User login
Cutaneous Gummatous Tuberculosis in a Kidney Transplant Patient
Case Report
A 60-year-old Cambodian woman presented with recurrent fever (temperature, up to 38.8°C) 7 months after receiving a kidney transplant secondary to polycystic kidney disease. Fever was attributed to recurrent pyelonephritis of the native kidneys while on mycophenolate mofetil, tacrolimus, and prednisone. As a result, she underwent a bilateral native nephrectomy and was found to have peritoneal nodules. Pathology of both native kidneys and peritoneal tissue revealed caseating granulomas and acid-fast bacilli (AFB) diagnostic for kidney and peritoneal tuberculosis (TB). She had no history of TB, and a TB skin test (purified protein derivative [PPD]) upon entering the United States from Cambodia a decade earlier was negative. Additionally, her pretransplantation PPD was negative.
Treatment with isoniazid, ethambutol, pyrazinamide, and levofloxacin was initiated immediately upon diagnosis, and all of her immunosuppressive medications—mycophenolate mofetil, tacrolimus, and prednisone—were discontinued. Her symptoms subsided within 1 week, and she was discharged from the hospital. Over the next 2 months, her immunosuppressive medications were restarted, and her TB medications were periodically discontinued by the Tuberculosis Control Program at the Department of Health (Philadelphia, Pennsylvania) due to severe thrombocytopenia. During this time, she was closely monitored twice weekly in the clinic with blood draws performed weekly.
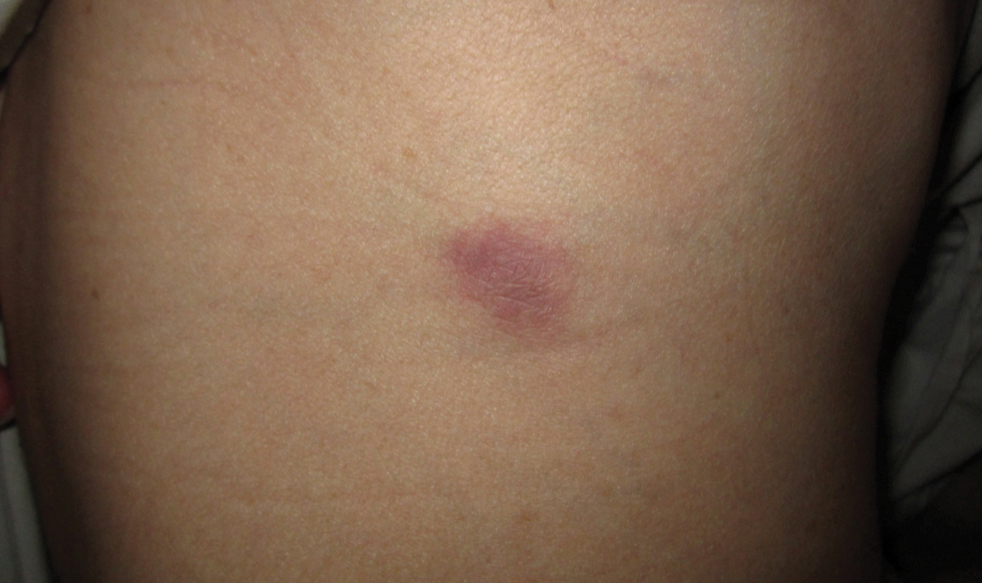
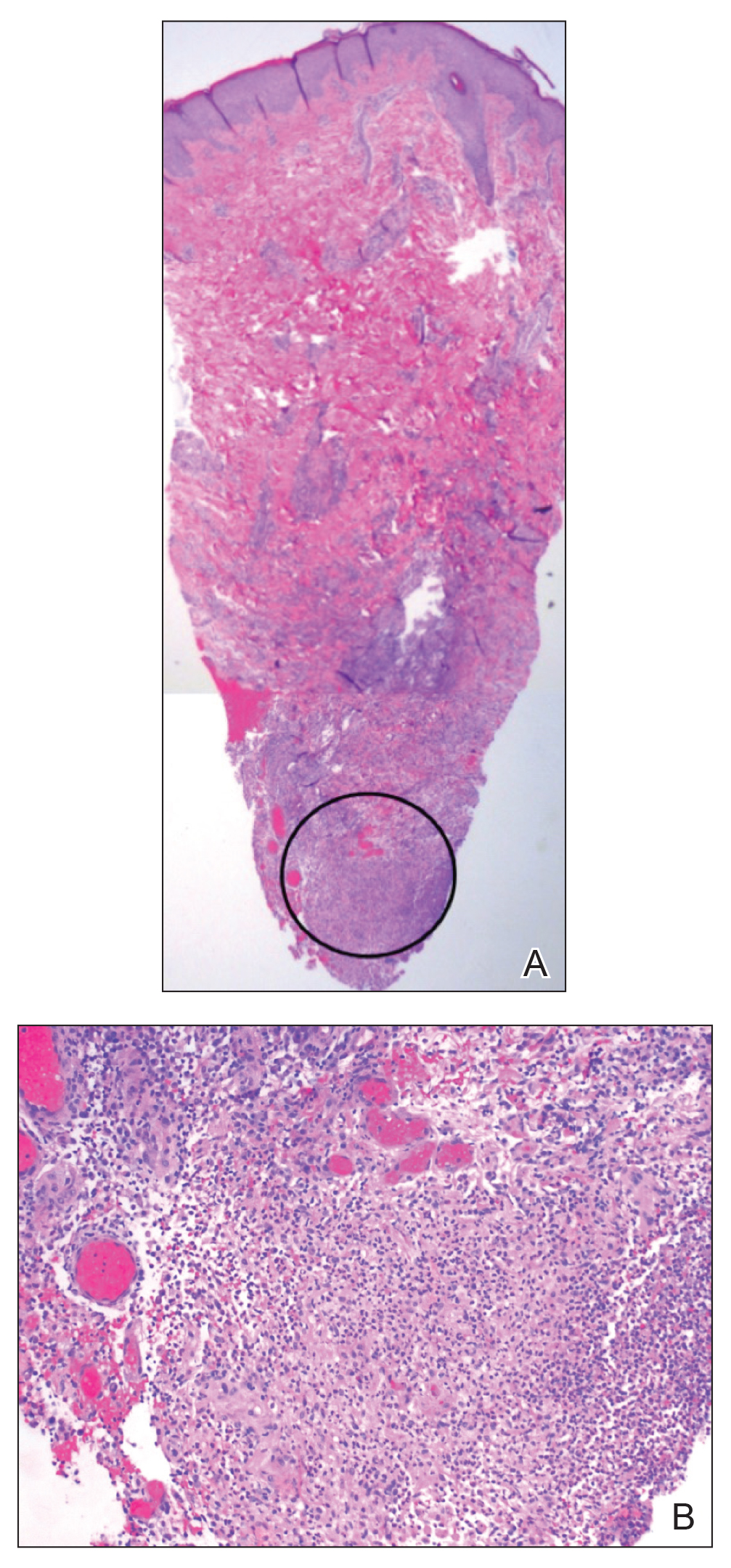
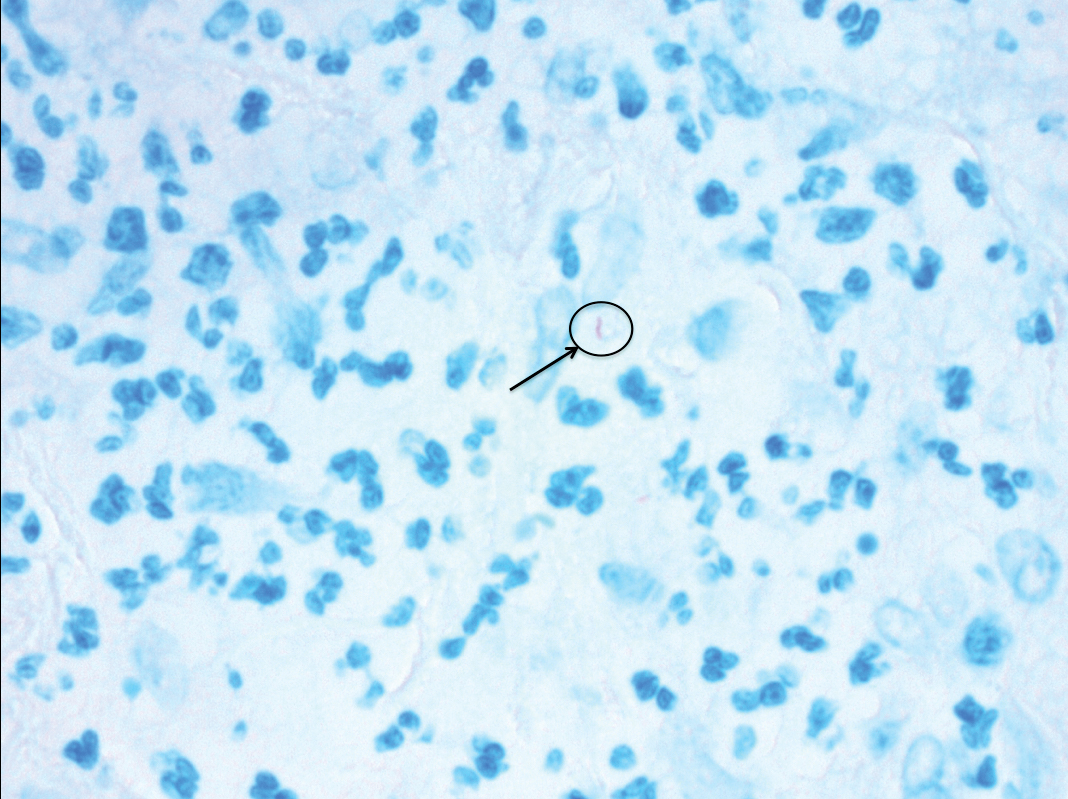
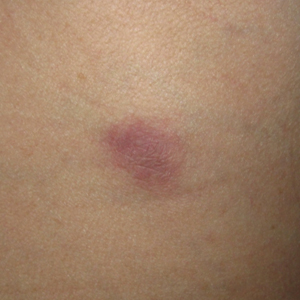
Approximately 10 weeks after initiation of treatment, she noted recurrent subjective fever (temperature, up to 38.8°C) and painful lesions on the right side of the flank, left breast, and left arm of 3 days’ duration. Physical examination revealed a warm, dull red, tender nodule on the right side of the flank (Figure 1) and subcutaneous nodules with no overlying skin changes on the left breast and left arm. A biopsy of the lesion on the right side of the flank was performed, which resulted in substantial purulent drainage. Histologic analysis showed an inflammatory infiltrate within the deep dermis composed of neutrophils, macrophages, and giant cells, indicative of suppurative granulomatous dermatitis (Figure 2). Ziehl-Neelsen stain demonstrated rare AFB within the cytoplasm of macrophages, suggestive of Mycobacterium tuberculosis infection (Figure 3). A repeat chest radiograph was normal.
Based on the patient’s history and clinical presentation, she was continued on isoniazid, ethambutol, and levofloxacin, with complete resolution of symptoms and cutaneous lesions. Over the subsequent 2 months, the therapy was modified to rifabutin, pyrazinamide, and levofloxacin, and subsequently pyrazinamide was stopped. A subsequent biopsy of the left breast and histologic analysis indicated that the specimen was benign; stains for AFB were negative. Currently, both the fever and skin lesions have completely resolved, and she remains on anti-TB therapy.
Comment
Clinical Presentation
Cutaneous TB is an uncommon manifestation of TB that can occur either exogenously or endogenously.1 It tends to occur primarily in previously infected TB patients through hematogenous, lymphatic, or contiguous spread.2 Due to their immunocompromised state, solid organ transplant recipients have an increased incidence of primary and reactivated latent TB reported to be 20 to 74 times greater than the general population.3,4 One report stated the total incidence of posttransplant TB as 0.48% in the West and 11.8% in endemic regions such as India.5 The occurrence of cutaneous TB is rare among solid organ transplant recipients.1 On average, a diagnosis of latent TB is made 9 months after transplantation because of the opportunistic nature of M tuberculosis in an immunosuppressed environment.6
TB Subtypes
Cutaneous TB can be in the form of localized disease (eg, primary tuberculous chancre, TB verrucosa cutis, lupus vulgaris, smear-negative scrofuloderma), disseminated disease (eg, disseminated TB, TB gumma, orificial TB, miliary cutaneous TB), or tuberculids (eg, papulonecrotic tuberculid, lichen scrofulosorum, erythema induratum).7 Due to the pustular epithelioid cell granulomas and AFB positivity of the involved cutaneous lesions, our patient’s TB can be classified as a metastatic TB abscess or gummatous TB.7
Metastatic TB abscess, an uncommon subtype of cutaneous TB, generally is only seen in malnourished children and notably immunocompromised individuals.2,8,9 In these individuals, systemic failure of cell-mediated immunity enables M tuberculosis to hematogenously infect various organs of the body, resulting in alternative forms of TB, such as gummatous-type TB.10 One study reported that of the 0.1% of dermatology patients presenting with cutaneous TB, only 5.4% of these individuals had the rarer gummatous form.7 These metastatic TB abscesses begin as a single or multiple nontender subcutaneous nodule(s), which breaks down and softens to form a draining sinus abscess.2,8,9 Abscesses are most commonly seen on the trunk and extremities; however, they can be found nearly anywhere on the body.8 The pathology of cutaneous TB lesions demonstrates caseating necrosis with epithelioid and giant cells forming a surrounding rim.9
Diagnosis
Diagnosis may be difficult because of the vast number of dermatologic conditions that resemble cutaneous TB, including mycoses, sarcoidosis, leishmaniasis, leprosy, syphilis, other non-TB mycobacteria, and Wegener granulomatosis.9 Thus, confirmatory diagnosis is made via clinical presentation, detailed history and physical examination, and laboratory tests.11 These tests include the Mantoux tuberculin skin test (PPD or TST) or IFN-γ release assays (QuantiFERON-TB Gold test), identification of AFB on skin biopsy, and isolation of M tuberculosis from tissue culture or polymerase chain reaction.11
At-Risk Populations
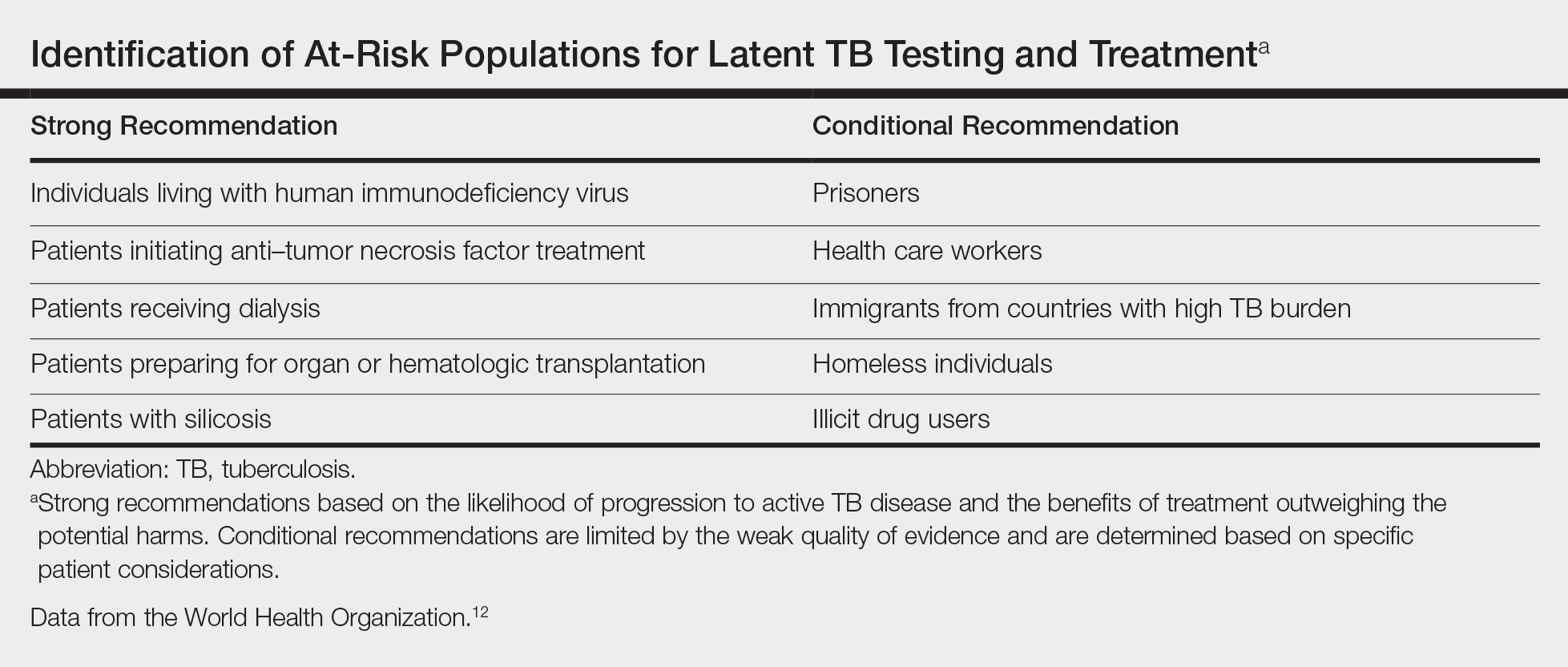
The recommendation for the identification of at-risk populations for latent TB testing and treatment have been clearly defined by the World Health Organization (Table).12 Our patient met 2 of these criteria: she had been preparing for organ transplantation and was from a country with high TB burden. Such at-risk patients should be tested for a latent TB infection with either IFN-γ release assays or PPD.12
Treatment
The recommended treatment of active TB in transplant recipients is based on randomized trials in immunocompetent hosts, and thus the same as that used by the general population.16 This anti-TB regimen includes the use of 4 drugs—typically rifampicin, isoniazid, ethambutol, and pyrazinamide—for a 6-month duration.11 Unfortunately, the management of TB in an immunocompromised patient is more challenging due to the potential side effects and drug interactions.
Finally, thrombocytopenia is an infrequent, life-threatening complication that can be acquired by immunocompromised patients on anti-TB therapy.17 Drug-induced thrombocytopenia can be caused by a variety of medications, including rifampicin, isoniazid, ethambutol, and pyrazinamide. Diagnosis of drug-induced thrombocytopenia can be confirmed only after discontinuation of the suspected drug and subsequent resolution of the thrombocytopenia.17 Our patient initially became thrombocytopenic while taking isoniazid, ethambutol, pyrazinamide, and levofloxacin. However, her platelet levels improved once the pyrazinamide was discontinued, thereby suggesting pyrazinamide-induced thrombocytopenia.
Conclusion
The risk for infectious disease reactivation in an immunocompromised patient undergoing transplant surgery is notable. Our findings emphasize the value of a comprehensive pretransplant evaluation, vigilance even when test results appear negative, and treatment of latent TB within this population.16,18,19 Furthermore, this case illustrates a noteworthy example of a rare form of cutaneous TB, which should be considered and included in the differential for cutaneous lesions in an immunosuppressed patient.
- Sakhuja V, Jha V, Varma PP, et al. The high incidence of tuberculosis among renal transplant recipients in India. Transplantation. 1996;61:211-215.
- Frankel A, Penrose C, Emer J. Cutaneous tuberculosis: a practical case report and review for the dermatologist. J Clin Aesthet Dermatol. 2009;2:19-27.
- Schultz V, Marroni CA, Amorim CS, et al. Risk factors for hepatotoxicity in solid organ transplants recipients being treated for tuberculosis. Transplant Proc. 2014;46:3606-3610.
- Tabarsi P, Farshidpour M, Marjani M, et al. Mycobacterial infection and the impact of rifabutin treatment in organ transplant recipients: a single-center study. Saudi J Kidney Dis Transpl. 2015;26:6-11.
- Rathi M, Gundlapalli S, Ramachandran R, et al. A rare case of cytomegalovirus, scedosporium apiospermum and mycobacterium tuberculosis in a renal transplant recipient. BMC Infect Dis. 2014;14:259.
- Hickey MD, Quan DJ, Chin-Hong PV, et al. Use of rifabutin for the treatment of a latent tuberculosis infection in a patient after solid organ transplantation. Liver Transpl. 2013;19:457-461.
- Kumar B, Muralidhar S. Cutaneous tuberculosis: a twenty-year prospective study. Int J Tuberc Lung Dis. 1999;3:494-500.
- Dekeyzer S, Moerman F, Callens S, et al. Cutaneous metastatic tuberculous abscess in patient with cervico-mediastinal lymphatic tuberculosis. Acta Clin Belg. 2013;68:34-36.
- Ko M, Wu C, Chiu H. Tuberculous gumma (cutaneous metastatic tuberculous abscess). Dermatol Sinica. 2005;23:27-31.
- Steger JW, Barrett TL. Cutaneous tuberculosis. In: James WD, ed. Textbook of Military Medicine: Military Dermatology. Washington, DC: Borden Institute; 1994:355-389.
- Santos JB, Figueiredo AR, Ferraz CE, et al. Cutaneous tuberculosis: diagnosis, histopathology and treatment - part II. An Bras Dermatol. 2014;89:545-555.
- Guidelines on the Management of Latent Tuberculosis Infection. Geneva, Switzerland: World Health Organization; 2015.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000;161(4 pt 2):S221-S247.
- Mycobacterium tuberculosis. Am J Transplant. 2004;4(suppl 10):37-41.
- Aguado JM, Torre-Cisneros J, Fortún J, et al. Tuberculosis in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis. 2009;48:1276-1284.
- Blumberg HM, Burman WJ, Chaisson RE, et al; American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603-662.
- Kant S, Verma SK, Gupta V, et al. Pyrazinamide induced thrombocytopenia. Indian J Pharmacol. 2010;42:108-109.
- Screening for tuberculosis and tuberculosis infection in high-risk populations. recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR Recomm Rep. 1995;44:19-34.
- Fischer SA, Avery RK; AST Infectious Disease Community of Practice. Screening of donor and recipient prior to solid organ transplantation. Am J Transplant. 2009;9(suppl 4):S7-S18.
Case Report
A 60-year-old Cambodian woman presented with recurrent fever (temperature, up to 38.8°C) 7 months after receiving a kidney transplant secondary to polycystic kidney disease. Fever was attributed to recurrent pyelonephritis of the native kidneys while on mycophenolate mofetil, tacrolimus, and prednisone. As a result, she underwent a bilateral native nephrectomy and was found to have peritoneal nodules. Pathology of both native kidneys and peritoneal tissue revealed caseating granulomas and acid-fast bacilli (AFB) diagnostic for kidney and peritoneal tuberculosis (TB). She had no history of TB, and a TB skin test (purified protein derivative [PPD]) upon entering the United States from Cambodia a decade earlier was negative. Additionally, her pretransplantation PPD was negative.
Treatment with isoniazid, ethambutol, pyrazinamide, and levofloxacin was initiated immediately upon diagnosis, and all of her immunosuppressive medications—mycophenolate mofetil, tacrolimus, and prednisone—were discontinued. Her symptoms subsided within 1 week, and she was discharged from the hospital. Over the next 2 months, her immunosuppressive medications were restarted, and her TB medications were periodically discontinued by the Tuberculosis Control Program at the Department of Health (Philadelphia, Pennsylvania) due to severe thrombocytopenia. During this time, she was closely monitored twice weekly in the clinic with blood draws performed weekly.
Approximately 10 weeks after initiation of treatment, she noted recurrent subjective fever (temperature, up to 38.8°C) and painful lesions on the right side of the flank, left breast, and left arm of 3 days’ duration. Physical examination revealed a warm, dull red, tender nodule on the right side of the flank (Figure 1) and subcutaneous nodules with no overlying skin changes on the left breast and left arm. A biopsy of the lesion on the right side of the flank was performed, which resulted in substantial purulent drainage. Histologic analysis showed an inflammatory infiltrate within the deep dermis composed of neutrophils, macrophages, and giant cells, indicative of suppurative granulomatous dermatitis (Figure 2). Ziehl-Neelsen stain demonstrated rare AFB within the cytoplasm of macrophages, suggestive of Mycobacterium tuberculosis infection (Figure 3). A repeat chest radiograph was normal.
Based on the patient’s history and clinical presentation, she was continued on isoniazid, ethambutol, and levofloxacin, with complete resolution of symptoms and cutaneous lesions. Over the subsequent 2 months, the therapy was modified to rifabutin, pyrazinamide, and levofloxacin, and subsequently pyrazinamide was stopped. A subsequent biopsy of the left breast and histologic analysis indicated that the specimen was benign; stains for AFB were negative. Currently, both the fever and skin lesions have completely resolved, and she remains on anti-TB therapy.
Comment
Clinical Presentation
Cutaneous TB is an uncommon manifestation of TB that can occur either exogenously or endogenously.1 It tends to occur primarily in previously infected TB patients through hematogenous, lymphatic, or contiguous spread.2 Due to their immunocompromised state, solid organ transplant recipients have an increased incidence of primary and reactivated latent TB reported to be 20 to 74 times greater than the general population.3,4 One report stated the total incidence of posttransplant TB as 0.48% in the West and 11.8% in endemic regions such as India.5 The occurrence of cutaneous TB is rare among solid organ transplant recipients.1 On average, a diagnosis of latent TB is made 9 months after transplantation because of the opportunistic nature of M tuberculosis in an immunosuppressed environment.6
TB Subtypes
Cutaneous TB can be in the form of localized disease (eg, primary tuberculous chancre, TB verrucosa cutis, lupus vulgaris, smear-negative scrofuloderma), disseminated disease (eg, disseminated TB, TB gumma, orificial TB, miliary cutaneous TB), or tuberculids (eg, papulonecrotic tuberculid, lichen scrofulosorum, erythema induratum).7 Due to the pustular epithelioid cell granulomas and AFB positivity of the involved cutaneous lesions, our patient’s TB can be classified as a metastatic TB abscess or gummatous TB.7
Metastatic TB abscess, an uncommon subtype of cutaneous TB, generally is only seen in malnourished children and notably immunocompromised individuals.2,8,9 In these individuals, systemic failure of cell-mediated immunity enables M tuberculosis to hematogenously infect various organs of the body, resulting in alternative forms of TB, such as gummatous-type TB.10 One study reported that of the 0.1% of dermatology patients presenting with cutaneous TB, only 5.4% of these individuals had the rarer gummatous form.7 These metastatic TB abscesses begin as a single or multiple nontender subcutaneous nodule(s), which breaks down and softens to form a draining sinus abscess.2,8,9 Abscesses are most commonly seen on the trunk and extremities; however, they can be found nearly anywhere on the body.8 The pathology of cutaneous TB lesions demonstrates caseating necrosis with epithelioid and giant cells forming a surrounding rim.9
Diagnosis
Diagnosis may be difficult because of the vast number of dermatologic conditions that resemble cutaneous TB, including mycoses, sarcoidosis, leishmaniasis, leprosy, syphilis, other non-TB mycobacteria, and Wegener granulomatosis.9 Thus, confirmatory diagnosis is made via clinical presentation, detailed history and physical examination, and laboratory tests.11 These tests include the Mantoux tuberculin skin test (PPD or TST) or IFN-γ release assays (QuantiFERON-TB Gold test), identification of AFB on skin biopsy, and isolation of M tuberculosis from tissue culture or polymerase chain reaction.11
At-Risk Populations
The recommendation for the identification of at-risk populations for latent TB testing and treatment have been clearly defined by the World Health Organization (Table).12 Our patient met 2 of these criteria: she had been preparing for organ transplantation and was from a country with high TB burden. Such at-risk patients should be tested for a latent TB infection with either IFN-γ release assays or PPD.12
Treatment
The recommended treatment of active TB in transplant recipients is based on randomized trials in immunocompetent hosts, and thus the same as that used by the general population.16 This anti-TB regimen includes the use of 4 drugs—typically rifampicin, isoniazid, ethambutol, and pyrazinamide—for a 6-month duration.11 Unfortunately, the management of TB in an immunocompromised patient is more challenging due to the potential side effects and drug interactions.
Finally, thrombocytopenia is an infrequent, life-threatening complication that can be acquired by immunocompromised patients on anti-TB therapy.17 Drug-induced thrombocytopenia can be caused by a variety of medications, including rifampicin, isoniazid, ethambutol, and pyrazinamide. Diagnosis of drug-induced thrombocytopenia can be confirmed only after discontinuation of the suspected drug and subsequent resolution of the thrombocytopenia.17 Our patient initially became thrombocytopenic while taking isoniazid, ethambutol, pyrazinamide, and levofloxacin. However, her platelet levels improved once the pyrazinamide was discontinued, thereby suggesting pyrazinamide-induced thrombocytopenia.
Conclusion
The risk for infectious disease reactivation in an immunocompromised patient undergoing transplant surgery is notable. Our findings emphasize the value of a comprehensive pretransplant evaluation, vigilance even when test results appear negative, and treatment of latent TB within this population.16,18,19 Furthermore, this case illustrates a noteworthy example of a rare form of cutaneous TB, which should be considered and included in the differential for cutaneous lesions in an immunosuppressed patient.
Case Report
A 60-year-old Cambodian woman presented with recurrent fever (temperature, up to 38.8°C) 7 months after receiving a kidney transplant secondary to polycystic kidney disease. Fever was attributed to recurrent pyelonephritis of the native kidneys while on mycophenolate mofetil, tacrolimus, and prednisone. As a result, she underwent a bilateral native nephrectomy and was found to have peritoneal nodules. Pathology of both native kidneys and peritoneal tissue revealed caseating granulomas and acid-fast bacilli (AFB) diagnostic for kidney and peritoneal tuberculosis (TB). She had no history of TB, and a TB skin test (purified protein derivative [PPD]) upon entering the United States from Cambodia a decade earlier was negative. Additionally, her pretransplantation PPD was negative.
Treatment with isoniazid, ethambutol, pyrazinamide, and levofloxacin was initiated immediately upon diagnosis, and all of her immunosuppressive medications—mycophenolate mofetil, tacrolimus, and prednisone—were discontinued. Her symptoms subsided within 1 week, and she was discharged from the hospital. Over the next 2 months, her immunosuppressive medications were restarted, and her TB medications were periodically discontinued by the Tuberculosis Control Program at the Department of Health (Philadelphia, Pennsylvania) due to severe thrombocytopenia. During this time, she was closely monitored twice weekly in the clinic with blood draws performed weekly.
Approximately 10 weeks after initiation of treatment, she noted recurrent subjective fever (temperature, up to 38.8°C) and painful lesions on the right side of the flank, left breast, and left arm of 3 days’ duration. Physical examination revealed a warm, dull red, tender nodule on the right side of the flank (Figure 1) and subcutaneous nodules with no overlying skin changes on the left breast and left arm. A biopsy of the lesion on the right side of the flank was performed, which resulted in substantial purulent drainage. Histologic analysis showed an inflammatory infiltrate within the deep dermis composed of neutrophils, macrophages, and giant cells, indicative of suppurative granulomatous dermatitis (Figure 2). Ziehl-Neelsen stain demonstrated rare AFB within the cytoplasm of macrophages, suggestive of Mycobacterium tuberculosis infection (Figure 3). A repeat chest radiograph was normal.
Based on the patient’s history and clinical presentation, she was continued on isoniazid, ethambutol, and levofloxacin, with complete resolution of symptoms and cutaneous lesions. Over the subsequent 2 months, the therapy was modified to rifabutin, pyrazinamide, and levofloxacin, and subsequently pyrazinamide was stopped. A subsequent biopsy of the left breast and histologic analysis indicated that the specimen was benign; stains for AFB were negative. Currently, both the fever and skin lesions have completely resolved, and she remains on anti-TB therapy.
Comment
Clinical Presentation
Cutaneous TB is an uncommon manifestation of TB that can occur either exogenously or endogenously.1 It tends to occur primarily in previously infected TB patients through hematogenous, lymphatic, or contiguous spread.2 Due to their immunocompromised state, solid organ transplant recipients have an increased incidence of primary and reactivated latent TB reported to be 20 to 74 times greater than the general population.3,4 One report stated the total incidence of posttransplant TB as 0.48% in the West and 11.8% in endemic regions such as India.5 The occurrence of cutaneous TB is rare among solid organ transplant recipients.1 On average, a diagnosis of latent TB is made 9 months after transplantation because of the opportunistic nature of M tuberculosis in an immunosuppressed environment.6
TB Subtypes
Cutaneous TB can be in the form of localized disease (eg, primary tuberculous chancre, TB verrucosa cutis, lupus vulgaris, smear-negative scrofuloderma), disseminated disease (eg, disseminated TB, TB gumma, orificial TB, miliary cutaneous TB), or tuberculids (eg, papulonecrotic tuberculid, lichen scrofulosorum, erythema induratum).7 Due to the pustular epithelioid cell granulomas and AFB positivity of the involved cutaneous lesions, our patient’s TB can be classified as a metastatic TB abscess or gummatous TB.7
Metastatic TB abscess, an uncommon subtype of cutaneous TB, generally is only seen in malnourished children and notably immunocompromised individuals.2,8,9 In these individuals, systemic failure of cell-mediated immunity enables M tuberculosis to hematogenously infect various organs of the body, resulting in alternative forms of TB, such as gummatous-type TB.10 One study reported that of the 0.1% of dermatology patients presenting with cutaneous TB, only 5.4% of these individuals had the rarer gummatous form.7 These metastatic TB abscesses begin as a single or multiple nontender subcutaneous nodule(s), which breaks down and softens to form a draining sinus abscess.2,8,9 Abscesses are most commonly seen on the trunk and extremities; however, they can be found nearly anywhere on the body.8 The pathology of cutaneous TB lesions demonstrates caseating necrosis with epithelioid and giant cells forming a surrounding rim.9
Diagnosis
Diagnosis may be difficult because of the vast number of dermatologic conditions that resemble cutaneous TB, including mycoses, sarcoidosis, leishmaniasis, leprosy, syphilis, other non-TB mycobacteria, and Wegener granulomatosis.9 Thus, confirmatory diagnosis is made via clinical presentation, detailed history and physical examination, and laboratory tests.11 These tests include the Mantoux tuberculin skin test (PPD or TST) or IFN-γ release assays (QuantiFERON-TB Gold test), identification of AFB on skin biopsy, and isolation of M tuberculosis from tissue culture or polymerase chain reaction.11
At-Risk Populations
The recommendation for the identification of at-risk populations for latent TB testing and treatment have been clearly defined by the World Health Organization (Table).12 Our patient met 2 of these criteria: she had been preparing for organ transplantation and was from a country with high TB burden. Such at-risk patients should be tested for a latent TB infection with either IFN-γ release assays or PPD.12
Treatment
The recommended treatment of active TB in transplant recipients is based on randomized trials in immunocompetent hosts, and thus the same as that used by the general population.16 This anti-TB regimen includes the use of 4 drugs—typically rifampicin, isoniazid, ethambutol, and pyrazinamide—for a 6-month duration.11 Unfortunately, the management of TB in an immunocompromised patient is more challenging due to the potential side effects and drug interactions.
Finally, thrombocytopenia is an infrequent, life-threatening complication that can be acquired by immunocompromised patients on anti-TB therapy.17 Drug-induced thrombocytopenia can be caused by a variety of medications, including rifampicin, isoniazid, ethambutol, and pyrazinamide. Diagnosis of drug-induced thrombocytopenia can be confirmed only after discontinuation of the suspected drug and subsequent resolution of the thrombocytopenia.17 Our patient initially became thrombocytopenic while taking isoniazid, ethambutol, pyrazinamide, and levofloxacin. However, her platelet levels improved once the pyrazinamide was discontinued, thereby suggesting pyrazinamide-induced thrombocytopenia.
Conclusion
The risk for infectious disease reactivation in an immunocompromised patient undergoing transplant surgery is notable. Our findings emphasize the value of a comprehensive pretransplant evaluation, vigilance even when test results appear negative, and treatment of latent TB within this population.16,18,19 Furthermore, this case illustrates a noteworthy example of a rare form of cutaneous TB, which should be considered and included in the differential for cutaneous lesions in an immunosuppressed patient.
- Sakhuja V, Jha V, Varma PP, et al. The high incidence of tuberculosis among renal transplant recipients in India. Transplantation. 1996;61:211-215.
- Frankel A, Penrose C, Emer J. Cutaneous tuberculosis: a practical case report and review for the dermatologist. J Clin Aesthet Dermatol. 2009;2:19-27.
- Schultz V, Marroni CA, Amorim CS, et al. Risk factors for hepatotoxicity in solid organ transplants recipients being treated for tuberculosis. Transplant Proc. 2014;46:3606-3610.
- Tabarsi P, Farshidpour M, Marjani M, et al. Mycobacterial infection and the impact of rifabutin treatment in organ transplant recipients: a single-center study. Saudi J Kidney Dis Transpl. 2015;26:6-11.
- Rathi M, Gundlapalli S, Ramachandran R, et al. A rare case of cytomegalovirus, scedosporium apiospermum and mycobacterium tuberculosis in a renal transplant recipient. BMC Infect Dis. 2014;14:259.
- Hickey MD, Quan DJ, Chin-Hong PV, et al. Use of rifabutin for the treatment of a latent tuberculosis infection in a patient after solid organ transplantation. Liver Transpl. 2013;19:457-461.
- Kumar B, Muralidhar S. Cutaneous tuberculosis: a twenty-year prospective study. Int J Tuberc Lung Dis. 1999;3:494-500.
- Dekeyzer S, Moerman F, Callens S, et al. Cutaneous metastatic tuberculous abscess in patient with cervico-mediastinal lymphatic tuberculosis. Acta Clin Belg. 2013;68:34-36.
- Ko M, Wu C, Chiu H. Tuberculous gumma (cutaneous metastatic tuberculous abscess). Dermatol Sinica. 2005;23:27-31.
- Steger JW, Barrett TL. Cutaneous tuberculosis. In: James WD, ed. Textbook of Military Medicine: Military Dermatology. Washington, DC: Borden Institute; 1994:355-389.
- Santos JB, Figueiredo AR, Ferraz CE, et al. Cutaneous tuberculosis: diagnosis, histopathology and treatment - part II. An Bras Dermatol. 2014;89:545-555.
- Guidelines on the Management of Latent Tuberculosis Infection. Geneva, Switzerland: World Health Organization; 2015.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000;161(4 pt 2):S221-S247.
- Mycobacterium tuberculosis. Am J Transplant. 2004;4(suppl 10):37-41.
- Aguado JM, Torre-Cisneros J, Fortún J, et al. Tuberculosis in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis. 2009;48:1276-1284.
- Blumberg HM, Burman WJ, Chaisson RE, et al; American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603-662.
- Kant S, Verma SK, Gupta V, et al. Pyrazinamide induced thrombocytopenia. Indian J Pharmacol. 2010;42:108-109.
- Screening for tuberculosis and tuberculosis infection in high-risk populations. recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR Recomm Rep. 1995;44:19-34.
- Fischer SA, Avery RK; AST Infectious Disease Community of Practice. Screening of donor and recipient prior to solid organ transplantation. Am J Transplant. 2009;9(suppl 4):S7-S18.
- Sakhuja V, Jha V, Varma PP, et al. The high incidence of tuberculosis among renal transplant recipients in India. Transplantation. 1996;61:211-215.
- Frankel A, Penrose C, Emer J. Cutaneous tuberculosis: a practical case report and review for the dermatologist. J Clin Aesthet Dermatol. 2009;2:19-27.
- Schultz V, Marroni CA, Amorim CS, et al. Risk factors for hepatotoxicity in solid organ transplants recipients being treated for tuberculosis. Transplant Proc. 2014;46:3606-3610.
- Tabarsi P, Farshidpour M, Marjani M, et al. Mycobacterial infection and the impact of rifabutin treatment in organ transplant recipients: a single-center study. Saudi J Kidney Dis Transpl. 2015;26:6-11.
- Rathi M, Gundlapalli S, Ramachandran R, et al. A rare case of cytomegalovirus, scedosporium apiospermum and mycobacterium tuberculosis in a renal transplant recipient. BMC Infect Dis. 2014;14:259.
- Hickey MD, Quan DJ, Chin-Hong PV, et al. Use of rifabutin for the treatment of a latent tuberculosis infection in a patient after solid organ transplantation. Liver Transpl. 2013;19:457-461.
- Kumar B, Muralidhar S. Cutaneous tuberculosis: a twenty-year prospective study. Int J Tuberc Lung Dis. 1999;3:494-500.
- Dekeyzer S, Moerman F, Callens S, et al. Cutaneous metastatic tuberculous abscess in patient with cervico-mediastinal lymphatic tuberculosis. Acta Clin Belg. 2013;68:34-36.
- Ko M, Wu C, Chiu H. Tuberculous gumma (cutaneous metastatic tuberculous abscess). Dermatol Sinica. 2005;23:27-31.
- Steger JW, Barrett TL. Cutaneous tuberculosis. In: James WD, ed. Textbook of Military Medicine: Military Dermatology. Washington, DC: Borden Institute; 1994:355-389.
- Santos JB, Figueiredo AR, Ferraz CE, et al. Cutaneous tuberculosis: diagnosis, histopathology and treatment - part II. An Bras Dermatol. 2014;89:545-555.
- Guidelines on the Management of Latent Tuberculosis Infection. Geneva, Switzerland: World Health Organization; 2015.
- Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000;161(4 pt 2):S221-S247.
- Mycobacterium tuberculosis. Am J Transplant. 2004;4(suppl 10):37-41.
- Aguado JM, Torre-Cisneros J, Fortún J, et al. Tuberculosis in solid-organ transplant recipients: consensus statement of the group for the study of infection in transplant recipients (GESITRA) of the Spanish Society of Infectious Diseases and Clinical Microbiology. Clin Infect Dis. 2009;48:1276-1284.
- Blumberg HM, Burman WJ, Chaisson RE, et al; American Thoracic Society, Centers for Disease Control and Prevention, Infectious Diseases Society. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603-662.
- Kant S, Verma SK, Gupta V, et al. Pyrazinamide induced thrombocytopenia. Indian J Pharmacol. 2010;42:108-109.
- Screening for tuberculosis and tuberculosis infection in high-risk populations. recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR Recomm Rep. 1995;44:19-34.
- Fischer SA, Avery RK; AST Infectious Disease Community of Practice. Screening of donor and recipient prior to solid organ transplantation. Am J Transplant. 2009;9(suppl 4):S7-S18.
Practice Points
- Transplant patients are at increased risk for infection given their immunosuppressed state.
- Although rare, cutaneous tuberculosis should be considered in the differential for cutaneous lesions in an immunosuppressed patient.
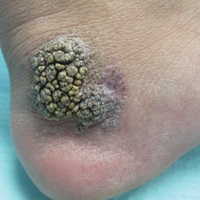
Friable Warty Plaque on the Heel
The Diagnosis: Verrucous Hemangioma
Verrucous hemangioma (VH) is a rare vascular anomaly that has not been definitively delineated as a malformation or a tumor, as it has features of both. Verrucous hemangioma presents at birth as a compressible soft mass with a red violaceous hue favoring the legs.1,2 Over time VH will develop a warty, friable, and keratotic surface that can begin to evolve as early as 6 months or as late as 34 years of age.3 Verrucous hemangioma does not involute and tends to grow proportionally with the patient. Thus, VH classically has been considered a vascular malformation.
On histopathology VH shows collections of uniform, thin-walled vessels with a multilamellated basement membrane throughout the dermis, similar to an infantile hemangioma (IH). These lesions extend deep into the subcutaneous tissue and often involve the underlying fascia. The papillary dermis has large ectatic vessels, while the epidermis displays verrucous hyperkeratosis, papillomatosis, and irregular acanthosis without viral change (Figure).4,5 The superficial component can resemble an angiokeratoma; however, VH is differentiated by a deeper component that is often larger in size and has a more protracted clinical course.
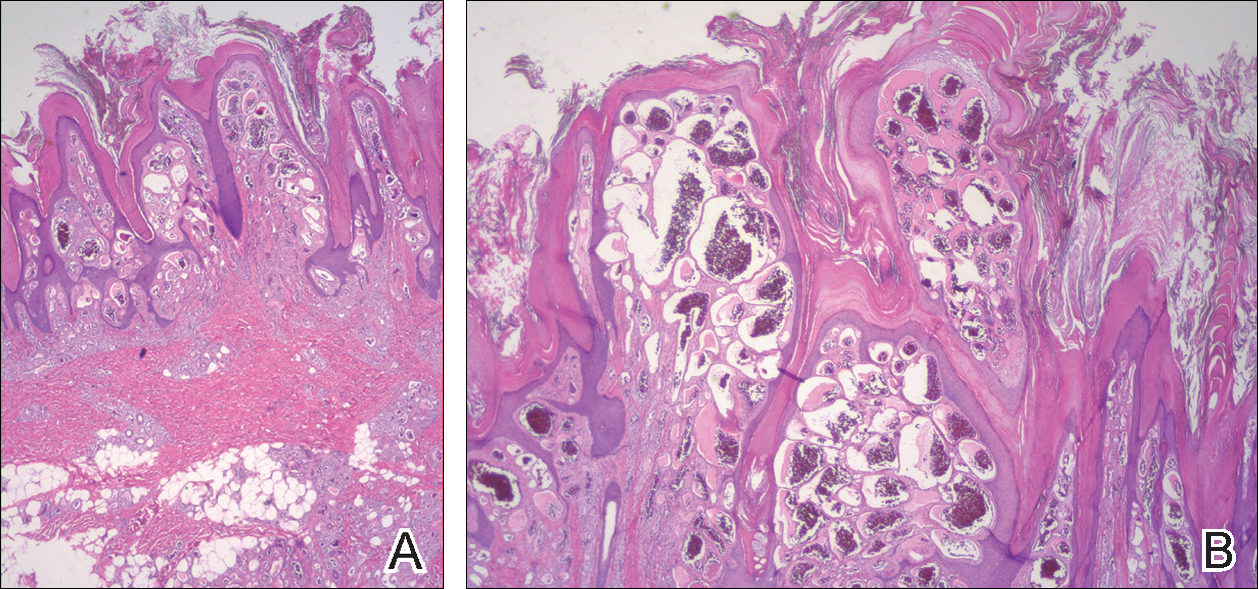
Similar to IH, immunohistochemical studies have shown that VH expresses Wilms tumor 1 and glucose transporter 1 but is negative for D2-40.4 These findings suggest that VH is a vascular tumor rather than a vascular malformation, as was previously reported.6 Additional research has shown that the immunohistochemical staining profile of VH is nearly identical to IH, which has led to postulation that VH may be of placental mesodermal origin, as has been hypothesized for IH.5
Due to its deep infiltration and tendency for recurrence, VH is most effectively treated with wide local excision.3,6-8 Preoperative planning with magnetic resonance imaging may be indicated. Although laser monotherapy and other local destructive therapies have been largely unsuccessful, postsurgical laser therapy with CO2 lasers as well as dual pulsed dye laser and Nd:YAG laser have shown promise in preventing recurrence.3
- Tennant LB, Mulliken JB, Perez-Atayde AR, et al. Verrucous hemangioma revisited. Pediatr Dermatol. 2006;23:208-215.
- Koc M, Kavala M, Kocatür E, et al. An unusual vascular tumor: verrucous hemangioma. Dermatol Online J. 2009;15:7.
- Yang CH, Ohara K. Successful surgical treatment of verrucous hemangioma: a combined approach. Dermatol Surg. 2002;28:913-919; discussion 920.
- Trindade F, Torrelo A, Requena L, et al. An immunohistochemical study of verrucous hemangiomas. J Cutan Pathol. 2013;40:472-476.
- Laing EL, Brasch HD, Steel R, et al. Verrucous hemangioma expresses primitive markers. J Cutan Pathol. 2013;40:391-396.
- Mankani MH, Dufresne CR. Verrucous malformations: their presentation and management. Ann Plast Surg. 2000;45:31-36.
- Clairwood MQ, Bruckner AL, Dadras SS. Verrucous hemangioma: a report of two cases and review of the literature. J Cutan Pathol. 2011;38:740-746.
- Segura Palacios JM, Boixeda P, Rocha J, et al. Laser treatment for verrucous hemangioma. Laser Med Sci. 2012;27:681-684.
The Diagnosis: Verrucous Hemangioma
Verrucous hemangioma (VH) is a rare vascular anomaly that has not been definitively delineated as a malformation or a tumor, as it has features of both. Verrucous hemangioma presents at birth as a compressible soft mass with a red violaceous hue favoring the legs.1,2 Over time VH will develop a warty, friable, and keratotic surface that can begin to evolve as early as 6 months or as late as 34 years of age.3 Verrucous hemangioma does not involute and tends to grow proportionally with the patient. Thus, VH classically has been considered a vascular malformation.
On histopathology VH shows collections of uniform, thin-walled vessels with a multilamellated basement membrane throughout the dermis, similar to an infantile hemangioma (IH). These lesions extend deep into the subcutaneous tissue and often involve the underlying fascia. The papillary dermis has large ectatic vessels, while the epidermis displays verrucous hyperkeratosis, papillomatosis, and irregular acanthosis without viral change (Figure).4,5 The superficial component can resemble an angiokeratoma; however, VH is differentiated by a deeper component that is often larger in size and has a more protracted clinical course.

Similar to IH, immunohistochemical studies have shown that VH expresses Wilms tumor 1 and glucose transporter 1 but is negative for D2-40.4 These findings suggest that VH is a vascular tumor rather than a vascular malformation, as was previously reported.6 Additional research has shown that the immunohistochemical staining profile of VH is nearly identical to IH, which has led to postulation that VH may be of placental mesodermal origin, as has been hypothesized for IH.5
Due to its deep infiltration and tendency for recurrence, VH is most effectively treated with wide local excision.3,6-8 Preoperative planning with magnetic resonance imaging may be indicated. Although laser monotherapy and other local destructive therapies have been largely unsuccessful, postsurgical laser therapy with CO2 lasers as well as dual pulsed dye laser and Nd:YAG laser have shown promise in preventing recurrence.3
The Diagnosis: Verrucous Hemangioma
Verrucous hemangioma (VH) is a rare vascular anomaly that has not been definitively delineated as a malformation or a tumor, as it has features of both. Verrucous hemangioma presents at birth as a compressible soft mass with a red violaceous hue favoring the legs.1,2 Over time VH will develop a warty, friable, and keratotic surface that can begin to evolve as early as 6 months or as late as 34 years of age.3 Verrucous hemangioma does not involute and tends to grow proportionally with the patient. Thus, VH classically has been considered a vascular malformation.
On histopathology VH shows collections of uniform, thin-walled vessels with a multilamellated basement membrane throughout the dermis, similar to an infantile hemangioma (IH). These lesions extend deep into the subcutaneous tissue and often involve the underlying fascia. The papillary dermis has large ectatic vessels, while the epidermis displays verrucous hyperkeratosis, papillomatosis, and irregular acanthosis without viral change (Figure).4,5 The superficial component can resemble an angiokeratoma; however, VH is differentiated by a deeper component that is often larger in size and has a more protracted clinical course.

Similar to IH, immunohistochemical studies have shown that VH expresses Wilms tumor 1 and glucose transporter 1 but is negative for D2-40.4 These findings suggest that VH is a vascular tumor rather than a vascular malformation, as was previously reported.6 Additional research has shown that the immunohistochemical staining profile of VH is nearly identical to IH, which has led to postulation that VH may be of placental mesodermal origin, as has been hypothesized for IH.5
Due to its deep infiltration and tendency for recurrence, VH is most effectively treated with wide local excision.3,6-8 Preoperative planning with magnetic resonance imaging may be indicated. Although laser monotherapy and other local destructive therapies have been largely unsuccessful, postsurgical laser therapy with CO2 lasers as well as dual pulsed dye laser and Nd:YAG laser have shown promise in preventing recurrence.3
- Tennant LB, Mulliken JB, Perez-Atayde AR, et al. Verrucous hemangioma revisited. Pediatr Dermatol. 2006;23:208-215.
- Koc M, Kavala M, Kocatür E, et al. An unusual vascular tumor: verrucous hemangioma. Dermatol Online J. 2009;15:7.
- Yang CH, Ohara K. Successful surgical treatment of verrucous hemangioma: a combined approach. Dermatol Surg. 2002;28:913-919; discussion 920.
- Trindade F, Torrelo A, Requena L, et al. An immunohistochemical study of verrucous hemangiomas. J Cutan Pathol. 2013;40:472-476.
- Laing EL, Brasch HD, Steel R, et al. Verrucous hemangioma expresses primitive markers. J Cutan Pathol. 2013;40:391-396.
- Mankani MH, Dufresne CR. Verrucous malformations: their presentation and management. Ann Plast Surg. 2000;45:31-36.
- Clairwood MQ, Bruckner AL, Dadras SS. Verrucous hemangioma: a report of two cases and review of the literature. J Cutan Pathol. 2011;38:740-746.
- Segura Palacios JM, Boixeda P, Rocha J, et al. Laser treatment for verrucous hemangioma. Laser Med Sci. 2012;27:681-684.
- Tennant LB, Mulliken JB, Perez-Atayde AR, et al. Verrucous hemangioma revisited. Pediatr Dermatol. 2006;23:208-215.
- Koc M, Kavala M, Kocatür E, et al. An unusual vascular tumor: verrucous hemangioma. Dermatol Online J. 2009;15:7.
- Yang CH, Ohara K. Successful surgical treatment of verrucous hemangioma: a combined approach. Dermatol Surg. 2002;28:913-919; discussion 920.
- Trindade F, Torrelo A, Requena L, et al. An immunohistochemical study of verrucous hemangiomas. J Cutan Pathol. 2013;40:472-476.
- Laing EL, Brasch HD, Steel R, et al. Verrucous hemangioma expresses primitive markers. J Cutan Pathol. 2013;40:391-396.
- Mankani MH, Dufresne CR. Verrucous malformations: their presentation and management. Ann Plast Surg. 2000;45:31-36.
- Clairwood MQ, Bruckner AL, Dadras SS. Verrucous hemangioma: a report of two cases and review of the literature. J Cutan Pathol. 2011;38:740-746.
- Segura Palacios JM, Boixeda P, Rocha J, et al. Laser treatment for verrucous hemangioma. Laser Med Sci. 2012;27:681-684.
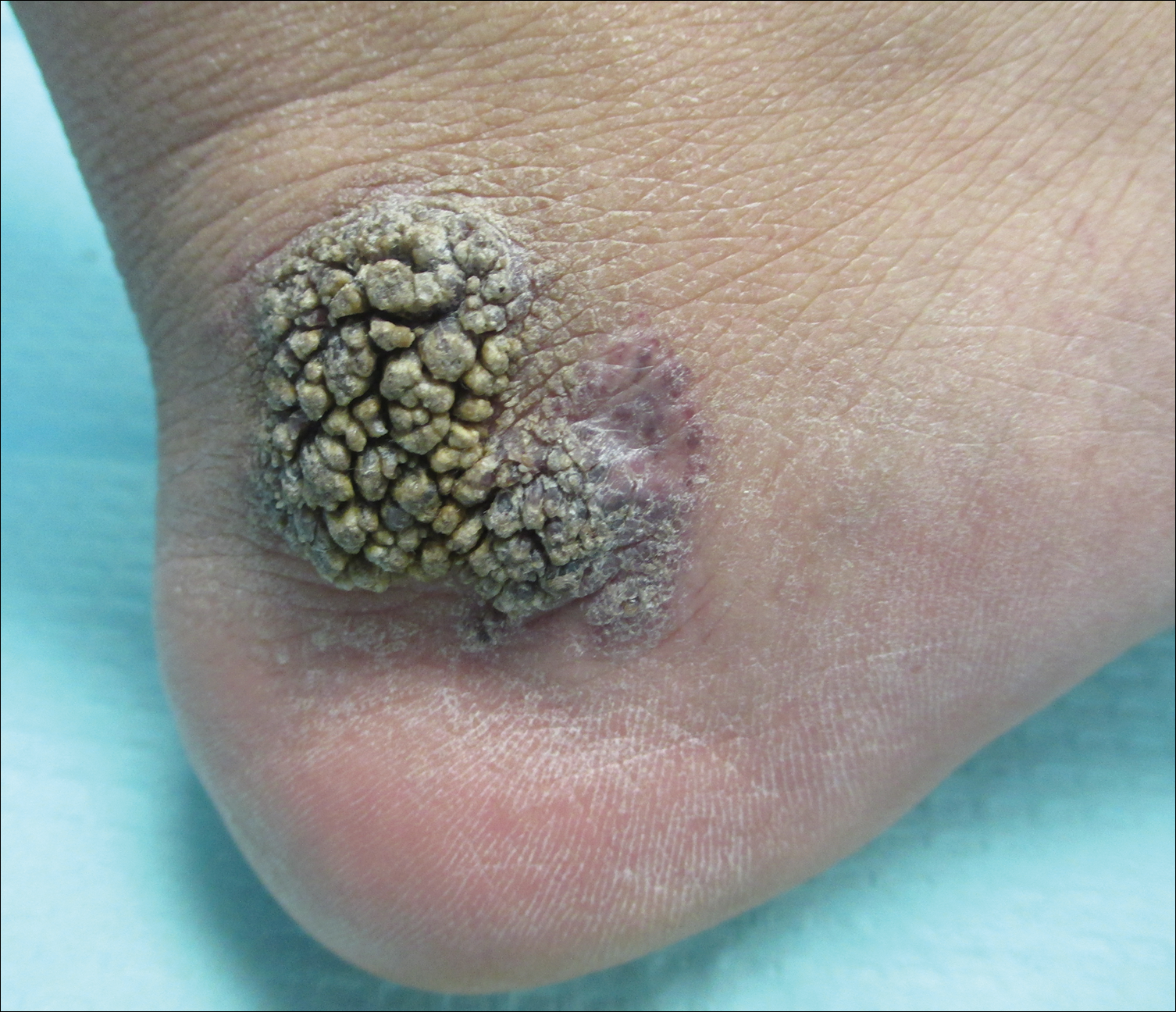
A 31-year-old man presented with a large friable and warty plaque on the left heel. He recalled that the lesion had been present since birth as a flat red birthmark that grew proportionally with him. Throughout his adolescence its surface became increasingly rough and bumpy. The patient described receiving laser treatment twice in his early 20s without notable improvement. He wanted the lesion removed because it was easily traumatized, resulting in bleeding, pain, and infection. The patient reported being otherwise healthy.