training curriculum, dermatologists can gain knowledge independently by reading literature and studies on cosmeceutical active ingredients and experimenting with consumer products. I look forward to regularly contributing to this column to further our awareness and understanding of the mechanisms of and data supporting cosmeceuticals so that we can better guide our patients.
Please feel free to email me at chloe@derm.net or message me on Instagram @DrChloeGoldman with ideas that you would like me to address in this column.
Dr. Goldman is a dermatologist in private practice in Miami, and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a new general dermatology practice. Dr. Goldman receives compensation to create social media content for Replenix, a skin care company. She has no other relevant disclosures.
References
1. Kirby JS et al. J Am Acad Dermatol. 2013;68(2):e23-8.
2. Bauer et al. JAMA Dermatol. 2014;150(2):125-9.
3. Group A et al. Dermatol Surg. 2012;38(12):1975-80.
4. Feetham HJ et al. J Cosmet Dermatol. 2018;17(2):220-6.
5. de Lucas R et al. BMC Dermatol. 2015;15:17.
6. Araviiskaia E and Dreno BJ. Eur Acad Dermatol Venereol. 2016;30(6):926-35.
7. Leyden JJ et al. J Eur Acad Dermatol Venereol. 2011;25(10):1140-5.
Starting this month, I will be joining Dr. Leslie S. Baumann as a cocontributor to the Cosmeceutical Critique column, and since this is my first column, I would like to formally introduce myself. I am a cosmetic and general dermatologist in private practice in Miami and a longtime skin care enthusiast. My path toward becoming a dermatologist began when I was working in New York City, my hometown, as a scientific researcher, fulfilling my passion for scientific inquiry. After realizing that I most enjoyed applying discoveries made in the lab directly to patient care, I decided to pursue medical school at New York University before completing a dermatology residency at the University of Miami, serving as Chief Resident during my final year. Although I was born and raised in New York, staying in Miami was an obvious decision for me. In addition to the tropical weather and amazing lifestyle, the medical community in Miami supports adventure, creativity, and innovation, which are key aspects that drew me to the University of Miami and continue to drive my personal evolution in private practice.
I now practice at Baumann Cosmetic & Research Institute alongside my mentor, Dr. Baumann. I truly have my dream job – I get to talk skin care and do a wide array of cosmetics procedures, perform skin surgeries, and solve complex medical dermatology cases all in a day’s work. My career sits at the intersection of my passions for science, critical thinking, beauty, aesthetics, and most importantly, engaging with patients.
For my first column, I want to , and I will provide a simple framework to approach the design of skin care regimens and utilization of cosmeceuticals in practice.
The focus of a dermatology residency is on medical and surgical skills. We become experts in diagnosing and treating conditions ranging from life-threatening drug reactions like Stevens-Johnson Syndrome to complex diseases like dermatomyositis, utilizing medications and treatments ranging from cyclosporine and methotrexate to biologics and intravenous immunoglobulin, and performing advanced skin surgeries utilizing flaps and grafts to repair defects.
The discipline of cosmetic dermatology, let alone cosmeceuticals, accounts for a fraction of our didactic and hands-on training. I completed a top dermatology residency program that prepared me to treat any dermatologic condition; however, I honestly felt like I didn’t have a strong understanding of cosmeceuticals and skin care and how to integrate them with prescription therapies when I completed residency, which is a sentiment shared by residents across the country. I remember a study break while preparing for my final board exam when I went into a tailspin for an entire day trying to decode an ingredient list of a new “antiaging serum” and researching its mechanisms of action and the clinical data supporting the active ingredients in the serum, which included bakuchiol and a blend of peptides. As a dermatologist who likes to treat and provide recommendations based on scientific rationale and data to deliver the highest level of care, I admit that I felt insecure not being as knowledgeable about cosmeceuticals as I was about more complex dermatology treatments. As both a cosmetic and general dermatologist, discussing skin care and cosmeceuticals independent of or in conjunction with medical management occurs daily, and I recognized that becoming an expert in this area is essential to becoming a top, well-rounded dermatologist.
A gap in cosmeceutical education in dermatology residency
Multiple studies have established that the field of cosmetic dermatology comprises a fraction of dermatology residency training. In 2013, Kirby et al. published a survey of dermatology instructors and chief residents across the country and found that only 67% of responders reported having received formal lectures on cosmetic dermatology.1 In 2014, Bauer et al. published a survey of dermatology program directors assessing attitudes toward cosmetic dermatology and reported that only 38% of program directors believed that cosmetic dermatology should be a necessary aspect of residency training.2 A survey sent to dermatology residents published in 2012 found that among respondents, more than 58% of residency programs have an “encouraging or somewhat encouraging” attitude toward teaching cosmetic dermatology, yet 22% of programs had a “somewhat discouraging” or “discouraging” attitude.3 While these noted studies have focused on procedural aspects of cosmetic dermatology training, Feetham et al. surveyed dermatology residents and faculty to assess attitudes toward and training on skin care and cosmeceuticals specifically. Among resident respondents, most (74.5%) reported their education on skin care and cosmeceuticals has been “too little or nonexistent” during residency and 76.5% “agree or strongly agree” that it should be part of their education.4 In contrast, 60% of faculty reported resident education on skin care and cosmeceuticals is “just the right amount or too much” (P < .001).
In my personal experience as a resident, discussing skin care was emphasized when treating patients with eczema, contact dermatitis, acne, and hair disorders, but otherwise, the majority of skin care discussions relied on having a stock list of recommended cleansers, moisturizers, and sunscreens. In regards to cosmeceuticals for facial skin specifically, there were only a handful of instances in which alternative ingredients, such as vitamin C for hyperpigmentation, were discussed and specific brands were mentioned. Upon reflection, I wish I had more opportunity to see the clinical benefits of cosmeceuticals first hand, just like when I observe dupilumab clear patients with severe atopic dermatitis, rather than reading about it in textbooks and journals.
While one hypothesis for programs’ limited attention given to cosmetic training may be that it detracts from medical training, the survey by Bauer et al. found that residents did not feel less prepared (94.9%) or less interested (97.4%) in medical dermatology as a result of their cosmetic training.2 In addition, providers in an academic dermatology residency may limit discussions of skin care because of the high patient volume and because extensive skin care discussions will not impact insurance billings. Academic dermatology programs often service patients with more financial constraints, which further limits OTC cosmeceutical discussions. In my residency experience, I had the opportunity to regularly treat more severe and rare dermatologic cases than those I encounter in private practice; therefore, I spent more time focusing on systemic therapies, with fewer opportunities to dedicate time to cosmeceuticals.
Why skin care and cosmeceuticals should be an essential aspect of residency training
Discussing skin care and cosmeceuticals is a valuable aspect of medical and general dermatology, not just aesthetic dermatology. When treating general dermatologic conditions, guidance on proper skin care can improve both adherence and efficacy of medical treatments. For example, an acne study by de Lucas et al. demonstrated that adherence to adjuvant treatment of acne (such as the use of moisturizers) was associated not only with a 2.4-fold increase in the probability of adherence to pharmacological treatment, but also with a significant reduction in acne severity.5 Aside from skin care, cosmeceuticals themselves have efficacy in treating general dermatologic conditions. In the treatment of acne, topical niacinamide, a popular cosmeceutical ingredient, has been shown to have sebosuppressive and anti-inflammatory effects, addressing key aspects of acne pathogenesis.6 A double-blind study by Draelos et al. reported topical 2% niacinamide was effective in reducing the rate of sebum excretion in 50 Japanese patients over 4 weeks.6 In several double-blind studies that have compared twice daily application of 4% nicotinamide gel with the same application of 1% clindamycin gel in moderate inflammatory acne over 8 weeks, nicotinamide gel reduced the number of inflammatory papules and acne lesions to a level comparable with clindamycin gel.6 These studies support the use of niacinamide cosmeceutical products as an adjunctive treatment for acne.
With increased clinical data supporting cosmeceuticals, it can be expected that some cosmeceuticals will substitute traditional prescription medications in the dermatologists’ arsenal. For example, hydroquinone – both prescription strength and OTC 2% – is a workhorse in treating melasma; however, there is increasing interest in hydroquinone-free treatments, especially since OTC cosmeceuticals containing 2% hydroquinone were banned in 2020 because of safety concerns. Dermatologists will therefore need to provide guidance about hydroquinone alternatives for skin lightening, including soy, licorice extracts, kojic acid, arbutin, niacinamide, N-acetylglucosamine, and vitamin C, among others.7 Utilizing knowledge of a cosmeceutical’s mechanisms of action and clinical data, the dermatologist is in the best position to guide patients toward optimal ingredients and dispel cosmeceutical myths. Given that cosmeceuticals are not regulated by the Food and Drug Administration, it is even more important that the dermatologist serves as an authority on cosmeceuticals.
How to become a master skin care and cosmeceutical prescriber
A common pitfall I have observed among practitioners less experienced with aesthetic-focused skin care and cosmeceuticals is adapting a one-size-fits-all approach. In the one-size-fits-all approach, every patient concerned about aging gets the same vitamin C serum and retinoid, and every patient with hyperpigmentation gets the same hydroquinone prescription, for example. This approach, however, does not take into account unique differences in patients’ skin. Below
is the basic skin care framework that I follow, taught to me by Dr. Baumann. It utilizes an individualized approach based on the patient’s skin qualities to achieve optimal results.
Determine the patient’s skin type (dry vs. oily; sensitive vs. not sensitive; pigmentation issues vs. no hyperpigmentation; wrinkled and mature vs. nonwrinkled) and identify concerns (e.g., dark spots, redness, acne, dehydration).
Separate products into categories of cleansers, eye creams, moisturizers, sun protection, and treatments. Treatments refers to any additional products in a skin care regimen intended to ameliorate a particular condition (e.g., vitamin C for hyperpigmentation, retinoids for fine lines).
Choose products for each category in step 2 (cleansers, eye creams, moisturizers, sun protection, treatments) that are complementary to the patient’s skin type (determined in step 1) and aid the patient in meeting their particular skin goals. For example, a salicylic acid cleanser would be beneficial for a patient with oily skin and acne, but this same cleanser may be too drying and irritating for an acne patient with dry skin.
Ensure that chosen ingredients and products work together harmoniously. For example, while the acne patient may benefit from a salicylic acid cleanser and retinoid cream, using them in succession initially may be overly drying for some patients.
Spend the time to make sure patients understand the appropriate order of application and recognize when efficacy of a product is impacted by another product in the regimen. For example, a low pH cleanser can increase penetration of an ascorbic acid product that follows it in the regimen.
After establishing a basic skin care framework, the next step for beginners is learning about ingredients and their mechanisms of action and familiarizing themselves with scientific and clinical studies. Until cosmeceuticals become an integral part of the training curriculum, dermatologists can gain knowledge independently by reading literature and studies on cosmeceutical active ingredients and experimenting with consumer products. I look forward to regularly contributing to this column to further our awareness and understanding of the mechanisms of and data supporting cosmeceuticals so that we can better guide our patients.
Please feel free to email me at chloe@derm.net or message me on Instagram @DrChloeGoldman with ideas that you would like me to address in this column.
Dr. Goldman is a dermatologist in private practice in Miami, and specializes in cosmetic and general dermatology. She practices at Baumann Cosmetic & Research Institute and is also opening a new general dermatology practice. Dr. Goldman receives compensation to create social media content for Replenix, a skin care company. She has no other relevant disclosures.
References
1. Kirby JS et al. J Am Acad Dermatol. 2013;68(2):e23-8.
2. Bauer et al. JAMA Dermatol. 2014;150(2):125-9.
3. Group A et al. Dermatol Surg. 2012;38(12):1975-80.
4. Feetham HJ et al. J Cosmet Dermatol. 2018;17(2):220-6.
5. de Lucas R et al. BMC Dermatol. 2015;15:17.
6. Araviiskaia E and Dreno BJ. Eur Acad Dermatol Venereol. 2016;30(6):926-35.
7. Leyden JJ et al. J Eur Acad Dermatol Venereol. 2011;25(10):1140-5.