User login
Discontinuing an antidepressant?
Most psychiatrists have encountered patients who report distressing symptoms when they have forgotten to take their antidepressant for a few days or during changes in the medication regimen. A discontinuation syndrome can occur with almost any antidepressant, highlighting the need to slowly taper these medications when discontinuation is part of a treatment plan.
This article discusses antidepressant discontinuation syndrome (ADS) in a patient who experienced substantial distress after a rapid antidepressant taper in preparation for electroconvulsive therapy (ECT). My goal is to raise awareness of ADS, promote early detection of the syndrome, and address proper prevention and management strategies.
CASE REPORT: Feeling ‘worse than ever’
Mr. J, a 32-year-old tax accountant, is hospitalized for a major depressive episode (MDE) associated with deteriorating function and suicidal ideation. This second lifetime MDE started 8 months before his admission to an inpatient mood disorders unit.
Mr. J initially was treated with fluoxetine, up to 40 mg/d across 14 weeks, with good tolerability but no significant benefit. His psychiatrist switched Mr. J to bupropion but stopped it after 4 weeks because of side effects—including headaches, insomnia, and tremor—and limited antidepressant benefit. Venlafaxine XR was initiated next, at 150 mg/d within the first 2 weeks, increased to 225 mg/d at week 6, then titrated to 300 mg/d at week 10. After 10 weeks, aripiprazole, 5 mg/d, was added because Mr. J showed only partial, limited response to venlafaxine XR and this antipsychotic is indicated for adjunctive treatment of major depressive disorder.
Mr. J reported mild, transient restlessness but otherwise he tolerated the medications well, and he claimed excellent adherence. After 6 additional weeks of treatment, however, Mr. J was hospitalized because of persistent severely depressed mood, increasing suicidal ideation, and inability to function at work.
On admission, Mr. J is evaluated and agrees to ECT. To meet the ECT service’s protocol, venlafaxine XR is reduced to 150 mg/d for 2 days and then stopped when ECT is started. Aripiprazole is continued at 5 mg/d.
Mr. J tolerates the first ECT treatment well, but the morning before his second treatment he complains of feeling “worse than ever.” An agitated Mr. J reports dramatically intensified suicidal ideation—much more intrusive than before he was hospitalized. He also complains of diffuse muscle aches and cramps, runny nose, nausea, headache, and burning sensations in both arms and hands. He withdraws consent for ECT and returns to the mood disorders unit for ongoing treatment.
Could this be ADS?
Yes, it could. In this case, the inpatient psychiatrist and treatment team were lulled into a false sense of security by Mr. J’s history of few side effects with various treatments and medication changes. The ECT service wanted the patient off venlafaxine XR before beginning ECT, and the treatment team believed a quick taper would not cause discontinuation symptoms because Mr. J was taking an “extended-release” medication.
Within 72 hours, Mr. J went from taking 300 mg/d of venlafaxine XR to none. Within 2 days of cessation, he complained of symptoms that could characterize a discontinuation syndrome. A potential red herring in this case is that the patient complained of feeling worse after his first ECT treatment, and one might erroneously think the myalgias, headache, and other somatic symptoms were side effects of ECT and/or anesthesia.
Typical ADS symptoms
Nearly all antidepressant classes are associated with ADS. Symptoms vary from patient to patient but typically include the “FINISH” syndrome: flu-like symptoms, ###bold/bold###nsomnia, nausea, ###bold/bold###mbalance, sensory disturbances, and hyperarousal (anxiety/agitation) (Table 1).1
Adverse effects after stopping tricyclic antidepressants have been well documented. They may include FINISH syndrome features as well as cholinergic overdrive or “rebound” such as abdominal cramping and diarrhea.2-4 Reports of ADS after patients stopped selective serotonin reuptake inhibitors (SSRIs) emerged soon after these agents were introduced.5-7 Similarly, ADS has been reported with serotonin-norepinephrine reuptake inhibitors (SNRIs), including venlafaxine,8-10 venlafaxine XR,11 and duloxetine.12 ADS symptoms are similar with SSRIs and SNRIs, generally without the anticholinergic effects associated with tricyclic antidepressant discontinuation.
Fewer reports of discontinuation syndrome exist for bupropion, mirtazapine, monoamine oxidase inhibitors (MAOIs), and nefazodone.13-17 Discontinuation-emergent syndromes with these non-SSRI/non-SNRI antidepressants tend to present differently. With MAOIs, for example, neuropsychiatric symptoms such as severe anxiety, agitation, pressured speech, sleeplessness or drowsiness, hallucinations, delirium, and paranoid psychosis can be prominent.17
The prevalence of ADS is unclear, and published estimates vary widely because of the lack of large controlled studies. ADS rates with SSRIs/SNRIs have been reported from as low as 0% for fluoxetine to higher rates for shorter half-life antidepressants:
- 2.2% with sertraline
- 14% with fluvoxamine
- 20% with paroxetine
- 30.8% with clomipramine.
These rates come from a retrospective case note review of patients who discontinued antidepressants under supervision.18 In a small cohort of outpatients being treated for major depressive disorder, stopping venlafaxine XR was associated with discontinuation symptoms for the next 3 days in 7 of 9 patients (78%), compared with 2 of 9 patients (22%) stopping placebo.11
Diagnostic criteria have been proposed for ADS associated with serotonin (5-HT) reuptake inhibitors.19-22 Proposed ADS definitions differ somewhat, but essentially 3 features guide the diagnosis:
- appearance of characteristic symptoms (Table 2)21,23
- timing of those symptoms, which usually emerge within 1 week of abrupt cessation or marked reduction of the antidepressant
- symptoms generally are mild, short-lived, self-limiting, and/or rapidly reversed by restarting the original antidepressant.
Evidence suggests shorter half-life antidepressants may be associated with the highest risk for ADS, but other risk factors remain presumptive (Table 3).
Table 1
FINISH: Symptoms of antidepressant discontinuation syndrome
| Flu-like symptoms |
| Insomnia |
| Nausea |
| Imbalance |
| Sensory disturbances |
| Hyperarousal (anxiety/agitation) |
| Source: Reference 1 |
Table 2
ADS symptoms can range across a variety of system clusters
| System cluster | Symptoms |
|---|---|
| Neurosensory | Vertigo, paresthesias, shock-like reactions, myalgias, numbness, sensitivity to sound, unusual visual sensations, ringing in the ears |
| Neuromotor | Tremor, myoclonus, ataxia/gait instability, visual changes, restless legs, problems with speech, tongue movements |
| Gastrointestinal | Nausea, vomiting, cramps/bloating, diarrhea, anorexia |
| Neuropsychiatric | Anxiety/panic, depression, mood swings, suicidal ideation, irritability, impulsivity, confusion, psychosis |
| Vasomotor | Diaphoresis, flushing, temperature intolerance |
| Other | Headache, insomnia, vivid dreams, nightmares, lethargy/fatigue, flu-like symptoms |
| ADS: antidepressant discontinuation syndrome | |
| Source: Construct suggested by Shelton,21 with additional symptoms added from other sources, including the discontinuation symptom checklist of Rosenbaum et al23 | |
Table 3
Possible patient risk factors for developing ADS*
| Abrupt antidepressant discontinuation |
| Shorter half-life antidepressants |
| Intermittent nonadherence/noncompliance |
| Interrupted treatment or use of ‘drug holiday’ |
| Specific antidepressant properties (such as potent [5-HT] receptor antagonism, cholinergic effects) |
| Younger patient age (including children and adolescents) |
| Female gender |
| Pregnancy |
| Neonate/breast-fed infant (mother on antidepressant therapy) |
| History of ADS |
| Vulnerability to depressive relapse |
| Duration of treatment (possible increased risk with more than 4 to 6 weeks of antidepressant exposure) |
| Switches to or between generic antidepressant formulations (related to variations in bioequivalence) |
| History of early adverse reactions when the antidepressant was initiated |
| ADS: antidepressant discontinuation syndrome |
| *Risk factors for ADS have not been rigorously studied in randomized controlled trials. Possible risk factors in this table were found in case reports |
What causes ADS?
Although the exact cause of ADS is unknown, the literature proposes several theories.
Because of the central serotonin system’s complex connections, acute reduction in synaptic serotonin when an SSRI or SNRI is abruptly or too quickly stopped may be the first in a cascade of steps affecting transmission of multiple monoamines. Parallels have been drawn between the phenomenon observed with rapid depletion of tryptophan—the essential amino acid precursor for the synthesis of 5-HT—and ADS seen with abrupt discontinuation of serotonergic antidepressants. This suggests that acute drops in neurotransmitter levels can precipitate neuropsychiatric and somatic manifestations of ADS.24
Patients’ uncomfortable symptoms likely are caused by the serotonin, norepinephrine, and cholinergic systems and their complex interactions.25 Individual genetic factors may influence patients’ vulnerability for ADS.
Managing ADS
Awareness and prevention. ADS can be misinterpreted as side effects of newly started treatment after an antidepressant is stopped. In Mr. J’s case, the appearance of muscle aches, headaches, and other ADS symptoms after ECT was started easily could have been perceived as adverse effects of ECT. Mr. J’s agitation and increased suicidal ideation could lead a clinician to mistakenly think that MDE was worsening because the antidepressant was stopped before ECT became effective. Being aware of ADS can prevent misdiagnosis and allow you to quickly identify the condition, manage the reversible syndrome, and continue with new treatment plan—in this case, ECT.
You can help prevent ADS by educating patients about the need to adhere to antidepressant regimens and to avoid missing doses. Consider ADS risk factors—particularly medications’ half-lives—before you start, change, or stop antidepressant therapy. Gradually taper all antidepressants being discontinued, with the possible exception of fluoxetine (which, including its active metabolite, has an elimination half-life of approximately 1 to 2 weeks).
Tapering antidepressants is more art than science because we have no controlled data to support any particular tapering regimen. Tailor the taper duration based on each patient’s response to sequential dosage reductions. Antidepressants with shorter half-lives—such as venlafaxine or paroxetine—may need to be tapered more slowly, perhaps by reducing the dosage by 25% every 4 to 6 weeks. If you plan to switch medications, this process may be expedited during a cross-taper to another antidepressant. You still may see discontinuation symptoms, however, depending on which new agent is chosen and which is being stopped.
Treating ADS. Appropriately recognizing ADS risk and slowly tapering antidepressants as needed usually prevents clinically significant distress associated with discontinuation. For some patients, however, ADS may be particularly severe or prolonged, or may emerge at the end of a slow taper.
Challenging cases may be more likely with paroxetine or venlafaxine—even the extended-release or controlled-release preparations. The elimination half-life of paroxetine is 15 to 20 hours, and the half-lives of venlafaxine and venlafaxine XR are 5 to 11 hours. Desvenlafaxine’s half-life is 11 hours, and product labeling of this enantiomer of racemic venlafaxine notes that discontinuation symptoms have occurred.26 ADS treatment depends on the severity of the reaction and whether or not further antidepressant therapy is necessary.
For mild ADS, reassurance and treatment focused on specific symptoms—such as sedative-hypnotics for insomnia or benzodiazepines for anxiety—may be all that is needed, because ADS tends to gradually resolve over an average of 10 days.27
For more severe ADS, or when ongoing antidepressant therapy is indicated, restarting the recently withdrawn antidepressant at the pre-ADS dosage typically resolves the syndrome within 24 hours. Then employ a slower, more cautious taper when next attempting to discontinue that antidepressant.
Another option. An alternate management strategy is to substitute fluoxetine to suppress ADS associated with shorter half-life SSRIs or SNRIs. Case reports18,20,28 suggest that fluoxetine, 5 to 20 mg/d, can be used to ameliorate venlafaxine-induced ADS. Fluoxetine can be tried as monotherapy for 1 to 2 weeks and then rapidly tapered or stopped. Others have suggested combination therapy, such as:
- restarting venlafaxine at the pre-ADS dose plus fluoxetine, 20 mg/d
- tapering venlafaxine by 50% every 5 days until stopped
- reducing fluoxetine 1 week later to 10 mg/d for 5 days
- then stopping fluoxetine.28
In general, SSRIs should not be co-administered with SNRIs long-term because of potential additive adverse effects such as serotonin syndrome. Combining fluoxetine with an SNRI such as venlafaxine for the purpose of tapering off venlafaxine and reducing ADS risk probably is safe, however, as long as the fluoxetine dose is low (5 to 20 mg) and SNRI reduction begins immediately, with a plan for complete tapering.
CASE CONTINUED: ECT treatment proceeds
Venlafaxine XR is not restarted to address Mr. J’s suspected ADS because of concerns about potential increased risk for cardiac events (asystole, prolonged bradycardia) during ECT with concomitant venlafaxine use.29,30 Fluoxetine, which rarely may prolong ECT-induced seizures, is deemed a safer choice and is started immediately at 20 mg/d.
Because of Mr. J’s other symptoms, we prescribe lorazepam, 0.5 mg bid, for anxiety for 2 days; increase aripiprazole to 5 mg bid for agitation; and add zolpidem, 10 mg at bedtime, for insomnia. The following day, Mr. J reports substantial relief from ADS symptoms, including myalgias, paresthesias, and suicidal ideation.21,23
His second ECT treatment is administered the next day, followed by a successful course of 9 treatments and partial remission of the MDE within 3 weeks. Fluoxetine is reduced to 10 mg/d one week into the ECT series, then discontinued one week later. No signs of emergent ADS are seen at discharge or 2-week outpatient follow-up. Mr. J achieves full remission with maintenance ECT plus bedtime doses of mirtazapine, 30 mg, and aripiprazole, 7.5 mg, across 6 months of follow-up care.
Related resources
- Schatzberg AF, Blier P, Delgado PL, et al. Antidepressant discontinuation syndrome: consensus panel recommendations for clinical management and additional research. J Clin Psychiatry. 2006;67(suppl 4):27-30.
- American Family Physician. Patient handout on antidepressant discontinuation. www.aafp.org/afp/2006/0801/p457.html.
- Rosenbaum JF, Fava M, Hoog SL, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77-87. See appendix for discontinuation-emergent signs and symptoms checklist.
Drug brand names
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Clomipramine • Anafranil
- Desvenlafaxine • Pristiq
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Lorazepam • Ativan
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
- Venlafaxine extended-release • Effexor XR
- Zolpidem • Ambien
Disclosure
Dr. Muzina reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
When Dr. Muzina submitted this article to Current Psychiatry, he was director, Center for Mood Disorders Treatment and Research, Cleveland Clinic Neurological Institute, Cleveland, OH.
1. Berber MJ. FINISH: remembering the discontinuation syndrome. Flu-like symptoms, insomnia, nausea, imbalance, sensory disturbances, and hyperarousal (anxiety/agitation). J Clin Psychiatry. 1998;59(5):255.-
2. Ceccherini-Nelli A, Bardellini L, Cur A, et al. Antidepressant withdrawal: prospective findings. Am J Psychiatry. 1993;150(1):165.-
3. Dilsaver SC, Kronfol Z, Sackellares JC, et al. Antidepressant withdrawal syndromes: evidence supporting the cholinergic overdrive hypothesis. J Clin Psychopharmacol. 1983;3(3):157-164.
4. Garner EM, Kelly MW, Thompson DF. Tricyclic antidepressant withdrawal syndrome. Ann Pharmacother. 1993;27(9):1068-1072.
5. Barr LC, Goodman WK, Price LH. Physical symptoms associated with paroxetine discontinuation. Am J Psychiatry. 1994;151(2):289.-
6. Frost L, Lal S. Shock-like sensations after discontinuation of selective serotonin reuptake inhibitors. Am J Psychiatry. 1995;152(5):810.-
7. Louie AK, Lannon RA, Ajari LJ. Withdrawal reaction after sertraline discontinuation. Am J Psychiatry. 1994;151(3):450-451.
8. Benazzi F. Venlafaxine withdrawal symptoms. Can J Psychiatry. 1996;41(7):487.-
9. Farah A, Lauer TE. Possible venlafaxine withdrawal syndrome. Am J Psychiatry. 1996;153(4):576.-
10. Louie AK, Lannon RA, Kirsch MA, et al. Venlafaxine withdrawal reactions. Am J Psychiatry. 1996;153(12):1652.-
11. Fava M, Mulroy R, Alpert J, et al. Emergence of adverse events following discontinuation of treatment with extended-release venlafaxine. Am J Psychiatry. 1997;154(12):1760-1762.
12. Perahia DG, Kajdasz DK, Desaiah D, et al. Symptoms following abrupt discontinuation of duloxetine treatment in patients with major depressive disorder. J Affect Disord. 2005;89(1-3):207-212.
13. Benazzi F. Mirtazapine withdrawal symptoms. Can J Psychiatry. 1998;43(5):525.-
14. Benazzi F. Nefazodone withdrawal symptoms. Can J Psychiatry. 1998;43(2):194-195.
15. Berigan TR. Bupropion-associated withdrawal symptoms revisited: a case report. Prim Care Companion J Clin Psychiatry. 2002;4(2):78.-
16. Berigan TR, Harazin JS. Bupropion-associated withdrawal symptoms: a case report. Prim Care Companion J Clin Psychiatry. 1999;1(2):50-51.
17. Dilsaver SC. Monoamine oxidase inhibitor withdrawal phenomena: symptoms and pathophysiology. Acta Psychiatr Scand. 1988;78(1):1-7.
18. Coupland NJ, Bell CJ, Potokar JP. Serotonin reuptake inhibitor withdrawal. J Clin Psychopharmacol. 1996;16(5):356-362.
19. Black K, Shea C, Dursun S, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: proposed diagnostic criteria. J Psychiatry Neurosci. 2000;25(3):255-261.
20. Haddad PM. Antidepressant discontinuation syndromes. Drug Saf. 2001;24(3):183-197.
21. Shelton RC. The nature of the discontinuation syndrome associated with antidepressant drugs. J Clin Psychiatry. 2006;67(suppl 4):3-7.
22. Schatzberg AF, Haddad P, Kaplan EM, et al. Serotonin reuptake inhibitor discontinuation syndrome: a hypothetical definition. Discontinuation consensus panel. J Clin Psychiatry. 1997;58(suppl 7):5-10.
23. Rosenbaum JF, Fava M, Hoog SL, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77-87.
24. Delgado PL. Monoamine depletion studies: implications for antidepressant discontinuation syndrome. J Clin Psychiatry. 2006;67(suppl 4):22-26.
25. Blier P, Tremblay P. Physiologic mechanisms underlying the antidepressant discontinuation syndrome. J Clin Psychiatry. 2006;67(suppl 4):8-13.
26. Wyeth. Desvenlafaxine (Pristiq) prescribing information. Available at: http://www.wyeth.com/content/showlabeling.asp?id=497. Accessed January 27, 2010.
27. Price JS, Waller PC, Wood SM, et al. A comparison of the post-marketing safety of four selective serotonin re-uptake inhibitors including the investigation of symptoms occurring on withdrawal. Br J Clin Pharmacol. 1996;42(6):757-763.
28. Benazzi F. SSRI discontinuation syndrome treated with fluoxetine. Int J Geriatr Psychiatry. 1998;13(6):421-422.
29. Agelink MM, Zeit T, Klieser E. Prolonged bradycardia complicates antidepressive treatment with venlafaxine and ECT. Br J Psychiatry. 1998;173:441.-
30. Gonzalez-Pinto A, Gutierrez M, Gonzalez N, et al. Efficacy and safety of venlafaxine-ECT combination in treatment-resistant depression. J Neuropsychiatry Clin Neurosci. 2002;14(2):206-209.
Most psychiatrists have encountered patients who report distressing symptoms when they have forgotten to take their antidepressant for a few days or during changes in the medication regimen. A discontinuation syndrome can occur with almost any antidepressant, highlighting the need to slowly taper these medications when discontinuation is part of a treatment plan.
This article discusses antidepressant discontinuation syndrome (ADS) in a patient who experienced substantial distress after a rapid antidepressant taper in preparation for electroconvulsive therapy (ECT). My goal is to raise awareness of ADS, promote early detection of the syndrome, and address proper prevention and management strategies.
CASE REPORT: Feeling ‘worse than ever’
Mr. J, a 32-year-old tax accountant, is hospitalized for a major depressive episode (MDE) associated with deteriorating function and suicidal ideation. This second lifetime MDE started 8 months before his admission to an inpatient mood disorders unit.
Mr. J initially was treated with fluoxetine, up to 40 mg/d across 14 weeks, with good tolerability but no significant benefit. His psychiatrist switched Mr. J to bupropion but stopped it after 4 weeks because of side effects—including headaches, insomnia, and tremor—and limited antidepressant benefit. Venlafaxine XR was initiated next, at 150 mg/d within the first 2 weeks, increased to 225 mg/d at week 6, then titrated to 300 mg/d at week 10. After 10 weeks, aripiprazole, 5 mg/d, was added because Mr. J showed only partial, limited response to venlafaxine XR and this antipsychotic is indicated for adjunctive treatment of major depressive disorder.
Mr. J reported mild, transient restlessness but otherwise he tolerated the medications well, and he claimed excellent adherence. After 6 additional weeks of treatment, however, Mr. J was hospitalized because of persistent severely depressed mood, increasing suicidal ideation, and inability to function at work.
On admission, Mr. J is evaluated and agrees to ECT. To meet the ECT service’s protocol, venlafaxine XR is reduced to 150 mg/d for 2 days and then stopped when ECT is started. Aripiprazole is continued at 5 mg/d.
Mr. J tolerates the first ECT treatment well, but the morning before his second treatment he complains of feeling “worse than ever.” An agitated Mr. J reports dramatically intensified suicidal ideation—much more intrusive than before he was hospitalized. He also complains of diffuse muscle aches and cramps, runny nose, nausea, headache, and burning sensations in both arms and hands. He withdraws consent for ECT and returns to the mood disorders unit for ongoing treatment.
Could this be ADS?
Yes, it could. In this case, the inpatient psychiatrist and treatment team were lulled into a false sense of security by Mr. J’s history of few side effects with various treatments and medication changes. The ECT service wanted the patient off venlafaxine XR before beginning ECT, and the treatment team believed a quick taper would not cause discontinuation symptoms because Mr. J was taking an “extended-release” medication.
Within 72 hours, Mr. J went from taking 300 mg/d of venlafaxine XR to none. Within 2 days of cessation, he complained of symptoms that could characterize a discontinuation syndrome. A potential red herring in this case is that the patient complained of feeling worse after his first ECT treatment, and one might erroneously think the myalgias, headache, and other somatic symptoms were side effects of ECT and/or anesthesia.
Typical ADS symptoms
Nearly all antidepressant classes are associated with ADS. Symptoms vary from patient to patient but typically include the “FINISH” syndrome: flu-like symptoms, ###bold/bold###nsomnia, nausea, ###bold/bold###mbalance, sensory disturbances, and hyperarousal (anxiety/agitation) (Table 1).1
Adverse effects after stopping tricyclic antidepressants have been well documented. They may include FINISH syndrome features as well as cholinergic overdrive or “rebound” such as abdominal cramping and diarrhea.2-4 Reports of ADS after patients stopped selective serotonin reuptake inhibitors (SSRIs) emerged soon after these agents were introduced.5-7 Similarly, ADS has been reported with serotonin-norepinephrine reuptake inhibitors (SNRIs), including venlafaxine,8-10 venlafaxine XR,11 and duloxetine.12 ADS symptoms are similar with SSRIs and SNRIs, generally without the anticholinergic effects associated with tricyclic antidepressant discontinuation.
Fewer reports of discontinuation syndrome exist for bupropion, mirtazapine, monoamine oxidase inhibitors (MAOIs), and nefazodone.13-17 Discontinuation-emergent syndromes with these non-SSRI/non-SNRI antidepressants tend to present differently. With MAOIs, for example, neuropsychiatric symptoms such as severe anxiety, agitation, pressured speech, sleeplessness or drowsiness, hallucinations, delirium, and paranoid psychosis can be prominent.17
The prevalence of ADS is unclear, and published estimates vary widely because of the lack of large controlled studies. ADS rates with SSRIs/SNRIs have been reported from as low as 0% for fluoxetine to higher rates for shorter half-life antidepressants:
- 2.2% with sertraline
- 14% with fluvoxamine
- 20% with paroxetine
- 30.8% with clomipramine.
These rates come from a retrospective case note review of patients who discontinued antidepressants under supervision.18 In a small cohort of outpatients being treated for major depressive disorder, stopping venlafaxine XR was associated with discontinuation symptoms for the next 3 days in 7 of 9 patients (78%), compared with 2 of 9 patients (22%) stopping placebo.11
Diagnostic criteria have been proposed for ADS associated with serotonin (5-HT) reuptake inhibitors.19-22 Proposed ADS definitions differ somewhat, but essentially 3 features guide the diagnosis:
- appearance of characteristic symptoms (Table 2)21,23
- timing of those symptoms, which usually emerge within 1 week of abrupt cessation or marked reduction of the antidepressant
- symptoms generally are mild, short-lived, self-limiting, and/or rapidly reversed by restarting the original antidepressant.
Evidence suggests shorter half-life antidepressants may be associated with the highest risk for ADS, but other risk factors remain presumptive (Table 3).
Table 1
FINISH: Symptoms of antidepressant discontinuation syndrome
| Flu-like symptoms |
| Insomnia |
| Nausea |
| Imbalance |
| Sensory disturbances |
| Hyperarousal (anxiety/agitation) |
| Source: Reference 1 |
Table 2
ADS symptoms can range across a variety of system clusters
| System cluster | Symptoms |
|---|---|
| Neurosensory | Vertigo, paresthesias, shock-like reactions, myalgias, numbness, sensitivity to sound, unusual visual sensations, ringing in the ears |
| Neuromotor | Tremor, myoclonus, ataxia/gait instability, visual changes, restless legs, problems with speech, tongue movements |
| Gastrointestinal | Nausea, vomiting, cramps/bloating, diarrhea, anorexia |
| Neuropsychiatric | Anxiety/panic, depression, mood swings, suicidal ideation, irritability, impulsivity, confusion, psychosis |
| Vasomotor | Diaphoresis, flushing, temperature intolerance |
| Other | Headache, insomnia, vivid dreams, nightmares, lethargy/fatigue, flu-like symptoms |
| ADS: antidepressant discontinuation syndrome | |
| Source: Construct suggested by Shelton,21 with additional symptoms added from other sources, including the discontinuation symptom checklist of Rosenbaum et al23 | |
Table 3
Possible patient risk factors for developing ADS*
| Abrupt antidepressant discontinuation |
| Shorter half-life antidepressants |
| Intermittent nonadherence/noncompliance |
| Interrupted treatment or use of ‘drug holiday’ |
| Specific antidepressant properties (such as potent [5-HT] receptor antagonism, cholinergic effects) |
| Younger patient age (including children and adolescents) |
| Female gender |
| Pregnancy |
| Neonate/breast-fed infant (mother on antidepressant therapy) |
| History of ADS |
| Vulnerability to depressive relapse |
| Duration of treatment (possible increased risk with more than 4 to 6 weeks of antidepressant exposure) |
| Switches to or between generic antidepressant formulations (related to variations in bioequivalence) |
| History of early adverse reactions when the antidepressant was initiated |
| ADS: antidepressant discontinuation syndrome |
| *Risk factors for ADS have not been rigorously studied in randomized controlled trials. Possible risk factors in this table were found in case reports |
What causes ADS?
Although the exact cause of ADS is unknown, the literature proposes several theories.
Because of the central serotonin system’s complex connections, acute reduction in synaptic serotonin when an SSRI or SNRI is abruptly or too quickly stopped may be the first in a cascade of steps affecting transmission of multiple monoamines. Parallels have been drawn between the phenomenon observed with rapid depletion of tryptophan—the essential amino acid precursor for the synthesis of 5-HT—and ADS seen with abrupt discontinuation of serotonergic antidepressants. This suggests that acute drops in neurotransmitter levels can precipitate neuropsychiatric and somatic manifestations of ADS.24
Patients’ uncomfortable symptoms likely are caused by the serotonin, norepinephrine, and cholinergic systems and their complex interactions.25 Individual genetic factors may influence patients’ vulnerability for ADS.
Managing ADS
Awareness and prevention. ADS can be misinterpreted as side effects of newly started treatment after an antidepressant is stopped. In Mr. J’s case, the appearance of muscle aches, headaches, and other ADS symptoms after ECT was started easily could have been perceived as adverse effects of ECT. Mr. J’s agitation and increased suicidal ideation could lead a clinician to mistakenly think that MDE was worsening because the antidepressant was stopped before ECT became effective. Being aware of ADS can prevent misdiagnosis and allow you to quickly identify the condition, manage the reversible syndrome, and continue with new treatment plan—in this case, ECT.
You can help prevent ADS by educating patients about the need to adhere to antidepressant regimens and to avoid missing doses. Consider ADS risk factors—particularly medications’ half-lives—before you start, change, or stop antidepressant therapy. Gradually taper all antidepressants being discontinued, with the possible exception of fluoxetine (which, including its active metabolite, has an elimination half-life of approximately 1 to 2 weeks).
Tapering antidepressants is more art than science because we have no controlled data to support any particular tapering regimen. Tailor the taper duration based on each patient’s response to sequential dosage reductions. Antidepressants with shorter half-lives—such as venlafaxine or paroxetine—may need to be tapered more slowly, perhaps by reducing the dosage by 25% every 4 to 6 weeks. If you plan to switch medications, this process may be expedited during a cross-taper to another antidepressant. You still may see discontinuation symptoms, however, depending on which new agent is chosen and which is being stopped.
Treating ADS. Appropriately recognizing ADS risk and slowly tapering antidepressants as needed usually prevents clinically significant distress associated with discontinuation. For some patients, however, ADS may be particularly severe or prolonged, or may emerge at the end of a slow taper.
Challenging cases may be more likely with paroxetine or venlafaxine—even the extended-release or controlled-release preparations. The elimination half-life of paroxetine is 15 to 20 hours, and the half-lives of venlafaxine and venlafaxine XR are 5 to 11 hours. Desvenlafaxine’s half-life is 11 hours, and product labeling of this enantiomer of racemic venlafaxine notes that discontinuation symptoms have occurred.26 ADS treatment depends on the severity of the reaction and whether or not further antidepressant therapy is necessary.
For mild ADS, reassurance and treatment focused on specific symptoms—such as sedative-hypnotics for insomnia or benzodiazepines for anxiety—may be all that is needed, because ADS tends to gradually resolve over an average of 10 days.27
For more severe ADS, or when ongoing antidepressant therapy is indicated, restarting the recently withdrawn antidepressant at the pre-ADS dosage typically resolves the syndrome within 24 hours. Then employ a slower, more cautious taper when next attempting to discontinue that antidepressant.
Another option. An alternate management strategy is to substitute fluoxetine to suppress ADS associated with shorter half-life SSRIs or SNRIs. Case reports18,20,28 suggest that fluoxetine, 5 to 20 mg/d, can be used to ameliorate venlafaxine-induced ADS. Fluoxetine can be tried as monotherapy for 1 to 2 weeks and then rapidly tapered or stopped. Others have suggested combination therapy, such as:
- restarting venlafaxine at the pre-ADS dose plus fluoxetine, 20 mg/d
- tapering venlafaxine by 50% every 5 days until stopped
- reducing fluoxetine 1 week later to 10 mg/d for 5 days
- then stopping fluoxetine.28
In general, SSRIs should not be co-administered with SNRIs long-term because of potential additive adverse effects such as serotonin syndrome. Combining fluoxetine with an SNRI such as venlafaxine for the purpose of tapering off venlafaxine and reducing ADS risk probably is safe, however, as long as the fluoxetine dose is low (5 to 20 mg) and SNRI reduction begins immediately, with a plan for complete tapering.
CASE CONTINUED: ECT treatment proceeds
Venlafaxine XR is not restarted to address Mr. J’s suspected ADS because of concerns about potential increased risk for cardiac events (asystole, prolonged bradycardia) during ECT with concomitant venlafaxine use.29,30 Fluoxetine, which rarely may prolong ECT-induced seizures, is deemed a safer choice and is started immediately at 20 mg/d.
Because of Mr. J’s other symptoms, we prescribe lorazepam, 0.5 mg bid, for anxiety for 2 days; increase aripiprazole to 5 mg bid for agitation; and add zolpidem, 10 mg at bedtime, for insomnia. The following day, Mr. J reports substantial relief from ADS symptoms, including myalgias, paresthesias, and suicidal ideation.21,23
His second ECT treatment is administered the next day, followed by a successful course of 9 treatments and partial remission of the MDE within 3 weeks. Fluoxetine is reduced to 10 mg/d one week into the ECT series, then discontinued one week later. No signs of emergent ADS are seen at discharge or 2-week outpatient follow-up. Mr. J achieves full remission with maintenance ECT plus bedtime doses of mirtazapine, 30 mg, and aripiprazole, 7.5 mg, across 6 months of follow-up care.
Related resources
- Schatzberg AF, Blier P, Delgado PL, et al. Antidepressant discontinuation syndrome: consensus panel recommendations for clinical management and additional research. J Clin Psychiatry. 2006;67(suppl 4):27-30.
- American Family Physician. Patient handout on antidepressant discontinuation. www.aafp.org/afp/2006/0801/p457.html.
- Rosenbaum JF, Fava M, Hoog SL, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77-87. See appendix for discontinuation-emergent signs and symptoms checklist.
Drug brand names
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Clomipramine • Anafranil
- Desvenlafaxine • Pristiq
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Lorazepam • Ativan
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
- Venlafaxine extended-release • Effexor XR
- Zolpidem • Ambien
Disclosure
Dr. Muzina reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
When Dr. Muzina submitted this article to Current Psychiatry, he was director, Center for Mood Disorders Treatment and Research, Cleveland Clinic Neurological Institute, Cleveland, OH.
Most psychiatrists have encountered patients who report distressing symptoms when they have forgotten to take their antidepressant for a few days or during changes in the medication regimen. A discontinuation syndrome can occur with almost any antidepressant, highlighting the need to slowly taper these medications when discontinuation is part of a treatment plan.
This article discusses antidepressant discontinuation syndrome (ADS) in a patient who experienced substantial distress after a rapid antidepressant taper in preparation for electroconvulsive therapy (ECT). My goal is to raise awareness of ADS, promote early detection of the syndrome, and address proper prevention and management strategies.
CASE REPORT: Feeling ‘worse than ever’
Mr. J, a 32-year-old tax accountant, is hospitalized for a major depressive episode (MDE) associated with deteriorating function and suicidal ideation. This second lifetime MDE started 8 months before his admission to an inpatient mood disorders unit.
Mr. J initially was treated with fluoxetine, up to 40 mg/d across 14 weeks, with good tolerability but no significant benefit. His psychiatrist switched Mr. J to bupropion but stopped it after 4 weeks because of side effects—including headaches, insomnia, and tremor—and limited antidepressant benefit. Venlafaxine XR was initiated next, at 150 mg/d within the first 2 weeks, increased to 225 mg/d at week 6, then titrated to 300 mg/d at week 10. After 10 weeks, aripiprazole, 5 mg/d, was added because Mr. J showed only partial, limited response to venlafaxine XR and this antipsychotic is indicated for adjunctive treatment of major depressive disorder.
Mr. J reported mild, transient restlessness but otherwise he tolerated the medications well, and he claimed excellent adherence. After 6 additional weeks of treatment, however, Mr. J was hospitalized because of persistent severely depressed mood, increasing suicidal ideation, and inability to function at work.
On admission, Mr. J is evaluated and agrees to ECT. To meet the ECT service’s protocol, venlafaxine XR is reduced to 150 mg/d for 2 days and then stopped when ECT is started. Aripiprazole is continued at 5 mg/d.
Mr. J tolerates the first ECT treatment well, but the morning before his second treatment he complains of feeling “worse than ever.” An agitated Mr. J reports dramatically intensified suicidal ideation—much more intrusive than before he was hospitalized. He also complains of diffuse muscle aches and cramps, runny nose, nausea, headache, and burning sensations in both arms and hands. He withdraws consent for ECT and returns to the mood disorders unit for ongoing treatment.
Could this be ADS?
Yes, it could. In this case, the inpatient psychiatrist and treatment team were lulled into a false sense of security by Mr. J’s history of few side effects with various treatments and medication changes. The ECT service wanted the patient off venlafaxine XR before beginning ECT, and the treatment team believed a quick taper would not cause discontinuation symptoms because Mr. J was taking an “extended-release” medication.
Within 72 hours, Mr. J went from taking 300 mg/d of venlafaxine XR to none. Within 2 days of cessation, he complained of symptoms that could characterize a discontinuation syndrome. A potential red herring in this case is that the patient complained of feeling worse after his first ECT treatment, and one might erroneously think the myalgias, headache, and other somatic symptoms were side effects of ECT and/or anesthesia.
Typical ADS symptoms
Nearly all antidepressant classes are associated with ADS. Symptoms vary from patient to patient but typically include the “FINISH” syndrome: flu-like symptoms, ###bold/bold###nsomnia, nausea, ###bold/bold###mbalance, sensory disturbances, and hyperarousal (anxiety/agitation) (Table 1).1
Adverse effects after stopping tricyclic antidepressants have been well documented. They may include FINISH syndrome features as well as cholinergic overdrive or “rebound” such as abdominal cramping and diarrhea.2-4 Reports of ADS after patients stopped selective serotonin reuptake inhibitors (SSRIs) emerged soon after these agents were introduced.5-7 Similarly, ADS has been reported with serotonin-norepinephrine reuptake inhibitors (SNRIs), including venlafaxine,8-10 venlafaxine XR,11 and duloxetine.12 ADS symptoms are similar with SSRIs and SNRIs, generally without the anticholinergic effects associated with tricyclic antidepressant discontinuation.
Fewer reports of discontinuation syndrome exist for bupropion, mirtazapine, monoamine oxidase inhibitors (MAOIs), and nefazodone.13-17 Discontinuation-emergent syndromes with these non-SSRI/non-SNRI antidepressants tend to present differently. With MAOIs, for example, neuropsychiatric symptoms such as severe anxiety, agitation, pressured speech, sleeplessness or drowsiness, hallucinations, delirium, and paranoid psychosis can be prominent.17
The prevalence of ADS is unclear, and published estimates vary widely because of the lack of large controlled studies. ADS rates with SSRIs/SNRIs have been reported from as low as 0% for fluoxetine to higher rates for shorter half-life antidepressants:
- 2.2% with sertraline
- 14% with fluvoxamine
- 20% with paroxetine
- 30.8% with clomipramine.
These rates come from a retrospective case note review of patients who discontinued antidepressants under supervision.18 In a small cohort of outpatients being treated for major depressive disorder, stopping venlafaxine XR was associated with discontinuation symptoms for the next 3 days in 7 of 9 patients (78%), compared with 2 of 9 patients (22%) stopping placebo.11
Diagnostic criteria have been proposed for ADS associated with serotonin (5-HT) reuptake inhibitors.19-22 Proposed ADS definitions differ somewhat, but essentially 3 features guide the diagnosis:
- appearance of characteristic symptoms (Table 2)21,23
- timing of those symptoms, which usually emerge within 1 week of abrupt cessation or marked reduction of the antidepressant
- symptoms generally are mild, short-lived, self-limiting, and/or rapidly reversed by restarting the original antidepressant.
Evidence suggests shorter half-life antidepressants may be associated with the highest risk for ADS, but other risk factors remain presumptive (Table 3).
Table 1
FINISH: Symptoms of antidepressant discontinuation syndrome
| Flu-like symptoms |
| Insomnia |
| Nausea |
| Imbalance |
| Sensory disturbances |
| Hyperarousal (anxiety/agitation) |
| Source: Reference 1 |
Table 2
ADS symptoms can range across a variety of system clusters
| System cluster | Symptoms |
|---|---|
| Neurosensory | Vertigo, paresthesias, shock-like reactions, myalgias, numbness, sensitivity to sound, unusual visual sensations, ringing in the ears |
| Neuromotor | Tremor, myoclonus, ataxia/gait instability, visual changes, restless legs, problems with speech, tongue movements |
| Gastrointestinal | Nausea, vomiting, cramps/bloating, diarrhea, anorexia |
| Neuropsychiatric | Anxiety/panic, depression, mood swings, suicidal ideation, irritability, impulsivity, confusion, psychosis |
| Vasomotor | Diaphoresis, flushing, temperature intolerance |
| Other | Headache, insomnia, vivid dreams, nightmares, lethargy/fatigue, flu-like symptoms |
| ADS: antidepressant discontinuation syndrome | |
| Source: Construct suggested by Shelton,21 with additional symptoms added from other sources, including the discontinuation symptom checklist of Rosenbaum et al23 | |
Table 3
Possible patient risk factors for developing ADS*
| Abrupt antidepressant discontinuation |
| Shorter half-life antidepressants |
| Intermittent nonadherence/noncompliance |
| Interrupted treatment or use of ‘drug holiday’ |
| Specific antidepressant properties (such as potent [5-HT] receptor antagonism, cholinergic effects) |
| Younger patient age (including children and adolescents) |
| Female gender |
| Pregnancy |
| Neonate/breast-fed infant (mother on antidepressant therapy) |
| History of ADS |
| Vulnerability to depressive relapse |
| Duration of treatment (possible increased risk with more than 4 to 6 weeks of antidepressant exposure) |
| Switches to or between generic antidepressant formulations (related to variations in bioequivalence) |
| History of early adverse reactions when the antidepressant was initiated |
| ADS: antidepressant discontinuation syndrome |
| *Risk factors for ADS have not been rigorously studied in randomized controlled trials. Possible risk factors in this table were found in case reports |
What causes ADS?
Although the exact cause of ADS is unknown, the literature proposes several theories.
Because of the central serotonin system’s complex connections, acute reduction in synaptic serotonin when an SSRI or SNRI is abruptly or too quickly stopped may be the first in a cascade of steps affecting transmission of multiple monoamines. Parallels have been drawn between the phenomenon observed with rapid depletion of tryptophan—the essential amino acid precursor for the synthesis of 5-HT—and ADS seen with abrupt discontinuation of serotonergic antidepressants. This suggests that acute drops in neurotransmitter levels can precipitate neuropsychiatric and somatic manifestations of ADS.24
Patients’ uncomfortable symptoms likely are caused by the serotonin, norepinephrine, and cholinergic systems and their complex interactions.25 Individual genetic factors may influence patients’ vulnerability for ADS.
Managing ADS
Awareness and prevention. ADS can be misinterpreted as side effects of newly started treatment after an antidepressant is stopped. In Mr. J’s case, the appearance of muscle aches, headaches, and other ADS symptoms after ECT was started easily could have been perceived as adverse effects of ECT. Mr. J’s agitation and increased suicidal ideation could lead a clinician to mistakenly think that MDE was worsening because the antidepressant was stopped before ECT became effective. Being aware of ADS can prevent misdiagnosis and allow you to quickly identify the condition, manage the reversible syndrome, and continue with new treatment plan—in this case, ECT.
You can help prevent ADS by educating patients about the need to adhere to antidepressant regimens and to avoid missing doses. Consider ADS risk factors—particularly medications’ half-lives—before you start, change, or stop antidepressant therapy. Gradually taper all antidepressants being discontinued, with the possible exception of fluoxetine (which, including its active metabolite, has an elimination half-life of approximately 1 to 2 weeks).
Tapering antidepressants is more art than science because we have no controlled data to support any particular tapering regimen. Tailor the taper duration based on each patient’s response to sequential dosage reductions. Antidepressants with shorter half-lives—such as venlafaxine or paroxetine—may need to be tapered more slowly, perhaps by reducing the dosage by 25% every 4 to 6 weeks. If you plan to switch medications, this process may be expedited during a cross-taper to another antidepressant. You still may see discontinuation symptoms, however, depending on which new agent is chosen and which is being stopped.
Treating ADS. Appropriately recognizing ADS risk and slowly tapering antidepressants as needed usually prevents clinically significant distress associated with discontinuation. For some patients, however, ADS may be particularly severe or prolonged, or may emerge at the end of a slow taper.
Challenging cases may be more likely with paroxetine or venlafaxine—even the extended-release or controlled-release preparations. The elimination half-life of paroxetine is 15 to 20 hours, and the half-lives of venlafaxine and venlafaxine XR are 5 to 11 hours. Desvenlafaxine’s half-life is 11 hours, and product labeling of this enantiomer of racemic venlafaxine notes that discontinuation symptoms have occurred.26 ADS treatment depends on the severity of the reaction and whether or not further antidepressant therapy is necessary.
For mild ADS, reassurance and treatment focused on specific symptoms—such as sedative-hypnotics for insomnia or benzodiazepines for anxiety—may be all that is needed, because ADS tends to gradually resolve over an average of 10 days.27
For more severe ADS, or when ongoing antidepressant therapy is indicated, restarting the recently withdrawn antidepressant at the pre-ADS dosage typically resolves the syndrome within 24 hours. Then employ a slower, more cautious taper when next attempting to discontinue that antidepressant.
Another option. An alternate management strategy is to substitute fluoxetine to suppress ADS associated with shorter half-life SSRIs or SNRIs. Case reports18,20,28 suggest that fluoxetine, 5 to 20 mg/d, can be used to ameliorate venlafaxine-induced ADS. Fluoxetine can be tried as monotherapy for 1 to 2 weeks and then rapidly tapered or stopped. Others have suggested combination therapy, such as:
- restarting venlafaxine at the pre-ADS dose plus fluoxetine, 20 mg/d
- tapering venlafaxine by 50% every 5 days until stopped
- reducing fluoxetine 1 week later to 10 mg/d for 5 days
- then stopping fluoxetine.28
In general, SSRIs should not be co-administered with SNRIs long-term because of potential additive adverse effects such as serotonin syndrome. Combining fluoxetine with an SNRI such as venlafaxine for the purpose of tapering off venlafaxine and reducing ADS risk probably is safe, however, as long as the fluoxetine dose is low (5 to 20 mg) and SNRI reduction begins immediately, with a plan for complete tapering.
CASE CONTINUED: ECT treatment proceeds
Venlafaxine XR is not restarted to address Mr. J’s suspected ADS because of concerns about potential increased risk for cardiac events (asystole, prolonged bradycardia) during ECT with concomitant venlafaxine use.29,30 Fluoxetine, which rarely may prolong ECT-induced seizures, is deemed a safer choice and is started immediately at 20 mg/d.
Because of Mr. J’s other symptoms, we prescribe lorazepam, 0.5 mg bid, for anxiety for 2 days; increase aripiprazole to 5 mg bid for agitation; and add zolpidem, 10 mg at bedtime, for insomnia. The following day, Mr. J reports substantial relief from ADS symptoms, including myalgias, paresthesias, and suicidal ideation.21,23
His second ECT treatment is administered the next day, followed by a successful course of 9 treatments and partial remission of the MDE within 3 weeks. Fluoxetine is reduced to 10 mg/d one week into the ECT series, then discontinued one week later. No signs of emergent ADS are seen at discharge or 2-week outpatient follow-up. Mr. J achieves full remission with maintenance ECT plus bedtime doses of mirtazapine, 30 mg, and aripiprazole, 7.5 mg, across 6 months of follow-up care.
Related resources
- Schatzberg AF, Blier P, Delgado PL, et al. Antidepressant discontinuation syndrome: consensus panel recommendations for clinical management and additional research. J Clin Psychiatry. 2006;67(suppl 4):27-30.
- American Family Physician. Patient handout on antidepressant discontinuation. www.aafp.org/afp/2006/0801/p457.html.
- Rosenbaum JF, Fava M, Hoog SL, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77-87. See appendix for discontinuation-emergent signs and symptoms checklist.
Drug brand names
- Aripiprazole • Abilify
- Bupropion • Wellbutrin
- Clomipramine • Anafranil
- Desvenlafaxine • Pristiq
- Duloxetine • Cymbalta
- Fluoxetine • Prozac
- Fluvoxamine • Luvox
- Lorazepam • Ativan
- Mirtazapine • Remeron
- Nefazodone • Serzone
- Paroxetine • Paxil
- Sertraline • Zoloft
- Venlafaxine • Effexor
- Venlafaxine extended-release • Effexor XR
- Zolpidem • Ambien
Disclosure
Dr. Muzina reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Acknowledgment
When Dr. Muzina submitted this article to Current Psychiatry, he was director, Center for Mood Disorders Treatment and Research, Cleveland Clinic Neurological Institute, Cleveland, OH.
1. Berber MJ. FINISH: remembering the discontinuation syndrome. Flu-like symptoms, insomnia, nausea, imbalance, sensory disturbances, and hyperarousal (anxiety/agitation). J Clin Psychiatry. 1998;59(5):255.-
2. Ceccherini-Nelli A, Bardellini L, Cur A, et al. Antidepressant withdrawal: prospective findings. Am J Psychiatry. 1993;150(1):165.-
3. Dilsaver SC, Kronfol Z, Sackellares JC, et al. Antidepressant withdrawal syndromes: evidence supporting the cholinergic overdrive hypothesis. J Clin Psychopharmacol. 1983;3(3):157-164.
4. Garner EM, Kelly MW, Thompson DF. Tricyclic antidepressant withdrawal syndrome. Ann Pharmacother. 1993;27(9):1068-1072.
5. Barr LC, Goodman WK, Price LH. Physical symptoms associated with paroxetine discontinuation. Am J Psychiatry. 1994;151(2):289.-
6. Frost L, Lal S. Shock-like sensations after discontinuation of selective serotonin reuptake inhibitors. Am J Psychiatry. 1995;152(5):810.-
7. Louie AK, Lannon RA, Ajari LJ. Withdrawal reaction after sertraline discontinuation. Am J Psychiatry. 1994;151(3):450-451.
8. Benazzi F. Venlafaxine withdrawal symptoms. Can J Psychiatry. 1996;41(7):487.-
9. Farah A, Lauer TE. Possible venlafaxine withdrawal syndrome. Am J Psychiatry. 1996;153(4):576.-
10. Louie AK, Lannon RA, Kirsch MA, et al. Venlafaxine withdrawal reactions. Am J Psychiatry. 1996;153(12):1652.-
11. Fava M, Mulroy R, Alpert J, et al. Emergence of adverse events following discontinuation of treatment with extended-release venlafaxine. Am J Psychiatry. 1997;154(12):1760-1762.
12. Perahia DG, Kajdasz DK, Desaiah D, et al. Symptoms following abrupt discontinuation of duloxetine treatment in patients with major depressive disorder. J Affect Disord. 2005;89(1-3):207-212.
13. Benazzi F. Mirtazapine withdrawal symptoms. Can J Psychiatry. 1998;43(5):525.-
14. Benazzi F. Nefazodone withdrawal symptoms. Can J Psychiatry. 1998;43(2):194-195.
15. Berigan TR. Bupropion-associated withdrawal symptoms revisited: a case report. Prim Care Companion J Clin Psychiatry. 2002;4(2):78.-
16. Berigan TR, Harazin JS. Bupropion-associated withdrawal symptoms: a case report. Prim Care Companion J Clin Psychiatry. 1999;1(2):50-51.
17. Dilsaver SC. Monoamine oxidase inhibitor withdrawal phenomena: symptoms and pathophysiology. Acta Psychiatr Scand. 1988;78(1):1-7.
18. Coupland NJ, Bell CJ, Potokar JP. Serotonin reuptake inhibitor withdrawal. J Clin Psychopharmacol. 1996;16(5):356-362.
19. Black K, Shea C, Dursun S, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: proposed diagnostic criteria. J Psychiatry Neurosci. 2000;25(3):255-261.
20. Haddad PM. Antidepressant discontinuation syndromes. Drug Saf. 2001;24(3):183-197.
21. Shelton RC. The nature of the discontinuation syndrome associated with antidepressant drugs. J Clin Psychiatry. 2006;67(suppl 4):3-7.
22. Schatzberg AF, Haddad P, Kaplan EM, et al. Serotonin reuptake inhibitor discontinuation syndrome: a hypothetical definition. Discontinuation consensus panel. J Clin Psychiatry. 1997;58(suppl 7):5-10.
23. Rosenbaum JF, Fava M, Hoog SL, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77-87.
24. Delgado PL. Monoamine depletion studies: implications for antidepressant discontinuation syndrome. J Clin Psychiatry. 2006;67(suppl 4):22-26.
25. Blier P, Tremblay P. Physiologic mechanisms underlying the antidepressant discontinuation syndrome. J Clin Psychiatry. 2006;67(suppl 4):8-13.
26. Wyeth. Desvenlafaxine (Pristiq) prescribing information. Available at: http://www.wyeth.com/content/showlabeling.asp?id=497. Accessed January 27, 2010.
27. Price JS, Waller PC, Wood SM, et al. A comparison of the post-marketing safety of four selective serotonin re-uptake inhibitors including the investigation of symptoms occurring on withdrawal. Br J Clin Pharmacol. 1996;42(6):757-763.
28. Benazzi F. SSRI discontinuation syndrome treated with fluoxetine. Int J Geriatr Psychiatry. 1998;13(6):421-422.
29. Agelink MM, Zeit T, Klieser E. Prolonged bradycardia complicates antidepressive treatment with venlafaxine and ECT. Br J Psychiatry. 1998;173:441.-
30. Gonzalez-Pinto A, Gutierrez M, Gonzalez N, et al. Efficacy and safety of venlafaxine-ECT combination in treatment-resistant depression. J Neuropsychiatry Clin Neurosci. 2002;14(2):206-209.
1. Berber MJ. FINISH: remembering the discontinuation syndrome. Flu-like symptoms, insomnia, nausea, imbalance, sensory disturbances, and hyperarousal (anxiety/agitation). J Clin Psychiatry. 1998;59(5):255.-
2. Ceccherini-Nelli A, Bardellini L, Cur A, et al. Antidepressant withdrawal: prospective findings. Am J Psychiatry. 1993;150(1):165.-
3. Dilsaver SC, Kronfol Z, Sackellares JC, et al. Antidepressant withdrawal syndromes: evidence supporting the cholinergic overdrive hypothesis. J Clin Psychopharmacol. 1983;3(3):157-164.
4. Garner EM, Kelly MW, Thompson DF. Tricyclic antidepressant withdrawal syndrome. Ann Pharmacother. 1993;27(9):1068-1072.
5. Barr LC, Goodman WK, Price LH. Physical symptoms associated with paroxetine discontinuation. Am J Psychiatry. 1994;151(2):289.-
6. Frost L, Lal S. Shock-like sensations after discontinuation of selective serotonin reuptake inhibitors. Am J Psychiatry. 1995;152(5):810.-
7. Louie AK, Lannon RA, Ajari LJ. Withdrawal reaction after sertraline discontinuation. Am J Psychiatry. 1994;151(3):450-451.
8. Benazzi F. Venlafaxine withdrawal symptoms. Can J Psychiatry. 1996;41(7):487.-
9. Farah A, Lauer TE. Possible venlafaxine withdrawal syndrome. Am J Psychiatry. 1996;153(4):576.-
10. Louie AK, Lannon RA, Kirsch MA, et al. Venlafaxine withdrawal reactions. Am J Psychiatry. 1996;153(12):1652.-
11. Fava M, Mulroy R, Alpert J, et al. Emergence of adverse events following discontinuation of treatment with extended-release venlafaxine. Am J Psychiatry. 1997;154(12):1760-1762.
12. Perahia DG, Kajdasz DK, Desaiah D, et al. Symptoms following abrupt discontinuation of duloxetine treatment in patients with major depressive disorder. J Affect Disord. 2005;89(1-3):207-212.
13. Benazzi F. Mirtazapine withdrawal symptoms. Can J Psychiatry. 1998;43(5):525.-
14. Benazzi F. Nefazodone withdrawal symptoms. Can J Psychiatry. 1998;43(2):194-195.
15. Berigan TR. Bupropion-associated withdrawal symptoms revisited: a case report. Prim Care Companion J Clin Psychiatry. 2002;4(2):78.-
16. Berigan TR, Harazin JS. Bupropion-associated withdrawal symptoms: a case report. Prim Care Companion J Clin Psychiatry. 1999;1(2):50-51.
17. Dilsaver SC. Monoamine oxidase inhibitor withdrawal phenomena: symptoms and pathophysiology. Acta Psychiatr Scand. 1988;78(1):1-7.
18. Coupland NJ, Bell CJ, Potokar JP. Serotonin reuptake inhibitor withdrawal. J Clin Psychopharmacol. 1996;16(5):356-362.
19. Black K, Shea C, Dursun S, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: proposed diagnostic criteria. J Psychiatry Neurosci. 2000;25(3):255-261.
20. Haddad PM. Antidepressant discontinuation syndromes. Drug Saf. 2001;24(3):183-197.
21. Shelton RC. The nature of the discontinuation syndrome associated with antidepressant drugs. J Clin Psychiatry. 2006;67(suppl 4):3-7.
22. Schatzberg AF, Haddad P, Kaplan EM, et al. Serotonin reuptake inhibitor discontinuation syndrome: a hypothetical definition. Discontinuation consensus panel. J Clin Psychiatry. 1997;58(suppl 7):5-10.
23. Rosenbaum JF, Fava M, Hoog SL, et al. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77-87.
24. Delgado PL. Monoamine depletion studies: implications for antidepressant discontinuation syndrome. J Clin Psychiatry. 2006;67(suppl 4):22-26.
25. Blier P, Tremblay P. Physiologic mechanisms underlying the antidepressant discontinuation syndrome. J Clin Psychiatry. 2006;67(suppl 4):8-13.
26. Wyeth. Desvenlafaxine (Pristiq) prescribing information. Available at: http://www.wyeth.com/content/showlabeling.asp?id=497. Accessed January 27, 2010.
27. Price JS, Waller PC, Wood SM, et al. A comparison of the post-marketing safety of four selective serotonin re-uptake inhibitors including the investigation of symptoms occurring on withdrawal. Br J Clin Pharmacol. 1996;42(6):757-763.
28. Benazzi F. SSRI discontinuation syndrome treated with fluoxetine. Int J Geriatr Psychiatry. 1998;13(6):421-422.
29. Agelink MM, Zeit T, Klieser E. Prolonged bradycardia complicates antidepressive treatment with venlafaxine and ECT. Br J Psychiatry. 1998;173:441.-
30. Gonzalez-Pinto A, Gutierrez M, Gonzalez N, et al. Efficacy and safety of venlafaxine-ECT combination in treatment-resistant depression. J Neuropsychiatry Clin Neurosci. 2002;14(2):206-209.
Suicide intervention: How to recognize risk, focus on patient safety
More than 50% of psychiatrists have experienced the death of a patient by suicide.1 For many of us, suicide represents the most feared outcome of a patient’s mental illness and makes managing suicide risk critical to everyday practice.
Unfortunately, we have little ability to predict suicide. Research into risk factors and the use of suicide rating scales have produced no consistently definitive methods to determine who will and who will not attempt or complete suicide.2 The purpose of suicide assessment, then, is not to predict suicide but to help us understand the sources of a patient’s suicidality and develop an informed intervention.
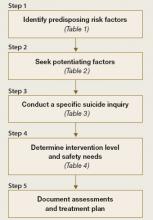
This article describes a practical, commonly accepted approach to suicide risk assessment and intervention, based on the B-SAFE model (Basic Suicide Assessment Five-step Evaluation) proposed by Jacobs et al (Figure).3 Using this method to assess suicide risk can help you answer questions such as:
- Which factors are most important to consider when evaluating suicide risk in my patient?
- What questions should I ask my patient to find out if he or she is suicidal?
- How do I know if a patient is at risk for suicide?
- What emergent interventions are called for when managing the acutely suicidal patient?
- How should I document a suicide risk assessment?
Figure Basic Suicide Assessment Five-step Evaluation (B-SAFE)
Source: Created from information in references 2,11
Why ask about suicide?
No single risk factor or combination of risk factors can predict or preclude suicide. Even so, attempting to evaluate an individual’s risk by asking about suicidal thinking, reviewing risk factors, or using clinical rating scales helps you determine the next appropriate action (discharge, medication, psychiatric referral, consultation, or hospitalization).
While talking to patients and evaluating their risk for suicide, you may begin to understand their suffering—described as the most common denominator in suicide2 and perhaps the most important clue to heightened suicide risk. Such an exploration allows you to identify potential:
- risk factors that can be modified
- preventative factors to promote.
Don’t be afraid to ask. Asking about suicidal thoughts is necessary—but not enough—to understand an individual’s potential for suicide. Never be afraid to ask patients about suicide, believing that doing so will “put ideas into their heads.” By the same token, a patient who denies thoughts or plans for suicide may still be at risk.
Identifying at-risk patients is much more difficult than just asking if they are considering suicide. Opening a concerned dialogue can provide a sense of relief to the patient while allowing you to explore:
- the extent and seriousness of the suicidal thoughts
- associated risk factors or conditions, such as depression.
Stepwise risk assessment
The first 3 steps of Jacobs’ B-SAFE model focus on identifying predisposing and protective factors for suicide.3 For an in-depth discussion, consult the American Psychiatric Association practice guideline for the assessment and treatment of patients with suicidal behaviors4 (available at http://www.psych.org/psych_pract/treatg/pg/suicidalbehavior_05-15-06.pdf).
STEP 1: Risk factors. Use the patient interview, medical records, and collateral information to uncover potential suicide risk factors (Table 1).2
Psychopathology. Focus on depression, bipolar disorder, schizophrenia, substance abuse, and personality disorders, which are strongly associated with suicide. These disorders are considered modifiable risk factors—diagnosis and appropriate treatment can diminish suicide risk.
Suicidality has been associated with early depression or bipolar disorder, often before patients receive a diagnosis or effective treatment. Recovery and immediate post-discharge periods also are thought to be times of heightened suicide risk.
Psychosocial variables. Demographic and psychosocial variables may influence suicide risk estimation. A retrospective study of 100 patients who attempted suicide suggests that the most predictive factors for suicide are:
- living alone
- being aged 17 to 35 (although in other studies, more advanced age also has been linked to increased suicide risk3)
- complaints of severe hopelessness, anhedonia, and insomnia.5
Physical illness may potentiate suicide risk. Medical illnesses that produce great pain, disfigurement, limited function, or fear of dependence may reduce a person’s will to live and increase suicide risk.6 Epilepsy has been associated with a 4- to 5-fold increase in suicide risk7 and is the only medical diagnosis to carry a documented increase in suicide among children and adolescents.8 Often these medical disorders coexist with psychiatric disorders, complicating the task of determining independent risk.
Severity of attempts or self-mutilation. When evaluating self-injurious or suicidal behavior in the emergency setting, consider the severity of the attempt as part of overall suicide assessment. Self-injurious behavior (cutting or burning) or impulsive suicide attempts (planned for <3 hours, committed in the presence others, or where discovery is very probable) appear to carry less severity or intent to die than do carefully planned and/or hidden suicide attempts.9 However, consider at high risk for suicide any patient with self-mutilating or suicidal behavior who expresses persistent intent to die; acute stabilization on an in-patient unit may be necessary.
STEP 2: ‘Protective’ factors. Discover and discuss internal and external factors that might help prevent the individual with suicidal thoughts from converting those thoughts into action (Table 2).2 When discussing these potentially protective effects, emphasize the patient’s:
- resilience during past personal crises
- family responsibilities
- religious or spiritual beliefs.
‘No-harm contracts.’ Suicide (or “no-harm”) contracts with patients might help open communication about factors that promote or mitigate suicide risk. Such contacts do not prevent suicide or lessen medicolegal risk in the event of a patient suicide, however.10
STEP 3: Suicide plans. Ask about suicide thoughts, plans, and behaviors (Table 3).11 Probe gently to allow the individual to discuss his or her feelings and to explore the next appropriate avenue of care.
In my experience, patients who reveal passive suicidal ideation (such as, “I sometimes wish I would just die in my sleep”) and strong deterrents to acting on thoughts of suicide (such as, “My children need me,” or “It’s against my religion”) should continue outpatient treatment. Those without deterrents or who discuss active and imminent thoughts and recent actions—writing suicide notes, buying a weapon, stockpiling pills—require emergent evaluation for psychiatric admission. Ask about thoughts of self-injury or mutilation (such as cutting or burning), as well as homicidal ideation.
Recognizing that patients with suicidal thoughts are almost always ambivalent about suicide to some extent—conflicted by simultaneous desires to live and to die—gives you the opportunity to intervene by allying with the part of the patient that wants to live. Creating a therapeutic connection also will help you determine the level of intervention required.
STEP 4: Intervention. Understanding why a patient feels suicidal—gathered in Steps 1 to 3—can help you choose the appropriate intervention. Among the 5 steps, Step 4 relies most heavily on clinical judgment:
- Is the suicidality acute or chronic?
- How great is the risk for suicide?
- To keep the patient safe, how urgent is the required intervention?
Acute risk. Suicidality related to Axis I psychiatric disorders tends to be acute, with prominent pain, anguish, and a desire to escape. Patients may describe a driven quality to the suicidality, which commands a treatment plan that maintains patient safety until suicidal feelings remit.
Hospitalization is often needed, plus focused treatments such as medication, psychotherapy, or electroconvulsive therapy. Intensive outpatient follow-up or partial hospitalization programs might be considered for patients:
- with whom you have a strong therapeutic alliance
- who have sturdy psychosocial support
- whose precipitating factors for suicidality have resolved.
Chronic risk. Suicide risk tends to be more chronic and has an impulsive quality for patients with suicidality related to personality disorders and environmental factors. Personality disordered patients may report feelings of anger, rage, or vengeance connected with their suicidal thoughts.
Hospitalization might become necessary, although multiple hospitalizations can be counter-therapeutic. Attempting in therapy to teach the patient to cope with suicidal thoughts and feelings might be a more effective intervention.
Malingering. Use your best judgment when patients make suicide threats that could represent malingering to achieve hospitalization.
Step 5: Documentation. Document your assessment of the suicidal patient and decision making to:
- clarify the treatment plan
- communicate to other caregivers
- manage medicolegal risk.
Include a brief summary (Box) that is timely, legible, and communicates the estimated degree of risk, known data, diagnosis, and planned interventions such as medications, tests, consultations, and follow-up reassessments.
This 46-year-old, recently divorced man is experiencing his second episode of major depression associated with clear-cut panic attacks and suspected psychotic features. Although he denies current suicidal ideation, the treatment team believes he is at moderate to high risk for suicide because of known past history of serious suicide attempt with first depression, the presence of panic/anxiety, and possibly psychotic features. Additional risk is posed by loss of marital support and his inability to verbalize meaningful protective factors.
The plan is to convert from observation status on the inpatient unit to full admission, as the suicide risk precludes discharge at present. Further medication management and consideration for electroconvulsive therapy will take place, with daily reassessments. Suicide precautions ordered.
Table 1
Factors associated with potential for increased suicide risk
| Variable | Risk Factors |
|---|---|
| Demographic | Male gender, Caucasian race, rural residence, possibly age (varies among studies) |
| Imprisoned; widowed, divorced, or separated; living alone; no children or no children living in the home | |
| Psychosocial | Lack or loss of social supports, recent loss of employment, decrease in socioeconomic status or poverty, hopelessness |
| History of victimization (physical or sexual abuse), psychological turmoil, severe relationship conflict, aggressive or impulsive traits | |
| Writing suicide notes; family history of suicide, previous attempts, ‘imitation’ suicide, gun ownership | |
| Occupational risk (physicians, dentists, nurses, pharmacists, veterinarians, farmers) | |
| Psychiatric | Psychiatric diagnosis of recent onset |
| Mood disorder, particularly major depression and bipolar disorder | |
| Schizophrenia; alcohol or other substance abuse or addiction; personality disorder; panic attacks or severe psychic anxiety | |
| Insomnia; poor concentration or confusion; anhedonia | |
| Medical | Huntington’s disease, stroke, multiple sclerosis, head injury, spinal cord injury, systemic lupus erythematosus, AIDS |
| Epilepsy, pain, malignant neoplasms, peptic ulcer disease, renal disease | |
| Source: Adapted with permission from reference 3 | |
Table 2
Potentially protective factors against suicide
| Internal |
| Successful past responses to stress |
| Positive coping skills |
| Spirituality |
| Capacity for reality testing |
| Frustration tolerance/optimism |
| Overall individual resiliency |
| External |
| Children or pets in the home |
| Religious prohibition or beliefs |
| Positive therapeutic relationships |
| Sense of responsibility to family |
| Social supports and connections |
| Financial incentives or deterrents |
| Source: Adapted from reference 11 |
Table 3
Evaluating suicide risk: Questions to ask patients
| Have you felt so sad or depressed that you thought life is not worth living? |
| Have you thought about hurting yourself or taking your own life? |
| Have you thought about a way or plan to kill yourself? |
| Do you have the means to complete the plan? (such as, do you have access to weapons or pills?) |
| Have you practiced or rehearsed this plan to end your own life? |
| Do you have a location picked out? |
| What has stopped you from following through with the plan? |
| Have you ever attempted suicide? |
| Has anyone in your family ever attempted or committed suicide? |
| Source: Adapted with permission from reference 3 |
Interventions for suicidal patients
Physical protection. Take decisive action when you determine that suicide risk is elevated and imminent. Pursue urgent psychiatric hospitalization, with or without patient consent, in accordance with local probate and involuntary commitment statutes.
The logistics of protective action can be challenging; transportation is often required, and the patient is not always cooperative with admission. Table 4 lists measures and precautions that can help keep the suicidal patient safe.
Disease-specific interventions. Because suicidal ideation is often symptomatic of a primary psychiatric disorder, rapidly identifying major depression, bipolar disorder, or a psychotic illness is crucial to reducing suicidal thoughts and behaviors. Prescribe appropriate antidepressants, mood stabilizers, and antipsychotics at adequate doses and for sufficient duration.
Be vigilant for distressing symptoms that may be elevating the patient’s suicide risk, such as anxiety, panic, agitation, insomnia, or pain. Pharmacotherapies—such as anxiolytics, sedative-hypnotics, antipsychotics, or analgesics—may rapidly reduce suffering.
Impulsivity associated with substance use disorders—particularly during intoxication and withdrawal syndromes—requires aggressive attempts by the treatment team to engage the patient in detoxification and rehabilitation.
Direct antisuicide therapy. Clozapine carries an FDA-approved indication for preventing suicide in patients with schizophrenia or schizoaffective disorder. The mechanism by which clozapine helps prevent suicide is not known, but its anti-suicidal effects appear to be independent of its antipsychotic effects.12
Lithium has been reported to reduce risk of suicide and suicide attempts in patients with bipolar disorder, perhaps by as much as 80%.13 Such benefit has not been observed with other mood stabilizers, suggesting that lithium confers protective effects against suicide beyond its mood-stabilizing effects. Suicide risk is known to increase after lithium is discontinued.14
Lithium’s antisuicidal effects may arise from its ability to enhance serotonin. This theory, although unproven, is consistent with observations associating central serotonergic deficiency with suicidal and aggressive behaviors.
Psychosocial measures. Address psychosocial variables that may increase suicide risk (Table 1). Recruit and involve the patient’s support system, augmented with a close follow-up plan. Case management to explore housing and job opportunities can help. Work with the patient’s family or others to remove guns from the patient’s access. Individual, marital, and family therapies can reduce conflicts and strengthen coping skills.
Table 4
Safety measures to protect the suicidal patient
| Hospitalize—voluntarily or involuntarily—on a locked psychiatric unit |
| Provide constant 1-to-1 observation by staff |
| Transport the patient, accompanied by adequate personnel |
| Use physical restraints or seclusion while maintaining continuous observation |
| Employ metal detector to remove dangerous, hidden objects |
| Remove and secure patient’s belongings (bags, coats, purses may contain pills or weapons) |
| Search visitors’ belongings before allowing access to unit |
| Ensure that inpatient unit meets all coded safety regulations |
Related resources
- National Suicide Prevention Lifeline, sponsored by the Substance Abuse & Mental Health Services Administration: 1-800-SUICIDE or 1-800-273-TALK (8255); www.suicidepreventionlifeline.org.
- American Foundation for Suicide Prevention (AFSP) 1-888-333-AFSP; www.afsp.org.
- Simon RI, Hales RE. Textbook of suicide assessment and management. Washington, DC: American Psychiatric Publishing; 2006.
Drug brand names
- Clozapine • Clozaril
- Lithium • Eskalith, Lithobid, others
Disclosure
Dr. Muzina has received grants from or served as a consultant to Abbott Laboratories, AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Novartis Pharmaceuticals Corp., Pfizer, and Repligen.
1. Chemtob CM, Hamada RS, Bauer G, et al. Patients’ suicides: frequency and impact on psychiatrists. Am J Psychiatry 1988;145(2):224-8.
2. Muzina DJ. What physicians can do to prevent suicide. Cleve Clin J Med 2004;71(3):242-50.
3. Jacobs DG, Brewer ML, Klein-Benheim M. Suicide assessment: an overview and recommended protocol. In: Jacobs DG, ed. The Harvard Medical School guide to suicide assessment and intervention.. San Francisco, CA: Jossey-Bass Publishers; 1999:3-39.
4. Jacobs DG, Baldessarini RJ, Conwell Y, et al. American Psychiatric Association practice guideline for the assessment and treatment of patients with suicidal behaviors. Available at: http://www.psych.org/psych_pract/treatg/pg/suicidalbehavior_05-15-06.pdf. Accessed May 14, 2007.
5. Hall RC, Platt DE, Hall RC. Suicide risk assessment: a review of risk factors for suicide in 100 patients who made severe suicide attempts. Evaluation of suicide risk in a time of managed care. Psychosomatics 1999;40(1):18-27.
6. Mackenzie TB, Popkin MK. Suicide in the medical patient. Int J Psychiatry Med 1987;17(1):3-22.
7. Barraclough BM. The suicide rate of epilepsy. Acta Psychiatr Scand 1987;76(4):339-45
8. Brent DA, Kolko DJ, Allan MJ, Brown RV. Suicidality in affectively disordered adolescent inpatients. J Am Acad Child Adolesc Psychiatry 1990;29(4):586-93.
9. Polewka A, Mikolaszek-Boba M, Chrostek Maj J, Groszek B. The characteristics of suicide attempts based on the suicidal intent scale scores. Przegl Lek 2005;62(6):415-8.
10. Lewis LM. No-harm contracts: a review of what we know. Suicide Life Threat Behav 2007;37(1):50-7.
11. Jacobs DG. A resource guide for implementing the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) 2007 patient safety goals on suicide. Available at: http://www.sprc.org/library/jcahosafetygoals.pdf. Accessed May 14, 2007.
12. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry 2003;60(1):82-91.
13. Baldessarini RJ, Tondo L, Davis P, et al. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord 2006;8(5 Pt 2):625-39.
14. Baldessarini RJ, Tondo L, Viguera AC. Discontinuing lithium maintenance treatment in bipolar disorders: risks and implications. Bipolar Disord 1999;1(1):17-24.
More than 50% of psychiatrists have experienced the death of a patient by suicide.1 For many of us, suicide represents the most feared outcome of a patient’s mental illness and makes managing suicide risk critical to everyday practice.
Unfortunately, we have little ability to predict suicide. Research into risk factors and the use of suicide rating scales have produced no consistently definitive methods to determine who will and who will not attempt or complete suicide.2 The purpose of suicide assessment, then, is not to predict suicide but to help us understand the sources of a patient’s suicidality and develop an informed intervention.
This article describes a practical, commonly accepted approach to suicide risk assessment and intervention, based on the B-SAFE model (Basic Suicide Assessment Five-step Evaluation) proposed by Jacobs et al (Figure).3 Using this method to assess suicide risk can help you answer questions such as:
- Which factors are most important to consider when evaluating suicide risk in my patient?
- What questions should I ask my patient to find out if he or she is suicidal?
- How do I know if a patient is at risk for suicide?
- What emergent interventions are called for when managing the acutely suicidal patient?
- How should I document a suicide risk assessment?
Figure Basic Suicide Assessment Five-step Evaluation (B-SAFE)
Source: Created from information in references 2,11
Why ask about suicide?
No single risk factor or combination of risk factors can predict or preclude suicide. Even so, attempting to evaluate an individual’s risk by asking about suicidal thinking, reviewing risk factors, or using clinical rating scales helps you determine the next appropriate action (discharge, medication, psychiatric referral, consultation, or hospitalization).
While talking to patients and evaluating their risk for suicide, you may begin to understand their suffering—described as the most common denominator in suicide2 and perhaps the most important clue to heightened suicide risk. Such an exploration allows you to identify potential:
- risk factors that can be modified
- preventative factors to promote.
Don’t be afraid to ask. Asking about suicidal thoughts is necessary—but not enough—to understand an individual’s potential for suicide. Never be afraid to ask patients about suicide, believing that doing so will “put ideas into their heads.” By the same token, a patient who denies thoughts or plans for suicide may still be at risk.
Identifying at-risk patients is much more difficult than just asking if they are considering suicide. Opening a concerned dialogue can provide a sense of relief to the patient while allowing you to explore:
- the extent and seriousness of the suicidal thoughts
- associated risk factors or conditions, such as depression.
Stepwise risk assessment
The first 3 steps of Jacobs’ B-SAFE model focus on identifying predisposing and protective factors for suicide.3 For an in-depth discussion, consult the American Psychiatric Association practice guideline for the assessment and treatment of patients with suicidal behaviors4 (available at http://www.psych.org/psych_pract/treatg/pg/suicidalbehavior_05-15-06.pdf).
STEP 1: Risk factors. Use the patient interview, medical records, and collateral information to uncover potential suicide risk factors (Table 1).2
Psychopathology. Focus on depression, bipolar disorder, schizophrenia, substance abuse, and personality disorders, which are strongly associated with suicide. These disorders are considered modifiable risk factors—diagnosis and appropriate treatment can diminish suicide risk.
Suicidality has been associated with early depression or bipolar disorder, often before patients receive a diagnosis or effective treatment. Recovery and immediate post-discharge periods also are thought to be times of heightened suicide risk.
Psychosocial variables. Demographic and psychosocial variables may influence suicide risk estimation. A retrospective study of 100 patients who attempted suicide suggests that the most predictive factors for suicide are:
- living alone
- being aged 17 to 35 (although in other studies, more advanced age also has been linked to increased suicide risk3)
- complaints of severe hopelessness, anhedonia, and insomnia.5
Physical illness may potentiate suicide risk. Medical illnesses that produce great pain, disfigurement, limited function, or fear of dependence may reduce a person’s will to live and increase suicide risk.6 Epilepsy has been associated with a 4- to 5-fold increase in suicide risk7 and is the only medical diagnosis to carry a documented increase in suicide among children and adolescents.8 Often these medical disorders coexist with psychiatric disorders, complicating the task of determining independent risk.
Severity of attempts or self-mutilation. When evaluating self-injurious or suicidal behavior in the emergency setting, consider the severity of the attempt as part of overall suicide assessment. Self-injurious behavior (cutting or burning) or impulsive suicide attempts (planned for <3 hours, committed in the presence others, or where discovery is very probable) appear to carry less severity or intent to die than do carefully planned and/or hidden suicide attempts.9 However, consider at high risk for suicide any patient with self-mutilating or suicidal behavior who expresses persistent intent to die; acute stabilization on an in-patient unit may be necessary.
STEP 2: ‘Protective’ factors. Discover and discuss internal and external factors that might help prevent the individual with suicidal thoughts from converting those thoughts into action (Table 2).2 When discussing these potentially protective effects, emphasize the patient’s:
- resilience during past personal crises
- family responsibilities
- religious or spiritual beliefs.
‘No-harm contracts.’ Suicide (or “no-harm”) contracts with patients might help open communication about factors that promote or mitigate suicide risk. Such contacts do not prevent suicide or lessen medicolegal risk in the event of a patient suicide, however.10
STEP 3: Suicide plans. Ask about suicide thoughts, plans, and behaviors (Table 3).11 Probe gently to allow the individual to discuss his or her feelings and to explore the next appropriate avenue of care.
In my experience, patients who reveal passive suicidal ideation (such as, “I sometimes wish I would just die in my sleep”) and strong deterrents to acting on thoughts of suicide (such as, “My children need me,” or “It’s against my religion”) should continue outpatient treatment. Those without deterrents or who discuss active and imminent thoughts and recent actions—writing suicide notes, buying a weapon, stockpiling pills—require emergent evaluation for psychiatric admission. Ask about thoughts of self-injury or mutilation (such as cutting or burning), as well as homicidal ideation.
Recognizing that patients with suicidal thoughts are almost always ambivalent about suicide to some extent—conflicted by simultaneous desires to live and to die—gives you the opportunity to intervene by allying with the part of the patient that wants to live. Creating a therapeutic connection also will help you determine the level of intervention required.
STEP 4: Intervention. Understanding why a patient feels suicidal—gathered in Steps 1 to 3—can help you choose the appropriate intervention. Among the 5 steps, Step 4 relies most heavily on clinical judgment:
- Is the suicidality acute or chronic?
- How great is the risk for suicide?
- To keep the patient safe, how urgent is the required intervention?
Acute risk. Suicidality related to Axis I psychiatric disorders tends to be acute, with prominent pain, anguish, and a desire to escape. Patients may describe a driven quality to the suicidality, which commands a treatment plan that maintains patient safety until suicidal feelings remit.
Hospitalization is often needed, plus focused treatments such as medication, psychotherapy, or electroconvulsive therapy. Intensive outpatient follow-up or partial hospitalization programs might be considered for patients:
- with whom you have a strong therapeutic alliance
- who have sturdy psychosocial support
- whose precipitating factors for suicidality have resolved.
Chronic risk. Suicide risk tends to be more chronic and has an impulsive quality for patients with suicidality related to personality disorders and environmental factors. Personality disordered patients may report feelings of anger, rage, or vengeance connected with their suicidal thoughts.
Hospitalization might become necessary, although multiple hospitalizations can be counter-therapeutic. Attempting in therapy to teach the patient to cope with suicidal thoughts and feelings might be a more effective intervention.
Malingering. Use your best judgment when patients make suicide threats that could represent malingering to achieve hospitalization.
Step 5: Documentation. Document your assessment of the suicidal patient and decision making to:
- clarify the treatment plan
- communicate to other caregivers
- manage medicolegal risk.
Include a brief summary (Box) that is timely, legible, and communicates the estimated degree of risk, known data, diagnosis, and planned interventions such as medications, tests, consultations, and follow-up reassessments.
This 46-year-old, recently divorced man is experiencing his second episode of major depression associated with clear-cut panic attacks and suspected psychotic features. Although he denies current suicidal ideation, the treatment team believes he is at moderate to high risk for suicide because of known past history of serious suicide attempt with first depression, the presence of panic/anxiety, and possibly psychotic features. Additional risk is posed by loss of marital support and his inability to verbalize meaningful protective factors.
The plan is to convert from observation status on the inpatient unit to full admission, as the suicide risk precludes discharge at present. Further medication management and consideration for electroconvulsive therapy will take place, with daily reassessments. Suicide precautions ordered.
Table 1
Factors associated with potential for increased suicide risk
| Variable | Risk Factors |
|---|---|
| Demographic | Male gender, Caucasian race, rural residence, possibly age (varies among studies) |
| Imprisoned; widowed, divorced, or separated; living alone; no children or no children living in the home | |
| Psychosocial | Lack or loss of social supports, recent loss of employment, decrease in socioeconomic status or poverty, hopelessness |
| History of victimization (physical or sexual abuse), psychological turmoil, severe relationship conflict, aggressive or impulsive traits | |
| Writing suicide notes; family history of suicide, previous attempts, ‘imitation’ suicide, gun ownership | |
| Occupational risk (physicians, dentists, nurses, pharmacists, veterinarians, farmers) | |
| Psychiatric | Psychiatric diagnosis of recent onset |
| Mood disorder, particularly major depression and bipolar disorder | |
| Schizophrenia; alcohol or other substance abuse or addiction; personality disorder; panic attacks or severe psychic anxiety | |
| Insomnia; poor concentration or confusion; anhedonia | |
| Medical | Huntington’s disease, stroke, multiple sclerosis, head injury, spinal cord injury, systemic lupus erythematosus, AIDS |
| Epilepsy, pain, malignant neoplasms, peptic ulcer disease, renal disease | |
| Source: Adapted with permission from reference 3 | |
Table 2
Potentially protective factors against suicide
| Internal |
| Successful past responses to stress |
| Positive coping skills |
| Spirituality |
| Capacity for reality testing |
| Frustration tolerance/optimism |
| Overall individual resiliency |
| External |
| Children or pets in the home |
| Religious prohibition or beliefs |
| Positive therapeutic relationships |
| Sense of responsibility to family |
| Social supports and connections |
| Financial incentives or deterrents |
| Source: Adapted from reference 11 |
Table 3
Evaluating suicide risk: Questions to ask patients
| Have you felt so sad or depressed that you thought life is not worth living? |
| Have you thought about hurting yourself or taking your own life? |
| Have you thought about a way or plan to kill yourself? |
| Do you have the means to complete the plan? (such as, do you have access to weapons or pills?) |
| Have you practiced or rehearsed this plan to end your own life? |
| Do you have a location picked out? |
| What has stopped you from following through with the plan? |
| Have you ever attempted suicide? |
| Has anyone in your family ever attempted or committed suicide? |
| Source: Adapted with permission from reference 3 |
Interventions for suicidal patients
Physical protection. Take decisive action when you determine that suicide risk is elevated and imminent. Pursue urgent psychiatric hospitalization, with or without patient consent, in accordance with local probate and involuntary commitment statutes.
The logistics of protective action can be challenging; transportation is often required, and the patient is not always cooperative with admission. Table 4 lists measures and precautions that can help keep the suicidal patient safe.
Disease-specific interventions. Because suicidal ideation is often symptomatic of a primary psychiatric disorder, rapidly identifying major depression, bipolar disorder, or a psychotic illness is crucial to reducing suicidal thoughts and behaviors. Prescribe appropriate antidepressants, mood stabilizers, and antipsychotics at adequate doses and for sufficient duration.
Be vigilant for distressing symptoms that may be elevating the patient’s suicide risk, such as anxiety, panic, agitation, insomnia, or pain. Pharmacotherapies—such as anxiolytics, sedative-hypnotics, antipsychotics, or analgesics—may rapidly reduce suffering.
Impulsivity associated with substance use disorders—particularly during intoxication and withdrawal syndromes—requires aggressive attempts by the treatment team to engage the patient in detoxification and rehabilitation.
Direct antisuicide therapy. Clozapine carries an FDA-approved indication for preventing suicide in patients with schizophrenia or schizoaffective disorder. The mechanism by which clozapine helps prevent suicide is not known, but its anti-suicidal effects appear to be independent of its antipsychotic effects.12
Lithium has been reported to reduce risk of suicide and suicide attempts in patients with bipolar disorder, perhaps by as much as 80%.13 Such benefit has not been observed with other mood stabilizers, suggesting that lithium confers protective effects against suicide beyond its mood-stabilizing effects. Suicide risk is known to increase after lithium is discontinued.14
Lithium’s antisuicidal effects may arise from its ability to enhance serotonin. This theory, although unproven, is consistent with observations associating central serotonergic deficiency with suicidal and aggressive behaviors.
Psychosocial measures. Address psychosocial variables that may increase suicide risk (Table 1). Recruit and involve the patient’s support system, augmented with a close follow-up plan. Case management to explore housing and job opportunities can help. Work with the patient’s family or others to remove guns from the patient’s access. Individual, marital, and family therapies can reduce conflicts and strengthen coping skills.
Table 4
Safety measures to protect the suicidal patient
| Hospitalize—voluntarily or involuntarily—on a locked psychiatric unit |
| Provide constant 1-to-1 observation by staff |
| Transport the patient, accompanied by adequate personnel |
| Use physical restraints or seclusion while maintaining continuous observation |
| Employ metal detector to remove dangerous, hidden objects |
| Remove and secure patient’s belongings (bags, coats, purses may contain pills or weapons) |
| Search visitors’ belongings before allowing access to unit |
| Ensure that inpatient unit meets all coded safety regulations |
Related resources
- National Suicide Prevention Lifeline, sponsored by the Substance Abuse & Mental Health Services Administration: 1-800-SUICIDE or 1-800-273-TALK (8255); www.suicidepreventionlifeline.org.
- American Foundation for Suicide Prevention (AFSP) 1-888-333-AFSP; www.afsp.org.
- Simon RI, Hales RE. Textbook of suicide assessment and management. Washington, DC: American Psychiatric Publishing; 2006.
Drug brand names
- Clozapine • Clozaril
- Lithium • Eskalith, Lithobid, others
Disclosure
Dr. Muzina has received grants from or served as a consultant to Abbott Laboratories, AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Novartis Pharmaceuticals Corp., Pfizer, and Repligen.
More than 50% of psychiatrists have experienced the death of a patient by suicide.1 For many of us, suicide represents the most feared outcome of a patient’s mental illness and makes managing suicide risk critical to everyday practice.
Unfortunately, we have little ability to predict suicide. Research into risk factors and the use of suicide rating scales have produced no consistently definitive methods to determine who will and who will not attempt or complete suicide.2 The purpose of suicide assessment, then, is not to predict suicide but to help us understand the sources of a patient’s suicidality and develop an informed intervention.
This article describes a practical, commonly accepted approach to suicide risk assessment and intervention, based on the B-SAFE model (Basic Suicide Assessment Five-step Evaluation) proposed by Jacobs et al (Figure).3 Using this method to assess suicide risk can help you answer questions such as:
- Which factors are most important to consider when evaluating suicide risk in my patient?
- What questions should I ask my patient to find out if he or she is suicidal?
- How do I know if a patient is at risk for suicide?
- What emergent interventions are called for when managing the acutely suicidal patient?
- How should I document a suicide risk assessment?
Figure Basic Suicide Assessment Five-step Evaluation (B-SAFE)
Source: Created from information in references 2,11
Why ask about suicide?
No single risk factor or combination of risk factors can predict or preclude suicide. Even so, attempting to evaluate an individual’s risk by asking about suicidal thinking, reviewing risk factors, or using clinical rating scales helps you determine the next appropriate action (discharge, medication, psychiatric referral, consultation, or hospitalization).
While talking to patients and evaluating their risk for suicide, you may begin to understand their suffering—described as the most common denominator in suicide2 and perhaps the most important clue to heightened suicide risk. Such an exploration allows you to identify potential:
- risk factors that can be modified
- preventative factors to promote.
Don’t be afraid to ask. Asking about suicidal thoughts is necessary—but not enough—to understand an individual’s potential for suicide. Never be afraid to ask patients about suicide, believing that doing so will “put ideas into their heads.” By the same token, a patient who denies thoughts or plans for suicide may still be at risk.
Identifying at-risk patients is much more difficult than just asking if they are considering suicide. Opening a concerned dialogue can provide a sense of relief to the patient while allowing you to explore:
- the extent and seriousness of the suicidal thoughts
- associated risk factors or conditions, such as depression.
Stepwise risk assessment
The first 3 steps of Jacobs’ B-SAFE model focus on identifying predisposing and protective factors for suicide.3 For an in-depth discussion, consult the American Psychiatric Association practice guideline for the assessment and treatment of patients with suicidal behaviors4 (available at http://www.psych.org/psych_pract/treatg/pg/suicidalbehavior_05-15-06.pdf).
STEP 1: Risk factors. Use the patient interview, medical records, and collateral information to uncover potential suicide risk factors (Table 1).2
Psychopathology. Focus on depression, bipolar disorder, schizophrenia, substance abuse, and personality disorders, which are strongly associated with suicide. These disorders are considered modifiable risk factors—diagnosis and appropriate treatment can diminish suicide risk.
Suicidality has been associated with early depression or bipolar disorder, often before patients receive a diagnosis or effective treatment. Recovery and immediate post-discharge periods also are thought to be times of heightened suicide risk.
Psychosocial variables. Demographic and psychosocial variables may influence suicide risk estimation. A retrospective study of 100 patients who attempted suicide suggests that the most predictive factors for suicide are:
- living alone
- being aged 17 to 35 (although in other studies, more advanced age also has been linked to increased suicide risk3)
- complaints of severe hopelessness, anhedonia, and insomnia.5
Physical illness may potentiate suicide risk. Medical illnesses that produce great pain, disfigurement, limited function, or fear of dependence may reduce a person’s will to live and increase suicide risk.6 Epilepsy has been associated with a 4- to 5-fold increase in suicide risk7 and is the only medical diagnosis to carry a documented increase in suicide among children and adolescents.8 Often these medical disorders coexist with psychiatric disorders, complicating the task of determining independent risk.
Severity of attempts or self-mutilation. When evaluating self-injurious or suicidal behavior in the emergency setting, consider the severity of the attempt as part of overall suicide assessment. Self-injurious behavior (cutting or burning) or impulsive suicide attempts (planned for <3 hours, committed in the presence others, or where discovery is very probable) appear to carry less severity or intent to die than do carefully planned and/or hidden suicide attempts.9 However, consider at high risk for suicide any patient with self-mutilating or suicidal behavior who expresses persistent intent to die; acute stabilization on an in-patient unit may be necessary.
STEP 2: ‘Protective’ factors. Discover and discuss internal and external factors that might help prevent the individual with suicidal thoughts from converting those thoughts into action (Table 2).2 When discussing these potentially protective effects, emphasize the patient’s:
- resilience during past personal crises
- family responsibilities
- religious or spiritual beliefs.
‘No-harm contracts.’ Suicide (or “no-harm”) contracts with patients might help open communication about factors that promote or mitigate suicide risk. Such contacts do not prevent suicide or lessen medicolegal risk in the event of a patient suicide, however.10
STEP 3: Suicide plans. Ask about suicide thoughts, plans, and behaviors (Table 3).11 Probe gently to allow the individual to discuss his or her feelings and to explore the next appropriate avenue of care.
In my experience, patients who reveal passive suicidal ideation (such as, “I sometimes wish I would just die in my sleep”) and strong deterrents to acting on thoughts of suicide (such as, “My children need me,” or “It’s against my religion”) should continue outpatient treatment. Those without deterrents or who discuss active and imminent thoughts and recent actions—writing suicide notes, buying a weapon, stockpiling pills—require emergent evaluation for psychiatric admission. Ask about thoughts of self-injury or mutilation (such as cutting or burning), as well as homicidal ideation.
Recognizing that patients with suicidal thoughts are almost always ambivalent about suicide to some extent—conflicted by simultaneous desires to live and to die—gives you the opportunity to intervene by allying with the part of the patient that wants to live. Creating a therapeutic connection also will help you determine the level of intervention required.
STEP 4: Intervention. Understanding why a patient feels suicidal—gathered in Steps 1 to 3—can help you choose the appropriate intervention. Among the 5 steps, Step 4 relies most heavily on clinical judgment:
- Is the suicidality acute or chronic?
- How great is the risk for suicide?
- To keep the patient safe, how urgent is the required intervention?
Acute risk. Suicidality related to Axis I psychiatric disorders tends to be acute, with prominent pain, anguish, and a desire to escape. Patients may describe a driven quality to the suicidality, which commands a treatment plan that maintains patient safety until suicidal feelings remit.
Hospitalization is often needed, plus focused treatments such as medication, psychotherapy, or electroconvulsive therapy. Intensive outpatient follow-up or partial hospitalization programs might be considered for patients:
- with whom you have a strong therapeutic alliance
- who have sturdy psychosocial support
- whose precipitating factors for suicidality have resolved.
Chronic risk. Suicide risk tends to be more chronic and has an impulsive quality for patients with suicidality related to personality disorders and environmental factors. Personality disordered patients may report feelings of anger, rage, or vengeance connected with their suicidal thoughts.
Hospitalization might become necessary, although multiple hospitalizations can be counter-therapeutic. Attempting in therapy to teach the patient to cope with suicidal thoughts and feelings might be a more effective intervention.
Malingering. Use your best judgment when patients make suicide threats that could represent malingering to achieve hospitalization.
Step 5: Documentation. Document your assessment of the suicidal patient and decision making to:
- clarify the treatment plan
- communicate to other caregivers
- manage medicolegal risk.
Include a brief summary (Box) that is timely, legible, and communicates the estimated degree of risk, known data, diagnosis, and planned interventions such as medications, tests, consultations, and follow-up reassessments.
This 46-year-old, recently divorced man is experiencing his second episode of major depression associated with clear-cut panic attacks and suspected psychotic features. Although he denies current suicidal ideation, the treatment team believes he is at moderate to high risk for suicide because of known past history of serious suicide attempt with first depression, the presence of panic/anxiety, and possibly psychotic features. Additional risk is posed by loss of marital support and his inability to verbalize meaningful protective factors.
The plan is to convert from observation status on the inpatient unit to full admission, as the suicide risk precludes discharge at present. Further medication management and consideration for electroconvulsive therapy will take place, with daily reassessments. Suicide precautions ordered.
Table 1
Factors associated with potential for increased suicide risk
| Variable | Risk Factors |
|---|---|
| Demographic | Male gender, Caucasian race, rural residence, possibly age (varies among studies) |
| Imprisoned; widowed, divorced, or separated; living alone; no children or no children living in the home | |
| Psychosocial | Lack or loss of social supports, recent loss of employment, decrease in socioeconomic status or poverty, hopelessness |
| History of victimization (physical or sexual abuse), psychological turmoil, severe relationship conflict, aggressive or impulsive traits | |
| Writing suicide notes; family history of suicide, previous attempts, ‘imitation’ suicide, gun ownership | |
| Occupational risk (physicians, dentists, nurses, pharmacists, veterinarians, farmers) | |
| Psychiatric | Psychiatric diagnosis of recent onset |
| Mood disorder, particularly major depression and bipolar disorder | |
| Schizophrenia; alcohol or other substance abuse or addiction; personality disorder; panic attacks or severe psychic anxiety | |
| Insomnia; poor concentration or confusion; anhedonia | |
| Medical | Huntington’s disease, stroke, multiple sclerosis, head injury, spinal cord injury, systemic lupus erythematosus, AIDS |
| Epilepsy, pain, malignant neoplasms, peptic ulcer disease, renal disease | |
| Source: Adapted with permission from reference 3 | |
Table 2
Potentially protective factors against suicide
| Internal |
| Successful past responses to stress |
| Positive coping skills |
| Spirituality |
| Capacity for reality testing |
| Frustration tolerance/optimism |
| Overall individual resiliency |
| External |
| Children or pets in the home |
| Religious prohibition or beliefs |
| Positive therapeutic relationships |
| Sense of responsibility to family |
| Social supports and connections |
| Financial incentives or deterrents |
| Source: Adapted from reference 11 |
Table 3
Evaluating suicide risk: Questions to ask patients
| Have you felt so sad or depressed that you thought life is not worth living? |
| Have you thought about hurting yourself or taking your own life? |
| Have you thought about a way or plan to kill yourself? |
| Do you have the means to complete the plan? (such as, do you have access to weapons or pills?) |
| Have you practiced or rehearsed this plan to end your own life? |
| Do you have a location picked out? |
| What has stopped you from following through with the plan? |
| Have you ever attempted suicide? |
| Has anyone in your family ever attempted or committed suicide? |
| Source: Adapted with permission from reference 3 |
Interventions for suicidal patients
Physical protection. Take decisive action when you determine that suicide risk is elevated and imminent. Pursue urgent psychiatric hospitalization, with or without patient consent, in accordance with local probate and involuntary commitment statutes.
The logistics of protective action can be challenging; transportation is often required, and the patient is not always cooperative with admission. Table 4 lists measures and precautions that can help keep the suicidal patient safe.
Disease-specific interventions. Because suicidal ideation is often symptomatic of a primary psychiatric disorder, rapidly identifying major depression, bipolar disorder, or a psychotic illness is crucial to reducing suicidal thoughts and behaviors. Prescribe appropriate antidepressants, mood stabilizers, and antipsychotics at adequate doses and for sufficient duration.
Be vigilant for distressing symptoms that may be elevating the patient’s suicide risk, such as anxiety, panic, agitation, insomnia, or pain. Pharmacotherapies—such as anxiolytics, sedative-hypnotics, antipsychotics, or analgesics—may rapidly reduce suffering.
Impulsivity associated with substance use disorders—particularly during intoxication and withdrawal syndromes—requires aggressive attempts by the treatment team to engage the patient in detoxification and rehabilitation.
Direct antisuicide therapy. Clozapine carries an FDA-approved indication for preventing suicide in patients with schizophrenia or schizoaffective disorder. The mechanism by which clozapine helps prevent suicide is not known, but its anti-suicidal effects appear to be independent of its antipsychotic effects.12
Lithium has been reported to reduce risk of suicide and suicide attempts in patients with bipolar disorder, perhaps by as much as 80%.13 Such benefit has not been observed with other mood stabilizers, suggesting that lithium confers protective effects against suicide beyond its mood-stabilizing effects. Suicide risk is known to increase after lithium is discontinued.14
Lithium’s antisuicidal effects may arise from its ability to enhance serotonin. This theory, although unproven, is consistent with observations associating central serotonergic deficiency with suicidal and aggressive behaviors.
Psychosocial measures. Address psychosocial variables that may increase suicide risk (Table 1). Recruit and involve the patient’s support system, augmented with a close follow-up plan. Case management to explore housing and job opportunities can help. Work with the patient’s family or others to remove guns from the patient’s access. Individual, marital, and family therapies can reduce conflicts and strengthen coping skills.
Table 4
Safety measures to protect the suicidal patient
| Hospitalize—voluntarily or involuntarily—on a locked psychiatric unit |
| Provide constant 1-to-1 observation by staff |
| Transport the patient, accompanied by adequate personnel |
| Use physical restraints or seclusion while maintaining continuous observation |
| Employ metal detector to remove dangerous, hidden objects |
| Remove and secure patient’s belongings (bags, coats, purses may contain pills or weapons) |
| Search visitors’ belongings before allowing access to unit |
| Ensure that inpatient unit meets all coded safety regulations |
Related resources
- National Suicide Prevention Lifeline, sponsored by the Substance Abuse & Mental Health Services Administration: 1-800-SUICIDE or 1-800-273-TALK (8255); www.suicidepreventionlifeline.org.
- American Foundation for Suicide Prevention (AFSP) 1-888-333-AFSP; www.afsp.org.
- Simon RI, Hales RE. Textbook of suicide assessment and management. Washington, DC: American Psychiatric Publishing; 2006.
Drug brand names
- Clozapine • Clozaril
- Lithium • Eskalith, Lithobid, others
Disclosure
Dr. Muzina has received grants from or served as a consultant to Abbott Laboratories, AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Novartis Pharmaceuticals Corp., Pfizer, and Repligen.
1. Chemtob CM, Hamada RS, Bauer G, et al. Patients’ suicides: frequency and impact on psychiatrists. Am J Psychiatry 1988;145(2):224-8.
2. Muzina DJ. What physicians can do to prevent suicide. Cleve Clin J Med 2004;71(3):242-50.
3. Jacobs DG, Brewer ML, Klein-Benheim M. Suicide assessment: an overview and recommended protocol. In: Jacobs DG, ed. The Harvard Medical School guide to suicide assessment and intervention.. San Francisco, CA: Jossey-Bass Publishers; 1999:3-39.
4. Jacobs DG, Baldessarini RJ, Conwell Y, et al. American Psychiatric Association practice guideline for the assessment and treatment of patients with suicidal behaviors. Available at: http://www.psych.org/psych_pract/treatg/pg/suicidalbehavior_05-15-06.pdf. Accessed May 14, 2007.
5. Hall RC, Platt DE, Hall RC. Suicide risk assessment: a review of risk factors for suicide in 100 patients who made severe suicide attempts. Evaluation of suicide risk in a time of managed care. Psychosomatics 1999;40(1):18-27.
6. Mackenzie TB, Popkin MK. Suicide in the medical patient. Int J Psychiatry Med 1987;17(1):3-22.
7. Barraclough BM. The suicide rate of epilepsy. Acta Psychiatr Scand 1987;76(4):339-45
8. Brent DA, Kolko DJ, Allan MJ, Brown RV. Suicidality in affectively disordered adolescent inpatients. J Am Acad Child Adolesc Psychiatry 1990;29(4):586-93.
9. Polewka A, Mikolaszek-Boba M, Chrostek Maj J, Groszek B. The characteristics of suicide attempts based on the suicidal intent scale scores. Przegl Lek 2005;62(6):415-8.
10. Lewis LM. No-harm contracts: a review of what we know. Suicide Life Threat Behav 2007;37(1):50-7.
11. Jacobs DG. A resource guide for implementing the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) 2007 patient safety goals on suicide. Available at: http://www.sprc.org/library/jcahosafetygoals.pdf. Accessed May 14, 2007.
12. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry 2003;60(1):82-91.
13. Baldessarini RJ, Tondo L, Davis P, et al. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord 2006;8(5 Pt 2):625-39.
14. Baldessarini RJ, Tondo L, Viguera AC. Discontinuing lithium maintenance treatment in bipolar disorders: risks and implications. Bipolar Disord 1999;1(1):17-24.
1. Chemtob CM, Hamada RS, Bauer G, et al. Patients’ suicides: frequency and impact on psychiatrists. Am J Psychiatry 1988;145(2):224-8.
2. Muzina DJ. What physicians can do to prevent suicide. Cleve Clin J Med 2004;71(3):242-50.
3. Jacobs DG, Brewer ML, Klein-Benheim M. Suicide assessment: an overview and recommended protocol. In: Jacobs DG, ed. The Harvard Medical School guide to suicide assessment and intervention.. San Francisco, CA: Jossey-Bass Publishers; 1999:3-39.
4. Jacobs DG, Baldessarini RJ, Conwell Y, et al. American Psychiatric Association practice guideline for the assessment and treatment of patients with suicidal behaviors. Available at: http://www.psych.org/psych_pract/treatg/pg/suicidalbehavior_05-15-06.pdf. Accessed May 14, 2007.
5. Hall RC, Platt DE, Hall RC. Suicide risk assessment: a review of risk factors for suicide in 100 patients who made severe suicide attempts. Evaluation of suicide risk in a time of managed care. Psychosomatics 1999;40(1):18-27.
6. Mackenzie TB, Popkin MK. Suicide in the medical patient. Int J Psychiatry Med 1987;17(1):3-22.
7. Barraclough BM. The suicide rate of epilepsy. Acta Psychiatr Scand 1987;76(4):339-45
8. Brent DA, Kolko DJ, Allan MJ, Brown RV. Suicidality in affectively disordered adolescent inpatients. J Am Acad Child Adolesc Psychiatry 1990;29(4):586-93.
9. Polewka A, Mikolaszek-Boba M, Chrostek Maj J, Groszek B. The characteristics of suicide attempts based on the suicidal intent scale scores. Przegl Lek 2005;62(6):415-8.
10. Lewis LM. No-harm contracts: a review of what we know. Suicide Life Threat Behav 2007;37(1):50-7.
11. Jacobs DG. A resource guide for implementing the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) 2007 patient safety goals on suicide. Available at: http://www.sprc.org/library/jcahosafetygoals.pdf. Accessed May 14, 2007.
12. Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry 2003;60(1):82-91.
13. Baldessarini RJ, Tondo L, Davis P, et al. Decreased risk of suicides and attempts during long-term lithium treatment: a meta-analytic review. Bipolar Disord 2006;8(5 Pt 2):625-39.
14. Baldessarini RJ, Tondo L, Viguera AC. Discontinuing lithium maintenance treatment in bipolar disorders: risks and implications. Bipolar Disord 1999;1(1):17-24.
Atypical antipsychotics: New drugs, new challenges
Differentiating bipolar disorder from depression in primary care
Bipolar maintenance: Are atypical antipsychotics really ‘mood stabilizers’?
Maintenance therapy with mood stabilizers is the most critical phase of bipolar disorder treatment but the stage with the least available evidence about medication risks and benefits. The FDA’s recent approval of olanzapine for bipolar maintenance raises the question of whether atypical antipsychotics are really mood stabilizers. This article attempts to answer that question by:
- describing the “ideal” mood stabilizer
- discussing atypicals’ advantages over conventional antipsychotics in bipolar patients
- comparing efficacy data for the six available atypicals
- recommending strategies to prevent and treat atypicals’ potentially serious side effects during long-term therapy.
What is a ‘mood stabilizer’?
Successful mood stabilizer maintenance therapy decreases the time patients are sick and disabled. Although somewhat dated after only 2 years, the most recent American Psychiatric Association (APA) practice guidelines1 support using mood stabilizers for patients with bipolar I and bipolar II disorders.
Table 1
Bipolar maintenance treatment goals
|
| Adapted from American Psychiatric Association practice guidelines for treating patients with bipolar disorder (reference 1) |
The goals of maintenance therapy are listed in Table 1. The ideal mood stabilizer would work in maintenance and all bipolar phases and treatment stages—from treating acute depression, mania, hypomania, and mixed states to preventing abnormal mood elevations and depressions. It would not precipitate depression or mania, rapid cycling, or cycle acceleration.
In other words, the best “mood stabilizer” would work in all four treatment roles of bipolar disorder: treating highs and lows, and preventing highs and lows. No such mood stabilizer exists, although lithium may come closest to the ideal.2
Most U.S. psychiatrists use combination therapies for bipolar disorder, particularly when treating acute manic states. The most common combination is a “known” mood stabilizer—such as lithium or divalproex—plus an antipsychotic to quickly control mania.
After mania remits, clinicians often try to eliminate the antipsychotic in hopes of maintaining mood stability and euthymia with the mood stabilizer alone. This was especially true before atypical antipsychotics were approved, given the risk for tardive dyskinesia (TD) associated with long-term use of conventional antipsychotics.
Unfortunately, patients frequently relapse with this strategy, so psychiatrists may leave their bipolar patients on atypical antipsychotics during long-term maintenance. But how good are atypicals as mood stabilizers? Perhaps more importantly, how safe is long-term use of atypicals in bipolar patients?
Antipsychotics as mood stabilizers
The 2002 APA practice guidelines discuss efficacy data for using lithium, divalproex or valproate, lamotrigine, carbamazepine, and electroconvulsive therapy for bipolar maintenance treatment. Two sentences on antipsychotic drug use note:
- one placebo-controlled study of a conventional antipsychotic showing no efficacy
- some data supporting clozapine as a prophylactic bipolar treatment.1
A 1998 review of five open trials3 touched on conventional depot antipsychotics’ value in reducing manic or affective illness. However, the authors warned:
- no controlled trials existed
- maintenance antipsychotic treatment may be associated with increased risk for tardive movement disorders
- conventional agents can exacerbate depressive symptoms in some patients.
Using conventional antipsychotics long-term in bipolar disorder is not advisable, with the possible exception of depot preparations in nonadhering patients with severe illness. Long-acting injectable atypicals—such as the recently approved IM risperidone—may displace any use of conventional antipsychotics in bipolar patients.
Atypical antipsychotics hold several advantages over conventional agents:
- significantly reduced risk for TD and extrapyramidal symptoms (EPS)
- lack of serum prolactin elevation (except with risperidone)
- improved cognition
- possible decreased suicidality, particularly with clozapine.4
Table 2
Tips for managing atypicals’ potentially serious side-effect risks
| Weight gain/obesity | ||
| Assessment | Prevention | Treatment |
| Evaluate comorbid conditions such as eating disorders or substance abuse Take nutritional and exercise history | Check weight and waist circumference at baseline and every visit Calculate body mass index at every visit Prescribe healthy diet and exercise | Patient education, careful monitoring, and prevention are most-effective treatments Drug therapy for persistent weight gain or early rapid gain (>7% in first 6 months). Agents of potential benefit include topiramate, sibutramine, metformin, zonisamide, and orlistat (see Table 3) |
| Glucose control/type 2 diabetes | ||
| Assessment | Prevention | Treatment |
| Take history of glucose intolerance or diabetes Ask about family history of diabetes, obesity, hypertension, heart disease | Check baseline weight and plasma glucose Obtain fasting plasma glucose every 3 months for first year, then annually Prescribe healthy diet and exercise | Primary prevention through careful monitoring is most effective Discontinue atypical antipsychotic; use other mood stabilizer unless atypical is only effective drug for that patient Oral hypoglycemics (metformin, others) |
| Hyperlipidemia | ||
| Assessment | Prevention | Treatment |
| Take history of hyperlipidemia or cardiovascular disease Ask about family history of hyperlipidemia | Check fasting lipid profile including triglycerides at baseline and every 3 months in first year Prescribe healthy diet and exercise | Monitor diet, exercise, weight, lipids regularly Change atypical antipsychotic or use other mood stabilizer (as described above) Oral antilipemics (simvastatin, others) |
Evidence for atypicals
Olanzapine is the only atypical FDA-approved for relapse prevention in bipolar disorder. This approval is supported by several studies, most notably two 1-year, double-blind trials:
- Mean time to any mood relapse was 174 days in patients taking olanzapine, mean 12.5 mg/d (±5 mg), compared with 22 days in a placebo group (Eli Lilly and Co., data on file).
- Manic relapse rate was 14.3% in patients treated with olanzapine, ~12 mg/d, compared with 28% in patients treated with lithium, ~1,100 mg/d (mean 0.76 mEq/L). The two treatments were similarly effective in preventing depressive relapse.5
As a mood stabilizer, olanzapine was as effective as divalproex in a 47-week randomized, double-blind study of 251 adults with bipolar I disorder.6 Patients treated with olanzapine improved more rapidly and had fewer manic symptoms than those treated with divalproex, but bipolar relapse rates were similar in both treatment groups.
Risperidone appears to have a role as a potential maintenance mood stabilizer in bipolar patients, although double-blind trials are lacking.
In a 6-month, open-label investigation, relapse rates were 16% for depression and 7% for mania in bipolar patients receiving risperidone (average 4 mg/d) combined with mood-stabilizing medications.7 These relapse rates are lower than those typically reported for mood-stabilizing monotherapy.
In another 6-month, open-label study, risperidone monotherapy (average 4 mg/d) was effective for treating mania and maintaining euthymia.8
IM risperidone is a useful option for bipolar patients chronically nonadherent with oral medications; it also substantially reduces the risk of neuroleptic side effects compared with older depot antipsychotics.
Quetiapine was recently approved as an antimanic agent and may possess mood-stabilizing properties. In a preliminary study of 10 patients with bipolar disorder, adding quetiapine (mean 200 mg/d) to existing mood stabilizer therapy for 12 weeks improved psychopathology, mania, and depression rating scale scores.9
More-recent unpublished data suggest dosing quetiapine to approximately 600 mg/d as monotherapy or an adjunct to treat acute mania, though controlled maintenance studies are lacking (AstraZeneca Pharmaceuticals, data on file).
Others. Some early evidence supports using ziprasidone and aripiprazole for bipolar mania:
- Ziprasidone monotherapy, 40 to 80 mg bid, was significantly more effective than placebo in reducing acute mania symptoms in a 3-week, double-blind, randomized trial of 197 patients with bipolar I disorder.10
- Aripiprazole monotherapy, 15 to 30 mg/d, had a significantly greater effect than placebo in a 3-week, double-blind, randomized trial of 262 patients in acute manic or mixed bipolar episodes. Response rates among patients with mania were 40% with aripiprazole and 19% with placebo.11
Both ziprasidone and aripiprazole were well-tolerated in these brief trials, although their efficacy as long-term mood-stabilizers in bipolar disorder is unclear.
Using clozapine raises concerns about potentially serious adverse events, although it remains the only agent with proven efficacy in treatment-refractory mania.12,13 Clozapine also appears to reduce hospitalization and affective relapse rates and improve symptoms and quality of life.14,15
Long-term safety
Compared with conventional antipsychotics, EPS are not a major concern with the atypical agents. Except for risperidone, atypicals’ effect on prolactin levels generally is not clinically meaningful. Atypicals appear to be “mood-friendly,” whereas conventional antipsychotics seem to contribute to dysphoria or cause depression in some patients.
Sedation or other annoying side effects such as dry mouth or dizziness can occur with any atypical. Other more-serious side effects may complicate antipsychotic treatment, as we are coming to understand from using atypicals for long-term schizophrenia management.
Table 3
Weight-loss medications for bipolar patients taking atypical antipsychotics
| Drug | Dosage | Side effects | Recommendations |
|---|---|---|---|
| Metformin | 500 to 1,000 mg bid | Hypoglycemia Diarrhea Nausea/vomiting | First-line in patients with comorbid type 2 diabetes |
| Orlistat | 120 mg tid | GI distress Change in bowel habits | Second-line For patients with BMI >27 Supplement fat-soluble vitamins |
| Sibutramine | 5 to 15 mg/d | Dry mouth Anorexia Insomnia Constipation | Second-line For patients with BMI 27 to 30 Risk of serotonin syndrome if given with serotonergic drugs |
| Topiramate | 50 to 250 mg/d | Somnolence Fatigue Paresthesias | Consider first-line for its potential additive mood-stabilizing effect May help comorbid binge-eating or seizure disorders |
| Zonisamide | 100 to 600 mg/d | Somnolence Dizziness Anorexia | Consider first-line for its potential additive mood-stabilizing effect May help comorbid binge-eating or seizure disorders |
Movement disorders. Antipsychotics appear more likely to cause EPS in patients with mood disorders than with schizophrenia. In one study using conventional antipsychotics, bipolar patients were 4 to 5 times more likely than schizophrenia patients to experience acute dystonia.16
Although atypicals pose some small risk for acute EPS and TD, the risk is near placebo-level with clinically relevant and comparable dosages.17 Even so, it is important to educate patients to watch for emerging signs of TD during long-term treatment with any antipsychotic. EPS risk may be dose-dependent, particularly with risperidone.18
Weight gain and obesity. Patients with bipolar disorder are more likely to be overweight or obese (body mass index [BMI] > 30) than the general population,17,19 though the reasons are unknown. Studies suggest an obesity prevalence of 32% to 35% in bipolar patients, compared with 18% in the general population.20,21
All atypicals can cause weight gain, although olanzapine and clozapine are associated with the greatest mean weight gains. In three long-term trials (47 weeks to 18 months), bipolar patients who received olanzapine gained significantly more weight (mean 2 to 3 kg) than those receiving lithium or divalproex.19
Cases with much greater weight gain—even leading to clinical obesity—have been observed, particularly with olanzapine. Although evidence from registration trials and clinical experience show lesser weight gains with risperidone, quetiapine, ziprasidone, and aripiprazole, some of our patients do gain weight while taking these agents—either alone or in combination with lithium or divalproex.
Weight management. Because patients with bipolar disorder may be at increased risk for weight gain and obesity, weight management techniques may improve their health by:
- decreasing morbidity and mortality tied to weight-related physical illnesses
- enhancing psychological well-being.1
In addition to diet and exercise counseling, some bipolar patients taking long-term atypical antipsychotics may benefit from adjunctive weight-loss medications (Table 3). We generally use such medications for bipolar patients who:
- persistently gain weight despite best dietary practices
- gain substantial weight early in treatment with an atypical antipsychotic that is providing effective symptomatic relief.
Early weight gain—particularly gains of >7% within the first 6 weeks—might predict large weight gain over time.
Diabetes. In September 2003, the FDA requested a class-wide labeling change to warn about a possible link between atypical antipsychotics and diabetes. The FDA recommended blood sugar monitoring of patients taking atypicals, especially those with obesity risk factors or family history of diabetes.
Type 2 diabetes develops in some patients taking atypicals, whether or not they gain substantial weight.22 This suggests that weight gain associated with bipolar disorder and the use of atypical antipsychotics may be independent risk factors for diabetes—a clear concern when treating bipolar patients.
Evidence provides no clear answer as to which atypicals may increase diabetes risk. Cautious use and vigilant monitoring of blood glucose are therefore recommended for every patient taking an atypical for long-term therapy. Also watch for increases in triglycerides and cholesterol17 in patients taking atypicals as bipolar maintenance therapy.
Conclusion
Atypical antipsychotics are valuable therapies in preventing bipolar relapses, although olanzapine is the only atypical with this indication so far. Collective data and clinical experience suggest that atypicals are indeed mood stabilizers, although—like other mood stabilizers such as lithium or divalproex—they have limitations. None achieve ideal efficacy in all four bipolar treatment roles: treating the highs and lows, and preventing the highs and lows. Atypicals seem more effective in treating and preventing the highs than the lows, reminding us that effective depression treatment is the greatest unmet need in bipolar disorder.
More double-blind, randomized, controlled trials are needed to fully understand whether all atypicals are mood stabilizers and to determine their safety and side effects in long-term therapy for patients with bipolar disorder.
Related resources
- Depression and Bipolar Support Alliance. www.dbsalliance.org
- Muzina DJ, Calabrese JR. Guidelines for treatment of bipolar disorder.In: Stein DJ, Kupfer DJ, Schatzberg AF (eds). Textbook of mood disorders Washington, DC: American Psychiatric Publishing, 2004 (in press).
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Divalproex/valproate • Depakote, Depakene
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid, et al
- Metformin • Glucophage
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal, Risperdal Consta
- Sibutramine • Meridia
- Simvastatin • Zocor
- Topiramate • Topamax
- Ziprasidone • Geodon
- Zonisamide • Zonegran
Disclosure
Dr. Muzina receives research grants from AstraZeneca Pharmaceuticals, Eli Lilly and Co., and Abbott Laboratories, is a consultant to AstraZeneca Pharmaceuticals and Pfizer, Inc., and a speaker for AstraZeneca Pharmaceuticals, Pfizer Inc., Eli Lilly and Co., and GlaxoSmithKline.
1. Hirschfeld RM, Bowden CL, Gitlin MJ, et al. Practice guideline for the treatment of patients with bipolar disorder (rev). Am J Psychiatry 2002;159:1-50.
2. Bauer MS, Mitchner L. What is a “mood stabilizer?” An evidence-based response. Am J Psychiatry 2004;161(1):3-18.
3. Keck PE, Jr, McElroy SL, Strakowski SM. Anticonvulsants and antipsychotics in the treatment of bipolar disorder. J Clin Psychiatry 1998;59(suppl 6):74-81.
4. Sharma V. Atypical antipsychotics and suicide in mood and anxiety disorders. Bipolar Disord 2003;5(suppl 2):48-52.
5. Tohen M, Marneros A, Bowden C, et al. Olanzapine versus lithium in relapse prevention in bipolar disorder: a randomized double-blind controlled 12-month clinical trial (presentation). Freiberg, Germany: Stanley Foundation Bipolar Network, Sept. 11-14, 2002.
6. Tohen M, Ketter TA, Zarate CA, et al. Olanzapine versus divalproex sodium for the treatment of acute mania and maintenance of remission: a 47-week study. Am J Psychiatry 2003;160(7):1263-71.
7. Vieta E, Goikolea JM, Corbella B, et al. Risperidone safety and efficacy in the treatment of bipolar and schizoaffective disorders: results from a 6-month, multicenter, open study. J Clin Psychiatry 2001;62(10):818-25.
8. Vieta E, Brugue E, Goikolea JM, et al. Acute and continuation risperidone monotherapy in mania. Hum Psychopharmacol 2004;19(1):41-5.
9. Sajatovic M, Brescan DW, Perez DE, et al. Quetiapine alone and added to a mood stabilizer for serious mood disorders. J Clin Psychiatry 2001;62(9):728-32.
10. Keck PE, Jr, Versiani M, Potkin S, et al. Ziprasidone in the treatment of acute bipolar mania: a three-week, placebo-controlled, double-blind, randomized trial. Am J Psychiatry 2003;160(4):741-8.
11. Keck PE, Jr, Marcus R, Tourkodimitris S, et al. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry 2003;160(9):1651-8.
12. Calabrese JR, Kimmel SE, Woyshville MJ, et al. Clozapine for treatment-refractory mania. Am J Psychiatry 1996;153(6):759-64.
13. Green AI, Tohen M, Patel JK, et al. Clozapine in the treatment of refractory psychotic mania. Am J Psychiatry 2000;157(6):982-6.
14. Zarate CA, Jr, Tohen M, Banov MD, et al. Is clozapine a mood stabilizer? J Clin Psychiatry 1995;56(3):108-12.
15. Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry 1999;156(8):1164-9.
16. Nasrallah HA, Churchill CM, Hamdan-Allan GA. Higher frequency of neuroleptic-induced dystonia in mania than in schizophrenia. Am J Psychiatry 1988;145(11):1455-6.
17. Chue P, Kovacs CS. Safety and tolerability of atypical antipsychotics in patients with bipolar disorder: prevalence, monitoring and management. Bipolar Disord 2003;5(suppl 2):62-79.
18. Simpson GM, Lindenmayer JP. Extrapyramidal symptoms in patients treated with risperidone. J Clin Psychopharmacol 1997;17(3):194-201.
19. Keck PE, Jr, McElroy SL. Bipolar disorder, obesity, and pharmacotherapy-associated weight gain. J Clin Psychiatry 2003;64(12):1426-35.
20. Fagiolini A, Frank E, Houck PR, et al. Prevalence of obesity and weight change during treatment in patients with bipolar I disorder. J Clin Psychiatry 2002;63(6):528-33.
21. Fagiolini A, Kupfer DJ, Houck PR, et al. Obesity as a correlate of outcome in patients with bipolar I disorder. Am J Psychiatry 2003;160(1):112-17.
22. Haupt DW, Newcomer JW. Abnormalities in glucose regulation associated with mental illness and treatment. J Psychosom Res 2002;53(4):925-33.
Maintenance therapy with mood stabilizers is the most critical phase of bipolar disorder treatment but the stage with the least available evidence about medication risks and benefits. The FDA’s recent approval of olanzapine for bipolar maintenance raises the question of whether atypical antipsychotics are really mood stabilizers. This article attempts to answer that question by:
- describing the “ideal” mood stabilizer
- discussing atypicals’ advantages over conventional antipsychotics in bipolar patients
- comparing efficacy data for the six available atypicals
- recommending strategies to prevent and treat atypicals’ potentially serious side effects during long-term therapy.
What is a ‘mood stabilizer’?
Successful mood stabilizer maintenance therapy decreases the time patients are sick and disabled. Although somewhat dated after only 2 years, the most recent American Psychiatric Association (APA) practice guidelines1 support using mood stabilizers for patients with bipolar I and bipolar II disorders.
Table 1
Bipolar maintenance treatment goals
|
| Adapted from American Psychiatric Association practice guidelines for treating patients with bipolar disorder (reference 1) |
The goals of maintenance therapy are listed in Table 1. The ideal mood stabilizer would work in maintenance and all bipolar phases and treatment stages—from treating acute depression, mania, hypomania, and mixed states to preventing abnormal mood elevations and depressions. It would not precipitate depression or mania, rapid cycling, or cycle acceleration.
In other words, the best “mood stabilizer” would work in all four treatment roles of bipolar disorder: treating highs and lows, and preventing highs and lows. No such mood stabilizer exists, although lithium may come closest to the ideal.2
Most U.S. psychiatrists use combination therapies for bipolar disorder, particularly when treating acute manic states. The most common combination is a “known” mood stabilizer—such as lithium or divalproex—plus an antipsychotic to quickly control mania.
After mania remits, clinicians often try to eliminate the antipsychotic in hopes of maintaining mood stability and euthymia with the mood stabilizer alone. This was especially true before atypical antipsychotics were approved, given the risk for tardive dyskinesia (TD) associated with long-term use of conventional antipsychotics.
Unfortunately, patients frequently relapse with this strategy, so psychiatrists may leave their bipolar patients on atypical antipsychotics during long-term maintenance. But how good are atypicals as mood stabilizers? Perhaps more importantly, how safe is long-term use of atypicals in bipolar patients?
Antipsychotics as mood stabilizers
The 2002 APA practice guidelines discuss efficacy data for using lithium, divalproex or valproate, lamotrigine, carbamazepine, and electroconvulsive therapy for bipolar maintenance treatment. Two sentences on antipsychotic drug use note:
- one placebo-controlled study of a conventional antipsychotic showing no efficacy
- some data supporting clozapine as a prophylactic bipolar treatment.1
A 1998 review of five open trials3 touched on conventional depot antipsychotics’ value in reducing manic or affective illness. However, the authors warned:
- no controlled trials existed
- maintenance antipsychotic treatment may be associated with increased risk for tardive movement disorders
- conventional agents can exacerbate depressive symptoms in some patients.
Using conventional antipsychotics long-term in bipolar disorder is not advisable, with the possible exception of depot preparations in nonadhering patients with severe illness. Long-acting injectable atypicals—such as the recently approved IM risperidone—may displace any use of conventional antipsychotics in bipolar patients.
Atypical antipsychotics hold several advantages over conventional agents:
- significantly reduced risk for TD and extrapyramidal symptoms (EPS)
- lack of serum prolactin elevation (except with risperidone)
- improved cognition
- possible decreased suicidality, particularly with clozapine.4
Table 2
Tips for managing atypicals’ potentially serious side-effect risks
| Weight gain/obesity | ||
| Assessment | Prevention | Treatment |
| Evaluate comorbid conditions such as eating disorders or substance abuse Take nutritional and exercise history | Check weight and waist circumference at baseline and every visit Calculate body mass index at every visit Prescribe healthy diet and exercise | Patient education, careful monitoring, and prevention are most-effective treatments Drug therapy for persistent weight gain or early rapid gain (>7% in first 6 months). Agents of potential benefit include topiramate, sibutramine, metformin, zonisamide, and orlistat (see Table 3) |
| Glucose control/type 2 diabetes | ||
| Assessment | Prevention | Treatment |
| Take history of glucose intolerance or diabetes Ask about family history of diabetes, obesity, hypertension, heart disease | Check baseline weight and plasma glucose Obtain fasting plasma glucose every 3 months for first year, then annually Prescribe healthy diet and exercise | Primary prevention through careful monitoring is most effective Discontinue atypical antipsychotic; use other mood stabilizer unless atypical is only effective drug for that patient Oral hypoglycemics (metformin, others) |
| Hyperlipidemia | ||
| Assessment | Prevention | Treatment |
| Take history of hyperlipidemia or cardiovascular disease Ask about family history of hyperlipidemia | Check fasting lipid profile including triglycerides at baseline and every 3 months in first year Prescribe healthy diet and exercise | Monitor diet, exercise, weight, lipids regularly Change atypical antipsychotic or use other mood stabilizer (as described above) Oral antilipemics (simvastatin, others) |
Evidence for atypicals
Olanzapine is the only atypical FDA-approved for relapse prevention in bipolar disorder. This approval is supported by several studies, most notably two 1-year, double-blind trials:
- Mean time to any mood relapse was 174 days in patients taking olanzapine, mean 12.5 mg/d (±5 mg), compared with 22 days in a placebo group (Eli Lilly and Co., data on file).
- Manic relapse rate was 14.3% in patients treated with olanzapine, ~12 mg/d, compared with 28% in patients treated with lithium, ~1,100 mg/d (mean 0.76 mEq/L). The two treatments were similarly effective in preventing depressive relapse.5
As a mood stabilizer, olanzapine was as effective as divalproex in a 47-week randomized, double-blind study of 251 adults with bipolar I disorder.6 Patients treated with olanzapine improved more rapidly and had fewer manic symptoms than those treated with divalproex, but bipolar relapse rates were similar in both treatment groups.
Risperidone appears to have a role as a potential maintenance mood stabilizer in bipolar patients, although double-blind trials are lacking.
In a 6-month, open-label investigation, relapse rates were 16% for depression and 7% for mania in bipolar patients receiving risperidone (average 4 mg/d) combined with mood-stabilizing medications.7 These relapse rates are lower than those typically reported for mood-stabilizing monotherapy.
In another 6-month, open-label study, risperidone monotherapy (average 4 mg/d) was effective for treating mania and maintaining euthymia.8
IM risperidone is a useful option for bipolar patients chronically nonadherent with oral medications; it also substantially reduces the risk of neuroleptic side effects compared with older depot antipsychotics.
Quetiapine was recently approved as an antimanic agent and may possess mood-stabilizing properties. In a preliminary study of 10 patients with bipolar disorder, adding quetiapine (mean 200 mg/d) to existing mood stabilizer therapy for 12 weeks improved psychopathology, mania, and depression rating scale scores.9
More-recent unpublished data suggest dosing quetiapine to approximately 600 mg/d as monotherapy or an adjunct to treat acute mania, though controlled maintenance studies are lacking (AstraZeneca Pharmaceuticals, data on file).
Others. Some early evidence supports using ziprasidone and aripiprazole for bipolar mania:
- Ziprasidone monotherapy, 40 to 80 mg bid, was significantly more effective than placebo in reducing acute mania symptoms in a 3-week, double-blind, randomized trial of 197 patients with bipolar I disorder.10
- Aripiprazole monotherapy, 15 to 30 mg/d, had a significantly greater effect than placebo in a 3-week, double-blind, randomized trial of 262 patients in acute manic or mixed bipolar episodes. Response rates among patients with mania were 40% with aripiprazole and 19% with placebo.11
Both ziprasidone and aripiprazole were well-tolerated in these brief trials, although their efficacy as long-term mood-stabilizers in bipolar disorder is unclear.
Using clozapine raises concerns about potentially serious adverse events, although it remains the only agent with proven efficacy in treatment-refractory mania.12,13 Clozapine also appears to reduce hospitalization and affective relapse rates and improve symptoms and quality of life.14,15
Long-term safety
Compared with conventional antipsychotics, EPS are not a major concern with the atypical agents. Except for risperidone, atypicals’ effect on prolactin levels generally is not clinically meaningful. Atypicals appear to be “mood-friendly,” whereas conventional antipsychotics seem to contribute to dysphoria or cause depression in some patients.
Sedation or other annoying side effects such as dry mouth or dizziness can occur with any atypical. Other more-serious side effects may complicate antipsychotic treatment, as we are coming to understand from using atypicals for long-term schizophrenia management.
Table 3
Weight-loss medications for bipolar patients taking atypical antipsychotics
| Drug | Dosage | Side effects | Recommendations |
|---|---|---|---|
| Metformin | 500 to 1,000 mg bid | Hypoglycemia Diarrhea Nausea/vomiting | First-line in patients with comorbid type 2 diabetes |
| Orlistat | 120 mg tid | GI distress Change in bowel habits | Second-line For patients with BMI >27 Supplement fat-soluble vitamins |
| Sibutramine | 5 to 15 mg/d | Dry mouth Anorexia Insomnia Constipation | Second-line For patients with BMI 27 to 30 Risk of serotonin syndrome if given with serotonergic drugs |
| Topiramate | 50 to 250 mg/d | Somnolence Fatigue Paresthesias | Consider first-line for its potential additive mood-stabilizing effect May help comorbid binge-eating or seizure disorders |
| Zonisamide | 100 to 600 mg/d | Somnolence Dizziness Anorexia | Consider first-line for its potential additive mood-stabilizing effect May help comorbid binge-eating or seizure disorders |
Movement disorders. Antipsychotics appear more likely to cause EPS in patients with mood disorders than with schizophrenia. In one study using conventional antipsychotics, bipolar patients were 4 to 5 times more likely than schizophrenia patients to experience acute dystonia.16
Although atypicals pose some small risk for acute EPS and TD, the risk is near placebo-level with clinically relevant and comparable dosages.17 Even so, it is important to educate patients to watch for emerging signs of TD during long-term treatment with any antipsychotic. EPS risk may be dose-dependent, particularly with risperidone.18
Weight gain and obesity. Patients with bipolar disorder are more likely to be overweight or obese (body mass index [BMI] > 30) than the general population,17,19 though the reasons are unknown. Studies suggest an obesity prevalence of 32% to 35% in bipolar patients, compared with 18% in the general population.20,21
All atypicals can cause weight gain, although olanzapine and clozapine are associated with the greatest mean weight gains. In three long-term trials (47 weeks to 18 months), bipolar patients who received olanzapine gained significantly more weight (mean 2 to 3 kg) than those receiving lithium or divalproex.19
Cases with much greater weight gain—even leading to clinical obesity—have been observed, particularly with olanzapine. Although evidence from registration trials and clinical experience show lesser weight gains with risperidone, quetiapine, ziprasidone, and aripiprazole, some of our patients do gain weight while taking these agents—either alone or in combination with lithium or divalproex.
Weight management. Because patients with bipolar disorder may be at increased risk for weight gain and obesity, weight management techniques may improve their health by:
- decreasing morbidity and mortality tied to weight-related physical illnesses
- enhancing psychological well-being.1
In addition to diet and exercise counseling, some bipolar patients taking long-term atypical antipsychotics may benefit from adjunctive weight-loss medications (Table 3). We generally use such medications for bipolar patients who:
- persistently gain weight despite best dietary practices
- gain substantial weight early in treatment with an atypical antipsychotic that is providing effective symptomatic relief.
Early weight gain—particularly gains of >7% within the first 6 weeks—might predict large weight gain over time.
Diabetes. In September 2003, the FDA requested a class-wide labeling change to warn about a possible link between atypical antipsychotics and diabetes. The FDA recommended blood sugar monitoring of patients taking atypicals, especially those with obesity risk factors or family history of diabetes.
Type 2 diabetes develops in some patients taking atypicals, whether or not they gain substantial weight.22 This suggests that weight gain associated with bipolar disorder and the use of atypical antipsychotics may be independent risk factors for diabetes—a clear concern when treating bipolar patients.
Evidence provides no clear answer as to which atypicals may increase diabetes risk. Cautious use and vigilant monitoring of blood glucose are therefore recommended for every patient taking an atypical for long-term therapy. Also watch for increases in triglycerides and cholesterol17 in patients taking atypicals as bipolar maintenance therapy.
Conclusion
Atypical antipsychotics are valuable therapies in preventing bipolar relapses, although olanzapine is the only atypical with this indication so far. Collective data and clinical experience suggest that atypicals are indeed mood stabilizers, although—like other mood stabilizers such as lithium or divalproex—they have limitations. None achieve ideal efficacy in all four bipolar treatment roles: treating the highs and lows, and preventing the highs and lows. Atypicals seem more effective in treating and preventing the highs than the lows, reminding us that effective depression treatment is the greatest unmet need in bipolar disorder.
More double-blind, randomized, controlled trials are needed to fully understand whether all atypicals are mood stabilizers and to determine their safety and side effects in long-term therapy for patients with bipolar disorder.
Related resources
- Depression and Bipolar Support Alliance. www.dbsalliance.org
- Muzina DJ, Calabrese JR. Guidelines for treatment of bipolar disorder.In: Stein DJ, Kupfer DJ, Schatzberg AF (eds). Textbook of mood disorders Washington, DC: American Psychiatric Publishing, 2004 (in press).
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Divalproex/valproate • Depakote, Depakene
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid, et al
- Metformin • Glucophage
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal, Risperdal Consta
- Sibutramine • Meridia
- Simvastatin • Zocor
- Topiramate • Topamax
- Ziprasidone • Geodon
- Zonisamide • Zonegran
Disclosure
Dr. Muzina receives research grants from AstraZeneca Pharmaceuticals, Eli Lilly and Co., and Abbott Laboratories, is a consultant to AstraZeneca Pharmaceuticals and Pfizer, Inc., and a speaker for AstraZeneca Pharmaceuticals, Pfizer Inc., Eli Lilly and Co., and GlaxoSmithKline.
Maintenance therapy with mood stabilizers is the most critical phase of bipolar disorder treatment but the stage with the least available evidence about medication risks and benefits. The FDA’s recent approval of olanzapine for bipolar maintenance raises the question of whether atypical antipsychotics are really mood stabilizers. This article attempts to answer that question by:
- describing the “ideal” mood stabilizer
- discussing atypicals’ advantages over conventional antipsychotics in bipolar patients
- comparing efficacy data for the six available atypicals
- recommending strategies to prevent and treat atypicals’ potentially serious side effects during long-term therapy.
What is a ‘mood stabilizer’?
Successful mood stabilizer maintenance therapy decreases the time patients are sick and disabled. Although somewhat dated after only 2 years, the most recent American Psychiatric Association (APA) practice guidelines1 support using mood stabilizers for patients with bipolar I and bipolar II disorders.
Table 1
Bipolar maintenance treatment goals
|
| Adapted from American Psychiatric Association practice guidelines for treating patients with bipolar disorder (reference 1) |
The goals of maintenance therapy are listed in Table 1. The ideal mood stabilizer would work in maintenance and all bipolar phases and treatment stages—from treating acute depression, mania, hypomania, and mixed states to preventing abnormal mood elevations and depressions. It would not precipitate depression or mania, rapid cycling, or cycle acceleration.
In other words, the best “mood stabilizer” would work in all four treatment roles of bipolar disorder: treating highs and lows, and preventing highs and lows. No such mood stabilizer exists, although lithium may come closest to the ideal.2
Most U.S. psychiatrists use combination therapies for bipolar disorder, particularly when treating acute manic states. The most common combination is a “known” mood stabilizer—such as lithium or divalproex—plus an antipsychotic to quickly control mania.
After mania remits, clinicians often try to eliminate the antipsychotic in hopes of maintaining mood stability and euthymia with the mood stabilizer alone. This was especially true before atypical antipsychotics were approved, given the risk for tardive dyskinesia (TD) associated with long-term use of conventional antipsychotics.
Unfortunately, patients frequently relapse with this strategy, so psychiatrists may leave their bipolar patients on atypical antipsychotics during long-term maintenance. But how good are atypicals as mood stabilizers? Perhaps more importantly, how safe is long-term use of atypicals in bipolar patients?
Antipsychotics as mood stabilizers
The 2002 APA practice guidelines discuss efficacy data for using lithium, divalproex or valproate, lamotrigine, carbamazepine, and electroconvulsive therapy for bipolar maintenance treatment. Two sentences on antipsychotic drug use note:
- one placebo-controlled study of a conventional antipsychotic showing no efficacy
- some data supporting clozapine as a prophylactic bipolar treatment.1
A 1998 review of five open trials3 touched on conventional depot antipsychotics’ value in reducing manic or affective illness. However, the authors warned:
- no controlled trials existed
- maintenance antipsychotic treatment may be associated with increased risk for tardive movement disorders
- conventional agents can exacerbate depressive symptoms in some patients.
Using conventional antipsychotics long-term in bipolar disorder is not advisable, with the possible exception of depot preparations in nonadhering patients with severe illness. Long-acting injectable atypicals—such as the recently approved IM risperidone—may displace any use of conventional antipsychotics in bipolar patients.
Atypical antipsychotics hold several advantages over conventional agents:
- significantly reduced risk for TD and extrapyramidal symptoms (EPS)
- lack of serum prolactin elevation (except with risperidone)
- improved cognition
- possible decreased suicidality, particularly with clozapine.4
Table 2
Tips for managing atypicals’ potentially serious side-effect risks
| Weight gain/obesity | ||
| Assessment | Prevention | Treatment |
| Evaluate comorbid conditions such as eating disorders or substance abuse Take nutritional and exercise history | Check weight and waist circumference at baseline and every visit Calculate body mass index at every visit Prescribe healthy diet and exercise | Patient education, careful monitoring, and prevention are most-effective treatments Drug therapy for persistent weight gain or early rapid gain (>7% in first 6 months). Agents of potential benefit include topiramate, sibutramine, metformin, zonisamide, and orlistat (see Table 3) |
| Glucose control/type 2 diabetes | ||
| Assessment | Prevention | Treatment |
| Take history of glucose intolerance or diabetes Ask about family history of diabetes, obesity, hypertension, heart disease | Check baseline weight and plasma glucose Obtain fasting plasma glucose every 3 months for first year, then annually Prescribe healthy diet and exercise | Primary prevention through careful monitoring is most effective Discontinue atypical antipsychotic; use other mood stabilizer unless atypical is only effective drug for that patient Oral hypoglycemics (metformin, others) |
| Hyperlipidemia | ||
| Assessment | Prevention | Treatment |
| Take history of hyperlipidemia or cardiovascular disease Ask about family history of hyperlipidemia | Check fasting lipid profile including triglycerides at baseline and every 3 months in first year Prescribe healthy diet and exercise | Monitor diet, exercise, weight, lipids regularly Change atypical antipsychotic or use other mood stabilizer (as described above) Oral antilipemics (simvastatin, others) |
Evidence for atypicals
Olanzapine is the only atypical FDA-approved for relapse prevention in bipolar disorder. This approval is supported by several studies, most notably two 1-year, double-blind trials:
- Mean time to any mood relapse was 174 days in patients taking olanzapine, mean 12.5 mg/d (±5 mg), compared with 22 days in a placebo group (Eli Lilly and Co., data on file).
- Manic relapse rate was 14.3% in patients treated with olanzapine, ~12 mg/d, compared with 28% in patients treated with lithium, ~1,100 mg/d (mean 0.76 mEq/L). The two treatments were similarly effective in preventing depressive relapse.5
As a mood stabilizer, olanzapine was as effective as divalproex in a 47-week randomized, double-blind study of 251 adults with bipolar I disorder.6 Patients treated with olanzapine improved more rapidly and had fewer manic symptoms than those treated with divalproex, but bipolar relapse rates were similar in both treatment groups.
Risperidone appears to have a role as a potential maintenance mood stabilizer in bipolar patients, although double-blind trials are lacking.
In a 6-month, open-label investigation, relapse rates were 16% for depression and 7% for mania in bipolar patients receiving risperidone (average 4 mg/d) combined with mood-stabilizing medications.7 These relapse rates are lower than those typically reported for mood-stabilizing monotherapy.
In another 6-month, open-label study, risperidone monotherapy (average 4 mg/d) was effective for treating mania and maintaining euthymia.8
IM risperidone is a useful option for bipolar patients chronically nonadherent with oral medications; it also substantially reduces the risk of neuroleptic side effects compared with older depot antipsychotics.
Quetiapine was recently approved as an antimanic agent and may possess mood-stabilizing properties. In a preliminary study of 10 patients with bipolar disorder, adding quetiapine (mean 200 mg/d) to existing mood stabilizer therapy for 12 weeks improved psychopathology, mania, and depression rating scale scores.9
More-recent unpublished data suggest dosing quetiapine to approximately 600 mg/d as monotherapy or an adjunct to treat acute mania, though controlled maintenance studies are lacking (AstraZeneca Pharmaceuticals, data on file).
Others. Some early evidence supports using ziprasidone and aripiprazole for bipolar mania:
- Ziprasidone monotherapy, 40 to 80 mg bid, was significantly more effective than placebo in reducing acute mania symptoms in a 3-week, double-blind, randomized trial of 197 patients with bipolar I disorder.10
- Aripiprazole monotherapy, 15 to 30 mg/d, had a significantly greater effect than placebo in a 3-week, double-blind, randomized trial of 262 patients in acute manic or mixed bipolar episodes. Response rates among patients with mania were 40% with aripiprazole and 19% with placebo.11
Both ziprasidone and aripiprazole were well-tolerated in these brief trials, although their efficacy as long-term mood-stabilizers in bipolar disorder is unclear.
Using clozapine raises concerns about potentially serious adverse events, although it remains the only agent with proven efficacy in treatment-refractory mania.12,13 Clozapine also appears to reduce hospitalization and affective relapse rates and improve symptoms and quality of life.14,15
Long-term safety
Compared with conventional antipsychotics, EPS are not a major concern with the atypical agents. Except for risperidone, atypicals’ effect on prolactin levels generally is not clinically meaningful. Atypicals appear to be “mood-friendly,” whereas conventional antipsychotics seem to contribute to dysphoria or cause depression in some patients.
Sedation or other annoying side effects such as dry mouth or dizziness can occur with any atypical. Other more-serious side effects may complicate antipsychotic treatment, as we are coming to understand from using atypicals for long-term schizophrenia management.
Table 3
Weight-loss medications for bipolar patients taking atypical antipsychotics
| Drug | Dosage | Side effects | Recommendations |
|---|---|---|---|
| Metformin | 500 to 1,000 mg bid | Hypoglycemia Diarrhea Nausea/vomiting | First-line in patients with comorbid type 2 diabetes |
| Orlistat | 120 mg tid | GI distress Change in bowel habits | Second-line For patients with BMI >27 Supplement fat-soluble vitamins |
| Sibutramine | 5 to 15 mg/d | Dry mouth Anorexia Insomnia Constipation | Second-line For patients with BMI 27 to 30 Risk of serotonin syndrome if given with serotonergic drugs |
| Topiramate | 50 to 250 mg/d | Somnolence Fatigue Paresthesias | Consider first-line for its potential additive mood-stabilizing effect May help comorbid binge-eating or seizure disorders |
| Zonisamide | 100 to 600 mg/d | Somnolence Dizziness Anorexia | Consider first-line for its potential additive mood-stabilizing effect May help comorbid binge-eating or seizure disorders |
Movement disorders. Antipsychotics appear more likely to cause EPS in patients with mood disorders than with schizophrenia. In one study using conventional antipsychotics, bipolar patients were 4 to 5 times more likely than schizophrenia patients to experience acute dystonia.16
Although atypicals pose some small risk for acute EPS and TD, the risk is near placebo-level with clinically relevant and comparable dosages.17 Even so, it is important to educate patients to watch for emerging signs of TD during long-term treatment with any antipsychotic. EPS risk may be dose-dependent, particularly with risperidone.18
Weight gain and obesity. Patients with bipolar disorder are more likely to be overweight or obese (body mass index [BMI] > 30) than the general population,17,19 though the reasons are unknown. Studies suggest an obesity prevalence of 32% to 35% in bipolar patients, compared with 18% in the general population.20,21
All atypicals can cause weight gain, although olanzapine and clozapine are associated with the greatest mean weight gains. In three long-term trials (47 weeks to 18 months), bipolar patients who received olanzapine gained significantly more weight (mean 2 to 3 kg) than those receiving lithium or divalproex.19
Cases with much greater weight gain—even leading to clinical obesity—have been observed, particularly with olanzapine. Although evidence from registration trials and clinical experience show lesser weight gains with risperidone, quetiapine, ziprasidone, and aripiprazole, some of our patients do gain weight while taking these agents—either alone or in combination with lithium or divalproex.
Weight management. Because patients with bipolar disorder may be at increased risk for weight gain and obesity, weight management techniques may improve their health by:
- decreasing morbidity and mortality tied to weight-related physical illnesses
- enhancing psychological well-being.1
In addition to diet and exercise counseling, some bipolar patients taking long-term atypical antipsychotics may benefit from adjunctive weight-loss medications (Table 3). We generally use such medications for bipolar patients who:
- persistently gain weight despite best dietary practices
- gain substantial weight early in treatment with an atypical antipsychotic that is providing effective symptomatic relief.
Early weight gain—particularly gains of >7% within the first 6 weeks—might predict large weight gain over time.
Diabetes. In September 2003, the FDA requested a class-wide labeling change to warn about a possible link between atypical antipsychotics and diabetes. The FDA recommended blood sugar monitoring of patients taking atypicals, especially those with obesity risk factors or family history of diabetes.
Type 2 diabetes develops in some patients taking atypicals, whether or not they gain substantial weight.22 This suggests that weight gain associated with bipolar disorder and the use of atypical antipsychotics may be independent risk factors for diabetes—a clear concern when treating bipolar patients.
Evidence provides no clear answer as to which atypicals may increase diabetes risk. Cautious use and vigilant monitoring of blood glucose are therefore recommended for every patient taking an atypical for long-term therapy. Also watch for increases in triglycerides and cholesterol17 in patients taking atypicals as bipolar maintenance therapy.
Conclusion
Atypical antipsychotics are valuable therapies in preventing bipolar relapses, although olanzapine is the only atypical with this indication so far. Collective data and clinical experience suggest that atypicals are indeed mood stabilizers, although—like other mood stabilizers such as lithium or divalproex—they have limitations. None achieve ideal efficacy in all four bipolar treatment roles: treating the highs and lows, and preventing the highs and lows. Atypicals seem more effective in treating and preventing the highs than the lows, reminding us that effective depression treatment is the greatest unmet need in bipolar disorder.
More double-blind, randomized, controlled trials are needed to fully understand whether all atypicals are mood stabilizers and to determine their safety and side effects in long-term therapy for patients with bipolar disorder.
Related resources
- Depression and Bipolar Support Alliance. www.dbsalliance.org
- Muzina DJ, Calabrese JR. Guidelines for treatment of bipolar disorder.In: Stein DJ, Kupfer DJ, Schatzberg AF (eds). Textbook of mood disorders Washington, DC: American Psychiatric Publishing, 2004 (in press).
Drug brand names
- Aripiprazole • Abilify
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Divalproex/valproate • Depakote, Depakene
- Lamotrigine • Lamictal
- Lithium • Eskalith, Lithobid, et al
- Metformin • Glucophage
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal, Risperdal Consta
- Sibutramine • Meridia
- Simvastatin • Zocor
- Topiramate • Topamax
- Ziprasidone • Geodon
- Zonisamide • Zonegran
Disclosure
Dr. Muzina receives research grants from AstraZeneca Pharmaceuticals, Eli Lilly and Co., and Abbott Laboratories, is a consultant to AstraZeneca Pharmaceuticals and Pfizer, Inc., and a speaker for AstraZeneca Pharmaceuticals, Pfizer Inc., Eli Lilly and Co., and GlaxoSmithKline.
1. Hirschfeld RM, Bowden CL, Gitlin MJ, et al. Practice guideline for the treatment of patients with bipolar disorder (rev). Am J Psychiatry 2002;159:1-50.
2. Bauer MS, Mitchner L. What is a “mood stabilizer?” An evidence-based response. Am J Psychiatry 2004;161(1):3-18.
3. Keck PE, Jr, McElroy SL, Strakowski SM. Anticonvulsants and antipsychotics in the treatment of bipolar disorder. J Clin Psychiatry 1998;59(suppl 6):74-81.
4. Sharma V. Atypical antipsychotics and suicide in mood and anxiety disorders. Bipolar Disord 2003;5(suppl 2):48-52.
5. Tohen M, Marneros A, Bowden C, et al. Olanzapine versus lithium in relapse prevention in bipolar disorder: a randomized double-blind controlled 12-month clinical trial (presentation). Freiberg, Germany: Stanley Foundation Bipolar Network, Sept. 11-14, 2002.
6. Tohen M, Ketter TA, Zarate CA, et al. Olanzapine versus divalproex sodium for the treatment of acute mania and maintenance of remission: a 47-week study. Am J Psychiatry 2003;160(7):1263-71.
7. Vieta E, Goikolea JM, Corbella B, et al. Risperidone safety and efficacy in the treatment of bipolar and schizoaffective disorders: results from a 6-month, multicenter, open study. J Clin Psychiatry 2001;62(10):818-25.
8. Vieta E, Brugue E, Goikolea JM, et al. Acute and continuation risperidone monotherapy in mania. Hum Psychopharmacol 2004;19(1):41-5.
9. Sajatovic M, Brescan DW, Perez DE, et al. Quetiapine alone and added to a mood stabilizer for serious mood disorders. J Clin Psychiatry 2001;62(9):728-32.
10. Keck PE, Jr, Versiani M, Potkin S, et al. Ziprasidone in the treatment of acute bipolar mania: a three-week, placebo-controlled, double-blind, randomized trial. Am J Psychiatry 2003;160(4):741-8.
11. Keck PE, Jr, Marcus R, Tourkodimitris S, et al. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry 2003;160(9):1651-8.
12. Calabrese JR, Kimmel SE, Woyshville MJ, et al. Clozapine for treatment-refractory mania. Am J Psychiatry 1996;153(6):759-64.
13. Green AI, Tohen M, Patel JK, et al. Clozapine in the treatment of refractory psychotic mania. Am J Psychiatry 2000;157(6):982-6.
14. Zarate CA, Jr, Tohen M, Banov MD, et al. Is clozapine a mood stabilizer? J Clin Psychiatry 1995;56(3):108-12.
15. Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry 1999;156(8):1164-9.
16. Nasrallah HA, Churchill CM, Hamdan-Allan GA. Higher frequency of neuroleptic-induced dystonia in mania than in schizophrenia. Am J Psychiatry 1988;145(11):1455-6.
17. Chue P, Kovacs CS. Safety and tolerability of atypical antipsychotics in patients with bipolar disorder: prevalence, monitoring and management. Bipolar Disord 2003;5(suppl 2):62-79.
18. Simpson GM, Lindenmayer JP. Extrapyramidal symptoms in patients treated with risperidone. J Clin Psychopharmacol 1997;17(3):194-201.
19. Keck PE, Jr, McElroy SL. Bipolar disorder, obesity, and pharmacotherapy-associated weight gain. J Clin Psychiatry 2003;64(12):1426-35.
20. Fagiolini A, Frank E, Houck PR, et al. Prevalence of obesity and weight change during treatment in patients with bipolar I disorder. J Clin Psychiatry 2002;63(6):528-33.
21. Fagiolini A, Kupfer DJ, Houck PR, et al. Obesity as a correlate of outcome in patients with bipolar I disorder. Am J Psychiatry 2003;160(1):112-17.
22. Haupt DW, Newcomer JW. Abnormalities in glucose regulation associated with mental illness and treatment. J Psychosom Res 2002;53(4):925-33.
1. Hirschfeld RM, Bowden CL, Gitlin MJ, et al. Practice guideline for the treatment of patients with bipolar disorder (rev). Am J Psychiatry 2002;159:1-50.
2. Bauer MS, Mitchner L. What is a “mood stabilizer?” An evidence-based response. Am J Psychiatry 2004;161(1):3-18.
3. Keck PE, Jr, McElroy SL, Strakowski SM. Anticonvulsants and antipsychotics in the treatment of bipolar disorder. J Clin Psychiatry 1998;59(suppl 6):74-81.
4. Sharma V. Atypical antipsychotics and suicide in mood and anxiety disorders. Bipolar Disord 2003;5(suppl 2):48-52.
5. Tohen M, Marneros A, Bowden C, et al. Olanzapine versus lithium in relapse prevention in bipolar disorder: a randomized double-blind controlled 12-month clinical trial (presentation). Freiberg, Germany: Stanley Foundation Bipolar Network, Sept. 11-14, 2002.
6. Tohen M, Ketter TA, Zarate CA, et al. Olanzapine versus divalproex sodium for the treatment of acute mania and maintenance of remission: a 47-week study. Am J Psychiatry 2003;160(7):1263-71.
7. Vieta E, Goikolea JM, Corbella B, et al. Risperidone safety and efficacy in the treatment of bipolar and schizoaffective disorders: results from a 6-month, multicenter, open study. J Clin Psychiatry 2001;62(10):818-25.
8. Vieta E, Brugue E, Goikolea JM, et al. Acute and continuation risperidone monotherapy in mania. Hum Psychopharmacol 2004;19(1):41-5.
9. Sajatovic M, Brescan DW, Perez DE, et al. Quetiapine alone and added to a mood stabilizer for serious mood disorders. J Clin Psychiatry 2001;62(9):728-32.
10. Keck PE, Jr, Versiani M, Potkin S, et al. Ziprasidone in the treatment of acute bipolar mania: a three-week, placebo-controlled, double-blind, randomized trial. Am J Psychiatry 2003;160(4):741-8.
11. Keck PE, Jr, Marcus R, Tourkodimitris S, et al. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. Am J Psychiatry 2003;160(9):1651-8.
12. Calabrese JR, Kimmel SE, Woyshville MJ, et al. Clozapine for treatment-refractory mania. Am J Psychiatry 1996;153(6):759-64.
13. Green AI, Tohen M, Patel JK, et al. Clozapine in the treatment of refractory psychotic mania. Am J Psychiatry 2000;157(6):982-6.
14. Zarate CA, Jr, Tohen M, Banov MD, et al. Is clozapine a mood stabilizer? J Clin Psychiatry 1995;56(3):108-12.
15. Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry 1999;156(8):1164-9.
16. Nasrallah HA, Churchill CM, Hamdan-Allan GA. Higher frequency of neuroleptic-induced dystonia in mania than in schizophrenia. Am J Psychiatry 1988;145(11):1455-6.
17. Chue P, Kovacs CS. Safety and tolerability of atypical antipsychotics in patients with bipolar disorder: prevalence, monitoring and management. Bipolar Disord 2003;5(suppl 2):62-79.
18. Simpson GM, Lindenmayer JP. Extrapyramidal symptoms in patients treated with risperidone. J Clin Psychopharmacol 1997;17(3):194-201.
19. Keck PE, Jr, McElroy SL. Bipolar disorder, obesity, and pharmacotherapy-associated weight gain. J Clin Psychiatry 2003;64(12):1426-35.
20. Fagiolini A, Frank E, Houck PR, et al. Prevalence of obesity and weight change during treatment in patients with bipolar I disorder. J Clin Psychiatry 2002;63(6):528-33.
21. Fagiolini A, Kupfer DJ, Houck PR, et al. Obesity as a correlate of outcome in patients with bipolar I disorder. Am J Psychiatry 2003;160(1):112-17.
22. Haupt DW, Newcomer JW. Abnormalities in glucose regulation associated with mental illness and treatment. J Psychosom Res 2002;53(4):925-33.
What physicians can do to prevent suicide
Rapid-cycling bipolar disorder: Which therapies are most effective?
Patients with rapid-cycling bipolar disorder (RCBD) can be frustrating to treat. Despite growing research and data, knowledge and effective therapies remain limited. How do you manage patients with rapid cycling who do not respond robustly to lithium, divalproex, or carbamazepine monotherapy? Are combination therapies likely to be more effective? Where does lamotrigine fit in? Is there a role for conventional antidepressants?
We’ll explore these and related questions—but the final answers are not yet in. Recognition of RCBD is important because it presents such difficult treatment challenges. Available evidence does suggest that rapid cycling as defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (Box 1), describes a clinically specific course of illness that may require treatments different from currently used traditional drug therapies for nonrapid cycling bipolar disorder, particularly as no one agent appears to provide ideal bimodal treatment and prophylaxis of this bipolar disorder variant.
Rapid cycling is a specifier of the longitudinal course of illness presentation that is seen almost exclusively in bipolar disorder and is associated with a greater morbidity. Dunner and Fieve1 originally coined the term when evaluating clinical factors associated with lithium prophylaxis failure. Since that time the validity of rapid cycling as a distinct course modifier for bipolar disorder has been supported by multiple studies, leading to its inclusion in the fourth edition of the Diagnostic and Statistical Manual of the APA (1994).
According to DSM-IV, the course specifier of rapid cycling applies to “at least 4 episodes of a mood disturbance in the previous 12 months that meet criteria for a manic episode, a hypomanic episode, or a major depressive episode.” The episodes must be demarcated by a full or partial remission lasting at least 2 months or by a switch to a mood state of opposite polarity.
Early reports noted that patients suffering from RCBD did not respond adequately when treated with lithium.1 Other observations indicated that divalproex was more effective in this patient population, particularly for the illness’ hypomanic or manic phases.2 We hope that the following evaluation of these and other drug therapies will prove helpful.
Watch out for antidepressants
Most concerning has been the frequency and severity of treatment-refractory depressive phases of RCBD that may be exacerbated by antidepressant use (cycle induction or acceleration). Indeed, the frequent recurrence of refractory depression has been described as the hallmark of this bipolar disorder variant.3
Lithium: the scale weighs against it
Although an excellent mood stabilizer for most patients with bipolar disorder, lithium monotherapy is less than ideal for patients with the rapid-cycling variant, particularly in treatment or prevention of depressive or mixed episodes. The efficacy of lithium is likely decreased by the concurrent administration of antidepressant medication and increased when administered with other mood stabilizers.
The landmark article by Dunner and Fieve,1 which described a placebo-controlled, double-blind maintenance study in a general cohort of 55 patients, tried to clarify factors associated with the failure of lithium prophylaxis in bipolar disorder. Rapid cyclers comprised 20% of the subjects and 80% were nonrapid cyclers. Rapid cyclers were disproportionately represented in the lithium failure group. Lithium failures included 82% (9 of 11) of rapid cyclers compared to 41% (18 of 44) of nonrapid cyclers. Lithium failure was defined as (1) hospitalization for, or (2) treatment of, mania or (3) depression during lithium therapy, or as mood symptoms that, as documented by rating scales, were sufficient to warrant a diagnosis of mild depression, hypomania, or mania persisting for at least 2 weeks.
Kukopulos et al4 replicated the findings of Dunner and Fieve in a study of the longitudinal clinical course of 434 bipolar patients. Of these patients, 50 were rapid cyclers and had received continuous lithium therapy for more than a year, with good to partial prophylaxis in only 28%. Maj and colleagues5 published a 5-year prospective study of lithium therapy in 402 patients with bipolar disorder and noted the absence of rapid cycling in good responders to lithium but an incidence rate of 26% in nonresponders to lithium.
Other investigators have reported better response in RCBD. In a select cohort of lithium-responsive bipolar I and II patients, Tondo et al6 concluded that lithium maintenance yields striking long-term reductions in depressive and manic morbidity, more so in rapid cycling type II patients. This study, however, was in a cohort of lithium responders and excluded patients who had been exposed to antipsychotic or antidepressant medications for more than 3 months, those on chronic anticonvulsant therapy, and those with substance abuse disorders.
Although most studies do report poor response to lithium therapy in RCBD, Wehr and colleagues7 suggest that in some patients with rapid cycling, the discontinuation of antidepressant drugs may allow lithium to act as a more effective anticycling mood-stabilizing agent.
Divalproex: effective in manic phase
In contrast to lithium, an open trial of a homogenous cohort of patients with RCBD by Calabrese and colleagues3,8 found divalproex to possess moderate to marked acute and prophylactic antimanic properties with only modest antidepressant effects (Table 1). Data from 6 open studies involving 147 patients with rapid cycling suggest that divalproex possesses moderate to marked efficacy in the manic phase, but poor to moderate efficacy in the depressed phase. Positive outcome predictors were bipolar II and mixed states, no prior lithium therapy, and a positive family history of affective disorder. Predictors of negative response included increase in frequency and severity of mania, and borderline personality disorder.
Divalproex therapy in combination with lithium may improve response rates.9 Calabrese and colleagues, however, have examined large cohorts of patients, including those comorbid with alcohol, cocaine, and/or cannabis abuse, treated with a lithium-divalproex combination over 6-month study periods. The researchers found that only 25% to 50% of patients stabilized, and that of those not exhibiting a response, the majority (75%) did not respond because of treatment-refractory depression in the context of RCBD.3
Although experts believe divalproex to be more effective than lithium in preventing episodes associated with RCBD, such a conclusion awaits confirmation with the near completion of a double-blind, 20-month maintenance trial sponsored by the National Institute of Mental Health (NIMH).
Carbamazepine’s role in combination therapy
Early reports by Post and colleagues in 1987 suggested that rapid cycling predicted positive response to carbamazepine, but later findings by Okuma in 1993 refuted this. Other collective open and controlled studies suggest that this anticonvulsant possesses moderate to marked efficacy in the manic phase, and poor to moderate efficacy in the depressed phase of RCBD. Again, combination therapy with lithium may offer greater efficacy. Of significance, carbamazepine treatment outcomes have not been prospectively evaluated in a homogeneous cohort of rapid cyclers.
The limitations of carbamazepine therapy are well known and available evidence also does not seem to support monotherapy with this agent as being useful in RCBD, especially in the treatment and prophylaxis of depressive or mixed phases of the disorder. Thus, further controlled studies are needed to examine the agent’s potential role and safety in combination therapies for RCBD.
Table 1
SPECTRUM OF ACUTE AND PROPHYLACTIC EFFICACY OF DIVALPROEX IN RAPID-CYCLING BIPOLAR DISORDER
| Spectrum of marked responses to divalproex in bipolar rapid cycling | ||
|---|---|---|
| Acute | Prophylactic | |
| Dysphoric hypomania/mania | 87% | 89% |
| Elated hypomania/mania | 64% | 77% |
| Depression (n = 101, mean follow-up 15 months) | 21% | 38% |
| Adapted from Calabrese and Delucchi. Am J Psychiatry. 1990;147:431-434 and Calabrese et al. J Clin Psychopharmacol. 1993;13:280-283. | ||
Lamotrigine: best hope for monotherapy
Lamotrigine monotherapy has been reported to be effective in some RCBD cases. The data suggest that it possesses both antidepressant and mood-stabilizing properties.10
An open, naturalistic study of 5 women with treatment-refractory rapid cycling by Fatemi et al11 demonstrated both mood-stabilizing and antidepressant effects from lamotrigine monotherapy or augmentation at a mean dose of 185 ±33.5 mg/d. In 14 clinical reports involving 207 patients with bipolar disorder, 66 of whom had rapid cycling, lamotrigine was observed to possess moderate to marked efficacy in depression and hypomania, but only moderate efficacy in mania.
An open, prospective study compared the efficacy of lamotrigine add-on or monotherapy in 41 rapid cyclers to 34 nonrapid cyclers across 48 weeks. Improvement from baseline to last visit was significant between both subgroups for depressive and hypomanic symptoms. Patients presenting with more severe manic symptoms did less well.13
In the first double-blind, placebo-controlled study of lamotrigine in RCBD,12 182 of 324 patients with rapid cycling responded to treatment with open-label lamotrigine and were then randomized to the study’s double-blind phase. Forty-one percent of lamotrigine-treated vs. 26% of placebo-treated patients were stable without relapse during 6 months of monotherapy. Patients with rapid-cycling bipolar II disorder consistently experienced more improvement than their bipolar I counterparts (Figure 1). The results of this only prospective, placebo-controlled, acute-treatment study of rapid-cycling bipolar patients to date indicate that lamotrigine monotherapy is useful for some patients with RCBD, particularly those with bipolar II.
Frye et al14 conducted a double-blind, placebo-controlled study of 23 patients with rapid cycling utilizing a crossover series of three 6-week monotherapy evaluations of lamotrigine, gabapentin, and placebo. Marked antidepressant response on lamotrigine was seen in 45% of the participants compared with 19% of patients on placebo and a similar response rate among those on gabapentin.
A study evaluating the safety and efficacy of 2 dosages of lamotrigine (50 mg/d or 200 mg/d) compared with placebo in the treatment of a major depressive episode in patients with bipolar I disorder over 7 weeks demonstrated significant antidepressant efficacy.15 These bipolar outpatients displayed clinical improvement as early as the third week of treatment, and switch rates for both dosages did not exceed that of placebo. Patients with RCBD, however, were excluded from this initial trial. Subsequent studies have demonstrated similar magnitudes of efficacy in patients with RCBD, primarily in the prevention of depressive episodes, including the 6-month RCBD maintenance study10 and 2 recently completed 18-month maintenance studies of patients with bipolar I disorder, either recently manic or recently depressed.
Lamotrigine thus may have a special role in RCBD treatment. Its most significant side effect in bipolar disorder is benign rash, which has occurred in 9.0% (108 of 1,198) of patients randomized to lamotrigine vs. 7.6% (80 of 1,056) of those randomized to placebo in pivotal multicenter, double-blind, placebo-controlled bipolar trials.
In mood disorder trials conducted to date, the rate of serious rash, defined as requiring both drug discontinuation and hospitalization, has been 0.06% (2 of 3,153) on lamotrigine and 0.09% (1 out of 1,053) on placebo. No cases of Steven’s Johnson syndrome or toxic epidermal necrolysis were observed.16 A low starting dose and gradual titration of lamotrigine (Table 2) appear to minimize the risk of serious rash.
Levothyroxine: possible add-on therapy
Levothyroxine should be considered as add-on therapy in patients with known hypothyroidism, borderline hypothyroidism, or otherwise treatment-refractory cases.
The strategy of thyroid supplementation is derived from Gjessing’s 1936 report of success in administering hypermetabolic doses of thyroid hormone to patients with periodic catatonia. Stancer and Persad17 initially reported the potential efficacy of this therapeutic maneuver in rapid cyclers; remissions were induced by hypermetabolic thyroid doses in 5 of 7 patients with treatment-refractory bipolar disorder. No data currently support any prophylactic efficacy of thyroid supplementation monotherapy for RCBD treatment.
Bauer and Whybrow18 suggest that thyroid supplementation with T4 added to mood stabilizers augments efficacy independent of pre-existing thyroid function. The potential side effects of long-term levothryoxine administration, namely osteoporosis and cardiac arrhythmias, limit the usefulness of thyroid augmentation in RCBD.
Atypical antipsychotics: perhaps in combination
Coadministering atypical antipsychotics in other mood stabilizers may help rapid cyclers with current or past psychotic symptoms during their mood episodes, but further study is clearly needed.
Atypical antipsychotic medications may have specific mood-stabilizing properties, particularly in the management of mixed and manic states. In the first prospective trial of clozapine monotherapy in bipolar disorder, Calabrese and colleagues in 1994 reported that rapid cycling did not appear to predict nonresponse to treatment.
In the first randomized, controlled trial involving clozapine in bipolar disorder, Suppes et al19 noted significant improvement for symptoms of mania, psychosis, and global improvement. Subjects with nonpsychotic bipolar disorder showed a degree of improvement similar to that seen in the entire clozapine-treated group. These results support a mood-stabilizing role for clozapine.
Preliminary studies of risperidone and olanzapine further suggest therapeutic utility for atypical antipsychotics.
Figure 1 LAMOTRIGINE VS. PLACEBO IN RAPID-CYCLING BIPOLAR DISORDER
Table 2
DOSAGE PLAN FOR LAMOTRIGINE IN MONOTHERAPY AND COMBINATION
| Treatment period | Type of therapy | Daily dosage |
|---|---|---|
| Weeks 1-2 | Monotherapy With divalproex With carbamazepine | 25 mg 12.5 mg 50 mg |
| Weeks 3-4 | Monotherapy With divalproex With carbamazepine | 50 mg 25 mg 100 mg |
| Week 5 | Monotherapy With divalproex With carbamazepine | 100 mg 50 mg 200 mg |
| Thereafter | Monotherapy With divalproex With carbamazepine | 200 mg 100 mg 400 mg |
Topiramate: in patients with weight problems
Evidence from 12 open studies of 223 patients with bipolar disorder suggest that this anticonvulsant may possess mood-stabilizing properties while sparing patients the weight gain commonly seen with other pharmacotherapies.
Two studies of topiramate as an add-on therapy come to mind. Marcotte in 1998 retrospectively studied 44 patients with RCBD, 23 (52%) of whom demonstrated moderate or marked improvement on a mean topiramate dosage of 200 mg/d.
Sachs also reported that symptoms for some patients with a treatment-refractory bipolar disorder could be improved when topiramate was added to their medication regimen. Available data encourage further controlled studies of topiramate add-on therapy for RCBD, especially in obese patients.
Gabapentin: contradictory reports
Preliminary data from 14 open-label studies of gabapentin in 302 bipolar patients reported a response rate of around 67% when used as an add-on therapy, usually in mania or mixed states. A moderate antimanic effect was observed among a total of 23 rapid cyclers in 9 of the studies.
The reports are contradictory, however, and available data do not firmly support the agent’s efficacy in RCBD treatment.
Other potential uses for gabapentin are being investigated, such as antidepressant augmentation, anxiety, and chronic pain. Thus, patients with RCBD and a comorbid illness may benefit from add-on gabapentin.
ECT: Some limited success
Electroconvulsive therapy (ECT) has been implicated less frequently than antidepressants in the induction of rapid cycling, and when this does occur it is usually in the context of combined ECT/antidepressant therapies.
Berma and Wolpert in 1987 reported a case of successful ECT treatment of rapid cycling in an adolescent who had been treated with trimipramine for depression. Vanelle et al in 1994 suggested that maintenance ECT works well in 22 treatment-resistant bipolar patients, including 4 with rapid cycling, over an 18-month treatment period.
Behavioral intervention: changing sleep routines
NIMH research of 15 rapid cyclers who were studied for 3 months looked at behavioral interventions and their effect on switching (Feldman-Naim 1997). This study suggested that patients were more likely to switch from depression into hypomania/mania during daytime hours and from mania/hypomania into depression during nighttime.
The use of light therapy or activity and exercise during depression and the use of induced sleep or exposure to darkness during mania/hypomania may be therapeutic. Wehr and colleagues supported this in a 1998 report of one patient studied over several years, comparing this rapid-cycling patient’s regular sleep routine with prolonged (10 to 14 hours per night) and enforced bed rest in the dark.7 The promotion of sleep by scheduling regular nighttime periods of enforced bed rest in the dark may help prevent mania and stabilize mood in rapid cyclers.
Other add-on possibilities
Haykal in 1990 reported bupropion to be an effective add-on treatment in 5 of 6 patients with refractory, rapid-cycling bipolar II disorder. Benefit has also been reported from clorgyline, clonidine, magnesium, primidone, and acetazolamide.
Calcium-channel blockers may also offer clinical utility, although supportive evidence is limited. Nimodipine was evaluated for efficacy in 30 patients with treatment-refractory affective illness by the NIMH and Passaglia et al in 1998. Patients who improved on this agent had ultradian rapid cycling, defined in the study as those with affective episodes lasting as short as a week.
A recommended treatment strategy
Based on the available data in bipolar I rapid cycling, we recommend initial treatment with divalproex followed by augmentation with lithium if hypomanic or manic episodes persist, lamotrigine if breakthrough episodes are predominantly depressive, and atypical antipsychotics if psychotic symptoms or true mixed states remain.
For patients presenting with bipolar II rapid cycling, we recommend starting with lamotrigine, then augmenting with divalproex or lithium for breakthrough episodes. Lamotrigine shows more promise because of its reportedly greater antidepressant properties and lack of cycle induction or switching, but offers only modest antimanic therapy.
Pending further investigations, current application of the data suggests that when treating patients with RCBD, conventional antidepressants should be avoided and, if first-line therapies are not effective, the clinician should consider moving to combination drug therapy with 2 or more agents.
Related resources
- Bauer MS, Calabrese JR, Dunner DL, et al. Multi-site data reanalysis: validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry. 1994;151:506-515.
- Calabrese JR, Kimmel SE, Woyshville MJ, et al. Clozapine in treatment refractory mania. Am J Psychiatry. 1996;153(6):759-764.
- Calabrese JR, Bowden CL, McElroy SL, et al. Spectrum of activity of lamotrigine in treatment refractory bipolar disorder. Am J Psychiatry. 1999;156(7):1019-1023.
- Sachs GS. Printz DJ. Kahn DA. Carpenter D. Docherty JP. The expert consensus guideline series: medication treatment of bipolar disorder 2000. Postgraduate Medicine. April 2000; Spec No:1-104.
Drug brand names
- Buproprion • Wellbutrin
- Carbamazepine • Tegretol
- Clonidine • Catapres
- Clozapine • Clozaril
- Divalproex sodium • Depakote
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levothyroxine • Synthroid, Levothroid, Levoxyl
- Olanzapine • Zyprexa
- Nimodipine • Nimotop
- Primidonem • Mysoline
- Topiramate • Topamax
Disclosure
Dr. Muzina reports that he is a member of the Eli Lilly and Co. speaker’s bureau.
Dr. Calabrese reports that he receives research/grant support from Abbott Laboratories, GlaxoSmithKline, and Wyeth-Ayerst Pharmaceuticals, and serves as a consultant to Abbott Pharmaceuticals, Eli Lilly and Co., GlaxoSmithKline, Novartis Pharmaceuticals Corp., and Parke Davis/Warner Lambert.
1. Dunner DL, Fieve RR. Clinical factors in lithium carbonate prophylaxis failure. Arch Gen Psychiatry. 1974;20:229-233.
2. Calabrese JR, Delucchi GA. Spectrum of efficacy of valproate in 55 rapid-cycling manic depressives. Am J Psychiatry. 1990;147(4):431-434.
3. Calabrese JR, Shelton MD, Bowden CL, et al. Bipolar rapid cycling: Focus on depression as its hallmark. J Clin Psychiatry. 2001;62(Suppl 14):34-41.
4. Kukopulos A, Reginaldi D, Laddomada P, et al. Course of the manic depressive cycle and changes caused by treatment. Pharmacopsychiatry. 1980;13:156-167.
5. Maj M, et al. Long-term outcome of lithium prophylaxis in bipolar disorder: a 5-year prospective study of 402 patients at a lithium clinic. Am J Psychiatry. 1998;155:30-35.
6. Tondo L, Baldessarini RJ, et al. Lithium maintenance treatment of depression and mania in bipolar I and bipolar II disorders. Am J Psychiatry. 1998;155:638-645.
7. Wehr TA, Sack DA, Rosenthal NE, et al. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry. 1988;145:179-84.
8. Calabrese JR, Woyshville MJ, Kimmel SE. Rapport DJ: Predictors of valproate response in bipolar rapid cycling. J Clin Psychopharmacology. 1993;13(4):280-283.
9. Sharma V, Persad E, et al. Treatment of rapid cycling bipolar disorder with combination therapy of valproate and lithium. Can J Psychiatry. 1993;38:137-139.
10. Calabrese JR, Suppes T, Bowden CL, et al. for the Lamictal 614 Study Group. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry. 2000;61(11):841-850.
11. Fatemi SH, Rapport DJ, Calabrese JR, et al. Lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry 1997;58:522-527.
12. Calabrese JR, Bowden CL, et al. for the Lamictal 602 Study Group. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60(2):79-88.
13. Bowden CL, Calabrese JR, McElroy SL, et al. Comparison of the efficacy of lamotrigine in rapid cycling and non-rapid cycling bipolar disorder. Biol Psychiatry. 1999;45(8):953-958.
14. Frye MA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol. 2000;20:607-614.
15. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry. 1999;60(2):79-88.
16. Calabrese JR, Bowden CL, DeVeaugh-Geiss J, et al. Lamotrigine demonstrates long term mood stabilization in recently manic patients. Annual meeting of the American Psychiatric Association, New Orleans, 2001.
17. Stancer HC, Persad E. Treatment of intractable rapid-cycling manic-depressive disorder with levothyroxine. Clinical observations. Arch Gen Psychiatry. 1982;39(3):311-312.
18. Bauer MS, Whybrow PC. Rapid cycling bipolar affective disorder. II. Treatment of refractory rapid cycling with high-dose levothyroxine: a preliminary study.. Arch Gen Psychiatry. 1990;47(5):435-40.
19. Suppes T, Webb A, Paul B, Carmody T, et al:. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156:1164-1169.
Patients with rapid-cycling bipolar disorder (RCBD) can be frustrating to treat. Despite growing research and data, knowledge and effective therapies remain limited. How do you manage patients with rapid cycling who do not respond robustly to lithium, divalproex, or carbamazepine monotherapy? Are combination therapies likely to be more effective? Where does lamotrigine fit in? Is there a role for conventional antidepressants?
We’ll explore these and related questions—but the final answers are not yet in. Recognition of RCBD is important because it presents such difficult treatment challenges. Available evidence does suggest that rapid cycling as defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (Box 1), describes a clinically specific course of illness that may require treatments different from currently used traditional drug therapies for nonrapid cycling bipolar disorder, particularly as no one agent appears to provide ideal bimodal treatment and prophylaxis of this bipolar disorder variant.
Rapid cycling is a specifier of the longitudinal course of illness presentation that is seen almost exclusively in bipolar disorder and is associated with a greater morbidity. Dunner and Fieve1 originally coined the term when evaluating clinical factors associated with lithium prophylaxis failure. Since that time the validity of rapid cycling as a distinct course modifier for bipolar disorder has been supported by multiple studies, leading to its inclusion in the fourth edition of the Diagnostic and Statistical Manual of the APA (1994).
According to DSM-IV, the course specifier of rapid cycling applies to “at least 4 episodes of a mood disturbance in the previous 12 months that meet criteria for a manic episode, a hypomanic episode, or a major depressive episode.” The episodes must be demarcated by a full or partial remission lasting at least 2 months or by a switch to a mood state of opposite polarity.
Early reports noted that patients suffering from RCBD did not respond adequately when treated with lithium.1 Other observations indicated that divalproex was more effective in this patient population, particularly for the illness’ hypomanic or manic phases.2 We hope that the following evaluation of these and other drug therapies will prove helpful.
Watch out for antidepressants
Most concerning has been the frequency and severity of treatment-refractory depressive phases of RCBD that may be exacerbated by antidepressant use (cycle induction or acceleration). Indeed, the frequent recurrence of refractory depression has been described as the hallmark of this bipolar disorder variant.3
Lithium: the scale weighs against it
Although an excellent mood stabilizer for most patients with bipolar disorder, lithium monotherapy is less than ideal for patients with the rapid-cycling variant, particularly in treatment or prevention of depressive or mixed episodes. The efficacy of lithium is likely decreased by the concurrent administration of antidepressant medication and increased when administered with other mood stabilizers.
The landmark article by Dunner and Fieve,1 which described a placebo-controlled, double-blind maintenance study in a general cohort of 55 patients, tried to clarify factors associated with the failure of lithium prophylaxis in bipolar disorder. Rapid cyclers comprised 20% of the subjects and 80% were nonrapid cyclers. Rapid cyclers were disproportionately represented in the lithium failure group. Lithium failures included 82% (9 of 11) of rapid cyclers compared to 41% (18 of 44) of nonrapid cyclers. Lithium failure was defined as (1) hospitalization for, or (2) treatment of, mania or (3) depression during lithium therapy, or as mood symptoms that, as documented by rating scales, were sufficient to warrant a diagnosis of mild depression, hypomania, or mania persisting for at least 2 weeks.
Kukopulos et al4 replicated the findings of Dunner and Fieve in a study of the longitudinal clinical course of 434 bipolar patients. Of these patients, 50 were rapid cyclers and had received continuous lithium therapy for more than a year, with good to partial prophylaxis in only 28%. Maj and colleagues5 published a 5-year prospective study of lithium therapy in 402 patients with bipolar disorder and noted the absence of rapid cycling in good responders to lithium but an incidence rate of 26% in nonresponders to lithium.
Other investigators have reported better response in RCBD. In a select cohort of lithium-responsive bipolar I and II patients, Tondo et al6 concluded that lithium maintenance yields striking long-term reductions in depressive and manic morbidity, more so in rapid cycling type II patients. This study, however, was in a cohort of lithium responders and excluded patients who had been exposed to antipsychotic or antidepressant medications for more than 3 months, those on chronic anticonvulsant therapy, and those with substance abuse disorders.
Although most studies do report poor response to lithium therapy in RCBD, Wehr and colleagues7 suggest that in some patients with rapid cycling, the discontinuation of antidepressant drugs may allow lithium to act as a more effective anticycling mood-stabilizing agent.
Divalproex: effective in manic phase
In contrast to lithium, an open trial of a homogenous cohort of patients with RCBD by Calabrese and colleagues3,8 found divalproex to possess moderate to marked acute and prophylactic antimanic properties with only modest antidepressant effects (Table 1). Data from 6 open studies involving 147 patients with rapid cycling suggest that divalproex possesses moderate to marked efficacy in the manic phase, but poor to moderate efficacy in the depressed phase. Positive outcome predictors were bipolar II and mixed states, no prior lithium therapy, and a positive family history of affective disorder. Predictors of negative response included increase in frequency and severity of mania, and borderline personality disorder.
Divalproex therapy in combination with lithium may improve response rates.9 Calabrese and colleagues, however, have examined large cohorts of patients, including those comorbid with alcohol, cocaine, and/or cannabis abuse, treated with a lithium-divalproex combination over 6-month study periods. The researchers found that only 25% to 50% of patients stabilized, and that of those not exhibiting a response, the majority (75%) did not respond because of treatment-refractory depression in the context of RCBD.3
Although experts believe divalproex to be more effective than lithium in preventing episodes associated with RCBD, such a conclusion awaits confirmation with the near completion of a double-blind, 20-month maintenance trial sponsored by the National Institute of Mental Health (NIMH).
Carbamazepine’s role in combination therapy
Early reports by Post and colleagues in 1987 suggested that rapid cycling predicted positive response to carbamazepine, but later findings by Okuma in 1993 refuted this. Other collective open and controlled studies suggest that this anticonvulsant possesses moderate to marked efficacy in the manic phase, and poor to moderate efficacy in the depressed phase of RCBD. Again, combination therapy with lithium may offer greater efficacy. Of significance, carbamazepine treatment outcomes have not been prospectively evaluated in a homogeneous cohort of rapid cyclers.
The limitations of carbamazepine therapy are well known and available evidence also does not seem to support monotherapy with this agent as being useful in RCBD, especially in the treatment and prophylaxis of depressive or mixed phases of the disorder. Thus, further controlled studies are needed to examine the agent’s potential role and safety in combination therapies for RCBD.
Table 1
SPECTRUM OF ACUTE AND PROPHYLACTIC EFFICACY OF DIVALPROEX IN RAPID-CYCLING BIPOLAR DISORDER
| Spectrum of marked responses to divalproex in bipolar rapid cycling | ||
|---|---|---|
| Acute | Prophylactic | |
| Dysphoric hypomania/mania | 87% | 89% |
| Elated hypomania/mania | 64% | 77% |
| Depression (n = 101, mean follow-up 15 months) | 21% | 38% |
| Adapted from Calabrese and Delucchi. Am J Psychiatry. 1990;147:431-434 and Calabrese et al. J Clin Psychopharmacol. 1993;13:280-283. | ||
Lamotrigine: best hope for monotherapy
Lamotrigine monotherapy has been reported to be effective in some RCBD cases. The data suggest that it possesses both antidepressant and mood-stabilizing properties.10
An open, naturalistic study of 5 women with treatment-refractory rapid cycling by Fatemi et al11 demonstrated both mood-stabilizing and antidepressant effects from lamotrigine monotherapy or augmentation at a mean dose of 185 ±33.5 mg/d. In 14 clinical reports involving 207 patients with bipolar disorder, 66 of whom had rapid cycling, lamotrigine was observed to possess moderate to marked efficacy in depression and hypomania, but only moderate efficacy in mania.
An open, prospective study compared the efficacy of lamotrigine add-on or monotherapy in 41 rapid cyclers to 34 nonrapid cyclers across 48 weeks. Improvement from baseline to last visit was significant between both subgroups for depressive and hypomanic symptoms. Patients presenting with more severe manic symptoms did less well.13
In the first double-blind, placebo-controlled study of lamotrigine in RCBD,12 182 of 324 patients with rapid cycling responded to treatment with open-label lamotrigine and were then randomized to the study’s double-blind phase. Forty-one percent of lamotrigine-treated vs. 26% of placebo-treated patients were stable without relapse during 6 months of monotherapy. Patients with rapid-cycling bipolar II disorder consistently experienced more improvement than their bipolar I counterparts (Figure 1). The results of this only prospective, placebo-controlled, acute-treatment study of rapid-cycling bipolar patients to date indicate that lamotrigine monotherapy is useful for some patients with RCBD, particularly those with bipolar II.
Frye et al14 conducted a double-blind, placebo-controlled study of 23 patients with rapid cycling utilizing a crossover series of three 6-week monotherapy evaluations of lamotrigine, gabapentin, and placebo. Marked antidepressant response on lamotrigine was seen in 45% of the participants compared with 19% of patients on placebo and a similar response rate among those on gabapentin.
A study evaluating the safety and efficacy of 2 dosages of lamotrigine (50 mg/d or 200 mg/d) compared with placebo in the treatment of a major depressive episode in patients with bipolar I disorder over 7 weeks demonstrated significant antidepressant efficacy.15 These bipolar outpatients displayed clinical improvement as early as the third week of treatment, and switch rates for both dosages did not exceed that of placebo. Patients with RCBD, however, were excluded from this initial trial. Subsequent studies have demonstrated similar magnitudes of efficacy in patients with RCBD, primarily in the prevention of depressive episodes, including the 6-month RCBD maintenance study10 and 2 recently completed 18-month maintenance studies of patients with bipolar I disorder, either recently manic or recently depressed.
Lamotrigine thus may have a special role in RCBD treatment. Its most significant side effect in bipolar disorder is benign rash, which has occurred in 9.0% (108 of 1,198) of patients randomized to lamotrigine vs. 7.6% (80 of 1,056) of those randomized to placebo in pivotal multicenter, double-blind, placebo-controlled bipolar trials.
In mood disorder trials conducted to date, the rate of serious rash, defined as requiring both drug discontinuation and hospitalization, has been 0.06% (2 of 3,153) on lamotrigine and 0.09% (1 out of 1,053) on placebo. No cases of Steven’s Johnson syndrome or toxic epidermal necrolysis were observed.16 A low starting dose and gradual titration of lamotrigine (Table 2) appear to minimize the risk of serious rash.
Levothyroxine: possible add-on therapy
Levothyroxine should be considered as add-on therapy in patients with known hypothyroidism, borderline hypothyroidism, or otherwise treatment-refractory cases.
The strategy of thyroid supplementation is derived from Gjessing’s 1936 report of success in administering hypermetabolic doses of thyroid hormone to patients with periodic catatonia. Stancer and Persad17 initially reported the potential efficacy of this therapeutic maneuver in rapid cyclers; remissions were induced by hypermetabolic thyroid doses in 5 of 7 patients with treatment-refractory bipolar disorder. No data currently support any prophylactic efficacy of thyroid supplementation monotherapy for RCBD treatment.
Bauer and Whybrow18 suggest that thyroid supplementation with T4 added to mood stabilizers augments efficacy independent of pre-existing thyroid function. The potential side effects of long-term levothryoxine administration, namely osteoporosis and cardiac arrhythmias, limit the usefulness of thyroid augmentation in RCBD.
Atypical antipsychotics: perhaps in combination
Coadministering atypical antipsychotics in other mood stabilizers may help rapid cyclers with current or past psychotic symptoms during their mood episodes, but further study is clearly needed.
Atypical antipsychotic medications may have specific mood-stabilizing properties, particularly in the management of mixed and manic states. In the first prospective trial of clozapine monotherapy in bipolar disorder, Calabrese and colleagues in 1994 reported that rapid cycling did not appear to predict nonresponse to treatment.
In the first randomized, controlled trial involving clozapine in bipolar disorder, Suppes et al19 noted significant improvement for symptoms of mania, psychosis, and global improvement. Subjects with nonpsychotic bipolar disorder showed a degree of improvement similar to that seen in the entire clozapine-treated group. These results support a mood-stabilizing role for clozapine.
Preliminary studies of risperidone and olanzapine further suggest therapeutic utility for atypical antipsychotics.
Figure 1 LAMOTRIGINE VS. PLACEBO IN RAPID-CYCLING BIPOLAR DISORDER
Table 2
DOSAGE PLAN FOR LAMOTRIGINE IN MONOTHERAPY AND COMBINATION
| Treatment period | Type of therapy | Daily dosage |
|---|---|---|
| Weeks 1-2 | Monotherapy With divalproex With carbamazepine | 25 mg 12.5 mg 50 mg |
| Weeks 3-4 | Monotherapy With divalproex With carbamazepine | 50 mg 25 mg 100 mg |
| Week 5 | Monotherapy With divalproex With carbamazepine | 100 mg 50 mg 200 mg |
| Thereafter | Monotherapy With divalproex With carbamazepine | 200 mg 100 mg 400 mg |
Topiramate: in patients with weight problems
Evidence from 12 open studies of 223 patients with bipolar disorder suggest that this anticonvulsant may possess mood-stabilizing properties while sparing patients the weight gain commonly seen with other pharmacotherapies.
Two studies of topiramate as an add-on therapy come to mind. Marcotte in 1998 retrospectively studied 44 patients with RCBD, 23 (52%) of whom demonstrated moderate or marked improvement on a mean topiramate dosage of 200 mg/d.
Sachs also reported that symptoms for some patients with a treatment-refractory bipolar disorder could be improved when topiramate was added to their medication regimen. Available data encourage further controlled studies of topiramate add-on therapy for RCBD, especially in obese patients.
Gabapentin: contradictory reports
Preliminary data from 14 open-label studies of gabapentin in 302 bipolar patients reported a response rate of around 67% when used as an add-on therapy, usually in mania or mixed states. A moderate antimanic effect was observed among a total of 23 rapid cyclers in 9 of the studies.
The reports are contradictory, however, and available data do not firmly support the agent’s efficacy in RCBD treatment.
Other potential uses for gabapentin are being investigated, such as antidepressant augmentation, anxiety, and chronic pain. Thus, patients with RCBD and a comorbid illness may benefit from add-on gabapentin.
ECT: Some limited success
Electroconvulsive therapy (ECT) has been implicated less frequently than antidepressants in the induction of rapid cycling, and when this does occur it is usually in the context of combined ECT/antidepressant therapies.
Berma and Wolpert in 1987 reported a case of successful ECT treatment of rapid cycling in an adolescent who had been treated with trimipramine for depression. Vanelle et al in 1994 suggested that maintenance ECT works well in 22 treatment-resistant bipolar patients, including 4 with rapid cycling, over an 18-month treatment period.
Behavioral intervention: changing sleep routines
NIMH research of 15 rapid cyclers who were studied for 3 months looked at behavioral interventions and their effect on switching (Feldman-Naim 1997). This study suggested that patients were more likely to switch from depression into hypomania/mania during daytime hours and from mania/hypomania into depression during nighttime.
The use of light therapy or activity and exercise during depression and the use of induced sleep or exposure to darkness during mania/hypomania may be therapeutic. Wehr and colleagues supported this in a 1998 report of one patient studied over several years, comparing this rapid-cycling patient’s regular sleep routine with prolonged (10 to 14 hours per night) and enforced bed rest in the dark.7 The promotion of sleep by scheduling regular nighttime periods of enforced bed rest in the dark may help prevent mania and stabilize mood in rapid cyclers.
Other add-on possibilities
Haykal in 1990 reported bupropion to be an effective add-on treatment in 5 of 6 patients with refractory, rapid-cycling bipolar II disorder. Benefit has also been reported from clorgyline, clonidine, magnesium, primidone, and acetazolamide.
Calcium-channel blockers may also offer clinical utility, although supportive evidence is limited. Nimodipine was evaluated for efficacy in 30 patients with treatment-refractory affective illness by the NIMH and Passaglia et al in 1998. Patients who improved on this agent had ultradian rapid cycling, defined in the study as those with affective episodes lasting as short as a week.
A recommended treatment strategy
Based on the available data in bipolar I rapid cycling, we recommend initial treatment with divalproex followed by augmentation with lithium if hypomanic or manic episodes persist, lamotrigine if breakthrough episodes are predominantly depressive, and atypical antipsychotics if psychotic symptoms or true mixed states remain.
For patients presenting with bipolar II rapid cycling, we recommend starting with lamotrigine, then augmenting with divalproex or lithium for breakthrough episodes. Lamotrigine shows more promise because of its reportedly greater antidepressant properties and lack of cycle induction or switching, but offers only modest antimanic therapy.
Pending further investigations, current application of the data suggests that when treating patients with RCBD, conventional antidepressants should be avoided and, if first-line therapies are not effective, the clinician should consider moving to combination drug therapy with 2 or more agents.
Related resources
- Bauer MS, Calabrese JR, Dunner DL, et al. Multi-site data reanalysis: validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry. 1994;151:506-515.
- Calabrese JR, Kimmel SE, Woyshville MJ, et al. Clozapine in treatment refractory mania. Am J Psychiatry. 1996;153(6):759-764.
- Calabrese JR, Bowden CL, McElroy SL, et al. Spectrum of activity of lamotrigine in treatment refractory bipolar disorder. Am J Psychiatry. 1999;156(7):1019-1023.
- Sachs GS. Printz DJ. Kahn DA. Carpenter D. Docherty JP. The expert consensus guideline series: medication treatment of bipolar disorder 2000. Postgraduate Medicine. April 2000; Spec No:1-104.
Drug brand names
- Buproprion • Wellbutrin
- Carbamazepine • Tegretol
- Clonidine • Catapres
- Clozapine • Clozaril
- Divalproex sodium • Depakote
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levothyroxine • Synthroid, Levothroid, Levoxyl
- Olanzapine • Zyprexa
- Nimodipine • Nimotop
- Primidonem • Mysoline
- Topiramate • Topamax
Disclosure
Dr. Muzina reports that he is a member of the Eli Lilly and Co. speaker’s bureau.
Dr. Calabrese reports that he receives research/grant support from Abbott Laboratories, GlaxoSmithKline, and Wyeth-Ayerst Pharmaceuticals, and serves as a consultant to Abbott Pharmaceuticals, Eli Lilly and Co., GlaxoSmithKline, Novartis Pharmaceuticals Corp., and Parke Davis/Warner Lambert.
Patients with rapid-cycling bipolar disorder (RCBD) can be frustrating to treat. Despite growing research and data, knowledge and effective therapies remain limited. How do you manage patients with rapid cycling who do not respond robustly to lithium, divalproex, or carbamazepine monotherapy? Are combination therapies likely to be more effective? Where does lamotrigine fit in? Is there a role for conventional antidepressants?
We’ll explore these and related questions—but the final answers are not yet in. Recognition of RCBD is important because it presents such difficult treatment challenges. Available evidence does suggest that rapid cycling as defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (Box 1), describes a clinically specific course of illness that may require treatments different from currently used traditional drug therapies for nonrapid cycling bipolar disorder, particularly as no one agent appears to provide ideal bimodal treatment and prophylaxis of this bipolar disorder variant.
Rapid cycling is a specifier of the longitudinal course of illness presentation that is seen almost exclusively in bipolar disorder and is associated with a greater morbidity. Dunner and Fieve1 originally coined the term when evaluating clinical factors associated with lithium prophylaxis failure. Since that time the validity of rapid cycling as a distinct course modifier for bipolar disorder has been supported by multiple studies, leading to its inclusion in the fourth edition of the Diagnostic and Statistical Manual of the APA (1994).
According to DSM-IV, the course specifier of rapid cycling applies to “at least 4 episodes of a mood disturbance in the previous 12 months that meet criteria for a manic episode, a hypomanic episode, or a major depressive episode.” The episodes must be demarcated by a full or partial remission lasting at least 2 months or by a switch to a mood state of opposite polarity.
Early reports noted that patients suffering from RCBD did not respond adequately when treated with lithium.1 Other observations indicated that divalproex was more effective in this patient population, particularly for the illness’ hypomanic or manic phases.2 We hope that the following evaluation of these and other drug therapies will prove helpful.
Watch out for antidepressants
Most concerning has been the frequency and severity of treatment-refractory depressive phases of RCBD that may be exacerbated by antidepressant use (cycle induction or acceleration). Indeed, the frequent recurrence of refractory depression has been described as the hallmark of this bipolar disorder variant.3
Lithium: the scale weighs against it
Although an excellent mood stabilizer for most patients with bipolar disorder, lithium monotherapy is less than ideal for patients with the rapid-cycling variant, particularly in treatment or prevention of depressive or mixed episodes. The efficacy of lithium is likely decreased by the concurrent administration of antidepressant medication and increased when administered with other mood stabilizers.
The landmark article by Dunner and Fieve,1 which described a placebo-controlled, double-blind maintenance study in a general cohort of 55 patients, tried to clarify factors associated with the failure of lithium prophylaxis in bipolar disorder. Rapid cyclers comprised 20% of the subjects and 80% were nonrapid cyclers. Rapid cyclers were disproportionately represented in the lithium failure group. Lithium failures included 82% (9 of 11) of rapid cyclers compared to 41% (18 of 44) of nonrapid cyclers. Lithium failure was defined as (1) hospitalization for, or (2) treatment of, mania or (3) depression during lithium therapy, or as mood symptoms that, as documented by rating scales, were sufficient to warrant a diagnosis of mild depression, hypomania, or mania persisting for at least 2 weeks.
Kukopulos et al4 replicated the findings of Dunner and Fieve in a study of the longitudinal clinical course of 434 bipolar patients. Of these patients, 50 were rapid cyclers and had received continuous lithium therapy for more than a year, with good to partial prophylaxis in only 28%. Maj and colleagues5 published a 5-year prospective study of lithium therapy in 402 patients with bipolar disorder and noted the absence of rapid cycling in good responders to lithium but an incidence rate of 26% in nonresponders to lithium.
Other investigators have reported better response in RCBD. In a select cohort of lithium-responsive bipolar I and II patients, Tondo et al6 concluded that lithium maintenance yields striking long-term reductions in depressive and manic morbidity, more so in rapid cycling type II patients. This study, however, was in a cohort of lithium responders and excluded patients who had been exposed to antipsychotic or antidepressant medications for more than 3 months, those on chronic anticonvulsant therapy, and those with substance abuse disorders.
Although most studies do report poor response to lithium therapy in RCBD, Wehr and colleagues7 suggest that in some patients with rapid cycling, the discontinuation of antidepressant drugs may allow lithium to act as a more effective anticycling mood-stabilizing agent.
Divalproex: effective in manic phase
In contrast to lithium, an open trial of a homogenous cohort of patients with RCBD by Calabrese and colleagues3,8 found divalproex to possess moderate to marked acute and prophylactic antimanic properties with only modest antidepressant effects (Table 1). Data from 6 open studies involving 147 patients with rapid cycling suggest that divalproex possesses moderate to marked efficacy in the manic phase, but poor to moderate efficacy in the depressed phase. Positive outcome predictors were bipolar II and mixed states, no prior lithium therapy, and a positive family history of affective disorder. Predictors of negative response included increase in frequency and severity of mania, and borderline personality disorder.
Divalproex therapy in combination with lithium may improve response rates.9 Calabrese and colleagues, however, have examined large cohorts of patients, including those comorbid with alcohol, cocaine, and/or cannabis abuse, treated with a lithium-divalproex combination over 6-month study periods. The researchers found that only 25% to 50% of patients stabilized, and that of those not exhibiting a response, the majority (75%) did not respond because of treatment-refractory depression in the context of RCBD.3
Although experts believe divalproex to be more effective than lithium in preventing episodes associated with RCBD, such a conclusion awaits confirmation with the near completion of a double-blind, 20-month maintenance trial sponsored by the National Institute of Mental Health (NIMH).
Carbamazepine’s role in combination therapy
Early reports by Post and colleagues in 1987 suggested that rapid cycling predicted positive response to carbamazepine, but later findings by Okuma in 1993 refuted this. Other collective open and controlled studies suggest that this anticonvulsant possesses moderate to marked efficacy in the manic phase, and poor to moderate efficacy in the depressed phase of RCBD. Again, combination therapy with lithium may offer greater efficacy. Of significance, carbamazepine treatment outcomes have not been prospectively evaluated in a homogeneous cohort of rapid cyclers.
The limitations of carbamazepine therapy are well known and available evidence also does not seem to support monotherapy with this agent as being useful in RCBD, especially in the treatment and prophylaxis of depressive or mixed phases of the disorder. Thus, further controlled studies are needed to examine the agent’s potential role and safety in combination therapies for RCBD.
Table 1
SPECTRUM OF ACUTE AND PROPHYLACTIC EFFICACY OF DIVALPROEX IN RAPID-CYCLING BIPOLAR DISORDER
| Spectrum of marked responses to divalproex in bipolar rapid cycling | ||
|---|---|---|
| Acute | Prophylactic | |
| Dysphoric hypomania/mania | 87% | 89% |
| Elated hypomania/mania | 64% | 77% |
| Depression (n = 101, mean follow-up 15 months) | 21% | 38% |
| Adapted from Calabrese and Delucchi. Am J Psychiatry. 1990;147:431-434 and Calabrese et al. J Clin Psychopharmacol. 1993;13:280-283. | ||
Lamotrigine: best hope for monotherapy
Lamotrigine monotherapy has been reported to be effective in some RCBD cases. The data suggest that it possesses both antidepressant and mood-stabilizing properties.10
An open, naturalistic study of 5 women with treatment-refractory rapid cycling by Fatemi et al11 demonstrated both mood-stabilizing and antidepressant effects from lamotrigine monotherapy or augmentation at a mean dose of 185 ±33.5 mg/d. In 14 clinical reports involving 207 patients with bipolar disorder, 66 of whom had rapid cycling, lamotrigine was observed to possess moderate to marked efficacy in depression and hypomania, but only moderate efficacy in mania.
An open, prospective study compared the efficacy of lamotrigine add-on or monotherapy in 41 rapid cyclers to 34 nonrapid cyclers across 48 weeks. Improvement from baseline to last visit was significant between both subgroups for depressive and hypomanic symptoms. Patients presenting with more severe manic symptoms did less well.13
In the first double-blind, placebo-controlled study of lamotrigine in RCBD,12 182 of 324 patients with rapid cycling responded to treatment with open-label lamotrigine and were then randomized to the study’s double-blind phase. Forty-one percent of lamotrigine-treated vs. 26% of placebo-treated patients were stable without relapse during 6 months of monotherapy. Patients with rapid-cycling bipolar II disorder consistently experienced more improvement than their bipolar I counterparts (Figure 1). The results of this only prospective, placebo-controlled, acute-treatment study of rapid-cycling bipolar patients to date indicate that lamotrigine monotherapy is useful for some patients with RCBD, particularly those with bipolar II.
Frye et al14 conducted a double-blind, placebo-controlled study of 23 patients with rapid cycling utilizing a crossover series of three 6-week monotherapy evaluations of lamotrigine, gabapentin, and placebo. Marked antidepressant response on lamotrigine was seen in 45% of the participants compared with 19% of patients on placebo and a similar response rate among those on gabapentin.
A study evaluating the safety and efficacy of 2 dosages of lamotrigine (50 mg/d or 200 mg/d) compared with placebo in the treatment of a major depressive episode in patients with bipolar I disorder over 7 weeks demonstrated significant antidepressant efficacy.15 These bipolar outpatients displayed clinical improvement as early as the third week of treatment, and switch rates for both dosages did not exceed that of placebo. Patients with RCBD, however, were excluded from this initial trial. Subsequent studies have demonstrated similar magnitudes of efficacy in patients with RCBD, primarily in the prevention of depressive episodes, including the 6-month RCBD maintenance study10 and 2 recently completed 18-month maintenance studies of patients with bipolar I disorder, either recently manic or recently depressed.
Lamotrigine thus may have a special role in RCBD treatment. Its most significant side effect in bipolar disorder is benign rash, which has occurred in 9.0% (108 of 1,198) of patients randomized to lamotrigine vs. 7.6% (80 of 1,056) of those randomized to placebo in pivotal multicenter, double-blind, placebo-controlled bipolar trials.
In mood disorder trials conducted to date, the rate of serious rash, defined as requiring both drug discontinuation and hospitalization, has been 0.06% (2 of 3,153) on lamotrigine and 0.09% (1 out of 1,053) on placebo. No cases of Steven’s Johnson syndrome or toxic epidermal necrolysis were observed.16 A low starting dose and gradual titration of lamotrigine (Table 2) appear to minimize the risk of serious rash.
Levothyroxine: possible add-on therapy
Levothyroxine should be considered as add-on therapy in patients with known hypothyroidism, borderline hypothyroidism, or otherwise treatment-refractory cases.
The strategy of thyroid supplementation is derived from Gjessing’s 1936 report of success in administering hypermetabolic doses of thyroid hormone to patients with periodic catatonia. Stancer and Persad17 initially reported the potential efficacy of this therapeutic maneuver in rapid cyclers; remissions were induced by hypermetabolic thyroid doses in 5 of 7 patients with treatment-refractory bipolar disorder. No data currently support any prophylactic efficacy of thyroid supplementation monotherapy for RCBD treatment.
Bauer and Whybrow18 suggest that thyroid supplementation with T4 added to mood stabilizers augments efficacy independent of pre-existing thyroid function. The potential side effects of long-term levothryoxine administration, namely osteoporosis and cardiac arrhythmias, limit the usefulness of thyroid augmentation in RCBD.
Atypical antipsychotics: perhaps in combination
Coadministering atypical antipsychotics in other mood stabilizers may help rapid cyclers with current or past psychotic symptoms during their mood episodes, but further study is clearly needed.
Atypical antipsychotic medications may have specific mood-stabilizing properties, particularly in the management of mixed and manic states. In the first prospective trial of clozapine monotherapy in bipolar disorder, Calabrese and colleagues in 1994 reported that rapid cycling did not appear to predict nonresponse to treatment.
In the first randomized, controlled trial involving clozapine in bipolar disorder, Suppes et al19 noted significant improvement for symptoms of mania, psychosis, and global improvement. Subjects with nonpsychotic bipolar disorder showed a degree of improvement similar to that seen in the entire clozapine-treated group. These results support a mood-stabilizing role for clozapine.
Preliminary studies of risperidone and olanzapine further suggest therapeutic utility for atypical antipsychotics.
Figure 1 LAMOTRIGINE VS. PLACEBO IN RAPID-CYCLING BIPOLAR DISORDER
Table 2
DOSAGE PLAN FOR LAMOTRIGINE IN MONOTHERAPY AND COMBINATION
| Treatment period | Type of therapy | Daily dosage |
|---|---|---|
| Weeks 1-2 | Monotherapy With divalproex With carbamazepine | 25 mg 12.5 mg 50 mg |
| Weeks 3-4 | Monotherapy With divalproex With carbamazepine | 50 mg 25 mg 100 mg |
| Week 5 | Monotherapy With divalproex With carbamazepine | 100 mg 50 mg 200 mg |
| Thereafter | Monotherapy With divalproex With carbamazepine | 200 mg 100 mg 400 mg |
Topiramate: in patients with weight problems
Evidence from 12 open studies of 223 patients with bipolar disorder suggest that this anticonvulsant may possess mood-stabilizing properties while sparing patients the weight gain commonly seen with other pharmacotherapies.
Two studies of topiramate as an add-on therapy come to mind. Marcotte in 1998 retrospectively studied 44 patients with RCBD, 23 (52%) of whom demonstrated moderate or marked improvement on a mean topiramate dosage of 200 mg/d.
Sachs also reported that symptoms for some patients with a treatment-refractory bipolar disorder could be improved when topiramate was added to their medication regimen. Available data encourage further controlled studies of topiramate add-on therapy for RCBD, especially in obese patients.
Gabapentin: contradictory reports
Preliminary data from 14 open-label studies of gabapentin in 302 bipolar patients reported a response rate of around 67% when used as an add-on therapy, usually in mania or mixed states. A moderate antimanic effect was observed among a total of 23 rapid cyclers in 9 of the studies.
The reports are contradictory, however, and available data do not firmly support the agent’s efficacy in RCBD treatment.
Other potential uses for gabapentin are being investigated, such as antidepressant augmentation, anxiety, and chronic pain. Thus, patients with RCBD and a comorbid illness may benefit from add-on gabapentin.
ECT: Some limited success
Electroconvulsive therapy (ECT) has been implicated less frequently than antidepressants in the induction of rapid cycling, and when this does occur it is usually in the context of combined ECT/antidepressant therapies.
Berma and Wolpert in 1987 reported a case of successful ECT treatment of rapid cycling in an adolescent who had been treated with trimipramine for depression. Vanelle et al in 1994 suggested that maintenance ECT works well in 22 treatment-resistant bipolar patients, including 4 with rapid cycling, over an 18-month treatment period.
Behavioral intervention: changing sleep routines
NIMH research of 15 rapid cyclers who were studied for 3 months looked at behavioral interventions and their effect on switching (Feldman-Naim 1997). This study suggested that patients were more likely to switch from depression into hypomania/mania during daytime hours and from mania/hypomania into depression during nighttime.
The use of light therapy or activity and exercise during depression and the use of induced sleep or exposure to darkness during mania/hypomania may be therapeutic. Wehr and colleagues supported this in a 1998 report of one patient studied over several years, comparing this rapid-cycling patient’s regular sleep routine with prolonged (10 to 14 hours per night) and enforced bed rest in the dark.7 The promotion of sleep by scheduling regular nighttime periods of enforced bed rest in the dark may help prevent mania and stabilize mood in rapid cyclers.
Other add-on possibilities
Haykal in 1990 reported bupropion to be an effective add-on treatment in 5 of 6 patients with refractory, rapid-cycling bipolar II disorder. Benefit has also been reported from clorgyline, clonidine, magnesium, primidone, and acetazolamide.
Calcium-channel blockers may also offer clinical utility, although supportive evidence is limited. Nimodipine was evaluated for efficacy in 30 patients with treatment-refractory affective illness by the NIMH and Passaglia et al in 1998. Patients who improved on this agent had ultradian rapid cycling, defined in the study as those with affective episodes lasting as short as a week.
A recommended treatment strategy
Based on the available data in bipolar I rapid cycling, we recommend initial treatment with divalproex followed by augmentation with lithium if hypomanic or manic episodes persist, lamotrigine if breakthrough episodes are predominantly depressive, and atypical antipsychotics if psychotic symptoms or true mixed states remain.
For patients presenting with bipolar II rapid cycling, we recommend starting with lamotrigine, then augmenting with divalproex or lithium for breakthrough episodes. Lamotrigine shows more promise because of its reportedly greater antidepressant properties and lack of cycle induction or switching, but offers only modest antimanic therapy.
Pending further investigations, current application of the data suggests that when treating patients with RCBD, conventional antidepressants should be avoided and, if first-line therapies are not effective, the clinician should consider moving to combination drug therapy with 2 or more agents.
Related resources
- Bauer MS, Calabrese JR, Dunner DL, et al. Multi-site data reanalysis: validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry. 1994;151:506-515.
- Calabrese JR, Kimmel SE, Woyshville MJ, et al. Clozapine in treatment refractory mania. Am J Psychiatry. 1996;153(6):759-764.
- Calabrese JR, Bowden CL, McElroy SL, et al. Spectrum of activity of lamotrigine in treatment refractory bipolar disorder. Am J Psychiatry. 1999;156(7):1019-1023.
- Sachs GS. Printz DJ. Kahn DA. Carpenter D. Docherty JP. The expert consensus guideline series: medication treatment of bipolar disorder 2000. Postgraduate Medicine. April 2000; Spec No:1-104.
Drug brand names
- Buproprion • Wellbutrin
- Carbamazepine • Tegretol
- Clonidine • Catapres
- Clozapine • Clozaril
- Divalproex sodium • Depakote
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levothyroxine • Synthroid, Levothroid, Levoxyl
- Olanzapine • Zyprexa
- Nimodipine • Nimotop
- Primidonem • Mysoline
- Topiramate • Topamax
Disclosure
Dr. Muzina reports that he is a member of the Eli Lilly and Co. speaker’s bureau.
Dr. Calabrese reports that he receives research/grant support from Abbott Laboratories, GlaxoSmithKline, and Wyeth-Ayerst Pharmaceuticals, and serves as a consultant to Abbott Pharmaceuticals, Eli Lilly and Co., GlaxoSmithKline, Novartis Pharmaceuticals Corp., and Parke Davis/Warner Lambert.
1. Dunner DL, Fieve RR. Clinical factors in lithium carbonate prophylaxis failure. Arch Gen Psychiatry. 1974;20:229-233.
2. Calabrese JR, Delucchi GA. Spectrum of efficacy of valproate in 55 rapid-cycling manic depressives. Am J Psychiatry. 1990;147(4):431-434.
3. Calabrese JR, Shelton MD, Bowden CL, et al. Bipolar rapid cycling: Focus on depression as its hallmark. J Clin Psychiatry. 2001;62(Suppl 14):34-41.
4. Kukopulos A, Reginaldi D, Laddomada P, et al. Course of the manic depressive cycle and changes caused by treatment. Pharmacopsychiatry. 1980;13:156-167.
5. Maj M, et al. Long-term outcome of lithium prophylaxis in bipolar disorder: a 5-year prospective study of 402 patients at a lithium clinic. Am J Psychiatry. 1998;155:30-35.
6. Tondo L, Baldessarini RJ, et al. Lithium maintenance treatment of depression and mania in bipolar I and bipolar II disorders. Am J Psychiatry. 1998;155:638-645.
7. Wehr TA, Sack DA, Rosenthal NE, et al. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry. 1988;145:179-84.
8. Calabrese JR, Woyshville MJ, Kimmel SE. Rapport DJ: Predictors of valproate response in bipolar rapid cycling. J Clin Psychopharmacology. 1993;13(4):280-283.
9. Sharma V, Persad E, et al. Treatment of rapid cycling bipolar disorder with combination therapy of valproate and lithium. Can J Psychiatry. 1993;38:137-139.
10. Calabrese JR, Suppes T, Bowden CL, et al. for the Lamictal 614 Study Group. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry. 2000;61(11):841-850.
11. Fatemi SH, Rapport DJ, Calabrese JR, et al. Lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry 1997;58:522-527.
12. Calabrese JR, Bowden CL, et al. for the Lamictal 602 Study Group. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60(2):79-88.
13. Bowden CL, Calabrese JR, McElroy SL, et al. Comparison of the efficacy of lamotrigine in rapid cycling and non-rapid cycling bipolar disorder. Biol Psychiatry. 1999;45(8):953-958.
14. Frye MA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol. 2000;20:607-614.
15. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry. 1999;60(2):79-88.
16. Calabrese JR, Bowden CL, DeVeaugh-Geiss J, et al. Lamotrigine demonstrates long term mood stabilization in recently manic patients. Annual meeting of the American Psychiatric Association, New Orleans, 2001.
17. Stancer HC, Persad E. Treatment of intractable rapid-cycling manic-depressive disorder with levothyroxine. Clinical observations. Arch Gen Psychiatry. 1982;39(3):311-312.
18. Bauer MS, Whybrow PC. Rapid cycling bipolar affective disorder. II. Treatment of refractory rapid cycling with high-dose levothyroxine: a preliminary study.. Arch Gen Psychiatry. 1990;47(5):435-40.
19. Suppes T, Webb A, Paul B, Carmody T, et al:. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156:1164-1169.
1. Dunner DL, Fieve RR. Clinical factors in lithium carbonate prophylaxis failure. Arch Gen Psychiatry. 1974;20:229-233.
2. Calabrese JR, Delucchi GA. Spectrum of efficacy of valproate in 55 rapid-cycling manic depressives. Am J Psychiatry. 1990;147(4):431-434.
3. Calabrese JR, Shelton MD, Bowden CL, et al. Bipolar rapid cycling: Focus on depression as its hallmark. J Clin Psychiatry. 2001;62(Suppl 14):34-41.
4. Kukopulos A, Reginaldi D, Laddomada P, et al. Course of the manic depressive cycle and changes caused by treatment. Pharmacopsychiatry. 1980;13:156-167.
5. Maj M, et al. Long-term outcome of lithium prophylaxis in bipolar disorder: a 5-year prospective study of 402 patients at a lithium clinic. Am J Psychiatry. 1998;155:30-35.
6. Tondo L, Baldessarini RJ, et al. Lithium maintenance treatment of depression and mania in bipolar I and bipolar II disorders. Am J Psychiatry. 1998;155:638-645.
7. Wehr TA, Sack DA, Rosenthal NE, et al. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry. 1988;145:179-84.
8. Calabrese JR, Woyshville MJ, Kimmel SE. Rapport DJ: Predictors of valproate response in bipolar rapid cycling. J Clin Psychopharmacology. 1993;13(4):280-283.
9. Sharma V, Persad E, et al. Treatment of rapid cycling bipolar disorder with combination therapy of valproate and lithium. Can J Psychiatry. 1993;38:137-139.
10. Calabrese JR, Suppes T, Bowden CL, et al. for the Lamictal 614 Study Group. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry. 2000;61(11):841-850.
11. Fatemi SH, Rapport DJ, Calabrese JR, et al. Lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry 1997;58:522-527.
12. Calabrese JR, Bowden CL, et al. for the Lamictal 602 Study Group. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60(2):79-88.
13. Bowden CL, Calabrese JR, McElroy SL, et al. Comparison of the efficacy of lamotrigine in rapid cycling and non-rapid cycling bipolar disorder. Biol Psychiatry. 1999;45(8):953-958.
14. Frye MA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol. 2000;20:607-614.
15. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry. 1999;60(2):79-88.
16. Calabrese JR, Bowden CL, DeVeaugh-Geiss J, et al. Lamotrigine demonstrates long term mood stabilization in recently manic patients. Annual meeting of the American Psychiatric Association, New Orleans, 2001.
17. Stancer HC, Persad E. Treatment of intractable rapid-cycling manic-depressive disorder with levothyroxine. Clinical observations. Arch Gen Psychiatry. 1982;39(3):311-312.
18. Bauer MS, Whybrow PC. Rapid cycling bipolar affective disorder. II. Treatment of refractory rapid cycling with high-dose levothyroxine: a preliminary study.. Arch Gen Psychiatry. 1990;47(5):435-40.
19. Suppes T, Webb A, Paul B, Carmody T, et al:. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156:1164-1169.
Rapid-cycling bipolar disorder: Which therapies are most effective?
Patients with rapid-cycling bipolar disorder (RCBD) can be frustrating to treat. Despite growing research and data, knowledge and effective therapies remain limited. How do you manage patients with rapid cycling who do not respond robustly to lithium, divalproex, or carbamazepine monotherapy? Are combination therapies likely to be more effective? Where does lamotrigine fit in? Is there a role for conventional antidepressants?
We’ll explore these and related questions—but the final answers are not yet in. Recognition of RCBD is important because it presents such difficult treatment challenges. Available evidence does suggest that rapid cycling as defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (Box 1), describes a clinically specific course of illness that may require treatments different from currently used traditional drug therapies for nonrapid cycling bipolar disorder, particularly as no one agent appears to provide ideal bimodal treatment and prophylaxis of this bipolar disorder variant.
Rapid cycling is a specifier of the longitudinal course of illness presentation that is seen almost exclusively in bipolar disorder and is associated with a greater morbidity. Dunner and Fieve1 originally coined the term when evaluating clinical factors associated with lithium prophylaxis failure. Since that time the validity of rapid cycling as a distinct course modifier for bipolar disorder has been supported by multiple studies, leading to its inclusion in the fourth edition of the Diagnostic and Statistical Manual of the APA (1994).
According to DSM-IV, the course specifier of rapid cycling applies to “at least 4 episodes of a mood disturbance in the previous 12 months that meet criteria for a manic episode, a hypomanic episode, or a major depressive episode.” The episodes must be demarcated by a full or partial remission lasting at least 2 months or by a switch to a mood state of opposite polarity.
Early reports noted that patients suffering from RCBD did not respond adequately when treated with lithium.1 Other observations indicated that divalproex was more effective in this patient population, particularly for the illness’ hypomanic or manic phases.2 We hope that the following evaluation of these and other drug therapies will prove helpful.
Watch out for antidepressants
Most concerning has been the frequency and severity of treatment-refractory depressive phases of RCBD that may be exacerbated by antidepressant use (cycle induction or acceleration). Indeed, the frequent recurrence of refractory depression has been described as the hallmark of this bipolar disorder variant.3
Lithium: the scale weighs against it
Although an excellent mood stabilizer for most patients with bipolar disorder, lithium monotherapy is less than ideal for patients with the rapid-cycling variant, particularly in treatment or prevention of depressive or mixed episodes. The efficacy of lithium is likely decreased by the concurrent administration of antidepressant medication and increased when administered with other mood stabilizers.
The landmark article by Dunner and Fieve,1 which described a placebo-controlled, double-blind maintenance study in a general cohort of 55 patients, tried to clarify factors associated with the failure of lithium prophylaxis in bipolar disorder. Rapid cyclers comprised 20% of the subjects and 80% were nonrapid cyclers. Rapid cyclers were disproportionately represented in the lithium failure group. Lithium failures included 82% (9 of 11) of rapid cyclers compared to 41% (18 of 44) of nonrapid cyclers. Lithium failure was defined as (1) hospitalization for, or (2) treatment of, mania or (3) depression during lithium therapy, or as mood symptoms that, as documented by rating scales, were sufficient to warrant a diagnosis of mild depression, hypomania, or mania persisting for at least 2 weeks.
Kukopulos et al4 replicated the findings of Dunner and Fieve in a study of the longitudinal clinical course of 434 bipolar patients. Of these patients, 50 were rapid cyclers and had received continuous lithium therapy for more than a year, with good to partial prophylaxis in only 28%. Maj and colleagues5 published a 5-year prospective study of lithium therapy in 402 patients with bipolar disorder and noted the absence of rapid cycling in good responders to lithium but an incidence rate of 26% in nonresponders to lithium.
Other investigators have reported better response in RCBD. In a select cohort of lithium-responsive bipolar I and II patients, Tondo et al6 concluded that lithium maintenance yields striking long-term reductions in depressive and manic morbidity, more so in rapid cycling type II patients. This study, however, was in a cohort of lithium responders and excluded patients who had been exposed to antipsychotic or antidepressant medications for more than 3 months, those on chronic anticonvulsant therapy, and those with substance abuse disorders.
Although most studies do report poor response to lithium therapy in RCBD, Wehr and colleagues7 suggest that in some patients with rapid cycling, the discontinuation of antidepressant drugs may allow lithium to act as a more effective anticycling mood-stabilizing agent.
Divalproex: effective in manic phase
In contrast to lithium, an open trial of a homogenous cohort of patients with RCBD by Calabrese and colleagues3,8 found divalproex to possess moderate to marked acute and prophylactic antimanic properties with only modest antidepressant effects (Table 1). Data from 6 open studies involving 147 patients with rapid cycling suggest that divalproex possesses moderate to marked efficacy in the manic phase, but poor to moderate efficacy in the depressed phase. Positive outcome predictors were bipolar II and mixed states, no prior lithium therapy, and a positive family history of affective disorder. Predictors of negative response included increase in frequency and severity of mania, and borderline personality disorder.
Divalproex therapy in combination with lithium may improve response rates.9 Calabrese and colleagues, however, have examined large cohorts of patients, including those comorbid with alcohol, cocaine, and/or cannabis abuse, treated with a lithium-divalproex combination over 6-month study periods. The researchers found that only 25% to 50% of patients stabilized, and that of those not exhibiting a response, the majority (75%) did not respond because of treatment-refractory depression in the context of RCBD.3
Although experts believe divalproex to be more effective than lithium in preventing episodes associated with RCBD, such a conclusion awaits confirmation with the near completion of a double-blind, 20-month maintenance trial sponsored by the National Institute of Mental Health (NIMH).
Carbamazepine’s role in combination therapy
Early reports by Post and colleagues in 1987 suggested that rapid cycling predicted positive response to carbamazepine, but later findings by Okuma in 1993 refuted this. Other collective open and controlled studies suggest that this anticonvulsant possesses moderate to marked efficacy in the manic phase, and poor to moderate efficacy in the depressed phase of RCBD. Again, combination therapy with lithium may offer greater efficacy. Of significance, carbamazepine treatment outcomes have not been prospectively evaluated in a homogeneous cohort of rapid cyclers.
The limitations of carbamazepine therapy are well known and available evidence also does not seem to support monotherapy with this agent as being useful in RCBD, especially in the treatment and prophylaxis of depressive or mixed phases of the disorder. Thus, further controlled studies are needed to examine the agent’s potential role and safety in combination therapies for RCBD.
Table 1
SPECTRUM OF ACUTE AND PROPHYLACTIC EFFICACY OF DIVALPROEX IN RAPID-CYCLING BIPOLAR DISORDER
| Spectrum of marked responses to divalproex in bipolar rapid cycling | ||
|---|---|---|
| Acute | Prophylactic | |
| Dysphoric hypomania/mania | 87% | 89% |
| Elated hypomania/mania | 64% | 77% |
| Depression (n = 101, mean follow-up 15 months) | 21% | 38% |
| Adapted from Calabrese and Delucchi. Am J Psychiatry. 1990;147:431-434 and Calabrese et al. J Clin Psychopharmacol. 1993;13:280-283. | ||
Lamotrigine: best hope for monotherapy
Lamotrigine monotherapy has been reported to be effective in some RCBD cases. The data suggest that it possesses both antidepressant and mood-stabilizing properties.10
An open, naturalistic study of 5 women with treatment-refractory rapid cycling by Fatemi et al11 demonstrated both mood-stabilizing and antidepressant effects from lamotrigine monotherapy or augmentation at a mean dose of 185 ±33.5 mg/d. In 14 clinical reports involving 207 patients with bipolar disorder, 66 of whom had rapid cycling, lamotrigine was observed to possess moderate to marked efficacy in depression and hypomania, but only moderate efficacy in mania.
An open, prospective study compared the efficacy of lamotrigine add-on or monotherapy in 41 rapid cyclers to 34 nonrapid cyclers across 48 weeks. Improvement from baseline to last visit was significant between both subgroups for depressive and hypomanic symptoms. Patients presenting with more severe manic symptoms did less well.13
In the first double-blind, placebo-controlled study of lamotrigine in RCBD,12 182 of 324 patients with rapid cycling responded to treatment with open-label lamotrigine and were then randomized to the study’s double-blind phase. Forty-one percent of lamotrigine-treated vs. 26% of placebo-treated patients were stable without relapse during 6 months of monotherapy. Patients with rapid-cycling bipolar II disorder consistently experienced more improvement than their bipolar I counterparts (Figure 1). The results of this only prospective, placebo-controlled, acute-treatment study of rapid-cycling bipolar patients to date indicate that lamotrigine monotherapy is useful for some patients with RCBD, particularly those with bipolar II.
Frye et al14 conducted a double-blind, placebo-controlled study of 23 patients with rapid cycling utilizing a crossover series of three 6-week monotherapy evaluations of lamotrigine, gabapentin, and placebo. Marked antidepressant response on lamotrigine was seen in 45% of the participants compared with 19% of patients on placebo and a similar response rate among those on gabapentin.
A study evaluating the safety and efficacy of 2 dosages of lamotrigine (50 mg/d or 200 mg/d) compared with placebo in the treatment of a major depressive episode in patients with bipolar I disorder over 7 weeks demonstrated significant antidepressant efficacy.15 These bipolar outpatients displayed clinical improvement as early as the third week of treatment, and switch rates for both dosages did not exceed that of placebo. Patients with RCBD, however, were excluded from this initial trial. Subsequent studies have demonstrated similar magnitudes of efficacy in patients with RCBD, primarily in the prevention of depressive episodes, including the 6-month RCBD maintenance study10 and 2 recently completed 18-month maintenance studies of patients with bipolar I disorder, either recently manic or recently depressed.
Lamotrigine thus may have a special role in RCBD treatment. Its most significant side effect in bipolar disorder is benign rash, which has occurred in 9.0% (108 of 1,198) of patients randomized to lamotrigine vs. 7.6% (80 of 1,056) of those randomized to placebo in pivotal multicenter, double-blind, placebo-controlled bipolar trials.
In mood disorder trials conducted to date, the rate of serious rash, defined as requiring both drug discontinuation and hospitalization, has been 0.06% (2 of 3,153) on lamotrigine and 0.09% (1 out of 1,053) on placebo. No cases of Steven’s Johnson syndrome or toxic epidermal necrolysis were observed.16 A low starting dose and gradual titration of lamotrigine (Table 2) appear to minimize the risk of serious rash.
Levothyroxine: possible add-on therapy
Levothyroxine should be considered as add-on therapy in patients with known hypothyroidism, borderline hypothyroidism, or otherwise treatment-refractory cases.
The strategy of thyroid supplementation is derived from Gjessing’s 1936 report of success in administering hypermetabolic doses of thyroid hormone to patients with periodic catatonia. Stancer and Persad17 initially reported the potential efficacy of this therapeutic maneuver in rapid cyclers; remissions were induced by hypermetabolic thyroid doses in 5 of 7 patients with treatment-refractory bipolar disorder. No data currently support any prophylactic efficacy of thyroid supplementation monotherapy for RCBD treatment.
Bauer and Whybrow18 suggest that thyroid supplementation with T4 added to mood stabilizers augments efficacy independent of pre-existing thyroid function. The potential side effects of long-term levothryoxine administration, namely osteoporosis and cardiac arrhythmias, limit the usefulness of thyroid augmentation in RCBD.
Atypical antipsychotics: perhaps in combination
Coadministering atypical antipsychotics in other mood stabilizers may help rapid cyclers with current or past psychotic symptoms during their mood episodes, but further study is clearly needed.
Atypical antipsychotic medications may have specific mood-stabilizing properties, particularly in the management of mixed and manic states. In the first prospective trial of clozapine monotherapy in bipolar disorder, Calabrese and colleagues in 1994 reported that rapid cycling did not appear to predict nonresponse to treatment.
In the first randomized, controlled trial involving clozapine in bipolar disorder, Suppes et al19 noted significant improvement for symptoms of mania, psychosis, and global improvement. Subjects with nonpsychotic bipolar disorder showed a degree of improvement similar to that seen in the entire clozapine-treated group. These results support a mood-stabilizing role for clozapine.
Preliminary studies of risperidone and olanzapine further suggest therapeutic utility for atypical antipsychotics.
Figure 1 LAMOTRIGINE VS. PLACEBO IN RAPID-CYCLING BIPOLAR DISORDER
Table 2
DOSAGE PLAN FOR LAMOTRIGINE IN MONOTHERAPY AND COMBINATION
| Treatment period | Type of therapy | Daily dosage |
|---|---|---|
| Weeks 1-2 | Monotherapy With divalproex With carbamazepine | 25 mg 12.5 mg 50 mg |
| Weeks 3-4 | Monotherapy With divalproex With carbamazepine | 50 mg 25 mg 100 mg |
| Week 5 | Monotherapy With divalproex With carbamazepine | 100 mg 50 mg 200 mg |
| Thereafter | Monotherapy With divalproex With carbamazepine | 200 mg 100 mg 400 mg |
Topiramate: in patients with weight problems
Evidence from 12 open studies of 223 patients with bipolar disorder suggest that this anticonvulsant may possess mood-stabilizing properties while sparing patients the weight gain commonly seen with other pharmacotherapies.
Two studies of topiramate as an add-on therapy come to mind. Marcotte in 1998 retrospectively studied 44 patients with RCBD, 23 (52%) of whom demonstrated moderate or marked improvement on a mean topiramate dosage of 200 mg/d.
Sachs also reported that symptoms for some patients with a treatment-refractory bipolar disorder could be improved when topiramate was added to their medication regimen. Available data encourage further controlled studies of topiramate add-on therapy for RCBD, especially in obese patients.
Gabapentin: contradictory reports
Preliminary data from 14 open-label studies of gabapentin in 302 bipolar patients reported a response rate of around 67% when used as an add-on therapy, usually in mania or mixed states. A moderate antimanic effect was observed among a total of 23 rapid cyclers in 9 of the studies.
The reports are contradictory, however, and available data do not firmly support the agent’s efficacy in RCBD treatment.
Other potential uses for gabapentin are being investigated, such as antidepressant augmentation, anxiety, and chronic pain. Thus, patients with RCBD and a comorbid illness may benefit from add-on gabapentin.
ECT: Some limited success
Electroconvulsive therapy (ECT) has been implicated less frequently than antidepressants in the induction of rapid cycling, and when this does occur it is usually in the context of combined ECT/antidepressant therapies.
Berma and Wolpert in 1987 reported a case of successful ECT treatment of rapid cycling in an adolescent who had been treated with trimipramine for depression. Vanelle et al in 1994 suggested that maintenance ECT works well in 22 treatment-resistant bipolar patients, including 4 with rapid cycling, over an 18-month treatment period.
Behavioral intervention: changing sleep routines
NIMH research of 15 rapid cyclers who were studied for 3 months looked at behavioral interventions and their effect on switching (Feldman-Naim 1997). This study suggested that patients were more likely to switch from depression into hypomania/mania during daytime hours and from mania/hypomania into depression during nighttime.
The use of light therapy or activity and exercise during depression and the use of induced sleep or exposure to darkness during mania/hypomania may be therapeutic. Wehr and colleagues supported this in a 1998 report of one patient studied over several years, comparing this rapid-cycling patient’s regular sleep routine with prolonged (10 to 14 hours per night) and enforced bed rest in the dark.7 The promotion of sleep by scheduling regular nighttime periods of enforced bed rest in the dark may help prevent mania and stabilize mood in rapid cyclers.
Other add-on possibilities
Haykal in 1990 reported bupropion to be an effective add-on treatment in 5 of 6 patients with refractory, rapid-cycling bipolar II disorder. Benefit has also been reported from clorgyline, clonidine, magnesium, primidone, and acetazolamide.
Calcium-channel blockers may also offer clinical utility, although supportive evidence is limited. Nimodipine was evaluated for efficacy in 30 patients with treatment-refractory affective illness by the NIMH and Passaglia et al in 1998. Patients who improved on this agent had ultradian rapid cycling, defined in the study as those with affective episodes lasting as short as a week.
A recommended treatment strategy
Based on the available data in bipolar I rapid cycling, we recommend initial treatment with divalproex followed by augmentation with lithium if hypomanic or manic episodes persist, lamotrigine if breakthrough episodes are predominantly depressive, and atypical antipsychotics if psychotic symptoms or true mixed states remain.
For patients presenting with bipolar II rapid cycling, we recommend starting with lamotrigine, then augmenting with divalproex or lithium for breakthrough episodes. Lamotrigine shows more promise because of its reportedly greater antidepressant properties and lack of cycle induction or switching, but offers only modest antimanic therapy.
Pending further investigations, current application of the data suggests that when treating patients with RCBD, conventional antidepressants should be avoided and, if first-line therapies are not effective, the clinician should consider moving to combination drug therapy with 2 or more agents.
Related resources
- Bauer MS, Calabrese JR, Dunner DL, et al. Multi-site data reanalysis: validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry. 1994;151:506-515.
- Calabrese JR, Kimmel SE, Woyshville MJ, et al. Clozapine in treatment refractory mania. Am J Psychiatry. 1996;153(6):759-764.
- Calabrese JR, Bowden CL, McElroy SL, et al. Spectrum of activity of lamotrigine in treatment refractory bipolar disorder. Am J Psychiatry. 1999;156(7):1019-1023.
- Sachs GS. Printz DJ. Kahn DA. Carpenter D. Docherty JP. The expert consensus guideline series: medication treatment of bipolar disorder 2000. Postgraduate Medicine. April 2000; Spec No:1-104.
Drug brand names
- Buproprion • Wellbutrin
- Carbamazepine • Tegretol
- Clonidine • Catapres
- Clozapine • Clozaril
- Divalproex sodium • Depakote
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levothyroxine • Synthroid, Levothroid, Levoxyl
- Olanzapine • Zyprexa
- Nimodipine • Nimotop
- Primidonem • Mysoline
- Topiramate • Topamax
1. Dunner DL, Fieve RR. Clinical factors in lithium carbonate prophylaxis failure. Arch Gen Psychiatry. 1974;20:229-233.
2. Calabrese JR, Delucchi GA. Spectrum of efficacy of valproate in 55 rapid-cycling manic depressives. Am J Psychiatry. 1990;147(4):431-434.
3. Calabrese JR, Shelton MD, Bowden CL, et al. Bipolar rapid cycling: Focus on depression as its hallmark. J Clin Psychiatry. 2001;62(Suppl 14):34-41.
4. Kukopulos A, Reginaldi D, Laddomada P, et al. Course of the manic depressive cycle and changes caused by treatment. Pharmacopsychiatry. 1980;13:156-167.
5. Maj M, et al. Long-term outcome of lithium prophylaxis in bipolar disorder: a 5-year prospective study of 402 patients at a lithium clinic. Am J Psychiatry. 1998;155:30-35.
6. Tondo L, Baldessarini RJ, et al. Lithium maintenance treatment of depression and mania in bipolar I and bipolar II disorders. Am J Psychiatry. 1998;155:638-645.
7. Wehr TA, Sack DA, Rosenthal NE, et al. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry. 1988;145:179-84.
8. Calabrese JR, Woyshville MJ, Kimmel SE. Rapport DJ: Predictors of valproate response in bipolar rapid cycling. J Clin Psychopharmacology. 1993;13(4):280-283.
9. Sharma V, Persad E, et al. Treatment of rapid cycling bipolar disorder with combination therapy of valproate and lithium. Can J Psychiatry. 1993;38:137-139.
10. Calabrese JR, Suppes T, Bowden CL, et al. for the Lamictal 614 Study Group. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry. 2000;61(11):841-850.
11. Fatemi SH, Rapport DJ, Calabrese JR, et al. Lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry 1997;58:522-527.
12. Calabrese JR, Bowden CL, et al. for the Lamictal 602 Study Group. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60(2):79-88.
13. Bowden CL, Calabrese JR, McElroy SL, et al. Comparison of the efficacy of lamotrigine in rapid cycling and non-rapid cycling bipolar disorder. Biol Psychiatry. 1999;45(8):953-958.
14. Frye MA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol. 2000;20:607-614.
15. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry. 1999;60(2):79-88.
16. Calabrese JR, Bowden CL, DeVeaugh-Geiss J, et al. Lamotrigine demonstrates long term mood stabilization in recently manic patients. Annual meeting of the American Psychiatric Association, New Orleans, 2001.
17. Stancer HC, Persad E. Treatment of intractable rapid-cycling manic-depressive disorder with levothyroxine. Clinical observations. Arch Gen Psychiatry. 1982;39(3):311-312.
18. Bauer MS, Whybrow PC. Rapid cycling bipolar affective disorder. II. Treatment of refractory rapid cycling with high-dose levothyroxine: a preliminary study.. Arch Gen Psychiatry. 1990;47(5):435-40.
19. Suppes T, Webb A, Paul B, Carmody T, et al:. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156:1164-1169.
Patients with rapid-cycling bipolar disorder (RCBD) can be frustrating to treat. Despite growing research and data, knowledge and effective therapies remain limited. How do you manage patients with rapid cycling who do not respond robustly to lithium, divalproex, or carbamazepine monotherapy? Are combination therapies likely to be more effective? Where does lamotrigine fit in? Is there a role for conventional antidepressants?
We’ll explore these and related questions—but the final answers are not yet in. Recognition of RCBD is important because it presents such difficult treatment challenges. Available evidence does suggest that rapid cycling as defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (Box 1), describes a clinically specific course of illness that may require treatments different from currently used traditional drug therapies for nonrapid cycling bipolar disorder, particularly as no one agent appears to provide ideal bimodal treatment and prophylaxis of this bipolar disorder variant.
Rapid cycling is a specifier of the longitudinal course of illness presentation that is seen almost exclusively in bipolar disorder and is associated with a greater morbidity. Dunner and Fieve1 originally coined the term when evaluating clinical factors associated with lithium prophylaxis failure. Since that time the validity of rapid cycling as a distinct course modifier for bipolar disorder has been supported by multiple studies, leading to its inclusion in the fourth edition of the Diagnostic and Statistical Manual of the APA (1994).
According to DSM-IV, the course specifier of rapid cycling applies to “at least 4 episodes of a mood disturbance in the previous 12 months that meet criteria for a manic episode, a hypomanic episode, or a major depressive episode.” The episodes must be demarcated by a full or partial remission lasting at least 2 months or by a switch to a mood state of opposite polarity.
Early reports noted that patients suffering from RCBD did not respond adequately when treated with lithium.1 Other observations indicated that divalproex was more effective in this patient population, particularly for the illness’ hypomanic or manic phases.2 We hope that the following evaluation of these and other drug therapies will prove helpful.
Watch out for antidepressants
Most concerning has been the frequency and severity of treatment-refractory depressive phases of RCBD that may be exacerbated by antidepressant use (cycle induction or acceleration). Indeed, the frequent recurrence of refractory depression has been described as the hallmark of this bipolar disorder variant.3
Lithium: the scale weighs against it
Although an excellent mood stabilizer for most patients with bipolar disorder, lithium monotherapy is less than ideal for patients with the rapid-cycling variant, particularly in treatment or prevention of depressive or mixed episodes. The efficacy of lithium is likely decreased by the concurrent administration of antidepressant medication and increased when administered with other mood stabilizers.
The landmark article by Dunner and Fieve,1 which described a placebo-controlled, double-blind maintenance study in a general cohort of 55 patients, tried to clarify factors associated with the failure of lithium prophylaxis in bipolar disorder. Rapid cyclers comprised 20% of the subjects and 80% were nonrapid cyclers. Rapid cyclers were disproportionately represented in the lithium failure group. Lithium failures included 82% (9 of 11) of rapid cyclers compared to 41% (18 of 44) of nonrapid cyclers. Lithium failure was defined as (1) hospitalization for, or (2) treatment of, mania or (3) depression during lithium therapy, or as mood symptoms that, as documented by rating scales, were sufficient to warrant a diagnosis of mild depression, hypomania, or mania persisting for at least 2 weeks.
Kukopulos et al4 replicated the findings of Dunner and Fieve in a study of the longitudinal clinical course of 434 bipolar patients. Of these patients, 50 were rapid cyclers and had received continuous lithium therapy for more than a year, with good to partial prophylaxis in only 28%. Maj and colleagues5 published a 5-year prospective study of lithium therapy in 402 patients with bipolar disorder and noted the absence of rapid cycling in good responders to lithium but an incidence rate of 26% in nonresponders to lithium.
Other investigators have reported better response in RCBD. In a select cohort of lithium-responsive bipolar I and II patients, Tondo et al6 concluded that lithium maintenance yields striking long-term reductions in depressive and manic morbidity, more so in rapid cycling type II patients. This study, however, was in a cohort of lithium responders and excluded patients who had been exposed to antipsychotic or antidepressant medications for more than 3 months, those on chronic anticonvulsant therapy, and those with substance abuse disorders.
Although most studies do report poor response to lithium therapy in RCBD, Wehr and colleagues7 suggest that in some patients with rapid cycling, the discontinuation of antidepressant drugs may allow lithium to act as a more effective anticycling mood-stabilizing agent.
Divalproex: effective in manic phase
In contrast to lithium, an open trial of a homogenous cohort of patients with RCBD by Calabrese and colleagues3,8 found divalproex to possess moderate to marked acute and prophylactic antimanic properties with only modest antidepressant effects (Table 1). Data from 6 open studies involving 147 patients with rapid cycling suggest that divalproex possesses moderate to marked efficacy in the manic phase, but poor to moderate efficacy in the depressed phase. Positive outcome predictors were bipolar II and mixed states, no prior lithium therapy, and a positive family history of affective disorder. Predictors of negative response included increase in frequency and severity of mania, and borderline personality disorder.
Divalproex therapy in combination with lithium may improve response rates.9 Calabrese and colleagues, however, have examined large cohorts of patients, including those comorbid with alcohol, cocaine, and/or cannabis abuse, treated with a lithium-divalproex combination over 6-month study periods. The researchers found that only 25% to 50% of patients stabilized, and that of those not exhibiting a response, the majority (75%) did not respond because of treatment-refractory depression in the context of RCBD.3
Although experts believe divalproex to be more effective than lithium in preventing episodes associated with RCBD, such a conclusion awaits confirmation with the near completion of a double-blind, 20-month maintenance trial sponsored by the National Institute of Mental Health (NIMH).
Carbamazepine’s role in combination therapy
Early reports by Post and colleagues in 1987 suggested that rapid cycling predicted positive response to carbamazepine, but later findings by Okuma in 1993 refuted this. Other collective open and controlled studies suggest that this anticonvulsant possesses moderate to marked efficacy in the manic phase, and poor to moderate efficacy in the depressed phase of RCBD. Again, combination therapy with lithium may offer greater efficacy. Of significance, carbamazepine treatment outcomes have not been prospectively evaluated in a homogeneous cohort of rapid cyclers.
The limitations of carbamazepine therapy are well known and available evidence also does not seem to support monotherapy with this agent as being useful in RCBD, especially in the treatment and prophylaxis of depressive or mixed phases of the disorder. Thus, further controlled studies are needed to examine the agent’s potential role and safety in combination therapies for RCBD.
Table 1
SPECTRUM OF ACUTE AND PROPHYLACTIC EFFICACY OF DIVALPROEX IN RAPID-CYCLING BIPOLAR DISORDER
| Spectrum of marked responses to divalproex in bipolar rapid cycling | ||
|---|---|---|
| Acute | Prophylactic | |
| Dysphoric hypomania/mania | 87% | 89% |
| Elated hypomania/mania | 64% | 77% |
| Depression (n = 101, mean follow-up 15 months) | 21% | 38% |
| Adapted from Calabrese and Delucchi. Am J Psychiatry. 1990;147:431-434 and Calabrese et al. J Clin Psychopharmacol. 1993;13:280-283. | ||
Lamotrigine: best hope for monotherapy
Lamotrigine monotherapy has been reported to be effective in some RCBD cases. The data suggest that it possesses both antidepressant and mood-stabilizing properties.10
An open, naturalistic study of 5 women with treatment-refractory rapid cycling by Fatemi et al11 demonstrated both mood-stabilizing and antidepressant effects from lamotrigine monotherapy or augmentation at a mean dose of 185 ±33.5 mg/d. In 14 clinical reports involving 207 patients with bipolar disorder, 66 of whom had rapid cycling, lamotrigine was observed to possess moderate to marked efficacy in depression and hypomania, but only moderate efficacy in mania.
An open, prospective study compared the efficacy of lamotrigine add-on or monotherapy in 41 rapid cyclers to 34 nonrapid cyclers across 48 weeks. Improvement from baseline to last visit was significant between both subgroups for depressive and hypomanic symptoms. Patients presenting with more severe manic symptoms did less well.13
In the first double-blind, placebo-controlled study of lamotrigine in RCBD,12 182 of 324 patients with rapid cycling responded to treatment with open-label lamotrigine and were then randomized to the study’s double-blind phase. Forty-one percent of lamotrigine-treated vs. 26% of placebo-treated patients were stable without relapse during 6 months of monotherapy. Patients with rapid-cycling bipolar II disorder consistently experienced more improvement than their bipolar I counterparts (Figure 1). The results of this only prospective, placebo-controlled, acute-treatment study of rapid-cycling bipolar patients to date indicate that lamotrigine monotherapy is useful for some patients with RCBD, particularly those with bipolar II.
Frye et al14 conducted a double-blind, placebo-controlled study of 23 patients with rapid cycling utilizing a crossover series of three 6-week monotherapy evaluations of lamotrigine, gabapentin, and placebo. Marked antidepressant response on lamotrigine was seen in 45% of the participants compared with 19% of patients on placebo and a similar response rate among those on gabapentin.
A study evaluating the safety and efficacy of 2 dosages of lamotrigine (50 mg/d or 200 mg/d) compared with placebo in the treatment of a major depressive episode in patients with bipolar I disorder over 7 weeks demonstrated significant antidepressant efficacy.15 These bipolar outpatients displayed clinical improvement as early as the third week of treatment, and switch rates for both dosages did not exceed that of placebo. Patients with RCBD, however, were excluded from this initial trial. Subsequent studies have demonstrated similar magnitudes of efficacy in patients with RCBD, primarily in the prevention of depressive episodes, including the 6-month RCBD maintenance study10 and 2 recently completed 18-month maintenance studies of patients with bipolar I disorder, either recently manic or recently depressed.
Lamotrigine thus may have a special role in RCBD treatment. Its most significant side effect in bipolar disorder is benign rash, which has occurred in 9.0% (108 of 1,198) of patients randomized to lamotrigine vs. 7.6% (80 of 1,056) of those randomized to placebo in pivotal multicenter, double-blind, placebo-controlled bipolar trials.
In mood disorder trials conducted to date, the rate of serious rash, defined as requiring both drug discontinuation and hospitalization, has been 0.06% (2 of 3,153) on lamotrigine and 0.09% (1 out of 1,053) on placebo. No cases of Steven’s Johnson syndrome or toxic epidermal necrolysis were observed.16 A low starting dose and gradual titration of lamotrigine (Table 2) appear to minimize the risk of serious rash.
Levothyroxine: possible add-on therapy
Levothyroxine should be considered as add-on therapy in patients with known hypothyroidism, borderline hypothyroidism, or otherwise treatment-refractory cases.
The strategy of thyroid supplementation is derived from Gjessing’s 1936 report of success in administering hypermetabolic doses of thyroid hormone to patients with periodic catatonia. Stancer and Persad17 initially reported the potential efficacy of this therapeutic maneuver in rapid cyclers; remissions were induced by hypermetabolic thyroid doses in 5 of 7 patients with treatment-refractory bipolar disorder. No data currently support any prophylactic efficacy of thyroid supplementation monotherapy for RCBD treatment.
Bauer and Whybrow18 suggest that thyroid supplementation with T4 added to mood stabilizers augments efficacy independent of pre-existing thyroid function. The potential side effects of long-term levothryoxine administration, namely osteoporosis and cardiac arrhythmias, limit the usefulness of thyroid augmentation in RCBD.
Atypical antipsychotics: perhaps in combination
Coadministering atypical antipsychotics in other mood stabilizers may help rapid cyclers with current or past psychotic symptoms during their mood episodes, but further study is clearly needed.
Atypical antipsychotic medications may have specific mood-stabilizing properties, particularly in the management of mixed and manic states. In the first prospective trial of clozapine monotherapy in bipolar disorder, Calabrese and colleagues in 1994 reported that rapid cycling did not appear to predict nonresponse to treatment.
In the first randomized, controlled trial involving clozapine in bipolar disorder, Suppes et al19 noted significant improvement for symptoms of mania, psychosis, and global improvement. Subjects with nonpsychotic bipolar disorder showed a degree of improvement similar to that seen in the entire clozapine-treated group. These results support a mood-stabilizing role for clozapine.
Preliminary studies of risperidone and olanzapine further suggest therapeutic utility for atypical antipsychotics.
Figure 1 LAMOTRIGINE VS. PLACEBO IN RAPID-CYCLING BIPOLAR DISORDER
Table 2
DOSAGE PLAN FOR LAMOTRIGINE IN MONOTHERAPY AND COMBINATION
| Treatment period | Type of therapy | Daily dosage |
|---|---|---|
| Weeks 1-2 | Monotherapy With divalproex With carbamazepine | 25 mg 12.5 mg 50 mg |
| Weeks 3-4 | Monotherapy With divalproex With carbamazepine | 50 mg 25 mg 100 mg |
| Week 5 | Monotherapy With divalproex With carbamazepine | 100 mg 50 mg 200 mg |
| Thereafter | Monotherapy With divalproex With carbamazepine | 200 mg 100 mg 400 mg |
Topiramate: in patients with weight problems
Evidence from 12 open studies of 223 patients with bipolar disorder suggest that this anticonvulsant may possess mood-stabilizing properties while sparing patients the weight gain commonly seen with other pharmacotherapies.
Two studies of topiramate as an add-on therapy come to mind. Marcotte in 1998 retrospectively studied 44 patients with RCBD, 23 (52%) of whom demonstrated moderate or marked improvement on a mean topiramate dosage of 200 mg/d.
Sachs also reported that symptoms for some patients with a treatment-refractory bipolar disorder could be improved when topiramate was added to their medication regimen. Available data encourage further controlled studies of topiramate add-on therapy for RCBD, especially in obese patients.
Gabapentin: contradictory reports
Preliminary data from 14 open-label studies of gabapentin in 302 bipolar patients reported a response rate of around 67% when used as an add-on therapy, usually in mania or mixed states. A moderate antimanic effect was observed among a total of 23 rapid cyclers in 9 of the studies.
The reports are contradictory, however, and available data do not firmly support the agent’s efficacy in RCBD treatment.
Other potential uses for gabapentin are being investigated, such as antidepressant augmentation, anxiety, and chronic pain. Thus, patients with RCBD and a comorbid illness may benefit from add-on gabapentin.
ECT: Some limited success
Electroconvulsive therapy (ECT) has been implicated less frequently than antidepressants in the induction of rapid cycling, and when this does occur it is usually in the context of combined ECT/antidepressant therapies.
Berma and Wolpert in 1987 reported a case of successful ECT treatment of rapid cycling in an adolescent who had been treated with trimipramine for depression. Vanelle et al in 1994 suggested that maintenance ECT works well in 22 treatment-resistant bipolar patients, including 4 with rapid cycling, over an 18-month treatment period.
Behavioral intervention: changing sleep routines
NIMH research of 15 rapid cyclers who were studied for 3 months looked at behavioral interventions and their effect on switching (Feldman-Naim 1997). This study suggested that patients were more likely to switch from depression into hypomania/mania during daytime hours and from mania/hypomania into depression during nighttime.
The use of light therapy or activity and exercise during depression and the use of induced sleep or exposure to darkness during mania/hypomania may be therapeutic. Wehr and colleagues supported this in a 1998 report of one patient studied over several years, comparing this rapid-cycling patient’s regular sleep routine with prolonged (10 to 14 hours per night) and enforced bed rest in the dark.7 The promotion of sleep by scheduling regular nighttime periods of enforced bed rest in the dark may help prevent mania and stabilize mood in rapid cyclers.
Other add-on possibilities
Haykal in 1990 reported bupropion to be an effective add-on treatment in 5 of 6 patients with refractory, rapid-cycling bipolar II disorder. Benefit has also been reported from clorgyline, clonidine, magnesium, primidone, and acetazolamide.
Calcium-channel blockers may also offer clinical utility, although supportive evidence is limited. Nimodipine was evaluated for efficacy in 30 patients with treatment-refractory affective illness by the NIMH and Passaglia et al in 1998. Patients who improved on this agent had ultradian rapid cycling, defined in the study as those with affective episodes lasting as short as a week.
A recommended treatment strategy
Based on the available data in bipolar I rapid cycling, we recommend initial treatment with divalproex followed by augmentation with lithium if hypomanic or manic episodes persist, lamotrigine if breakthrough episodes are predominantly depressive, and atypical antipsychotics if psychotic symptoms or true mixed states remain.
For patients presenting with bipolar II rapid cycling, we recommend starting with lamotrigine, then augmenting with divalproex or lithium for breakthrough episodes. Lamotrigine shows more promise because of its reportedly greater antidepressant properties and lack of cycle induction or switching, but offers only modest antimanic therapy.
Pending further investigations, current application of the data suggests that when treating patients with RCBD, conventional antidepressants should be avoided and, if first-line therapies are not effective, the clinician should consider moving to combination drug therapy with 2 or more agents.
Related resources
- Bauer MS, Calabrese JR, Dunner DL, et al. Multi-site data reanalysis: validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry. 1994;151:506-515.
- Calabrese JR, Kimmel SE, Woyshville MJ, et al. Clozapine in treatment refractory mania. Am J Psychiatry. 1996;153(6):759-764.
- Calabrese JR, Bowden CL, McElroy SL, et al. Spectrum of activity of lamotrigine in treatment refractory bipolar disorder. Am J Psychiatry. 1999;156(7):1019-1023.
- Sachs GS. Printz DJ. Kahn DA. Carpenter D. Docherty JP. The expert consensus guideline series: medication treatment of bipolar disorder 2000. Postgraduate Medicine. April 2000; Spec No:1-104.
Drug brand names
- Buproprion • Wellbutrin
- Carbamazepine • Tegretol
- Clonidine • Catapres
- Clozapine • Clozaril
- Divalproex sodium • Depakote
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levothyroxine • Synthroid, Levothroid, Levoxyl
- Olanzapine • Zyprexa
- Nimodipine • Nimotop
- Primidonem • Mysoline
- Topiramate • Topamax
Patients with rapid-cycling bipolar disorder (RCBD) can be frustrating to treat. Despite growing research and data, knowledge and effective therapies remain limited. How do you manage patients with rapid cycling who do not respond robustly to lithium, divalproex, or carbamazepine monotherapy? Are combination therapies likely to be more effective? Where does lamotrigine fit in? Is there a role for conventional antidepressants?
We’ll explore these and related questions—but the final answers are not yet in. Recognition of RCBD is important because it presents such difficult treatment challenges. Available evidence does suggest that rapid cycling as defined in the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (Box 1), describes a clinically specific course of illness that may require treatments different from currently used traditional drug therapies for nonrapid cycling bipolar disorder, particularly as no one agent appears to provide ideal bimodal treatment and prophylaxis of this bipolar disorder variant.
Rapid cycling is a specifier of the longitudinal course of illness presentation that is seen almost exclusively in bipolar disorder and is associated with a greater morbidity. Dunner and Fieve1 originally coined the term when evaluating clinical factors associated with lithium prophylaxis failure. Since that time the validity of rapid cycling as a distinct course modifier for bipolar disorder has been supported by multiple studies, leading to its inclusion in the fourth edition of the Diagnostic and Statistical Manual of the APA (1994).
According to DSM-IV, the course specifier of rapid cycling applies to “at least 4 episodes of a mood disturbance in the previous 12 months that meet criteria for a manic episode, a hypomanic episode, or a major depressive episode.” The episodes must be demarcated by a full or partial remission lasting at least 2 months or by a switch to a mood state of opposite polarity.
Early reports noted that patients suffering from RCBD did not respond adequately when treated with lithium.1 Other observations indicated that divalproex was more effective in this patient population, particularly for the illness’ hypomanic or manic phases.2 We hope that the following evaluation of these and other drug therapies will prove helpful.
Watch out for antidepressants
Most concerning has been the frequency and severity of treatment-refractory depressive phases of RCBD that may be exacerbated by antidepressant use (cycle induction or acceleration). Indeed, the frequent recurrence of refractory depression has been described as the hallmark of this bipolar disorder variant.3
Lithium: the scale weighs against it
Although an excellent mood stabilizer for most patients with bipolar disorder, lithium monotherapy is less than ideal for patients with the rapid-cycling variant, particularly in treatment or prevention of depressive or mixed episodes. The efficacy of lithium is likely decreased by the concurrent administration of antidepressant medication and increased when administered with other mood stabilizers.
The landmark article by Dunner and Fieve,1 which described a placebo-controlled, double-blind maintenance study in a general cohort of 55 patients, tried to clarify factors associated with the failure of lithium prophylaxis in bipolar disorder. Rapid cyclers comprised 20% of the subjects and 80% were nonrapid cyclers. Rapid cyclers were disproportionately represented in the lithium failure group. Lithium failures included 82% (9 of 11) of rapid cyclers compared to 41% (18 of 44) of nonrapid cyclers. Lithium failure was defined as (1) hospitalization for, or (2) treatment of, mania or (3) depression during lithium therapy, or as mood symptoms that, as documented by rating scales, were sufficient to warrant a diagnosis of mild depression, hypomania, or mania persisting for at least 2 weeks.
Kukopulos et al4 replicated the findings of Dunner and Fieve in a study of the longitudinal clinical course of 434 bipolar patients. Of these patients, 50 were rapid cyclers and had received continuous lithium therapy for more than a year, with good to partial prophylaxis in only 28%. Maj and colleagues5 published a 5-year prospective study of lithium therapy in 402 patients with bipolar disorder and noted the absence of rapid cycling in good responders to lithium but an incidence rate of 26% in nonresponders to lithium.
Other investigators have reported better response in RCBD. In a select cohort of lithium-responsive bipolar I and II patients, Tondo et al6 concluded that lithium maintenance yields striking long-term reductions in depressive and manic morbidity, more so in rapid cycling type II patients. This study, however, was in a cohort of lithium responders and excluded patients who had been exposed to antipsychotic or antidepressant medications for more than 3 months, those on chronic anticonvulsant therapy, and those with substance abuse disorders.
Although most studies do report poor response to lithium therapy in RCBD, Wehr and colleagues7 suggest that in some patients with rapid cycling, the discontinuation of antidepressant drugs may allow lithium to act as a more effective anticycling mood-stabilizing agent.
Divalproex: effective in manic phase
In contrast to lithium, an open trial of a homogenous cohort of patients with RCBD by Calabrese and colleagues3,8 found divalproex to possess moderate to marked acute and prophylactic antimanic properties with only modest antidepressant effects (Table 1). Data from 6 open studies involving 147 patients with rapid cycling suggest that divalproex possesses moderate to marked efficacy in the manic phase, but poor to moderate efficacy in the depressed phase. Positive outcome predictors were bipolar II and mixed states, no prior lithium therapy, and a positive family history of affective disorder. Predictors of negative response included increase in frequency and severity of mania, and borderline personality disorder.
Divalproex therapy in combination with lithium may improve response rates.9 Calabrese and colleagues, however, have examined large cohorts of patients, including those comorbid with alcohol, cocaine, and/or cannabis abuse, treated with a lithium-divalproex combination over 6-month study periods. The researchers found that only 25% to 50% of patients stabilized, and that of those not exhibiting a response, the majority (75%) did not respond because of treatment-refractory depression in the context of RCBD.3
Although experts believe divalproex to be more effective than lithium in preventing episodes associated with RCBD, such a conclusion awaits confirmation with the near completion of a double-blind, 20-month maintenance trial sponsored by the National Institute of Mental Health (NIMH).
Carbamazepine’s role in combination therapy
Early reports by Post and colleagues in 1987 suggested that rapid cycling predicted positive response to carbamazepine, but later findings by Okuma in 1993 refuted this. Other collective open and controlled studies suggest that this anticonvulsant possesses moderate to marked efficacy in the manic phase, and poor to moderate efficacy in the depressed phase of RCBD. Again, combination therapy with lithium may offer greater efficacy. Of significance, carbamazepine treatment outcomes have not been prospectively evaluated in a homogeneous cohort of rapid cyclers.
The limitations of carbamazepine therapy are well known and available evidence also does not seem to support monotherapy with this agent as being useful in RCBD, especially in the treatment and prophylaxis of depressive or mixed phases of the disorder. Thus, further controlled studies are needed to examine the agent’s potential role and safety in combination therapies for RCBD.
Table 1
SPECTRUM OF ACUTE AND PROPHYLACTIC EFFICACY OF DIVALPROEX IN RAPID-CYCLING BIPOLAR DISORDER
| Spectrum of marked responses to divalproex in bipolar rapid cycling | ||
|---|---|---|
| Acute | Prophylactic | |
| Dysphoric hypomania/mania | 87% | 89% |
| Elated hypomania/mania | 64% | 77% |
| Depression (n = 101, mean follow-up 15 months) | 21% | 38% |
| Adapted from Calabrese and Delucchi. Am J Psychiatry. 1990;147:431-434 and Calabrese et al. J Clin Psychopharmacol. 1993;13:280-283. | ||
Lamotrigine: best hope for monotherapy
Lamotrigine monotherapy has been reported to be effective in some RCBD cases. The data suggest that it possesses both antidepressant and mood-stabilizing properties.10
An open, naturalistic study of 5 women with treatment-refractory rapid cycling by Fatemi et al11 demonstrated both mood-stabilizing and antidepressant effects from lamotrigine monotherapy or augmentation at a mean dose of 185 ±33.5 mg/d. In 14 clinical reports involving 207 patients with bipolar disorder, 66 of whom had rapid cycling, lamotrigine was observed to possess moderate to marked efficacy in depression and hypomania, but only moderate efficacy in mania.
An open, prospective study compared the efficacy of lamotrigine add-on or monotherapy in 41 rapid cyclers to 34 nonrapid cyclers across 48 weeks. Improvement from baseline to last visit was significant between both subgroups for depressive and hypomanic symptoms. Patients presenting with more severe manic symptoms did less well.13
In the first double-blind, placebo-controlled study of lamotrigine in RCBD,12 182 of 324 patients with rapid cycling responded to treatment with open-label lamotrigine and were then randomized to the study’s double-blind phase. Forty-one percent of lamotrigine-treated vs. 26% of placebo-treated patients were stable without relapse during 6 months of monotherapy. Patients with rapid-cycling bipolar II disorder consistently experienced more improvement than their bipolar I counterparts (Figure 1). The results of this only prospective, placebo-controlled, acute-treatment study of rapid-cycling bipolar patients to date indicate that lamotrigine monotherapy is useful for some patients with RCBD, particularly those with bipolar II.
Frye et al14 conducted a double-blind, placebo-controlled study of 23 patients with rapid cycling utilizing a crossover series of three 6-week monotherapy evaluations of lamotrigine, gabapentin, and placebo. Marked antidepressant response on lamotrigine was seen in 45% of the participants compared with 19% of patients on placebo and a similar response rate among those on gabapentin.
A study evaluating the safety and efficacy of 2 dosages of lamotrigine (50 mg/d or 200 mg/d) compared with placebo in the treatment of a major depressive episode in patients with bipolar I disorder over 7 weeks demonstrated significant antidepressant efficacy.15 These bipolar outpatients displayed clinical improvement as early as the third week of treatment, and switch rates for both dosages did not exceed that of placebo. Patients with RCBD, however, were excluded from this initial trial. Subsequent studies have demonstrated similar magnitudes of efficacy in patients with RCBD, primarily in the prevention of depressive episodes, including the 6-month RCBD maintenance study10 and 2 recently completed 18-month maintenance studies of patients with bipolar I disorder, either recently manic or recently depressed.
Lamotrigine thus may have a special role in RCBD treatment. Its most significant side effect in bipolar disorder is benign rash, which has occurred in 9.0% (108 of 1,198) of patients randomized to lamotrigine vs. 7.6% (80 of 1,056) of those randomized to placebo in pivotal multicenter, double-blind, placebo-controlled bipolar trials.
In mood disorder trials conducted to date, the rate of serious rash, defined as requiring both drug discontinuation and hospitalization, has been 0.06% (2 of 3,153) on lamotrigine and 0.09% (1 out of 1,053) on placebo. No cases of Steven’s Johnson syndrome or toxic epidermal necrolysis were observed.16 A low starting dose and gradual titration of lamotrigine (Table 2) appear to minimize the risk of serious rash.
Levothyroxine: possible add-on therapy
Levothyroxine should be considered as add-on therapy in patients with known hypothyroidism, borderline hypothyroidism, or otherwise treatment-refractory cases.
The strategy of thyroid supplementation is derived from Gjessing’s 1936 report of success in administering hypermetabolic doses of thyroid hormone to patients with periodic catatonia. Stancer and Persad17 initially reported the potential efficacy of this therapeutic maneuver in rapid cyclers; remissions were induced by hypermetabolic thyroid doses in 5 of 7 patients with treatment-refractory bipolar disorder. No data currently support any prophylactic efficacy of thyroid supplementation monotherapy for RCBD treatment.
Bauer and Whybrow18 suggest that thyroid supplementation with T4 added to mood stabilizers augments efficacy independent of pre-existing thyroid function. The potential side effects of long-term levothryoxine administration, namely osteoporosis and cardiac arrhythmias, limit the usefulness of thyroid augmentation in RCBD.
Atypical antipsychotics: perhaps in combination
Coadministering atypical antipsychotics in other mood stabilizers may help rapid cyclers with current or past psychotic symptoms during their mood episodes, but further study is clearly needed.
Atypical antipsychotic medications may have specific mood-stabilizing properties, particularly in the management of mixed and manic states. In the first prospective trial of clozapine monotherapy in bipolar disorder, Calabrese and colleagues in 1994 reported that rapid cycling did not appear to predict nonresponse to treatment.
In the first randomized, controlled trial involving clozapine in bipolar disorder, Suppes et al19 noted significant improvement for symptoms of mania, psychosis, and global improvement. Subjects with nonpsychotic bipolar disorder showed a degree of improvement similar to that seen in the entire clozapine-treated group. These results support a mood-stabilizing role for clozapine.
Preliminary studies of risperidone and olanzapine further suggest therapeutic utility for atypical antipsychotics.
Figure 1 LAMOTRIGINE VS. PLACEBO IN RAPID-CYCLING BIPOLAR DISORDER
Table 2
DOSAGE PLAN FOR LAMOTRIGINE IN MONOTHERAPY AND COMBINATION
| Treatment period | Type of therapy | Daily dosage |
|---|---|---|
| Weeks 1-2 | Monotherapy With divalproex With carbamazepine | 25 mg 12.5 mg 50 mg |
| Weeks 3-4 | Monotherapy With divalproex With carbamazepine | 50 mg 25 mg 100 mg |
| Week 5 | Monotherapy With divalproex With carbamazepine | 100 mg 50 mg 200 mg |
| Thereafter | Monotherapy With divalproex With carbamazepine | 200 mg 100 mg 400 mg |
Topiramate: in patients with weight problems
Evidence from 12 open studies of 223 patients with bipolar disorder suggest that this anticonvulsant may possess mood-stabilizing properties while sparing patients the weight gain commonly seen with other pharmacotherapies.
Two studies of topiramate as an add-on therapy come to mind. Marcotte in 1998 retrospectively studied 44 patients with RCBD, 23 (52%) of whom demonstrated moderate or marked improvement on a mean topiramate dosage of 200 mg/d.
Sachs also reported that symptoms for some patients with a treatment-refractory bipolar disorder could be improved when topiramate was added to their medication regimen. Available data encourage further controlled studies of topiramate add-on therapy for RCBD, especially in obese patients.
Gabapentin: contradictory reports
Preliminary data from 14 open-label studies of gabapentin in 302 bipolar patients reported a response rate of around 67% when used as an add-on therapy, usually in mania or mixed states. A moderate antimanic effect was observed among a total of 23 rapid cyclers in 9 of the studies.
The reports are contradictory, however, and available data do not firmly support the agent’s efficacy in RCBD treatment.
Other potential uses for gabapentin are being investigated, such as antidepressant augmentation, anxiety, and chronic pain. Thus, patients with RCBD and a comorbid illness may benefit from add-on gabapentin.
ECT: Some limited success
Electroconvulsive therapy (ECT) has been implicated less frequently than antidepressants in the induction of rapid cycling, and when this does occur it is usually in the context of combined ECT/antidepressant therapies.
Berma and Wolpert in 1987 reported a case of successful ECT treatment of rapid cycling in an adolescent who had been treated with trimipramine for depression. Vanelle et al in 1994 suggested that maintenance ECT works well in 22 treatment-resistant bipolar patients, including 4 with rapid cycling, over an 18-month treatment period.
Behavioral intervention: changing sleep routines
NIMH research of 15 rapid cyclers who were studied for 3 months looked at behavioral interventions and their effect on switching (Feldman-Naim 1997). This study suggested that patients were more likely to switch from depression into hypomania/mania during daytime hours and from mania/hypomania into depression during nighttime.
The use of light therapy or activity and exercise during depression and the use of induced sleep or exposure to darkness during mania/hypomania may be therapeutic. Wehr and colleagues supported this in a 1998 report of one patient studied over several years, comparing this rapid-cycling patient’s regular sleep routine with prolonged (10 to 14 hours per night) and enforced bed rest in the dark.7 The promotion of sleep by scheduling regular nighttime periods of enforced bed rest in the dark may help prevent mania and stabilize mood in rapid cyclers.
Other add-on possibilities
Haykal in 1990 reported bupropion to be an effective add-on treatment in 5 of 6 patients with refractory, rapid-cycling bipolar II disorder. Benefit has also been reported from clorgyline, clonidine, magnesium, primidone, and acetazolamide.
Calcium-channel blockers may also offer clinical utility, although supportive evidence is limited. Nimodipine was evaluated for efficacy in 30 patients with treatment-refractory affective illness by the NIMH and Passaglia et al in 1998. Patients who improved on this agent had ultradian rapid cycling, defined in the study as those with affective episodes lasting as short as a week.
A recommended treatment strategy
Based on the available data in bipolar I rapid cycling, we recommend initial treatment with divalproex followed by augmentation with lithium if hypomanic or manic episodes persist, lamotrigine if breakthrough episodes are predominantly depressive, and atypical antipsychotics if psychotic symptoms or true mixed states remain.
For patients presenting with bipolar II rapid cycling, we recommend starting with lamotrigine, then augmenting with divalproex or lithium for breakthrough episodes. Lamotrigine shows more promise because of its reportedly greater antidepressant properties and lack of cycle induction or switching, but offers only modest antimanic therapy.
Pending further investigations, current application of the data suggests that when treating patients with RCBD, conventional antidepressants should be avoided and, if first-line therapies are not effective, the clinician should consider moving to combination drug therapy with 2 or more agents.
Related resources
- Bauer MS, Calabrese JR, Dunner DL, et al. Multi-site data reanalysis: validity of rapid cycling as a course modifier for bipolar disorder in DSM-IV. Am J Psychiatry. 1994;151:506-515.
- Calabrese JR, Kimmel SE, Woyshville MJ, et al. Clozapine in treatment refractory mania. Am J Psychiatry. 1996;153(6):759-764.
- Calabrese JR, Bowden CL, McElroy SL, et al. Spectrum of activity of lamotrigine in treatment refractory bipolar disorder. Am J Psychiatry. 1999;156(7):1019-1023.
- Sachs GS. Printz DJ. Kahn DA. Carpenter D. Docherty JP. The expert consensus guideline series: medication treatment of bipolar disorder 2000. Postgraduate Medicine. April 2000; Spec No:1-104.
Drug brand names
- Buproprion • Wellbutrin
- Carbamazepine • Tegretol
- Clonidine • Catapres
- Clozapine • Clozaril
- Divalproex sodium • Depakote
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levothyroxine • Synthroid, Levothroid, Levoxyl
- Olanzapine • Zyprexa
- Nimodipine • Nimotop
- Primidonem • Mysoline
- Topiramate • Topamax
1. Dunner DL, Fieve RR. Clinical factors in lithium carbonate prophylaxis failure. Arch Gen Psychiatry. 1974;20:229-233.
2. Calabrese JR, Delucchi GA. Spectrum of efficacy of valproate in 55 rapid-cycling manic depressives. Am J Psychiatry. 1990;147(4):431-434.
3. Calabrese JR, Shelton MD, Bowden CL, et al. Bipolar rapid cycling: Focus on depression as its hallmark. J Clin Psychiatry. 2001;62(Suppl 14):34-41.
4. Kukopulos A, Reginaldi D, Laddomada P, et al. Course of the manic depressive cycle and changes caused by treatment. Pharmacopsychiatry. 1980;13:156-167.
5. Maj M, et al. Long-term outcome of lithium prophylaxis in bipolar disorder: a 5-year prospective study of 402 patients at a lithium clinic. Am J Psychiatry. 1998;155:30-35.
6. Tondo L, Baldessarini RJ, et al. Lithium maintenance treatment of depression and mania in bipolar I and bipolar II disorders. Am J Psychiatry. 1998;155:638-645.
7. Wehr TA, Sack DA, Rosenthal NE, et al. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry. 1988;145:179-84.
8. Calabrese JR, Woyshville MJ, Kimmel SE. Rapport DJ: Predictors of valproate response in bipolar rapid cycling. J Clin Psychopharmacology. 1993;13(4):280-283.
9. Sharma V, Persad E, et al. Treatment of rapid cycling bipolar disorder with combination therapy of valproate and lithium. Can J Psychiatry. 1993;38:137-139.
10. Calabrese JR, Suppes T, Bowden CL, et al. for the Lamictal 614 Study Group. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry. 2000;61(11):841-850.
11. Fatemi SH, Rapport DJ, Calabrese JR, et al. Lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry 1997;58:522-527.
12. Calabrese JR, Bowden CL, et al. for the Lamictal 602 Study Group. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60(2):79-88.
13. Bowden CL, Calabrese JR, McElroy SL, et al. Comparison of the efficacy of lamotrigine in rapid cycling and non-rapid cycling bipolar disorder. Biol Psychiatry. 1999;45(8):953-958.
14. Frye MA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol. 2000;20:607-614.
15. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry. 1999;60(2):79-88.
16. Calabrese JR, Bowden CL, DeVeaugh-Geiss J, et al. Lamotrigine demonstrates long term mood stabilization in recently manic patients. Annual meeting of the American Psychiatric Association, New Orleans, 2001.
17. Stancer HC, Persad E. Treatment of intractable rapid-cycling manic-depressive disorder with levothyroxine. Clinical observations. Arch Gen Psychiatry. 1982;39(3):311-312.
18. Bauer MS, Whybrow PC. Rapid cycling bipolar affective disorder. II. Treatment of refractory rapid cycling with high-dose levothyroxine: a preliminary study.. Arch Gen Psychiatry. 1990;47(5):435-40.
19. Suppes T, Webb A, Paul B, Carmody T, et al:. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156:1164-1169.
1. Dunner DL, Fieve RR. Clinical factors in lithium carbonate prophylaxis failure. Arch Gen Psychiatry. 1974;20:229-233.
2. Calabrese JR, Delucchi GA. Spectrum of efficacy of valproate in 55 rapid-cycling manic depressives. Am J Psychiatry. 1990;147(4):431-434.
3. Calabrese JR, Shelton MD, Bowden CL, et al. Bipolar rapid cycling: Focus on depression as its hallmark. J Clin Psychiatry. 2001;62(Suppl 14):34-41.
4. Kukopulos A, Reginaldi D, Laddomada P, et al. Course of the manic depressive cycle and changes caused by treatment. Pharmacopsychiatry. 1980;13:156-167.
5. Maj M, et al. Long-term outcome of lithium prophylaxis in bipolar disorder: a 5-year prospective study of 402 patients at a lithium clinic. Am J Psychiatry. 1998;155:30-35.
6. Tondo L, Baldessarini RJ, et al. Lithium maintenance treatment of depression and mania in bipolar I and bipolar II disorders. Am J Psychiatry. 1998;155:638-645.
7. Wehr TA, Sack DA, Rosenthal NE, et al. Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry. 1988;145:179-84.
8. Calabrese JR, Woyshville MJ, Kimmel SE. Rapport DJ: Predictors of valproate response in bipolar rapid cycling. J Clin Psychopharmacology. 1993;13(4):280-283.
9. Sharma V, Persad E, et al. Treatment of rapid cycling bipolar disorder with combination therapy of valproate and lithium. Can J Psychiatry. 1993;38:137-139.
10. Calabrese JR, Suppes T, Bowden CL, et al. for the Lamictal 614 Study Group. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry. 2000;61(11):841-850.
11. Fatemi SH, Rapport DJ, Calabrese JR, et al. Lamotrigine in rapid cycling bipolar disorder. J Clin Psychiatry 1997;58:522-527.
12. Calabrese JR, Bowden CL, et al. for the Lamictal 602 Study Group. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60(2):79-88.
13. Bowden CL, Calabrese JR, McElroy SL, et al. Comparison of the efficacy of lamotrigine in rapid cycling and non-rapid cycling bipolar disorder. Biol Psychiatry. 1999;45(8):953-958.
14. Frye MA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol. 2000;20:607-614.
15. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry. 1999;60(2):79-88.
16. Calabrese JR, Bowden CL, DeVeaugh-Geiss J, et al. Lamotrigine demonstrates long term mood stabilization in recently manic patients. Annual meeting of the American Psychiatric Association, New Orleans, 2001.
17. Stancer HC, Persad E. Treatment of intractable rapid-cycling manic-depressive disorder with levothyroxine. Clinical observations. Arch Gen Psychiatry. 1982;39(3):311-312.
18. Bauer MS, Whybrow PC. Rapid cycling bipolar affective disorder. II. Treatment of refractory rapid cycling with high-dose levothyroxine: a preliminary study.. Arch Gen Psychiatry. 1990;47(5):435-40.
19. Suppes T, Webb A, Paul B, Carmody T, et al:. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156:1164-1169.