User login
Hospital Unit‐Based Leadership Models
Hospital‐based care has become more complex over time. Patients are sicker, with more chronic comorbid conditions requiring greater collaboration to provide coordinated patient care.[1, 2] Care coordination requires an interdisciplinary approach during hospitalization and especially during transitions of care.[3, 4] In addition, hospitals are tasked with managing and improving clinical workflow efficiencies, and implementing electronic health records (EHR)[5] that require healthcare professionals to learn new systems of care and technology. Payment models have also started to shift toward an incentive and penalty‐based structure in the form of value‐based purchasing, readmission penalties, hospital‐acquired conditions, and meaningful use.[4, 6]
In response to these pressures, hospitals are searching for ways to reliably deliver quality care that is safe, effective, patient centered, timely, efficient, and equitable.[7] Previous efforts to improve quality in the general medical inpatient setting have included redesign of the clinical work environment and new workflows through the use of checklists and whiteboards to enhance communication, patient‐centered bedside rounds, standardized protocols and handovers, and integrated clinical decision support using health information technology.[8, 9, 10, 11, 12, 13] Although each of these care coordination activities has potential value, integrating them at the unit level often remains a challenge. Some hospitals have addressed this challenge by establishing and supporting a unit‐based leadership model, where a medical director and nurse manager work together to assess and improve the quality, safety, efficiency, and patient experience‐based mission of the organization.[14, 15] However, there are few descriptions of this leadership model in the current literature. Herein, we present the unit‐based leadership model that has been developed and implemented at 6 hospitals.
MODELS OF UNIT‐BASED LEADERSHIP
The unit‐based leadership model is grounded on the idea that culture and clinical care are products of frontline structure, process, and relationships, and that leaders at the site of care can have the greatest influence on the local work environment.[16, 17] The objective is to influence care and culture at the bedside and the unit, where care is delivered and where alignment with organizational vision and mission must occur. The concept of the inpatient unit medical director is not new, and hospitals in the past have recruited physician leaders to become clinical champions for quality improvement and help establish a collaborative work environment for physicians and unit‐based staff.[18, 19, 20, 21, 22] These studies report on the challenges and benefits of incorporating a medical director to inpatient psychiatry or general care units, but do not provide specific details about the recruitment and responsibilities for unit‐based dyad partnerships, which are critical factors for success on multidisciplinary inpatient care units.
There are several logistical matters to consider when instituting a unit‐based leadership model. These include the composition of the leadership team, selection process of the leaders, the presence of trainees and permanent faculty, and whether the units are able to geographically cohort patients. Other considerations include a clear role description with established shared goals and expectations, and a compensation model that includes effort and incentives. In addition, there should be a clearly established reporting structure to senior leadership, and the unit leaders should be given opportunities for professional growth and development. Table 1 provides a summary overview of 6 hospitals' experiences to date.
| Structure | Hospital of the University of Pennsylvania | Northwestern Memorial Hospital | Emory University Hospital | University of Michigan Health System | Christiana Care Health System | St. Joseph Mercy Health System/Integrated Health Associates |
|---|---|---|---|---|---|---|
| ||||||
| Description of hospital(s) | Academic medical center, 784 beds, 40,000 annual admissions | Academic medical center, 897 beds, 53,000 annual admissions | Academic medical center, 579 beds, 24,000 annual admissions | Academic medical center, 839 beds, 45,000 annual admissions | Independent academic medical center, 1,100 beds, 53,000 annual admissions | Tertiary community hospital that is part of a larger health care system (Trinity Health), 579 beds, 33,000 annual admissions |
| Unit leadership model | Triad of medical director, nurse manager, and quality improvement specialist/project manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager |
| Percent effort time supported for unit medical director | 10% | 17% | 10% | 20% | 20% | 10% |
| Incentives built into unit leaders' performance in outcomes metrics | No | Yes | No | No | No | Yes |
| Professional development/leadership training | Quality improvement method: PDSA, Six Sigma, Lean Healthcare | Quality improvement method: Six Sigma | Situational leadership training with 1:1 mentoring | Quality improvement method: Lean Healthcare, service excellence program | Quality Improvement method: Six Sigma, Lean Healthcare | Quality improvement method: Six Sigma |
| Additional leadership development through Penn Medicine Leadership Academy and Wharton Executive Education | Additional leadership development through Northwestern's professional development center and simulation training center | Conflict resolution skill development | Attend patient and Family Centered Care conference | Additional leadership development through Christiana Care Learning Institute | Attend educational course on Crucial Conversations | |
| Personality profile with coaching | Additional leadership development through University of Michigan Health System's human resources group | |||||
| Outcomes metrics monitored | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction |
| Efficiency of multidisciplinary rounds | Teamwork climate (survey) | Teamwork and implementation of structured interdisciplinary bedside rounds | Multidisciplinary rounds | Interdisciplinary rounds | Participation in interdisciplinary rounds | |
| RNMD work environment surveys | Adverse events | Unit‐based patient safety culture survey | Patient‐centered, bedside rounds | Readmission rates | ||
| Hospital‐acquired conditions (CAUTI, CLABSI, VAP, DVT, pressure ulcers) | Hospital‐acquired conditions (fall rates, pressure ulcers | Hospital‐acquired conditions (CAUTI, CLABSI, fall rates, pressure ulcers) | Hospital‐acquired conditions (CAUTI) | Hospital‐acquired conditions (fall rates, pressure ulcers) | Core measures | |
| Readmission rates | Readmission rates | Mortality | Readmission rates | Readmission rates | Medication reconciliation | |
| Core measures, patient safety indicators | Core measures | Length of stay | DVT prophylaxis | Hand hygiene | Discharge by 11 am | |
| Mortality (observed to expected, transfer, inpatient) | Hand hygiene | Glycemic control | Meeting attendance | Length of stay | Use of patient teach‐back | |
| Medication reconciliation | Restraint use | Communication with PCPs | ||||
| Home care, hospice, post‐acute care referral rates | ||||||
| Organizational leadership structure support for clinical unit partnership program | CMO, CNO, vice president of quality/patient safety, directors of medical and surgical nursing | Associate chair of medicine, director of medicine nursing; all medical directors are members of the department of medicine quality management committee | CMO, CNO, CEO, CQO | CMO, CNO | All teams report to and are supported by 3 overarching, system‐wide committees: (1) safety first, (2) think of yourself as a patient, (3) clinical excellence. Those committees, in turn, report up to the senior management quality/safety coordinating council. | Director of hospitalist program (reports to CMO); nursing director of acute care (reports to CNO) |
DISCUSSION
In reviewing our 6 organization's collective experiences, we identified several common themes and some notable differences across sites. The core of the leadership team was primarily composed of the medical director and nurse manager on the unit. Across all 6 organizations, medical directors had a portion of their effort supported for their leadership work on the unit. Leadership development training was provided at all of our sites, with particular emphasis on quality improvement (QI) methods such as Six‐Sigma, Lean, or Plan, Do, Study, Act (PDSA). Additional leadership development sessions were provided through the organization's human resources or affiliated university. Common outcome measures of interest include patient satisfaction, interdisciplinary practice, and collaboration on the unit, and some hospital‐acquired condition measures. Last, there is a direct reporting relationship to a chief or senior nurse or physician leader within each organization. These commonalities and variances are further detailed below.
Establishing the Unit‐Based Leadership Model
The composition of the unit‐based leadership model in our 6 organizations is predominantly a dyad partnership of medical directors and nurse managers. Although informal physician‐nurse collaborative practices have likely been in existence at many hospitals, formalizing this dyad partnership is an important step to fostering collaborative efforts to improve quality of care. It is also essential for hospital leadership to clearly articulate the need for this unit‐based leadership model. Whether the motivation for change is from a previously untenable practice environment, or part of an ongoing improvement program, the model should be presented in a manner that supports the organization's commitment to improve collaborative practices for better patient care. One of our 6 hospitals initiated this leadership model based on troubling relationships between nurses and physicians on some of their inpatient care units, which threatened to stall the organization's Magnet application. Implementation of the leadership model at the unit level yielded improvements in nursephysician interactions, patient satisfaction, and staff turnover.[15, 23] Another of the hospitals first evaluated why a previous attempt at this model did not deliver the intended outcomes, and redesigned the model based on its analysis.[14]
Across all of the organizations featured here, a common driver behind the adoption of the unit‐based leadership model was to bridge the divide between physician services and nursing and other allied health providers. We found that many of the physicians routinely had patients on multiple units, limiting the quantity and quality of collaborative practices between unit‐based staff and physician teams. The unit‐based dyad leaders are ideally positioned to build and foster a culture of collaboration, and our organizations have been inclusive to ensure the participation of a multidisciplinary group of providers, including representatives from pharmacy, environmental services, physical therapy, respiratory therapy, social work, case management, and nutrition at leadership meetings or in daily patient‐care discussions. In addition, 2 of the organizations have added quality improvement specialist/project managers to their teams to support the physiciannurse manager leaders on the unit.
Selection Process and Professional Development
The traditional approach to hiring a physician leader or a nurse manager has been an isolated process of drafting a job description for each position and hiring within their respective departments. For the dyad partnership to be successful, there should be established goals and expectations that require shared responsibilities between the 2 partners, which should guide the selection of these leaders. Other leadership attributes and essential character traits that should be modeled by the unit‐based leaders include good communication skills, respect among coworkers, and a collaborative approach to decision making and action. In addition, both physician leaders and nurse managers in these roles should have the ability to take a system's view, recognizing that within the complex network of healthcare providers and processes on their unit, these elements interact with each other, which lead to the outcomes achieved on their units.[24, 25] Table 2 lists some general shared responsibilities, highlighting specific activities that can be used to achieve the established outcomes. As the unit's dyad leadership works together to address these shared responsibilities, they should keep their sights focused on the overall strategic goals of the healthcare organization. Bohmer has defined 4 habits of the high‐value healthcare organization that in turn can be reflected through the inpatient unit leadership model to capture these activities at the local level: (1) planning care for specific patient populations, (2) microsystem design, (3) measurement and oversight, and (4) self‐study.[26] In determining specific shared responsibilities for each dyad partner, it is important for these leaders to understand the clinical microsystem of their unit such as their patient population, interdisciplinary care team, approach to process improvement, and performance patterns over time.[27]
| General Shared Responsibilities of Physician and Nurse Unit Directors | Examples of Specific Activities |
|---|---|
| |
| Serve as management partners to enhance culture of the unit | Co‐craft and deliver consistent leadership message |
| Co‐establish and enforce unit processes and protocols | |
| Co‐lead recruitment and retention efforts | |
| Co‐orient trainees and faculty rotating through unit | |
| Co‐educate on the management of common medical and surgical conditions | |
| Facilitate interstaff conflict resolution sessions | |
| Regular leadership meetings | |
| Actively manage unit processes and outcomes | Quality: improve core quality measure performance |
| Safety: improve culture of patient safety within the unit as measured by surveys and incident reporting systems | |
| Efficiency: reduce unnecessary length of stay and variability in resource use | |
| Patient experience: focus on improving patient‐family experience with targeted outcomes in patient experience metrics (eg, HCAHPS) | |
| Education: develop trainee and staff clinical and teamwork competencies | |
| Continuous process improvement initiatives (eg, PDSA cycles) | Improve the discharge transitions process, tailoring the process to each individual patient's identified risk factors |
| Focus improvement efforts on reduction in specific hospital acquired conditions such as CAUTI, VTE, CLABSI, pressure ulcers, falls | |
| Measure, analyze, reassess, and improve in all described areas of shared responsibilities | |
| Perform unit level chart reviews to evaluate readmissions and LOS and identify improvement opportunities | |
In our collective experience, the dyad leaders bring passion and commitment to improving care; however, many (the medical directors in particular) have minimal prior formal training in leadership, quality improvement, or hospital management. Recognizing that unit leaders require specialized knowledge and skills, each of our organizations has enrolled unit medical directors and nurse managers in leadership development courses or educational programs. Many healthcare organizations have become more grounded in a QI methodology including Six‐Sigma, Lean Healthcare, PDSA, and other scientifically based methods, and the unit‐based leaders should receive advanced training in the preferred methods of their institution. Additional training in quality improvement, patient safety, and physician leadership can also be obtained through supplemental coursework specifically designed to train hospital leaders, with some programs leading to a certification or additional credentials.[28]
Beyond such formal educational opportunities, hospitals should not overlook the opportunity to learn from and share experiences with the other dyad leadership units within the hospital. One of the organizations described here holds monthly meetings with all of the unit dyad leaders, and 2 other organizations conduct quarterly meetings to share experiences and best practices related to specific improvement initiatives in a learning network model. Those units with more experience in specific initiatives are asked to share their lessons learned with others, as well as support each other in their efforts to collectively meet the strategic goals of the hospital.
Time and Organizational Support
In addition to leadership development, hospitals and the clinical department leadership need to support the medical directors with dedicated time away from their usual clinical duties. Some organizations in this report are providing up to 20% effort for the medical director's unit‐based leadership work; however, there is some variation in practice with regard to physician effort across sites. The University of Pennsylvania has a smaller effort support at 10%; however, some of that effort differential may be offset through the allocation of the quality improvement specialist/project manager assigned to work with the medical director and nurse manager dyad. St. Joseph Mercy Hospital also has a lower allocation, as there is additional financial compensation for the role that is at risk and not included in this 10% allocation.
It is also important to assure that the medical directors have institutional support to carry out their work in partnership with their nursing leadership. The 6 health systems described here report that although most of the physicians have appointments within a physician group or clinical department, there is hospital leadership oversight from a chief medical, nursing, or operating officer. This organizational structure may be an important aspect of the model as the unit‐based leaders seek to align their efforts with that of the hospital. Further, this form of organizational oversight can ensure that the unit leaders will receive timely and essential unit‐ and hospital‐based performance measures to manage local improvement efforts. These measures may include some components of patient experiences as reported in the Hospital Consumer Assessment of Healthcare Providers and Systems survey, readmission rates, hospital‐acquired condition rates, length of stay, observed to expected mortality rates, and results of staff satisfaction and safety culture surveys. As highlighted by several studies and commentaries, our collective experiences also identified interdisciplinary teamwork, collaboration, and communication as desirable outcome measures through the unit‐based leadership structure.[21, 22, 24, 29, 30] The medical director and nurse manager dyads can prioritize their improvement efforts based on the data provided to them, and mobilize the appropriate group of multidisciplinary practitioners and support staff on the unit.
OTHER CONSIDERATIONS
Other infrastructure variables that may increase the effectiveness of the unit leadership dyad include unit‐based clinical services (geographic localization), engaging the frontline team members in the design and implementation of change innovations, a commitment to patient and family centered practices on the unit, and enhancing clinical workflow through the support of EHR functions such as concurrent documentation and provider order entry. Geographic localization, placing the fewest possible clinical service providers on the unit to work alongside unit‐based staff, allows for a cohesive interdisciplinary unit‐based team to develop under the dyad leadership, and has been shown to improve communication practices.[9, 31] Beyond geographic localization of patients, it is critical to ensure team members are committed to the changes in workflow by directly involving them through the design and implementation of new models of care taking place on the unit. This commitment starts from the top senior nurse and physician leaders in the organization, and extends to the unit‐based dyad partners, and down to each individual interdisciplinary team member on the unit.[1] Thus, it is critical to clarify roles and responsibilities and how team members on the unit will interact with each other. For some situations, conflict management training will be helpful to the unit‐based leaders to resolve issues. To appreciate potential barriers to successful rollout of this unit leadership model, a phased implementation of pilot units, followed by successive waves, should be considered. Many of the units that instituted unit‐based interdisciplinary team rounds solicited and implemented direct feedback from frontline team members in efforts to improve communication and be more patient centered. Conversely, there are also likely to be situations where the unit‐based leaders will be confronted with hindrances to their unit‐based collaborative improvement efforts. To help prepare the dyad leaders, many of our unit‐based leaders have received specific training on how to coach and conduct difficult conversations with individuals who have performance gaps or are perceived to be hindering the progress of the unit's work. These crucial negotiation skills are not innate among most managers and should be explicitly provided to new leaders across organizations.
The goals and merits of patient‐ and family‐centered care (PFCC) have been well described.[32, 33, 34] Organizational support to teach and disseminate PFCC practices throughout all settings of care may help the leadership dyads implement rounding strategies that engage all staff, patients, and family members throughout the hospital course and during the transitions out of the hospital.
Clinical workflow has become heavily dependent on the EHR systems. For those organizations that have yet to adopt a particular EHR system, the leadership dyads should be involved throughout the EHR design process to help ensure that the technological solutions will be built to assist the clinical workflow, and once the system has been built, the leadership dyad should monitor and enhance the interface between workflow and EHR system so that it can support the creation and advancement of interdisciplinary plans of care on the unit.
CONCLUSION
The care of the hospitalized patient has become more complex over time. Interdisciplinary teamwork needs to be improved at the unit level to achieve the strategic goals of the hospital. Although quality improvement is an organizational goal, change takes place locally. Physician leaders, in partnership with nurse managers, are needed now more than ever to take on this task to improve the hospital‐care experience for patients by functioning as the primary effector arms for changing the landscape of hospital‐based care. We have described characteristics of unit‐based leadership programs adopted across 6 organizations. Hospitalists with clinical experience as the principal providers of inpatient‐based care and quality improvement experience and training, have been key participants in the development and implementation of the local leadership models in each of these hospital systems. We hope the comparison of the various models featured in this article serves as a valuable reference to hospitals and healthcare organizations who are contemplating the incorporation of this model into their strategic plan.
- , , , et al. Organizational predictors of coordination in inpatient medicine [published online ahead of print February 26, 2014]. Health Care Manage Rev. doi: 10.1097/HMR.0000000000000004.
- . Trends in case‐mix in the medicare population. Paper presented at: American Hospital Association, Federation of American Hospitals, Association of American Medical Colleges; http://www.aha.org/content/00‐10/100715‐CMItrends.pdf. July 15, 2010.
- . A requirement to reduce readmissions: take care of the patient, not just the disease. JAMA. 2013;309(4):394–396.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- Medicare and Medicaid programs; electronic health record incentive program. Final rule. Fed Regist. 2010;75(144):44313–44588.
- . The Center for Medicare and Medicaid innovation's blueprint for rapid‐cycle evaluation of new care and payment models. Health Aff (Millwood). 2013;32(4):807–812.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit. J Gen Intern Med. 2010;25(8):826–832.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , . A review on systematic reviews of health information system studies. J Am Med Inform Assoc. 2010;17(6):637–645.
- , , , , . Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. Apr 2010;5(4):234–239.
- , , . Development of a checklist for documenting team and collaborative behaviors during multidisciplinary bedside rounds. J Nurs Adm. 2013;43(5):280–285.
- , , , , . Assessment of teamwork during structured interdisciplinary rounds on medical units. J Hosp Med. 2012;7(9):679–683.
- , , , et al. Leadership at the front line: a clinical partnership model on general care inpatient units. Am J Med Qual. 2012;27(2):106–111.
- , . AHRQ health care innovations exchange: improvement projects led by unit‐based teams of nurse, physician, and quality leaders reduce infections, lower costs, improve patient satisfaction, and nurse‐physician communication. Available at: http://www.innovations.ahrq.gov/content.aspx?id=2719. Published April 14, 2010. Accessed November 26, 2011.
- , , , , , . Microsystems in health care: part 8. Developing people and improving work life: what front‐line staff told us. Jt Comm J Qual Saf. 2003;29(10):512–522.
- , , , et al. Microsystems in health care: part 5. How leaders are leading. Jt Comm J Qual Saf. 2003;29(6):297–308.
- , , . The academic dilemma of the inpatient unit director. Am J Psychiatry. 1989;146(1):73–76.
- , , , , . Improving and sustaining core measure performance through effective accountability of clinical microsystems in an academic medical center. Jt Comm J Qual Patient Saf. 2010;36(9):387–398.
- , , . Physician leadership and quality improvement in the acute child and adolescent psychiatric care setting. Child Adolesc Psychiatr Clin N Am. 2010;19(1):1–19; table of contents.
- , , , . Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses. Am J Crit Care. 2005;14(1):71–77.
- , . Nurse‐physician leadership: insights into interprofessional collaboration. J Nurs Adm. 2013;43(12):653–659.
- The Advisory Board. University of Pennsylvania Health System pilots unit clinical leadership model to spur quality gains. Nurs Exec Watch. 2008;9(2):4–6.
- , . Physicians as leaders in improving health care: a new series in Annals of Internal Medicine. Ann Intern Med. 1998;128(4):289–292.
- . Understanding medical systems. Ann Intern Med. 1998;128(4):293–298.
- . The four habits of high‐value health care organizations. N Engl J Med. 2011;365(22):2045–2047.
- , , , et al. Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units. Jt Comm J Qual Improv. 2002;28(9):472–493.
- , , , et al. The quality and safety educators academy: fulfilling an unmet need for faculty development. Am J Med Qual. 2014;29(1):5–12.
- , , , . Cooperation: the foundation of improvement. Ann Intern Med. 1998;128(12 pt 1):1004–1009.
- , , , , , . Ten principles of good interdisciplinary team work. Hum Resour Health 2013;11(1):19.
- , , , et al. Impact of localizing general medical teams to a single nursing unit. J Hosp Med. 2012;7(7):551–556.
- , , , . Integrating patient‐ and family‐centered care with health policy: four proposed policy approaches. Qual Manag Health Care. 2013;22(2):137–145.
- , , . Incorporating patient‐ and family‐centered care into resident education: approaches, benefits, and challenges. J Grad Med Educ. 2011;3(2):272–278.
- Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
Hospital‐based care has become more complex over time. Patients are sicker, with more chronic comorbid conditions requiring greater collaboration to provide coordinated patient care.[1, 2] Care coordination requires an interdisciplinary approach during hospitalization and especially during transitions of care.[3, 4] In addition, hospitals are tasked with managing and improving clinical workflow efficiencies, and implementing electronic health records (EHR)[5] that require healthcare professionals to learn new systems of care and technology. Payment models have also started to shift toward an incentive and penalty‐based structure in the form of value‐based purchasing, readmission penalties, hospital‐acquired conditions, and meaningful use.[4, 6]
In response to these pressures, hospitals are searching for ways to reliably deliver quality care that is safe, effective, patient centered, timely, efficient, and equitable.[7] Previous efforts to improve quality in the general medical inpatient setting have included redesign of the clinical work environment and new workflows through the use of checklists and whiteboards to enhance communication, patient‐centered bedside rounds, standardized protocols and handovers, and integrated clinical decision support using health information technology.[8, 9, 10, 11, 12, 13] Although each of these care coordination activities has potential value, integrating them at the unit level often remains a challenge. Some hospitals have addressed this challenge by establishing and supporting a unit‐based leadership model, where a medical director and nurse manager work together to assess and improve the quality, safety, efficiency, and patient experience‐based mission of the organization.[14, 15] However, there are few descriptions of this leadership model in the current literature. Herein, we present the unit‐based leadership model that has been developed and implemented at 6 hospitals.
MODELS OF UNIT‐BASED LEADERSHIP
The unit‐based leadership model is grounded on the idea that culture and clinical care are products of frontline structure, process, and relationships, and that leaders at the site of care can have the greatest influence on the local work environment.[16, 17] The objective is to influence care and culture at the bedside and the unit, where care is delivered and where alignment with organizational vision and mission must occur. The concept of the inpatient unit medical director is not new, and hospitals in the past have recruited physician leaders to become clinical champions for quality improvement and help establish a collaborative work environment for physicians and unit‐based staff.[18, 19, 20, 21, 22] These studies report on the challenges and benefits of incorporating a medical director to inpatient psychiatry or general care units, but do not provide specific details about the recruitment and responsibilities for unit‐based dyad partnerships, which are critical factors for success on multidisciplinary inpatient care units.
There are several logistical matters to consider when instituting a unit‐based leadership model. These include the composition of the leadership team, selection process of the leaders, the presence of trainees and permanent faculty, and whether the units are able to geographically cohort patients. Other considerations include a clear role description with established shared goals and expectations, and a compensation model that includes effort and incentives. In addition, there should be a clearly established reporting structure to senior leadership, and the unit leaders should be given opportunities for professional growth and development. Table 1 provides a summary overview of 6 hospitals' experiences to date.
| Structure | Hospital of the University of Pennsylvania | Northwestern Memorial Hospital | Emory University Hospital | University of Michigan Health System | Christiana Care Health System | St. Joseph Mercy Health System/Integrated Health Associates |
|---|---|---|---|---|---|---|
| ||||||
| Description of hospital(s) | Academic medical center, 784 beds, 40,000 annual admissions | Academic medical center, 897 beds, 53,000 annual admissions | Academic medical center, 579 beds, 24,000 annual admissions | Academic medical center, 839 beds, 45,000 annual admissions | Independent academic medical center, 1,100 beds, 53,000 annual admissions | Tertiary community hospital that is part of a larger health care system (Trinity Health), 579 beds, 33,000 annual admissions |
| Unit leadership model | Triad of medical director, nurse manager, and quality improvement specialist/project manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager |
| Percent effort time supported for unit medical director | 10% | 17% | 10% | 20% | 20% | 10% |
| Incentives built into unit leaders' performance in outcomes metrics | No | Yes | No | No | No | Yes |
| Professional development/leadership training | Quality improvement method: PDSA, Six Sigma, Lean Healthcare | Quality improvement method: Six Sigma | Situational leadership training with 1:1 mentoring | Quality improvement method: Lean Healthcare, service excellence program | Quality Improvement method: Six Sigma, Lean Healthcare | Quality improvement method: Six Sigma |
| Additional leadership development through Penn Medicine Leadership Academy and Wharton Executive Education | Additional leadership development through Northwestern's professional development center and simulation training center | Conflict resolution skill development | Attend patient and Family Centered Care conference | Additional leadership development through Christiana Care Learning Institute | Attend educational course on Crucial Conversations | |
| Personality profile with coaching | Additional leadership development through University of Michigan Health System's human resources group | |||||
| Outcomes metrics monitored | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction |
| Efficiency of multidisciplinary rounds | Teamwork climate (survey) | Teamwork and implementation of structured interdisciplinary bedside rounds | Multidisciplinary rounds | Interdisciplinary rounds | Participation in interdisciplinary rounds | |
| RNMD work environment surveys | Adverse events | Unit‐based patient safety culture survey | Patient‐centered, bedside rounds | Readmission rates | ||
| Hospital‐acquired conditions (CAUTI, CLABSI, VAP, DVT, pressure ulcers) | Hospital‐acquired conditions (fall rates, pressure ulcers | Hospital‐acquired conditions (CAUTI, CLABSI, fall rates, pressure ulcers) | Hospital‐acquired conditions (CAUTI) | Hospital‐acquired conditions (fall rates, pressure ulcers) | Core measures | |
| Readmission rates | Readmission rates | Mortality | Readmission rates | Readmission rates | Medication reconciliation | |
| Core measures, patient safety indicators | Core measures | Length of stay | DVT prophylaxis | Hand hygiene | Discharge by 11 am | |
| Mortality (observed to expected, transfer, inpatient) | Hand hygiene | Glycemic control | Meeting attendance | Length of stay | Use of patient teach‐back | |
| Medication reconciliation | Restraint use | Communication with PCPs | ||||
| Home care, hospice, post‐acute care referral rates | ||||||
| Organizational leadership structure support for clinical unit partnership program | CMO, CNO, vice president of quality/patient safety, directors of medical and surgical nursing | Associate chair of medicine, director of medicine nursing; all medical directors are members of the department of medicine quality management committee | CMO, CNO, CEO, CQO | CMO, CNO | All teams report to and are supported by 3 overarching, system‐wide committees: (1) safety first, (2) think of yourself as a patient, (3) clinical excellence. Those committees, in turn, report up to the senior management quality/safety coordinating council. | Director of hospitalist program (reports to CMO); nursing director of acute care (reports to CNO) |
DISCUSSION
In reviewing our 6 organization's collective experiences, we identified several common themes and some notable differences across sites. The core of the leadership team was primarily composed of the medical director and nurse manager on the unit. Across all 6 organizations, medical directors had a portion of their effort supported for their leadership work on the unit. Leadership development training was provided at all of our sites, with particular emphasis on quality improvement (QI) methods such as Six‐Sigma, Lean, or Plan, Do, Study, Act (PDSA). Additional leadership development sessions were provided through the organization's human resources or affiliated university. Common outcome measures of interest include patient satisfaction, interdisciplinary practice, and collaboration on the unit, and some hospital‐acquired condition measures. Last, there is a direct reporting relationship to a chief or senior nurse or physician leader within each organization. These commonalities and variances are further detailed below.
Establishing the Unit‐Based Leadership Model
The composition of the unit‐based leadership model in our 6 organizations is predominantly a dyad partnership of medical directors and nurse managers. Although informal physician‐nurse collaborative practices have likely been in existence at many hospitals, formalizing this dyad partnership is an important step to fostering collaborative efforts to improve quality of care. It is also essential for hospital leadership to clearly articulate the need for this unit‐based leadership model. Whether the motivation for change is from a previously untenable practice environment, or part of an ongoing improvement program, the model should be presented in a manner that supports the organization's commitment to improve collaborative practices for better patient care. One of our 6 hospitals initiated this leadership model based on troubling relationships between nurses and physicians on some of their inpatient care units, which threatened to stall the organization's Magnet application. Implementation of the leadership model at the unit level yielded improvements in nursephysician interactions, patient satisfaction, and staff turnover.[15, 23] Another of the hospitals first evaluated why a previous attempt at this model did not deliver the intended outcomes, and redesigned the model based on its analysis.[14]
Across all of the organizations featured here, a common driver behind the adoption of the unit‐based leadership model was to bridge the divide between physician services and nursing and other allied health providers. We found that many of the physicians routinely had patients on multiple units, limiting the quantity and quality of collaborative practices between unit‐based staff and physician teams. The unit‐based dyad leaders are ideally positioned to build and foster a culture of collaboration, and our organizations have been inclusive to ensure the participation of a multidisciplinary group of providers, including representatives from pharmacy, environmental services, physical therapy, respiratory therapy, social work, case management, and nutrition at leadership meetings or in daily patient‐care discussions. In addition, 2 of the organizations have added quality improvement specialist/project managers to their teams to support the physiciannurse manager leaders on the unit.
Selection Process and Professional Development
The traditional approach to hiring a physician leader or a nurse manager has been an isolated process of drafting a job description for each position and hiring within their respective departments. For the dyad partnership to be successful, there should be established goals and expectations that require shared responsibilities between the 2 partners, which should guide the selection of these leaders. Other leadership attributes and essential character traits that should be modeled by the unit‐based leaders include good communication skills, respect among coworkers, and a collaborative approach to decision making and action. In addition, both physician leaders and nurse managers in these roles should have the ability to take a system's view, recognizing that within the complex network of healthcare providers and processes on their unit, these elements interact with each other, which lead to the outcomes achieved on their units.[24, 25] Table 2 lists some general shared responsibilities, highlighting specific activities that can be used to achieve the established outcomes. As the unit's dyad leadership works together to address these shared responsibilities, they should keep their sights focused on the overall strategic goals of the healthcare organization. Bohmer has defined 4 habits of the high‐value healthcare organization that in turn can be reflected through the inpatient unit leadership model to capture these activities at the local level: (1) planning care for specific patient populations, (2) microsystem design, (3) measurement and oversight, and (4) self‐study.[26] In determining specific shared responsibilities for each dyad partner, it is important for these leaders to understand the clinical microsystem of their unit such as their patient population, interdisciplinary care team, approach to process improvement, and performance patterns over time.[27]
| General Shared Responsibilities of Physician and Nurse Unit Directors | Examples of Specific Activities |
|---|---|
| |
| Serve as management partners to enhance culture of the unit | Co‐craft and deliver consistent leadership message |
| Co‐establish and enforce unit processes and protocols | |
| Co‐lead recruitment and retention efforts | |
| Co‐orient trainees and faculty rotating through unit | |
| Co‐educate on the management of common medical and surgical conditions | |
| Facilitate interstaff conflict resolution sessions | |
| Regular leadership meetings | |
| Actively manage unit processes and outcomes | Quality: improve core quality measure performance |
| Safety: improve culture of patient safety within the unit as measured by surveys and incident reporting systems | |
| Efficiency: reduce unnecessary length of stay and variability in resource use | |
| Patient experience: focus on improving patient‐family experience with targeted outcomes in patient experience metrics (eg, HCAHPS) | |
| Education: develop trainee and staff clinical and teamwork competencies | |
| Continuous process improvement initiatives (eg, PDSA cycles) | Improve the discharge transitions process, tailoring the process to each individual patient's identified risk factors |
| Focus improvement efforts on reduction in specific hospital acquired conditions such as CAUTI, VTE, CLABSI, pressure ulcers, falls | |
| Measure, analyze, reassess, and improve in all described areas of shared responsibilities | |
| Perform unit level chart reviews to evaluate readmissions and LOS and identify improvement opportunities | |
In our collective experience, the dyad leaders bring passion and commitment to improving care; however, many (the medical directors in particular) have minimal prior formal training in leadership, quality improvement, or hospital management. Recognizing that unit leaders require specialized knowledge and skills, each of our organizations has enrolled unit medical directors and nurse managers in leadership development courses or educational programs. Many healthcare organizations have become more grounded in a QI methodology including Six‐Sigma, Lean Healthcare, PDSA, and other scientifically based methods, and the unit‐based leaders should receive advanced training in the preferred methods of their institution. Additional training in quality improvement, patient safety, and physician leadership can also be obtained through supplemental coursework specifically designed to train hospital leaders, with some programs leading to a certification or additional credentials.[28]
Beyond such formal educational opportunities, hospitals should not overlook the opportunity to learn from and share experiences with the other dyad leadership units within the hospital. One of the organizations described here holds monthly meetings with all of the unit dyad leaders, and 2 other organizations conduct quarterly meetings to share experiences and best practices related to specific improvement initiatives in a learning network model. Those units with more experience in specific initiatives are asked to share their lessons learned with others, as well as support each other in their efforts to collectively meet the strategic goals of the hospital.
Time and Organizational Support
In addition to leadership development, hospitals and the clinical department leadership need to support the medical directors with dedicated time away from their usual clinical duties. Some organizations in this report are providing up to 20% effort for the medical director's unit‐based leadership work; however, there is some variation in practice with regard to physician effort across sites. The University of Pennsylvania has a smaller effort support at 10%; however, some of that effort differential may be offset through the allocation of the quality improvement specialist/project manager assigned to work with the medical director and nurse manager dyad. St. Joseph Mercy Hospital also has a lower allocation, as there is additional financial compensation for the role that is at risk and not included in this 10% allocation.
It is also important to assure that the medical directors have institutional support to carry out their work in partnership with their nursing leadership. The 6 health systems described here report that although most of the physicians have appointments within a physician group or clinical department, there is hospital leadership oversight from a chief medical, nursing, or operating officer. This organizational structure may be an important aspect of the model as the unit‐based leaders seek to align their efforts with that of the hospital. Further, this form of organizational oversight can ensure that the unit leaders will receive timely and essential unit‐ and hospital‐based performance measures to manage local improvement efforts. These measures may include some components of patient experiences as reported in the Hospital Consumer Assessment of Healthcare Providers and Systems survey, readmission rates, hospital‐acquired condition rates, length of stay, observed to expected mortality rates, and results of staff satisfaction and safety culture surveys. As highlighted by several studies and commentaries, our collective experiences also identified interdisciplinary teamwork, collaboration, and communication as desirable outcome measures through the unit‐based leadership structure.[21, 22, 24, 29, 30] The medical director and nurse manager dyads can prioritize their improvement efforts based on the data provided to them, and mobilize the appropriate group of multidisciplinary practitioners and support staff on the unit.
OTHER CONSIDERATIONS
Other infrastructure variables that may increase the effectiveness of the unit leadership dyad include unit‐based clinical services (geographic localization), engaging the frontline team members in the design and implementation of change innovations, a commitment to patient and family centered practices on the unit, and enhancing clinical workflow through the support of EHR functions such as concurrent documentation and provider order entry. Geographic localization, placing the fewest possible clinical service providers on the unit to work alongside unit‐based staff, allows for a cohesive interdisciplinary unit‐based team to develop under the dyad leadership, and has been shown to improve communication practices.[9, 31] Beyond geographic localization of patients, it is critical to ensure team members are committed to the changes in workflow by directly involving them through the design and implementation of new models of care taking place on the unit. This commitment starts from the top senior nurse and physician leaders in the organization, and extends to the unit‐based dyad partners, and down to each individual interdisciplinary team member on the unit.[1] Thus, it is critical to clarify roles and responsibilities and how team members on the unit will interact with each other. For some situations, conflict management training will be helpful to the unit‐based leaders to resolve issues. To appreciate potential barriers to successful rollout of this unit leadership model, a phased implementation of pilot units, followed by successive waves, should be considered. Many of the units that instituted unit‐based interdisciplinary team rounds solicited and implemented direct feedback from frontline team members in efforts to improve communication and be more patient centered. Conversely, there are also likely to be situations where the unit‐based leaders will be confronted with hindrances to their unit‐based collaborative improvement efforts. To help prepare the dyad leaders, many of our unit‐based leaders have received specific training on how to coach and conduct difficult conversations with individuals who have performance gaps or are perceived to be hindering the progress of the unit's work. These crucial negotiation skills are not innate among most managers and should be explicitly provided to new leaders across organizations.
The goals and merits of patient‐ and family‐centered care (PFCC) have been well described.[32, 33, 34] Organizational support to teach and disseminate PFCC practices throughout all settings of care may help the leadership dyads implement rounding strategies that engage all staff, patients, and family members throughout the hospital course and during the transitions out of the hospital.
Clinical workflow has become heavily dependent on the EHR systems. For those organizations that have yet to adopt a particular EHR system, the leadership dyads should be involved throughout the EHR design process to help ensure that the technological solutions will be built to assist the clinical workflow, and once the system has been built, the leadership dyad should monitor and enhance the interface between workflow and EHR system so that it can support the creation and advancement of interdisciplinary plans of care on the unit.
CONCLUSION
The care of the hospitalized patient has become more complex over time. Interdisciplinary teamwork needs to be improved at the unit level to achieve the strategic goals of the hospital. Although quality improvement is an organizational goal, change takes place locally. Physician leaders, in partnership with nurse managers, are needed now more than ever to take on this task to improve the hospital‐care experience for patients by functioning as the primary effector arms for changing the landscape of hospital‐based care. We have described characteristics of unit‐based leadership programs adopted across 6 organizations. Hospitalists with clinical experience as the principal providers of inpatient‐based care and quality improvement experience and training, have been key participants in the development and implementation of the local leadership models in each of these hospital systems. We hope the comparison of the various models featured in this article serves as a valuable reference to hospitals and healthcare organizations who are contemplating the incorporation of this model into their strategic plan.
Hospital‐based care has become more complex over time. Patients are sicker, with more chronic comorbid conditions requiring greater collaboration to provide coordinated patient care.[1, 2] Care coordination requires an interdisciplinary approach during hospitalization and especially during transitions of care.[3, 4] In addition, hospitals are tasked with managing and improving clinical workflow efficiencies, and implementing electronic health records (EHR)[5] that require healthcare professionals to learn new systems of care and technology. Payment models have also started to shift toward an incentive and penalty‐based structure in the form of value‐based purchasing, readmission penalties, hospital‐acquired conditions, and meaningful use.[4, 6]
In response to these pressures, hospitals are searching for ways to reliably deliver quality care that is safe, effective, patient centered, timely, efficient, and equitable.[7] Previous efforts to improve quality in the general medical inpatient setting have included redesign of the clinical work environment and new workflows through the use of checklists and whiteboards to enhance communication, patient‐centered bedside rounds, standardized protocols and handovers, and integrated clinical decision support using health information technology.[8, 9, 10, 11, 12, 13] Although each of these care coordination activities has potential value, integrating them at the unit level often remains a challenge. Some hospitals have addressed this challenge by establishing and supporting a unit‐based leadership model, where a medical director and nurse manager work together to assess and improve the quality, safety, efficiency, and patient experience‐based mission of the organization.[14, 15] However, there are few descriptions of this leadership model in the current literature. Herein, we present the unit‐based leadership model that has been developed and implemented at 6 hospitals.
MODELS OF UNIT‐BASED LEADERSHIP
The unit‐based leadership model is grounded on the idea that culture and clinical care are products of frontline structure, process, and relationships, and that leaders at the site of care can have the greatest influence on the local work environment.[16, 17] The objective is to influence care and culture at the bedside and the unit, where care is delivered and where alignment with organizational vision and mission must occur. The concept of the inpatient unit medical director is not new, and hospitals in the past have recruited physician leaders to become clinical champions for quality improvement and help establish a collaborative work environment for physicians and unit‐based staff.[18, 19, 20, 21, 22] These studies report on the challenges and benefits of incorporating a medical director to inpatient psychiatry or general care units, but do not provide specific details about the recruitment and responsibilities for unit‐based dyad partnerships, which are critical factors for success on multidisciplinary inpatient care units.
There are several logistical matters to consider when instituting a unit‐based leadership model. These include the composition of the leadership team, selection process of the leaders, the presence of trainees and permanent faculty, and whether the units are able to geographically cohort patients. Other considerations include a clear role description with established shared goals and expectations, and a compensation model that includes effort and incentives. In addition, there should be a clearly established reporting structure to senior leadership, and the unit leaders should be given opportunities for professional growth and development. Table 1 provides a summary overview of 6 hospitals' experiences to date.
| Structure | Hospital of the University of Pennsylvania | Northwestern Memorial Hospital | Emory University Hospital | University of Michigan Health System | Christiana Care Health System | St. Joseph Mercy Health System/Integrated Health Associates |
|---|---|---|---|---|---|---|
| ||||||
| Description of hospital(s) | Academic medical center, 784 beds, 40,000 annual admissions | Academic medical center, 897 beds, 53,000 annual admissions | Academic medical center, 579 beds, 24,000 annual admissions | Academic medical center, 839 beds, 45,000 annual admissions | Independent academic medical center, 1,100 beds, 53,000 annual admissions | Tertiary community hospital that is part of a larger health care system (Trinity Health), 579 beds, 33,000 annual admissions |
| Unit leadership model | Triad of medical director, nurse manager, and quality improvement specialist/project manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager | Dyad of medical director and nurse manager |
| Percent effort time supported for unit medical director | 10% | 17% | 10% | 20% | 20% | 10% |
| Incentives built into unit leaders' performance in outcomes metrics | No | Yes | No | No | No | Yes |
| Professional development/leadership training | Quality improvement method: PDSA, Six Sigma, Lean Healthcare | Quality improvement method: Six Sigma | Situational leadership training with 1:1 mentoring | Quality improvement method: Lean Healthcare, service excellence program | Quality Improvement method: Six Sigma, Lean Healthcare | Quality improvement method: Six Sigma |
| Additional leadership development through Penn Medicine Leadership Academy and Wharton Executive Education | Additional leadership development through Northwestern's professional development center and simulation training center | Conflict resolution skill development | Attend patient and Family Centered Care conference | Additional leadership development through Christiana Care Learning Institute | Attend educational course on Crucial Conversations | |
| Personality profile with coaching | Additional leadership development through University of Michigan Health System's human resources group | |||||
| Outcomes metrics monitored | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction | Patient satisfaction |
| Efficiency of multidisciplinary rounds | Teamwork climate (survey) | Teamwork and implementation of structured interdisciplinary bedside rounds | Multidisciplinary rounds | Interdisciplinary rounds | Participation in interdisciplinary rounds | |
| RNMD work environment surveys | Adverse events | Unit‐based patient safety culture survey | Patient‐centered, bedside rounds | Readmission rates | ||
| Hospital‐acquired conditions (CAUTI, CLABSI, VAP, DVT, pressure ulcers) | Hospital‐acquired conditions (fall rates, pressure ulcers | Hospital‐acquired conditions (CAUTI, CLABSI, fall rates, pressure ulcers) | Hospital‐acquired conditions (CAUTI) | Hospital‐acquired conditions (fall rates, pressure ulcers) | Core measures | |
| Readmission rates | Readmission rates | Mortality | Readmission rates | Readmission rates | Medication reconciliation | |
| Core measures, patient safety indicators | Core measures | Length of stay | DVT prophylaxis | Hand hygiene | Discharge by 11 am | |
| Mortality (observed to expected, transfer, inpatient) | Hand hygiene | Glycemic control | Meeting attendance | Length of stay | Use of patient teach‐back | |
| Medication reconciliation | Restraint use | Communication with PCPs | ||||
| Home care, hospice, post‐acute care referral rates | ||||||
| Organizational leadership structure support for clinical unit partnership program | CMO, CNO, vice president of quality/patient safety, directors of medical and surgical nursing | Associate chair of medicine, director of medicine nursing; all medical directors are members of the department of medicine quality management committee | CMO, CNO, CEO, CQO | CMO, CNO | All teams report to and are supported by 3 overarching, system‐wide committees: (1) safety first, (2) think of yourself as a patient, (3) clinical excellence. Those committees, in turn, report up to the senior management quality/safety coordinating council. | Director of hospitalist program (reports to CMO); nursing director of acute care (reports to CNO) |
DISCUSSION
In reviewing our 6 organization's collective experiences, we identified several common themes and some notable differences across sites. The core of the leadership team was primarily composed of the medical director and nurse manager on the unit. Across all 6 organizations, medical directors had a portion of their effort supported for their leadership work on the unit. Leadership development training was provided at all of our sites, with particular emphasis on quality improvement (QI) methods such as Six‐Sigma, Lean, or Plan, Do, Study, Act (PDSA). Additional leadership development sessions were provided through the organization's human resources or affiliated university. Common outcome measures of interest include patient satisfaction, interdisciplinary practice, and collaboration on the unit, and some hospital‐acquired condition measures. Last, there is a direct reporting relationship to a chief or senior nurse or physician leader within each organization. These commonalities and variances are further detailed below.
Establishing the Unit‐Based Leadership Model
The composition of the unit‐based leadership model in our 6 organizations is predominantly a dyad partnership of medical directors and nurse managers. Although informal physician‐nurse collaborative practices have likely been in existence at many hospitals, formalizing this dyad partnership is an important step to fostering collaborative efforts to improve quality of care. It is also essential for hospital leadership to clearly articulate the need for this unit‐based leadership model. Whether the motivation for change is from a previously untenable practice environment, or part of an ongoing improvement program, the model should be presented in a manner that supports the organization's commitment to improve collaborative practices for better patient care. One of our 6 hospitals initiated this leadership model based on troubling relationships between nurses and physicians on some of their inpatient care units, which threatened to stall the organization's Magnet application. Implementation of the leadership model at the unit level yielded improvements in nursephysician interactions, patient satisfaction, and staff turnover.[15, 23] Another of the hospitals first evaluated why a previous attempt at this model did not deliver the intended outcomes, and redesigned the model based on its analysis.[14]
Across all of the organizations featured here, a common driver behind the adoption of the unit‐based leadership model was to bridge the divide between physician services and nursing and other allied health providers. We found that many of the physicians routinely had patients on multiple units, limiting the quantity and quality of collaborative practices between unit‐based staff and physician teams. The unit‐based dyad leaders are ideally positioned to build and foster a culture of collaboration, and our organizations have been inclusive to ensure the participation of a multidisciplinary group of providers, including representatives from pharmacy, environmental services, physical therapy, respiratory therapy, social work, case management, and nutrition at leadership meetings or in daily patient‐care discussions. In addition, 2 of the organizations have added quality improvement specialist/project managers to their teams to support the physiciannurse manager leaders on the unit.
Selection Process and Professional Development
The traditional approach to hiring a physician leader or a nurse manager has been an isolated process of drafting a job description for each position and hiring within their respective departments. For the dyad partnership to be successful, there should be established goals and expectations that require shared responsibilities between the 2 partners, which should guide the selection of these leaders. Other leadership attributes and essential character traits that should be modeled by the unit‐based leaders include good communication skills, respect among coworkers, and a collaborative approach to decision making and action. In addition, both physician leaders and nurse managers in these roles should have the ability to take a system's view, recognizing that within the complex network of healthcare providers and processes on their unit, these elements interact with each other, which lead to the outcomes achieved on their units.[24, 25] Table 2 lists some general shared responsibilities, highlighting specific activities that can be used to achieve the established outcomes. As the unit's dyad leadership works together to address these shared responsibilities, they should keep their sights focused on the overall strategic goals of the healthcare organization. Bohmer has defined 4 habits of the high‐value healthcare organization that in turn can be reflected through the inpatient unit leadership model to capture these activities at the local level: (1) planning care for specific patient populations, (2) microsystem design, (3) measurement and oversight, and (4) self‐study.[26] In determining specific shared responsibilities for each dyad partner, it is important for these leaders to understand the clinical microsystem of their unit such as their patient population, interdisciplinary care team, approach to process improvement, and performance patterns over time.[27]
| General Shared Responsibilities of Physician and Nurse Unit Directors | Examples of Specific Activities |
|---|---|
| |
| Serve as management partners to enhance culture of the unit | Co‐craft and deliver consistent leadership message |
| Co‐establish and enforce unit processes and protocols | |
| Co‐lead recruitment and retention efforts | |
| Co‐orient trainees and faculty rotating through unit | |
| Co‐educate on the management of common medical and surgical conditions | |
| Facilitate interstaff conflict resolution sessions | |
| Regular leadership meetings | |
| Actively manage unit processes and outcomes | Quality: improve core quality measure performance |
| Safety: improve culture of patient safety within the unit as measured by surveys and incident reporting systems | |
| Efficiency: reduce unnecessary length of stay and variability in resource use | |
| Patient experience: focus on improving patient‐family experience with targeted outcomes in patient experience metrics (eg, HCAHPS) | |
| Education: develop trainee and staff clinical and teamwork competencies | |
| Continuous process improvement initiatives (eg, PDSA cycles) | Improve the discharge transitions process, tailoring the process to each individual patient's identified risk factors |
| Focus improvement efforts on reduction in specific hospital acquired conditions such as CAUTI, VTE, CLABSI, pressure ulcers, falls | |
| Measure, analyze, reassess, and improve in all described areas of shared responsibilities | |
| Perform unit level chart reviews to evaluate readmissions and LOS and identify improvement opportunities | |
In our collective experience, the dyad leaders bring passion and commitment to improving care; however, many (the medical directors in particular) have minimal prior formal training in leadership, quality improvement, or hospital management. Recognizing that unit leaders require specialized knowledge and skills, each of our organizations has enrolled unit medical directors and nurse managers in leadership development courses or educational programs. Many healthcare organizations have become more grounded in a QI methodology including Six‐Sigma, Lean Healthcare, PDSA, and other scientifically based methods, and the unit‐based leaders should receive advanced training in the preferred methods of their institution. Additional training in quality improvement, patient safety, and physician leadership can also be obtained through supplemental coursework specifically designed to train hospital leaders, with some programs leading to a certification or additional credentials.[28]
Beyond such formal educational opportunities, hospitals should not overlook the opportunity to learn from and share experiences with the other dyad leadership units within the hospital. One of the organizations described here holds monthly meetings with all of the unit dyad leaders, and 2 other organizations conduct quarterly meetings to share experiences and best practices related to specific improvement initiatives in a learning network model. Those units with more experience in specific initiatives are asked to share their lessons learned with others, as well as support each other in their efforts to collectively meet the strategic goals of the hospital.
Time and Organizational Support
In addition to leadership development, hospitals and the clinical department leadership need to support the medical directors with dedicated time away from their usual clinical duties. Some organizations in this report are providing up to 20% effort for the medical director's unit‐based leadership work; however, there is some variation in practice with regard to physician effort across sites. The University of Pennsylvania has a smaller effort support at 10%; however, some of that effort differential may be offset through the allocation of the quality improvement specialist/project manager assigned to work with the medical director and nurse manager dyad. St. Joseph Mercy Hospital also has a lower allocation, as there is additional financial compensation for the role that is at risk and not included in this 10% allocation.
It is also important to assure that the medical directors have institutional support to carry out their work in partnership with their nursing leadership. The 6 health systems described here report that although most of the physicians have appointments within a physician group or clinical department, there is hospital leadership oversight from a chief medical, nursing, or operating officer. This organizational structure may be an important aspect of the model as the unit‐based leaders seek to align their efforts with that of the hospital. Further, this form of organizational oversight can ensure that the unit leaders will receive timely and essential unit‐ and hospital‐based performance measures to manage local improvement efforts. These measures may include some components of patient experiences as reported in the Hospital Consumer Assessment of Healthcare Providers and Systems survey, readmission rates, hospital‐acquired condition rates, length of stay, observed to expected mortality rates, and results of staff satisfaction and safety culture surveys. As highlighted by several studies and commentaries, our collective experiences also identified interdisciplinary teamwork, collaboration, and communication as desirable outcome measures through the unit‐based leadership structure.[21, 22, 24, 29, 30] The medical director and nurse manager dyads can prioritize their improvement efforts based on the data provided to them, and mobilize the appropriate group of multidisciplinary practitioners and support staff on the unit.
OTHER CONSIDERATIONS
Other infrastructure variables that may increase the effectiveness of the unit leadership dyad include unit‐based clinical services (geographic localization), engaging the frontline team members in the design and implementation of change innovations, a commitment to patient and family centered practices on the unit, and enhancing clinical workflow through the support of EHR functions such as concurrent documentation and provider order entry. Geographic localization, placing the fewest possible clinical service providers on the unit to work alongside unit‐based staff, allows for a cohesive interdisciplinary unit‐based team to develop under the dyad leadership, and has been shown to improve communication practices.[9, 31] Beyond geographic localization of patients, it is critical to ensure team members are committed to the changes in workflow by directly involving them through the design and implementation of new models of care taking place on the unit. This commitment starts from the top senior nurse and physician leaders in the organization, and extends to the unit‐based dyad partners, and down to each individual interdisciplinary team member on the unit.[1] Thus, it is critical to clarify roles and responsibilities and how team members on the unit will interact with each other. For some situations, conflict management training will be helpful to the unit‐based leaders to resolve issues. To appreciate potential barriers to successful rollout of this unit leadership model, a phased implementation of pilot units, followed by successive waves, should be considered. Many of the units that instituted unit‐based interdisciplinary team rounds solicited and implemented direct feedback from frontline team members in efforts to improve communication and be more patient centered. Conversely, there are also likely to be situations where the unit‐based leaders will be confronted with hindrances to their unit‐based collaborative improvement efforts. To help prepare the dyad leaders, many of our unit‐based leaders have received specific training on how to coach and conduct difficult conversations with individuals who have performance gaps or are perceived to be hindering the progress of the unit's work. These crucial negotiation skills are not innate among most managers and should be explicitly provided to new leaders across organizations.
The goals and merits of patient‐ and family‐centered care (PFCC) have been well described.[32, 33, 34] Organizational support to teach and disseminate PFCC practices throughout all settings of care may help the leadership dyads implement rounding strategies that engage all staff, patients, and family members throughout the hospital course and during the transitions out of the hospital.
Clinical workflow has become heavily dependent on the EHR systems. For those organizations that have yet to adopt a particular EHR system, the leadership dyads should be involved throughout the EHR design process to help ensure that the technological solutions will be built to assist the clinical workflow, and once the system has been built, the leadership dyad should monitor and enhance the interface between workflow and EHR system so that it can support the creation and advancement of interdisciplinary plans of care on the unit.
CONCLUSION
The care of the hospitalized patient has become more complex over time. Interdisciplinary teamwork needs to be improved at the unit level to achieve the strategic goals of the hospital. Although quality improvement is an organizational goal, change takes place locally. Physician leaders, in partnership with nurse managers, are needed now more than ever to take on this task to improve the hospital‐care experience for patients by functioning as the primary effector arms for changing the landscape of hospital‐based care. We have described characteristics of unit‐based leadership programs adopted across 6 organizations. Hospitalists with clinical experience as the principal providers of inpatient‐based care and quality improvement experience and training, have been key participants in the development and implementation of the local leadership models in each of these hospital systems. We hope the comparison of the various models featured in this article serves as a valuable reference to hospitals and healthcare organizations who are contemplating the incorporation of this model into their strategic plan.
- , , , et al. Organizational predictors of coordination in inpatient medicine [published online ahead of print February 26, 2014]. Health Care Manage Rev. doi: 10.1097/HMR.0000000000000004.
- . Trends in case‐mix in the medicare population. Paper presented at: American Hospital Association, Federation of American Hospitals, Association of American Medical Colleges; http://www.aha.org/content/00‐10/100715‐CMItrends.pdf. July 15, 2010.
- . A requirement to reduce readmissions: take care of the patient, not just the disease. JAMA. 2013;309(4):394–396.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- Medicare and Medicaid programs; electronic health record incentive program. Final rule. Fed Regist. 2010;75(144):44313–44588.
- . The Center for Medicare and Medicaid innovation's blueprint for rapid‐cycle evaluation of new care and payment models. Health Aff (Millwood). 2013;32(4):807–812.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit. J Gen Intern Med. 2010;25(8):826–832.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , . A review on systematic reviews of health information system studies. J Am Med Inform Assoc. 2010;17(6):637–645.
- , , , , . Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. Apr 2010;5(4):234–239.
- , , . Development of a checklist for documenting team and collaborative behaviors during multidisciplinary bedside rounds. J Nurs Adm. 2013;43(5):280–285.
- , , , , . Assessment of teamwork during structured interdisciplinary rounds on medical units. J Hosp Med. 2012;7(9):679–683.
- , , , et al. Leadership at the front line: a clinical partnership model on general care inpatient units. Am J Med Qual. 2012;27(2):106–111.
- , . AHRQ health care innovations exchange: improvement projects led by unit‐based teams of nurse, physician, and quality leaders reduce infections, lower costs, improve patient satisfaction, and nurse‐physician communication. Available at: http://www.innovations.ahrq.gov/content.aspx?id=2719. Published April 14, 2010. Accessed November 26, 2011.
- , , , , , . Microsystems in health care: part 8. Developing people and improving work life: what front‐line staff told us. Jt Comm J Qual Saf. 2003;29(10):512–522.
- , , , et al. Microsystems in health care: part 5. How leaders are leading. Jt Comm J Qual Saf. 2003;29(6):297–308.
- , , . The academic dilemma of the inpatient unit director. Am J Psychiatry. 1989;146(1):73–76.
- , , , , . Improving and sustaining core measure performance through effective accountability of clinical microsystems in an academic medical center. Jt Comm J Qual Patient Saf. 2010;36(9):387–398.
- , , . Physician leadership and quality improvement in the acute child and adolescent psychiatric care setting. Child Adolesc Psychiatr Clin N Am. 2010;19(1):1–19; table of contents.
- , , , . Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses. Am J Crit Care. 2005;14(1):71–77.
- , . Nurse‐physician leadership: insights into interprofessional collaboration. J Nurs Adm. 2013;43(12):653–659.
- The Advisory Board. University of Pennsylvania Health System pilots unit clinical leadership model to spur quality gains. Nurs Exec Watch. 2008;9(2):4–6.
- , . Physicians as leaders in improving health care: a new series in Annals of Internal Medicine. Ann Intern Med. 1998;128(4):289–292.
- . Understanding medical systems. Ann Intern Med. 1998;128(4):293–298.
- . The four habits of high‐value health care organizations. N Engl J Med. 2011;365(22):2045–2047.
- , , , et al. Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units. Jt Comm J Qual Improv. 2002;28(9):472–493.
- , , , et al. The quality and safety educators academy: fulfilling an unmet need for faculty development. Am J Med Qual. 2014;29(1):5–12.
- , , , . Cooperation: the foundation of improvement. Ann Intern Med. 1998;128(12 pt 1):1004–1009.
- , , , , , . Ten principles of good interdisciplinary team work. Hum Resour Health 2013;11(1):19.
- , , , et al. Impact of localizing general medical teams to a single nursing unit. J Hosp Med. 2012;7(7):551–556.
- , , , . Integrating patient‐ and family‐centered care with health policy: four proposed policy approaches. Qual Manag Health Care. 2013;22(2):137–145.
- , , . Incorporating patient‐ and family‐centered care into resident education: approaches, benefits, and challenges. J Grad Med Educ. 2011;3(2):272–278.
- Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
- , , , et al. Organizational predictors of coordination in inpatient medicine [published online ahead of print February 26, 2014]. Health Care Manage Rev. doi: 10.1097/HMR.0000000000000004.
- . Trends in case‐mix in the medicare population. Paper presented at: American Hospital Association, Federation of American Hospitals, Association of American Medical Colleges; http://www.aha.org/content/00‐10/100715‐CMItrends.pdf. July 15, 2010.
- . A requirement to reduce readmissions: take care of the patient, not just the disease. JAMA. 2013;309(4):394–396.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- Medicare and Medicaid programs; electronic health record incentive program. Final rule. Fed Regist. 2010;75(144):44313–44588.
- . The Center for Medicare and Medicaid innovation's blueprint for rapid‐cycle evaluation of new care and payment models. Health Aff (Millwood). 2013;32(4):807–812.
- Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academies Press; 2001.
- , , , , , . Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit. J Gen Intern Med. 2010;25(8):826–832.
- , , , et al. Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care. J Gen Intern Med. 2009;24(11):1223–1227.
- , , , . A review on systematic reviews of health information system studies. J Am Med Inform Assoc. 2010;17(6):637–645.
- , , , , . Patient whiteboards as a communication tool in the hospital setting: a survey of practices and recommendations. J Hosp Med. Apr 2010;5(4):234–239.
- , , . Development of a checklist for documenting team and collaborative behaviors during multidisciplinary bedside rounds. J Nurs Adm. 2013;43(5):280–285.
- , , , , . Assessment of teamwork during structured interdisciplinary rounds on medical units. J Hosp Med. 2012;7(9):679–683.
- , , , et al. Leadership at the front line: a clinical partnership model on general care inpatient units. Am J Med Qual. 2012;27(2):106–111.
- , . AHRQ health care innovations exchange: improvement projects led by unit‐based teams of nurse, physician, and quality leaders reduce infections, lower costs, improve patient satisfaction, and nurse‐physician communication. Available at: http://www.innovations.ahrq.gov/content.aspx?id=2719. Published April 14, 2010. Accessed November 26, 2011.
- , , , , , . Microsystems in health care: part 8. Developing people and improving work life: what front‐line staff told us. Jt Comm J Qual Saf. 2003;29(10):512–522.
- , , , et al. Microsystems in health care: part 5. How leaders are leading. Jt Comm J Qual Saf. 2003;29(6):297–308.
- , , . The academic dilemma of the inpatient unit director. Am J Psychiatry. 1989;146(1):73–76.
- , , , , . Improving and sustaining core measure performance through effective accountability of clinical microsystems in an academic medical center. Jt Comm J Qual Patient Saf. 2010;36(9):387–398.
- , , . Physician leadership and quality improvement in the acute child and adolescent psychiatric care setting. Child Adolesc Psychiatr Clin N Am. 2010;19(1):1–19; table of contents.
- , , , . Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses. Am J Crit Care. 2005;14(1):71–77.
- , . Nurse‐physician leadership: insights into interprofessional collaboration. J Nurs Adm. 2013;43(12):653–659.
- The Advisory Board. University of Pennsylvania Health System pilots unit clinical leadership model to spur quality gains. Nurs Exec Watch. 2008;9(2):4–6.
- , . Physicians as leaders in improving health care: a new series in Annals of Internal Medicine. Ann Intern Med. 1998;128(4):289–292.
- . Understanding medical systems. Ann Intern Med. 1998;128(4):293–298.
- . The four habits of high‐value health care organizations. N Engl J Med. 2011;365(22):2045–2047.
- , , , et al. Microsystems in health care: Part 1. Learning from high‐performing front‐line clinical units. Jt Comm J Qual Improv. 2002;28(9):472–493.
- , , , et al. The quality and safety educators academy: fulfilling an unmet need for faculty development. Am J Med Qual. 2014;29(1):5–12.
- , , , . Cooperation: the foundation of improvement. Ann Intern Med. 1998;128(12 pt 1):1004–1009.
- , , , , , . Ten principles of good interdisciplinary team work. Hum Resour Health 2013;11(1):19.
- , , , et al. Impact of localizing general medical teams to a single nursing unit. J Hosp Med. 2012;7(7):551–556.
- , , , . Integrating patient‐ and family‐centered care with health policy: four proposed policy approaches. Qual Manag Health Care. 2013;22(2):137–145.
- , , . Incorporating patient‐ and family‐centered care into resident education: approaches, benefits, and challenges. J Grad Med Educ. 2011;3(2):272–278.
- Smith M, Saunders R, Stuckhardt L, McGinnis JM, eds. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
Severe babesiosis
An 85‐year‐old male from rural southern New Jersey with history of innumerable tick bites over many years was admitted for fever of unknown origin. On hospital day 2, his hemoglobin dropped from 11.3 g/dL to 7.5 g/dL, with an associated elevated indirect bilirubin and lactate dehydrogenase. Blood smear showed numerous intracellular and extracellular trophozoites, with approximately 10% to 30% of red cells infected, consistent with severe babesiosis. Given his high parasitemia, new hypoxia, lethargy, and advanced age, treatment was initiated with intravenous antibiotics and red cell exchange transfusion.
Babesiosis should be considered on the differential diagnosis of hemolytic anemia in patients that live in or have traveled to endemic areas, especially with history of tick bites. The most common appearance on blood smear is round to oval rings with pale blue cytoplasm and a red‐staining nucleus (Fig. 1). Exoerythrocytic parasites or the pathognomonic Maltese Cross tetrad forms (not present in our patient's smear) help to differentiate from falciparum malaria.
The patient's parasite burden and clinical status markedly improved with treatment and he was discharged home. 0
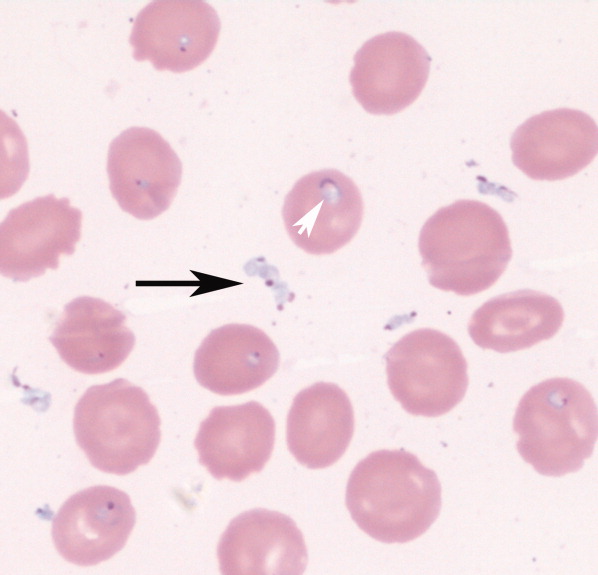
An 85‐year‐old male from rural southern New Jersey with history of innumerable tick bites over many years was admitted for fever of unknown origin. On hospital day 2, his hemoglobin dropped from 11.3 g/dL to 7.5 g/dL, with an associated elevated indirect bilirubin and lactate dehydrogenase. Blood smear showed numerous intracellular and extracellular trophozoites, with approximately 10% to 30% of red cells infected, consistent with severe babesiosis. Given his high parasitemia, new hypoxia, lethargy, and advanced age, treatment was initiated with intravenous antibiotics and red cell exchange transfusion.
Babesiosis should be considered on the differential diagnosis of hemolytic anemia in patients that live in or have traveled to endemic areas, especially with history of tick bites. The most common appearance on blood smear is round to oval rings with pale blue cytoplasm and a red‐staining nucleus (Fig. 1). Exoerythrocytic parasites or the pathognomonic Maltese Cross tetrad forms (not present in our patient's smear) help to differentiate from falciparum malaria.
The patient's parasite burden and clinical status markedly improved with treatment and he was discharged home. 0

An 85‐year‐old male from rural southern New Jersey with history of innumerable tick bites over many years was admitted for fever of unknown origin. On hospital day 2, his hemoglobin dropped from 11.3 g/dL to 7.5 g/dL, with an associated elevated indirect bilirubin and lactate dehydrogenase. Blood smear showed numerous intracellular and extracellular trophozoites, with approximately 10% to 30% of red cells infected, consistent with severe babesiosis. Given his high parasitemia, new hypoxia, lethargy, and advanced age, treatment was initiated with intravenous antibiotics and red cell exchange transfusion.
Babesiosis should be considered on the differential diagnosis of hemolytic anemia in patients that live in or have traveled to endemic areas, especially with history of tick bites. The most common appearance on blood smear is round to oval rings with pale blue cytoplasm and a red‐staining nucleus (Fig. 1). Exoerythrocytic parasites or the pathognomonic Maltese Cross tetrad forms (not present in our patient's smear) help to differentiate from falciparum malaria.
The patient's parasite burden and clinical status markedly improved with treatment and he was discharged home. 0

Establishing a Rapid Response Team
Medical emergency teams (METs) were introduced more than a decade ago in Australia and the United Kingdom to rapidly identify and manage seriously ill patients at risk of cardiopulmonary arrest and other high‐risk conditions.1 METs, known in the United States as rapid response teams (RRTs), have been slow to be adopted thus far but are quickly gaining ground. Despite numerous studies indicating long‐term patient outcomes are poor following cardiac resuscitation in the hospital, the benefits of early intervention have sometimes been overlooked.25 Several observational studies and a retrospective analysis that included the Medical Emergency Response Improvement Team (MERIT) in Pittsburgh showed that introduction of a MET apparently has the potential to decrease the incidence of unanticipated intensive care unit (ICU) admissions and in‐hospital morbidity and mortality from unexpected cardiopulmonary arrest.69 Furthermore, the use of a MET as a quality improvement tool to detect medical errors and effect systemwide interventions is promising.10 Most recently, the Institute for Healthcare Improvement (IHI) and the American Hospital Association challenged health care organizations to redesign patient safety systems to prevent avoidable deaths in its 100K Lives Campaign. One of the 6 proposed core interventions was the deployment of rapid response teams at the first sign of patient decline.11
Despite these reports of success, a recent large cluster‐randomized controlled trial did not yield the same positive results. In this well‐designed study of 23 Australian hospitals, the Medical Early Response, Intervention and Therapy (MERIT) study investigators found the incidence of cardiac arrest, unplanned ICU admissions, and unexpected death essentially unchanged despite large increases in how often the emergency team was called.12 One possible explanation why these findings conflicted with previous favorable results is that the ultimate impact of a MET may depend on the effectiveness of implementation strategies. To derive the benefits of a MET/RRT, hospitals must increasingly focus on identifying barriers to implementation and address practical issues that may undermine their long‐term effectiveness.
In this article we describe in detail the process of establishing an RRT at our urban, academic hospital and the modifications that became necessary as we rolled out the intervention and encountered obstacles. This analysis was undertaken as a quality improvement (QI) activity. To our knowledge, this is one of the few recent published descriptions of the experiences of implementing an RRT in the United States since earlier work in Pittsburgh.9, 13
METHODS
Temple University Hospital is a tertiary care academic hospital in urban Philadelphia, Pennsylvania. Our RRT was first implemented July 1, 2004, and in the first 12 months of initiation, it was activated 307 times. The RRT at Temple University Hospital was designed to be accessible 24 hours a day, 7 days a week. The daytime team (8 am‐5 pm) is composed of an attending physician (a hospitalist trained as a general internist), a senior internal medicine resident, a critical care nurse, a nurse manager, a pharmacist, and a respiratory therapist. In addition, both a transporter and a member of the admissions office respond to all rapid response team calls but do not get clinically involved in patient care. For nighttime (5 pm‐8 am) and weekend coverage the hospitalist is replaced by an on‐site pulmonary critical care physician, but the remainder of the team is unchanged. All RRT members carry beepers synchronized to provide the location of an RRT activation. In addition, all RRT calls are simultaneously announced on the overhead paging system. No changes were made to the existing cardiac arrest team (code team) at the hospital, which remained a 24‐hour response team for patients found to be in true cardiopulmonary arrest and was comprised of on‐call internal medicine house staff (but no hospitalist attending physician), a respiratory therapist, a pharmacist, a critical care nurse, a nurse manager, and, most notably, an anesthesiologist for emergent intubation and airway management.
The RRT was intended for use within the physical confines of Temple University Hospital and its immediately adjacent grounds. Within the hospital the main locations defined were: inpatient areas, including patient rooms and hallways of the medical‐surgical units of the inpatient tower, as well as the burn, coronary, medical, neurological, neurosurgical, and surgical intensive care units; off‐unit/procedural areas, including diagnostic/emnterventional radiology, the gastroenterology endoscopy suite, the pulmonary procedure suite and pulmonary function lab, the cardiac catheterization/ECHO/stress Lab, the inpatient dialysis unit, and the physical therapy gym, all areas where inpatients are routinely transported during their hospital admission for workup/treatment and where outpatients go for scheduled procedures and therapies; and outpatient/common areas, including all the general medical and subspecialty outpatient clinics in 2 separate outpatient towers (Outpatient Building and Parkinson Pavilion) with direct access from the main hospital building, the outpatient pharmacy, the elevators, the hallways in the outpatient sections of the hospital, all lobbies, and the immediately adjacent outside grounds.
Prior to the launch date of the RRT, clinical criteria were established to help guide staff about when an RRT might be called (Fig. 1). These were based in part on early literature on the clinical markers that most often precede clinical deterioration.14, 15 In addition, 2 much broader categories for RRT activation were added (Inability to reach the patient's primary team of treating physicians for any of the above and Any potentially serious medical errors or adverse events) in order to minimize the need for a very specific physiologic definition to be met in order to activate the team. Physicians, nurses, and other staff with significant daily contact with inpatients and outpatients were in‐serviced about the purpose of the RRT and how to activate the system via the hospital paging operator. Laminated cards with RRT criteria were distributed to all hospital personnel, and educational posters were displayed prominently throughout the hospital.

Each RRT event was to be assessed by team members using a standardized evaluation form (Fig. 2), with primary responsibility going to the physician team leader. In the initial phases of implementation, these forms were kept in the offices of the Section of Hospital Medicine for the use of hospitalist attending physician team leaders. Later on in the year they were kept in the pharmacist's RRT medication bag. These forms were collected at the completion of each RRT event or faxed to a central location and then entered into a database maintained by the hospital's Department of Patient Safety Operations. Weekly debriefing meetings to review all RRT events from the preceding week were attended by representatives from patient safety, respiratory, nursing, hospital medicine, and the pharmacy. Attempts were made to identify the issues that led to selected RRT activations, to obtain patient follow‐up from the clinical event, and to evaluate the performance of the team. Throughout these weekly meetings, QI strategies for improving the effectiveness of the RRT were identified and implemented.
The core outcome measures that were used to assess RRT performance were: appropriateness of the RRT activation, percentage of patients who were stabilized, percentage of patients who were transferred to a higher level of care, and overall team performance.
In the weekly meeting of the RRT evaluation committee, at which each RRT was reviewed by the clinical team, each scenario and details of the event were reviewed to determine whether the RRT activation was appropriate, whether the intervention was successful, and whether there were any issues with the team performance. After a thorough discussion of each case and review of additional data from the chart if necessary, the RRT evaluation committee reached a consensus about each of these measures.
We also tracked the number of code team activations from the year preceding establishment of the RRT (2003‐2004) through the year during which the RRT was established (2004‐2005). Because all calls for both the RRT and the code team go first to the hospital operator, we reviewed the hospital paging operators' logs for the entire 12‐month period to track the rate of code team events to RRT events on a monthly basis.
RESULTS
In a 12‐month period, the RRT was activated 307 times, as recorded in the hospital operator logs. In the year preceding inception of the RRT, there were 272 code team activations. In the first 12 months concurrent with RRT implementation, the code team was activated 258 times. Overall, at their discretion the team leaders converted 13% of the 307 RRT activations to traditional code team activations.
There were 11 RRT activations in July, the first month of implementation, and 14 activations in the second month. At that point, the internal hospital newsletter released a feature on the new RRT, and our patient safety officer/director of patient safety operations made a concerted effort to educate hospital administration and the Graduate Medical Education Committee (GMEC); as a result, utilization picked up. From September onward through the remainder of the academic year, an average of 28 RRT activations occurred each month (range 20‐37), whereas an average of 22 codes took place each month (range 12‐27). The numbers of RRT versus code team activations are plotted in Figure 3. A trend line for the number of code team activations per month in 2003, the year prior to implementation of the RRT, was added for comparison; it conveys the slight overall decrease in the number of codes as the RRT took effect (average of 23 codes per month, range 15‐31).
Physician evaluation forms were returned for 170 of the 307 RRT events (55%). The main inpatient tower was the site of 42% of these RRT activations, followed by the outpatient/common areas, where 19% of the activations occurred, and off‐unit/procedural areas, the site of 18%. Table 2 provides information on specific location, reason for call, and disposition of a sample of the RRT activations in the non‐inpatient areas. Time of day was noted in 76.8% of events. Of these, 82.9% occurred during the traditional day shift (7 am‐7 pm) and 17.1% on night shift (7 pm‐7 am). Most RRT activations occurred between 8 am and 4 pm. Daytime events heavily outnumbered nighttime events regardless of location.
Physician team leaders largely believed a specific underlying clinical diagnosis was responsible for 59% of the RRT activations, followed by adverse drug reactions (3.5%), physician error (1.8%), and nursing error (0.6%). When an underlying clinical diagnosis or organ system was suspected, it was most frequently pulmonary (32%), followed by neurological (14%) and cardiac (11%). It was believed that 32% of events were for other reason not listed. Table 1 provides the breakdown of other underlying diagnoses in RRT events.
| Pulmonary | 32% |
| Hypoxia/Respiratory Distress (32%) | |
| Neurological | 14% |
| Change of mental status (7%) | |
| Syncope (7%) | |
| Cardiac | 11% |
| Hypotension (8%) | |
| Arrhythmia (2%) | |
| Hypertension (1%) | |
| Hematologic | 2% |
| Bleeding (2%) | |
| Endocrine | 1% |
| Hypoglycemia (1%) | |
| Other reason not listed | 32% |
| No reason given | 9% |
| Location | Reason for RRT call | Disposition | |
|---|---|---|---|
| Outpatient clinical | Outpatient orthopedics | Dysrhythmia | ED |
| Outpatient medicine clinic | Hypoxia/respiratory Distress | Stabilized | |
| Outpatient urology | Vomiting | ED | |
| Outpatient Parkinson | Asthma | ED | |
| Outpatient Parkinson | Seizure | ED | |
| Common area/nonclinical | Preadmissions testing | Changed mental status | Unknown |
| Admissions | Changed mental status | Stabilized | |
| Hypoxia/respiratory distress | Stabilized | ||
| Syncope/bradycardia | ED | ||
| Security | Syncope | Improved | |
| Lobby | Hypoxia/respiratory distress | Unknown | |
| Changed mental status | ED | ||
| Hypoxia/respiratory distress | Improved | ||
| Procedures/Off‐unit clinical | Stress test lab | Hypoxia/respiratory distress | Improved |
| Cardiac catheterization lab | Chest pain | ED | |
| Diagnostic imaging | Changed mental status | Improved | |
| Mucus plug in tracheostomy | Improved | ||
| Seizure | ICU | ||
| Syncope | ED | ||
| Hypoxia/respiratory distress | Unknown | ||
| Hypoglycemia | ED | ||
| Dialysis | Bleeding | Stabilized | |
| Gastroenterology procedures | Hypoxia/respiratory distress | ICU | |
| Hypoxia/respiratory distress | Stabilized | ||
| Hypoxia/respiratory distress | ICU | ||
| Interventional radiology | Hypotension/dehydration | Unknown | |
| Hypoxia/respiratory distress | ICU | ||
| Changed mental status | Stabilized | ||
| Hypoxia/Respiratory distress | ICU | ||
| Hypoxia/Respiratory distress | ICU | ||
| Changed mental status | ED | ||
| Hypoxia/Respiratory distress | ICU | ||
| MRI | Hypoxia/Respiratory distress | ED | |
| Hypoxia/respiratory distress | ED | ||
| Hypoxia/respiratory distress | ED | ||
| Changed mental status | ED | ||
| Occupational therapy | Hypotension | ED | |
| Physical therapy | Hypotension | Stabilized | |
| Physical medicine/rehab | Hypoxia/respiratory distress | Unknown | |
| Short procedure unit | Syncope | Stabilized | |
| Hypotension | ICU |
In the judgment of evaluators, the system was utilized appropriately in 98% of the evaluated events. Eighty‐five percent of RRT activations were believed to have prevented further clinical deterioration, though it was also thought that 3% of patients deteriorated despite the efforts of the team. Disposition of the patient following an RRT event was noted 87% of the time, and it was believed that 88% of the patients were stabilized. Of the formally evaluated RRT events, team members were largely satisfied with the response and the functioning of the team, stating for 68% of the events that the team performed without a problem.
Problems Identified and Addressed During Implementation
Though it was encouraging that those surveyed believed the team performed without a problem in 68% of the activations, another way to look at it is that team performance was inadequate in 32% of the cases. Any issues cited on the evaluation sheets, ranging from delays in arrival of team members to missing/delayed arrival of equipment, were seen as opportunities for improvement. For example, very early on in the implementation process, team leaders specifically noted repeatedly encountering a diagnosis of suspected hypoglycemia in patients with a known history of diabetes found with altered mental status. Early clinical assessments by the RRT were severely limited and judged problematic without a simple way to objectively rule out this possibility and/or to attempt immediate treatment, especially because this frequently occurred in non‐inpatient settings. Team members suggested and quickly obtained approval to carry both glucometers and glucose tablets and Glucagon in the pharmacist's fanny pack. In another case, our respiratory therapists arrived promptly to the scene of an RRT call for shortness of breath but were hampered by lack of readily available oxygen tanks. This was promptly remedied, at the recommendation of the committee, by placing additional oxygen tanks near all hospital security stations. Placement of code (crash) carts has also been modified to increase accessibility, especially in nonclinical areas, where delays were perceived to have contributed to poor outcomes. In the future, alphanumeric pagers will be used to allow for more specific and efficient deployment of the team.
Other changes that have been made include the addition to respiratory/pharmacy fanny packs of other key medications such as lorazepam for seizures, equipment such as peripheral catheters for intravenous access, and syringes/needles. It is hoped that in the near future, a state‐of‐the‐art point‐of‐care blood‐testing device, I‐stat, capable of quickly analyzing a blood sample for basic stat lab tests will be added to the pack to expedite triage.16 Perhaps most important, the committee reached a consensus that to improve and encourage real‐time evaluations, it might be best to have the RRT evaluation forms and other paperwork at the point of care to increase yield. The pharmacist now carries blank forms in the fanny pack for convenience. Early on in our RRT implementation process, all these items were noted to be lacking at various times and were requested by team leaders, nurses, and pharmacists in order to be better prepared for various clinical scenarios. In addition, ongoing analysis of the most common RRT diagnoses in the database guided our final decisions in order to keep the size of the fanny pack down to a minimum while providing crucial equipment.
DISCUSSION
We have found the RRT to be an effective but challenging‐to‐implement QI intervention to increase patient safety at our academic institution. The Australian MERIT investigators recently suggested that despite growing evidence of the benefits of MET/RRT systems, long‐term success may depend most on effective implementation strategies.12 We experienced firsthand these challenges in the first year of our new RRT system.
Large system changes in a hospital are especially fraught with danger because of the unique aspects of health care delivery systems. As Reid commented in an editorial about the emerging use of the MET system in the United Kingdom, Despite potential advantages to patients, ensuring appropriate utilization was difficult because of cultural barriers. Traditional hierarchical behaviors that dictate how doctors and nurses react and work got in the way of people calling these life saving teams.17
Our weekly multidisciplinary RRT debriefings were the most crucial component of our implementation strategy. Many latent systems issues were uncovered, as well as more subtle problems such as lack of coordination of care, communication errors, gaps in patient handoffs or sign‐out. Previous studies by the Pittsburgh MERIT team have validated such retrospective categorization of errors uncovered by MET responses.10
However, neither that group nor the Australian MERIT study investigators specifically addressed the importance of the feedback process in RRT implementation. A strength of our system is that modifications to the RRT are made prospectively and in real time based on feedback from active RRT members during debriefing. In fact, the success of our RRT underscores the importance of open communication among hospitalists, house staff, nurses, pharmacists, and ancillary staff in multidisciplinary patient safety and QI endeavors. Everything from the responsibilities of team members to equipment evolved over the 12‐month period in order to improve the function and effectiveness of the team and was almost entirely based on feedback from the RRT doctors and nurses on the front lines. Suggestions from the evaluation forms were given serious consideration at every RRT evaluation committee debriefing. By optimizing the efficient operation of the RRT, we hope to continue to improve outcomes.
We believe a key to the success of our debriefing process was the constant attendance of our patient safety officer/chief medical officer and director of patient safety operations, who both encouraged active participation. Early on in the process, comments were made principally by physician and critical care nurse RRT members, and the dynamic was a bit one‐sided. However, we quickly saw a noticeable and sustained increase in participation by pharmacists and respiratory therapists, and by year's end, they had offered some of the most valuable practical suggestions, which resulted in a more efficient response. As the year went on and real changes were made quickly, all groups were much more vocal and willing to bounce ideas around the room, and the team dynamic and spirit of the group effort improved substantially.
Previous studies have focused on the impact of METs/RRTs on the rate of inpatient cardiac arrests. However, we found that nearly as many RRT events occurred off the inpatient units, for instance, when admitted patients were transported to other areas such as radiology, procedural suites, physical therapy, or dialysis and when scheduled outpatients arrived for their appointments. In addition, a large number of RRT calls came from outpatient departments and common areas of the hospital such as lobbies, hallways, and waiting rooms, mostly involving outpatients and visitors, but not infrequently hospital employees were involved as well. This unexpected and, to our knowledge, previously unreported finding is mirrored in the distribution of RRT activations throughout the course of the day. Most events occurred during the traditional day shift of 7 am‐7 pm, and were heavily clustered between 8 am and 4 pm. In most American hospitals, these are the hours during which outpatients and visitors make up a significant proportion of the hospital population and during which most elective procedures on inpatients occur. Prior to the introduction of our RRT, no specific system was in place for emergent triage, assessment, and expedited treatment of off‐unit patients, outpatients, and visitors. Most often, the code team was mobilized, sometimes taking them to remote locations and making them unavailable for true inpatient cardiopulmonary arrests. Our RRT seems to have the potential to fill a much‐needed gap in patient safety, offering off‐unit patients, outpatients, and visitors a safety net while in our hospital. No prior descriptions of RRT or MET implementation have touched on this area. It would be interesting to see if other hospitals with RRTs have had a similar experience in order to determine whether having an RRT dedicated specifically to the outpatient and common areas of the hospital might provide even more targeted efforts and efficient response times. Thus, the benefits of our RRT seemed to extend beyond a simple reduction in the number of in‐hospital cardiopulmonary arrests and into an unanticipated patient safety black hole.
Implementation of the RRT specifically in academic medical centers has been limited to date. In our opinion, the academic environment is an ideal area for RRTs (because the most critically ill patients often are cared for on teaching services by junior house officers), but it is also a challenging arena in which to make change (because of the complex hierarchy of teaching hospitals). We chose to have an attending physician lead our RRT efforts for the most part. However, residents always participated, and not infrequently led, as key team members. As a commentator on the Australian RRT system pointed out, it is important that junior medical staff [feel empowered] to call for immediate assistance when they are concerned about their patient, but may not have the experience, knowledge, confidence or skills necessary to manage them appropriately.18 We believe that the RRT serves as a valuable educational forum for resident education. Academic centers that develop RRTs must work to integrate the teams into an educational context while simultaneously providing patients with the most experienced and knowledgeable clinical team to address their needs at a time when appropriate clinical decision making is critical. Therefore, the residents who participate in our RRT are formally evaluated by the hospitalists using a standard program evaluation form that encompasses the Accreditation Council for Graduate Medical Education (ACGME) core competencies.19
Through the first year of our RRT system and beyond, activation of the code team and RRT shifted as more RRT activations were recorded and fewer codes were called. Concerted educational efforts and reinforcement of the criteria for calling the RRT had a definite sustained impact of helping staff to become comfortable with using the system. At our institution, it has been difficult to definitively conclude whether RRT calls prevented codes or merely substituted for them at times, especially because 13% of all RRT activations were subsequently converted to code team calls. The Australian MERIT study investigators, despite an excellent study design of a large multicenter trial, also were unable to demonstrate a true decrease in the cardiac arrest rate.12 Much more significant to us, especially in the first year of implementation, was learning that the vast majority of physician RRT leaders perceived activation of the team to occur appropriately and to play a role in preventing clinical deterioration of patients. None of the other RRT or MET implementation studies that we reviewed commented specifically on these areas. It will be interesting to continue to follow these trends, as we expect the use of RRTs to become even more defined. Over time, we will no doubt be better able to determine whether RRTs have a true, sustained impact on preventing patient deterioration and inpatient cardiopulmonary arrests while maintaining a high rate of physician satisfaction that the team is being activated for legitimate reasons.
Our descriptive study had some limitations. The number of RRT evaluations received, while adequate for preliminary analysis, may not accurately represent the 307 activations of the system that occurred in the first 12 months. We suspect that this underreporting, especially in the first half of the year, was in large part a result of relying on team leaders to voluntarily return data forms at the conclusion of each RRT event. RRT evaluations in the second half of the year were more actively distributed at the point of care to the team leader directly by the pharmacist and were more diligently followed up on. Forms are now readily available in the team pharmacist's fanny pack, which was done because of quality improvement feedback from physicians at a debriefing meeting. Since those interventions, there has been a dramatic improvement in the capture of event data and the timely submission of forms. We expect and have demanded close to a 100% return of the forms in the second year of our RRT system, which will vastly improve our analysis. We were also surprised that despite the comprehensiveness of our RRT activation criteria, 32% of physicians were unable to find a match with a clinical indication on the list, indicating unanticipated reasons for calling an RRT. We will continually strive to improve the specificity of future data for planning purposes and training initiatives. However, in some way this confirms our belief that RRTs occur for such a wide variety of reasons that they cannot always be limited to the major clinical categories. On a similar note, we regret not adding a specific category under Outcomes on the evaluation form to include the possibility that RRT members might have offered palliative care or changes in code/do not resuscitate (DNR) status to patients or families. Given that our hospital has both a code team and an RRT begs the question of whether mortality rates might be affected if patients who prior to the RRT might have had a full resuscitation effort were made DNR. In the future, this would be an interesting issue to consider in analysis. Carefully categorizing RRT events is critical to continued success. Further work involving formal team skills training for RRT members, including use of the medical school's clinical simulators for mock RRT scenarios, is planned. These sessions are planned to review performance and clinical decision making for the most common scenarios that we have found to be involved in RRT activations. The 307 activations of the RRT in our first year have clearly set us on the path toward defining predictive rules and directed skills training for earlier identification of patient problems. Further outcome analyses of these efforts will be crucial.
CONCLUSIONS
An RRT was successfully introduced into an academic medical center. The team was heavily utilized in the first 12 months after the program was initiated, especially for off‐unit inpatients and those in outpatient/common areas, perhaps filling a gap in hospital patient safety. The keys to the early success of implementation of our RRT were multidisciplinary input and improvements made in real time. The long‐term effects of the RRT on the culture of patient safety in our institution and throughout the United States remain to be seen but are promising.
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,, et al.Quality of cardiopulmonary resuscitation during in‐hospital cardiac arrest.JAMA.2005;293:363–365.
- ,,.In‐hospital cardiopulmonary resuscitation.Medicine.1995;74:163–175.
- ,,, et al.In‐hospital cardiac arrest: survival depends mainly on the effectiveness of the emergency response.Resuscitation.2004;62:291–297.
- ,,.Factors influencing survival after in‐hospital cardiopulmonary resuscitation.Resuscitation.2005;66:317–321.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.Br Med J.2002;324:1–5.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–204.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,,,,.Use of medical emergency team (MET) responses to detect medical errors.Qual Saf Health Care.2004;13:255–259.
- Institute for Healthcare Improvement. 100K Lives Campaign [IHI website]. Available at: http://www.ihi.org/IHI/Programs/campaign. Accessed November 10,2005.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,, et al.Improving the utilization of medical crisis teams (condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,.Developing strategies to prevent in‐hospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22:244–247.
- ,,,,.Clinical Antecedents to In‐Hospital Cardiopulmonary Arrest.Chest.1990;98:1388–1392.
- Abbot Point of Care: Abbot Laboratories Online. Available at: http://www.istat.com/website/www/products/analyzers.htm. Accessed November 10,2005.
- .Developing and implementing organisational practice that delivers better, safer care.Qual Saf Health Care.2004;13:247.
- ,.The medical emergency team: does it really make a difference?Intern Med J.2003;33:511–514.
- Accreditation Council for Graduate Medical Education (ACGME). Program requirements for residency education in internal medicine. Effective July 2003; revised July 1, 2004. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/140pr703_u704.pdf. Accessed February 17,2006.
Medical emergency teams (METs) were introduced more than a decade ago in Australia and the United Kingdom to rapidly identify and manage seriously ill patients at risk of cardiopulmonary arrest and other high‐risk conditions.1 METs, known in the United States as rapid response teams (RRTs), have been slow to be adopted thus far but are quickly gaining ground. Despite numerous studies indicating long‐term patient outcomes are poor following cardiac resuscitation in the hospital, the benefits of early intervention have sometimes been overlooked.25 Several observational studies and a retrospective analysis that included the Medical Emergency Response Improvement Team (MERIT) in Pittsburgh showed that introduction of a MET apparently has the potential to decrease the incidence of unanticipated intensive care unit (ICU) admissions and in‐hospital morbidity and mortality from unexpected cardiopulmonary arrest.69 Furthermore, the use of a MET as a quality improvement tool to detect medical errors and effect systemwide interventions is promising.10 Most recently, the Institute for Healthcare Improvement (IHI) and the American Hospital Association challenged health care organizations to redesign patient safety systems to prevent avoidable deaths in its 100K Lives Campaign. One of the 6 proposed core interventions was the deployment of rapid response teams at the first sign of patient decline.11
Despite these reports of success, a recent large cluster‐randomized controlled trial did not yield the same positive results. In this well‐designed study of 23 Australian hospitals, the Medical Early Response, Intervention and Therapy (MERIT) study investigators found the incidence of cardiac arrest, unplanned ICU admissions, and unexpected death essentially unchanged despite large increases in how often the emergency team was called.12 One possible explanation why these findings conflicted with previous favorable results is that the ultimate impact of a MET may depend on the effectiveness of implementation strategies. To derive the benefits of a MET/RRT, hospitals must increasingly focus on identifying barriers to implementation and address practical issues that may undermine their long‐term effectiveness.
In this article we describe in detail the process of establishing an RRT at our urban, academic hospital and the modifications that became necessary as we rolled out the intervention and encountered obstacles. This analysis was undertaken as a quality improvement (QI) activity. To our knowledge, this is one of the few recent published descriptions of the experiences of implementing an RRT in the United States since earlier work in Pittsburgh.9, 13
METHODS
Temple University Hospital is a tertiary care academic hospital in urban Philadelphia, Pennsylvania. Our RRT was first implemented July 1, 2004, and in the first 12 months of initiation, it was activated 307 times. The RRT at Temple University Hospital was designed to be accessible 24 hours a day, 7 days a week. The daytime team (8 am‐5 pm) is composed of an attending physician (a hospitalist trained as a general internist), a senior internal medicine resident, a critical care nurse, a nurse manager, a pharmacist, and a respiratory therapist. In addition, both a transporter and a member of the admissions office respond to all rapid response team calls but do not get clinically involved in patient care. For nighttime (5 pm‐8 am) and weekend coverage the hospitalist is replaced by an on‐site pulmonary critical care physician, but the remainder of the team is unchanged. All RRT members carry beepers synchronized to provide the location of an RRT activation. In addition, all RRT calls are simultaneously announced on the overhead paging system. No changes were made to the existing cardiac arrest team (code team) at the hospital, which remained a 24‐hour response team for patients found to be in true cardiopulmonary arrest and was comprised of on‐call internal medicine house staff (but no hospitalist attending physician), a respiratory therapist, a pharmacist, a critical care nurse, a nurse manager, and, most notably, an anesthesiologist for emergent intubation and airway management.
The RRT was intended for use within the physical confines of Temple University Hospital and its immediately adjacent grounds. Within the hospital the main locations defined were: inpatient areas, including patient rooms and hallways of the medical‐surgical units of the inpatient tower, as well as the burn, coronary, medical, neurological, neurosurgical, and surgical intensive care units; off‐unit/procedural areas, including diagnostic/emnterventional radiology, the gastroenterology endoscopy suite, the pulmonary procedure suite and pulmonary function lab, the cardiac catheterization/ECHO/stress Lab, the inpatient dialysis unit, and the physical therapy gym, all areas where inpatients are routinely transported during their hospital admission for workup/treatment and where outpatients go for scheduled procedures and therapies; and outpatient/common areas, including all the general medical and subspecialty outpatient clinics in 2 separate outpatient towers (Outpatient Building and Parkinson Pavilion) with direct access from the main hospital building, the outpatient pharmacy, the elevators, the hallways in the outpatient sections of the hospital, all lobbies, and the immediately adjacent outside grounds.
Prior to the launch date of the RRT, clinical criteria were established to help guide staff about when an RRT might be called (Fig. 1). These were based in part on early literature on the clinical markers that most often precede clinical deterioration.14, 15 In addition, 2 much broader categories for RRT activation were added (Inability to reach the patient's primary team of treating physicians for any of the above and Any potentially serious medical errors or adverse events) in order to minimize the need for a very specific physiologic definition to be met in order to activate the team. Physicians, nurses, and other staff with significant daily contact with inpatients and outpatients were in‐serviced about the purpose of the RRT and how to activate the system via the hospital paging operator. Laminated cards with RRT criteria were distributed to all hospital personnel, and educational posters were displayed prominently throughout the hospital.

Each RRT event was to be assessed by team members using a standardized evaluation form (Fig. 2), with primary responsibility going to the physician team leader. In the initial phases of implementation, these forms were kept in the offices of the Section of Hospital Medicine for the use of hospitalist attending physician team leaders. Later on in the year they were kept in the pharmacist's RRT medication bag. These forms were collected at the completion of each RRT event or faxed to a central location and then entered into a database maintained by the hospital's Department of Patient Safety Operations. Weekly debriefing meetings to review all RRT events from the preceding week were attended by representatives from patient safety, respiratory, nursing, hospital medicine, and the pharmacy. Attempts were made to identify the issues that led to selected RRT activations, to obtain patient follow‐up from the clinical event, and to evaluate the performance of the team. Throughout these weekly meetings, QI strategies for improving the effectiveness of the RRT were identified and implemented.

The core outcome measures that were used to assess RRT performance were: appropriateness of the RRT activation, percentage of patients who were stabilized, percentage of patients who were transferred to a higher level of care, and overall team performance.
In the weekly meeting of the RRT evaluation committee, at which each RRT was reviewed by the clinical team, each scenario and details of the event were reviewed to determine whether the RRT activation was appropriate, whether the intervention was successful, and whether there were any issues with the team performance. After a thorough discussion of each case and review of additional data from the chart if necessary, the RRT evaluation committee reached a consensus about each of these measures.
We also tracked the number of code team activations from the year preceding establishment of the RRT (2003‐2004) through the year during which the RRT was established (2004‐2005). Because all calls for both the RRT and the code team go first to the hospital operator, we reviewed the hospital paging operators' logs for the entire 12‐month period to track the rate of code team events to RRT events on a monthly basis.
RESULTS
In a 12‐month period, the RRT was activated 307 times, as recorded in the hospital operator logs. In the year preceding inception of the RRT, there were 272 code team activations. In the first 12 months concurrent with RRT implementation, the code team was activated 258 times. Overall, at their discretion the team leaders converted 13% of the 307 RRT activations to traditional code team activations.
There were 11 RRT activations in July, the first month of implementation, and 14 activations in the second month. At that point, the internal hospital newsletter released a feature on the new RRT, and our patient safety officer/director of patient safety operations made a concerted effort to educate hospital administration and the Graduate Medical Education Committee (GMEC); as a result, utilization picked up. From September onward through the remainder of the academic year, an average of 28 RRT activations occurred each month (range 20‐37), whereas an average of 22 codes took place each month (range 12‐27). The numbers of RRT versus code team activations are plotted in Figure 3. A trend line for the number of code team activations per month in 2003, the year prior to implementation of the RRT, was added for comparison; it conveys the slight overall decrease in the number of codes as the RRT took effect (average of 23 codes per month, range 15‐31).

Physician evaluation forms were returned for 170 of the 307 RRT events (55%). The main inpatient tower was the site of 42% of these RRT activations, followed by the outpatient/common areas, where 19% of the activations occurred, and off‐unit/procedural areas, the site of 18%. Table 2 provides information on specific location, reason for call, and disposition of a sample of the RRT activations in the non‐inpatient areas. Time of day was noted in 76.8% of events. Of these, 82.9% occurred during the traditional day shift (7 am‐7 pm) and 17.1% on night shift (7 pm‐7 am). Most RRT activations occurred between 8 am and 4 pm. Daytime events heavily outnumbered nighttime events regardless of location.
Physician team leaders largely believed a specific underlying clinical diagnosis was responsible for 59% of the RRT activations, followed by adverse drug reactions (3.5%), physician error (1.8%), and nursing error (0.6%). When an underlying clinical diagnosis or organ system was suspected, it was most frequently pulmonary (32%), followed by neurological (14%) and cardiac (11%). It was believed that 32% of events were for other reason not listed. Table 1 provides the breakdown of other underlying diagnoses in RRT events.
| Pulmonary | 32% |
| Hypoxia/Respiratory Distress (32%) | |
| Neurological | 14% |
| Change of mental status (7%) | |
| Syncope (7%) | |
| Cardiac | 11% |
| Hypotension (8%) | |
| Arrhythmia (2%) | |
| Hypertension (1%) | |
| Hematologic | 2% |
| Bleeding (2%) | |
| Endocrine | 1% |
| Hypoglycemia (1%) | |
| Other reason not listed | 32% |
| No reason given | 9% |
| Location | Reason for RRT call | Disposition | |
|---|---|---|---|
| Outpatient clinical | Outpatient orthopedics | Dysrhythmia | ED |
| Outpatient medicine clinic | Hypoxia/respiratory Distress | Stabilized | |
| Outpatient urology | Vomiting | ED | |
| Outpatient Parkinson | Asthma | ED | |
| Outpatient Parkinson | Seizure | ED | |
| Common area/nonclinical | Preadmissions testing | Changed mental status | Unknown |
| Admissions | Changed mental status | Stabilized | |
| Hypoxia/respiratory distress | Stabilized | ||
| Syncope/bradycardia | ED | ||
| Security | Syncope | Improved | |
| Lobby | Hypoxia/respiratory distress | Unknown | |
| Changed mental status | ED | ||
| Hypoxia/respiratory distress | Improved | ||
| Procedures/Off‐unit clinical | Stress test lab | Hypoxia/respiratory distress | Improved |
| Cardiac catheterization lab | Chest pain | ED | |
| Diagnostic imaging | Changed mental status | Improved | |
| Mucus plug in tracheostomy | Improved | ||
| Seizure | ICU | ||
| Syncope | ED | ||
| Hypoxia/respiratory distress | Unknown | ||
| Hypoglycemia | ED | ||
| Dialysis | Bleeding | Stabilized | |
| Gastroenterology procedures | Hypoxia/respiratory distress | ICU | |
| Hypoxia/respiratory distress | Stabilized | ||
| Hypoxia/respiratory distress | ICU | ||
| Interventional radiology | Hypotension/dehydration | Unknown | |
| Hypoxia/respiratory distress | ICU | ||
| Changed mental status | Stabilized | ||
| Hypoxia/Respiratory distress | ICU | ||
| Hypoxia/Respiratory distress | ICU | ||
| Changed mental status | ED | ||
| Hypoxia/Respiratory distress | ICU | ||
| MRI | Hypoxia/Respiratory distress | ED | |
| Hypoxia/respiratory distress | ED | ||
| Hypoxia/respiratory distress | ED | ||
| Changed mental status | ED | ||
| Occupational therapy | Hypotension | ED | |
| Physical therapy | Hypotension | Stabilized | |
| Physical medicine/rehab | Hypoxia/respiratory distress | Unknown | |
| Short procedure unit | Syncope | Stabilized | |
| Hypotension | ICU |
In the judgment of evaluators, the system was utilized appropriately in 98% of the evaluated events. Eighty‐five percent of RRT activations were believed to have prevented further clinical deterioration, though it was also thought that 3% of patients deteriorated despite the efforts of the team. Disposition of the patient following an RRT event was noted 87% of the time, and it was believed that 88% of the patients were stabilized. Of the formally evaluated RRT events, team members were largely satisfied with the response and the functioning of the team, stating for 68% of the events that the team performed without a problem.
Problems Identified and Addressed During Implementation
Though it was encouraging that those surveyed believed the team performed without a problem in 68% of the activations, another way to look at it is that team performance was inadequate in 32% of the cases. Any issues cited on the evaluation sheets, ranging from delays in arrival of team members to missing/delayed arrival of equipment, were seen as opportunities for improvement. For example, very early on in the implementation process, team leaders specifically noted repeatedly encountering a diagnosis of suspected hypoglycemia in patients with a known history of diabetes found with altered mental status. Early clinical assessments by the RRT were severely limited and judged problematic without a simple way to objectively rule out this possibility and/or to attempt immediate treatment, especially because this frequently occurred in non‐inpatient settings. Team members suggested and quickly obtained approval to carry both glucometers and glucose tablets and Glucagon in the pharmacist's fanny pack. In another case, our respiratory therapists arrived promptly to the scene of an RRT call for shortness of breath but were hampered by lack of readily available oxygen tanks. This was promptly remedied, at the recommendation of the committee, by placing additional oxygen tanks near all hospital security stations. Placement of code (crash) carts has also been modified to increase accessibility, especially in nonclinical areas, where delays were perceived to have contributed to poor outcomes. In the future, alphanumeric pagers will be used to allow for more specific and efficient deployment of the team.
Other changes that have been made include the addition to respiratory/pharmacy fanny packs of other key medications such as lorazepam for seizures, equipment such as peripheral catheters for intravenous access, and syringes/needles. It is hoped that in the near future, a state‐of‐the‐art point‐of‐care blood‐testing device, I‐stat, capable of quickly analyzing a blood sample for basic stat lab tests will be added to the pack to expedite triage.16 Perhaps most important, the committee reached a consensus that to improve and encourage real‐time evaluations, it might be best to have the RRT evaluation forms and other paperwork at the point of care to increase yield. The pharmacist now carries blank forms in the fanny pack for convenience. Early on in our RRT implementation process, all these items were noted to be lacking at various times and were requested by team leaders, nurses, and pharmacists in order to be better prepared for various clinical scenarios. In addition, ongoing analysis of the most common RRT diagnoses in the database guided our final decisions in order to keep the size of the fanny pack down to a minimum while providing crucial equipment.
DISCUSSION
We have found the RRT to be an effective but challenging‐to‐implement QI intervention to increase patient safety at our academic institution. The Australian MERIT investigators recently suggested that despite growing evidence of the benefits of MET/RRT systems, long‐term success may depend most on effective implementation strategies.12 We experienced firsthand these challenges in the first year of our new RRT system.
Large system changes in a hospital are especially fraught with danger because of the unique aspects of health care delivery systems. As Reid commented in an editorial about the emerging use of the MET system in the United Kingdom, Despite potential advantages to patients, ensuring appropriate utilization was difficult because of cultural barriers. Traditional hierarchical behaviors that dictate how doctors and nurses react and work got in the way of people calling these life saving teams.17
Our weekly multidisciplinary RRT debriefings were the most crucial component of our implementation strategy. Many latent systems issues were uncovered, as well as more subtle problems such as lack of coordination of care, communication errors, gaps in patient handoffs or sign‐out. Previous studies by the Pittsburgh MERIT team have validated such retrospective categorization of errors uncovered by MET responses.10
However, neither that group nor the Australian MERIT study investigators specifically addressed the importance of the feedback process in RRT implementation. A strength of our system is that modifications to the RRT are made prospectively and in real time based on feedback from active RRT members during debriefing. In fact, the success of our RRT underscores the importance of open communication among hospitalists, house staff, nurses, pharmacists, and ancillary staff in multidisciplinary patient safety and QI endeavors. Everything from the responsibilities of team members to equipment evolved over the 12‐month period in order to improve the function and effectiveness of the team and was almost entirely based on feedback from the RRT doctors and nurses on the front lines. Suggestions from the evaluation forms were given serious consideration at every RRT evaluation committee debriefing. By optimizing the efficient operation of the RRT, we hope to continue to improve outcomes.
We believe a key to the success of our debriefing process was the constant attendance of our patient safety officer/chief medical officer and director of patient safety operations, who both encouraged active participation. Early on in the process, comments were made principally by physician and critical care nurse RRT members, and the dynamic was a bit one‐sided. However, we quickly saw a noticeable and sustained increase in participation by pharmacists and respiratory therapists, and by year's end, they had offered some of the most valuable practical suggestions, which resulted in a more efficient response. As the year went on and real changes were made quickly, all groups were much more vocal and willing to bounce ideas around the room, and the team dynamic and spirit of the group effort improved substantially.
Previous studies have focused on the impact of METs/RRTs on the rate of inpatient cardiac arrests. However, we found that nearly as many RRT events occurred off the inpatient units, for instance, when admitted patients were transported to other areas such as radiology, procedural suites, physical therapy, or dialysis and when scheduled outpatients arrived for their appointments. In addition, a large number of RRT calls came from outpatient departments and common areas of the hospital such as lobbies, hallways, and waiting rooms, mostly involving outpatients and visitors, but not infrequently hospital employees were involved as well. This unexpected and, to our knowledge, previously unreported finding is mirrored in the distribution of RRT activations throughout the course of the day. Most events occurred during the traditional day shift of 7 am‐7 pm, and were heavily clustered between 8 am and 4 pm. In most American hospitals, these are the hours during which outpatients and visitors make up a significant proportion of the hospital population and during which most elective procedures on inpatients occur. Prior to the introduction of our RRT, no specific system was in place for emergent triage, assessment, and expedited treatment of off‐unit patients, outpatients, and visitors. Most often, the code team was mobilized, sometimes taking them to remote locations and making them unavailable for true inpatient cardiopulmonary arrests. Our RRT seems to have the potential to fill a much‐needed gap in patient safety, offering off‐unit patients, outpatients, and visitors a safety net while in our hospital. No prior descriptions of RRT or MET implementation have touched on this area. It would be interesting to see if other hospitals with RRTs have had a similar experience in order to determine whether having an RRT dedicated specifically to the outpatient and common areas of the hospital might provide even more targeted efforts and efficient response times. Thus, the benefits of our RRT seemed to extend beyond a simple reduction in the number of in‐hospital cardiopulmonary arrests and into an unanticipated patient safety black hole.
Implementation of the RRT specifically in academic medical centers has been limited to date. In our opinion, the academic environment is an ideal area for RRTs (because the most critically ill patients often are cared for on teaching services by junior house officers), but it is also a challenging arena in which to make change (because of the complex hierarchy of teaching hospitals). We chose to have an attending physician lead our RRT efforts for the most part. However, residents always participated, and not infrequently led, as key team members. As a commentator on the Australian RRT system pointed out, it is important that junior medical staff [feel empowered] to call for immediate assistance when they are concerned about their patient, but may not have the experience, knowledge, confidence or skills necessary to manage them appropriately.18 We believe that the RRT serves as a valuable educational forum for resident education. Academic centers that develop RRTs must work to integrate the teams into an educational context while simultaneously providing patients with the most experienced and knowledgeable clinical team to address their needs at a time when appropriate clinical decision making is critical. Therefore, the residents who participate in our RRT are formally evaluated by the hospitalists using a standard program evaluation form that encompasses the Accreditation Council for Graduate Medical Education (ACGME) core competencies.19
Through the first year of our RRT system and beyond, activation of the code team and RRT shifted as more RRT activations were recorded and fewer codes were called. Concerted educational efforts and reinforcement of the criteria for calling the RRT had a definite sustained impact of helping staff to become comfortable with using the system. At our institution, it has been difficult to definitively conclude whether RRT calls prevented codes or merely substituted for them at times, especially because 13% of all RRT activations were subsequently converted to code team calls. The Australian MERIT study investigators, despite an excellent study design of a large multicenter trial, also were unable to demonstrate a true decrease in the cardiac arrest rate.12 Much more significant to us, especially in the first year of implementation, was learning that the vast majority of physician RRT leaders perceived activation of the team to occur appropriately and to play a role in preventing clinical deterioration of patients. None of the other RRT or MET implementation studies that we reviewed commented specifically on these areas. It will be interesting to continue to follow these trends, as we expect the use of RRTs to become even more defined. Over time, we will no doubt be better able to determine whether RRTs have a true, sustained impact on preventing patient deterioration and inpatient cardiopulmonary arrests while maintaining a high rate of physician satisfaction that the team is being activated for legitimate reasons.
Our descriptive study had some limitations. The number of RRT evaluations received, while adequate for preliminary analysis, may not accurately represent the 307 activations of the system that occurred in the first 12 months. We suspect that this underreporting, especially in the first half of the year, was in large part a result of relying on team leaders to voluntarily return data forms at the conclusion of each RRT event. RRT evaluations in the second half of the year were more actively distributed at the point of care to the team leader directly by the pharmacist and were more diligently followed up on. Forms are now readily available in the team pharmacist's fanny pack, which was done because of quality improvement feedback from physicians at a debriefing meeting. Since those interventions, there has been a dramatic improvement in the capture of event data and the timely submission of forms. We expect and have demanded close to a 100% return of the forms in the second year of our RRT system, which will vastly improve our analysis. We were also surprised that despite the comprehensiveness of our RRT activation criteria, 32% of physicians were unable to find a match with a clinical indication on the list, indicating unanticipated reasons for calling an RRT. We will continually strive to improve the specificity of future data for planning purposes and training initiatives. However, in some way this confirms our belief that RRTs occur for such a wide variety of reasons that they cannot always be limited to the major clinical categories. On a similar note, we regret not adding a specific category under Outcomes on the evaluation form to include the possibility that RRT members might have offered palliative care or changes in code/do not resuscitate (DNR) status to patients or families. Given that our hospital has both a code team and an RRT begs the question of whether mortality rates might be affected if patients who prior to the RRT might have had a full resuscitation effort were made DNR. In the future, this would be an interesting issue to consider in analysis. Carefully categorizing RRT events is critical to continued success. Further work involving formal team skills training for RRT members, including use of the medical school's clinical simulators for mock RRT scenarios, is planned. These sessions are planned to review performance and clinical decision making for the most common scenarios that we have found to be involved in RRT activations. The 307 activations of the RRT in our first year have clearly set us on the path toward defining predictive rules and directed skills training for earlier identification of patient problems. Further outcome analyses of these efforts will be crucial.
CONCLUSIONS
An RRT was successfully introduced into an academic medical center. The team was heavily utilized in the first 12 months after the program was initiated, especially for off‐unit inpatients and those in outpatient/common areas, perhaps filling a gap in hospital patient safety. The keys to the early success of implementation of our RRT were multidisciplinary input and improvements made in real time. The long‐term effects of the RRT on the culture of patient safety in our institution and throughout the United States remain to be seen but are promising.
Medical emergency teams (METs) were introduced more than a decade ago in Australia and the United Kingdom to rapidly identify and manage seriously ill patients at risk of cardiopulmonary arrest and other high‐risk conditions.1 METs, known in the United States as rapid response teams (RRTs), have been slow to be adopted thus far but are quickly gaining ground. Despite numerous studies indicating long‐term patient outcomes are poor following cardiac resuscitation in the hospital, the benefits of early intervention have sometimes been overlooked.25 Several observational studies and a retrospective analysis that included the Medical Emergency Response Improvement Team (MERIT) in Pittsburgh showed that introduction of a MET apparently has the potential to decrease the incidence of unanticipated intensive care unit (ICU) admissions and in‐hospital morbidity and mortality from unexpected cardiopulmonary arrest.69 Furthermore, the use of a MET as a quality improvement tool to detect medical errors and effect systemwide interventions is promising.10 Most recently, the Institute for Healthcare Improvement (IHI) and the American Hospital Association challenged health care organizations to redesign patient safety systems to prevent avoidable deaths in its 100K Lives Campaign. One of the 6 proposed core interventions was the deployment of rapid response teams at the first sign of patient decline.11
Despite these reports of success, a recent large cluster‐randomized controlled trial did not yield the same positive results. In this well‐designed study of 23 Australian hospitals, the Medical Early Response, Intervention and Therapy (MERIT) study investigators found the incidence of cardiac arrest, unplanned ICU admissions, and unexpected death essentially unchanged despite large increases in how often the emergency team was called.12 One possible explanation why these findings conflicted with previous favorable results is that the ultimate impact of a MET may depend on the effectiveness of implementation strategies. To derive the benefits of a MET/RRT, hospitals must increasingly focus on identifying barriers to implementation and address practical issues that may undermine their long‐term effectiveness.
In this article we describe in detail the process of establishing an RRT at our urban, academic hospital and the modifications that became necessary as we rolled out the intervention and encountered obstacles. This analysis was undertaken as a quality improvement (QI) activity. To our knowledge, this is one of the few recent published descriptions of the experiences of implementing an RRT in the United States since earlier work in Pittsburgh.9, 13
METHODS
Temple University Hospital is a tertiary care academic hospital in urban Philadelphia, Pennsylvania. Our RRT was first implemented July 1, 2004, and in the first 12 months of initiation, it was activated 307 times. The RRT at Temple University Hospital was designed to be accessible 24 hours a day, 7 days a week. The daytime team (8 am‐5 pm) is composed of an attending physician (a hospitalist trained as a general internist), a senior internal medicine resident, a critical care nurse, a nurse manager, a pharmacist, and a respiratory therapist. In addition, both a transporter and a member of the admissions office respond to all rapid response team calls but do not get clinically involved in patient care. For nighttime (5 pm‐8 am) and weekend coverage the hospitalist is replaced by an on‐site pulmonary critical care physician, but the remainder of the team is unchanged. All RRT members carry beepers synchronized to provide the location of an RRT activation. In addition, all RRT calls are simultaneously announced on the overhead paging system. No changes were made to the existing cardiac arrest team (code team) at the hospital, which remained a 24‐hour response team for patients found to be in true cardiopulmonary arrest and was comprised of on‐call internal medicine house staff (but no hospitalist attending physician), a respiratory therapist, a pharmacist, a critical care nurse, a nurse manager, and, most notably, an anesthesiologist for emergent intubation and airway management.
The RRT was intended for use within the physical confines of Temple University Hospital and its immediately adjacent grounds. Within the hospital the main locations defined were: inpatient areas, including patient rooms and hallways of the medical‐surgical units of the inpatient tower, as well as the burn, coronary, medical, neurological, neurosurgical, and surgical intensive care units; off‐unit/procedural areas, including diagnostic/emnterventional radiology, the gastroenterology endoscopy suite, the pulmonary procedure suite and pulmonary function lab, the cardiac catheterization/ECHO/stress Lab, the inpatient dialysis unit, and the physical therapy gym, all areas where inpatients are routinely transported during their hospital admission for workup/treatment and where outpatients go for scheduled procedures and therapies; and outpatient/common areas, including all the general medical and subspecialty outpatient clinics in 2 separate outpatient towers (Outpatient Building and Parkinson Pavilion) with direct access from the main hospital building, the outpatient pharmacy, the elevators, the hallways in the outpatient sections of the hospital, all lobbies, and the immediately adjacent outside grounds.
Prior to the launch date of the RRT, clinical criteria were established to help guide staff about when an RRT might be called (Fig. 1). These were based in part on early literature on the clinical markers that most often precede clinical deterioration.14, 15 In addition, 2 much broader categories for RRT activation were added (Inability to reach the patient's primary team of treating physicians for any of the above and Any potentially serious medical errors or adverse events) in order to minimize the need for a very specific physiologic definition to be met in order to activate the team. Physicians, nurses, and other staff with significant daily contact with inpatients and outpatients were in‐serviced about the purpose of the RRT and how to activate the system via the hospital paging operator. Laminated cards with RRT criteria were distributed to all hospital personnel, and educational posters were displayed prominently throughout the hospital.

Each RRT event was to be assessed by team members using a standardized evaluation form (Fig. 2), with primary responsibility going to the physician team leader. In the initial phases of implementation, these forms were kept in the offices of the Section of Hospital Medicine for the use of hospitalist attending physician team leaders. Later on in the year they were kept in the pharmacist's RRT medication bag. These forms were collected at the completion of each RRT event or faxed to a central location and then entered into a database maintained by the hospital's Department of Patient Safety Operations. Weekly debriefing meetings to review all RRT events from the preceding week were attended by representatives from patient safety, respiratory, nursing, hospital medicine, and the pharmacy. Attempts were made to identify the issues that led to selected RRT activations, to obtain patient follow‐up from the clinical event, and to evaluate the performance of the team. Throughout these weekly meetings, QI strategies for improving the effectiveness of the RRT were identified and implemented.

The core outcome measures that were used to assess RRT performance were: appropriateness of the RRT activation, percentage of patients who were stabilized, percentage of patients who were transferred to a higher level of care, and overall team performance.
In the weekly meeting of the RRT evaluation committee, at which each RRT was reviewed by the clinical team, each scenario and details of the event were reviewed to determine whether the RRT activation was appropriate, whether the intervention was successful, and whether there were any issues with the team performance. After a thorough discussion of each case and review of additional data from the chart if necessary, the RRT evaluation committee reached a consensus about each of these measures.
We also tracked the number of code team activations from the year preceding establishment of the RRT (2003‐2004) through the year during which the RRT was established (2004‐2005). Because all calls for both the RRT and the code team go first to the hospital operator, we reviewed the hospital paging operators' logs for the entire 12‐month period to track the rate of code team events to RRT events on a monthly basis.
RESULTS
In a 12‐month period, the RRT was activated 307 times, as recorded in the hospital operator logs. In the year preceding inception of the RRT, there were 272 code team activations. In the first 12 months concurrent with RRT implementation, the code team was activated 258 times. Overall, at their discretion the team leaders converted 13% of the 307 RRT activations to traditional code team activations.
There were 11 RRT activations in July, the first month of implementation, and 14 activations in the second month. At that point, the internal hospital newsletter released a feature on the new RRT, and our patient safety officer/director of patient safety operations made a concerted effort to educate hospital administration and the Graduate Medical Education Committee (GMEC); as a result, utilization picked up. From September onward through the remainder of the academic year, an average of 28 RRT activations occurred each month (range 20‐37), whereas an average of 22 codes took place each month (range 12‐27). The numbers of RRT versus code team activations are plotted in Figure 3. A trend line for the number of code team activations per month in 2003, the year prior to implementation of the RRT, was added for comparison; it conveys the slight overall decrease in the number of codes as the RRT took effect (average of 23 codes per month, range 15‐31).

Physician evaluation forms were returned for 170 of the 307 RRT events (55%). The main inpatient tower was the site of 42% of these RRT activations, followed by the outpatient/common areas, where 19% of the activations occurred, and off‐unit/procedural areas, the site of 18%. Table 2 provides information on specific location, reason for call, and disposition of a sample of the RRT activations in the non‐inpatient areas. Time of day was noted in 76.8% of events. Of these, 82.9% occurred during the traditional day shift (7 am‐7 pm) and 17.1% on night shift (7 pm‐7 am). Most RRT activations occurred between 8 am and 4 pm. Daytime events heavily outnumbered nighttime events regardless of location.
Physician team leaders largely believed a specific underlying clinical diagnosis was responsible for 59% of the RRT activations, followed by adverse drug reactions (3.5%), physician error (1.8%), and nursing error (0.6%). When an underlying clinical diagnosis or organ system was suspected, it was most frequently pulmonary (32%), followed by neurological (14%) and cardiac (11%). It was believed that 32% of events were for other reason not listed. Table 1 provides the breakdown of other underlying diagnoses in RRT events.
| Pulmonary | 32% |
| Hypoxia/Respiratory Distress (32%) | |
| Neurological | 14% |
| Change of mental status (7%) | |
| Syncope (7%) | |
| Cardiac | 11% |
| Hypotension (8%) | |
| Arrhythmia (2%) | |
| Hypertension (1%) | |
| Hematologic | 2% |
| Bleeding (2%) | |
| Endocrine | 1% |
| Hypoglycemia (1%) | |
| Other reason not listed | 32% |
| No reason given | 9% |
| Location | Reason for RRT call | Disposition | |
|---|---|---|---|
| Outpatient clinical | Outpatient orthopedics | Dysrhythmia | ED |
| Outpatient medicine clinic | Hypoxia/respiratory Distress | Stabilized | |
| Outpatient urology | Vomiting | ED | |
| Outpatient Parkinson | Asthma | ED | |
| Outpatient Parkinson | Seizure | ED | |
| Common area/nonclinical | Preadmissions testing | Changed mental status | Unknown |
| Admissions | Changed mental status | Stabilized | |
| Hypoxia/respiratory distress | Stabilized | ||
| Syncope/bradycardia | ED | ||
| Security | Syncope | Improved | |
| Lobby | Hypoxia/respiratory distress | Unknown | |
| Changed mental status | ED | ||
| Hypoxia/respiratory distress | Improved | ||
| Procedures/Off‐unit clinical | Stress test lab | Hypoxia/respiratory distress | Improved |
| Cardiac catheterization lab | Chest pain | ED | |
| Diagnostic imaging | Changed mental status | Improved | |
| Mucus plug in tracheostomy | Improved | ||
| Seizure | ICU | ||
| Syncope | ED | ||
| Hypoxia/respiratory distress | Unknown | ||
| Hypoglycemia | ED | ||
| Dialysis | Bleeding | Stabilized | |
| Gastroenterology procedures | Hypoxia/respiratory distress | ICU | |
| Hypoxia/respiratory distress | Stabilized | ||
| Hypoxia/respiratory distress | ICU | ||
| Interventional radiology | Hypotension/dehydration | Unknown | |
| Hypoxia/respiratory distress | ICU | ||
| Changed mental status | Stabilized | ||
| Hypoxia/Respiratory distress | ICU | ||
| Hypoxia/Respiratory distress | ICU | ||
| Changed mental status | ED | ||
| Hypoxia/Respiratory distress | ICU | ||
| MRI | Hypoxia/Respiratory distress | ED | |
| Hypoxia/respiratory distress | ED | ||
| Hypoxia/respiratory distress | ED | ||
| Changed mental status | ED | ||
| Occupational therapy | Hypotension | ED | |
| Physical therapy | Hypotension | Stabilized | |
| Physical medicine/rehab | Hypoxia/respiratory distress | Unknown | |
| Short procedure unit | Syncope | Stabilized | |
| Hypotension | ICU |
In the judgment of evaluators, the system was utilized appropriately in 98% of the evaluated events. Eighty‐five percent of RRT activations were believed to have prevented further clinical deterioration, though it was also thought that 3% of patients deteriorated despite the efforts of the team. Disposition of the patient following an RRT event was noted 87% of the time, and it was believed that 88% of the patients were stabilized. Of the formally evaluated RRT events, team members were largely satisfied with the response and the functioning of the team, stating for 68% of the events that the team performed without a problem.
Problems Identified and Addressed During Implementation
Though it was encouraging that those surveyed believed the team performed without a problem in 68% of the activations, another way to look at it is that team performance was inadequate in 32% of the cases. Any issues cited on the evaluation sheets, ranging from delays in arrival of team members to missing/delayed arrival of equipment, were seen as opportunities for improvement. For example, very early on in the implementation process, team leaders specifically noted repeatedly encountering a diagnosis of suspected hypoglycemia in patients with a known history of diabetes found with altered mental status. Early clinical assessments by the RRT were severely limited and judged problematic without a simple way to objectively rule out this possibility and/or to attempt immediate treatment, especially because this frequently occurred in non‐inpatient settings. Team members suggested and quickly obtained approval to carry both glucometers and glucose tablets and Glucagon in the pharmacist's fanny pack. In another case, our respiratory therapists arrived promptly to the scene of an RRT call for shortness of breath but were hampered by lack of readily available oxygen tanks. This was promptly remedied, at the recommendation of the committee, by placing additional oxygen tanks near all hospital security stations. Placement of code (crash) carts has also been modified to increase accessibility, especially in nonclinical areas, where delays were perceived to have contributed to poor outcomes. In the future, alphanumeric pagers will be used to allow for more specific and efficient deployment of the team.
Other changes that have been made include the addition to respiratory/pharmacy fanny packs of other key medications such as lorazepam for seizures, equipment such as peripheral catheters for intravenous access, and syringes/needles. It is hoped that in the near future, a state‐of‐the‐art point‐of‐care blood‐testing device, I‐stat, capable of quickly analyzing a blood sample for basic stat lab tests will be added to the pack to expedite triage.16 Perhaps most important, the committee reached a consensus that to improve and encourage real‐time evaluations, it might be best to have the RRT evaluation forms and other paperwork at the point of care to increase yield. The pharmacist now carries blank forms in the fanny pack for convenience. Early on in our RRT implementation process, all these items were noted to be lacking at various times and were requested by team leaders, nurses, and pharmacists in order to be better prepared for various clinical scenarios. In addition, ongoing analysis of the most common RRT diagnoses in the database guided our final decisions in order to keep the size of the fanny pack down to a minimum while providing crucial equipment.
DISCUSSION
We have found the RRT to be an effective but challenging‐to‐implement QI intervention to increase patient safety at our academic institution. The Australian MERIT investigators recently suggested that despite growing evidence of the benefits of MET/RRT systems, long‐term success may depend most on effective implementation strategies.12 We experienced firsthand these challenges in the first year of our new RRT system.
Large system changes in a hospital are especially fraught with danger because of the unique aspects of health care delivery systems. As Reid commented in an editorial about the emerging use of the MET system in the United Kingdom, Despite potential advantages to patients, ensuring appropriate utilization was difficult because of cultural barriers. Traditional hierarchical behaviors that dictate how doctors and nurses react and work got in the way of people calling these life saving teams.17
Our weekly multidisciplinary RRT debriefings were the most crucial component of our implementation strategy. Many latent systems issues were uncovered, as well as more subtle problems such as lack of coordination of care, communication errors, gaps in patient handoffs or sign‐out. Previous studies by the Pittsburgh MERIT team have validated such retrospective categorization of errors uncovered by MET responses.10
However, neither that group nor the Australian MERIT study investigators specifically addressed the importance of the feedback process in RRT implementation. A strength of our system is that modifications to the RRT are made prospectively and in real time based on feedback from active RRT members during debriefing. In fact, the success of our RRT underscores the importance of open communication among hospitalists, house staff, nurses, pharmacists, and ancillary staff in multidisciplinary patient safety and QI endeavors. Everything from the responsibilities of team members to equipment evolved over the 12‐month period in order to improve the function and effectiveness of the team and was almost entirely based on feedback from the RRT doctors and nurses on the front lines. Suggestions from the evaluation forms were given serious consideration at every RRT evaluation committee debriefing. By optimizing the efficient operation of the RRT, we hope to continue to improve outcomes.
We believe a key to the success of our debriefing process was the constant attendance of our patient safety officer/chief medical officer and director of patient safety operations, who both encouraged active participation. Early on in the process, comments were made principally by physician and critical care nurse RRT members, and the dynamic was a bit one‐sided. However, we quickly saw a noticeable and sustained increase in participation by pharmacists and respiratory therapists, and by year's end, they had offered some of the most valuable practical suggestions, which resulted in a more efficient response. As the year went on and real changes were made quickly, all groups were much more vocal and willing to bounce ideas around the room, and the team dynamic and spirit of the group effort improved substantially.
Previous studies have focused on the impact of METs/RRTs on the rate of inpatient cardiac arrests. However, we found that nearly as many RRT events occurred off the inpatient units, for instance, when admitted patients were transported to other areas such as radiology, procedural suites, physical therapy, or dialysis and when scheduled outpatients arrived for their appointments. In addition, a large number of RRT calls came from outpatient departments and common areas of the hospital such as lobbies, hallways, and waiting rooms, mostly involving outpatients and visitors, but not infrequently hospital employees were involved as well. This unexpected and, to our knowledge, previously unreported finding is mirrored in the distribution of RRT activations throughout the course of the day. Most events occurred during the traditional day shift of 7 am‐7 pm, and were heavily clustered between 8 am and 4 pm. In most American hospitals, these are the hours during which outpatients and visitors make up a significant proportion of the hospital population and during which most elective procedures on inpatients occur. Prior to the introduction of our RRT, no specific system was in place for emergent triage, assessment, and expedited treatment of off‐unit patients, outpatients, and visitors. Most often, the code team was mobilized, sometimes taking them to remote locations and making them unavailable for true inpatient cardiopulmonary arrests. Our RRT seems to have the potential to fill a much‐needed gap in patient safety, offering off‐unit patients, outpatients, and visitors a safety net while in our hospital. No prior descriptions of RRT or MET implementation have touched on this area. It would be interesting to see if other hospitals with RRTs have had a similar experience in order to determine whether having an RRT dedicated specifically to the outpatient and common areas of the hospital might provide even more targeted efforts and efficient response times. Thus, the benefits of our RRT seemed to extend beyond a simple reduction in the number of in‐hospital cardiopulmonary arrests and into an unanticipated patient safety black hole.
Implementation of the RRT specifically in academic medical centers has been limited to date. In our opinion, the academic environment is an ideal area for RRTs (because the most critically ill patients often are cared for on teaching services by junior house officers), but it is also a challenging arena in which to make change (because of the complex hierarchy of teaching hospitals). We chose to have an attending physician lead our RRT efforts for the most part. However, residents always participated, and not infrequently led, as key team members. As a commentator on the Australian RRT system pointed out, it is important that junior medical staff [feel empowered] to call for immediate assistance when they are concerned about their patient, but may not have the experience, knowledge, confidence or skills necessary to manage them appropriately.18 We believe that the RRT serves as a valuable educational forum for resident education. Academic centers that develop RRTs must work to integrate the teams into an educational context while simultaneously providing patients with the most experienced and knowledgeable clinical team to address their needs at a time when appropriate clinical decision making is critical. Therefore, the residents who participate in our RRT are formally evaluated by the hospitalists using a standard program evaluation form that encompasses the Accreditation Council for Graduate Medical Education (ACGME) core competencies.19
Through the first year of our RRT system and beyond, activation of the code team and RRT shifted as more RRT activations were recorded and fewer codes were called. Concerted educational efforts and reinforcement of the criteria for calling the RRT had a definite sustained impact of helping staff to become comfortable with using the system. At our institution, it has been difficult to definitively conclude whether RRT calls prevented codes or merely substituted for them at times, especially because 13% of all RRT activations were subsequently converted to code team calls. The Australian MERIT study investigators, despite an excellent study design of a large multicenter trial, also were unable to demonstrate a true decrease in the cardiac arrest rate.12 Much more significant to us, especially in the first year of implementation, was learning that the vast majority of physician RRT leaders perceived activation of the team to occur appropriately and to play a role in preventing clinical deterioration of patients. None of the other RRT or MET implementation studies that we reviewed commented specifically on these areas. It will be interesting to continue to follow these trends, as we expect the use of RRTs to become even more defined. Over time, we will no doubt be better able to determine whether RRTs have a true, sustained impact on preventing patient deterioration and inpatient cardiopulmonary arrests while maintaining a high rate of physician satisfaction that the team is being activated for legitimate reasons.
Our descriptive study had some limitations. The number of RRT evaluations received, while adequate for preliminary analysis, may not accurately represent the 307 activations of the system that occurred in the first 12 months. We suspect that this underreporting, especially in the first half of the year, was in large part a result of relying on team leaders to voluntarily return data forms at the conclusion of each RRT event. RRT evaluations in the second half of the year were more actively distributed at the point of care to the team leader directly by the pharmacist and were more diligently followed up on. Forms are now readily available in the team pharmacist's fanny pack, which was done because of quality improvement feedback from physicians at a debriefing meeting. Since those interventions, there has been a dramatic improvement in the capture of event data and the timely submission of forms. We expect and have demanded close to a 100% return of the forms in the second year of our RRT system, which will vastly improve our analysis. We were also surprised that despite the comprehensiveness of our RRT activation criteria, 32% of physicians were unable to find a match with a clinical indication on the list, indicating unanticipated reasons for calling an RRT. We will continually strive to improve the specificity of future data for planning purposes and training initiatives. However, in some way this confirms our belief that RRTs occur for such a wide variety of reasons that they cannot always be limited to the major clinical categories. On a similar note, we regret not adding a specific category under Outcomes on the evaluation form to include the possibility that RRT members might have offered palliative care or changes in code/do not resuscitate (DNR) status to patients or families. Given that our hospital has both a code team and an RRT begs the question of whether mortality rates might be affected if patients who prior to the RRT might have had a full resuscitation effort were made DNR. In the future, this would be an interesting issue to consider in analysis. Carefully categorizing RRT events is critical to continued success. Further work involving formal team skills training for RRT members, including use of the medical school's clinical simulators for mock RRT scenarios, is planned. These sessions are planned to review performance and clinical decision making for the most common scenarios that we have found to be involved in RRT activations. The 307 activations of the RRT in our first year have clearly set us on the path toward defining predictive rules and directed skills training for earlier identification of patient problems. Further outcome analyses of these efforts will be crucial.
CONCLUSIONS
An RRT was successfully introduced into an academic medical center. The team was heavily utilized in the first 12 months after the program was initiated, especially for off‐unit inpatients and those in outpatient/common areas, perhaps filling a gap in hospital patient safety. The keys to the early success of implementation of our RRT were multidisciplinary input and improvements made in real time. The long‐term effects of the RRT on the culture of patient safety in our institution and throughout the United States remain to be seen but are promising.
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,, et al.Quality of cardiopulmonary resuscitation during in‐hospital cardiac arrest.JAMA.2005;293:363–365.
- ,,.In‐hospital cardiopulmonary resuscitation.Medicine.1995;74:163–175.
- ,,, et al.In‐hospital cardiac arrest: survival depends mainly on the effectiveness of the emergency response.Resuscitation.2004;62:291–297.
- ,,.Factors influencing survival after in‐hospital cardiopulmonary resuscitation.Resuscitation.2005;66:317–321.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.Br Med J.2002;324:1–5.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–204.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,,,,.Use of medical emergency team (MET) responses to detect medical errors.Qual Saf Health Care.2004;13:255–259.
- Institute for Healthcare Improvement. 100K Lives Campaign [IHI website]. Available at: http://www.ihi.org/IHI/Programs/campaign. Accessed November 10,2005.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,, et al.Improving the utilization of medical crisis teams (condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,.Developing strategies to prevent in‐hospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22:244–247.
- ,,,,.Clinical Antecedents to In‐Hospital Cardiopulmonary Arrest.Chest.1990;98:1388–1392.
- Abbot Point of Care: Abbot Laboratories Online. Available at: http://www.istat.com/website/www/products/analyzers.htm. Accessed November 10,2005.
- .Developing and implementing organisational practice that delivers better, safer care.Qual Saf Health Care.2004;13:247.
- ,.The medical emergency team: does it really make a difference?Intern Med J.2003;33:511–514.
- Accreditation Council for Graduate Medical Education (ACGME). Program requirements for residency education in internal medicine. Effective July 2003; revised July 1, 2004. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/140pr703_u704.pdf. Accessed February 17,2006.
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,, et al.Quality of cardiopulmonary resuscitation during in‐hospital cardiac arrest.JAMA.2005;293:363–365.
- ,,.In‐hospital cardiopulmonary resuscitation.Medicine.1995;74:163–175.
- ,,, et al.In‐hospital cardiac arrest: survival depends mainly on the effectiveness of the emergency response.Resuscitation.2004;62:291–297.
- ,,.Factors influencing survival after in‐hospital cardiopulmonary resuscitation.Resuscitation.2005;66:317–321.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.Br Med J.2002;324:1–5.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–204.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,,,,.Use of medical emergency team (MET) responses to detect medical errors.Qual Saf Health Care.2004;13:255–259.
- Institute for Healthcare Improvement. 100K Lives Campaign [IHI website]. Available at: http://www.ihi.org/IHI/Programs/campaign. Accessed November 10,2005.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,, et al.Improving the utilization of medical crisis teams (condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,.Developing strategies to prevent in‐hospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22:244–247.
- ,,,,.Clinical Antecedents to In‐Hospital Cardiopulmonary Arrest.Chest.1990;98:1388–1392.
- Abbot Point of Care: Abbot Laboratories Online. Available at: http://www.istat.com/website/www/products/analyzers.htm. Accessed November 10,2005.
- .Developing and implementing organisational practice that delivers better, safer care.Qual Saf Health Care.2004;13:247.
- ,.The medical emergency team: does it really make a difference?Intern Med J.2003;33:511–514.
- Accreditation Council for Graduate Medical Education (ACGME). Program requirements for residency education in internal medicine. Effective July 2003; revised July 1, 2004. Available at: http://www.acgme.org/acWebsite/downloads/RRC_progReq/140pr703_u704.pdf. Accessed February 17,2006.