User login
Closed Reduction of Subacute Patellar Dislocation Using Saline Joint Insufflation: A Technical Trick
As the largest sesamoid bone in the human body, the patella acts as a fulcrum to enhance the biomechanical advantage of the quadriceps in extension.1 It is subject to a variety of forces while improving distribution of forces along the extensor mechanism.2 With sufficient force, the patella can be dislocated. Acute patellar dislocations are the most common knee injury, encompassing 2% to 3% of all knee injuries3 and occurring in 5.8 per 100,000 individuals.4-5 These injuries are associated with acute trauma, frequently from sports and physical activities, occurring while in terminal extension with an axial-valgus stress on the knee during rotation.6
With acute patellar dislocations, patients are usually in significant discomfort. Often, the patella may spontaneously reduce; if not, closed reduction is usually successful with pressure applied anteromedially on the lateral patellar margin, while simultaneously attempting gentle extension of the leg.7 Closed reduction is almost universally successful, and there have only been case reports of irreducible, mainly fixed vertical axis patellar dislocations.8-11 No reports in the literature have described subacute patellar dislocations because of their rarity. Patients present immediately after dislocation, spontaneously reduce, or have a painless, chronically dislocated patella.
We present a case of an elderly man with dementia and a subacute fixed irreducible patellar dislocation, which was reduced using a technique not described in the literature. The patient and the patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 68-year-old nonambulatory man with a history of dementia and stroke presented to the emergency department with complaints of left knee pain and his knee locked in flexion. The patient’s knee had been in that fixed hyperflexed position for at least 10 days after he sustained a twisting injury to his knee while attempting to get out of bed. At baseline, the patient was mostly bedbound and could walk minimally with maximum support, but, given his dementia, he would often attempt to ambulate by himself. After the injury, the patient did not complain of much pain at rest, but attempts at his group home to straighten his leg had caused severe pain. As a result, the patient was brought to the emergency department to be evaluated for fractures.
Physical examination in the emergency department revealed atrophy of the lower extremity musculature and a left knee fixed at 120º in flexion. The skin was intact, and there was minimal effusion of the knee joint. The patella was noted to be laterally subluxated and tender to palpation over the lateral and medial facets. He was neurovascularly intact distally and had painless range of motion of his hips. His contralateral right knee had full range of motion with good patellar tracking.
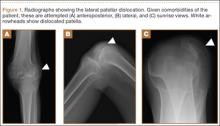
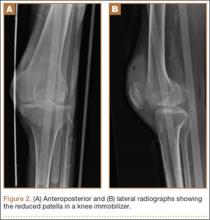
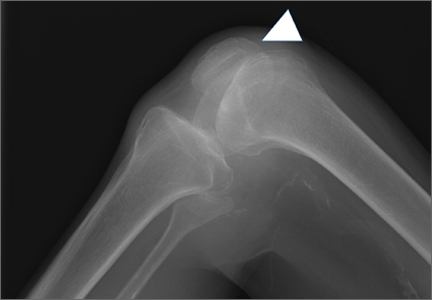
Radiographs of the patient’s knee confirmed a lateral dislocation of the patella (Figures 1A-1C). After oral and intravenous administration of pain medication, a reduction was attempted without success. Next, an intra-articular knee injection of 10 mL of 1% lidocaine was given. After waiting 15 minutes, another reduction was tried. While the pain control was sufficient, the reduction was again unsuccessful. The knee was insufflated with 120 mL of sterile saline and reduction attempted again. By extending the knee and applying a medially directed force to the patella, reduction was successful. The patient was placed into a knee immobilizer and postreduction radiographs were taken (Figures 2A, 2B). Saline was extracted from the knee. The patient was admitted to the hospital where repeat examination of his knees during the next week revealed markedly less pain. The patient was lost to follow-up.
Discussion
Our patient presumably had a low-energy mechanism of injury, resulting in an undiagnosed patellar dislocation with delayed treatment. This subacute patellar dislocation was irreducible using the standard techniques. Alternatively, insufflation of the joint with saline provided the necessary impetus to allow for successful patellar reduction. The history of the patient reveals clues about the mechanism of injury. It is likely that the patient’s nonambulatory status resulted in a weak vastus medialis muscle that placed the patella at risk for dislocation. While the exact mechanism of dislocation is unknown, the patella was unable to be reduced spontaneously because our patient’s knee was maintained in a state of flexion secondary to pain and muscle contraction. The combination of weak quadriceps musculature, increased Q angle, and forced hyperflexion of the knee prevented closed reduction of the patella.
Fixed, irreducible patellar dislocations are rare and discussed infrequently in the literature.9,11-12 Reported mechanisms are mostly high energy, including blows during athletics and impacts from motor vehicle collisions.9,13 Vertical axis rotation, as first described by Cooper,14 is commonly implicated in irreducible patellar dislocations. This occurs when the patella internally rotates 180º on its vertical axis, associated with a large tear of the medial retinaculum but intact quadriceps tendon. The patella is fixed over the lateral femoral condyle with the articular surface pointing anterolaterally. Despite adequate sedation and analgesia, these are notoriously difficult to close-reduce and may necessitate open reduction.3 Our patient, while having a fixed dislocation, did not have a vertical axis component and, therefore, was amenable to our closed reduction attempt.
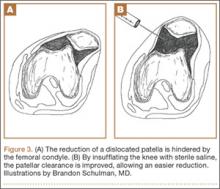
Our first reduction attempts were unsuccessful, likely because the patient continued to be tense, firing his quadriceps. Even after injecting the knee with lidocaine and eliminating the pain component, the patella was still impinging on the lateral femoral condyle (Figure 3A). By insufflating the knee with saline, we were able to increase the distance from the patella to the trochlea (Figure 3B). This is comparable to a knee arthroscopy, in which joint fluid pressure allows passage of arthroscopic instruments into the patellofemoral joint. We postulate that the farther the patella is anterior to the trochlea, the higher the likelihood that the patella can be reduced to its anatomic position.
Insufflation of the knee with sterile saline is a novel technique that involves minimal risk compared with the alternatives. Sometimes, for closed reduction to be successful, individuals need to be consciously sedated to relax their muscles and eliminate pain. While conscious sedation is generally considered low risk, complications have been noted, including hypotension, apnea, and retrograde amnesia.15 Manual closed reduction may also cause additional chondral damage when the medial patellar facet contacts the lateral femoral trochlea. When closed reduction of the patella fails, open reduction is required; this inherently includes all the risks of surgery, such as bleeding, infection, neurovascular injury, and wound complications.
Our insufflation technique does not require sedation and is minimally invasive. The saline creates space and provides lubrication to allow for easier manipulation of the patella. This theoretically protects the cartilage as the patella passes over the lateral trochlea. In addition to the intended effect of providing more space and lubrication for the reduction of the patella, insufflation of the joint may also relax the vastus musculature.16 In their study, Torry and colleagues16 injected 13 knees with 20 mL sterile saline and noted reduction in electromyography readings in the vastus medialis and lateralis muscles. This inhibition of vastus musculature may provide enough relaxation to aid in the successful reduction of the patella.
Our study is limited by our sample size of 1. Because acute patellar dislocations are often easily reduced, our technical trick is not frequently used. Additionally, while we were able to monitor his progress during his inpatient stay, our patient was lost to follow-up after his discharge from the hospital.
If successful, the insufflation technique eliminates the need for urgent open reduction in the operating room. As a result, we recommend attempting closed reduction using insufflation of the knee with sterile saline for irreducible patellar dislocations before proceeding with open reduction.
Conclusion
Saline insufflation of the knee can be safely and easily performed to aid in the reduction of subacute, difficult patellar dislocations.
1. Fu FH, Seel M, Berger RA. Patellofemoral biomechanics. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:49.
2. Dye SF. Patellofemoral anatomy. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:2-3.
3. Li X, Nielsen NM, Zhou H, Stein BS, Shelton YA, Busconi BD. Surgical treatment of a chronically fixed lateral patella dislocation in an adolescent patient. Orthop Rev (Pavia). 2013;5(2):45-47.
4. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
5. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90(12):2751-2762.
6. Panni AS, Vasso M, Cerciello S. Acute patellar dislocation. What to do? Knee Surg Sports Traumatol Arthrosc. 2013;21(2):275-278.
7. Lu DW, Wang EE, Self WH, Kharasch M. Patellar dislocation reduction. Acad Emerg Med. 2010;17(2):226.
8. Michels F, Pouliart N, Oosterlinck D. Locked patellar dislocation: a case report. J Med Case Rep. 2008;2:371.
9. ElMaraghy AW, Berry GK, Kreder HJ. Irreducible lateral patellar dislocation with vertical axis rotation: case report and review of the literature. J Trauma. 2002;53(1):131-132.
10. Wajid MA, Cheema MQ, Siddique MS. Vertical axis patellar dislocation with ipsilateral femoral fracture: use of a closed percutaneous technique for reduction of the dislocation. J Orthop Trauma. 2006;20(2):143-146.
11. Shetty S, Ramesh B, Gul A, Madhusudan TR, Altayeb T. Vertical dislocation of the patella: report of 2 cases. Orthopedics. 2009;32(10). doi: 10.3928/01477447-20090818-25.
12. Hackl W, Benedetto KP, Fink C, Sailer R, Rieger M. Locked lateral patellar dislocation: a rare case of irreducible patellar dislocation requiring open reduction. Knee Surg Sports Traumatol Arthrosc. 1999;7(6):352-355.
13. Gidden DJ, Bell KM. An unusual case of irreducible intra-articular patellar dislocation with vertical axis rotation. Injury. 1995;26(9):643-644.
14. Cooper A. Dislocation of the patella. In: Cooper A, ed. A Treatise on the Dislocations and Fractures of the Joints. Philadelphia, PA: Lea & Febiger; 1844:195.
15. Swanson ER, Seaberg DC, Mathias S. The use of propofol for sedation in the emergency department. Acad Emerg Med. 2008;3(3):234-238.
16. Torry MR, Decker MJ, Millett PJ, Steadman JR, Sterett WI. The effects of knee joint effusion on quadriceps electromyography during jogging. J Sports Sci Med. 2005;4(1):1-8.
As the largest sesamoid bone in the human body, the patella acts as a fulcrum to enhance the biomechanical advantage of the quadriceps in extension.1 It is subject to a variety of forces while improving distribution of forces along the extensor mechanism.2 With sufficient force, the patella can be dislocated. Acute patellar dislocations are the most common knee injury, encompassing 2% to 3% of all knee injuries3 and occurring in 5.8 per 100,000 individuals.4-5 These injuries are associated with acute trauma, frequently from sports and physical activities, occurring while in terminal extension with an axial-valgus stress on the knee during rotation.6
With acute patellar dislocations, patients are usually in significant discomfort. Often, the patella may spontaneously reduce; if not, closed reduction is usually successful with pressure applied anteromedially on the lateral patellar margin, while simultaneously attempting gentle extension of the leg.7 Closed reduction is almost universally successful, and there have only been case reports of irreducible, mainly fixed vertical axis patellar dislocations.8-11 No reports in the literature have described subacute patellar dislocations because of their rarity. Patients present immediately after dislocation, spontaneously reduce, or have a painless, chronically dislocated patella.
We present a case of an elderly man with dementia and a subacute fixed irreducible patellar dislocation, which was reduced using a technique not described in the literature. The patient and the patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 68-year-old nonambulatory man with a history of dementia and stroke presented to the emergency department with complaints of left knee pain and his knee locked in flexion. The patient’s knee had been in that fixed hyperflexed position for at least 10 days after he sustained a twisting injury to his knee while attempting to get out of bed. At baseline, the patient was mostly bedbound and could walk minimally with maximum support, but, given his dementia, he would often attempt to ambulate by himself. After the injury, the patient did not complain of much pain at rest, but attempts at his group home to straighten his leg had caused severe pain. As a result, the patient was brought to the emergency department to be evaluated for fractures.
Physical examination in the emergency department revealed atrophy of the lower extremity musculature and a left knee fixed at 120º in flexion. The skin was intact, and there was minimal effusion of the knee joint. The patella was noted to be laterally subluxated and tender to palpation over the lateral and medial facets. He was neurovascularly intact distally and had painless range of motion of his hips. His contralateral right knee had full range of motion with good patellar tracking.
Radiographs of the patient’s knee confirmed a lateral dislocation of the patella (Figures 1A-1C). After oral and intravenous administration of pain medication, a reduction was attempted without success. Next, an intra-articular knee injection of 10 mL of 1% lidocaine was given. After waiting 15 minutes, another reduction was tried. While the pain control was sufficient, the reduction was again unsuccessful. The knee was insufflated with 120 mL of sterile saline and reduction attempted again. By extending the knee and applying a medially directed force to the patella, reduction was successful. The patient was placed into a knee immobilizer and postreduction radiographs were taken (Figures 2A, 2B). Saline was extracted from the knee. The patient was admitted to the hospital where repeat examination of his knees during the next week revealed markedly less pain. The patient was lost to follow-up.
Discussion
Our patient presumably had a low-energy mechanism of injury, resulting in an undiagnosed patellar dislocation with delayed treatment. This subacute patellar dislocation was irreducible using the standard techniques. Alternatively, insufflation of the joint with saline provided the necessary impetus to allow for successful patellar reduction. The history of the patient reveals clues about the mechanism of injury. It is likely that the patient’s nonambulatory status resulted in a weak vastus medialis muscle that placed the patella at risk for dislocation. While the exact mechanism of dislocation is unknown, the patella was unable to be reduced spontaneously because our patient’s knee was maintained in a state of flexion secondary to pain and muscle contraction. The combination of weak quadriceps musculature, increased Q angle, and forced hyperflexion of the knee prevented closed reduction of the patella.
Fixed, irreducible patellar dislocations are rare and discussed infrequently in the literature.9,11-12 Reported mechanisms are mostly high energy, including blows during athletics and impacts from motor vehicle collisions.9,13 Vertical axis rotation, as first described by Cooper,14 is commonly implicated in irreducible patellar dislocations. This occurs when the patella internally rotates 180º on its vertical axis, associated with a large tear of the medial retinaculum but intact quadriceps tendon. The patella is fixed over the lateral femoral condyle with the articular surface pointing anterolaterally. Despite adequate sedation and analgesia, these are notoriously difficult to close-reduce and may necessitate open reduction.3 Our patient, while having a fixed dislocation, did not have a vertical axis component and, therefore, was amenable to our closed reduction attempt.
Our first reduction attempts were unsuccessful, likely because the patient continued to be tense, firing his quadriceps. Even after injecting the knee with lidocaine and eliminating the pain component, the patella was still impinging on the lateral femoral condyle (Figure 3A). By insufflating the knee with saline, we were able to increase the distance from the patella to the trochlea (Figure 3B). This is comparable to a knee arthroscopy, in which joint fluid pressure allows passage of arthroscopic instruments into the patellofemoral joint. We postulate that the farther the patella is anterior to the trochlea, the higher the likelihood that the patella can be reduced to its anatomic position.
Insufflation of the knee with sterile saline is a novel technique that involves minimal risk compared with the alternatives. Sometimes, for closed reduction to be successful, individuals need to be consciously sedated to relax their muscles and eliminate pain. While conscious sedation is generally considered low risk, complications have been noted, including hypotension, apnea, and retrograde amnesia.15 Manual closed reduction may also cause additional chondral damage when the medial patellar facet contacts the lateral femoral trochlea. When closed reduction of the patella fails, open reduction is required; this inherently includes all the risks of surgery, such as bleeding, infection, neurovascular injury, and wound complications.
Our insufflation technique does not require sedation and is minimally invasive. The saline creates space and provides lubrication to allow for easier manipulation of the patella. This theoretically protects the cartilage as the patella passes over the lateral trochlea. In addition to the intended effect of providing more space and lubrication for the reduction of the patella, insufflation of the joint may also relax the vastus musculature.16 In their study, Torry and colleagues16 injected 13 knees with 20 mL sterile saline and noted reduction in electromyography readings in the vastus medialis and lateralis muscles. This inhibition of vastus musculature may provide enough relaxation to aid in the successful reduction of the patella.
Our study is limited by our sample size of 1. Because acute patellar dislocations are often easily reduced, our technical trick is not frequently used. Additionally, while we were able to monitor his progress during his inpatient stay, our patient was lost to follow-up after his discharge from the hospital.
If successful, the insufflation technique eliminates the need for urgent open reduction in the operating room. As a result, we recommend attempting closed reduction using insufflation of the knee with sterile saline for irreducible patellar dislocations before proceeding with open reduction.
Conclusion
Saline insufflation of the knee can be safely and easily performed to aid in the reduction of subacute, difficult patellar dislocations.
As the largest sesamoid bone in the human body, the patella acts as a fulcrum to enhance the biomechanical advantage of the quadriceps in extension.1 It is subject to a variety of forces while improving distribution of forces along the extensor mechanism.2 With sufficient force, the patella can be dislocated. Acute patellar dislocations are the most common knee injury, encompassing 2% to 3% of all knee injuries3 and occurring in 5.8 per 100,000 individuals.4-5 These injuries are associated with acute trauma, frequently from sports and physical activities, occurring while in terminal extension with an axial-valgus stress on the knee during rotation.6
With acute patellar dislocations, patients are usually in significant discomfort. Often, the patella may spontaneously reduce; if not, closed reduction is usually successful with pressure applied anteromedially on the lateral patellar margin, while simultaneously attempting gentle extension of the leg.7 Closed reduction is almost universally successful, and there have only been case reports of irreducible, mainly fixed vertical axis patellar dislocations.8-11 No reports in the literature have described subacute patellar dislocations because of their rarity. Patients present immediately after dislocation, spontaneously reduce, or have a painless, chronically dislocated patella.
We present a case of an elderly man with dementia and a subacute fixed irreducible patellar dislocation, which was reduced using a technique not described in the literature. The patient and the patient’s guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 68-year-old nonambulatory man with a history of dementia and stroke presented to the emergency department with complaints of left knee pain and his knee locked in flexion. The patient’s knee had been in that fixed hyperflexed position for at least 10 days after he sustained a twisting injury to his knee while attempting to get out of bed. At baseline, the patient was mostly bedbound and could walk minimally with maximum support, but, given his dementia, he would often attempt to ambulate by himself. After the injury, the patient did not complain of much pain at rest, but attempts at his group home to straighten his leg had caused severe pain. As a result, the patient was brought to the emergency department to be evaluated for fractures.
Physical examination in the emergency department revealed atrophy of the lower extremity musculature and a left knee fixed at 120º in flexion. The skin was intact, and there was minimal effusion of the knee joint. The patella was noted to be laterally subluxated and tender to palpation over the lateral and medial facets. He was neurovascularly intact distally and had painless range of motion of his hips. His contralateral right knee had full range of motion with good patellar tracking.
Radiographs of the patient’s knee confirmed a lateral dislocation of the patella (Figures 1A-1C). After oral and intravenous administration of pain medication, a reduction was attempted without success. Next, an intra-articular knee injection of 10 mL of 1% lidocaine was given. After waiting 15 minutes, another reduction was tried. While the pain control was sufficient, the reduction was again unsuccessful. The knee was insufflated with 120 mL of sterile saline and reduction attempted again. By extending the knee and applying a medially directed force to the patella, reduction was successful. The patient was placed into a knee immobilizer and postreduction radiographs were taken (Figures 2A, 2B). Saline was extracted from the knee. The patient was admitted to the hospital where repeat examination of his knees during the next week revealed markedly less pain. The patient was lost to follow-up.
Discussion
Our patient presumably had a low-energy mechanism of injury, resulting in an undiagnosed patellar dislocation with delayed treatment. This subacute patellar dislocation was irreducible using the standard techniques. Alternatively, insufflation of the joint with saline provided the necessary impetus to allow for successful patellar reduction. The history of the patient reveals clues about the mechanism of injury. It is likely that the patient’s nonambulatory status resulted in a weak vastus medialis muscle that placed the patella at risk for dislocation. While the exact mechanism of dislocation is unknown, the patella was unable to be reduced spontaneously because our patient’s knee was maintained in a state of flexion secondary to pain and muscle contraction. The combination of weak quadriceps musculature, increased Q angle, and forced hyperflexion of the knee prevented closed reduction of the patella.
Fixed, irreducible patellar dislocations are rare and discussed infrequently in the literature.9,11-12 Reported mechanisms are mostly high energy, including blows during athletics and impacts from motor vehicle collisions.9,13 Vertical axis rotation, as first described by Cooper,14 is commonly implicated in irreducible patellar dislocations. This occurs when the patella internally rotates 180º on its vertical axis, associated with a large tear of the medial retinaculum but intact quadriceps tendon. The patella is fixed over the lateral femoral condyle with the articular surface pointing anterolaterally. Despite adequate sedation and analgesia, these are notoriously difficult to close-reduce and may necessitate open reduction.3 Our patient, while having a fixed dislocation, did not have a vertical axis component and, therefore, was amenable to our closed reduction attempt.
Our first reduction attempts were unsuccessful, likely because the patient continued to be tense, firing his quadriceps. Even after injecting the knee with lidocaine and eliminating the pain component, the patella was still impinging on the lateral femoral condyle (Figure 3A). By insufflating the knee with saline, we were able to increase the distance from the patella to the trochlea (Figure 3B). This is comparable to a knee arthroscopy, in which joint fluid pressure allows passage of arthroscopic instruments into the patellofemoral joint. We postulate that the farther the patella is anterior to the trochlea, the higher the likelihood that the patella can be reduced to its anatomic position.
Insufflation of the knee with sterile saline is a novel technique that involves minimal risk compared with the alternatives. Sometimes, for closed reduction to be successful, individuals need to be consciously sedated to relax their muscles and eliminate pain. While conscious sedation is generally considered low risk, complications have been noted, including hypotension, apnea, and retrograde amnesia.15 Manual closed reduction may also cause additional chondral damage when the medial patellar facet contacts the lateral femoral trochlea. When closed reduction of the patella fails, open reduction is required; this inherently includes all the risks of surgery, such as bleeding, infection, neurovascular injury, and wound complications.
Our insufflation technique does not require sedation and is minimally invasive. The saline creates space and provides lubrication to allow for easier manipulation of the patella. This theoretically protects the cartilage as the patella passes over the lateral trochlea. In addition to the intended effect of providing more space and lubrication for the reduction of the patella, insufflation of the joint may also relax the vastus musculature.16 In their study, Torry and colleagues16 injected 13 knees with 20 mL sterile saline and noted reduction in electromyography readings in the vastus medialis and lateralis muscles. This inhibition of vastus musculature may provide enough relaxation to aid in the successful reduction of the patella.
Our study is limited by our sample size of 1. Because acute patellar dislocations are often easily reduced, our technical trick is not frequently used. Additionally, while we were able to monitor his progress during his inpatient stay, our patient was lost to follow-up after his discharge from the hospital.
If successful, the insufflation technique eliminates the need for urgent open reduction in the operating room. As a result, we recommend attempting closed reduction using insufflation of the knee with sterile saline for irreducible patellar dislocations before proceeding with open reduction.
Conclusion
Saline insufflation of the knee can be safely and easily performed to aid in the reduction of subacute, difficult patellar dislocations.
1. Fu FH, Seel M, Berger RA. Patellofemoral biomechanics. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:49.
2. Dye SF. Patellofemoral anatomy. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:2-3.
3. Li X, Nielsen NM, Zhou H, Stein BS, Shelton YA, Busconi BD. Surgical treatment of a chronically fixed lateral patella dislocation in an adolescent patient. Orthop Rev (Pavia). 2013;5(2):45-47.
4. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
5. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90(12):2751-2762.
6. Panni AS, Vasso M, Cerciello S. Acute patellar dislocation. What to do? Knee Surg Sports Traumatol Arthrosc. 2013;21(2):275-278.
7. Lu DW, Wang EE, Self WH, Kharasch M. Patellar dislocation reduction. Acad Emerg Med. 2010;17(2):226.
8. Michels F, Pouliart N, Oosterlinck D. Locked patellar dislocation: a case report. J Med Case Rep. 2008;2:371.
9. ElMaraghy AW, Berry GK, Kreder HJ. Irreducible lateral patellar dislocation with vertical axis rotation: case report and review of the literature. J Trauma. 2002;53(1):131-132.
10. Wajid MA, Cheema MQ, Siddique MS. Vertical axis patellar dislocation with ipsilateral femoral fracture: use of a closed percutaneous technique for reduction of the dislocation. J Orthop Trauma. 2006;20(2):143-146.
11. Shetty S, Ramesh B, Gul A, Madhusudan TR, Altayeb T. Vertical dislocation of the patella: report of 2 cases. Orthopedics. 2009;32(10). doi: 10.3928/01477447-20090818-25.
12. Hackl W, Benedetto KP, Fink C, Sailer R, Rieger M. Locked lateral patellar dislocation: a rare case of irreducible patellar dislocation requiring open reduction. Knee Surg Sports Traumatol Arthrosc. 1999;7(6):352-355.
13. Gidden DJ, Bell KM. An unusual case of irreducible intra-articular patellar dislocation with vertical axis rotation. Injury. 1995;26(9):643-644.
14. Cooper A. Dislocation of the patella. In: Cooper A, ed. A Treatise on the Dislocations and Fractures of the Joints. Philadelphia, PA: Lea & Febiger; 1844:195.
15. Swanson ER, Seaberg DC, Mathias S. The use of propofol for sedation in the emergency department. Acad Emerg Med. 2008;3(3):234-238.
16. Torry MR, Decker MJ, Millett PJ, Steadman JR, Sterett WI. The effects of knee joint effusion on quadriceps electromyography during jogging. J Sports Sci Med. 2005;4(1):1-8.
1. Fu FH, Seel M, Berger RA. Patellofemoral biomechanics. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:49.
2. Dye SF. Patellofemoral anatomy. In: Fox J, del Pizzo W, eds. The Patellofemoral Joint. New York, NY: McGraw-Hill; 1993:2-3.
3. Li X, Nielsen NM, Zhou H, Stein BS, Shelton YA, Busconi BD. Surgical treatment of a chronically fixed lateral patella dislocation in an adolescent patient. Orthop Rev (Pavia). 2013;5(2):45-47.
4. Fithian DC, Paxton EW, Stone ML, et al. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32(5):1114-1121.
5. Colvin AC, West RV. Patellar instability. J Bone Joint Surg Am. 2008;90(12):2751-2762.
6. Panni AS, Vasso M, Cerciello S. Acute patellar dislocation. What to do? Knee Surg Sports Traumatol Arthrosc. 2013;21(2):275-278.
7. Lu DW, Wang EE, Self WH, Kharasch M. Patellar dislocation reduction. Acad Emerg Med. 2010;17(2):226.
8. Michels F, Pouliart N, Oosterlinck D. Locked patellar dislocation: a case report. J Med Case Rep. 2008;2:371.
9. ElMaraghy AW, Berry GK, Kreder HJ. Irreducible lateral patellar dislocation with vertical axis rotation: case report and review of the literature. J Trauma. 2002;53(1):131-132.
10. Wajid MA, Cheema MQ, Siddique MS. Vertical axis patellar dislocation with ipsilateral femoral fracture: use of a closed percutaneous technique for reduction of the dislocation. J Orthop Trauma. 2006;20(2):143-146.
11. Shetty S, Ramesh B, Gul A, Madhusudan TR, Altayeb T. Vertical dislocation of the patella: report of 2 cases. Orthopedics. 2009;32(10). doi: 10.3928/01477447-20090818-25.
12. Hackl W, Benedetto KP, Fink C, Sailer R, Rieger M. Locked lateral patellar dislocation: a rare case of irreducible patellar dislocation requiring open reduction. Knee Surg Sports Traumatol Arthrosc. 1999;7(6):352-355.
13. Gidden DJ, Bell KM. An unusual case of irreducible intra-articular patellar dislocation with vertical axis rotation. Injury. 1995;26(9):643-644.
14. Cooper A. Dislocation of the patella. In: Cooper A, ed. A Treatise on the Dislocations and Fractures of the Joints. Philadelphia, PA: Lea & Febiger; 1844:195.
15. Swanson ER, Seaberg DC, Mathias S. The use of propofol for sedation in the emergency department. Acad Emerg Med. 2008;3(3):234-238.
16. Torry MR, Decker MJ, Millett PJ, Steadman JR, Sterett WI. The effects of knee joint effusion on quadriceps electromyography during jogging. J Sports Sci Med. 2005;4(1):1-8.
Total Hip Arthroplasty for Posttraumatic Osteoarthritis of the Hip Fares Worse Than THA for Primary Osteoarthritis
The incidence of hip fractures decreased between 1995 and 2005, but these injuries continue to occur in large numbers. Between 1986 and 2005, the mean annual number of hip fractures was 957.3/100,000, and the majority of these occurred in patients 75 to 84 years old.1 Investigators have described total hip arthroplasty (THA) performed after initial surgical treatment in patients who developed osteoarthritis (OA) of the hip secondary to a fracture.2-7 Only 1 of these studies compared these patients with a control group of patients who had THA for primary hip OA.2 No study included both previous proximal femur and acetabular fractures.
Postfracture OA may occur when there is residual articular incongruity after fracture or osteonecrosis of the femoral head. THA is commonly used to treat OA when more conservative treatments have failed.6 Other indications for conversion to THA include femoral neck nonunion, significant leg-length discrepancy, and femoral head damage caused by previous internal fixation.4
Given these conditions and previous study findings, THA performed in patients with previous hip fracture fixation is potentially more complicated than THA for primary OA. We therefore conducted a study to evaluate differences in sociodemographic factors, surgical details, and outcomes between patients who had THA for posttraumatic OA and patients who had THA for primary OA.
Materials and Methods
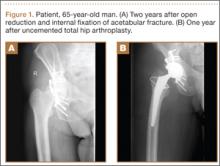
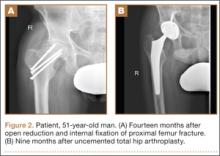
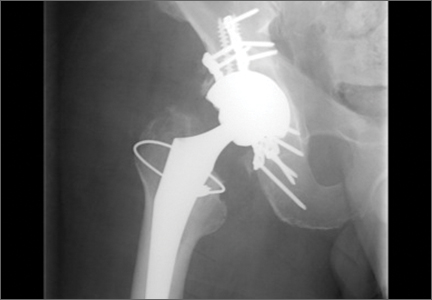
After obtaining institutional review board approval and patient consent, we used a prospective database to follow 3844 patients who had THA performed for OA by 1 of 17 different surgeons at a single center over an 8-year period. Patients who had THA for secondary causes of hip OA, developmental hip dysplasia, or inflammatory processes were excluded. Of the remaining 1199 patients, 62 (5.2%) had THA for posttraumatic OA after previous acetabular or proximal femur fracture fixation (Figures 1, 2) (no THA was performed at time of initial fracture treatment), and 1137 had THA for primary OA and served as the control group.
We collected data on age, sex, fracture location, reason for THA, time between open reduction and internal fixation (ORIF) and THA, type of components, cement use, leg-length discrepancy, intraoperative complications, blood loss, operating room time, and postoperative complications. All patients were aseptic at time of THA. All posttraumatic OA patients had previous hardware removed; the extent of hardware removal was dictated by the exposure required for prosthesis implantation. These patients were contacted, and clinical follow-up was assessed with modified Harris Hip Score (HHS).8 HHS was determined by Dr. Khurana. Statistical analysis was performed with Student t test and Pearson χ2 test using PASW Statistics 18 (SPSS, Chicago, Illinois).
The 62 posttraumatic OA patients had 63 fractures, 41 of the proximal femur (femoral neck and intertrochanteric; 65%) and 22 acetabular (35%). This group consisted of 33 females and 29 males. Their mean age at time of THA surgery was 58 years (range, 31-90 years). Mean age of the control patients was 59.4 years (range, 18-95 years). There were 35 right hips and 27 left hips in the posttrauma group. Mean body mass index (BMI) was 28.4 for the posttrauma group and 28.9 for the control group. There were no differences in age (P = .451), sex (P = .674), or BMI (P = .592) between the 2 groups (Table 1).
All 62 posttraumatic OA patients had complete hospital data, and 32 (52%) of the 62 underwent long-term follow-up (mean, 4.3 years; range, 4 months–10.5 years). At time of attempted contact (mean, 6.79 years after THA), 7 patients were deceased; cause of death was an unrelated medical condition (1) or unknown (6). The rest of the patients did not respond to multiple telephone and mail summons. Primary reasons for conversion to THA included OA (34 patients, 54%), development of osteonecrosis (12 patients, 19%), and nonunion (12 patients, 19%). The rest of the patients had fixation failure. The mechanisms of injury were motor vehicle accidents (30 patients), falls (20), and other causes (15).
Results
Thirty-two (52%) of the posttraumatic OA patients had a preoperative leg-length discrepancy. For these patients, mean time between initial fracture fixation and conversion to THA was 74 months (range, 1-480 months). Four patients required grafting with cancellous autogenous bone graft or allograft chips to fill a bony defect. Mean acetabular component diameter was 54 mm. Nineteen patients had acetabular fixation supplemented with screws. (Screw supplementation data were not recorded for control patients.) Three patients (4.7%) with an acetabular fracture had heterotopic bone removed at time of THA. Two patients underwent neurolysis of the sciatic nerve at time of surgery for preexisting nerve palsy.
Mean postoperative hemoglobin was 109 g/L in the posttraumatic OA group and 121 g/L in the control group (P <. 001). Mean postoperative hematocrit was 0.327 and 0.367, respectively (P < .001). Mean amount of Cell Saver (Haemonetics) used by patients was 176.2 and 72.9 mL, respectively (P < .001). Posttrauma patients lost a mean of 360 mL of blood more than control patients did (P < .001) and were transfused a mean of 1.59 units of blood, compared with 0.85 unit in the controls (P < .001). Patients with acetabular fractures required a mean of only 0.65 unit of transfused blood. Mean operating room time was 240.5 minutes for posttrauma patients and 135.6 minutes for control patients (P < .001). In the posttrauma group, mean size of the head of the femoral component was 29 mm (head size was not recorded for the control group). Posttrauma patients had 18 (29%) hybrid cemented hip replacements (femoral component only) and 44 uncemented hip replacements. Data on femoral stem size and type were not reported for either group.
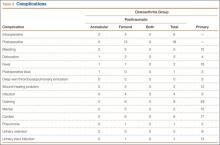
Twenty-four posttrauma patients (39%) had a total of 63 perioperative complications, and 131 control patients (11.5%) had a total of 160 complications (P < .001). Complications in posttrauma patients with proximal femur fractures included excess bleeding (5 patients), in-hospital dislocations (2), and postoperative infections (4: 2 superficial wound infections, 1 implant infection requiring explant, 1 Clostridium difficile infection); in patients with acetabular fractures, there was only 1 dislocation (no infections). The posttraumatic OA group did not develop any symptomatic venous thromboembolic complications. One patient developed a sciatic nerve palsy after surgery. Of the 3 patients who sustained dislocations, 2 were treated with closed reduction and maintenance of implants, and 1 with revision THA. Complications in the control group included 3 infections, 4 dislocations, and 12 cases of extensive blood loss (Table 2).
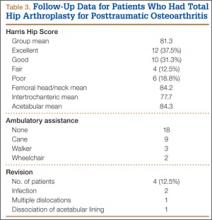
In patients with long-term follow-up, mean postoperative modified HHS was 81.33 (range, 34.1-100.1). Twelve patients had an excellent score (>90), 10 a good score (80-89), 4 a fair score (70-79), and 6 a poor score (<70). Mean HHS was 84.2 for the 16 patients with a femoral head or neck fracture, 77.7 for the 6 patients with an intertrochanteric fracture, and 84.3 for the 9 patients with an acetabular fracture. Nine patients reported using a cane, 3 required walkers, 2 required wheelchairs, and 18 did not require any walking support. Four (12.5%) of the 32 patients required THA revision a mean of 3.5 years (range, 2 months–8 years) after initial arthroplasty. Reasons for revision were infections (2 patients), multiple dislocations (1), and dissociation of acetabular lining (1) (Table 3). Two of the patients who underwent THA revision had a cemented femoral stem, and 2 did not have any cemented implants. Additional details of the femoral stem components were not available for either group.
Discussion
Patients who develop posttraumatic OA of the hip have limited options. THA has emerged as an excellent option in cases of failed repair of fractures about the hip joint. The results of the present study are consistent with earlier findings of the effectiveness of THA in salvaging posttraumatic hips.2-7 THA for patients with posttraumatic arthritis of the hip after acetabular or proximal femur fracture is longer and more complicated than THA for primary OA, and there is significantly more blood loss. In addition, the rate of early failure appears to be higher.9
In this study, mean amount of blood transfused for patients with previous acetabular fracture was 0.65 unit, much less than the mean of 3.5 units noted by Weber and colleagues.6 In their study, complications associated with THA were increased in patients with posttraumatic OA from acetabular fractures. The authors attributed these complications to scarring from previous surgery, retained hardware, heterotopic bone, and residual osseous deformity and deficiency. Our results support their conclusion. Operating times were longer, as well as blood loss and the need for blood transfusions and other blood products were increased in the patients with posttraumatic OA, as compared with patients with primary OA. Fifteen percent of patients with an acetabular fracture had undergone removal of heterotopic bone at time of surgery—similar to the rate of 18% noted in the Weber study.6
Our results showed that the rate of revision THA was also higher than in patients with primary THA within the general population—reported to be about 4%.9 The higher rate may be the result of the additional surgeries performed on patients with fractures, or hardware retention increasing the infection risk over the years. Our revision rate of 12.5% was similar to the 19% found by Ranawat and colleagues7 in their study.
A majority of the patients in our study had favorable long-term HHS. Mean overall HHS was 83, slightly better than the 79 reported by Srivastav and colleagues.4 We found that patients with intertrochanteric fractures ultimately had worse outcome scores than patients with acetabular or femoral neck fractures. These results are consistent with findings reported by Mehlhoff and colleagues5 in a study comparing patients with femoral neck and intertrochanteric fractures. Mean HHS for the intertrochanteric fracture patients in our study was 77.7, comparable to the mean of 78 reported by Mehlhoff and colleagues.5 Mean HHS for the femoral neck or head fractures in our study was 84.2, similar to the mean of 81 they noted. Patients with a previous acetabular fracture in our study had a mean HHS of 84.3, consistent with the 84 reported by Ranawat and colleagues7 for patients who had initially undergone ORIF for acetabular fracture. Mean HHS in our study (83) was slightly less than the 88.5 reported by Shi and colleagues10 in their study of primary THAs.
Few studies have been conducted exclusively on one type of hip fracture (acetabular) or another (proximal femur), and all except 1 did not perform a comparison. Tabsh and colleagues2 compared similar cohorts but focused solely on patients with previous proximal femur fractures. The present study included a control group and both acetabular and proximal femur fractures, which allowed us to compare patients with and without previous fracture fixation and to consider the 2 different fracture types and see if they affected outcomes.
The strengths of this study include the large control group and the relatively short data-collection period. The shorter period decreased the influence of improvements in implants on patient outcomes. In addition, the control group was our own population, as we did not compare our cohort of patients with previous internal fixation and patients who had primary THAs in other studies, aside from comparisons for revision rates and HHS.
Although the ultimate long-term follow-up rate for patients with previous internal fixation was 50%, our sample size was still larger than that in most reported studies. Another weakness of our study was the large number of surgeons (17), representing an array of techniques, approaches, and surgical experience. All these factors could have influenced patient outcomes and operative data. In addition, data on revision rates and HHS were not available for our control group, so we could not directly compare these outcomes with those of the posttraumatic group. However, we used previously reported data on revision rates and HHS in primary THAs for comparison with the posttraumatic group.9,10
Conclusion
In this study, THA was a viable option for patients with posttraumatic arthritis from a previous acetabular or proximal femur fracture. The outcomes, however, were less reliable than the outcomes of primary THA for degenerative arthritis, and the complication rates were higher. Surgeons should counsel patients about the complexity of the procedure as well as its ultimately favorable outcomes. Surgeons should expect additional technical difficulties in the operating room when treating this patient population.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573-1579.
2. Tabsh I, Waddell JP, Morton J. Total hip arthroplasty for complications of proximal femoral fractures. J Orthop Trauma. 1997;11(3):166-169.
3. Haidukewych GJ, Berry DJ. Hip arthroplasty for salvage of failed treatment of intertrochanteric hip fractures. J Bone Joint Surg Am. 2003;85(5):899-904.
4. Srivastav S, Mittal V, Agarwal S. Total hip arthroplasty following failed fixation of proximal hip fractures. Indian J Orthop. 2008;42(3):279-286.
5. Mehlhoff T, Landon GC, Tullos HS. Total hip arthroplasty following failed internal fixation of hip fractures. Clin Orthop Relat Res. 1991;(269):32-37.
6. Weber M, Berry DJ, Harmsen WS. Total hip arthroplasty after operative treatment of an acetabular fracture. J Bone Joint Surg Am. 1998;80(9):1295-1305.
7. Ranawat A, Zelken J, Helfet D, Buly R. Total hip arthroplasty for posttraumatic arthritis after acetabular fracture. J Arthroplasty. 2009;24(5):759-767.
8. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
9. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
10. Shi HY, Mau LW, Chang JK, Wang JW, Chiu HC. Responsiveness of the Harris Hip Score and the SF-36: five years after total hip arthroplasty. Qual Life Res. 2009;18(8):1053-1060.
The incidence of hip fractures decreased between 1995 and 2005, but these injuries continue to occur in large numbers. Between 1986 and 2005, the mean annual number of hip fractures was 957.3/100,000, and the majority of these occurred in patients 75 to 84 years old.1 Investigators have described total hip arthroplasty (THA) performed after initial surgical treatment in patients who developed osteoarthritis (OA) of the hip secondary to a fracture.2-7 Only 1 of these studies compared these patients with a control group of patients who had THA for primary hip OA.2 No study included both previous proximal femur and acetabular fractures.
Postfracture OA may occur when there is residual articular incongruity after fracture or osteonecrosis of the femoral head. THA is commonly used to treat OA when more conservative treatments have failed.6 Other indications for conversion to THA include femoral neck nonunion, significant leg-length discrepancy, and femoral head damage caused by previous internal fixation.4
Given these conditions and previous study findings, THA performed in patients with previous hip fracture fixation is potentially more complicated than THA for primary OA. We therefore conducted a study to evaluate differences in sociodemographic factors, surgical details, and outcomes between patients who had THA for posttraumatic OA and patients who had THA for primary OA.
Materials and Methods
After obtaining institutional review board approval and patient consent, we used a prospective database to follow 3844 patients who had THA performed for OA by 1 of 17 different surgeons at a single center over an 8-year period. Patients who had THA for secondary causes of hip OA, developmental hip dysplasia, or inflammatory processes were excluded. Of the remaining 1199 patients, 62 (5.2%) had THA for posttraumatic OA after previous acetabular or proximal femur fracture fixation (Figures 1, 2) (no THA was performed at time of initial fracture treatment), and 1137 had THA for primary OA and served as the control group.
We collected data on age, sex, fracture location, reason for THA, time between open reduction and internal fixation (ORIF) and THA, type of components, cement use, leg-length discrepancy, intraoperative complications, blood loss, operating room time, and postoperative complications. All patients were aseptic at time of THA. All posttraumatic OA patients had previous hardware removed; the extent of hardware removal was dictated by the exposure required for prosthesis implantation. These patients were contacted, and clinical follow-up was assessed with modified Harris Hip Score (HHS).8 HHS was determined by Dr. Khurana. Statistical analysis was performed with Student t test and Pearson χ2 test using PASW Statistics 18 (SPSS, Chicago, Illinois).
The 62 posttraumatic OA patients had 63 fractures, 41 of the proximal femur (femoral neck and intertrochanteric; 65%) and 22 acetabular (35%). This group consisted of 33 females and 29 males. Their mean age at time of THA surgery was 58 years (range, 31-90 years). Mean age of the control patients was 59.4 years (range, 18-95 years). There were 35 right hips and 27 left hips in the posttrauma group. Mean body mass index (BMI) was 28.4 for the posttrauma group and 28.9 for the control group. There were no differences in age (P = .451), sex (P = .674), or BMI (P = .592) between the 2 groups (Table 1).
All 62 posttraumatic OA patients had complete hospital data, and 32 (52%) of the 62 underwent long-term follow-up (mean, 4.3 years; range, 4 months–10.5 years). At time of attempted contact (mean, 6.79 years after THA), 7 patients were deceased; cause of death was an unrelated medical condition (1) or unknown (6). The rest of the patients did not respond to multiple telephone and mail summons. Primary reasons for conversion to THA included OA (34 patients, 54%), development of osteonecrosis (12 patients, 19%), and nonunion (12 patients, 19%). The rest of the patients had fixation failure. The mechanisms of injury were motor vehicle accidents (30 patients), falls (20), and other causes (15).
Results
Thirty-two (52%) of the posttraumatic OA patients had a preoperative leg-length discrepancy. For these patients, mean time between initial fracture fixation and conversion to THA was 74 months (range, 1-480 months). Four patients required grafting with cancellous autogenous bone graft or allograft chips to fill a bony defect. Mean acetabular component diameter was 54 mm. Nineteen patients had acetabular fixation supplemented with screws. (Screw supplementation data were not recorded for control patients.) Three patients (4.7%) with an acetabular fracture had heterotopic bone removed at time of THA. Two patients underwent neurolysis of the sciatic nerve at time of surgery for preexisting nerve palsy.
Mean postoperative hemoglobin was 109 g/L in the posttraumatic OA group and 121 g/L in the control group (P <. 001). Mean postoperative hematocrit was 0.327 and 0.367, respectively (P < .001). Mean amount of Cell Saver (Haemonetics) used by patients was 176.2 and 72.9 mL, respectively (P < .001). Posttrauma patients lost a mean of 360 mL of blood more than control patients did (P < .001) and were transfused a mean of 1.59 units of blood, compared with 0.85 unit in the controls (P < .001). Patients with acetabular fractures required a mean of only 0.65 unit of transfused blood. Mean operating room time was 240.5 minutes for posttrauma patients and 135.6 minutes for control patients (P < .001). In the posttrauma group, mean size of the head of the femoral component was 29 mm (head size was not recorded for the control group). Posttrauma patients had 18 (29%) hybrid cemented hip replacements (femoral component only) and 44 uncemented hip replacements. Data on femoral stem size and type were not reported for either group.
Twenty-four posttrauma patients (39%) had a total of 63 perioperative complications, and 131 control patients (11.5%) had a total of 160 complications (P < .001). Complications in posttrauma patients with proximal femur fractures included excess bleeding (5 patients), in-hospital dislocations (2), and postoperative infections (4: 2 superficial wound infections, 1 implant infection requiring explant, 1 Clostridium difficile infection); in patients with acetabular fractures, there was only 1 dislocation (no infections). The posttraumatic OA group did not develop any symptomatic venous thromboembolic complications. One patient developed a sciatic nerve palsy after surgery. Of the 3 patients who sustained dislocations, 2 were treated with closed reduction and maintenance of implants, and 1 with revision THA. Complications in the control group included 3 infections, 4 dislocations, and 12 cases of extensive blood loss (Table 2).
In patients with long-term follow-up, mean postoperative modified HHS was 81.33 (range, 34.1-100.1). Twelve patients had an excellent score (>90), 10 a good score (80-89), 4 a fair score (70-79), and 6 a poor score (<70). Mean HHS was 84.2 for the 16 patients with a femoral head or neck fracture, 77.7 for the 6 patients with an intertrochanteric fracture, and 84.3 for the 9 patients with an acetabular fracture. Nine patients reported using a cane, 3 required walkers, 2 required wheelchairs, and 18 did not require any walking support. Four (12.5%) of the 32 patients required THA revision a mean of 3.5 years (range, 2 months–8 years) after initial arthroplasty. Reasons for revision were infections (2 patients), multiple dislocations (1), and dissociation of acetabular lining (1) (Table 3). Two of the patients who underwent THA revision had a cemented femoral stem, and 2 did not have any cemented implants. Additional details of the femoral stem components were not available for either group.
Discussion
Patients who develop posttraumatic OA of the hip have limited options. THA has emerged as an excellent option in cases of failed repair of fractures about the hip joint. The results of the present study are consistent with earlier findings of the effectiveness of THA in salvaging posttraumatic hips.2-7 THA for patients with posttraumatic arthritis of the hip after acetabular or proximal femur fracture is longer and more complicated than THA for primary OA, and there is significantly more blood loss. In addition, the rate of early failure appears to be higher.9
In this study, mean amount of blood transfused for patients with previous acetabular fracture was 0.65 unit, much less than the mean of 3.5 units noted by Weber and colleagues.6 In their study, complications associated with THA were increased in patients with posttraumatic OA from acetabular fractures. The authors attributed these complications to scarring from previous surgery, retained hardware, heterotopic bone, and residual osseous deformity and deficiency. Our results support their conclusion. Operating times were longer, as well as blood loss and the need for blood transfusions and other blood products were increased in the patients with posttraumatic OA, as compared with patients with primary OA. Fifteen percent of patients with an acetabular fracture had undergone removal of heterotopic bone at time of surgery—similar to the rate of 18% noted in the Weber study.6
Our results showed that the rate of revision THA was also higher than in patients with primary THA within the general population—reported to be about 4%.9 The higher rate may be the result of the additional surgeries performed on patients with fractures, or hardware retention increasing the infection risk over the years. Our revision rate of 12.5% was similar to the 19% found by Ranawat and colleagues7 in their study.
A majority of the patients in our study had favorable long-term HHS. Mean overall HHS was 83, slightly better than the 79 reported by Srivastav and colleagues.4 We found that patients with intertrochanteric fractures ultimately had worse outcome scores than patients with acetabular or femoral neck fractures. These results are consistent with findings reported by Mehlhoff and colleagues5 in a study comparing patients with femoral neck and intertrochanteric fractures. Mean HHS for the intertrochanteric fracture patients in our study was 77.7, comparable to the mean of 78 reported by Mehlhoff and colleagues.5 Mean HHS for the femoral neck or head fractures in our study was 84.2, similar to the mean of 81 they noted. Patients with a previous acetabular fracture in our study had a mean HHS of 84.3, consistent with the 84 reported by Ranawat and colleagues7 for patients who had initially undergone ORIF for acetabular fracture. Mean HHS in our study (83) was slightly less than the 88.5 reported by Shi and colleagues10 in their study of primary THAs.
Few studies have been conducted exclusively on one type of hip fracture (acetabular) or another (proximal femur), and all except 1 did not perform a comparison. Tabsh and colleagues2 compared similar cohorts but focused solely on patients with previous proximal femur fractures. The present study included a control group and both acetabular and proximal femur fractures, which allowed us to compare patients with and without previous fracture fixation and to consider the 2 different fracture types and see if they affected outcomes.
The strengths of this study include the large control group and the relatively short data-collection period. The shorter period decreased the influence of improvements in implants on patient outcomes. In addition, the control group was our own population, as we did not compare our cohort of patients with previous internal fixation and patients who had primary THAs in other studies, aside from comparisons for revision rates and HHS.
Although the ultimate long-term follow-up rate for patients with previous internal fixation was 50%, our sample size was still larger than that in most reported studies. Another weakness of our study was the large number of surgeons (17), representing an array of techniques, approaches, and surgical experience. All these factors could have influenced patient outcomes and operative data. In addition, data on revision rates and HHS were not available for our control group, so we could not directly compare these outcomes with those of the posttraumatic group. However, we used previously reported data on revision rates and HHS in primary THAs for comparison with the posttraumatic group.9,10
Conclusion
In this study, THA was a viable option for patients with posttraumatic arthritis from a previous acetabular or proximal femur fracture. The outcomes, however, were less reliable than the outcomes of primary THA for degenerative arthritis, and the complication rates were higher. Surgeons should counsel patients about the complexity of the procedure as well as its ultimately favorable outcomes. Surgeons should expect additional technical difficulties in the operating room when treating this patient population.
The incidence of hip fractures decreased between 1995 and 2005, but these injuries continue to occur in large numbers. Between 1986 and 2005, the mean annual number of hip fractures was 957.3/100,000, and the majority of these occurred in patients 75 to 84 years old.1 Investigators have described total hip arthroplasty (THA) performed after initial surgical treatment in patients who developed osteoarthritis (OA) of the hip secondary to a fracture.2-7 Only 1 of these studies compared these patients with a control group of patients who had THA for primary hip OA.2 No study included both previous proximal femur and acetabular fractures.
Postfracture OA may occur when there is residual articular incongruity after fracture or osteonecrosis of the femoral head. THA is commonly used to treat OA when more conservative treatments have failed.6 Other indications for conversion to THA include femoral neck nonunion, significant leg-length discrepancy, and femoral head damage caused by previous internal fixation.4
Given these conditions and previous study findings, THA performed in patients with previous hip fracture fixation is potentially more complicated than THA for primary OA. We therefore conducted a study to evaluate differences in sociodemographic factors, surgical details, and outcomes between patients who had THA for posttraumatic OA and patients who had THA for primary OA.
Materials and Methods
After obtaining institutional review board approval and patient consent, we used a prospective database to follow 3844 patients who had THA performed for OA by 1 of 17 different surgeons at a single center over an 8-year period. Patients who had THA for secondary causes of hip OA, developmental hip dysplasia, or inflammatory processes were excluded. Of the remaining 1199 patients, 62 (5.2%) had THA for posttraumatic OA after previous acetabular or proximal femur fracture fixation (Figures 1, 2) (no THA was performed at time of initial fracture treatment), and 1137 had THA for primary OA and served as the control group.
We collected data on age, sex, fracture location, reason for THA, time between open reduction and internal fixation (ORIF) and THA, type of components, cement use, leg-length discrepancy, intraoperative complications, blood loss, operating room time, and postoperative complications. All patients were aseptic at time of THA. All posttraumatic OA patients had previous hardware removed; the extent of hardware removal was dictated by the exposure required for prosthesis implantation. These patients were contacted, and clinical follow-up was assessed with modified Harris Hip Score (HHS).8 HHS was determined by Dr. Khurana. Statistical analysis was performed with Student t test and Pearson χ2 test using PASW Statistics 18 (SPSS, Chicago, Illinois).
The 62 posttraumatic OA patients had 63 fractures, 41 of the proximal femur (femoral neck and intertrochanteric; 65%) and 22 acetabular (35%). This group consisted of 33 females and 29 males. Their mean age at time of THA surgery was 58 years (range, 31-90 years). Mean age of the control patients was 59.4 years (range, 18-95 years). There were 35 right hips and 27 left hips in the posttrauma group. Mean body mass index (BMI) was 28.4 for the posttrauma group and 28.9 for the control group. There were no differences in age (P = .451), sex (P = .674), or BMI (P = .592) between the 2 groups (Table 1).
All 62 posttraumatic OA patients had complete hospital data, and 32 (52%) of the 62 underwent long-term follow-up (mean, 4.3 years; range, 4 months–10.5 years). At time of attempted contact (mean, 6.79 years after THA), 7 patients were deceased; cause of death was an unrelated medical condition (1) or unknown (6). The rest of the patients did not respond to multiple telephone and mail summons. Primary reasons for conversion to THA included OA (34 patients, 54%), development of osteonecrosis (12 patients, 19%), and nonunion (12 patients, 19%). The rest of the patients had fixation failure. The mechanisms of injury were motor vehicle accidents (30 patients), falls (20), and other causes (15).
Results
Thirty-two (52%) of the posttraumatic OA patients had a preoperative leg-length discrepancy. For these patients, mean time between initial fracture fixation and conversion to THA was 74 months (range, 1-480 months). Four patients required grafting with cancellous autogenous bone graft or allograft chips to fill a bony defect. Mean acetabular component diameter was 54 mm. Nineteen patients had acetabular fixation supplemented with screws. (Screw supplementation data were not recorded for control patients.) Three patients (4.7%) with an acetabular fracture had heterotopic bone removed at time of THA. Two patients underwent neurolysis of the sciatic nerve at time of surgery for preexisting nerve palsy.
Mean postoperative hemoglobin was 109 g/L in the posttraumatic OA group and 121 g/L in the control group (P <. 001). Mean postoperative hematocrit was 0.327 and 0.367, respectively (P < .001). Mean amount of Cell Saver (Haemonetics) used by patients was 176.2 and 72.9 mL, respectively (P < .001). Posttrauma patients lost a mean of 360 mL of blood more than control patients did (P < .001) and were transfused a mean of 1.59 units of blood, compared with 0.85 unit in the controls (P < .001). Patients with acetabular fractures required a mean of only 0.65 unit of transfused blood. Mean operating room time was 240.5 minutes for posttrauma patients and 135.6 minutes for control patients (P < .001). In the posttrauma group, mean size of the head of the femoral component was 29 mm (head size was not recorded for the control group). Posttrauma patients had 18 (29%) hybrid cemented hip replacements (femoral component only) and 44 uncemented hip replacements. Data on femoral stem size and type were not reported for either group.
Twenty-four posttrauma patients (39%) had a total of 63 perioperative complications, and 131 control patients (11.5%) had a total of 160 complications (P < .001). Complications in posttrauma patients with proximal femur fractures included excess bleeding (5 patients), in-hospital dislocations (2), and postoperative infections (4: 2 superficial wound infections, 1 implant infection requiring explant, 1 Clostridium difficile infection); in patients with acetabular fractures, there was only 1 dislocation (no infections). The posttraumatic OA group did not develop any symptomatic venous thromboembolic complications. One patient developed a sciatic nerve palsy after surgery. Of the 3 patients who sustained dislocations, 2 were treated with closed reduction and maintenance of implants, and 1 with revision THA. Complications in the control group included 3 infections, 4 dislocations, and 12 cases of extensive blood loss (Table 2).
In patients with long-term follow-up, mean postoperative modified HHS was 81.33 (range, 34.1-100.1). Twelve patients had an excellent score (>90), 10 a good score (80-89), 4 a fair score (70-79), and 6 a poor score (<70). Mean HHS was 84.2 for the 16 patients with a femoral head or neck fracture, 77.7 for the 6 patients with an intertrochanteric fracture, and 84.3 for the 9 patients with an acetabular fracture. Nine patients reported using a cane, 3 required walkers, 2 required wheelchairs, and 18 did not require any walking support. Four (12.5%) of the 32 patients required THA revision a mean of 3.5 years (range, 2 months–8 years) after initial arthroplasty. Reasons for revision were infections (2 patients), multiple dislocations (1), and dissociation of acetabular lining (1) (Table 3). Two of the patients who underwent THA revision had a cemented femoral stem, and 2 did not have any cemented implants. Additional details of the femoral stem components were not available for either group.
Discussion
Patients who develop posttraumatic OA of the hip have limited options. THA has emerged as an excellent option in cases of failed repair of fractures about the hip joint. The results of the present study are consistent with earlier findings of the effectiveness of THA in salvaging posttraumatic hips.2-7 THA for patients with posttraumatic arthritis of the hip after acetabular or proximal femur fracture is longer and more complicated than THA for primary OA, and there is significantly more blood loss. In addition, the rate of early failure appears to be higher.9
In this study, mean amount of blood transfused for patients with previous acetabular fracture was 0.65 unit, much less than the mean of 3.5 units noted by Weber and colleagues.6 In their study, complications associated with THA were increased in patients with posttraumatic OA from acetabular fractures. The authors attributed these complications to scarring from previous surgery, retained hardware, heterotopic bone, and residual osseous deformity and deficiency. Our results support their conclusion. Operating times were longer, as well as blood loss and the need for blood transfusions and other blood products were increased in the patients with posttraumatic OA, as compared with patients with primary OA. Fifteen percent of patients with an acetabular fracture had undergone removal of heterotopic bone at time of surgery—similar to the rate of 18% noted in the Weber study.6
Our results showed that the rate of revision THA was also higher than in patients with primary THA within the general population—reported to be about 4%.9 The higher rate may be the result of the additional surgeries performed on patients with fractures, or hardware retention increasing the infection risk over the years. Our revision rate of 12.5% was similar to the 19% found by Ranawat and colleagues7 in their study.
A majority of the patients in our study had favorable long-term HHS. Mean overall HHS was 83, slightly better than the 79 reported by Srivastav and colleagues.4 We found that patients with intertrochanteric fractures ultimately had worse outcome scores than patients with acetabular or femoral neck fractures. These results are consistent with findings reported by Mehlhoff and colleagues5 in a study comparing patients with femoral neck and intertrochanteric fractures. Mean HHS for the intertrochanteric fracture patients in our study was 77.7, comparable to the mean of 78 reported by Mehlhoff and colleagues.5 Mean HHS for the femoral neck or head fractures in our study was 84.2, similar to the mean of 81 they noted. Patients with a previous acetabular fracture in our study had a mean HHS of 84.3, consistent with the 84 reported by Ranawat and colleagues7 for patients who had initially undergone ORIF for acetabular fracture. Mean HHS in our study (83) was slightly less than the 88.5 reported by Shi and colleagues10 in their study of primary THAs.
Few studies have been conducted exclusively on one type of hip fracture (acetabular) or another (proximal femur), and all except 1 did not perform a comparison. Tabsh and colleagues2 compared similar cohorts but focused solely on patients with previous proximal femur fractures. The present study included a control group and both acetabular and proximal femur fractures, which allowed us to compare patients with and without previous fracture fixation and to consider the 2 different fracture types and see if they affected outcomes.
The strengths of this study include the large control group and the relatively short data-collection period. The shorter period decreased the influence of improvements in implants on patient outcomes. In addition, the control group was our own population, as we did not compare our cohort of patients with previous internal fixation and patients who had primary THAs in other studies, aside from comparisons for revision rates and HHS.
Although the ultimate long-term follow-up rate for patients with previous internal fixation was 50%, our sample size was still larger than that in most reported studies. Another weakness of our study was the large number of surgeons (17), representing an array of techniques, approaches, and surgical experience. All these factors could have influenced patient outcomes and operative data. In addition, data on revision rates and HHS were not available for our control group, so we could not directly compare these outcomes with those of the posttraumatic group. However, we used previously reported data on revision rates and HHS in primary THAs for comparison with the posttraumatic group.9,10
Conclusion
In this study, THA was a viable option for patients with posttraumatic arthritis from a previous acetabular or proximal femur fracture. The outcomes, however, were less reliable than the outcomes of primary THA for degenerative arthritis, and the complication rates were higher. Surgeons should counsel patients about the complexity of the procedure as well as its ultimately favorable outcomes. Surgeons should expect additional technical difficulties in the operating room when treating this patient population.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573-1579.
2. Tabsh I, Waddell JP, Morton J. Total hip arthroplasty for complications of proximal femoral fractures. J Orthop Trauma. 1997;11(3):166-169.
3. Haidukewych GJ, Berry DJ. Hip arthroplasty for salvage of failed treatment of intertrochanteric hip fractures. J Bone Joint Surg Am. 2003;85(5):899-904.
4. Srivastav S, Mittal V, Agarwal S. Total hip arthroplasty following failed fixation of proximal hip fractures. Indian J Orthop. 2008;42(3):279-286.
5. Mehlhoff T, Landon GC, Tullos HS. Total hip arthroplasty following failed internal fixation of hip fractures. Clin Orthop Relat Res. 1991;(269):32-37.
6. Weber M, Berry DJ, Harmsen WS. Total hip arthroplasty after operative treatment of an acetabular fracture. J Bone Joint Surg Am. 1998;80(9):1295-1305.
7. Ranawat A, Zelken J, Helfet D, Buly R. Total hip arthroplasty for posttraumatic arthritis after acetabular fracture. J Arthroplasty. 2009;24(5):759-767.
8. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
9. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
10. Shi HY, Mau LW, Chang JK, Wang JW, Chiu HC. Responsiveness of the Harris Hip Score and the SF-36: five years after total hip arthroplasty. Qual Life Res. 2009;18(8):1053-1060.
1. Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573-1579.
2. Tabsh I, Waddell JP, Morton J. Total hip arthroplasty for complications of proximal femoral fractures. J Orthop Trauma. 1997;11(3):166-169.
3. Haidukewych GJ, Berry DJ. Hip arthroplasty for salvage of failed treatment of intertrochanteric hip fractures. J Bone Joint Surg Am. 2003;85(5):899-904.
4. Srivastav S, Mittal V, Agarwal S. Total hip arthroplasty following failed fixation of proximal hip fractures. Indian J Orthop. 2008;42(3):279-286.
5. Mehlhoff T, Landon GC, Tullos HS. Total hip arthroplasty following failed internal fixation of hip fractures. Clin Orthop Relat Res. 1991;(269):32-37.
6. Weber M, Berry DJ, Harmsen WS. Total hip arthroplasty after operative treatment of an acetabular fracture. J Bone Joint Surg Am. 1998;80(9):1295-1305.
7. Ranawat A, Zelken J, Helfet D, Buly R. Total hip arthroplasty for posttraumatic arthritis after acetabular fracture. J Arthroplasty. 2009;24(5):759-767.
8. Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51(4):737-755.
9. Mahomed NN, Barrett JA, Katz JN, et al. Rates and outcomes of primary and revision total hip replacement in the United States Medicare population. J Bone Joint Surg Am. 2003;85(1):27-32.
10. Shi HY, Mau LW, Chang JK, Wang JW, Chiu HC. Responsiveness of the Harris Hip Score and the SF-36: five years after total hip arthroplasty. Qual Life Res. 2009;18(8):1053-1060.