User login
Ovarian tumor markers: What to draw and when
Tumor markers are serum measures that are valuable in the discrimination of an adnexal mass. However, given the long list from which to choose, it can be confusing to know exactly which might best serve your diagnostic needs. I am commonly asked by obstetrician/gynecologists and primary care doctors for guidance on this subject. In this column I will explore some of the decision making that I use when determining which markers might be most helpful for individual patients.
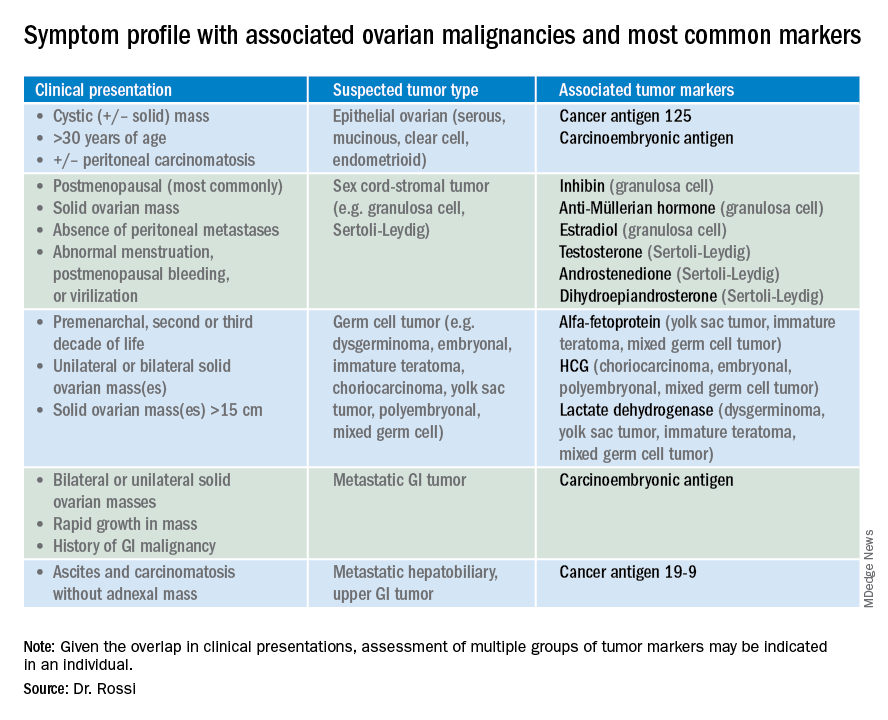
So which tumor markers should you order when you have diagnosed an adnexal mass? Because tumor marker profiles can differ dramatically based on the cell type of the neoplasm, perhaps the first question to ask is what is the most likely category of neoplasm based on other clinical data? Ovarian neoplasms fit into the following subgroups: epithelial (including the most common cell type, serous ovarian cancer, but also the less common mucinous and low malignant potential tumors), sex cord-stromal tumors, germ cell tumors, and metastatic tumors. Table 1 summarizes which tumor markers should be considered based on the clinical setting.
You should suspect an epithelial tumor if there is an adnexal mass with significant cystic components in older, postmenopausal patients, or the presence of peritoneal carcinomatosis on imaging. The tumor markers most commonly elevated in this clinical setting are cancer antigen 125 (CA 125), carcinoembryonic antigen (CEA), and possibly CA 19-9. The CA 125 antigen is a glycoprotein derived from the epithelium of peritoneum, pleura, pericardium, and Müllerian tissues. The multiple sites of origin of this glycoprotein speaks to the poor specificity associated with its elevation, as it is well known to be elevated in both benign conditions such as endometriosis, fibroids, pregnancy, ovulation, cirrhosis, and pericarditis as well as in nongynecologic malignancies, particularly those metastatic to the peritoneal cavity. Multiple different assays are available to measure CA 125, and each is associated with a slightly different reference range. Therefore, if measuring serial values, it is best to have these assessed by the same laboratory. Similarly, as it can be physiologically elevated during the menstrual cycle, premenopausal women should have serial assessments at the same point in their menstrual cycle or ideally within the first 2 weeks of their cycle.
The sensitivity of CA 125 in detecting ovarian cancer is only 78%, which is limited by the fact that not all epithelial ovarian cancer cell types (including some clear cell, carcinosarcoma, and mucinous) express elevations in this tumor marker, and because CA 125 is elevated in less than half of stage I ovarian cancers.1 Therefore, given the lack of sensitivity and specificity for this tumor marker, you should integrate other clinical data, such as imaging findings, age of the patient, and associated benign medical conditions, when evaluating the likelihood of cancer. The American College of Obstetricians and Gynecologists (ACOG) recommends that in the setting of an adnexal mass, referral to gynecologic oncology is recommended when the CA 125 value is greater than 200 U/mL in premenopausal women, or greater than 35U/mL in postmenopausal women.2
CEA is a protein that can be expressed in the colon but not in other normal tissues after birth, and therefore its elevation is commonly associated with metastatic GI tumors to the ovary and peritoneum, or mucinous ovarian tumors, including borderline tumors. Metastatic GI tumors typically are suspected when there are bilateral ovarian solid masses. Right-sided ovarian cysts also can be associated with appendiceal pathology and checking a CEA level can be considered in these cases. I will commonly draw both CA 125 and CEA tumor markers in the setting of cystic +/– solid ovarian masses. This allows the recognition of CA 125-negative/CEA-positive ovarian cancers, such as mucinous tumors, which aids in later surveillance or increases my suspicion for an occult GI tumor (particularly if there is a disproportionately higher elevation in CEA than CA 125).3 If tumor marker profiles are suggestive of an occult GI tumor, I often will consider a preoperative colonoscopy and upper GI endoscopic assessment.
CA 19-9 is a much less specific tumor marker which can be elevated in a variety of solid organ tumors including pancreatic, hepatobiliary, gastric and ovarian tumors. I typically reserve adding this marker for atypical clinical presentations of ovarian cancer, such as carcinomatosis in the absence of pelvic masses.
Ovarian sex cord-stromal neoplasms most commonly present as solid tumors in the ovary. The ovarian stroma includes the bland fibroblasts and the hormone-producing sex-cord granulosa, Sertoli and Leydig cells. Therefore the sex cord-stromal tumors commonly are associated with elevations in serum inhibin, anti-Müllerian hormone, and potentially androstenedione and dehydroepiandrosterone.4 These tumors rarely have advanced disease at diagnosis. Granulosa cell tumors should be suspected in women with a solid ovarian mass and abnormal uterine bleeding (including postmenopausal bleeding), and the appropriate tumor markers (inhibin and anti-Müllerian hormone) can guide this diagnosis preoperatively.4 Androgen-secreting stromal tumors such as Sertoli-Leydig tumors often present with virilization or menstrual irregularities. Interestingly, these patients may have dramatic clinical symptoms with corresponding nonvisible or very small solid adnexal lesions seen on imaging. In the case of fibromas, these solid tumors have normal hormonal tumor markers but may present with ascites and pleural effusions as part of Meigs syndrome, which can confuse the clinician who may suspect advanced-stage epithelial cancer especially as this condition may be associated with elevated CA 125.
Germ cell tumors make up the other main group of primary ovarian tumors, and typically strongly express tumor markers. These tumors typically are solid and highly vascularized on imaging, can be bilateral, and may be very large at the time of diagnosis.5 They most commonly are unilateral and arise among younger women (including usually in the second and third decades of life). Table 1 demonstrates the different tumor markers associated with different germ cell tumors. It is my practice to order a panel of all of these germ cell markers in young women with solid adnexal masses in whom germ cell tumors are suspected, but I will not routinely draw this expansive panel for older women with cystic lesions.
Tumor marker panels (such as OVA 1, Overa, Risk of Malignancy Algorithm or ROMA) have become popular in recent years. These panels include multiple serum markers (such as CA 125, beta-2 microglobulin, human epididymis secretory protein 4, transferrin, etc.) evaluated in concert with the goal being a more nuanced assessment of likelihood for malignancy.6,7 These assays typically are stratified by age or menopausal status given the physiologic differences in normal reference ranges that occur between these groups. While these studies do improve upon the sensitivity and specificity for identifying malignancy, compared with single-assay tests, they are not definitively diagnostic for this purpose. Therefore, I typically recommend these assays if a referring doctor needs additional risk stratification to guide whether or not to refer to an oncologist for surgery.
Not all tumor markers are of equal value in all patients with an adnexal mass. I recommend careful consideration of other clinical factors such as age, menopausal status, ultrasonographic features, and associated findings such as GI symptoms or manifestations of hormonal alterations when considering which markers to assess.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Hum Reprod. 1989 Jan;4(1):1-12.
2. Obstet Gynecol. 2016 Nov;128(5):e210-e26.
3. Dan Med Bull. 2011 Nov;58(11):A4331.
4. Int J Cancer. 2015 Oct 1;137(7):1661-71.
5. Obstet Gynecol. 2000 Jan;95(1):128-33.
6. Obstet Gynecol. 2011 Jun;117(6):1289-97.
7. Obstet Gynecol. 2011 Aug;118(2 Pt 1):280-8.
Tumor markers are serum measures that are valuable in the discrimination of an adnexal mass. However, given the long list from which to choose, it can be confusing to know exactly which might best serve your diagnostic needs. I am commonly asked by obstetrician/gynecologists and primary care doctors for guidance on this subject. In this column I will explore some of the decision making that I use when determining which markers might be most helpful for individual patients.

So which tumor markers should you order when you have diagnosed an adnexal mass? Because tumor marker profiles can differ dramatically based on the cell type of the neoplasm, perhaps the first question to ask is what is the most likely category of neoplasm based on other clinical data? Ovarian neoplasms fit into the following subgroups: epithelial (including the most common cell type, serous ovarian cancer, but also the less common mucinous and low malignant potential tumors), sex cord-stromal tumors, germ cell tumors, and metastatic tumors. Table 1 summarizes which tumor markers should be considered based on the clinical setting.
You should suspect an epithelial tumor if there is an adnexal mass with significant cystic components in older, postmenopausal patients, or the presence of peritoneal carcinomatosis on imaging. The tumor markers most commonly elevated in this clinical setting are cancer antigen 125 (CA 125), carcinoembryonic antigen (CEA), and possibly CA 19-9. The CA 125 antigen is a glycoprotein derived from the epithelium of peritoneum, pleura, pericardium, and Müllerian tissues. The multiple sites of origin of this glycoprotein speaks to the poor specificity associated with its elevation, as it is well known to be elevated in both benign conditions such as endometriosis, fibroids, pregnancy, ovulation, cirrhosis, and pericarditis as well as in nongynecologic malignancies, particularly those metastatic to the peritoneal cavity. Multiple different assays are available to measure CA 125, and each is associated with a slightly different reference range. Therefore, if measuring serial values, it is best to have these assessed by the same laboratory. Similarly, as it can be physiologically elevated during the menstrual cycle, premenopausal women should have serial assessments at the same point in their menstrual cycle or ideally within the first 2 weeks of their cycle.
The sensitivity of CA 125 in detecting ovarian cancer is only 78%, which is limited by the fact that not all epithelial ovarian cancer cell types (including some clear cell, carcinosarcoma, and mucinous) express elevations in this tumor marker, and because CA 125 is elevated in less than half of stage I ovarian cancers.1 Therefore, given the lack of sensitivity and specificity for this tumor marker, you should integrate other clinical data, such as imaging findings, age of the patient, and associated benign medical conditions, when evaluating the likelihood of cancer. The American College of Obstetricians and Gynecologists (ACOG) recommends that in the setting of an adnexal mass, referral to gynecologic oncology is recommended when the CA 125 value is greater than 200 U/mL in premenopausal women, or greater than 35U/mL in postmenopausal women.2
CEA is a protein that can be expressed in the colon but not in other normal tissues after birth, and therefore its elevation is commonly associated with metastatic GI tumors to the ovary and peritoneum, or mucinous ovarian tumors, including borderline tumors. Metastatic GI tumors typically are suspected when there are bilateral ovarian solid masses. Right-sided ovarian cysts also can be associated with appendiceal pathology and checking a CEA level can be considered in these cases. I will commonly draw both CA 125 and CEA tumor markers in the setting of cystic +/– solid ovarian masses. This allows the recognition of CA 125-negative/CEA-positive ovarian cancers, such as mucinous tumors, which aids in later surveillance or increases my suspicion for an occult GI tumor (particularly if there is a disproportionately higher elevation in CEA than CA 125).3 If tumor marker profiles are suggestive of an occult GI tumor, I often will consider a preoperative colonoscopy and upper GI endoscopic assessment.
CA 19-9 is a much less specific tumor marker which can be elevated in a variety of solid organ tumors including pancreatic, hepatobiliary, gastric and ovarian tumors. I typically reserve adding this marker for atypical clinical presentations of ovarian cancer, such as carcinomatosis in the absence of pelvic masses.
Ovarian sex cord-stromal neoplasms most commonly present as solid tumors in the ovary. The ovarian stroma includes the bland fibroblasts and the hormone-producing sex-cord granulosa, Sertoli and Leydig cells. Therefore the sex cord-stromal tumors commonly are associated with elevations in serum inhibin, anti-Müllerian hormone, and potentially androstenedione and dehydroepiandrosterone.4 These tumors rarely have advanced disease at diagnosis. Granulosa cell tumors should be suspected in women with a solid ovarian mass and abnormal uterine bleeding (including postmenopausal bleeding), and the appropriate tumor markers (inhibin and anti-Müllerian hormone) can guide this diagnosis preoperatively.4 Androgen-secreting stromal tumors such as Sertoli-Leydig tumors often present with virilization or menstrual irregularities. Interestingly, these patients may have dramatic clinical symptoms with corresponding nonvisible or very small solid adnexal lesions seen on imaging. In the case of fibromas, these solid tumors have normal hormonal tumor markers but may present with ascites and pleural effusions as part of Meigs syndrome, which can confuse the clinician who may suspect advanced-stage epithelial cancer especially as this condition may be associated with elevated CA 125.
Germ cell tumors make up the other main group of primary ovarian tumors, and typically strongly express tumor markers. These tumors typically are solid and highly vascularized on imaging, can be bilateral, and may be very large at the time of diagnosis.5 They most commonly are unilateral and arise among younger women (including usually in the second and third decades of life). Table 1 demonstrates the different tumor markers associated with different germ cell tumors. It is my practice to order a panel of all of these germ cell markers in young women with solid adnexal masses in whom germ cell tumors are suspected, but I will not routinely draw this expansive panel for older women with cystic lesions.
Tumor marker panels (such as OVA 1, Overa, Risk of Malignancy Algorithm or ROMA) have become popular in recent years. These panels include multiple serum markers (such as CA 125, beta-2 microglobulin, human epididymis secretory protein 4, transferrin, etc.) evaluated in concert with the goal being a more nuanced assessment of likelihood for malignancy.6,7 These assays typically are stratified by age or menopausal status given the physiologic differences in normal reference ranges that occur between these groups. While these studies do improve upon the sensitivity and specificity for identifying malignancy, compared with single-assay tests, they are not definitively diagnostic for this purpose. Therefore, I typically recommend these assays if a referring doctor needs additional risk stratification to guide whether or not to refer to an oncologist for surgery.
Not all tumor markers are of equal value in all patients with an adnexal mass. I recommend careful consideration of other clinical factors such as age, menopausal status, ultrasonographic features, and associated findings such as GI symptoms or manifestations of hormonal alterations when considering which markers to assess.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Hum Reprod. 1989 Jan;4(1):1-12.
2. Obstet Gynecol. 2016 Nov;128(5):e210-e26.
3. Dan Med Bull. 2011 Nov;58(11):A4331.
4. Int J Cancer. 2015 Oct 1;137(7):1661-71.
5. Obstet Gynecol. 2000 Jan;95(1):128-33.
6. Obstet Gynecol. 2011 Jun;117(6):1289-97.
7. Obstet Gynecol. 2011 Aug;118(2 Pt 1):280-8.
Tumor markers are serum measures that are valuable in the discrimination of an adnexal mass. However, given the long list from which to choose, it can be confusing to know exactly which might best serve your diagnostic needs. I am commonly asked by obstetrician/gynecologists and primary care doctors for guidance on this subject. In this column I will explore some of the decision making that I use when determining which markers might be most helpful for individual patients.

So which tumor markers should you order when you have diagnosed an adnexal mass? Because tumor marker profiles can differ dramatically based on the cell type of the neoplasm, perhaps the first question to ask is what is the most likely category of neoplasm based on other clinical data? Ovarian neoplasms fit into the following subgroups: epithelial (including the most common cell type, serous ovarian cancer, but also the less common mucinous and low malignant potential tumors), sex cord-stromal tumors, germ cell tumors, and metastatic tumors. Table 1 summarizes which tumor markers should be considered based on the clinical setting.
You should suspect an epithelial tumor if there is an adnexal mass with significant cystic components in older, postmenopausal patients, or the presence of peritoneal carcinomatosis on imaging. The tumor markers most commonly elevated in this clinical setting are cancer antigen 125 (CA 125), carcinoembryonic antigen (CEA), and possibly CA 19-9. The CA 125 antigen is a glycoprotein derived from the epithelium of peritoneum, pleura, pericardium, and Müllerian tissues. The multiple sites of origin of this glycoprotein speaks to the poor specificity associated with its elevation, as it is well known to be elevated in both benign conditions such as endometriosis, fibroids, pregnancy, ovulation, cirrhosis, and pericarditis as well as in nongynecologic malignancies, particularly those metastatic to the peritoneal cavity. Multiple different assays are available to measure CA 125, and each is associated with a slightly different reference range. Therefore, if measuring serial values, it is best to have these assessed by the same laboratory. Similarly, as it can be physiologically elevated during the menstrual cycle, premenopausal women should have serial assessments at the same point in their menstrual cycle or ideally within the first 2 weeks of their cycle.
The sensitivity of CA 125 in detecting ovarian cancer is only 78%, which is limited by the fact that not all epithelial ovarian cancer cell types (including some clear cell, carcinosarcoma, and mucinous) express elevations in this tumor marker, and because CA 125 is elevated in less than half of stage I ovarian cancers.1 Therefore, given the lack of sensitivity and specificity for this tumor marker, you should integrate other clinical data, such as imaging findings, age of the patient, and associated benign medical conditions, when evaluating the likelihood of cancer. The American College of Obstetricians and Gynecologists (ACOG) recommends that in the setting of an adnexal mass, referral to gynecologic oncology is recommended when the CA 125 value is greater than 200 U/mL in premenopausal women, or greater than 35U/mL in postmenopausal women.2
CEA is a protein that can be expressed in the colon but not in other normal tissues after birth, and therefore its elevation is commonly associated with metastatic GI tumors to the ovary and peritoneum, or mucinous ovarian tumors, including borderline tumors. Metastatic GI tumors typically are suspected when there are bilateral ovarian solid masses. Right-sided ovarian cysts also can be associated with appendiceal pathology and checking a CEA level can be considered in these cases. I will commonly draw both CA 125 and CEA tumor markers in the setting of cystic +/– solid ovarian masses. This allows the recognition of CA 125-negative/CEA-positive ovarian cancers, such as mucinous tumors, which aids in later surveillance or increases my suspicion for an occult GI tumor (particularly if there is a disproportionately higher elevation in CEA than CA 125).3 If tumor marker profiles are suggestive of an occult GI tumor, I often will consider a preoperative colonoscopy and upper GI endoscopic assessment.
CA 19-9 is a much less specific tumor marker which can be elevated in a variety of solid organ tumors including pancreatic, hepatobiliary, gastric and ovarian tumors. I typically reserve adding this marker for atypical clinical presentations of ovarian cancer, such as carcinomatosis in the absence of pelvic masses.
Ovarian sex cord-stromal neoplasms most commonly present as solid tumors in the ovary. The ovarian stroma includes the bland fibroblasts and the hormone-producing sex-cord granulosa, Sertoli and Leydig cells. Therefore the sex cord-stromal tumors commonly are associated with elevations in serum inhibin, anti-Müllerian hormone, and potentially androstenedione and dehydroepiandrosterone.4 These tumors rarely have advanced disease at diagnosis. Granulosa cell tumors should be suspected in women with a solid ovarian mass and abnormal uterine bleeding (including postmenopausal bleeding), and the appropriate tumor markers (inhibin and anti-Müllerian hormone) can guide this diagnosis preoperatively.4 Androgen-secreting stromal tumors such as Sertoli-Leydig tumors often present with virilization or menstrual irregularities. Interestingly, these patients may have dramatic clinical symptoms with corresponding nonvisible or very small solid adnexal lesions seen on imaging. In the case of fibromas, these solid tumors have normal hormonal tumor markers but may present with ascites and pleural effusions as part of Meigs syndrome, which can confuse the clinician who may suspect advanced-stage epithelial cancer especially as this condition may be associated with elevated CA 125.
Germ cell tumors make up the other main group of primary ovarian tumors, and typically strongly express tumor markers. These tumors typically are solid and highly vascularized on imaging, can be bilateral, and may be very large at the time of diagnosis.5 They most commonly are unilateral and arise among younger women (including usually in the second and third decades of life). Table 1 demonstrates the different tumor markers associated with different germ cell tumors. It is my practice to order a panel of all of these germ cell markers in young women with solid adnexal masses in whom germ cell tumors are suspected, but I will not routinely draw this expansive panel for older women with cystic lesions.
Tumor marker panels (such as OVA 1, Overa, Risk of Malignancy Algorithm or ROMA) have become popular in recent years. These panels include multiple serum markers (such as CA 125, beta-2 microglobulin, human epididymis secretory protein 4, transferrin, etc.) evaluated in concert with the goal being a more nuanced assessment of likelihood for malignancy.6,7 These assays typically are stratified by age or menopausal status given the physiologic differences in normal reference ranges that occur between these groups. While these studies do improve upon the sensitivity and specificity for identifying malignancy, compared with single-assay tests, they are not definitively diagnostic for this purpose. Therefore, I typically recommend these assays if a referring doctor needs additional risk stratification to guide whether or not to refer to an oncologist for surgery.
Not all tumor markers are of equal value in all patients with an adnexal mass. I recommend careful consideration of other clinical factors such as age, menopausal status, ultrasonographic features, and associated findings such as GI symptoms or manifestations of hormonal alterations when considering which markers to assess.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no relevant financial disclosures. Email her at obnews@mdedge.com.
References
1. Hum Reprod. 1989 Jan;4(1):1-12.
2. Obstet Gynecol. 2016 Nov;128(5):e210-e26.
3. Dan Med Bull. 2011 Nov;58(11):A4331.
4. Int J Cancer. 2015 Oct 1;137(7):1661-71.
5. Obstet Gynecol. 2000 Jan;95(1):128-33.
6. Obstet Gynecol. 2011 Jun;117(6):1289-97.
7. Obstet Gynecol. 2011 Aug;118(2 Pt 1):280-8.
What is the future of para-aortic lymphadenectomy for endometrial cancer?
A landmark study of advanced endometrial cancer, GOG 258, was published in the New England Journal of Medicine this summer.1 This clinical trial compared the use of carboplatin and paclitaxel chemotherapy with a combination of chemotherapy with external beam radiation, exploring the notion of “more is better.” The results of the trial revealed that the “more” (chemotherapy with external beam radiation) was no better than chemotherapy alone with respect to overall survival. These results have challenged a creeping dogma in gynecologic oncology, which has seen many providers embrace combination therapy, particularly for patients with stage III (node-positive) endometrial cancer, a group of patients who made up approximately three-quarters of GOG 258’s study population. Many have been left searching for justification of their early adoption of combination therapy before the results of a trial such as this were available. For me it also raised a slightly different question: In the light of these results, what IS the role of para-aortic lymphadenectomy in the staging of endometrial cancers? If radiation to the nodal basins is no longer part of adjuvant therapy, then
It was in the 1980s that the removal of clinically normal para-aortic lymph nodes (those residing between the renal and proximal common iliac vessels) became a part of surgical staging. This practice was endorsed by the International Federation of Gynecology and Obstetrics (FIGO) and the Gynecologic Oncology Group (GOG) surgical committee in response to findings that 11% of women with clinical stage I endometrial cancer had microscopic lymph node metastases which were discovered only with routine pathologic evaluation of these tissues. Among those with pelvic lymph node metastases (stage IIIC disease), approximately one-third also harbored disease in para-aortic nodal regions.2 Among all patients with endometrial cancer, including those with low-grade disease, only a small fraction (approximately 2%) have isolated para-aortic lymph nodes (positive para-aortic nodes, but negative pelvic nodes). However, among patients with deeply invasive higher-grade tumors, the likelihood of discovering isolated para-aortic metastases is higher at approximately 16%.3 Therefore, the dominant pattern of lymph node metastases and lymphatic dissemination of endometrial cancer appears to be via the parametrial channels laterally towards the pelvic basins, and then sequentially to the para-aortic regions. The direct lymphatic pathway to the para-aortic basins from the uterine fundus through the adnexal lymphatics misses the pelvic regions altogether and may seen logical, but actually is observed fairly infrequently.4
Over the subsequent decades, there have been debates and schools of thought regarding what is the optimal degree of lymphatic dissection for endometrial cancer staging. Some advocated for full pelvic and infrarenal para-aortic nodal dissections in all patients, including even those in the lowest risk for metastases. Others advocated for a more limited, inframesenteric para-aortic nodal dissection, although the origins of such a distinction appear to be largely arbitrary. The inferior mesenteric artery is not a physiologic landmark for lymphatic pathways, and approximately half of para-aortic metastases are located above the level of the inferior mesenteric artery. This limited sampling may have been preferred because of relative ease of dissection rather than diagnostic or therapeutic efficacy.
As the population became more obese, making para-aortic nodal dissections less feasible, and laparoscopic staging became accepted as the standard of care in endometrial cancer staging, there was a further push towards limiting the scope of lymphadenectomy. Selective algorithms, such as the so-called “Mayo clinic criteria,” were widely adopted. In this approach, gynecologic oncologists perform full pelvic and infrarenal para-aortic lymphadenectomies but only in the presence of a high-risk uterine feature such as tumor size greater than 2 cm, deep myometrial invasion, or grade 3 histology.3 While this reduced the number of para-aortic dissections being performed, it did not eliminate them, as approximately 40% of patients with endometrial cancer meet at least one of those criteria.
At this same time, we learned something else critical about the benefits, or lack thereof, of lymphadenectomy. Two landmark surgical-staging trials were published in 2009 which randomly assigned women to hysterectomy with lymphadenectomy or hysterectomy alone, and found no survival benefit for lymphadenectomy.5,6 While these trial results initially were met with noisy backlash, particularly from those who had legitimate concerns regarding study design and conclusions that reach beyond the scope of this column, ultimately their findings (that there is no therapeutic benefit to surgically removing clinically normal lymph nodes) has become largely accepted. The subsequent findings of the Laparoscopic Approach to Cancer of the Endometrium (LACE) trial further support this, as there was no difference in survival found between patients who were randomly assigned to laparoscopic versus open staging for endometrial cancer, even despite a significantly lower rate of lymphadenectomy among the laparoscopic arm.7
SLN biopsy, in which the specific nodes which drain the uterus are selectively removed, represents the most recent development in lymph node assessment for endometrial cancer. On average, only three lymph nodes are removed per patient, and para-aortic nodes infrequently are removed, because it is rare that lymphatic pathways drain directly into the aortic basins after cervical injection. Yet despite this more limited dissection of lymph nodes, especially para-aortic, with SLN biopsy, surgeons still observe similar rates of IIIC disease, compared with full lymphadenectomy, suggesting that the presence or absence of lymphatic metastases still is able to be adequately determined. SLN biopsy misses only 3% of lymphatic disease.8 What is of particular interest to practitioners of the SLN approach is that “atypical” pathways are discovered approximately 20% of the time, and nodes are harvested from locations such as the presacral space or medial to the internal iliac vessels. These nodes are in locations previously overlooked by even the most comprehensive pelvic and para-aortic lymphadenectomy. Therefore, while the para-aortic nodes may not be systematically removed with SLN biopsy, new and arguably more relevant regions are interrogated, which might explain its equivalent diagnostic virtue.
With this evolution in surgical-staging practice, what we have come to recognize is that the role of lymph node assessment is predominantly, if not exclusively, diagnostic. It can help us determine which patients are at risk for distant relapse and therefore candidates for systemic therapy (chemotherapy), versus those whose risk is predominantly of local relapse and can be adequately treated with local therapies alone, such as vaginal radiation. This brings us to the results of GOG 258. If defining the specific and complete extent of lymph node metastases (as if that was ever truly what surgeons were doing) is no longer necessary to guide the prescription and extent of external beam radiation, then lymph node dissection need only inform us of whether or not there are nodal metastases, not specifically the location of those nodal metastases. The prescription of chemotherapy is the same whether the disease is limited to the pelvic nodes or also includes the para-aortic nodes. While GOG 258 discovered more para-aortic failures among the chemotherapy-alone group, suggesting there may be some therapeutic role of radiation in preventing this, it should be noted that these para-aortic relapses did not negatively impact relapse-free survival, and these patients still can presumably be salvaged with external beam radiation to the site of para-aortic relapse.
It would seem logical that the results of GOG 258 further limit the potential role of para-aortic lymphadenectomy in women with clinical stage I disease. Perhaps para-aortic dissection can be limited to women who are at highest risk for isolated para-aortic disease, such as those with deeply invasive high-grade tumors not successfully mapped with the highly targeted sentinel node biopsy technique? Most clinicians look forward to an era in which we no longer rely on crude dissections of disease-free tissue just to prove they are disease free, but instead can utilize more sophisticated diagnostic methods to recognize disseminated disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Email her at obnews@mdedge.com.
References
1. N Engl J Med. 2019 Jun 13;380(24):2317-26.
2. Cancer. 1987 Oct 15;60(8 Suppl):2035-41.
3. Gynecol Oncol. 2008;109(1):11-8.
4. Int J Gynecol Cancer. 2019 Mar;29(3):613-21.
5. J Natl Cancer Inst. 2008 Dec 3;100(23):1707-16.
6. Lancet. 2009 Jan 10;373(9658):125-36.
7. JAMA. 2017 Mar 28;317(12):1224-33.
8. Lancet Oncol. 2017 Mar;18(3):384-92.
A landmark study of advanced endometrial cancer, GOG 258, was published in the New England Journal of Medicine this summer.1 This clinical trial compared the use of carboplatin and paclitaxel chemotherapy with a combination of chemotherapy with external beam radiation, exploring the notion of “more is better.” The results of the trial revealed that the “more” (chemotherapy with external beam radiation) was no better than chemotherapy alone with respect to overall survival. These results have challenged a creeping dogma in gynecologic oncology, which has seen many providers embrace combination therapy, particularly for patients with stage III (node-positive) endometrial cancer, a group of patients who made up approximately three-quarters of GOG 258’s study population. Many have been left searching for justification of their early adoption of combination therapy before the results of a trial such as this were available. For me it also raised a slightly different question: In the light of these results, what IS the role of para-aortic lymphadenectomy in the staging of endometrial cancers? If radiation to the nodal basins is no longer part of adjuvant therapy, then
It was in the 1980s that the removal of clinically normal para-aortic lymph nodes (those residing between the renal and proximal common iliac vessels) became a part of surgical staging. This practice was endorsed by the International Federation of Gynecology and Obstetrics (FIGO) and the Gynecologic Oncology Group (GOG) surgical committee in response to findings that 11% of women with clinical stage I endometrial cancer had microscopic lymph node metastases which were discovered only with routine pathologic evaluation of these tissues. Among those with pelvic lymph node metastases (stage IIIC disease), approximately one-third also harbored disease in para-aortic nodal regions.2 Among all patients with endometrial cancer, including those with low-grade disease, only a small fraction (approximately 2%) have isolated para-aortic lymph nodes (positive para-aortic nodes, but negative pelvic nodes). However, among patients with deeply invasive higher-grade tumors, the likelihood of discovering isolated para-aortic metastases is higher at approximately 16%.3 Therefore, the dominant pattern of lymph node metastases and lymphatic dissemination of endometrial cancer appears to be via the parametrial channels laterally towards the pelvic basins, and then sequentially to the para-aortic regions. The direct lymphatic pathway to the para-aortic basins from the uterine fundus through the adnexal lymphatics misses the pelvic regions altogether and may seen logical, but actually is observed fairly infrequently.4
Over the subsequent decades, there have been debates and schools of thought regarding what is the optimal degree of lymphatic dissection for endometrial cancer staging. Some advocated for full pelvic and infrarenal para-aortic nodal dissections in all patients, including even those in the lowest risk for metastases. Others advocated for a more limited, inframesenteric para-aortic nodal dissection, although the origins of such a distinction appear to be largely arbitrary. The inferior mesenteric artery is not a physiologic landmark for lymphatic pathways, and approximately half of para-aortic metastases are located above the level of the inferior mesenteric artery. This limited sampling may have been preferred because of relative ease of dissection rather than diagnostic or therapeutic efficacy.
As the population became more obese, making para-aortic nodal dissections less feasible, and laparoscopic staging became accepted as the standard of care in endometrial cancer staging, there was a further push towards limiting the scope of lymphadenectomy. Selective algorithms, such as the so-called “Mayo clinic criteria,” were widely adopted. In this approach, gynecologic oncologists perform full pelvic and infrarenal para-aortic lymphadenectomies but only in the presence of a high-risk uterine feature such as tumor size greater than 2 cm, deep myometrial invasion, or grade 3 histology.3 While this reduced the number of para-aortic dissections being performed, it did not eliminate them, as approximately 40% of patients with endometrial cancer meet at least one of those criteria.
At this same time, we learned something else critical about the benefits, or lack thereof, of lymphadenectomy. Two landmark surgical-staging trials were published in 2009 which randomly assigned women to hysterectomy with lymphadenectomy or hysterectomy alone, and found no survival benefit for lymphadenectomy.5,6 While these trial results initially were met with noisy backlash, particularly from those who had legitimate concerns regarding study design and conclusions that reach beyond the scope of this column, ultimately their findings (that there is no therapeutic benefit to surgically removing clinically normal lymph nodes) has become largely accepted. The subsequent findings of the Laparoscopic Approach to Cancer of the Endometrium (LACE) trial further support this, as there was no difference in survival found between patients who were randomly assigned to laparoscopic versus open staging for endometrial cancer, even despite a significantly lower rate of lymphadenectomy among the laparoscopic arm.7
SLN biopsy, in which the specific nodes which drain the uterus are selectively removed, represents the most recent development in lymph node assessment for endometrial cancer. On average, only three lymph nodes are removed per patient, and para-aortic nodes infrequently are removed, because it is rare that lymphatic pathways drain directly into the aortic basins after cervical injection. Yet despite this more limited dissection of lymph nodes, especially para-aortic, with SLN biopsy, surgeons still observe similar rates of IIIC disease, compared with full lymphadenectomy, suggesting that the presence or absence of lymphatic metastases still is able to be adequately determined. SLN biopsy misses only 3% of lymphatic disease.8 What is of particular interest to practitioners of the SLN approach is that “atypical” pathways are discovered approximately 20% of the time, and nodes are harvested from locations such as the presacral space or medial to the internal iliac vessels. These nodes are in locations previously overlooked by even the most comprehensive pelvic and para-aortic lymphadenectomy. Therefore, while the para-aortic nodes may not be systematically removed with SLN biopsy, new and arguably more relevant regions are interrogated, which might explain its equivalent diagnostic virtue.
With this evolution in surgical-staging practice, what we have come to recognize is that the role of lymph node assessment is predominantly, if not exclusively, diagnostic. It can help us determine which patients are at risk for distant relapse and therefore candidates for systemic therapy (chemotherapy), versus those whose risk is predominantly of local relapse and can be adequately treated with local therapies alone, such as vaginal radiation. This brings us to the results of GOG 258. If defining the specific and complete extent of lymph node metastases (as if that was ever truly what surgeons were doing) is no longer necessary to guide the prescription and extent of external beam radiation, then lymph node dissection need only inform us of whether or not there are nodal metastases, not specifically the location of those nodal metastases. The prescription of chemotherapy is the same whether the disease is limited to the pelvic nodes or also includes the para-aortic nodes. While GOG 258 discovered more para-aortic failures among the chemotherapy-alone group, suggesting there may be some therapeutic role of radiation in preventing this, it should be noted that these para-aortic relapses did not negatively impact relapse-free survival, and these patients still can presumably be salvaged with external beam radiation to the site of para-aortic relapse.
It would seem logical that the results of GOG 258 further limit the potential role of para-aortic lymphadenectomy in women with clinical stage I disease. Perhaps para-aortic dissection can be limited to women who are at highest risk for isolated para-aortic disease, such as those with deeply invasive high-grade tumors not successfully mapped with the highly targeted sentinel node biopsy technique? Most clinicians look forward to an era in which we no longer rely on crude dissections of disease-free tissue just to prove they are disease free, but instead can utilize more sophisticated diagnostic methods to recognize disseminated disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Email her at obnews@mdedge.com.
References
1. N Engl J Med. 2019 Jun 13;380(24):2317-26.
2. Cancer. 1987 Oct 15;60(8 Suppl):2035-41.
3. Gynecol Oncol. 2008;109(1):11-8.
4. Int J Gynecol Cancer. 2019 Mar;29(3):613-21.
5. J Natl Cancer Inst. 2008 Dec 3;100(23):1707-16.
6. Lancet. 2009 Jan 10;373(9658):125-36.
7. JAMA. 2017 Mar 28;317(12):1224-33.
8. Lancet Oncol. 2017 Mar;18(3):384-92.
A landmark study of advanced endometrial cancer, GOG 258, was published in the New England Journal of Medicine this summer.1 This clinical trial compared the use of carboplatin and paclitaxel chemotherapy with a combination of chemotherapy with external beam radiation, exploring the notion of “more is better.” The results of the trial revealed that the “more” (chemotherapy with external beam radiation) was no better than chemotherapy alone with respect to overall survival. These results have challenged a creeping dogma in gynecologic oncology, which has seen many providers embrace combination therapy, particularly for patients with stage III (node-positive) endometrial cancer, a group of patients who made up approximately three-quarters of GOG 258’s study population. Many have been left searching for justification of their early adoption of combination therapy before the results of a trial such as this were available. For me it also raised a slightly different question: In the light of these results, what IS the role of para-aortic lymphadenectomy in the staging of endometrial cancers? If radiation to the nodal basins is no longer part of adjuvant therapy, then
It was in the 1980s that the removal of clinically normal para-aortic lymph nodes (those residing between the renal and proximal common iliac vessels) became a part of surgical staging. This practice was endorsed by the International Federation of Gynecology and Obstetrics (FIGO) and the Gynecologic Oncology Group (GOG) surgical committee in response to findings that 11% of women with clinical stage I endometrial cancer had microscopic lymph node metastases which were discovered only with routine pathologic evaluation of these tissues. Among those with pelvic lymph node metastases (stage IIIC disease), approximately one-third also harbored disease in para-aortic nodal regions.2 Among all patients with endometrial cancer, including those with low-grade disease, only a small fraction (approximately 2%) have isolated para-aortic lymph nodes (positive para-aortic nodes, but negative pelvic nodes). However, among patients with deeply invasive higher-grade tumors, the likelihood of discovering isolated para-aortic metastases is higher at approximately 16%.3 Therefore, the dominant pattern of lymph node metastases and lymphatic dissemination of endometrial cancer appears to be via the parametrial channels laterally towards the pelvic basins, and then sequentially to the para-aortic regions. The direct lymphatic pathway to the para-aortic basins from the uterine fundus through the adnexal lymphatics misses the pelvic regions altogether and may seen logical, but actually is observed fairly infrequently.4
Over the subsequent decades, there have been debates and schools of thought regarding what is the optimal degree of lymphatic dissection for endometrial cancer staging. Some advocated for full pelvic and infrarenal para-aortic nodal dissections in all patients, including even those in the lowest risk for metastases. Others advocated for a more limited, inframesenteric para-aortic nodal dissection, although the origins of such a distinction appear to be largely arbitrary. The inferior mesenteric artery is not a physiologic landmark for lymphatic pathways, and approximately half of para-aortic metastases are located above the level of the inferior mesenteric artery. This limited sampling may have been preferred because of relative ease of dissection rather than diagnostic or therapeutic efficacy.
As the population became more obese, making para-aortic nodal dissections less feasible, and laparoscopic staging became accepted as the standard of care in endometrial cancer staging, there was a further push towards limiting the scope of lymphadenectomy. Selective algorithms, such as the so-called “Mayo clinic criteria,” were widely adopted. In this approach, gynecologic oncologists perform full pelvic and infrarenal para-aortic lymphadenectomies but only in the presence of a high-risk uterine feature such as tumor size greater than 2 cm, deep myometrial invasion, or grade 3 histology.3 While this reduced the number of para-aortic dissections being performed, it did not eliminate them, as approximately 40% of patients with endometrial cancer meet at least one of those criteria.
At this same time, we learned something else critical about the benefits, or lack thereof, of lymphadenectomy. Two landmark surgical-staging trials were published in 2009 which randomly assigned women to hysterectomy with lymphadenectomy or hysterectomy alone, and found no survival benefit for lymphadenectomy.5,6 While these trial results initially were met with noisy backlash, particularly from those who had legitimate concerns regarding study design and conclusions that reach beyond the scope of this column, ultimately their findings (that there is no therapeutic benefit to surgically removing clinically normal lymph nodes) has become largely accepted. The subsequent findings of the Laparoscopic Approach to Cancer of the Endometrium (LACE) trial further support this, as there was no difference in survival found between patients who were randomly assigned to laparoscopic versus open staging for endometrial cancer, even despite a significantly lower rate of lymphadenectomy among the laparoscopic arm.7
SLN biopsy, in which the specific nodes which drain the uterus are selectively removed, represents the most recent development in lymph node assessment for endometrial cancer. On average, only three lymph nodes are removed per patient, and para-aortic nodes infrequently are removed, because it is rare that lymphatic pathways drain directly into the aortic basins after cervical injection. Yet despite this more limited dissection of lymph nodes, especially para-aortic, with SLN biopsy, surgeons still observe similar rates of IIIC disease, compared with full lymphadenectomy, suggesting that the presence or absence of lymphatic metastases still is able to be adequately determined. SLN biopsy misses only 3% of lymphatic disease.8 What is of particular interest to practitioners of the SLN approach is that “atypical” pathways are discovered approximately 20% of the time, and nodes are harvested from locations such as the presacral space or medial to the internal iliac vessels. These nodes are in locations previously overlooked by even the most comprehensive pelvic and para-aortic lymphadenectomy. Therefore, while the para-aortic nodes may not be systematically removed with SLN biopsy, new and arguably more relevant regions are interrogated, which might explain its equivalent diagnostic virtue.
With this evolution in surgical-staging practice, what we have come to recognize is that the role of lymph node assessment is predominantly, if not exclusively, diagnostic. It can help us determine which patients are at risk for distant relapse and therefore candidates for systemic therapy (chemotherapy), versus those whose risk is predominantly of local relapse and can be adequately treated with local therapies alone, such as vaginal radiation. This brings us to the results of GOG 258. If defining the specific and complete extent of lymph node metastases (as if that was ever truly what surgeons were doing) is no longer necessary to guide the prescription and extent of external beam radiation, then lymph node dissection need only inform us of whether or not there are nodal metastases, not specifically the location of those nodal metastases. The prescription of chemotherapy is the same whether the disease is limited to the pelvic nodes or also includes the para-aortic nodes. While GOG 258 discovered more para-aortic failures among the chemotherapy-alone group, suggesting there may be some therapeutic role of radiation in preventing this, it should be noted that these para-aortic relapses did not negatively impact relapse-free survival, and these patients still can presumably be salvaged with external beam radiation to the site of para-aortic relapse.
It would seem logical that the results of GOG 258 further limit the potential role of para-aortic lymphadenectomy in women with clinical stage I disease. Perhaps para-aortic dissection can be limited to women who are at highest risk for isolated para-aortic disease, such as those with deeply invasive high-grade tumors not successfully mapped with the highly targeted sentinel node biopsy technique? Most clinicians look forward to an era in which we no longer rely on crude dissections of disease-free tissue just to prove they are disease free, but instead can utilize more sophisticated diagnostic methods to recognize disseminated disease.
Dr. Rossi is assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Email her at obnews@mdedge.com.
References
1. N Engl J Med. 2019 Jun 13;380(24):2317-26.
2. Cancer. 1987 Oct 15;60(8 Suppl):2035-41.
3. Gynecol Oncol. 2008;109(1):11-8.
4. Int J Gynecol Cancer. 2019 Mar;29(3):613-21.
5. J Natl Cancer Inst. 2008 Dec 3;100(23):1707-16.
6. Lancet. 2009 Jan 10;373(9658):125-36.
7. JAMA. 2017 Mar 28;317(12):1224-33.
8. Lancet Oncol. 2017 Mar;18(3):384-92.
What is the ‘microbiome’ and how may it influence gynecologic cancers?
Bacteria are everywhere, good and bad alike! It is well known in the scientific community that microbes significantly outnumber the cells in the human body by at least 10 times. Joshua Lederberg, PhD, gave meaning to the term “microbiome” in 2001 as the “ecological community of commensal, symbiotic, and pathogenic microorganisms that literally share our body space.”1 This community of microorganisms comprises bacteria, fungi, viruses, archaea, and protists.
In 2007, the National Institutes of Health Human Microbiome Project was established to study the human microbiome starting with five specific sites – the gastrointestinal tract, the mouth, the vagina, the skin, and nasal cavity. The goal was not only to identify the microbes inhabiting a specific body site but also to establish a range of “normal” for resident microbes as well as sequence the genomes of these microbes.2 Much of the research predating this era focused on microorganisms in terms of disease potential rather than a focus on the benefits of resident microorganisms.
The richness – the number of microorganisms in an area – and diversity – the relative proportion of microorganisms in an environment – can vary regionally. The microbiota that contribute to the class of resident microorganisms in a specific body habitat can be described broadly as commensals or mutualistic. With commensal microorganisms, one partner benefits and the other is unaffected. On the other hand, mutualistic microorganisms allow both parties to derive benefit. For example, resident microorganisms in the gut aid in the absorption of nutrients and in the production of vitamin K. On mucosal surfaces and the skin, it is possible that these resident microorganisms prevent colonization of pathogenic microbes, which could aid in prevention of disease.3
The microbiota composition can be influenced by multiple factors such as age, diet, medications, environment, early microbial exposure, and host genetics. The gut microbiota, for example, can be significantly altered by dietary intake or antibiotic use. Alterations in the diversity of microbes in certain body habitats has been linked to several human diseases such as obesity, inflammatory bowel disease, and bacterial vaginosis.4
In women, there are differences noted in the composition of resident microorganisms soon after birth as well as at prepubertal, postpubertal, and postmenopausal transitions. At puberty, anaerobic and aerobic lactobacilli aid in maintaining vaginal pH. If the normal microbiota is suppressed, it allows for yeast and other bacteria to grow causing vaginitis, and dramatic shifts in the makeup of the vaginal microbiota can lead to bacterial vaginosis. Interestingly, research has shown that the pH and microbiome of the vagina differs by ethnicity. These differences in composition of the vaginal microbiome likely contribute to known differences in the acquisition of sexually transmitted infections and development of bacterial vaginosis. The microbiome is believed to have a complex role in regulating human health and disease, including cancer.
There is growing evidence to suggest the gut microbiome may play an important role in the pathogenesis of both obesity and cancer. Two divisions of bacteria predominate in the gut in humans and mice, Bacteroidetes and Firmicutes, and the relative ratio of these two divisions is dramatically affected by obesity, such that Bacteroidetes levels decrease and Firmicutes levels increase.5 The change in the microbial environment leads to a greater ability to harvest dietary energy, which would be conducive to cancer development.
The microbiome and gynecologic cancers
The presence and relative abundance of bacterial species in the vagina are affected by unique factors such as hormonal contraception, pregnancy, and menopause. There are researchers investigating alterations in the microbiome of the vagina and implications in persistence of high-risk human papillomavirus infections and HPV-induced carcinogenesis. There were significant differences found in the composition of the vaginal microbiota in healthy women, compared with women with low-grade squamous intraepithelial neoplasm and high-grade squamous intraepithelial neoplasm.6
Conceivably, the subsequent clinical questions are: Can we apply this data to diagnose women at risk for dysplasia or can we alter the vaginal microbiome to impact the clearance rate of the HPV virus in susceptible or infected women to decrease the long-term risk of cervical dysplasia or malignancy?
The upper reproductive tract in women – the uterus, fallopian tubes, and ovaries – had been presumed to be a sterile environment. However, we know that bacteria have been isolated in the pre- and postmenopausal uterus of healthy women. Therefore, there also are investigators seeking to establish the microbiome of normal uteri to accurately compare it with malignant uteri. Notably, there also is interest in how treatments for cancer – chemotherapy and radiation – ultimately can affect a woman’s vaginal and gut microbiome.
Currently, microbiome research has an expansive range. Women will greatly benefit from research seeking to define improved prevention, diagnosis, and treatment based on alterations of the microbiome for common gynecologic premalignant and malignant conditions.
Dr. Hawkins is a fellow of gynecologic oncology and Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. They had no conflicts of interest to disclose.
References
1. “ ’Ome Sweet ’Omics – a genealogical treasury of words,” by Joshua Lederberg, The Scientist, Apr 2, 2001.
2. Genome Res. 2009 Dec;19(12):2317-23.
3. “Normal Human Microbiota,” Jawetz, Melnick & Adelberg’s Medical Microbiology, 27th edition (New York, NY: McGraw-Hill, 2016).
4. Nature. 2012 Jun 13;486(7402):207-14.
5. Nature. 2006 Dec 21;444(7122):1027-31.
6. Oncol Lett. 2018 Dec; 16(6): 7035-47.
Bacteria are everywhere, good and bad alike! It is well known in the scientific community that microbes significantly outnumber the cells in the human body by at least 10 times. Joshua Lederberg, PhD, gave meaning to the term “microbiome” in 2001 as the “ecological community of commensal, symbiotic, and pathogenic microorganisms that literally share our body space.”1 This community of microorganisms comprises bacteria, fungi, viruses, archaea, and protists.
In 2007, the National Institutes of Health Human Microbiome Project was established to study the human microbiome starting with five specific sites – the gastrointestinal tract, the mouth, the vagina, the skin, and nasal cavity. The goal was not only to identify the microbes inhabiting a specific body site but also to establish a range of “normal” for resident microbes as well as sequence the genomes of these microbes.2 Much of the research predating this era focused on microorganisms in terms of disease potential rather than a focus on the benefits of resident microorganisms.
The richness – the number of microorganisms in an area – and diversity – the relative proportion of microorganisms in an environment – can vary regionally. The microbiota that contribute to the class of resident microorganisms in a specific body habitat can be described broadly as commensals or mutualistic. With commensal microorganisms, one partner benefits and the other is unaffected. On the other hand, mutualistic microorganisms allow both parties to derive benefit. For example, resident microorganisms in the gut aid in the absorption of nutrients and in the production of vitamin K. On mucosal surfaces and the skin, it is possible that these resident microorganisms prevent colonization of pathogenic microbes, which could aid in prevention of disease.3
The microbiota composition can be influenced by multiple factors such as age, diet, medications, environment, early microbial exposure, and host genetics. The gut microbiota, for example, can be significantly altered by dietary intake or antibiotic use. Alterations in the diversity of microbes in certain body habitats has been linked to several human diseases such as obesity, inflammatory bowel disease, and bacterial vaginosis.4
In women, there are differences noted in the composition of resident microorganisms soon after birth as well as at prepubertal, postpubertal, and postmenopausal transitions. At puberty, anaerobic and aerobic lactobacilli aid in maintaining vaginal pH. If the normal microbiota is suppressed, it allows for yeast and other bacteria to grow causing vaginitis, and dramatic shifts in the makeup of the vaginal microbiota can lead to bacterial vaginosis. Interestingly, research has shown that the pH and microbiome of the vagina differs by ethnicity. These differences in composition of the vaginal microbiome likely contribute to known differences in the acquisition of sexually transmitted infections and development of bacterial vaginosis. The microbiome is believed to have a complex role in regulating human health and disease, including cancer.
There is growing evidence to suggest the gut microbiome may play an important role in the pathogenesis of both obesity and cancer. Two divisions of bacteria predominate in the gut in humans and mice, Bacteroidetes and Firmicutes, and the relative ratio of these two divisions is dramatically affected by obesity, such that Bacteroidetes levels decrease and Firmicutes levels increase.5 The change in the microbial environment leads to a greater ability to harvest dietary energy, which would be conducive to cancer development.
The microbiome and gynecologic cancers
The presence and relative abundance of bacterial species in the vagina are affected by unique factors such as hormonal contraception, pregnancy, and menopause. There are researchers investigating alterations in the microbiome of the vagina and implications in persistence of high-risk human papillomavirus infections and HPV-induced carcinogenesis. There were significant differences found in the composition of the vaginal microbiota in healthy women, compared with women with low-grade squamous intraepithelial neoplasm and high-grade squamous intraepithelial neoplasm.6
Conceivably, the subsequent clinical questions are: Can we apply this data to diagnose women at risk for dysplasia or can we alter the vaginal microbiome to impact the clearance rate of the HPV virus in susceptible or infected women to decrease the long-term risk of cervical dysplasia or malignancy?
The upper reproductive tract in women – the uterus, fallopian tubes, and ovaries – had been presumed to be a sterile environment. However, we know that bacteria have been isolated in the pre- and postmenopausal uterus of healthy women. Therefore, there also are investigators seeking to establish the microbiome of normal uteri to accurately compare it with malignant uteri. Notably, there also is interest in how treatments for cancer – chemotherapy and radiation – ultimately can affect a woman’s vaginal and gut microbiome.
Currently, microbiome research has an expansive range. Women will greatly benefit from research seeking to define improved prevention, diagnosis, and treatment based on alterations of the microbiome for common gynecologic premalignant and malignant conditions.
Dr. Hawkins is a fellow of gynecologic oncology and Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. They had no conflicts of interest to disclose.
References
1. “ ’Ome Sweet ’Omics – a genealogical treasury of words,” by Joshua Lederberg, The Scientist, Apr 2, 2001.
2. Genome Res. 2009 Dec;19(12):2317-23.
3. “Normal Human Microbiota,” Jawetz, Melnick & Adelberg’s Medical Microbiology, 27th edition (New York, NY: McGraw-Hill, 2016).
4. Nature. 2012 Jun 13;486(7402):207-14.
5. Nature. 2006 Dec 21;444(7122):1027-31.
6. Oncol Lett. 2018 Dec; 16(6): 7035-47.
Bacteria are everywhere, good and bad alike! It is well known in the scientific community that microbes significantly outnumber the cells in the human body by at least 10 times. Joshua Lederberg, PhD, gave meaning to the term “microbiome” in 2001 as the “ecological community of commensal, symbiotic, and pathogenic microorganisms that literally share our body space.”1 This community of microorganisms comprises bacteria, fungi, viruses, archaea, and protists.
In 2007, the National Institutes of Health Human Microbiome Project was established to study the human microbiome starting with five specific sites – the gastrointestinal tract, the mouth, the vagina, the skin, and nasal cavity. The goal was not only to identify the microbes inhabiting a specific body site but also to establish a range of “normal” for resident microbes as well as sequence the genomes of these microbes.2 Much of the research predating this era focused on microorganisms in terms of disease potential rather than a focus on the benefits of resident microorganisms.
The richness – the number of microorganisms in an area – and diversity – the relative proportion of microorganisms in an environment – can vary regionally. The microbiota that contribute to the class of resident microorganisms in a specific body habitat can be described broadly as commensals or mutualistic. With commensal microorganisms, one partner benefits and the other is unaffected. On the other hand, mutualistic microorganisms allow both parties to derive benefit. For example, resident microorganisms in the gut aid in the absorption of nutrients and in the production of vitamin K. On mucosal surfaces and the skin, it is possible that these resident microorganisms prevent colonization of pathogenic microbes, which could aid in prevention of disease.3
The microbiota composition can be influenced by multiple factors such as age, diet, medications, environment, early microbial exposure, and host genetics. The gut microbiota, for example, can be significantly altered by dietary intake or antibiotic use. Alterations in the diversity of microbes in certain body habitats has been linked to several human diseases such as obesity, inflammatory bowel disease, and bacterial vaginosis.4
In women, there are differences noted in the composition of resident microorganisms soon after birth as well as at prepubertal, postpubertal, and postmenopausal transitions. At puberty, anaerobic and aerobic lactobacilli aid in maintaining vaginal pH. If the normal microbiota is suppressed, it allows for yeast and other bacteria to grow causing vaginitis, and dramatic shifts in the makeup of the vaginal microbiota can lead to bacterial vaginosis. Interestingly, research has shown that the pH and microbiome of the vagina differs by ethnicity. These differences in composition of the vaginal microbiome likely contribute to known differences in the acquisition of sexually transmitted infections and development of bacterial vaginosis. The microbiome is believed to have a complex role in regulating human health and disease, including cancer.
There is growing evidence to suggest the gut microbiome may play an important role in the pathogenesis of both obesity and cancer. Two divisions of bacteria predominate in the gut in humans and mice, Bacteroidetes and Firmicutes, and the relative ratio of these two divisions is dramatically affected by obesity, such that Bacteroidetes levels decrease and Firmicutes levels increase.5 The change in the microbial environment leads to a greater ability to harvest dietary energy, which would be conducive to cancer development.
The microbiome and gynecologic cancers
The presence and relative abundance of bacterial species in the vagina are affected by unique factors such as hormonal contraception, pregnancy, and menopause. There are researchers investigating alterations in the microbiome of the vagina and implications in persistence of high-risk human papillomavirus infections and HPV-induced carcinogenesis. There were significant differences found in the composition of the vaginal microbiota in healthy women, compared with women with low-grade squamous intraepithelial neoplasm and high-grade squamous intraepithelial neoplasm.6
Conceivably, the subsequent clinical questions are: Can we apply this data to diagnose women at risk for dysplasia or can we alter the vaginal microbiome to impact the clearance rate of the HPV virus in susceptible or infected women to decrease the long-term risk of cervical dysplasia or malignancy?
The upper reproductive tract in women – the uterus, fallopian tubes, and ovaries – had been presumed to be a sterile environment. However, we know that bacteria have been isolated in the pre- and postmenopausal uterus of healthy women. Therefore, there also are investigators seeking to establish the microbiome of normal uteri to accurately compare it with malignant uteri. Notably, there also is interest in how treatments for cancer – chemotherapy and radiation – ultimately can affect a woman’s vaginal and gut microbiome.
Currently, microbiome research has an expansive range. Women will greatly benefit from research seeking to define improved prevention, diagnosis, and treatment based on alterations of the microbiome for common gynecologic premalignant and malignant conditions.
Dr. Hawkins is a fellow of gynecologic oncology and Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. They had no conflicts of interest to disclose.
References
1. “ ’Ome Sweet ’Omics – a genealogical treasury of words,” by Joshua Lederberg, The Scientist, Apr 2, 2001.
2. Genome Res. 2009 Dec;19(12):2317-23.
3. “Normal Human Microbiota,” Jawetz, Melnick & Adelberg’s Medical Microbiology, 27th edition (New York, NY: McGraw-Hill, 2016).
4. Nature. 2012 Jun 13;486(7402):207-14.
5. Nature. 2006 Dec 21;444(7122):1027-31.
6. Oncol Lett. 2018 Dec; 16(6): 7035-47.
Oophorectomy for premenopausal breast cancer
One-quarter of patients with breast cancer are diagnosed at a premenopausal age and these young women may be directed to discuss oophorectomy with their ob.gyn. This may be because of the discovery of a deleterious BRCA gene mutation, which places them at increased risk for ovarian cancer, but oophorectomy may also be a therapeutic option for their breast cancer: 60% of premenopausal breast cancers are hormone receptor–positive. Ovarian ablation has been associated with improved overall survival and disease-free survival among these patients.1
Estrogen is an important promoter of breast cancer and is predominantly derived from ovarian tissue in premenopausal women. However, in postmenopausal women, the majority of estrogen is produced peripherally through the conversion of androgens to estrogen via the enzyme aromatase. Aromatase inhibitors, such as exemestane, anastrazole, and letrazole, are drugs which block this conversion in peripheral tissues. They are contraindicated in premenopausal women with intact ovarian function, because there is a reflex pituitary stimulation of ovarian estrogen release in response to suppression of peripheral conversion of androgens. For such patients, ovarian function must be ablated either with surgery or with gonadotropin-releasing hormone (GnRH) analogues such as leuprorelin and goserelin if aromatase inhibitors are desired.
In these trials, ovarian ablation was achieved either reversibly with GnRH analogues or permanently and irreversibly with oophorectomy. No studies have compared the survival benefit of these two approaches; however, surgical ovarian ablation is immediate, reliable, and has been shown to be the most cost-effective method.4 It is a good option for women who struggle with adherence to repeated appointments for injections. It also substantially reduces the risk for ovarian cancer, which is elevated among this population of patients, even among those without a deleterious BRCA gene mutation.
BRCA populations
For women with BRCA germline mutations and a history of breast cancer, oophorectomy is associated with a 70% risk of all-cause mortality, including a 60% reduction in breast cancer mortality. This effect is inclusive of patients with “triple-negative,” hormone receptor–negative tumors. The positive effect on breast cancer mortality is predominantly seen among BRCA-1 mutation carriers, and if the oophorectomy is performed within 2 years of diagnosis.5
Technique
When performing oophorectomy either for breast cancer or because of a hereditary cancer syndrome such as BRCA mutation, it is important to ensure that the ovarian vessel pedicle is transected at least 2 cm from its insertion in the ovary. This prevents leaving a residual ovarian remnant. In order to do this, it may be necessary to skeletonize the ovarian vessels free from their physiological attachments to the sigmoid colon on the left, and terminal ileum and cecum on the right. It is also important to ensure that the ureter is not invested in this more proximal segment of ovarian vessels. To prevent this, the retroperitoneal space can be opened lateral to and parallel with the ovarian vessels, and the “medial leaf” of the broad ligament swept medially to expose the ureter as it crosses the bifurcation of the external and internal iliac arteries at the pelvic brim. With the ureter in view, a window can then be made in the “medial leaf” above the ureter and below the ovary and ovarian vessels, in doing so creating a skeletonized ovarian vessel segment which can be sealed and cut 2 cm or more from its insertion in the ovary.
The fallopian tubes should be removed with the ovarian specimens, with attention made to removing the fallopian tube at its junction with the uterine cornua. It should be noted that the majority of fallopian tube cancers arise in the fimbriated end of the tube, and cornual tubal malignancies are fairly uncommon.
The decision about whether or not to perform hysterectomy at the time of salpingo-oophorectomy is complex. In patients without hereditary cancer syndromes, such as BRCA or Lynch syndrome, hysterectomy likely offers no benefit to the patient who is undergoing a procedure for the purpose of ovarian ablation. An argument has been made that hysterectomy can eliminate the increased endometrial cancer risk associated with tamoxifen. However, given the previously discussed data, after oophorectomy, aromatase inhibitors are the preferred treatment option, and tamoxifen can be avoided. If a patient has unrelated underlying uterine pathology a hysterectomy might be indicated. Women with BRCA germline mutations, particularly women with BRCA-1 mutations, may be at increased risk for uterine serous carcinoma, and in these patients, hysterectomy at the time of oophorectomy can be discussed and offered, though as yet, it is not a guideline recommendation for all patients.6 Patients who ask to “just take everything out while you are there” without a clear indication for hysterectomy should be counseled that hysterectomy is associated with increased risk, recovery, and cost, compared with bilateral salpingo-oophorectomy. Among patients with elevated surgical risk (such as morbid obesity, known adhesive disease, increased venous thromboembolism risk, diabetes, and so on) it may not always be appropriate to extend the complexity of the procedure given the limited benefit.
Consequences of ovarian ablation
It should be noted that ovarian ablation in the TEXT and SOFT trials was not associated with an increase in overall survival for women with premenopausal breast cancer. Alternatively, large, observational studies such as the Nurses’ Health Study have shown that premenopausal oophorectomy without hormone replacement therapy is associated with increased all-cause mortality. This is primarily driven by the increased cardiopulmonary risk (heart attack and stroke), deaths after osteoporotic hip fractures, and the increased risk for lung and colon cancer.7,8
It is normal for young patients to have heightened concerns regarding their risk of recurrence from their cancer, and less concerned by threats to their health in decades to come. However, it is important to discuss this data with the patient and allow for her to make an informed decision about her immediate versus future risks. If she determines that she is not interested in permanent ovarian ablation with oophorectomy because of either surgical risks, concerns regarding permanent infertility, or increased all-cause mortality, she still has an option for medical ovarian ablation with GnRH analogues in the treatment of her breast cancer.
Hormone replacement therapy postoperatively
Women who undergo oophorectomy for the treatment of breast cancer should not be offered hormone replacement therapy. This is true even for “triple-negative” or hormone receptor–negative breast cancers as there is still some observed benefit of ovarian ablation, and risk from exogenous hormone administration in these women. Alternatively, postoperative hormone replacement therapy remains safe until the age of natural menopause among premenopausal patients with BRCA germline mutations without a preceding breast cancer diagnosis.
Surgical ovarian ablation with bilateral salpingo-oophorectomy is a valuable strategy in the adjuvant therapy of premenopausal breast cancer, particularly among BRCA mutation carriers and women with hormone receptor–positive disease, or among women who find adherence to medical ablation difficult. Patients should be carefully counseled that this may introduce increased long-term cardiovascular risks for them.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1996 Nov 2;348:1189-96.
2. Pagani O et al. N Engl J Med. 2014 Jul 10;371(12):107-18.
3. Francis PA et al. N Engl J Med. 2015 Jan 29;372(5):436-46.
4. Ferrandina G et al. Clin Drug Investig. 2017 Nov;37(11):1093-102.
5. Finch AP et al. J Clin Oncol. 2014 May 20;32(15):1547-53.
6. Shu CA et al. JAMA Oncol. 2016 Nov 1;2(11):1434-40.
7. Parker WH et al. Obstet Gynecol. 2013 Apr;121(4):709-16.
8. Rivera CM et al. Menopause. 2009 Jan-Feb;16:15-23.
One-quarter of patients with breast cancer are diagnosed at a premenopausal age and these young women may be directed to discuss oophorectomy with their ob.gyn. This may be because of the discovery of a deleterious BRCA gene mutation, which places them at increased risk for ovarian cancer, but oophorectomy may also be a therapeutic option for their breast cancer: 60% of premenopausal breast cancers are hormone receptor–positive. Ovarian ablation has been associated with improved overall survival and disease-free survival among these patients.1
Estrogen is an important promoter of breast cancer and is predominantly derived from ovarian tissue in premenopausal women. However, in postmenopausal women, the majority of estrogen is produced peripherally through the conversion of androgens to estrogen via the enzyme aromatase. Aromatase inhibitors, such as exemestane, anastrazole, and letrazole, are drugs which block this conversion in peripheral tissues. They are contraindicated in premenopausal women with intact ovarian function, because there is a reflex pituitary stimulation of ovarian estrogen release in response to suppression of peripheral conversion of androgens. For such patients, ovarian function must be ablated either with surgery or with gonadotropin-releasing hormone (GnRH) analogues such as leuprorelin and goserelin if aromatase inhibitors are desired.
In these trials, ovarian ablation was achieved either reversibly with GnRH analogues or permanently and irreversibly with oophorectomy. No studies have compared the survival benefit of these two approaches; however, surgical ovarian ablation is immediate, reliable, and has been shown to be the most cost-effective method.4 It is a good option for women who struggle with adherence to repeated appointments for injections. It also substantially reduces the risk for ovarian cancer, which is elevated among this population of patients, even among those without a deleterious BRCA gene mutation.
BRCA populations
For women with BRCA germline mutations and a history of breast cancer, oophorectomy is associated with a 70% risk of all-cause mortality, including a 60% reduction in breast cancer mortality. This effect is inclusive of patients with “triple-negative,” hormone receptor–negative tumors. The positive effect on breast cancer mortality is predominantly seen among BRCA-1 mutation carriers, and if the oophorectomy is performed within 2 years of diagnosis.5
Technique
When performing oophorectomy either for breast cancer or because of a hereditary cancer syndrome such as BRCA mutation, it is important to ensure that the ovarian vessel pedicle is transected at least 2 cm from its insertion in the ovary. This prevents leaving a residual ovarian remnant. In order to do this, it may be necessary to skeletonize the ovarian vessels free from their physiological attachments to the sigmoid colon on the left, and terminal ileum and cecum on the right. It is also important to ensure that the ureter is not invested in this more proximal segment of ovarian vessels. To prevent this, the retroperitoneal space can be opened lateral to and parallel with the ovarian vessels, and the “medial leaf” of the broad ligament swept medially to expose the ureter as it crosses the bifurcation of the external and internal iliac arteries at the pelvic brim. With the ureter in view, a window can then be made in the “medial leaf” above the ureter and below the ovary and ovarian vessels, in doing so creating a skeletonized ovarian vessel segment which can be sealed and cut 2 cm or more from its insertion in the ovary.
The fallopian tubes should be removed with the ovarian specimens, with attention made to removing the fallopian tube at its junction with the uterine cornua. It should be noted that the majority of fallopian tube cancers arise in the fimbriated end of the tube, and cornual tubal malignancies are fairly uncommon.
The decision about whether or not to perform hysterectomy at the time of salpingo-oophorectomy is complex. In patients without hereditary cancer syndromes, such as BRCA or Lynch syndrome, hysterectomy likely offers no benefit to the patient who is undergoing a procedure for the purpose of ovarian ablation. An argument has been made that hysterectomy can eliminate the increased endometrial cancer risk associated with tamoxifen. However, given the previously discussed data, after oophorectomy, aromatase inhibitors are the preferred treatment option, and tamoxifen can be avoided. If a patient has unrelated underlying uterine pathology a hysterectomy might be indicated. Women with BRCA germline mutations, particularly women with BRCA-1 mutations, may be at increased risk for uterine serous carcinoma, and in these patients, hysterectomy at the time of oophorectomy can be discussed and offered, though as yet, it is not a guideline recommendation for all patients.6 Patients who ask to “just take everything out while you are there” without a clear indication for hysterectomy should be counseled that hysterectomy is associated with increased risk, recovery, and cost, compared with bilateral salpingo-oophorectomy. Among patients with elevated surgical risk (such as morbid obesity, known adhesive disease, increased venous thromboembolism risk, diabetes, and so on) it may not always be appropriate to extend the complexity of the procedure given the limited benefit.
Consequences of ovarian ablation
It should be noted that ovarian ablation in the TEXT and SOFT trials was not associated with an increase in overall survival for women with premenopausal breast cancer. Alternatively, large, observational studies such as the Nurses’ Health Study have shown that premenopausal oophorectomy without hormone replacement therapy is associated with increased all-cause mortality. This is primarily driven by the increased cardiopulmonary risk (heart attack and stroke), deaths after osteoporotic hip fractures, and the increased risk for lung and colon cancer.7,8
It is normal for young patients to have heightened concerns regarding their risk of recurrence from their cancer, and less concerned by threats to their health in decades to come. However, it is important to discuss this data with the patient and allow for her to make an informed decision about her immediate versus future risks. If she determines that she is not interested in permanent ovarian ablation with oophorectomy because of either surgical risks, concerns regarding permanent infertility, or increased all-cause mortality, she still has an option for medical ovarian ablation with GnRH analogues in the treatment of her breast cancer.
Hormone replacement therapy postoperatively
Women who undergo oophorectomy for the treatment of breast cancer should not be offered hormone replacement therapy. This is true even for “triple-negative” or hormone receptor–negative breast cancers as there is still some observed benefit of ovarian ablation, and risk from exogenous hormone administration in these women. Alternatively, postoperative hormone replacement therapy remains safe until the age of natural menopause among premenopausal patients with BRCA germline mutations without a preceding breast cancer diagnosis.
Surgical ovarian ablation with bilateral salpingo-oophorectomy is a valuable strategy in the adjuvant therapy of premenopausal breast cancer, particularly among BRCA mutation carriers and women with hormone receptor–positive disease, or among women who find adherence to medical ablation difficult. Patients should be carefully counseled that this may introduce increased long-term cardiovascular risks for them.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1996 Nov 2;348:1189-96.
2. Pagani O et al. N Engl J Med. 2014 Jul 10;371(12):107-18.
3. Francis PA et al. N Engl J Med. 2015 Jan 29;372(5):436-46.
4. Ferrandina G et al. Clin Drug Investig. 2017 Nov;37(11):1093-102.
5. Finch AP et al. J Clin Oncol. 2014 May 20;32(15):1547-53.
6. Shu CA et al. JAMA Oncol. 2016 Nov 1;2(11):1434-40.
7. Parker WH et al. Obstet Gynecol. 2013 Apr;121(4):709-16.
8. Rivera CM et al. Menopause. 2009 Jan-Feb;16:15-23.
One-quarter of patients with breast cancer are diagnosed at a premenopausal age and these young women may be directed to discuss oophorectomy with their ob.gyn. This may be because of the discovery of a deleterious BRCA gene mutation, which places them at increased risk for ovarian cancer, but oophorectomy may also be a therapeutic option for their breast cancer: 60% of premenopausal breast cancers are hormone receptor–positive. Ovarian ablation has been associated with improved overall survival and disease-free survival among these patients.1
Estrogen is an important promoter of breast cancer and is predominantly derived from ovarian tissue in premenopausal women. However, in postmenopausal women, the majority of estrogen is produced peripherally through the conversion of androgens to estrogen via the enzyme aromatase. Aromatase inhibitors, such as exemestane, anastrazole, and letrazole, are drugs which block this conversion in peripheral tissues. They are contraindicated in premenopausal women with intact ovarian function, because there is a reflex pituitary stimulation of ovarian estrogen release in response to suppression of peripheral conversion of androgens. For such patients, ovarian function must be ablated either with surgery or with gonadotropin-releasing hormone (GnRH) analogues such as leuprorelin and goserelin if aromatase inhibitors are desired.
In these trials, ovarian ablation was achieved either reversibly with GnRH analogues or permanently and irreversibly with oophorectomy. No studies have compared the survival benefit of these two approaches; however, surgical ovarian ablation is immediate, reliable, and has been shown to be the most cost-effective method.4 It is a good option for women who struggle with adherence to repeated appointments for injections. It also substantially reduces the risk for ovarian cancer, which is elevated among this population of patients, even among those without a deleterious BRCA gene mutation.
BRCA populations
For women with BRCA germline mutations and a history of breast cancer, oophorectomy is associated with a 70% risk of all-cause mortality, including a 60% reduction in breast cancer mortality. This effect is inclusive of patients with “triple-negative,” hormone receptor–negative tumors. The positive effect on breast cancer mortality is predominantly seen among BRCA-1 mutation carriers, and if the oophorectomy is performed within 2 years of diagnosis.5
Technique
When performing oophorectomy either for breast cancer or because of a hereditary cancer syndrome such as BRCA mutation, it is important to ensure that the ovarian vessel pedicle is transected at least 2 cm from its insertion in the ovary. This prevents leaving a residual ovarian remnant. In order to do this, it may be necessary to skeletonize the ovarian vessels free from their physiological attachments to the sigmoid colon on the left, and terminal ileum and cecum on the right. It is also important to ensure that the ureter is not invested in this more proximal segment of ovarian vessels. To prevent this, the retroperitoneal space can be opened lateral to and parallel with the ovarian vessels, and the “medial leaf” of the broad ligament swept medially to expose the ureter as it crosses the bifurcation of the external and internal iliac arteries at the pelvic brim. With the ureter in view, a window can then be made in the “medial leaf” above the ureter and below the ovary and ovarian vessels, in doing so creating a skeletonized ovarian vessel segment which can be sealed and cut 2 cm or more from its insertion in the ovary.
The fallopian tubes should be removed with the ovarian specimens, with attention made to removing the fallopian tube at its junction with the uterine cornua. It should be noted that the majority of fallopian tube cancers arise in the fimbriated end of the tube, and cornual tubal malignancies are fairly uncommon.
The decision about whether or not to perform hysterectomy at the time of salpingo-oophorectomy is complex. In patients without hereditary cancer syndromes, such as BRCA or Lynch syndrome, hysterectomy likely offers no benefit to the patient who is undergoing a procedure for the purpose of ovarian ablation. An argument has been made that hysterectomy can eliminate the increased endometrial cancer risk associated with tamoxifen. However, given the previously discussed data, after oophorectomy, aromatase inhibitors are the preferred treatment option, and tamoxifen can be avoided. If a patient has unrelated underlying uterine pathology a hysterectomy might be indicated. Women with BRCA germline mutations, particularly women with BRCA-1 mutations, may be at increased risk for uterine serous carcinoma, and in these patients, hysterectomy at the time of oophorectomy can be discussed and offered, though as yet, it is not a guideline recommendation for all patients.6 Patients who ask to “just take everything out while you are there” without a clear indication for hysterectomy should be counseled that hysterectomy is associated with increased risk, recovery, and cost, compared with bilateral salpingo-oophorectomy. Among patients with elevated surgical risk (such as morbid obesity, known adhesive disease, increased venous thromboembolism risk, diabetes, and so on) it may not always be appropriate to extend the complexity of the procedure given the limited benefit.
Consequences of ovarian ablation
It should be noted that ovarian ablation in the TEXT and SOFT trials was not associated with an increase in overall survival for women with premenopausal breast cancer. Alternatively, large, observational studies such as the Nurses’ Health Study have shown that premenopausal oophorectomy without hormone replacement therapy is associated with increased all-cause mortality. This is primarily driven by the increased cardiopulmonary risk (heart attack and stroke), deaths after osteoporotic hip fractures, and the increased risk for lung and colon cancer.7,8
It is normal for young patients to have heightened concerns regarding their risk of recurrence from their cancer, and less concerned by threats to their health in decades to come. However, it is important to discuss this data with the patient and allow for her to make an informed decision about her immediate versus future risks. If she determines that she is not interested in permanent ovarian ablation with oophorectomy because of either surgical risks, concerns regarding permanent infertility, or increased all-cause mortality, she still has an option for medical ovarian ablation with GnRH analogues in the treatment of her breast cancer.
Hormone replacement therapy postoperatively
Women who undergo oophorectomy for the treatment of breast cancer should not be offered hormone replacement therapy. This is true even for “triple-negative” or hormone receptor–negative breast cancers as there is still some observed benefit of ovarian ablation, and risk from exogenous hormone administration in these women. Alternatively, postoperative hormone replacement therapy remains safe until the age of natural menopause among premenopausal patients with BRCA germline mutations without a preceding breast cancer diagnosis.
Surgical ovarian ablation with bilateral salpingo-oophorectomy is a valuable strategy in the adjuvant therapy of premenopausal breast cancer, particularly among BRCA mutation carriers and women with hormone receptor–positive disease, or among women who find adherence to medical ablation difficult. Patients should be carefully counseled that this may introduce increased long-term cardiovascular risks for them.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill.
References
1. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1996 Nov 2;348:1189-96.
2. Pagani O et al. N Engl J Med. 2014 Jul 10;371(12):107-18.
3. Francis PA et al. N Engl J Med. 2015 Jan 29;372(5):436-46.
4. Ferrandina G et al. Clin Drug Investig. 2017 Nov;37(11):1093-102.
5. Finch AP et al. J Clin Oncol. 2014 May 20;32(15):1547-53.
6. Shu CA et al. JAMA Oncol. 2016 Nov 1;2(11):1434-40.
7. Parker WH et al. Obstet Gynecol. 2013 Apr;121(4):709-16.
8. Rivera CM et al. Menopause. 2009 Jan-Feb;16:15-23.
Approach to the asymptomatic adnexal mass: When to operate, refer, or observe
Adnexal masses are common findings in women. While the decision to operate on symptomatic adnexal masses is straightforward, the decision-making process for asymptomatic masses is more complicated. Here we address how to approach an asymptomatic adnexal mass, including how to decide when to operate, when to refer, or how to monitor.
It is important to minimize the number of surgeries for benign, asymptomatic adnexal masses because complications are reported in 2%-15% of surgeries for adnexal masses and these can range from minimal to devastating.1 In addition, unnecessary surgery is associated with a burden of cost to the health care system. Therefore, there is a paradigm shift in the management of asymptomatic adnexal masses trending toward surveillance of any masses that are likely to be benign. What becomes critical in this approach is the ability to accurately classify these masses preoperatively.
Determining the malignant potential of a mass
Guidance is provided by the ACOG Practice Bulletin Number 174, which was published in 2016: “Evaluation and Management of Adnexal Masses.”2 These guidelines remind clinicians that:
- Most adnexal masses are benign, even in postmenopausal patients.
- The recommended imaging modality is quality transvaginal ultrasonography with an ultrasonographer accredited through the American Registry of Diagnostic Medical Sonographers.
- Simple cysts up to 10 cm can be monitored using repeat imaging every 6 months without surgical intervention, even in postmenopausal patients. In prospective studies, no cases of malignancy were diagnosed over 6 years of surveillance and most resolved. Those that persist are likely to be serous cystadenomas.
- Many benign lesions such as endometriomas and cystic teratomas have characteristic radiologic features. Surgery for these lesions is warranted for large size, symptoms, or growth in size.
- Ultrasound characteristics of malignant masses include:
1. Cyst size greater than 10 cm
2. Papillary or solid components
3. Septations
4. Internal blood flow on color Doppler.
An alternative approach that has been proposed is an ultrasound scoring system devised by International Ovarian Tumor Analysis Group. The scoring system uses 10 ultrasound findings that are characteristic of malignant and benign and is designed to characterize masses as either benign or malignant.3 This approach is able to correctly classify 77% of masses. The remaining masses with features that do not fit the “simple rules” are considered potentially malignant and should be referred to an oncology specialist for further decision making.
Decision to operate
After referral to gynecologic oncologists, surgery is not always inevitable, particularly for women with indeterminate masses. The gynecologic oncologist uses a decision-making process that factors in the underlying surgical risks for that patient with the likelihood of malignancy based on the features of the mass. The threshold to operate is higher in women with underlying major comorbidities, such as morbid obesity, complex prior surgical history, or cardiopulmonary disease. Healthier surgical candidates are more likely to be considered for a surgery, even if the suspicion for malignancy is lower. However, low surgical risk does not equate to no surgical risk. Therefore, even in apparently “good” surgical candidates, the suspicion for underlying malignancy needs to be reasonably high in order to justify the cost and risk of surgery in an asymptomatic patient. Sometimes it is patient anxiety and a desire to avoid repeated surveillance that prompts a decision to operate.
How to monitor
The role of surveillance and monitoring is to establish a natural history of the lesion or to allow it to reveal itself to be stable or regressive. Surveillance with serial sonography has shown that most asymptomatic adnexal masses with low risk features will resolve over time. Lack of resolution in the setting of stable findings is not a worrisome feature and is not suggestive of malignancy. The mere persistence of an otherwise benign-appearing lesion is not a reason to intervene with surgery.
Unfortunately, there is no clear guidance on the surveillance intervals. Some experts recommend an initial repeat scan in 3 months. If at that point the morphologic features and size are stable or decreasing, ultrasounds can be repeated at annual intervals for 5 years. In one study, masses that became malignant demonstrated growth by 7 months. Other experts recommend limiting the period of surveillance of cystic lesions to 1 year and lesions with solid components to 2 years.
Conclusions
Many asymptomatic adnexal masses discovered on imaging can be monitored with serial sonography. Lesions with more worrisome morphology that’s suggestive of malignancy should prompt referral to a gynecologic oncologist. Surgery on benign masses can be avoided. Outcome data is needed to advise the optimal timing intervals and the limit of follow-up serial ultrasonography. A caveat of this watch-and-see approach is having to allay the patient’s fears of the malignant potential of the mass. This requires conversations with the patient informing them that the stability of the mass will be shown over time and that surgery can be safely avoided.
References
1. Glanc P et al. J Ultrasound Med. 2017;36:849-63.
2. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins – Gynecology. Obstet Gynecol. 2016 Nov;128(5):e210-26.
3. Timmerman D et al. Ultrasound Obstet Gynecol. 2008 Jun;31(6):681-90.
Dr. Jackson-Moore is an associate professor in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC. They reported having no relevant financial disclosures.
Adnexal masses are common findings in women. While the decision to operate on symptomatic adnexal masses is straightforward, the decision-making process for asymptomatic masses is more complicated. Here we address how to approach an asymptomatic adnexal mass, including how to decide when to operate, when to refer, or how to monitor.
It is important to minimize the number of surgeries for benign, asymptomatic adnexal masses because complications are reported in 2%-15% of surgeries for adnexal masses and these can range from minimal to devastating.1 In addition, unnecessary surgery is associated with a burden of cost to the health care system. Therefore, there is a paradigm shift in the management of asymptomatic adnexal masses trending toward surveillance of any masses that are likely to be benign. What becomes critical in this approach is the ability to accurately classify these masses preoperatively.
Determining the malignant potential of a mass
Guidance is provided by the ACOG Practice Bulletin Number 174, which was published in 2016: “Evaluation and Management of Adnexal Masses.”2 These guidelines remind clinicians that:
- Most adnexal masses are benign, even in postmenopausal patients.
- The recommended imaging modality is quality transvaginal ultrasonography with an ultrasonographer accredited through the American Registry of Diagnostic Medical Sonographers.
- Simple cysts up to 10 cm can be monitored using repeat imaging every 6 months without surgical intervention, even in postmenopausal patients. In prospective studies, no cases of malignancy were diagnosed over 6 years of surveillance and most resolved. Those that persist are likely to be serous cystadenomas.
- Many benign lesions such as endometriomas and cystic teratomas have characteristic radiologic features. Surgery for these lesions is warranted for large size, symptoms, or growth in size.
- Ultrasound characteristics of malignant masses include:
1. Cyst size greater than 10 cm
2. Papillary or solid components
3. Septations
4. Internal blood flow on color Doppler.
An alternative approach that has been proposed is an ultrasound scoring system devised by International Ovarian Tumor Analysis Group. The scoring system uses 10 ultrasound findings that are characteristic of malignant and benign and is designed to characterize masses as either benign or malignant.3 This approach is able to correctly classify 77% of masses. The remaining masses with features that do not fit the “simple rules” are considered potentially malignant and should be referred to an oncology specialist for further decision making.
Decision to operate
After referral to gynecologic oncologists, surgery is not always inevitable, particularly for women with indeterminate masses. The gynecologic oncologist uses a decision-making process that factors in the underlying surgical risks for that patient with the likelihood of malignancy based on the features of the mass. The threshold to operate is higher in women with underlying major comorbidities, such as morbid obesity, complex prior surgical history, or cardiopulmonary disease. Healthier surgical candidates are more likely to be considered for a surgery, even if the suspicion for malignancy is lower. However, low surgical risk does not equate to no surgical risk. Therefore, even in apparently “good” surgical candidates, the suspicion for underlying malignancy needs to be reasonably high in order to justify the cost and risk of surgery in an asymptomatic patient. Sometimes it is patient anxiety and a desire to avoid repeated surveillance that prompts a decision to operate.
How to monitor
The role of surveillance and monitoring is to establish a natural history of the lesion or to allow it to reveal itself to be stable or regressive. Surveillance with serial sonography has shown that most asymptomatic adnexal masses with low risk features will resolve over time. Lack of resolution in the setting of stable findings is not a worrisome feature and is not suggestive of malignancy. The mere persistence of an otherwise benign-appearing lesion is not a reason to intervene with surgery.
Unfortunately, there is no clear guidance on the surveillance intervals. Some experts recommend an initial repeat scan in 3 months. If at that point the morphologic features and size are stable or decreasing, ultrasounds can be repeated at annual intervals for 5 years. In one study, masses that became malignant demonstrated growth by 7 months. Other experts recommend limiting the period of surveillance of cystic lesions to 1 year and lesions with solid components to 2 years.
Conclusions
Many asymptomatic adnexal masses discovered on imaging can be monitored with serial sonography. Lesions with more worrisome morphology that’s suggestive of malignancy should prompt referral to a gynecologic oncologist. Surgery on benign masses can be avoided. Outcome data is needed to advise the optimal timing intervals and the limit of follow-up serial ultrasonography. A caveat of this watch-and-see approach is having to allay the patient’s fears of the malignant potential of the mass. This requires conversations with the patient informing them that the stability of the mass will be shown over time and that surgery can be safely avoided.
References
1. Glanc P et al. J Ultrasound Med. 2017;36:849-63.
2. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins – Gynecology. Obstet Gynecol. 2016 Nov;128(5):e210-26.
3. Timmerman D et al. Ultrasound Obstet Gynecol. 2008 Jun;31(6):681-90.
Dr. Jackson-Moore is an associate professor in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC. They reported having no relevant financial disclosures.
Adnexal masses are common findings in women. While the decision to operate on symptomatic adnexal masses is straightforward, the decision-making process for asymptomatic masses is more complicated. Here we address how to approach an asymptomatic adnexal mass, including how to decide when to operate, when to refer, or how to monitor.
It is important to minimize the number of surgeries for benign, asymptomatic adnexal masses because complications are reported in 2%-15% of surgeries for adnexal masses and these can range from minimal to devastating.1 In addition, unnecessary surgery is associated with a burden of cost to the health care system. Therefore, there is a paradigm shift in the management of asymptomatic adnexal masses trending toward surveillance of any masses that are likely to be benign. What becomes critical in this approach is the ability to accurately classify these masses preoperatively.
Determining the malignant potential of a mass
Guidance is provided by the ACOG Practice Bulletin Number 174, which was published in 2016: “Evaluation and Management of Adnexal Masses.”2 These guidelines remind clinicians that:
- Most adnexal masses are benign, even in postmenopausal patients.
- The recommended imaging modality is quality transvaginal ultrasonography with an ultrasonographer accredited through the American Registry of Diagnostic Medical Sonographers.
- Simple cysts up to 10 cm can be monitored using repeat imaging every 6 months without surgical intervention, even in postmenopausal patients. In prospective studies, no cases of malignancy were diagnosed over 6 years of surveillance and most resolved. Those that persist are likely to be serous cystadenomas.
- Many benign lesions such as endometriomas and cystic teratomas have characteristic radiologic features. Surgery for these lesions is warranted for large size, symptoms, or growth in size.
- Ultrasound characteristics of malignant masses include:
1. Cyst size greater than 10 cm
2. Papillary or solid components
3. Septations
4. Internal blood flow on color Doppler.
An alternative approach that has been proposed is an ultrasound scoring system devised by International Ovarian Tumor Analysis Group. The scoring system uses 10 ultrasound findings that are characteristic of malignant and benign and is designed to characterize masses as either benign or malignant.3 This approach is able to correctly classify 77% of masses. The remaining masses with features that do not fit the “simple rules” are considered potentially malignant and should be referred to an oncology specialist for further decision making.
Decision to operate
After referral to gynecologic oncologists, surgery is not always inevitable, particularly for women with indeterminate masses. The gynecologic oncologist uses a decision-making process that factors in the underlying surgical risks for that patient with the likelihood of malignancy based on the features of the mass. The threshold to operate is higher in women with underlying major comorbidities, such as morbid obesity, complex prior surgical history, or cardiopulmonary disease. Healthier surgical candidates are more likely to be considered for a surgery, even if the suspicion for malignancy is lower. However, low surgical risk does not equate to no surgical risk. Therefore, even in apparently “good” surgical candidates, the suspicion for underlying malignancy needs to be reasonably high in order to justify the cost and risk of surgery in an asymptomatic patient. Sometimes it is patient anxiety and a desire to avoid repeated surveillance that prompts a decision to operate.
How to monitor
The role of surveillance and monitoring is to establish a natural history of the lesion or to allow it to reveal itself to be stable or regressive. Surveillance with serial sonography has shown that most asymptomatic adnexal masses with low risk features will resolve over time. Lack of resolution in the setting of stable findings is not a worrisome feature and is not suggestive of malignancy. The mere persistence of an otherwise benign-appearing lesion is not a reason to intervene with surgery.
Unfortunately, there is no clear guidance on the surveillance intervals. Some experts recommend an initial repeat scan in 3 months. If at that point the morphologic features and size are stable or decreasing, ultrasounds can be repeated at annual intervals for 5 years. In one study, masses that became malignant demonstrated growth by 7 months. Other experts recommend limiting the period of surveillance of cystic lesions to 1 year and lesions with solid components to 2 years.
Conclusions
Many asymptomatic adnexal masses discovered on imaging can be monitored with serial sonography. Lesions with more worrisome morphology that’s suggestive of malignancy should prompt referral to a gynecologic oncologist. Surgery on benign masses can be avoided. Outcome data is needed to advise the optimal timing intervals and the limit of follow-up serial ultrasonography. A caveat of this watch-and-see approach is having to allay the patient’s fears of the malignant potential of the mass. This requires conversations with the patient informing them that the stability of the mass will be shown over time and that surgery can be safely avoided.
References
1. Glanc P et al. J Ultrasound Med. 2017;36:849-63.
2. American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins – Gynecology. Obstet Gynecol. 2016 Nov;128(5):e210-26.
3. Timmerman D et al. Ultrasound Obstet Gynecol. 2008 Jun;31(6):681-90.
Dr. Jackson-Moore is an associate professor in gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC. They reported having no relevant financial disclosures.
Risk for obstetric complications when treating cervical dysplasia
Cervical dysplasia is a condition commonly encountered by the gynecologist. It is either treated (with excision or ablation) or monitored, depending on the lesion grade, cytologic history, medical history, and reproductive goals. Cervical dysplasia commonly arises in women of reproductive age. Therefore, consider reproductive effects when deciding whether to treat or monitor, as well as when choosing the treatment modality.
Background
Approximately two-thirds of human papillomavirus infections resolve within a year, and more than 90% resolve within 2 years. Similarly, low-grade cervical intraepithelial neoplasia (CIN 1) lesions frequently resolve. High-grade (CIN 2 and CIN 3) lesions regress less commonly, with 5% and 12%-40% progressing to invasive cancer, respectively. Therefore, treatment is typically recommended.
Obstetric implications
Potential obstetric risks of treatment for CIN include infertility, spontaneous abortion, preterm premature rupture of membranes (PPROM), preterm delivery, and perinatal/neonatal mortality. These risks are discussed individually below. Mechanisms that have been suggested for such complications include decreased cervical mucous, cervical scarring impeding conception or dilation, loss of cervical volume, collagen breakdown, and immunologic processes due to decreased physical defenses or microbiome shifts.
Fertility
Studies have shown that treatment does not appear to impede conception. The overall pregnancy rate is higher among treated women than untreated women. Pregnancy rates are not different among women intending to conceive or among women attempting conception for more than 12 months, with the caveat being that these studies are heterogenous.2,3
Miscarriage
No difference has been observed in total (less than 24 weeks) miscarriage rate or first trimester (less than 12 weeks) miscarriage rate among treated and untreated women. However, the second trimester miscarriage rate is significantly higher among treated women (risk ratio, 2.60).2 This risk is most notable following laser conization or LEEP.4 There may also be an association between ablation and pregnancy loss.
Preterm birth and PPROM
Several studies and meta-analyses show an association between preterm birth and treatment for CIN using LEEP or CKC. There is an increased risk of severe preterm delivery (relative risk, 2.78), extreme preterm delivery (relative risk, 5.33), and low birth weight (relative risk, 2.86) with CKC.5 LEEP is associated with the same outcomes, albeit the risk is lower than with CKC.6 The risk of preterm birth is even lower for ablation.7
The risk of PPROM is approximately two times higher among those treated with LEEP, and PPROM rates are higher among those treated with CKC, compared with LEEP.9,10
Other complications
Ectopic pregnancy and termination rates may be higher in treated women, compared with untreated women.2 However, there does not appear to be an increased risk for perinatal/neonatal mortality, cesarean section, or neonatal intensive care unit admission among women treated with excisional procedures.6
Pointers for practice
- Due to the potential for adverse obstetric complications following excisional procedures for cervical dysplasia, gynecologists should closely adhere to the American Society for Colposcopy and Cervical Pathology guidelines when determining the appropriateness of dysplasia interventions. The decision to treat, versus monitor, dysplasia in a woman who plans future childbearing should be made with the patient after thorough discussion of the risks and benefits of each path.
- Women younger than age 30 years should not be screened for high-risk human papillomavirus because of both its high incidence and its high rate of spontaneous resolution.
- For reproductive-aged women with CIN 2 and adequate colposcopy, the American Society for Colposcopy and Cervical Pathology supports either monitoring with cytology and colposcopy every 6 months for a year or excisional treatment. However, women with CIN 3, inadequate colposcopy, prior cervical cancer, diethylstilbestrol exposure, or decreased immunity should undergo excisional treatment.
- When selecting an excisional method (LEEP or CKC), surgeons should choose the most appropriate technique for the patient’s pathology but should acknowledge the observed higher rates of PPROM, preterm birth, and low-birth-weight infants among those receiving CKC, and tailor the size of the excision to the specific lesion.
- Consider recommending a 12-month interval between treatment and pregnancy to ensure resolution of high-grade dysplasia. Furthermore, obstetric risk may be increased within 12 months following treatment.
Dr. Robbins is a resident in the department of obstetrics and gynecology at the University of North Carolina, Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC, Chapel Hill. They reported having no relevant financial disclosures.
References
1. Am J Obstet Gynecol. 2011 Jul;205(1):19-27.
2. Cochrane Database Syst Rev. 2015 Sep 29;(9):CD008478.
3. BMJ. 2014 Oct 28;349:g6192.
4. Obstet Gynecol. 2016 Dec;128(6):1265-73.
5. BMJ. 2008 Sep 18;337:a1284.
6. Arch Gynecol Obstet. 2014 Jan;289(1):85-99.
7. BJOG. 2011 Aug;118(9):1031-41.
8. Obstet Gynecol. 2013 May;121(5):1063-8.
9. Lancet. 2006 Feb 11;367(9509):489-98.
10. Gynecol Obstet Invest. 2014;77(4):240-4.
Cervical dysplasia is a condition commonly encountered by the gynecologist. It is either treated (with excision or ablation) or monitored, depending on the lesion grade, cytologic history, medical history, and reproductive goals. Cervical dysplasia commonly arises in women of reproductive age. Therefore, consider reproductive effects when deciding whether to treat or monitor, as well as when choosing the treatment modality.
Background
Approximately two-thirds of human papillomavirus infections resolve within a year, and more than 90% resolve within 2 years. Similarly, low-grade cervical intraepithelial neoplasia (CIN 1) lesions frequently resolve. High-grade (CIN 2 and CIN 3) lesions regress less commonly, with 5% and 12%-40% progressing to invasive cancer, respectively. Therefore, treatment is typically recommended.
Obstetric implications
Potential obstetric risks of treatment for CIN include infertility, spontaneous abortion, preterm premature rupture of membranes (PPROM), preterm delivery, and perinatal/neonatal mortality. These risks are discussed individually below. Mechanisms that have been suggested for such complications include decreased cervical mucous, cervical scarring impeding conception or dilation, loss of cervical volume, collagen breakdown, and immunologic processes due to decreased physical defenses or microbiome shifts.
Fertility
Studies have shown that treatment does not appear to impede conception. The overall pregnancy rate is higher among treated women than untreated women. Pregnancy rates are not different among women intending to conceive or among women attempting conception for more than 12 months, with the caveat being that these studies are heterogenous.2,3
Miscarriage
No difference has been observed in total (less than 24 weeks) miscarriage rate or first trimester (less than 12 weeks) miscarriage rate among treated and untreated women. However, the second trimester miscarriage rate is significantly higher among treated women (risk ratio, 2.60).2 This risk is most notable following laser conization or LEEP.4 There may also be an association between ablation and pregnancy loss.
Preterm birth and PPROM
Several studies and meta-analyses show an association between preterm birth and treatment for CIN using LEEP or CKC. There is an increased risk of severe preterm delivery (relative risk, 2.78), extreme preterm delivery (relative risk, 5.33), and low birth weight (relative risk, 2.86) with CKC.5 LEEP is associated with the same outcomes, albeit the risk is lower than with CKC.6 The risk of preterm birth is even lower for ablation.7
The risk of PPROM is approximately two times higher among those treated with LEEP, and PPROM rates are higher among those treated with CKC, compared with LEEP.9,10
Other complications
Ectopic pregnancy and termination rates may be higher in treated women, compared with untreated women.2 However, there does not appear to be an increased risk for perinatal/neonatal mortality, cesarean section, or neonatal intensive care unit admission among women treated with excisional procedures.6
Pointers for practice
- Due to the potential for adverse obstetric complications following excisional procedures for cervical dysplasia, gynecologists should closely adhere to the American Society for Colposcopy and Cervical Pathology guidelines when determining the appropriateness of dysplasia interventions. The decision to treat, versus monitor, dysplasia in a woman who plans future childbearing should be made with the patient after thorough discussion of the risks and benefits of each path.
- Women younger than age 30 years should not be screened for high-risk human papillomavirus because of both its high incidence and its high rate of spontaneous resolution.
- For reproductive-aged women with CIN 2 and adequate colposcopy, the American Society for Colposcopy and Cervical Pathology supports either monitoring with cytology and colposcopy every 6 months for a year or excisional treatment. However, women with CIN 3, inadequate colposcopy, prior cervical cancer, diethylstilbestrol exposure, or decreased immunity should undergo excisional treatment.
- When selecting an excisional method (LEEP or CKC), surgeons should choose the most appropriate technique for the patient’s pathology but should acknowledge the observed higher rates of PPROM, preterm birth, and low-birth-weight infants among those receiving CKC, and tailor the size of the excision to the specific lesion.
- Consider recommending a 12-month interval between treatment and pregnancy to ensure resolution of high-grade dysplasia. Furthermore, obstetric risk may be increased within 12 months following treatment.
Dr. Robbins is a resident in the department of obstetrics and gynecology at the University of North Carolina, Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC, Chapel Hill. They reported having no relevant financial disclosures.
References
1. Am J Obstet Gynecol. 2011 Jul;205(1):19-27.
2. Cochrane Database Syst Rev. 2015 Sep 29;(9):CD008478.
3. BMJ. 2014 Oct 28;349:g6192.
4. Obstet Gynecol. 2016 Dec;128(6):1265-73.
5. BMJ. 2008 Sep 18;337:a1284.
6. Arch Gynecol Obstet. 2014 Jan;289(1):85-99.
7. BJOG. 2011 Aug;118(9):1031-41.
8. Obstet Gynecol. 2013 May;121(5):1063-8.
9. Lancet. 2006 Feb 11;367(9509):489-98.
10. Gynecol Obstet Invest. 2014;77(4):240-4.
Cervical dysplasia is a condition commonly encountered by the gynecologist. It is either treated (with excision or ablation) or monitored, depending on the lesion grade, cytologic history, medical history, and reproductive goals. Cervical dysplasia commonly arises in women of reproductive age. Therefore, consider reproductive effects when deciding whether to treat or monitor, as well as when choosing the treatment modality.
Background
Approximately two-thirds of human papillomavirus infections resolve within a year, and more than 90% resolve within 2 years. Similarly, low-grade cervical intraepithelial neoplasia (CIN 1) lesions frequently resolve. High-grade (CIN 2 and CIN 3) lesions regress less commonly, with 5% and 12%-40% progressing to invasive cancer, respectively. Therefore, treatment is typically recommended.
Obstetric implications
Potential obstetric risks of treatment for CIN include infertility, spontaneous abortion, preterm premature rupture of membranes (PPROM), preterm delivery, and perinatal/neonatal mortality. These risks are discussed individually below. Mechanisms that have been suggested for such complications include decreased cervical mucous, cervical scarring impeding conception or dilation, loss of cervical volume, collagen breakdown, and immunologic processes due to decreased physical defenses or microbiome shifts.
Fertility
Studies have shown that treatment does not appear to impede conception. The overall pregnancy rate is higher among treated women than untreated women. Pregnancy rates are not different among women intending to conceive or among women attempting conception for more than 12 months, with the caveat being that these studies are heterogenous.2,3
Miscarriage
No difference has been observed in total (less than 24 weeks) miscarriage rate or first trimester (less than 12 weeks) miscarriage rate among treated and untreated women. However, the second trimester miscarriage rate is significantly higher among treated women (risk ratio, 2.60).2 This risk is most notable following laser conization or LEEP.4 There may also be an association between ablation and pregnancy loss.
Preterm birth and PPROM
Several studies and meta-analyses show an association between preterm birth and treatment for CIN using LEEP or CKC. There is an increased risk of severe preterm delivery (relative risk, 2.78), extreme preterm delivery (relative risk, 5.33), and low birth weight (relative risk, 2.86) with CKC.5 LEEP is associated with the same outcomes, albeit the risk is lower than with CKC.6 The risk of preterm birth is even lower for ablation.7
The risk of PPROM is approximately two times higher among those treated with LEEP, and PPROM rates are higher among those treated with CKC, compared with LEEP.9,10
Other complications
Ectopic pregnancy and termination rates may be higher in treated women, compared with untreated women.2 However, there does not appear to be an increased risk for perinatal/neonatal mortality, cesarean section, or neonatal intensive care unit admission among women treated with excisional procedures.6
Pointers for practice
- Due to the potential for adverse obstetric complications following excisional procedures for cervical dysplasia, gynecologists should closely adhere to the American Society for Colposcopy and Cervical Pathology guidelines when determining the appropriateness of dysplasia interventions. The decision to treat, versus monitor, dysplasia in a woman who plans future childbearing should be made with the patient after thorough discussion of the risks and benefits of each path.
- Women younger than age 30 years should not be screened for high-risk human papillomavirus because of both its high incidence and its high rate of spontaneous resolution.
- For reproductive-aged women with CIN 2 and adequate colposcopy, the American Society for Colposcopy and Cervical Pathology supports either monitoring with cytology and colposcopy every 6 months for a year or excisional treatment. However, women with CIN 3, inadequate colposcopy, prior cervical cancer, diethylstilbestrol exposure, or decreased immunity should undergo excisional treatment.
- When selecting an excisional method (LEEP or CKC), surgeons should choose the most appropriate technique for the patient’s pathology but should acknowledge the observed higher rates of PPROM, preterm birth, and low-birth-weight infants among those receiving CKC, and tailor the size of the excision to the specific lesion.
- Consider recommending a 12-month interval between treatment and pregnancy to ensure resolution of high-grade dysplasia. Furthermore, obstetric risk may be increased within 12 months following treatment.
Dr. Robbins is a resident in the department of obstetrics and gynecology at the University of North Carolina, Chapel Hill. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC, Chapel Hill. They reported having no relevant financial disclosures.
References
1. Am J Obstet Gynecol. 2011 Jul;205(1):19-27.
2. Cochrane Database Syst Rev. 2015 Sep 29;(9):CD008478.
3. BMJ. 2014 Oct 28;349:g6192.
4. Obstet Gynecol. 2016 Dec;128(6):1265-73.
5. BMJ. 2008 Sep 18;337:a1284.
6. Arch Gynecol Obstet. 2014 Jan;289(1):85-99.
7. BJOG. 2011 Aug;118(9):1031-41.
8. Obstet Gynecol. 2013 May;121(5):1063-8.
9. Lancet. 2006 Feb 11;367(9509):489-98.
10. Gynecol Obstet Invest. 2014;77(4):240-4.
Postoperative pain in women with preexisting chronic pain
Chronic pain disorders have reached epidemic levels in the United States, with the Institute of Medicine reporting more than 100 million Americans affected and health care costs more than $500 billion annually.1 Although many pain disorders are confined to the abdomen or pelvis (chronic pelvic pain, vulvodynia, irritable bowel syndrome, and bladder pain syndrome), others present with global symptoms (fibromyalgia and chronic fatigue syndrome). Women are more likely to be diagnosed with a chronic pain condition and more likely to seek treatment for chronic pain, including undergoing a surgical intervention. In fact, chronic pelvic pain alone affects upward of 20% of women in the United States, and, of the 400,000 hysterectomies performed each year (54.2%, abdominal; 16.7%, vaginal; and 16.8%, laparoscopic/robotic assisted), approximately 15% are for chronic pain.2
Neurobiology of pain
Perioperative pain control, specifically in women with preexisting pain disorders, can provide an additional challenge. Unlike acute pain, chronic pain (lasting more than 6 months) is associated with an amplified pain response of the central nervous system. This abnormal pain processing, known as centralization of pain, may result in a decrease of the inhibitory pain pathways and/or an increase of the amplification pathways, often augmenting the pain response of the original peripheral insult, specifically surgery. Because of these physiologic changes, a multimodal approach to perioperative pain should be offered, especially in women with preexisting pain. The approach ideally ought to target the different mechanisms of actions in both the peripheral and central nervous systems to provide an overall reduction in pain perception.
Preoperative visit
Perhaps the most underutilized opportunity to optimize postoperative pain is a proactive, preoperative approach. Preoperative education, including goal setting of postoperative pain expectations, has been associated with a significant reduction in postoperative opioid use, less preoperative anxiety, and a decreased length of surgical stay.3 While it is unknown exactly when this should be provided to the patient in the treatment course, it should occur prior to the day of surgery to allow for appropriate intervention.
The use of a shared decision-making model between the clinician and the chronic pain patient in the development of a pain management plan has been highly successful in improving pain outcomes in the outpatient setting.4 A similar method can be applied to the preoperative course as well. A detailed history (including the use of an opioid risk assessment tool) allows the clinician to identify patients at risk for opioid misuse and abuse. This is also an opportunity to review a plan for an opioid taper with the patient and the prescriber, if the postoperative plan includes opioid reduction/cessation. The preoperative visit may be an opportunity to adjust centrally acting medications (antidepressants, anticonvulsants) before surgery or to reduce the dose or frequency of high-risk medications, such as benzodiazepines.
Perioperative strategy
One of the most impactful ways for us, as surgeons, to reduce tissue injury and decrease pain from surgery is by offering a minimally invasive approach. The benefits of minimally invasive surgery are well established, resulting in improved perioperative pain control, decreased blood loss, lower infection rates, decreased length of hospital stay, and a faster recovery, compared with laparotomy. Because patients with chronic pain disorders are at increased risk of greater acute postoperative pain and have an elevated risk for the development of chronic postsurgical pain, a minimally invasive surgical approach should be prioritized, when available.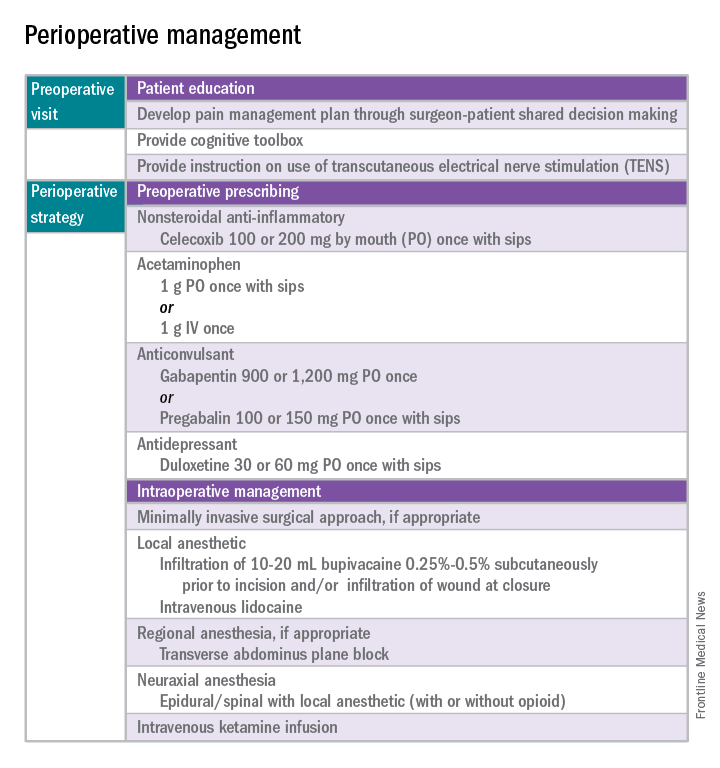
Perioperative multimodal drug therapy is associated with significant decreases in opioid consumption and reductions in acute postoperative pain.6 Recently, a multidisciplinary expert panel from the American Pain Society devised an evidence-based clinical practice guideline for postoperative pain.7 While there is no consensus as to the best regimen specific to gynecologic surgery, the general principles are similar across disciplines.
The postoperative period
Opioid-tolerant patients may experience greater pain during the first 24 hours postoperatively and require an increase in opioids, compared with opioid-naive patients.8 In the event that a postoperative patient does not respond as expected to the usual course, that patient should be evaluated for barriers to routine postoperative care, such as a surgical complication, opioid tolerance, or psychological distress. Surgeons should be aggressive with pain management immediately after surgery, even in the opioid-tolerant patient, and make short-term adjustments as needed based on the pain response. These patients will require pain medications beyond their baseline dose. Additionally, if an opioid taper is not planned in a chronic opioid user, work with the patient and the long-term opioid prescriber in restarting baseline opioid therapy outside of the acute surgical window. 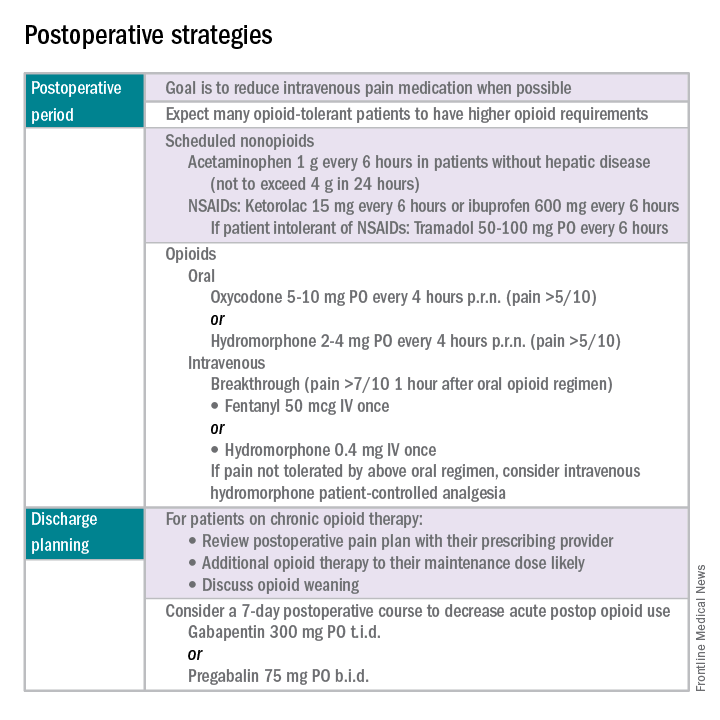
References
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press, 2011.
2. Obstet Gynecol. 2013 Aug;122(2 Pt 1):233-41.
3. N Engl J Med. 1964 Apr 16;270:825-7.
4. J Pain Symptom Manage. 1999 Jul;18(1):38-48.
5. Pain. 2010 Dec;151(3):694-702.
6. Anesthesiology. 2005 Dec;103(6):1296-304.
7. J Pain. 2016 Feb;17(2):131-57.
8. Pharmacotherapy. 2008 Dec;28(12):1453-60.
Dr. Carey is the director of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, and specializes in the medical and surgical management of pelvic pain disorders. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no relevant financial disclosures.
Chronic pain disorders have reached epidemic levels in the United States, with the Institute of Medicine reporting more than 100 million Americans affected and health care costs more than $500 billion annually.1 Although many pain disorders are confined to the abdomen or pelvis (chronic pelvic pain, vulvodynia, irritable bowel syndrome, and bladder pain syndrome), others present with global symptoms (fibromyalgia and chronic fatigue syndrome). Women are more likely to be diagnosed with a chronic pain condition and more likely to seek treatment for chronic pain, including undergoing a surgical intervention. In fact, chronic pelvic pain alone affects upward of 20% of women in the United States, and, of the 400,000 hysterectomies performed each year (54.2%, abdominal; 16.7%, vaginal; and 16.8%, laparoscopic/robotic assisted), approximately 15% are for chronic pain.2
Neurobiology of pain
Perioperative pain control, specifically in women with preexisting pain disorders, can provide an additional challenge. Unlike acute pain, chronic pain (lasting more than 6 months) is associated with an amplified pain response of the central nervous system. This abnormal pain processing, known as centralization of pain, may result in a decrease of the inhibitory pain pathways and/or an increase of the amplification pathways, often augmenting the pain response of the original peripheral insult, specifically surgery. Because of these physiologic changes, a multimodal approach to perioperative pain should be offered, especially in women with preexisting pain. The approach ideally ought to target the different mechanisms of actions in both the peripheral and central nervous systems to provide an overall reduction in pain perception.
Preoperative visit
Perhaps the most underutilized opportunity to optimize postoperative pain is a proactive, preoperative approach. Preoperative education, including goal setting of postoperative pain expectations, has been associated with a significant reduction in postoperative opioid use, less preoperative anxiety, and a decreased length of surgical stay.3 While it is unknown exactly when this should be provided to the patient in the treatment course, it should occur prior to the day of surgery to allow for appropriate intervention.
The use of a shared decision-making model between the clinician and the chronic pain patient in the development of a pain management plan has been highly successful in improving pain outcomes in the outpatient setting.4 A similar method can be applied to the preoperative course as well. A detailed history (including the use of an opioid risk assessment tool) allows the clinician to identify patients at risk for opioid misuse and abuse. This is also an opportunity to review a plan for an opioid taper with the patient and the prescriber, if the postoperative plan includes opioid reduction/cessation. The preoperative visit may be an opportunity to adjust centrally acting medications (antidepressants, anticonvulsants) before surgery or to reduce the dose or frequency of high-risk medications, such as benzodiazepines.
Perioperative strategy
One of the most impactful ways for us, as surgeons, to reduce tissue injury and decrease pain from surgery is by offering a minimally invasive approach. The benefits of minimally invasive surgery are well established, resulting in improved perioperative pain control, decreased blood loss, lower infection rates, decreased length of hospital stay, and a faster recovery, compared with laparotomy. Because patients with chronic pain disorders are at increased risk of greater acute postoperative pain and have an elevated risk for the development of chronic postsurgical pain, a minimally invasive surgical approach should be prioritized, when available.
Perioperative multimodal drug therapy is associated with significant decreases in opioid consumption and reductions in acute postoperative pain.6 Recently, a multidisciplinary expert panel from the American Pain Society devised an evidence-based clinical practice guideline for postoperative pain.7 While there is no consensus as to the best regimen specific to gynecologic surgery, the general principles are similar across disciplines.
The postoperative period
Opioid-tolerant patients may experience greater pain during the first 24 hours postoperatively and require an increase in opioids, compared with opioid-naive patients.8 In the event that a postoperative patient does not respond as expected to the usual course, that patient should be evaluated for barriers to routine postoperative care, such as a surgical complication, opioid tolerance, or psychological distress. Surgeons should be aggressive with pain management immediately after surgery, even in the opioid-tolerant patient, and make short-term adjustments as needed based on the pain response. These patients will require pain medications beyond their baseline dose. Additionally, if an opioid taper is not planned in a chronic opioid user, work with the patient and the long-term opioid prescriber in restarting baseline opioid therapy outside of the acute surgical window. 
References
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press, 2011.
2. Obstet Gynecol. 2013 Aug;122(2 Pt 1):233-41.
3. N Engl J Med. 1964 Apr 16;270:825-7.
4. J Pain Symptom Manage. 1999 Jul;18(1):38-48.
5. Pain. 2010 Dec;151(3):694-702.
6. Anesthesiology. 2005 Dec;103(6):1296-304.
7. J Pain. 2016 Feb;17(2):131-57.
8. Pharmacotherapy. 2008 Dec;28(12):1453-60.
Dr. Carey is the director of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, and specializes in the medical and surgical management of pelvic pain disorders. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no relevant financial disclosures.
Chronic pain disorders have reached epidemic levels in the United States, with the Institute of Medicine reporting more than 100 million Americans affected and health care costs more than $500 billion annually.1 Although many pain disorders are confined to the abdomen or pelvis (chronic pelvic pain, vulvodynia, irritable bowel syndrome, and bladder pain syndrome), others present with global symptoms (fibromyalgia and chronic fatigue syndrome). Women are more likely to be diagnosed with a chronic pain condition and more likely to seek treatment for chronic pain, including undergoing a surgical intervention. In fact, chronic pelvic pain alone affects upward of 20% of women in the United States, and, of the 400,000 hysterectomies performed each year (54.2%, abdominal; 16.7%, vaginal; and 16.8%, laparoscopic/robotic assisted), approximately 15% are for chronic pain.2
Neurobiology of pain
Perioperative pain control, specifically in women with preexisting pain disorders, can provide an additional challenge. Unlike acute pain, chronic pain (lasting more than 6 months) is associated with an amplified pain response of the central nervous system. This abnormal pain processing, known as centralization of pain, may result in a decrease of the inhibitory pain pathways and/or an increase of the amplification pathways, often augmenting the pain response of the original peripheral insult, specifically surgery. Because of these physiologic changes, a multimodal approach to perioperative pain should be offered, especially in women with preexisting pain. The approach ideally ought to target the different mechanisms of actions in both the peripheral and central nervous systems to provide an overall reduction in pain perception.
Preoperative visit
Perhaps the most underutilized opportunity to optimize postoperative pain is a proactive, preoperative approach. Preoperative education, including goal setting of postoperative pain expectations, has been associated with a significant reduction in postoperative opioid use, less preoperative anxiety, and a decreased length of surgical stay.3 While it is unknown exactly when this should be provided to the patient in the treatment course, it should occur prior to the day of surgery to allow for appropriate intervention.
The use of a shared decision-making model between the clinician and the chronic pain patient in the development of a pain management plan has been highly successful in improving pain outcomes in the outpatient setting.4 A similar method can be applied to the preoperative course as well. A detailed history (including the use of an opioid risk assessment tool) allows the clinician to identify patients at risk for opioid misuse and abuse. This is also an opportunity to review a plan for an opioid taper with the patient and the prescriber, if the postoperative plan includes opioid reduction/cessation. The preoperative visit may be an opportunity to adjust centrally acting medications (antidepressants, anticonvulsants) before surgery or to reduce the dose or frequency of high-risk medications, such as benzodiazepines.
Perioperative strategy
One of the most impactful ways for us, as surgeons, to reduce tissue injury and decrease pain from surgery is by offering a minimally invasive approach. The benefits of minimally invasive surgery are well established, resulting in improved perioperative pain control, decreased blood loss, lower infection rates, decreased length of hospital stay, and a faster recovery, compared with laparotomy. Because patients with chronic pain disorders are at increased risk of greater acute postoperative pain and have an elevated risk for the development of chronic postsurgical pain, a minimally invasive surgical approach should be prioritized, when available.
Perioperative multimodal drug therapy is associated with significant decreases in opioid consumption and reductions in acute postoperative pain.6 Recently, a multidisciplinary expert panel from the American Pain Society devised an evidence-based clinical practice guideline for postoperative pain.7 While there is no consensus as to the best regimen specific to gynecologic surgery, the general principles are similar across disciplines.
The postoperative period
Opioid-tolerant patients may experience greater pain during the first 24 hours postoperatively and require an increase in opioids, compared with opioid-naive patients.8 In the event that a postoperative patient does not respond as expected to the usual course, that patient should be evaluated for barriers to routine postoperative care, such as a surgical complication, opioid tolerance, or psychological distress. Surgeons should be aggressive with pain management immediately after surgery, even in the opioid-tolerant patient, and make short-term adjustments as needed based on the pain response. These patients will require pain medications beyond their baseline dose. Additionally, if an opioid taper is not planned in a chronic opioid user, work with the patient and the long-term opioid prescriber in restarting baseline opioid therapy outside of the acute surgical window. 
References
1. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press, 2011.
2. Obstet Gynecol. 2013 Aug;122(2 Pt 1):233-41.
3. N Engl J Med. 1964 Apr 16;270:825-7.
4. J Pain Symptom Manage. 1999 Jul;18(1):38-48.
5. Pain. 2010 Dec;151(3):694-702.
6. Anesthesiology. 2005 Dec;103(6):1296-304.
7. J Pain. 2016 Feb;17(2):131-57.
8. Pharmacotherapy. 2008 Dec;28(12):1453-60.
Dr. Carey is the director of minimally invasive gynecologic surgery at the University of North Carolina at Chapel Hill, and specializes in the medical and surgical management of pelvic pain disorders. Dr. Rossi is an assistant professor in the division of gynecologic oncology at UNC–Chapel Hill. They reported having no relevant financial disclosures.
Preventing surgical site infections in hysterectomy
Surgical site infections are a major source of patient morbidity. They are also an important quality metric for surgeons and hospital systems, and are increasingly being linked to reimbursement.
They occur in approximately 2% of the 600,000 women undergoing hysterectomy in the United States each year. The U.S. Centers for Disease Control and Prevention defines surgical site infection (SSI) as an infection that occurs within 30 days of a procedure in the part of the body where the surgery took place. Most SSIs are superficial incisional, but they also include deep incisional or organ or space infections.
Classification
The incidence of SSI varies according to the classification of the wound, as defined by the National Academy of Sciences.1 Most hysterectomies are classified as clean-contaminated wounds because they involve entry into the mucosa of the genitourinary tract. However, hysterectomy with contamination of bowel flora, or in the setting of acute infection (such as suppurative pelvic inflammatory disease) are considered a contaminated wound class, and are associated with even higher rates of SSI.
Risk factors
The risk factors associated with SSI are both modifiable and unmodifiable. Broadly speaking, they include increased risk to endogenous flora (e.g., wound classification), increased exposure to exogenous flora (e.g., inadequate protection of a wound from external pathogens), and impairment of the body’s immune mechanisms to prevent and overcome infection (e.g., hypothermia and hypoglycemia).
Unmodifiable risk factors include increasing age, a history of radiation exposure, vascular disease, and a history of prior SSIs. Modifiable risk factors include obesity, tobacco use, immunosuppressive medications, hypoalbuminemia, route of hysterectomy, hair removal, preoperative infections (such as bacterial vaginosis), surgical scrub, skin and vaginal preparation, antimicrobial prophylaxis (inappropriate choice or timing, inadequate dosing or redosing), operative time, blood transfusion, surgical skill, and operating room characteristics (ventilation, increased OR traffic, and sterilization of surgical equipment).
Antimicrobial prophylaxis
The CDC and the American College of Obstetricians and Gynecologists (ACOG) have provided clear guidelines regarding methods to reduce SSI in hysterectomy.3,4 There is strong evidence for using antimicrobial prophylaxis for hysterectomy.
It is important that physicians confirm the validity of beta-lactam allergies with patients because there are higher rates of SSI with the use of non–beta-lactam regimens, even those endorsed by the CDC and ACOG.5
Antibiotics should be administered within 1 hour of skin incision, and ideally within 30 minutes. They should be discontinued within 24 hours. Dosing should be adjusted to weight, and antimicrobials should be redosed for long procedures (at intervals of two half-lives), and for increased blood loss.
Skin preparation
Hair removal should be avoided unless necessary for technical reasons. If it is required, it should be performed outside of the operative space using clippers, not razors. For patients colonized with methicillin-resistant S. aureus, there is supporting evidence for pretreatment with mupirocin ointment to the nares, and chlorhexidine showers for 5-10 days. Patients who have bacterial vaginosis should be treated before surgery to decrease the rate of vaginal cuff SSI.

If there is a planned or potential gastrointestinal procedure as part of the hysterectomy, the surgeon should consider using an impervious plastic wound protector in place of, or in addition to, other retractors. Preoperative oral antimicrobials with mechanical bowel preparation have been associated with decreased SSIs; however, this benefit is not observed with mechanical bowel preparation alone.
Wound closure
Surgical technique and wound closure techniques also impact SSI. Minimally invasive and vaginal hysterectomy routes are preferred, as these are associated with the lowest rates of SSI. Antimicrobial-impregnated suture materials appear to be unnecessary. Surgeons should ensure that there is delicate handling of tissues and closure of dead spaces. If the subcutaneous fat space depth measures more than 2.5 cm, it should be reapproximated with a rapidly-absorbing suture material.
Use of electrosurgery versus a scalpel when creating the incision does not appear to influence infection rates, nor does use of staples versus subcuticular suture during closure.7
Using a dilute iodine lavage in the subcutaneous space, opening a sterile closing tray, and having surgeons change gloves prior to skin closure should be considered. The CDC recommends keeping the skin dressing in place for 24 hours postoperatively.
Other strategies
Hyperglycemia is associated with impaired neutrophil response, and therefore blood glucose should be controlled before surgery (hemoglobin A1c levels of less than 7% preoperatively) and immediately postoperatively (less than 180 mg/dL within 18-24 hours after the end of anesthesia).
It is also important to minimize perioperative hypothermia (less than 35.5° F), as this also impairs the body’s immune response. Keeping operative room ambient temperatures higher, minimizing incision size, warming CO2 gas in minimally invasive procedures, warming fluids, and using extrinsic body warmers can help achieve this.
Excessive blood loss should be minimized because blood transfusion is associated with impaired macrophage function and increased risk for SSI.
In addition to teamwide (including nonsurgeon) strict adherence to hand hygiene, OR personnel should avoid unnecessary operating room traffic. Hospital officials should ensure that the facility’s ventilator systems are well maintained and that there is care and maintenance of air handlers.
Many strategies can be employed perioperatively to decrease SSI rates for hysterectomy. We advocate for a protocol-based approach (known as “bundling” strategies) to achieve consistency of practice and to maximize surgeon and institutional improvements in SSI rates. This is similar to the approach outlined in a recent consensus statement from the Council on Patient Safety in Women’s Health Care.8
A comprehensive multidisciplinary approach throughout the perioperative period is necessary. It is imperative that good communication exist with patients regarding SSIs after hysterectomy and how patients, surgeons, and hospitals can together minimize the risks of SSIs.
References
1. Altemeier WA. “Manual on Control of Infection in Surgical Patients” (Philadelphia: Lippincott Williams & Wilkins, 1984).
2. Rev Infect Dis. 1991 Sep-Oct;13(Suppl 10):S821-41.
3. Infect Control Hosp Epidemiol. 2014 Jun;35(6):605-27.
4. Obstet Gynecol. 2009 May;113(5):1180-9.
5. Obstet Gynecol. 2016 Feb;127(2):321-9.
6. Am J Obstet Gynecol. 2005 Feb;192(2):422-5.
7. J Gastrointest Surg. 2016 Dec;20(12):2083-92.
8. Obstet Gynecol. 2016 Dec 7. doi: 10.1097/AOG.0000000000001751.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Jackson-Moore is an associate professor in gynecologic oncology at UNC. They reported having no relevant financial disclosures.
Surgical site infections are a major source of patient morbidity. They are also an important quality metric for surgeons and hospital systems, and are increasingly being linked to reimbursement.
They occur in approximately 2% of the 600,000 women undergoing hysterectomy in the United States each year. The U.S. Centers for Disease Control and Prevention defines surgical site infection (SSI) as an infection that occurs within 30 days of a procedure in the part of the body where the surgery took place. Most SSIs are superficial incisional, but they also include deep incisional or organ or space infections.
Classification
The incidence of SSI varies according to the classification of the wound, as defined by the National Academy of Sciences.1 Most hysterectomies are classified as clean-contaminated wounds because they involve entry into the mucosa of the genitourinary tract. However, hysterectomy with contamination of bowel flora, or in the setting of acute infection (such as suppurative pelvic inflammatory disease) are considered a contaminated wound class, and are associated with even higher rates of SSI.
Risk factors
The risk factors associated with SSI are both modifiable and unmodifiable. Broadly speaking, they include increased risk to endogenous flora (e.g., wound classification), increased exposure to exogenous flora (e.g., inadequate protection of a wound from external pathogens), and impairment of the body’s immune mechanisms to prevent and overcome infection (e.g., hypothermia and hypoglycemia).
Unmodifiable risk factors include increasing age, a history of radiation exposure, vascular disease, and a history of prior SSIs. Modifiable risk factors include obesity, tobacco use, immunosuppressive medications, hypoalbuminemia, route of hysterectomy, hair removal, preoperative infections (such as bacterial vaginosis), surgical scrub, skin and vaginal preparation, antimicrobial prophylaxis (inappropriate choice or timing, inadequate dosing or redosing), operative time, blood transfusion, surgical skill, and operating room characteristics (ventilation, increased OR traffic, and sterilization of surgical equipment).
Antimicrobial prophylaxis
The CDC and the American College of Obstetricians and Gynecologists (ACOG) have provided clear guidelines regarding methods to reduce SSI in hysterectomy.3,4 There is strong evidence for using antimicrobial prophylaxis for hysterectomy.
It is important that physicians confirm the validity of beta-lactam allergies with patients because there are higher rates of SSI with the use of non–beta-lactam regimens, even those endorsed by the CDC and ACOG.5
Antibiotics should be administered within 1 hour of skin incision, and ideally within 30 minutes. They should be discontinued within 24 hours. Dosing should be adjusted to weight, and antimicrobials should be redosed for long procedures (at intervals of two half-lives), and for increased blood loss.
Skin preparation
Hair removal should be avoided unless necessary for technical reasons. If it is required, it should be performed outside of the operative space using clippers, not razors. For patients colonized with methicillin-resistant S. aureus, there is supporting evidence for pretreatment with mupirocin ointment to the nares, and chlorhexidine showers for 5-10 days. Patients who have bacterial vaginosis should be treated before surgery to decrease the rate of vaginal cuff SSI.

If there is a planned or potential gastrointestinal procedure as part of the hysterectomy, the surgeon should consider using an impervious plastic wound protector in place of, or in addition to, other retractors. Preoperative oral antimicrobials with mechanical bowel preparation have been associated with decreased SSIs; however, this benefit is not observed with mechanical bowel preparation alone.
Wound closure
Surgical technique and wound closure techniques also impact SSI. Minimally invasive and vaginal hysterectomy routes are preferred, as these are associated with the lowest rates of SSI. Antimicrobial-impregnated suture materials appear to be unnecessary. Surgeons should ensure that there is delicate handling of tissues and closure of dead spaces. If the subcutaneous fat space depth measures more than 2.5 cm, it should be reapproximated with a rapidly-absorbing suture material.
Use of electrosurgery versus a scalpel when creating the incision does not appear to influence infection rates, nor does use of staples versus subcuticular suture during closure.7
Using a dilute iodine lavage in the subcutaneous space, opening a sterile closing tray, and having surgeons change gloves prior to skin closure should be considered. The CDC recommends keeping the skin dressing in place for 24 hours postoperatively.
Other strategies
Hyperglycemia is associated with impaired neutrophil response, and therefore blood glucose should be controlled before surgery (hemoglobin A1c levels of less than 7% preoperatively) and immediately postoperatively (less than 180 mg/dL within 18-24 hours after the end of anesthesia).
It is also important to minimize perioperative hypothermia (less than 35.5° F), as this also impairs the body’s immune response. Keeping operative room ambient temperatures higher, minimizing incision size, warming CO2 gas in minimally invasive procedures, warming fluids, and using extrinsic body warmers can help achieve this.
Excessive blood loss should be minimized because blood transfusion is associated with impaired macrophage function and increased risk for SSI.
In addition to teamwide (including nonsurgeon) strict adherence to hand hygiene, OR personnel should avoid unnecessary operating room traffic. Hospital officials should ensure that the facility’s ventilator systems are well maintained and that there is care and maintenance of air handlers.
Many strategies can be employed perioperatively to decrease SSI rates for hysterectomy. We advocate for a protocol-based approach (known as “bundling” strategies) to achieve consistency of practice and to maximize surgeon and institutional improvements in SSI rates. This is similar to the approach outlined in a recent consensus statement from the Council on Patient Safety in Women’s Health Care.8
A comprehensive multidisciplinary approach throughout the perioperative period is necessary. It is imperative that good communication exist with patients regarding SSIs after hysterectomy and how patients, surgeons, and hospitals can together minimize the risks of SSIs.
References
1. Altemeier WA. “Manual on Control of Infection in Surgical Patients” (Philadelphia: Lippincott Williams & Wilkins, 1984).
2. Rev Infect Dis. 1991 Sep-Oct;13(Suppl 10):S821-41.
3. Infect Control Hosp Epidemiol. 2014 Jun;35(6):605-27.
4. Obstet Gynecol. 2009 May;113(5):1180-9.
5. Obstet Gynecol. 2016 Feb;127(2):321-9.
6. Am J Obstet Gynecol. 2005 Feb;192(2):422-5.
7. J Gastrointest Surg. 2016 Dec;20(12):2083-92.
8. Obstet Gynecol. 2016 Dec 7. doi: 10.1097/AOG.0000000000001751.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Jackson-Moore is an associate professor in gynecologic oncology at UNC. They reported having no relevant financial disclosures.
Surgical site infections are a major source of patient morbidity. They are also an important quality metric for surgeons and hospital systems, and are increasingly being linked to reimbursement.
They occur in approximately 2% of the 600,000 women undergoing hysterectomy in the United States each year. The U.S. Centers for Disease Control and Prevention defines surgical site infection (SSI) as an infection that occurs within 30 days of a procedure in the part of the body where the surgery took place. Most SSIs are superficial incisional, but they also include deep incisional or organ or space infections.
Classification
The incidence of SSI varies according to the classification of the wound, as defined by the National Academy of Sciences.1 Most hysterectomies are classified as clean-contaminated wounds because they involve entry into the mucosa of the genitourinary tract. However, hysterectomy with contamination of bowel flora, or in the setting of acute infection (such as suppurative pelvic inflammatory disease) are considered a contaminated wound class, and are associated with even higher rates of SSI.
Risk factors
The risk factors associated with SSI are both modifiable and unmodifiable. Broadly speaking, they include increased risk to endogenous flora (e.g., wound classification), increased exposure to exogenous flora (e.g., inadequate protection of a wound from external pathogens), and impairment of the body’s immune mechanisms to prevent and overcome infection (e.g., hypothermia and hypoglycemia).
Unmodifiable risk factors include increasing age, a history of radiation exposure, vascular disease, and a history of prior SSIs. Modifiable risk factors include obesity, tobacco use, immunosuppressive medications, hypoalbuminemia, route of hysterectomy, hair removal, preoperative infections (such as bacterial vaginosis), surgical scrub, skin and vaginal preparation, antimicrobial prophylaxis (inappropriate choice or timing, inadequate dosing or redosing), operative time, blood transfusion, surgical skill, and operating room characteristics (ventilation, increased OR traffic, and sterilization of surgical equipment).
Antimicrobial prophylaxis
The CDC and the American College of Obstetricians and Gynecologists (ACOG) have provided clear guidelines regarding methods to reduce SSI in hysterectomy.3,4 There is strong evidence for using antimicrobial prophylaxis for hysterectomy.
It is important that physicians confirm the validity of beta-lactam allergies with patients because there are higher rates of SSI with the use of non–beta-lactam regimens, even those endorsed by the CDC and ACOG.5
Antibiotics should be administered within 1 hour of skin incision, and ideally within 30 minutes. They should be discontinued within 24 hours. Dosing should be adjusted to weight, and antimicrobials should be redosed for long procedures (at intervals of two half-lives), and for increased blood loss.
Skin preparation
Hair removal should be avoided unless necessary for technical reasons. If it is required, it should be performed outside of the operative space using clippers, not razors. For patients colonized with methicillin-resistant S. aureus, there is supporting evidence for pretreatment with mupirocin ointment to the nares, and chlorhexidine showers for 5-10 days. Patients who have bacterial vaginosis should be treated before surgery to decrease the rate of vaginal cuff SSI.

If there is a planned or potential gastrointestinal procedure as part of the hysterectomy, the surgeon should consider using an impervious plastic wound protector in place of, or in addition to, other retractors. Preoperative oral antimicrobials with mechanical bowel preparation have been associated with decreased SSIs; however, this benefit is not observed with mechanical bowel preparation alone.
Wound closure
Surgical technique and wound closure techniques also impact SSI. Minimally invasive and vaginal hysterectomy routes are preferred, as these are associated with the lowest rates of SSI. Antimicrobial-impregnated suture materials appear to be unnecessary. Surgeons should ensure that there is delicate handling of tissues and closure of dead spaces. If the subcutaneous fat space depth measures more than 2.5 cm, it should be reapproximated with a rapidly-absorbing suture material.
Use of electrosurgery versus a scalpel when creating the incision does not appear to influence infection rates, nor does use of staples versus subcuticular suture during closure.7
Using a dilute iodine lavage in the subcutaneous space, opening a sterile closing tray, and having surgeons change gloves prior to skin closure should be considered. The CDC recommends keeping the skin dressing in place for 24 hours postoperatively.
Other strategies
Hyperglycemia is associated with impaired neutrophil response, and therefore blood glucose should be controlled before surgery (hemoglobin A1c levels of less than 7% preoperatively) and immediately postoperatively (less than 180 mg/dL within 18-24 hours after the end of anesthesia).
It is also important to minimize perioperative hypothermia (less than 35.5° F), as this also impairs the body’s immune response. Keeping operative room ambient temperatures higher, minimizing incision size, warming CO2 gas in minimally invasive procedures, warming fluids, and using extrinsic body warmers can help achieve this.
Excessive blood loss should be minimized because blood transfusion is associated with impaired macrophage function and increased risk for SSI.
In addition to teamwide (including nonsurgeon) strict adherence to hand hygiene, OR personnel should avoid unnecessary operating room traffic. Hospital officials should ensure that the facility’s ventilator systems are well maintained and that there is care and maintenance of air handlers.
Many strategies can be employed perioperatively to decrease SSI rates for hysterectomy. We advocate for a protocol-based approach (known as “bundling” strategies) to achieve consistency of practice and to maximize surgeon and institutional improvements in SSI rates. This is similar to the approach outlined in a recent consensus statement from the Council on Patient Safety in Women’s Health Care.8
A comprehensive multidisciplinary approach throughout the perioperative period is necessary. It is imperative that good communication exist with patients regarding SSIs after hysterectomy and how patients, surgeons, and hospitals can together minimize the risks of SSIs.
References
1. Altemeier WA. “Manual on Control of Infection in Surgical Patients” (Philadelphia: Lippincott Williams & Wilkins, 1984).
2. Rev Infect Dis. 1991 Sep-Oct;13(Suppl 10):S821-41.
3. Infect Control Hosp Epidemiol. 2014 Jun;35(6):605-27.
4. Obstet Gynecol. 2009 May;113(5):1180-9.
5. Obstet Gynecol. 2016 Feb;127(2):321-9.
6. Am J Obstet Gynecol. 2005 Feb;192(2):422-5.
7. J Gastrointest Surg. 2016 Dec;20(12):2083-92.
8. Obstet Gynecol. 2016 Dec 7. doi: 10.1097/AOG.0000000000001751.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. Dr. Jackson-Moore is an associate professor in gynecologic oncology at UNC. They reported having no relevant financial disclosures.