User login
Sleep and Circadian Misalignment
For hospitalists, patient care is 24 hours a day. To provide continual patient care, shift work has become a way of life for hospitalists, similar to hospital nurses, residents in training, and emergency medicine physicians. Notably, they belong to a substantial minority of the workforce as shift workers, starting after 6 PM or before 6 AM, approximately one‐fifth of the total work force in industrialized nations.1, 2 Unfortunately, shift workers suffer from misalignment of their endogenous circadian system, which regulates daily sleep and alertness patterns, and work obligations beyond daylight hours. Such a misalignment can lead to fatigue, sleep loss, and excessive sleepiness, which can adversely affect personal health and safety, as well as the quality of medical care delivered.3
The relationship between shift work, extended work hours, and medical safety is a topic currently under intense scrutiny, as reviewed in the Institute of Medicine's (IOM) controversial report on residents and sleep.4 This publication led the Accreditation Council of Graduate Medical Education (ACGME) to mandate more changes to residents' work hours,5 adding to those first implemented in 2003.6 These restrictions forbid residents from working more than 30 consecutive hours, and required at least 10 hours off between shifts and an average of 1 day off in 7. Subsequent studies suggested that the reduction in resident work hours led to greater resident well‐being, fewer attention failures. and fewer medical errors.3, 7
In 2007, amid growing public concern over sleep‐deprived residents and patient safety, Congress requested the IOM investigate additional safeguards for residents.8 In 2008, the IOM published a report calling for more protection against resident fatigue.4 They recommended integrating a protected sleep period into any 24‐hour shift. If residents cannot get protected sleep time, then the maximal shift duration should not exceed 16 hoursreduced from the previous ACGME recommendation of 30. Further provisions to allow adequate sleep include capping the number of consecutive night shifts at 4, and extending the time off after a night shift. In response, the ACGME recently updated their recommendations effective July 1, 2011,5 though not following all the IOM's recommendations (Table 1).
| 2003 ACGME Limits | 2008 IOM Recommendation | 2010 ACGME Limits | |
|---|---|---|---|
| |||
| Maximum work hours per week | 80 hr, averaged over 4 wk | No change | No change |
| Maximum shift length | 30 hr (admitting patients up to 24 hr, with 6 hr of transition activities) | 30 hr (admitting patients up to 16 hr, with 5 hr protected sleep between 10 PM to 8 AM, and remaining hours for transition activities) | PGY‐1: 16 hr; PGY‐2 and above: 28 hr (admitting patients up to 24 hr, with 4 hr of transition activities) |
| Minimum time off between shifts | 10 hr after shift | 10 hr after day shift; 12 hr after night shift; 14 hr after any extended shift of 30 hr and not return until 6 AM the next day | 10 hr after shift; 14 hr free after 24‐hr shift for intermediate level residents |
| Maximum frequency of in‐hospital night shifts | No limits | 4 night maximum, with 48 hr off after 3 or 4 consecutive shifts | 6 consecutive night maximum |
The growing nationwide emphasis on fatigue prevention within healthcare settings now clearly impacts residents and their training schedule. But why focus only on residents? Why not other physicians, such as hospitalists, who work shifts to cover 24 hours each day? Are they any less prone to making medical errors when fatigued? Given that hospitalists' represent the fastest growing specialty in the history of American medicine,9 we sought to inform decisions about their scheduling by reviewing normal regulation of sleep and wake patterns, addressing the problems associated with misalignment between sleep and work, and identifying strategies to realign circadian schedules.
NORMAL SLEEP AND CIRCADIAN RHYTHMS
An understanding of sleep physiology begins with the endogenous circadian timekeeping system. At the center of this timekeeping system is a master circadian clock, located in the suprachiasmatic nucleus (SCN) of the hypothalamus. Cells within the SCN generate a near 24‐hour rhythm, transmitted through neural connections, to rhythmically influence the entire central nervous system and other bodily systems.10
The SCN and the circadian rhythm interact with the need to sleep (sleep homeostasis) to form the 2‐process model of sleepwakefulness.11 In this model, progression of biological day (a time when wakefulness and its associated functions are promoted) coincides with a rise in homeostatic pressure to sleep (see Figure 1). Daytime alertness is maintained by increasing SCN neuronal activity to counterbalance rising sleep pressure. After peaking in the early evening, SCN activity falls to begin biological night (a time when sleep and its associated functions are promoted). To facilitate the onset of biological night, the SCN coordinates the activity of sleep‐promoting centers and the release of melatonin from the pineal gland which promotes sleep.
This endogenous circadian clock runs slightly longer than 24 hours and must be resynchronized daily to the 24‐hour day, a process known as entrainment. This occurs primarily through environmental exposure of retinalhypothalamic links to the lightdark cycle. The intensity, duration, and wave length of light all influence the circadian system,12 but perhaps most importantly is the timing. In general, light exposure in the evening will shift the circadian clock later (phase delay shift), whereas light exposure in the morning will shift the clock earlier (phase advance shift). Exogenous melatonin can also shift the circadian system. However, when endogenous levels of melatonin are high, ingested melatonin has little influence on sleep.13
Balancing sleep and wakefulness requires an interweaving of endogenous and exogenous factors. This balance is disturbed if we try to sleep or be wakeful during incorrect endogenous biological times, a process called circadian misalignment.
DELETERIOUS EFFECTS OF CIRCADIAN MISALIGNMENT
Hospitalists and other shift workers required to work during the biological night risk circadian misalignment and, consequently, poor sleep, shift work disorder, errors on the job, and possibly long‐term health consequences.
Chronic Sleep Loss
When working at night or in the early morning, nearly 75% of shift workers encounter some amount of at‐work fatigue and sleepiness.14 After the shift is over, objective assessments among rotating shift workers15, 16 and interns7 demonstrated that day sleep is 1 to 4 hours shorter than night sleep. Chronic or recurring night shifts can therefore lead to chronic sleep loss. While it seems reasonable that permanent night shift workers have greater circadian adjustment to suit their work schedule, little evidence supports this argument.17 Permanent night shift workers may sleep a little longer during the day than rotating shift workers. Yet, the sleep quality does not match night sleep, presumably from conflict between external factors, such as light and activity, and the scheduled sleep period.
Shift Work Disorder
If severe and chronic, sleepiness and impaired performance during work hours and poor sleep during the day can be enough to warrant a diagnosis of shift work disorder (SWD), one of the several circadian rhythm sleep disorders (CRSD). The prevalence of SWD among rotating and night workers is estimated to be 10%25%.18 Patients with SWD can experience similar levels of nighttime sleepiness as patients with narcolepsy and sleep apnea.19 These patients experience reduced satisfaction with the work schedule, and suffer higher rates of depression, ulcers, and sleepiness‐related accidents, compared to other shift workers.18 What distinguishes those shift workers who suffer from normal fatigue and those with SWD is not easily identified. The International Classification of Sleep Disorders‐2 (ICSD‐2) lists the diagnostic criteria for SWD20:
-
Symptoms of insomnia associated with a work schedule that overlaps the usual time for sleep.
-
Symptoms are directly associated with shift work schedule over the course of at least 1 month.
-
Sleep log monitoring for at least 7 days demonstrates circadian and sleep‐time misalignment.
-
Sleep disturbance is not better explained by another sleep disorder or by a medical, neurological, or mental disorder; medication use; or substance‐use disorder.
Symptoms must be present for at least 1 month, and comorbid mood or sleep/wake disorders (commonly found in this disorder) need to be treated. SWD is more common among night shift workers, although those workers starting shifts between 4 AM and 7 AM (early morning shift) are also subject to SWD.21 Type of work schedule, along with physical or mental disorders, domestic responsibilities, and commute times are examples of factors that may increase vulnerability for SWD.18 In addition, genetic factors may explain the considerable inter‐individual differences in susceptibility to SWD. For example, a polymorphism in the circadian gene, PER 3, present in 10%15% of the population, is believed to decrease tolerability to acute sleep loss,22 while genetic variation in the adenosine A2A receptor may be associated with resistance to the effects of sleep loss.23 If a hospitalist suspects a diagnosis SWD, they should seek evaluation by a physician specializing in sleep medicine.
Errors
Disruption of the circadian rhythm influences neurocognitive and psychomotor function, and can lead to human error. Human errors that result in serious accidents or injuries typically result from interaction of circadian rhythm misalignment with multiple other factors, including task duration and complexity, motivation and proficiency, and level of sleep deprivation.24 Though difficult to isolate from the environmental and work experience factors, consistent evidence identifies circadian misalignment as a cause of errors and serious accidents. Most evidence comes from night shift workers trying to remain awake when the circadian signal for alertness is low, or attempting sleep when the circadian alerting signal is high. Compared to day workers, night shift workers are 1.63 times more likely to suffer a fatal accident.25 A study of critical care nurses revealed a prominent circadian pattern of inadvertent sleep episodes during work with the highest peak between 2 AM and 6 AM.26 In addition, nurses working the night shift have been shown to commit more medication administration errors than day workers.27
Medical errors among resident physicians during extended shift durations is well documented.28 On the other hand, not much research has examined error rates among attending physicians. In 1 small study, attending surgeons made more cognitive errors using a simulated laparoscopic exercise as the amount of on‐call overnight sleep decreased.29 A large, single‐center review reported an increased rate of complications among post‐nighttime surgical procedures performed by attendings who slept 6 hours or less the preceding night.30 Notably, proposed legislation would require physicians who have been awake 22 of the preceding 24 hours to inform patients of the potential safety impact of their sleep deprivation prior to providing clinical care.31
Chronic Health Morbidity
Several studies reveal the effect of shift work on chronic health conditions among healthcare workers, such as obesity, cardiovascular disease, and certain cancers (eg, breast, colorectal). These results are summarized in Table 2, with the largest evaluation of healthcare shift workers coming from the Nurses' Health Study.3234
| Disease | Study Design | Population | Comparison | Health Risk | Adjusted Risk Factors |
|---|---|---|---|---|---|
| |||||
| Acute myocardial infarction | Prospective cohort32 | 79,109 US nurses | Working 3 night shifts/mo for 6 yr | RR 1.51 | CAD risk factors, aspirin use, hormone replacement therapy |
| 95% CI (1.12‐2.03) | |||||
| Obesity (BMI 30) | Cross‐sectional72 | 27,485 Swedish workers | Shift‐workers vs day workers | OR 1.41 | Age, socioeconomic status |
| 95% CI (1.25‐1.59) | |||||
| Breast cancer | Prospective cohort33 | 116,087 US nurses | Working 3 night shifts/mo for 20 yr | RR 1.79 | Breast cancer risk factors |
| 95% CI (1.06‐3.01) | |||||
| Colon cancer | Prospective cohort34 | 78,586 US nurses | Working 3 night shifts/mo for 15 yr | RR 1.35 | Family history of colon cancer, dietary intake, activity |
| 95% CI (1.03‐1.77) | |||||
Some believe that adverse health outcomes in shift workers derive from circadian stressan alteration of psychosocial and physiological homeostasis (eg, increased cortisol and catecholamine output) resulting from circadian misalignment.35 Based on data suggesting an increased risk for certain cancers among shift workers, the International Agency for Research of Cancer, a unit of the World Health Organization, announced that shift work resulting in circadian misalignment is probably carcinogenic.36 Researchers propose several biologic mechanisms to explain the increased cancer riskmost revolve around the alteration of the melatonin circadian cycle, found in night shift workers,37 and subsequent disruption of its believed cancer‐protective biologic pathways.
Overall, however, the heterogeneous nature of shift work limits conclusions regarding the long‐term health of shift workers. That is, as the shift work intensity and composition varies, and as the number and timing of these shifts change, so too can the adverse health consequences.
HOSPITALISTS AND NIGHT SHIFTS
Hospital medicine is the fastest growing specialty in the history of medicine, with an estimated 30,000 practicing hospitalists in 2010.38 Survey results from 2009 indicate that hospitalists staff 58% of hospitals; 89% of hospitals with more than 200 beds (J. Miller, Society of Hospital Medicine, personal communication). One reason for the growth in the number of hospitalists at academic medical centers has been the imposed work‐hour restrictions for residents.39
Across the county, hospitalist programs use a variety of shift work systems to ensure 24‐hour patient care. Among those programs that provide continuous on‐site coverage, many staff 3 shiftsday, late afternoon/evening (swing), and night shifts. Some permanently partition the scheduling, with dedicated night hospitalists or nocturnists.40
Hospitalists do not have mandated work‐hour restrictions and, in general, are older than resident physicians. Whether or not hospitalists who trained before the era of work‐hour regulations are better prepared for practicing in a real‐world, after‐hours scenario than hospitalists with previous work‐hour restrictions is a matter of debate. That said, hospitalists who are fatigued, just like residents, may be at increased risk for committing medical errors, particularly when the fatigue is unrecognized. Yet, limiting hospitalists' work hours would have obvious financial implications, likely similar those from resident work‐hour reductions.41 As part of the ACGME 2011 recommendations, faculty and residents now must be trained to recognize signs of fatigue and sleep deprivation, and adopt management strategies such as naps or backup call schedules. Fatigue that results in excessive sleepiness while at work may manifest as weariness, difficulty concentrating, headache, irritability or depressed mood, and feeling unrefreshed after sleeping.42
STRATEGIES TO IMPROVE CIRCADIAN ADAPTATION
Hospitalists can help limit fatigue and improve performance and safety through circadian adaptation: a multimodal approach to realign work and circadian schedules. Depending on whether the shift starts at night or in the early morning (4 AM to 7 AM), circadian adaptation aims may differ. For night shift workers, the overall aim is to delay the timing of circadian rhythms such that the highest propensity of wakefulness occurs during the night work period, while the highest propensity for sleep occurs during the day.17, 43 For early morning shift workers, circadian rhythms for wakefulness and sleep propensity should be shifted earlier. Circadian adaptation involves not only sleeping well before work, but also preventing dips in wakefulness during work. Adaptation strategies are listed in Table 3.
| Night Shift60 | Early Day Shift (Starting at 4 AM‐7 AM) | |
|---|---|---|
| Prior to shift | Avoid sleep debt | Avoid sleep debt |
| Proper sleep hygiene | Proper sleep hygiene | |
| Planned napping | Bright light exposure | |
| Caffeine use | ||
| During the shift | Bright light exposure | Caffeine use |
| Planned napping | ||
| After the shift | Avoid bright light | Avoid late evening bright light (when applicable) |
| Melatonin prior to sleep | ||
| Careful use of other hypnotics | Initiate sleep early |
Improved Sleep Before Work
As an essential first step, hospitalists must get a full night's rest before starting a night shift, as sleep debt will worsen fatigue while at work. Tips for proper sleep hygiene are listed in Table 4. Some shift workers stay up late the night before a scheduled night shift, in order to sleep during the day and awaken shortly before their scheduled night shift, to combat fatigue at work. Such an approach to shift work is typically met with 3 barriers. First, environmental factors often prevent 6 hours of uninterrupted day sleep. Second, 6 hours of continual day sleep is typically difficult because rising circadian activity often limits the sleep period to just a few hours. Third, an adequate amount of sleep prior to a night shift will itself not be enough to prevent sleepiness from occurring after midnightreducing the fall in circadian activity is also essential to maintaining alertness and performance.
| Physical | Adhere to regular wake and sleep schedule |
| activities73 | Engage in regular exercise early in the day |
| Avoid caffeine, nicotine, and alcohol use 6 hr prior to sleeping | |
| Avoid stimulating or stressful activities 30 min prior to sleeping | |
| Proper sleep | Well ventilated, temperature‐controlled bedroom |
| environment | Use heavy curtains to provide as much darkness as possible |
| Comfortable mattress and pillow | |
| Remove television and pets from the bedroom | |
| Housemates should help provide quiet sleep environment |
Napping
Napping prior to a night shift, or during the work shift, can improve alertness and performance and decrease accident rates.44, 45 During shift work, naps of 20 to 50 minutes in duration have demonstrated improvements in reaction time, and restoration of performance to that seen at the start of the shift. Napping early in the night shift can improve objective measures of alertness.44 To avoid increased drowsiness that sometimes occurs when waking from a nap, naps should not be longer than 50 minutes, and can be as short as 10 to 15 minutes.44, 46 Although effective, napping may be impractical for many workers due to time or space constraints. To facilitate brief naps, hospitalist practices should ensure they have a dark, quiet call room for use by overnight hospitalists.
Bright Light Exposure
Studies demonstrate that light exposure during the night shift improves circadian alignment, mood, and performance during the night shift.47, 48 Light exposure ranged from 6 hours to 5 light treatments of 15 minutes each, with brightness ranging from 2,500 to 10,000 lux (approximating outdoor daylight; typical office lighting provides 200‐500 lux).47, 49 Results demonstrate that bright light exposure during the night shift acutely improves alertness and performance, though not to daytime levels.50 The greatest circadian adjustments occur in groups using both bright light during the night shift and light avoidance the following morning.51 Dark sunglasses and a dark home environment can decrease bright light exposure during the day. Though little evidence exists to support widespread application of bright light devices in hospitalists' call‐rooms, a hospitalist practice should consider installing one to promote circadian adaptation if physicians working overnight have multiple consecutive shifts. Likewise, these physicians should be vigilant and wear dark sunglasses during the day after their night shifteven a few minutes of light exposure at the wrong time of the day may disrupt the intended circadian adaptation.
Wake‐Promoting Agents
Numerous studies demonstrate that 150 mg to 400 mg of caffeine (a 16 oz grande cup of coffee from Starbucks contains between 200 to 500 mg of caffeine52; a Diet Coke contains 46 mg/12 oz53) reduces sleepiness, increases alertness, and improves performance during the night shift.54, 55 Thus, judicious use of caffeine may be recommended in hospital practices during extended work hours. Other wake‐promoting agents, such as modafinil and armodafinil, are US Food and Drug Administration (FDA)‐approved in the treatment of excessive sleepiness associated with SWD. Typically taken 3060 minutes before the start of the night shift, these medications have been shown in trials, enrolling mostly permanent night shift workers, to reduce excessive nighttime sleepiness and improve performance.19, 56 Armodafinil used to treat SWD‐associated excessive sleepiness, has been safely tolerated for durations of 1 year or more.57 However, these agents are not approved for use in patients without a diagnosed sleep disorder.
Melatonin
Exogenous melatonin has been used to reset circadian rhythms in patients with CRSDs.58 Melatonin administered in the late afternoon to early evening directs the largest phase advance. In contrast, melatonin given in the morning produces the largest phase delays.59 When taken after a night shift, melatonin (at a dose 1.8 to 3.0 mg) can improve day sleep quality and duration.60 Despite this result, melatonin's effectiveness in improving circadian adaptation has been mixed.61 For example, improvements in nighttime alertness during the night shift were not seen, despite the use of melatonin to facilitate daytime sleep beforehand.62 Hospitalists may consider a trial of melatonin to improve circadian alignment and facilitate daytime sleep, but its chronic use and long‐term safety has not been adequately studied.
Hypnotics After Work
Hypnotics such as temazepam 20 mg,63 triazolam,64 and zolpidem65 taken after night shift work have been shown to improve day sleep quality under simulated conditions, but do not improve shift work performance. These medications should be reserved for judicious short‐term use in patients with insomnia associated with SWD.
NIGHT SHIFT SCHEDULING TO REDUCE CIRCADIAN MISALIGNMENT
When providing 24‐hour, on‐site medical care, questions may arise about how to incorporate circadian adaptation into the daily schedule.
How Should Shifts Be Rotated?
When scheduling shifts with different start times, evidence suggests that sleep disturbance is reduced with a clockwise progression in shifts (eg, day shift to evening shift to night shift). This reduction in sleep disturbance is thought due to increased time between shifts and the circadian timekeeping tendency to extend beyond 24 hours.66
When Should the Night Shift Start?
Those hospitalist programs using an evening swing shift from afternoon to late evening may have the option of using a 12‐hour night shift starting around 7 PM, or a shorter night shift beginning later at night. Though there are no data among hospitalists to suggest which night shift start time and duration would lead to the least amount of fatigue and errors, healthcare providers working a 12‐hour night shift may have increased morale due to fewer shifts, but may suffer a reduction in the quality of care provided compared with working an 8‐hour night shift.67 In either case, shift workers given flexibility in scheduling have been shown to have positive effects on sleep.68
Should Night Shifts Be Bunched?
The decision to bunch night shifts together depends on how many night shifts are required, and how quickly circadian adaptation can occur. Under simulated conditions, circadian adaptation can yield significant changes in sleep/wake cycles in as little as 4 days.48 In real‐world settings, more time may be required to achieve significant shifts in the circadian cycle. Therefore, hospitalists who have 7 or fewer night shifts during the academic year may want to space the shifts out to prevent sleep debt on consecutive shifts, since significant circadian adaptation would be difficult to achieve in less than a week. In this situation, after a night shift, the hospitalist should have at least one 9‐hour sleep period at night to relieve their sleep debt before staffing another night shift.69 Consecutive night shifts require at least 2 nighttime sleep periods of 9 hours to recover from sleep debt.70 The IOM recommends setting a limit of 4 consecutive night float shifts for resident physicians, however, a recent systematic review of resident night float models did not find data supporting use of a 4‐night‐maximum model.28
If more than 7 night shifts are required, then scheduling the shifts close together with use of circadian adaptation techniques may result in increased nighttime alertness, less fatigue, and fewer errors while at work than widely spacing out the shifts. For example, 1 recent study simulated 8 night shifts over a 10‐day period, and compared circadian schedules and work performance between those subjects who used circadian adaptation strategies and those that did not.71 Circadian adaptation techniques included: four 15‐minute bright light pulses during each night shift, dark sunglasses when outside, dark bedrooms and delayed sleeping until 3 AM on the nights off in between the night shift blocks. The group who shifted their circadian schedule improved night shift alertness and performance as measured by neurophysiological testing, while permitting sufficient daytime sleep after work, as well as late‐night sleep on days off. The group without circadian interventions did not shift their circadian schedule as significantly as the intervention group, and performed worse on the performance testing.
CONCLUSIONS
The nationwide use of hospitalists to provide 24‐hour patient care continues to expand, thus subjecting more hospitalists to work hours asynchronous with the lightdark cycle. Resultant circadian misalignment can result in fatigue while at work, shift work disorder, and, potentially, an increased rate of medical errors. Recognition of these dangers among resident physicians has prompted the ACGME to intensify their regulations on work hours, shift schedules, and time off between shifts. However, no such recommendations exist for hospitalists or emergency physicians and nurses.
Given the potential risk to both physicians and patients, we recommend more research examining the effects of circadian misalignment within the hospitalist community. Sample research questions are offered in Table 5. More information is urgently needed to provide evidence‐based practice guidelines to ensure the safety of this growing workforce and the patients they treat.
| Are hospitalists more immune to fatigue than resident physicians? |
| Are hospitalists better able to recognize fatigue while at work than resident physicians? |
| Does 1 shift work schedule promote better circadian alignment than other shift schedules? |
| Do consistent nighttime hours of nocturnists make them more prone to commit medical errors than hospitalists rotating their shifts? |
- .Job, family, and gender: determinants of nonstandard work schedules among employed Americans in 1991.Demography.1995;32:577–598.
- ,.Shift work among dual‐earner couples with children.Science.1983;219:876–879.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units.N Engl J Med.2004;351:1838–1848.
- Institute of Medicine. Resident Duty Hours: Enhancing Sleep, Supervision and Safety. Released December 15,2008. Available at: http://www.iom.edu/Reports/2008/Resident‐Duty‐Hours‐Enhancing‐Sleep‐Supervision‐and‐Safety.aspx. Accessed on October 20, 2010.
- ACGME. ACGME Approved Standards. Effective July2011. Available at: http://acgme‐2010standards.org/. Accessed on January 6, 2011
- ACGME. Common Program Requirements: Resident Duty Hours in the Learning and Work Environment. Effective July 1, 2007. Available at: http://www.acgme.org/acWebsite/dutyHours/dh_ComProgrRequirmentsDutyHours0707.pdf. Accessed October 20,2010.
- ,,, et al.Effect of reducing interns' weekly work hours on sleep and attentional failures.N Engl J Med.2004;351:1829–1837.
- Letter written to William Munier, Agency for Healthcare Research and Quality.Washington, DC:US House of Representatives, Committee on Energy and Commerce, March 29,2007. Available at: http://energycommerce.house.gov/Press_110/110‐ltr.032907.HHS.Munier.pdf. Accessed on October 24, 2010.
- ,.The evolution and future of hospital medicine.Mt Sinai J Med.2008;75:418–423.
- .Brain structures and receptors involved in alertness.Sleep Med.2005;6(suppl 1):S3–S7.
- .A two process model of sleep regulation.Hum Neurobiol.1982;1:195–204.
- ,.Entrainment of the human circadian system by light.J Biol Rhythms.2005;20:326–338.
- ,.Sleep‐promoting and hypothermic effects of daytime melatonin administration in humans.Sleep.1997;20:124–131.
- .Sleepiness as a consequence of shift work.Sleep.1988;11:17–34.
- ,,,,.The sleep of long‐haul truck drivers.N Engl J Med.1997;337:755–761.
- ,,,.Sleep on the night shift: 24‐hour EEG monitoring of spontaneous sleep/wake behavior.Psychophysiology.1989;26:352–358.
- .Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm.Chronobiol Int.2008;25:215–224.
- ,,,,.Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers.Sleep.2004;27:1453–1462.
- ,,, et al.Modafinil for excessive sleepiness associated with shift‐work sleep disorder.N Engl J Med.2005;353:476–486.
- American Academy of Sleep Medicine.The International Classification of Sleep Disorders (ICSD).2nd ed.Chicago, IL:American Academy of Sleep Medicine;2005.
- ,.Circadian rhythm sleep disorders.Chest.2006;130:1915–1923.
- ,,,,,.Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism.Sleep.2008;31:1159–1167.
- ,,,,,.Genetic variation in the adenosine A2A receptor gene modulates performance on the psychomotor vigilance task.Sleep Biol Rhythms.2007;5:A47.
- ,.Circadian rhythms in cognitive performance: methodological constraints, protocols, theoretical underpinnings.Physiol Behav.2007;90:196–208.
- ,,,.A prospective study of fatal occupational accidents—relationship to sleeping difficulties and occupational factors.J Sleep Res.2002;11:69–71.
- ,,,.Effects of critical care nurses' work hours on vigilance and patients' safety.Am J Crit Care.2006;15:30–37.
- ,,,,.Daytime sleepiness, sleep habits and occupational accidents among hospital nurses.J Adv Nurs.2005;52:445–453.
- ,,.Systematic review: association of shift length, protected sleep time, and night float with patient care, residents' health, and education.Ann Intern Med.2010;153:829–842.
- ,,,,.Jack Barney Award: the effect of fatigue on cognitive and psychomotor skills of trauma residents and attending surgeons.Am J Surg.2008;196:813–819; discussion 9–20.
- ,,, et al.Risks of complications by attending physicians after performing nighttime procedures.JAMA.2009;302:1565–1572.
- ,,.Sleep deprivation, elective surgical procedures, and informed consent.N Engl J Med.2010;363:2577–2579.
- ,,,,.A prospective study of anger and coronary heart disease. The Normative Aging Study.Circulation.1996;94:2090–2095.
- ,,,.Night work and risk of breast cancer.Epidemiology.2006;17:108–111.
- ,,, et al.Night‐shift work and risk of colorectal cancer in the nurses' health study.J Natl Cancer Inst.2003;95:825–828.
- ,,.Shift work and cardiovascular disease—pathways from circadian stress to morbidity.Scand J Work Environ Health.2010;36:96–108.
- IARC. IARC monographs on the evalutaion of carcinogenic risks to humans. Vol 98. Painting, firefighting, and shiftwork. 2007. Available at: monographs.iarc.fr/ENG/Monographs/vol98/mono98.pdf. Accessed January 16,2011.
- ,,,.Influence of melatonin on invasive and metastatic properties of MCF‐7 human breast cancer cells.Cancer Res.1998;58:4383–4390.
- Society of Hospital Medicine. Society of Hospital Medicine releases results of the 2007–2008 survey on the state of the hospital medicine movement. 2008. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Press_Releases3:247–255.
- ,,.Hospitalists: A Guide to Building and Sustaining a Successful Program.Chicago, IL:Health Administration Press;2008.
- ,,,,.The increased financial burden of further proposed orthopaedic resident work‐hour reductions.J Bone Joint Surg Am.2011;93:e31.
- ,,,,,.Fatigue and shift work.J Sleep Res.2006;15:1–5.
- ,,, et al.Rapid shift in peak melatonin secretion associated with improved performance in short shift work schedule.Sleep.1997;20:1145–1150.
- ,,.The impact of a nap opportunity during the night shift on the performance and alertness of 12‐h shift workers.J Sleep Res.2002;11:219–227.
- ,,, et al.Improving alertness and performance in emergency department physicians and nurses: the use of planned naps.Ann Emerg Med.2006;48:596–604, e1‐e3.
- ,,,,.Promoting alertness with a short nap during a night shift.J Sleep Res.1998;7:240–247.
- ,.Circadian adaptation to night‐shift work by judicious light and darkness exposure.J Biol Rhythms.2002;17:556–567.
- ,,,,,.Exposure to bright light and darkness to treat physiologic maladaptation to night work.N Engl J Med.1990;322:1253–1259.
- ,.Night shift performance is improved by a compromise circadian phase position: study 3. Circadian phase after 7 night shifts with an intervening weekend off.Sleep.2008;31:1639–1645.
- ,,,.Dose‐response relationship for light intensity and ocular and electroencephalographic correlates of human alertness.Behav Brain Res.2000;115:75–83.
- ,,,,.Dark goggles and bright light improve circadian rhythm adaptation to night‐shift work.Sleep.1994;17:535–543.
- ,,.Caffeine content of specialty coffees.J Anal Toxicol.2003;27:520–522.
- ,.Caffeine content of prepackaged national‐brand and private‐label carbonated beverages.J Food Sci.2007;72:C337–C342.
- ,,, et al.The effects of coffee and napping on nighttime highway driving: a randomized trial.Ann Intern Med.2006;144:785–791.
- ,,,,.Laboratory and field studies of naps and caffeine as practical countermeasures for sleep‐wake problems associated with night work.Sleep.2006;29:39–50.
- ,,,,.Armodafinil for treatment of excessive sleepiness associated with shift work disorder: a randomized controlled study.Mayo Clin Proc.2009;84:958–972.
- ,,,,.The long‐term tolerability and efficacy of armodafinil in patients with excessive sleepiness associated with treated obstructive sleep apnea, shift work disorder, or narcolepsy: an open‐label extension study.J Clin Sleep Med.2010;6:458–466.
- ,,.Circadian rhythm sleep disorders.Med Clin North Am.2004;88:631–651, viii.
- ,,.A three pulse phase response curve to three milligrams of melatonin in humans.J Physiol.2008;586:639–647.
- ,,, et al.Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report.Sleep.2007;30:1445–1459.
- ,,, et al.Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta‐analysis.BMJ.2006;332:385–393.
- ,,.Effects of melatonin administration on daytime sleep after simulated night shift work.J Sleep Res.2001;10:181–192.
- ,,,.Performance, ability to stay awake, and tendency to fall asleep during the night after a diurnal sleep with temazepam or placebo.Sleep.1997;20:535–541.
- ,,,,,.Sleepiness/alertness on a simulated night shift following sleep at home with triazolam.Sleep.1991;14:140–146.
- ,,,.Zolpidem‐related effects on performance and mood during simulated night‐shift work.Exp Clin Psychopharmacol.2003;11:259–268.
- ,,,,.Intrinsic near‐24‐h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans.Proc Natl Acad Sci USA.2001;98:14027–14032.
- ,,,.Work shift duration: a review comparing eight hour and 12 hour shift systems.Occup Environ Med.1998;55:217–229.
- ,,.Influence of flexibility and variability of working hours on health and well‐being.Chronobiol Int.2006;23:1125–1137.
- ,,.Impact of night‐float rotation on sleep, mood, and alertness: the resident's perception.Chronobiol Int.2002;19:893–902.
- ,,,,,.The characteristics of recovery sleep when recovery opportunity is restricted.Sleep.2007;30:353–360.
- ,,.Practical interventions to promote circadian adaptation to permanent night shift work: study 4.J Biol Rhythms.2009;24:161–172.
- ,,.Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people.Occup Environ Med.2001;58:747–752.
- ,.Use of sleep hygiene in the treatment of insomnia.Sleep Med Rev.2003;7:215–225.
For hospitalists, patient care is 24 hours a day. To provide continual patient care, shift work has become a way of life for hospitalists, similar to hospital nurses, residents in training, and emergency medicine physicians. Notably, they belong to a substantial minority of the workforce as shift workers, starting after 6 PM or before 6 AM, approximately one‐fifth of the total work force in industrialized nations.1, 2 Unfortunately, shift workers suffer from misalignment of their endogenous circadian system, which regulates daily sleep and alertness patterns, and work obligations beyond daylight hours. Such a misalignment can lead to fatigue, sleep loss, and excessive sleepiness, which can adversely affect personal health and safety, as well as the quality of medical care delivered.3
The relationship between shift work, extended work hours, and medical safety is a topic currently under intense scrutiny, as reviewed in the Institute of Medicine's (IOM) controversial report on residents and sleep.4 This publication led the Accreditation Council of Graduate Medical Education (ACGME) to mandate more changes to residents' work hours,5 adding to those first implemented in 2003.6 These restrictions forbid residents from working more than 30 consecutive hours, and required at least 10 hours off between shifts and an average of 1 day off in 7. Subsequent studies suggested that the reduction in resident work hours led to greater resident well‐being, fewer attention failures. and fewer medical errors.3, 7
In 2007, amid growing public concern over sleep‐deprived residents and patient safety, Congress requested the IOM investigate additional safeguards for residents.8 In 2008, the IOM published a report calling for more protection against resident fatigue.4 They recommended integrating a protected sleep period into any 24‐hour shift. If residents cannot get protected sleep time, then the maximal shift duration should not exceed 16 hoursreduced from the previous ACGME recommendation of 30. Further provisions to allow adequate sleep include capping the number of consecutive night shifts at 4, and extending the time off after a night shift. In response, the ACGME recently updated their recommendations effective July 1, 2011,5 though not following all the IOM's recommendations (Table 1).
| 2003 ACGME Limits | 2008 IOM Recommendation | 2010 ACGME Limits | |
|---|---|---|---|
| |||
| Maximum work hours per week | 80 hr, averaged over 4 wk | No change | No change |
| Maximum shift length | 30 hr (admitting patients up to 24 hr, with 6 hr of transition activities) | 30 hr (admitting patients up to 16 hr, with 5 hr protected sleep between 10 PM to 8 AM, and remaining hours for transition activities) | PGY‐1: 16 hr; PGY‐2 and above: 28 hr (admitting patients up to 24 hr, with 4 hr of transition activities) |
| Minimum time off between shifts | 10 hr after shift | 10 hr after day shift; 12 hr after night shift; 14 hr after any extended shift of 30 hr and not return until 6 AM the next day | 10 hr after shift; 14 hr free after 24‐hr shift for intermediate level residents |
| Maximum frequency of in‐hospital night shifts | No limits | 4 night maximum, with 48 hr off after 3 or 4 consecutive shifts | 6 consecutive night maximum |
The growing nationwide emphasis on fatigue prevention within healthcare settings now clearly impacts residents and their training schedule. But why focus only on residents? Why not other physicians, such as hospitalists, who work shifts to cover 24 hours each day? Are they any less prone to making medical errors when fatigued? Given that hospitalists' represent the fastest growing specialty in the history of American medicine,9 we sought to inform decisions about their scheduling by reviewing normal regulation of sleep and wake patterns, addressing the problems associated with misalignment between sleep and work, and identifying strategies to realign circadian schedules.
NORMAL SLEEP AND CIRCADIAN RHYTHMS
An understanding of sleep physiology begins with the endogenous circadian timekeeping system. At the center of this timekeeping system is a master circadian clock, located in the suprachiasmatic nucleus (SCN) of the hypothalamus. Cells within the SCN generate a near 24‐hour rhythm, transmitted through neural connections, to rhythmically influence the entire central nervous system and other bodily systems.10
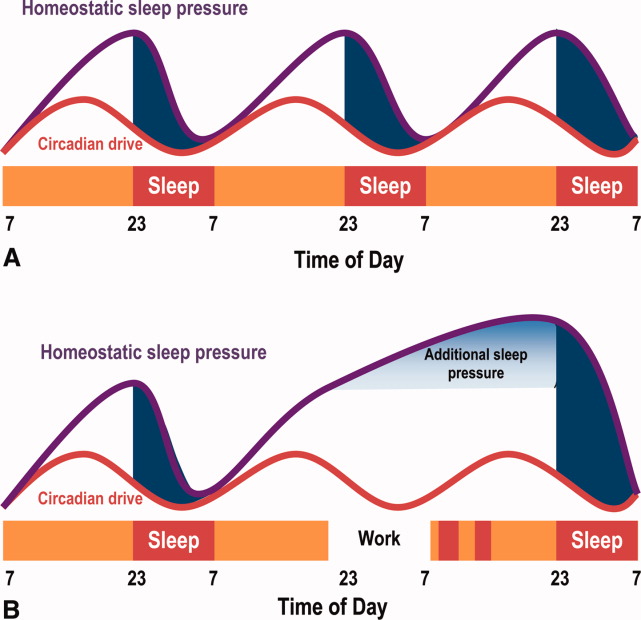
The SCN and the circadian rhythm interact with the need to sleep (sleep homeostasis) to form the 2‐process model of sleepwakefulness.11 In this model, progression of biological day (a time when wakefulness and its associated functions are promoted) coincides with a rise in homeostatic pressure to sleep (see Figure 1). Daytime alertness is maintained by increasing SCN neuronal activity to counterbalance rising sleep pressure. After peaking in the early evening, SCN activity falls to begin biological night (a time when sleep and its associated functions are promoted). To facilitate the onset of biological night, the SCN coordinates the activity of sleep‐promoting centers and the release of melatonin from the pineal gland which promotes sleep.

This endogenous circadian clock runs slightly longer than 24 hours and must be resynchronized daily to the 24‐hour day, a process known as entrainment. This occurs primarily through environmental exposure of retinalhypothalamic links to the lightdark cycle. The intensity, duration, and wave length of light all influence the circadian system,12 but perhaps most importantly is the timing. In general, light exposure in the evening will shift the circadian clock later (phase delay shift), whereas light exposure in the morning will shift the clock earlier (phase advance shift). Exogenous melatonin can also shift the circadian system. However, when endogenous levels of melatonin are high, ingested melatonin has little influence on sleep.13
Balancing sleep and wakefulness requires an interweaving of endogenous and exogenous factors. This balance is disturbed if we try to sleep or be wakeful during incorrect endogenous biological times, a process called circadian misalignment.
DELETERIOUS EFFECTS OF CIRCADIAN MISALIGNMENT
Hospitalists and other shift workers required to work during the biological night risk circadian misalignment and, consequently, poor sleep, shift work disorder, errors on the job, and possibly long‐term health consequences.
Chronic Sleep Loss
When working at night or in the early morning, nearly 75% of shift workers encounter some amount of at‐work fatigue and sleepiness.14 After the shift is over, objective assessments among rotating shift workers15, 16 and interns7 demonstrated that day sleep is 1 to 4 hours shorter than night sleep. Chronic or recurring night shifts can therefore lead to chronic sleep loss. While it seems reasonable that permanent night shift workers have greater circadian adjustment to suit their work schedule, little evidence supports this argument.17 Permanent night shift workers may sleep a little longer during the day than rotating shift workers. Yet, the sleep quality does not match night sleep, presumably from conflict between external factors, such as light and activity, and the scheduled sleep period.
Shift Work Disorder
If severe and chronic, sleepiness and impaired performance during work hours and poor sleep during the day can be enough to warrant a diagnosis of shift work disorder (SWD), one of the several circadian rhythm sleep disorders (CRSD). The prevalence of SWD among rotating and night workers is estimated to be 10%25%.18 Patients with SWD can experience similar levels of nighttime sleepiness as patients with narcolepsy and sleep apnea.19 These patients experience reduced satisfaction with the work schedule, and suffer higher rates of depression, ulcers, and sleepiness‐related accidents, compared to other shift workers.18 What distinguishes those shift workers who suffer from normal fatigue and those with SWD is not easily identified. The International Classification of Sleep Disorders‐2 (ICSD‐2) lists the diagnostic criteria for SWD20:
-
Symptoms of insomnia associated with a work schedule that overlaps the usual time for sleep.
-
Symptoms are directly associated with shift work schedule over the course of at least 1 month.
-
Sleep log monitoring for at least 7 days demonstrates circadian and sleep‐time misalignment.
-
Sleep disturbance is not better explained by another sleep disorder or by a medical, neurological, or mental disorder; medication use; or substance‐use disorder.
Symptoms must be present for at least 1 month, and comorbid mood or sleep/wake disorders (commonly found in this disorder) need to be treated. SWD is more common among night shift workers, although those workers starting shifts between 4 AM and 7 AM (early morning shift) are also subject to SWD.21 Type of work schedule, along with physical or mental disorders, domestic responsibilities, and commute times are examples of factors that may increase vulnerability for SWD.18 In addition, genetic factors may explain the considerable inter‐individual differences in susceptibility to SWD. For example, a polymorphism in the circadian gene, PER 3, present in 10%15% of the population, is believed to decrease tolerability to acute sleep loss,22 while genetic variation in the adenosine A2A receptor may be associated with resistance to the effects of sleep loss.23 If a hospitalist suspects a diagnosis SWD, they should seek evaluation by a physician specializing in sleep medicine.
Errors
Disruption of the circadian rhythm influences neurocognitive and psychomotor function, and can lead to human error. Human errors that result in serious accidents or injuries typically result from interaction of circadian rhythm misalignment with multiple other factors, including task duration and complexity, motivation and proficiency, and level of sleep deprivation.24 Though difficult to isolate from the environmental and work experience factors, consistent evidence identifies circadian misalignment as a cause of errors and serious accidents. Most evidence comes from night shift workers trying to remain awake when the circadian signal for alertness is low, or attempting sleep when the circadian alerting signal is high. Compared to day workers, night shift workers are 1.63 times more likely to suffer a fatal accident.25 A study of critical care nurses revealed a prominent circadian pattern of inadvertent sleep episodes during work with the highest peak between 2 AM and 6 AM.26 In addition, nurses working the night shift have been shown to commit more medication administration errors than day workers.27
Medical errors among resident physicians during extended shift durations is well documented.28 On the other hand, not much research has examined error rates among attending physicians. In 1 small study, attending surgeons made more cognitive errors using a simulated laparoscopic exercise as the amount of on‐call overnight sleep decreased.29 A large, single‐center review reported an increased rate of complications among post‐nighttime surgical procedures performed by attendings who slept 6 hours or less the preceding night.30 Notably, proposed legislation would require physicians who have been awake 22 of the preceding 24 hours to inform patients of the potential safety impact of their sleep deprivation prior to providing clinical care.31
Chronic Health Morbidity
Several studies reveal the effect of shift work on chronic health conditions among healthcare workers, such as obesity, cardiovascular disease, and certain cancers (eg, breast, colorectal). These results are summarized in Table 2, with the largest evaluation of healthcare shift workers coming from the Nurses' Health Study.3234
| Disease | Study Design | Population | Comparison | Health Risk | Adjusted Risk Factors |
|---|---|---|---|---|---|
| |||||
| Acute myocardial infarction | Prospective cohort32 | 79,109 US nurses | Working 3 night shifts/mo for 6 yr | RR 1.51 | CAD risk factors, aspirin use, hormone replacement therapy |
| 95% CI (1.12‐2.03) | |||||
| Obesity (BMI 30) | Cross‐sectional72 | 27,485 Swedish workers | Shift‐workers vs day workers | OR 1.41 | Age, socioeconomic status |
| 95% CI (1.25‐1.59) | |||||
| Breast cancer | Prospective cohort33 | 116,087 US nurses | Working 3 night shifts/mo for 20 yr | RR 1.79 | Breast cancer risk factors |
| 95% CI (1.06‐3.01) | |||||
| Colon cancer | Prospective cohort34 | 78,586 US nurses | Working 3 night shifts/mo for 15 yr | RR 1.35 | Family history of colon cancer, dietary intake, activity |
| 95% CI (1.03‐1.77) | |||||
Some believe that adverse health outcomes in shift workers derive from circadian stressan alteration of psychosocial and physiological homeostasis (eg, increased cortisol and catecholamine output) resulting from circadian misalignment.35 Based on data suggesting an increased risk for certain cancers among shift workers, the International Agency for Research of Cancer, a unit of the World Health Organization, announced that shift work resulting in circadian misalignment is probably carcinogenic.36 Researchers propose several biologic mechanisms to explain the increased cancer riskmost revolve around the alteration of the melatonin circadian cycle, found in night shift workers,37 and subsequent disruption of its believed cancer‐protective biologic pathways.
Overall, however, the heterogeneous nature of shift work limits conclusions regarding the long‐term health of shift workers. That is, as the shift work intensity and composition varies, and as the number and timing of these shifts change, so too can the adverse health consequences.
HOSPITALISTS AND NIGHT SHIFTS
Hospital medicine is the fastest growing specialty in the history of medicine, with an estimated 30,000 practicing hospitalists in 2010.38 Survey results from 2009 indicate that hospitalists staff 58% of hospitals; 89% of hospitals with more than 200 beds (J. Miller, Society of Hospital Medicine, personal communication). One reason for the growth in the number of hospitalists at academic medical centers has been the imposed work‐hour restrictions for residents.39
Across the county, hospitalist programs use a variety of shift work systems to ensure 24‐hour patient care. Among those programs that provide continuous on‐site coverage, many staff 3 shiftsday, late afternoon/evening (swing), and night shifts. Some permanently partition the scheduling, with dedicated night hospitalists or nocturnists.40
Hospitalists do not have mandated work‐hour restrictions and, in general, are older than resident physicians. Whether or not hospitalists who trained before the era of work‐hour regulations are better prepared for practicing in a real‐world, after‐hours scenario than hospitalists with previous work‐hour restrictions is a matter of debate. That said, hospitalists who are fatigued, just like residents, may be at increased risk for committing medical errors, particularly when the fatigue is unrecognized. Yet, limiting hospitalists' work hours would have obvious financial implications, likely similar those from resident work‐hour reductions.41 As part of the ACGME 2011 recommendations, faculty and residents now must be trained to recognize signs of fatigue and sleep deprivation, and adopt management strategies such as naps or backup call schedules. Fatigue that results in excessive sleepiness while at work may manifest as weariness, difficulty concentrating, headache, irritability or depressed mood, and feeling unrefreshed after sleeping.42
STRATEGIES TO IMPROVE CIRCADIAN ADAPTATION
Hospitalists can help limit fatigue and improve performance and safety through circadian adaptation: a multimodal approach to realign work and circadian schedules. Depending on whether the shift starts at night or in the early morning (4 AM to 7 AM), circadian adaptation aims may differ. For night shift workers, the overall aim is to delay the timing of circadian rhythms such that the highest propensity of wakefulness occurs during the night work period, while the highest propensity for sleep occurs during the day.17, 43 For early morning shift workers, circadian rhythms for wakefulness and sleep propensity should be shifted earlier. Circadian adaptation involves not only sleeping well before work, but also preventing dips in wakefulness during work. Adaptation strategies are listed in Table 3.
| Night Shift60 | Early Day Shift (Starting at 4 AM‐7 AM) | |
|---|---|---|
| Prior to shift | Avoid sleep debt | Avoid sleep debt |
| Proper sleep hygiene | Proper sleep hygiene | |
| Planned napping | Bright light exposure | |
| Caffeine use | ||
| During the shift | Bright light exposure | Caffeine use |
| Planned napping | ||
| After the shift | Avoid bright light | Avoid late evening bright light (when applicable) |
| Melatonin prior to sleep | ||
| Careful use of other hypnotics | Initiate sleep early |
Improved Sleep Before Work
As an essential first step, hospitalists must get a full night's rest before starting a night shift, as sleep debt will worsen fatigue while at work. Tips for proper sleep hygiene are listed in Table 4. Some shift workers stay up late the night before a scheduled night shift, in order to sleep during the day and awaken shortly before their scheduled night shift, to combat fatigue at work. Such an approach to shift work is typically met with 3 barriers. First, environmental factors often prevent 6 hours of uninterrupted day sleep. Second, 6 hours of continual day sleep is typically difficult because rising circadian activity often limits the sleep period to just a few hours. Third, an adequate amount of sleep prior to a night shift will itself not be enough to prevent sleepiness from occurring after midnightreducing the fall in circadian activity is also essential to maintaining alertness and performance.
| Physical | Adhere to regular wake and sleep schedule |
| activities73 | Engage in regular exercise early in the day |
| Avoid caffeine, nicotine, and alcohol use 6 hr prior to sleeping | |
| Avoid stimulating or stressful activities 30 min prior to sleeping | |
| Proper sleep | Well ventilated, temperature‐controlled bedroom |
| environment | Use heavy curtains to provide as much darkness as possible |
| Comfortable mattress and pillow | |
| Remove television and pets from the bedroom | |
| Housemates should help provide quiet sleep environment |
Napping
Napping prior to a night shift, or during the work shift, can improve alertness and performance and decrease accident rates.44, 45 During shift work, naps of 20 to 50 minutes in duration have demonstrated improvements in reaction time, and restoration of performance to that seen at the start of the shift. Napping early in the night shift can improve objective measures of alertness.44 To avoid increased drowsiness that sometimes occurs when waking from a nap, naps should not be longer than 50 minutes, and can be as short as 10 to 15 minutes.44, 46 Although effective, napping may be impractical for many workers due to time or space constraints. To facilitate brief naps, hospitalist practices should ensure they have a dark, quiet call room for use by overnight hospitalists.
Bright Light Exposure
Studies demonstrate that light exposure during the night shift improves circadian alignment, mood, and performance during the night shift.47, 48 Light exposure ranged from 6 hours to 5 light treatments of 15 minutes each, with brightness ranging from 2,500 to 10,000 lux (approximating outdoor daylight; typical office lighting provides 200‐500 lux).47, 49 Results demonstrate that bright light exposure during the night shift acutely improves alertness and performance, though not to daytime levels.50 The greatest circadian adjustments occur in groups using both bright light during the night shift and light avoidance the following morning.51 Dark sunglasses and a dark home environment can decrease bright light exposure during the day. Though little evidence exists to support widespread application of bright light devices in hospitalists' call‐rooms, a hospitalist practice should consider installing one to promote circadian adaptation if physicians working overnight have multiple consecutive shifts. Likewise, these physicians should be vigilant and wear dark sunglasses during the day after their night shifteven a few minutes of light exposure at the wrong time of the day may disrupt the intended circadian adaptation.
Wake‐Promoting Agents
Numerous studies demonstrate that 150 mg to 400 mg of caffeine (a 16 oz grande cup of coffee from Starbucks contains between 200 to 500 mg of caffeine52; a Diet Coke contains 46 mg/12 oz53) reduces sleepiness, increases alertness, and improves performance during the night shift.54, 55 Thus, judicious use of caffeine may be recommended in hospital practices during extended work hours. Other wake‐promoting agents, such as modafinil and armodafinil, are US Food and Drug Administration (FDA)‐approved in the treatment of excessive sleepiness associated with SWD. Typically taken 3060 minutes before the start of the night shift, these medications have been shown in trials, enrolling mostly permanent night shift workers, to reduce excessive nighttime sleepiness and improve performance.19, 56 Armodafinil used to treat SWD‐associated excessive sleepiness, has been safely tolerated for durations of 1 year or more.57 However, these agents are not approved for use in patients without a diagnosed sleep disorder.
Melatonin
Exogenous melatonin has been used to reset circadian rhythms in patients with CRSDs.58 Melatonin administered in the late afternoon to early evening directs the largest phase advance. In contrast, melatonin given in the morning produces the largest phase delays.59 When taken after a night shift, melatonin (at a dose 1.8 to 3.0 mg) can improve day sleep quality and duration.60 Despite this result, melatonin's effectiveness in improving circadian adaptation has been mixed.61 For example, improvements in nighttime alertness during the night shift were not seen, despite the use of melatonin to facilitate daytime sleep beforehand.62 Hospitalists may consider a trial of melatonin to improve circadian alignment and facilitate daytime sleep, but its chronic use and long‐term safety has not been adequately studied.
Hypnotics After Work
Hypnotics such as temazepam 20 mg,63 triazolam,64 and zolpidem65 taken after night shift work have been shown to improve day sleep quality under simulated conditions, but do not improve shift work performance. These medications should be reserved for judicious short‐term use in patients with insomnia associated with SWD.
NIGHT SHIFT SCHEDULING TO REDUCE CIRCADIAN MISALIGNMENT
When providing 24‐hour, on‐site medical care, questions may arise about how to incorporate circadian adaptation into the daily schedule.
How Should Shifts Be Rotated?
When scheduling shifts with different start times, evidence suggests that sleep disturbance is reduced with a clockwise progression in shifts (eg, day shift to evening shift to night shift). This reduction in sleep disturbance is thought due to increased time between shifts and the circadian timekeeping tendency to extend beyond 24 hours.66
When Should the Night Shift Start?
Those hospitalist programs using an evening swing shift from afternoon to late evening may have the option of using a 12‐hour night shift starting around 7 PM, or a shorter night shift beginning later at night. Though there are no data among hospitalists to suggest which night shift start time and duration would lead to the least amount of fatigue and errors, healthcare providers working a 12‐hour night shift may have increased morale due to fewer shifts, but may suffer a reduction in the quality of care provided compared with working an 8‐hour night shift.67 In either case, shift workers given flexibility in scheduling have been shown to have positive effects on sleep.68
Should Night Shifts Be Bunched?
The decision to bunch night shifts together depends on how many night shifts are required, and how quickly circadian adaptation can occur. Under simulated conditions, circadian adaptation can yield significant changes in sleep/wake cycles in as little as 4 days.48 In real‐world settings, more time may be required to achieve significant shifts in the circadian cycle. Therefore, hospitalists who have 7 or fewer night shifts during the academic year may want to space the shifts out to prevent sleep debt on consecutive shifts, since significant circadian adaptation would be difficult to achieve in less than a week. In this situation, after a night shift, the hospitalist should have at least one 9‐hour sleep period at night to relieve their sleep debt before staffing another night shift.69 Consecutive night shifts require at least 2 nighttime sleep periods of 9 hours to recover from sleep debt.70 The IOM recommends setting a limit of 4 consecutive night float shifts for resident physicians, however, a recent systematic review of resident night float models did not find data supporting use of a 4‐night‐maximum model.28
If more than 7 night shifts are required, then scheduling the shifts close together with use of circadian adaptation techniques may result in increased nighttime alertness, less fatigue, and fewer errors while at work than widely spacing out the shifts. For example, 1 recent study simulated 8 night shifts over a 10‐day period, and compared circadian schedules and work performance between those subjects who used circadian adaptation strategies and those that did not.71 Circadian adaptation techniques included: four 15‐minute bright light pulses during each night shift, dark sunglasses when outside, dark bedrooms and delayed sleeping until 3 AM on the nights off in between the night shift blocks. The group who shifted their circadian schedule improved night shift alertness and performance as measured by neurophysiological testing, while permitting sufficient daytime sleep after work, as well as late‐night sleep on days off. The group without circadian interventions did not shift their circadian schedule as significantly as the intervention group, and performed worse on the performance testing.
CONCLUSIONS
The nationwide use of hospitalists to provide 24‐hour patient care continues to expand, thus subjecting more hospitalists to work hours asynchronous with the lightdark cycle. Resultant circadian misalignment can result in fatigue while at work, shift work disorder, and, potentially, an increased rate of medical errors. Recognition of these dangers among resident physicians has prompted the ACGME to intensify their regulations on work hours, shift schedules, and time off between shifts. However, no such recommendations exist for hospitalists or emergency physicians and nurses.
Given the potential risk to both physicians and patients, we recommend more research examining the effects of circadian misalignment within the hospitalist community. Sample research questions are offered in Table 5. More information is urgently needed to provide evidence‐based practice guidelines to ensure the safety of this growing workforce and the patients they treat.
| Are hospitalists more immune to fatigue than resident physicians? |
| Are hospitalists better able to recognize fatigue while at work than resident physicians? |
| Does 1 shift work schedule promote better circadian alignment than other shift schedules? |
| Do consistent nighttime hours of nocturnists make them more prone to commit medical errors than hospitalists rotating their shifts? |
For hospitalists, patient care is 24 hours a day. To provide continual patient care, shift work has become a way of life for hospitalists, similar to hospital nurses, residents in training, and emergency medicine physicians. Notably, they belong to a substantial minority of the workforce as shift workers, starting after 6 PM or before 6 AM, approximately one‐fifth of the total work force in industrialized nations.1, 2 Unfortunately, shift workers suffer from misalignment of their endogenous circadian system, which regulates daily sleep and alertness patterns, and work obligations beyond daylight hours. Such a misalignment can lead to fatigue, sleep loss, and excessive sleepiness, which can adversely affect personal health and safety, as well as the quality of medical care delivered.3
The relationship between shift work, extended work hours, and medical safety is a topic currently under intense scrutiny, as reviewed in the Institute of Medicine's (IOM) controversial report on residents and sleep.4 This publication led the Accreditation Council of Graduate Medical Education (ACGME) to mandate more changes to residents' work hours,5 adding to those first implemented in 2003.6 These restrictions forbid residents from working more than 30 consecutive hours, and required at least 10 hours off between shifts and an average of 1 day off in 7. Subsequent studies suggested that the reduction in resident work hours led to greater resident well‐being, fewer attention failures. and fewer medical errors.3, 7
In 2007, amid growing public concern over sleep‐deprived residents and patient safety, Congress requested the IOM investigate additional safeguards for residents.8 In 2008, the IOM published a report calling for more protection against resident fatigue.4 They recommended integrating a protected sleep period into any 24‐hour shift. If residents cannot get protected sleep time, then the maximal shift duration should not exceed 16 hoursreduced from the previous ACGME recommendation of 30. Further provisions to allow adequate sleep include capping the number of consecutive night shifts at 4, and extending the time off after a night shift. In response, the ACGME recently updated their recommendations effective July 1, 2011,5 though not following all the IOM's recommendations (Table 1).
| 2003 ACGME Limits | 2008 IOM Recommendation | 2010 ACGME Limits | |
|---|---|---|---|
| |||
| Maximum work hours per week | 80 hr, averaged over 4 wk | No change | No change |
| Maximum shift length | 30 hr (admitting patients up to 24 hr, with 6 hr of transition activities) | 30 hr (admitting patients up to 16 hr, with 5 hr protected sleep between 10 PM to 8 AM, and remaining hours for transition activities) | PGY‐1: 16 hr; PGY‐2 and above: 28 hr (admitting patients up to 24 hr, with 4 hr of transition activities) |
| Minimum time off between shifts | 10 hr after shift | 10 hr after day shift; 12 hr after night shift; 14 hr after any extended shift of 30 hr and not return until 6 AM the next day | 10 hr after shift; 14 hr free after 24‐hr shift for intermediate level residents |
| Maximum frequency of in‐hospital night shifts | No limits | 4 night maximum, with 48 hr off after 3 or 4 consecutive shifts | 6 consecutive night maximum |
The growing nationwide emphasis on fatigue prevention within healthcare settings now clearly impacts residents and their training schedule. But why focus only on residents? Why not other physicians, such as hospitalists, who work shifts to cover 24 hours each day? Are they any less prone to making medical errors when fatigued? Given that hospitalists' represent the fastest growing specialty in the history of American medicine,9 we sought to inform decisions about their scheduling by reviewing normal regulation of sleep and wake patterns, addressing the problems associated with misalignment between sleep and work, and identifying strategies to realign circadian schedules.
NORMAL SLEEP AND CIRCADIAN RHYTHMS
An understanding of sleep physiology begins with the endogenous circadian timekeeping system. At the center of this timekeeping system is a master circadian clock, located in the suprachiasmatic nucleus (SCN) of the hypothalamus. Cells within the SCN generate a near 24‐hour rhythm, transmitted through neural connections, to rhythmically influence the entire central nervous system and other bodily systems.10
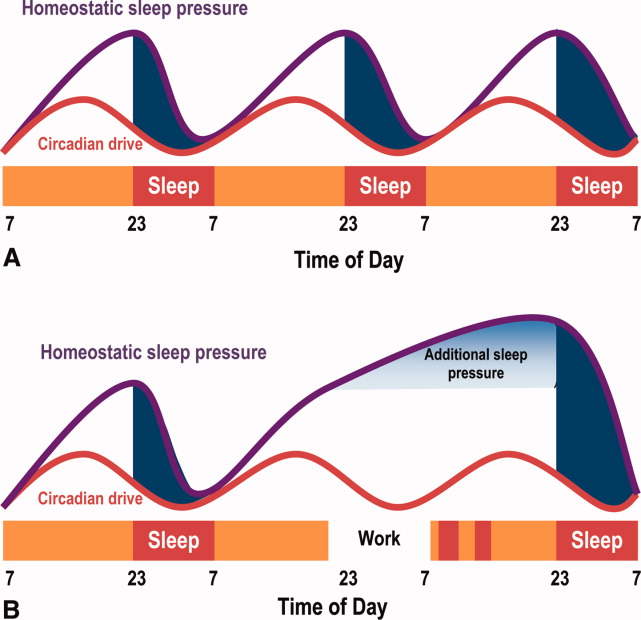
The SCN and the circadian rhythm interact with the need to sleep (sleep homeostasis) to form the 2‐process model of sleepwakefulness.11 In this model, progression of biological day (a time when wakefulness and its associated functions are promoted) coincides with a rise in homeostatic pressure to sleep (see Figure 1). Daytime alertness is maintained by increasing SCN neuronal activity to counterbalance rising sleep pressure. After peaking in the early evening, SCN activity falls to begin biological night (a time when sleep and its associated functions are promoted). To facilitate the onset of biological night, the SCN coordinates the activity of sleep‐promoting centers and the release of melatonin from the pineal gland which promotes sleep.

This endogenous circadian clock runs slightly longer than 24 hours and must be resynchronized daily to the 24‐hour day, a process known as entrainment. This occurs primarily through environmental exposure of retinalhypothalamic links to the lightdark cycle. The intensity, duration, and wave length of light all influence the circadian system,12 but perhaps most importantly is the timing. In general, light exposure in the evening will shift the circadian clock later (phase delay shift), whereas light exposure in the morning will shift the clock earlier (phase advance shift). Exogenous melatonin can also shift the circadian system. However, when endogenous levels of melatonin are high, ingested melatonin has little influence on sleep.13
Balancing sleep and wakefulness requires an interweaving of endogenous and exogenous factors. This balance is disturbed if we try to sleep or be wakeful during incorrect endogenous biological times, a process called circadian misalignment.
DELETERIOUS EFFECTS OF CIRCADIAN MISALIGNMENT
Hospitalists and other shift workers required to work during the biological night risk circadian misalignment and, consequently, poor sleep, shift work disorder, errors on the job, and possibly long‐term health consequences.
Chronic Sleep Loss
When working at night or in the early morning, nearly 75% of shift workers encounter some amount of at‐work fatigue and sleepiness.14 After the shift is over, objective assessments among rotating shift workers15, 16 and interns7 demonstrated that day sleep is 1 to 4 hours shorter than night sleep. Chronic or recurring night shifts can therefore lead to chronic sleep loss. While it seems reasonable that permanent night shift workers have greater circadian adjustment to suit their work schedule, little evidence supports this argument.17 Permanent night shift workers may sleep a little longer during the day than rotating shift workers. Yet, the sleep quality does not match night sleep, presumably from conflict between external factors, such as light and activity, and the scheduled sleep period.
Shift Work Disorder
If severe and chronic, sleepiness and impaired performance during work hours and poor sleep during the day can be enough to warrant a diagnosis of shift work disorder (SWD), one of the several circadian rhythm sleep disorders (CRSD). The prevalence of SWD among rotating and night workers is estimated to be 10%25%.18 Patients with SWD can experience similar levels of nighttime sleepiness as patients with narcolepsy and sleep apnea.19 These patients experience reduced satisfaction with the work schedule, and suffer higher rates of depression, ulcers, and sleepiness‐related accidents, compared to other shift workers.18 What distinguishes those shift workers who suffer from normal fatigue and those with SWD is not easily identified. The International Classification of Sleep Disorders‐2 (ICSD‐2) lists the diagnostic criteria for SWD20:
-
Symptoms of insomnia associated with a work schedule that overlaps the usual time for sleep.
-
Symptoms are directly associated with shift work schedule over the course of at least 1 month.
-
Sleep log monitoring for at least 7 days demonstrates circadian and sleep‐time misalignment.
-
Sleep disturbance is not better explained by another sleep disorder or by a medical, neurological, or mental disorder; medication use; or substance‐use disorder.
Symptoms must be present for at least 1 month, and comorbid mood or sleep/wake disorders (commonly found in this disorder) need to be treated. SWD is more common among night shift workers, although those workers starting shifts between 4 AM and 7 AM (early morning shift) are also subject to SWD.21 Type of work schedule, along with physical or mental disorders, domestic responsibilities, and commute times are examples of factors that may increase vulnerability for SWD.18 In addition, genetic factors may explain the considerable inter‐individual differences in susceptibility to SWD. For example, a polymorphism in the circadian gene, PER 3, present in 10%15% of the population, is believed to decrease tolerability to acute sleep loss,22 while genetic variation in the adenosine A2A receptor may be associated with resistance to the effects of sleep loss.23 If a hospitalist suspects a diagnosis SWD, they should seek evaluation by a physician specializing in sleep medicine.
Errors
Disruption of the circadian rhythm influences neurocognitive and psychomotor function, and can lead to human error. Human errors that result in serious accidents or injuries typically result from interaction of circadian rhythm misalignment with multiple other factors, including task duration and complexity, motivation and proficiency, and level of sleep deprivation.24 Though difficult to isolate from the environmental and work experience factors, consistent evidence identifies circadian misalignment as a cause of errors and serious accidents. Most evidence comes from night shift workers trying to remain awake when the circadian signal for alertness is low, or attempting sleep when the circadian alerting signal is high. Compared to day workers, night shift workers are 1.63 times more likely to suffer a fatal accident.25 A study of critical care nurses revealed a prominent circadian pattern of inadvertent sleep episodes during work with the highest peak between 2 AM and 6 AM.26 In addition, nurses working the night shift have been shown to commit more medication administration errors than day workers.27
Medical errors among resident physicians during extended shift durations is well documented.28 On the other hand, not much research has examined error rates among attending physicians. In 1 small study, attending surgeons made more cognitive errors using a simulated laparoscopic exercise as the amount of on‐call overnight sleep decreased.29 A large, single‐center review reported an increased rate of complications among post‐nighttime surgical procedures performed by attendings who slept 6 hours or less the preceding night.30 Notably, proposed legislation would require physicians who have been awake 22 of the preceding 24 hours to inform patients of the potential safety impact of their sleep deprivation prior to providing clinical care.31
Chronic Health Morbidity
Several studies reveal the effect of shift work on chronic health conditions among healthcare workers, such as obesity, cardiovascular disease, and certain cancers (eg, breast, colorectal). These results are summarized in Table 2, with the largest evaluation of healthcare shift workers coming from the Nurses' Health Study.3234
| Disease | Study Design | Population | Comparison | Health Risk | Adjusted Risk Factors |
|---|---|---|---|---|---|
| |||||
| Acute myocardial infarction | Prospective cohort32 | 79,109 US nurses | Working 3 night shifts/mo for 6 yr | RR 1.51 | CAD risk factors, aspirin use, hormone replacement therapy |
| 95% CI (1.12‐2.03) | |||||
| Obesity (BMI 30) | Cross‐sectional72 | 27,485 Swedish workers | Shift‐workers vs day workers | OR 1.41 | Age, socioeconomic status |
| 95% CI (1.25‐1.59) | |||||
| Breast cancer | Prospective cohort33 | 116,087 US nurses | Working 3 night shifts/mo for 20 yr | RR 1.79 | Breast cancer risk factors |
| 95% CI (1.06‐3.01) | |||||
| Colon cancer | Prospective cohort34 | 78,586 US nurses | Working 3 night shifts/mo for 15 yr | RR 1.35 | Family history of colon cancer, dietary intake, activity |
| 95% CI (1.03‐1.77) | |||||
Some believe that adverse health outcomes in shift workers derive from circadian stressan alteration of psychosocial and physiological homeostasis (eg, increased cortisol and catecholamine output) resulting from circadian misalignment.35 Based on data suggesting an increased risk for certain cancers among shift workers, the International Agency for Research of Cancer, a unit of the World Health Organization, announced that shift work resulting in circadian misalignment is probably carcinogenic.36 Researchers propose several biologic mechanisms to explain the increased cancer riskmost revolve around the alteration of the melatonin circadian cycle, found in night shift workers,37 and subsequent disruption of its believed cancer‐protective biologic pathways.
Overall, however, the heterogeneous nature of shift work limits conclusions regarding the long‐term health of shift workers. That is, as the shift work intensity and composition varies, and as the number and timing of these shifts change, so too can the adverse health consequences.
HOSPITALISTS AND NIGHT SHIFTS
Hospital medicine is the fastest growing specialty in the history of medicine, with an estimated 30,000 practicing hospitalists in 2010.38 Survey results from 2009 indicate that hospitalists staff 58% of hospitals; 89% of hospitals with more than 200 beds (J. Miller, Society of Hospital Medicine, personal communication). One reason for the growth in the number of hospitalists at academic medical centers has been the imposed work‐hour restrictions for residents.39
Across the county, hospitalist programs use a variety of shift work systems to ensure 24‐hour patient care. Among those programs that provide continuous on‐site coverage, many staff 3 shiftsday, late afternoon/evening (swing), and night shifts. Some permanently partition the scheduling, with dedicated night hospitalists or nocturnists.40
Hospitalists do not have mandated work‐hour restrictions and, in general, are older than resident physicians. Whether or not hospitalists who trained before the era of work‐hour regulations are better prepared for practicing in a real‐world, after‐hours scenario than hospitalists with previous work‐hour restrictions is a matter of debate. That said, hospitalists who are fatigued, just like residents, may be at increased risk for committing medical errors, particularly when the fatigue is unrecognized. Yet, limiting hospitalists' work hours would have obvious financial implications, likely similar those from resident work‐hour reductions.41 As part of the ACGME 2011 recommendations, faculty and residents now must be trained to recognize signs of fatigue and sleep deprivation, and adopt management strategies such as naps or backup call schedules. Fatigue that results in excessive sleepiness while at work may manifest as weariness, difficulty concentrating, headache, irritability or depressed mood, and feeling unrefreshed after sleeping.42
STRATEGIES TO IMPROVE CIRCADIAN ADAPTATION
Hospitalists can help limit fatigue and improve performance and safety through circadian adaptation: a multimodal approach to realign work and circadian schedules. Depending on whether the shift starts at night or in the early morning (4 AM to 7 AM), circadian adaptation aims may differ. For night shift workers, the overall aim is to delay the timing of circadian rhythms such that the highest propensity of wakefulness occurs during the night work period, while the highest propensity for sleep occurs during the day.17, 43 For early morning shift workers, circadian rhythms for wakefulness and sleep propensity should be shifted earlier. Circadian adaptation involves not only sleeping well before work, but also preventing dips in wakefulness during work. Adaptation strategies are listed in Table 3.
| Night Shift60 | Early Day Shift (Starting at 4 AM‐7 AM) | |
|---|---|---|
| Prior to shift | Avoid sleep debt | Avoid sleep debt |
| Proper sleep hygiene | Proper sleep hygiene | |
| Planned napping | Bright light exposure | |
| Caffeine use | ||
| During the shift | Bright light exposure | Caffeine use |
| Planned napping | ||
| After the shift | Avoid bright light | Avoid late evening bright light (when applicable) |
| Melatonin prior to sleep | ||
| Careful use of other hypnotics | Initiate sleep early |
Improved Sleep Before Work
As an essential first step, hospitalists must get a full night's rest before starting a night shift, as sleep debt will worsen fatigue while at work. Tips for proper sleep hygiene are listed in Table 4. Some shift workers stay up late the night before a scheduled night shift, in order to sleep during the day and awaken shortly before their scheduled night shift, to combat fatigue at work. Such an approach to shift work is typically met with 3 barriers. First, environmental factors often prevent 6 hours of uninterrupted day sleep. Second, 6 hours of continual day sleep is typically difficult because rising circadian activity often limits the sleep period to just a few hours. Third, an adequate amount of sleep prior to a night shift will itself not be enough to prevent sleepiness from occurring after midnightreducing the fall in circadian activity is also essential to maintaining alertness and performance.
| Physical | Adhere to regular wake and sleep schedule |
| activities73 | Engage in regular exercise early in the day |
| Avoid caffeine, nicotine, and alcohol use 6 hr prior to sleeping | |
| Avoid stimulating or stressful activities 30 min prior to sleeping | |
| Proper sleep | Well ventilated, temperature‐controlled bedroom |
| environment | Use heavy curtains to provide as much darkness as possible |
| Comfortable mattress and pillow | |
| Remove television and pets from the bedroom | |
| Housemates should help provide quiet sleep environment |
Napping
Napping prior to a night shift, or during the work shift, can improve alertness and performance and decrease accident rates.44, 45 During shift work, naps of 20 to 50 minutes in duration have demonstrated improvements in reaction time, and restoration of performance to that seen at the start of the shift. Napping early in the night shift can improve objective measures of alertness.44 To avoid increased drowsiness that sometimes occurs when waking from a nap, naps should not be longer than 50 minutes, and can be as short as 10 to 15 minutes.44, 46 Although effective, napping may be impractical for many workers due to time or space constraints. To facilitate brief naps, hospitalist practices should ensure they have a dark, quiet call room for use by overnight hospitalists.
Bright Light Exposure
Studies demonstrate that light exposure during the night shift improves circadian alignment, mood, and performance during the night shift.47, 48 Light exposure ranged from 6 hours to 5 light treatments of 15 minutes each, with brightness ranging from 2,500 to 10,000 lux (approximating outdoor daylight; typical office lighting provides 200‐500 lux).47, 49 Results demonstrate that bright light exposure during the night shift acutely improves alertness and performance, though not to daytime levels.50 The greatest circadian adjustments occur in groups using both bright light during the night shift and light avoidance the following morning.51 Dark sunglasses and a dark home environment can decrease bright light exposure during the day. Though little evidence exists to support widespread application of bright light devices in hospitalists' call‐rooms, a hospitalist practice should consider installing one to promote circadian adaptation if physicians working overnight have multiple consecutive shifts. Likewise, these physicians should be vigilant and wear dark sunglasses during the day after their night shifteven a few minutes of light exposure at the wrong time of the day may disrupt the intended circadian adaptation.
Wake‐Promoting Agents
Numerous studies demonstrate that 150 mg to 400 mg of caffeine (a 16 oz grande cup of coffee from Starbucks contains between 200 to 500 mg of caffeine52; a Diet Coke contains 46 mg/12 oz53) reduces sleepiness, increases alertness, and improves performance during the night shift.54, 55 Thus, judicious use of caffeine may be recommended in hospital practices during extended work hours. Other wake‐promoting agents, such as modafinil and armodafinil, are US Food and Drug Administration (FDA)‐approved in the treatment of excessive sleepiness associated with SWD. Typically taken 3060 minutes before the start of the night shift, these medications have been shown in trials, enrolling mostly permanent night shift workers, to reduce excessive nighttime sleepiness and improve performance.19, 56 Armodafinil used to treat SWD‐associated excessive sleepiness, has been safely tolerated for durations of 1 year or more.57 However, these agents are not approved for use in patients without a diagnosed sleep disorder.
Melatonin
Exogenous melatonin has been used to reset circadian rhythms in patients with CRSDs.58 Melatonin administered in the late afternoon to early evening directs the largest phase advance. In contrast, melatonin given in the morning produces the largest phase delays.59 When taken after a night shift, melatonin (at a dose 1.8 to 3.0 mg) can improve day sleep quality and duration.60 Despite this result, melatonin's effectiveness in improving circadian adaptation has been mixed.61 For example, improvements in nighttime alertness during the night shift were not seen, despite the use of melatonin to facilitate daytime sleep beforehand.62 Hospitalists may consider a trial of melatonin to improve circadian alignment and facilitate daytime sleep, but its chronic use and long‐term safety has not been adequately studied.
Hypnotics After Work
Hypnotics such as temazepam 20 mg,63 triazolam,64 and zolpidem65 taken after night shift work have been shown to improve day sleep quality under simulated conditions, but do not improve shift work performance. These medications should be reserved for judicious short‐term use in patients with insomnia associated with SWD.
NIGHT SHIFT SCHEDULING TO REDUCE CIRCADIAN MISALIGNMENT
When providing 24‐hour, on‐site medical care, questions may arise about how to incorporate circadian adaptation into the daily schedule.
How Should Shifts Be Rotated?
When scheduling shifts with different start times, evidence suggests that sleep disturbance is reduced with a clockwise progression in shifts (eg, day shift to evening shift to night shift). This reduction in sleep disturbance is thought due to increased time between shifts and the circadian timekeeping tendency to extend beyond 24 hours.66
When Should the Night Shift Start?
Those hospitalist programs using an evening swing shift from afternoon to late evening may have the option of using a 12‐hour night shift starting around 7 PM, or a shorter night shift beginning later at night. Though there are no data among hospitalists to suggest which night shift start time and duration would lead to the least amount of fatigue and errors, healthcare providers working a 12‐hour night shift may have increased morale due to fewer shifts, but may suffer a reduction in the quality of care provided compared with working an 8‐hour night shift.67 In either case, shift workers given flexibility in scheduling have been shown to have positive effects on sleep.68
Should Night Shifts Be Bunched?
The decision to bunch night shifts together depends on how many night shifts are required, and how quickly circadian adaptation can occur. Under simulated conditions, circadian adaptation can yield significant changes in sleep/wake cycles in as little as 4 days.48 In real‐world settings, more time may be required to achieve significant shifts in the circadian cycle. Therefore, hospitalists who have 7 or fewer night shifts during the academic year may want to space the shifts out to prevent sleep debt on consecutive shifts, since significant circadian adaptation would be difficult to achieve in less than a week. In this situation, after a night shift, the hospitalist should have at least one 9‐hour sleep period at night to relieve their sleep debt before staffing another night shift.69 Consecutive night shifts require at least 2 nighttime sleep periods of 9 hours to recover from sleep debt.70 The IOM recommends setting a limit of 4 consecutive night float shifts for resident physicians, however, a recent systematic review of resident night float models did not find data supporting use of a 4‐night‐maximum model.28
If more than 7 night shifts are required, then scheduling the shifts close together with use of circadian adaptation techniques may result in increased nighttime alertness, less fatigue, and fewer errors while at work than widely spacing out the shifts. For example, 1 recent study simulated 8 night shifts over a 10‐day period, and compared circadian schedules and work performance between those subjects who used circadian adaptation strategies and those that did not.71 Circadian adaptation techniques included: four 15‐minute bright light pulses during each night shift, dark sunglasses when outside, dark bedrooms and delayed sleeping until 3 AM on the nights off in between the night shift blocks. The group who shifted their circadian schedule improved night shift alertness and performance as measured by neurophysiological testing, while permitting sufficient daytime sleep after work, as well as late‐night sleep on days off. The group without circadian interventions did not shift their circadian schedule as significantly as the intervention group, and performed worse on the performance testing.
CONCLUSIONS
The nationwide use of hospitalists to provide 24‐hour patient care continues to expand, thus subjecting more hospitalists to work hours asynchronous with the lightdark cycle. Resultant circadian misalignment can result in fatigue while at work, shift work disorder, and, potentially, an increased rate of medical errors. Recognition of these dangers among resident physicians has prompted the ACGME to intensify their regulations on work hours, shift schedules, and time off between shifts. However, no such recommendations exist for hospitalists or emergency physicians and nurses.
Given the potential risk to both physicians and patients, we recommend more research examining the effects of circadian misalignment within the hospitalist community. Sample research questions are offered in Table 5. More information is urgently needed to provide evidence‐based practice guidelines to ensure the safety of this growing workforce and the patients they treat.
| Are hospitalists more immune to fatigue than resident physicians? |
| Are hospitalists better able to recognize fatigue while at work than resident physicians? |
| Does 1 shift work schedule promote better circadian alignment than other shift schedules? |
| Do consistent nighttime hours of nocturnists make them more prone to commit medical errors than hospitalists rotating their shifts? |
- .Job, family, and gender: determinants of nonstandard work schedules among employed Americans in 1991.Demography.1995;32:577–598.
- ,.Shift work among dual‐earner couples with children.Science.1983;219:876–879.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units.N Engl J Med.2004;351:1838–1848.
- Institute of Medicine. Resident Duty Hours: Enhancing Sleep, Supervision and Safety. Released December 15,2008. Available at: http://www.iom.edu/Reports/2008/Resident‐Duty‐Hours‐Enhancing‐Sleep‐Supervision‐and‐Safety.aspx. Accessed on October 20, 2010.
- ACGME. ACGME Approved Standards. Effective July2011. Available at: http://acgme‐2010standards.org/. Accessed on January 6, 2011
- ACGME. Common Program Requirements: Resident Duty Hours in the Learning and Work Environment. Effective July 1, 2007. Available at: http://www.acgme.org/acWebsite/dutyHours/dh_ComProgrRequirmentsDutyHours0707.pdf. Accessed October 20,2010.
- ,,, et al.Effect of reducing interns' weekly work hours on sleep and attentional failures.N Engl J Med.2004;351:1829–1837.
- Letter written to William Munier, Agency for Healthcare Research and Quality.Washington, DC:US House of Representatives, Committee on Energy and Commerce, March 29,2007. Available at: http://energycommerce.house.gov/Press_110/110‐ltr.032907.HHS.Munier.pdf. Accessed on October 24, 2010.
- ,.The evolution and future of hospital medicine.Mt Sinai J Med.2008;75:418–423.
- .Brain structures and receptors involved in alertness.Sleep Med.2005;6(suppl 1):S3–S7.
- .A two process model of sleep regulation.Hum Neurobiol.1982;1:195–204.
- ,.Entrainment of the human circadian system by light.J Biol Rhythms.2005;20:326–338.
- ,.Sleep‐promoting and hypothermic effects of daytime melatonin administration in humans.Sleep.1997;20:124–131.
- .Sleepiness as a consequence of shift work.Sleep.1988;11:17–34.
- ,,,,.The sleep of long‐haul truck drivers.N Engl J Med.1997;337:755–761.
- ,,,.Sleep on the night shift: 24‐hour EEG monitoring of spontaneous sleep/wake behavior.Psychophysiology.1989;26:352–358.
- .Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm.Chronobiol Int.2008;25:215–224.
- ,,,,.Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers.Sleep.2004;27:1453–1462.
- ,,, et al.Modafinil for excessive sleepiness associated with shift‐work sleep disorder.N Engl J Med.2005;353:476–486.
- American Academy of Sleep Medicine.The International Classification of Sleep Disorders (ICSD).2nd ed.Chicago, IL:American Academy of Sleep Medicine;2005.
- ,.Circadian rhythm sleep disorders.Chest.2006;130:1915–1923.
- ,,,,,.Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism.Sleep.2008;31:1159–1167.
- ,,,,,.Genetic variation in the adenosine A2A receptor gene modulates performance on the psychomotor vigilance task.Sleep Biol Rhythms.2007;5:A47.
- ,.Circadian rhythms in cognitive performance: methodological constraints, protocols, theoretical underpinnings.Physiol Behav.2007;90:196–208.
- ,,,.A prospective study of fatal occupational accidents—relationship to sleeping difficulties and occupational factors.J Sleep Res.2002;11:69–71.
- ,,,.Effects of critical care nurses' work hours on vigilance and patients' safety.Am J Crit Care.2006;15:30–37.
- ,,,,.Daytime sleepiness, sleep habits and occupational accidents among hospital nurses.J Adv Nurs.2005;52:445–453.
- ,,.Systematic review: association of shift length, protected sleep time, and night float with patient care, residents' health, and education.Ann Intern Med.2010;153:829–842.
- ,,,,.Jack Barney Award: the effect of fatigue on cognitive and psychomotor skills of trauma residents and attending surgeons.Am J Surg.2008;196:813–819; discussion 9–20.
- ,,, et al.Risks of complications by attending physicians after performing nighttime procedures.JAMA.2009;302:1565–1572.
- ,,.Sleep deprivation, elective surgical procedures, and informed consent.N Engl J Med.2010;363:2577–2579.
- ,,,,.A prospective study of anger and coronary heart disease. The Normative Aging Study.Circulation.1996;94:2090–2095.
- ,,,.Night work and risk of breast cancer.Epidemiology.2006;17:108–111.
- ,,, et al.Night‐shift work and risk of colorectal cancer in the nurses' health study.J Natl Cancer Inst.2003;95:825–828.
- ,,.Shift work and cardiovascular disease—pathways from circadian stress to morbidity.Scand J Work Environ Health.2010;36:96–108.
- IARC. IARC monographs on the evalutaion of carcinogenic risks to humans. Vol 98. Painting, firefighting, and shiftwork. 2007. Available at: monographs.iarc.fr/ENG/Monographs/vol98/mono98.pdf. Accessed January 16,2011.
- ,,,.Influence of melatonin on invasive and metastatic properties of MCF‐7 human breast cancer cells.Cancer Res.1998;58:4383–4390.
- Society of Hospital Medicine. Society of Hospital Medicine releases results of the 2007–2008 survey on the state of the hospital medicine movement. 2008. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Press_Releases3:247–255.
- ,,.Hospitalists: A Guide to Building and Sustaining a Successful Program.Chicago, IL:Health Administration Press;2008.
- ,,,,.The increased financial burden of further proposed orthopaedic resident work‐hour reductions.J Bone Joint Surg Am.2011;93:e31.
- ,,,,,.Fatigue and shift work.J Sleep Res.2006;15:1–5.
- ,,, et al.Rapid shift in peak melatonin secretion associated with improved performance in short shift work schedule.Sleep.1997;20:1145–1150.
- ,,.The impact of a nap opportunity during the night shift on the performance and alertness of 12‐h shift workers.J Sleep Res.2002;11:219–227.
- ,,, et al.Improving alertness and performance in emergency department physicians and nurses: the use of planned naps.Ann Emerg Med.2006;48:596–604, e1‐e3.
- ,,,,.Promoting alertness with a short nap during a night shift.J Sleep Res.1998;7:240–247.
- ,.Circadian adaptation to night‐shift work by judicious light and darkness exposure.J Biol Rhythms.2002;17:556–567.
- ,,,,,.Exposure to bright light and darkness to treat physiologic maladaptation to night work.N Engl J Med.1990;322:1253–1259.
- ,.Night shift performance is improved by a compromise circadian phase position: study 3. Circadian phase after 7 night shifts with an intervening weekend off.Sleep.2008;31:1639–1645.
- ,,,.Dose‐response relationship for light intensity and ocular and electroencephalographic correlates of human alertness.Behav Brain Res.2000;115:75–83.
- ,,,,.Dark goggles and bright light improve circadian rhythm adaptation to night‐shift work.Sleep.1994;17:535–543.
- ,,.Caffeine content of specialty coffees.J Anal Toxicol.2003;27:520–522.
- ,.Caffeine content of prepackaged national‐brand and private‐label carbonated beverages.J Food Sci.2007;72:C337–C342.
- ,,, et al.The effects of coffee and napping on nighttime highway driving: a randomized trial.Ann Intern Med.2006;144:785–791.
- ,,,,.Laboratory and field studies of naps and caffeine as practical countermeasures for sleep‐wake problems associated with night work.Sleep.2006;29:39–50.
- ,,,,.Armodafinil for treatment of excessive sleepiness associated with shift work disorder: a randomized controlled study.Mayo Clin Proc.2009;84:958–972.
- ,,,,.The long‐term tolerability and efficacy of armodafinil in patients with excessive sleepiness associated with treated obstructive sleep apnea, shift work disorder, or narcolepsy: an open‐label extension study.J Clin Sleep Med.2010;6:458–466.
- ,,.Circadian rhythm sleep disorders.Med Clin North Am.2004;88:631–651, viii.
- ,,.A three pulse phase response curve to three milligrams of melatonin in humans.J Physiol.2008;586:639–647.
- ,,, et al.Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report.Sleep.2007;30:1445–1459.
- ,,, et al.Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta‐analysis.BMJ.2006;332:385–393.
- ,,.Effects of melatonin administration on daytime sleep after simulated night shift work.J Sleep Res.2001;10:181–192.
- ,,,.Performance, ability to stay awake, and tendency to fall asleep during the night after a diurnal sleep with temazepam or placebo.Sleep.1997;20:535–541.
- ,,,,,.Sleepiness/alertness on a simulated night shift following sleep at home with triazolam.Sleep.1991;14:140–146.
- ,,,.Zolpidem‐related effects on performance and mood during simulated night‐shift work.Exp Clin Psychopharmacol.2003;11:259–268.
- ,,,,.Intrinsic near‐24‐h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans.Proc Natl Acad Sci USA.2001;98:14027–14032.
- ,,,.Work shift duration: a review comparing eight hour and 12 hour shift systems.Occup Environ Med.1998;55:217–229.
- ,,.Influence of flexibility and variability of working hours on health and well‐being.Chronobiol Int.2006;23:1125–1137.
- ,,.Impact of night‐float rotation on sleep, mood, and alertness: the resident's perception.Chronobiol Int.2002;19:893–902.
- ,,,,,.The characteristics of recovery sleep when recovery opportunity is restricted.Sleep.2007;30:353–360.
- ,,.Practical interventions to promote circadian adaptation to permanent night shift work: study 4.J Biol Rhythms.2009;24:161–172.
- ,,.Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people.Occup Environ Med.2001;58:747–752.
- ,.Use of sleep hygiene in the treatment of insomnia.Sleep Med Rev.2003;7:215–225.
- .Job, family, and gender: determinants of nonstandard work schedules among employed Americans in 1991.Demography.1995;32:577–598.
- ,.Shift work among dual‐earner couples with children.Science.1983;219:876–879.
- ,,, et al.Effect of reducing interns' work hours on serious medical errors in intensive care units.N Engl J Med.2004;351:1838–1848.
- Institute of Medicine. Resident Duty Hours: Enhancing Sleep, Supervision and Safety. Released December 15,2008. Available at: http://www.iom.edu/Reports/2008/Resident‐Duty‐Hours‐Enhancing‐Sleep‐Supervision‐and‐Safety.aspx. Accessed on October 20, 2010.
- ACGME. ACGME Approved Standards. Effective July2011. Available at: http://acgme‐2010standards.org/. Accessed on January 6, 2011
- ACGME. Common Program Requirements: Resident Duty Hours in the Learning and Work Environment. Effective July 1, 2007. Available at: http://www.acgme.org/acWebsite/dutyHours/dh_ComProgrRequirmentsDutyHours0707.pdf. Accessed October 20,2010.
- ,,, et al.Effect of reducing interns' weekly work hours on sleep and attentional failures.N Engl J Med.2004;351:1829–1837.
- Letter written to William Munier, Agency for Healthcare Research and Quality.Washington, DC:US House of Representatives, Committee on Energy and Commerce, March 29,2007. Available at: http://energycommerce.house.gov/Press_110/110‐ltr.032907.HHS.Munier.pdf. Accessed on October 24, 2010.
- ,.The evolution and future of hospital medicine.Mt Sinai J Med.2008;75:418–423.
- .Brain structures and receptors involved in alertness.Sleep Med.2005;6(suppl 1):S3–S7.
- .A two process model of sleep regulation.Hum Neurobiol.1982;1:195–204.
- ,.Entrainment of the human circadian system by light.J Biol Rhythms.2005;20:326–338.
- ,.Sleep‐promoting and hypothermic effects of daytime melatonin administration in humans.Sleep.1997;20:124–131.
- .Sleepiness as a consequence of shift work.Sleep.1988;11:17–34.
- ,,,,.The sleep of long‐haul truck drivers.N Engl J Med.1997;337:755–761.
- ,,,.Sleep on the night shift: 24‐hour EEG monitoring of spontaneous sleep/wake behavior.Psychophysiology.1989;26:352–358.
- .Do permanent night workers show circadian adjustment? A review based on the endogenous melatonin rhythm.Chronobiol Int.2008;25:215–224.
- ,,,,.Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers.Sleep.2004;27:1453–1462.
- ,,, et al.Modafinil for excessive sleepiness associated with shift‐work sleep disorder.N Engl J Med.2005;353:476–486.
- American Academy of Sleep Medicine.The International Classification of Sleep Disorders (ICSD).2nd ed.Chicago, IL:American Academy of Sleep Medicine;2005.
- ,.Circadian rhythm sleep disorders.Chest.2006;130:1915–1923.
- ,,,,,.Early morning executive functioning during sleep deprivation is compromised by a PERIOD3 polymorphism.Sleep.2008;31:1159–1167.
- ,,,,,.Genetic variation in the adenosine A2A receptor gene modulates performance on the psychomotor vigilance task.Sleep Biol Rhythms.2007;5:A47.
- ,.Circadian rhythms in cognitive performance: methodological constraints, protocols, theoretical underpinnings.Physiol Behav.2007;90:196–208.
- ,,,.A prospective study of fatal occupational accidents—relationship to sleeping difficulties and occupational factors.J Sleep Res.2002;11:69–71.
- ,,,.Effects of critical care nurses' work hours on vigilance and patients' safety.Am J Crit Care.2006;15:30–37.
- ,,,,.Daytime sleepiness, sleep habits and occupational accidents among hospital nurses.J Adv Nurs.2005;52:445–453.
- ,,.Systematic review: association of shift length, protected sleep time, and night float with patient care, residents' health, and education.Ann Intern Med.2010;153:829–842.
- ,,,,.Jack Barney Award: the effect of fatigue on cognitive and psychomotor skills of trauma residents and attending surgeons.Am J Surg.2008;196:813–819; discussion 9–20.
- ,,, et al.Risks of complications by attending physicians after performing nighttime procedures.JAMA.2009;302:1565–1572.
- ,,.Sleep deprivation, elective surgical procedures, and informed consent.N Engl J Med.2010;363:2577–2579.
- ,,,,.A prospective study of anger and coronary heart disease. The Normative Aging Study.Circulation.1996;94:2090–2095.
- ,,,.Night work and risk of breast cancer.Epidemiology.2006;17:108–111.
- ,,, et al.Night‐shift work and risk of colorectal cancer in the nurses' health study.J Natl Cancer Inst.2003;95:825–828.
- ,,.Shift work and cardiovascular disease—pathways from circadian stress to morbidity.Scand J Work Environ Health.2010;36:96–108.
- IARC. IARC monographs on the evalutaion of carcinogenic risks to humans. Vol 98. Painting, firefighting, and shiftwork. 2007. Available at: monographs.iarc.fr/ENG/Monographs/vol98/mono98.pdf. Accessed January 16,2011.
- ,,,.Influence of melatonin on invasive and metastatic properties of MCF‐7 human breast cancer cells.Cancer Res.1998;58:4383–4390.
- Society of Hospital Medicine. Society of Hospital Medicine releases results of the 2007–2008 survey on the state of the hospital medicine movement. 2008. Available at: http://www.hospitalmedicine.org/AM/Template.cfm?Section=Press_Releases3:247–255.
- ,,.Hospitalists: A Guide to Building and Sustaining a Successful Program.Chicago, IL:Health Administration Press;2008.
- ,,,,.The increased financial burden of further proposed orthopaedic resident work‐hour reductions.J Bone Joint Surg Am.2011;93:e31.
- ,,,,,.Fatigue and shift work.J Sleep Res.2006;15:1–5.
- ,,, et al.Rapid shift in peak melatonin secretion associated with improved performance in short shift work schedule.Sleep.1997;20:1145–1150.
- ,,.The impact of a nap opportunity during the night shift on the performance and alertness of 12‐h shift workers.J Sleep Res.2002;11:219–227.
- ,,, et al.Improving alertness and performance in emergency department physicians and nurses: the use of planned naps.Ann Emerg Med.2006;48:596–604, e1‐e3.
- ,,,,.Promoting alertness with a short nap during a night shift.J Sleep Res.1998;7:240–247.
- ,.Circadian adaptation to night‐shift work by judicious light and darkness exposure.J Biol Rhythms.2002;17:556–567.
- ,,,,,.Exposure to bright light and darkness to treat physiologic maladaptation to night work.N Engl J Med.1990;322:1253–1259.
- ,.Night shift performance is improved by a compromise circadian phase position: study 3. Circadian phase after 7 night shifts with an intervening weekend off.Sleep.2008;31:1639–1645.
- ,,,.Dose‐response relationship for light intensity and ocular and electroencephalographic correlates of human alertness.Behav Brain Res.2000;115:75–83.
- ,,,,.Dark goggles and bright light improve circadian rhythm adaptation to night‐shift work.Sleep.1994;17:535–543.
- ,,.Caffeine content of specialty coffees.J Anal Toxicol.2003;27:520–522.
- ,.Caffeine content of prepackaged national‐brand and private‐label carbonated beverages.J Food Sci.2007;72:C337–C342.
- ,,, et al.The effects of coffee and napping on nighttime highway driving: a randomized trial.Ann Intern Med.2006;144:785–791.
- ,,,,.Laboratory and field studies of naps and caffeine as practical countermeasures for sleep‐wake problems associated with night work.Sleep.2006;29:39–50.
- ,,,,.Armodafinil for treatment of excessive sleepiness associated with shift work disorder: a randomized controlled study.Mayo Clin Proc.2009;84:958–972.
- ,,,,.The long‐term tolerability and efficacy of armodafinil in patients with excessive sleepiness associated with treated obstructive sleep apnea, shift work disorder, or narcolepsy: an open‐label extension study.J Clin Sleep Med.2010;6:458–466.
- ,,.Circadian rhythm sleep disorders.Med Clin North Am.2004;88:631–651, viii.
- ,,.A three pulse phase response curve to three milligrams of melatonin in humans.J Physiol.2008;586:639–647.
- ,,, et al.Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report.Sleep.2007;30:1445–1459.
- ,,, et al.Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta‐analysis.BMJ.2006;332:385–393.
- ,,.Effects of melatonin administration on daytime sleep after simulated night shift work.J Sleep Res.2001;10:181–192.
- ,,,.Performance, ability to stay awake, and tendency to fall asleep during the night after a diurnal sleep with temazepam or placebo.Sleep.1997;20:535–541.
- ,,,,,.Sleepiness/alertness on a simulated night shift following sleep at home with triazolam.Sleep.1991;14:140–146.
- ,,,.Zolpidem‐related effects on performance and mood during simulated night‐shift work.Exp Clin Psychopharmacol.2003;11:259–268.
- ,,,,.Intrinsic near‐24‐h pacemaker period determines limits of circadian entrainment to a weak synchronizer in humans.Proc Natl Acad Sci USA.2001;98:14027–14032.
- ,,,.Work shift duration: a review comparing eight hour and 12 hour shift systems.Occup Environ Med.1998;55:217–229.
- ,,.Influence of flexibility and variability of working hours on health and well‐being.Chronobiol Int.2006;23:1125–1137.
- ,,.Impact of night‐float rotation on sleep, mood, and alertness: the resident's perception.Chronobiol Int.2002;19:893–902.
- ,,,,,.The characteristics of recovery sleep when recovery opportunity is restricted.Sleep.2007;30:353–360.
- ,,.Practical interventions to promote circadian adaptation to permanent night shift work: study 4.J Biol Rhythms.2009;24:161–172.
- ,,.Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27,485 people.Occup Environ Med.2001;58:747–752.
- ,.Use of sleep hygiene in the treatment of insomnia.Sleep Med Rev.2003;7:215–225.
Severe Hypertriglyceridemia: A Review
The patient with a markedly high serum triglyceride (TG) level poses an interesting challenge for hospitalists. Hypertriglyceridemia (HTG) is defined as a fasting plasma TG level that is above the 95th percentile for age and sex.1 TG levels are commonly classified into categories according to Adult Treatment Panel III guidelines with desirable levels <150 mg/dL (1.7 mmol/L), borderline levels 150199, high levels 200499 mg/dL, and very high levels >500 mg/dL (5.6 mmol/L).2 A TG level exceeding an arbitrary threshold of >1000 mg/dL (11.3 mmol/L) is referred to as severe HTG. The Lipid Research Clinics Program Prevalence Study found that 1.79 per 10,000 outpatients (<0.02%) had TG levels > 2000 mg/dL.3 Chylomicronemia syndrome occurs when severe HTG is accompanied by 1 or more of the following: symptoms of abdominal pain or acute pancreatitis or physical examination findings such as eruptive xanthomas or lipemia retinalis. There is no TG level above which pancreatitis invariably occurs, making the decision to hospitalize difficult. The goal of this review is to discuss the causes of severe HTG; the clinical assessment, including criteria for hospitalization; and the available treatment options for this infrequent but serious condition. We begin with a clinical case of severe HTG.
CASE PRESENTATION
A 47‐year‐old woman with a history of chronic myelogenous leukemia was admitted to the hospital with a serum triglyceride level of 17,393 mg/dL. Two years prior to admission, she underwent allogenic stem cell transplantation for chronic myelogenous leukemia and has since remained in remission. Six months prior to admission, severe diarrhea from intestinal graft‐versus‐host disease required the use of total parenteral nutrition (TPN) and immunosuppressive therapy consisting of prednisone 20 mg/day, mycophenolate mofetil 250 mg thrice daily, and sirolimus 0.3 mg/day. During treatment with steroids, she developed diabetes mellitus requiring insulin, with a subsequent hemoglobin A1c level of 7.7% (normal, <7%). The serum TG level prior to transplantation was unknown but was 343 mg/dL prior to TPN initiation. One month prior to admission, the diarrhea resolved and TPN was stopped. The TG level was 7463 mg/dL 1 week prior to admission, and despite use of fenofibrate, it rose to 17,393 mg/dL. The patient denied abdominal pain, and did not have abdominal tenderness or eruptive xanthomas. She denied a family history of dyslipidemia or recent medication changes. Given the extreme TG elevation, the lack of response to outpatient treatment and the concern for developing acute pancreatitis, the patient was admitted to the hospital for inpatient TG‐lowering treatment.
Upon admission, serum lipase and amylase were within normal limits, but the blood glucose level was 243 mg/dL. Insulin infusion and oral fenofibrate 145 mg/day was started, and the patient was kept non per os (NPO). Six hours later, despite insulin infusion, the TG level rose to 26,250 mg/dL. Therapeutic plasma exchange (TPE) was performed on 2 consecutive days with a resultant decrease in TG level to 530 mg/dL. The patient was later discharged home on fenofibrate and omega‐3 ethyl esters, her same immunosuppressive and insulin regimen, and instructions for a very low‐fat diet. In the next 3 months, her serum TG level did not rise above 530 mg/dL. The cause of our patient's extreme TG elevation was likely a combination of genetic factors exacerbated by immunosuppressive and glucocorticoid therapy.
This case featured dramatic elevations in serum TG levels that the managing doctors believed merited a hospital admission. Management of patients with severe HTG first requires an understanding of TG metabolism.
ETIOLOGY
Serum TGs produced by the liver are carried by very low‐density lipoproteins (VLDLs), whereas TGs derived from dietary fat are carried by chylomicrons. Both chylomicrons and VLDLs are hydrolyzed by the same enzymelipoprotein lipase (LPL). TGs are hydrolyzed into fatty acids for uptake by muscle and adipose tissue, whereas remnants of VLDL and chylomicrons are removed by the liver. More details on TG pathophysiology may be found in a recent review.4 When LPL is saturated with VLDL, ingestion of a fatty meal may cause chylomicrons to linger in circulation for days instead of hours. Asking the laboratory to spin down the blood of a patient with severe HTG and keep the test tube upright at 4C may reveal a large creamy supernatant layer demonstrating chylomicronemia.
A fasting TG level drawn 12 hours after the last meal reflects hepatic TG production. Although a nonfasting TG level may reflect postprandial chylomicrons, values above 1000 mg/dL strongly suggest true HTG, particularly in the setting of acute pancreatitis. Treatment should not be delayed to obtain a fasting TG level.
HTG may result from increased VLDL production, reduced VLDL/chylomicron clearance, or more likely a combination of the two. The causes of these metabolic derangements are classified as primary (genetic) or secondary (acquired) (Table 1). In adult patients, HTG is usually the result of a combination of primary and secondary causes. A study of 123 patients with TG levels >2000 mg/dL found that all patients had a primary metabolic defect and 110/123 had a coexistent secondary cause.3 An underlying genetic lipoprotein metabolism derangement is often clinically silent until coupled with a secondary cause of HTG that together raise TG levels high enough to cause the chylomicronemia syndrome.
|
| Primary |
| Familial lipid disorders |
| Lipoprotein pattern type I |
| Familial chylomicronemia |
| Deficiency in LPL and/or apo‐CII |
| Autosomal recessive; presents in childhood |
| Rare functional disorders in LPL |
| Lipoprotein pattern type III |
| Familial dysbetalipoproteinemia |
| Inadequate VLDL clearance from apo‐E2 |
| Autosomal recessive; presents in adulthood |
| Lipoprotein pattern type IV |
| Familial HTG: increased VLDL |
| Autosomal dominant; presents in adulthood |
| Familial combined hyperlipidemia |
| Multiple phenotypes seen; increased apo‐B levels |
| Lipoprotein pattern type V |
| Mixed HTG: increased VLDL and chylomicrons; presents in adulthood |
| Secondary |
| Disease |
| Poorly controlled diabetes mellitus; hypothyroid; SLE; Cushing syndrome; HIV infection; sarcoid multiple myeloma; obesity; renal disease (nephrotic) |
| Disorder of metabolism |
| Pregnancy |
| Diet |
| Excessive alcohol, especially with high‐fat diet |
| Drugs |
| Estrogen; tamoxifen; glucocorticoids; protease inhibitors; nonselective beta‐blockers; propofol; isotretinoin; some antipsychotic medications (clozapine, olanzapine); tacrolimus; sirolimus; cyclosporine; bexarotene; all‐trans retinoic acid; L‐asparaginase; interferon‐ |
The most common primary cause of HTG in adults is familial HTG, an autosomal dominant condition with a population prevalence ranging from 1%2% to 5%10% and age‐dependent penetrance.5, 6 Other genetic causes are much rarer, such as LPL deficiency (1 in 1 million patients), apolipoprotein‐CII, and other mutations resulting in impaired binding to LPL.79 Primary causes of HTG are often listed as Fredrickson phenotypes (Table 1). Recent genome‐wide association studies reveal a complex polygenic basis to the Fredrickson categories and suggest additional undefined genes or nongenetic factors may significantly contribute to the final phenotype.10 Diagnosis of familial lipid disorders requires an accurate family history that may be difficult to obtain.
Secondary causes of HTG can be categorized using a four‐D mnemonic: Diseases, Diet, Disorder of Metabolism, and Drugs.11 The most common condition associated with HTG is obesity.6 The mechanism between obesity and HTG is complex and likely involves an increase in fatty acid flux from adipose tissue to other tissues and insulin resistance.12 Asking whether a patient's current weight is close to the heaviest lifetime weight is a clue to diagnosing obesity‐driven HTG. One case series of hypertriglyceridemic pancreatitis found that diabetes or excessive alcohol intake account for the majority of secondary causes of HTG.13 The cause of HTG among patients with diabetes is multifactorial: insulin deficiency reduces LPL levels (insulin is required for synthesis of LPL), whereas insulin resistance attenuates the ability of insulin to decrease hepatic cholesterol synthesis and thus increases hepatic secretion of VLDL.14 Alcohol impairs lipolysis and increases VLDL production that can lead to severe HTG, particularly in those patients with an underlying functional deficiency in LPL. Other secondary etiologies of severe HTG may be elicited through careful attention to medical and medication history.
CLINICAL ASSESSMENT
Table 2 proposes a reasonable initial assessment of the history and physical and laboratory tests in patients with severe HTG.
|
| History |
| Family history of lipid disorders |
| Maximal weight and when achieved |
| Detailed medication history (including those recently stopped) |
| Alcohol consumption |
| Diabetes mellitus |
| Possible physical examination findings |
| Eruptive xanthomas |
| Lipemia retinalis |
| Hepatomegaly |
| Lymphadenopathy |
| Laboratory tests |
| Basic Metabolic Panel* with glucose |
| Lipid panel |
| Thyroid‐stimulating hormone, free T4 |
| Liver function, amylase, lipase |
| Hemoglobin A1c |
| Urinalysis |
Distinguishing physical examination findings may arise when serum TG levels exceed 1000 mg/dL. Eruptive xanthoma form when large amounts of TG are sequestered in cutaneous histiocytes, resulting in small yellow‐orange papules with an erythematous base. This finding is seen in a minority of HTG patients and may be missed altogether without a careful examination of the extensor surfaces of arms, legs, back, and buttocks.15 Effective TG‐lowering treatment will result in resolution of these xanthomas. An ophthalmologic examination may reveal lipemia retinalis, a condition that occurs when retinal vessels appear white from lipemic serum and contrast against a pale salmon‐colored retina. Although a dramatic finding, these changes do not result in vision impairment. Hepatomegaly from fatty infiltration of the liver occurs frequently,16 and diffuse lymphadenopathy may also be found.17
Laboratory tests are also essential in the clinical assessment of severe HTG. Important tests include thyroid‐stimulating hormone, creatinine, serum urea nitrogen, and a urinalysis. A hemoglobin A1c test provides information on the level of glycemic control and is now recognized by the American Diabetes Association to diagnose diabetes mellitus.18 Liver function tests commonly reveal a transaminase elevation from underlying steatohepatitis and also provide a baseline value prior to initiating any lipid‐lowering medications. Additional diagnostic tests may be useful in selected patients: HIV testing, serum protein electrophoresis, and urine protein electrophoresis to help diagnose paraproteinemias such as multiple myeloma, and an antinuclear antibody and double‐stranded DNA for systemic lupus erythematosus.
Severe HTG may interfere with the result of 2 commonly obtained laboratory tests. The sodium concentration can be falsely low (pseudohyponatremia) due to the high levels of TG displacing sodium containing water from the plasma.19 Due to interference by plasma lipids, amylase levels may be near normal in up to 50% of patients with hypertriglyceridemic pancreatitis at the time of admission.20 Thus, if the suspicion for pancreatitis is high, it is reasonable to proceed to imaging if amylase or lipase levels are not confirmatory. Abdominal imaging with computed tomography or magnetic resonance imaging may be used to diagnose acute pancreatitis.
Behind excessive alcohol consumption and gallstone disease, HTG is the third leading cause of pancreatitis, accounting for up to 10% of cases in the general population.21 The exact mechanism by which HTG causes pancreatitis is unclear. One theory is that elevated plasma TG levels are hydrolyzed in the pancreas to cause an increase in local free fatty acids, which in turn may cause inflammation and overt pancreatitis.22 Another theory proposes that elevated levels of chylomicrons lead to plasma hyperviscosity, which causes ischemia and local acidosis in pancreatic capillaries.23 Whatever the cause, it is unclear why only some patients with severe HTG develop acute pancreatitis. One study of 129 patients with severe HTG found mean serum TG levels to be higher in patients with acute pancreatitis than in those without (4470 versus 2450 mg/dL), suggesting the threshold to develop acute pancreatitis is higher than previously thought.24 Without a firm TG threshold above which patients develop pancreatitis, the decision to hospitalize can be difficult.
WHEN TO HOSPITALIZE?
The choice of whether to hospitalize a patient with severe HTG is first based on the presence or absence of abdominal pain and/or acute pancreatitis. Figure 1 diagrams a suggested admission and treatment algorithm. If abdominal pain is present, the patient should be hospitalized and assessed for possible triggers with prompt initiation of pharmacologic treatment. In the absence of abdominal pain, the decision to admit the patient with severe HTG requires clinical judgment. In these cases, prompt consultation with a physician experienced in the management of lipid disorders is recommended. In our experience, admission is usually driven by factors such as (1) severe hyperglycemia requiring inpatient insulin therapy; (2) severe HTG at or near a level where pancreatitis has occurred in the past in a patient for whom adherence is suspect (mindful of the great variability at the levels where patients develop pancreatitis); (3) unremitting triggers of severe HTG such as ongoing use of essential medications also known to exacerbate HTG (such as some forms of chemotherapy) or pregnancy in the third trimester. TG levels rise continuously throughout pregnancy and peak during the third trimester, when hypertriglyceridemic pancreatitis most often occurs. Asymptomatic patients with severe HTG not requiring hospitalization need close outpatient follow‐up to prevent the onset of chylomicronemia syndrome.
INPATIENT MANAGEMENT
No professional recommendations exist regarding a standardized treatment plan for severe HTG. The treatment regimen is first based on the presence or absence of symptoms. Treatment of hypertriglyceridemic pancreatitis should target a serum TG level <1000 mg/dL and resolution of abdominal pain. The initial goal for asymptomatic patients is a TG level <1000 mg/dL, as this level represents a significant reduction in the risk of developing chylomicronemia syndrome. In either case, the first 2 components of the treatment regimen are dietary changes and oral medications.
Dietary Changes
Patients with hypertriglyceridemic pancreatitis should be made NPO, with the exception of necessary medications taken only with water to provide bowel rest and eliminate fat intake. As chylomicron production in the intestine falls, TG levels will fall dramatically within 12 days of NPO status regardless of other treatments. Once TG levels approach 1000 mg/dL and there is no residual abdominal pain, a no‐fat diet can be given. Patients with persistent abdominal pain requiring a prolonged fast (>57 days) may require nutrition through alternate means such as an enteral formula through a feeding tube or the use of TPN. If a feeding tube is required, we suggest beginning with an elemental, peptide‐based, fat‐free formula, with help from a nutrition consult to assist with individual tube‐feeding options.25 Enteral formula can be supplemented with medium‐chain triglyceride oils (found in coconut and palm kernel) to provide some additional nutritional support. MCTs do not raise serum TG levels, as they are absorbed directly into the portal vein for prompt oxidation by the liver, whereas long‐chain TGs are converted into chylomicrons for peripheral transport. One case report describes a dramatic therapeutic response to medium‐chain triglyceride oils in a patient with familial chylomicronemia,26 although we do not routinely recommend these oils as therapy given lack of long‐term safety data. Lastly, if TPN is required, it is crucial to avoid lipid emulsions to prevent a rise in serum TG levels.
Asymptomatic patients with severe HTG can be fed upon admission, but should be placed on a fat‐free diet. Fat is added back into the diet when TG levels fall below 1000 mg/dL and is slowly increased to a target fat content of 10% of the total calories, usually not exceeding 25 g/day.
Oral Medications
Oral medications should be initiated to lower TG levels for both symptomatic and asymptomatic patients. Table 3 lists the different classes of medications. In our experience, oral fibrates are a recommended first‐line treatment, with other agents used as adjunctive therapy. Through complex mechanisms, fibrates reduce hepatic VLDL secretion and increase serum lipolysis of TG.27 In patients who do not have diabetes and are at low risk for coronary heart disease (CHD), either gemfibrozil or fenofibrate may be used to lower serum TG levels. However, in patients with diabetes, CHD, or a CHD risk equivalent, use of fenofibrate is preferred as an HMG‐CoA reductase inhibitor (a statin) and will almost always be necessary to reach low‐density lipoprotein (LDL)‐cholesterol goals. Fenofibrate, unlike gemfibrozil, does not interfere with the glucuronidation of statins by the liver.28
| Drug | Usual Dose | TG Reduction | Cautions or Contraindications | Comments |
|---|---|---|---|---|
| ||||
| Fenofibrate | 130200 mg/day | 50% | Hepatic or renal insufficiency | Best fibrate to use with HMG‐CoA reductase Inhibitors |
| Gemfibrozil | 600 mg BID | 50% | Hepatic or renal insufficiency | Avoid combination with statins |
| HMG‐CoA reductase inhibitors or statins | Variable; the more potent LDL lowering, the more TG lowering | 25% | Decompensated cirrhosis; end‐stage renal disease | Not the primary treatment for patients with TG levels >1000 mg/dL; some statins such as atorvastatin and fluvastatin are favored for patients with renal insufficiency due to less renal excretion than other statins |
| Omega‐3 fatty acids | 2 g BID Lovaza (840 mg DHA/EPA per dose) | 25%50% with monotherapy (the higher the TG level, the greater the reduction); 30% with combination therapy | Allergy to fish | Fishy aftertaste; may cause flatulence; may increase serum glucose and LDL; low risk of clinical bleeding |
| Nicotinic acid | 12 g/day ER; up‐titrate from lowest dose | 15%35% | Active liver disease; active peptic ulcer disease; arterial bleeding | Can increase blood sugar levels by increasing insulin resistance |
| Orlistat (Xenical) | 120 mg TID | 15%35% | Cases of serious liver dysfunction have been reported | Can interfere with drug absorption, especially fat‐soluble vitamins; oily rectal discharge |
| Insulin | IV 0.10.3 U/kg/hr; titrate to serum glucose 140180 mg/dL OR basal/bolus subcutaneously | Variable; >50% in some cases | Hypoglycemia | Useful in patients who have diabetes |
If a fibrate fails to achieve an acceptable serum TG level, we recommend adjunctive therapy with omega‐3 fatty acid esters. Omega‐3 fatty acids lower serum TG levels by decreasing VLDL production and can lower TG levels by as much as 45% in cases of severe HTG.29 This medication is typically the first adjunctive medication chosen due to its low side effect profile.
If additional TG lowering is needed, niacin or nicotinic acid (vitamin B3) may be added next. This medication decreases VLDL production, lowers LDL, and increases high‐density lipoprotein, but invariably with initiation, patients exhibit prominent skin flushing, burning, or itching. This prostaglandin‐mediated effect may be prevented or at least reduced in severity by taking 325 mg aspirin 1 hour before niacin administration. If the TG level is still not at goal, orlistat, a lipase or fat blocker, may be useful.30 Orlistat improves postprandial lipemia through reduction of dietary fat absorption. Finally, although potent statins such as atorvastatin and rosuvastatin can lower TGs derived from VLDL substantially,31 their use should be prompted by the patient's risk for atherosclerotic cardiovascular disease. Because all of the hypotriglyceridemic medications can affect the liver, regular liver function testing is prudent, as is a periodic re‐evaluation of the ongoing need for these medications. Statin use causing mild transaminase elevation (up to 3 times the upper limit of normal) may be safely tolerated.32
Additional Inpatient Management
Insulin
Insulin increases lipoprotein lipase activity, thereby accelerating chylomicron degradation.33 Insulin (along with glucose if necessary to maintain euglycemia) is therefore a useful adjunctive TG‐lowering medication to oral medications, even in nondiabetic patients. Insulin administered intravenously should follow a titration protocol with hourly monitoring of blood glucose. The goal of the insulin protocol with severe HTG is not maintaining strict euglycemia but rather maintenance of LPL activation by exogenous insulin with avoidance of hypoglycemia. In hospitals where an insulin infusion protocol for diabetic ketoacidosis or postsurgical hyperglycemia already exists, the protocol can be applied for HTG management with minor modifications: introduce dextrose‐containing fluids at higher blood glucoses (180 mg/dL or less) and eliminate insulin boluses. A suggested dose is a continuous intravenous insulin drip at 0.10.3 U/kg/hr with glucose to maintain blood glucose levels between 140 and 180 mg/dL, although there are no guidelines from professional societies. Subcutaneous insulin has also been used to successfully lower TG levels.34, 35 The major limitation of subcutaneous administration is the inability to rapidly adjust the dosing when needed, which is particularly concerning when treating patients who do not have diabetes. We prefer to use subcutaneous basal insulin in patients requiring long‐term use of insulin after a significant TG reduction with intravenous insulin. Subcutaneous bolus prandial insulin should not be used until the patient has resumed a solid diet, because a liquid diet may not reliably contain enough carbohydrates for bolus therapy.
Intravenous Heparin
Heparin has been used in case reports as adjunctive treatment for hypertriglyceridemic pancreatitis.36, 37 Although heparin may increase circulating LPL levels, this effect is short‐lived and is quickly followed by increased hepatic LPL degradation.38 Therefore, the use of heparin to treat severe HTG cannot be routinely recommended.
Therapeutic Plasma Exchange
First used in 1978, therapeutic plasma exchange (TPE) has been demonstrated to quickly and dramatically lower serum TG levels.39 Since its first use, TPE has been used in several small case studies.4042 Without data from larger studies, the optimal frequency and duration of TPE remains unclear. One review suggests the use of TPE as first‐line therapy provided the patient is euglycemic, apheresis can be started within 48 hours of diagnosis, and the patient can tolerate the central venous access.43 On the other hand, guidelines from the American Society of Apheresis incorporating low‐quality evidence do not recommend TPE as routine first‐ or second‐line treatment for hypertriglyceridemic pancreatitis, but rather suggest the use of TPE on a case‐by‐case basis.44 When TPE is needed, this society recommends daily treatment for 13 days until an adequate postapheresis TG level is obtained. Although TPE rapidly lowers TG levels, it is also aggressive (requires placement of a pheresis catheter), expensive, and may not be readily available. We believe it remains an option for patients with severe HTG who do not respond readily to fat restriction, glycemic control with insulin, and pharmacologic treatment with a fibrate and omega‐3 fatty acids. In our judgment, routine use of TPE cannot be recommended without data from a randomized clinical trial examining the value of immediate lowering of TG levels with TPE versus the usually prompt fall in TG levels with less aggressive measures.
Discharge Planning
Typically, asymptomatic patients are discharged once their TG levels approach 1000 mg/dL. Patients recovering from hypertriglyceridemic pancreatitis may be discharged once they tolerate a no‐fat diet without recurrence of abdominal pain and without a significant TG increase above 1000 mg/dL.
Discharge Diet and Activity
At discharge, the diet should be high in fiber, fruits, vegetables, and lean protein, with fat intake restricted to approximately 10% of total calories. Insulin‐resistant patients and patients with diabetes should avoid sugar‐sweetened foods and drinks. Specifically, the daily amount of fructose intake should be no more than 50 mg to avoid a dose‐dependent increase in plasma TG levels compared with other sugars.4 At least 2 servings per week of marine foods naturally rich in omega‐3 fatty acids (fatty fish such as salmon or trout) are recommended. Nonmarine forms of omega‐3 fatty acids (walnuts, flaxseed), which have not demonstrated consistent reductions in TG, cannot be routinely recommended.4 In addition, alcohol consumption should be eliminated.
Once patients maintain a TG level near 500 mg/dL, we allow for dietary flexibility by slowly increasing the amount of dietary unsaturated fat. For patients with TG levels <500 mg/dL, Adult Treatment Panel III advocated restriction of daily dietary saturated fat levels to <7% and keeping the total fat level between 25% and 35%.2 A range in total fat was provided so that unsaturated fat could be increased to limit dietary carbohydrates if glycemic control was needed. In addition to dietary changes, counseling patients about the importance of physical activity and weight loss is crucial for long‐term management of severe HTG. TG lowering in response to diet and weight loss varies, but typically approximates 25%.45
Outpatient Medications
Patients without significant contraindications should be discharged on a fibrate and omega‐3 fatty acids. As mentioned, niacin, orlistat, and/or a statin may be used as adjunctive therapy. Despite use of these hypotriglyceridemic medications, secondary causes of HTG should be modified (such as removal of aggravating medications or appropriately treating uncontrolled diabetes) to yield lasting improvements in TG levels.
CONCLUSIONS
As the prevalence of obesity and diabetes continues to rise, so too does the clinical importance of proper management of severe HTG. Recognizing chylomicronemia syndromeone of the most dramatic consequences of lipid disordersand the underlying primary and secondary causes of HTG is required before starting treatment. Patients with severe HTG may require hospitalization for immediate reduction in TG levels and relief of abdominal pain, if present. Treatment involves modifying secondary causes, if possible, and eliminating dietary fat intake. Although use of medications such as an oral fibrate, omega‐3 fatty acids, and insulin are routine, the use of a more invasive procedure such as TPE should be considered on a case‐by‐case basis and may be limited by availability. Upon hospital discharge, careful follow‐up should promote lifestyle changes and medication adherence to prevent recurrence of severe HTG.
- ,,,.Pathophysiology of triglyceride‐rich lipoproteins in atherothrombosis: clinical aspects.Clin Cardiol.1999;22:II15–II20.
- Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III).JAMA.2001;285:2486–2497.
- ,.Chylomicronemia syndrome. Interaction of genetic and acquired hypertriglyceridemia.Med Clin North Am.1982;66:455–468.
- ,,, et al.Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association.Circulation.2011;123:2292–2333.
- .Familial lipoprotein lipase deficiency and other causes of the chylomicronemia syndrome. In: Scriver C, Beaudet A, Sly W, Valle D, eds.The Metabolic and Molecular Basis of Inherited Disease.7th ed.New York:McGraw‐Hill;1995:1913–1932.
- ,,.Hypertriglyceridemia: its etiology, effects and treatment.CMAJ.2007;176:1113–1120.
- ,,, et al.Chylomicronemia with a mutant GPIHBP1 (Q115P) that cannot bind lipoprotein lipase.Arterioscler Thromb Vasc Biol.2009;29:956–962.
- ,,, et al.A mutation in the human lipoprotein lipase gene as the most common cause of familial chylomicronemia in French Canadians.N Engl J Med.1991;324:1761–1766.
- ,,, et al.Inherited apolipoprotein A‐V deficiency in severe hypertriglyceridemia.Arterioscler Thromb Vasc Biol.2005;25:411–417.
- ,,, et al.A polygenic basis for four classical Fredrickson hyperlipoproteinemia phenotypes that are characterized by hypertriglyceridemia.Hum Mol Genet.2009;18:4189–4194.
- .Secondary causes of hyperlipidemia.Med Clin North Am.1994;78:117–141.
- ,,.Hypertriglyceridemic hyperapob: the unappreciated atherogenic dyslipoproteinemia in type 2 diabetes mellitus.Ann Intern Med.2001;135:447–459.
- .Hyperlipidemic pancreatitis.Gastroenterol Clin North Am.1990;19:783–791.
- ,,, et al.Effects of insulin on cholesterol synthesis in type II diabetes patients.Diabetes Care.1995;18:1362–1369.
- ,,,.Evidence for the chylomicron origin of lipids accumulating in diabetic eruptive xanthomas: a correlative lipid biochemical, histochemical, and electron microscopic study.J Clin Invest.1970;49:2172–2187.
- .Dyslipidaemia.Lancet.2003;362:717–731.
- ,,.Lymphadenopathy associated with severe hypertriglyceridemia.JAMA.1990;264:727–728.
- Diagnosis and classification of diabetes mellitus.Diabetes Care.2010;33(suppl 1):S62–S69.
- ,.Pseudohyponatremia in acute hyperlipemic pancreatitis. A potential pitfall in therapy.Arch Surg.1985;120:1053–1055.
- ,,.Suppression of amylase activity by hypertriglyceridemia.JAMA.1973;225:1331–1334.
- ,,,.Dyslipidaemic pancreatitis clinical assessment and analysis of disease severity and outcomes.Pancreatology.2009;9:252–257.
- .Pathogenesis, differentiation and management of hypertriglyceridemia.Adv Intern Med.1969;15:117–154.
- ,.Role of hypertriglyceridemia in the pathogenesis of experimental acute pancreatitis in rats.Int J Pancreatol.1996;20:177–184.
- ,,, et al.Acute pancreatitis in a cohort of 129 patients referred for severe hypertriglyceridemia.Pancreas.2008;37:13–12.
- ,,, et al.ESPEN Guidelines on Enteral Nutrition: pancreas.Clin Nutr.2006;25:275–284.
- ,,, et al.Therapeutic response to medium‐chain triglycerides and omega‐3 fatty acids in a patient with the familial chylomicronemia syndrome.Arterioscler Thromb Vasc Biol.1997;17:1400–1406.
- ,,,,,.Mechanism of action of fibrates on lipid and lipoprotein metabolism.Circulation.1998;98:2088–2093.
- ,,.Drug interactions with lipid‐lowering drugs: mechanisms and clinical relevance.Clin Pharmacol Ther.2006;80:565–81.
- ,,, et al.Safety and efficacy of Omacor in severe hypertriglyceridemia.J Cardiovasc Risk.1997;4:385–391.
- ,,.Usefulness of Orlistat in the treatment of severe hypertriglyceridemia.Am J Cardiol.2002;89:229–231.
- ,,, et al.Effects of intensive atorvastatin and rosuvastatin treatment on apolipoprotein B‐48 and remnant lipoprotein cholesterol levels.Atherosclerosis.2009;205:197–201.
- ,,,.Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force.Am J Cardiol.2006;97:89C–94C.
- .Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases.N Engl J Med.1989;320:1060–1068.
- ,,.Insulin therapy for a non‐diabetic patient with severe hypertriglyceridemia.J Am Coll Nutr.1998;17:458–461.
- ,,,,.Treatment of severe hypertriglyceridemia in nondiabetic patients with insulin.Am J Emerg Med.2005;23:415–417.
- ,.Decreasing the plasma triglyceride level in hypertriglyceridemia‐induced pancreatitis in pregnancy: a case report.Am J Obstet Gynecol.2002;187:241–242.
- ,,,,,.Pancreatitis may occur with a normal amylase concentration in hypertriglyceridaemia.BMJ.1996;313:1265.
- ,,,.Lipoprotein lipase during continuous heparin infusion: tissue stores become partially depleted.J Lab Clin Med.2001;138:206–13.
- ,,,,,.Treatment of severe diabetic hypertriglyceridaemia by plasma exchange.Lancet.1978;1:1368.
- ,,,.Therapeutic plasma exchange in patients with hyperlipidemic pancreatitis.World J Gastroenterol.2004;10:2272–2274.
- ,,.Plasma exchange in severe hypertriglyceridemia a clinical study.Transfus Apher Sci.2006;34:253–257.
- ,,, et al.Management of acute severe hyperlipidemic pancreatitis.Digestion.2006;73:259–264.
- ,,,,.Hypertriglyceridemic pancreatitis: presentation and management.Am J Gastroenterol.2009;104:984–991.
- ,,, et al.Guidelines on the use of therapeutic apheresis in clinical practice—evidence‐based approach from the Apheresis Applications Committee of the American Society for Apheresis.J Clin Apher.2010;25:83–177.
- ,,,,,.Effects of a low‐fat diet compared with those of a high‐monounsaturated fat diet on body weight, plasma lipids and lipoproteins, and glycemic control in type 2 diabetes.Am J Clin Nutr.2004;80:668–673.
The patient with a markedly high serum triglyceride (TG) level poses an interesting challenge for hospitalists. Hypertriglyceridemia (HTG) is defined as a fasting plasma TG level that is above the 95th percentile for age and sex.1 TG levels are commonly classified into categories according to Adult Treatment Panel III guidelines with desirable levels <150 mg/dL (1.7 mmol/L), borderline levels 150199, high levels 200499 mg/dL, and very high levels >500 mg/dL (5.6 mmol/L).2 A TG level exceeding an arbitrary threshold of >1000 mg/dL (11.3 mmol/L) is referred to as severe HTG. The Lipid Research Clinics Program Prevalence Study found that 1.79 per 10,000 outpatients (<0.02%) had TG levels > 2000 mg/dL.3 Chylomicronemia syndrome occurs when severe HTG is accompanied by 1 or more of the following: symptoms of abdominal pain or acute pancreatitis or physical examination findings such as eruptive xanthomas or lipemia retinalis. There is no TG level above which pancreatitis invariably occurs, making the decision to hospitalize difficult. The goal of this review is to discuss the causes of severe HTG; the clinical assessment, including criteria for hospitalization; and the available treatment options for this infrequent but serious condition. We begin with a clinical case of severe HTG.
CASE PRESENTATION
A 47‐year‐old woman with a history of chronic myelogenous leukemia was admitted to the hospital with a serum triglyceride level of 17,393 mg/dL. Two years prior to admission, she underwent allogenic stem cell transplantation for chronic myelogenous leukemia and has since remained in remission. Six months prior to admission, severe diarrhea from intestinal graft‐versus‐host disease required the use of total parenteral nutrition (TPN) and immunosuppressive therapy consisting of prednisone 20 mg/day, mycophenolate mofetil 250 mg thrice daily, and sirolimus 0.3 mg/day. During treatment with steroids, she developed diabetes mellitus requiring insulin, with a subsequent hemoglobin A1c level of 7.7% (normal, <7%). The serum TG level prior to transplantation was unknown but was 343 mg/dL prior to TPN initiation. One month prior to admission, the diarrhea resolved and TPN was stopped. The TG level was 7463 mg/dL 1 week prior to admission, and despite use of fenofibrate, it rose to 17,393 mg/dL. The patient denied abdominal pain, and did not have abdominal tenderness or eruptive xanthomas. She denied a family history of dyslipidemia or recent medication changes. Given the extreme TG elevation, the lack of response to outpatient treatment and the concern for developing acute pancreatitis, the patient was admitted to the hospital for inpatient TG‐lowering treatment.
Upon admission, serum lipase and amylase were within normal limits, but the blood glucose level was 243 mg/dL. Insulin infusion and oral fenofibrate 145 mg/day was started, and the patient was kept non per os (NPO). Six hours later, despite insulin infusion, the TG level rose to 26,250 mg/dL. Therapeutic plasma exchange (TPE) was performed on 2 consecutive days with a resultant decrease in TG level to 530 mg/dL. The patient was later discharged home on fenofibrate and omega‐3 ethyl esters, her same immunosuppressive and insulin regimen, and instructions for a very low‐fat diet. In the next 3 months, her serum TG level did not rise above 530 mg/dL. The cause of our patient's extreme TG elevation was likely a combination of genetic factors exacerbated by immunosuppressive and glucocorticoid therapy.
This case featured dramatic elevations in serum TG levels that the managing doctors believed merited a hospital admission. Management of patients with severe HTG first requires an understanding of TG metabolism.
ETIOLOGY
Serum TGs produced by the liver are carried by very low‐density lipoproteins (VLDLs), whereas TGs derived from dietary fat are carried by chylomicrons. Both chylomicrons and VLDLs are hydrolyzed by the same enzymelipoprotein lipase (LPL). TGs are hydrolyzed into fatty acids for uptake by muscle and adipose tissue, whereas remnants of VLDL and chylomicrons are removed by the liver. More details on TG pathophysiology may be found in a recent review.4 When LPL is saturated with VLDL, ingestion of a fatty meal may cause chylomicrons to linger in circulation for days instead of hours. Asking the laboratory to spin down the blood of a patient with severe HTG and keep the test tube upright at 4C may reveal a large creamy supernatant layer demonstrating chylomicronemia.
A fasting TG level drawn 12 hours after the last meal reflects hepatic TG production. Although a nonfasting TG level may reflect postprandial chylomicrons, values above 1000 mg/dL strongly suggest true HTG, particularly in the setting of acute pancreatitis. Treatment should not be delayed to obtain a fasting TG level.
HTG may result from increased VLDL production, reduced VLDL/chylomicron clearance, or more likely a combination of the two. The causes of these metabolic derangements are classified as primary (genetic) or secondary (acquired) (Table 1). In adult patients, HTG is usually the result of a combination of primary and secondary causes. A study of 123 patients with TG levels >2000 mg/dL found that all patients had a primary metabolic defect and 110/123 had a coexistent secondary cause.3 An underlying genetic lipoprotein metabolism derangement is often clinically silent until coupled with a secondary cause of HTG that together raise TG levels high enough to cause the chylomicronemia syndrome.
|
| Primary |
| Familial lipid disorders |
| Lipoprotein pattern type I |
| Familial chylomicronemia |
| Deficiency in LPL and/or apo‐CII |
| Autosomal recessive; presents in childhood |
| Rare functional disorders in LPL |
| Lipoprotein pattern type III |
| Familial dysbetalipoproteinemia |
| Inadequate VLDL clearance from apo‐E2 |
| Autosomal recessive; presents in adulthood |
| Lipoprotein pattern type IV |
| Familial HTG: increased VLDL |
| Autosomal dominant; presents in adulthood |
| Familial combined hyperlipidemia |
| Multiple phenotypes seen; increased apo‐B levels |
| Lipoprotein pattern type V |
| Mixed HTG: increased VLDL and chylomicrons; presents in adulthood |
| Secondary |
| Disease |
| Poorly controlled diabetes mellitus; hypothyroid; SLE; Cushing syndrome; HIV infection; sarcoid multiple myeloma; obesity; renal disease (nephrotic) |
| Disorder of metabolism |
| Pregnancy |
| Diet |
| Excessive alcohol, especially with high‐fat diet |
| Drugs |
| Estrogen; tamoxifen; glucocorticoids; protease inhibitors; nonselective beta‐blockers; propofol; isotretinoin; some antipsychotic medications (clozapine, olanzapine); tacrolimus; sirolimus; cyclosporine; bexarotene; all‐trans retinoic acid; L‐asparaginase; interferon‐ |
The most common primary cause of HTG in adults is familial HTG, an autosomal dominant condition with a population prevalence ranging from 1%2% to 5%10% and age‐dependent penetrance.5, 6 Other genetic causes are much rarer, such as LPL deficiency (1 in 1 million patients), apolipoprotein‐CII, and other mutations resulting in impaired binding to LPL.79 Primary causes of HTG are often listed as Fredrickson phenotypes (Table 1). Recent genome‐wide association studies reveal a complex polygenic basis to the Fredrickson categories and suggest additional undefined genes or nongenetic factors may significantly contribute to the final phenotype.10 Diagnosis of familial lipid disorders requires an accurate family history that may be difficult to obtain.
Secondary causes of HTG can be categorized using a four‐D mnemonic: Diseases, Diet, Disorder of Metabolism, and Drugs.11 The most common condition associated with HTG is obesity.6 The mechanism between obesity and HTG is complex and likely involves an increase in fatty acid flux from adipose tissue to other tissues and insulin resistance.12 Asking whether a patient's current weight is close to the heaviest lifetime weight is a clue to diagnosing obesity‐driven HTG. One case series of hypertriglyceridemic pancreatitis found that diabetes or excessive alcohol intake account for the majority of secondary causes of HTG.13 The cause of HTG among patients with diabetes is multifactorial: insulin deficiency reduces LPL levels (insulin is required for synthesis of LPL), whereas insulin resistance attenuates the ability of insulin to decrease hepatic cholesterol synthesis and thus increases hepatic secretion of VLDL.14 Alcohol impairs lipolysis and increases VLDL production that can lead to severe HTG, particularly in those patients with an underlying functional deficiency in LPL. Other secondary etiologies of severe HTG may be elicited through careful attention to medical and medication history.
CLINICAL ASSESSMENT
Table 2 proposes a reasonable initial assessment of the history and physical and laboratory tests in patients with severe HTG.
|
| History |
| Family history of lipid disorders |
| Maximal weight and when achieved |
| Detailed medication history (including those recently stopped) |
| Alcohol consumption |
| Diabetes mellitus |
| Possible physical examination findings |
| Eruptive xanthomas |
| Lipemia retinalis |
| Hepatomegaly |
| Lymphadenopathy |
| Laboratory tests |
| Basic Metabolic Panel* with glucose |
| Lipid panel |
| Thyroid‐stimulating hormone, free T4 |
| Liver function, amylase, lipase |
| Hemoglobin A1c |
| Urinalysis |
Distinguishing physical examination findings may arise when serum TG levels exceed 1000 mg/dL. Eruptive xanthoma form when large amounts of TG are sequestered in cutaneous histiocytes, resulting in small yellow‐orange papules with an erythematous base. This finding is seen in a minority of HTG patients and may be missed altogether without a careful examination of the extensor surfaces of arms, legs, back, and buttocks.15 Effective TG‐lowering treatment will result in resolution of these xanthomas. An ophthalmologic examination may reveal lipemia retinalis, a condition that occurs when retinal vessels appear white from lipemic serum and contrast against a pale salmon‐colored retina. Although a dramatic finding, these changes do not result in vision impairment. Hepatomegaly from fatty infiltration of the liver occurs frequently,16 and diffuse lymphadenopathy may also be found.17
Laboratory tests are also essential in the clinical assessment of severe HTG. Important tests include thyroid‐stimulating hormone, creatinine, serum urea nitrogen, and a urinalysis. A hemoglobin A1c test provides information on the level of glycemic control and is now recognized by the American Diabetes Association to diagnose diabetes mellitus.18 Liver function tests commonly reveal a transaminase elevation from underlying steatohepatitis and also provide a baseline value prior to initiating any lipid‐lowering medications. Additional diagnostic tests may be useful in selected patients: HIV testing, serum protein electrophoresis, and urine protein electrophoresis to help diagnose paraproteinemias such as multiple myeloma, and an antinuclear antibody and double‐stranded DNA for systemic lupus erythematosus.
Severe HTG may interfere with the result of 2 commonly obtained laboratory tests. The sodium concentration can be falsely low (pseudohyponatremia) due to the high levels of TG displacing sodium containing water from the plasma.19 Due to interference by plasma lipids, amylase levels may be near normal in up to 50% of patients with hypertriglyceridemic pancreatitis at the time of admission.20 Thus, if the suspicion for pancreatitis is high, it is reasonable to proceed to imaging if amylase or lipase levels are not confirmatory. Abdominal imaging with computed tomography or magnetic resonance imaging may be used to diagnose acute pancreatitis.
Behind excessive alcohol consumption and gallstone disease, HTG is the third leading cause of pancreatitis, accounting for up to 10% of cases in the general population.21 The exact mechanism by which HTG causes pancreatitis is unclear. One theory is that elevated plasma TG levels are hydrolyzed in the pancreas to cause an increase in local free fatty acids, which in turn may cause inflammation and overt pancreatitis.22 Another theory proposes that elevated levels of chylomicrons lead to plasma hyperviscosity, which causes ischemia and local acidosis in pancreatic capillaries.23 Whatever the cause, it is unclear why only some patients with severe HTG develop acute pancreatitis. One study of 129 patients with severe HTG found mean serum TG levels to be higher in patients with acute pancreatitis than in those without (4470 versus 2450 mg/dL), suggesting the threshold to develop acute pancreatitis is higher than previously thought.24 Without a firm TG threshold above which patients develop pancreatitis, the decision to hospitalize can be difficult.
WHEN TO HOSPITALIZE?
The choice of whether to hospitalize a patient with severe HTG is first based on the presence or absence of abdominal pain and/or acute pancreatitis. Figure 1 diagrams a suggested admission and treatment algorithm. If abdominal pain is present, the patient should be hospitalized and assessed for possible triggers with prompt initiation of pharmacologic treatment. In the absence of abdominal pain, the decision to admit the patient with severe HTG requires clinical judgment. In these cases, prompt consultation with a physician experienced in the management of lipid disorders is recommended. In our experience, admission is usually driven by factors such as (1) severe hyperglycemia requiring inpatient insulin therapy; (2) severe HTG at or near a level where pancreatitis has occurred in the past in a patient for whom adherence is suspect (mindful of the great variability at the levels where patients develop pancreatitis); (3) unremitting triggers of severe HTG such as ongoing use of essential medications also known to exacerbate HTG (such as some forms of chemotherapy) or pregnancy in the third trimester. TG levels rise continuously throughout pregnancy and peak during the third trimester, when hypertriglyceridemic pancreatitis most often occurs. Asymptomatic patients with severe HTG not requiring hospitalization need close outpatient follow‐up to prevent the onset of chylomicronemia syndrome.

INPATIENT MANAGEMENT
No professional recommendations exist regarding a standardized treatment plan for severe HTG. The treatment regimen is first based on the presence or absence of symptoms. Treatment of hypertriglyceridemic pancreatitis should target a serum TG level <1000 mg/dL and resolution of abdominal pain. The initial goal for asymptomatic patients is a TG level <1000 mg/dL, as this level represents a significant reduction in the risk of developing chylomicronemia syndrome. In either case, the first 2 components of the treatment regimen are dietary changes and oral medications.
Dietary Changes
Patients with hypertriglyceridemic pancreatitis should be made NPO, with the exception of necessary medications taken only with water to provide bowel rest and eliminate fat intake. As chylomicron production in the intestine falls, TG levels will fall dramatically within 12 days of NPO status regardless of other treatments. Once TG levels approach 1000 mg/dL and there is no residual abdominal pain, a no‐fat diet can be given. Patients with persistent abdominal pain requiring a prolonged fast (>57 days) may require nutrition through alternate means such as an enteral formula through a feeding tube or the use of TPN. If a feeding tube is required, we suggest beginning with an elemental, peptide‐based, fat‐free formula, with help from a nutrition consult to assist with individual tube‐feeding options.25 Enteral formula can be supplemented with medium‐chain triglyceride oils (found in coconut and palm kernel) to provide some additional nutritional support. MCTs do not raise serum TG levels, as they are absorbed directly into the portal vein for prompt oxidation by the liver, whereas long‐chain TGs are converted into chylomicrons for peripheral transport. One case report describes a dramatic therapeutic response to medium‐chain triglyceride oils in a patient with familial chylomicronemia,26 although we do not routinely recommend these oils as therapy given lack of long‐term safety data. Lastly, if TPN is required, it is crucial to avoid lipid emulsions to prevent a rise in serum TG levels.
Asymptomatic patients with severe HTG can be fed upon admission, but should be placed on a fat‐free diet. Fat is added back into the diet when TG levels fall below 1000 mg/dL and is slowly increased to a target fat content of 10% of the total calories, usually not exceeding 25 g/day.
Oral Medications
Oral medications should be initiated to lower TG levels for both symptomatic and asymptomatic patients. Table 3 lists the different classes of medications. In our experience, oral fibrates are a recommended first‐line treatment, with other agents used as adjunctive therapy. Through complex mechanisms, fibrates reduce hepatic VLDL secretion and increase serum lipolysis of TG.27 In patients who do not have diabetes and are at low risk for coronary heart disease (CHD), either gemfibrozil or fenofibrate may be used to lower serum TG levels. However, in patients with diabetes, CHD, or a CHD risk equivalent, use of fenofibrate is preferred as an HMG‐CoA reductase inhibitor (a statin) and will almost always be necessary to reach low‐density lipoprotein (LDL)‐cholesterol goals. Fenofibrate, unlike gemfibrozil, does not interfere with the glucuronidation of statins by the liver.28
| Drug | Usual Dose | TG Reduction | Cautions or Contraindications | Comments |
|---|---|---|---|---|
| ||||
| Fenofibrate | 130200 mg/day | 50% | Hepatic or renal insufficiency | Best fibrate to use with HMG‐CoA reductase Inhibitors |
| Gemfibrozil | 600 mg BID | 50% | Hepatic or renal insufficiency | Avoid combination with statins |
| HMG‐CoA reductase inhibitors or statins | Variable; the more potent LDL lowering, the more TG lowering | 25% | Decompensated cirrhosis; end‐stage renal disease | Not the primary treatment for patients with TG levels >1000 mg/dL; some statins such as atorvastatin and fluvastatin are favored for patients with renal insufficiency due to less renal excretion than other statins |
| Omega‐3 fatty acids | 2 g BID Lovaza (840 mg DHA/EPA per dose) | 25%50% with monotherapy (the higher the TG level, the greater the reduction); 30% with combination therapy | Allergy to fish | Fishy aftertaste; may cause flatulence; may increase serum glucose and LDL; low risk of clinical bleeding |
| Nicotinic acid | 12 g/day ER; up‐titrate from lowest dose | 15%35% | Active liver disease; active peptic ulcer disease; arterial bleeding | Can increase blood sugar levels by increasing insulin resistance |
| Orlistat (Xenical) | 120 mg TID | 15%35% | Cases of serious liver dysfunction have been reported | Can interfere with drug absorption, especially fat‐soluble vitamins; oily rectal discharge |
| Insulin | IV 0.10.3 U/kg/hr; titrate to serum glucose 140180 mg/dL OR basal/bolus subcutaneously | Variable; >50% in some cases | Hypoglycemia | Useful in patients who have diabetes |
If a fibrate fails to achieve an acceptable serum TG level, we recommend adjunctive therapy with omega‐3 fatty acid esters. Omega‐3 fatty acids lower serum TG levels by decreasing VLDL production and can lower TG levels by as much as 45% in cases of severe HTG.29 This medication is typically the first adjunctive medication chosen due to its low side effect profile.
If additional TG lowering is needed, niacin or nicotinic acid (vitamin B3) may be added next. This medication decreases VLDL production, lowers LDL, and increases high‐density lipoprotein, but invariably with initiation, patients exhibit prominent skin flushing, burning, or itching. This prostaglandin‐mediated effect may be prevented or at least reduced in severity by taking 325 mg aspirin 1 hour before niacin administration. If the TG level is still not at goal, orlistat, a lipase or fat blocker, may be useful.30 Orlistat improves postprandial lipemia through reduction of dietary fat absorption. Finally, although potent statins such as atorvastatin and rosuvastatin can lower TGs derived from VLDL substantially,31 their use should be prompted by the patient's risk for atherosclerotic cardiovascular disease. Because all of the hypotriglyceridemic medications can affect the liver, regular liver function testing is prudent, as is a periodic re‐evaluation of the ongoing need for these medications. Statin use causing mild transaminase elevation (up to 3 times the upper limit of normal) may be safely tolerated.32
Additional Inpatient Management
Insulin
Insulin increases lipoprotein lipase activity, thereby accelerating chylomicron degradation.33 Insulin (along with glucose if necessary to maintain euglycemia) is therefore a useful adjunctive TG‐lowering medication to oral medications, even in nondiabetic patients. Insulin administered intravenously should follow a titration protocol with hourly monitoring of blood glucose. The goal of the insulin protocol with severe HTG is not maintaining strict euglycemia but rather maintenance of LPL activation by exogenous insulin with avoidance of hypoglycemia. In hospitals where an insulin infusion protocol for diabetic ketoacidosis or postsurgical hyperglycemia already exists, the protocol can be applied for HTG management with minor modifications: introduce dextrose‐containing fluids at higher blood glucoses (180 mg/dL or less) and eliminate insulin boluses. A suggested dose is a continuous intravenous insulin drip at 0.10.3 U/kg/hr with glucose to maintain blood glucose levels between 140 and 180 mg/dL, although there are no guidelines from professional societies. Subcutaneous insulin has also been used to successfully lower TG levels.34, 35 The major limitation of subcutaneous administration is the inability to rapidly adjust the dosing when needed, which is particularly concerning when treating patients who do not have diabetes. We prefer to use subcutaneous basal insulin in patients requiring long‐term use of insulin after a significant TG reduction with intravenous insulin. Subcutaneous bolus prandial insulin should not be used until the patient has resumed a solid diet, because a liquid diet may not reliably contain enough carbohydrates for bolus therapy.
Intravenous Heparin
Heparin has been used in case reports as adjunctive treatment for hypertriglyceridemic pancreatitis.36, 37 Although heparin may increase circulating LPL levels, this effect is short‐lived and is quickly followed by increased hepatic LPL degradation.38 Therefore, the use of heparin to treat severe HTG cannot be routinely recommended.
Therapeutic Plasma Exchange
First used in 1978, therapeutic plasma exchange (TPE) has been demonstrated to quickly and dramatically lower serum TG levels.39 Since its first use, TPE has been used in several small case studies.4042 Without data from larger studies, the optimal frequency and duration of TPE remains unclear. One review suggests the use of TPE as first‐line therapy provided the patient is euglycemic, apheresis can be started within 48 hours of diagnosis, and the patient can tolerate the central venous access.43 On the other hand, guidelines from the American Society of Apheresis incorporating low‐quality evidence do not recommend TPE as routine first‐ or second‐line treatment for hypertriglyceridemic pancreatitis, but rather suggest the use of TPE on a case‐by‐case basis.44 When TPE is needed, this society recommends daily treatment for 13 days until an adequate postapheresis TG level is obtained. Although TPE rapidly lowers TG levels, it is also aggressive (requires placement of a pheresis catheter), expensive, and may not be readily available. We believe it remains an option for patients with severe HTG who do not respond readily to fat restriction, glycemic control with insulin, and pharmacologic treatment with a fibrate and omega‐3 fatty acids. In our judgment, routine use of TPE cannot be recommended without data from a randomized clinical trial examining the value of immediate lowering of TG levels with TPE versus the usually prompt fall in TG levels with less aggressive measures.
Discharge Planning
Typically, asymptomatic patients are discharged once their TG levels approach 1000 mg/dL. Patients recovering from hypertriglyceridemic pancreatitis may be discharged once they tolerate a no‐fat diet without recurrence of abdominal pain and without a significant TG increase above 1000 mg/dL.
Discharge Diet and Activity
At discharge, the diet should be high in fiber, fruits, vegetables, and lean protein, with fat intake restricted to approximately 10% of total calories. Insulin‐resistant patients and patients with diabetes should avoid sugar‐sweetened foods and drinks. Specifically, the daily amount of fructose intake should be no more than 50 mg to avoid a dose‐dependent increase in plasma TG levels compared with other sugars.4 At least 2 servings per week of marine foods naturally rich in omega‐3 fatty acids (fatty fish such as salmon or trout) are recommended. Nonmarine forms of omega‐3 fatty acids (walnuts, flaxseed), which have not demonstrated consistent reductions in TG, cannot be routinely recommended.4 In addition, alcohol consumption should be eliminated.
Once patients maintain a TG level near 500 mg/dL, we allow for dietary flexibility by slowly increasing the amount of dietary unsaturated fat. For patients with TG levels <500 mg/dL, Adult Treatment Panel III advocated restriction of daily dietary saturated fat levels to <7% and keeping the total fat level between 25% and 35%.2 A range in total fat was provided so that unsaturated fat could be increased to limit dietary carbohydrates if glycemic control was needed. In addition to dietary changes, counseling patients about the importance of physical activity and weight loss is crucial for long‐term management of severe HTG. TG lowering in response to diet and weight loss varies, but typically approximates 25%.45
Outpatient Medications
Patients without significant contraindications should be discharged on a fibrate and omega‐3 fatty acids. As mentioned, niacin, orlistat, and/or a statin may be used as adjunctive therapy. Despite use of these hypotriglyceridemic medications, secondary causes of HTG should be modified (such as removal of aggravating medications or appropriately treating uncontrolled diabetes) to yield lasting improvements in TG levels.
CONCLUSIONS
As the prevalence of obesity and diabetes continues to rise, so too does the clinical importance of proper management of severe HTG. Recognizing chylomicronemia syndromeone of the most dramatic consequences of lipid disordersand the underlying primary and secondary causes of HTG is required before starting treatment. Patients with severe HTG may require hospitalization for immediate reduction in TG levels and relief of abdominal pain, if present. Treatment involves modifying secondary causes, if possible, and eliminating dietary fat intake. Although use of medications such as an oral fibrate, omega‐3 fatty acids, and insulin are routine, the use of a more invasive procedure such as TPE should be considered on a case‐by‐case basis and may be limited by availability. Upon hospital discharge, careful follow‐up should promote lifestyle changes and medication adherence to prevent recurrence of severe HTG.
The patient with a markedly high serum triglyceride (TG) level poses an interesting challenge for hospitalists. Hypertriglyceridemia (HTG) is defined as a fasting plasma TG level that is above the 95th percentile for age and sex.1 TG levels are commonly classified into categories according to Adult Treatment Panel III guidelines with desirable levels <150 mg/dL (1.7 mmol/L), borderline levels 150199, high levels 200499 mg/dL, and very high levels >500 mg/dL (5.6 mmol/L).2 A TG level exceeding an arbitrary threshold of >1000 mg/dL (11.3 mmol/L) is referred to as severe HTG. The Lipid Research Clinics Program Prevalence Study found that 1.79 per 10,000 outpatients (<0.02%) had TG levels > 2000 mg/dL.3 Chylomicronemia syndrome occurs when severe HTG is accompanied by 1 or more of the following: symptoms of abdominal pain or acute pancreatitis or physical examination findings such as eruptive xanthomas or lipemia retinalis. There is no TG level above which pancreatitis invariably occurs, making the decision to hospitalize difficult. The goal of this review is to discuss the causes of severe HTG; the clinical assessment, including criteria for hospitalization; and the available treatment options for this infrequent but serious condition. We begin with a clinical case of severe HTG.
CASE PRESENTATION
A 47‐year‐old woman with a history of chronic myelogenous leukemia was admitted to the hospital with a serum triglyceride level of 17,393 mg/dL. Two years prior to admission, she underwent allogenic stem cell transplantation for chronic myelogenous leukemia and has since remained in remission. Six months prior to admission, severe diarrhea from intestinal graft‐versus‐host disease required the use of total parenteral nutrition (TPN) and immunosuppressive therapy consisting of prednisone 20 mg/day, mycophenolate mofetil 250 mg thrice daily, and sirolimus 0.3 mg/day. During treatment with steroids, she developed diabetes mellitus requiring insulin, with a subsequent hemoglobin A1c level of 7.7% (normal, <7%). The serum TG level prior to transplantation was unknown but was 343 mg/dL prior to TPN initiation. One month prior to admission, the diarrhea resolved and TPN was stopped. The TG level was 7463 mg/dL 1 week prior to admission, and despite use of fenofibrate, it rose to 17,393 mg/dL. The patient denied abdominal pain, and did not have abdominal tenderness or eruptive xanthomas. She denied a family history of dyslipidemia or recent medication changes. Given the extreme TG elevation, the lack of response to outpatient treatment and the concern for developing acute pancreatitis, the patient was admitted to the hospital for inpatient TG‐lowering treatment.
Upon admission, serum lipase and amylase were within normal limits, but the blood glucose level was 243 mg/dL. Insulin infusion and oral fenofibrate 145 mg/day was started, and the patient was kept non per os (NPO). Six hours later, despite insulin infusion, the TG level rose to 26,250 mg/dL. Therapeutic plasma exchange (TPE) was performed on 2 consecutive days with a resultant decrease in TG level to 530 mg/dL. The patient was later discharged home on fenofibrate and omega‐3 ethyl esters, her same immunosuppressive and insulin regimen, and instructions for a very low‐fat diet. In the next 3 months, her serum TG level did not rise above 530 mg/dL. The cause of our patient's extreme TG elevation was likely a combination of genetic factors exacerbated by immunosuppressive and glucocorticoid therapy.
This case featured dramatic elevations in serum TG levels that the managing doctors believed merited a hospital admission. Management of patients with severe HTG first requires an understanding of TG metabolism.
ETIOLOGY
Serum TGs produced by the liver are carried by very low‐density lipoproteins (VLDLs), whereas TGs derived from dietary fat are carried by chylomicrons. Both chylomicrons and VLDLs are hydrolyzed by the same enzymelipoprotein lipase (LPL). TGs are hydrolyzed into fatty acids for uptake by muscle and adipose tissue, whereas remnants of VLDL and chylomicrons are removed by the liver. More details on TG pathophysiology may be found in a recent review.4 When LPL is saturated with VLDL, ingestion of a fatty meal may cause chylomicrons to linger in circulation for days instead of hours. Asking the laboratory to spin down the blood of a patient with severe HTG and keep the test tube upright at 4C may reveal a large creamy supernatant layer demonstrating chylomicronemia.
A fasting TG level drawn 12 hours after the last meal reflects hepatic TG production. Although a nonfasting TG level may reflect postprandial chylomicrons, values above 1000 mg/dL strongly suggest true HTG, particularly in the setting of acute pancreatitis. Treatment should not be delayed to obtain a fasting TG level.
HTG may result from increased VLDL production, reduced VLDL/chylomicron clearance, or more likely a combination of the two. The causes of these metabolic derangements are classified as primary (genetic) or secondary (acquired) (Table 1). In adult patients, HTG is usually the result of a combination of primary and secondary causes. A study of 123 patients with TG levels >2000 mg/dL found that all patients had a primary metabolic defect and 110/123 had a coexistent secondary cause.3 An underlying genetic lipoprotein metabolism derangement is often clinically silent until coupled with a secondary cause of HTG that together raise TG levels high enough to cause the chylomicronemia syndrome.
|
| Primary |
| Familial lipid disorders |
| Lipoprotein pattern type I |
| Familial chylomicronemia |
| Deficiency in LPL and/or apo‐CII |
| Autosomal recessive; presents in childhood |
| Rare functional disorders in LPL |
| Lipoprotein pattern type III |
| Familial dysbetalipoproteinemia |
| Inadequate VLDL clearance from apo‐E2 |
| Autosomal recessive; presents in adulthood |
| Lipoprotein pattern type IV |
| Familial HTG: increased VLDL |
| Autosomal dominant; presents in adulthood |
| Familial combined hyperlipidemia |
| Multiple phenotypes seen; increased apo‐B levels |
| Lipoprotein pattern type V |
| Mixed HTG: increased VLDL and chylomicrons; presents in adulthood |
| Secondary |
| Disease |
| Poorly controlled diabetes mellitus; hypothyroid; SLE; Cushing syndrome; HIV infection; sarcoid multiple myeloma; obesity; renal disease (nephrotic) |
| Disorder of metabolism |
| Pregnancy |
| Diet |
| Excessive alcohol, especially with high‐fat diet |
| Drugs |
| Estrogen; tamoxifen; glucocorticoids; protease inhibitors; nonselective beta‐blockers; propofol; isotretinoin; some antipsychotic medications (clozapine, olanzapine); tacrolimus; sirolimus; cyclosporine; bexarotene; all‐trans retinoic acid; L‐asparaginase; interferon‐ |
The most common primary cause of HTG in adults is familial HTG, an autosomal dominant condition with a population prevalence ranging from 1%2% to 5%10% and age‐dependent penetrance.5, 6 Other genetic causes are much rarer, such as LPL deficiency (1 in 1 million patients), apolipoprotein‐CII, and other mutations resulting in impaired binding to LPL.79 Primary causes of HTG are often listed as Fredrickson phenotypes (Table 1). Recent genome‐wide association studies reveal a complex polygenic basis to the Fredrickson categories and suggest additional undefined genes or nongenetic factors may significantly contribute to the final phenotype.10 Diagnosis of familial lipid disorders requires an accurate family history that may be difficult to obtain.
Secondary causes of HTG can be categorized using a four‐D mnemonic: Diseases, Diet, Disorder of Metabolism, and Drugs.11 The most common condition associated with HTG is obesity.6 The mechanism between obesity and HTG is complex and likely involves an increase in fatty acid flux from adipose tissue to other tissues and insulin resistance.12 Asking whether a patient's current weight is close to the heaviest lifetime weight is a clue to diagnosing obesity‐driven HTG. One case series of hypertriglyceridemic pancreatitis found that diabetes or excessive alcohol intake account for the majority of secondary causes of HTG.13 The cause of HTG among patients with diabetes is multifactorial: insulin deficiency reduces LPL levels (insulin is required for synthesis of LPL), whereas insulin resistance attenuates the ability of insulin to decrease hepatic cholesterol synthesis and thus increases hepatic secretion of VLDL.14 Alcohol impairs lipolysis and increases VLDL production that can lead to severe HTG, particularly in those patients with an underlying functional deficiency in LPL. Other secondary etiologies of severe HTG may be elicited through careful attention to medical and medication history.
CLINICAL ASSESSMENT
Table 2 proposes a reasonable initial assessment of the history and physical and laboratory tests in patients with severe HTG.
|
| History |
| Family history of lipid disorders |
| Maximal weight and when achieved |
| Detailed medication history (including those recently stopped) |
| Alcohol consumption |
| Diabetes mellitus |
| Possible physical examination findings |
| Eruptive xanthomas |
| Lipemia retinalis |
| Hepatomegaly |
| Lymphadenopathy |
| Laboratory tests |
| Basic Metabolic Panel* with glucose |
| Lipid panel |
| Thyroid‐stimulating hormone, free T4 |
| Liver function, amylase, lipase |
| Hemoglobin A1c |
| Urinalysis |
Distinguishing physical examination findings may arise when serum TG levels exceed 1000 mg/dL. Eruptive xanthoma form when large amounts of TG are sequestered in cutaneous histiocytes, resulting in small yellow‐orange papules with an erythematous base. This finding is seen in a minority of HTG patients and may be missed altogether without a careful examination of the extensor surfaces of arms, legs, back, and buttocks.15 Effective TG‐lowering treatment will result in resolution of these xanthomas. An ophthalmologic examination may reveal lipemia retinalis, a condition that occurs when retinal vessels appear white from lipemic serum and contrast against a pale salmon‐colored retina. Although a dramatic finding, these changes do not result in vision impairment. Hepatomegaly from fatty infiltration of the liver occurs frequently,16 and diffuse lymphadenopathy may also be found.17
Laboratory tests are also essential in the clinical assessment of severe HTG. Important tests include thyroid‐stimulating hormone, creatinine, serum urea nitrogen, and a urinalysis. A hemoglobin A1c test provides information on the level of glycemic control and is now recognized by the American Diabetes Association to diagnose diabetes mellitus.18 Liver function tests commonly reveal a transaminase elevation from underlying steatohepatitis and also provide a baseline value prior to initiating any lipid‐lowering medications. Additional diagnostic tests may be useful in selected patients: HIV testing, serum protein electrophoresis, and urine protein electrophoresis to help diagnose paraproteinemias such as multiple myeloma, and an antinuclear antibody and double‐stranded DNA for systemic lupus erythematosus.
Severe HTG may interfere with the result of 2 commonly obtained laboratory tests. The sodium concentration can be falsely low (pseudohyponatremia) due to the high levels of TG displacing sodium containing water from the plasma.19 Due to interference by plasma lipids, amylase levels may be near normal in up to 50% of patients with hypertriglyceridemic pancreatitis at the time of admission.20 Thus, if the suspicion for pancreatitis is high, it is reasonable to proceed to imaging if amylase or lipase levels are not confirmatory. Abdominal imaging with computed tomography or magnetic resonance imaging may be used to diagnose acute pancreatitis.
Behind excessive alcohol consumption and gallstone disease, HTG is the third leading cause of pancreatitis, accounting for up to 10% of cases in the general population.21 The exact mechanism by which HTG causes pancreatitis is unclear. One theory is that elevated plasma TG levels are hydrolyzed in the pancreas to cause an increase in local free fatty acids, which in turn may cause inflammation and overt pancreatitis.22 Another theory proposes that elevated levels of chylomicrons lead to plasma hyperviscosity, which causes ischemia and local acidosis in pancreatic capillaries.23 Whatever the cause, it is unclear why only some patients with severe HTG develop acute pancreatitis. One study of 129 patients with severe HTG found mean serum TG levels to be higher in patients with acute pancreatitis than in those without (4470 versus 2450 mg/dL), suggesting the threshold to develop acute pancreatitis is higher than previously thought.24 Without a firm TG threshold above which patients develop pancreatitis, the decision to hospitalize can be difficult.
WHEN TO HOSPITALIZE?
The choice of whether to hospitalize a patient with severe HTG is first based on the presence or absence of abdominal pain and/or acute pancreatitis. Figure 1 diagrams a suggested admission and treatment algorithm. If abdominal pain is present, the patient should be hospitalized and assessed for possible triggers with prompt initiation of pharmacologic treatment. In the absence of abdominal pain, the decision to admit the patient with severe HTG requires clinical judgment. In these cases, prompt consultation with a physician experienced in the management of lipid disorders is recommended. In our experience, admission is usually driven by factors such as (1) severe hyperglycemia requiring inpatient insulin therapy; (2) severe HTG at or near a level where pancreatitis has occurred in the past in a patient for whom adherence is suspect (mindful of the great variability at the levels where patients develop pancreatitis); (3) unremitting triggers of severe HTG such as ongoing use of essential medications also known to exacerbate HTG (such as some forms of chemotherapy) or pregnancy in the third trimester. TG levels rise continuously throughout pregnancy and peak during the third trimester, when hypertriglyceridemic pancreatitis most often occurs. Asymptomatic patients with severe HTG not requiring hospitalization need close outpatient follow‐up to prevent the onset of chylomicronemia syndrome.

INPATIENT MANAGEMENT
No professional recommendations exist regarding a standardized treatment plan for severe HTG. The treatment regimen is first based on the presence or absence of symptoms. Treatment of hypertriglyceridemic pancreatitis should target a serum TG level <1000 mg/dL and resolution of abdominal pain. The initial goal for asymptomatic patients is a TG level <1000 mg/dL, as this level represents a significant reduction in the risk of developing chylomicronemia syndrome. In either case, the first 2 components of the treatment regimen are dietary changes and oral medications.
Dietary Changes
Patients with hypertriglyceridemic pancreatitis should be made NPO, with the exception of necessary medications taken only with water to provide bowel rest and eliminate fat intake. As chylomicron production in the intestine falls, TG levels will fall dramatically within 12 days of NPO status regardless of other treatments. Once TG levels approach 1000 mg/dL and there is no residual abdominal pain, a no‐fat diet can be given. Patients with persistent abdominal pain requiring a prolonged fast (>57 days) may require nutrition through alternate means such as an enteral formula through a feeding tube or the use of TPN. If a feeding tube is required, we suggest beginning with an elemental, peptide‐based, fat‐free formula, with help from a nutrition consult to assist with individual tube‐feeding options.25 Enteral formula can be supplemented with medium‐chain triglyceride oils (found in coconut and palm kernel) to provide some additional nutritional support. MCTs do not raise serum TG levels, as they are absorbed directly into the portal vein for prompt oxidation by the liver, whereas long‐chain TGs are converted into chylomicrons for peripheral transport. One case report describes a dramatic therapeutic response to medium‐chain triglyceride oils in a patient with familial chylomicronemia,26 although we do not routinely recommend these oils as therapy given lack of long‐term safety data. Lastly, if TPN is required, it is crucial to avoid lipid emulsions to prevent a rise in serum TG levels.
Asymptomatic patients with severe HTG can be fed upon admission, but should be placed on a fat‐free diet. Fat is added back into the diet when TG levels fall below 1000 mg/dL and is slowly increased to a target fat content of 10% of the total calories, usually not exceeding 25 g/day.
Oral Medications
Oral medications should be initiated to lower TG levels for both symptomatic and asymptomatic patients. Table 3 lists the different classes of medications. In our experience, oral fibrates are a recommended first‐line treatment, with other agents used as adjunctive therapy. Through complex mechanisms, fibrates reduce hepatic VLDL secretion and increase serum lipolysis of TG.27 In patients who do not have diabetes and are at low risk for coronary heart disease (CHD), either gemfibrozil or fenofibrate may be used to lower serum TG levels. However, in patients with diabetes, CHD, or a CHD risk equivalent, use of fenofibrate is preferred as an HMG‐CoA reductase inhibitor (a statin) and will almost always be necessary to reach low‐density lipoprotein (LDL)‐cholesterol goals. Fenofibrate, unlike gemfibrozil, does not interfere with the glucuronidation of statins by the liver.28
| Drug | Usual Dose | TG Reduction | Cautions or Contraindications | Comments |
|---|---|---|---|---|
| ||||
| Fenofibrate | 130200 mg/day | 50% | Hepatic or renal insufficiency | Best fibrate to use with HMG‐CoA reductase Inhibitors |
| Gemfibrozil | 600 mg BID | 50% | Hepatic or renal insufficiency | Avoid combination with statins |
| HMG‐CoA reductase inhibitors or statins | Variable; the more potent LDL lowering, the more TG lowering | 25% | Decompensated cirrhosis; end‐stage renal disease | Not the primary treatment for patients with TG levels >1000 mg/dL; some statins such as atorvastatin and fluvastatin are favored for patients with renal insufficiency due to less renal excretion than other statins |
| Omega‐3 fatty acids | 2 g BID Lovaza (840 mg DHA/EPA per dose) | 25%50% with monotherapy (the higher the TG level, the greater the reduction); 30% with combination therapy | Allergy to fish | Fishy aftertaste; may cause flatulence; may increase serum glucose and LDL; low risk of clinical bleeding |
| Nicotinic acid | 12 g/day ER; up‐titrate from lowest dose | 15%35% | Active liver disease; active peptic ulcer disease; arterial bleeding | Can increase blood sugar levels by increasing insulin resistance |
| Orlistat (Xenical) | 120 mg TID | 15%35% | Cases of serious liver dysfunction have been reported | Can interfere with drug absorption, especially fat‐soluble vitamins; oily rectal discharge |
| Insulin | IV 0.10.3 U/kg/hr; titrate to serum glucose 140180 mg/dL OR basal/bolus subcutaneously | Variable; >50% in some cases | Hypoglycemia | Useful in patients who have diabetes |
If a fibrate fails to achieve an acceptable serum TG level, we recommend adjunctive therapy with omega‐3 fatty acid esters. Omega‐3 fatty acids lower serum TG levels by decreasing VLDL production and can lower TG levels by as much as 45% in cases of severe HTG.29 This medication is typically the first adjunctive medication chosen due to its low side effect profile.
If additional TG lowering is needed, niacin or nicotinic acid (vitamin B3) may be added next. This medication decreases VLDL production, lowers LDL, and increases high‐density lipoprotein, but invariably with initiation, patients exhibit prominent skin flushing, burning, or itching. This prostaglandin‐mediated effect may be prevented or at least reduced in severity by taking 325 mg aspirin 1 hour before niacin administration. If the TG level is still not at goal, orlistat, a lipase or fat blocker, may be useful.30 Orlistat improves postprandial lipemia through reduction of dietary fat absorption. Finally, although potent statins such as atorvastatin and rosuvastatin can lower TGs derived from VLDL substantially,31 their use should be prompted by the patient's risk for atherosclerotic cardiovascular disease. Because all of the hypotriglyceridemic medications can affect the liver, regular liver function testing is prudent, as is a periodic re‐evaluation of the ongoing need for these medications. Statin use causing mild transaminase elevation (up to 3 times the upper limit of normal) may be safely tolerated.32
Additional Inpatient Management
Insulin
Insulin increases lipoprotein lipase activity, thereby accelerating chylomicron degradation.33 Insulin (along with glucose if necessary to maintain euglycemia) is therefore a useful adjunctive TG‐lowering medication to oral medications, even in nondiabetic patients. Insulin administered intravenously should follow a titration protocol with hourly monitoring of blood glucose. The goal of the insulin protocol with severe HTG is not maintaining strict euglycemia but rather maintenance of LPL activation by exogenous insulin with avoidance of hypoglycemia. In hospitals where an insulin infusion protocol for diabetic ketoacidosis or postsurgical hyperglycemia already exists, the protocol can be applied for HTG management with minor modifications: introduce dextrose‐containing fluids at higher blood glucoses (180 mg/dL or less) and eliminate insulin boluses. A suggested dose is a continuous intravenous insulin drip at 0.10.3 U/kg/hr with glucose to maintain blood glucose levels between 140 and 180 mg/dL, although there are no guidelines from professional societies. Subcutaneous insulin has also been used to successfully lower TG levels.34, 35 The major limitation of subcutaneous administration is the inability to rapidly adjust the dosing when needed, which is particularly concerning when treating patients who do not have diabetes. We prefer to use subcutaneous basal insulin in patients requiring long‐term use of insulin after a significant TG reduction with intravenous insulin. Subcutaneous bolus prandial insulin should not be used until the patient has resumed a solid diet, because a liquid diet may not reliably contain enough carbohydrates for bolus therapy.
Intravenous Heparin
Heparin has been used in case reports as adjunctive treatment for hypertriglyceridemic pancreatitis.36, 37 Although heparin may increase circulating LPL levels, this effect is short‐lived and is quickly followed by increased hepatic LPL degradation.38 Therefore, the use of heparin to treat severe HTG cannot be routinely recommended.
Therapeutic Plasma Exchange
First used in 1978, therapeutic plasma exchange (TPE) has been demonstrated to quickly and dramatically lower serum TG levels.39 Since its first use, TPE has been used in several small case studies.4042 Without data from larger studies, the optimal frequency and duration of TPE remains unclear. One review suggests the use of TPE as first‐line therapy provided the patient is euglycemic, apheresis can be started within 48 hours of diagnosis, and the patient can tolerate the central venous access.43 On the other hand, guidelines from the American Society of Apheresis incorporating low‐quality evidence do not recommend TPE as routine first‐ or second‐line treatment for hypertriglyceridemic pancreatitis, but rather suggest the use of TPE on a case‐by‐case basis.44 When TPE is needed, this society recommends daily treatment for 13 days until an adequate postapheresis TG level is obtained. Although TPE rapidly lowers TG levels, it is also aggressive (requires placement of a pheresis catheter), expensive, and may not be readily available. We believe it remains an option for patients with severe HTG who do not respond readily to fat restriction, glycemic control with insulin, and pharmacologic treatment with a fibrate and omega‐3 fatty acids. In our judgment, routine use of TPE cannot be recommended without data from a randomized clinical trial examining the value of immediate lowering of TG levels with TPE versus the usually prompt fall in TG levels with less aggressive measures.
Discharge Planning
Typically, asymptomatic patients are discharged once their TG levels approach 1000 mg/dL. Patients recovering from hypertriglyceridemic pancreatitis may be discharged once they tolerate a no‐fat diet without recurrence of abdominal pain and without a significant TG increase above 1000 mg/dL.
Discharge Diet and Activity
At discharge, the diet should be high in fiber, fruits, vegetables, and lean protein, with fat intake restricted to approximately 10% of total calories. Insulin‐resistant patients and patients with diabetes should avoid sugar‐sweetened foods and drinks. Specifically, the daily amount of fructose intake should be no more than 50 mg to avoid a dose‐dependent increase in plasma TG levels compared with other sugars.4 At least 2 servings per week of marine foods naturally rich in omega‐3 fatty acids (fatty fish such as salmon or trout) are recommended. Nonmarine forms of omega‐3 fatty acids (walnuts, flaxseed), which have not demonstrated consistent reductions in TG, cannot be routinely recommended.4 In addition, alcohol consumption should be eliminated.
Once patients maintain a TG level near 500 mg/dL, we allow for dietary flexibility by slowly increasing the amount of dietary unsaturated fat. For patients with TG levels <500 mg/dL, Adult Treatment Panel III advocated restriction of daily dietary saturated fat levels to <7% and keeping the total fat level between 25% and 35%.2 A range in total fat was provided so that unsaturated fat could be increased to limit dietary carbohydrates if glycemic control was needed. In addition to dietary changes, counseling patients about the importance of physical activity and weight loss is crucial for long‐term management of severe HTG. TG lowering in response to diet and weight loss varies, but typically approximates 25%.45
Outpatient Medications
Patients without significant contraindications should be discharged on a fibrate and omega‐3 fatty acids. As mentioned, niacin, orlistat, and/or a statin may be used as adjunctive therapy. Despite use of these hypotriglyceridemic medications, secondary causes of HTG should be modified (such as removal of aggravating medications or appropriately treating uncontrolled diabetes) to yield lasting improvements in TG levels.
CONCLUSIONS
As the prevalence of obesity and diabetes continues to rise, so too does the clinical importance of proper management of severe HTG. Recognizing chylomicronemia syndromeone of the most dramatic consequences of lipid disordersand the underlying primary and secondary causes of HTG is required before starting treatment. Patients with severe HTG may require hospitalization for immediate reduction in TG levels and relief of abdominal pain, if present. Treatment involves modifying secondary causes, if possible, and eliminating dietary fat intake. Although use of medications such as an oral fibrate, omega‐3 fatty acids, and insulin are routine, the use of a more invasive procedure such as TPE should be considered on a case‐by‐case basis and may be limited by availability. Upon hospital discharge, careful follow‐up should promote lifestyle changes and medication adherence to prevent recurrence of severe HTG.
- ,,,.Pathophysiology of triglyceride‐rich lipoproteins in atherothrombosis: clinical aspects.Clin Cardiol.1999;22:II15–II20.
- Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III).JAMA.2001;285:2486–2497.
- ,.Chylomicronemia syndrome. Interaction of genetic and acquired hypertriglyceridemia.Med Clin North Am.1982;66:455–468.
- ,,, et al.Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association.Circulation.2011;123:2292–2333.
- .Familial lipoprotein lipase deficiency and other causes of the chylomicronemia syndrome. In: Scriver C, Beaudet A, Sly W, Valle D, eds.The Metabolic and Molecular Basis of Inherited Disease.7th ed.New York:McGraw‐Hill;1995:1913–1932.
- ,,.Hypertriglyceridemia: its etiology, effects and treatment.CMAJ.2007;176:1113–1120.
- ,,, et al.Chylomicronemia with a mutant GPIHBP1 (Q115P) that cannot bind lipoprotein lipase.Arterioscler Thromb Vasc Biol.2009;29:956–962.
- ,,, et al.A mutation in the human lipoprotein lipase gene as the most common cause of familial chylomicronemia in French Canadians.N Engl J Med.1991;324:1761–1766.
- ,,, et al.Inherited apolipoprotein A‐V deficiency in severe hypertriglyceridemia.Arterioscler Thromb Vasc Biol.2005;25:411–417.
- ,,, et al.A polygenic basis for four classical Fredrickson hyperlipoproteinemia phenotypes that are characterized by hypertriglyceridemia.Hum Mol Genet.2009;18:4189–4194.
- .Secondary causes of hyperlipidemia.Med Clin North Am.1994;78:117–141.
- ,,.Hypertriglyceridemic hyperapob: the unappreciated atherogenic dyslipoproteinemia in type 2 diabetes mellitus.Ann Intern Med.2001;135:447–459.
- .Hyperlipidemic pancreatitis.Gastroenterol Clin North Am.1990;19:783–791.
- ,,, et al.Effects of insulin on cholesterol synthesis in type II diabetes patients.Diabetes Care.1995;18:1362–1369.
- ,,,.Evidence for the chylomicron origin of lipids accumulating in diabetic eruptive xanthomas: a correlative lipid biochemical, histochemical, and electron microscopic study.J Clin Invest.1970;49:2172–2187.
- .Dyslipidaemia.Lancet.2003;362:717–731.
- ,,.Lymphadenopathy associated with severe hypertriglyceridemia.JAMA.1990;264:727–728.
- Diagnosis and classification of diabetes mellitus.Diabetes Care.2010;33(suppl 1):S62–S69.
- ,.Pseudohyponatremia in acute hyperlipemic pancreatitis. A potential pitfall in therapy.Arch Surg.1985;120:1053–1055.
- ,,.Suppression of amylase activity by hypertriglyceridemia.JAMA.1973;225:1331–1334.
- ,,,.Dyslipidaemic pancreatitis clinical assessment and analysis of disease severity and outcomes.Pancreatology.2009;9:252–257.
- .Pathogenesis, differentiation and management of hypertriglyceridemia.Adv Intern Med.1969;15:117–154.
- ,.Role of hypertriglyceridemia in the pathogenesis of experimental acute pancreatitis in rats.Int J Pancreatol.1996;20:177–184.
- ,,, et al.Acute pancreatitis in a cohort of 129 patients referred for severe hypertriglyceridemia.Pancreas.2008;37:13–12.
- ,,, et al.ESPEN Guidelines on Enteral Nutrition: pancreas.Clin Nutr.2006;25:275–284.
- ,,, et al.Therapeutic response to medium‐chain triglycerides and omega‐3 fatty acids in a patient with the familial chylomicronemia syndrome.Arterioscler Thromb Vasc Biol.1997;17:1400–1406.
- ,,,,,.Mechanism of action of fibrates on lipid and lipoprotein metabolism.Circulation.1998;98:2088–2093.
- ,,.Drug interactions with lipid‐lowering drugs: mechanisms and clinical relevance.Clin Pharmacol Ther.2006;80:565–81.
- ,,, et al.Safety and efficacy of Omacor in severe hypertriglyceridemia.J Cardiovasc Risk.1997;4:385–391.
- ,,.Usefulness of Orlistat in the treatment of severe hypertriglyceridemia.Am J Cardiol.2002;89:229–231.
- ,,, et al.Effects of intensive atorvastatin and rosuvastatin treatment on apolipoprotein B‐48 and remnant lipoprotein cholesterol levels.Atherosclerosis.2009;205:197–201.
- ,,,.Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force.Am J Cardiol.2006;97:89C–94C.
- .Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases.N Engl J Med.1989;320:1060–1068.
- ,,.Insulin therapy for a non‐diabetic patient with severe hypertriglyceridemia.J Am Coll Nutr.1998;17:458–461.
- ,,,,.Treatment of severe hypertriglyceridemia in nondiabetic patients with insulin.Am J Emerg Med.2005;23:415–417.
- ,.Decreasing the plasma triglyceride level in hypertriglyceridemia‐induced pancreatitis in pregnancy: a case report.Am J Obstet Gynecol.2002;187:241–242.
- ,,,,,.Pancreatitis may occur with a normal amylase concentration in hypertriglyceridaemia.BMJ.1996;313:1265.
- ,,,.Lipoprotein lipase during continuous heparin infusion: tissue stores become partially depleted.J Lab Clin Med.2001;138:206–13.
- ,,,,,.Treatment of severe diabetic hypertriglyceridaemia by plasma exchange.Lancet.1978;1:1368.
- ,,,.Therapeutic plasma exchange in patients with hyperlipidemic pancreatitis.World J Gastroenterol.2004;10:2272–2274.
- ,,.Plasma exchange in severe hypertriglyceridemia a clinical study.Transfus Apher Sci.2006;34:253–257.
- ,,, et al.Management of acute severe hyperlipidemic pancreatitis.Digestion.2006;73:259–264.
- ,,,,.Hypertriglyceridemic pancreatitis: presentation and management.Am J Gastroenterol.2009;104:984–991.
- ,,, et al.Guidelines on the use of therapeutic apheresis in clinical practice—evidence‐based approach from the Apheresis Applications Committee of the American Society for Apheresis.J Clin Apher.2010;25:83–177.
- ,,,,,.Effects of a low‐fat diet compared with those of a high‐monounsaturated fat diet on body weight, plasma lipids and lipoproteins, and glycemic control in type 2 diabetes.Am J Clin Nutr.2004;80:668–673.
- ,,,.Pathophysiology of triglyceride‐rich lipoproteins in atherothrombosis: clinical aspects.Clin Cardiol.1999;22:II15–II20.
- Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III).JAMA.2001;285:2486–2497.
- ,.Chylomicronemia syndrome. Interaction of genetic and acquired hypertriglyceridemia.Med Clin North Am.1982;66:455–468.
- ,,, et al.Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association.Circulation.2011;123:2292–2333.
- .Familial lipoprotein lipase deficiency and other causes of the chylomicronemia syndrome. In: Scriver C, Beaudet A, Sly W, Valle D, eds.The Metabolic and Molecular Basis of Inherited Disease.7th ed.New York:McGraw‐Hill;1995:1913–1932.
- ,,.Hypertriglyceridemia: its etiology, effects and treatment.CMAJ.2007;176:1113–1120.
- ,,, et al.Chylomicronemia with a mutant GPIHBP1 (Q115P) that cannot bind lipoprotein lipase.Arterioscler Thromb Vasc Biol.2009;29:956–962.
- ,,, et al.A mutation in the human lipoprotein lipase gene as the most common cause of familial chylomicronemia in French Canadians.N Engl J Med.1991;324:1761–1766.
- ,,, et al.Inherited apolipoprotein A‐V deficiency in severe hypertriglyceridemia.Arterioscler Thromb Vasc Biol.2005;25:411–417.
- ,,, et al.A polygenic basis for four classical Fredrickson hyperlipoproteinemia phenotypes that are characterized by hypertriglyceridemia.Hum Mol Genet.2009;18:4189–4194.
- .Secondary causes of hyperlipidemia.Med Clin North Am.1994;78:117–141.
- ,,.Hypertriglyceridemic hyperapob: the unappreciated atherogenic dyslipoproteinemia in type 2 diabetes mellitus.Ann Intern Med.2001;135:447–459.
- .Hyperlipidemic pancreatitis.Gastroenterol Clin North Am.1990;19:783–791.
- ,,, et al.Effects of insulin on cholesterol synthesis in type II diabetes patients.Diabetes Care.1995;18:1362–1369.
- ,,,.Evidence for the chylomicron origin of lipids accumulating in diabetic eruptive xanthomas: a correlative lipid biochemical, histochemical, and electron microscopic study.J Clin Invest.1970;49:2172–2187.
- .Dyslipidaemia.Lancet.2003;362:717–731.
- ,,.Lymphadenopathy associated with severe hypertriglyceridemia.JAMA.1990;264:727–728.
- Diagnosis and classification of diabetes mellitus.Diabetes Care.2010;33(suppl 1):S62–S69.
- ,.Pseudohyponatremia in acute hyperlipemic pancreatitis. A potential pitfall in therapy.Arch Surg.1985;120:1053–1055.
- ,,.Suppression of amylase activity by hypertriglyceridemia.JAMA.1973;225:1331–1334.
- ,,,.Dyslipidaemic pancreatitis clinical assessment and analysis of disease severity and outcomes.Pancreatology.2009;9:252–257.
- .Pathogenesis, differentiation and management of hypertriglyceridemia.Adv Intern Med.1969;15:117–154.
- ,.Role of hypertriglyceridemia in the pathogenesis of experimental acute pancreatitis in rats.Int J Pancreatol.1996;20:177–184.
- ,,, et al.Acute pancreatitis in a cohort of 129 patients referred for severe hypertriglyceridemia.Pancreas.2008;37:13–12.
- ,,, et al.ESPEN Guidelines on Enteral Nutrition: pancreas.Clin Nutr.2006;25:275–284.
- ,,, et al.Therapeutic response to medium‐chain triglycerides and omega‐3 fatty acids in a patient with the familial chylomicronemia syndrome.Arterioscler Thromb Vasc Biol.1997;17:1400–1406.
- ,,,,,.Mechanism of action of fibrates on lipid and lipoprotein metabolism.Circulation.1998;98:2088–2093.
- ,,.Drug interactions with lipid‐lowering drugs: mechanisms and clinical relevance.Clin Pharmacol Ther.2006;80:565–81.
- ,,, et al.Safety and efficacy of Omacor in severe hypertriglyceridemia.J Cardiovasc Risk.1997;4:385–391.
- ,,.Usefulness of Orlistat in the treatment of severe hypertriglyceridemia.Am J Cardiol.2002;89:229–231.
- ,,, et al.Effects of intensive atorvastatin and rosuvastatin treatment on apolipoprotein B‐48 and remnant lipoprotein cholesterol levels.Atherosclerosis.2009;205:197–201.
- ,,,.Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force.Am J Cardiol.2006;97:89C–94C.
- .Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases.N Engl J Med.1989;320:1060–1068.
- ,,.Insulin therapy for a non‐diabetic patient with severe hypertriglyceridemia.J Am Coll Nutr.1998;17:458–461.
- ,,,,.Treatment of severe hypertriglyceridemia in nondiabetic patients with insulin.Am J Emerg Med.2005;23:415–417.
- ,.Decreasing the plasma triglyceride level in hypertriglyceridemia‐induced pancreatitis in pregnancy: a case report.Am J Obstet Gynecol.2002;187:241–242.
- ,,,,,.Pancreatitis may occur with a normal amylase concentration in hypertriglyceridaemia.BMJ.1996;313:1265.
- ,,,.Lipoprotein lipase during continuous heparin infusion: tissue stores become partially depleted.J Lab Clin Med.2001;138:206–13.
- ,,,,,.Treatment of severe diabetic hypertriglyceridaemia by plasma exchange.Lancet.1978;1:1368.
- ,,,.Therapeutic plasma exchange in patients with hyperlipidemic pancreatitis.World J Gastroenterol.2004;10:2272–2274.
- ,,.Plasma exchange in severe hypertriglyceridemia a clinical study.Transfus Apher Sci.2006;34:253–257.
- ,,, et al.Management of acute severe hyperlipidemic pancreatitis.Digestion.2006;73:259–264.
- ,,,,.Hypertriglyceridemic pancreatitis: presentation and management.Am J Gastroenterol.2009;104:984–991.
- ,,, et al.Guidelines on the use of therapeutic apheresis in clinical practice—evidence‐based approach from the Apheresis Applications Committee of the American Society for Apheresis.J Clin Apher.2010;25:83–177.
- ,,,,,.Effects of a low‐fat diet compared with those of a high‐monounsaturated fat diet on body weight, plasma lipids and lipoproteins, and glycemic control in type 2 diabetes.Am J Clin Nutr.2004;80:668–673.