User login
Treatment Trends and Outcomes in Healthcare-Associated Pneumonia
Bacterial pneumonia remains an important cause of morbidity and mortality in the United States, and is the 8th leading cause of death with 55,227 deaths among adults annually.1 In 2005, the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) collaborated to update guidelines for hospital-acquired pneumonia (HAP), ventilator-associated pneumonia, and healthcare-associated pneumonia (HCAP).2 This broad document outlines an evidence-based approach to diagnostic testing and antibiotic management based on the epidemiology and risk factors for these conditions. The guideline specifies the following criteria for HCAP: hospitalization in the past 90 days, residence in a skilled nursing facility (SNF), home infusion therapy, hemodialysis, home wound care, family members with multidrug resistant organisms (MDRO), and immunosuppressive diseases or medications, with the presumption that these patients are more likely to be harboring MDRO and should thus be treated empirically with broad-spectrum antibiotic therapy. Prior studies have shown that patients with HCAP have a more severe illness, are more likely to have MDRO, are more likely to be inadequately treated, and are at a higher risk for mortality than patients with community-acquired pneumonia (CAP).3,4
These guidelines are controversial, especially in regard to the recommendations to empirically treat broadly with 2 antibiotics targeting Pseudomonas species, whether patients with HCAP merit broader spectrum coverage than patients with CAP, and whether the criteria for defining HCAP are adequate to predict which patients are harboring MDRO. It has subsequently been proposed that HCAP is more related to CAP than to HAP, and a recent update to the guideline removed recommendations for treatment of HCAP and will be placing HCAP into the guidelines for CAP instead.5 We sought to investigate the degree of uptake of the ATS and IDSA guideline recommendations by physicians over time, and whether this led to a change in outcomes among patients who met the criteria for HCAP.
METHODS
Setting and Patients
We identified patients discharged between July 1, 2007, and November 30, 2011, from 488 US hospitals that participated in the Premier database (Premier Inc., Charlotte, North Carolina), an inpatient database developed for measuring quality and healthcare utilization. The database is frequently used for healthcare research and has been described previously.6 Member hospitals are in all regions of the US and are generally reflective of US hospitals. This database contains multiple data elements, including sociodemographic information, International Classification of Diseases, 9th Revision-Clinical Modification (ICD-9-CM) diagnosis and procedure codes, hospital and physician information, source of admission, and discharge status. It also includes a date-stamped log of all billed items and services, including diagnostic tests, medications, and other treatments. Because the data do not contain identifiable information, the institutional review board at our medical center determined that this study did not constitute human subjects research.
We included all patients aged ≥18 years with a principal diagnosis of pneumonia or with a secondary diagnosis of pneumonia paired with a principal diagnosis of respiratory failure, acute respiratory distress syndrome, respiratory arrest, sepsis, or influenza. Patients were excluded if they were transferred to or from another acute care institution, had a length of stay of 1 day or less, had cystic fibrosis, did not have a chest radiograph, or did not receive antibiotics within 48 hours of admission.
For each patient, we extracted age, gender, principal diagnosis, comorbidities, and the specialty of the attending physician. Comorbidities were identified from ICD-9-CM secondary diagnosis codes and Diagnosis Related Groups by using Healthcare Cost and Utilization Project Comorbidity Software, version 3.1, based on the work of Elixhauser (Agency for Healthcare Research and Quality, Rockville, Maryland).7 In order to ensure that patients had HCAP, we required the presence of ≥1 HCAP criteria, including hospitalization in the past 90 days, hemodialysis, admission from an SNF, or immune suppression (which was derived from either a secondary diagnosis for neutropenia, hematological malignancy, organ transplant, acquired immunodeficiency virus, or receiving immunosuppressant drugs or corticosteroids [equivalent to ≥20 mg/day of prednisone]).
Definitions of Guideline-Concordant and Discordant Antibiotic Therapy
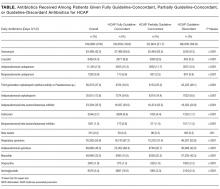
The ATS and IDSA guidelines recommended the following antibiotic combinations for HCAP: an antipseudomonal cephalosporin or carbapenem or a beta-lactam/lactamase inhibitor, plus an antipseudomonal quinolone or aminoglycoside, plus an antibiotic with activity versus methicillin resistant Staphylococcus aureus (MRSA), such as vancomycin or linezolid. Based on these guidelines, we defined the receipt of fully guideline-concordant antibiotics as 2 recommended antibiotics for Pseudomonas species plus 1 for MRSA administered by the second day of admission. Partially guideline-concordant antibiotics were defined as 1 recommended antibiotic for Pseudomonas species plus 1 for MRSA by the second day of hospitalization. Guideline-discordant antibiotics were defined as all other combinations.
Statistical Analysis
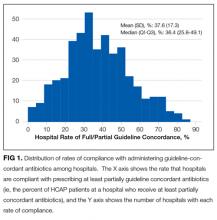
Descriptive statistics on patient characteristics are presented as frequency, proportions for categorical factors, and median with interquartile range (IQR) for continuous variables for the full cohort and by treatment group, defined as fully or partially guideline-concordant antibiotic therapy or discordant therapy. Hospital rates of fully guideline-concordant treatment are presented overall and by hospital characteristics. The association of hospital characteristics with rates of fully guideline-concordant therapy were assessed by using 1-way analysis of variance tests.
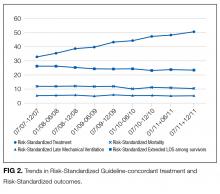
To assess trends across hospitals for the association between the use of guideline-concordant therapy and mortality, progression to respiratory failure as measured by the late initiation of invasive mechanical ventilation (day 3 or later), and the length of stay among survivors, we divided the 4.5-year study period into 9 intervals of 6 months each; 292 hospitals that submitted data for all 9 time points were examined in this analysis. Based on the distribution of length of stay in the first time period, we created an indicator variable for extended length of stay with length of stay at or above the 75th percentile, defined as extended. For each hospital at each 6-month interval, we then computed risk-standardized guideline-concordant treatment (RS-treatment) rates and risk-standardized in-hospital outcome rates similar to methods used by the Centers for Medicare and Medicaid Services for public reporting.8 For each hospital at each time interval, we estimated a predicted rate of guideline-concordant treatment as the sum of predicted probabilities of guideline-concordant treatment from patient factors and the random intercept for the hospital in which they were admitted. We then calculated the expected rate of guideline-concordant treatment as the sum of expected probabilities of treatment received from patient factors only. RS-treatment was then calculated as the ratio of predicted to expected rates multiplied by the overall unadjusted mean treatment rate from all patients.9 We repeated the same modeling strategy to calculate risk-standardized outcome (RS-outcome) rates for each hospital across all time points. All models were adjusted for patient demographics and comorbidities. Similar models using administrative data have moderate discrimination for mortality.10
We then fit mixed-effects linear models with random hospital intercept and slope across time for the RS-treatment and outcome rates, respectively. From these models, we estimated the mean slope for RS-treatment and for RS-outcome over time. In addition, we estimated a slope or trend over time for each hospital for treatment and for outcome and evaluated the correlation between the treatment and outcome trends.
All analyses were performed using the Statistical Analysis System version 9.4 (SAS Institute Inc., Cary, NC) and STATA release 13 (StataCorp, LLC, College Station, Texas).
RESULTS
DISCUSSION
In this large, retrospective cohort study, we found that there was a substantial gap between the empiric antibiotics recommended by the ATS and IDSA guidelines and the empiric antibiotics that patients actually received. Over the study period, we saw an increased adherence to guidelines, in spite of growing evidence that HCAP risk factors do not adequately predict which patients are at risk for infection with an MDRO.11 We used this change in antibiotic prescribing behavior over time to determine if there was a clinical impact on patient outcomes and found that at the hospital level, there were no improvements in mortality, excess length of stay, or progression to respiratory failure despite a doubling in guideline-concordant antibiotic use.
At least 2 other large studies have assessed the association between guideline-concordant therapy and outcomes in HCAP.12,13 Both found that guideline-concordant therapy was associated with increased mortality, despite propensity matching. Both were conducted at the individual patient level by using administrative data, and results were likely affected by unmeasured clinical confounders, with sicker patients being more likely to receive guideline-concordant therapy. Our focus on the outcomes at the hospital level avoids this selection bias because the overall severity of illness of patients at any given hospital would not be expected to change over the study period, while physician uptake of antibiotic prescribing guidelines would be expected to increase over time. Determining the correlation between increases in guideline adherence and changes in patient outcome may offer a better assessment of the impact of guideline adherence. In this regard, our results are similar to those achieved by 1 quality improvement collaborative that was aimed at increasing guideline concordant therapy in ICUs. Despite an increase in guideline concordance from 33% to 47% of patients, they found no change in overall mortality.14
There were several limitations to our study. We did not have access to microbiologic data, so we were unable to determine which patients had MDRO infection or determine antibiotic-pathogen matching. However, the treating physicians in our study population presumably did not have access to this data at the time of treatment either because the time period we examined was within the first 48 hours of hospitalization, the interval during which cultures are incubating and the patients are being treated empirically. In addition, there may have been HCAP patients that we failed to identify, such as patients who were admitted in the past 90 days to a hospital that does not submit data to Premier. However, it is unlikely that prescribing for such patients should differ systematically from what we observed. While the database draws from 488 hospitals nationwide, it is possible that practices may be different at facilities that are not contained within the Premier database, such as Veterans Administration Hospitals. Similarly, we did not have readings for chest x-rays; hence, there could be some patients in the dataset who did not have pneumonia. However, we tried to overcome this by including only those patients with a principal diagnosis of pneumonia or sepsis with a secondary pneumonia diagnosis, a chest x-ray, and antibiotics administered within the first 48 hours of admission.
There are likely several reasons why so few HCAP patients in our study received guideline-concordant antibiotics. A lack of knowledge about the ATS and IDSA guidelines may have impacted the physicians in our study population. El-Solh et al.15 surveyed physicians about the ATS-IDSA guidelines 4 years after publication and found that only 45% were familiar with the document. We found that the rate of prescribing at least partially guideline-concordant antibiotics rose steadily over time, supporting the idea that the newness of the guidelines was 1 barrier. Additionally, prior studies have shown that many physicians may not agree with or choose to follow guidelines, with only 20% of physicians indicating that guidelines have a major impact on their clinical decision making,16 and the majority do not choose HCAP guideline-concordant antibiotics when tested.17 Alternatively, clinicians may not follow the guidelines because of a belief that the HCAP criteria do not adequately indicate patients who are at risk for MDRO. Previous studies have demonstrated the relative inability of HCAP risk factors to predict patients who harbor MDRO18 and suggest that better tools such as clinical scoring systems, which include not only the traditional HCAP risk factors but also prior exposure to antibiotics, prior culture data, and a cumulative assessment of both intrinsic and extrinsic factors, could more accurately predict MDRO and lead to a more judicious use of broad-spectrum antimicrobial agents.19-25 Indeed, these collective findings have led the authors of the recently updated guidelines to remove HCAP as a clinical entity from the hospital-acquired or ventilator-associated pneumonia guidelines and place them instead in the upcoming updated guidelines on the management of CAP.5 Of these 3 explanations, the lack of familiarity fits best with our observation that guideline-concordant therapy increased steadily over time with no evidence of reaching a plateau. Ironically, as consensus was building that HCAP is a poor marker for MDROs, routine empiric treatment with vancomycin and piperacillin-tazobactam (“vanco and zosyn”) have become routine in many hospitals. Additional studies are needed to know if this trend has stabilized or reversed.
CONCLUSIONS
In conclusion, clinicians in our large, nationally representative sample treated the majority of HCAP patients as though they had CAP. Although there was an increase in the administration of guideline-concordant therapy over time, this increase was not associated with improved outcomes. This study supports the growing consensus that HCAP criteria do not accurately predict which patients benefit from broad-spectrum antibiotics for pneumonia, and most patients fare well with antibiotics targeting common community-acquired organisms.
Disclosure
This work was supported by grant # R01HS018723 from the Agency for Healthcare Research and Quality. Dr. Lagu is also supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. Dr. Lindenauer is supported by grant K24HL132008 from the National Heart, Lung, and Blood Institute. The funding agency had no role in the data acquisition, analysis, or manuscript preparation for this study. Drs. Haessler and Rothberg had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Haessler, Lagu, Lindenauer, Skiest, Zilberberg, Higgins, and Rothberg conceived of the study and analyzed and interpreted the data. Dr. Lindenauer acquired the data. Dr. Pekow and Ms. Priya carried out the statistical analyses. Dr. Haessler drafted the manuscript. All authors critically reviewed the manuscript for accuracy and integrity. All authors certify no potential conflicts of interest. Preliminary results from this study were presented in oral and poster format at IDWeek in 2012 and 2013.
1. Kochanek KD, Murphy SL, Xu JQ, Tejada-Vera B. Deaths: Final data for 2014. National vital statistics reports; vol 65 no 4. Hyattsville, MD: National Center for Health Statistics. 2016. PubMed
2. American Thoracic Society, Infectious Diseases Society of America. Guidelines for the Management of Adults with Hospital-acquired, Ventilator-associated, and Healthcare-associated Pneumonia. Am J Respir Crit Care Med. 2005;171(4):388-416. PubMed
3. Zilberberg MD, Shorr A. Healthcare-associated pneumonia: the state of the evidence to date. Curr Opin Pulm Med. 2011;17(3):142-147. PubMed
4. Kollef MK, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and Outcomes of Health-care-associated pneumonia. Chest. 2005;128(6):3854-3862. PubMed
5. Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):575-582. PubMed
6. Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010;303(23):2359-2367. PubMed
7. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. PubMed
8. Centers for Medicare & Medicaid Services. Frequently asked questions (FAQs): Implementation and maintenance of CMS mortality measures for AMI & HF. 2007. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/downloads/HospitalMortalityAboutAMI_HF.pdf. Accessed November 1, 2016.
9. Normand SL, Shahian DM. Statistical and Clinical Aspects of Hospital Outcomes Profiling. Stat Sci. 2007;22(2):206-226.
10. Rothberg MB, Pekow PS, Priya A, et al. Using highly detailed administrative data to predict pneumonia mortality. PLoS One. 2014;9(1):e87382. PubMed
11. Jones BE, Jones MM, Huttner B, et al. Trends in antibiotic use and nosocomial pathogens in hospitalized veterans with pneumonia at 128 medical centers, 2006-2010. Clin Infect Dis. 2015;61(9):1403-1410. PubMed
12. Attridge RT, Frei CR, Restrepo MI, et al. Guideline-concordant therapy and outcomes in healthcare-associated pneumonia. Eur Respir J. 2011;38(4):878-887. PubMed
13. Rothberg MB, Zilberberg MD, Pekow PS, et al. Association of Guideline-based Antimicrobial Therapy and Outcomes in Healthcare-Associated Pneumonia. J Antimicrob Chemother. 2015;70(5):1573-1579. PubMed
14. Kett DH, Cano E, Quartin AA, et al. Improving Medicine through Pathway Assessment of Critical Therapy of Hospital-Acquired Pneumonia (IMPACT-HAP) Investigators. Implementation of guidelines for management of possible multidrug-resistant pneumonia in intensive care: an observational, multicentre cohort study. Lancet Infect Dis. 2011;11(3):181-189. PubMed
15. El-Solh AA, Alhajhusain A, Saliba RG, Drinka P. Physicians’ Attitudes Toward Guidelines for the Treatment of Hospitalized Nursing-Home -Acquired Pneumonia. J Am Med Dir Assoc. 2011;12(4):270-276. PubMed
16. Tunis S, Hayward R, Wilson M, et al. Internists’ Attitudes about Clinical Practice Guidelines. Ann Intern Med. 1994;120(11):956-963. PubMed
17. Seymann GB, Di Francesco L, Sharpe B, et al. The HCAP Gap: Differences between Self-Reported Practice Patterns and Published Guidelines for Health Care-Associated Pneumonia. Clin Infect Dis. 2009;49(12):1868-1874. PubMed
18. Chalmers JD, Rother C, Salih W, Ewig S. Healthcare associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis. 2014;58(3):330-339. PubMed
19. Shorr A, Zilberberg MD, Reichley R, et al. Validation of a Clinical Score for Assessing the Risk of Resistant Pathogens in Patients with Pneumonia Presenting to the Emergency Department. Clin Infect Dis. 2012;54(2):193-198. PubMed
20. Aliberti S, Pasquale MD, Zanaboni AM, et al. Stratifying Risk Factors for Multidrug-Resistant Pathogens in Hospitalized Patients Coming from the Community with Pneumonia. Clin Infect Dis. 2012;54(4):470-478. PubMed
21. Schreiber MP, Chan CM, Shorr AF. Resistant Pathogens in Nonnosocomial Pneumonia and Respiratory Failure: Is it Time to Refine the Definition of Health-care-Associated Pneumonia? Chest. 2010;137(6):1283-1288. PubMed
22. Madaras-Kelly KJ, Remington RE, Fan VS, Sloan KL. Predicting antibiotic resistance to community-acquired pneumonia antibiotics in culture-positive patients with healthcare-associated pneumonia. J Hosp Med. 2012;7(3):195-202. PubMed
23. Shindo Y, Ito R, Kobayashi D, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188(8):985-995. PubMed
24. Metersky ML, Frei CR, Mortensen EM. Predictors of Pseudomonas and methicillin-resistant Staphylococcus aureus in hospitalized patients with healthcare-associated pneumonia. Respirology. 2016;21(1):157-163. PubMed
25. Webb BJ, Dascomb K, Stenehjem E, Dean N. Predicting risk of drug-resistant organisms in pneumonia: moving beyond the HCAP model. Respir Med. 2015;109(1):1-10. PubMed
Bacterial pneumonia remains an important cause of morbidity and mortality in the United States, and is the 8th leading cause of death with 55,227 deaths among adults annually.1 In 2005, the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) collaborated to update guidelines for hospital-acquired pneumonia (HAP), ventilator-associated pneumonia, and healthcare-associated pneumonia (HCAP).2 This broad document outlines an evidence-based approach to diagnostic testing and antibiotic management based on the epidemiology and risk factors for these conditions. The guideline specifies the following criteria for HCAP: hospitalization in the past 90 days, residence in a skilled nursing facility (SNF), home infusion therapy, hemodialysis, home wound care, family members with multidrug resistant organisms (MDRO), and immunosuppressive diseases or medications, with the presumption that these patients are more likely to be harboring MDRO and should thus be treated empirically with broad-spectrum antibiotic therapy. Prior studies have shown that patients with HCAP have a more severe illness, are more likely to have MDRO, are more likely to be inadequately treated, and are at a higher risk for mortality than patients with community-acquired pneumonia (CAP).3,4
These guidelines are controversial, especially in regard to the recommendations to empirically treat broadly with 2 antibiotics targeting Pseudomonas species, whether patients with HCAP merit broader spectrum coverage than patients with CAP, and whether the criteria for defining HCAP are adequate to predict which patients are harboring MDRO. It has subsequently been proposed that HCAP is more related to CAP than to HAP, and a recent update to the guideline removed recommendations for treatment of HCAP and will be placing HCAP into the guidelines for CAP instead.5 We sought to investigate the degree of uptake of the ATS and IDSA guideline recommendations by physicians over time, and whether this led to a change in outcomes among patients who met the criteria for HCAP.
METHODS
Setting and Patients
We identified patients discharged between July 1, 2007, and November 30, 2011, from 488 US hospitals that participated in the Premier database (Premier Inc., Charlotte, North Carolina), an inpatient database developed for measuring quality and healthcare utilization. The database is frequently used for healthcare research and has been described previously.6 Member hospitals are in all regions of the US and are generally reflective of US hospitals. This database contains multiple data elements, including sociodemographic information, International Classification of Diseases, 9th Revision-Clinical Modification (ICD-9-CM) diagnosis and procedure codes, hospital and physician information, source of admission, and discharge status. It also includes a date-stamped log of all billed items and services, including diagnostic tests, medications, and other treatments. Because the data do not contain identifiable information, the institutional review board at our medical center determined that this study did not constitute human subjects research.
We included all patients aged ≥18 years with a principal diagnosis of pneumonia or with a secondary diagnosis of pneumonia paired with a principal diagnosis of respiratory failure, acute respiratory distress syndrome, respiratory arrest, sepsis, or influenza. Patients were excluded if they were transferred to or from another acute care institution, had a length of stay of 1 day or less, had cystic fibrosis, did not have a chest radiograph, or did not receive antibiotics within 48 hours of admission.
For each patient, we extracted age, gender, principal diagnosis, comorbidities, and the specialty of the attending physician. Comorbidities were identified from ICD-9-CM secondary diagnosis codes and Diagnosis Related Groups by using Healthcare Cost and Utilization Project Comorbidity Software, version 3.1, based on the work of Elixhauser (Agency for Healthcare Research and Quality, Rockville, Maryland).7 In order to ensure that patients had HCAP, we required the presence of ≥1 HCAP criteria, including hospitalization in the past 90 days, hemodialysis, admission from an SNF, or immune suppression (which was derived from either a secondary diagnosis for neutropenia, hematological malignancy, organ transplant, acquired immunodeficiency virus, or receiving immunosuppressant drugs or corticosteroids [equivalent to ≥20 mg/day of prednisone]).
Definitions of Guideline-Concordant and Discordant Antibiotic Therapy
The ATS and IDSA guidelines recommended the following antibiotic combinations for HCAP: an antipseudomonal cephalosporin or carbapenem or a beta-lactam/lactamase inhibitor, plus an antipseudomonal quinolone or aminoglycoside, plus an antibiotic with activity versus methicillin resistant Staphylococcus aureus (MRSA), such as vancomycin or linezolid. Based on these guidelines, we defined the receipt of fully guideline-concordant antibiotics as 2 recommended antibiotics for Pseudomonas species plus 1 for MRSA administered by the second day of admission. Partially guideline-concordant antibiotics were defined as 1 recommended antibiotic for Pseudomonas species plus 1 for MRSA by the second day of hospitalization. Guideline-discordant antibiotics were defined as all other combinations.
Statistical Analysis
Descriptive statistics on patient characteristics are presented as frequency, proportions for categorical factors, and median with interquartile range (IQR) for continuous variables for the full cohort and by treatment group, defined as fully or partially guideline-concordant antibiotic therapy or discordant therapy. Hospital rates of fully guideline-concordant treatment are presented overall and by hospital characteristics. The association of hospital characteristics with rates of fully guideline-concordant therapy were assessed by using 1-way analysis of variance tests.
To assess trends across hospitals for the association between the use of guideline-concordant therapy and mortality, progression to respiratory failure as measured by the late initiation of invasive mechanical ventilation (day 3 or later), and the length of stay among survivors, we divided the 4.5-year study period into 9 intervals of 6 months each; 292 hospitals that submitted data for all 9 time points were examined in this analysis. Based on the distribution of length of stay in the first time period, we created an indicator variable for extended length of stay with length of stay at or above the 75th percentile, defined as extended. For each hospital at each 6-month interval, we then computed risk-standardized guideline-concordant treatment (RS-treatment) rates and risk-standardized in-hospital outcome rates similar to methods used by the Centers for Medicare and Medicaid Services for public reporting.8 For each hospital at each time interval, we estimated a predicted rate of guideline-concordant treatment as the sum of predicted probabilities of guideline-concordant treatment from patient factors and the random intercept for the hospital in which they were admitted. We then calculated the expected rate of guideline-concordant treatment as the sum of expected probabilities of treatment received from patient factors only. RS-treatment was then calculated as the ratio of predicted to expected rates multiplied by the overall unadjusted mean treatment rate from all patients.9 We repeated the same modeling strategy to calculate risk-standardized outcome (RS-outcome) rates for each hospital across all time points. All models were adjusted for patient demographics and comorbidities. Similar models using administrative data have moderate discrimination for mortality.10
We then fit mixed-effects linear models with random hospital intercept and slope across time for the RS-treatment and outcome rates, respectively. From these models, we estimated the mean slope for RS-treatment and for RS-outcome over time. In addition, we estimated a slope or trend over time for each hospital for treatment and for outcome and evaluated the correlation between the treatment and outcome trends.
All analyses were performed using the Statistical Analysis System version 9.4 (SAS Institute Inc., Cary, NC) and STATA release 13 (StataCorp, LLC, College Station, Texas).
RESULTS
DISCUSSION
In this large, retrospective cohort study, we found that there was a substantial gap between the empiric antibiotics recommended by the ATS and IDSA guidelines and the empiric antibiotics that patients actually received. Over the study period, we saw an increased adherence to guidelines, in spite of growing evidence that HCAP risk factors do not adequately predict which patients are at risk for infection with an MDRO.11 We used this change in antibiotic prescribing behavior over time to determine if there was a clinical impact on patient outcomes and found that at the hospital level, there were no improvements in mortality, excess length of stay, or progression to respiratory failure despite a doubling in guideline-concordant antibiotic use.
At least 2 other large studies have assessed the association between guideline-concordant therapy and outcomes in HCAP.12,13 Both found that guideline-concordant therapy was associated with increased mortality, despite propensity matching. Both were conducted at the individual patient level by using administrative data, and results were likely affected by unmeasured clinical confounders, with sicker patients being more likely to receive guideline-concordant therapy. Our focus on the outcomes at the hospital level avoids this selection bias because the overall severity of illness of patients at any given hospital would not be expected to change over the study period, while physician uptake of antibiotic prescribing guidelines would be expected to increase over time. Determining the correlation between increases in guideline adherence and changes in patient outcome may offer a better assessment of the impact of guideline adherence. In this regard, our results are similar to those achieved by 1 quality improvement collaborative that was aimed at increasing guideline concordant therapy in ICUs. Despite an increase in guideline concordance from 33% to 47% of patients, they found no change in overall mortality.14
There were several limitations to our study. We did not have access to microbiologic data, so we were unable to determine which patients had MDRO infection or determine antibiotic-pathogen matching. However, the treating physicians in our study population presumably did not have access to this data at the time of treatment either because the time period we examined was within the first 48 hours of hospitalization, the interval during which cultures are incubating and the patients are being treated empirically. In addition, there may have been HCAP patients that we failed to identify, such as patients who were admitted in the past 90 days to a hospital that does not submit data to Premier. However, it is unlikely that prescribing for such patients should differ systematically from what we observed. While the database draws from 488 hospitals nationwide, it is possible that practices may be different at facilities that are not contained within the Premier database, such as Veterans Administration Hospitals. Similarly, we did not have readings for chest x-rays; hence, there could be some patients in the dataset who did not have pneumonia. However, we tried to overcome this by including only those patients with a principal diagnosis of pneumonia or sepsis with a secondary pneumonia diagnosis, a chest x-ray, and antibiotics administered within the first 48 hours of admission.
There are likely several reasons why so few HCAP patients in our study received guideline-concordant antibiotics. A lack of knowledge about the ATS and IDSA guidelines may have impacted the physicians in our study population. El-Solh et al.15 surveyed physicians about the ATS-IDSA guidelines 4 years after publication and found that only 45% were familiar with the document. We found that the rate of prescribing at least partially guideline-concordant antibiotics rose steadily over time, supporting the idea that the newness of the guidelines was 1 barrier. Additionally, prior studies have shown that many physicians may not agree with or choose to follow guidelines, with only 20% of physicians indicating that guidelines have a major impact on their clinical decision making,16 and the majority do not choose HCAP guideline-concordant antibiotics when tested.17 Alternatively, clinicians may not follow the guidelines because of a belief that the HCAP criteria do not adequately indicate patients who are at risk for MDRO. Previous studies have demonstrated the relative inability of HCAP risk factors to predict patients who harbor MDRO18 and suggest that better tools such as clinical scoring systems, which include not only the traditional HCAP risk factors but also prior exposure to antibiotics, prior culture data, and a cumulative assessment of both intrinsic and extrinsic factors, could more accurately predict MDRO and lead to a more judicious use of broad-spectrum antimicrobial agents.19-25 Indeed, these collective findings have led the authors of the recently updated guidelines to remove HCAP as a clinical entity from the hospital-acquired or ventilator-associated pneumonia guidelines and place them instead in the upcoming updated guidelines on the management of CAP.5 Of these 3 explanations, the lack of familiarity fits best with our observation that guideline-concordant therapy increased steadily over time with no evidence of reaching a plateau. Ironically, as consensus was building that HCAP is a poor marker for MDROs, routine empiric treatment with vancomycin and piperacillin-tazobactam (“vanco and zosyn”) have become routine in many hospitals. Additional studies are needed to know if this trend has stabilized or reversed.
CONCLUSIONS
In conclusion, clinicians in our large, nationally representative sample treated the majority of HCAP patients as though they had CAP. Although there was an increase in the administration of guideline-concordant therapy over time, this increase was not associated with improved outcomes. This study supports the growing consensus that HCAP criteria do not accurately predict which patients benefit from broad-spectrum antibiotics for pneumonia, and most patients fare well with antibiotics targeting common community-acquired organisms.
Disclosure
This work was supported by grant # R01HS018723 from the Agency for Healthcare Research and Quality. Dr. Lagu is also supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. Dr. Lindenauer is supported by grant K24HL132008 from the National Heart, Lung, and Blood Institute. The funding agency had no role in the data acquisition, analysis, or manuscript preparation for this study. Drs. Haessler and Rothberg had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Haessler, Lagu, Lindenauer, Skiest, Zilberberg, Higgins, and Rothberg conceived of the study and analyzed and interpreted the data. Dr. Lindenauer acquired the data. Dr. Pekow and Ms. Priya carried out the statistical analyses. Dr. Haessler drafted the manuscript. All authors critically reviewed the manuscript for accuracy and integrity. All authors certify no potential conflicts of interest. Preliminary results from this study were presented in oral and poster format at IDWeek in 2012 and 2013.
Bacterial pneumonia remains an important cause of morbidity and mortality in the United States, and is the 8th leading cause of death with 55,227 deaths among adults annually.1 In 2005, the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) collaborated to update guidelines for hospital-acquired pneumonia (HAP), ventilator-associated pneumonia, and healthcare-associated pneumonia (HCAP).2 This broad document outlines an evidence-based approach to diagnostic testing and antibiotic management based on the epidemiology and risk factors for these conditions. The guideline specifies the following criteria for HCAP: hospitalization in the past 90 days, residence in a skilled nursing facility (SNF), home infusion therapy, hemodialysis, home wound care, family members with multidrug resistant organisms (MDRO), and immunosuppressive diseases or medications, with the presumption that these patients are more likely to be harboring MDRO and should thus be treated empirically with broad-spectrum antibiotic therapy. Prior studies have shown that patients with HCAP have a more severe illness, are more likely to have MDRO, are more likely to be inadequately treated, and are at a higher risk for mortality than patients with community-acquired pneumonia (CAP).3,4
These guidelines are controversial, especially in regard to the recommendations to empirically treat broadly with 2 antibiotics targeting Pseudomonas species, whether patients with HCAP merit broader spectrum coverage than patients with CAP, and whether the criteria for defining HCAP are adequate to predict which patients are harboring MDRO. It has subsequently been proposed that HCAP is more related to CAP than to HAP, and a recent update to the guideline removed recommendations for treatment of HCAP and will be placing HCAP into the guidelines for CAP instead.5 We sought to investigate the degree of uptake of the ATS and IDSA guideline recommendations by physicians over time, and whether this led to a change in outcomes among patients who met the criteria for HCAP.
METHODS
Setting and Patients
We identified patients discharged between July 1, 2007, and November 30, 2011, from 488 US hospitals that participated in the Premier database (Premier Inc., Charlotte, North Carolina), an inpatient database developed for measuring quality and healthcare utilization. The database is frequently used for healthcare research and has been described previously.6 Member hospitals are in all regions of the US and are generally reflective of US hospitals. This database contains multiple data elements, including sociodemographic information, International Classification of Diseases, 9th Revision-Clinical Modification (ICD-9-CM) diagnosis and procedure codes, hospital and physician information, source of admission, and discharge status. It also includes a date-stamped log of all billed items and services, including diagnostic tests, medications, and other treatments. Because the data do not contain identifiable information, the institutional review board at our medical center determined that this study did not constitute human subjects research.
We included all patients aged ≥18 years with a principal diagnosis of pneumonia or with a secondary diagnosis of pneumonia paired with a principal diagnosis of respiratory failure, acute respiratory distress syndrome, respiratory arrest, sepsis, or influenza. Patients were excluded if they were transferred to or from another acute care institution, had a length of stay of 1 day or less, had cystic fibrosis, did not have a chest radiograph, or did not receive antibiotics within 48 hours of admission.
For each patient, we extracted age, gender, principal diagnosis, comorbidities, and the specialty of the attending physician. Comorbidities were identified from ICD-9-CM secondary diagnosis codes and Diagnosis Related Groups by using Healthcare Cost and Utilization Project Comorbidity Software, version 3.1, based on the work of Elixhauser (Agency for Healthcare Research and Quality, Rockville, Maryland).7 In order to ensure that patients had HCAP, we required the presence of ≥1 HCAP criteria, including hospitalization in the past 90 days, hemodialysis, admission from an SNF, or immune suppression (which was derived from either a secondary diagnosis for neutropenia, hematological malignancy, organ transplant, acquired immunodeficiency virus, or receiving immunosuppressant drugs or corticosteroids [equivalent to ≥20 mg/day of prednisone]).
Definitions of Guideline-Concordant and Discordant Antibiotic Therapy
The ATS and IDSA guidelines recommended the following antibiotic combinations for HCAP: an antipseudomonal cephalosporin or carbapenem or a beta-lactam/lactamase inhibitor, plus an antipseudomonal quinolone or aminoglycoside, plus an antibiotic with activity versus methicillin resistant Staphylococcus aureus (MRSA), such as vancomycin or linezolid. Based on these guidelines, we defined the receipt of fully guideline-concordant antibiotics as 2 recommended antibiotics for Pseudomonas species plus 1 for MRSA administered by the second day of admission. Partially guideline-concordant antibiotics were defined as 1 recommended antibiotic for Pseudomonas species plus 1 for MRSA by the second day of hospitalization. Guideline-discordant antibiotics were defined as all other combinations.
Statistical Analysis
Descriptive statistics on patient characteristics are presented as frequency, proportions for categorical factors, and median with interquartile range (IQR) for continuous variables for the full cohort and by treatment group, defined as fully or partially guideline-concordant antibiotic therapy or discordant therapy. Hospital rates of fully guideline-concordant treatment are presented overall and by hospital characteristics. The association of hospital characteristics with rates of fully guideline-concordant therapy were assessed by using 1-way analysis of variance tests.
To assess trends across hospitals for the association between the use of guideline-concordant therapy and mortality, progression to respiratory failure as measured by the late initiation of invasive mechanical ventilation (day 3 or later), and the length of stay among survivors, we divided the 4.5-year study period into 9 intervals of 6 months each; 292 hospitals that submitted data for all 9 time points were examined in this analysis. Based on the distribution of length of stay in the first time period, we created an indicator variable for extended length of stay with length of stay at or above the 75th percentile, defined as extended. For each hospital at each 6-month interval, we then computed risk-standardized guideline-concordant treatment (RS-treatment) rates and risk-standardized in-hospital outcome rates similar to methods used by the Centers for Medicare and Medicaid Services for public reporting.8 For each hospital at each time interval, we estimated a predicted rate of guideline-concordant treatment as the sum of predicted probabilities of guideline-concordant treatment from patient factors and the random intercept for the hospital in which they were admitted. We then calculated the expected rate of guideline-concordant treatment as the sum of expected probabilities of treatment received from patient factors only. RS-treatment was then calculated as the ratio of predicted to expected rates multiplied by the overall unadjusted mean treatment rate from all patients.9 We repeated the same modeling strategy to calculate risk-standardized outcome (RS-outcome) rates for each hospital across all time points. All models were adjusted for patient demographics and comorbidities. Similar models using administrative data have moderate discrimination for mortality.10
We then fit mixed-effects linear models with random hospital intercept and slope across time for the RS-treatment and outcome rates, respectively. From these models, we estimated the mean slope for RS-treatment and for RS-outcome over time. In addition, we estimated a slope or trend over time for each hospital for treatment and for outcome and evaluated the correlation between the treatment and outcome trends.
All analyses were performed using the Statistical Analysis System version 9.4 (SAS Institute Inc., Cary, NC) and STATA release 13 (StataCorp, LLC, College Station, Texas).
RESULTS
DISCUSSION
In this large, retrospective cohort study, we found that there was a substantial gap between the empiric antibiotics recommended by the ATS and IDSA guidelines and the empiric antibiotics that patients actually received. Over the study period, we saw an increased adherence to guidelines, in spite of growing evidence that HCAP risk factors do not adequately predict which patients are at risk for infection with an MDRO.11 We used this change in antibiotic prescribing behavior over time to determine if there was a clinical impact on patient outcomes and found that at the hospital level, there were no improvements in mortality, excess length of stay, or progression to respiratory failure despite a doubling in guideline-concordant antibiotic use.
At least 2 other large studies have assessed the association between guideline-concordant therapy and outcomes in HCAP.12,13 Both found that guideline-concordant therapy was associated with increased mortality, despite propensity matching. Both were conducted at the individual patient level by using administrative data, and results were likely affected by unmeasured clinical confounders, with sicker patients being more likely to receive guideline-concordant therapy. Our focus on the outcomes at the hospital level avoids this selection bias because the overall severity of illness of patients at any given hospital would not be expected to change over the study period, while physician uptake of antibiotic prescribing guidelines would be expected to increase over time. Determining the correlation between increases in guideline adherence and changes in patient outcome may offer a better assessment of the impact of guideline adherence. In this regard, our results are similar to those achieved by 1 quality improvement collaborative that was aimed at increasing guideline concordant therapy in ICUs. Despite an increase in guideline concordance from 33% to 47% of patients, they found no change in overall mortality.14
There were several limitations to our study. We did not have access to microbiologic data, so we were unable to determine which patients had MDRO infection or determine antibiotic-pathogen matching. However, the treating physicians in our study population presumably did not have access to this data at the time of treatment either because the time period we examined was within the first 48 hours of hospitalization, the interval during which cultures are incubating and the patients are being treated empirically. In addition, there may have been HCAP patients that we failed to identify, such as patients who were admitted in the past 90 days to a hospital that does not submit data to Premier. However, it is unlikely that prescribing for such patients should differ systematically from what we observed. While the database draws from 488 hospitals nationwide, it is possible that practices may be different at facilities that are not contained within the Premier database, such as Veterans Administration Hospitals. Similarly, we did not have readings for chest x-rays; hence, there could be some patients in the dataset who did not have pneumonia. However, we tried to overcome this by including only those patients with a principal diagnosis of pneumonia or sepsis with a secondary pneumonia diagnosis, a chest x-ray, and antibiotics administered within the first 48 hours of admission.
There are likely several reasons why so few HCAP patients in our study received guideline-concordant antibiotics. A lack of knowledge about the ATS and IDSA guidelines may have impacted the physicians in our study population. El-Solh et al.15 surveyed physicians about the ATS-IDSA guidelines 4 years after publication and found that only 45% were familiar with the document. We found that the rate of prescribing at least partially guideline-concordant antibiotics rose steadily over time, supporting the idea that the newness of the guidelines was 1 barrier. Additionally, prior studies have shown that many physicians may not agree with or choose to follow guidelines, with only 20% of physicians indicating that guidelines have a major impact on their clinical decision making,16 and the majority do not choose HCAP guideline-concordant antibiotics when tested.17 Alternatively, clinicians may not follow the guidelines because of a belief that the HCAP criteria do not adequately indicate patients who are at risk for MDRO. Previous studies have demonstrated the relative inability of HCAP risk factors to predict patients who harbor MDRO18 and suggest that better tools such as clinical scoring systems, which include not only the traditional HCAP risk factors but also prior exposure to antibiotics, prior culture data, and a cumulative assessment of both intrinsic and extrinsic factors, could more accurately predict MDRO and lead to a more judicious use of broad-spectrum antimicrobial agents.19-25 Indeed, these collective findings have led the authors of the recently updated guidelines to remove HCAP as a clinical entity from the hospital-acquired or ventilator-associated pneumonia guidelines and place them instead in the upcoming updated guidelines on the management of CAP.5 Of these 3 explanations, the lack of familiarity fits best with our observation that guideline-concordant therapy increased steadily over time with no evidence of reaching a plateau. Ironically, as consensus was building that HCAP is a poor marker for MDROs, routine empiric treatment with vancomycin and piperacillin-tazobactam (“vanco and zosyn”) have become routine in many hospitals. Additional studies are needed to know if this trend has stabilized or reversed.
CONCLUSIONS
In conclusion, clinicians in our large, nationally representative sample treated the majority of HCAP patients as though they had CAP. Although there was an increase in the administration of guideline-concordant therapy over time, this increase was not associated with improved outcomes. This study supports the growing consensus that HCAP criteria do not accurately predict which patients benefit from broad-spectrum antibiotics for pneumonia, and most patients fare well with antibiotics targeting common community-acquired organisms.
Disclosure
This work was supported by grant # R01HS018723 from the Agency for Healthcare Research and Quality. Dr. Lagu is also supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number K01HL114745. Dr. Lindenauer is supported by grant K24HL132008 from the National Heart, Lung, and Blood Institute. The funding agency had no role in the data acquisition, analysis, or manuscript preparation for this study. Drs. Haessler and Rothberg had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs. Haessler, Lagu, Lindenauer, Skiest, Zilberberg, Higgins, and Rothberg conceived of the study and analyzed and interpreted the data. Dr. Lindenauer acquired the data. Dr. Pekow and Ms. Priya carried out the statistical analyses. Dr. Haessler drafted the manuscript. All authors critically reviewed the manuscript for accuracy and integrity. All authors certify no potential conflicts of interest. Preliminary results from this study were presented in oral and poster format at IDWeek in 2012 and 2013.
1. Kochanek KD, Murphy SL, Xu JQ, Tejada-Vera B. Deaths: Final data for 2014. National vital statistics reports; vol 65 no 4. Hyattsville, MD: National Center for Health Statistics. 2016. PubMed
2. American Thoracic Society, Infectious Diseases Society of America. Guidelines for the Management of Adults with Hospital-acquired, Ventilator-associated, and Healthcare-associated Pneumonia. Am J Respir Crit Care Med. 2005;171(4):388-416. PubMed
3. Zilberberg MD, Shorr A. Healthcare-associated pneumonia: the state of the evidence to date. Curr Opin Pulm Med. 2011;17(3):142-147. PubMed
4. Kollef MK, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and Outcomes of Health-care-associated pneumonia. Chest. 2005;128(6):3854-3862. PubMed
5. Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):575-582. PubMed
6. Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010;303(23):2359-2367. PubMed
7. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. PubMed
8. Centers for Medicare & Medicaid Services. Frequently asked questions (FAQs): Implementation and maintenance of CMS mortality measures for AMI & HF. 2007. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/downloads/HospitalMortalityAboutAMI_HF.pdf. Accessed November 1, 2016.
9. Normand SL, Shahian DM. Statistical and Clinical Aspects of Hospital Outcomes Profiling. Stat Sci. 2007;22(2):206-226.
10. Rothberg MB, Pekow PS, Priya A, et al. Using highly detailed administrative data to predict pneumonia mortality. PLoS One. 2014;9(1):e87382. PubMed
11. Jones BE, Jones MM, Huttner B, et al. Trends in antibiotic use and nosocomial pathogens in hospitalized veterans with pneumonia at 128 medical centers, 2006-2010. Clin Infect Dis. 2015;61(9):1403-1410. PubMed
12. Attridge RT, Frei CR, Restrepo MI, et al. Guideline-concordant therapy and outcomes in healthcare-associated pneumonia. Eur Respir J. 2011;38(4):878-887. PubMed
13. Rothberg MB, Zilberberg MD, Pekow PS, et al. Association of Guideline-based Antimicrobial Therapy and Outcomes in Healthcare-Associated Pneumonia. J Antimicrob Chemother. 2015;70(5):1573-1579. PubMed
14. Kett DH, Cano E, Quartin AA, et al. Improving Medicine through Pathway Assessment of Critical Therapy of Hospital-Acquired Pneumonia (IMPACT-HAP) Investigators. Implementation of guidelines for management of possible multidrug-resistant pneumonia in intensive care: an observational, multicentre cohort study. Lancet Infect Dis. 2011;11(3):181-189. PubMed
15. El-Solh AA, Alhajhusain A, Saliba RG, Drinka P. Physicians’ Attitudes Toward Guidelines for the Treatment of Hospitalized Nursing-Home -Acquired Pneumonia. J Am Med Dir Assoc. 2011;12(4):270-276. PubMed
16. Tunis S, Hayward R, Wilson M, et al. Internists’ Attitudes about Clinical Practice Guidelines. Ann Intern Med. 1994;120(11):956-963. PubMed
17. Seymann GB, Di Francesco L, Sharpe B, et al. The HCAP Gap: Differences between Self-Reported Practice Patterns and Published Guidelines for Health Care-Associated Pneumonia. Clin Infect Dis. 2009;49(12):1868-1874. PubMed
18. Chalmers JD, Rother C, Salih W, Ewig S. Healthcare associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis. 2014;58(3):330-339. PubMed
19. Shorr A, Zilberberg MD, Reichley R, et al. Validation of a Clinical Score for Assessing the Risk of Resistant Pathogens in Patients with Pneumonia Presenting to the Emergency Department. Clin Infect Dis. 2012;54(2):193-198. PubMed
20. Aliberti S, Pasquale MD, Zanaboni AM, et al. Stratifying Risk Factors for Multidrug-Resistant Pathogens in Hospitalized Patients Coming from the Community with Pneumonia. Clin Infect Dis. 2012;54(4):470-478. PubMed
21. Schreiber MP, Chan CM, Shorr AF. Resistant Pathogens in Nonnosocomial Pneumonia and Respiratory Failure: Is it Time to Refine the Definition of Health-care-Associated Pneumonia? Chest. 2010;137(6):1283-1288. PubMed
22. Madaras-Kelly KJ, Remington RE, Fan VS, Sloan KL. Predicting antibiotic resistance to community-acquired pneumonia antibiotics in culture-positive patients with healthcare-associated pneumonia. J Hosp Med. 2012;7(3):195-202. PubMed
23. Shindo Y, Ito R, Kobayashi D, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188(8):985-995. PubMed
24. Metersky ML, Frei CR, Mortensen EM. Predictors of Pseudomonas and methicillin-resistant Staphylococcus aureus in hospitalized patients with healthcare-associated pneumonia. Respirology. 2016;21(1):157-163. PubMed
25. Webb BJ, Dascomb K, Stenehjem E, Dean N. Predicting risk of drug-resistant organisms in pneumonia: moving beyond the HCAP model. Respir Med. 2015;109(1):1-10. PubMed
1. Kochanek KD, Murphy SL, Xu JQ, Tejada-Vera B. Deaths: Final data for 2014. National vital statistics reports; vol 65 no 4. Hyattsville, MD: National Center for Health Statistics. 2016. PubMed
2. American Thoracic Society, Infectious Diseases Society of America. Guidelines for the Management of Adults with Hospital-acquired, Ventilator-associated, and Healthcare-associated Pneumonia. Am J Respir Crit Care Med. 2005;171(4):388-416. PubMed
3. Zilberberg MD, Shorr A. Healthcare-associated pneumonia: the state of the evidence to date. Curr Opin Pulm Med. 2011;17(3):142-147. PubMed
4. Kollef MK, Shorr A, Tabak YP, Gupta V, Liu LZ, Johannes RS. Epidemiology and Outcomes of Health-care-associated pneumonia. Chest. 2005;128(6):3854-3862. PubMed
5. Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5):575-582. PubMed
6. Lindenauer PK, Pekow PS, Lahti MC, Lee Y, Benjamin EM, Rothberg MB. Association of corticosteroid dose and route of administration with risk of treatment failure in acute exacerbation of chronic obstructive pulmonary disease. JAMA. 2010;303(23):2359-2367. PubMed
7. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8-27. PubMed
8. Centers for Medicare & Medicaid Services. Frequently asked questions (FAQs): Implementation and maintenance of CMS mortality measures for AMI & HF. 2007. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/downloads/HospitalMortalityAboutAMI_HF.pdf. Accessed November 1, 2016.
9. Normand SL, Shahian DM. Statistical and Clinical Aspects of Hospital Outcomes Profiling. Stat Sci. 2007;22(2):206-226.
10. Rothberg MB, Pekow PS, Priya A, et al. Using highly detailed administrative data to predict pneumonia mortality. PLoS One. 2014;9(1):e87382. PubMed
11. Jones BE, Jones MM, Huttner B, et al. Trends in antibiotic use and nosocomial pathogens in hospitalized veterans with pneumonia at 128 medical centers, 2006-2010. Clin Infect Dis. 2015;61(9):1403-1410. PubMed
12. Attridge RT, Frei CR, Restrepo MI, et al. Guideline-concordant therapy and outcomes in healthcare-associated pneumonia. Eur Respir J. 2011;38(4):878-887. PubMed
13. Rothberg MB, Zilberberg MD, Pekow PS, et al. Association of Guideline-based Antimicrobial Therapy and Outcomes in Healthcare-Associated Pneumonia. J Antimicrob Chemother. 2015;70(5):1573-1579. PubMed
14. Kett DH, Cano E, Quartin AA, et al. Improving Medicine through Pathway Assessment of Critical Therapy of Hospital-Acquired Pneumonia (IMPACT-HAP) Investigators. Implementation of guidelines for management of possible multidrug-resistant pneumonia in intensive care: an observational, multicentre cohort study. Lancet Infect Dis. 2011;11(3):181-189. PubMed
15. El-Solh AA, Alhajhusain A, Saliba RG, Drinka P. Physicians’ Attitudes Toward Guidelines for the Treatment of Hospitalized Nursing-Home -Acquired Pneumonia. J Am Med Dir Assoc. 2011;12(4):270-276. PubMed
16. Tunis S, Hayward R, Wilson M, et al. Internists’ Attitudes about Clinical Practice Guidelines. Ann Intern Med. 1994;120(11):956-963. PubMed
17. Seymann GB, Di Francesco L, Sharpe B, et al. The HCAP Gap: Differences between Self-Reported Practice Patterns and Published Guidelines for Health Care-Associated Pneumonia. Clin Infect Dis. 2009;49(12):1868-1874. PubMed
18. Chalmers JD, Rother C, Salih W, Ewig S. Healthcare associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis. 2014;58(3):330-339. PubMed
19. Shorr A, Zilberberg MD, Reichley R, et al. Validation of a Clinical Score for Assessing the Risk of Resistant Pathogens in Patients with Pneumonia Presenting to the Emergency Department. Clin Infect Dis. 2012;54(2):193-198. PubMed
20. Aliberti S, Pasquale MD, Zanaboni AM, et al. Stratifying Risk Factors for Multidrug-Resistant Pathogens in Hospitalized Patients Coming from the Community with Pneumonia. Clin Infect Dis. 2012;54(4):470-478. PubMed
21. Schreiber MP, Chan CM, Shorr AF. Resistant Pathogens in Nonnosocomial Pneumonia and Respiratory Failure: Is it Time to Refine the Definition of Health-care-Associated Pneumonia? Chest. 2010;137(6):1283-1288. PubMed
22. Madaras-Kelly KJ, Remington RE, Fan VS, Sloan KL. Predicting antibiotic resistance to community-acquired pneumonia antibiotics in culture-positive patients with healthcare-associated pneumonia. J Hosp Med. 2012;7(3):195-202. PubMed
23. Shindo Y, Ito R, Kobayashi D, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188(8):985-995. PubMed
24. Metersky ML, Frei CR, Mortensen EM. Predictors of Pseudomonas and methicillin-resistant Staphylococcus aureus in hospitalized patients with healthcare-associated pneumonia. Respirology. 2016;21(1):157-163. PubMed
25. Webb BJ, Dascomb K, Stenehjem E, Dean N. Predicting risk of drug-resistant organisms in pneumonia: moving beyond the HCAP model. Respir Med. 2015;109(1):1-10. PubMed
© 2017 Society of Hospital Medicine
Macrolides and Quinolones for AECOPD
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are responsible for more than 600,000 hospitalizations annually, resulting in direct costs of over $20 billion.1 Bacterial infections appear responsible for 50% of such exacerbations,25 and current COPD guidelines recommend treatment with antibiotics for patients with severe exacerbations or a change in sputum.1,69 These recommendations are based on a number of small randomized trials, most of which were conducted more than 20 years ago using narrow spectrum antibiotics that are no longer commonly used.10 Only 4 studies, totaling 321 subjects, included hospitalized patients, and most studies excluded patients who required steroids. Because no clinical trials have compared different antibiotic regimens for AECOPD, existing guidelines offer a range of treatment options, including amoxicillin‐clavulonate, macrolides, quinolones, cephalosporins, aminopenicillins, and tetracyclines.
Among hospitalized patients, macrolides and quinolones appear to be the most frequently prescribed antibiotics.11 Both are available in oral formulations, have excellent bioavailability, and are administered once daily. In addition to their antimicrobial activity, macrolides are believed to have antiinflammatory effects, which could be especially advantageous in AECOPD.1214 In trials of chronic bronchitis, however, fluoroquinolones have been shown to reduce the risk of recurrent exacerbation when compared to macrolides.15 The wide variation that has been observed in antibiotic selection for patients hospitalized for AECOPD suggests a high degree of uncertainty among clinicians about the benefits of different treatment options.11 Given the limited evidence from randomized trials, we sought to evaluate the comparative effectiveness of macrolides and quinolones among a large, representative sample of patients hospitalized with AECOPD.
Subjects and Methods
Setting and Subjects
We conducted a retrospective cohort study of all patients hospitalized between January 1 and December 31, 2001 for AECOPD at any 1 of 375 acute care facilities in the United States that participated in Premier's Perspective, a voluntary, fee‐supported database developed for measuring quality and healthcare utilization. Participating hospitals represent all geographical regions, and are primarily small‐sized to medium‐sized nonteaching hospitals located mostly in urban areas. In addition to the information contained in the standard hospital discharge file (Uniform Billing 92) such as patient age, International Classification of Disease, 9th Edition, Clinical Modification (ICD‐9‐CM) codes, the Perspective database includes a date‐stamped log of all billed items, including diagnostic tests, medications, and other treatments, as well as costs, for individual patients. The study was approved by the Institutional Review Board of Baystate Medical Center.
Patients were included if they had a primary diagnosis consistent with AECOPD (ICD‐9 codes 491.21 and 493.22) or a primary diagnosis of respiratory failure (ICD‐9 codes 518.81 and 518.84) paired with secondary diagnosis of AECOPD; they also had to receive at least 2 consecutive days of either a macrolide or a quinolone, started within 48 hours of hospitalization. Patients receiving both antibiotics were excluded, but patients who received additional antibiotics were included. To enhance the specificity of our diagnosis codes, we limited our study to patients age 40 years.16 Because mechanical ventilation initiated after hospital day 2 was an outcome measure, we excluded patients admitted directly to the intensive care unit. We also excluded: those with other bacterial infections, such as pneumonia or cellulitis, who might have another indication for antibiotics; those with a length of stay <2 days, because we could not ascertain whether they received a full course of antibiotics; patients with a secondary diagnosis of pulmonary embolism or pneumothorax; and those whose attending physicians were not internists, family practitioners, hospitalists, pulmonologists, or intensivists. For patients with more than 1 admission during the study period, we included only the first admission.
Data Elements
For each patient, we assessed age, gender, race, marital and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9 secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software (version 3.1), based on the work of Elixhauser et al.17 In addition, to assess disease severity we recorded the presence of chronic pulmonary heart disease, the number of admissions for COPD during the 12 months prior to the index admission, and arterial blood gas testing.18,19 We also identified pharmacy or diagnostic charges for interventions that were recommended in current guidelines (beta‐adrenergic and anticholinergic bronchodilators, steroids, and noninvasive positive‐pressure ventilation); those that were not recommended or were of uncertain benefit (methylxanthine bronchodilators, spirometry/pulmonary function testing, mucolytic medications, chest physiotherapy, and sputum testing); and drugs that might be associated with severe exacerbations or end‐stage COPD (loop diuretics, morphine, and nutritional supplements).1,69 Hospitals were categorized by region (Northeast, South, Midwest, or West), bed size, setting (urban vs. rural), ownership, and teaching status.
Antibiotic Class and Outcome Variables
Our primary predictor variable was the antibiotic initiated during the first 2 hospital days and continued for at least 2 days, regardless of other antibiotics the patient may have received during the course of hospitalization. Because we anticipated low in‐hospital mortality, our primary outcome was a composite measure of treatment failure, defined as initiation of mechanical ventilation after hospital day 2, in‐hospital mortality, or readmission for COPD within 30 days of discharge.20 Secondary outcomes included hospital costs and length of stay, as well as allergic reactions identified by ICD‐9 code, and antibiotic‐associated diarrhea, defined as treatment with either metronidazole or oral vancomycin begun after hospital day 3.
Statistical Analysis
Summary statistics were computed using frequencies and percents for categorical variables; and means, medians, standard deviations, and interquartile ranges for continuous variables. Associations between antibiotic selection and patient and hospital characteristics were assessed using chi‐square tests for categorical variables and z‐tests for continuous variables.
We developed a series of multivariable models to evaluate the impact of initial antibiotic selection on the risk of treatment failure, length of stay, and total cost. In order to account for the effects of within‐hospital correlation, generalized estimating equation (GEE) models with a logit link were used to assess the effect of antibiotic selection on the risk of treatment failure, and identity link models were used for analyses of length of stay and cost. Unadjusted and covariate‐adjusted models for treatment failure were evaluated with and without adjustments for propensity score. A propensity score is the probability that a given patient would receive treatment with a macrolide, derived from a nonparsimonious model in which treatment with a macrolide was considered the outcome. The propensity model included all patient characteristics, other early treatments and tests, comorbidities, hospital and physician characteristics, and selected interaction terms.21 Length of stay and cost were trimmed at 3 standard deviations above the mean, and natural log‐transformed values were modeled due to extreme positive skew. In addition, we carried out matched analyses in which we compared the outcomes of patients who were treated with a macrolide to those with similar propensity scores (ie, with similar likelihood of receiving a macrolide) who received a quinolone.22
Finally, to reduce the threat of residual confounding by indication, which can occur if sicker patients are more likely to receive a particular antibiotic, we developed a grouped treatment model, in which all patients treated at the same hospital were assigned a probability of treatment with a macrolide equal to the overall treatment rate at that hospital.23 This is an adaptation of instrumental variable analysis, a well‐accepted technique in econometrics with growing use in health care.24,25 It attempts to assess whether patients treated at a hospital at which quinolones are used more frequently have better outcomes than patients treated at hospitals at which macrolides are used more frequently, while adjusting for other patient, physician, and hospital variables. It ignores the actual treatment the patient received, and instead substitutes the hospital's rate of macrolide use. By grouping treatment at the hospital level, this method greatly reduces the possibility of residual selection bias, unless hospitals that use a lot of macrolides have patients who differ in a consistent way from hospitals which use mostly quinolones.
All analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC).
Results
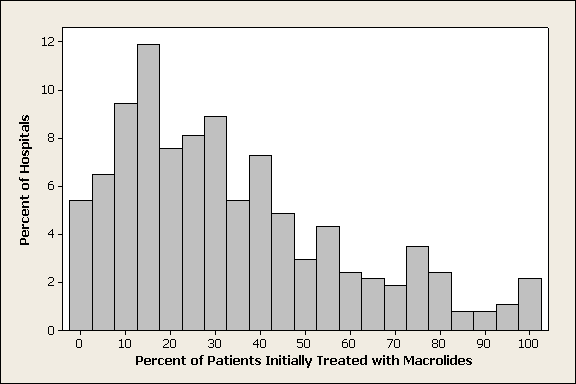
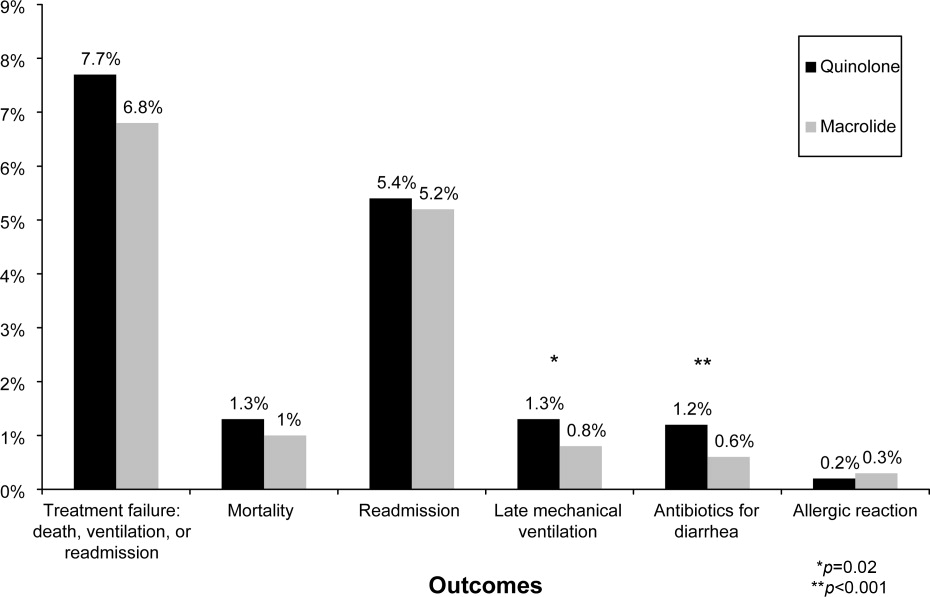
Of 26,248 AECOPD patients treated with antibiotics, 19,608 patients met the inclusion criteria; of these, 6139 (31%) were treated initially with a macrolide; the median age was 70 years; 60% were female; and 78% were white. A total of 86% of patients had a primary diagnosis of obstructive chronic bronchitis with acute exacerbation, and 6% had respiratory failure. The most common comorbidities were hypertension, diabetes, and congestive heart failure. Twenty‐two percent had been admitted at least once in the preceding 12 months. Treatment failure occurred in 7.7% of patients, and 1.3% died in the hospital. Mean length of stay was 4.8 days. Hospital prescribing rates for macrolides varied from 0% to 100%, with a mean of 33% and an interquartile range of 14% to 46% (Supporting Appendix Figure 1).
Compared to patients receiving macrolides, those receiving quinolones were older, more likely to have respiratory failure, to be cared for by a pulmonologist, and to have an admission in the previous year (Table 1). They were also more likely to be treated with bronchodilators, methylxanthines, steroids, diuretics, and noninvasive positive pressure ventilation, and to have an arterial blood gas, but less likely to receive concomitant treatment with a cephalosporin (11% vs. 57%). With the exception of cephalosporin treatment, these differences were small, but due to the large sample were statistically significant. Comorbidities were similar in both groups. Patients in the quinolone group were also more likely to experience treatment failure (8.1% vs. 6.8%), death (1.5% vs. 1.0%), and antibiotic‐associated diarrhea (1.1% vs. 0.5%).
| Complete Cohort | Propensity‐matched Subsample | |||||
|---|---|---|---|---|---|---|
| Characteristic | Quinolone (n = 13469) | Macrolide (n = 6139) | P Value | Quinolone (n = 5610) | Macrolide (n = 5610) | P Value |
| ||||||
| Antibiotics received during hospitalization* [n (%)] | ||||||
| Macrolide | 264 (2) | 6139 (100) | 119 (2) | 5610 (100) | ||
| Quinolone | 13469 (100) | 459 (8) | 5610 (100) | 424 (8) | ||
| Cephalosporin | 1696 (13) | 3579 (59) | <0.001 | 726 (13) | 3305 (59) | <0.001 |
| Tetracycline | 231 (2) | 75 (2) | 0.01 | 101 (2) | 73 (2) | 0.06 |
| Other antibiotics | 397 (3) | 220 (4) | 0.02 | 166 (3) | 193 (3) | 0.03 |
| Age (years) (mean [SD]) | 69.1 (11.4) | 68.2 (11.8) | <0.001 | 68.6 (11.7) | 68.5 (11.7) | 0.58 |
| Male sex (n [%]) | 5447 (40) | 2440 (40) | 0.36 | 2207 (39) | 2196 (39) | 0.85 |
| Race/ethnic group (n [%]) | <0.001 | 0.44 | ||||
| White | 10454 (78) | 4758 (78) | 4359 (78) | 4368 (78) | ||
| Black | 1060 (8) | 540 (9) | 470 (8) | 455 (8) | ||
| Hispanic | 463 (3) | 144 (2) | 157 (3) | 134 (2) | ||
| Other | 1492 (11) | 697 (11) | 624 (11) | 653 (12) | ||
| Primary diagnosis (n [%]) | <0.001 | 0.78 | ||||
| Obstructive chronic bronchitis with acute exacerbation | 11650 (87) | 5298 (86) | 4884 (87) | 4860 (87) | ||
| Chronic obstructive asthma/asthma with COPD | 908 (7) | 569 (9) | 466 (8) | 486 (9) | ||
| Respiratory failure | 911 (7) | 272 (4) | 260 (5) | 264 (5) | ||
| Admissions in the prior year (n [%]) | <0.001 | 0.84 | ||||
| 0 | 9846 (73) | 4654 (76) | 4249 (76) | 4231 (75) | ||
| 1 | 1918 (14) | 816 (13) | 747 (13) | 750 (13) | ||
| 2+ | 1085 (8) | 445 (7) | 397 (7) | 420 (8) | ||
| Missing | 620 (5) | 224 (4) | 217 (4) | 209 (4) | ||
| Physician specialty (n [%]) | <0.001 | 0.84 | ||||
| Internal medicine/hospitalist | 7069 (53) | 3321 (54) | 3032 (54) | 3072 (55) | ||
| Family/general medicine | 3569 (27) | 2074 (34) | 1824 (33) | 1812 (32) | ||
| Pulmonologist | 2776 (21) | 727 (12) | 738 (13) | 711 (13) | ||
| Critical care/emntensivist | 55 (0) | 17 (0) | 16 (0) | 15 (0) | ||
| Tests on hospital day 1 or 2 (n [%]) | ||||||
| Arterial blood gas | 8084 (60) | 3377 (55) | <0.001 | 3195 (57) | 3129 (56) | 0.22 |
| Sputum test | 1741 (13) | 766 (13) | 0.39 | 20 (0) | 16 (0) | 0.62 |
| Medications/therapies on hospital day 1 or 2 (n [%]) | ||||||
| Short‐acting bronchodilators | 7555 (56) | 3242 (53) | <0.001 | 2969 (53) | 2820 (50) | 0.005 |
| Long‐acting beta‐2 agonists | 2068 (15) | 748 (12) | <0.001 | 704 (13) | 719 (13) | 0.69 |
| Methylxanthine bronchodilators | 3051 (23) | 1149 (19) | <0.001 | 1102 (20) | 1093 (20) | 0.85 |
| Steroids | 0.04 | 0.68 | ||||
| Intravenous | 11148 (83) | 4989 (81) | 4547 (81) | 4581 (82) | ||
| Oral | 772 (6) | 376 (6) | 334 (6) | 330 (6) | ||
| Severity indicators (n [%]) | ||||||
| Chronic pulmonary heart disease | 890 (7) | 401 (7) | 0.85 | 337 (6) | 368 (7) | 0.24 |
| Sleep apnea | 586 (4) | 234 (4) | 0.08 | 211 (4) | 218 (4) | 0.77 |
| Noninvasive positive pressure ventilation | 391 (3) | 128 (2) | <0.001 | 128 (2) | 114 (2) | 0.40 |
| Loop diuretics | 4838 (36) | 1971 (32) | <0.001 | 1884 (34) | 1862 (33) | 0.67 |
| Hospital characteristics (n [%]) | ||||||
| Staffed beds | <0.001 | 0.71 | ||||
| 6200 | 3483 (26) | 1688 (28) | 1610 (29) | 1586 (28) | ||
| 201300 | 3132 (23) | 1198 (20) | 1174 (21) | 1154 (21) | ||
| 301500 | 4265 (32) | 2047 (33) | 1809 (32) | 1867 (33) | ||
| 500+ | 2589 (19) | 1206 (20) | 1017 (18) | 1003 (18) | ||
| Hospital region (n [%]) | <0.001 | 0.65 | ||||
| South | 8562 (64) | 3270 (53) | 3212 (57) | 3160 (56) | ||
| Midwest | 2602 (19) | 1444 (24) | 1170 (21) | 1216 (22) | ||
| Northeast | 1163 (9) | 871 (14) | 687 (12) | 704 (13) | ||
| West | 1142 (9) | 554 (9) | 541 (10) | 530 (9) | ||
| Teaching hospital | <0.001 | 0.63 | ||||
| No | 12090 (90) | 5037 (82) | 4896 (87) | 4878 (87) | ||
| Yes | 1379 (10) | 1102 (18) | 714 (13) | 732 (13) | ||
| Comorbidities (n [%]) | ||||||
| Congestive heart failure | 2673 (20) | 1147 (19) | 0.06 | 1081 (19) | 1060 (19) | 0.63 |
| Metastatic cancer | 134 (1) | 27 (0) | <0.001 | 34 (1) | 38 (1) | 0.72 |
| Depression | 1419 (11) | 669 (11) | 0.45 | 598 (11) | 603 (11) | 0.90 |
| Deficiency anemias | 1155 (9) | 476 (8) | 0.05 | 426 (8) | 432 (8) | 0.86 |
| Solid tumor without metastasis | 1487 (11) | 586 (10) | 0.002 | 550 (10) | 552 (10) | 0.97 |
| Hypothyroidism | 1267 (9) | 527 (9) | 0.07 | 481 (9) | 482 (9) | 1.00 |
| Peripheral vascular disease | 821 (6) | 312 (5) | 0.005 | 287 (5) | 288 (5) | 1.00 |
| Paralysis | 165 (1) | 46 (1) | 0.003 | 49 (1) | 51 (1) | 0.92 |
| Obesity | 957 (7) | 435 (7) | 0.98 | 386 (7) | 398 (7) | 0.68 |
| Hypertension | 5793 (43) | 2688 (44) | 0.31 | 2474 (44) | 2468 (44) | 0.92 |
| Diabetes | 0.04 | 0.45 | ||||
| Without chronic complications | 2630 (20) | 1127 (18) | 1057 (19) | 1066 (19) | ||
| With chronic complications | 298 (2) | 116 (2) | 115 (2) | 97 (2) | ||
In the unadjusted analysis, compared to patients receiving quinolones, those treated with macrolides were less likely to experience treatment failure (OR, 0.83; 95% CI, 0.740.94) (Table 2). Adjusting for all patient, hospital, and physician covariates, including the propensity for treatment with macrolides, increased the OR to 0.89 and the results were no longer significant (95% CI, 0.781.01). Propensity matching successfully balanced all measured covariates except for the use of short‐acting bronchodilators and additional antibiotics (Table 1). In the propensity‐matched sample (Figure 1), quinolone‐treated patients were more likely to experience antibiotic‐associated diarrhea (1.2% vs. 0.6%; P = 0.0003) and late mechanical ventilation (1.3% vs. 0.8%; P = 0.02). There were no differences in adjusted cost or length of stay between the 2 groups. The results of the grouped treatment analysis, substituting the hospital's specific rate of macrolide use in place of the actual treatment that each patient received suggested that the 2 antibiotics were associated with similar rates of treatment failure. The OR for a 100% hospital rate of macrolide treatment vs. a 0% rate was 1.01 (95% CI, 0.751.35).
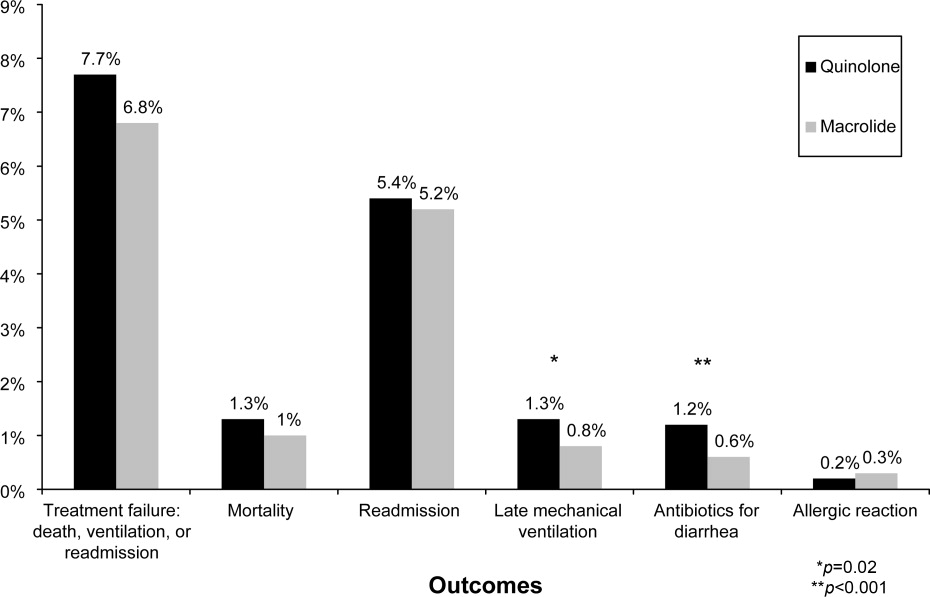
| Treatment Failure | Cost | LOS | ||||
|---|---|---|---|---|---|---|
| Models | OR | 95% CI | Ratio | 95% CI | Ratio | 95% CI |
| ||||||
| Unadjusted | 0.83 | 0.730.93 | 0.98 | 0.971.00 | 0.96 | 0.950.98 |
| Adjusted for propensity score only* | 0.89 | 0.791.01 | 1.00 | 0.981.01 | 0.98 | 0.971.00 |
| Adjusted for covariates | 0.87 | 0.770.99 | 1.00 | 0.991.02 | 0.99 | 0.971.00 |
| Adjusted for covariates and propensity score | 0.89 | 0.781.01 | 1.00 | 0.991.02 | 0.98 | 0.971.00 |
| Matched sample, unadjusted | 0.87 | 0.751.00 | 0.99 | 0.981.01 | 0.99 | 0.971.01 |
| Matched sample, adjusted for unbalanced variables | 0.87 | 0.751.01 | 1.00 | 0.981.02 | 0.99 | 0.971.01 |
| Grouped treatment model, unadjusted | 0.90 | 0.681.19 | 0.97 | 0.891.06 | 0.92 | 0.870.96 |
| Group treatment model, adjusted for covariates∥ | 1.01 | 0.751.35 | 0.96 | 0.881.05 | 0.96 | 0.911.00 |
Discussion
In this large observational study conducted at 375 hospitals, we took advantage of a natural experiment in which antibiotic prescribing patterns varied widely across hospitals to compare the effectiveness of 2 common antibiotic regimens for AECOPD. Treatment with macrolides and quinolones were associated with a similar risk of treatment failure, costs, and length of stay; however, patients treated with macrolides were less likely to experience late mechanical ventilation or treatment for antibiotic‐associated diarrhea.
Despite broad consensus in COPD guidelines that patients with severe acute exacerbations should receive antibiotics, there is little agreement about the preferred empiric agent. Controversy exists regarding antibiotics' comparative effectiveness, and even over which pathogens cause COPD exacerbations. Given the frequency of hospitalization for AECOPD, understanding the comparative effectiveness of treatments in this setting could have important implications for health outcomes and costs. Unfortunately, most antibiotic studies in AECOPD were conducted >20 years ago, using antibiotics that rarely appeared in our sample.26 Consequently, clinical practice guidelines offer conflicting recommendations. For example, the National Institute for Clinical Excellence recommends empirical treatment with an aminopenicillin, a macrolide, or a tetracycline,8 while the American Thoracic Society recommends amoxicillin‐clavulonate or a fluoroquinolone.9
As might be expected in light of so much uncertainty, we found wide variation in prescribing patterns across hospitals. Overall, approximately one‐third of patients received a macrolide and two‐thirds a quinolone. Both regimens provide adequate coverage of H. influenza, S. pneumoniae, and M. catarrhalis, and conform to at least 1 COPD guideline. Nevertheless, patients receiving macrolides often received a cephalosporin as well; this pattern of treatment suggests that antibiotic selection is likely to have been influenced more by guidelines for the treatment of community‐acquired pneumonia than COPD.
Previous studies comparing antibiotic effectiveness suffer from shortcomings that limit their application to patients hospitalized with AECOPD. First, they enrolled patients with chronic bronchitis, and included patients without obstructive lung disease, and most studies included patients as young as 18 years old. Second, many either did not include treatment with steroids or excluded patients receiving more than 10 mg of prednisone daily. Third, almost all enrolled only ambulatory patients.
While there are no studies comparing quinolones and macrolides in patients hospitalized for AECOPD, a meta‐analysis comparing quinolones, macrolides, and amoxicillin‐clavulonate identified 19 trials of ambulatory patients with chronic bronchitis. That study found that all 3 drugs had similar efficacy initially, but that quinolones resulted in the fewest relapses over a 26‐week period.27 Macrolides and quinolones had similar rates of adverse effects. In contrast, we did not find a difference in treatment failure, cost, or length of stay, but did find a higher rate of diarrhea associated with quinolones. Others have also documented an association between fluoroquinolones and C. difficile diarrhea.2830 This trend, first noted in 2001, is of particular concern because the fluoroquinolone‐resistant strains appear to be hypervirulent and have been associated with nosocomial epidemics.3134
Our study has several limitations. First, its observational design leaves open the possibility of selection bias. For this reason we analyzed our data in several ways, including using a grouped treatment approach, an adaptation of the instrumental variable technique, and accepted only those differences which were consistent across all models. Second, our study used claims data, and therefore we could not directly adjust for physiological measures of severity. However, the highly detailed nature of the data allowed us to adjust for numerous tests and treatments that reflected the clinician's assessment of the patient's severity, as well as the number of prior COPD admissions. Third, we cannot exclude the possibility that some patients may have had concurrent pneumonia without an ICD‐9 code. We think that the number would be small because reimbursement for pneumonia is generally higher than for COPD, so hospitals have an incentive to code pneumonia as the principal diagnosis when present. Finally, we compared initial antibiotics only. More than one‐quarter of our patients received an additional antibiotic before discharge. In particular, patients receiving macrolides were often prescribed a concomitant cephalosporin. We do not know to what extent these additional antibiotics may have affected the outcomes.
Despite the large number of patients hospitalized annually for AECOPD, there are no randomized trials comparing different antibiotics in this population. Studies comparing antibiotics in chronic bronchitis can offer little guidance, since they have primarily focused on proving equivalence between existing antibiotics and newer, more expensive formulations.35 Because many of the patients enrolled in such trials do not benefit from antibiotics at all, either because they do not have COPD or because their exacerbation is not caused by bacteria, it is relatively easy to prove equivalence. Given that AECOPD is one of the leading causes of hospitalization in the United States, large, randomized trials comparing the effectiveness of different antibiotics should be a high priority. In the meantime, macrolides (often given together with cephalosporins) and quinolones appear to be equally effective initial antibiotic choices; considering antibiotic‐associated diarrhea, macrolides appear to be the safer of the 2.
- ,,.Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2001;134:595–599.
- ,,, et al.Infectious etiologies in acute exacerbation of COPD.Diagn Microbiol Infect Dis.2001;40:95–102.
- ,.Bacterial infections in patients requiring admission for an acute exacerbation of COPD; a 1‐year prospective study.Respir Med.2003;97:770–777.
- ,,, et al.Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease.Arch Intern Med.2005;165:891–897.
- ,.Infection in the pathogenesis and course of chronic obstructive pulmonary disease.N Engl J Med.2008;359:2355–2365.
- ,,, et al.Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary.Am J Respir Crit Care Med.2007;176:532–555.
- ,,, et al.Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2008 update—highlights for primary care.Can Respir J.2008;15(suppl A):1A–8A.
- Chronic obstructive pulmonary disease.National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care.Thorax.2004;59(suppl 1):1–232.
- ,,, et al.Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper.Eur Respir J.2004;23:932–946.
- ,,,,.Antibiotics for exacerbations of chronic obstructive pulmonary disease.Cochrane Database Syst Rev.2006:CD004403.
- ,,,,,.Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2006;144:894–903.
- ,,, et al.Modulation of neutrophil and inflammation markers in chronic obstructive pulmonary disease by short‐term azithromycin treatment.Eur J Pharmacol.2005;517:132–143.
- ,,, et al.Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects.Eur J Pharmacol.2002;450:277–289.
- ,,,,,.The effect of clarithromycin on inflammatory markers in chronic obstructive pulmonary disease: preliminary data.Ann Pharmacother.2004;38:1400–1405.
- ,,,.A comparison of gemifloxacin and clarithromycin in acute exacerbations of chronic bronchitis and long‐term clinical outcomes.Clin Ther.2002;24:639–652.
- ,,,.In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease.Arch Intern Med.2003;163:1180–1186.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36:8–27.
- ,,, et al.Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments).Am J Respir Crit Care Med.1996;154:959–967.
- ,,.Mortality and mortality‐related factors after hospitalization for acute exacerbation of COPD.Chest.2003;124:459–467.
- ,,, et al.Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease.N Engl J Med.1999;340:1941–1947.
- .Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group.Stat Med.1998;17:2265–2281.
- .Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. Proceedings of the Twenty‐Sixth Annual SAS Users Group International Conference. Cary, NC: SAS Institute;2001,214–216.
- ,,,.Modeling treatment effects on binary outcomes with grouped‐treatment variables and individual covariates.Am J Epidemiol.2002;156:753–760.
- ,,.Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. [see Comment].JAMA.1994;272:859–866.
- ,,,,,.Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods.JAMA.2007;297:278–285.
- ,,,.Antibiotics in chronic obstructive pulmonary disease exacerbations. A meta‐analysis.JAMA.1995;273:957–960.
- ,,,,.Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta‐analysis.Eur Respir J.2007;29:1127–1137.
- ,,,,.Antimicrobial‐associated risk factors for Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S19–S31.
- ,,, et al.Short‐term and long‐term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis.Chest.2004;125:953–964.
- ,,,,.Oral gemifloxacin once daily for 5 days compared with sequential therapy with i.v. ceftriaxone/oral cefuroxime (maximum of 10 days) in the treatment of hospitalized patients with acute exacerbations of chronic bronchitis.Respir Med.2003;97:242–249.
- ,,, et al.A large outbreak of Clostridium difficile‐associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use.Infect Control Hosp Epidemiol.2005;26:273–280.
- ,,, et al.An epidemic, toxin gene‐variant strain of Clostridium difficile.N Engl J Med.2005;353:2433–2441.
- ,,, et al.Outbreak of Clostridium difficile infection in a long‐term care facility: association with gatifloxacin use.Clin Infect Dis.2004;38:640–645.
- ,,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,.No more equivalence trials for antibiotics in exacerbations of COPD, please.Chest.2004;125:811–813.
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are responsible for more than 600,000 hospitalizations annually, resulting in direct costs of over $20 billion.1 Bacterial infections appear responsible for 50% of such exacerbations,25 and current COPD guidelines recommend treatment with antibiotics for patients with severe exacerbations or a change in sputum.1,69 These recommendations are based on a number of small randomized trials, most of which were conducted more than 20 years ago using narrow spectrum antibiotics that are no longer commonly used.10 Only 4 studies, totaling 321 subjects, included hospitalized patients, and most studies excluded patients who required steroids. Because no clinical trials have compared different antibiotic regimens for AECOPD, existing guidelines offer a range of treatment options, including amoxicillin‐clavulonate, macrolides, quinolones, cephalosporins, aminopenicillins, and tetracyclines.
Among hospitalized patients, macrolides and quinolones appear to be the most frequently prescribed antibiotics.11 Both are available in oral formulations, have excellent bioavailability, and are administered once daily. In addition to their antimicrobial activity, macrolides are believed to have antiinflammatory effects, which could be especially advantageous in AECOPD.1214 In trials of chronic bronchitis, however, fluoroquinolones have been shown to reduce the risk of recurrent exacerbation when compared to macrolides.15 The wide variation that has been observed in antibiotic selection for patients hospitalized for AECOPD suggests a high degree of uncertainty among clinicians about the benefits of different treatment options.11 Given the limited evidence from randomized trials, we sought to evaluate the comparative effectiveness of macrolides and quinolones among a large, representative sample of patients hospitalized with AECOPD.
Subjects and Methods
Setting and Subjects
We conducted a retrospective cohort study of all patients hospitalized between January 1 and December 31, 2001 for AECOPD at any 1 of 375 acute care facilities in the United States that participated in Premier's Perspective, a voluntary, fee‐supported database developed for measuring quality and healthcare utilization. Participating hospitals represent all geographical regions, and are primarily small‐sized to medium‐sized nonteaching hospitals located mostly in urban areas. In addition to the information contained in the standard hospital discharge file (Uniform Billing 92) such as patient age, International Classification of Disease, 9th Edition, Clinical Modification (ICD‐9‐CM) codes, the Perspective database includes a date‐stamped log of all billed items, including diagnostic tests, medications, and other treatments, as well as costs, for individual patients. The study was approved by the Institutional Review Board of Baystate Medical Center.
Patients were included if they had a primary diagnosis consistent with AECOPD (ICD‐9 codes 491.21 and 493.22) or a primary diagnosis of respiratory failure (ICD‐9 codes 518.81 and 518.84) paired with secondary diagnosis of AECOPD; they also had to receive at least 2 consecutive days of either a macrolide or a quinolone, started within 48 hours of hospitalization. Patients receiving both antibiotics were excluded, but patients who received additional antibiotics were included. To enhance the specificity of our diagnosis codes, we limited our study to patients age 40 years.16 Because mechanical ventilation initiated after hospital day 2 was an outcome measure, we excluded patients admitted directly to the intensive care unit. We also excluded: those with other bacterial infections, such as pneumonia or cellulitis, who might have another indication for antibiotics; those with a length of stay <2 days, because we could not ascertain whether they received a full course of antibiotics; patients with a secondary diagnosis of pulmonary embolism or pneumothorax; and those whose attending physicians were not internists, family practitioners, hospitalists, pulmonologists, or intensivists. For patients with more than 1 admission during the study period, we included only the first admission.
Data Elements
For each patient, we assessed age, gender, race, marital and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9 secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software (version 3.1), based on the work of Elixhauser et al.17 In addition, to assess disease severity we recorded the presence of chronic pulmonary heart disease, the number of admissions for COPD during the 12 months prior to the index admission, and arterial blood gas testing.18,19 We also identified pharmacy or diagnostic charges for interventions that were recommended in current guidelines (beta‐adrenergic and anticholinergic bronchodilators, steroids, and noninvasive positive‐pressure ventilation); those that were not recommended or were of uncertain benefit (methylxanthine bronchodilators, spirometry/pulmonary function testing, mucolytic medications, chest physiotherapy, and sputum testing); and drugs that might be associated with severe exacerbations or end‐stage COPD (loop diuretics, morphine, and nutritional supplements).1,69 Hospitals were categorized by region (Northeast, South, Midwest, or West), bed size, setting (urban vs. rural), ownership, and teaching status.
Antibiotic Class and Outcome Variables
Our primary predictor variable was the antibiotic initiated during the first 2 hospital days and continued for at least 2 days, regardless of other antibiotics the patient may have received during the course of hospitalization. Because we anticipated low in‐hospital mortality, our primary outcome was a composite measure of treatment failure, defined as initiation of mechanical ventilation after hospital day 2, in‐hospital mortality, or readmission for COPD within 30 days of discharge.20 Secondary outcomes included hospital costs and length of stay, as well as allergic reactions identified by ICD‐9 code, and antibiotic‐associated diarrhea, defined as treatment with either metronidazole or oral vancomycin begun after hospital day 3.
Statistical Analysis
Summary statistics were computed using frequencies and percents for categorical variables; and means, medians, standard deviations, and interquartile ranges for continuous variables. Associations between antibiotic selection and patient and hospital characteristics were assessed using chi‐square tests for categorical variables and z‐tests for continuous variables.
We developed a series of multivariable models to evaluate the impact of initial antibiotic selection on the risk of treatment failure, length of stay, and total cost. In order to account for the effects of within‐hospital correlation, generalized estimating equation (GEE) models with a logit link were used to assess the effect of antibiotic selection on the risk of treatment failure, and identity link models were used for analyses of length of stay and cost. Unadjusted and covariate‐adjusted models for treatment failure were evaluated with and without adjustments for propensity score. A propensity score is the probability that a given patient would receive treatment with a macrolide, derived from a nonparsimonious model in which treatment with a macrolide was considered the outcome. The propensity model included all patient characteristics, other early treatments and tests, comorbidities, hospital and physician characteristics, and selected interaction terms.21 Length of stay and cost were trimmed at 3 standard deviations above the mean, and natural log‐transformed values were modeled due to extreme positive skew. In addition, we carried out matched analyses in which we compared the outcomes of patients who were treated with a macrolide to those with similar propensity scores (ie, with similar likelihood of receiving a macrolide) who received a quinolone.22
Finally, to reduce the threat of residual confounding by indication, which can occur if sicker patients are more likely to receive a particular antibiotic, we developed a grouped treatment model, in which all patients treated at the same hospital were assigned a probability of treatment with a macrolide equal to the overall treatment rate at that hospital.23 This is an adaptation of instrumental variable analysis, a well‐accepted technique in econometrics with growing use in health care.24,25 It attempts to assess whether patients treated at a hospital at which quinolones are used more frequently have better outcomes than patients treated at hospitals at which macrolides are used more frequently, while adjusting for other patient, physician, and hospital variables. It ignores the actual treatment the patient received, and instead substitutes the hospital's rate of macrolide use. By grouping treatment at the hospital level, this method greatly reduces the possibility of residual selection bias, unless hospitals that use a lot of macrolides have patients who differ in a consistent way from hospitals which use mostly quinolones.
All analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Of 26,248 AECOPD patients treated with antibiotics, 19,608 patients met the inclusion criteria; of these, 6139 (31%) were treated initially with a macrolide; the median age was 70 years; 60% were female; and 78% were white. A total of 86% of patients had a primary diagnosis of obstructive chronic bronchitis with acute exacerbation, and 6% had respiratory failure. The most common comorbidities were hypertension, diabetes, and congestive heart failure. Twenty‐two percent had been admitted at least once in the preceding 12 months. Treatment failure occurred in 7.7% of patients, and 1.3% died in the hospital. Mean length of stay was 4.8 days. Hospital prescribing rates for macrolides varied from 0% to 100%, with a mean of 33% and an interquartile range of 14% to 46% (Supporting Appendix Figure 1).
Compared to patients receiving macrolides, those receiving quinolones were older, more likely to have respiratory failure, to be cared for by a pulmonologist, and to have an admission in the previous year (Table 1). They were also more likely to be treated with bronchodilators, methylxanthines, steroids, diuretics, and noninvasive positive pressure ventilation, and to have an arterial blood gas, but less likely to receive concomitant treatment with a cephalosporin (11% vs. 57%). With the exception of cephalosporin treatment, these differences were small, but due to the large sample were statistically significant. Comorbidities were similar in both groups. Patients in the quinolone group were also more likely to experience treatment failure (8.1% vs. 6.8%), death (1.5% vs. 1.0%), and antibiotic‐associated diarrhea (1.1% vs. 0.5%).
| Complete Cohort | Propensity‐matched Subsample | |||||
|---|---|---|---|---|---|---|
| Characteristic | Quinolone (n = 13469) | Macrolide (n = 6139) | P Value | Quinolone (n = 5610) | Macrolide (n = 5610) | P Value |
| ||||||
| Antibiotics received during hospitalization* [n (%)] | ||||||
| Macrolide | 264 (2) | 6139 (100) | 119 (2) | 5610 (100) | ||
| Quinolone | 13469 (100) | 459 (8) | 5610 (100) | 424 (8) | ||
| Cephalosporin | 1696 (13) | 3579 (59) | <0.001 | 726 (13) | 3305 (59) | <0.001 |
| Tetracycline | 231 (2) | 75 (2) | 0.01 | 101 (2) | 73 (2) | 0.06 |
| Other antibiotics | 397 (3) | 220 (4) | 0.02 | 166 (3) | 193 (3) | 0.03 |
| Age (years) (mean [SD]) | 69.1 (11.4) | 68.2 (11.8) | <0.001 | 68.6 (11.7) | 68.5 (11.7) | 0.58 |
| Male sex (n [%]) | 5447 (40) | 2440 (40) | 0.36 | 2207 (39) | 2196 (39) | 0.85 |
| Race/ethnic group (n [%]) | <0.001 | 0.44 | ||||
| White | 10454 (78) | 4758 (78) | 4359 (78) | 4368 (78) | ||
| Black | 1060 (8) | 540 (9) | 470 (8) | 455 (8) | ||
| Hispanic | 463 (3) | 144 (2) | 157 (3) | 134 (2) | ||
| Other | 1492 (11) | 697 (11) | 624 (11) | 653 (12) | ||
| Primary diagnosis (n [%]) | <0.001 | 0.78 | ||||
| Obstructive chronic bronchitis with acute exacerbation | 11650 (87) | 5298 (86) | 4884 (87) | 4860 (87) | ||
| Chronic obstructive asthma/asthma with COPD | 908 (7) | 569 (9) | 466 (8) | 486 (9) | ||
| Respiratory failure | 911 (7) | 272 (4) | 260 (5) | 264 (5) | ||
| Admissions in the prior year (n [%]) | <0.001 | 0.84 | ||||
| 0 | 9846 (73) | 4654 (76) | 4249 (76) | 4231 (75) | ||
| 1 | 1918 (14) | 816 (13) | 747 (13) | 750 (13) | ||
| 2+ | 1085 (8) | 445 (7) | 397 (7) | 420 (8) | ||
| Missing | 620 (5) | 224 (4) | 217 (4) | 209 (4) | ||
| Physician specialty (n [%]) | <0.001 | 0.84 | ||||
| Internal medicine/hospitalist | 7069 (53) | 3321 (54) | 3032 (54) | 3072 (55) | ||
| Family/general medicine | 3569 (27) | 2074 (34) | 1824 (33) | 1812 (32) | ||
| Pulmonologist | 2776 (21) | 727 (12) | 738 (13) | 711 (13) | ||
| Critical care/emntensivist | 55 (0) | 17 (0) | 16 (0) | 15 (0) | ||
| Tests on hospital day 1 or 2 (n [%]) | ||||||
| Arterial blood gas | 8084 (60) | 3377 (55) | <0.001 | 3195 (57) | 3129 (56) | 0.22 |
| Sputum test | 1741 (13) | 766 (13) | 0.39 | 20 (0) | 16 (0) | 0.62 |
| Medications/therapies on hospital day 1 or 2 (n [%]) | ||||||
| Short‐acting bronchodilators | 7555 (56) | 3242 (53) | <0.001 | 2969 (53) | 2820 (50) | 0.005 |
| Long‐acting beta‐2 agonists | 2068 (15) | 748 (12) | <0.001 | 704 (13) | 719 (13) | 0.69 |
| Methylxanthine bronchodilators | 3051 (23) | 1149 (19) | <0.001 | 1102 (20) | 1093 (20) | 0.85 |
| Steroids | 0.04 | 0.68 | ||||
| Intravenous | 11148 (83) | 4989 (81) | 4547 (81) | 4581 (82) | ||
| Oral | 772 (6) | 376 (6) | 334 (6) | 330 (6) | ||
| Severity indicators (n [%]) | ||||||
| Chronic pulmonary heart disease | 890 (7) | 401 (7) | 0.85 | 337 (6) | 368 (7) | 0.24 |
| Sleep apnea | 586 (4) | 234 (4) | 0.08 | 211 (4) | 218 (4) | 0.77 |
| Noninvasive positive pressure ventilation | 391 (3) | 128 (2) | <0.001 | 128 (2) | 114 (2) | 0.40 |
| Loop diuretics | 4838 (36) | 1971 (32) | <0.001 | 1884 (34) | 1862 (33) | 0.67 |
| Hospital characteristics (n [%]) | ||||||
| Staffed beds | <0.001 | 0.71 | ||||
| 6200 | 3483 (26) | 1688 (28) | 1610 (29) | 1586 (28) | ||
| 201300 | 3132 (23) | 1198 (20) | 1174 (21) | 1154 (21) | ||
| 301500 | 4265 (32) | 2047 (33) | 1809 (32) | 1867 (33) | ||
| 500+ | 2589 (19) | 1206 (20) | 1017 (18) | 1003 (18) | ||
| Hospital region (n [%]) | <0.001 | 0.65 | ||||
| South | 8562 (64) | 3270 (53) | 3212 (57) | 3160 (56) | ||
| Midwest | 2602 (19) | 1444 (24) | 1170 (21) | 1216 (22) | ||
| Northeast | 1163 (9) | 871 (14) | 687 (12) | 704 (13) | ||
| West | 1142 (9) | 554 (9) | 541 (10) | 530 (9) | ||
| Teaching hospital | <0.001 | 0.63 | ||||
| No | 12090 (90) | 5037 (82) | 4896 (87) | 4878 (87) | ||
| Yes | 1379 (10) | 1102 (18) | 714 (13) | 732 (13) | ||
| Comorbidities (n [%]) | ||||||
| Congestive heart failure | 2673 (20) | 1147 (19) | 0.06 | 1081 (19) | 1060 (19) | 0.63 |
| Metastatic cancer | 134 (1) | 27 (0) | <0.001 | 34 (1) | 38 (1) | 0.72 |
| Depression | 1419 (11) | 669 (11) | 0.45 | 598 (11) | 603 (11) | 0.90 |
| Deficiency anemias | 1155 (9) | 476 (8) | 0.05 | 426 (8) | 432 (8) | 0.86 |
| Solid tumor without metastasis | 1487 (11) | 586 (10) | 0.002 | 550 (10) | 552 (10) | 0.97 |
| Hypothyroidism | 1267 (9) | 527 (9) | 0.07 | 481 (9) | 482 (9) | 1.00 |
| Peripheral vascular disease | 821 (6) | 312 (5) | 0.005 | 287 (5) | 288 (5) | 1.00 |
| Paralysis | 165 (1) | 46 (1) | 0.003 | 49 (1) | 51 (1) | 0.92 |
| Obesity | 957 (7) | 435 (7) | 0.98 | 386 (7) | 398 (7) | 0.68 |
| Hypertension | 5793 (43) | 2688 (44) | 0.31 | 2474 (44) | 2468 (44) | 0.92 |
| Diabetes | 0.04 | 0.45 | ||||
| Without chronic complications | 2630 (20) | 1127 (18) | 1057 (19) | 1066 (19) | ||
| With chronic complications | 298 (2) | 116 (2) | 115 (2) | 97 (2) | ||
In the unadjusted analysis, compared to patients receiving quinolones, those treated with macrolides were less likely to experience treatment failure (OR, 0.83; 95% CI, 0.740.94) (Table 2). Adjusting for all patient, hospital, and physician covariates, including the propensity for treatment with macrolides, increased the OR to 0.89 and the results were no longer significant (95% CI, 0.781.01). Propensity matching successfully balanced all measured covariates except for the use of short‐acting bronchodilators and additional antibiotics (Table 1). In the propensity‐matched sample (Figure 1), quinolone‐treated patients were more likely to experience antibiotic‐associated diarrhea (1.2% vs. 0.6%; P = 0.0003) and late mechanical ventilation (1.3% vs. 0.8%; P = 0.02). There were no differences in adjusted cost or length of stay between the 2 groups. The results of the grouped treatment analysis, substituting the hospital's specific rate of macrolide use in place of the actual treatment that each patient received suggested that the 2 antibiotics were associated with similar rates of treatment failure. The OR for a 100% hospital rate of macrolide treatment vs. a 0% rate was 1.01 (95% CI, 0.751.35).

| Treatment Failure | Cost | LOS | ||||
|---|---|---|---|---|---|---|
| Models | OR | 95% CI | Ratio | 95% CI | Ratio | 95% CI |
| ||||||
| Unadjusted | 0.83 | 0.730.93 | 0.98 | 0.971.00 | 0.96 | 0.950.98 |
| Adjusted for propensity score only* | 0.89 | 0.791.01 | 1.00 | 0.981.01 | 0.98 | 0.971.00 |
| Adjusted for covariates | 0.87 | 0.770.99 | 1.00 | 0.991.02 | 0.99 | 0.971.00 |
| Adjusted for covariates and propensity score | 0.89 | 0.781.01 | 1.00 | 0.991.02 | 0.98 | 0.971.00 |
| Matched sample, unadjusted | 0.87 | 0.751.00 | 0.99 | 0.981.01 | 0.99 | 0.971.01 |
| Matched sample, adjusted for unbalanced variables | 0.87 | 0.751.01 | 1.00 | 0.981.02 | 0.99 | 0.971.01 |
| Grouped treatment model, unadjusted | 0.90 | 0.681.19 | 0.97 | 0.891.06 | 0.92 | 0.870.96 |
| Group treatment model, adjusted for covariates∥ | 1.01 | 0.751.35 | 0.96 | 0.881.05 | 0.96 | 0.911.00 |
Discussion
In this large observational study conducted at 375 hospitals, we took advantage of a natural experiment in which antibiotic prescribing patterns varied widely across hospitals to compare the effectiveness of 2 common antibiotic regimens for AECOPD. Treatment with macrolides and quinolones were associated with a similar risk of treatment failure, costs, and length of stay; however, patients treated with macrolides were less likely to experience late mechanical ventilation or treatment for antibiotic‐associated diarrhea.
Despite broad consensus in COPD guidelines that patients with severe acute exacerbations should receive antibiotics, there is little agreement about the preferred empiric agent. Controversy exists regarding antibiotics' comparative effectiveness, and even over which pathogens cause COPD exacerbations. Given the frequency of hospitalization for AECOPD, understanding the comparative effectiveness of treatments in this setting could have important implications for health outcomes and costs. Unfortunately, most antibiotic studies in AECOPD were conducted >20 years ago, using antibiotics that rarely appeared in our sample.26 Consequently, clinical practice guidelines offer conflicting recommendations. For example, the National Institute for Clinical Excellence recommends empirical treatment with an aminopenicillin, a macrolide, or a tetracycline,8 while the American Thoracic Society recommends amoxicillin‐clavulonate or a fluoroquinolone.9
As might be expected in light of so much uncertainty, we found wide variation in prescribing patterns across hospitals. Overall, approximately one‐third of patients received a macrolide and two‐thirds a quinolone. Both regimens provide adequate coverage of H. influenza, S. pneumoniae, and M. catarrhalis, and conform to at least 1 COPD guideline. Nevertheless, patients receiving macrolides often received a cephalosporin as well; this pattern of treatment suggests that antibiotic selection is likely to have been influenced more by guidelines for the treatment of community‐acquired pneumonia than COPD.
Previous studies comparing antibiotic effectiveness suffer from shortcomings that limit their application to patients hospitalized with AECOPD. First, they enrolled patients with chronic bronchitis, and included patients without obstructive lung disease, and most studies included patients as young as 18 years old. Second, many either did not include treatment with steroids or excluded patients receiving more than 10 mg of prednisone daily. Third, almost all enrolled only ambulatory patients.
While there are no studies comparing quinolones and macrolides in patients hospitalized for AECOPD, a meta‐analysis comparing quinolones, macrolides, and amoxicillin‐clavulonate identified 19 trials of ambulatory patients with chronic bronchitis. That study found that all 3 drugs had similar efficacy initially, but that quinolones resulted in the fewest relapses over a 26‐week period.27 Macrolides and quinolones had similar rates of adverse effects. In contrast, we did not find a difference in treatment failure, cost, or length of stay, but did find a higher rate of diarrhea associated with quinolones. Others have also documented an association between fluoroquinolones and C. difficile diarrhea.2830 This trend, first noted in 2001, is of particular concern because the fluoroquinolone‐resistant strains appear to be hypervirulent and have been associated with nosocomial epidemics.3134
Our study has several limitations. First, its observational design leaves open the possibility of selection bias. For this reason we analyzed our data in several ways, including using a grouped treatment approach, an adaptation of the instrumental variable technique, and accepted only those differences which were consistent across all models. Second, our study used claims data, and therefore we could not directly adjust for physiological measures of severity. However, the highly detailed nature of the data allowed us to adjust for numerous tests and treatments that reflected the clinician's assessment of the patient's severity, as well as the number of prior COPD admissions. Third, we cannot exclude the possibility that some patients may have had concurrent pneumonia without an ICD‐9 code. We think that the number would be small because reimbursement for pneumonia is generally higher than for COPD, so hospitals have an incentive to code pneumonia as the principal diagnosis when present. Finally, we compared initial antibiotics only. More than one‐quarter of our patients received an additional antibiotic before discharge. In particular, patients receiving macrolides were often prescribed a concomitant cephalosporin. We do not know to what extent these additional antibiotics may have affected the outcomes.
Despite the large number of patients hospitalized annually for AECOPD, there are no randomized trials comparing different antibiotics in this population. Studies comparing antibiotics in chronic bronchitis can offer little guidance, since they have primarily focused on proving equivalence between existing antibiotics and newer, more expensive formulations.35 Because many of the patients enrolled in such trials do not benefit from antibiotics at all, either because they do not have COPD or because their exacerbation is not caused by bacteria, it is relatively easy to prove equivalence. Given that AECOPD is one of the leading causes of hospitalization in the United States, large, randomized trials comparing the effectiveness of different antibiotics should be a high priority. In the meantime, macrolides (often given together with cephalosporins) and quinolones appear to be equally effective initial antibiotic choices; considering antibiotic‐associated diarrhea, macrolides appear to be the safer of the 2.
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are responsible for more than 600,000 hospitalizations annually, resulting in direct costs of over $20 billion.1 Bacterial infections appear responsible for 50% of such exacerbations,25 and current COPD guidelines recommend treatment with antibiotics for patients with severe exacerbations or a change in sputum.1,69 These recommendations are based on a number of small randomized trials, most of which were conducted more than 20 years ago using narrow spectrum antibiotics that are no longer commonly used.10 Only 4 studies, totaling 321 subjects, included hospitalized patients, and most studies excluded patients who required steroids. Because no clinical trials have compared different antibiotic regimens for AECOPD, existing guidelines offer a range of treatment options, including amoxicillin‐clavulonate, macrolides, quinolones, cephalosporins, aminopenicillins, and tetracyclines.
Among hospitalized patients, macrolides and quinolones appear to be the most frequently prescribed antibiotics.11 Both are available in oral formulations, have excellent bioavailability, and are administered once daily. In addition to their antimicrobial activity, macrolides are believed to have antiinflammatory effects, which could be especially advantageous in AECOPD.1214 In trials of chronic bronchitis, however, fluoroquinolones have been shown to reduce the risk of recurrent exacerbation when compared to macrolides.15 The wide variation that has been observed in antibiotic selection for patients hospitalized for AECOPD suggests a high degree of uncertainty among clinicians about the benefits of different treatment options.11 Given the limited evidence from randomized trials, we sought to evaluate the comparative effectiveness of macrolides and quinolones among a large, representative sample of patients hospitalized with AECOPD.
Subjects and Methods
Setting and Subjects
We conducted a retrospective cohort study of all patients hospitalized between January 1 and December 31, 2001 for AECOPD at any 1 of 375 acute care facilities in the United States that participated in Premier's Perspective, a voluntary, fee‐supported database developed for measuring quality and healthcare utilization. Participating hospitals represent all geographical regions, and are primarily small‐sized to medium‐sized nonteaching hospitals located mostly in urban areas. In addition to the information contained in the standard hospital discharge file (Uniform Billing 92) such as patient age, International Classification of Disease, 9th Edition, Clinical Modification (ICD‐9‐CM) codes, the Perspective database includes a date‐stamped log of all billed items, including diagnostic tests, medications, and other treatments, as well as costs, for individual patients. The study was approved by the Institutional Review Board of Baystate Medical Center.
Patients were included if they had a primary diagnosis consistent with AECOPD (ICD‐9 codes 491.21 and 493.22) or a primary diagnosis of respiratory failure (ICD‐9 codes 518.81 and 518.84) paired with secondary diagnosis of AECOPD; they also had to receive at least 2 consecutive days of either a macrolide or a quinolone, started within 48 hours of hospitalization. Patients receiving both antibiotics were excluded, but patients who received additional antibiotics were included. To enhance the specificity of our diagnosis codes, we limited our study to patients age 40 years.16 Because mechanical ventilation initiated after hospital day 2 was an outcome measure, we excluded patients admitted directly to the intensive care unit. We also excluded: those with other bacterial infections, such as pneumonia or cellulitis, who might have another indication for antibiotics; those with a length of stay <2 days, because we could not ascertain whether they received a full course of antibiotics; patients with a secondary diagnosis of pulmonary embolism or pneumothorax; and those whose attending physicians were not internists, family practitioners, hospitalists, pulmonologists, or intensivists. For patients with more than 1 admission during the study period, we included only the first admission.
Data Elements
For each patient, we assessed age, gender, race, marital and insurance status, principal diagnosis, comorbidities, and specialty of the attending physician. Comorbidities were identified from ICD‐9 secondary diagnosis codes and Diagnosis Related Groups using Healthcare Cost and Utilization Project Comorbidity Software (version 3.1), based on the work of Elixhauser et al.17 In addition, to assess disease severity we recorded the presence of chronic pulmonary heart disease, the number of admissions for COPD during the 12 months prior to the index admission, and arterial blood gas testing.18,19 We also identified pharmacy or diagnostic charges for interventions that were recommended in current guidelines (beta‐adrenergic and anticholinergic bronchodilators, steroids, and noninvasive positive‐pressure ventilation); those that were not recommended or were of uncertain benefit (methylxanthine bronchodilators, spirometry/pulmonary function testing, mucolytic medications, chest physiotherapy, and sputum testing); and drugs that might be associated with severe exacerbations or end‐stage COPD (loop diuretics, morphine, and nutritional supplements).1,69 Hospitals were categorized by region (Northeast, South, Midwest, or West), bed size, setting (urban vs. rural), ownership, and teaching status.
Antibiotic Class and Outcome Variables
Our primary predictor variable was the antibiotic initiated during the first 2 hospital days and continued for at least 2 days, regardless of other antibiotics the patient may have received during the course of hospitalization. Because we anticipated low in‐hospital mortality, our primary outcome was a composite measure of treatment failure, defined as initiation of mechanical ventilation after hospital day 2, in‐hospital mortality, or readmission for COPD within 30 days of discharge.20 Secondary outcomes included hospital costs and length of stay, as well as allergic reactions identified by ICD‐9 code, and antibiotic‐associated diarrhea, defined as treatment with either metronidazole or oral vancomycin begun after hospital day 3.
Statistical Analysis
Summary statistics were computed using frequencies and percents for categorical variables; and means, medians, standard deviations, and interquartile ranges for continuous variables. Associations between antibiotic selection and patient and hospital characteristics were assessed using chi‐square tests for categorical variables and z‐tests for continuous variables.
We developed a series of multivariable models to evaluate the impact of initial antibiotic selection on the risk of treatment failure, length of stay, and total cost. In order to account for the effects of within‐hospital correlation, generalized estimating equation (GEE) models with a logit link were used to assess the effect of antibiotic selection on the risk of treatment failure, and identity link models were used for analyses of length of stay and cost. Unadjusted and covariate‐adjusted models for treatment failure were evaluated with and without adjustments for propensity score. A propensity score is the probability that a given patient would receive treatment with a macrolide, derived from a nonparsimonious model in which treatment with a macrolide was considered the outcome. The propensity model included all patient characteristics, other early treatments and tests, comorbidities, hospital and physician characteristics, and selected interaction terms.21 Length of stay and cost were trimmed at 3 standard deviations above the mean, and natural log‐transformed values were modeled due to extreme positive skew. In addition, we carried out matched analyses in which we compared the outcomes of patients who were treated with a macrolide to those with similar propensity scores (ie, with similar likelihood of receiving a macrolide) who received a quinolone.22
Finally, to reduce the threat of residual confounding by indication, which can occur if sicker patients are more likely to receive a particular antibiotic, we developed a grouped treatment model, in which all patients treated at the same hospital were assigned a probability of treatment with a macrolide equal to the overall treatment rate at that hospital.23 This is an adaptation of instrumental variable analysis, a well‐accepted technique in econometrics with growing use in health care.24,25 It attempts to assess whether patients treated at a hospital at which quinolones are used more frequently have better outcomes than patients treated at hospitals at which macrolides are used more frequently, while adjusting for other patient, physician, and hospital variables. It ignores the actual treatment the patient received, and instead substitutes the hospital's rate of macrolide use. By grouping treatment at the hospital level, this method greatly reduces the possibility of residual selection bias, unless hospitals that use a lot of macrolides have patients who differ in a consistent way from hospitals which use mostly quinolones.
All analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC).
Results
Of 26,248 AECOPD patients treated with antibiotics, 19,608 patients met the inclusion criteria; of these, 6139 (31%) were treated initially with a macrolide; the median age was 70 years; 60% were female; and 78% were white. A total of 86% of patients had a primary diagnosis of obstructive chronic bronchitis with acute exacerbation, and 6% had respiratory failure. The most common comorbidities were hypertension, diabetes, and congestive heart failure. Twenty‐two percent had been admitted at least once in the preceding 12 months. Treatment failure occurred in 7.7% of patients, and 1.3% died in the hospital. Mean length of stay was 4.8 days. Hospital prescribing rates for macrolides varied from 0% to 100%, with a mean of 33% and an interquartile range of 14% to 46% (Supporting Appendix Figure 1).
Compared to patients receiving macrolides, those receiving quinolones were older, more likely to have respiratory failure, to be cared for by a pulmonologist, and to have an admission in the previous year (Table 1). They were also more likely to be treated with bronchodilators, methylxanthines, steroids, diuretics, and noninvasive positive pressure ventilation, and to have an arterial blood gas, but less likely to receive concomitant treatment with a cephalosporin (11% vs. 57%). With the exception of cephalosporin treatment, these differences were small, but due to the large sample were statistically significant. Comorbidities were similar in both groups. Patients in the quinolone group were also more likely to experience treatment failure (8.1% vs. 6.8%), death (1.5% vs. 1.0%), and antibiotic‐associated diarrhea (1.1% vs. 0.5%).
| Complete Cohort | Propensity‐matched Subsample | |||||
|---|---|---|---|---|---|---|
| Characteristic | Quinolone (n = 13469) | Macrolide (n = 6139) | P Value | Quinolone (n = 5610) | Macrolide (n = 5610) | P Value |
| ||||||
| Antibiotics received during hospitalization* [n (%)] | ||||||
| Macrolide | 264 (2) | 6139 (100) | 119 (2) | 5610 (100) | ||
| Quinolone | 13469 (100) | 459 (8) | 5610 (100) | 424 (8) | ||
| Cephalosporin | 1696 (13) | 3579 (59) | <0.001 | 726 (13) | 3305 (59) | <0.001 |
| Tetracycline | 231 (2) | 75 (2) | 0.01 | 101 (2) | 73 (2) | 0.06 |
| Other antibiotics | 397 (3) | 220 (4) | 0.02 | 166 (3) | 193 (3) | 0.03 |
| Age (years) (mean [SD]) | 69.1 (11.4) | 68.2 (11.8) | <0.001 | 68.6 (11.7) | 68.5 (11.7) | 0.58 |
| Male sex (n [%]) | 5447 (40) | 2440 (40) | 0.36 | 2207 (39) | 2196 (39) | 0.85 |
| Race/ethnic group (n [%]) | <0.001 | 0.44 | ||||
| White | 10454 (78) | 4758 (78) | 4359 (78) | 4368 (78) | ||
| Black | 1060 (8) | 540 (9) | 470 (8) | 455 (8) | ||
| Hispanic | 463 (3) | 144 (2) | 157 (3) | 134 (2) | ||
| Other | 1492 (11) | 697 (11) | 624 (11) | 653 (12) | ||
| Primary diagnosis (n [%]) | <0.001 | 0.78 | ||||
| Obstructive chronic bronchitis with acute exacerbation | 11650 (87) | 5298 (86) | 4884 (87) | 4860 (87) | ||
| Chronic obstructive asthma/asthma with COPD | 908 (7) | 569 (9) | 466 (8) | 486 (9) | ||
| Respiratory failure | 911 (7) | 272 (4) | 260 (5) | 264 (5) | ||
| Admissions in the prior year (n [%]) | <0.001 | 0.84 | ||||
| 0 | 9846 (73) | 4654 (76) | 4249 (76) | 4231 (75) | ||
| 1 | 1918 (14) | 816 (13) | 747 (13) | 750 (13) | ||
| 2+ | 1085 (8) | 445 (7) | 397 (7) | 420 (8) | ||
| Missing | 620 (5) | 224 (4) | 217 (4) | 209 (4) | ||
| Physician specialty (n [%]) | <0.001 | 0.84 | ||||
| Internal medicine/hospitalist | 7069 (53) | 3321 (54) | 3032 (54) | 3072 (55) | ||
| Family/general medicine | 3569 (27) | 2074 (34) | 1824 (33) | 1812 (32) | ||
| Pulmonologist | 2776 (21) | 727 (12) | 738 (13) | 711 (13) | ||
| Critical care/emntensivist | 55 (0) | 17 (0) | 16 (0) | 15 (0) | ||
| Tests on hospital day 1 or 2 (n [%]) | ||||||
| Arterial blood gas | 8084 (60) | 3377 (55) | <0.001 | 3195 (57) | 3129 (56) | 0.22 |
| Sputum test | 1741 (13) | 766 (13) | 0.39 | 20 (0) | 16 (0) | 0.62 |
| Medications/therapies on hospital day 1 or 2 (n [%]) | ||||||
| Short‐acting bronchodilators | 7555 (56) | 3242 (53) | <0.001 | 2969 (53) | 2820 (50) | 0.005 |
| Long‐acting beta‐2 agonists | 2068 (15) | 748 (12) | <0.001 | 704 (13) | 719 (13) | 0.69 |
| Methylxanthine bronchodilators | 3051 (23) | 1149 (19) | <0.001 | 1102 (20) | 1093 (20) | 0.85 |
| Steroids | 0.04 | 0.68 | ||||
| Intravenous | 11148 (83) | 4989 (81) | 4547 (81) | 4581 (82) | ||
| Oral | 772 (6) | 376 (6) | 334 (6) | 330 (6) | ||
| Severity indicators (n [%]) | ||||||
| Chronic pulmonary heart disease | 890 (7) | 401 (7) | 0.85 | 337 (6) | 368 (7) | 0.24 |
| Sleep apnea | 586 (4) | 234 (4) | 0.08 | 211 (4) | 218 (4) | 0.77 |
| Noninvasive positive pressure ventilation | 391 (3) | 128 (2) | <0.001 | 128 (2) | 114 (2) | 0.40 |
| Loop diuretics | 4838 (36) | 1971 (32) | <0.001 | 1884 (34) | 1862 (33) | 0.67 |
| Hospital characteristics (n [%]) | ||||||
| Staffed beds | <0.001 | 0.71 | ||||
| 6200 | 3483 (26) | 1688 (28) | 1610 (29) | 1586 (28) | ||
| 201300 | 3132 (23) | 1198 (20) | 1174 (21) | 1154 (21) | ||
| 301500 | 4265 (32) | 2047 (33) | 1809 (32) | 1867 (33) | ||
| 500+ | 2589 (19) | 1206 (20) | 1017 (18) | 1003 (18) | ||
| Hospital region (n [%]) | <0.001 | 0.65 | ||||
| South | 8562 (64) | 3270 (53) | 3212 (57) | 3160 (56) | ||
| Midwest | 2602 (19) | 1444 (24) | 1170 (21) | 1216 (22) | ||
| Northeast | 1163 (9) | 871 (14) | 687 (12) | 704 (13) | ||
| West | 1142 (9) | 554 (9) | 541 (10) | 530 (9) | ||
| Teaching hospital | <0.001 | 0.63 | ||||
| No | 12090 (90) | 5037 (82) | 4896 (87) | 4878 (87) | ||
| Yes | 1379 (10) | 1102 (18) | 714 (13) | 732 (13) | ||
| Comorbidities (n [%]) | ||||||
| Congestive heart failure | 2673 (20) | 1147 (19) | 0.06 | 1081 (19) | 1060 (19) | 0.63 |
| Metastatic cancer | 134 (1) | 27 (0) | <0.001 | 34 (1) | 38 (1) | 0.72 |
| Depression | 1419 (11) | 669 (11) | 0.45 | 598 (11) | 603 (11) | 0.90 |
| Deficiency anemias | 1155 (9) | 476 (8) | 0.05 | 426 (8) | 432 (8) | 0.86 |
| Solid tumor without metastasis | 1487 (11) | 586 (10) | 0.002 | 550 (10) | 552 (10) | 0.97 |
| Hypothyroidism | 1267 (9) | 527 (9) | 0.07 | 481 (9) | 482 (9) | 1.00 |
| Peripheral vascular disease | 821 (6) | 312 (5) | 0.005 | 287 (5) | 288 (5) | 1.00 |
| Paralysis | 165 (1) | 46 (1) | 0.003 | 49 (1) | 51 (1) | 0.92 |
| Obesity | 957 (7) | 435 (7) | 0.98 | 386 (7) | 398 (7) | 0.68 |
| Hypertension | 5793 (43) | 2688 (44) | 0.31 | 2474 (44) | 2468 (44) | 0.92 |
| Diabetes | 0.04 | 0.45 | ||||
| Without chronic complications | 2630 (20) | 1127 (18) | 1057 (19) | 1066 (19) | ||
| With chronic complications | 298 (2) | 116 (2) | 115 (2) | 97 (2) | ||
In the unadjusted analysis, compared to patients receiving quinolones, those treated with macrolides were less likely to experience treatment failure (OR, 0.83; 95% CI, 0.740.94) (Table 2). Adjusting for all patient, hospital, and physician covariates, including the propensity for treatment with macrolides, increased the OR to 0.89 and the results were no longer significant (95% CI, 0.781.01). Propensity matching successfully balanced all measured covariates except for the use of short‐acting bronchodilators and additional antibiotics (Table 1). In the propensity‐matched sample (Figure 1), quinolone‐treated patients were more likely to experience antibiotic‐associated diarrhea (1.2% vs. 0.6%; P = 0.0003) and late mechanical ventilation (1.3% vs. 0.8%; P = 0.02). There were no differences in adjusted cost or length of stay between the 2 groups. The results of the grouped treatment analysis, substituting the hospital's specific rate of macrolide use in place of the actual treatment that each patient received suggested that the 2 antibiotics were associated with similar rates of treatment failure. The OR for a 100% hospital rate of macrolide treatment vs. a 0% rate was 1.01 (95% CI, 0.751.35).

| Treatment Failure | Cost | LOS | ||||
|---|---|---|---|---|---|---|
| Models | OR | 95% CI | Ratio | 95% CI | Ratio | 95% CI |
| ||||||
| Unadjusted | 0.83 | 0.730.93 | 0.98 | 0.971.00 | 0.96 | 0.950.98 |
| Adjusted for propensity score only* | 0.89 | 0.791.01 | 1.00 | 0.981.01 | 0.98 | 0.971.00 |
| Adjusted for covariates | 0.87 | 0.770.99 | 1.00 | 0.991.02 | 0.99 | 0.971.00 |
| Adjusted for covariates and propensity score | 0.89 | 0.781.01 | 1.00 | 0.991.02 | 0.98 | 0.971.00 |
| Matched sample, unadjusted | 0.87 | 0.751.00 | 0.99 | 0.981.01 | 0.99 | 0.971.01 |
| Matched sample, adjusted for unbalanced variables | 0.87 | 0.751.01 | 1.00 | 0.981.02 | 0.99 | 0.971.01 |
| Grouped treatment model, unadjusted | 0.90 | 0.681.19 | 0.97 | 0.891.06 | 0.92 | 0.870.96 |
| Group treatment model, adjusted for covariates∥ | 1.01 | 0.751.35 | 0.96 | 0.881.05 | 0.96 | 0.911.00 |
Discussion
In this large observational study conducted at 375 hospitals, we took advantage of a natural experiment in which antibiotic prescribing patterns varied widely across hospitals to compare the effectiveness of 2 common antibiotic regimens for AECOPD. Treatment with macrolides and quinolones were associated with a similar risk of treatment failure, costs, and length of stay; however, patients treated with macrolides were less likely to experience late mechanical ventilation or treatment for antibiotic‐associated diarrhea.
Despite broad consensus in COPD guidelines that patients with severe acute exacerbations should receive antibiotics, there is little agreement about the preferred empiric agent. Controversy exists regarding antibiotics' comparative effectiveness, and even over which pathogens cause COPD exacerbations. Given the frequency of hospitalization for AECOPD, understanding the comparative effectiveness of treatments in this setting could have important implications for health outcomes and costs. Unfortunately, most antibiotic studies in AECOPD were conducted >20 years ago, using antibiotics that rarely appeared in our sample.26 Consequently, clinical practice guidelines offer conflicting recommendations. For example, the National Institute for Clinical Excellence recommends empirical treatment with an aminopenicillin, a macrolide, or a tetracycline,8 while the American Thoracic Society recommends amoxicillin‐clavulonate or a fluoroquinolone.9
As might be expected in light of so much uncertainty, we found wide variation in prescribing patterns across hospitals. Overall, approximately one‐third of patients received a macrolide and two‐thirds a quinolone. Both regimens provide adequate coverage of H. influenza, S. pneumoniae, and M. catarrhalis, and conform to at least 1 COPD guideline. Nevertheless, patients receiving macrolides often received a cephalosporin as well; this pattern of treatment suggests that antibiotic selection is likely to have been influenced more by guidelines for the treatment of community‐acquired pneumonia than COPD.
Previous studies comparing antibiotic effectiveness suffer from shortcomings that limit their application to patients hospitalized with AECOPD. First, they enrolled patients with chronic bronchitis, and included patients without obstructive lung disease, and most studies included patients as young as 18 years old. Second, many either did not include treatment with steroids or excluded patients receiving more than 10 mg of prednisone daily. Third, almost all enrolled only ambulatory patients.
While there are no studies comparing quinolones and macrolides in patients hospitalized for AECOPD, a meta‐analysis comparing quinolones, macrolides, and amoxicillin‐clavulonate identified 19 trials of ambulatory patients with chronic bronchitis. That study found that all 3 drugs had similar efficacy initially, but that quinolones resulted in the fewest relapses over a 26‐week period.27 Macrolides and quinolones had similar rates of adverse effects. In contrast, we did not find a difference in treatment failure, cost, or length of stay, but did find a higher rate of diarrhea associated with quinolones. Others have also documented an association between fluoroquinolones and C. difficile diarrhea.2830 This trend, first noted in 2001, is of particular concern because the fluoroquinolone‐resistant strains appear to be hypervirulent and have been associated with nosocomial epidemics.3134
Our study has several limitations. First, its observational design leaves open the possibility of selection bias. For this reason we analyzed our data in several ways, including using a grouped treatment approach, an adaptation of the instrumental variable technique, and accepted only those differences which were consistent across all models. Second, our study used claims data, and therefore we could not directly adjust for physiological measures of severity. However, the highly detailed nature of the data allowed us to adjust for numerous tests and treatments that reflected the clinician's assessment of the patient's severity, as well as the number of prior COPD admissions. Third, we cannot exclude the possibility that some patients may have had concurrent pneumonia without an ICD‐9 code. We think that the number would be small because reimbursement for pneumonia is generally higher than for COPD, so hospitals have an incentive to code pneumonia as the principal diagnosis when present. Finally, we compared initial antibiotics only. More than one‐quarter of our patients received an additional antibiotic before discharge. In particular, patients receiving macrolides were often prescribed a concomitant cephalosporin. We do not know to what extent these additional antibiotics may have affected the outcomes.
Despite the large number of patients hospitalized annually for AECOPD, there are no randomized trials comparing different antibiotics in this population. Studies comparing antibiotics in chronic bronchitis can offer little guidance, since they have primarily focused on proving equivalence between existing antibiotics and newer, more expensive formulations.35 Because many of the patients enrolled in such trials do not benefit from antibiotics at all, either because they do not have COPD or because their exacerbation is not caused by bacteria, it is relatively easy to prove equivalence. Given that AECOPD is one of the leading causes of hospitalization in the United States, large, randomized trials comparing the effectiveness of different antibiotics should be a high priority. In the meantime, macrolides (often given together with cephalosporins) and quinolones appear to be equally effective initial antibiotic choices; considering antibiotic‐associated diarrhea, macrolides appear to be the safer of the 2.
- ,,.Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2001;134:595–599.
- ,,, et al.Infectious etiologies in acute exacerbation of COPD.Diagn Microbiol Infect Dis.2001;40:95–102.
- ,.Bacterial infections in patients requiring admission for an acute exacerbation of COPD; a 1‐year prospective study.Respir Med.2003;97:770–777.
- ,,, et al.Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease.Arch Intern Med.2005;165:891–897.
- ,.Infection in the pathogenesis and course of chronic obstructive pulmonary disease.N Engl J Med.2008;359:2355–2365.
- ,,, et al.Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary.Am J Respir Crit Care Med.2007;176:532–555.
- ,,, et al.Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2008 update—highlights for primary care.Can Respir J.2008;15(suppl A):1A–8A.
- Chronic obstructive pulmonary disease.National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care.Thorax.2004;59(suppl 1):1–232.
- ,,, et al.Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper.Eur Respir J.2004;23:932–946.
- ,,,,.Antibiotics for exacerbations of chronic obstructive pulmonary disease.Cochrane Database Syst Rev.2006:CD004403.
- ,,,,,.Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2006;144:894–903.
- ,,, et al.Modulation of neutrophil and inflammation markers in chronic obstructive pulmonary disease by short‐term azithromycin treatment.Eur J Pharmacol.2005;517:132–143.
- ,,, et al.Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects.Eur J Pharmacol.2002;450:277–289.
- ,,,,,.The effect of clarithromycin on inflammatory markers in chronic obstructive pulmonary disease: preliminary data.Ann Pharmacother.2004;38:1400–1405.
- ,,,.A comparison of gemifloxacin and clarithromycin in acute exacerbations of chronic bronchitis and long‐term clinical outcomes.Clin Ther.2002;24:639–652.
- ,,,.In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease.Arch Intern Med.2003;163:1180–1186.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36:8–27.
- ,,, et al.Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments).Am J Respir Crit Care Med.1996;154:959–967.
- ,,.Mortality and mortality‐related factors after hospitalization for acute exacerbation of COPD.Chest.2003;124:459–467.
- ,,, et al.Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease.N Engl J Med.1999;340:1941–1947.
- .Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group.Stat Med.1998;17:2265–2281.
- .Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. Proceedings of the Twenty‐Sixth Annual SAS Users Group International Conference. Cary, NC: SAS Institute;2001,214–216.
- ,,,.Modeling treatment effects on binary outcomes with grouped‐treatment variables and individual covariates.Am J Epidemiol.2002;156:753–760.
- ,,.Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. [see Comment].JAMA.1994;272:859–866.
- ,,,,,.Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods.JAMA.2007;297:278–285.
- ,,,.Antibiotics in chronic obstructive pulmonary disease exacerbations. A meta‐analysis.JAMA.1995;273:957–960.
- ,,,,.Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta‐analysis.Eur Respir J.2007;29:1127–1137.
- ,,,,.Antimicrobial‐associated risk factors for Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S19–S31.
- ,,, et al.Short‐term and long‐term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis.Chest.2004;125:953–964.
- ,,,,.Oral gemifloxacin once daily for 5 days compared with sequential therapy with i.v. ceftriaxone/oral cefuroxime (maximum of 10 days) in the treatment of hospitalized patients with acute exacerbations of chronic bronchitis.Respir Med.2003;97:242–249.
- ,,, et al.A large outbreak of Clostridium difficile‐associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use.Infect Control Hosp Epidemiol.2005;26:273–280.
- ,,, et al.An epidemic, toxin gene‐variant strain of Clostridium difficile.N Engl J Med.2005;353:2433–2441.
- ,,, et al.Outbreak of Clostridium difficile infection in a long‐term care facility: association with gatifloxacin use.Clin Infect Dis.2004;38:640–645.
- ,,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,.No more equivalence trials for antibiotics in exacerbations of COPD, please.Chest.2004;125:811–813.
- ,,.Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2001;134:595–599.
- ,,, et al.Infectious etiologies in acute exacerbation of COPD.Diagn Microbiol Infect Dis.2001;40:95–102.
- ,.Bacterial infections in patients requiring admission for an acute exacerbation of COPD; a 1‐year prospective study.Respir Med.2003;97:770–777.
- ,,, et al.Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease.Arch Intern Med.2005;165:891–897.
- ,.Infection in the pathogenesis and course of chronic obstructive pulmonary disease.N Engl J Med.2008;359:2355–2365.
- ,,, et al.Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary.Am J Respir Crit Care Med.2007;176:532–555.
- ,,, et al.Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2008 update—highlights for primary care.Can Respir J.2008;15(suppl A):1A–8A.
- Chronic obstructive pulmonary disease.National clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care.Thorax.2004;59(suppl 1):1–232.
- ,,, et al.Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper.Eur Respir J.2004;23:932–946.
- ,,,,.Antibiotics for exacerbations of chronic obstructive pulmonary disease.Cochrane Database Syst Rev.2006:CD004403.
- ,,,,,.Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease.Ann Intern Med.2006;144:894–903.
- ,,, et al.Modulation of neutrophil and inflammation markers in chronic obstructive pulmonary disease by short‐term azithromycin treatment.Eur J Pharmacol.2005;517:132–143.
- ,,, et al.Azithromycin modulates neutrophil function and circulating inflammatory mediators in healthy human subjects.Eur J Pharmacol.2002;450:277–289.
- ,,,,,.The effect of clarithromycin on inflammatory markers in chronic obstructive pulmonary disease: preliminary data.Ann Pharmacother.2004;38:1400–1405.
- ,,,.A comparison of gemifloxacin and clarithromycin in acute exacerbations of chronic bronchitis and long‐term clinical outcomes.Clin Ther.2002;24:639–652.
- ,,,.In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease.Arch Intern Med.2003;163:1180–1186.
- ,,,.Comorbidity measures for use with administrative data.Med Care.1998;36:8–27.
- ,,, et al.Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments).Am J Respir Crit Care Med.1996;154:959–967.
- ,,.Mortality and mortality‐related factors after hospitalization for acute exacerbation of COPD.Chest.2003;124:459–467.
- ,,, et al.Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease.N Engl J Med.1999;340:1941–1947.
- .Propensity score methods for bias reduction in the comparison of a treatment to a non‐randomized control group.Stat Med.1998;17:2265–2281.
- .Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. Proceedings of the Twenty‐Sixth Annual SAS Users Group International Conference. Cary, NC: SAS Institute;2001,214–216.
- ,,,.Modeling treatment effects on binary outcomes with grouped‐treatment variables and individual covariates.Am J Epidemiol.2002;156:753–760.
- ,,.Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. [see Comment].JAMA.1994;272:859–866.
- ,,,,,.Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods.JAMA.2007;297:278–285.
- ,,,.Antibiotics in chronic obstructive pulmonary disease exacerbations. A meta‐analysis.JAMA.1995;273:957–960.
- ,,,,.Macrolides, quinolones and amoxicillin/clavulanate for chronic bronchitis: a meta‐analysis.Eur Respir J.2007;29:1127–1137.
- ,,,,.Antimicrobial‐associated risk factors for Clostridium difficile infection.Clin Infect Dis.2008;46(suppl 1):S19–S31.
- ,,, et al.Short‐term and long‐term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis.Chest.2004;125:953–964.
- ,,,,.Oral gemifloxacin once daily for 5 days compared with sequential therapy with i.v. ceftriaxone/oral cefuroxime (maximum of 10 days) in the treatment of hospitalized patients with acute exacerbations of chronic bronchitis.Respir Med.2003;97:242–249.
- ,,, et al.A large outbreak of Clostridium difficile‐associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use.Infect Control Hosp Epidemiol.2005;26:273–280.
- ,,, et al.An epidemic, toxin gene‐variant strain of Clostridium difficile.N Engl J Med.2005;353:2433–2441.
- ,,, et al.Outbreak of Clostridium difficile infection in a long‐term care facility: association with gatifloxacin use.Clin Infect Dis.2004;38:640–645.
- ,,, et al.A predominantly clonal multi‐institutional outbreak of Clostridium difficile‐associated diarrhea with high morbidity and mortality.N Engl J Med.2005;353:2442–2449.
- ,.No more equivalence trials for antibiotics in exacerbations of COPD, please.Chest.2004;125:811–813.
Copyright © 2010 Society of Hospital Medicine