User login
Calciphylaxis in Renal Failure
Narrative Description
This Case Report reviews the presentation, the differential diagnosis and the treatment modalities used to treat calciphylaxis. It emphasizes the poor prognosis and that there is inadequate clinical experience to guide a physician as to what the most appropriate treatment is despite promising anecdotal reports about a variety of agents. The report demonstrates that intravenous sodium thiosulfate is tolerated.
Key Points
-
Calciphylaxis occurs in 1 to 4% of patients with end stage renal failure.
-
Two patterns of presentation are generally recognizedcentral and peripheral.
-
Pain is a prominent symptom and eschar formation is usually present.
-
The role of surgery is controversial.
-
Several promising modalities for the treatment of this condition have been described in anecdotal reports.
Calciphylaxis is a rare condition. It is seen most frequently in patients with chronic kidney disease and can affect any part of the body.14 Calciphylaxis is increasingly being referred to as calcific uremic arteriolopathy as this term more accurately reflects the histology of vascular calcification in small‐ and medium‐sized arteries, intimal arterial hypertrophy, and small vessel thrombosis associated with panniculitis, dermal necrosis, and eschar formation.5 Pain is a prominent symptom. The most effective treatment for this condition remains uncertain.
Case Report
A 68‐year‐old female presented with an 8‐month history of increasing lower extremity edema, and numerous large, painful, necrotic ulcers with an associated foul odor. She had a past medical history of type 2 diabetes mellitus, hypertension, end stage renal disease requiring hemodialysis 3 times a week for the previous 6 months, severe peripheral vascular disease, coronary artery disease for which she had previously undergone coronary artery bypass surgery, multiple myocardial infarctions, and congestive heart failure with an ejection fraction of 20%. She had also suffered from numerous infections including septicemia, endocarditis, and a sternal wound infection in the past with no current evidence of septicemia or endocarditis. The patient was not on calcium supplements, Vitamin D, warfarin or calcium‐containing phosphate binders.
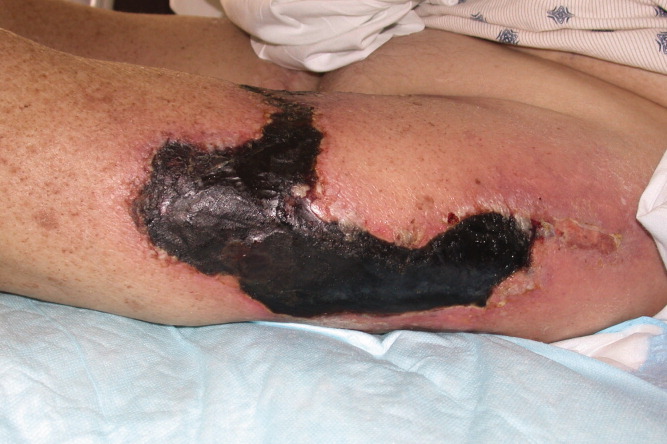
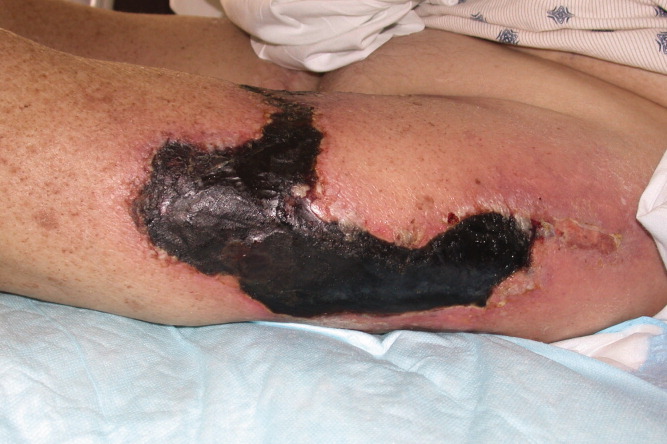
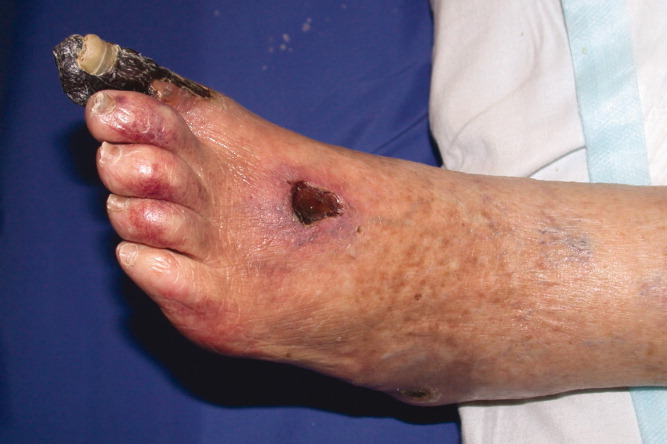
Eight months prior to admission she developed vesicles on the left thigh that slowly progressed to large, extremely painful, violaceous, indurated plaques with central ulceration and eschar (Figure 1). She subsequently developed several smaller lesions with similar morphology on her legs and feet and gangrene of her left big toe (Figure 2). A biopsy from the left thigh was consistent with calciphylaxis with associated necrosis of the deep dermis and subcutaneous tissues. The patient's lesions were aggressively debrided, broad spectrum antibiotics given, and the patient dialyzed with low calcium dialysates. Ultimately intravenous sodium thiosulfate (25 g intravenously, over 60 minutes), was given which she tolerated with no side effect. Sodium thiosulfate is thought to act by forming highly soluble calcium thiosulfate salts and therefore mobilizing tissue calcium.5 Hyperbaric oxygen was contraindicated because of the patient's left ventricular ejection fraction (LVEF) of 20% and previous history of congestive heart failure as this treatment modality may precipitate congestive heart failure in a patient with a low LVEF particularly with a past history of congestive heart failure. Her condition continued to deteriorate and she died a few days after initiation of intravenous sodium thiosulfate infusion secondary to a massive gastrointestinal bleed.
Discussion
The differential diagnosis for painful necrotic cutaneous ulcerations with eschar formation includes: calciphylaxis, cryoglobulinemia, cryofibrinogenema, peripheral vascular disease, embolic phenomenon (endocarditis, septic, cholesterol), warfarin skin necrosis, brown recluse spider bites, hypercoagulable states, hyperoxaluria, and necrotizing vasculitis.14
Calciphylaxis is a rare entity that affects approximately 1% to 4% of end stage renal failure patients.1, 3 The typical patient is a morbidly obese, female with longstanding end stage renal disease, diabetes, hyperphosphatemia and an elevated calcium‐phosphate product usually greater than 60 mg2/dL2.1, 3 It has also been described in patients with alcoholic cirrhosis and acute reversible renal failure,6 primary and secondary hyperparathyroidism,7 and metastatic breast cancer.8
Patients typically present with symmetric lesions that evolve from erythematous to violaceous, livedo‐reticularis like patches or plaques with occasional bullae to painful, indurated, necrotic plaques that subsequently ulcerate. The ulcerations are slow to heal and covered with eschar.4, 9
There are 2 patterns of involvement. The central/proximal pattern involves the abdomen, gluteal region, and thighs while the peripheral/distal pattern involves the extremities distal to the elbows and knees.1, 2, 4 The central pattern tends to carry a worse prognosis,9, 10 though this has not been validated in all reports and recent literature suggests that patients with both patterns have the worst prognosis.11
A biopsy may be required to exclude other diagnoses. The histology demonstrates an obliterative vasculopathy secondary to the vascular intimal changes and endovascular fibrosis.12 A suggestive finding is calcification of the medial wall of small‐ and medium‐sized arteries and arterioles with associated intimal hyperplasia and fibrosis. Necrosis of the surrounding tissue, panniculitis, and soft tissue calcification are often present.9, 13 The trauma of the biopsy can lead to worsening of the disease.
Secondary to its association with end stage renal disease, laboratory data often reveals elevated blood urea nitrogen (BUN), creatinine, parathyroid hormone, and calcium‐phosphate product. Bone scans show increased uptake in the subcutaneous calcified plaques.14 X‐rays utilizing mammogram technique have demonstrated arteriolar calcification.15
Besides chronic kidney disease, other potential risk factors include protein C and S deficiencies, obesity, warfarin use, high calcium containing dialysates, liver disease, and systemic corticosteroids.4, 9, 11, 16
Calciphylaxis is a difficult disease to treat with a mortality of 60% to 70%9 and a 1‐year survival rate of 45.8%.11 There is no consistently effective treatment.5 Therapy therefore, is focused on symptom control, debridement, and treatment of infection. Mortality is most commonly due to wound infections and resulting septicemia. Meticulous wound care is important with any infection treated early and aggressively. Though trauma and surgical procedures have been known to precipitate ulcerations, given their high rate of infection early surgical debridement of wounds is often required and has been shown to improve mortality.11, 17 Because of the poor healing of the involved tissues, wounds are often left to close by secondary intention or in some circumstances with vacuum assistance.2
As secondary hyperparathyroidism is common, attempts are often made to lower the calcium‐phosphate product. This often requires parathyroidectomy.18 Calcium containing phosphate binders are avoided and low calcium dialysate used.19 However, the above interventions do not consistently improve mortality.5, 11
Other potential treatments include: hyperbaric oxygen therapy,20 intravenous sodium thiosulfate,14 low‐dose tissue plasminogen activator,21 cinacalcet,22 etidronate disodium,23 and maggots.24 Because of the rarity of the condition, most of the literature to date is anecdotal and based on case reports and small retrospective studies.
Conclusions
As the number of patients who develop chronic kidney disease and require hemodialysis increases, it is likely that the number of patients who develop calciphylaxis will also increase. Hospitalists, besides nephrologists, should therefore become familiar with the presentation of this disease as it is possible, although unproven, that treatment in the early stage of the disease may result in a better response. Although several treatment modalities have been used to treat calcific uremic arteriolopathy or calciphylaxis, it remains unclear what is the best treatment for these patients. Carefully done clinical trials using some of the treatment modalities mentioned will help physicians decide what the most appropriate treatment is for patients with this debilitating and often fatal disease.
- ,,,.Early recognition and treatment of calciphylaxis.South Med J.2003;96:53–55.
- ,,,.Calciphylaxis: medical and surgical management of chronic extensive wounds in a renal dialysis population.Plast Reconstr Surg.2004;113:304–312.
- ,,, et al.Cutaneous necrosis by calcific uremic arteriolopathy.Int J Dermatol.2005;44:101–106.
- ,.Calciphylaxis.Int J Dermatol.2007;46:231–236.
- ,,.Calcific uremic arteriolopathy: Advances in pathogenesis and treatment.Semin Dial.2007;20:150–157.
- ,,, et al.Calciphylaxis associated with acute, reversible renal failure in the setting of alcoholic cirrhosis.J Am Acad Dermatol2004;50:S125–S128.
- ,,, et al.An unusual presentation of calciphylaxis due to primary hyperparathyroidism.Arch Pathol Lab Med.2001;125:1351–1353.
- ,,, et al.Calciphylaxis associated with metastatic breast carcinoma.J Am Acad Dermatol.1999;41:295–298.
- ,.Panniculitis. In: Freeberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, eds.Fitzpatrick's Dermatology in General Medicine.6th ed.McGraw‐Hill,New York, NY.2003:1051–1052.
- ,,,.The vascular lesions associated with skin necrosis in renal disease.Br J Dermatol.1983;109:85–95.
- ,,, et al.Calciphylaxis: Natural history, risk factor analysis, and outcome.J Am Acad Dermatol.2007;56:569–579.
- ,.Calciphylaxis: emerging concepts in prevention, diagnosis, and treatment.Semin Dial.2002;15:172–186.
- ,,,.Lever's Histopathology of the Skin.8th ed.Lippincott, Williams 43:1104–1108.
- ,,.A case report comparing various radiological tests in the diagnosis of calcific uremic arteriolopathy.Am J Kidney Dis.2006;48:659–661.
- .Ever‐changing concepts of calciphylaxis.Intern Med.2004;43:7–8.
- ,,, et al.Is calciphylaxis best treated surgically or medically?Surgery2000;128:967–971.
- ,,, et al.Therapy for calciphylaxis: an outcome analysis.Surgery.2003;134:941–945.
- ,,.Successful treatment of severe calciphylaxis in a hemodialysis patient using low‐calcium dialysate and medical parathyroidectomy: case report and literature review.Ren Fail.2004;26:77–82.
- ,,, et al.Hyperbaric oxygen therapy for calcific uremic arteriolopathy: a case series.J Nephrol.2002;15:676–680.
- ,,, et al.Low‐dose tissue plasminogen activator for calciphylaxis.Arch Dermatol.2004;140:1045–1048.
- ,,.Cinacalcet for the treatment of calciphylaxis.Arch Dermatol.2007;143:152–154.
- ,,, et al.Successful treatment of a patient with severe calcific uremic arteriolopathy (calciphylaxis) by etidronate disodium.Am J Kidney Dis.2006;48:151–154.
- ,,, et al.Sterile maggots as adjuvant procedure for local treatment in a patient with proximal calciphylaxis.Nefrologia.2005;25:559–562.
Narrative Description
This Case Report reviews the presentation, the differential diagnosis and the treatment modalities used to treat calciphylaxis. It emphasizes the poor prognosis and that there is inadequate clinical experience to guide a physician as to what the most appropriate treatment is despite promising anecdotal reports about a variety of agents. The report demonstrates that intravenous sodium thiosulfate is tolerated.
Key Points
-
Calciphylaxis occurs in 1 to 4% of patients with end stage renal failure.
-
Two patterns of presentation are generally recognizedcentral and peripheral.
-
Pain is a prominent symptom and eschar formation is usually present.
-
The role of surgery is controversial.
-
Several promising modalities for the treatment of this condition have been described in anecdotal reports.
Calciphylaxis is a rare condition. It is seen most frequently in patients with chronic kidney disease and can affect any part of the body.14 Calciphylaxis is increasingly being referred to as calcific uremic arteriolopathy as this term more accurately reflects the histology of vascular calcification in small‐ and medium‐sized arteries, intimal arterial hypertrophy, and small vessel thrombosis associated with panniculitis, dermal necrosis, and eschar formation.5 Pain is a prominent symptom. The most effective treatment for this condition remains uncertain.
Case Report
A 68‐year‐old female presented with an 8‐month history of increasing lower extremity edema, and numerous large, painful, necrotic ulcers with an associated foul odor. She had a past medical history of type 2 diabetes mellitus, hypertension, end stage renal disease requiring hemodialysis 3 times a week for the previous 6 months, severe peripheral vascular disease, coronary artery disease for which she had previously undergone coronary artery bypass surgery, multiple myocardial infarctions, and congestive heart failure with an ejection fraction of 20%. She had also suffered from numerous infections including septicemia, endocarditis, and a sternal wound infection in the past with no current evidence of septicemia or endocarditis. The patient was not on calcium supplements, Vitamin D, warfarin or calcium‐containing phosphate binders.
Eight months prior to admission she developed vesicles on the left thigh that slowly progressed to large, extremely painful, violaceous, indurated plaques with central ulceration and eschar (Figure 1). She subsequently developed several smaller lesions with similar morphology on her legs and feet and gangrene of her left big toe (Figure 2). A biopsy from the left thigh was consistent with calciphylaxis with associated necrosis of the deep dermis and subcutaneous tissues. The patient's lesions were aggressively debrided, broad spectrum antibiotics given, and the patient dialyzed with low calcium dialysates. Ultimately intravenous sodium thiosulfate (25 g intravenously, over 60 minutes), was given which she tolerated with no side effect. Sodium thiosulfate is thought to act by forming highly soluble calcium thiosulfate salts and therefore mobilizing tissue calcium.5 Hyperbaric oxygen was contraindicated because of the patient's left ventricular ejection fraction (LVEF) of 20% and previous history of congestive heart failure as this treatment modality may precipitate congestive heart failure in a patient with a low LVEF particularly with a past history of congestive heart failure. Her condition continued to deteriorate and she died a few days after initiation of intravenous sodium thiosulfate infusion secondary to a massive gastrointestinal bleed.


Discussion
The differential diagnosis for painful necrotic cutaneous ulcerations with eschar formation includes: calciphylaxis, cryoglobulinemia, cryofibrinogenema, peripheral vascular disease, embolic phenomenon (endocarditis, septic, cholesterol), warfarin skin necrosis, brown recluse spider bites, hypercoagulable states, hyperoxaluria, and necrotizing vasculitis.14
Calciphylaxis is a rare entity that affects approximately 1% to 4% of end stage renal failure patients.1, 3 The typical patient is a morbidly obese, female with longstanding end stage renal disease, diabetes, hyperphosphatemia and an elevated calcium‐phosphate product usually greater than 60 mg2/dL2.1, 3 It has also been described in patients with alcoholic cirrhosis and acute reversible renal failure,6 primary and secondary hyperparathyroidism,7 and metastatic breast cancer.8
Patients typically present with symmetric lesions that evolve from erythematous to violaceous, livedo‐reticularis like patches or plaques with occasional bullae to painful, indurated, necrotic plaques that subsequently ulcerate. The ulcerations are slow to heal and covered with eschar.4, 9
There are 2 patterns of involvement. The central/proximal pattern involves the abdomen, gluteal region, and thighs while the peripheral/distal pattern involves the extremities distal to the elbows and knees.1, 2, 4 The central pattern tends to carry a worse prognosis,9, 10 though this has not been validated in all reports and recent literature suggests that patients with both patterns have the worst prognosis.11
A biopsy may be required to exclude other diagnoses. The histology demonstrates an obliterative vasculopathy secondary to the vascular intimal changes and endovascular fibrosis.12 A suggestive finding is calcification of the medial wall of small‐ and medium‐sized arteries and arterioles with associated intimal hyperplasia and fibrosis. Necrosis of the surrounding tissue, panniculitis, and soft tissue calcification are often present.9, 13 The trauma of the biopsy can lead to worsening of the disease.
Secondary to its association with end stage renal disease, laboratory data often reveals elevated blood urea nitrogen (BUN), creatinine, parathyroid hormone, and calcium‐phosphate product. Bone scans show increased uptake in the subcutaneous calcified plaques.14 X‐rays utilizing mammogram technique have demonstrated arteriolar calcification.15
Besides chronic kidney disease, other potential risk factors include protein C and S deficiencies, obesity, warfarin use, high calcium containing dialysates, liver disease, and systemic corticosteroids.4, 9, 11, 16
Calciphylaxis is a difficult disease to treat with a mortality of 60% to 70%9 and a 1‐year survival rate of 45.8%.11 There is no consistently effective treatment.5 Therapy therefore, is focused on symptom control, debridement, and treatment of infection. Mortality is most commonly due to wound infections and resulting septicemia. Meticulous wound care is important with any infection treated early and aggressively. Though trauma and surgical procedures have been known to precipitate ulcerations, given their high rate of infection early surgical debridement of wounds is often required and has been shown to improve mortality.11, 17 Because of the poor healing of the involved tissues, wounds are often left to close by secondary intention or in some circumstances with vacuum assistance.2
As secondary hyperparathyroidism is common, attempts are often made to lower the calcium‐phosphate product. This often requires parathyroidectomy.18 Calcium containing phosphate binders are avoided and low calcium dialysate used.19 However, the above interventions do not consistently improve mortality.5, 11
Other potential treatments include: hyperbaric oxygen therapy,20 intravenous sodium thiosulfate,14 low‐dose tissue plasminogen activator,21 cinacalcet,22 etidronate disodium,23 and maggots.24 Because of the rarity of the condition, most of the literature to date is anecdotal and based on case reports and small retrospective studies.
Conclusions
As the number of patients who develop chronic kidney disease and require hemodialysis increases, it is likely that the number of patients who develop calciphylaxis will also increase. Hospitalists, besides nephrologists, should therefore become familiar with the presentation of this disease as it is possible, although unproven, that treatment in the early stage of the disease may result in a better response. Although several treatment modalities have been used to treat calcific uremic arteriolopathy or calciphylaxis, it remains unclear what is the best treatment for these patients. Carefully done clinical trials using some of the treatment modalities mentioned will help physicians decide what the most appropriate treatment is for patients with this debilitating and often fatal disease.
Narrative Description
This Case Report reviews the presentation, the differential diagnosis and the treatment modalities used to treat calciphylaxis. It emphasizes the poor prognosis and that there is inadequate clinical experience to guide a physician as to what the most appropriate treatment is despite promising anecdotal reports about a variety of agents. The report demonstrates that intravenous sodium thiosulfate is tolerated.
Key Points
-
Calciphylaxis occurs in 1 to 4% of patients with end stage renal failure.
-
Two patterns of presentation are generally recognizedcentral and peripheral.
-
Pain is a prominent symptom and eschar formation is usually present.
-
The role of surgery is controversial.
-
Several promising modalities for the treatment of this condition have been described in anecdotal reports.
Calciphylaxis is a rare condition. It is seen most frequently in patients with chronic kidney disease and can affect any part of the body.14 Calciphylaxis is increasingly being referred to as calcific uremic arteriolopathy as this term more accurately reflects the histology of vascular calcification in small‐ and medium‐sized arteries, intimal arterial hypertrophy, and small vessel thrombosis associated with panniculitis, dermal necrosis, and eschar formation.5 Pain is a prominent symptom. The most effective treatment for this condition remains uncertain.
Case Report
A 68‐year‐old female presented with an 8‐month history of increasing lower extremity edema, and numerous large, painful, necrotic ulcers with an associated foul odor. She had a past medical history of type 2 diabetes mellitus, hypertension, end stage renal disease requiring hemodialysis 3 times a week for the previous 6 months, severe peripheral vascular disease, coronary artery disease for which she had previously undergone coronary artery bypass surgery, multiple myocardial infarctions, and congestive heart failure with an ejection fraction of 20%. She had also suffered from numerous infections including septicemia, endocarditis, and a sternal wound infection in the past with no current evidence of septicemia or endocarditis. The patient was not on calcium supplements, Vitamin D, warfarin or calcium‐containing phosphate binders.
Eight months prior to admission she developed vesicles on the left thigh that slowly progressed to large, extremely painful, violaceous, indurated plaques with central ulceration and eschar (Figure 1). She subsequently developed several smaller lesions with similar morphology on her legs and feet and gangrene of her left big toe (Figure 2). A biopsy from the left thigh was consistent with calciphylaxis with associated necrosis of the deep dermis and subcutaneous tissues. The patient's lesions were aggressively debrided, broad spectrum antibiotics given, and the patient dialyzed with low calcium dialysates. Ultimately intravenous sodium thiosulfate (25 g intravenously, over 60 minutes), was given which she tolerated with no side effect. Sodium thiosulfate is thought to act by forming highly soluble calcium thiosulfate salts and therefore mobilizing tissue calcium.5 Hyperbaric oxygen was contraindicated because of the patient's left ventricular ejection fraction (LVEF) of 20% and previous history of congestive heart failure as this treatment modality may precipitate congestive heart failure in a patient with a low LVEF particularly with a past history of congestive heart failure. Her condition continued to deteriorate and she died a few days after initiation of intravenous sodium thiosulfate infusion secondary to a massive gastrointestinal bleed.


Discussion
The differential diagnosis for painful necrotic cutaneous ulcerations with eschar formation includes: calciphylaxis, cryoglobulinemia, cryofibrinogenema, peripheral vascular disease, embolic phenomenon (endocarditis, septic, cholesterol), warfarin skin necrosis, brown recluse spider bites, hypercoagulable states, hyperoxaluria, and necrotizing vasculitis.14
Calciphylaxis is a rare entity that affects approximately 1% to 4% of end stage renal failure patients.1, 3 The typical patient is a morbidly obese, female with longstanding end stage renal disease, diabetes, hyperphosphatemia and an elevated calcium‐phosphate product usually greater than 60 mg2/dL2.1, 3 It has also been described in patients with alcoholic cirrhosis and acute reversible renal failure,6 primary and secondary hyperparathyroidism,7 and metastatic breast cancer.8
Patients typically present with symmetric lesions that evolve from erythematous to violaceous, livedo‐reticularis like patches or plaques with occasional bullae to painful, indurated, necrotic plaques that subsequently ulcerate. The ulcerations are slow to heal and covered with eschar.4, 9
There are 2 patterns of involvement. The central/proximal pattern involves the abdomen, gluteal region, and thighs while the peripheral/distal pattern involves the extremities distal to the elbows and knees.1, 2, 4 The central pattern tends to carry a worse prognosis,9, 10 though this has not been validated in all reports and recent literature suggests that patients with both patterns have the worst prognosis.11
A biopsy may be required to exclude other diagnoses. The histology demonstrates an obliterative vasculopathy secondary to the vascular intimal changes and endovascular fibrosis.12 A suggestive finding is calcification of the medial wall of small‐ and medium‐sized arteries and arterioles with associated intimal hyperplasia and fibrosis. Necrosis of the surrounding tissue, panniculitis, and soft tissue calcification are often present.9, 13 The trauma of the biopsy can lead to worsening of the disease.
Secondary to its association with end stage renal disease, laboratory data often reveals elevated blood urea nitrogen (BUN), creatinine, parathyroid hormone, and calcium‐phosphate product. Bone scans show increased uptake in the subcutaneous calcified plaques.14 X‐rays utilizing mammogram technique have demonstrated arteriolar calcification.15
Besides chronic kidney disease, other potential risk factors include protein C and S deficiencies, obesity, warfarin use, high calcium containing dialysates, liver disease, and systemic corticosteroids.4, 9, 11, 16
Calciphylaxis is a difficult disease to treat with a mortality of 60% to 70%9 and a 1‐year survival rate of 45.8%.11 There is no consistently effective treatment.5 Therapy therefore, is focused on symptom control, debridement, and treatment of infection. Mortality is most commonly due to wound infections and resulting septicemia. Meticulous wound care is important with any infection treated early and aggressively. Though trauma and surgical procedures have been known to precipitate ulcerations, given their high rate of infection early surgical debridement of wounds is often required and has been shown to improve mortality.11, 17 Because of the poor healing of the involved tissues, wounds are often left to close by secondary intention or in some circumstances with vacuum assistance.2
As secondary hyperparathyroidism is common, attempts are often made to lower the calcium‐phosphate product. This often requires parathyroidectomy.18 Calcium containing phosphate binders are avoided and low calcium dialysate used.19 However, the above interventions do not consistently improve mortality.5, 11
Other potential treatments include: hyperbaric oxygen therapy,20 intravenous sodium thiosulfate,14 low‐dose tissue plasminogen activator,21 cinacalcet,22 etidronate disodium,23 and maggots.24 Because of the rarity of the condition, most of the literature to date is anecdotal and based on case reports and small retrospective studies.
Conclusions
As the number of patients who develop chronic kidney disease and require hemodialysis increases, it is likely that the number of patients who develop calciphylaxis will also increase. Hospitalists, besides nephrologists, should therefore become familiar with the presentation of this disease as it is possible, although unproven, that treatment in the early stage of the disease may result in a better response. Although several treatment modalities have been used to treat calcific uremic arteriolopathy or calciphylaxis, it remains unclear what is the best treatment for these patients. Carefully done clinical trials using some of the treatment modalities mentioned will help physicians decide what the most appropriate treatment is for patients with this debilitating and often fatal disease.
- ,,,.Early recognition and treatment of calciphylaxis.South Med J.2003;96:53–55.
- ,,,.Calciphylaxis: medical and surgical management of chronic extensive wounds in a renal dialysis population.Plast Reconstr Surg.2004;113:304–312.
- ,,, et al.Cutaneous necrosis by calcific uremic arteriolopathy.Int J Dermatol.2005;44:101–106.
- ,.Calciphylaxis.Int J Dermatol.2007;46:231–236.
- ,,.Calcific uremic arteriolopathy: Advances in pathogenesis and treatment.Semin Dial.2007;20:150–157.
- ,,, et al.Calciphylaxis associated with acute, reversible renal failure in the setting of alcoholic cirrhosis.J Am Acad Dermatol2004;50:S125–S128.
- ,,, et al.An unusual presentation of calciphylaxis due to primary hyperparathyroidism.Arch Pathol Lab Med.2001;125:1351–1353.
- ,,, et al.Calciphylaxis associated with metastatic breast carcinoma.J Am Acad Dermatol.1999;41:295–298.
- ,.Panniculitis. In: Freeberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, eds.Fitzpatrick's Dermatology in General Medicine.6th ed.McGraw‐Hill,New York, NY.2003:1051–1052.
- ,,,.The vascular lesions associated with skin necrosis in renal disease.Br J Dermatol.1983;109:85–95.
- ,,, et al.Calciphylaxis: Natural history, risk factor analysis, and outcome.J Am Acad Dermatol.2007;56:569–579.
- ,.Calciphylaxis: emerging concepts in prevention, diagnosis, and treatment.Semin Dial.2002;15:172–186.
- ,,,.Lever's Histopathology of the Skin.8th ed.Lippincott, Williams 43:1104–1108.
- ,,.A case report comparing various radiological tests in the diagnosis of calcific uremic arteriolopathy.Am J Kidney Dis.2006;48:659–661.
- .Ever‐changing concepts of calciphylaxis.Intern Med.2004;43:7–8.
- ,,, et al.Is calciphylaxis best treated surgically or medically?Surgery2000;128:967–971.
- ,,, et al.Therapy for calciphylaxis: an outcome analysis.Surgery.2003;134:941–945.
- ,,.Successful treatment of severe calciphylaxis in a hemodialysis patient using low‐calcium dialysate and medical parathyroidectomy: case report and literature review.Ren Fail.2004;26:77–82.
- ,,, et al.Hyperbaric oxygen therapy for calcific uremic arteriolopathy: a case series.J Nephrol.2002;15:676–680.
- ,,, et al.Low‐dose tissue plasminogen activator for calciphylaxis.Arch Dermatol.2004;140:1045–1048.
- ,,.Cinacalcet for the treatment of calciphylaxis.Arch Dermatol.2007;143:152–154.
- ,,, et al.Successful treatment of a patient with severe calcific uremic arteriolopathy (calciphylaxis) by etidronate disodium.Am J Kidney Dis.2006;48:151–154.
- ,,, et al.Sterile maggots as adjuvant procedure for local treatment in a patient with proximal calciphylaxis.Nefrologia.2005;25:559–562.
- ,,,.Early recognition and treatment of calciphylaxis.South Med J.2003;96:53–55.
- ,,,.Calciphylaxis: medical and surgical management of chronic extensive wounds in a renal dialysis population.Plast Reconstr Surg.2004;113:304–312.
- ,,, et al.Cutaneous necrosis by calcific uremic arteriolopathy.Int J Dermatol.2005;44:101–106.
- ,.Calciphylaxis.Int J Dermatol.2007;46:231–236.
- ,,.Calcific uremic arteriolopathy: Advances in pathogenesis and treatment.Semin Dial.2007;20:150–157.
- ,,, et al.Calciphylaxis associated with acute, reversible renal failure in the setting of alcoholic cirrhosis.J Am Acad Dermatol2004;50:S125–S128.
- ,,, et al.An unusual presentation of calciphylaxis due to primary hyperparathyroidism.Arch Pathol Lab Med.2001;125:1351–1353.
- ,,, et al.Calciphylaxis associated with metastatic breast carcinoma.J Am Acad Dermatol.1999;41:295–298.
- ,.Panniculitis. In: Freeberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, eds.Fitzpatrick's Dermatology in General Medicine.6th ed.McGraw‐Hill,New York, NY.2003:1051–1052.
- ,,,.The vascular lesions associated with skin necrosis in renal disease.Br J Dermatol.1983;109:85–95.
- ,,, et al.Calciphylaxis: Natural history, risk factor analysis, and outcome.J Am Acad Dermatol.2007;56:569–579.
- ,.Calciphylaxis: emerging concepts in prevention, diagnosis, and treatment.Semin Dial.2002;15:172–186.
- ,,,.Lever's Histopathology of the Skin.8th ed.Lippincott, Williams 43:1104–1108.
- ,,.A case report comparing various radiological tests in the diagnosis of calcific uremic arteriolopathy.Am J Kidney Dis.2006;48:659–661.
- .Ever‐changing concepts of calciphylaxis.Intern Med.2004;43:7–8.
- ,,, et al.Is calciphylaxis best treated surgically or medically?Surgery2000;128:967–971.
- ,,, et al.Therapy for calciphylaxis: an outcome analysis.Surgery.2003;134:941–945.
- ,,.Successful treatment of severe calciphylaxis in a hemodialysis patient using low‐calcium dialysate and medical parathyroidectomy: case report and literature review.Ren Fail.2004;26:77–82.
- ,,, et al.Hyperbaric oxygen therapy for calcific uremic arteriolopathy: a case series.J Nephrol.2002;15:676–680.
- ,,, et al.Low‐dose tissue plasminogen activator for calciphylaxis.Arch Dermatol.2004;140:1045–1048.
- ,,.Cinacalcet for the treatment of calciphylaxis.Arch Dermatol.2007;143:152–154.
- ,,, et al.Successful treatment of a patient with severe calcific uremic arteriolopathy (calciphylaxis) by etidronate disodium.Am J Kidney Dis.2006;48:151–154.
- ,,, et al.Sterile maggots as adjuvant procedure for local treatment in a patient with proximal calciphylaxis.Nefrologia.2005;25:559–562.
Should patients on long-term warfarin take aspirin for heart disease?
The literature on this topic is limited, but it suggests that the decision to prescribe aspirin to patients already taking warfarin (Coumadin) should be individualized. On one hand, the cardiovascular benefit of starting or continuing aspirin in patients already on warfarin outweighs the increased risk of bleeding in patients presenting with an acute coronary syndrome or those with mechanical heart valves or coronary stents. However, for patients with stable coronary artery disease or at risk of coronary disease, the benefit of adding aspirin is not substantial, and continuing warfarin alone may be the preferred strategy.
In patients with coronary artery disease, aspirin has been shown to reduce the rate of death due to all causes by about 18% and the rate of vascular events by about 25% to 30%.1,2 Warfarin is at least as effective as aspirin in reducing the rate of future cardiovascular events (especially if the target international normalized ratio [INR] is greater than 2.5), albeit with a higher bleeding risk.3–6
The decision to prescribe or continue aspirin in patients with coronary artery disease who also need long-term anticoagulation with warfarin for an unrelated medical problem, such as pulmonary emboli, requires careful assessment of the individual patient’s bleeding risk and cardiovascular benefit.
ESTIMATING THE BLEEDING RISK FOR PATIENTS ON WARFARIN
In patients taking warfarin, the risk of major bleeding (defined in most studies as hospitalization because of bleeding and requiring transfusion of at least two units of packed red cells, or an intracranial, intraperitoneal, or fatal bleeding episode) is reported to be about 2.0% to 3.8% per person-year.7–11 The risk of major bleeding with aspirin alone is estimated to be 0.13% per person-year,12 but when aspirin is combined with warfarin, the risk increases significantly.13 In a meta-analysis of randomized controlled trials,14 the risk of major bleeding was calculated to be about 1.5 times higher with combination therapy with aspirin and warfarin than with warfarin alone.
The individual’s bleeding risk depends on specific risk factors and the intensity of anticoagulation.15 The outpatient Bleeding Risk Index (BRI) can be used to estimate the bleeding risk for patients on warfarin.16 The BRI includes four risk factors for major bleeding, each scored as 1 point:
- Age 65 or older
- History of gastrointestinal bleeding
- History of stroke
- One or more comorbid conditions—recent myocardial infarction, anemia (hematocrit < 30%), renal impairment (serum creatinine level > 1.5 mg/dL), or diabetes mellitus.
The risk is low if the score is 0, moderate if the score is 1 or 2, and high if the score is 3 or more. In a validation study of the BRI, the rate of major bleeding was found to be 0.8%, 2.5%, and 10.6% per person-year on warfarin in the low, intermediate, and high-risk groups, respectively.17 In addition, compared with patients with a target INR of 2.5, those with a target INR higher than 3.0 have a higher frequency of bleeding episodes.10,15
CONDITIONS IN WHICH ADDING ASPIRIN TO WARFARIN IS FAVORABLE
Acute coronary syndromes
Drugs that inhibit platelet function are the mainstay of medical treatment for acute coronary syndromes. The American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend that aspirin be started in patients who have an acute myocardial infarction even if they have been receiving warfarin long-term and their INR is in the therapeutic range, especially if a percutaneous coronary intervention is anticipated.4
After percutaneous coronary intervention
In patients who have undergone percutaneous coronary intervention with stent implantation, dual antiplatelet therapy with aspirin and a thienopyridine—ie, clopidogrel (Plavix) or ticlopidine (Ticlid)—is superior to aspirin or warfarin alone in reducing the risk of stent thrombosis and major adverse cardiovascular events such as myocardial infarction or urgent revascularization.18,19 If patients have an indication for long-term anticoagulation, triple therapy with aspirin, warfarin, and clopidogrel or ticlopidine may be considered in order to reduce the likelihood of stent thrombosis.4,20,21 In such patients the INR should be maintained between 2.0 and 3.0 to reduce the risk of bleeding.
The duration of triple therapy is guided by the type of stent used. For bare metal stents, aspirin, clopidogrel or ticlopidine, and warfarin should be given for at least 1 month, after which clopidogrel or ticlopidine may be discontinued. If drug-eluting stents are used, the duration of clopidogrel or ticlopidine therapy should be extended to 1 year or more.4,22
Mechanical heart valves
In patients with mechanical heart valves, the combination of aspirin and warfarin has been shown to decrease the frequency of thromboembolism.23 Guidelines recommend adding aspirin (75 to 100 mg per day) to warfarin in all patients with mechanical valves, especially in patients who have had an embolus while on warfarin therapy or who have a history of cerebrovascular or peripheral vascular disease, a hypercoagulable state, or coronary artery disease.24
CONDITIONS IN WHICH WARFARIN ALONE MAY BE SUFFICIENT
At risk of coronary artery disease
Aspirin therapy is generally recommended as primary prevention for patients whose estimated risk of coronary events is 1.5% per year or higher.25 However, warfarin has also been shown to be effective in the primary prevention of coronary artery disease in men,26 and for patients already taking warfarin, the possible benefit of adding aspirin for primary prevention is outweighed by the increased risk of major bleeding.14 The Medical Research Council directly compared low-intensity warfarin therapy (mean INR 1.47), aspirin, and placebo in a two-by-two factorial study of primary prevention of ischemic heart disease in men.26 Warfarin was more effective than aspirin, and men who received warfarin plus aspirin or warfarin plus placebo had a rate of ischemic heart disease that was 21% lower than those who received aspirin plus placebo or double placebo, and their rate of all-cause mortality was 17% lower. Combining aspirin and warfarin for patients at risk of coronary disease led to a higher rate of major bleeding but no difference in cardiovascular events or all-cause mortality (odds ratio 0.98; 95% confidence interval 0.77–1.25).14
Stable coronary artery disease without mechanical heart valves or stents
Large randomized trials have found warfarin to be effective in secondary prevention of coronary artery disease.4–6 For most patients with stable coronary artery disease (ie, who have had no ischemic events or coronary interventions in the last 6 months) who need anticoagulation because of atrial fibrillation or venous thromboembolism, warfarin alone (target INR 2.0–3.0) should provide satisfactory antithrombotic prophylaxis against both cerebral and myocardial ischemic events.27 The addition of an antiplatelet agent is not required unless a patient has a coronary stent, a mechanical valve, or an excessive thrombotic risk.4,24,27
TAKE-HOME POINTS
For patients receiving warfarin therapy, whether to add or continue aspirin to their treatment is a common clinical question. The risk of bleeding is greater with combination therapy than with warfarin alone. The cardiovascular benefit varies depending on the clinical situation:
- In patients who have had an acute coronary syndrome or who have a coronary stent or mechanical valve, combination therapy is usually recommended because the benefits outweigh the risks.
- In patients with stable coronary artery disease or those without coronary artery disease who are at risk of coronary events, the risks outweigh the benefits. Combination therapy is usually not indicated in these patients.
- Weisman SM, Graham DY. Evaluation of the benefits and risks of low-dose aspirin in the secondary prevention of cardiovascular and cerebrovascular events. Arch Intern Med 2002; 162:2197–2202.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med 2002; 347:969–974.
- Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction). J Am Coll Cardiol 2004; 44:E1–E211.
- Van Es RF, Jonker JJ, Verheugt FW, et al. Antithrombotics in the Secondary Prevention of Events in Coronary Thrombosis-2 (ASPECT-2) Research Group. Aspirin and coumadin after acute coronary syndromes (the ASPECT-2 study): a randomised controlled trial. Lancet 2002; 360:109–113.
- Anand SS, Yusuf S. Oral anticoagulant therapy in patients with coronary artery disease: a meta-analysis. JAMA 1999; 282:2058–2067.
- Schulman S, Granqvist S, Holmstrom M, et al. The duration of oral anticoagulant therapy after a second episode of venous thromboembolism. The Duration of Anticoagulation Trial Study Group. N Engl J Med 1997; 336:393–398.
- Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999; 340:901–907.
- Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med 2001; 345:165–169.
- Levine MN, Raskob G, Beyth RJ, Kearon C, Schulman S. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126 suppl:287S–310S.
- Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med 2003; 139:893–900.
- McQuaid KR, Laine L. Systematic review and meta-analysis of adverse events of low-dose aspirin and clopidogrel in randomized controlled trials. Am J Med 2006; 119:624–638.
- Rothberg MB, Celestin C, Fiore LD, Lawler E, Cook JR. Warfarin plus aspirin after myocardial infarction or the acute coronary syndrome: meta-analysis with estimates of risk and benefit. Ann Intern Med 2005; 143:241–250.
- Dentali F, Douketis JD, Lim W, Crowther M. Combined aspirin-oral anticoagulant therapy compared with oral anticoagulant therapy alone among patients at risk for cardiovascular disease: a meta-analysis of randomized trials. Arch Intern Med 2007; 167:117–124.
- Hirsh J, Fuster V, Ansell J, Halperin JL. American Heart Association; American College of Cardiology Foundation. American Heart Association/American College of Cardiology Foundation guide to warfarin therapy. Circulation 2003; 107:1692–1711.
- Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med 1998; 105:91–99.
- Aspinall SL, DeSanzo BE, Trilli LE, Good CB. Bleeding Risk Index in an anticoagulation clinic. Assessment by indication and implications for care. J Gen Intern Med 2005; 20:1008–1013.
- Mehta SR, Yusuf S, Peters RJ, et al. Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCICURE study. Lancet 2001; 358:527–533.
- Bertrand ME, Legrand V, Boland J, et al. Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. The Full Anticoagulation versus Aspirin and Ticlopidine (FANTASTIC) study. Circulation 1998; 98:1597–1603.
- Kushner FG, Antman EM. Oral anticoagulation for atrial fibrillation after ST-elevation myocardial infarction: new evidence to guide clinical practice. Circulation 2005; 112:3212–3214.
- Porter A, Konstantino Y, Iakobishvili Z, Shachar L, Battler A, Hasdai D. Short-term triple therapy with aspirin, warfarin, and a thienopyridine among patients undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv 2006; 68:56–61.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 Guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction—executive summary. A report of the ACC-AHA Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction). J Am Coll Cardiol 2007; 50:652–726.
- Turpie AG, Gent M, Laupacis A, et al. A comparison of aspirin with placebo in patients treated with warfarin after heart-valve replacement. N Engl J Med 1993; 329:524–529.
- Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the ACC/AHA Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease). Circulation 2006; 114:e84–e231.
- Lauer MS. Clinical practice. Aspirin for primary prevention of coronary events. N Engl J Med 2002; 346:1468–1474.
- The Medical Research Council’s General Practice Research Framework. Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. Lancet 1998; 351:233–241.
- Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation. Circulation 2006; 114:260–335.
The literature on this topic is limited, but it suggests that the decision to prescribe aspirin to patients already taking warfarin (Coumadin) should be individualized. On one hand, the cardiovascular benefit of starting or continuing aspirin in patients already on warfarin outweighs the increased risk of bleeding in patients presenting with an acute coronary syndrome or those with mechanical heart valves or coronary stents. However, for patients with stable coronary artery disease or at risk of coronary disease, the benefit of adding aspirin is not substantial, and continuing warfarin alone may be the preferred strategy.
In patients with coronary artery disease, aspirin has been shown to reduce the rate of death due to all causes by about 18% and the rate of vascular events by about 25% to 30%.1,2 Warfarin is at least as effective as aspirin in reducing the rate of future cardiovascular events (especially if the target international normalized ratio [INR] is greater than 2.5), albeit with a higher bleeding risk.3–6
The decision to prescribe or continue aspirin in patients with coronary artery disease who also need long-term anticoagulation with warfarin for an unrelated medical problem, such as pulmonary emboli, requires careful assessment of the individual patient’s bleeding risk and cardiovascular benefit.
ESTIMATING THE BLEEDING RISK FOR PATIENTS ON WARFARIN
In patients taking warfarin, the risk of major bleeding (defined in most studies as hospitalization because of bleeding and requiring transfusion of at least two units of packed red cells, or an intracranial, intraperitoneal, or fatal bleeding episode) is reported to be about 2.0% to 3.8% per person-year.7–11 The risk of major bleeding with aspirin alone is estimated to be 0.13% per person-year,12 but when aspirin is combined with warfarin, the risk increases significantly.13 In a meta-analysis of randomized controlled trials,14 the risk of major bleeding was calculated to be about 1.5 times higher with combination therapy with aspirin and warfarin than with warfarin alone.
The individual’s bleeding risk depends on specific risk factors and the intensity of anticoagulation.15 The outpatient Bleeding Risk Index (BRI) can be used to estimate the bleeding risk for patients on warfarin.16 The BRI includes four risk factors for major bleeding, each scored as 1 point:
- Age 65 or older
- History of gastrointestinal bleeding
- History of stroke
- One or more comorbid conditions—recent myocardial infarction, anemia (hematocrit < 30%), renal impairment (serum creatinine level > 1.5 mg/dL), or diabetes mellitus.
The risk is low if the score is 0, moderate if the score is 1 or 2, and high if the score is 3 or more. In a validation study of the BRI, the rate of major bleeding was found to be 0.8%, 2.5%, and 10.6% per person-year on warfarin in the low, intermediate, and high-risk groups, respectively.17 In addition, compared with patients with a target INR of 2.5, those with a target INR higher than 3.0 have a higher frequency of bleeding episodes.10,15
CONDITIONS IN WHICH ADDING ASPIRIN TO WARFARIN IS FAVORABLE
Acute coronary syndromes
Drugs that inhibit platelet function are the mainstay of medical treatment for acute coronary syndromes. The American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend that aspirin be started in patients who have an acute myocardial infarction even if they have been receiving warfarin long-term and their INR is in the therapeutic range, especially if a percutaneous coronary intervention is anticipated.4
After percutaneous coronary intervention
In patients who have undergone percutaneous coronary intervention with stent implantation, dual antiplatelet therapy with aspirin and a thienopyridine—ie, clopidogrel (Plavix) or ticlopidine (Ticlid)—is superior to aspirin or warfarin alone in reducing the risk of stent thrombosis and major adverse cardiovascular events such as myocardial infarction or urgent revascularization.18,19 If patients have an indication for long-term anticoagulation, triple therapy with aspirin, warfarin, and clopidogrel or ticlopidine may be considered in order to reduce the likelihood of stent thrombosis.4,20,21 In such patients the INR should be maintained between 2.0 and 3.0 to reduce the risk of bleeding.
The duration of triple therapy is guided by the type of stent used. For bare metal stents, aspirin, clopidogrel or ticlopidine, and warfarin should be given for at least 1 month, after which clopidogrel or ticlopidine may be discontinued. If drug-eluting stents are used, the duration of clopidogrel or ticlopidine therapy should be extended to 1 year or more.4,22
Mechanical heart valves
In patients with mechanical heart valves, the combination of aspirin and warfarin has been shown to decrease the frequency of thromboembolism.23 Guidelines recommend adding aspirin (75 to 100 mg per day) to warfarin in all patients with mechanical valves, especially in patients who have had an embolus while on warfarin therapy or who have a history of cerebrovascular or peripheral vascular disease, a hypercoagulable state, or coronary artery disease.24
CONDITIONS IN WHICH WARFARIN ALONE MAY BE SUFFICIENT
At risk of coronary artery disease
Aspirin therapy is generally recommended as primary prevention for patients whose estimated risk of coronary events is 1.5% per year or higher.25 However, warfarin has also been shown to be effective in the primary prevention of coronary artery disease in men,26 and for patients already taking warfarin, the possible benefit of adding aspirin for primary prevention is outweighed by the increased risk of major bleeding.14 The Medical Research Council directly compared low-intensity warfarin therapy (mean INR 1.47), aspirin, and placebo in a two-by-two factorial study of primary prevention of ischemic heart disease in men.26 Warfarin was more effective than aspirin, and men who received warfarin plus aspirin or warfarin plus placebo had a rate of ischemic heart disease that was 21% lower than those who received aspirin plus placebo or double placebo, and their rate of all-cause mortality was 17% lower. Combining aspirin and warfarin for patients at risk of coronary disease led to a higher rate of major bleeding but no difference in cardiovascular events or all-cause mortality (odds ratio 0.98; 95% confidence interval 0.77–1.25).14
Stable coronary artery disease without mechanical heart valves or stents
Large randomized trials have found warfarin to be effective in secondary prevention of coronary artery disease.4–6 For most patients with stable coronary artery disease (ie, who have had no ischemic events or coronary interventions in the last 6 months) who need anticoagulation because of atrial fibrillation or venous thromboembolism, warfarin alone (target INR 2.0–3.0) should provide satisfactory antithrombotic prophylaxis against both cerebral and myocardial ischemic events.27 The addition of an antiplatelet agent is not required unless a patient has a coronary stent, a mechanical valve, or an excessive thrombotic risk.4,24,27
TAKE-HOME POINTS
For patients receiving warfarin therapy, whether to add or continue aspirin to their treatment is a common clinical question. The risk of bleeding is greater with combination therapy than with warfarin alone. The cardiovascular benefit varies depending on the clinical situation:
- In patients who have had an acute coronary syndrome or who have a coronary stent or mechanical valve, combination therapy is usually recommended because the benefits outweigh the risks.
- In patients with stable coronary artery disease or those without coronary artery disease who are at risk of coronary events, the risks outweigh the benefits. Combination therapy is usually not indicated in these patients.
The literature on this topic is limited, but it suggests that the decision to prescribe aspirin to patients already taking warfarin (Coumadin) should be individualized. On one hand, the cardiovascular benefit of starting or continuing aspirin in patients already on warfarin outweighs the increased risk of bleeding in patients presenting with an acute coronary syndrome or those with mechanical heart valves or coronary stents. However, for patients with stable coronary artery disease or at risk of coronary disease, the benefit of adding aspirin is not substantial, and continuing warfarin alone may be the preferred strategy.
In patients with coronary artery disease, aspirin has been shown to reduce the rate of death due to all causes by about 18% and the rate of vascular events by about 25% to 30%.1,2 Warfarin is at least as effective as aspirin in reducing the rate of future cardiovascular events (especially if the target international normalized ratio [INR] is greater than 2.5), albeit with a higher bleeding risk.3–6
The decision to prescribe or continue aspirin in patients with coronary artery disease who also need long-term anticoagulation with warfarin for an unrelated medical problem, such as pulmonary emboli, requires careful assessment of the individual patient’s bleeding risk and cardiovascular benefit.
ESTIMATING THE BLEEDING RISK FOR PATIENTS ON WARFARIN
In patients taking warfarin, the risk of major bleeding (defined in most studies as hospitalization because of bleeding and requiring transfusion of at least two units of packed red cells, or an intracranial, intraperitoneal, or fatal bleeding episode) is reported to be about 2.0% to 3.8% per person-year.7–11 The risk of major bleeding with aspirin alone is estimated to be 0.13% per person-year,12 but when aspirin is combined with warfarin, the risk increases significantly.13 In a meta-analysis of randomized controlled trials,14 the risk of major bleeding was calculated to be about 1.5 times higher with combination therapy with aspirin and warfarin than with warfarin alone.
The individual’s bleeding risk depends on specific risk factors and the intensity of anticoagulation.15 The outpatient Bleeding Risk Index (BRI) can be used to estimate the bleeding risk for patients on warfarin.16 The BRI includes four risk factors for major bleeding, each scored as 1 point:
- Age 65 or older
- History of gastrointestinal bleeding
- History of stroke
- One or more comorbid conditions—recent myocardial infarction, anemia (hematocrit < 30%), renal impairment (serum creatinine level > 1.5 mg/dL), or diabetes mellitus.
The risk is low if the score is 0, moderate if the score is 1 or 2, and high if the score is 3 or more. In a validation study of the BRI, the rate of major bleeding was found to be 0.8%, 2.5%, and 10.6% per person-year on warfarin in the low, intermediate, and high-risk groups, respectively.17 In addition, compared with patients with a target INR of 2.5, those with a target INR higher than 3.0 have a higher frequency of bleeding episodes.10,15
CONDITIONS IN WHICH ADDING ASPIRIN TO WARFARIN IS FAVORABLE
Acute coronary syndromes
Drugs that inhibit platelet function are the mainstay of medical treatment for acute coronary syndromes. The American College of Cardiology/American Heart Association (ACC/AHA) guidelines recommend that aspirin be started in patients who have an acute myocardial infarction even if they have been receiving warfarin long-term and their INR is in the therapeutic range, especially if a percutaneous coronary intervention is anticipated.4
After percutaneous coronary intervention
In patients who have undergone percutaneous coronary intervention with stent implantation, dual antiplatelet therapy with aspirin and a thienopyridine—ie, clopidogrel (Plavix) or ticlopidine (Ticlid)—is superior to aspirin or warfarin alone in reducing the risk of stent thrombosis and major adverse cardiovascular events such as myocardial infarction or urgent revascularization.18,19 If patients have an indication for long-term anticoagulation, triple therapy with aspirin, warfarin, and clopidogrel or ticlopidine may be considered in order to reduce the likelihood of stent thrombosis.4,20,21 In such patients the INR should be maintained between 2.0 and 3.0 to reduce the risk of bleeding.
The duration of triple therapy is guided by the type of stent used. For bare metal stents, aspirin, clopidogrel or ticlopidine, and warfarin should be given for at least 1 month, after which clopidogrel or ticlopidine may be discontinued. If drug-eluting stents are used, the duration of clopidogrel or ticlopidine therapy should be extended to 1 year or more.4,22
Mechanical heart valves
In patients with mechanical heart valves, the combination of aspirin and warfarin has been shown to decrease the frequency of thromboembolism.23 Guidelines recommend adding aspirin (75 to 100 mg per day) to warfarin in all patients with mechanical valves, especially in patients who have had an embolus while on warfarin therapy or who have a history of cerebrovascular or peripheral vascular disease, a hypercoagulable state, or coronary artery disease.24
CONDITIONS IN WHICH WARFARIN ALONE MAY BE SUFFICIENT
At risk of coronary artery disease
Aspirin therapy is generally recommended as primary prevention for patients whose estimated risk of coronary events is 1.5% per year or higher.25 However, warfarin has also been shown to be effective in the primary prevention of coronary artery disease in men,26 and for patients already taking warfarin, the possible benefit of adding aspirin for primary prevention is outweighed by the increased risk of major bleeding.14 The Medical Research Council directly compared low-intensity warfarin therapy (mean INR 1.47), aspirin, and placebo in a two-by-two factorial study of primary prevention of ischemic heart disease in men.26 Warfarin was more effective than aspirin, and men who received warfarin plus aspirin or warfarin plus placebo had a rate of ischemic heart disease that was 21% lower than those who received aspirin plus placebo or double placebo, and their rate of all-cause mortality was 17% lower. Combining aspirin and warfarin for patients at risk of coronary disease led to a higher rate of major bleeding but no difference in cardiovascular events or all-cause mortality (odds ratio 0.98; 95% confidence interval 0.77–1.25).14
Stable coronary artery disease without mechanical heart valves or stents
Large randomized trials have found warfarin to be effective in secondary prevention of coronary artery disease.4–6 For most patients with stable coronary artery disease (ie, who have had no ischemic events or coronary interventions in the last 6 months) who need anticoagulation because of atrial fibrillation or venous thromboembolism, warfarin alone (target INR 2.0–3.0) should provide satisfactory antithrombotic prophylaxis against both cerebral and myocardial ischemic events.27 The addition of an antiplatelet agent is not required unless a patient has a coronary stent, a mechanical valve, or an excessive thrombotic risk.4,24,27
TAKE-HOME POINTS
For patients receiving warfarin therapy, whether to add or continue aspirin to their treatment is a common clinical question. The risk of bleeding is greater with combination therapy than with warfarin alone. The cardiovascular benefit varies depending on the clinical situation:
- In patients who have had an acute coronary syndrome or who have a coronary stent or mechanical valve, combination therapy is usually recommended because the benefits outweigh the risks.
- In patients with stable coronary artery disease or those without coronary artery disease who are at risk of coronary events, the risks outweigh the benefits. Combination therapy is usually not indicated in these patients.
- Weisman SM, Graham DY. Evaluation of the benefits and risks of low-dose aspirin in the secondary prevention of cardiovascular and cerebrovascular events. Arch Intern Med 2002; 162:2197–2202.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med 2002; 347:969–974.
- Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction). J Am Coll Cardiol 2004; 44:E1–E211.
- Van Es RF, Jonker JJ, Verheugt FW, et al. Antithrombotics in the Secondary Prevention of Events in Coronary Thrombosis-2 (ASPECT-2) Research Group. Aspirin and coumadin after acute coronary syndromes (the ASPECT-2 study): a randomised controlled trial. Lancet 2002; 360:109–113.
- Anand SS, Yusuf S. Oral anticoagulant therapy in patients with coronary artery disease: a meta-analysis. JAMA 1999; 282:2058–2067.
- Schulman S, Granqvist S, Holmstrom M, et al. The duration of oral anticoagulant therapy after a second episode of venous thromboembolism. The Duration of Anticoagulation Trial Study Group. N Engl J Med 1997; 336:393–398.
- Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999; 340:901–907.
- Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med 2001; 345:165–169.
- Levine MN, Raskob G, Beyth RJ, Kearon C, Schulman S. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126 suppl:287S–310S.
- Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med 2003; 139:893–900.
- McQuaid KR, Laine L. Systematic review and meta-analysis of adverse events of low-dose aspirin and clopidogrel in randomized controlled trials. Am J Med 2006; 119:624–638.
- Rothberg MB, Celestin C, Fiore LD, Lawler E, Cook JR. Warfarin plus aspirin after myocardial infarction or the acute coronary syndrome: meta-analysis with estimates of risk and benefit. Ann Intern Med 2005; 143:241–250.
- Dentali F, Douketis JD, Lim W, Crowther M. Combined aspirin-oral anticoagulant therapy compared with oral anticoagulant therapy alone among patients at risk for cardiovascular disease: a meta-analysis of randomized trials. Arch Intern Med 2007; 167:117–124.
- Hirsh J, Fuster V, Ansell J, Halperin JL. American Heart Association; American College of Cardiology Foundation. American Heart Association/American College of Cardiology Foundation guide to warfarin therapy. Circulation 2003; 107:1692–1711.
- Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med 1998; 105:91–99.
- Aspinall SL, DeSanzo BE, Trilli LE, Good CB. Bleeding Risk Index in an anticoagulation clinic. Assessment by indication and implications for care. J Gen Intern Med 2005; 20:1008–1013.
- Mehta SR, Yusuf S, Peters RJ, et al. Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCICURE study. Lancet 2001; 358:527–533.
- Bertrand ME, Legrand V, Boland J, et al. Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. The Full Anticoagulation versus Aspirin and Ticlopidine (FANTASTIC) study. Circulation 1998; 98:1597–1603.
- Kushner FG, Antman EM. Oral anticoagulation for atrial fibrillation after ST-elevation myocardial infarction: new evidence to guide clinical practice. Circulation 2005; 112:3212–3214.
- Porter A, Konstantino Y, Iakobishvili Z, Shachar L, Battler A, Hasdai D. Short-term triple therapy with aspirin, warfarin, and a thienopyridine among patients undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv 2006; 68:56–61.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 Guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction—executive summary. A report of the ACC-AHA Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction). J Am Coll Cardiol 2007; 50:652–726.
- Turpie AG, Gent M, Laupacis A, et al. A comparison of aspirin with placebo in patients treated with warfarin after heart-valve replacement. N Engl J Med 1993; 329:524–529.
- Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the ACC/AHA Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease). Circulation 2006; 114:e84–e231.
- Lauer MS. Clinical practice. Aspirin for primary prevention of coronary events. N Engl J Med 2002; 346:1468–1474.
- The Medical Research Council’s General Practice Research Framework. Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. Lancet 1998; 351:233–241.
- Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation. Circulation 2006; 114:260–335.
- Weisman SM, Graham DY. Evaluation of the benefits and risks of low-dose aspirin in the secondary prevention of cardiovascular and cerebrovascular events. Arch Intern Med 2002; 162:2197–2202.
- Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002; 324:71–86.
- Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med 2002; 347:969–974.
- Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction; a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1999 Guidelines for the Management of patients with acute myocardial infarction). J Am Coll Cardiol 2004; 44:E1–E211.
- Van Es RF, Jonker JJ, Verheugt FW, et al. Antithrombotics in the Secondary Prevention of Events in Coronary Thrombosis-2 (ASPECT-2) Research Group. Aspirin and coumadin after acute coronary syndromes (the ASPECT-2 study): a randomised controlled trial. Lancet 2002; 360:109–113.
- Anand SS, Yusuf S. Oral anticoagulant therapy in patients with coronary artery disease: a meta-analysis. JAMA 1999; 282:2058–2067.
- Schulman S, Granqvist S, Holmstrom M, et al. The duration of oral anticoagulant therapy after a second episode of venous thromboembolism. The Duration of Anticoagulation Trial Study Group. N Engl J Med 1997; 336:393–398.
- Kearon C, Gent M, Hirsh J, et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. N Engl J Med 1999; 340:901–907.
- Agnelli G, Prandoni P, Santamaria MG, et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med 2001; 345:165–169.
- Levine MN, Raskob G, Beyth RJ, Kearon C, Schulman S. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126 suppl:287S–310S.
- Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med 2003; 139:893–900.
- McQuaid KR, Laine L. Systematic review and meta-analysis of adverse events of low-dose aspirin and clopidogrel in randomized controlled trials. Am J Med 2006; 119:624–638.
- Rothberg MB, Celestin C, Fiore LD, Lawler E, Cook JR. Warfarin plus aspirin after myocardial infarction or the acute coronary syndrome: meta-analysis with estimates of risk and benefit. Ann Intern Med 2005; 143:241–250.
- Dentali F, Douketis JD, Lim W, Crowther M. Combined aspirin-oral anticoagulant therapy compared with oral anticoagulant therapy alone among patients at risk for cardiovascular disease: a meta-analysis of randomized trials. Arch Intern Med 2007; 167:117–124.
- Hirsh J, Fuster V, Ansell J, Halperin JL. American Heart Association; American College of Cardiology Foundation. American Heart Association/American College of Cardiology Foundation guide to warfarin therapy. Circulation 2003; 107:1692–1711.
- Beyth RJ, Quinn LM, Landefeld CS. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med 1998; 105:91–99.
- Aspinall SL, DeSanzo BE, Trilli LE, Good CB. Bleeding Risk Index in an anticoagulation clinic. Assessment by indication and implications for care. J Gen Intern Med 2005; 20:1008–1013.
- Mehta SR, Yusuf S, Peters RJ, et al. Clopidogrel in Unstable angina to prevent Recurrent Events trial (CURE) Investigators. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCICURE study. Lancet 2001; 358:527–533.
- Bertrand ME, Legrand V, Boland J, et al. Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. The Full Anticoagulation versus Aspirin and Ticlopidine (FANTASTIC) study. Circulation 1998; 98:1597–1603.
- Kushner FG, Antman EM. Oral anticoagulation for atrial fibrillation after ST-elevation myocardial infarction: new evidence to guide clinical practice. Circulation 2005; 112:3212–3214.
- Porter A, Konstantino Y, Iakobishvili Z, Shachar L, Battler A, Hasdai D. Short-term triple therapy with aspirin, warfarin, and a thienopyridine among patients undergoing percutaneous coronary intervention. Catheter Cardiovasc Interv 2006; 68:56–61.
- Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 Guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction—executive summary. A report of the ACC-AHA Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction). J Am Coll Cardiol 2007; 50:652–726.
- Turpie AG, Gent M, Laupacis A, et al. A comparison of aspirin with placebo in patients treated with warfarin after heart-valve replacement. N Engl J Med 1993; 329:524–529.
- Bonow RO, Carabello BA, Kanu C, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the ACC/AHA Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease). Circulation 2006; 114:e84–e231.
- Lauer MS. Clinical practice. Aspirin for primary prevention of coronary events. N Engl J Med 2002; 346:1468–1474.
- The Medical Research Council’s General Practice Research Framework. Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. Lancet 1998; 351:233–241.
- Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation. Circulation 2006; 114:260–335.