User login
Unusual Congenital Pulmonary Anomaly in an Adult Patient With Dyspnea
Anatomic variations may result in abnormal return from the pulmonary veins to the right side of the heart. This group of congenital anomalies, also known as partial anomalous pulmonary venous return (PAPVR), may connect oxygenated blood from the pulmonary vein to a systemic vein before reaching the right atrium. The most common PAPVR is derived from the left upper pulmonary vein, which then connects to the left innominate vein and drains into the superior vena cava (SVC).
Scimitar syndrome is a rare PAPVR variant in which part of or the entire right lung is drained by the pulmonary vein into the inferior vena cava (IVC), giving the curvilinear dimension the appearance of a Middle Eastern sword (scimitar). The syndrome is frequently associated with other abnormalities, such as right lung hypoplasia and abnormal right lung lobation, dextroposition of the heart, right pulmonary artery hypoplasia, systemic arterial blood supply to the right lower lung from the infradiaphragmatic aorta, atrial septal defects of the secundum type, right-sided diaphragmatic hernia, and horseshoe lung.1,2 The syndrome was first described in 1836 by Cooper during an autopsy of an infant, and Dotter diagnosed the first symptomatic patient in 1949.3,4
Case Report
A 62-year-old man, former smoker (40 pack-year), with a past medical history of arterial hypertension and asthma visited the clinic, reporting exertional dyspnea. He also reported oppressive, retrosternally located exertional chest pain, 6/10 in intensity, of 3 minutes’ duration that radiated to the right chest and ameliorated with rest. Symptoms had occurred every other day for the past year. His physical exam was remarkable for central obesity. Lung auscultation was essentially clear. There was no jugular vein distention. The patient’s heart showed a regular rate and rhythm without evidence of murmurs or gallops. There was no evidence of leg edema or cyanosis. The patient’s resting oxygen saturation of 98% remained unchanged after exercise.
Related: Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
An electrocardiogram showed normal sinus rhythm with no ischemic changes. A pulmonary function test showed a forced expiratory volume (FEV1) of 1.44 L (61% of predicted), forced vital capacity (FVC) of 1.99 L (68% of predicted), and slow vital capacity (SVC) of 2.09 L (60% of predicted), with an FEV1/SVC ratio of 68% of predicted. These results suggested moderate-to-severe obstructive ventilatory impairment.
There was no response to bronchodilator therapy. Lung volumes were measured by plethysmography. The residual volume (RV), total lung capacity (TLC), and RV/TLC ratio were 2.57 L (147% of predicted), 4.66 L (88% of predicted), and 55%, respectively, suggesting severe air trapping. Diffusion lung capacity (DLCO) testing revealed 16.95 mL/min/mm Hg (73% of predicted) when corrected by hemoglobin and DLCO/alveolar volume of 4.97 mL/min/mm Hg/L (114% of predicted). This result was consistent with a mild reduction of gas transfer, which normalized when corrected by alveolar volume.
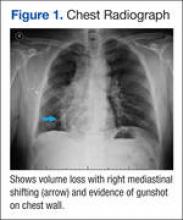
A posteroanterior chest radiograph image was remarkable for mediastinal shifting toward the right side, volume loss of the right lung, and evidence of a previous gunshot on the right chest wall (Figure 1). Previous chest imaging done in October 2009 showed an opacification of the right lower lung with indistinctness of the right cardiac border and partial obliteration of the right hemidiaphragm. The patient was treated with inhaled steroids and long- acting bronchodilators with partial improvement in dyspnea symptoms.
Myocardial perfusion imaging revealed scintigraphic evidence of heart rate-induced ischemia on the inferior and apical wall segments of the left ventricular myocardium. A transthoracic echocardiogram showed a very poor echocardiographic window. Left ventricular function seemed preserved. Transesophageal echocardiography was scheduled, but the patient missed the appointment.
Cardiac catheterization was only remarkable for 40% to 50% obstruction of the mid-left anterior descending artery, which did not explain the patient’s dyspnea or chest pain. Right side pressures were described as follows: right atrial mean, 10 mm Hg; right ventricle, 36/8 mm Hg; pulmonary artery, 33/16 mm Hg; pulmonary artery mean, 23 mm Hg; pulmonary capillary wedge pressure, 12 mm Hg; and a mean arterial pressure of 100 mm Hg. He had a left ventricle ejection fraction of 60%.
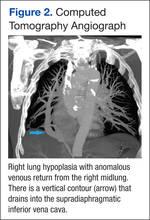
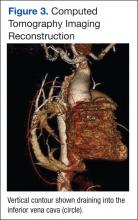
Because images suggested dextroposition of the heart and right lung hypoplasia, a chest computed tomography (CT) and angiography were done (Figure 2). The images showed hypoplasia of the right lung field with an anomalous venous return from the right midlung, having a vertical contour that drained into the supradiaphragmatic IVC. In addition, CT reconstruction demarcated the last mentioned contour draining into the IVC, consistent with scimitar syndrome (Figure 3). The patient was treated conservatively due to age, optimizing therapy for obstructive lung and cardiovascular disease.
Discussion
Partial anomalous pulmonary venous return is a relatively uncommon congenital anomaly, accounting for 0.5% to 1% of congenital heart disease.4,5 The characteristic abnormality is PAPVR of part of or the entire right lung to the IVC, either below the diaphragm or at the junction of the IVC and the right atrium. The rare combination (3%-5%) of an association of PAPVR, right lung hypoplasia, and dextroposition of the heart is designated scimitar syndrome. The scimitar vein sign is a characteristic chest roentgenographic finding of a crescentlike shadow in the right lower lung field where the curvilinear dimension gives the appearance of a scimitar sword.
Related: Another Reason Not to Smoke: Acute Eosinophilic Pneunomia
Normally, the pulmonary veins from the right and left lung carry oxygenated blood into the left atrium, then to the left ventricle, and then flowing out systemically. The SCV and IVC return the deoxygenated blood from the body system to the right atrium. From the right atrium, blood flows into the right ventricle, and then through pulmonary arteries, reaching the lungs where oxygenation occurs. In this syndrome, a left-to-right shunt is established when the anomalous pulmonary vein drains blood from the right lung into the IVC, resulting in an increased risk of developing right ventricular failure due to long-standing right ventricular volume overload.
Presentation and Diagnosis
There are two clinical presentations of scimitar syndrome: infantile and pediatric/adult. Infantile scimitar syndrome has a clinical presentation of tachypnea and heart failure within the first 2 months of life, with a high mortality rate. The pediatric/adult type is milder and frequently asymptomatic, and the diagnosis is usually incidental after performing an imaging study. Scimitar vein sign appears in 70% of the noninfantile cases, and lung hypoplasia is less severe. A spirometry may reveal mild deficits in vital capacity and FEV1. An electrocardiogram may show right ventricular hypertrophy.
Cardiac catheterization is required to confirm the diagnosis. Additionally, this procedure can help in the assessment of the pulmonary venous drainage course, pulmonary artery anatomy and pressure, scimitar vein stenosis, and presence of left-to-right shunt or other cardiac anomalies, if present. Other modalities have been suggested as alternative methods for diagnosing this condition, including the use of coronary CT and 3D echocardiography.6,7 However, these diagnostic tests are not available in all facilities and are very costly.
Treatment and Prognosis
Vida and colleagues conducted a multicentric study for the European Congenital Heart Surgeons Association on scimitar syndrome.8 Data were collected from 1997 to 2007 for 68 patients who underwent a surgical procedure. A total of 11 patients were categorized as late onset, and when compared with the infantile category, they had fewer postoperatory complications, hospital mortality, late mortality, and were less likely to develop pulmonary hypertension. Both pulmonary stenosis and pulmonary hypertension were linked with poor outcomes. It seems the younger the patient (infantile), the higher the possibility of complications and mortality. Adults who are incidentally diagnosed have a better outcome if asymptomatic. Findings such as hypoplastic lungs may predispose these patients to developing recurrent pneumonias.8,9
Related: Prevention of Venous Thromboembolism After Total Joint Replacement: A Rivaroxaban Update
Dusenbery and colleagues documented in a cohort study the relationship between poor survival and other variables. Significant variables included age at presentation, nonatrial septal defect (non-ASD) congenital heart disease, left pulmonary vein stenosis, and pulmonary artery pressure (PAP) at the time of presentation. Predictors of survival for nonsurgical patients were directly related to PAP at presentation and absence of non-ASD congenital heart disease. If the patient’s PAP is less than half of the systemic pressure, the survival is near 100% at 5 years from initial presentation.9
Surgery is the definitive treatment for PAPVR. However, asymptomatic patients with PAPVR with small left-to-right shunt do not require intervention, as the defect has no significant clinical impact, and patients have a normal life expectancy without correction.10
Surgical treatment may be considered in the following circumstances:
- A hemodynamically significant left-to-right shunt (a ratio of pulmonary to systemic blood flow is greater than 2:1), often manifested as right ventricular volume overload
- Recurrent pulmonary infections
- Compression or obstruction of surrounding structures caused by the anomalous vein
- During surgical repair of other major cardiac lesions, depending on the surgical risk of a repair and level and degree of shunting
Surgical options include redirecting the venous drainage to the left atria, ligation/embolization of vascular supply to the sequestered lobe, and pneumonectomy. The procedure complications may include thrombosis of the scimitar vein, lung infarct, hemoptysis, and pulmonary hypertension, which may lead to resection of the lung.11,12 Surgical procedures are recommended in cases where the patient has had recurrent lung infections or a significant degree of shunting. Studies have compared both approaches, demonstrating a better outcome after 10 years for those patients who were medically treated considering the aforementioned surgical indications.
Conclusion
Scimitar syndrome is a rare but welldescribed constellation of cardiopulmonary anomalies, accounting for 0.5% to 1% of congenital heart disease. It is a variant of PAPVR, in which part of or even the entire right lung is drained by right pulmonary veins that connect anomalously to the IVC. Although a diagnosis can be made by chest radiograph, further imaging is needed to corroborate the diagnosis and demonstrate other associated abnormalities.
Additional tests have been described in the literature, but these procedures are not available in all facilities and may incur a higher cost. Therefore, CT angiographic reconstruction is an alternative, noninvasive procedure. Surgery is the definitive treatment; however, asymptomatic patients with PAPVR and small left-to-right shunt do not require intervention.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cooper G. Case of malformation of the thoracic viscera: consisting of imperfect development of the right lung, and transposition of the heart. London Med Gaz. 1836;18:600-602.
2. Spentzouris G, Zandian A, Cesmebasi A, et al. The clinical anatomy of the inferior vena cava: a review of common congenital anomalies and the considerations for clinicians. Clin Anat. 2014;27(8):1234-1243.
3. Neill CA, Ferencz C, Sabiston DC, Sheldon H. The familial occurrence of hypoplastic right lung with systemic arterial supply and venous drainage “scimitar syndrome.” Bull Johns Hopkins Hosp. 1960;107:1-21.
4. Ward KE, Mullins CE. Anomalous pulmonary venous connections, pulmonary vein stenosis, and atresia of the common pulmonary vein. In: Garson A, Bricker JT, Fisher DJ, Neish SR, eds. The Science and Practice of Pediatric Cardiology. 2nd ed. Baltimore, MD: Williams and Wilkins; 1998:1431-1461.
5. Garcia-Barreto L, Vega W, Deliz R, Rodriguez W. Right hilar abnormality in a young man. Respiration. 1996;63(4):246-250.
6. Simmons DB, Menon RS, Pomeroy WL, Batts TC, Slim AM. An unusual presentation of scimitar syndrome in a military service member. Case Rep Vasc Med. 2013;2013:632402.
7. Palios J, Pernetz MA, Clements S Jr, Lerakis S. Three-dimensional echocardiography images showing anomalous pulmonary venous return in an adult with scimitar syndrome. Echocardiography. 2014;31(3):E103.
8. Vida VL, Padalino MA, Boccuzzo G, et al. Scimitar syndrome: a European Congenital Heart Surgeons Association (ECHSA) multicentric study. Circulation. 2010;122(12):1159-1166.
9. Dusenbery SM, Geva T, Seale A, et al. Outcome predictors and implications for management of scimitar syndrome. Am Heart J. 2013;165(5):770-777.
10. Sehgal A, Loughran-Fowlds A. Scimitar syndrome. Indian J Pediatr. 2005;72(3):249-251.
11. Najm HK, Williams WG, Coles JG, Rebeyka IM, Freedom RM. Scimitar syndrome: twenty years’ experience and results of repair. J Thorac Cardiovasc Surg. 1996;112(5):1161-1169.
12. Dupuis C, Charaf LA, Brevière GM, Abou P, Rémy-Jardin M, Helmius G. The “adult” form of the scimitar syndrome. Am J Cardiol. 1992;70(4):502-507.
Anatomic variations may result in abnormal return from the pulmonary veins to the right side of the heart. This group of congenital anomalies, also known as partial anomalous pulmonary venous return (PAPVR), may connect oxygenated blood from the pulmonary vein to a systemic vein before reaching the right atrium. The most common PAPVR is derived from the left upper pulmonary vein, which then connects to the left innominate vein and drains into the superior vena cava (SVC).
Scimitar syndrome is a rare PAPVR variant in which part of or the entire right lung is drained by the pulmonary vein into the inferior vena cava (IVC), giving the curvilinear dimension the appearance of a Middle Eastern sword (scimitar). The syndrome is frequently associated with other abnormalities, such as right lung hypoplasia and abnormal right lung lobation, dextroposition of the heart, right pulmonary artery hypoplasia, systemic arterial blood supply to the right lower lung from the infradiaphragmatic aorta, atrial septal defects of the secundum type, right-sided diaphragmatic hernia, and horseshoe lung.1,2 The syndrome was first described in 1836 by Cooper during an autopsy of an infant, and Dotter diagnosed the first symptomatic patient in 1949.3,4
Case Report
A 62-year-old man, former smoker (40 pack-year), with a past medical history of arterial hypertension and asthma visited the clinic, reporting exertional dyspnea. He also reported oppressive, retrosternally located exertional chest pain, 6/10 in intensity, of 3 minutes’ duration that radiated to the right chest and ameliorated with rest. Symptoms had occurred every other day for the past year. His physical exam was remarkable for central obesity. Lung auscultation was essentially clear. There was no jugular vein distention. The patient’s heart showed a regular rate and rhythm without evidence of murmurs or gallops. There was no evidence of leg edema or cyanosis. The patient’s resting oxygen saturation of 98% remained unchanged after exercise.
Related: Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
An electrocardiogram showed normal sinus rhythm with no ischemic changes. A pulmonary function test showed a forced expiratory volume (FEV1) of 1.44 L (61% of predicted), forced vital capacity (FVC) of 1.99 L (68% of predicted), and slow vital capacity (SVC) of 2.09 L (60% of predicted), with an FEV1/SVC ratio of 68% of predicted. These results suggested moderate-to-severe obstructive ventilatory impairment.
There was no response to bronchodilator therapy. Lung volumes were measured by plethysmography. The residual volume (RV), total lung capacity (TLC), and RV/TLC ratio were 2.57 L (147% of predicted), 4.66 L (88% of predicted), and 55%, respectively, suggesting severe air trapping. Diffusion lung capacity (DLCO) testing revealed 16.95 mL/min/mm Hg (73% of predicted) when corrected by hemoglobin and DLCO/alveolar volume of 4.97 mL/min/mm Hg/L (114% of predicted). This result was consistent with a mild reduction of gas transfer, which normalized when corrected by alveolar volume.
A posteroanterior chest radiograph image was remarkable for mediastinal shifting toward the right side, volume loss of the right lung, and evidence of a previous gunshot on the right chest wall (Figure 1). Previous chest imaging done in October 2009 showed an opacification of the right lower lung with indistinctness of the right cardiac border and partial obliteration of the right hemidiaphragm. The patient was treated with inhaled steroids and long- acting bronchodilators with partial improvement in dyspnea symptoms.
Myocardial perfusion imaging revealed scintigraphic evidence of heart rate-induced ischemia on the inferior and apical wall segments of the left ventricular myocardium. A transthoracic echocardiogram showed a very poor echocardiographic window. Left ventricular function seemed preserved. Transesophageal echocardiography was scheduled, but the patient missed the appointment.
Cardiac catheterization was only remarkable for 40% to 50% obstruction of the mid-left anterior descending artery, which did not explain the patient’s dyspnea or chest pain. Right side pressures were described as follows: right atrial mean, 10 mm Hg; right ventricle, 36/8 mm Hg; pulmonary artery, 33/16 mm Hg; pulmonary artery mean, 23 mm Hg; pulmonary capillary wedge pressure, 12 mm Hg; and a mean arterial pressure of 100 mm Hg. He had a left ventricle ejection fraction of 60%.
Because images suggested dextroposition of the heart and right lung hypoplasia, a chest computed tomography (CT) and angiography were done (Figure 2). The images showed hypoplasia of the right lung field with an anomalous venous return from the right midlung, having a vertical contour that drained into the supradiaphragmatic IVC. In addition, CT reconstruction demarcated the last mentioned contour draining into the IVC, consistent with scimitar syndrome (Figure 3). The patient was treated conservatively due to age, optimizing therapy for obstructive lung and cardiovascular disease.
Discussion
Partial anomalous pulmonary venous return is a relatively uncommon congenital anomaly, accounting for 0.5% to 1% of congenital heart disease.4,5 The characteristic abnormality is PAPVR of part of or the entire right lung to the IVC, either below the diaphragm or at the junction of the IVC and the right atrium. The rare combination (3%-5%) of an association of PAPVR, right lung hypoplasia, and dextroposition of the heart is designated scimitar syndrome. The scimitar vein sign is a characteristic chest roentgenographic finding of a crescentlike shadow in the right lower lung field where the curvilinear dimension gives the appearance of a scimitar sword.
Related: Another Reason Not to Smoke: Acute Eosinophilic Pneunomia
Normally, the pulmonary veins from the right and left lung carry oxygenated blood into the left atrium, then to the left ventricle, and then flowing out systemically. The SCV and IVC return the deoxygenated blood from the body system to the right atrium. From the right atrium, blood flows into the right ventricle, and then through pulmonary arteries, reaching the lungs where oxygenation occurs. In this syndrome, a left-to-right shunt is established when the anomalous pulmonary vein drains blood from the right lung into the IVC, resulting in an increased risk of developing right ventricular failure due to long-standing right ventricular volume overload.
Presentation and Diagnosis
There are two clinical presentations of scimitar syndrome: infantile and pediatric/adult. Infantile scimitar syndrome has a clinical presentation of tachypnea and heart failure within the first 2 months of life, with a high mortality rate. The pediatric/adult type is milder and frequently asymptomatic, and the diagnosis is usually incidental after performing an imaging study. Scimitar vein sign appears in 70% of the noninfantile cases, and lung hypoplasia is less severe. A spirometry may reveal mild deficits in vital capacity and FEV1. An electrocardiogram may show right ventricular hypertrophy.
Cardiac catheterization is required to confirm the diagnosis. Additionally, this procedure can help in the assessment of the pulmonary venous drainage course, pulmonary artery anatomy and pressure, scimitar vein stenosis, and presence of left-to-right shunt or other cardiac anomalies, if present. Other modalities have been suggested as alternative methods for diagnosing this condition, including the use of coronary CT and 3D echocardiography.6,7 However, these diagnostic tests are not available in all facilities and are very costly.
Treatment and Prognosis
Vida and colleagues conducted a multicentric study for the European Congenital Heart Surgeons Association on scimitar syndrome.8 Data were collected from 1997 to 2007 for 68 patients who underwent a surgical procedure. A total of 11 patients were categorized as late onset, and when compared with the infantile category, they had fewer postoperatory complications, hospital mortality, late mortality, and were less likely to develop pulmonary hypertension. Both pulmonary stenosis and pulmonary hypertension were linked with poor outcomes. It seems the younger the patient (infantile), the higher the possibility of complications and mortality. Adults who are incidentally diagnosed have a better outcome if asymptomatic. Findings such as hypoplastic lungs may predispose these patients to developing recurrent pneumonias.8,9
Related: Prevention of Venous Thromboembolism After Total Joint Replacement: A Rivaroxaban Update
Dusenbery and colleagues documented in a cohort study the relationship between poor survival and other variables. Significant variables included age at presentation, nonatrial septal defect (non-ASD) congenital heart disease, left pulmonary vein stenosis, and pulmonary artery pressure (PAP) at the time of presentation. Predictors of survival for nonsurgical patients were directly related to PAP at presentation and absence of non-ASD congenital heart disease. If the patient’s PAP is less than half of the systemic pressure, the survival is near 100% at 5 years from initial presentation.9
Surgery is the definitive treatment for PAPVR. However, asymptomatic patients with PAPVR with small left-to-right shunt do not require intervention, as the defect has no significant clinical impact, and patients have a normal life expectancy without correction.10
Surgical treatment may be considered in the following circumstances:
- A hemodynamically significant left-to-right shunt (a ratio of pulmonary to systemic blood flow is greater than 2:1), often manifested as right ventricular volume overload
- Recurrent pulmonary infections
- Compression or obstruction of surrounding structures caused by the anomalous vein
- During surgical repair of other major cardiac lesions, depending on the surgical risk of a repair and level and degree of shunting
Surgical options include redirecting the venous drainage to the left atria, ligation/embolization of vascular supply to the sequestered lobe, and pneumonectomy. The procedure complications may include thrombosis of the scimitar vein, lung infarct, hemoptysis, and pulmonary hypertension, which may lead to resection of the lung.11,12 Surgical procedures are recommended in cases where the patient has had recurrent lung infections or a significant degree of shunting. Studies have compared both approaches, demonstrating a better outcome after 10 years for those patients who were medically treated considering the aforementioned surgical indications.
Conclusion
Scimitar syndrome is a rare but welldescribed constellation of cardiopulmonary anomalies, accounting for 0.5% to 1% of congenital heart disease. It is a variant of PAPVR, in which part of or even the entire right lung is drained by right pulmonary veins that connect anomalously to the IVC. Although a diagnosis can be made by chest radiograph, further imaging is needed to corroborate the diagnosis and demonstrate other associated abnormalities.
Additional tests have been described in the literature, but these procedures are not available in all facilities and may incur a higher cost. Therefore, CT angiographic reconstruction is an alternative, noninvasive procedure. Surgery is the definitive treatment; however, asymptomatic patients with PAPVR and small left-to-right shunt do not require intervention.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Anatomic variations may result in abnormal return from the pulmonary veins to the right side of the heart. This group of congenital anomalies, also known as partial anomalous pulmonary venous return (PAPVR), may connect oxygenated blood from the pulmonary vein to a systemic vein before reaching the right atrium. The most common PAPVR is derived from the left upper pulmonary vein, which then connects to the left innominate vein and drains into the superior vena cava (SVC).
Scimitar syndrome is a rare PAPVR variant in which part of or the entire right lung is drained by the pulmonary vein into the inferior vena cava (IVC), giving the curvilinear dimension the appearance of a Middle Eastern sword (scimitar). The syndrome is frequently associated with other abnormalities, such as right lung hypoplasia and abnormal right lung lobation, dextroposition of the heart, right pulmonary artery hypoplasia, systemic arterial blood supply to the right lower lung from the infradiaphragmatic aorta, atrial septal defects of the secundum type, right-sided diaphragmatic hernia, and horseshoe lung.1,2 The syndrome was first described in 1836 by Cooper during an autopsy of an infant, and Dotter diagnosed the first symptomatic patient in 1949.3,4
Case Report
A 62-year-old man, former smoker (40 pack-year), with a past medical history of arterial hypertension and asthma visited the clinic, reporting exertional dyspnea. He also reported oppressive, retrosternally located exertional chest pain, 6/10 in intensity, of 3 minutes’ duration that radiated to the right chest and ameliorated with rest. Symptoms had occurred every other day for the past year. His physical exam was remarkable for central obesity. Lung auscultation was essentially clear. There was no jugular vein distention. The patient’s heart showed a regular rate and rhythm without evidence of murmurs or gallops. There was no evidence of leg edema or cyanosis. The patient’s resting oxygen saturation of 98% remained unchanged after exercise.
Related: Venous Thromboembolism Prophylaxis in Acutely Ill Veterans With Respiratory Disease
An electrocardiogram showed normal sinus rhythm with no ischemic changes. A pulmonary function test showed a forced expiratory volume (FEV1) of 1.44 L (61% of predicted), forced vital capacity (FVC) of 1.99 L (68% of predicted), and slow vital capacity (SVC) of 2.09 L (60% of predicted), with an FEV1/SVC ratio of 68% of predicted. These results suggested moderate-to-severe obstructive ventilatory impairment.
There was no response to bronchodilator therapy. Lung volumes were measured by plethysmography. The residual volume (RV), total lung capacity (TLC), and RV/TLC ratio were 2.57 L (147% of predicted), 4.66 L (88% of predicted), and 55%, respectively, suggesting severe air trapping. Diffusion lung capacity (DLCO) testing revealed 16.95 mL/min/mm Hg (73% of predicted) when corrected by hemoglobin and DLCO/alveolar volume of 4.97 mL/min/mm Hg/L (114% of predicted). This result was consistent with a mild reduction of gas transfer, which normalized when corrected by alveolar volume.
A posteroanterior chest radiograph image was remarkable for mediastinal shifting toward the right side, volume loss of the right lung, and evidence of a previous gunshot on the right chest wall (Figure 1). Previous chest imaging done in October 2009 showed an opacification of the right lower lung with indistinctness of the right cardiac border and partial obliteration of the right hemidiaphragm. The patient was treated with inhaled steroids and long- acting bronchodilators with partial improvement in dyspnea symptoms.
Myocardial perfusion imaging revealed scintigraphic evidence of heart rate-induced ischemia on the inferior and apical wall segments of the left ventricular myocardium. A transthoracic echocardiogram showed a very poor echocardiographic window. Left ventricular function seemed preserved. Transesophageal echocardiography was scheduled, but the patient missed the appointment.
Cardiac catheterization was only remarkable for 40% to 50% obstruction of the mid-left anterior descending artery, which did not explain the patient’s dyspnea or chest pain. Right side pressures were described as follows: right atrial mean, 10 mm Hg; right ventricle, 36/8 mm Hg; pulmonary artery, 33/16 mm Hg; pulmonary artery mean, 23 mm Hg; pulmonary capillary wedge pressure, 12 mm Hg; and a mean arterial pressure of 100 mm Hg. He had a left ventricle ejection fraction of 60%.
Because images suggested dextroposition of the heart and right lung hypoplasia, a chest computed tomography (CT) and angiography were done (Figure 2). The images showed hypoplasia of the right lung field with an anomalous venous return from the right midlung, having a vertical contour that drained into the supradiaphragmatic IVC. In addition, CT reconstruction demarcated the last mentioned contour draining into the IVC, consistent with scimitar syndrome (Figure 3). The patient was treated conservatively due to age, optimizing therapy for obstructive lung and cardiovascular disease.
Discussion
Partial anomalous pulmonary venous return is a relatively uncommon congenital anomaly, accounting for 0.5% to 1% of congenital heart disease.4,5 The characteristic abnormality is PAPVR of part of or the entire right lung to the IVC, either below the diaphragm or at the junction of the IVC and the right atrium. The rare combination (3%-5%) of an association of PAPVR, right lung hypoplasia, and dextroposition of the heart is designated scimitar syndrome. The scimitar vein sign is a characteristic chest roentgenographic finding of a crescentlike shadow in the right lower lung field where the curvilinear dimension gives the appearance of a scimitar sword.
Related: Another Reason Not to Smoke: Acute Eosinophilic Pneunomia
Normally, the pulmonary veins from the right and left lung carry oxygenated blood into the left atrium, then to the left ventricle, and then flowing out systemically. The SCV and IVC return the deoxygenated blood from the body system to the right atrium. From the right atrium, blood flows into the right ventricle, and then through pulmonary arteries, reaching the lungs where oxygenation occurs. In this syndrome, a left-to-right shunt is established when the anomalous pulmonary vein drains blood from the right lung into the IVC, resulting in an increased risk of developing right ventricular failure due to long-standing right ventricular volume overload.
Presentation and Diagnosis
There are two clinical presentations of scimitar syndrome: infantile and pediatric/adult. Infantile scimitar syndrome has a clinical presentation of tachypnea and heart failure within the first 2 months of life, with a high mortality rate. The pediatric/adult type is milder and frequently asymptomatic, and the diagnosis is usually incidental after performing an imaging study. Scimitar vein sign appears in 70% of the noninfantile cases, and lung hypoplasia is less severe. A spirometry may reveal mild deficits in vital capacity and FEV1. An electrocardiogram may show right ventricular hypertrophy.
Cardiac catheterization is required to confirm the diagnosis. Additionally, this procedure can help in the assessment of the pulmonary venous drainage course, pulmonary artery anatomy and pressure, scimitar vein stenosis, and presence of left-to-right shunt or other cardiac anomalies, if present. Other modalities have been suggested as alternative methods for diagnosing this condition, including the use of coronary CT and 3D echocardiography.6,7 However, these diagnostic tests are not available in all facilities and are very costly.
Treatment and Prognosis
Vida and colleagues conducted a multicentric study for the European Congenital Heart Surgeons Association on scimitar syndrome.8 Data were collected from 1997 to 2007 for 68 patients who underwent a surgical procedure. A total of 11 patients were categorized as late onset, and when compared with the infantile category, they had fewer postoperatory complications, hospital mortality, late mortality, and were less likely to develop pulmonary hypertension. Both pulmonary stenosis and pulmonary hypertension were linked with poor outcomes. It seems the younger the patient (infantile), the higher the possibility of complications and mortality. Adults who are incidentally diagnosed have a better outcome if asymptomatic. Findings such as hypoplastic lungs may predispose these patients to developing recurrent pneumonias.8,9
Related: Prevention of Venous Thromboembolism After Total Joint Replacement: A Rivaroxaban Update
Dusenbery and colleagues documented in a cohort study the relationship between poor survival and other variables. Significant variables included age at presentation, nonatrial septal defect (non-ASD) congenital heart disease, left pulmonary vein stenosis, and pulmonary artery pressure (PAP) at the time of presentation. Predictors of survival for nonsurgical patients were directly related to PAP at presentation and absence of non-ASD congenital heart disease. If the patient’s PAP is less than half of the systemic pressure, the survival is near 100% at 5 years from initial presentation.9
Surgery is the definitive treatment for PAPVR. However, asymptomatic patients with PAPVR with small left-to-right shunt do not require intervention, as the defect has no significant clinical impact, and patients have a normal life expectancy without correction.10
Surgical treatment may be considered in the following circumstances:
- A hemodynamically significant left-to-right shunt (a ratio of pulmonary to systemic blood flow is greater than 2:1), often manifested as right ventricular volume overload
- Recurrent pulmonary infections
- Compression or obstruction of surrounding structures caused by the anomalous vein
- During surgical repair of other major cardiac lesions, depending on the surgical risk of a repair and level and degree of shunting
Surgical options include redirecting the venous drainage to the left atria, ligation/embolization of vascular supply to the sequestered lobe, and pneumonectomy. The procedure complications may include thrombosis of the scimitar vein, lung infarct, hemoptysis, and pulmonary hypertension, which may lead to resection of the lung.11,12 Surgical procedures are recommended in cases where the patient has had recurrent lung infections or a significant degree of shunting. Studies have compared both approaches, demonstrating a better outcome after 10 years for those patients who were medically treated considering the aforementioned surgical indications.
Conclusion
Scimitar syndrome is a rare but welldescribed constellation of cardiopulmonary anomalies, accounting for 0.5% to 1% of congenital heart disease. It is a variant of PAPVR, in which part of or even the entire right lung is drained by right pulmonary veins that connect anomalously to the IVC. Although a diagnosis can be made by chest radiograph, further imaging is needed to corroborate the diagnosis and demonstrate other associated abnormalities.
Additional tests have been described in the literature, but these procedures are not available in all facilities and may incur a higher cost. Therefore, CT angiographic reconstruction is an alternative, noninvasive procedure. Surgery is the definitive treatment; however, asymptomatic patients with PAPVR and small left-to-right shunt do not require intervention.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cooper G. Case of malformation of the thoracic viscera: consisting of imperfect development of the right lung, and transposition of the heart. London Med Gaz. 1836;18:600-602.
2. Spentzouris G, Zandian A, Cesmebasi A, et al. The clinical anatomy of the inferior vena cava: a review of common congenital anomalies and the considerations for clinicians. Clin Anat. 2014;27(8):1234-1243.
3. Neill CA, Ferencz C, Sabiston DC, Sheldon H. The familial occurrence of hypoplastic right lung with systemic arterial supply and venous drainage “scimitar syndrome.” Bull Johns Hopkins Hosp. 1960;107:1-21.
4. Ward KE, Mullins CE. Anomalous pulmonary venous connections, pulmonary vein stenosis, and atresia of the common pulmonary vein. In: Garson A, Bricker JT, Fisher DJ, Neish SR, eds. The Science and Practice of Pediatric Cardiology. 2nd ed. Baltimore, MD: Williams and Wilkins; 1998:1431-1461.
5. Garcia-Barreto L, Vega W, Deliz R, Rodriguez W. Right hilar abnormality in a young man. Respiration. 1996;63(4):246-250.
6. Simmons DB, Menon RS, Pomeroy WL, Batts TC, Slim AM. An unusual presentation of scimitar syndrome in a military service member. Case Rep Vasc Med. 2013;2013:632402.
7. Palios J, Pernetz MA, Clements S Jr, Lerakis S. Three-dimensional echocardiography images showing anomalous pulmonary venous return in an adult with scimitar syndrome. Echocardiography. 2014;31(3):E103.
8. Vida VL, Padalino MA, Boccuzzo G, et al. Scimitar syndrome: a European Congenital Heart Surgeons Association (ECHSA) multicentric study. Circulation. 2010;122(12):1159-1166.
9. Dusenbery SM, Geva T, Seale A, et al. Outcome predictors and implications for management of scimitar syndrome. Am Heart J. 2013;165(5):770-777.
10. Sehgal A, Loughran-Fowlds A. Scimitar syndrome. Indian J Pediatr. 2005;72(3):249-251.
11. Najm HK, Williams WG, Coles JG, Rebeyka IM, Freedom RM. Scimitar syndrome: twenty years’ experience and results of repair. J Thorac Cardiovasc Surg. 1996;112(5):1161-1169.
12. Dupuis C, Charaf LA, Brevière GM, Abou P, Rémy-Jardin M, Helmius G. The “adult” form of the scimitar syndrome. Am J Cardiol. 1992;70(4):502-507.
1. Cooper G. Case of malformation of the thoracic viscera: consisting of imperfect development of the right lung, and transposition of the heart. London Med Gaz. 1836;18:600-602.
2. Spentzouris G, Zandian A, Cesmebasi A, et al. The clinical anatomy of the inferior vena cava: a review of common congenital anomalies and the considerations for clinicians. Clin Anat. 2014;27(8):1234-1243.
3. Neill CA, Ferencz C, Sabiston DC, Sheldon H. The familial occurrence of hypoplastic right lung with systemic arterial supply and venous drainage “scimitar syndrome.” Bull Johns Hopkins Hosp. 1960;107:1-21.
4. Ward KE, Mullins CE. Anomalous pulmonary venous connections, pulmonary vein stenosis, and atresia of the common pulmonary vein. In: Garson A, Bricker JT, Fisher DJ, Neish SR, eds. The Science and Practice of Pediatric Cardiology. 2nd ed. Baltimore, MD: Williams and Wilkins; 1998:1431-1461.
5. Garcia-Barreto L, Vega W, Deliz R, Rodriguez W. Right hilar abnormality in a young man. Respiration. 1996;63(4):246-250.
6. Simmons DB, Menon RS, Pomeroy WL, Batts TC, Slim AM. An unusual presentation of scimitar syndrome in a military service member. Case Rep Vasc Med. 2013;2013:632402.
7. Palios J, Pernetz MA, Clements S Jr, Lerakis S. Three-dimensional echocardiography images showing anomalous pulmonary venous return in an adult with scimitar syndrome. Echocardiography. 2014;31(3):E103.
8. Vida VL, Padalino MA, Boccuzzo G, et al. Scimitar syndrome: a European Congenital Heart Surgeons Association (ECHSA) multicentric study. Circulation. 2010;122(12):1159-1166.
9. Dusenbery SM, Geva T, Seale A, et al. Outcome predictors and implications for management of scimitar syndrome. Am Heart J. 2013;165(5):770-777.
10. Sehgal A, Loughran-Fowlds A. Scimitar syndrome. Indian J Pediatr. 2005;72(3):249-251.
11. Najm HK, Williams WG, Coles JG, Rebeyka IM, Freedom RM. Scimitar syndrome: twenty years’ experience and results of repair. J Thorac Cardiovasc Surg. 1996;112(5):1161-1169.
12. Dupuis C, Charaf LA, Brevière GM, Abou P, Rémy-Jardin M, Helmius G. The “adult” form of the scimitar syndrome. Am J Cardiol. 1992;70(4):502-507.