User login
Dysphagia, Dizziness, and Dysarthria
Brief history: A 32-year-old female presents with dysphagia, dizziness, and dysarthria.
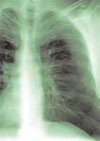
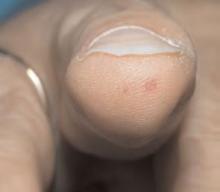
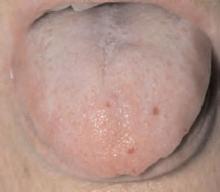
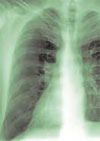
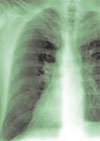
Salient findings: Chest X-ray demonstrates many embolization coils within both lungs. Photographs demonstrate superficial telangiectasias of the tongue and distal phalanx. These findings indicate the patient’s diagnosis: hereditary hemorrhagic telangiectasia (HHT), previously known as Osler-Weber-Rendu syndrome.
Patient population/natural history of disease: HHT is an autosomal dominant trait, so family members should be counseled on the implications of having a relative with the disease. HHT patients have abnormal vessels prone to bleeding and often develop arteriovenous malformations (AVMs). Diagnosis is made with 75% of the following symptoms:
- Epistaxis;
- Mucocutaneous telangiectasias;
- GI, pulmonary, or hepatic AVMs; and/or
- A first-degree relative with HHT.
Patients often present with dyspnea and hemoptysis. With pulmonary AVMs, the oxygenation and filtration functions of the lungs are bypassed, placing the patient at risk for hypoxia, polycythemia, paradoxical strokes, and brain abscesses.
Management: AVMs can be diagnosed and treated with angiography and embolization. In this patient the coils had been placed elsewhere. The use of coils larger than 3 mm in AVMs should be treated because they are associated with significantly increased morbidity and mortality. Steel coils are covered with thrombogenic fibers that induce clotting and sealing of the AVM; blood is no longer shunted through the right-to-left shunt. Unfortunately, a long-term complication of pulmonary AVMs treated by embolization therapy is the development of new pulmonary AVMs.
The patient in this case had many metallic coils visible on chest X-ray and because she had required multiple pulmonary angiograms and embolizations over the years.
It’s important to administer an ECG to all HHT patients prior to treatment; those with a left bundle branch block must have pacing mechanisms in place or at hand because catheter placement and manipulation within the right heart can induce right heart blockage. Take care to avoid air emboli in all lines due to right-to-left shunting in these patients.
Take-Home Points:
- Untreated pulmonary AVMs are associated with paradoxical strokes, brain abscesses, and hypoxia;
- HHT is associated with pulmonary AVMs;
- Coil embolization of pulmonary AVMs has been shown to improve dyspnea and oxygen saturation while decreasing right-to-left shunt fraction in HHT patients.
- Complications of embolization therapy may include development of new pulmonary AVMs; and
- All patients should undergo ECG prior to pulmonary angiography to screen for left bundle branch block. TH
Helena Summers is a radiology resident and Erik Summers is a hospitalist at the Mayo Clinic College of Medicine, Rochester, Minn.
References
- Swanson KL, Prakash UB, Stanson AW. Pulmonary arteriovenous fistulas: Mayo Clinic experience, 1982-1997. Mayo Clin Proc. 1999 Jul;74(7):671-680.
- Cottin V, Plauchu H, Bayle JY, et al. Pulmonary arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia. Am J Respir Crit Care Med. 2004 May 1;169(9):994-1000. Epub 2004 Jan 23.
Brief history: A 32-year-old female presents with dysphagia, dizziness, and dysarthria.
Salient findings: Chest X-ray demonstrates many embolization coils within both lungs. Photographs demonstrate superficial telangiectasias of the tongue and distal phalanx. These findings indicate the patient’s diagnosis: hereditary hemorrhagic telangiectasia (HHT), previously known as Osler-Weber-Rendu syndrome.
Patient population/natural history of disease: HHT is an autosomal dominant trait, so family members should be counseled on the implications of having a relative with the disease. HHT patients have abnormal vessels prone to bleeding and often develop arteriovenous malformations (AVMs). Diagnosis is made with 75% of the following symptoms:
- Epistaxis;
- Mucocutaneous telangiectasias;
- GI, pulmonary, or hepatic AVMs; and/or
- A first-degree relative with HHT.
Patients often present with dyspnea and hemoptysis. With pulmonary AVMs, the oxygenation and filtration functions of the lungs are bypassed, placing the patient at risk for hypoxia, polycythemia, paradoxical strokes, and brain abscesses.
Management: AVMs can be diagnosed and treated with angiography and embolization. In this patient the coils had been placed elsewhere. The use of coils larger than 3 mm in AVMs should be treated because they are associated with significantly increased morbidity and mortality. Steel coils are covered with thrombogenic fibers that induce clotting and sealing of the AVM; blood is no longer shunted through the right-to-left shunt. Unfortunately, a long-term complication of pulmonary AVMs treated by embolization therapy is the development of new pulmonary AVMs.
The patient in this case had many metallic coils visible on chest X-ray and because she had required multiple pulmonary angiograms and embolizations over the years.
It’s important to administer an ECG to all HHT patients prior to treatment; those with a left bundle branch block must have pacing mechanisms in place or at hand because catheter placement and manipulation within the right heart can induce right heart blockage. Take care to avoid air emboli in all lines due to right-to-left shunting in these patients.
Take-Home Points:
- Untreated pulmonary AVMs are associated with paradoxical strokes, brain abscesses, and hypoxia;
- HHT is associated with pulmonary AVMs;
- Coil embolization of pulmonary AVMs has been shown to improve dyspnea and oxygen saturation while decreasing right-to-left shunt fraction in HHT patients.
- Complications of embolization therapy may include development of new pulmonary AVMs; and
- All patients should undergo ECG prior to pulmonary angiography to screen for left bundle branch block. TH
Helena Summers is a radiology resident and Erik Summers is a hospitalist at the Mayo Clinic College of Medicine, Rochester, Minn.
References
- Swanson KL, Prakash UB, Stanson AW. Pulmonary arteriovenous fistulas: Mayo Clinic experience, 1982-1997. Mayo Clin Proc. 1999 Jul;74(7):671-680.
- Cottin V, Plauchu H, Bayle JY, et al. Pulmonary arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia. Am J Respir Crit Care Med. 2004 May 1;169(9):994-1000. Epub 2004 Jan 23.
Brief history: A 32-year-old female presents with dysphagia, dizziness, and dysarthria.
Salient findings: Chest X-ray demonstrates many embolization coils within both lungs. Photographs demonstrate superficial telangiectasias of the tongue and distal phalanx. These findings indicate the patient’s diagnosis: hereditary hemorrhagic telangiectasia (HHT), previously known as Osler-Weber-Rendu syndrome.
Patient population/natural history of disease: HHT is an autosomal dominant trait, so family members should be counseled on the implications of having a relative with the disease. HHT patients have abnormal vessels prone to bleeding and often develop arteriovenous malformations (AVMs). Diagnosis is made with 75% of the following symptoms:
- Epistaxis;
- Mucocutaneous telangiectasias;
- GI, pulmonary, or hepatic AVMs; and/or
- A first-degree relative with HHT.
Patients often present with dyspnea and hemoptysis. With pulmonary AVMs, the oxygenation and filtration functions of the lungs are bypassed, placing the patient at risk for hypoxia, polycythemia, paradoxical strokes, and brain abscesses.
Management: AVMs can be diagnosed and treated with angiography and embolization. In this patient the coils had been placed elsewhere. The use of coils larger than 3 mm in AVMs should be treated because they are associated with significantly increased morbidity and mortality. Steel coils are covered with thrombogenic fibers that induce clotting and sealing of the AVM; blood is no longer shunted through the right-to-left shunt. Unfortunately, a long-term complication of pulmonary AVMs treated by embolization therapy is the development of new pulmonary AVMs.
The patient in this case had many metallic coils visible on chest X-ray and because she had required multiple pulmonary angiograms and embolizations over the years.
It’s important to administer an ECG to all HHT patients prior to treatment; those with a left bundle branch block must have pacing mechanisms in place or at hand because catheter placement and manipulation within the right heart can induce right heart blockage. Take care to avoid air emboli in all lines due to right-to-left shunting in these patients.
Take-Home Points:
- Untreated pulmonary AVMs are associated with paradoxical strokes, brain abscesses, and hypoxia;
- HHT is associated with pulmonary AVMs;
- Coil embolization of pulmonary AVMs has been shown to improve dyspnea and oxygen saturation while decreasing right-to-left shunt fraction in HHT patients.
- Complications of embolization therapy may include development of new pulmonary AVMs; and
- All patients should undergo ECG prior to pulmonary angiography to screen for left bundle branch block. TH
Helena Summers is a radiology resident and Erik Summers is a hospitalist at the Mayo Clinic College of Medicine, Rochester, Minn.
References
- Swanson KL, Prakash UB, Stanson AW. Pulmonary arteriovenous fistulas: Mayo Clinic experience, 1982-1997. Mayo Clin Proc. 1999 Jul;74(7):671-680.
- Cottin V, Plauchu H, Bayle JY, et al. Pulmonary arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia. Am J Respir Crit Care Med. 2004 May 1;169(9):994-1000. Epub 2004 Jan 23.
Fibromuscular Dysplasia
Brief history: 52-year-old female with uncontrolled hypertension.
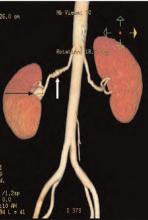
Salient findings: The middle third of the arteries are involved with a “string of pearls” appearance of alternating webs and stenoses. This appearance is classic for fibromuscular dysplasia (FMD) (white arrow, above). The patient also has a 1.8-cm right renal artery aneurysm at the trifurcation of her first order renal artery branches (black arrow, above).
Patient population and natural history of disease: FMD is most common in young adult females, and its etiology is unknown. An association with alpha-1 antitrypsin deficiency has been reported in the literature. FMD is a leading cause of curable hypertension. Clinical manifestations of FMD include distal embolization of thrombus formed in small aneurysms, hypertension/ischemia due to obstruction by webs, and occlusion/infarct via spontaneous dissection. The natural prevalence of renal artery aneurysms is low—0.1% in all angiography patients—and its natural course is not well established. Renal artery aneurysms are most common in FMD, vasculitides, neoplasm, trauma, and Ehlers-Danlos Syndrome; they may be iatrogenic or idiopathic.
Management: Symptomatic medial fibroplasia-type FMD responds well to balloon angioplasty. Renal artery aneurysms may be managed medically or surgically, depending on risk factors. Indications for repair of renal artery aneurysms include a size of 2 cm or greater, pregnancy, expansion, renovascular hypertension, distal embolization, and rupture. Mortality from ruptured renal artery aneurysms is 10% in nonpregnant patients and 55% during pregnancy.
This patient had a good response to balloon angioplasty of the left renal artery. The right renal artery could not be angioplastied secondary to increased risk of aneurysm rupture with restoration of arterial blood flow due to increased pressure on the walls of the aneurysm. Hence, physicians surgically resected the right renal artery aneurysm and performed a bypass to the aorta.
Take Home Points
- FMD is most common in young or middle-age women;
- FMD is a type of curable hypertension, treated by renal artery angioplasty;
- FMD is diagnosed by an angiographic study—in classic cases, the involved artery has a string of pearls appearance; and
- FMD is associated with renal artery aneurysms. Consider surgical intervention in aneurysms greater than 2 cm. TH
Helena Summers is a radiology resident and Erik Summers is a hospitalist at the Mayo Clinic College of Medicine, Rochester, Minn.
Bibliography
- Kaufman JA, Lee MJ. Vascular and Interventional Radiology: The Requisites. Philadelphia: Mosby; 2004.
- Bisschops RH, Popma JJ, Meyerovitz MF. Treatment of fibromuscular dysplasia and renal artery aneurysm with use of a stent-graft. J Vasc Interv Radiol. 2001 Jun;12(6):757-760.
- Luscher TF, Lie JT, Stanson AW, et al. Arterial fibromuscular dysplasia. Mayo Clin Proc. 1987;62:931-952.
Brief history: 52-year-old female with uncontrolled hypertension.
Salient findings: The middle third of the arteries are involved with a “string of pearls” appearance of alternating webs and stenoses. This appearance is classic for fibromuscular dysplasia (FMD) (white arrow, above). The patient also has a 1.8-cm right renal artery aneurysm at the trifurcation of her first order renal artery branches (black arrow, above).
Patient population and natural history of disease: FMD is most common in young adult females, and its etiology is unknown. An association with alpha-1 antitrypsin deficiency has been reported in the literature. FMD is a leading cause of curable hypertension. Clinical manifestations of FMD include distal embolization of thrombus formed in small aneurysms, hypertension/ischemia due to obstruction by webs, and occlusion/infarct via spontaneous dissection. The natural prevalence of renal artery aneurysms is low—0.1% in all angiography patients—and its natural course is not well established. Renal artery aneurysms are most common in FMD, vasculitides, neoplasm, trauma, and Ehlers-Danlos Syndrome; they may be iatrogenic or idiopathic.
Management: Symptomatic medial fibroplasia-type FMD responds well to balloon angioplasty. Renal artery aneurysms may be managed medically or surgically, depending on risk factors. Indications for repair of renal artery aneurysms include a size of 2 cm or greater, pregnancy, expansion, renovascular hypertension, distal embolization, and rupture. Mortality from ruptured renal artery aneurysms is 10% in nonpregnant patients and 55% during pregnancy.
This patient had a good response to balloon angioplasty of the left renal artery. The right renal artery could not be angioplastied secondary to increased risk of aneurysm rupture with restoration of arterial blood flow due to increased pressure on the walls of the aneurysm. Hence, physicians surgically resected the right renal artery aneurysm and performed a bypass to the aorta.
Take Home Points
- FMD is most common in young or middle-age women;
- FMD is a type of curable hypertension, treated by renal artery angioplasty;
- FMD is diagnosed by an angiographic study—in classic cases, the involved artery has a string of pearls appearance; and
- FMD is associated with renal artery aneurysms. Consider surgical intervention in aneurysms greater than 2 cm. TH
Helena Summers is a radiology resident and Erik Summers is a hospitalist at the Mayo Clinic College of Medicine, Rochester, Minn.
Bibliography
- Kaufman JA, Lee MJ. Vascular and Interventional Radiology: The Requisites. Philadelphia: Mosby; 2004.
- Bisschops RH, Popma JJ, Meyerovitz MF. Treatment of fibromuscular dysplasia and renal artery aneurysm with use of a stent-graft. J Vasc Interv Radiol. 2001 Jun;12(6):757-760.
- Luscher TF, Lie JT, Stanson AW, et al. Arterial fibromuscular dysplasia. Mayo Clin Proc. 1987;62:931-952.
Brief history: 52-year-old female with uncontrolled hypertension.
Salient findings: The middle third of the arteries are involved with a “string of pearls” appearance of alternating webs and stenoses. This appearance is classic for fibromuscular dysplasia (FMD) (white arrow, above). The patient also has a 1.8-cm right renal artery aneurysm at the trifurcation of her first order renal artery branches (black arrow, above).
Patient population and natural history of disease: FMD is most common in young adult females, and its etiology is unknown. An association with alpha-1 antitrypsin deficiency has been reported in the literature. FMD is a leading cause of curable hypertension. Clinical manifestations of FMD include distal embolization of thrombus formed in small aneurysms, hypertension/ischemia due to obstruction by webs, and occlusion/infarct via spontaneous dissection. The natural prevalence of renal artery aneurysms is low—0.1% in all angiography patients—and its natural course is not well established. Renal artery aneurysms are most common in FMD, vasculitides, neoplasm, trauma, and Ehlers-Danlos Syndrome; they may be iatrogenic or idiopathic.
Management: Symptomatic medial fibroplasia-type FMD responds well to balloon angioplasty. Renal artery aneurysms may be managed medically or surgically, depending on risk factors. Indications for repair of renal artery aneurysms include a size of 2 cm or greater, pregnancy, expansion, renovascular hypertension, distal embolization, and rupture. Mortality from ruptured renal artery aneurysms is 10% in nonpregnant patients and 55% during pregnancy.
This patient had a good response to balloon angioplasty of the left renal artery. The right renal artery could not be angioplastied secondary to increased risk of aneurysm rupture with restoration of arterial blood flow due to increased pressure on the walls of the aneurysm. Hence, physicians surgically resected the right renal artery aneurysm and performed a bypass to the aorta.
Take Home Points
- FMD is most common in young or middle-age women;
- FMD is a type of curable hypertension, treated by renal artery angioplasty;
- FMD is diagnosed by an angiographic study—in classic cases, the involved artery has a string of pearls appearance; and
- FMD is associated with renal artery aneurysms. Consider surgical intervention in aneurysms greater than 2 cm. TH
Helena Summers is a radiology resident and Erik Summers is a hospitalist at the Mayo Clinic College of Medicine, Rochester, Minn.
Bibliography
- Kaufman JA, Lee MJ. Vascular and Interventional Radiology: The Requisites. Philadelphia: Mosby; 2004.
- Bisschops RH, Popma JJ, Meyerovitz MF. Treatment of fibromuscular dysplasia and renal artery aneurysm with use of a stent-graft. J Vasc Interv Radiol. 2001 Jun;12(6):757-760.
- Luscher TF, Lie JT, Stanson AW, et al. Arterial fibromuscular dysplasia. Mayo Clin Proc. 1987;62:931-952.
A Case of Emphysematous Cystitis
Patient history: The patient is a 53-year-old woman, 70 days status post-allogenic stem-cell transplantation for multiple myeloma. She presents with fever, mental status changes, and abdominal distension. Abdominal films are ordered.
Salient Findings
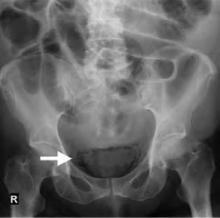
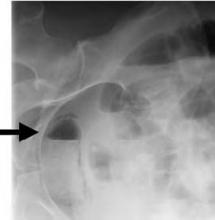
The patient has air within the wall of the bladder (see Figure 1 below, white arrow) and within the bladder lumen, diagnostic for emphysematous cystitis. On the lateral film the intravesicular air layers with a gravity dependent air-fluid level (see Figure 2 below, black arrow). The gas in the bladder wall is seen circumferentially. Because the lucency (air) remains in the dependent portions of the bladder, it must be in the bladder wall itself.
Patient population and disease history
Fifty to 80% of patients with emphysematous cystitis are diabetic. Other predisposing factors include immunosuppression, indwelling catheters, neutropenic bladders, female gender, and bladder outlet obstruction.
Most cases are caused by E. coli; less common pathogens are Enterobacter aerogenes and Klebsiella pneumoniae.
Management
Emphysematous infections are gas-forming infections of unknown mechanism. Common symptoms of emphysematous cystitis include abdominal pain, dysuria, and pneumaturia. Most patients with emphysematous cystitis (involvement of bladder only) respond well to systemic antibiotics, good bladder drainage, and excellent glycemic control.
On occasion, patients require cystectomy if unresponsive to medical management alone. Emphysematous pyelonephritis (involvement of renal parenchymal, intrarenal collecting system, and/or perinephric tissues), on the other hand, requires antibiotics along with surgical intervention. Percutaneous catheter placement is indicated for gas in the collecting system or renal parenchyma. Further extension of the gas or abscess formation may require surgical resection and/or total nephrectomy. Patients with emphysematous pyelonephritis should have a CT scan to define the extent of involvement.
Take-home points:
- Emphysematous cystitis is most common in diabetics;
- Pathogenesis is unclear at present;
- Emphysematous cystitis typically responds to IV antibiotics; and
- Emphysematous pyelonephritis requires systemic antibiotics and surgical intervention (percutaneous catheterization, resection of involved tissue, or total nephrectomy). CT can characterize the extent of tissue involvement and guide treatment. TH
Helena Summers is a radiology resident and Erik Summers is a hospitalist at the Mayo Clinic College of Medicine, Rochester, Minn.
References
- Stamm WE; Harrison’s Textbook of Internal Medicine, 16th ed; 2005, McGraw-Hill. Chapter 269.
- Huang JJ, Tseng CC. Emphysematous pyelonephritis: clinicoradiological classification, management, prognosis, and pathogenesis. Arch Intern Med. 2000;Mar 27;160(6):797-805.
- Evanoff GV, Thompson CS, Foley R, Weinman EJ. Spectrum of gas within the kidney. Emphysematous pyelonephritis and emphysematous pyelitis. Am J Med. 1987 Jul;83(1):149-154.
Patient history: The patient is a 53-year-old woman, 70 days status post-allogenic stem-cell transplantation for multiple myeloma. She presents with fever, mental status changes, and abdominal distension. Abdominal films are ordered.
Salient Findings
The patient has air within the wall of the bladder (see Figure 1 below, white arrow) and within the bladder lumen, diagnostic for emphysematous cystitis. On the lateral film the intravesicular air layers with a gravity dependent air-fluid level (see Figure 2 below, black arrow). The gas in the bladder wall is seen circumferentially. Because the lucency (air) remains in the dependent portions of the bladder, it must be in the bladder wall itself.
Patient population and disease history
Fifty to 80% of patients with emphysematous cystitis are diabetic. Other predisposing factors include immunosuppression, indwelling catheters, neutropenic bladders, female gender, and bladder outlet obstruction.
Most cases are caused by E. coli; less common pathogens are Enterobacter aerogenes and Klebsiella pneumoniae.
Management
Emphysematous infections are gas-forming infections of unknown mechanism. Common symptoms of emphysematous cystitis include abdominal pain, dysuria, and pneumaturia. Most patients with emphysematous cystitis (involvement of bladder only) respond well to systemic antibiotics, good bladder drainage, and excellent glycemic control.
On occasion, patients require cystectomy if unresponsive to medical management alone. Emphysematous pyelonephritis (involvement of renal parenchymal, intrarenal collecting system, and/or perinephric tissues), on the other hand, requires antibiotics along with surgical intervention. Percutaneous catheter placement is indicated for gas in the collecting system or renal parenchyma. Further extension of the gas or abscess formation may require surgical resection and/or total nephrectomy. Patients with emphysematous pyelonephritis should have a CT scan to define the extent of involvement.
Take-home points:
- Emphysematous cystitis is most common in diabetics;
- Pathogenesis is unclear at present;
- Emphysematous cystitis typically responds to IV antibiotics; and
- Emphysematous pyelonephritis requires systemic antibiotics and surgical intervention (percutaneous catheterization, resection of involved tissue, or total nephrectomy). CT can characterize the extent of tissue involvement and guide treatment. TH
Helena Summers is a radiology resident and Erik Summers is a hospitalist at the Mayo Clinic College of Medicine, Rochester, Minn.
References
- Stamm WE; Harrison’s Textbook of Internal Medicine, 16th ed; 2005, McGraw-Hill. Chapter 269.
- Huang JJ, Tseng CC. Emphysematous pyelonephritis: clinicoradiological classification, management, prognosis, and pathogenesis. Arch Intern Med. 2000;Mar 27;160(6):797-805.
- Evanoff GV, Thompson CS, Foley R, Weinman EJ. Spectrum of gas within the kidney. Emphysematous pyelonephritis and emphysematous pyelitis. Am J Med. 1987 Jul;83(1):149-154.
Patient history: The patient is a 53-year-old woman, 70 days status post-allogenic stem-cell transplantation for multiple myeloma. She presents with fever, mental status changes, and abdominal distension. Abdominal films are ordered.
Salient Findings
The patient has air within the wall of the bladder (see Figure 1 below, white arrow) and within the bladder lumen, diagnostic for emphysematous cystitis. On the lateral film the intravesicular air layers with a gravity dependent air-fluid level (see Figure 2 below, black arrow). The gas in the bladder wall is seen circumferentially. Because the lucency (air) remains in the dependent portions of the bladder, it must be in the bladder wall itself.
Patient population and disease history
Fifty to 80% of patients with emphysematous cystitis are diabetic. Other predisposing factors include immunosuppression, indwelling catheters, neutropenic bladders, female gender, and bladder outlet obstruction.
Most cases are caused by E. coli; less common pathogens are Enterobacter aerogenes and Klebsiella pneumoniae.
Management
Emphysematous infections are gas-forming infections of unknown mechanism. Common symptoms of emphysematous cystitis include abdominal pain, dysuria, and pneumaturia. Most patients with emphysematous cystitis (involvement of bladder only) respond well to systemic antibiotics, good bladder drainage, and excellent glycemic control.
On occasion, patients require cystectomy if unresponsive to medical management alone. Emphysematous pyelonephritis (involvement of renal parenchymal, intrarenal collecting system, and/or perinephric tissues), on the other hand, requires antibiotics along with surgical intervention. Percutaneous catheter placement is indicated for gas in the collecting system or renal parenchyma. Further extension of the gas or abscess formation may require surgical resection and/or total nephrectomy. Patients with emphysematous pyelonephritis should have a CT scan to define the extent of involvement.
Take-home points:
- Emphysematous cystitis is most common in diabetics;
- Pathogenesis is unclear at present;
- Emphysematous cystitis typically responds to IV antibiotics; and
- Emphysematous pyelonephritis requires systemic antibiotics and surgical intervention (percutaneous catheterization, resection of involved tissue, or total nephrectomy). CT can characterize the extent of tissue involvement and guide treatment. TH
Helena Summers is a radiology resident and Erik Summers is a hospitalist at the Mayo Clinic College of Medicine, Rochester, Minn.
References
- Stamm WE; Harrison’s Textbook of Internal Medicine, 16th ed; 2005, McGraw-Hill. Chapter 269.
- Huang JJ, Tseng CC. Emphysematous pyelonephritis: clinicoradiological classification, management, prognosis, and pathogenesis. Arch Intern Med. 2000;Mar 27;160(6):797-805.
- Evanoff GV, Thompson CS, Foley R, Weinman EJ. Spectrum of gas within the kidney. Emphysematous pyelonephritis and emphysematous pyelitis. Am J Med. 1987 Jul;83(1):149-154.