User login
Total Hip Arthroplasty After Proximal Femoral Osteotomy: A Technique That Can Be Used to Address Presence of a Retained Intracortical Plate
Total hip arthroplasty (THA) is an effective treatment for advanced hip arthritis from a variety of causes, including osteoarthritis, inflammatory arthritis, posttraumatic arthritis, and sequelae of developmental disorders. It is not uncommon to perform THA in the presence of a previous proximal femoral osteotomy that may have been performed for slipped capital femoral epiphysis (SCFE), Legg-Calvé-Perthes disease, or developmental dysplasia of the hip, among other conditions. These osteotomies are commonly combined with internal fixation, a plate-and-screw device. These patients are at risk for developing degenerative arthritis at an earlier age than patients with other types of arthritis and subsequently may undergo THA at a younger age.1-3 Presence of a plate can pose a technical challenge during THA surgery. THA performed after intertrochanteric osteotomy has higher rates of perioperative and postoperative complications.4 Ferguson and colleagues4 noted difficulty during hardware removal in 24% of cases. Among the complications encountered were broken hardware, stripped screws, greater trochanteric fracture, stress risers from previous screw holes, canal narrowing from endosteal hypertrophy around hardware, and lateral cortical deficiency after removal of the side plate. As intertrochanteric osteotomies are often performed in patients who have yet to reach skeletal maturity, cortical hypertrophy can lead to complete coverage of the side plate and an “intracortical” position.
This article reports on 2 THA cases in which a technique was used to avoid intracortical plate removal and the resulting problems of lateral cortical deficiency. During each THA, the plate was left in place to avoid compromise of the lateral femoral cortex. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
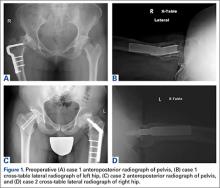
An adolescent with bilateral SCFE was treated first with internal fixation of the right hip and subsequently with left proximal femoral osteotomy with internal fixation. He did well until age 31 years, when he developed progressively worsening pain about the left hip. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the left hip. Radiographs showed a sliding hip screw in place, with proximal femoral deformity consisting of femoral neck shortening and posterior angulation (Figures 1A, 1B). Preoperative Harris Hip Score was 54.5.
Case 2
A 51-year-old woman presented with a history of right hip problems dating back to age 13 years, when she sustained a fracture of the right hip and was treated with internal fixation. At age 15 years, she underwent proximal femoral osteotomy to correct residual deformity. She did well until age 45 years, when she developed worsening hip symptoms. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the right hip. Radiographs showed a fixed-angle blade plate in the proximal femur, with significant proximal femoral deformity (Figures 1C, 1D). Preoperative Harris Hip Score was 53.6.
Surgical Technique
In both cases, a standard series of radiographs was obtained—an anteroposterior (AP) radiograph of the pelvis and AP and cross-table lateral radiographs of the operative hip (Figure 1). Computed tomography (CT) with a metal-artifact-reducing technique may be useful in determining amount of cortical bone remaining under the plate. CT showed limited lateral cortex beneath the side plate and bony overgrowth covering the side plate. Preoperative templating was performed using previously described techniques.5
During THA, before removing any portion of any retained hardware, the surgeon should perform 3 important actions: Dislocate the hip, perform all appropriate capsular releases, and reduce the hip. Dislocating the hip before hardware removal significantly decreases the risk for fracture caused by stress risers, as the force required for dislocation is much more controlled because of the capsular releases. After hardware removal, the hip can be easily redislocated, and the femoral neck osteotomy can be performed.
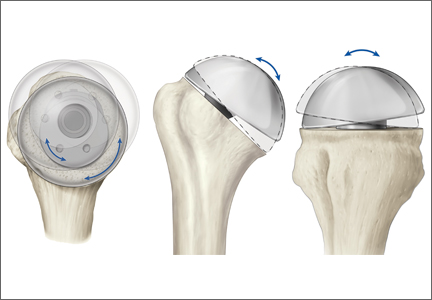
When plate and screws are in an intracortical position, the screws can be removed only after removing the small shell of cortical bone covering them. The amount of bone to be removed is minimal. After the screws are removed, the plate remains in place. A motorized device with a metal-cutting attachment is used to transect the construct at the junction of the plate and barrel (case 1) or at the bend of a fixed-angle device (case 2). Laparotomy sponges are placed around the proximal femur to minimize the amount of soft tissue that could be exposed to metal shavings. Copious irrigation is used throughout this part of the procedure. Osteotomes are used to elevate the proximal portion of the plate and the barrel, preserving the distal portion of the plate on the lateral cortex of the femoral shaft.
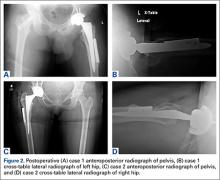
After the head is removed, the rest of the THA can be performed using standard press-fit insertion technique (Figures 2A-2D). Care must be taken to ensure that the distal aspect of the femoral stem bypasses the most distal screw hole by at least 2 cortical diameters in order to reduce the risk for periprosthetic fracture.
By 2-year follow-up, both patients had regained excellent range of motion, ambulation, and overall function. Postoperative Harris Hip Scores were 86.6 and 83.8, respectively. There were no radiographic signs of complications.
Discussion
THA can be challenging in the setting of previously placed internal fixation devices, particularly devices inserted during a patient’s adolescence, as significant bony overgrowth can occur. The standard approach has been to remove the internal fixation device and then perform the THA. In most cases, and particularly when the internal fixation device is in an intracortical position, the result is significant compromise of bone. This article describes a technique in which a portion of the hardware is retained to avoid compromise of the lateral femoral cortex, thereby allowing insertion of a noncemented femoral component.
THA is the most effective procedure for reducing hip pain and disability in the setting of degenerative changes.6 Patients with SCFE, Legg-Calvé-Perthes disease, or developmental dysplasia of the hip generally are younger at the time they may be sufficiently symptomatic to consider THA.7,8 Many have had previous surgery using internal fixation devices. THAs after previous osteotomies with internal fixation devices are more technically demanding, require more operative time, are subject to more blood loss, and have a higher rate of complications, including femoral fracture. Ferguson and colleagues4 and Boos and colleagues9 found these surgeries were more difficult 33.8% and 36.8% of the time, respectively. For these reasons, some authors have recommended removing the internal fixation device as soon as the osteotomy is healed.4 However, this has not become the standard of care, and surgeons continue to perform THAs in the presence of a previous osteotomy with an internal fixation device in place.
The technique described in this article was used successfully in 2 cases. In each case, leaving the intracortical plate in place avoided compromise of the lateral femoral cortex and allowed insertion of a noncemented femoral component without complication. Of course, with the screw holes representing stress risers, careful insertion of the femoral component was required. Retaining the intracortical plate allowed it to function as part of the lateral femoral cortex, thereby maintaining the structural integrity of the femoral canal. As has been described for the 2 cases, a blade plate and plate and barrel were converted to a limited intracortical plate by removing the proximal portion of the plates—a modification that could be applied to other types of internal fixation devices that extend into the femoral neck as long as appropriate cutting tools are available.
Conclusion
THA in the setting of a retained internal fixation device is relatively common. This article describes a technique that can be used when a plate applied to the lateral femoral cortex has become intracortical as a result of extensive bony overgrowth. In using this technique to avoid plate removal, the surgeon eliminates the need for more extensive procedures aimed at compensating for deficiency of the femoral cortex in the area of plate removal. Although only 2 cases are presented here, this technique potentially can be used more broadly in these specific clinical situations.
1. Engesæter LB, Engesæter IØ, Fenstad AM, et al. Low revision rate after total hip arthroplasty in patients with pediatric hip diseases. Acta Orthop. 2012;83(5):436-441.
2. Froberg L, Christensen F, Pedersen NW, Overgaard S. The need for total hip arthroplasty in Perthes disease: a long-term study. Clin Orthop Relat Res. 2011;469(4):1134-1140.
3. Furnes O, Lie SA, Espehaug B, Vollset SE, Engesæter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586.
4. Ferguson GM, Cabanela ME, Ilstrup DM. Total hip arthroplasty after failed intertrochanteric osteotomy. J Bone Joint Surg Br. 1994;76(2):252-257.
5. Scheerlinck T. Primary hip arthroplasty templating on standard radiographs. A stepwise approach. Acta Orthop Belg. 2010;76(4):432-442.
6. Wroblewski BM, Siney PD. Charnley low-friction arthroplasty of the hip. Long-term results. Clin Orthop Relat Res. 1993;(292):191-201.
7. Chandler HP, Reineck FT, Wixson RL, McCarthy JC. Total hip replacement in patients younger than thirty years old. A five-year follow-up study. J Bone Joint Surg Am. 1981;63(9):1426-1434.
8. Dorr LD, Luckett M, Conaty JP. Total hip arthroplasties in patients younger than 45 years. A nine- to ten-year follow-up study. Clin Orthop Relat Res. 1990;(260):215-219.
9. Boos N, Krushell R, Ganz R, Müller ME. Total hip arthroplasty after previous proximal femoral osteotomy. J Bone Joint Surg Br. 1997;79(2):247-253.
Total hip arthroplasty (THA) is an effective treatment for advanced hip arthritis from a variety of causes, including osteoarthritis, inflammatory arthritis, posttraumatic arthritis, and sequelae of developmental disorders. It is not uncommon to perform THA in the presence of a previous proximal femoral osteotomy that may have been performed for slipped capital femoral epiphysis (SCFE), Legg-Calvé-Perthes disease, or developmental dysplasia of the hip, among other conditions. These osteotomies are commonly combined with internal fixation, a plate-and-screw device. These patients are at risk for developing degenerative arthritis at an earlier age than patients with other types of arthritis and subsequently may undergo THA at a younger age.1-3 Presence of a plate can pose a technical challenge during THA surgery. THA performed after intertrochanteric osteotomy has higher rates of perioperative and postoperative complications.4 Ferguson and colleagues4 noted difficulty during hardware removal in 24% of cases. Among the complications encountered were broken hardware, stripped screws, greater trochanteric fracture, stress risers from previous screw holes, canal narrowing from endosteal hypertrophy around hardware, and lateral cortical deficiency after removal of the side plate. As intertrochanteric osteotomies are often performed in patients who have yet to reach skeletal maturity, cortical hypertrophy can lead to complete coverage of the side plate and an “intracortical” position.
This article reports on 2 THA cases in which a technique was used to avoid intracortical plate removal and the resulting problems of lateral cortical deficiency. During each THA, the plate was left in place to avoid compromise of the lateral femoral cortex. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
An adolescent with bilateral SCFE was treated first with internal fixation of the right hip and subsequently with left proximal femoral osteotomy with internal fixation. He did well until age 31 years, when he developed progressively worsening pain about the left hip. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the left hip. Radiographs showed a sliding hip screw in place, with proximal femoral deformity consisting of femoral neck shortening and posterior angulation (Figures 1A, 1B). Preoperative Harris Hip Score was 54.5.
Case 2
A 51-year-old woman presented with a history of right hip problems dating back to age 13 years, when she sustained a fracture of the right hip and was treated with internal fixation. At age 15 years, she underwent proximal femoral osteotomy to correct residual deformity. She did well until age 45 years, when she developed worsening hip symptoms. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the right hip. Radiographs showed a fixed-angle blade plate in the proximal femur, with significant proximal femoral deformity (Figures 1C, 1D). Preoperative Harris Hip Score was 53.6.
Surgical Technique
In both cases, a standard series of radiographs was obtained—an anteroposterior (AP) radiograph of the pelvis and AP and cross-table lateral radiographs of the operative hip (Figure 1). Computed tomography (CT) with a metal-artifact-reducing technique may be useful in determining amount of cortical bone remaining under the plate. CT showed limited lateral cortex beneath the side plate and bony overgrowth covering the side plate. Preoperative templating was performed using previously described techniques.5
During THA, before removing any portion of any retained hardware, the surgeon should perform 3 important actions: Dislocate the hip, perform all appropriate capsular releases, and reduce the hip. Dislocating the hip before hardware removal significantly decreases the risk for fracture caused by stress risers, as the force required for dislocation is much more controlled because of the capsular releases. After hardware removal, the hip can be easily redislocated, and the femoral neck osteotomy can be performed.
When plate and screws are in an intracortical position, the screws can be removed only after removing the small shell of cortical bone covering them. The amount of bone to be removed is minimal. After the screws are removed, the plate remains in place. A motorized device with a metal-cutting attachment is used to transect the construct at the junction of the plate and barrel (case 1) or at the bend of a fixed-angle device (case 2). Laparotomy sponges are placed around the proximal femur to minimize the amount of soft tissue that could be exposed to metal shavings. Copious irrigation is used throughout this part of the procedure. Osteotomes are used to elevate the proximal portion of the plate and the barrel, preserving the distal portion of the plate on the lateral cortex of the femoral shaft.
After the head is removed, the rest of the THA can be performed using standard press-fit insertion technique (Figures 2A-2D). Care must be taken to ensure that the distal aspect of the femoral stem bypasses the most distal screw hole by at least 2 cortical diameters in order to reduce the risk for periprosthetic fracture.
By 2-year follow-up, both patients had regained excellent range of motion, ambulation, and overall function. Postoperative Harris Hip Scores were 86.6 and 83.8, respectively. There were no radiographic signs of complications.
Discussion
THA can be challenging in the setting of previously placed internal fixation devices, particularly devices inserted during a patient’s adolescence, as significant bony overgrowth can occur. The standard approach has been to remove the internal fixation device and then perform the THA. In most cases, and particularly when the internal fixation device is in an intracortical position, the result is significant compromise of bone. This article describes a technique in which a portion of the hardware is retained to avoid compromise of the lateral femoral cortex, thereby allowing insertion of a noncemented femoral component.
THA is the most effective procedure for reducing hip pain and disability in the setting of degenerative changes.6 Patients with SCFE, Legg-Calvé-Perthes disease, or developmental dysplasia of the hip generally are younger at the time they may be sufficiently symptomatic to consider THA.7,8 Many have had previous surgery using internal fixation devices. THAs after previous osteotomies with internal fixation devices are more technically demanding, require more operative time, are subject to more blood loss, and have a higher rate of complications, including femoral fracture. Ferguson and colleagues4 and Boos and colleagues9 found these surgeries were more difficult 33.8% and 36.8% of the time, respectively. For these reasons, some authors have recommended removing the internal fixation device as soon as the osteotomy is healed.4 However, this has not become the standard of care, and surgeons continue to perform THAs in the presence of a previous osteotomy with an internal fixation device in place.
The technique described in this article was used successfully in 2 cases. In each case, leaving the intracortical plate in place avoided compromise of the lateral femoral cortex and allowed insertion of a noncemented femoral component without complication. Of course, with the screw holes representing stress risers, careful insertion of the femoral component was required. Retaining the intracortical plate allowed it to function as part of the lateral femoral cortex, thereby maintaining the structural integrity of the femoral canal. As has been described for the 2 cases, a blade plate and plate and barrel were converted to a limited intracortical plate by removing the proximal portion of the plates—a modification that could be applied to other types of internal fixation devices that extend into the femoral neck as long as appropriate cutting tools are available.
Conclusion
THA in the setting of a retained internal fixation device is relatively common. This article describes a technique that can be used when a plate applied to the lateral femoral cortex has become intracortical as a result of extensive bony overgrowth. In using this technique to avoid plate removal, the surgeon eliminates the need for more extensive procedures aimed at compensating for deficiency of the femoral cortex in the area of plate removal. Although only 2 cases are presented here, this technique potentially can be used more broadly in these specific clinical situations.
Total hip arthroplasty (THA) is an effective treatment for advanced hip arthritis from a variety of causes, including osteoarthritis, inflammatory arthritis, posttraumatic arthritis, and sequelae of developmental disorders. It is not uncommon to perform THA in the presence of a previous proximal femoral osteotomy that may have been performed for slipped capital femoral epiphysis (SCFE), Legg-Calvé-Perthes disease, or developmental dysplasia of the hip, among other conditions. These osteotomies are commonly combined with internal fixation, a plate-and-screw device. These patients are at risk for developing degenerative arthritis at an earlier age than patients with other types of arthritis and subsequently may undergo THA at a younger age.1-3 Presence of a plate can pose a technical challenge during THA surgery. THA performed after intertrochanteric osteotomy has higher rates of perioperative and postoperative complications.4 Ferguson and colleagues4 noted difficulty during hardware removal in 24% of cases. Among the complications encountered were broken hardware, stripped screws, greater trochanteric fracture, stress risers from previous screw holes, canal narrowing from endosteal hypertrophy around hardware, and lateral cortical deficiency after removal of the side plate. As intertrochanteric osteotomies are often performed in patients who have yet to reach skeletal maturity, cortical hypertrophy can lead to complete coverage of the side plate and an “intracortical” position.
This article reports on 2 THA cases in which a technique was used to avoid intracortical plate removal and the resulting problems of lateral cortical deficiency. During each THA, the plate was left in place to avoid compromise of the lateral femoral cortex. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
An adolescent with bilateral SCFE was treated first with internal fixation of the right hip and subsequently with left proximal femoral osteotomy with internal fixation. He did well until age 31 years, when he developed progressively worsening pain about the left hip. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the left hip. Radiographs showed a sliding hip screw in place, with proximal femoral deformity consisting of femoral neck shortening and posterior angulation (Figures 1A, 1B). Preoperative Harris Hip Score was 54.5.
Case 2
A 51-year-old woman presented with a history of right hip problems dating back to age 13 years, when she sustained a fracture of the right hip and was treated with internal fixation. At age 15 years, she underwent proximal femoral osteotomy to correct residual deformity. She did well until age 45 years, when she developed worsening hip symptoms. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the right hip. Radiographs showed a fixed-angle blade plate in the proximal femur, with significant proximal femoral deformity (Figures 1C, 1D). Preoperative Harris Hip Score was 53.6.
Surgical Technique
In both cases, a standard series of radiographs was obtained—an anteroposterior (AP) radiograph of the pelvis and AP and cross-table lateral radiographs of the operative hip (Figure 1). Computed tomography (CT) with a metal-artifact-reducing technique may be useful in determining amount of cortical bone remaining under the plate. CT showed limited lateral cortex beneath the side plate and bony overgrowth covering the side plate. Preoperative templating was performed using previously described techniques.5
During THA, before removing any portion of any retained hardware, the surgeon should perform 3 important actions: Dislocate the hip, perform all appropriate capsular releases, and reduce the hip. Dislocating the hip before hardware removal significantly decreases the risk for fracture caused by stress risers, as the force required for dislocation is much more controlled because of the capsular releases. After hardware removal, the hip can be easily redislocated, and the femoral neck osteotomy can be performed.
When plate and screws are in an intracortical position, the screws can be removed only after removing the small shell of cortical bone covering them. The amount of bone to be removed is minimal. After the screws are removed, the plate remains in place. A motorized device with a metal-cutting attachment is used to transect the construct at the junction of the plate and barrel (case 1) or at the bend of a fixed-angle device (case 2). Laparotomy sponges are placed around the proximal femur to minimize the amount of soft tissue that could be exposed to metal shavings. Copious irrigation is used throughout this part of the procedure. Osteotomes are used to elevate the proximal portion of the plate and the barrel, preserving the distal portion of the plate on the lateral cortex of the femoral shaft.
After the head is removed, the rest of the THA can be performed using standard press-fit insertion technique (Figures 2A-2D). Care must be taken to ensure that the distal aspect of the femoral stem bypasses the most distal screw hole by at least 2 cortical diameters in order to reduce the risk for periprosthetic fracture.
By 2-year follow-up, both patients had regained excellent range of motion, ambulation, and overall function. Postoperative Harris Hip Scores were 86.6 and 83.8, respectively. There were no radiographic signs of complications.
Discussion
THA can be challenging in the setting of previously placed internal fixation devices, particularly devices inserted during a patient’s adolescence, as significant bony overgrowth can occur. The standard approach has been to remove the internal fixation device and then perform the THA. In most cases, and particularly when the internal fixation device is in an intracortical position, the result is significant compromise of bone. This article describes a technique in which a portion of the hardware is retained to avoid compromise of the lateral femoral cortex, thereby allowing insertion of a noncemented femoral component.
THA is the most effective procedure for reducing hip pain and disability in the setting of degenerative changes.6 Patients with SCFE, Legg-Calvé-Perthes disease, or developmental dysplasia of the hip generally are younger at the time they may be sufficiently symptomatic to consider THA.7,8 Many have had previous surgery using internal fixation devices. THAs after previous osteotomies with internal fixation devices are more technically demanding, require more operative time, are subject to more blood loss, and have a higher rate of complications, including femoral fracture. Ferguson and colleagues4 and Boos and colleagues9 found these surgeries were more difficult 33.8% and 36.8% of the time, respectively. For these reasons, some authors have recommended removing the internal fixation device as soon as the osteotomy is healed.4 However, this has not become the standard of care, and surgeons continue to perform THAs in the presence of a previous osteotomy with an internal fixation device in place.
The technique described in this article was used successfully in 2 cases. In each case, leaving the intracortical plate in place avoided compromise of the lateral femoral cortex and allowed insertion of a noncemented femoral component without complication. Of course, with the screw holes representing stress risers, careful insertion of the femoral component was required. Retaining the intracortical plate allowed it to function as part of the lateral femoral cortex, thereby maintaining the structural integrity of the femoral canal. As has been described for the 2 cases, a blade plate and plate and barrel were converted to a limited intracortical plate by removing the proximal portion of the plates—a modification that could be applied to other types of internal fixation devices that extend into the femoral neck as long as appropriate cutting tools are available.
Conclusion
THA in the setting of a retained internal fixation device is relatively common. This article describes a technique that can be used when a plate applied to the lateral femoral cortex has become intracortical as a result of extensive bony overgrowth. In using this technique to avoid plate removal, the surgeon eliminates the need for more extensive procedures aimed at compensating for deficiency of the femoral cortex in the area of plate removal. Although only 2 cases are presented here, this technique potentially can be used more broadly in these specific clinical situations.
1. Engesæter LB, Engesæter IØ, Fenstad AM, et al. Low revision rate after total hip arthroplasty in patients with pediatric hip diseases. Acta Orthop. 2012;83(5):436-441.
2. Froberg L, Christensen F, Pedersen NW, Overgaard S. The need for total hip arthroplasty in Perthes disease: a long-term study. Clin Orthop Relat Res. 2011;469(4):1134-1140.
3. Furnes O, Lie SA, Espehaug B, Vollset SE, Engesæter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586.
4. Ferguson GM, Cabanela ME, Ilstrup DM. Total hip arthroplasty after failed intertrochanteric osteotomy. J Bone Joint Surg Br. 1994;76(2):252-257.
5. Scheerlinck T. Primary hip arthroplasty templating on standard radiographs. A stepwise approach. Acta Orthop Belg. 2010;76(4):432-442.
6. Wroblewski BM, Siney PD. Charnley low-friction arthroplasty of the hip. Long-term results. Clin Orthop Relat Res. 1993;(292):191-201.
7. Chandler HP, Reineck FT, Wixson RL, McCarthy JC. Total hip replacement in patients younger than thirty years old. A five-year follow-up study. J Bone Joint Surg Am. 1981;63(9):1426-1434.
8. Dorr LD, Luckett M, Conaty JP. Total hip arthroplasties in patients younger than 45 years. A nine- to ten-year follow-up study. Clin Orthop Relat Res. 1990;(260):215-219.
9. Boos N, Krushell R, Ganz R, Müller ME. Total hip arthroplasty after previous proximal femoral osteotomy. J Bone Joint Surg Br. 1997;79(2):247-253.
1. Engesæter LB, Engesæter IØ, Fenstad AM, et al. Low revision rate after total hip arthroplasty in patients with pediatric hip diseases. Acta Orthop. 2012;83(5):436-441.
2. Froberg L, Christensen F, Pedersen NW, Overgaard S. The need for total hip arthroplasty in Perthes disease: a long-term study. Clin Orthop Relat Res. 2011;469(4):1134-1140.
3. Furnes O, Lie SA, Espehaug B, Vollset SE, Engesæter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586.
4. Ferguson GM, Cabanela ME, Ilstrup DM. Total hip arthroplasty after failed intertrochanteric osteotomy. J Bone Joint Surg Br. 1994;76(2):252-257.
5. Scheerlinck T. Primary hip arthroplasty templating on standard radiographs. A stepwise approach. Acta Orthop Belg. 2010;76(4):432-442.
6. Wroblewski BM, Siney PD. Charnley low-friction arthroplasty of the hip. Long-term results. Clin Orthop Relat Res. 1993;(292):191-201.
7. Chandler HP, Reineck FT, Wixson RL, McCarthy JC. Total hip replacement in patients younger than thirty years old. A five-year follow-up study. J Bone Joint Surg Am. 1981;63(9):1426-1434.
8. Dorr LD, Luckett M, Conaty JP. Total hip arthroplasties in patients younger than 45 years. A nine- to ten-year follow-up study. Clin Orthop Relat Res. 1990;(260):215-219.
9. Boos N, Krushell R, Ganz R, Müller ME. Total hip arthroplasty after previous proximal femoral osteotomy. J Bone Joint Surg Br. 1997;79(2):247-253.
Orthopedics in US Health Care
In the United States, the landscape of health care is changing. Health care reform and fluctuating political and economic climates have affected and will continue to affect the practice of orthopedic surgery. Demand for musculoskeletal care and the costs of providing this care are exceeding available resources—which has led to an evolution in how we practice as individuals and in the institutions where we provide care. Patient safety, quality, and value have become the outcomes of importance. Orthopedic surgeons, as experts in musculoskeletal care, must be a part of these changes. In this review, we offer perspective on the changing face of orthopedic surgery in the modern US health care system.
1. Meeting the demand
Musculoskeletal conditions represent one of the most common and costly health issues in the United States, affecting individuals medically and economically and compromising their quality of life.1,2 In 2008, more than 110 million US adults (1 in 2) reported having a musculoskeletal condition for more than 3 months, and almost 7% reported that a chronic musculoskeletal condition made routine activities of daily living significantly difficult.1 Overall, in the United States, some of the most common chronic conditions are musculoskeletal in origin. These conditions include osteoarthritis and back pain.
Osteoarthritis is the leading cause of chronic pain and disability. Physician-diagnosed arthritis is expected to affect 25% of US adults by 2030,3 and in more than one-third of these patients arthritis limits work or other activity.4 Back pain is another of the most common debilitating conditions in the United States.3,5 St Sauver and colleagues6 found that back pain is the third most common condition (23.9%) that prompts patients to seek health care—following skin-related problems (42.7%) and osteoarthritis/joint pain (33.6%).
As life expectancy increases, so do expectations of enjoying higher levels of activity into the later years. Patients expect to be as active in their geriatric years as they were in middle age, and many are able to do so. Amid the growing obesity epidemic and increased incidence of chronic comorbidities, however, the aging population not only is at substantial risk for developing a chronic musculoskeletal disorder but may face new challenges in accessing care.
Although orthopedic surgeons specialize in treating musculoskeletal conditions, up to 90% of common nonsurgical musculoskeletal complaints are thought to be manageable in the primary care setting.7 With a disproportionate increase in musculoskeletal demand against a relatively constant number of orthopedic providers,8 it is becoming increasingly important for nonorthopedists to adequately manage musculoskeletal conditions. Physiatrists, rheumatologists, internists, family practitioners, and the expanding field of sports medicine specialists provide primary care of musculoskeletal conditions. To meet the growing demand and to ensure that patients receive quality, sustainable, effective, and efficient care, orthopedic surgeons should be actively involved in training these providers. As high as the cost of managing musculoskeletal conditions can be, it is far less than the cost resulting from inadequate or improper management. There is already justification for formal development of a specialization in nonoperative management of musculoskeletal care. Establishing this specialization requires a multidisciplinary approach, with orthopedic surgery taking a lead role.
2. The cost equation
As the prevalence of orthopedic conditions increases, so does the cost of delivering musculoskeletal care. The economic implications of meeting this growing demand are an important area of concern for our health care system. Steadily increasing hospital expenses for personnel and services, rising costs of pharmaceuticals and laboratory tests, constant evolution of costly technology, and insurance/reimbursement rates that do not keep pace with rising costs all contribute to the rapid escalation of the “cost of care.”
Health care expenditures accounted for 17.2% of the US gross domestic product (GDP) in 2012 and are expected to represent 19.3% by 2023.9 For musculoskeletal disease, direct costs alone are expected to approach $510 billion, equaling 5% of GDP and representing almost 30% of all health care expenditures. In Medicare patients, osteoarthritis is the most expensive condition to treat overall, and 3 other musculoskeletal problems rank highly as well: femoral neck fractures (3rd), back pain (10th), and fractures of all types (16th).10 Clearly, musculoskeletal care is one of the most prevalent and expensive health conditions in the United States.
Part of the direct costs of care that consistently increase each year are the steadily increasing costs of technology, which is often considered synonymous with orthopedic care. Promotion of new and more costly implants is common in the absence of evidence supporting their use. However, use of new implants and technology is being scrutinized in an effort to strike the proper cost–benefit balance.
To change the slope of the cost curve, orthopedic surgeons should utilize technological advances that are proven to be clinically significant and economically feasible and should avoid modest improvements with limited clinical benefit and higher price tags. Unfortunately, this approach is not being taken. Minor modifications of implant designs are often marketed as “new and improved” to justify increased costs, and these implants often gain widespread use. A few may prove to be clinically better, but most will be only comparable to older, less expensive designs, and some may end up being clinical failures, discovered at great cost to patients and the health care system.11,12
Orthopedic surgeons have an important role in this decision-making. We should strive for the best, most cost-effective outcomes for our patients. We should reject new technology that does not clearly improve outcomes. At the least, we should use the technology in a manufacturer-supported clinical trial to determine its superiority. Whether the improvement is in technique, implant design, or workflow efficiency, orthopedic surgeons must be actively involved in researching and developing the latest innovations and must help determine their prospective value by considering not only their potential clinical benefits but also their economic implications.
As the political and economic environment becomes more directed at the cost-containment and sustainability of care, there has been a clear shift in focus to quality and value rather than volume, giving rise to the “value-based care” approach. The “value equation,” in which value equals quality divided by cost, requires a clear measure of outcomes and an equally clear understanding of costs. Delivering high-quality care in a cost-conscious environment is an approach that every orthopedic surgeon should adopt. Widespread adoption of the value-based strategy by hospital systems and insurance companies is resulting in a paradigm shift away from more traditional volume-based metrics and in favor of value-based metrics, including quality measures, patient-reported outcomes, Hospital Consumer Assessment of Healthcare Providers and Systems, and physician-specific outcome measures.
The new paradigm has brought the bundled payment initiative (BPI), a strategy included in the Patient Protection and Affordable Care Act. The philosophy behind the BPI model is for hospital systems and physicians to control costs while maintaining and improving the quality of care. Measured by patient metrics (eg, clinical outcomes, patient satisfaction) and hospital metrics (eg, readmission rates, cost of care), bundled payments reimburse hospitals on the basis of cost of an entire episode of care rather than on the basis of individual procedures and services. This approach provides incentives for both physicians and hospitals to promote value-based care while emphasizing coordination of care among all members of the health care team.
Providing the best possible care for our patients while holding our practice to the highest standards is a central tenet of the practice of orthopedic surgery and should be independent of reimbursement strategies. Thus, to increase the value of care, we must establish practice models and strategies to optimize cost-efficiency while improving outcomes. As explained by Porter and Teisberg,13 it is important to be conscientious about cost, but above all we must not allow quality of health care delivery to be compromised when trying to improve the “value” of care. Through evidence-based management and a clear understanding of costs, we must develop cost-efficient practice models that sustainably deliver the highest value of care.
3. Evolving practice models
As the health care landscape continues to change, physician practice models evolve accordingly. Although the private practice model once dominated the physician workforce, this is no longer true, as there has been a significant shift to employer-based practice models. The multiple factors at work relate to changing patterns of reimbursement, increasing government regulations, and a general change in recent residency graduates’ expectations regarding work–life balance. Other catalysts are the shift from volume- to value-based care and the recognition that cost-effective health care is more easily achieved when physicians and their institutions are in alignment. Ultimately, physician–institution alignment is crucial in improving care and outcomes.
Physician–institution alignment requires further discussion. Ideally, it should strike the proper balance between physician autonomy and institutional priorities to ensure the highest quality care. Physicians and their institutions should align their interests in terms of patient safety, quality, and economics to create a work environment conducive to both patient/physician satisfaction and institutional success.14 As identified by Page and colleagues,15 the primary drivers of physician–institution alignment, specific to orthopedic surgery, are economic, regulatory, and cultural. In economics, implant selection and ancillary services are the important issues; in the regulatory area, cooperative efforts to address expanding state and federal requirements are needed; last, the primary cultural driver is delivery of care to an expanding, diverse patient population.
Physician–institution alignment brings opportunities for “gainsharing,” which can directly benefit individual physicians, physician groups, and departments. Gainsharing is classically defined as “arrangements in which a hospital gives physicians a percentage share of any reduction in the hospital’s costs for patient care attributable in part to the physicians’ efforts.”16 Modern gainsharing programs can be used by institutions to align the economic interests of physicians and hospitals, with the ultimate goal being to achieve a sustainable increase in the value and quality of care delivered to patients.13 Examples include efforts to reduce the cost of orthopedic implants, which is a major cost driver in orthopedic surgery. Our institution realized significant savings when surgeons were directly involved in the implant contracting process with strategic sourcing personnel. These savings were shared with the department to enhance research and education programs. BPI, a risk-sharing program in which Medicare and hospitals participate, incorporates gainsharing opportunities in which each participating physician can receive up to 50% of his or her previous Medicare billings when specific targets are achieved. BPI included 27 musculoskeletal diagnosis–related groups that could be developed into a bundled payment proposal. Our institution participated in a 90-day episode, for primary hip and knee arthroplasty and non–cervical spine fusion, that had very promising results.
Gainsharing offers physicians incentives to meet institution goals of improved outcomes and increased patient satisfaction while increasing oversight and accountability. When physician-specific outcomes do not meet the established goals in key areas (readmissions, thromboembolic complications, infections), it is only logical that steps will be taken to improve outcomes. Although physicians may not be used to this increased scrutiny, the goal of improving outcomes, even if it necessitates a change in an established approach to care, should be welcomed.
Physicians should be rewarded for good outcomes but not suboptimal outcomes. When outcomes are suboptimal, physicians should take a constructive approach to improve them. On the other hand, not being rewarded for unachieved goals can be perceived as being penalized. Additional monitoring may paradoxically lead physicians to avoid more “complex” cases, such as those of patients at higher risk for complications and poorer outcomes. An example is found in patient selection for surgery, in which issues like obesity, diabetes, and heart disease are known to negatively affect outcomes. In these models, “cherry-picking” is a well-recognized risk17,18 that can compromise our ethical obligation to provide equal access for all patients. To offset this tendency, we should use a risk-stratification model in which all patients are not considered equal in the risks they present. A risk-adjustment approach benefits both patients and providers by identifying modifiable risk factors that can be addressed to positively affect outcomes. This risk-stratification approach further incentivizes the orthopedist to closely work with other health care providers to address the medical comorbidities that may negatively affect surgical outcomes.
4. Patient and physician expectations
Living in a technology-driven society in the age of information has had a major impact on patients’ attitudes and expectations about their care—and therefore on physicians’ practice methods. It is uncommon to evaluate a patient who has not already consulted the Internet about a problem. Patients now have much more information they can use to make decisions about their treatment, and, though many question the accuracy of Internet information, there is no argument that being more informed is beneficial. In this time of shared decision-making, it is absolutely essential that patients keep themselves informed.
It is crucial to align the expectations of both physicians and patients in order to achieve the best outcomes. Gaining a clear understanding of treatment goals, management, and potential complications consistently leads to improved patient satisfaction, more favorable clinical outcomes, and reduced risk of litigation.19-22 Addressing patient concerns and expectations is significantly enhanced by a strong patient–physician relationship through clinical models focused on patient-centered care.
Now considered a standard of care, the patient-centered model has changed the way we practice. The foundation of the patient-centered approach is to strengthen the patient–physician relationship by empowering patients to become active decision-makers in the management of their own health. The role of orthopedists in this model is to provide patients with information and insight into their conditions in order to facilitate shared decision-making. Our role should be to guide patients to make educated and informed decisions. Doing so enhances communication, thereby strengthening the patient–physician relationship, and places both patient and physician expectations in perspective. Patient-reported outcomes, satisfaction rates, symptomatic burdens, and costs of care are all positively correlated with strong communication and realistic expectations achieved through a patient-centered approach.21,23
The evolution of clinical practice has been influenced by factors ranging from external forces (eg, changing political and economic climates) to social trends (use of social media and the Internet). Technology has been a driving force in our rapidly changing clinical environment, significantly altering the way we practice. Although we must be careful in how we use it, new technology can certainly work to our advantage. We have a plethora of medical information at our fingertips, and, with physician-directed guidance, our patients can become more informed than ever before. This is the principle of patient-centered medicine and shared decision-making, and its utility will only increase in importance.
5. The role of advocacy
The central tenet of orthopedic practice has always been a focus on patients. We continually strive to improve patient outcomes, reduce costs, and work efficiently in our practices and facilities. Although we can focus on our individual practices, we cannot ignore the influence and impact of the political system on our performance. Federal and state regulations give physicians and insurance companies an uneven playing field. This imbalance requires that physicians be more active in health care policymaking and advocacy. Although we are more involved than ever before, our influence is far less than what we would like it to be, perhaps partly because of the nature of the political process but perhaps also because of physicians’ resistance to becoming involved.
As experts in the treatment of musculoskeletal conditions, we should be at the forefront of health care policy development—a position we have not been able to attain. Although many factors contribute to our lack of a “seat at the table,” we must recognize our reluctance as a group to support advocacy, either financially or through personal time commitment. The American Association of Orthopaedic Surgeons (AAOS) Orthopaedic Political Action Committee has never been able to obtain donations from more than 30% of AAOS members. Although this committee historically has been successful, we could be much more so if we had financial support from 90% of members. There are many ways to be actively involved in advocacy. One way is to join local and state orthopedic societies and support their advocacy efforts. State orthopedic societies work closely with the AAOS Office of Government Relations to coordinate advocacy and direct efforts and resources to areas of greatest need. Knowing local congressional representatives and communicating with them about issues we face in our practices make our issues “real.” Some of our colleagues have even successfully run for office in Congress, and they certainly deserve our support. Advocacy will absolutely play an increasingly important role as federal and state governments expand their involvement in health care. Our role should be to get involved, at least to some degree. We need to recognize that our strength is in our numbers, as the few cannot accomplish nearly as much as the many.
Summary
Orthopedic surgeons are practicing in the midst of almost constant change—evolving patient care, shifts in employment models, advances in technology, modern patient expectations, and an increasingly complex regulatory environment. Even in this context, however, our goal remains unchanged: to give our patients the highest-quality care possible. Our core values as orthopedic surgeons and physicians are dedication, commitment, and service to patients and to our profession. As US health care continues to evolve, we must evolve as well, with an emphasis on expanding our role in the health care policy debate.
1. US Bone and Joint Initiative. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal, and Economic Cost. Rosemont, IL: US Bone and Joint Initiative; 2008. http://www.boneandjointburden.org. Accessed October 26, 2015.
2. US Bone and Joint Initiative. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal, and Economic Cost. 2nd ed. Rosemont, IL: US Bone and Joint Initiative; 2011. http://www.boneandjointburden.org. Accessed October 26, 2015.
3. Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil. 2014;95(5):986-995.e1.
4. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226-229.
5. Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169(3):251-258.
6. St Sauver JL, Warner DO, Yawn BP, et al. Why patients visit their doctors: assessing the most prevalent conditions in a defined American population. Mayo Clin Proc. 2013;88(1):56-67.
7. Anderson BC. Office Orthopedics for Primary Care: Diagnosis and Treatment. 2nd ed. Philadelphia, PA: Saunders; 1999.
8. American Academy of Orthopaedic Surgeons, Department of Research and Scientific Affairs. Orthopaedic Practice in the U.S. 2012 [2012 Orthopaedic Surgeon Census Report]. Rosemont, IL: American Academy of Orthopaedic Surgeons; January 2013.
9. US Department of Health and Human Services, Centers for Medicare & Medicaid Services, Office of the Actuary, National Health Statistics Group. NHE [National Health Expenditure] Fact Sheet, 2014. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet.html. Updated July 28, 2015. Accessed October 26, 2015.
10. Cutler DM, Ghosh K. The potential for cost savings through bundled episode payments. N Engl J Med. 2012;366(12):1075-1077.
11. Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92(1):38-46.
12. Dahlstrand H, Stark A, Anissian L, Hailer NP. Elevated serum concentrations of cobalt, chromium, nickel, and manganese after metal-on-metal alloarthroplasty of the hip: a prospective randomized study. J Arthroplasty. 2009;24(6):837-845.
13. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
14. American Association of Orthopaedic Surgeons. Alignment of physician and facility payment and incentives. Position statement 1171. American Association of Orthopaedic Surgeons website. http://www.aaos.org/about/papers/position/1171.asp. Published September 2006. Revised February 2009. Accessed October 26, 2015.
15. Page AE, Butler CA, Bozic KJ. Factors driving physician–hospital alignment in orthopaedic surgery. Clin Orthop Relat Res. 2013;471(6):1809-1817.
16. US Department of Health and Human Services, Office of Inspector General. Gainsharing arrangements and CMPs for hospital payments to physicians to reduce or limit services to beneficiaries [special advisory bulletin]. Office of Inspector General website. http://oig.hhs.gov/fraud/docs/alertsandbulletins/gainsh.htm. Published July 1999. Accessed October 26, 2015.
17. Bronson WH, Fewer M, Godlewski K, et al. The ethics of patient risk modification prior to elective joint replacement surgery. J Bone Joint Surg Am. 2014;96(13):e113.
18. Bosco J. To cherry pick or not: the unintended ethical consequences of pay for performance. Presented at: New York University Colloquium on Medical Ethics; New York, NY; November 2014.
19. Hageman MG, Briët JP, Bossen JK, Blok RD, Ring DC, Vranceanu AM. Do previsit expectations correlate with satisfaction of new patients presenting for evaluation with an orthopaedic surgical practice? Clin Orthop Relat Res. 2015;473(2):716-721.
20. Jourdan C, Poiraudeau S, Descamps S, et al. Comparison of patient and surgeon expectations of total hip arthroplasty. PLoS One. 2012;7(1):e30195.
21. McMillan S, Kendall E, Sav A, et al. Patient-centered approaches to health care: a systematic review of randomized controlled trials. Med Care Res Rev. 2013;70(6):567-596.
22. Forster HP, Schwartz J, DeRenzo E. Reducing legal risk by practicing patient-centered medicine. Arch Intern Med. 2002;162(11):1217-1219.
23. Van Citters AD, Fahlman C, Goldmann DA, et al. Developing a pathway for high-value, patient-centered total joint arthroplasty. Clin Orthop Relat Res. 2014;472(5):1619-1635.
In the United States, the landscape of health care is changing. Health care reform and fluctuating political and economic climates have affected and will continue to affect the practice of orthopedic surgery. Demand for musculoskeletal care and the costs of providing this care are exceeding available resources—which has led to an evolution in how we practice as individuals and in the institutions where we provide care. Patient safety, quality, and value have become the outcomes of importance. Orthopedic surgeons, as experts in musculoskeletal care, must be a part of these changes. In this review, we offer perspective on the changing face of orthopedic surgery in the modern US health care system.
1. Meeting the demand
Musculoskeletal conditions represent one of the most common and costly health issues in the United States, affecting individuals medically and economically and compromising their quality of life.1,2 In 2008, more than 110 million US adults (1 in 2) reported having a musculoskeletal condition for more than 3 months, and almost 7% reported that a chronic musculoskeletal condition made routine activities of daily living significantly difficult.1 Overall, in the United States, some of the most common chronic conditions are musculoskeletal in origin. These conditions include osteoarthritis and back pain.
Osteoarthritis is the leading cause of chronic pain and disability. Physician-diagnosed arthritis is expected to affect 25% of US adults by 2030,3 and in more than one-third of these patients arthritis limits work or other activity.4 Back pain is another of the most common debilitating conditions in the United States.3,5 St Sauver and colleagues6 found that back pain is the third most common condition (23.9%) that prompts patients to seek health care—following skin-related problems (42.7%) and osteoarthritis/joint pain (33.6%).
As life expectancy increases, so do expectations of enjoying higher levels of activity into the later years. Patients expect to be as active in their geriatric years as they were in middle age, and many are able to do so. Amid the growing obesity epidemic and increased incidence of chronic comorbidities, however, the aging population not only is at substantial risk for developing a chronic musculoskeletal disorder but may face new challenges in accessing care.
Although orthopedic surgeons specialize in treating musculoskeletal conditions, up to 90% of common nonsurgical musculoskeletal complaints are thought to be manageable in the primary care setting.7 With a disproportionate increase in musculoskeletal demand against a relatively constant number of orthopedic providers,8 it is becoming increasingly important for nonorthopedists to adequately manage musculoskeletal conditions. Physiatrists, rheumatologists, internists, family practitioners, and the expanding field of sports medicine specialists provide primary care of musculoskeletal conditions. To meet the growing demand and to ensure that patients receive quality, sustainable, effective, and efficient care, orthopedic surgeons should be actively involved in training these providers. As high as the cost of managing musculoskeletal conditions can be, it is far less than the cost resulting from inadequate or improper management. There is already justification for formal development of a specialization in nonoperative management of musculoskeletal care. Establishing this specialization requires a multidisciplinary approach, with orthopedic surgery taking a lead role.
2. The cost equation
As the prevalence of orthopedic conditions increases, so does the cost of delivering musculoskeletal care. The economic implications of meeting this growing demand are an important area of concern for our health care system. Steadily increasing hospital expenses for personnel and services, rising costs of pharmaceuticals and laboratory tests, constant evolution of costly technology, and insurance/reimbursement rates that do not keep pace with rising costs all contribute to the rapid escalation of the “cost of care.”
Health care expenditures accounted for 17.2% of the US gross domestic product (GDP) in 2012 and are expected to represent 19.3% by 2023.9 For musculoskeletal disease, direct costs alone are expected to approach $510 billion, equaling 5% of GDP and representing almost 30% of all health care expenditures. In Medicare patients, osteoarthritis is the most expensive condition to treat overall, and 3 other musculoskeletal problems rank highly as well: femoral neck fractures (3rd), back pain (10th), and fractures of all types (16th).10 Clearly, musculoskeletal care is one of the most prevalent and expensive health conditions in the United States.
Part of the direct costs of care that consistently increase each year are the steadily increasing costs of technology, which is often considered synonymous with orthopedic care. Promotion of new and more costly implants is common in the absence of evidence supporting their use. However, use of new implants and technology is being scrutinized in an effort to strike the proper cost–benefit balance.
To change the slope of the cost curve, orthopedic surgeons should utilize technological advances that are proven to be clinically significant and economically feasible and should avoid modest improvements with limited clinical benefit and higher price tags. Unfortunately, this approach is not being taken. Minor modifications of implant designs are often marketed as “new and improved” to justify increased costs, and these implants often gain widespread use. A few may prove to be clinically better, but most will be only comparable to older, less expensive designs, and some may end up being clinical failures, discovered at great cost to patients and the health care system.11,12
Orthopedic surgeons have an important role in this decision-making. We should strive for the best, most cost-effective outcomes for our patients. We should reject new technology that does not clearly improve outcomes. At the least, we should use the technology in a manufacturer-supported clinical trial to determine its superiority. Whether the improvement is in technique, implant design, or workflow efficiency, orthopedic surgeons must be actively involved in researching and developing the latest innovations and must help determine their prospective value by considering not only their potential clinical benefits but also their economic implications.
As the political and economic environment becomes more directed at the cost-containment and sustainability of care, there has been a clear shift in focus to quality and value rather than volume, giving rise to the “value-based care” approach. The “value equation,” in which value equals quality divided by cost, requires a clear measure of outcomes and an equally clear understanding of costs. Delivering high-quality care in a cost-conscious environment is an approach that every orthopedic surgeon should adopt. Widespread adoption of the value-based strategy by hospital systems and insurance companies is resulting in a paradigm shift away from more traditional volume-based metrics and in favor of value-based metrics, including quality measures, patient-reported outcomes, Hospital Consumer Assessment of Healthcare Providers and Systems, and physician-specific outcome measures.
The new paradigm has brought the bundled payment initiative (BPI), a strategy included in the Patient Protection and Affordable Care Act. The philosophy behind the BPI model is for hospital systems and physicians to control costs while maintaining and improving the quality of care. Measured by patient metrics (eg, clinical outcomes, patient satisfaction) and hospital metrics (eg, readmission rates, cost of care), bundled payments reimburse hospitals on the basis of cost of an entire episode of care rather than on the basis of individual procedures and services. This approach provides incentives for both physicians and hospitals to promote value-based care while emphasizing coordination of care among all members of the health care team.
Providing the best possible care for our patients while holding our practice to the highest standards is a central tenet of the practice of orthopedic surgery and should be independent of reimbursement strategies. Thus, to increase the value of care, we must establish practice models and strategies to optimize cost-efficiency while improving outcomes. As explained by Porter and Teisberg,13 it is important to be conscientious about cost, but above all we must not allow quality of health care delivery to be compromised when trying to improve the “value” of care. Through evidence-based management and a clear understanding of costs, we must develop cost-efficient practice models that sustainably deliver the highest value of care.
3. Evolving practice models
As the health care landscape continues to change, physician practice models evolve accordingly. Although the private practice model once dominated the physician workforce, this is no longer true, as there has been a significant shift to employer-based practice models. The multiple factors at work relate to changing patterns of reimbursement, increasing government regulations, and a general change in recent residency graduates’ expectations regarding work–life balance. Other catalysts are the shift from volume- to value-based care and the recognition that cost-effective health care is more easily achieved when physicians and their institutions are in alignment. Ultimately, physician–institution alignment is crucial in improving care and outcomes.
Physician–institution alignment requires further discussion. Ideally, it should strike the proper balance between physician autonomy and institutional priorities to ensure the highest quality care. Physicians and their institutions should align their interests in terms of patient safety, quality, and economics to create a work environment conducive to both patient/physician satisfaction and institutional success.14 As identified by Page and colleagues,15 the primary drivers of physician–institution alignment, specific to orthopedic surgery, are economic, regulatory, and cultural. In economics, implant selection and ancillary services are the important issues; in the regulatory area, cooperative efforts to address expanding state and federal requirements are needed; last, the primary cultural driver is delivery of care to an expanding, diverse patient population.
Physician–institution alignment brings opportunities for “gainsharing,” which can directly benefit individual physicians, physician groups, and departments. Gainsharing is classically defined as “arrangements in which a hospital gives physicians a percentage share of any reduction in the hospital’s costs for patient care attributable in part to the physicians’ efforts.”16 Modern gainsharing programs can be used by institutions to align the economic interests of physicians and hospitals, with the ultimate goal being to achieve a sustainable increase in the value and quality of care delivered to patients.13 Examples include efforts to reduce the cost of orthopedic implants, which is a major cost driver in orthopedic surgery. Our institution realized significant savings when surgeons were directly involved in the implant contracting process with strategic sourcing personnel. These savings were shared with the department to enhance research and education programs. BPI, a risk-sharing program in which Medicare and hospitals participate, incorporates gainsharing opportunities in which each participating physician can receive up to 50% of his or her previous Medicare billings when specific targets are achieved. BPI included 27 musculoskeletal diagnosis–related groups that could be developed into a bundled payment proposal. Our institution participated in a 90-day episode, for primary hip and knee arthroplasty and non–cervical spine fusion, that had very promising results.
Gainsharing offers physicians incentives to meet institution goals of improved outcomes and increased patient satisfaction while increasing oversight and accountability. When physician-specific outcomes do not meet the established goals in key areas (readmissions, thromboembolic complications, infections), it is only logical that steps will be taken to improve outcomes. Although physicians may not be used to this increased scrutiny, the goal of improving outcomes, even if it necessitates a change in an established approach to care, should be welcomed.
Physicians should be rewarded for good outcomes but not suboptimal outcomes. When outcomes are suboptimal, physicians should take a constructive approach to improve them. On the other hand, not being rewarded for unachieved goals can be perceived as being penalized. Additional monitoring may paradoxically lead physicians to avoid more “complex” cases, such as those of patients at higher risk for complications and poorer outcomes. An example is found in patient selection for surgery, in which issues like obesity, diabetes, and heart disease are known to negatively affect outcomes. In these models, “cherry-picking” is a well-recognized risk17,18 that can compromise our ethical obligation to provide equal access for all patients. To offset this tendency, we should use a risk-stratification model in which all patients are not considered equal in the risks they present. A risk-adjustment approach benefits both patients and providers by identifying modifiable risk factors that can be addressed to positively affect outcomes. This risk-stratification approach further incentivizes the orthopedist to closely work with other health care providers to address the medical comorbidities that may negatively affect surgical outcomes.
4. Patient and physician expectations
Living in a technology-driven society in the age of information has had a major impact on patients’ attitudes and expectations about their care—and therefore on physicians’ practice methods. It is uncommon to evaluate a patient who has not already consulted the Internet about a problem. Patients now have much more information they can use to make decisions about their treatment, and, though many question the accuracy of Internet information, there is no argument that being more informed is beneficial. In this time of shared decision-making, it is absolutely essential that patients keep themselves informed.
It is crucial to align the expectations of both physicians and patients in order to achieve the best outcomes. Gaining a clear understanding of treatment goals, management, and potential complications consistently leads to improved patient satisfaction, more favorable clinical outcomes, and reduced risk of litigation.19-22 Addressing patient concerns and expectations is significantly enhanced by a strong patient–physician relationship through clinical models focused on patient-centered care.
Now considered a standard of care, the patient-centered model has changed the way we practice. The foundation of the patient-centered approach is to strengthen the patient–physician relationship by empowering patients to become active decision-makers in the management of their own health. The role of orthopedists in this model is to provide patients with information and insight into their conditions in order to facilitate shared decision-making. Our role should be to guide patients to make educated and informed decisions. Doing so enhances communication, thereby strengthening the patient–physician relationship, and places both patient and physician expectations in perspective. Patient-reported outcomes, satisfaction rates, symptomatic burdens, and costs of care are all positively correlated with strong communication and realistic expectations achieved through a patient-centered approach.21,23
The evolution of clinical practice has been influenced by factors ranging from external forces (eg, changing political and economic climates) to social trends (use of social media and the Internet). Technology has been a driving force in our rapidly changing clinical environment, significantly altering the way we practice. Although we must be careful in how we use it, new technology can certainly work to our advantage. We have a plethora of medical information at our fingertips, and, with physician-directed guidance, our patients can become more informed than ever before. This is the principle of patient-centered medicine and shared decision-making, and its utility will only increase in importance.
5. The role of advocacy
The central tenet of orthopedic practice has always been a focus on patients. We continually strive to improve patient outcomes, reduce costs, and work efficiently in our practices and facilities. Although we can focus on our individual practices, we cannot ignore the influence and impact of the political system on our performance. Federal and state regulations give physicians and insurance companies an uneven playing field. This imbalance requires that physicians be more active in health care policymaking and advocacy. Although we are more involved than ever before, our influence is far less than what we would like it to be, perhaps partly because of the nature of the political process but perhaps also because of physicians’ resistance to becoming involved.
As experts in the treatment of musculoskeletal conditions, we should be at the forefront of health care policy development—a position we have not been able to attain. Although many factors contribute to our lack of a “seat at the table,” we must recognize our reluctance as a group to support advocacy, either financially or through personal time commitment. The American Association of Orthopaedic Surgeons (AAOS) Orthopaedic Political Action Committee has never been able to obtain donations from more than 30% of AAOS members. Although this committee historically has been successful, we could be much more so if we had financial support from 90% of members. There are many ways to be actively involved in advocacy. One way is to join local and state orthopedic societies and support their advocacy efforts. State orthopedic societies work closely with the AAOS Office of Government Relations to coordinate advocacy and direct efforts and resources to areas of greatest need. Knowing local congressional representatives and communicating with them about issues we face in our practices make our issues “real.” Some of our colleagues have even successfully run for office in Congress, and they certainly deserve our support. Advocacy will absolutely play an increasingly important role as federal and state governments expand their involvement in health care. Our role should be to get involved, at least to some degree. We need to recognize that our strength is in our numbers, as the few cannot accomplish nearly as much as the many.
Summary
Orthopedic surgeons are practicing in the midst of almost constant change—evolving patient care, shifts in employment models, advances in technology, modern patient expectations, and an increasingly complex regulatory environment. Even in this context, however, our goal remains unchanged: to give our patients the highest-quality care possible. Our core values as orthopedic surgeons and physicians are dedication, commitment, and service to patients and to our profession. As US health care continues to evolve, we must evolve as well, with an emphasis on expanding our role in the health care policy debate.
In the United States, the landscape of health care is changing. Health care reform and fluctuating political and economic climates have affected and will continue to affect the practice of orthopedic surgery. Demand for musculoskeletal care and the costs of providing this care are exceeding available resources—which has led to an evolution in how we practice as individuals and in the institutions where we provide care. Patient safety, quality, and value have become the outcomes of importance. Orthopedic surgeons, as experts in musculoskeletal care, must be a part of these changes. In this review, we offer perspective on the changing face of orthopedic surgery in the modern US health care system.
1. Meeting the demand
Musculoskeletal conditions represent one of the most common and costly health issues in the United States, affecting individuals medically and economically and compromising their quality of life.1,2 In 2008, more than 110 million US adults (1 in 2) reported having a musculoskeletal condition for more than 3 months, and almost 7% reported that a chronic musculoskeletal condition made routine activities of daily living significantly difficult.1 Overall, in the United States, some of the most common chronic conditions are musculoskeletal in origin. These conditions include osteoarthritis and back pain.
Osteoarthritis is the leading cause of chronic pain and disability. Physician-diagnosed arthritis is expected to affect 25% of US adults by 2030,3 and in more than one-third of these patients arthritis limits work or other activity.4 Back pain is another of the most common debilitating conditions in the United States.3,5 St Sauver and colleagues6 found that back pain is the third most common condition (23.9%) that prompts patients to seek health care—following skin-related problems (42.7%) and osteoarthritis/joint pain (33.6%).
As life expectancy increases, so do expectations of enjoying higher levels of activity into the later years. Patients expect to be as active in their geriatric years as they were in middle age, and many are able to do so. Amid the growing obesity epidemic and increased incidence of chronic comorbidities, however, the aging population not only is at substantial risk for developing a chronic musculoskeletal disorder but may face new challenges in accessing care.
Although orthopedic surgeons specialize in treating musculoskeletal conditions, up to 90% of common nonsurgical musculoskeletal complaints are thought to be manageable in the primary care setting.7 With a disproportionate increase in musculoskeletal demand against a relatively constant number of orthopedic providers,8 it is becoming increasingly important for nonorthopedists to adequately manage musculoskeletal conditions. Physiatrists, rheumatologists, internists, family practitioners, and the expanding field of sports medicine specialists provide primary care of musculoskeletal conditions. To meet the growing demand and to ensure that patients receive quality, sustainable, effective, and efficient care, orthopedic surgeons should be actively involved in training these providers. As high as the cost of managing musculoskeletal conditions can be, it is far less than the cost resulting from inadequate or improper management. There is already justification for formal development of a specialization in nonoperative management of musculoskeletal care. Establishing this specialization requires a multidisciplinary approach, with orthopedic surgery taking a lead role.
2. The cost equation
As the prevalence of orthopedic conditions increases, so does the cost of delivering musculoskeletal care. The economic implications of meeting this growing demand are an important area of concern for our health care system. Steadily increasing hospital expenses for personnel and services, rising costs of pharmaceuticals and laboratory tests, constant evolution of costly technology, and insurance/reimbursement rates that do not keep pace with rising costs all contribute to the rapid escalation of the “cost of care.”
Health care expenditures accounted for 17.2% of the US gross domestic product (GDP) in 2012 and are expected to represent 19.3% by 2023.9 For musculoskeletal disease, direct costs alone are expected to approach $510 billion, equaling 5% of GDP and representing almost 30% of all health care expenditures. In Medicare patients, osteoarthritis is the most expensive condition to treat overall, and 3 other musculoskeletal problems rank highly as well: femoral neck fractures (3rd), back pain (10th), and fractures of all types (16th).10 Clearly, musculoskeletal care is one of the most prevalent and expensive health conditions in the United States.
Part of the direct costs of care that consistently increase each year are the steadily increasing costs of technology, which is often considered synonymous with orthopedic care. Promotion of new and more costly implants is common in the absence of evidence supporting their use. However, use of new implants and technology is being scrutinized in an effort to strike the proper cost–benefit balance.
To change the slope of the cost curve, orthopedic surgeons should utilize technological advances that are proven to be clinically significant and economically feasible and should avoid modest improvements with limited clinical benefit and higher price tags. Unfortunately, this approach is not being taken. Minor modifications of implant designs are often marketed as “new and improved” to justify increased costs, and these implants often gain widespread use. A few may prove to be clinically better, but most will be only comparable to older, less expensive designs, and some may end up being clinical failures, discovered at great cost to patients and the health care system.11,12
Orthopedic surgeons have an important role in this decision-making. We should strive for the best, most cost-effective outcomes for our patients. We should reject new technology that does not clearly improve outcomes. At the least, we should use the technology in a manufacturer-supported clinical trial to determine its superiority. Whether the improvement is in technique, implant design, or workflow efficiency, orthopedic surgeons must be actively involved in researching and developing the latest innovations and must help determine their prospective value by considering not only their potential clinical benefits but also their economic implications.
As the political and economic environment becomes more directed at the cost-containment and sustainability of care, there has been a clear shift in focus to quality and value rather than volume, giving rise to the “value-based care” approach. The “value equation,” in which value equals quality divided by cost, requires a clear measure of outcomes and an equally clear understanding of costs. Delivering high-quality care in a cost-conscious environment is an approach that every orthopedic surgeon should adopt. Widespread adoption of the value-based strategy by hospital systems and insurance companies is resulting in a paradigm shift away from more traditional volume-based metrics and in favor of value-based metrics, including quality measures, patient-reported outcomes, Hospital Consumer Assessment of Healthcare Providers and Systems, and physician-specific outcome measures.
The new paradigm has brought the bundled payment initiative (BPI), a strategy included in the Patient Protection and Affordable Care Act. The philosophy behind the BPI model is for hospital systems and physicians to control costs while maintaining and improving the quality of care. Measured by patient metrics (eg, clinical outcomes, patient satisfaction) and hospital metrics (eg, readmission rates, cost of care), bundled payments reimburse hospitals on the basis of cost of an entire episode of care rather than on the basis of individual procedures and services. This approach provides incentives for both physicians and hospitals to promote value-based care while emphasizing coordination of care among all members of the health care team.
Providing the best possible care for our patients while holding our practice to the highest standards is a central tenet of the practice of orthopedic surgery and should be independent of reimbursement strategies. Thus, to increase the value of care, we must establish practice models and strategies to optimize cost-efficiency while improving outcomes. As explained by Porter and Teisberg,13 it is important to be conscientious about cost, but above all we must not allow quality of health care delivery to be compromised when trying to improve the “value” of care. Through evidence-based management and a clear understanding of costs, we must develop cost-efficient practice models that sustainably deliver the highest value of care.
3. Evolving practice models
As the health care landscape continues to change, physician practice models evolve accordingly. Although the private practice model once dominated the physician workforce, this is no longer true, as there has been a significant shift to employer-based practice models. The multiple factors at work relate to changing patterns of reimbursement, increasing government regulations, and a general change in recent residency graduates’ expectations regarding work–life balance. Other catalysts are the shift from volume- to value-based care and the recognition that cost-effective health care is more easily achieved when physicians and their institutions are in alignment. Ultimately, physician–institution alignment is crucial in improving care and outcomes.
Physician–institution alignment requires further discussion. Ideally, it should strike the proper balance between physician autonomy and institutional priorities to ensure the highest quality care. Physicians and their institutions should align their interests in terms of patient safety, quality, and economics to create a work environment conducive to both patient/physician satisfaction and institutional success.14 As identified by Page and colleagues,15 the primary drivers of physician–institution alignment, specific to orthopedic surgery, are economic, regulatory, and cultural. In economics, implant selection and ancillary services are the important issues; in the regulatory area, cooperative efforts to address expanding state and federal requirements are needed; last, the primary cultural driver is delivery of care to an expanding, diverse patient population.
Physician–institution alignment brings opportunities for “gainsharing,” which can directly benefit individual physicians, physician groups, and departments. Gainsharing is classically defined as “arrangements in which a hospital gives physicians a percentage share of any reduction in the hospital’s costs for patient care attributable in part to the physicians’ efforts.”16 Modern gainsharing programs can be used by institutions to align the economic interests of physicians and hospitals, with the ultimate goal being to achieve a sustainable increase in the value and quality of care delivered to patients.13 Examples include efforts to reduce the cost of orthopedic implants, which is a major cost driver in orthopedic surgery. Our institution realized significant savings when surgeons were directly involved in the implant contracting process with strategic sourcing personnel. These savings were shared with the department to enhance research and education programs. BPI, a risk-sharing program in which Medicare and hospitals participate, incorporates gainsharing opportunities in which each participating physician can receive up to 50% of his or her previous Medicare billings when specific targets are achieved. BPI included 27 musculoskeletal diagnosis–related groups that could be developed into a bundled payment proposal. Our institution participated in a 90-day episode, for primary hip and knee arthroplasty and non–cervical spine fusion, that had very promising results.
Gainsharing offers physicians incentives to meet institution goals of improved outcomes and increased patient satisfaction while increasing oversight and accountability. When physician-specific outcomes do not meet the established goals in key areas (readmissions, thromboembolic complications, infections), it is only logical that steps will be taken to improve outcomes. Although physicians may not be used to this increased scrutiny, the goal of improving outcomes, even if it necessitates a change in an established approach to care, should be welcomed.
Physicians should be rewarded for good outcomes but not suboptimal outcomes. When outcomes are suboptimal, physicians should take a constructive approach to improve them. On the other hand, not being rewarded for unachieved goals can be perceived as being penalized. Additional monitoring may paradoxically lead physicians to avoid more “complex” cases, such as those of patients at higher risk for complications and poorer outcomes. An example is found in patient selection for surgery, in which issues like obesity, diabetes, and heart disease are known to negatively affect outcomes. In these models, “cherry-picking” is a well-recognized risk17,18 that can compromise our ethical obligation to provide equal access for all patients. To offset this tendency, we should use a risk-stratification model in which all patients are not considered equal in the risks they present. A risk-adjustment approach benefits both patients and providers by identifying modifiable risk factors that can be addressed to positively affect outcomes. This risk-stratification approach further incentivizes the orthopedist to closely work with other health care providers to address the medical comorbidities that may negatively affect surgical outcomes.
4. Patient and physician expectations
Living in a technology-driven society in the age of information has had a major impact on patients’ attitudes and expectations about their care—and therefore on physicians’ practice methods. It is uncommon to evaluate a patient who has not already consulted the Internet about a problem. Patients now have much more information they can use to make decisions about their treatment, and, though many question the accuracy of Internet information, there is no argument that being more informed is beneficial. In this time of shared decision-making, it is absolutely essential that patients keep themselves informed.
It is crucial to align the expectations of both physicians and patients in order to achieve the best outcomes. Gaining a clear understanding of treatment goals, management, and potential complications consistently leads to improved patient satisfaction, more favorable clinical outcomes, and reduced risk of litigation.19-22 Addressing patient concerns and expectations is significantly enhanced by a strong patient–physician relationship through clinical models focused on patient-centered care.
Now considered a standard of care, the patient-centered model has changed the way we practice. The foundation of the patient-centered approach is to strengthen the patient–physician relationship by empowering patients to become active decision-makers in the management of their own health. The role of orthopedists in this model is to provide patients with information and insight into their conditions in order to facilitate shared decision-making. Our role should be to guide patients to make educated and informed decisions. Doing so enhances communication, thereby strengthening the patient–physician relationship, and places both patient and physician expectations in perspective. Patient-reported outcomes, satisfaction rates, symptomatic burdens, and costs of care are all positively correlated with strong communication and realistic expectations achieved through a patient-centered approach.21,23
The evolution of clinical practice has been influenced by factors ranging from external forces (eg, changing political and economic climates) to social trends (use of social media and the Internet). Technology has been a driving force in our rapidly changing clinical environment, significantly altering the way we practice. Although we must be careful in how we use it, new technology can certainly work to our advantage. We have a plethora of medical information at our fingertips, and, with physician-directed guidance, our patients can become more informed than ever before. This is the principle of patient-centered medicine and shared decision-making, and its utility will only increase in importance.
5. The role of advocacy
The central tenet of orthopedic practice has always been a focus on patients. We continually strive to improve patient outcomes, reduce costs, and work efficiently in our practices and facilities. Although we can focus on our individual practices, we cannot ignore the influence and impact of the political system on our performance. Federal and state regulations give physicians and insurance companies an uneven playing field. This imbalance requires that physicians be more active in health care policymaking and advocacy. Although we are more involved than ever before, our influence is far less than what we would like it to be, perhaps partly because of the nature of the political process but perhaps also because of physicians’ resistance to becoming involved.
As experts in the treatment of musculoskeletal conditions, we should be at the forefront of health care policy development—a position we have not been able to attain. Although many factors contribute to our lack of a “seat at the table,” we must recognize our reluctance as a group to support advocacy, either financially or through personal time commitment. The American Association of Orthopaedic Surgeons (AAOS) Orthopaedic Political Action Committee has never been able to obtain donations from more than 30% of AAOS members. Although this committee historically has been successful, we could be much more so if we had financial support from 90% of members. There are many ways to be actively involved in advocacy. One way is to join local and state orthopedic societies and support their advocacy efforts. State orthopedic societies work closely with the AAOS Office of Government Relations to coordinate advocacy and direct efforts and resources to areas of greatest need. Knowing local congressional representatives and communicating with them about issues we face in our practices make our issues “real.” Some of our colleagues have even successfully run for office in Congress, and they certainly deserve our support. Advocacy will absolutely play an increasingly important role as federal and state governments expand their involvement in health care. Our role should be to get involved, at least to some degree. We need to recognize that our strength is in our numbers, as the few cannot accomplish nearly as much as the many.
Summary
Orthopedic surgeons are practicing in the midst of almost constant change—evolving patient care, shifts in employment models, advances in technology, modern patient expectations, and an increasingly complex regulatory environment. Even in this context, however, our goal remains unchanged: to give our patients the highest-quality care possible. Our core values as orthopedic surgeons and physicians are dedication, commitment, and service to patients and to our profession. As US health care continues to evolve, we must evolve as well, with an emphasis on expanding our role in the health care policy debate.
1. US Bone and Joint Initiative. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal, and Economic Cost. Rosemont, IL: US Bone and Joint Initiative; 2008. http://www.boneandjointburden.org. Accessed October 26, 2015.
2. US Bone and Joint Initiative. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal, and Economic Cost. 2nd ed. Rosemont, IL: US Bone and Joint Initiative; 2011. http://www.boneandjointburden.org. Accessed October 26, 2015.
3. Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil. 2014;95(5):986-995.e1.
4. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226-229.
5. Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169(3):251-258.
6. St Sauver JL, Warner DO, Yawn BP, et al. Why patients visit their doctors: assessing the most prevalent conditions in a defined American population. Mayo Clin Proc. 2013;88(1):56-67.
7. Anderson BC. Office Orthopedics for Primary Care: Diagnosis and Treatment. 2nd ed. Philadelphia, PA: Saunders; 1999.
8. American Academy of Orthopaedic Surgeons, Department of Research and Scientific Affairs. Orthopaedic Practice in the U.S. 2012 [2012 Orthopaedic Surgeon Census Report]. Rosemont, IL: American Academy of Orthopaedic Surgeons; January 2013.
9. US Department of Health and Human Services, Centers for Medicare & Medicaid Services, Office of the Actuary, National Health Statistics Group. NHE [National Health Expenditure] Fact Sheet, 2014. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet.html. Updated July 28, 2015. Accessed October 26, 2015.
10. Cutler DM, Ghosh K. The potential for cost savings through bundled episode payments. N Engl J Med. 2012;366(12):1075-1077.
11. Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92(1):38-46.
12. Dahlstrand H, Stark A, Anissian L, Hailer NP. Elevated serum concentrations of cobalt, chromium, nickel, and manganese after metal-on-metal alloarthroplasty of the hip: a prospective randomized study. J Arthroplasty. 2009;24(6):837-845.
13. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
14. American Association of Orthopaedic Surgeons. Alignment of physician and facility payment and incentives. Position statement 1171. American Association of Orthopaedic Surgeons website. http://www.aaos.org/about/papers/position/1171.asp. Published September 2006. Revised February 2009. Accessed October 26, 2015.
15. Page AE, Butler CA, Bozic KJ. Factors driving physician–hospital alignment in orthopaedic surgery. Clin Orthop Relat Res. 2013;471(6):1809-1817.
16. US Department of Health and Human Services, Office of Inspector General. Gainsharing arrangements and CMPs for hospital payments to physicians to reduce or limit services to beneficiaries [special advisory bulletin]. Office of Inspector General website. http://oig.hhs.gov/fraud/docs/alertsandbulletins/gainsh.htm. Published July 1999. Accessed October 26, 2015.
17. Bronson WH, Fewer M, Godlewski K, et al. The ethics of patient risk modification prior to elective joint replacement surgery. J Bone Joint Surg Am. 2014;96(13):e113.
18. Bosco J. To cherry pick or not: the unintended ethical consequences of pay for performance. Presented at: New York University Colloquium on Medical Ethics; New York, NY; November 2014.
19. Hageman MG, Briët JP, Bossen JK, Blok RD, Ring DC, Vranceanu AM. Do previsit expectations correlate with satisfaction of new patients presenting for evaluation with an orthopaedic surgical practice? Clin Orthop Relat Res. 2015;473(2):716-721.
20. Jourdan C, Poiraudeau S, Descamps S, et al. Comparison of patient and surgeon expectations of total hip arthroplasty. PLoS One. 2012;7(1):e30195.
21. McMillan S, Kendall E, Sav A, et al. Patient-centered approaches to health care: a systematic review of randomized controlled trials. Med Care Res Rev. 2013;70(6):567-596.
22. Forster HP, Schwartz J, DeRenzo E. Reducing legal risk by practicing patient-centered medicine. Arch Intern Med. 2002;162(11):1217-1219.
23. Van Citters AD, Fahlman C, Goldmann DA, et al. Developing a pathway for high-value, patient-centered total joint arthroplasty. Clin Orthop Relat Res. 2014;472(5):1619-1635.
1. US Bone and Joint Initiative. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal, and Economic Cost. Rosemont, IL: US Bone and Joint Initiative; 2008. http://www.boneandjointburden.org. Accessed October 26, 2015.
2. US Bone and Joint Initiative. Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal, and Economic Cost. 2nd ed. Rosemont, IL: US Bone and Joint Initiative; 2011. http://www.boneandjointburden.org. Accessed October 26, 2015.
3. Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil. 2014;95(5):986-995.e1.
4. Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226-229.
5. Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169(3):251-258.
6. St Sauver JL, Warner DO, Yawn BP, et al. Why patients visit their doctors: assessing the most prevalent conditions in a defined American population. Mayo Clin Proc. 2013;88(1):56-67.
7. Anderson BC. Office Orthopedics for Primary Care: Diagnosis and Treatment. 2nd ed. Philadelphia, PA: Saunders; 1999.
8. American Academy of Orthopaedic Surgeons, Department of Research and Scientific Affairs. Orthopaedic Practice in the U.S. 2012 [2012 Orthopaedic Surgeon Census Report]. Rosemont, IL: American Academy of Orthopaedic Surgeons; January 2013.
9. US Department of Health and Human Services, Centers for Medicare & Medicaid Services, Office of the Actuary, National Health Statistics Group. NHE [National Health Expenditure] Fact Sheet, 2014. Centers for Medicare & Medicaid Services website. http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet.html. Updated July 28, 2015. Accessed October 26, 2015.
10. Cutler DM, Ghosh K. The potential for cost savings through bundled episode payments. N Engl J Med. 2012;366(12):1075-1077.
11. Langton DJ, Jameson SS, Joyce TJ, Hallab NJ, Natu S, Nargol AV. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: a consequence of excess wear. J Bone Joint Surg Br. 2010;92(1):38-46.
12. Dahlstrand H, Stark A, Anissian L, Hailer NP. Elevated serum concentrations of cobalt, chromium, nickel, and manganese after metal-on-metal alloarthroplasty of the hip: a prospective randomized study. J Arthroplasty. 2009;24(6):837-845.
13. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Boston, MA: Harvard Business School Press; 2006.
14. American Association of Orthopaedic Surgeons. Alignment of physician and facility payment and incentives. Position statement 1171. American Association of Orthopaedic Surgeons website. http://www.aaos.org/about/papers/position/1171.asp. Published September 2006. Revised February 2009. Accessed October 26, 2015.
15. Page AE, Butler CA, Bozic KJ. Factors driving physician–hospital alignment in orthopaedic surgery. Clin Orthop Relat Res. 2013;471(6):1809-1817.
16. US Department of Health and Human Services, Office of Inspector General. Gainsharing arrangements and CMPs for hospital payments to physicians to reduce or limit services to beneficiaries [special advisory bulletin]. Office of Inspector General website. http://oig.hhs.gov/fraud/docs/alertsandbulletins/gainsh.htm. Published July 1999. Accessed October 26, 2015.
17. Bronson WH, Fewer M, Godlewski K, et al. The ethics of patient risk modification prior to elective joint replacement surgery. J Bone Joint Surg Am. 2014;96(13):e113.
18. Bosco J. To cherry pick or not: the unintended ethical consequences of pay for performance. Presented at: New York University Colloquium on Medical Ethics; New York, NY; November 2014.
19. Hageman MG, Briët JP, Bossen JK, Blok RD, Ring DC, Vranceanu AM. Do previsit expectations correlate with satisfaction of new patients presenting for evaluation with an orthopaedic surgical practice? Clin Orthop Relat Res. 2015;473(2):716-721.
20. Jourdan C, Poiraudeau S, Descamps S, et al. Comparison of patient and surgeon expectations of total hip arthroplasty. PLoS One. 2012;7(1):e30195.
21. McMillan S, Kendall E, Sav A, et al. Patient-centered approaches to health care: a systematic review of randomized controlled trials. Med Care Res Rev. 2013;70(6):567-596.
22. Forster HP, Schwartz J, DeRenzo E. Reducing legal risk by practicing patient-centered medicine. Arch Intern Med. 2002;162(11):1217-1219.
23. Van Citters AD, Fahlman C, Goldmann DA, et al. Developing a pathway for high-value, patient-centered total joint arthroplasty. Clin Orthop Relat Res. 2014;472(5):1619-1635.
Total Shoulder Arthroplasty Outcome for Treatment of Osteoarthritis: A Multicenter Study Using a Contemporary Implant
Anatomical total shoulder arthroplasty (TSA) is an effective treatment for advanced osteoarthritis (OA) of the glenohumeral joint.1-4 Over the past 40 years, since the early reports appeared, the implants have evolved from the early monoblock humeral component to modular components, variable neck angled components with eccentric heads, and components that can provide variable neck angles, version angles, and dual eccentricity to match the anatomy of the proximal humerus. The goal of the new implants is to replicate the individual patient’s native anatomy using a combination of modularity, multiple neck and version angles, and dual eccentricity of the neck and head. The flexibility of the implant system is made possible by a replicator plate. There are few reports on outcomes of using these new implants for OA.
In this article, we report outcomes of using a dual eccentric, variable neck angle, variable version angle implant with a replicator plate for the treatment of OA of the shoulder at 4 centers.
Materials and Methods
The Western Institutional Review Board approved this study, and consent was prospectively obtained and retrospectively reviewed.
The data banks of a 4-center consortium were queried. Only primary TSA patients treated for OA with a fourth-generation Exactech Equinoxe implant (Exactech, Inc.) were included. For the center to be included, it had to have an 80% patient follow-up rate at a minimum of 2 years. Four centers qualified for inclusion: University of Florida, Medical College of Georgia, New York University, and Bordeaux-Merignac Clinic. Data were obtained on surgeries sequentially performed between August 1, 2006, and December 31, 2010. All data were obtained prospectively using a common data collection format.
The Equinoxe anatomical TSA allows for independent adaptation of neck angle and humeral version and provides 2 variable offset times (1 on replicator plate, 1 on humeral head) for matching the native anatomy in more than 99% of cases5 (Figure). The replicator plate is eccentric and can be angled 7.5° in any direction and rotated 360° to provide humeral head coverage. Once its optimal position is obtained, the plate is permanently fixed to the humeral stem using a breakaway screw. Some contemporary implants have similar features.
There were 218 primary shoulder arthroplasties performed on 201 patients (98 male, 103 female). Mean age at time of surgery was 67 years (range, 31-87 years), and mean follow-up was 36 months (range, 24-72 months). The collective follow-up rate at the 3-year mean follow-up and 2-year minimal follow-up was 81%. Eleven shoulders had a cemented stem, and 207 had an uncemented stem. Forty-eight shoulders used the 1.5-mm replicator plate, and 170 used the 4.5-mm offset replicator plate. The patients in this study were typically not very healthy: mean American Society of Anesthesiologists (ASA) score was 2.57 (range, 1-3).
Five outcome scores were calculated from the prospectively obtained data: Constant normalized, Shoulder Pain and Disability Index (SPADI), Simple Shoulder Test (SST), UCLA Shoulder Rating Scale (UCLA), and American Shoulder and Elbow Surgeons Shoulder Assessment (ASES). Before initiating data collection, we developed the Metric Form6 so we could calculate multiple scores while asking the minimal possible number of questions. This could be done for all 5 outcome scores, as their questions have significant overlap.
Objective outcomes included active external rotation, active scaption, active abduction, and active internal rotation. Complications, including revisions, were noted and analyzed. We focus on functional outcomes and do not present radiographic outcomes.
Results
A 2-tailed unpaired t test was used to compare preoperative values with final outcome values (P < .05). Four objective outcomes were significantly improved over preoperative levels: active external rotation (preoperative, 15°; postoperative, 42°), active scaption (pre, 92°; post, 137°), active abduction (pre, 80°; post, 121°), and active internal rotation (pre, S3; post, L2). The functional outcome scores that were significantly (P < .05) improved at final follow-up were Constant normalized (pre, 39; post, 79), SPADI (pre, 86; post, 20), SST (pre, 3.3; post, 10), UCLA (pre, 13; post, 31), and ASES (pre, 33; post, 85).
The outcome improvements at latest follow-up were active external rotation (+28), active scaption (+45), active abduction (+42), active internal rotation (+6 anatomical segments), Constant normalized (+40), SPADI (–66), SST (+6.7), UCLA (+18), and ASES (+52).
There were 32 complications in 25 shoulders. There were no bilateral complications. Seven shoulders had multiple complications, of which many were not independent events. For example, rotator cuff deficiency was associated with instability, and infection was associated with glenoid loosening. One patient had 2 procedures, the first an arthroscopic release and the second a revision shoulder arthroplasty for glenoid loosening. The most common postoperative complication was rotator cuff failure (RCF) or suspected RCF (13 shoulders, including 8 treated with revision arthroplasty). RCF occurred most commonly at the rotator cuff interval, followed by the subscapularis and the supraspinatus. RCF location was based on computed tomography scan or intraoperative observation. The few subscapularis failures occurred with both subscapularis tendon repair and osteotomy. The high RCF rate may derive from scrutinizing postoperative radiographs and was not necessarily confirmed with repeat surgery. We think this represents a more realistic estimate of true postoperative rotator cuff dysfunction, rather than including only reoperated cases. The second most common complication was infection (6 shoulders, 1 with a superficial suture abscess and 5 with deep infections). Other complications were instability (4, with 2 caused by rotator cuff insufficiency), glenoid loosening (4, with 2 caused by infection), stiffness (3), nerve issue (1), and hematoma evacuation (1).
In 21 shoulders, these complications were treated with revision shoulder arthroplasty (16 shoulders), arthroscopic capsular release (3), evacuation of postoperative hematoma (1), and débridement of suture abscess (1). The 16 revision shoulder arthroplasties performed were conversion to reverse shoulder arthroplasty (11 shoulders) and placement of an antibiotic spacer for infection (5). The stem was left in place for all revisions, excluding those for infection. This is a significant advantage of the modular platform stem. Details of the complications and treatments are listed in the Table. There was no difference in health status between patients with a complication (ASA, 2.57) and those without one (ASA, 2.56).
Discussion
The implant described in this article consists of a metaphyseal press-fit stem, a replicator plate, multiple eccentric humeral heads, and a glenoid of multiple sizes with 2 radii of curvatures used to match the patient’s native anatomy and still maintain the appropriate radius of curvature mismatch between the humeral head and the glenoid. Between the eccentricity in the replicator plate and the eccentricity in the humeral head, almost any humeral head cut can be covered, more than 99% of the time.1 However, it remains to be seen if a versatile implant that comes close to matching the patient’s native anatomy will make a difference clinically.
The objective and functional outcomes in this study compare well with those of other, large TSA studies using older prostheses.1-4 There are few reports on contemporary implants with sufficient follow-up numbers for the single diagnosis of OA. Norris and Iannotti2 reported on a multicenter study of 176 patients with a Depuy Global TSA. The design of their study comes closest to that of our clinical outcome study. Nineteen surgeons were involved in their study. The follow-up rate is not clear. Their outcomes (with ours in parentheses for comparison) were active external rotation of 45° (42°), active elevation of 138° (137°), ASES of 84 (85), and SST of 9.2 (10). Norris and Iannotti2 noted an overall complication rate of 13% (12% in our series). Their most common postoperative complications were RCF and glenoid loosening; ours were RCF and infection. Another multicenter study with short-term results using a contemporary prosthesis included 268 shoulders followed for a minimum of 12 months.1 At final follow-up, Constant score was 97, active elevation was 145°, and the complication rate was 8.6%. Godenèche and colleagues1 also noted a glenoid lucent-line rate of 58% and reported that rotator cuff pathology adversely affected outcome.
Although the overall clinical outcome results are encouraging and the complication rate is in the reported range, we believe that a focus on the major complication categories may have a significant positive impact on our patients. The present article places significant importance on reporting complications prospectively, which is more accurate than retrospective reporting. The rates of both RCF and infection, the most common complications in our study, need to be decreased. Aldinger and colleagues7 reported a 12% complication rate in 485 primary shoulder arthroplasties—a rate identical to ours here. In their study, nerve injuries and humeral fractures were both more common than rotator cuff tears. We think that rotator cuff deficiency after TSA is underreported because it is often based on revision surgery alone. It is also interesting that the majority of the cuff deficiencies were through the upper subscapularis rotator interval and were not a complete failure of the subscapularis repair. Not all these patients will undergo revision surgery. In the future, the RCF rate may drop with the increasingly common use of reverse shoulder arthroplasty for substandard rotator cuffs.
Use of this contemporary variable neck angle, variable version angle, dual eccentric shoulder arthroplasty with a replicator plate provides satisfying short-term clinical outcomes. Patients with less than optimal health (mean ASA, 2.57) seem to tolerate the procedure well. Continued focus on RCF and infection will have the greatest impact on the overall complication rate.
1. Godenèche A, Boileau P, Favard L, et al. Prosthetic replacement in the treatment of osteoarthritis of the shoulder: early results of 268 cases. J Shoulder Elbow Surg. 2002;11(1):11-18.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
3. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
4. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
5. Irlenbusch U, Rott O, Gebhardt K, Werner A. Reconstruction of the rotational centre of the humeral head with double eccentric adaptable shoulder prosthesis [abstract]. In: Proceedings of the European Federation of National Associations of Orthopaedics and Traumatology (EFORT); May 29-June 1, 2008; Nice, France.
6. Flurin PH, Roche CP, Wright TW, Zuckerman J, Johnson D, Christensen M. A correlation of five commonly used clinical metrics to measure outcomes in shoulder arthroplasty. In: Transactions of the 58th Annual Meeting of the Orthopaedic Research Society (ORS); February 4-7, 2012; San Francisco, CA.
7. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
Anatomical total shoulder arthroplasty (TSA) is an effective treatment for advanced osteoarthritis (OA) of the glenohumeral joint.1-4 Over the past 40 years, since the early reports appeared, the implants have evolved from the early monoblock humeral component to modular components, variable neck angled components with eccentric heads, and components that can provide variable neck angles, version angles, and dual eccentricity to match the anatomy of the proximal humerus. The goal of the new implants is to replicate the individual patient’s native anatomy using a combination of modularity, multiple neck and version angles, and dual eccentricity of the neck and head. The flexibility of the implant system is made possible by a replicator plate. There are few reports on outcomes of using these new implants for OA.
In this article, we report outcomes of using a dual eccentric, variable neck angle, variable version angle implant with a replicator plate for the treatment of OA of the shoulder at 4 centers.
Materials and Methods
The Western Institutional Review Board approved this study, and consent was prospectively obtained and retrospectively reviewed.
The data banks of a 4-center consortium were queried. Only primary TSA patients treated for OA with a fourth-generation Exactech Equinoxe implant (Exactech, Inc.) were included. For the center to be included, it had to have an 80% patient follow-up rate at a minimum of 2 years. Four centers qualified for inclusion: University of Florida, Medical College of Georgia, New York University, and Bordeaux-Merignac Clinic. Data were obtained on surgeries sequentially performed between August 1, 2006, and December 31, 2010. All data were obtained prospectively using a common data collection format.
The Equinoxe anatomical TSA allows for independent adaptation of neck angle and humeral version and provides 2 variable offset times (1 on replicator plate, 1 on humeral head) for matching the native anatomy in more than 99% of cases5 (Figure). The replicator plate is eccentric and can be angled 7.5° in any direction and rotated 360° to provide humeral head coverage. Once its optimal position is obtained, the plate is permanently fixed to the humeral stem using a breakaway screw. Some contemporary implants have similar features.
There were 218 primary shoulder arthroplasties performed on 201 patients (98 male, 103 female). Mean age at time of surgery was 67 years (range, 31-87 years), and mean follow-up was 36 months (range, 24-72 months). The collective follow-up rate at the 3-year mean follow-up and 2-year minimal follow-up was 81%. Eleven shoulders had a cemented stem, and 207 had an uncemented stem. Forty-eight shoulders used the 1.5-mm replicator plate, and 170 used the 4.5-mm offset replicator plate. The patients in this study were typically not very healthy: mean American Society of Anesthesiologists (ASA) score was 2.57 (range, 1-3).
Five outcome scores were calculated from the prospectively obtained data: Constant normalized, Shoulder Pain and Disability Index (SPADI), Simple Shoulder Test (SST), UCLA Shoulder Rating Scale (UCLA), and American Shoulder and Elbow Surgeons Shoulder Assessment (ASES). Before initiating data collection, we developed the Metric Form6 so we could calculate multiple scores while asking the minimal possible number of questions. This could be done for all 5 outcome scores, as their questions have significant overlap.
Objective outcomes included active external rotation, active scaption, active abduction, and active internal rotation. Complications, including revisions, were noted and analyzed. We focus on functional outcomes and do not present radiographic outcomes.
Results
A 2-tailed unpaired t test was used to compare preoperative values with final outcome values (P < .05). Four objective outcomes were significantly improved over preoperative levels: active external rotation (preoperative, 15°; postoperative, 42°), active scaption (pre, 92°; post, 137°), active abduction (pre, 80°; post, 121°), and active internal rotation (pre, S3; post, L2). The functional outcome scores that were significantly (P < .05) improved at final follow-up were Constant normalized (pre, 39; post, 79), SPADI (pre, 86; post, 20), SST (pre, 3.3; post, 10), UCLA (pre, 13; post, 31), and ASES (pre, 33; post, 85).
The outcome improvements at latest follow-up were active external rotation (+28), active scaption (+45), active abduction (+42), active internal rotation (+6 anatomical segments), Constant normalized (+40), SPADI (–66), SST (+6.7), UCLA (+18), and ASES (+52).
There were 32 complications in 25 shoulders. There were no bilateral complications. Seven shoulders had multiple complications, of which many were not independent events. For example, rotator cuff deficiency was associated with instability, and infection was associated with glenoid loosening. One patient had 2 procedures, the first an arthroscopic release and the second a revision shoulder arthroplasty for glenoid loosening. The most common postoperative complication was rotator cuff failure (RCF) or suspected RCF (13 shoulders, including 8 treated with revision arthroplasty). RCF occurred most commonly at the rotator cuff interval, followed by the subscapularis and the supraspinatus. RCF location was based on computed tomography scan or intraoperative observation. The few subscapularis failures occurred with both subscapularis tendon repair and osteotomy. The high RCF rate may derive from scrutinizing postoperative radiographs and was not necessarily confirmed with repeat surgery. We think this represents a more realistic estimate of true postoperative rotator cuff dysfunction, rather than including only reoperated cases. The second most common complication was infection (6 shoulders, 1 with a superficial suture abscess and 5 with deep infections). Other complications were instability (4, with 2 caused by rotator cuff insufficiency), glenoid loosening (4, with 2 caused by infection), stiffness (3), nerve issue (1), and hematoma evacuation (1).
In 21 shoulders, these complications were treated with revision shoulder arthroplasty (16 shoulders), arthroscopic capsular release (3), evacuation of postoperative hematoma (1), and débridement of suture abscess (1). The 16 revision shoulder arthroplasties performed were conversion to reverse shoulder arthroplasty (11 shoulders) and placement of an antibiotic spacer for infection (5). The stem was left in place for all revisions, excluding those for infection. This is a significant advantage of the modular platform stem. Details of the complications and treatments are listed in the Table. There was no difference in health status between patients with a complication (ASA, 2.57) and those without one (ASA, 2.56).
Discussion
The implant described in this article consists of a metaphyseal press-fit stem, a replicator plate, multiple eccentric humeral heads, and a glenoid of multiple sizes with 2 radii of curvatures used to match the patient’s native anatomy and still maintain the appropriate radius of curvature mismatch between the humeral head and the glenoid. Between the eccentricity in the replicator plate and the eccentricity in the humeral head, almost any humeral head cut can be covered, more than 99% of the time.1 However, it remains to be seen if a versatile implant that comes close to matching the patient’s native anatomy will make a difference clinically.
The objective and functional outcomes in this study compare well with those of other, large TSA studies using older prostheses.1-4 There are few reports on contemporary implants with sufficient follow-up numbers for the single diagnosis of OA. Norris and Iannotti2 reported on a multicenter study of 176 patients with a Depuy Global TSA. The design of their study comes closest to that of our clinical outcome study. Nineteen surgeons were involved in their study. The follow-up rate is not clear. Their outcomes (with ours in parentheses for comparison) were active external rotation of 45° (42°), active elevation of 138° (137°), ASES of 84 (85), and SST of 9.2 (10). Norris and Iannotti2 noted an overall complication rate of 13% (12% in our series). Their most common postoperative complications were RCF and glenoid loosening; ours were RCF and infection. Another multicenter study with short-term results using a contemporary prosthesis included 268 shoulders followed for a minimum of 12 months.1 At final follow-up, Constant score was 97, active elevation was 145°, and the complication rate was 8.6%. Godenèche and colleagues1 also noted a glenoid lucent-line rate of 58% and reported that rotator cuff pathology adversely affected outcome.
Although the overall clinical outcome results are encouraging and the complication rate is in the reported range, we believe that a focus on the major complication categories may have a significant positive impact on our patients. The present article places significant importance on reporting complications prospectively, which is more accurate than retrospective reporting. The rates of both RCF and infection, the most common complications in our study, need to be decreased. Aldinger and colleagues7 reported a 12% complication rate in 485 primary shoulder arthroplasties—a rate identical to ours here. In their study, nerve injuries and humeral fractures were both more common than rotator cuff tears. We think that rotator cuff deficiency after TSA is underreported because it is often based on revision surgery alone. It is also interesting that the majority of the cuff deficiencies were through the upper subscapularis rotator interval and were not a complete failure of the subscapularis repair. Not all these patients will undergo revision surgery. In the future, the RCF rate may drop with the increasingly common use of reverse shoulder arthroplasty for substandard rotator cuffs.
Use of this contemporary variable neck angle, variable version angle, dual eccentric shoulder arthroplasty with a replicator plate provides satisfying short-term clinical outcomes. Patients with less than optimal health (mean ASA, 2.57) seem to tolerate the procedure well. Continued focus on RCF and infection will have the greatest impact on the overall complication rate.
Anatomical total shoulder arthroplasty (TSA) is an effective treatment for advanced osteoarthritis (OA) of the glenohumeral joint.1-4 Over the past 40 years, since the early reports appeared, the implants have evolved from the early monoblock humeral component to modular components, variable neck angled components with eccentric heads, and components that can provide variable neck angles, version angles, and dual eccentricity to match the anatomy of the proximal humerus. The goal of the new implants is to replicate the individual patient’s native anatomy using a combination of modularity, multiple neck and version angles, and dual eccentricity of the neck and head. The flexibility of the implant system is made possible by a replicator plate. There are few reports on outcomes of using these new implants for OA.
In this article, we report outcomes of using a dual eccentric, variable neck angle, variable version angle implant with a replicator plate for the treatment of OA of the shoulder at 4 centers.
Materials and Methods
The Western Institutional Review Board approved this study, and consent was prospectively obtained and retrospectively reviewed.
The data banks of a 4-center consortium were queried. Only primary TSA patients treated for OA with a fourth-generation Exactech Equinoxe implant (Exactech, Inc.) were included. For the center to be included, it had to have an 80% patient follow-up rate at a minimum of 2 years. Four centers qualified for inclusion: University of Florida, Medical College of Georgia, New York University, and Bordeaux-Merignac Clinic. Data were obtained on surgeries sequentially performed between August 1, 2006, and December 31, 2010. All data were obtained prospectively using a common data collection format.
The Equinoxe anatomical TSA allows for independent adaptation of neck angle and humeral version and provides 2 variable offset times (1 on replicator plate, 1 on humeral head) for matching the native anatomy in more than 99% of cases5 (Figure). The replicator plate is eccentric and can be angled 7.5° in any direction and rotated 360° to provide humeral head coverage. Once its optimal position is obtained, the plate is permanently fixed to the humeral stem using a breakaway screw. Some contemporary implants have similar features.
There were 218 primary shoulder arthroplasties performed on 201 patients (98 male, 103 female). Mean age at time of surgery was 67 years (range, 31-87 years), and mean follow-up was 36 months (range, 24-72 months). The collective follow-up rate at the 3-year mean follow-up and 2-year minimal follow-up was 81%. Eleven shoulders had a cemented stem, and 207 had an uncemented stem. Forty-eight shoulders used the 1.5-mm replicator plate, and 170 used the 4.5-mm offset replicator plate. The patients in this study were typically not very healthy: mean American Society of Anesthesiologists (ASA) score was 2.57 (range, 1-3).
Five outcome scores were calculated from the prospectively obtained data: Constant normalized, Shoulder Pain and Disability Index (SPADI), Simple Shoulder Test (SST), UCLA Shoulder Rating Scale (UCLA), and American Shoulder and Elbow Surgeons Shoulder Assessment (ASES). Before initiating data collection, we developed the Metric Form6 so we could calculate multiple scores while asking the minimal possible number of questions. This could be done for all 5 outcome scores, as their questions have significant overlap.
Objective outcomes included active external rotation, active scaption, active abduction, and active internal rotation. Complications, including revisions, were noted and analyzed. We focus on functional outcomes and do not present radiographic outcomes.
Results
A 2-tailed unpaired t test was used to compare preoperative values with final outcome values (P < .05). Four objective outcomes were significantly improved over preoperative levels: active external rotation (preoperative, 15°; postoperative, 42°), active scaption (pre, 92°; post, 137°), active abduction (pre, 80°; post, 121°), and active internal rotation (pre, S3; post, L2). The functional outcome scores that were significantly (P < .05) improved at final follow-up were Constant normalized (pre, 39; post, 79), SPADI (pre, 86; post, 20), SST (pre, 3.3; post, 10), UCLA (pre, 13; post, 31), and ASES (pre, 33; post, 85).
The outcome improvements at latest follow-up were active external rotation (+28), active scaption (+45), active abduction (+42), active internal rotation (+6 anatomical segments), Constant normalized (+40), SPADI (–66), SST (+6.7), UCLA (+18), and ASES (+52).
There were 32 complications in 25 shoulders. There were no bilateral complications. Seven shoulders had multiple complications, of which many were not independent events. For example, rotator cuff deficiency was associated with instability, and infection was associated with glenoid loosening. One patient had 2 procedures, the first an arthroscopic release and the second a revision shoulder arthroplasty for glenoid loosening. The most common postoperative complication was rotator cuff failure (RCF) or suspected RCF (13 shoulders, including 8 treated with revision arthroplasty). RCF occurred most commonly at the rotator cuff interval, followed by the subscapularis and the supraspinatus. RCF location was based on computed tomography scan or intraoperative observation. The few subscapularis failures occurred with both subscapularis tendon repair and osteotomy. The high RCF rate may derive from scrutinizing postoperative radiographs and was not necessarily confirmed with repeat surgery. We think this represents a more realistic estimate of true postoperative rotator cuff dysfunction, rather than including only reoperated cases. The second most common complication was infection (6 shoulders, 1 with a superficial suture abscess and 5 with deep infections). Other complications were instability (4, with 2 caused by rotator cuff insufficiency), glenoid loosening (4, with 2 caused by infection), stiffness (3), nerve issue (1), and hematoma evacuation (1).
In 21 shoulders, these complications were treated with revision shoulder arthroplasty (16 shoulders), arthroscopic capsular release (3), evacuation of postoperative hematoma (1), and débridement of suture abscess (1). The 16 revision shoulder arthroplasties performed were conversion to reverse shoulder arthroplasty (11 shoulders) and placement of an antibiotic spacer for infection (5). The stem was left in place for all revisions, excluding those for infection. This is a significant advantage of the modular platform stem. Details of the complications and treatments are listed in the Table. There was no difference in health status between patients with a complication (ASA, 2.57) and those without one (ASA, 2.56).
Discussion
The implant described in this article consists of a metaphyseal press-fit stem, a replicator plate, multiple eccentric humeral heads, and a glenoid of multiple sizes with 2 radii of curvatures used to match the patient’s native anatomy and still maintain the appropriate radius of curvature mismatch between the humeral head and the glenoid. Between the eccentricity in the replicator plate and the eccentricity in the humeral head, almost any humeral head cut can be covered, more than 99% of the time.1 However, it remains to be seen if a versatile implant that comes close to matching the patient’s native anatomy will make a difference clinically.
The objective and functional outcomes in this study compare well with those of other, large TSA studies using older prostheses.1-4 There are few reports on contemporary implants with sufficient follow-up numbers for the single diagnosis of OA. Norris and Iannotti2 reported on a multicenter study of 176 patients with a Depuy Global TSA. The design of their study comes closest to that of our clinical outcome study. Nineteen surgeons were involved in their study. The follow-up rate is not clear. Their outcomes (with ours in parentheses for comparison) were active external rotation of 45° (42°), active elevation of 138° (137°), ASES of 84 (85), and SST of 9.2 (10). Norris and Iannotti2 noted an overall complication rate of 13% (12% in our series). Their most common postoperative complications were RCF and glenoid loosening; ours were RCF and infection. Another multicenter study with short-term results using a contemporary prosthesis included 268 shoulders followed for a minimum of 12 months.1 At final follow-up, Constant score was 97, active elevation was 145°, and the complication rate was 8.6%. Godenèche and colleagues1 also noted a glenoid lucent-line rate of 58% and reported that rotator cuff pathology adversely affected outcome.
Although the overall clinical outcome results are encouraging and the complication rate is in the reported range, we believe that a focus on the major complication categories may have a significant positive impact on our patients. The present article places significant importance on reporting complications prospectively, which is more accurate than retrospective reporting. The rates of both RCF and infection, the most common complications in our study, need to be decreased. Aldinger and colleagues7 reported a 12% complication rate in 485 primary shoulder arthroplasties—a rate identical to ours here. In their study, nerve injuries and humeral fractures were both more common than rotator cuff tears. We think that rotator cuff deficiency after TSA is underreported because it is often based on revision surgery alone. It is also interesting that the majority of the cuff deficiencies were through the upper subscapularis rotator interval and were not a complete failure of the subscapularis repair. Not all these patients will undergo revision surgery. In the future, the RCF rate may drop with the increasingly common use of reverse shoulder arthroplasty for substandard rotator cuffs.
Use of this contemporary variable neck angle, variable version angle, dual eccentric shoulder arthroplasty with a replicator plate provides satisfying short-term clinical outcomes. Patients with less than optimal health (mean ASA, 2.57) seem to tolerate the procedure well. Continued focus on RCF and infection will have the greatest impact on the overall complication rate.
1. Godenèche A, Boileau P, Favard L, et al. Prosthetic replacement in the treatment of osteoarthritis of the shoulder: early results of 268 cases. J Shoulder Elbow Surg. 2002;11(1):11-18.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
3. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
4. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
5. Irlenbusch U, Rott O, Gebhardt K, Werner A. Reconstruction of the rotational centre of the humeral head with double eccentric adaptable shoulder prosthesis [abstract]. In: Proceedings of the European Federation of National Associations of Orthopaedics and Traumatology (EFORT); May 29-June 1, 2008; Nice, France.
6. Flurin PH, Roche CP, Wright TW, Zuckerman J, Johnson D, Christensen M. A correlation of five commonly used clinical metrics to measure outcomes in shoulder arthroplasty. In: Transactions of the 58th Annual Meeting of the Orthopaedic Research Society (ORS); February 4-7, 2012; San Francisco, CA.
7. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.
1. Godenèche A, Boileau P, Favard L, et al. Prosthetic replacement in the treatment of osteoarthritis of the shoulder: early results of 268 cases. J Shoulder Elbow Surg. 2002;11(1):11-18.
2. Norris TR, Iannotti JP. Functional outcome after shoulder arthroplasty for primary osteoarthritis: a multicenter study. J Shoulder Elbow Surg. 2002;11(2):130-135.
3. Razmjou H, Holtby R, Christakis M, Axelrod T, Richards R. Impact of prosthetic design on clinical and radiologic outcomes of total shoulder arthroplasty: a prospective study. J Shoulder Elbow Surg. 2013;22(2):206-214.
4. Walch G, Young AA, Melis B, Gazielly D, Loew M, Boileau P. Results of a convex-back cemented keeled glenoid component in primary osteoarthritis: multicenter study with a follow-up greater than 5 years. J Shoulder Elbow Surg. 2011;20(3):385-394.
5. Irlenbusch U, Rott O, Gebhardt K, Werner A. Reconstruction of the rotational centre of the humeral head with double eccentric adaptable shoulder prosthesis [abstract]. In: Proceedings of the European Federation of National Associations of Orthopaedics and Traumatology (EFORT); May 29-June 1, 2008; Nice, France.
6. Flurin PH, Roche CP, Wright TW, Zuckerman J, Johnson D, Christensen M. A correlation of five commonly used clinical metrics to measure outcomes in shoulder arthroplasty. In: Transactions of the 58th Annual Meeting of the Orthopaedic Research Society (ORS); February 4-7, 2012; San Francisco, CA.
7. Aldinger PR, Raiss P, Rickert M, Loew M. Complications in shoulder arthroplasty: an analysis of 485 cases. Int Orthop. 2010;34(4):517-524.