User login
Establishing an Orthopedic Excess Hospital Days in Acute Care Program
Total joint arthroplasty (TJA) procedures currently account for more Medicare expenses than any other inpatient procedure.1 In 2015, Centers for Medicare & Medicaid Services (CMS) announced the Comprehensive Care for Joint Replacement (CJR) model in which hospitals are paid one bundled payment for all related items and services utilized within a 90-day episode of care.2 Recent studies have suggested that the best opportunity to lower episode costs appears to be in the post-acute care setting and reducing readmissions.1,3
Surgical comanagement, which provides shared management of surgical patients between surgeons and hospitalists, is typically used in orthopedic surgery, neurosurgery, vascular surgery, and general surgery.4 Among patients with at least one medical comorbidity, surgical comanagement decreases length of stay (LOS), 30-day readmission rate for medical causes, and the proportion of patients with at least two medical consultants.5,6 Not all studies have shown that comanagement is beneficial. Maxwell et al found no significant differences in mortality or morbidity among hip fracture patients who did or did not receive comanagement7; however, comanaged patients were older and had more significant comorbidities, and there was no standard definition of comanagement among the participating institutions.
Comanagement after patients are discharged is a concept that has not been previously published but may become important with the Bundled Payments for Care Improvement initiative and high costs of excess days in acute care (EDAC). Hospitalists may be able to continue their work after discharge as part of the 90-day episode of care.8 TJA patients often have comorbidities, and surgical site infections and cardiovascular events are the most common causes of 30-day TJA readmissions.9
At our institution, 25% of TJA patients who presented to the Emergency Department (ED) within 90 days of surgery required a stay of less than 48 hours for conditions that did not require inpatient level of care. In addition, 50% of readmissions were secondary to medical complications. We also found significant variation in the management of common postoperative complications, such as postoperative fever, dislocation, anemia, and shortness of breath, especially among the different service lines caring for these patients. Therefore, we developed an Orthopedic EDAC program to reduce readmissions and to implement standardized admission algorithms and evidenced-based treatment protocols for common postoperative problems.
METHODS
Setting/Participants
We included patients who underwent total knee arthroplasty (TKA), total hip arthroplasty (THA), revision TKA, or revision THA from April 1, 2017, to September 30, 2018, at an urban teaching hospital. Patients were followed for 90 days after discharge. Factors such as age, sex, race, primary payer, Medicare Severity-Diagnosis Related Group (MS-DRG), discharge destination (home, home with home health, skilled nursing facility [SNF], acute rehab, other), and EDAC LOS were compared. An interdisciplinary committee comprising representatives from orthopedic surgery, hospital medicine, emergency medicine, and case management formulated observation criteria for the Orthopedic EDAC program. To be eligible for inclusion, observation patients had to have re-presented within 90 days from their initial surgery, could not be safely discharged home immediately from the ED, and did not require inpatient level of care. Patients qualifying for orthopedic observation were assigned rooms on the orthopedic wards to maintain continuity with nursing, physical therapy/occupational therapy, and case management staff. The University of Pennsylvania institutional review board reviewed this study and determined the project to be exempt.
Study Design
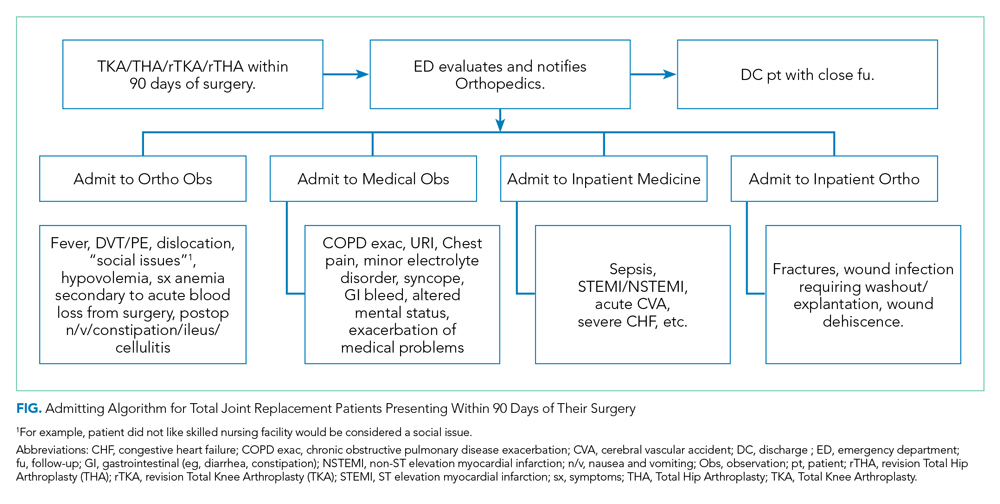
The Figure shows the admitting algorithm for TJA patients re-presenting within 90 days of their surgery. The ED evaluated the eligible patients; if they were not able to discharge the patient home, they notified the orthopedic resident on call for evaluation. Eligible diagnoses for the orthopedic observation in which orthopedics was the primary service included the need for postoperative pain control, fever (without signs or symptoms of sepsis), deep venous thrombosis or pulmonary embolism without hemodynamic instability, hemodynamically stable hypovolemia, symptomatic anemia secondary to acute blood loss anemia following surgery, and postoperative nausea, vomiting, constipation, ileus, and cellulitis. Eligible diagnoses for medical observation on the Medicine service included mild exacerbations of chronic obstructive pulmonary disease (COPD), syncope, upper respiratory tract infections, chest pain, delirium, and other exacerbation of medical problems. Full admission to Orthopedics included patients with wound infections requiring surgical washout, periprosthetic fractures/hematoma requiring operative management, and wound dehiscence requiring repair. All other readmissions requiring a stay of 48 or more hours were admitted to the medical or subspecialty medical service lines (eg, internal medicine, family medicine, geriatrics, cardiology, or pulmonary critical care).
Development of Evidence-Based Algorithms
Patients who re-presented to acute care (for either observation stays or readmissions) were treated based on standardized algorithms. The interdisciplinary work group developed evidence-based evaluation and treatment plans for common postoperative problems, including postoperative fever, postoperative shortness of breath, and postoperative septic joints. This was based on a comprehensive literature review and consensus among emergency medicine, hospital medicine, and orthopedic surgery. Appendix 1 illustrates an example of a standardized algorithm for the workup of hypoxia.
Definition of Readmissions and EDAC
Readmission and observation stays were flagged on re-presentation, and reasons for readmission or observation status were analyzed. Observation cutoffs of “successful” (<48 hours) vs “unsuccessful” (≥48 hours and/or conversion to inpatient status) were based on the CMS Two-Midnight Rule in accordance with past studies.10 Readmissions were defined as patients who required an acute stay of 48 or more hours within 90 days of discharge from their original surgical stay. Patients admitted under observation status who required a stay of less than 48 hours did not count as a readmission but did count toward EDAC.
We acknowledge that our definition of Orthopedic EDAC is not the same as CMS’s definition of EDAC for other conditions such as congestive heart failure, which includes hours in observation, readmissions, and ED visits. We focused on studying and reducing days in the hospital (observation status and readmissions), and our intervention was not intended to prevent issues that would cause patients to present to the ED. Therefore, including ED visits in our operational definition of EDAC would add an unnecessary source of confounding that would bias our results toward the null hypothesis.
Data Collection and Data Analysis
The Orthopedic EDAC program was implemented on October 1, 2017, based on the above triage and treatment plans. We analyzed demographic and outcome data (readmissions, LOS, time in observation status, reason for readmission/observation status) for 6 months prior (April 1, 2017, to September 30, 2017) and 1 year after (October 1, 2017, to September 30, 2018). Microsoft Excel (Jones, 2013) was used for data analysis. Paired t-test with P < .05 was predefined as significant.
Eligible patients were identified from previous admission diagnoses obtained through Vizient, which is a collaboration of academic medical centers that maintains a hospital discharge data set (the Clinical Data Base/Resource Manager CDB/RM). It included patient demographics, discharge diagnoses, procedures, and outcomes.11 The Vizient database is a respected source of data and has been used for several scholarly studies.10-12 We queried the Vizient Clinical Data Base/Resource Manager v. 8.12.0.11 (Vizient Inc., Irvine, TX) for the following data from both before and after the program’s implementation: disposition, LOS, insurance information, gender, type of surgery, MS-DRG, and race.
The five included MS-DRGs represented major hip and knee joint replacements with and without major comorbid conditions (MCCs; MS-DRG 469 and MS-DRG 470, respectively) and revision hip or knee replacement with MCCs, with comorbid conditions (CCs), and without MCCs or CCs (MS-DRG 466, MS-DRG 467, and MS-DRG 468, respectively). MCCs included but were not limited to decubitus ulcer, severe malnutrition, quadriplegia, and end-stage renal disease. Examples of CCs included transplant patients, lymphoma, leukemia, and malignancies (except breast or prostate), based on CMS definitions.13
RESULTS
Table 1 compares the demographics of the pre-implementation and post-implementation periods. There were a total of 2,662 admissions (799 before program implementation and 1,863 after). TKA and THA patients without MCCs (MS-DRG 470) accounted for 80% of patients during both periods. In both periods, approximately 60% of patients were female, 50% of patients were White, 40% were Black, and 10% were another race. The mean age was 63.6 years old. Most patients had Medicare or commercial insurance. Discharge destinations were similar during both periods.
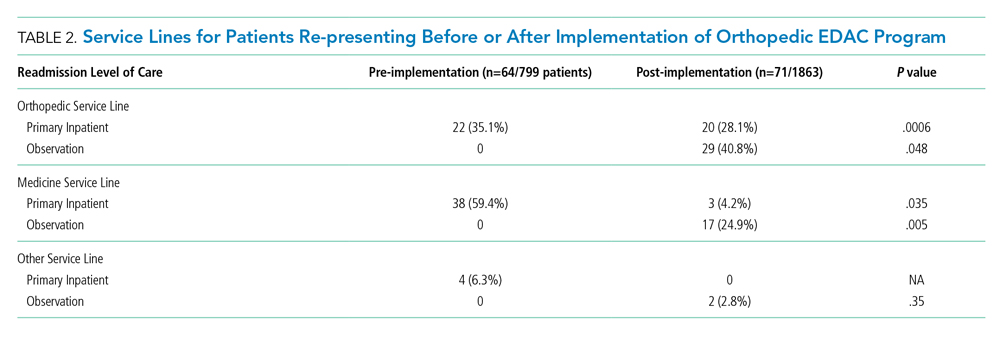
Table 2 illustrates how the patients who re-presented to acute care were triaged based on the algorithm described in the Figure. Among the 64 patients who re-presented during the pre-implementation period, there were no observation stays; there were 38 patients who were placed under medicine inpatient services. During post-implementation, there were 48 patients (29 on orthopedics, 17 on medicine, and 2 on other service lines) who were admitted under observation status. Twenty-three patients were discharged on observation status. Of those patients, 20 were admitted to orthopedic observation and 3 patients to medicine observation. Among the 71 patients who re-presented during the post-implementation period, 40.8% (29 patients) were admitted to inpatient orthopedic services, and 17 patients were readmitted to medicine services (24.9%). Among re-presenting patients, 70% were admitted to orthopedics inpatient and observation combined, in contrast to just 35% during the pre-implementation period.
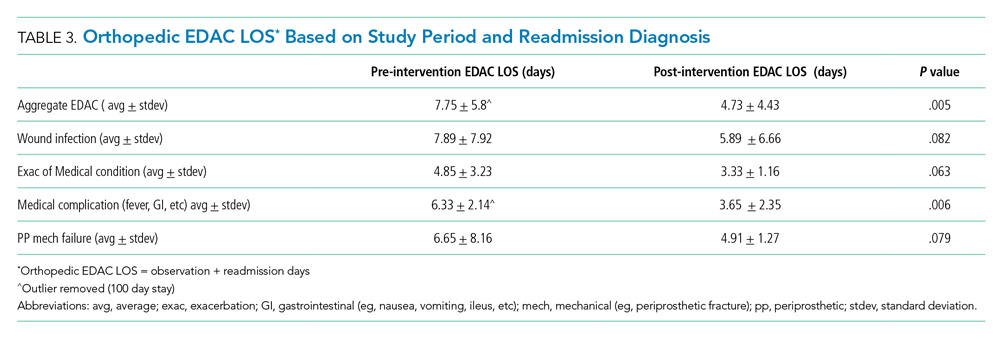
Readmissions decreased from 6.1% during pre-implementation to 2% during post-implementation (P = .004). In addition, the LOS for patients re-presenting during post-implementation was significantly lower than it was during pre-implementation. Table 3 details the associated LOS based on study period and readmission diagnosis. The aggregate LOS for all readmissions decreased from 7.75 days to 4.73 days (P = .005). The LOS decreased across all realms of readmission diagnoses. An outlier with an LOS greater than 100 days was removed from the pre-implementation group.
Appendix 2 further looked at patients who had observation orders, reasons for observation stay, and which patients were able to be discharged on observation status. Patients with medical complications such as fever and urinary tract infection were more likely to be discharged on observation status than were patients with wound drainage or redness that was concerning for a periprosthetic joint infection.
DISCUSSION
To our knowledge, this is the first description of a published Orthopedic EDAC program using orthopedic observation, standardized admitting and treatment algorithms, and comanagement of patients who re-presented after their original surgery. The development of an Orthopedic EDAC program at our hospital with comanagement was successful in reducing readmissions, decreasing LOS for readmitted patients, and increasing continuity of care. A number of points require more elaboration.
The Orthopedic EDAC program’s improvement in both reducing readmissions and decreasing LOS for EDAC (including days for observation and readmissions) was not caused by simply shifting patients with shorter LOS from inpatient to observation because the inpatients did not have a longer LOS. We had lower Orthopedic EDAC during the post-implementation vs pre-implementation even when considering EDAC in terms of both observation and readmissions. The decrease in readmissions is not only from the patients that were discharged on observation status, but also a result of other concurrent interventions, such as encouraging discharge to home rather than to rehabilitation facilities and more rigorous preoperative optimization.
The national rates of 30- and 90-day readmissions after primary TKA were 4% (95% CI, 3.8%-4.0%) and 7% (95% CI, 6.8%-7.2%), respectively,10 and the average cost of readmission for medical causes was $22,775 for THA and $11,682 for TKA.12 If one considers the 23 “saved readmissions” with 12 surgical complications and 11 medical complications, we “saved” roughly $591,105. Also, with the decrease in LOS for each readmission for any cause from 7.75 days to 4.73 days, the 48 readmissions had a 150 day lower LOS overall. With the average hospital day costing $2,289/day at nonprofit hospitals,13 there are additional cost savings of $343,350 overall. Therefore, the grand total estimated savings during this pilot was $934,455.
The decrease in post-implementation LOS vs pre-implementation LOS was likely multifactorial. The Orthopedic EDAC program improved continuity of care with orthopedic surgery and support staff (registered nurses, social workers, physical therapists) and utilized standardized protocols for work-up of common postoperative problems. These evidence-based protocols reduced waste that resulted in less testing with fewer incidental findings and side effects. The clinical history and patient circumstance did not need to be reestablished and tests did not need to be duplicated, which led to decreased LOS. Observation status allowed us to return patients to SNFs without the tedious procedure of insurance reauthorization and reevaluation by physical therapy and occupational therapy. Other factors such as “discharge before noon” and early physical therapy services ongoing during post-implementation also contributed to the decreased LOS.
Our Orthopedic EDAC program did not deliberately place patients on observation status who met full inpatient criteria solely to decrease the readmission rate. Our average LOS on observation status was 26 hours. In contrast, a study of observation stays at another tertiary academic medical center showed longer LOS: The average observation LOS was 33.3 hours with 44.4% of stays less than 24 hours and 16.5% greater than 48 hours.11 The use of EDAC hours in our study, which included both observation hours and readmission hours, made our impact more than simply a shifting of readmissions to observation stays.
It is important to utilize observation stays as they were intended—ie, stays requiring less than 48 hours. Over the past 10 years, the incidence and duration of observation stays has increased significantly while readmissions have decreased.14,15 Observation status has serious financial implications, and it is estimated that 10% of observation stays end up costing the patient more than an inpatient stay would and patients must pay 20% of services after the Part B deductible.16,17 In addition, Medicare beneficiaries have no cap on costs for an observation stay.16 Therefore, it is important to determine which patients and diagnoses are best suited for observation status. We found that younger patients without comorbidities who came from home and presented with complications such as fever and syncope were most likely to be successfully discharged on observation status with the Orthopedic EDAC program. SNF patients on observation status in particular may have large hospital bills because they often require 3 midnight stays but do not meet inpatient level of care and are thus not covered as inpatients.18
The Orthopedic EDAC program emphasized continuity of care with the primary orthopedic surgery team. Prior to implementation, orthopedics was often not even notified when their patients were in the ED or readmitted because the prevailing practice was that once surgery was completed, the surgeon’s job was done. Post-implementation, orthopedics was called for every bundled patient re-presenting within 90 days after a TJA. The triage protocol (Figure) was agreed upon prior to implementation by orthopedics, hospital medicine, and emergency medicine. Orthopedic attendings wanted to play a larger role and more strongly influence care of their patients on re-presentation because these attendings had become frustrated with the great disparities in work-up when patients went to various other services instead. Pre-implementation, many patients admitted to the primary orthopedic service had lower acuity, and they tended to be younger and have less medical complexity. Post-implementation, primary orthopedic services took care of more patients under observation status and those with “mechanical” complications that required surgery.
It is important to note that, while comanagement is common preoperatively and immediately postoperatively, studies of comanaged patients on re-presentation have apparently not been previously published. In addition, a recent study by Maxwell et al found that patients who were comanaged perioperatively had higher mortality and morbidity than did patients who were not comanaged.7 These findings reflect the need for more studies to be done to best optimize the use of comanagement. Comanagement as part of the Orthopedic EDAC program at our institution was successful in keeping patients who re-presented on the orthopedic service, decreasing LOS, and decreasing readmissions.
The study has some limitations. First, this was a retrospective study, so confounding variables may not be completely eliminated. Second, our study was conducted at a single center for total joint arthroplasty and did not consider other orthopedic conditions; however, our readmission numbers and demographics are similar to past studies. Third, we had small numbers of readmissions and observation patients, which resulted in a small effect size; however, our intervention demonstrated significant changes in LOS and readmissions. Fourth, our data is based on prior billing and coding, which may not always be accurate or inclusive. Fifth, we did not have THA or TKA patients on overnight recovery status or same day surgeries during either period studied; however, we are developing infrastructure to implement this in the future. Finally, ED visit data was not readily available to us, so we were not able to calculate the traditional EDAC. Despite these limitations, this study provides an important look at how an Orthopedic EDAC program can decrease readmissions, decrease LOS, and improve continuity of care in patients undergoing TJA.
CONCLUSION
An Orthopedic EDAC program with comanagement may decrease readmissions, improve continuity of care on re-presentation, and decrease LOS for total joint arthroplasty patients who presented after initial surgery and lead to substantial cost savings.
Disclosures
The authors have no potential conflicts to disclose. Dr Greysen was supported by a career development award from the National Institute on Aging (K23AG045338).
1. Hawker GA, Badley EM, Croxford R, et al. A population based nested case-control study of the costs of hip and knee replacement surgery. Med Care. 2009;47(7):732-741. https://doi.org/10.1097/MLR.0b013e3181934553
2. Kilgore M, Patel HK, Kielhorn A, Maya JF, Sharma P. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy. 2017;10:63-70. https://doi.org/10.2147/RMHP.S130341
3. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskeletal Med. 2017;10(3):370-377. https://doi.org/10.1007/s12178-017-9423-6
4. The Society of Hospital Medicine. The Evolution of Co-Management. 2017. Accessed October 30, 2019. https://www.hospitalmedicine.org/globalassets/practice-management/practice-management-pdf/pm-19-0004-co-management-white-paper_minor-update-m.pdf
5. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: a propensity score analysis. Ann Surg. 2016;264(2):275-282. https://doi.org/10.1097/SLA.0000000000001629
6. Fitzgerald SJ, Palmer TC, Kraay MJ. Improved perioperative care of elective joint replacement patients: the impact of an orthopedic perioperative hospitalist. J Arthroplasty. 2018;33(8):2387-2391. https://doi,org/10.1016/j.arth.2018.03.029
7. Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: a propensity score-matched retrospective cohort analysis of the National Surgical Quality Improvement Project. J Hosp Med. 2019;14:E1-E7. https://doi.org/10.12788/jhm.3343
8. Centers for Medicare & Medicaid Services. Medicare Program; Comprehensive Care for Joint Replacement Payment Model for Acute Care Hospitals Furnishing Lower Extremity Joint Replacement Services; Final Rule. November 24, 2015. https://www.govinfo.gov/content/pkg/FR-2015-11-24/pdf/2015-29438.pdf
9. Avram V, Petruccelli D, Winemaker M, de Beer J. Total joint arthroplasty readmission rates and reasons for 30-day hospital readmission. J Arthroplasty. 2014;29(3):465-468. https://doi.org/10.1016/j.arth.2013.07.039
10. ICD-10-CM/PCS MS-DRG v37.0 Definitions Manual. Accessed April 27, 2020. https://www.cms.gov/icd10m/version37-fullcode-cms/fullcode_cms/P0031.html
11. Chaudhary NS, Donnelly JP, Wang HE. Racial differences in sepsis mortality at United States academic medical center-affiliated hospitals. Crit Care Med. 2018;46(6):878-883. https://doi.org/10.1097/CCM.0000000000003020
12. Clair AJ, Evangelista PJ, Lajam CM, Slover JD, Bosco JA, Iorio R. Cost analysis of total joint arthroplasty readmissions in a Bundled Payment Care Improvement Initiative. J Arthroplasty. 2016;31(9):1862-1865.
13. Kaiser Family Foundation. Hospital Adjusted Expenses per Inpatient Day by Ownership. Kaiser Family Foundation. Accessed April 27, 2020. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D
14. Goldstein JN, Zhang Z, Schwartz JS, Hicks LS. Observation status, poverty, and high financial liability among Medicare beneficiaries. Am J Med. 2018;131(1):101.e9-101.e15. https://doi.org/10.1016/j.amjmed.2017.07.013
15. Lind KD, Noel-Miller CM, Sangaralingham LR, et al. Increasing trends in the use of hospital observation services for older Medicare Advantage and privately insured patients. Med Care Res Rev. 2019;76(2):229-239. https://doi.org/10.1177/1077558717718026
16. Sabbatini AK, Wright B. Excluding observation stays from readmission rates - what quality measures are missing. N Engl J Med. 2018;378(22):2062-2065. https://doi.org/10.1056/NEJMp1800732
17. Gabayan GZ, Doyle B, Liang, L, Donkor K, Huang, D, Sarkisian CA. Who has an unsuccessful observation care stay? Healthcare (Basel). 2018;6(4):138. https://doi.org/10.3390/healthcare6040138
18. Fang M, Hume E, Ibrahim S. Race, Bundled payment policy, and discharge destination after TKA: the experience of an urban academic hospital. Geriatr Orthop Surg Rehabil. 2018. https://doi.org/10.1177/2151459318803222
Total joint arthroplasty (TJA) procedures currently account for more Medicare expenses than any other inpatient procedure.1 In 2015, Centers for Medicare & Medicaid Services (CMS) announced the Comprehensive Care for Joint Replacement (CJR) model in which hospitals are paid one bundled payment for all related items and services utilized within a 90-day episode of care.2 Recent studies have suggested that the best opportunity to lower episode costs appears to be in the post-acute care setting and reducing readmissions.1,3
Surgical comanagement, which provides shared management of surgical patients between surgeons and hospitalists, is typically used in orthopedic surgery, neurosurgery, vascular surgery, and general surgery.4 Among patients with at least one medical comorbidity, surgical comanagement decreases length of stay (LOS), 30-day readmission rate for medical causes, and the proportion of patients with at least two medical consultants.5,6 Not all studies have shown that comanagement is beneficial. Maxwell et al found no significant differences in mortality or morbidity among hip fracture patients who did or did not receive comanagement7; however, comanaged patients were older and had more significant comorbidities, and there was no standard definition of comanagement among the participating institutions.
Comanagement after patients are discharged is a concept that has not been previously published but may become important with the Bundled Payments for Care Improvement initiative and high costs of excess days in acute care (EDAC). Hospitalists may be able to continue their work after discharge as part of the 90-day episode of care.8 TJA patients often have comorbidities, and surgical site infections and cardiovascular events are the most common causes of 30-day TJA readmissions.9
At our institution, 25% of TJA patients who presented to the Emergency Department (ED) within 90 days of surgery required a stay of less than 48 hours for conditions that did not require inpatient level of care. In addition, 50% of readmissions were secondary to medical complications. We also found significant variation in the management of common postoperative complications, such as postoperative fever, dislocation, anemia, and shortness of breath, especially among the different service lines caring for these patients. Therefore, we developed an Orthopedic EDAC program to reduce readmissions and to implement standardized admission algorithms and evidenced-based treatment protocols for common postoperative problems.
METHODS
Setting/Participants
We included patients who underwent total knee arthroplasty (TKA), total hip arthroplasty (THA), revision TKA, or revision THA from April 1, 2017, to September 30, 2018, at an urban teaching hospital. Patients were followed for 90 days after discharge. Factors such as age, sex, race, primary payer, Medicare Severity-Diagnosis Related Group (MS-DRG), discharge destination (home, home with home health, skilled nursing facility [SNF], acute rehab, other), and EDAC LOS were compared. An interdisciplinary committee comprising representatives from orthopedic surgery, hospital medicine, emergency medicine, and case management formulated observation criteria for the Orthopedic EDAC program. To be eligible for inclusion, observation patients had to have re-presented within 90 days from their initial surgery, could not be safely discharged home immediately from the ED, and did not require inpatient level of care. Patients qualifying for orthopedic observation were assigned rooms on the orthopedic wards to maintain continuity with nursing, physical therapy/occupational therapy, and case management staff. The University of Pennsylvania institutional review board reviewed this study and determined the project to be exempt.
Study Design
The Figure shows the admitting algorithm for TJA patients re-presenting within 90 days of their surgery. The ED evaluated the eligible patients; if they were not able to discharge the patient home, they notified the orthopedic resident on call for evaluation. Eligible diagnoses for the orthopedic observation in which orthopedics was the primary service included the need for postoperative pain control, fever (without signs or symptoms of sepsis), deep venous thrombosis or pulmonary embolism without hemodynamic instability, hemodynamically stable hypovolemia, symptomatic anemia secondary to acute blood loss anemia following surgery, and postoperative nausea, vomiting, constipation, ileus, and cellulitis. Eligible diagnoses for medical observation on the Medicine service included mild exacerbations of chronic obstructive pulmonary disease (COPD), syncope, upper respiratory tract infections, chest pain, delirium, and other exacerbation of medical problems. Full admission to Orthopedics included patients with wound infections requiring surgical washout, periprosthetic fractures/hematoma requiring operative management, and wound dehiscence requiring repair. All other readmissions requiring a stay of 48 or more hours were admitted to the medical or subspecialty medical service lines (eg, internal medicine, family medicine, geriatrics, cardiology, or pulmonary critical care).

Development of Evidence-Based Algorithms
Patients who re-presented to acute care (for either observation stays or readmissions) were treated based on standardized algorithms. The interdisciplinary work group developed evidence-based evaluation and treatment plans for common postoperative problems, including postoperative fever, postoperative shortness of breath, and postoperative septic joints. This was based on a comprehensive literature review and consensus among emergency medicine, hospital medicine, and orthopedic surgery. Appendix 1 illustrates an example of a standardized algorithm for the workup of hypoxia.
Definition of Readmissions and EDAC
Readmission and observation stays were flagged on re-presentation, and reasons for readmission or observation status were analyzed. Observation cutoffs of “successful” (<48 hours) vs “unsuccessful” (≥48 hours and/or conversion to inpatient status) were based on the CMS Two-Midnight Rule in accordance with past studies.10 Readmissions were defined as patients who required an acute stay of 48 or more hours within 90 days of discharge from their original surgical stay. Patients admitted under observation status who required a stay of less than 48 hours did not count as a readmission but did count toward EDAC.
We acknowledge that our definition of Orthopedic EDAC is not the same as CMS’s definition of EDAC for other conditions such as congestive heart failure, which includes hours in observation, readmissions, and ED visits. We focused on studying and reducing days in the hospital (observation status and readmissions), and our intervention was not intended to prevent issues that would cause patients to present to the ED. Therefore, including ED visits in our operational definition of EDAC would add an unnecessary source of confounding that would bias our results toward the null hypothesis.
Data Collection and Data Analysis
The Orthopedic EDAC program was implemented on October 1, 2017, based on the above triage and treatment plans. We analyzed demographic and outcome data (readmissions, LOS, time in observation status, reason for readmission/observation status) for 6 months prior (April 1, 2017, to September 30, 2017) and 1 year after (October 1, 2017, to September 30, 2018). Microsoft Excel (Jones, 2013) was used for data analysis. Paired t-test with P < .05 was predefined as significant.
Eligible patients were identified from previous admission diagnoses obtained through Vizient, which is a collaboration of academic medical centers that maintains a hospital discharge data set (the Clinical Data Base/Resource Manager CDB/RM). It included patient demographics, discharge diagnoses, procedures, and outcomes.11 The Vizient database is a respected source of data and has been used for several scholarly studies.10-12 We queried the Vizient Clinical Data Base/Resource Manager v. 8.12.0.11 (Vizient Inc., Irvine, TX) for the following data from both before and after the program’s implementation: disposition, LOS, insurance information, gender, type of surgery, MS-DRG, and race.
The five included MS-DRGs represented major hip and knee joint replacements with and without major comorbid conditions (MCCs; MS-DRG 469 and MS-DRG 470, respectively) and revision hip or knee replacement with MCCs, with comorbid conditions (CCs), and without MCCs or CCs (MS-DRG 466, MS-DRG 467, and MS-DRG 468, respectively). MCCs included but were not limited to decubitus ulcer, severe malnutrition, quadriplegia, and end-stage renal disease. Examples of CCs included transplant patients, lymphoma, leukemia, and malignancies (except breast or prostate), based on CMS definitions.13
RESULTS
Table 1 compares the demographics of the pre-implementation and post-implementation periods. There were a total of 2,662 admissions (799 before program implementation and 1,863 after). TKA and THA patients without MCCs (MS-DRG 470) accounted for 80% of patients during both periods. In both periods, approximately 60% of patients were female, 50% of patients were White, 40% were Black, and 10% were another race. The mean age was 63.6 years old. Most patients had Medicare or commercial insurance. Discharge destinations were similar during both periods.
Table 2 illustrates how the patients who re-presented to acute care were triaged based on the algorithm described in the Figure. Among the 64 patients who re-presented during the pre-implementation period, there were no observation stays; there were 38 patients who were placed under medicine inpatient services. During post-implementation, there were 48 patients (29 on orthopedics, 17 on medicine, and 2 on other service lines) who were admitted under observation status. Twenty-three patients were discharged on observation status. Of those patients, 20 were admitted to orthopedic observation and 3 patients to medicine observation. Among the 71 patients who re-presented during the post-implementation period, 40.8% (29 patients) were admitted to inpatient orthopedic services, and 17 patients were readmitted to medicine services (24.9%). Among re-presenting patients, 70% were admitted to orthopedics inpatient and observation combined, in contrast to just 35% during the pre-implementation period.

Readmissions decreased from 6.1% during pre-implementation to 2% during post-implementation (P = .004). In addition, the LOS for patients re-presenting during post-implementation was significantly lower than it was during pre-implementation. Table 3 details the associated LOS based on study period and readmission diagnosis. The aggregate LOS for all readmissions decreased from 7.75 days to 4.73 days (P = .005). The LOS decreased across all realms of readmission diagnoses. An outlier with an LOS greater than 100 days was removed from the pre-implementation group.

Appendix 2 further looked at patients who had observation orders, reasons for observation stay, and which patients were able to be discharged on observation status. Patients with medical complications such as fever and urinary tract infection were more likely to be discharged on observation status than were patients with wound drainage or redness that was concerning for a periprosthetic joint infection.
DISCUSSION
To our knowledge, this is the first description of a published Orthopedic EDAC program using orthopedic observation, standardized admitting and treatment algorithms, and comanagement of patients who re-presented after their original surgery. The development of an Orthopedic EDAC program at our hospital with comanagement was successful in reducing readmissions, decreasing LOS for readmitted patients, and increasing continuity of care. A number of points require more elaboration.
The Orthopedic EDAC program’s improvement in both reducing readmissions and decreasing LOS for EDAC (including days for observation and readmissions) was not caused by simply shifting patients with shorter LOS from inpatient to observation because the inpatients did not have a longer LOS. We had lower Orthopedic EDAC during the post-implementation vs pre-implementation even when considering EDAC in terms of both observation and readmissions. The decrease in readmissions is not only from the patients that were discharged on observation status, but also a result of other concurrent interventions, such as encouraging discharge to home rather than to rehabilitation facilities and more rigorous preoperative optimization.
The national rates of 30- and 90-day readmissions after primary TKA were 4% (95% CI, 3.8%-4.0%) and 7% (95% CI, 6.8%-7.2%), respectively,10 and the average cost of readmission for medical causes was $22,775 for THA and $11,682 for TKA.12 If one considers the 23 “saved readmissions” with 12 surgical complications and 11 medical complications, we “saved” roughly $591,105. Also, with the decrease in LOS for each readmission for any cause from 7.75 days to 4.73 days, the 48 readmissions had a 150 day lower LOS overall. With the average hospital day costing $2,289/day at nonprofit hospitals,13 there are additional cost savings of $343,350 overall. Therefore, the grand total estimated savings during this pilot was $934,455.
The decrease in post-implementation LOS vs pre-implementation LOS was likely multifactorial. The Orthopedic EDAC program improved continuity of care with orthopedic surgery and support staff (registered nurses, social workers, physical therapists) and utilized standardized protocols for work-up of common postoperative problems. These evidence-based protocols reduced waste that resulted in less testing with fewer incidental findings and side effects. The clinical history and patient circumstance did not need to be reestablished and tests did not need to be duplicated, which led to decreased LOS. Observation status allowed us to return patients to SNFs without the tedious procedure of insurance reauthorization and reevaluation by physical therapy and occupational therapy. Other factors such as “discharge before noon” and early physical therapy services ongoing during post-implementation also contributed to the decreased LOS.
Our Orthopedic EDAC program did not deliberately place patients on observation status who met full inpatient criteria solely to decrease the readmission rate. Our average LOS on observation status was 26 hours. In contrast, a study of observation stays at another tertiary academic medical center showed longer LOS: The average observation LOS was 33.3 hours with 44.4% of stays less than 24 hours and 16.5% greater than 48 hours.11 The use of EDAC hours in our study, which included both observation hours and readmission hours, made our impact more than simply a shifting of readmissions to observation stays.
It is important to utilize observation stays as they were intended—ie, stays requiring less than 48 hours. Over the past 10 years, the incidence and duration of observation stays has increased significantly while readmissions have decreased.14,15 Observation status has serious financial implications, and it is estimated that 10% of observation stays end up costing the patient more than an inpatient stay would and patients must pay 20% of services after the Part B deductible.16,17 In addition, Medicare beneficiaries have no cap on costs for an observation stay.16 Therefore, it is important to determine which patients and diagnoses are best suited for observation status. We found that younger patients without comorbidities who came from home and presented with complications such as fever and syncope were most likely to be successfully discharged on observation status with the Orthopedic EDAC program. SNF patients on observation status in particular may have large hospital bills because they often require 3 midnight stays but do not meet inpatient level of care and are thus not covered as inpatients.18
The Orthopedic EDAC program emphasized continuity of care with the primary orthopedic surgery team. Prior to implementation, orthopedics was often not even notified when their patients were in the ED or readmitted because the prevailing practice was that once surgery was completed, the surgeon’s job was done. Post-implementation, orthopedics was called for every bundled patient re-presenting within 90 days after a TJA. The triage protocol (Figure) was agreed upon prior to implementation by orthopedics, hospital medicine, and emergency medicine. Orthopedic attendings wanted to play a larger role and more strongly influence care of their patients on re-presentation because these attendings had become frustrated with the great disparities in work-up when patients went to various other services instead. Pre-implementation, many patients admitted to the primary orthopedic service had lower acuity, and they tended to be younger and have less medical complexity. Post-implementation, primary orthopedic services took care of more patients under observation status and those with “mechanical” complications that required surgery.
It is important to note that, while comanagement is common preoperatively and immediately postoperatively, studies of comanaged patients on re-presentation have apparently not been previously published. In addition, a recent study by Maxwell et al found that patients who were comanaged perioperatively had higher mortality and morbidity than did patients who were not comanaged.7 These findings reflect the need for more studies to be done to best optimize the use of comanagement. Comanagement as part of the Orthopedic EDAC program at our institution was successful in keeping patients who re-presented on the orthopedic service, decreasing LOS, and decreasing readmissions.
The study has some limitations. First, this was a retrospective study, so confounding variables may not be completely eliminated. Second, our study was conducted at a single center for total joint arthroplasty and did not consider other orthopedic conditions; however, our readmission numbers and demographics are similar to past studies. Third, we had small numbers of readmissions and observation patients, which resulted in a small effect size; however, our intervention demonstrated significant changes in LOS and readmissions. Fourth, our data is based on prior billing and coding, which may not always be accurate or inclusive. Fifth, we did not have THA or TKA patients on overnight recovery status or same day surgeries during either period studied; however, we are developing infrastructure to implement this in the future. Finally, ED visit data was not readily available to us, so we were not able to calculate the traditional EDAC. Despite these limitations, this study provides an important look at how an Orthopedic EDAC program can decrease readmissions, decrease LOS, and improve continuity of care in patients undergoing TJA.
CONCLUSION
An Orthopedic EDAC program with comanagement may decrease readmissions, improve continuity of care on re-presentation, and decrease LOS for total joint arthroplasty patients who presented after initial surgery and lead to substantial cost savings.
Disclosures
The authors have no potential conflicts to disclose. Dr Greysen was supported by a career development award from the National Institute on Aging (K23AG045338).
Total joint arthroplasty (TJA) procedures currently account for more Medicare expenses than any other inpatient procedure.1 In 2015, Centers for Medicare & Medicaid Services (CMS) announced the Comprehensive Care for Joint Replacement (CJR) model in which hospitals are paid one bundled payment for all related items and services utilized within a 90-day episode of care.2 Recent studies have suggested that the best opportunity to lower episode costs appears to be in the post-acute care setting and reducing readmissions.1,3
Surgical comanagement, which provides shared management of surgical patients between surgeons and hospitalists, is typically used in orthopedic surgery, neurosurgery, vascular surgery, and general surgery.4 Among patients with at least one medical comorbidity, surgical comanagement decreases length of stay (LOS), 30-day readmission rate for medical causes, and the proportion of patients with at least two medical consultants.5,6 Not all studies have shown that comanagement is beneficial. Maxwell et al found no significant differences in mortality or morbidity among hip fracture patients who did or did not receive comanagement7; however, comanaged patients were older and had more significant comorbidities, and there was no standard definition of comanagement among the participating institutions.
Comanagement after patients are discharged is a concept that has not been previously published but may become important with the Bundled Payments for Care Improvement initiative and high costs of excess days in acute care (EDAC). Hospitalists may be able to continue their work after discharge as part of the 90-day episode of care.8 TJA patients often have comorbidities, and surgical site infections and cardiovascular events are the most common causes of 30-day TJA readmissions.9
At our institution, 25% of TJA patients who presented to the Emergency Department (ED) within 90 days of surgery required a stay of less than 48 hours for conditions that did not require inpatient level of care. In addition, 50% of readmissions were secondary to medical complications. We also found significant variation in the management of common postoperative complications, such as postoperative fever, dislocation, anemia, and shortness of breath, especially among the different service lines caring for these patients. Therefore, we developed an Orthopedic EDAC program to reduce readmissions and to implement standardized admission algorithms and evidenced-based treatment protocols for common postoperative problems.
METHODS
Setting/Participants
We included patients who underwent total knee arthroplasty (TKA), total hip arthroplasty (THA), revision TKA, or revision THA from April 1, 2017, to September 30, 2018, at an urban teaching hospital. Patients were followed for 90 days after discharge. Factors such as age, sex, race, primary payer, Medicare Severity-Diagnosis Related Group (MS-DRG), discharge destination (home, home with home health, skilled nursing facility [SNF], acute rehab, other), and EDAC LOS were compared. An interdisciplinary committee comprising representatives from orthopedic surgery, hospital medicine, emergency medicine, and case management formulated observation criteria for the Orthopedic EDAC program. To be eligible for inclusion, observation patients had to have re-presented within 90 days from their initial surgery, could not be safely discharged home immediately from the ED, and did not require inpatient level of care. Patients qualifying for orthopedic observation were assigned rooms on the orthopedic wards to maintain continuity with nursing, physical therapy/occupational therapy, and case management staff. The University of Pennsylvania institutional review board reviewed this study and determined the project to be exempt.
Study Design
The Figure shows the admitting algorithm for TJA patients re-presenting within 90 days of their surgery. The ED evaluated the eligible patients; if they were not able to discharge the patient home, they notified the orthopedic resident on call for evaluation. Eligible diagnoses for the orthopedic observation in which orthopedics was the primary service included the need for postoperative pain control, fever (without signs or symptoms of sepsis), deep venous thrombosis or pulmonary embolism without hemodynamic instability, hemodynamically stable hypovolemia, symptomatic anemia secondary to acute blood loss anemia following surgery, and postoperative nausea, vomiting, constipation, ileus, and cellulitis. Eligible diagnoses for medical observation on the Medicine service included mild exacerbations of chronic obstructive pulmonary disease (COPD), syncope, upper respiratory tract infections, chest pain, delirium, and other exacerbation of medical problems. Full admission to Orthopedics included patients with wound infections requiring surgical washout, periprosthetic fractures/hematoma requiring operative management, and wound dehiscence requiring repair. All other readmissions requiring a stay of 48 or more hours were admitted to the medical or subspecialty medical service lines (eg, internal medicine, family medicine, geriatrics, cardiology, or pulmonary critical care).

Development of Evidence-Based Algorithms
Patients who re-presented to acute care (for either observation stays or readmissions) were treated based on standardized algorithms. The interdisciplinary work group developed evidence-based evaluation and treatment plans for common postoperative problems, including postoperative fever, postoperative shortness of breath, and postoperative septic joints. This was based on a comprehensive literature review and consensus among emergency medicine, hospital medicine, and orthopedic surgery. Appendix 1 illustrates an example of a standardized algorithm for the workup of hypoxia.
Definition of Readmissions and EDAC
Readmission and observation stays were flagged on re-presentation, and reasons for readmission or observation status were analyzed. Observation cutoffs of “successful” (<48 hours) vs “unsuccessful” (≥48 hours and/or conversion to inpatient status) were based on the CMS Two-Midnight Rule in accordance with past studies.10 Readmissions were defined as patients who required an acute stay of 48 or more hours within 90 days of discharge from their original surgical stay. Patients admitted under observation status who required a stay of less than 48 hours did not count as a readmission but did count toward EDAC.
We acknowledge that our definition of Orthopedic EDAC is not the same as CMS’s definition of EDAC for other conditions such as congestive heart failure, which includes hours in observation, readmissions, and ED visits. We focused on studying and reducing days in the hospital (observation status and readmissions), and our intervention was not intended to prevent issues that would cause patients to present to the ED. Therefore, including ED visits in our operational definition of EDAC would add an unnecessary source of confounding that would bias our results toward the null hypothesis.
Data Collection and Data Analysis
The Orthopedic EDAC program was implemented on October 1, 2017, based on the above triage and treatment plans. We analyzed demographic and outcome data (readmissions, LOS, time in observation status, reason for readmission/observation status) for 6 months prior (April 1, 2017, to September 30, 2017) and 1 year after (October 1, 2017, to September 30, 2018). Microsoft Excel (Jones, 2013) was used for data analysis. Paired t-test with P < .05 was predefined as significant.
Eligible patients were identified from previous admission diagnoses obtained through Vizient, which is a collaboration of academic medical centers that maintains a hospital discharge data set (the Clinical Data Base/Resource Manager CDB/RM). It included patient demographics, discharge diagnoses, procedures, and outcomes.11 The Vizient database is a respected source of data and has been used for several scholarly studies.10-12 We queried the Vizient Clinical Data Base/Resource Manager v. 8.12.0.11 (Vizient Inc., Irvine, TX) for the following data from both before and after the program’s implementation: disposition, LOS, insurance information, gender, type of surgery, MS-DRG, and race.
The five included MS-DRGs represented major hip and knee joint replacements with and without major comorbid conditions (MCCs; MS-DRG 469 and MS-DRG 470, respectively) and revision hip or knee replacement with MCCs, with comorbid conditions (CCs), and without MCCs or CCs (MS-DRG 466, MS-DRG 467, and MS-DRG 468, respectively). MCCs included but were not limited to decubitus ulcer, severe malnutrition, quadriplegia, and end-stage renal disease. Examples of CCs included transplant patients, lymphoma, leukemia, and malignancies (except breast or prostate), based on CMS definitions.13
RESULTS
Table 1 compares the demographics of the pre-implementation and post-implementation periods. There were a total of 2,662 admissions (799 before program implementation and 1,863 after). TKA and THA patients without MCCs (MS-DRG 470) accounted for 80% of patients during both periods. In both periods, approximately 60% of patients were female, 50% of patients were White, 40% were Black, and 10% were another race. The mean age was 63.6 years old. Most patients had Medicare or commercial insurance. Discharge destinations were similar during both periods.
Table 2 illustrates how the patients who re-presented to acute care were triaged based on the algorithm described in the Figure. Among the 64 patients who re-presented during the pre-implementation period, there were no observation stays; there were 38 patients who were placed under medicine inpatient services. During post-implementation, there were 48 patients (29 on orthopedics, 17 on medicine, and 2 on other service lines) who were admitted under observation status. Twenty-three patients were discharged on observation status. Of those patients, 20 were admitted to orthopedic observation and 3 patients to medicine observation. Among the 71 patients who re-presented during the post-implementation period, 40.8% (29 patients) were admitted to inpatient orthopedic services, and 17 patients were readmitted to medicine services (24.9%). Among re-presenting patients, 70% were admitted to orthopedics inpatient and observation combined, in contrast to just 35% during the pre-implementation period.

Readmissions decreased from 6.1% during pre-implementation to 2% during post-implementation (P = .004). In addition, the LOS for patients re-presenting during post-implementation was significantly lower than it was during pre-implementation. Table 3 details the associated LOS based on study period and readmission diagnosis. The aggregate LOS for all readmissions decreased from 7.75 days to 4.73 days (P = .005). The LOS decreased across all realms of readmission diagnoses. An outlier with an LOS greater than 100 days was removed from the pre-implementation group.

Appendix 2 further looked at patients who had observation orders, reasons for observation stay, and which patients were able to be discharged on observation status. Patients with medical complications such as fever and urinary tract infection were more likely to be discharged on observation status than were patients with wound drainage or redness that was concerning for a periprosthetic joint infection.
DISCUSSION
To our knowledge, this is the first description of a published Orthopedic EDAC program using orthopedic observation, standardized admitting and treatment algorithms, and comanagement of patients who re-presented after their original surgery. The development of an Orthopedic EDAC program at our hospital with comanagement was successful in reducing readmissions, decreasing LOS for readmitted patients, and increasing continuity of care. A number of points require more elaboration.
The Orthopedic EDAC program’s improvement in both reducing readmissions and decreasing LOS for EDAC (including days for observation and readmissions) was not caused by simply shifting patients with shorter LOS from inpatient to observation because the inpatients did not have a longer LOS. We had lower Orthopedic EDAC during the post-implementation vs pre-implementation even when considering EDAC in terms of both observation and readmissions. The decrease in readmissions is not only from the patients that were discharged on observation status, but also a result of other concurrent interventions, such as encouraging discharge to home rather than to rehabilitation facilities and more rigorous preoperative optimization.
The national rates of 30- and 90-day readmissions after primary TKA were 4% (95% CI, 3.8%-4.0%) and 7% (95% CI, 6.8%-7.2%), respectively,10 and the average cost of readmission for medical causes was $22,775 for THA and $11,682 for TKA.12 If one considers the 23 “saved readmissions” with 12 surgical complications and 11 medical complications, we “saved” roughly $591,105. Also, with the decrease in LOS for each readmission for any cause from 7.75 days to 4.73 days, the 48 readmissions had a 150 day lower LOS overall. With the average hospital day costing $2,289/day at nonprofit hospitals,13 there are additional cost savings of $343,350 overall. Therefore, the grand total estimated savings during this pilot was $934,455.
The decrease in post-implementation LOS vs pre-implementation LOS was likely multifactorial. The Orthopedic EDAC program improved continuity of care with orthopedic surgery and support staff (registered nurses, social workers, physical therapists) and utilized standardized protocols for work-up of common postoperative problems. These evidence-based protocols reduced waste that resulted in less testing with fewer incidental findings and side effects. The clinical history and patient circumstance did not need to be reestablished and tests did not need to be duplicated, which led to decreased LOS. Observation status allowed us to return patients to SNFs without the tedious procedure of insurance reauthorization and reevaluation by physical therapy and occupational therapy. Other factors such as “discharge before noon” and early physical therapy services ongoing during post-implementation also contributed to the decreased LOS.
Our Orthopedic EDAC program did not deliberately place patients on observation status who met full inpatient criteria solely to decrease the readmission rate. Our average LOS on observation status was 26 hours. In contrast, a study of observation stays at another tertiary academic medical center showed longer LOS: The average observation LOS was 33.3 hours with 44.4% of stays less than 24 hours and 16.5% greater than 48 hours.11 The use of EDAC hours in our study, which included both observation hours and readmission hours, made our impact more than simply a shifting of readmissions to observation stays.
It is important to utilize observation stays as they were intended—ie, stays requiring less than 48 hours. Over the past 10 years, the incidence and duration of observation stays has increased significantly while readmissions have decreased.14,15 Observation status has serious financial implications, and it is estimated that 10% of observation stays end up costing the patient more than an inpatient stay would and patients must pay 20% of services after the Part B deductible.16,17 In addition, Medicare beneficiaries have no cap on costs for an observation stay.16 Therefore, it is important to determine which patients and diagnoses are best suited for observation status. We found that younger patients without comorbidities who came from home and presented with complications such as fever and syncope were most likely to be successfully discharged on observation status with the Orthopedic EDAC program. SNF patients on observation status in particular may have large hospital bills because they often require 3 midnight stays but do not meet inpatient level of care and are thus not covered as inpatients.18
The Orthopedic EDAC program emphasized continuity of care with the primary orthopedic surgery team. Prior to implementation, orthopedics was often not even notified when their patients were in the ED or readmitted because the prevailing practice was that once surgery was completed, the surgeon’s job was done. Post-implementation, orthopedics was called for every bundled patient re-presenting within 90 days after a TJA. The triage protocol (Figure) was agreed upon prior to implementation by orthopedics, hospital medicine, and emergency medicine. Orthopedic attendings wanted to play a larger role and more strongly influence care of their patients on re-presentation because these attendings had become frustrated with the great disparities in work-up when patients went to various other services instead. Pre-implementation, many patients admitted to the primary orthopedic service had lower acuity, and they tended to be younger and have less medical complexity. Post-implementation, primary orthopedic services took care of more patients under observation status and those with “mechanical” complications that required surgery.
It is important to note that, while comanagement is common preoperatively and immediately postoperatively, studies of comanaged patients on re-presentation have apparently not been previously published. In addition, a recent study by Maxwell et al found that patients who were comanaged perioperatively had higher mortality and morbidity than did patients who were not comanaged.7 These findings reflect the need for more studies to be done to best optimize the use of comanagement. Comanagement as part of the Orthopedic EDAC program at our institution was successful in keeping patients who re-presented on the orthopedic service, decreasing LOS, and decreasing readmissions.
The study has some limitations. First, this was a retrospective study, so confounding variables may not be completely eliminated. Second, our study was conducted at a single center for total joint arthroplasty and did not consider other orthopedic conditions; however, our readmission numbers and demographics are similar to past studies. Third, we had small numbers of readmissions and observation patients, which resulted in a small effect size; however, our intervention demonstrated significant changes in LOS and readmissions. Fourth, our data is based on prior billing and coding, which may not always be accurate or inclusive. Fifth, we did not have THA or TKA patients on overnight recovery status or same day surgeries during either period studied; however, we are developing infrastructure to implement this in the future. Finally, ED visit data was not readily available to us, so we were not able to calculate the traditional EDAC. Despite these limitations, this study provides an important look at how an Orthopedic EDAC program can decrease readmissions, decrease LOS, and improve continuity of care in patients undergoing TJA.
CONCLUSION
An Orthopedic EDAC program with comanagement may decrease readmissions, improve continuity of care on re-presentation, and decrease LOS for total joint arthroplasty patients who presented after initial surgery and lead to substantial cost savings.
Disclosures
The authors have no potential conflicts to disclose. Dr Greysen was supported by a career development award from the National Institute on Aging (K23AG045338).
1. Hawker GA, Badley EM, Croxford R, et al. A population based nested case-control study of the costs of hip and knee replacement surgery. Med Care. 2009;47(7):732-741. https://doi.org/10.1097/MLR.0b013e3181934553
2. Kilgore M, Patel HK, Kielhorn A, Maya JF, Sharma P. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy. 2017;10:63-70. https://doi.org/10.2147/RMHP.S130341
3. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskeletal Med. 2017;10(3):370-377. https://doi.org/10.1007/s12178-017-9423-6
4. The Society of Hospital Medicine. The Evolution of Co-Management. 2017. Accessed October 30, 2019. https://www.hospitalmedicine.org/globalassets/practice-management/practice-management-pdf/pm-19-0004-co-management-white-paper_minor-update-m.pdf
5. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: a propensity score analysis. Ann Surg. 2016;264(2):275-282. https://doi.org/10.1097/SLA.0000000000001629
6. Fitzgerald SJ, Palmer TC, Kraay MJ. Improved perioperative care of elective joint replacement patients: the impact of an orthopedic perioperative hospitalist. J Arthroplasty. 2018;33(8):2387-2391. https://doi,org/10.1016/j.arth.2018.03.029
7. Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: a propensity score-matched retrospective cohort analysis of the National Surgical Quality Improvement Project. J Hosp Med. 2019;14:E1-E7. https://doi.org/10.12788/jhm.3343
8. Centers for Medicare & Medicaid Services. Medicare Program; Comprehensive Care for Joint Replacement Payment Model for Acute Care Hospitals Furnishing Lower Extremity Joint Replacement Services; Final Rule. November 24, 2015. https://www.govinfo.gov/content/pkg/FR-2015-11-24/pdf/2015-29438.pdf
9. Avram V, Petruccelli D, Winemaker M, de Beer J. Total joint arthroplasty readmission rates and reasons for 30-day hospital readmission. J Arthroplasty. 2014;29(3):465-468. https://doi.org/10.1016/j.arth.2013.07.039
10. ICD-10-CM/PCS MS-DRG v37.0 Definitions Manual. Accessed April 27, 2020. https://www.cms.gov/icd10m/version37-fullcode-cms/fullcode_cms/P0031.html
11. Chaudhary NS, Donnelly JP, Wang HE. Racial differences in sepsis mortality at United States academic medical center-affiliated hospitals. Crit Care Med. 2018;46(6):878-883. https://doi.org/10.1097/CCM.0000000000003020
12. Clair AJ, Evangelista PJ, Lajam CM, Slover JD, Bosco JA, Iorio R. Cost analysis of total joint arthroplasty readmissions in a Bundled Payment Care Improvement Initiative. J Arthroplasty. 2016;31(9):1862-1865.
13. Kaiser Family Foundation. Hospital Adjusted Expenses per Inpatient Day by Ownership. Kaiser Family Foundation. Accessed April 27, 2020. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D
14. Goldstein JN, Zhang Z, Schwartz JS, Hicks LS. Observation status, poverty, and high financial liability among Medicare beneficiaries. Am J Med. 2018;131(1):101.e9-101.e15. https://doi.org/10.1016/j.amjmed.2017.07.013
15. Lind KD, Noel-Miller CM, Sangaralingham LR, et al. Increasing trends in the use of hospital observation services for older Medicare Advantage and privately insured patients. Med Care Res Rev. 2019;76(2):229-239. https://doi.org/10.1177/1077558717718026
16. Sabbatini AK, Wright B. Excluding observation stays from readmission rates - what quality measures are missing. N Engl J Med. 2018;378(22):2062-2065. https://doi.org/10.1056/NEJMp1800732
17. Gabayan GZ, Doyle B, Liang, L, Donkor K, Huang, D, Sarkisian CA. Who has an unsuccessful observation care stay? Healthcare (Basel). 2018;6(4):138. https://doi.org/10.3390/healthcare6040138
18. Fang M, Hume E, Ibrahim S. Race, Bundled payment policy, and discharge destination after TKA: the experience of an urban academic hospital. Geriatr Orthop Surg Rehabil. 2018. https://doi.org/10.1177/2151459318803222
1. Hawker GA, Badley EM, Croxford R, et al. A population based nested case-control study of the costs of hip and knee replacement surgery. Med Care. 2009;47(7):732-741. https://doi.org/10.1097/MLR.0b013e3181934553
2. Kilgore M, Patel HK, Kielhorn A, Maya JF, Sharma P. Economic burden of hospitalizations of Medicare beneficiaries with heart failure. Risk Manag Healthc Policy. 2017;10:63-70. https://doi.org/10.2147/RMHP.S130341
3. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskeletal Med. 2017;10(3):370-377. https://doi.org/10.1007/s12178-017-9423-6
4. The Society of Hospital Medicine. The Evolution of Co-Management. 2017. Accessed October 30, 2019. https://www.hospitalmedicine.org/globalassets/practice-management/practice-management-pdf/pm-19-0004-co-management-white-paper_minor-update-m.pdf
5. Rohatgi N, Loftus P, Grujic O, Cullen M, Hopkins J, Ahuja N. Surgical comanagement by hospitalists improves patient outcomes: a propensity score analysis. Ann Surg. 2016;264(2):275-282. https://doi.org/10.1097/SLA.0000000000001629
6. Fitzgerald SJ, Palmer TC, Kraay MJ. Improved perioperative care of elective joint replacement patients: the impact of an orthopedic perioperative hospitalist. J Arthroplasty. 2018;33(8):2387-2391. https://doi,org/10.1016/j.arth.2018.03.029
7. Maxwell BG, Mirza A. Medical comanagement of hip fracture patients is not associated with superior perioperative outcomes: a propensity score-matched retrospective cohort analysis of the National Surgical Quality Improvement Project. J Hosp Med. 2019;14:E1-E7. https://doi.org/10.12788/jhm.3343
8. Centers for Medicare & Medicaid Services. Medicare Program; Comprehensive Care for Joint Replacement Payment Model for Acute Care Hospitals Furnishing Lower Extremity Joint Replacement Services; Final Rule. November 24, 2015. https://www.govinfo.gov/content/pkg/FR-2015-11-24/pdf/2015-29438.pdf
9. Avram V, Petruccelli D, Winemaker M, de Beer J. Total joint arthroplasty readmission rates and reasons for 30-day hospital readmission. J Arthroplasty. 2014;29(3):465-468. https://doi.org/10.1016/j.arth.2013.07.039
10. ICD-10-CM/PCS MS-DRG v37.0 Definitions Manual. Accessed April 27, 2020. https://www.cms.gov/icd10m/version37-fullcode-cms/fullcode_cms/P0031.html
11. Chaudhary NS, Donnelly JP, Wang HE. Racial differences in sepsis mortality at United States academic medical center-affiliated hospitals. Crit Care Med. 2018;46(6):878-883. https://doi.org/10.1097/CCM.0000000000003020
12. Clair AJ, Evangelista PJ, Lajam CM, Slover JD, Bosco JA, Iorio R. Cost analysis of total joint arthroplasty readmissions in a Bundled Payment Care Improvement Initiative. J Arthroplasty. 2016;31(9):1862-1865.
13. Kaiser Family Foundation. Hospital Adjusted Expenses per Inpatient Day by Ownership. Kaiser Family Foundation. Accessed April 27, 2020. https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D
14. Goldstein JN, Zhang Z, Schwartz JS, Hicks LS. Observation status, poverty, and high financial liability among Medicare beneficiaries. Am J Med. 2018;131(1):101.e9-101.e15. https://doi.org/10.1016/j.amjmed.2017.07.013
15. Lind KD, Noel-Miller CM, Sangaralingham LR, et al. Increasing trends in the use of hospital observation services for older Medicare Advantage and privately insured patients. Med Care Res Rev. 2019;76(2):229-239. https://doi.org/10.1177/1077558717718026
16. Sabbatini AK, Wright B. Excluding observation stays from readmission rates - what quality measures are missing. N Engl J Med. 2018;378(22):2062-2065. https://doi.org/10.1056/NEJMp1800732
17. Gabayan GZ, Doyle B, Liang, L, Donkor K, Huang, D, Sarkisian CA. Who has an unsuccessful observation care stay? Healthcare (Basel). 2018;6(4):138. https://doi.org/10.3390/healthcare6040138
18. Fang M, Hume E, Ibrahim S. Race, Bundled payment policy, and discharge destination after TKA: the experience of an urban academic hospital. Geriatr Orthop Surg Rehabil. 2018. https://doi.org/10.1177/2151459318803222
© 2020 Society of Hospital Medicine
Quantifying the Risks of Hospitalization—Is It Really as Safe as We Believe?
Even though I could not remember her name, I remembered her story, and I would bet that my colleagues did as well. She was someone that we had all cared for at one time or another. She frequently presented to the hospital with chest pain or shortness of breath attributable to a combination of longstanding congestive heart failure, chronic obstructive pulmonary disease, and cocaine abuse. But most tragic of all, she was homeless, which meant that she was frequently hospitalized not only for medical complaints but also for a night’s shelter and a bite of food. Even though she often refused medical treatment and social workers’ efforts to stabilize her housing situation, the staff in the emergency room and observation unit all knew her by name and greeted her like an old friend. And then one day she stopped showing up to the hospital. Sitting in the emergency department (ED), I overheard that she was found outside of a storefront and had passed away. Saddened by her death, which was not unexpected given her medical issues, I still wondered if we had done right by her during the hundreds of times that she had come to our hospital. Clinicians at busy safety-net hospitals face these questions every day, and it would seem beyond doubt that our duty is to address both medical and nonmedical determinants of health of everyone that walks through our door. But is this in fact the right thing to do? Is it possible that we unwittingly expose these vulnerable patients to risks from hospitalization alone?
In this month’s Journal of Hospital Medicine, Sekijima et al. sought to quantify precisely the risks of hospitalization, particularly among the subset of patients whose “severity” of medical problems alone might not have warranted hospital admission, a scenario known colloquially as a “social” admission.1 In real time, an inhouse triage physician classified patients as being admitted with or without “definite medical acuity.” Investigators retrospectively identified adverse events and illness acuity using standardized instruments, the Institute for Healthcare Improvement Global Trigger Tool and Emergency Severity Index, respectively. Despite the acknowledged differences in the patient population and the inherent subjectivity within the designation process, Sekijima et al. found no statistically significant difference in the percentage of admissions with an adverse event nor in the rate of adverse events per 1,000 patient days. Falls, oversedation/hypotension and code/arrest/rapid response activation were the most frequently encountered adverse events.
Delving deeper into the origin of admissions without definite medical acuity, the authors identified homelessness, lack of outpatient social support, substance use disorder, and lack of outpatient medical support as the most common reasons for “nonmedical” admissions. As healthcare providers, we recognize that these factors are generally long-term, chronic socioeconomic determinants of health. Despite our objective knowledge that we are limited in our ability to fix these problems on a short-term basis, the authors’ observations reflect our compulsion to try and help in any way possible. Patients admitted without definite medical acuity were more vulnerable and had higher rates of public insurance and housing insecurity. However, they were less acutely ill, as indicated by lower Emergency Severity Index scores. These factors were not associated with statistically significant differences in either 48-hour ED readmission or 30-day hospital readmission rates.
The process of appropriately triaging patients to an inpatient setting is challenging because of wide variability in both patients and ED providers. Hospitalists are increasingly recognized as an additional resource to assist in the triage process, as we are uniquely in a position to view the patient’s clinical presentation within the context of their anticipated clinical trajectory, promote effective utilization of inpatient bed availability, and anticipate potential barriers to discharge. Graduate medical education now identifies the triage process as a specific milestone within the transitions of care competency, as it requires mastery of interpersonal communication, professionalism, systems-based thinking, and patient-centered care.2 However, many institutions lack a dedicated faculty member to perform the triage role. Our institution recently examined the feasibility of instituting a daily “huddle” between the admitting hospitalist and the ED to facilitate interdepartmental communication to create care plans in patient triage and to promote patient throughput. Available admission beds are valuable commodities, and one challenge is that the ED makes disposition decisions without knowledge of the number of available beds in the hospital. The goal of the huddle was to quickly discuss all patients potentially requiring admission prior to the final disposition decision and to address any modifiable factors to potentially prevent a “social” admission with social work early in the day. Further work is in progress to determine if introducing flexibility within existing provider roles can improve the triage process in a measurable and efficient manner.
Many challenges remain as we balance the medical needs of patients with any potential social drivers that necessitate admission to the inpatient hospital setting. From an ED perspective, social support and community follow-up were “universally considered powerful influences on admission,” and other factors such as time of day, clinical volume, and the four-hour waiting time target also played a significant role in the decision to admit.3 Hunter et al. found that admissions with moderate to low acuity may be shorter or less costly,4 which presents an interesting question of cost-effectiveness as an avenue for further study. As clinicians, we are intuitively aware of the subjective risk of hospitalization itself, and this work provides new objective evidence that hospitalization confers specific and quantifiable risks. Though we can undoubtedly use this knowledge to guide internal decisions about admissions and discharges, do we also have an obligation to inform our patients about these risks in real time? Ultimately, hospitalization itself might be viewed as a “procedure” or intervention that has inherent risks for all who receive it, regardless of the individual patient or hospital characteristics. As hospitalists, we should continue to strive to reduce these risks, but we should also initiate a conversation about the risks and benefits of hospitalization similarly to how we discuss other procedures with patients and their families.
1. Sekijima A, Sunga C, Bann M. Adverse events experienced by patients hospitalized without definite medical acuity: A retrospective cohort study. J Hosp Med. 2020;15(1):42-45. https://doi.org/10.12788/jhm.3235.
2. Wang ES, Velásquez ST, Smith CJ, et al. Triaging inpatient admissions : An opportunity for resident education. J Gen Intern Med. 2019;34(5):754-757. https://doi.org/10.1007/s11606-019-04882-2.
3. Pope I, Burn H, Ismail SA, et al. A qualitative study exploring the factors influencing admission to hospital from the emergency department. BMJ Open. 2017;7(8):e011543. https://doi.org/10.1136/bmjopen-2016-011543.
4. Lewis Hunter AE, Spatz ES, Bernstein SL, Rosenthal MS. Factors influencing hospital admission of non-critically ill patients presenting to the emergency department: a cross-sectional study. J Gen Intern Med. 2016;31(1):37-44. https://doi.org/10.1007/s11606-015-3438-8.
Even though I could not remember her name, I remembered her story, and I would bet that my colleagues did as well. She was someone that we had all cared for at one time or another. She frequently presented to the hospital with chest pain or shortness of breath attributable to a combination of longstanding congestive heart failure, chronic obstructive pulmonary disease, and cocaine abuse. But most tragic of all, she was homeless, which meant that she was frequently hospitalized not only for medical complaints but also for a night’s shelter and a bite of food. Even though she often refused medical treatment and social workers’ efforts to stabilize her housing situation, the staff in the emergency room and observation unit all knew her by name and greeted her like an old friend. And then one day she stopped showing up to the hospital. Sitting in the emergency department (ED), I overheard that she was found outside of a storefront and had passed away. Saddened by her death, which was not unexpected given her medical issues, I still wondered if we had done right by her during the hundreds of times that she had come to our hospital. Clinicians at busy safety-net hospitals face these questions every day, and it would seem beyond doubt that our duty is to address both medical and nonmedical determinants of health of everyone that walks through our door. But is this in fact the right thing to do? Is it possible that we unwittingly expose these vulnerable patients to risks from hospitalization alone?
In this month’s Journal of Hospital Medicine, Sekijima et al. sought to quantify precisely the risks of hospitalization, particularly among the subset of patients whose “severity” of medical problems alone might not have warranted hospital admission, a scenario known colloquially as a “social” admission.1 In real time, an inhouse triage physician classified patients as being admitted with or without “definite medical acuity.” Investigators retrospectively identified adverse events and illness acuity using standardized instruments, the Institute for Healthcare Improvement Global Trigger Tool and Emergency Severity Index, respectively. Despite the acknowledged differences in the patient population and the inherent subjectivity within the designation process, Sekijima et al. found no statistically significant difference in the percentage of admissions with an adverse event nor in the rate of adverse events per 1,000 patient days. Falls, oversedation/hypotension and code/arrest/rapid response activation were the most frequently encountered adverse events.
Delving deeper into the origin of admissions without definite medical acuity, the authors identified homelessness, lack of outpatient social support, substance use disorder, and lack of outpatient medical support as the most common reasons for “nonmedical” admissions. As healthcare providers, we recognize that these factors are generally long-term, chronic socioeconomic determinants of health. Despite our objective knowledge that we are limited in our ability to fix these problems on a short-term basis, the authors’ observations reflect our compulsion to try and help in any way possible. Patients admitted without definite medical acuity were more vulnerable and had higher rates of public insurance and housing insecurity. However, they were less acutely ill, as indicated by lower Emergency Severity Index scores. These factors were not associated with statistically significant differences in either 48-hour ED readmission or 30-day hospital readmission rates.
The process of appropriately triaging patients to an inpatient setting is challenging because of wide variability in both patients and ED providers. Hospitalists are increasingly recognized as an additional resource to assist in the triage process, as we are uniquely in a position to view the patient’s clinical presentation within the context of their anticipated clinical trajectory, promote effective utilization of inpatient bed availability, and anticipate potential barriers to discharge. Graduate medical education now identifies the triage process as a specific milestone within the transitions of care competency, as it requires mastery of interpersonal communication, professionalism, systems-based thinking, and patient-centered care.2 However, many institutions lack a dedicated faculty member to perform the triage role. Our institution recently examined the feasibility of instituting a daily “huddle” between the admitting hospitalist and the ED to facilitate interdepartmental communication to create care plans in patient triage and to promote patient throughput. Available admission beds are valuable commodities, and one challenge is that the ED makes disposition decisions without knowledge of the number of available beds in the hospital. The goal of the huddle was to quickly discuss all patients potentially requiring admission prior to the final disposition decision and to address any modifiable factors to potentially prevent a “social” admission with social work early in the day. Further work is in progress to determine if introducing flexibility within existing provider roles can improve the triage process in a measurable and efficient manner.
Many challenges remain as we balance the medical needs of patients with any potential social drivers that necessitate admission to the inpatient hospital setting. From an ED perspective, social support and community follow-up were “universally considered powerful influences on admission,” and other factors such as time of day, clinical volume, and the four-hour waiting time target also played a significant role in the decision to admit.3 Hunter et al. found that admissions with moderate to low acuity may be shorter or less costly,4 which presents an interesting question of cost-effectiveness as an avenue for further study. As clinicians, we are intuitively aware of the subjective risk of hospitalization itself, and this work provides new objective evidence that hospitalization confers specific and quantifiable risks. Though we can undoubtedly use this knowledge to guide internal decisions about admissions and discharges, do we also have an obligation to inform our patients about these risks in real time? Ultimately, hospitalization itself might be viewed as a “procedure” or intervention that has inherent risks for all who receive it, regardless of the individual patient or hospital characteristics. As hospitalists, we should continue to strive to reduce these risks, but we should also initiate a conversation about the risks and benefits of hospitalization similarly to how we discuss other procedures with patients and their families.
Even though I could not remember her name, I remembered her story, and I would bet that my colleagues did as well. She was someone that we had all cared for at one time or another. She frequently presented to the hospital with chest pain or shortness of breath attributable to a combination of longstanding congestive heart failure, chronic obstructive pulmonary disease, and cocaine abuse. But most tragic of all, she was homeless, which meant that she was frequently hospitalized not only for medical complaints but also for a night’s shelter and a bite of food. Even though she often refused medical treatment and social workers’ efforts to stabilize her housing situation, the staff in the emergency room and observation unit all knew her by name and greeted her like an old friend. And then one day she stopped showing up to the hospital. Sitting in the emergency department (ED), I overheard that she was found outside of a storefront and had passed away. Saddened by her death, which was not unexpected given her medical issues, I still wondered if we had done right by her during the hundreds of times that she had come to our hospital. Clinicians at busy safety-net hospitals face these questions every day, and it would seem beyond doubt that our duty is to address both medical and nonmedical determinants of health of everyone that walks through our door. But is this in fact the right thing to do? Is it possible that we unwittingly expose these vulnerable patients to risks from hospitalization alone?
In this month’s Journal of Hospital Medicine, Sekijima et al. sought to quantify precisely the risks of hospitalization, particularly among the subset of patients whose “severity” of medical problems alone might not have warranted hospital admission, a scenario known colloquially as a “social” admission.1 In real time, an inhouse triage physician classified patients as being admitted with or without “definite medical acuity.” Investigators retrospectively identified adverse events and illness acuity using standardized instruments, the Institute for Healthcare Improvement Global Trigger Tool and Emergency Severity Index, respectively. Despite the acknowledged differences in the patient population and the inherent subjectivity within the designation process, Sekijima et al. found no statistically significant difference in the percentage of admissions with an adverse event nor in the rate of adverse events per 1,000 patient days. Falls, oversedation/hypotension and code/arrest/rapid response activation were the most frequently encountered adverse events.
Delving deeper into the origin of admissions without definite medical acuity, the authors identified homelessness, lack of outpatient social support, substance use disorder, and lack of outpatient medical support as the most common reasons for “nonmedical” admissions. As healthcare providers, we recognize that these factors are generally long-term, chronic socioeconomic determinants of health. Despite our objective knowledge that we are limited in our ability to fix these problems on a short-term basis, the authors’ observations reflect our compulsion to try and help in any way possible. Patients admitted without definite medical acuity were more vulnerable and had higher rates of public insurance and housing insecurity. However, they were less acutely ill, as indicated by lower Emergency Severity Index scores. These factors were not associated with statistically significant differences in either 48-hour ED readmission or 30-day hospital readmission rates.
The process of appropriately triaging patients to an inpatient setting is challenging because of wide variability in both patients and ED providers. Hospitalists are increasingly recognized as an additional resource to assist in the triage process, as we are uniquely in a position to view the patient’s clinical presentation within the context of their anticipated clinical trajectory, promote effective utilization of inpatient bed availability, and anticipate potential barriers to discharge. Graduate medical education now identifies the triage process as a specific milestone within the transitions of care competency, as it requires mastery of interpersonal communication, professionalism, systems-based thinking, and patient-centered care.2 However, many institutions lack a dedicated faculty member to perform the triage role. Our institution recently examined the feasibility of instituting a daily “huddle” between the admitting hospitalist and the ED to facilitate interdepartmental communication to create care plans in patient triage and to promote patient throughput. Available admission beds are valuable commodities, and one challenge is that the ED makes disposition decisions without knowledge of the number of available beds in the hospital. The goal of the huddle was to quickly discuss all patients potentially requiring admission prior to the final disposition decision and to address any modifiable factors to potentially prevent a “social” admission with social work early in the day. Further work is in progress to determine if introducing flexibility within existing provider roles can improve the triage process in a measurable and efficient manner.
Many challenges remain as we balance the medical needs of patients with any potential social drivers that necessitate admission to the inpatient hospital setting. From an ED perspective, social support and community follow-up were “universally considered powerful influences on admission,” and other factors such as time of day, clinical volume, and the four-hour waiting time target also played a significant role in the decision to admit.3 Hunter et al. found that admissions with moderate to low acuity may be shorter or less costly,4 which presents an interesting question of cost-effectiveness as an avenue for further study. As clinicians, we are intuitively aware of the subjective risk of hospitalization itself, and this work provides new objective evidence that hospitalization confers specific and quantifiable risks. Though we can undoubtedly use this knowledge to guide internal decisions about admissions and discharges, do we also have an obligation to inform our patients about these risks in real time? Ultimately, hospitalization itself might be viewed as a “procedure” or intervention that has inherent risks for all who receive it, regardless of the individual patient or hospital characteristics. As hospitalists, we should continue to strive to reduce these risks, but we should also initiate a conversation about the risks and benefits of hospitalization similarly to how we discuss other procedures with patients and their families.
1. Sekijima A, Sunga C, Bann M. Adverse events experienced by patients hospitalized without definite medical acuity: A retrospective cohort study. J Hosp Med. 2020;15(1):42-45. https://doi.org/10.12788/jhm.3235.
2. Wang ES, Velásquez ST, Smith CJ, et al. Triaging inpatient admissions : An opportunity for resident education. J Gen Intern Med. 2019;34(5):754-757. https://doi.org/10.1007/s11606-019-04882-2.
3. Pope I, Burn H, Ismail SA, et al. A qualitative study exploring the factors influencing admission to hospital from the emergency department. BMJ Open. 2017;7(8):e011543. https://doi.org/10.1136/bmjopen-2016-011543.
4. Lewis Hunter AE, Spatz ES, Bernstein SL, Rosenthal MS. Factors influencing hospital admission of non-critically ill patients presenting to the emergency department: a cross-sectional study. J Gen Intern Med. 2016;31(1):37-44. https://doi.org/10.1007/s11606-015-3438-8.
1. Sekijima A, Sunga C, Bann M. Adverse events experienced by patients hospitalized without definite medical acuity: A retrospective cohort study. J Hosp Med. 2020;15(1):42-45. https://doi.org/10.12788/jhm.3235.
2. Wang ES, Velásquez ST, Smith CJ, et al. Triaging inpatient admissions : An opportunity for resident education. J Gen Intern Med. 2019;34(5):754-757. https://doi.org/10.1007/s11606-019-04882-2.
3. Pope I, Burn H, Ismail SA, et al. A qualitative study exploring the factors influencing admission to hospital from the emergency department. BMJ Open. 2017;7(8):e011543. https://doi.org/10.1136/bmjopen-2016-011543.
4. Lewis Hunter AE, Spatz ES, Bernstein SL, Rosenthal MS. Factors influencing hospital admission of non-critically ill patients presenting to the emergency department: a cross-sectional study. J Gen Intern Med. 2016;31(1):37-44. https://doi.org/10.1007/s11606-015-3438-8.
© 2020 Society of Hospital Medicine
Is Hospital Discharge the Rube Goldberg Machine of Academic Internal Medicine?
One of the least taught yet most complicated tasks confronting new trainees is the bewildering process of discharging a patient. On an internal medicine service, this process can often resemble a Rube Goldberg machine, in which a “simple” task is accomplished through a series of interconnected, almost comically convoluted, yet separate steps that are triggered one after another and must be executed perfectly in sequence for success. It seems easy at first; just tap out a few sentences in the discharge paperwork, do a quick medication reconciliation, and a click of a button later, voila! The patient magically falls off the list and is on their merry way home. In reality, it only takes one wrench thrown into the Rube Goldberg machine to take down the whole operation. Much to the chagrin of internal medicine interns across the country, residents quickly learn that discharge planning is usually far from straightforward and that a myriad of obstacles (often dynamic and frustratingly unpredictable) can stand in the way of a successful discharge.
While some surgical services can streamline discharge processes to target defined lengths of stay based on a particular diagnosis, general medicine patients tend to have greater numbers of comorbid conditions, complex hospital courses, and wider variation in access to posthospital healthcare. In addition, there is very little formal instruction in transitions of care, and most residents identify direct patient care (learning by doing) as the primary mode of education.1,2 Struggling through the process of finding an appropriate placement, ensuring adequate outpatient follow-up, and untangling a jumbled mess of a medication reconciliation is often the only way that housestaff learn the Sisyphean task of transitioning care out of the hospital. The unpredictability and intensity of patient care adds to the ever growing list of competing demands on the time and attention of residents. Attendings face pressure on all sides to not only provide exemplary patient care and an educational experience but also to optimize hospital throughput by discharging patients as soon as possible (and ideally before noon). No wonder that the discharge process can threaten to unravel at any time, with delays and complications in discharge metamorphosing into increased length of stay (LOS), poorer outcomes, and increased 30-day readmission rates. As on-the-ground providers, what realities do we face when challenging ourselves to discharge patients before noon, and what practical changes in our workflow can we make to reach this goal?
In this month’s Journal of Hospital Medicine, Zoucha et al. examine these questions in real time by identifying barriers preventing both “definite” and “possible” discharges at three representative time points over the course of randomly chosen weekdays. They surveyed both housestaff and attendings at five academic hospitals across the United States, and the majority of patients were cared for on teaching services.3 Reflecting the inherent differences in workflow between teaching and nonteaching services, delays in definite discharges on teaching services were most often hindered by completing rounds and the need to staff the patient with the attending, whereas nonresident services identified other patient-care-related (both urgent and nonurgent) issues to be the culprits. Late-afternoon discharges were delayed on teaching services due to outstanding paperwork and follow-up arrangements, both of which most senior residents are keenly aware of and make their best effort to complete ahead of time. Patients designated as “possible” discharges were awaiting clinical improvement and resolution of disposition issues dependent on social work and safe placement, which reasonably seemed independent of service type. These descriptive findings suggest that nonresident services are more efficient than resident teams, and we are keen to identify novel solutions, such as dedicated discharge coordinators,4 to facilitate the discharge process on resident teams without detracting from the educational value of the rotation.
Zoucha et al. also found that factors beyond our control (having a lower daily census, attending on a nonresident service) were significantly associated with both earlier discharge order entry times and the actual time of patient discharge.3 While it is tempting to foist the entirety of the blame on extrinsic factors such as discharge placement and insurance issues, the reality is there might be some workflow changes that could expedite the discharge process. The authors are correct to emphasize that rounding style, in which discharges are prioritized to be seen first, is a behavior modification worth targeting. The percentage of teams that routinely see discharges first is not well studied, as other factors, such as clinically unstable patients, new admissions from overnight, and even mundane characteristics such as geographic location in the hospital, can also compete for prioritization in rounding order. Given the authors’ findings, we are eager to see further work in this area as prioritization of discharges during rounds could conceivably be studied within the context of a randomized controlled trial. Other innovations in rounding styles such as rounding-in-flow5 (in which all tasks are completed for a single patient before rounding on the next patient) can also significantly reduce the time to discharge order placement.
With help from the Penn Medicine Center for Health Care Innovation, we are actively studying bottlenecks in the discharge process by developing an interactive platform focused on delivering real-time information to all members of the healthcare team. Rapid rounds are held every morning with the attending physician, floor nursing leadership, physical therapy, social worker, and case management to quickly identify pending tasks, anticipated disposition, and a target date of discharge. Efficiency is key, as each team is limited to approximately 5-10 minutes. Previous studies (mostly pre–post studies) have shown that this simple intervention significantly reduced LOS,6,7 increased rates of discharge before noon,8 and was improved by electronic tracking tools.9 Our multidisciplinary rounds are unique in that information is then entered into an intuitive, web-based platform, which allows consolidation and analysis that permits generation of real-time statistics. By standardizing the discharge planning process, we hope to streamline a previously fragmented process and maximize the efficiency of hospital resource utilization.
Ultimately, high-quality care of complex patients on internal medicine services from admission to discharge requires hard work, smart utilization of resources, and a little bit of luck. There may not be a secret ingredient that guarantees perfectly efficient discharges 100% of the time, but this study inspires us to ponder additional approaches to this longstanding problem. The authors are to be congratulated for a rigorous study that illuminates where we as healthcare providers are able to realistically intervene to expedite the discharge process. First, having a lower census cap may not be possible in this era of maximal hospital usage, but this work suggests that thoughtful management of time on rounds may be a way to address the underlying problem. Secondly, the superior efficiency of nonteaching services may merely reflect the increased experience of the providers, and a realistic solution could be to implement a formal curriculum to educate housestaff about the discharge process, which would simultaneously address residency competency standards for transitions of care. Finally, the role of innovative informatics tools will surely open further avenues of investigation, as we continually evolve in response to intensifying standards of modern, efficient healthcare delivery in the 21st century. It may not be possible to eliminate the complexity from this particular Rube Goldberg machine, but taking the steps above may allow us to implement as many fail-safes as we can.
Disclosures
The authors have nothing to disclose.
1. Young E, Stickrath C, McNulty M, et al. Residents’ exposure to educational experiences in facilitating hospital discharges. J Grad Med Educ. 2017;9(2):184-189. doi: 10.4300/JGME-D-16-00503.1. PubMed
2. Greysen SR, Schiliro D, Curry L, et al. “Learning by doing” - Resident perspectives on developing competency in high-quality discharge care. J Gen Intern Med. 2012;27(9):1188-1194. doi: 10.1007/s11606-012-2094-5. PubMed
3. Zoucha J, Hull M, Keniston A, et al. Barriers to Early Hospital Discharge: A Cross-Sectional Study at Five Academic Hospitals. J Hosp Med. 2018;13(12):816-822. doi: 10.12788/jhm.3074. PubMed
4. Finn KM, Heffner R, Chang Y, et al. Improving the discharge process by embedding a discharge facilitator in a resident team. J Hosp Med. 2011;6(9):494-500. doi: 10.1002/jhm.924. PubMed
5. Calderon AS, Blackmore CC, Williams BL, et al. Transforming ward rounds through rounding-in-flow. J Grad Med Educ. 2014;6(4):750-755. doi: 10.4300/JGME-D-13-00324.1. PubMed
6. Kane M, Rohatgi N, Heidenreich PA, et al. Lean-based redesign of multidisciplinary rounds on general medicine service. J Hosp Med. 2018;13(7):482-485. doi: 10.12788/jhm.2908. PubMed
7. Gonçalves-Bradley D, Lannin N, Clemson L, Cameron ID, Shepperd S. Discharge planning from hospital. Cochrane Database Syst Rev. 2016;1-3. doi: 10.1002/14651858.CD000313.pub5.www.cochranelibrary.com. PubMed
8. Wertheimer B, Jacobs REA, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi: 10.1002/jhm.2154. PubMed
9. Meo N, Paul E, Wilson C, Powers J, Magbual M, Miles KM. Introducing an electronic tracking tool into daily multidisciplinary discharge rounds on a medicine service: a quality improvement project to reduce length of stay. BMJ Open Qual. 2018;7(3):e000174. doi: 10.1136/bmjoq-2017-000174. PubMed
One of the least taught yet most complicated tasks confronting new trainees is the bewildering process of discharging a patient. On an internal medicine service, this process can often resemble a Rube Goldberg machine, in which a “simple” task is accomplished through a series of interconnected, almost comically convoluted, yet separate steps that are triggered one after another and must be executed perfectly in sequence for success. It seems easy at first; just tap out a few sentences in the discharge paperwork, do a quick medication reconciliation, and a click of a button later, voila! The patient magically falls off the list and is on their merry way home. In reality, it only takes one wrench thrown into the Rube Goldberg machine to take down the whole operation. Much to the chagrin of internal medicine interns across the country, residents quickly learn that discharge planning is usually far from straightforward and that a myriad of obstacles (often dynamic and frustratingly unpredictable) can stand in the way of a successful discharge.
While some surgical services can streamline discharge processes to target defined lengths of stay based on a particular diagnosis, general medicine patients tend to have greater numbers of comorbid conditions, complex hospital courses, and wider variation in access to posthospital healthcare. In addition, there is very little formal instruction in transitions of care, and most residents identify direct patient care (learning by doing) as the primary mode of education.1,2 Struggling through the process of finding an appropriate placement, ensuring adequate outpatient follow-up, and untangling a jumbled mess of a medication reconciliation is often the only way that housestaff learn the Sisyphean task of transitioning care out of the hospital. The unpredictability and intensity of patient care adds to the ever growing list of competing demands on the time and attention of residents. Attendings face pressure on all sides to not only provide exemplary patient care and an educational experience but also to optimize hospital throughput by discharging patients as soon as possible (and ideally before noon). No wonder that the discharge process can threaten to unravel at any time, with delays and complications in discharge metamorphosing into increased length of stay (LOS), poorer outcomes, and increased 30-day readmission rates. As on-the-ground providers, what realities do we face when challenging ourselves to discharge patients before noon, and what practical changes in our workflow can we make to reach this goal?
In this month’s Journal of Hospital Medicine, Zoucha et al. examine these questions in real time by identifying barriers preventing both “definite” and “possible” discharges at three representative time points over the course of randomly chosen weekdays. They surveyed both housestaff and attendings at five academic hospitals across the United States, and the majority of patients were cared for on teaching services.3 Reflecting the inherent differences in workflow between teaching and nonteaching services, delays in definite discharges on teaching services were most often hindered by completing rounds and the need to staff the patient with the attending, whereas nonresident services identified other patient-care-related (both urgent and nonurgent) issues to be the culprits. Late-afternoon discharges were delayed on teaching services due to outstanding paperwork and follow-up arrangements, both of which most senior residents are keenly aware of and make their best effort to complete ahead of time. Patients designated as “possible” discharges were awaiting clinical improvement and resolution of disposition issues dependent on social work and safe placement, which reasonably seemed independent of service type. These descriptive findings suggest that nonresident services are more efficient than resident teams, and we are keen to identify novel solutions, such as dedicated discharge coordinators,4 to facilitate the discharge process on resident teams without detracting from the educational value of the rotation.
Zoucha et al. also found that factors beyond our control (having a lower daily census, attending on a nonresident service) were significantly associated with both earlier discharge order entry times and the actual time of patient discharge.3 While it is tempting to foist the entirety of the blame on extrinsic factors such as discharge placement and insurance issues, the reality is there might be some workflow changes that could expedite the discharge process. The authors are correct to emphasize that rounding style, in which discharges are prioritized to be seen first, is a behavior modification worth targeting. The percentage of teams that routinely see discharges first is not well studied, as other factors, such as clinically unstable patients, new admissions from overnight, and even mundane characteristics such as geographic location in the hospital, can also compete for prioritization in rounding order. Given the authors’ findings, we are eager to see further work in this area as prioritization of discharges during rounds could conceivably be studied within the context of a randomized controlled trial. Other innovations in rounding styles such as rounding-in-flow5 (in which all tasks are completed for a single patient before rounding on the next patient) can also significantly reduce the time to discharge order placement.
With help from the Penn Medicine Center for Health Care Innovation, we are actively studying bottlenecks in the discharge process by developing an interactive platform focused on delivering real-time information to all members of the healthcare team. Rapid rounds are held every morning with the attending physician, floor nursing leadership, physical therapy, social worker, and case management to quickly identify pending tasks, anticipated disposition, and a target date of discharge. Efficiency is key, as each team is limited to approximately 5-10 minutes. Previous studies (mostly pre–post studies) have shown that this simple intervention significantly reduced LOS,6,7 increased rates of discharge before noon,8 and was improved by electronic tracking tools.9 Our multidisciplinary rounds are unique in that information is then entered into an intuitive, web-based platform, which allows consolidation and analysis that permits generation of real-time statistics. By standardizing the discharge planning process, we hope to streamline a previously fragmented process and maximize the efficiency of hospital resource utilization.
Ultimately, high-quality care of complex patients on internal medicine services from admission to discharge requires hard work, smart utilization of resources, and a little bit of luck. There may not be a secret ingredient that guarantees perfectly efficient discharges 100% of the time, but this study inspires us to ponder additional approaches to this longstanding problem. The authors are to be congratulated for a rigorous study that illuminates where we as healthcare providers are able to realistically intervene to expedite the discharge process. First, having a lower census cap may not be possible in this era of maximal hospital usage, but this work suggests that thoughtful management of time on rounds may be a way to address the underlying problem. Secondly, the superior efficiency of nonteaching services may merely reflect the increased experience of the providers, and a realistic solution could be to implement a formal curriculum to educate housestaff about the discharge process, which would simultaneously address residency competency standards for transitions of care. Finally, the role of innovative informatics tools will surely open further avenues of investigation, as we continually evolve in response to intensifying standards of modern, efficient healthcare delivery in the 21st century. It may not be possible to eliminate the complexity from this particular Rube Goldberg machine, but taking the steps above may allow us to implement as many fail-safes as we can.
Disclosures
The authors have nothing to disclose.
One of the least taught yet most complicated tasks confronting new trainees is the bewildering process of discharging a patient. On an internal medicine service, this process can often resemble a Rube Goldberg machine, in which a “simple” task is accomplished through a series of interconnected, almost comically convoluted, yet separate steps that are triggered one after another and must be executed perfectly in sequence for success. It seems easy at first; just tap out a few sentences in the discharge paperwork, do a quick medication reconciliation, and a click of a button later, voila! The patient magically falls off the list and is on their merry way home. In reality, it only takes one wrench thrown into the Rube Goldberg machine to take down the whole operation. Much to the chagrin of internal medicine interns across the country, residents quickly learn that discharge planning is usually far from straightforward and that a myriad of obstacles (often dynamic and frustratingly unpredictable) can stand in the way of a successful discharge.
While some surgical services can streamline discharge processes to target defined lengths of stay based on a particular diagnosis, general medicine patients tend to have greater numbers of comorbid conditions, complex hospital courses, and wider variation in access to posthospital healthcare. In addition, there is very little formal instruction in transitions of care, and most residents identify direct patient care (learning by doing) as the primary mode of education.1,2 Struggling through the process of finding an appropriate placement, ensuring adequate outpatient follow-up, and untangling a jumbled mess of a medication reconciliation is often the only way that housestaff learn the Sisyphean task of transitioning care out of the hospital. The unpredictability and intensity of patient care adds to the ever growing list of competing demands on the time and attention of residents. Attendings face pressure on all sides to not only provide exemplary patient care and an educational experience but also to optimize hospital throughput by discharging patients as soon as possible (and ideally before noon). No wonder that the discharge process can threaten to unravel at any time, with delays and complications in discharge metamorphosing into increased length of stay (LOS), poorer outcomes, and increased 30-day readmission rates. As on-the-ground providers, what realities do we face when challenging ourselves to discharge patients before noon, and what practical changes in our workflow can we make to reach this goal?
In this month’s Journal of Hospital Medicine, Zoucha et al. examine these questions in real time by identifying barriers preventing both “definite” and “possible” discharges at three representative time points over the course of randomly chosen weekdays. They surveyed both housestaff and attendings at five academic hospitals across the United States, and the majority of patients were cared for on teaching services.3 Reflecting the inherent differences in workflow between teaching and nonteaching services, delays in definite discharges on teaching services were most often hindered by completing rounds and the need to staff the patient with the attending, whereas nonresident services identified other patient-care-related (both urgent and nonurgent) issues to be the culprits. Late-afternoon discharges were delayed on teaching services due to outstanding paperwork and follow-up arrangements, both of which most senior residents are keenly aware of and make their best effort to complete ahead of time. Patients designated as “possible” discharges were awaiting clinical improvement and resolution of disposition issues dependent on social work and safe placement, which reasonably seemed independent of service type. These descriptive findings suggest that nonresident services are more efficient than resident teams, and we are keen to identify novel solutions, such as dedicated discharge coordinators,4 to facilitate the discharge process on resident teams without detracting from the educational value of the rotation.
Zoucha et al. also found that factors beyond our control (having a lower daily census, attending on a nonresident service) were significantly associated with both earlier discharge order entry times and the actual time of patient discharge.3 While it is tempting to foist the entirety of the blame on extrinsic factors such as discharge placement and insurance issues, the reality is there might be some workflow changes that could expedite the discharge process. The authors are correct to emphasize that rounding style, in which discharges are prioritized to be seen first, is a behavior modification worth targeting. The percentage of teams that routinely see discharges first is not well studied, as other factors, such as clinically unstable patients, new admissions from overnight, and even mundane characteristics such as geographic location in the hospital, can also compete for prioritization in rounding order. Given the authors’ findings, we are eager to see further work in this area as prioritization of discharges during rounds could conceivably be studied within the context of a randomized controlled trial. Other innovations in rounding styles such as rounding-in-flow5 (in which all tasks are completed for a single patient before rounding on the next patient) can also significantly reduce the time to discharge order placement.
With help from the Penn Medicine Center for Health Care Innovation, we are actively studying bottlenecks in the discharge process by developing an interactive platform focused on delivering real-time information to all members of the healthcare team. Rapid rounds are held every morning with the attending physician, floor nursing leadership, physical therapy, social worker, and case management to quickly identify pending tasks, anticipated disposition, and a target date of discharge. Efficiency is key, as each team is limited to approximately 5-10 minutes. Previous studies (mostly pre–post studies) have shown that this simple intervention significantly reduced LOS,6,7 increased rates of discharge before noon,8 and was improved by electronic tracking tools.9 Our multidisciplinary rounds are unique in that information is then entered into an intuitive, web-based platform, which allows consolidation and analysis that permits generation of real-time statistics. By standardizing the discharge planning process, we hope to streamline a previously fragmented process and maximize the efficiency of hospital resource utilization.
Ultimately, high-quality care of complex patients on internal medicine services from admission to discharge requires hard work, smart utilization of resources, and a little bit of luck. There may not be a secret ingredient that guarantees perfectly efficient discharges 100% of the time, but this study inspires us to ponder additional approaches to this longstanding problem. The authors are to be congratulated for a rigorous study that illuminates where we as healthcare providers are able to realistically intervene to expedite the discharge process. First, having a lower census cap may not be possible in this era of maximal hospital usage, but this work suggests that thoughtful management of time on rounds may be a way to address the underlying problem. Secondly, the superior efficiency of nonteaching services may merely reflect the increased experience of the providers, and a realistic solution could be to implement a formal curriculum to educate housestaff about the discharge process, which would simultaneously address residency competency standards for transitions of care. Finally, the role of innovative informatics tools will surely open further avenues of investigation, as we continually evolve in response to intensifying standards of modern, efficient healthcare delivery in the 21st century. It may not be possible to eliminate the complexity from this particular Rube Goldberg machine, but taking the steps above may allow us to implement as many fail-safes as we can.
Disclosures
The authors have nothing to disclose.
1. Young E, Stickrath C, McNulty M, et al. Residents’ exposure to educational experiences in facilitating hospital discharges. J Grad Med Educ. 2017;9(2):184-189. doi: 10.4300/JGME-D-16-00503.1. PubMed
2. Greysen SR, Schiliro D, Curry L, et al. “Learning by doing” - Resident perspectives on developing competency in high-quality discharge care. J Gen Intern Med. 2012;27(9):1188-1194. doi: 10.1007/s11606-012-2094-5. PubMed
3. Zoucha J, Hull M, Keniston A, et al. Barriers to Early Hospital Discharge: A Cross-Sectional Study at Five Academic Hospitals. J Hosp Med. 2018;13(12):816-822. doi: 10.12788/jhm.3074. PubMed
4. Finn KM, Heffner R, Chang Y, et al. Improving the discharge process by embedding a discharge facilitator in a resident team. J Hosp Med. 2011;6(9):494-500. doi: 10.1002/jhm.924. PubMed
5. Calderon AS, Blackmore CC, Williams BL, et al. Transforming ward rounds through rounding-in-flow. J Grad Med Educ. 2014;6(4):750-755. doi: 10.4300/JGME-D-13-00324.1. PubMed
6. Kane M, Rohatgi N, Heidenreich PA, et al. Lean-based redesign of multidisciplinary rounds on general medicine service. J Hosp Med. 2018;13(7):482-485. doi: 10.12788/jhm.2908. PubMed
7. Gonçalves-Bradley D, Lannin N, Clemson L, Cameron ID, Shepperd S. Discharge planning from hospital. Cochrane Database Syst Rev. 2016;1-3. doi: 10.1002/14651858.CD000313.pub5.www.cochranelibrary.com. PubMed
8. Wertheimer B, Jacobs REA, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi: 10.1002/jhm.2154. PubMed
9. Meo N, Paul E, Wilson C, Powers J, Magbual M, Miles KM. Introducing an electronic tracking tool into daily multidisciplinary discharge rounds on a medicine service: a quality improvement project to reduce length of stay. BMJ Open Qual. 2018;7(3):e000174. doi: 10.1136/bmjoq-2017-000174. PubMed
1. Young E, Stickrath C, McNulty M, et al. Residents’ exposure to educational experiences in facilitating hospital discharges. J Grad Med Educ. 2017;9(2):184-189. doi: 10.4300/JGME-D-16-00503.1. PubMed
2. Greysen SR, Schiliro D, Curry L, et al. “Learning by doing” - Resident perspectives on developing competency in high-quality discharge care. J Gen Intern Med. 2012;27(9):1188-1194. doi: 10.1007/s11606-012-2094-5. PubMed
3. Zoucha J, Hull M, Keniston A, et al. Barriers to Early Hospital Discharge: A Cross-Sectional Study at Five Academic Hospitals. J Hosp Med. 2018;13(12):816-822. doi: 10.12788/jhm.3074. PubMed
4. Finn KM, Heffner R, Chang Y, et al. Improving the discharge process by embedding a discharge facilitator in a resident team. J Hosp Med. 2011;6(9):494-500. doi: 10.1002/jhm.924. PubMed
5. Calderon AS, Blackmore CC, Williams BL, et al. Transforming ward rounds through rounding-in-flow. J Grad Med Educ. 2014;6(4):750-755. doi: 10.4300/JGME-D-13-00324.1. PubMed
6. Kane M, Rohatgi N, Heidenreich PA, et al. Lean-based redesign of multidisciplinary rounds on general medicine service. J Hosp Med. 2018;13(7):482-485. doi: 10.12788/jhm.2908. PubMed
7. Gonçalves-Bradley D, Lannin N, Clemson L, Cameron ID, Shepperd S. Discharge planning from hospital. Cochrane Database Syst Rev. 2016;1-3. doi: 10.1002/14651858.CD000313.pub5.www.cochranelibrary.com. PubMed
8. Wertheimer B, Jacobs REA, Bailey M, et al. Discharge before noon: an achievable hospital goal. J Hosp Med. 2014;9(4):210-214. doi: 10.1002/jhm.2154. PubMed
9. Meo N, Paul E, Wilson C, Powers J, Magbual M, Miles KM. Introducing an electronic tracking tool into daily multidisciplinary discharge rounds on a medicine service: a quality improvement project to reduce length of stay. BMJ Open Qual. 2018;7(3):e000174. doi: 10.1136/bmjoq-2017-000174. PubMed
© 2018 Society of Hospital Medicine